User login
followed over a 4-year extension of a randomized trial.
Patients from a previous study of ixekizumab were treated with 120 mg at the start of the extension, and then 80 mg subcutaneously every 4 weeks, Claus Zachariae, MD, of the University Hospital of Copenhagen Gentofte, and his coauthors reported in the Journal of the American Academy of Dermatology.
At week 208, 82% of the patients achieved Psoriasis Area and Severity Index (PASI) 75, 65% achieved PASI 90, and 45% achieved PASI 100; 65% scored a 0 or 1 on the Physician’s Global Assessment Scale. In addition, 45% of patients reported a score of 0 on the Physician Global Assessment. Patients also reported a decrease in itching from baseline.
A total of 17% of patients experienced a serious adverse event and 87% of the patients experienced at least one treatment-related adverse event by the end of the 4-year extension period. Most of the reported events were mild to moderate; the most common were nasopharyngitis (23%), sinusitis (13%), upper respiratory tract infection (13%), and headache (10%).
The study findings were limited by several factors including the lack of blinding and lack of a placebo, the researchers noted.
However, the results demonstrate “that efficacy can be maintained at high levels for up to 4 years of ixekizumab therapy without apparent increases in health risks or safety issues,” for psoriasis patients, Dr. Zachariae and his associates said. “Longer treatment periods in larger numbers of patients will be reported for patients enrolled in the 5-year phase 3 ixekizumab studies.”
The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
SOURCE: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.
followed over a 4-year extension of a randomized trial.
Patients from a previous study of ixekizumab were treated with 120 mg at the start of the extension, and then 80 mg subcutaneously every 4 weeks, Claus Zachariae, MD, of the University Hospital of Copenhagen Gentofte, and his coauthors reported in the Journal of the American Academy of Dermatology.
At week 208, 82% of the patients achieved Psoriasis Area and Severity Index (PASI) 75, 65% achieved PASI 90, and 45% achieved PASI 100; 65% scored a 0 or 1 on the Physician’s Global Assessment Scale. In addition, 45% of patients reported a score of 0 on the Physician Global Assessment. Patients also reported a decrease in itching from baseline.
A total of 17% of patients experienced a serious adverse event and 87% of the patients experienced at least one treatment-related adverse event by the end of the 4-year extension period. Most of the reported events were mild to moderate; the most common were nasopharyngitis (23%), sinusitis (13%), upper respiratory tract infection (13%), and headache (10%).
The study findings were limited by several factors including the lack of blinding and lack of a placebo, the researchers noted.
However, the results demonstrate “that efficacy can be maintained at high levels for up to 4 years of ixekizumab therapy without apparent increases in health risks or safety issues,” for psoriasis patients, Dr. Zachariae and his associates said. “Longer treatment periods in larger numbers of patients will be reported for patients enrolled in the 5-year phase 3 ixekizumab studies.”
The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
SOURCE: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.
followed over a 4-year extension of a randomized trial.
Patients from a previous study of ixekizumab were treated with 120 mg at the start of the extension, and then 80 mg subcutaneously every 4 weeks, Claus Zachariae, MD, of the University Hospital of Copenhagen Gentofte, and his coauthors reported in the Journal of the American Academy of Dermatology.
At week 208, 82% of the patients achieved Psoriasis Area and Severity Index (PASI) 75, 65% achieved PASI 90, and 45% achieved PASI 100; 65% scored a 0 or 1 on the Physician’s Global Assessment Scale. In addition, 45% of patients reported a score of 0 on the Physician Global Assessment. Patients also reported a decrease in itching from baseline.
A total of 17% of patients experienced a serious adverse event and 87% of the patients experienced at least one treatment-related adverse event by the end of the 4-year extension period. Most of the reported events were mild to moderate; the most common were nasopharyngitis (23%), sinusitis (13%), upper respiratory tract infection (13%), and headache (10%).
The study findings were limited by several factors including the lack of blinding and lack of a placebo, the researchers noted.
However, the results demonstrate “that efficacy can be maintained at high levels for up to 4 years of ixekizumab therapy without apparent increases in health risks or safety issues,” for psoriasis patients, Dr. Zachariae and his associates said. “Longer treatment periods in larger numbers of patients will be reported for patients enrolled in the 5-year phase 3 ixekizumab studies.”
The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
SOURCE: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.
FROM JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
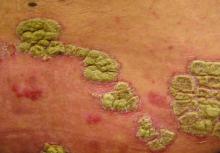
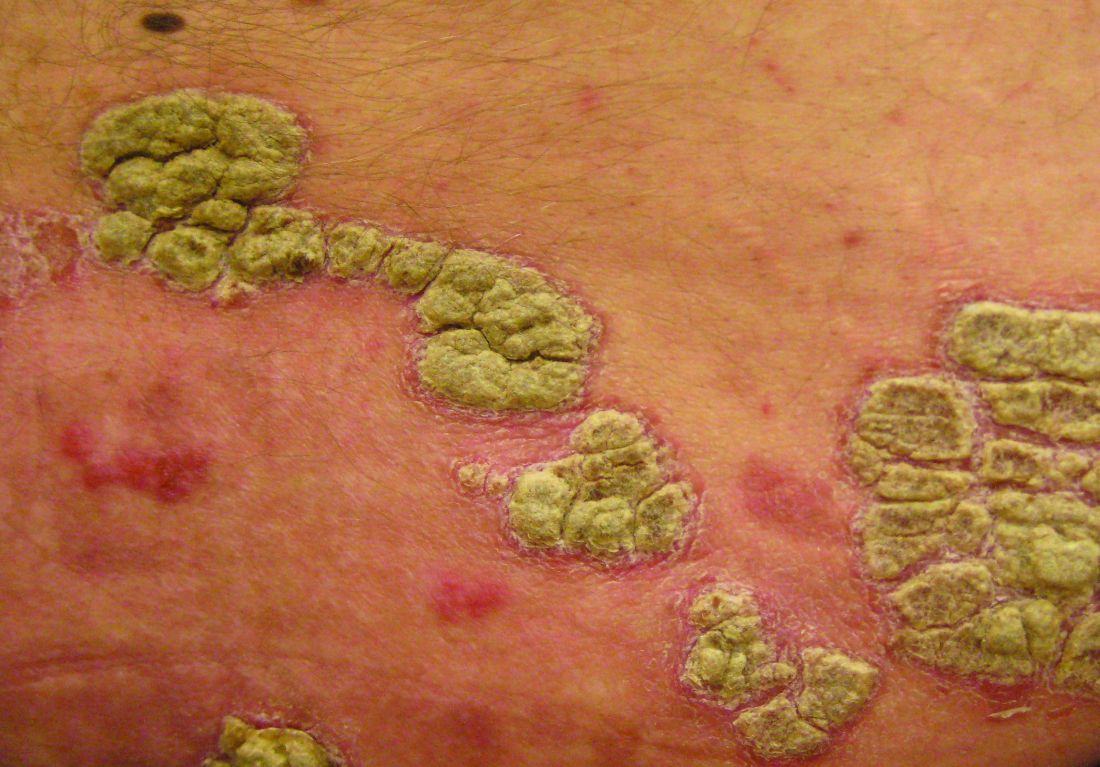
Key clinical point: Ixekizumab appears to be an effective option for long-term treatment of patients with moderate to severe psoriasis.
Major finding: A total of 82% of patients achieved PASI 75 at week 208 of the open-label extension study.
Study details: The data come from a 4-year open-label extension of a phase 2 randomized, placebo-controlled trial including 120 adults with psoriasis.
Disclosures: The study was supported by Eli Lilly. Dr. Zachariae disclosed relationships with Eli Lilly and other companies including Janssen, Novartis, AbbVie, and Amgen. His coauthors had financial relationships with multiple companies.
Source: Zachariae C et al. J Am Acad Dermatol. 2018 Aug;79(2):294-301.e6.