User login
Case
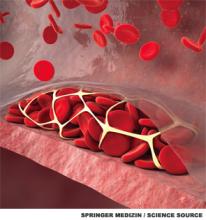
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
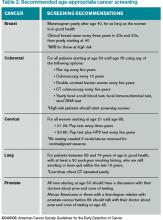
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
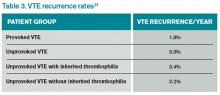
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.
Case
A 56-year-old woman with hypertension and diabetes presents to the hospital with acute onset of painful swelling in her right calf. She has had no recent surgeries, trauma, or travel, and takes lisinopril and metformin. An ultrasound of her right lower extremity demonstrates a venous thromboembolism (VTE). The patient’s last mammogram was three years ago, and she’s never undergone a screening colonoscopy. On lab workup, she is noted to have a microcytic anemia.
Should this patient be screened for an underlying hypercoagulable state or malignancy?
Background
An estimated 550,000 hospitalized adults are diagnosed with VTE each year.1 VTE can occur in the absence of known precipitants (unprovoked) or can be temporally associated with a known major risk factor (provoked). This practical division has implications for both treatment duration and risk of recurrence. A VTE is considered provoked if it occurs in the setting of surgery, leg trauma, fracture, pregnancy within the previous three months, estrogen therapy, immobility from an acute illness for more than one week, travel lasting more than six hours, or active malignancy.2 If none of these provoking factors is present, the VTE is considered unprovoked.2
Nearly 20% of first-time VTE events can be attributed to malignancy.3 Additionally, patients presenting with an unprovoked VTE possess a higher risk of being diagnosed with a cancer, raising the question of whether unprovoked VTEs should compel aggressive malignancy screening.4
Before the discovery of antithrombin deficiency in 1965, most unprovoked VTE events remained unexplained. Since then, numerous inherited coagulation abnormalities have been identified. It is now estimated that coagulation abnormalities can be found in up to half of patients with unprovoked thrombi.5
The increase in availability of molecular and genetic assays for hypercoagulability has been accompanied by a dramatic rise in the rate of testing for these disorders.6 Despite increased testing available for inherited thrombophilias, disagreement exists over the utility of this workup.6
Review of the Data
Hypercoagulability leading to venous thrombosis can be broadly divided into two groups: acquired and hereditary (see Table 1). First, let’s examine acquired hypercoagulable states.
Malignancy: Armand Trousseau first suggested an association between thrombotic events and malignancy in 1865. Malignancy causes a hypercoagulable state; additionally, tumors can cause thromboemboli by other mechanisms, such as vascular invasion or external compression of vasculature.7
Multiple studies demonstrate that malignancy increases the chance of developing a VTE. A Danish cohort study of nearly 60,000 cancer patients compared with over 280,000 controls over nine years offered twice the incidence of VTE in patients with cancer.8 Other studies reveal that VTE rates peak in the first year after a cancer diagnosis; moreover, VTE events are associated with more advanced disease and worse prognosis.9 Approximately 11% of cancer patients will develop a clinically evident VTE during the course of their disease.10,11
The majority of cancers associated with VTE events are clinically evident; however, some patients with thrombi have an occult malignancy. During the two years following an unprovoked VTE, the rate of discovering a previously undiagnosed malignancy was three times higher when compared with provoked VTE.6
This potential to diagnose occult malignancy in patients with idiopathic thromboembolic events stimulates debate around the usefulness of extensive cancer screening for these patients. One large systematic review compared routine and extensive cancer screening strategies following an unprovoked VTE. An extensive screening strategy consisting of CT scans of the abdomen and pelvis significantly increased the proportion of previously undiagnosed cancers; however, the authors did not determine complication rates, cost effectiveness, or difference in morbidity and mortality associated with extensive screening strategies.7
Other studies have demonstrated that extensive screening with CT, endoscopy, and tumor markers finds more previously undetected cancers; however, up to half of these malignancies could have been identified without resorting to such expensive and invasive workups.12 Additionally, no prospective data demonstrate improved outcomes or increased survival from these diagnoses. Likewise, no cost-effectiveness data exist to support this expensive and aggressive screening approach.7
All patients with an idiopathic VTE should undergo a complete history and physical examination with attention to common areas of malignancy. Patients should have basic lab work and be recommended for age-appropriate cancer screening (see Table 2). Any abnormalities uncovered on this initial workup should be aggressively investigated.13 If overt cancer is detected, then low molecular weight heparin would be preferred over oral anticoagulation as treatment for the VTE.14 Extensive malignancy evaluation in all patients with unprovoked VTE is not warranted, however, given the lack of data regarding efficacy of extensive screening, the potential for increased harms, and the costs associated with this approach.
Antiphospholipid syndrome: Antiphospholipid syndrome is the most common acquired cause of thrombophilia.15 Characterized by the presence of antiphospholipid antibodies (e.g. lupus anticoagulant antibodies or anticardiolipin antibodies), this syndrome is usually secondary to cancer or an autoimmune disease.
Antiphospholipid antibody syndrome is a thrombophilic disorder in which both venous and arterial thrombosis may occur. Patients with this disorder are considered at high risk for thrombotic events. Data suggest that antiphospholipid antibody syndrome also increases the risk of VTE recurrence. In one retrospective study, cessation of warfarin therapy in patients with antiphospholipid antibodies after a VTE resulted in 69% of patients having recurrent thrombosis in the first year.16 Given this substantial risk, antiphospholipid antibody testing is recommended in those with a suggestive history, including patients with 1) recurrent fetal loss, 2) fetal loss after 10 weeks, or 3) known collagen vascular disease.16 Lifelong anticoagulation is recommended for these patients.
Inherited hypercoagulable states: The most frequent causes of an inherited hypercoagulable state are the factor V Leiden mutation and the prothrombin gene mutation, accounting for 50% to 60% of hereditary thrombophilias. Protein S, protein C, and antithrombin defects account for most of the remaining cases of inherited thrombophilias.15
Currently, there is no consensus regarding who should be tested for inherited thrombophilia. Testing for an inherited thrombophilia would be indicated if the results added prognostic information or changed management. Arguments against testing hinge on the fact that neither prognosis nor management is affected by the presence of an inherited thrombophilia.
The presence of a thrombophilia also does not change the method or intensity of anticoagulation.17 The risk of recurrence after discontinuing anticoagulation therapy is not affected.17,18 The strongest predictor of VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia.15 Recurrent VTE is nearly twice as frequent in patients with idiopathic VTE compared to those with provoked VTE.15
The American College of Chest Physicians (ACCP) recommends treating a provoked VTE for three months.19 According to the same guidelines, an unprovoked VTE should be treated for a minimum of three months, and lifelong anticoagulation should be considered.19
Overall, the rate of recurrence after a first VTE is considerable after completion of anticoagulation, especially for an unprovoked thrombotic event. Studies show a 7%-15% recurrence rate during the two years following the index VTE (see Table 3).17,20,21 Currently, no data suggest that a hereditary thrombophilia substantially changes this baseline high recurrent risk. ACCP recommendations state that the presence of hereditary thrombophilia should not be used as a major factor to guide duration of anticoagulation.19
Back to the Case
Our patient presented with an unprovoked VTE. She should be started on anticoagulation therapy with low molecular weight heparin and transitioned to oral anticoagulation.
Her highest risk for VTE recurrence is the unprovoked VTE itself, regardless of an underlying thrombophilia. Since the presence of an inherited thrombophilia will not change duration or intensity of management, our patient should not be tested.
There are no prospective trials showing improved outcomes from aggressive workup for occult malignancy. Given this information, an extensive workup for occult malignancy should not be undertaken; however, this patient has an idiopathic VTE and should undergo a complete history, physical examination, and basic lab work, with attention to common areas of malignancy. Any abnormalities uncovered on this initial workup should be investigated more aggressively. Screening with mammography and Pap smear should be arranged in outpatient follow-up and communicated to the primary care physician, because she is not up to date with these age-appropriate screening tests.
Based on new evidence, a low-dose chest CT would be a consideration if she had a smoking history of at least 30 pack-years.22 Her microcytic anemia uncovered on routine lab work should be investigated further for a possible underlying gastrointestinal malignancy.
Bottom Line
An initial diagnosis of unprovoked VTE remains the strongest risk factor for recurrent thromboembolic events. The presence of an inherited thrombophilia does not significantly alter management. Aggressive workup for occult malignancy has not prospectively improved outcomes, but age-appropriate malignancy screening should be recommended.
Drs. Czernik and Anderson are hospitalists and instructors of medicine at the University of Colorado Denver (UCD). Dr. Wolfe is a hospitalist and assistant professor of medicine at UCD. Dr. Cumbler is a hospitalist and associate professor of medicine at UCD.
References
- Centers for Disease Control and Prevention. Venous thromboembolism in adult hospitalizations—United States, 2007–2009. MMWR Morb Mortal Wkly Rep. 2012;61(22);401-404.
- Baglin T, Gray E, Greaves M, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149(2):209-220.
- Heit, JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162(11):1245-1248.
- Iodice S, Gandini S, Löhr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost. 2008;6(9):1474-1477.
- Coppens M, van Mourik JA, Eckmann CM, Büller HR, Middeldorp S. Current practise of testing for inherited thrombophilia. J Thromb Haemost. 2007;5(9):1979-1981.
- Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149(5):323-333.
- Cronin-Fenton DP, Søndergaard F, Pedersen LA, et al. Hospitalisation for venous thromboembolism in cancer patients and the general population: a population-based cohort study in Denmark, 1997-2006. Br J Cancer. 2010;103(7):947-953.
- Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464.
- Lee JL, Lee JH, Kim MK, et al. A case of bone marrow necrosis with thrombotic thrombocytopenic purpura as a manifestation of occult colon cancer. Jpn J Clin Oncol. 2004;34(8):476-480.
- Sack GH Jr, Levin J, Bell WR. Trousseau’s syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine (Baltimore). 1977;56(1):1-37.
- Prins MH, Hettiarachchi RJ, Lensing AW, Hirsh J. Newly diagnosed malignancy in patients with venous thromboembolism. Search or wait and see? Thromb Haemost. 1997;78(1):121-125.
- Cornuz J, Pearson SD, Creager MA, Cook EF, Goldman L. Importance of findings on the initial evaluation for cancer in patients with symptomatic idiopathic deep venous thrombosis. Ann Intern Med. 1996;125(10):785-793.
- Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
- Dalen JE. Should patients with venous thromboembolism be screened for thrombophilia? Am J Med. 2008;121(6):458-463.
- Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993-997.
- Ridker PM, Goldhaber SZ, Danielson E, et al. Long-term, low-intensity warfarin therapy for the prevention of recurrent venous thromboembolism. N Engl J Med. 2003;348(15):1425-1434.
- Hron G, Eichinger S, Weltermann A, et al. Family history for venous thromboembolism and the risk for recurrence. Am J Med. 2006;119(1):50-53.
- Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e419S-e494S.
- Douketis, James, Tosetto A, Marcucci M, et al. Risk of recurrence after venous thromboembolism in men and women: patient level meta-analysis. BMJ. 2011;342:d813.
- Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293(19):2352-2361.
- American Cancer Society Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Accessed November 15, 2014.