User login
CASE: Low-risk patient requests cell-free DNA screening
Ms. Smith is a 25-year-old woman (G1P0) presenting at 10 weeks’ gestation for her first prenatal visit. She requests cell-free DNA (cfDNA) screening to test for fetal aneuploidy. You explain that the current recommendations are for traditional screening, and inform her that her insurance may not cover the cost of cfDNA screening. She is anxious to learn the sex of her fetus as early as possible, and indicates that she would like to pursue cfDNA. After further discussion of the pros and cons, you order the test.
Prenatal screening is currently recommended in pregnancy for a number of genetic disorders, chromosomal aneuploidy, and structural birth defects in the fetus, regardless of maternal age or family history. There is a broad range of sonographic and maternal serum-based options available for carrying out aneuploidy risk assessment in the first and/or second trimester.
In addition, cfDNA screening for fetal aneuploidy has been clinically available since 2011 and has seen tremendous uptake, particularly in the high-risk population. Recent data indicate that cfDNA screening likewise has very high sensitivity and specificity for trisomy 21 in the low-risk population.1,2
Many low-risk patients are asking providers about the pros and cons of cfDNA screening, and the appropriateness of this test as a primary screen, including in low-risk patients, is the focus of this article.
What is cfDNA?
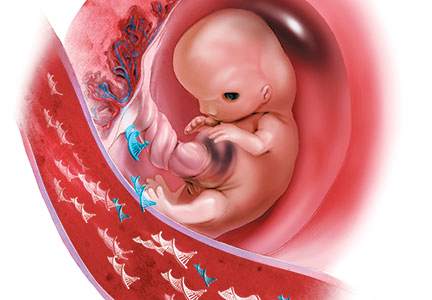
cfDNA consists of small (<200 base pairs) fragments of DNA that are present in the maternal serum. After 10 weeks of gestation, about 10% of the total circulating cfDNA in the maternal serum is derived from the placenta and can therefore be used to test for fetal disorders (FIGURE).3
Although cfDNA screening has been reported to be possible for many different types of genetic conditions, such as RhD type and single-gene disorders such as achondroplasia,4 most clinical testing is done for fetal chromosomal disorders, including trisomies 13, 18, and 21 and the sex chromosomes. In addition, some laboratories provide testing for other trisomies (16 and 22), as well as some of the microdeletion syndromes (22q11.2, 1p36, Prader Willi syndrome, and others).5
Analysis of cfDNA to assess the risk for aneuploidy is done using a number of different approaches; these generally all include next-generation sequencing with advanced bioinformatics analyses.3,6–9 Although the laboratories use somewhat different techniques, all of them share very high sensitivity and specificity for detection of trisomy 21 (TABLE 1).10
Sensitivities for trisomy 13 and sex chromosomal abnormalities are somewhat lower, but the specificity is greater than 99% for each condition, meaning that false-positive rates are very low.
The accuracy of cfDNA in identifying chromosomal aneuploidy depends on several factors, including the relative amount of fetal to maternal DNA, the chance that a chromosome abnormality is present (that is, the risk based on maternal age or results of other screening), and other factors such as the presence of twins or a nonviable second fetus, or the presence of placental mosaicism.
Because of these variables, both false-positive and false-negative results can occur, and the test is not diagnostic but rather is considered a screening test. A positive result does not mean that the fetus is definitely affected with aneuploidy.
What are the advantages of cfDNA screening for low-risk patients?
There are several benefits of cfDNA screening versus traditional screening or diagnostic testing, which are the other options available (TABLE 2). For Down syndrome, the detection rate is higher and the false-positive rate is lower than that seen with traditional aneuploidy screening using serum analytes and nuchal translucency ultrasonography.1,2
TABLE 2 Pros and cons of cfDNA screening in low-risk patients
Pros
- High detection rate and very low false-positive rate
- Can be performed any time after 10 weeks’ gestation
- Requires a single blood test at any gestational age
- Results presented in simple “Yes” or “No” format
- As with other screening tests, cfDNA provides a noninvasive determination of risk
Cons
- Tests for a limited range of conditions, which are rare in low-risk patients
- Is not as comprehensive or definitive as diagnostic testing with amniocentesis or chorionic villus sampling
- Results do not adjust for patient’s prior risk
- Positive results require calculation and interpretation of positive predictive value by provider
- Low fetal DNA and other factors can lead to test failure in some cases
- Cannot be used with vanishing twin
- Can reveal unsuspected maternal conditions of uncertain significance
The test can be done any time after 10 weeks’ gestation without the narrow gestational-age windows required or the need for accurate gestational age determination using traditional screening to accurately interpret results. cfDNA screening involves a single blood test that does not require integration with multiple serum markers or ultrasound findings. Finally, results are generally presented in a simple “Yes” or “No” format that is easy for providers and patients to understand.
CASE Continued
Your patient’s results are positive for trisomy 13. Her understanding is that the test is more than 99% accurate, and she interprets this to mean that the chance of trisomy 13 in her fetus is more than 99%. She is distraught and asks about pregnancy termination.
What are the limitations of cfDNA screening?
Similar to other noninvasive screening tests, cfDNA screening does not carry direct risk to the pregnancy. However, there are limitations to this testing. As a result, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recently have stated that traditional screening is the most appropriate option for most women.11,12
One reason that cfDNA screening may not be the best choice for low-risk women is that Down syndrome is quite uncommon in this group, so cfDNA screening is a very precise test for a rare condition. Traditional multiple marker screening, on the other hand, is more effective at signaling risk for the broad range of adverse perinatal outcomes that can affect a pregnancy, including other structural birth defects, as well as such obstetric complications as preterm birth, preeclampsia, and fetal growth restriction.13,14
Many women who undergo cfDNA screening are under the impression that they have had a definitive test for all birth defects when, in fact, the coverage of cfDNA for all possible birth defects in a low-risk woman is very limited; her residual risk for a birth defect is little changed by a normal cfDNA result.
The ease of obtaining a blood sample for cfDNA screening is an advantage of the test. However, because it is simple to perform, it often is done with inadequate pretest counseling or consideration. Just because the test is easily obtained does not negate the need for adequate discussion to assure that each woman understands what the test can and cannot measure, and the possible outcomes of testing.
Another perceived benefit of cfDNA screening is the simple presentation of results. While reports vary, they generally provide very dichotomized results. Aneuploidy risk is reported as “Positive” or “Negative,” or as “Detected” or “Not detected.”
Some laboratories report the chance of aneuploidy; this is almost always stated to be more than 99% in patients at increased risk, and less than 1 in 10,000 in patients at low risk.
All of these results suggest a near diagnostic certainty. However, this reporting is oversimplified and misleading, as it does not account for each patient’s prior or background risk. The chance that a positive result is a true positive is very different in a 20-year-old versus a 35-year-old woman, yet the reports do not reflect this difference in positive predictive value (PPV). See TABLE 1.
Accurate interpretation of risk for the individual patient, therefore, requires calculation by the provider; this can be done through an online calculator available through the Perinatal Quality Foundation (www.perina talquality.org).
CASE Continued
You explain to your patient that the chance her fetus has trisomy 13 is far lower than 99%, based in part on the very low prior risk given her age. You calculate the PPV using an online calculator, which estimates that there is only a 7% chance that this is a true positive result.
As mentioned earlier, there has been a tremendously rapid uptake of cfDNA screening. Given wider use by practitioners not as familiar with the complexities of genetic testing and statistical analysis, misunderstanding of the test characteristics carries risks if inappropriate recommendations or decisions are made or actions taken.
Most low-risk patients do not request or desire diagnostic testing. It is important during pretest counseling to explain that cfDNA cannot detect all significant chromosomal aneuploidies. Some serious abnormalities will be undetected; therefore, some women may prefer more comprehensive prenatal testing (TABLE 3).
TABLE 3 Checklist for pretest counseling for cfDNA28
- cfDNA screening is the most accurate screening test for trisomy 21
- cfDNA is a screening test, and false-positive and false-negative results can and do occur
- Diagnostic confirmation with chorionic villus sampling or amniocentesis is recommended for women with abnormal cfDNA results
- A negative cfDNA result decreases risk but does not rule out trisomy 21 and other chromosomal conditions
- cfDNA does not test for all chromosomal conditions
- Women who desire definitive information about chromosome conditions in the pregnancy should consider diagnostic testing with chorionic villus sampling or amniocentesis
- All genetic testing is optional. Whether a woman chooses to have a screening test, a diagnostic test, or no testing is a personal decision; any are reasonable options for any pregnant woman.
ACOG recommends that diagnostic testing should be available to all pregnant women, regardless of age.15 In prenatal series, trisomies 13, 18, and 21 make up approximately two-thirds of all clinically significant aneuploidies.16,17 Given that cfDNA detects only these aneuploidies, the other third will not be identified prenatally in patients who choose cfDNA. Traditional aneuploidy screening has been demonstrated to detect a broader range of these less common but clinically important chromosomal abnormalities.18
In one study of women found to be at increased risk based on traditional multiple marker screening, if cfDNA were chosen instead of diagnostic testing, 17% of the aneuploidies present in this group would not have been detected.18 Of all high-risk women in this study, 2%, or 1 in 50, had a chromosomal abnormality detectable by amniocentesis but not with cfDNA.
Successful tests require adequate placental DNA
Accurate interpretation of cfDNA screening also requires that an adequate quantity of placental DNA be present; this is often referred to as the “fetal fraction.” In some cases, the placental DNA volume is too low for accurate analysis, particularly in obese patients and women with specific chromosomal abnormalities.
Some laboratories measure this and do not report a result if the fetal fraction is below a specific cut-off, typically about 4%. Other laboratories do not measure or exclude cases with too little fetal DNA, raising concern that this could result in missing cases of aneuploidy. It has been noted that a placental DNA fraction of less than 8% is associated with less accurate test results, even if results are returned.8
Low fetal fraction also has been associated with maternal obesity, and in one study cfDNA failed to provide a result in 20% of women weighing more than 250 lb and 50% of women weighing more than 350 lb.19 Therefore, cfDNA is not the best option for obese women (TABLE 4).
TABLE 4 Appropriateness of cfDNA screeningin specific clinical circumstances
Optimal candidates for cfDNA screening
- High risk for trisomy based on maternal age (≥35 years)
- Ultrasound findings suggesting trisomy 13, 18, or 21
- History of prior pregnancy with trisomy 13, 18, or 21
- Positive traditional screening test
- Parental balanced Robertsonian translocation associated with risk for trisomy 13 or 21
Less optimal candidates
- Low risk for trisomy based on age and/or low risk traditional screening
- Ultrasound structural anomalies other than those specifically suggesting trisomy 13, 18, or 21
- High risk for nonchromosomal genetic disorder
- Comprehensive genetic diagnosis desired
- Maternal malignancy
- Maternal organ transplant
- Maternal sex chromosomal mosaicism or other chromosomal abnormality
- Maternal obesity
- Gestational age <10 weeks
While the free fraction is relatively constant from 10 to 22 weeks’ gestation, it is lower earlier than 10 weeks’ gestation and less likely to provide a result. For this reason, the test should not be attempted before 10 weeks’ gestation.
Recent evidence indicates that low fetal DNA fraction is associated with some chromosome abnormalities. Given this association, women with failed cfDNA results should be counseled and offered appropriate follow-up. As the association appears to be greater for trisomies 13 and 18, and triploidy, a careful ultrasound is likely to detect abnormalities in many such cases. However, it also is appropriate to offer the option of diagnostic testing, given the very high risk.
A repeat cfDNA test will be successful in some cases. Whether the patient chooses to attempt cfDNA again may depend in part on maternal body mass index (BMI), as well as gestational age—a patient at a more advanced gestation may not wish to delay obtaining definitive information given the high risk.
cfDNA screening has a low false-positive rate
One of the greatest benefits of cfDNA screening is a lower false-positive rate than is reported with traditional screening. However, when “no results” cases are also considered, the percentage of patients who require follow-up after cfDNA is close to that of traditional screening.
The chance of test failure is reported to be 0.9% to 8.1%,7,9,10 and varies in part by whether the laboratory measures fetal fraction and requires a minimum concentration.
A recent meta-analysis estimated the overall test failure rate at 3%.10 When comparing cfDNA to traditional screening, if “no results” cases are included with the “screen positive” group, the benefits of cfDNA over traditional screening are much less clear, particularly in a low-risk population.
ACOG: Offer traditional multiple-marker screening first
While multiple marker and cfDNA screening have differing performance characteristics, there are no data to support doing both tests concurrently. In fact, in a recent survey of nearly 200 women presented with different testing scenarios, women found it preferable and more reassuring to have a positive traditional screen followed by normal cfDNA results, rather that discrepant results of the 2 tests done concurrently.20
For many reasons, the approach recommended by ACOG and SMFM is to offer traditional multiple-marker screening first, and cfDNA screening or diagnostic testing as a follow-up for patients that screen positive. In that scenario, the benefits and limitations of diagnostic testing versus follow-up with cfDNA screening should be explained carefully.
In all patients who have a positive cfDNA result, diagnostic testing for confirmation should be offered and strongly recommended prior to pregnancy termination if that is considered. Even if a structural abnormality is present and a true positive result is highly likely, karyotyping is important to determine if there may be an inherited translocation putting subsequent pregnancies at higher risk.
Components of pretest counseling
A woman of any age can have a fetus with trisomy or another chromosomal abnormality, and some women prefer diagnostic testing or no testing regardless of age. It is therefore appropriate to offer diagnostic testing, screening, or the option of no testing to all women.
Recent studies have demonstrated that providing well-informed access to all prenatal tests results in more informed choices and no increase in uptake of invasive testing.22 However, the offer of prenatal testing requires discussion of the pros and cons of all test options, including the detection rates of all significant abnormalities, the screen positive rates, and recommended follow-up if an abnormal result is obtained. See TABLE 4.
Cost-effectiveness
Although the detection rate of cfDNA for trisomy 21 is higher than that of traditional screening, the detection rate of traditional screening is also quite high at lower cost. For low-risk women, therefore, traditional screening provides a less expensive alternative to cfDNA. Because aneuploidy is rare in low-risk patients, the residual chance of aneuploidy after a normal traditional screen is very low, and the cost per additional case of Down syndrome detected by cfDNA is very high.
In one study, this was estimated at $3.6 million.23 These authors suggested that, at present, cfDNA is optimally used as a secondary screen for high-risk women. Other cost analyses also have demonstrated that the most cost-effective strategy is a model in which cfDNA is used as a follow-up test in patients found to be screen positive by traditional screening.15,24 A recent cost utility analysis compared outcomes of 6 approaches to prenatal screening, including sequential screening, cfDNA screening, nuchal translucency only, and diagnostic testing with microarray (alone, in combination, or in sequence).
The clinical outcomes included fetal abnormalities detected, taking into account all chromosomal abnormalities, as well as failed cfDNA tests. For younger women (<40 yr), traditional sequential screening provided the highest detection of all abnormalities and was the optimal testing strategy, while cfDNA was preferable for women aged 40 or older, given the higher prevalence of trisomy 21.20
Incidental findings
Given that the cfDNA in maternal serum is a mixture of maternal and placental DNA, a number of biologic phenomena can cause a false-positive cfDNA result. In many cases, these false-positives reveal unanticipated or unexpected maternal conditions and information that the woman may have preferred not to know. A few cases of maternal malignancies with chromosomal abnormalities within the tumor have been reported in patients with false-positive cfDNA results.26
These case reports have raised the question about the need for further evaluation for maternal malignancy in women with false-positive results. Maternal genetic disorders also can cause false-positive results, and may lead to unanticipated detection of adult-onset conditions. In some cases, positive results for sex chromosomal aneuploidy can occur in pregnant women who themselves have a sex chromosomal abnormality, often in mosaic form and previously undiagnosed.27
Again, this has led to discussion of the possible health benefit of karyotyping women who have a false-positive cfDNA result to rule out a mosaic chromosomal abnormality in the mother.
At this time, the clinical utility of such investigations is unknown and there are no recommendations regarding appropriate follow-up for such cases.
CASE Resolved
Given the results of her cfDNA screening, your patient opts to undergo diagnostic testing. In that testing, trisomy 13 is ruled out and she goes on to have a healthy daughter.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597.
- Norton ME, Brar H, Weiss J, et al. Non-Invasive Chromosomal Evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(2):137.e1–e8.
- Chitty LS, Mason S, Barrett AN, et al. Non-invasive prenatal diagnosis of achondroplasia and thanatophoric dysplasia: next-generation sequencing allows for a safer, more accurate, and comprehensive approach. Prenat Diagn. 2015;35(7):656–662.
- Wapner RJ, Babiarz JE, Levy B, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212(3):332.e1–e9.
- Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med. 2011;13(11):913–920.
- Sparks AB, Wang ET, Struble CA, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study [abstract]. Proceedings of the ISPD 16th International Conference on Prenatal Diagnosis and Therapy; Miami, Florida; June 3–6, 2012. Prenat Diagn. 2012;32(suppl 1):s3–s9.
- Zimmermann B, Hill M, Gemelos G, et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat Diagn. 2012;32(13):1233–1241.
- Gil MM, Quezada MS, Revello R, Akolekar R, Niclaides KH. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2015;45(3):249–266.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 640: cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126(3):e31–e37.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(6):711–716.
- Baer RJ, Currier RJ, Norton ME, et al. Obstetric, perinatal, and fetal outcomes in pregnancies with false-positive integrated screening results. Obstet Gynecol. 2014;123(3):603–609.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115(5):1052–1061.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 88: invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110(6):1459–1467.
- Alamillo CM, Krantz D, Evans M, Fiddler M, Pergament E. Nearly a third of abnormalities found after first-trimester screening are different than expected: 10-year experience from a single center. Prenat Diagn. 2013;33(3):251–256.
- Wellesley D, Dolk H, Boyd PA, et al. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet. 2012;20(5):521–526.
- Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124(5):979–986.
- Ashoor G, Syngelaki A, Poon LC, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11-13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41(1):26–32.
- Kaimal AJ, Norton ME, Kuppermann M. Prenatal testing in the genomic age: clinical outcomes, quality of life, and costs. Obstet Gynecol. 2015;126(4):737–746.
- Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367(23):2175–2184.
- Kuppermann M, Pena S, Bishop JT, et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized clinical trial. JAMA. 2014;312(12):1210–1217.
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
- Beulen L, Grutters JPC, Faas BH, Feenstra I, van Vugt JMG, Bekker MN. The consequences of implementing non-invasive prenatal testing in Dutch national health care: a cost-effectiveness analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:53–61.
- Okun N, Teitelbaum M, Huang T, Dewa CS, Hoch JS. The price of performance: a cost and performance analysis of the implementation of cell-free fetal DNA testing for Down syndrome in Ontario, Canada: Cost and performance analysis of cfDNA testing for Down syndrome in Ontario. Prenat Diagn. 2014;34(4):350–356.
- Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA. 2015;314(2):162–169.
- Wang Y, Chen Y, Tian F, et al. Maternal mosaicism is a significant contributor to discordant sex chromosomal aneuploidies associated with noninvasive prenatal testing. Clin Chem. 2014;60(1):251–259.
- Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124(5):979–986Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(6):711–716.
CASE: Low-risk patient requests cell-free DNA screening
Ms. Smith is a 25-year-old woman (G1P0) presenting at 10 weeks’ gestation for her first prenatal visit. She requests cell-free DNA (cfDNA) screening to test for fetal aneuploidy. You explain that the current recommendations are for traditional screening, and inform her that her insurance may not cover the cost of cfDNA screening. She is anxious to learn the sex of her fetus as early as possible, and indicates that she would like to pursue cfDNA. After further discussion of the pros and cons, you order the test.
Prenatal screening is currently recommended in pregnancy for a number of genetic disorders, chromosomal aneuploidy, and structural birth defects in the fetus, regardless of maternal age or family history. There is a broad range of sonographic and maternal serum-based options available for carrying out aneuploidy risk assessment in the first and/or second trimester.
In addition, cfDNA screening for fetal aneuploidy has been clinically available since 2011 and has seen tremendous uptake, particularly in the high-risk population. Recent data indicate that cfDNA screening likewise has very high sensitivity and specificity for trisomy 21 in the low-risk population.1,2
Many low-risk patients are asking providers about the pros and cons of cfDNA screening, and the appropriateness of this test as a primary screen, including in low-risk patients, is the focus of this article.
What is cfDNA?
cfDNA consists of small (<200 base pairs) fragments of DNA that are present in the maternal serum. After 10 weeks of gestation, about 10% of the total circulating cfDNA in the maternal serum is derived from the placenta and can therefore be used to test for fetal disorders (FIGURE).3
Although cfDNA screening has been reported to be possible for many different types of genetic conditions, such as RhD type and single-gene disorders such as achondroplasia,4 most clinical testing is done for fetal chromosomal disorders, including trisomies 13, 18, and 21 and the sex chromosomes. In addition, some laboratories provide testing for other trisomies (16 and 22), as well as some of the microdeletion syndromes (22q11.2, 1p36, Prader Willi syndrome, and others).5
Analysis of cfDNA to assess the risk for aneuploidy is done using a number of different approaches; these generally all include next-generation sequencing with advanced bioinformatics analyses.3,6–9 Although the laboratories use somewhat different techniques, all of them share very high sensitivity and specificity for detection of trisomy 21 (TABLE 1).10
Sensitivities for trisomy 13 and sex chromosomal abnormalities are somewhat lower, but the specificity is greater than 99% for each condition, meaning that false-positive rates are very low.
The accuracy of cfDNA in identifying chromosomal aneuploidy depends on several factors, including the relative amount of fetal to maternal DNA, the chance that a chromosome abnormality is present (that is, the risk based on maternal age or results of other screening), and other factors such as the presence of twins or a nonviable second fetus, or the presence of placental mosaicism.
Because of these variables, both false-positive and false-negative results can occur, and the test is not diagnostic but rather is considered a screening test. A positive result does not mean that the fetus is definitely affected with aneuploidy.
What are the advantages of cfDNA screening for low-risk patients?
There are several benefits of cfDNA screening versus traditional screening or diagnostic testing, which are the other options available (TABLE 2). For Down syndrome, the detection rate is higher and the false-positive rate is lower than that seen with traditional aneuploidy screening using serum analytes and nuchal translucency ultrasonography.1,2
TABLE 2 Pros and cons of cfDNA screening in low-risk patients
Pros
- High detection rate and very low false-positive rate
- Can be performed any time after 10 weeks’ gestation
- Requires a single blood test at any gestational age
- Results presented in simple “Yes” or “No” format
- As with other screening tests, cfDNA provides a noninvasive determination of risk
Cons
- Tests for a limited range of conditions, which are rare in low-risk patients
- Is not as comprehensive or definitive as diagnostic testing with amniocentesis or chorionic villus sampling
- Results do not adjust for patient’s prior risk
- Positive results require calculation and interpretation of positive predictive value by provider
- Low fetal DNA and other factors can lead to test failure in some cases
- Cannot be used with vanishing twin
- Can reveal unsuspected maternal conditions of uncertain significance
The test can be done any time after 10 weeks’ gestation without the narrow gestational-age windows required or the need for accurate gestational age determination using traditional screening to accurately interpret results. cfDNA screening involves a single blood test that does not require integration with multiple serum markers or ultrasound findings. Finally, results are generally presented in a simple “Yes” or “No” format that is easy for providers and patients to understand.
CASE Continued
Your patient’s results are positive for trisomy 13. Her understanding is that the test is more than 99% accurate, and she interprets this to mean that the chance of trisomy 13 in her fetus is more than 99%. She is distraught and asks about pregnancy termination.
What are the limitations of cfDNA screening?
Similar to other noninvasive screening tests, cfDNA screening does not carry direct risk to the pregnancy. However, there are limitations to this testing. As a result, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recently have stated that traditional screening is the most appropriate option for most women.11,12
One reason that cfDNA screening may not be the best choice for low-risk women is that Down syndrome is quite uncommon in this group, so cfDNA screening is a very precise test for a rare condition. Traditional multiple marker screening, on the other hand, is more effective at signaling risk for the broad range of adverse perinatal outcomes that can affect a pregnancy, including other structural birth defects, as well as such obstetric complications as preterm birth, preeclampsia, and fetal growth restriction.13,14
Many women who undergo cfDNA screening are under the impression that they have had a definitive test for all birth defects when, in fact, the coverage of cfDNA for all possible birth defects in a low-risk woman is very limited; her residual risk for a birth defect is little changed by a normal cfDNA result.
The ease of obtaining a blood sample for cfDNA screening is an advantage of the test. However, because it is simple to perform, it often is done with inadequate pretest counseling or consideration. Just because the test is easily obtained does not negate the need for adequate discussion to assure that each woman understands what the test can and cannot measure, and the possible outcomes of testing.
Another perceived benefit of cfDNA screening is the simple presentation of results. While reports vary, they generally provide very dichotomized results. Aneuploidy risk is reported as “Positive” or “Negative,” or as “Detected” or “Not detected.”
Some laboratories report the chance of aneuploidy; this is almost always stated to be more than 99% in patients at increased risk, and less than 1 in 10,000 in patients at low risk.
All of these results suggest a near diagnostic certainty. However, this reporting is oversimplified and misleading, as it does not account for each patient’s prior or background risk. The chance that a positive result is a true positive is very different in a 20-year-old versus a 35-year-old woman, yet the reports do not reflect this difference in positive predictive value (PPV). See TABLE 1.
Accurate interpretation of risk for the individual patient, therefore, requires calculation by the provider; this can be done through an online calculator available through the Perinatal Quality Foundation (www.perina talquality.org).
CASE Continued
You explain to your patient that the chance her fetus has trisomy 13 is far lower than 99%, based in part on the very low prior risk given her age. You calculate the PPV using an online calculator, which estimates that there is only a 7% chance that this is a true positive result.
As mentioned earlier, there has been a tremendously rapid uptake of cfDNA screening. Given wider use by practitioners not as familiar with the complexities of genetic testing and statistical analysis, misunderstanding of the test characteristics carries risks if inappropriate recommendations or decisions are made or actions taken.
Most low-risk patients do not request or desire diagnostic testing. It is important during pretest counseling to explain that cfDNA cannot detect all significant chromosomal aneuploidies. Some serious abnormalities will be undetected; therefore, some women may prefer more comprehensive prenatal testing (TABLE 3).
TABLE 3 Checklist for pretest counseling for cfDNA28
- cfDNA screening is the most accurate screening test for trisomy 21
- cfDNA is a screening test, and false-positive and false-negative results can and do occur
- Diagnostic confirmation with chorionic villus sampling or amniocentesis is recommended for women with abnormal cfDNA results
- A negative cfDNA result decreases risk but does not rule out trisomy 21 and other chromosomal conditions
- cfDNA does not test for all chromosomal conditions
- Women who desire definitive information about chromosome conditions in the pregnancy should consider diagnostic testing with chorionic villus sampling or amniocentesis
- All genetic testing is optional. Whether a woman chooses to have a screening test, a diagnostic test, or no testing is a personal decision; any are reasonable options for any pregnant woman.
ACOG recommends that diagnostic testing should be available to all pregnant women, regardless of age.15 In prenatal series, trisomies 13, 18, and 21 make up approximately two-thirds of all clinically significant aneuploidies.16,17 Given that cfDNA detects only these aneuploidies, the other third will not be identified prenatally in patients who choose cfDNA. Traditional aneuploidy screening has been demonstrated to detect a broader range of these less common but clinically important chromosomal abnormalities.18
In one study of women found to be at increased risk based on traditional multiple marker screening, if cfDNA were chosen instead of diagnostic testing, 17% of the aneuploidies present in this group would not have been detected.18 Of all high-risk women in this study, 2%, or 1 in 50, had a chromosomal abnormality detectable by amniocentesis but not with cfDNA.
Successful tests require adequate placental DNA
Accurate interpretation of cfDNA screening also requires that an adequate quantity of placental DNA be present; this is often referred to as the “fetal fraction.” In some cases, the placental DNA volume is too low for accurate analysis, particularly in obese patients and women with specific chromosomal abnormalities.
Some laboratories measure this and do not report a result if the fetal fraction is below a specific cut-off, typically about 4%. Other laboratories do not measure or exclude cases with too little fetal DNA, raising concern that this could result in missing cases of aneuploidy. It has been noted that a placental DNA fraction of less than 8% is associated with less accurate test results, even if results are returned.8
Low fetal fraction also has been associated with maternal obesity, and in one study cfDNA failed to provide a result in 20% of women weighing more than 250 lb and 50% of women weighing more than 350 lb.19 Therefore, cfDNA is not the best option for obese women (TABLE 4).
TABLE 4 Appropriateness of cfDNA screeningin specific clinical circumstances
Optimal candidates for cfDNA screening
- High risk for trisomy based on maternal age (≥35 years)
- Ultrasound findings suggesting trisomy 13, 18, or 21
- History of prior pregnancy with trisomy 13, 18, or 21
- Positive traditional screening test
- Parental balanced Robertsonian translocation associated with risk for trisomy 13 or 21
Less optimal candidates
- Low risk for trisomy based on age and/or low risk traditional screening
- Ultrasound structural anomalies other than those specifically suggesting trisomy 13, 18, or 21
- High risk for nonchromosomal genetic disorder
- Comprehensive genetic diagnosis desired
- Maternal malignancy
- Maternal organ transplant
- Maternal sex chromosomal mosaicism or other chromosomal abnormality
- Maternal obesity
- Gestational age <10 weeks
While the free fraction is relatively constant from 10 to 22 weeks’ gestation, it is lower earlier than 10 weeks’ gestation and less likely to provide a result. For this reason, the test should not be attempted before 10 weeks’ gestation.
Recent evidence indicates that low fetal DNA fraction is associated with some chromosome abnormalities. Given this association, women with failed cfDNA results should be counseled and offered appropriate follow-up. As the association appears to be greater for trisomies 13 and 18, and triploidy, a careful ultrasound is likely to detect abnormalities in many such cases. However, it also is appropriate to offer the option of diagnostic testing, given the very high risk.
A repeat cfDNA test will be successful in some cases. Whether the patient chooses to attempt cfDNA again may depend in part on maternal body mass index (BMI), as well as gestational age—a patient at a more advanced gestation may not wish to delay obtaining definitive information given the high risk.
cfDNA screening has a low false-positive rate
One of the greatest benefits of cfDNA screening is a lower false-positive rate than is reported with traditional screening. However, when “no results” cases are also considered, the percentage of patients who require follow-up after cfDNA is close to that of traditional screening.
The chance of test failure is reported to be 0.9% to 8.1%,7,9,10 and varies in part by whether the laboratory measures fetal fraction and requires a minimum concentration.
A recent meta-analysis estimated the overall test failure rate at 3%.10 When comparing cfDNA to traditional screening, if “no results” cases are included with the “screen positive” group, the benefits of cfDNA over traditional screening are much less clear, particularly in a low-risk population.
ACOG: Offer traditional multiple-marker screening first
While multiple marker and cfDNA screening have differing performance characteristics, there are no data to support doing both tests concurrently. In fact, in a recent survey of nearly 200 women presented with different testing scenarios, women found it preferable and more reassuring to have a positive traditional screen followed by normal cfDNA results, rather that discrepant results of the 2 tests done concurrently.20
For many reasons, the approach recommended by ACOG and SMFM is to offer traditional multiple-marker screening first, and cfDNA screening or diagnostic testing as a follow-up for patients that screen positive. In that scenario, the benefits and limitations of diagnostic testing versus follow-up with cfDNA screening should be explained carefully.
In all patients who have a positive cfDNA result, diagnostic testing for confirmation should be offered and strongly recommended prior to pregnancy termination if that is considered. Even if a structural abnormality is present and a true positive result is highly likely, karyotyping is important to determine if there may be an inherited translocation putting subsequent pregnancies at higher risk.
Components of pretest counseling
A woman of any age can have a fetus with trisomy or another chromosomal abnormality, and some women prefer diagnostic testing or no testing regardless of age. It is therefore appropriate to offer diagnostic testing, screening, or the option of no testing to all women.
Recent studies have demonstrated that providing well-informed access to all prenatal tests results in more informed choices and no increase in uptake of invasive testing.22 However, the offer of prenatal testing requires discussion of the pros and cons of all test options, including the detection rates of all significant abnormalities, the screen positive rates, and recommended follow-up if an abnormal result is obtained. See TABLE 4.
Cost-effectiveness
Although the detection rate of cfDNA for trisomy 21 is higher than that of traditional screening, the detection rate of traditional screening is also quite high at lower cost. For low-risk women, therefore, traditional screening provides a less expensive alternative to cfDNA. Because aneuploidy is rare in low-risk patients, the residual chance of aneuploidy after a normal traditional screen is very low, and the cost per additional case of Down syndrome detected by cfDNA is very high.
In one study, this was estimated at $3.6 million.23 These authors suggested that, at present, cfDNA is optimally used as a secondary screen for high-risk women. Other cost analyses also have demonstrated that the most cost-effective strategy is a model in which cfDNA is used as a follow-up test in patients found to be screen positive by traditional screening.15,24 A recent cost utility analysis compared outcomes of 6 approaches to prenatal screening, including sequential screening, cfDNA screening, nuchal translucency only, and diagnostic testing with microarray (alone, in combination, or in sequence).
The clinical outcomes included fetal abnormalities detected, taking into account all chromosomal abnormalities, as well as failed cfDNA tests. For younger women (<40 yr), traditional sequential screening provided the highest detection of all abnormalities and was the optimal testing strategy, while cfDNA was preferable for women aged 40 or older, given the higher prevalence of trisomy 21.20
Incidental findings
Given that the cfDNA in maternal serum is a mixture of maternal and placental DNA, a number of biologic phenomena can cause a false-positive cfDNA result. In many cases, these false-positives reveal unanticipated or unexpected maternal conditions and information that the woman may have preferred not to know. A few cases of maternal malignancies with chromosomal abnormalities within the tumor have been reported in patients with false-positive cfDNA results.26
These case reports have raised the question about the need for further evaluation for maternal malignancy in women with false-positive results. Maternal genetic disorders also can cause false-positive results, and may lead to unanticipated detection of adult-onset conditions. In some cases, positive results for sex chromosomal aneuploidy can occur in pregnant women who themselves have a sex chromosomal abnormality, often in mosaic form and previously undiagnosed.27
Again, this has led to discussion of the possible health benefit of karyotyping women who have a false-positive cfDNA result to rule out a mosaic chromosomal abnormality in the mother.
At this time, the clinical utility of such investigations is unknown and there are no recommendations regarding appropriate follow-up for such cases.
CASE Resolved
Given the results of her cfDNA screening, your patient opts to undergo diagnostic testing. In that testing, trisomy 13 is ruled out and she goes on to have a healthy daughter.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE: Low-risk patient requests cell-free DNA screening
Ms. Smith is a 25-year-old woman (G1P0) presenting at 10 weeks’ gestation for her first prenatal visit. She requests cell-free DNA (cfDNA) screening to test for fetal aneuploidy. You explain that the current recommendations are for traditional screening, and inform her that her insurance may not cover the cost of cfDNA screening. She is anxious to learn the sex of her fetus as early as possible, and indicates that she would like to pursue cfDNA. After further discussion of the pros and cons, you order the test.
Prenatal screening is currently recommended in pregnancy for a number of genetic disorders, chromosomal aneuploidy, and structural birth defects in the fetus, regardless of maternal age or family history. There is a broad range of sonographic and maternal serum-based options available for carrying out aneuploidy risk assessment in the first and/or second trimester.
In addition, cfDNA screening for fetal aneuploidy has been clinically available since 2011 and has seen tremendous uptake, particularly in the high-risk population. Recent data indicate that cfDNA screening likewise has very high sensitivity and specificity for trisomy 21 in the low-risk population.1,2
Many low-risk patients are asking providers about the pros and cons of cfDNA screening, and the appropriateness of this test as a primary screen, including in low-risk patients, is the focus of this article.
What is cfDNA?
cfDNA consists of small (<200 base pairs) fragments of DNA that are present in the maternal serum. After 10 weeks of gestation, about 10% of the total circulating cfDNA in the maternal serum is derived from the placenta and can therefore be used to test for fetal disorders (FIGURE).3
Although cfDNA screening has been reported to be possible for many different types of genetic conditions, such as RhD type and single-gene disorders such as achondroplasia,4 most clinical testing is done for fetal chromosomal disorders, including trisomies 13, 18, and 21 and the sex chromosomes. In addition, some laboratories provide testing for other trisomies (16 and 22), as well as some of the microdeletion syndromes (22q11.2, 1p36, Prader Willi syndrome, and others).5
Analysis of cfDNA to assess the risk for aneuploidy is done using a number of different approaches; these generally all include next-generation sequencing with advanced bioinformatics analyses.3,6–9 Although the laboratories use somewhat different techniques, all of them share very high sensitivity and specificity for detection of trisomy 21 (TABLE 1).10
Sensitivities for trisomy 13 and sex chromosomal abnormalities are somewhat lower, but the specificity is greater than 99% for each condition, meaning that false-positive rates are very low.
The accuracy of cfDNA in identifying chromosomal aneuploidy depends on several factors, including the relative amount of fetal to maternal DNA, the chance that a chromosome abnormality is present (that is, the risk based on maternal age or results of other screening), and other factors such as the presence of twins or a nonviable second fetus, or the presence of placental mosaicism.
Because of these variables, both false-positive and false-negative results can occur, and the test is not diagnostic but rather is considered a screening test. A positive result does not mean that the fetus is definitely affected with aneuploidy.
What are the advantages of cfDNA screening for low-risk patients?
There are several benefits of cfDNA screening versus traditional screening or diagnostic testing, which are the other options available (TABLE 2). For Down syndrome, the detection rate is higher and the false-positive rate is lower than that seen with traditional aneuploidy screening using serum analytes and nuchal translucency ultrasonography.1,2
TABLE 2 Pros and cons of cfDNA screening in low-risk patients
Pros
- High detection rate and very low false-positive rate
- Can be performed any time after 10 weeks’ gestation
- Requires a single blood test at any gestational age
- Results presented in simple “Yes” or “No” format
- As with other screening tests, cfDNA provides a noninvasive determination of risk
Cons
- Tests for a limited range of conditions, which are rare in low-risk patients
- Is not as comprehensive or definitive as diagnostic testing with amniocentesis or chorionic villus sampling
- Results do not adjust for patient’s prior risk
- Positive results require calculation and interpretation of positive predictive value by provider
- Low fetal DNA and other factors can lead to test failure in some cases
- Cannot be used with vanishing twin
- Can reveal unsuspected maternal conditions of uncertain significance
The test can be done any time after 10 weeks’ gestation without the narrow gestational-age windows required or the need for accurate gestational age determination using traditional screening to accurately interpret results. cfDNA screening involves a single blood test that does not require integration with multiple serum markers or ultrasound findings. Finally, results are generally presented in a simple “Yes” or “No” format that is easy for providers and patients to understand.
CASE Continued
Your patient’s results are positive for trisomy 13. Her understanding is that the test is more than 99% accurate, and she interprets this to mean that the chance of trisomy 13 in her fetus is more than 99%. She is distraught and asks about pregnancy termination.
What are the limitations of cfDNA screening?
Similar to other noninvasive screening tests, cfDNA screening does not carry direct risk to the pregnancy. However, there are limitations to this testing. As a result, the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine (SMFM) recently have stated that traditional screening is the most appropriate option for most women.11,12
One reason that cfDNA screening may not be the best choice for low-risk women is that Down syndrome is quite uncommon in this group, so cfDNA screening is a very precise test for a rare condition. Traditional multiple marker screening, on the other hand, is more effective at signaling risk for the broad range of adverse perinatal outcomes that can affect a pregnancy, including other structural birth defects, as well as such obstetric complications as preterm birth, preeclampsia, and fetal growth restriction.13,14
Many women who undergo cfDNA screening are under the impression that they have had a definitive test for all birth defects when, in fact, the coverage of cfDNA for all possible birth defects in a low-risk woman is very limited; her residual risk for a birth defect is little changed by a normal cfDNA result.
The ease of obtaining a blood sample for cfDNA screening is an advantage of the test. However, because it is simple to perform, it often is done with inadequate pretest counseling or consideration. Just because the test is easily obtained does not negate the need for adequate discussion to assure that each woman understands what the test can and cannot measure, and the possible outcomes of testing.
Another perceived benefit of cfDNA screening is the simple presentation of results. While reports vary, they generally provide very dichotomized results. Aneuploidy risk is reported as “Positive” or “Negative,” or as “Detected” or “Not detected.”
Some laboratories report the chance of aneuploidy; this is almost always stated to be more than 99% in patients at increased risk, and less than 1 in 10,000 in patients at low risk.
All of these results suggest a near diagnostic certainty. However, this reporting is oversimplified and misleading, as it does not account for each patient’s prior or background risk. The chance that a positive result is a true positive is very different in a 20-year-old versus a 35-year-old woman, yet the reports do not reflect this difference in positive predictive value (PPV). See TABLE 1.
Accurate interpretation of risk for the individual patient, therefore, requires calculation by the provider; this can be done through an online calculator available through the Perinatal Quality Foundation (www.perina talquality.org).
CASE Continued
You explain to your patient that the chance her fetus has trisomy 13 is far lower than 99%, based in part on the very low prior risk given her age. You calculate the PPV using an online calculator, which estimates that there is only a 7% chance that this is a true positive result.
As mentioned earlier, there has been a tremendously rapid uptake of cfDNA screening. Given wider use by practitioners not as familiar with the complexities of genetic testing and statistical analysis, misunderstanding of the test characteristics carries risks if inappropriate recommendations or decisions are made or actions taken.
Most low-risk patients do not request or desire diagnostic testing. It is important during pretest counseling to explain that cfDNA cannot detect all significant chromosomal aneuploidies. Some serious abnormalities will be undetected; therefore, some women may prefer more comprehensive prenatal testing (TABLE 3).
TABLE 3 Checklist for pretest counseling for cfDNA28
- cfDNA screening is the most accurate screening test for trisomy 21
- cfDNA is a screening test, and false-positive and false-negative results can and do occur
- Diagnostic confirmation with chorionic villus sampling or amniocentesis is recommended for women with abnormal cfDNA results
- A negative cfDNA result decreases risk but does not rule out trisomy 21 and other chromosomal conditions
- cfDNA does not test for all chromosomal conditions
- Women who desire definitive information about chromosome conditions in the pregnancy should consider diagnostic testing with chorionic villus sampling or amniocentesis
- All genetic testing is optional. Whether a woman chooses to have a screening test, a diagnostic test, or no testing is a personal decision; any are reasonable options for any pregnant woman.
ACOG recommends that diagnostic testing should be available to all pregnant women, regardless of age.15 In prenatal series, trisomies 13, 18, and 21 make up approximately two-thirds of all clinically significant aneuploidies.16,17 Given that cfDNA detects only these aneuploidies, the other third will not be identified prenatally in patients who choose cfDNA. Traditional aneuploidy screening has been demonstrated to detect a broader range of these less common but clinically important chromosomal abnormalities.18
In one study of women found to be at increased risk based on traditional multiple marker screening, if cfDNA were chosen instead of diagnostic testing, 17% of the aneuploidies present in this group would not have been detected.18 Of all high-risk women in this study, 2%, or 1 in 50, had a chromosomal abnormality detectable by amniocentesis but not with cfDNA.
Successful tests require adequate placental DNA
Accurate interpretation of cfDNA screening also requires that an adequate quantity of placental DNA be present; this is often referred to as the “fetal fraction.” In some cases, the placental DNA volume is too low for accurate analysis, particularly in obese patients and women with specific chromosomal abnormalities.
Some laboratories measure this and do not report a result if the fetal fraction is below a specific cut-off, typically about 4%. Other laboratories do not measure or exclude cases with too little fetal DNA, raising concern that this could result in missing cases of aneuploidy. It has been noted that a placental DNA fraction of less than 8% is associated with less accurate test results, even if results are returned.8
Low fetal fraction also has been associated with maternal obesity, and in one study cfDNA failed to provide a result in 20% of women weighing more than 250 lb and 50% of women weighing more than 350 lb.19 Therefore, cfDNA is not the best option for obese women (TABLE 4).
TABLE 4 Appropriateness of cfDNA screeningin specific clinical circumstances
Optimal candidates for cfDNA screening
- High risk for trisomy based on maternal age (≥35 years)
- Ultrasound findings suggesting trisomy 13, 18, or 21
- History of prior pregnancy with trisomy 13, 18, or 21
- Positive traditional screening test
- Parental balanced Robertsonian translocation associated with risk for trisomy 13 or 21
Less optimal candidates
- Low risk for trisomy based on age and/or low risk traditional screening
- Ultrasound structural anomalies other than those specifically suggesting trisomy 13, 18, or 21
- High risk for nonchromosomal genetic disorder
- Comprehensive genetic diagnosis desired
- Maternal malignancy
- Maternal organ transplant
- Maternal sex chromosomal mosaicism or other chromosomal abnormality
- Maternal obesity
- Gestational age <10 weeks
While the free fraction is relatively constant from 10 to 22 weeks’ gestation, it is lower earlier than 10 weeks’ gestation and less likely to provide a result. For this reason, the test should not be attempted before 10 weeks’ gestation.
Recent evidence indicates that low fetal DNA fraction is associated with some chromosome abnormalities. Given this association, women with failed cfDNA results should be counseled and offered appropriate follow-up. As the association appears to be greater for trisomies 13 and 18, and triploidy, a careful ultrasound is likely to detect abnormalities in many such cases. However, it also is appropriate to offer the option of diagnostic testing, given the very high risk.
A repeat cfDNA test will be successful in some cases. Whether the patient chooses to attempt cfDNA again may depend in part on maternal body mass index (BMI), as well as gestational age—a patient at a more advanced gestation may not wish to delay obtaining definitive information given the high risk.
cfDNA screening has a low false-positive rate
One of the greatest benefits of cfDNA screening is a lower false-positive rate than is reported with traditional screening. However, when “no results” cases are also considered, the percentage of patients who require follow-up after cfDNA is close to that of traditional screening.
The chance of test failure is reported to be 0.9% to 8.1%,7,9,10 and varies in part by whether the laboratory measures fetal fraction and requires a minimum concentration.
A recent meta-analysis estimated the overall test failure rate at 3%.10 When comparing cfDNA to traditional screening, if “no results” cases are included with the “screen positive” group, the benefits of cfDNA over traditional screening are much less clear, particularly in a low-risk population.
ACOG: Offer traditional multiple-marker screening first
While multiple marker and cfDNA screening have differing performance characteristics, there are no data to support doing both tests concurrently. In fact, in a recent survey of nearly 200 women presented with different testing scenarios, women found it preferable and more reassuring to have a positive traditional screen followed by normal cfDNA results, rather that discrepant results of the 2 tests done concurrently.20
For many reasons, the approach recommended by ACOG and SMFM is to offer traditional multiple-marker screening first, and cfDNA screening or diagnostic testing as a follow-up for patients that screen positive. In that scenario, the benefits and limitations of diagnostic testing versus follow-up with cfDNA screening should be explained carefully.
In all patients who have a positive cfDNA result, diagnostic testing for confirmation should be offered and strongly recommended prior to pregnancy termination if that is considered. Even if a structural abnormality is present and a true positive result is highly likely, karyotyping is important to determine if there may be an inherited translocation putting subsequent pregnancies at higher risk.
Components of pretest counseling
A woman of any age can have a fetus with trisomy or another chromosomal abnormality, and some women prefer diagnostic testing or no testing regardless of age. It is therefore appropriate to offer diagnostic testing, screening, or the option of no testing to all women.
Recent studies have demonstrated that providing well-informed access to all prenatal tests results in more informed choices and no increase in uptake of invasive testing.22 However, the offer of prenatal testing requires discussion of the pros and cons of all test options, including the detection rates of all significant abnormalities, the screen positive rates, and recommended follow-up if an abnormal result is obtained. See TABLE 4.
Cost-effectiveness
Although the detection rate of cfDNA for trisomy 21 is higher than that of traditional screening, the detection rate of traditional screening is also quite high at lower cost. For low-risk women, therefore, traditional screening provides a less expensive alternative to cfDNA. Because aneuploidy is rare in low-risk patients, the residual chance of aneuploidy after a normal traditional screen is very low, and the cost per additional case of Down syndrome detected by cfDNA is very high.
In one study, this was estimated at $3.6 million.23 These authors suggested that, at present, cfDNA is optimally used as a secondary screen for high-risk women. Other cost analyses also have demonstrated that the most cost-effective strategy is a model in which cfDNA is used as a follow-up test in patients found to be screen positive by traditional screening.15,24 A recent cost utility analysis compared outcomes of 6 approaches to prenatal screening, including sequential screening, cfDNA screening, nuchal translucency only, and diagnostic testing with microarray (alone, in combination, or in sequence).
The clinical outcomes included fetal abnormalities detected, taking into account all chromosomal abnormalities, as well as failed cfDNA tests. For younger women (<40 yr), traditional sequential screening provided the highest detection of all abnormalities and was the optimal testing strategy, while cfDNA was preferable for women aged 40 or older, given the higher prevalence of trisomy 21.20
Incidental findings
Given that the cfDNA in maternal serum is a mixture of maternal and placental DNA, a number of biologic phenomena can cause a false-positive cfDNA result. In many cases, these false-positives reveal unanticipated or unexpected maternal conditions and information that the woman may have preferred not to know. A few cases of maternal malignancies with chromosomal abnormalities within the tumor have been reported in patients with false-positive cfDNA results.26
These case reports have raised the question about the need for further evaluation for maternal malignancy in women with false-positive results. Maternal genetic disorders also can cause false-positive results, and may lead to unanticipated detection of adult-onset conditions. In some cases, positive results for sex chromosomal aneuploidy can occur in pregnant women who themselves have a sex chromosomal abnormality, often in mosaic form and previously undiagnosed.27
Again, this has led to discussion of the possible health benefit of karyotyping women who have a false-positive cfDNA result to rule out a mosaic chromosomal abnormality in the mother.
At this time, the clinical utility of such investigations is unknown and there are no recommendations regarding appropriate follow-up for such cases.
CASE Resolved
Given the results of her cfDNA screening, your patient opts to undergo diagnostic testing. In that testing, trisomy 13 is ruled out and she goes on to have a healthy daughter.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597.
- Norton ME, Brar H, Weiss J, et al. Non-Invasive Chromosomal Evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(2):137.e1–e8.
- Chitty LS, Mason S, Barrett AN, et al. Non-invasive prenatal diagnosis of achondroplasia and thanatophoric dysplasia: next-generation sequencing allows for a safer, more accurate, and comprehensive approach. Prenat Diagn. 2015;35(7):656–662.
- Wapner RJ, Babiarz JE, Levy B, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212(3):332.e1–e9.
- Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med. 2011;13(11):913–920.
- Sparks AB, Wang ET, Struble CA, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study [abstract]. Proceedings of the ISPD 16th International Conference on Prenatal Diagnosis and Therapy; Miami, Florida; June 3–6, 2012. Prenat Diagn. 2012;32(suppl 1):s3–s9.
- Zimmermann B, Hill M, Gemelos G, et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat Diagn. 2012;32(13):1233–1241.
- Gil MM, Quezada MS, Revello R, Akolekar R, Niclaides KH. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2015;45(3):249–266.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 640: cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126(3):e31–e37.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(6):711–716.
- Baer RJ, Currier RJ, Norton ME, et al. Obstetric, perinatal, and fetal outcomes in pregnancies with false-positive integrated screening results. Obstet Gynecol. 2014;123(3):603–609.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115(5):1052–1061.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 88: invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110(6):1459–1467.
- Alamillo CM, Krantz D, Evans M, Fiddler M, Pergament E. Nearly a third of abnormalities found after first-trimester screening are different than expected: 10-year experience from a single center. Prenat Diagn. 2013;33(3):251–256.
- Wellesley D, Dolk H, Boyd PA, et al. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet. 2012;20(5):521–526.
- Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124(5):979–986.
- Ashoor G, Syngelaki A, Poon LC, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11-13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41(1):26–32.
- Kaimal AJ, Norton ME, Kuppermann M. Prenatal testing in the genomic age: clinical outcomes, quality of life, and costs. Obstet Gynecol. 2015;126(4):737–746.
- Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367(23):2175–2184.
- Kuppermann M, Pena S, Bishop JT, et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized clinical trial. JAMA. 2014;312(12):1210–1217.
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
- Beulen L, Grutters JPC, Faas BH, Feenstra I, van Vugt JMG, Bekker MN. The consequences of implementing non-invasive prenatal testing in Dutch national health care: a cost-effectiveness analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:53–61.
- Okun N, Teitelbaum M, Huang T, Dewa CS, Hoch JS. The price of performance: a cost and performance analysis of the implementation of cell-free fetal DNA testing for Down syndrome in Ontario, Canada: Cost and performance analysis of cfDNA testing for Down syndrome in Ontario. Prenat Diagn. 2014;34(4):350–356.
- Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA. 2015;314(2):162–169.
- Wang Y, Chen Y, Tian F, et al. Maternal mosaicism is a significant contributor to discordant sex chromosomal aneuploidies associated with noninvasive prenatal testing. Clin Chem. 2014;60(1):251–259.
- Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124(5):979–986Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(6):711–716.
- Bianchi DW, Parker RL, Wentworth J, et al. DNA sequencing versus standard prenatal aneuploidy screening. N Engl J Med. 2014;370(9):799–808.
- Norton ME, Jacobsson B, Swamy GK, et al. Cell-free DNA analysis for noninvasive examination of trisomy. N Engl J Med. 2015;372(17):1589–1597.
- Norton ME, Brar H, Weiss J, et al. Non-Invasive Chromosomal Evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. Am J Obstet Gynecol. 2012;207(2):137.e1–e8.
- Chitty LS, Mason S, Barrett AN, et al. Non-invasive prenatal diagnosis of achondroplasia and thanatophoric dysplasia: next-generation sequencing allows for a safer, more accurate, and comprehensive approach. Prenat Diagn. 2015;35(7):656–662.
- Wapner RJ, Babiarz JE, Levy B, et al. Expanding the scope of noninvasive prenatal testing: detection of fetal microdeletion syndromes. Am J Obstet Gynecol. 2015;212(3):332.e1–e9.
- Bianchi DW, Platt LD, Goldberg JD, Abuhamad AZ, Sehnert AJ, Rava RP. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstet Gynecol. 2012;119(5):890–901.
- Palomaki GE, Kloza EM, Lambert-Messerlian GM, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study. Genet Med. 2011;13(11):913–920.
- Sparks AB, Wang ET, Struble CA, et al. DNA sequencing of maternal plasma to detect Down syndrome: an international clinical validation study [abstract]. Proceedings of the ISPD 16th International Conference on Prenatal Diagnosis and Therapy; Miami, Florida; June 3–6, 2012. Prenat Diagn. 2012;32(suppl 1):s3–s9.
- Zimmermann B, Hill M, Gemelos G, et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenat Diagn. 2012;32(13):1233–1241.
- Gil MM, Quezada MS, Revello R, Akolekar R, Niclaides KH. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2015;45(3):249–266.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 640: cell-free DNA screening for fetal aneuploidy. Obstet Gynecol. 2015;126(3):e31–e37.
- Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(6):711–716.
- Baer RJ, Currier RJ, Norton ME, et al. Obstetric, perinatal, and fetal outcomes in pregnancies with false-positive integrated screening results. Obstet Gynecol. 2014;123(3):603–609.
- Dugoff L; Society for Maternal-Fetal Medicine. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115(5):1052–1061.
- American College of Obstetricians and Gynecologists. Practice bulletin No. 88: invasive prenatal testing for aneuploidy. Obstet Gynecol. 2007;110(6):1459–1467.
- Alamillo CM, Krantz D, Evans M, Fiddler M, Pergament E. Nearly a third of abnormalities found after first-trimester screening are different than expected: 10-year experience from a single center. Prenat Diagn. 2013;33(3):251–256.
- Wellesley D, Dolk H, Boyd PA, et al. Rare chromosome abnormalities, prevalence and prenatal diagnosis rates from population-based congenital anomaly registers in Europe. Eur J Hum Genet. 2012;20(5):521–526.
- Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124(5):979–986.
- Ashoor G, Syngelaki A, Poon LC, Rezende JC, Nicolaides KH. Fetal fraction in maternal plasma cell-free DNA at 11-13 weeks’ gestation: relation to maternal and fetal characteristics. Ultrasound Obstet Gynecol. 2013;41(1):26–32.
- Kaimal AJ, Norton ME, Kuppermann M. Prenatal testing in the genomic age: clinical outcomes, quality of life, and costs. Obstet Gynecol. 2015;126(4):737–746.
- Wapner RJ, Martin CL, Levy B, et al. Chromosomal microarray versus karyotyping for prenatal diagnosis. N Engl J Med. 2012;367(23):2175–2184.
- Kuppermann M, Pena S, Bishop JT, et al. Effect of enhanced information, values clarification, and removal of financial barriers on use of prenatal genetic testing: a randomized clinical trial. JAMA. 2014;312(12):1210–1217.
- Cuckle H, Benn P, Pergament E. Maternal cfDNA screening for Down syndrome—a cost sensitivity analysis. Prenat Diagn. 2013;33(7):636–642.
- Beulen L, Grutters JPC, Faas BH, Feenstra I, van Vugt JMG, Bekker MN. The consequences of implementing non-invasive prenatal testing in Dutch national health care: a cost-effectiveness analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:53–61.
- Okun N, Teitelbaum M, Huang T, Dewa CS, Hoch JS. The price of performance: a cost and performance analysis of the implementation of cell-free fetal DNA testing for Down syndrome in Ontario, Canada: Cost and performance analysis of cfDNA testing for Down syndrome in Ontario. Prenat Diagn. 2014;34(4):350–356.
- Bianchi DW, Chudova D, Sehnert AJ, et al. Noninvasive prenatal testing and incidental detection of occult maternal malignancies. JAMA. 2015;314(2):162–169.
- Wang Y, Chen Y, Tian F, et al. Maternal mosaicism is a significant contributor to discordant sex chromosomal aneuploidies associated with noninvasive prenatal testing. Clin Chem. 2014;60(1):251–259.
- Norton ME, Jelliffe-Pawlowski LL, Currier RJ. Chromosome abnormalities detected by current prenatal screening and noninvasive prenatal testing. Obstet Gynecol. 2014;124(5):979–986Society for Maternal-Fetal Medicine (SMFM) Publications Committee. Prenatal aneuploidy screening using cell-free DNA. Am J Obstet Gynecol. 2015;212(6):711–716.
In this Article
- Pros and cons of cfDNA in low-risk patients
- Optimal and less optimal candidates for cfDNA screening
- Checklist for pretest counseling for cfDNA