User login
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.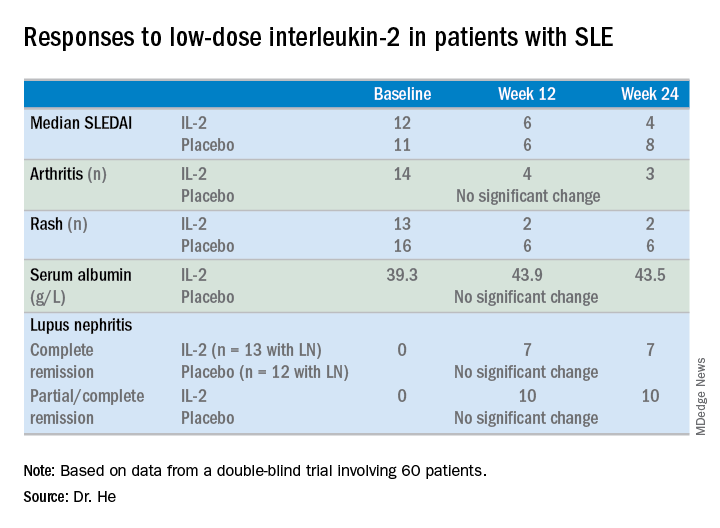
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
SAN FRANCISCO – , according to the first randomized, double-blind, placebo-controlled clinical trial of the novel therapy.
Of note, more than half of the study participants with lupus nephritis experienced complete remission of their renal impairment, and another quarter had partial remission, Jing He, MD, PhD, reported at an international congress on systemic lupus erythematosus.
The mechanism of benefit appears to be the same as previously shown for low-dose interleukin-2 in patients with chronic graft versus host disease refractory to glucocorticoids (N Engl J Med. 2011 Dec 1;365[22]:2055-66): expansion of the deficient population of T regulatory cells, which is a hallmark of both inflammatory diseases.
“Low-dose IL-2 can reinstate the imbalance of T regulatory/T effector cells and improve immune homeostasis, which is critical in clinical remission of SLE,” said Dr. He of Peking University People’s Hospital in Beijing.
For nearly 20 years it has been known that SLE is characterized by very low levels of endogenous IL-2. Dr. He was lead author of the first proof-of-concept study, which showed low-dose subcutaneous IL-2 therapy resulted in markedly reduced SLE disease activity accompanied by expansion of the T regulatory cell population and suppression of follicular helper T cells and IL-17–producing helper T cells (Nat Med. 2016 Sep;22[9]:991-3). However, that was a small, single-center, uncontrolled study, so she and her coworkers have now carried out a 60-patient, double-blind, placebo-controlled randomized trial. In addition to hydroxychloroquine and other standard background medications, the patients in the active treatment arm received 1 million IU of IL-2 every other day for 2 weeks, followed by a 2-week hiatus, for a total of three courses.
At week 24 – 12 weeks after the last injection – the IL-2 recipients showed significantly greater improvement on numerous endpoints.
For example, the median SLE Disease Activity Index (SLEDAI) in the IL-2 group improved from 12 at baseline to 6 at week 12 and to 4 at week 24.
The marked improvement in renal impairment in the IL-2 recipients with lupus nephritis at baseline was accompanied by a significant increase in serum albumin and reduced 24-hour urinary protein, compared with controls.
The treatment was safe, with no increase in infections, severe or otherwise, and indeed with no serious adverse events of any kind, although nine patients in the IL-2 group experienced mild injection site reactions and three developed flu-like symptoms.
Dr. He reported having no financial conflicts regarding her study.
REPORTING FROM LUPUS 2019

