User login
The typical patient voids 16 times a day and 2 or more times at night. In later stages of the disease, she may urinate as often as 60 times a day and every half hour at night, severely eroding her ability to hold a job, travel, or lead a normal life. In fact, her quality of life may be impaired as much as that of a person with end-stage renal disease.1,2 She sees an average of 4 doctors and endures irritative voiding symptoms for 4 years before her disease is identified. The cause is unknown.
Interstitial cystitis produces a wide spectrum of symptom severity, occurring episodically with spontaneous flare-ups and remission, or with continuous, intractable urinary urgency and pain. Until recently, women presenting with urinary urgency, frequency, and pain were presumed to have a urinary tract infection (UTI) or overactive bladder, and were often treated—to no avail—with multiple courses of antibiotics or anticholinergics.
Fortunately, interstitial cystitis is gaining recognition, and effective treatments are emerging. Usually the ObGyn—often the first physician a woman consults—need refer only the refractory cases to a specialist. This article describes the components of diagnosis and the most effective treatments, including use of the first-line agents amitriptyline (Elavil) and pentosan polysulfate sodium (Elmiron).
CASE Is overactive bladder the cause of stubborn symptoms?
“R.H.,” a healthy 48-year-old G2P2 with a 5-year history of urinary urgency and frequency, reports that she voids “at least 15 times per day.” She denies any urge incontinence, but says she experiences occasional stress incontinence if she has a bad cold. Four years ago, she saw a urologist for these symptoms, after her husband said he was tired of having to stop the car so she could go to the bathroom. The urologist diagnosed a “small bladder,” performed urethral “stretching,” and prescribed oxybutynin.
Her symptoms improved for about 6 months, but then progressed and have now worsened. She began taking tolterodine, 4 mg daily, 2 months ago, as prescribed by her primary care physician. The sensation of painful urgency has eased, but there has been no change in frequency. R.H. used to wake as often as 4 times a night with the urge to urinate, but since she began taking zolpidem tartrate (Ambien) as a sleep aid, she now wakes only 2 times every night.
Why are her symptoms so persistent?
This woman’s case is a classic example of interstitial cystitis masquerading as overactive bladder. Treatment with anticholinergic drugs may ease urgency symptoms slightly, but has no real effect on frequency.
This case has 5 hallmarks of the syndrome of interstitial cystitis:
- Urgency
- Frequency (more than 8 voids/day, taking fluid intake into account)
- Bladder pain
- Nocturia (more than twice)
- Absence of a genitourinary tract infection
Patients show signs of “battle fatigue”
Women with interstitial cystitis may be anxious, depressed, angry, and sleep-deprived. In some women, stress exacerbates the urinary symptoms and pain (as do certain common foods and beverages, especially citrus, tomatoes, and caffeine).
Approximately 60% of patients report dyspareunia, and many report chronic pelvic pain. In fact, 75% of women who report chronic pelvic pain also have irritative voiding symptoms. Therefore, it is important to ask about lower urinary tract symptoms whenever a woman presents with pelvic pain.3,4
Pain may be suprapubic, vaginal, perineal, or originate in the groin or lower back. Although 16% of patients present solely with pain, and 30% have only urinary frequency, most patients suffer from both symptoms.
Approximately 40% report premenstrual or ovulatory exacerbation of symptoms, although symptoms may improve during pregnancy.5 Voided volumes are usually small, despite the strong urgency, which does not always resolve. Pelvic pain may ease after voiding but recurs shortly.
Insidious, worsening course
Symptoms appear insidiously and worsen to a “final” stage within 5 to 15 years, at which point a plateau is reached with little further progression.6 Some experts suggest that the disease be classified as “early non-ulcerous” or “classic ulcerous.”
- In early disease, bladder capacity exceeds 450 cc under anesthesia, with glomerulations and hemorrhage.
- In classic disease, bladder capacity is less than 450 cc under anesthesia, and Hunner’s ulcers and fissures are evident. Hunner’s ulcers are described as “a central scar with small fibrin deposits before distension, and post-distension edema.”7
For now, however, there are no agreed-upon markers to distinguish the 2 types of disease.
The female-to-male ratio is 9:1, and about 500,000 to more than 1 million adults in the United States are thought to have interstitial cystitis.8 Caucasian women constitute 95% of patients, and the average age at diagnosis is 45 years. Thirty percent of women with interstitial cystitis are 30 years old or younger. Significantly more women with interstitial cystitis have had a hysterectomy than controls.9
For a diagnosis, skip the NIH criteria
Symptoms
Although the National Institutes of Health (NIH) established diagnostic criteria for research subjects, the criteria are overly stringent—60% of women with symptoms typical of interstitial cystitis do not qualify, but should not necessarily be excluded from diagnosis and treatment.
When a woman has the hallmark symptoms listed on page 57, but also reports continuous pain or dysmenorrhea, other pelvic pathology such as endometriosis should be considered, although interstitial cystitis should be included in the differential diagnosis of any woman reporting pelvic pain.
Incontinence is atypical. If present, it merits an incontinence evaluation to detect detrusor hyperreflexia or detrusor-sphincter dyssynergia.
Dysuria suggests a UTI, urethral diverticulum, urogenital atrophy, or vaginitis. Many patients present with an erroneous diagnosis of “recurrent UTIs.”
Diagnostic tools
Voiding diaries are useful and can be revealing. The Pelvic Pain and Urinary Frequency (PUF) scale, developed by Parsons, is helpful in predicting interstitial cystitis (see the Clip-and-save chart). The higher the score, the greater the likelihood of interstitial cystitis, particularly with a score of more than 8.
Another tool is the O’Leary-Sant Index, which measures pain, voiding symptoms, and quality of life.
Physical examination and laboratory studies
Perform a pelvic exam to rule out other diseases and pelvic pathology, including sexually transmitted diseases, urethral diverticulum, and pelvic masses. Typically, the pelvic exam in women with interstitial cystitis is negative except for suprapubic and/or trigonal tenderness.
Urinalysis, culture, and sensitivity are warranted but are usually negative.
Cytology should be analyzed if microscopic hematuria is present, or with other risk factors such as a history of smoking or age over 40.
Obtain cultures for sexually transmitted diseases if clinically indicated.
Urodynamic studies are not necessary to diagnose interstitial cystitis. However, if incontinence is present, a cystometrogram can confirm detrusor hyperreflexia. Otherwise the cystometrogram is normal except for heightened sensation or pain with bladder filling, or a bladder capacity of less than 350 cc.
The potassium sensitivity test: Useful but painful
Women with interstitial cystitis are thought to have increased bladder permeability that allows potassium to pass through to the detrusor muscle. Thus, the potassium sensitivity test often is used to diagnose the condition. The test is an office procedure in which 2 separate solutions are instilled into the bladder: 40 cc sterile water followed by 40 cc of a solution of 400 mEq potassium per liter of water. After each solution is instilled, the patient is monitored for symptoms. The test is positive when the patient responds only to the potassium.
The response may be marked and painful, and the bladder should be emptied immediately. Subsequent irrigation with sterile water may be necessary to alleviate the discomfort caused by the potassium solution. Symptoms provoked by the test generally subside after bladder emptying, but can persist and cause moderate distress, which limits the utility of this office-based test.
Parsons et al10 demonstrated an 81% positive response (197 of 244 women) to the test among women with pelvic pain, compared with 0 of 47 patients with no pelvic pain. They also found that 70% of patients with interstitial cystitis and 4% of controls had a positive response.
If a woman is extremely volume-sensitive during the water phase, the potassium phase may not be accurate. A false-positive response can be caused by infection or prior exposure to radiation or chemotherapy. A thorough history is imperative.
The gold standard: Cystoscopy under anesthesia
Cystoscopy with hydrodistention under general anesthesia is the surest way to diagnose interstitial cystitis or rule it out. Sterile water or saline is infused until bladder capacity is reached. Bladder rupture occurs in up to 10% of patients, so careful inspection during filling is crucial. After 5 minutes of distension, bladder volume is measured into a calibrated beaker. Terminal hematuria (the last 50 cc of effluent) often is noted.
Normal bladder capacity under anesthesia is 1,000 cc, but it is reduced in women with interstitial cystitis. Bladder capacity of 450 cc or less under anesthesia indicates a more contracted bladder and a later-stage disease. Glomerulations, petechiae, fissures, or (rarely) Hunner’s ulcers typically are visible, regardless of bladder volume. However, the presence of glomerulations does not necessarily make the diagnosis, because they can be found in asymptomatic women. Further, cystoscopic observations do not always correlate with the severity of symptoms (nor does positive biopsy always reflect interstitial cystitis).
Hydrodistention is not only diagnostic, but also can be therapeutic, as sympathetic nerve fiber density decreases afterward.11 However, the need for this procedure is under debate, due to the limitations described above. A bladder capacity less than 1,000 cc with the presence of glomerulations or petechiae and fissures, with or without the Hunner’s ulcers, constitutes a definitive diagnosis.
Cystoscopy under anesthesia is recommended because medical treatment can be costly and cause significant side effects. An accurate diagnosis should precede therapy to avoid misdirected therapy in a patient who does not have interstitial cystitis. Moreover, cystoscopy can rule out bladder neoplasms or other diseases. Some bladder carcinomas have been missed in women treated empirically for interstitial cystitis.12
Cystoscopic images “paint a thousand words.” When a woman sees her cystoscopy images, the picture indeed “paints a thousand words.” For many women, the images “justify” their symptoms and confirm that the disease is real.
CASE Don’t treat a UTI without a positive culture
“M.P.” is a healthy 44-year-old G2P2 with a history of recurrent UTIs. Approximately 14 months ago, while on vacation, she began having symptoms of urinary frequency, urgency, and lower abdominal pain that were relieved with voiding. She called her primary care physician, who prescribed levofloxacin and phenazopyridine over the phone for a presumed UTI. Since the patient was out of town, a urine culture was not obtained.
When M.P. returned from vacation, her symptoms recurred, so she underwent urinalysis, including culture and sensitivity, and began a 7-day course of nitrofurantoin (100 mg twice daily). When her symptoms did not improve by day 4, a second course of levofloxacin was given. The urine culture was sterile. As her physician recommended, M.P. increased her fluid intake, including water and cranberry juice. She also avoided sexual relations, since they exacerbated her symptoms, which improved overall but did not clearly abate.
Three months later her symptoms returned in full force.
How would you treat this patient?
Interstitial cystitis can produce symptoms consistent with a lower UTI, but urine cultures will be negative and the response to antibiotics will be minimal. Many patients call their physicians and report “another UTI.” However, if the woman is healthy with no history of renal disease or diabetes, consider interstitial cystitis. Obtain urine culture results from other physicians, if possible, to determine whether bacterial infection was ever confirmed.
Cranberry juice is acidic and may exacerbate urgency and pain.
What to tell patients
The Interstitial Cystitis Association (ICA) encourages patients to become involved in their own care. The ICA was formed in 1984 by women with painful bladder symptoms who had been told by their physicians that nothing was wrong. The organization provides patients with clinical research updates, clinical trial opportunities, and literature and information.
Once the diagnosis is confirmed, patient education and counseling are imperative. Compliance is critical.
There is no cure for interstitial cystitis; the disease is chronic, with relapses and remissions. Although it does not progress once it develops fully, improvement is slow, usually occurring after 3 months or more of treatment. No single treatment works for all patients, so empiric trials with various agents may be needed. Treatment often is multimodal, and the rationale for each therapy should be explained.
Have the patient keep a voiding diary before and after treatment, as well as during any flare-up, to provide evidence of improvement and identify triggers. Also instruct her to pay attention to any foods or activities that exacerbate her symptoms (eg, caffeine, sexual activity).
Treatment
Does a change in diet help?
Some foods and beverages apparently exacerbate symptoms, although the link between foods and symptoms has not been fully investigated. About 53% of patients with interstitial cystitis associate symptom aggravation with dietary factors, especially acidic foods and beverages.5 Dietary restrictions should be attempted for 1 to 2 weeks to determine which foods to avoid.
Gillespie13 found elevated urine levels of tryptophan metabolites in women with hypersensitive bladders, compared with controls.
Tryptophan metabolites may disrupt the glycosaminoglycan layer of the bladder epithelium, as seen in a study involving rabbit bladders.14
Urge suppression
Another helpful strategy is having the patient increase the time between voids using distraction techniques and by contracting the pelvic floor muscles and overriding the first urge to void.
Oral medications
Pentosan polysulfate sodium is a glycosaminoglycan with an affinity for mucosal membranes. It is approved by the Food and Drug Administration (FDA) for the treatment of interstitial cystitis. The mechanism by which it reduces pain and urinary frequency is unclear, but it may replace the deficient glycosaminoglycan layer in the bladder epithelium.
Pain relief occurs in approximately 40% to 60% of patients after 3 months of therapy (100 mg orally 3 times daily).15
The patient should clearly understand that beneficial effects may not occur for 3 to 6 months, and that patience is necessary to give the drug an adequate trial. The response is maintained over the long term, and the drug should be used indefinitely. Pentosan polysulfate sodium is well tolerated, although gastrointestinal side effects and reversible alopecia occur in 4% of patients.
Performing a cystoscopy under anesthesia with hydrodistension is not always necessary prior to starting pentosan polysulfate, as long as the patient is not at risk for bladder neoplasms. However, prior to starting the drug the minimal evaluation should include a voiding diary and either the PUF questionnaire or the potassium sensitivity test.
Antihistamines. If the patient has a history of allergies, or mast cells were confirmed on bladder biopsy, an antihistamine such as hydroxyzine should be given along with pentosan polysulfate sodium. Hydroxyzine has an inhibitory effect on bladder mast cells, as well as anticholinergic and analgesic properties, which improve typical symptoms of interstitial cystitis.
Initiate hydroxyzine at a dose of 10 to 25 mg at bedtime for 1 week, gradually increasing to 50 to 75 mg. Side effects include drowsiness, which is beneficial for women who have nocturia. Other effects are dry mouth and a bitter taste.
Amitriptyline hydrochloride also has analgesic, antihistaminic, anticholinergic, and sedative effects. Amitriptyline is a noradrenaline and serotonin reuptake inhibitor that blocks nociception in the central nervous system.
Compared with placebo, amitriptyline significantly improved symptom scores, pain, and urgency intensity. In a study by van Ophoven and colleagues,16 50 patients (44 women, 6 men) were randomly assigned to amitriptyline at self-titrating doses or placebo. O’Leary-Sant symptom scores, pain, and urgency intensity improved significantly in the amitriptyline group, compared with placebo.
Anticholinergic side effects (eg, dry mouth, constipation), weight gain, and sedation occur in 20% to 80% of patients. In an open-label study of amitriptyline for interstitial cystitis,17 long-term efficacy (mean of 17 months) revealed a 64% response rate (60 of 94 patients) using the global response assessment questionnaire.
Start amitriptyline at a dose of 10 to 25 mg at bedtime, gradually increasing to 75 mg as tolerated. Sedation becomes a limiting factor in the higher doses. Other tricyclic antidepressants have not been studied to any significant extent in treating interstitial cystitis. When used as part of a multimodal treatment in addition to pentosan polysulfate sodium, amitriptyline may be tapered off once remission is attained. No studies have compared treatment response using pentosan polysulfate sodium with and without amitriptyline.
Calcium channel blockers (nifedipine) and drugs for neuropathic pain (gabapentin) are being investigated.
Anticholinergic and antispasmodic agents are typically ineffective in women with interstitial cystitis. In fact, if a patient has no improvement in her symptoms after these drugs are tried, interstitial cystitis should be strongly considered.
Oral L-arginine (1,500–3,000 mg per day, divided doses) improved symptoms in a small study by increasing nitric oxide synthase activity.18
NSAIDs are used adjunctively and may help reduce pain.
Intravesical therapy
Patients unable to tolerate oral medications may benefit from intravesical therapy. It can also be used as an adjunct to oral therapy. Intravesical therapy delivers drugs directly to the bladder wall with a low incidence of side effects. Risks include a potential for UTI via catheterization, as well as transient chemical cystitis, which exacerbates symptoms. A variety of therapeutic “cocktails” are used.
Dimethyl sulfoxide (DMSO) is the only other drug, besides pentosan polysulfate, approved by the FDA for treatment of interstitial cystitis. DMSO has antiinflammatory, analgesic, and muscle-relaxant effects, and inhibits mast-cell activity. DMSO induces remission in 50% to 70% of patients for up to 24 months.19
Lidocaine jelly is injected intraurethrally, followed by instillation of 50 cc of DMSO (alone or with heparin, sodium bicarbonate, and Solu-Cortef). This solution is held in the bladder for 20 to 30 minutes before voiding.
DMSO is secreted through the lungs and skin and has a garlic-like odor. Treatments are administered every 1 to 2 weeks for a total of 4 to 8 treatments. If the condition relapses, DMSO can be reinstituted on a long-term basis. Motivated patients can be taught to administer this treatment themselves.
Heparin is another option. It is administered at a dose of 10,000 U thrice weekly.
Hyaluronic acid. In a small study involving 20 patients, weekly intravesical hyaluronic acid improved symptoms in 65% of patients, with a 40% and 30% decrease in nocturia and pain, respectively.20
Intravesical pentosan polysulfate sodium is another option that improves symptoms and increases bladder capacity,21 although larger studies of its efficacy are lacking.
Bacillus Calmette-Guérin (BCG) solution intravesically had a 60% response rate in 1 study (versus 27% for placebo).22 The mechanism of action is unknown, but the solution may modulate the bladder immune response. Additional studies are pending.
Although their link to interstitial cystitis has not been proven, these foods are thought to be implicated and should be limited or avoided
ACIDIC FOODS
All alcoholic beverages
Apples
Apple juice
Cantaloupe
Carbonated drinks
Chilies/spicy foods
Citrus fruits (lemons, limes, oranges, etc)
Coffee
Cranberries
Grapes
Guava
Lemon juice
Peaches
Pineapples
Plums
Strawberries
Tea
Tomatoes
Vinegar
FOODS HIGH IN TYROSINE, TRYPTOPHAN, OR ASPARTATE
Aspartame
Avocado
Bananas
Beer
Brewer’s yeast
Canned figs
Champagne
Cheese
Chicken livers
Chocolate
Corned beef
Cranberries
Fava beans
Lima beans
Mayonnaise
Nuts
Onions
Pickled herring
Pineapple
Prunes
Raisins
Rye bread
Saccharine
Sour cream
Soy sauce
Wines
Yogurt
Vitamins buffered with aspartate
Adapted from You Don’t Have to Live with Cystitis! by Larrian Gillespie, MD (revised and updated 1996)
1. Altered bladder permeability
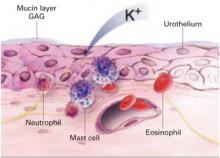
In interstitial cystitis, the protective glycosaminoglycan layer of the bladder epithelium may be deficient, increasing bladder permeability. Antiproliferative factor in the urine may impair the proliferation and repair of urothelium, increasing bladder permeability further.28 This breakdown allows potassium to penetrate the urothelium, stimulating pain receptors and causing an inflammatory response in the detrusor muscle.
Loss of the normal protective layer
In a normal bladder, the epithelium is protected by an ionic, hydrophilic, glycosaminoglycan (GAG) layer that serves as a barrier against urine, which is hyperosmolar and rich in acid and potassium. When the GAG layer is deficient, as it is thought to be in interstitial cystitis, bladder permeability increases, triggering an inflammatory response that inhibits repair of the GAG layer, creating a vicious cycle of exposure, inflammation, and pain.
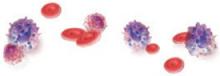
2. Mast cell activation
Mast cell degranulation may cause or contribute to interstitial cystitis and produce its hallmark symptoms. Mast cells also may be activated in response to a noxious factor. These cells secrete histamine, prostaglandins, leukotrienes, cytokines, and chemotactic factors. Urine from women with interstitial cystitis contains histamine, histamine metabolites, and tryptase,29,30 and electron microscopy of bladder biopsies from affected women shows degranulating mast cells adjacent to sensory nerve fibers. When these fibers are stimulated, they release neuropeptides (such as substance P) and may promote inflammation by activating mast cells and nearby nerve terminals.31
3. Inflammation
Inflammation clearly plays a role in interstitial cystitis. Bladder biopsies reveal mild to severe inflammation, and the presence of T cells, B cells, plasma cells, neutrophils, eosinophils, and mast cells. Inflammatory mediators such as kallikrein, interleukin-6, interleukin-2 inhibitor, and neutrophil chemotactic factor are increased in the urine of individuals with the disease.
4. Autoimmunity
Clinical features of interstitial cystitis that mimic those of other autoimmune diseases include chronic symptoms that wax and wane; higher prevalence in women; immunological deposits in bladder biopsies with mononuclear cell infiltrates, which suggests the presence of bladder autoantigens; association with other autoimmune disorders such as Sjögren’s syndrome and lupus; and, in some cases, a positive response to steroids or other immunosuppressants.32
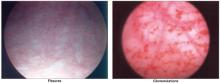
Glomerulations and fissures are telltale signs
When the bladder is distended under anesthesia, examination may reveal a pattern of fissures and glomerulations, which are hallmarks of interstitial cystitis. These findings are sometimes present in asymptomatic women as well, and do not always correlate with severity of symptoms.
5. Infection
Some experts have postulated that occult infection with fastidious organisms, fungi, or viruses plays a role in the development of interstitial cystitis. However, special cultures, serology, and electron microscopy have not shown any organisms consistently associated with the disease.33
The use of polymerase chain reaction techniques to test for bacterial DNA in bladder biopsies of patients with interstitial cystitis has yielded conflicting results.34,35 Infection may be the inciting event that injures the bladder epithelium, causing a cascade of inflammation.
6. Neurologic changes
Studies have revealed increased sympathetic nerve fiber density in the bladders of patients with interstitial cystitis.31,36 The disease may also be a type of reflex-sympathetic dystrophy with increased and abnormal spinal sympathetic activity. Butrick describes interstitial cystitis as a “visceral pain syndrome.”37 C-fibers (silent afferents) transmit pain when activated by a prolonged or noxious stimulus. This leads to neuroplastic changes that lower the threshold of nociceptive nerves, thus reducing the amount of stimulus needed to provoke pain (allodynia). Pelvic viscera share innervation, which may explain the association between
interstitial cystitis and irritable bowel syndrome and endometriosis. Interstitial cystitis is not limited to the bladder; it involves chronic neuropathic inflammation, afferent overactivity, and central sensitization. Increased pain perception may cause pelvic floor muscle instability, spasm, and hypertonic state.38
Alternative therapies
Electrical nerve stimulation
This transcutaneous modality improves symptoms in 25% to 50% of patients.23 It is thought to stimulate the afferent nerves, thereby activating the inhibitory circuits and decreasing the sensation of pain.
Sacral neuromodulation is another modality being studied for the treatment of interstitial cystitis. So far it has significantly reduced urinary urgency-frequency symptoms as well as pain.24
Other new therapies under investigation include intravesical injection of botulinum toxin, resiniferatoxin, gene therapy, and nerve growth-factor inhibitors.
Surgery: High relapse rates make it a last resort
The treatment of visible ulcers by resection or laser ablation improves symptoms but carries a relapse rate of more than 50%.25 More aggressive surgeries, with cure rates ranging from 50% to 80%, include denervation procedures, augmentation cystoplasty for severely contracted bladders (not necessarily due to interstitial cystitis), cecocystoplasty (where a segment of cecum is excised and reanastomosed with the bladder to increase bladder capacity), and total cystourethrectomy and urinary diversion. However, persistent pain has been reported after these invasive procedures, and permanent intermittent self-catheterization and/or reoperation is often required.26,27
Dr. LaSala is a speaker for Pfizer, Inc.
1. Held PJ, Janno PM, Wein AJ, et al. Epidemiology of IC. In: Hanno P, Staskin DR, Krane RJ, et al, eds. Interstitial Cystitis. New York: Springer-Verlag; 1990:29–48.
2. Ratner V, Slade D, Greene G. Interstitial cystitis: a patient’s perspective. Urol Clin North Am. 1994;21:1-5.
3. Parson CL, Bullen M, Kahn BS, et al. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98:127-132.
4. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002;100:337-341.
5. Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994;21:121-130.
6. Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465-469.
7. Parsons CL. Interstitial cystitis: clinical manifestations and diagnostic criteria in over 200 cases. Neurourol Urodyn. 1990;9:241-250.
8. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2-9.
9. Kozial JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7-20.
10. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187:1395-1400.
11. Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am. 1994;21:163-176.
12. Tissot WD, Diokno AC, Peters KM. A referral center’s experience with transitional cell carcinoma misdiag-nosed as interstitial cystitis. J Urol. 2004;172:478-480.
13. Gillespie L. Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br J Urol. 1993;72:293-297.
14. Kaufman JE, Anderson K, Parsons CL. Inactivation of antiadherence effect of bladder surface glycosaminoglycans as possible mechanism for carcinogenesis. Urology. 1987;30:255-258.
15. Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(suppl 5A):93-99.
16. Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533-536.
17. van Ophoven, Hertle L. Long-term results of amitripty-line treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
18. Smity SD, Ehheler MA, Foster HE, Weiss RM. Improvement in interstitial cystitis symptom scores during treatment with L-arginine. J Urol. 1997;158:703-708.
19. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36-39.
20. Kallestrup EB, Jorgensen SS, Nordling J, et al. Treatment of interstitial cystitis with Cystistat: a hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143-147.
21. Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosan polysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168-171.
22. Peters K, Diokno A, Steinart B, et al. The efficacy of intravesical Tice strain Bacillus Calmette-Guérin in the treatment of interstitial cystitis: a double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090-2094.
23. Fall M, Lindstrom S. Transcutaneous electrical nerve stimulation in classic and nonulcer interstitial cystitis. Urol Clin North Am. 1994;21:133-139.
24. Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
25. Malloy TR, Shanberg AM. Laser therapy for interstitial cystitis. Urol Clin North Am. 1994;21:141-144.
26. Smith RB, Van Caugh P, Skinner DG, et al. Augmentation enterocystoplasty: a critical review. J Urol. 1977;118:35-39.
27. Chakravarti A, Ganta S, Somani B, Jones MA. Caecocystoplasty for intractable interstitial cystitis: long-term results. Eur Urol. 2004;46:114-117.
28. Erickson DR, Zie SX, Bhavanandan VP, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461.-
29. El-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:250-353.
30. Boucher W, El-Mansoury M, Pang X, Sant GR. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol. 1995;76:94-100.
31. Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744-750.
32. Oravisto KJ. Interstitial cystitis as an autoimmune disease. Eur Urol. 1980;6:10-13.
33. Duncan JL, Schaeffer AJ. Do infectious agents cause interstitial cystitis? Urology. 1997;49:48-51.
34. Haarala M, Jalava J, Laato M, Kilholma P, Nurmi M, Alanen A. Absence of bacterial DNA in the bladder of patients with interstitial cystitis. J Urol. 1996;156:1843-1845.
35. Keay S, Zhang C-O, Bladwin BR, Jacobs SC, Warren JW. Polymerase chain reaction amplification of bacterial 16S rRNA genes in interstitial cystitis and control patient bladder biopsies. J Urol. 1998;159:280-283.
36. Hohenfeller M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587-591.
37. Butrick CW. Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis and treatment. Clin Obstet Gynecol. 2003;46:812-814.
38. Moldwin RM, Fried-Siegel J, Mendelowitz F. Pelvic floor dysfunction and interstitial cystitis. J Urol. 1994;151:285A.-
The typical patient voids 16 times a day and 2 or more times at night. In later stages of the disease, she may urinate as often as 60 times a day and every half hour at night, severely eroding her ability to hold a job, travel, or lead a normal life. In fact, her quality of life may be impaired as much as that of a person with end-stage renal disease.1,2 She sees an average of 4 doctors and endures irritative voiding symptoms for 4 years before her disease is identified. The cause is unknown.
Interstitial cystitis produces a wide spectrum of symptom severity, occurring episodically with spontaneous flare-ups and remission, or with continuous, intractable urinary urgency and pain. Until recently, women presenting with urinary urgency, frequency, and pain were presumed to have a urinary tract infection (UTI) or overactive bladder, and were often treated—to no avail—with multiple courses of antibiotics or anticholinergics.
Fortunately, interstitial cystitis is gaining recognition, and effective treatments are emerging. Usually the ObGyn—often the first physician a woman consults—need refer only the refractory cases to a specialist. This article describes the components of diagnosis and the most effective treatments, including use of the first-line agents amitriptyline (Elavil) and pentosan polysulfate sodium (Elmiron).
CASE Is overactive bladder the cause of stubborn symptoms?
“R.H.,” a healthy 48-year-old G2P2 with a 5-year history of urinary urgency and frequency, reports that she voids “at least 15 times per day.” She denies any urge incontinence, but says she experiences occasional stress incontinence if she has a bad cold. Four years ago, she saw a urologist for these symptoms, after her husband said he was tired of having to stop the car so she could go to the bathroom. The urologist diagnosed a “small bladder,” performed urethral “stretching,” and prescribed oxybutynin.
Her symptoms improved for about 6 months, but then progressed and have now worsened. She began taking tolterodine, 4 mg daily, 2 months ago, as prescribed by her primary care physician. The sensation of painful urgency has eased, but there has been no change in frequency. R.H. used to wake as often as 4 times a night with the urge to urinate, but since she began taking zolpidem tartrate (Ambien) as a sleep aid, she now wakes only 2 times every night.
Why are her symptoms so persistent?
This woman’s case is a classic example of interstitial cystitis masquerading as overactive bladder. Treatment with anticholinergic drugs may ease urgency symptoms slightly, but has no real effect on frequency.
This case has 5 hallmarks of the syndrome of interstitial cystitis:
- Urgency
- Frequency (more than 8 voids/day, taking fluid intake into account)
- Bladder pain
- Nocturia (more than twice)
- Absence of a genitourinary tract infection
Patients show signs of “battle fatigue”
Women with interstitial cystitis may be anxious, depressed, angry, and sleep-deprived. In some women, stress exacerbates the urinary symptoms and pain (as do certain common foods and beverages, especially citrus, tomatoes, and caffeine).
Approximately 60% of patients report dyspareunia, and many report chronic pelvic pain. In fact, 75% of women who report chronic pelvic pain also have irritative voiding symptoms. Therefore, it is important to ask about lower urinary tract symptoms whenever a woman presents with pelvic pain.3,4
Pain may be suprapubic, vaginal, perineal, or originate in the groin or lower back. Although 16% of patients present solely with pain, and 30% have only urinary frequency, most patients suffer from both symptoms.
Approximately 40% report premenstrual or ovulatory exacerbation of symptoms, although symptoms may improve during pregnancy.5 Voided volumes are usually small, despite the strong urgency, which does not always resolve. Pelvic pain may ease after voiding but recurs shortly.
Insidious, worsening course
Symptoms appear insidiously and worsen to a “final” stage within 5 to 15 years, at which point a plateau is reached with little further progression.6 Some experts suggest that the disease be classified as “early non-ulcerous” or “classic ulcerous.”
- In early disease, bladder capacity exceeds 450 cc under anesthesia, with glomerulations and hemorrhage.
- In classic disease, bladder capacity is less than 450 cc under anesthesia, and Hunner’s ulcers and fissures are evident. Hunner’s ulcers are described as “a central scar with small fibrin deposits before distension, and post-distension edema.”7
For now, however, there are no agreed-upon markers to distinguish the 2 types of disease.
The female-to-male ratio is 9:1, and about 500,000 to more than 1 million adults in the United States are thought to have interstitial cystitis.8 Caucasian women constitute 95% of patients, and the average age at diagnosis is 45 years. Thirty percent of women with interstitial cystitis are 30 years old or younger. Significantly more women with interstitial cystitis have had a hysterectomy than controls.9
For a diagnosis, skip the NIH criteria
Symptoms
Although the National Institutes of Health (NIH) established diagnostic criteria for research subjects, the criteria are overly stringent—60% of women with symptoms typical of interstitial cystitis do not qualify, but should not necessarily be excluded from diagnosis and treatment.
When a woman has the hallmark symptoms listed on page 57, but also reports continuous pain or dysmenorrhea, other pelvic pathology such as endometriosis should be considered, although interstitial cystitis should be included in the differential diagnosis of any woman reporting pelvic pain.
Incontinence is atypical. If present, it merits an incontinence evaluation to detect detrusor hyperreflexia or detrusor-sphincter dyssynergia.
Dysuria suggests a UTI, urethral diverticulum, urogenital atrophy, or vaginitis. Many patients present with an erroneous diagnosis of “recurrent UTIs.”
Diagnostic tools
Voiding diaries are useful and can be revealing. The Pelvic Pain and Urinary Frequency (PUF) scale, developed by Parsons, is helpful in predicting interstitial cystitis (see the Clip-and-save chart). The higher the score, the greater the likelihood of interstitial cystitis, particularly with a score of more than 8.
Another tool is the O’Leary-Sant Index, which measures pain, voiding symptoms, and quality of life.
Physical examination and laboratory studies
Perform a pelvic exam to rule out other diseases and pelvic pathology, including sexually transmitted diseases, urethral diverticulum, and pelvic masses. Typically, the pelvic exam in women with interstitial cystitis is negative except for suprapubic and/or trigonal tenderness.
Urinalysis, culture, and sensitivity are warranted but are usually negative.
Cytology should be analyzed if microscopic hematuria is present, or with other risk factors such as a history of smoking or age over 40.
Obtain cultures for sexually transmitted diseases if clinically indicated.
Urodynamic studies are not necessary to diagnose interstitial cystitis. However, if incontinence is present, a cystometrogram can confirm detrusor hyperreflexia. Otherwise the cystometrogram is normal except for heightened sensation or pain with bladder filling, or a bladder capacity of less than 350 cc.
The potassium sensitivity test: Useful but painful
Women with interstitial cystitis are thought to have increased bladder permeability that allows potassium to pass through to the detrusor muscle. Thus, the potassium sensitivity test often is used to diagnose the condition. The test is an office procedure in which 2 separate solutions are instilled into the bladder: 40 cc sterile water followed by 40 cc of a solution of 400 mEq potassium per liter of water. After each solution is instilled, the patient is monitored for symptoms. The test is positive when the patient responds only to the potassium.
The response may be marked and painful, and the bladder should be emptied immediately. Subsequent irrigation with sterile water may be necessary to alleviate the discomfort caused by the potassium solution. Symptoms provoked by the test generally subside after bladder emptying, but can persist and cause moderate distress, which limits the utility of this office-based test.
Parsons et al10 demonstrated an 81% positive response (197 of 244 women) to the test among women with pelvic pain, compared with 0 of 47 patients with no pelvic pain. They also found that 70% of patients with interstitial cystitis and 4% of controls had a positive response.
If a woman is extremely volume-sensitive during the water phase, the potassium phase may not be accurate. A false-positive response can be caused by infection or prior exposure to radiation or chemotherapy. A thorough history is imperative.
The gold standard: Cystoscopy under anesthesia
Cystoscopy with hydrodistention under general anesthesia is the surest way to diagnose interstitial cystitis or rule it out. Sterile water or saline is infused until bladder capacity is reached. Bladder rupture occurs in up to 10% of patients, so careful inspection during filling is crucial. After 5 minutes of distension, bladder volume is measured into a calibrated beaker. Terminal hematuria (the last 50 cc of effluent) often is noted.
Normal bladder capacity under anesthesia is 1,000 cc, but it is reduced in women with interstitial cystitis. Bladder capacity of 450 cc or less under anesthesia indicates a more contracted bladder and a later-stage disease. Glomerulations, petechiae, fissures, or (rarely) Hunner’s ulcers typically are visible, regardless of bladder volume. However, the presence of glomerulations does not necessarily make the diagnosis, because they can be found in asymptomatic women. Further, cystoscopic observations do not always correlate with the severity of symptoms (nor does positive biopsy always reflect interstitial cystitis).
Hydrodistention is not only diagnostic, but also can be therapeutic, as sympathetic nerve fiber density decreases afterward.11 However, the need for this procedure is under debate, due to the limitations described above. A bladder capacity less than 1,000 cc with the presence of glomerulations or petechiae and fissures, with or without the Hunner’s ulcers, constitutes a definitive diagnosis.
Cystoscopy under anesthesia is recommended because medical treatment can be costly and cause significant side effects. An accurate diagnosis should precede therapy to avoid misdirected therapy in a patient who does not have interstitial cystitis. Moreover, cystoscopy can rule out bladder neoplasms or other diseases. Some bladder carcinomas have been missed in women treated empirically for interstitial cystitis.12
Cystoscopic images “paint a thousand words.” When a woman sees her cystoscopy images, the picture indeed “paints a thousand words.” For many women, the images “justify” their symptoms and confirm that the disease is real.
CASE Don’t treat a UTI without a positive culture
“M.P.” is a healthy 44-year-old G2P2 with a history of recurrent UTIs. Approximately 14 months ago, while on vacation, she began having symptoms of urinary frequency, urgency, and lower abdominal pain that were relieved with voiding. She called her primary care physician, who prescribed levofloxacin and phenazopyridine over the phone for a presumed UTI. Since the patient was out of town, a urine culture was not obtained.
When M.P. returned from vacation, her symptoms recurred, so she underwent urinalysis, including culture and sensitivity, and began a 7-day course of nitrofurantoin (100 mg twice daily). When her symptoms did not improve by day 4, a second course of levofloxacin was given. The urine culture was sterile. As her physician recommended, M.P. increased her fluid intake, including water and cranberry juice. She also avoided sexual relations, since they exacerbated her symptoms, which improved overall but did not clearly abate.
Three months later her symptoms returned in full force.
How would you treat this patient?
Interstitial cystitis can produce symptoms consistent with a lower UTI, but urine cultures will be negative and the response to antibiotics will be minimal. Many patients call their physicians and report “another UTI.” However, if the woman is healthy with no history of renal disease or diabetes, consider interstitial cystitis. Obtain urine culture results from other physicians, if possible, to determine whether bacterial infection was ever confirmed.
Cranberry juice is acidic and may exacerbate urgency and pain.
What to tell patients
The Interstitial Cystitis Association (ICA) encourages patients to become involved in their own care. The ICA was formed in 1984 by women with painful bladder symptoms who had been told by their physicians that nothing was wrong. The organization provides patients with clinical research updates, clinical trial opportunities, and literature and information.
Once the diagnosis is confirmed, patient education and counseling are imperative. Compliance is critical.
There is no cure for interstitial cystitis; the disease is chronic, with relapses and remissions. Although it does not progress once it develops fully, improvement is slow, usually occurring after 3 months or more of treatment. No single treatment works for all patients, so empiric trials with various agents may be needed. Treatment often is multimodal, and the rationale for each therapy should be explained.
Have the patient keep a voiding diary before and after treatment, as well as during any flare-up, to provide evidence of improvement and identify triggers. Also instruct her to pay attention to any foods or activities that exacerbate her symptoms (eg, caffeine, sexual activity).
Treatment
Does a change in diet help?
Some foods and beverages apparently exacerbate symptoms, although the link between foods and symptoms has not been fully investigated. About 53% of patients with interstitial cystitis associate symptom aggravation with dietary factors, especially acidic foods and beverages.5 Dietary restrictions should be attempted for 1 to 2 weeks to determine which foods to avoid.
Gillespie13 found elevated urine levels of tryptophan metabolites in women with hypersensitive bladders, compared with controls.
Tryptophan metabolites may disrupt the glycosaminoglycan layer of the bladder epithelium, as seen in a study involving rabbit bladders.14
Urge suppression
Another helpful strategy is having the patient increase the time between voids using distraction techniques and by contracting the pelvic floor muscles and overriding the first urge to void.
Oral medications
Pentosan polysulfate sodium is a glycosaminoglycan with an affinity for mucosal membranes. It is approved by the Food and Drug Administration (FDA) for the treatment of interstitial cystitis. The mechanism by which it reduces pain and urinary frequency is unclear, but it may replace the deficient glycosaminoglycan layer in the bladder epithelium.
Pain relief occurs in approximately 40% to 60% of patients after 3 months of therapy (100 mg orally 3 times daily).15
The patient should clearly understand that beneficial effects may not occur for 3 to 6 months, and that patience is necessary to give the drug an adequate trial. The response is maintained over the long term, and the drug should be used indefinitely. Pentosan polysulfate sodium is well tolerated, although gastrointestinal side effects and reversible alopecia occur in 4% of patients.
Performing a cystoscopy under anesthesia with hydrodistension is not always necessary prior to starting pentosan polysulfate, as long as the patient is not at risk for bladder neoplasms. However, prior to starting the drug the minimal evaluation should include a voiding diary and either the PUF questionnaire or the potassium sensitivity test.
Antihistamines. If the patient has a history of allergies, or mast cells were confirmed on bladder biopsy, an antihistamine such as hydroxyzine should be given along with pentosan polysulfate sodium. Hydroxyzine has an inhibitory effect on bladder mast cells, as well as anticholinergic and analgesic properties, which improve typical symptoms of interstitial cystitis.
Initiate hydroxyzine at a dose of 10 to 25 mg at bedtime for 1 week, gradually increasing to 50 to 75 mg. Side effects include drowsiness, which is beneficial for women who have nocturia. Other effects are dry mouth and a bitter taste.
Amitriptyline hydrochloride also has analgesic, antihistaminic, anticholinergic, and sedative effects. Amitriptyline is a noradrenaline and serotonin reuptake inhibitor that blocks nociception in the central nervous system.
Compared with placebo, amitriptyline significantly improved symptom scores, pain, and urgency intensity. In a study by van Ophoven and colleagues,16 50 patients (44 women, 6 men) were randomly assigned to amitriptyline at self-titrating doses or placebo. O’Leary-Sant symptom scores, pain, and urgency intensity improved significantly in the amitriptyline group, compared with placebo.
Anticholinergic side effects (eg, dry mouth, constipation), weight gain, and sedation occur in 20% to 80% of patients. In an open-label study of amitriptyline for interstitial cystitis,17 long-term efficacy (mean of 17 months) revealed a 64% response rate (60 of 94 patients) using the global response assessment questionnaire.
Start amitriptyline at a dose of 10 to 25 mg at bedtime, gradually increasing to 75 mg as tolerated. Sedation becomes a limiting factor in the higher doses. Other tricyclic antidepressants have not been studied to any significant extent in treating interstitial cystitis. When used as part of a multimodal treatment in addition to pentosan polysulfate sodium, amitriptyline may be tapered off once remission is attained. No studies have compared treatment response using pentosan polysulfate sodium with and without amitriptyline.
Calcium channel blockers (nifedipine) and drugs for neuropathic pain (gabapentin) are being investigated.
Anticholinergic and antispasmodic agents are typically ineffective in women with interstitial cystitis. In fact, if a patient has no improvement in her symptoms after these drugs are tried, interstitial cystitis should be strongly considered.
Oral L-arginine (1,500–3,000 mg per day, divided doses) improved symptoms in a small study by increasing nitric oxide synthase activity.18
NSAIDs are used adjunctively and may help reduce pain.
Intravesical therapy
Patients unable to tolerate oral medications may benefit from intravesical therapy. It can also be used as an adjunct to oral therapy. Intravesical therapy delivers drugs directly to the bladder wall with a low incidence of side effects. Risks include a potential for UTI via catheterization, as well as transient chemical cystitis, which exacerbates symptoms. A variety of therapeutic “cocktails” are used.
Dimethyl sulfoxide (DMSO) is the only other drug, besides pentosan polysulfate, approved by the FDA for treatment of interstitial cystitis. DMSO has antiinflammatory, analgesic, and muscle-relaxant effects, and inhibits mast-cell activity. DMSO induces remission in 50% to 70% of patients for up to 24 months.19
Lidocaine jelly is injected intraurethrally, followed by instillation of 50 cc of DMSO (alone or with heparin, sodium bicarbonate, and Solu-Cortef). This solution is held in the bladder for 20 to 30 minutes before voiding.
DMSO is secreted through the lungs and skin and has a garlic-like odor. Treatments are administered every 1 to 2 weeks for a total of 4 to 8 treatments. If the condition relapses, DMSO can be reinstituted on a long-term basis. Motivated patients can be taught to administer this treatment themselves.
Heparin is another option. It is administered at a dose of 10,000 U thrice weekly.
Hyaluronic acid. In a small study involving 20 patients, weekly intravesical hyaluronic acid improved symptoms in 65% of patients, with a 40% and 30% decrease in nocturia and pain, respectively.20
Intravesical pentosan polysulfate sodium is another option that improves symptoms and increases bladder capacity,21 although larger studies of its efficacy are lacking.
Bacillus Calmette-Guérin (BCG) solution intravesically had a 60% response rate in 1 study (versus 27% for placebo).22 The mechanism of action is unknown, but the solution may modulate the bladder immune response. Additional studies are pending.
Although their link to interstitial cystitis has not been proven, these foods are thought to be implicated and should be limited or avoided
ACIDIC FOODS
All alcoholic beverages
Apples
Apple juice
Cantaloupe
Carbonated drinks
Chilies/spicy foods
Citrus fruits (lemons, limes, oranges, etc)
Coffee
Cranberries
Grapes
Guava
Lemon juice
Peaches
Pineapples
Plums
Strawberries
Tea
Tomatoes
Vinegar
FOODS HIGH IN TYROSINE, TRYPTOPHAN, OR ASPARTATE
Aspartame
Avocado
Bananas
Beer
Brewer’s yeast
Canned figs
Champagne
Cheese
Chicken livers
Chocolate
Corned beef
Cranberries
Fava beans
Lima beans
Mayonnaise
Nuts
Onions
Pickled herring
Pineapple
Prunes
Raisins
Rye bread
Saccharine
Sour cream
Soy sauce
Wines
Yogurt
Vitamins buffered with aspartate
Adapted from You Don’t Have to Live with Cystitis! by Larrian Gillespie, MD (revised and updated 1996)
1. Altered bladder permeability
In interstitial cystitis, the protective glycosaminoglycan layer of the bladder epithelium may be deficient, increasing bladder permeability. Antiproliferative factor in the urine may impair the proliferation and repair of urothelium, increasing bladder permeability further.28 This breakdown allows potassium to penetrate the urothelium, stimulating pain receptors and causing an inflammatory response in the detrusor muscle.
Loss of the normal protective layer
In a normal bladder, the epithelium is protected by an ionic, hydrophilic, glycosaminoglycan (GAG) layer that serves as a barrier against urine, which is hyperosmolar and rich in acid and potassium. When the GAG layer is deficient, as it is thought to be in interstitial cystitis, bladder permeability increases, triggering an inflammatory response that inhibits repair of the GAG layer, creating a vicious cycle of exposure, inflammation, and pain.
2. Mast cell activation
Mast cell degranulation may cause or contribute to interstitial cystitis and produce its hallmark symptoms. Mast cells also may be activated in response to a noxious factor. These cells secrete histamine, prostaglandins, leukotrienes, cytokines, and chemotactic factors. Urine from women with interstitial cystitis contains histamine, histamine metabolites, and tryptase,29,30 and electron microscopy of bladder biopsies from affected women shows degranulating mast cells adjacent to sensory nerve fibers. When these fibers are stimulated, they release neuropeptides (such as substance P) and may promote inflammation by activating mast cells and nearby nerve terminals.31
3. Inflammation
Inflammation clearly plays a role in interstitial cystitis. Bladder biopsies reveal mild to severe inflammation, and the presence of T cells, B cells, plasma cells, neutrophils, eosinophils, and mast cells. Inflammatory mediators such as kallikrein, interleukin-6, interleukin-2 inhibitor, and neutrophil chemotactic factor are increased in the urine of individuals with the disease.
4. Autoimmunity
Clinical features of interstitial cystitis that mimic those of other autoimmune diseases include chronic symptoms that wax and wane; higher prevalence in women; immunological deposits in bladder biopsies with mononuclear cell infiltrates, which suggests the presence of bladder autoantigens; association with other autoimmune disorders such as Sjögren’s syndrome and lupus; and, in some cases, a positive response to steroids or other immunosuppressants.32
Glomerulations and fissures are telltale signs
When the bladder is distended under anesthesia, examination may reveal a pattern of fissures and glomerulations, which are hallmarks of interstitial cystitis. These findings are sometimes present in asymptomatic women as well, and do not always correlate with severity of symptoms.
5. Infection
Some experts have postulated that occult infection with fastidious organisms, fungi, or viruses plays a role in the development of interstitial cystitis. However, special cultures, serology, and electron microscopy have not shown any organisms consistently associated with the disease.33
The use of polymerase chain reaction techniques to test for bacterial DNA in bladder biopsies of patients with interstitial cystitis has yielded conflicting results.34,35 Infection may be the inciting event that injures the bladder epithelium, causing a cascade of inflammation.
6. Neurologic changes
Studies have revealed increased sympathetic nerve fiber density in the bladders of patients with interstitial cystitis.31,36 The disease may also be a type of reflex-sympathetic dystrophy with increased and abnormal spinal sympathetic activity. Butrick describes interstitial cystitis as a “visceral pain syndrome.”37 C-fibers (silent afferents) transmit pain when activated by a prolonged or noxious stimulus. This leads to neuroplastic changes that lower the threshold of nociceptive nerves, thus reducing the amount of stimulus needed to provoke pain (allodynia). Pelvic viscera share innervation, which may explain the association between
interstitial cystitis and irritable bowel syndrome and endometriosis. Interstitial cystitis is not limited to the bladder; it involves chronic neuropathic inflammation, afferent overactivity, and central sensitization. Increased pain perception may cause pelvic floor muscle instability, spasm, and hypertonic state.38
Alternative therapies
Electrical nerve stimulation
This transcutaneous modality improves symptoms in 25% to 50% of patients.23 It is thought to stimulate the afferent nerves, thereby activating the inhibitory circuits and decreasing the sensation of pain.
Sacral neuromodulation is another modality being studied for the treatment of interstitial cystitis. So far it has significantly reduced urinary urgency-frequency symptoms as well as pain.24
Other new therapies under investigation include intravesical injection of botulinum toxin, resiniferatoxin, gene therapy, and nerve growth-factor inhibitors.
Surgery: High relapse rates make it a last resort
The treatment of visible ulcers by resection or laser ablation improves symptoms but carries a relapse rate of more than 50%.25 More aggressive surgeries, with cure rates ranging from 50% to 80%, include denervation procedures, augmentation cystoplasty for severely contracted bladders (not necessarily due to interstitial cystitis), cecocystoplasty (where a segment of cecum is excised and reanastomosed with the bladder to increase bladder capacity), and total cystourethrectomy and urinary diversion. However, persistent pain has been reported after these invasive procedures, and permanent intermittent self-catheterization and/or reoperation is often required.26,27
Dr. LaSala is a speaker for Pfizer, Inc.
The typical patient voids 16 times a day and 2 or more times at night. In later stages of the disease, she may urinate as often as 60 times a day and every half hour at night, severely eroding her ability to hold a job, travel, or lead a normal life. In fact, her quality of life may be impaired as much as that of a person with end-stage renal disease.1,2 She sees an average of 4 doctors and endures irritative voiding symptoms for 4 years before her disease is identified. The cause is unknown.
Interstitial cystitis produces a wide spectrum of symptom severity, occurring episodically with spontaneous flare-ups and remission, or with continuous, intractable urinary urgency and pain. Until recently, women presenting with urinary urgency, frequency, and pain were presumed to have a urinary tract infection (UTI) or overactive bladder, and were often treated—to no avail—with multiple courses of antibiotics or anticholinergics.
Fortunately, interstitial cystitis is gaining recognition, and effective treatments are emerging. Usually the ObGyn—often the first physician a woman consults—need refer only the refractory cases to a specialist. This article describes the components of diagnosis and the most effective treatments, including use of the first-line agents amitriptyline (Elavil) and pentosan polysulfate sodium (Elmiron).
CASE Is overactive bladder the cause of stubborn symptoms?
“R.H.,” a healthy 48-year-old G2P2 with a 5-year history of urinary urgency and frequency, reports that she voids “at least 15 times per day.” She denies any urge incontinence, but says she experiences occasional stress incontinence if she has a bad cold. Four years ago, she saw a urologist for these symptoms, after her husband said he was tired of having to stop the car so she could go to the bathroom. The urologist diagnosed a “small bladder,” performed urethral “stretching,” and prescribed oxybutynin.
Her symptoms improved for about 6 months, but then progressed and have now worsened. She began taking tolterodine, 4 mg daily, 2 months ago, as prescribed by her primary care physician. The sensation of painful urgency has eased, but there has been no change in frequency. R.H. used to wake as often as 4 times a night with the urge to urinate, but since she began taking zolpidem tartrate (Ambien) as a sleep aid, she now wakes only 2 times every night.
Why are her symptoms so persistent?
This woman’s case is a classic example of interstitial cystitis masquerading as overactive bladder. Treatment with anticholinergic drugs may ease urgency symptoms slightly, but has no real effect on frequency.
This case has 5 hallmarks of the syndrome of interstitial cystitis:
- Urgency
- Frequency (more than 8 voids/day, taking fluid intake into account)
- Bladder pain
- Nocturia (more than twice)
- Absence of a genitourinary tract infection
Patients show signs of “battle fatigue”
Women with interstitial cystitis may be anxious, depressed, angry, and sleep-deprived. In some women, stress exacerbates the urinary symptoms and pain (as do certain common foods and beverages, especially citrus, tomatoes, and caffeine).
Approximately 60% of patients report dyspareunia, and many report chronic pelvic pain. In fact, 75% of women who report chronic pelvic pain also have irritative voiding symptoms. Therefore, it is important to ask about lower urinary tract symptoms whenever a woman presents with pelvic pain.3,4
Pain may be suprapubic, vaginal, perineal, or originate in the groin or lower back. Although 16% of patients present solely with pain, and 30% have only urinary frequency, most patients suffer from both symptoms.
Approximately 40% report premenstrual or ovulatory exacerbation of symptoms, although symptoms may improve during pregnancy.5 Voided volumes are usually small, despite the strong urgency, which does not always resolve. Pelvic pain may ease after voiding but recurs shortly.
Insidious, worsening course
Symptoms appear insidiously and worsen to a “final” stage within 5 to 15 years, at which point a plateau is reached with little further progression.6 Some experts suggest that the disease be classified as “early non-ulcerous” or “classic ulcerous.”
- In early disease, bladder capacity exceeds 450 cc under anesthesia, with glomerulations and hemorrhage.
- In classic disease, bladder capacity is less than 450 cc under anesthesia, and Hunner’s ulcers and fissures are evident. Hunner’s ulcers are described as “a central scar with small fibrin deposits before distension, and post-distension edema.”7
For now, however, there are no agreed-upon markers to distinguish the 2 types of disease.
The female-to-male ratio is 9:1, and about 500,000 to more than 1 million adults in the United States are thought to have interstitial cystitis.8 Caucasian women constitute 95% of patients, and the average age at diagnosis is 45 years. Thirty percent of women with interstitial cystitis are 30 years old or younger. Significantly more women with interstitial cystitis have had a hysterectomy than controls.9
For a diagnosis, skip the NIH criteria
Symptoms
Although the National Institutes of Health (NIH) established diagnostic criteria for research subjects, the criteria are overly stringent—60% of women with symptoms typical of interstitial cystitis do not qualify, but should not necessarily be excluded from diagnosis and treatment.
When a woman has the hallmark symptoms listed on page 57, but also reports continuous pain or dysmenorrhea, other pelvic pathology such as endometriosis should be considered, although interstitial cystitis should be included in the differential diagnosis of any woman reporting pelvic pain.
Incontinence is atypical. If present, it merits an incontinence evaluation to detect detrusor hyperreflexia or detrusor-sphincter dyssynergia.
Dysuria suggests a UTI, urethral diverticulum, urogenital atrophy, or vaginitis. Many patients present with an erroneous diagnosis of “recurrent UTIs.”
Diagnostic tools
Voiding diaries are useful and can be revealing. The Pelvic Pain and Urinary Frequency (PUF) scale, developed by Parsons, is helpful in predicting interstitial cystitis (see the Clip-and-save chart). The higher the score, the greater the likelihood of interstitial cystitis, particularly with a score of more than 8.
Another tool is the O’Leary-Sant Index, which measures pain, voiding symptoms, and quality of life.
Physical examination and laboratory studies
Perform a pelvic exam to rule out other diseases and pelvic pathology, including sexually transmitted diseases, urethral diverticulum, and pelvic masses. Typically, the pelvic exam in women with interstitial cystitis is negative except for suprapubic and/or trigonal tenderness.
Urinalysis, culture, and sensitivity are warranted but are usually negative.
Cytology should be analyzed if microscopic hematuria is present, or with other risk factors such as a history of smoking or age over 40.
Obtain cultures for sexually transmitted diseases if clinically indicated.
Urodynamic studies are not necessary to diagnose interstitial cystitis. However, if incontinence is present, a cystometrogram can confirm detrusor hyperreflexia. Otherwise the cystometrogram is normal except for heightened sensation or pain with bladder filling, or a bladder capacity of less than 350 cc.
The potassium sensitivity test: Useful but painful
Women with interstitial cystitis are thought to have increased bladder permeability that allows potassium to pass through to the detrusor muscle. Thus, the potassium sensitivity test often is used to diagnose the condition. The test is an office procedure in which 2 separate solutions are instilled into the bladder: 40 cc sterile water followed by 40 cc of a solution of 400 mEq potassium per liter of water. After each solution is instilled, the patient is monitored for symptoms. The test is positive when the patient responds only to the potassium.
The response may be marked and painful, and the bladder should be emptied immediately. Subsequent irrigation with sterile water may be necessary to alleviate the discomfort caused by the potassium solution. Symptoms provoked by the test generally subside after bladder emptying, but can persist and cause moderate distress, which limits the utility of this office-based test.
Parsons et al10 demonstrated an 81% positive response (197 of 244 women) to the test among women with pelvic pain, compared with 0 of 47 patients with no pelvic pain. They also found that 70% of patients with interstitial cystitis and 4% of controls had a positive response.
If a woman is extremely volume-sensitive during the water phase, the potassium phase may not be accurate. A false-positive response can be caused by infection or prior exposure to radiation or chemotherapy. A thorough history is imperative.
The gold standard: Cystoscopy under anesthesia
Cystoscopy with hydrodistention under general anesthesia is the surest way to diagnose interstitial cystitis or rule it out. Sterile water or saline is infused until bladder capacity is reached. Bladder rupture occurs in up to 10% of patients, so careful inspection during filling is crucial. After 5 minutes of distension, bladder volume is measured into a calibrated beaker. Terminal hematuria (the last 50 cc of effluent) often is noted.
Normal bladder capacity under anesthesia is 1,000 cc, but it is reduced in women with interstitial cystitis. Bladder capacity of 450 cc or less under anesthesia indicates a more contracted bladder and a later-stage disease. Glomerulations, petechiae, fissures, or (rarely) Hunner’s ulcers typically are visible, regardless of bladder volume. However, the presence of glomerulations does not necessarily make the diagnosis, because they can be found in asymptomatic women. Further, cystoscopic observations do not always correlate with the severity of symptoms (nor does positive biopsy always reflect interstitial cystitis).
Hydrodistention is not only diagnostic, but also can be therapeutic, as sympathetic nerve fiber density decreases afterward.11 However, the need for this procedure is under debate, due to the limitations described above. A bladder capacity less than 1,000 cc with the presence of glomerulations or petechiae and fissures, with or without the Hunner’s ulcers, constitutes a definitive diagnosis.
Cystoscopy under anesthesia is recommended because medical treatment can be costly and cause significant side effects. An accurate diagnosis should precede therapy to avoid misdirected therapy in a patient who does not have interstitial cystitis. Moreover, cystoscopy can rule out bladder neoplasms or other diseases. Some bladder carcinomas have been missed in women treated empirically for interstitial cystitis.12
Cystoscopic images “paint a thousand words.” When a woman sees her cystoscopy images, the picture indeed “paints a thousand words.” For many women, the images “justify” their symptoms and confirm that the disease is real.
CASE Don’t treat a UTI without a positive culture
“M.P.” is a healthy 44-year-old G2P2 with a history of recurrent UTIs. Approximately 14 months ago, while on vacation, she began having symptoms of urinary frequency, urgency, and lower abdominal pain that were relieved with voiding. She called her primary care physician, who prescribed levofloxacin and phenazopyridine over the phone for a presumed UTI. Since the patient was out of town, a urine culture was not obtained.
When M.P. returned from vacation, her symptoms recurred, so she underwent urinalysis, including culture and sensitivity, and began a 7-day course of nitrofurantoin (100 mg twice daily). When her symptoms did not improve by day 4, a second course of levofloxacin was given. The urine culture was sterile. As her physician recommended, M.P. increased her fluid intake, including water and cranberry juice. She also avoided sexual relations, since they exacerbated her symptoms, which improved overall but did not clearly abate.
Three months later her symptoms returned in full force.
How would you treat this patient?
Interstitial cystitis can produce symptoms consistent with a lower UTI, but urine cultures will be negative and the response to antibiotics will be minimal. Many patients call their physicians and report “another UTI.” However, if the woman is healthy with no history of renal disease or diabetes, consider interstitial cystitis. Obtain urine culture results from other physicians, if possible, to determine whether bacterial infection was ever confirmed.
Cranberry juice is acidic and may exacerbate urgency and pain.
What to tell patients
The Interstitial Cystitis Association (ICA) encourages patients to become involved in their own care. The ICA was formed in 1984 by women with painful bladder symptoms who had been told by their physicians that nothing was wrong. The organization provides patients with clinical research updates, clinical trial opportunities, and literature and information.
Once the diagnosis is confirmed, patient education and counseling are imperative. Compliance is critical.
There is no cure for interstitial cystitis; the disease is chronic, with relapses and remissions. Although it does not progress once it develops fully, improvement is slow, usually occurring after 3 months or more of treatment. No single treatment works for all patients, so empiric trials with various agents may be needed. Treatment often is multimodal, and the rationale for each therapy should be explained.
Have the patient keep a voiding diary before and after treatment, as well as during any flare-up, to provide evidence of improvement and identify triggers. Also instruct her to pay attention to any foods or activities that exacerbate her symptoms (eg, caffeine, sexual activity).
Treatment
Does a change in diet help?
Some foods and beverages apparently exacerbate symptoms, although the link between foods and symptoms has not been fully investigated. About 53% of patients with interstitial cystitis associate symptom aggravation with dietary factors, especially acidic foods and beverages.5 Dietary restrictions should be attempted for 1 to 2 weeks to determine which foods to avoid.
Gillespie13 found elevated urine levels of tryptophan metabolites in women with hypersensitive bladders, compared with controls.
Tryptophan metabolites may disrupt the glycosaminoglycan layer of the bladder epithelium, as seen in a study involving rabbit bladders.14
Urge suppression
Another helpful strategy is having the patient increase the time between voids using distraction techniques and by contracting the pelvic floor muscles and overriding the first urge to void.
Oral medications
Pentosan polysulfate sodium is a glycosaminoglycan with an affinity for mucosal membranes. It is approved by the Food and Drug Administration (FDA) for the treatment of interstitial cystitis. The mechanism by which it reduces pain and urinary frequency is unclear, but it may replace the deficient glycosaminoglycan layer in the bladder epithelium.
Pain relief occurs in approximately 40% to 60% of patients after 3 months of therapy (100 mg orally 3 times daily).15
The patient should clearly understand that beneficial effects may not occur for 3 to 6 months, and that patience is necessary to give the drug an adequate trial. The response is maintained over the long term, and the drug should be used indefinitely. Pentosan polysulfate sodium is well tolerated, although gastrointestinal side effects and reversible alopecia occur in 4% of patients.
Performing a cystoscopy under anesthesia with hydrodistension is not always necessary prior to starting pentosan polysulfate, as long as the patient is not at risk for bladder neoplasms. However, prior to starting the drug the minimal evaluation should include a voiding diary and either the PUF questionnaire or the potassium sensitivity test.
Antihistamines. If the patient has a history of allergies, or mast cells were confirmed on bladder biopsy, an antihistamine such as hydroxyzine should be given along with pentosan polysulfate sodium. Hydroxyzine has an inhibitory effect on bladder mast cells, as well as anticholinergic and analgesic properties, which improve typical symptoms of interstitial cystitis.
Initiate hydroxyzine at a dose of 10 to 25 mg at bedtime for 1 week, gradually increasing to 50 to 75 mg. Side effects include drowsiness, which is beneficial for women who have nocturia. Other effects are dry mouth and a bitter taste.
Amitriptyline hydrochloride also has analgesic, antihistaminic, anticholinergic, and sedative effects. Amitriptyline is a noradrenaline and serotonin reuptake inhibitor that blocks nociception in the central nervous system.
Compared with placebo, amitriptyline significantly improved symptom scores, pain, and urgency intensity. In a study by van Ophoven and colleagues,16 50 patients (44 women, 6 men) were randomly assigned to amitriptyline at self-titrating doses or placebo. O’Leary-Sant symptom scores, pain, and urgency intensity improved significantly in the amitriptyline group, compared with placebo.
Anticholinergic side effects (eg, dry mouth, constipation), weight gain, and sedation occur in 20% to 80% of patients. In an open-label study of amitriptyline for interstitial cystitis,17 long-term efficacy (mean of 17 months) revealed a 64% response rate (60 of 94 patients) using the global response assessment questionnaire.
Start amitriptyline at a dose of 10 to 25 mg at bedtime, gradually increasing to 75 mg as tolerated. Sedation becomes a limiting factor in the higher doses. Other tricyclic antidepressants have not been studied to any significant extent in treating interstitial cystitis. When used as part of a multimodal treatment in addition to pentosan polysulfate sodium, amitriptyline may be tapered off once remission is attained. No studies have compared treatment response using pentosan polysulfate sodium with and without amitriptyline.
Calcium channel blockers (nifedipine) and drugs for neuropathic pain (gabapentin) are being investigated.
Anticholinergic and antispasmodic agents are typically ineffective in women with interstitial cystitis. In fact, if a patient has no improvement in her symptoms after these drugs are tried, interstitial cystitis should be strongly considered.
Oral L-arginine (1,500–3,000 mg per day, divided doses) improved symptoms in a small study by increasing nitric oxide synthase activity.18
NSAIDs are used adjunctively and may help reduce pain.
Intravesical therapy
Patients unable to tolerate oral medications may benefit from intravesical therapy. It can also be used as an adjunct to oral therapy. Intravesical therapy delivers drugs directly to the bladder wall with a low incidence of side effects. Risks include a potential for UTI via catheterization, as well as transient chemical cystitis, which exacerbates symptoms. A variety of therapeutic “cocktails” are used.
Dimethyl sulfoxide (DMSO) is the only other drug, besides pentosan polysulfate, approved by the FDA for treatment of interstitial cystitis. DMSO has antiinflammatory, analgesic, and muscle-relaxant effects, and inhibits mast-cell activity. DMSO induces remission in 50% to 70% of patients for up to 24 months.19
Lidocaine jelly is injected intraurethrally, followed by instillation of 50 cc of DMSO (alone or with heparin, sodium bicarbonate, and Solu-Cortef). This solution is held in the bladder for 20 to 30 minutes before voiding.
DMSO is secreted through the lungs and skin and has a garlic-like odor. Treatments are administered every 1 to 2 weeks for a total of 4 to 8 treatments. If the condition relapses, DMSO can be reinstituted on a long-term basis. Motivated patients can be taught to administer this treatment themselves.
Heparin is another option. It is administered at a dose of 10,000 U thrice weekly.
Hyaluronic acid. In a small study involving 20 patients, weekly intravesical hyaluronic acid improved symptoms in 65% of patients, with a 40% and 30% decrease in nocturia and pain, respectively.20
Intravesical pentosan polysulfate sodium is another option that improves symptoms and increases bladder capacity,21 although larger studies of its efficacy are lacking.
Bacillus Calmette-Guérin (BCG) solution intravesically had a 60% response rate in 1 study (versus 27% for placebo).22 The mechanism of action is unknown, but the solution may modulate the bladder immune response. Additional studies are pending.
Although their link to interstitial cystitis has not been proven, these foods are thought to be implicated and should be limited or avoided
ACIDIC FOODS
All alcoholic beverages
Apples
Apple juice
Cantaloupe
Carbonated drinks
Chilies/spicy foods
Citrus fruits (lemons, limes, oranges, etc)
Coffee
Cranberries
Grapes
Guava
Lemon juice
Peaches
Pineapples
Plums
Strawberries
Tea
Tomatoes
Vinegar
FOODS HIGH IN TYROSINE, TRYPTOPHAN, OR ASPARTATE
Aspartame
Avocado
Bananas
Beer
Brewer’s yeast
Canned figs
Champagne
Cheese
Chicken livers
Chocolate
Corned beef
Cranberries
Fava beans
Lima beans
Mayonnaise
Nuts
Onions
Pickled herring
Pineapple
Prunes
Raisins
Rye bread
Saccharine
Sour cream
Soy sauce
Wines
Yogurt
Vitamins buffered with aspartate
Adapted from You Don’t Have to Live with Cystitis! by Larrian Gillespie, MD (revised and updated 1996)
1. Altered bladder permeability
In interstitial cystitis, the protective glycosaminoglycan layer of the bladder epithelium may be deficient, increasing bladder permeability. Antiproliferative factor in the urine may impair the proliferation and repair of urothelium, increasing bladder permeability further.28 This breakdown allows potassium to penetrate the urothelium, stimulating pain receptors and causing an inflammatory response in the detrusor muscle.
Loss of the normal protective layer
In a normal bladder, the epithelium is protected by an ionic, hydrophilic, glycosaminoglycan (GAG) layer that serves as a barrier against urine, which is hyperosmolar and rich in acid and potassium. When the GAG layer is deficient, as it is thought to be in interstitial cystitis, bladder permeability increases, triggering an inflammatory response that inhibits repair of the GAG layer, creating a vicious cycle of exposure, inflammation, and pain.
2. Mast cell activation
Mast cell degranulation may cause or contribute to interstitial cystitis and produce its hallmark symptoms. Mast cells also may be activated in response to a noxious factor. These cells secrete histamine, prostaglandins, leukotrienes, cytokines, and chemotactic factors. Urine from women with interstitial cystitis contains histamine, histamine metabolites, and tryptase,29,30 and electron microscopy of bladder biopsies from affected women shows degranulating mast cells adjacent to sensory nerve fibers. When these fibers are stimulated, they release neuropeptides (such as substance P) and may promote inflammation by activating mast cells and nearby nerve terminals.31
3. Inflammation
Inflammation clearly plays a role in interstitial cystitis. Bladder biopsies reveal mild to severe inflammation, and the presence of T cells, B cells, plasma cells, neutrophils, eosinophils, and mast cells. Inflammatory mediators such as kallikrein, interleukin-6, interleukin-2 inhibitor, and neutrophil chemotactic factor are increased in the urine of individuals with the disease.
4. Autoimmunity
Clinical features of interstitial cystitis that mimic those of other autoimmune diseases include chronic symptoms that wax and wane; higher prevalence in women; immunological deposits in bladder biopsies with mononuclear cell infiltrates, which suggests the presence of bladder autoantigens; association with other autoimmune disorders such as Sjögren’s syndrome and lupus; and, in some cases, a positive response to steroids or other immunosuppressants.32
Glomerulations and fissures are telltale signs
When the bladder is distended under anesthesia, examination may reveal a pattern of fissures and glomerulations, which are hallmarks of interstitial cystitis. These findings are sometimes present in asymptomatic women as well, and do not always correlate with severity of symptoms.
5. Infection
Some experts have postulated that occult infection with fastidious organisms, fungi, or viruses plays a role in the development of interstitial cystitis. However, special cultures, serology, and electron microscopy have not shown any organisms consistently associated with the disease.33
The use of polymerase chain reaction techniques to test for bacterial DNA in bladder biopsies of patients with interstitial cystitis has yielded conflicting results.34,35 Infection may be the inciting event that injures the bladder epithelium, causing a cascade of inflammation.
6. Neurologic changes
Studies have revealed increased sympathetic nerve fiber density in the bladders of patients with interstitial cystitis.31,36 The disease may also be a type of reflex-sympathetic dystrophy with increased and abnormal spinal sympathetic activity. Butrick describes interstitial cystitis as a “visceral pain syndrome.”37 C-fibers (silent afferents) transmit pain when activated by a prolonged or noxious stimulus. This leads to neuroplastic changes that lower the threshold of nociceptive nerves, thus reducing the amount of stimulus needed to provoke pain (allodynia). Pelvic viscera share innervation, which may explain the association between
interstitial cystitis and irritable bowel syndrome and endometriosis. Interstitial cystitis is not limited to the bladder; it involves chronic neuropathic inflammation, afferent overactivity, and central sensitization. Increased pain perception may cause pelvic floor muscle instability, spasm, and hypertonic state.38
Alternative therapies
Electrical nerve stimulation
This transcutaneous modality improves symptoms in 25% to 50% of patients.23 It is thought to stimulate the afferent nerves, thereby activating the inhibitory circuits and decreasing the sensation of pain.
Sacral neuromodulation is another modality being studied for the treatment of interstitial cystitis. So far it has significantly reduced urinary urgency-frequency symptoms as well as pain.24
Other new therapies under investigation include intravesical injection of botulinum toxin, resiniferatoxin, gene therapy, and nerve growth-factor inhibitors.
Surgery: High relapse rates make it a last resort
The treatment of visible ulcers by resection or laser ablation improves symptoms but carries a relapse rate of more than 50%.25 More aggressive surgeries, with cure rates ranging from 50% to 80%, include denervation procedures, augmentation cystoplasty for severely contracted bladders (not necessarily due to interstitial cystitis), cecocystoplasty (where a segment of cecum is excised and reanastomosed with the bladder to increase bladder capacity), and total cystourethrectomy and urinary diversion. However, persistent pain has been reported after these invasive procedures, and permanent intermittent self-catheterization and/or reoperation is often required.26,27
Dr. LaSala is a speaker for Pfizer, Inc.
1. Held PJ, Janno PM, Wein AJ, et al. Epidemiology of IC. In: Hanno P, Staskin DR, Krane RJ, et al, eds. Interstitial Cystitis. New York: Springer-Verlag; 1990:29–48.
2. Ratner V, Slade D, Greene G. Interstitial cystitis: a patient’s perspective. Urol Clin North Am. 1994;21:1-5.
3. Parson CL, Bullen M, Kahn BS, et al. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98:127-132.
4. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002;100:337-341.
5. Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994;21:121-130.
6. Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465-469.
7. Parsons CL. Interstitial cystitis: clinical manifestations and diagnostic criteria in over 200 cases. Neurourol Urodyn. 1990;9:241-250.
8. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2-9.
9. Kozial JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7-20.
10. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187:1395-1400.
11. Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am. 1994;21:163-176.
12. Tissot WD, Diokno AC, Peters KM. A referral center’s experience with transitional cell carcinoma misdiag-nosed as interstitial cystitis. J Urol. 2004;172:478-480.
13. Gillespie L. Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br J Urol. 1993;72:293-297.
14. Kaufman JE, Anderson K, Parsons CL. Inactivation of antiadherence effect of bladder surface glycosaminoglycans as possible mechanism for carcinogenesis. Urology. 1987;30:255-258.
15. Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(suppl 5A):93-99.
16. Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533-536.
17. van Ophoven, Hertle L. Long-term results of amitripty-line treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
18. Smity SD, Ehheler MA, Foster HE, Weiss RM. Improvement in interstitial cystitis symptom scores during treatment with L-arginine. J Urol. 1997;158:703-708.
19. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36-39.
20. Kallestrup EB, Jorgensen SS, Nordling J, et al. Treatment of interstitial cystitis with Cystistat: a hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143-147.
21. Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosan polysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168-171.
22. Peters K, Diokno A, Steinart B, et al. The efficacy of intravesical Tice strain Bacillus Calmette-Guérin in the treatment of interstitial cystitis: a double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090-2094.
23. Fall M, Lindstrom S. Transcutaneous electrical nerve stimulation in classic and nonulcer interstitial cystitis. Urol Clin North Am. 1994;21:133-139.
24. Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
25. Malloy TR, Shanberg AM. Laser therapy for interstitial cystitis. Urol Clin North Am. 1994;21:141-144.
26. Smith RB, Van Caugh P, Skinner DG, et al. Augmentation enterocystoplasty: a critical review. J Urol. 1977;118:35-39.
27. Chakravarti A, Ganta S, Somani B, Jones MA. Caecocystoplasty for intractable interstitial cystitis: long-term results. Eur Urol. 2004;46:114-117.
28. Erickson DR, Zie SX, Bhavanandan VP, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461.-
29. El-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:250-353.
30. Boucher W, El-Mansoury M, Pang X, Sant GR. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol. 1995;76:94-100.
31. Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744-750.
32. Oravisto KJ. Interstitial cystitis as an autoimmune disease. Eur Urol. 1980;6:10-13.
33. Duncan JL, Schaeffer AJ. Do infectious agents cause interstitial cystitis? Urology. 1997;49:48-51.
34. Haarala M, Jalava J, Laato M, Kilholma P, Nurmi M, Alanen A. Absence of bacterial DNA in the bladder of patients with interstitial cystitis. J Urol. 1996;156:1843-1845.
35. Keay S, Zhang C-O, Bladwin BR, Jacobs SC, Warren JW. Polymerase chain reaction amplification of bacterial 16S rRNA genes in interstitial cystitis and control patient bladder biopsies. J Urol. 1998;159:280-283.
36. Hohenfeller M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587-591.
37. Butrick CW. Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis and treatment. Clin Obstet Gynecol. 2003;46:812-814.
38. Moldwin RM, Fried-Siegel J, Mendelowitz F. Pelvic floor dysfunction and interstitial cystitis. J Urol. 1994;151:285A.-
1. Held PJ, Janno PM, Wein AJ, et al. Epidemiology of IC. In: Hanno P, Staskin DR, Krane RJ, et al, eds. Interstitial Cystitis. New York: Springer-Verlag; 1990:29–48.
2. Ratner V, Slade D, Greene G. Interstitial cystitis: a patient’s perspective. Urol Clin North Am. 1994;21:1-5.
3. Parson CL, Bullen M, Kahn BS, et al. Gynecologic presentation of interstitial cystitis as detected by intravesical potassium sensitivity. Obstet Gynecol. 2001;98:127-132.
4. Clemons JL, Arya LA, Myers DL. Diagnosing interstitial cystitis in women with chronic pelvic pain. Obstet Gynecol. 2002;100:337-341.
5. Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am. 1994;21:121-130.
6. Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465-469.
7. Parsons CL. Interstitial cystitis: clinical manifestations and diagnostic criteria in over 200 cases. Neurourol Urodyn. 1990;9:241-250.
8. Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2-9.
9. Kozial JA. Epidemiology of interstitial cystitis. Urol Clin North Am. 1994;21:7-20.
10. Parsons CL, Dell J, Stanford EJ, Bullen M, Kahn BS, Willems JJ. The prevalence of interstitial cystitis in gynecologic patients with pelvic pain, as detected by intravesical potassium sensitivity. Am J Obstet Gynecol. 2002;187:1395-1400.
11. Ruggieri MR, Chelsky MJ, Rosen SI, Shickley TJ, Hanno PM. Current findings and future research avenues in the study of interstitial cystitis. Urol Clin North Am. 1994;21:163-176.
12. Tissot WD, Diokno AC, Peters KM. A referral center’s experience with transitional cell carcinoma misdiag-nosed as interstitial cystitis. J Urol. 2004;172:478-480.
13. Gillespie L. Metabolic appraisal of the effects of dietary modification on hypersensitive bladder symptoms. Br J Urol. 1993;72:293-297.
14. Kaufman JE, Anderson K, Parsons CL. Inactivation of antiadherence effect of bladder surface glycosaminoglycans as possible mechanism for carcinogenesis. Urology. 1987;30:255-258.
15. Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(suppl 5A):93-99.
16. Van Ophoven A, Pokupic S, Heinecke A, Hertle L. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172:533-536.
17. van Ophoven, Hertle L. Long-term results of amitripty-line treatment for interstitial cystitis. J Urol. 2005;174:1837-1840.
18. Smity SD, Ehheler MA, Foster HE, Weiss RM. Improvement in interstitial cystitis symptom scores during treatment with L-arginine. J Urol. 1997;158:703-708.
19. Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. J Urol. 1988;140:36-39.
20. Kallestrup EB, Jorgensen SS, Nordling J, et al. Treatment of interstitial cystitis with Cystistat: a hyaluronic acid product. Scand J Urol Nephrol. 2005;39:143-147.
21. Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosan polysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168-171.
22. Peters K, Diokno A, Steinart B, et al. The efficacy of intravesical Tice strain Bacillus Calmette-Guérin in the treatment of interstitial cystitis: a double-blind, prospective, placebo controlled trial. J Urol. 1997;157:2090-2094.
23. Fall M, Lindstrom S. Transcutaneous electrical nerve stimulation in classic and nonulcer interstitial cystitis. Urol Clin North Am. 1994;21:133-139.
24. Comiter CV. Sacral neuromodulation for the symptomatic treatment of refractory interstitial cystitis: a prospective study. J Urol. 2003;169:1369-1373.
25. Malloy TR, Shanberg AM. Laser therapy for interstitial cystitis. Urol Clin North Am. 1994;21:141-144.
26. Smith RB, Van Caugh P, Skinner DG, et al. Augmentation enterocystoplasty: a critical review. J Urol. 1977;118:35-39.
27. Chakravarti A, Ganta S, Somani B, Jones MA. Caecocystoplasty for intractable interstitial cystitis: long-term results. Eur Urol. 2004;46:114-117.
28. Erickson DR, Zie SX, Bhavanandan VP, et al. A comparison of multiple urine markers for interstitial cystitis. J Urol. 2002;167:2461.-
29. El-Mansoury M, Boucher W, Sant GR, Theoharides TC. Increased urine histamine and methylhistamine in interstitial cystitis. J Urol. 1994;152:250-353.
30. Boucher W, El-Mansoury M, Pang X, Sant GR. Elevated mast cell tryptase in the urine of patients with interstitial cystitis. Br J Urol. 1995;76:94-100.
31. Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol. 1995;75:744-750.
32. Oravisto KJ. Interstitial cystitis as an autoimmune disease. Eur Urol. 1980;6:10-13.
33. Duncan JL, Schaeffer AJ. Do infectious agents cause interstitial cystitis? Urology. 1997;49:48-51.
34. Haarala M, Jalava J, Laato M, Kilholma P, Nurmi M, Alanen A. Absence of bacterial DNA in the bladder of patients with interstitial cystitis. J Urol. 1996;156:1843-1845.
35. Keay S, Zhang C-O, Bladwin BR, Jacobs SC, Warren JW. Polymerase chain reaction amplification of bacterial 16S rRNA genes in interstitial cystitis and control patient bladder biopsies. J Urol. 1998;159:280-283.
36. Hohenfeller M, Nunes L, Schmidt RA, Lampel A, Thuroff JW, Tanagho EA. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol. 1992;147:587-591.
37. Butrick CW. Interstitial cystitis and chronic pelvic pain: new insights in neuropathology, diagnosis and treatment. Clin Obstet Gynecol. 2003;46:812-814.
38. Moldwin RM, Fried-Siegel J, Mendelowitz F. Pelvic floor dysfunction and interstitial cystitis. J Urol. 1994;151:285A.-