User login
- The hepatitis C virus (HCV) is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States.
- In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%. In females, prevalence is highest during the childbearing years, peaking at age 35.
- Injection-drug use accounts for 60% of infections, while the transfusion of blood or blood products accounts for another 10%.
- About 15% of people with acute HCV infection clear the virus; the rest develop chronic infection.
- During chronic infection, most patients are asymptomatic or have mild, nonspecific symptoms such as fatigue.
- Combination therapy with interferon alpha and ribavirin elicits a sustained virologic response rate of 40%, and newer therapy with pegylated interferon alpha and ribavirin improves the rate to 54%.
- Perinatal transmission in women with chronic HCV infection occurs at an average rate of 5%.
The hepatitis C virus (HCV) was first identified in 1989 as the cause of non-A, non-B hepatitis infections. Since its discovery, HCV has become the most common chronic blood-borne infection in the United States: Approximately 2.7 to 3.5 million people have chronic HCV infection,1,2 as compared with 1.25 million people with chronic hepatitis B virus infection and 1 million with the human immunodeficiency virus (HIV). In addition, HCV infection is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States. Some call HCV infection “the silent epidemic,” since 75% of people infected are asymptomatic and chronic manifestations don’t appear for 1 to 2 decades.
In 1998, the Centers for Disease Control and Prevention (CDC) issued guidelines for screening for HCV infection ( Table 1).3 The American College of Obstetricians and Gynecologists (ACOG) advocates screening for HCV infection at the annual exam if the patient belongs to one of the CDC’s routine-screening categories.4 Although ACOG has not issued separate screening guidelines for obstetric patients, some practitioners have advocated screening based on risk factors, as listed in the CDC’s routine-screening categories.5
HCV accounts for about 20% of acute hepatitis cases in the U.S.
Because Ob/Gyns are increasingly likely to encounter patients with positive HCV blood-screening results, they should be prepared to answer the following inquiries: What is hepatitis C? How is it diagnosed and transmitted? What is the natural history of the infection? Is there a treatment for it? How will HCV infection affect pregnancy?
This review addresses those questions.
What is hepatitis C?
Hepatitis C is a liver disease caused by the HCV, an RNA virus of the Flavivirus family, which includes the dengue and yellow fever viruses. Worldwide, approximately 170 million people are infected with HCV, with a prevalence ranging from a low of 0.15% in Scandinavia to a high of 38% in northern Egypt.6,7 In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%.1 U.S. prevalence rates by gender and age reveal that more males are infected than females and that, in females, prevalence is highest during the childbearing years, peaking at age 35.
TABLE 1
Recommendations for hepatitis C virus screening
| PEOPLE WHO SHOULD BE TESTED ROUTINELY |
|---|
|
| PEOPLE FOR WHOM ROUTINE TESTING IS OF UNCERTAIN NEED |
|
| PEOPLE FOR WHOM ROUTINE TESTING IS NOT RECOMMENDED* |
|
| * Except in cases where risk factors are present ALT=alanine aminotransferase; HCV=hepatitis C virus |
| Source: Centers for Disease Control and Prevention |
How is HCV infection diagnosed?
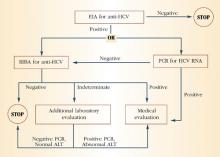
The initial screening test is an enzyme immunoassay (EIA) for the antibody to HCV. Currently, a third-generation EIA is used, with a sensitivity and specificity of 99% in immunocompetent people. If the EIA is positive, the practitioner may proceed to a confirmatory recombinant immunoblot assay (RIBA) in individuals with a low pretest probability, or to direct measurement of HCV RNA by reverse-transcription polymerase chain reaction (PCR) in individuals with a high pretest probability. Figure 1 depicts the HCV testing algorithm recommended by the CDC.
FIGURE 1 HCV infection testing algorithm
ALT=alanine aminotransferase; EIA=enzyme immunoassay; PCR=polymerase chain reaction; RIBA=recombinant immunoblast assay Source: Centers for Disease Control and Prevention
How is HCV transmitted?
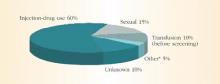
HCV spreads primarily through blood or fluids containing blood. Injection-drug use accounts for 60% of infections;1 a person who has used injection drugs for 5 years has a 60% to 90% chance of becoming infected with HCV. In fact, among injection-drug users, HCV infection is 4 times more common than HIV infection.8 The transfusion of blood or blood products accounts for another 10% of HCV cases. However, the transfusion-related risk has decreased markedly since the introduction of HCV screening in the blood banks in 1992. Figure 2 shows additional sources of HCV infection.
Although sexual transmission of HCV does occur, the virus is inefficiently spread in this manner. Evidence for this route of transmission has been accumulated from case-control and partner studies. Case-control studies have reported an association between HCV infection and exposure to a sex partner with a history of hepatitis or exposure to multiple sex partners.9,10 Cross-sectional studies suggest that the probability of HCV infection in the sexual partner of an HCV-positive patient is 0% to 3% in northern Europe or North America. Higher probabilities are found in southern Europe and the Far East.11 One prospective study showed no cases of sexual transmission of HCV from 94 HCV-positive females to their male part-ners.12 Another prospective study found a low incidence—12 infections per 1,000 per-son-years—among 449 sexual partners of HCV-positive individuals.13
Since the risk of spreading HCV through sexual contact is relatively low, individuals in long-term, stable relationships with an infected partner need not change their sexual practices. However, they may choose to use barrier methods to lower the chance of transmission even further. Individuals with high-risk sexual practices, such as having multiple sexual partners, should definitely use barrier methods.
FIGURE 2 Sources of infection for persons with hepatitis C
*Nosocomial, occupational, perinatal
Source: Centers for Disease Control and Prevention
What is the natural history of HCV infection?
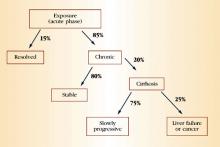
HCV accounts for approximately 20% of cases of acute hepatitis in the United States.14 The incubation period is approximately 6 weeks. Children and adults who acquire the infection usually are asymptomatic or have nonspecific symptoms of fatigue, malaise, anorexia, and weight loss. Some patients may present with jaundice. Serum aminotransferases can fluctuate during acute infection, and normalization occurs in 40% of individuals. However, this normalization does not necessarily represent a clearance of infection. Only 15% of patients clear the virus; the rest develop chronic infection.
Only 15% of patients clear the virus; the rest develop chronic infection.
During chronic infection, most patients are unaware that they are HCV-positive because, as with acute infection, they are asymptomatic or have mild, nonspecific symptoms such as fatigue. Again, serum aminotransferases typically fluctuate and are normal 30% of the time. Clinical progression to cirrhosis occurs in 20% of cases over a span of about 20 years. Once cirrhosis develops, the risk of decompensated cirrhosis or liver failure is about 3% to 4% per year. The annual risk of primary hepatocellular carcinoma in patients with cirrhosis is 1% to 4%. Data suggest that alcohol use is a predictive factor for progressive liver disease and liver cancer. Figure 3 summarizes the natural history of HCV infection.
FIGURE 3 Natural history of HCV infection
Is there a treatment for hepatitis C?
Over the past decade, there have been incremental improvements in hepatitis C therapy. The original mainstay of treatment was interferon alpha, with an initial response rate of 50% and a sustained response rate of only 10% to 15%.15-17 Later, combination therapy with interferon alpha and ribavirin raised the sustained response rate to 40%.18,19 However, both these therapies have substantial side effects and require frequent dosing schedules.
In January 2001, a new therapy, pegylated interferon alpha, was introduced. This modified form of traditional interferon alpha has fewer side effects and requires less frequent dosing. In addition, initial studies showed that it had a greater sustained-response rate than traditional interferon alpha alone.20 The Food and Drug Administration (FDA) recently approved it for the treatment of chronic hepatitis not previously treated with standard interferon alpha. A recent trial showed that the combination of pegylated interferon alpha with ribavirin had a sustained virologic response rate of 54%.21 None of these medications are approved for use in pregnancy or childhood.
To prevent progressive liver disease, patients chronically infected with HCV should avoid alcohol and other hepatotoxins and get vaccinated against hepatitis A and B. To reduce the risk of transmission to others, they should be advised not to donate blood and tissues; not to share toothbrushes, razors, or other personal-care items that may have blood on them; and to cover cuts and sores on the skin.
TABLE 2
Characteristics of hepatitis viral infection in the U.S. population
| HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | |
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Source/route of transmission | Feces/Fecal-oral | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Feces/Fecal-oral |
| No. of acute infections (per year) | 125,000-200,000 | 140,000-320,000 | 35,000-180,000 | 6,000-13,000 | Rare in the the United States |
| No. of chronic infections | 0 | 1-1.25 million | 3.5 million | 70,000 | 0 |
| Deaths from chronic liver disease (per year) | 0 | 5,000 | 8,000-10,000 | 1,000 | NA |
| Symptoms | Fatigue, nausea, pain near liver or in upper-abdominal area, dark urine, light stools, fever, jaundice | Same as hepatitis A. However, almost 50% of people infected with hepatitis B are asymptomatic. | Same as hepatitis A. However,most people are asymptomatic | Similar to other types of viral hepatitis | Similar to other types of viral hepatitis |
| Prevention | Good personal hygiene and proper sanitation. Also pre- or postexposure immunization | Pre- or post-exposure immunization | Blood donor screening; risk behavior modification | Pre- or post-exposure immunization | Ensure safe drinking water |
| Comments | Typically lasts about 3 weeks, but may persist for as long as 6 months | — | Originally defined in the 1970s as non-A non-B hepatitis. Renamed hepatitis C in 1989. | Requires the hepatitis B virus to exist* | Usually associated with fecally contaminated drinking water. U.S. cases usually involve a history of travel to areas with endemic hepatitis E infection |
| *Hepatitis D infection can be acquired either as a co-infection with the hepatitis B virus or as a superinfection in persons with chronic hepatitis B infection. Persons coinfected with the B and D viruses may have more severe acute disease and a higher risk of fulminant hepatitis (2% to 20%) compared with those infected with hepatitis B alone. However, chronic hepatitis B infection appears to occur less frequently in persons coinfected with the B and D viruses. Chronic hepatitis B carriers who acquire hepatitis D superinfection usually develop chronic hepatitis D infection. In long-term studies of chronic hepatitis B carriers with hepatitis D superinfection, 70% to 80% have developed evidence of chronic liver diseases with cirrhosis compared with 15% to 30% of patients with chronic hepatitis B infection alone. | |||||
| Source: American Liver Foundation, Centers for Disease Control and Prevention | |||||
How will hepatitis C affect my pregnancy?
Perinatal transmission occurs in women with chronic HCV infection at an average rate of 5%.22-24 Small studies suggest that the level of HCV viremia may affect the risk of viral transmission.22,25 However, in 2 large studies, no significant difference in HCV viremia levels was found between women who transmitted the virus to their children and women who did not.23,24 Women co-infected with HIV have 2 to 3 times the rate of HCV transmission compared with women infected only with HCV.26,27 The natural history of HCV infection in children is not well understood, but 50% to 80% of HCV-positive children develop chronic infection.28
Currently, no intervention is available to decrease perinatal HCV transmission.
Currently, no intervention is available to decrease perinatal HCV transmission, although an unclear association with mode of delivery exists. One study of 441 mother-infant pairs showed that elective cesarean delivery may be associated with a lower transmission risk.29 However, another study of 370 mother-infant pairs found no difference in risk.24
The effect of pregnancy on the progression of hepatitis C infection also is unclear. In studies of HCV-positive pregnant women—as compared to HCV-positive non-pregnant women—researchers reported a decrease in serum alanine aminotransferase (ALT) and an increase in HCV RNA during pregnancy,30 as well as a deterioration of hepatitis C disease after pregnancy, as confirmed by liver biopsy.31 In prospective cohort studies comparing infected and uninfected pregnant women, serum aminotrans-ferases flared postpartum in one study32 but remained normal in another.33
Not much is known regarding the effect of chronic hepatitis C infection on pregnancy outcome, although 1 case-control study reported an association between hepatitis C infection and cholestasis of pregnancy.34
Although HCV RNA has been detected in colostrum,35 studies of breastfed infants with HCV-positive mothers have found no cases of transmission secondary to breastfeeding.35,36 Thus, both the American Academy of Pediatrics and ACOG conclude that breastfeeding does not appreciably increase the risk of transmission to the neonate and should not be prohibited.37,38 However, pending further studies, practitioners may choose to advise against breastfeeding if nipples are cracked and bleeding.
- Approximately 25% of people with chronic HCV infection are asymptomatic with normal liver function tests and benign histology.
- Only 10% of people recognize acute infection when they acquire HCV. In the vast majority of cases, the disease is subclinical.
- The “epidemic” of HCV infection represents greater identification of chronic cases rather than increasing numbers of new outbreaks.
- Most new cases of hepatitis C occur in young adults, ages 25 to 40, who may not learn for years or even decades that they are infected.
- African Americans are twice as likely as non-Hispanic whites to be infected with HCV. Hispanics, too, are more likely than non-Hispanic whites to be infected.
- Infection with the human immunodeficiency virus (HIV) appears to accelerate the course of HCV, as does co-infection with the hepatitis B virus.
- Due to improvements in blood bank screening and a decrease in the use of IV drugs, the number of new cases of HCV diagnosed in 1997 was only about 37,000—an 80% decline. Nevertheless, over the next 1 to 2 decades, the HCV mortality rate is expected to double.
- Total expenditures for HCV therapy are thought to range from $10,000 to $12,000 when the costs of interferon, ribavirin, laboratory studies, and office visits are taken into account.
Acute infection with the hepatitis C virus (HCV) progresses to chronic disease in 50% to 84% of cases. Once HCV infection is chronic, combination therapy with interferon alfa-2b and ribavirin elicits a sustained virologic response in only 41% of cases. Even the latest therapy for chronic HCV—a combination of pegylated interferon alfa-2a or 2b and ribavirin—eradicates the virus in only 54% of patients.1 For these reasons, a team of researchers in Germany explored the effectiveness of treating HCV during the acute phase of infection using high-dose interferon alfa-2b to prevent progression.2
‘Peginterferons’ elicit a greater sustained virologic response.
Because the viral load of patients with HCV infection starts to rise within 24 hours after a single dose of inter-feron alfa-2b, investigators used a daily dosing regimen—rather than the standard thrice-weekly therapy—for the first 4 weeks of treatment. They then followed the standard schedule for another 20 weeks, measuring serum HCV RNA levels before, during, and 24 weeks after the termination of therapy.
All 44 patients in the study were given 5 million units of subcutaneous interferon alfa-2b daily for 4 weeks, after which time they received the same dosage 3 times a week. Twenty-four weeks after the end of therapy, 43 patients (98%) had normal serum alanine aminotransferase (ALT) and undetectable levels of serum HCV RNA. Side effects caused 1 patient to stop treatment after 12 weeks.
Although the investigators concluded that the treatment of acute HCV infection with interferon alfa-2b prevents progression to chronic disease, a number of issues remain unresolved. The primary one is whether all patients with acute disease should undergo treatment with a costly therapy that can be hard to tolerate, when many of these patients would recover without it. Further, since pegylated interferon has proven more effective than standard interferon in treating chronic HCV, it also may be more effective in treating acute disease. (“Peginterferons,” as they are called, can be administered weekly and elicit a greater sustained virologic response.) Finally, since acute infection is no longer common in the United States—dropping from 250,000 to 500,000 cases annually in the 1980s to less than 40,000 today—and since not all infections are clinically acute, this approach has limited applicability.3
These and other issues require further exploration. —Katherine Chen, MD, MPH
REFERENCES
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
2. Jaeckel E, Cornberg M, Wedemeyer H, et al. for the German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345(20):1452-1457.
3. Hoofnagle JH. Therapy for acute hepatitis C [editorial]. N Engl J Med. 2001;345(20):1495-1497.
Conclusion
Chronic HCV infection can lead to serious medical consequences, such as cirrhosis, liver failure, hepatocellular cancer, and death. Unfortunately, 75% of individuals infected with HCV are asymptomatic. By the time symptoms do occur, the disease often is in its advanced stages. Thus, both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups. The early identification of chronic HCV infection is important, as it enables women to take advantage of increasingly effective treatments and alerts them to the need for preconception counseling.
Both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups.
Although perinatal HCV transmission is a known risk, we lack strategies to prevent it. In addition, the effects of pregnancy on the progression of chronic HCV infection, and the effects of chronic HCV infection on pregnancy outcome, are unknown. However, the identification of HCV-positive pregnant women will lead to screening of their infants and earlier identification of infected children.
1. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562.
2. National Center for Infectious Diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncidod/dis-eases/hepatitis/slideset/intro/slide_5.htm. Accessed January 11, 2002.
3. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47:1-39.
4. American College of Obstetricians and Gynecologists. Primary and preventive care: periodic assessments. ACOG Committee Opinion #229. Washington, DC: ACOG; 1999.
5. Burns DN, Minkoff H. Hepatitis C: screening in pregnancy. Obstet Gynecol. 1999;94:1044-1048.
6. World Health Organization. Global prevalence of hepatitis C, 1999. Available at: http://www.who.int/emc/images/hepacmap.jpg. Accessed August 31, 2001.
7. Arthur RR, Hassan NF, Abdallah MY, et al. Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91:271-274.
8. Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lym-photropic viruses. Am J Public Health. 1996;86:655-661.
9. Alter MJ, Gerety RJ, Smallwood LA, Sampliner RE, Tabor E, Deinhardt F, et al. Sporadic non-A, non-B hepatitis: frequency and epidemiology in an urban U.S. population. J Infect Dis. 1982;145:886-893.
10. Alter MJ, Coleman PJ, Alexander WJ, et al. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA. 1989;262:1201-1205.
11. Rooney G, Gilson RJ. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399-404.
12. Meisel H, Reip A, Faltus B, et al. Transmission of hepatitis C virus to children and husbands by women infected with contaminated anti-D immunoglobulin. Lancet. 1995;345:1209-1211.
13. Piazza M, Sagliocca L, Tosone G, et al. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537-1544.
14. Cheney CP, Chopra S, Graham C, Hepatitis C. Infect Dis Clin North Am. 2000;14:633-667.
15. Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506.
16. Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interfer-on alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510.
17. Marcellin P, Boyer N, Giostra E, et al. Recombinant human alpha-interferon in patients with chronic non-A, non-B hepatitis: a multicenter randomized controlled trial from France. Hepatology. 1991;13:393-397.
18. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492.
19. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432.
20. Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438.
21. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
22. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;30:744-750.
23. Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441.
24. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
25. Lin HH, Kao JH, Hsu HY, et al. Possible role of high-titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis. 1994;169:638-641.
26. Thomas DL, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480-1488.
27. Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis. 1997;25:1121-1124.
28. Bortolotti F, Faggion S, Con P. Natural history of chronic viral hepatitis in childhood. Acta Gastroenterol Belg. 1998;61:198-201.
29. Lin HH, Kao JH, Hsu HY, et al. Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. J Pediatr. 1995;126:589-591.
30. Polywka S, Schroter M, Feucht HH, Zollner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29:1327-1329.
31. Hepatitis C virus infection. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics. 1998;101:481-485.
32. American College of Obstetricians and Gynecologists. Breastfeeding and the risk of hepatitis C virus transmission. ACOG Committee Opinion #220. Washington, DC: ACOG; 1999.
33. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
34. Gervais A, Bacq Y, Bernuau J, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol. 2000;32:293-299.
35. Fontaine H, Nalpas B, Carnot F, Brechot C, Pol S. Effect of pregnancy on chronic hepatitis C: a case-control study. Lancet. 2000;356:1328-1329.
36. Latt NC, Spencer JD, Beeby PJ, et al. Hepatitis C in injecting drug-using women during and after pregnancy. J Gastroenterol Hepatol. 2000;15:175-181.
37. Floreani A, Paternoster D, Zappala F, et al. Hepatitis C virus infection in pregnancy. Br J Obstet Gynaecol. 1996;103:325-329.
38. Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498-500.
- The hepatitis C virus (HCV) is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States.
- In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%. In females, prevalence is highest during the childbearing years, peaking at age 35.
- Injection-drug use accounts for 60% of infections, while the transfusion of blood or blood products accounts for another 10%.
- About 15% of people with acute HCV infection clear the virus; the rest develop chronic infection.
- During chronic infection, most patients are asymptomatic or have mild, nonspecific symptoms such as fatigue.
- Combination therapy with interferon alpha and ribavirin elicits a sustained virologic response rate of 40%, and newer therapy with pegylated interferon alpha and ribavirin improves the rate to 54%.
- Perinatal transmission in women with chronic HCV infection occurs at an average rate of 5%.
The hepatitis C virus (HCV) was first identified in 1989 as the cause of non-A, non-B hepatitis infections. Since its discovery, HCV has become the most common chronic blood-borne infection in the United States: Approximately 2.7 to 3.5 million people have chronic HCV infection,1,2 as compared with 1.25 million people with chronic hepatitis B virus infection and 1 million with the human immunodeficiency virus (HIV). In addition, HCV infection is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States. Some call HCV infection “the silent epidemic,” since 75% of people infected are asymptomatic and chronic manifestations don’t appear for 1 to 2 decades.
In 1998, the Centers for Disease Control and Prevention (CDC) issued guidelines for screening for HCV infection ( Table 1).3 The American College of Obstetricians and Gynecologists (ACOG) advocates screening for HCV infection at the annual exam if the patient belongs to one of the CDC’s routine-screening categories.4 Although ACOG has not issued separate screening guidelines for obstetric patients, some practitioners have advocated screening based on risk factors, as listed in the CDC’s routine-screening categories.5
HCV accounts for about 20% of acute hepatitis cases in the U.S.
Because Ob/Gyns are increasingly likely to encounter patients with positive HCV blood-screening results, they should be prepared to answer the following inquiries: What is hepatitis C? How is it diagnosed and transmitted? What is the natural history of the infection? Is there a treatment for it? How will HCV infection affect pregnancy?
This review addresses those questions.
What is hepatitis C?
Hepatitis C is a liver disease caused by the HCV, an RNA virus of the Flavivirus family, which includes the dengue and yellow fever viruses. Worldwide, approximately 170 million people are infected with HCV, with a prevalence ranging from a low of 0.15% in Scandinavia to a high of 38% in northern Egypt.6,7 In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%.1 U.S. prevalence rates by gender and age reveal that more males are infected than females and that, in females, prevalence is highest during the childbearing years, peaking at age 35.
TABLE 1
Recommendations for hepatitis C virus screening
| PEOPLE WHO SHOULD BE TESTED ROUTINELY |
|---|
|
| PEOPLE FOR WHOM ROUTINE TESTING IS OF UNCERTAIN NEED |
|
| PEOPLE FOR WHOM ROUTINE TESTING IS NOT RECOMMENDED* |
|
| * Except in cases where risk factors are present ALT=alanine aminotransferase; HCV=hepatitis C virus |
| Source: Centers for Disease Control and Prevention |
How is HCV infection diagnosed?
The initial screening test is an enzyme immunoassay (EIA) for the antibody to HCV. Currently, a third-generation EIA is used, with a sensitivity and specificity of 99% in immunocompetent people. If the EIA is positive, the practitioner may proceed to a confirmatory recombinant immunoblot assay (RIBA) in individuals with a low pretest probability, or to direct measurement of HCV RNA by reverse-transcription polymerase chain reaction (PCR) in individuals with a high pretest probability. Figure 1 depicts the HCV testing algorithm recommended by the CDC.
FIGURE 1 HCV infection testing algorithm
ALT=alanine aminotransferase; EIA=enzyme immunoassay; PCR=polymerase chain reaction; RIBA=recombinant immunoblast assay Source: Centers for Disease Control and Prevention
How is HCV transmitted?
HCV spreads primarily through blood or fluids containing blood. Injection-drug use accounts for 60% of infections;1 a person who has used injection drugs for 5 years has a 60% to 90% chance of becoming infected with HCV. In fact, among injection-drug users, HCV infection is 4 times more common than HIV infection.8 The transfusion of blood or blood products accounts for another 10% of HCV cases. However, the transfusion-related risk has decreased markedly since the introduction of HCV screening in the blood banks in 1992. Figure 2 shows additional sources of HCV infection.
Although sexual transmission of HCV does occur, the virus is inefficiently spread in this manner. Evidence for this route of transmission has been accumulated from case-control and partner studies. Case-control studies have reported an association between HCV infection and exposure to a sex partner with a history of hepatitis or exposure to multiple sex partners.9,10 Cross-sectional studies suggest that the probability of HCV infection in the sexual partner of an HCV-positive patient is 0% to 3% in northern Europe or North America. Higher probabilities are found in southern Europe and the Far East.11 One prospective study showed no cases of sexual transmission of HCV from 94 HCV-positive females to their male part-ners.12 Another prospective study found a low incidence—12 infections per 1,000 per-son-years—among 449 sexual partners of HCV-positive individuals.13
Since the risk of spreading HCV through sexual contact is relatively low, individuals in long-term, stable relationships with an infected partner need not change their sexual practices. However, they may choose to use barrier methods to lower the chance of transmission even further. Individuals with high-risk sexual practices, such as having multiple sexual partners, should definitely use barrier methods.
FIGURE 2 Sources of infection for persons with hepatitis C
*Nosocomial, occupational, perinatal
Source: Centers for Disease Control and Prevention
What is the natural history of HCV infection?
HCV accounts for approximately 20% of cases of acute hepatitis in the United States.14 The incubation period is approximately 6 weeks. Children and adults who acquire the infection usually are asymptomatic or have nonspecific symptoms of fatigue, malaise, anorexia, and weight loss. Some patients may present with jaundice. Serum aminotransferases can fluctuate during acute infection, and normalization occurs in 40% of individuals. However, this normalization does not necessarily represent a clearance of infection. Only 15% of patients clear the virus; the rest develop chronic infection.
Only 15% of patients clear the virus; the rest develop chronic infection.
During chronic infection, most patients are unaware that they are HCV-positive because, as with acute infection, they are asymptomatic or have mild, nonspecific symptoms such as fatigue. Again, serum aminotransferases typically fluctuate and are normal 30% of the time. Clinical progression to cirrhosis occurs in 20% of cases over a span of about 20 years. Once cirrhosis develops, the risk of decompensated cirrhosis or liver failure is about 3% to 4% per year. The annual risk of primary hepatocellular carcinoma in patients with cirrhosis is 1% to 4%. Data suggest that alcohol use is a predictive factor for progressive liver disease and liver cancer. Figure 3 summarizes the natural history of HCV infection.
FIGURE 3 Natural history of HCV infection
Is there a treatment for hepatitis C?
Over the past decade, there have been incremental improvements in hepatitis C therapy. The original mainstay of treatment was interferon alpha, with an initial response rate of 50% and a sustained response rate of only 10% to 15%.15-17 Later, combination therapy with interferon alpha and ribavirin raised the sustained response rate to 40%.18,19 However, both these therapies have substantial side effects and require frequent dosing schedules.
In January 2001, a new therapy, pegylated interferon alpha, was introduced. This modified form of traditional interferon alpha has fewer side effects and requires less frequent dosing. In addition, initial studies showed that it had a greater sustained-response rate than traditional interferon alpha alone.20 The Food and Drug Administration (FDA) recently approved it for the treatment of chronic hepatitis not previously treated with standard interferon alpha. A recent trial showed that the combination of pegylated interferon alpha with ribavirin had a sustained virologic response rate of 54%.21 None of these medications are approved for use in pregnancy or childhood.
To prevent progressive liver disease, patients chronically infected with HCV should avoid alcohol and other hepatotoxins and get vaccinated against hepatitis A and B. To reduce the risk of transmission to others, they should be advised not to donate blood and tissues; not to share toothbrushes, razors, or other personal-care items that may have blood on them; and to cover cuts and sores on the skin.
TABLE 2
Characteristics of hepatitis viral infection in the U.S. population
| HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | |
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Source/route of transmission | Feces/Fecal-oral | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Feces/Fecal-oral |
| No. of acute infections (per year) | 125,000-200,000 | 140,000-320,000 | 35,000-180,000 | 6,000-13,000 | Rare in the the United States |
| No. of chronic infections | 0 | 1-1.25 million | 3.5 million | 70,000 | 0 |
| Deaths from chronic liver disease (per year) | 0 | 5,000 | 8,000-10,000 | 1,000 | NA |
| Symptoms | Fatigue, nausea, pain near liver or in upper-abdominal area, dark urine, light stools, fever, jaundice | Same as hepatitis A. However, almost 50% of people infected with hepatitis B are asymptomatic. | Same as hepatitis A. However,most people are asymptomatic | Similar to other types of viral hepatitis | Similar to other types of viral hepatitis |
| Prevention | Good personal hygiene and proper sanitation. Also pre- or postexposure immunization | Pre- or post-exposure immunization | Blood donor screening; risk behavior modification | Pre- or post-exposure immunization | Ensure safe drinking water |
| Comments | Typically lasts about 3 weeks, but may persist for as long as 6 months | — | Originally defined in the 1970s as non-A non-B hepatitis. Renamed hepatitis C in 1989. | Requires the hepatitis B virus to exist* | Usually associated with fecally contaminated drinking water. U.S. cases usually involve a history of travel to areas with endemic hepatitis E infection |
| *Hepatitis D infection can be acquired either as a co-infection with the hepatitis B virus or as a superinfection in persons with chronic hepatitis B infection. Persons coinfected with the B and D viruses may have more severe acute disease and a higher risk of fulminant hepatitis (2% to 20%) compared with those infected with hepatitis B alone. However, chronic hepatitis B infection appears to occur less frequently in persons coinfected with the B and D viruses. Chronic hepatitis B carriers who acquire hepatitis D superinfection usually develop chronic hepatitis D infection. In long-term studies of chronic hepatitis B carriers with hepatitis D superinfection, 70% to 80% have developed evidence of chronic liver diseases with cirrhosis compared with 15% to 30% of patients with chronic hepatitis B infection alone. | |||||
| Source: American Liver Foundation, Centers for Disease Control and Prevention | |||||
How will hepatitis C affect my pregnancy?
Perinatal transmission occurs in women with chronic HCV infection at an average rate of 5%.22-24 Small studies suggest that the level of HCV viremia may affect the risk of viral transmission.22,25 However, in 2 large studies, no significant difference in HCV viremia levels was found between women who transmitted the virus to their children and women who did not.23,24 Women co-infected with HIV have 2 to 3 times the rate of HCV transmission compared with women infected only with HCV.26,27 The natural history of HCV infection in children is not well understood, but 50% to 80% of HCV-positive children develop chronic infection.28
Currently, no intervention is available to decrease perinatal HCV transmission.
Currently, no intervention is available to decrease perinatal HCV transmission, although an unclear association with mode of delivery exists. One study of 441 mother-infant pairs showed that elective cesarean delivery may be associated with a lower transmission risk.29 However, another study of 370 mother-infant pairs found no difference in risk.24
The effect of pregnancy on the progression of hepatitis C infection also is unclear. In studies of HCV-positive pregnant women—as compared to HCV-positive non-pregnant women—researchers reported a decrease in serum alanine aminotransferase (ALT) and an increase in HCV RNA during pregnancy,30 as well as a deterioration of hepatitis C disease after pregnancy, as confirmed by liver biopsy.31 In prospective cohort studies comparing infected and uninfected pregnant women, serum aminotrans-ferases flared postpartum in one study32 but remained normal in another.33
Not much is known regarding the effect of chronic hepatitis C infection on pregnancy outcome, although 1 case-control study reported an association between hepatitis C infection and cholestasis of pregnancy.34
Although HCV RNA has been detected in colostrum,35 studies of breastfed infants with HCV-positive mothers have found no cases of transmission secondary to breastfeeding.35,36 Thus, both the American Academy of Pediatrics and ACOG conclude that breastfeeding does not appreciably increase the risk of transmission to the neonate and should not be prohibited.37,38 However, pending further studies, practitioners may choose to advise against breastfeeding if nipples are cracked and bleeding.
- Approximately 25% of people with chronic HCV infection are asymptomatic with normal liver function tests and benign histology.
- Only 10% of people recognize acute infection when they acquire HCV. In the vast majority of cases, the disease is subclinical.
- The “epidemic” of HCV infection represents greater identification of chronic cases rather than increasing numbers of new outbreaks.
- Most new cases of hepatitis C occur in young adults, ages 25 to 40, who may not learn for years or even decades that they are infected.
- African Americans are twice as likely as non-Hispanic whites to be infected with HCV. Hispanics, too, are more likely than non-Hispanic whites to be infected.
- Infection with the human immunodeficiency virus (HIV) appears to accelerate the course of HCV, as does co-infection with the hepatitis B virus.
- Due to improvements in blood bank screening and a decrease in the use of IV drugs, the number of new cases of HCV diagnosed in 1997 was only about 37,000—an 80% decline. Nevertheless, over the next 1 to 2 decades, the HCV mortality rate is expected to double.
- Total expenditures for HCV therapy are thought to range from $10,000 to $12,000 when the costs of interferon, ribavirin, laboratory studies, and office visits are taken into account.
Acute infection with the hepatitis C virus (HCV) progresses to chronic disease in 50% to 84% of cases. Once HCV infection is chronic, combination therapy with interferon alfa-2b and ribavirin elicits a sustained virologic response in only 41% of cases. Even the latest therapy for chronic HCV—a combination of pegylated interferon alfa-2a or 2b and ribavirin—eradicates the virus in only 54% of patients.1 For these reasons, a team of researchers in Germany explored the effectiveness of treating HCV during the acute phase of infection using high-dose interferon alfa-2b to prevent progression.2
‘Peginterferons’ elicit a greater sustained virologic response.
Because the viral load of patients with HCV infection starts to rise within 24 hours after a single dose of inter-feron alfa-2b, investigators used a daily dosing regimen—rather than the standard thrice-weekly therapy—for the first 4 weeks of treatment. They then followed the standard schedule for another 20 weeks, measuring serum HCV RNA levels before, during, and 24 weeks after the termination of therapy.
All 44 patients in the study were given 5 million units of subcutaneous interferon alfa-2b daily for 4 weeks, after which time they received the same dosage 3 times a week. Twenty-four weeks after the end of therapy, 43 patients (98%) had normal serum alanine aminotransferase (ALT) and undetectable levels of serum HCV RNA. Side effects caused 1 patient to stop treatment after 12 weeks.
Although the investigators concluded that the treatment of acute HCV infection with interferon alfa-2b prevents progression to chronic disease, a number of issues remain unresolved. The primary one is whether all patients with acute disease should undergo treatment with a costly therapy that can be hard to tolerate, when many of these patients would recover without it. Further, since pegylated interferon has proven more effective than standard interferon in treating chronic HCV, it also may be more effective in treating acute disease. (“Peginterferons,” as they are called, can be administered weekly and elicit a greater sustained virologic response.) Finally, since acute infection is no longer common in the United States—dropping from 250,000 to 500,000 cases annually in the 1980s to less than 40,000 today—and since not all infections are clinically acute, this approach has limited applicability.3
These and other issues require further exploration. —Katherine Chen, MD, MPH
REFERENCES
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
2. Jaeckel E, Cornberg M, Wedemeyer H, et al. for the German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345(20):1452-1457.
3. Hoofnagle JH. Therapy for acute hepatitis C [editorial]. N Engl J Med. 2001;345(20):1495-1497.
Conclusion
Chronic HCV infection can lead to serious medical consequences, such as cirrhosis, liver failure, hepatocellular cancer, and death. Unfortunately, 75% of individuals infected with HCV are asymptomatic. By the time symptoms do occur, the disease often is in its advanced stages. Thus, both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups. The early identification of chronic HCV infection is important, as it enables women to take advantage of increasingly effective treatments and alerts them to the need for preconception counseling.
Both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups.
Although perinatal HCV transmission is a known risk, we lack strategies to prevent it. In addition, the effects of pregnancy on the progression of chronic HCV infection, and the effects of chronic HCV infection on pregnancy outcome, are unknown. However, the identification of HCV-positive pregnant women will lead to screening of their infants and earlier identification of infected children.
- The hepatitis C virus (HCV) is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States.
- In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%. In females, prevalence is highest during the childbearing years, peaking at age 35.
- Injection-drug use accounts for 60% of infections, while the transfusion of blood or blood products accounts for another 10%.
- About 15% of people with acute HCV infection clear the virus; the rest develop chronic infection.
- During chronic infection, most patients are asymptomatic or have mild, nonspecific symptoms such as fatigue.
- Combination therapy with interferon alpha and ribavirin elicits a sustained virologic response rate of 40%, and newer therapy with pegylated interferon alpha and ribavirin improves the rate to 54%.
- Perinatal transmission in women with chronic HCV infection occurs at an average rate of 5%.
The hepatitis C virus (HCV) was first identified in 1989 as the cause of non-A, non-B hepatitis infections. Since its discovery, HCV has become the most common chronic blood-borne infection in the United States: Approximately 2.7 to 3.5 million people have chronic HCV infection,1,2 as compared with 1.25 million people with chronic hepatitis B virus infection and 1 million with the human immunodeficiency virus (HIV). In addition, HCV infection is the leading cause of chronic liver disease and the leading indication for liver transplantation in the United States. Some call HCV infection “the silent epidemic,” since 75% of people infected are asymptomatic and chronic manifestations don’t appear for 1 to 2 decades.
In 1998, the Centers for Disease Control and Prevention (CDC) issued guidelines for screening for HCV infection ( Table 1).3 The American College of Obstetricians and Gynecologists (ACOG) advocates screening for HCV infection at the annual exam if the patient belongs to one of the CDC’s routine-screening categories.4 Although ACOG has not issued separate screening guidelines for obstetric patients, some practitioners have advocated screening based on risk factors, as listed in the CDC’s routine-screening categories.5
HCV accounts for about 20% of acute hepatitis cases in the U.S.
Because Ob/Gyns are increasingly likely to encounter patients with positive HCV blood-screening results, they should be prepared to answer the following inquiries: What is hepatitis C? How is it diagnosed and transmitted? What is the natural history of the infection? Is there a treatment for it? How will HCV infection affect pregnancy?
This review addresses those questions.
What is hepatitis C?
Hepatitis C is a liver disease caused by the HCV, an RNA virus of the Flavivirus family, which includes the dengue and yellow fever viruses. Worldwide, approximately 170 million people are infected with HCV, with a prevalence ranging from a low of 0.15% in Scandinavia to a high of 38% in northern Egypt.6,7 In the United States, 3.9 million people have been infected with HCV, with an overall prevalence of 1.8%.1 U.S. prevalence rates by gender and age reveal that more males are infected than females and that, in females, prevalence is highest during the childbearing years, peaking at age 35.
TABLE 1
Recommendations for hepatitis C virus screening
| PEOPLE WHO SHOULD BE TESTED ROUTINELY |
|---|
|
| PEOPLE FOR WHOM ROUTINE TESTING IS OF UNCERTAIN NEED |
|
| PEOPLE FOR WHOM ROUTINE TESTING IS NOT RECOMMENDED* |
|
| * Except in cases where risk factors are present ALT=alanine aminotransferase; HCV=hepatitis C virus |
| Source: Centers for Disease Control and Prevention |
How is HCV infection diagnosed?
The initial screening test is an enzyme immunoassay (EIA) for the antibody to HCV. Currently, a third-generation EIA is used, with a sensitivity and specificity of 99% in immunocompetent people. If the EIA is positive, the practitioner may proceed to a confirmatory recombinant immunoblot assay (RIBA) in individuals with a low pretest probability, or to direct measurement of HCV RNA by reverse-transcription polymerase chain reaction (PCR) in individuals with a high pretest probability. Figure 1 depicts the HCV testing algorithm recommended by the CDC.
FIGURE 1 HCV infection testing algorithm
ALT=alanine aminotransferase; EIA=enzyme immunoassay; PCR=polymerase chain reaction; RIBA=recombinant immunoblast assay Source: Centers for Disease Control and Prevention
How is HCV transmitted?
HCV spreads primarily through blood or fluids containing blood. Injection-drug use accounts for 60% of infections;1 a person who has used injection drugs for 5 years has a 60% to 90% chance of becoming infected with HCV. In fact, among injection-drug users, HCV infection is 4 times more common than HIV infection.8 The transfusion of blood or blood products accounts for another 10% of HCV cases. However, the transfusion-related risk has decreased markedly since the introduction of HCV screening in the blood banks in 1992. Figure 2 shows additional sources of HCV infection.
Although sexual transmission of HCV does occur, the virus is inefficiently spread in this manner. Evidence for this route of transmission has been accumulated from case-control and partner studies. Case-control studies have reported an association between HCV infection and exposure to a sex partner with a history of hepatitis or exposure to multiple sex partners.9,10 Cross-sectional studies suggest that the probability of HCV infection in the sexual partner of an HCV-positive patient is 0% to 3% in northern Europe or North America. Higher probabilities are found in southern Europe and the Far East.11 One prospective study showed no cases of sexual transmission of HCV from 94 HCV-positive females to their male part-ners.12 Another prospective study found a low incidence—12 infections per 1,000 per-son-years—among 449 sexual partners of HCV-positive individuals.13
Since the risk of spreading HCV through sexual contact is relatively low, individuals in long-term, stable relationships with an infected partner need not change their sexual practices. However, they may choose to use barrier methods to lower the chance of transmission even further. Individuals with high-risk sexual practices, such as having multiple sexual partners, should definitely use barrier methods.
FIGURE 2 Sources of infection for persons with hepatitis C
*Nosocomial, occupational, perinatal
Source: Centers for Disease Control and Prevention
What is the natural history of HCV infection?
HCV accounts for approximately 20% of cases of acute hepatitis in the United States.14 The incubation period is approximately 6 weeks. Children and adults who acquire the infection usually are asymptomatic or have nonspecific symptoms of fatigue, malaise, anorexia, and weight loss. Some patients may present with jaundice. Serum aminotransferases can fluctuate during acute infection, and normalization occurs in 40% of individuals. However, this normalization does not necessarily represent a clearance of infection. Only 15% of patients clear the virus; the rest develop chronic infection.
Only 15% of patients clear the virus; the rest develop chronic infection.
During chronic infection, most patients are unaware that they are HCV-positive because, as with acute infection, they are asymptomatic or have mild, nonspecific symptoms such as fatigue. Again, serum aminotransferases typically fluctuate and are normal 30% of the time. Clinical progression to cirrhosis occurs in 20% of cases over a span of about 20 years. Once cirrhosis develops, the risk of decompensated cirrhosis or liver failure is about 3% to 4% per year. The annual risk of primary hepatocellular carcinoma in patients with cirrhosis is 1% to 4%. Data suggest that alcohol use is a predictive factor for progressive liver disease and liver cancer. Figure 3 summarizes the natural history of HCV infection.
FIGURE 3 Natural history of HCV infection
Is there a treatment for hepatitis C?
Over the past decade, there have been incremental improvements in hepatitis C therapy. The original mainstay of treatment was interferon alpha, with an initial response rate of 50% and a sustained response rate of only 10% to 15%.15-17 Later, combination therapy with interferon alpha and ribavirin raised the sustained response rate to 40%.18,19 However, both these therapies have substantial side effects and require frequent dosing schedules.
In January 2001, a new therapy, pegylated interferon alpha, was introduced. This modified form of traditional interferon alpha has fewer side effects and requires less frequent dosing. In addition, initial studies showed that it had a greater sustained-response rate than traditional interferon alpha alone.20 The Food and Drug Administration (FDA) recently approved it for the treatment of chronic hepatitis not previously treated with standard interferon alpha. A recent trial showed that the combination of pegylated interferon alpha with ribavirin had a sustained virologic response rate of 54%.21 None of these medications are approved for use in pregnancy or childhood.
To prevent progressive liver disease, patients chronically infected with HCV should avoid alcohol and other hepatotoxins and get vaccinated against hepatitis A and B. To reduce the risk of transmission to others, they should be advised not to donate blood and tissues; not to share toothbrushes, razors, or other personal-care items that may have blood on them; and to cover cuts and sores on the skin.
TABLE 2
Characteristics of hepatitis viral infection in the U.S. population
| HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | HEPATITIS | |
|---|---|---|---|---|---|
| A | B | C | D | E | |
| Source/route of transmission | Feces/Fecal-oral | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Blood and blood-derived body fluids/Percutaneous, permucosal | Feces/Fecal-oral |
| No. of acute infections (per year) | 125,000-200,000 | 140,000-320,000 | 35,000-180,000 | 6,000-13,000 | Rare in the the United States |
| No. of chronic infections | 0 | 1-1.25 million | 3.5 million | 70,000 | 0 |
| Deaths from chronic liver disease (per year) | 0 | 5,000 | 8,000-10,000 | 1,000 | NA |
| Symptoms | Fatigue, nausea, pain near liver or in upper-abdominal area, dark urine, light stools, fever, jaundice | Same as hepatitis A. However, almost 50% of people infected with hepatitis B are asymptomatic. | Same as hepatitis A. However,most people are asymptomatic | Similar to other types of viral hepatitis | Similar to other types of viral hepatitis |
| Prevention | Good personal hygiene and proper sanitation. Also pre- or postexposure immunization | Pre- or post-exposure immunization | Blood donor screening; risk behavior modification | Pre- or post-exposure immunization | Ensure safe drinking water |
| Comments | Typically lasts about 3 weeks, but may persist for as long as 6 months | — | Originally defined in the 1970s as non-A non-B hepatitis. Renamed hepatitis C in 1989. | Requires the hepatitis B virus to exist* | Usually associated with fecally contaminated drinking water. U.S. cases usually involve a history of travel to areas with endemic hepatitis E infection |
| *Hepatitis D infection can be acquired either as a co-infection with the hepatitis B virus or as a superinfection in persons with chronic hepatitis B infection. Persons coinfected with the B and D viruses may have more severe acute disease and a higher risk of fulminant hepatitis (2% to 20%) compared with those infected with hepatitis B alone. However, chronic hepatitis B infection appears to occur less frequently in persons coinfected with the B and D viruses. Chronic hepatitis B carriers who acquire hepatitis D superinfection usually develop chronic hepatitis D infection. In long-term studies of chronic hepatitis B carriers with hepatitis D superinfection, 70% to 80% have developed evidence of chronic liver diseases with cirrhosis compared with 15% to 30% of patients with chronic hepatitis B infection alone. | |||||
| Source: American Liver Foundation, Centers for Disease Control and Prevention | |||||
How will hepatitis C affect my pregnancy?
Perinatal transmission occurs in women with chronic HCV infection at an average rate of 5%.22-24 Small studies suggest that the level of HCV viremia may affect the risk of viral transmission.22,25 However, in 2 large studies, no significant difference in HCV viremia levels was found between women who transmitted the virus to their children and women who did not.23,24 Women co-infected with HIV have 2 to 3 times the rate of HCV transmission compared with women infected only with HCV.26,27 The natural history of HCV infection in children is not well understood, but 50% to 80% of HCV-positive children develop chronic infection.28
Currently, no intervention is available to decrease perinatal HCV transmission.
Currently, no intervention is available to decrease perinatal HCV transmission, although an unclear association with mode of delivery exists. One study of 441 mother-infant pairs showed that elective cesarean delivery may be associated with a lower transmission risk.29 However, another study of 370 mother-infant pairs found no difference in risk.24
The effect of pregnancy on the progression of hepatitis C infection also is unclear. In studies of HCV-positive pregnant women—as compared to HCV-positive non-pregnant women—researchers reported a decrease in serum alanine aminotransferase (ALT) and an increase in HCV RNA during pregnancy,30 as well as a deterioration of hepatitis C disease after pregnancy, as confirmed by liver biopsy.31 In prospective cohort studies comparing infected and uninfected pregnant women, serum aminotrans-ferases flared postpartum in one study32 but remained normal in another.33
Not much is known regarding the effect of chronic hepatitis C infection on pregnancy outcome, although 1 case-control study reported an association between hepatitis C infection and cholestasis of pregnancy.34
Although HCV RNA has been detected in colostrum,35 studies of breastfed infants with HCV-positive mothers have found no cases of transmission secondary to breastfeeding.35,36 Thus, both the American Academy of Pediatrics and ACOG conclude that breastfeeding does not appreciably increase the risk of transmission to the neonate and should not be prohibited.37,38 However, pending further studies, practitioners may choose to advise against breastfeeding if nipples are cracked and bleeding.
- Approximately 25% of people with chronic HCV infection are asymptomatic with normal liver function tests and benign histology.
- Only 10% of people recognize acute infection when they acquire HCV. In the vast majority of cases, the disease is subclinical.
- The “epidemic” of HCV infection represents greater identification of chronic cases rather than increasing numbers of new outbreaks.
- Most new cases of hepatitis C occur in young adults, ages 25 to 40, who may not learn for years or even decades that they are infected.
- African Americans are twice as likely as non-Hispanic whites to be infected with HCV. Hispanics, too, are more likely than non-Hispanic whites to be infected.
- Infection with the human immunodeficiency virus (HIV) appears to accelerate the course of HCV, as does co-infection with the hepatitis B virus.
- Due to improvements in blood bank screening and a decrease in the use of IV drugs, the number of new cases of HCV diagnosed in 1997 was only about 37,000—an 80% decline. Nevertheless, over the next 1 to 2 decades, the HCV mortality rate is expected to double.
- Total expenditures for HCV therapy are thought to range from $10,000 to $12,000 when the costs of interferon, ribavirin, laboratory studies, and office visits are taken into account.
Acute infection with the hepatitis C virus (HCV) progresses to chronic disease in 50% to 84% of cases. Once HCV infection is chronic, combination therapy with interferon alfa-2b and ribavirin elicits a sustained virologic response in only 41% of cases. Even the latest therapy for chronic HCV—a combination of pegylated interferon alfa-2a or 2b and ribavirin—eradicates the virus in only 54% of patients.1 For these reasons, a team of researchers in Germany explored the effectiveness of treating HCV during the acute phase of infection using high-dose interferon alfa-2b to prevent progression.2
‘Peginterferons’ elicit a greater sustained virologic response.
Because the viral load of patients with HCV infection starts to rise within 24 hours after a single dose of inter-feron alfa-2b, investigators used a daily dosing regimen—rather than the standard thrice-weekly therapy—for the first 4 weeks of treatment. They then followed the standard schedule for another 20 weeks, measuring serum HCV RNA levels before, during, and 24 weeks after the termination of therapy.
All 44 patients in the study were given 5 million units of subcutaneous interferon alfa-2b daily for 4 weeks, after which time they received the same dosage 3 times a week. Twenty-four weeks after the end of therapy, 43 patients (98%) had normal serum alanine aminotransferase (ALT) and undetectable levels of serum HCV RNA. Side effects caused 1 patient to stop treatment after 12 weeks.
Although the investigators concluded that the treatment of acute HCV infection with interferon alfa-2b prevents progression to chronic disease, a number of issues remain unresolved. The primary one is whether all patients with acute disease should undergo treatment with a costly therapy that can be hard to tolerate, when many of these patients would recover without it. Further, since pegylated interferon has proven more effective than standard interferon in treating chronic HCV, it also may be more effective in treating acute disease. (“Peginterferons,” as they are called, can be administered weekly and elicit a greater sustained virologic response.) Finally, since acute infection is no longer common in the United States—dropping from 250,000 to 500,000 cases annually in the 1980s to less than 40,000 today—and since not all infections are clinically acute, this approach has limited applicability.3
These and other issues require further exploration. —Katherine Chen, MD, MPH
REFERENCES
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
2. Jaeckel E, Cornberg M, Wedemeyer H, et al. for the German Acute Hepatitis C Therapy Group. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345(20):1452-1457.
3. Hoofnagle JH. Therapy for acute hepatitis C [editorial]. N Engl J Med. 2001;345(20):1495-1497.
Conclusion
Chronic HCV infection can lead to serious medical consequences, such as cirrhosis, liver failure, hepatocellular cancer, and death. Unfortunately, 75% of individuals infected with HCV are asymptomatic. By the time symptoms do occur, the disease often is in its advanced stages. Thus, both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups. The early identification of chronic HCV infection is important, as it enables women to take advantage of increasingly effective treatments and alerts them to the need for preconception counseling.
Both the CDC and ACOG recommend that Ob/Gyns screen women in high-risk groups.
Although perinatal HCV transmission is a known risk, we lack strategies to prevent it. In addition, the effects of pregnancy on the progression of chronic HCV infection, and the effects of chronic HCV infection on pregnancy outcome, are unknown. However, the identification of HCV-positive pregnant women will lead to screening of their infants and earlier identification of infected children.
1. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562.
2. National Center for Infectious Diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncidod/dis-eases/hepatitis/slideset/intro/slide_5.htm. Accessed January 11, 2002.
3. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47:1-39.
4. American College of Obstetricians and Gynecologists. Primary and preventive care: periodic assessments. ACOG Committee Opinion #229. Washington, DC: ACOG; 1999.
5. Burns DN, Minkoff H. Hepatitis C: screening in pregnancy. Obstet Gynecol. 1999;94:1044-1048.
6. World Health Organization. Global prevalence of hepatitis C, 1999. Available at: http://www.who.int/emc/images/hepacmap.jpg. Accessed August 31, 2001.
7. Arthur RR, Hassan NF, Abdallah MY, et al. Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91:271-274.
8. Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lym-photropic viruses. Am J Public Health. 1996;86:655-661.
9. Alter MJ, Gerety RJ, Smallwood LA, Sampliner RE, Tabor E, Deinhardt F, et al. Sporadic non-A, non-B hepatitis: frequency and epidemiology in an urban U.S. population. J Infect Dis. 1982;145:886-893.
10. Alter MJ, Coleman PJ, Alexander WJ, et al. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA. 1989;262:1201-1205.
11. Rooney G, Gilson RJ. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399-404.
12. Meisel H, Reip A, Faltus B, et al. Transmission of hepatitis C virus to children and husbands by women infected with contaminated anti-D immunoglobulin. Lancet. 1995;345:1209-1211.
13. Piazza M, Sagliocca L, Tosone G, et al. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537-1544.
14. Cheney CP, Chopra S, Graham C, Hepatitis C. Infect Dis Clin North Am. 2000;14:633-667.
15. Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506.
16. Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interfer-on alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510.
17. Marcellin P, Boyer N, Giostra E, et al. Recombinant human alpha-interferon in patients with chronic non-A, non-B hepatitis: a multicenter randomized controlled trial from France. Hepatology. 1991;13:393-397.
18. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492.
19. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432.
20. Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438.
21. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
22. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;30:744-750.
23. Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441.
24. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
25. Lin HH, Kao JH, Hsu HY, et al. Possible role of high-titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis. 1994;169:638-641.
26. Thomas DL, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480-1488.
27. Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis. 1997;25:1121-1124.
28. Bortolotti F, Faggion S, Con P. Natural history of chronic viral hepatitis in childhood. Acta Gastroenterol Belg. 1998;61:198-201.
29. Lin HH, Kao JH, Hsu HY, et al. Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. J Pediatr. 1995;126:589-591.
30. Polywka S, Schroter M, Feucht HH, Zollner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29:1327-1329.
31. Hepatitis C virus infection. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics. 1998;101:481-485.
32. American College of Obstetricians and Gynecologists. Breastfeeding and the risk of hepatitis C virus transmission. ACOG Committee Opinion #220. Washington, DC: ACOG; 1999.
33. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
34. Gervais A, Bacq Y, Bernuau J, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol. 2000;32:293-299.
35. Fontaine H, Nalpas B, Carnot F, Brechot C, Pol S. Effect of pregnancy on chronic hepatitis C: a case-control study. Lancet. 2000;356:1328-1329.
36. Latt NC, Spencer JD, Beeby PJ, et al. Hepatitis C in injecting drug-using women during and after pregnancy. J Gastroenterol Hepatol. 2000;15:175-181.
37. Floreani A, Paternoster D, Zappala F, et al. Hepatitis C virus infection in pregnancy. Br J Obstet Gynaecol. 1996;103:325-329.
38. Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498-500.
1. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556-562.
2. National Center for Infectious Diseases. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncidod/dis-eases/hepatitis/slideset/intro/slide_5.htm. Accessed January 11, 2002.
3. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Morb Mortal Wkly Rep. 1998;47:1-39.
4. American College of Obstetricians and Gynecologists. Primary and preventive care: periodic assessments. ACOG Committee Opinion #229. Washington, DC: ACOG; 1999.
5. Burns DN, Minkoff H. Hepatitis C: screening in pregnancy. Obstet Gynecol. 1999;94:1044-1048.
6. World Health Organization. Global prevalence of hepatitis C, 1999. Available at: http://www.who.int/emc/images/hepacmap.jpg. Accessed August 31, 2001.
7. Arthur RR, Hassan NF, Abdallah MY, et al. Hepatitis C antibody prevalence in blood donors in different governorates in Egypt. Trans R Soc Trop Med Hyg. 1997;91:271-274.
8. Garfein RS, Vlahov D, Galai N, Doherty MC, Nelson KE. Viral infections in short-term injection drug users: the prevalence of the hepatitis C, hepatitis B, human immunodeficiency, and human T-lym-photropic viruses. Am J Public Health. 1996;86:655-661.
9. Alter MJ, Gerety RJ, Smallwood LA, Sampliner RE, Tabor E, Deinhardt F, et al. Sporadic non-A, non-B hepatitis: frequency and epidemiology in an urban U.S. population. J Infect Dis. 1982;145:886-893.
10. Alter MJ, Coleman PJ, Alexander WJ, et al. Importance of heterosexual activity in the transmission of hepatitis B and non-A, non-B hepatitis. JAMA. 1989;262:1201-1205.
11. Rooney G, Gilson RJ. Sexual transmission of hepatitis C virus infection. Sex Transm Infect. 1998;74:399-404.
12. Meisel H, Reip A, Faltus B, et al. Transmission of hepatitis C virus to children and husbands by women infected with contaminated anti-D immunoglobulin. Lancet. 1995;345:1209-1211.
13. Piazza M, Sagliocca L, Tosone G, et al. Sexual transmission of the hepatitis C virus and efficacy of prophylaxis with intramuscular immune serum globulin. A randomized controlled trial. Arch Intern Med. 1997;157:1537-1544.
14. Cheney CP, Chopra S, Graham C, Hepatitis C. Infect Dis Clin North Am. 2000;14:633-667.
15. Davis GL, Balart LA, Schiff ER, et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N Engl J Med. 1989;321:1501-1506.
16. Di Bisceglie AM, Martin P, Kassianides C, et al. Recombinant interfer-on alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510.
17. Marcellin P, Boyer N, Giostra E, et al. Recombinant human alpha-interferon in patients with chronic non-A, non-B hepatitis: a multicenter randomized controlled trial from France. Hepatology. 1991;13:393-397.
18. McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492.
19. Poynard T, Marcellin P, Lee SS, et al. Randomised trial of interferon alpha-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426-1432.
20. Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433-438.
21. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b in combination with ribavirin compared with interferon alfa-2b plus rib-avirin for initial treatment of chronic hepatitis C: results of a randomized trial. Lancet. 2001;358:958-965.
22. Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N Engl J Med. 1994;30:744-750.
23. Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437-441.
24. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751-755.
25. Lin HH, Kao JH, Hsu HY, et al. Possible role of high-titer maternal viremia in perinatal transmission of hepatitis C virus. J Infect Dis. 1994;169:638-641.
26. Thomas DL, et al. Perinatal transmission of hepatitis C virus from human immunodeficiency virus type 1-infected mothers. Women and Infants Transmission Study. J Infect Dis. 1998;177:1480-1488.
27. Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis. 1997;25:1121-1124.
28. Bortolotti F, Faggion S, Con P. Natural history of chronic viral hepatitis in childhood. Acta Gastroenterol Belg. 1998;61:198-201.
29. Lin HH, Kao JH, Hsu HY, et al. Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. J Pediatr. 1995;126:589-591.
30. Polywka S, Schroter M, Feucht HH, Zollner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis. 1999;29:1327-1329.
31. Hepatitis C virus infection. American Academy of Pediatrics. Committee on Infectious Diseases. Pediatrics. 1998;101:481-485.
32. American College of Obstetricians and Gynecologists. Breastfeeding and the risk of hepatitis C virus transmission. ACOG Committee Opinion #220. Washington, DC: ACOG; 1999.
33. Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904-907.
34. Gervais A, Bacq Y, Bernuau J, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. J Hepatol. 2000;32:293-299.
35. Fontaine H, Nalpas B, Carnot F, Brechot C, Pol S. Effect of pregnancy on chronic hepatitis C: a case-control study. Lancet. 2000;356:1328-1329.
36. Latt NC, Spencer JD, Beeby PJ, et al. Hepatitis C in injecting drug-using women during and after pregnancy. J Gastroenterol Hepatol. 2000;15:175-181.
37. Floreani A, Paternoster D, Zappala F, et al. Hepatitis C virus infection in pregnancy. Br J Obstet Gynaecol. 1996;103:325-329.
38. Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. Br J Obstet Gynaecol. 1999;106:498-500.