User login
In the United States, >50% of psychiatric inpatients have vitamin D deficiency—<30 nmol/L (<12 ng/mL).1 A growing body of literature has found associations between vitamin D deficiency and psychiatric illnesses, particularly depression. Several randomized controlled trials (RCTs) have demonstrated that vitamin D supplementation can benefit depression symptoms. In this article, we discuss the current literature on vitamin D and psychiatric illness, and provide practical information for clinicians on the use of vitamin D supplementation.
Biosynthesis of vitamin D
Biosynthesis of vitamin D begins with the sterol provitamin D3 molecule 7-dehydrocholesterol (Figure).2 When skin is exposed to sunlight, 7-dehydrocholesterol absorbs UV radiation and forms provitamin D3, which undergoes rapid transformation to vitamin D3.2 Vitamin D3 is released from the plasma membrane and enters systemic circulation in a protein-bound form that has a serum half-life of 36 to 78 hours.3 Vitamin D3 can be taken up by adipocytes and stored in fat deposits, where it has a half-life of approximately 2 months.4
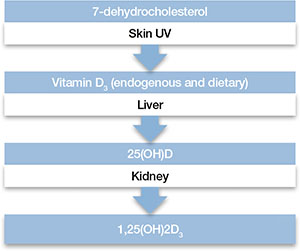
Figure: Biosynthesis of vitamin D
Provitamin D3 (7-dehydrocholesterol) in the skin absorbs UV radiation and undergoes isomerization to form vitamin D3. Endogenously produced vitamin D3 along with dietary vitamin D2 and vitamin D3 absorbed in the gastrointestinal tract are metabolized in the liver to 25-hydroxyvitamin D (25[OH]D), which re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D). Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P450 (CYP) enzymes CYP24 and CYP3A4.
Source: Reference 2Circulating vitamin D3 is metabolized in the liver by the enzyme vitamin D-25-hydroxylase to 25-hydroxyvitamin D (25[OH]D3), which has a serum half-life of approximately 15 days.4 Circulating 25(OH)D3 is not biologically active at the physiological level, and requires activation by conversion to 1,25-dihydroxyvitamin D (1,25[OH]2D3) in the kidneys by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is regulated by serum phosphorus and parathyroid hormone levels and other factors.5 Catabolism of 1,25(OH)2D3 is rapid, with a serum half-life of 3.5 to 21 hours.6 Vitamin D2 is structurally similar to vitamin D3, but occurs primarily in fungi, yeasts, and some invertebrates.
Risk factors for deficiency
A patient’s vitamin D status is determined by measuring 25(OH)D (Box 1). Risk factors for vitamin D deficiency include conditions that affect cutaneous production (insufficient sunlight exposure), obesity, gastrointestinal disorders, aging, renal disorders, and medications (Table 1). 2,5,7,8 The link between sunscreen use, either alone or in cosmetics, and vitamin D deficiency continues to be debated. While controlled studies have found that application of sunscreen with high sun protection factor can significantly reduce vitamin D production, 9 studies in clinical populations have failed to confirm these findings. 10,11 See Box 2 for a discussion of these risk factors and Box 3 for a discussion of acute and long-term medical manifestations of deficiency.
Although 1,25-dihydroxyvitamin D (1,25[OH]2D3) is the biologically active form of vitamin D, its circulating half-life is only 4 to 6 hours.a,b Therefore, 25-hydroxyvitamin D (25[OH]D) is the principal vitamin D metabolite measured to determine vitamin D status. Vitamin D levels commonly are expressed as ng/mL or nmol/L; the conversion factor from ng/mL to nmol/L is 2.496. The Institute of Medicine has defined vitamin D deficiency as a serum 25(OH)D level of <30 nmol/L (<12 ng/mL).c However, many experts define vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/ml, and deficiency as <20 ng/mL.a,d The upper limit is more difficult to define, but symptoms of vitamin D intoxication appear with blood levels >150 to 200 ng/mL.a
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96(10):2987-2996.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
Table 1
Risk factors associated with vitamin D deficiency
| Age (>65) |
| Insufficient sunlight |
| Breastfeeding |
| Dark skin |
| Malabsorption diseases |
| Obesity (BMI >30 kg/m2) |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Hepatobiliary disease |
| Renal disease |
| BMI: body mass index Source: References 2,5,7,8 |
Any factor that diminishes UV radiation penetration into the skin will affect cutaneous synthesis of vitamin D.a,b For example, sunscreen with a sun protection factor of 15 can decrease vitamin D synthesis by 98%.c Geography and its impact on yearly sunlight exposure is a well-known factor in vitamin D deficiency. Individuals who live below a latitude of approximately 35° North—approximately the southern border of Tennessee and through Albuquerque, NM—receive sufficient UV radiation exposure to ensure adequate vitamin D production throughout the year, but at higher latitudes, adequate vitamin D is not produced during winter months.d Melanin affects UV radiation absorption in a manner that prevents vitamin D production, and increased skin pigmentation markedly reduces vitamin D synthesis.e African Americans with very dark skin have significantly diminished cutaneous production of vitamin D.e,f
Renal 1α-hydroxylase activity decreases with aging in parallel with age-related decreases in glomerular filtration.g In addition, aging is associated with increased clearance of 1,25-dihydroxyvitamin D (1,25[OH]2D3).h However, vitamin D absorption generally is adequate even at older ages.i Studies have shown that obese individuals tend to have lower serum concentrations of vitamin D and 25-hydroxyvitamin D (25[OH]D) than those at a normal weight.j,k Obese patients have been shown to have lower cutaneous production of vitamin D3 and display lower bioavailability of orally administered vitamin D2.j
For patients with chronic renal insufficiency, creatinine clearance is positively correlated with serum 1,25(OH)2D levels.l Any process that results in malabsorption of intestinal fat may impair vitamin D absorption. In patients with celiac disease, biliary obstruction, or chronic pancreatitis, absorption consistently is reduced.m Individuals taking bile acid-binding medications, such as cholestyramine for hypercholesterolemia, also may have impaired vitamin D absorption.n In addition, hepatobiliary disease is associated with low levels of 25(OH)D.o Some drugs that alter hepatic metabolism are associated with vitamin D deficiency, including anticonvulsants or glucocorticoids, which can increase catabolism or vitamin D.p
References
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361-368.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
- Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213-217.
- Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
- Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J Clin Invest. 1980;66(5):1118-1123.
- Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644-650.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
- Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370-373.
- Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67(5):876-881.
- Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94-102.
- Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644-649.
- Pappa HM, Bern E, Kamin D, et al. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24(2):176-183.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Acute effects. Vitamin D deficiency produces a range of clinical effects.a-c One well-documented consequence of vitamin D deficiency is osteomalacia—bone demineralization—which produces characteristic bone deformity and growth retardation in children.d,e In adults, osteomalacia may manifest as diffuse pain bone discomfort and muscle aches that may resemble fibromyalgia or arthritis.f Because vitamin D receptors are present in skeletal muscle, deficiency also may lead to proximal muscle weakness; an increased risk of falls; global bone discomfort, often elicited with pressure over the sternum or tibia; and low back pain in older women.c,f
Long-term effects. A large epidemiologic study found that adults with 25-hydroxyvitamin D (25[OH]D) levels <21 ng/mL had an increased risk of hypertension, diabetes, obesity, and dyslipidemia.g Cardiovascular mortality was higher in individuals with 25(OH)D levels <10 ng/mL compared with those with >40 ng/mL.h Adolescents in the National Health and Nutrition Examination Survey-III with serum 25(OH)D levels <15 ng/mL were more likely to have elevated blood glucose levels than those with >26 ng/mL.i Other epidemiologic data have demonstrated associations of vitamin D deficiency with multiple sclerosis, seasonal allergies, asthma, and various infectious diseases.j,k
Because vitamin D is known to promote cellular differentiation and inhibit cellular proliferation, its role in cancer has been studied extensively. A recent meta-analysis of case-control studies found that the odds of colon cancer were reduced by >40% for each 20 ng/mL increase in serum 25(OH)D levels.l Another meta-analysis reported a lower risk of breast cancer among women in the highest quartile of 25(OH)D values compared with the lowest quartile.m
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841-846.
- Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56(5):785-791.
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159-1165.
- Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595-1603.
- Reis JP, von Mühlen D, Miller ER 3rd, et al. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371-379.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
- Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838.
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113-125.
- Chen P, Hu P, Xie D, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469-477.
Vitamin D’s role in the brain
Vitamin D’s role in psychiatric illnesses is suggested by region-specific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, amygdala, and hippocampus.12 Most of these regions also express 1α-hydroxylase enzymes capable of metabolizing 25(OH)D to 1,25(OH)2D3, which suggests that vitamin D may have an autocrine or paracrine function in brain.13
Vitamin D regulates expression of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine, norepinephrine, and epinephrine.14 Vitamin D also promotes survival of monoaminergic neurons through upregulation of glial cell line-derived neurotrophic factor, which supports survival of midbrain dopaminergic neurons and confers resistance to neurotoxins that deplete dopaminergic neurons in Parkinson’s disease.15 Vitamin D also promotes neuronal survival by inhibiting oxidative pathways in the brain through inhibition of inducible nitric oxide synthase (reducing free radical formation)16 and upregulation of γ-glutamyl transpeptidase (increasing antioxidant production).17 Vitamin D may play a neuroprotective role through regulation of calcium channels. In vitro studies have shown that vitamin D downregulates expression of L-type calcium channels, conferring protection against excitatory neurotoxins in cultured neurons.18 Proteomic analysis of brain tissue in a rat model of developmental vitamin D revealed dysregulation of 36 brain proteins involved in many biologic pathways involved in calcium homeostasis, synaptic plasticity, and neurotransmission.19 Taken together, these findings suggest vitamin D has a neurosteroid-like role in the CNS.
Psychotic disorders
Several epidemiologic studies have linked low vitamin D levels to schizophrenia and other psychotic disorders. Researchers in Norway who used a structured clinical interview to identify psychosis consistently found low levels of 25(OH)D among immigrants and native Norwegians with psychotic symptoms.20 A study of 8,411 Swedish women found low vitamin D levels were associated with psychotic symptoms.21 The Finnish birth cohort study found that use of vitamin D supplementation during the first year of life reduced the incidence of schizophrenia.22 In another pilot study, researchers measured third-trimester serum 25(OH)D levels and found that low levels of maternal vitamin D may be associated with an increased risk of schizophrenia.23 These studies suggest that low prenatal vitamin D levels may adversely impact the developing brain, increasing the risk for adult-onset schizophrenia.
Cognitive dysfunction
Low vitamin D concentrations have been associated with impairments in cognitive functions such as memory and orientation,24 executive function impairments,25 and Alzheimer’s disease (AD).26 A large study conducted from 1998 to 2006 in Italy concluded that persons with severe vitamin D deficiency (<25 nmol/L) had a higher risk of substantial decline on Mini-Mental State Examination than those with sufficient levels (≥75 nmol/L).27 Other studies have linked low vitamin D levels to poor cognitive performance in depressed older adults.28 Low vitamin D levels in older women have been associated with risk of AD, but not with other dementias.29 Polymorphisms of VDR have been associated with depression and poor cognitive performance.30
Depression
Epidemiologic studies evaluating vitamin D deficiency have had conflicting results. The Third National Health and Nutrition Examination Survey, which used a sample of 7,970 non-institutionalized U.S. residents age 15 to 39, demonstrated that individuals with serum vitamin D ≤50 nmol/L are at a significantly higher risk of developing depression than those with vitamin D ≥75 nmol/L.31 A study of 1,282 adults age 65 to 95 in the Netherlands found that 25(OH)D levels were 14% lower in depressed patients compared with controls.32 However, a large epidemiologic study in China did not detect a relationship between vitamin D and depression in 3,262 men and women age 50 to 70.33 After researchers adjusted for geography, body mass index, physical activity, and smoking, 25(OH)D levels did not correlate significantly with the presence or severity of depression. In a case series,34 after 48 vitamin D-deficient depressed adolescents were given vitamin D3 over 3 months, there was a significant improvement in well-being, depressive symptoms, irritability, and fatigue.34 Other small, cross-sectional studies have examined associations between vitamin D status and depression with divergent results, which may reflect differences in population and methodology.
Prospective interventional studies. Although direct causal relationships are difficult to establish, several prospective studies have tested the hypothesis that treating vitamin D deficiency can improve depressive symptoms.
In a double-blind, controlled trial, Jorde et al35 randomized 441 individuals age 21 to 70 to vitamin D, 20,000 IU per week; vitamin D, 40,000 IU per week; or placebo for 1 year. Individuals with serum 25(OH)D levels <40 nmol/L scored significantly higher on depression rating scales than those with serum 25(OH)D levels ≥40 nmol/L at the end of the study. There was no significant improvement in depression ratings in the placebo group (Table 2).35 These results must be interpreted with care because depressive symptoms were secondary endpoints in this study.
Table 2
Effect of vitamin D supplementation on depressive symptoms in a controlled trial
| Vitamin D Supplementation | Serum 25(OH)D levels at baseline | BDI total score, Median and range at end of study | After 1 year of vitamin D supplementation |
|---|---|---|---|
| 20,000 IU/week | <40 nmol/L | Significantly higher (more depressive traits), 6.0 (0 to 23) | Significantly improved BDI score |
| 40,000 IU/week | ≥40 nmol/L | 4.5 (0 to 28) | Significantly improved BDI score |
| Placebo | - | - | No improvement in BDI score |
| 25(OH)D: 25-hydroxyvitamin D; BDI: Beck Depression Inventory Source: Reference 35 | |||
Kjærgaard et al36 systematically examined vitamin D levels in a case-control study followed by a randomized controlled trial (RCT) of vitamin D supplementation. In the case-control phase, participants with low 25(OH)D levels at baseline were significantly more depressed than participants with high 25(OH)D levels. Participants with low 25(OH)D levels were randomized to placebo or 40,000 IU vitamin D3 per week for 6 months. Low levels of vitamin D were strongly associated with depressive symptoms, but vitamin D supplementation did not have a significant effect on depressive symptom scores.
Seasonal affective disorder (SAD). Seasonal variation in vitamin D levels suggests that supplementation may help patients who have seasonal mood disturbances. In a randomized, double-blind study, 44 healthy individuals received vitamin D3, 400 IU/d, 800 IU/d, or no vitamin D3 for 5 days during late winter. Based on self-reports, vitamin D3 significantly enhanced positive affect and there was some evidence it reduced negative affect.37 In a pilot study of 9 women with serum vitamin D levels <40 ng/ml, vitamin D supplementation during winter was associated with an average 10-point decline in Beck Depression Inventory-II scores.38 In a prospective RCT of 15 individuals with SAD, all patients who received vitamin D improved in all outcome measures.39 Vieth40 randomized 82 adults with vitamin D deficiency to 600 IU/d or 4,000 IU/d of vitamin D3 for 3 months over 2 consecutive winters. Patients taking the higher dose showed some evidence of improved well-being compared with those taking the lower dose, although results were not significant for all comparisons. Two other trials did not observe any improvement in SAD symptoms with vitamin D treatment.41,42
Treating vitamin D deficiency
The Endocrine Society recently developed consensus guidelines for diagnosing and managing vitamin D deficiency.43 In addition, the Institute of Medicine of the National Academies recommends daily vitamin D supplementation to prevent deficiency:
- age <70: 400 IU/d
- age >70: 800 IU/d
- pregnant or lactating women: 600 IU/d
- upper limit: 4,000 IU/d.7
Higher doses may be used for patients deprived of sun exposure.8 A typical replacement regimen consists of oral ergocalciferol, 50,000 IU per week for 8 weeks.44 The optimal time for rechecking serum levels after repletion has not been clearly defined, but serum 25(OH)D levels should be measured again after therapy is completed. If values have not reached or exceeded 20 ng/mL, consider a second 8-week course of ergocalciferol (see the Box 1 for a discussion of measuring vitamin D levels). If serum 25(OH)D levels have not increased, the most likely cause is nonadherence or malabsorption.
Contraindications and toxicity. Contraindications to vitamin D supplementation include granulomatous diseases, sarcoidosis, metastatic bone disease, and Williams syndrome.45Table 345 lists signs of vitamin D toxicity. There is little risk of toxicity at dosages of up to 2,000 IU/d.46
Table 3
Signs of vitamin D toxicity
| Headache |
| Metallic taste |
| Nephrocalcinosis or vascular calcinosis |
| Pancreatitis |
| Nausea |
| Vomiting |
| Source: Reference 45 |
Related Resources
- LaFerney MC. Vitamin D deficiency in older adults. Current Psychiatry. 2012;11(11):63.
- National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional.
Drug Brand Names
- Cholestyramine • Questran
- Ergocalciferol • Calciferol, Drisdol
Disclosures
Dr. Harris is an employee of Rho, Chapel Hill, NC.
Dr. Jaiswal reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Holmes receives research support from Bristol-Myers Squibb, Elan, Merck, Otsuka, Shire, Takeda, and Theravance, and is on the speaker’s bureau for Forest Pharmaceuticals and PamLab.
Dr. Patkar is a consultant for Dey Pharmaceuticals, Forest, Gilead, and TTK Pharma and is on the speaker’s bureau and received honoraria from Alkermes, Bristol-Myers Squibb, Dey Pharmaceuticals, Pfizer, and Sunovion; and has received grant support from the Duke Endowment, Dey Pharmaceuticals, Envivo, Forest, Janssen, Lundbeck, The National Institutes of Health, the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, Pfizer Inc., Shire, Sunovion, and Titan.
Dr. Weisler has been a consultant to, on the speaker’s bureaus of, and/or received research support from Abbott, Agency for Toxic Substances and Disease Registry, AstraZeneca, Biovail, Bristol-Myers Squibb, Burroughs Wellcome, Cenerx, Centers for Disease Control and Prevention, Cephalon, Ciba Geigy, CoMentis, Corcept, Cortex, Dainippon Sumitomo Pharma America, Eisai, Elan, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, McNeil Pharmaceuticals, Medicinova, Medscape Advisory Board, Merck, National Institute of Mental Health, Neurochem, New River Pharmaceuticals, Novartis, Organon, Otsuka America Pharma, Pfizer Inc., Pharmacia, Repligen, Saegis, Sandoz, Sanofi, Sanofi-Synthelabo, Schwabe/Ingenix, Sepracor, Shire, Solvay, Sunovion, Synaptic, Takeda, TAP, Theravance, Transcept Pharma, TransTech, UCB Pharma, Validus, Vela, and Wyeth.
1. McCue RE, Charles RA, Orendain GC, et al. Vitamin D deficiency among psychiatric inpatients [published online April 19, 2012]. Prim Care Companion CNS Disord. doi: 10.4088/PCC.11m01230.
2. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115-124.
3. Adams JS, Clemens TL, Parrish JA, et al. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306(12):722-725.
4. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
5. Holick MF. Vitamin D: importance in the prevention of cancers type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
6. Fakih MG, Trump DL, Muindi JR, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216-1223.
7. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
9. Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
10. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732-736.
11. Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003-2006. Cancer Causes Control. 2012;23(1):133-140.
12. Prüfer K, Veenstra TD, Jirikowski GF, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135-145.
13. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
14. Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100-105.
15. Smith MP, Fletcher-Turner A, Yurek DM, et al. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31(4):533-539.
16. Garcion E, Nataf S, Berod A, et al. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255-267.
17. Baas D, Prüfer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000;31(1):59-68.
18. Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98-108.
19. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769-780.
20. Berg AO, Melle I, Torjesen PA, et al. A cross-sectional study of vitamin D deficiency among immigrants and Norwegians with psychosis compared to the general population. J Clin Psychiatry. 2010;71(12):1598-1604.
21. Hedelin M, Löf M, Olsson M, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry. 2010;10:38.-
22. McGrath J, Saari K, Hakko H, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67(2-3):237-245.
23. McGrath J, Eyles D, Mowry B, et al. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63(1-2):73-78.
24. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
25. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
26. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18-26.
27. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-1141.
28. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
29. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205-1211.
30. Kuningas M, Mooijaart SP, Jolles J, et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466-473.
31. Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29.-
32. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
33. Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1-3):240-243.
34. Högberg G, Gustafsson SA, Hällström T, et al. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779-783.
35. Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599-609.
36. Kjærgaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360-368.
37. Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl). 1998;135(4):319-223.
38. Shipowick CD, Moore CB, Corbett C, et al. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res. 2009;22(3):221-225.
39. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5-7.
40. Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8.-
41. Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Res. 1993;49(1):77-87.
42. Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10(2):151-153.
43. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
44. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
45. Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Can Fam Physician. 2007;53(5):841-854.
46. Norman AW, Bouillon R, Whiting SJ, et al. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3-5):204-205.
In the United States, >50% of psychiatric inpatients have vitamin D deficiency—<30 nmol/L (<12 ng/mL).1 A growing body of literature has found associations between vitamin D deficiency and psychiatric illnesses, particularly depression. Several randomized controlled trials (RCTs) have demonstrated that vitamin D supplementation can benefit depression symptoms. In this article, we discuss the current literature on vitamin D and psychiatric illness, and provide practical information for clinicians on the use of vitamin D supplementation.
Biosynthesis of vitamin D
Biosynthesis of vitamin D begins with the sterol provitamin D3 molecule 7-dehydrocholesterol (Figure).2 When skin is exposed to sunlight, 7-dehydrocholesterol absorbs UV radiation and forms provitamin D3, which undergoes rapid transformation to vitamin D3.2 Vitamin D3 is released from the plasma membrane and enters systemic circulation in a protein-bound form that has a serum half-life of 36 to 78 hours.3 Vitamin D3 can be taken up by adipocytes and stored in fat deposits, where it has a half-life of approximately 2 months.4

Figure: Biosynthesis of vitamin D
Provitamin D3 (7-dehydrocholesterol) in the skin absorbs UV radiation and undergoes isomerization to form vitamin D3. Endogenously produced vitamin D3 along with dietary vitamin D2 and vitamin D3 absorbed in the gastrointestinal tract are metabolized in the liver to 25-hydroxyvitamin D (25[OH]D), which re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D). Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P450 (CYP) enzymes CYP24 and CYP3A4.
Source: Reference 2Circulating vitamin D3 is metabolized in the liver by the enzyme vitamin D-25-hydroxylase to 25-hydroxyvitamin D (25[OH]D3), which has a serum half-life of approximately 15 days.4 Circulating 25(OH)D3 is not biologically active at the physiological level, and requires activation by conversion to 1,25-dihydroxyvitamin D (1,25[OH]2D3) in the kidneys by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is regulated by serum phosphorus and parathyroid hormone levels and other factors.5 Catabolism of 1,25(OH)2D3 is rapid, with a serum half-life of 3.5 to 21 hours.6 Vitamin D2 is structurally similar to vitamin D3, but occurs primarily in fungi, yeasts, and some invertebrates.
Risk factors for deficiency
A patient’s vitamin D status is determined by measuring 25(OH)D (Box 1). Risk factors for vitamin D deficiency include conditions that affect cutaneous production (insufficient sunlight exposure), obesity, gastrointestinal disorders, aging, renal disorders, and medications (Table 1). 2,5,7,8 The link between sunscreen use, either alone or in cosmetics, and vitamin D deficiency continues to be debated. While controlled studies have found that application of sunscreen with high sun protection factor can significantly reduce vitamin D production, 9 studies in clinical populations have failed to confirm these findings. 10,11 See Box 2 for a discussion of these risk factors and Box 3 for a discussion of acute and long-term medical manifestations of deficiency.
Although 1,25-dihydroxyvitamin D (1,25[OH]2D3) is the biologically active form of vitamin D, its circulating half-life is only 4 to 6 hours.a,b Therefore, 25-hydroxyvitamin D (25[OH]D) is the principal vitamin D metabolite measured to determine vitamin D status. Vitamin D levels commonly are expressed as ng/mL or nmol/L; the conversion factor from ng/mL to nmol/L is 2.496. The Institute of Medicine has defined vitamin D deficiency as a serum 25(OH)D level of <30 nmol/L (<12 ng/mL).c However, many experts define vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/ml, and deficiency as <20 ng/mL.a,d The upper limit is more difficult to define, but symptoms of vitamin D intoxication appear with blood levels >150 to 200 ng/mL.a
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96(10):2987-2996.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
Table 1
Risk factors associated with vitamin D deficiency
| Age (>65) |
| Insufficient sunlight |
| Breastfeeding |
| Dark skin |
| Malabsorption diseases |
| Obesity (BMI >30 kg/m2) |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Hepatobiliary disease |
| Renal disease |
| BMI: body mass index Source: References 2,5,7,8 |
Any factor that diminishes UV radiation penetration into the skin will affect cutaneous synthesis of vitamin D.a,b For example, sunscreen with a sun protection factor of 15 can decrease vitamin D synthesis by 98%.c Geography and its impact on yearly sunlight exposure is a well-known factor in vitamin D deficiency. Individuals who live below a latitude of approximately 35° North—approximately the southern border of Tennessee and through Albuquerque, NM—receive sufficient UV radiation exposure to ensure adequate vitamin D production throughout the year, but at higher latitudes, adequate vitamin D is not produced during winter months.d Melanin affects UV radiation absorption in a manner that prevents vitamin D production, and increased skin pigmentation markedly reduces vitamin D synthesis.e African Americans with very dark skin have significantly diminished cutaneous production of vitamin D.e,f
Renal 1α-hydroxylase activity decreases with aging in parallel with age-related decreases in glomerular filtration.g In addition, aging is associated with increased clearance of 1,25-dihydroxyvitamin D (1,25[OH]2D3).h However, vitamin D absorption generally is adequate even at older ages.i Studies have shown that obese individuals tend to have lower serum concentrations of vitamin D and 25-hydroxyvitamin D (25[OH]D) than those at a normal weight.j,k Obese patients have been shown to have lower cutaneous production of vitamin D3 and display lower bioavailability of orally administered vitamin D2.j
For patients with chronic renal insufficiency, creatinine clearance is positively correlated with serum 1,25(OH)2D levels.l Any process that results in malabsorption of intestinal fat may impair vitamin D absorption. In patients with celiac disease, biliary obstruction, or chronic pancreatitis, absorption consistently is reduced.m Individuals taking bile acid-binding medications, such as cholestyramine for hypercholesterolemia, also may have impaired vitamin D absorption.n In addition, hepatobiliary disease is associated with low levels of 25(OH)D.o Some drugs that alter hepatic metabolism are associated with vitamin D deficiency, including anticonvulsants or glucocorticoids, which can increase catabolism or vitamin D.p
References
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361-368.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
- Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213-217.
- Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
- Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J Clin Invest. 1980;66(5):1118-1123.
- Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644-650.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
- Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370-373.
- Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67(5):876-881.
- Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94-102.
- Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644-649.
- Pappa HM, Bern E, Kamin D, et al. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24(2):176-183.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Acute effects. Vitamin D deficiency produces a range of clinical effects.a-c One well-documented consequence of vitamin D deficiency is osteomalacia—bone demineralization—which produces characteristic bone deformity and growth retardation in children.d,e In adults, osteomalacia may manifest as diffuse pain bone discomfort and muscle aches that may resemble fibromyalgia or arthritis.f Because vitamin D receptors are present in skeletal muscle, deficiency also may lead to proximal muscle weakness; an increased risk of falls; global bone discomfort, often elicited with pressure over the sternum or tibia; and low back pain in older women.c,f
Long-term effects. A large epidemiologic study found that adults with 25-hydroxyvitamin D (25[OH]D) levels <21 ng/mL had an increased risk of hypertension, diabetes, obesity, and dyslipidemia.g Cardiovascular mortality was higher in individuals with 25(OH)D levels <10 ng/mL compared with those with >40 ng/mL.h Adolescents in the National Health and Nutrition Examination Survey-III with serum 25(OH)D levels <15 ng/mL were more likely to have elevated blood glucose levels than those with >26 ng/mL.i Other epidemiologic data have demonstrated associations of vitamin D deficiency with multiple sclerosis, seasonal allergies, asthma, and various infectious diseases.j,k
Because vitamin D is known to promote cellular differentiation and inhibit cellular proliferation, its role in cancer has been studied extensively. A recent meta-analysis of case-control studies found that the odds of colon cancer were reduced by >40% for each 20 ng/mL increase in serum 25(OH)D levels.l Another meta-analysis reported a lower risk of breast cancer among women in the highest quartile of 25(OH)D values compared with the lowest quartile.m
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841-846.
- Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56(5):785-791.
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159-1165.
- Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595-1603.
- Reis JP, von Mühlen D, Miller ER 3rd, et al. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371-379.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
- Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838.
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113-125.
- Chen P, Hu P, Xie D, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469-477.
Vitamin D’s role in the brain
Vitamin D’s role in psychiatric illnesses is suggested by region-specific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, amygdala, and hippocampus.12 Most of these regions also express 1α-hydroxylase enzymes capable of metabolizing 25(OH)D to 1,25(OH)2D3, which suggests that vitamin D may have an autocrine or paracrine function in brain.13
Vitamin D regulates expression of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine, norepinephrine, and epinephrine.14 Vitamin D also promotes survival of monoaminergic neurons through upregulation of glial cell line-derived neurotrophic factor, which supports survival of midbrain dopaminergic neurons and confers resistance to neurotoxins that deplete dopaminergic neurons in Parkinson’s disease.15 Vitamin D also promotes neuronal survival by inhibiting oxidative pathways in the brain through inhibition of inducible nitric oxide synthase (reducing free radical formation)16 and upregulation of γ-glutamyl transpeptidase (increasing antioxidant production).17 Vitamin D may play a neuroprotective role through regulation of calcium channels. In vitro studies have shown that vitamin D downregulates expression of L-type calcium channels, conferring protection against excitatory neurotoxins in cultured neurons.18 Proteomic analysis of brain tissue in a rat model of developmental vitamin D revealed dysregulation of 36 brain proteins involved in many biologic pathways involved in calcium homeostasis, synaptic plasticity, and neurotransmission.19 Taken together, these findings suggest vitamin D has a neurosteroid-like role in the CNS.
Psychotic disorders
Several epidemiologic studies have linked low vitamin D levels to schizophrenia and other psychotic disorders. Researchers in Norway who used a structured clinical interview to identify psychosis consistently found low levels of 25(OH)D among immigrants and native Norwegians with psychotic symptoms.20 A study of 8,411 Swedish women found low vitamin D levels were associated with psychotic symptoms.21 The Finnish birth cohort study found that use of vitamin D supplementation during the first year of life reduced the incidence of schizophrenia.22 In another pilot study, researchers measured third-trimester serum 25(OH)D levels and found that low levels of maternal vitamin D may be associated with an increased risk of schizophrenia.23 These studies suggest that low prenatal vitamin D levels may adversely impact the developing brain, increasing the risk for adult-onset schizophrenia.
Cognitive dysfunction
Low vitamin D concentrations have been associated with impairments in cognitive functions such as memory and orientation,24 executive function impairments,25 and Alzheimer’s disease (AD).26 A large study conducted from 1998 to 2006 in Italy concluded that persons with severe vitamin D deficiency (<25 nmol/L) had a higher risk of substantial decline on Mini-Mental State Examination than those with sufficient levels (≥75 nmol/L).27 Other studies have linked low vitamin D levels to poor cognitive performance in depressed older adults.28 Low vitamin D levels in older women have been associated with risk of AD, but not with other dementias.29 Polymorphisms of VDR have been associated with depression and poor cognitive performance.30
Depression
Epidemiologic studies evaluating vitamin D deficiency have had conflicting results. The Third National Health and Nutrition Examination Survey, which used a sample of 7,970 non-institutionalized U.S. residents age 15 to 39, demonstrated that individuals with serum vitamin D ≤50 nmol/L are at a significantly higher risk of developing depression than those with vitamin D ≥75 nmol/L.31 A study of 1,282 adults age 65 to 95 in the Netherlands found that 25(OH)D levels were 14% lower in depressed patients compared with controls.32 However, a large epidemiologic study in China did not detect a relationship between vitamin D and depression in 3,262 men and women age 50 to 70.33 After researchers adjusted for geography, body mass index, physical activity, and smoking, 25(OH)D levels did not correlate significantly with the presence or severity of depression. In a case series,34 after 48 vitamin D-deficient depressed adolescents were given vitamin D3 over 3 months, there was a significant improvement in well-being, depressive symptoms, irritability, and fatigue.34 Other small, cross-sectional studies have examined associations between vitamin D status and depression with divergent results, which may reflect differences in population and methodology.
Prospective interventional studies. Although direct causal relationships are difficult to establish, several prospective studies have tested the hypothesis that treating vitamin D deficiency can improve depressive symptoms.
In a double-blind, controlled trial, Jorde et al35 randomized 441 individuals age 21 to 70 to vitamin D, 20,000 IU per week; vitamin D, 40,000 IU per week; or placebo for 1 year. Individuals with serum 25(OH)D levels <40 nmol/L scored significantly higher on depression rating scales than those with serum 25(OH)D levels ≥40 nmol/L at the end of the study. There was no significant improvement in depression ratings in the placebo group (Table 2).35 These results must be interpreted with care because depressive symptoms were secondary endpoints in this study.
Table 2
Effect of vitamin D supplementation on depressive symptoms in a controlled trial
| Vitamin D Supplementation | Serum 25(OH)D levels at baseline | BDI total score, Median and range at end of study | After 1 year of vitamin D supplementation |
|---|---|---|---|
| 20,000 IU/week | <40 nmol/L | Significantly higher (more depressive traits), 6.0 (0 to 23) | Significantly improved BDI score |
| 40,000 IU/week | ≥40 nmol/L | 4.5 (0 to 28) | Significantly improved BDI score |
| Placebo | - | - | No improvement in BDI score |
| 25(OH)D: 25-hydroxyvitamin D; BDI: Beck Depression Inventory Source: Reference 35 | |||
Kjærgaard et al36 systematically examined vitamin D levels in a case-control study followed by a randomized controlled trial (RCT) of vitamin D supplementation. In the case-control phase, participants with low 25(OH)D levels at baseline were significantly more depressed than participants with high 25(OH)D levels. Participants with low 25(OH)D levels were randomized to placebo or 40,000 IU vitamin D3 per week for 6 months. Low levels of vitamin D were strongly associated with depressive symptoms, but vitamin D supplementation did not have a significant effect on depressive symptom scores.
Seasonal affective disorder (SAD). Seasonal variation in vitamin D levels suggests that supplementation may help patients who have seasonal mood disturbances. In a randomized, double-blind study, 44 healthy individuals received vitamin D3, 400 IU/d, 800 IU/d, or no vitamin D3 for 5 days during late winter. Based on self-reports, vitamin D3 significantly enhanced positive affect and there was some evidence it reduced negative affect.37 In a pilot study of 9 women with serum vitamin D levels <40 ng/ml, vitamin D supplementation during winter was associated with an average 10-point decline in Beck Depression Inventory-II scores.38 In a prospective RCT of 15 individuals with SAD, all patients who received vitamin D improved in all outcome measures.39 Vieth40 randomized 82 adults with vitamin D deficiency to 600 IU/d or 4,000 IU/d of vitamin D3 for 3 months over 2 consecutive winters. Patients taking the higher dose showed some evidence of improved well-being compared with those taking the lower dose, although results were not significant for all comparisons. Two other trials did not observe any improvement in SAD symptoms with vitamin D treatment.41,42
Treating vitamin D deficiency
The Endocrine Society recently developed consensus guidelines for diagnosing and managing vitamin D deficiency.43 In addition, the Institute of Medicine of the National Academies recommends daily vitamin D supplementation to prevent deficiency:
- age <70: 400 IU/d
- age >70: 800 IU/d
- pregnant or lactating women: 600 IU/d
- upper limit: 4,000 IU/d.7
Higher doses may be used for patients deprived of sun exposure.8 A typical replacement regimen consists of oral ergocalciferol, 50,000 IU per week for 8 weeks.44 The optimal time for rechecking serum levels after repletion has not been clearly defined, but serum 25(OH)D levels should be measured again after therapy is completed. If values have not reached or exceeded 20 ng/mL, consider a second 8-week course of ergocalciferol (see the Box 1 for a discussion of measuring vitamin D levels). If serum 25(OH)D levels have not increased, the most likely cause is nonadherence or malabsorption.
Contraindications and toxicity. Contraindications to vitamin D supplementation include granulomatous diseases, sarcoidosis, metastatic bone disease, and Williams syndrome.45Table 345 lists signs of vitamin D toxicity. There is little risk of toxicity at dosages of up to 2,000 IU/d.46
Table 3
Signs of vitamin D toxicity
| Headache |
| Metallic taste |
| Nephrocalcinosis or vascular calcinosis |
| Pancreatitis |
| Nausea |
| Vomiting |
| Source: Reference 45 |
Related Resources
- LaFerney MC. Vitamin D deficiency in older adults. Current Psychiatry. 2012;11(11):63.
- National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional.
Drug Brand Names
- Cholestyramine • Questran
- Ergocalciferol • Calciferol, Drisdol
Disclosures
Dr. Harris is an employee of Rho, Chapel Hill, NC.
Dr. Jaiswal reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Holmes receives research support from Bristol-Myers Squibb, Elan, Merck, Otsuka, Shire, Takeda, and Theravance, and is on the speaker’s bureau for Forest Pharmaceuticals and PamLab.
Dr. Patkar is a consultant for Dey Pharmaceuticals, Forest, Gilead, and TTK Pharma and is on the speaker’s bureau and received honoraria from Alkermes, Bristol-Myers Squibb, Dey Pharmaceuticals, Pfizer, and Sunovion; and has received grant support from the Duke Endowment, Dey Pharmaceuticals, Envivo, Forest, Janssen, Lundbeck, The National Institutes of Health, the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, Pfizer Inc., Shire, Sunovion, and Titan.
Dr. Weisler has been a consultant to, on the speaker’s bureaus of, and/or received research support from Abbott, Agency for Toxic Substances and Disease Registry, AstraZeneca, Biovail, Bristol-Myers Squibb, Burroughs Wellcome, Cenerx, Centers for Disease Control and Prevention, Cephalon, Ciba Geigy, CoMentis, Corcept, Cortex, Dainippon Sumitomo Pharma America, Eisai, Elan, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, McNeil Pharmaceuticals, Medicinova, Medscape Advisory Board, Merck, National Institute of Mental Health, Neurochem, New River Pharmaceuticals, Novartis, Organon, Otsuka America Pharma, Pfizer Inc., Pharmacia, Repligen, Saegis, Sandoz, Sanofi, Sanofi-Synthelabo, Schwabe/Ingenix, Sepracor, Shire, Solvay, Sunovion, Synaptic, Takeda, TAP, Theravance, Transcept Pharma, TransTech, UCB Pharma, Validus, Vela, and Wyeth.
In the United States, >50% of psychiatric inpatients have vitamin D deficiency—<30 nmol/L (<12 ng/mL).1 A growing body of literature has found associations between vitamin D deficiency and psychiatric illnesses, particularly depression. Several randomized controlled trials (RCTs) have demonstrated that vitamin D supplementation can benefit depression symptoms. In this article, we discuss the current literature on vitamin D and psychiatric illness, and provide practical information for clinicians on the use of vitamin D supplementation.
Biosynthesis of vitamin D
Biosynthesis of vitamin D begins with the sterol provitamin D3 molecule 7-dehydrocholesterol (Figure).2 When skin is exposed to sunlight, 7-dehydrocholesterol absorbs UV radiation and forms provitamin D3, which undergoes rapid transformation to vitamin D3.2 Vitamin D3 is released from the plasma membrane and enters systemic circulation in a protein-bound form that has a serum half-life of 36 to 78 hours.3 Vitamin D3 can be taken up by adipocytes and stored in fat deposits, where it has a half-life of approximately 2 months.4

Figure: Biosynthesis of vitamin D
Provitamin D3 (7-dehydrocholesterol) in the skin absorbs UV radiation and undergoes isomerization to form vitamin D3. Endogenously produced vitamin D3 along with dietary vitamin D2 and vitamin D3 absorbed in the gastrointestinal tract are metabolized in the liver to 25-hydroxyvitamin D (25[OH]D), which re-enters the circulation and is metabolized in the kidney and other tissues to the active metabolite 1,25-dihydroxyvitamin D (1,25[OH]2D). Catabolism of 25(OH)D and 1,25(OH)2D into biologically-inactive molecules is primarily mediated by the cytochrome P450 (CYP) enzymes CYP24 and CYP3A4.
Source: Reference 2Circulating vitamin D3 is metabolized in the liver by the enzyme vitamin D-25-hydroxylase to 25-hydroxyvitamin D (25[OH]D3), which has a serum half-life of approximately 15 days.4 Circulating 25(OH)D3 is not biologically active at the physiological level, and requires activation by conversion to 1,25-dihydroxyvitamin D (1,25[OH]2D3) in the kidneys by the enzyme 25(OH)D-1α-hydroxylase. Production of 1,25(OH)2D3 is regulated by serum phosphorus and parathyroid hormone levels and other factors.5 Catabolism of 1,25(OH)2D3 is rapid, with a serum half-life of 3.5 to 21 hours.6 Vitamin D2 is structurally similar to vitamin D3, but occurs primarily in fungi, yeasts, and some invertebrates.
Risk factors for deficiency
A patient’s vitamin D status is determined by measuring 25(OH)D (Box 1). Risk factors for vitamin D deficiency include conditions that affect cutaneous production (insufficient sunlight exposure), obesity, gastrointestinal disorders, aging, renal disorders, and medications (Table 1). 2,5,7,8 The link between sunscreen use, either alone or in cosmetics, and vitamin D deficiency continues to be debated. While controlled studies have found that application of sunscreen with high sun protection factor can significantly reduce vitamin D production, 9 studies in clinical populations have failed to confirm these findings. 10,11 See Box 2 for a discussion of these risk factors and Box 3 for a discussion of acute and long-term medical manifestations of deficiency.
Although 1,25-dihydroxyvitamin D (1,25[OH]2D3) is the biologically active form of vitamin D, its circulating half-life is only 4 to 6 hours.a,b Therefore, 25-hydroxyvitamin D (25[OH]D) is the principal vitamin D metabolite measured to determine vitamin D status. Vitamin D levels commonly are expressed as ng/mL or nmol/L; the conversion factor from ng/mL to nmol/L is 2.496. The Institute of Medicine has defined vitamin D deficiency as a serum 25(OH)D level of <30 nmol/L (<12 ng/mL).c However, many experts define vitamin D insufficiency as a 25(OH)D level of 21 to 29 ng/ml, and deficiency as <20 ng/mL.a,d The upper limit is more difficult to define, but symptoms of vitamin D intoxication appear with blood levels >150 to 200 ng/mL.a
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Aloia JF. Clinical review: the 2011 report on dietary reference intake for vitamin D: where do we go from here? J Clin Endocrinol Metab. 2011;96(10):2987-2996.
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18-28.
Table 1
Risk factors associated with vitamin D deficiency
| Age (>65) |
| Insufficient sunlight |
| Breastfeeding |
| Dark skin |
| Malabsorption diseases |
| Obesity (BMI >30 kg/m2) |
| Use of medications that alter vitamin D metabolism (eg, anticonvulsants, glucocorticoids) |
| Hepatobiliary disease |
| Renal disease |
| BMI: body mass index Source: References 2,5,7,8 |
Any factor that diminishes UV radiation penetration into the skin will affect cutaneous synthesis of vitamin D.a,b For example, sunscreen with a sun protection factor of 15 can decrease vitamin D synthesis by 98%.c Geography and its impact on yearly sunlight exposure is a well-known factor in vitamin D deficiency. Individuals who live below a latitude of approximately 35° North—approximately the southern border of Tennessee and through Albuquerque, NM—receive sufficient UV radiation exposure to ensure adequate vitamin D production throughout the year, but at higher latitudes, adequate vitamin D is not produced during winter months.d Melanin affects UV radiation absorption in a manner that prevents vitamin D production, and increased skin pigmentation markedly reduces vitamin D synthesis.e African Americans with very dark skin have significantly diminished cutaneous production of vitamin D.e,f
Renal 1α-hydroxylase activity decreases with aging in parallel with age-related decreases in glomerular filtration.g In addition, aging is associated with increased clearance of 1,25-dihydroxyvitamin D (1,25[OH]2D3).h However, vitamin D absorption generally is adequate even at older ages.i Studies have shown that obese individuals tend to have lower serum concentrations of vitamin D and 25-hydroxyvitamin D (25[OH]D) than those at a normal weight.j,k Obese patients have been shown to have lower cutaneous production of vitamin D3 and display lower bioavailability of orally administered vitamin D2.j
For patients with chronic renal insufficiency, creatinine clearance is positively correlated with serum 1,25(OH)2D levels.l Any process that results in malabsorption of intestinal fat may impair vitamin D absorption. In patients with celiac disease, biliary obstruction, or chronic pancreatitis, absorption consistently is reduced.m Individuals taking bile acid-binding medications, such as cholestyramine for hypercholesterolemia, also may have impaired vitamin D absorption.n In addition, hepatobiliary disease is associated with low levels of 25(OH)D.o Some drugs that alter hepatic metabolism are associated with vitamin D deficiency, including anticonvulsants or glucocorticoids, which can increase catabolism or vitamin D.p
References
- Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med. 2008;29(6):361-368.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
- Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
- Clemens TL, Adams JS, Henderson SL, et al. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1(8263):74-76.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213-217.
- Slovik DM, Adams JS, Neer RM, et al. Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305(7):372-374.
- Armbrecht HJ, Zenser TV, Davis BB. Effect of age on the conversion of 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 by kidney of rat. J Clin Invest. 1980;66(5):1118-1123.
- Lips P, Wiersinga A, van Ginkel FC, et al. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab. 1988;67(4):644-650.
- Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690-693.
- Bell NH, Epstein S, Greene A, et al. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370-373.
- Pitts TO, Piraino BH, Mitro R, et al. Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab. 1988;67(5):876-881.
- Thompson GR, Lewis B, Booth CC. Absorption of vitamin D3-3H in control subjects and patients with intestinal malabsorption. J Clin Invest. 1966;45(1):94-102.
- Lo CW, Paris PW, Clemens TL, et al. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985;42(4):644-649.
- Pappa HM, Bern E, Kamin D, et al. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24(2):176-183.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
Acute effects. Vitamin D deficiency produces a range of clinical effects.a-c One well-documented consequence of vitamin D deficiency is osteomalacia—bone demineralization—which produces characteristic bone deformity and growth retardation in children.d,e In adults, osteomalacia may manifest as diffuse pain bone discomfort and muscle aches that may resemble fibromyalgia or arthritis.f Because vitamin D receptors are present in skeletal muscle, deficiency also may lead to proximal muscle weakness; an increased risk of falls; global bone discomfort, often elicited with pressure over the sternum or tibia; and low back pain in older women.c,f
Long-term effects. A large epidemiologic study found that adults with 25-hydroxyvitamin D (25[OH]D) levels <21 ng/mL had an increased risk of hypertension, diabetes, obesity, and dyslipidemia.g Cardiovascular mortality was higher in individuals with 25(OH)D levels <10 ng/mL compared with those with >40 ng/mL.h Adolescents in the National Health and Nutrition Examination Survey-III with serum 25(OH)D levels <15 ng/mL were more likely to have elevated blood glucose levels than those with >26 ng/mL.i Other epidemiologic data have demonstrated associations of vitamin D deficiency with multiple sclerosis, seasonal allergies, asthma, and various infectious diseases.j,k
Because vitamin D is known to promote cellular differentiation and inhibit cellular proliferation, its role in cancer has been studied extensively. A recent meta-analysis of case-control studies found that the odds of colon cancer were reduced by >40% for each 20 ng/mL increase in serum 25(OH)D levels.l Another meta-analysis reported a lower risk of breast cancer among women in the highest quartile of 25(OH)D values compared with the lowest quartile.m
References
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353-373.
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73-78.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
- Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
- Bordelon P, Ghetu MV, Langan RC. Recognition and management of vitamin D deficiency. Am Fam Physician. 2009;80(8):841-846.
- Hicks GE, Shardell M, Miller RR, et al. Associations between vitamin D status and pain in older adults: the Invecchiare in Chianti study. J Am Geriatr Soc. 2008;56(5):785-791.
- Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167(11):1159-1165.
- Ginde AA, Scragg R, Schwartz RS, et al. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57(9):1595-1603.
- Reis JP, von Mühlen D, Miller ER 3rd, et al. Vitamin D status and cardiometabolic risk factors in the United States adolescent population. Pediatrics. 2009;124(3):e371-379.
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
- Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832-2838.
- Yin L, Grandi N, Raum E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113-125.
- Chen P, Hu P, Xie D, et al. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469-477.
Vitamin D’s role in the brain
Vitamin D’s role in psychiatric illnesses is suggested by region-specific expression of vitamin D receptors (VDR) in the cingulate cortex, thalamus, cerebellum, amygdala, and hippocampus.12 Most of these regions also express 1α-hydroxylase enzymes capable of metabolizing 25(OH)D to 1,25(OH)2D3, which suggests that vitamin D may have an autocrine or paracrine function in brain.13
Vitamin D regulates expression of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of dopamine, norepinephrine, and epinephrine.14 Vitamin D also promotes survival of monoaminergic neurons through upregulation of glial cell line-derived neurotrophic factor, which supports survival of midbrain dopaminergic neurons and confers resistance to neurotoxins that deplete dopaminergic neurons in Parkinson’s disease.15 Vitamin D also promotes neuronal survival by inhibiting oxidative pathways in the brain through inhibition of inducible nitric oxide synthase (reducing free radical formation)16 and upregulation of γ-glutamyl transpeptidase (increasing antioxidant production).17 Vitamin D may play a neuroprotective role through regulation of calcium channels. In vitro studies have shown that vitamin D downregulates expression of L-type calcium channels, conferring protection against excitatory neurotoxins in cultured neurons.18 Proteomic analysis of brain tissue in a rat model of developmental vitamin D revealed dysregulation of 36 brain proteins involved in many biologic pathways involved in calcium homeostasis, synaptic plasticity, and neurotransmission.19 Taken together, these findings suggest vitamin D has a neurosteroid-like role in the CNS.
Psychotic disorders
Several epidemiologic studies have linked low vitamin D levels to schizophrenia and other psychotic disorders. Researchers in Norway who used a structured clinical interview to identify psychosis consistently found low levels of 25(OH)D among immigrants and native Norwegians with psychotic symptoms.20 A study of 8,411 Swedish women found low vitamin D levels were associated with psychotic symptoms.21 The Finnish birth cohort study found that use of vitamin D supplementation during the first year of life reduced the incidence of schizophrenia.22 In another pilot study, researchers measured third-trimester serum 25(OH)D levels and found that low levels of maternal vitamin D may be associated with an increased risk of schizophrenia.23 These studies suggest that low prenatal vitamin D levels may adversely impact the developing brain, increasing the risk for adult-onset schizophrenia.
Cognitive dysfunction
Low vitamin D concentrations have been associated with impairments in cognitive functions such as memory and orientation,24 executive function impairments,25 and Alzheimer’s disease (AD).26 A large study conducted from 1998 to 2006 in Italy concluded that persons with severe vitamin D deficiency (<25 nmol/L) had a higher risk of substantial decline on Mini-Mental State Examination than those with sufficient levels (≥75 nmol/L).27 Other studies have linked low vitamin D levels to poor cognitive performance in depressed older adults.28 Low vitamin D levels in older women have been associated with risk of AD, but not with other dementias.29 Polymorphisms of VDR have been associated with depression and poor cognitive performance.30
Depression
Epidemiologic studies evaluating vitamin D deficiency have had conflicting results. The Third National Health and Nutrition Examination Survey, which used a sample of 7,970 non-institutionalized U.S. residents age 15 to 39, demonstrated that individuals with serum vitamin D ≤50 nmol/L are at a significantly higher risk of developing depression than those with vitamin D ≥75 nmol/L.31 A study of 1,282 adults age 65 to 95 in the Netherlands found that 25(OH)D levels were 14% lower in depressed patients compared with controls.32 However, a large epidemiologic study in China did not detect a relationship between vitamin D and depression in 3,262 men and women age 50 to 70.33 After researchers adjusted for geography, body mass index, physical activity, and smoking, 25(OH)D levels did not correlate significantly with the presence or severity of depression. In a case series,34 after 48 vitamin D-deficient depressed adolescents were given vitamin D3 over 3 months, there was a significant improvement in well-being, depressive symptoms, irritability, and fatigue.34 Other small, cross-sectional studies have examined associations between vitamin D status and depression with divergent results, which may reflect differences in population and methodology.
Prospective interventional studies. Although direct causal relationships are difficult to establish, several prospective studies have tested the hypothesis that treating vitamin D deficiency can improve depressive symptoms.
In a double-blind, controlled trial, Jorde et al35 randomized 441 individuals age 21 to 70 to vitamin D, 20,000 IU per week; vitamin D, 40,000 IU per week; or placebo for 1 year. Individuals with serum 25(OH)D levels <40 nmol/L scored significantly higher on depression rating scales than those with serum 25(OH)D levels ≥40 nmol/L at the end of the study. There was no significant improvement in depression ratings in the placebo group (Table 2).35 These results must be interpreted with care because depressive symptoms were secondary endpoints in this study.
Table 2
Effect of vitamin D supplementation on depressive symptoms in a controlled trial
| Vitamin D Supplementation | Serum 25(OH)D levels at baseline | BDI total score, Median and range at end of study | After 1 year of vitamin D supplementation |
|---|---|---|---|
| 20,000 IU/week | <40 nmol/L | Significantly higher (more depressive traits), 6.0 (0 to 23) | Significantly improved BDI score |
| 40,000 IU/week | ≥40 nmol/L | 4.5 (0 to 28) | Significantly improved BDI score |
| Placebo | - | - | No improvement in BDI score |
| 25(OH)D: 25-hydroxyvitamin D; BDI: Beck Depression Inventory Source: Reference 35 | |||
Kjærgaard et al36 systematically examined vitamin D levels in a case-control study followed by a randomized controlled trial (RCT) of vitamin D supplementation. In the case-control phase, participants with low 25(OH)D levels at baseline were significantly more depressed than participants with high 25(OH)D levels. Participants with low 25(OH)D levels were randomized to placebo or 40,000 IU vitamin D3 per week for 6 months. Low levels of vitamin D were strongly associated with depressive symptoms, but vitamin D supplementation did not have a significant effect on depressive symptom scores.
Seasonal affective disorder (SAD). Seasonal variation in vitamin D levels suggests that supplementation may help patients who have seasonal mood disturbances. In a randomized, double-blind study, 44 healthy individuals received vitamin D3, 400 IU/d, 800 IU/d, or no vitamin D3 for 5 days during late winter. Based on self-reports, vitamin D3 significantly enhanced positive affect and there was some evidence it reduced negative affect.37 In a pilot study of 9 women with serum vitamin D levels <40 ng/ml, vitamin D supplementation during winter was associated with an average 10-point decline in Beck Depression Inventory-II scores.38 In a prospective RCT of 15 individuals with SAD, all patients who received vitamin D improved in all outcome measures.39 Vieth40 randomized 82 adults with vitamin D deficiency to 600 IU/d or 4,000 IU/d of vitamin D3 for 3 months over 2 consecutive winters. Patients taking the higher dose showed some evidence of improved well-being compared with those taking the lower dose, although results were not significant for all comparisons. Two other trials did not observe any improvement in SAD symptoms with vitamin D treatment.41,42
Treating vitamin D deficiency
The Endocrine Society recently developed consensus guidelines for diagnosing and managing vitamin D deficiency.43 In addition, the Institute of Medicine of the National Academies recommends daily vitamin D supplementation to prevent deficiency:
- age <70: 400 IU/d
- age >70: 800 IU/d
- pregnant or lactating women: 600 IU/d
- upper limit: 4,000 IU/d.7
Higher doses may be used for patients deprived of sun exposure.8 A typical replacement regimen consists of oral ergocalciferol, 50,000 IU per week for 8 weeks.44 The optimal time for rechecking serum levels after repletion has not been clearly defined, but serum 25(OH)D levels should be measured again after therapy is completed. If values have not reached or exceeded 20 ng/mL, consider a second 8-week course of ergocalciferol (see the Box 1 for a discussion of measuring vitamin D levels). If serum 25(OH)D levels have not increased, the most likely cause is nonadherence or malabsorption.
Contraindications and toxicity. Contraindications to vitamin D supplementation include granulomatous diseases, sarcoidosis, metastatic bone disease, and Williams syndrome.45Table 345 lists signs of vitamin D toxicity. There is little risk of toxicity at dosages of up to 2,000 IU/d.46
Table 3
Signs of vitamin D toxicity
| Headache |
| Metallic taste |
| Nephrocalcinosis or vascular calcinosis |
| Pancreatitis |
| Nausea |
| Vomiting |
| Source: Reference 45 |
Related Resources
- LaFerney MC. Vitamin D deficiency in older adults. Current Psychiatry. 2012;11(11):63.
- National Institutes of Health Office of Dietary Supplements. Dietary supplement fact sheet: vitamin D. http://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional.
Drug Brand Names
- Cholestyramine • Questran
- Ergocalciferol • Calciferol, Drisdol
Disclosures
Dr. Harris is an employee of Rho, Chapel Hill, NC.
Dr. Jaiswal reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Holmes receives research support from Bristol-Myers Squibb, Elan, Merck, Otsuka, Shire, Takeda, and Theravance, and is on the speaker’s bureau for Forest Pharmaceuticals and PamLab.
Dr. Patkar is a consultant for Dey Pharmaceuticals, Forest, Gilead, and TTK Pharma and is on the speaker’s bureau and received honoraria from Alkermes, Bristol-Myers Squibb, Dey Pharmaceuticals, Pfizer, and Sunovion; and has received grant support from the Duke Endowment, Dey Pharmaceuticals, Envivo, Forest, Janssen, Lundbeck, The National Institutes of Health, the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism, Pfizer Inc., Shire, Sunovion, and Titan.
Dr. Weisler has been a consultant to, on the speaker’s bureaus of, and/or received research support from Abbott, Agency for Toxic Substances and Disease Registry, AstraZeneca, Biovail, Bristol-Myers Squibb, Burroughs Wellcome, Cenerx, Centers for Disease Control and Prevention, Cephalon, Ciba Geigy, CoMentis, Corcept, Cortex, Dainippon Sumitomo Pharma America, Eisai, Elan, Eli Lilly and Company, Forest Pharmaceuticals, GlaxoSmithKline, Janssen, Johnson & Johnson, Lundbeck, McNeil Pharmaceuticals, Medicinova, Medscape Advisory Board, Merck, National Institute of Mental Health, Neurochem, New River Pharmaceuticals, Novartis, Organon, Otsuka America Pharma, Pfizer Inc., Pharmacia, Repligen, Saegis, Sandoz, Sanofi, Sanofi-Synthelabo, Schwabe/Ingenix, Sepracor, Shire, Solvay, Sunovion, Synaptic, Takeda, TAP, Theravance, Transcept Pharma, TransTech, UCB Pharma, Validus, Vela, and Wyeth.
1. McCue RE, Charles RA, Orendain GC, et al. Vitamin D deficiency among psychiatric inpatients [published online April 19, 2012]. Prim Care Companion CNS Disord. doi: 10.4088/PCC.11m01230.
2. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115-124.
3. Adams JS, Clemens TL, Parrish JA, et al. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306(12):722-725.
4. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
5. Holick MF. Vitamin D: importance in the prevention of cancers type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
6. Fakih MG, Trump DL, Muindi JR, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216-1223.
7. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
9. Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
10. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732-736.
11. Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003-2006. Cancer Causes Control. 2012;23(1):133-140.
12. Prüfer K, Veenstra TD, Jirikowski GF, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135-145.
13. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
14. Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100-105.
15. Smith MP, Fletcher-Turner A, Yurek DM, et al. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31(4):533-539.
16. Garcion E, Nataf S, Berod A, et al. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255-267.
17. Baas D, Prüfer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000;31(1):59-68.
18. Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98-108.
19. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769-780.
20. Berg AO, Melle I, Torjesen PA, et al. A cross-sectional study of vitamin D deficiency among immigrants and Norwegians with psychosis compared to the general population. J Clin Psychiatry. 2010;71(12):1598-1604.
21. Hedelin M, Löf M, Olsson M, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry. 2010;10:38.-
22. McGrath J, Saari K, Hakko H, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67(2-3):237-245.
23. McGrath J, Eyles D, Mowry B, et al. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63(1-2):73-78.
24. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
25. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
26. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18-26.
27. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-1141.
28. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
29. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205-1211.
30. Kuningas M, Mooijaart SP, Jolles J, et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466-473.
31. Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29.-
32. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
33. Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1-3):240-243.
34. Högberg G, Gustafsson SA, Hällström T, et al. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779-783.
35. Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599-609.
36. Kjærgaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360-368.
37. Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl). 1998;135(4):319-223.
38. Shipowick CD, Moore CB, Corbett C, et al. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res. 2009;22(3):221-225.
39. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5-7.
40. Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8.-
41. Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Res. 1993;49(1):77-87.
42. Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10(2):151-153.
43. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
44. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
45. Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Can Fam Physician. 2007;53(5):841-854.
46. Norman AW, Bouillon R, Whiting SJ, et al. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3-5):204-205.
1. McCue RE, Charles RA, Orendain GC, et al. Vitamin D deficiency among psychiatric inpatients [published online April 19, 2012]. Prim Care Companion CNS Disord. doi: 10.4088/PCC.11m01230.
2. Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115-124.
3. Adams JS, Clemens TL, Parrish JA, et al. Vitamin-D synthesis and metabolism after ultraviolet irradiation of normal and vitamin-D-deficient subjects. N Engl J Med. 1982;306(12):722-725.
4. Jones G. Pharmacokinetics of vitamin D toxicity. Am J Clin Nutr. 2008;88(2):582S-586S.
5. Holick MF. Vitamin D: importance in the prevention of cancers type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362-371.
6. Fakih MG, Trump DL, Muindi JR, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216-1223.
7. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S-1086S.
8. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-281.
9. Matsuoka LY, Ide L, Wortsman J, et al. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64(6):1165-1168.
10. Norval M, Wulf HC. Does chronic sunscreen use reduce vitamin D production to insufficient levels? Br J Dermatol. 2009;161(4):732-736.
11. Linos E, Keiser E, Kanzler M, et al. Sun protective behaviors and vitamin D levels in the US population: NHANES 2003-2006. Cancer Causes Control. 2012;23(1):133-140.
12. Prüfer K, Veenstra TD, Jirikowski GF, et al. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16(2):135-145.
13. Eyles DW, Smith S, Kinobe R, et al. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21-30.
14. Garcion E, Wion-Barbot N, Montero-Menei CN, et al. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100-105.
15. Smith MP, Fletcher-Turner A, Yurek DM, et al. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31(4):533-539.
16. Garcion E, Nataf S, Berod A, et al. 1,25-dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Brain Res Mol Brain Res. 1997;45(2):255-267.
17. Baas D, Prüfer K, Ittel ME, et al. Rat oligodendrocytes express the vitamin D(3) receptor and respond to 1,25-dihydroxyvitamin D(3). Glia. 2000;31(1):59-68.
18. Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21(1):98-108.
19. Almeras L, Eyles D, Benech P, et al. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics. 2007;7(5):769-780.
20. Berg AO, Melle I, Torjesen PA, et al. A cross-sectional study of vitamin D deficiency among immigrants and Norwegians with psychosis compared to the general population. J Clin Psychiatry. 2010;71(12):1598-1604.
21. Hedelin M, Löf M, Olsson M, et al. Dietary intake of fish, omega-3, omega-6 polyunsaturated fatty acids and vitamin D and the prevalence of psychotic-like symptoms in a cohort of 33,000 women from the general population. BMC Psychiatry. 2010;10:38.-
22. McGrath J, Saari K, Hakko H, et al. Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophr Res. 2004;67(2-3):237-245.
23. McGrath J, Eyles D, Mowry B, et al. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res. 2003;63(1-2):73-78.
24. Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460(2):202-205.
25. Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80(7):722-729.
26. Buell JS, Dawson-Hughes B, Scott TM, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18-26.
27. Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170(13):1135-1141.
28. Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14(12):1032-1040.
29. Annweiler C, Rolland Y, Schott AM, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer’s disease: a 7-year follow-up. J Gerontol A Biol Sci Med Sci. 2012;67(11):1205-1211.
30. Kuningas M, Mooijaart SP, Jolles J, et al. VDR gene variants associate with cognitive function and depressive symptoms in old age. Neurobiol Aging. 2009;30(3):466-473.
31. Ganji V, Milone C, Cody MM, et al. Serum vitamin D concentrations are related to depression in young adult US population: the Third National Health and Nutrition Examination Survey. Int Arch Med. 2010;3:29.-
32. Hoogendijk WJ, Lips P, Dik MG, et al. Depression is associated with decreased 25-hydroxyvitamin D and increased parathyroid hormone levels in older adults. Arch Gen Psychiatry. 2008;65(5):508-512.
33. Pan A, Lu L, Franco OH, et al. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J Affect Disord. 2009;118(1-3):240-243.
34. Högberg G, Gustafsson SA, Hällström T, et al. Depressed adolescents in a case-series were low in vitamin D and depression was ameliorated by vitamin D supplementation. Acta Paediatr. 2012;101(7):779-783.
35. Jorde R, Sneve M, Figenschau Y, et al. Effects of vitamin D supplementation on symptoms of depression in overweight and obese subjects: randomized double blind trial. J Intern Med. 2008;264(6):599-609.
36. Kjærgaard M, Waterloo K, Wang CE, et al. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: nested case-control study and randomised clinical trial. Br J Psychiatry. 2012;201(5):360-368.
37. Lansdowne AT, Provost SC. Vitamin D3 enhances mood in healthy subjects during winter. Psychopharmacology (Berl). 1998;135(4):319-223.
38. Shipowick CD, Moore CB, Corbett C, et al. Vitamin D and depressive symptoms in women during the winter: a pilot study. Appl Nurs Res. 2009;22(3):221-225.
39. Gloth FM 3rd, Alam W, Hollis B. Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging. 1999;3(1):5-7.
40. Vieth R, Kimball S, Hu A, et al. Randomized comparison of the effects of the vitamin D3 adequate intake versus 100 mcg (4000 IU) per day on biochemical responses and the wellbeing of patients. Nutr J. 2004;3:8.-
41. Harris S, Dawson-Hughes B. Seasonal mood changes in 250 normal women. Psychiatry Res. 1993;49(1):77-87.
42. Dumville JC, Miles JN, Porthouse J, et al. Can vitamin D supplementation prevent winter-time blues? A randomised trial among older women. J Nutr Health Aging. 2006;10(2):151-153.
43. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
44. Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86(1):50-60.
45. Schwalfenberg G. Not enough vitamin D: health consequences for Canadians. Can Fam Physician. 2007;53(5):841-854.
46. Norman AW, Bouillon R, Whiting SJ, et al. 13th Workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103(3-5):204-205.