User login
CASE: PROM at 22 weeks
J.S. is a 22-year-old woman at 22 weeks’ gestation in her second pregnancy. Her first gestation ended in spontaneous abortion at 10 weeks, followed by dilation and curettage. She has been referred to you by her midwife, who is concerned about J.S.’s complaints of loss of fluid over the past 2 weeks and who cannot document rupture of membranes by the usual means.
In your office, J.S. continues to complain of intermittent leakage of clear fluid. She says there has been no vaginal bleeding, foul-smelling discharge, fever, chills, or abdominal tenderness. You find a normal abdomen. A sterile speculum exam is equivocal, without evidence of pooling or ferning; a nitrazine test is positive, however. A complete blood count reveals no evidence of leukocytosis. Urinalysis is negative.
You suspect preterm premature rupture of membranes (PROM) when bedside ultrasonography (US) documents oligohydramnios with an amniotic fluid index of less than 5 cm. The kidneys, bladder, and stomach all appear normal.
What is the best way to confirm the diagnosis? What is the most appropriate management at this gestational age? And how do you counsel J.S. about the risk to her, and her baby, of continuing the pregnancy?
Given the very poor prognosis of many cases of early PROM, accurate diagnosis is critical to determine the best management strategy. The gold standard of diagnosis is sterile vaginal examination with a speculum to identify clear fluid leaking from the cervix or pooling in the posterior fornix. Use nitrazine paper to assess the fluid collected from the posterior fornix for alkaline pH; this method has a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.1 The appearance of “ferning”—a crystalline pattern that occurs when the saline amniotic fluid dries—carries a PPV of 98% to 99% and a NPV of 90% to 99%.2
One must also consider the patient’s history. When that history and the physical exam fail to render a clear result, use US to assess the amniotic fluid volume. A low volume in the presence of a convincing clinical history is very suspicious for PROM, as in the case just described.
Tinting the amniotic fluid may help
In equivocal cases, mix 1 to 3 mL of indigo carmine with 5 mL of sterile saline and insert it into the amniotic fluid under US guidance. This dye will make any leaking amniotic fluid obvious. Be aware, however, that instillation of the dye is very difficult in cases of severe oligohydramnios or anhydramnios. In this setting, amniocentesis can also cause contractions or vaginal bleeding.
New diagnostic tool on the horizon
Recent studies have focused on a new rapid test (AmniSure) that uses immunochromatography to detect trace amounts of placental α-microglobulin-1 protein.3 This protein is specific to amniotic fluid and present in vaginal secretions only when amniotic fluid is leaking through the cervix. One study of 203 patients suspected of having ruptured membranes found the AmniSure test to have a PPV of 100% and NPV of 99.1%.3 Although these findings are promising, further confirmatory studies are needed before this product can be recommended for widespread use.
CASE continued: Leakage of tinted fluid confirms PROM
Because the diagnostic steps taken so far have been inconclusive, J.S. undergoes amniocentesis with infusion of indigo carmine. Within 2 hours, blue dye is observed leaking from the cervix, confirming PROM. A sample of amniotic fluid obtained at the time of amniocentesis produces a negative gram stain and reveals a normal glucose level and leukocyte count. Amniotic fluid cultures are pending.
What is your next step?
Determining the best management strategy is next. The treatment plan should be based on gestational age, presence or absence of infection or labor, and fetal status. Therefore, the initial evaluation of a patient with PROM should focus on the collection of this clinical information.
I recommend these measures:
- Document the exact gestational age by careful review of available records and ultrasound biometry
- Identify indicators of infection, such as maternal fever and tachycardia, fundal tenderness, fetal tachycardia, and an elevated white blood cell count
- Amniocentesis may be required to rule out amnionitis in cases where the diagnosis is clinically unclear
- Document fetal presentation
- Initiate fetal heart rate (FHR) monitoring at the time of diagnosis and perform a biophysical profile (BPP).
Midtrimester PROM: 16 to 24 weeks’ gestation
Management differs for each gestational age.
Midtrimester PROM occurs in approximately 0.7% of all pregnancies and is a significant source of morbidity and mortality.4,5 It may be iatrogenic in nature when it follows an invasive procedure such as amniocentesis or fetoscopy. It also may occur spontaneously, with causes similar to those of PROM at later gestational ages. At this early gestational age, PROM is more likely to be associated with cervical incompetence and inflammation.6,7
Infection is a risk—and may be the underlying cause
Infection is associated with as many as 30% to 50% of cases of PROM.4,8-10 Half of the cases of intra-amniotic infection develop within 7 days after PROM. That’s because many cases of early PROM have infection or inflammation as their cause.
Intrauterine demise is common at early gestational ages
The risk of intrauterine fetal demise is inversely related to gestational age at the time of rupture. That is, the earlier the gestational age, the higher the rate of fetal death. One study found that the rate of intrauterine fetal demise was 33% when PROM occurred before 20 weeks’ gestation and 20% when it occurred between 20 and 24 weeks; it was rare after 25 weeks.10
Pulmonary hypoplasia is more common at this critical juncture
The midtrimester is a critical time for fetal lung development. During the canalicular stage (between 17 and 24 weeks’ gestation), the gas-exchanging acini and pulmonary capillaries are forming, so they are more susceptible to injury. The incidence of pulmonary hypoplasia is approximately 10% when PROM occurs earlier than 20 weeks’ gestation, although a wide range of rates has been reported.4,8-10 Pulmonary hypoplasia remains a significant cause of neonatal mortality and is found in as many as 77% of autopsies of infants from pregnancies complicated by midtrimester PROM.11
The incidence of pulmonary hypoplasia decreases by as much as 46% with each week of gestational age at the time of PROM.12 After 26 weeks, when the terminal sac stage of development occurs, the rate of pulmonary hypoplasia complicating PROM drops to less than 2%.12-14
The degree of oligohydramnios also affects the rate of pulmonary hypoplasia, which increases significantly when the amniotic fluid index is less than 5 cm.15
Limb deformity may be related to restricted movement
Although limb development occurs in the embryonic period, most limb growth takes place during the second and third trimesters.16 The restriction in movement and increased pressure associated with prolonged periods of oligohydramnios can lead to skeletal deformity in otherwise normal extremities.
The frequency of deformity varies widely among studies, but the mean incidence is 7%.4,8-11 A twofold higher incidence of skeletal abnormality occurs when midtrimester PROM is accompanied by severe oligohydramnios. In one study, the rate of skeletal abnormality was 54% when the deepest pocket of amniotic fluid was less than 1 cm, compared with 26% for matched pregnancies with a normal or mildly reduced volume.16
Maternal complications include retained placenta, endometritis
Maternal complications associated with very early PROM include a higher rate of cesarean section due to fetal malpresentation and FHR abnormalities, which often accompany oligohydramnios and intraamniotic infection.10 A classical incision is more likely in these cases due to the poorly developed lower uterine segment. Retained placenta necessitating postpartum curettage occurs in 9% to 18% of cases of PROM at less than 20 weeks’ gestation. In addition, postpartum endometritis complicates as many as 40% of cases of midtrimester PROM.4,8-11
General prognosis
The outcome of midtrimester PROM depends on the underlying cause. If it is iatrogenic, the outcome is usually favorable, with frequent resealing of the membranes; most cases end in a normal term delivery. The outcome of spontaneous PROM is more grim.
Midtrimester PROM has the same relatively short latency (approximately 17 days on average) as PROM that occurs later in pregnancy. Less than 50% of women with midtrimester PROM remain pregnant at the end of the first week, and as many as 75% of these women will have delivered by 28 days after PROM.11 These percentages indicate that most women with midtrimester PROM deliver before fetal viability can be attained, or in the risky periviable period.
Overall, midtrimester PROM is associated with significant fetal, neonatal, and maternal morbidity. The risks must be explained to the patient along with any management plan.
Given the very poor prognosis and small chance of prolonged latency, induction of labor and pregnancy termination are reasonable options at the time of presentation. The patient needs to know that expectant management can be associated with significant long-term morbidity and a higher rate of neonatal mortality.
CASE continued: Patient is apprised of the risks
After a frank discussion of the risks involved in continuing her pregnancy, J.S. chooses expectant management. Given the early gestational age and absence of any sign of infection, she is sent home for bed rest and instructed to check her temperature twice daily. She is told to return for evaluation if fever (>100°F) or symptoms of infection develop. Because of the very early gestational age, no steroid or antibiotic will be given until 24 weeks’ gestation, when she will be admitted for inpatient care.
PROM at 24 to 32 weeks’ gestation
Selecting a management strategy for a pregnancy at this gestational age means weighing the potential morbidity and mortality of immediate delivery against the morbidity and mortality of expectant management. At this gestational age, the principal source of fetal morbidity is prematurity itself. As many as 40% of infants delivered before 26 weeks’ gestation experience some type of long-term morbidity such as intraventricular hemorrhage (IVH), retinopathy of prematurity, necrotizing enterocolitis (NEC), or, most commonly, respiratory distress syndrome (RDS).16,17 It is true that fetal morbidity increases when chorioamnionitis is present, but the potential benefit of prolonging the pregnancy by 7 to 14 days is believed to outweigh the risk of infection at these gestational ages. Therefore, in the absence of contraindications, expectant management is the usual course of action.
Select patients carefully for expectant management
Consider expectant management only when fetal well-being can be documented, without evidence of infection. Abruption is a contraindication to expectant management, although the clinical nature of this diagnosis can make it difficult to identify. If abruption is diagnosed, aggressive management with labor augmentation or cesarean section and intravenous (IV) antibiotics is appropriate.
Repetitive fetal heart decelerations in the presence of active vaginal bleeding and uterine tenderness indicate placental insufficiency and are an indication for delivery.
More than 50% of patients deliver within the first week after PROM is diagnosed.18 At least 30% experience chorioamnionitis some time after the diagnosis of PROM, and 1% to 2% suffer cord prolapse.10,18,19 As many as 4% to 12% of cases of PROM will also be complicated by abruption,20,21 and a rate of intrauterine fetal demise as high as 1% has been documented.18 Therefore, if expectant management is selected, it should include close monitoring for these complications.
Hospitalization is warranted. Women who are stable and being managed expectantly should probably be hospitalized. One prospective trial comparing outcomes between women managed at home and women who were hospitalized found no significant difference in latency period or the rate of infection.22 However, the strict inclusion criteria for this study make it difficult to generalize the results. Only 18% of the 349 women screened for enrollment met these criteria.
The high rate of precipitous labor, frequent onset of infection, and need for frequent maternal and neonatal evaluation at this gestational age make hospitalization a prudent choice.
Fetal surveillance is mandatory
Most investigators would agree that a regular schedule of fetal surveillance is necessary during expectant management. But there is no clear evidence indicating which type, and what timing, of surveillance are best. It is clear that changes in the FHR pattern and BPP precede the onset of chorioamnionitis and intrauterine demise due to cord accidents.23-25 However, no studies have demonstrated a significant improvement in neonatal outcomes with daily or even twice-daily antenatal surveillance.
At our institution, we follow a regimen of daily surveillance, which consists of a nonstress test and/or BPP to confirm fetal well-being.
Tocolysis won’t prolong gestation beyond 48 hours…
There is no evidence that prolonged tocolysis with any therapy significantly increases long-term latency or improves any type of neonatal morbidity in pregnancies complicated by PROM. Tocolysis may prolong pregnancy over the short term (<48 hours),26,27 but its widespread use is not supported by the evidence.
Tocolysis is appropriate to achieve safe maternal transport or administer steroids.
…but corticosteroids are highly beneficial
Antenatal corticosteroids clearly improve neonatal outcomes when PROM occurs before 32 weeks’ gestation. Two large meta-analyses have found such benefits to be a decrease in the rates of RDS, IVH, NEC, and neonatal death.28,29 A recent prospective study confirmed these findings.30 The rate of RDS declined 26%—from 44% to 18%.
A consensus panel of the National Institutes of Health (NIH) recommended use of corticosteroids in cases of PROM between 24 and 32 weeks’ gestation in which there is no clinical evidence of infection.31 Any of the standard steroid regimens is appropriate. At our institution, we give an intramuscular injection of 12 mg of betamethasone and repeat this one time in 24 hours.
Are prophylactic antibiotics warranted?
The fact that infection is the most commonly identified cause of PROM prompts the question: Does treatment with IV antibiotics improve outcomes and prolong latency even in the absence of clinically apparent infection? Mercer and colleagues32 reported a significant reduction in chorioamnionitis, endometritis, and neonatal infection, including sepsis and pneumonia, in pregnancies treated with prophylactic antibiotics, compared with expectant management alone. In that study, latency also increased significantly following antibiotic therapy. Other meta-analyses confirm the benefits of prophylactic antibiotics, demonstrating a lower rate of neonatal sepsis and IVH following treatment.33,34
One large multicenter randomized trial found a reduced rate of IVH and RDS after treatment with IV erythromycin and ampicillin for 48 hours, followed by a 5-day course of amoxicillin and erythromycin.35 A Cochrane review of the use of prophylactic antibiotics in the setting of PROM included 19 studies with various antibiotic regimens.36 It concluded that antibiotic therapy prolongs latency (at both 48 hours and 7 days), decreases maternal infection, and reduces the incidence of neonatal complications, including infection, need for oxygen, IVH, and periventricular leukomalacia.
No superior regimen, but avoid amoxicillin-clavulanate. Although no single antibiotic regimen is clearly superior to the others, erythromycin has been associated with benefits most consistently. The most common dosage for erythromycin is 250 mg every 6 hours for a total of 48 hours and then an additional 5 days of oral treatment. Amoxicillin-clavulanate has been associated with an increased risk of NEC in at least two trials, and should probably be avoided. A Cochrane review confirms these conclusions.36
Choice of delivery route is flexible
Once the need for delivery arises, choose the route according to normal obstetric indications. In the setting of PROM with malpresentation, cesarean delivery is probably the best approach. However, in very-low-birth-weight infants, the best mode of delivery remains unclear.37 If the fetus is in cephalic presentation, an attempt at vaginal delivery does not appear to have a worse neonatal outcome.
If spontaneous labor does not occur or if induction is not indicated for maternal or fetal reasons, one may choose to deliver the patient at 32 weeks’ gestation or continue expectant management until 34 weeks’ gestation. This decision is discussed in more detail in the next section.
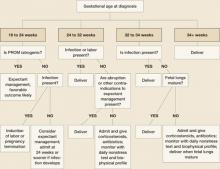
ALGORITHM
Management of PROM varies with gestational age
PROM at 32 to 34 weeks’ gestation
Although it is generally accepted that the fetus benefits from expectant management in pregnancies complicated by PROM before 32 weeks’ gestation, the management of PROM that arises between 32 and 34 weeks remains controversial and a focus of ongoing research. Because most neonatal morbidity is caused by prematurity, and the rate of prematurity-related complications decreases with increasing gestational age, some argue that the potential benefit of prolonging latency after 32 weeks’ gestation does not outweigh the risk of chorioamnionitis.
Continue the gestation? Or deliver?
Mercer and colleagues randomized 97 women with PROM between 32 and 36 weeks’ gestation and a mature lung profile to expectant management or immediate induction.38 Although expectant management did prolong pregnancy, no neonatal benefit was observed, and the rate of chorioamnionitis was higher with expectant management, with a longer hospital stay.
Cox and associates found a higher rate of chorioamnionitis among 68 women with PROM between 30 and 34 weeks’ gestation who were managed expectantly, compared with 61 women assigned to immediate induction.39 Neonatal morbidity was similar in both groups.
These studies suggest that expectant management after 32 weeks leads only to an increased rate of chorioamnionitis and longer maternal and neonatal hospitalization, without any demonstrable neonatal benefit. However, one significant limitation of these studies is the fact that patients managed expectantly received neither corticosteroids nor prophylactic antibiotics.
Are corticosteroids appropriate at this gestational age?
We lack sufficient evidence to support the routine use of corticosteroids after 32 weeks in pregnancies complicated by PROM. The NIH consensus panel suggested that they may be an option in patients without contraindications up to 34 weeks’ gestation.31
Some experts recommend testing for fetal lung maturity when PROM occurs between 32 and 34 weeks’ gestation. In this group, the rate of fetal lung maturity is between 50% and 60%.40,41 There is no clear benefit in prolonging a pregnancy when fetal lung maturity can be documented. However, in the setting of immature fetal lungs, expectant management may be appropriate following treatment with corticosteroids and a prophylactic antibiotic regimen. Patients who present at 34 weeks’ gestation or beyond are likely to benefit most from immediate delivery.
When expectant management is chosen between 32 and 34 weeks, inpatient hospitalization with daily monitoring is also recommended. The mode of delivery depends on the usual obstetric indications.
CASE resolved: Patient develops fever and spontaneous labor
Ten days after the documentation of PROM, J.S. reports a fever and abdominal tenderness, as well as frequent uterine contractions that began early in the day. She is admitted to the hospital. A physical examination confirms clinically apparent intra-amniotic infection and labor. The patient is started on IV antibiotics and, after a short labor, delivers a nonviable male infant weighing 500 g. Pathologic examination of the fetus and placenta reveals a normal, immature fetus with evidence of acute chorioamnionitis on placental sections.
1. Abe T. The detection of rupture of fetal membranes with the nitrazine indicator. Am J Obstet Gynecol. 1940;39:400.-
2. Davidson KM. Detection of premature rupture of the membranes. Clin Obstet Gynecol. 1991;34:715-722.
3. Cousins LM, Smok D, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of ruptured membranes. Am J Perinatol. 2005;22(6):317-320.
4. Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol. 1984;64:615-620.
5. Schucker JL, Mercer BM. Midtrimester premature rupture of the membranes. Semin Perinatol. 1996;20:389-400.
6. Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972-978.
7. Morales WJ. The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol. 1987;70:183-186.
8. Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390-396.
9. Beydoun SN, Yasin SY. Premature rupture of the membranes before 28 weeks: conservative management. Am J Obstet Gynecol. 1986;155:471-479.
10. Major CA, Kitzmiller JL. Perinatal survival with expectant management of midtrimester rupture of membranes. Am J Obstet Gynecol. 1990;163:838-844.
11. Falk SJ, Campbell LJ, Lee-Parritz A, et al. Expectant management in spontaneous preterm premature rupture of membranes between 14 and 24 weeks’ gestation. J Perinatol. 2004;24:611-616.
12. Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996;175:675-681.
13. Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46-52.
14. Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second-trimester rupture of membranes. Obstet Gynecol. 1998;92:895-901.
15. Vergani P, Ghidini A, Locatelli A, et al. Risk factors for pulmonary hypoplasia in second-trimester premature rupture of membranes. Am J Obstet Gynecol. 1994;170:1359-1364.
16. Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child. 1988;63:1146-1150.
17. Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632-1639.
18. Nelson LH, Anderson RL, O’Shea TM, Swain M. Expectant management of preterm premature rupture of membranes. Am J Obstet Gynecol. 1994;171:350-356.
19. Belady PH, Farkouh LJ, Gibbs RS. Intra-amniotic infection and premature rupture of the membranes. Clin Perinatol. 1997;24:43-57.
20. Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88:309-318.
21. Gonen R, Hannah ME, Milligan JE. Does prolonged preterm premature rupture of the membranes predispose to abruptio placentae? Obstet Gynecol. 1989;74:347-350.
22. Carlan SJ, O’Brien WF, Parsons MT, Lense JJ. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
23. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol. 1986;155:149-153.
24. Smith CV, Greenspoon J, Phelan JP, Platt LD. Clinical utility of the nonstress test in the conservative management of women with preterm spontaneous premature rupture of the membranes. J Reprod Med. 1987;32:1-4.
25. Hanley ML, Vintzileos AM. Biophysical testing in premature rupture of the membranes. Semin Perinatol. 1996;20:418-425.
26. Levy DL, Warsof SL. Oral ritodrine and preterm premature rupture of membranes. Obstet Gynecol. 1985;66:621-623.
27. Weiner CP, Renk K, Klugman M. The therapeutic efficacy and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:216-222.
28. Ohlsson A. Treatments of preterm premature rupture of the membranes: a meta-analysis. Am J Obstet Gynecol. 1989;160:890-906.
29. Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322-335.
30. Lewis DF, Brody K, Edwards MS, et al. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
31. National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413-418.
32. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989-995.
33. Egarter C, Leitich H, Karas H, et al. Antibiotic treatment in preterm premature rupture of membranes and neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 1996;174:589-597.
34. Ananth CV, Guise JM, Thorp JM, Jr. Utility of antibiotic therapy in preterm premature rupture of membranes: a meta-analysis. Obstet Gynecol Surv. 1996;51:324-328.
35. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979-988.
36. Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2003;(2):CD001058.-
37. Bottoms SF, Paul RH, Iams JD, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960-966.
38. Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
39. Cox SM, Leveno KJ. Intentional delivery versus expectant management with preterm ruptured membranes at 30-34 weeks’ gestation. Obstet Gynecol. 1995;86:875-879.
40. Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331-336.
41. Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316-321.
Dr. Esplin receives support from the National Institute of Child Health and Human Development and is a speaker for Adeza.
CASE: PROM at 22 weeks
J.S. is a 22-year-old woman at 22 weeks’ gestation in her second pregnancy. Her first gestation ended in spontaneous abortion at 10 weeks, followed by dilation and curettage. She has been referred to you by her midwife, who is concerned about J.S.’s complaints of loss of fluid over the past 2 weeks and who cannot document rupture of membranes by the usual means.
In your office, J.S. continues to complain of intermittent leakage of clear fluid. She says there has been no vaginal bleeding, foul-smelling discharge, fever, chills, or abdominal tenderness. You find a normal abdomen. A sterile speculum exam is equivocal, without evidence of pooling or ferning; a nitrazine test is positive, however. A complete blood count reveals no evidence of leukocytosis. Urinalysis is negative.
You suspect preterm premature rupture of membranes (PROM) when bedside ultrasonography (US) documents oligohydramnios with an amniotic fluid index of less than 5 cm. The kidneys, bladder, and stomach all appear normal.
What is the best way to confirm the diagnosis? What is the most appropriate management at this gestational age? And how do you counsel J.S. about the risk to her, and her baby, of continuing the pregnancy?
Given the very poor prognosis of many cases of early PROM, accurate diagnosis is critical to determine the best management strategy. The gold standard of diagnosis is sterile vaginal examination with a speculum to identify clear fluid leaking from the cervix or pooling in the posterior fornix. Use nitrazine paper to assess the fluid collected from the posterior fornix for alkaline pH; this method has a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.1 The appearance of “ferning”—a crystalline pattern that occurs when the saline amniotic fluid dries—carries a PPV of 98% to 99% and a NPV of 90% to 99%.2
One must also consider the patient’s history. When that history and the physical exam fail to render a clear result, use US to assess the amniotic fluid volume. A low volume in the presence of a convincing clinical history is very suspicious for PROM, as in the case just described.
Tinting the amniotic fluid may help
In equivocal cases, mix 1 to 3 mL of indigo carmine with 5 mL of sterile saline and insert it into the amniotic fluid under US guidance. This dye will make any leaking amniotic fluid obvious. Be aware, however, that instillation of the dye is very difficult in cases of severe oligohydramnios or anhydramnios. In this setting, amniocentesis can also cause contractions or vaginal bleeding.
New diagnostic tool on the horizon
Recent studies have focused on a new rapid test (AmniSure) that uses immunochromatography to detect trace amounts of placental α-microglobulin-1 protein.3 This protein is specific to amniotic fluid and present in vaginal secretions only when amniotic fluid is leaking through the cervix. One study of 203 patients suspected of having ruptured membranes found the AmniSure test to have a PPV of 100% and NPV of 99.1%.3 Although these findings are promising, further confirmatory studies are needed before this product can be recommended for widespread use.
CASE continued: Leakage of tinted fluid confirms PROM
Because the diagnostic steps taken so far have been inconclusive, J.S. undergoes amniocentesis with infusion of indigo carmine. Within 2 hours, blue dye is observed leaking from the cervix, confirming PROM. A sample of amniotic fluid obtained at the time of amniocentesis produces a negative gram stain and reveals a normal glucose level and leukocyte count. Amniotic fluid cultures are pending.
What is your next step?
Determining the best management strategy is next. The treatment plan should be based on gestational age, presence or absence of infection or labor, and fetal status. Therefore, the initial evaluation of a patient with PROM should focus on the collection of this clinical information.
I recommend these measures:
- Document the exact gestational age by careful review of available records and ultrasound biometry
- Identify indicators of infection, such as maternal fever and tachycardia, fundal tenderness, fetal tachycardia, and an elevated white blood cell count
- Amniocentesis may be required to rule out amnionitis in cases where the diagnosis is clinically unclear
- Document fetal presentation
- Initiate fetal heart rate (FHR) monitoring at the time of diagnosis and perform a biophysical profile (BPP).
Midtrimester PROM: 16 to 24 weeks’ gestation
Management differs for each gestational age.
Midtrimester PROM occurs in approximately 0.7% of all pregnancies and is a significant source of morbidity and mortality.4,5 It may be iatrogenic in nature when it follows an invasive procedure such as amniocentesis or fetoscopy. It also may occur spontaneously, with causes similar to those of PROM at later gestational ages. At this early gestational age, PROM is more likely to be associated with cervical incompetence and inflammation.6,7
Infection is a risk—and may be the underlying cause
Infection is associated with as many as 30% to 50% of cases of PROM.4,8-10 Half of the cases of intra-amniotic infection develop within 7 days after PROM. That’s because many cases of early PROM have infection or inflammation as their cause.
Intrauterine demise is common at early gestational ages
The risk of intrauterine fetal demise is inversely related to gestational age at the time of rupture. That is, the earlier the gestational age, the higher the rate of fetal death. One study found that the rate of intrauterine fetal demise was 33% when PROM occurred before 20 weeks’ gestation and 20% when it occurred between 20 and 24 weeks; it was rare after 25 weeks.10
Pulmonary hypoplasia is more common at this critical juncture
The midtrimester is a critical time for fetal lung development. During the canalicular stage (between 17 and 24 weeks’ gestation), the gas-exchanging acini and pulmonary capillaries are forming, so they are more susceptible to injury. The incidence of pulmonary hypoplasia is approximately 10% when PROM occurs earlier than 20 weeks’ gestation, although a wide range of rates has been reported.4,8-10 Pulmonary hypoplasia remains a significant cause of neonatal mortality and is found in as many as 77% of autopsies of infants from pregnancies complicated by midtrimester PROM.11
The incidence of pulmonary hypoplasia decreases by as much as 46% with each week of gestational age at the time of PROM.12 After 26 weeks, when the terminal sac stage of development occurs, the rate of pulmonary hypoplasia complicating PROM drops to less than 2%.12-14
The degree of oligohydramnios also affects the rate of pulmonary hypoplasia, which increases significantly when the amniotic fluid index is less than 5 cm.15
Limb deformity may be related to restricted movement
Although limb development occurs in the embryonic period, most limb growth takes place during the second and third trimesters.16 The restriction in movement and increased pressure associated with prolonged periods of oligohydramnios can lead to skeletal deformity in otherwise normal extremities.
The frequency of deformity varies widely among studies, but the mean incidence is 7%.4,8-11 A twofold higher incidence of skeletal abnormality occurs when midtrimester PROM is accompanied by severe oligohydramnios. In one study, the rate of skeletal abnormality was 54% when the deepest pocket of amniotic fluid was less than 1 cm, compared with 26% for matched pregnancies with a normal or mildly reduced volume.16
Maternal complications include retained placenta, endometritis
Maternal complications associated with very early PROM include a higher rate of cesarean section due to fetal malpresentation and FHR abnormalities, which often accompany oligohydramnios and intraamniotic infection.10 A classical incision is more likely in these cases due to the poorly developed lower uterine segment. Retained placenta necessitating postpartum curettage occurs in 9% to 18% of cases of PROM at less than 20 weeks’ gestation. In addition, postpartum endometritis complicates as many as 40% of cases of midtrimester PROM.4,8-11
General prognosis
The outcome of midtrimester PROM depends on the underlying cause. If it is iatrogenic, the outcome is usually favorable, with frequent resealing of the membranes; most cases end in a normal term delivery. The outcome of spontaneous PROM is more grim.
Midtrimester PROM has the same relatively short latency (approximately 17 days on average) as PROM that occurs later in pregnancy. Less than 50% of women with midtrimester PROM remain pregnant at the end of the first week, and as many as 75% of these women will have delivered by 28 days after PROM.11 These percentages indicate that most women with midtrimester PROM deliver before fetal viability can be attained, or in the risky periviable period.
Overall, midtrimester PROM is associated with significant fetal, neonatal, and maternal morbidity. The risks must be explained to the patient along with any management plan.
Given the very poor prognosis and small chance of prolonged latency, induction of labor and pregnancy termination are reasonable options at the time of presentation. The patient needs to know that expectant management can be associated with significant long-term morbidity and a higher rate of neonatal mortality.
CASE continued: Patient is apprised of the risks
After a frank discussion of the risks involved in continuing her pregnancy, J.S. chooses expectant management. Given the early gestational age and absence of any sign of infection, she is sent home for bed rest and instructed to check her temperature twice daily. She is told to return for evaluation if fever (>100°F) or symptoms of infection develop. Because of the very early gestational age, no steroid or antibiotic will be given until 24 weeks’ gestation, when she will be admitted for inpatient care.
PROM at 24 to 32 weeks’ gestation
Selecting a management strategy for a pregnancy at this gestational age means weighing the potential morbidity and mortality of immediate delivery against the morbidity and mortality of expectant management. At this gestational age, the principal source of fetal morbidity is prematurity itself. As many as 40% of infants delivered before 26 weeks’ gestation experience some type of long-term morbidity such as intraventricular hemorrhage (IVH), retinopathy of prematurity, necrotizing enterocolitis (NEC), or, most commonly, respiratory distress syndrome (RDS).16,17 It is true that fetal morbidity increases when chorioamnionitis is present, but the potential benefit of prolonging the pregnancy by 7 to 14 days is believed to outweigh the risk of infection at these gestational ages. Therefore, in the absence of contraindications, expectant management is the usual course of action.
Select patients carefully for expectant management
Consider expectant management only when fetal well-being can be documented, without evidence of infection. Abruption is a contraindication to expectant management, although the clinical nature of this diagnosis can make it difficult to identify. If abruption is diagnosed, aggressive management with labor augmentation or cesarean section and intravenous (IV) antibiotics is appropriate.
Repetitive fetal heart decelerations in the presence of active vaginal bleeding and uterine tenderness indicate placental insufficiency and are an indication for delivery.
More than 50% of patients deliver within the first week after PROM is diagnosed.18 At least 30% experience chorioamnionitis some time after the diagnosis of PROM, and 1% to 2% suffer cord prolapse.10,18,19 As many as 4% to 12% of cases of PROM will also be complicated by abruption,20,21 and a rate of intrauterine fetal demise as high as 1% has been documented.18 Therefore, if expectant management is selected, it should include close monitoring for these complications.
Hospitalization is warranted. Women who are stable and being managed expectantly should probably be hospitalized. One prospective trial comparing outcomes between women managed at home and women who were hospitalized found no significant difference in latency period or the rate of infection.22 However, the strict inclusion criteria for this study make it difficult to generalize the results. Only 18% of the 349 women screened for enrollment met these criteria.
The high rate of precipitous labor, frequent onset of infection, and need for frequent maternal and neonatal evaluation at this gestational age make hospitalization a prudent choice.
Fetal surveillance is mandatory
Most investigators would agree that a regular schedule of fetal surveillance is necessary during expectant management. But there is no clear evidence indicating which type, and what timing, of surveillance are best. It is clear that changes in the FHR pattern and BPP precede the onset of chorioamnionitis and intrauterine demise due to cord accidents.23-25 However, no studies have demonstrated a significant improvement in neonatal outcomes with daily or even twice-daily antenatal surveillance.
At our institution, we follow a regimen of daily surveillance, which consists of a nonstress test and/or BPP to confirm fetal well-being.
Tocolysis won’t prolong gestation beyond 48 hours…
There is no evidence that prolonged tocolysis with any therapy significantly increases long-term latency or improves any type of neonatal morbidity in pregnancies complicated by PROM. Tocolysis may prolong pregnancy over the short term (<48 hours),26,27 but its widespread use is not supported by the evidence.
Tocolysis is appropriate to achieve safe maternal transport or administer steroids.
…but corticosteroids are highly beneficial
Antenatal corticosteroids clearly improve neonatal outcomes when PROM occurs before 32 weeks’ gestation. Two large meta-analyses have found such benefits to be a decrease in the rates of RDS, IVH, NEC, and neonatal death.28,29 A recent prospective study confirmed these findings.30 The rate of RDS declined 26%—from 44% to 18%.
A consensus panel of the National Institutes of Health (NIH) recommended use of corticosteroids in cases of PROM between 24 and 32 weeks’ gestation in which there is no clinical evidence of infection.31 Any of the standard steroid regimens is appropriate. At our institution, we give an intramuscular injection of 12 mg of betamethasone and repeat this one time in 24 hours.
Are prophylactic antibiotics warranted?
The fact that infection is the most commonly identified cause of PROM prompts the question: Does treatment with IV antibiotics improve outcomes and prolong latency even in the absence of clinically apparent infection? Mercer and colleagues32 reported a significant reduction in chorioamnionitis, endometritis, and neonatal infection, including sepsis and pneumonia, in pregnancies treated with prophylactic antibiotics, compared with expectant management alone. In that study, latency also increased significantly following antibiotic therapy. Other meta-analyses confirm the benefits of prophylactic antibiotics, demonstrating a lower rate of neonatal sepsis and IVH following treatment.33,34
One large multicenter randomized trial found a reduced rate of IVH and RDS after treatment with IV erythromycin and ampicillin for 48 hours, followed by a 5-day course of amoxicillin and erythromycin.35 A Cochrane review of the use of prophylactic antibiotics in the setting of PROM included 19 studies with various antibiotic regimens.36 It concluded that antibiotic therapy prolongs latency (at both 48 hours and 7 days), decreases maternal infection, and reduces the incidence of neonatal complications, including infection, need for oxygen, IVH, and periventricular leukomalacia.
No superior regimen, but avoid amoxicillin-clavulanate. Although no single antibiotic regimen is clearly superior to the others, erythromycin has been associated with benefits most consistently. The most common dosage for erythromycin is 250 mg every 6 hours for a total of 48 hours and then an additional 5 days of oral treatment. Amoxicillin-clavulanate has been associated with an increased risk of NEC in at least two trials, and should probably be avoided. A Cochrane review confirms these conclusions.36
Choice of delivery route is flexible
Once the need for delivery arises, choose the route according to normal obstetric indications. In the setting of PROM with malpresentation, cesarean delivery is probably the best approach. However, in very-low-birth-weight infants, the best mode of delivery remains unclear.37 If the fetus is in cephalic presentation, an attempt at vaginal delivery does not appear to have a worse neonatal outcome.
If spontaneous labor does not occur or if induction is not indicated for maternal or fetal reasons, one may choose to deliver the patient at 32 weeks’ gestation or continue expectant management until 34 weeks’ gestation. This decision is discussed in more detail in the next section.
ALGORITHM
Management of PROM varies with gestational age
PROM at 32 to 34 weeks’ gestation
Although it is generally accepted that the fetus benefits from expectant management in pregnancies complicated by PROM before 32 weeks’ gestation, the management of PROM that arises between 32 and 34 weeks remains controversial and a focus of ongoing research. Because most neonatal morbidity is caused by prematurity, and the rate of prematurity-related complications decreases with increasing gestational age, some argue that the potential benefit of prolonging latency after 32 weeks’ gestation does not outweigh the risk of chorioamnionitis.
Continue the gestation? Or deliver?
Mercer and colleagues randomized 97 women with PROM between 32 and 36 weeks’ gestation and a mature lung profile to expectant management or immediate induction.38 Although expectant management did prolong pregnancy, no neonatal benefit was observed, and the rate of chorioamnionitis was higher with expectant management, with a longer hospital stay.
Cox and associates found a higher rate of chorioamnionitis among 68 women with PROM between 30 and 34 weeks’ gestation who were managed expectantly, compared with 61 women assigned to immediate induction.39 Neonatal morbidity was similar in both groups.
These studies suggest that expectant management after 32 weeks leads only to an increased rate of chorioamnionitis and longer maternal and neonatal hospitalization, without any demonstrable neonatal benefit. However, one significant limitation of these studies is the fact that patients managed expectantly received neither corticosteroids nor prophylactic antibiotics.
Are corticosteroids appropriate at this gestational age?
We lack sufficient evidence to support the routine use of corticosteroids after 32 weeks in pregnancies complicated by PROM. The NIH consensus panel suggested that they may be an option in patients without contraindications up to 34 weeks’ gestation.31
Some experts recommend testing for fetal lung maturity when PROM occurs between 32 and 34 weeks’ gestation. In this group, the rate of fetal lung maturity is between 50% and 60%.40,41 There is no clear benefit in prolonging a pregnancy when fetal lung maturity can be documented. However, in the setting of immature fetal lungs, expectant management may be appropriate following treatment with corticosteroids and a prophylactic antibiotic regimen. Patients who present at 34 weeks’ gestation or beyond are likely to benefit most from immediate delivery.
When expectant management is chosen between 32 and 34 weeks, inpatient hospitalization with daily monitoring is also recommended. The mode of delivery depends on the usual obstetric indications.
CASE resolved: Patient develops fever and spontaneous labor
Ten days after the documentation of PROM, J.S. reports a fever and abdominal tenderness, as well as frequent uterine contractions that began early in the day. She is admitted to the hospital. A physical examination confirms clinically apparent intra-amniotic infection and labor. The patient is started on IV antibiotics and, after a short labor, delivers a nonviable male infant weighing 500 g. Pathologic examination of the fetus and placenta reveals a normal, immature fetus with evidence of acute chorioamnionitis on placental sections.
CASE: PROM at 22 weeks
J.S. is a 22-year-old woman at 22 weeks’ gestation in her second pregnancy. Her first gestation ended in spontaneous abortion at 10 weeks, followed by dilation and curettage. She has been referred to you by her midwife, who is concerned about J.S.’s complaints of loss of fluid over the past 2 weeks and who cannot document rupture of membranes by the usual means.
In your office, J.S. continues to complain of intermittent leakage of clear fluid. She says there has been no vaginal bleeding, foul-smelling discharge, fever, chills, or abdominal tenderness. You find a normal abdomen. A sterile speculum exam is equivocal, without evidence of pooling or ferning; a nitrazine test is positive, however. A complete blood count reveals no evidence of leukocytosis. Urinalysis is negative.
You suspect preterm premature rupture of membranes (PROM) when bedside ultrasonography (US) documents oligohydramnios with an amniotic fluid index of less than 5 cm. The kidneys, bladder, and stomach all appear normal.
What is the best way to confirm the diagnosis? What is the most appropriate management at this gestational age? And how do you counsel J.S. about the risk to her, and her baby, of continuing the pregnancy?
Given the very poor prognosis of many cases of early PROM, accurate diagnosis is critical to determine the best management strategy. The gold standard of diagnosis is sterile vaginal examination with a speculum to identify clear fluid leaking from the cervix or pooling in the posterior fornix. Use nitrazine paper to assess the fluid collected from the posterior fornix for alkaline pH; this method has a positive predictive value (PPV) of 99% and negative predictive value (NPV) of 96%.1 The appearance of “ferning”—a crystalline pattern that occurs when the saline amniotic fluid dries—carries a PPV of 98% to 99% and a NPV of 90% to 99%.2
One must also consider the patient’s history. When that history and the physical exam fail to render a clear result, use US to assess the amniotic fluid volume. A low volume in the presence of a convincing clinical history is very suspicious for PROM, as in the case just described.
Tinting the amniotic fluid may help
In equivocal cases, mix 1 to 3 mL of indigo carmine with 5 mL of sterile saline and insert it into the amniotic fluid under US guidance. This dye will make any leaking amniotic fluid obvious. Be aware, however, that instillation of the dye is very difficult in cases of severe oligohydramnios or anhydramnios. In this setting, amniocentesis can also cause contractions or vaginal bleeding.
New diagnostic tool on the horizon
Recent studies have focused on a new rapid test (AmniSure) that uses immunochromatography to detect trace amounts of placental α-microglobulin-1 protein.3 This protein is specific to amniotic fluid and present in vaginal secretions only when amniotic fluid is leaking through the cervix. One study of 203 patients suspected of having ruptured membranes found the AmniSure test to have a PPV of 100% and NPV of 99.1%.3 Although these findings are promising, further confirmatory studies are needed before this product can be recommended for widespread use.
CASE continued: Leakage of tinted fluid confirms PROM
Because the diagnostic steps taken so far have been inconclusive, J.S. undergoes amniocentesis with infusion of indigo carmine. Within 2 hours, blue dye is observed leaking from the cervix, confirming PROM. A sample of amniotic fluid obtained at the time of amniocentesis produces a negative gram stain and reveals a normal glucose level and leukocyte count. Amniotic fluid cultures are pending.
What is your next step?
Determining the best management strategy is next. The treatment plan should be based on gestational age, presence or absence of infection or labor, and fetal status. Therefore, the initial evaluation of a patient with PROM should focus on the collection of this clinical information.
I recommend these measures:
- Document the exact gestational age by careful review of available records and ultrasound biometry
- Identify indicators of infection, such as maternal fever and tachycardia, fundal tenderness, fetal tachycardia, and an elevated white blood cell count
- Amniocentesis may be required to rule out amnionitis in cases where the diagnosis is clinically unclear
- Document fetal presentation
- Initiate fetal heart rate (FHR) monitoring at the time of diagnosis and perform a biophysical profile (BPP).
Midtrimester PROM: 16 to 24 weeks’ gestation
Management differs for each gestational age.
Midtrimester PROM occurs in approximately 0.7% of all pregnancies and is a significant source of morbidity and mortality.4,5 It may be iatrogenic in nature when it follows an invasive procedure such as amniocentesis or fetoscopy. It also may occur spontaneously, with causes similar to those of PROM at later gestational ages. At this early gestational age, PROM is more likely to be associated with cervical incompetence and inflammation.6,7
Infection is a risk—and may be the underlying cause
Infection is associated with as many as 30% to 50% of cases of PROM.4,8-10 Half of the cases of intra-amniotic infection develop within 7 days after PROM. That’s because many cases of early PROM have infection or inflammation as their cause.
Intrauterine demise is common at early gestational ages
The risk of intrauterine fetal demise is inversely related to gestational age at the time of rupture. That is, the earlier the gestational age, the higher the rate of fetal death. One study found that the rate of intrauterine fetal demise was 33% when PROM occurred before 20 weeks’ gestation and 20% when it occurred between 20 and 24 weeks; it was rare after 25 weeks.10
Pulmonary hypoplasia is more common at this critical juncture
The midtrimester is a critical time for fetal lung development. During the canalicular stage (between 17 and 24 weeks’ gestation), the gas-exchanging acini and pulmonary capillaries are forming, so they are more susceptible to injury. The incidence of pulmonary hypoplasia is approximately 10% when PROM occurs earlier than 20 weeks’ gestation, although a wide range of rates has been reported.4,8-10 Pulmonary hypoplasia remains a significant cause of neonatal mortality and is found in as many as 77% of autopsies of infants from pregnancies complicated by midtrimester PROM.11
The incidence of pulmonary hypoplasia decreases by as much as 46% with each week of gestational age at the time of PROM.12 After 26 weeks, when the terminal sac stage of development occurs, the rate of pulmonary hypoplasia complicating PROM drops to less than 2%.12-14
The degree of oligohydramnios also affects the rate of pulmonary hypoplasia, which increases significantly when the amniotic fluid index is less than 5 cm.15
Limb deformity may be related to restricted movement
Although limb development occurs in the embryonic period, most limb growth takes place during the second and third trimesters.16 The restriction in movement and increased pressure associated with prolonged periods of oligohydramnios can lead to skeletal deformity in otherwise normal extremities.
The frequency of deformity varies widely among studies, but the mean incidence is 7%.4,8-11 A twofold higher incidence of skeletal abnormality occurs when midtrimester PROM is accompanied by severe oligohydramnios. In one study, the rate of skeletal abnormality was 54% when the deepest pocket of amniotic fluid was less than 1 cm, compared with 26% for matched pregnancies with a normal or mildly reduced volume.16
Maternal complications include retained placenta, endometritis
Maternal complications associated with very early PROM include a higher rate of cesarean section due to fetal malpresentation and FHR abnormalities, which often accompany oligohydramnios and intraamniotic infection.10 A classical incision is more likely in these cases due to the poorly developed lower uterine segment. Retained placenta necessitating postpartum curettage occurs in 9% to 18% of cases of PROM at less than 20 weeks’ gestation. In addition, postpartum endometritis complicates as many as 40% of cases of midtrimester PROM.4,8-11
General prognosis
The outcome of midtrimester PROM depends on the underlying cause. If it is iatrogenic, the outcome is usually favorable, with frequent resealing of the membranes; most cases end in a normal term delivery. The outcome of spontaneous PROM is more grim.
Midtrimester PROM has the same relatively short latency (approximately 17 days on average) as PROM that occurs later in pregnancy. Less than 50% of women with midtrimester PROM remain pregnant at the end of the first week, and as many as 75% of these women will have delivered by 28 days after PROM.11 These percentages indicate that most women with midtrimester PROM deliver before fetal viability can be attained, or in the risky periviable period.
Overall, midtrimester PROM is associated with significant fetal, neonatal, and maternal morbidity. The risks must be explained to the patient along with any management plan.
Given the very poor prognosis and small chance of prolonged latency, induction of labor and pregnancy termination are reasonable options at the time of presentation. The patient needs to know that expectant management can be associated with significant long-term morbidity and a higher rate of neonatal mortality.
CASE continued: Patient is apprised of the risks
After a frank discussion of the risks involved in continuing her pregnancy, J.S. chooses expectant management. Given the early gestational age and absence of any sign of infection, she is sent home for bed rest and instructed to check her temperature twice daily. She is told to return for evaluation if fever (>100°F) or symptoms of infection develop. Because of the very early gestational age, no steroid or antibiotic will be given until 24 weeks’ gestation, when she will be admitted for inpatient care.
PROM at 24 to 32 weeks’ gestation
Selecting a management strategy for a pregnancy at this gestational age means weighing the potential morbidity and mortality of immediate delivery against the morbidity and mortality of expectant management. At this gestational age, the principal source of fetal morbidity is prematurity itself. As many as 40% of infants delivered before 26 weeks’ gestation experience some type of long-term morbidity such as intraventricular hemorrhage (IVH), retinopathy of prematurity, necrotizing enterocolitis (NEC), or, most commonly, respiratory distress syndrome (RDS).16,17 It is true that fetal morbidity increases when chorioamnionitis is present, but the potential benefit of prolonging the pregnancy by 7 to 14 days is believed to outweigh the risk of infection at these gestational ages. Therefore, in the absence of contraindications, expectant management is the usual course of action.
Select patients carefully for expectant management
Consider expectant management only when fetal well-being can be documented, without evidence of infection. Abruption is a contraindication to expectant management, although the clinical nature of this diagnosis can make it difficult to identify. If abruption is diagnosed, aggressive management with labor augmentation or cesarean section and intravenous (IV) antibiotics is appropriate.
Repetitive fetal heart decelerations in the presence of active vaginal bleeding and uterine tenderness indicate placental insufficiency and are an indication for delivery.
More than 50% of patients deliver within the first week after PROM is diagnosed.18 At least 30% experience chorioamnionitis some time after the diagnosis of PROM, and 1% to 2% suffer cord prolapse.10,18,19 As many as 4% to 12% of cases of PROM will also be complicated by abruption,20,21 and a rate of intrauterine fetal demise as high as 1% has been documented.18 Therefore, if expectant management is selected, it should include close monitoring for these complications.
Hospitalization is warranted. Women who are stable and being managed expectantly should probably be hospitalized. One prospective trial comparing outcomes between women managed at home and women who were hospitalized found no significant difference in latency period or the rate of infection.22 However, the strict inclusion criteria for this study make it difficult to generalize the results. Only 18% of the 349 women screened for enrollment met these criteria.
The high rate of precipitous labor, frequent onset of infection, and need for frequent maternal and neonatal evaluation at this gestational age make hospitalization a prudent choice.
Fetal surveillance is mandatory
Most investigators would agree that a regular schedule of fetal surveillance is necessary during expectant management. But there is no clear evidence indicating which type, and what timing, of surveillance are best. It is clear that changes in the FHR pattern and BPP precede the onset of chorioamnionitis and intrauterine demise due to cord accidents.23-25 However, no studies have demonstrated a significant improvement in neonatal outcomes with daily or even twice-daily antenatal surveillance.
At our institution, we follow a regimen of daily surveillance, which consists of a nonstress test and/or BPP to confirm fetal well-being.
Tocolysis won’t prolong gestation beyond 48 hours…
There is no evidence that prolonged tocolysis with any therapy significantly increases long-term latency or improves any type of neonatal morbidity in pregnancies complicated by PROM. Tocolysis may prolong pregnancy over the short term (<48 hours),26,27 but its widespread use is not supported by the evidence.
Tocolysis is appropriate to achieve safe maternal transport or administer steroids.
…but corticosteroids are highly beneficial
Antenatal corticosteroids clearly improve neonatal outcomes when PROM occurs before 32 weeks’ gestation. Two large meta-analyses have found such benefits to be a decrease in the rates of RDS, IVH, NEC, and neonatal death.28,29 A recent prospective study confirmed these findings.30 The rate of RDS declined 26%—from 44% to 18%.
A consensus panel of the National Institutes of Health (NIH) recommended use of corticosteroids in cases of PROM between 24 and 32 weeks’ gestation in which there is no clinical evidence of infection.31 Any of the standard steroid regimens is appropriate. At our institution, we give an intramuscular injection of 12 mg of betamethasone and repeat this one time in 24 hours.
Are prophylactic antibiotics warranted?
The fact that infection is the most commonly identified cause of PROM prompts the question: Does treatment with IV antibiotics improve outcomes and prolong latency even in the absence of clinically apparent infection? Mercer and colleagues32 reported a significant reduction in chorioamnionitis, endometritis, and neonatal infection, including sepsis and pneumonia, in pregnancies treated with prophylactic antibiotics, compared with expectant management alone. In that study, latency also increased significantly following antibiotic therapy. Other meta-analyses confirm the benefits of prophylactic antibiotics, demonstrating a lower rate of neonatal sepsis and IVH following treatment.33,34
One large multicenter randomized trial found a reduced rate of IVH and RDS after treatment with IV erythromycin and ampicillin for 48 hours, followed by a 5-day course of amoxicillin and erythromycin.35 A Cochrane review of the use of prophylactic antibiotics in the setting of PROM included 19 studies with various antibiotic regimens.36 It concluded that antibiotic therapy prolongs latency (at both 48 hours and 7 days), decreases maternal infection, and reduces the incidence of neonatal complications, including infection, need for oxygen, IVH, and periventricular leukomalacia.
No superior regimen, but avoid amoxicillin-clavulanate. Although no single antibiotic regimen is clearly superior to the others, erythromycin has been associated with benefits most consistently. The most common dosage for erythromycin is 250 mg every 6 hours for a total of 48 hours and then an additional 5 days of oral treatment. Amoxicillin-clavulanate has been associated with an increased risk of NEC in at least two trials, and should probably be avoided. A Cochrane review confirms these conclusions.36
Choice of delivery route is flexible
Once the need for delivery arises, choose the route according to normal obstetric indications. In the setting of PROM with malpresentation, cesarean delivery is probably the best approach. However, in very-low-birth-weight infants, the best mode of delivery remains unclear.37 If the fetus is in cephalic presentation, an attempt at vaginal delivery does not appear to have a worse neonatal outcome.
If spontaneous labor does not occur or if induction is not indicated for maternal or fetal reasons, one may choose to deliver the patient at 32 weeks’ gestation or continue expectant management until 34 weeks’ gestation. This decision is discussed in more detail in the next section.
ALGORITHM
Management of PROM varies with gestational age
PROM at 32 to 34 weeks’ gestation
Although it is generally accepted that the fetus benefits from expectant management in pregnancies complicated by PROM before 32 weeks’ gestation, the management of PROM that arises between 32 and 34 weeks remains controversial and a focus of ongoing research. Because most neonatal morbidity is caused by prematurity, and the rate of prematurity-related complications decreases with increasing gestational age, some argue that the potential benefit of prolonging latency after 32 weeks’ gestation does not outweigh the risk of chorioamnionitis.
Continue the gestation? Or deliver?
Mercer and colleagues randomized 97 women with PROM between 32 and 36 weeks’ gestation and a mature lung profile to expectant management or immediate induction.38 Although expectant management did prolong pregnancy, no neonatal benefit was observed, and the rate of chorioamnionitis was higher with expectant management, with a longer hospital stay.
Cox and associates found a higher rate of chorioamnionitis among 68 women with PROM between 30 and 34 weeks’ gestation who were managed expectantly, compared with 61 women assigned to immediate induction.39 Neonatal morbidity was similar in both groups.
These studies suggest that expectant management after 32 weeks leads only to an increased rate of chorioamnionitis and longer maternal and neonatal hospitalization, without any demonstrable neonatal benefit. However, one significant limitation of these studies is the fact that patients managed expectantly received neither corticosteroids nor prophylactic antibiotics.
Are corticosteroids appropriate at this gestational age?
We lack sufficient evidence to support the routine use of corticosteroids after 32 weeks in pregnancies complicated by PROM. The NIH consensus panel suggested that they may be an option in patients without contraindications up to 34 weeks’ gestation.31
Some experts recommend testing for fetal lung maturity when PROM occurs between 32 and 34 weeks’ gestation. In this group, the rate of fetal lung maturity is between 50% and 60%.40,41 There is no clear benefit in prolonging a pregnancy when fetal lung maturity can be documented. However, in the setting of immature fetal lungs, expectant management may be appropriate following treatment with corticosteroids and a prophylactic antibiotic regimen. Patients who present at 34 weeks’ gestation or beyond are likely to benefit most from immediate delivery.
When expectant management is chosen between 32 and 34 weeks, inpatient hospitalization with daily monitoring is also recommended. The mode of delivery depends on the usual obstetric indications.
CASE resolved: Patient develops fever and spontaneous labor
Ten days after the documentation of PROM, J.S. reports a fever and abdominal tenderness, as well as frequent uterine contractions that began early in the day. She is admitted to the hospital. A physical examination confirms clinically apparent intra-amniotic infection and labor. The patient is started on IV antibiotics and, after a short labor, delivers a nonviable male infant weighing 500 g. Pathologic examination of the fetus and placenta reveals a normal, immature fetus with evidence of acute chorioamnionitis on placental sections.
1. Abe T. The detection of rupture of fetal membranes with the nitrazine indicator. Am J Obstet Gynecol. 1940;39:400.-
2. Davidson KM. Detection of premature rupture of the membranes. Clin Obstet Gynecol. 1991;34:715-722.
3. Cousins LM, Smok D, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of ruptured membranes. Am J Perinatol. 2005;22(6):317-320.
4. Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol. 1984;64:615-620.
5. Schucker JL, Mercer BM. Midtrimester premature rupture of the membranes. Semin Perinatol. 1996;20:389-400.
6. Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972-978.
7. Morales WJ. The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol. 1987;70:183-186.
8. Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390-396.
9. Beydoun SN, Yasin SY. Premature rupture of the membranes before 28 weeks: conservative management. Am J Obstet Gynecol. 1986;155:471-479.
10. Major CA, Kitzmiller JL. Perinatal survival with expectant management of midtrimester rupture of membranes. Am J Obstet Gynecol. 1990;163:838-844.
11. Falk SJ, Campbell LJ, Lee-Parritz A, et al. Expectant management in spontaneous preterm premature rupture of membranes between 14 and 24 weeks’ gestation. J Perinatol. 2004;24:611-616.
12. Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996;175:675-681.
13. Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46-52.
14. Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second-trimester rupture of membranes. Obstet Gynecol. 1998;92:895-901.
15. Vergani P, Ghidini A, Locatelli A, et al. Risk factors for pulmonary hypoplasia in second-trimester premature rupture of membranes. Am J Obstet Gynecol. 1994;170:1359-1364.
16. Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child. 1988;63:1146-1150.
17. Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632-1639.
18. Nelson LH, Anderson RL, O’Shea TM, Swain M. Expectant management of preterm premature rupture of membranes. Am J Obstet Gynecol. 1994;171:350-356.
19. Belady PH, Farkouh LJ, Gibbs RS. Intra-amniotic infection and premature rupture of the membranes. Clin Perinatol. 1997;24:43-57.
20. Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88:309-318.
21. Gonen R, Hannah ME, Milligan JE. Does prolonged preterm premature rupture of the membranes predispose to abruptio placentae? Obstet Gynecol. 1989;74:347-350.
22. Carlan SJ, O’Brien WF, Parsons MT, Lense JJ. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
23. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol. 1986;155:149-153.
24. Smith CV, Greenspoon J, Phelan JP, Platt LD. Clinical utility of the nonstress test in the conservative management of women with preterm spontaneous premature rupture of the membranes. J Reprod Med. 1987;32:1-4.
25. Hanley ML, Vintzileos AM. Biophysical testing in premature rupture of the membranes. Semin Perinatol. 1996;20:418-425.
26. Levy DL, Warsof SL. Oral ritodrine and preterm premature rupture of membranes. Obstet Gynecol. 1985;66:621-623.
27. Weiner CP, Renk K, Klugman M. The therapeutic efficacy and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:216-222.
28. Ohlsson A. Treatments of preterm premature rupture of the membranes: a meta-analysis. Am J Obstet Gynecol. 1989;160:890-906.
29. Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322-335.
30. Lewis DF, Brody K, Edwards MS, et al. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
31. National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413-418.
32. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989-995.
33. Egarter C, Leitich H, Karas H, et al. Antibiotic treatment in preterm premature rupture of membranes and neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 1996;174:589-597.
34. Ananth CV, Guise JM, Thorp JM, Jr. Utility of antibiotic therapy in preterm premature rupture of membranes: a meta-analysis. Obstet Gynecol Surv. 1996;51:324-328.
35. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979-988.
36. Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2003;(2):CD001058.-
37. Bottoms SF, Paul RH, Iams JD, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960-966.
38. Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
39. Cox SM, Leveno KJ. Intentional delivery versus expectant management with preterm ruptured membranes at 30-34 weeks’ gestation. Obstet Gynecol. 1995;86:875-879.
40. Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331-336.
41. Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316-321.
Dr. Esplin receives support from the National Institute of Child Health and Human Development and is a speaker for Adeza.
1. Abe T. The detection of rupture of fetal membranes with the nitrazine indicator. Am J Obstet Gynecol. 1940;39:400.-
2. Davidson KM. Detection of premature rupture of the membranes. Clin Obstet Gynecol. 1991;34:715-722.
3. Cousins LM, Smok D, Lovett SM, Poeltler DM. AmniSure placental alpha microglobulin-1 rapid immunoassay versus standard diagnostic methods for detection of ruptured membranes. Am J Perinatol. 2005;22(6):317-320.
4. Taylor J, Garite TJ. Premature rupture of membranes before fetal viability. Obstet Gynecol. 1984;64:615-620.
5. Schucker JL, Mercer BM. Midtrimester premature rupture of the membranes. Semin Perinatol. 1996;20:389-400.
6. Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972-978.
7. Morales WJ. The effect of chorioamnionitis on the developmental outcome of preterm infants at one year. Obstet Gynecol. 1987;70:183-186.
8. Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390-396.
9. Beydoun SN, Yasin SY. Premature rupture of the membranes before 28 weeks: conservative management. Am J Obstet Gynecol. 1986;155:471-479.
10. Major CA, Kitzmiller JL. Perinatal survival with expectant management of midtrimester rupture of membranes. Am J Obstet Gynecol. 1990;163:838-844.
11. Falk SJ, Campbell LJ, Lee-Parritz A, et al. Expectant management in spontaneous preterm premature rupture of membranes between 14 and 24 weeks’ gestation. J Perinatol. 2004;24:611-616.
12. Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996;175:675-681.
13. Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46-52.
14. Farooqi A, Holmgren PA, Engberg S, Serenius F. Survival and 2-year outcome with expectant management of second-trimester rupture of membranes. Obstet Gynecol. 1998;92:895-901.
15. Vergani P, Ghidini A, Locatelli A, et al. Risk factors for pulmonary hypoplasia in second-trimester premature rupture of membranes. Am J Obstet Gynecol. 1994;170:1359-1364.
16. Blott M, Greenough A. Neonatal outcome after prolonged rupture of the membranes starting in the second trimester. Arch Dis Child. 1988;63:1146-1150.
17. Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179:1632-1639.
18. Nelson LH, Anderson RL, O’Shea TM, Swain M. Expectant management of preterm premature rupture of membranes. Am J Obstet Gynecol. 1994;171:350-356.
19. Belady PH, Farkouh LJ, Gibbs RS. Intra-amniotic infection and premature rupture of the membranes. Clin Perinatol. 1997;24:43-57.
20. Ananth CV, Savitz DA, Williams MA. Placental abruption and its association with hypertension and prolonged rupture of membranes: a methodologic review and meta-analysis. Obstet Gynecol. 1996;88:309-318.
21. Gonen R, Hannah ME, Milligan JE. Does prolonged preterm premature rupture of the membranes predispose to abruptio placentae? Obstet Gynecol. 1989;74:347-350.
22. Carlan SJ, O’Brien WF, Parsons MT, Lense JJ. Preterm premature rupture of membranes: a randomized study of home versus hospital management. Obstet Gynecol. 1993;81:61-64.
23. Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. The use of the nonstress test in patients with premature rupture of the membranes. Am J Obstet Gynecol. 1986;155:149-153.
24. Smith CV, Greenspoon J, Phelan JP, Platt LD. Clinical utility of the nonstress test in the conservative management of women with preterm spontaneous premature rupture of the membranes. J Reprod Med. 1987;32:1-4.
25. Hanley ML, Vintzileos AM. Biophysical testing in premature rupture of the membranes. Semin Perinatol. 1996;20:418-425.
26. Levy DL, Warsof SL. Oral ritodrine and preterm premature rupture of membranes. Obstet Gynecol. 1985;66:621-623.
27. Weiner CP, Renk K, Klugman M. The therapeutic efficacy and cost-effectiveness of aggressive tocolysis for premature labor associated with premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:216-222.
28. Ohlsson A. Treatments of preterm premature rupture of the membranes: a meta-analysis. Am J Obstet Gynecol. 1989;160:890-906.
29. Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322-335.
30. Lewis DF, Brody K, Edwards MS, et al. Preterm premature ruptured membranes: a randomized trial of steroids after treatment with antibiotics. Obstet Gynecol. 1996;88:801-805.
31. National Institutes of Health. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413-418.
32. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction of infant morbidity after preterm premature rupture of the membranes. A randomized controlled trial. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. JAMA. 1997;278:989-995.
33. Egarter C, Leitich H, Karas H, et al. Antibiotic treatment in preterm premature rupture of membranes and neonatal morbidity: a metaanalysis. Am J Obstet Gynecol. 1996;174:589-597.
34. Ananth CV, Guise JM, Thorp JM, Jr. Utility of antibiotic therapy in preterm premature rupture of membranes: a meta-analysis. Obstet Gynecol Surv. 1996;51:324-328.
35. Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979-988.
36. Kenyon S, Boulvain M, Neilson J. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2003;(2):CD001058.-
37. Bottoms SF, Paul RH, Iams JD, et al. Obstetric determinants of neonatal survival: influence of willingness to perform cesarean delivery on survival of extremely low-birth-weight infants. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1997;176:960-966.
38. Mercer BM, Crocker LG, Boe NM, Sibai BM. Induction versus expectant management in premature rupture of the membranes with mature amniotic fluid at 32 to 36 weeks: a randomized trial. Am J Obstet Gynecol. 1993;169:775-782.
39. Cox SM, Leveno KJ. Intentional delivery versus expectant management with preterm ruptured membranes at 30-34 weeks’ gestation. Obstet Gynecol. 1995;86:875-879.
40. Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331-336.
41. Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316-321.
Dr. Esplin receives support from the National Institute of Child Health and Human Development and is a speaker for Adeza.