User login
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.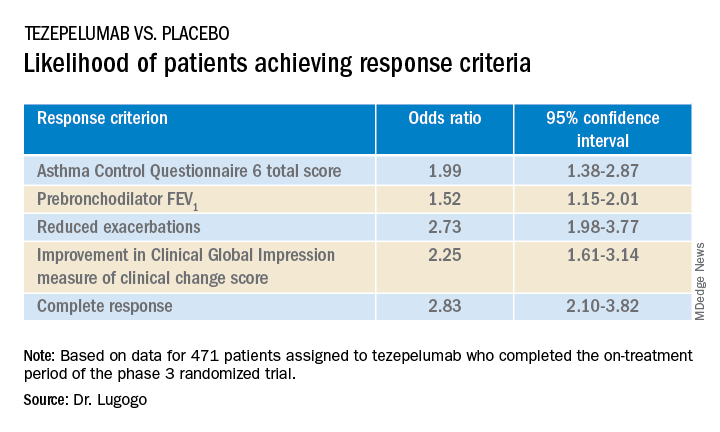
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
SAN FRANCISCO – Nearly half of all patients with severe, uncontrolled asthma who received a full course of the biologic agent tezepelumab (Tezspire) in the NAVIGATOR trial had a complete response to treatment at 1 year, results of a prespecified exploratory analysis indicated.
Among 471 patients assigned to tezepelumab who completed the on-treatment period of the phase 3 randomized trial, 46% had a complete response at 52 weeks, compared with 24% of patients assigned to placebo.
Complete response was defined as reduction in exacerbations of at least 50% over the previous year, improvement from baseline in Asthma Control Questionnaire 6 (ACQ-6) total score of at least 0.5 points, improvement in prebronchodilator forced expiratory volume in 1 second (pre-BD FEV1), and physician-assessed Clinical Global Impression measure of clinical change (CGI-C) score.
“These data further support the efficacy of tezepelumab in a broad population of patients with severe, uncontrolled asthma,” said Njira Lugogo, MD, of the division of pulmonary and critical care medicine at the University of Michigan, Ann Arbor.
Dr. Lugogo presented results of the exploratory analysis at the American Thoracic Society’s international conference.
Exacerbations reduced, lung function improved
Primary results from NAVIGATOR, published in The New England Journal of Medicine, showed that patients with severe, uncontrolled asthma randomly assigned to tezepelumab had fewer exacerbations and better lung function, asthma control, and health-related quality of life compared with patients assigned to placebo.
The investigators noted that approximately 10% of patients with asthma have symptoms and exacerbations despite maximal standard-of-care controller therapy.
Tezepelumab is a human monoclonal antibody that inhibits action of thymic stromal lymphopoietin (TSLP), an epithelial cytokine that is released in response to airborne triggers of asthma. TSLP is a major contributor to initiation and persistence of airway inflammation, Dr. Lugogo said.
The on-treatment analysis looked at all patients in the trial who completed 52 weeks of treatment and had complete data for all criteria studied.
The odds ratios (OR) for patients on tezepelumab achieving each of the response criteria are shown in the table.
Exacerbations explored
In a separate presentation, Christopher S. Ambrose, MD, MBA, of AstraZeneca in Gaithersburg, Md., presented information from investigator-narrative descriptions of all hospitalization events related to asthma exacerbations (mild, moderate, or severe) that occurred while the investigator was blinded to each patient’s treatment assignment in NAVIGATOR.
In all, 39 of 531 patients (7.3%) assigned to placebo had a total of 78 exacerbations requiring hospitalization, compared with 13 of 528 patients (2.5%) assigned to tezepelumab. The latter group had a total of 14 exacerbations requiring hospitalization during the study.
Among hospitalized patients, 32 of the 39 assigned to placebo had severe, incapacitating exacerbations, compared with 5 of 13 assigned to tezepelumab.
Reported symptoms were generally similar between hospitalized patients in the two treatment groups, although there appeared to be trends toward lower incidence of dyspnea, fever, and tachycardia with tezepelumab.
Health care resource utilization, a surrogate marker for disease burden, was substantially lower for patients assigned to tezepelumab.
Infections were the most common triggers of exacerbations in both groups.
“These data provide further evidence that tezepelumab can reduce the burden of disease of severe uncontrolled asthma, both to patients and to health care systems,” Dr. Ambrose said.
Head-to-head studies needed
Although there have been no head-to-head comparisons of biologic agents for asthma to date, results of these studies suggest that tezepelumab has efficacy similar to that of other agents for reducing exacerbation, said Fernando Holguin, MD, MPH, from the University of Colorado at Denver, Aurora, who comoderated the oral session where the data were presented but was not involved in the study.
Biologic agents appear to be slightly more effective against type 2 inflammation in asthma, “but in general I think we give it to a broader severe population, so that’s exciting,” he told this news organization.
Comoderator Amisha Barochia, MBBS, MHS, of the National Institutes of Health, Bethesda, Md., told this news organization that head-to-head trials of biologic agents would provide important clinical information going forward.
“Should we switch to a different biologic or add a second biologic? Those are questions we need answers for,” she said.
The NAVIGATOR trial is funded by AstraZeneca and Amgen. Dr. Lugogo disclosed financial relationships with both companies. Dr. Holguin and Dr. Barochia have disclosed no financial relationships relevant to the studies presented.
A version of this article first appeared on Medscape.com.
AT ATS 2022