User login
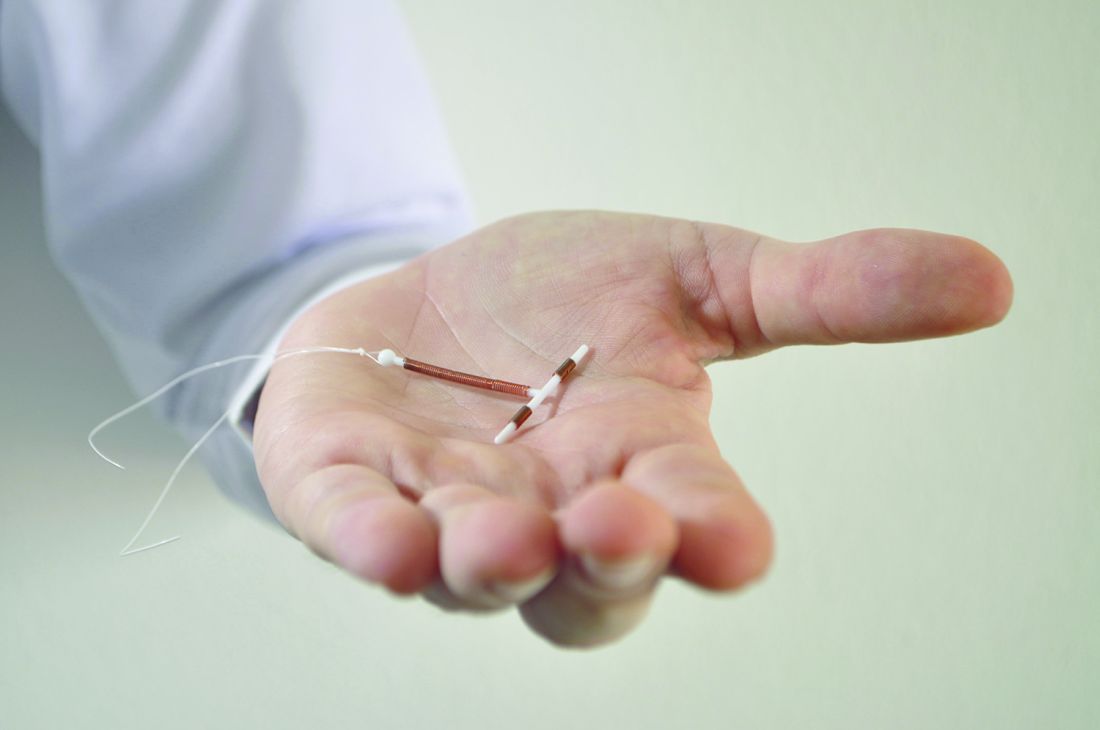
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
A new study of Medicaid-covered women who sought long-acting reversible contraception via subdermal etonogestrel implants or IUDs found that levels of continuation at 1 year were high, and complication rates were low.
There was little difference between the groups on both fronts.
“This study demonstrates high rates of continued use of subdermal and intrauterine contraceptives by Medicaid clients, particularly among adolescents, and therefore, interventions that increase adolescent access to these contraceptives are likely to be cost effective from the payer perspective,” the study authors wrote in Contraception.
The study, led by Max J. Romano, MD, MPH, of MedStar Franklin Square Medical Center in Baltimore, retrospectively examined the medical claims of 3,305 women treated via MedStar Family Choice, a Medicaid managed-choice insurer.
The women, who lived in Maryland and the District of Columbia, were aged 15-44 years when they underwent contraceptive treatment during 2012-2015. Of the women, 1,335 had subdermal etonogestrel implants inserted, and 1,970 had IUDs inserted. Researchers followed the women for a mean 344 days.
The implant users were younger than the IUD group (mean age, 25 years vs. 28 years; P = less than .001), and women under age 20 years were more than more than twice as likely to get implants than IUDs (71% vs. 29%).
Women older than 20 years were more likely to get IUDs than implants, with those older than 25 preferring them by a ratio of 70% to 30%.
After researchers controlled for such factors as age group and year of insertion, they found that implant recipients were slightly more likely to still have the contraception tool in place at 1 year: 81% (implants) vs. IUDs (77%; P = .01). It’s not clear why women had their implants or IUDs removed.
Claims for complications were similar between the groups (8% for implants, 7% for IUDs), and researchers didn’t find a statistically significant difference after they controlled for various factors. Dysfunctional uterine bleeding was the most common complication, followed by excessive or frequent menstruation.
Few women reported pregnancy among the implant (0.82%) and IUD (0.86%) groups, and there was no statistically significant difference between the groups.
The researchers reported that their study has various limitations: The insurance claims information may provide misleading information about minor complications, and it doesn’t include details about abortions. The claims also may not report IUD expulsions and self-removals.
The authors also noted that they didn’t control for factors like socioeconomic status, psychological conditions, and multimorbidity.
MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen disclosed serving on the Diclegis speakers bureau.
FROM CONTRACEPTION
Key clinical point:
Major finding: For implants and IUDS, respectively, 1-year continuation rates were similar (adjusted 81% vs. 77%, P = .01), as were complication rates (unadjusted 8% for implants, 7% for IUDs).
Study details: Retrospective analysis of 3,305 Medicaid-covered women treated with the two types of contraceptives during 2012-2015 in the District of Columbia and Maryland.
Disclosures: MedStar Family Choice provided study funding. Dr. Romano and Loral Patchen, PhD, disclosed receiving free CME trainings and insertion certification regarding the long-acting contraceptive devices discussed in the study from Merck, Bayer, and Teva. Dr. Patchen discloses serving on the Diclegis speakers bureau.
Source: Romano MJ et al. Contraception. 2018 Aug;98:125-9.