User login
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
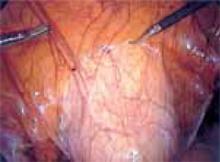
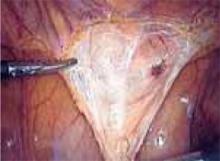
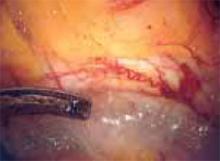
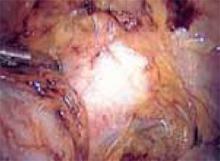
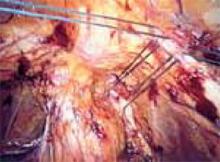
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
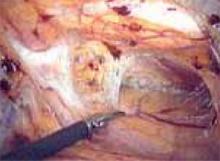
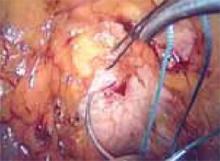
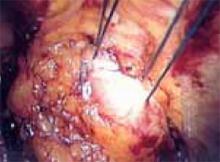
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
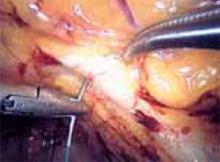
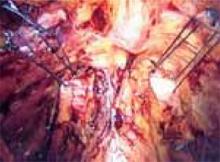
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
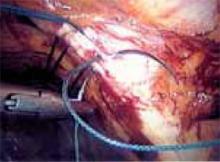
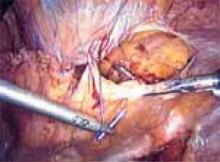
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
- Laparoscopic Burch colposuspension provides high long-term success rates, reduced morbidity, and accelerated convalescence.
- A growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.
- When we limit the discussion to 2 comparable techniques—a laparoscopic versus open 2-suture procedure—there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.
- The selection of suture material and the total number and placement of sutures are crucial to the long-term cure rate.
Despite the growing body of medical knowledge on stress urinary incontinence (SUI), controversies over its management remain.
SUI is the most common type of inconti-nence and occurs almost exclusively in females. A recent survey by the National Association for Continence revealed that SUI affects approximately 16.5 million women in the United States.1 Nearly two thirds of these women are under 50 years of age.
Still, there is no surgical procedure of choice for women with this condition. In fact, a recent systematic review of the literature by Black and Downs could not determine the “best procedure” based on scientific clinical evidence.2
Among the large number of surgical options for SUI treatment is bladder-neck suspension via a laparoscopic Burch colposus-pension. When it is properly executed, this procedure offers high long-term success rates, reduced morbidity, and accelerated convalescence. In this article, we describe how to perform the laparoscopic Burch procedure (reviewing both conventional suturing techniques and the Tanagho modification)3 and discuss when to consider a laparoscopic approach. In addition, we explain why the procedure should be part of your surgical options for female SUI.
Retropubic versus transvaginal suspensions
With so many surgeries to choose from, determining which procedure would be best for a woman with genuine stress urinary incontinence is a challenge. In 1997, the American Urological Association published a report designed to offer some guidance.
An 8-member panel reviewed data from 282 articles, all of which followed patients for a minimum of 12 months for short-term cure/dry results, and 48 months for long-term results. The Report on the Surgical Management of Female Stress Urinary Incontinence—based on expert opinion and evidence from the literature (as determined by probability estimates)—stated that retropubic suspensions and slings are the most efficacious procedures for long-term success.
Still, the panel noted that these interventions are associated with slightly higher complication rates—including an increased incidence of voiding dysfunction—and longer convalescence than other SUI procedures. For patients willing to accept these complication rates for the sake of improved long-term success, the panel concluded, retropubic suspension and slings are appropriate. However, for patients valuing a decreased hospital stay, reduced morbidity, and an earlier return to normal activity, transvaginal suspensions—the only minimally invasive option widely offered at that time—were the better option.4
The Burch procedure
In the classic Burch colposuspension, a physician places 2 bilateral nonabsorbable sutures through the pubocervical fascia—1 at the level of the midurethra, the other at the urethrovesical junction (UVJ)—and fixes them to Cooper’s ligament. But since 1991, when Vancaille and Schuessler introduced a laparoscopic approach to a retropubic colposuspension (MMK technique),5 a growing number of studies have shown the laparoscopic Burch to have results similar to traditional laparotomy when conventional surgical techniques and suture materials are used.6-24
A number of reports have also described modifications or alternatives to the classic laparoscopic Burch.25-28 These variations—which use stapling devices, mesh placement, bone anchors, and even fibrin glue—avoid laparoscopic suturing, thereby reducing the surgical complexity and shortening the learning curve. They also may lower the cost per procedure by decreasing time in the operating room.29-32 Still, an experienced laparoscopist who has mastered endoscopic suturing can perform a laparoscopic Burch using “standard” suturing in a time frame comparable to that of one of the modifications.33
Retropubic suspensions and slings are associated with slightly higher complication rates than other SUI procedures.
As far as outcomes go, it is the selection of suture material, the total number of sutures used, and their proper placement that are crucial to an optimal long-term cure rate—regardless of the surgical access to the space of Retzius.34-36 In fact, if a surgeon laparoscopically employs the identical operative technique, “suture for suture,” that he or she would use via laparotomy, there is no biological reason why the continence cure rates would be any different.
When? Burch procedure versus the TVT sling
Several studies have demonstrated that for patients with intrinsic sphincter deficiency (ISD) the Burch colposuspension cure/dry rate is less than that of a standard sling procedure.37 We therefore obtain urodynamic studies on all patients presenting with SUI who we feel are at risk for ISD ( TABLE). For patients with ISD and urethral hypermobility, we recommend the minimally invasive pubovaginal sling tension-free vaginal tape (TVT) procedure. In the absence of urethral hypermobility, we first utilize periurethral bulking agents to correct the ISD. If this is not successful, we proceed with urethrolysis and a traditional sling procedure.
For women who have concomitant pelvic-support defects such as uterine prolapse, vaginal vault inversion, or lateral cystoceles, we routinely perform laparoscopic reconstructive surgery, including the laparoscopic Burch for correction of the SUI.
Still, the difficult question remains: Which minimally invasive procedure—a laparoscopic Burch or a TVT—is preferable for the patient with genuine SUI without ISD or any additional pelvic-support defects aside from urethral hypermobility? Only a few studies comparing the clinical outcomes of the TVT and laparoscopic Burch procedures have reported preliminary findings. One retrospective study of 74 women followed for at least 1 year demonstrated an overall objective cure rate of 88% for the laparoscopic Burch versus 92% for the TVT procedure.38 There were no significant differences in time to resumption of normal voiding or in irritative symptoms such as frequency, urgency, and urge incontinence. The TVT group, however, was noted to have a shorter operative time and hospital stay.
Another study reported a higher cure or improvement rate (94%) among patients undergoing the laparoscopic Burch than the TVT (82%). Postoperative voiding difficulty was also significantly less in the laparoscopic group (0% versus 18%).39
Although these early studies suggest that laparoscopic Burch and TVT are comparable, we anxiously await the results of welldesigned, prospective, randomized clinical trials currently under way. One recent report (level I evidence) has demonstrated that the open Burch and the TVT procedure have equivalent results.40
TABLE
Patients at risk for intrinsic sphincter deficiency
|
How? The laparoscopic Burch technique
Preparing the patient. As always, obtain informed consent prior to the procedure. Beyond the usual surgical risks of blood loss, infection, surgical injury, failure rate, and thromboembolic complications, patients also face potential postoperative voiding dysfunction, as mentioned earlier, as well as de novo detrusor instability. Also inform your patients of the possible conversion to laparotomy.
Administer a single intravenous dose of an appropriate broad-spectrum antibiotic no more than 1 hour prior to surgery. For patients undergoing additional laparoscopic reconstructive surgery, we recommend a modified bowel preparation to improve visualization by decompressing the sigmoid colon.
Administer general anesthesia and place the patient in a dorsal lithotomy position with both arms tucked. Support the patient’s lower extremities with Allen Universal Stirrups (Allen Medical Systems, Mayfield, Ohio) and avoid excessive flexion of the knees or hips. Insert a 16F 3-way Foley catheter into the bladder—this allows intermittent bladder filling during the procedure—and inflate the bulb to 10 cc to facilitate identification of the UVJ throughout surgery.
Entering the space of Retzius. We routinely perform operative laparoscopy after Veress needle insertion and insufflation through an umbilical incision. (Use open laparoscopy for patients with prior abdominal surgery and paraumbilical scarring.)
Under direct visualization, place 2 additional accessory 10-mm trocars in the lower quadrants, just lateral to the inferior epigastric arteries. Brief insufflation to greater than 20 mm Hg intra-abdominal pressure facilitates safe entry for these secondary trocars. Although you may opt for smaller trocars, the 10-mm size allows unhindered passage of suture, thus providing more options for maximizing favorable ergonomics with future suture placement.
Although a preperitoneal, or extraperitoneal, approach has been described, we favor a transperitoneal entrance into the space of Retzius. The extraperitoneal approach allows the use of regional anesthesia, avoids intraabdominal adhesions, and eliminates the associated risks of peritoneal entry.31 The disadvantages, however, are significant, including failure to enter the retropubic space secondary to abdominal wall scarring, the inability to perform concomitant vault suspension, and the cost of commercially available dissecting balloons. With experience, a transperitoneal approach will not prolong operative time.
With experience, a transperitoneal approach into the space of Retzius will not prolong operative time.
Approaching the bladder. Distend the bladder in a retrograde fashion with 300 mL to 400 mL of normal saline. This allows identification of the superior margin of the bladder dome and provides mass traction posteriorly. Use the urachus to identify the midline; then, grasp the anterior abdominal wall peritoneum and apply downward traction ( FIGURE 1). Next, create a transverse incision 3 cm to 4 cm above the bladder reflection, using monopolar endoscopic scissors on a 70-watt pure-cut setting ( FIGURE 2). The incision should be within the obliterated umbilical ligaments, but can be extended slightly beyond for patients undergoing a combined laparoscopic Burch-paravaginal repair.41 Using a combination of blunt and electrocautery dissection, you then can easily dissect the loose areolar tissue of the prevesicle space down to the level of the pubic symphysis and ramus ( FIGURE 3).
FIGURE 1
Pictured is the distended bladder, forceps (right) at the bladder margin, endoshears (left) at level of incision, and urachus.
FIGURE 2
Create a transverse incision using monopolar scissors. Note the loose areolar tissue of prevesicle space.
FIGURE 3
Locate the pubic symphysis and ramus using the pelvic brim as a landmark.As the paravesical space is further developed, the pubocervical fascia will become exposed at the level of the UVJ. You must carefully protect the urethra, avoiding aggressive midline dissection as well as the obturator neurovascular bundle laterally. Medial traction on the bladder, perpendicular to the slope of the pubic ramus, encourages identification of the proper surgical plane. Use electrocautery to maintain meticulous hemostasis at all times. Identify Cooper’s ligament, and bluntly dissect away any obstructing fat or areolar tissue (FIGURE 4). To encourage scarification, gently remove excessive overlying periurethral and perivesical fat from pubocervical fascia at the level of the bladder neck, while avoiding any dissection within 1 cm lateral to the urethra ( FIGURE 5).
Placing the sutures. Using an extra-long (36-in), doubled-armed, nonabsorbable suture on an SH needle, place the sutures in the consistent sequence outlined below (note that sturdy needle drivers will facilitate secure needle placement):
First, introduce a needle from the contralateral port and pass it through the pubocervical fascia at the level of the midurethra, using your index finger for transvaginal guidance. If you think the tissue bite will not purchase nearly the entire thickness of the anterior vaginal wall, place a second helical throw (FIGURE 6). Next, bring the suture up through Cooper’s ligament and “store” it by hooking the anterior wall peritoneum (FIGURE 7). Bring up the second arm (needle) of the suture through Cooper’s ligament, but at a different depth than the first pass so ligament fibers are truly encircled by the suture (FIGURE 8). Retrieve both needles, bringing them out through the same port, but do not yet tie the suture.
Introduce the second suture through the ipsilateral port and place it in the same fashion at the level of the UVJ. Again, use helical throws as necessary. Once both sutures have been placed, tie them extracorporeally in sequence using a closed-loop knot pusher. (Waiting until both sutures are placed before tying allows exposure for easy placement of the second suture [FIGURE 9].) The appropriate tension should create a small, localized “knuckle” of pubocervical fascia that approximates laterally to the obturator internus fascia ( FIGURE 10).
Repeat this procedure in the same sequence on the opposite side of the pelvis ( FIGURE 11), then close the retropubic space using a running continuous 2-0 suture reapproximating the peritoneum (FIGURE 12). Close the laparoscopic ports at the fascia level using a Veress needle threaded with a 0-Vicryl. Both ends of the suture are passed on either side of the fascial incision. Using a contralateral grasping forceps, the suture end is freed from the Veress needle, then retrieved using an ipsilateral forceps. This port closure technique is easy to perform as well as cost-effective.
Postoperative care. Place a suprapubic catheter with a 2-way stop clock; this makes postoperative voiding trials easier for both patients and nursing staff.
Most patients will be discharged the day after surgery. If the patient still has an elevated postvoid residual, she’ll likely find going home with the suprapubic catheter more acceptable than intermittent self-catheterization or an indwelling Foley running to a leg bag. For postoperative discomfort, acetaminophen and nonsteroidal anti-inflammatory preparations are generally sufficient. Patients can resume normal living activities within days, but should be cautioned to delay strenuous work or exercise for at least 8 weeks.
FIGURE 4
Apply medial traction to the bladder as the paravesical space is developed down to the level of pubocervical fascia. Note Cooper’s ligament, seen anteriorly.
FIGURE 5
Gently remove the overlying periurethral and perivesical fat to expose white pubocervical fascia.
FIGURE 6
Apply counter traction on the suture from the first pass to facilitate better tissue purchase on the second pass of the helical suture.
FIGURE 7
Place the needle through Cooper’s ligament.
FIGURE 8
Pass the second needle at a deeper depth through Cooper’s ligament to encircle fibers within the suture. This will minimize the risk of “pull out.”
FIGURE 9
Place both sutures before extracorporeal tying.
FIGURE 10
Suture tension should create only a small knuckle of pubocervical fascia, approximating obturator fascia laterally.
FIGURE 11
Both sides completed.
FIGURE 12
Close the retropubic space.
Why? A look at the evidence
The learning curve for laparoscopic Burch is steep and somewhat long—approximately 20 cases. The real question, therefore, is whether the benefits justify the time needed to master this procedure. In other words, is there clinical evidence that, once the plateau of this curve has been reached, we can reduce patient morbidity while maintaining efficacy compared to the traditional open technique? If not, there’s little reason for surgeons to learn the technique. If there is, however, more physicians should include the laparoscopic Burch in their surgical arsenal.
The real question is whether the benefits of the laparoscopic Burch justify the time needed to master the procedure.
When we limit the discussion to 2 comparable techniques, a laparoscopic versus open 2-suture procedure, there is moderately strong evidence that the laparoscopic approach maintains efficacy while modestly reducing morbidity.42 The strength of this evidence is established in 6 studies, including 3 randomized trials (level I evidence).8,18,23,24,43,44
Currently, data are insufficient regarding a laparoscopic approach to make concomitant site-specific defect repairs.45-51 However, if the procedures are performed in the same fashion, “suture for suture,” as their abdominal counterparts, we should expect to see, as we have with the laparoscopic Burch, similar efficacy rates between the laparoscopic and open approaches.
So, in closing, we encourage our colleagues to reignite their interest in learning laparoscopic reconstructive surgery and recommit to reaching that learning curve plateau. Why? We owe it to our patients.
Dr. Lucente is a consultant for GyneCare. Dr. Murphy reports no financial relationship with any companies whose products are mentioned in this article.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.
1. National Association for Incontinence Quarterly Report. Summer 2002, Vol 20 No 3.
2. Black NA, Down SH. The effectiveness of surgery for stress incontinence in women: A systematic review. Br J Urol. 1996;78:497.-
3. Tanagho E. Colpocystourethropexy: The way we do it. J Urol. 1976;116:751-753.
4. Leach GE, Dinochowski RR, Appell RA, et al. Female stress urinary incontinence clinical guidelines panel: Report on the surgical management of female stress urinary incontinence. Baltimore, Md: American Urological Association, Inc.; 1997.
5. Vancaille TG, Schuessler W. Laparoscopic bladder neck suspension. J Laparoendosc Surg. 1991;1:169-173.
6. Albala DM, Schuessler WW, Vancaillie TG. Laparoscopic bladder suspension for the treatment of stress incontinence. Semin Urol. 1992;10:222-225.
7. Burton G. A randomized comparison of laparoscopic and open colposuspension [abstract]. Neurourol Urodyn. 1993;16:353-354.
8. Polascik TJ, Moore RG, Roseberg MT, Kavoussi LR. Comparison of laparoscopic and open retropubic colposuspension for treatment of stress urinary incontinence. Urology. 1995;45:647-652.
9. Liu CY. Laparoscopic treatment of genuine urinary stress incontinence. Clin Obstet Gynecol. 1994;8:789-798.
10. Gunn GC, Cooper RP, Gordon NS, Gragnon L. Use of a new device for endoscopic suturing in the laparoscopic Burch procedure. J Am Assoc Gynecol Laparosc. 1994;2:65-70.
11. Nezhat CH, Nezhat F, Nezhat CR, et al. Laparoscopic retropubic cystocolposuspension. J Am Assoc Gynecol Laparosc. 1994;1:339-349.
12. Lyons TL, Winer WK. Clinical outcomes with laparoscopic approaches and open Burch procedures for urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1995;2:193-197.
13. McDougal EM, Klutke CG, Cornell T. Comparison of transvaginal versus laparoscopic bladder neck suspension for stress urinary incontinence. Adult Urol. 1995;45:641-645.
14. Ross JW. Laparoscopic Burch repair compared to laparotomy Burch for cure of urinary stress incontinence. Int Urogynecol. 1995;6:323-328.
15. Langebrekke A, Dahlstrom B, Eraker R, Urnes A. The laparoscopic Burch procedure: a preliminary report. Acta Obstet Gynecol Scand. 1995;74:153-155.
16. Radomski SB, Herschorn S. Laparoscopic Burch bladder neck suspension: early results. J Urol. 1996;155:515-518.
17. Ross JW. Two techniques of laparoscopic Burch repair for stress incontinence: a prospective randomized study. J Am Assoc Gynecol Laparoscopists. 1996;3:351-357.
18. Lam AM, Jenkins GJ, Hyslop RS. Laparoscopic results. Med J Aust. 1995;162:18-22.
19. Su TH, Wang KG, Hsu CY, Wei H, Hong BK. Prospective comparison of laparoscopic and traditional colposuspensions in the treatment of genuine stress inconti-nence. Acta Obstet Gynecol. 1997;76:576-582.
20. Papasakelariou C, Papasakelariou B. Laparoscopic bladder neck suspension. J Am Assoc Gynecol Laparosc. 1997;4:185-188.
21. Lobel RW, Davis GD. Long-term results of laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1997;4:341-345.
22. Ross JW. Multichannel urodynamic evaluation of laparoscopic Burch colposuspension for genuine stress incontinence. Obstet Gynecol. 1998;91:55-59.
23. Miannay E, Cosson M, Querleu D, et al. [Comparison of laparoscopic and laparotomy colposuspension in the treatment of urinary stress incontinence. Comparative study of 72 matched cases][French]. Contracept Fertil Sex. 1998;26:376-385.
24. Saidi MH, Gallagher MS, Skop IP, et al. Extrperitoneal laparoscopic colposuspension: short-term cure rate, complications and duration of hospital stay in comparison with Burch colposuspension. Obstet Gynecol. 1998;92:619-625.
25. Henley C. The Henley staple-suture technique for laparoscopic Burch colposuspension. J Am Assoc Gynecol Laparosc. 1995;2:441-444.
26. Das S, Palmer JK. Laparoscopic colposuspension. J Urol. 1995;154:1119-1121.
27. Ou CS, Presthus J, Beadle E. Laparoscopic bladder neck suspension using hernia mesh and surgical staples. J Laparoendosc Surg. 1993;3:563-566.
28. Kiilholma P, Haarala M, Polvi II, Makinen J, Chancellor MB. Sutureless colposuspension with fibrin sealant. Tech Urol. 1995;1:81-83.
29. Lose G. Laparoscopic Burch colposuspension. Acta Obstet Gynecol Scand Suppl. 1998;168:29-33.
30. Miannay E, Cosson M, Lanvin D, et al. Comparison of open retropubic and laparoscopic colposuspension for treatment of stress urinary incontinence. Eur J Obstet Gynecol Reprod Biol. 1998;79:159-166.
31. Saidi MH, Sadler RK, Saidi JA. Extraperitoneal laparoscopic colposuspension for genuine urinary stress incontinence. J Am Assoc Gynecol Laparosc. 1998;9:249-252.
32. Ou CS, Rowbotham R. Five-year follow-up of a laparoscopic bladder neck suspension using synthetic mesh and surgical staples. J Laparoendosc Adv Surg Techn A. 1999;9:249-252.
33. Zullo F, Morelli M, Russo T, et al. Two techniques of laparoscopic retropubic urethropexy. J Am Assoc Gynecol Laparosc. 2001;12:323-327.
34. Langer R, Lipshitz Y, Halperin R, et al. Long-term (10-15 years) follow-up after Burch colposuspension for urinary stress incontinence. Int Urogynecol J. 2001;12:323-327.
35. Herbertsson G, Iosif CS. Surgical results and urodynamic studies 10 years after retropubic colpurethropexy. Acta Obstet Gynecol Scand. 1993;72:298-301.
36. Parasio M, Falcone T, Walters M. Laparoscopic surgery for genuine stress incontinence. Int Urogynecol J. 1999;10:237-247.
37. Horbach NS. Suburethral sling procedures. Urogynecology and Urodynamics: Theory and Practice. 4th ed. Baltimore, Md: Williams and Wilkins 1996;569-579.
38. Vassallo B, Murphy M, Lucente V, Karram M. TVT versus Laparoscopic Burch: a retrospective review at 1-4 years. 27th Scientific Meeting of the Society of Gynecologic Surgeons. March 5-7, 2001.
39. Fotte A. Which is the best minimally invasive procedure? TVT versus laparoscopic colposuspension. Presented at the 31st annual meeting of the International Continence Society; September 18-21, 2001; Seoul, Korea.
40. Ward KL, Hilton P. Prospective multicentre randomized trial of tension-free vaginal tape and colposuspension as primary treatment for stress incontinence. BMJ. 2002;326:67.-
41. Miklos J, Kohli N. Laparoscopic paravaginal repair plus Burch colposuspension: review and descriptive technique. Urology. 2000;56:64-68.
42. Walter A. Laparoscopic repairs for stress urinary incontinence and pelvic organ prolapse: “show me the evidence.” Presented at the annual meeting of the American Urogynecologic Society; 2002; San Diego, Calif.
43. Summitt RL, Lucente V, Karram MM, Shull BL, Bent AE. Randomized comparison of laparoscopic and transabdominal Burch urethropexy for the treatment of genuine stress incontinence. Obstet Gynecol. 2000;95:(suppl):2.-
44. Fatthy H, El Hao M, Samaha I, Abdallah K. Modified Burch colposuspension: laparoscopy versus laparotomy. J Am Assoc Gynecol Laparosc. 2001;8:99-106.
45. Ross JW. Techniques of laparoscopic repair for total vault eversion after hysterectomy. J Am Assoc Gynecol Laparosc. 1997;4:173-183.
46. Ross JW. Laparoscopic approach for severe pelvic vault prolapse. J Am Assoc Gynecol Laparosc. 1996;3(suppl):43.-
47. Lyons TL, Winer WK. Laparoscopic rectocele repair using polyglactin mesh. J Am Assoc Gynecol Laparosc. 1997;4:381-384.
48. Ostrzenski A. Laparoscopic colposuspension for total vaginal prolapse. Int J Gynaecol Obstet. 1996;55:147-152.
49. Carter JE, Winter M, Mendehlsohn S, Saye W, Richardson AC. Vaginal vault suspension and enterocele repair by Richardson-Saye laparoscopic technique: description of training technique and results. JSLS. 2001;5:29-36.
50. Cosson M, Rajabally R, Bogaert E, Querleu D, Crepin G. Laparoscopic sacrocolpopexy, hysterectomy and Burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS. 2002;6:115-119.
51. Miklos JR, Moore RD, Kohli N. Laparoscopic surgery for pelvic support defects. Curr Opin Obstet Gynecol. 2002;14:387-395.