User login
GAITHERSBURG, MD. – The first human studies evaluating the use of genetically modified oocytes to prevent the transmission of mitochondrial diseases could enroll women with diseases that are the most severe, tend to present in early childhood, and are relatively common for a mitochondrial disease, according to panelists at a meeting convened by the Food and Drug Administration to discuss the design of such trials and related issues.
At a meeting on Feb. 25 and 26, members of the FDA Cellular, Tissue, and Gene Therapies Advisory Committee mentioned two mitochondrial diseases in particular, Leigh’s disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes), that could be included in initial clinical trials evaluating the safety and efficacy of what the FDA refers to as "mitochondrial manipulation technologies."
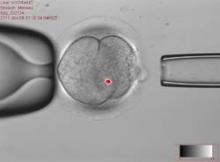
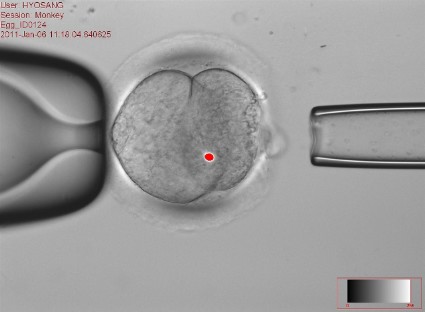
This controversial approach, which is being developed to prevent maternal transmission of debilitating and often fatal mitochondrial diseases, entails removing the mitochondrial DNA from an affected woman’s oocyte or embryo and replacing it via assisted reproductive technologies with the mitochondrial DNA from the egg of a healthy donor.
The approach has been studied in animal and in vitro studies, but not yet in humans. However, researchers at Oregon Health and Science University (OHSU), Portland, say they are ready to start a clinical trial based on their results in macaque monkeys.
The FDA called the 2-day meeting to discuss potential clinical trials and to focus on the scientific, technologic, and clinical aspects of the technologies, but not to address public policy or ethical issues. In briefing documents posted before the meeting, the agency acknowledged that there are ethical and policy issues related to genetic modification of eggs and embryos, which can affect regulatory decisions, but added that these issues were "outside the scope" of this meeting.
Regarding clinical trial design and execution, panelists recommended that studies should closely monitor the fetus through gestation, and after birth and long-term follow-up, should include future generations, if female offspring are included. Several panelists supported including only male embryos to minimize the risk of a female passing on damaged DNA to future generations, while others said this would result in a lost opportunity to study the transgenerational risks of the technology.
Other recommendations included avoiding the enrollment of people at high risk of having a baby with a birth defect, or with comorbidities that could affect birth outcome, which would make it more difficult to evaluate the risks of the technologies. The use of controls, panelists said, was problematic, because of the variability in when and how mitochondrial diseases present and because of the relatively small populations of patients affected by these diseases. Historical controls could be used, but larger patient registries are needed, they said.
Panelists also recommended screening egg donors for mitochondrial diseases, and providing informed consent to children born to mothers in the trials when they turn age 18.
It is clear that there is a "deft group of creative, innovative investigators" who can perform these techniques, "which is a good start, but there are so many things we don’t know" that must be evaluated further in animal studies, said panelist Dr. David Keefe, who referred to thalidomide and diethylstilbestrol (DES) as historical examples of therapies that were thought to be promising but proved to have devastating effects.
Another concern Dr. Keefe raised was the possibility that a woman whose risk of having a baby without the inherited defect might be as high as 95% and that she might choose mitochondrial manipulation over preimplantation genetic diagnosis.
"A woman could be led down the primrose path towards a procedure that’s experimental and miss the opportunity to pursue a relatively well-established procedure," said Dr. Keefe, the Stanley H. Kaplan Professor, department of obstetrics and gynecology, New York University.
Another panelist, Dr. Katharine Wenstrom, professor of obstetrics and gynecology, Brown University, Providence, R.I., said that based on her experience with women with genetic diseases, these women "are very vulnerable, and my concern would be how to [provide] consent [for] somebody for whom a pregnancy would be very dangerous and [who] might not consider a pregnancy, but then given the opportunity to have this technique, might agree to a pregnancy that could actually be life threatening."
She also said that she was concerned about whether the technique could deplete mitochondria, which has been associated with several forms of cancer, and about the "inability to ensure that the technique has not inflicted some new abnormality" on the child.
Shoukhrat Mitalipov, Ph.D., whose research group at the Oregon Stem Cell Center at OHSU has tested the technology in macaque monkeys, said that their research cohort currently includes four subjects born through mitochondrial manipulation that are almost adults. To date, they have been healthy, with normal blood test results, and are no different from controls, showing that mitochondrial DNA in oocytes can be replaced.
The next step in their research is to recruit families who are carriers of early-onset mitochondrial DNA diseases who have had at least one affected child, recruit healthy egg donors, and then perform the procedure, followed by preimplantation genetic diagnosis of the embryo and/or prenatal diagnosis to "ensure complete mitochondrial DNA replacement and chromosomal normalcy," he said.
The panel was also asked to discuss the use of mitochondrial manipulation as a treatment for infertility. However, members considered this indication a far different type of application than preventing mitochondrial disease, which would have different inclusion criteria, controls, and risk-benefit evaluations, and several panelists raised particular concerns about the use of this technology for infertility.
"The idea we’re going to do anything to infertility patients involving mitochondria I think should be off the table," Dr. Keefe said, noting that there is "a very, very slippery slope when you’re dealing with human reproduction" in the United States, where licensure of infertility clinics is not required.
The controversies of this area of research, which some critics point out would result in a child with three genetically related parents, were not off limits to the open public hearing speakers, including Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society.
"We want to avoid waking up in a world" where researchers, infertility clinics, governments, insurance companies, "or parents decide that they are going to try to engineer children with specific traits and even possibly [put] in motion a regime of high-tech consumer eugenics," she said.
GAITHERSBURG, MD. – The first human studies evaluating the use of genetically modified oocytes to prevent the transmission of mitochondrial diseases could enroll women with diseases that are the most severe, tend to present in early childhood, and are relatively common for a mitochondrial disease, according to panelists at a meeting convened by the Food and Drug Administration to discuss the design of such trials and related issues.
At a meeting on Feb. 25 and 26, members of the FDA Cellular, Tissue, and Gene Therapies Advisory Committee mentioned two mitochondrial diseases in particular, Leigh’s disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes), that could be included in initial clinical trials evaluating the safety and efficacy of what the FDA refers to as "mitochondrial manipulation technologies."
This controversial approach, which is being developed to prevent maternal transmission of debilitating and often fatal mitochondrial diseases, entails removing the mitochondrial DNA from an affected woman’s oocyte or embryo and replacing it via assisted reproductive technologies with the mitochondrial DNA from the egg of a healthy donor.
The approach has been studied in animal and in vitro studies, but not yet in humans. However, researchers at Oregon Health and Science University (OHSU), Portland, say they are ready to start a clinical trial based on their results in macaque monkeys.
The FDA called the 2-day meeting to discuss potential clinical trials and to focus on the scientific, technologic, and clinical aspects of the technologies, but not to address public policy or ethical issues. In briefing documents posted before the meeting, the agency acknowledged that there are ethical and policy issues related to genetic modification of eggs and embryos, which can affect regulatory decisions, but added that these issues were "outside the scope" of this meeting.
Regarding clinical trial design and execution, panelists recommended that studies should closely monitor the fetus through gestation, and after birth and long-term follow-up, should include future generations, if female offspring are included. Several panelists supported including only male embryos to minimize the risk of a female passing on damaged DNA to future generations, while others said this would result in a lost opportunity to study the transgenerational risks of the technology.
Other recommendations included avoiding the enrollment of people at high risk of having a baby with a birth defect, or with comorbidities that could affect birth outcome, which would make it more difficult to evaluate the risks of the technologies. The use of controls, panelists said, was problematic, because of the variability in when and how mitochondrial diseases present and because of the relatively small populations of patients affected by these diseases. Historical controls could be used, but larger patient registries are needed, they said.
Panelists also recommended screening egg donors for mitochondrial diseases, and providing informed consent to children born to mothers in the trials when they turn age 18.
It is clear that there is a "deft group of creative, innovative investigators" who can perform these techniques, "which is a good start, but there are so many things we don’t know" that must be evaluated further in animal studies, said panelist Dr. David Keefe, who referred to thalidomide and diethylstilbestrol (DES) as historical examples of therapies that were thought to be promising but proved to have devastating effects.
Another concern Dr. Keefe raised was the possibility that a woman whose risk of having a baby without the inherited defect might be as high as 95% and that she might choose mitochondrial manipulation over preimplantation genetic diagnosis.
"A woman could be led down the primrose path towards a procedure that’s experimental and miss the opportunity to pursue a relatively well-established procedure," said Dr. Keefe, the Stanley H. Kaplan Professor, department of obstetrics and gynecology, New York University.
Another panelist, Dr. Katharine Wenstrom, professor of obstetrics and gynecology, Brown University, Providence, R.I., said that based on her experience with women with genetic diseases, these women "are very vulnerable, and my concern would be how to [provide] consent [for] somebody for whom a pregnancy would be very dangerous and [who] might not consider a pregnancy, but then given the opportunity to have this technique, might agree to a pregnancy that could actually be life threatening."
She also said that she was concerned about whether the technique could deplete mitochondria, which has been associated with several forms of cancer, and about the "inability to ensure that the technique has not inflicted some new abnormality" on the child.
Shoukhrat Mitalipov, Ph.D., whose research group at the Oregon Stem Cell Center at OHSU has tested the technology in macaque monkeys, said that their research cohort currently includes four subjects born through mitochondrial manipulation that are almost adults. To date, they have been healthy, with normal blood test results, and are no different from controls, showing that mitochondrial DNA in oocytes can be replaced.
The next step in their research is to recruit families who are carriers of early-onset mitochondrial DNA diseases who have had at least one affected child, recruit healthy egg donors, and then perform the procedure, followed by preimplantation genetic diagnosis of the embryo and/or prenatal diagnosis to "ensure complete mitochondrial DNA replacement and chromosomal normalcy," he said.
The panel was also asked to discuss the use of mitochondrial manipulation as a treatment for infertility. However, members considered this indication a far different type of application than preventing mitochondrial disease, which would have different inclusion criteria, controls, and risk-benefit evaluations, and several panelists raised particular concerns about the use of this technology for infertility.
"The idea we’re going to do anything to infertility patients involving mitochondria I think should be off the table," Dr. Keefe said, noting that there is "a very, very slippery slope when you’re dealing with human reproduction" in the United States, where licensure of infertility clinics is not required.
The controversies of this area of research, which some critics point out would result in a child with three genetically related parents, were not off limits to the open public hearing speakers, including Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society.
"We want to avoid waking up in a world" where researchers, infertility clinics, governments, insurance companies, "or parents decide that they are going to try to engineer children with specific traits and even possibly [put] in motion a regime of high-tech consumer eugenics," she said.
GAITHERSBURG, MD. – The first human studies evaluating the use of genetically modified oocytes to prevent the transmission of mitochondrial diseases could enroll women with diseases that are the most severe, tend to present in early childhood, and are relatively common for a mitochondrial disease, according to panelists at a meeting convened by the Food and Drug Administration to discuss the design of such trials and related issues.
At a meeting on Feb. 25 and 26, members of the FDA Cellular, Tissue, and Gene Therapies Advisory Committee mentioned two mitochondrial diseases in particular, Leigh’s disease and MELAS (mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes), that could be included in initial clinical trials evaluating the safety and efficacy of what the FDA refers to as "mitochondrial manipulation technologies."
This controversial approach, which is being developed to prevent maternal transmission of debilitating and often fatal mitochondrial diseases, entails removing the mitochondrial DNA from an affected woman’s oocyte or embryo and replacing it via assisted reproductive technologies with the mitochondrial DNA from the egg of a healthy donor.
The approach has been studied in animal and in vitro studies, but not yet in humans. However, researchers at Oregon Health and Science University (OHSU), Portland, say they are ready to start a clinical trial based on their results in macaque monkeys.
The FDA called the 2-day meeting to discuss potential clinical trials and to focus on the scientific, technologic, and clinical aspects of the technologies, but not to address public policy or ethical issues. In briefing documents posted before the meeting, the agency acknowledged that there are ethical and policy issues related to genetic modification of eggs and embryos, which can affect regulatory decisions, but added that these issues were "outside the scope" of this meeting.
Regarding clinical trial design and execution, panelists recommended that studies should closely monitor the fetus through gestation, and after birth and long-term follow-up, should include future generations, if female offspring are included. Several panelists supported including only male embryos to minimize the risk of a female passing on damaged DNA to future generations, while others said this would result in a lost opportunity to study the transgenerational risks of the technology.
Other recommendations included avoiding the enrollment of people at high risk of having a baby with a birth defect, or with comorbidities that could affect birth outcome, which would make it more difficult to evaluate the risks of the technologies. The use of controls, panelists said, was problematic, because of the variability in when and how mitochondrial diseases present and because of the relatively small populations of patients affected by these diseases. Historical controls could be used, but larger patient registries are needed, they said.
Panelists also recommended screening egg donors for mitochondrial diseases, and providing informed consent to children born to mothers in the trials when they turn age 18.
It is clear that there is a "deft group of creative, innovative investigators" who can perform these techniques, "which is a good start, but there are so many things we don’t know" that must be evaluated further in animal studies, said panelist Dr. David Keefe, who referred to thalidomide and diethylstilbestrol (DES) as historical examples of therapies that were thought to be promising but proved to have devastating effects.
Another concern Dr. Keefe raised was the possibility that a woman whose risk of having a baby without the inherited defect might be as high as 95% and that she might choose mitochondrial manipulation over preimplantation genetic diagnosis.
"A woman could be led down the primrose path towards a procedure that’s experimental and miss the opportunity to pursue a relatively well-established procedure," said Dr. Keefe, the Stanley H. Kaplan Professor, department of obstetrics and gynecology, New York University.
Another panelist, Dr. Katharine Wenstrom, professor of obstetrics and gynecology, Brown University, Providence, R.I., said that based on her experience with women with genetic diseases, these women "are very vulnerable, and my concern would be how to [provide] consent [for] somebody for whom a pregnancy would be very dangerous and [who] might not consider a pregnancy, but then given the opportunity to have this technique, might agree to a pregnancy that could actually be life threatening."
She also said that she was concerned about whether the technique could deplete mitochondria, which has been associated with several forms of cancer, and about the "inability to ensure that the technique has not inflicted some new abnormality" on the child.
Shoukhrat Mitalipov, Ph.D., whose research group at the Oregon Stem Cell Center at OHSU has tested the technology in macaque monkeys, said that their research cohort currently includes four subjects born through mitochondrial manipulation that are almost adults. To date, they have been healthy, with normal blood test results, and are no different from controls, showing that mitochondrial DNA in oocytes can be replaced.
The next step in their research is to recruit families who are carriers of early-onset mitochondrial DNA diseases who have had at least one affected child, recruit healthy egg donors, and then perform the procedure, followed by preimplantation genetic diagnosis of the embryo and/or prenatal diagnosis to "ensure complete mitochondrial DNA replacement and chromosomal normalcy," he said.
The panel was also asked to discuss the use of mitochondrial manipulation as a treatment for infertility. However, members considered this indication a far different type of application than preventing mitochondrial disease, which would have different inclusion criteria, controls, and risk-benefit evaluations, and several panelists raised particular concerns about the use of this technology for infertility.
"The idea we’re going to do anything to infertility patients involving mitochondria I think should be off the table," Dr. Keefe said, noting that there is "a very, very slippery slope when you’re dealing with human reproduction" in the United States, where licensure of infertility clinics is not required.
The controversies of this area of research, which some critics point out would result in a child with three genetically related parents, were not off limits to the open public hearing speakers, including Marcy Darnovsky, Ph.D., executive director of the Center for Genetics and Society.
"We want to avoid waking up in a world" where researchers, infertility clinics, governments, insurance companies, "or parents decide that they are going to try to engineer children with specific traits and even possibly [put] in motion a regime of high-tech consumer eugenics," she said.
AT AN FDA ADVISORY COMMITTEE MEETING