User login
DENVER – Uninterrupted warfarin therapy during pacemaker or implantable cardioverter-defibrillator surgery in patients at high thromboembolic risk proved superior to the guideline-recommended practice of discontinuing warfarin and bridging with heparin, according to a large, multicenter, randomized clinical trial.
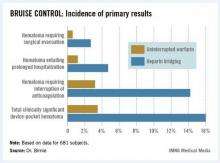
The primary outcome in the 17-center, 681-patient BRUISE CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) was the incidence of clinically significant device-pocket hematoma. The rate was 16% in patients randomized to heparin bridging, compared with 3.5% with uninterrupted warfarin, Dr. David H. Birnie reported at the annual meeting of the Heart Rhythm Society.
These results are clearly practice changing. Heparin bridging has been the standard of care. It is recommended in this common clinical scenario in all of the major guidelines, but that’s bound to change as a result of BRUISE CONTROL, predicted Dr. Birnie of the University of Ottawa Heart Institute.
"This trial was a home run. It was unequivocally positive," he commented. "For sure, our clinical practice changed as soon as we saw those results."
Device-pocket hematoma is a "very nasty" complication of cardiac device surgery, Dr. Birnie noted. It is quite painful, can cause device infection, and is difficult to treat. Clinically significant device-pocket hematoma was defined in this trial as a hematoma resulting in prolonged hospitalization for an additional day or more, or interruption of oral anticoagulation for at least 24 hours, and/or requiring additional surgery. All three components of the primary endpoint were significantly less frequent in the uninterrupted warfarin group (see chart).
Performing device surgery in patients on uninterrupted warfarin with a median international normalized ratio (INR) of 2.3 was not associated with any increase in major perioperative bleeding or other surgical or thromboembolic complications. And patient satisfaction surveys indicated subjects greatly preferred having their procedure without stopping their warfarin.
BRUISE CONTROL was planned as a definitive 1,000-patient clinical trial. However, the Data and Safety Monitoring Board halted the study after an interim analysis involving the first 681 subjects.
Of note, this was a study restricted to patients at high stroke risk – greater than 5% annually – as defined by the presence of atrial fibrillation and a CHADS2 score of 3 or more or the presence of a mechanical heart valve.
Dr. Birnie said that although it seems counterintuitive to have less bleeding when cardiac device surgery is performed on a fully anticoagulated patient, as occurred in BRUISE CONTROL, he and his coinvestigators have an explanatory hypothesis: "When bleeding occurs in a fully anticoagulated patient, the operator can readily see it and address it. On the contrary, with bridging you get hemostasis at the time of surgery, but you may have missed a tiny little thing, and then 24 hours later when you start up your heparin bridging again, that’s when the bleeding occurs. It’s a physiologically plausible explanation," he said.
In a multivariate analysis, neither the use of pressure dressings, nor the use of sandbags, nor injection of antibleeding agents into the device pocket before closure had any significant impact on the incidence of clinically significant hematomas. Only three factors did: uninterrupted warfarin therapy, which reduced the risk by 84%; aspirin therapy, which doubled the risk; and the presence of diabetes mellitus, which was inexplicably associated with a 52% reduction in hematoma risk.
Each year roughly 1.6 million people worldwide undergo pacemaker or implantable cardioverter-defibrillator (ICD) implantation. Up to one-third of them are on long-term oral anticoagulation, most commonly with warfarin.
Dr. Birnie stressed that the BRUISE CONTROL findings have no relevance to patients on one of the new oral anticoagulants.
"The whole risk/benefit ratio of the new agents is completely different from warfarin. Their onset and offset of action is hours as opposed to the 5 days for warfarin," he noted.
With that point in mind, he and his coinvestigators have just started the BRUISE CONTROL-2 trial, which is examining whether it’s better to stop the new agents around the time of device surgery or continue the medication uninterrupted.
Simultaneous with Dr. Birnie’s presentation of the BRUISE CONTROL data in Denver, the study results were published online (N. Engl. J. Med. 2013 May 9 [doi: 10.1056/NEJMoa1302946]).
BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.
DENVER – Uninterrupted warfarin therapy during pacemaker or implantable cardioverter-defibrillator surgery in patients at high thromboembolic risk proved superior to the guideline-recommended practice of discontinuing warfarin and bridging with heparin, according to a large, multicenter, randomized clinical trial.
The primary outcome in the 17-center, 681-patient BRUISE CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) was the incidence of clinically significant device-pocket hematoma. The rate was 16% in patients randomized to heparin bridging, compared with 3.5% with uninterrupted warfarin, Dr. David H. Birnie reported at the annual meeting of the Heart Rhythm Society.
These results are clearly practice changing. Heparin bridging has been the standard of care. It is recommended in this common clinical scenario in all of the major guidelines, but that’s bound to change as a result of BRUISE CONTROL, predicted Dr. Birnie of the University of Ottawa Heart Institute.
"This trial was a home run. It was unequivocally positive," he commented. "For sure, our clinical practice changed as soon as we saw those results."
Device-pocket hematoma is a "very nasty" complication of cardiac device surgery, Dr. Birnie noted. It is quite painful, can cause device infection, and is difficult to treat. Clinically significant device-pocket hematoma was defined in this trial as a hematoma resulting in prolonged hospitalization for an additional day or more, or interruption of oral anticoagulation for at least 24 hours, and/or requiring additional surgery. All three components of the primary endpoint were significantly less frequent in the uninterrupted warfarin group (see chart).
Performing device surgery in patients on uninterrupted warfarin with a median international normalized ratio (INR) of 2.3 was not associated with any increase in major perioperative bleeding or other surgical or thromboembolic complications. And patient satisfaction surveys indicated subjects greatly preferred having their procedure without stopping their warfarin.
BRUISE CONTROL was planned as a definitive 1,000-patient clinical trial. However, the Data and Safety Monitoring Board halted the study after an interim analysis involving the first 681 subjects.
Of note, this was a study restricted to patients at high stroke risk – greater than 5% annually – as defined by the presence of atrial fibrillation and a CHADS2 score of 3 or more or the presence of a mechanical heart valve.
Dr. Birnie said that although it seems counterintuitive to have less bleeding when cardiac device surgery is performed on a fully anticoagulated patient, as occurred in BRUISE CONTROL, he and his coinvestigators have an explanatory hypothesis: "When bleeding occurs in a fully anticoagulated patient, the operator can readily see it and address it. On the contrary, with bridging you get hemostasis at the time of surgery, but you may have missed a tiny little thing, and then 24 hours later when you start up your heparin bridging again, that’s when the bleeding occurs. It’s a physiologically plausible explanation," he said.
In a multivariate analysis, neither the use of pressure dressings, nor the use of sandbags, nor injection of antibleeding agents into the device pocket before closure had any significant impact on the incidence of clinically significant hematomas. Only three factors did: uninterrupted warfarin therapy, which reduced the risk by 84%; aspirin therapy, which doubled the risk; and the presence of diabetes mellitus, which was inexplicably associated with a 52% reduction in hematoma risk.
Each year roughly 1.6 million people worldwide undergo pacemaker or implantable cardioverter-defibrillator (ICD) implantation. Up to one-third of them are on long-term oral anticoagulation, most commonly with warfarin.
Dr. Birnie stressed that the BRUISE CONTROL findings have no relevance to patients on one of the new oral anticoagulants.
"The whole risk/benefit ratio of the new agents is completely different from warfarin. Their onset and offset of action is hours as opposed to the 5 days for warfarin," he noted.
With that point in mind, he and his coinvestigators have just started the BRUISE CONTROL-2 trial, which is examining whether it’s better to stop the new agents around the time of device surgery or continue the medication uninterrupted.
Simultaneous with Dr. Birnie’s presentation of the BRUISE CONTROL data in Denver, the study results were published online (N. Engl. J. Med. 2013 May 9 [doi: 10.1056/NEJMoa1302946]).
BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.
DENVER – Uninterrupted warfarin therapy during pacemaker or implantable cardioverter-defibrillator surgery in patients at high thromboembolic risk proved superior to the guideline-recommended practice of discontinuing warfarin and bridging with heparin, according to a large, multicenter, randomized clinical trial.
The primary outcome in the 17-center, 681-patient BRUISE CONTROL (Bridge or Continue Coumadin for Device Surgery Randomized Controlled Trial) was the incidence of clinically significant device-pocket hematoma. The rate was 16% in patients randomized to heparin bridging, compared with 3.5% with uninterrupted warfarin, Dr. David H. Birnie reported at the annual meeting of the Heart Rhythm Society.
These results are clearly practice changing. Heparin bridging has been the standard of care. It is recommended in this common clinical scenario in all of the major guidelines, but that’s bound to change as a result of BRUISE CONTROL, predicted Dr. Birnie of the University of Ottawa Heart Institute.
"This trial was a home run. It was unequivocally positive," he commented. "For sure, our clinical practice changed as soon as we saw those results."
Device-pocket hematoma is a "very nasty" complication of cardiac device surgery, Dr. Birnie noted. It is quite painful, can cause device infection, and is difficult to treat. Clinically significant device-pocket hematoma was defined in this trial as a hematoma resulting in prolonged hospitalization for an additional day or more, or interruption of oral anticoagulation for at least 24 hours, and/or requiring additional surgery. All three components of the primary endpoint were significantly less frequent in the uninterrupted warfarin group (see chart).
Performing device surgery in patients on uninterrupted warfarin with a median international normalized ratio (INR) of 2.3 was not associated with any increase in major perioperative bleeding or other surgical or thromboembolic complications. And patient satisfaction surveys indicated subjects greatly preferred having their procedure without stopping their warfarin.
BRUISE CONTROL was planned as a definitive 1,000-patient clinical trial. However, the Data and Safety Monitoring Board halted the study after an interim analysis involving the first 681 subjects.
Of note, this was a study restricted to patients at high stroke risk – greater than 5% annually – as defined by the presence of atrial fibrillation and a CHADS2 score of 3 or more or the presence of a mechanical heart valve.
Dr. Birnie said that although it seems counterintuitive to have less bleeding when cardiac device surgery is performed on a fully anticoagulated patient, as occurred in BRUISE CONTROL, he and his coinvestigators have an explanatory hypothesis: "When bleeding occurs in a fully anticoagulated patient, the operator can readily see it and address it. On the contrary, with bridging you get hemostasis at the time of surgery, but you may have missed a tiny little thing, and then 24 hours later when you start up your heparin bridging again, that’s when the bleeding occurs. It’s a physiologically plausible explanation," he said.
In a multivariate analysis, neither the use of pressure dressings, nor the use of sandbags, nor injection of antibleeding agents into the device pocket before closure had any significant impact on the incidence of clinically significant hematomas. Only three factors did: uninterrupted warfarin therapy, which reduced the risk by 84%; aspirin therapy, which doubled the risk; and the presence of diabetes mellitus, which was inexplicably associated with a 52% reduction in hematoma risk.
Each year roughly 1.6 million people worldwide undergo pacemaker or implantable cardioverter-defibrillator (ICD) implantation. Up to one-third of them are on long-term oral anticoagulation, most commonly with warfarin.
Dr. Birnie stressed that the BRUISE CONTROL findings have no relevance to patients on one of the new oral anticoagulants.
"The whole risk/benefit ratio of the new agents is completely different from warfarin. Their onset and offset of action is hours as opposed to the 5 days for warfarin," he noted.
With that point in mind, he and his coinvestigators have just started the BRUISE CONTROL-2 trial, which is examining whether it’s better to stop the new agents around the time of device surgery or continue the medication uninterrupted.
Simultaneous with Dr. Birnie’s presentation of the BRUISE CONTROL data in Denver, the study results were published online (N. Engl. J. Med. 2013 May 9 [doi: 10.1056/NEJMoa1302946]).
BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.
Major finding: The incidence of clinically significant device-pocket hematoma in patients at high thromboembolic risk who underwent pacemaker or implantable cardioverter-defibrillator surgery was 3.5% if they remained on full-dose warfarin, compared with 16% with the guideline-recommended, standard-of-care practice of interrupting warfarin in favor of heparin bridging.
Data source: BRUISE CONTROL was a 681-patient, 17-center randomized clinical trial.
Disclosures: BRUISE CONTROL was funded by the Canadian Institutes of Health Research and the Ministry of Health and Long-Term Care of Ontario. Dr. Birnie reported having no conflicts of interest.