User login
A microscopic colitis treatment guideline from the American Gastroenterological Association was published in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2015.11.008).
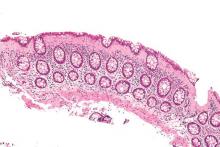
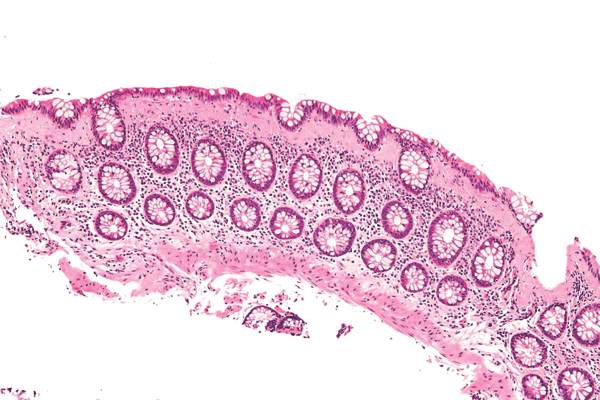
Microscopic colitis is most common in persons aged 60 years and over, typically presents as chronic watery diarrhea with nausea or abdominal pain caused by inflammation in the colon, and is diagnosed by colonic biopsy. It is more of a nuisance than life threatening, with no evidence that the persistent histologic inflammation predicts colorectal cancer or the need for surgical intervention.
The guideline strongly recommends, with moderate-quality evidence, treating symptomatic patients with budesonide rather than foregoing any treatment. The AGA also strongly recommends, with high-quality evidence, that budesonide be used over mesalamine to induce remission, unless such treatment is not feasible in a patient because of a drug reaction or its high price. In that case, the AGA recommends (with moderate-quality evidence) the use of mesalamine. Once induction therapy is discontinued, the AGA strongly recommends, with moderate-quality evidence, maintenance treatment using budesonide, if possible.
In patients for whom budesonide is not feasible, the AGA recommends that rather than forgo treatment, patients may be induced with bismuth subsalicylate (low-quality evidence) or prednisone (very-low-quality evidence).
Other recommendations made with low-quality evidence for treating symptomatic microscopic colitis include not using combination therapy with cholestyramine and mesalamine over mesalamine alone for induction therapy, nor Boswellia serrata to induce remission. The AGA also does not recommend (low-quality evidence) using probiotics over no treatment for the condition.
Because the meta-analysis of evidence conducted by the AGA only included data derived from clinical trials, the guideline does not include antidiarrheals, cholestyramine monotherapy, a variety of combination therapies, nor the medical treatment of steroid-refractory microscopic colitis, although the AGA did note in the guideline that very limited evidence from a series of cases suggests certain immunosuppressive therapies may benefit these patients.
On Twitter @whitneymcknight
A microscopic colitis treatment guideline from the American Gastroenterological Association was published in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2015.11.008).
Microscopic colitis is most common in persons aged 60 years and over, typically presents as chronic watery diarrhea with nausea or abdominal pain caused by inflammation in the colon, and is diagnosed by colonic biopsy. It is more of a nuisance than life threatening, with no evidence that the persistent histologic inflammation predicts colorectal cancer or the need for surgical intervention.
The guideline strongly recommends, with moderate-quality evidence, treating symptomatic patients with budesonide rather than foregoing any treatment. The AGA also strongly recommends, with high-quality evidence, that budesonide be used over mesalamine to induce remission, unless such treatment is not feasible in a patient because of a drug reaction or its high price. In that case, the AGA recommends (with moderate-quality evidence) the use of mesalamine. Once induction therapy is discontinued, the AGA strongly recommends, with moderate-quality evidence, maintenance treatment using budesonide, if possible.
In patients for whom budesonide is not feasible, the AGA recommends that rather than forgo treatment, patients may be induced with bismuth subsalicylate (low-quality evidence) or prednisone (very-low-quality evidence).
Other recommendations made with low-quality evidence for treating symptomatic microscopic colitis include not using combination therapy with cholestyramine and mesalamine over mesalamine alone for induction therapy, nor Boswellia serrata to induce remission. The AGA also does not recommend (low-quality evidence) using probiotics over no treatment for the condition.
Because the meta-analysis of evidence conducted by the AGA only included data derived from clinical trials, the guideline does not include antidiarrheals, cholestyramine monotherapy, a variety of combination therapies, nor the medical treatment of steroid-refractory microscopic colitis, although the AGA did note in the guideline that very limited evidence from a series of cases suggests certain immunosuppressive therapies may benefit these patients.
On Twitter @whitneymcknight
A microscopic colitis treatment guideline from the American Gastroenterological Association was published in the January issue of Gastroenterology (doi: 10.1053/j.gastro.2015.11.008).
Microscopic colitis is most common in persons aged 60 years and over, typically presents as chronic watery diarrhea with nausea or abdominal pain caused by inflammation in the colon, and is diagnosed by colonic biopsy. It is more of a nuisance than life threatening, with no evidence that the persistent histologic inflammation predicts colorectal cancer or the need for surgical intervention.
The guideline strongly recommends, with moderate-quality evidence, treating symptomatic patients with budesonide rather than foregoing any treatment. The AGA also strongly recommends, with high-quality evidence, that budesonide be used over mesalamine to induce remission, unless such treatment is not feasible in a patient because of a drug reaction or its high price. In that case, the AGA recommends (with moderate-quality evidence) the use of mesalamine. Once induction therapy is discontinued, the AGA strongly recommends, with moderate-quality evidence, maintenance treatment using budesonide, if possible.
In patients for whom budesonide is not feasible, the AGA recommends that rather than forgo treatment, patients may be induced with bismuth subsalicylate (low-quality evidence) or prednisone (very-low-quality evidence).
Other recommendations made with low-quality evidence for treating symptomatic microscopic colitis include not using combination therapy with cholestyramine and mesalamine over mesalamine alone for induction therapy, nor Boswellia serrata to induce remission. The AGA also does not recommend (low-quality evidence) using probiotics over no treatment for the condition.
Because the meta-analysis of evidence conducted by the AGA only included data derived from clinical trials, the guideline does not include antidiarrheals, cholestyramine monotherapy, a variety of combination therapies, nor the medical treatment of steroid-refractory microscopic colitis, although the AGA did note in the guideline that very limited evidence from a series of cases suggests certain immunosuppressive therapies may benefit these patients.
On Twitter @whitneymcknight
FROM THE AMERICAN GASTROENTEROLOGICAL ASSOCIATION