User login
Adoption of Choosing Wisely Recommendations Slow to Catch On
Clinical question: Have the Choosing Wisely campaign recommendations led to changes in practice?
Background: The Choosing Wisely campaign aims to reduce the incidence of low-value care by providing evidence-based recommendations for common clinical situations. The rate of adoption of these recommendations is unknown.
Study design: Retrospective review.
Setting: Anthem insurance members.
Synopsis: The study examined the claims data from 25 million Anthem insurance members to compare the rate of services that were targeted by seven Choosing Wisely campaign recommendations before and after the recommendations were published in 2012.
Investigators found the incidence of two of the services declined after the Choosing Wisely recommendations were published; the other five services remained stable or increased slightly. Furthermore, the declines were statistically significant but not a marked absolute difference, with the incidence of head imaging in patients with uncomplicated headaches going down to 13.4% from 14.9% and the use of cardiac imaging in the absence of cardiac disease declining to 9.7% from 10.8%.
The main limitations are the narrow population of Anthem insurance members and the lack of specific data that could help answer why clinical practice has not changed, but that could be the aim of future studies.
Bottom line: Choosing Wisely recommendations have not been adopted on a population level; widespread implementation likely will require financial incentives, provider-level data feedback, and systems interventions.
Citation: Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. doi:10.1001/jamainternmed.2015.5441.
Clinical question: Have the Choosing Wisely campaign recommendations led to changes in practice?
Background: The Choosing Wisely campaign aims to reduce the incidence of low-value care by providing evidence-based recommendations for common clinical situations. The rate of adoption of these recommendations is unknown.
Study design: Retrospective review.
Setting: Anthem insurance members.
Synopsis: The study examined the claims data from 25 million Anthem insurance members to compare the rate of services that were targeted by seven Choosing Wisely campaign recommendations before and after the recommendations were published in 2012.
Investigators found the incidence of two of the services declined after the Choosing Wisely recommendations were published; the other five services remained stable or increased slightly. Furthermore, the declines were statistically significant but not a marked absolute difference, with the incidence of head imaging in patients with uncomplicated headaches going down to 13.4% from 14.9% and the use of cardiac imaging in the absence of cardiac disease declining to 9.7% from 10.8%.
The main limitations are the narrow population of Anthem insurance members and the lack of specific data that could help answer why clinical practice has not changed, but that could be the aim of future studies.
Bottom line: Choosing Wisely recommendations have not been adopted on a population level; widespread implementation likely will require financial incentives, provider-level data feedback, and systems interventions.
Citation: Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. doi:10.1001/jamainternmed.2015.5441.
Clinical question: Have the Choosing Wisely campaign recommendations led to changes in practice?
Background: The Choosing Wisely campaign aims to reduce the incidence of low-value care by providing evidence-based recommendations for common clinical situations. The rate of adoption of these recommendations is unknown.
Study design: Retrospective review.
Setting: Anthem insurance members.
Synopsis: The study examined the claims data from 25 million Anthem insurance members to compare the rate of services that were targeted by seven Choosing Wisely campaign recommendations before and after the recommendations were published in 2012.
Investigators found the incidence of two of the services declined after the Choosing Wisely recommendations were published; the other five services remained stable or increased slightly. Furthermore, the declines were statistically significant but not a marked absolute difference, with the incidence of head imaging in patients with uncomplicated headaches going down to 13.4% from 14.9% and the use of cardiac imaging in the absence of cardiac disease declining to 9.7% from 10.8%.
The main limitations are the narrow population of Anthem insurance members and the lack of specific data that could help answer why clinical practice has not changed, but that could be the aim of future studies.
Bottom line: Choosing Wisely recommendations have not been adopted on a population level; widespread implementation likely will require financial incentives, provider-level data feedback, and systems interventions.
Citation: Rosenberg A, Agiro A, Gottlieb M, et al. Early trends among seven recommendations from the Choosing Wisely campaign. JAMA Intern Med. 2015;175(12):1913-1920. doi:10.1001/jamainternmed.2015.5441.
Overall Patient Satisfaction Better on Hospitalist Teams Compared with Teaching Teams
Clinical question: Is there a difference in patient experience on hospitalist teams compared with teaching teams?
Background: Hospitalist-intensive hospitals tend to perform better on patient-satisfaction measures on HCAHPS survey; however, little is known about the difference in patient experience between patients cared for by hospitalist and trainee teams.
Study design: Retrospective cohort analysis.
Setting: University of Chicago Medical Center.
Synopsis: A 30-day post-discharge survey was sent to 14,855 patients cared for by hospitalist and teaching teams, with 57% of teaching and 31% of hospitalist team patients returning fully completed surveys. A higher percentage of hospitalist team patients reported satisfaction with their overall care (73% vs. 67%; P<0.001; regression model odds ratio = 1.33; 95% CI, 1.15–1.47). There was no statistically significant difference in patient satisfaction with the teamwork of their providers, confidence in identifying their provider, or ability to understand the role of their provider.
Other than the inability to mitigate response-selection bias, the main limitation of this study is the single-center setting, which impacts the generalizability of the findings. Hospital-specific factors like different services and structures (hospitalists at their institution care for renal and lung transplant and oncology patients) could influence patients’ perception of their care. More research needs to be done to determine the specific factors that lead to a better patient experience.
Bottom line: At a single academic center, overall patient satisfaction was higher on a hospitalist service compared with teaching teams.
Citation: Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire [published online ahead of print September 18, 2015]. J Hosp Med. doi:10.1002/jhm.2485.
Clinical question: Is there a difference in patient experience on hospitalist teams compared with teaching teams?
Background: Hospitalist-intensive hospitals tend to perform better on patient-satisfaction measures on HCAHPS survey; however, little is known about the difference in patient experience between patients cared for by hospitalist and trainee teams.
Study design: Retrospective cohort analysis.
Setting: University of Chicago Medical Center.
Synopsis: A 30-day post-discharge survey was sent to 14,855 patients cared for by hospitalist and teaching teams, with 57% of teaching and 31% of hospitalist team patients returning fully completed surveys. A higher percentage of hospitalist team patients reported satisfaction with their overall care (73% vs. 67%; P<0.001; regression model odds ratio = 1.33; 95% CI, 1.15–1.47). There was no statistically significant difference in patient satisfaction with the teamwork of their providers, confidence in identifying their provider, or ability to understand the role of their provider.
Other than the inability to mitigate response-selection bias, the main limitation of this study is the single-center setting, which impacts the generalizability of the findings. Hospital-specific factors like different services and structures (hospitalists at their institution care for renal and lung transplant and oncology patients) could influence patients’ perception of their care. More research needs to be done to determine the specific factors that lead to a better patient experience.
Bottom line: At a single academic center, overall patient satisfaction was higher on a hospitalist service compared with teaching teams.
Citation: Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire [published online ahead of print September 18, 2015]. J Hosp Med. doi:10.1002/jhm.2485.
Clinical question: Is there a difference in patient experience on hospitalist teams compared with teaching teams?
Background: Hospitalist-intensive hospitals tend to perform better on patient-satisfaction measures on HCAHPS survey; however, little is known about the difference in patient experience between patients cared for by hospitalist and trainee teams.
Study design: Retrospective cohort analysis.
Setting: University of Chicago Medical Center.
Synopsis: A 30-day post-discharge survey was sent to 14,855 patients cared for by hospitalist and teaching teams, with 57% of teaching and 31% of hospitalist team patients returning fully completed surveys. A higher percentage of hospitalist team patients reported satisfaction with their overall care (73% vs. 67%; P<0.001; regression model odds ratio = 1.33; 95% CI, 1.15–1.47). There was no statistically significant difference in patient satisfaction with the teamwork of their providers, confidence in identifying their provider, or ability to understand the role of their provider.
Other than the inability to mitigate response-selection bias, the main limitation of this study is the single-center setting, which impacts the generalizability of the findings. Hospital-specific factors like different services and structures (hospitalists at their institution care for renal and lung transplant and oncology patients) could influence patients’ perception of their care. More research needs to be done to determine the specific factors that lead to a better patient experience.
Bottom line: At a single academic center, overall patient satisfaction was higher on a hospitalist service compared with teaching teams.
Citation: Wray CM, Flores A, Padula WV, Prochaska MT, Meltzer DO, Arora VM. Measuring patient experiences on hospitalist and teaching services: patient responses to a 30-day postdischarge questionnaire [published online ahead of print September 18, 2015]. J Hosp Med. doi:10.1002/jhm.2485.
Caprini Score Accurately Predicts Risk of Venous Thromboembolism in Critically Ill Surgical Patients
Clinical question: Is the Caprini risk assessment model (RAM) a valid tool to predict venous thromboembolism (VTE) risk in critically ill surgical patients?
Background: VTE is a major source of morbidity and mortality among hospitalized patients; prevention is critical to reduce morbidity and cut healthcare costs. Risk assessment is important to determine thromboprophylaxis, yet data are lacking regarding an appropriate tool for risk stratification in the critically ill.
Study design: Retrospective cohort.
Setting: University of Michigan Health System; 20-bed surgical ICU at an academic hospital.
Synopsis: This study included 4,844 surgical ICU patients. Primary outcome was VTE during the patient’s hospital admission. A retrospective risk scoring method based on the 2005 Caprini RAM was used to calculate the risk for all patients at the time of ICU admission. Patients were divided into low (Caprini score 0–2), moderate, high, highest, and super-high (Caprini score > 8) risk levels. The incidence of VTE increased in linear fashion with increasing Caprini score.
This study was limited to one academic medical center. The retrospective scoring model limits the ability to identify all patient risk factors. VTE outcomes were reported only for the length of hospitalization and did not include post-discharge follow-up. Replicating this study across a larger patient population and performing a prospective study with follow-up after discharge would address these limitations.
Bottom line: The Caprini risk assessment model is a valid instrument to assess VTE risk in critically ill surgical patients.
Citation: Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150(10):941-948.
Clinical question: Is the Caprini risk assessment model (RAM) a valid tool to predict venous thromboembolism (VTE) risk in critically ill surgical patients?
Background: VTE is a major source of morbidity and mortality among hospitalized patients; prevention is critical to reduce morbidity and cut healthcare costs. Risk assessment is important to determine thromboprophylaxis, yet data are lacking regarding an appropriate tool for risk stratification in the critically ill.
Study design: Retrospective cohort.
Setting: University of Michigan Health System; 20-bed surgical ICU at an academic hospital.
Synopsis: This study included 4,844 surgical ICU patients. Primary outcome was VTE during the patient’s hospital admission. A retrospective risk scoring method based on the 2005 Caprini RAM was used to calculate the risk for all patients at the time of ICU admission. Patients were divided into low (Caprini score 0–2), moderate, high, highest, and super-high (Caprini score > 8) risk levels. The incidence of VTE increased in linear fashion with increasing Caprini score.
This study was limited to one academic medical center. The retrospective scoring model limits the ability to identify all patient risk factors. VTE outcomes were reported only for the length of hospitalization and did not include post-discharge follow-up. Replicating this study across a larger patient population and performing a prospective study with follow-up after discharge would address these limitations.
Bottom line: The Caprini risk assessment model is a valid instrument to assess VTE risk in critically ill surgical patients.
Citation: Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150(10):941-948.
Clinical question: Is the Caprini risk assessment model (RAM) a valid tool to predict venous thromboembolism (VTE) risk in critically ill surgical patients?
Background: VTE is a major source of morbidity and mortality among hospitalized patients; prevention is critical to reduce morbidity and cut healthcare costs. Risk assessment is important to determine thromboprophylaxis, yet data are lacking regarding an appropriate tool for risk stratification in the critically ill.
Study design: Retrospective cohort.
Setting: University of Michigan Health System; 20-bed surgical ICU at an academic hospital.
Synopsis: This study included 4,844 surgical ICU patients. Primary outcome was VTE during the patient’s hospital admission. A retrospective risk scoring method based on the 2005 Caprini RAM was used to calculate the risk for all patients at the time of ICU admission. Patients were divided into low (Caprini score 0–2), moderate, high, highest, and super-high (Caprini score > 8) risk levels. The incidence of VTE increased in linear fashion with increasing Caprini score.
This study was limited to one academic medical center. The retrospective scoring model limits the ability to identify all patient risk factors. VTE outcomes were reported only for the length of hospitalization and did not include post-discharge follow-up. Replicating this study across a larger patient population and performing a prospective study with follow-up after discharge would address these limitations.
Bottom line: The Caprini risk assessment model is a valid instrument to assess VTE risk in critically ill surgical patients.
Citation: Obi AT, Pannucci CJ, Nackashi A, et al. Validation of the Caprini venous thromboembolism risk assessment model in critically ill surgical patients. JAMA Surg. 2015;150(10):941-948.
Total Knee Replacement Superior to Non-Surgical Intervention
Clinical question: Does total knee replacement followed by a 12-week non-surgical treatment program provide greater pain relief and improvement in function and quality of life than non-surgical treatment alone?
Background: The number of total knee replacements in the U.S. has increased dramatically since the 1970s and is expected to continue to rise. To date, evidence to support the effectiveness of surgical intervention compared to non-surgical intervention is lacking.
Study design: Randomized, controlled trial.
Setting: Aalborg University Hospital Outpatient Clinics, Denmark.
Synopsis: One hundred patients with osteoarthritis were randomly assigned to undergo total knee replacement followed by 12 weeks of non-surgical treatment or to receive only 12 weeks of non-surgical treatment. The non-surgical treatment program consisted of exercise, education, dietary advice, insoles, and pain medication. Change from baseline to 12 months was assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS).
The total knee replacement group had a significantly greater improvement in the KOOS score than did the non-surgical group. Serious adverse events were more common in the total knee replacement group.
The study did not include a sham-surgery control group. It is unknown whether the KOOS pain subscale is generalizable to patients with severe pain. Additionally, the intensity of non-surgical treatment may have differed between groups.
Bottom line: Total knee replacement followed by non-surgical treatment is more efficacious than non-surgical treatment alone in providing pain relief and improving function and quality of life, but it is associated with higher number of adverse events.
Citation: Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(17):1597-1606.
Clinical question: Does total knee replacement followed by a 12-week non-surgical treatment program provide greater pain relief and improvement in function and quality of life than non-surgical treatment alone?
Background: The number of total knee replacements in the U.S. has increased dramatically since the 1970s and is expected to continue to rise. To date, evidence to support the effectiveness of surgical intervention compared to non-surgical intervention is lacking.
Study design: Randomized, controlled trial.
Setting: Aalborg University Hospital Outpatient Clinics, Denmark.
Synopsis: One hundred patients with osteoarthritis were randomly assigned to undergo total knee replacement followed by 12 weeks of non-surgical treatment or to receive only 12 weeks of non-surgical treatment. The non-surgical treatment program consisted of exercise, education, dietary advice, insoles, and pain medication. Change from baseline to 12 months was assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS).
The total knee replacement group had a significantly greater improvement in the KOOS score than did the non-surgical group. Serious adverse events were more common in the total knee replacement group.
The study did not include a sham-surgery control group. It is unknown whether the KOOS pain subscale is generalizable to patients with severe pain. Additionally, the intensity of non-surgical treatment may have differed between groups.
Bottom line: Total knee replacement followed by non-surgical treatment is more efficacious than non-surgical treatment alone in providing pain relief and improving function and quality of life, but it is associated with higher number of adverse events.
Citation: Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(17):1597-1606.
Clinical question: Does total knee replacement followed by a 12-week non-surgical treatment program provide greater pain relief and improvement in function and quality of life than non-surgical treatment alone?
Background: The number of total knee replacements in the U.S. has increased dramatically since the 1970s and is expected to continue to rise. To date, evidence to support the effectiveness of surgical intervention compared to non-surgical intervention is lacking.
Study design: Randomized, controlled trial.
Setting: Aalborg University Hospital Outpatient Clinics, Denmark.
Synopsis: One hundred patients with osteoarthritis were randomly assigned to undergo total knee replacement followed by 12 weeks of non-surgical treatment or to receive only 12 weeks of non-surgical treatment. The non-surgical treatment program consisted of exercise, education, dietary advice, insoles, and pain medication. Change from baseline to 12 months was assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS).
The total knee replacement group had a significantly greater improvement in the KOOS score than did the non-surgical group. Serious adverse events were more common in the total knee replacement group.
The study did not include a sham-surgery control group. It is unknown whether the KOOS pain subscale is generalizable to patients with severe pain. Additionally, the intensity of non-surgical treatment may have differed between groups.
Bottom line: Total knee replacement followed by non-surgical treatment is more efficacious than non-surgical treatment alone in providing pain relief and improving function and quality of life, but it is associated with higher number of adverse events.
Citation: Skou ST, Roos EM, Laursen MB, et al. A randomized, controlled trial of total knee replacement. N Engl J Med. 2015;373(17):1597-1606.
Patients with Postoperative Myocardial Infarction May Benefit from Higher Transfusion Threshold
Clinical question: Is there an improved 30-day mortality rate if patients receive blood transfusion at higher hematocrit values after postoperative myocardial infarction (MI)?
Background: Prior studies evaluating patients with a history of coronary artery disease (CAD) who undergo non-cardiac surgery have shown similar mortality outcomes with liberal and restrictive transfusion strategies. Data are lacking for transfusion strategies in patients with CAD who experience postoperative MI after non-cardiac surgeries.
Study design: Retrospective cohort.
Setting: Veterans Affairs health system.
Synopsis: The study included 7,361 patients with a history of CAD who underwent non-cardiac surgery whose postoperative hematocrit was between 20% and 30%. Patients were stratified by postoperative hematocrit nadir and presence of postoperative MI. In patients with postoperative MI, transfusion was associated with lower mortality with hematocrit nadir of 20%–24% but not with hematocrit of 24%–27% or 27%–30%. In patients without postoperative MI, transfusion was associated with higher mortality in patients with hematocrit of 27%–30%.
This retrospective study was limited to the VA population of mostly male patients. The sample size was limited. The study was unable to determine if postoperative blood transfusion is a risk for developing MI.
Bottom line: Patients with a history of CAD and MI who have a postoperative MI following non-cardiac surgery may benefit from higher blood transfusion thresholds; however, further controlled studies are needed.
Citation: Hollis RH, Singeltary BA, McMurtrie JT, et al. Blood transfusion and 30-day mortality in patients with coronary artery disease and anemia following noncardiac surgery [published online ahead of print October 7, 2015]. JAMA Surg. doi:10.1001/jamasurg.2015.3420.
Clinical question: Is there an improved 30-day mortality rate if patients receive blood transfusion at higher hematocrit values after postoperative myocardial infarction (MI)?
Background: Prior studies evaluating patients with a history of coronary artery disease (CAD) who undergo non-cardiac surgery have shown similar mortality outcomes with liberal and restrictive transfusion strategies. Data are lacking for transfusion strategies in patients with CAD who experience postoperative MI after non-cardiac surgeries.
Study design: Retrospective cohort.
Setting: Veterans Affairs health system.
Synopsis: The study included 7,361 patients with a history of CAD who underwent non-cardiac surgery whose postoperative hematocrit was between 20% and 30%. Patients were stratified by postoperative hematocrit nadir and presence of postoperative MI. In patients with postoperative MI, transfusion was associated with lower mortality with hematocrit nadir of 20%–24% but not with hematocrit of 24%–27% or 27%–30%. In patients without postoperative MI, transfusion was associated with higher mortality in patients with hematocrit of 27%–30%.
This retrospective study was limited to the VA population of mostly male patients. The sample size was limited. The study was unable to determine if postoperative blood transfusion is a risk for developing MI.
Bottom line: Patients with a history of CAD and MI who have a postoperative MI following non-cardiac surgery may benefit from higher blood transfusion thresholds; however, further controlled studies are needed.
Citation: Hollis RH, Singeltary BA, McMurtrie JT, et al. Blood transfusion and 30-day mortality in patients with coronary artery disease and anemia following noncardiac surgery [published online ahead of print October 7, 2015]. JAMA Surg. doi:10.1001/jamasurg.2015.3420.
Clinical question: Is there an improved 30-day mortality rate if patients receive blood transfusion at higher hematocrit values after postoperative myocardial infarction (MI)?
Background: Prior studies evaluating patients with a history of coronary artery disease (CAD) who undergo non-cardiac surgery have shown similar mortality outcomes with liberal and restrictive transfusion strategies. Data are lacking for transfusion strategies in patients with CAD who experience postoperative MI after non-cardiac surgeries.
Study design: Retrospective cohort.
Setting: Veterans Affairs health system.
Synopsis: The study included 7,361 patients with a history of CAD who underwent non-cardiac surgery whose postoperative hematocrit was between 20% and 30%. Patients were stratified by postoperative hematocrit nadir and presence of postoperative MI. In patients with postoperative MI, transfusion was associated with lower mortality with hematocrit nadir of 20%–24% but not with hematocrit of 24%–27% or 27%–30%. In patients without postoperative MI, transfusion was associated with higher mortality in patients with hematocrit of 27%–30%.
This retrospective study was limited to the VA population of mostly male patients. The sample size was limited. The study was unable to determine if postoperative blood transfusion is a risk for developing MI.
Bottom line: Patients with a history of CAD and MI who have a postoperative MI following non-cardiac surgery may benefit from higher blood transfusion thresholds; however, further controlled studies are needed.
Citation: Hollis RH, Singeltary BA, McMurtrie JT, et al. Blood transfusion and 30-day mortality in patients with coronary artery disease and anemia following noncardiac surgery [published online ahead of print October 7, 2015]. JAMA Surg. doi:10.1001/jamasurg.2015.3420.
Beta-Blockers May Increase Risk of Perioperative MACEs in Patients with Uncomplicated Hypertension
Clinical question: Does taking a perioperative beta-blocker increase the risk of major adverse cardiovascular events (MACEs) and all-cause mortality in low-risk patients with essential hypertension (HTN)?
Background: Guidelines for the use of perioperative beta-blockers are being reevaluated due to concerns about validity of prior studies that supported the use of perioperative beta-blockers. This study sought to evaluate effectiveness and safety of beta-blockers in patients with uncomplicated HTN.
Study design: Observational cohort study.
Setting: Denmark.
Synopsis: This study included 55,320 hypertensive patients using at least two antihypertensive drugs who underwent non-cardiac surgery. Of these, 14,644 patients were treated with a beta-blocker. Patients with secondary cardiovascular conditions, renal disease, or liver disease were excluded; 30-day MACEs and all-cause mortality were analyzed.
In patients treated with a beta-blocker, the incidence of 30-day MACEs was 1.32% compared with 0.84% in the non-beta-blockers group; 30-day mortality in those treated with beta-blocker was 1.9% compared with 1.3% in the non-beta-blocker group. Risk of beta-blocker-associated MACEs was higher in patients 70 and older. Causality cannot be concluded based on observational data.
Bottom line: In patients with uncomplicated HTN, treatment with a beta-blocker may be associated with increased 30-day risk of perioperative MACEs after non-cardiac surgery.
Citation: Jorgensen ME, Hlatky MA, Kober L, et al. Beta-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med. 2015;175(12):1923-1931.
Clinical question: Does taking a perioperative beta-blocker increase the risk of major adverse cardiovascular events (MACEs) and all-cause mortality in low-risk patients with essential hypertension (HTN)?
Background: Guidelines for the use of perioperative beta-blockers are being reevaluated due to concerns about validity of prior studies that supported the use of perioperative beta-blockers. This study sought to evaluate effectiveness and safety of beta-blockers in patients with uncomplicated HTN.
Study design: Observational cohort study.
Setting: Denmark.
Synopsis: This study included 55,320 hypertensive patients using at least two antihypertensive drugs who underwent non-cardiac surgery. Of these, 14,644 patients were treated with a beta-blocker. Patients with secondary cardiovascular conditions, renal disease, or liver disease were excluded; 30-day MACEs and all-cause mortality were analyzed.
In patients treated with a beta-blocker, the incidence of 30-day MACEs was 1.32% compared with 0.84% in the non-beta-blockers group; 30-day mortality in those treated with beta-blocker was 1.9% compared with 1.3% in the non-beta-blocker group. Risk of beta-blocker-associated MACEs was higher in patients 70 and older. Causality cannot be concluded based on observational data.
Bottom line: In patients with uncomplicated HTN, treatment with a beta-blocker may be associated with increased 30-day risk of perioperative MACEs after non-cardiac surgery.
Citation: Jorgensen ME, Hlatky MA, Kober L, et al. Beta-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med. 2015;175(12):1923-1931.
Clinical question: Does taking a perioperative beta-blocker increase the risk of major adverse cardiovascular events (MACEs) and all-cause mortality in low-risk patients with essential hypertension (HTN)?
Background: Guidelines for the use of perioperative beta-blockers are being reevaluated due to concerns about validity of prior studies that supported the use of perioperative beta-blockers. This study sought to evaluate effectiveness and safety of beta-blockers in patients with uncomplicated HTN.
Study design: Observational cohort study.
Setting: Denmark.
Synopsis: This study included 55,320 hypertensive patients using at least two antihypertensive drugs who underwent non-cardiac surgery. Of these, 14,644 patients were treated with a beta-blocker. Patients with secondary cardiovascular conditions, renal disease, or liver disease were excluded; 30-day MACEs and all-cause mortality were analyzed.
In patients treated with a beta-blocker, the incidence of 30-day MACEs was 1.32% compared with 0.84% in the non-beta-blockers group; 30-day mortality in those treated with beta-blocker was 1.9% compared with 1.3% in the non-beta-blocker group. Risk of beta-blocker-associated MACEs was higher in patients 70 and older. Causality cannot be concluded based on observational data.
Bottom line: In patients with uncomplicated HTN, treatment with a beta-blocker may be associated with increased 30-day risk of perioperative MACEs after non-cardiac surgery.
Citation: Jorgensen ME, Hlatky MA, Kober L, et al. Beta-blocker-associated risks in patients with uncomplicated hypertension undergoing noncardiac surgery. JAMA Intern Med. 2015;175(12):1923-1931.
Pharmacist Involvement in Transitional Care Can Reduce Return ED Visits, Inpatient Readmissions
Clinical question: Does pharmacist involvement in transitions of care decrease medication errors (MEs), adverse drug events (ADEs), and 30-day ED visits and inpatient readmissions?
Background: Previous studies show pharmacist involvement in discharge can reduce ADEs and improve patient satisfaction, but there have been inconsistent data on the impact of pharmacist involvement on readmissions, ADEs, and MEs.
Study design: Prospective, randomized, single-period, longitudinal study.
Setting: Northwestern Memorial Hospital, Chicago.
Synopsis: Investigators included 278 patients (137 in study arm, 141 in control arm) in the final analysis. The study arm received intensive pharmacist involvement on admission and discharge, followed by phone calls at three, 14, and 30 days post-discharge. The study arm had lower composite 30-day ED visits and inpatient readmission rates compared to the control group (25% vs. 39%; P=0.001) but did not have lower isolated inpatient readmission rates (20% vs. 24%; P=0.43). ADEs and MEs were not significantly different between the two groups.
This study had extensive exclusion criteria, limiting the patient population to which these results can be applied. It was underpowered, which could have prevented the detection of a significant improvement in readmission rates.
Care transitions are high-risk periods in patient care, and there is benefit to continuity of care of an interdisciplinary team, including pharmacists.
Bottom line: Pharmacist involvement in transitions of care was shown to reduce the composite of ED visits and inpatient readmissions.
Citation: Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016;11(1):39-44. doi:10.1002/jhm.2493.
Clinical question: Does pharmacist involvement in transitions of care decrease medication errors (MEs), adverse drug events (ADEs), and 30-day ED visits and inpatient readmissions?
Background: Previous studies show pharmacist involvement in discharge can reduce ADEs and improve patient satisfaction, but there have been inconsistent data on the impact of pharmacist involvement on readmissions, ADEs, and MEs.
Study design: Prospective, randomized, single-period, longitudinal study.
Setting: Northwestern Memorial Hospital, Chicago.
Synopsis: Investigators included 278 patients (137 in study arm, 141 in control arm) in the final analysis. The study arm received intensive pharmacist involvement on admission and discharge, followed by phone calls at three, 14, and 30 days post-discharge. The study arm had lower composite 30-day ED visits and inpatient readmission rates compared to the control group (25% vs. 39%; P=0.001) but did not have lower isolated inpatient readmission rates (20% vs. 24%; P=0.43). ADEs and MEs were not significantly different between the two groups.
This study had extensive exclusion criteria, limiting the patient population to which these results can be applied. It was underpowered, which could have prevented the detection of a significant improvement in readmission rates.
Care transitions are high-risk periods in patient care, and there is benefit to continuity of care of an interdisciplinary team, including pharmacists.
Bottom line: Pharmacist involvement in transitions of care was shown to reduce the composite of ED visits and inpatient readmissions.
Citation: Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016;11(1):39-44. doi:10.1002/jhm.2493.
Clinical question: Does pharmacist involvement in transitions of care decrease medication errors (MEs), adverse drug events (ADEs), and 30-day ED visits and inpatient readmissions?
Background: Previous studies show pharmacist involvement in discharge can reduce ADEs and improve patient satisfaction, but there have been inconsistent data on the impact of pharmacist involvement on readmissions, ADEs, and MEs.
Study design: Prospective, randomized, single-period, longitudinal study.
Setting: Northwestern Memorial Hospital, Chicago.
Synopsis: Investigators included 278 patients (137 in study arm, 141 in control arm) in the final analysis. The study arm received intensive pharmacist involvement on admission and discharge, followed by phone calls at three, 14, and 30 days post-discharge. The study arm had lower composite 30-day ED visits and inpatient readmission rates compared to the control group (25% vs. 39%; P=0.001) but did not have lower isolated inpatient readmission rates (20% vs. 24%; P=0.43). ADEs and MEs were not significantly different between the two groups.
This study had extensive exclusion criteria, limiting the patient population to which these results can be applied. It was underpowered, which could have prevented the detection of a significant improvement in readmission rates.
Care transitions are high-risk periods in patient care, and there is benefit to continuity of care of an interdisciplinary team, including pharmacists.
Bottom line: Pharmacist involvement in transitions of care was shown to reduce the composite of ED visits and inpatient readmissions.
Citation: Phatak A, Prusi R, Ward B, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH Study). J Hosp Med. 2016;11(1):39-44. doi:10.1002/jhm.2493.
Displaying Prices to Providers May Reduce Overall Ordering Costs
Clinical question: Does price display impact order costs and volume as well as patient safety outcomes, and is it acceptable to providers?
Background: Up to one-third of national healthcare expenditures are wasteful, with physicians playing a central role in overall cost, purchasing almost all tests and therapies for patients. Increasing the transparency of costs for physicians is one strategy to reduce unnecessary spending.
Study design: Systematic review.
Setting: Yale School of Medicine, New Haven, Conn.
Synopsis: Nineteen publications were selected for final analysis. Thirteen studies reported the impact of price display on costs, nine of which showed a statistically significant decrease in order costs. Only three of eight studies reporting the impact of price display on order volume showed statistically significant decreases in order volume. One study showed adverse safety findings in the form of higher rates of unscheduled follow-up care in a pediatric ED. Physicians were overall satisfied with price display in the five studies reporting this.
There was high heterogeneity among studies, which did not allow for pooling of data. Furthermore, more than half of the studies were conducted more than 15 years ago, limiting their generalizability to the modern era of electronic health records (EHRs).
Overall, this review supports the conclusion that price display has a modest effect on order costs. Additional studies utilizing EHR systems are required to more definitively confirm these findings.
Bottom line: Displaying prices to physicians can have a modest effect on overall order costs.
Citation: Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. doi:10.1002/jhm.2500.
Clinical question: Does price display impact order costs and volume as well as patient safety outcomes, and is it acceptable to providers?
Background: Up to one-third of national healthcare expenditures are wasteful, with physicians playing a central role in overall cost, purchasing almost all tests and therapies for patients. Increasing the transparency of costs for physicians is one strategy to reduce unnecessary spending.
Study design: Systematic review.
Setting: Yale School of Medicine, New Haven, Conn.
Synopsis: Nineteen publications were selected for final analysis. Thirteen studies reported the impact of price display on costs, nine of which showed a statistically significant decrease in order costs. Only three of eight studies reporting the impact of price display on order volume showed statistically significant decreases in order volume. One study showed adverse safety findings in the form of higher rates of unscheduled follow-up care in a pediatric ED. Physicians were overall satisfied with price display in the five studies reporting this.
There was high heterogeneity among studies, which did not allow for pooling of data. Furthermore, more than half of the studies were conducted more than 15 years ago, limiting their generalizability to the modern era of electronic health records (EHRs).
Overall, this review supports the conclusion that price display has a modest effect on order costs. Additional studies utilizing EHR systems are required to more definitively confirm these findings.
Bottom line: Displaying prices to physicians can have a modest effect on overall order costs.
Citation: Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. doi:10.1002/jhm.2500.
Clinical question: Does price display impact order costs and volume as well as patient safety outcomes, and is it acceptable to providers?
Background: Up to one-third of national healthcare expenditures are wasteful, with physicians playing a central role in overall cost, purchasing almost all tests and therapies for patients. Increasing the transparency of costs for physicians is one strategy to reduce unnecessary spending.
Study design: Systematic review.
Setting: Yale School of Medicine, New Haven, Conn.
Synopsis: Nineteen publications were selected for final analysis. Thirteen studies reported the impact of price display on costs, nine of which showed a statistically significant decrease in order costs. Only three of eight studies reporting the impact of price display on order volume showed statistically significant decreases in order volume. One study showed adverse safety findings in the form of higher rates of unscheduled follow-up care in a pediatric ED. Physicians were overall satisfied with price display in the five studies reporting this.
There was high heterogeneity among studies, which did not allow for pooling of data. Furthermore, more than half of the studies were conducted more than 15 years ago, limiting their generalizability to the modern era of electronic health records (EHRs).
Overall, this review supports the conclusion that price display has a modest effect on order costs. Additional studies utilizing EHR systems are required to more definitively confirm these findings.
Bottom line: Displaying prices to physicians can have a modest effect on overall order costs.
Citation: Silvestri MT, Bongiovanni TR, Glover JG, Gross CP. Impact of price display on provider ordering: a systematic review. J Hosp Med. 2016;11(1):65-76. doi:10.1002/jhm.2500.
How Should a Patient with Pulmonary Hypertension Be Evaluated, Managed?
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
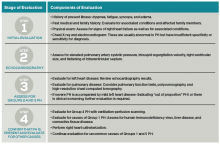
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
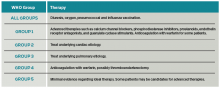
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Case
A 62-year-old female with no significant past medical history presents with three weeks of progressive dyspnea on exertion and bilateral lower extremity edema. Family members report that the patient often snores and “gasps for air” during sleep. B-type natriuretic peptide is elevated at 2,261 pg/ml. Due to concern for congestive heart failure, transthoracic echocardiography (TTE) is performed and shows normal left ventricular systolic function, mild left ventricular diastolic dysfunction, severely elevated right ventricular systolic pressure of 74 mm Hg, and right ventricular dilatation and hypokinesis.
How should this patient with newfound pulmonary hypertension (PH) be evaluated and managed?
Background
PH is a progressive disease that presents with nonspecific signs and symptoms and can be fatal if untreated. Ernst von Romberg first identified the disease in 1891, and efforts have been made through the last century to understand its etiology and mechanisms.1
PH is defined as an elevated mean pulmonary arterial pressure (mPAP) of ≥25 mmHg at rest; a mPAP of ≤20 mmHg is considered normal, and a mPAP of 21-24 mmHg is borderline.2 This elevation of the mPAP can be due to a primary elevation of pressures in the pulmonary arterial system alone (pulmonary arterial hypertension) or secondary to elevation in pressures in the pulmonary venous and pulmonary capillary systems (pulmonary venous hypertension).
PH classification has endured many modifications through the years with better understanding of its pathophysiology. Currently, the World Health Organization (WHO) classification system includes five groups based on etiology (see Table 1):3,4
- Group 1: Pulmonary arterial hypertension (PAH);
- Group 2: PH due to left heart disease;
- Group 3: PH due to chronic lung disease and hypoxemia;
- Group 4: Chronic thromboembolic PH (CTEPH); and
- Group 5: PH due to unclear multifactorial mechanisms.
The pathophysiology differs among the groups, and much of what is known has come from studies performed in patients with idiopathic PAH. It is a proliferative vasculopathy characterized by vasoconstriction, cell proliferation, fibrosis, and thrombosis. Both genetic predisposition and modifiers that include drugs and toxins, human immunodeficiency virus (HIV), congenital heart disease with left-to-right shunting, and potassium channel dysfunction play a role in the pathogenesis.3,5,6 Although many processes underlying the pathophysiology of PH groups 2, 3, 4, and 5 are not fully understood, vascular remodeling and increased vascular resistance are common to all of them.
PH affects both genders and all age groups and races. Due to its broad classification and multiple etiologies, it is difficult to assess PH prevalence in the general population. There are wide ranges among different populations, with PH prevalence in sickle cell disease ranging from 20% to 40%, in systemic sclerosis from 10% to 15%, and in portal hypertension from 2% to 16%.7,8,9 PH in COPD is usually mild to moderate, with preserved cardiac output, although a minority of patients develop severe PH.10-12 PH is present in approximately 20% of patients with moderate to severe sleep apnea.13 The prevalence of PH in left heart disease is unknown due to variability in populations assessed and methods used in various studies; estimates have ranged from 25-100%.14
Evaluation
Initial evaluation: A thorough history and physical examination can help determine PH etiology, identify associated conditions, and determine the severity of disease. Dyspnea on exertion is the most common presenting complaint; weakness, fatigue, and angina may be present.15 Lower extremity edema and ascites are indicative of more advanced disease.
A patient’s symptoms may suggest the presence of undiagnosed conditions that are associated with PH, and past medical history should evaluate for previous diagnoses of these conditions (see Table 1).
Family history may reveal relatives with PH, given the genetic predisposition to development of Group 1 PH. Physical exam findings include a prominent pulmonic valve closure during the second heart sound, a palpable left parasternal heave, and a tricuspid regurgitation murmur.
Electrocardiogram (ECG) and chest X-ray (CXR) are not sufficiently sensitive or specific to diagnose PH but may provide initial supporting evidence that prompts further testing. Signs of right ventricular hypertrophy and right atrial enlargement may be present on ECG. The CXR may show pruning (prominent hilar vasculature with reduced vasculature peripherally) and right ventricular hypertrophy, as evidenced by shrinking of the retrosternal window on lateral CXR. An unremarkable ECG or normal CXR does not rule out PH.
Echocardiography: TTE allows estimation of pulmonary artery systolic pressure (PASP) via measurement of tricuspid regurgitation jet velocity and estimation of right atrial pressure. Although results of TTE do correlate with measurements from right heart catheterization (RHC), underestimation and overestimation commonly occur. PASP thresholds for diagnosing or ruling out PH cannot thus be defined easily. An elevated PASP less than 36 mmHg, tricuspid regurgitation velocity <2.8 m/s, and no additional echocardiographic variables suggestive of PH may indicate that PH is unlikely, based on arbitrary criteria from one clinical practice guideline.16
The guideline suggested that tricuspid regurgitation velocity >3.4 m/s or estimated PASP >50 mmHg indicated that PH was likely. Other echocardiographic variables that may suggest the presence of PH include right ventricular enlargement or intraventricular septal flattening. Finally, TTE should also be used to assess for possible causes of PH, such as left heart disease or cardiac shunts.
Further evaluation: Following identification of PH via TTE, further testing can confirm the diagnosis, determine the etiology of the PH, and allow appropriate treatment (see Table 2). Much of this evaluation may occur after hospital discharge and, in cases of unexplained PH, referral to a pulmonologist for further evaluation and management is appropriate. Depending on patient stability, test availability, and patient ability to follow up, some testing may be reasonable during the inpatient stay.
Patients should undergo a stepwise series of testing that initially may be guided by clinical suspicion for underlying conditions.15-19 Polysomnography can identify sleep-disordered breathing, and pulmonary function tests and high-resolution chest CT can assess for chronic pulmonary diseases. Patients with groups 2 and 3 PH, whose PH can be explained by left heart disease or lung disease, do not necessarily require RHC or extensive evaluation for other etiologies of PH.2,17 These patients may be monitored while their underlying conditions are managed.
Patients with worsening clinical course or PH that is “out of proportion” to their lung disease or heart disease, however, do require further evaluation, including RHC. “Out of proportion” has not been consistently defined but generally refers to severe PH observed in patients with mild left heart or lung disease.18 More precise terminology and criteria to define patients with out of proportion PH have been proposed.14
Ventilation-perfusion scanning is required in all cases of PH of unknown etiology to evaluate for CTEPH (Group 4 PH). CT angiography, while appropriate to use in testing for acute pulmonary embolism, is not sufficiently sensitive to evaluate for CTEPH. Tests for liver function, HIV, and connective tissue disease may identify conditions associated with Group 1 PH. Ultimately, RHC is required to confirm the diagnosis of PH, given the shortcomings of TTE. A vasodilator study during RHC allows identification of candidates for advanced therapies, such as patients with Group 1 PH.
Management
The prognosis and treatment of PH varies by WHO Group. The hospitalist will often undertake initial management of symptomatic patients (see Table 3). Intravenous loop diuretics will successfully treat peripheral edema and hepatic congestion in all PH patients.20 Due to the possibility of decreased cardiac output or worsened hypotension in some PH groups, patients should be monitored closely during initial diuresis.
All patients with PH should be assessed for hypoxia during rest, ambulation, and sleep during their hospitalization. Supplemental oxygen therapy should be initiated in all patients with evidence of persistent hypoxia (arterial oxygen blood pressure <60 mmHg).20 Vaccination against pneumococcus and influenza should also be performed during the initial hospitalization. Pregnant patients diagnosed with PH require urgent maternal-fetal medicine consultation.
Further management should be guided by the underlying etiology of the PH:17,18
- Group 1 PH. These patients should be evaluated by a pulmonology consultant, if one is available, as they require intense outpatient follow-up with a pulmonologist. Specialized treatment regimens include calcium channel blockers, phosphodiesterase inhibitors, prostanoids, endothelin receptor antagonists, or newly approved guanylate cyclase stimulants. In previously diagnosed patients, these medications should be continued during a patient’s admission unless the medication is clearly causing the patient harm (such as worsening hypotension) or preventing improvement. Many of these patients are placed on chronic anticoagulation with warfarin, with a goal international normalized ratio (INR) of 1.5 to 2.5.
- Group 2 PH. Patients with left heart or valvular dysfunction and PH have a worse prognosis than similar patients without PH. Management of these patients should focus on treating the underlying etiology. Use of prostanoids may be harmful in this patient population.18
- Group 3 PH. Patients whose PH is fully explained by pulmonary disease should be started on continuous oxygen therapy to treat persistent hypoxemia, and their underlying disorder should be treated, with pulmonologist consultation and referral if necessary.
- Group 4 PH. Patients with newly diagnosed CTEPH should be initiated on warfarin with a goal INR of 2.0 to 3.0. They should undergo evaluation by a pulmonologist for thromboendarterectomy and possibly advanced medical therapies.
- Group 5 PH. Patients with sarcoidosis as the cause of their PH may benefit from prostanoid or endothelin receptor antagonist therapy and should undergo evaluation by a pulmonologist.21,22
Patients with sickle cell anemia, metabolic disorders, and other causes should undergo further subspecialist evaluation prior to initiating therapy to treat their PH.
Back to the Case
The patient underwent diuresis with intravenous furosemide over several days, with gradual improvement in her lower extremity edema and dyspnea. She was placed on oxygen therapy for persistent hypoxemia. As her highly elevated pulmonary artery pressure appeared to be “out of proportion” to her mild left ventricular diastolic dysfunction, further evaluation was pursued. Ventilation-perfusion scanning was performed and showed no mismatch of perfusion and ventilation, effectively ruling out CTEPH. Liver function, HIV, and connective tissue disease testing yielded unremarkable results.
The patient was euvolemic after one week of diuresis and was discharged home with plans for PH specialist follow-up, polysomnography to evaluate for sleep-disordered breathing, and likely RHC. The etiology of her PH was not clear at discharge.
Bottom Line
Evaluation of PH is a step-wise process that starts with history and physical exam and may require extensive evaluation, including right heart catheterization to confirm the diagnosis and define the etiology. A primary goal of evaluation is to define the appropriate therapy for a given patient, which may include advanced therapies in some cases.
Dr. Griffith is a quality improvement fellow and instructor of medicine in the Hospital Medicine Division at the University of Colorado Denver. Drs. McFarland and Smolkin are hospitalists and instructors of medicine at the University of Colorado Denver.
References
- von Romberg E. Über sklerose der lungenarterie. Dtsch Arch Klin Med. 1891;48:197-206.
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42-D50.
- Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34-D41.
- Rich S, Rubin L, Abenhail L, et al. Executive summary from the World Symposium on primary pulmonary hypertension. Evian, France: The World Health Organization; 1998.
- Newman JH, Wheeler L, Lane KB, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. New Engl J Med. 2001;345(5):319-24.'
- Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation. 1994;89(6):2722-2727.
- Gladwin MT, Vichinsky E. Pulmonary complications of sickle cell disease. New Engl J Med. 2008;359(21):2254-2265.
- Wigley FM, Lima JA, Mayes M, McLain D, Chapin JL, Ward-Able C. The prevalence of undiagnosed pulmonary arterial hypertension in subjects with connective tissue disease at the secondary health care level of community-based rheumatologists (the UNCOVER study). Arthritis Rheum. 2005;52(7):2125-2132.
- Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3(5):494-500.
- Kessler R, Faller M, Weitzenblum E, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164(2):219-24.
- Chaouat A, Bugnet AS, Kadaoui N, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):189-94.
- Thabut G, Dauriat G, Stern JB, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127(5):1531-1536.
- Yamakawa H, Shiomi T, Sasanabe R, et al. Pulmonary hypertension in patients with severe obstructive sleep apnea. Psychiatry Clin Neurosci. 2002;56(3):311-312.
- Vachiery JL, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100-D108.
- McGoon M, Gutterman D, Steen V, et al. Screening, early detection, and diagnosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126(1 Suppl):14S-34S.
- Grünig E, Barner A, Bell M, et al. Non-invasive diagnosis of pulmonary hypertension: ESC/ERS Guidelines with Updated Commentary of the Cologne Consensus Conference 2011. Int J Cardiol. 2011;154 Suppl 1:S3-12.
- Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537.
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250-2294.
- Brown K, Gutierrez AJ, Mohammed TL, et al. ACR Appropriateness Criteria(R) pulmonary hypertension. J Thorac Imaging. 2013;28(4):W57-60.
- Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60-72.
- Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481-1488.
- Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest. 2006;130(5):1471-1480.
Infectious Diseases Society of America 2014 Practice Guidelines To Diagnose, Manage Skin, Soft Tissue Infections
Background
Surveillance studies in the U.S. have shown an increase in the number of hospitalizations for skin and soft tissue infections (SSTIs) by 29% from 2000 to 2004.1 Moreover, recent studies on the inpatient management of SSTIs have shown significant deviation from recommended therapy, with the majority of patients receiving excessively long treatment courses or unnecessarily broad antimicrobial coverage.2,3
With the ever-increasing threat of antibiotic resistance and rising rates of Clostridium difficile colitis, this update provides clinicians with a set of recommendations to apply antibiotic stewardship while effectively managing SSTIs.4
Guideline Update
In June 2014, the Infectious Diseases Society of America (IDSA) published an update to its 2005 guidelines for the treatment of SSTIs.5 For purulent SSTIs (cutaneous abscesses, furuncles, carbuncles, and inflamed epidermoid cysts), incision and drainage is primary therapy. The use of systemic antimicrobial therapy is unnecessary for mild cases, even those caused by methicillin-resistant Staphylococcus aureus (MRSA). The use of empiric adjunctive antibiotics should be reserved for those with impaired host defenses or signs of systemic inflammatory response syndrome (SIRS). The recommended antibiotics in such patients have anti-MRSA activity and include trimethoprim-sulfamethoxazole or doxycycline for moderate infections and vancomycin, daptomycin, linezolid, telavancin, or ceftaroline for severe infections. Antibiotics should subsequently be adjusted based on susceptibilities of the organism cultured from purulent drainage.
Nonpurulent cellulitis without SIRS may be treated on an outpatient basis with an oral antibiotic targeted against streptococci, including penicillin VK, cephalosporins, dicloxacillin, or clindamycin. Cellulitis with SIRS may be treated with an intravenous antibiotic with methicillin-susceptible Staphylococcus aureus (MSSA) activity, including penicillin, ceftriaxone, cefazolin, or clindamycin.
The use of antibiotics with MRSA activity should be reserved for those at highest risk, such as patients with impaired immunity or signs of a deep space infection. Cultures of blood, cutaneous biopsies, or swabs are not routinely recommended; however, prompt surgical consultation is recommended for patients suspected of having a necrotizing infection or gangrene.
The recommended duration of antimicrobial therapy for uncomplicated cellulitis is five days, and therapy should only be extended in those who have not shown clinical improvement. Elevation of the affected area and the use of systemic corticosteroids in nondiabetic adults may lead to a more rapid resolution of cellulitis, although the clinician must ensure that a deeper space infection is not present prior to initiating steroids.
Preventing the recurrence of cellulitis is an integral part of routine patient care and includes the treatment of interdigital toe space fissuring, scaling, and maceration, which may act as a reservoir for streptococci. Likewise, treatment of predisposing conditions such as eczema, venous insufficiency, and lymphedema may reduce the recurrence of infection. In patients who have three to four episodes of cellulitis despite attempts to treat or control predisposing risk factors, the use of prophylactic antibiotics with erythromycin or penicillin may be considered.
For patients with an SSTI during the first episode of febrile neutropenia, hospitalization and empiric therapy with vancomycin and an antipseudomonal beta-lactam are recommended. Antibiotics should subsequently be adjusted based on the antimicrobial susceptibilities of isolated organisms.
For patients with SSTIs in the presence of persistent or recurrent febrile neutropenia, empirically adding antifungal therapy is recommended. Such patients should be aggressively evaluated with blood cultures and biopsy with tissue culture of the skin lesions. The recommended duration of therapy is seven to 14 days for most bacterial SSTIs in the immunocompromised patient.
Analysis
The updated SSTI guidelines provide hospitalists with a practical algorithm for the management of SSTIs, focusing on the presence or absence of purulence, systemic signs of infection, and host immune status to guide therapy. Whereas the 2005 guidelines provided clinicians with a list of recommended antibiotics based on spectrum of activity, the updated guidelines provide a short list of empiric antibiotics based on the type and severity of infection.6
The list of recommended antibiotics with MRSA activity has been updated to include ceftaroline and telavancin. Of note, since these guidelines have been published, three new antibiotics with MRSA activity (tedizolid, oritavancin, and dalbavancin) have been approved by the FDA for the treatment of SSTIs, although their specific role in routine clinical practice is not yet determined.
The treatment algorithm for surgical site infections remains largely unchanged, which reinforces the concept that fever in the first 48 hours is unlikely to represent infection unless accompanied by purulent wound drainage with a positive culture. Likewise, the guidelines recommend risk-stratifying patients with fever and a suspected wound infection more than four days after surgery by the presence or absence of systemic infection or evidence of surrounding cellulitis.
A comprehensive guide to the management of specific pathogens or conditions, such as tularemia, cutaneous anthrax, and bite wounds, is largely unchanged, although the update now includes focused summary statements to navigate through these recommendations more easily.
The updated guidelines provide a more robust yet focused set of recommendations for the diagnosis and treatment of bacterial, fungal, and viral skin infections in immunocompromised hosts, especially those with neutropenia.
HM Takeaways
The 2014 update to the IDSA practice guidelines for SSTIs contains a chart to help clinicians diagnose and manage common skin infections more effectively. The guidelines’ algorithm stratifies the severity of illness according to whether or not the patient has SIRS or is immunocompromised. The authors recommend against the use of antibiotics for mild purulent SSTIs and reserve the use of anti-MRSA therapy mainly for patients with moderate purulent SSTIs, those with severe SSTIs, or those at high risk for MRSA. Likewise, the use of broad spectrum gram-negative coverage is not recommended in most common, uncomplicated SSTIs and should be reserved for special populations, such as those with immune compromise.
The guidelines strongly recommend a short, five-day course of therapy for uncomplicated cellulitis. Longer treatment courses (i.e., 10 days) are unnecessary and do not improve efficacy for those exhibiting clinical improvement by day five.
Drs. Yogo and Saveli work in the division of infectious disease in the department of medicine at the University of Colorado School of Medicine in Aurora.
References
- Edelsberg J, Taneja C, Zervos M, et al. Trends in the US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009;15(9):1516-1518.
- Jenkins TC, Sabel AL, Sacrone EE, Price CS, Mehler PS, Burman WJ. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895-903.
- Jenkins TC, Knepper BC, Moore SJ, et al. Antibiotic prescribing practices in a multicenter cohort of patients hospitalized for acute bacterial skin and skin structure infection. Infect Control Hosp Epidemiol. 2014;35(10):1241-1250.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed February 8, 2015.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-52. Stevens DL, Bisno AL, Chambers HF, et al.
- Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373-1406.
Background
Surveillance studies in the U.S. have shown an increase in the number of hospitalizations for skin and soft tissue infections (SSTIs) by 29% from 2000 to 2004.1 Moreover, recent studies on the inpatient management of SSTIs have shown significant deviation from recommended therapy, with the majority of patients receiving excessively long treatment courses or unnecessarily broad antimicrobial coverage.2,3
With the ever-increasing threat of antibiotic resistance and rising rates of Clostridium difficile colitis, this update provides clinicians with a set of recommendations to apply antibiotic stewardship while effectively managing SSTIs.4
Guideline Update
In June 2014, the Infectious Diseases Society of America (IDSA) published an update to its 2005 guidelines for the treatment of SSTIs.5 For purulent SSTIs (cutaneous abscesses, furuncles, carbuncles, and inflamed epidermoid cysts), incision and drainage is primary therapy. The use of systemic antimicrobial therapy is unnecessary for mild cases, even those caused by methicillin-resistant Staphylococcus aureus (MRSA). The use of empiric adjunctive antibiotics should be reserved for those with impaired host defenses or signs of systemic inflammatory response syndrome (SIRS). The recommended antibiotics in such patients have anti-MRSA activity and include trimethoprim-sulfamethoxazole or doxycycline for moderate infections and vancomycin, daptomycin, linezolid, telavancin, or ceftaroline for severe infections. Antibiotics should subsequently be adjusted based on susceptibilities of the organism cultured from purulent drainage.
Nonpurulent cellulitis without SIRS may be treated on an outpatient basis with an oral antibiotic targeted against streptococci, including penicillin VK, cephalosporins, dicloxacillin, or clindamycin. Cellulitis with SIRS may be treated with an intravenous antibiotic with methicillin-susceptible Staphylococcus aureus (MSSA) activity, including penicillin, ceftriaxone, cefazolin, or clindamycin.
The use of antibiotics with MRSA activity should be reserved for those at highest risk, such as patients with impaired immunity or signs of a deep space infection. Cultures of blood, cutaneous biopsies, or swabs are not routinely recommended; however, prompt surgical consultation is recommended for patients suspected of having a necrotizing infection or gangrene.
The recommended duration of antimicrobial therapy for uncomplicated cellulitis is five days, and therapy should only be extended in those who have not shown clinical improvement. Elevation of the affected area and the use of systemic corticosteroids in nondiabetic adults may lead to a more rapid resolution of cellulitis, although the clinician must ensure that a deeper space infection is not present prior to initiating steroids.
Preventing the recurrence of cellulitis is an integral part of routine patient care and includes the treatment of interdigital toe space fissuring, scaling, and maceration, which may act as a reservoir for streptococci. Likewise, treatment of predisposing conditions such as eczema, venous insufficiency, and lymphedema may reduce the recurrence of infection. In patients who have three to four episodes of cellulitis despite attempts to treat or control predisposing risk factors, the use of prophylactic antibiotics with erythromycin or penicillin may be considered.
For patients with an SSTI during the first episode of febrile neutropenia, hospitalization and empiric therapy with vancomycin and an antipseudomonal beta-lactam are recommended. Antibiotics should subsequently be adjusted based on the antimicrobial susceptibilities of isolated organisms.
For patients with SSTIs in the presence of persistent or recurrent febrile neutropenia, empirically adding antifungal therapy is recommended. Such patients should be aggressively evaluated with blood cultures and biopsy with tissue culture of the skin lesions. The recommended duration of therapy is seven to 14 days for most bacterial SSTIs in the immunocompromised patient.
Analysis
The updated SSTI guidelines provide hospitalists with a practical algorithm for the management of SSTIs, focusing on the presence or absence of purulence, systemic signs of infection, and host immune status to guide therapy. Whereas the 2005 guidelines provided clinicians with a list of recommended antibiotics based on spectrum of activity, the updated guidelines provide a short list of empiric antibiotics based on the type and severity of infection.6
The list of recommended antibiotics with MRSA activity has been updated to include ceftaroline and telavancin. Of note, since these guidelines have been published, three new antibiotics with MRSA activity (tedizolid, oritavancin, and dalbavancin) have been approved by the FDA for the treatment of SSTIs, although their specific role in routine clinical practice is not yet determined.
The treatment algorithm for surgical site infections remains largely unchanged, which reinforces the concept that fever in the first 48 hours is unlikely to represent infection unless accompanied by purulent wound drainage with a positive culture. Likewise, the guidelines recommend risk-stratifying patients with fever and a suspected wound infection more than four days after surgery by the presence or absence of systemic infection or evidence of surrounding cellulitis.
A comprehensive guide to the management of specific pathogens or conditions, such as tularemia, cutaneous anthrax, and bite wounds, is largely unchanged, although the update now includes focused summary statements to navigate through these recommendations more easily.
The updated guidelines provide a more robust yet focused set of recommendations for the diagnosis and treatment of bacterial, fungal, and viral skin infections in immunocompromised hosts, especially those with neutropenia.
HM Takeaways
The 2014 update to the IDSA practice guidelines for SSTIs contains a chart to help clinicians diagnose and manage common skin infections more effectively. The guidelines’ algorithm stratifies the severity of illness according to whether or not the patient has SIRS or is immunocompromised. The authors recommend against the use of antibiotics for mild purulent SSTIs and reserve the use of anti-MRSA therapy mainly for patients with moderate purulent SSTIs, those with severe SSTIs, or those at high risk for MRSA. Likewise, the use of broad spectrum gram-negative coverage is not recommended in most common, uncomplicated SSTIs and should be reserved for special populations, such as those with immune compromise.
The guidelines strongly recommend a short, five-day course of therapy for uncomplicated cellulitis. Longer treatment courses (i.e., 10 days) are unnecessary and do not improve efficacy for those exhibiting clinical improvement by day five.
Drs. Yogo and Saveli work in the division of infectious disease in the department of medicine at the University of Colorado School of Medicine in Aurora.
References
- Edelsberg J, Taneja C, Zervos M, et al. Trends in the US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009;15(9):1516-1518.
- Jenkins TC, Sabel AL, Sacrone EE, Price CS, Mehler PS, Burman WJ. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895-903.
- Jenkins TC, Knepper BC, Moore SJ, et al. Antibiotic prescribing practices in a multicenter cohort of patients hospitalized for acute bacterial skin and skin structure infection. Infect Control Hosp Epidemiol. 2014;35(10):1241-1250.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed February 8, 2015.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-52. Stevens DL, Bisno AL, Chambers HF, et al.
- Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373-1406.
Background
Surveillance studies in the U.S. have shown an increase in the number of hospitalizations for skin and soft tissue infections (SSTIs) by 29% from 2000 to 2004.1 Moreover, recent studies on the inpatient management of SSTIs have shown significant deviation from recommended therapy, with the majority of patients receiving excessively long treatment courses or unnecessarily broad antimicrobial coverage.2,3
With the ever-increasing threat of antibiotic resistance and rising rates of Clostridium difficile colitis, this update provides clinicians with a set of recommendations to apply antibiotic stewardship while effectively managing SSTIs.4
Guideline Update
In June 2014, the Infectious Diseases Society of America (IDSA) published an update to its 2005 guidelines for the treatment of SSTIs.5 For purulent SSTIs (cutaneous abscesses, furuncles, carbuncles, and inflamed epidermoid cysts), incision and drainage is primary therapy. The use of systemic antimicrobial therapy is unnecessary for mild cases, even those caused by methicillin-resistant Staphylococcus aureus (MRSA). The use of empiric adjunctive antibiotics should be reserved for those with impaired host defenses or signs of systemic inflammatory response syndrome (SIRS). The recommended antibiotics in such patients have anti-MRSA activity and include trimethoprim-sulfamethoxazole or doxycycline for moderate infections and vancomycin, daptomycin, linezolid, telavancin, or ceftaroline for severe infections. Antibiotics should subsequently be adjusted based on susceptibilities of the organism cultured from purulent drainage.
Nonpurulent cellulitis without SIRS may be treated on an outpatient basis with an oral antibiotic targeted against streptococci, including penicillin VK, cephalosporins, dicloxacillin, or clindamycin. Cellulitis with SIRS may be treated with an intravenous antibiotic with methicillin-susceptible Staphylococcus aureus (MSSA) activity, including penicillin, ceftriaxone, cefazolin, or clindamycin.
The use of antibiotics with MRSA activity should be reserved for those at highest risk, such as patients with impaired immunity or signs of a deep space infection. Cultures of blood, cutaneous biopsies, or swabs are not routinely recommended; however, prompt surgical consultation is recommended for patients suspected of having a necrotizing infection or gangrene.
The recommended duration of antimicrobial therapy for uncomplicated cellulitis is five days, and therapy should only be extended in those who have not shown clinical improvement. Elevation of the affected area and the use of systemic corticosteroids in nondiabetic adults may lead to a more rapid resolution of cellulitis, although the clinician must ensure that a deeper space infection is not present prior to initiating steroids.
Preventing the recurrence of cellulitis is an integral part of routine patient care and includes the treatment of interdigital toe space fissuring, scaling, and maceration, which may act as a reservoir for streptococci. Likewise, treatment of predisposing conditions such as eczema, venous insufficiency, and lymphedema may reduce the recurrence of infection. In patients who have three to four episodes of cellulitis despite attempts to treat or control predisposing risk factors, the use of prophylactic antibiotics with erythromycin or penicillin may be considered.
For patients with an SSTI during the first episode of febrile neutropenia, hospitalization and empiric therapy with vancomycin and an antipseudomonal beta-lactam are recommended. Antibiotics should subsequently be adjusted based on the antimicrobial susceptibilities of isolated organisms.
For patients with SSTIs in the presence of persistent or recurrent febrile neutropenia, empirically adding antifungal therapy is recommended. Such patients should be aggressively evaluated with blood cultures and biopsy with tissue culture of the skin lesions. The recommended duration of therapy is seven to 14 days for most bacterial SSTIs in the immunocompromised patient.
Analysis
The updated SSTI guidelines provide hospitalists with a practical algorithm for the management of SSTIs, focusing on the presence or absence of purulence, systemic signs of infection, and host immune status to guide therapy. Whereas the 2005 guidelines provided clinicians with a list of recommended antibiotics based on spectrum of activity, the updated guidelines provide a short list of empiric antibiotics based on the type and severity of infection.6
The list of recommended antibiotics with MRSA activity has been updated to include ceftaroline and telavancin. Of note, since these guidelines have been published, three new antibiotics with MRSA activity (tedizolid, oritavancin, and dalbavancin) have been approved by the FDA for the treatment of SSTIs, although their specific role in routine clinical practice is not yet determined.
The treatment algorithm for surgical site infections remains largely unchanged, which reinforces the concept that fever in the first 48 hours is unlikely to represent infection unless accompanied by purulent wound drainage with a positive culture. Likewise, the guidelines recommend risk-stratifying patients with fever and a suspected wound infection more than four days after surgery by the presence or absence of systemic infection or evidence of surrounding cellulitis.
A comprehensive guide to the management of specific pathogens or conditions, such as tularemia, cutaneous anthrax, and bite wounds, is largely unchanged, although the update now includes focused summary statements to navigate through these recommendations more easily.
The updated guidelines provide a more robust yet focused set of recommendations for the diagnosis and treatment of bacterial, fungal, and viral skin infections in immunocompromised hosts, especially those with neutropenia.
HM Takeaways
The 2014 update to the IDSA practice guidelines for SSTIs contains a chart to help clinicians diagnose and manage common skin infections more effectively. The guidelines’ algorithm stratifies the severity of illness according to whether or not the patient has SIRS or is immunocompromised. The authors recommend against the use of antibiotics for mild purulent SSTIs and reserve the use of anti-MRSA therapy mainly for patients with moderate purulent SSTIs, those with severe SSTIs, or those at high risk for MRSA. Likewise, the use of broad spectrum gram-negative coverage is not recommended in most common, uncomplicated SSTIs and should be reserved for special populations, such as those with immune compromise.
The guidelines strongly recommend a short, five-day course of therapy for uncomplicated cellulitis. Longer treatment courses (i.e., 10 days) are unnecessary and do not improve efficacy for those exhibiting clinical improvement by day five.
Drs. Yogo and Saveli work in the division of infectious disease in the department of medicine at the University of Colorado School of Medicine in Aurora.
References
- Edelsberg J, Taneja C, Zervos M, et al. Trends in the US hospital admissions for skin and soft tissue infections. Emerg Infect Dis. 2009;15(9):1516-1518.
- Jenkins TC, Sabel AL, Sacrone EE, Price CS, Mehler PS, Burman WJ. Skin and soft-tissue infections requiring hospitalization at an academic medical center: opportunities for antimicrobial stewardship. Clin Infect Dis. 2010;51(8):895-903.
- Jenkins TC, Knepper BC, Moore SJ, et al. Antibiotic prescribing practices in a multicenter cohort of patients hospitalized for acute bacterial skin and skin structure infection. Infect Control Hosp Epidemiol. 2014;35(10):1241-1250.
- U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2013. Available at: http://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf. Accessed February 8, 2015.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10-52. Stevens DL, Bisno AL, Chambers HF, et al.
- Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373-1406.