User login
CASE: Heavy bleeding and apprehension over hormones
Your patient, R.L., 34 years old, has been bothered by gradually worsening menorrhagia for 2 years when she schedules an appointment to discuss treatment options. She prefers to avoid surgery, if at all possible, and is reluctant to use hormonal medications because she’s concerned about side effects. Nonsteroidal anti-inflammatory drugs provide inadequate relief.
R.L. asks if there is any other agent that can reduce her menstrual blood loss.
What can you offer to her?
Heavy menstrual bleeding (menorrhagia) impairs quality of life and prompts many women such as R.L. to seek a gynecologic consultation—many of them hoping to avoid surgery.
In the United States, medical-management options for the treatment of heavy menstrual bleeding include nonsteroidal anti-inflammatory drugs (NSAIDs) and off-label use of oral contraceptives, injectable depot medroxyprogesterone acetate, and high-dose oral progestin (e.g., norethindrone acetate, 5-mg tablets). The levonorgestrel intrauterine system (LNG-IUS; Mirena) is also widely used to treat heavy menstrual bleeding in women and was approved for this indication in October 2009. FDA approval of the LNG-IUS for heavy menstrual bleeding was based on a pivotal randomized trial demonstrating high efficacy.1,2
Very soon an additional option will be available—the antifibrinolytic agent tranexamic acid (to be sold by Ferring Pharmaceuticals under the brand name Lysteda). Although the LNG-IUS remains first-line treatment for heavy menstrual bleeding, tranexamic acid is appropriate for women who need to, or prefer to, avoid hormones.
How tranexamic acid works
Women who experience heavy menstrual bleeding have an elevated level of plasminogen activators in the endometrium, enzymes that facilitate the dissolution of clots. These enzymes can be inhibited by antifibrinolytic agents such as tranexamic acid, which competitively blocks the conversion of plasminogen to plasmin, thereby reducing fibrinolysis (FIGURE).3
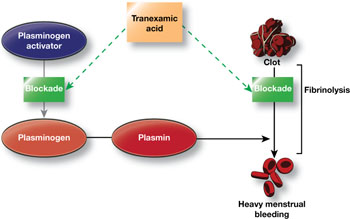
FIGURE How tranexamic acid stanches heavy menstrual bleeding
Tranexamic acid (Lysteda) competitively blocks the conversion of plasminogen to plasmin, thereby reducing fibrinolysis.Clinical trials of tranexamic acid to treat menorrhagia were first performed in the 1960s. The agent proved to be superior to placebo, NSAIDs, and cyclic oral progestin.4 However, the LNG-IUS has been found to be more effective than tranexamic acid in reducing menstrual blood loss.5
In the United States, until very recently, tranexamic acid was FDA-indicated only as an injectable agent for use at the time of dental extraction in hemophilic patients. However, an oral formulation has been widely used abroad, including in Canada, to treat heavy menstrual bleeding. In Sweden, the agent is available over the counter.
In November 2009, the FDA approved 650-mg tablets of tranexamic acid as a treatment for heavy menstrual bleeding.6
Recommended dosage
In women who have normal renal function, the recommended dosage of tranexamic acid is 1,300 mg (two 650-mg tablets) three times daily (a total of 3,900 mg) for as long as 5 days during menstruation. In women who have impaired renal function, the dosage should be adjusted according to the package insert.
In randomized clinical trials, tranexamic acid did not produce more side effects than placebo did. However, because of concerns that tranexamic acid may increase risk of venous thromboembolism (VTE), the agent is contraindicated in women who have a history of VTE.
Although women who use hormonal contraception have not been included in clinical trials of tranexamic acid, the FDA recommends that they take tranexamic acid only if there is “a strong medical need and the benefit of treatment will outweigh the potential increased risk of a thrombotic event.”6
Tranexamic acid will likely be of particular utility for women who desire treatment for heavy menstrual bleeding but who should, or would prefer to, avoid hormonal agents. British guidelines recommend that, when medical management is appropriate in the treatment of heavy menstrual bleeding, the LNG-IUS be considered first-line treatment, with tranexamic acid representing a second-line strategy.7
CASE: Resolved
When she hears about tranexamic acid, R.L. is eager to try it. She is pleased that it does not contain hormones and needs to be taken only 5 days or fewer each month. You give her a prescription and schedule a follow-up visit 3 months later, at which time she reports being satisfied with the drug.
1. FDA approves additional use for IUD Mirena to treat heavy menstrual bleeding in IUD users [press release]. Rockville, Md: FDA; Oct. 1, 2009.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 3, 2010.
2. Kaunitz AM, Bissonette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen J. Levonorgestrel intrauterine system and medroxyprogesterone acetate for treatment of heavy menstrual bleeding. Poster No. 49 Presented at Reproductive Health 2009, Association of Reproductive Health Professionals annual meeting, September 30–October 3, 2009, Los Angeles, Calif. Contraception. 2009;80(2):212.-
3. Grimes DA, Davis AR, Ramos DE. Heavy menstrual bleeding: Assessing impact, evaluating management options. OBG Management. 2009;21(10)(supple).-http://www.obgmanagement.com/article_pages.asp?filename="2206OBG_Article2" aid=7971. Accessed May 3, 2010.
4. Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(4):CD000249.-
5. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164(3):879-883.
6. FDA approves Lysteda to treat heavy menstrual bleeding [press release]. Rockville, Md: FDA; Nov. 13, 2009.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm190551.htm. Accessed May 3, 2010.
7. National Institute for Health and Clinical Excellence. Heavy menstrual bleeding. January 2007.http://www.nice.org.uk/CG44. Accessed May 3, 2010.
CASE: Heavy bleeding and apprehension over hormones
Your patient, R.L., 34 years old, has been bothered by gradually worsening menorrhagia for 2 years when she schedules an appointment to discuss treatment options. She prefers to avoid surgery, if at all possible, and is reluctant to use hormonal medications because she’s concerned about side effects. Nonsteroidal anti-inflammatory drugs provide inadequate relief.
R.L. asks if there is any other agent that can reduce her menstrual blood loss.
What can you offer to her?
Heavy menstrual bleeding (menorrhagia) impairs quality of life and prompts many women such as R.L. to seek a gynecologic consultation—many of them hoping to avoid surgery.
In the United States, medical-management options for the treatment of heavy menstrual bleeding include nonsteroidal anti-inflammatory drugs (NSAIDs) and off-label use of oral contraceptives, injectable depot medroxyprogesterone acetate, and high-dose oral progestin (e.g., norethindrone acetate, 5-mg tablets). The levonorgestrel intrauterine system (LNG-IUS; Mirena) is also widely used to treat heavy menstrual bleeding in women and was approved for this indication in October 2009. FDA approval of the LNG-IUS for heavy menstrual bleeding was based on a pivotal randomized trial demonstrating high efficacy.1,2
Very soon an additional option will be available—the antifibrinolytic agent tranexamic acid (to be sold by Ferring Pharmaceuticals under the brand name Lysteda). Although the LNG-IUS remains first-line treatment for heavy menstrual bleeding, tranexamic acid is appropriate for women who need to, or prefer to, avoid hormones.
How tranexamic acid works
Women who experience heavy menstrual bleeding have an elevated level of plasminogen activators in the endometrium, enzymes that facilitate the dissolution of clots. These enzymes can be inhibited by antifibrinolytic agents such as tranexamic acid, which competitively blocks the conversion of plasminogen to plasmin, thereby reducing fibrinolysis (FIGURE).3

FIGURE How tranexamic acid stanches heavy menstrual bleeding
Tranexamic acid (Lysteda) competitively blocks the conversion of plasminogen to plasmin, thereby reducing fibrinolysis.Clinical trials of tranexamic acid to treat menorrhagia were first performed in the 1960s. The agent proved to be superior to placebo, NSAIDs, and cyclic oral progestin.4 However, the LNG-IUS has been found to be more effective than tranexamic acid in reducing menstrual blood loss.5
In the United States, until very recently, tranexamic acid was FDA-indicated only as an injectable agent for use at the time of dental extraction in hemophilic patients. However, an oral formulation has been widely used abroad, including in Canada, to treat heavy menstrual bleeding. In Sweden, the agent is available over the counter.
In November 2009, the FDA approved 650-mg tablets of tranexamic acid as a treatment for heavy menstrual bleeding.6
Recommended dosage
In women who have normal renal function, the recommended dosage of tranexamic acid is 1,300 mg (two 650-mg tablets) three times daily (a total of 3,900 mg) for as long as 5 days during menstruation. In women who have impaired renal function, the dosage should be adjusted according to the package insert.
In randomized clinical trials, tranexamic acid did not produce more side effects than placebo did. However, because of concerns that tranexamic acid may increase risk of venous thromboembolism (VTE), the agent is contraindicated in women who have a history of VTE.
Although women who use hormonal contraception have not been included in clinical trials of tranexamic acid, the FDA recommends that they take tranexamic acid only if there is “a strong medical need and the benefit of treatment will outweigh the potential increased risk of a thrombotic event.”6
Tranexamic acid will likely be of particular utility for women who desire treatment for heavy menstrual bleeding but who should, or would prefer to, avoid hormonal agents. British guidelines recommend that, when medical management is appropriate in the treatment of heavy menstrual bleeding, the LNG-IUS be considered first-line treatment, with tranexamic acid representing a second-line strategy.7
CASE: Resolved
When she hears about tranexamic acid, R.L. is eager to try it. She is pleased that it does not contain hormones and needs to be taken only 5 days or fewer each month. You give her a prescription and schedule a follow-up visit 3 months later, at which time she reports being satisfied with the drug.
CASE: Heavy bleeding and apprehension over hormones
Your patient, R.L., 34 years old, has been bothered by gradually worsening menorrhagia for 2 years when she schedules an appointment to discuss treatment options. She prefers to avoid surgery, if at all possible, and is reluctant to use hormonal medications because she’s concerned about side effects. Nonsteroidal anti-inflammatory drugs provide inadequate relief.
R.L. asks if there is any other agent that can reduce her menstrual blood loss.
What can you offer to her?
Heavy menstrual bleeding (menorrhagia) impairs quality of life and prompts many women such as R.L. to seek a gynecologic consultation—many of them hoping to avoid surgery.
In the United States, medical-management options for the treatment of heavy menstrual bleeding include nonsteroidal anti-inflammatory drugs (NSAIDs) and off-label use of oral contraceptives, injectable depot medroxyprogesterone acetate, and high-dose oral progestin (e.g., norethindrone acetate, 5-mg tablets). The levonorgestrel intrauterine system (LNG-IUS; Mirena) is also widely used to treat heavy menstrual bleeding in women and was approved for this indication in October 2009. FDA approval of the LNG-IUS for heavy menstrual bleeding was based on a pivotal randomized trial demonstrating high efficacy.1,2
Very soon an additional option will be available—the antifibrinolytic agent tranexamic acid (to be sold by Ferring Pharmaceuticals under the brand name Lysteda). Although the LNG-IUS remains first-line treatment for heavy menstrual bleeding, tranexamic acid is appropriate for women who need to, or prefer to, avoid hormones.
How tranexamic acid works
Women who experience heavy menstrual bleeding have an elevated level of plasminogen activators in the endometrium, enzymes that facilitate the dissolution of clots. These enzymes can be inhibited by antifibrinolytic agents such as tranexamic acid, which competitively blocks the conversion of plasminogen to plasmin, thereby reducing fibrinolysis (FIGURE).3

FIGURE How tranexamic acid stanches heavy menstrual bleeding
Tranexamic acid (Lysteda) competitively blocks the conversion of plasminogen to plasmin, thereby reducing fibrinolysis.Clinical trials of tranexamic acid to treat menorrhagia were first performed in the 1960s. The agent proved to be superior to placebo, NSAIDs, and cyclic oral progestin.4 However, the LNG-IUS has been found to be more effective than tranexamic acid in reducing menstrual blood loss.5
In the United States, until very recently, tranexamic acid was FDA-indicated only as an injectable agent for use at the time of dental extraction in hemophilic patients. However, an oral formulation has been widely used abroad, including in Canada, to treat heavy menstrual bleeding. In Sweden, the agent is available over the counter.
In November 2009, the FDA approved 650-mg tablets of tranexamic acid as a treatment for heavy menstrual bleeding.6
Recommended dosage
In women who have normal renal function, the recommended dosage of tranexamic acid is 1,300 mg (two 650-mg tablets) three times daily (a total of 3,900 mg) for as long as 5 days during menstruation. In women who have impaired renal function, the dosage should be adjusted according to the package insert.
In randomized clinical trials, tranexamic acid did not produce more side effects than placebo did. However, because of concerns that tranexamic acid may increase risk of venous thromboembolism (VTE), the agent is contraindicated in women who have a history of VTE.
Although women who use hormonal contraception have not been included in clinical trials of tranexamic acid, the FDA recommends that they take tranexamic acid only if there is “a strong medical need and the benefit of treatment will outweigh the potential increased risk of a thrombotic event.”6
Tranexamic acid will likely be of particular utility for women who desire treatment for heavy menstrual bleeding but who should, or would prefer to, avoid hormonal agents. British guidelines recommend that, when medical management is appropriate in the treatment of heavy menstrual bleeding, the LNG-IUS be considered first-line treatment, with tranexamic acid representing a second-line strategy.7
CASE: Resolved
When she hears about tranexamic acid, R.L. is eager to try it. She is pleased that it does not contain hormones and needs to be taken only 5 days or fewer each month. You give her a prescription and schedule a follow-up visit 3 months later, at which time she reports being satisfied with the drug.
1. FDA approves additional use for IUD Mirena to treat heavy menstrual bleeding in IUD users [press release]. Rockville, Md: FDA; Oct. 1, 2009.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 3, 2010.
2. Kaunitz AM, Bissonette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen J. Levonorgestrel intrauterine system and medroxyprogesterone acetate for treatment of heavy menstrual bleeding. Poster No. 49 Presented at Reproductive Health 2009, Association of Reproductive Health Professionals annual meeting, September 30–October 3, 2009, Los Angeles, Calif. Contraception. 2009;80(2):212.-
3. Grimes DA, Davis AR, Ramos DE. Heavy menstrual bleeding: Assessing impact, evaluating management options. OBG Management. 2009;21(10)(supple).-http://www.obgmanagement.com/article_pages.asp?filename="2206OBG_Article2" aid=7971. Accessed May 3, 2010.
4. Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(4):CD000249.-
5. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164(3):879-883.
6. FDA approves Lysteda to treat heavy menstrual bleeding [press release]. Rockville, Md: FDA; Nov. 13, 2009.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm190551.htm. Accessed May 3, 2010.
7. National Institute for Health and Clinical Excellence. Heavy menstrual bleeding. January 2007.http://www.nice.org.uk/CG44. Accessed May 3, 2010.
1. FDA approves additional use for IUD Mirena to treat heavy menstrual bleeding in IUD users [press release]. Rockville, Md: FDA; Oct. 1, 2009.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm184747.htm. Accessed May 3, 2010.
2. Kaunitz AM, Bissonette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen J. Levonorgestrel intrauterine system and medroxyprogesterone acetate for treatment of heavy menstrual bleeding. Poster No. 49 Presented at Reproductive Health 2009, Association of Reproductive Health Professionals annual meeting, September 30–October 3, 2009, Los Angeles, Calif. Contraception. 2009;80(2):212.-
3. Grimes DA, Davis AR, Ramos DE. Heavy menstrual bleeding: Assessing impact, evaluating management options. OBG Management. 2009;21(10)(supple).-http://www.obgmanagement.com/article_pages.asp?filename="2206OBG_Article2" aid=7971. Accessed May 3, 2010.
4. Lethaby A, Farquhar C, Cooke I. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2000;(4):CD000249.-
5. Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164(3):879-883.
6. FDA approves Lysteda to treat heavy menstrual bleeding [press release]. Rockville, Md: FDA; Nov. 13, 2009.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm190551.htm. Accessed May 3, 2010.
7. National Institute for Health and Clinical Excellence. Heavy menstrual bleeding. January 2007.http://www.nice.org.uk/CG44. Accessed May 3, 2010.
IN THIS ARTICLE