User login
- 4 case studies
- Drug treatment based on T-scores and risk factors Reasonable options if T-score is borderline
- When is a follow-up in 1 year vital? When is a 2- or 3-year interval safe?
- BMD test techniques, sites, and T-scores
This question begs for a simple yes or no, but it is best answered by asking a second question, “Do I need to know my patient’s bone density to give her the best care possible at menopause?” If the answer is yes, then bone density testing is a must, because there is no other way to know what her bone density actually is.
How, then, does this knowledge affect clinical decision-making?
Our concern, of course, is whether we need to intervene pharmacologically to preserve the strength of the skeleton. Even though bone mineral density (BMD) does not completely account for bone strength, it does determine some 60% to 80% of bone strength, and it is still the best predictor of an initial fracture.
Of immediate concern to the physician caring for a woman entering the postmenopausal period is whether she has sufficient bone mass to withstand the bone loss that estrogen deficiency will impose—without developing a dangerously fragile skeletal structure.
Women start losing bone mass years before menopause. While she is still in her mid-40s, a woman’s spinal bone density begins to diminish due to accumulating dietary calcium deficiency, declining physical activity, and declining estradiol levels. (Unless menopause occurs earlier for any reason, however, bone density in the spine is thought to remain relatively stable from the time peak bone mass is attained, before age 30 in most skeletal sites,1 until the mid-40s.) The exact age at which the proximal femur begins to lose bone is more controversial. Cross-sectional studies have suggested that bone loss may in fact begin in a woman’s 20s, almost immediately after reaching peak bone mass. Others have suggested that bone loss does not begin until later, in her 30s.2
A variety of risk factors are modifiable, but one that we cannot modify—genetics— may play the predominant role in determining peak bone mass. Other factors include nutrition, physical activity, intervening illnesses, medications, and lifestyle factors like smoking and alcohol use.
Expect bone loss with any cause of estrogen decline
Postmenopausal bone loss is inexorable in the absence of estrogen replacement, as well as after stopping estrogen replacement therapy (ERT) or hormone replacement therapy (HRT). If your patient stops ERT or HRT, from a skeletal perspective she has just become postmenopausal again. By measuring her bone density, you can ascertain whether bone loss— which will certainly occur—will further deplete bone mass that is already less than ideal. If so, immediate intervention to prevent bone loss is appropriate.
One key longitudinal study,3 for example, found that perimenopausal women lost an average of 2.3% per year from the spine; postmenopausal women, 0.5%. The authors observed these losses in peri- and postmenopausal women, assessed over an average of 27 months. (Women were classified as perimenopausal if they became postmenopausal during the study.)
Calcium intake of 1,000 mg/day or more does not stop bone loss
In a study designed to evaluate the effectiveness of alendronate compared with placebo in preventing bone loss in women within 3 years of menopause, McClung et al4 found a 3% to 4% bone loss at the end of 3 years in the placebo group, despite total calcium intakes of 1,000 mg per day or more.
Stopping HT merits equal concern
Estrogen deficiency precipitated by stopping hormone therapy is due the same concern as that created by menopause itself. Although the exact rates vary in studies, it is clear that bone loss begins when ERT or HRT stops, just as it does with onset of menopause. Hysterectomized postmenopausal women who received ERT for 2 years were found to have a 4.5% decline in posterior-anterior (PA) lumbar spine bone density and a 1.2% decline in total hip bone density only 1 year after estrogen withdrawal.5 This loss occurred despite calcium supplementation.
Trémollieres et al found a 1.64% per year loss of bone density from the spine for the first 2 years after discontinuing HRT, which was similar to that seen in estrogen-deficient women for the first 2 years immediately after menopause.6 In the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, women who stopped HRT after 3 years lost bone density at an annual rate of 1.04% from the spine and 1.01% from the hip during 4 years of follow-up.7
A conservative assessment of the rate of bone loss in the first few years after menopause or cessation of hormone therapy is about 1% per year from the spine and proximal femur. At first glance, 1% per year does not appear worrisome. But within 10 years of menopause, at or about the age of only 60, 10% of the bone mass that was present at menopause is gone. In 15 years, at least 15% is gone because of estrogen deficiency.
Unquestionably, many women have stopped ERT or HRT or are choosing not to begin, due to media attention on negative findings from trials such as the combined-continuous HRT arm of the Wo men’s Health Initiative (WHI) and the Heart and Estrogen Replacement Study (HERS-I). Reviews of the National Prescription Audit database and National Disease and Therapeutic Index database confirmed a subsequent marked drop in prescriptions for ERT or HRT,8 despite WHI findings showing that combined-continuous HRT significantly reduces the risk of spine and hip fracture.9
Anne:Onset of hot flashes is a “teachable moment”
“Anne,” a 53-year-old Caucasian woman, has come to see you because of hot flashes that have begun to trouble her since her menstrual periods stopped 8 months ago.
Although she knows that estrogen replacement would help relieve her hot flashes, she is uncertain whether to use it, having heard negative media reports about WHI findings. She has no family or personal history of breast cancer, but is very frightened at even the slightest possibility of increasing her personal risk for breast cancer. She is 5’5” tall and weighs 120 lb. She broke her right wrist in a fall at age 46.
Don’t miss this opportunity!
Though Anne’s visit was prompted by distress over hot flashes, night sweats, and related symptoms of sleep disruption, daytime fatigue, mental lapses, and irritability, it’s a “teachable moment” to discuss osteoporosis prevention and testing. As is typical, her primary desire is relief from hot flashes, yet bone loss is a more serious threat.
If long-term inter vention starts early, bone loss and osteoporosis are preventable; in that context, onset of hot flashes can be seen as a positive force, since they prompted her to seek medical help.
Beth:Concerned because of her mother’s hip fracture
Occasionally a patient will raise the issue of osteoporosis herself. “Beth” is a 49-year-old woman who reports that her last menstrual period 3 months ago was very light in comparison to what she considers normal. Her periods have become irregular over the last year, initially being about 21 days apart, but now 10 to 12 weeks apart. She says she may have noticed an occasional hot flash, but it was not troublesome. She is concerned about the menstrual irregularity and wonders if she is close to menopause.
While she is not psychologically troubled about cessation of menstrual periods, she is concerned about potential bone loss due to estrogen deficiency. With additional questioning, you discover that her mother had a hip fracture.
Carol:Believes her risk low and refuses BMD test
“Carol,” on the other hand, says she doesn’t need bone density testing, because she is not interested in taking any medication to prevent or treat osteoporosis.
If Carol truly will not consider preventive medications, then bone density testing is certainly not indicated. The few patients who refuse to consider medications or testing tend to think their risk is slight. Careful questioning often elicits this belief. They may exercise, avoid cigarette smoke, and consume more than adequate amounts of calcium supplements or dairy products.
Unfortunately, such admirable habits in no way prevent estrogen-deficient bone loss.
Genetically determined low BMD
And no woman can overcome the effects of a genetically determined lower-than-average peak bone density, which may exist without the patient’s knowledge. Without a bone density test, the patient is making an uninformed decision and it is from this perspective that this situation is best approached. Her decision should always be respected, but it is our responsibility to insure that it is an informed decision.
Drug intervention based on T-score
By measuring the bone density at menopause, we can determine if pharmacologic intervention to prevent bone loss needs to start immediately. According to the National Osteoporosis Foundation (NOF) and the American Association of Clinical Endocrinologists (AACE) guidelines, if a woman’s T-score is below -1.5 and she has even 1 other risk factor, pharmacologic intervention is warranted.12,13
This level of bone density is clearly above the threshold for a diagnosis of osteoporosis based on the WHO criteria. Nevertheless, this patient’s estrogen deficiency will further deplete her already lower-than-normal bone density, and could be rapidly devastating. Knowledge of her T-score gives us potential to prevent fractures, now that we have drugs to prevent such devastation.
Three guidelines (TABLE)12-14 recommend pharmacologic intervention if the T-score is -2.5 or lower, and these guidelines differ only in the intervention threshold that also requires an additional risk factor. Note that all 3 recommend pharmacologic intervention when there is only a single risk factor in addition to a bone density level that would not be considered osteoporotic by WHO criteria.
The Food and Drug Administration (FDA) has approved drugs for prevention or treatment of postmenopausal osteoporosis, or both, based on whether data demonstrate that a drug:
- inhibits or stops bone loss, for the prevention indication, or
- reduces fracture risk, for the treatment indication.
The FDA-approved dosages of nonestrogen agents may vary by indication (TABLE). In clinical practice, however, the distinction between prevention and treatment is often less clear, leaving the dosage to the judgment of the clinician.
The complete list of clinical risk factors to consider in initiating therapy based on the T-score is lengthy; furthermore, an ever-increasing number of medications and diseases are now known to contribute to bone loss. The 5 major risk factors listed in the margin below are some of the most important to consider along with the T-score.
TABLE
Drug intervention is appropriate when there are…
| NO RISK FACTORS AND A T-SCORE: | RISK FACTORS AND A T-SCORE: | |
|---|---|---|
| National Osteoporosis Foundation | Below -2.0 | Below -1.5 |
| American Association of Clinical Endocrinologists | At or below -2.5 | -1.5 or poorer |
| North American Menopause Society | Below -2.5 | -2.0 or poorer |
| FDA-approved agents for prevention and treatment of postmenopausal osteoporosis* | ||
| Alendronate | ||
| Prevention | 5 mg po qd or 35 mg po qw | |
| Treatment | 10 mg po qd or 70 mg po qw | |
| Ibandronate† | 2.5 mg po qd | |
| Risedronate | 5 mg po qd or 35 mg po qw | |
| Raloxifene | 60 mg po qd | |
| *Unless otherwise noted, doses are the same for prevention or treatment | ||
| †Although FDA-approved, ibandronate is not currently marketed in the United States | ||
Follow-up testing intervals
Once your patient begins drug therapy, it is appropriate to follow up periodically with bone densitometry. The skeletal site measured at follow-up and the intervals between are dictated by reimbursement, as well as scientific issues. Many insurers, including Medicare, reimburse only once every 2 years.16 Exceptions are few.
From a scientific standpoint, BMD increases at the PA lumbar spine may be sufficiently great to be detected in only 1 year, with potent agents like the bisphosphonates or teriparatide. Since changes in PA lumbar spine density are generally less with raloxifene or salmon calcitonin, waiting 2 years to remeasure the PA lumbar spine is entirely appropriate here.
The PA lumbar spine is the preferred site for monitoring therapy because its higher percentage of trabecular bone generally results in a greater magnitude of change than at the proximal femur.
However, the slower rate of change at the proximal femur means that it need not be measured more often than 2 or even 3 years.
If your patient’s bone density is above the pharmacologic intervention threshold, it is always appropriate to counsel her on nonpharmacologic measures to preserve her skeleton: adequate dietary or supplemental calcium and vitamin D, regular weight-bearing or resistance exercise, and avoidance of cigarette smoke.
But we cannot assume that these are sufficient to protect her skeleton. Follow-up bone density studies are recommended to identify women who will lose bone despite these measures, and for whom pharmacologic intervention is warranted. AACE and the North American Menopause Society (NAMS) recommend follow-up bone density studies every 3 to 5 years in postmenopausal women in whom pharmacologic intervention is not deemed immediately necessary.13,14
A more specific approach based on the patient’s lowest T-score at either the PA lumbar spine or femoral neck has been suggested.17 If her T-score is greater than 0, a repeat study is suggested in 5 years. If however, the T-score is 0 to -0.5, a repeat study is suggested in 3 years. A repeat study should be done in only 1 year if the T-score is -0.5 to -1.
This approach assumes that you wish to know when the patient’s T-score might fall below the normal range established by the WHO, that is, below a T-score of -1, and assumes a rate of bone loss of approximately 0.5 SD (or 0.5 T-score units) per year. If you know you would not intervene until the T-score reaches -1.5 or -2, you can adjust the interval accordingly.
Precision in bone density testing is integral to accurate drug therapy monitoring. When properly performed, dual energy x-ray absorptiometry (DXA) bone density measurements are highly, but not perfectly, reproducible. To reflect actual biologic changes, a measured change i n BMD must have sufficient magnitude.
In general, for measurements at the PA lumbar spine or total hip, a change of 2.77% is needed for 95% confidence. The bone densitometry testing facility should provide the clinician with the exact magnitude of the change necessary for a given level of statistical confidence.18 It is also important to remember that while increases in BMD are desirable and reassuring, no loss of BMD may also be considered efficacious.
The guidelines
The NOF, AACE, the American College of Obstetricians and Gynecologists (ACOG), NAMS and the United States Preventive Services Task Force (USPSTF) guidelines12-14,19,20 agree that all postmenopausal women age 65 and older should have bone density testing. With the exception of the USPSTF, they also agree that all postmenopausal women under age 65 with risk factors should be tested. (The USPSTF limits this recommendation to women age 60 to 64.)
In reality, all postmenopausal women should have bone density testing because the list of risk factors is so comprehensive that it is unusual to find a woman who does not have at least 1 risk factor for osteoporosis.
Anne:Test again in 1 year
With this information in mind, let’s again consider Anne, the 53-year-old woman who sought help for hot flashes. Her visit was an opportunity to discuss osteoporosis prevention. Of the major risk factors, Anne has 2: weight less than 127 lb and a fracture after age 40. Based on the recommendations from the NOF, AACE, ACOG, and NAMS, bone density testing is appropriate.
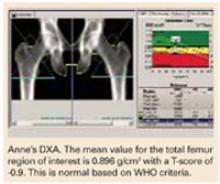
A DXA study of both proximal femurs shows bone density data for each femur individually as well as the mean BMD value for each region of interest for both femurs. There are 5 regions of interest in the proximal femur: the total hip (or total femur), the femoral neck, Wa rd’s area, the trochanter, and the shaft. The total hip or femoral neck is preferred for diagnosis. Based on her normal T-scores, Anne does not meet any of the pharmacologic intervention guidelines. She should nevertheless be counseled on nonpharmacologic interventions to prevent bone loss.
She should have another bone density study in 1 year. Anne’s PA lumbar spine DXA study is not shown, but it provided no additional information. The PA lumbar spine would be the preferred site for follow-up in 1 year, however.
The recommendation for follow-up in 1 year would not change even if you elected to begin low-dose combined-continuous HRT for relief of hot flashes. Although HRT would be expected to preserve her skeleton, follow-up testing in 1 year for confirmation is appropriate.21
Beth:Treat now, test in 1 yr
Beth has an important risk factor: her mother’s hip fracture. This raises the possibility that she is genetically predisposed to lowerthan-average peak bone density. She meets NOF, AACE, NAMS, and ACOG guidelines for bone density testing.
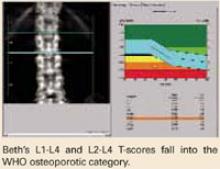
At the PA lumbar spine, it is preferable to use either the L1-L4 BMD or the L2-L4 BMD and the corresponding T-score. In either case, Beth’s T-score is disturbingly low at -3.7 and -3.6, respectively. Either T-score meets the diagnosis of osteoporosis based on WHO criteria.
This single bone density study does not reveal whether she has lost bone density from a previously higher level or whether her current bone density represents her peak bone density. It is incumbent on the physician to evaluate her medically to exclude possible causes of bone loss other than estrogen deficiency, which might require a different or additional therapy.
Beth certainly meets NOF, AACE, and NAMS guidelines for drug intervention.
A follow-up PA lumbar spine DXA study is indicated in 1 year. Although she has osteoporosis, she has not yet had a fracture. For now, her diagnosis is nothing more than a test result. An osteoporotic fracture will change that. Immediate intervention with drug therapy can preserve her skeletal mass and her quality of life.
Donna:Borderline T-scores
Treatment decisions are not always as clear as in the cases of Anne, Beth, and Carol. Consider Donna, age 54, who is 2 years postmenopausal and in good health. However, her mother reportedly had a dowager’s hump at the time of her death. Although Donna was never told that her mother had osteoporosis, you suspect that she did because of the kyphosis. Donna is fairly sedentary, thin, and continues to smoke. She is not using HRT and rarely takes nutritional supplements of any kind. L 1 - L 4 PA lumbar spine T-score is -1.4; total hip is -1.3.
The dilemma is that Donna does not meet any guideline for pharmacologic intervention based on T-score, even in the presence of risk factors. Both T-scores are just above the NOF and AACE cutoff points, even in the presence of risk factors.
But the guidelines are not hard and fast rules. T- score cutoff points, with or without other risk factors, were chosen to balance the potential benefit and any potential harm of pharmacologic therapy with the risk of fracture if untreated. So, while it may seem arbitrary to recommend treatment when the T- score is -1.5 with risk factors, yet not if the T-score is -1.4 or -1.3, there is a substantive rationale behind the recommendation. Still, there is no substitute for your judgment.
What is a reasonable course?
She has 6 risk factors for bone loss and osteoporosis: estrogen deficiency, current smoking, probable family history, thinness, sedentary lifestyle, and probable calcium deficiency. Every attempt to modify the risk factors that can be modified is worth the effort—smoking cessation, exercise, and calcium and vitamin D supplementation would benefit her skeleton.
Important: Test again in 1 year. It is extremely important to repeat bone density testing at the lumbar spine in 1 year. If the nonpharmacologic interventions you recommend prove insufficient to radically slow the anticipated bone loss, she will fall below a T-score of -1.5 in the next year.
On the other hand, if she demonstrates that she can maintain her bone density with nonpharmacologic measures, a prescription may not be warranted. It would not be unreasonable to allow her this 1 year, because at her relatively young age of 54, at this bone density, her short-term risk of fracture is actually quite low.
“Yes” to both questions
If bone density is low—particularly if it is low and a woman has risk factors for osteoporosis—pharmacologic intervention can be reasonably expected to prevent the devastating consequences of osteoporosis. The question, “Does this menopausal woman need pharmacologic intervention to prevent or treat osteoporosis now, or might she need it later?” can be answered by measuring bone density. It is a question we would be remiss not to ask. Bone density measurement, preferably at the PA lumbar spine and proximal femur by DXA, is the only way to answer this all-important question. To provide the best care possible for a woman who has just become menopausal, you do need to know her bone density. The simple answer to both original questions then, is yes.
Techniques and sites
Bone densitometry can be performed using any of several techniques: dual energy x-ray absorptiometry (DXA), quantitative computerized tomography (QCT), radiographic absorptiometry (RA), or quantitative ultrasound (QUS).
Similarly, bone densitometry can be performed at a myriad of skeletal sites such as the PA lumbar spine, lateral lumbar spine, proximal femur, forearm, phalanges, calcaneus, and total body.
Guidelines are based on PA lumbar or proximal femur by DXA. It is correct that virtually all sites, measured by any technique, predict an individual’s fracture risk, but guidelines for diagnosis of osteoporosis and pharmacologic intervention to prevent or treat osteoporosis are overwhelmingly based on measurements of the PA lumbar spine or proximal femur by DXA.10-14 This is not because of any inadequacy or inaccuracy of the other technologies at these or other skeletal sites. It is because of the use of the World Health Organization (WHO) criteria for diagnosis of osteoporosis and the reliance upon the T-score in intervention guidelines.
WHO diagnosis based on T-score
| DIAGNOSTIC CATEGORY | T-SCORE CRITERIA |
|---|---|
| Normal | -1 or better |
| Osteopenia (low bone mass) | Between -1 and -2.5 |
| Osteoporosis | -2.5 or poorer |
| Severe osteoporosis | -2.5 or poorer, with a fragility fracture |
In its sentinel 1994 guidelines, the WHO defined osteoporosis as a bone density of 2.5 standard deviations (SD) or more below the average bone density for a young adult.15 This threshold was chosen in an attempt to reconcile the prevalence of the disease created by the threshold and the observed lifetime fracture risks. The data used to reach this conclusion were largely based on single-photon absorptiometry (SPA) data from the mid-radius, dual-photon absorptiometry (DXA’s predecessor) and DXA data from the PA lumbar spine and proximal femur.
The WHO warned that applying these criteria in persons measured by other technologies or at other skeletal sites could result in a different diagnostic category. When physicians did apply the criteria in clinical practice, WHO’s prediction became a reality that was quickly recognized and discussed in the literature.
It became clear that we could not apply the WHO criteria to all technologies and all skeletal sites.
Consequentially, major osteoporosis-related medical organizations issued guidelines calling for restricting the diagnosis of osteoporosis based on the WHO criteria to bone density studies performed at the PA lumbar spine and proximal femur using DXA.
T-score means above or below “average”
The T- score on modern bone density reports, although not a technically correct use of the term, indicates your patient’s number of SDs above or below that of the average value for a young adult. If your patient’s BMD is below the average value for a young adult, a minus sign is placed in front of the T- score. The young-adult average value is always assigned a T- score value of 0. For example, a BMD that is 2.2 SD below the average value for a young adult of the same sex would be assigned a T- score of -2.2. Because the WHO defined osteoporosis based on the number of SDs below the average for a young adult, the WHO criteria readily translate to a T- score.
Dr. Bonnick reports research support from Merck and Roche/GSK; consultant fees from Merck, Roche/GSK, and Wyeth; and speaking fees from Merck, Roche/GSK, and Procter & Gamble.
1. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16:393S-399S.
2. Hui SL, Perkins AJ, Zhous L, et al. Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab. 2002;87:1539-1543.
3. Pouilles JM, Trémollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int. 1993;53:340-343.
4. McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med. 1998;128:253-261.
5. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
6. Trémollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int. 2001;12:385-390.
7. Greendale GA, Espeland M, Slone S, Marcus R, BarrettConnor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
8. Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trend response to recent evidence. JAMA. 2004;291:104-106.
9. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259.
11. Miller PD, Siris ES, Barrett-Connor E, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222-2230.
12. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: NOF; 2003.
13. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001edition, with selected updates for 2003. Endocr Pract. 2003;9:545-564.
14. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
15. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994.
16. Department of Health and Human Services. Health Care Finance Administration. Medicare program; Medicare coverage of and payment for bone mass measurements. 42 CFR Part 410, Federal Register 63:June 24, 1989.
17. Abrahamsen B, Nissen N, Hermann AP, et al. When should densitometry be repeated in healthy peri- and postmenopausal women: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2002;17:2061-2067.
18. Bonnick SL, Johnston CC, Kleerekoper M, et al. The importance of precision in bone density measurements. J Clin Densitom. 2001;4:105-110.
19. American College of Obstetricians and Gynecologists. ACOG releases recommendations for bone density screening for osteoporosis. Washington, DC: ACOG; 2002.
20. US Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
21. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
- 4 case studies
- Drug treatment based on T-scores and risk factors Reasonable options if T-score is borderline
- When is a follow-up in 1 year vital? When is a 2- or 3-year interval safe?
- BMD test techniques, sites, and T-scores
This question begs for a simple yes or no, but it is best answered by asking a second question, “Do I need to know my patient’s bone density to give her the best care possible at menopause?” If the answer is yes, then bone density testing is a must, because there is no other way to know what her bone density actually is.
How, then, does this knowledge affect clinical decision-making?
Our concern, of course, is whether we need to intervene pharmacologically to preserve the strength of the skeleton. Even though bone mineral density (BMD) does not completely account for bone strength, it does determine some 60% to 80% of bone strength, and it is still the best predictor of an initial fracture.
Of immediate concern to the physician caring for a woman entering the postmenopausal period is whether she has sufficient bone mass to withstand the bone loss that estrogen deficiency will impose—without developing a dangerously fragile skeletal structure.
Women start losing bone mass years before menopause. While she is still in her mid-40s, a woman’s spinal bone density begins to diminish due to accumulating dietary calcium deficiency, declining physical activity, and declining estradiol levels. (Unless menopause occurs earlier for any reason, however, bone density in the spine is thought to remain relatively stable from the time peak bone mass is attained, before age 30 in most skeletal sites,1 until the mid-40s.) The exact age at which the proximal femur begins to lose bone is more controversial. Cross-sectional studies have suggested that bone loss may in fact begin in a woman’s 20s, almost immediately after reaching peak bone mass. Others have suggested that bone loss does not begin until later, in her 30s.2
A variety of risk factors are modifiable, but one that we cannot modify—genetics— may play the predominant role in determining peak bone mass. Other factors include nutrition, physical activity, intervening illnesses, medications, and lifestyle factors like smoking and alcohol use.
Expect bone loss with any cause of estrogen decline
Postmenopausal bone loss is inexorable in the absence of estrogen replacement, as well as after stopping estrogen replacement therapy (ERT) or hormone replacement therapy (HRT). If your patient stops ERT or HRT, from a skeletal perspective she has just become postmenopausal again. By measuring her bone density, you can ascertain whether bone loss— which will certainly occur—will further deplete bone mass that is already less than ideal. If so, immediate intervention to prevent bone loss is appropriate.
One key longitudinal study,3 for example, found that perimenopausal women lost an average of 2.3% per year from the spine; postmenopausal women, 0.5%. The authors observed these losses in peri- and postmenopausal women, assessed over an average of 27 months. (Women were classified as perimenopausal if they became postmenopausal during the study.)
Calcium intake of 1,000 mg/day or more does not stop bone loss
In a study designed to evaluate the effectiveness of alendronate compared with placebo in preventing bone loss in women within 3 years of menopause, McClung et al4 found a 3% to 4% bone loss at the end of 3 years in the placebo group, despite total calcium intakes of 1,000 mg per day or more.
Stopping HT merits equal concern
Estrogen deficiency precipitated by stopping hormone therapy is due the same concern as that created by menopause itself. Although the exact rates vary in studies, it is clear that bone loss begins when ERT or HRT stops, just as it does with onset of menopause. Hysterectomized postmenopausal women who received ERT for 2 years were found to have a 4.5% decline in posterior-anterior (PA) lumbar spine bone density and a 1.2% decline in total hip bone density only 1 year after estrogen withdrawal.5 This loss occurred despite calcium supplementation.
Trémollieres et al found a 1.64% per year loss of bone density from the spine for the first 2 years after discontinuing HRT, which was similar to that seen in estrogen-deficient women for the first 2 years immediately after menopause.6 In the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, women who stopped HRT after 3 years lost bone density at an annual rate of 1.04% from the spine and 1.01% from the hip during 4 years of follow-up.7
A conservative assessment of the rate of bone loss in the first few years after menopause or cessation of hormone therapy is about 1% per year from the spine and proximal femur. At first glance, 1% per year does not appear worrisome. But within 10 years of menopause, at or about the age of only 60, 10% of the bone mass that was present at menopause is gone. In 15 years, at least 15% is gone because of estrogen deficiency.
Unquestionably, many women have stopped ERT or HRT or are choosing not to begin, due to media attention on negative findings from trials such as the combined-continuous HRT arm of the Wo men’s Health Initiative (WHI) and the Heart and Estrogen Replacement Study (HERS-I). Reviews of the National Prescription Audit database and National Disease and Therapeutic Index database confirmed a subsequent marked drop in prescriptions for ERT or HRT,8 despite WHI findings showing that combined-continuous HRT significantly reduces the risk of spine and hip fracture.9
Anne:Onset of hot flashes is a “teachable moment”
“Anne,” a 53-year-old Caucasian woman, has come to see you because of hot flashes that have begun to trouble her since her menstrual periods stopped 8 months ago.
Although she knows that estrogen replacement would help relieve her hot flashes, she is uncertain whether to use it, having heard negative media reports about WHI findings. She has no family or personal history of breast cancer, but is very frightened at even the slightest possibility of increasing her personal risk for breast cancer. She is 5’5” tall and weighs 120 lb. She broke her right wrist in a fall at age 46.
Don’t miss this opportunity!
Though Anne’s visit was prompted by distress over hot flashes, night sweats, and related symptoms of sleep disruption, daytime fatigue, mental lapses, and irritability, it’s a “teachable moment” to discuss osteoporosis prevention and testing. As is typical, her primary desire is relief from hot flashes, yet bone loss is a more serious threat.
If long-term inter vention starts early, bone loss and osteoporosis are preventable; in that context, onset of hot flashes can be seen as a positive force, since they prompted her to seek medical help.
Beth:Concerned because of her mother’s hip fracture
Occasionally a patient will raise the issue of osteoporosis herself. “Beth” is a 49-year-old woman who reports that her last menstrual period 3 months ago was very light in comparison to what she considers normal. Her periods have become irregular over the last year, initially being about 21 days apart, but now 10 to 12 weeks apart. She says she may have noticed an occasional hot flash, but it was not troublesome. She is concerned about the menstrual irregularity and wonders if she is close to menopause.
While she is not psychologically troubled about cessation of menstrual periods, she is concerned about potential bone loss due to estrogen deficiency. With additional questioning, you discover that her mother had a hip fracture.
Carol:Believes her risk low and refuses BMD test
“Carol,” on the other hand, says she doesn’t need bone density testing, because she is not interested in taking any medication to prevent or treat osteoporosis.
If Carol truly will not consider preventive medications, then bone density testing is certainly not indicated. The few patients who refuse to consider medications or testing tend to think their risk is slight. Careful questioning often elicits this belief. They may exercise, avoid cigarette smoke, and consume more than adequate amounts of calcium supplements or dairy products.
Unfortunately, such admirable habits in no way prevent estrogen-deficient bone loss.
Genetically determined low BMD
And no woman can overcome the effects of a genetically determined lower-than-average peak bone density, which may exist without the patient’s knowledge. Without a bone density test, the patient is making an uninformed decision and it is from this perspective that this situation is best approached. Her decision should always be respected, but it is our responsibility to insure that it is an informed decision.
Drug intervention based on T-score
By measuring the bone density at menopause, we can determine if pharmacologic intervention to prevent bone loss needs to start immediately. According to the National Osteoporosis Foundation (NOF) and the American Association of Clinical Endocrinologists (AACE) guidelines, if a woman’s T-score is below -1.5 and she has even 1 other risk factor, pharmacologic intervention is warranted.12,13
This level of bone density is clearly above the threshold for a diagnosis of osteoporosis based on the WHO criteria. Nevertheless, this patient’s estrogen deficiency will further deplete her already lower-than-normal bone density, and could be rapidly devastating. Knowledge of her T-score gives us potential to prevent fractures, now that we have drugs to prevent such devastation.
Three guidelines (TABLE)12-14 recommend pharmacologic intervention if the T-score is -2.5 or lower, and these guidelines differ only in the intervention threshold that also requires an additional risk factor. Note that all 3 recommend pharmacologic intervention when there is only a single risk factor in addition to a bone density level that would not be considered osteoporotic by WHO criteria.
The Food and Drug Administration (FDA) has approved drugs for prevention or treatment of postmenopausal osteoporosis, or both, based on whether data demonstrate that a drug:
- inhibits or stops bone loss, for the prevention indication, or
- reduces fracture risk, for the treatment indication.
The FDA-approved dosages of nonestrogen agents may vary by indication (TABLE). In clinical practice, however, the distinction between prevention and treatment is often less clear, leaving the dosage to the judgment of the clinician.
The complete list of clinical risk factors to consider in initiating therapy based on the T-score is lengthy; furthermore, an ever-increasing number of medications and diseases are now known to contribute to bone loss. The 5 major risk factors listed in the margin below are some of the most important to consider along with the T-score.
TABLE
Drug intervention is appropriate when there are…
| NO RISK FACTORS AND A T-SCORE: | RISK FACTORS AND A T-SCORE: | |
|---|---|---|
| National Osteoporosis Foundation | Below -2.0 | Below -1.5 |
| American Association of Clinical Endocrinologists | At or below -2.5 | -1.5 or poorer |
| North American Menopause Society | Below -2.5 | -2.0 or poorer |
| FDA-approved agents for prevention and treatment of postmenopausal osteoporosis* | ||
| Alendronate | ||
| Prevention | 5 mg po qd or 35 mg po qw | |
| Treatment | 10 mg po qd or 70 mg po qw | |
| Ibandronate† | 2.5 mg po qd | |
| Risedronate | 5 mg po qd or 35 mg po qw | |
| Raloxifene | 60 mg po qd | |
| *Unless otherwise noted, doses are the same for prevention or treatment | ||
| †Although FDA-approved, ibandronate is not currently marketed in the United States | ||
Follow-up testing intervals
Once your patient begins drug therapy, it is appropriate to follow up periodically with bone densitometry. The skeletal site measured at follow-up and the intervals between are dictated by reimbursement, as well as scientific issues. Many insurers, including Medicare, reimburse only once every 2 years.16 Exceptions are few.
From a scientific standpoint, BMD increases at the PA lumbar spine may be sufficiently great to be detected in only 1 year, with potent agents like the bisphosphonates or teriparatide. Since changes in PA lumbar spine density are generally less with raloxifene or salmon calcitonin, waiting 2 years to remeasure the PA lumbar spine is entirely appropriate here.
The PA lumbar spine is the preferred site for monitoring therapy because its higher percentage of trabecular bone generally results in a greater magnitude of change than at the proximal femur.
However, the slower rate of change at the proximal femur means that it need not be measured more often than 2 or even 3 years.
If your patient’s bone density is above the pharmacologic intervention threshold, it is always appropriate to counsel her on nonpharmacologic measures to preserve her skeleton: adequate dietary or supplemental calcium and vitamin D, regular weight-bearing or resistance exercise, and avoidance of cigarette smoke.
But we cannot assume that these are sufficient to protect her skeleton. Follow-up bone density studies are recommended to identify women who will lose bone despite these measures, and for whom pharmacologic intervention is warranted. AACE and the North American Menopause Society (NAMS) recommend follow-up bone density studies every 3 to 5 years in postmenopausal women in whom pharmacologic intervention is not deemed immediately necessary.13,14
A more specific approach based on the patient’s lowest T-score at either the PA lumbar spine or femoral neck has been suggested.17 If her T-score is greater than 0, a repeat study is suggested in 5 years. If however, the T-score is 0 to -0.5, a repeat study is suggested in 3 years. A repeat study should be done in only 1 year if the T-score is -0.5 to -1.
This approach assumes that you wish to know when the patient’s T-score might fall below the normal range established by the WHO, that is, below a T-score of -1, and assumes a rate of bone loss of approximately 0.5 SD (or 0.5 T-score units) per year. If you know you would not intervene until the T-score reaches -1.5 or -2, you can adjust the interval accordingly.
Precision in bone density testing is integral to accurate drug therapy monitoring. When properly performed, dual energy x-ray absorptiometry (DXA) bone density measurements are highly, but not perfectly, reproducible. To reflect actual biologic changes, a measured change i n BMD must have sufficient magnitude.
In general, for measurements at the PA lumbar spine or total hip, a change of 2.77% is needed for 95% confidence. The bone densitometry testing facility should provide the clinician with the exact magnitude of the change necessary for a given level of statistical confidence.18 It is also important to remember that while increases in BMD are desirable and reassuring, no loss of BMD may also be considered efficacious.
The guidelines
The NOF, AACE, the American College of Obstetricians and Gynecologists (ACOG), NAMS and the United States Preventive Services Task Force (USPSTF) guidelines12-14,19,20 agree that all postmenopausal women age 65 and older should have bone density testing. With the exception of the USPSTF, they also agree that all postmenopausal women under age 65 with risk factors should be tested. (The USPSTF limits this recommendation to women age 60 to 64.)
In reality, all postmenopausal women should have bone density testing because the list of risk factors is so comprehensive that it is unusual to find a woman who does not have at least 1 risk factor for osteoporosis.
Anne:Test again in 1 year
With this information in mind, let’s again consider Anne, the 53-year-old woman who sought help for hot flashes. Her visit was an opportunity to discuss osteoporosis prevention. Of the major risk factors, Anne has 2: weight less than 127 lb and a fracture after age 40. Based on the recommendations from the NOF, AACE, ACOG, and NAMS, bone density testing is appropriate.
A DXA study of both proximal femurs shows bone density data for each femur individually as well as the mean BMD value for each region of interest for both femurs. There are 5 regions of interest in the proximal femur: the total hip (or total femur), the femoral neck, Wa rd’s area, the trochanter, and the shaft. The total hip or femoral neck is preferred for diagnosis. Based on her normal T-scores, Anne does not meet any of the pharmacologic intervention guidelines. She should nevertheless be counseled on nonpharmacologic interventions to prevent bone loss.
She should have another bone density study in 1 year. Anne’s PA lumbar spine DXA study is not shown, but it provided no additional information. The PA lumbar spine would be the preferred site for follow-up in 1 year, however.
The recommendation for follow-up in 1 year would not change even if you elected to begin low-dose combined-continuous HRT for relief of hot flashes. Although HRT would be expected to preserve her skeleton, follow-up testing in 1 year for confirmation is appropriate.21
Beth:Treat now, test in 1 yr
Beth has an important risk factor: her mother’s hip fracture. This raises the possibility that she is genetically predisposed to lowerthan-average peak bone density. She meets NOF, AACE, NAMS, and ACOG guidelines for bone density testing.
At the PA lumbar spine, it is preferable to use either the L1-L4 BMD or the L2-L4 BMD and the corresponding T-score. In either case, Beth’s T-score is disturbingly low at -3.7 and -3.6, respectively. Either T-score meets the diagnosis of osteoporosis based on WHO criteria.
This single bone density study does not reveal whether she has lost bone density from a previously higher level or whether her current bone density represents her peak bone density. It is incumbent on the physician to evaluate her medically to exclude possible causes of bone loss other than estrogen deficiency, which might require a different or additional therapy.
Beth certainly meets NOF, AACE, and NAMS guidelines for drug intervention.
A follow-up PA lumbar spine DXA study is indicated in 1 year. Although she has osteoporosis, she has not yet had a fracture. For now, her diagnosis is nothing more than a test result. An osteoporotic fracture will change that. Immediate intervention with drug therapy can preserve her skeletal mass and her quality of life.
Donna:Borderline T-scores
Treatment decisions are not always as clear as in the cases of Anne, Beth, and Carol. Consider Donna, age 54, who is 2 years postmenopausal and in good health. However, her mother reportedly had a dowager’s hump at the time of her death. Although Donna was never told that her mother had osteoporosis, you suspect that she did because of the kyphosis. Donna is fairly sedentary, thin, and continues to smoke. She is not using HRT and rarely takes nutritional supplements of any kind. L 1 - L 4 PA lumbar spine T-score is -1.4; total hip is -1.3.
The dilemma is that Donna does not meet any guideline for pharmacologic intervention based on T-score, even in the presence of risk factors. Both T-scores are just above the NOF and AACE cutoff points, even in the presence of risk factors.
But the guidelines are not hard and fast rules. T- score cutoff points, with or without other risk factors, were chosen to balance the potential benefit and any potential harm of pharmacologic therapy with the risk of fracture if untreated. So, while it may seem arbitrary to recommend treatment when the T- score is -1.5 with risk factors, yet not if the T-score is -1.4 or -1.3, there is a substantive rationale behind the recommendation. Still, there is no substitute for your judgment.
What is a reasonable course?
She has 6 risk factors for bone loss and osteoporosis: estrogen deficiency, current smoking, probable family history, thinness, sedentary lifestyle, and probable calcium deficiency. Every attempt to modify the risk factors that can be modified is worth the effort—smoking cessation, exercise, and calcium and vitamin D supplementation would benefit her skeleton.
Important: Test again in 1 year. It is extremely important to repeat bone density testing at the lumbar spine in 1 year. If the nonpharmacologic interventions you recommend prove insufficient to radically slow the anticipated bone loss, she will fall below a T-score of -1.5 in the next year.
On the other hand, if she demonstrates that she can maintain her bone density with nonpharmacologic measures, a prescription may not be warranted. It would not be unreasonable to allow her this 1 year, because at her relatively young age of 54, at this bone density, her short-term risk of fracture is actually quite low.
“Yes” to both questions
If bone density is low—particularly if it is low and a woman has risk factors for osteoporosis—pharmacologic intervention can be reasonably expected to prevent the devastating consequences of osteoporosis. The question, “Does this menopausal woman need pharmacologic intervention to prevent or treat osteoporosis now, or might she need it later?” can be answered by measuring bone density. It is a question we would be remiss not to ask. Bone density measurement, preferably at the PA lumbar spine and proximal femur by DXA, is the only way to answer this all-important question. To provide the best care possible for a woman who has just become menopausal, you do need to know her bone density. The simple answer to both original questions then, is yes.
Techniques and sites
Bone densitometry can be performed using any of several techniques: dual energy x-ray absorptiometry (DXA), quantitative computerized tomography (QCT), radiographic absorptiometry (RA), or quantitative ultrasound (QUS).
Similarly, bone densitometry can be performed at a myriad of skeletal sites such as the PA lumbar spine, lateral lumbar spine, proximal femur, forearm, phalanges, calcaneus, and total body.
Guidelines are based on PA lumbar or proximal femur by DXA. It is correct that virtually all sites, measured by any technique, predict an individual’s fracture risk, but guidelines for diagnosis of osteoporosis and pharmacologic intervention to prevent or treat osteoporosis are overwhelmingly based on measurements of the PA lumbar spine or proximal femur by DXA.10-14 This is not because of any inadequacy or inaccuracy of the other technologies at these or other skeletal sites. It is because of the use of the World Health Organization (WHO) criteria for diagnosis of osteoporosis and the reliance upon the T-score in intervention guidelines.
WHO diagnosis based on T-score
| DIAGNOSTIC CATEGORY | T-SCORE CRITERIA |
|---|---|
| Normal | -1 or better |
| Osteopenia (low bone mass) | Between -1 and -2.5 |
| Osteoporosis | -2.5 or poorer |
| Severe osteoporosis | -2.5 or poorer, with a fragility fracture |
In its sentinel 1994 guidelines, the WHO defined osteoporosis as a bone density of 2.5 standard deviations (SD) or more below the average bone density for a young adult.15 This threshold was chosen in an attempt to reconcile the prevalence of the disease created by the threshold and the observed lifetime fracture risks. The data used to reach this conclusion were largely based on single-photon absorptiometry (SPA) data from the mid-radius, dual-photon absorptiometry (DXA’s predecessor) and DXA data from the PA lumbar spine and proximal femur.
The WHO warned that applying these criteria in persons measured by other technologies or at other skeletal sites could result in a different diagnostic category. When physicians did apply the criteria in clinical practice, WHO’s prediction became a reality that was quickly recognized and discussed in the literature.
It became clear that we could not apply the WHO criteria to all technologies and all skeletal sites.
Consequentially, major osteoporosis-related medical organizations issued guidelines calling for restricting the diagnosis of osteoporosis based on the WHO criteria to bone density studies performed at the PA lumbar spine and proximal femur using DXA.
T-score means above or below “average”
The T- score on modern bone density reports, although not a technically correct use of the term, indicates your patient’s number of SDs above or below that of the average value for a young adult. If your patient’s BMD is below the average value for a young adult, a minus sign is placed in front of the T- score. The young-adult average value is always assigned a T- score value of 0. For example, a BMD that is 2.2 SD below the average value for a young adult of the same sex would be assigned a T- score of -2.2. Because the WHO defined osteoporosis based on the number of SDs below the average for a young adult, the WHO criteria readily translate to a T- score.
Dr. Bonnick reports research support from Merck and Roche/GSK; consultant fees from Merck, Roche/GSK, and Wyeth; and speaking fees from Merck, Roche/GSK, and Procter & Gamble.
- 4 case studies
- Drug treatment based on T-scores and risk factors Reasonable options if T-score is borderline
- When is a follow-up in 1 year vital? When is a 2- or 3-year interval safe?
- BMD test techniques, sites, and T-scores
This question begs for a simple yes or no, but it is best answered by asking a second question, “Do I need to know my patient’s bone density to give her the best care possible at menopause?” If the answer is yes, then bone density testing is a must, because there is no other way to know what her bone density actually is.
How, then, does this knowledge affect clinical decision-making?
Our concern, of course, is whether we need to intervene pharmacologically to preserve the strength of the skeleton. Even though bone mineral density (BMD) does not completely account for bone strength, it does determine some 60% to 80% of bone strength, and it is still the best predictor of an initial fracture.
Of immediate concern to the physician caring for a woman entering the postmenopausal period is whether she has sufficient bone mass to withstand the bone loss that estrogen deficiency will impose—without developing a dangerously fragile skeletal structure.
Women start losing bone mass years before menopause. While she is still in her mid-40s, a woman’s spinal bone density begins to diminish due to accumulating dietary calcium deficiency, declining physical activity, and declining estradiol levels. (Unless menopause occurs earlier for any reason, however, bone density in the spine is thought to remain relatively stable from the time peak bone mass is attained, before age 30 in most skeletal sites,1 until the mid-40s.) The exact age at which the proximal femur begins to lose bone is more controversial. Cross-sectional studies have suggested that bone loss may in fact begin in a woman’s 20s, almost immediately after reaching peak bone mass. Others have suggested that bone loss does not begin until later, in her 30s.2
A variety of risk factors are modifiable, but one that we cannot modify—genetics— may play the predominant role in determining peak bone mass. Other factors include nutrition, physical activity, intervening illnesses, medications, and lifestyle factors like smoking and alcohol use.
Expect bone loss with any cause of estrogen decline
Postmenopausal bone loss is inexorable in the absence of estrogen replacement, as well as after stopping estrogen replacement therapy (ERT) or hormone replacement therapy (HRT). If your patient stops ERT or HRT, from a skeletal perspective she has just become postmenopausal again. By measuring her bone density, you can ascertain whether bone loss— which will certainly occur—will further deplete bone mass that is already less than ideal. If so, immediate intervention to prevent bone loss is appropriate.
One key longitudinal study,3 for example, found that perimenopausal women lost an average of 2.3% per year from the spine; postmenopausal women, 0.5%. The authors observed these losses in peri- and postmenopausal women, assessed over an average of 27 months. (Women were classified as perimenopausal if they became postmenopausal during the study.)
Calcium intake of 1,000 mg/day or more does not stop bone loss
In a study designed to evaluate the effectiveness of alendronate compared with placebo in preventing bone loss in women within 3 years of menopause, McClung et al4 found a 3% to 4% bone loss at the end of 3 years in the placebo group, despite total calcium intakes of 1,000 mg per day or more.
Stopping HT merits equal concern
Estrogen deficiency precipitated by stopping hormone therapy is due the same concern as that created by menopause itself. Although the exact rates vary in studies, it is clear that bone loss begins when ERT or HRT stops, just as it does with onset of menopause. Hysterectomized postmenopausal women who received ERT for 2 years were found to have a 4.5% decline in posterior-anterior (PA) lumbar spine bone density and a 1.2% decline in total hip bone density only 1 year after estrogen withdrawal.5 This loss occurred despite calcium supplementation.
Trémollieres et al found a 1.64% per year loss of bone density from the spine for the first 2 years after discontinuing HRT, which was similar to that seen in estrogen-deficient women for the first 2 years immediately after menopause.6 In the Postmenopausal Estrogen/Progestin Interventions (PEPI) trial, women who stopped HRT after 3 years lost bone density at an annual rate of 1.04% from the spine and 1.01% from the hip during 4 years of follow-up.7
A conservative assessment of the rate of bone loss in the first few years after menopause or cessation of hormone therapy is about 1% per year from the spine and proximal femur. At first glance, 1% per year does not appear worrisome. But within 10 years of menopause, at or about the age of only 60, 10% of the bone mass that was present at menopause is gone. In 15 years, at least 15% is gone because of estrogen deficiency.
Unquestionably, many women have stopped ERT or HRT or are choosing not to begin, due to media attention on negative findings from trials such as the combined-continuous HRT arm of the Wo men’s Health Initiative (WHI) and the Heart and Estrogen Replacement Study (HERS-I). Reviews of the National Prescription Audit database and National Disease and Therapeutic Index database confirmed a subsequent marked drop in prescriptions for ERT or HRT,8 despite WHI findings showing that combined-continuous HRT significantly reduces the risk of spine and hip fracture.9
Anne:Onset of hot flashes is a “teachable moment”
“Anne,” a 53-year-old Caucasian woman, has come to see you because of hot flashes that have begun to trouble her since her menstrual periods stopped 8 months ago.
Although she knows that estrogen replacement would help relieve her hot flashes, she is uncertain whether to use it, having heard negative media reports about WHI findings. She has no family or personal history of breast cancer, but is very frightened at even the slightest possibility of increasing her personal risk for breast cancer. She is 5’5” tall and weighs 120 lb. She broke her right wrist in a fall at age 46.
Don’t miss this opportunity!
Though Anne’s visit was prompted by distress over hot flashes, night sweats, and related symptoms of sleep disruption, daytime fatigue, mental lapses, and irritability, it’s a “teachable moment” to discuss osteoporosis prevention and testing. As is typical, her primary desire is relief from hot flashes, yet bone loss is a more serious threat.
If long-term inter vention starts early, bone loss and osteoporosis are preventable; in that context, onset of hot flashes can be seen as a positive force, since they prompted her to seek medical help.
Beth:Concerned because of her mother’s hip fracture
Occasionally a patient will raise the issue of osteoporosis herself. “Beth” is a 49-year-old woman who reports that her last menstrual period 3 months ago was very light in comparison to what she considers normal. Her periods have become irregular over the last year, initially being about 21 days apart, but now 10 to 12 weeks apart. She says she may have noticed an occasional hot flash, but it was not troublesome. She is concerned about the menstrual irregularity and wonders if she is close to menopause.
While she is not psychologically troubled about cessation of menstrual periods, she is concerned about potential bone loss due to estrogen deficiency. With additional questioning, you discover that her mother had a hip fracture.
Carol:Believes her risk low and refuses BMD test
“Carol,” on the other hand, says she doesn’t need bone density testing, because she is not interested in taking any medication to prevent or treat osteoporosis.
If Carol truly will not consider preventive medications, then bone density testing is certainly not indicated. The few patients who refuse to consider medications or testing tend to think their risk is slight. Careful questioning often elicits this belief. They may exercise, avoid cigarette smoke, and consume more than adequate amounts of calcium supplements or dairy products.
Unfortunately, such admirable habits in no way prevent estrogen-deficient bone loss.
Genetically determined low BMD
And no woman can overcome the effects of a genetically determined lower-than-average peak bone density, which may exist without the patient’s knowledge. Without a bone density test, the patient is making an uninformed decision and it is from this perspective that this situation is best approached. Her decision should always be respected, but it is our responsibility to insure that it is an informed decision.
Drug intervention based on T-score
By measuring the bone density at menopause, we can determine if pharmacologic intervention to prevent bone loss needs to start immediately. According to the National Osteoporosis Foundation (NOF) and the American Association of Clinical Endocrinologists (AACE) guidelines, if a woman’s T-score is below -1.5 and she has even 1 other risk factor, pharmacologic intervention is warranted.12,13
This level of bone density is clearly above the threshold for a diagnosis of osteoporosis based on the WHO criteria. Nevertheless, this patient’s estrogen deficiency will further deplete her already lower-than-normal bone density, and could be rapidly devastating. Knowledge of her T-score gives us potential to prevent fractures, now that we have drugs to prevent such devastation.
Three guidelines (TABLE)12-14 recommend pharmacologic intervention if the T-score is -2.5 or lower, and these guidelines differ only in the intervention threshold that also requires an additional risk factor. Note that all 3 recommend pharmacologic intervention when there is only a single risk factor in addition to a bone density level that would not be considered osteoporotic by WHO criteria.
The Food and Drug Administration (FDA) has approved drugs for prevention or treatment of postmenopausal osteoporosis, or both, based on whether data demonstrate that a drug:
- inhibits or stops bone loss, for the prevention indication, or
- reduces fracture risk, for the treatment indication.
The FDA-approved dosages of nonestrogen agents may vary by indication (TABLE). In clinical practice, however, the distinction between prevention and treatment is often less clear, leaving the dosage to the judgment of the clinician.
The complete list of clinical risk factors to consider in initiating therapy based on the T-score is lengthy; furthermore, an ever-increasing number of medications and diseases are now known to contribute to bone loss. The 5 major risk factors listed in the margin below are some of the most important to consider along with the T-score.
TABLE
Drug intervention is appropriate when there are…
| NO RISK FACTORS AND A T-SCORE: | RISK FACTORS AND A T-SCORE: | |
|---|---|---|
| National Osteoporosis Foundation | Below -2.0 | Below -1.5 |
| American Association of Clinical Endocrinologists | At or below -2.5 | -1.5 or poorer |
| North American Menopause Society | Below -2.5 | -2.0 or poorer |
| FDA-approved agents for prevention and treatment of postmenopausal osteoporosis* | ||
| Alendronate | ||
| Prevention | 5 mg po qd or 35 mg po qw | |
| Treatment | 10 mg po qd or 70 mg po qw | |
| Ibandronate† | 2.5 mg po qd | |
| Risedronate | 5 mg po qd or 35 mg po qw | |
| Raloxifene | 60 mg po qd | |
| *Unless otherwise noted, doses are the same for prevention or treatment | ||
| †Although FDA-approved, ibandronate is not currently marketed in the United States | ||
Follow-up testing intervals
Once your patient begins drug therapy, it is appropriate to follow up periodically with bone densitometry. The skeletal site measured at follow-up and the intervals between are dictated by reimbursement, as well as scientific issues. Many insurers, including Medicare, reimburse only once every 2 years.16 Exceptions are few.
From a scientific standpoint, BMD increases at the PA lumbar spine may be sufficiently great to be detected in only 1 year, with potent agents like the bisphosphonates or teriparatide. Since changes in PA lumbar spine density are generally less with raloxifene or salmon calcitonin, waiting 2 years to remeasure the PA lumbar spine is entirely appropriate here.
The PA lumbar spine is the preferred site for monitoring therapy because its higher percentage of trabecular bone generally results in a greater magnitude of change than at the proximal femur.
However, the slower rate of change at the proximal femur means that it need not be measured more often than 2 or even 3 years.
If your patient’s bone density is above the pharmacologic intervention threshold, it is always appropriate to counsel her on nonpharmacologic measures to preserve her skeleton: adequate dietary or supplemental calcium and vitamin D, regular weight-bearing or resistance exercise, and avoidance of cigarette smoke.
But we cannot assume that these are sufficient to protect her skeleton. Follow-up bone density studies are recommended to identify women who will lose bone despite these measures, and for whom pharmacologic intervention is warranted. AACE and the North American Menopause Society (NAMS) recommend follow-up bone density studies every 3 to 5 years in postmenopausal women in whom pharmacologic intervention is not deemed immediately necessary.13,14
A more specific approach based on the patient’s lowest T-score at either the PA lumbar spine or femoral neck has been suggested.17 If her T-score is greater than 0, a repeat study is suggested in 5 years. If however, the T-score is 0 to -0.5, a repeat study is suggested in 3 years. A repeat study should be done in only 1 year if the T-score is -0.5 to -1.
This approach assumes that you wish to know when the patient’s T-score might fall below the normal range established by the WHO, that is, below a T-score of -1, and assumes a rate of bone loss of approximately 0.5 SD (or 0.5 T-score units) per year. If you know you would not intervene until the T-score reaches -1.5 or -2, you can adjust the interval accordingly.
Precision in bone density testing is integral to accurate drug therapy monitoring. When properly performed, dual energy x-ray absorptiometry (DXA) bone density measurements are highly, but not perfectly, reproducible. To reflect actual biologic changes, a measured change i n BMD must have sufficient magnitude.
In general, for measurements at the PA lumbar spine or total hip, a change of 2.77% is needed for 95% confidence. The bone densitometry testing facility should provide the clinician with the exact magnitude of the change necessary for a given level of statistical confidence.18 It is also important to remember that while increases in BMD are desirable and reassuring, no loss of BMD may also be considered efficacious.
The guidelines
The NOF, AACE, the American College of Obstetricians and Gynecologists (ACOG), NAMS and the United States Preventive Services Task Force (USPSTF) guidelines12-14,19,20 agree that all postmenopausal women age 65 and older should have bone density testing. With the exception of the USPSTF, they also agree that all postmenopausal women under age 65 with risk factors should be tested. (The USPSTF limits this recommendation to women age 60 to 64.)
In reality, all postmenopausal women should have bone density testing because the list of risk factors is so comprehensive that it is unusual to find a woman who does not have at least 1 risk factor for osteoporosis.
Anne:Test again in 1 year
With this information in mind, let’s again consider Anne, the 53-year-old woman who sought help for hot flashes. Her visit was an opportunity to discuss osteoporosis prevention. Of the major risk factors, Anne has 2: weight less than 127 lb and a fracture after age 40. Based on the recommendations from the NOF, AACE, ACOG, and NAMS, bone density testing is appropriate.
A DXA study of both proximal femurs shows bone density data for each femur individually as well as the mean BMD value for each region of interest for both femurs. There are 5 regions of interest in the proximal femur: the total hip (or total femur), the femoral neck, Wa rd’s area, the trochanter, and the shaft. The total hip or femoral neck is preferred for diagnosis. Based on her normal T-scores, Anne does not meet any of the pharmacologic intervention guidelines. She should nevertheless be counseled on nonpharmacologic interventions to prevent bone loss.
She should have another bone density study in 1 year. Anne’s PA lumbar spine DXA study is not shown, but it provided no additional information. The PA lumbar spine would be the preferred site for follow-up in 1 year, however.
The recommendation for follow-up in 1 year would not change even if you elected to begin low-dose combined-continuous HRT for relief of hot flashes. Although HRT would be expected to preserve her skeleton, follow-up testing in 1 year for confirmation is appropriate.21
Beth:Treat now, test in 1 yr
Beth has an important risk factor: her mother’s hip fracture. This raises the possibility that she is genetically predisposed to lowerthan-average peak bone density. She meets NOF, AACE, NAMS, and ACOG guidelines for bone density testing.
At the PA lumbar spine, it is preferable to use either the L1-L4 BMD or the L2-L4 BMD and the corresponding T-score. In either case, Beth’s T-score is disturbingly low at -3.7 and -3.6, respectively. Either T-score meets the diagnosis of osteoporosis based on WHO criteria.
This single bone density study does not reveal whether she has lost bone density from a previously higher level or whether her current bone density represents her peak bone density. It is incumbent on the physician to evaluate her medically to exclude possible causes of bone loss other than estrogen deficiency, which might require a different or additional therapy.
Beth certainly meets NOF, AACE, and NAMS guidelines for drug intervention.
A follow-up PA lumbar spine DXA study is indicated in 1 year. Although she has osteoporosis, she has not yet had a fracture. For now, her diagnosis is nothing more than a test result. An osteoporotic fracture will change that. Immediate intervention with drug therapy can preserve her skeletal mass and her quality of life.
Donna:Borderline T-scores
Treatment decisions are not always as clear as in the cases of Anne, Beth, and Carol. Consider Donna, age 54, who is 2 years postmenopausal and in good health. However, her mother reportedly had a dowager’s hump at the time of her death. Although Donna was never told that her mother had osteoporosis, you suspect that she did because of the kyphosis. Donna is fairly sedentary, thin, and continues to smoke. She is not using HRT and rarely takes nutritional supplements of any kind. L 1 - L 4 PA lumbar spine T-score is -1.4; total hip is -1.3.
The dilemma is that Donna does not meet any guideline for pharmacologic intervention based on T-score, even in the presence of risk factors. Both T-scores are just above the NOF and AACE cutoff points, even in the presence of risk factors.
But the guidelines are not hard and fast rules. T- score cutoff points, with or without other risk factors, were chosen to balance the potential benefit and any potential harm of pharmacologic therapy with the risk of fracture if untreated. So, while it may seem arbitrary to recommend treatment when the T- score is -1.5 with risk factors, yet not if the T-score is -1.4 or -1.3, there is a substantive rationale behind the recommendation. Still, there is no substitute for your judgment.
What is a reasonable course?
She has 6 risk factors for bone loss and osteoporosis: estrogen deficiency, current smoking, probable family history, thinness, sedentary lifestyle, and probable calcium deficiency. Every attempt to modify the risk factors that can be modified is worth the effort—smoking cessation, exercise, and calcium and vitamin D supplementation would benefit her skeleton.
Important: Test again in 1 year. It is extremely important to repeat bone density testing at the lumbar spine in 1 year. If the nonpharmacologic interventions you recommend prove insufficient to radically slow the anticipated bone loss, she will fall below a T-score of -1.5 in the next year.
On the other hand, if she demonstrates that she can maintain her bone density with nonpharmacologic measures, a prescription may not be warranted. It would not be unreasonable to allow her this 1 year, because at her relatively young age of 54, at this bone density, her short-term risk of fracture is actually quite low.
“Yes” to both questions
If bone density is low—particularly if it is low and a woman has risk factors for osteoporosis—pharmacologic intervention can be reasonably expected to prevent the devastating consequences of osteoporosis. The question, “Does this menopausal woman need pharmacologic intervention to prevent or treat osteoporosis now, or might she need it later?” can be answered by measuring bone density. It is a question we would be remiss not to ask. Bone density measurement, preferably at the PA lumbar spine and proximal femur by DXA, is the only way to answer this all-important question. To provide the best care possible for a woman who has just become menopausal, you do need to know her bone density. The simple answer to both original questions then, is yes.
Techniques and sites
Bone densitometry can be performed using any of several techniques: dual energy x-ray absorptiometry (DXA), quantitative computerized tomography (QCT), radiographic absorptiometry (RA), or quantitative ultrasound (QUS).
Similarly, bone densitometry can be performed at a myriad of skeletal sites such as the PA lumbar spine, lateral lumbar spine, proximal femur, forearm, phalanges, calcaneus, and total body.
Guidelines are based on PA lumbar or proximal femur by DXA. It is correct that virtually all sites, measured by any technique, predict an individual’s fracture risk, but guidelines for diagnosis of osteoporosis and pharmacologic intervention to prevent or treat osteoporosis are overwhelmingly based on measurements of the PA lumbar spine or proximal femur by DXA.10-14 This is not because of any inadequacy or inaccuracy of the other technologies at these or other skeletal sites. It is because of the use of the World Health Organization (WHO) criteria for diagnosis of osteoporosis and the reliance upon the T-score in intervention guidelines.
WHO diagnosis based on T-score
| DIAGNOSTIC CATEGORY | T-SCORE CRITERIA |
|---|---|
| Normal | -1 or better |
| Osteopenia (low bone mass) | Between -1 and -2.5 |
| Osteoporosis | -2.5 or poorer |
| Severe osteoporosis | -2.5 or poorer, with a fragility fracture |
In its sentinel 1994 guidelines, the WHO defined osteoporosis as a bone density of 2.5 standard deviations (SD) or more below the average bone density for a young adult.15 This threshold was chosen in an attempt to reconcile the prevalence of the disease created by the threshold and the observed lifetime fracture risks. The data used to reach this conclusion were largely based on single-photon absorptiometry (SPA) data from the mid-radius, dual-photon absorptiometry (DXA’s predecessor) and DXA data from the PA lumbar spine and proximal femur.
The WHO warned that applying these criteria in persons measured by other technologies or at other skeletal sites could result in a different diagnostic category. When physicians did apply the criteria in clinical practice, WHO’s prediction became a reality that was quickly recognized and discussed in the literature.
It became clear that we could not apply the WHO criteria to all technologies and all skeletal sites.
Consequentially, major osteoporosis-related medical organizations issued guidelines calling for restricting the diagnosis of osteoporosis based on the WHO criteria to bone density studies performed at the PA lumbar spine and proximal femur using DXA.
T-score means above or below “average”
The T- score on modern bone density reports, although not a technically correct use of the term, indicates your patient’s number of SDs above or below that of the average value for a young adult. If your patient’s BMD is below the average value for a young adult, a minus sign is placed in front of the T- score. The young-adult average value is always assigned a T- score value of 0. For example, a BMD that is 2.2 SD below the average value for a young adult of the same sex would be assigned a T- score of -2.2. Because the WHO defined osteoporosis based on the number of SDs below the average for a young adult, the WHO criteria readily translate to a T- score.
Dr. Bonnick reports research support from Merck and Roche/GSK; consultant fees from Merck, Roche/GSK, and Wyeth; and speaking fees from Merck, Roche/GSK, and Procter & Gamble.
1. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16:393S-399S.
2. Hui SL, Perkins AJ, Zhous L, et al. Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab. 2002;87:1539-1543.
3. Pouilles JM, Trémollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int. 1993;53:340-343.
4. McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med. 1998;128:253-261.
5. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
6. Trémollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int. 2001;12:385-390.
7. Greendale GA, Espeland M, Slone S, Marcus R, BarrettConnor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
8. Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trend response to recent evidence. JAMA. 2004;291:104-106.
9. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259.
11. Miller PD, Siris ES, Barrett-Connor E, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222-2230.
12. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: NOF; 2003.
13. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001edition, with selected updates for 2003. Endocr Pract. 2003;9:545-564.
14. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
15. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994.
16. Department of Health and Human Services. Health Care Finance Administration. Medicare program; Medicare coverage of and payment for bone mass measurements. 42 CFR Part 410, Federal Register 63:June 24, 1989.
17. Abrahamsen B, Nissen N, Hermann AP, et al. When should densitometry be repeated in healthy peri- and postmenopausal women: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2002;17:2061-2067.
18. Bonnick SL, Johnston CC, Kleerekoper M, et al. The importance of precision in bone density measurements. J Clin Densitom. 2001;4:105-110.
19. American College of Obstetricians and Gynecologists. ACOG releases recommendations for bone density screening for osteoporosis. Washington, DC: ACOG; 2002.
20. US Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
21. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.
1. Zanchetta JR, Plotkin H, Alvarez Filgueira ML. Bone mass in children: normative values for the 2-20-year-old population. Bone. 1995;16:393S-399S.
2. Hui SL, Perkins AJ, Zhous L, et al. Bone loss at the femoral neck in premenopausal white women: effects of weight change and sex-hormone levels. J Clin Endocrinol Metab. 2002;87:1539-1543.
3. Pouilles JM, Trémollieres F, Ribot C. The effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int. 1993;53:340-343.
4. McClung M, Clemmesen B, Daifotis A, et al. Alendronate prevents postmenopausal bone loss in women without osteoporosis. Ann Intern Med. 1998;128:253-261.
5. Greenspan SL, Emkey RD, Bone HG, et al. Significant differential effects of alendronate, estrogen, or combination therapy on the rate of bone loss after discontinuation of treatment of postmenopausal osteoporosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2002;137:875-883.
6. Trémollieres FA, Pouilles JM, Ribot C. Withdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int. 2001;12:385-390.
7. Greendale GA, Espeland M, Slone S, Marcus R, BarrettConnor E. Bone mass response to discontinuation of long-term hormone replacement therapy: results from the Postmenopausal Estrogen/Progestin Interventions (PEPI) Safety Follow-up Study. Arch Intern Med. 2002;162:665-672.
8. Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trend response to recent evidence. JAMA. 2004;291:104-106.
9. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
10. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254-1259.
11. Miller PD, Siris ES, Barrett-Connor E, et al. Prediction of fracture risk in postmenopausal white women with peripheral bone densitometry: evidence from the National Osteoporosis Risk Assessment. J Bone Miner Res. 2002;17:2222-2230.
12. National Osteoporosis Foundation. Physician’s guide to prevention and treatment of osteoporosis. Washington, DC: NOF; 2003.
13. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of postmenopausal osteoporosis: 2001edition, with selected updates for 2003. Endocr Pract. 2003;9:545-564.
14. Management of postmenopausal osteoporosis: position statement of The North American Menopause Society. Menopause. 2002;9:84-101.
15. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994.
16. Department of Health and Human Services. Health Care Finance Administration. Medicare program; Medicare coverage of and payment for bone mass measurements. 42 CFR Part 410, Federal Register 63:June 24, 1989.
17. Abrahamsen B, Nissen N, Hermann AP, et al. When should densitometry be repeated in healthy peri- and postmenopausal women: the Danish Osteoporosis Prevention Study. J Bone Miner Res. 2002;17:2061-2067.
18. Bonnick SL, Johnston CC, Kleerekoper M, et al. The importance of precision in bone density measurements. J Clin Densitom. 2001;4:105-110.
19. American College of Obstetricians and Gynecologists. ACOG releases recommendations for bone density screening for osteoporosis. Washington, DC: ACOG; 2002.
20. US Preventive Services Task Force. Screening for osteoporosis in postmenopausal women: recommendations and rationale. Ann Intern Med. 2002;137:526-528.
21. Lindsay R, Gallagher JC, Kleerekoper M, Pickar JH. Effect of lower doses of conjugated equine estrogens with and without medroxyprogesterone acetate on bone in early postmenopausal women. JAMA. 2002;287:2668-2676.