User login
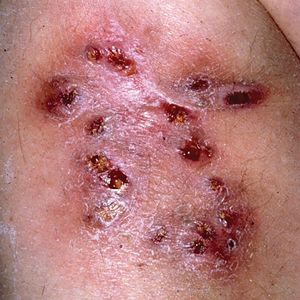
Multiple Draining Sinus Tracts on the Thigh
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
The Diagnosis: Mycobacterial Infection
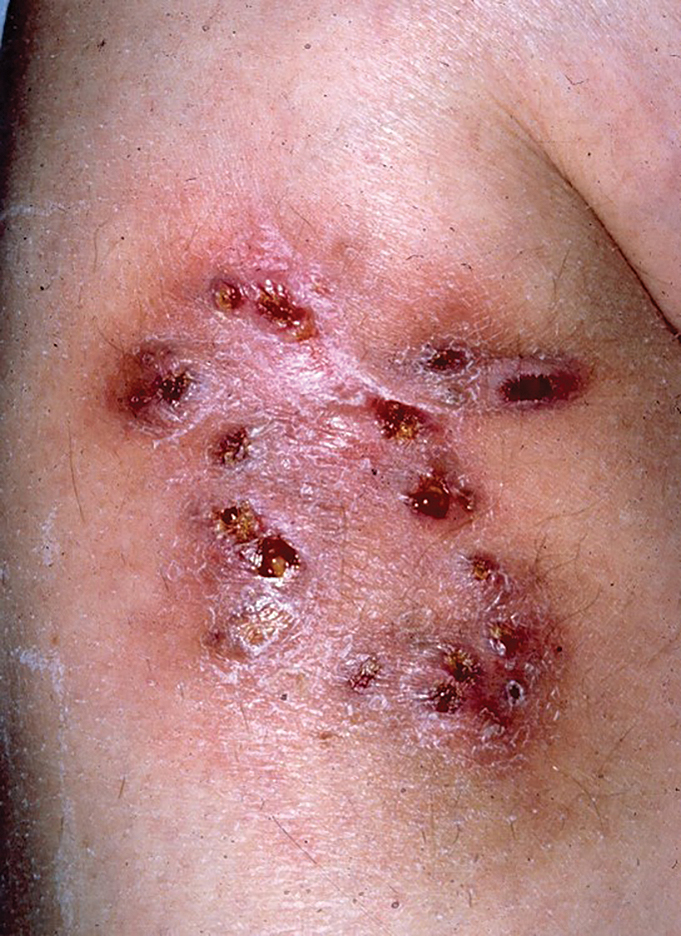
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
The Diagnosis: Mycobacterial Infection
An injury sustained in a wet environment that results in chronic indolent abscesses, nodules, or draining sinus tracts suggests a mycobacterial infection. In our patient, a culture revealed MycobacteriuM fortuitum, which is classified in the rapid grower nontuberculous mycobacteria (NTM) group, along with Mycobacterium chelonae and Mycobacterium abscessus.1 The patient’s history of skin injury while cutting wet grass and the common presence of M fortuitum in the environment suggested that the organism entered the wound. The patient healed completely following surgical excision and a 2-month course of clarithromycin 1 g daily and rifampin 600 mg daily.
MycobacteriuM fortuitum was first isolated from an amphibian source in 1905 and later identified in a human with cutaneous infection in 1938. It commonly is found in soil and water.2 Skin and soft-tissue infections with M fortuitum usually are acquired from direct entry of the organism through a damaged skin barrier from trauma, medical injection, surgery, or tattoo placement.2,3
Skin lesions caused by NTM often are nonspecific and can mimic a variety of other dermatologic conditions, making clinical diagnosis challenging. As such, cutaneous manifestations of M fortuitum infection can include recurrent cutaneous abscesses, nodular lesions, chronic discharging sinuses, cellulitis, and surgical site infections.4 Although cutaneous infection with M fortuitum classically manifests with a single subcutaneous nodule at the site of trauma or surgery,5 it also can manifest as multiple draining sinus tracts, as seen in our patient. Hence, the diagnosis and treatment of cutaneous NTM infection is challenging, especially when M fortuitum skin manifestations can take up to 4 to 6 weeks to develop after inoculation. Diagnosis often requires a detailed patient history, tissue cultures, and histopathology.5
In recent years, rapid detection with polymerase chain reaction (PCR) techniques has been employed more widely. Notably, a molecular system based on multiplex real-time PCR with high-resolution melting was shown to have a sensitivity of up to 54% for distinguishing M fortuitum from other NTM.6 More recently, a 2-step real-time PCR method has demonstrated diagnostic sensitivity and specificity for differentiating NTM from Mycobacterium tuberculosis infections and identifying the causative NTM agent.7
Compared to immunocompetent individuals, those who are immunocompromised are more susceptible to less pathogenic strains of NTM, which can cause dissemination and lead to tenosynovitis, myositis, osteomyelitis, and septic arthritis.8-12 Nonetheless, cases of infections with NTM—including M fortuitum—are becoming harder to treat. Several single nucleotide polymorphisms and point mutations have been demonstrated in the ribosomal RNA methylase gene erm(39) related to clarithromycin resistance and in the rrl gene related to linezolid resistance.13 Due to increasing inducible resistance to common classes of antibiotics, such as macrolides and linezolid, treatment of M fortuitum requires multidrug regimens.13,14 Drug susceptibility testing also may be required, as M fortuitum has shown low resistance to tigecycline, tetracycline, cefmetazole, imipenem, and aminoglycosides (eg, amikacin, tobramycin, neomycin, gentamycin). Surgery is an important adjunctive tool in treating M fortuitum infections; patients with a single lesion are more likely to undergo surgical treatment alone or in combination with antibiotic therapy.15 More recently, antimicrobial photodynamic therapy has been explored as an alternative to eliminate NTM, including M fortuitum.16
The differential diagnosis for skin lesions manifesting with draining fistulae and sinus tracts includes conditions with infectious (cellulitis and chromomycosis) and inflammatory (pyoderma gangrenosum [PG] and hidradenitis suppurativa [HS]) causes.
Cellulitis is a common infection of the skin and subcutaneous tissue that predominantly is caused by gram-positive organisms such as β-hemolytic streptococci.17 Clinical manifestations include acute skin erythema, swelling, tenderness, and warmth. The legs are the most common sites of infection, but any area of the skin can be involved.17 Cellulitis comprises 10% of all infectious disease hospitalizations and up to 11% of all dermatologic admissions.18,19 It frequently is misdiagnosed, perhaps due to the lack of a reliable confirmatory laboratory test or imaging study, in addition to the plethora of diseases that mimic cellulitis, such as stasis dermatitis, lipodermatosclerosis, contact dermatitis, lymphedema, eosinophilic cellulitis, and papular urticaria.20,21 The consequences of misdiagnosis include but are not limited to unnecessary hospitalizations, inappropriate antibiotic use, and delayed management of the disease; thus, there is an urgent need for a reliable standard test to confirm the diagnosis, especially among nonspecialist physicians. 20 Most patients with uncomplicated cellulitis can be treated with empiric oral antibiotics that target β-hemolytic streptococci (ie, penicillin V potassium, amoxicillin).17 Methicillin-resistant Staphylococcus aureus coverage generally is unnecessary for nonpurulent cellulitis, but clinicians can consider adding amoxicillin-clavulanate, dicloxacillin, and cephalexin to the regimen. For purulent cellulitis, incision and drainage should be performed. In severe cases that manifest with sepsis, altered mental status, or hemodynamic instability, inpatient management is required.17
Chromomycosis (also known as chromoblastomycosis) is a chronic, indolent, granulomatous, suppurative mycosis of the skin and subcutaneous tissue22 that is caused by traumatic inoculation of various fungi of the order Chaetothyriales and family Herpotrichiellaceae, which are present in soil, plants, and decomposing wood. Chromomycosis is prevalent in tropical and subtropical regions.23,24 Clinically, it manifests as oligosymptomatic or asymptomatic lesions around an infection site that can manifest as papules with centrifugal growth evolving into nodular, verrucous, plaque, tumoral, or atrophic forms.22 Diagnosis is made with direct microscopy using potassium hydroxide, which reveals muriform bodies. Fungal culture in Sabouraud agar also can be used to isolate the causative pathogen.22 Unfortunately, chromomycosis is difficult to treat, with low cure rates and high relapse rates. Antifungal agents combined with surgery, cryotherapy, or thermotherapy often are used, with cure rates ranging from 15% to 80%.22,25
Pyoderma gangrenosum is a reactive noninfectious inflammatory dermatosis associated with inflammatory bowel disease and rheumatoid arthritis. The exact etiology is not clearly understood, but it generally is considered an autoinflammatory disorder.26 The most common form—classical PG—occurs in approximately 85% of cases and manifests as a painful erythematous lesion that progresses to a blistered or necrotic ulcer. It primarily affects the lower legs but can occur in other body sites.27 The diagnosis is based on clinical symptoms after excluding other similar conditions; histopathology of biopsied wound tissues often are required for confirmation. Treatment of PG starts with fast-acting immunosuppressive drugs (corticosteroids and/or cyclosporine) followed by slowacting immunosuppressive drugs (biologics).26
Hidradenitis suppurativa is a chronic recurrent disease of the hair follicle unit that develops after puberty.28 Clinically, HS manifests with painful nodules, abscesses, chronically draining fistulas, and scarring in areas of the body rich in apocrine glands.29,30 Treatment of HS is challenging due to its diverse clinical manifestations and unclear etiology. Topical therapy, systemic treatments, biologic agents, surgery, and light therapy have shown variable results.28,31
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
- Franco-Paredes C, Marcos LA, Henao-Martínez AF, et al. Cutaneous mycobacterial infections. Clin Microbiol Rev. 2018;32: E00069-18. doi:10.1128/CMR.00069-18
- Brown TH. The rapidly growing mycobacteria—MycobacteriuM fortuitum and Mycobacterium chelonae. Infect Control. 1985;6:283-238. doi:10.1017/s0195941700061762
- Hooper J; Beltrami EJ; Santoro F; et al. Remember the fite: a case of cutaneous MycobacteriuM fortuitum infection. Am J Dermatopathol. 2023;45:214-215. doi:10.1097/DAD.0000000000002336
- Franco-Paredes C, Chastain DB, Allen L, et al. Overview of cutaneous mycobacterial infections. Curr Trop Med Rep. 2018;5:228-232. doi:10.1007/s40475-018-0161-7
- Gonzalez-Santiago TM, Drage LA. Nontuberculous mycobacteria: skin and soft tissue infections. Dermatol Clin. 2015;33:563-77. doi:10.1016/j.det.2015.03.017
- Peixoto ADS, Montenegro LML, Lima AS, et al. Identification of nontuberculous mycobacteria species by multiplex real-time PCR with high-resolution melting. Rev Soc Bras Med Trop. 2020;53:E20200211. doi:10.1590/0037-8682-0211-2020
- Park J, Kwak N, Chae JC, et al. A two-step real-time PCR method to identify Mycobacterium tuberculosis infections and six dominant nontuberculous mycobacterial infections from clinical specimens. Microbiol Spectr. 2023:E0160623. doi:10.1128/spectrum.01606-23
- Fowler J, Mahlen SD. Localized cutaneous infections in immunocompetent individuals due to rapidly growing mycobacteria. Arch Pathol Lab Med. 2014;138:1106-1109. doi:10.5858/arpa.2012-0203-RS
- Gardini G, Gregori N, Matteelli A, et al. Mycobacterial skin infection. Curr Opin Infect Dis. 2022;35:79-87. doi:10.1097/QCO.0000000000000820
- Wang SH, Pancholi P. Mycobacterial skin and soft tissue infection. Curr Infect Dis Rep. 2014;16:438. doi:10.1007/s11908-014-0438-5
- Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Disease Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Mougari F, Guglielmetti L, Raskine L, et al. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Expert Rev Anti Infect Ther. 2016;14:1139-1154. doi:10.1080/14787210.201 6.1238304
- Tu HZ, Lee HS, Chen YS, et al. High rates of antimicrobial resistance in rapidly growing mycobacterial infections in Taiwan. Pathogens. 2022;11:969. doi:10.3390/pathogens11090969
- Hashemzadeh M, Zadegan Dezfuli AA, Khosravi AD, et al. F requency of mutations in erm(39) related to clarithromycin resistance and in rrl related to linezolid resistance in clinical isolates of MycobacteriuM fortuitum in Iran. Acta Microbiol Immunol Hung. 2023;70:167-176. doi:10.1556/030.2023.02020
- Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292. doi:10.1001/archderm.142.10.1287
- Miretti M, Juri L, Peralta A, et al. Photoinactivation of non-tuberculous mycobacteria using Zn-phthalocyanine loaded into liposomes. Tuberculosis (Edinb). 2022;136:102247. doi:10.1016/j.tube.2022.102247
- Bystritsky RJ. Cellulitis. Infect Dis Clin North Am. 2021;35:49-60. doi:10.1016/j.idc.2020.10.002
- Christensen K, Holman R, Steiner C, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49:1025-1035. doi:10.1086/605562
- Yang JJ, Maloney NJ, Bach DQ, et al. Dermatology in the emergency department: prescriptions, rates of inpatient admission, and predictors of high utilization in the United States from 1996 to 2012. J Am Acad Dermatol. 2021;84:1480-1483. doi:10.1016/J.JAAD.2020.07.055
- Cutler TS, Jannat-Khah DP, Kam B, et al. Prevalence of misdiagnosis of cellulitis: a systematic review and meta-analysis. J Hosp Med. 2023;18:254-261. doi:10.1002/jhm.12977
- Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79:547-52. doi:10.3949/ccjm.79a.11121
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- McGinnis MR. Chromoblastomycosis and phaeohyphomycosis: new concepts, diagnosis, and mycology. J Am Acad Dermatol. 1983;8:1-16.
- Rubin HA, Bruce S, Rosen T, et al. Evidence for percutaneous inoculation as the mode of transmission for chromoblastomycosis. J Am Acad Dermatol. 1991;25:951-954.
- Bonifaz A, Paredes-Solís V, Saúl A. Treating chromoblastomycosis with systemic antifungals. Expert Opin Pharmacother. 2004;5:247-254.
- Maverakis E, Marzano AV, Le ST, et al. Pyoderma gangrenosum. Nat Rev Dis Primers. 2020;6:81. doi:10.1038/s41572-020-0213-x
- George C, Deroide F, Rustin M. Pyoderma gangrenosum—a guide to diagnosis and management. Clin Med (Lond). 2019;19:224-228. doi:10.7861/clinmedicine.19-3-224
- Narla S, Lyons AB, Hamzavi IH. The most recent advances in understanding and managing hidradenitis suppurativa. F1000Res. 2020;9:F1000 Faculty Rev-1049. doi:10.12688/f1000research.26083.1
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118-122. doi:10.1016/j.jaad.2017.02.005
- Daxhelet M, Suppa M, White J, et al. Proposed definitions of typical lesions in hidradenitis suppurativa. Dermatology. 2020;236:431-438. doi:10.1159/000507348
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, et al. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;12:20406223211055920. doi:10.1177/20406223211055920
A 40-year-old woman presented with multiple draining sinus tracts on the right thigh following an injury sustained weeks earlier while mowing wet grass.
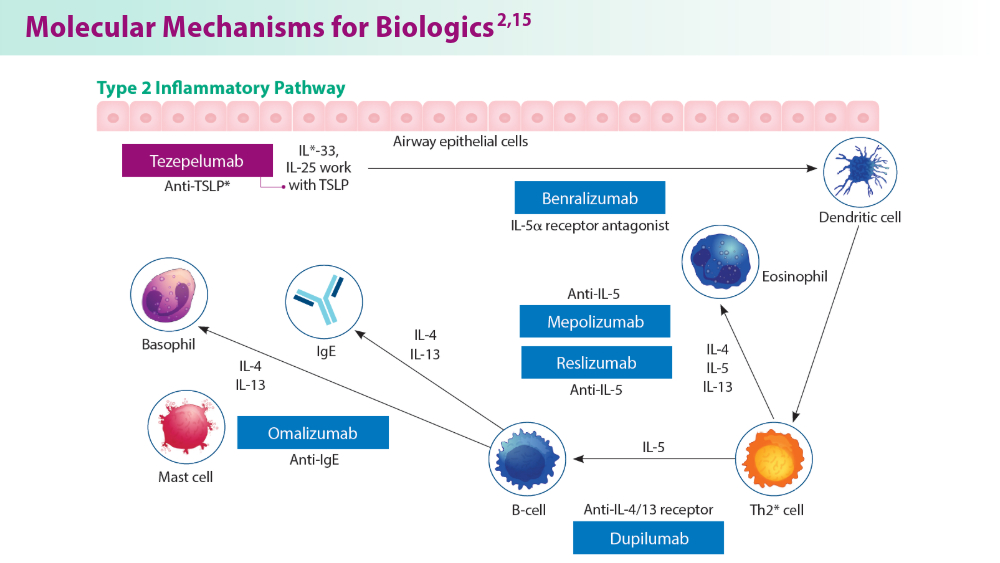
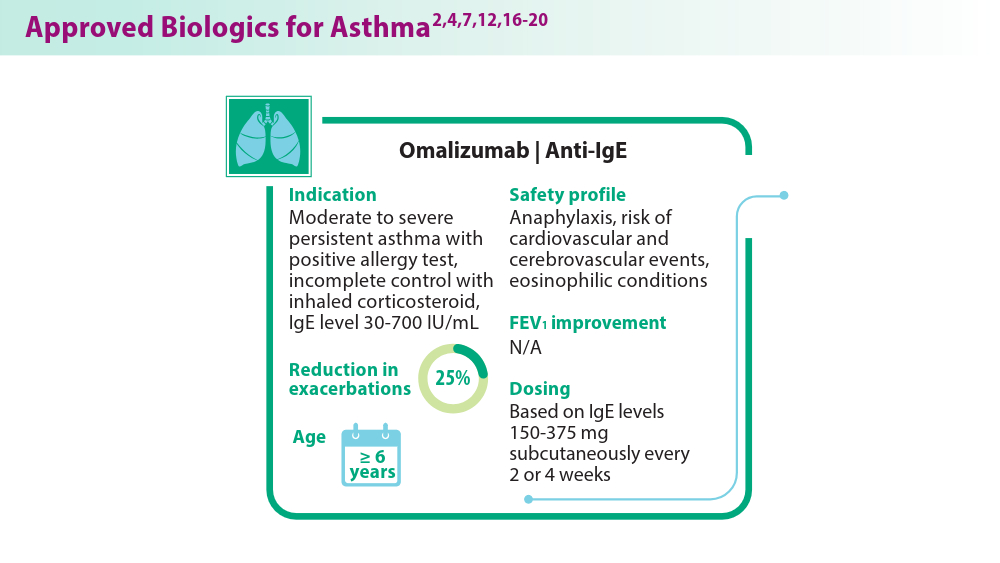
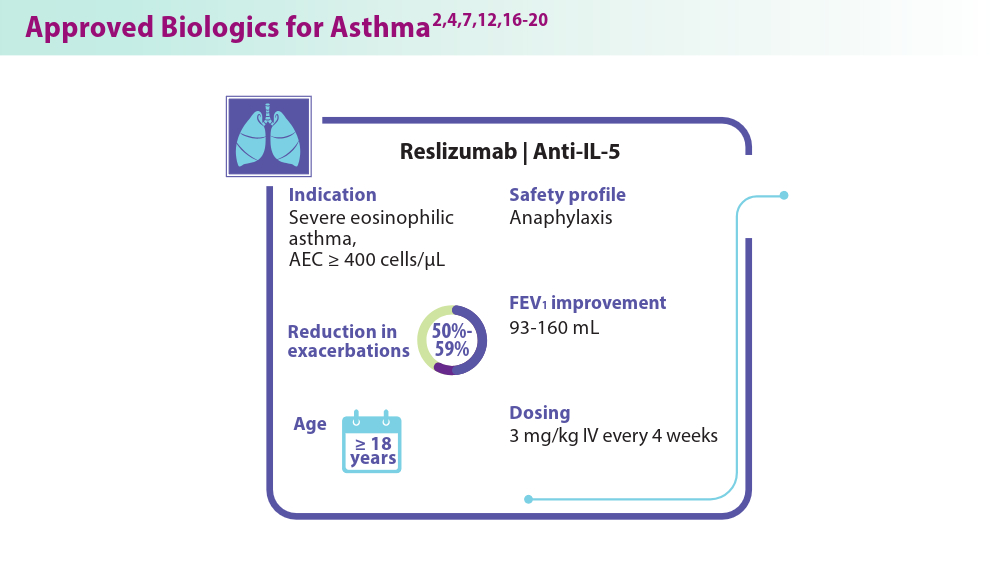
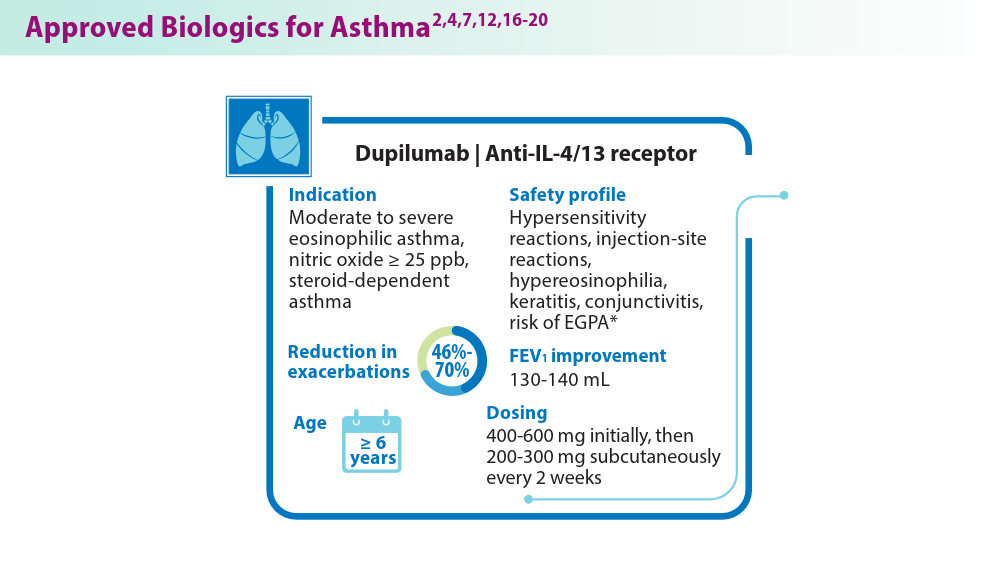
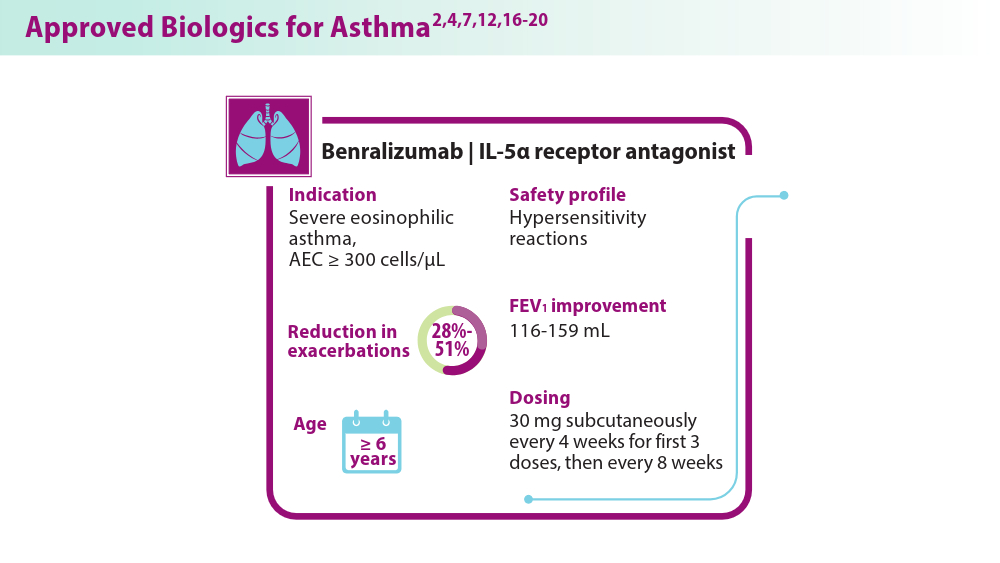
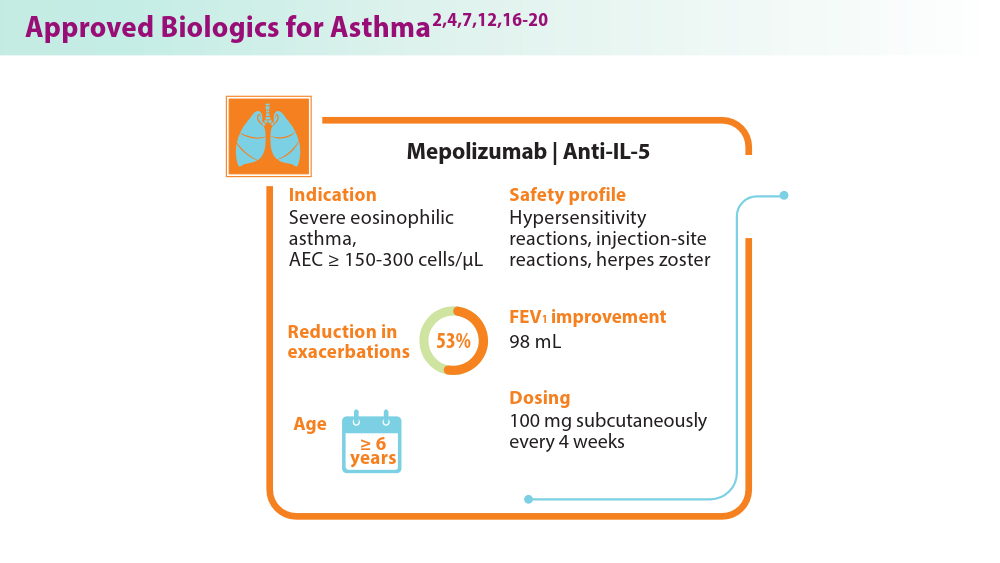
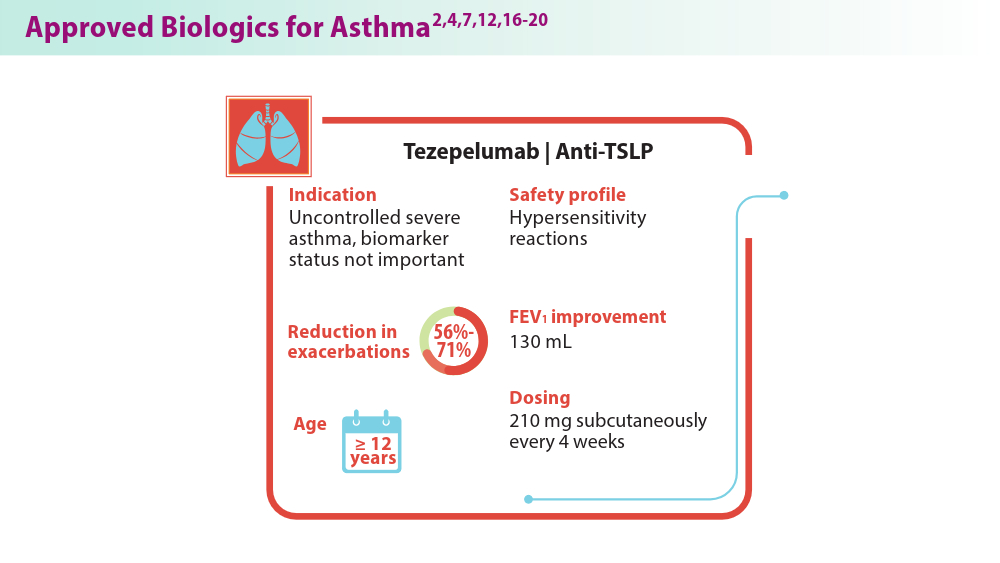
Biologics in Asthma: Changing the Severe Asthma Paradigm
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
- Shah PA, Brightling C. Biologics for severe asthma—which, when and why? Respirology. 2023;28(8):709-721. doi:10.1111/resp.14520
- Rogers L, Jesenak M, Bjermer L, Hanania NA, Seys SF, Diamant Z. Biologics in severe asthma: a pragmatic approach for choosing the right treatment for the right patient. Respir Med. 2023;218:107414. doi:10.1016/j.rmed.2023.107414
- Frøssing L, Silberbrandt A, Von Bülow A, Backer V, Porsbjerg C. The Prevalence of Subtypes of Type 2 Inflammation in an Unselected Population of Patients with Severe Asthma. J Allergy Clin Immunol Pract. 2021;9(3):1267-1275. doi:10.1016/j.jaip.2020.09.051
- McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199(4):433-445. doi:10.1164/rccm.201810-1944CI
- d'Ancona G, Kavanagh J, Roxas C, et al. Adherence to corticosteroids and clinical outcomes in mepolizumab therapy for severe asthma. Eur Respir J. 2020;55(5):1902259. Published 2020 May 7. doi:10.1183/13993003.02259-2019
- Exacerbation reduction & other clinical information | TEZSPIRE® (tezepelumab-Ekko) for hcps. Accessed July 25, 2024. https://www.tezspirehcp.com/efficacy-and-clinical-data/exacerbation-reductions-and-clinical-in-formation.html
- Exacerbation reduction in patients 12+ years. DUPIXENT® (dupilumab) for healthcare providers. Accessed June 18, 2024. https://www.dupixenthcp.com/asthma/efficacy/exacerbations
- Korn S, Bourdin A, Chupp G, et al. Integrated Safety and Efficacy Among Patients Receiving Benralizumab for Up to 5 Years. J Allergy Clin Immunol Pract. 2021;9(12):4381-4392.e4. doi:10.1016/j.jaip.2021.07.058
- Jackson DJ, Heaney LG, Humbert M, et al; for the SHAMAL Investigators. Reduction of daily maintenance inhaled corticosteroids in patients with severe eosinophilic asthma treated with benralizumab (SHAMAL): a randomised, multicentre, open-label, phase 4 study [published correction appears in Lancet. 2024;403(10432):1140]. Lancet. 2024;403(10423):271-281. doi:10.1016/S0140-6736(23)02284-5
- Thomas D, McDonald VM, Stevens S, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy. 2024;79(2):384-392. doi:10.1111/all.15867
- Hansen S, Baastrup Søndergaard M, von Bülow A, et al. Clinical response and remission in patients with severe asthma treated with biologic therapies. Chest. 2024;165(2):253-266. doi:10.1016/j.chest.2023.10.046
- Bagnasco D, Savarino EV, Yacoub MR, et al. Personalized and precision medicine in asthma and eosinophilic esophagitis: the role of T2 target therapy. Pharmaceutics. 2023;15(9):2359. doi:10.3390/pharmaceutics15092359
- Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry [published correction appears in Chest. 2021;160(5):1989.]. Chest. 2020;157(4):790-804. doi:10.1016/j.chest.2019.10.053
- Inselman JW, Jeffery MM, Maddux JT, Shah NS, Rank MA. Trends and Disparities in Asthma Biologic Use in the United States. J Allergy Clin Immunol Pract. 2020;8(2):549-554.e1. doi:10.1016/j.jaip.2019.08.024
- Pelaia C, Crimi C, Vatrella A, Tinello C, Terracciano R, Pelaia G. Molecular targets for biological therapies of severe asthma. Front Immunol. 2020;11:603312. doi:10.3389/fimmu.2020.603312
- Biologics for the treatment of asthma. Asthma and Allergy Foundation of America. Reviewed November 2023. Accessed June 18, 2024. https://aafa.org/asthma/asthma-treatment/biologics-asthma-treatment/
- Safety profile. TEZSPIRE® (tezepelumab-ekko) for healthcare providers. Accessed June 18, 2024. https://www.tezspirehcp.com/safety-profile.html
- Nucala (mepolizumab) for hcps. Severe Eosinophilic Asthma | NUCALA (mepolizumab) for HCPs. Accessed August 1, 2024. https://nucalahcp.com/severe-eosinophilic-asthma/.
- Xolair® (omalizumab). xolair. Accessed August 1, 2024. https://www.xolairhcp.com/allergic-asthma/side-effects/summary.html.
- Cinqair. Cinqairhcp.com. Accessed August 1, 2024. https://www.cinqairhcp.com/efficacy-and-safety-profiles/.
The Genetic Side of Interstitial Lung Disease
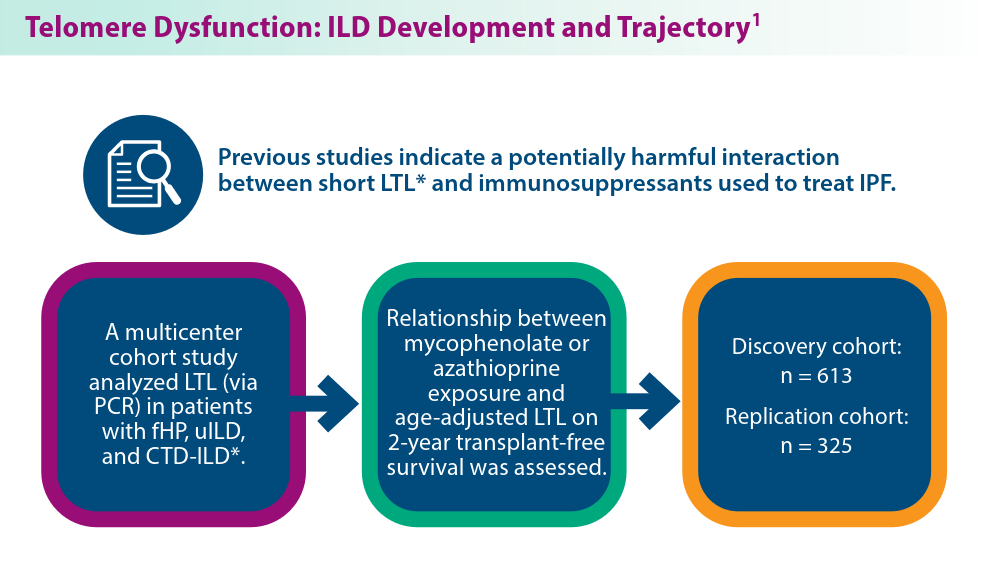
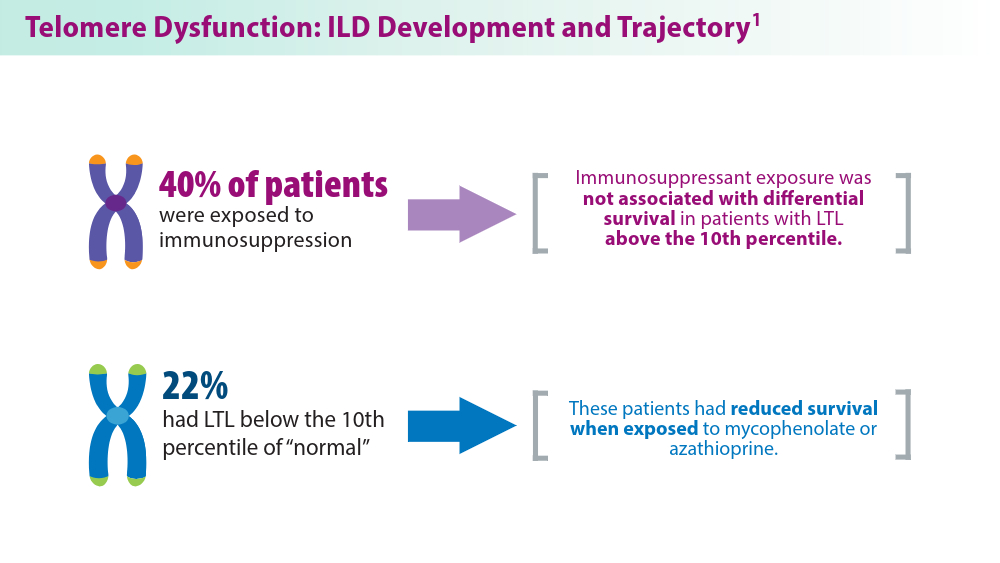
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
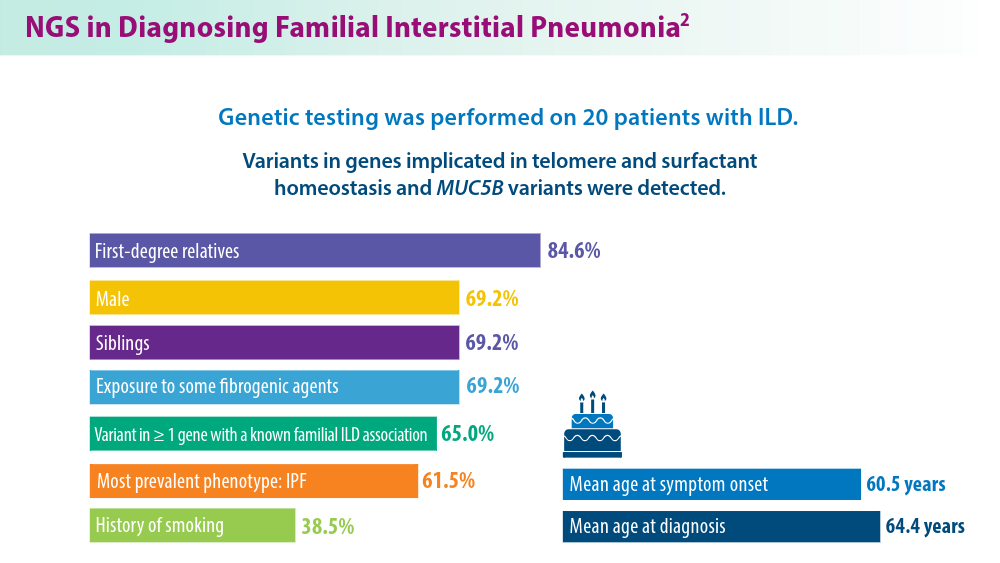
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
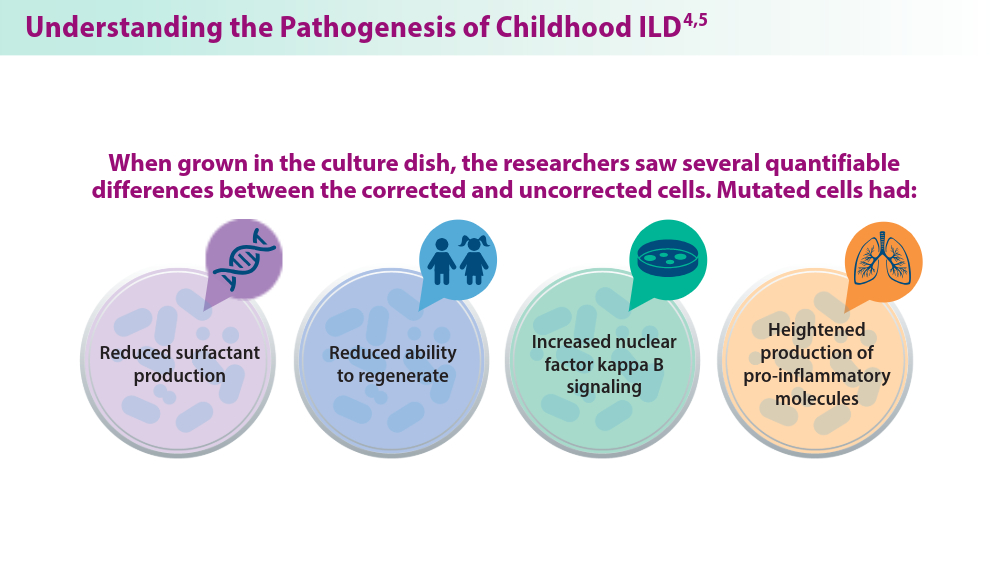
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
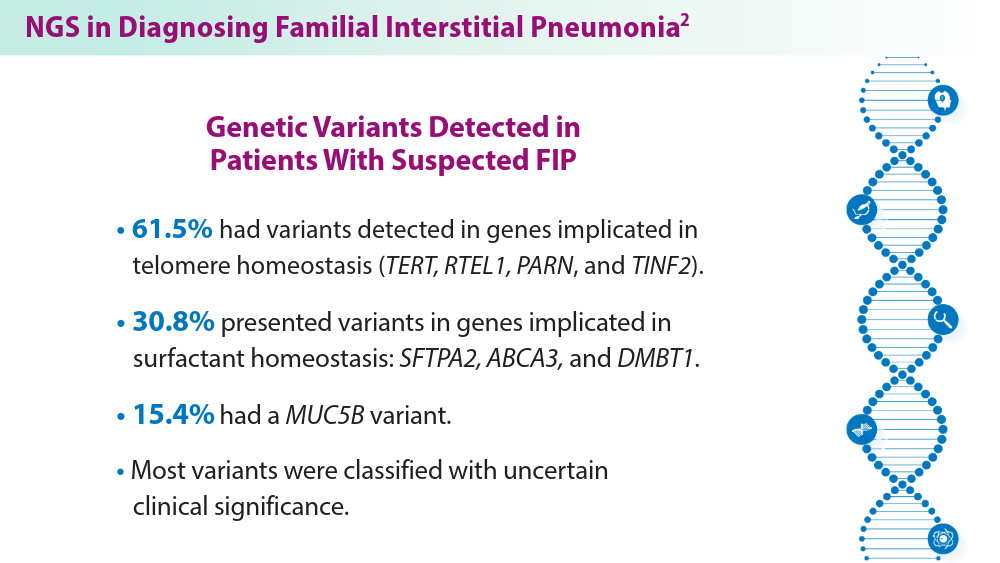
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
- Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441. doi:10.1183/13993003.00441-2023
- Gigante AR, Tinoco EM, Fonseca A, et al. Use of next-generation sequencing to support the diagnosis of familial interstitial pneumonia. Genes (Basel). 2023;14(2):326. doi:10.3390/genes14020326
- Adegunsoye A, Kropski JA, Behr J, et al. Genetics and genomics of pulmonary fibrosis: charting the molecular landscape and shaping precision medicine. Am J Respir Crit Care Med. Published online April 4, 2024. doi:10.1164/rccm.202401-0238SO
- Sun YL, Hennessey EE, Heins H, et al. Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations. J Clin Invest. 2024;134(2):e164274. doi:10.1172/JCI164274
- Raghu G, Torres JM, Bennett RL. Genetic factors for ILD—the path of precision medicine. Lancet Respir Med. Published online March 20, 2024. doi:10.1016/S2213-2600(24)00071-7
PTSD: The Basics
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Editor's Note: This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
Severe Community-Acquired Pneumonia: Diagnostic Criteria, Treatment, and COVID-19
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
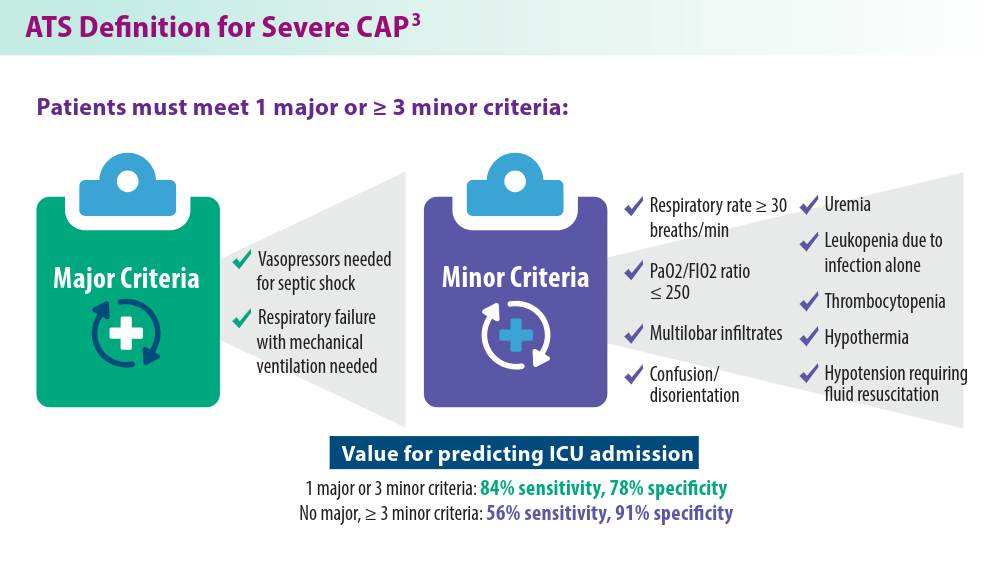
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
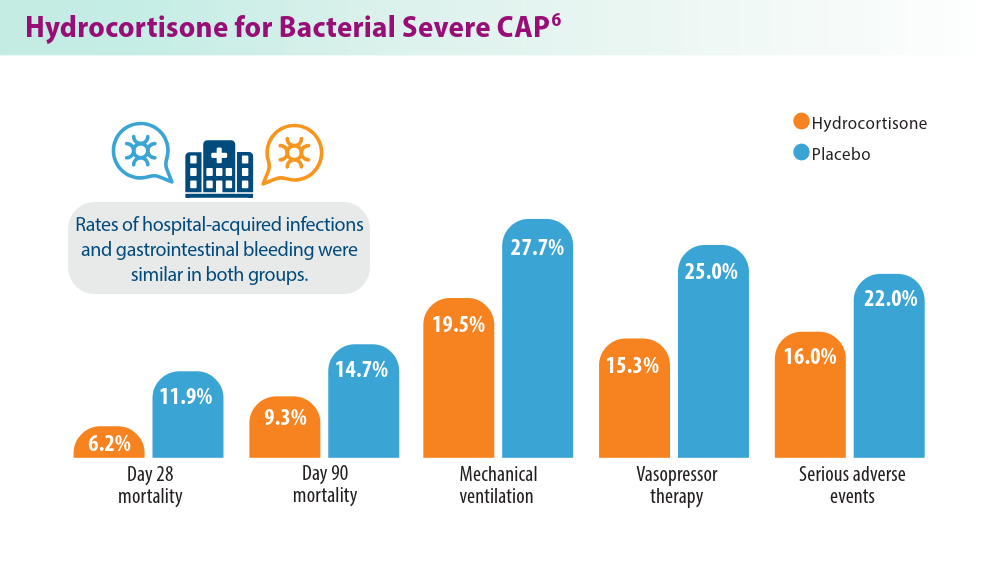
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
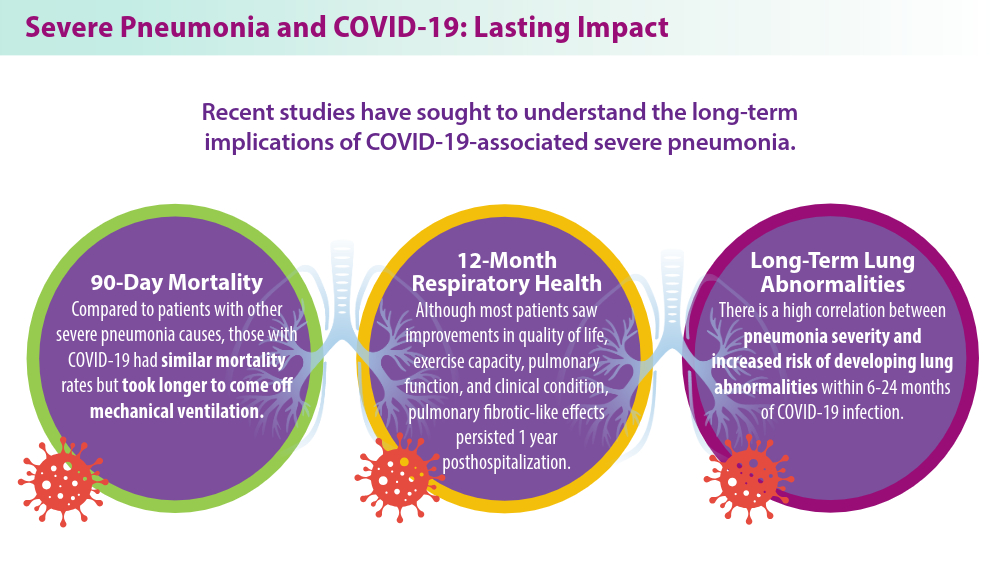
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
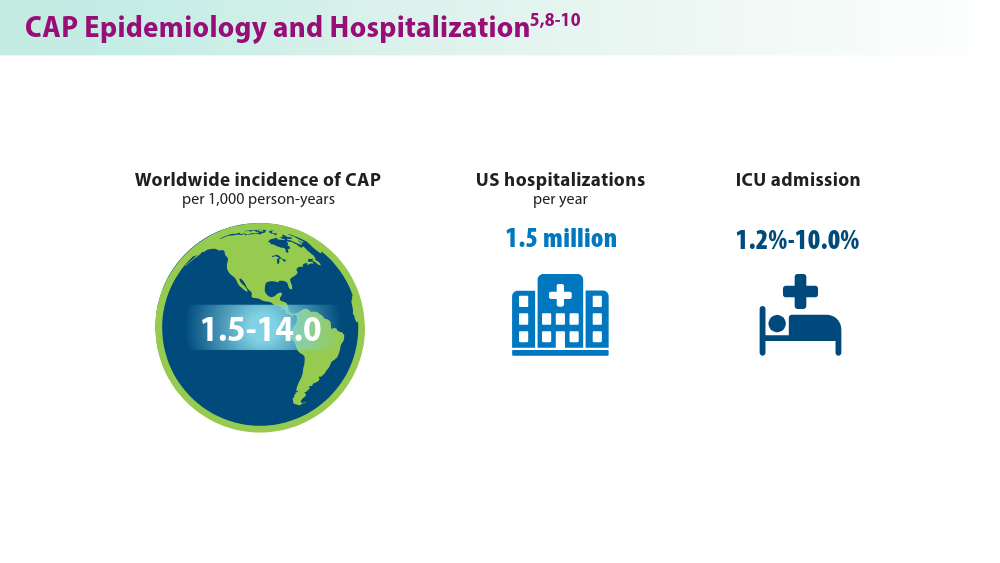
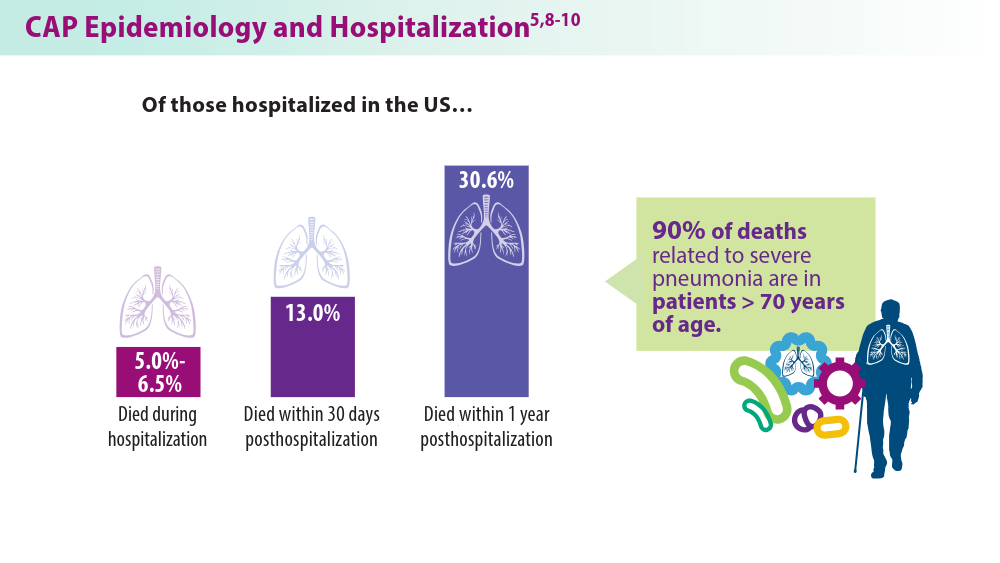
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
- Torres A, Cilloniz C, Niederman MS, et al. Pneumonia. Nat Rev Dis Primers. 2021;7(1):25. doi:10.1038/s41572-021-00259-0
- Niederman MS, Torres A. Severe community-acquired pneumonia. Eur Respir Rev. 2022;31(166):220123. doi:10.1183/16000617.0123-2022
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. doi:10.1164/rccm.201908-1581ST
- Memon RA, Rashid MA, Avva S, et al. The use of the SMART-COP score in predicting severity outcomes among patients with community-acquired pneumonia: a meta-analysis. Cureus. 2022;14(7):e27248. doi:10.7759/cureus.27248
- Regunath H, Oba Y. Community-acquired pneumonia. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2024. Updated January 26, 2024. Accessed May 14, 2024. https://www.ncbi.nlm.nih.gov/books/NBK430749/
- Dequin PF, Meziani F, Quenot JP, et al; for the CRICS-TriGGERSep Network. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931-1941. doi:10.1056/NEJMoa2215145
- Eizaguirre S, Sabater G, Belda S, et al. Long-term respiratory consequences of COVID-19 related pneumonia: a cohort study. BMC Pulm Med. 2023;23(1):439. doi:10.1186/s12890-023-02627-w
- Ramirez JA, Wiemken TL, Peyrani P, et al; for the University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65(11):1806-1812. doi:10.1093/cid/cix647
- Morgan AJ, Glossop AJ. Severe community-acquired pneumonia. BJA Educ. 2016;16(5):167-172. doi:10.1093/bjaed/mkv052
- Haessler S, Guo N, Deshpande A, et al. Etiology, treatments, and outcomes of patients with severe community-acquired pneumonia in a large U.S. sample. Crit Care Med. 2022;50(7):1063-1071. doi:10.1097/CCM.0000000000005498
- Nolley EP, Sahetya SK, Hochberg CH, et al. Outcomes among mechanically ventilated patients with severe pneumonia and acute hypoxemic respiratory failure from SARS-CoV-2 and other etiologies. JAMA Netw Open. 2023;6(1):e2250401. doi:10.1001/jamanetworkopen.2022.50401
- Hino T, Nishino M, Valtchinov VI, et al. Severe COVID-19 pneumonia leads to post-COVID-19 lung abnormalities on follow-up CT scans. Eur J Radiol Open. 2023;10:100483. doi:10.1016/j.ejro.2023.100483
Artificial Intelligence in Sleep Apnea
Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/S2213-2600(19)30198-5
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hia KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
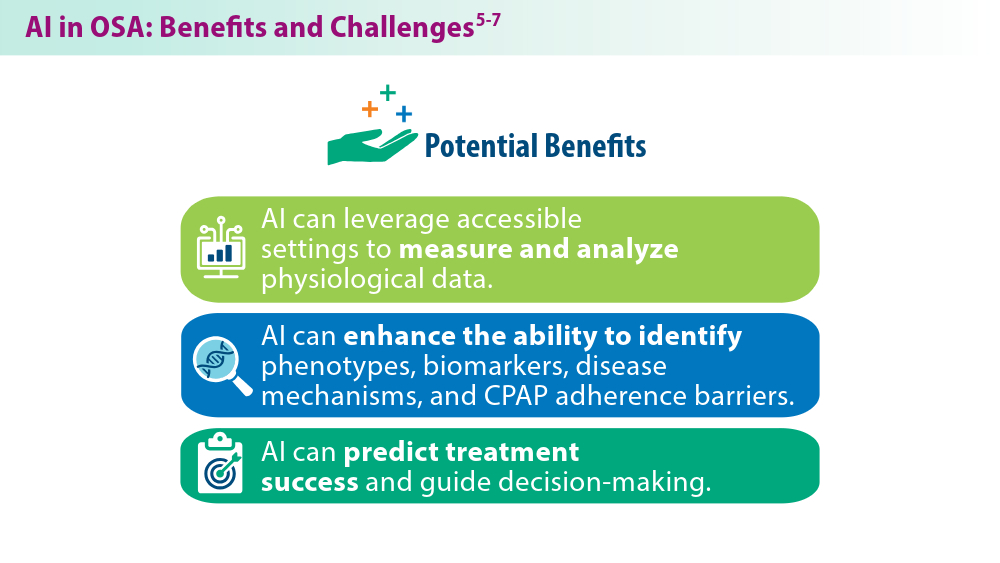
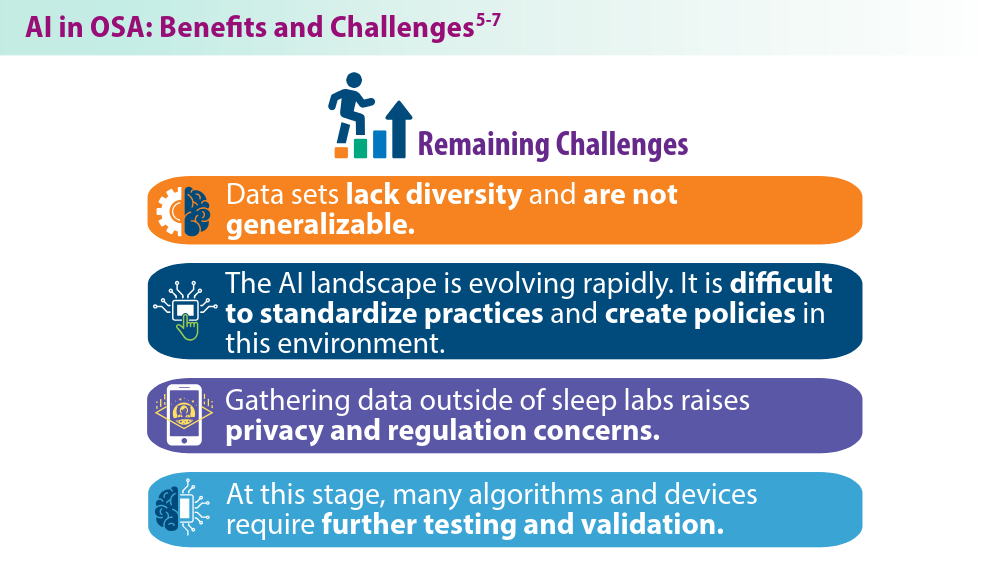
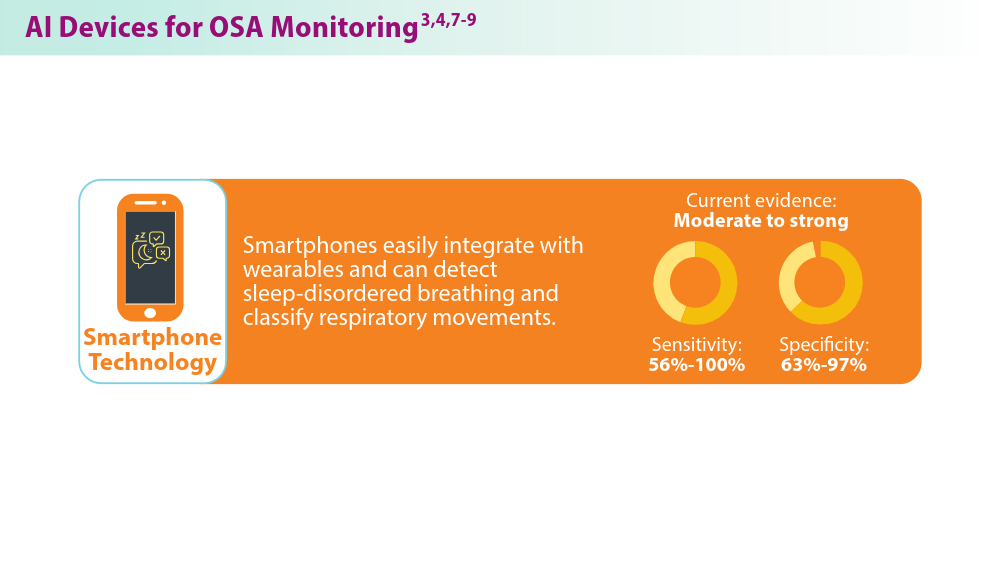
Nag DS, Swain A, Sahu S, Chatterjee A, Swain BP. Relevance of sleep for wellness: new trends in using artificial intelligence and machine learning. World J Clin Cases. 2024;12(7):1196-1199. doi:10.12998/wjcc.v12.i7.1196
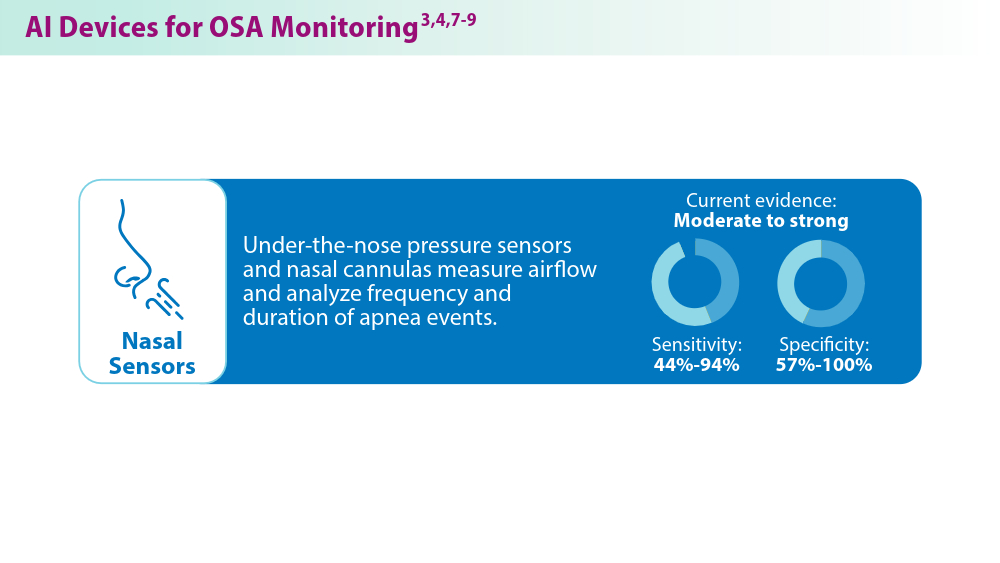
Duarte M, Pereira-Rodrigues P, Ferreira-Santos D. The role of novel digital clinical tools in the screening or diagnosis of obstructive sleep apnea: systematic review. J Med Internet Res. 2023;25:e47735. doi:10.2196/47735
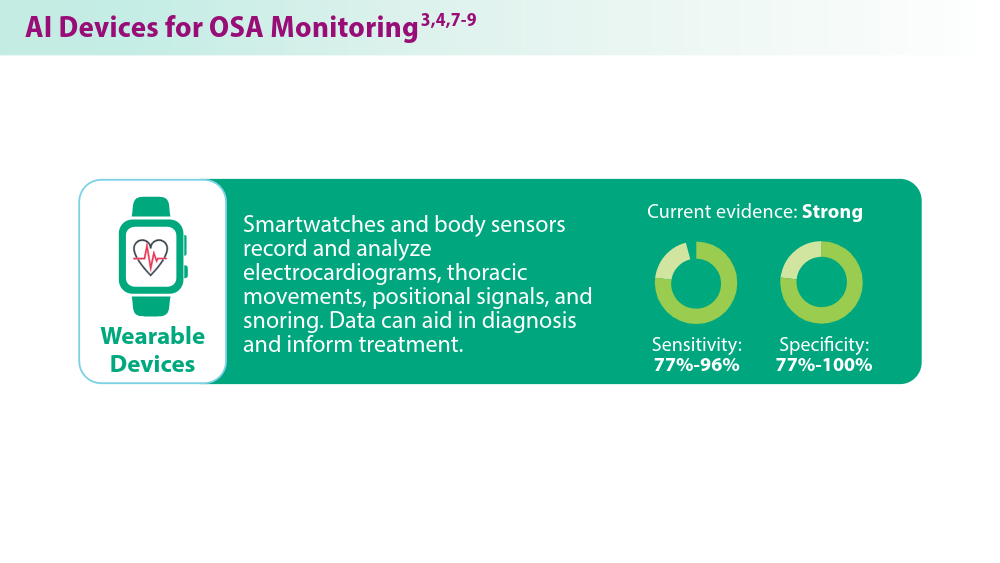
Bandyopadhyay A, Goldstein C. Clinical applications of artificial intelligence in sleep medicine: a sleep clinician's perspective. Sleep Breath. 2023;27(1):39-55. doi:10.1007/s11325-022-02592-4
Verma RK, Dhillon G, Grewal H, et al. Artificial intelligence in sleep medicine: present and future. World J Clin Cases. 2023;11(34):8106-8110. doi:10.12998/wjcc.v11.i34.8106
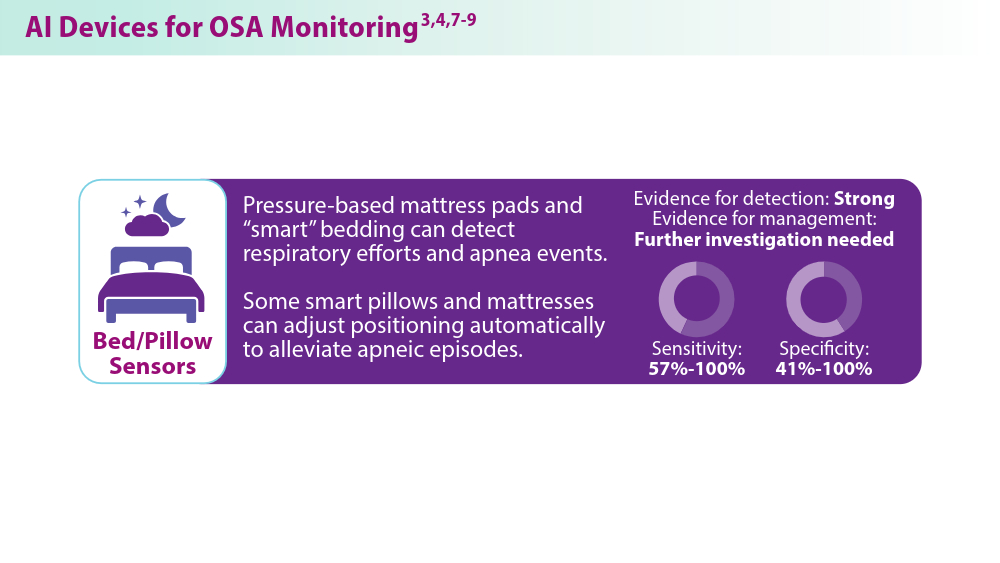
Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol Head Neck Surg. 2023;52(1):7. doi:10.1186/s40463-023-00621-0
Chung TT, Lee MT, Ku MC, Yang KC, Wei CY. Efficacy of a smart antisnore pillow in patients with obstructive sleep apnea syndrome. Behav Neurol. 2021;2021:8824011. doi:10.1155/2021/8824011
Rusk S, Nygate YN, Fernandez C, et al. 0463 Deep learning classification of future PAP adherence based on CMS and other adherence criteria. Sleep. 2023;46(suppl 1):A206. doi:10.1093/sleep/zsad077.0463
Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/S2213-2600(19)30198-5
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hia KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
Nag DS, Swain A, Sahu S, Chatterjee A, Swain BP. Relevance of sleep for wellness: new trends in using artificial intelligence and machine learning. World J Clin Cases. 2024;12(7):1196-1199. doi:10.12998/wjcc.v12.i7.1196
Duarte M, Pereira-Rodrigues P, Ferreira-Santos D. The role of novel digital clinical tools in the screening or diagnosis of obstructive sleep apnea: systematic review. J Med Internet Res. 2023;25:e47735. doi:10.2196/47735
Bandyopadhyay A, Goldstein C. Clinical applications of artificial intelligence in sleep medicine: a sleep clinician's perspective. Sleep Breath. 2023;27(1):39-55. doi:10.1007/s11325-022-02592-4
Verma RK, Dhillon G, Grewal H, et al. Artificial intelligence in sleep medicine: present and future. World J Clin Cases. 2023;11(34):8106-8110. doi:10.12998/wjcc.v11.i34.8106
Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol Head Neck Surg. 2023;52(1):7. doi:10.1186/s40463-023-00621-0
Chung TT, Lee MT, Ku MC, Yang KC, Wei CY. Efficacy of a smart antisnore pillow in patients with obstructive sleep apnea syndrome. Behav Neurol. 2021;2021:8824011. doi:10.1155/2021/8824011
Rusk S, Nygate YN, Fernandez C, et al. 0463 Deep learning classification of future PAP adherence based on CMS and other adherence criteria. Sleep. 2023;46(suppl 1):A206. doi:10.1093/sleep/zsad077.0463
Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. 2019;7(8):687-698. doi:10.1016/S2213-2600(19)30198-5
Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hia KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014. doi:10.1093/aje/kws342
Nag DS, Swain A, Sahu S, Chatterjee A, Swain BP. Relevance of sleep for wellness: new trends in using artificial intelligence and machine learning. World J Clin Cases. 2024;12(7):1196-1199. doi:10.12998/wjcc.v12.i7.1196
Duarte M, Pereira-Rodrigues P, Ferreira-Santos D. The role of novel digital clinical tools in the screening or diagnosis of obstructive sleep apnea: systematic review. J Med Internet Res. 2023;25:e47735. doi:10.2196/47735
Bandyopadhyay A, Goldstein C. Clinical applications of artificial intelligence in sleep medicine: a sleep clinician's perspective. Sleep Breath. 2023;27(1):39-55. doi:10.1007/s11325-022-02592-4
Verma RK, Dhillon G, Grewal H, et al. Artificial intelligence in sleep medicine: present and future. World J Clin Cases. 2023;11(34):8106-8110. doi:10.12998/wjcc.v11.i34.8106
Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol Head Neck Surg. 2023;52(1):7. doi:10.1186/s40463-023-00621-0
Chung TT, Lee MT, Ku MC, Yang KC, Wei CY. Efficacy of a smart antisnore pillow in patients with obstructive sleep apnea syndrome. Behav Neurol. 2021;2021:8824011. doi:10.1155/2021/8824011
Rusk S, Nygate YN, Fernandez C, et al. 0463 Deep learning classification of future PAP adherence based on CMS and other adherence criteria. Sleep. 2023;46(suppl 1):A206. doi:10.1093/sleep/zsad077.0463
Acute Tender Papules on the Arms and Legs
The Diagnosis: Erythema Nodosum Leprosum
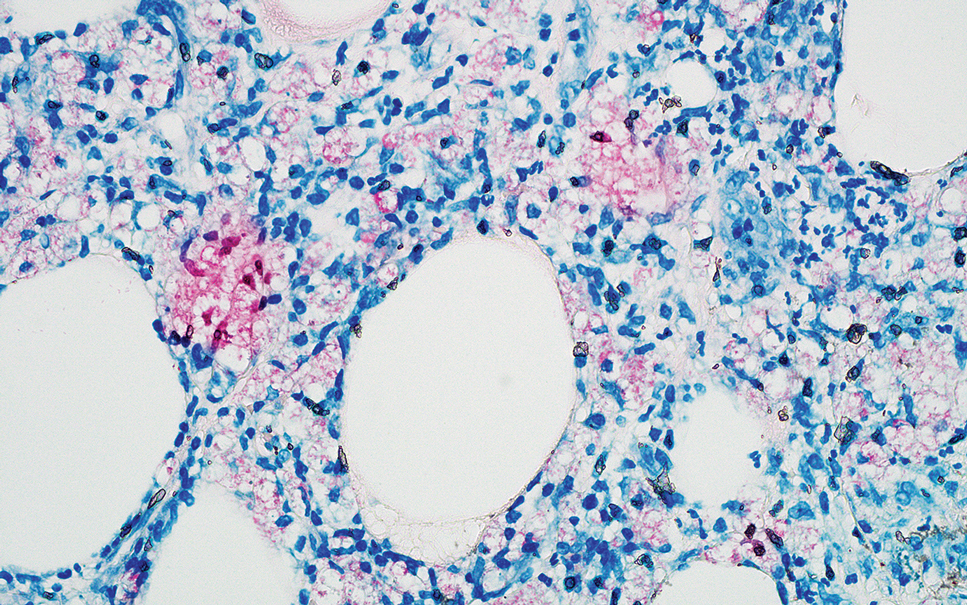
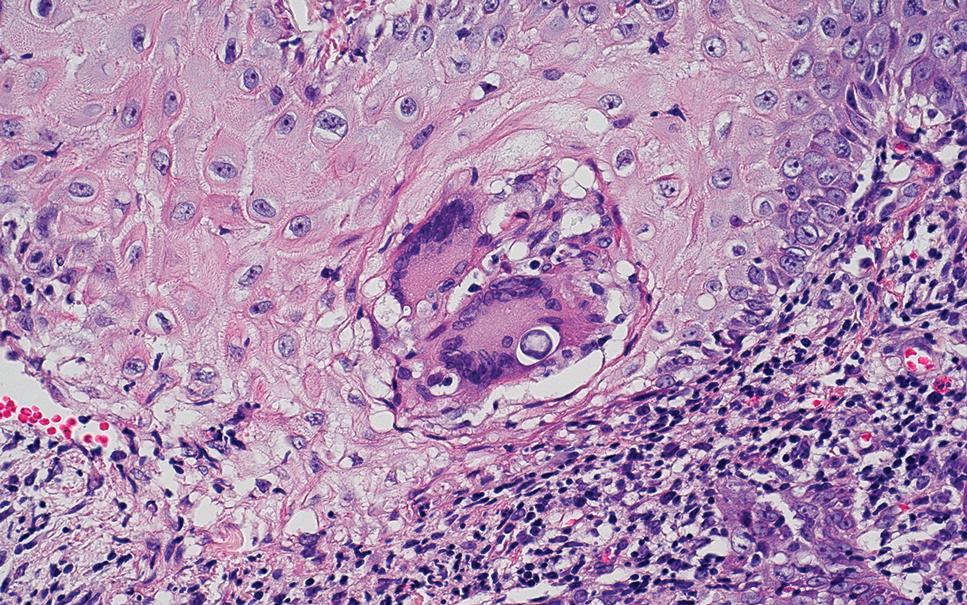
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).
Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
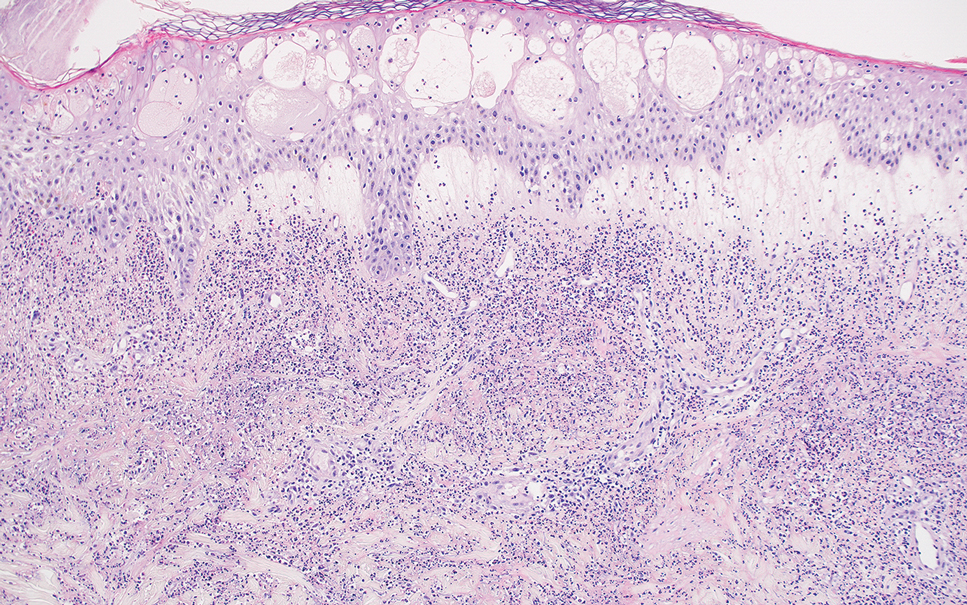
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.
Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.
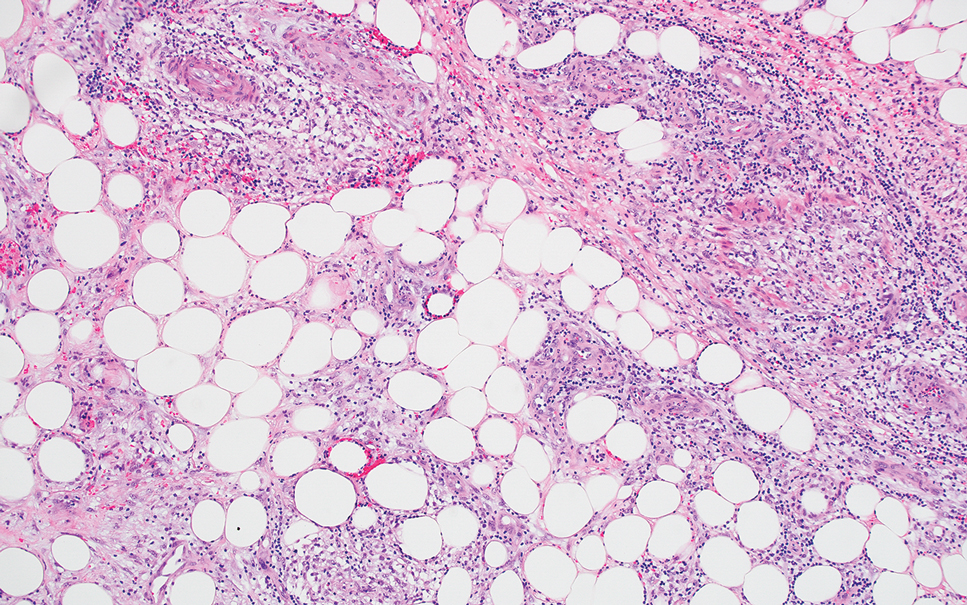
Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9
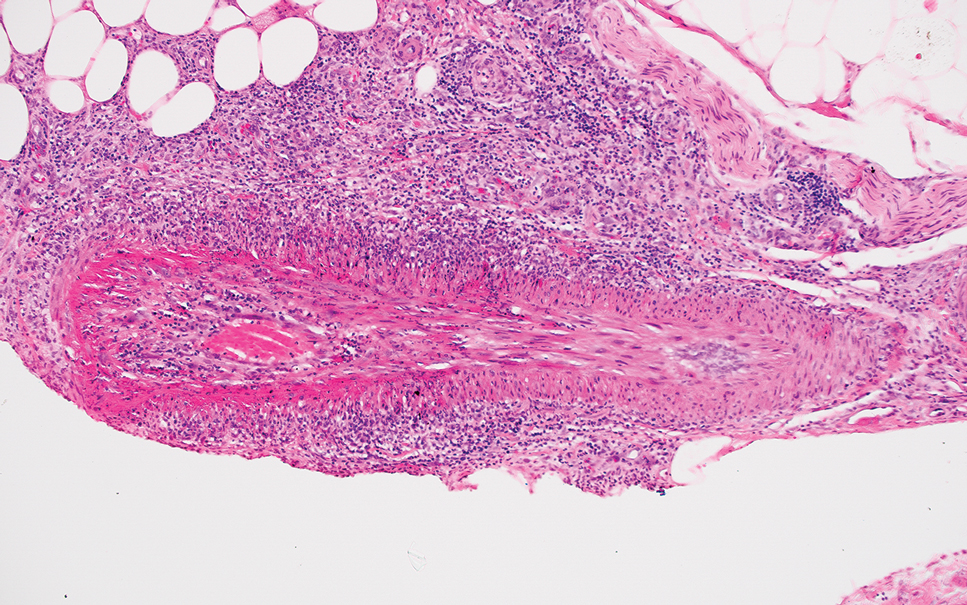
Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

The Diagnosis: Erythema Nodosum Leprosum
Erythema nodosum leprosum (ENL) is a type 2 reaction sometimes seen in patients infected with Mycobacterium leprae—primarily those with lepromatous or borderline lepromatous subtypes. Clinically, ENL manifests with abrupt onset of tender erythematous papules with associated fevers and general malaise. Studies have demonstrated a complex immune system reaction in ENL, but the detailed pathophysiology is not fully understood.1 Biopsies conducted within 24 hours of lesion formation are most elucidating. Foamy histiocytes admixed with neutrophils are seen in the subcutis, often causing a lobular panniculitis (quiz image).2 Neutrophils rarely are seen in other types of leprosy and thus are a useful diagnostic clue for ENL. Vasculitis of small- to medium-sized vessels can be seen but is not a necessary diagnostic criterion. Fite staining will highlight many acid-fast bacilli within the histiocytes (Figure 1).

Erythema nodosum leprosum is treated with a combination of immunosuppressants such as prednisone and thalidomide. Our patient was taking triple-antibiotic therapy—dapsone, rifampin, and clofazimine—for lepromatous leprosy when the erythematous papules developed on the arms and legs. After a skin biopsy confirmed the diagnosis of ENL, he was started on prednisone 20 mg daily with plans for close follow-up. Unfortunately, the patient was subsequently lost to follow-up.
Acute febrile neutrophilic dermatosis (also known as Sweet syndrome) is an acute inflammatory disease characterized by abrupt onset of painful erythematous papules, plaques, or nodules on the skin. It often is seen in association with preceding infections (especially those in the upper respiratory or gastrointestinal tracts), hematologic malignancies, inflammatory bowel disease, or exposure to certain classes of medications (eg, granulocyte colony-stimulating factor, tyrosine kinase inhibitors, various antibiotics).3 Histologically, acute febrile neutrophilic dermatosis is characterized by dense neutrophilic infiltrates, often with notable dermal edema (Figure 2).4 Many cases also show leukocytoclastic vasculitis; however, foamy histiocytes are not a notable component of the inflammatory infiltrate, though a histiocytoid form of acute febrile neutrophilic dermatosis has been described.5 Infections must be rigorously ruled out prior to diagnosing a patient with acute febrile neutrophilic dermatosis, making it a diagnosis of exclusion.

Cutaneous coccidioidomycosis is an infection caused by the dimorphic fungi Coccidioides immitis or Coccidioides posadasii. Cutaneous disease is rare but can occur from direct inoculation or dissemination from pulmonary disease in immunocompetent or immunocompromised patients. Papules, pustules, or plaques are seen clinically. Histologically, cutaneous coccidioidomycosis shows spherules that vary from 10 to 100 μm and are filled with multiple smaller endospores (Figure 3).6 Pseudoepitheliomatous hyperplasia with dense suppurative and granulomatous infiltrates also is seen.

Erythema induratum is characterized by tender nodules on the lower extremities and has a substantial female predominance. Many cases are associated with Mycobacterium tuberculosis infection. The bacteria are not seen directly in the skin but are instead detectable through DNA polymerase chain reaction testing or investigation of other organ systems.7,8 Histologically, lesions show a lobular panniculitis with a mixed infiltrate. Vasculitis is seen in approximately 90% of erythema induratum cases vs approximately 25% of classic ENL cases (Figure 4),2,9 which has led some to use the term nodular vasculitis to describe this disease entity. Nodular vasculitis is considered by others to be a distinct disease entity in which there are clinical and histologic features similar to erythema induratum but no evidence of M tuberculosis infection.9

Polyarteritis nodosa is a vasculitis that affects medium- sized vessels of various organ systems. The presenting signs and symptoms vary based on the affected organ systems. Palpable to retiform purpura, livedo racemosa, subcutaneous nodules, or ulcers are seen when the skin is involved. The histologic hallmark is necrotizing vasculitis of medium-sized arterioles (Figure 5), although leukocytoclastic vasculitis of small-caliber vessels also can be seen in biopsies of affected skin.10 The vascular changes are said to be segmental, with uninvolved segments interspersed with involved segments. Antineutrophil cytoplasmic antibody (ANCA)– associated vasculitis also must be considered when one sees leukocytoclastic vasculitis of small-caliber vessels in the skin, as it can be distinguished most readily by detecting circulating antibodies specific for myeloperoxidase (MPO-ANCA) or proteinase 3 (PR3-ANCA).

- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
- Polycarpou A, Walker SL, Lockwood DNJ. A systematic review of immunological studies of erythema nodosum leprosum. Front Immunol. 2017;8:233. doi:10.3389/fimmu.2017.00233
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45. doi:10.1016/j.clindermatol.2014.10.003
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:1-28. doi:10.1186/1750-1172-2-34
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133. doi:10.1097/01.dad.0000249887.59810.76
- Wilson TC, Stone MS, Swick BL. Histiocytoid Sweet syndrome with haloed myeloid cells masquerading as a cryptococcal infection. Am J Dermatopathology. 2014;36:264-269. doi:10.1097/DAD.0b013e31828b811b
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Schneider JW, Jordaan HF, Geiger DH, et al. Erythema induratum of Bazin: a clinicopathological study of 20 cases of Mycobacterium tuberculosis DNA in skin lesions by polymerase chain reaction. Am J Dermatopathol. 1995;17:350-356. doi:10.1097/00000372-199508000-00008
- Boonchai W, Suthipinittharm P, Mahaisavariya P. Panniculitis in tuberculosis: a clinicopathologic study of nodular panniculitis associated with tuberculosis. Int J Dermatol. 1998;37:361-363. doi:10.1046/j.1365-4362.1998.00299.x
- Segura S, Pujol RM, Trindade F, et al. Vasculitis in erythema induratum of Bazin: a histopathologic study of 101 biopsy specimens from 86 patients. J Am Acad Dermatol. 2008;59:839-851. doi:10.1016/j.jaad.2008.07.030
- Ishiguro N, Kawashima M. Cutaneous polyarteritis nodosa: a report of 16 cases with clinical and histopathological analysis and a review of the published work. J Dermatol. 2010;37:85-93. doi:10.1111/j.1346-8138.2009.00752.x
A 66-year-old man presented with new tender erythematous papules scattered over the arms and legs. A biopsy of a lesion on the left thigh was performed.
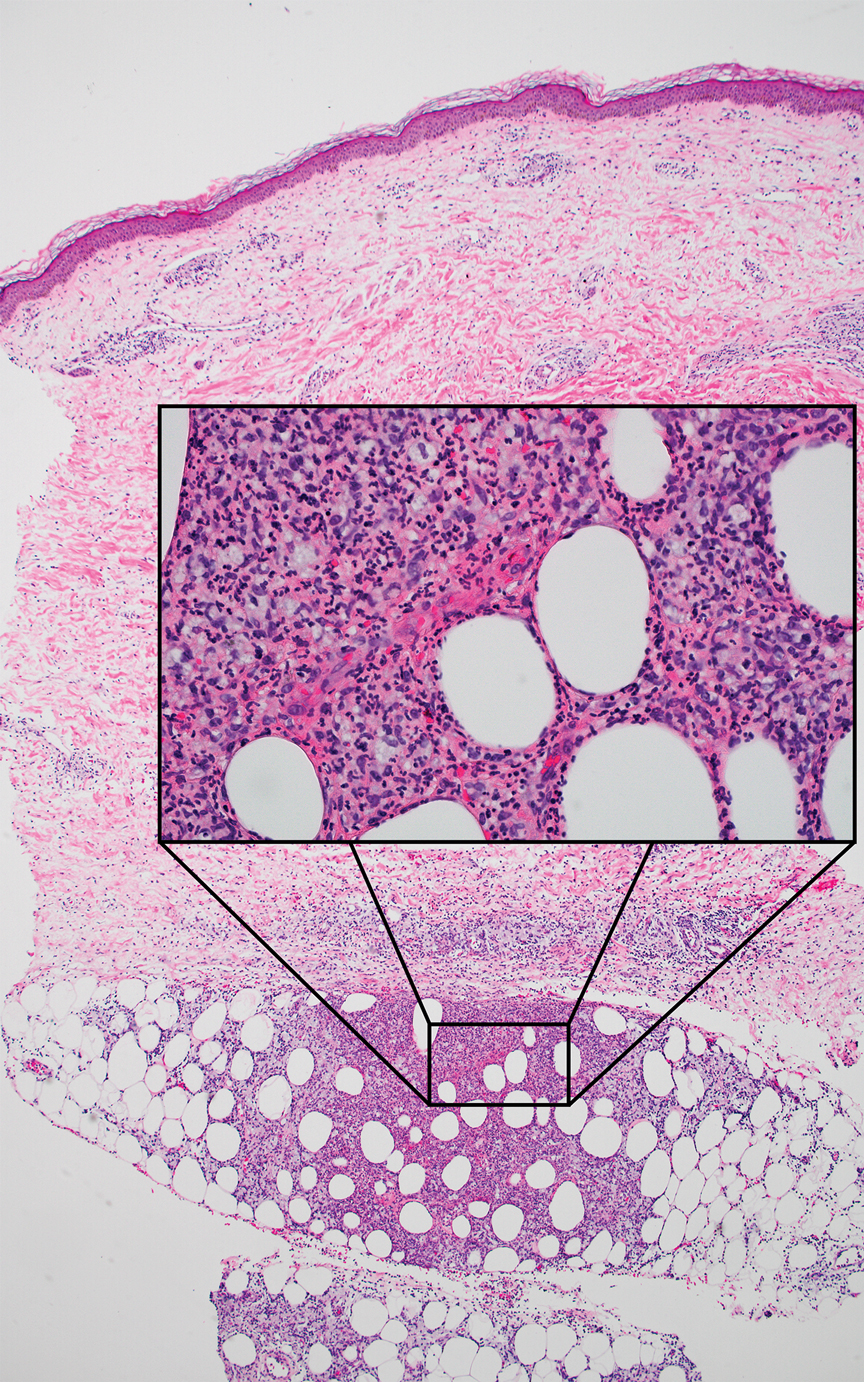
Beyond Borders: Tonsillar Squamous Cell Carcinoma with Intriguing Liver Metastasis
Background
Oropharyngeal squamous cell carcinoma (OPSCC) arises in the middle pharynx, including the tonsils, base of the tongue, and surrounding tissues. While OPSCC commonly metastasizes to regional lymph nodes, distant metastases to sites like the liver are rare, occurring in about 1-4% of cases with advanced disease.
Case Presentation
A 66-year-old male presented to the emergency department with recurrent right-sided facial swelling and a two-week history of sore throat. CT imaging revealed a large right tonsillar mass extending to the base of the tongue. Further evaluation with PET scan showed hypermetabolic activity in the right tonsil, multiple hypermetabolic lymph nodes in the right neck (stations 1B, 2, 3, 4, 5), right supraclavicular fossa, and small retropharyngeal nodes. Additionally, PET scan detected a hypermetabolic lesion in the liver and focal activity at T10 suggestive of bone metastasis. Fine needle aspiration (FNA) confirmed squamous cell carcinoma. Biopsy of the liver lesion revealed metastatic squamous cell carcinoma with basaloid differentiation, positive for p40 and p63 stains. Clinical staging was T2b cN2 cM1. The patient’s case was discussed in tumor boards, leading to a treatment plan of palliative radiotherapy with radiosensitizer (weekly carboplatin/paclitaxel) due to recent myocardial infarction, precluding cisplatin or 5FU use. Post-radiotherapy, Pembrolizumab was planned based on 60% PD-L1 expression. The patient opted to forego additional systemic chemotherapy and currently receives Keytruda every three weeks.
Discussion
Liver metastases from head and neck SCC are rare, highlighting the complexity of treatment decisions in such cases. Effective management requires a multidisciplinary approach to optimize therapeutic outcomes while considering patient-specific factors and comorbidities.
Conclusions
This case underscores the challenges and poor prognosis associated with tonsillar SCC with liver metastases. It underscores the need for personalized treatment strategies tailored to the unique characteristics of each patient’s disease.
Background
Oropharyngeal squamous cell carcinoma (OPSCC) arises in the middle pharynx, including the tonsils, base of the tongue, and surrounding tissues. While OPSCC commonly metastasizes to regional lymph nodes, distant metastases to sites like the liver are rare, occurring in about 1-4% of cases with advanced disease.
Case Presentation
A 66-year-old male presented to the emergency department with recurrent right-sided facial swelling and a two-week history of sore throat. CT imaging revealed a large right tonsillar mass extending to the base of the tongue. Further evaluation with PET scan showed hypermetabolic activity in the right tonsil, multiple hypermetabolic lymph nodes in the right neck (stations 1B, 2, 3, 4, 5), right supraclavicular fossa, and small retropharyngeal nodes. Additionally, PET scan detected a hypermetabolic lesion in the liver and focal activity at T10 suggestive of bone metastasis. Fine needle aspiration (FNA) confirmed squamous cell carcinoma. Biopsy of the liver lesion revealed metastatic squamous cell carcinoma with basaloid differentiation, positive for p40 and p63 stains. Clinical staging was T2b cN2 cM1. The patient’s case was discussed in tumor boards, leading to a treatment plan of palliative radiotherapy with radiosensitizer (weekly carboplatin/paclitaxel) due to recent myocardial infarction, precluding cisplatin or 5FU use. Post-radiotherapy, Pembrolizumab was planned based on 60% PD-L1 expression. The patient opted to forego additional systemic chemotherapy and currently receives Keytruda every three weeks.
Discussion
Liver metastases from head and neck SCC are rare, highlighting the complexity of treatment decisions in such cases. Effective management requires a multidisciplinary approach to optimize therapeutic outcomes while considering patient-specific factors and comorbidities.
Conclusions
This case underscores the challenges and poor prognosis associated with tonsillar SCC with liver metastases. It underscores the need for personalized treatment strategies tailored to the unique characteristics of each patient’s disease.
Background
Oropharyngeal squamous cell carcinoma (OPSCC) arises in the middle pharynx, including the tonsils, base of the tongue, and surrounding tissues. While OPSCC commonly metastasizes to regional lymph nodes, distant metastases to sites like the liver are rare, occurring in about 1-4% of cases with advanced disease.
Case Presentation
A 66-year-old male presented to the emergency department with recurrent right-sided facial swelling and a two-week history of sore throat. CT imaging revealed a large right tonsillar mass extending to the base of the tongue. Further evaluation with PET scan showed hypermetabolic activity in the right tonsil, multiple hypermetabolic lymph nodes in the right neck (stations 1B, 2, 3, 4, 5), right supraclavicular fossa, and small retropharyngeal nodes. Additionally, PET scan detected a hypermetabolic lesion in the liver and focal activity at T10 suggestive of bone metastasis. Fine needle aspiration (FNA) confirmed squamous cell carcinoma. Biopsy of the liver lesion revealed metastatic squamous cell carcinoma with basaloid differentiation, positive for p40 and p63 stains. Clinical staging was T2b cN2 cM1. The patient’s case was discussed in tumor boards, leading to a treatment plan of palliative radiotherapy with radiosensitizer (weekly carboplatin/paclitaxel) due to recent myocardial infarction, precluding cisplatin or 5FU use. Post-radiotherapy, Pembrolizumab was planned based on 60% PD-L1 expression. The patient opted to forego additional systemic chemotherapy and currently receives Keytruda every three weeks.
Discussion
Liver metastases from head and neck SCC are rare, highlighting the complexity of treatment decisions in such cases. Effective management requires a multidisciplinary approach to optimize therapeutic outcomes while considering patient-specific factors and comorbidities.
Conclusions
This case underscores the challenges and poor prognosis associated with tonsillar SCC with liver metastases. It underscores the need for personalized treatment strategies tailored to the unique characteristics of each patient’s disease.
ENT Multidisciplinary Workgroup
Background
The care of veterans with head and neck cancers requires a team approach among multiple disciplines throughout the entire trajectory of their cancer treatment course. Veterans with head and neck cancer have complicated treatments including surgery, radiation, chemotherapy and reconstructive surgery which can affect swallow function, speech, taste and physical appearance. Many patients who get treated for head and neck cancer will have lasting side effects of treatment. Veterans with cancer are more likely than the general population to have mental health comorbidities such as anxiety, depression and PTSD. Many head and neck cancer patients have used tobacco and/or alcohol as coping mechanisms for these issues. A new diagnosis of cancer may exacerbate their mental illness. Tobacco cessation may exacerbate anxiety for patients who have used tobacco as a coping mechanism. Ongoing alcohol use can complicate treatment. All of these issues can create delays in care.
Methods
In August 2019, a task force (“the ENT Multidisciplinary Workgroup”) was formed at VA Connecticut Healthcare System (“VACHS”) including representatives from ENT, Speech Pathology, Nutrition, Palliative Care and Oncology with the specific goal of improved coordination of care for head and neck cancer patients. Regular weekly meetings began in September 2019 to identify and track patients and to make referrals for appropriate diagnostic testing, treatment and supportive care.
Discussion
Weekly meeting among the core members of the ENT workgroup led to identification of patient needs earlier in the illness course than was observed prior to this workgroup initiative. Each week several opportunities are identified to improve patient care. This is a dynamic, ongoing process that has improved communication among key members of the interdisciplinary team that cares for these very complex patients and has led to the development of quality improvement initiatives that are reproducible at other VA sites.
Background
The care of veterans with head and neck cancers requires a team approach among multiple disciplines throughout the entire trajectory of their cancer treatment course. Veterans with head and neck cancer have complicated treatments including surgery, radiation, chemotherapy and reconstructive surgery which can affect swallow function, speech, taste and physical appearance. Many patients who get treated for head and neck cancer will have lasting side effects of treatment. Veterans with cancer are more likely than the general population to have mental health comorbidities such as anxiety, depression and PTSD. Many head and neck cancer patients have used tobacco and/or alcohol as coping mechanisms for these issues. A new diagnosis of cancer may exacerbate their mental illness. Tobacco cessation may exacerbate anxiety for patients who have used tobacco as a coping mechanism. Ongoing alcohol use can complicate treatment. All of these issues can create delays in care.
Methods
In August 2019, a task force (“the ENT Multidisciplinary Workgroup”) was formed at VA Connecticut Healthcare System (“VACHS”) including representatives from ENT, Speech Pathology, Nutrition, Palliative Care and Oncology with the specific goal of improved coordination of care for head and neck cancer patients. Regular weekly meetings began in September 2019 to identify and track patients and to make referrals for appropriate diagnostic testing, treatment and supportive care.
Discussion
Weekly meeting among the core members of the ENT workgroup led to identification of patient needs earlier in the illness course than was observed prior to this workgroup initiative. Each week several opportunities are identified to improve patient care. This is a dynamic, ongoing process that has improved communication among key members of the interdisciplinary team that cares for these very complex patients and has led to the development of quality improvement initiatives that are reproducible at other VA sites.
Background
The care of veterans with head and neck cancers requires a team approach among multiple disciplines throughout the entire trajectory of their cancer treatment course. Veterans with head and neck cancer have complicated treatments including surgery, radiation, chemotherapy and reconstructive surgery which can affect swallow function, speech, taste and physical appearance. Many patients who get treated for head and neck cancer will have lasting side effects of treatment. Veterans with cancer are more likely than the general population to have mental health comorbidities such as anxiety, depression and PTSD. Many head and neck cancer patients have used tobacco and/or alcohol as coping mechanisms for these issues. A new diagnosis of cancer may exacerbate their mental illness. Tobacco cessation may exacerbate anxiety for patients who have used tobacco as a coping mechanism. Ongoing alcohol use can complicate treatment. All of these issues can create delays in care.
Methods
In August 2019, a task force (“the ENT Multidisciplinary Workgroup”) was formed at VA Connecticut Healthcare System (“VACHS”) including representatives from ENT, Speech Pathology, Nutrition, Palliative Care and Oncology with the specific goal of improved coordination of care for head and neck cancer patients. Regular weekly meetings began in September 2019 to identify and track patients and to make referrals for appropriate diagnostic testing, treatment and supportive care.
Discussion
Weekly meeting among the core members of the ENT workgroup led to identification of patient needs earlier in the illness course than was observed prior to this workgroup initiative. Each week several opportunities are identified to improve patient care. This is a dynamic, ongoing process that has improved communication among key members of the interdisciplinary team that cares for these very complex patients and has led to the development of quality improvement initiatives that are reproducible at other VA sites.
Multimodal Treatment Approaches for Basaloid Squamous Cell Carcinoma of the Larynx
Background
Basaloid squamous cell carcinoma (BSCC) is an aggressive laryngeal cancer with high recurrence and metastasis rates. Its rarity complicates diagnosis and optimal treatment selection, underscoring the significance of comprehensive data collection through national cancer registries. Historically, surgical intervention has been the primary approach to management.The RTOG 91-11 randomized trial catalyzed a paradigm shift, prioritizing laryngealpreserving treatments. The study provided evidence for radiotherapy in early-stage disease (stages 1-2) and combined chemoradiotherapy in advanced disease (stages 3-4). Consequently, organ preservation protocols gained traction, maintaining laryngeal anatomy while achieving comparable oncologic outcomes to total laryngectomy. This shift emphasizes exploring multimodal, laryngeal-sparing regimens to optimize quality of life without compromising disease control. However, further research utilizing large databases is needed to elucidate survival outcomes associated with these approaches.
Methods
We used the National Cancer Database to identify patients diagnosed with BSCC of the larynx (ICD-O-3 histology code 8083) between 2004-2019 (Nf1487). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p< 0.05.
Results
For early-stage patients, the estimated survival was 93.179 months. Surgery demonstrated the most favorable outcome with a median survival of 100.957 months, significantly higher than non-surgical patients (85.895 months, p=0.028). Survival did not differ between patients who received only chemotherapy (p=0.281), radiation (p=0.326), or chemoradiation (p=0.919) and those received other treatment modalities. In late-stage patients, the estimated survival was 61.993 months. Surgery yielded the most favorable outcome with a median survival of 70.484 months, significantly higher than non-surgical patients (54.153 months, p< 0.001). Patients who received only chemotherapy (p< 0.001), radiation (p< 0.001) and chemoradiation (p=0.24) had a worse survival outcome compared to those who received other treatment modalities.
Conclusions
The study results indicate that surgical resection could potentially improve survival outcomes for patients diagnosed with advanced-stage laryngeal BSCC. Conversely, for those with earlystage BSCC, larynx-preserving treatment modalities such as radiation, chemotherapy or concurrent chemoradiation appear to achieve comparable survival rates to primary surgical management. These results highlight the importance of careful consideration of treatment modalities based on disease staging at initial presentation.
Background
Basaloid squamous cell carcinoma (BSCC) is an aggressive laryngeal cancer with high recurrence and metastasis rates. Its rarity complicates diagnosis and optimal treatment selection, underscoring the significance of comprehensive data collection through national cancer registries. Historically, surgical intervention has been the primary approach to management.The RTOG 91-11 randomized trial catalyzed a paradigm shift, prioritizing laryngealpreserving treatments. The study provided evidence for radiotherapy in early-stage disease (stages 1-2) and combined chemoradiotherapy in advanced disease (stages 3-4). Consequently, organ preservation protocols gained traction, maintaining laryngeal anatomy while achieving comparable oncologic outcomes to total laryngectomy. This shift emphasizes exploring multimodal, laryngeal-sparing regimens to optimize quality of life without compromising disease control. However, further research utilizing large databases is needed to elucidate survival outcomes associated with these approaches.
Methods
We used the National Cancer Database to identify patients diagnosed with BSCC of the larynx (ICD-O-3 histology code 8083) between 2004-2019 (Nf1487). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p< 0.05.
Results
For early-stage patients, the estimated survival was 93.179 months. Surgery demonstrated the most favorable outcome with a median survival of 100.957 months, significantly higher than non-surgical patients (85.895 months, p=0.028). Survival did not differ between patients who received only chemotherapy (p=0.281), radiation (p=0.326), or chemoradiation (p=0.919) and those received other treatment modalities. In late-stage patients, the estimated survival was 61.993 months. Surgery yielded the most favorable outcome with a median survival of 70.484 months, significantly higher than non-surgical patients (54.153 months, p< 0.001). Patients who received only chemotherapy (p< 0.001), radiation (p< 0.001) and chemoradiation (p=0.24) had a worse survival outcome compared to those who received other treatment modalities.
Conclusions
The study results indicate that surgical resection could potentially improve survival outcomes for patients diagnosed with advanced-stage laryngeal BSCC. Conversely, for those with earlystage BSCC, larynx-preserving treatment modalities such as radiation, chemotherapy or concurrent chemoradiation appear to achieve comparable survival rates to primary surgical management. These results highlight the importance of careful consideration of treatment modalities based on disease staging at initial presentation.
Background
Basaloid squamous cell carcinoma (BSCC) is an aggressive laryngeal cancer with high recurrence and metastasis rates. Its rarity complicates diagnosis and optimal treatment selection, underscoring the significance of comprehensive data collection through national cancer registries. Historically, surgical intervention has been the primary approach to management.The RTOG 91-11 randomized trial catalyzed a paradigm shift, prioritizing laryngealpreserving treatments. The study provided evidence for radiotherapy in early-stage disease (stages 1-2) and combined chemoradiotherapy in advanced disease (stages 3-4). Consequently, organ preservation protocols gained traction, maintaining laryngeal anatomy while achieving comparable oncologic outcomes to total laryngectomy. This shift emphasizes exploring multimodal, laryngeal-sparing regimens to optimize quality of life without compromising disease control. However, further research utilizing large databases is needed to elucidate survival outcomes associated with these approaches.
Methods
We used the National Cancer Database to identify patients diagnosed with BSCC of the larynx (ICD-O-3 histology code 8083) between 2004-2019 (Nf1487). General patient characteristics were assessed using descriptive statistics. Survival was evaluated using Kaplan-Meier curves and log-rank tests. Significance was set at p< 0.05.
Results
For early-stage patients, the estimated survival was 93.179 months. Surgery demonstrated the most favorable outcome with a median survival of 100.957 months, significantly higher than non-surgical patients (85.895 months, p=0.028). Survival did not differ between patients who received only chemotherapy (p=0.281), radiation (p=0.326), or chemoradiation (p=0.919) and those received other treatment modalities. In late-stage patients, the estimated survival was 61.993 months. Surgery yielded the most favorable outcome with a median survival of 70.484 months, significantly higher than non-surgical patients (54.153 months, p< 0.001). Patients who received only chemotherapy (p< 0.001), radiation (p< 0.001) and chemoradiation (p=0.24) had a worse survival outcome compared to those who received other treatment modalities.
Conclusions
The study results indicate that surgical resection could potentially improve survival outcomes for patients diagnosed with advanced-stage laryngeal BSCC. Conversely, for those with earlystage BSCC, larynx-preserving treatment modalities such as radiation, chemotherapy or concurrent chemoradiation appear to achieve comparable survival rates to primary surgical management. These results highlight the importance of careful consideration of treatment modalities based on disease staging at initial presentation.