User login
Baby Boomers have transformed attitudes toward many aspects of aging. Menopause is no exception. Once a taboo topic, menopause is now openly discussed among women who seek information about vasomotor symptoms, hormones and their alternatives, and ways to maintain health as they move past midlife. ObGyns are treating more and more of these women, and fielding their many questions.
In this Update, I examine recent data on three important aspects of menopause:
- how to reduce the risk of cardiovascular disease among women who enter menopause surgically, through oophorectomy
- what to offer women who ask for nonhormonal relief from vasomotor symptoms
- a new drug on the horizon to combat osteoporosis.
Bilateral oophorectomy raises young women’s risk of cardiovascular death
Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23.
Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16:1–2.
Cardiovascular mortality does not increase among women who undergo unilateral oophorectomy, but it does rise among women who undergo bilateral oophorectomy before 45 years of age. However, among women who initiate estrogen therapy at the time of bilateral oophorectomy and continue that therapy through at least 45 years of age, no excess cardiovascular mortality occurs.
Those are the findings of a unique retrospective cohort study performed by investigators from the Mayo Clinic. In the study, investigators reviewed the death certificates of more than 2,300 women who underwent unilateral or bilateral oophorectomy for benign disease before menopause in Olmstead County, Minnesota, from 1950 to 1987. They also followed a similar number of age-matched women for several decades.
These results support the findings of other studies that have observed that menopausal hormone therapy is associated with a lower incidence of cardiovascular death in “young” menopausal women, including those in their 50s or within one decade of the onset of menopause.1,2
More than 500,000 women undergo bilateral oophorectomy each year in the United States, usually in association with hysterectomy for benign disease.
Induced menopause merits special attention
Spontaneous menopause is physiologic. In contrast, induced menopause (whether associated with surgery, radiation therapy, or chemotherapy) and premature ovarian failure are pathologic conditions.3 Unless they are managed appropriately, induced menopause and premature ovarian failure raise the risk of cardiovascular disease.
Since the initial findings of the Women’s Health Initiative trial of estrogen–progestin therapy were published in 2002, many women and clinicians have become wary about the use of hormone therapy, even among young women who have no ovarian function and who lack a contraindication to hormone therapy.4 Unless hormonal management is contraindicated, it is recommended in this setting.
In addition, Parker and Manson recommend that gynecologic surgeons who routinely perform bilateral oophorectomy at the time of hysterectomy for benign disease in premenopausal women who do not have an elevated risk of ovarian cancer should consider updating their therapeutic recommendations and, whenever possible, preserving the ovaries.
Interest in nonhormonal therapies for hot flashes remains high
Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopausal transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15:32–43.
Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized trial. Menopause. 2008;15:310–318.
More than three quarters of women use some type of complementary and alternative medicine (CAM) during the menopausal transition. So found a survey conducted as part of the Study of Women’s Health Across the Nation (SWAN), which involved more than 2,000 premenopausal and perimenopausal women.
More than one third of all US women use one or more forms of CAM, spending more than $600 million a year.
In the SWAN survey, Japanese and white women were significantly more likely to report use of CAM than were Chinese, African-American, and Hispanic women during menopause.
Some women use both CAM and hormones
A notable finding of this report from SWAN is that concomitant use of menopausal hormone therapy and CAM is common among symptomatic women, even though herbal therapies have not been proved to be more effective than placebo in the treatment of vasomotor symptoms.5
Given the high prevalence of use of CAM, ObGyns should recognize that symptomatic patients especially bothered by vasomotor symptoms may seek relief with both CAM and a prescription medication. For this reason, it is wise to ask perimenopausal and postmenopausal women to list all the remedies they use—both prescription and over the counter.
Gabapentin eases symptoms in some women
Along with the antidepressants paroxetine and venlafaxine, gabapentin is the most widely studied prescription agent used in the nonhormonal treatment of menopausal vasomotor symptoms.
Canadian investigators performed a double-blind, placebo-controlled trial involving 197 symptomatic, spontaneously menopausal women (age range: 45 to 65 years). Participants were randomized to gabapentin (300 mg) or placebo, given three times daily for 4 weeks.
Ten of the 99 women who were randomized to gabapentin and six of the 98 women who were randomized to placebo dropped out of the study because of adverse effects. Hot-flash scores decreased by 51% among women taking gabapentin and by 26% among women in the placebo arm (p<.001). Women who used gabapentin were significantly more likely to report dizziness, drowsiness, and unsteadiness than those who received placebo.
Despite its proven efficacy, gabapentin does not appear to equal estrogen in its ability to alleviate vasomotor symptoms. Further, gabapentin’s side-effect profile and the need for thrice-daily administration also limit its appeal. Nevertheless, symptomatic women who are interested in nonhormonal treatment may wish to consider this option.
Anti-osteoporosis arsenal may gain a new weapon
Cummings S, McClung M, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 16, 2008; Montreal, Canada.
Miller PD, Bolognese MA, Lewiecki EM, et al; AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized, blinded phase 2 clinical trial. Bone. 2008;43:222–229.
In a 3-year study in which more than 7,800 women 60 to 90 years old were randomized to denosumab (60 mg) or placebo, denosumab reduced the risk of vertebral fracture by 68% and the risk of hip fracture by 40%.
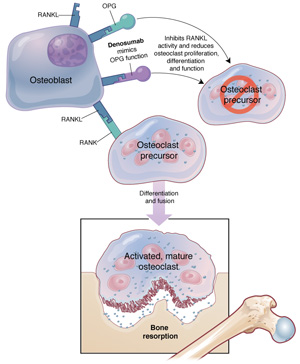
FIGURE A new agent to inhibit bone resorption
RankL is a protein that controls osteoclast maturation, activity, and survival. Denosumab mimics the endogenous decoy receptor OPG to inhibit RankL and reduce osteoclast proliferation, differentiation, and function. Denosumab is a monoclonal antibody to RankL, a mediator of osteoclast function ( FIGURE ). In contrast to alendronate, which enhances trabecular bone density (e.g., that found in the spine), denosumab increases cortical (as in long bones) as well as trabecular bone density.6 If the agent obtains approval by the US Food and Drug Administration, it will be an important new approach to the treatment of osteoporosis in menopausal women.7
The drug is administered every 6 months by subcutaneous injection.
Like estrogen, denosumab has rapidly reversible effects
The trial by Miller and associates found that the effects of denosumab are rapidly reversible after discontinuation of therapy, in contrast to alendronate. As Goldstein noted in “Update on Osteoporosis” in the November 2008 issue of OBG Management, clinicians are still exploring the relationships between alendronate and osteonecrosis of the jaw and proximal femoral-shaft fracture.7 Only further research and surveillance will clarify the clinical pros and cons of denosumab’s swift reversibility.
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. WHI and WHICACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606-614.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009; Jan 21 [Epub ahead of print].
6. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821-832.
7. Goldstein SR. Update on osteoporosis. OBG Management. 2008;20(11):41-46.
Baby Boomers have transformed attitudes toward many aspects of aging. Menopause is no exception. Once a taboo topic, menopause is now openly discussed among women who seek information about vasomotor symptoms, hormones and their alternatives, and ways to maintain health as they move past midlife. ObGyns are treating more and more of these women, and fielding their many questions.
In this Update, I examine recent data on three important aspects of menopause:
- how to reduce the risk of cardiovascular disease among women who enter menopause surgically, through oophorectomy
- what to offer women who ask for nonhormonal relief from vasomotor symptoms
- a new drug on the horizon to combat osteoporosis.
Bilateral oophorectomy raises young women’s risk of cardiovascular death
Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23.
Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16:1–2.
Cardiovascular mortality does not increase among women who undergo unilateral oophorectomy, but it does rise among women who undergo bilateral oophorectomy before 45 years of age. However, among women who initiate estrogen therapy at the time of bilateral oophorectomy and continue that therapy through at least 45 years of age, no excess cardiovascular mortality occurs.
Those are the findings of a unique retrospective cohort study performed by investigators from the Mayo Clinic. In the study, investigators reviewed the death certificates of more than 2,300 women who underwent unilateral or bilateral oophorectomy for benign disease before menopause in Olmstead County, Minnesota, from 1950 to 1987. They also followed a similar number of age-matched women for several decades.
These results support the findings of other studies that have observed that menopausal hormone therapy is associated with a lower incidence of cardiovascular death in “young” menopausal women, including those in their 50s or within one decade of the onset of menopause.1,2
More than 500,000 women undergo bilateral oophorectomy each year in the United States, usually in association with hysterectomy for benign disease.
Induced menopause merits special attention
Spontaneous menopause is physiologic. In contrast, induced menopause (whether associated with surgery, radiation therapy, or chemotherapy) and premature ovarian failure are pathologic conditions.3 Unless they are managed appropriately, induced menopause and premature ovarian failure raise the risk of cardiovascular disease.
Since the initial findings of the Women’s Health Initiative trial of estrogen–progestin therapy were published in 2002, many women and clinicians have become wary about the use of hormone therapy, even among young women who have no ovarian function and who lack a contraindication to hormone therapy.4 Unless hormonal management is contraindicated, it is recommended in this setting.
In addition, Parker and Manson recommend that gynecologic surgeons who routinely perform bilateral oophorectomy at the time of hysterectomy for benign disease in premenopausal women who do not have an elevated risk of ovarian cancer should consider updating their therapeutic recommendations and, whenever possible, preserving the ovaries.
Interest in nonhormonal therapies for hot flashes remains high
Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopausal transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15:32–43.
Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized trial. Menopause. 2008;15:310–318.
More than three quarters of women use some type of complementary and alternative medicine (CAM) during the menopausal transition. So found a survey conducted as part of the Study of Women’s Health Across the Nation (SWAN), which involved more than 2,000 premenopausal and perimenopausal women.
More than one third of all US women use one or more forms of CAM, spending more than $600 million a year.
In the SWAN survey, Japanese and white women were significantly more likely to report use of CAM than were Chinese, African-American, and Hispanic women during menopause.
Some women use both CAM and hormones
A notable finding of this report from SWAN is that concomitant use of menopausal hormone therapy and CAM is common among symptomatic women, even though herbal therapies have not been proved to be more effective than placebo in the treatment of vasomotor symptoms.5
Given the high prevalence of use of CAM, ObGyns should recognize that symptomatic patients especially bothered by vasomotor symptoms may seek relief with both CAM and a prescription medication. For this reason, it is wise to ask perimenopausal and postmenopausal women to list all the remedies they use—both prescription and over the counter.
Gabapentin eases symptoms in some women
Along with the antidepressants paroxetine and venlafaxine, gabapentin is the most widely studied prescription agent used in the nonhormonal treatment of menopausal vasomotor symptoms.
Canadian investigators performed a double-blind, placebo-controlled trial involving 197 symptomatic, spontaneously menopausal women (age range: 45 to 65 years). Participants were randomized to gabapentin (300 mg) or placebo, given three times daily for 4 weeks.
Ten of the 99 women who were randomized to gabapentin and six of the 98 women who were randomized to placebo dropped out of the study because of adverse effects. Hot-flash scores decreased by 51% among women taking gabapentin and by 26% among women in the placebo arm (p<.001). Women who used gabapentin were significantly more likely to report dizziness, drowsiness, and unsteadiness than those who received placebo.
Despite its proven efficacy, gabapentin does not appear to equal estrogen in its ability to alleviate vasomotor symptoms. Further, gabapentin’s side-effect profile and the need for thrice-daily administration also limit its appeal. Nevertheless, symptomatic women who are interested in nonhormonal treatment may wish to consider this option.
Anti-osteoporosis arsenal may gain a new weapon
Cummings S, McClung M, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 16, 2008; Montreal, Canada.
Miller PD, Bolognese MA, Lewiecki EM, et al; AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized, blinded phase 2 clinical trial. Bone. 2008;43:222–229.
In a 3-year study in which more than 7,800 women 60 to 90 years old were randomized to denosumab (60 mg) or placebo, denosumab reduced the risk of vertebral fracture by 68% and the risk of hip fracture by 40%.

FIGURE A new agent to inhibit bone resorption
RankL is a protein that controls osteoclast maturation, activity, and survival. Denosumab mimics the endogenous decoy receptor OPG to inhibit RankL and reduce osteoclast proliferation, differentiation, and function. Denosumab is a monoclonal antibody to RankL, a mediator of osteoclast function ( FIGURE ). In contrast to alendronate, which enhances trabecular bone density (e.g., that found in the spine), denosumab increases cortical (as in long bones) as well as trabecular bone density.6 If the agent obtains approval by the US Food and Drug Administration, it will be an important new approach to the treatment of osteoporosis in menopausal women.7
The drug is administered every 6 months by subcutaneous injection.
Like estrogen, denosumab has rapidly reversible effects
The trial by Miller and associates found that the effects of denosumab are rapidly reversible after discontinuation of therapy, in contrast to alendronate. As Goldstein noted in “Update on Osteoporosis” in the November 2008 issue of OBG Management, clinicians are still exploring the relationships between alendronate and osteonecrosis of the jaw and proximal femoral-shaft fracture.7 Only further research and surveillance will clarify the clinical pros and cons of denosumab’s swift reversibility.
Baby Boomers have transformed attitudes toward many aspects of aging. Menopause is no exception. Once a taboo topic, menopause is now openly discussed among women who seek information about vasomotor symptoms, hormones and their alternatives, and ways to maintain health as they move past midlife. ObGyns are treating more and more of these women, and fielding their many questions.
In this Update, I examine recent data on three important aspects of menopause:
- how to reduce the risk of cardiovascular disease among women who enter menopause surgically, through oophorectomy
- what to offer women who ask for nonhormonal relief from vasomotor symptoms
- a new drug on the horizon to combat osteoporosis.
Bilateral oophorectomy raises young women’s risk of cardiovascular death
Rivera CM, Grossardt BR, Rhodes DJ, et al. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause. 2009;16:15–23.
Parker WH, Manson JE. Oophorectomy and cardiovascular mortality: is there a link? Menopause. 2009;16:1–2.
Cardiovascular mortality does not increase among women who undergo unilateral oophorectomy, but it does rise among women who undergo bilateral oophorectomy before 45 years of age. However, among women who initiate estrogen therapy at the time of bilateral oophorectomy and continue that therapy through at least 45 years of age, no excess cardiovascular mortality occurs.
Those are the findings of a unique retrospective cohort study performed by investigators from the Mayo Clinic. In the study, investigators reviewed the death certificates of more than 2,300 women who underwent unilateral or bilateral oophorectomy for benign disease before menopause in Olmstead County, Minnesota, from 1950 to 1987. They also followed a similar number of age-matched women for several decades.
These results support the findings of other studies that have observed that menopausal hormone therapy is associated with a lower incidence of cardiovascular death in “young” menopausal women, including those in their 50s or within one decade of the onset of menopause.1,2
More than 500,000 women undergo bilateral oophorectomy each year in the United States, usually in association with hysterectomy for benign disease.
Induced menopause merits special attention
Spontaneous menopause is physiologic. In contrast, induced menopause (whether associated with surgery, radiation therapy, or chemotherapy) and premature ovarian failure are pathologic conditions.3 Unless they are managed appropriately, induced menopause and premature ovarian failure raise the risk of cardiovascular disease.
Since the initial findings of the Women’s Health Initiative trial of estrogen–progestin therapy were published in 2002, many women and clinicians have become wary about the use of hormone therapy, even among young women who have no ovarian function and who lack a contraindication to hormone therapy.4 Unless hormonal management is contraindicated, it is recommended in this setting.
In addition, Parker and Manson recommend that gynecologic surgeons who routinely perform bilateral oophorectomy at the time of hysterectomy for benign disease in premenopausal women who do not have an elevated risk of ovarian cancer should consider updating their therapeutic recommendations and, whenever possible, preserving the ovaries.
Interest in nonhormonal therapies for hot flashes remains high
Bair YA, Gold EB, Zhang G, et al. Use of complementary and alternative medicine during the menopausal transition: longitudinal results from the Study of Women’s Health Across the Nation. Menopause. 2008;15:32–43.
Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized trial. Menopause. 2008;15:310–318.
More than three quarters of women use some type of complementary and alternative medicine (CAM) during the menopausal transition. So found a survey conducted as part of the Study of Women’s Health Across the Nation (SWAN), which involved more than 2,000 premenopausal and perimenopausal women.
More than one third of all US women use one or more forms of CAM, spending more than $600 million a year.
In the SWAN survey, Japanese and white women were significantly more likely to report use of CAM than were Chinese, African-American, and Hispanic women during menopause.
Some women use both CAM and hormones
A notable finding of this report from SWAN is that concomitant use of menopausal hormone therapy and CAM is common among symptomatic women, even though herbal therapies have not been proved to be more effective than placebo in the treatment of vasomotor symptoms.5
Given the high prevalence of use of CAM, ObGyns should recognize that symptomatic patients especially bothered by vasomotor symptoms may seek relief with both CAM and a prescription medication. For this reason, it is wise to ask perimenopausal and postmenopausal women to list all the remedies they use—both prescription and over the counter.
Gabapentin eases symptoms in some women
Along with the antidepressants paroxetine and venlafaxine, gabapentin is the most widely studied prescription agent used in the nonhormonal treatment of menopausal vasomotor symptoms.
Canadian investigators performed a double-blind, placebo-controlled trial involving 197 symptomatic, spontaneously menopausal women (age range: 45 to 65 years). Participants were randomized to gabapentin (300 mg) or placebo, given three times daily for 4 weeks.
Ten of the 99 women who were randomized to gabapentin and six of the 98 women who were randomized to placebo dropped out of the study because of adverse effects. Hot-flash scores decreased by 51% among women taking gabapentin and by 26% among women in the placebo arm (p<.001). Women who used gabapentin were significantly more likely to report dizziness, drowsiness, and unsteadiness than those who received placebo.
Despite its proven efficacy, gabapentin does not appear to equal estrogen in its ability to alleviate vasomotor symptoms. Further, gabapentin’s side-effect profile and the need for thrice-daily administration also limit its appeal. Nevertheless, symptomatic women who are interested in nonhormonal treatment may wish to consider this option.
Anti-osteoporosis arsenal may gain a new weapon
Cummings S, McClung M, Christiansen C, et al. A phase III study of the effects of denosumab on vertebral, nonvertebral, and hip fracture in women with osteoporosis: results from the FREEDOM Trial. Paper presented at: Annual Meeting of the American Society for Bone and Mineral Research; September 16, 2008; Montreal, Canada.
Miller PD, Bolognese MA, Lewiecki EM, et al; AMG Bone Loss Study Group. Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized, blinded phase 2 clinical trial. Bone. 2008;43:222–229.
In a 3-year study in which more than 7,800 women 60 to 90 years old were randomized to denosumab (60 mg) or placebo, denosumab reduced the risk of vertebral fracture by 68% and the risk of hip fracture by 40%.

FIGURE A new agent to inhibit bone resorption
RankL is a protein that controls osteoclast maturation, activity, and survival. Denosumab mimics the endogenous decoy receptor OPG to inhibit RankL and reduce osteoclast proliferation, differentiation, and function. Denosumab is a monoclonal antibody to RankL, a mediator of osteoclast function ( FIGURE ). In contrast to alendronate, which enhances trabecular bone density (e.g., that found in the spine), denosumab increases cortical (as in long bones) as well as trabecular bone density.6 If the agent obtains approval by the US Food and Drug Administration, it will be an important new approach to the treatment of osteoporosis in menopausal women.7
The drug is administered every 6 months by subcutaneous injection.
Like estrogen, denosumab has rapidly reversible effects
The trial by Miller and associates found that the effects of denosumab are rapidly reversible after discontinuation of therapy, in contrast to alendronate. As Goldstein noted in “Update on Osteoporosis” in the November 2008 issue of OBG Management, clinicians are still exploring the relationships between alendronate and osteonecrosis of the jaw and proximal femoral-shaft fracture.7 Only further research and surveillance will clarify the clinical pros and cons of denosumab’s swift reversibility.
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. WHI and WHICACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606-614.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009; Jan 21 [Epub ahead of print].
6. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821-832.
7. Goldstein SR. Update on osteoporosis. OBG Management. 2008;20(11):41-46.
1. Rossouw JE, Prentice RL, Manson JE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465-1477.
2. Manson JE, Allison MA, Rossouw JE, et al. WHI and WHICACS Investigators. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591-2602.
3. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360:606-614.
4. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321-333.
5. Kaunitz AM. Effective herbal treatment of vasomotor symptoms—are we any closer? Menopause. 2009; Jan 21 [Epub ahead of print].
6. McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354:821-832.
7. Goldstein SR. Update on osteoporosis. OBG Management. 2008;20(11):41-46.