User login
Children of alcoholics have a 40% to 60% increased risk of developing severe alcohol-related problems1—a harsh legacy recognized for >30 years. Now, as the result of rapidly growing evidence, we can explain in greater detail why alcoholism runs in families when discussing alcohol dependence with patients.
Individuals vary in response to medications and substances of abuse, and genetic research is revealing the heritable origins. Numerous genetic variations are known to influence response to alcohol, as well as alcoholism’s pathophysiology, clinical manifestations, and treatment. Pieces are still missing from this complex picture, but investigators are identifying possible risk factors for alcoholism and matching potential responders with treatments such as naltrexone and acamprosate.
This article provides a progress report on contemporary genetic research of alcoholism. Our goal is to inform your clinical practice by describing:
- new understandings of the genetics of alcoholism
- how researchers identify relationships between genetic variations and clinical/behavioral phenomena
- practical implications of this knowledge.
Genetic variations and risk of addiction
No single gene appears to cause alcoholism. Many genetic variations that accumulated during evolution likely contribute to individual differences in response to alcohol and susceptibility to developing alcohol-related problems. A growing number of genetic variations have been associated with increased alcohol tolerance, consumption, and other related phenotypes.
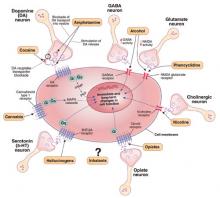
Like other addictive substances, alcohol triggers pharmacodynamic effects by interacting with a variety of molecular targets (Figure 1).2 These target proteins in turn interact with specific signaling proteins and trigger responses in complex functional pathways. Genetic variations may affect the structure of genes coding for proteins that constitute pathways involved in alcohol’s effects on target proteins (pharmacodynamics) or its metabolism (pharmacokinetics). If such variations alter the production, function, or stability of these proteins, the pathway’s function also may be altered and produce behavioral phenotypes—such as high or low sensitivity to alcohol’s effects.
Alcoholism-related phenotypes. DSM-IV-TR diagnostic criteria include some but not all of the multiple phenotypes within alcoholism’s clinical presentation. Researchers in the Collaborative Studies on Genetics of Alcoholism (COGA)3 identified chromosome regions linked to alcoholism-related phenotypes, including:
- alcohol dependence4
- later age of drinking onset and increased harm avoidance5
- alcoholism and depression6
- alcohol sensitivity7
- alcohol consumption.8
To identify genes of interest within these chromosome regions, researchers used transitional phenotypes (endophenotypes) that “lie on the pathway between genes and disease,”9,10 and allowed them to characterize the neural systems affected by gene risk variants.11 As a result, they found associations among variations in the GABRA2 gene, (encoding the alpha-2 subunit of the GABAA receptor), specific brain oscillations (electrophysiologic endophenotype), and predisposition to alcohol dependence.12,13 By this same strategy, researchers discovered an association between the endophenotype (low-level of response to alcohol) and some genotypes, including at least 1 short allele of the serotonin transporter gene SLC6A4.14
Figure 1
Known molecular targets for alcohol and other drugs of abuse
Effects of drugs of abuse (black arrows) influence intracellular signaling pathways (blue arrows) to produce immediate and long-term changes in cell function.
cAMP: cyclic adenosine monophosphate; GABAA: gamma-aminobutyric acid A receptor; Gi, Gs, Gq: G proteins (heterotrimeric guanine nucleotide-binding proteins) MAPK: mitogen-activated protein kinase; N-cholino receptor: nicotinic cholinoreceptor; NMDA: N-methyl-D-aspartate; PKA: protein kinase A
Source: Created for CURRENT PSYCHIATRY from information in reference 2
Alcohol metabolism
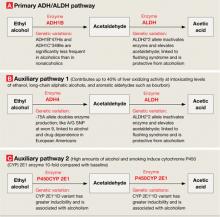
Alcohol is metabolized to acetic acid through primary and auxiliary pathways involving alcohol and acetaldehyde dehydrogenases (ADH/ALDH) and the microsomal ethanol oxidizing system (cytochrome P-450 [CYP] 2E1)15 (Figure 2). Auxiliary pathways become involved when the primary pathway is overwhelmed by the amount of alcohol needed to be metabolized. Catalase and fatty acid ethyl ester synthases play a minor role under normal conditions but may be implicated in alcohol-induced organ damage.16
ADH/ALDH pathway. From one individual to another, the ability to metabolize ethyl alcohol varies up to 3- to 4-fold.17 In European and Amerindian samples, a genetic link has been identified between alcoholism and the 4q21-23 region on chromosome 4.18 This region contains a cluster of 7 genes encoding for alcohol dehydrogenases (ADH), including 3 Class I genes—ADH1A, ADH1B, and ADH1C—coding for the corresponding proteins that play a major role in alcohol metabolism.19 In eastern Asian samples, alleles encoding high activity enzymes (ADH1B*47His and ADH1C*349Ile) are significantly less frequent in alcoholics compared with nonalcoholic controls.20
Mitochondrial ALDH2 protein plays the central role in acetaldehyde metabolism and is highly expressed in the liver, stomach, and other tissues—including the brain.21 The ALDH2*2 gene variant encodes for a catalytically inactive enzyme, thus inhibiting acetaldehyde metabolism and causing a facial flushing reaction.22 The ALDH2*2 allele has a relatively high frequency in Asians but also is found in other populations.23 Meta-analyses of published data indicate that possessing either of the variant alleles in the ADH1B and ALDH2 genes is protective against alcohol dependence in Asians.24
The ADH4 enzyme catalyzes oxidation or reduction of numerous substrates—including long-chain aliphatic alcohols and aromatic aldehydes—and becomes involved in alcohol metabolism at moderate to high concentrations. The -75A allele of the ADH4 gene has promoter activity more than twice that of the -75C allele and significantly affects its expression.25 This substitution in the promoter region, as well as A/G SNP (rs1042364)—a single nucleotide polymorphism (SNP)—at exon 9, has been associated with an increased risk for alcohol and drug dependence in European Americans.26
Finally, variations in the ADH7 gene may play a protective role against alcoholism through epistatic effects.27
The CYP 2E1 pathway has low initial catalytic efficiency compared with the ADH/ALDH pathway, but it may metabolize alcohol up to 10 times faster after chronic alcohol consumption or cigarette smoking and accounts for metabolic tolerance.28 CYP 2E1 is involved in metabolizing both alcohol and acetaldehyde.29 The CYP 2E1*1D polymorphism has been associated with greater inducibility as well as alcohol and nicotine dependence.30
Thus, linkage and association studies support the association of phenotypes related to alcohol response and dependence with variations in genes that code for proteins involved in alcohol’s pharmacodynamic and pharmacokinetic effects. Each of these findings is important, but conceptual models organizing them all and explaining their role in alcohol’s effects and predisposition to alcoholism have yet to be constructed.
Figure 2
Genetic variations that affect alcohol metabolism pathways
Genetic variations affect the efficiency of primary and auxiliary pathways by which ethyl alcohol is metabolized to acetaldehyde and acetic acid. Auxiliary pathways become involved when the primary pathway is overwhelmed by the amount of alcohol to be metabolized.
ADH: alcohol dehydrogenase; ALDH: acetaldehyde dehydrogenase; P450CYP 2E1: cytochrome P450, family 2, subfamily E, polypeptide 1; A/G SNP: adenine/guanine single nucleotide polymorphism
Phenotype-genotype relationships
Alcohol—unlike most other addictive substances—does not have a specific receptor and is believed to act by disturbing the balance between excitatory and inhibitory neurotransmission in the neural system. Consequently, researchers explore relationships between genetics and alcohol-related problems using 2 approaches:
- Forward genetics (discovering disease-related genes via genome-wide studies and then studying their function; examples include linkage and genome-wide association studies [GWAS]).
- Reverse genetics (testing whether candidate genes and polymorphisms identified in animal studies as relevant for biological effects also exist in humans and are relevant to the phenotype).31
When searching for relationships between genotypes and phenotypes, both approaches must take into account a framework of functional anatomic and physiologic connections (Figure 3).
Linkage studies. The goal of linkage studies is to find a link between a phenotypic variation (ideally a measurable trait, such as number of drinks necessary for intoxication) and the chromosomal marker expected to be in the vicinity of the disease-specific gene variation. An advantage of linkage studies is that they can be started without knowing specific DNA sequences. Their limitations include:
- limited power when applied to complex diseases such as alcoholism
- they do not yield gene-specific information
- their success is highly dependent on family members’ willingness to participate.
Association studies. Candidate gene-based association studies are designed to directly test a potential association between the phenotype of interest and a known genomic sequence variation. This approach provides adequate power to study variations with modest effects and allows use of DNA from unrelated individuals. The candidate gene approach has revealed associations between specific genomic variations and phenotypes related to alcohol misuse and alcoholism (Table).
Like linkage studies, association studies have their own challenges and limitations, such as:
- historically high false-positive rates
- confounding risks (allele frequencies may vary because of ethnic stratification rather than disease predisposition).
To address these challenges, researchers must carefully choose behavioral, physiologic, or intermediate phenotypes and genotype variations, as well as control subjects and sample sizes. Replication studies are necessary to rule out false-positive associations. In fact, only some of the findings depicted in the Table—those related to GABRA2 and GABRA6 and few other genes—have been replicated.
Genome-wide association studies are a powerful new method for studying relationships between genomic variability and behavior. With GWAS, thousands of DNA samples can be scanned for thousands of SNPs throughout the human genome, with the goal of identifying variations that modestly increase the risk of developing common diseases.
Unlike the candidate gene approach—which focuses on preselected genomic variations—GWAS scans the whole genome and may identify unexpected susceptibility factors. Unlike the family-based linkage approach, GWAS is not limited to specific families and can address all recombination events in a population.
Challenges are associated with GWAS, however, and include:
- need for substantial numbers (2,000 to 5,000) of rigorously described cases and matched controls
- need for accurate, high-throughput genotyping technologies and sophisticated algorithms for analyzing data
- risk of high false-positive rates related to multiple testing
- inability to scan 100% of the genome, which may lead to false-negative findings.
Table
Examples of gene variations related to alcohol misuse and alcoholism phenotypes
| Phenotype | Gene variation(s) |
|---|---|
| Related to alcohol misuse | |
| Low response/high tolerance to alcohol | L allele of serotonin transporter gene (SLC6A4) and Ser385 allele of alpha-6 subunit of GABAA receptor gene (GABRA6) in adolescents and healthy adult men;a,b 600G allele of alpha-2 subunit of GABAA receptor gene (GABRA2) in healthy social drinkersc |
| Cue-related craving for alcohol | 118G allele of opioid μ-receptor gene (OMPR) and L allele of dopamine receptor type 4 gene (DRD4) in young problem drinkersd,e |
| Binge drinking | S allele of serotonin transporter gene in college students; combination of SS genotype of serotonin transporter gene and HH genotype of MAOA gene in young womenf |
| Related to alcoholism | |
| Alcohol dependence | Two common haplotypes of GABRA2 geneg |
| Liver cirrhosis in alcoholics | -238A allele of tumor necrosis factor gene (TNFA)h |
| Protects against withdrawal symptoms in alcoholics | -141C Del variant of the dopamine receptor type 2 gene (DRD2)i |
| Associated with delirium tremens | 8 genetic polymorphisms in 3 candidate genes involved in the dopamine transmission (DRD2, DRD3, and SLC6A3), 1 gene involved in the glutamate pathway (GRIK3), 1 neuropeptide gene (BDNF), and 1 cannabinoid gene (CNR1)j |
| Associated with alcohol withdrawal seizures | 9 repeat allele of dopamine transporter (SLC6A3); 10 repeat allele of tyrosine hydroxylase gene (TH); Ser9 allele of dopamine receptor type 3 gene (DRD3); SS genotype of serotonin transporter gene; 2108A allele in NR1 subunit of NMDA receptor gene (GRIN1)k-m |
| GABAA: gamma-aminobutyric acid A; MAOA: monoamine oxidase A; NMDA: N-methyl-D-aspartate | |
| Source: Click here to view references | |
Figure 3
2 ways to seek relationships between genes and behavior
Researchers use ‘forward’ and ‘reverse’ genetics to connect behavioral phenotypes with predisposing genotypes. Each approach must consider intermediary functional anatomic and physiologic levels (black box), as shown in this conceptual framework.
DA: dopamine; EEG: electroencephalography; 5-HT: serotonin; LTD: long-term depression; LTP: long-term potentiation; RNA: ribonucleic acid; SNPs: single nucleotide polymorphisms; VNTRs: variable number tandem (triplet) repeats
Clinical implications
Genomic research is increasing our understanding of alcohol’s pharmacokinetic and pharmacodynamic interactions and of potential genetic associations with alcoholic phenotypes. These insights may lead to discovery of new therapies to compensate for specific physiologic and behavioral dysfunctions. For example, medications with pharmacologic profiles complimentary to addiction-related physiologic/behavioral deficits might be designed in the future.
Likewise, new understandings about genetic variability may allow us to predict an individual’s ability to tolerate and respond to existing medications used to treat alcohol dependence. For example, in studies of alcoholic and nonalcoholic subjects:
- Individuals with the 118G variant allele of the μ-opioid receptor may experience a stronger subjective response to alcohol and respond more robustly to naltrexone treatment than do carriers of the more common 118A allele.32
- Persons with a functional variation in the DRD4 gene (7 repeat allele–DRD4L) coding for type 4 dopamine receptor reported greater euphoria and reward while drinking alcohol and reduced alcohol consumption during 12-week treatment with olanzapine, a DRD2/DRD4 blocker.33
Applying this approach to the study of acamprosate has been difficult because of uncertainty about which protein molecules it targets. Variation in the Per2 gene (coding for protein involved in the circadian cycle) has been shown to be associated with brain glutamate levels, alcohol consumption, and the effects of acamprosate, although these interactions require further investigation.34
Pharmacogenomics of alcoholism treatment is in a very early stage of development. Findings require replication in different clinical samples and functional analysis. At the same time, if the reported association between the μ-opioid receptor genetic variation and naltrexone’s treatment efficacy is confirmed, this finding could help to guide clinical practice. Studies of genomic predictors of other medications’ efficacy and tolerability for alcoholism treatment would be expected to follow.
Related resources
- National Institute on Alcohol Abuse and Alcoholism. Collaborative Studies on Genetics of Alcoholism (COGA). www.niaaa.nih.gov (search NIAAA-funded collaborative research programs).
- Dick DM, Jones K, Saccone N, et al. Endophenotypes successfully lead to gene identification: results from the Collaborative Study on the Genetics of Alcoholism. Behav Genet 2006;36(1):112-26.
Drug brand names
- Acamprosate • Campral
- Naltrexone • ReVia, Vivitrol
- Olanzapine • Zyprexa
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work has been supported by the S.C. Johnson Genomic of Addictions Program. The authors thank Ms. Barbara Hall for expert technical assistance in preparing this manuscript.
1. Schuckit MA, Goodwin DA, Winokur G. A study of alcoholism in half siblings. Am J Psychiatry 1972;128(9):1132-6.
2. Nestler E. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2001;2(2):119-28.
3. National Institute of Alcohol Abuse and Alcoholism. Collaborative Studies on Genetics of Alcoholism (COGA). Available at: http://zork.wustl.edu/niaaa. Accessed January 28, 2008.
4. Foroud T, Edenberg HJ, Goate A, et al. Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcohol Clin Exp Res 2000;24(7):933-45.
5. Dick DM, Nurnberger J, Jr, Edenberg HJ, et al. Suggestive linkage on chromosome 1 for a quantitative alcohol-related phenotype. Alcohol Clin Exp Res 2002;26(10):1453-60.
6. Nurnberger JI, Jr, Foroud T, Flury L, et al. Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. Am J Psychiatry 2001;158(5):718-24.
7. Schuckit MA, Edenberg HJ, Kalmijn J, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res 2001;25(3):323-9.
8. Saccone NL, Kwon JM, Corbett J, et al. A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet 2000;96(5):632-7.
9. Gottesman II, Shields J. Schizophrenia and genetics;a twin study vantage point. New York, NY: Academic Press;1972.
10. Rieder RO, Gershon ES. Genetic strategies in biological psychiatry. Arch Gen Psychiatry 1978;35(7):866-73.
11. Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 2006;7(10):818-27.
12. Dick DM, Jones K, Saccone N, et al. Endophenotypes successfully lead to gene identification: results from the Collaborative Study on Genetics of Alcoholism. Behav Genet 2006;36(1):112-26.
13. Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet 2004;74(4):705-14.
14. Hinckers AS, Laucht M, Schmidt MH, et al. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry 2006;60(3):282-7.
15. Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol (Paris) 2001;49(9):676-82.
16. Beckemeier ME, Bora PS. Fatty acid ethyl esters: potentially toxic products of myocardial ethanol metabolism. J Mol Cell Cardiol 1998;30(11):2487-94.
17. Li TK, Yin SJ, Crabb DW, et al. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res 2001;25(1):136-44.
18. Long JC, Knowler WC, Hanson RL, et al. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 1998;81(3):216-21.
19. Edenberg HJ. Regulation of the mammalian alcohol dehydrogenase genes. Prog Nucleic Acid Res Mol Biol 2000;64:295-341.
20. Osier M, Pakstis A, Soodyall H, et al. A global perspective on genetic variation at the ADH genes reveals unusual patterns of linkage disequilibrium and diversity. Am J Hum Genet 2002;71(1):84-99.
21. Yoshida A, Rzhetsky A, Hsu LC, Chang C. Human aldehyde dehydrogenase gene family. Eur J Biochem 1998;251(3):549-57.
22. Harada S, Agarwal DP, Goedde HW. Aldehyde dehydrogenase deficiency as a cause of facial flashing reaction to alcohol in Japanese. Lancet 1981;2(8253):982.-
23. Goedde HW, Agarwal DP, Fritze G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet 1992;88(3):344-6.
24. Luczak SE, Glatt SJ, Wall TJ. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull 2006;132(4):607-21.
25. Edenberg HJ, Jerome RE, Li M. Polymorphism of the human alcohol dehydrogenase 4 (ADH4) promoter affects gene expression. Pharmacogenetics 1999;9(1):25-30.
26. Luo X, Kranzler HR, Zuo L, et al. ADH4 gene variation is associated with alcohol dependence and drug dependence in European Americans: results from HWD tests and case-control association studies. Neuropsychopharmacology 2006;31(5):1085-95.
27. Osier MV, Lu RB, Pakstis AJ, et al. Possible epistatic role of ADH7 in the protection against alcoholism. Am J Med Genet B Neuropsychiatr Genet 2004;126(1):19-22.
28. Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968-1998)—a review. Alcohol Clin Exp Res 1999;23(6):991-1007.
29. Kunitoh S, Imaoka S, Hiroi T, et al. Acetaldehyde as well as ethanol is metabolized by human CYP2E1. J Pharmacol Exp Ther 1997;280(2):527-32.
30. Howard LA, Ahluwalia JS, Lin SK, et al. CYP2E1*1D regulatory polymorphism: association with alcohol and nicotine dependence [erratum in Pharmacogenetics. 2003;13(7):441-2]. Pharmacogenetics 2003;13(6):321-8.
31. Risch NJ. Searching for genetic determinants in the new millennium. Nature 2000;405(6788):847-56.
32. Oslin DW, Berrettini W, Kranzler HR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology 2003;28:1546-52.
33. Hutchison KE, Ray L, Sandman E, et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology 2006;31(6):1310-7.
34. Spanagel R, Pendyala G, Abarca C, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 2005;11(1):35-42.
Children of alcoholics have a 40% to 60% increased risk of developing severe alcohol-related problems1—a harsh legacy recognized for >30 years. Now, as the result of rapidly growing evidence, we can explain in greater detail why alcoholism runs in families when discussing alcohol dependence with patients.
Individuals vary in response to medications and substances of abuse, and genetic research is revealing the heritable origins. Numerous genetic variations are known to influence response to alcohol, as well as alcoholism’s pathophysiology, clinical manifestations, and treatment. Pieces are still missing from this complex picture, but investigators are identifying possible risk factors for alcoholism and matching potential responders with treatments such as naltrexone and acamprosate.
This article provides a progress report on contemporary genetic research of alcoholism. Our goal is to inform your clinical practice by describing:
- new understandings of the genetics of alcoholism
- how researchers identify relationships between genetic variations and clinical/behavioral phenomena
- practical implications of this knowledge.
Genetic variations and risk of addiction
No single gene appears to cause alcoholism. Many genetic variations that accumulated during evolution likely contribute to individual differences in response to alcohol and susceptibility to developing alcohol-related problems. A growing number of genetic variations have been associated with increased alcohol tolerance, consumption, and other related phenotypes.
Like other addictive substances, alcohol triggers pharmacodynamic effects by interacting with a variety of molecular targets (Figure 1).2 These target proteins in turn interact with specific signaling proteins and trigger responses in complex functional pathways. Genetic variations may affect the structure of genes coding for proteins that constitute pathways involved in alcohol’s effects on target proteins (pharmacodynamics) or its metabolism (pharmacokinetics). If such variations alter the production, function, or stability of these proteins, the pathway’s function also may be altered and produce behavioral phenotypes—such as high or low sensitivity to alcohol’s effects.
Alcoholism-related phenotypes. DSM-IV-TR diagnostic criteria include some but not all of the multiple phenotypes within alcoholism’s clinical presentation. Researchers in the Collaborative Studies on Genetics of Alcoholism (COGA)3 identified chromosome regions linked to alcoholism-related phenotypes, including:
- alcohol dependence4
- later age of drinking onset and increased harm avoidance5
- alcoholism and depression6
- alcohol sensitivity7
- alcohol consumption.8
To identify genes of interest within these chromosome regions, researchers used transitional phenotypes (endophenotypes) that “lie on the pathway between genes and disease,”9,10 and allowed them to characterize the neural systems affected by gene risk variants.11 As a result, they found associations among variations in the GABRA2 gene, (encoding the alpha-2 subunit of the GABAA receptor), specific brain oscillations (electrophysiologic endophenotype), and predisposition to alcohol dependence.12,13 By this same strategy, researchers discovered an association between the endophenotype (low-level of response to alcohol) and some genotypes, including at least 1 short allele of the serotonin transporter gene SLC6A4.14
Figure 1
Known molecular targets for alcohol and other drugs of abuse
Effects of drugs of abuse (black arrows) influence intracellular signaling pathways (blue arrows) to produce immediate and long-term changes in cell function.
cAMP: cyclic adenosine monophosphate; GABAA: gamma-aminobutyric acid A receptor; Gi, Gs, Gq: G proteins (heterotrimeric guanine nucleotide-binding proteins) MAPK: mitogen-activated protein kinase; N-cholino receptor: nicotinic cholinoreceptor; NMDA: N-methyl-D-aspartate; PKA: protein kinase A
Source: Created for CURRENT PSYCHIATRY from information in reference 2
Alcohol metabolism
Alcohol is metabolized to acetic acid through primary and auxiliary pathways involving alcohol and acetaldehyde dehydrogenases (ADH/ALDH) and the microsomal ethanol oxidizing system (cytochrome P-450 [CYP] 2E1)15 (Figure 2). Auxiliary pathways become involved when the primary pathway is overwhelmed by the amount of alcohol needed to be metabolized. Catalase and fatty acid ethyl ester synthases play a minor role under normal conditions but may be implicated in alcohol-induced organ damage.16
ADH/ALDH pathway. From one individual to another, the ability to metabolize ethyl alcohol varies up to 3- to 4-fold.17 In European and Amerindian samples, a genetic link has been identified between alcoholism and the 4q21-23 region on chromosome 4.18 This region contains a cluster of 7 genes encoding for alcohol dehydrogenases (ADH), including 3 Class I genes—ADH1A, ADH1B, and ADH1C—coding for the corresponding proteins that play a major role in alcohol metabolism.19 In eastern Asian samples, alleles encoding high activity enzymes (ADH1B*47His and ADH1C*349Ile) are significantly less frequent in alcoholics compared with nonalcoholic controls.20
Mitochondrial ALDH2 protein plays the central role in acetaldehyde metabolism and is highly expressed in the liver, stomach, and other tissues—including the brain.21 The ALDH2*2 gene variant encodes for a catalytically inactive enzyme, thus inhibiting acetaldehyde metabolism and causing a facial flushing reaction.22 The ALDH2*2 allele has a relatively high frequency in Asians but also is found in other populations.23 Meta-analyses of published data indicate that possessing either of the variant alleles in the ADH1B and ALDH2 genes is protective against alcohol dependence in Asians.24
The ADH4 enzyme catalyzes oxidation or reduction of numerous substrates—including long-chain aliphatic alcohols and aromatic aldehydes—and becomes involved in alcohol metabolism at moderate to high concentrations. The -75A allele of the ADH4 gene has promoter activity more than twice that of the -75C allele and significantly affects its expression.25 This substitution in the promoter region, as well as A/G SNP (rs1042364)—a single nucleotide polymorphism (SNP)—at exon 9, has been associated with an increased risk for alcohol and drug dependence in European Americans.26
Finally, variations in the ADH7 gene may play a protective role against alcoholism through epistatic effects.27
The CYP 2E1 pathway has low initial catalytic efficiency compared with the ADH/ALDH pathway, but it may metabolize alcohol up to 10 times faster after chronic alcohol consumption or cigarette smoking and accounts for metabolic tolerance.28 CYP 2E1 is involved in metabolizing both alcohol and acetaldehyde.29 The CYP 2E1*1D polymorphism has been associated with greater inducibility as well as alcohol and nicotine dependence.30
Thus, linkage and association studies support the association of phenotypes related to alcohol response and dependence with variations in genes that code for proteins involved in alcohol’s pharmacodynamic and pharmacokinetic effects. Each of these findings is important, but conceptual models organizing them all and explaining their role in alcohol’s effects and predisposition to alcoholism have yet to be constructed.
Figure 2
Genetic variations that affect alcohol metabolism pathways
Genetic variations affect the efficiency of primary and auxiliary pathways by which ethyl alcohol is metabolized to acetaldehyde and acetic acid. Auxiliary pathways become involved when the primary pathway is overwhelmed by the amount of alcohol to be metabolized.
ADH: alcohol dehydrogenase; ALDH: acetaldehyde dehydrogenase; P450CYP 2E1: cytochrome P450, family 2, subfamily E, polypeptide 1; A/G SNP: adenine/guanine single nucleotide polymorphism
Phenotype-genotype relationships
Alcohol—unlike most other addictive substances—does not have a specific receptor and is believed to act by disturbing the balance between excitatory and inhibitory neurotransmission in the neural system. Consequently, researchers explore relationships between genetics and alcohol-related problems using 2 approaches:
- Forward genetics (discovering disease-related genes via genome-wide studies and then studying their function; examples include linkage and genome-wide association studies [GWAS]).
- Reverse genetics (testing whether candidate genes and polymorphisms identified in animal studies as relevant for biological effects also exist in humans and are relevant to the phenotype).31
When searching for relationships between genotypes and phenotypes, both approaches must take into account a framework of functional anatomic and physiologic connections (Figure 3).
Linkage studies. The goal of linkage studies is to find a link between a phenotypic variation (ideally a measurable trait, such as number of drinks necessary for intoxication) and the chromosomal marker expected to be in the vicinity of the disease-specific gene variation. An advantage of linkage studies is that they can be started without knowing specific DNA sequences. Their limitations include:
- limited power when applied to complex diseases such as alcoholism
- they do not yield gene-specific information
- their success is highly dependent on family members’ willingness to participate.
Association studies. Candidate gene-based association studies are designed to directly test a potential association between the phenotype of interest and a known genomic sequence variation. This approach provides adequate power to study variations with modest effects and allows use of DNA from unrelated individuals. The candidate gene approach has revealed associations between specific genomic variations and phenotypes related to alcohol misuse and alcoholism (Table).
Like linkage studies, association studies have their own challenges and limitations, such as:
- historically high false-positive rates
- confounding risks (allele frequencies may vary because of ethnic stratification rather than disease predisposition).
To address these challenges, researchers must carefully choose behavioral, physiologic, or intermediate phenotypes and genotype variations, as well as control subjects and sample sizes. Replication studies are necessary to rule out false-positive associations. In fact, only some of the findings depicted in the Table—those related to GABRA2 and GABRA6 and few other genes—have been replicated.
Genome-wide association studies are a powerful new method for studying relationships between genomic variability and behavior. With GWAS, thousands of DNA samples can be scanned for thousands of SNPs throughout the human genome, with the goal of identifying variations that modestly increase the risk of developing common diseases.
Unlike the candidate gene approach—which focuses on preselected genomic variations—GWAS scans the whole genome and may identify unexpected susceptibility factors. Unlike the family-based linkage approach, GWAS is not limited to specific families and can address all recombination events in a population.
Challenges are associated with GWAS, however, and include:
- need for substantial numbers (2,000 to 5,000) of rigorously described cases and matched controls
- need for accurate, high-throughput genotyping technologies and sophisticated algorithms for analyzing data
- risk of high false-positive rates related to multiple testing
- inability to scan 100% of the genome, which may lead to false-negative findings.
Table
Examples of gene variations related to alcohol misuse and alcoholism phenotypes
| Phenotype | Gene variation(s) |
|---|---|
| Related to alcohol misuse | |
| Low response/high tolerance to alcohol | L allele of serotonin transporter gene (SLC6A4) and Ser385 allele of alpha-6 subunit of GABAA receptor gene (GABRA6) in adolescents and healthy adult men;a,b 600G allele of alpha-2 subunit of GABAA receptor gene (GABRA2) in healthy social drinkersc |
| Cue-related craving for alcohol | 118G allele of opioid μ-receptor gene (OMPR) and L allele of dopamine receptor type 4 gene (DRD4) in young problem drinkersd,e |
| Binge drinking | S allele of serotonin transporter gene in college students; combination of SS genotype of serotonin transporter gene and HH genotype of MAOA gene in young womenf |
| Related to alcoholism | |
| Alcohol dependence | Two common haplotypes of GABRA2 geneg |
| Liver cirrhosis in alcoholics | -238A allele of tumor necrosis factor gene (TNFA)h |
| Protects against withdrawal symptoms in alcoholics | -141C Del variant of the dopamine receptor type 2 gene (DRD2)i |
| Associated with delirium tremens | 8 genetic polymorphisms in 3 candidate genes involved in the dopamine transmission (DRD2, DRD3, and SLC6A3), 1 gene involved in the glutamate pathway (GRIK3), 1 neuropeptide gene (BDNF), and 1 cannabinoid gene (CNR1)j |
| Associated with alcohol withdrawal seizures | 9 repeat allele of dopamine transporter (SLC6A3); 10 repeat allele of tyrosine hydroxylase gene (TH); Ser9 allele of dopamine receptor type 3 gene (DRD3); SS genotype of serotonin transporter gene; 2108A allele in NR1 subunit of NMDA receptor gene (GRIN1)k-m |
| GABAA: gamma-aminobutyric acid A; MAOA: monoamine oxidase A; NMDA: N-methyl-D-aspartate | |
| Source: Click here to view references | |
Figure 3
2 ways to seek relationships between genes and behavior
Researchers use ‘forward’ and ‘reverse’ genetics to connect behavioral phenotypes with predisposing genotypes. Each approach must consider intermediary functional anatomic and physiologic levels (black box), as shown in this conceptual framework.
DA: dopamine; EEG: electroencephalography; 5-HT: serotonin; LTD: long-term depression; LTP: long-term potentiation; RNA: ribonucleic acid; SNPs: single nucleotide polymorphisms; VNTRs: variable number tandem (triplet) repeats
Clinical implications
Genomic research is increasing our understanding of alcohol’s pharmacokinetic and pharmacodynamic interactions and of potential genetic associations with alcoholic phenotypes. These insights may lead to discovery of new therapies to compensate for specific physiologic and behavioral dysfunctions. For example, medications with pharmacologic profiles complimentary to addiction-related physiologic/behavioral deficits might be designed in the future.
Likewise, new understandings about genetic variability may allow us to predict an individual’s ability to tolerate and respond to existing medications used to treat alcohol dependence. For example, in studies of alcoholic and nonalcoholic subjects:
- Individuals with the 118G variant allele of the μ-opioid receptor may experience a stronger subjective response to alcohol and respond more robustly to naltrexone treatment than do carriers of the more common 118A allele.32
- Persons with a functional variation in the DRD4 gene (7 repeat allele–DRD4L) coding for type 4 dopamine receptor reported greater euphoria and reward while drinking alcohol and reduced alcohol consumption during 12-week treatment with olanzapine, a DRD2/DRD4 blocker.33
Applying this approach to the study of acamprosate has been difficult because of uncertainty about which protein molecules it targets. Variation in the Per2 gene (coding for protein involved in the circadian cycle) has been shown to be associated with brain glutamate levels, alcohol consumption, and the effects of acamprosate, although these interactions require further investigation.34
Pharmacogenomics of alcoholism treatment is in a very early stage of development. Findings require replication in different clinical samples and functional analysis. At the same time, if the reported association between the μ-opioid receptor genetic variation and naltrexone’s treatment efficacy is confirmed, this finding could help to guide clinical practice. Studies of genomic predictors of other medications’ efficacy and tolerability for alcoholism treatment would be expected to follow.
Related resources
- National Institute on Alcohol Abuse and Alcoholism. Collaborative Studies on Genetics of Alcoholism (COGA). www.niaaa.nih.gov (search NIAAA-funded collaborative research programs).
- Dick DM, Jones K, Saccone N, et al. Endophenotypes successfully lead to gene identification: results from the Collaborative Study on the Genetics of Alcoholism. Behav Genet 2006;36(1):112-26.
Drug brand names
- Acamprosate • Campral
- Naltrexone • ReVia, Vivitrol
- Olanzapine • Zyprexa
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work has been supported by the S.C. Johnson Genomic of Addictions Program. The authors thank Ms. Barbara Hall for expert technical assistance in preparing this manuscript.
Children of alcoholics have a 40% to 60% increased risk of developing severe alcohol-related problems1—a harsh legacy recognized for >30 years. Now, as the result of rapidly growing evidence, we can explain in greater detail why alcoholism runs in families when discussing alcohol dependence with patients.
Individuals vary in response to medications and substances of abuse, and genetic research is revealing the heritable origins. Numerous genetic variations are known to influence response to alcohol, as well as alcoholism’s pathophysiology, clinical manifestations, and treatment. Pieces are still missing from this complex picture, but investigators are identifying possible risk factors for alcoholism and matching potential responders with treatments such as naltrexone and acamprosate.
This article provides a progress report on contemporary genetic research of alcoholism. Our goal is to inform your clinical practice by describing:
- new understandings of the genetics of alcoholism
- how researchers identify relationships between genetic variations and clinical/behavioral phenomena
- practical implications of this knowledge.
Genetic variations and risk of addiction
No single gene appears to cause alcoholism. Many genetic variations that accumulated during evolution likely contribute to individual differences in response to alcohol and susceptibility to developing alcohol-related problems. A growing number of genetic variations have been associated with increased alcohol tolerance, consumption, and other related phenotypes.
Like other addictive substances, alcohol triggers pharmacodynamic effects by interacting with a variety of molecular targets (Figure 1).2 These target proteins in turn interact with specific signaling proteins and trigger responses in complex functional pathways. Genetic variations may affect the structure of genes coding for proteins that constitute pathways involved in alcohol’s effects on target proteins (pharmacodynamics) or its metabolism (pharmacokinetics). If such variations alter the production, function, or stability of these proteins, the pathway’s function also may be altered and produce behavioral phenotypes—such as high or low sensitivity to alcohol’s effects.
Alcoholism-related phenotypes. DSM-IV-TR diagnostic criteria include some but not all of the multiple phenotypes within alcoholism’s clinical presentation. Researchers in the Collaborative Studies on Genetics of Alcoholism (COGA)3 identified chromosome regions linked to alcoholism-related phenotypes, including:
- alcohol dependence4
- later age of drinking onset and increased harm avoidance5
- alcoholism and depression6
- alcohol sensitivity7
- alcohol consumption.8
To identify genes of interest within these chromosome regions, researchers used transitional phenotypes (endophenotypes) that “lie on the pathway between genes and disease,”9,10 and allowed them to characterize the neural systems affected by gene risk variants.11 As a result, they found associations among variations in the GABRA2 gene, (encoding the alpha-2 subunit of the GABAA receptor), specific brain oscillations (electrophysiologic endophenotype), and predisposition to alcohol dependence.12,13 By this same strategy, researchers discovered an association between the endophenotype (low-level of response to alcohol) and some genotypes, including at least 1 short allele of the serotonin transporter gene SLC6A4.14
Figure 1
Known molecular targets for alcohol and other drugs of abuse
Effects of drugs of abuse (black arrows) influence intracellular signaling pathways (blue arrows) to produce immediate and long-term changes in cell function.
cAMP: cyclic adenosine monophosphate; GABAA: gamma-aminobutyric acid A receptor; Gi, Gs, Gq: G proteins (heterotrimeric guanine nucleotide-binding proteins) MAPK: mitogen-activated protein kinase; N-cholino receptor: nicotinic cholinoreceptor; NMDA: N-methyl-D-aspartate; PKA: protein kinase A
Source: Created for CURRENT PSYCHIATRY from information in reference 2
Alcohol metabolism
Alcohol is metabolized to acetic acid through primary and auxiliary pathways involving alcohol and acetaldehyde dehydrogenases (ADH/ALDH) and the microsomal ethanol oxidizing system (cytochrome P-450 [CYP] 2E1)15 (Figure 2). Auxiliary pathways become involved when the primary pathway is overwhelmed by the amount of alcohol needed to be metabolized. Catalase and fatty acid ethyl ester synthases play a minor role under normal conditions but may be implicated in alcohol-induced organ damage.16
ADH/ALDH pathway. From one individual to another, the ability to metabolize ethyl alcohol varies up to 3- to 4-fold.17 In European and Amerindian samples, a genetic link has been identified between alcoholism and the 4q21-23 region on chromosome 4.18 This region contains a cluster of 7 genes encoding for alcohol dehydrogenases (ADH), including 3 Class I genes—ADH1A, ADH1B, and ADH1C—coding for the corresponding proteins that play a major role in alcohol metabolism.19 In eastern Asian samples, alleles encoding high activity enzymes (ADH1B*47His and ADH1C*349Ile) are significantly less frequent in alcoholics compared with nonalcoholic controls.20
Mitochondrial ALDH2 protein plays the central role in acetaldehyde metabolism and is highly expressed in the liver, stomach, and other tissues—including the brain.21 The ALDH2*2 gene variant encodes for a catalytically inactive enzyme, thus inhibiting acetaldehyde metabolism and causing a facial flushing reaction.22 The ALDH2*2 allele has a relatively high frequency in Asians but also is found in other populations.23 Meta-analyses of published data indicate that possessing either of the variant alleles in the ADH1B and ALDH2 genes is protective against alcohol dependence in Asians.24
The ADH4 enzyme catalyzes oxidation or reduction of numerous substrates—including long-chain aliphatic alcohols and aromatic aldehydes—and becomes involved in alcohol metabolism at moderate to high concentrations. The -75A allele of the ADH4 gene has promoter activity more than twice that of the -75C allele and significantly affects its expression.25 This substitution in the promoter region, as well as A/G SNP (rs1042364)—a single nucleotide polymorphism (SNP)—at exon 9, has been associated with an increased risk for alcohol and drug dependence in European Americans.26
Finally, variations in the ADH7 gene may play a protective role against alcoholism through epistatic effects.27
The CYP 2E1 pathway has low initial catalytic efficiency compared with the ADH/ALDH pathway, but it may metabolize alcohol up to 10 times faster after chronic alcohol consumption or cigarette smoking and accounts for metabolic tolerance.28 CYP 2E1 is involved in metabolizing both alcohol and acetaldehyde.29 The CYP 2E1*1D polymorphism has been associated with greater inducibility as well as alcohol and nicotine dependence.30
Thus, linkage and association studies support the association of phenotypes related to alcohol response and dependence with variations in genes that code for proteins involved in alcohol’s pharmacodynamic and pharmacokinetic effects. Each of these findings is important, but conceptual models organizing them all and explaining their role in alcohol’s effects and predisposition to alcoholism have yet to be constructed.
Figure 2
Genetic variations that affect alcohol metabolism pathways
Genetic variations affect the efficiency of primary and auxiliary pathways by which ethyl alcohol is metabolized to acetaldehyde and acetic acid. Auxiliary pathways become involved when the primary pathway is overwhelmed by the amount of alcohol to be metabolized.
ADH: alcohol dehydrogenase; ALDH: acetaldehyde dehydrogenase; P450CYP 2E1: cytochrome P450, family 2, subfamily E, polypeptide 1; A/G SNP: adenine/guanine single nucleotide polymorphism
Phenotype-genotype relationships
Alcohol—unlike most other addictive substances—does not have a specific receptor and is believed to act by disturbing the balance between excitatory and inhibitory neurotransmission in the neural system. Consequently, researchers explore relationships between genetics and alcohol-related problems using 2 approaches:
- Forward genetics (discovering disease-related genes via genome-wide studies and then studying their function; examples include linkage and genome-wide association studies [GWAS]).
- Reverse genetics (testing whether candidate genes and polymorphisms identified in animal studies as relevant for biological effects also exist in humans and are relevant to the phenotype).31
When searching for relationships between genotypes and phenotypes, both approaches must take into account a framework of functional anatomic and physiologic connections (Figure 3).
Linkage studies. The goal of linkage studies is to find a link between a phenotypic variation (ideally a measurable trait, such as number of drinks necessary for intoxication) and the chromosomal marker expected to be in the vicinity of the disease-specific gene variation. An advantage of linkage studies is that they can be started without knowing specific DNA sequences. Their limitations include:
- limited power when applied to complex diseases such as alcoholism
- they do not yield gene-specific information
- their success is highly dependent on family members’ willingness to participate.
Association studies. Candidate gene-based association studies are designed to directly test a potential association between the phenotype of interest and a known genomic sequence variation. This approach provides adequate power to study variations with modest effects and allows use of DNA from unrelated individuals. The candidate gene approach has revealed associations between specific genomic variations and phenotypes related to alcohol misuse and alcoholism (Table).
Like linkage studies, association studies have their own challenges and limitations, such as:
- historically high false-positive rates
- confounding risks (allele frequencies may vary because of ethnic stratification rather than disease predisposition).
To address these challenges, researchers must carefully choose behavioral, physiologic, or intermediate phenotypes and genotype variations, as well as control subjects and sample sizes. Replication studies are necessary to rule out false-positive associations. In fact, only some of the findings depicted in the Table—those related to GABRA2 and GABRA6 and few other genes—have been replicated.
Genome-wide association studies are a powerful new method for studying relationships between genomic variability and behavior. With GWAS, thousands of DNA samples can be scanned for thousands of SNPs throughout the human genome, with the goal of identifying variations that modestly increase the risk of developing common diseases.
Unlike the candidate gene approach—which focuses on preselected genomic variations—GWAS scans the whole genome and may identify unexpected susceptibility factors. Unlike the family-based linkage approach, GWAS is not limited to specific families and can address all recombination events in a population.
Challenges are associated with GWAS, however, and include:
- need for substantial numbers (2,000 to 5,000) of rigorously described cases and matched controls
- need for accurate, high-throughput genotyping technologies and sophisticated algorithms for analyzing data
- risk of high false-positive rates related to multiple testing
- inability to scan 100% of the genome, which may lead to false-negative findings.
Table
Examples of gene variations related to alcohol misuse and alcoholism phenotypes
| Phenotype | Gene variation(s) |
|---|---|
| Related to alcohol misuse | |
| Low response/high tolerance to alcohol | L allele of serotonin transporter gene (SLC6A4) and Ser385 allele of alpha-6 subunit of GABAA receptor gene (GABRA6) in adolescents and healthy adult men;a,b 600G allele of alpha-2 subunit of GABAA receptor gene (GABRA2) in healthy social drinkersc |
| Cue-related craving for alcohol | 118G allele of opioid μ-receptor gene (OMPR) and L allele of dopamine receptor type 4 gene (DRD4) in young problem drinkersd,e |
| Binge drinking | S allele of serotonin transporter gene in college students; combination of SS genotype of serotonin transporter gene and HH genotype of MAOA gene in young womenf |
| Related to alcoholism | |
| Alcohol dependence | Two common haplotypes of GABRA2 geneg |
| Liver cirrhosis in alcoholics | -238A allele of tumor necrosis factor gene (TNFA)h |
| Protects against withdrawal symptoms in alcoholics | -141C Del variant of the dopamine receptor type 2 gene (DRD2)i |
| Associated with delirium tremens | 8 genetic polymorphisms in 3 candidate genes involved in the dopamine transmission (DRD2, DRD3, and SLC6A3), 1 gene involved in the glutamate pathway (GRIK3), 1 neuropeptide gene (BDNF), and 1 cannabinoid gene (CNR1)j |
| Associated with alcohol withdrawal seizures | 9 repeat allele of dopamine transporter (SLC6A3); 10 repeat allele of tyrosine hydroxylase gene (TH); Ser9 allele of dopamine receptor type 3 gene (DRD3); SS genotype of serotonin transporter gene; 2108A allele in NR1 subunit of NMDA receptor gene (GRIN1)k-m |
| GABAA: gamma-aminobutyric acid A; MAOA: monoamine oxidase A; NMDA: N-methyl-D-aspartate | |
| Source: Click here to view references | |
Figure 3
2 ways to seek relationships between genes and behavior
Researchers use ‘forward’ and ‘reverse’ genetics to connect behavioral phenotypes with predisposing genotypes. Each approach must consider intermediary functional anatomic and physiologic levels (black box), as shown in this conceptual framework.
DA: dopamine; EEG: electroencephalography; 5-HT: serotonin; LTD: long-term depression; LTP: long-term potentiation; RNA: ribonucleic acid; SNPs: single nucleotide polymorphisms; VNTRs: variable number tandem (triplet) repeats
Clinical implications
Genomic research is increasing our understanding of alcohol’s pharmacokinetic and pharmacodynamic interactions and of potential genetic associations with alcoholic phenotypes. These insights may lead to discovery of new therapies to compensate for specific physiologic and behavioral dysfunctions. For example, medications with pharmacologic profiles complimentary to addiction-related physiologic/behavioral deficits might be designed in the future.
Likewise, new understandings about genetic variability may allow us to predict an individual’s ability to tolerate and respond to existing medications used to treat alcohol dependence. For example, in studies of alcoholic and nonalcoholic subjects:
- Individuals with the 118G variant allele of the μ-opioid receptor may experience a stronger subjective response to alcohol and respond more robustly to naltrexone treatment than do carriers of the more common 118A allele.32
- Persons with a functional variation in the DRD4 gene (7 repeat allele–DRD4L) coding for type 4 dopamine receptor reported greater euphoria and reward while drinking alcohol and reduced alcohol consumption during 12-week treatment with olanzapine, a DRD2/DRD4 blocker.33
Applying this approach to the study of acamprosate has been difficult because of uncertainty about which protein molecules it targets. Variation in the Per2 gene (coding for protein involved in the circadian cycle) has been shown to be associated with brain glutamate levels, alcohol consumption, and the effects of acamprosate, although these interactions require further investigation.34
Pharmacogenomics of alcoholism treatment is in a very early stage of development. Findings require replication in different clinical samples and functional analysis. At the same time, if the reported association between the μ-opioid receptor genetic variation and naltrexone’s treatment efficacy is confirmed, this finding could help to guide clinical practice. Studies of genomic predictors of other medications’ efficacy and tolerability for alcoholism treatment would be expected to follow.
Related resources
- National Institute on Alcohol Abuse and Alcoholism. Collaborative Studies on Genetics of Alcoholism (COGA). www.niaaa.nih.gov (search NIAAA-funded collaborative research programs).
- Dick DM, Jones K, Saccone N, et al. Endophenotypes successfully lead to gene identification: results from the Collaborative Study on the Genetics of Alcoholism. Behav Genet 2006;36(1):112-26.
Drug brand names
- Acamprosate • Campral
- Naltrexone • ReVia, Vivitrol
- Olanzapine • Zyprexa
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgments
This work has been supported by the S.C. Johnson Genomic of Addictions Program. The authors thank Ms. Barbara Hall for expert technical assistance in preparing this manuscript.
1. Schuckit MA, Goodwin DA, Winokur G. A study of alcoholism in half siblings. Am J Psychiatry 1972;128(9):1132-6.
2. Nestler E. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2001;2(2):119-28.
3. National Institute of Alcohol Abuse and Alcoholism. Collaborative Studies on Genetics of Alcoholism (COGA). Available at: http://zork.wustl.edu/niaaa. Accessed January 28, 2008.
4. Foroud T, Edenberg HJ, Goate A, et al. Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcohol Clin Exp Res 2000;24(7):933-45.
5. Dick DM, Nurnberger J, Jr, Edenberg HJ, et al. Suggestive linkage on chromosome 1 for a quantitative alcohol-related phenotype. Alcohol Clin Exp Res 2002;26(10):1453-60.
6. Nurnberger JI, Jr, Foroud T, Flury L, et al. Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. Am J Psychiatry 2001;158(5):718-24.
7. Schuckit MA, Edenberg HJ, Kalmijn J, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res 2001;25(3):323-9.
8. Saccone NL, Kwon JM, Corbett J, et al. A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet 2000;96(5):632-7.
9. Gottesman II, Shields J. Schizophrenia and genetics;a twin study vantage point. New York, NY: Academic Press;1972.
10. Rieder RO, Gershon ES. Genetic strategies in biological psychiatry. Arch Gen Psychiatry 1978;35(7):866-73.
11. Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 2006;7(10):818-27.
12. Dick DM, Jones K, Saccone N, et al. Endophenotypes successfully lead to gene identification: results from the Collaborative Study on Genetics of Alcoholism. Behav Genet 2006;36(1):112-26.
13. Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet 2004;74(4):705-14.
14. Hinckers AS, Laucht M, Schmidt MH, et al. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry 2006;60(3):282-7.
15. Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol (Paris) 2001;49(9):676-82.
16. Beckemeier ME, Bora PS. Fatty acid ethyl esters: potentially toxic products of myocardial ethanol metabolism. J Mol Cell Cardiol 1998;30(11):2487-94.
17. Li TK, Yin SJ, Crabb DW, et al. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res 2001;25(1):136-44.
18. Long JC, Knowler WC, Hanson RL, et al. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 1998;81(3):216-21.
19. Edenberg HJ. Regulation of the mammalian alcohol dehydrogenase genes. Prog Nucleic Acid Res Mol Biol 2000;64:295-341.
20. Osier M, Pakstis A, Soodyall H, et al. A global perspective on genetic variation at the ADH genes reveals unusual patterns of linkage disequilibrium and diversity. Am J Hum Genet 2002;71(1):84-99.
21. Yoshida A, Rzhetsky A, Hsu LC, Chang C. Human aldehyde dehydrogenase gene family. Eur J Biochem 1998;251(3):549-57.
22. Harada S, Agarwal DP, Goedde HW. Aldehyde dehydrogenase deficiency as a cause of facial flashing reaction to alcohol in Japanese. Lancet 1981;2(8253):982.-
23. Goedde HW, Agarwal DP, Fritze G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet 1992;88(3):344-6.
24. Luczak SE, Glatt SJ, Wall TJ. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull 2006;132(4):607-21.
25. Edenberg HJ, Jerome RE, Li M. Polymorphism of the human alcohol dehydrogenase 4 (ADH4) promoter affects gene expression. Pharmacogenetics 1999;9(1):25-30.
26. Luo X, Kranzler HR, Zuo L, et al. ADH4 gene variation is associated with alcohol dependence and drug dependence in European Americans: results from HWD tests and case-control association studies. Neuropsychopharmacology 2006;31(5):1085-95.
27. Osier MV, Lu RB, Pakstis AJ, et al. Possible epistatic role of ADH7 in the protection against alcoholism. Am J Med Genet B Neuropsychiatr Genet 2004;126(1):19-22.
28. Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968-1998)—a review. Alcohol Clin Exp Res 1999;23(6):991-1007.
29. Kunitoh S, Imaoka S, Hiroi T, et al. Acetaldehyde as well as ethanol is metabolized by human CYP2E1. J Pharmacol Exp Ther 1997;280(2):527-32.
30. Howard LA, Ahluwalia JS, Lin SK, et al. CYP2E1*1D regulatory polymorphism: association with alcohol and nicotine dependence [erratum in Pharmacogenetics. 2003;13(7):441-2]. Pharmacogenetics 2003;13(6):321-8.
31. Risch NJ. Searching for genetic determinants in the new millennium. Nature 2000;405(6788):847-56.
32. Oslin DW, Berrettini W, Kranzler HR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology 2003;28:1546-52.
33. Hutchison KE, Ray L, Sandman E, et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology 2006;31(6):1310-7.
34. Spanagel R, Pendyala G, Abarca C, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 2005;11(1):35-42.
1. Schuckit MA, Goodwin DA, Winokur G. A study of alcoholism in half siblings. Am J Psychiatry 1972;128(9):1132-6.
2. Nestler E. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2001;2(2):119-28.
3. National Institute of Alcohol Abuse and Alcoholism. Collaborative Studies on Genetics of Alcoholism (COGA). Available at: http://zork.wustl.edu/niaaa. Accessed January 28, 2008.
4. Foroud T, Edenberg HJ, Goate A, et al. Alcoholism susceptibility loci: confirmation studies in a replicate sample and further mapping. Alcohol Clin Exp Res 2000;24(7):933-45.
5. Dick DM, Nurnberger J, Jr, Edenberg HJ, et al. Suggestive linkage on chromosome 1 for a quantitative alcohol-related phenotype. Alcohol Clin Exp Res 2002;26(10):1453-60.
6. Nurnberger JI, Jr, Foroud T, Flury L, et al. Evidence for a locus on chromosome 1 that influences vulnerability to alcoholism and affective disorder. Am J Psychiatry 2001;158(5):718-24.
7. Schuckit MA, Edenberg HJ, Kalmijn J, et al. A genome-wide search for genes that relate to a low level of response to alcohol. Alcohol Clin Exp Res 2001;25(3):323-9.
8. Saccone NL, Kwon JM, Corbett J, et al. A genome screen of maximum number of drinks as an alcoholism phenotype. Am J Med Genet 2000;96(5):632-7.
9. Gottesman II, Shields J. Schizophrenia and genetics;a twin study vantage point. New York, NY: Academic Press;1972.
10. Rieder RO, Gershon ES. Genetic strategies in biological psychiatry. Arch Gen Psychiatry 1978;35(7):866-73.
11. Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 2006;7(10):818-27.
12. Dick DM, Jones K, Saccone N, et al. Endophenotypes successfully lead to gene identification: results from the Collaborative Study on Genetics of Alcoholism. Behav Genet 2006;36(1):112-26.
13. Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet 2004;74(4):705-14.
14. Hinckers AS, Laucht M, Schmidt MH, et al. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biol Psychiatry 2006;60(3):282-7.
15. Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol (Paris) 2001;49(9):676-82.
16. Beckemeier ME, Bora PS. Fatty acid ethyl esters: potentially toxic products of myocardial ethanol metabolism. J Mol Cell Cardiol 1998;30(11):2487-94.
17. Li TK, Yin SJ, Crabb DW, et al. Genetic and environmental influences on alcohol metabolism in humans. Alcohol Clin Exp Res 2001;25(1):136-44.
18. Long JC, Knowler WC, Hanson RL, et al. Evidence for genetic linkage to alcohol dependence on chromosomes 4 and 11 from an autosome-wide scan in an American Indian population. Am J Med Genet 1998;81(3):216-21.
19. Edenberg HJ. Regulation of the mammalian alcohol dehydrogenase genes. Prog Nucleic Acid Res Mol Biol 2000;64:295-341.
20. Osier M, Pakstis A, Soodyall H, et al. A global perspective on genetic variation at the ADH genes reveals unusual patterns of linkage disequilibrium and diversity. Am J Hum Genet 2002;71(1):84-99.
21. Yoshida A, Rzhetsky A, Hsu LC, Chang C. Human aldehyde dehydrogenase gene family. Eur J Biochem 1998;251(3):549-57.
22. Harada S, Agarwal DP, Goedde HW. Aldehyde dehydrogenase deficiency as a cause of facial flashing reaction to alcohol in Japanese. Lancet 1981;2(8253):982.-
23. Goedde HW, Agarwal DP, Fritze G, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum Genet 1992;88(3):344-6.
24. Luczak SE, Glatt SJ, Wall TJ. Meta-analyses of ALDH2 and ADH1B with alcohol dependence in Asians. Psychol Bull 2006;132(4):607-21.
25. Edenberg HJ, Jerome RE, Li M. Polymorphism of the human alcohol dehydrogenase 4 (ADH4) promoter affects gene expression. Pharmacogenetics 1999;9(1):25-30.
26. Luo X, Kranzler HR, Zuo L, et al. ADH4 gene variation is associated with alcohol dependence and drug dependence in European Americans: results from HWD tests and case-control association studies. Neuropsychopharmacology 2006;31(5):1085-95.
27. Osier MV, Lu RB, Pakstis AJ, et al. Possible epistatic role of ADH7 in the protection against alcoholism. Am J Med Genet B Neuropsychiatr Genet 2004;126(1):19-22.
28. Lieber CS. Microsomal ethanol-oxidizing system (MEOS): the first 30 years (1968-1998)—a review. Alcohol Clin Exp Res 1999;23(6):991-1007.
29. Kunitoh S, Imaoka S, Hiroi T, et al. Acetaldehyde as well as ethanol is metabolized by human CYP2E1. J Pharmacol Exp Ther 1997;280(2):527-32.
30. Howard LA, Ahluwalia JS, Lin SK, et al. CYP2E1*1D regulatory polymorphism: association with alcohol and nicotine dependence [erratum in Pharmacogenetics. 2003;13(7):441-2]. Pharmacogenetics 2003;13(6):321-8.
31. Risch NJ. Searching for genetic determinants in the new millennium. Nature 2000;405(6788):847-56.
32. Oslin DW, Berrettini W, Kranzler HR, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology 2003;28:1546-52.
33. Hutchison KE, Ray L, Sandman E, et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology 2006;31(6):1310-7.
34. Spanagel R, Pendyala G, Abarca C, et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 2005;11(1):35-42.