User login
CASE: Depressed, irritable — and smoking
Michael, age 16, is admitted to a psychiatric unit for severe depressive symptoms and suicidal ideation. The next day, he is irritable and refuses to cooperate with the interview. During group therapy he is distractible and unable to focus. The treating psychiatrist learns that before admission Michael had been smoking 10 to 15 cigarettes per day and now feels a strong craving for cigarettes.
Unrecognized nicotine dependence can be problematic on inpatient psychiatric units, where adolescents such as Michael are not permitted to smoke and rarely are offered nicotine replacement therapy (NRT). Unfortunately, psychiatrists seldom diagnose and treat nicotine dependence—particularly in adolescents—whether in outpatient or inpatient settings.1,2
Do adolescent smokers need help quitting? Do they experience withdrawal symptoms when they stop smoking? Are pharmacologic interventions appropriate? For each question, the answer is a resounding yes.
To help you treat young smokers, this article offers:
- tools for assessing adolescent tobacco use and dependence
- evidence-based treatment options
- an algorithm to guide treatment choice.
Not just a ‘phase’
Early smoking—especially among those younger than age 13—is associated with adolescent psychopathology, including depressive disorders and other substance use disorders.3 Compared with nonsmoking teens, those who smoke at least monthly are significantly more likely to smoke as adults.4 Among high school seniors:
- >20% report smoking cigarettes in the past 30 days
- 12% smoke daily
- 6% smoke ≥10 cigarettes per day.5
Nicotine dependence can develop very rapidly: nearly 25% of adolescents have ≥1 symptom within 2 weeks of starting to smoke at least once a month.6
Role of parents. Early intervention for teen nicotine addiction is particularly important because of the long-term health risks associated with tobacco use.7 In our experience, however, teen smokers’ parents’ attitudes can make addressing adolescent nicotine dependence a therapeutic challenge.
Modified Fagerstrom Tolerance Questionnaire8 (7 items)
Stanford Dependence Inventory (SDI)9 (5 items)
Hooked on Nicotine Checklist (HONC)10 (10 items)
Nicotine Dependence Scale for Adolescents11 (6 items)
Cigarette Dependence Scale (CDS-5 and CDS-12)12 (5 or 12 items)
Parents may be unaware of their teens’ smoking, and those who are aware may:
- not know what help is available
- dismiss teen smoking as “just a phase”
- feel that smoking cigarettes is preferable to smoking marijuana or using other illicit drugs.
Other parents have no objections because they themselves smoke. Some permit their teens to smoke and may even give them cigarettes.
Parents who want their teens to stop smoking often believe erroneously that the best method is to quit “cold turkey.”
Assessing use and dependence
Teen smokers’ nicotine withdrawal symptoms—such as irritability, anxiety, and impaired concentration—can imitate or exacerbate other psychiatric symptoms, thus complicating diagnosis and treatment. Ask all adolescent patients about the quantity, frequency, pattern, and duration of use of all forms of tobacco, including:
- cigarettes
- cigars
- cigarillos (short, narrow cigars)
- bidis (thin, flavored South Asian cigarettes wrapped in leaf)
- smokeless tobacco.
Dependence. Establishing nicotine dependence in young smokers is more complicated than in adults because of teen smokers’ variable smoking patterns. Several self-rating scales have been developed to assess nicotine dependence in adolescents (Box 1).8-12 Although some of these tools have been used primarily in research, outpatient psychiatrists may find these scales useful for evaluating adolescents’ smoking.
Some DSM-IV-TR criteria for substance dependence may not apply to nicotine dependence or correlate with other validated measures of nicotine dependence. For example, “significant time spent obtaining, using, or recovering from the effects of a substance” might not apply to all adolescent smokers.13 Based on our clinical experience, daily smoking for an extended period of time (several months) is a marker of dependence for almost all adolescents.
The Timeline Follow Back method can help you capture a more complete picture of adolescent tobacco use over time.14 This involves asking teens about tobacco use over the past 30 or 90 days, beginning with the assessment day and working backward. Record tobacco use on a calendar, using holidays, weekends, and events as anchor points to help teens recall their smoking.
Biomarker tests can be used to measure nicotine use. The 2 most common are:
- expired carbon monoxide (CO) level (essentially a “breathalyzer” for smoking)
- cotinine level—a metabolite of nicotine.
Expired CO testing is simple to conduct but requires specialized equipment that costs approximately $1,000. Marijuana use may affect CO results, but NRT will not. Measuring CO levels provides information about cigarette smoking over the past several hours, compared with the past several days with cotinine.15
Cotinine can be tested in serum, saliva, or urine. Serum testing can be expensive and may require shipping samples to a specialized laboratory for processing. Testing saliva or urine is less expensive and may be conducted in an office. Cotinine testing in teens who use NRT may be unreliable because the nicotine in these products will be metabolized to cotinine and yield a positive result.
CASE CONTINUED: Wanting to quit
Michael was placed on a 21-mg transdermal nicotine patch, which greatly reduced his craving and irritability. He expressed an interest in quitting smoking. Given Michael’s depressive symptoms, bupropion SR was initiated to treat his depression and assist with smoking cessation.
Treatment options
Optimal smoking cessation treatment includes a combination of medication and behavioral counseling.16
Pharmacologic treatments. FDA-approved medications for adult smoking cessation include NRT—available as a gum, inhaler, nasal spray, lozenge, or transdermal patch—bupropion SR, and varenicline. Although not FDA-approved for patients younger than age 18, NRT and bupropion SR have been evaluated for smoking in adolescents.
NRT helps smokers by reducing nicotine withdrawal symptoms during cessation. Only nicotine gum and transdermal nicotine patch have been studied in adolescents. Results are modest at best, although in some studies including behavioral treatments may have obscured any medication effect (Table 1).17-20
Bupropion SR. How bupropion SR helps patients stop smoking is not completely clear. Three studies have evaluated bupropion SR in adolescents; 2 had positive results, but all 3 had important limitations (Table 2).21-23
One of the 2 positive studies included only 16 patients and had an open-label design.22 The second—a larger randomized, placebo-controlled trial23—found that bupropion SR improved nicotine abstinence compared with placebo at 6 weeks, but this effect did not last after subjects stopped taking the drug.
The third bupropion SR study used 150 mg/d (the recommend adult dose is 300 mg/d) and had poor medication adherence.21 The difference in abstinence rate compared with placebo was not statistically significant.
Other medications. Varenicline—a partial nicotine receptor agonist recently approved for adult smoking cessation—has not been studied in adolescents. Nortriptyline, doxepin, selegiline, clonidine, and mecamylamine have shown promise in adult smokers but are not approved for smoking cessation and require further study, especially in young smokers.24-27
Pharmacotherapy risks. NRT can cause nicotine overdose symptoms, such as rapid heart rate or nausea, especially if used while smoking. Transdermal NRT can cause a local reaction at the application site and can cause burns if worn while undergoing magnetic resonance imaging.28
Adverse effects associated with bupropion SR include a small risk of seizure, weight loss, and insomnia. This drug is contraindicated for patients who:
- have a seizure disorder
- have ever been diagnosed with bulimia or anorexia nervosa
- are taking other bupropion formulations.
Patients should not take bupropion SR during abrupt discontinuation of alcohol or sedatives or within 14 days of taking a monoamine oxidase inhibitor.29
Table 1
Can the nicotine patch help teens quit smoking?
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Smith et al, 199617 | 13- to 17-year-olds (N=22) who smoked ≥20 cpd | 8 weeks of open-label treatment with transdermal NRT plus behavioral counseling and group support | 14% at 8 weeks, 4.5% at 3 and 6 months |
| Hurt et al, 200418 | 13- to 17-year-olds (N=101) who smoked ≥10 cpd | 6 weeks of open-label treatment with transdermal NRT plus self-help material and brief individual counseling if requested | 11% at 6 weeks, 5% at 6 months |
| Hanson et al, 200319 | 13- to 19-year-olds (N=100) who smoked ≥10 cpd | 10 weeks of double-blind treatment with transdermal NRT or placebo plus CBT and contingency management | 20% (active) vs 18% (placebo); not statistically significant |
| Moolchan et al, 200520 | 13- to 17-year-olds (N=120) who smoked ≥10 cpd | 12 weeks of double-blind treatment with:
| 17.7% (active transdermal NRT)* vs 6.5% (active gum) vs 2.5% (placebo only) |
| *P=0.04 for transdermal NRT vs placebo | |||
| CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
Table 2
Teen smoking cessation: Evidence for bupropion SR
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Upadhyaya et al, 200421 | 12- to 19-year-olds (N=16, 11 of whom had ADHD) who smoked ≥5 cpd | 7 weeks of open-label treatment with bupropion SR, 150 mg bid, with brief smoking cessation counseling | 31.3% after 4 weeks of medication |
| Killen et al, 200422 | 15- to 18-year-olds (N=211) who smoked ≥10 cpd | 9 weeks of double-blind treatment with bupropion SR, 150 mg/d, or placebo; subjects received 8 weeks of transdermal NRT and group skills training | 23% (active) vs 28% (placebo) at 10 weeks; 8% (active) vs 7% placebo) at 26 weeks; not statistically significant |
| Muramoto et al, 200523 | 14- to 17-year-olds (N=312) who smoked ≥6 cpd | 6 weeks of double-blind treatment with bupropion SR, 150 mg/d; 150 mg bid; or placebo, with CBT and motivational enhancement | 16.9% (150 mg bid) vs 10.3% (150 mg/d) vs 6.7% (placebo) at 6 weeks* No differences at 26 weeks |
| ADHD: attention-deficit/hyperactivity disorder; CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
| *P=0.019 for 150 mg bid vs placebo | |||
Behavioral therapy. Specialized treatments developed specifically for teen smoking cessation—such as Not On Tobacco (see Related Resources)—often are delivered in schools or other group settings. The most successful consist of ≥5 sessions and include motivational enhancement, cognitive-behavioral, and social influence-oriented approaches.30
Other behavioral treatments. Most psychiatrists are not equipped to deliver these specialized behavioral treatments. Instead, you can use simple yet effective behavioral treatments during routine office visits as adjuncts to pharmacotherapy. At the very least, we recommend the U.S. Public Health Service’s “5 As” strategy (Box 2).16
Educate patients about what to expect during withdrawal, how long withdrawal will last, and medication side effects. To help adolescents develop appropriate treatment expectations:
- discuss the difference between a “slip” (having 1 cigarette) and a “relapse” (returning to daily smoking)
- explain that many individuals need multiple attempts before they quit.
Encourage adolescent smokers to contact the National Network of Tobacco Cessation Quitlines (1-800-784-8669; www.smokefree.gov), which provides free access to telephone-based counseling services.
Ask every patient about tobacco use during every visit, and have a system for recording and tracking tobacco use in the chart
Advise patients clearly and unambiguously to stop smoking, and tailor that advice to each patient’s needs
Assess every patient’s readiness to quit
Assist patients who are ready to quit through self-help materials, referral, and/or smoking cessation treatment
Arrange follow-up visits for relapse prevention or to reassess readiness to quit
Source: Reference 16
Choosing treatment
Evidence guiding treatment choice for teen smoking cessation is limited but growing. Most studies examined daily cigarette smoking, with significantly less evidence to support treatment decisions for light (non-daily) smokers and teens who use other tobacco products.
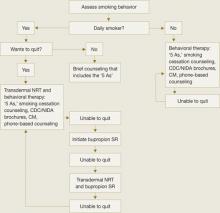
Recommendations.We have developed a strategy to guide treatment of adolescent smokers (Algorithm). We recommend using pharmacologic interventions only for teens who smoke daily because:
- most studies have focused on daily smoking
- efficacy data are limited
- pharmacologic interventions carry potential risks.
Because of bupropion SR’s contraindications and potential side effect profile, we suggest NRT in combination with smoking cessation counseling as a first-line treatment for young smokers. We recommend beginning with transdermal NRT because of the low likelihood of underdosing with the patch’s once-daily application.20 With either NRT or bupropion SR, schedule follow-up appointments to target relapse prevention and solve any issues that arise.
Help your patient choose a “quit date,” preferably 1 to 2 weeks after your initial assessment. We recommend encouraging young smokers to reduce their smoking by 1 cigarette per day to help minimize withdrawal symptoms from “cold turkey” cessation.31
Some physicians have found it helpful to see the patient on the quit day—or 2 days after when withdrawal symptoms tend to be most robust—to provide support and encouragement. Ask the adolescent to bring and discard during the visit all smoking paraphernalia as a symbol of his or her new smoke-free status.
Step 1: NRT. Initiate smoking cessation counseling plus transdermal NRT using the dosing guidelines in Table 3,28,31 and adjust the dose depending on severity of withdrawal symptoms. Ideally, the patch delivery will be used for 12 weeks, with at least 3 and ideally 6 weeks on the initial dose, followed by a gradual taper.28 We strongly recommend using transdermal NRT on adolescent inpatient units, especially for daily smokers and those who exhibit nicotine withdrawal symptoms.
Step 2: Bupropion SR. If NRT fails, the next step is bupropion SR plus smoking cessation counseling, assuming the adolescent does not have contraindications to bupropion SR. Start the medication based on the dosing guidelines in Table 3,28,31 and set a quit date for 2 weeks after starting bupropion SR.
Again, encourage adolescents to reduce by 1 cigarette per day over the 2 weeks before the quit day to minimize withdrawal symptoms.31 Continue bupropion SR for at least 8 and optimally 12 weeks.31
Combination therapy? If teens are unable to successfully quit smoking with NRT or bupropion SR alone, experience with adults suggests combining the 2 therapies might be beneficial. However, no evidence supports combination therapy in adolescents. Instead, consider referring adolescents who can’t quit to a smoking cessation specialist.
AlgorithmA strategy to initiate smoking cessation in adolescents
CDC: Centers for Disease Control and Prevention; CM: contingency management; NIDA: National Institute of Drug Abuse; NRT: nicotine replacement therapy
Source: Adapted from Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6 with permission of Lippincott Williams & Wilkins.Table 3
Dosing guidelines for adolescent smoking cessation therapy
| Medication | Recommended regimen |
|---|---|
| Bupropion SR31 | Patient weight ≥90 lbs: 150 mg once in the morning for 3 to 6 days, then 150 mg bid for 12 weeks Patient weight <90 lbs: Maximum 150 mg once in the morning, if tolerated |
| Transdermal NRT28 | Patient smokes ≥10 cpd: 21 mg/d for 6 weeks, then 14 mg/d for 2 weeks, then 7 mg/d for 2 weeks, then discontinue Patient smokes <10 cpd: 14 mg/d for 6 weeks, then 7 mg/d for 2 weeks, then discontinue |
| cpd: cigarettes per day; NRT: nicotine replacement therapy | |
CASE CONTINUED: Staying smoke-free
Upon discharge, Michael discontinued NRT and followed up with his outpatient psychiatrist, who provided brief smoking cessation counseling in addition to bupropion SR, 150 mg bid. Michael’s depressive symptoms improved with the medication. He was able to stop smoking within 3 months with the combination of medication and behavioral therapy.
- Centers for Disease Control and Prevention, Youth Tobacco Prevention. www.cdc.gov/tobacco/youth/index.htm.
- Not On Tobacco model program. Substance Abuse and Mental Health Services Administration.www.modelprograms.samhsa.gov/pdfs/model/Not_On_Tobacco.pdf.
Drug brand names
- Bupropion SR • Zyban
- Clonidine • Catapres
- Doxepin • Sinequan
- Mecamylamine • Inversine
- Nicotine/inhalation system • Nicotrol Inhaler
- Nicotine/lozenge • Commit
- Nicotine/nasal spray • Nicotrol NS
- Nicotine/polacrilex • Nicorette
- Nicotine/transdermal • Nicotrol, Prostep
- Nortriptyline • Pamelor
- Selegiline • Eldepryl
- Varenicline • Chantix
Disclosures
Dr. Verduin has received research/grant support from the National Institute of Drug Abuse.
Dr. Upadhyaya is a consultant and speaker for Shire Pharmaceuticals and has received grant/research support from and is a consultant to Eli Lilly and Company.
1. Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol 1990;9(6):701-16.
2. Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings 2006. Bethesda, MD: National Institute on Drug Abuse; 2007. NIH publication no. 07-6202.
3. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescence. Tob Control 2000;9(3):313-9.
4. Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry 2002;41(11):1294-1305.
5. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):222-830.
7. Upadhyaya HP, Brady KT, Wharton M, Liao J. Psychiatric disorders and cigarette smoking among child and adolescent psychiatry inpatients. Am J Addict 2003;12(2):144-52.
8. Prokhorov AV, Pallonen UE, Fava JL, et al. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996;21(1):117-27.
9. Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 1998;152(2):151-6.
10. O’Loughlin J, DiFranza J, Tarasuk J, et al. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control 2002;11(4):354-60.
11. Nonnemaker J, Mowery P, Hersey J, et al. Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res 2004;6(2):295-301.
12. Etter JF, LeHouezec J, Perneger TV. A self-administered questionnaire to measure addiction to cigarettes: the Cigarette Dependence Scale. Neuropsychopharmacology 2003;28(2):359-70.
13. Hughes JR, Oliveto AH, Riggs R, et al. Concordance of different measures of nicotine dependence: two pilot studies. Addict Behav 2004;29(8):1527-39.
14. Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, eds. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: The Humana Press Inc.; 1992.
15. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4(2):149-59.
16. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Quick reference guide for clinicians. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
17. Smith TA, House RF, Croghan IT, et al. Nicotine patch therapy in adolescent smokers. Pediatrics 1996;98(4):659-67.
18. Hurt RD, Croghan GA, Beede SD, et al. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Arch Pediatr Adolesc Med 2000;154(1):31-7.
19. Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tob Res 2003;5(4):515-26.
20. Moolchan ET, Robinson ML, Ernst M, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407-14.
21. Upadhyaya HP, Brady KT, Wang W. Bupropion SR in adolescents with comorbid ADHD and nicotine dependence: a pilot study. J Am Acad Child Adolesc Psychiatry 2004;43(2):199-205.
22. Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol 2004;72(4):729-35.
23. Muramoto ML, Leischow SJ, Sherrill D. A randomized trial of the efficacy of bupropion for adolescent smoking cessation. Paper presented at: Annual Meeting of the Society for Research on Nicotine and Tobacco; March 20-23, 2005; Prague, Czech Republic.
24. Hall SM. Tricyclic antidepressants in the treatment of nicotine dependence. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:95-107.
25. Berlin I. Monoamine oxidase inhibitors for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:109-21.
26. Weinberger AH, Reutenauer EL, George TP. Other nonapproved agents for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:137-48.
27. Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev 2005;2:CD001009.-
28. Nicoderm CQ [package insert]. Bridgewater, NJ: Sanofi Aventis; 2006.
29. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
30. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006;25(5):549-57.
31. Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6.
CASE: Depressed, irritable — and smoking
Michael, age 16, is admitted to a psychiatric unit for severe depressive symptoms and suicidal ideation. The next day, he is irritable and refuses to cooperate with the interview. During group therapy he is distractible and unable to focus. The treating psychiatrist learns that before admission Michael had been smoking 10 to 15 cigarettes per day and now feels a strong craving for cigarettes.
Unrecognized nicotine dependence can be problematic on inpatient psychiatric units, where adolescents such as Michael are not permitted to smoke and rarely are offered nicotine replacement therapy (NRT). Unfortunately, psychiatrists seldom diagnose and treat nicotine dependence—particularly in adolescents—whether in outpatient or inpatient settings.1,2
Do adolescent smokers need help quitting? Do they experience withdrawal symptoms when they stop smoking? Are pharmacologic interventions appropriate? For each question, the answer is a resounding yes.
To help you treat young smokers, this article offers:
- tools for assessing adolescent tobacco use and dependence
- evidence-based treatment options
- an algorithm to guide treatment choice.
Not just a ‘phase’
Early smoking—especially among those younger than age 13—is associated with adolescent psychopathology, including depressive disorders and other substance use disorders.3 Compared with nonsmoking teens, those who smoke at least monthly are significantly more likely to smoke as adults.4 Among high school seniors:
- >20% report smoking cigarettes in the past 30 days
- 12% smoke daily
- 6% smoke ≥10 cigarettes per day.5
Nicotine dependence can develop very rapidly: nearly 25% of adolescents have ≥1 symptom within 2 weeks of starting to smoke at least once a month.6
Role of parents. Early intervention for teen nicotine addiction is particularly important because of the long-term health risks associated with tobacco use.7 In our experience, however, teen smokers’ parents’ attitudes can make addressing adolescent nicotine dependence a therapeutic challenge.
Modified Fagerstrom Tolerance Questionnaire8 (7 items)
Stanford Dependence Inventory (SDI)9 (5 items)
Hooked on Nicotine Checklist (HONC)10 (10 items)
Nicotine Dependence Scale for Adolescents11 (6 items)
Cigarette Dependence Scale (CDS-5 and CDS-12)12 (5 or 12 items)
Parents may be unaware of their teens’ smoking, and those who are aware may:
- not know what help is available
- dismiss teen smoking as “just a phase”
- feel that smoking cigarettes is preferable to smoking marijuana or using other illicit drugs.
Other parents have no objections because they themselves smoke. Some permit their teens to smoke and may even give them cigarettes.
Parents who want their teens to stop smoking often believe erroneously that the best method is to quit “cold turkey.”
Assessing use and dependence
Teen smokers’ nicotine withdrawal symptoms—such as irritability, anxiety, and impaired concentration—can imitate or exacerbate other psychiatric symptoms, thus complicating diagnosis and treatment. Ask all adolescent patients about the quantity, frequency, pattern, and duration of use of all forms of tobacco, including:
- cigarettes
- cigars
- cigarillos (short, narrow cigars)
- bidis (thin, flavored South Asian cigarettes wrapped in leaf)
- smokeless tobacco.
Dependence. Establishing nicotine dependence in young smokers is more complicated than in adults because of teen smokers’ variable smoking patterns. Several self-rating scales have been developed to assess nicotine dependence in adolescents (Box 1).8-12 Although some of these tools have been used primarily in research, outpatient psychiatrists may find these scales useful for evaluating adolescents’ smoking.
Some DSM-IV-TR criteria for substance dependence may not apply to nicotine dependence or correlate with other validated measures of nicotine dependence. For example, “significant time spent obtaining, using, or recovering from the effects of a substance” might not apply to all adolescent smokers.13 Based on our clinical experience, daily smoking for an extended period of time (several months) is a marker of dependence for almost all adolescents.
The Timeline Follow Back method can help you capture a more complete picture of adolescent tobacco use over time.14 This involves asking teens about tobacco use over the past 30 or 90 days, beginning with the assessment day and working backward. Record tobacco use on a calendar, using holidays, weekends, and events as anchor points to help teens recall their smoking.
Biomarker tests can be used to measure nicotine use. The 2 most common are:
- expired carbon monoxide (CO) level (essentially a “breathalyzer” for smoking)
- cotinine level—a metabolite of nicotine.
Expired CO testing is simple to conduct but requires specialized equipment that costs approximately $1,000. Marijuana use may affect CO results, but NRT will not. Measuring CO levels provides information about cigarette smoking over the past several hours, compared with the past several days with cotinine.15
Cotinine can be tested in serum, saliva, or urine. Serum testing can be expensive and may require shipping samples to a specialized laboratory for processing. Testing saliva or urine is less expensive and may be conducted in an office. Cotinine testing in teens who use NRT may be unreliable because the nicotine in these products will be metabolized to cotinine and yield a positive result.
CASE CONTINUED: Wanting to quit
Michael was placed on a 21-mg transdermal nicotine patch, which greatly reduced his craving and irritability. He expressed an interest in quitting smoking. Given Michael’s depressive symptoms, bupropion SR was initiated to treat his depression and assist with smoking cessation.
Treatment options
Optimal smoking cessation treatment includes a combination of medication and behavioral counseling.16
Pharmacologic treatments. FDA-approved medications for adult smoking cessation include NRT—available as a gum, inhaler, nasal spray, lozenge, or transdermal patch—bupropion SR, and varenicline. Although not FDA-approved for patients younger than age 18, NRT and bupropion SR have been evaluated for smoking in adolescents.
NRT helps smokers by reducing nicotine withdrawal symptoms during cessation. Only nicotine gum and transdermal nicotine patch have been studied in adolescents. Results are modest at best, although in some studies including behavioral treatments may have obscured any medication effect (Table 1).17-20
Bupropion SR. How bupropion SR helps patients stop smoking is not completely clear. Three studies have evaluated bupropion SR in adolescents; 2 had positive results, but all 3 had important limitations (Table 2).21-23
One of the 2 positive studies included only 16 patients and had an open-label design.22 The second—a larger randomized, placebo-controlled trial23—found that bupropion SR improved nicotine abstinence compared with placebo at 6 weeks, but this effect did not last after subjects stopped taking the drug.
The third bupropion SR study used 150 mg/d (the recommend adult dose is 300 mg/d) and had poor medication adherence.21 The difference in abstinence rate compared with placebo was not statistically significant.
Other medications. Varenicline—a partial nicotine receptor agonist recently approved for adult smoking cessation—has not been studied in adolescents. Nortriptyline, doxepin, selegiline, clonidine, and mecamylamine have shown promise in adult smokers but are not approved for smoking cessation and require further study, especially in young smokers.24-27
Pharmacotherapy risks. NRT can cause nicotine overdose symptoms, such as rapid heart rate or nausea, especially if used while smoking. Transdermal NRT can cause a local reaction at the application site and can cause burns if worn while undergoing magnetic resonance imaging.28
Adverse effects associated with bupropion SR include a small risk of seizure, weight loss, and insomnia. This drug is contraindicated for patients who:
- have a seizure disorder
- have ever been diagnosed with bulimia or anorexia nervosa
- are taking other bupropion formulations.
Patients should not take bupropion SR during abrupt discontinuation of alcohol or sedatives or within 14 days of taking a monoamine oxidase inhibitor.29
Table 1
Can the nicotine patch help teens quit smoking?
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Smith et al, 199617 | 13- to 17-year-olds (N=22) who smoked ≥20 cpd | 8 weeks of open-label treatment with transdermal NRT plus behavioral counseling and group support | 14% at 8 weeks, 4.5% at 3 and 6 months |
| Hurt et al, 200418 | 13- to 17-year-olds (N=101) who smoked ≥10 cpd | 6 weeks of open-label treatment with transdermal NRT plus self-help material and brief individual counseling if requested | 11% at 6 weeks, 5% at 6 months |
| Hanson et al, 200319 | 13- to 19-year-olds (N=100) who smoked ≥10 cpd | 10 weeks of double-blind treatment with transdermal NRT or placebo plus CBT and contingency management | 20% (active) vs 18% (placebo); not statistically significant |
| Moolchan et al, 200520 | 13- to 17-year-olds (N=120) who smoked ≥10 cpd | 12 weeks of double-blind treatment with:
| 17.7% (active transdermal NRT)* vs 6.5% (active gum) vs 2.5% (placebo only) |
| *P=0.04 for transdermal NRT vs placebo | |||
| CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
Table 2
Teen smoking cessation: Evidence for bupropion SR
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Upadhyaya et al, 200421 | 12- to 19-year-olds (N=16, 11 of whom had ADHD) who smoked ≥5 cpd | 7 weeks of open-label treatment with bupropion SR, 150 mg bid, with brief smoking cessation counseling | 31.3% after 4 weeks of medication |
| Killen et al, 200422 | 15- to 18-year-olds (N=211) who smoked ≥10 cpd | 9 weeks of double-blind treatment with bupropion SR, 150 mg/d, or placebo; subjects received 8 weeks of transdermal NRT and group skills training | 23% (active) vs 28% (placebo) at 10 weeks; 8% (active) vs 7% placebo) at 26 weeks; not statistically significant |
| Muramoto et al, 200523 | 14- to 17-year-olds (N=312) who smoked ≥6 cpd | 6 weeks of double-blind treatment with bupropion SR, 150 mg/d; 150 mg bid; or placebo, with CBT and motivational enhancement | 16.9% (150 mg bid) vs 10.3% (150 mg/d) vs 6.7% (placebo) at 6 weeks* No differences at 26 weeks |
| ADHD: attention-deficit/hyperactivity disorder; CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
| *P=0.019 for 150 mg bid vs placebo | |||
Behavioral therapy. Specialized treatments developed specifically for teen smoking cessation—such as Not On Tobacco (see Related Resources)—often are delivered in schools or other group settings. The most successful consist of ≥5 sessions and include motivational enhancement, cognitive-behavioral, and social influence-oriented approaches.30
Other behavioral treatments. Most psychiatrists are not equipped to deliver these specialized behavioral treatments. Instead, you can use simple yet effective behavioral treatments during routine office visits as adjuncts to pharmacotherapy. At the very least, we recommend the U.S. Public Health Service’s “5 As” strategy (Box 2).16
Educate patients about what to expect during withdrawal, how long withdrawal will last, and medication side effects. To help adolescents develop appropriate treatment expectations:
- discuss the difference between a “slip” (having 1 cigarette) and a “relapse” (returning to daily smoking)
- explain that many individuals need multiple attempts before they quit.
Encourage adolescent smokers to contact the National Network of Tobacco Cessation Quitlines (1-800-784-8669; www.smokefree.gov), which provides free access to telephone-based counseling services.
Ask every patient about tobacco use during every visit, and have a system for recording and tracking tobacco use in the chart
Advise patients clearly and unambiguously to stop smoking, and tailor that advice to each patient’s needs
Assess every patient’s readiness to quit
Assist patients who are ready to quit through self-help materials, referral, and/or smoking cessation treatment
Arrange follow-up visits for relapse prevention or to reassess readiness to quit
Source: Reference 16
Choosing treatment
Evidence guiding treatment choice for teen smoking cessation is limited but growing. Most studies examined daily cigarette smoking, with significantly less evidence to support treatment decisions for light (non-daily) smokers and teens who use other tobacco products.
Recommendations.We have developed a strategy to guide treatment of adolescent smokers (Algorithm). We recommend using pharmacologic interventions only for teens who smoke daily because:
- most studies have focused on daily smoking
- efficacy data are limited
- pharmacologic interventions carry potential risks.
Because of bupropion SR’s contraindications and potential side effect profile, we suggest NRT in combination with smoking cessation counseling as a first-line treatment for young smokers. We recommend beginning with transdermal NRT because of the low likelihood of underdosing with the patch’s once-daily application.20 With either NRT or bupropion SR, schedule follow-up appointments to target relapse prevention and solve any issues that arise.
Help your patient choose a “quit date,” preferably 1 to 2 weeks after your initial assessment. We recommend encouraging young smokers to reduce their smoking by 1 cigarette per day to help minimize withdrawal symptoms from “cold turkey” cessation.31
Some physicians have found it helpful to see the patient on the quit day—or 2 days after when withdrawal symptoms tend to be most robust—to provide support and encouragement. Ask the adolescent to bring and discard during the visit all smoking paraphernalia as a symbol of his or her new smoke-free status.
Step 1: NRT. Initiate smoking cessation counseling plus transdermal NRT using the dosing guidelines in Table 3,28,31 and adjust the dose depending on severity of withdrawal symptoms. Ideally, the patch delivery will be used for 12 weeks, with at least 3 and ideally 6 weeks on the initial dose, followed by a gradual taper.28 We strongly recommend using transdermal NRT on adolescent inpatient units, especially for daily smokers and those who exhibit nicotine withdrawal symptoms.
Step 2: Bupropion SR. If NRT fails, the next step is bupropion SR plus smoking cessation counseling, assuming the adolescent does not have contraindications to bupropion SR. Start the medication based on the dosing guidelines in Table 3,28,31 and set a quit date for 2 weeks after starting bupropion SR.
Again, encourage adolescents to reduce by 1 cigarette per day over the 2 weeks before the quit day to minimize withdrawal symptoms.31 Continue bupropion SR for at least 8 and optimally 12 weeks.31
Combination therapy? If teens are unable to successfully quit smoking with NRT or bupropion SR alone, experience with adults suggests combining the 2 therapies might be beneficial. However, no evidence supports combination therapy in adolescents. Instead, consider referring adolescents who can’t quit to a smoking cessation specialist.
AlgorithmA strategy to initiate smoking cessation in adolescents
CDC: Centers for Disease Control and Prevention; CM: contingency management; NIDA: National Institute of Drug Abuse; NRT: nicotine replacement therapy
Source: Adapted from Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6 with permission of Lippincott Williams & Wilkins.Table 3
Dosing guidelines for adolescent smoking cessation therapy
| Medication | Recommended regimen |
|---|---|
| Bupropion SR31 | Patient weight ≥90 lbs: 150 mg once in the morning for 3 to 6 days, then 150 mg bid for 12 weeks Patient weight <90 lbs: Maximum 150 mg once in the morning, if tolerated |
| Transdermal NRT28 | Patient smokes ≥10 cpd: 21 mg/d for 6 weeks, then 14 mg/d for 2 weeks, then 7 mg/d for 2 weeks, then discontinue Patient smokes <10 cpd: 14 mg/d for 6 weeks, then 7 mg/d for 2 weeks, then discontinue |
| cpd: cigarettes per day; NRT: nicotine replacement therapy | |
CASE CONTINUED: Staying smoke-free
Upon discharge, Michael discontinued NRT and followed up with his outpatient psychiatrist, who provided brief smoking cessation counseling in addition to bupropion SR, 150 mg bid. Michael’s depressive symptoms improved with the medication. He was able to stop smoking within 3 months with the combination of medication and behavioral therapy.
- Centers for Disease Control and Prevention, Youth Tobacco Prevention. www.cdc.gov/tobacco/youth/index.htm.
- Not On Tobacco model program. Substance Abuse and Mental Health Services Administration.www.modelprograms.samhsa.gov/pdfs/model/Not_On_Tobacco.pdf.
Drug brand names
- Bupropion SR • Zyban
- Clonidine • Catapres
- Doxepin • Sinequan
- Mecamylamine • Inversine
- Nicotine/inhalation system • Nicotrol Inhaler
- Nicotine/lozenge • Commit
- Nicotine/nasal spray • Nicotrol NS
- Nicotine/polacrilex • Nicorette
- Nicotine/transdermal • Nicotrol, Prostep
- Nortriptyline • Pamelor
- Selegiline • Eldepryl
- Varenicline • Chantix
Disclosures
Dr. Verduin has received research/grant support from the National Institute of Drug Abuse.
Dr. Upadhyaya is a consultant and speaker for Shire Pharmaceuticals and has received grant/research support from and is a consultant to Eli Lilly and Company.
CASE: Depressed, irritable — and smoking
Michael, age 16, is admitted to a psychiatric unit for severe depressive symptoms and suicidal ideation. The next day, he is irritable and refuses to cooperate with the interview. During group therapy he is distractible and unable to focus. The treating psychiatrist learns that before admission Michael had been smoking 10 to 15 cigarettes per day and now feels a strong craving for cigarettes.
Unrecognized nicotine dependence can be problematic on inpatient psychiatric units, where adolescents such as Michael are not permitted to smoke and rarely are offered nicotine replacement therapy (NRT). Unfortunately, psychiatrists seldom diagnose and treat nicotine dependence—particularly in adolescents—whether in outpatient or inpatient settings.1,2
Do adolescent smokers need help quitting? Do they experience withdrawal symptoms when they stop smoking? Are pharmacologic interventions appropriate? For each question, the answer is a resounding yes.
To help you treat young smokers, this article offers:
- tools for assessing adolescent tobacco use and dependence
- evidence-based treatment options
- an algorithm to guide treatment choice.
Not just a ‘phase’
Early smoking—especially among those younger than age 13—is associated with adolescent psychopathology, including depressive disorders and other substance use disorders.3 Compared with nonsmoking teens, those who smoke at least monthly are significantly more likely to smoke as adults.4 Among high school seniors:
- >20% report smoking cigarettes in the past 30 days
- 12% smoke daily
- 6% smoke ≥10 cigarettes per day.5
Nicotine dependence can develop very rapidly: nearly 25% of adolescents have ≥1 symptom within 2 weeks of starting to smoke at least once a month.6
Role of parents. Early intervention for teen nicotine addiction is particularly important because of the long-term health risks associated with tobacco use.7 In our experience, however, teen smokers’ parents’ attitudes can make addressing adolescent nicotine dependence a therapeutic challenge.
Modified Fagerstrom Tolerance Questionnaire8 (7 items)
Stanford Dependence Inventory (SDI)9 (5 items)
Hooked on Nicotine Checklist (HONC)10 (10 items)
Nicotine Dependence Scale for Adolescents11 (6 items)
Cigarette Dependence Scale (CDS-5 and CDS-12)12 (5 or 12 items)
Parents may be unaware of their teens’ smoking, and those who are aware may:
- not know what help is available
- dismiss teen smoking as “just a phase”
- feel that smoking cigarettes is preferable to smoking marijuana or using other illicit drugs.
Other parents have no objections because they themselves smoke. Some permit their teens to smoke and may even give them cigarettes.
Parents who want their teens to stop smoking often believe erroneously that the best method is to quit “cold turkey.”
Assessing use and dependence
Teen smokers’ nicotine withdrawal symptoms—such as irritability, anxiety, and impaired concentration—can imitate or exacerbate other psychiatric symptoms, thus complicating diagnosis and treatment. Ask all adolescent patients about the quantity, frequency, pattern, and duration of use of all forms of tobacco, including:
- cigarettes
- cigars
- cigarillos (short, narrow cigars)
- bidis (thin, flavored South Asian cigarettes wrapped in leaf)
- smokeless tobacco.
Dependence. Establishing nicotine dependence in young smokers is more complicated than in adults because of teen smokers’ variable smoking patterns. Several self-rating scales have been developed to assess nicotine dependence in adolescents (Box 1).8-12 Although some of these tools have been used primarily in research, outpatient psychiatrists may find these scales useful for evaluating adolescents’ smoking.
Some DSM-IV-TR criteria for substance dependence may not apply to nicotine dependence or correlate with other validated measures of nicotine dependence. For example, “significant time spent obtaining, using, or recovering from the effects of a substance” might not apply to all adolescent smokers.13 Based on our clinical experience, daily smoking for an extended period of time (several months) is a marker of dependence for almost all adolescents.
The Timeline Follow Back method can help you capture a more complete picture of adolescent tobacco use over time.14 This involves asking teens about tobacco use over the past 30 or 90 days, beginning with the assessment day and working backward. Record tobacco use on a calendar, using holidays, weekends, and events as anchor points to help teens recall their smoking.
Biomarker tests can be used to measure nicotine use. The 2 most common are:
- expired carbon monoxide (CO) level (essentially a “breathalyzer” for smoking)
- cotinine level—a metabolite of nicotine.
Expired CO testing is simple to conduct but requires specialized equipment that costs approximately $1,000. Marijuana use may affect CO results, but NRT will not. Measuring CO levels provides information about cigarette smoking over the past several hours, compared with the past several days with cotinine.15
Cotinine can be tested in serum, saliva, or urine. Serum testing can be expensive and may require shipping samples to a specialized laboratory for processing. Testing saliva or urine is less expensive and may be conducted in an office. Cotinine testing in teens who use NRT may be unreliable because the nicotine in these products will be metabolized to cotinine and yield a positive result.
CASE CONTINUED: Wanting to quit
Michael was placed on a 21-mg transdermal nicotine patch, which greatly reduced his craving and irritability. He expressed an interest in quitting smoking. Given Michael’s depressive symptoms, bupropion SR was initiated to treat his depression and assist with smoking cessation.
Treatment options
Optimal smoking cessation treatment includes a combination of medication and behavioral counseling.16
Pharmacologic treatments. FDA-approved medications for adult smoking cessation include NRT—available as a gum, inhaler, nasal spray, lozenge, or transdermal patch—bupropion SR, and varenicline. Although not FDA-approved for patients younger than age 18, NRT and bupropion SR have been evaluated for smoking in adolescents.
NRT helps smokers by reducing nicotine withdrawal symptoms during cessation. Only nicotine gum and transdermal nicotine patch have been studied in adolescents. Results are modest at best, although in some studies including behavioral treatments may have obscured any medication effect (Table 1).17-20
Bupropion SR. How bupropion SR helps patients stop smoking is not completely clear. Three studies have evaluated bupropion SR in adolescents; 2 had positive results, but all 3 had important limitations (Table 2).21-23
One of the 2 positive studies included only 16 patients and had an open-label design.22 The second—a larger randomized, placebo-controlled trial23—found that bupropion SR improved nicotine abstinence compared with placebo at 6 weeks, but this effect did not last after subjects stopped taking the drug.
The third bupropion SR study used 150 mg/d (the recommend adult dose is 300 mg/d) and had poor medication adherence.21 The difference in abstinence rate compared with placebo was not statistically significant.
Other medications. Varenicline—a partial nicotine receptor agonist recently approved for adult smoking cessation—has not been studied in adolescents. Nortriptyline, doxepin, selegiline, clonidine, and mecamylamine have shown promise in adult smokers but are not approved for smoking cessation and require further study, especially in young smokers.24-27
Pharmacotherapy risks. NRT can cause nicotine overdose symptoms, such as rapid heart rate or nausea, especially if used while smoking. Transdermal NRT can cause a local reaction at the application site and can cause burns if worn while undergoing magnetic resonance imaging.28
Adverse effects associated with bupropion SR include a small risk of seizure, weight loss, and insomnia. This drug is contraindicated for patients who:
- have a seizure disorder
- have ever been diagnosed with bulimia or anorexia nervosa
- are taking other bupropion formulations.
Patients should not take bupropion SR during abrupt discontinuation of alcohol or sedatives or within 14 days of taking a monoamine oxidase inhibitor.29
Table 1
Can the nicotine patch help teens quit smoking?
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Smith et al, 199617 | 13- to 17-year-olds (N=22) who smoked ≥20 cpd | 8 weeks of open-label treatment with transdermal NRT plus behavioral counseling and group support | 14% at 8 weeks, 4.5% at 3 and 6 months |
| Hurt et al, 200418 | 13- to 17-year-olds (N=101) who smoked ≥10 cpd | 6 weeks of open-label treatment with transdermal NRT plus self-help material and brief individual counseling if requested | 11% at 6 weeks, 5% at 6 months |
| Hanson et al, 200319 | 13- to 19-year-olds (N=100) who smoked ≥10 cpd | 10 weeks of double-blind treatment with transdermal NRT or placebo plus CBT and contingency management | 20% (active) vs 18% (placebo); not statistically significant |
| Moolchan et al, 200520 | 13- to 17-year-olds (N=120) who smoked ≥10 cpd | 12 weeks of double-blind treatment with:
| 17.7% (active transdermal NRT)* vs 6.5% (active gum) vs 2.5% (placebo only) |
| *P=0.04 for transdermal NRT vs placebo | |||
| CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
Table 2
Teen smoking cessation: Evidence for bupropion SR
| Authors | Study population | Study design | Abstinence rate |
|---|---|---|---|
| Upadhyaya et al, 200421 | 12- to 19-year-olds (N=16, 11 of whom had ADHD) who smoked ≥5 cpd | 7 weeks of open-label treatment with bupropion SR, 150 mg bid, with brief smoking cessation counseling | 31.3% after 4 weeks of medication |
| Killen et al, 200422 | 15- to 18-year-olds (N=211) who smoked ≥10 cpd | 9 weeks of double-blind treatment with bupropion SR, 150 mg/d, or placebo; subjects received 8 weeks of transdermal NRT and group skills training | 23% (active) vs 28% (placebo) at 10 weeks; 8% (active) vs 7% placebo) at 26 weeks; not statistically significant |
| Muramoto et al, 200523 | 14- to 17-year-olds (N=312) who smoked ≥6 cpd | 6 weeks of double-blind treatment with bupropion SR, 150 mg/d; 150 mg bid; or placebo, with CBT and motivational enhancement | 16.9% (150 mg bid) vs 10.3% (150 mg/d) vs 6.7% (placebo) at 6 weeks* No differences at 26 weeks |
| ADHD: attention-deficit/hyperactivity disorder; CBT: cognitive-behavioral therapy; cpd: cigarettes per day; NRT: nicotine replacement therapy | |||
| *P=0.019 for 150 mg bid vs placebo | |||
Behavioral therapy. Specialized treatments developed specifically for teen smoking cessation—such as Not On Tobacco (see Related Resources)—often are delivered in schools or other group settings. The most successful consist of ≥5 sessions and include motivational enhancement, cognitive-behavioral, and social influence-oriented approaches.30
Other behavioral treatments. Most psychiatrists are not equipped to deliver these specialized behavioral treatments. Instead, you can use simple yet effective behavioral treatments during routine office visits as adjuncts to pharmacotherapy. At the very least, we recommend the U.S. Public Health Service’s “5 As” strategy (Box 2).16
Educate patients about what to expect during withdrawal, how long withdrawal will last, and medication side effects. To help adolescents develop appropriate treatment expectations:
- discuss the difference between a “slip” (having 1 cigarette) and a “relapse” (returning to daily smoking)
- explain that many individuals need multiple attempts before they quit.
Encourage adolescent smokers to contact the National Network of Tobacco Cessation Quitlines (1-800-784-8669; www.smokefree.gov), which provides free access to telephone-based counseling services.
Ask every patient about tobacco use during every visit, and have a system for recording and tracking tobacco use in the chart
Advise patients clearly and unambiguously to stop smoking, and tailor that advice to each patient’s needs
Assess every patient’s readiness to quit
Assist patients who are ready to quit through self-help materials, referral, and/or smoking cessation treatment
Arrange follow-up visits for relapse prevention or to reassess readiness to quit
Source: Reference 16
Choosing treatment
Evidence guiding treatment choice for teen smoking cessation is limited but growing. Most studies examined daily cigarette smoking, with significantly less evidence to support treatment decisions for light (non-daily) smokers and teens who use other tobacco products.
Recommendations.We have developed a strategy to guide treatment of adolescent smokers (Algorithm). We recommend using pharmacologic interventions only for teens who smoke daily because:
- most studies have focused on daily smoking
- efficacy data are limited
- pharmacologic interventions carry potential risks.
Because of bupropion SR’s contraindications and potential side effect profile, we suggest NRT in combination with smoking cessation counseling as a first-line treatment for young smokers. We recommend beginning with transdermal NRT because of the low likelihood of underdosing with the patch’s once-daily application.20 With either NRT or bupropion SR, schedule follow-up appointments to target relapse prevention and solve any issues that arise.
Help your patient choose a “quit date,” preferably 1 to 2 weeks after your initial assessment. We recommend encouraging young smokers to reduce their smoking by 1 cigarette per day to help minimize withdrawal symptoms from “cold turkey” cessation.31
Some physicians have found it helpful to see the patient on the quit day—or 2 days after when withdrawal symptoms tend to be most robust—to provide support and encouragement. Ask the adolescent to bring and discard during the visit all smoking paraphernalia as a symbol of his or her new smoke-free status.
Step 1: NRT. Initiate smoking cessation counseling plus transdermal NRT using the dosing guidelines in Table 3,28,31 and adjust the dose depending on severity of withdrawal symptoms. Ideally, the patch delivery will be used for 12 weeks, with at least 3 and ideally 6 weeks on the initial dose, followed by a gradual taper.28 We strongly recommend using transdermal NRT on adolescent inpatient units, especially for daily smokers and those who exhibit nicotine withdrawal symptoms.
Step 2: Bupropion SR. If NRT fails, the next step is bupropion SR plus smoking cessation counseling, assuming the adolescent does not have contraindications to bupropion SR. Start the medication based on the dosing guidelines in Table 3,28,31 and set a quit date for 2 weeks after starting bupropion SR.
Again, encourage adolescents to reduce by 1 cigarette per day over the 2 weeks before the quit day to minimize withdrawal symptoms.31 Continue bupropion SR for at least 8 and optimally 12 weeks.31
Combination therapy? If teens are unable to successfully quit smoking with NRT or bupropion SR alone, experience with adults suggests combining the 2 therapies might be beneficial. However, no evidence supports combination therapy in adolescents. Instead, consider referring adolescents who can’t quit to a smoking cessation specialist.
AlgorithmA strategy to initiate smoking cessation in adolescents
CDC: Centers for Disease Control and Prevention; CM: contingency management; NIDA: National Institute of Drug Abuse; NRT: nicotine replacement therapy
Source: Adapted from Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6 with permission of Lippincott Williams & Wilkins.Table 3
Dosing guidelines for adolescent smoking cessation therapy
| Medication | Recommended regimen |
|---|---|
| Bupropion SR31 | Patient weight ≥90 lbs: 150 mg once in the morning for 3 to 6 days, then 150 mg bid for 12 weeks Patient weight <90 lbs: Maximum 150 mg once in the morning, if tolerated |
| Transdermal NRT28 | Patient smokes ≥10 cpd: 21 mg/d for 6 weeks, then 14 mg/d for 2 weeks, then 7 mg/d for 2 weeks, then discontinue Patient smokes <10 cpd: 14 mg/d for 6 weeks, then 7 mg/d for 2 weeks, then discontinue |
| cpd: cigarettes per day; NRT: nicotine replacement therapy | |
CASE CONTINUED: Staying smoke-free
Upon discharge, Michael discontinued NRT and followed up with his outpatient psychiatrist, who provided brief smoking cessation counseling in addition to bupropion SR, 150 mg bid. Michael’s depressive symptoms improved with the medication. He was able to stop smoking within 3 months with the combination of medication and behavioral therapy.
- Centers for Disease Control and Prevention, Youth Tobacco Prevention. www.cdc.gov/tobacco/youth/index.htm.
- Not On Tobacco model program. Substance Abuse and Mental Health Services Administration.www.modelprograms.samhsa.gov/pdfs/model/Not_On_Tobacco.pdf.
Drug brand names
- Bupropion SR • Zyban
- Clonidine • Catapres
- Doxepin • Sinequan
- Mecamylamine • Inversine
- Nicotine/inhalation system • Nicotrol Inhaler
- Nicotine/lozenge • Commit
- Nicotine/nasal spray • Nicotrol NS
- Nicotine/polacrilex • Nicorette
- Nicotine/transdermal • Nicotrol, Prostep
- Nortriptyline • Pamelor
- Selegiline • Eldepryl
- Varenicline • Chantix
Disclosures
Dr. Verduin has received research/grant support from the National Institute of Drug Abuse.
Dr. Upadhyaya is a consultant and speaker for Shire Pharmaceuticals and has received grant/research support from and is a consultant to Eli Lilly and Company.
1. Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol 1990;9(6):701-16.
2. Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings 2006. Bethesda, MD: National Institute on Drug Abuse; 2007. NIH publication no. 07-6202.
3. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescence. Tob Control 2000;9(3):313-9.
4. Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry 2002;41(11):1294-1305.
5. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):222-830.
7. Upadhyaya HP, Brady KT, Wharton M, Liao J. Psychiatric disorders and cigarette smoking among child and adolescent psychiatry inpatients. Am J Addict 2003;12(2):144-52.
8. Prokhorov AV, Pallonen UE, Fava JL, et al. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996;21(1):117-27.
9. Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 1998;152(2):151-6.
10. O’Loughlin J, DiFranza J, Tarasuk J, et al. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control 2002;11(4):354-60.
11. Nonnemaker J, Mowery P, Hersey J, et al. Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res 2004;6(2):295-301.
12. Etter JF, LeHouezec J, Perneger TV. A self-administered questionnaire to measure addiction to cigarettes: the Cigarette Dependence Scale. Neuropsychopharmacology 2003;28(2):359-70.
13. Hughes JR, Oliveto AH, Riggs R, et al. Concordance of different measures of nicotine dependence: two pilot studies. Addict Behav 2004;29(8):1527-39.
14. Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, eds. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: The Humana Press Inc.; 1992.
15. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4(2):149-59.
16. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Quick reference guide for clinicians. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
17. Smith TA, House RF, Croghan IT, et al. Nicotine patch therapy in adolescent smokers. Pediatrics 1996;98(4):659-67.
18. Hurt RD, Croghan GA, Beede SD, et al. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Arch Pediatr Adolesc Med 2000;154(1):31-7.
19. Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tob Res 2003;5(4):515-26.
20. Moolchan ET, Robinson ML, Ernst M, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407-14.
21. Upadhyaya HP, Brady KT, Wang W. Bupropion SR in adolescents with comorbid ADHD and nicotine dependence: a pilot study. J Am Acad Child Adolesc Psychiatry 2004;43(2):199-205.
22. Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol 2004;72(4):729-35.
23. Muramoto ML, Leischow SJ, Sherrill D. A randomized trial of the efficacy of bupropion for adolescent smoking cessation. Paper presented at: Annual Meeting of the Society for Research on Nicotine and Tobacco; March 20-23, 2005; Prague, Czech Republic.
24. Hall SM. Tricyclic antidepressants in the treatment of nicotine dependence. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:95-107.
25. Berlin I. Monoamine oxidase inhibitors for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:109-21.
26. Weinberger AH, Reutenauer EL, George TP. Other nonapproved agents for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:137-48.
27. Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev 2005;2:CD001009.-
28. Nicoderm CQ [package insert]. Bridgewater, NJ: Sanofi Aventis; 2006.
29. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
30. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006;25(5):549-57.
31. Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6.
1. Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol 1990;9(6):701-16.
2. Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: overview of key findings 2006. Bethesda, MD: National Institute on Drug Abuse; 2007. NIH publication no. 07-6202.
3. DiFranza JR, Rigotti NA, McNeill AD, et al. Initial symptoms of nicotine dependence in adolescence. Tob Control 2000;9(3):313-9.
4. Upadhyaya HP, Deas D, Brady KT, Kruesi M. Cigarette smoking and psychiatric comorbidity in children and adolescents. J Am Acad Child Adolesc Psychiatry 2002;41(11):1294-1305.
5. U.S. Department of Health and Human Services. The health consequences of smoking: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004.
6. Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling? Am J Psychiatry 2003;160(12):222-830.
7. Upadhyaya HP, Brady KT, Wharton M, Liao J. Psychiatric disorders and cigarette smoking among child and adolescent psychiatry inpatients. Am J Addict 2003;12(2):144-52.
8. Prokhorov AV, Pallonen UE, Fava JL, et al. Measuring nicotine dependence among high-risk adolescent smokers. Addict Behav 1996;21(1):117-27.
9. Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Arch Pediatr Adolesc Med 1998;152(2):151-6.
10. O’Loughlin J, DiFranza J, Tarasuk J, et al. Assessment of nicotine dependence symptoms in adolescents: a comparison of five indicators. Tob Control 2002;11(4):354-60.
11. Nonnemaker J, Mowery P, Hersey J, et al. Measurement properties of a nicotine dependence scale for adolescents. Nicotine Tob Res 2004;6(2):295-301.
12. Etter JF, LeHouezec J, Perneger TV. A self-administered questionnaire to measure addiction to cigarettes: the Cigarette Dependence Scale. Neuropsychopharmacology 2003;28(2):359-70.
13. Hughes JR, Oliveto AH, Riggs R, et al. Concordance of different measures of nicotine dependence: two pilot studies. Addict Behav 2004;29(8):1527-39.
14. Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, eds. Measuring alcohol consumption: psychosocial and biochemical methods. Totowa, NJ: The Humana Press Inc.; 1992.
15. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res 2002;4(2):149-59.
16. Fiore MC, Bailey WC, Cohen SJ, et al. Treating tobacco use and dependence. Quick reference guide for clinicians. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service; 2000.
17. Smith TA, House RF, Croghan IT, et al. Nicotine patch therapy in adolescent smokers. Pediatrics 1996;98(4):659-67.
18. Hurt RD, Croghan GA, Beede SD, et al. Nicotine patch therapy in 101 adolescent smokers: efficacy, withdrawal symptom relief, and carbon monoxide and plasma cotinine levels. Arch Pediatr Adolesc Med 2000;154(1):31-7.
19. Hanson K, Allen S, Jensen S, Hatsukami D. Treatment of adolescent smokers with the nicotine patch. Nicotine Tob Res 2003;5(4):515-26.
20. Moolchan ET, Robinson ML, Ernst M, et al. Safety and efficacy of the nicotine patch and gum for the treatment of adolescent tobacco addiction. Pediatrics 2005;115(4):e407-14.
21. Upadhyaya HP, Brady KT, Wang W. Bupropion SR in adolescents with comorbid ADHD and nicotine dependence: a pilot study. J Am Acad Child Adolesc Psychiatry 2004;43(2):199-205.
22. Killen JD, Robinson TN, Ammerman S, et al. Randomized clinical trial of the efficacy of bupropion combined with nicotine patch in the treatment of adolescent smokers. J Consult Clin Psychol 2004;72(4):729-35.
23. Muramoto ML, Leischow SJ, Sherrill D. A randomized trial of the efficacy of bupropion for adolescent smoking cessation. Paper presented at: Annual Meeting of the Society for Research on Nicotine and Tobacco; March 20-23, 2005; Prague, Czech Republic.
24. Hall SM. Tricyclic antidepressants in the treatment of nicotine dependence. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:95-107.
25. Berlin I. Monoamine oxidase inhibitors for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:109-21.
26. Weinberger AH, Reutenauer EL, George TP. Other nonapproved agents for smoking cessation. In: George TP, ed. Medication treatments for nicotine dependence. Boca Raton, FL: CRC Press; 2007:137-48.
27. Lancaster T, Stead LF. Mecamylamine (a nicotine antagonist) for smoking cessation. Cochrane Database Syst Rev 2005;2:CD001009.-
28. Nicoderm CQ [package insert]. Bridgewater, NJ: Sanofi Aventis; 2006.
29. Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2007.
30. Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychol 2006;25(5):549-57.
31. Upadhyaya H, Deas D, Brady K. A practical clinical approach to the treatment of nicotine dependence in adolescents. J Am Acad Child Adolesc Psychiatry 2005;44(9):942-6.