User login
Vedolizumab-Induced Acne Fulminans: An Uncommon and Severe Adverse Effect
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
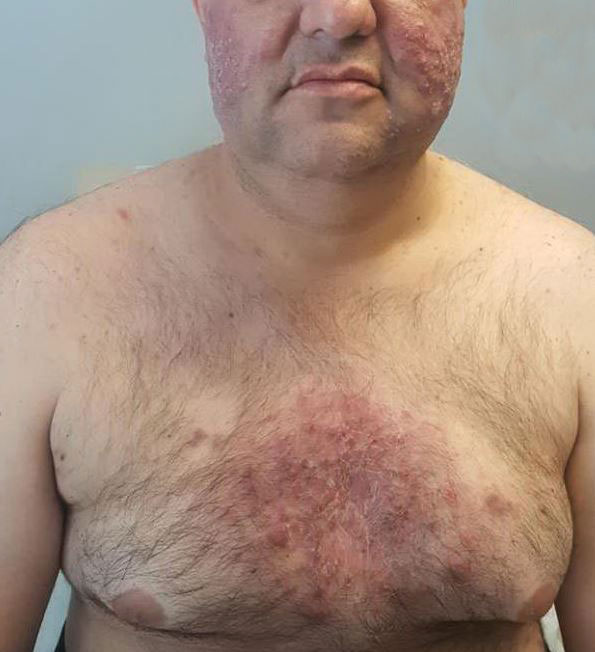
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.
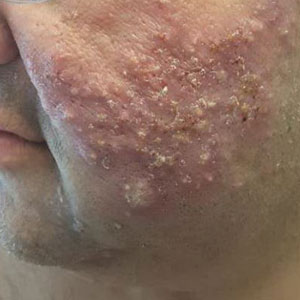
Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.
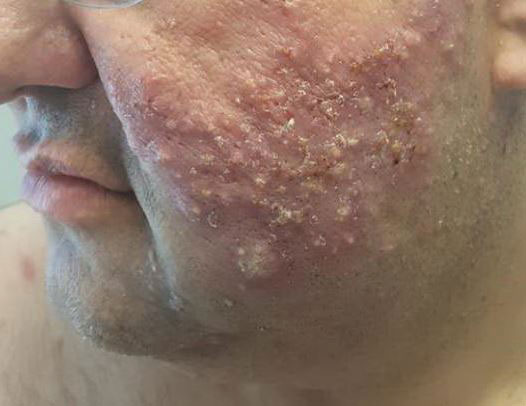
The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
To the Editor:
Vedolizumab is an innovative monoclonal antibody targeted against the α4β7 integrin that is approved for treatment of moderate to severe ulcerative colitis and Crohn disease refractory to standard treatment.1 Vedolizumab is thought to be gut specific, blocking integrins specific to T lymphocytes destined for the gastrointestinal tract and their interaction with endothelial cells, thereby modulating the adaptive immune system in the gut without systemic immunosuppression.2 It generally is well tolerated, and acne rarely has been reported as an adverse event.3,4 We present a case of acne fulminans without systemic symptoms (AF-WOSS) as a severe side effect of vedolizumab that responded very well to systemic steroids and oral isotretinoin in addition to the discontinuation of treatment.
A 46-year-old obese man presented to our dermatology clinic with a chief complaint of rapidly progressive tender skin lesions. The patient had a long-standing history of severe fistulating and stricturing Crohn disease status post–bowel resection with ileostomy and had recently started treatment with vedolizumab after failing treatment with infliximab, adalimumab, certolizumab pegol, ustekinumab, and methotrexate. Several weeks after beginning infusions of vedolizumab, the patient began to develop many erythematous papules and pustules on the face, chest (Figure 1), and buttocks that rapidly progressed into painful and coalescing nodules and cysts over the next several months. He was prescribed benzoyl peroxide wash 10% as well as several weeks of oral doxycycline 100 mg twice daily with no improvement. The patient denied any other new medications or triggers, fever, chills, bone pain, headache, fatigue, or myalgia. The skin involvement continued to worsen with successive vedolizumab infusions over a period of 8 weeks, which ultimately resulted in cessation of vedolizumab.

Physical examination revealed large, tender, pink, erythematous, and indurated plaques that were heavily studded with pink papules, pustules, and nodules on the cheeks (Figure 2), central chest, and buttocks. A punch biopsy of a pustule on the cheek showed ruptured suppurative folliculitis. The patient subsequently was diagnosed with AF-WOSS.

The patient then completed a 7-day course of sulfamethoxazole-trimethoprim followed by a 10-day course of amoxicillin-clavulanic acid, neither of which led to improvement of the lesions. He then was started on an oral prednisone taper (1 mg/kg starting dose) that ultimately totaled 14 weeks in length due to his frequent flares any time prednisone was decreased below 40 mg daily. After 3 weeks on the oral prednisone, the patient was started on 0.3 mg/kg of concomitant oral isotretinoin every other day, which slowly was increased as tolerated until he reached a goal dose of roughly 150 mg/kg, which resolved the acneform papules and pustules and allowed for successful tapering off the prednisone.
Many studies have been published regarding the safety and side-effect profile of vedolizumab, but most do not report acne as an adverse event.3-5 A German cohort study by Baumgart et al3 reported acne as a side effect in 15 of 212 (7.1%) patients but did not classify the severity. Another case report noted nodulocystic acne in a patient receiving vedolizumab for treatment of inflammatory bowel disease; however, this patient responded well to the use of a tetracycline antibiotic and was able to continue therapy with vedolizumab.5 Our patient demonstrated a severe and uncommon case of acne classified as AF-WOSS following initiation of therapy with vedolizumab, which required treatment with systemic steroids plus oral isotretinoin and resulted in cessation of vedolizumab.
As new therapies emerge, it is important to document new or severe adverse effects so providers can choose an appropriate therapy and adequately counsel patients regarding the side effects. Although vedolizumab was thought to have gut-specific action, there is new evidence to suggest that the principal ligand of the α4β7 integrin, mucosal addressin cell adhesion molecule-1, is not only expressed on gut endothelial cells but also on fibroblasts and melanomas, which may provide insight into the observed extraintestinal side effects of vedolizumab.6
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
- Smith MA, Mohammad RA. Vedolizumab: an α4β7 integrin inhibitor for inflammatory bowel diseases. Ann Pharmacother. 2014;48:1629-1635.
- Singh H, Grewal N, Arora E, et al. Vedolizumab: a novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Bio Med. 2016;7:4-9.
- Baumgart DC, Bokemeyer B, Drabik A, et al. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice: a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43:1090-1102.
- Bye WA, Jairath V, Travis SPL. Systematic review: the safety of vedolizumab for the treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46:3-15.
- Gilhooley E, Doherty G, Lally A. Vedolizumab-induced acne in inflammatory bowel disease. Int J Dermatol. 2018;57:752-753.
- Leung E, Kanwar RK, Kanwar JR, et al. Mucosal vascular addressin cell adhesion molecule-1 is expressed outside the endothelial lineage on fibroblasts and melanoma cells. Immunol Cell Biol. 2003;81:320-327.
Practice Points
- Vedolizumab, a monoclonal antibody for the treatment of refractory inflammatory bowel disease, was found to cause acne fulminans without systemic symptoms.
- Vedolizumab previously was believed to be a gut-limited immune modulator.
- Off-target cutaneous effects may indicate wider expression of the target integrin of vedolizumab and should be recognized as the drug becomes more widely used.