User login
CASE-BASED LEARNING
Her risk is high, but how high? Is invasive testing the only answer?
Mrs. S, a 37-year-old primigravida, has an age-related risk of having a baby with Down syndrome of 1 in 250. Before deciding whether to undergo an invasive diagnostic procedure based on her age alone, she wants to learn more about her risk by having a prenatal screening test. The 2 safest and most informative options: first-trimester combined screening and fully integrated screening. About 5% of women who have the first test are found to be high-risk, and the test identifies about 85% of all cases of Down syndrome. As for fully integrated screening, it identifies about 85% of all Down syndrome cases at a 1% screen-positive rate. Since it offers a faster result, Mrs. S opts for first-trimester screening.
At 11 weeks, 0 days (according to crown-rump length), she undergoes nuchal translucency imaging and has a blood sample drawn to measure pregnancy-associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG). Her test results are reported in multiples of the median (MoM):
| Nuchal translucency | 2.3 mm | 1.62 MoM |
| PAPP-A | 0.5 mIU/mL | 0.79 MoM |
| hCG | 45 IU/mL | 1.25 MoM |
These values suggest that Mrs. S has a risk of having a pregnancy affected by Down syndrome of 1 in 170. In other words, her results are screen-positive.
Can her risk be more precisely quantified without invasive testing?
Had Mrs. S chosen fully integrated screening, the answer to that question would be yes, but it would have meant waiting until the second trimester for the result.
This article describes the 2 screening approaches—first-trimester combined screening and fully integrated screening—as well as the serum-only variant of the integrated test and the established quad marker test. Also discussed are the findings of recent studies, including 2 key trials:
- the First and Second Trimester Evaluation of Risk (FASTER) of aneuploidy trial, published in November1
- the Serum, Urine, and Ultrasound Screening Study (SURUSS), published in 2003 in the United Kingdom2
These 2 trials are the only ones to compare screening markers at different times during pregnancy in the same women—the only way to fairly assess the quality of various marker combinations within and across gestational weeks.
How good is current practice?
In 1995, about 2.5 million of the approximately 4 million gravidas in the United States had maternal serum screening for Down syndrome and open neural tube defects.3 Today, this practice usually involves a serum sample drawn early during the second trimester (15–20 weeks), measurement of 3 or 4 serum markers (the triple or quad test), and calculation and reporting of risk.
Second-trimester serum screening is a relatively easy procedure involving a single blood sample and established risk-calculation methods. Further, the follow-up when a woman is screen-positive—ie, at increased risk—is clear: amniocentesis in the case of Down syndrome risk and targeted ultrasound in the case of open neural tube defects (with amniocentesis backup). For the triple test, we can expect a detection rate of about 70%, and for the quad test, 80%, by identifying 5% of screened women with the highest calculated risk (the effective screen-positive rate).1,2,4
Why the newer options are better
Optimally, prenatal screening should minimize the number of women identified as screen-positive (ie, women at sufficient risk to be offered amniocentesis or comparable procedures) while maximizing the overall detection rate. This point is important because screen-positive status leads to follow-up diagnostic procedures that are necessarily invasive and risky.
First-trimester screening slightly better than the quad test
The option Mrs. S selected entails measuring 3 markers during the late first trimester (11–13 gestational weeks): nuchal translucency, PAPP-A, and hCG or its free βsubunit. These markers constitute a screening test that performs as well as, or slightly better than, the second-trimester quad test. The best estimate of first-trimester screening is an 85% detection rate at a 5% screen-positive rate (compared with about 80% detection rate at a 5% screen-positive rate for the quad test).1,2,5,6
Serum markers or ultrasound alone not enough in first trimester
Two serum markers together, without nuchal translucency, or nuchal translucency alone, without the serum markers, do not constitute a sufficient first-trimester screening test, since they each detect about 60% to 65% of Down syndrome cases with a 5% false-positive rate. This is clearly inferior to the best we can do during the second trimester (about 80% detection rate for a 5% false-positive rate). Only when nuchal translucency and serum markers are used together is first-trimester screening a viable option.
Timing is important in integrated screening
For the integrated screening option, instead of requiring that screening be offered in the late first or early second trimester, each marker is measured when it is most informative. The optimal time for nuchal translucency and PAPP-A measurement is at 10 to 11 weeks, while the optimal time for the measurement of hCG (or its free β subunit), inhibin A, alpha fetoprotein (AFP), and unconjugated estriol (uE3) is at 15 to 20 weeks.
Therefore, the integrated test is accomplished in 2 steps. At about 11 weeks, a woman undergoes nuchal translucency ultrasound imaging and has a blood sample drawn for PAPP-A measurement. At about 15 weeks (the earlier in the second-trimester window the better), she has a second sample drawn for measurement of the quad markers. A risk report then is generated, using all 6 markers to calculate the woman’s new risk. Such a test has to be superior to any test that uses fewer markers or the same markers at less than the optimal time.
The integrated test can also be carried out without nuchal translucency, by measuring PAPP-A during the first trimester and the quad serum markers during the second trimester, for an estimated detection rate of 85% with a 5% false-positive rate.
Integrated option has 1% screen-positive rate
Integrated screening reduces the screen-positive rate by as much as fourfold—to 1% or less. That is, only 1 in 100 women undergoing screening will be called screen-positive, and, in that 1%, approximately 85% of all Down syndrome pregnancies will be found.1,2,7
First-trimester serum markers
The most informative serum marker during the first trimester is PAPP-A, a large glycoprotein complex made by the placenta. In pregnancies affected by Down syndrome, PAPP-A levels tend to be low: about 0.4 MoM on average, or about 2.5 times lower than in unaffected pregnancies.
The second most commonly used serum marker is the free βsubunit of hCG, which is, on average, 1.8 MoM in pregnancies affected by Down syndrome, or almost twice as high as in normal pregnancies.4,8 Studies indicate that hCG and inhibin A are also effective serum markers during the late first trimester, providing screening performance equivalent to that of the free β-hCG when combined with nuchal translucency and PAPP-A.4
Nuchal translucency: A powerful marker
Both the fully integrated and first-trimester screening approaches necessitate ultrasound measurement of nuchal translucency, which is always measured along with fetal crown-rump length. The nuchal translucency value—initially measured in tenths of millimeters—then is normalized for gestational age based on crown-rump length, and reported in multiples of the median, the same unit used to normalize serum screening markers.
FIGURE 1 shows how nuchal translucency values (in millimeters) measured in a general population increase with gestation. The most commonly accepted period of gestation to measure nuchal translucency is between 10 and 13 completed weeks.
Why nuchal translucency is more informative
Nuchal translucency values tend to be increased in Down syndrome pregnancies, as are certain serum markers such as hCG or its free β subunit and inhibin A. However, nuchal translucency is more informative than these markers because there is less overlap between Down syndrome and unaffected case values. This is not because nuchal translucency values tend to be higher in affected pregnancies. In fact, all 3 markers are, on average, about twice as high in cases of Down syndrome as in controls. However, because the distribution of nuchal translucency values in unaffected fetuses is much narrower (or tighter) than is true for hCG (or its free β subunit) or inhibin A, very few unaffected fetuses have increased nuchal translucency values. Therefore, when nuchal translucency is elevated, it is more likely to be associated with an affected pregnancy than is either of the other 2 markers.
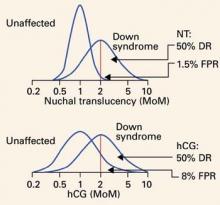
FIGURE 2 shows the overlapping distributions in cases and controls for both hCG and nuchal translucency. In unaffected pregnancies, the distribution of values centers around 1 MoM, while in Down syndrome pregnancies, the values center around 2 MoM. About 8% of hCG values in unaffected pregnancies exceed 2 MoM, but only about 1.5% of nuchal translucency values do. Thus, for a detection rate of 50%, the false-positive rate using nuchal translucency is 1.5%, much smaller than the false-positive rate of 8% using hCG.
Imaging expertise is key
While it is beyond the scope of this article to detail the methodology of nuchal translucency measurement, specialized training and ongoing quality assurance are necessary to get the measurement right. Both the Society for Maternal-Fetal Medicine in the United States and the Fetal Medicine Foundation in the United Kingdom provide training and credentialing in nuchal translucency sonography. If performed correctly, it is an excellent screening marker. However, if attempted with no hands-on training, this imaging method yields unreliable results.
FIGURE 1 Nuchal translucency values increase with gestational age
Nuchal translucency values, in millimeters, in 561 pregnancies between 10 and 13 completed weeks of gestation, as estimated by crown-rump length. Reprinted with permission from Drs. Wald and Schucter; data from Schuchter K, et al.9
FIGURE 2 Very few unaffected fetuses have elevated nuchal translucency values
Distribution of values of second-trimester human chorionic gonadotropin (hCG) and first-trimester nuchal translucency among unaffected and Down syndrome pregnancies, both given in multiples of the median (MoM). Note that the unaffected nuchal translucency distribution is much narrower and taller than the unaffected hCG distribution. The scale is a log progression of increasing MoM values because both markers are log-normally distributed in unaffected and Down syndrome gestations. DR=detection rate; FPR=false-positive rate; NT=nuchal translucency.
2 important studies
The SURUSS trial, conducted mainly in the United Kingdom, was an observational study in which all women underwent first-trimester ultrasound measurement of nuchal translucency, as well as first- and second-trimester blood and urine sampling, with all samples stored. After all outcomes were chronicled, case-control sets of first- and second-trimester samples were constructed and assayed for a wide variety of known and potential screening markers. Nuchal translucency data also were analyzed. More than 48,000 women were enrolled, and 101 Down syndrome pregnancies were identified and studied. 2,10
The FASTER trial, completed more recently, was an observational study conducted at 15 enrollment centers in the US and involving more than 38,000 women, among whom 117 Down syndrome cases were identified.1 All women in the trial underwent first-trimester ultrasound examination and blood sampling between 10 weeks 3 days, and 13 weeks 6 days, and were asked to return for a second-trimester blood draw between 15 and 18 completed weeks, after which a report was issued detailing the separate results. In addition, combinations of markers across the trimesters were modeled and compared.
Perhaps the most interesting and surprising finding from the 2 studies is the remarkable similarity of results (TABLE). For 2 large populations on separate continents with a different ethnic mix, the primary findings were almost identical:
- 86% to 87% detection rate at a 5% false-positive rate for first-trimester combined screening (nuchal translucency, PAPP-A, and free β-hCG) and 80% to 83% detection rate at a 5% false-positive rate for second-trimester quad screening (AFP, uE3 , hCG, and inhibin A). In both trials, first-trimester screening was incrementally better than second-trimester screening, but the difference was not statistically significant.
- For integrated screening, a detection rate of 86% to 88% at a false-positive rate of 1%. Nuchal translucency and PAPP-A were measured during the first trimester, and quad markers during the second trimester. At a 5% false-positive rate, the detection rate in both trials was about 95%. With a serum-only integrated test, detection rates were 87% to 88% with a 5% false-positive rate.
- Nuchal translucency was more informative than any of the serum markers tested. This corroborates the rich literature on nuchal translucency published over the past decade. Both trials demonstrated that nuchal translucency measurement is effective when training is adequate, and that ongoing monitoring of quality is essential. However, in both studies, a satisfactory nuchal translucency measurement was not attained in about 7% of all women scanned between 10 and 13 weeks, so a small but significant number of women will not have nuchal translucency included in their risk assessment.
Other notable FASTER findings
Other findings from the FASTER trial that merit special attention:
- An ultrasound finding of cystic hygroma warrants an immediate prenatal diagnostic workup and was associated with an aneuploidy rate of 50% (one third of which was Down syndrome) and adverse outcomes in the great majority of cases.11 Cystic hygroma is uncommon, occurring in about 1 in 300 pregnancies in the first trimester.
- The value of the fetal nasal bone as a first-trimester ultrasound marker is unclear. In the FASTER trial, the nasal bone was studied in about 6,000 of the 38,000 ultrasound examinations, with no detected benefit. Thus, in a nonselected pregnant population, the nasal bone may not be a reasonable marker.12
- Various first- and second-trimester markers are modestly informative about adverse pregnancy outcomes other than aneuploidy (eg, fetal growth restriction, early delivery, and preeclampsia). Only a small proportion of affected pregnancies will be identified by these markers, alone or in combination.13,14
TABLE
5 screening approaches: What the SURUSS and FASTER trials reveal
| TEST | NUCHAL TRANSLUCENCY MEASURED? | METHOD OF PRENATAL DIAGNOSIS | DETECTION RATE (%) AT 1% FALSE-POSITIVE RATE | DETECTION RATE (%) AT 5% FALSE-POSITIVE RATE | FALSE-POSITIVE RATE* (%) TO ACHIEVE: | |||
|---|---|---|---|---|---|---|---|---|
| SURUSS | FASTER | SURUSS | FASTER | 85% DETECTION | 95% DETECTION | |||
| 2nd-trimester triple marker | No | 2nd-trimester amniocentesis | 56 | 45 | 77 | 70 | 14 | 32 |
| 2nd-trimester quad marker | No | 2nd-trimester amniocentesis | 64 | 60 | 83 | 80 | 7.3 | 22 |
| 1st-trimester combined | Yes | 1st-trimester chorionic villus sampling | 72 | 73 | 86 | 87 | 3.8 | 18 |
| Serum integrated test | No | 2nd-trimester amniocentesis | 73 | 73 | 87 | 88 | 3.6 | 15 |
| Full integrated test | Yes | 2nd-trimester amniocentesis | 86 | 88 | 94 | 96 | 0.6 | 4 |
| FASTER=First- and Second-Trimester Evaluation of Risk[1]; SURUSS=Serum, Urine, and Ultrasound Screening Study.2 | ||||||||
| *Based on data from FASTER trial only. | ||||||||
Clinical considerations
Integrated screening
A number of considerations are important:
Tell the patient screening for neural tube defects is included in the integrated test, since it spans the first and second trimesters and includes AFP as one of the markers measured. Thus, women having the integrated test will also be screened for open spina bifida and anencephaly, in addition to Down syndrome.
Hold individual measurements until all results are in. First-trimester nuchal translucency and PAPP-A results are the first to become available. After these tests are performed, a waiting period of about 2 to 5 weeks is required to allow for second-trimester testing, tabulation, and integration into a single risk estimate.
In the case of ultrasound imaging, it is important for the sonographer to explain what is being measured without conveying special import regarding the nuchal translucency measurement—whether it is large or small. If asked, the sonographer should explain that nuchal translucency is only 1 of 6 measures that will determine the patient’s risk.
Advise the patient that definitive diagnosis will not occur until the second trimester. Because the integrated test is reported after the second-trimester serum sample is drawn and assayed, any follow-up diagnostic testing will not be available any sooner than is typical for second-trimester screening (ie, 16–18 gestational weeks).
A very large nuchal translucency measurement may be cause for concern and points to the need for early diagnosis. If a woman having the integrated test is found to have a nuchal translucency measurement of 3 to 4 mm or more (or any cystic hygroma), a clinically reasonable strategy is to offer immediate prenatal diagnosis by CVS rather than continue with the screening test.
Nuchal translucency values of 3 or 4 mm or more are seen in fewer than 1% of women scanned, and are associated with a very high risk of fetal aneuploidy and adverse pregnancy outcomes.
If nuchal translucency ultrasound is not available in your region, the integrated test using serum markers will provide better screening performance than any other serum-only test.
First-trimester combined screening
Offer it as early as possible. The benefit of first-trimester screening is the prospect of early prenatal diagnosis. Therefore, the earlier the test is offered within the accepted time frame of 11 to 13 completed weeks, the more apparent the benefit.
Early screening requires nuchal translucency measurement. First-trimester screening involves the measurement of serum analytes and ultrasound measurement of nuchal translucency. Serum markers without nuchal translucency or nuchal translucency without serum markers provide insufficient screening. If nuchal translucency is unavailable, offer the serum-only form of the integrated test or the second-trimester quad marker test.
Early diagnosis is requisite. Patients who are screen-positive in the first trimester should have CVS as an option for the earliest possible diagnosis. If amniocentesis at 15 weeks or beyond is the only invasive diagnostic procedure available, first-trimester combined screening is not appropriate.
An additional screen for neural tube defects is needed. First-trimester combined screening does not test for risk of open neural tube defects. Most commonly, a second-trimester serum AFP measurement is recommended for all women who have had first-trimester screening. Alternatively, a second-trimester ultrasound scan for fetal anomalies is highly indicative of neural tube defects if it includes the cranial lemon and cerebellar banana signs.
Even newer choices
Within the past year, 2 new screening methods have been proposed: sequential testing and contingent testing.15,16 They are essentially hybrids of the first-trimester and integrated tests. Both identify a very small, very high-risk group based on first-trimester nuchal translucency and serum markers. This group (eg, women having a risk of 1 in 25 and higher) would account for less than 1% of the total screened population and would ultimately be found to have more than 50% of Down syndrome cases.
In sequential testing, all women whose risk is less than the high-risk cutoff (eg, a possible cutoff of less than 1 in 25 or higher) would go on to have the full integrated test. In contingent testing, a second group would be identified as very low-risk based on first-trimester markers (eg, a possible risk cutoff of 1 in 2,000 or lower). Such low-risk women would have a small chance of having their results become high-risk based on the completed integrated test; therefore, they would be identified as screen-negative early and would not have to go on to integrated testing. In contingent testing, only the intermediate group (eg, those between, say, 1 in 25 and 1 in 2,000) would complete the integrated test.
The terms screen-negative and screen-positive are applied to test results to convey the most accurate balance between detection and false-positive rates. However, these terms indicate only risk categories; within these categories, the specific risk assigned is of the greatest value in counseling the patient. For example, a woman with a screen-positive result may have a risk of 1 in 200 or a risk of 1 in 10.
These patient-specific risks are extremely accurate. Thus, a woman with the lower risk (ie, 1 in 200) may choose to forego invasive testing, whereas a woman with the much higher risk (1 in 10) may want definitive diagnosis.
Similarly, women with screen-negative results can have very different risks—as low as 1 in 50,000 or less and as high as being almost screen-positive. Again, it is helpful to counsel each woman using the specific risk calculated for her.
Clinical guidelines
Clinicians have an obligation to help pregnant women choose the best and safest options in diagnosis and treatment.It is not enough to simply offer a menu of therapies or tests and let the patient choose. A clinician would never let the patient decide which medications are safest and most effective, and the same should hold true for screening tests.
The following guidelines may help the clinician and patient make the most informed decision:
- If nuchal translucency ultrasound is available and the gravida presents by 11 to 13 gestational weeks, the integrated test is the safest, most effective screening method to assess Down syndrome risk.
- If a woman wants the earliest prenatal diagnosis, first-trimester combined screening, using nuchal translucency and serum markers, is appropriate.
- If nuchal translucency is unavailable, the serumonly version of the integrated test is the best screening method.
- If a woman presents after 13 weeks’ gestation, the second-trimester quad marker test is best.
Each hybrid test makes sense in theory. However, no one knows yet whether they will work as anticipated once they are implemented clinically, and the appropriate risk cutoffs have not yet been determined. It also is unclear whether women whose risks fall on the edge of the various groupings would be interested in waiting for the integrated test to be completed.
These tests remain investigational.
Dr. Canick is a consultant to and receives grant/research support from Diagnostic Systems. He holds patents on use of estriol in prenatal screening (nos. 5506150 and 5605843).
1. Malone FD, Canick JA, Ball RH, et al. A comparison of first trimester screening, second trimester screening, and the combination of both for evaluation of risk of Down syndrome. N Engl J Med. 2005;353:2001-2011.
2. Wald NJ, Rodeck C, et al. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003;10:56-104.
3. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM. Maternal serum screening for Down syndrome in the United States: a 1995 survey. Am J Obstet Gynecol. 1997;176:1046-1051.
4. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome [published corrections appear in J Med Screen. 1998;5:110; J Med Screen. 1998;5:166]. J Med Screen. 1997;4:181-246.
5. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down’s syndrome. Prenat Diagn. 1997;17:821-829.
6. Malone FD, D’Alton ME. Society for Maternal-Fetal Medicine. First-trimester sonographic screening for Down syndrome. Obstet Gynecol. 2003;102:1066-1079.
7. Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341:461-467.
8. Canick JA, Kellner LH. First trimester screening for aneuploidy: serum biochemical markers. Semin Perinatol. 1999;23:359-368.
9. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10–13 weeks of pregnancy. Prenat Diagn. 1998;18:281-286.
10. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Br J Obstet Gynaecol. 2004;111:521-531.
11. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Trial Research Consortium. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005;106:288-294.
12. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Research Consortium. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004;104:1222-1228.
13. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446-1451.
14. Dugoff L, Hobbins JC, Malone FD, et al. for the FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260-267.
15. Maymon R, Betser M, Dreazen E, et al. A model for disclosing the first trimester part of an integrated Down’s syndrome screening test. Clin Genet. 2004;65:113-119.
16. Wright D, Bradbury I, Benn P, Cuckle H, Ritchie K. Contingent screening for Down syndrome is an efficient alternative to non-disclosure sequential screening. Prenat Diagn. 2004;24:762-766.
CASE-BASED LEARNING
Her risk is high, but how high? Is invasive testing the only answer?
Mrs. S, a 37-year-old primigravida, has an age-related risk of having a baby with Down syndrome of 1 in 250. Before deciding whether to undergo an invasive diagnostic procedure based on her age alone, she wants to learn more about her risk by having a prenatal screening test. The 2 safest and most informative options: first-trimester combined screening and fully integrated screening. About 5% of women who have the first test are found to be high-risk, and the test identifies about 85% of all cases of Down syndrome. As for fully integrated screening, it identifies about 85% of all Down syndrome cases at a 1% screen-positive rate. Since it offers a faster result, Mrs. S opts for first-trimester screening.
At 11 weeks, 0 days (according to crown-rump length), she undergoes nuchal translucency imaging and has a blood sample drawn to measure pregnancy-associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG). Her test results are reported in multiples of the median (MoM):
| Nuchal translucency | 2.3 mm | 1.62 MoM |
| PAPP-A | 0.5 mIU/mL | 0.79 MoM |
| hCG | 45 IU/mL | 1.25 MoM |
These values suggest that Mrs. S has a risk of having a pregnancy affected by Down syndrome of 1 in 170. In other words, her results are screen-positive.
Can her risk be more precisely quantified without invasive testing?
Had Mrs. S chosen fully integrated screening, the answer to that question would be yes, but it would have meant waiting until the second trimester for the result.
This article describes the 2 screening approaches—first-trimester combined screening and fully integrated screening—as well as the serum-only variant of the integrated test and the established quad marker test. Also discussed are the findings of recent studies, including 2 key trials:
- the First and Second Trimester Evaluation of Risk (FASTER) of aneuploidy trial, published in November1
- the Serum, Urine, and Ultrasound Screening Study (SURUSS), published in 2003 in the United Kingdom2
These 2 trials are the only ones to compare screening markers at different times during pregnancy in the same women—the only way to fairly assess the quality of various marker combinations within and across gestational weeks.
How good is current practice?
In 1995, about 2.5 million of the approximately 4 million gravidas in the United States had maternal serum screening for Down syndrome and open neural tube defects.3 Today, this practice usually involves a serum sample drawn early during the second trimester (15–20 weeks), measurement of 3 or 4 serum markers (the triple or quad test), and calculation and reporting of risk.
Second-trimester serum screening is a relatively easy procedure involving a single blood sample and established risk-calculation methods. Further, the follow-up when a woman is screen-positive—ie, at increased risk—is clear: amniocentesis in the case of Down syndrome risk and targeted ultrasound in the case of open neural tube defects (with amniocentesis backup). For the triple test, we can expect a detection rate of about 70%, and for the quad test, 80%, by identifying 5% of screened women with the highest calculated risk (the effective screen-positive rate).1,2,4
Why the newer options are better
Optimally, prenatal screening should minimize the number of women identified as screen-positive (ie, women at sufficient risk to be offered amniocentesis or comparable procedures) while maximizing the overall detection rate. This point is important because screen-positive status leads to follow-up diagnostic procedures that are necessarily invasive and risky.
First-trimester screening slightly better than the quad test
The option Mrs. S selected entails measuring 3 markers during the late first trimester (11–13 gestational weeks): nuchal translucency, PAPP-A, and hCG or its free βsubunit. These markers constitute a screening test that performs as well as, or slightly better than, the second-trimester quad test. The best estimate of first-trimester screening is an 85% detection rate at a 5% screen-positive rate (compared with about 80% detection rate at a 5% screen-positive rate for the quad test).1,2,5,6
Serum markers or ultrasound alone not enough in first trimester
Two serum markers together, without nuchal translucency, or nuchal translucency alone, without the serum markers, do not constitute a sufficient first-trimester screening test, since they each detect about 60% to 65% of Down syndrome cases with a 5% false-positive rate. This is clearly inferior to the best we can do during the second trimester (about 80% detection rate for a 5% false-positive rate). Only when nuchal translucency and serum markers are used together is first-trimester screening a viable option.
Timing is important in integrated screening
For the integrated screening option, instead of requiring that screening be offered in the late first or early second trimester, each marker is measured when it is most informative. The optimal time for nuchal translucency and PAPP-A measurement is at 10 to 11 weeks, while the optimal time for the measurement of hCG (or its free β subunit), inhibin A, alpha fetoprotein (AFP), and unconjugated estriol (uE3) is at 15 to 20 weeks.
Therefore, the integrated test is accomplished in 2 steps. At about 11 weeks, a woman undergoes nuchal translucency ultrasound imaging and has a blood sample drawn for PAPP-A measurement. At about 15 weeks (the earlier in the second-trimester window the better), she has a second sample drawn for measurement of the quad markers. A risk report then is generated, using all 6 markers to calculate the woman’s new risk. Such a test has to be superior to any test that uses fewer markers or the same markers at less than the optimal time.
The integrated test can also be carried out without nuchal translucency, by measuring PAPP-A during the first trimester and the quad serum markers during the second trimester, for an estimated detection rate of 85% with a 5% false-positive rate.
Integrated option has 1% screen-positive rate
Integrated screening reduces the screen-positive rate by as much as fourfold—to 1% or less. That is, only 1 in 100 women undergoing screening will be called screen-positive, and, in that 1%, approximately 85% of all Down syndrome pregnancies will be found.1,2,7
First-trimester serum markers
The most informative serum marker during the first trimester is PAPP-A, a large glycoprotein complex made by the placenta. In pregnancies affected by Down syndrome, PAPP-A levels tend to be low: about 0.4 MoM on average, or about 2.5 times lower than in unaffected pregnancies.
The second most commonly used serum marker is the free βsubunit of hCG, which is, on average, 1.8 MoM in pregnancies affected by Down syndrome, or almost twice as high as in normal pregnancies.4,8 Studies indicate that hCG and inhibin A are also effective serum markers during the late first trimester, providing screening performance equivalent to that of the free β-hCG when combined with nuchal translucency and PAPP-A.4
Nuchal translucency: A powerful marker
Both the fully integrated and first-trimester screening approaches necessitate ultrasound measurement of nuchal translucency, which is always measured along with fetal crown-rump length. The nuchal translucency value—initially measured in tenths of millimeters—then is normalized for gestational age based on crown-rump length, and reported in multiples of the median, the same unit used to normalize serum screening markers.
FIGURE 1 shows how nuchal translucency values (in millimeters) measured in a general population increase with gestation. The most commonly accepted period of gestation to measure nuchal translucency is between 10 and 13 completed weeks.
Why nuchal translucency is more informative
Nuchal translucency values tend to be increased in Down syndrome pregnancies, as are certain serum markers such as hCG or its free β subunit and inhibin A. However, nuchal translucency is more informative than these markers because there is less overlap between Down syndrome and unaffected case values. This is not because nuchal translucency values tend to be higher in affected pregnancies. In fact, all 3 markers are, on average, about twice as high in cases of Down syndrome as in controls. However, because the distribution of nuchal translucency values in unaffected fetuses is much narrower (or tighter) than is true for hCG (or its free β subunit) or inhibin A, very few unaffected fetuses have increased nuchal translucency values. Therefore, when nuchal translucency is elevated, it is more likely to be associated with an affected pregnancy than is either of the other 2 markers.
FIGURE 2 shows the overlapping distributions in cases and controls for both hCG and nuchal translucency. In unaffected pregnancies, the distribution of values centers around 1 MoM, while in Down syndrome pregnancies, the values center around 2 MoM. About 8% of hCG values in unaffected pregnancies exceed 2 MoM, but only about 1.5% of nuchal translucency values do. Thus, for a detection rate of 50%, the false-positive rate using nuchal translucency is 1.5%, much smaller than the false-positive rate of 8% using hCG.
Imaging expertise is key
While it is beyond the scope of this article to detail the methodology of nuchal translucency measurement, specialized training and ongoing quality assurance are necessary to get the measurement right. Both the Society for Maternal-Fetal Medicine in the United States and the Fetal Medicine Foundation in the United Kingdom provide training and credentialing in nuchal translucency sonography. If performed correctly, it is an excellent screening marker. However, if attempted with no hands-on training, this imaging method yields unreliable results.
FIGURE 1 Nuchal translucency values increase with gestational age
Nuchal translucency values, in millimeters, in 561 pregnancies between 10 and 13 completed weeks of gestation, as estimated by crown-rump length. Reprinted with permission from Drs. Wald and Schucter; data from Schuchter K, et al.9
FIGURE 2 Very few unaffected fetuses have elevated nuchal translucency values
Distribution of values of second-trimester human chorionic gonadotropin (hCG) and first-trimester nuchal translucency among unaffected and Down syndrome pregnancies, both given in multiples of the median (MoM). Note that the unaffected nuchal translucency distribution is much narrower and taller than the unaffected hCG distribution. The scale is a log progression of increasing MoM values because both markers are log-normally distributed in unaffected and Down syndrome gestations. DR=detection rate; FPR=false-positive rate; NT=nuchal translucency.
2 important studies
The SURUSS trial, conducted mainly in the United Kingdom, was an observational study in which all women underwent first-trimester ultrasound measurement of nuchal translucency, as well as first- and second-trimester blood and urine sampling, with all samples stored. After all outcomes were chronicled, case-control sets of first- and second-trimester samples were constructed and assayed for a wide variety of known and potential screening markers. Nuchal translucency data also were analyzed. More than 48,000 women were enrolled, and 101 Down syndrome pregnancies were identified and studied. 2,10
The FASTER trial, completed more recently, was an observational study conducted at 15 enrollment centers in the US and involving more than 38,000 women, among whom 117 Down syndrome cases were identified.1 All women in the trial underwent first-trimester ultrasound examination and blood sampling between 10 weeks 3 days, and 13 weeks 6 days, and were asked to return for a second-trimester blood draw between 15 and 18 completed weeks, after which a report was issued detailing the separate results. In addition, combinations of markers across the trimesters were modeled and compared.
Perhaps the most interesting and surprising finding from the 2 studies is the remarkable similarity of results (TABLE). For 2 large populations on separate continents with a different ethnic mix, the primary findings were almost identical:
- 86% to 87% detection rate at a 5% false-positive rate for first-trimester combined screening (nuchal translucency, PAPP-A, and free β-hCG) and 80% to 83% detection rate at a 5% false-positive rate for second-trimester quad screening (AFP, uE3 , hCG, and inhibin A). In both trials, first-trimester screening was incrementally better than second-trimester screening, but the difference was not statistically significant.
- For integrated screening, a detection rate of 86% to 88% at a false-positive rate of 1%. Nuchal translucency and PAPP-A were measured during the first trimester, and quad markers during the second trimester. At a 5% false-positive rate, the detection rate in both trials was about 95%. With a serum-only integrated test, detection rates were 87% to 88% with a 5% false-positive rate.
- Nuchal translucency was more informative than any of the serum markers tested. This corroborates the rich literature on nuchal translucency published over the past decade. Both trials demonstrated that nuchal translucency measurement is effective when training is adequate, and that ongoing monitoring of quality is essential. However, in both studies, a satisfactory nuchal translucency measurement was not attained in about 7% of all women scanned between 10 and 13 weeks, so a small but significant number of women will not have nuchal translucency included in their risk assessment.
Other notable FASTER findings
Other findings from the FASTER trial that merit special attention:
- An ultrasound finding of cystic hygroma warrants an immediate prenatal diagnostic workup and was associated with an aneuploidy rate of 50% (one third of which was Down syndrome) and adverse outcomes in the great majority of cases.11 Cystic hygroma is uncommon, occurring in about 1 in 300 pregnancies in the first trimester.
- The value of the fetal nasal bone as a first-trimester ultrasound marker is unclear. In the FASTER trial, the nasal bone was studied in about 6,000 of the 38,000 ultrasound examinations, with no detected benefit. Thus, in a nonselected pregnant population, the nasal bone may not be a reasonable marker.12
- Various first- and second-trimester markers are modestly informative about adverse pregnancy outcomes other than aneuploidy (eg, fetal growth restriction, early delivery, and preeclampsia). Only a small proportion of affected pregnancies will be identified by these markers, alone or in combination.13,14
TABLE
5 screening approaches: What the SURUSS and FASTER trials reveal
| TEST | NUCHAL TRANSLUCENCY MEASURED? | METHOD OF PRENATAL DIAGNOSIS | DETECTION RATE (%) AT 1% FALSE-POSITIVE RATE | DETECTION RATE (%) AT 5% FALSE-POSITIVE RATE | FALSE-POSITIVE RATE* (%) TO ACHIEVE: | |||
|---|---|---|---|---|---|---|---|---|
| SURUSS | FASTER | SURUSS | FASTER | 85% DETECTION | 95% DETECTION | |||
| 2nd-trimester triple marker | No | 2nd-trimester amniocentesis | 56 | 45 | 77 | 70 | 14 | 32 |
| 2nd-trimester quad marker | No | 2nd-trimester amniocentesis | 64 | 60 | 83 | 80 | 7.3 | 22 |
| 1st-trimester combined | Yes | 1st-trimester chorionic villus sampling | 72 | 73 | 86 | 87 | 3.8 | 18 |
| Serum integrated test | No | 2nd-trimester amniocentesis | 73 | 73 | 87 | 88 | 3.6 | 15 |
| Full integrated test | Yes | 2nd-trimester amniocentesis | 86 | 88 | 94 | 96 | 0.6 | 4 |
| FASTER=First- and Second-Trimester Evaluation of Risk[1]; SURUSS=Serum, Urine, and Ultrasound Screening Study.2 | ||||||||
| *Based on data from FASTER trial only. | ||||||||
Clinical considerations
Integrated screening
A number of considerations are important:
Tell the patient screening for neural tube defects is included in the integrated test, since it spans the first and second trimesters and includes AFP as one of the markers measured. Thus, women having the integrated test will also be screened for open spina bifida and anencephaly, in addition to Down syndrome.
Hold individual measurements until all results are in. First-trimester nuchal translucency and PAPP-A results are the first to become available. After these tests are performed, a waiting period of about 2 to 5 weeks is required to allow for second-trimester testing, tabulation, and integration into a single risk estimate.
In the case of ultrasound imaging, it is important for the sonographer to explain what is being measured without conveying special import regarding the nuchal translucency measurement—whether it is large or small. If asked, the sonographer should explain that nuchal translucency is only 1 of 6 measures that will determine the patient’s risk.
Advise the patient that definitive diagnosis will not occur until the second trimester. Because the integrated test is reported after the second-trimester serum sample is drawn and assayed, any follow-up diagnostic testing will not be available any sooner than is typical for second-trimester screening (ie, 16–18 gestational weeks).
A very large nuchal translucency measurement may be cause for concern and points to the need for early diagnosis. If a woman having the integrated test is found to have a nuchal translucency measurement of 3 to 4 mm or more (or any cystic hygroma), a clinically reasonable strategy is to offer immediate prenatal diagnosis by CVS rather than continue with the screening test.
Nuchal translucency values of 3 or 4 mm or more are seen in fewer than 1% of women scanned, and are associated with a very high risk of fetal aneuploidy and adverse pregnancy outcomes.
If nuchal translucency ultrasound is not available in your region, the integrated test using serum markers will provide better screening performance than any other serum-only test.
First-trimester combined screening
Offer it as early as possible. The benefit of first-trimester screening is the prospect of early prenatal diagnosis. Therefore, the earlier the test is offered within the accepted time frame of 11 to 13 completed weeks, the more apparent the benefit.
Early screening requires nuchal translucency measurement. First-trimester screening involves the measurement of serum analytes and ultrasound measurement of nuchal translucency. Serum markers without nuchal translucency or nuchal translucency without serum markers provide insufficient screening. If nuchal translucency is unavailable, offer the serum-only form of the integrated test or the second-trimester quad marker test.
Early diagnosis is requisite. Patients who are screen-positive in the first trimester should have CVS as an option for the earliest possible diagnosis. If amniocentesis at 15 weeks or beyond is the only invasive diagnostic procedure available, first-trimester combined screening is not appropriate.
An additional screen for neural tube defects is needed. First-trimester combined screening does not test for risk of open neural tube defects. Most commonly, a second-trimester serum AFP measurement is recommended for all women who have had first-trimester screening. Alternatively, a second-trimester ultrasound scan for fetal anomalies is highly indicative of neural tube defects if it includes the cranial lemon and cerebellar banana signs.
Even newer choices
Within the past year, 2 new screening methods have been proposed: sequential testing and contingent testing.15,16 They are essentially hybrids of the first-trimester and integrated tests. Both identify a very small, very high-risk group based on first-trimester nuchal translucency and serum markers. This group (eg, women having a risk of 1 in 25 and higher) would account for less than 1% of the total screened population and would ultimately be found to have more than 50% of Down syndrome cases.
In sequential testing, all women whose risk is less than the high-risk cutoff (eg, a possible cutoff of less than 1 in 25 or higher) would go on to have the full integrated test. In contingent testing, a second group would be identified as very low-risk based on first-trimester markers (eg, a possible risk cutoff of 1 in 2,000 or lower). Such low-risk women would have a small chance of having their results become high-risk based on the completed integrated test; therefore, they would be identified as screen-negative early and would not have to go on to integrated testing. In contingent testing, only the intermediate group (eg, those between, say, 1 in 25 and 1 in 2,000) would complete the integrated test.
The terms screen-negative and screen-positive are applied to test results to convey the most accurate balance between detection and false-positive rates. However, these terms indicate only risk categories; within these categories, the specific risk assigned is of the greatest value in counseling the patient. For example, a woman with a screen-positive result may have a risk of 1 in 200 or a risk of 1 in 10.
These patient-specific risks are extremely accurate. Thus, a woman with the lower risk (ie, 1 in 200) may choose to forego invasive testing, whereas a woman with the much higher risk (1 in 10) may want definitive diagnosis.
Similarly, women with screen-negative results can have very different risks—as low as 1 in 50,000 or less and as high as being almost screen-positive. Again, it is helpful to counsel each woman using the specific risk calculated for her.
Clinical guidelines
Clinicians have an obligation to help pregnant women choose the best and safest options in diagnosis and treatment.It is not enough to simply offer a menu of therapies or tests and let the patient choose. A clinician would never let the patient decide which medications are safest and most effective, and the same should hold true for screening tests.
The following guidelines may help the clinician and patient make the most informed decision:
- If nuchal translucency ultrasound is available and the gravida presents by 11 to 13 gestational weeks, the integrated test is the safest, most effective screening method to assess Down syndrome risk.
- If a woman wants the earliest prenatal diagnosis, first-trimester combined screening, using nuchal translucency and serum markers, is appropriate.
- If nuchal translucency is unavailable, the serumonly version of the integrated test is the best screening method.
- If a woman presents after 13 weeks’ gestation, the second-trimester quad marker test is best.
Each hybrid test makes sense in theory. However, no one knows yet whether they will work as anticipated once they are implemented clinically, and the appropriate risk cutoffs have not yet been determined. It also is unclear whether women whose risks fall on the edge of the various groupings would be interested in waiting for the integrated test to be completed.
These tests remain investigational.
Dr. Canick is a consultant to and receives grant/research support from Diagnostic Systems. He holds patents on use of estriol in prenatal screening (nos. 5506150 and 5605843).
CASE-BASED LEARNING
Her risk is high, but how high? Is invasive testing the only answer?
Mrs. S, a 37-year-old primigravida, has an age-related risk of having a baby with Down syndrome of 1 in 250. Before deciding whether to undergo an invasive diagnostic procedure based on her age alone, she wants to learn more about her risk by having a prenatal screening test. The 2 safest and most informative options: first-trimester combined screening and fully integrated screening. About 5% of women who have the first test are found to be high-risk, and the test identifies about 85% of all cases of Down syndrome. As for fully integrated screening, it identifies about 85% of all Down syndrome cases at a 1% screen-positive rate. Since it offers a faster result, Mrs. S opts for first-trimester screening.
At 11 weeks, 0 days (according to crown-rump length), she undergoes nuchal translucency imaging and has a blood sample drawn to measure pregnancy-associated plasma protein A (PAPP-A) and human chorionic gonadotropin (hCG). Her test results are reported in multiples of the median (MoM):
| Nuchal translucency | 2.3 mm | 1.62 MoM |
| PAPP-A | 0.5 mIU/mL | 0.79 MoM |
| hCG | 45 IU/mL | 1.25 MoM |
These values suggest that Mrs. S has a risk of having a pregnancy affected by Down syndrome of 1 in 170. In other words, her results are screen-positive.
Can her risk be more precisely quantified without invasive testing?
Had Mrs. S chosen fully integrated screening, the answer to that question would be yes, but it would have meant waiting until the second trimester for the result.
This article describes the 2 screening approaches—first-trimester combined screening and fully integrated screening—as well as the serum-only variant of the integrated test and the established quad marker test. Also discussed are the findings of recent studies, including 2 key trials:
- the First and Second Trimester Evaluation of Risk (FASTER) of aneuploidy trial, published in November1
- the Serum, Urine, and Ultrasound Screening Study (SURUSS), published in 2003 in the United Kingdom2
These 2 trials are the only ones to compare screening markers at different times during pregnancy in the same women—the only way to fairly assess the quality of various marker combinations within and across gestational weeks.
How good is current practice?
In 1995, about 2.5 million of the approximately 4 million gravidas in the United States had maternal serum screening for Down syndrome and open neural tube defects.3 Today, this practice usually involves a serum sample drawn early during the second trimester (15–20 weeks), measurement of 3 or 4 serum markers (the triple or quad test), and calculation and reporting of risk.
Second-trimester serum screening is a relatively easy procedure involving a single blood sample and established risk-calculation methods. Further, the follow-up when a woman is screen-positive—ie, at increased risk—is clear: amniocentesis in the case of Down syndrome risk and targeted ultrasound in the case of open neural tube defects (with amniocentesis backup). For the triple test, we can expect a detection rate of about 70%, and for the quad test, 80%, by identifying 5% of screened women with the highest calculated risk (the effective screen-positive rate).1,2,4
Why the newer options are better
Optimally, prenatal screening should minimize the number of women identified as screen-positive (ie, women at sufficient risk to be offered amniocentesis or comparable procedures) while maximizing the overall detection rate. This point is important because screen-positive status leads to follow-up diagnostic procedures that are necessarily invasive and risky.
First-trimester screening slightly better than the quad test
The option Mrs. S selected entails measuring 3 markers during the late first trimester (11–13 gestational weeks): nuchal translucency, PAPP-A, and hCG or its free βsubunit. These markers constitute a screening test that performs as well as, or slightly better than, the second-trimester quad test. The best estimate of first-trimester screening is an 85% detection rate at a 5% screen-positive rate (compared with about 80% detection rate at a 5% screen-positive rate for the quad test).1,2,5,6
Serum markers or ultrasound alone not enough in first trimester
Two serum markers together, without nuchal translucency, or nuchal translucency alone, without the serum markers, do not constitute a sufficient first-trimester screening test, since they each detect about 60% to 65% of Down syndrome cases with a 5% false-positive rate. This is clearly inferior to the best we can do during the second trimester (about 80% detection rate for a 5% false-positive rate). Only when nuchal translucency and serum markers are used together is first-trimester screening a viable option.
Timing is important in integrated screening
For the integrated screening option, instead of requiring that screening be offered in the late first or early second trimester, each marker is measured when it is most informative. The optimal time for nuchal translucency and PAPP-A measurement is at 10 to 11 weeks, while the optimal time for the measurement of hCG (or its free β subunit), inhibin A, alpha fetoprotein (AFP), and unconjugated estriol (uE3) is at 15 to 20 weeks.
Therefore, the integrated test is accomplished in 2 steps. At about 11 weeks, a woman undergoes nuchal translucency ultrasound imaging and has a blood sample drawn for PAPP-A measurement. At about 15 weeks (the earlier in the second-trimester window the better), she has a second sample drawn for measurement of the quad markers. A risk report then is generated, using all 6 markers to calculate the woman’s new risk. Such a test has to be superior to any test that uses fewer markers or the same markers at less than the optimal time.
The integrated test can also be carried out without nuchal translucency, by measuring PAPP-A during the first trimester and the quad serum markers during the second trimester, for an estimated detection rate of 85% with a 5% false-positive rate.
Integrated option has 1% screen-positive rate
Integrated screening reduces the screen-positive rate by as much as fourfold—to 1% or less. That is, only 1 in 100 women undergoing screening will be called screen-positive, and, in that 1%, approximately 85% of all Down syndrome pregnancies will be found.1,2,7
First-trimester serum markers
The most informative serum marker during the first trimester is PAPP-A, a large glycoprotein complex made by the placenta. In pregnancies affected by Down syndrome, PAPP-A levels tend to be low: about 0.4 MoM on average, or about 2.5 times lower than in unaffected pregnancies.
The second most commonly used serum marker is the free βsubunit of hCG, which is, on average, 1.8 MoM in pregnancies affected by Down syndrome, or almost twice as high as in normal pregnancies.4,8 Studies indicate that hCG and inhibin A are also effective serum markers during the late first trimester, providing screening performance equivalent to that of the free β-hCG when combined with nuchal translucency and PAPP-A.4
Nuchal translucency: A powerful marker
Both the fully integrated and first-trimester screening approaches necessitate ultrasound measurement of nuchal translucency, which is always measured along with fetal crown-rump length. The nuchal translucency value—initially measured in tenths of millimeters—then is normalized for gestational age based on crown-rump length, and reported in multiples of the median, the same unit used to normalize serum screening markers.
FIGURE 1 shows how nuchal translucency values (in millimeters) measured in a general population increase with gestation. The most commonly accepted period of gestation to measure nuchal translucency is between 10 and 13 completed weeks.
Why nuchal translucency is more informative
Nuchal translucency values tend to be increased in Down syndrome pregnancies, as are certain serum markers such as hCG or its free β subunit and inhibin A. However, nuchal translucency is more informative than these markers because there is less overlap between Down syndrome and unaffected case values. This is not because nuchal translucency values tend to be higher in affected pregnancies. In fact, all 3 markers are, on average, about twice as high in cases of Down syndrome as in controls. However, because the distribution of nuchal translucency values in unaffected fetuses is much narrower (or tighter) than is true for hCG (or its free β subunit) or inhibin A, very few unaffected fetuses have increased nuchal translucency values. Therefore, when nuchal translucency is elevated, it is more likely to be associated with an affected pregnancy than is either of the other 2 markers.
FIGURE 2 shows the overlapping distributions in cases and controls for both hCG and nuchal translucency. In unaffected pregnancies, the distribution of values centers around 1 MoM, while in Down syndrome pregnancies, the values center around 2 MoM. About 8% of hCG values in unaffected pregnancies exceed 2 MoM, but only about 1.5% of nuchal translucency values do. Thus, for a detection rate of 50%, the false-positive rate using nuchal translucency is 1.5%, much smaller than the false-positive rate of 8% using hCG.
Imaging expertise is key
While it is beyond the scope of this article to detail the methodology of nuchal translucency measurement, specialized training and ongoing quality assurance are necessary to get the measurement right. Both the Society for Maternal-Fetal Medicine in the United States and the Fetal Medicine Foundation in the United Kingdom provide training and credentialing in nuchal translucency sonography. If performed correctly, it is an excellent screening marker. However, if attempted with no hands-on training, this imaging method yields unreliable results.
FIGURE 1 Nuchal translucency values increase with gestational age
Nuchal translucency values, in millimeters, in 561 pregnancies between 10 and 13 completed weeks of gestation, as estimated by crown-rump length. Reprinted with permission from Drs. Wald and Schucter; data from Schuchter K, et al.9
FIGURE 2 Very few unaffected fetuses have elevated nuchal translucency values
Distribution of values of second-trimester human chorionic gonadotropin (hCG) and first-trimester nuchal translucency among unaffected and Down syndrome pregnancies, both given in multiples of the median (MoM). Note that the unaffected nuchal translucency distribution is much narrower and taller than the unaffected hCG distribution. The scale is a log progression of increasing MoM values because both markers are log-normally distributed in unaffected and Down syndrome gestations. DR=detection rate; FPR=false-positive rate; NT=nuchal translucency.
2 important studies
The SURUSS trial, conducted mainly in the United Kingdom, was an observational study in which all women underwent first-trimester ultrasound measurement of nuchal translucency, as well as first- and second-trimester blood and urine sampling, with all samples stored. After all outcomes were chronicled, case-control sets of first- and second-trimester samples were constructed and assayed for a wide variety of known and potential screening markers. Nuchal translucency data also were analyzed. More than 48,000 women were enrolled, and 101 Down syndrome pregnancies were identified and studied. 2,10
The FASTER trial, completed more recently, was an observational study conducted at 15 enrollment centers in the US and involving more than 38,000 women, among whom 117 Down syndrome cases were identified.1 All women in the trial underwent first-trimester ultrasound examination and blood sampling between 10 weeks 3 days, and 13 weeks 6 days, and were asked to return for a second-trimester blood draw between 15 and 18 completed weeks, after which a report was issued detailing the separate results. In addition, combinations of markers across the trimesters were modeled and compared.
Perhaps the most interesting and surprising finding from the 2 studies is the remarkable similarity of results (TABLE). For 2 large populations on separate continents with a different ethnic mix, the primary findings were almost identical:
- 86% to 87% detection rate at a 5% false-positive rate for first-trimester combined screening (nuchal translucency, PAPP-A, and free β-hCG) and 80% to 83% detection rate at a 5% false-positive rate for second-trimester quad screening (AFP, uE3 , hCG, and inhibin A). In both trials, first-trimester screening was incrementally better than second-trimester screening, but the difference was not statistically significant.
- For integrated screening, a detection rate of 86% to 88% at a false-positive rate of 1%. Nuchal translucency and PAPP-A were measured during the first trimester, and quad markers during the second trimester. At a 5% false-positive rate, the detection rate in both trials was about 95%. With a serum-only integrated test, detection rates were 87% to 88% with a 5% false-positive rate.
- Nuchal translucency was more informative than any of the serum markers tested. This corroborates the rich literature on nuchal translucency published over the past decade. Both trials demonstrated that nuchal translucency measurement is effective when training is adequate, and that ongoing monitoring of quality is essential. However, in both studies, a satisfactory nuchal translucency measurement was not attained in about 7% of all women scanned between 10 and 13 weeks, so a small but significant number of women will not have nuchal translucency included in their risk assessment.
Other notable FASTER findings
Other findings from the FASTER trial that merit special attention:
- An ultrasound finding of cystic hygroma warrants an immediate prenatal diagnostic workup and was associated with an aneuploidy rate of 50% (one third of which was Down syndrome) and adverse outcomes in the great majority of cases.11 Cystic hygroma is uncommon, occurring in about 1 in 300 pregnancies in the first trimester.
- The value of the fetal nasal bone as a first-trimester ultrasound marker is unclear. In the FASTER trial, the nasal bone was studied in about 6,000 of the 38,000 ultrasound examinations, with no detected benefit. Thus, in a nonselected pregnant population, the nasal bone may not be a reasonable marker.12
- Various first- and second-trimester markers are modestly informative about adverse pregnancy outcomes other than aneuploidy (eg, fetal growth restriction, early delivery, and preeclampsia). Only a small proportion of affected pregnancies will be identified by these markers, alone or in combination.13,14
TABLE
5 screening approaches: What the SURUSS and FASTER trials reveal
| TEST | NUCHAL TRANSLUCENCY MEASURED? | METHOD OF PRENATAL DIAGNOSIS | DETECTION RATE (%) AT 1% FALSE-POSITIVE RATE | DETECTION RATE (%) AT 5% FALSE-POSITIVE RATE | FALSE-POSITIVE RATE* (%) TO ACHIEVE: | |||
|---|---|---|---|---|---|---|---|---|
| SURUSS | FASTER | SURUSS | FASTER | 85% DETECTION | 95% DETECTION | |||
| 2nd-trimester triple marker | No | 2nd-trimester amniocentesis | 56 | 45 | 77 | 70 | 14 | 32 |
| 2nd-trimester quad marker | No | 2nd-trimester amniocentesis | 64 | 60 | 83 | 80 | 7.3 | 22 |
| 1st-trimester combined | Yes | 1st-trimester chorionic villus sampling | 72 | 73 | 86 | 87 | 3.8 | 18 |
| Serum integrated test | No | 2nd-trimester amniocentesis | 73 | 73 | 87 | 88 | 3.6 | 15 |
| Full integrated test | Yes | 2nd-trimester amniocentesis | 86 | 88 | 94 | 96 | 0.6 | 4 |
| FASTER=First- and Second-Trimester Evaluation of Risk[1]; SURUSS=Serum, Urine, and Ultrasound Screening Study.2 | ||||||||
| *Based on data from FASTER trial only. | ||||||||
Clinical considerations
Integrated screening
A number of considerations are important:
Tell the patient screening for neural tube defects is included in the integrated test, since it spans the first and second trimesters and includes AFP as one of the markers measured. Thus, women having the integrated test will also be screened for open spina bifida and anencephaly, in addition to Down syndrome.
Hold individual measurements until all results are in. First-trimester nuchal translucency and PAPP-A results are the first to become available. After these tests are performed, a waiting period of about 2 to 5 weeks is required to allow for second-trimester testing, tabulation, and integration into a single risk estimate.
In the case of ultrasound imaging, it is important for the sonographer to explain what is being measured without conveying special import regarding the nuchal translucency measurement—whether it is large or small. If asked, the sonographer should explain that nuchal translucency is only 1 of 6 measures that will determine the patient’s risk.
Advise the patient that definitive diagnosis will not occur until the second trimester. Because the integrated test is reported after the second-trimester serum sample is drawn and assayed, any follow-up diagnostic testing will not be available any sooner than is typical for second-trimester screening (ie, 16–18 gestational weeks).
A very large nuchal translucency measurement may be cause for concern and points to the need for early diagnosis. If a woman having the integrated test is found to have a nuchal translucency measurement of 3 to 4 mm or more (or any cystic hygroma), a clinically reasonable strategy is to offer immediate prenatal diagnosis by CVS rather than continue with the screening test.
Nuchal translucency values of 3 or 4 mm or more are seen in fewer than 1% of women scanned, and are associated with a very high risk of fetal aneuploidy and adverse pregnancy outcomes.
If nuchal translucency ultrasound is not available in your region, the integrated test using serum markers will provide better screening performance than any other serum-only test.
First-trimester combined screening
Offer it as early as possible. The benefit of first-trimester screening is the prospect of early prenatal diagnosis. Therefore, the earlier the test is offered within the accepted time frame of 11 to 13 completed weeks, the more apparent the benefit.
Early screening requires nuchal translucency measurement. First-trimester screening involves the measurement of serum analytes and ultrasound measurement of nuchal translucency. Serum markers without nuchal translucency or nuchal translucency without serum markers provide insufficient screening. If nuchal translucency is unavailable, offer the serum-only form of the integrated test or the second-trimester quad marker test.
Early diagnosis is requisite. Patients who are screen-positive in the first trimester should have CVS as an option for the earliest possible diagnosis. If amniocentesis at 15 weeks or beyond is the only invasive diagnostic procedure available, first-trimester combined screening is not appropriate.
An additional screen for neural tube defects is needed. First-trimester combined screening does not test for risk of open neural tube defects. Most commonly, a second-trimester serum AFP measurement is recommended for all women who have had first-trimester screening. Alternatively, a second-trimester ultrasound scan for fetal anomalies is highly indicative of neural tube defects if it includes the cranial lemon and cerebellar banana signs.
Even newer choices
Within the past year, 2 new screening methods have been proposed: sequential testing and contingent testing.15,16 They are essentially hybrids of the first-trimester and integrated tests. Both identify a very small, very high-risk group based on first-trimester nuchal translucency and serum markers. This group (eg, women having a risk of 1 in 25 and higher) would account for less than 1% of the total screened population and would ultimately be found to have more than 50% of Down syndrome cases.
In sequential testing, all women whose risk is less than the high-risk cutoff (eg, a possible cutoff of less than 1 in 25 or higher) would go on to have the full integrated test. In contingent testing, a second group would be identified as very low-risk based on first-trimester markers (eg, a possible risk cutoff of 1 in 2,000 or lower). Such low-risk women would have a small chance of having their results become high-risk based on the completed integrated test; therefore, they would be identified as screen-negative early and would not have to go on to integrated testing. In contingent testing, only the intermediate group (eg, those between, say, 1 in 25 and 1 in 2,000) would complete the integrated test.
The terms screen-negative and screen-positive are applied to test results to convey the most accurate balance between detection and false-positive rates. However, these terms indicate only risk categories; within these categories, the specific risk assigned is of the greatest value in counseling the patient. For example, a woman with a screen-positive result may have a risk of 1 in 200 or a risk of 1 in 10.
These patient-specific risks are extremely accurate. Thus, a woman with the lower risk (ie, 1 in 200) may choose to forego invasive testing, whereas a woman with the much higher risk (1 in 10) may want definitive diagnosis.
Similarly, women with screen-negative results can have very different risks—as low as 1 in 50,000 or less and as high as being almost screen-positive. Again, it is helpful to counsel each woman using the specific risk calculated for her.
Clinical guidelines
Clinicians have an obligation to help pregnant women choose the best and safest options in diagnosis and treatment.It is not enough to simply offer a menu of therapies or tests and let the patient choose. A clinician would never let the patient decide which medications are safest and most effective, and the same should hold true for screening tests.
The following guidelines may help the clinician and patient make the most informed decision:
- If nuchal translucency ultrasound is available and the gravida presents by 11 to 13 gestational weeks, the integrated test is the safest, most effective screening method to assess Down syndrome risk.
- If a woman wants the earliest prenatal diagnosis, first-trimester combined screening, using nuchal translucency and serum markers, is appropriate.
- If nuchal translucency is unavailable, the serumonly version of the integrated test is the best screening method.
- If a woman presents after 13 weeks’ gestation, the second-trimester quad marker test is best.
Each hybrid test makes sense in theory. However, no one knows yet whether they will work as anticipated once they are implemented clinically, and the appropriate risk cutoffs have not yet been determined. It also is unclear whether women whose risks fall on the edge of the various groupings would be interested in waiting for the integrated test to be completed.
These tests remain investigational.
Dr. Canick is a consultant to and receives grant/research support from Diagnostic Systems. He holds patents on use of estriol in prenatal screening (nos. 5506150 and 5605843).
1. Malone FD, Canick JA, Ball RH, et al. A comparison of first trimester screening, second trimester screening, and the combination of both for evaluation of risk of Down syndrome. N Engl J Med. 2005;353:2001-2011.
2. Wald NJ, Rodeck C, et al. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003;10:56-104.
3. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM. Maternal serum screening for Down syndrome in the United States: a 1995 survey. Am J Obstet Gynecol. 1997;176:1046-1051.
4. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome [published corrections appear in J Med Screen. 1998;5:110; J Med Screen. 1998;5:166]. J Med Screen. 1997;4:181-246.
5. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down’s syndrome. Prenat Diagn. 1997;17:821-829.
6. Malone FD, D’Alton ME. Society for Maternal-Fetal Medicine. First-trimester sonographic screening for Down syndrome. Obstet Gynecol. 2003;102:1066-1079.
7. Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341:461-467.
8. Canick JA, Kellner LH. First trimester screening for aneuploidy: serum biochemical markers. Semin Perinatol. 1999;23:359-368.
9. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10–13 weeks of pregnancy. Prenat Diagn. 1998;18:281-286.
10. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Br J Obstet Gynaecol. 2004;111:521-531.
11. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Trial Research Consortium. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005;106:288-294.
12. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Research Consortium. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004;104:1222-1228.
13. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446-1451.
14. Dugoff L, Hobbins JC, Malone FD, et al. for the FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260-267.
15. Maymon R, Betser M, Dreazen E, et al. A model for disclosing the first trimester part of an integrated Down’s syndrome screening test. Clin Genet. 2004;65:113-119.
16. Wright D, Bradbury I, Benn P, Cuckle H, Ritchie K. Contingent screening for Down syndrome is an efficient alternative to non-disclosure sequential screening. Prenat Diagn. 2004;24:762-766.
1. Malone FD, Canick JA, Ball RH, et al. A comparison of first trimester screening, second trimester screening, and the combination of both for evaluation of risk of Down syndrome. N Engl J Med. 2005;353:2001-2011.
2. Wald NJ, Rodeck C, et al. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003;10:56-104.
3. Palomaki GE, Knight GJ, McCarthy JE, Haddow JE, Donhowe JM. Maternal serum screening for Down syndrome in the United States: a 1995 survey. Am J Obstet Gynecol. 1997;176:1046-1051.
4. Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome [published corrections appear in J Med Screen. 1998;5:110; J Med Screen. 1998;5:166]. J Med Screen. 1997;4:181-246.
5. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down’s syndrome. Prenat Diagn. 1997;17:821-829.
6. Malone FD, D’Alton ME. Society for Maternal-Fetal Medicine. First-trimester sonographic screening for Down syndrome. Obstet Gynecol. 2003;102:1066-1079.
7. Wald NJ, Watt HC, Hackshaw AK. Integrated screening for Down’s syndrome on the basis of tests performed during the first and second trimesters. N Engl J Med. 1999;341:461-467.
8. Canick JA, Kellner LH. First trimester screening for aneuploidy: serum biochemical markers. Semin Perinatol. 1999;23:359-368.
9. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10–13 weeks of pregnancy. Prenat Diagn. 1998;18:281-286.
10. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Br J Obstet Gynaecol. 2004;111:521-531.
11. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Trial Research Consortium. First-trimester septated cystic hygroma: prevalence, natural history, and pediatric outcome. Obstet Gynecol. 2005;106:288-294.
12. Malone FD, Ball RH, Nyberg DA, et al. for the FASTER Research Consortium. First-trimester nasal bone evaluation for aneuploidy in the general population. Obstet Gynecol. 2004;104:1222-1228.
13. Dugoff L, Hobbins JC, Malone FD, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER Trial). Am J Obstet Gynecol. 2004;191:1446-1451.
14. Dugoff L, Hobbins JC, Malone FD, et al. for the FASTER Trial Research Consortium. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106:260-267.
15. Maymon R, Betser M, Dreazen E, et al. A model for disclosing the first trimester part of an integrated Down’s syndrome screening test. Clin Genet. 2004;65:113-119.
16. Wright D, Bradbury I, Benn P, Cuckle H, Ritchie K. Contingent screening for Down syndrome is an efficient alternative to non-disclosure sequential screening. Prenat Diagn. 2004;24:762-766.