User login
Placement of a left ventricular assist device (LVAD) after a heart attack has been found to suppress certain cellular signaling pathways that protect coronary tissue, but at the same time LVAD placement seemed to help normalize other protective properties in areas of the heart closest to the infarcted region, investigators reported in a recent study.
The findings could have implications in determining the best method for unloading and other medical therapies in the aftermath of a heart attack, Dr. Keshava Rajagopal of the University of Texas, Houston, and associates reported in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1332-41).
To study the effect of LVAD on cardiac tissue, the investigators induced myocardial infarction in sheep and then placed the animals on LVAD support for 2 weeks. After 10 more weeks of observation, the investigators harvested and analyzed the myocardial specimens. The principal goal of the study was to investigate how heart attack and subsequent short-term mechanical support of the left ventricle can influence signaling controlled by the protein beta-arrestin.
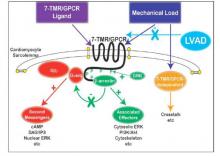
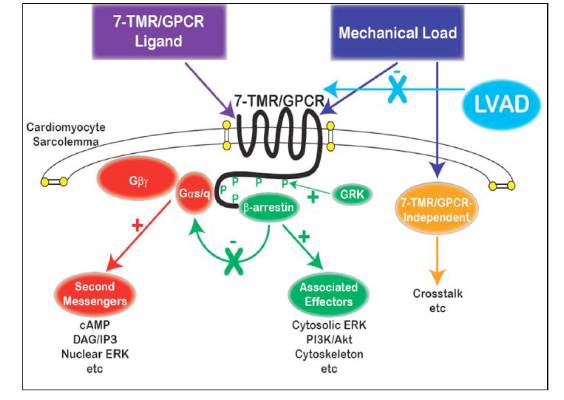
They found that an infarction of myocardial tissue caused activation of the beta-arrestin protein that regulates cellular pathways that can benefit cardiac cells. At the same time, LVAD support inhibited beta-arrestin activation, specifically in regulating pathways of two cardioprotective proteins: Akt, also called protein kinase B (PKB), and, to a lesser extent, ERK-1 and -2.
They also found that MI resulted in regional activation of load-induced signaling of cardiac G protein-coupled receptor (GPCR) via G proteins.
“These studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective beta-arrestin–mediated signal transduction, while normalizing the signaling networks of G-alpha-q–coupled cardiac GPCRS in the MI-adjacent zone,” Dr. Rajagopal and colleagues said.
They acknowledged that further studies are needed to better understand the roles that specific GPCRs in beta-arrestin–regulated signaling play in left ventricle dysfunction after a heart attack and to help define the optimal timing for LVAD based on signaling and genetic markers along with standard LV functional endpoints.
The authors had no disclosures.
The University of Maryland investigators in this study have joined the ranks of other investigators who have begun to unravel the consequences of mechanical unloading at the cellular level as well as its effect on the heart’s ability to handle calcium after infarction, Dr. William Hiesinger and Dr. Pavan Atluri of the University of Pennsylvania wrote in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1342-3).
“More broadly, these investigations are building the foundation of what will likely be the best platform for an efficacious bridge to recovery: multimodal therapy utilizing the titration of mechanical myocardial unloading,” they said. Dr. Hiesinger and Dr. Atluri commented on the limitations of the University of Maryland study, namely its small sample size and narrow scope. “This is, however, reflective more of the amazing complexity of the biologic and mechanical interactions between the heart and VAD and the need for further investigations of this kind than the quality of the research,” they said.
Understanding the molecular basis and metabolic function cardiac dysfunction after a heart attack is in the “nascent stages,” and even less is known about the effect ventricular loading has on these pathways, Dr. Hiesinger and Dr. Atluri said. “This study offers a concrete platform for both specific treatment and further study,” they wrote.
The University of Maryland investigators in this study have joined the ranks of other investigators who have begun to unravel the consequences of mechanical unloading at the cellular level as well as its effect on the heart’s ability to handle calcium after infarction, Dr. William Hiesinger and Dr. Pavan Atluri of the University of Pennsylvania wrote in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1342-3).
“More broadly, these investigations are building the foundation of what will likely be the best platform for an efficacious bridge to recovery: multimodal therapy utilizing the titration of mechanical myocardial unloading,” they said. Dr. Hiesinger and Dr. Atluri commented on the limitations of the University of Maryland study, namely its small sample size and narrow scope. “This is, however, reflective more of the amazing complexity of the biologic and mechanical interactions between the heart and VAD and the need for further investigations of this kind than the quality of the research,” they said.
Understanding the molecular basis and metabolic function cardiac dysfunction after a heart attack is in the “nascent stages,” and even less is known about the effect ventricular loading has on these pathways, Dr. Hiesinger and Dr. Atluri said. “This study offers a concrete platform for both specific treatment and further study,” they wrote.
The University of Maryland investigators in this study have joined the ranks of other investigators who have begun to unravel the consequences of mechanical unloading at the cellular level as well as its effect on the heart’s ability to handle calcium after infarction, Dr. William Hiesinger and Dr. Pavan Atluri of the University of Pennsylvania wrote in their invited commentary (J Thorac Cardiovasc Surg. 2015;150:1342-3).
“More broadly, these investigations are building the foundation of what will likely be the best platform for an efficacious bridge to recovery: multimodal therapy utilizing the titration of mechanical myocardial unloading,” they said. Dr. Hiesinger and Dr. Atluri commented on the limitations of the University of Maryland study, namely its small sample size and narrow scope. “This is, however, reflective more of the amazing complexity of the biologic and mechanical interactions between the heart and VAD and the need for further investigations of this kind than the quality of the research,” they said.
Understanding the molecular basis and metabolic function cardiac dysfunction after a heart attack is in the “nascent stages,” and even less is known about the effect ventricular loading has on these pathways, Dr. Hiesinger and Dr. Atluri said. “This study offers a concrete platform for both specific treatment and further study,” they wrote.
Placement of a left ventricular assist device (LVAD) after a heart attack has been found to suppress certain cellular signaling pathways that protect coronary tissue, but at the same time LVAD placement seemed to help normalize other protective properties in areas of the heart closest to the infarcted region, investigators reported in a recent study.
The findings could have implications in determining the best method for unloading and other medical therapies in the aftermath of a heart attack, Dr. Keshava Rajagopal of the University of Texas, Houston, and associates reported in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1332-41).
To study the effect of LVAD on cardiac tissue, the investigators induced myocardial infarction in sheep and then placed the animals on LVAD support for 2 weeks. After 10 more weeks of observation, the investigators harvested and analyzed the myocardial specimens. The principal goal of the study was to investigate how heart attack and subsequent short-term mechanical support of the left ventricle can influence signaling controlled by the protein beta-arrestin.
They found that an infarction of myocardial tissue caused activation of the beta-arrestin protein that regulates cellular pathways that can benefit cardiac cells. At the same time, LVAD support inhibited beta-arrestin activation, specifically in regulating pathways of two cardioprotective proteins: Akt, also called protein kinase B (PKB), and, to a lesser extent, ERK-1 and -2.
They also found that MI resulted in regional activation of load-induced signaling of cardiac G protein-coupled receptor (GPCR) via G proteins.
“These studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective beta-arrestin–mediated signal transduction, while normalizing the signaling networks of G-alpha-q–coupled cardiac GPCRS in the MI-adjacent zone,” Dr. Rajagopal and colleagues said.
They acknowledged that further studies are needed to better understand the roles that specific GPCRs in beta-arrestin–regulated signaling play in left ventricle dysfunction after a heart attack and to help define the optimal timing for LVAD based on signaling and genetic markers along with standard LV functional endpoints.
The authors had no disclosures.
Placement of a left ventricular assist device (LVAD) after a heart attack has been found to suppress certain cellular signaling pathways that protect coronary tissue, but at the same time LVAD placement seemed to help normalize other protective properties in areas of the heart closest to the infarcted region, investigators reported in a recent study.
The findings could have implications in determining the best method for unloading and other medical therapies in the aftermath of a heart attack, Dr. Keshava Rajagopal of the University of Texas, Houston, and associates reported in the November issue of the Journal of Thoracic and Cardiovascular Surgery (2015;150:1332-41).
To study the effect of LVAD on cardiac tissue, the investigators induced myocardial infarction in sheep and then placed the animals on LVAD support for 2 weeks. After 10 more weeks of observation, the investigators harvested and analyzed the myocardial specimens. The principal goal of the study was to investigate how heart attack and subsequent short-term mechanical support of the left ventricle can influence signaling controlled by the protein beta-arrestin.
They found that an infarction of myocardial tissue caused activation of the beta-arrestin protein that regulates cellular pathways that can benefit cardiac cells. At the same time, LVAD support inhibited beta-arrestin activation, specifically in regulating pathways of two cardioprotective proteins: Akt, also called protein kinase B (PKB), and, to a lesser extent, ERK-1 and -2.
They also found that MI resulted in regional activation of load-induced signaling of cardiac G protein-coupled receptor (GPCR) via G proteins.
“These studies demonstrate that small platform catheter-based LVAD support exerts suppressive effects on cardioprotective beta-arrestin–mediated signal transduction, while normalizing the signaling networks of G-alpha-q–coupled cardiac GPCRS in the MI-adjacent zone,” Dr. Rajagopal and colleagues said.
They acknowledged that further studies are needed to better understand the roles that specific GPCRs in beta-arrestin–regulated signaling play in left ventricle dysfunction after a heart attack and to help define the optimal timing for LVAD based on signaling and genetic markers along with standard LV functional endpoints.
The authors had no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Left ventricular assist device (LVAD) support inhibits pathologic responses to mechanical loading but also can inhibit adaptive responses after myocardial infarction.
Major finding: LVAD support inhibited cardioprotective beta-arrestin–mediated signaling, but net benefits of normalization of load-induced G protein-coupled receptor (GPCR) signaling were observed in the MI-adjacent zone.
Data source: Sheep were induced with myocardial infarction and then placed on LVAD support for 2 weeks and observed for a total of 12 weeks. Then myocardial specimens were harvested and analyzed.
Disclosures: The study authors had no relationships to disclose.