User login
AMSTERDAM – , but the study failed to meet the specified more rigorous definition of significance (P = .01) on the primary hierarchical composite of death, hospitalizations for heart failure, or 6-minute walk distance.
The trial, which investigated intravenous ferric carboxymaltose treatment vs. placebo, also showed no statistical difference in the main secondary endpoint: time to cardiovascular death or first heart failure hospitalization.
It was hoped that HEART-FID, the largest study to date to look at intravenous iron supplementation in heart failure, would confirm benefits suggested in previous smaller studies, but its modest results seem to have, if anything, caused more uncertainly on whether supplementing iron is actually worthwhile.
The HEART-FID trial was presented at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Another presentation at the ESC Congress reported a pooled meta-analysis of all the intravenous iron supplementation studies, including HEART-FID. This showed a significant reduction in one coprimary endpoint (cardiovascular hospitalization/CV death) but not in the other (heart failure hospitalization/CV death), which is the more traditional and well-recognized endpoint in heart failure trials.
The meta-analysis was also published online in the European Heart Journal.
HEART-FID lead investigator, Robert J. Mentz, MD, Duke University, Durham, N.C., said the totality of the evidence showed clinical benefits of intravenous iron supplementation with intravenous ferric carboxymaltose.
“I worry that people will focus on a P value rather than the actual clinical benefits seen across all the studies,” Dr. Mentz said in an interview. “Technically, this study was neutral in respect to the primary endpoint, but when we look at all the evidence with respect to ferric carboxymaltose, including this new pooled analysis, this does support clinical benefits.”
Comoderator of the ESC Hotline session at which the trial was presented, John McMurray, MD, University of Glasgow (Scotland), thought the trial had “muddied the waters a bit” on the issue of iron supplementation in heart failure.
“I would say we are in a less clear position on iron supplementation now than we were a few months ago. Those clinicians who have believed that checking iron levels and supplementing iron in those who are low is the right thing to do may now be wondering about that,” he told this news organization.
Dr. McMurray noted that initial impressions of the data from both HEART-FID and the meta-analysis suggested some benefit of intravenous iron on CV death/heart failure hospitalization in the first year, but on longer term follow-up, that benefit was less evident.
“We need to look further into why there is that discrepancy,” he said. “This could be a statistical phenomenon or could be something to do with the frequency of redosing over the longer term.”
He explained that several previous studies of intravenous iron supplementation in heart failure have reported apparent convincing benefits on quality of life and functional capacity, but there has been some uncertainty on this because of the difficulty in producing a placebo for intravenous iron.
“So, it would have been great to have some additional confirmation of these benefits and on harder endpoints,” he said, “but even in HEART-FID, there was only a small nonsignificant benefit in walking distance.”
HEART-FID
The HEART-FID trial randomly assigned 3,065 ambulatory patients with heart failure, a left ventricular ejection fraction of 40% or less, and iron deficiency to intravenous ferric carboxymaltose or placebo, given every 6 months as needed on the basis of iron indexes and hemoglobin levels, in addition to standard therapy for heart failure.
The primary outcome was a hierarchical composite of death within 12 months after randomization, hospitalizations for heart failure within 12 months after randomization, or change from baseline to 6 months in the 6-minute walk distance. The significance level was set at .01.
Results showed that death by month 12 occurred in 8.6% of the ferric carboxymaltose group and 10.3% of the placebo group; a total of 297 and 332 hospitalizations for heart failure, respectively, occurred by month 12; and the mean change from baseline to 6 months in the 6-minute walk distance was 8 meters in the ferric carboxymaltose group and 4 meters with placebo. The P value for the primary composite was .02.
The trial also used another method (unmatched win ratio) to analyze the hierarchical composite outcome in the ferric carboxymaltose group as compared with the placebo group that gave a result of 1.10 (99% confidence interval, 0.99-1.23).
During the follow-up period, CV death or hospitalization for heart failure (the main secondary outcome) occurred in 31.0% of the ferric carboxymaltose group and in 32.2% of the placebo group (hazard ratio, 0.93; 96% CI, 0.81-1.06).
Repeated dosing of ferric carboxymaltose appeared to be safe, with an acceptable adverse-event profile in most patients. The number of patients with serious adverse events occurring during the treatment period was similar in the two groups (27.0% in the ferric carboxymaltose group and 26.2% in the placebo group).
‘It’s hard to argue that we are not disappointed’
Designated discussant of the HEART-FID study at the ESC HOTLINE session, Scott Solomon, MD, Brigham and Women’s Hospital, Boston, described HEART-FID as “an extremely important and well-conducted trial.”
He noted that iron deficiency is extremely common in patients with heart failure, affecting at least about a third of patients, and is associated with reduced New York Heart Association class and reduced survival. Previous smaller studies have suggested benefit but have narrowly missed their primary endpoints. HEART-FID was a larger and sufficiently well-powered trial to test the hypothesis that iron supplementation can improve harder clinical endpoints.
Dr. Solomon said that the primary endpoint could be difficult to interpret, with a hierarchical composite, and a win ratio. “But I think it’s fair to say that the results are modest at best,” he added.
“When we look at the traditional cardiovascular death/heart failure hospitalization endpoint, one of the hard endpoints that we care about most in heart failure, it’s hard to argue that we are not disappointed,” he commented.
Referring to the P value of .01 threshold set for significance, which is based on new U.S. Food and Drug Administration regulatory standards, Dr. Solomon noted, “If they had used a standard P = .05 threshold, then they would be able to claim that this trial had met its primary endpoint. But, nevertheless, whatever threshold for significance we look at, the benefit was clearly modest.”
“As with all trials that show modest results, it will be useful to look at subgroups that are most likely to respond to the greatest extent to this therapy, and I look forward to learning more on this from further analyses,” Dr. Solomon concluded.
In an accompanying editorial in the New England Journal of Medicine, Pieter Martens, MD, and Wilfried Mullens, MD, PhD, Ziekenhuis Oost-Limburg, Genk, Belgium, and Hasselt (Belgium) University, point out that analyses from previous trials have suggested that intravenous iron did not have a treatment effect in patients with a transferrin saturation of more than 20%.
They note that, in the ferric carboxymaltose group in the HEART-FID trial, the mean transferrin saturation was 23.9% at baseline, higher than in previous studies.
Future analyses should assess the importance of the transferrin saturation value at baseline, which “could help redefine the definition of iron deficiency in patients with heart failure and, we hope, help clinicians determine which patients might benefit from intravenous iron supplementation,” they write.
Meta-analysis of trials
The meta-analysis of intravenous iron supplementation trials in heart failure was presented by Piotr Ponikowski, MD, Medical University Wroclaw (Poland).
The analysis pooled individual patient data from three double-blind, placebo-controlled trials – CONFIRM-HF 2, AFFIRM-AHF 3, and HEART-FID – giving a total of 4,475 patients, with 2,241 receiving ferric carboxymaltose and 2,234 receiving placebo.
The two prespecified composite primary endpoints were CV hospitalizations/CV death and heart failure hospitalizations/CV death.
These showed similar 13%-14% relative risk reductions with ferric carboxymaltose, but only the former was statistically significant.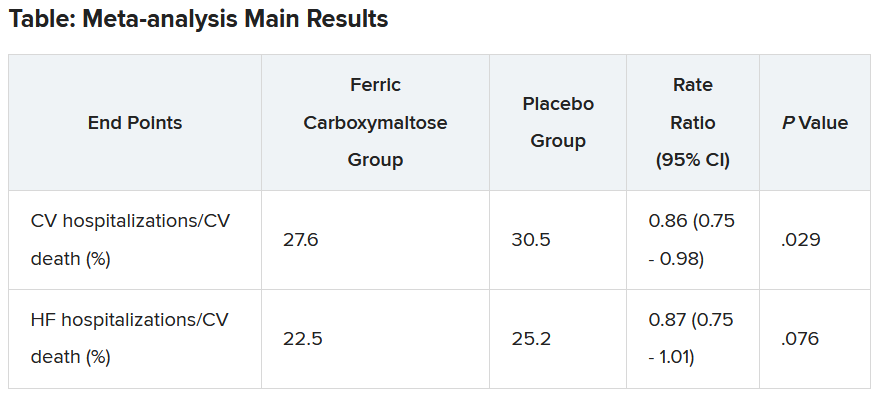
Similar results were seen when a fourth trial – IRONMAN (an open-label trial) – was included. In this case, the heart failure hospitalization/CV death endpoint was also nonsignificantly reduced with ferric carboxymaltose (rate ratio, 0.82; 95% CI, 0.58-1.07).
Subgroup analysis suggested that patients with higher transferrin saturation levels appeared to have a lack of treatment effect, whereas those with lower transferrin saturation (< 15%) showed significant treatment benefits.
A higher 6-month cumulative dose of ferric carboxymaltose – likely the result of redosing – may be associated with a slightly greater treatment effect after 6 months, Dr. Ponikowski reported.
He concluded: “These data support the use of intravenous ferric carboxymaltose to treat iron deficiency among patients with heart failure with reduced/mildly reduced LVEF [left ventricular ejection fraction] to reduce the risk of future hospitalization.”
“Our findings support additional research to challenge the current definition of iron deficiency in heart failure as an indication for IV iron therapy and to identify eligibility criteria for optimal redosing strategy,” Dr. Ponikowski added.
Discussant of the meta-analysis presentation at the ESC Hotline session, Pardeep Jhund, MD, University of Glasgow, suggested that the endpoint of most interest would be heart failure hospitalization/CV death in the analysis that included the IRONMAN trial, “which unfortunately did not meet statistical significance.”
In answer to the question “Where does this leave clinicians when treating patients?”Dr. Jhund said, “After yet another meta-analysis, I think the role of IV iron in reducing morbidity and mortality outcomes in heart failure remains questionable.”
“While the absence of evidence is not evidence of absence, the wide confidence intervals of the treatment effect on heart failure hospitalization/CV death leaves a lot of room for doubt about the efficacy of IV iron for reducing HF hospitalizations,” he concluded.
The HEART-FID trial was funded by American Regent, a Daiichi Sankyo Group company. Dr. Mentz reports receiving research support from American Regent and honoraria from American Regent, Vifor, and Pharmacosmos. Dr. Ponikowski reports consultancy fees/honoraria from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Bayer, MSD, Pfizer, Moderna, Sanofi, and Radcliffe Group.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , but the study failed to meet the specified more rigorous definition of significance (P = .01) on the primary hierarchical composite of death, hospitalizations for heart failure, or 6-minute walk distance.
The trial, which investigated intravenous ferric carboxymaltose treatment vs. placebo, also showed no statistical difference in the main secondary endpoint: time to cardiovascular death or first heart failure hospitalization.
It was hoped that HEART-FID, the largest study to date to look at intravenous iron supplementation in heart failure, would confirm benefits suggested in previous smaller studies, but its modest results seem to have, if anything, caused more uncertainly on whether supplementing iron is actually worthwhile.
The HEART-FID trial was presented at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Another presentation at the ESC Congress reported a pooled meta-analysis of all the intravenous iron supplementation studies, including HEART-FID. This showed a significant reduction in one coprimary endpoint (cardiovascular hospitalization/CV death) but not in the other (heart failure hospitalization/CV death), which is the more traditional and well-recognized endpoint in heart failure trials.
The meta-analysis was also published online in the European Heart Journal.
HEART-FID lead investigator, Robert J. Mentz, MD, Duke University, Durham, N.C., said the totality of the evidence showed clinical benefits of intravenous iron supplementation with intravenous ferric carboxymaltose.
“I worry that people will focus on a P value rather than the actual clinical benefits seen across all the studies,” Dr. Mentz said in an interview. “Technically, this study was neutral in respect to the primary endpoint, but when we look at all the evidence with respect to ferric carboxymaltose, including this new pooled analysis, this does support clinical benefits.”
Comoderator of the ESC Hotline session at which the trial was presented, John McMurray, MD, University of Glasgow (Scotland), thought the trial had “muddied the waters a bit” on the issue of iron supplementation in heart failure.
“I would say we are in a less clear position on iron supplementation now than we were a few months ago. Those clinicians who have believed that checking iron levels and supplementing iron in those who are low is the right thing to do may now be wondering about that,” he told this news organization.
Dr. McMurray noted that initial impressions of the data from both HEART-FID and the meta-analysis suggested some benefit of intravenous iron on CV death/heart failure hospitalization in the first year, but on longer term follow-up, that benefit was less evident.
“We need to look further into why there is that discrepancy,” he said. “This could be a statistical phenomenon or could be something to do with the frequency of redosing over the longer term.”
He explained that several previous studies of intravenous iron supplementation in heart failure have reported apparent convincing benefits on quality of life and functional capacity, but there has been some uncertainty on this because of the difficulty in producing a placebo for intravenous iron.
“So, it would have been great to have some additional confirmation of these benefits and on harder endpoints,” he said, “but even in HEART-FID, there was only a small nonsignificant benefit in walking distance.”
HEART-FID
The HEART-FID trial randomly assigned 3,065 ambulatory patients with heart failure, a left ventricular ejection fraction of 40% or less, and iron deficiency to intravenous ferric carboxymaltose or placebo, given every 6 months as needed on the basis of iron indexes and hemoglobin levels, in addition to standard therapy for heart failure.
The primary outcome was a hierarchical composite of death within 12 months after randomization, hospitalizations for heart failure within 12 months after randomization, or change from baseline to 6 months in the 6-minute walk distance. The significance level was set at .01.
Results showed that death by month 12 occurred in 8.6% of the ferric carboxymaltose group and 10.3% of the placebo group; a total of 297 and 332 hospitalizations for heart failure, respectively, occurred by month 12; and the mean change from baseline to 6 months in the 6-minute walk distance was 8 meters in the ferric carboxymaltose group and 4 meters with placebo. The P value for the primary composite was .02.
The trial also used another method (unmatched win ratio) to analyze the hierarchical composite outcome in the ferric carboxymaltose group as compared with the placebo group that gave a result of 1.10 (99% confidence interval, 0.99-1.23).
During the follow-up period, CV death or hospitalization for heart failure (the main secondary outcome) occurred in 31.0% of the ferric carboxymaltose group and in 32.2% of the placebo group (hazard ratio, 0.93; 96% CI, 0.81-1.06).
Repeated dosing of ferric carboxymaltose appeared to be safe, with an acceptable adverse-event profile in most patients. The number of patients with serious adverse events occurring during the treatment period was similar in the two groups (27.0% in the ferric carboxymaltose group and 26.2% in the placebo group).
‘It’s hard to argue that we are not disappointed’
Designated discussant of the HEART-FID study at the ESC HOTLINE session, Scott Solomon, MD, Brigham and Women’s Hospital, Boston, described HEART-FID as “an extremely important and well-conducted trial.”
He noted that iron deficiency is extremely common in patients with heart failure, affecting at least about a third of patients, and is associated with reduced New York Heart Association class and reduced survival. Previous smaller studies have suggested benefit but have narrowly missed their primary endpoints. HEART-FID was a larger and sufficiently well-powered trial to test the hypothesis that iron supplementation can improve harder clinical endpoints.
Dr. Solomon said that the primary endpoint could be difficult to interpret, with a hierarchical composite, and a win ratio. “But I think it’s fair to say that the results are modest at best,” he added.
“When we look at the traditional cardiovascular death/heart failure hospitalization endpoint, one of the hard endpoints that we care about most in heart failure, it’s hard to argue that we are not disappointed,” he commented.
Referring to the P value of .01 threshold set for significance, which is based on new U.S. Food and Drug Administration regulatory standards, Dr. Solomon noted, “If they had used a standard P = .05 threshold, then they would be able to claim that this trial had met its primary endpoint. But, nevertheless, whatever threshold for significance we look at, the benefit was clearly modest.”
“As with all trials that show modest results, it will be useful to look at subgroups that are most likely to respond to the greatest extent to this therapy, and I look forward to learning more on this from further analyses,” Dr. Solomon concluded.
In an accompanying editorial in the New England Journal of Medicine, Pieter Martens, MD, and Wilfried Mullens, MD, PhD, Ziekenhuis Oost-Limburg, Genk, Belgium, and Hasselt (Belgium) University, point out that analyses from previous trials have suggested that intravenous iron did not have a treatment effect in patients with a transferrin saturation of more than 20%.
They note that, in the ferric carboxymaltose group in the HEART-FID trial, the mean transferrin saturation was 23.9% at baseline, higher than in previous studies.
Future analyses should assess the importance of the transferrin saturation value at baseline, which “could help redefine the definition of iron deficiency in patients with heart failure and, we hope, help clinicians determine which patients might benefit from intravenous iron supplementation,” they write.
Meta-analysis of trials
The meta-analysis of intravenous iron supplementation trials in heart failure was presented by Piotr Ponikowski, MD, Medical University Wroclaw (Poland).
The analysis pooled individual patient data from three double-blind, placebo-controlled trials – CONFIRM-HF 2, AFFIRM-AHF 3, and HEART-FID – giving a total of 4,475 patients, with 2,241 receiving ferric carboxymaltose and 2,234 receiving placebo.
The two prespecified composite primary endpoints were CV hospitalizations/CV death and heart failure hospitalizations/CV death.
These showed similar 13%-14% relative risk reductions with ferric carboxymaltose, but only the former was statistically significant.
Similar results were seen when a fourth trial – IRONMAN (an open-label trial) – was included. In this case, the heart failure hospitalization/CV death endpoint was also nonsignificantly reduced with ferric carboxymaltose (rate ratio, 0.82; 95% CI, 0.58-1.07).
Subgroup analysis suggested that patients with higher transferrin saturation levels appeared to have a lack of treatment effect, whereas those with lower transferrin saturation (< 15%) showed significant treatment benefits.
A higher 6-month cumulative dose of ferric carboxymaltose – likely the result of redosing – may be associated with a slightly greater treatment effect after 6 months, Dr. Ponikowski reported.
He concluded: “These data support the use of intravenous ferric carboxymaltose to treat iron deficiency among patients with heart failure with reduced/mildly reduced LVEF [left ventricular ejection fraction] to reduce the risk of future hospitalization.”
“Our findings support additional research to challenge the current definition of iron deficiency in heart failure as an indication for IV iron therapy and to identify eligibility criteria for optimal redosing strategy,” Dr. Ponikowski added.
Discussant of the meta-analysis presentation at the ESC Hotline session, Pardeep Jhund, MD, University of Glasgow, suggested that the endpoint of most interest would be heart failure hospitalization/CV death in the analysis that included the IRONMAN trial, “which unfortunately did not meet statistical significance.”
In answer to the question “Where does this leave clinicians when treating patients?”Dr. Jhund said, “After yet another meta-analysis, I think the role of IV iron in reducing morbidity and mortality outcomes in heart failure remains questionable.”
“While the absence of evidence is not evidence of absence, the wide confidence intervals of the treatment effect on heart failure hospitalization/CV death leaves a lot of room for doubt about the efficacy of IV iron for reducing HF hospitalizations,” he concluded.
The HEART-FID trial was funded by American Regent, a Daiichi Sankyo Group company. Dr. Mentz reports receiving research support from American Regent and honoraria from American Regent, Vifor, and Pharmacosmos. Dr. Ponikowski reports consultancy fees/honoraria from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Bayer, MSD, Pfizer, Moderna, Sanofi, and Radcliffe Group.
A version of this article first appeared on Medscape.com.
AMSTERDAM – , but the study failed to meet the specified more rigorous definition of significance (P = .01) on the primary hierarchical composite of death, hospitalizations for heart failure, or 6-minute walk distance.
The trial, which investigated intravenous ferric carboxymaltose treatment vs. placebo, also showed no statistical difference in the main secondary endpoint: time to cardiovascular death or first heart failure hospitalization.
It was hoped that HEART-FID, the largest study to date to look at intravenous iron supplementation in heart failure, would confirm benefits suggested in previous smaller studies, but its modest results seem to have, if anything, caused more uncertainly on whether supplementing iron is actually worthwhile.
The HEART-FID trial was presented at the annual congress of the European Society of Cardiology and simultaneously published online in the New England Journal of Medicine.
Another presentation at the ESC Congress reported a pooled meta-analysis of all the intravenous iron supplementation studies, including HEART-FID. This showed a significant reduction in one coprimary endpoint (cardiovascular hospitalization/CV death) but not in the other (heart failure hospitalization/CV death), which is the more traditional and well-recognized endpoint in heart failure trials.
The meta-analysis was also published online in the European Heart Journal.
HEART-FID lead investigator, Robert J. Mentz, MD, Duke University, Durham, N.C., said the totality of the evidence showed clinical benefits of intravenous iron supplementation with intravenous ferric carboxymaltose.
“I worry that people will focus on a P value rather than the actual clinical benefits seen across all the studies,” Dr. Mentz said in an interview. “Technically, this study was neutral in respect to the primary endpoint, but when we look at all the evidence with respect to ferric carboxymaltose, including this new pooled analysis, this does support clinical benefits.”
Comoderator of the ESC Hotline session at which the trial was presented, John McMurray, MD, University of Glasgow (Scotland), thought the trial had “muddied the waters a bit” on the issue of iron supplementation in heart failure.
“I would say we are in a less clear position on iron supplementation now than we were a few months ago. Those clinicians who have believed that checking iron levels and supplementing iron in those who are low is the right thing to do may now be wondering about that,” he told this news organization.
Dr. McMurray noted that initial impressions of the data from both HEART-FID and the meta-analysis suggested some benefit of intravenous iron on CV death/heart failure hospitalization in the first year, but on longer term follow-up, that benefit was less evident.
“We need to look further into why there is that discrepancy,” he said. “This could be a statistical phenomenon or could be something to do with the frequency of redosing over the longer term.”
He explained that several previous studies of intravenous iron supplementation in heart failure have reported apparent convincing benefits on quality of life and functional capacity, but there has been some uncertainty on this because of the difficulty in producing a placebo for intravenous iron.
“So, it would have been great to have some additional confirmation of these benefits and on harder endpoints,” he said, “but even in HEART-FID, there was only a small nonsignificant benefit in walking distance.”
HEART-FID
The HEART-FID trial randomly assigned 3,065 ambulatory patients with heart failure, a left ventricular ejection fraction of 40% or less, and iron deficiency to intravenous ferric carboxymaltose or placebo, given every 6 months as needed on the basis of iron indexes and hemoglobin levels, in addition to standard therapy for heart failure.
The primary outcome was a hierarchical composite of death within 12 months after randomization, hospitalizations for heart failure within 12 months after randomization, or change from baseline to 6 months in the 6-minute walk distance. The significance level was set at .01.
Results showed that death by month 12 occurred in 8.6% of the ferric carboxymaltose group and 10.3% of the placebo group; a total of 297 and 332 hospitalizations for heart failure, respectively, occurred by month 12; and the mean change from baseline to 6 months in the 6-minute walk distance was 8 meters in the ferric carboxymaltose group and 4 meters with placebo. The P value for the primary composite was .02.
The trial also used another method (unmatched win ratio) to analyze the hierarchical composite outcome in the ferric carboxymaltose group as compared with the placebo group that gave a result of 1.10 (99% confidence interval, 0.99-1.23).
During the follow-up period, CV death or hospitalization for heart failure (the main secondary outcome) occurred in 31.0% of the ferric carboxymaltose group and in 32.2% of the placebo group (hazard ratio, 0.93; 96% CI, 0.81-1.06).
Repeated dosing of ferric carboxymaltose appeared to be safe, with an acceptable adverse-event profile in most patients. The number of patients with serious adverse events occurring during the treatment period was similar in the two groups (27.0% in the ferric carboxymaltose group and 26.2% in the placebo group).
‘It’s hard to argue that we are not disappointed’
Designated discussant of the HEART-FID study at the ESC HOTLINE session, Scott Solomon, MD, Brigham and Women’s Hospital, Boston, described HEART-FID as “an extremely important and well-conducted trial.”
He noted that iron deficiency is extremely common in patients with heart failure, affecting at least about a third of patients, and is associated with reduced New York Heart Association class and reduced survival. Previous smaller studies have suggested benefit but have narrowly missed their primary endpoints. HEART-FID was a larger and sufficiently well-powered trial to test the hypothesis that iron supplementation can improve harder clinical endpoints.
Dr. Solomon said that the primary endpoint could be difficult to interpret, with a hierarchical composite, and a win ratio. “But I think it’s fair to say that the results are modest at best,” he added.
“When we look at the traditional cardiovascular death/heart failure hospitalization endpoint, one of the hard endpoints that we care about most in heart failure, it’s hard to argue that we are not disappointed,” he commented.
Referring to the P value of .01 threshold set for significance, which is based on new U.S. Food and Drug Administration regulatory standards, Dr. Solomon noted, “If they had used a standard P = .05 threshold, then they would be able to claim that this trial had met its primary endpoint. But, nevertheless, whatever threshold for significance we look at, the benefit was clearly modest.”
“As with all trials that show modest results, it will be useful to look at subgroups that are most likely to respond to the greatest extent to this therapy, and I look forward to learning more on this from further analyses,” Dr. Solomon concluded.
In an accompanying editorial in the New England Journal of Medicine, Pieter Martens, MD, and Wilfried Mullens, MD, PhD, Ziekenhuis Oost-Limburg, Genk, Belgium, and Hasselt (Belgium) University, point out that analyses from previous trials have suggested that intravenous iron did not have a treatment effect in patients with a transferrin saturation of more than 20%.
They note that, in the ferric carboxymaltose group in the HEART-FID trial, the mean transferrin saturation was 23.9% at baseline, higher than in previous studies.
Future analyses should assess the importance of the transferrin saturation value at baseline, which “could help redefine the definition of iron deficiency in patients with heart failure and, we hope, help clinicians determine which patients might benefit from intravenous iron supplementation,” they write.
Meta-analysis of trials
The meta-analysis of intravenous iron supplementation trials in heart failure was presented by Piotr Ponikowski, MD, Medical University Wroclaw (Poland).
The analysis pooled individual patient data from three double-blind, placebo-controlled trials – CONFIRM-HF 2, AFFIRM-AHF 3, and HEART-FID – giving a total of 4,475 patients, with 2,241 receiving ferric carboxymaltose and 2,234 receiving placebo.
The two prespecified composite primary endpoints were CV hospitalizations/CV death and heart failure hospitalizations/CV death.
These showed similar 13%-14% relative risk reductions with ferric carboxymaltose, but only the former was statistically significant.
Similar results were seen when a fourth trial – IRONMAN (an open-label trial) – was included. In this case, the heart failure hospitalization/CV death endpoint was also nonsignificantly reduced with ferric carboxymaltose (rate ratio, 0.82; 95% CI, 0.58-1.07).
Subgroup analysis suggested that patients with higher transferrin saturation levels appeared to have a lack of treatment effect, whereas those with lower transferrin saturation (< 15%) showed significant treatment benefits.
A higher 6-month cumulative dose of ferric carboxymaltose – likely the result of redosing – may be associated with a slightly greater treatment effect after 6 months, Dr. Ponikowski reported.
He concluded: “These data support the use of intravenous ferric carboxymaltose to treat iron deficiency among patients with heart failure with reduced/mildly reduced LVEF [left ventricular ejection fraction] to reduce the risk of future hospitalization.”
“Our findings support additional research to challenge the current definition of iron deficiency in heart failure as an indication for IV iron therapy and to identify eligibility criteria for optimal redosing strategy,” Dr. Ponikowski added.
Discussant of the meta-analysis presentation at the ESC Hotline session, Pardeep Jhund, MD, University of Glasgow, suggested that the endpoint of most interest would be heart failure hospitalization/CV death in the analysis that included the IRONMAN trial, “which unfortunately did not meet statistical significance.”
In answer to the question “Where does this leave clinicians when treating patients?”Dr. Jhund said, “After yet another meta-analysis, I think the role of IV iron in reducing morbidity and mortality outcomes in heart failure remains questionable.”
“While the absence of evidence is not evidence of absence, the wide confidence intervals of the treatment effect on heart failure hospitalization/CV death leaves a lot of room for doubt about the efficacy of IV iron for reducing HF hospitalizations,” he concluded.
The HEART-FID trial was funded by American Regent, a Daiichi Sankyo Group company. Dr. Mentz reports receiving research support from American Regent and honoraria from American Regent, Vifor, and Pharmacosmos. Dr. Ponikowski reports consultancy fees/honoraria from Vifor Pharma, Boehringer Ingelheim, AstraZeneca, Servier, Novartis, Bayer, MSD, Pfizer, Moderna, Sanofi, and Radcliffe Group.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023