User login
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
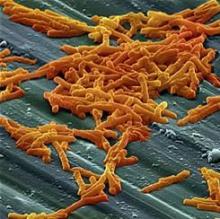
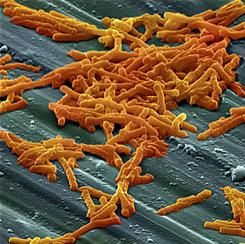
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
Clostridium difficile infection (CDI) patients treated with bezlotoxumab were less likely to be readmitted for recurring symptoms within 30 days of discharge, according to a phase 3 trial funded by Merck.
Recurrent CDI is a burden on both patients and providers, increasing health risks with each recurrence and eating through hospital resources, according to Vimalanand S. Prabhu, PhD, associate principal scientist for Merck.
In a randomized, double-blind, placebo-controlled, study of 1,050 CDI patients, a total of 27 (5%) of 530 of those given bezlotoxumab were re-hospitalized 30 days after discharge, compared with 58 (11%) of 520 patients in the placebo group (Clin Infect Dis. 2017 Aug 11. doi. 10.1093/cid/cix523).
Patients were gathered from 322 sites across 30 countries between November 2011 and May 2015.
When measuring CDI-related readmissions, the investigators found use of bezlotoxumab reduced rCDI hospitalizations by 6%, and by approximately 8% in high-risk patients, such as those over 65 years old or with severe CDI.
Bezlotoxumab works by binding to CDI toxin B, a primary cause of CDI symptoms, according to Dr. Prabhu and fellow investigators. The researchers suggested that bezlotoxumab could be a prevailing factor in fighting the rate of CDI infections, which accounted for 29,000 deaths in 2011 (N Engl J Med. 2015 Jun 11;372[24]:2368-9).
Investigators acknowledged that patients admitted for the study may be healthier than the real-world CDI population.
All investigators reported some financial involvement, whether being a full-time employee or acting as a consultant, for Merck, which funded the study. Individually, investigators reported financial ties to similar medical companies, such as Pfizer and AstraZeneca.
[email protected]
On Twitter @eaztweets
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point:
Major finding: A total of 27 of 530 (5%) bezlotoxumab patients were readmitted within 30 days of discharge compared with 58 of 520 (11%) placebo patients.
Data source: Randomized, double-blind, placebo-controlled, multicenter, global phase 3 trials conducted from November 2011-May 2015 at 322 sites in 30 countries.
Disclosures: All investigators report employment or financial support with Merck and have individually reported financial ties to similar companies like Astellas, AstraZeneca, Pfizer, and others.