User login
Patients with axial spondyloarthritis showed reduced spinal radiographic progression after treatment with tumor necrosis factor (TNF) inhibitors, based on data from 314 adults in a prospective cohort study.
Evidence of a link between inflammation and axial damage in patients with axial spondyloarthritis (axSpA) has been reported, and these patients are routinely treated with NSAIDs and TNF inhibitors (TNFi), wrote Alexandre Sepriano, MD, PhD, of Leiden (the Netherlands) University Medical Center, and colleagues.
“However, and despite significant efforts, it remains to be clarified whether there is also an effect of these drugs on axial damage accrual,” they noted.
In a study published in Arthritis & Rheumatology, the researchers recruited consecutive patients from rheumatology practices in Northern Alberta to enroll in the Follow Up Research Cohort in Ankylosing Spondylitis Treatment (ALBERTA FORCAST) observational cohort study. The average age of the patients was 41 years, 74% were men, 83% were HLA-B27 positive, and the average duration of symptoms was 18 years.
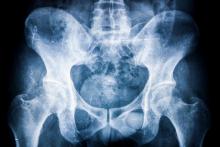
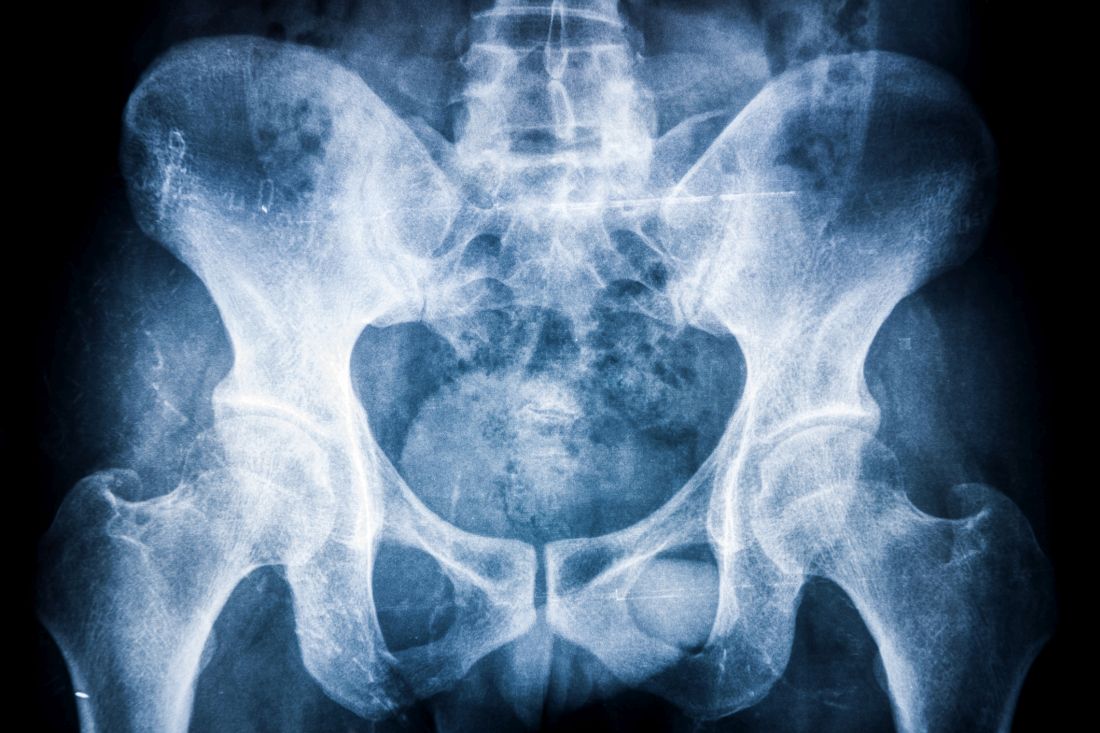
Progression was measured via spine radiographs every 2 years for up to 10 years; the radiographs were scored using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). In addition, the researchers assessed the interaction between TNFi exposure and clinical disease activity using the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the impact on mSASSS every 2 years. The analysis included 442 2-year intervals.
Overall, the researchers found a significant interaction between ASDAS and TNFi at the start of the interval, followed by gradient effect of ASDAS at the start of the interval on mSASSS 2 years later, which was more than twice as high in patients never treated with TNFi (beta = 0.41), compared with patients who were continuously treated with a TNFi (beta = 0.16).
“Similarly, patients treated with TNFi were 30% less likely to develop a new syndesmophyte 2 years later compared to those not treated,” the researchers said.
TNFi also directly slowed progression, as treated patients averaged 0.85 mSASSS units less 2 years later, compared with untreated patients.
Of note, “treatment with NSAIDs during follow-up was neither associated with the outcome nor did it modify or confound the association between TNFi and mSASSS,” the researchers said. In addition, “the direct effect of TNFi on mSASSS was still present after adjusting for a propensity score,” they wrote.
The study results were limited by several factors including the observational design, lack of data on long-term treatment effects, and inability to assess individual TNFi drugs separately, the researchers noted.
However, “the present study informs the rheumatology community by addressing the question as to whether or not TNFi inhibit radiographic progression in axSpA and if this effect is mediated solely by their effects on inflammation, as measured by the ASDAS, or whether additional mechanisms may be relevant,” they emphasized.
“A better understanding of these mechanisms might open avenues to further treatment strategies that might finally lead to effective disease modification in axial SpA,” they concluded.
The ALBERTA FORCAST study was supported by AbbVie. Several authors disclosed financial relationships with AbbVie and other manufacturers of TNFi.
Patients with axial spondyloarthritis showed reduced spinal radiographic progression after treatment with tumor necrosis factor (TNF) inhibitors, based on data from 314 adults in a prospective cohort study.
Evidence of a link between inflammation and axial damage in patients with axial spondyloarthritis (axSpA) has been reported, and these patients are routinely treated with NSAIDs and TNF inhibitors (TNFi), wrote Alexandre Sepriano, MD, PhD, of Leiden (the Netherlands) University Medical Center, and colleagues.
“However, and despite significant efforts, it remains to be clarified whether there is also an effect of these drugs on axial damage accrual,” they noted.
In a study published in Arthritis & Rheumatology, the researchers recruited consecutive patients from rheumatology practices in Northern Alberta to enroll in the Follow Up Research Cohort in Ankylosing Spondylitis Treatment (ALBERTA FORCAST) observational cohort study. The average age of the patients was 41 years, 74% were men, 83% were HLA-B27 positive, and the average duration of symptoms was 18 years.
Progression was measured via spine radiographs every 2 years for up to 10 years; the radiographs were scored using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). In addition, the researchers assessed the interaction between TNFi exposure and clinical disease activity using the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the impact on mSASSS every 2 years. The analysis included 442 2-year intervals.
Overall, the researchers found a significant interaction between ASDAS and TNFi at the start of the interval, followed by gradient effect of ASDAS at the start of the interval on mSASSS 2 years later, which was more than twice as high in patients never treated with TNFi (beta = 0.41), compared with patients who were continuously treated with a TNFi (beta = 0.16).
“Similarly, patients treated with TNFi were 30% less likely to develop a new syndesmophyte 2 years later compared to those not treated,” the researchers said.
TNFi also directly slowed progression, as treated patients averaged 0.85 mSASSS units less 2 years later, compared with untreated patients.
Of note, “treatment with NSAIDs during follow-up was neither associated with the outcome nor did it modify or confound the association between TNFi and mSASSS,” the researchers said. In addition, “the direct effect of TNFi on mSASSS was still present after adjusting for a propensity score,” they wrote.
The study results were limited by several factors including the observational design, lack of data on long-term treatment effects, and inability to assess individual TNFi drugs separately, the researchers noted.
However, “the present study informs the rheumatology community by addressing the question as to whether or not TNFi inhibit radiographic progression in axSpA and if this effect is mediated solely by their effects on inflammation, as measured by the ASDAS, or whether additional mechanisms may be relevant,” they emphasized.
“A better understanding of these mechanisms might open avenues to further treatment strategies that might finally lead to effective disease modification in axial SpA,” they concluded.
The ALBERTA FORCAST study was supported by AbbVie. Several authors disclosed financial relationships with AbbVie and other manufacturers of TNFi.
Patients with axial spondyloarthritis showed reduced spinal radiographic progression after treatment with tumor necrosis factor (TNF) inhibitors, based on data from 314 adults in a prospective cohort study.
Evidence of a link between inflammation and axial damage in patients with axial spondyloarthritis (axSpA) has been reported, and these patients are routinely treated with NSAIDs and TNF inhibitors (TNFi), wrote Alexandre Sepriano, MD, PhD, of Leiden (the Netherlands) University Medical Center, and colleagues.
“However, and despite significant efforts, it remains to be clarified whether there is also an effect of these drugs on axial damage accrual,” they noted.
In a study published in Arthritis & Rheumatology, the researchers recruited consecutive patients from rheumatology practices in Northern Alberta to enroll in the Follow Up Research Cohort in Ankylosing Spondylitis Treatment (ALBERTA FORCAST) observational cohort study. The average age of the patients was 41 years, 74% were men, 83% were HLA-B27 positive, and the average duration of symptoms was 18 years.
Progression was measured via spine radiographs every 2 years for up to 10 years; the radiographs were scored using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). In addition, the researchers assessed the interaction between TNFi exposure and clinical disease activity using the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the impact on mSASSS every 2 years. The analysis included 442 2-year intervals.
Overall, the researchers found a significant interaction between ASDAS and TNFi at the start of the interval, followed by gradient effect of ASDAS at the start of the interval on mSASSS 2 years later, which was more than twice as high in patients never treated with TNFi (beta = 0.41), compared with patients who were continuously treated with a TNFi (beta = 0.16).
“Similarly, patients treated with TNFi were 30% less likely to develop a new syndesmophyte 2 years later compared to those not treated,” the researchers said.
TNFi also directly slowed progression, as treated patients averaged 0.85 mSASSS units less 2 years later, compared with untreated patients.
Of note, “treatment with NSAIDs during follow-up was neither associated with the outcome nor did it modify or confound the association between TNFi and mSASSS,” the researchers said. In addition, “the direct effect of TNFi on mSASSS was still present after adjusting for a propensity score,” they wrote.
The study results were limited by several factors including the observational design, lack of data on long-term treatment effects, and inability to assess individual TNFi drugs separately, the researchers noted.
However, “the present study informs the rheumatology community by addressing the question as to whether or not TNFi inhibit radiographic progression in axSpA and if this effect is mediated solely by their effects on inflammation, as measured by the ASDAS, or whether additional mechanisms may be relevant,” they emphasized.
“A better understanding of these mechanisms might open avenues to further treatment strategies that might finally lead to effective disease modification in axial SpA,” they concluded.
The ALBERTA FORCAST study was supported by AbbVie. Several authors disclosed financial relationships with AbbVie and other manufacturers of TNFi.
FROM ARTHRITIS & RHEUMATOLOGY