User login
- Distances between the entry trocar and the aorta bifurcation increase directly with body mass index, mainly because of the commensurate increase in abdominal wall thickness.
- The mean thrusting force for insertion of a disposable trocar is 10.2 lb versus 17.53 lb for a reusable device, and the time to penetrate is shorter for the disposable trocar: mean of 3.54 seconds versus 11.64 seconds. Thus, greater caution is warranted when inserting a disposable trocar.
- Thrust the primary trocar into the midline of the abdomen at a 45° to 60° angle relative to the plane of the abdominal wall, with the trocar pointing toward the uterus, to avoid injuring the iliac vessels.
- When injury occurs, call for a vascular surgeon immediately, perform a laparotomy using a vertical incision, and get accurate inputs, outputs, and blood-loss estimates.
Major vessel injury is a two-sided coin: It can occur with alarming speed, but it is preventable.
Fortunately, the laparoscopic surgeon can avoid the problem by following simple precautions and steering clear of scenarios that increase the risk of injury. This article tells how to accomplish both objectives.
In the process, it reviews the evidence, details management for any injuries that occur, and includes a comprehensive table listing typical distances between the entry trocar and vascular structures, to help the surgeon adjust entry strategy.
Adequate prevention depends on:
- familiarity with the vascular anatomy, particularly in relation to the umbilicus, presacral space, infundibulopelvic ligament, and ovarian fossa.
- creating a proper pneumoperitoneum, especially when using disposable trocars.
- careful attention to primary trocar thrusting techniques to ensure midline insertion at the proper angle. Also exercise caution when placing secondary trocars. Specifically, during far lateral insertion, avoid cleaving the inferior epigastric artery from the external iliac or directly hitting the external artery or vein.
- avoiding long trocars, which are unnecessary to penetrate the peritoneal cavity.
- reliance on laparotomy if trocar insertion proves too difficult, vision is obscured, or appropriate anatomic dissection planes cannot be developed.
- when injury occurs, performing laparotomy using a vertical incision.
A 36-year-old woman with a body mass index of 38.2, indicating severe obesity, is scheduled to undergo hysteroscopy and dilatation and curettage for irregular bleeding, as well as laparoscopic bilateral partial salpingectomy for elective sterilization. The setting is an outpatient surgery center without a blood bank.
After general anesthesia, the surgeon makes a 1.5-cm incision just below the umbilicus, inserts a Verres needle, and insufflates carbon dioxide gas to a volume of approximately 3.4 L. He then inserts a disposable trocar and places a laparoscope, but views fat. Unbeknownst to him, he has insufflated the properitoneal fat space rather than the peritoneum.
The surgeon finally enters the peritoneum with a “long” trocar after several more attempts. Since the uterus and adnexa appear to be normal, he inserts a second trocar and places a probe. As he is moving the intestines, however, he observes blood, and the field suddenly becomes unclear. He removes the probe and, when the gas-pressure valve of the secondary trocar is opened, blood spews from the site.
The surgeon removes all trocars and performs an emergency laparotomy using a Pfannenstiel incision. He and 2 general surgeons, who arrive within 20 to 30 minutes, work for 2 hours to repair what they believe is a hole in the inferior vena cava. The woman is brought out of anesthesia and transferred to the local community hospital, where she goes into cardiac arrest and dies. A postmortem reveals injury to the right common iliac artery and vein. No sutures were observed in either vessel. Cause of death: exsanguination.
What went wrong?
Three serious errors contributed to the patient’s death:
- He made multiple attempts to insert the trocar without considering the possibility that the wrong space had been insufflated.
- He inserted the trocar off the midline and at the wrong angle relative to the abdominal wall.
- In his frustration, he switched to a “long” trocar, which made it more likely that vascular structures would be injured.
Operating on an obese patient in a center without a blood bank also was unwise, as obese women of short stature are at greatest risk for vascular injury.
How big is the problem?
A French study1 of 103,852 laparoscopic procedures—of which 15.7%, or 16,000 operations, were gynecologic—reported 47 cases of major vascular injury for an incidence of 0.5 per 1,000 cases and a mortality rate of 17%. Several additional articles2-8 reported a range of vascular complications of between 0.1 and 6.4 per 1,000 laparoscopies.
In a study9 conducted in 7 gynecologic laparoscopy surgery centers in France over 9 years and involving 29,966 diagnostic and operative cases, the overall complication rate was 4.64 per 1,000 laparoscopies (n = 139). Of the 21 major vascular injuries associated with gynecologic surgery, the majority occurred during set-up, and 84.6% during insertion of the primary trocar. Two patients died from their injuries.
Bhoyrul and colleagues10 analyzed data reported to the US Food and Drug Administration and found that 408 of 629 trocar-related injuries involved major blood vessels, as did 26 of 32 deaths (81%). Most of the deaths (87%) were linked to the use of disposable trocars equipped with safety shields; 9% with direct-view trocars. Although surgeons asserted that the trocar malfunctioned in 41 cases, that claim was confirmed in only 1 case (2%).
Another study found that 37 of 79 (46.8%) serious complications involving optical-access trocars between 1994 and 2002 involved major vessels, injuring the aorta, iliac vessels, or vena cava.11
A study12 carried out in the Netherlands in 1994 evaluated the relative number of complications that occurred within a total of 25,764 laparoscopic procedures. The study divided complications into those occurring as the result of the laparoscopic approach (eg, trocar insertion) versus those happening during the performance of the operation. Fifty-seven percent of the 145 complications were caused by the laparoscopic approach; the 2 reported deaths also were secondary to that approach.
Snapshot of vascular injury: A series of 31 patients
In 2003, I published data13 on 31 cases of major vessel injury associated with gynecologic laparoscopy (see). These cases were collected from a variety of sources: medicolegal case files, hospital morbidity-mortality presentations, and quality-assurance departments. Eight cases involved diagnostic procedures, while 23 involved operative laparoscopy.
The medical records of these cases provided details on the nature of the injury. The cases were categorized by body mass index (BMI) and cause, ie, whether they occurred as the result of the laparoscopic approach (ie, entry-related) or arose during surgery.
Of the 31 cases, 22 (71%) involved women with BMIs from 25 to more than 30 (overweight or obese). A large majority—28 cases (90%)—were related to entry. Only 3 injuries occurred during surgery.
In several women, more than 1 vessel was damaged. Of the 49 total injuries, 38 (78%) involved the iliac vessels. Seven (23%) women died as a result of their injuries, all of which involved venous trauma.
Damage to structures in the vicinity of the injured vessels was substantial in 16 cases. Major morbidity included ureteral, nerve, and intestinal injury; arterial and venous thrombosis; compartment syndrome; and suturing of the wrong vessel.
Some patients also experienced edema or pain in an extremity (vascular insufficiency); infection; diffuse intravascular coagulation and/or adult respiratory distress; cardiac arrest; central nervous system injury (stroke); or hospitalization of more than 1 week. Cases also were categorized as early or late diagnosis, depending on whether shock had supervened. Diagnosis was early in 8 cases (26%) and late in 21 (68%). Two patients were diagnosed postoperatively; ie, they had gone to the recovery room prior to developing shock.
The volume of blood loss ranged from 1,000 mL to 7,000 mL, with a mean loss of 3,400 mL. All patients received packed red blood cells and/or a mixture of other blood products. The time required for cross-matching and receiving blood ranged from 10 to 120 minutes.
In all cases, a vascular or general surgeon was called to consult on the case.
Mapping vascular structures to ensure safe trocar entry
Knowing the distances between blood vessels and laparoscopic entry trocars is critical if injury is to be avoided. In pursuit of this goal, Hurd and colleagues14 performed a retrospective study involving women who had undergone magnetic resonance imaging or computed tomography scans of the abdomen. Investigators measured the distance between the lower abdominal wall and the aortic bifurcation in these women, who were all unanesthetized and in the supine position.
Distances increased with BMI
This occurred in the study by Hurd et al,14 as well as in a prospective study by Narendran and Baggish,15 who calculated body mass index in 101 consecutive women who were undergoing diagnostic or operative laparoscopy. These women were anesthetized, with pneumoperitoneum established and a laparoscope inserted; all were in the lithotomy position.
In this study, Narendran and Baggish measured the following distances from the entry trocar:
- perpendicular distance to aortic bifurcation,
- oblique distance to the right and left common iliac vessels,
- oblique distance to the superior margin of the bladder,
- perpendicular distance from the peritoneum to skin at the umbilicus (abdominal wall thickness), and
- oblique distance from the subumbilical peritoneal opening to the right and left common iliac vessels.
Wide range of BMIs
In the study by Narendran and Baggish, successful measurement panels were created for 99 of the 101 cases. Of these, 49 women had a BMI of less than 25 (normal), 29 had a BMI greater than 25 but less than 30 (overweight), and 21 had a BMI greater than 30 (obese).
A significant difference was observed in the perpendicular distance from the entry trocar to aortic bifurcation (TABLE 1). Specifically, as the BMI increased, so did the distance. The only other significant BMI-related increase was the abdominal wall thickness, which also varied directly with the BMI.
Other distances increase with height
The distance between the primary trocar and the iliac vessels and urinary bladder consistently increased with the patient’s height.
However, no significant change in distance between the great vessels and the primary trocar site occurred when the patient’s position changed from level to Trendelenburg.
Trocar insertion: Disposable devices require less force
Laparoscopic trocar thrusting is a dynamic process, and we observed that process in our study.15 When force is applied via trocar to the anterior abdominal wall, that structure is displaced toward the abdominal cavity in the direction of the posterior abdominal wall—even when countertraction is taken into consideration. The movement is more apparent in obese women because of greater elasticity created by the larger mass of properitoneal and subcutaneous fat. We measured the distortion and determined that the depression can be 5 cm or more.
In contrast, thin women have rigid, relatively unyielding anterior abdominal walls and therefore experience minimal displacement. In thin women, the greater risk is the shorter passive distance between the anterior abdominal wall and the great vessels.
Comparing force curves
We16 calculated the force required to thrust a disposable or reusable trocar through the anterior abdominal wall during actual laparoscopic surgery. We used a 25-lb compression load cell connected to the trocar by an Ultem handle, which could be sterilized between cases. A linear variable displacement transducer detected displacement, and the measuring apparata fed data into a computer. Ten women were randomized to a disposable trocar and 10 to a reusable device.
The mean thrusting force for disposable trocars was 10.2 lb versus 17.53 lb for the reusable device. The time to penetrate was likewise significantly shortened for disposable trocars: mean time of 3.54 seconds versus 11.64 seconds. Overall work tilted in favor of disposable trocars: 14.34 pound-seconds versus 103.88 pound-seconds.
The disposable trocar has the advantage for 2 reasons: its razor-sharp cutting edge and streamlined design.
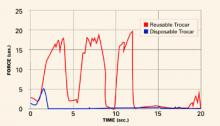
FIGURE 1 shows typical force curves of disposable and reusable trocars.
FIGURE 1 Reusable trocar requires more force than disposable trocar
A considerable difference in force is required for insertion, depending on type of device, as this graph of typical force curves shows. The reusable trocar requires 18 to 20 lb of force over 12 seconds; the disposable, only 5 lb over 2 seconds.
Safe trocar insertion begins with pneumoperitoneum
McDougall et al17 demonstrated that adequate pneumoperitoneum lessens the force required to drive a trocar through the anterior abdominal wall. Although the differences were small, the forces required with an intraperitoneal gas pressure of 30 mm Hg were smaller than those required with a pressure of 15 mm Hg.17
Manufacturers of disposable trocars also recommend creating an adequate pneumoperitoneum prior to aiming and inserting the razor-sharp device. The goal is creating a carbon dioxide gas pocket large enough to permit rapid deployment of the “safety shield” after the trocar tip clears the properitoneal fat and peritoneal membrane.
Slow-motion video sequences of disposable trocar entry show the sharp trocar tip penetrating the parietal peritoneum of the anterior abdominal wall for 1 cm before the spring-loaded shield advances and locks over the blade. During this insertion, the anterior abdominal wall has an elastic reaction to the applied force; this reaction pushes it toward the posterior abdominal wall.
Direct insertions (ie, without adequate pneumoperitoneum) involve less space for the trocar’s safety shield to deploy. Thus, there is a greater risk of the armed trocar tip coming into direct contact with underlying viscera and blood vessels.
Delayed diagnosis
The earlier a major vessel incident can be diagnosed, the better for patient, physician, and hospital. Diagnosis after the onset of hypotension, tachycardia, or tachypnia constitutes “late” diagnosis. Dark venous blood pooling in the abdomen, bright red pulsatile blood emitting from a trocar sleeve, or a retroperitoneal hematoma lateral to the iliacs or at the level of the presacral space suggests major vessel injury. Signs of hypovolemic shock or sudden appearance of profound shock places the possibility of major vessel injury at the top of the differential diagnosis.
Relying on observation when a retroperitoneal hematoma develops
Unfortunately, with observation, the surgeon cannot determine the identity or nature of the damaged vessel, know whether the hematoma is expanding beyond the view of the laparoscope, or predict when the patient will go into shock.
Leaving an armed trocar in place in a vessel
Assuming that the trocar is plugging a hole and preventing hemorrhage is a recipe for disaster. The movement of the sharp device against a vessel wall is most likely to create greater trauma to the vessel. In the case of partial penetration, the device may cut the rest of the way through the vessel.
Laparoscopic exploration
Attempts to locate the injury via laparoscopy usually are unsuccessful, and laparoscopic attempts to sew up the injury limit accuracy and efficacy.
Use of the Pfannenstiel incision during emergency laparotomy
Unfortunately, in 1 study,13 27 of 31 women with vascular injuries received this incision. A vertical incision is preferred because it affords greater access and visibility.
Underestimating blood loss
In the case of a major vessel injury, underestimation of blood volume requirements can be fatal. In 1 study,13 19 of 31 women were under-transfused and/or inadequately cross-matched.
Clamping injured vessels
This can lead to arterial or venous thrombosis. Nonvascular clamps can tear large vessels, adding to the damage and complicating the vascular surgeon’s attempt at repair. Rather, apply direct pressure with a sponge stick.
Delay in calling for help
This translates into greater blood loss and a less stable patient. In 1 study,13 the mean time for a vascular surgeon to intercede was 23 minutes.
Use a shorter insufflation needle
Our data on women with a BMI greater than 30 (obese range) indicate that the mean thickness of the anterior abdominal wall is 5.05 cm and the distance to the aorta is 15.14 cm.15 A standard Verres needle measures 12.5 cm from the tip of the shaft to the point where the shaft joins the hub of the needle. This is clearly excessive length, since women with a BMI above 30 have an abdominal wall thickness of approximately 5.05 cm and women with BMIs between 25 and 30 have a thickness of only 3.85 cm.
I prefer a Touhy epidural needle for subumbilical insertion and creation of the pneumoperitoneum, since it is a relatively short 8.5 cm. Thus, it is less hazardous than the Verres needle. It also is less likely to clog with tissue fragments because of its curved tip, and more likely to create a successful pneumoperitoneum on the first try.
Fortunately, large-vessel injuries caused by the insufflation needle are rare.
Proper insertion technique
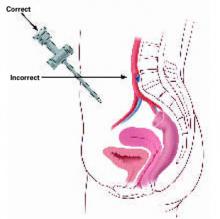
I have residents draw a straight line with a marking pen from the lower margin of the umbilicus to the superior margin of the pubic symphysis. This serves as a guide to keep the trocar pointing toward the middle of the abdomen, away from the iliac vessels. I also teach residents to thrust the trocar in the midline at a 45° to 60° angle in relation to the plane of the abdominal wall, with the trocar pointing toward the uterus (FIGURES 2 AND 3).
Many residents twist disposable trocars during insertion. This “door knob” movement works against the design of the trocar and traumatizes tissue. The correct approach is thrusting the device into the abdominal cavity, or holding the trocar (only for disposable trocar devices) like a dart and thrusting it into the abdomen as though throwing a dart. The only trocar designed for twisting is the conical reusable device; the sharp pyramidal reusable trocar should be thrust rather than twisted.
Avoid “long” trocars
These are a full 5 cm longer than the 20-cm standard device (hub of handle to tip of shaft). Abdominal wall measurements indicate that these devices are never required to simply penetrate the anterior abdominal wall; these trocars also carry the risk of hitting the iliac vessels.
Open laparoscopy is not foolproof
Although open laparoscopy would seem to guarantee safe entry of the primary trocar, reports of aortic injuries have recently been published. Similar data have been reported for optical access trocars.11,18
FIGURE 2 Insert the trocar at 45° to 60° angle
At insertion, the trocar should be at a 45°to 60°angle relative to the abdominal wall, with the tip of the device tilted in the direction of the uterus and bladder. A 90°angle of insertion is dangerous.
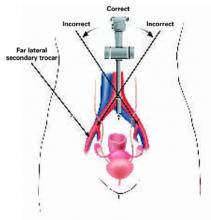
FIGURE 3 Midline insertion is safest
Insert the primary trocar in the midline pointing toward the uterus; deviation to the right or left is dangerous. Also avoid injuring the inferior epigastric and external iliac vessels with far lateral trocar insertion.
Body habitus and vascular injury
The obese patient of short stature is at the greatest risk for vascular injury. Although the relative distances between the anterior abdominal wall and the aorta are greater at the highest BMI levels, short stature means that the iliac vessels are closer. Significantly, large vessel injuries in the series cited herein were associated with the use of disposable trocars 90% of the time.
I believe high-risk conditions are created when carbon dioxide gas is inadvertently infused into the properitoneal fat space (FIGURE 4). As the volume of gas grows, the anterior wall parietal peritoneum dissects free from the remainder of the anterior abdominal fat, creating a pseudo-pneumoperitoneum. The operator fails to realize that the true peritoneal cavity has not been entered and, in fact, has paradoxically constricted in size because of the enlarging pseudoperitoneal space. Careful attention to the pressure gauges would have aroused suspicion that gas was being infused into the wrong space, since pressures tend to be higher and flow erratic in such situations.
Nevertheless, the surgeon places a trocar into the space, looks through the laparoscope, sees red or yellow, and realizes that the peritoneal cavity has not been entered. More gas is insufflated and the trocar is tried again.
Typically, the duller, reusable trocar pushes the leading edge of the peritoneum rather than penetrating it, further enlarging the properitoneal space and bringing the anterior and posterior peritoneal walls very close together.
In another scenario, the same set of circumstances exists except, rather than employing a reusable trocar, the surgeon selects a disposable device or even, after 2 failures to enter the peritoneal cavity with the reusable device, an extra-long (11-inch) disposable trocar (FIGURE 4).
In this scenario, an armed trocar enters the pseudospace—without the safety shield deployed—because no resistance was encountered during penetration of the incision, owing to the fact that two 10–12-mm trocars have previously traversed the same skin incision.
As the tip of the trocar comes into contact with the leading edge of the peritoneum, it encounters resistance, and the razor-sharp blade cuts through the anterior peritoneum, traverses the narrow peritoneal space, and cuts through the posterior peritoneum and the underlying great vessel.
Often, the trocar’s knife edge injures an artery by glancing off the curved surface of the vessels and embedding itself in the neighboring or underlying vein.
The best technique to manage a pseudo-pneumoperitoneal pocket is to abandon the subumbilical site, insert a Touhy needle in the left upper quadrant, and enter and overinflate the peritoneal cavity, thereby obliterating the properitoneal gas space.
When injury occurs: 7 recommended management steps
In the event of a vascular injury, early diagnosis and treatment are vital. Do not observe retroperitoneal hematomas. The following steps are recommended:
- Call for a vascular surgeon immediately and indicate that the situation is an emergency. Do not waste time trying to locate the injury before calling for help.
- Get emergency type and cross-match for at least 6 U of whole blood.
- Obtain baseline lab measurements, including hemoglobin, hematocrit, platelets, fibrinogen, and fibrin split products.
- Open the abdomen using a vertical incision for maximum access and visibility.
- Get accurate outputs and blood-loss estimates and have anesthesia keep careful records of fluids given.
- Advise anesthesia staff to obtain additional help. This will facilitate starting additional IV sites, rapidly infusing blood products, obtaining key samples for laboratory data, and maintaining accurate and detailed records of blood gases, blood loss, replacement fluids, and blood products.
- Use a circulator to manage urgent medications or laboratory tests.
The author reports no financial relationships relevant to this article.
1. Champault G, Cazacu F, Taffinader N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367.
2. Baadsgaard SE, Bille S, Egelblad K. Major vascular injury during gynecologic laparoscopy: report of a case and review of published cases. Acta Obstet Gynecol Scand. 1989;68:283.
3. Chamberlain G, Brown JD. Gynecologic laparoscopy: report of the working party of the confidential enquiry into gynecologic laparoscopy. Br J Obstet Gynaecol. 1978;85:401.
4. Mintz M. Risk and prophylaxis in laparoscopy: a survey of 100,000 cases. J Reprod Med. 1977;18:269.
5. Phillips JM, Hulka JF, Peterson HB. American Association of Gynecologic Laparoscopists’ 1982 Membership Survey. J Reprod Med. 1984;29:592.
6. Saidi MH, Vancaillie TG, White AJ, et al. Complications of major operative laparoscopy: a review of 452 cases. J Reprod Med. 1996;41:471.
7. Loffer F, Pent D. Indications, contraindications, and complications of laparoscopy. Obstet Gynecol Surv. 1975;30:407.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108.
9. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynecologic laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
10. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
11. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
12. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Herman J, Trimbos JB. Complications of laparoscopy: a prospective multi-center observational study. Br J Obstet Gynaecol. 1997;104:595-600.
13. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
14. Hurd WW, Bude RO, DeLancey JOL, Gauvin JM, Aisen AM. Abdominal wall characterization by MRI and CT imaging: the effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473.
15. Narendran M, Baggish MS. Mean distance between primary trocar insertion site and major retroperitoneal vessels during routine laparoscopy. J Gynecol Surg. 2002;18:121-127.
16. Baggish MS, Gandhi S, Kasper G. Force required by laparoscopic trocar devices to penetrate the human female’s anterior abdominal wall. J Gynecol Surg. 2003;19:1-11.
17. McDougall EM, Figenshau RS, Clayman RV, Monk TG, Smith DS. Laparoscopic pneumoperitoneum: impact of body habitus. J Laparosc Endosc Surg. 1994;4:385-391.
18. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of Hassan cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
- Distances between the entry trocar and the aorta bifurcation increase directly with body mass index, mainly because of the commensurate increase in abdominal wall thickness.
- The mean thrusting force for insertion of a disposable trocar is 10.2 lb versus 17.53 lb for a reusable device, and the time to penetrate is shorter for the disposable trocar: mean of 3.54 seconds versus 11.64 seconds. Thus, greater caution is warranted when inserting a disposable trocar.
- Thrust the primary trocar into the midline of the abdomen at a 45° to 60° angle relative to the plane of the abdominal wall, with the trocar pointing toward the uterus, to avoid injuring the iliac vessels.
- When injury occurs, call for a vascular surgeon immediately, perform a laparotomy using a vertical incision, and get accurate inputs, outputs, and blood-loss estimates.
Major vessel injury is a two-sided coin: It can occur with alarming speed, but it is preventable.
Fortunately, the laparoscopic surgeon can avoid the problem by following simple precautions and steering clear of scenarios that increase the risk of injury. This article tells how to accomplish both objectives.
In the process, it reviews the evidence, details management for any injuries that occur, and includes a comprehensive table listing typical distances between the entry trocar and vascular structures, to help the surgeon adjust entry strategy.
Adequate prevention depends on:
- familiarity with the vascular anatomy, particularly in relation to the umbilicus, presacral space, infundibulopelvic ligament, and ovarian fossa.
- creating a proper pneumoperitoneum, especially when using disposable trocars.
- careful attention to primary trocar thrusting techniques to ensure midline insertion at the proper angle. Also exercise caution when placing secondary trocars. Specifically, during far lateral insertion, avoid cleaving the inferior epigastric artery from the external iliac or directly hitting the external artery or vein.
- avoiding long trocars, which are unnecessary to penetrate the peritoneal cavity.
- reliance on laparotomy if trocar insertion proves too difficult, vision is obscured, or appropriate anatomic dissection planes cannot be developed.
- when injury occurs, performing laparotomy using a vertical incision.
A 36-year-old woman with a body mass index of 38.2, indicating severe obesity, is scheduled to undergo hysteroscopy and dilatation and curettage for irregular bleeding, as well as laparoscopic bilateral partial salpingectomy for elective sterilization. The setting is an outpatient surgery center without a blood bank.
After general anesthesia, the surgeon makes a 1.5-cm incision just below the umbilicus, inserts a Verres needle, and insufflates carbon dioxide gas to a volume of approximately 3.4 L. He then inserts a disposable trocar and places a laparoscope, but views fat. Unbeknownst to him, he has insufflated the properitoneal fat space rather than the peritoneum.
The surgeon finally enters the peritoneum with a “long” trocar after several more attempts. Since the uterus and adnexa appear to be normal, he inserts a second trocar and places a probe. As he is moving the intestines, however, he observes blood, and the field suddenly becomes unclear. He removes the probe and, when the gas-pressure valve of the secondary trocar is opened, blood spews from the site.
The surgeon removes all trocars and performs an emergency laparotomy using a Pfannenstiel incision. He and 2 general surgeons, who arrive within 20 to 30 minutes, work for 2 hours to repair what they believe is a hole in the inferior vena cava. The woman is brought out of anesthesia and transferred to the local community hospital, where she goes into cardiac arrest and dies. A postmortem reveals injury to the right common iliac artery and vein. No sutures were observed in either vessel. Cause of death: exsanguination.
What went wrong?
Three serious errors contributed to the patient’s death:
- He made multiple attempts to insert the trocar without considering the possibility that the wrong space had been insufflated.
- He inserted the trocar off the midline and at the wrong angle relative to the abdominal wall.
- In his frustration, he switched to a “long” trocar, which made it more likely that vascular structures would be injured.
Operating on an obese patient in a center without a blood bank also was unwise, as obese women of short stature are at greatest risk for vascular injury.
How big is the problem?
A French study1 of 103,852 laparoscopic procedures—of which 15.7%, or 16,000 operations, were gynecologic—reported 47 cases of major vascular injury for an incidence of 0.5 per 1,000 cases and a mortality rate of 17%. Several additional articles2-8 reported a range of vascular complications of between 0.1 and 6.4 per 1,000 laparoscopies.
In a study9 conducted in 7 gynecologic laparoscopy surgery centers in France over 9 years and involving 29,966 diagnostic and operative cases, the overall complication rate was 4.64 per 1,000 laparoscopies (n = 139). Of the 21 major vascular injuries associated with gynecologic surgery, the majority occurred during set-up, and 84.6% during insertion of the primary trocar. Two patients died from their injuries.
Bhoyrul and colleagues10 analyzed data reported to the US Food and Drug Administration and found that 408 of 629 trocar-related injuries involved major blood vessels, as did 26 of 32 deaths (81%). Most of the deaths (87%) were linked to the use of disposable trocars equipped with safety shields; 9% with direct-view trocars. Although surgeons asserted that the trocar malfunctioned in 41 cases, that claim was confirmed in only 1 case (2%).
Another study found that 37 of 79 (46.8%) serious complications involving optical-access trocars between 1994 and 2002 involved major vessels, injuring the aorta, iliac vessels, or vena cava.11
A study12 carried out in the Netherlands in 1994 evaluated the relative number of complications that occurred within a total of 25,764 laparoscopic procedures. The study divided complications into those occurring as the result of the laparoscopic approach (eg, trocar insertion) versus those happening during the performance of the operation. Fifty-seven percent of the 145 complications were caused by the laparoscopic approach; the 2 reported deaths also were secondary to that approach.
Snapshot of vascular injury: A series of 31 patients
In 2003, I published data13 on 31 cases of major vessel injury associated with gynecologic laparoscopy (see). These cases were collected from a variety of sources: medicolegal case files, hospital morbidity-mortality presentations, and quality-assurance departments. Eight cases involved diagnostic procedures, while 23 involved operative laparoscopy.
The medical records of these cases provided details on the nature of the injury. The cases were categorized by body mass index (BMI) and cause, ie, whether they occurred as the result of the laparoscopic approach (ie, entry-related) or arose during surgery.
Of the 31 cases, 22 (71%) involved women with BMIs from 25 to more than 30 (overweight or obese). A large majority—28 cases (90%)—were related to entry. Only 3 injuries occurred during surgery.
In several women, more than 1 vessel was damaged. Of the 49 total injuries, 38 (78%) involved the iliac vessels. Seven (23%) women died as a result of their injuries, all of which involved venous trauma.
Damage to structures in the vicinity of the injured vessels was substantial in 16 cases. Major morbidity included ureteral, nerve, and intestinal injury; arterial and venous thrombosis; compartment syndrome; and suturing of the wrong vessel.
Some patients also experienced edema or pain in an extremity (vascular insufficiency); infection; diffuse intravascular coagulation and/or adult respiratory distress; cardiac arrest; central nervous system injury (stroke); or hospitalization of more than 1 week. Cases also were categorized as early or late diagnosis, depending on whether shock had supervened. Diagnosis was early in 8 cases (26%) and late in 21 (68%). Two patients were diagnosed postoperatively; ie, they had gone to the recovery room prior to developing shock.
The volume of blood loss ranged from 1,000 mL to 7,000 mL, with a mean loss of 3,400 mL. All patients received packed red blood cells and/or a mixture of other blood products. The time required for cross-matching and receiving blood ranged from 10 to 120 minutes.
In all cases, a vascular or general surgeon was called to consult on the case.
Mapping vascular structures to ensure safe trocar entry
Knowing the distances between blood vessels and laparoscopic entry trocars is critical if injury is to be avoided. In pursuit of this goal, Hurd and colleagues14 performed a retrospective study involving women who had undergone magnetic resonance imaging or computed tomography scans of the abdomen. Investigators measured the distance between the lower abdominal wall and the aortic bifurcation in these women, who were all unanesthetized and in the supine position.
Distances increased with BMI
This occurred in the study by Hurd et al,14 as well as in a prospective study by Narendran and Baggish,15 who calculated body mass index in 101 consecutive women who were undergoing diagnostic or operative laparoscopy. These women were anesthetized, with pneumoperitoneum established and a laparoscope inserted; all were in the lithotomy position.
In this study, Narendran and Baggish measured the following distances from the entry trocar:
- perpendicular distance to aortic bifurcation,
- oblique distance to the right and left common iliac vessels,
- oblique distance to the superior margin of the bladder,
- perpendicular distance from the peritoneum to skin at the umbilicus (abdominal wall thickness), and
- oblique distance from the subumbilical peritoneal opening to the right and left common iliac vessels.
Wide range of BMIs
In the study by Narendran and Baggish, successful measurement panels were created for 99 of the 101 cases. Of these, 49 women had a BMI of less than 25 (normal), 29 had a BMI greater than 25 but less than 30 (overweight), and 21 had a BMI greater than 30 (obese).
A significant difference was observed in the perpendicular distance from the entry trocar to aortic bifurcation (TABLE 1). Specifically, as the BMI increased, so did the distance. The only other significant BMI-related increase was the abdominal wall thickness, which also varied directly with the BMI.
Other distances increase with height
The distance between the primary trocar and the iliac vessels and urinary bladder consistently increased with the patient’s height.
However, no significant change in distance between the great vessels and the primary trocar site occurred when the patient’s position changed from level to Trendelenburg.
Trocar insertion: Disposable devices require less force
Laparoscopic trocar thrusting is a dynamic process, and we observed that process in our study.15 When force is applied via trocar to the anterior abdominal wall, that structure is displaced toward the abdominal cavity in the direction of the posterior abdominal wall—even when countertraction is taken into consideration. The movement is more apparent in obese women because of greater elasticity created by the larger mass of properitoneal and subcutaneous fat. We measured the distortion and determined that the depression can be 5 cm or more.
In contrast, thin women have rigid, relatively unyielding anterior abdominal walls and therefore experience minimal displacement. In thin women, the greater risk is the shorter passive distance between the anterior abdominal wall and the great vessels.
Comparing force curves
We16 calculated the force required to thrust a disposable or reusable trocar through the anterior abdominal wall during actual laparoscopic surgery. We used a 25-lb compression load cell connected to the trocar by an Ultem handle, which could be sterilized between cases. A linear variable displacement transducer detected displacement, and the measuring apparata fed data into a computer. Ten women were randomized to a disposable trocar and 10 to a reusable device.
The mean thrusting force for disposable trocars was 10.2 lb versus 17.53 lb for the reusable device. The time to penetrate was likewise significantly shortened for disposable trocars: mean time of 3.54 seconds versus 11.64 seconds. Overall work tilted in favor of disposable trocars: 14.34 pound-seconds versus 103.88 pound-seconds.
The disposable trocar has the advantage for 2 reasons: its razor-sharp cutting edge and streamlined design.
FIGURE 1 shows typical force curves of disposable and reusable trocars.
FIGURE 1 Reusable trocar requires more force than disposable trocar
A considerable difference in force is required for insertion, depending on type of device, as this graph of typical force curves shows. The reusable trocar requires 18 to 20 lb of force over 12 seconds; the disposable, only 5 lb over 2 seconds.
Safe trocar insertion begins with pneumoperitoneum
McDougall et al17 demonstrated that adequate pneumoperitoneum lessens the force required to drive a trocar through the anterior abdominal wall. Although the differences were small, the forces required with an intraperitoneal gas pressure of 30 mm Hg were smaller than those required with a pressure of 15 mm Hg.17
Manufacturers of disposable trocars also recommend creating an adequate pneumoperitoneum prior to aiming and inserting the razor-sharp device. The goal is creating a carbon dioxide gas pocket large enough to permit rapid deployment of the “safety shield” after the trocar tip clears the properitoneal fat and peritoneal membrane.
Slow-motion video sequences of disposable trocar entry show the sharp trocar tip penetrating the parietal peritoneum of the anterior abdominal wall for 1 cm before the spring-loaded shield advances and locks over the blade. During this insertion, the anterior abdominal wall has an elastic reaction to the applied force; this reaction pushes it toward the posterior abdominal wall.
Direct insertions (ie, without adequate pneumoperitoneum) involve less space for the trocar’s safety shield to deploy. Thus, there is a greater risk of the armed trocar tip coming into direct contact with underlying viscera and blood vessels.
Delayed diagnosis
The earlier a major vessel incident can be diagnosed, the better for patient, physician, and hospital. Diagnosis after the onset of hypotension, tachycardia, or tachypnia constitutes “late” diagnosis. Dark venous blood pooling in the abdomen, bright red pulsatile blood emitting from a trocar sleeve, or a retroperitoneal hematoma lateral to the iliacs or at the level of the presacral space suggests major vessel injury. Signs of hypovolemic shock or sudden appearance of profound shock places the possibility of major vessel injury at the top of the differential diagnosis.
Relying on observation when a retroperitoneal hematoma develops
Unfortunately, with observation, the surgeon cannot determine the identity or nature of the damaged vessel, know whether the hematoma is expanding beyond the view of the laparoscope, or predict when the patient will go into shock.
Leaving an armed trocar in place in a vessel
Assuming that the trocar is plugging a hole and preventing hemorrhage is a recipe for disaster. The movement of the sharp device against a vessel wall is most likely to create greater trauma to the vessel. In the case of partial penetration, the device may cut the rest of the way through the vessel.
Laparoscopic exploration
Attempts to locate the injury via laparoscopy usually are unsuccessful, and laparoscopic attempts to sew up the injury limit accuracy and efficacy.
Use of the Pfannenstiel incision during emergency laparotomy
Unfortunately, in 1 study,13 27 of 31 women with vascular injuries received this incision. A vertical incision is preferred because it affords greater access and visibility.
Underestimating blood loss
In the case of a major vessel injury, underestimation of blood volume requirements can be fatal. In 1 study,13 19 of 31 women were under-transfused and/or inadequately cross-matched.
Clamping injured vessels
This can lead to arterial or venous thrombosis. Nonvascular clamps can tear large vessels, adding to the damage and complicating the vascular surgeon’s attempt at repair. Rather, apply direct pressure with a sponge stick.
Delay in calling for help
This translates into greater blood loss and a less stable patient. In 1 study,13 the mean time for a vascular surgeon to intercede was 23 minutes.
Use a shorter insufflation needle
Our data on women with a BMI greater than 30 (obese range) indicate that the mean thickness of the anterior abdominal wall is 5.05 cm and the distance to the aorta is 15.14 cm.15 A standard Verres needle measures 12.5 cm from the tip of the shaft to the point where the shaft joins the hub of the needle. This is clearly excessive length, since women with a BMI above 30 have an abdominal wall thickness of approximately 5.05 cm and women with BMIs between 25 and 30 have a thickness of only 3.85 cm.
I prefer a Touhy epidural needle for subumbilical insertion and creation of the pneumoperitoneum, since it is a relatively short 8.5 cm. Thus, it is less hazardous than the Verres needle. It also is less likely to clog with tissue fragments because of its curved tip, and more likely to create a successful pneumoperitoneum on the first try.
Fortunately, large-vessel injuries caused by the insufflation needle are rare.
Proper insertion technique
I have residents draw a straight line with a marking pen from the lower margin of the umbilicus to the superior margin of the pubic symphysis. This serves as a guide to keep the trocar pointing toward the middle of the abdomen, away from the iliac vessels. I also teach residents to thrust the trocar in the midline at a 45° to 60° angle in relation to the plane of the abdominal wall, with the trocar pointing toward the uterus (FIGURES 2 AND 3).
Many residents twist disposable trocars during insertion. This “door knob” movement works against the design of the trocar and traumatizes tissue. The correct approach is thrusting the device into the abdominal cavity, or holding the trocar (only for disposable trocar devices) like a dart and thrusting it into the abdomen as though throwing a dart. The only trocar designed for twisting is the conical reusable device; the sharp pyramidal reusable trocar should be thrust rather than twisted.
Avoid “long” trocars
These are a full 5 cm longer than the 20-cm standard device (hub of handle to tip of shaft). Abdominal wall measurements indicate that these devices are never required to simply penetrate the anterior abdominal wall; these trocars also carry the risk of hitting the iliac vessels.
Open laparoscopy is not foolproof
Although open laparoscopy would seem to guarantee safe entry of the primary trocar, reports of aortic injuries have recently been published. Similar data have been reported for optical access trocars.11,18
FIGURE 2 Insert the trocar at 45° to 60° angle
At insertion, the trocar should be at a 45°to 60°angle relative to the abdominal wall, with the tip of the device tilted in the direction of the uterus and bladder. A 90°angle of insertion is dangerous.
FIGURE 3 Midline insertion is safest
Insert the primary trocar in the midline pointing toward the uterus; deviation to the right or left is dangerous. Also avoid injuring the inferior epigastric and external iliac vessels with far lateral trocar insertion.
Body habitus and vascular injury
The obese patient of short stature is at the greatest risk for vascular injury. Although the relative distances between the anterior abdominal wall and the aorta are greater at the highest BMI levels, short stature means that the iliac vessels are closer. Significantly, large vessel injuries in the series cited herein were associated with the use of disposable trocars 90% of the time.
I believe high-risk conditions are created when carbon dioxide gas is inadvertently infused into the properitoneal fat space (FIGURE 4). As the volume of gas grows, the anterior wall parietal peritoneum dissects free from the remainder of the anterior abdominal fat, creating a pseudo-pneumoperitoneum. The operator fails to realize that the true peritoneal cavity has not been entered and, in fact, has paradoxically constricted in size because of the enlarging pseudoperitoneal space. Careful attention to the pressure gauges would have aroused suspicion that gas was being infused into the wrong space, since pressures tend to be higher and flow erratic in such situations.
Nevertheless, the surgeon places a trocar into the space, looks through the laparoscope, sees red or yellow, and realizes that the peritoneal cavity has not been entered. More gas is insufflated and the trocar is tried again.
Typically, the duller, reusable trocar pushes the leading edge of the peritoneum rather than penetrating it, further enlarging the properitoneal space and bringing the anterior and posterior peritoneal walls very close together.
In another scenario, the same set of circumstances exists except, rather than employing a reusable trocar, the surgeon selects a disposable device or even, after 2 failures to enter the peritoneal cavity with the reusable device, an extra-long (11-inch) disposable trocar (FIGURE 4).
In this scenario, an armed trocar enters the pseudospace—without the safety shield deployed—because no resistance was encountered during penetration of the incision, owing to the fact that two 10–12-mm trocars have previously traversed the same skin incision.
As the tip of the trocar comes into contact with the leading edge of the peritoneum, it encounters resistance, and the razor-sharp blade cuts through the anterior peritoneum, traverses the narrow peritoneal space, and cuts through the posterior peritoneum and the underlying great vessel.
Often, the trocar’s knife edge injures an artery by glancing off the curved surface of the vessels and embedding itself in the neighboring or underlying vein.
The best technique to manage a pseudo-pneumoperitoneal pocket is to abandon the subumbilical site, insert a Touhy needle in the left upper quadrant, and enter and overinflate the peritoneal cavity, thereby obliterating the properitoneal gas space.
When injury occurs: 7 recommended management steps
In the event of a vascular injury, early diagnosis and treatment are vital. Do not observe retroperitoneal hematomas. The following steps are recommended:
- Call for a vascular surgeon immediately and indicate that the situation is an emergency. Do not waste time trying to locate the injury before calling for help.
- Get emergency type and cross-match for at least 6 U of whole blood.
- Obtain baseline lab measurements, including hemoglobin, hematocrit, platelets, fibrinogen, and fibrin split products.
- Open the abdomen using a vertical incision for maximum access and visibility.
- Get accurate outputs and blood-loss estimates and have anesthesia keep careful records of fluids given.
- Advise anesthesia staff to obtain additional help. This will facilitate starting additional IV sites, rapidly infusing blood products, obtaining key samples for laboratory data, and maintaining accurate and detailed records of blood gases, blood loss, replacement fluids, and blood products.
- Use a circulator to manage urgent medications or laboratory tests.
The author reports no financial relationships relevant to this article.
- Distances between the entry trocar and the aorta bifurcation increase directly with body mass index, mainly because of the commensurate increase in abdominal wall thickness.
- The mean thrusting force for insertion of a disposable trocar is 10.2 lb versus 17.53 lb for a reusable device, and the time to penetrate is shorter for the disposable trocar: mean of 3.54 seconds versus 11.64 seconds. Thus, greater caution is warranted when inserting a disposable trocar.
- Thrust the primary trocar into the midline of the abdomen at a 45° to 60° angle relative to the plane of the abdominal wall, with the trocar pointing toward the uterus, to avoid injuring the iliac vessels.
- When injury occurs, call for a vascular surgeon immediately, perform a laparotomy using a vertical incision, and get accurate inputs, outputs, and blood-loss estimates.
Major vessel injury is a two-sided coin: It can occur with alarming speed, but it is preventable.
Fortunately, the laparoscopic surgeon can avoid the problem by following simple precautions and steering clear of scenarios that increase the risk of injury. This article tells how to accomplish both objectives.
In the process, it reviews the evidence, details management for any injuries that occur, and includes a comprehensive table listing typical distances between the entry trocar and vascular structures, to help the surgeon adjust entry strategy.
Adequate prevention depends on:
- familiarity with the vascular anatomy, particularly in relation to the umbilicus, presacral space, infundibulopelvic ligament, and ovarian fossa.
- creating a proper pneumoperitoneum, especially when using disposable trocars.
- careful attention to primary trocar thrusting techniques to ensure midline insertion at the proper angle. Also exercise caution when placing secondary trocars. Specifically, during far lateral insertion, avoid cleaving the inferior epigastric artery from the external iliac or directly hitting the external artery or vein.
- avoiding long trocars, which are unnecessary to penetrate the peritoneal cavity.
- reliance on laparotomy if trocar insertion proves too difficult, vision is obscured, or appropriate anatomic dissection planes cannot be developed.
- when injury occurs, performing laparotomy using a vertical incision.
A 36-year-old woman with a body mass index of 38.2, indicating severe obesity, is scheduled to undergo hysteroscopy and dilatation and curettage for irregular bleeding, as well as laparoscopic bilateral partial salpingectomy for elective sterilization. The setting is an outpatient surgery center without a blood bank.
After general anesthesia, the surgeon makes a 1.5-cm incision just below the umbilicus, inserts a Verres needle, and insufflates carbon dioxide gas to a volume of approximately 3.4 L. He then inserts a disposable trocar and places a laparoscope, but views fat. Unbeknownst to him, he has insufflated the properitoneal fat space rather than the peritoneum.
The surgeon finally enters the peritoneum with a “long” trocar after several more attempts. Since the uterus and adnexa appear to be normal, he inserts a second trocar and places a probe. As he is moving the intestines, however, he observes blood, and the field suddenly becomes unclear. He removes the probe and, when the gas-pressure valve of the secondary trocar is opened, blood spews from the site.
The surgeon removes all trocars and performs an emergency laparotomy using a Pfannenstiel incision. He and 2 general surgeons, who arrive within 20 to 30 minutes, work for 2 hours to repair what they believe is a hole in the inferior vena cava. The woman is brought out of anesthesia and transferred to the local community hospital, where she goes into cardiac arrest and dies. A postmortem reveals injury to the right common iliac artery and vein. No sutures were observed in either vessel. Cause of death: exsanguination.
What went wrong?
Three serious errors contributed to the patient’s death:
- He made multiple attempts to insert the trocar without considering the possibility that the wrong space had been insufflated.
- He inserted the trocar off the midline and at the wrong angle relative to the abdominal wall.
- In his frustration, he switched to a “long” trocar, which made it more likely that vascular structures would be injured.
Operating on an obese patient in a center without a blood bank also was unwise, as obese women of short stature are at greatest risk for vascular injury.
How big is the problem?
A French study1 of 103,852 laparoscopic procedures—of which 15.7%, or 16,000 operations, were gynecologic—reported 47 cases of major vascular injury for an incidence of 0.5 per 1,000 cases and a mortality rate of 17%. Several additional articles2-8 reported a range of vascular complications of between 0.1 and 6.4 per 1,000 laparoscopies.
In a study9 conducted in 7 gynecologic laparoscopy surgery centers in France over 9 years and involving 29,966 diagnostic and operative cases, the overall complication rate was 4.64 per 1,000 laparoscopies (n = 139). Of the 21 major vascular injuries associated with gynecologic surgery, the majority occurred during set-up, and 84.6% during insertion of the primary trocar. Two patients died from their injuries.
Bhoyrul and colleagues10 analyzed data reported to the US Food and Drug Administration and found that 408 of 629 trocar-related injuries involved major blood vessels, as did 26 of 32 deaths (81%). Most of the deaths (87%) were linked to the use of disposable trocars equipped with safety shields; 9% with direct-view trocars. Although surgeons asserted that the trocar malfunctioned in 41 cases, that claim was confirmed in only 1 case (2%).
Another study found that 37 of 79 (46.8%) serious complications involving optical-access trocars between 1994 and 2002 involved major vessels, injuring the aorta, iliac vessels, or vena cava.11
A study12 carried out in the Netherlands in 1994 evaluated the relative number of complications that occurred within a total of 25,764 laparoscopic procedures. The study divided complications into those occurring as the result of the laparoscopic approach (eg, trocar insertion) versus those happening during the performance of the operation. Fifty-seven percent of the 145 complications were caused by the laparoscopic approach; the 2 reported deaths also were secondary to that approach.
Snapshot of vascular injury: A series of 31 patients
In 2003, I published data13 on 31 cases of major vessel injury associated with gynecologic laparoscopy (see). These cases were collected from a variety of sources: medicolegal case files, hospital morbidity-mortality presentations, and quality-assurance departments. Eight cases involved diagnostic procedures, while 23 involved operative laparoscopy.
The medical records of these cases provided details on the nature of the injury. The cases were categorized by body mass index (BMI) and cause, ie, whether they occurred as the result of the laparoscopic approach (ie, entry-related) or arose during surgery.
Of the 31 cases, 22 (71%) involved women with BMIs from 25 to more than 30 (overweight or obese). A large majority—28 cases (90%)—were related to entry. Only 3 injuries occurred during surgery.
In several women, more than 1 vessel was damaged. Of the 49 total injuries, 38 (78%) involved the iliac vessels. Seven (23%) women died as a result of their injuries, all of which involved venous trauma.
Damage to structures in the vicinity of the injured vessels was substantial in 16 cases. Major morbidity included ureteral, nerve, and intestinal injury; arterial and venous thrombosis; compartment syndrome; and suturing of the wrong vessel.
Some patients also experienced edema or pain in an extremity (vascular insufficiency); infection; diffuse intravascular coagulation and/or adult respiratory distress; cardiac arrest; central nervous system injury (stroke); or hospitalization of more than 1 week. Cases also were categorized as early or late diagnosis, depending on whether shock had supervened. Diagnosis was early in 8 cases (26%) and late in 21 (68%). Two patients were diagnosed postoperatively; ie, they had gone to the recovery room prior to developing shock.
The volume of blood loss ranged from 1,000 mL to 7,000 mL, with a mean loss of 3,400 mL. All patients received packed red blood cells and/or a mixture of other blood products. The time required for cross-matching and receiving blood ranged from 10 to 120 minutes.
In all cases, a vascular or general surgeon was called to consult on the case.
Mapping vascular structures to ensure safe trocar entry
Knowing the distances between blood vessels and laparoscopic entry trocars is critical if injury is to be avoided. In pursuit of this goal, Hurd and colleagues14 performed a retrospective study involving women who had undergone magnetic resonance imaging or computed tomography scans of the abdomen. Investigators measured the distance between the lower abdominal wall and the aortic bifurcation in these women, who were all unanesthetized and in the supine position.
Distances increased with BMI
This occurred in the study by Hurd et al,14 as well as in a prospective study by Narendran and Baggish,15 who calculated body mass index in 101 consecutive women who were undergoing diagnostic or operative laparoscopy. These women were anesthetized, with pneumoperitoneum established and a laparoscope inserted; all were in the lithotomy position.
In this study, Narendran and Baggish measured the following distances from the entry trocar:
- perpendicular distance to aortic bifurcation,
- oblique distance to the right and left common iliac vessels,
- oblique distance to the superior margin of the bladder,
- perpendicular distance from the peritoneum to skin at the umbilicus (abdominal wall thickness), and
- oblique distance from the subumbilical peritoneal opening to the right and left common iliac vessels.
Wide range of BMIs
In the study by Narendran and Baggish, successful measurement panels were created for 99 of the 101 cases. Of these, 49 women had a BMI of less than 25 (normal), 29 had a BMI greater than 25 but less than 30 (overweight), and 21 had a BMI greater than 30 (obese).
A significant difference was observed in the perpendicular distance from the entry trocar to aortic bifurcation (TABLE 1). Specifically, as the BMI increased, so did the distance. The only other significant BMI-related increase was the abdominal wall thickness, which also varied directly with the BMI.
Other distances increase with height
The distance between the primary trocar and the iliac vessels and urinary bladder consistently increased with the patient’s height.
However, no significant change in distance between the great vessels and the primary trocar site occurred when the patient’s position changed from level to Trendelenburg.
Trocar insertion: Disposable devices require less force
Laparoscopic trocar thrusting is a dynamic process, and we observed that process in our study.15 When force is applied via trocar to the anterior abdominal wall, that structure is displaced toward the abdominal cavity in the direction of the posterior abdominal wall—even when countertraction is taken into consideration. The movement is more apparent in obese women because of greater elasticity created by the larger mass of properitoneal and subcutaneous fat. We measured the distortion and determined that the depression can be 5 cm or more.
In contrast, thin women have rigid, relatively unyielding anterior abdominal walls and therefore experience minimal displacement. In thin women, the greater risk is the shorter passive distance between the anterior abdominal wall and the great vessels.
Comparing force curves
We16 calculated the force required to thrust a disposable or reusable trocar through the anterior abdominal wall during actual laparoscopic surgery. We used a 25-lb compression load cell connected to the trocar by an Ultem handle, which could be sterilized between cases. A linear variable displacement transducer detected displacement, and the measuring apparata fed data into a computer. Ten women were randomized to a disposable trocar and 10 to a reusable device.
The mean thrusting force for disposable trocars was 10.2 lb versus 17.53 lb for the reusable device. The time to penetrate was likewise significantly shortened for disposable trocars: mean time of 3.54 seconds versus 11.64 seconds. Overall work tilted in favor of disposable trocars: 14.34 pound-seconds versus 103.88 pound-seconds.
The disposable trocar has the advantage for 2 reasons: its razor-sharp cutting edge and streamlined design.
FIGURE 1 shows typical force curves of disposable and reusable trocars.
FIGURE 1 Reusable trocar requires more force than disposable trocar
A considerable difference in force is required for insertion, depending on type of device, as this graph of typical force curves shows. The reusable trocar requires 18 to 20 lb of force over 12 seconds; the disposable, only 5 lb over 2 seconds.
Safe trocar insertion begins with pneumoperitoneum
McDougall et al17 demonstrated that adequate pneumoperitoneum lessens the force required to drive a trocar through the anterior abdominal wall. Although the differences were small, the forces required with an intraperitoneal gas pressure of 30 mm Hg were smaller than those required with a pressure of 15 mm Hg.17
Manufacturers of disposable trocars also recommend creating an adequate pneumoperitoneum prior to aiming and inserting the razor-sharp device. The goal is creating a carbon dioxide gas pocket large enough to permit rapid deployment of the “safety shield” after the trocar tip clears the properitoneal fat and peritoneal membrane.
Slow-motion video sequences of disposable trocar entry show the sharp trocar tip penetrating the parietal peritoneum of the anterior abdominal wall for 1 cm before the spring-loaded shield advances and locks over the blade. During this insertion, the anterior abdominal wall has an elastic reaction to the applied force; this reaction pushes it toward the posterior abdominal wall.
Direct insertions (ie, without adequate pneumoperitoneum) involve less space for the trocar’s safety shield to deploy. Thus, there is a greater risk of the armed trocar tip coming into direct contact with underlying viscera and blood vessels.
Delayed diagnosis
The earlier a major vessel incident can be diagnosed, the better for patient, physician, and hospital. Diagnosis after the onset of hypotension, tachycardia, or tachypnia constitutes “late” diagnosis. Dark venous blood pooling in the abdomen, bright red pulsatile blood emitting from a trocar sleeve, or a retroperitoneal hematoma lateral to the iliacs or at the level of the presacral space suggests major vessel injury. Signs of hypovolemic shock or sudden appearance of profound shock places the possibility of major vessel injury at the top of the differential diagnosis.
Relying on observation when a retroperitoneal hematoma develops
Unfortunately, with observation, the surgeon cannot determine the identity or nature of the damaged vessel, know whether the hematoma is expanding beyond the view of the laparoscope, or predict when the patient will go into shock.
Leaving an armed trocar in place in a vessel
Assuming that the trocar is plugging a hole and preventing hemorrhage is a recipe for disaster. The movement of the sharp device against a vessel wall is most likely to create greater trauma to the vessel. In the case of partial penetration, the device may cut the rest of the way through the vessel.
Laparoscopic exploration
Attempts to locate the injury via laparoscopy usually are unsuccessful, and laparoscopic attempts to sew up the injury limit accuracy and efficacy.
Use of the Pfannenstiel incision during emergency laparotomy
Unfortunately, in 1 study,13 27 of 31 women with vascular injuries received this incision. A vertical incision is preferred because it affords greater access and visibility.
Underestimating blood loss
In the case of a major vessel injury, underestimation of blood volume requirements can be fatal. In 1 study,13 19 of 31 women were under-transfused and/or inadequately cross-matched.
Clamping injured vessels
This can lead to arterial or venous thrombosis. Nonvascular clamps can tear large vessels, adding to the damage and complicating the vascular surgeon’s attempt at repair. Rather, apply direct pressure with a sponge stick.
Delay in calling for help
This translates into greater blood loss and a less stable patient. In 1 study,13 the mean time for a vascular surgeon to intercede was 23 minutes.
Use a shorter insufflation needle
Our data on women with a BMI greater than 30 (obese range) indicate that the mean thickness of the anterior abdominal wall is 5.05 cm and the distance to the aorta is 15.14 cm.15 A standard Verres needle measures 12.5 cm from the tip of the shaft to the point where the shaft joins the hub of the needle. This is clearly excessive length, since women with a BMI above 30 have an abdominal wall thickness of approximately 5.05 cm and women with BMIs between 25 and 30 have a thickness of only 3.85 cm.
I prefer a Touhy epidural needle for subumbilical insertion and creation of the pneumoperitoneum, since it is a relatively short 8.5 cm. Thus, it is less hazardous than the Verres needle. It also is less likely to clog with tissue fragments because of its curved tip, and more likely to create a successful pneumoperitoneum on the first try.
Fortunately, large-vessel injuries caused by the insufflation needle are rare.
Proper insertion technique
I have residents draw a straight line with a marking pen from the lower margin of the umbilicus to the superior margin of the pubic symphysis. This serves as a guide to keep the trocar pointing toward the middle of the abdomen, away from the iliac vessels. I also teach residents to thrust the trocar in the midline at a 45° to 60° angle in relation to the plane of the abdominal wall, with the trocar pointing toward the uterus (FIGURES 2 AND 3).
Many residents twist disposable trocars during insertion. This “door knob” movement works against the design of the trocar and traumatizes tissue. The correct approach is thrusting the device into the abdominal cavity, or holding the trocar (only for disposable trocar devices) like a dart and thrusting it into the abdomen as though throwing a dart. The only trocar designed for twisting is the conical reusable device; the sharp pyramidal reusable trocar should be thrust rather than twisted.
Avoid “long” trocars
These are a full 5 cm longer than the 20-cm standard device (hub of handle to tip of shaft). Abdominal wall measurements indicate that these devices are never required to simply penetrate the anterior abdominal wall; these trocars also carry the risk of hitting the iliac vessels.
Open laparoscopy is not foolproof
Although open laparoscopy would seem to guarantee safe entry of the primary trocar, reports of aortic injuries have recently been published. Similar data have been reported for optical access trocars.11,18
FIGURE 2 Insert the trocar at 45° to 60° angle
At insertion, the trocar should be at a 45°to 60°angle relative to the abdominal wall, with the tip of the device tilted in the direction of the uterus and bladder. A 90°angle of insertion is dangerous.
FIGURE 3 Midline insertion is safest
Insert the primary trocar in the midline pointing toward the uterus; deviation to the right or left is dangerous. Also avoid injuring the inferior epigastric and external iliac vessels with far lateral trocar insertion.
Body habitus and vascular injury
The obese patient of short stature is at the greatest risk for vascular injury. Although the relative distances between the anterior abdominal wall and the aorta are greater at the highest BMI levels, short stature means that the iliac vessels are closer. Significantly, large vessel injuries in the series cited herein were associated with the use of disposable trocars 90% of the time.
I believe high-risk conditions are created when carbon dioxide gas is inadvertently infused into the properitoneal fat space (FIGURE 4). As the volume of gas grows, the anterior wall parietal peritoneum dissects free from the remainder of the anterior abdominal fat, creating a pseudo-pneumoperitoneum. The operator fails to realize that the true peritoneal cavity has not been entered and, in fact, has paradoxically constricted in size because of the enlarging pseudoperitoneal space. Careful attention to the pressure gauges would have aroused suspicion that gas was being infused into the wrong space, since pressures tend to be higher and flow erratic in such situations.
Nevertheless, the surgeon places a trocar into the space, looks through the laparoscope, sees red or yellow, and realizes that the peritoneal cavity has not been entered. More gas is insufflated and the trocar is tried again.
Typically, the duller, reusable trocar pushes the leading edge of the peritoneum rather than penetrating it, further enlarging the properitoneal space and bringing the anterior and posterior peritoneal walls very close together.
In another scenario, the same set of circumstances exists except, rather than employing a reusable trocar, the surgeon selects a disposable device or even, after 2 failures to enter the peritoneal cavity with the reusable device, an extra-long (11-inch) disposable trocar (FIGURE 4).
In this scenario, an armed trocar enters the pseudospace—without the safety shield deployed—because no resistance was encountered during penetration of the incision, owing to the fact that two 10–12-mm trocars have previously traversed the same skin incision.
As the tip of the trocar comes into contact with the leading edge of the peritoneum, it encounters resistance, and the razor-sharp blade cuts through the anterior peritoneum, traverses the narrow peritoneal space, and cuts through the posterior peritoneum and the underlying great vessel.
Often, the trocar’s knife edge injures an artery by glancing off the curved surface of the vessels and embedding itself in the neighboring or underlying vein.
The best technique to manage a pseudo-pneumoperitoneal pocket is to abandon the subumbilical site, insert a Touhy needle in the left upper quadrant, and enter and overinflate the peritoneal cavity, thereby obliterating the properitoneal gas space.
When injury occurs: 7 recommended management steps
In the event of a vascular injury, early diagnosis and treatment are vital. Do not observe retroperitoneal hematomas. The following steps are recommended:
- Call for a vascular surgeon immediately and indicate that the situation is an emergency. Do not waste time trying to locate the injury before calling for help.
- Get emergency type and cross-match for at least 6 U of whole blood.
- Obtain baseline lab measurements, including hemoglobin, hematocrit, platelets, fibrinogen, and fibrin split products.
- Open the abdomen using a vertical incision for maximum access and visibility.
- Get accurate outputs and blood-loss estimates and have anesthesia keep careful records of fluids given.
- Advise anesthesia staff to obtain additional help. This will facilitate starting additional IV sites, rapidly infusing blood products, obtaining key samples for laboratory data, and maintaining accurate and detailed records of blood gases, blood loss, replacement fluids, and blood products.
- Use a circulator to manage urgent medications or laboratory tests.
The author reports no financial relationships relevant to this article.
1. Champault G, Cazacu F, Taffinader N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367.
2. Baadsgaard SE, Bille S, Egelblad K. Major vascular injury during gynecologic laparoscopy: report of a case and review of published cases. Acta Obstet Gynecol Scand. 1989;68:283.
3. Chamberlain G, Brown JD. Gynecologic laparoscopy: report of the working party of the confidential enquiry into gynecologic laparoscopy. Br J Obstet Gynaecol. 1978;85:401.
4. Mintz M. Risk and prophylaxis in laparoscopy: a survey of 100,000 cases. J Reprod Med. 1977;18:269.
5. Phillips JM, Hulka JF, Peterson HB. American Association of Gynecologic Laparoscopists’ 1982 Membership Survey. J Reprod Med. 1984;29:592.
6. Saidi MH, Vancaillie TG, White AJ, et al. Complications of major operative laparoscopy: a review of 452 cases. J Reprod Med. 1996;41:471.
7. Loffer F, Pent D. Indications, contraindications, and complications of laparoscopy. Obstet Gynecol Surv. 1975;30:407.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108.
9. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynecologic laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
10. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
11. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
12. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Herman J, Trimbos JB. Complications of laparoscopy: a prospective multi-center observational study. Br J Obstet Gynaecol. 1997;104:595-600.
13. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
14. Hurd WW, Bude RO, DeLancey JOL, Gauvin JM, Aisen AM. Abdominal wall characterization by MRI and CT imaging: the effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473.
15. Narendran M, Baggish MS. Mean distance between primary trocar insertion site and major retroperitoneal vessels during routine laparoscopy. J Gynecol Surg. 2002;18:121-127.
16. Baggish MS, Gandhi S, Kasper G. Force required by laparoscopic trocar devices to penetrate the human female’s anterior abdominal wall. J Gynecol Surg. 2003;19:1-11.
17. McDougall EM, Figenshau RS, Clayman RV, Monk TG, Smith DS. Laparoscopic pneumoperitoneum: impact of body habitus. J Laparosc Endosc Surg. 1994;4:385-391.
18. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of Hassan cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
1. Champault G, Cazacu F, Taffinader N. Serious trocar accidents in laparoscopic surgery: a French survey of 103,852 operations. Surg Laparosc Endosc. 1996;6:367.
2. Baadsgaard SE, Bille S, Egelblad K. Major vascular injury during gynecologic laparoscopy: report of a case and review of published cases. Acta Obstet Gynecol Scand. 1989;68:283.
3. Chamberlain G, Brown JD. Gynecologic laparoscopy: report of the working party of the confidential enquiry into gynecologic laparoscopy. Br J Obstet Gynaecol. 1978;85:401.
4. Mintz M. Risk and prophylaxis in laparoscopy: a survey of 100,000 cases. J Reprod Med. 1977;18:269.
5. Phillips JM, Hulka JF, Peterson HB. American Association of Gynecologic Laparoscopists’ 1982 Membership Survey. J Reprod Med. 1984;29:592.
6. Saidi MH, Vancaillie TG, White AJ, et al. Complications of major operative laparoscopy: a review of 452 cases. J Reprod Med. 1996;41:471.
7. Loffer F, Pent D. Indications, contraindications, and complications of laparoscopy. Obstet Gynecol Surv. 1975;30:407.
8. Härkki-Sirén P, Kurki T. A nationwide analysis of laparoscopic complications. Obstet Gynecol. 1997;89:108.
9. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynecologic laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
10. Bhoyrul S, Vierra MA, Nezhat CR, Krummel TM, Way LW. Trocar injuries in laparoscopic surgery. J Am Coll Surg. 2001;192:677-683.
11. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette RC, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
12. Jansen FW, Kapiteyn K, Trimbos-Kemper T, Herman J, Trimbos JB. Complications of laparoscopy: a prospective multi-center observational study. Br J Obstet Gynaecol. 1997;104:595-600.
13. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
14. Hurd WW, Bude RO, DeLancey JOL, Gauvin JM, Aisen AM. Abdominal wall characterization by MRI and CT imaging: the effect of obesity on laparoscopic approach. J Reprod Med. 1991;36:473.
15. Narendran M, Baggish MS. Mean distance between primary trocar insertion site and major retroperitoneal vessels during routine laparoscopy. J Gynecol Surg. 2002;18:121-127.
16. Baggish MS, Gandhi S, Kasper G. Force required by laparoscopic trocar devices to penetrate the human female’s anterior abdominal wall. J Gynecol Surg. 2003;19:1-11.
17. McDougall EM, Figenshau RS, Clayman RV, Monk TG, Smith DS. Laparoscopic pneumoperitoneum: impact of body habitus. J Laparosc Endosc Surg. 1994;4:385-391.
18. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of Hassan cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.