User login
- In young women, many cases of osteoporosis are caused by hypoestrogenism resulting from hormone treatment (eg, GnRH agonists, aromatase inhibitors) or lifestyle adaptations (elite athletics, eating disorders).
- Treatment of osteoporosis in young women can often be successful with the use of estrogen or “androgenic” progestins.
- Chronic glucocorticoid treatment is a common cause of clinically significant osteoporosis in young women. Glucocorticoid-induced osteoporosis is an important cause of premenopausal osteoporotic fractures.
- ObGyns play a key role in ensuring that women enter midlife with strong bones. A focus on young women at very high risk for osteoporosis will help to ensure that our patients build their future bone health on a strong foundation.
ObGyns are very well trained to diagnose and treat women with osteoporosis, most of whom are perimenopausal and menopausal. We are also treating a significant number of young women at risk for osteoporosis because of lifestyle choices or medical treatment of endometriosis or rheumatic diseases. Treatment of this population poses unique challenges and requires specialized approaches.
An important caveat in any discussion of bone loss in young women: Few randomized clinical trials have assessed the efficacy of the various treatments available. In most treatment studies of osteoporosis in young women, bone mineral density (BMD)—an intermediate biometric endpoint—is the primary treatment outcome. In contrast, in the best studies in the menopausal population, the primary treatment outcome is bone fracture—a clinically important endpoint.
In addition, fewer consensus recommendations are available on the management of osteoporosis in young women than for its treatment in menopausal women. In this article, 3 different kinds of cases of bone loss in young women are used to develop key clinical points.
CASE 1 HISTORY
A teen with pelvic pain, bone loss
Therapy eased pain but decreased BMD
A 19-year-old woman has a 3-year history of severe, disabling dysmenorrhea. For 2 years she was treated with nonsteroidal anti-inflammatory drugs and cyclic estrogen-progestin contraceptives before switching to continuous oral contraceptives. These interventions did not relieve her pain. When she was 18, laparoscopy revealed stage I endometriosis, which was resected, providing 6 months of relief. When her pain recurred, the patient was started on leuprolide acetate depot, which caused amenorrhea and provided excellent pain relief. The patient said the leuprolide had “given back her life”. After 6 months of leuprolide therapy, a DXA bone scan demonstrated osteoporosis with a lumbar spine Z score of–2.6.
What treatment do you recommend?
Under age 25, use Z score, not T score
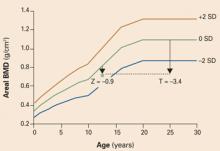
The diagnosis of osteoporosis in very young women is complex and continues to evolve. Physicians have been educated to use the T score (comparison to the mean peak bone mass of young adults) to assess BMD in menopausal women, in whom a decrease in T score of 1 standard deviation is associated with a 2- to 4-fold increase in fracture risk. However, teenage women are often still gaining bone mineral mass as the skeleton develops, so their T scores are normally below that of the peak bone mass of an adult woman. The Z score gives a good comparison of their bone mass with that of teens of the same sex at a similar developmental stage. Most experts agree that, when using DXA test results to assess bone density in women younger than 25, the Z score should be used.1 In one recent study, peak bone mass in the spine was achieved at approximately 23 years of age.2 As women reach age 25, the T and Z scores converge (FIGURE 1).
FIGURE 1 The Z score is more informative when the patient is under 25
The Z score compares the patient with persons of the same age and sex. The T score compares the patient with young normal adults of the same sex. Since bone mass increases until approximately age 20 to 25 years, it is best to use the Z score to evaluate the bone mineral density of women younger than 25 years.1 Reprinted from Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253–257. ©2004 with permission from Elsevier.
Treatment
For this patient with osteoporosis, there are several approaches to treatment:
Ensure adequate vitamin D and calcium. Vitamin D should be prescribed in doses of 400 to 800 IU daily. A recent meta-analysis reported that 800 IU vitamin D daily appears to provide better protection against osteoporotic fractures than 400 IU in menopausal women.3 Consider measuring 25-hydroxy vitamin D levels to assess the patient’s stores of this important pre-vitamin. Many young women are vitamin D-deficient.
Calcium intake should be in the range of 1,500 mg daily. To achieve this level of calcium intake, a calcium supplement, 500 to 1,000 mg daily, will probably need to be prescribed.
Discontinue leuprolide treatment. Typically, menses and ovarian estrogen production resume once leuprolide is stopped, and bone mass begins to recover. If pain recurs, the patient could be treated with cyclic or continuous estrogen-progestin contraceptives, depot-subQ Provera, a progestinreleasing intrauterine device, or a second laparoscopy procedure.
Continue leuprolide therapy and initiate steroid add-back treatment. Options shown to be effective in preserving bone mass in young women taking a gonadotropin-releasing hormone (GnRH) analogue include:
- norethindrone acetate, 5 mg daily,
- conjugated equine estrogen (Premarin), 0.3 or 0.625 mg daily, plus a progestin, and
- low-dose transdermal estrogen, 25 μg daily, plus a progestin.
Key trials
Lupron Add-Back Study. Women with endometriosis and chronic pelvic pain were randomly assigned to 1 of 4 treatment groups:
- leuprolide alone,
- leuprolide plus an oral synthetic progestin, norethindrone acetate, 5 mg daily,
- leuprolide plus “low-dose” conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, or
- leuprolide plus “high-dose” conjugated equine estrogen, 1.25 mg, plus norethindrone acetate, 5 mg daily.4
Women in all 4 groups received depotleuprolide, 3.75 mg intramuscularly every 4 weeks for 1 year. Over 1 year of treatment, BMD decreased significantly in the women who received the GnRH agonist alone. Bone density was preserved in the 3 groups that received steroid add-back.
Vasomotor symptoms were significantly reduced in all 3 groups receiving steroid add-back therapy, compared with the placebo group. However, more women in the group that received the larger dose of estrogen dropped out of the study than from the other groups because of more significant pelvic pain.
In summary, norethindrone, 5 mg daily, or low-dose conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, were both effective steroid add-back regimens for prevention of bone loss in young women with endometriosis being treated with long-term GnRH analogues (TABLE 1).
GnRH analogue vs GnRH plus estradiolprogestin. In another trial, women who experienced endometriosis and pelvic pain were randomized to receive a GnRH agonist alone or a GnRH agonist plus low-dose transdermal estradiol, 25 μg daily, plus medroxyprogesterone acetate, 2.5 mg daily, for 6 months. Women in both groups had similar improvement in pelvic pain symptoms and a similar decrease in endometriosis surgical staging scores as determined by laparoscopy before and after treatment. However, the women who received the GnRH agonist alone, without add-back therapy, had more vasomotor symptoms and a greater decline in BMD than the women who received the GnRH agonist plus low-dose transdermal estradiol plus progestin.5
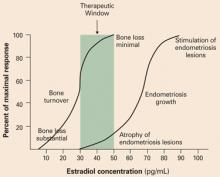
This study supports the estrogenthreshold hypothesis6 that there is a “sweet spot” in estradiol concentration where vasomotor symptoms and bone loss can be attenuated, but where endometriosis lesion activity is suppressed. This “sweet spot” appears to be at an estradiol concentration of about 30 pg/mL (FIGURE 2).
GnRH analogue vs GnRH plus teriparatide. A synthetic parathyroid analogue (PTH 1-34, teriparatide, Forteo) has been demonstrated to prevent bone loss in hypoestrogenic women. In this study, women with pelvic pain and endometriosis were randomized to treatment with a GnRH agonist alone or a GnRH agonist plus teriparatide for 6 months. Women receiving the GnRH agonist alone had a decrease in BMD at the spine of 3.5%, when measured in the lateral plane. Women receiving combined treatment had an increase in BMD at the spine of 3.4%.7
The Food and Drug Administration (FDA) has approved teriparatide at a dose of 20 μg daily by subcutaneous injection for the treatment of osteoporosis. A major advantage of PTH is that it appears to stimulate both osteoblasts and osteoclasts. In contrast, estrogen may preferentially block osteoclast activity without significantly stimulating osteoblasts. A major disadvantage of PTH: It is currently available only as a daily injection. It also is more expensive than estrogen or a synthetic progestin.
CASE 1 MANAGEMENT
A teen with pelvic pain, bone loss
Long-term combined therapy improved her BMD
This patient strongly preferred to continue the leuprolide treatment because it was so effective in treating her pelvic pain. She continued her leuprolide treatment and started norethindrone acetate 5 mg daily. She reported continued excellent control of her pelvic pain on the combined regimen of leuprolide plus norethindrone acetate. Follow-up bone density measurement demonstrated significant improvement. She continues on long-term combined therapy and is scheduled for annual BMD measurements.
CASE 2 HISTORY
The female athlete triad
A college-age dancer with an eating disorder
A 21-year-old elite ballet dancer, height 5’4” and weight 104 lb (BMI 17.8 kg/m2), presents with a history of a stress fracture of her foot. Besides being amenorrheic, she is on a high-fiber macrobiotic diet. DXA measurement demonstrates a Z score consistent with osteoporosis. She refuses to take estrogen-progestin contraceptives because she claims they impair her ability to train for her dance performances. She wonders if she should start alendronate therapy.
The female athlete triad is the combination of amenorrhea, disordered eating, and osteoporosis.8 In young female athletes, exercise and disordered eating may cause menstrual irregularity, and either disordered eating or menstrual irregularity, or both, may cause decreased BMD.9
Strenuous exercise has divergent effects on trabecular and cortical bone. By causing hypoestrogenism, strenuous exercise decreases bone density at cortical sites (vertebral spine). However, the weight-bearing exercise itself can lead to an increase in bone density at weight-bearing sites.10
Many elite athletes with the female athlete triad do not want to alter the intensity of their training or their diet, which might result in weight gain and resolution of the osteoporosis. Some elite athletes report that they do not want to take standard birth control pills because they believe the pill may impair their training and peak competitive performance. The clinician is often challenged by these strongly held beliefs to identify a treatment plan that will reverse the bone loss.
Is systemic disease involved? Occasionally, systemic disease causes or contributes to low bone mass in a young woman. Laboratory tests may help in screening some women for these diseases (TABLE 2).
FIGURE 2 Aim for the “sweet spot” in estradiol concentrations
According to the estrogen-threshold hypothesis, there is a range, or “sweet spot,” of estradiol concentrations where endometriosis lesion activity is suppressed and vasomotor symptoms and bone loss are not excessive. In humans, estrogen concentrations in the range of 30 pg/mL appear to be associated with decreased endometriosis pain and minimal degrees of bone loss. At estradiol levels of less than 10 pg/mL, bone loss is markedly accelerated.6 Reprinted from Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. ©1992 with permission from Elsevier.TABLE 1
Effective steroid hormone add-back regimens
Regimens that suppressed vasomotor symptoms and bone loss associated with long-term GnRH analogue use
| INVESTIGATOR | RANDOMIZATION SCHEME | STEROID HORMONE ADD-BACK REGIMEN |
|---|---|---|
| Howell et al5 | 50 women with endometriosis and pelvic pain randomized to GnRH agonist alone or GnRH agonist plus add-back regimen | Transdermal estradiol, 25 μg/d, plus oral medroxyprogesterone acetate, 2.5 mg/d |
| Lupron Add-Back Study Group4 | 201 women with endometriosis and pelvic pain randomized to GnRH agonist plus placebo or GnRH agonist plus 1 of 3 add-back regimens | Oral norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 0.625 mg/d plus norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 1.25 mg/d plus norethindrone, 5 mg/d |
TABLE 2
Screening for secondary causes of bone loss
| The following serum tests may be useful in screening women with osteoporosis for secondary causes of disordered bone metabolism |
| Calcium and albumin |
| Phosphorus |
| 25-Hydroxy vitamin D |
| Thyroid-stimulating hormone |
| Alkaline phosphatase |
| Creatinine |
Treatment of elite athletes
Peak bone mass depends on many factors, including genetics11; ovarian estrogen production12; adequate intake of calories, protein, vitamin D, and calcium9; exercise13; and achievement of target height and weight.14 When combinations of risk factors for osteoporosis are present, such as an eating disorder plus amenorrhea, correction of only one factor may not result in improved BMD.15
There are few clinical trials of the treatment of osteoporosis in women with the female athlete triad or an eating disorder. Treatment recommendations are largely based on clinical experience. If the woman resists lifestyle and dietary changes that will result in weight gain, other treatment recommendations to pursue include:
- vitamin D and calcium supplements,
- increased dietary protein,
- weight training,16 and
- smoking cessation.
Psychological counseling may help many young women with disordered eating.
Hormonal therapy has not been documented to reliably increase bone density in young amenorrheic women with an eating disorder. In 3 clinical trials, the effect of estrogen replacement on bone density in such patients was negligible or minimal.17-19 Given our understanding of the positive effect of estrogen on spinal BMD, it is difficult to understand these findings. Two possibilities are that compliance with estrogen replacement was modest because the women did not want a return of menses, or an insufficient dose of estrogen was prescribed. Another is that being underweight blocks the positive effect of estrogen on bone density. One small clinical trial did report that treatment of hypoestrogenic women with an estrogen-progestin contraceptive containing 35 μg of ethinyl estradiol resulted in a significant increase in lumbar spine BMD (5.4%) and a nonsignificant increase in femur BMD (3.6%).20 A logical recommendation, based on clinical experience, would be to prescribe an estrogen-progestin contraceptive to this woman to help preserve or improve her bone density.
Bisphosphonate therapy has been studied in small groups of women with disordered eating and osteoporosis. In one small clinical trial, 32 women with anorexia nervosa were given 10 mg alendronate daily or placebo for 1 year. Alendronate treatment was associated with a nonsignificant increase in femoral neck and spine bone density of 4.4% and 3.5%, respectively. Weight gain was an important predictor of improved BMD.21
The young woman in this case probably should not be prescribed alendronate. The FDA drug information for alendronate warns: “Safety and efficacy have not been established in pregnant women. Animal studies have shown delays in delivery and fetal/neonatal death (secondary to hypocalcemia). Bisphosphonates are incorporated into the bone matrix and gradually released over time. Theoretically, there may be a risk of fetal harm when pregnancy follows the completion of therapy. Based on limited case reports with pamidronate, serum calcium levels in the newborn may be altered if administered during pregnancy.”
Although there are minimal data in humans that alendronate will have adverse effects on fetal bone development and function, findings in rats treated with very high doses of alendronate leave some concern about the potential risk for human fetuses.
The young woman in this case may want to have children in the near future. Bisphosphonates may be incorporated into the bone and have a long residual half-life, resulting in potential exposure of the fetus to low doses of the compound. Very few malformations in human pregnancy have been reported after treatment with bisphosphonates.22 Based on a cautious approach to this situation, however, I would recommend that this woman of reproductive age who plans future child-bearing probably should not be treated with alendronate.
CASE 2 MANAGEMENT
The female athlete triad
Estrogen-progestin ring and mental health evaluation
Many women with the female athlete triad do not meet formal criteria for anorexia nervosa, but have disordered eating patterns. Questions in the medical history such as “Do you think you should be dieting?”23 may help initiate a conversation about eating practices and attitudes. The woman in Case 2 was screened for clinical depression. She reported that she was not blue over the past 2 weeks, but noted that she worried that she measured too much of her self-worth by her body image—a diagnostic criterion for anorexia nervosa. After extensive counseling, she was willing to start an estrogen-progestin contraceptive ring. She was also referred to a mental health provider for further evaluation.
CASE 3 HISTORY
Glucocorticoid-related bone loss
A 35-year-old woman with rheumatoid arthritis
This woman (G1P1) has been treated with various doses of prednisone over the past 10 years. Currently she is taking prednisone, 20 mg daily. She is oligomenorrheic with menstrual cycles every 45 days. She has undergone both bilateral hip replacement and bilateral tubal ligation. Her BMI is 20.5 kg/m2. DXA shows osteoporosis at her lumbar spine.
Glucocorticoids are a common cause of osteoporosis in young women. These drugs inhibit osteoblast activity and increase osteoclast activity. They also increase renal calcium excretion and decrease intestinal calcium absorption and adrenal androgen production. A meta-analysis of 89 publications reported that chronic glucocorticoid use increased both the risk of osteoporosis and clinically significant fractures.24 Chronic glucocorticoid treatment was associated with an increased relative risk of vertebral and hip fracture of 2.60 (95% confidence interval [CI], 2.31–2.92) and 1.61 (95% CI, 1.47–1.76), respectively. Daily doses of prednisone 10 mg or greater were clearly associated with an increased risk of fracture. The onset of the increased fracture risk occurred within 3 months of initiating therapy.
Treatment
Treatment for this woman should include:
- vitamin D and calcium supplementation,25 and
- consideration of estrogen-progestin therapy26 and/or bisphosphonates.
PTH analogue treatment could also be utilized if neither estrogen replacement nor bisphosphonate treatment were possible.27 If the patient could discontinue the glucocorticoid therapy and begin another treatment for rheumatoid arthritis, such as etanercept (Enbrel), her BMD might improve.
Bisphosphonates are a first-line option, since she has had a bilateral tubal ligation and is at very low risk for a future pregnancy. Many clinical trials demonstrate the efficacy of bisphosphonates in this clinical situation. Bisphosphonates are approved by the FDA for the prevention and treatment of osteoporosis in women receiving glucocorticoids.
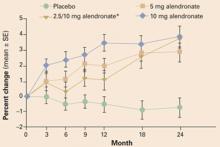
In a study of alendronate, the rate of new vertebral fractures was 0.7% in the alendronate group and 6.8% in the placebo group.28 Subjects who were taking at least 7.5 mg prednisone daily were randomized to receive alendronate, 5 or 10 mg daily, or a placebo. After 2 years of treatment, there was a 3.7% increase in lumbar spine BMD in the group treated with alendronate, 10 mg daily, and a 0.8% loss in bone denisty in the placebo group (FIGURE 3).
In a study of risedronate, 5 mg daily versus placebo, bisphosphonate treatment was associated with a 70% decrease in fracture risk.29
These clinical trials were completed before the development of once-weekly bisphosphonate treatment. Now that such treatment is available, it makes sense to prescribe either alendronate, 70 mg once weekly, or risedronate, 35 mg once weekly. Ibandronate, 150 mg once monthly, is also likely to be effective.
FIGURE 3 Alendronate improves BMD in women taking glucocorticoids
Effects of 2 years of alendronate treatment on bone mineral density of the lumbar spine in patients receiving average daily doses of prednisone of at least 7.5 mg. Alendronate treatment significantly improved bone mineral density (P<.0001). *Alendronate 2.5 mg was switched to 10 mg after it was determined that 2.5 mg was a suboptimal dose.
28 From Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, doubleblind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. Copyright ©2001. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Do US women get adequate treatment?
Surveys of community-based practices report that approximately 40% of women taking chronic glucocorticoid therapy are not receiving any intervention to prevent osteoporosis.30
Gynecologists are well positioned to ensure that these young women are fully apprised of their options and receive appropriate preventive intervention.
CASE 3 MANAGEMENT
Glucocorticoid-related bone loss
Estrogen-progestin patch, a bisphosphonate, and a rheumatology consult
This patient was started on an estrogen-progestin contraceptive patch and a bisphosphonate. An annual BMD measurement is planned.
In addition, a rheumatologist was consulted to determine if etanercept (Enbrel) could be initiated for the treatment of her rheumatoid arthritis, with a concomitant reduction in her prednisone dosage.
SUMMARYAim treatment toward future bone health
Many controversies remain unresolved concerning the best approach to bone loss in young women. On the one hand, most young women with osteoporosis are at low risk of vertebral and hip fracture, so a large number would need to be treated to prevent one fracture. On the other hand, most hope to become middle-aged; if young women begin adult life with osteoporosis, it is possible that they will have a poor foundation upon which to achieve optimal bone health. Until this key issue is resolved, it is probably best to try to help young women with osteoporosis achieve the best bone health possible. Bone health in young women is likely to be the foundation determining their future bone health and fracture risk.
1. Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253-257.
2. Lin YC, Lyle RM, Weaver CM, et al. Peak spine and femoral neck bone mass in young women. Bone. 2003;32:546-253.
3. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation. JAMA. 2005;293:2257-2264.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
5. Howell R, Edmonds D, Dowsett M. Gonadotropin releasing hormone analogue plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril. 1996;66:666-668.
6. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
7. Finkelstein JS, Klibanski A, Shaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331:1618-1623.
8. Warren MP, Brooks-Gunn J, Fox RP, Holderness C, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162-3168.
9. Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711-719.
10. Young N, Formica C, Szmukler, Seeman E. Bone density at weight-bearing and non weight-bearing sites in ballet dancers: the effects of exercise, hypogonadism and body weight. J Clin Endocrinol Metab. 1994;78:449-454.
11. Young D, Hopper JL, Nowson CA, et al. Determinants of bone mineral mass in 10- to 26- year old females: a twin study. J Bone Miner Res. 1995;10:558-567.
12. Drinkwater BL, Bruemner B, Chestnut CH. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545-548.
13. Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579-586.
14. Lloyd T, Rollings N, Andon MB, et al. Determinants of bone density in young women. J Clin Endocrinol Metab. 1992;75:383-387.
15. Jonnavithula S, Warren MP, Fox RP, Lazaro MI. Bone mineral density is compromised in amenorrheic women despite return of menses: a 2-year study. Obstet Gynecol. 1993;81:669-674.
16. Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Mineral Res. 1995;10:574-585.
17. Klibanski A, Biller BMK, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898-904.
18. Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135-143.
19. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935-4941.
20. Hergenroeder AC, Smith EO, Shypailo R, et al. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone acetate or placebo over 12 months. Am J Obstet Gynecol. 1997;176:1017-1025.
21. Golden NH, Iglesias EA, Jacobsen MS, et al. Alendronate for the treatment of osteopenia in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:3179-3185.
22. Ott SM. Letter re: alendronate in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:5508.-
23. Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Phys. 2003;67:297-304.
24. van Staa TP, Leufkens HGM, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int. 2002;13:624-629.
25. Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292-299.
26. Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499-1505.
27. Lane NE, Roe B, Genant HK, Arnaud C. Parathyroid hormone treatment reverses glucocorticoid-induced osteoporosis: results of a randomized controlled trial. J Clin Invest. 1998;102:1627-1633.
28. Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202-211.
29. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006-1013.
30. Yood RA, Harrold L, Fish L, et al. Prevention of glucocorticoid-induced osteoporosis: experience in a managed care setting. Arch Intern Med. 2001;161:1322-1327.
The author reports no financial relationships relevant to this article.
- In young women, many cases of osteoporosis are caused by hypoestrogenism resulting from hormone treatment (eg, GnRH agonists, aromatase inhibitors) or lifestyle adaptations (elite athletics, eating disorders).
- Treatment of osteoporosis in young women can often be successful with the use of estrogen or “androgenic” progestins.
- Chronic glucocorticoid treatment is a common cause of clinically significant osteoporosis in young women. Glucocorticoid-induced osteoporosis is an important cause of premenopausal osteoporotic fractures.
- ObGyns play a key role in ensuring that women enter midlife with strong bones. A focus on young women at very high risk for osteoporosis will help to ensure that our patients build their future bone health on a strong foundation.
ObGyns are very well trained to diagnose and treat women with osteoporosis, most of whom are perimenopausal and menopausal. We are also treating a significant number of young women at risk for osteoporosis because of lifestyle choices or medical treatment of endometriosis or rheumatic diseases. Treatment of this population poses unique challenges and requires specialized approaches.
An important caveat in any discussion of bone loss in young women: Few randomized clinical trials have assessed the efficacy of the various treatments available. In most treatment studies of osteoporosis in young women, bone mineral density (BMD)—an intermediate biometric endpoint—is the primary treatment outcome. In contrast, in the best studies in the menopausal population, the primary treatment outcome is bone fracture—a clinically important endpoint.
In addition, fewer consensus recommendations are available on the management of osteoporosis in young women than for its treatment in menopausal women. In this article, 3 different kinds of cases of bone loss in young women are used to develop key clinical points.
CASE 1 HISTORY
A teen with pelvic pain, bone loss
Therapy eased pain but decreased BMD
A 19-year-old woman has a 3-year history of severe, disabling dysmenorrhea. For 2 years she was treated with nonsteroidal anti-inflammatory drugs and cyclic estrogen-progestin contraceptives before switching to continuous oral contraceptives. These interventions did not relieve her pain. When she was 18, laparoscopy revealed stage I endometriosis, which was resected, providing 6 months of relief. When her pain recurred, the patient was started on leuprolide acetate depot, which caused amenorrhea and provided excellent pain relief. The patient said the leuprolide had “given back her life”. After 6 months of leuprolide therapy, a DXA bone scan demonstrated osteoporosis with a lumbar spine Z score of–2.6.
What treatment do you recommend?
Under age 25, use Z score, not T score
The diagnosis of osteoporosis in very young women is complex and continues to evolve. Physicians have been educated to use the T score (comparison to the mean peak bone mass of young adults) to assess BMD in menopausal women, in whom a decrease in T score of 1 standard deviation is associated with a 2- to 4-fold increase in fracture risk. However, teenage women are often still gaining bone mineral mass as the skeleton develops, so their T scores are normally below that of the peak bone mass of an adult woman. The Z score gives a good comparison of their bone mass with that of teens of the same sex at a similar developmental stage. Most experts agree that, when using DXA test results to assess bone density in women younger than 25, the Z score should be used.1 In one recent study, peak bone mass in the spine was achieved at approximately 23 years of age.2 As women reach age 25, the T and Z scores converge (FIGURE 1).
FIGURE 1 The Z score is more informative when the patient is under 25
The Z score compares the patient with persons of the same age and sex. The T score compares the patient with young normal adults of the same sex. Since bone mass increases until approximately age 20 to 25 years, it is best to use the Z score to evaluate the bone mineral density of women younger than 25 years.1 Reprinted from Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253–257. ©2004 with permission from Elsevier.
Treatment
For this patient with osteoporosis, there are several approaches to treatment:
Ensure adequate vitamin D and calcium. Vitamin D should be prescribed in doses of 400 to 800 IU daily. A recent meta-analysis reported that 800 IU vitamin D daily appears to provide better protection against osteoporotic fractures than 400 IU in menopausal women.3 Consider measuring 25-hydroxy vitamin D levels to assess the patient’s stores of this important pre-vitamin. Many young women are vitamin D-deficient.
Calcium intake should be in the range of 1,500 mg daily. To achieve this level of calcium intake, a calcium supplement, 500 to 1,000 mg daily, will probably need to be prescribed.
Discontinue leuprolide treatment. Typically, menses and ovarian estrogen production resume once leuprolide is stopped, and bone mass begins to recover. If pain recurs, the patient could be treated with cyclic or continuous estrogen-progestin contraceptives, depot-subQ Provera, a progestinreleasing intrauterine device, or a second laparoscopy procedure.
Continue leuprolide therapy and initiate steroid add-back treatment. Options shown to be effective in preserving bone mass in young women taking a gonadotropin-releasing hormone (GnRH) analogue include:
- norethindrone acetate, 5 mg daily,
- conjugated equine estrogen (Premarin), 0.3 or 0.625 mg daily, plus a progestin, and
- low-dose transdermal estrogen, 25 μg daily, plus a progestin.
Key trials
Lupron Add-Back Study. Women with endometriosis and chronic pelvic pain were randomly assigned to 1 of 4 treatment groups:
- leuprolide alone,
- leuprolide plus an oral synthetic progestin, norethindrone acetate, 5 mg daily,
- leuprolide plus “low-dose” conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, or
- leuprolide plus “high-dose” conjugated equine estrogen, 1.25 mg, plus norethindrone acetate, 5 mg daily.4
Women in all 4 groups received depotleuprolide, 3.75 mg intramuscularly every 4 weeks for 1 year. Over 1 year of treatment, BMD decreased significantly in the women who received the GnRH agonist alone. Bone density was preserved in the 3 groups that received steroid add-back.
Vasomotor symptoms were significantly reduced in all 3 groups receiving steroid add-back therapy, compared with the placebo group. However, more women in the group that received the larger dose of estrogen dropped out of the study than from the other groups because of more significant pelvic pain.
In summary, norethindrone, 5 mg daily, or low-dose conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, were both effective steroid add-back regimens for prevention of bone loss in young women with endometriosis being treated with long-term GnRH analogues (TABLE 1).
GnRH analogue vs GnRH plus estradiolprogestin. In another trial, women who experienced endometriosis and pelvic pain were randomized to receive a GnRH agonist alone or a GnRH agonist plus low-dose transdermal estradiol, 25 μg daily, plus medroxyprogesterone acetate, 2.5 mg daily, for 6 months. Women in both groups had similar improvement in pelvic pain symptoms and a similar decrease in endometriosis surgical staging scores as determined by laparoscopy before and after treatment. However, the women who received the GnRH agonist alone, without add-back therapy, had more vasomotor symptoms and a greater decline in BMD than the women who received the GnRH agonist plus low-dose transdermal estradiol plus progestin.5
This study supports the estrogenthreshold hypothesis6 that there is a “sweet spot” in estradiol concentration where vasomotor symptoms and bone loss can be attenuated, but where endometriosis lesion activity is suppressed. This “sweet spot” appears to be at an estradiol concentration of about 30 pg/mL (FIGURE 2).
GnRH analogue vs GnRH plus teriparatide. A synthetic parathyroid analogue (PTH 1-34, teriparatide, Forteo) has been demonstrated to prevent bone loss in hypoestrogenic women. In this study, women with pelvic pain and endometriosis were randomized to treatment with a GnRH agonist alone or a GnRH agonist plus teriparatide for 6 months. Women receiving the GnRH agonist alone had a decrease in BMD at the spine of 3.5%, when measured in the lateral plane. Women receiving combined treatment had an increase in BMD at the spine of 3.4%.7
The Food and Drug Administration (FDA) has approved teriparatide at a dose of 20 μg daily by subcutaneous injection for the treatment of osteoporosis. A major advantage of PTH is that it appears to stimulate both osteoblasts and osteoclasts. In contrast, estrogen may preferentially block osteoclast activity without significantly stimulating osteoblasts. A major disadvantage of PTH: It is currently available only as a daily injection. It also is more expensive than estrogen or a synthetic progestin.
CASE 1 MANAGEMENT
A teen with pelvic pain, bone loss
Long-term combined therapy improved her BMD
This patient strongly preferred to continue the leuprolide treatment because it was so effective in treating her pelvic pain. She continued her leuprolide treatment and started norethindrone acetate 5 mg daily. She reported continued excellent control of her pelvic pain on the combined regimen of leuprolide plus norethindrone acetate. Follow-up bone density measurement demonstrated significant improvement. She continues on long-term combined therapy and is scheduled for annual BMD measurements.
CASE 2 HISTORY
The female athlete triad
A college-age dancer with an eating disorder
A 21-year-old elite ballet dancer, height 5’4” and weight 104 lb (BMI 17.8 kg/m2), presents with a history of a stress fracture of her foot. Besides being amenorrheic, she is on a high-fiber macrobiotic diet. DXA measurement demonstrates a Z score consistent with osteoporosis. She refuses to take estrogen-progestin contraceptives because she claims they impair her ability to train for her dance performances. She wonders if she should start alendronate therapy.
The female athlete triad is the combination of amenorrhea, disordered eating, and osteoporosis.8 In young female athletes, exercise and disordered eating may cause menstrual irregularity, and either disordered eating or menstrual irregularity, or both, may cause decreased BMD.9
Strenuous exercise has divergent effects on trabecular and cortical bone. By causing hypoestrogenism, strenuous exercise decreases bone density at cortical sites (vertebral spine). However, the weight-bearing exercise itself can lead to an increase in bone density at weight-bearing sites.10
Many elite athletes with the female athlete triad do not want to alter the intensity of their training or their diet, which might result in weight gain and resolution of the osteoporosis. Some elite athletes report that they do not want to take standard birth control pills because they believe the pill may impair their training and peak competitive performance. The clinician is often challenged by these strongly held beliefs to identify a treatment plan that will reverse the bone loss.
Is systemic disease involved? Occasionally, systemic disease causes or contributes to low bone mass in a young woman. Laboratory tests may help in screening some women for these diseases (TABLE 2).
FIGURE 2 Aim for the “sweet spot” in estradiol concentrations
According to the estrogen-threshold hypothesis, there is a range, or “sweet spot,” of estradiol concentrations where endometriosis lesion activity is suppressed and vasomotor symptoms and bone loss are not excessive. In humans, estrogen concentrations in the range of 30 pg/mL appear to be associated with decreased endometriosis pain and minimal degrees of bone loss. At estradiol levels of less than 10 pg/mL, bone loss is markedly accelerated.6 Reprinted from Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. ©1992 with permission from Elsevier.TABLE 1
Effective steroid hormone add-back regimens
Regimens that suppressed vasomotor symptoms and bone loss associated with long-term GnRH analogue use
| INVESTIGATOR | RANDOMIZATION SCHEME | STEROID HORMONE ADD-BACK REGIMEN |
|---|---|---|
| Howell et al5 | 50 women with endometriosis and pelvic pain randomized to GnRH agonist alone or GnRH agonist plus add-back regimen | Transdermal estradiol, 25 μg/d, plus oral medroxyprogesterone acetate, 2.5 mg/d |
| Lupron Add-Back Study Group4 | 201 women with endometriosis and pelvic pain randomized to GnRH agonist plus placebo or GnRH agonist plus 1 of 3 add-back regimens | Oral norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 0.625 mg/d plus norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 1.25 mg/d plus norethindrone, 5 mg/d |
TABLE 2
Screening for secondary causes of bone loss
| The following serum tests may be useful in screening women with osteoporosis for secondary causes of disordered bone metabolism |
| Calcium and albumin |
| Phosphorus |
| 25-Hydroxy vitamin D |
| Thyroid-stimulating hormone |
| Alkaline phosphatase |
| Creatinine |
Treatment of elite athletes
Peak bone mass depends on many factors, including genetics11; ovarian estrogen production12; adequate intake of calories, protein, vitamin D, and calcium9; exercise13; and achievement of target height and weight.14 When combinations of risk factors for osteoporosis are present, such as an eating disorder plus amenorrhea, correction of only one factor may not result in improved BMD.15
There are few clinical trials of the treatment of osteoporosis in women with the female athlete triad or an eating disorder. Treatment recommendations are largely based on clinical experience. If the woman resists lifestyle and dietary changes that will result in weight gain, other treatment recommendations to pursue include:
- vitamin D and calcium supplements,
- increased dietary protein,
- weight training,16 and
- smoking cessation.
Psychological counseling may help many young women with disordered eating.
Hormonal therapy has not been documented to reliably increase bone density in young amenorrheic women with an eating disorder. In 3 clinical trials, the effect of estrogen replacement on bone density in such patients was negligible or minimal.17-19 Given our understanding of the positive effect of estrogen on spinal BMD, it is difficult to understand these findings. Two possibilities are that compliance with estrogen replacement was modest because the women did not want a return of menses, or an insufficient dose of estrogen was prescribed. Another is that being underweight blocks the positive effect of estrogen on bone density. One small clinical trial did report that treatment of hypoestrogenic women with an estrogen-progestin contraceptive containing 35 μg of ethinyl estradiol resulted in a significant increase in lumbar spine BMD (5.4%) and a nonsignificant increase in femur BMD (3.6%).20 A logical recommendation, based on clinical experience, would be to prescribe an estrogen-progestin contraceptive to this woman to help preserve or improve her bone density.
Bisphosphonate therapy has been studied in small groups of women with disordered eating and osteoporosis. In one small clinical trial, 32 women with anorexia nervosa were given 10 mg alendronate daily or placebo for 1 year. Alendronate treatment was associated with a nonsignificant increase in femoral neck and spine bone density of 4.4% and 3.5%, respectively. Weight gain was an important predictor of improved BMD.21
The young woman in this case probably should not be prescribed alendronate. The FDA drug information for alendronate warns: “Safety and efficacy have not been established in pregnant women. Animal studies have shown delays in delivery and fetal/neonatal death (secondary to hypocalcemia). Bisphosphonates are incorporated into the bone matrix and gradually released over time. Theoretically, there may be a risk of fetal harm when pregnancy follows the completion of therapy. Based on limited case reports with pamidronate, serum calcium levels in the newborn may be altered if administered during pregnancy.”
Although there are minimal data in humans that alendronate will have adverse effects on fetal bone development and function, findings in rats treated with very high doses of alendronate leave some concern about the potential risk for human fetuses.
The young woman in this case may want to have children in the near future. Bisphosphonates may be incorporated into the bone and have a long residual half-life, resulting in potential exposure of the fetus to low doses of the compound. Very few malformations in human pregnancy have been reported after treatment with bisphosphonates.22 Based on a cautious approach to this situation, however, I would recommend that this woman of reproductive age who plans future child-bearing probably should not be treated with alendronate.
CASE 2 MANAGEMENT
The female athlete triad
Estrogen-progestin ring and mental health evaluation
Many women with the female athlete triad do not meet formal criteria for anorexia nervosa, but have disordered eating patterns. Questions in the medical history such as “Do you think you should be dieting?”23 may help initiate a conversation about eating practices and attitudes. The woman in Case 2 was screened for clinical depression. She reported that she was not blue over the past 2 weeks, but noted that she worried that she measured too much of her self-worth by her body image—a diagnostic criterion for anorexia nervosa. After extensive counseling, she was willing to start an estrogen-progestin contraceptive ring. She was also referred to a mental health provider for further evaluation.
CASE 3 HISTORY
Glucocorticoid-related bone loss
A 35-year-old woman with rheumatoid arthritis
This woman (G1P1) has been treated with various doses of prednisone over the past 10 years. Currently she is taking prednisone, 20 mg daily. She is oligomenorrheic with menstrual cycles every 45 days. She has undergone both bilateral hip replacement and bilateral tubal ligation. Her BMI is 20.5 kg/m2. DXA shows osteoporosis at her lumbar spine.
Glucocorticoids are a common cause of osteoporosis in young women. These drugs inhibit osteoblast activity and increase osteoclast activity. They also increase renal calcium excretion and decrease intestinal calcium absorption and adrenal androgen production. A meta-analysis of 89 publications reported that chronic glucocorticoid use increased both the risk of osteoporosis and clinically significant fractures.24 Chronic glucocorticoid treatment was associated with an increased relative risk of vertebral and hip fracture of 2.60 (95% confidence interval [CI], 2.31–2.92) and 1.61 (95% CI, 1.47–1.76), respectively. Daily doses of prednisone 10 mg or greater were clearly associated with an increased risk of fracture. The onset of the increased fracture risk occurred within 3 months of initiating therapy.
Treatment
Treatment for this woman should include:
- vitamin D and calcium supplementation,25 and
- consideration of estrogen-progestin therapy26 and/or bisphosphonates.
PTH analogue treatment could also be utilized if neither estrogen replacement nor bisphosphonate treatment were possible.27 If the patient could discontinue the glucocorticoid therapy and begin another treatment for rheumatoid arthritis, such as etanercept (Enbrel), her BMD might improve.
Bisphosphonates are a first-line option, since she has had a bilateral tubal ligation and is at very low risk for a future pregnancy. Many clinical trials demonstrate the efficacy of bisphosphonates in this clinical situation. Bisphosphonates are approved by the FDA for the prevention and treatment of osteoporosis in women receiving glucocorticoids.
In a study of alendronate, the rate of new vertebral fractures was 0.7% in the alendronate group and 6.8% in the placebo group.28 Subjects who were taking at least 7.5 mg prednisone daily were randomized to receive alendronate, 5 or 10 mg daily, or a placebo. After 2 years of treatment, there was a 3.7% increase in lumbar spine BMD in the group treated with alendronate, 10 mg daily, and a 0.8% loss in bone denisty in the placebo group (FIGURE 3).
In a study of risedronate, 5 mg daily versus placebo, bisphosphonate treatment was associated with a 70% decrease in fracture risk.29
These clinical trials were completed before the development of once-weekly bisphosphonate treatment. Now that such treatment is available, it makes sense to prescribe either alendronate, 70 mg once weekly, or risedronate, 35 mg once weekly. Ibandronate, 150 mg once monthly, is also likely to be effective.
FIGURE 3 Alendronate improves BMD in women taking glucocorticoids
Effects of 2 years of alendronate treatment on bone mineral density of the lumbar spine in patients receiving average daily doses of prednisone of at least 7.5 mg. Alendronate treatment significantly improved bone mineral density (P<.0001). *Alendronate 2.5 mg was switched to 10 mg after it was determined that 2.5 mg was a suboptimal dose.
28 From Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, doubleblind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. Copyright ©2001. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Do US women get adequate treatment?
Surveys of community-based practices report that approximately 40% of women taking chronic glucocorticoid therapy are not receiving any intervention to prevent osteoporosis.30
Gynecologists are well positioned to ensure that these young women are fully apprised of their options and receive appropriate preventive intervention.
CASE 3 MANAGEMENT
Glucocorticoid-related bone loss
Estrogen-progestin patch, a bisphosphonate, and a rheumatology consult
This patient was started on an estrogen-progestin contraceptive patch and a bisphosphonate. An annual BMD measurement is planned.
In addition, a rheumatologist was consulted to determine if etanercept (Enbrel) could be initiated for the treatment of her rheumatoid arthritis, with a concomitant reduction in her prednisone dosage.
SUMMARYAim treatment toward future bone health
Many controversies remain unresolved concerning the best approach to bone loss in young women. On the one hand, most young women with osteoporosis are at low risk of vertebral and hip fracture, so a large number would need to be treated to prevent one fracture. On the other hand, most hope to become middle-aged; if young women begin adult life with osteoporosis, it is possible that they will have a poor foundation upon which to achieve optimal bone health. Until this key issue is resolved, it is probably best to try to help young women with osteoporosis achieve the best bone health possible. Bone health in young women is likely to be the foundation determining their future bone health and fracture risk.
- In young women, many cases of osteoporosis are caused by hypoestrogenism resulting from hormone treatment (eg, GnRH agonists, aromatase inhibitors) or lifestyle adaptations (elite athletics, eating disorders).
- Treatment of osteoporosis in young women can often be successful with the use of estrogen or “androgenic” progestins.
- Chronic glucocorticoid treatment is a common cause of clinically significant osteoporosis in young women. Glucocorticoid-induced osteoporosis is an important cause of premenopausal osteoporotic fractures.
- ObGyns play a key role in ensuring that women enter midlife with strong bones. A focus on young women at very high risk for osteoporosis will help to ensure that our patients build their future bone health on a strong foundation.
ObGyns are very well trained to diagnose and treat women with osteoporosis, most of whom are perimenopausal and menopausal. We are also treating a significant number of young women at risk for osteoporosis because of lifestyle choices or medical treatment of endometriosis or rheumatic diseases. Treatment of this population poses unique challenges and requires specialized approaches.
An important caveat in any discussion of bone loss in young women: Few randomized clinical trials have assessed the efficacy of the various treatments available. In most treatment studies of osteoporosis in young women, bone mineral density (BMD)—an intermediate biometric endpoint—is the primary treatment outcome. In contrast, in the best studies in the menopausal population, the primary treatment outcome is bone fracture—a clinically important endpoint.
In addition, fewer consensus recommendations are available on the management of osteoporosis in young women than for its treatment in menopausal women. In this article, 3 different kinds of cases of bone loss in young women are used to develop key clinical points.
CASE 1 HISTORY
A teen with pelvic pain, bone loss
Therapy eased pain but decreased BMD
A 19-year-old woman has a 3-year history of severe, disabling dysmenorrhea. For 2 years she was treated with nonsteroidal anti-inflammatory drugs and cyclic estrogen-progestin contraceptives before switching to continuous oral contraceptives. These interventions did not relieve her pain. When she was 18, laparoscopy revealed stage I endometriosis, which was resected, providing 6 months of relief. When her pain recurred, the patient was started on leuprolide acetate depot, which caused amenorrhea and provided excellent pain relief. The patient said the leuprolide had “given back her life”. After 6 months of leuprolide therapy, a DXA bone scan demonstrated osteoporosis with a lumbar spine Z score of–2.6.
What treatment do you recommend?
Under age 25, use Z score, not T score
The diagnosis of osteoporosis in very young women is complex and continues to evolve. Physicians have been educated to use the T score (comparison to the mean peak bone mass of young adults) to assess BMD in menopausal women, in whom a decrease in T score of 1 standard deviation is associated with a 2- to 4-fold increase in fracture risk. However, teenage women are often still gaining bone mineral mass as the skeleton develops, so their T scores are normally below that of the peak bone mass of an adult woman. The Z score gives a good comparison of their bone mass with that of teens of the same sex at a similar developmental stage. Most experts agree that, when using DXA test results to assess bone density in women younger than 25, the Z score should be used.1 In one recent study, peak bone mass in the spine was achieved at approximately 23 years of age.2 As women reach age 25, the T and Z scores converge (FIGURE 1).
FIGURE 1 The Z score is more informative when the patient is under 25
The Z score compares the patient with persons of the same age and sex. The T score compares the patient with young normal adults of the same sex. Since bone mass increases until approximately age 20 to 25 years, it is best to use the Z score to evaluate the bone mineral density of women younger than 25 years.1 Reprinted from Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253–257. ©2004 with permission from Elsevier.
Treatment
For this patient with osteoporosis, there are several approaches to treatment:
Ensure adequate vitamin D and calcium. Vitamin D should be prescribed in doses of 400 to 800 IU daily. A recent meta-analysis reported that 800 IU vitamin D daily appears to provide better protection against osteoporotic fractures than 400 IU in menopausal women.3 Consider measuring 25-hydroxy vitamin D levels to assess the patient’s stores of this important pre-vitamin. Many young women are vitamin D-deficient.
Calcium intake should be in the range of 1,500 mg daily. To achieve this level of calcium intake, a calcium supplement, 500 to 1,000 mg daily, will probably need to be prescribed.
Discontinue leuprolide treatment. Typically, menses and ovarian estrogen production resume once leuprolide is stopped, and bone mass begins to recover. If pain recurs, the patient could be treated with cyclic or continuous estrogen-progestin contraceptives, depot-subQ Provera, a progestinreleasing intrauterine device, or a second laparoscopy procedure.
Continue leuprolide therapy and initiate steroid add-back treatment. Options shown to be effective in preserving bone mass in young women taking a gonadotropin-releasing hormone (GnRH) analogue include:
- norethindrone acetate, 5 mg daily,
- conjugated equine estrogen (Premarin), 0.3 or 0.625 mg daily, plus a progestin, and
- low-dose transdermal estrogen, 25 μg daily, plus a progestin.
Key trials
Lupron Add-Back Study. Women with endometriosis and chronic pelvic pain were randomly assigned to 1 of 4 treatment groups:
- leuprolide alone,
- leuprolide plus an oral synthetic progestin, norethindrone acetate, 5 mg daily,
- leuprolide plus “low-dose” conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, or
- leuprolide plus “high-dose” conjugated equine estrogen, 1.25 mg, plus norethindrone acetate, 5 mg daily.4
Women in all 4 groups received depotleuprolide, 3.75 mg intramuscularly every 4 weeks for 1 year. Over 1 year of treatment, BMD decreased significantly in the women who received the GnRH agonist alone. Bone density was preserved in the 3 groups that received steroid add-back.
Vasomotor symptoms were significantly reduced in all 3 groups receiving steroid add-back therapy, compared with the placebo group. However, more women in the group that received the larger dose of estrogen dropped out of the study than from the other groups because of more significant pelvic pain.
In summary, norethindrone, 5 mg daily, or low-dose conjugated equine estrogen, 0.625 mg, plus norethindrone acetate, 5 mg daily, were both effective steroid add-back regimens for prevention of bone loss in young women with endometriosis being treated with long-term GnRH analogues (TABLE 1).
GnRH analogue vs GnRH plus estradiolprogestin. In another trial, women who experienced endometriosis and pelvic pain were randomized to receive a GnRH agonist alone or a GnRH agonist plus low-dose transdermal estradiol, 25 μg daily, plus medroxyprogesterone acetate, 2.5 mg daily, for 6 months. Women in both groups had similar improvement in pelvic pain symptoms and a similar decrease in endometriosis surgical staging scores as determined by laparoscopy before and after treatment. However, the women who received the GnRH agonist alone, without add-back therapy, had more vasomotor symptoms and a greater decline in BMD than the women who received the GnRH agonist plus low-dose transdermal estradiol plus progestin.5
This study supports the estrogenthreshold hypothesis6 that there is a “sweet spot” in estradiol concentration where vasomotor symptoms and bone loss can be attenuated, but where endometriosis lesion activity is suppressed. This “sweet spot” appears to be at an estradiol concentration of about 30 pg/mL (FIGURE 2).
GnRH analogue vs GnRH plus teriparatide. A synthetic parathyroid analogue (PTH 1-34, teriparatide, Forteo) has been demonstrated to prevent bone loss in hypoestrogenic women. In this study, women with pelvic pain and endometriosis were randomized to treatment with a GnRH agonist alone or a GnRH agonist plus teriparatide for 6 months. Women receiving the GnRH agonist alone had a decrease in BMD at the spine of 3.5%, when measured in the lateral plane. Women receiving combined treatment had an increase in BMD at the spine of 3.4%.7
The Food and Drug Administration (FDA) has approved teriparatide at a dose of 20 μg daily by subcutaneous injection for the treatment of osteoporosis. A major advantage of PTH is that it appears to stimulate both osteoblasts and osteoclasts. In contrast, estrogen may preferentially block osteoclast activity without significantly stimulating osteoblasts. A major disadvantage of PTH: It is currently available only as a daily injection. It also is more expensive than estrogen or a synthetic progestin.
CASE 1 MANAGEMENT
A teen with pelvic pain, bone loss
Long-term combined therapy improved her BMD
This patient strongly preferred to continue the leuprolide treatment because it was so effective in treating her pelvic pain. She continued her leuprolide treatment and started norethindrone acetate 5 mg daily. She reported continued excellent control of her pelvic pain on the combined regimen of leuprolide plus norethindrone acetate. Follow-up bone density measurement demonstrated significant improvement. She continues on long-term combined therapy and is scheduled for annual BMD measurements.
CASE 2 HISTORY
The female athlete triad
A college-age dancer with an eating disorder
A 21-year-old elite ballet dancer, height 5’4” and weight 104 lb (BMI 17.8 kg/m2), presents with a history of a stress fracture of her foot. Besides being amenorrheic, she is on a high-fiber macrobiotic diet. DXA measurement demonstrates a Z score consistent with osteoporosis. She refuses to take estrogen-progestin contraceptives because she claims they impair her ability to train for her dance performances. She wonders if she should start alendronate therapy.
The female athlete triad is the combination of amenorrhea, disordered eating, and osteoporosis.8 In young female athletes, exercise and disordered eating may cause menstrual irregularity, and either disordered eating or menstrual irregularity, or both, may cause decreased BMD.9
Strenuous exercise has divergent effects on trabecular and cortical bone. By causing hypoestrogenism, strenuous exercise decreases bone density at cortical sites (vertebral spine). However, the weight-bearing exercise itself can lead to an increase in bone density at weight-bearing sites.10
Many elite athletes with the female athlete triad do not want to alter the intensity of their training or their diet, which might result in weight gain and resolution of the osteoporosis. Some elite athletes report that they do not want to take standard birth control pills because they believe the pill may impair their training and peak competitive performance. The clinician is often challenged by these strongly held beliefs to identify a treatment plan that will reverse the bone loss.
Is systemic disease involved? Occasionally, systemic disease causes or contributes to low bone mass in a young woman. Laboratory tests may help in screening some women for these diseases (TABLE 2).
FIGURE 2 Aim for the “sweet spot” in estradiol concentrations
According to the estrogen-threshold hypothesis, there is a range, or “sweet spot,” of estradiol concentrations where endometriosis lesion activity is suppressed and vasomotor symptoms and bone loss are not excessive. In humans, estrogen concentrations in the range of 30 pg/mL appear to be associated with decreased endometriosis pain and minimal degrees of bone loss. At estradiol levels of less than 10 pg/mL, bone loss is markedly accelerated.6 Reprinted from Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740–745. ©1992 with permission from Elsevier.TABLE 1
Effective steroid hormone add-back regimens
Regimens that suppressed vasomotor symptoms and bone loss associated with long-term GnRH analogue use
| INVESTIGATOR | RANDOMIZATION SCHEME | STEROID HORMONE ADD-BACK REGIMEN |
|---|---|---|
| Howell et al5 | 50 women with endometriosis and pelvic pain randomized to GnRH agonist alone or GnRH agonist plus add-back regimen | Transdermal estradiol, 25 μg/d, plus oral medroxyprogesterone acetate, 2.5 mg/d |
| Lupron Add-Back Study Group4 | 201 women with endometriosis and pelvic pain randomized to GnRH agonist plus placebo or GnRH agonist plus 1 of 3 add-back regimens | Oral norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 0.625 mg/d plus norethindrone acetate, 5 mg/d or Oral conjugated equine estrogen, 1.25 mg/d plus norethindrone, 5 mg/d |
TABLE 2
Screening for secondary causes of bone loss
| The following serum tests may be useful in screening women with osteoporosis for secondary causes of disordered bone metabolism |
| Calcium and albumin |
| Phosphorus |
| 25-Hydroxy vitamin D |
| Thyroid-stimulating hormone |
| Alkaline phosphatase |
| Creatinine |
Treatment of elite athletes
Peak bone mass depends on many factors, including genetics11; ovarian estrogen production12; adequate intake of calories, protein, vitamin D, and calcium9; exercise13; and achievement of target height and weight.14 When combinations of risk factors for osteoporosis are present, such as an eating disorder plus amenorrhea, correction of only one factor may not result in improved BMD.15
There are few clinical trials of the treatment of osteoporosis in women with the female athlete triad or an eating disorder. Treatment recommendations are largely based on clinical experience. If the woman resists lifestyle and dietary changes that will result in weight gain, other treatment recommendations to pursue include:
- vitamin D and calcium supplements,
- increased dietary protein,
- weight training,16 and
- smoking cessation.
Psychological counseling may help many young women with disordered eating.
Hormonal therapy has not been documented to reliably increase bone density in young amenorrheic women with an eating disorder. In 3 clinical trials, the effect of estrogen replacement on bone density in such patients was negligible or minimal.17-19 Given our understanding of the positive effect of estrogen on spinal BMD, it is difficult to understand these findings. Two possibilities are that compliance with estrogen replacement was modest because the women did not want a return of menses, or an insufficient dose of estrogen was prescribed. Another is that being underweight blocks the positive effect of estrogen on bone density. One small clinical trial did report that treatment of hypoestrogenic women with an estrogen-progestin contraceptive containing 35 μg of ethinyl estradiol resulted in a significant increase in lumbar spine BMD (5.4%) and a nonsignificant increase in femur BMD (3.6%).20 A logical recommendation, based on clinical experience, would be to prescribe an estrogen-progestin contraceptive to this woman to help preserve or improve her bone density.
Bisphosphonate therapy has been studied in small groups of women with disordered eating and osteoporosis. In one small clinical trial, 32 women with anorexia nervosa were given 10 mg alendronate daily or placebo for 1 year. Alendronate treatment was associated with a nonsignificant increase in femoral neck and spine bone density of 4.4% and 3.5%, respectively. Weight gain was an important predictor of improved BMD.21
The young woman in this case probably should not be prescribed alendronate. The FDA drug information for alendronate warns: “Safety and efficacy have not been established in pregnant women. Animal studies have shown delays in delivery and fetal/neonatal death (secondary to hypocalcemia). Bisphosphonates are incorporated into the bone matrix and gradually released over time. Theoretically, there may be a risk of fetal harm when pregnancy follows the completion of therapy. Based on limited case reports with pamidronate, serum calcium levels in the newborn may be altered if administered during pregnancy.”
Although there are minimal data in humans that alendronate will have adverse effects on fetal bone development and function, findings in rats treated with very high doses of alendronate leave some concern about the potential risk for human fetuses.
The young woman in this case may want to have children in the near future. Bisphosphonates may be incorporated into the bone and have a long residual half-life, resulting in potential exposure of the fetus to low doses of the compound. Very few malformations in human pregnancy have been reported after treatment with bisphosphonates.22 Based on a cautious approach to this situation, however, I would recommend that this woman of reproductive age who plans future child-bearing probably should not be treated with alendronate.
CASE 2 MANAGEMENT
The female athlete triad
Estrogen-progestin ring and mental health evaluation
Many women with the female athlete triad do not meet formal criteria for anorexia nervosa, but have disordered eating patterns. Questions in the medical history such as “Do you think you should be dieting?”23 may help initiate a conversation about eating practices and attitudes. The woman in Case 2 was screened for clinical depression. She reported that she was not blue over the past 2 weeks, but noted that she worried that she measured too much of her self-worth by her body image—a diagnostic criterion for anorexia nervosa. After extensive counseling, she was willing to start an estrogen-progestin contraceptive ring. She was also referred to a mental health provider for further evaluation.
CASE 3 HISTORY
Glucocorticoid-related bone loss
A 35-year-old woman with rheumatoid arthritis
This woman (G1P1) has been treated with various doses of prednisone over the past 10 years. Currently she is taking prednisone, 20 mg daily. She is oligomenorrheic with menstrual cycles every 45 days. She has undergone both bilateral hip replacement and bilateral tubal ligation. Her BMI is 20.5 kg/m2. DXA shows osteoporosis at her lumbar spine.
Glucocorticoids are a common cause of osteoporosis in young women. These drugs inhibit osteoblast activity and increase osteoclast activity. They also increase renal calcium excretion and decrease intestinal calcium absorption and adrenal androgen production. A meta-analysis of 89 publications reported that chronic glucocorticoid use increased both the risk of osteoporosis and clinically significant fractures.24 Chronic glucocorticoid treatment was associated with an increased relative risk of vertebral and hip fracture of 2.60 (95% confidence interval [CI], 2.31–2.92) and 1.61 (95% CI, 1.47–1.76), respectively. Daily doses of prednisone 10 mg or greater were clearly associated with an increased risk of fracture. The onset of the increased fracture risk occurred within 3 months of initiating therapy.
Treatment
Treatment for this woman should include:
- vitamin D and calcium supplementation,25 and
- consideration of estrogen-progestin therapy26 and/or bisphosphonates.
PTH analogue treatment could also be utilized if neither estrogen replacement nor bisphosphonate treatment were possible.27 If the patient could discontinue the glucocorticoid therapy and begin another treatment for rheumatoid arthritis, such as etanercept (Enbrel), her BMD might improve.
Bisphosphonates are a first-line option, since she has had a bilateral tubal ligation and is at very low risk for a future pregnancy. Many clinical trials demonstrate the efficacy of bisphosphonates in this clinical situation. Bisphosphonates are approved by the FDA for the prevention and treatment of osteoporosis in women receiving glucocorticoids.
In a study of alendronate, the rate of new vertebral fractures was 0.7% in the alendronate group and 6.8% in the placebo group.28 Subjects who were taking at least 7.5 mg prednisone daily were randomized to receive alendronate, 5 or 10 mg daily, or a placebo. After 2 years of treatment, there was a 3.7% increase in lumbar spine BMD in the group treated with alendronate, 10 mg daily, and a 0.8% loss in bone denisty in the placebo group (FIGURE 3).
In a study of risedronate, 5 mg daily versus placebo, bisphosphonate treatment was associated with a 70% decrease in fracture risk.29
These clinical trials were completed before the development of once-weekly bisphosphonate treatment. Now that such treatment is available, it makes sense to prescribe either alendronate, 70 mg once weekly, or risedronate, 35 mg once weekly. Ibandronate, 150 mg once monthly, is also likely to be effective.
FIGURE 3 Alendronate improves BMD in women taking glucocorticoids
Effects of 2 years of alendronate treatment on bone mineral density of the lumbar spine in patients receiving average daily doses of prednisone of at least 7.5 mg. Alendronate treatment significantly improved bone mineral density (P<.0001). *Alendronate 2.5 mg was switched to 10 mg after it was determined that 2.5 mg was a suboptimal dose.
28 From Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, doubleblind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202–211. Copyright ©2001. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc.
Do US women get adequate treatment?
Surveys of community-based practices report that approximately 40% of women taking chronic glucocorticoid therapy are not receiving any intervention to prevent osteoporosis.30
Gynecologists are well positioned to ensure that these young women are fully apprised of their options and receive appropriate preventive intervention.
CASE 3 MANAGEMENT
Glucocorticoid-related bone loss
Estrogen-progestin patch, a bisphosphonate, and a rheumatology consult
This patient was started on an estrogen-progestin contraceptive patch and a bisphosphonate. An annual BMD measurement is planned.
In addition, a rheumatologist was consulted to determine if etanercept (Enbrel) could be initiated for the treatment of her rheumatoid arthritis, with a concomitant reduction in her prednisone dosage.
SUMMARYAim treatment toward future bone health
Many controversies remain unresolved concerning the best approach to bone loss in young women. On the one hand, most young women with osteoporosis are at low risk of vertebral and hip fracture, so a large number would need to be treated to prevent one fracture. On the other hand, most hope to become middle-aged; if young women begin adult life with osteoporosis, it is possible that they will have a poor foundation upon which to achieve optimal bone health. Until this key issue is resolved, it is probably best to try to help young women with osteoporosis achieve the best bone health possible. Bone health in young women is likely to be the foundation determining their future bone health and fracture risk.
1. Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253-257.
2. Lin YC, Lyle RM, Weaver CM, et al. Peak spine and femoral neck bone mass in young women. Bone. 2003;32:546-253.
3. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation. JAMA. 2005;293:2257-2264.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
5. Howell R, Edmonds D, Dowsett M. Gonadotropin releasing hormone analogue plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril. 1996;66:666-668.
6. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
7. Finkelstein JS, Klibanski A, Shaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331:1618-1623.
8. Warren MP, Brooks-Gunn J, Fox RP, Holderness C, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162-3168.
9. Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711-719.
10. Young N, Formica C, Szmukler, Seeman E. Bone density at weight-bearing and non weight-bearing sites in ballet dancers: the effects of exercise, hypogonadism and body weight. J Clin Endocrinol Metab. 1994;78:449-454.
11. Young D, Hopper JL, Nowson CA, et al. Determinants of bone mineral mass in 10- to 26- year old females: a twin study. J Bone Miner Res. 1995;10:558-567.
12. Drinkwater BL, Bruemner B, Chestnut CH. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545-548.
13. Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579-586.
14. Lloyd T, Rollings N, Andon MB, et al. Determinants of bone density in young women. J Clin Endocrinol Metab. 1992;75:383-387.
15. Jonnavithula S, Warren MP, Fox RP, Lazaro MI. Bone mineral density is compromised in amenorrheic women despite return of menses: a 2-year study. Obstet Gynecol. 1993;81:669-674.
16. Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Mineral Res. 1995;10:574-585.
17. Klibanski A, Biller BMK, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898-904.
18. Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135-143.
19. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935-4941.
20. Hergenroeder AC, Smith EO, Shypailo R, et al. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone acetate or placebo over 12 months. Am J Obstet Gynecol. 1997;176:1017-1025.
21. Golden NH, Iglesias EA, Jacobsen MS, et al. Alendronate for the treatment of osteopenia in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:3179-3185.
22. Ott SM. Letter re: alendronate in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:5508.-
23. Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Phys. 2003;67:297-304.
24. van Staa TP, Leufkens HGM, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int. 2002;13:624-629.
25. Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292-299.
26. Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499-1505.
27. Lane NE, Roe B, Genant HK, Arnaud C. Parathyroid hormone treatment reverses glucocorticoid-induced osteoporosis: results of a randomized controlled trial. J Clin Invest. 1998;102:1627-1633.
28. Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202-211.
29. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006-1013.
30. Yood RA, Harrold L, Fish L, et al. Prevention of glucocorticoid-induced osteoporosis: experience in a managed care setting. Arch Intern Med. 2001;161:1322-1327.
The author reports no financial relationships relevant to this article.
1. Gafni RI, Baron J. Overdiagnosis of osteoporosis in children due to misinterpretation of dual energy x-ray absorptiometry. J Pediatr. 2004;144:253-257.
2. Lin YC, Lyle RM, Weaver CM, et al. Peak spine and femoral neck bone mass in young women. Bone. 2003;32:546-253.
3. Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation. JAMA. 2005;293:2257-2264.
4. Hornstein MD, Surrey ES, Weisberg GW, Casino LA. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet Gynecol. 1998;91:16-24.
5. Howell R, Edmonds D, Dowsett M. Gonadotropin releasing hormone analogue plus hormone replacement therapy for the treatment of endometriosis: a randomized controlled trial. Fertil Steril. 1996;66:666-668.
6. Barbieri RL. Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol. 1992;166:740-745.
7. Finkelstein JS, Klibanski A, Shaefer EH, Hornstein MD, Schiff I, Neer RM. Parathyroid hormone for the prevention of bone loss induced by estrogen deficiency. N Engl J Med. 1994;331:1618-1623.
8. Warren MP, Brooks-Gunn J, Fox RP, Holderness C, Hyle EP, Hamilton WG. Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: a longitudinal study. J Clin Endocrinol Metab. 2002;87:3162-3168.
9. Cobb KL, Bachrach LK, Greendale G, et al. Disordered eating, menstrual irregularity and bone mineral density in female runners. Med Sci Sports Exerc. 2003;35:711-719.
10. Young N, Formica C, Szmukler, Seeman E. Bone density at weight-bearing and non weight-bearing sites in ballet dancers: the effects of exercise, hypogonadism and body weight. J Clin Endocrinol Metab. 1994;78:449-454.
11. Young D, Hopper JL, Nowson CA, et al. Determinants of bone mineral mass in 10- to 26- year old females: a twin study. J Bone Miner Res. 1995;10:558-567.
12. Drinkwater BL, Bruemner B, Chestnut CH. Menstrual history as a determinant of current bone density in young athletes. JAMA. 1990;263:545-548.
13. Fehily AM, Coles RJ, Evans WD, Elwood PC. Factors affecting bone density in young adults. Am J Clin Nutr. 1992;56:579-586.
14. Lloyd T, Rollings N, Andon MB, et al. Determinants of bone density in young women. J Clin Endocrinol Metab. 1992;75:383-387.
15. Jonnavithula S, Warren MP, Fox RP, Lazaro MI. Bone mineral density is compromised in amenorrheic women despite return of menses: a 2-year study. Obstet Gynecol. 1993;81:669-674.
16. Friedlander AL, Genant HK, Sadowsky S, Byl NN, Gluer CC. A two-year program of aerobics and weight training enhances bone mineral density of young women. J Bone Mineral Res. 1995;10:574-585.
17. Klibanski A, Biller BMK, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898-904.
18. Golden NH, Lanzkowsky L, Schebendach J, et al. The effect of estrogen-progestin treatment on bone mineral density in anorexia nervosa. J Pediatr Adolesc Gynecol. 2002;15:135-143.
19. Gordon CM, Grace E, Emans SJ, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. J Clin Endocrinol Metab. 2002;87:4935-4941.
20. Hergenroeder AC, Smith EO, Shypailo R, et al. Bone mineral changes in young women with hypothalamic amenorrhea treated with oral contraceptives, medroxyprogesterone acetate or placebo over 12 months. Am J Obstet Gynecol. 1997;176:1017-1025.
21. Golden NH, Iglesias EA, Jacobsen MS, et al. Alendronate for the treatment of osteopenia in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:3179-3185.
22. Ott SM. Letter re: alendronate in anorexia nervosa. J Clin Endocrinol Metab. 2005;90:5508.-
23. Pritts SD, Susman J. Diagnosis of eating disorders in primary care. Am Fam Phys. 2003;67:297-304.
24. van Staa TP, Leufkens HGM, Cooper C. Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int. 2002;13:624-629.
25. Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. N Engl J Med. 1998;339:292-299.
26. Hall GM, Daniels M, Doyle DV, Spector TD. Effect of hormone replacement therapy on bone mass in rheumatoid arthritis patients treated with and without steroids. Arthritis Rheum. 1994;37:1499-1505.
27. Lane NE, Roe B, Genant HK, Arnaud C. Parathyroid hormone treatment reverses glucocorticoid-induced osteoporosis: results of a randomized controlled trial. J Clin Invest. 1998;102:1627-1633.
28. Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum. 2001;44:202-211.
29. Reid DM, Hughes RA, Laan RF, et al. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006-1013.
30. Yood RA, Harrold L, Fish L, et al. Prevention of glucocorticoid-induced osteoporosis: experience in a managed care setting. Arch Intern Med. 2001;161:1322-1327.
The author reports no financial relationships relevant to this article.