User login
An implantable device that stimulates the hypoglossal nerve during inspiration has been approved for treating a subgroup of adults with moderate to severe obstructive sleep apnea, the Food and Drug Administration has announced.
In a statement reviewing the approval, posted on the agency’s website on May 21, the FDA says that the device is indicated for adults, age 22 years and older, with moderate to severe OSA, "who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments," such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) machines, "and who do not have a complete concentric collapse (as seen during drug induced sleep endoscopy) at the soft palate level."
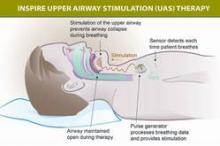
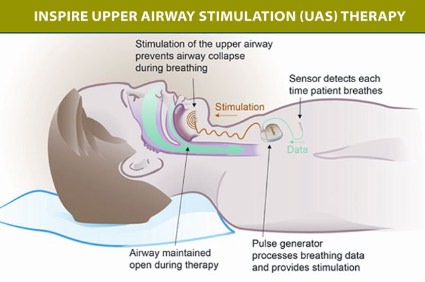
The components of the device include a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration-sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia. The pulse generator "detects the patient’s breathing pattern and maintains an open airway with mild stimulation of the hypoglossal nerve, which controls tongue movement, during inhaled breathing," according to the FDA statement. The stimulation settings are adjusted by physicians with an external programmer, and patients use a remote to turn the device on before they go to sleep and to turn it off when they wake up.
The device is manufactured by Inspire Medical Systems.
At a meeting in February 2014, the FDA’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, based on the results of the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, of 126 patients with moderate to severe OSA, which was published in January. Treatment with the device, which delivered unilateral stimulation of the hypoglossal nerve, "synchronous with ventilation, resulted in significant and clinically meaningful reductions in the severity of sleep apnea and self-reported sleepiness and improvement in quality of life measures at 1 year," the authors concluded (N. Engl. J. Med. 2014;370:139-49).
The device was approved on May 1. It will be commercially available during the second half of 2014, according to the manufacturer’s statement announcing the approval.
An implantable device that stimulates the hypoglossal nerve during inspiration has been approved for treating a subgroup of adults with moderate to severe obstructive sleep apnea, the Food and Drug Administration has announced.
In a statement reviewing the approval, posted on the agency’s website on May 21, the FDA says that the device is indicated for adults, age 22 years and older, with moderate to severe OSA, "who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments," such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) machines, "and who do not have a complete concentric collapse (as seen during drug induced sleep endoscopy) at the soft palate level."
The components of the device include a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration-sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia. The pulse generator "detects the patient’s breathing pattern and maintains an open airway with mild stimulation of the hypoglossal nerve, which controls tongue movement, during inhaled breathing," according to the FDA statement. The stimulation settings are adjusted by physicians with an external programmer, and patients use a remote to turn the device on before they go to sleep and to turn it off when they wake up.
The device is manufactured by Inspire Medical Systems.
At a meeting in February 2014, the FDA’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, based on the results of the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, of 126 patients with moderate to severe OSA, which was published in January. Treatment with the device, which delivered unilateral stimulation of the hypoglossal nerve, "synchronous with ventilation, resulted in significant and clinically meaningful reductions in the severity of sleep apnea and self-reported sleepiness and improvement in quality of life measures at 1 year," the authors concluded (N. Engl. J. Med. 2014;370:139-49).
The device was approved on May 1. It will be commercially available during the second half of 2014, according to the manufacturer’s statement announcing the approval.
An implantable device that stimulates the hypoglossal nerve during inspiration has been approved for treating a subgroup of adults with moderate to severe obstructive sleep apnea, the Food and Drug Administration has announced.
In a statement reviewing the approval, posted on the agency’s website on May 21, the FDA says that the device is indicated for adults, age 22 years and older, with moderate to severe OSA, "who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments," such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BPAP) machines, "and who do not have a complete concentric collapse (as seen during drug induced sleep endoscopy) at the soft palate level."
The components of the device include a stimulation lead placed around the distal hypoglossal nerve, a pulse generator implanted subcutaneously below the clavicle in the upper chest, and a respiration-sensing lead inserted between the fourth and fifth ribs – implanted under general anesthesia. The pulse generator "detects the patient’s breathing pattern and maintains an open airway with mild stimulation of the hypoglossal nerve, which controls tongue movement, during inhaled breathing," according to the FDA statement. The stimulation settings are adjusted by physicians with an external programmer, and patients use a remote to turn the device on before they go to sleep and to turn it off when they wake up.
The device is manufactured by Inspire Medical Systems.
At a meeting in February 2014, the FDA’s Anesthesiology and Respiratory Therapy Devices Panel supported approval of the Inspire Upper Airway Stimulation System, based on the results of the pivotal study, the STAR (Stimulation Therapy for Apnea Reduction) trial, of 126 patients with moderate to severe OSA, which was published in January. Treatment with the device, which delivered unilateral stimulation of the hypoglossal nerve, "synchronous with ventilation, resulted in significant and clinically meaningful reductions in the severity of sleep apnea and self-reported sleepiness and improvement in quality of life measures at 1 year," the authors concluded (N. Engl. J. Med. 2014;370:139-49).
The device was approved on May 1. It will be commercially available during the second half of 2014, according to the manufacturer’s statement announcing the approval.