User login
The urate-lowering therapy febuxostat reduced the number of disease flares that people with early gout experienced relative to placebo in a double-blind, placebo-controlled study.
Febuxostat (Uloric) produced a 12.1% reduction in the overall percentage of patients who experienced at least one gout flare in comparison to placebo (29.3% vs. 41.4%; P less than .05).
Treatment with febuxostat also significantly improved control of serum uric acid, compared with the placebo-treated patients (64.3% vs. 5.7%; P less than .001), as well as synovitis as measured via the rheumatoid arthritis magnetic resonance imaging score (RAMRIS) score (–0.43 vs. –0.07; P less than .001).
“This study indicates that even for people who have only had one or two prior gout flares, urate-lowering therapy to reduce serum urate below 6 mg/dL may have benefit in reduction future flares,” said Dr. Dalbeth of the University of Auckland (New Zealand).
Febuxostat is a xanthine oxidase inhibitor indicated for the chronic management of excess uric acid in the blood in people diagnosed with gout. It is not indicated for use in people who have high serum uric acid levels without a history of gout.
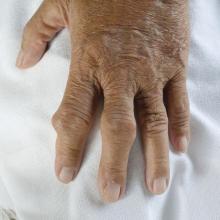
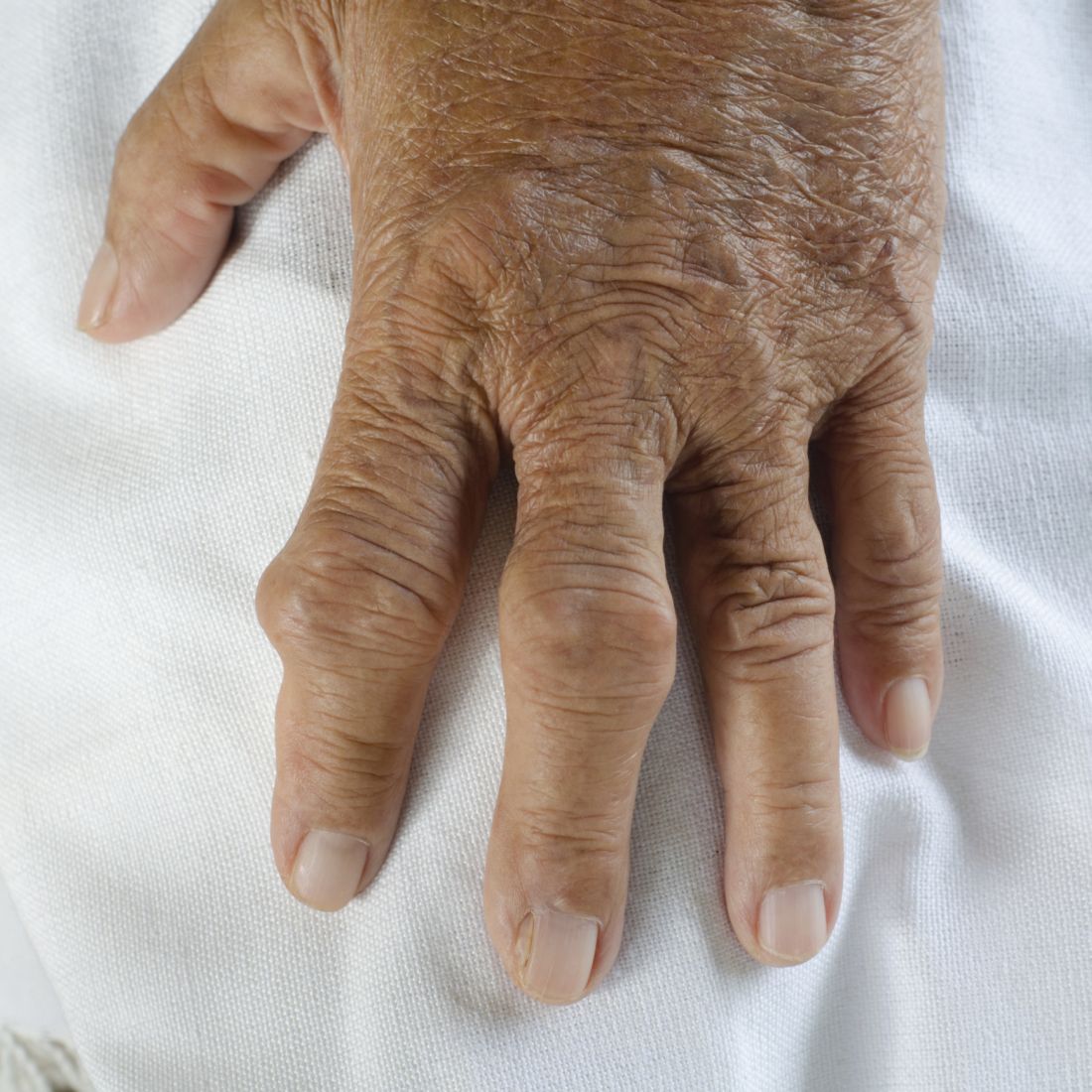
“Although acute self-limiting flares are the most common clinical presentation of gout, joint damage is a frequent complication of the disease,” Dr. Dalbeth and her associates wrote.
“Joint damage is typically a late feature of long-standing gout,” they added, but “longitudinal observational data have indicated that joint damage can occur in some patients with early disease.” Increased urate crystal deposition could be associated with bone erosions, they explained, and the primary aim of the study was to look the rate of bone erosions over the course of 2 years’ treatment.
However, despite this reasoning and some prior clinical evidence, the researchers found no noticeable differences between the study groups in terms of radiologic joint erosion. This was assessed over 2 years in a single-affected joint. The mean change from baseline to month 24 in modified Sharp-van der Heijde erosion scores was 0.01 for both febuxostat and placebo (P = .47).
This was a phase 2 study conducted in 56 centers in the United States that screened 798 subjects and enrolled 314 adults with early gout. For inclusion, patients had to have hyperuricemia, defined as a serum uric acid level of 7.0 mg/dL or higher, and they had to have experienced no more than two gout flares in the past year.
Patients were randomized to once-daily treatment with febuxostat or placebo, with those randomized to the active treatment started at a dose of 40 mg, which could be increased to 80 mg after 2 weeks if serum uric acid levels remained high, at 6.0 mg/dL or above.
“To our knowledge, this is the first randomized, controlled trial examining the effects of [urate-lowering therapy] in patients with early gout,” the authors observed.
Although the primary endpoint was not met, the study “provides important new information about the natural history of joint damage in subjects with gout,” they suggested. In contrast to other inflammatory joint diseases, such as rheumatoid arthritis, joint erosions on plain radiographs were “infrequently observed at baseline” and over the 2-year study period. The fact the there was a change in MRI synovitis suggests that perhaps there may be some underlying joint damage occurring, although the clinical significance of this is uncertain.
Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.
The urate-lowering therapy febuxostat reduced the number of disease flares that people with early gout experienced relative to placebo in a double-blind, placebo-controlled study.
Febuxostat (Uloric) produced a 12.1% reduction in the overall percentage of patients who experienced at least one gout flare in comparison to placebo (29.3% vs. 41.4%; P less than .05).
Treatment with febuxostat also significantly improved control of serum uric acid, compared with the placebo-treated patients (64.3% vs. 5.7%; P less than .001), as well as synovitis as measured via the rheumatoid arthritis magnetic resonance imaging score (RAMRIS) score (–0.43 vs. –0.07; P less than .001).
“This study indicates that even for people who have only had one or two prior gout flares, urate-lowering therapy to reduce serum urate below 6 mg/dL may have benefit in reduction future flares,” said Dr. Dalbeth of the University of Auckland (New Zealand).
Febuxostat is a xanthine oxidase inhibitor indicated for the chronic management of excess uric acid in the blood in people diagnosed with gout. It is not indicated for use in people who have high serum uric acid levels without a history of gout.
“Although acute self-limiting flares are the most common clinical presentation of gout, joint damage is a frequent complication of the disease,” Dr. Dalbeth and her associates wrote.
“Joint damage is typically a late feature of long-standing gout,” they added, but “longitudinal observational data have indicated that joint damage can occur in some patients with early disease.” Increased urate crystal deposition could be associated with bone erosions, they explained, and the primary aim of the study was to look the rate of bone erosions over the course of 2 years’ treatment.
However, despite this reasoning and some prior clinical evidence, the researchers found no noticeable differences between the study groups in terms of radiologic joint erosion. This was assessed over 2 years in a single-affected joint. The mean change from baseline to month 24 in modified Sharp-van der Heijde erosion scores was 0.01 for both febuxostat and placebo (P = .47).
This was a phase 2 study conducted in 56 centers in the United States that screened 798 subjects and enrolled 314 adults with early gout. For inclusion, patients had to have hyperuricemia, defined as a serum uric acid level of 7.0 mg/dL or higher, and they had to have experienced no more than two gout flares in the past year.
Patients were randomized to once-daily treatment with febuxostat or placebo, with those randomized to the active treatment started at a dose of 40 mg, which could be increased to 80 mg after 2 weeks if serum uric acid levels remained high, at 6.0 mg/dL or above.
“To our knowledge, this is the first randomized, controlled trial examining the effects of [urate-lowering therapy] in patients with early gout,” the authors observed.
Although the primary endpoint was not met, the study “provides important new information about the natural history of joint damage in subjects with gout,” they suggested. In contrast to other inflammatory joint diseases, such as rheumatoid arthritis, joint erosions on plain radiographs were “infrequently observed at baseline” and over the 2-year study period. The fact the there was a change in MRI synovitis suggests that perhaps there may be some underlying joint damage occurring, although the clinical significance of this is uncertain.
Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.
The urate-lowering therapy febuxostat reduced the number of disease flares that people with early gout experienced relative to placebo in a double-blind, placebo-controlled study.
Febuxostat (Uloric) produced a 12.1% reduction in the overall percentage of patients who experienced at least one gout flare in comparison to placebo (29.3% vs. 41.4%; P less than .05).
Treatment with febuxostat also significantly improved control of serum uric acid, compared with the placebo-treated patients (64.3% vs. 5.7%; P less than .001), as well as synovitis as measured via the rheumatoid arthritis magnetic resonance imaging score (RAMRIS) score (–0.43 vs. –0.07; P less than .001).
“This study indicates that even for people who have only had one or two prior gout flares, urate-lowering therapy to reduce serum urate below 6 mg/dL may have benefit in reduction future flares,” said Dr. Dalbeth of the University of Auckland (New Zealand).
Febuxostat is a xanthine oxidase inhibitor indicated for the chronic management of excess uric acid in the blood in people diagnosed with gout. It is not indicated for use in people who have high serum uric acid levels without a history of gout.
“Although acute self-limiting flares are the most common clinical presentation of gout, joint damage is a frequent complication of the disease,” Dr. Dalbeth and her associates wrote.
“Joint damage is typically a late feature of long-standing gout,” they added, but “longitudinal observational data have indicated that joint damage can occur in some patients with early disease.” Increased urate crystal deposition could be associated with bone erosions, they explained, and the primary aim of the study was to look the rate of bone erosions over the course of 2 years’ treatment.
However, despite this reasoning and some prior clinical evidence, the researchers found no noticeable differences between the study groups in terms of radiologic joint erosion. This was assessed over 2 years in a single-affected joint. The mean change from baseline to month 24 in modified Sharp-van der Heijde erosion scores was 0.01 for both febuxostat and placebo (P = .47).
This was a phase 2 study conducted in 56 centers in the United States that screened 798 subjects and enrolled 314 adults with early gout. For inclusion, patients had to have hyperuricemia, defined as a serum uric acid level of 7.0 mg/dL or higher, and they had to have experienced no more than two gout flares in the past year.
Patients were randomized to once-daily treatment with febuxostat or placebo, with those randomized to the active treatment started at a dose of 40 mg, which could be increased to 80 mg after 2 weeks if serum uric acid levels remained high, at 6.0 mg/dL or above.
“To our knowledge, this is the first randomized, controlled trial examining the effects of [urate-lowering therapy] in patients with early gout,” the authors observed.
Although the primary endpoint was not met, the study “provides important new information about the natural history of joint damage in subjects with gout,” they suggested. In contrast to other inflammatory joint diseases, such as rheumatoid arthritis, joint erosions on plain radiographs were “infrequently observed at baseline” and over the 2-year study period. The fact the there was a change in MRI synovitis suggests that perhaps there may be some underlying joint damage occurring, although the clinical significance of this is uncertain.
Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: Gout flares over 2 years occurred in 29.3% of subjects treated with febuxostat versus 41.4% of those given placebo (P less than .05)
Data source Randomized, double-blind, placebo-controlled study assessing febuxostat versus placebo effects on joint damage in 314 hyperuricemic subjects with early gout.
Disclosures: Takeda, which makes febuxostat, funded the study. Dr. Dalbeth has received consulting fees, speaker fees, or research grants from Takeda, CymaBay, Ardea Biosciences, AstraZeneca, and Horizon. A couple of her coauthors disclosed acting as consultants to or receiving research funding from Takeda, and three were current or past employees of the company.