User login
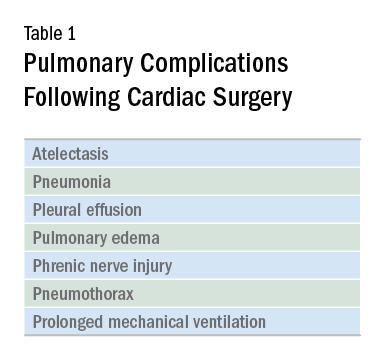
Cardiac surgery patients are sicker today than in previous decades due to an aging population and a rising complexity in medical care. There is an increasing reliance on noncardiac surgeons to care for these patients. The optimal postoperative providers and structure of the ICU where patients are cared for remain unclear, but what is irrefutable is patients’ increased postoperative morbidity. Pulmonary complications are a leading cause of morbidity in these patients, occurring in up to one-fifth of cases (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Common pulmonary complications of cardiac surgery are listed in Table 1. Those complications, captured by The Society of Thoracic Surgeons (STS) Cardiac Surgery Database, include receiving ventilation longer than 24 hours, pneumonia, pulmonary embolism, and pleural effusion requiring drainage (The Society of Thoracic Surgeons. STS National Database. https://www.sts.org/registries-research-center/sts-national-database. Accessed January 9, 2018).
It should come as no surprise that cardiac surgery can have pronounced effects on lung function. The anesthetic agents, chest wall alteration, and direct lung manipulation can all affect pulmonary parameters. Functional residual capacity (FRC) can decrease by up to 20% with anesthesia (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531), and the thoracic manipulation and alteration of rib cage mechanics with a classic median sternotomy approach can lead to decreases in forced vital capacity (FVC) and expiratory volume in the first second of forced expiration (FEV1) that can last for months after surgery. Use of the cardiopulmonary bypass circuit can also lead to bronchoconstriction. These changes in pulmonary function are less pronounced in alternative surgical approaches, such as partial sternotomies (Weissman C. Seminars in Cardiothoracic and Vascular Anesthesia: Pulmonary Complications After Cardiac Surgery. Glen Head, NY: Westminister Publications; 2004).
The most frequent pulmonary consequence of cardiac surgery is atelectasis, seen on postoperative chest radiographs in approximately 50% to 90% of patients (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Induction, apnea during cardiopulmonary bypass, manual compression of the lungs for surgical exposure, internal mammary harvesting, and pleurotomy can lead to atelectasis in the intraoperative setting while weak cough, poor inspiratory efforts, interstitial edema, and immobility further contribute postoperatively (Weissman 2004). While frequently seen, clinically significant pulmonary consequences from this radiographic finding alone are rare (Weissman 2004).
Pleural effusions are seen on immediate postoperative chest radiographs in the majority of patients. Additionally, 10% to 40% of patients develop pleural effusions 2 to 3 weeks after surgery secondary to postpericardiotomy syndrome. While some effusions require drainage and further intervention (eg, hemothorax), most effusions require no specific treatment and resolve over time (Weissman 2004).
The prevalence of pneumonia following cardiac surgery varies based on differences in study populations and diagnostic criteria, but it remains an important source of morbidity and mortality. In one series, postoperative pneumonia occurred in 3.1% of patients, with higher rates observed in patients who were older, had worse left ventricular ejection fraction, had COPD, experienced longer bypass times, and received more red blood cell transfusions in the operating room (Allou N, et al. Crit Care Med. 2014;42[5]:1150). A meta-analysis found that an average of 6.37% of patients developed ventilator-associated pneumonia (VAP), and this rose to 35.2% in those receiving ventilation for greater than 48 hours. Those who developed VAP had an odds ratio of dying of 15.18 (95% CI 5.81-39.68) compared with those who did not (He S, et al. J Thorac Cardiovasc Surg. 2014;148[6]:3148).
A small proportion of patients go on to develop ARDS. While relatively uncommon, ARDS carries a high mortality rate. Many possible etiologies for ARDS in cardiac surgery patients have been proposed, including an inflammatory response related to the cardiopulmonary bypass circuit, reperfusion injury secondary to reduced pulmonary blood flow during bypass, protamine administration, transfusion, hypothermia, and lack of ventilation during bypass (Weissman 2004); (Stephens RS, et al. Ann Thorac Surg. 2013;95[3]:1122). Type of surgery may also play a role, as patients who undergo aortic surgery are at an even greater risk (Stephens 2013). As with other cases of ARDS, treatment is supportive: low tidal volume ventilation and careful management of fluid balance, as well as paralysis, prone positioning, and consideration for extracorporeal membrane oxygenation (ECMO), as appropriate (Stephens 2013).
Therapies to prevent postoperative pulmonary complications have included early extubation, aggressive pain control, deep breathing, physical therapy, early mobilization, and noninvasive ventilation in the form of CPAP and intermittent positive pressure breathing. A meta-analysis of 18 trials looking at the use of various forms of prophylactic postoperative physiotherapy did not show a difference in any measured clinical outcome (Pasquina P, Walder B. Br Med J. 2003;327[7428]:1379).
However, the heterogeneity, short follow-up, and low quality of included studies made it difficult to draw meaningful conclusions on the benefit or lack thereof for these therapies. More recent studies have shown promise for chest physiotherapy started several weeks prior to elective coronary bypass graft surgery and extended CPAP via nasal CPAP mask immediately following extubation (Hulzebos EH. JAMA. 2006;296[15]:1851), (Stephens 2013).
Ongoing areas for improvement include further clarification and standardization of best practices for postcardiac surgery patients, including blood product transfusion, optimal tidal volumes for surgical and postsurgical ventilation, timing of extubation, and the use of preventive therapies in the pre- and postsurgical periods. As providers who care for these patients, understanding how we can improve their postoperative pulmonary recovery will allow us to enhance our patient’s experience.
Dr. Noel is a Critical Care Fellow, Cooper Medical School of Rowan University, Camden, New Jersey.
Cardiac surgery patients are sicker today than in previous decades due to an aging population and a rising complexity in medical care. There is an increasing reliance on noncardiac surgeons to care for these patients. The optimal postoperative providers and structure of the ICU where patients are cared for remain unclear, but what is irrefutable is patients’ increased postoperative morbidity. Pulmonary complications are a leading cause of morbidity in these patients, occurring in up to one-fifth of cases (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Common pulmonary complications of cardiac surgery are listed in Table 1. Those complications, captured by The Society of Thoracic Surgeons (STS) Cardiac Surgery Database, include receiving ventilation longer than 24 hours, pneumonia, pulmonary embolism, and pleural effusion requiring drainage (The Society of Thoracic Surgeons. STS National Database. https://www.sts.org/registries-research-center/sts-national-database. Accessed January 9, 2018).

It should come as no surprise that cardiac surgery can have pronounced effects on lung function. The anesthetic agents, chest wall alteration, and direct lung manipulation can all affect pulmonary parameters. Functional residual capacity (FRC) can decrease by up to 20% with anesthesia (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531), and the thoracic manipulation and alteration of rib cage mechanics with a classic median sternotomy approach can lead to decreases in forced vital capacity (FVC) and expiratory volume in the first second of forced expiration (FEV1) that can last for months after surgery. Use of the cardiopulmonary bypass circuit can also lead to bronchoconstriction. These changes in pulmonary function are less pronounced in alternative surgical approaches, such as partial sternotomies (Weissman C. Seminars in Cardiothoracic and Vascular Anesthesia: Pulmonary Complications After Cardiac Surgery. Glen Head, NY: Westminister Publications; 2004).
The most frequent pulmonary consequence of cardiac surgery is atelectasis, seen on postoperative chest radiographs in approximately 50% to 90% of patients (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Induction, apnea during cardiopulmonary bypass, manual compression of the lungs for surgical exposure, internal mammary harvesting, and pleurotomy can lead to atelectasis in the intraoperative setting while weak cough, poor inspiratory efforts, interstitial edema, and immobility further contribute postoperatively (Weissman 2004). While frequently seen, clinically significant pulmonary consequences from this radiographic finding alone are rare (Weissman 2004).
Pleural effusions are seen on immediate postoperative chest radiographs in the majority of patients. Additionally, 10% to 40% of patients develop pleural effusions 2 to 3 weeks after surgery secondary to postpericardiotomy syndrome. While some effusions require drainage and further intervention (eg, hemothorax), most effusions require no specific treatment and resolve over time (Weissman 2004).
The prevalence of pneumonia following cardiac surgery varies based on differences in study populations and diagnostic criteria, but it remains an important source of morbidity and mortality. In one series, postoperative pneumonia occurred in 3.1% of patients, with higher rates observed in patients who were older, had worse left ventricular ejection fraction, had COPD, experienced longer bypass times, and received more red blood cell transfusions in the operating room (Allou N, et al. Crit Care Med. 2014;42[5]:1150). A meta-analysis found that an average of 6.37% of patients developed ventilator-associated pneumonia (VAP), and this rose to 35.2% in those receiving ventilation for greater than 48 hours. Those who developed VAP had an odds ratio of dying of 15.18 (95% CI 5.81-39.68) compared with those who did not (He S, et al. J Thorac Cardiovasc Surg. 2014;148[6]:3148).
A small proportion of patients go on to develop ARDS. While relatively uncommon, ARDS carries a high mortality rate. Many possible etiologies for ARDS in cardiac surgery patients have been proposed, including an inflammatory response related to the cardiopulmonary bypass circuit, reperfusion injury secondary to reduced pulmonary blood flow during bypass, protamine administration, transfusion, hypothermia, and lack of ventilation during bypass (Weissman 2004); (Stephens RS, et al. Ann Thorac Surg. 2013;95[3]:1122). Type of surgery may also play a role, as patients who undergo aortic surgery are at an even greater risk (Stephens 2013). As with other cases of ARDS, treatment is supportive: low tidal volume ventilation and careful management of fluid balance, as well as paralysis, prone positioning, and consideration for extracorporeal membrane oxygenation (ECMO), as appropriate (Stephens 2013).
Therapies to prevent postoperative pulmonary complications have included early extubation, aggressive pain control, deep breathing, physical therapy, early mobilization, and noninvasive ventilation in the form of CPAP and intermittent positive pressure breathing. A meta-analysis of 18 trials looking at the use of various forms of prophylactic postoperative physiotherapy did not show a difference in any measured clinical outcome (Pasquina P, Walder B. Br Med J. 2003;327[7428]:1379).
However, the heterogeneity, short follow-up, and low quality of included studies made it difficult to draw meaningful conclusions on the benefit or lack thereof for these therapies. More recent studies have shown promise for chest physiotherapy started several weeks prior to elective coronary bypass graft surgery and extended CPAP via nasal CPAP mask immediately following extubation (Hulzebos EH. JAMA. 2006;296[15]:1851), (Stephens 2013).
Ongoing areas for improvement include further clarification and standardization of best practices for postcardiac surgery patients, including blood product transfusion, optimal tidal volumes for surgical and postsurgical ventilation, timing of extubation, and the use of preventive therapies in the pre- and postsurgical periods. As providers who care for these patients, understanding how we can improve their postoperative pulmonary recovery will allow us to enhance our patient’s experience.
Dr. Noel is a Critical Care Fellow, Cooper Medical School of Rowan University, Camden, New Jersey.
Cardiac surgery patients are sicker today than in previous decades due to an aging population and a rising complexity in medical care. There is an increasing reliance on noncardiac surgeons to care for these patients. The optimal postoperative providers and structure of the ICU where patients are cared for remain unclear, but what is irrefutable is patients’ increased postoperative morbidity. Pulmonary complications are a leading cause of morbidity in these patients, occurring in up to one-fifth of cases (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Common pulmonary complications of cardiac surgery are listed in Table 1. Those complications, captured by The Society of Thoracic Surgeons (STS) Cardiac Surgery Database, include receiving ventilation longer than 24 hours, pneumonia, pulmonary embolism, and pleural effusion requiring drainage (The Society of Thoracic Surgeons. STS National Database. https://www.sts.org/registries-research-center/sts-national-database. Accessed January 9, 2018).

It should come as no surprise that cardiac surgery can have pronounced effects on lung function. The anesthetic agents, chest wall alteration, and direct lung manipulation can all affect pulmonary parameters. Functional residual capacity (FRC) can decrease by up to 20% with anesthesia (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531), and the thoracic manipulation and alteration of rib cage mechanics with a classic median sternotomy approach can lead to decreases in forced vital capacity (FVC) and expiratory volume in the first second of forced expiration (FEV1) that can last for months after surgery. Use of the cardiopulmonary bypass circuit can also lead to bronchoconstriction. These changes in pulmonary function are less pronounced in alternative surgical approaches, such as partial sternotomies (Weissman C. Seminars in Cardiothoracic and Vascular Anesthesia: Pulmonary Complications After Cardiac Surgery. Glen Head, NY: Westminister Publications; 2004).
The most frequent pulmonary consequence of cardiac surgery is atelectasis, seen on postoperative chest radiographs in approximately 50% to 90% of patients (Szelowski LA, et al. Curr Probl Surg. 2015;52[1]:531). Induction, apnea during cardiopulmonary bypass, manual compression of the lungs for surgical exposure, internal mammary harvesting, and pleurotomy can lead to atelectasis in the intraoperative setting while weak cough, poor inspiratory efforts, interstitial edema, and immobility further contribute postoperatively (Weissman 2004). While frequently seen, clinically significant pulmonary consequences from this radiographic finding alone are rare (Weissman 2004).
Pleural effusions are seen on immediate postoperative chest radiographs in the majority of patients. Additionally, 10% to 40% of patients develop pleural effusions 2 to 3 weeks after surgery secondary to postpericardiotomy syndrome. While some effusions require drainage and further intervention (eg, hemothorax), most effusions require no specific treatment and resolve over time (Weissman 2004).
The prevalence of pneumonia following cardiac surgery varies based on differences in study populations and diagnostic criteria, but it remains an important source of morbidity and mortality. In one series, postoperative pneumonia occurred in 3.1% of patients, with higher rates observed in patients who were older, had worse left ventricular ejection fraction, had COPD, experienced longer bypass times, and received more red blood cell transfusions in the operating room (Allou N, et al. Crit Care Med. 2014;42[5]:1150). A meta-analysis found that an average of 6.37% of patients developed ventilator-associated pneumonia (VAP), and this rose to 35.2% in those receiving ventilation for greater than 48 hours. Those who developed VAP had an odds ratio of dying of 15.18 (95% CI 5.81-39.68) compared with those who did not (He S, et al. J Thorac Cardiovasc Surg. 2014;148[6]:3148).
A small proportion of patients go on to develop ARDS. While relatively uncommon, ARDS carries a high mortality rate. Many possible etiologies for ARDS in cardiac surgery patients have been proposed, including an inflammatory response related to the cardiopulmonary bypass circuit, reperfusion injury secondary to reduced pulmonary blood flow during bypass, protamine administration, transfusion, hypothermia, and lack of ventilation during bypass (Weissman 2004); (Stephens RS, et al. Ann Thorac Surg. 2013;95[3]:1122). Type of surgery may also play a role, as patients who undergo aortic surgery are at an even greater risk (Stephens 2013). As with other cases of ARDS, treatment is supportive: low tidal volume ventilation and careful management of fluid balance, as well as paralysis, prone positioning, and consideration for extracorporeal membrane oxygenation (ECMO), as appropriate (Stephens 2013).
Therapies to prevent postoperative pulmonary complications have included early extubation, aggressive pain control, deep breathing, physical therapy, early mobilization, and noninvasive ventilation in the form of CPAP and intermittent positive pressure breathing. A meta-analysis of 18 trials looking at the use of various forms of prophylactic postoperative physiotherapy did not show a difference in any measured clinical outcome (Pasquina P, Walder B. Br Med J. 2003;327[7428]:1379).
However, the heterogeneity, short follow-up, and low quality of included studies made it difficult to draw meaningful conclusions on the benefit or lack thereof for these therapies. More recent studies have shown promise for chest physiotherapy started several weeks prior to elective coronary bypass graft surgery and extended CPAP via nasal CPAP mask immediately following extubation (Hulzebos EH. JAMA. 2006;296[15]:1851), (Stephens 2013).
Ongoing areas for improvement include further clarification and standardization of best practices for postcardiac surgery patients, including blood product transfusion, optimal tidal volumes for surgical and postsurgical ventilation, timing of extubation, and the use of preventive therapies in the pre- and postsurgical periods. As providers who care for these patients, understanding how we can improve their postoperative pulmonary recovery will allow us to enhance our patient’s experience.
Dr. Noel is a Critical Care Fellow, Cooper Medical School of Rowan University, Camden, New Jersey.
