User login
Since it was approved more than 10 years ago, depot medroxyprogesterone acetate (DMPA; Depo-Provera) has gained popularity among US women, largely because it requires minimal user participation and has a failure rate of only 0.3% per year.1,2 The main limitation, from the patient’s point of view, has been the intramuscular (IM) route of injection, which requires an office visit every 12 to 14 weeks for administration.
Now a subcutaneous version of the drug is available (Depo-subQ Provera 104) that delivers a lower dose of medroxyprogesterone acetate (MPA) (104 mg versus 150 mg for the IM formulation). The subcutaneous route opens the possibility for home self-injections, and the lower dose could decrease suppression of pituitary function and ovarian estradiol production, though further study is needed.
This article reviews the indications, benefits, risks, and potential adverse reactions of subcutaneous DMPA, a pharmacologically unique formulation with 16% weight/volume and a final dose of 104 mg MPA/0.65 mL. The dose was selected after study showed 100 mg to be the lowest dose to effectively suppress ovulation for at least 91 days.
The formulation and composition of subcutaneous DMPA cannot be duplicated by diluting the original IM formulation.
A potent contraceptive
Tw o large open-label, phase 3 studies assessed the 1-year efficacy, safety, and patient satisfaction of subcutaneous DMPA.3 These studies, conducted in North and South America, Europe, and Asia, reported zero pregnancies in 16,023 women-cycles of exposure.
Women in these studies had a broad range of body weights, ranging from 86 to 364 lb in the Americas and 77 to 249 lb in the European/Asian trial. The absence of pregnancies across all categories of body mass index (BMI) suggests that no dosage adjustments are necessary for higher BMIs.
Noncontraceptive benefits
Besides the high efficacy and long duration, which free women from daily attention to contraception, DMPA protects against endometrial cancer. The fact that it contains no estrogen makes it suitable for women who cannot or will not take estrogen products. It also is safe for breastfeeding mothers.
Perhaps most important is its ameliorative effect on endometriosis-associated pain.
Adverse effects
Many women stop using DMPA during the first year due to problems with irregular uterine bleeding, such as spotting and prolonged bleeding, which are especially common during the first 3 months of use. However, this problem usually diminishes over time, with most users becoming amenorrheic. This is true of both IM and subcutaneous DMPA. In a study of the latter, amenorrhea increased from 26% during month 3 to 55% during month 12.
The bleeding abnormalities associated with progestin-only contraceptives are not fully understood. We do know that suppression of circulating estradiol and the potent effect of MPA on the endometrium lead to varying degrees of endometrial disruption and atrophy, which ultimately manifest as irregular bleeding and amenorrhea. Subcutaneous DMPA likely involves the same processes, even though it contains 30% less MPA than the IM formulation.
Importance of counseling about bleeding effects
Two studies have shown that women are more likely to continue DMPA if they are counseled about bleeding effects when they start the medication.4,5 Since many patients would prefer less frequent or no menses, they may be encouraged by the prospect of becoming amenorrheic.
Risk of breast cancer
It will be several years before the effect of the lower-dose MPA on breast cancer risk is known.
DMPA and bone loss: Should we worry?
Subcutaneous DMPA, like its IM counterpart, is associated with changes in bone mineral density and carries a “black box” warning regarding this risk.6 Because DMPA suppresses circulating estradiol levels, it causes reductions in bone mineral density (BMD) that have aroused concern among the lay and medical media, although studies suggest BMD levels generally change little and recover when the drug is discontinued— except during perimenopause.
A metaanalysis of 12 studies involving 1,039 DMPA users (IM formulation) and 2,086 controls found that the average Z-score in DMPA users decreased less than 1 standard deviation, compared with nonusers.7 These BMD reductions stabilized after 3 to 4 years of DMPA use, and the bone loss was reversed when the drug was discontinued.8,9 Thus, it appears that, in time, BMD returns to levels similar to those in women who have never used the drug.
IM versus subcutaneous DMPA
In a comparison of both formulations of DMPA, both caused decreases in BMD at the end of 1 and 2 years of treatment.10 Women using subcutaneous DMPA experienced smaller decrements in total hip, lumbar spine, and femoral neck BMD after 1 and 2 years of treatment. However, these differences were significant only in the lumbar spine at 1 year.
Uncertain value for adolescents
DMPA should be carefully considered for use in adolescent girls—and this proviso includes the subcutaneous formulation.
Adolescence is a critical period for bone mineralization. Thus, any agent that limits bone accretion should be prescribed only after weighing all the other options.
A prospective cohort study in adolescents found a 3.1% decrease in BMD after 2 years of DMPA use, versus a 9.5% increase among nonusers.11 More recent reports indicate significant gains in BMD and reversal in bone loss once the drug is discontinued.12
What the “black box” warning means
Based in part on results from these studies, the Food and Drug Administration (FDA) and the drug’s manufacturer issued a black box warning for both the IM and subcutaneous formulations of DMPA. This step was taken to highlight the fact that users of DMPA may lose significant BMD, and that this loss may increase with duration of use and may not be entirely reversible.
The warning recommends that the drugs be used as long-term birth control only if other methods are inadequate. It emphasizes the general lack of certainty about the effect of these drugs on peak bone mass (when used in adolescence or early adulthood) and the risk of osteoporotic fracture (later in life).
How to counsel patients
I discuss the black box warning with each patient in the larger context of contraceptive counseling. The lower efficacy and other problems associated with daily birth control methods must be weighed against the risk of bone loss in both adolescents and adults.
It also is important to consider other risk factors for osteoporosis, such as chronic alcohol or tobacco use, eating disorders, or chronic use of corticosteroids. Adolescents who have poor eating habits or who use alcohol or tobacco may be at heightened risk of BMD loss.
Once a woman chooses DMPA, she should be encouraged to maintain a healthy lifestyle, including adequate calcium intake, weight-bearing and musclestrengthening exercises, smoking cessation, and moderate to no alcohol intake.
BMD measurements are not recommended since they do not predict fracture risk in premenopausal women.
Other side effects
Though rare, serious thrombotic events have been reported in women using the IM formulation.
Also rare are ocular disorders (loss of vision, proptosis, diplopia, or migraine) and ectopic pregnancy.
Other possible side effects include injection site reactions, decreased libido, acne, headache, fatigue, gastrointestinal disorders (distention, abdominal pain, diarrhea, nausea), infection, arthralgia, back pain, limb pain, dizziness, insomnia, anxiety, depression, breast pain and/or tenderness, and hot flushes.
Return to ovulation
DMPA is associated with a prolonged return to ovulation once it is discontinued. In a large US study of women who discontinued intramuscular DMPA to become pregnant, 68% conceived within 12 months, 83% conceived within 15 months, and 93% conceived within 18 months of the last injection, with a median time to conception of 10 months.13
Though no studies have determined the median time to conception for subcutaneous DMPA, it is likely to be similar to the 10-month interval seen with the IM formulation.
Comparing drugs head to head
The IM and subcutaneous formulations were compared prospectively at a single US center.14 The study defined return to ovulation as the first time serum progesterone levels reached at least 4.7 ng/mL. At the end of 12 months (postinjection), the cumulative rate of ovulation was 97.4% for subcutaneous DMPA and 94.7% for the IM formulation.
Ovulation occurred at a median of approximately 7 months (subcutaneous route) and 6 months (IM).
Early ovulation is possible
One subject in the subcutaneous DMPA group ovulated 14 weeks after her last injection. Thus, it is important to adhere to the recommended dosing schedule of 12 to 14 weeks.
Weight gain: 0 to 7.5 lb
Reports of weight gain with DMPA have been highly variable. Many women who discontinue hormonal contraceptives cite weight gain as the reason. With one third of US women meeting the criteria for obesity—a number that is likely to rise—and with ethnic variations, it is difficult to determine the exact impact of DMPA.
A well-designed, placebo-controlled trial by Pelkman and colleagues15 found DMPA to have no effect on resting energy expenditure, food intake, or body weight. Three large clinical trials of subcutaneous DMPA found a mean weight gain of 3.5 lb during the first year of use, and a small 2-year study comparing IM and subcutaneous DMPA found mean weight gains of 7.6 and 7.5 lb, respectively.
Combating endometriosis pain
With the FDA’s approval of subcutaneous DMPA for treatment of endometriosis-associated pain, the drug expands the pharmacologic choices for endometriosis pain relief for the first time in 15 years, with less frequent side effects than the other widely used drug, leuprolide acetate.
In an 18-month clinical trial comparing the 2 drugs, researchers found similar efficacy, with DMPA causing less bone loss and less frequent and severe menopausal symptoms. The trial involved 274 women and measured pain across the following categories: pelvic pain, dysmenorrhea, dyspareunia, pelvic tenderness, and induration.
Clearing a woman for use
The manufacturer recommends that all women undergo an annual history and physical examination. The physical exam should include a blood pressure check; examination of the breasts, abdomen, and pelvic organs; cervical cytology; and any relevant laboratory studies.
Overall outlook
Subcutaneous DMPA offers women the same advantages as the IM formulation. Since we have long-term experience with MPA as a contraceptive agent, we know it offers many noncontraceptive benefits, safety, and excellent contraceptive efficacy.
As we gain experience specific to subcutaneous DMPA , and as data accumulate from additional trials, we will be able to further define its role as a contraceptive option.
Disclosure
Dr. Jain has disclosed that he has received grant/research support from Ferring, Organon, Pfizer, Serono, and TA P.
REFERENCES
1. Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera®): a highly effective contraceptive option with proven long-term safety. Contraception. 2003;68:75-87.
2. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart F, et al. Contraceptive Technology. 17th revised edition. New York: Irvington Publishers; 1998.
3. Jain JK, Jakimiuk AJ, Bode FR, et al. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
4. Canto De Cetina TE, Canto P, Luna MO. Effect of counseling to improve compliance in Mexican women receiving depo-medroxyprogesterone acetate. Contraception. 2001;63:143-146.
5. Lei ZW, Wu SC, Garceau RJ, et al. Effect of pretreatment counseling on discontinuation rates in Chinese women given depo-medroxyprogesterone acetate for contraception. Contraception. 1996;53:357-361.
6. Depo SubQ Provera 104 [package insert]. Cambridge, Mass: Pfizer; 2005.
7. Banks E, Berrington A, Casabonne D. Overview of the relationship between use of progestogen-only contraceptives and bone mineral density. Br J Obstet Gynecol. 2001;108:1214-1221.
8. Tang OS, Tang G, Yip PSF, et al. Further evaluation on long-term depot-medroxyprogesterone acetate use and bone mineral density: a longitudinal cohort study. Contraception. 2000;62:161-164.
9. Petitti DB, Piaggio G, Mehta S, et al. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. Obstet Gynecol. 2000;95:736-744.
10. Jain JK. Evaluation of bone mineral density in women treated with DMPA-SC 104 or DMPA-IM 150. Presented at: Annual Meeting of the American Academy of Nurse Practitioners; June 17, 2005; Fort Lauderdale, Fla.
11. Cromer BA, Blair JM, Mahan JD, et al. A prospective comparison of bone density in adolescent girls receiving depot medroxyprogesterone acetate (DMPA), levonorgestrel (Norplant), or oral contraceptives. J Pediatr. 1996;129:671-676.
12. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
13. Depo-Provera [package insert]. Cambridge, Mass: Pfizer; 1999.
14. Jain JK, Dutton C, Nicosia A, et al. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera®. Contraception. 2004;70:11-18.
15. Pelkman CL, Chow M, Heinbach RA, et al. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am J Clin Nutr. 2001;73:19-26.
CONTRACEPTIVES IN THE NEWS Plan B: Studies surprise both sides
Ian H. Thorneycroft
MD, PhD
OBG Management
Board of Editors
Professor of Obstetrics and Gynecology, University of South Alabama College of Medicine, Mobile, Ala
Marston C, Meltzer H, Majeed A. Impact on contraceptive practice of making emergency hormonal contraception available over the counter in Great Britain: repeated cross sectional surveys. BMJ doi;10.1136/bmj.38519.440266.8F (published July 11, 2005).
Raine TR, Harper CC, Rocca CH, Fischer R, Padian N, Klausner JD, Darney PD. Direct access to emergency contraception through pharmacies and effect of unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;292:54–62.
Litt IF. Placing emergency contraception in the hands of women. JAMA. 2005;293:98–99.
A quick succession of events in July seemed to clear the path to a Food and Drug Administration (FDA) decision on over-the-counter sale of levonorgestrel (Plan B), the emergency contraceptive.
A week after the publication of the large study led by Marston, Plan B was placed on the FDA calendar for a Sept. 1 decision. The authors concluded that their study “supports the case for lifting the ban on over-the-counter sales of emergency hormonal contraception in the United States and other countries.”
Plan B has been a point of contention, especially after the FDA, in 2004, rejected Barr Laboratories’ application for OTC access, on the basis of lack of long-term safety data on its use in young adolescent women, without medical supervision.
When the manufacturer changed its application, the agency declined to make a decision by the legal deadline, last January. Proponents of OTC sales of Plan B objected, citing the agency’s own staff endorsement, and that of an independent panel.
In a series of interactions between the Senate and the administration in early July, the U.S. secretary of health and human services promised a Sept. 1 decision on Barr’s application. Media reports related that announcement to the July 18 Senate confirmation of Dr. Lester Crawford to head the Food and Drug Administration.
Both sides of the controversy met with some surprises in the BMJ and JAMA reports of research on the effects of Plan B access. Opponents of OTC availability in the United States have predicted that such access might increase unprotected sex, especially in young women and girls. Advocates have predicted that it would reduce the number of unintended pregnancies and abortions.
Public health impact may be negligible”
The report in the British Medical Journal concluded, “Making emergency hormonal contraception available over the counter does not seem to have led to an increase in its use, to an increase in unprotected sex, or to a decrease in the use of more reliable methods of contraception.” The study used an Omnibus Survey of 7,600 adults (an annual multipurpose survey in Great Britain) to examine contraception use after OTC emergency contraception was legalized in 2001. Women aged 16 to 49 were surveyed.
Although the lack of any increase in use of emergency hormone contraception suggests that the predicted rise in unsafe sex has been overstated, so too have the predicted effects on unwanted pregnancy, the authors observed.Similarly, the January JAMA report of a US study found: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method.”
Barr requests OTC access of Plan B for women 16 and older. Prescriptions would still be required for younger women.
Plan B is often called the “morning-after pill,” but in fact the method can be used any time after intercourse for up to 72 hours. It is best used as soon as possible, but can even be used after 72 hours, although at reduced efficacy.
Kuyoh MA, Toroitich-Ruto C, Grimes DA, Schulz KF, Gallo MF. Sponge versus diaphragm for contraception: a Cochrane review. Contraception. 2003;67:15–18.
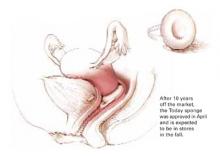
When the Today sponge was pulled off the market by its maker in 1995, the popular Jerry Seinfeld TV show aired an episode showing the character Elaine dashing from store to store, in search of the last few sponges on the shelves. She ultimately found a neighborhood pharmacy with 1 remaining case of 60 sponges. “Just give me the whole case and I’ll be on my way,” she tells the pharmacist. In the show, Elaine used the term “sponge-worthy” to characterize a potential date, and the term became a household word overnight. When it was available in the United States, the Today sponge was the most widely used form of OTC contraception, selling approximately 250 million sponges.
The sponge, which contains the spermicide nonoxynol-9, was sold from 1983 to 1995, when it was voluntarily withdrawn for safety reasons. The FDA found that water at the manufacturing plant was contaminated, and American Home Products, (now Wyeth), decided it was too costly to upgrade its plant, and voluntarily withdrew the Today sponge from the market. Allendale Pharmaceuticals bought the rights for the sponge in 1998, and has been working to secure approval for marketing. The new version has been available in Canada since 2003. According to the manufacturer, it should be in US drug stores sometime this fall, for about $2.50 to $3 per sponge.
The sponge is best compared to a diaphragm. Its failure rate is frequently quoted as approximately 9% to 11% per year, very comparable to condoms.
Efficacy. However, in a Cochrane review of sponges versus the diaphragm, the sponge failure rate was 17.4% in a US trial and 24.5% in a British trial. The rates for a diaphragm were 12.8% and 10.9%, respectively. Unlike a diaphragm, the sponge does not have to be fitted and had equal efficacy in multiparous as nulliparous women, in the 2 studies reviewed. The main advantage of the sponge over a diaphragm is its ready availability over-the-counter.
According the manufacturer, the sponge can be inserted up to 24 hours prior to intercourse, and provides continuous protection throughout that period, for as many acts of intercourse as desired. Although it is not necessary to keep the sponge inserted for a full 24 hours, it must be left in place for 6 hours after the last act of intercourse. It should not be worn for more than 30 consecutive hours. The women in the 2 studies included in the Cochrane review, however, left the sponge in place for 48 hours.
Same-day start-up for OCs improves compliance
Westhoff C, Kerns J, Morroni C, Cushman LF, Tiezzi L, Murphy PA. Quick Start: a novel oral contraceptive initiation method. Contraception. 2002;66:141–145.
Many women never start the oral contraceptive we prescribe. They have to wait for their next menses, and they may fail to fill the prescription; 25% of study participants never start their oral contraceptives.
Quick Start starts the contraceptive in the clinic, after a negative pregnancy test, regardless of the day of the patient’s cycle. She is given at least 1 pack so she does not have to go to the drug store to fill the first prescription. Emergency contraception is given to patients who may need it. The continuation rate to the second cycle was higher in women beginning the pill in the clinic than those who started at any point in their cycle but waited to fill the prescription.
The original oral contraceptives were started on the first Sunday of a menstrual cycle so that it was known the woman was not pregnant and so she would bleed midweek. Waiting until menses assured a possible pregnancy was not exposed to hormonal contraceptives; however, the components of oral contraceptives are not teratogenic. The contraceptive can therefore be started at anytime during the cycle and starting in the clinic increases compliance. A pregnancy test can be performed if the anticipated menses at the end of the pill pack does not occur.
Disclosure
The author is on the speakers bureaus for Barr, Berlex, and Wyeth-Ayerst.
Since it was approved more than 10 years ago, depot medroxyprogesterone acetate (DMPA; Depo-Provera) has gained popularity among US women, largely because it requires minimal user participation and has a failure rate of only 0.3% per year.1,2 The main limitation, from the patient’s point of view, has been the intramuscular (IM) route of injection, which requires an office visit every 12 to 14 weeks for administration.
Now a subcutaneous version of the drug is available (Depo-subQ Provera 104) that delivers a lower dose of medroxyprogesterone acetate (MPA) (104 mg versus 150 mg for the IM formulation). The subcutaneous route opens the possibility for home self-injections, and the lower dose could decrease suppression of pituitary function and ovarian estradiol production, though further study is needed.
This article reviews the indications, benefits, risks, and potential adverse reactions of subcutaneous DMPA, a pharmacologically unique formulation with 16% weight/volume and a final dose of 104 mg MPA/0.65 mL. The dose was selected after study showed 100 mg to be the lowest dose to effectively suppress ovulation for at least 91 days.
The formulation and composition of subcutaneous DMPA cannot be duplicated by diluting the original IM formulation.
A potent contraceptive
Tw o large open-label, phase 3 studies assessed the 1-year efficacy, safety, and patient satisfaction of subcutaneous DMPA.3 These studies, conducted in North and South America, Europe, and Asia, reported zero pregnancies in 16,023 women-cycles of exposure.
Women in these studies had a broad range of body weights, ranging from 86 to 364 lb in the Americas and 77 to 249 lb in the European/Asian trial. The absence of pregnancies across all categories of body mass index (BMI) suggests that no dosage adjustments are necessary for higher BMIs.
Noncontraceptive benefits
Besides the high efficacy and long duration, which free women from daily attention to contraception, DMPA protects against endometrial cancer. The fact that it contains no estrogen makes it suitable for women who cannot or will not take estrogen products. It also is safe for breastfeeding mothers.
Perhaps most important is its ameliorative effect on endometriosis-associated pain.
Adverse effects
Many women stop using DMPA during the first year due to problems with irregular uterine bleeding, such as spotting and prolonged bleeding, which are especially common during the first 3 months of use. However, this problem usually diminishes over time, with most users becoming amenorrheic. This is true of both IM and subcutaneous DMPA. In a study of the latter, amenorrhea increased from 26% during month 3 to 55% during month 12.
The bleeding abnormalities associated with progestin-only contraceptives are not fully understood. We do know that suppression of circulating estradiol and the potent effect of MPA on the endometrium lead to varying degrees of endometrial disruption and atrophy, which ultimately manifest as irregular bleeding and amenorrhea. Subcutaneous DMPA likely involves the same processes, even though it contains 30% less MPA than the IM formulation.
Importance of counseling about bleeding effects
Two studies have shown that women are more likely to continue DMPA if they are counseled about bleeding effects when they start the medication.4,5 Since many patients would prefer less frequent or no menses, they may be encouraged by the prospect of becoming amenorrheic.
Risk of breast cancer
It will be several years before the effect of the lower-dose MPA on breast cancer risk is known.
DMPA and bone loss: Should we worry?
Subcutaneous DMPA, like its IM counterpart, is associated with changes in bone mineral density and carries a “black box” warning regarding this risk.6 Because DMPA suppresses circulating estradiol levels, it causes reductions in bone mineral density (BMD) that have aroused concern among the lay and medical media, although studies suggest BMD levels generally change little and recover when the drug is discontinued— except during perimenopause.
A metaanalysis of 12 studies involving 1,039 DMPA users (IM formulation) and 2,086 controls found that the average Z-score in DMPA users decreased less than 1 standard deviation, compared with nonusers.7 These BMD reductions stabilized after 3 to 4 years of DMPA use, and the bone loss was reversed when the drug was discontinued.8,9 Thus, it appears that, in time, BMD returns to levels similar to those in women who have never used the drug.
IM versus subcutaneous DMPA
In a comparison of both formulations of DMPA, both caused decreases in BMD at the end of 1 and 2 years of treatment.10 Women using subcutaneous DMPA experienced smaller decrements in total hip, lumbar spine, and femoral neck BMD after 1 and 2 years of treatment. However, these differences were significant only in the lumbar spine at 1 year.
Uncertain value for adolescents
DMPA should be carefully considered for use in adolescent girls—and this proviso includes the subcutaneous formulation.
Adolescence is a critical period for bone mineralization. Thus, any agent that limits bone accretion should be prescribed only after weighing all the other options.
A prospective cohort study in adolescents found a 3.1% decrease in BMD after 2 years of DMPA use, versus a 9.5% increase among nonusers.11 More recent reports indicate significant gains in BMD and reversal in bone loss once the drug is discontinued.12
What the “black box” warning means
Based in part on results from these studies, the Food and Drug Administration (FDA) and the drug’s manufacturer issued a black box warning for both the IM and subcutaneous formulations of DMPA. This step was taken to highlight the fact that users of DMPA may lose significant BMD, and that this loss may increase with duration of use and may not be entirely reversible.
The warning recommends that the drugs be used as long-term birth control only if other methods are inadequate. It emphasizes the general lack of certainty about the effect of these drugs on peak bone mass (when used in adolescence or early adulthood) and the risk of osteoporotic fracture (later in life).
How to counsel patients
I discuss the black box warning with each patient in the larger context of contraceptive counseling. The lower efficacy and other problems associated with daily birth control methods must be weighed against the risk of bone loss in both adolescents and adults.
It also is important to consider other risk factors for osteoporosis, such as chronic alcohol or tobacco use, eating disorders, or chronic use of corticosteroids. Adolescents who have poor eating habits or who use alcohol or tobacco may be at heightened risk of BMD loss.
Once a woman chooses DMPA, she should be encouraged to maintain a healthy lifestyle, including adequate calcium intake, weight-bearing and musclestrengthening exercises, smoking cessation, and moderate to no alcohol intake.
BMD measurements are not recommended since they do not predict fracture risk in premenopausal women.
Other side effects
Though rare, serious thrombotic events have been reported in women using the IM formulation.
Also rare are ocular disorders (loss of vision, proptosis, diplopia, or migraine) and ectopic pregnancy.
Other possible side effects include injection site reactions, decreased libido, acne, headache, fatigue, gastrointestinal disorders (distention, abdominal pain, diarrhea, nausea), infection, arthralgia, back pain, limb pain, dizziness, insomnia, anxiety, depression, breast pain and/or tenderness, and hot flushes.
Return to ovulation
DMPA is associated with a prolonged return to ovulation once it is discontinued. In a large US study of women who discontinued intramuscular DMPA to become pregnant, 68% conceived within 12 months, 83% conceived within 15 months, and 93% conceived within 18 months of the last injection, with a median time to conception of 10 months.13
Though no studies have determined the median time to conception for subcutaneous DMPA, it is likely to be similar to the 10-month interval seen with the IM formulation.
Comparing drugs head to head
The IM and subcutaneous formulations were compared prospectively at a single US center.14 The study defined return to ovulation as the first time serum progesterone levels reached at least 4.7 ng/mL. At the end of 12 months (postinjection), the cumulative rate of ovulation was 97.4% for subcutaneous DMPA and 94.7% for the IM formulation.
Ovulation occurred at a median of approximately 7 months (subcutaneous route) and 6 months (IM).
Early ovulation is possible
One subject in the subcutaneous DMPA group ovulated 14 weeks after her last injection. Thus, it is important to adhere to the recommended dosing schedule of 12 to 14 weeks.
Weight gain: 0 to 7.5 lb
Reports of weight gain with DMPA have been highly variable. Many women who discontinue hormonal contraceptives cite weight gain as the reason. With one third of US women meeting the criteria for obesity—a number that is likely to rise—and with ethnic variations, it is difficult to determine the exact impact of DMPA.
A well-designed, placebo-controlled trial by Pelkman and colleagues15 found DMPA to have no effect on resting energy expenditure, food intake, or body weight. Three large clinical trials of subcutaneous DMPA found a mean weight gain of 3.5 lb during the first year of use, and a small 2-year study comparing IM and subcutaneous DMPA found mean weight gains of 7.6 and 7.5 lb, respectively.
Combating endometriosis pain
With the FDA’s approval of subcutaneous DMPA for treatment of endometriosis-associated pain, the drug expands the pharmacologic choices for endometriosis pain relief for the first time in 15 years, with less frequent side effects than the other widely used drug, leuprolide acetate.
In an 18-month clinical trial comparing the 2 drugs, researchers found similar efficacy, with DMPA causing less bone loss and less frequent and severe menopausal symptoms. The trial involved 274 women and measured pain across the following categories: pelvic pain, dysmenorrhea, dyspareunia, pelvic tenderness, and induration.
Clearing a woman for use
The manufacturer recommends that all women undergo an annual history and physical examination. The physical exam should include a blood pressure check; examination of the breasts, abdomen, and pelvic organs; cervical cytology; and any relevant laboratory studies.
Overall outlook
Subcutaneous DMPA offers women the same advantages as the IM formulation. Since we have long-term experience with MPA as a contraceptive agent, we know it offers many noncontraceptive benefits, safety, and excellent contraceptive efficacy.
As we gain experience specific to subcutaneous DMPA , and as data accumulate from additional trials, we will be able to further define its role as a contraceptive option.
Disclosure
Dr. Jain has disclosed that he has received grant/research support from Ferring, Organon, Pfizer, Serono, and TA P.
REFERENCES
1. Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera®): a highly effective contraceptive option with proven long-term safety. Contraception. 2003;68:75-87.
2. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart F, et al. Contraceptive Technology. 17th revised edition. New York: Irvington Publishers; 1998.
3. Jain JK, Jakimiuk AJ, Bode FR, et al. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
4. Canto De Cetina TE, Canto P, Luna MO. Effect of counseling to improve compliance in Mexican women receiving depo-medroxyprogesterone acetate. Contraception. 2001;63:143-146.
5. Lei ZW, Wu SC, Garceau RJ, et al. Effect of pretreatment counseling on discontinuation rates in Chinese women given depo-medroxyprogesterone acetate for contraception. Contraception. 1996;53:357-361.
6. Depo SubQ Provera 104 [package insert]. Cambridge, Mass: Pfizer; 2005.
7. Banks E, Berrington A, Casabonne D. Overview of the relationship between use of progestogen-only contraceptives and bone mineral density. Br J Obstet Gynecol. 2001;108:1214-1221.
8. Tang OS, Tang G, Yip PSF, et al. Further evaluation on long-term depot-medroxyprogesterone acetate use and bone mineral density: a longitudinal cohort study. Contraception. 2000;62:161-164.
9. Petitti DB, Piaggio G, Mehta S, et al. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. Obstet Gynecol. 2000;95:736-744.
10. Jain JK. Evaluation of bone mineral density in women treated with DMPA-SC 104 or DMPA-IM 150. Presented at: Annual Meeting of the American Academy of Nurse Practitioners; June 17, 2005; Fort Lauderdale, Fla.
11. Cromer BA, Blair JM, Mahan JD, et al. A prospective comparison of bone density in adolescent girls receiving depot medroxyprogesterone acetate (DMPA), levonorgestrel (Norplant), or oral contraceptives. J Pediatr. 1996;129:671-676.
12. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
13. Depo-Provera [package insert]. Cambridge, Mass: Pfizer; 1999.
14. Jain JK, Dutton C, Nicosia A, et al. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera®. Contraception. 2004;70:11-18.
15. Pelkman CL, Chow M, Heinbach RA, et al. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am J Clin Nutr. 2001;73:19-26.
CONTRACEPTIVES IN THE NEWS Plan B: Studies surprise both sides
Ian H. Thorneycroft
MD, PhD
OBG Management
Board of Editors
Professor of Obstetrics and Gynecology, University of South Alabama College of Medicine, Mobile, Ala
Marston C, Meltzer H, Majeed A. Impact on contraceptive practice of making emergency hormonal contraception available over the counter in Great Britain: repeated cross sectional surveys. BMJ doi;10.1136/bmj.38519.440266.8F (published July 11, 2005).
Raine TR, Harper CC, Rocca CH, Fischer R, Padian N, Klausner JD, Darney PD. Direct access to emergency contraception through pharmacies and effect of unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;292:54–62.
Litt IF. Placing emergency contraception in the hands of women. JAMA. 2005;293:98–99.
A quick succession of events in July seemed to clear the path to a Food and Drug Administration (FDA) decision on over-the-counter sale of levonorgestrel (Plan B), the emergency contraceptive.
A week after the publication of the large study led by Marston, Plan B was placed on the FDA calendar for a Sept. 1 decision. The authors concluded that their study “supports the case for lifting the ban on over-the-counter sales of emergency hormonal contraception in the United States and other countries.”
Plan B has been a point of contention, especially after the FDA, in 2004, rejected Barr Laboratories’ application for OTC access, on the basis of lack of long-term safety data on its use in young adolescent women, without medical supervision.
When the manufacturer changed its application, the agency declined to make a decision by the legal deadline, last January. Proponents of OTC sales of Plan B objected, citing the agency’s own staff endorsement, and that of an independent panel.
In a series of interactions between the Senate and the administration in early July, the U.S. secretary of health and human services promised a Sept. 1 decision on Barr’s application. Media reports related that announcement to the July 18 Senate confirmation of Dr. Lester Crawford to head the Food and Drug Administration.
Both sides of the controversy met with some surprises in the BMJ and JAMA reports of research on the effects of Plan B access. Opponents of OTC availability in the United States have predicted that such access might increase unprotected sex, especially in young women and girls. Advocates have predicted that it would reduce the number of unintended pregnancies and abortions.
Public health impact may be negligible”
The report in the British Medical Journal concluded, “Making emergency hormonal contraception available over the counter does not seem to have led to an increase in its use, to an increase in unprotected sex, or to a decrease in the use of more reliable methods of contraception.” The study used an Omnibus Survey of 7,600 adults (an annual multipurpose survey in Great Britain) to examine contraception use after OTC emergency contraception was legalized in 2001. Women aged 16 to 49 were surveyed.
Although the lack of any increase in use of emergency hormone contraception suggests that the predicted rise in unsafe sex has been overstated, so too have the predicted effects on unwanted pregnancy, the authors observed.Similarly, the January JAMA report of a US study found: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method.”
Barr requests OTC access of Plan B for women 16 and older. Prescriptions would still be required for younger women.
Plan B is often called the “morning-after pill,” but in fact the method can be used any time after intercourse for up to 72 hours. It is best used as soon as possible, but can even be used after 72 hours, although at reduced efficacy.
Kuyoh MA, Toroitich-Ruto C, Grimes DA, Schulz KF, Gallo MF. Sponge versus diaphragm for contraception: a Cochrane review. Contraception. 2003;67:15–18.
When the Today sponge was pulled off the market by its maker in 1995, the popular Jerry Seinfeld TV show aired an episode showing the character Elaine dashing from store to store, in search of the last few sponges on the shelves. She ultimately found a neighborhood pharmacy with 1 remaining case of 60 sponges. “Just give me the whole case and I’ll be on my way,” she tells the pharmacist. In the show, Elaine used the term “sponge-worthy” to characterize a potential date, and the term became a household word overnight. When it was available in the United States, the Today sponge was the most widely used form of OTC contraception, selling approximately 250 million sponges.
The sponge, which contains the spermicide nonoxynol-9, was sold from 1983 to 1995, when it was voluntarily withdrawn for safety reasons. The FDA found that water at the manufacturing plant was contaminated, and American Home Products, (now Wyeth), decided it was too costly to upgrade its plant, and voluntarily withdrew the Today sponge from the market. Allendale Pharmaceuticals bought the rights for the sponge in 1998, and has been working to secure approval for marketing. The new version has been available in Canada since 2003. According to the manufacturer, it should be in US drug stores sometime this fall, for about $2.50 to $3 per sponge.
The sponge is best compared to a diaphragm. Its failure rate is frequently quoted as approximately 9% to 11% per year, very comparable to condoms.
Efficacy. However, in a Cochrane review of sponges versus the diaphragm, the sponge failure rate was 17.4% in a US trial and 24.5% in a British trial. The rates for a diaphragm were 12.8% and 10.9%, respectively. Unlike a diaphragm, the sponge does not have to be fitted and had equal efficacy in multiparous as nulliparous women, in the 2 studies reviewed. The main advantage of the sponge over a diaphragm is its ready availability over-the-counter.
According the manufacturer, the sponge can be inserted up to 24 hours prior to intercourse, and provides continuous protection throughout that period, for as many acts of intercourse as desired. Although it is not necessary to keep the sponge inserted for a full 24 hours, it must be left in place for 6 hours after the last act of intercourse. It should not be worn for more than 30 consecutive hours. The women in the 2 studies included in the Cochrane review, however, left the sponge in place for 48 hours.
Same-day start-up for OCs improves compliance
Westhoff C, Kerns J, Morroni C, Cushman LF, Tiezzi L, Murphy PA. Quick Start: a novel oral contraceptive initiation method. Contraception. 2002;66:141–145.
Many women never start the oral contraceptive we prescribe. They have to wait for their next menses, and they may fail to fill the prescription; 25% of study participants never start their oral contraceptives.
Quick Start starts the contraceptive in the clinic, after a negative pregnancy test, regardless of the day of the patient’s cycle. She is given at least 1 pack so she does not have to go to the drug store to fill the first prescription. Emergency contraception is given to patients who may need it. The continuation rate to the second cycle was higher in women beginning the pill in the clinic than those who started at any point in their cycle but waited to fill the prescription.
The original oral contraceptives were started on the first Sunday of a menstrual cycle so that it was known the woman was not pregnant and so she would bleed midweek. Waiting until menses assured a possible pregnancy was not exposed to hormonal contraceptives; however, the components of oral contraceptives are not teratogenic. The contraceptive can therefore be started at anytime during the cycle and starting in the clinic increases compliance. A pregnancy test can be performed if the anticipated menses at the end of the pill pack does not occur.
Disclosure
The author is on the speakers bureaus for Barr, Berlex, and Wyeth-Ayerst.
Since it was approved more than 10 years ago, depot medroxyprogesterone acetate (DMPA; Depo-Provera) has gained popularity among US women, largely because it requires minimal user participation and has a failure rate of only 0.3% per year.1,2 The main limitation, from the patient’s point of view, has been the intramuscular (IM) route of injection, which requires an office visit every 12 to 14 weeks for administration.
Now a subcutaneous version of the drug is available (Depo-subQ Provera 104) that delivers a lower dose of medroxyprogesterone acetate (MPA) (104 mg versus 150 mg for the IM formulation). The subcutaneous route opens the possibility for home self-injections, and the lower dose could decrease suppression of pituitary function and ovarian estradiol production, though further study is needed.
This article reviews the indications, benefits, risks, and potential adverse reactions of subcutaneous DMPA, a pharmacologically unique formulation with 16% weight/volume and a final dose of 104 mg MPA/0.65 mL. The dose was selected after study showed 100 mg to be the lowest dose to effectively suppress ovulation for at least 91 days.
The formulation and composition of subcutaneous DMPA cannot be duplicated by diluting the original IM formulation.
A potent contraceptive
Tw o large open-label, phase 3 studies assessed the 1-year efficacy, safety, and patient satisfaction of subcutaneous DMPA.3 These studies, conducted in North and South America, Europe, and Asia, reported zero pregnancies in 16,023 women-cycles of exposure.
Women in these studies had a broad range of body weights, ranging from 86 to 364 lb in the Americas and 77 to 249 lb in the European/Asian trial. The absence of pregnancies across all categories of body mass index (BMI) suggests that no dosage adjustments are necessary for higher BMIs.
Noncontraceptive benefits
Besides the high efficacy and long duration, which free women from daily attention to contraception, DMPA protects against endometrial cancer. The fact that it contains no estrogen makes it suitable for women who cannot or will not take estrogen products. It also is safe for breastfeeding mothers.
Perhaps most important is its ameliorative effect on endometriosis-associated pain.
Adverse effects
Many women stop using DMPA during the first year due to problems with irregular uterine bleeding, such as spotting and prolonged bleeding, which are especially common during the first 3 months of use. However, this problem usually diminishes over time, with most users becoming amenorrheic. This is true of both IM and subcutaneous DMPA. In a study of the latter, amenorrhea increased from 26% during month 3 to 55% during month 12.
The bleeding abnormalities associated with progestin-only contraceptives are not fully understood. We do know that suppression of circulating estradiol and the potent effect of MPA on the endometrium lead to varying degrees of endometrial disruption and atrophy, which ultimately manifest as irregular bleeding and amenorrhea. Subcutaneous DMPA likely involves the same processes, even though it contains 30% less MPA than the IM formulation.
Importance of counseling about bleeding effects
Two studies have shown that women are more likely to continue DMPA if they are counseled about bleeding effects when they start the medication.4,5 Since many patients would prefer less frequent or no menses, they may be encouraged by the prospect of becoming amenorrheic.
Risk of breast cancer
It will be several years before the effect of the lower-dose MPA on breast cancer risk is known.
DMPA and bone loss: Should we worry?
Subcutaneous DMPA, like its IM counterpart, is associated with changes in bone mineral density and carries a “black box” warning regarding this risk.6 Because DMPA suppresses circulating estradiol levels, it causes reductions in bone mineral density (BMD) that have aroused concern among the lay and medical media, although studies suggest BMD levels generally change little and recover when the drug is discontinued— except during perimenopause.
A metaanalysis of 12 studies involving 1,039 DMPA users (IM formulation) and 2,086 controls found that the average Z-score in DMPA users decreased less than 1 standard deviation, compared with nonusers.7 These BMD reductions stabilized after 3 to 4 years of DMPA use, and the bone loss was reversed when the drug was discontinued.8,9 Thus, it appears that, in time, BMD returns to levels similar to those in women who have never used the drug.
IM versus subcutaneous DMPA
In a comparison of both formulations of DMPA, both caused decreases in BMD at the end of 1 and 2 years of treatment.10 Women using subcutaneous DMPA experienced smaller decrements in total hip, lumbar spine, and femoral neck BMD after 1 and 2 years of treatment. However, these differences were significant only in the lumbar spine at 1 year.
Uncertain value for adolescents
DMPA should be carefully considered for use in adolescent girls—and this proviso includes the subcutaneous formulation.
Adolescence is a critical period for bone mineralization. Thus, any agent that limits bone accretion should be prescribed only after weighing all the other options.
A prospective cohort study in adolescents found a 3.1% decrease in BMD after 2 years of DMPA use, versus a 9.5% increase among nonusers.11 More recent reports indicate significant gains in BMD and reversal in bone loss once the drug is discontinued.12
What the “black box” warning means
Based in part on results from these studies, the Food and Drug Administration (FDA) and the drug’s manufacturer issued a black box warning for both the IM and subcutaneous formulations of DMPA. This step was taken to highlight the fact that users of DMPA may lose significant BMD, and that this loss may increase with duration of use and may not be entirely reversible.
The warning recommends that the drugs be used as long-term birth control only if other methods are inadequate. It emphasizes the general lack of certainty about the effect of these drugs on peak bone mass (when used in adolescence or early adulthood) and the risk of osteoporotic fracture (later in life).
How to counsel patients
I discuss the black box warning with each patient in the larger context of contraceptive counseling. The lower efficacy and other problems associated with daily birth control methods must be weighed against the risk of bone loss in both adolescents and adults.
It also is important to consider other risk factors for osteoporosis, such as chronic alcohol or tobacco use, eating disorders, or chronic use of corticosteroids. Adolescents who have poor eating habits or who use alcohol or tobacco may be at heightened risk of BMD loss.
Once a woman chooses DMPA, she should be encouraged to maintain a healthy lifestyle, including adequate calcium intake, weight-bearing and musclestrengthening exercises, smoking cessation, and moderate to no alcohol intake.
BMD measurements are not recommended since they do not predict fracture risk in premenopausal women.
Other side effects
Though rare, serious thrombotic events have been reported in women using the IM formulation.
Also rare are ocular disorders (loss of vision, proptosis, diplopia, or migraine) and ectopic pregnancy.
Other possible side effects include injection site reactions, decreased libido, acne, headache, fatigue, gastrointestinal disorders (distention, abdominal pain, diarrhea, nausea), infection, arthralgia, back pain, limb pain, dizziness, insomnia, anxiety, depression, breast pain and/or tenderness, and hot flushes.
Return to ovulation
DMPA is associated with a prolonged return to ovulation once it is discontinued. In a large US study of women who discontinued intramuscular DMPA to become pregnant, 68% conceived within 12 months, 83% conceived within 15 months, and 93% conceived within 18 months of the last injection, with a median time to conception of 10 months.13
Though no studies have determined the median time to conception for subcutaneous DMPA, it is likely to be similar to the 10-month interval seen with the IM formulation.
Comparing drugs head to head
The IM and subcutaneous formulations were compared prospectively at a single US center.14 The study defined return to ovulation as the first time serum progesterone levels reached at least 4.7 ng/mL. At the end of 12 months (postinjection), the cumulative rate of ovulation was 97.4% for subcutaneous DMPA and 94.7% for the IM formulation.
Ovulation occurred at a median of approximately 7 months (subcutaneous route) and 6 months (IM).
Early ovulation is possible
One subject in the subcutaneous DMPA group ovulated 14 weeks after her last injection. Thus, it is important to adhere to the recommended dosing schedule of 12 to 14 weeks.
Weight gain: 0 to 7.5 lb
Reports of weight gain with DMPA have been highly variable. Many women who discontinue hormonal contraceptives cite weight gain as the reason. With one third of US women meeting the criteria for obesity—a number that is likely to rise—and with ethnic variations, it is difficult to determine the exact impact of DMPA.
A well-designed, placebo-controlled trial by Pelkman and colleagues15 found DMPA to have no effect on resting energy expenditure, food intake, or body weight. Three large clinical trials of subcutaneous DMPA found a mean weight gain of 3.5 lb during the first year of use, and a small 2-year study comparing IM and subcutaneous DMPA found mean weight gains of 7.6 and 7.5 lb, respectively.
Combating endometriosis pain
With the FDA’s approval of subcutaneous DMPA for treatment of endometriosis-associated pain, the drug expands the pharmacologic choices for endometriosis pain relief for the first time in 15 years, with less frequent side effects than the other widely used drug, leuprolide acetate.
In an 18-month clinical trial comparing the 2 drugs, researchers found similar efficacy, with DMPA causing less bone loss and less frequent and severe menopausal symptoms. The trial involved 274 women and measured pain across the following categories: pelvic pain, dysmenorrhea, dyspareunia, pelvic tenderness, and induration.
Clearing a woman for use
The manufacturer recommends that all women undergo an annual history and physical examination. The physical exam should include a blood pressure check; examination of the breasts, abdomen, and pelvic organs; cervical cytology; and any relevant laboratory studies.
Overall outlook
Subcutaneous DMPA offers women the same advantages as the IM formulation. Since we have long-term experience with MPA as a contraceptive agent, we know it offers many noncontraceptive benefits, safety, and excellent contraceptive efficacy.
As we gain experience specific to subcutaneous DMPA , and as data accumulate from additional trials, we will be able to further define its role as a contraceptive option.
Disclosure
Dr. Jain has disclosed that he has received grant/research support from Ferring, Organon, Pfizer, Serono, and TA P.
REFERENCES
1. Westhoff C. Depot-medroxyprogesterone acetate injection (Depo-Provera®): a highly effective contraceptive option with proven long-term safety. Contraception. 2003;68:75-87.
2. Trussell J. Contraceptive efficacy. In: Hatcher RA, Trussell J, Stewart F, et al. Contraceptive Technology. 17th revised edition. New York: Irvington Publishers; 1998.
3. Jain JK, Jakimiuk AJ, Bode FR, et al. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70:269-275.
4. Canto De Cetina TE, Canto P, Luna MO. Effect of counseling to improve compliance in Mexican women receiving depo-medroxyprogesterone acetate. Contraception. 2001;63:143-146.
5. Lei ZW, Wu SC, Garceau RJ, et al. Effect of pretreatment counseling on discontinuation rates in Chinese women given depo-medroxyprogesterone acetate for contraception. Contraception. 1996;53:357-361.
6. Depo SubQ Provera 104 [package insert]. Cambridge, Mass: Pfizer; 2005.
7. Banks E, Berrington A, Casabonne D. Overview of the relationship between use of progestogen-only contraceptives and bone mineral density. Br J Obstet Gynecol. 2001;108:1214-1221.
8. Tang OS, Tang G, Yip PSF, et al. Further evaluation on long-term depot-medroxyprogesterone acetate use and bone mineral density: a longitudinal cohort study. Contraception. 2000;62:161-164.
9. Petitti DB, Piaggio G, Mehta S, et al. Steroid hormone contraception and bone mineral density: a cross-sectional study in an international population. Obstet Gynecol. 2000;95:736-744.
10. Jain JK. Evaluation of bone mineral density in women treated with DMPA-SC 104 or DMPA-IM 150. Presented at: Annual Meeting of the American Academy of Nurse Practitioners; June 17, 2005; Fort Lauderdale, Fla.
11. Cromer BA, Blair JM, Mahan JD, et al. A prospective comparison of bone density in adolescent girls receiving depot medroxyprogesterone acetate (DMPA), levonorgestrel (Norplant), or oral contraceptives. J Pediatr. 1996;129:671-676.
12. Scholes D, LaCroix AZ, Ichikawa LE, et al. Change in bone mineral density among adolescent women using and discontinuing depot medroxyprogesterone acetate contraception. Arch Pediatr Adolesc Med. 2005;159:139-144.
13. Depo-Provera [package insert]. Cambridge, Mass: Pfizer; 1999.
14. Jain JK, Dutton C, Nicosia A, et al. Pharmacokinetics, ovulation suppression and return to ovulation following a lower dose subcutaneous formulation of Depo-Provera®. Contraception. 2004;70:11-18.
15. Pelkman CL, Chow M, Heinbach RA, et al. Short-term effects of a progestational contraceptive drug on food intake, resting energy expenditure, and body weight in young women. Am J Clin Nutr. 2001;73:19-26.
CONTRACEPTIVES IN THE NEWS Plan B: Studies surprise both sides
Ian H. Thorneycroft
MD, PhD
OBG Management
Board of Editors
Professor of Obstetrics and Gynecology, University of South Alabama College of Medicine, Mobile, Ala
Marston C, Meltzer H, Majeed A. Impact on contraceptive practice of making emergency hormonal contraception available over the counter in Great Britain: repeated cross sectional surveys. BMJ doi;10.1136/bmj.38519.440266.8F (published July 11, 2005).
Raine TR, Harper CC, Rocca CH, Fischer R, Padian N, Klausner JD, Darney PD. Direct access to emergency contraception through pharmacies and effect of unintended pregnancy and STIs: a randomized controlled trial. JAMA. 2005;292:54–62.
Litt IF. Placing emergency contraception in the hands of women. JAMA. 2005;293:98–99.
A quick succession of events in July seemed to clear the path to a Food and Drug Administration (FDA) decision on over-the-counter sale of levonorgestrel (Plan B), the emergency contraceptive.
A week after the publication of the large study led by Marston, Plan B was placed on the FDA calendar for a Sept. 1 decision. The authors concluded that their study “supports the case for lifting the ban on over-the-counter sales of emergency hormonal contraception in the United States and other countries.”
Plan B has been a point of contention, especially after the FDA, in 2004, rejected Barr Laboratories’ application for OTC access, on the basis of lack of long-term safety data on its use in young adolescent women, without medical supervision.
When the manufacturer changed its application, the agency declined to make a decision by the legal deadline, last January. Proponents of OTC sales of Plan B objected, citing the agency’s own staff endorsement, and that of an independent panel.
In a series of interactions between the Senate and the administration in early July, the U.S. secretary of health and human services promised a Sept. 1 decision on Barr’s application. Media reports related that announcement to the July 18 Senate confirmation of Dr. Lester Crawford to head the Food and Drug Administration.
Both sides of the controversy met with some surprises in the BMJ and JAMA reports of research on the effects of Plan B access. Opponents of OTC availability in the United States have predicted that such access might increase unprotected sex, especially in young women and girls. Advocates have predicted that it would reduce the number of unintended pregnancies and abortions.
Public health impact may be negligible”
The report in the British Medical Journal concluded, “Making emergency hormonal contraception available over the counter does not seem to have led to an increase in its use, to an increase in unprotected sex, or to a decrease in the use of more reliable methods of contraception.” The study used an Omnibus Survey of 7,600 adults (an annual multipurpose survey in Great Britain) to examine contraception use after OTC emergency contraception was legalized in 2001. Women aged 16 to 49 were surveyed.
Although the lack of any increase in use of emergency hormone contraception suggests that the predicted rise in unsafe sex has been overstated, so too have the predicted effects on unwanted pregnancy, the authors observed.Similarly, the January JAMA report of a US study found: “While removing the requirement to go through pharmacists or clinics to obtain emergency contraception increases use, the public health impact may be negligible because of high rates of unprotected intercourse and relative underutilization of the method.”
Barr requests OTC access of Plan B for women 16 and older. Prescriptions would still be required for younger women.
Plan B is often called the “morning-after pill,” but in fact the method can be used any time after intercourse for up to 72 hours. It is best used as soon as possible, but can even be used after 72 hours, although at reduced efficacy.
Kuyoh MA, Toroitich-Ruto C, Grimes DA, Schulz KF, Gallo MF. Sponge versus diaphragm for contraception: a Cochrane review. Contraception. 2003;67:15–18.
When the Today sponge was pulled off the market by its maker in 1995, the popular Jerry Seinfeld TV show aired an episode showing the character Elaine dashing from store to store, in search of the last few sponges on the shelves. She ultimately found a neighborhood pharmacy with 1 remaining case of 60 sponges. “Just give me the whole case and I’ll be on my way,” she tells the pharmacist. In the show, Elaine used the term “sponge-worthy” to characterize a potential date, and the term became a household word overnight. When it was available in the United States, the Today sponge was the most widely used form of OTC contraception, selling approximately 250 million sponges.
The sponge, which contains the spermicide nonoxynol-9, was sold from 1983 to 1995, when it was voluntarily withdrawn for safety reasons. The FDA found that water at the manufacturing plant was contaminated, and American Home Products, (now Wyeth), decided it was too costly to upgrade its plant, and voluntarily withdrew the Today sponge from the market. Allendale Pharmaceuticals bought the rights for the sponge in 1998, and has been working to secure approval for marketing. The new version has been available in Canada since 2003. According to the manufacturer, it should be in US drug stores sometime this fall, for about $2.50 to $3 per sponge.
The sponge is best compared to a diaphragm. Its failure rate is frequently quoted as approximately 9% to 11% per year, very comparable to condoms.
Efficacy. However, in a Cochrane review of sponges versus the diaphragm, the sponge failure rate was 17.4% in a US trial and 24.5% in a British trial. The rates for a diaphragm were 12.8% and 10.9%, respectively. Unlike a diaphragm, the sponge does not have to be fitted and had equal efficacy in multiparous as nulliparous women, in the 2 studies reviewed. The main advantage of the sponge over a diaphragm is its ready availability over-the-counter.
According the manufacturer, the sponge can be inserted up to 24 hours prior to intercourse, and provides continuous protection throughout that period, for as many acts of intercourse as desired. Although it is not necessary to keep the sponge inserted for a full 24 hours, it must be left in place for 6 hours after the last act of intercourse. It should not be worn for more than 30 consecutive hours. The women in the 2 studies included in the Cochrane review, however, left the sponge in place for 48 hours.
Same-day start-up for OCs improves compliance
Westhoff C, Kerns J, Morroni C, Cushman LF, Tiezzi L, Murphy PA. Quick Start: a novel oral contraceptive initiation method. Contraception. 2002;66:141–145.
Many women never start the oral contraceptive we prescribe. They have to wait for their next menses, and they may fail to fill the prescription; 25% of study participants never start their oral contraceptives.
Quick Start starts the contraceptive in the clinic, after a negative pregnancy test, regardless of the day of the patient’s cycle. She is given at least 1 pack so she does not have to go to the drug store to fill the first prescription. Emergency contraception is given to patients who may need it. The continuation rate to the second cycle was higher in women beginning the pill in the clinic than those who started at any point in their cycle but waited to fill the prescription.
The original oral contraceptives were started on the first Sunday of a menstrual cycle so that it was known the woman was not pregnant and so she would bleed midweek. Waiting until menses assured a possible pregnancy was not exposed to hormonal contraceptives; however, the components of oral contraceptives are not teratogenic. The contraceptive can therefore be started at anytime during the cycle and starting in the clinic increases compliance. A pregnancy test can be performed if the anticipated menses at the end of the pill pack does not occur.
Disclosure
The author is on the speakers bureaus for Barr, Berlex, and Wyeth-Ayerst.