User login
Dr. Mitwally holds patents licensed to Serono for use of aromatase inhibitors for infertility treatment.
Dr. Casper has a licensing agreement with Ares-Serono for use of aromatase inhibitors in assisted reproduction.
CASE 1 Ovulation begins, but pregnancy does not follow
U.Y. is a 32-year-old woman who has been trying to conceive for 3 years. Her infertility is caused by anovulation associated with polycystic ovary syndrome (PCOS). All other variables are within physiologic limits—she has patent tubes and an unremarkable uterus, and her partner has a normal semen analysis.
She has undergone six cycles of treatment with clomiphene citrate, with ovulation documented each time by ultrasonography (US) and measurement of luteal-phase progesterone levels. Her endometrial thickness is 4 to 6 mm around the day of ovulation.
Would an aromatase inhibitor increase her chances of conceiving?
This patient is an excellent candidate for ovulation induction using an aromatase inhibitor (AI).
The primary reason? She is unlikely to benefit from an increased dosage of clomiphene citrate because the dosage that triggers ovulation is believed to be most appropriate—an increase above that level is not expected to improve the chance of pregnancy. Moreover, conception is less likely after more than six cycles of clomiphene citrate.1,2
In this article, we describe the induction of ovulation using AIs—a relatively new, and off-label, application (TABLES 1 and 2). The strategies presented here are suitable for general ObGyns and do not require sophisticated technology such as rapid hormonal assays or transvaginal US.
Because this application is so new, with limited data published so far, much of the information presented here is based on our personal experience rather than level-1 evidence, which is sorely needed.
Of course, induction of ovulation is appropriate only after other specific causes of anovulation or ovulatory dysfunction are excluded, such as thyroid disorders, hyperprolactinemia, severe insulin resistance, and ovarian failure.
Concerns about teratogenicity of AIs appear to be largely unfounded (see below).
TABLE 1
Aromatase inhibitors work best in these applications
| APPLICATION | EVIDENCE |
|---|---|
Induction of ovulation, particularly in women with polycystic ovary syndrome:
See case 1 and case 2 |
|
| Ovarian stimulation (superovulation) in ovulatory women with unexplained or endometriosis-related infertility See case 3 | Strong evidence from several clinical trials |
| Use in conjunction with controlled ovarian hyperstimulation by gonadotropins with intrauterine insemination and assisted reproduction | Accumulating evidence of several advantages when used with gonadotropins:
|
TABLE 2
Avoid AIs in these situations
| SITUATION | JUSTIFICATION |
|---|---|
| When clomiphene citrate fails to induce ovulation in a woman with insulin resistance See case 2 | First try insulin sensitizers and other measures to improve insulin action (weight loss, exercise, and dietary modifications) |
| When other causes of infertility (besides ovulatory dysfunction) are likely | Pregnancy is unlikely |
| When the patient has hypothalamic/hypopituitary anovulation or ovarian failure | Ovarian stimulation is dependent on capacity to produce endogenous gonadotropins and presence of responding ovarian follicles |
Ovulation is good, but pregnancy is better
In women undergoing induction of ovulation, there are two levels of success: ovulation and pregnancy.
Clearly, the presence of other, nonovulatory infertility factors—e.g., male infertility and tubal-uterine problems—can prevent successful ovulation induction from translating into pregnancy.
We have reported3-9 on the successful use of AIs to stimulate the ovary and achieve pregnancy—even in women who fail to conceive after several treatment trials with clomiphene citrate.4
Other authors have conducted further investigations that have confirmed our findings and have recommended use of these agents for other aspects of infertility treatment, such as assisted reproduction.10-19
Latest generation of AIs is more benign
Many AIs have been developed over the past 30 years. The most recent are third-generation agents that were approved mainly to suppress estrogen production in postmenopausal women with breast cancer. Clinical failure of earlier generations of AIs for their approved indication was mainly due to significant adverse effects, lack of satisfactory potency, or lack of specificity in inhibiting the aromatase enzyme without inhibiting other enzymes of steroidogenesis.20
Third-generation AIs that are commercially available in North America, Europe, and other parts of the world include:
- two nonsteroidal preparations: anastrozole (Arimidex) and letrozole (Femara)
- one steroidal agent: exemestane (Aromasin).
Letrozole and anastrozole are reversible, competitive agents with considerably greater potency (more than 1,000 times greater) than the first-generation AI aminoglutethimide. At a dosage of 1 to 5 mg/day, they reduce estrogen levels by 97% to more than 99%.
AIs are completely absorbed after oral administration, with a mean terminal half-life of approximately 45 hours (range: 30–60 hours). Exemestane has a shorter circulating half-life of approximately 9 hours, but may have a longer effect because it is irreversible.21
Mild gastrointestinal (GI) disturbances account for most of the adverse events, and rarely limit therapy.
How AIs work
Although we continue to accrue data on the use of AIs to induce ovulation, the underlying mechanism of action has not been studied. However, we believe that AIs work both centrally (at the level of the hypothalamus and pituitary) and peripherally (at the level of the ovaries).22-28
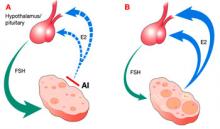
At the central level, AIs suppress estrogen production by directly, specifically, and potently inhibiting the aromatase enzyme (i.e., estrogen synthase, the enzyme responsible for the synthesis of estrogen). Because the aromatase enzyme is expressed in various tissues and organs—most notably, the ovaries, brain, and fat29—AIs suppress estrogen production in all of those tissues, leading to a low serum estrogen level and low local estrogen level. Low estrogen levels are thought to release the hypothalamus and pituitary gland from their negative-feedback mechanism, thereby increasing production of endogenous gonadotropins from the pituitary gland and stimulating ovarian follicular development and ovulation (FIGURE).
At the peripheral level, the aromatase enzyme catalyzes the terminal step in the steroidogenesis cascade that converts androgens into estrogen. When that enzyme is inhibited, enzyme substrate (androgens) is thought to accumulate. Contrary to the general belief that androgens are deleterious to ovarian follicles, studies in primates have demonstrated that androgens actually up-regulate the expression of gonadotropin receptors, particularly follicle-stimulating hormone (FSH) receptors.30 This renders the ovaries more sensitive to gonadotropin stimulation—whether the gonadotropins are endogenous or exogenous.22-28
FIGURE Aromatase inhibitors promote follicle development, then fade from the scene in time to prevent hyperovulation
Administration of an aromatase inhibitor (AI) on cycle days 3 to 7 suppresses ovarian estradiol (E2) secretion, as shown in A, which reduces estrogen-negative feedback at the hypothalamus and pituitary. As a result, follicle-stimulating hormone (FSH) secretion increases, fostering growth of multiple ovarian follicles. The growing follicles, shown in B, cause estrogen levels to rise again, depressing FSH, and leading to monofollicular ovulation in most cases.
Why AIs are superior to clomiphene
Clomiphene citrate is a selective estrogen receptor modulator (SERM) that is believed to induce ovulation through its antiestrogenic properties at the level of the hypothalamus or pituitary gland, or both. Clomiphene down-regulates estrogen receptors at this level, and the hypothalamus and pituitary gland react as though the estrogen level is very low. This reverses the suppression of endogenous gonadotropins by estrogen, and gonadotropin levels rise, stimulating ovarian follicular development.
The down-regulation of estrogen receptors with clomiphene administration is not limited to the hypothalamus and pituitary gland, but also occurs peripherally at the endometrium and cervix, where it is not so desirable. When the cervix is affected, it becomes an unfavorable environment for sperm to penetrate, and when the endometrium is affected, its hypoestrogenic status may reduce the likelihood of embryo implantation—or may increase the risk of pregnancy loss if implantation occurs.
These peripheral antiestrogenic prop erties of clomiphene citrate may account for the discrepancy between high ovulation and low pregnancy rates.22-28 Several strategies to overcome this problem—e.g., adding estrogen, starting clomiphene citrate earlier in the menstrual period, or using another SERM, such as tamoxifen—have been largely unsuccessful. With clomiphene citrate, depletion of estrogen receptors has long-term effects because of the drug’s relatively long half-life (several days).31
In contrast, AIs do not appear to affect the expression of estrogen receptors in different body tissues, such as the endometrium and cervix. AIs have a shorter half-life (8 hours to 2 days), and nonsteroidal third-generation agents have a reversible inhibitory effect on the aromatase enzyme. Moreover, the rise in endogenous gonadotropins stimulates the production of more aromatase enzyme. This newly formed aromatase enzyme, and the return of a normal aromatase level after a short half-life of AI, leads the maturing ovarian follicles to secrete estrogen, which reaches a physiologic level soon after the last administration of AI. The rising estrogen level allows development of a more hospitable uterine environment (endometrium and cervical mucus).22-28
Early evidence confirms efficacy of AIs
After our pioneering reports of successful ovulation induction3-9 and improved ovarian response to stimulation by gonadotropins5-7 using AIs in small, nonrandomized, controlled trials, several larger and better designed clinical trials followed and supported our findings.10-19
Clinical trials comparing AIs with clomiphene citrate have consistently reported a universal “trend” toward superiority of AIs in achieving pregnancy despite comparable levels of success in achieving ovulation.10,11,14,16-19 However, these published clinical trials lacked adequate sample size to definitively confirm the superiority of AIs in achieving clinical pregnancy. We believe AIs are superior because, in our experience, they have helped women achieve pregnancy even after failure of several cycles of clomiphene treatment.4,15
Should an AI follow a trial of clomiphene?
U.Y., the patient described at the opening of this article, has two main options now that she has completed six cycles of clomiphene citrate without conceiving. The usual strategy would be a shift to more sophisticated treatment using gonadotropin injection. However, exogenous gonadotropins have several disadvantages:
- the drugs must be injected (orally inactive)
- they are more expensive than clomiphene citrate and AIs
- they require close monitoring by an infertility specialist with expensive and sophisticated technology
- they carry a risk of severe ovarian hyperstimulation, which is unlikely with clomiphene citrate and unreported with AIs
- multiple pregnancy is likely, particularly in conjunction with intrauterine insemination
- the risk of ovarian hyperstimulation with gonadotropin injection is much higher in women with PCOS, such as U.Y., as is the likelihood of multiple pregnancy.
The reason U.Y. has not conceived after six cycles of clomiphene citrate is likely related to the drug’s antiestrogenic effects on the endometrium, which appeared to be very thin (4–6 mm) on US imaging around the day of ovulation. If she fails to conceive with AIs, she will probably not become pregnant after a switch to gonadotropin injection unless more advanced treatment is included, such as in vitro fertilization (IVF) and embryo transfer. Other causes of her infertility—besides ovulatory dysfunction—may explain the failure to conceive.
Comparable pregnancy rates have been observed for AIs and gonadotropin injection, although further study is needed—specifically, clinical trials comparing gonadotropin and AIs in conjunction with timed intercourse or intrauterine insemination, or both.
CASE 2 No response to clomiphene citrate
G.A., 28 years old, has been trying to conceive for 3 years. She reports having irregular menstrual periods indicative of anovulation, and body temperature charts and progesterone levels support that diagnosis. She undergoes three cycles of clomiphene citrate at dosages ranging from 50 to 150 mg/day for 5 days starting on day 3 of the menstrual cycle. Despite treatment, she fails to ovulate.
Would an AI increase her chance of ovulating and conceiving?
Failure to ovulate after treatment with clomiphene citrate may have any of several causes, including inappropriate patient selection and resistance to the drug.
An example of inappropriate patient selection would be a woman with hypothalamic/hypopituitary anovulation; this type of patient often has insufficient levels of endogenous gonadotropins (luteinizing hormone and FSH). Another example would be a woman with reduced ovarian reserve; this type of patient is often unresponsive to clomiphene citrate and may have substantially elevated gonadotropin levels, most notably high FSH on day 3 of the menstrual cycle.
AIs are unlikely to induce ovulation in either of these patients. For the first type of patient, exogenous gonadotropin injection would be appropriate, as would be a gonadotropin-releasing hormone (GnRH) pump. For a woman with reduced ovarian reserve, an oocyte donor and IVF are the best treatment option.
Success with an AI is unlikely when there is no appropriate indication for clomiphene citrate. For example, a woman with severe insulin resistance who fails to ovulate in response to clomiphene citrate is unlikely to ovulate in response to an AI. In that case, an insulin sensitizer—alone or in combination with clomiphene citrate or an AI—would be the appropriate option. Other measures to reduce insulin resistance, such as weight loss, exercise, and dietary modification, may also be helpful.
CASE 3 Ovulatory patient with endometriosis fails to conceive on clomiphene
R.C., 34 years old, has been trying to conceive for 2 years. Her basic infertility workup, which included a hysterosalpingogram and semen analysis, did not reveal any abnormalities. She has regular menstrual cycles suggestive of ovulation. In addition, luteal-phase progesterone levels and biphasic body temperature charts both indicate regular ovulation.
After six cycles of clomiphene citrate, her gynecologist performs diagnostic laparoscopy. Other than minimal, stage 1 endometriosis, confirmed by pathologic examination of peritoneal biopsies, there are no remarkable findings. Methylene blue tubal perfusion confirms patent fallopian tubes during the operation. The gynecologist fulgurates the minimal endometriotic implants using carbon dioxide laser. Two months after the procedure, the patient undergoes three more cycles of clomiphene citrate, without success.
Would an AI help her conceive?
Most of the data on successful treatment with clomiphene citrate come from anovulatory women with PCOS in whom anovulation is the main cause of infertility. Evidence is weaker when the patient is ovulatory and has unexplained or endometriosis-associated infertility.32
A recent nonrandomized, controlled study that included women with a medical history comparable to R.C.’s found treatment with clomiphene citrate to significantly reduce the chance of pregnancy, compared with timed intercourse without clomiphene or other forms of ovarian stimulation, following conservative laparoscopic surgery for their endometriosis.33 We believe that clomiphene citrate is in-appropriate in women with endometriosis-related infertility—and may activate underlying endometriotic lesions.
For R.C., treatment with an AI is a viable option, particularly in light of recent data showing that the aromatase enzyme is expressed in endometriotic lesions.34 An AI could also enhance conception by further suppressing endometriosis through its effects on circulating estrogen levels and local estrogen production. This is an unproven extrapolation that seems scientifically appropriate to us, but needs confirmation by randomized clinical trials.
CASE 4 Woman with unexplained—and uninvestigated—infertility
E.D., 31 years old, has been trying to conceive for 1 year. Neither she nor her husband has undergone any study of their infertility problem.
Would empiric treatment with an AI be appropriate?
No treatment should begin until the patient and her partner have undergone the basic workup (TABLE 3). If a specific cause of infertility is determined, the patient should be treated accordingly. If no explanation for the infertility can be found, or anovulation is the likely cause, empirical ovarian stimulation with timed intercourse or intrauterine insemination is reasonable, provided:
- semen analysis is within normal limits
- ovarian function is present—i.e., the patient is expected to ovulate in response to ovarian stimulation
- at least one tube is patent and functional
- uterus has no serious abnormalities.
If ovarian stimulation fails to trigger ovulation or pregnancy, consider the options listed in TABLE 4
TABLE 3
Basic infertility workup
|
|
|
|
|
TABLE 4
When ovarian stimulation fails, next step depends on several variables
| LEVEL OF FAILURE | CLOMIPHENE CITRATE | AROMATASE INHIBITORS |
|---|---|---|
| 1–No ovulation | Is indication appropriate? Neither clomiphene citrate nor AIs are appropriate for hypothalamic/hypopituitary anovulation or ovarian failure Is severe insulin resistance present? If so, consider insulin sensitizers and encourage exercise, dietary changes, and weight loss | |
| Other options: Change to AI or retry clomiphene citrate in conjunction with an insulin sensitizer. If treatment fails after 3 to 6 additional cycles, consider an injectable gonadotropin | Other options: Try adding an insulin sensitizer. If treatment fails after 3 to 6 additional cycles, consider an injectable gonadotropin | |
| 2–Ovulation but no pregnancy | Was another cause of infertility (besides ovulatory dysfunction) overlooked? Investigate further, if necessary Options: Consider AIs before injectable gonadotropins, especially when there is evidence, with clomiphene citrate, of a persistent antiestrogenic effect, such as thin endometrium around the time of ovulation; endometriosis; or unexplained infertility. Move to gonadotropins if AIs fail | |
Minimal adverse effects
AIs are generally well tolerated. The most common adverse effects are hot flushes, GI disturbances (nausea and vomiting), and leg cramps. In clinical trials involving postmenopausal women with breast cancer who were taking an AI, very few withdrew because of drug-related adverse effects.35 Those women took an AI on a daily basis over several months. Fewer adverse effects would be expected among usually healthy younger women administered a short course (a few days) for ovarian stimulation. In addition, our clinical experience has been that fewer women experience side effects such as mild hot flushes and symptoms similar to premenstrual syndrome when taking an AI, compared with clomiphene citrate.3-9
When any medication is given during pregnancy, there are concerns about its effects. Drugs used to induce ovulation are no exception. In fact, clomiphene citrate is classified as pregnancy category X—a fact frequently overlooked by treating physicians. As for AIs, recent studies found no evidence of teratogenicity or clastogenicity in animal embryos when anastrozole was given. The picture is murkier for letrozole.
When used for ovarian stimulation, the short half-life of AIs and administration in the early follicular phase (several days before ovulation and fertilization occur) should ensure clearance of the drugs before implantation. Nevertheless, it is important to confirm that the patient is not pregnant before an AI is given. We recommend a pregnancy test before administering an AI for ovulation induction.
Mixed bag of data on pregnancy outcomes
Three large studies recently reported on pregnancy outcomes after infertility treatment with AIs.9,36,37 The first was a cohort study comparing outcomes of 394 pregnancies achieved after treatment with letrozole (133 pregnancies) and other ovarian-stimulation agents, including clomiphene citrate (113 pregnancies) and gonadotropins (110 pregnancies), with a control group of 38 pregnancies achieved without ovarian stimulation.9 The study encompassed three tertiary referral centers over 2 years. Pregnancies conceived after treatment with an AI had rates of miscarriage and ectopic pregnancy comparable to all other groups. In addition, letrozole was associated with a significantly lower rate of multiple gestation than was clomiphene citrate.9
The second study, presented in abstract form, compared the outcome of 150 births after treatment with letrozole to a database of 36,050 normal deliveries.36 Although the authors themselves stated that there was no statistically significant difference in the overall incidence of congenital malformation, they reported a higher incidence of locomotor malformation and cardiac anomaly in the infants conceived after treatment with letrozole.36 They did not address this discrepancy or explain how locomotor malformation was assessed.
A closer look at the abstract reveals major methodological flaws that weaken the data and conclusions presented:
- The study was not well controlled. The treated patients (n=130) were infertile women, mainly suffering from PCOS and unexplained infertility, who had a mean age of 35.2 years. The control group included a database of spontaneously conceiving women who were significantly younger (mean age: 30.5 years). The control group also included deliveries in a low-risk hospital that refers out high-risk pregnancies to secondary and tertiary hospitals. These are important distinctions because women of advanced maternal age have an increased incidence of medical illnesses, making their pregnancies higher in risk.
- The incidence of multiple gestation was significantly higher among women treated for infertility than among women in the control group. It is well known that multiple gestations are at increased risk of fetal malformation compared with singleton pregnancies.
- The incidence of cardiac anomaly among women treated with letrozole did not differ significantly from the known incidence of cardiac malformation in the general population, but the authors concluded that the rate of cardiac malformation was significantly higher in the letrozole group than among controls. This is misleading because it was the control group that developed cardiac malformation at a significantly lower rate than in the general population. Such a low incidence of cardiac anomaly in a low-risk hospital setting is not surprising, because mothers would be transferred to a tertiary-care center once an anomaly was detected.
- Data on congenital malformation in the control group were collected from delivery records available in the maternity ward of the hospital. However, a significant percentage of congenital malformations, such as cardiac anomaly, are not detected until after the neonatal period.36
When using clomiphene citrate or an aromatase inhibitor (AI):
- avoid a dosage that exceeds 100 to 150 mg/day for clomiphene citrate or 2.5 to 5 mg/day for AIs or a treatment period longer than 5 days each cycle
- do not administer an AI beyond day 7 of the menstrual cycle
- stop after three to six cycles of treatment
- do not increase the dosage once ovulation occurs
- discontinue treatment when serious adverse effects are present, such as visual side effects.
It is also interesting that the results of this abstract have not been published in a peer-reviewed journal more than a year after its presentation.
The third study, which is more recent, compared the incidence of congenital malformation in 911 newborns conceived after treatment with letrozole (n=514) or clomiphene citrate (n=397).37 It found no statistically significant difference between the groups. Congenital malformation was diagnosed in 2.4% and 4.8% of the letrozole- and clomiphene-treated groups, respectively, and major malformation occurred in 1.2% and 3% of the letrozole- and clomiphene-treated groups, respectively. These differences were not statistically significant, but there was a sevenfold increase in overall cardiac anomalies in the clomiphene-treated group, compared with the letrozole-treated group—and this difference was statistically significant. These findings warrant further investigation into the use of clomiphene citrate for induction of ovulation.
1. Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Effect of diagnosis, age, sperm quality, and number of preovulatory follicles on the outcome of multiple cycles of clomiphene citrate-intrauterine insemination. Fertil Steril. 2002;78:1088-1095.
2. Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligomenorrheic infertility. J Clin Endocrinol Metab. 1999;84:1617-1622.
3. Mitwally MFM, Casper RF. Aromatase inhibition: a novel method of ovulation induction in women with polycystic ovarian syndrome. Reprod Technol. 2000;10:244-247.
4. Mitwally MFM, Casper RF. Use of an AI for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305-309.
5. Mitwally MFM, Casper RF. Aromatase inhibition improves ovarian response to follicle-stimulating hormone in poor responders. Fertil Steril. 2002;77:776-780.
6. Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotropin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;188:1588-1597.
7. Mitwally MF, Casper RF. Aromatase inhibition reduces the dose of gonadotropin required for controlled ovarian hyperstimulation. J Soc Gynecol Investig. 2004;11:406-415.
8. Mitwally MFM, Casper RF. Single dose administration of the aromatase inhibitor, letrozole: a simple and convenient effective method of ovulation induction. Fertil Steril. 2005;83:229-231.
9. Mitwally MFM, Casper RF. Pregnancy outcome after the use of an AI for induction of ovulation. Am J Obstet Gynecol. 2005;192:381-386.
10. Fatemi HM, Kolibianakis E, Tournaye H, et al. Clomiphene citrate versus letrozole for ovarian stimulation: a pilot study. Reprod Biomed Online. 2003;75:543-546.
11. Al-Fozan H, Al-Khadouri M, Tan SL, Tulandi T. A randomized trial of letrozole versus clomiphene citrate in women undergoing superovulation. Fertil Steril. 2004;82:1561-1563.
12. Goswami SK, Das T, Chattopadhyay R, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19:2031-2035.
13. Garcia-Velasco JA, Moreno L, Pacheco A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82-87.
14. Bayar U, Tanrierdi HA, Barut A, et al. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85:1045-1048.
15. Elnashar A, Fouad H, Eldosoky M, et al. Letrozole induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome may not depend on the period of infertility, the body mass index, or the luteinizing hormone/follicle stimulating hormone ratio. Fertil Steril. 2006;85:161-164.
16. Atay V, Cam C, Muhcu M, et al. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34:73-76.
17. Sohrabvand F, Ansari S, Bagheri M. Efficacy of combined metformin-letrozole in comparison with metformin-clomiphene citrate in clomiphene-resistant infertile women with polycystic ovarian disease. Hum Reprod. 2006;21:1432-1435.
18. Sipe CS, Davis WA, Maifeld M, Van Voorhis BJ. A prospective randomized trial comparing anastrozole and clomiphene citrate in an ovulation induction protocol using gonadotropins. Fertil Steril. 2006;86:1676-1681.
19. Bayar U, Basaran M, Kiran S, Coskun A, Gezer S. Use of an aromatase inhibitor in patients with polycystic ovary syndrome: a prospective randomized trial. Fertil Steril. 2006;86:1447-1451.
20. Buzdar A, Howell A. Advances in aromatase inhibition: clinical efficacy and tolerability in the treatment of breast cancer. Clin Cancer Res. 2001;7:2620-2635.
21. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology Technology Assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol. 2002;2015:3317-3327.
22. Mitwally MF, Casper RF. Potential of aromatase inhibitors for ovulation and superovulation induction in infertile women. Drugs. 2006;66:2149-2160.
23. Mitwally MFM, Casper RF. Letrozole for ovulation induction. Exp Rev Obstet Gynecol. 2006;1:15-27.
24. Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91:760-771.
25. Mitwally MF, Casper RF, Diamond MP. The role of aromatase inhibitors in ameliorating deleterious effects of ovarian stimulation on outcome of infertility treatment. Reprod Biol Endocrinol. 2005;3:54.-
26. Mitwally MF, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;22:61-78.
27. Mitwally MF, Casper RF. Aromatase inhibitors for the treatment of infertility. Expert Opin Investig Drugs. 2003;12:353-371.
28. Mitwally MF, Casper RF. Aromatase inhibition for ovarian stimulation: future avenues for infertility management. Curr Opin Obstet Gynecol. 2002;14:255-263.
29. Cole PA, Robinson CH. Mechanism and inhibition of cytochrome P-450 aromatase. J Med Chem. 1990;33:2933-2944.
30. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;848:2951-2956.
31. Mikkelson TJ, Kroboth PD, Cameron WJ. Single dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil Steril. 1986;46:392-396.
32. Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate for unexplained subfertility in women. Cochrane Database Syst Rev. 2000;(2):CD000057.-
33. Mitwally MF, Albuarki H, Ashraf M, Diamond MP, Abuzeid M. Clomiphene reduces chance of pregnancy in infertile women with endometriosis following laparoscopic surgery. J Soc Gynecol Investig. 2006;13(2) (suppl):abstract 646.-
34. Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49-56.
35. Goss PE. Risks versus benefits in the clinical application of aromatase inhibitors. Endocr Relat Cancer. 1999;6:325-332.
36. Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins [abstract no. 1033]. Fertil Steril. 2005;84 (suppl):abstract 1033.-
37. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85:1761-1765.
Dr. Mitwally holds patents licensed to Serono for use of aromatase inhibitors for infertility treatment.
Dr. Casper has a licensing agreement with Ares-Serono for use of aromatase inhibitors in assisted reproduction.
CASE 1 Ovulation begins, but pregnancy does not follow
U.Y. is a 32-year-old woman who has been trying to conceive for 3 years. Her infertility is caused by anovulation associated with polycystic ovary syndrome (PCOS). All other variables are within physiologic limits—she has patent tubes and an unremarkable uterus, and her partner has a normal semen analysis.
She has undergone six cycles of treatment with clomiphene citrate, with ovulation documented each time by ultrasonography (US) and measurement of luteal-phase progesterone levels. Her endometrial thickness is 4 to 6 mm around the day of ovulation.
Would an aromatase inhibitor increase her chances of conceiving?
This patient is an excellent candidate for ovulation induction using an aromatase inhibitor (AI).
The primary reason? She is unlikely to benefit from an increased dosage of clomiphene citrate because the dosage that triggers ovulation is believed to be most appropriate—an increase above that level is not expected to improve the chance of pregnancy. Moreover, conception is less likely after more than six cycles of clomiphene citrate.1,2
In this article, we describe the induction of ovulation using AIs—a relatively new, and off-label, application (TABLES 1 and 2). The strategies presented here are suitable for general ObGyns and do not require sophisticated technology such as rapid hormonal assays or transvaginal US.
Because this application is so new, with limited data published so far, much of the information presented here is based on our personal experience rather than level-1 evidence, which is sorely needed.
Of course, induction of ovulation is appropriate only after other specific causes of anovulation or ovulatory dysfunction are excluded, such as thyroid disorders, hyperprolactinemia, severe insulin resistance, and ovarian failure.
Concerns about teratogenicity of AIs appear to be largely unfounded (see below).
TABLE 1
Aromatase inhibitors work best in these applications
| APPLICATION | EVIDENCE |
|---|---|
Induction of ovulation, particularly in women with polycystic ovary syndrome:
See case 1 and case 2 |
|
| Ovarian stimulation (superovulation) in ovulatory women with unexplained or endometriosis-related infertility See case 3 | Strong evidence from several clinical trials |
| Use in conjunction with controlled ovarian hyperstimulation by gonadotropins with intrauterine insemination and assisted reproduction | Accumulating evidence of several advantages when used with gonadotropins:
|
TABLE 2
Avoid AIs in these situations
| SITUATION | JUSTIFICATION |
|---|---|
| When clomiphene citrate fails to induce ovulation in a woman with insulin resistance See case 2 | First try insulin sensitizers and other measures to improve insulin action (weight loss, exercise, and dietary modifications) |
| When other causes of infertility (besides ovulatory dysfunction) are likely | Pregnancy is unlikely |
| When the patient has hypothalamic/hypopituitary anovulation or ovarian failure | Ovarian stimulation is dependent on capacity to produce endogenous gonadotropins and presence of responding ovarian follicles |
Ovulation is good, but pregnancy is better
In women undergoing induction of ovulation, there are two levels of success: ovulation and pregnancy.
Clearly, the presence of other, nonovulatory infertility factors—e.g., male infertility and tubal-uterine problems—can prevent successful ovulation induction from translating into pregnancy.
We have reported3-9 on the successful use of AIs to stimulate the ovary and achieve pregnancy—even in women who fail to conceive after several treatment trials with clomiphene citrate.4
Other authors have conducted further investigations that have confirmed our findings and have recommended use of these agents for other aspects of infertility treatment, such as assisted reproduction.10-19
Latest generation of AIs is more benign
Many AIs have been developed over the past 30 years. The most recent are third-generation agents that were approved mainly to suppress estrogen production in postmenopausal women with breast cancer. Clinical failure of earlier generations of AIs for their approved indication was mainly due to significant adverse effects, lack of satisfactory potency, or lack of specificity in inhibiting the aromatase enzyme without inhibiting other enzymes of steroidogenesis.20
Third-generation AIs that are commercially available in North America, Europe, and other parts of the world include:
- two nonsteroidal preparations: anastrozole (Arimidex) and letrozole (Femara)
- one steroidal agent: exemestane (Aromasin).
Letrozole and anastrozole are reversible, competitive agents with considerably greater potency (more than 1,000 times greater) than the first-generation AI aminoglutethimide. At a dosage of 1 to 5 mg/day, they reduce estrogen levels by 97% to more than 99%.
AIs are completely absorbed after oral administration, with a mean terminal half-life of approximately 45 hours (range: 30–60 hours). Exemestane has a shorter circulating half-life of approximately 9 hours, but may have a longer effect because it is irreversible.21
Mild gastrointestinal (GI) disturbances account for most of the adverse events, and rarely limit therapy.
How AIs work
Although we continue to accrue data on the use of AIs to induce ovulation, the underlying mechanism of action has not been studied. However, we believe that AIs work both centrally (at the level of the hypothalamus and pituitary) and peripherally (at the level of the ovaries).22-28
At the central level, AIs suppress estrogen production by directly, specifically, and potently inhibiting the aromatase enzyme (i.e., estrogen synthase, the enzyme responsible for the synthesis of estrogen). Because the aromatase enzyme is expressed in various tissues and organs—most notably, the ovaries, brain, and fat29—AIs suppress estrogen production in all of those tissues, leading to a low serum estrogen level and low local estrogen level. Low estrogen levels are thought to release the hypothalamus and pituitary gland from their negative-feedback mechanism, thereby increasing production of endogenous gonadotropins from the pituitary gland and stimulating ovarian follicular development and ovulation (FIGURE).
At the peripheral level, the aromatase enzyme catalyzes the terminal step in the steroidogenesis cascade that converts androgens into estrogen. When that enzyme is inhibited, enzyme substrate (androgens) is thought to accumulate. Contrary to the general belief that androgens are deleterious to ovarian follicles, studies in primates have demonstrated that androgens actually up-regulate the expression of gonadotropin receptors, particularly follicle-stimulating hormone (FSH) receptors.30 This renders the ovaries more sensitive to gonadotropin stimulation—whether the gonadotropins are endogenous or exogenous.22-28
FIGURE Aromatase inhibitors promote follicle development, then fade from the scene in time to prevent hyperovulation
Administration of an aromatase inhibitor (AI) on cycle days 3 to 7 suppresses ovarian estradiol (E2) secretion, as shown in A, which reduces estrogen-negative feedback at the hypothalamus and pituitary. As a result, follicle-stimulating hormone (FSH) secretion increases, fostering growth of multiple ovarian follicles. The growing follicles, shown in B, cause estrogen levels to rise again, depressing FSH, and leading to monofollicular ovulation in most cases.
Why AIs are superior to clomiphene
Clomiphene citrate is a selective estrogen receptor modulator (SERM) that is believed to induce ovulation through its antiestrogenic properties at the level of the hypothalamus or pituitary gland, or both. Clomiphene down-regulates estrogen receptors at this level, and the hypothalamus and pituitary gland react as though the estrogen level is very low. This reverses the suppression of endogenous gonadotropins by estrogen, and gonadotropin levels rise, stimulating ovarian follicular development.
The down-regulation of estrogen receptors with clomiphene administration is not limited to the hypothalamus and pituitary gland, but also occurs peripherally at the endometrium and cervix, where it is not so desirable. When the cervix is affected, it becomes an unfavorable environment for sperm to penetrate, and when the endometrium is affected, its hypoestrogenic status may reduce the likelihood of embryo implantation—or may increase the risk of pregnancy loss if implantation occurs.
These peripheral antiestrogenic prop erties of clomiphene citrate may account for the discrepancy between high ovulation and low pregnancy rates.22-28 Several strategies to overcome this problem—e.g., adding estrogen, starting clomiphene citrate earlier in the menstrual period, or using another SERM, such as tamoxifen—have been largely unsuccessful. With clomiphene citrate, depletion of estrogen receptors has long-term effects because of the drug’s relatively long half-life (several days).31
In contrast, AIs do not appear to affect the expression of estrogen receptors in different body tissues, such as the endometrium and cervix. AIs have a shorter half-life (8 hours to 2 days), and nonsteroidal third-generation agents have a reversible inhibitory effect on the aromatase enzyme. Moreover, the rise in endogenous gonadotropins stimulates the production of more aromatase enzyme. This newly formed aromatase enzyme, and the return of a normal aromatase level after a short half-life of AI, leads the maturing ovarian follicles to secrete estrogen, which reaches a physiologic level soon after the last administration of AI. The rising estrogen level allows development of a more hospitable uterine environment (endometrium and cervical mucus).22-28
Early evidence confirms efficacy of AIs
After our pioneering reports of successful ovulation induction3-9 and improved ovarian response to stimulation by gonadotropins5-7 using AIs in small, nonrandomized, controlled trials, several larger and better designed clinical trials followed and supported our findings.10-19
Clinical trials comparing AIs with clomiphene citrate have consistently reported a universal “trend” toward superiority of AIs in achieving pregnancy despite comparable levels of success in achieving ovulation.10,11,14,16-19 However, these published clinical trials lacked adequate sample size to definitively confirm the superiority of AIs in achieving clinical pregnancy. We believe AIs are superior because, in our experience, they have helped women achieve pregnancy even after failure of several cycles of clomiphene treatment.4,15
Should an AI follow a trial of clomiphene?
U.Y., the patient described at the opening of this article, has two main options now that she has completed six cycles of clomiphene citrate without conceiving. The usual strategy would be a shift to more sophisticated treatment using gonadotropin injection. However, exogenous gonadotropins have several disadvantages:
- the drugs must be injected (orally inactive)
- they are more expensive than clomiphene citrate and AIs
- they require close monitoring by an infertility specialist with expensive and sophisticated technology
- they carry a risk of severe ovarian hyperstimulation, which is unlikely with clomiphene citrate and unreported with AIs
- multiple pregnancy is likely, particularly in conjunction with intrauterine insemination
- the risk of ovarian hyperstimulation with gonadotropin injection is much higher in women with PCOS, such as U.Y., as is the likelihood of multiple pregnancy.
The reason U.Y. has not conceived after six cycles of clomiphene citrate is likely related to the drug’s antiestrogenic effects on the endometrium, which appeared to be very thin (4–6 mm) on US imaging around the day of ovulation. If she fails to conceive with AIs, she will probably not become pregnant after a switch to gonadotropin injection unless more advanced treatment is included, such as in vitro fertilization (IVF) and embryo transfer. Other causes of her infertility—besides ovulatory dysfunction—may explain the failure to conceive.
Comparable pregnancy rates have been observed for AIs and gonadotropin injection, although further study is needed—specifically, clinical trials comparing gonadotropin and AIs in conjunction with timed intercourse or intrauterine insemination, or both.
CASE 2 No response to clomiphene citrate
G.A., 28 years old, has been trying to conceive for 3 years. She reports having irregular menstrual periods indicative of anovulation, and body temperature charts and progesterone levels support that diagnosis. She undergoes three cycles of clomiphene citrate at dosages ranging from 50 to 150 mg/day for 5 days starting on day 3 of the menstrual cycle. Despite treatment, she fails to ovulate.
Would an AI increase her chance of ovulating and conceiving?
Failure to ovulate after treatment with clomiphene citrate may have any of several causes, including inappropriate patient selection and resistance to the drug.
An example of inappropriate patient selection would be a woman with hypothalamic/hypopituitary anovulation; this type of patient often has insufficient levels of endogenous gonadotropins (luteinizing hormone and FSH). Another example would be a woman with reduced ovarian reserve; this type of patient is often unresponsive to clomiphene citrate and may have substantially elevated gonadotropin levels, most notably high FSH on day 3 of the menstrual cycle.
AIs are unlikely to induce ovulation in either of these patients. For the first type of patient, exogenous gonadotropin injection would be appropriate, as would be a gonadotropin-releasing hormone (GnRH) pump. For a woman with reduced ovarian reserve, an oocyte donor and IVF are the best treatment option.
Success with an AI is unlikely when there is no appropriate indication for clomiphene citrate. For example, a woman with severe insulin resistance who fails to ovulate in response to clomiphene citrate is unlikely to ovulate in response to an AI. In that case, an insulin sensitizer—alone or in combination with clomiphene citrate or an AI—would be the appropriate option. Other measures to reduce insulin resistance, such as weight loss, exercise, and dietary modification, may also be helpful.
CASE 3 Ovulatory patient with endometriosis fails to conceive on clomiphene
R.C., 34 years old, has been trying to conceive for 2 years. Her basic infertility workup, which included a hysterosalpingogram and semen analysis, did not reveal any abnormalities. She has regular menstrual cycles suggestive of ovulation. In addition, luteal-phase progesterone levels and biphasic body temperature charts both indicate regular ovulation.
After six cycles of clomiphene citrate, her gynecologist performs diagnostic laparoscopy. Other than minimal, stage 1 endometriosis, confirmed by pathologic examination of peritoneal biopsies, there are no remarkable findings. Methylene blue tubal perfusion confirms patent fallopian tubes during the operation. The gynecologist fulgurates the minimal endometriotic implants using carbon dioxide laser. Two months after the procedure, the patient undergoes three more cycles of clomiphene citrate, without success.
Would an AI help her conceive?
Most of the data on successful treatment with clomiphene citrate come from anovulatory women with PCOS in whom anovulation is the main cause of infertility. Evidence is weaker when the patient is ovulatory and has unexplained or endometriosis-associated infertility.32
A recent nonrandomized, controlled study that included women with a medical history comparable to R.C.’s found treatment with clomiphene citrate to significantly reduce the chance of pregnancy, compared with timed intercourse without clomiphene or other forms of ovarian stimulation, following conservative laparoscopic surgery for their endometriosis.33 We believe that clomiphene citrate is in-appropriate in women with endometriosis-related infertility—and may activate underlying endometriotic lesions.
For R.C., treatment with an AI is a viable option, particularly in light of recent data showing that the aromatase enzyme is expressed in endometriotic lesions.34 An AI could also enhance conception by further suppressing endometriosis through its effects on circulating estrogen levels and local estrogen production. This is an unproven extrapolation that seems scientifically appropriate to us, but needs confirmation by randomized clinical trials.
CASE 4 Woman with unexplained—and uninvestigated—infertility
E.D., 31 years old, has been trying to conceive for 1 year. Neither she nor her husband has undergone any study of their infertility problem.
Would empiric treatment with an AI be appropriate?
No treatment should begin until the patient and her partner have undergone the basic workup (TABLE 3). If a specific cause of infertility is determined, the patient should be treated accordingly. If no explanation for the infertility can be found, or anovulation is the likely cause, empirical ovarian stimulation with timed intercourse or intrauterine insemination is reasonable, provided:
- semen analysis is within normal limits
- ovarian function is present—i.e., the patient is expected to ovulate in response to ovarian stimulation
- at least one tube is patent and functional
- uterus has no serious abnormalities.
If ovarian stimulation fails to trigger ovulation or pregnancy, consider the options listed in TABLE 4
TABLE 3
Basic infertility workup
|
|
|
|
|
TABLE 4
When ovarian stimulation fails, next step depends on several variables
| LEVEL OF FAILURE | CLOMIPHENE CITRATE | AROMATASE INHIBITORS |
|---|---|---|
| 1–No ovulation | Is indication appropriate? Neither clomiphene citrate nor AIs are appropriate for hypothalamic/hypopituitary anovulation or ovarian failure Is severe insulin resistance present? If so, consider insulin sensitizers and encourage exercise, dietary changes, and weight loss | |
| Other options: Change to AI or retry clomiphene citrate in conjunction with an insulin sensitizer. If treatment fails after 3 to 6 additional cycles, consider an injectable gonadotropin | Other options: Try adding an insulin sensitizer. If treatment fails after 3 to 6 additional cycles, consider an injectable gonadotropin | |
| 2–Ovulation but no pregnancy | Was another cause of infertility (besides ovulatory dysfunction) overlooked? Investigate further, if necessary Options: Consider AIs before injectable gonadotropins, especially when there is evidence, with clomiphene citrate, of a persistent antiestrogenic effect, such as thin endometrium around the time of ovulation; endometriosis; or unexplained infertility. Move to gonadotropins if AIs fail | |
Minimal adverse effects
AIs are generally well tolerated. The most common adverse effects are hot flushes, GI disturbances (nausea and vomiting), and leg cramps. In clinical trials involving postmenopausal women with breast cancer who were taking an AI, very few withdrew because of drug-related adverse effects.35 Those women took an AI on a daily basis over several months. Fewer adverse effects would be expected among usually healthy younger women administered a short course (a few days) for ovarian stimulation. In addition, our clinical experience has been that fewer women experience side effects such as mild hot flushes and symptoms similar to premenstrual syndrome when taking an AI, compared with clomiphene citrate.3-9
When any medication is given during pregnancy, there are concerns about its effects. Drugs used to induce ovulation are no exception. In fact, clomiphene citrate is classified as pregnancy category X—a fact frequently overlooked by treating physicians. As for AIs, recent studies found no evidence of teratogenicity or clastogenicity in animal embryos when anastrozole was given. The picture is murkier for letrozole.
When used for ovarian stimulation, the short half-life of AIs and administration in the early follicular phase (several days before ovulation and fertilization occur) should ensure clearance of the drugs before implantation. Nevertheless, it is important to confirm that the patient is not pregnant before an AI is given. We recommend a pregnancy test before administering an AI for ovulation induction.
Mixed bag of data on pregnancy outcomes
Three large studies recently reported on pregnancy outcomes after infertility treatment with AIs.9,36,37 The first was a cohort study comparing outcomes of 394 pregnancies achieved after treatment with letrozole (133 pregnancies) and other ovarian-stimulation agents, including clomiphene citrate (113 pregnancies) and gonadotropins (110 pregnancies), with a control group of 38 pregnancies achieved without ovarian stimulation.9 The study encompassed three tertiary referral centers over 2 years. Pregnancies conceived after treatment with an AI had rates of miscarriage and ectopic pregnancy comparable to all other groups. In addition, letrozole was associated with a significantly lower rate of multiple gestation than was clomiphene citrate.9
The second study, presented in abstract form, compared the outcome of 150 births after treatment with letrozole to a database of 36,050 normal deliveries.36 Although the authors themselves stated that there was no statistically significant difference in the overall incidence of congenital malformation, they reported a higher incidence of locomotor malformation and cardiac anomaly in the infants conceived after treatment with letrozole.36 They did not address this discrepancy or explain how locomotor malformation was assessed.
A closer look at the abstract reveals major methodological flaws that weaken the data and conclusions presented:
- The study was not well controlled. The treated patients (n=130) were infertile women, mainly suffering from PCOS and unexplained infertility, who had a mean age of 35.2 years. The control group included a database of spontaneously conceiving women who were significantly younger (mean age: 30.5 years). The control group also included deliveries in a low-risk hospital that refers out high-risk pregnancies to secondary and tertiary hospitals. These are important distinctions because women of advanced maternal age have an increased incidence of medical illnesses, making their pregnancies higher in risk.
- The incidence of multiple gestation was significantly higher among women treated for infertility than among women in the control group. It is well known that multiple gestations are at increased risk of fetal malformation compared with singleton pregnancies.
- The incidence of cardiac anomaly among women treated with letrozole did not differ significantly from the known incidence of cardiac malformation in the general population, but the authors concluded that the rate of cardiac malformation was significantly higher in the letrozole group than among controls. This is misleading because it was the control group that developed cardiac malformation at a significantly lower rate than in the general population. Such a low incidence of cardiac anomaly in a low-risk hospital setting is not surprising, because mothers would be transferred to a tertiary-care center once an anomaly was detected.
- Data on congenital malformation in the control group were collected from delivery records available in the maternity ward of the hospital. However, a significant percentage of congenital malformations, such as cardiac anomaly, are not detected until after the neonatal period.36
When using clomiphene citrate or an aromatase inhibitor (AI):
- avoid a dosage that exceeds 100 to 150 mg/day for clomiphene citrate or 2.5 to 5 mg/day for AIs or a treatment period longer than 5 days each cycle
- do not administer an AI beyond day 7 of the menstrual cycle
- stop after three to six cycles of treatment
- do not increase the dosage once ovulation occurs
- discontinue treatment when serious adverse effects are present, such as visual side effects.
It is also interesting that the results of this abstract have not been published in a peer-reviewed journal more than a year after its presentation.
The third study, which is more recent, compared the incidence of congenital malformation in 911 newborns conceived after treatment with letrozole (n=514) or clomiphene citrate (n=397).37 It found no statistically significant difference between the groups. Congenital malformation was diagnosed in 2.4% and 4.8% of the letrozole- and clomiphene-treated groups, respectively, and major malformation occurred in 1.2% and 3% of the letrozole- and clomiphene-treated groups, respectively. These differences were not statistically significant, but there was a sevenfold increase in overall cardiac anomalies in the clomiphene-treated group, compared with the letrozole-treated group—and this difference was statistically significant. These findings warrant further investigation into the use of clomiphene citrate for induction of ovulation.
Dr. Mitwally holds patents licensed to Serono for use of aromatase inhibitors for infertility treatment.
Dr. Casper has a licensing agreement with Ares-Serono for use of aromatase inhibitors in assisted reproduction.
CASE 1 Ovulation begins, but pregnancy does not follow
U.Y. is a 32-year-old woman who has been trying to conceive for 3 years. Her infertility is caused by anovulation associated with polycystic ovary syndrome (PCOS). All other variables are within physiologic limits—she has patent tubes and an unremarkable uterus, and her partner has a normal semen analysis.
She has undergone six cycles of treatment with clomiphene citrate, with ovulation documented each time by ultrasonography (US) and measurement of luteal-phase progesterone levels. Her endometrial thickness is 4 to 6 mm around the day of ovulation.
Would an aromatase inhibitor increase her chances of conceiving?
This patient is an excellent candidate for ovulation induction using an aromatase inhibitor (AI).
The primary reason? She is unlikely to benefit from an increased dosage of clomiphene citrate because the dosage that triggers ovulation is believed to be most appropriate—an increase above that level is not expected to improve the chance of pregnancy. Moreover, conception is less likely after more than six cycles of clomiphene citrate.1,2
In this article, we describe the induction of ovulation using AIs—a relatively new, and off-label, application (TABLES 1 and 2). The strategies presented here are suitable for general ObGyns and do not require sophisticated technology such as rapid hormonal assays or transvaginal US.
Because this application is so new, with limited data published so far, much of the information presented here is based on our personal experience rather than level-1 evidence, which is sorely needed.
Of course, induction of ovulation is appropriate only after other specific causes of anovulation or ovulatory dysfunction are excluded, such as thyroid disorders, hyperprolactinemia, severe insulin resistance, and ovarian failure.
Concerns about teratogenicity of AIs appear to be largely unfounded (see below).
TABLE 1
Aromatase inhibitors work best in these applications
| APPLICATION | EVIDENCE |
|---|---|
Induction of ovulation, particularly in women with polycystic ovary syndrome:
See case 1 and case 2 |
|
| Ovarian stimulation (superovulation) in ovulatory women with unexplained or endometriosis-related infertility See case 3 | Strong evidence from several clinical trials |
| Use in conjunction with controlled ovarian hyperstimulation by gonadotropins with intrauterine insemination and assisted reproduction | Accumulating evidence of several advantages when used with gonadotropins:
|
TABLE 2
Avoid AIs in these situations
| SITUATION | JUSTIFICATION |
|---|---|
| When clomiphene citrate fails to induce ovulation in a woman with insulin resistance See case 2 | First try insulin sensitizers and other measures to improve insulin action (weight loss, exercise, and dietary modifications) |
| When other causes of infertility (besides ovulatory dysfunction) are likely | Pregnancy is unlikely |
| When the patient has hypothalamic/hypopituitary anovulation or ovarian failure | Ovarian stimulation is dependent on capacity to produce endogenous gonadotropins and presence of responding ovarian follicles |
Ovulation is good, but pregnancy is better
In women undergoing induction of ovulation, there are two levels of success: ovulation and pregnancy.
Clearly, the presence of other, nonovulatory infertility factors—e.g., male infertility and tubal-uterine problems—can prevent successful ovulation induction from translating into pregnancy.
We have reported3-9 on the successful use of AIs to stimulate the ovary and achieve pregnancy—even in women who fail to conceive after several treatment trials with clomiphene citrate.4
Other authors have conducted further investigations that have confirmed our findings and have recommended use of these agents for other aspects of infertility treatment, such as assisted reproduction.10-19
Latest generation of AIs is more benign
Many AIs have been developed over the past 30 years. The most recent are third-generation agents that were approved mainly to suppress estrogen production in postmenopausal women with breast cancer. Clinical failure of earlier generations of AIs for their approved indication was mainly due to significant adverse effects, lack of satisfactory potency, or lack of specificity in inhibiting the aromatase enzyme without inhibiting other enzymes of steroidogenesis.20
Third-generation AIs that are commercially available in North America, Europe, and other parts of the world include:
- two nonsteroidal preparations: anastrozole (Arimidex) and letrozole (Femara)
- one steroidal agent: exemestane (Aromasin).
Letrozole and anastrozole are reversible, competitive agents with considerably greater potency (more than 1,000 times greater) than the first-generation AI aminoglutethimide. At a dosage of 1 to 5 mg/day, they reduce estrogen levels by 97% to more than 99%.
AIs are completely absorbed after oral administration, with a mean terminal half-life of approximately 45 hours (range: 30–60 hours). Exemestane has a shorter circulating half-life of approximately 9 hours, but may have a longer effect because it is irreversible.21
Mild gastrointestinal (GI) disturbances account for most of the adverse events, and rarely limit therapy.
How AIs work
Although we continue to accrue data on the use of AIs to induce ovulation, the underlying mechanism of action has not been studied. However, we believe that AIs work both centrally (at the level of the hypothalamus and pituitary) and peripherally (at the level of the ovaries).22-28
At the central level, AIs suppress estrogen production by directly, specifically, and potently inhibiting the aromatase enzyme (i.e., estrogen synthase, the enzyme responsible for the synthesis of estrogen). Because the aromatase enzyme is expressed in various tissues and organs—most notably, the ovaries, brain, and fat29—AIs suppress estrogen production in all of those tissues, leading to a low serum estrogen level and low local estrogen level. Low estrogen levels are thought to release the hypothalamus and pituitary gland from their negative-feedback mechanism, thereby increasing production of endogenous gonadotropins from the pituitary gland and stimulating ovarian follicular development and ovulation (FIGURE).
At the peripheral level, the aromatase enzyme catalyzes the terminal step in the steroidogenesis cascade that converts androgens into estrogen. When that enzyme is inhibited, enzyme substrate (androgens) is thought to accumulate. Contrary to the general belief that androgens are deleterious to ovarian follicles, studies in primates have demonstrated that androgens actually up-regulate the expression of gonadotropin receptors, particularly follicle-stimulating hormone (FSH) receptors.30 This renders the ovaries more sensitive to gonadotropin stimulation—whether the gonadotropins are endogenous or exogenous.22-28
FIGURE Aromatase inhibitors promote follicle development, then fade from the scene in time to prevent hyperovulation
Administration of an aromatase inhibitor (AI) on cycle days 3 to 7 suppresses ovarian estradiol (E2) secretion, as shown in A, which reduces estrogen-negative feedback at the hypothalamus and pituitary. As a result, follicle-stimulating hormone (FSH) secretion increases, fostering growth of multiple ovarian follicles. The growing follicles, shown in B, cause estrogen levels to rise again, depressing FSH, and leading to monofollicular ovulation in most cases.
Why AIs are superior to clomiphene
Clomiphene citrate is a selective estrogen receptor modulator (SERM) that is believed to induce ovulation through its antiestrogenic properties at the level of the hypothalamus or pituitary gland, or both. Clomiphene down-regulates estrogen receptors at this level, and the hypothalamus and pituitary gland react as though the estrogen level is very low. This reverses the suppression of endogenous gonadotropins by estrogen, and gonadotropin levels rise, stimulating ovarian follicular development.
The down-regulation of estrogen receptors with clomiphene administration is not limited to the hypothalamus and pituitary gland, but also occurs peripherally at the endometrium and cervix, where it is not so desirable. When the cervix is affected, it becomes an unfavorable environment for sperm to penetrate, and when the endometrium is affected, its hypoestrogenic status may reduce the likelihood of embryo implantation—or may increase the risk of pregnancy loss if implantation occurs.
These peripheral antiestrogenic prop erties of clomiphene citrate may account for the discrepancy between high ovulation and low pregnancy rates.22-28 Several strategies to overcome this problem—e.g., adding estrogen, starting clomiphene citrate earlier in the menstrual period, or using another SERM, such as tamoxifen—have been largely unsuccessful. With clomiphene citrate, depletion of estrogen receptors has long-term effects because of the drug’s relatively long half-life (several days).31
In contrast, AIs do not appear to affect the expression of estrogen receptors in different body tissues, such as the endometrium and cervix. AIs have a shorter half-life (8 hours to 2 days), and nonsteroidal third-generation agents have a reversible inhibitory effect on the aromatase enzyme. Moreover, the rise in endogenous gonadotropins stimulates the production of more aromatase enzyme. This newly formed aromatase enzyme, and the return of a normal aromatase level after a short half-life of AI, leads the maturing ovarian follicles to secrete estrogen, which reaches a physiologic level soon after the last administration of AI. The rising estrogen level allows development of a more hospitable uterine environment (endometrium and cervical mucus).22-28
Early evidence confirms efficacy of AIs
After our pioneering reports of successful ovulation induction3-9 and improved ovarian response to stimulation by gonadotropins5-7 using AIs in small, nonrandomized, controlled trials, several larger and better designed clinical trials followed and supported our findings.10-19
Clinical trials comparing AIs with clomiphene citrate have consistently reported a universal “trend” toward superiority of AIs in achieving pregnancy despite comparable levels of success in achieving ovulation.10,11,14,16-19 However, these published clinical trials lacked adequate sample size to definitively confirm the superiority of AIs in achieving clinical pregnancy. We believe AIs are superior because, in our experience, they have helped women achieve pregnancy even after failure of several cycles of clomiphene treatment.4,15
Should an AI follow a trial of clomiphene?
U.Y., the patient described at the opening of this article, has two main options now that she has completed six cycles of clomiphene citrate without conceiving. The usual strategy would be a shift to more sophisticated treatment using gonadotropin injection. However, exogenous gonadotropins have several disadvantages:
- the drugs must be injected (orally inactive)
- they are more expensive than clomiphene citrate and AIs
- they require close monitoring by an infertility specialist with expensive and sophisticated technology
- they carry a risk of severe ovarian hyperstimulation, which is unlikely with clomiphene citrate and unreported with AIs
- multiple pregnancy is likely, particularly in conjunction with intrauterine insemination
- the risk of ovarian hyperstimulation with gonadotropin injection is much higher in women with PCOS, such as U.Y., as is the likelihood of multiple pregnancy.
The reason U.Y. has not conceived after six cycles of clomiphene citrate is likely related to the drug’s antiestrogenic effects on the endometrium, which appeared to be very thin (4–6 mm) on US imaging around the day of ovulation. If she fails to conceive with AIs, she will probably not become pregnant after a switch to gonadotropin injection unless more advanced treatment is included, such as in vitro fertilization (IVF) and embryo transfer. Other causes of her infertility—besides ovulatory dysfunction—may explain the failure to conceive.
Comparable pregnancy rates have been observed for AIs and gonadotropin injection, although further study is needed—specifically, clinical trials comparing gonadotropin and AIs in conjunction with timed intercourse or intrauterine insemination, or both.
CASE 2 No response to clomiphene citrate
G.A., 28 years old, has been trying to conceive for 3 years. She reports having irregular menstrual periods indicative of anovulation, and body temperature charts and progesterone levels support that diagnosis. She undergoes three cycles of clomiphene citrate at dosages ranging from 50 to 150 mg/day for 5 days starting on day 3 of the menstrual cycle. Despite treatment, she fails to ovulate.
Would an AI increase her chance of ovulating and conceiving?
Failure to ovulate after treatment with clomiphene citrate may have any of several causes, including inappropriate patient selection and resistance to the drug.
An example of inappropriate patient selection would be a woman with hypothalamic/hypopituitary anovulation; this type of patient often has insufficient levels of endogenous gonadotropins (luteinizing hormone and FSH). Another example would be a woman with reduced ovarian reserve; this type of patient is often unresponsive to clomiphene citrate and may have substantially elevated gonadotropin levels, most notably high FSH on day 3 of the menstrual cycle.
AIs are unlikely to induce ovulation in either of these patients. For the first type of patient, exogenous gonadotropin injection would be appropriate, as would be a gonadotropin-releasing hormone (GnRH) pump. For a woman with reduced ovarian reserve, an oocyte donor and IVF are the best treatment option.
Success with an AI is unlikely when there is no appropriate indication for clomiphene citrate. For example, a woman with severe insulin resistance who fails to ovulate in response to clomiphene citrate is unlikely to ovulate in response to an AI. In that case, an insulin sensitizer—alone or in combination with clomiphene citrate or an AI—would be the appropriate option. Other measures to reduce insulin resistance, such as weight loss, exercise, and dietary modification, may also be helpful.
CASE 3 Ovulatory patient with endometriosis fails to conceive on clomiphene
R.C., 34 years old, has been trying to conceive for 2 years. Her basic infertility workup, which included a hysterosalpingogram and semen analysis, did not reveal any abnormalities. She has regular menstrual cycles suggestive of ovulation. In addition, luteal-phase progesterone levels and biphasic body temperature charts both indicate regular ovulation.
After six cycles of clomiphene citrate, her gynecologist performs diagnostic laparoscopy. Other than minimal, stage 1 endometriosis, confirmed by pathologic examination of peritoneal biopsies, there are no remarkable findings. Methylene blue tubal perfusion confirms patent fallopian tubes during the operation. The gynecologist fulgurates the minimal endometriotic implants using carbon dioxide laser. Two months after the procedure, the patient undergoes three more cycles of clomiphene citrate, without success.
Would an AI help her conceive?
Most of the data on successful treatment with clomiphene citrate come from anovulatory women with PCOS in whom anovulation is the main cause of infertility. Evidence is weaker when the patient is ovulatory and has unexplained or endometriosis-associated infertility.32
A recent nonrandomized, controlled study that included women with a medical history comparable to R.C.’s found treatment with clomiphene citrate to significantly reduce the chance of pregnancy, compared with timed intercourse without clomiphene or other forms of ovarian stimulation, following conservative laparoscopic surgery for their endometriosis.33 We believe that clomiphene citrate is in-appropriate in women with endometriosis-related infertility—and may activate underlying endometriotic lesions.
For R.C., treatment with an AI is a viable option, particularly in light of recent data showing that the aromatase enzyme is expressed in endometriotic lesions.34 An AI could also enhance conception by further suppressing endometriosis through its effects on circulating estrogen levels and local estrogen production. This is an unproven extrapolation that seems scientifically appropriate to us, but needs confirmation by randomized clinical trials.
CASE 4 Woman with unexplained—and uninvestigated—infertility
E.D., 31 years old, has been trying to conceive for 1 year. Neither she nor her husband has undergone any study of their infertility problem.
Would empiric treatment with an AI be appropriate?
No treatment should begin until the patient and her partner have undergone the basic workup (TABLE 3). If a specific cause of infertility is determined, the patient should be treated accordingly. If no explanation for the infertility can be found, or anovulation is the likely cause, empirical ovarian stimulation with timed intercourse or intrauterine insemination is reasonable, provided:
- semen analysis is within normal limits
- ovarian function is present—i.e., the patient is expected to ovulate in response to ovarian stimulation
- at least one tube is patent and functional
- uterus has no serious abnormalities.
If ovarian stimulation fails to trigger ovulation or pregnancy, consider the options listed in TABLE 4
TABLE 3
Basic infertility workup
|
|
|
|
|
TABLE 4
When ovarian stimulation fails, next step depends on several variables
| LEVEL OF FAILURE | CLOMIPHENE CITRATE | AROMATASE INHIBITORS |
|---|---|---|
| 1–No ovulation | Is indication appropriate? Neither clomiphene citrate nor AIs are appropriate for hypothalamic/hypopituitary anovulation or ovarian failure Is severe insulin resistance present? If so, consider insulin sensitizers and encourage exercise, dietary changes, and weight loss | |
| Other options: Change to AI or retry clomiphene citrate in conjunction with an insulin sensitizer. If treatment fails after 3 to 6 additional cycles, consider an injectable gonadotropin | Other options: Try adding an insulin sensitizer. If treatment fails after 3 to 6 additional cycles, consider an injectable gonadotropin | |
| 2–Ovulation but no pregnancy | Was another cause of infertility (besides ovulatory dysfunction) overlooked? Investigate further, if necessary Options: Consider AIs before injectable gonadotropins, especially when there is evidence, with clomiphene citrate, of a persistent antiestrogenic effect, such as thin endometrium around the time of ovulation; endometriosis; or unexplained infertility. Move to gonadotropins if AIs fail | |
Minimal adverse effects
AIs are generally well tolerated. The most common adverse effects are hot flushes, GI disturbances (nausea and vomiting), and leg cramps. In clinical trials involving postmenopausal women with breast cancer who were taking an AI, very few withdrew because of drug-related adverse effects.35 Those women took an AI on a daily basis over several months. Fewer adverse effects would be expected among usually healthy younger women administered a short course (a few days) for ovarian stimulation. In addition, our clinical experience has been that fewer women experience side effects such as mild hot flushes and symptoms similar to premenstrual syndrome when taking an AI, compared with clomiphene citrate.3-9
When any medication is given during pregnancy, there are concerns about its effects. Drugs used to induce ovulation are no exception. In fact, clomiphene citrate is classified as pregnancy category X—a fact frequently overlooked by treating physicians. As for AIs, recent studies found no evidence of teratogenicity or clastogenicity in animal embryos when anastrozole was given. The picture is murkier for letrozole.
When used for ovarian stimulation, the short half-life of AIs and administration in the early follicular phase (several days before ovulation and fertilization occur) should ensure clearance of the drugs before implantation. Nevertheless, it is important to confirm that the patient is not pregnant before an AI is given. We recommend a pregnancy test before administering an AI for ovulation induction.
Mixed bag of data on pregnancy outcomes
Three large studies recently reported on pregnancy outcomes after infertility treatment with AIs.9,36,37 The first was a cohort study comparing outcomes of 394 pregnancies achieved after treatment with letrozole (133 pregnancies) and other ovarian-stimulation agents, including clomiphene citrate (113 pregnancies) and gonadotropins (110 pregnancies), with a control group of 38 pregnancies achieved without ovarian stimulation.9 The study encompassed three tertiary referral centers over 2 years. Pregnancies conceived after treatment with an AI had rates of miscarriage and ectopic pregnancy comparable to all other groups. In addition, letrozole was associated with a significantly lower rate of multiple gestation than was clomiphene citrate.9
The second study, presented in abstract form, compared the outcome of 150 births after treatment with letrozole to a database of 36,050 normal deliveries.36 Although the authors themselves stated that there was no statistically significant difference in the overall incidence of congenital malformation, they reported a higher incidence of locomotor malformation and cardiac anomaly in the infants conceived after treatment with letrozole.36 They did not address this discrepancy or explain how locomotor malformation was assessed.
A closer look at the abstract reveals major methodological flaws that weaken the data and conclusions presented:
- The study was not well controlled. The treated patients (n=130) were infertile women, mainly suffering from PCOS and unexplained infertility, who had a mean age of 35.2 years. The control group included a database of spontaneously conceiving women who were significantly younger (mean age: 30.5 years). The control group also included deliveries in a low-risk hospital that refers out high-risk pregnancies to secondary and tertiary hospitals. These are important distinctions because women of advanced maternal age have an increased incidence of medical illnesses, making their pregnancies higher in risk.
- The incidence of multiple gestation was significantly higher among women treated for infertility than among women in the control group. It is well known that multiple gestations are at increased risk of fetal malformation compared with singleton pregnancies.
- The incidence of cardiac anomaly among women treated with letrozole did not differ significantly from the known incidence of cardiac malformation in the general population, but the authors concluded that the rate of cardiac malformation was significantly higher in the letrozole group than among controls. This is misleading because it was the control group that developed cardiac malformation at a significantly lower rate than in the general population. Such a low incidence of cardiac anomaly in a low-risk hospital setting is not surprising, because mothers would be transferred to a tertiary-care center once an anomaly was detected.
- Data on congenital malformation in the control group were collected from delivery records available in the maternity ward of the hospital. However, a significant percentage of congenital malformations, such as cardiac anomaly, are not detected until after the neonatal period.36
When using clomiphene citrate or an aromatase inhibitor (AI):
- avoid a dosage that exceeds 100 to 150 mg/day for clomiphene citrate or 2.5 to 5 mg/day for AIs or a treatment period longer than 5 days each cycle
- do not administer an AI beyond day 7 of the menstrual cycle
- stop after three to six cycles of treatment
- do not increase the dosage once ovulation occurs
- discontinue treatment when serious adverse effects are present, such as visual side effects.
It is also interesting that the results of this abstract have not been published in a peer-reviewed journal more than a year after its presentation.
The third study, which is more recent, compared the incidence of congenital malformation in 911 newborns conceived after treatment with letrozole (n=514) or clomiphene citrate (n=397).37 It found no statistically significant difference between the groups. Congenital malformation was diagnosed in 2.4% and 4.8% of the letrozole- and clomiphene-treated groups, respectively, and major malformation occurred in 1.2% and 3% of the letrozole- and clomiphene-treated groups, respectively. These differences were not statistically significant, but there was a sevenfold increase in overall cardiac anomalies in the clomiphene-treated group, compared with the letrozole-treated group—and this difference was statistically significant. These findings warrant further investigation into the use of clomiphene citrate for induction of ovulation.
1. Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Effect of diagnosis, age, sperm quality, and number of preovulatory follicles on the outcome of multiple cycles of clomiphene citrate-intrauterine insemination. Fertil Steril. 2002;78:1088-1095.
2. Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligomenorrheic infertility. J Clin Endocrinol Metab. 1999;84:1617-1622.
3. Mitwally MFM, Casper RF. Aromatase inhibition: a novel method of ovulation induction in women with polycystic ovarian syndrome. Reprod Technol. 2000;10:244-247.
4. Mitwally MFM, Casper RF. Use of an AI for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305-309.
5. Mitwally MFM, Casper RF. Aromatase inhibition improves ovarian response to follicle-stimulating hormone in poor responders. Fertil Steril. 2002;77:776-780.
6. Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotropin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;188:1588-1597.
7. Mitwally MF, Casper RF. Aromatase inhibition reduces the dose of gonadotropin required for controlled ovarian hyperstimulation. J Soc Gynecol Investig. 2004;11:406-415.
8. Mitwally MFM, Casper RF. Single dose administration of the aromatase inhibitor, letrozole: a simple and convenient effective method of ovulation induction. Fertil Steril. 2005;83:229-231.
9. Mitwally MFM, Casper RF. Pregnancy outcome after the use of an AI for induction of ovulation. Am J Obstet Gynecol. 2005;192:381-386.
10. Fatemi HM, Kolibianakis E, Tournaye H, et al. Clomiphene citrate versus letrozole for ovarian stimulation: a pilot study. Reprod Biomed Online. 2003;75:543-546.
11. Al-Fozan H, Al-Khadouri M, Tan SL, Tulandi T. A randomized trial of letrozole versus clomiphene citrate in women undergoing superovulation. Fertil Steril. 2004;82:1561-1563.
12. Goswami SK, Das T, Chattopadhyay R, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19:2031-2035.
13. Garcia-Velasco JA, Moreno L, Pacheco A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82-87.
14. Bayar U, Tanrierdi HA, Barut A, et al. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85:1045-1048.
15. Elnashar A, Fouad H, Eldosoky M, et al. Letrozole induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome may not depend on the period of infertility, the body mass index, or the luteinizing hormone/follicle stimulating hormone ratio. Fertil Steril. 2006;85:161-164.
16. Atay V, Cam C, Muhcu M, et al. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34:73-76.
17. Sohrabvand F, Ansari S, Bagheri M. Efficacy of combined metformin-letrozole in comparison with metformin-clomiphene citrate in clomiphene-resistant infertile women with polycystic ovarian disease. Hum Reprod. 2006;21:1432-1435.
18. Sipe CS, Davis WA, Maifeld M, Van Voorhis BJ. A prospective randomized trial comparing anastrozole and clomiphene citrate in an ovulation induction protocol using gonadotropins. Fertil Steril. 2006;86:1676-1681.
19. Bayar U, Basaran M, Kiran S, Coskun A, Gezer S. Use of an aromatase inhibitor in patients with polycystic ovary syndrome: a prospective randomized trial. Fertil Steril. 2006;86:1447-1451.
20. Buzdar A, Howell A. Advances in aromatase inhibition: clinical efficacy and tolerability in the treatment of breast cancer. Clin Cancer Res. 2001;7:2620-2635.
21. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology Technology Assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol. 2002;2015:3317-3327.
22. Mitwally MF, Casper RF. Potential of aromatase inhibitors for ovulation and superovulation induction in infertile women. Drugs. 2006;66:2149-2160.
23. Mitwally MFM, Casper RF. Letrozole for ovulation induction. Exp Rev Obstet Gynecol. 2006;1:15-27.
24. Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91:760-771.
25. Mitwally MF, Casper RF, Diamond MP. The role of aromatase inhibitors in ameliorating deleterious effects of ovarian stimulation on outcome of infertility treatment. Reprod Biol Endocrinol. 2005;3:54.-
26. Mitwally MF, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;22:61-78.
27. Mitwally MF, Casper RF. Aromatase inhibitors for the treatment of infertility. Expert Opin Investig Drugs. 2003;12:353-371.
28. Mitwally MF, Casper RF. Aromatase inhibition for ovarian stimulation: future avenues for infertility management. Curr Opin Obstet Gynecol. 2002;14:255-263.
29. Cole PA, Robinson CH. Mechanism and inhibition of cytochrome P-450 aromatase. J Med Chem. 1990;33:2933-2944.
30. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;848:2951-2956.
31. Mikkelson TJ, Kroboth PD, Cameron WJ. Single dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil Steril. 1986;46:392-396.
32. Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate for unexplained subfertility in women. Cochrane Database Syst Rev. 2000;(2):CD000057.-
33. Mitwally MF, Albuarki H, Ashraf M, Diamond MP, Abuzeid M. Clomiphene reduces chance of pregnancy in infertile women with endometriosis following laparoscopic surgery. J Soc Gynecol Investig. 2006;13(2) (suppl):abstract 646.-
34. Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49-56.
35. Goss PE. Risks versus benefits in the clinical application of aromatase inhibitors. Endocr Relat Cancer. 1999;6:325-332.
36. Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins [abstract no. 1033]. Fertil Steril. 2005;84 (suppl):abstract 1033.-
37. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85:1761-1765.
1. Dickey RP, Taylor SN, Lu PY, Sartor BM, Rye PH, Pyrzak R. Effect of diagnosis, age, sperm quality, and number of preovulatory follicles on the outcome of multiple cycles of clomiphene citrate-intrauterine insemination. Fertil Steril. 2002;78:1088-1095.
2. Imani B, Eijkemans MJ, te Velde ER, Habbema JD, Fauser BC. Predictors of chances to conceive in ovulatory patients during clomiphene citrate induction of ovulation in normogonadotropic oligomenorrheic infertility. J Clin Endocrinol Metab. 1999;84:1617-1622.
3. Mitwally MFM, Casper RF. Aromatase inhibition: a novel method of ovulation induction in women with polycystic ovarian syndrome. Reprod Technol. 2000;10:244-247.
4. Mitwally MFM, Casper RF. Use of an AI for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305-309.
5. Mitwally MFM, Casper RF. Aromatase inhibition improves ovarian response to follicle-stimulating hormone in poor responders. Fertil Steril. 2002;77:776-780.
6. Mitwally MF, Casper RF. Aromatase inhibition reduces gonadotropin dose required for controlled ovarian stimulation in women with unexplained infertility. Hum Reprod. 2003;188:1588-1597.
7. Mitwally MF, Casper RF. Aromatase inhibition reduces the dose of gonadotropin required for controlled ovarian hyperstimulation. J Soc Gynecol Investig. 2004;11:406-415.
8. Mitwally MFM, Casper RF. Single dose administration of the aromatase inhibitor, letrozole: a simple and convenient effective method of ovulation induction. Fertil Steril. 2005;83:229-231.
9. Mitwally MFM, Casper RF. Pregnancy outcome after the use of an AI for induction of ovulation. Am J Obstet Gynecol. 2005;192:381-386.
10. Fatemi HM, Kolibianakis E, Tournaye H, et al. Clomiphene citrate versus letrozole for ovarian stimulation: a pilot study. Reprod Biomed Online. 2003;75:543-546.
11. Al-Fozan H, Al-Khadouri M, Tan SL, Tulandi T. A randomized trial of letrozole versus clomiphene citrate in women undergoing superovulation. Fertil Steril. 2004;82:1561-1563.
12. Goswami SK, Das T, Chattopadhyay R, et al. A randomized single-blind controlled trial of letrozole as a low-cost IVF protocol in women with poor ovarian response: a preliminary report. Hum Reprod. 2004;19:2031-2035.
13. Garcia-Velasco JA, Moreno L, Pacheco A, et al. The aromatase inhibitor letrozole increases the concentration of intraovarian androgens and improves in vitro fertilization outcome in low responder patients: a pilot study. Fertil Steril. 2005;84:82-87.
14. Bayar U, Tanrierdi HA, Barut A, et al. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85:1045-1048.
15. Elnashar A, Fouad H, Eldosoky M, et al. Letrozole induction of ovulation in women with clomiphene citrate-resistant polycystic ovary syndrome may not depend on the period of infertility, the body mass index, or the luteinizing hormone/follicle stimulating hormone ratio. Fertil Steril. 2006;85:161-164.
16. Atay V, Cam C, Muhcu M, et al. Comparison of letrozole and clomiphene citrate in women with polycystic ovaries undergoing ovarian stimulation. J Int Med Res. 2006;34:73-76.
17. Sohrabvand F, Ansari S, Bagheri M. Efficacy of combined metformin-letrozole in comparison with metformin-clomiphene citrate in clomiphene-resistant infertile women with polycystic ovarian disease. Hum Reprod. 2006;21:1432-1435.
18. Sipe CS, Davis WA, Maifeld M, Van Voorhis BJ. A prospective randomized trial comparing anastrozole and clomiphene citrate in an ovulation induction protocol using gonadotropins. Fertil Steril. 2006;86:1676-1681.
19. Bayar U, Basaran M, Kiran S, Coskun A, Gezer S. Use of an aromatase inhibitor in patients with polycystic ovary syndrome: a prospective randomized trial. Fertil Steril. 2006;86:1447-1451.
20. Buzdar A, Howell A. Advances in aromatase inhibition: clinical efficacy and tolerability in the treatment of breast cancer. Clin Cancer Res. 2001;7:2620-2635.
21. Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology Technology Assessment on the use of aromatase inhibitors as adjuvant therapy for women with hormone receptor-positive breast cancer: status report 2002. J Clin Oncol. 2002;2015:3317-3327.
22. Mitwally MF, Casper RF. Potential of aromatase inhibitors for ovulation and superovulation induction in infertile women. Drugs. 2006;66:2149-2160.
23. Mitwally MFM, Casper RF. Letrozole for ovulation induction. Exp Rev Obstet Gynecol. 2006;1:15-27.
24. Casper RF, Mitwally MF. Review: aromatase inhibitors for ovulation induction. J Clin Endocrinol Metab. 2006;91:760-771.
25. Mitwally MF, Casper RF, Diamond MP. The role of aromatase inhibitors in ameliorating deleterious effects of ovarian stimulation on outcome of infertility treatment. Reprod Biol Endocrinol. 2005;3:54.-
26. Mitwally MF, Casper RF. Aromatase inhibitors in ovulation induction. Semin Reprod Med. 2004;22:61-78.
27. Mitwally MF, Casper RF. Aromatase inhibitors for the treatment of infertility. Expert Opin Investig Drugs. 2003;12:353-371.
28. Mitwally MF, Casper RF. Aromatase inhibition for ovarian stimulation: future avenues for infertility management. Curr Opin Obstet Gynecol. 2002;14:255-263.
29. Cole PA, Robinson CH. Mechanism and inhibition of cytochrome P-450 aromatase. J Med Chem. 1990;33:2933-2944.
30. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;848:2951-2956.
31. Mikkelson TJ, Kroboth PD, Cameron WJ. Single dose pharmacokinetics of clomiphene citrate in normal volunteers. Fertil Steril. 1986;46:392-396.
32. Hughes E, Collins J, Vandekerckhove P. Clomiphene citrate for unexplained subfertility in women. Cochrane Database Syst Rev. 2000;(2):CD000057.-
33. Mitwally MF, Albuarki H, Ashraf M, Diamond MP, Abuzeid M. Clomiphene reduces chance of pregnancy in infertile women with endometriosis following laparoscopic surgery. J Soc Gynecol Investig. 2006;13(2) (suppl):abstract 646.-
34. Attar E, Bulun SE. Aromatase and other steroidogenic genes in endometriosis: translational aspects. Hum Reprod Update. 2006;12:49-56.
35. Goss PE. Risks versus benefits in the clinical application of aromatase inhibitors. Endocr Relat Cancer. 1999;6:325-332.
36. Biljan MM, Hemmings R, Brassard N. The outcome of 150 babies following the treatment with letrozole or letrozole and gonadotropins [abstract no. 1033]. Fertil Steril. 2005;84 (suppl):abstract 1033.-
37. Tulandi T, Martin J, Al-Fadhli R, et al. Congenital malformations among 911 newborns conceived after infertility treatment with letrozole or clomiphene citrate. Fertil Steril. 2006;85:1761-1765.