User login
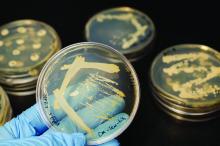
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
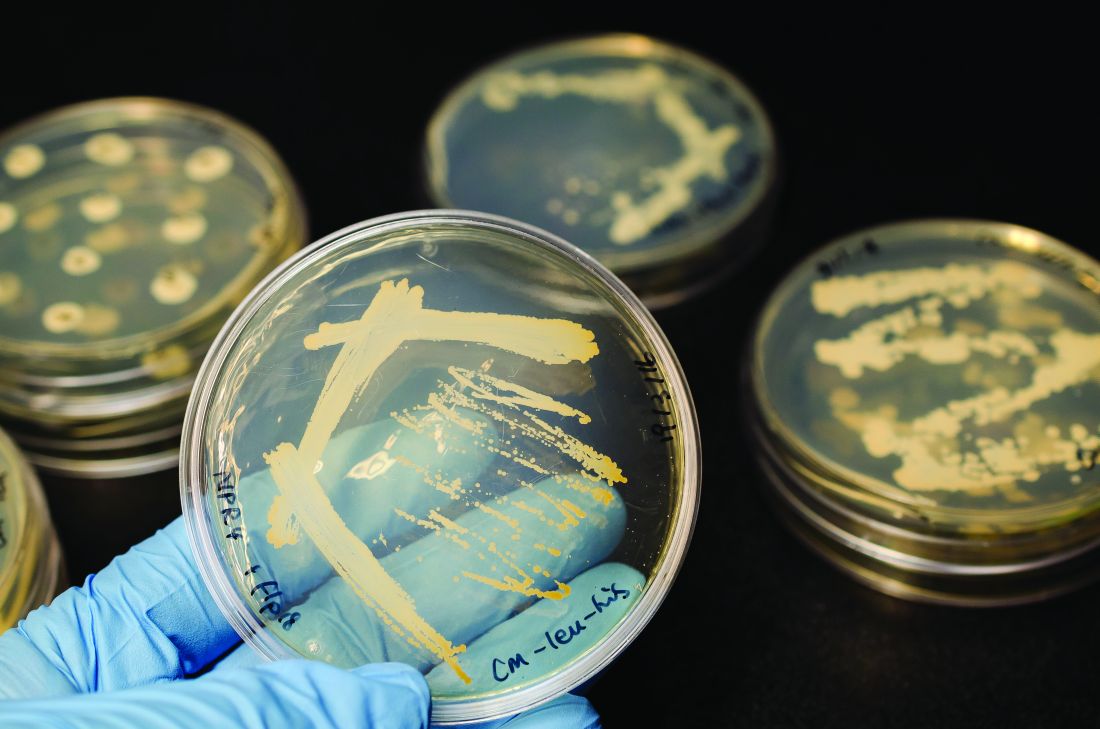
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
Intrabody 2H9-L, which can function as a hepatitis C virus (HCV) inhibitor in human hepatic cells, was expressed at high levels in Escherichia coli and silkworm pupae and was successfully purified in soluble form, according to a report published in Protein Expression and Purification by Tatsuya Kato, an assistant professor at Shizuoka (Japan) University, and his colleagues.
In their study, 171 mcg of purified intrabody was obtainable from 100 mL of E. coli culture, and 132 mcg could be obtained from 10 silkworm pupae.
The intrabodies were capable of binding to all HCV core protein variants tested. These purified intrabodies can be used in biochemical analyses and provide a potential pathway to developing a new type of therapy, according to the researchers.
“The structural basis of HCV core–intrabody interfaces would allow a novel strategy to design and generate chemical drugs with antiviral activities,” they stated. In addition, “to analyze the HCV core protein in detail, this intrabody can be used to keep the HCV core protein soluble, even when its concentration is high.”
No funding source or disclosures were reported in the paper.
SOURCE: Kato T et al. Protein Expression and Purification. 2018 October;150:61-6.
FROM PROTEIN EXPRESSION AND PURIFICATION