User login
Airways disorders
Updated guidelines on the use of home O2 in COPD: A much-needed respite
The use of long-term oxygen therapy (LTOT, oxygen prescribed for at least 15 h/day) in patients with COPD and chronic hypoxemia has been standard of care based on trials from the 1980s that conferred a survival benefit with the use of continuous oxygen (Ann Internal Med. 1980;93[3]:391-8). More recently, LTOT has not shown to improve survival or delay time to the hospitalization in patients with stable COPD and resting or exercise-induced moderate desaturation (N Engl J Med. 2016;375[17]:1617-27). Thus far, existing recommendations had been semi-inclusive in patient selection. A fundamental lack of evidence-based clinical practice guidelines prompted additional research into patient selection, portable oxygen technology, advocacy for improved oxygen therapy financing, and updating of policies (Jacobs et al., Ann Am Thorac Soc. 2018;15[12]:1369-81). With over a million patients in the United States being prescribed home oxygen and reported disconnect in-home oxygen needs/experiences across disease processes, lifestyles, and oxygen supply requirements, the 2020 American Thoracic Society (ATS) workshop on optimizing home oxygen therapy sought to answer critical questions in the use of LTOT for COPD patients (AlMutairi, et al. Respir Care. 2018;63[11]:1321-30; Jacobs, et al. Am J Respir Crit Care Med. 2020;202[10]:e121-e141).
Kadambari Vijaykumar, MD
Fellow-in-Training Member
Dharani Kumari Narendra, MBBS, FCCP
Steering Committee Member
Chest infections
Eradicating COVID-19 scourge: It is up to all of us— get vaccinated!
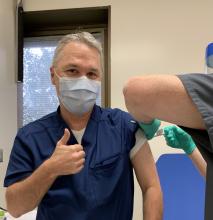
2021 brings hope, spurred by the availability of several effective COVID-19 vaccines – unprecedented scientific advances, considering that these vaccines were developed in record time. We have stark choices: while some individuals ignore scientific evidence and refuse to take the vaccine, we from the Chest Infections NetWork urge an alternative and imperative choice. As health providers caring for COVID-19 patients, we first-hand witness the horrors of dying alone in a hospital bed – far away from beloved ones. I have a sticker on my car that says: If you do not like your mask, you will not like my ventilator. With the advent of vaccines, I plan on replacing this sticker: If you do not want to get vaccinated, you will not like my ventilator. When the vaccine became available at my institution, I was the first to roll up my sleeve and feel the pinch in my upper arm. I urge you all to do the same. Make a difference, do your part – get vaccinated.
Marcos I. Restrepo, MD, MSc, PhD
Chair
Clinical pulmonary medicine
COVID-19 vaccines – mRNA and beyond
We currently have two COVID-19 mRNA vaccines with US FDA emergency use authorization (EUA) for use in individuals less than or equal to age 18 years – Pfizer and Moderna. They work by introducing mRNA into a muscle cell that instructs the host cell ribosomes to express Sars-CoV-2 spike proteins, thereby triggering a systemic immune response.
Both are two-dose regimens, with Pfizer’s 21 days apart and requires storage at -75 C, and Moderna’s 28 days apart, requiring storage at -20 C.
Presently in development are three more vaccines. AstraZeneca (AZ) and Johnson & Johnson (JnJ) use an adenovirus vector. Both vaccines are stable at standard refrigerator temperatures. AZ’s results were mixed – with two, full-size doses efficacy at 62% effective, but with a half-dose followed by a full dose, efficacy was 90%. Novavax candidate works differently - it’s a protein subunit vaccine and uses a lab-made version of the SARS-CoV-2 spike protein, mixed with an adjuvant to help trigger the immune system. Results from all trials are eagerly awaited.
Mary Jo S. Farmer, MD, PhD, FCCP
Steering Committee Member
Shyam Subramanian, MD, FCCP
Chair
Clinical research and quality improvement
COVID-19 treatment, so far!
COVID-19 has turned rapidly into a fatal illness, causing over 1.8 million deaths worldwide so far. The pandemic has also showed us the power of adaptive trials, multi-arm trials, and the role for collaboration across the global scientific community. A few significant studies are worth mentioning.
Possible future therapies include antiviral monoclonal antibodies, bamlanivimab (Chen P, et al. N Engl J Med. 2020; online ahead of print); early convalescent plasma (Libster R, et al. N Engl J Med. 2021 Jan 6. doi: 10.1056/NEJMoa2033700); and casirivimab-imdevimab (Baum A, et al. Science. 2020 Nov 27 doi: 10.1126/science.abe2402). Development of mRNA COVID-19 vaccines can help with primary prevention and herd immunity (Polack FP, et al. N Engl J Med. 2020;383[27]:2603; Baden LR, et al. N Engl J Med. 2020; Dec 30; doi: 10.1056/NEJMoa2035389).
We are starting to understand why COVID-19 infection is more pathogenic in some, how to predict development of severe disease, and how to best treat respiratory failure. Defeating the pandemic will require ongoing international collaboration in research, development, and resource allocation.
Muhammad Hayat Syed, MBBS
Ankita Agarwal, MD
Fellows-in-Training Members
Critical care
Awake proning in COVID-19
Prone positioning has been shown to improve pulmonary mechanics in intubated patients with acute respiratory distress syndrome (ARDS). Proposed mechanisms for these benefits include shape matching, reversing the pleural pressure gradient, homogenizing distribution of pleural pressures, reducing the impact of the heart and abdomen on the lungs, and maintaining distribution of perfusion. Application of prone positioning has also been shown to reduce mortality in severe ARDS (Guérin, et al. N Engl J Med. 2013;368(23):2159-68). With the COVID-19 pandemic, clinicians have extrapolated that nonintubated patients with severe hypoxia may benefit from awake proning in the hopes of improving oxygenation and decreasing need for intubation. But, what’s the evidence so far?
Kathryn Pendleton, MD
Viren Kaul, MD
Steering Committee Members
Home-Based Mechanical Ventilation and Neuromuscular Disease
New horizons in home ventilation
Phasing out a particular ventilator (Philips Respironics Trilogy 100 ventilator) has everyone on a steep learning curve with the replacement (Trilogy EVO). Most features are replicated in the EVO, including volume/pressure control and pressure-supported modes, mouthpiece ventilation, active/passive circuit capability, and portability (11.5 lb). Upgrades include longer battery life (15 hours; 7.5 hours internal/7.5 hours detachable) and use in pediatric patients now greater than or equal to 2.5 kg.
Other significant improvements include lower flow trigger sensitivity to accommodate patients with severe respiratory muscle weakness, a fast start AVAPS with rapid breath-to-breath 3 cm H20 increases for the first minute to rapidly reach target tidal volume, and breath-to-breath auto-EPAP sensing of upper airway resistance to maintain airway patency for patients with upper airway obstruction.
Internal bluetooth transmission to cloud-based monitoring (Care OrchestratorTM) expands access to patients without wi-fi or cellular service. New monitoring modules, SpO2 and EtCO2, and transcutaneous CO2 monitoring (Sentec), transmit to cloud-based monitoring (EVO EtCs2 spring 2021).
These welcome improvements allow clinicians to better match ventilator settings to the patients’ evolving physiology and provide flexibility and connectivity to optimize long-term care.
Karin Provost, DO, PhD
Steering Committee Member
Janet Hilbert, MD
NetWork Member
Online resources
EVO e-learning curriculum
Interprofessional team
Interprofessional team approach to palliative extubation
The emotional burden of caring for patients at the end of life affects all members of the care team. Palliative (or compassionate) extubation consists of the withdrawal of mechanical ventilation when the absolute priority in care delivery is to afford comfort and allow for natural death to occur. Rapid withdrawal of ventilatory support may lead to significant respiratory distress, and the critical care team has an obligation to ensure patient comfort during the dying process (Truog RD, et al. Crit Care Med. 2008;36[3]:953). Registered nurses (RN) are primarily responsible for the titration of sedation/analgesia and should be included in discussions regarding medication selection. It is imperative that neuromuscular blockade is absent, and benzodiazepines and/or opioids should be initiated prior to palliative extubation (Lanken PN, et al. Am J Respir Crit Care Med. 2008;177:912). Respiratory therapists (RT) are responsible for endotracheal tube removal despite rare participation in end-of-life discussions (Grandhige AP, et al. Respir Care. 2016;61[7]:891). It is recommended that an experienced physician, RN, and RT be readily available to respond quickly to any signs of distress (Downar J, et al. Intensive Care Med. 2016;42:1003). Regular debriefing sessions exploring team actions and communication dynamics are advised following end-of-life care (Ho A, et al. J Interprof Care. 2016;30[6]:795-803). Palliative extubation demands meticulous planning and clear communication among all team members (physician, RN, RT) and the patient’s family. Poor planning may result in physical and emotional suffering for the patient and difficult bereavement for the family (Coradazi A, et al. Hos Pal Med Int J. 2019;3[1]:10-14). Interprofessional team-based care results from intentional teams that exhibit collective identity and shared responsibility for the patients they serve (Core Competencies for Interprofessional Education Collaborative Practice, 2016). An inclusive and interprofessional approach to withdrawal of mechanical ventilation is key to both quality patient care and provider wellbeing.
Rebecca Anna Gersten, MD
Steering Committee Member
Samantha Davis, MS, RRT
Steering Committee Member
Munish Luthra, MD, FCCP
Vice-Chair Committee
Airways disorders
Updated guidelines on the use of home O2 in COPD: A much-needed respite
The use of long-term oxygen therapy (LTOT, oxygen prescribed for at least 15 h/day) in patients with COPD and chronic hypoxemia has been standard of care based on trials from the 1980s that conferred a survival benefit with the use of continuous oxygen (Ann Internal Med. 1980;93[3]:391-8). More recently, LTOT has not shown to improve survival or delay time to the hospitalization in patients with stable COPD and resting or exercise-induced moderate desaturation (N Engl J Med. 2016;375[17]:1617-27). Thus far, existing recommendations had been semi-inclusive in patient selection. A fundamental lack of evidence-based clinical practice guidelines prompted additional research into patient selection, portable oxygen technology, advocacy for improved oxygen therapy financing, and updating of policies (Jacobs et al., Ann Am Thorac Soc. 2018;15[12]:1369-81). With over a million patients in the United States being prescribed home oxygen and reported disconnect in-home oxygen needs/experiences across disease processes, lifestyles, and oxygen supply requirements, the 2020 American Thoracic Society (ATS) workshop on optimizing home oxygen therapy sought to answer critical questions in the use of LTOT for COPD patients (AlMutairi, et al. Respir Care. 2018;63[11]:1321-30; Jacobs, et al. Am J Respir Crit Care Med. 2020;202[10]:e121-e141).
Kadambari Vijaykumar, MD
Fellow-in-Training Member
Dharani Kumari Narendra, MBBS, FCCP
Steering Committee Member
Chest infections
Eradicating COVID-19 scourge: It is up to all of us— get vaccinated!
2021 brings hope, spurred by the availability of several effective COVID-19 vaccines – unprecedented scientific advances, considering that these vaccines were developed in record time. We have stark choices: while some individuals ignore scientific evidence and refuse to take the vaccine, we from the Chest Infections NetWork urge an alternative and imperative choice. As health providers caring for COVID-19 patients, we first-hand witness the horrors of dying alone in a hospital bed – far away from beloved ones. I have a sticker on my car that says: If you do not like your mask, you will not like my ventilator. With the advent of vaccines, I plan on replacing this sticker: If you do not want to get vaccinated, you will not like my ventilator. When the vaccine became available at my institution, I was the first to roll up my sleeve and feel the pinch in my upper arm. I urge you all to do the same. Make a difference, do your part – get vaccinated.
Marcos I. Restrepo, MD, MSc, PhD
Chair
Clinical pulmonary medicine
COVID-19 vaccines – mRNA and beyond
We currently have two COVID-19 mRNA vaccines with US FDA emergency use authorization (EUA) for use in individuals less than or equal to age 18 years – Pfizer and Moderna. They work by introducing mRNA into a muscle cell that instructs the host cell ribosomes to express Sars-CoV-2 spike proteins, thereby triggering a systemic immune response.
Both are two-dose regimens, with Pfizer’s 21 days apart and requires storage at -75 C, and Moderna’s 28 days apart, requiring storage at -20 C.
Presently in development are three more vaccines. AstraZeneca (AZ) and Johnson & Johnson (JnJ) use an adenovirus vector. Both vaccines are stable at standard refrigerator temperatures. AZ’s results were mixed – with two, full-size doses efficacy at 62% effective, but with a half-dose followed by a full dose, efficacy was 90%. Novavax candidate works differently - it’s a protein subunit vaccine and uses a lab-made version of the SARS-CoV-2 spike protein, mixed with an adjuvant to help trigger the immune system. Results from all trials are eagerly awaited.
Mary Jo S. Farmer, MD, PhD, FCCP
Steering Committee Member
Shyam Subramanian, MD, FCCP
Chair
Clinical research and quality improvement
COVID-19 treatment, so far!
COVID-19 has turned rapidly into a fatal illness, causing over 1.8 million deaths worldwide so far. The pandemic has also showed us the power of adaptive trials, multi-arm trials, and the role for collaboration across the global scientific community. A few significant studies are worth mentioning.
Possible future therapies include antiviral monoclonal antibodies, bamlanivimab (Chen P, et al. N Engl J Med. 2020; online ahead of print); early convalescent plasma (Libster R, et al. N Engl J Med. 2021 Jan 6. doi: 10.1056/NEJMoa2033700); and casirivimab-imdevimab (Baum A, et al. Science. 2020 Nov 27 doi: 10.1126/science.abe2402). Development of mRNA COVID-19 vaccines can help with primary prevention and herd immunity (Polack FP, et al. N Engl J Med. 2020;383[27]:2603; Baden LR, et al. N Engl J Med. 2020; Dec 30; doi: 10.1056/NEJMoa2035389).
We are starting to understand why COVID-19 infection is more pathogenic in some, how to predict development of severe disease, and how to best treat respiratory failure. Defeating the pandemic will require ongoing international collaboration in research, development, and resource allocation.
Muhammad Hayat Syed, MBBS
Ankita Agarwal, MD
Fellows-in-Training Members
Critical care
Awake proning in COVID-19
Prone positioning has been shown to improve pulmonary mechanics in intubated patients with acute respiratory distress syndrome (ARDS). Proposed mechanisms for these benefits include shape matching, reversing the pleural pressure gradient, homogenizing distribution of pleural pressures, reducing the impact of the heart and abdomen on the lungs, and maintaining distribution of perfusion. Application of prone positioning has also been shown to reduce mortality in severe ARDS (Guérin, et al. N Engl J Med. 2013;368(23):2159-68). With the COVID-19 pandemic, clinicians have extrapolated that nonintubated patients with severe hypoxia may benefit from awake proning in the hopes of improving oxygenation and decreasing need for intubation. But, what’s the evidence so far?
Kathryn Pendleton, MD
Viren Kaul, MD
Steering Committee Members
Home-Based Mechanical Ventilation and Neuromuscular Disease
New horizons in home ventilation
Phasing out a particular ventilator (Philips Respironics Trilogy 100 ventilator) has everyone on a steep learning curve with the replacement (Trilogy EVO). Most features are replicated in the EVO, including volume/pressure control and pressure-supported modes, mouthpiece ventilation, active/passive circuit capability, and portability (11.5 lb). Upgrades include longer battery life (15 hours; 7.5 hours internal/7.5 hours detachable) and use in pediatric patients now greater than or equal to 2.5 kg.
Other significant improvements include lower flow trigger sensitivity to accommodate patients with severe respiratory muscle weakness, a fast start AVAPS with rapid breath-to-breath 3 cm H20 increases for the first minute to rapidly reach target tidal volume, and breath-to-breath auto-EPAP sensing of upper airway resistance to maintain airway patency for patients with upper airway obstruction.
Internal bluetooth transmission to cloud-based monitoring (Care OrchestratorTM) expands access to patients without wi-fi or cellular service. New monitoring modules, SpO2 and EtCO2, and transcutaneous CO2 monitoring (Sentec), transmit to cloud-based monitoring (EVO EtCs2 spring 2021).
These welcome improvements allow clinicians to better match ventilator settings to the patients’ evolving physiology and provide flexibility and connectivity to optimize long-term care.
Karin Provost, DO, PhD
Steering Committee Member
Janet Hilbert, MD
NetWork Member
Online resources
EVO e-learning curriculum
Interprofessional team
Interprofessional team approach to palliative extubation
The emotional burden of caring for patients at the end of life affects all members of the care team. Palliative (or compassionate) extubation consists of the withdrawal of mechanical ventilation when the absolute priority in care delivery is to afford comfort and allow for natural death to occur. Rapid withdrawal of ventilatory support may lead to significant respiratory distress, and the critical care team has an obligation to ensure patient comfort during the dying process (Truog RD, et al. Crit Care Med. 2008;36[3]:953). Registered nurses (RN) are primarily responsible for the titration of sedation/analgesia and should be included in discussions regarding medication selection. It is imperative that neuromuscular blockade is absent, and benzodiazepines and/or opioids should be initiated prior to palliative extubation (Lanken PN, et al. Am J Respir Crit Care Med. 2008;177:912). Respiratory therapists (RT) are responsible for endotracheal tube removal despite rare participation in end-of-life discussions (Grandhige AP, et al. Respir Care. 2016;61[7]:891). It is recommended that an experienced physician, RN, and RT be readily available to respond quickly to any signs of distress (Downar J, et al. Intensive Care Med. 2016;42:1003). Regular debriefing sessions exploring team actions and communication dynamics are advised following end-of-life care (Ho A, et al. J Interprof Care. 2016;30[6]:795-803). Palliative extubation demands meticulous planning and clear communication among all team members (physician, RN, RT) and the patient’s family. Poor planning may result in physical and emotional suffering for the patient and difficult bereavement for the family (Coradazi A, et al. Hos Pal Med Int J. 2019;3[1]:10-14). Interprofessional team-based care results from intentional teams that exhibit collective identity and shared responsibility for the patients they serve (Core Competencies for Interprofessional Education Collaborative Practice, 2016). An inclusive and interprofessional approach to withdrawal of mechanical ventilation is key to both quality patient care and provider wellbeing.
Rebecca Anna Gersten, MD
Steering Committee Member
Samantha Davis, MS, RRT
Steering Committee Member
Munish Luthra, MD, FCCP
Vice-Chair Committee
Airways disorders
Updated guidelines on the use of home O2 in COPD: A much-needed respite
The use of long-term oxygen therapy (LTOT, oxygen prescribed for at least 15 h/day) in patients with COPD and chronic hypoxemia has been standard of care based on trials from the 1980s that conferred a survival benefit with the use of continuous oxygen (Ann Internal Med. 1980;93[3]:391-8). More recently, LTOT has not shown to improve survival or delay time to the hospitalization in patients with stable COPD and resting or exercise-induced moderate desaturation (N Engl J Med. 2016;375[17]:1617-27). Thus far, existing recommendations had been semi-inclusive in patient selection. A fundamental lack of evidence-based clinical practice guidelines prompted additional research into patient selection, portable oxygen technology, advocacy for improved oxygen therapy financing, and updating of policies (Jacobs et al., Ann Am Thorac Soc. 2018;15[12]:1369-81). With over a million patients in the United States being prescribed home oxygen and reported disconnect in-home oxygen needs/experiences across disease processes, lifestyles, and oxygen supply requirements, the 2020 American Thoracic Society (ATS) workshop on optimizing home oxygen therapy sought to answer critical questions in the use of LTOT for COPD patients (AlMutairi, et al. Respir Care. 2018;63[11]:1321-30; Jacobs, et al. Am J Respir Crit Care Med. 2020;202[10]:e121-e141).
Kadambari Vijaykumar, MD
Fellow-in-Training Member
Dharani Kumari Narendra, MBBS, FCCP
Steering Committee Member
Chest infections
Eradicating COVID-19 scourge: It is up to all of us— get vaccinated!
2021 brings hope, spurred by the availability of several effective COVID-19 vaccines – unprecedented scientific advances, considering that these vaccines were developed in record time. We have stark choices: while some individuals ignore scientific evidence and refuse to take the vaccine, we from the Chest Infections NetWork urge an alternative and imperative choice. As health providers caring for COVID-19 patients, we first-hand witness the horrors of dying alone in a hospital bed – far away from beloved ones. I have a sticker on my car that says: If you do not like your mask, you will not like my ventilator. With the advent of vaccines, I plan on replacing this sticker: If you do not want to get vaccinated, you will not like my ventilator. When the vaccine became available at my institution, I was the first to roll up my sleeve and feel the pinch in my upper arm. I urge you all to do the same. Make a difference, do your part – get vaccinated.
Marcos I. Restrepo, MD, MSc, PhD
Chair
Clinical pulmonary medicine
COVID-19 vaccines – mRNA and beyond
We currently have two COVID-19 mRNA vaccines with US FDA emergency use authorization (EUA) for use in individuals less than or equal to age 18 years – Pfizer and Moderna. They work by introducing mRNA into a muscle cell that instructs the host cell ribosomes to express Sars-CoV-2 spike proteins, thereby triggering a systemic immune response.
Both are two-dose regimens, with Pfizer’s 21 days apart and requires storage at -75 C, and Moderna’s 28 days apart, requiring storage at -20 C.
Presently in development are three more vaccines. AstraZeneca (AZ) and Johnson & Johnson (JnJ) use an adenovirus vector. Both vaccines are stable at standard refrigerator temperatures. AZ’s results were mixed – with two, full-size doses efficacy at 62% effective, but with a half-dose followed by a full dose, efficacy was 90%. Novavax candidate works differently - it’s a protein subunit vaccine and uses a lab-made version of the SARS-CoV-2 spike protein, mixed with an adjuvant to help trigger the immune system. Results from all trials are eagerly awaited.
Mary Jo S. Farmer, MD, PhD, FCCP
Steering Committee Member
Shyam Subramanian, MD, FCCP
Chair
Clinical research and quality improvement
COVID-19 treatment, so far!
COVID-19 has turned rapidly into a fatal illness, causing over 1.8 million deaths worldwide so far. The pandemic has also showed us the power of adaptive trials, multi-arm trials, and the role for collaboration across the global scientific community. A few significant studies are worth mentioning.
Possible future therapies include antiviral monoclonal antibodies, bamlanivimab (Chen P, et al. N Engl J Med. 2020; online ahead of print); early convalescent plasma (Libster R, et al. N Engl J Med. 2021 Jan 6. doi: 10.1056/NEJMoa2033700); and casirivimab-imdevimab (Baum A, et al. Science. 2020 Nov 27 doi: 10.1126/science.abe2402). Development of mRNA COVID-19 vaccines can help with primary prevention and herd immunity (Polack FP, et al. N Engl J Med. 2020;383[27]:2603; Baden LR, et al. N Engl J Med. 2020; Dec 30; doi: 10.1056/NEJMoa2035389).
We are starting to understand why COVID-19 infection is more pathogenic in some, how to predict development of severe disease, and how to best treat respiratory failure. Defeating the pandemic will require ongoing international collaboration in research, development, and resource allocation.
Muhammad Hayat Syed, MBBS
Ankita Agarwal, MD
Fellows-in-Training Members
Critical care
Awake proning in COVID-19
Prone positioning has been shown to improve pulmonary mechanics in intubated patients with acute respiratory distress syndrome (ARDS). Proposed mechanisms for these benefits include shape matching, reversing the pleural pressure gradient, homogenizing distribution of pleural pressures, reducing the impact of the heart and abdomen on the lungs, and maintaining distribution of perfusion. Application of prone positioning has also been shown to reduce mortality in severe ARDS (Guérin, et al. N Engl J Med. 2013;368(23):2159-68). With the COVID-19 pandemic, clinicians have extrapolated that nonintubated patients with severe hypoxia may benefit from awake proning in the hopes of improving oxygenation and decreasing need for intubation. But, what’s the evidence so far?
Kathryn Pendleton, MD
Viren Kaul, MD
Steering Committee Members
Home-Based Mechanical Ventilation and Neuromuscular Disease
New horizons in home ventilation
Phasing out a particular ventilator (Philips Respironics Trilogy 100 ventilator) has everyone on a steep learning curve with the replacement (Trilogy EVO). Most features are replicated in the EVO, including volume/pressure control and pressure-supported modes, mouthpiece ventilation, active/passive circuit capability, and portability (11.5 lb). Upgrades include longer battery life (15 hours; 7.5 hours internal/7.5 hours detachable) and use in pediatric patients now greater than or equal to 2.5 kg.
Other significant improvements include lower flow trigger sensitivity to accommodate patients with severe respiratory muscle weakness, a fast start AVAPS with rapid breath-to-breath 3 cm H20 increases for the first minute to rapidly reach target tidal volume, and breath-to-breath auto-EPAP sensing of upper airway resistance to maintain airway patency for patients with upper airway obstruction.
Internal bluetooth transmission to cloud-based monitoring (Care OrchestratorTM) expands access to patients without wi-fi or cellular service. New monitoring modules, SpO2 and EtCO2, and transcutaneous CO2 monitoring (Sentec), transmit to cloud-based monitoring (EVO EtCs2 spring 2021).
These welcome improvements allow clinicians to better match ventilator settings to the patients’ evolving physiology and provide flexibility and connectivity to optimize long-term care.
Karin Provost, DO, PhD
Steering Committee Member
Janet Hilbert, MD
NetWork Member
Online resources
EVO e-learning curriculum
Interprofessional team
Interprofessional team approach to palliative extubation
The emotional burden of caring for patients at the end of life affects all members of the care team. Palliative (or compassionate) extubation consists of the withdrawal of mechanical ventilation when the absolute priority in care delivery is to afford comfort and allow for natural death to occur. Rapid withdrawal of ventilatory support may lead to significant respiratory distress, and the critical care team has an obligation to ensure patient comfort during the dying process (Truog RD, et al. Crit Care Med. 2008;36[3]:953). Registered nurses (RN) are primarily responsible for the titration of sedation/analgesia and should be included in discussions regarding medication selection. It is imperative that neuromuscular blockade is absent, and benzodiazepines and/or opioids should be initiated prior to palliative extubation (Lanken PN, et al. Am J Respir Crit Care Med. 2008;177:912). Respiratory therapists (RT) are responsible for endotracheal tube removal despite rare participation in end-of-life discussions (Grandhige AP, et al. Respir Care. 2016;61[7]:891). It is recommended that an experienced physician, RN, and RT be readily available to respond quickly to any signs of distress (Downar J, et al. Intensive Care Med. 2016;42:1003). Regular debriefing sessions exploring team actions and communication dynamics are advised following end-of-life care (Ho A, et al. J Interprof Care. 2016;30[6]:795-803). Palliative extubation demands meticulous planning and clear communication among all team members (physician, RN, RT) and the patient’s family. Poor planning may result in physical and emotional suffering for the patient and difficult bereavement for the family (Coradazi A, et al. Hos Pal Med Int J. 2019;3[1]:10-14). Interprofessional team-based care results from intentional teams that exhibit collective identity and shared responsibility for the patients they serve (Core Competencies for Interprofessional Education Collaborative Practice, 2016). An inclusive and interprofessional approach to withdrawal of mechanical ventilation is key to both quality patient care and provider wellbeing.
Rebecca Anna Gersten, MD
Steering Committee Member
Samantha Davis, MS, RRT
Steering Committee Member
Munish Luthra, MD, FCCP
Vice-Chair Committee