User login
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
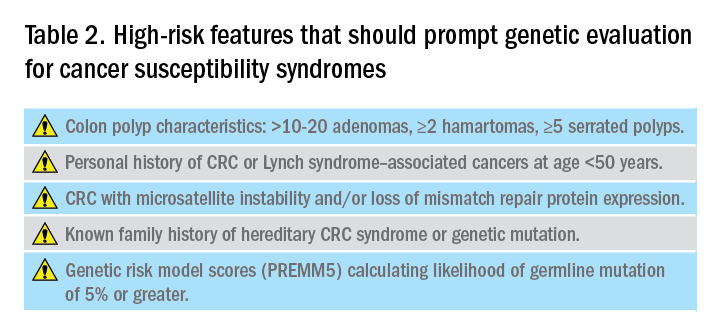
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.
As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.
Introduction
Genetic predisposition to colorectal polyps and colorectal cancer (CRC) is more common than previously recognized. Approximately 5%-10% of all individuals diagnosed with CRC have a known genetic association. However, among those with early-onset CRC (diagnosed at age less than 50 years), recent studies show that up to 20% have an associated genetic mutation.1,2 In addition, the risk of CRC in patients with certain hereditary syndromes, such as familial adenomatous polyposis (FAP), approaches 80%-90% without timely management.3 This overall high risk of CRC and extracolonic malignancies in patients with a hereditary syndrome, along with the rising rates of early-onset CRC, underscores the importance of early diagnosis and management of a hereditary condition.
Despite increasing awareness of hereditary polyposis and nonpolyposis syndromes, referral rates for genetic counseling and testing remain low.4 As gastroenterologists we have several unique opportunities, in clinic and in endoscopy, to identify patients at risk for hereditary syndromes.
Risk stratification
Personal and family history
Reviewing personal medical history and family history in detail should be a routine part of our practice. This is often when initial signs of a potential hereditary syndrome can be detected. For example, if a patient reports a personal or family history of colorectal polyps or CRC, additional information that becomes important includes age at time of diagnosis, polyp burden (number and histologic subtype), presence of inflammatory bowel disease, and history of any extracolonic malignancies. Patients with multiple colorectal polyps (e.g. more than 10-20 adenomas or more than 2 hamartomas) and those with CRC diagnosed at a young age (younger than 50 years) should be considered candidates for genetic evaluation.5
Lynch syndrome (LS), an autosomal dominant condition caused by loss of DNA mismatch repair (MMR) genes, is the most common hereditary CRC syndrome, accounting for 2%-4% of all CRCs.3,6 Extracolonic LS-associated cancers to keep in mind while reviewing personal and family histories include those involving the gastrointestinal (GI) tract such as gastric, pancreatic, biliary tract, and small intestine cancers, and also non-GI tract cancers including endometrial, ovarian, urinary tract, and renal cancers along with brain tumors, and skin lesions including sebaceous adenomas, sebaceous carcinomas, and keratoacanthomas. Notably, after CRC, endometrial cancer is the second most common cancer among women with LS. Prior diagnosis of endometrial cancer should also prompt additional history-taking and evaluation for LS.
As the National Comprehensive Cancer Network (NCCN) highlights in its recent guidelines, several key findings in family history that should prompt referral to genetics for evaluation and testing for LS include: one or more first-degree relatives (FDR) with CRC or endometrial cancer diagnosed at less than 50 years of age, one or more FDR with CRC or endometrial cancer and another synchronous or metachronous LS-related cancer, two or more FDR or second-degree relatives (SDR) with LS-related cancer (including at least one diagnosed at age less than 50 years), and three or more FDR or SDR with LS-related cancers (regardless of age).5
Comprehensive assessment of family history should include all cancer diagnoses in first- and second-degree relatives, including age at diagnosis and cancer type, as well as ethnicity, as these inform the likelihood that the patient harbors a germline pathogenic variant associated with cancer predisposition.5 Given the difficulty of eliciting this level of detail, the family histories elicited in clinical settings are often limited or incomplete. Unknown family history should not be mistaken for unremarkable family history. Alternatively, if family history is unimpressive, this is not necessarily reassuring, as there can be variability in disease penetrance, including autosomal recessive syndromes that may skip generations, and de novo mutations do occur. In fact, among individuals with early-onset CRC diagnosed at age less than 50, only half of mutation carriers reported a family history of CRC in an FDR.2 Thus, individuals with concerning personal histories should undergo a genetic evaluation even if family history is not concerning.
Polyp phenotype
In addition to personal and family history, colon polyp history (including number, size, and histology) can provide important clues to identifying individuals with genetic predisposition to CRC. Table 1 highlights hereditary syndromes and polyp phenotypes associated with increased CRC risk. Based on consensus guidelines, individuals with a history of greater than 10-20 adenomas, 2 or more hamartomas, or 5 or more sessile serrated polyps should be referred for genetic testing.5,7 Serrated polyposis syndrome (SPS) is diagnosed based on at least one of the following criteria: 1) 5 or more serrated polyps, all at least 5 mm in size, proximal to the rectum including at least 2 that are 10 mm or larger in size, or 2) more than 20 serrated polyps distributed throughout the colon with at least 5 proximal to the rectum.8 Pathogenic germline variants in RNF43, a tumor suppressor gene, have been associated with SPS in rare families; however, in most cases genetic testing is uninformative and further genetic and environmental discovery studies are needed to determine the underlying cause.8,9

Risk prediction models
Models have been developed that integrate family history and phenotype data to help identify patients who may be at risk for LS. The Amsterdam criteria (more than 3 relatives with LS-associated cancers, more than 2 generations involving LS-associated cancers, and more than 1 cancer diagnosed before the age of 50; “3:2:1” criteria) were initially developed for research purposes to identify individuals who were likely to be carriers of mutations of LS based on CRC and later revised to include extracolonic malignancies (Amsterdam II).11 However, they have limited sensitivity for identifying high-risk patients. Similarly, the Bethesda guidelines have also been modified and revised to identify patients at risk for LS whose tumors should be tested with microsatellite instability (MSI), but also with limited sensitivity.12
Several risk prediction models have been developed that perform better than the Amsterdam criteria or Bethesda guidelines for determining which patients should be referred for genetic testing for LS. These include MMRPredict, MMRpro, and PREMM5.13-16 These models use clinical data (personal and family history of cancer and tumor phenotypes) to calculate the probability of a germline mutation in one of the mismatch repair (MMR) genes associated with LS. The current threshold at which to refer a patient for genetic counseling and testing is a predicted probability of 5% or greater using any one of these models, though some have proposed lowering the threshold to 2.5%.16,17
Universal tumor testing
Because of the limitations of relying on clinical family history, such as with the Amsterdam criteria and the Bethesda guidelines,18,19 as of 2014 the NCCN recommended universal tumor screening for DNA MMR deficiency associated with LS. This approach, also known as “universal testing,” has been shown to be cost effective and more sensitive in identifying at-risk patients than clinical criteria alone.20,21 Specifically, the NCCN recommends that tumor specimens of all patients diagnosed with CRC undergo testing for microsatellite instability (MSI) or loss of MMR proteins (MLH1, MSH2, MSH6, PMS2) expression by immunohistochemistry (IHC).5 Loss of MMR proteins or MSI-high findings should prompt a referral to genetics for counseling and consideration of testing for germline mutations. Universal testing of CRC and endometrial cancers is considered the most reliable way to screen patients for LS.

Universal testing by MSI or IHC may be performed on premalignant or malignant lesions. However, it is important to recognize that DNA MMR deficiency testing may not be as reliable when applied to colorectal polyps. Using data from three cancer registries (Dana-Farber Cancer Institute, University of Michigan, MD Anderson Cancer Center), Yurgelun and colleagues investigated the yield of MSI and IHC in colorectal polyps removed from patients with known LS.22 Overall, high-level MSI was found in only 41% of Lynch-associated adenomas and loss of MMR protein expression was evident in only 50%. While adenomas 8 mm in size or greater were more likely to have MSI-high or loss of MMR protein expression compared with those less than 8 mm in size, MMR-deficiency phenotype was less reliable in smaller adenomas. Consequently, results of MSI and/or IHC should therefore be interpreted with caution and in the context of the specimen upon which they are performed.
Considerations for clinical genetic testing
Genetic testing for cancer susceptibility should include informed consent and counseling for patients regarding potential risks and benefits. Clinicians ordering genetic testing should have the expertise necessary to interpret test results, which may be positive (pathogenic or likely pathogenic germline variant identified), or negative (no variants identified), or may yield one or more variants of uncertain clinical significance. Individuals found to carry a pathogenic or likely pathogenic germline variant associated with cancer susceptibility should be referred for additional genetic counseling and may require additional expert consultation for management of extracolonic cancer risks. It is important that individuals diagnosed with a hereditary cancer syndrome be informed that this diagnosis has implications for family members, who may also be at risk for the condition and may benefit from genetic testing.
Practical considerations
Given the difficulty in obtaining a detailed family history while in clinic or in endoscopy, several studies have investigated strategies that may be integrated into practice to identify high-risk patients without substantial burden on providers or patients. Kastrinos and colleagues identified the following three high-yield questions as part of a CRC Risk Assessment Tool that can be used while performing a precolonoscopy assessment: 1) Do you have a first-degree relative with CRC or LS-related cancer diagnosed before age 50?; 2) Have you had CRC or polyps diagnosed prior to age 50?; and 3) Do you have three or more relatives with CRC? The authors found that these three questions alone identified 77% of high-risk individuals.23 In addition, implementation of family history screening instruments using standardized surveys or self-administered risk prediction models at the time of colonoscopy have been shown to improve ascertainment of high-risk patients.24,25 Such strategies may become increasingly easier to implement with integration into patients’ electronic medical records.
Conclusions
Hereditary CRC syndromes are becoming increasingly important to identify, especially in an era where we are seeing rising rates of early-onset CRC. Early identification of high-risk features (Table 2) can lead to timely diagnosis with the goal to implement preventive strategies for screening and/or surveillance, ideally prior to development of cancers.

As gastroenterologists, we have several unique opportunities to identify these individuals and must maintain a high level of suspicion with careful attention when obtaining personal and family history details in clinic and in endoscopy.
Dr. Maratt is assistant professor, Indiana University, Richard L. Roudebush VA Medical Center, Indianapolis. Dr. Stoffel is assistant professor, University of Michigan; director of Cancer Genetic Clinic, Rogel Cancer Center, Ann Arbor. They have no conflicts of interest.
References
1. Pearlman R et al. JAMA Oncol. 2017;3(4):464-71.
2. Stoffel EM et al. Gastroenterology. 2018;154(4):897-905.
3. Kanth P et al. Am J Gastroenterol. 2017;112:1509-25.
4. Brennan B et al. Ther Adv Gastroenterol. 2017;10:361-71.
5. National Comprehensive Cancer Network. Available at: nccn.org.
6. Lynch HT et al. Nat Rev Cancer. 2015;15:181-94.
7. Syngal S et al. Am J Gastroenterol. 2015;110:223-62.
8. Mankaney G et al. Clin Gastroenterol Hepatol. 2020:(in press)
9. Yan HHN et al. Gut 2017;66:1645-56.
10. Ma H et al. Pathology. 2018;50:49-59.
11. Vasen H et al. Gastroenterology 1999;116:1453-6.
12. Umar A et al. J Natl Cancer Inst. 2004;96:261-8.
13. Kastrinos F et al. J Natl Cancer Inst. 2015;108(2):1-9.
14. Chen S et al. JAMA. 2006;296(12):1479-87.
15. Barnetson RA et al. N Engl J Med. 2006;354(26):2751-63.
16. Kastrinos F et al. J Clin Oncol. 2017;35:2165-72.
17. Kastrinos F et al. Fam Cancer. 2018;17:567-67.
18. Cohen SA et al. Annu Rev Genomics Hum Genet. 2019;20:293-307.
19. Matloff J et al. J Natl Compr Canc Netw. 2013;11:1380-5.
20. Ladabaum U et al. Ann Intern Med. 2011;155(2):69-79.
21. Hampel H et al. N Engl J Med. 2005;352(18):1851-60.
22. Yurgelun MB et al. Cancer Prev Res. 2012;5:574-82.
23. Kastrinos F et al. Am J Gastroenterol. 2009;104:1508-18.
24. Luba DG et al. Clin Gastroenterol Hepatol. 2018;16:49-58.
25. Guivatchian T et al. Gastrointest Endosc. 2017;86:684-91.


