User login
What will the operating room of the future look like? Will it be a specialty-specific facility or a generic OR? Our Symposium panelists, each a primary investigator in an “OR of the Future” project at his own institution, share their ideas of what the future holds, from getting clutter off the floor and onto the ceiling, to integrating patient information, imaging, and robotic systems.
Panelists also share their visions of what the OR may look like in 25 years and what obstacles need to be overcome to make the futuristic vision a reality. Robotics, smart displays, information management and integration, and image-guided surgery may become as common as the stapler and the scalpel.
Dr. Rattner
The making of the “OR of the Future”
PARK: It’s been said that the operating room of the future means that we take the clutter off the floor and put it on the ceiling, but we need to have a much broader view.
“OR of the Future” embodies a vision of improved patient safety and outcomes—from evaluating and assimilating existing technologies, to identifying technologies that need to be developed or brought in, to integrating the technologies that will get us there.
SANDBERG: The “OR of the Future” is also about therapeutic effectiveness for our patients and organizational effectiveness for the hospital.
The OR of the future is a concept through which a big organization such as an academic hospital can foster innovative projects. It can designate a place within its own organization that’s politically, financially, and physically separate from the main workflow and objectives, where the hospital can test processes and technologies.
“Wall of Knowledge”
A matrix of real-time screens
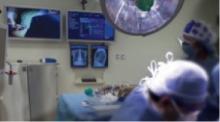
This operating room at Memorial Sloan-Kettering Cancer Center is one of 21 that employ the “Wall of Knowledge,” the matrix of screens in the background that show patient and OR data, high-resolution video of surgical and laboratory images, and two real-time monitors for viewing radiographs.
“Dashboard” data monitoring
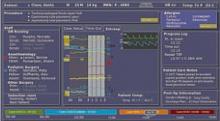
One component of the “Wall of Knowledge” is the OR-Dashboard (LiveData, Inc, Cambridge, MA) that captures data from multiple information systems, physiological monitors, and medical devices and displays it to the OR team.
PHOTOGRAPH BY RICHARD DEWITT, COURTESY MEMORIAL SLOAN-KETTERING CANCER CENTER, COURTESY LIVEDATA, INCThe goal is to develop safer systems, evaluate technology, and accumulate evidence to prove these technological innovations are effective. This also involves developing new processes for information management and patient flow.
Will Gyns share their OR?
SATAVA: I think of two kinds of OR of the future: The near-term, in the next 20 years or so, will concentrate an information-based system, such as imaging, robots, computers, and just-in-time inventory.
Shared, yet specialty-specific OR
We will continue to have multiple ORs in the near future because we will still need specialization. Neurosurgeons, gynecologists, and pediatric surgeons will still find it difficult to share the same OR—no matter how flexible it is. What these multi-specialty ORs will have in common is the way they’ll leverage information technologies.
To the cell level and beyond
The second kind of OR of the future is longer term and harder to describe. Here, biotechnology and energy-directed systems will replace mechanical and, to an extent, information-based systems. For example, in 30–50 years we’ll be using lasers for intracellular operations. Rather than physicians injecting various drugs or immunochemistry, they will actually use lasers and optical tweezers and manipulate the various organs—the mitochondria, operate on the DNA directly, and change cell biology rather than remove organs.
Will your office be your OR?
SATAVA: A capital-intensive facility such as a hospital or dedicated outpatient surgery center for complicated procedures will always have its place, although we’ve seen more minimally invasive surgery move out of the hospital. Beyond minimally invasive procedures are noninvasive procedures. High-intensity focused ultrasound and terahertz energy are the types of technologies that can safely move out of an operating room.
And a CT is not an imaging device, but an information system with eyes. Robotic machines and imaging devices can be integrated into “information space” in ways that are elusive in real space. “Information space” sees no difference between a process and an object.
Our product is our patient
Health care is the only industry that does not have an information representation of its product. Our product is our patient. We cannot use the hardware and software that all other industries use for virtual prototyping and testing.
Health care does not understand systems integration. We’ve spent a century dissecting the human into 26 different National Institutes of Health that don’t talk to each other.
We can reintegrate the human at the workstation and surgeon, where supply chain management, just-in-time inventory, robotics, and imaging for surgical rehearsal can come together.
Pre-op, literally
The robot can capture a surgeon’s hand motions and play them back with 10–12 times the accuracy at 7–15 times the speed. In the long-term, the surgeon would get the image of the patient in the OR just before the operation, perform the motions of the operation, and edit out the mistakes until the procedure is perfect.
When the surgeon is finished, he or she sends the edited, perfected operation to the robot, which performs it accurately and with greater speed. Appendectomies and cholecystectomies would take 10 minutes; Whipple procedures 45 minutes.
Richard M. Satava, MD
Big hospitals believe in generic ORs. Do we have scientific proof of what is best?
SANDBERG: OR design is not a science. No peer-reviewed literature in OR architecture exists. Few if any studies have actually tested the effectiveness of a given OR design.
The OR is a gigantic medical device that encloses the patient and staff. It ought to be studied in a rigorous and, ideally, prospective way, although such studies would be very expensive.
SATAVA: Moving toward noninvasive surgery doesn’t mean that open and minimally invasive surgery are going away. However, I don’t see anything in the near term that will make computed tomography (CT), magnetic resonance imaging (MRI), and new terahertz technology small enough to share space in one room.
On the other hand, robotics are moving us toward an OR with no staff. Put the patient in, and the surgeon on the outside will operate robotically. We could see that in the next 4–5 years. Biosurgery may create a need for quarantine or isolation. We’ll still have these multiple ORs in the future.
PARK: Look at the last 15 years and how long it has taken certain minimally invasive techniques to become mainstream. Only now are we getting the OR suites to accommodate them.
It’s a challenge that such little scholarly study has been undertaken. We need to push industry and hospital administrators to bring modularity to the ORs now on the drawing board. It’s ambitious, but it’s a way to start.
What outcomes matter the most?
SANDBERG: The commonly discussed outcome variables are procedure time, and hospital cost and revenue—but they’re not necessarily the right metrics.
What we’re looking for is a metric that captures the patient benefits and risks. Then we need to determine if the surgeons, the nurses, the anesthesiologists, the technologists, and the informationists can use this near-term OR of the future effectively for the patient’s benefit.
PARK: Designing studies is one of the real challenges in trying to generate data in this field. These studies tend to start out looking at global principles but end up fixating on operational issues—for example, how to get a pair of sharp scissors to an OR on a regular basis.
This is going to bring new metrics to the operating room—concepts such as process mapping, and workflow and workspace assessments.
Robotics gives us the chance to rehearse an operation. How practical are rehearsals?
No warm-ups for surgeons
SATAVA: I don’t know of any other profession that immediately starts to work without warming up or practicing.
I’ve never seen a baseball player run from the dugout to the batter’s box.
I’ve never seen a symphony orchestra run right out and start playing.
Why don’t we do a little rehearsing and practice the hardest parts before we actually start the incision?
Still, this won’t become cost-effective in the near term.
“Cognitive” rehearsals
PARK: You can rehearse selectively, based on the difficulty or complexity. We rehearse complex anti-reflux procedures and donor nephrectomies. Software models the patient’s data, and I will do a “fly-through” the afternoon before a laparoscopic donor nephrectomy.
Rehearsal at this stage has more to do with the cognitive processes than technical. So whether I need to practice taking that fat off the renal hilum is less of an issue than the position of lumbar veins or the relation of the renal vein to the aorta.
I feel immensely better prepared for the surgery when I’ve done that fly-through. I’m hard pressed to give you a good metric, to show the science to support it, but I believe there’s a role for it.
SANDBERG: This gets back to the question of metrics. Much of what we’re seeing as we look at ORs of the future actually improves the work experience for the surgeon, anesthesiologist, and nurses.
You can’t put a dollar value on that unless you’re talking about the cost of recruiting and training staff, but it does seem to improve outcomes for the people who work in the OR—and that must translate to patient outcomes. It’s difficult to justify to an administrator, but on a moral level it seems like the right thing to do.
The ideal would be a robotic anesthetist who would not be in the coffee lounge when you want to turn over your case.
“Robo-doc” anesthetist
SANDBERG: Making a business case for a robot for anesthesia is even harder. Functions such as vascular access and airway control are easy to an expert, but I don’t know yet of anyone who even attempted to make an IV-starting robot.
SATAVA: We have one.
SANDBERG: I’ve seen your prototypes. I don’t think you can program your “Robo-doc” to anesthetize the patient when you want, but the maintenance phase is one area in anesthesia ripe for automation.
Anesthesiology is actually in the driver’s seat for harvesting, controlling, and integrating information in the OR. Our project integrates data that anesthesiologists traditionally managed with that from surgical instruments and hospital information systems to create a total patient picture. That includes interventions and perturbations caused by surgery. Once that’s in place, you could imagine actually driving the anesthetic using closed-loop control.
SATAVA: Anesthesia is probably going to evolve more than surgery thanks to safer anesthetics and new nanotechnology. The new anesthetic autoregulators will make it almost impossible to overdose or under-dose a patient.
Are “smart” instruments equal to our own fingertips?
PARK: Today we can make a standard (open surgery) incision into which we insert one or both hands and have more than 20° of movement with the benefit of an innervated end effecter—our fingertips.
The OR of the future will put us in a situation where we’re disconnected in the tactile sense from the target anatomy and limit us to 4°–8° of movement. The stimulus is to get those degrees of movement back to compensate for the tactile feedback we’ve lost.
Hand-assisted laparoscopy is a lowtech way for getting around that loss of movement. It’s nothing other than a transition step, but it’s a clever solution.
How did you discover “parallel processing” to save costs and time?
SANDBERG: We tried to deconstruct the perioperative process immediately upstream and downstream of the OR itself, then we tested the time savings and cost effectiveness of the new process.1
ORs in America tend to do things sequentially via serial-processing work-flow: nurses and technologists set up the OR, the anesthesiologist brings in the patient and induces anesthetic, the surgery happens, the patient emerges from anesthesia, the anesthesiologist takes the patient to recovery.
We discovered that in any of these linear processes a team will be waiting for another team member to finish a task. That presented an opportunity to increase the effectiveness of the OR by finding a way for those team members to work in parallel rather than in sequence.
At Massachusetts General, we reduced the nonoperative time from when the surgeon steps back from the patient until the surgeon steps up to prep the next patient, from roughly 70 to 40 minutes by implementing a couple of parallel processing interventions. Other reports have verified this approach.2,3
In the MGH project, however, the interface between that particular OR and the hospital became problematic when the OR sent patients to the floor faster than the hospital could accept them.
What happened when the conveyor belt speeded up?
This gets us to the notion of perioperative systems design, which is basically a fantastic accident at most hospitals. In our OR of the Future project at MGH, we isolated part of the perioperative process, optimized it, and in so doing we broke the recovery room and upstream admission process. It was like transplanting an elephant heart into a mouse.
What is “plug-and-play”?
SATAVA: Plug-and-play would standardize interfacing between similar components that are interchangeable. Let’s say we have 6 different EKG machines, each with a different method for plugging in the leads. Our concept of plug-and-play goes from software that talks between devices to interchangeable tubing and electrical connections to the devices themselves. This standard is necessary to achieve even the most rudimentary information-based OR of the future.
Lack of plug-and-play connectivity has made it difficult even to get a patient’s allergy information from one database to another. At our institution, a couple software engineers took about 3 months to accomplish that. If this is what it takes to integrate 1 piece of information at 1 institution, we don’t have a chance.
Where do things stand with radiofrequency identification tagging?
PARK: Two types of RFID tags exist: A passive tag is a simple tag that when interrogated by a local node or cell just says it’s there. An active tag imparts a lot more information.
SANDBERG: An RFID tag would contain a patient’s critical medical data, but this vision has many problems at the moment. A tag with sufficient data storage is very expensive.
Security is another problem. The information is transmitted wirelessly and the encryption consumes tag memory. Passive RFID tags, which don’t require a battery and cost less to produce, simply can’t store enough information to be useful as a repository of medical data.
So we’ve discovered applications for RFID beyond the mobile patient record on the tag wristband. RFID can, at least in theory, be used for inventory management. Again, the problems with its costs and the need to scan the items limits its usefulness. Hospitals can use active RFID tags now for tracking high-value assets—not only expensive medical equipment but also personnel and patients.
PARK: We’ve been evaluating many different location RFID technologies in the operating room. We’ve found that the resolution of these technologies is miles from the manufacturers’ claims.
SATAVA: The trade-off for security is the inverse relationship between the level of security and the amount of computing power it demands, along with the amount of physical space available on the device.
Maybe in two years the computing power will increase and device size decrease, so we can get more on a smaller object, and its costs will decrease.
SANDBERG: Another problem with passive RFID is a high out-of-box failure rate. The antennae break off in manufacturing and shipping.
Besides cost, spatial resolution is another problem with active tags. On the other hand, passive RFID tags can have sub-centimeter resolution because the reader has to be so close to read the tag.
However, scanning the tag is another problem. Most of us in our workflows don’t have time to grab an RFID reader. Passive RFID reader technology is far from ideal for a busy OR.
Ideally, they should require no effort: objects brought into the OR would register on an as-yet-uninvented meter and localize in the room, ideally at 1 cm resolution or better.
What devices will be the stars of the OR of the Future?
LED Technology
SATAVA: With LED—light-emitting diode—technology and the small cameras now in cell phones, we have the opportunity to get rid of all the lights in the operating room.
The University of Barcelona has accomplished this.4 A sea of 100 to 150 cameras is placed around the room. You don’t have to move the cameras or lights. This clears up all the booms that are hanging down of these enormously inefficient lights that we have today.
This technology can leverage information systems that talk to objects and track instruments.
Wireless, miniaturized robots
PARK: Miniaturized robots, designed to be wireless in the near future, can actually rove the peritoneal cavity equipped with an end effecter or mobile camera. Dropped in through a 5- or 10-mm port, these devices can get to the target anatomy. Mechanical miniaturization is another way of getting around the loss of degrees of movement.
Better visualization with HD video
The minimally invasive revolution has moved us from having direct binocular visual contact with the target anatomy to working in a 3-dimensional space off a 2-dimensional image.
The next step is to take the vast amount of data we’re capturing, especially with HD video, from the operative field and use it to improve surgical visualization and ultimately outcomes.
This may involve real-time modeling and altering the visual perspective of the operative field as it is presented to the surgeon. We’re looking at new ways of making the data more intuitive.
Beyond the visible spectrum
SATAVA: There’s another dimension: Why are we only using cameras that see in the visible spectrum? Why aren’t we using infrared and ultraviolet cameras as other industries do?
We may not need the sense of touch because an infrared camera can show us all the blood vessels by temperature change. Health care hasn’t even looked at off-the-shelf technologies that other industries have used for 25 years.
PARK: Narrow-band imaging and confocal microscopy are now available, but we haven’t even started to look at how we could apply them intra-abdominally.
Will biotechnology bring down the curtain on surgeons?
SATAVA: We haven’t talked about the extraordinary revolution in biotechnology and how it’s either going to change the way a surgeon works or puts the surgeon out of business.
We may not have to do surgery if we can manipulate tissue on a molecular level. That’s 20–25 years out. It’s going to take that long for nanotechnology to develop usable products for health care.
The human element
RATTNER: We didn’t dwell on the human factor. The functioning of a highly skilled team and the interaction among team members is fertile ground for further research. Greater attention paid to team training and simulation crisis management will pay off in patient safety.
At the end of the day, the priority is that if we’re going to operate faster and more efficiently with more complex procedures, we have to get to Six Sigma or better safety levels.
Disclosures
Dr Park disclosed an affiliation with Stryker Endoscopy. Dr Sandberg disclosed affiliations with LiveData, Inc, Cambridge, MA; and Radianse, Inc, Lawrence, MA. Dr Satava disclosed an affiliation with Medical Education Technologies, Inc, Sarasota, FL. Dr Rattner had no affiliations to disclose.
1. Sandberg WS, Daily B, Egan M, et al. Deliberate perioperative systems design improves operating room throughput. Anesthesiology. 2005;103:406-418.
2. Hanss R, Buttgereit B, Tonner PH, et al. Overlapping induction of anesthesia: an analysis of benefits and costs. Anesthesiology. 2005;103:391-400.
3. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvela OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103:401-405.
4. LaPorte EN. Array of ceiling mounted LED lights for the OR of the Future. Paper presented at: 12th European Association of Endoscopic Surgeons (EAES) Congress; June 9-12, 2004, Barcelona, Spain.
What will the operating room of the future look like? Will it be a specialty-specific facility or a generic OR? Our Symposium panelists, each a primary investigator in an “OR of the Future” project at his own institution, share their ideas of what the future holds, from getting clutter off the floor and onto the ceiling, to integrating patient information, imaging, and robotic systems.
Panelists also share their visions of what the OR may look like in 25 years and what obstacles need to be overcome to make the futuristic vision a reality. Robotics, smart displays, information management and integration, and image-guided surgery may become as common as the stapler and the scalpel.
Dr. Rattner
The making of the “OR of the Future”
PARK: It’s been said that the operating room of the future means that we take the clutter off the floor and put it on the ceiling, but we need to have a much broader view.
“OR of the Future” embodies a vision of improved patient safety and outcomes—from evaluating and assimilating existing technologies, to identifying technologies that need to be developed or brought in, to integrating the technologies that will get us there.
SANDBERG: The “OR of the Future” is also about therapeutic effectiveness for our patients and organizational effectiveness for the hospital.
The OR of the future is a concept through which a big organization such as an academic hospital can foster innovative projects. It can designate a place within its own organization that’s politically, financially, and physically separate from the main workflow and objectives, where the hospital can test processes and technologies.
“Wall of Knowledge”
A matrix of real-time screens
This operating room at Memorial Sloan-Kettering Cancer Center is one of 21 that employ the “Wall of Knowledge,” the matrix of screens in the background that show patient and OR data, high-resolution video of surgical and laboratory images, and two real-time monitors for viewing radiographs.
“Dashboard” data monitoring
One component of the “Wall of Knowledge” is the OR-Dashboard (LiveData, Inc, Cambridge, MA) that captures data from multiple information systems, physiological monitors, and medical devices and displays it to the OR team.
PHOTOGRAPH BY RICHARD DEWITT, COURTESY MEMORIAL SLOAN-KETTERING CANCER CENTER, COURTESY LIVEDATA, INCThe goal is to develop safer systems, evaluate technology, and accumulate evidence to prove these technological innovations are effective. This also involves developing new processes for information management and patient flow.
Will Gyns share their OR?
SATAVA: I think of two kinds of OR of the future: The near-term, in the next 20 years or so, will concentrate an information-based system, such as imaging, robots, computers, and just-in-time inventory.
Shared, yet specialty-specific OR
We will continue to have multiple ORs in the near future because we will still need specialization. Neurosurgeons, gynecologists, and pediatric surgeons will still find it difficult to share the same OR—no matter how flexible it is. What these multi-specialty ORs will have in common is the way they’ll leverage information technologies.
To the cell level and beyond
The second kind of OR of the future is longer term and harder to describe. Here, biotechnology and energy-directed systems will replace mechanical and, to an extent, information-based systems. For example, in 30–50 years we’ll be using lasers for intracellular operations. Rather than physicians injecting various drugs or immunochemistry, they will actually use lasers and optical tweezers and manipulate the various organs—the mitochondria, operate on the DNA directly, and change cell biology rather than remove organs.
Will your office be your OR?
SATAVA: A capital-intensive facility such as a hospital or dedicated outpatient surgery center for complicated procedures will always have its place, although we’ve seen more minimally invasive surgery move out of the hospital. Beyond minimally invasive procedures are noninvasive procedures. High-intensity focused ultrasound and terahertz energy are the types of technologies that can safely move out of an operating room.
And a CT is not an imaging device, but an information system with eyes. Robotic machines and imaging devices can be integrated into “information space” in ways that are elusive in real space. “Information space” sees no difference between a process and an object.
Our product is our patient
Health care is the only industry that does not have an information representation of its product. Our product is our patient. We cannot use the hardware and software that all other industries use for virtual prototyping and testing.
Health care does not understand systems integration. We’ve spent a century dissecting the human into 26 different National Institutes of Health that don’t talk to each other.
We can reintegrate the human at the workstation and surgeon, where supply chain management, just-in-time inventory, robotics, and imaging for surgical rehearsal can come together.
Pre-op, literally
The robot can capture a surgeon’s hand motions and play them back with 10–12 times the accuracy at 7–15 times the speed. In the long-term, the surgeon would get the image of the patient in the OR just before the operation, perform the motions of the operation, and edit out the mistakes until the procedure is perfect.
When the surgeon is finished, he or she sends the edited, perfected operation to the robot, which performs it accurately and with greater speed. Appendectomies and cholecystectomies would take 10 minutes; Whipple procedures 45 minutes.
Richard M. Satava, MD
Big hospitals believe in generic ORs. Do we have scientific proof of what is best?
SANDBERG: OR design is not a science. No peer-reviewed literature in OR architecture exists. Few if any studies have actually tested the effectiveness of a given OR design.
The OR is a gigantic medical device that encloses the patient and staff. It ought to be studied in a rigorous and, ideally, prospective way, although such studies would be very expensive.
SATAVA: Moving toward noninvasive surgery doesn’t mean that open and minimally invasive surgery are going away. However, I don’t see anything in the near term that will make computed tomography (CT), magnetic resonance imaging (MRI), and new terahertz technology small enough to share space in one room.
On the other hand, robotics are moving us toward an OR with no staff. Put the patient in, and the surgeon on the outside will operate robotically. We could see that in the next 4–5 years. Biosurgery may create a need for quarantine or isolation. We’ll still have these multiple ORs in the future.
PARK: Look at the last 15 years and how long it has taken certain minimally invasive techniques to become mainstream. Only now are we getting the OR suites to accommodate them.
It’s a challenge that such little scholarly study has been undertaken. We need to push industry and hospital administrators to bring modularity to the ORs now on the drawing board. It’s ambitious, but it’s a way to start.
What outcomes matter the most?
SANDBERG: The commonly discussed outcome variables are procedure time, and hospital cost and revenue—but they’re not necessarily the right metrics.
What we’re looking for is a metric that captures the patient benefits and risks. Then we need to determine if the surgeons, the nurses, the anesthesiologists, the technologists, and the informationists can use this near-term OR of the future effectively for the patient’s benefit.
PARK: Designing studies is one of the real challenges in trying to generate data in this field. These studies tend to start out looking at global principles but end up fixating on operational issues—for example, how to get a pair of sharp scissors to an OR on a regular basis.
This is going to bring new metrics to the operating room—concepts such as process mapping, and workflow and workspace assessments.
Robotics gives us the chance to rehearse an operation. How practical are rehearsals?
No warm-ups for surgeons
SATAVA: I don’t know of any other profession that immediately starts to work without warming up or practicing.
I’ve never seen a baseball player run from the dugout to the batter’s box.
I’ve never seen a symphony orchestra run right out and start playing.
Why don’t we do a little rehearsing and practice the hardest parts before we actually start the incision?
Still, this won’t become cost-effective in the near term.
“Cognitive” rehearsals
PARK: You can rehearse selectively, based on the difficulty or complexity. We rehearse complex anti-reflux procedures and donor nephrectomies. Software models the patient’s data, and I will do a “fly-through” the afternoon before a laparoscopic donor nephrectomy.
Rehearsal at this stage has more to do with the cognitive processes than technical. So whether I need to practice taking that fat off the renal hilum is less of an issue than the position of lumbar veins or the relation of the renal vein to the aorta.
I feel immensely better prepared for the surgery when I’ve done that fly-through. I’m hard pressed to give you a good metric, to show the science to support it, but I believe there’s a role for it.
SANDBERG: This gets back to the question of metrics. Much of what we’re seeing as we look at ORs of the future actually improves the work experience for the surgeon, anesthesiologist, and nurses.
You can’t put a dollar value on that unless you’re talking about the cost of recruiting and training staff, but it does seem to improve outcomes for the people who work in the OR—and that must translate to patient outcomes. It’s difficult to justify to an administrator, but on a moral level it seems like the right thing to do.
The ideal would be a robotic anesthetist who would not be in the coffee lounge when you want to turn over your case.
“Robo-doc” anesthetist
SANDBERG: Making a business case for a robot for anesthesia is even harder. Functions such as vascular access and airway control are easy to an expert, but I don’t know yet of anyone who even attempted to make an IV-starting robot.
SATAVA: We have one.
SANDBERG: I’ve seen your prototypes. I don’t think you can program your “Robo-doc” to anesthetize the patient when you want, but the maintenance phase is one area in anesthesia ripe for automation.
Anesthesiology is actually in the driver’s seat for harvesting, controlling, and integrating information in the OR. Our project integrates data that anesthesiologists traditionally managed with that from surgical instruments and hospital information systems to create a total patient picture. That includes interventions and perturbations caused by surgery. Once that’s in place, you could imagine actually driving the anesthetic using closed-loop control.
SATAVA: Anesthesia is probably going to evolve more than surgery thanks to safer anesthetics and new nanotechnology. The new anesthetic autoregulators will make it almost impossible to overdose or under-dose a patient.
Are “smart” instruments equal to our own fingertips?
PARK: Today we can make a standard (open surgery) incision into which we insert one or both hands and have more than 20° of movement with the benefit of an innervated end effecter—our fingertips.
The OR of the future will put us in a situation where we’re disconnected in the tactile sense from the target anatomy and limit us to 4°–8° of movement. The stimulus is to get those degrees of movement back to compensate for the tactile feedback we’ve lost.
Hand-assisted laparoscopy is a lowtech way for getting around that loss of movement. It’s nothing other than a transition step, but it’s a clever solution.
How did you discover “parallel processing” to save costs and time?
SANDBERG: We tried to deconstruct the perioperative process immediately upstream and downstream of the OR itself, then we tested the time savings and cost effectiveness of the new process.1
ORs in America tend to do things sequentially via serial-processing work-flow: nurses and technologists set up the OR, the anesthesiologist brings in the patient and induces anesthetic, the surgery happens, the patient emerges from anesthesia, the anesthesiologist takes the patient to recovery.
We discovered that in any of these linear processes a team will be waiting for another team member to finish a task. That presented an opportunity to increase the effectiveness of the OR by finding a way for those team members to work in parallel rather than in sequence.
At Massachusetts General, we reduced the nonoperative time from when the surgeon steps back from the patient until the surgeon steps up to prep the next patient, from roughly 70 to 40 minutes by implementing a couple of parallel processing interventions. Other reports have verified this approach.2,3
In the MGH project, however, the interface between that particular OR and the hospital became problematic when the OR sent patients to the floor faster than the hospital could accept them.
What happened when the conveyor belt speeded up?
This gets us to the notion of perioperative systems design, which is basically a fantastic accident at most hospitals. In our OR of the Future project at MGH, we isolated part of the perioperative process, optimized it, and in so doing we broke the recovery room and upstream admission process. It was like transplanting an elephant heart into a mouse.
What is “plug-and-play”?
SATAVA: Plug-and-play would standardize interfacing between similar components that are interchangeable. Let’s say we have 6 different EKG machines, each with a different method for plugging in the leads. Our concept of plug-and-play goes from software that talks between devices to interchangeable tubing and electrical connections to the devices themselves. This standard is necessary to achieve even the most rudimentary information-based OR of the future.
Lack of plug-and-play connectivity has made it difficult even to get a patient’s allergy information from one database to another. At our institution, a couple software engineers took about 3 months to accomplish that. If this is what it takes to integrate 1 piece of information at 1 institution, we don’t have a chance.
Where do things stand with radiofrequency identification tagging?
PARK: Two types of RFID tags exist: A passive tag is a simple tag that when interrogated by a local node or cell just says it’s there. An active tag imparts a lot more information.
SANDBERG: An RFID tag would contain a patient’s critical medical data, but this vision has many problems at the moment. A tag with sufficient data storage is very expensive.
Security is another problem. The information is transmitted wirelessly and the encryption consumes tag memory. Passive RFID tags, which don’t require a battery and cost less to produce, simply can’t store enough information to be useful as a repository of medical data.
So we’ve discovered applications for RFID beyond the mobile patient record on the tag wristband. RFID can, at least in theory, be used for inventory management. Again, the problems with its costs and the need to scan the items limits its usefulness. Hospitals can use active RFID tags now for tracking high-value assets—not only expensive medical equipment but also personnel and patients.
PARK: We’ve been evaluating many different location RFID technologies in the operating room. We’ve found that the resolution of these technologies is miles from the manufacturers’ claims.
SATAVA: The trade-off for security is the inverse relationship between the level of security and the amount of computing power it demands, along with the amount of physical space available on the device.
Maybe in two years the computing power will increase and device size decrease, so we can get more on a smaller object, and its costs will decrease.
SANDBERG: Another problem with passive RFID is a high out-of-box failure rate. The antennae break off in manufacturing and shipping.
Besides cost, spatial resolution is another problem with active tags. On the other hand, passive RFID tags can have sub-centimeter resolution because the reader has to be so close to read the tag.
However, scanning the tag is another problem. Most of us in our workflows don’t have time to grab an RFID reader. Passive RFID reader technology is far from ideal for a busy OR.
Ideally, they should require no effort: objects brought into the OR would register on an as-yet-uninvented meter and localize in the room, ideally at 1 cm resolution or better.
What devices will be the stars of the OR of the Future?
LED Technology
SATAVA: With LED—light-emitting diode—technology and the small cameras now in cell phones, we have the opportunity to get rid of all the lights in the operating room.
The University of Barcelona has accomplished this.4 A sea of 100 to 150 cameras is placed around the room. You don’t have to move the cameras or lights. This clears up all the booms that are hanging down of these enormously inefficient lights that we have today.
This technology can leverage information systems that talk to objects and track instruments.
Wireless, miniaturized robots
PARK: Miniaturized robots, designed to be wireless in the near future, can actually rove the peritoneal cavity equipped with an end effecter or mobile camera. Dropped in through a 5- or 10-mm port, these devices can get to the target anatomy. Mechanical miniaturization is another way of getting around the loss of degrees of movement.
Better visualization with HD video
The minimally invasive revolution has moved us from having direct binocular visual contact with the target anatomy to working in a 3-dimensional space off a 2-dimensional image.
The next step is to take the vast amount of data we’re capturing, especially with HD video, from the operative field and use it to improve surgical visualization and ultimately outcomes.
This may involve real-time modeling and altering the visual perspective of the operative field as it is presented to the surgeon. We’re looking at new ways of making the data more intuitive.
Beyond the visible spectrum
SATAVA: There’s another dimension: Why are we only using cameras that see in the visible spectrum? Why aren’t we using infrared and ultraviolet cameras as other industries do?
We may not need the sense of touch because an infrared camera can show us all the blood vessels by temperature change. Health care hasn’t even looked at off-the-shelf technologies that other industries have used for 25 years.
PARK: Narrow-band imaging and confocal microscopy are now available, but we haven’t even started to look at how we could apply them intra-abdominally.
Will biotechnology bring down the curtain on surgeons?
SATAVA: We haven’t talked about the extraordinary revolution in biotechnology and how it’s either going to change the way a surgeon works or puts the surgeon out of business.
We may not have to do surgery if we can manipulate tissue on a molecular level. That’s 20–25 years out. It’s going to take that long for nanotechnology to develop usable products for health care.
The human element
RATTNER: We didn’t dwell on the human factor. The functioning of a highly skilled team and the interaction among team members is fertile ground for further research. Greater attention paid to team training and simulation crisis management will pay off in patient safety.
At the end of the day, the priority is that if we’re going to operate faster and more efficiently with more complex procedures, we have to get to Six Sigma or better safety levels.
Disclosures
Dr Park disclosed an affiliation with Stryker Endoscopy. Dr Sandberg disclosed affiliations with LiveData, Inc, Cambridge, MA; and Radianse, Inc, Lawrence, MA. Dr Satava disclosed an affiliation with Medical Education Technologies, Inc, Sarasota, FL. Dr Rattner had no affiliations to disclose.
What will the operating room of the future look like? Will it be a specialty-specific facility or a generic OR? Our Symposium panelists, each a primary investigator in an “OR of the Future” project at his own institution, share their ideas of what the future holds, from getting clutter off the floor and onto the ceiling, to integrating patient information, imaging, and robotic systems.
Panelists also share their visions of what the OR may look like in 25 years and what obstacles need to be overcome to make the futuristic vision a reality. Robotics, smart displays, information management and integration, and image-guided surgery may become as common as the stapler and the scalpel.
Dr. Rattner
The making of the “OR of the Future”
PARK: It’s been said that the operating room of the future means that we take the clutter off the floor and put it on the ceiling, but we need to have a much broader view.
“OR of the Future” embodies a vision of improved patient safety and outcomes—from evaluating and assimilating existing technologies, to identifying technologies that need to be developed or brought in, to integrating the technologies that will get us there.
SANDBERG: The “OR of the Future” is also about therapeutic effectiveness for our patients and organizational effectiveness for the hospital.
The OR of the future is a concept through which a big organization such as an academic hospital can foster innovative projects. It can designate a place within its own organization that’s politically, financially, and physically separate from the main workflow and objectives, where the hospital can test processes and technologies.
“Wall of Knowledge”
A matrix of real-time screens
This operating room at Memorial Sloan-Kettering Cancer Center is one of 21 that employ the “Wall of Knowledge,” the matrix of screens in the background that show patient and OR data, high-resolution video of surgical and laboratory images, and two real-time monitors for viewing radiographs.
“Dashboard” data monitoring
One component of the “Wall of Knowledge” is the OR-Dashboard (LiveData, Inc, Cambridge, MA) that captures data from multiple information systems, physiological monitors, and medical devices and displays it to the OR team.
PHOTOGRAPH BY RICHARD DEWITT, COURTESY MEMORIAL SLOAN-KETTERING CANCER CENTER, COURTESY LIVEDATA, INCThe goal is to develop safer systems, evaluate technology, and accumulate evidence to prove these technological innovations are effective. This also involves developing new processes for information management and patient flow.
Will Gyns share their OR?
SATAVA: I think of two kinds of OR of the future: The near-term, in the next 20 years or so, will concentrate an information-based system, such as imaging, robots, computers, and just-in-time inventory.
Shared, yet specialty-specific OR
We will continue to have multiple ORs in the near future because we will still need specialization. Neurosurgeons, gynecologists, and pediatric surgeons will still find it difficult to share the same OR—no matter how flexible it is. What these multi-specialty ORs will have in common is the way they’ll leverage information technologies.
To the cell level and beyond
The second kind of OR of the future is longer term and harder to describe. Here, biotechnology and energy-directed systems will replace mechanical and, to an extent, information-based systems. For example, in 30–50 years we’ll be using lasers for intracellular operations. Rather than physicians injecting various drugs or immunochemistry, they will actually use lasers and optical tweezers and manipulate the various organs—the mitochondria, operate on the DNA directly, and change cell biology rather than remove organs.
Will your office be your OR?
SATAVA: A capital-intensive facility such as a hospital or dedicated outpatient surgery center for complicated procedures will always have its place, although we’ve seen more minimally invasive surgery move out of the hospital. Beyond minimally invasive procedures are noninvasive procedures. High-intensity focused ultrasound and terahertz energy are the types of technologies that can safely move out of an operating room.
And a CT is not an imaging device, but an information system with eyes. Robotic machines and imaging devices can be integrated into “information space” in ways that are elusive in real space. “Information space” sees no difference between a process and an object.
Our product is our patient
Health care is the only industry that does not have an information representation of its product. Our product is our patient. We cannot use the hardware and software that all other industries use for virtual prototyping and testing.
Health care does not understand systems integration. We’ve spent a century dissecting the human into 26 different National Institutes of Health that don’t talk to each other.
We can reintegrate the human at the workstation and surgeon, where supply chain management, just-in-time inventory, robotics, and imaging for surgical rehearsal can come together.
Pre-op, literally
The robot can capture a surgeon’s hand motions and play them back with 10–12 times the accuracy at 7–15 times the speed. In the long-term, the surgeon would get the image of the patient in the OR just before the operation, perform the motions of the operation, and edit out the mistakes until the procedure is perfect.
When the surgeon is finished, he or she sends the edited, perfected operation to the robot, which performs it accurately and with greater speed. Appendectomies and cholecystectomies would take 10 minutes; Whipple procedures 45 minutes.
Richard M. Satava, MD
Big hospitals believe in generic ORs. Do we have scientific proof of what is best?
SANDBERG: OR design is not a science. No peer-reviewed literature in OR architecture exists. Few if any studies have actually tested the effectiveness of a given OR design.
The OR is a gigantic medical device that encloses the patient and staff. It ought to be studied in a rigorous and, ideally, prospective way, although such studies would be very expensive.
SATAVA: Moving toward noninvasive surgery doesn’t mean that open and minimally invasive surgery are going away. However, I don’t see anything in the near term that will make computed tomography (CT), magnetic resonance imaging (MRI), and new terahertz technology small enough to share space in one room.
On the other hand, robotics are moving us toward an OR with no staff. Put the patient in, and the surgeon on the outside will operate robotically. We could see that in the next 4–5 years. Biosurgery may create a need for quarantine or isolation. We’ll still have these multiple ORs in the future.
PARK: Look at the last 15 years and how long it has taken certain minimally invasive techniques to become mainstream. Only now are we getting the OR suites to accommodate them.
It’s a challenge that such little scholarly study has been undertaken. We need to push industry and hospital administrators to bring modularity to the ORs now on the drawing board. It’s ambitious, but it’s a way to start.
What outcomes matter the most?
SANDBERG: The commonly discussed outcome variables are procedure time, and hospital cost and revenue—but they’re not necessarily the right metrics.
What we’re looking for is a metric that captures the patient benefits and risks. Then we need to determine if the surgeons, the nurses, the anesthesiologists, the technologists, and the informationists can use this near-term OR of the future effectively for the patient’s benefit.
PARK: Designing studies is one of the real challenges in trying to generate data in this field. These studies tend to start out looking at global principles but end up fixating on operational issues—for example, how to get a pair of sharp scissors to an OR on a regular basis.
This is going to bring new metrics to the operating room—concepts such as process mapping, and workflow and workspace assessments.
Robotics gives us the chance to rehearse an operation. How practical are rehearsals?
No warm-ups for surgeons
SATAVA: I don’t know of any other profession that immediately starts to work without warming up or practicing.
I’ve never seen a baseball player run from the dugout to the batter’s box.
I’ve never seen a symphony orchestra run right out and start playing.
Why don’t we do a little rehearsing and practice the hardest parts before we actually start the incision?
Still, this won’t become cost-effective in the near term.
“Cognitive” rehearsals
PARK: You can rehearse selectively, based on the difficulty or complexity. We rehearse complex anti-reflux procedures and donor nephrectomies. Software models the patient’s data, and I will do a “fly-through” the afternoon before a laparoscopic donor nephrectomy.
Rehearsal at this stage has more to do with the cognitive processes than technical. So whether I need to practice taking that fat off the renal hilum is less of an issue than the position of lumbar veins or the relation of the renal vein to the aorta.
I feel immensely better prepared for the surgery when I’ve done that fly-through. I’m hard pressed to give you a good metric, to show the science to support it, but I believe there’s a role for it.
SANDBERG: This gets back to the question of metrics. Much of what we’re seeing as we look at ORs of the future actually improves the work experience for the surgeon, anesthesiologist, and nurses.
You can’t put a dollar value on that unless you’re talking about the cost of recruiting and training staff, but it does seem to improve outcomes for the people who work in the OR—and that must translate to patient outcomes. It’s difficult to justify to an administrator, but on a moral level it seems like the right thing to do.
The ideal would be a robotic anesthetist who would not be in the coffee lounge when you want to turn over your case.
“Robo-doc” anesthetist
SANDBERG: Making a business case for a robot for anesthesia is even harder. Functions such as vascular access and airway control are easy to an expert, but I don’t know yet of anyone who even attempted to make an IV-starting robot.
SATAVA: We have one.
SANDBERG: I’ve seen your prototypes. I don’t think you can program your “Robo-doc” to anesthetize the patient when you want, but the maintenance phase is one area in anesthesia ripe for automation.
Anesthesiology is actually in the driver’s seat for harvesting, controlling, and integrating information in the OR. Our project integrates data that anesthesiologists traditionally managed with that from surgical instruments and hospital information systems to create a total patient picture. That includes interventions and perturbations caused by surgery. Once that’s in place, you could imagine actually driving the anesthetic using closed-loop control.
SATAVA: Anesthesia is probably going to evolve more than surgery thanks to safer anesthetics and new nanotechnology. The new anesthetic autoregulators will make it almost impossible to overdose or under-dose a patient.
Are “smart” instruments equal to our own fingertips?
PARK: Today we can make a standard (open surgery) incision into which we insert one or both hands and have more than 20° of movement with the benefit of an innervated end effecter—our fingertips.
The OR of the future will put us in a situation where we’re disconnected in the tactile sense from the target anatomy and limit us to 4°–8° of movement. The stimulus is to get those degrees of movement back to compensate for the tactile feedback we’ve lost.
Hand-assisted laparoscopy is a lowtech way for getting around that loss of movement. It’s nothing other than a transition step, but it’s a clever solution.
How did you discover “parallel processing” to save costs and time?
SANDBERG: We tried to deconstruct the perioperative process immediately upstream and downstream of the OR itself, then we tested the time savings and cost effectiveness of the new process.1
ORs in America tend to do things sequentially via serial-processing work-flow: nurses and technologists set up the OR, the anesthesiologist brings in the patient and induces anesthetic, the surgery happens, the patient emerges from anesthesia, the anesthesiologist takes the patient to recovery.
We discovered that in any of these linear processes a team will be waiting for another team member to finish a task. That presented an opportunity to increase the effectiveness of the OR by finding a way for those team members to work in parallel rather than in sequence.
At Massachusetts General, we reduced the nonoperative time from when the surgeon steps back from the patient until the surgeon steps up to prep the next patient, from roughly 70 to 40 minutes by implementing a couple of parallel processing interventions. Other reports have verified this approach.2,3
In the MGH project, however, the interface between that particular OR and the hospital became problematic when the OR sent patients to the floor faster than the hospital could accept them.
What happened when the conveyor belt speeded up?
This gets us to the notion of perioperative systems design, which is basically a fantastic accident at most hospitals. In our OR of the Future project at MGH, we isolated part of the perioperative process, optimized it, and in so doing we broke the recovery room and upstream admission process. It was like transplanting an elephant heart into a mouse.
What is “plug-and-play”?
SATAVA: Plug-and-play would standardize interfacing between similar components that are interchangeable. Let’s say we have 6 different EKG machines, each with a different method for plugging in the leads. Our concept of plug-and-play goes from software that talks between devices to interchangeable tubing and electrical connections to the devices themselves. This standard is necessary to achieve even the most rudimentary information-based OR of the future.
Lack of plug-and-play connectivity has made it difficult even to get a patient’s allergy information from one database to another. At our institution, a couple software engineers took about 3 months to accomplish that. If this is what it takes to integrate 1 piece of information at 1 institution, we don’t have a chance.
Where do things stand with radiofrequency identification tagging?
PARK: Two types of RFID tags exist: A passive tag is a simple tag that when interrogated by a local node or cell just says it’s there. An active tag imparts a lot more information.
SANDBERG: An RFID tag would contain a patient’s critical medical data, but this vision has many problems at the moment. A tag with sufficient data storage is very expensive.
Security is another problem. The information is transmitted wirelessly and the encryption consumes tag memory. Passive RFID tags, which don’t require a battery and cost less to produce, simply can’t store enough information to be useful as a repository of medical data.
So we’ve discovered applications for RFID beyond the mobile patient record on the tag wristband. RFID can, at least in theory, be used for inventory management. Again, the problems with its costs and the need to scan the items limits its usefulness. Hospitals can use active RFID tags now for tracking high-value assets—not only expensive medical equipment but also personnel and patients.
PARK: We’ve been evaluating many different location RFID technologies in the operating room. We’ve found that the resolution of these technologies is miles from the manufacturers’ claims.
SATAVA: The trade-off for security is the inverse relationship between the level of security and the amount of computing power it demands, along with the amount of physical space available on the device.
Maybe in two years the computing power will increase and device size decrease, so we can get more on a smaller object, and its costs will decrease.
SANDBERG: Another problem with passive RFID is a high out-of-box failure rate. The antennae break off in manufacturing and shipping.
Besides cost, spatial resolution is another problem with active tags. On the other hand, passive RFID tags can have sub-centimeter resolution because the reader has to be so close to read the tag.
However, scanning the tag is another problem. Most of us in our workflows don’t have time to grab an RFID reader. Passive RFID reader technology is far from ideal for a busy OR.
Ideally, they should require no effort: objects brought into the OR would register on an as-yet-uninvented meter and localize in the room, ideally at 1 cm resolution or better.
What devices will be the stars of the OR of the Future?
LED Technology
SATAVA: With LED—light-emitting diode—technology and the small cameras now in cell phones, we have the opportunity to get rid of all the lights in the operating room.
The University of Barcelona has accomplished this.4 A sea of 100 to 150 cameras is placed around the room. You don’t have to move the cameras or lights. This clears up all the booms that are hanging down of these enormously inefficient lights that we have today.
This technology can leverage information systems that talk to objects and track instruments.
Wireless, miniaturized robots
PARK: Miniaturized robots, designed to be wireless in the near future, can actually rove the peritoneal cavity equipped with an end effecter or mobile camera. Dropped in through a 5- or 10-mm port, these devices can get to the target anatomy. Mechanical miniaturization is another way of getting around the loss of degrees of movement.
Better visualization with HD video
The minimally invasive revolution has moved us from having direct binocular visual contact with the target anatomy to working in a 3-dimensional space off a 2-dimensional image.
The next step is to take the vast amount of data we’re capturing, especially with HD video, from the operative field and use it to improve surgical visualization and ultimately outcomes.
This may involve real-time modeling and altering the visual perspective of the operative field as it is presented to the surgeon. We’re looking at new ways of making the data more intuitive.
Beyond the visible spectrum
SATAVA: There’s another dimension: Why are we only using cameras that see in the visible spectrum? Why aren’t we using infrared and ultraviolet cameras as other industries do?
We may not need the sense of touch because an infrared camera can show us all the blood vessels by temperature change. Health care hasn’t even looked at off-the-shelf technologies that other industries have used for 25 years.
PARK: Narrow-band imaging and confocal microscopy are now available, but we haven’t even started to look at how we could apply them intra-abdominally.
Will biotechnology bring down the curtain on surgeons?
SATAVA: We haven’t talked about the extraordinary revolution in biotechnology and how it’s either going to change the way a surgeon works or puts the surgeon out of business.
We may not have to do surgery if we can manipulate tissue on a molecular level. That’s 20–25 years out. It’s going to take that long for nanotechnology to develop usable products for health care.
The human element
RATTNER: We didn’t dwell on the human factor. The functioning of a highly skilled team and the interaction among team members is fertile ground for further research. Greater attention paid to team training and simulation crisis management will pay off in patient safety.
At the end of the day, the priority is that if we’re going to operate faster and more efficiently with more complex procedures, we have to get to Six Sigma or better safety levels.
Disclosures
Dr Park disclosed an affiliation with Stryker Endoscopy. Dr Sandberg disclosed affiliations with LiveData, Inc, Cambridge, MA; and Radianse, Inc, Lawrence, MA. Dr Satava disclosed an affiliation with Medical Education Technologies, Inc, Sarasota, FL. Dr Rattner had no affiliations to disclose.
1. Sandberg WS, Daily B, Egan M, et al. Deliberate perioperative systems design improves operating room throughput. Anesthesiology. 2005;103:406-418.
2. Hanss R, Buttgereit B, Tonner PH, et al. Overlapping induction of anesthesia: an analysis of benefits and costs. Anesthesiology. 2005;103:391-400.
3. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvela OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103:401-405.
4. LaPorte EN. Array of ceiling mounted LED lights for the OR of the Future. Paper presented at: 12th European Association of Endoscopic Surgeons (EAES) Congress; June 9-12, 2004, Barcelona, Spain.
1. Sandberg WS, Daily B, Egan M, et al. Deliberate perioperative systems design improves operating room throughput. Anesthesiology. 2005;103:406-418.
2. Hanss R, Buttgereit B, Tonner PH, et al. Overlapping induction of anesthesia: an analysis of benefits and costs. Anesthesiology. 2005;103:391-400.
3. Torkki PM, Marjamaa RA, Torkki MI, Kallio PE, Kirvela OA. Use of anesthesia induction rooms can increase the number of urgent orthopedic cases completed within 7 hours. Anesthesiology. 2005;103:401-405.
4. LaPorte EN. Array of ceiling mounted LED lights for the OR of the Future. Paper presented at: 12th European Association of Endoscopic Surgeons (EAES) Congress; June 9-12, 2004, Barcelona, Spain.