User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Two vastly underutilized interventions can improve schizophrenia outcomes
Discuss this article at http://currentpsychiatry.blogspot.com/2011/02/two-vastly-underutilized-interventions.html#comments
Many psychiatrists would agree that schizophrenia is the most devastating psychiatric brain disease. Its disabling effects result in stigma, unemployment, poverty, loneliness, homelessness, victimization, incarceration, malnutrition, infections, social isolation, ostracism, discrimination, suicide, poor health, medical neglect, and early death.
The consequences of schizophrenia are in many ways more malignant than those of cancer, where sympathy, prompt medical care, and preservation of friends and employment are assured. Also, unlike schizophrenia patients, persons with cancer are never hauled to jail, even when a slow-growing brain tumor causes erratic or violent behavior.
The outcome of schizophrenia has not improved much since patients were “freed” from state hospitals before the psychopharmacology era. Despite the availability of antipsychotics that can control delusions and hallucinations, few persons with schizophrenia achieve remission, let alone recovery. Although negative symptoms and cognitive deficits remain untreatable, the quality of life for persons with schizophrenia can be far less miserable if psychotic symptoms are fully remitted. So why do so many patients with schizophrenia suffer continuous or episodic psychosis that renders their lives a living hell and leads them to inexorable clinical, social, and functional deterioration?
Based on 3 decades of clinical and research work with thousands of patients afflicted with schizophrenia, I have observed that most psychiatrists are not exploiting 2 treatment options that could effectively alleviate continuous or relapsing psychosis. Let me elaborate:
- Continuous, unabated psychotic symptoms (delusions, hallucinations, bizarre behavior, and thought derailment) implies either refractory or treatment-resistant schizophrenia. This means that several antipsychotic trials already have failed to suppress psychotic symptoms. Only 1 drug has been proven to help such patients: clozapine. It was FDA-approved for refractory schizophrenia 22 years ago, and many studies—including the Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE) trial—show that it also is superior to other antipsychotics for treatment-resistant schizophrenia. Yet while about 30% to 35% of patients have refractory or treatment-resistant schizophrenia, only one-sixth of that eligible pool receive clozapine. Reasons include the “hassle” of monitoring, the fear of agranulocytosis (which is rare), and concern about serious side effects. As a result, hundreds of thousands of patients suffer needlessly because they have not received clozapine (full response may take 6 to 12 months in some patients, but only 3 months in others).
- For patients whose delusions and hallucinations do respond to nonclozapine antipsychotics, psychotic episodes are most likely caused by nonadherence to oral medications. All psychiatrists are aware that partial or complete nonadherence is ubiquitous in schizophrenia for multiple reasons, including lack of insight about being ill and needing treatment, suspiciousness (positive symptoms) about drugs, amotivation and avolition (negative symptoms), poor memory or planning ability (cognitive deficits), and toxic effects of alcohol and drugs. Therefore, many psychotic relapses occur needlessly because of inevitable nonadherence with oral medications. In my clinical estimate, this occurs in 50% to 60% of patients who had responded well to oral antipsychotics in the past. The tragic consequences of recurrent relapses is not only re-experiencing the anguish of reality-shattering psychotic states, but also progressive brain atrophy resulting from the “neurotoxic” effects of psychosis, and, unfortunately, progressive development of treatment resistance with each relapse.
The most logical solution to avoid psychotic relapses resulting from nonadherence is long-acting injectable (depot) antipsychotics, which have a large body of data (including FDA registration trials) documenting successful relapse prevention. It is incomprehensible why long-acting formulations are not used in at least 30% to 40% of patients instead of the 6% to 8% who currently receive them. They also should be given to all schizophrenia patients incarcerated for violent acts. Furthermore, there is no reason not to use injectable formulations early in the illness course—after 1 or 2 relapses—to halt or prevent the clinical and functional deterioration that occurs with multiple relapses. In my experience, the stabilization and full remission that occur after 18 to 24 months of continuous adherence with a long-acting injectable antipsychotic can be remarkable and gratifying to patients, families, and psychiatrists.
In summary, intensified research is needed to discover the next generation of pharmacotherapeutic agents that can treat all symptom domains of schizophrenia (positive, negative, cognitive, mood, and substance use). In the meantime, a wider use of those 2 available interventions—clozapine in refractory and treatment-resistant patients and long-acting injectable antipsychotics in nonadherent patients—can make a palpable impact on improving clinical and functional outcomes of patients suffering with schizophrenia. These 2 interventions might make a significant dent in the atrociously high rate of incarceration because of psychosis-induced illegal behaviors. The financial savings in hospitalization and forensic costs can be substantial. But most important, preventing psychosis and incarceration would mean a measurably better life for patients.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/02/two-vastly-underutilized-interventions.html#comments
Many psychiatrists would agree that schizophrenia is the most devastating psychiatric brain disease. Its disabling effects result in stigma, unemployment, poverty, loneliness, homelessness, victimization, incarceration, malnutrition, infections, social isolation, ostracism, discrimination, suicide, poor health, medical neglect, and early death.
The consequences of schizophrenia are in many ways more malignant than those of cancer, where sympathy, prompt medical care, and preservation of friends and employment are assured. Also, unlike schizophrenia patients, persons with cancer are never hauled to jail, even when a slow-growing brain tumor causes erratic or violent behavior.
The outcome of schizophrenia has not improved much since patients were “freed” from state hospitals before the psychopharmacology era. Despite the availability of antipsychotics that can control delusions and hallucinations, few persons with schizophrenia achieve remission, let alone recovery. Although negative symptoms and cognitive deficits remain untreatable, the quality of life for persons with schizophrenia can be far less miserable if psychotic symptoms are fully remitted. So why do so many patients with schizophrenia suffer continuous or episodic psychosis that renders their lives a living hell and leads them to inexorable clinical, social, and functional deterioration?
Based on 3 decades of clinical and research work with thousands of patients afflicted with schizophrenia, I have observed that most psychiatrists are not exploiting 2 treatment options that could effectively alleviate continuous or relapsing psychosis. Let me elaborate:
- Continuous, unabated psychotic symptoms (delusions, hallucinations, bizarre behavior, and thought derailment) implies either refractory or treatment-resistant schizophrenia. This means that several antipsychotic trials already have failed to suppress psychotic symptoms. Only 1 drug has been proven to help such patients: clozapine. It was FDA-approved for refractory schizophrenia 22 years ago, and many studies—including the Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE) trial—show that it also is superior to other antipsychotics for treatment-resistant schizophrenia. Yet while about 30% to 35% of patients have refractory or treatment-resistant schizophrenia, only one-sixth of that eligible pool receive clozapine. Reasons include the “hassle” of monitoring, the fear of agranulocytosis (which is rare), and concern about serious side effects. As a result, hundreds of thousands of patients suffer needlessly because they have not received clozapine (full response may take 6 to 12 months in some patients, but only 3 months in others).
- For patients whose delusions and hallucinations do respond to nonclozapine antipsychotics, psychotic episodes are most likely caused by nonadherence to oral medications. All psychiatrists are aware that partial or complete nonadherence is ubiquitous in schizophrenia for multiple reasons, including lack of insight about being ill and needing treatment, suspiciousness (positive symptoms) about drugs, amotivation and avolition (negative symptoms), poor memory or planning ability (cognitive deficits), and toxic effects of alcohol and drugs. Therefore, many psychotic relapses occur needlessly because of inevitable nonadherence with oral medications. In my clinical estimate, this occurs in 50% to 60% of patients who had responded well to oral antipsychotics in the past. The tragic consequences of recurrent relapses is not only re-experiencing the anguish of reality-shattering psychotic states, but also progressive brain atrophy resulting from the “neurotoxic” effects of psychosis, and, unfortunately, progressive development of treatment resistance with each relapse.
The most logical solution to avoid psychotic relapses resulting from nonadherence is long-acting injectable (depot) antipsychotics, which have a large body of data (including FDA registration trials) documenting successful relapse prevention. It is incomprehensible why long-acting formulations are not used in at least 30% to 40% of patients instead of the 6% to 8% who currently receive them. They also should be given to all schizophrenia patients incarcerated for violent acts. Furthermore, there is no reason not to use injectable formulations early in the illness course—after 1 or 2 relapses—to halt or prevent the clinical and functional deterioration that occurs with multiple relapses. In my experience, the stabilization and full remission that occur after 18 to 24 months of continuous adherence with a long-acting injectable antipsychotic can be remarkable and gratifying to patients, families, and psychiatrists.
In summary, intensified research is needed to discover the next generation of pharmacotherapeutic agents that can treat all symptom domains of schizophrenia (positive, negative, cognitive, mood, and substance use). In the meantime, a wider use of those 2 available interventions—clozapine in refractory and treatment-resistant patients and long-acting injectable antipsychotics in nonadherent patients—can make a palpable impact on improving clinical and functional outcomes of patients suffering with schizophrenia. These 2 interventions might make a significant dent in the atrociously high rate of incarceration because of psychosis-induced illegal behaviors. The financial savings in hospitalization and forensic costs can be substantial. But most important, preventing psychosis and incarceration would mean a measurably better life for patients.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/02/two-vastly-underutilized-interventions.html#comments
Many psychiatrists would agree that schizophrenia is the most devastating psychiatric brain disease. Its disabling effects result in stigma, unemployment, poverty, loneliness, homelessness, victimization, incarceration, malnutrition, infections, social isolation, ostracism, discrimination, suicide, poor health, medical neglect, and early death.
The consequences of schizophrenia are in many ways more malignant than those of cancer, where sympathy, prompt medical care, and preservation of friends and employment are assured. Also, unlike schizophrenia patients, persons with cancer are never hauled to jail, even when a slow-growing brain tumor causes erratic or violent behavior.
The outcome of schizophrenia has not improved much since patients were “freed” from state hospitals before the psychopharmacology era. Despite the availability of antipsychotics that can control delusions and hallucinations, few persons with schizophrenia achieve remission, let alone recovery. Although negative symptoms and cognitive deficits remain untreatable, the quality of life for persons with schizophrenia can be far less miserable if psychotic symptoms are fully remitted. So why do so many patients with schizophrenia suffer continuous or episodic psychosis that renders their lives a living hell and leads them to inexorable clinical, social, and functional deterioration?
Based on 3 decades of clinical and research work with thousands of patients afflicted with schizophrenia, I have observed that most psychiatrists are not exploiting 2 treatment options that could effectively alleviate continuous or relapsing psychosis. Let me elaborate:
- Continuous, unabated psychotic symptoms (delusions, hallucinations, bizarre behavior, and thought derailment) implies either refractory or treatment-resistant schizophrenia. This means that several antipsychotic trials already have failed to suppress psychotic symptoms. Only 1 drug has been proven to help such patients: clozapine. It was FDA-approved for refractory schizophrenia 22 years ago, and many studies—including the Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE) trial—show that it also is superior to other antipsychotics for treatment-resistant schizophrenia. Yet while about 30% to 35% of patients have refractory or treatment-resistant schizophrenia, only one-sixth of that eligible pool receive clozapine. Reasons include the “hassle” of monitoring, the fear of agranulocytosis (which is rare), and concern about serious side effects. As a result, hundreds of thousands of patients suffer needlessly because they have not received clozapine (full response may take 6 to 12 months in some patients, but only 3 months in others).
- For patients whose delusions and hallucinations do respond to nonclozapine antipsychotics, psychotic episodes are most likely caused by nonadherence to oral medications. All psychiatrists are aware that partial or complete nonadherence is ubiquitous in schizophrenia for multiple reasons, including lack of insight about being ill and needing treatment, suspiciousness (positive symptoms) about drugs, amotivation and avolition (negative symptoms), poor memory or planning ability (cognitive deficits), and toxic effects of alcohol and drugs. Therefore, many psychotic relapses occur needlessly because of inevitable nonadherence with oral medications. In my clinical estimate, this occurs in 50% to 60% of patients who had responded well to oral antipsychotics in the past. The tragic consequences of recurrent relapses is not only re-experiencing the anguish of reality-shattering psychotic states, but also progressive brain atrophy resulting from the “neurotoxic” effects of psychosis, and, unfortunately, progressive development of treatment resistance with each relapse.
The most logical solution to avoid psychotic relapses resulting from nonadherence is long-acting injectable (depot) antipsychotics, which have a large body of data (including FDA registration trials) documenting successful relapse prevention. It is incomprehensible why long-acting formulations are not used in at least 30% to 40% of patients instead of the 6% to 8% who currently receive them. They also should be given to all schizophrenia patients incarcerated for violent acts. Furthermore, there is no reason not to use injectable formulations early in the illness course—after 1 or 2 relapses—to halt or prevent the clinical and functional deterioration that occurs with multiple relapses. In my experience, the stabilization and full remission that occur after 18 to 24 months of continuous adherence with a long-acting injectable antipsychotic can be remarkable and gratifying to patients, families, and psychiatrists.
In summary, intensified research is needed to discover the next generation of pharmacotherapeutic agents that can treat all symptom domains of schizophrenia (positive, negative, cognitive, mood, and substance use). In the meantime, a wider use of those 2 available interventions—clozapine in refractory and treatment-resistant patients and long-acting injectable antipsychotics in nonadherent patients—can make a palpable impact on improving clinical and functional outcomes of patients suffering with schizophrenia. These 2 interventions might make a significant dent in the atrociously high rate of incarceration because of psychosis-induced illegal behaviors. The financial savings in hospitalization and forensic costs can be substantial. But most important, preventing psychosis and incarceration would mean a measurably better life for patients.
Not all mood swings are bipolar disorder
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
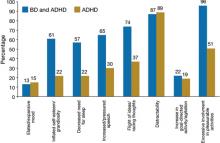
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
M, age 13, is referred by her pediatrician with the chief complaint of “severe mood swings, rule out bipolar disorder (BD).” In the past she was treated for attention-deficit/hyperactivity disorder (ADHD) with stimulants with mixed results. M’s parents are concerned about her “flipping out” whenever she is asked to do something she does not want to do. Her mother has a history of depression and anxiety; her father had a “drinking problem.” There is no history of BD in her first- or second-degree relatives. Are M’s rapid mood swings a sign of BD or another disorder?
The differential diagnosis of “mood swings” is important because they are a common presenting symptom of many children and adolescents with mood and behavioral disorders. Mood swings often occur in children and adolescents with ADHD, oppositional defiant disorder (ODD), developmental disorders, depressive disorders, BD, anxiety disorders, and conduct disorders. Mood swings are analogous to a fever in pediatrics—they indicate something potentially is wrong with the patient, but are not diagnostic as an isolated symptom.
Mood swings in children are common, nonspecific symptoms that more often are a sign of anxiety or behavioral disorders than BD. This article discusses the differential diagnosis of mood swings in children and adolescents and how to best screen and diagnose these patients.
What are ‘mood swings’?
Mood swings is a popular term that is nonspecific and not part of DSM-IV-TR diagnostic criteria for BD. The complaint of “mood swings” may reflect severe mood lability of pediatric patients with BD. This mood lability is best described by the Kiddie-Mania Rating Scale (K-MRS) developed by Axelson and colleagues as “rapid mood variation with several mood states within a brief period of time which appears internally driven without regard to the circumstance.”1 On K-MRS mood lability items, children with mania typically score:
- Moderate—many mood changes throughout the day, can vary from elevated mood to anger to sadness within a few hours; changes in mood are clearly out of proportion to circumstances and cause impairment in functioning
- Severe—rapid mood swings nearly all of the time, with mood intensity greatly out of proportion to circumstances
- Extreme—constant, explosive variability in mood, several mood changes occurring within minutes, difficult to identify a particular mood, changes in mood radically out of proportion to circumstances.
Patients with BD typically exhibit what is best described as a “mood cycle”—a pronounced shift in mood and energy from 1 extreme to another.2 An example of this would be a child who wakes up with extreme silliness, high energy, and intrusive behavior that persists for several hours and then later in the day becomes sad, depressed, and suicidal with no precipitant for either mood cycle. BD patients also will exhibit other symptoms of mania during these mood cycling periods.
Rapid cycling is a DSM-IV course specifier that indicates ≥4 mood episodes per year in patients with BD with a typical course of mania or hypomania followed by depression, or vice versa.3 The episodes must be demarcated by full or partial remission that lasts ≥2 months or by a switch to a mood state of opposite polarity. In the past, children with frequent mood swings were described incorrectly as “rapid cycling,” but this term has been dropped because it engenders confusion between adult and pediatric BD phenomenology.2
A more precise method of describing mood symptoms in a child or adolescent is to use the FIND criteria, which include:4
- Frequency of symptoms per week
- Intensity of mood symptoms
- Number of mood cycles per day
- Duration of symptoms per day.
Visit this article at CurrentPsychiatry.com to view a table that outlines what to look for when using the FIND criteria to evaluate common pediatric psychiatric disorders that include mood swings. Table 1
describes clinical characteristics and tools and resources used to differentiate these and other disorders.4
Table 1
Clinical characteristics of psychiatric disorders that often feature mood swings
| Disorder | Clinical description | Useful tools/resources |
|---|---|---|
| ADHD | Chronic symptoms of hyperactivity, distractibility, impulsivity, poor attentional skills, disorganization | Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L) |
| ODD | Chronic symptoms of oppositionality, negativity; short, frequent mood swings in response to being asked to do something they do not want to do | CPRS-R:L |
| Anxiety disorders | Excessive ‘worry,’ difficulty with transitions, increased mood swings during stressful periods, psychosomatic symptoms | Self-Report for Childhood Anxiety Related Disorders |
| ARND | History of exposure to alcohol in-utero; mild dysmorphia, attentional, mood, and executive functioning problems | National Organization on Fetal Alcohol Syndrome |
| Bipolar disorder | In children: clustering together of episodes or ‘mini-episodes’ (several days) of increased energy, decreased need for sleep, increased mood cycling, pressured speech, etc. In adolescents: depressive episodes with episodes of hypomania or mania | Mood Disorders Questionnaire Kiddie Schedule for Affective Disorders and Schizophrenia Mania Rating Scale |
| ADHD: attention-deficit/hyperactivity disorder; ARND: alcohol-related neurodevelopmental disorder; ODD: oppositional defiant disorder | ||
| Source: Reference 4 | ||
Mood swings: A chart review
We recently completed a retrospective chart review of 100 patients consecutively referred to our pediatric mood disorders clinic for evaluation of “mood swings, rule out BD.” These patients were self-referred, referred by a psychiatrist for a second opinion, or referred by their primary care physician. The mean age of these patients was 8±2.8 years and 68% were male.
Two experienced clinicians (RAK and EM) interviewed each patient and their caregivers and reviewed results of the Conners’ Parent Rating Scale-Revised: Long Form (CPRS-R:L)5 and other outside information.
Figure 1 illustrates these patients’ diagnoses. Diagnoses for each of these disorders were made using DSM-IV-TR criteria.3
The most common diagnoses among patients with the chief complaint of mood swings were ADHD (39%); ODD with ADHD (15%); an anxiety disorder, usually generalized anxiety disorder (GAD) (15%); BD (12%); and a secondary mood disorder, usually fetal alcohol spectrum disorder (10%). We were surprised at how often ADHD, ODD, and anxiety disorders were found to be responsible for these patients’ mood swings and how frequently the referring clinician did not recognize these disorders. In the following sections, we discuss each of these disorders and how they differ from BD.
Figure 1 Underlying diagnoses of 100 children/adolescents referred for ‘mood swings’
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder; MDD: major depressive disorder; ODD: oppositional defiant disorder; PDD: pervasive developmental disorder
ADHD and ODD
In our sample, patients with undiagnosed ADHD made up the largest group of those with frequent mood swings. ADHD inattentive type was missed frequently in adolescent girls who still had behavioral aspects of ADHD, including impulsivity and aggression.6
The CPRS-R:L is useful for screening and diagnosing children and adolescents with ADHD and ODD. It contains 80 items, can be used in males and females and patients age 3 to 17, and has validated norms by age and sex.5 It takes parents approximately 10 minutes to fill out this questionnaire and the results can be scored by hand. The CPRS-R:L includes the following scales: oppositional; cognitive problems/inattention; hyperactivity; anxious-shy; perfectionism; social problems; psychosomatic; Connors’ global index; DSM-IV symptom subscales; and an ADHD index. Patients with mood swings and ADHD combined typically score >2 standard deviations above their age/sex mean on the CPRS-R:L hyperactivity scale, Connors’ Global Index, and ADHD index.5
A common childhood disorder, ODD has multiple etiologies.7 The first DSM-IV criteria for ODD is “often loses temper”3—essentially mood swings that often are expressed behaviorally as anger and at times as aggressive outbursts.
Dodge and Cole8 categorized aggression as reactive (impulsivity with a high affective valence) or proactive (characterized by low arousal and premeditation, ie, predatory conduct disorder). Reactive aggression typically is an angry defensive response to frustration, threat, or provocation, whereas proactive aggression is deliberate, coercive behavior often used to obtain a goal.9 Reactive aggression is common among children with ADHD and ODD and typically begins as a mood swing that escalates into reactive aggressive behavior. In a study of 268 consecutively referred children and adolescents with ADHD and 100 community controls, Connor et al10 found significantly more reactive than proactive forms of aggression in ADHD patients.
It can be difficult to differentiate the moods swings and symptoms of ODD from those of pediatric BD. Mick et al11 found that severe irritability may be a diagnostic indicator of BD in children with ADHD. Using the Kiddie Schedule for Affective Disorders and Schizophrenia (epidemiologic version) structured diagnostic interview,12 they evaluated 274 children (mean age 10.8±3.2) with ADHD; 37% had no comorbid mood disorder, 36% had ADHD with depression, and 11% had ADHD with BD. Researchers characterized 3 types of irritability in these patients:
- ODD-type irritability characterized by a low frustration tolerance that is seen in ODD
- Mad/cranky irritability found in depressive disorders
- Super-angry/grouchy/cranky irritability with frequent, prolonged, and largely unprovoked anger episodes and characteristics of mania.
ODD-type irritability was common among all ADHD patients, was the least impairing type of irritability, and did not increase the risk of a mood disorder. Mad/cranky irritability was common only in children with ADHD and a mood disorder (depression or BD), was more impairing than ODD-type irritability, and was most predictive of unipolar depression. Super-angry/grouchy/cranky irritability was common only among children with ADHD and BD (77%), was the most impairing, and was predictive of both unipolar depression and BD. The type of irritability and clustering of DSM-IV manic symptoms best differentiated ADHD subjects from those with ADHD and BD. Figure 2 illustrates symptoms that differentiated patients with ADHD from those with ADHD and comorbid BD.11
A review of pharmacotherapy for aggression in children found the largest effects for methylphenidate for aggression in ADHD (mean effect size=0.9, combined N=844).13 Our clinical experience has been that pediatric patients with ADHD or ODD with ADHD often have high levels of reactive aggression that presents as mood swings, and aggressively treating ADHD often results in improved mood and other ADHD symptoms.
Figure 2 Symptoms that differentiate BD from BD with comorbid ADHD
ADHD: attention-deficit/hyperactivity disorder; BD: bipolar disorder
Source: Reference 11
Anxiety disorders
The estimated prevalence of child and adolescent anxiety disorders is 10% to 20%14; in our sample the prevalence was 15%. These disorders include GAD, separation anxiety disorder, social phobias, posttraumatic stress disorder (PTSD), and obsessive-compulsive disorder. Often, children with GAD worry excessively and become upset during transitions when things don’t proceed as they expect, with resultant angry outbursts and mood swings. Mood swings and difficulty sleeping are common in children with anxiety disorders or BD. Anxiety disorders often will be missed unless specific triggers of the mood swings or angry outbursts—as well as differentiating symptoms such as excessive fear, worry, and psychosomatic symptoms—are assessed.
In our clinical experience, simply asking a child if he or she is anxious is not sufficient to uncover an anxiety disorder. Although the CPRS-L:R will screen for anxiety disorders, we have found that the Self-Report for Childhood Anxiety Related Disorders (SCARED) developed by Birmaher et al15 is more specific. This tool can be used in patients age ≥8. The parent and child versions of the SCARED contain 41 items that measure 5 factors:
- general anxiety
- separation anxiety
- social phobia
- school phobia
- physical symptoms of anxiety.
The SCARED takes 5 minutes to fill out and is available in parent and child versions.
Secondary mood disorders
Many patients in our sample had a mood disorder secondary to the neurologic effects of alcohol on the developing brain. For more about maternal alcohol use, fetal alcohol spectrum disorders, and mood swings, visit this article at CurrentPsychiatry.com.
What BD looks like in children
In our sample, 12% of patients referred for mood swings were diagnosed with bipolar I disorder (BDI), bipolar II disorder (BDII), or bipolar disorder, not otherwise specified (BD-NOS). In the United States, lifetime prevalence of BDI and BDII in adolescents age 13 to 17 is 2.9%.16 No large epidemiologic studies have looked at the lifetime prevalence of BD in children age <13.
How often a clinician sees BD in children and adolescents largely depends on the type of setting in which he or she practices. Although in the general population BD is relatively rare compared with other childhood psychiatric disorders, on child/adolescent inpatient units it is common to find that 30% to 40% of patients have BD.17
The best longitudinal study to date of the phenomenology, comorbidity, and outcome of BD in children and adolescents is the National Institute of Mental Health-funded Course and Outcome of Bipolar Youth study (COBY).18 In this ongoing, longitudinal study, 413 youths (age 7 to 17) with BDI (N=244), BDII (N=28), or BD-NOS (N=141) were rigorously diagnosed using state-of-the-art measures, including the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present version19 and re-evaluated every 9.4 months for 4 years. When organizing this study, investigators found that DSM-IV criteria for BD-NOS were too vague to be useful and developed their own criteria (Table 2).18
For BDI patients in the COBY study, the mean age of onset for bipolar symptoms was 9.0±4.1 years and the mean duration of illness was 4.4±3.1 years. Researchers reported that at the 4-year assessment approximately 70% of patients with BD recovered from their index episode, and 50% had at least 1 syndromal recurrence, particularly depressive episodes.20 Analyses of these patients’ weekly mood symptoms showed that they had syndromal or subsyndromal symptoms with numerous changes in symptoms and shifts of mood polarity 60% of the time, and psychosis 3% of the time. During this study, 20% of BDII patients progressed to BDI, and 25% of BD-NOS patients converted to BDI or BDII.
Further analysis of the COBY data revealed that onset of mood symptoms preceded onset of clear bipolar episodes by an average of 1.0±1.7 years. Depression was the most common initial and most frequent episode for adolescents; mood lability was seen more often in childhood-onset and adolescents with early-onset BD. Depressed children had more severe irritability than depressed adolescents, and older age was associated with more severe and typical mood symptomatology.21
The clinical picture of a child with BD that emerges from the COBY study is:
- a fairly young child with the onset of mood symptoms between age 5 to 12
- subsyndromal and less frequently clear syndromal episodes
- primarily mixed and depressed symptoms with rapid mood cycles during these episodes.22
It is clear that there is a spectrum of bipolar disorders in children and adolescents with varying degrees of symptom expression and children differ from adolescents and adults in their initial presentation of BD.
Table 2
COBY criteria for bipolar disorder, not otherwise specified
| Presence of clinically relevant bipolar symptoms that do not fulfill DSM-IV criteria for BDI or BDII |
| In addition, patients are required to have elevated mood plus 2 associated DSM-IV symptoms or irritable mood plus 3 DSM-IV associated symptoms, along with a change in level of functioning |
| Duration of a minimum of 4 hours within a 24-hour period |
| At least 4 cumulative lifetime days meeting the criteria |
| BDI: bipolar I disorder; BDII: bipolar II disorder; COBY: Course and Outcome of Bipolar Youth study |
| Source: Reference 18 |
Related Resources
- Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Goodwin FK, Jamison KR. Manic-depressive illness. 2nd ed. Oxford, United Kingdom: Oxford University Press; 2007.
- Miklowitz DJ, Cicchetti D, eds. Understanding bipolar disorder: a developmental perspective. New York, NY: Guilford Press; 2010.
Drug Brand Name
- Methylphenidate • Ritalin, Concerta, others
Disclosures
Dr. Kowatch receives grant/research support from the National Institute of Child Health and Human Development and the National Institute of Mental Health and is a consultant to AstraZeneca, Forest Pharmaceuticals, Merck, and the REACH Foundation.
Dr. Delgado and Ms. Monroe report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Table
FIND criteria of disorders found to cause mood swings
| Criteria | BDI | BP-NOS | ODD | GAD | ARND |
|---|---|---|---|---|---|
| Frequency of symptoms/week | 7 days (more days than not in an average week) | 2 to 3 days/week | Daily (chronic) irritability and mood swings precipitated by ‘not getting their way’ | Greatest during times of change/stress | Daily |
| Intensity of symptoms | Severe—parents often are afraid to take the child out in public because of mood symptoms | Moderate | Mild/moderate | Mild/moderate when stressed | Mild/moderate |
| Number of mood cycles/day | Daily cycles of euphoria and depression | 3 to 4 | 5 to 10 | 2 to 3 | 8 to 10 |
| Duration of symptoms/day | Euphoria: 30 to 60 minutes Depression: 30 minutes to 6 hours | 4 hours total/day of mood symptoms | Short; 5 to 10 minutes | Short; 5 to 10 minutes | Short; 5 to 10 minutes |
| ARND: alcohol-related neurodevelopmental disorder; BDI: bipolar I disorder; BD-NOS: bipolar disorder, not otherwise specified; GAD: generalized anxiety disorder; ODD: oppositional defiant disorder | |||||
| Source: Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008 | |||||
Even small amounts of alcohol use by a pregnant woman can impact her child’s development. In a controlled study examining drinking behavior of 12,678 pregnant women and the effect this had on their children, Sayel et ala found that <1 drink per week during the first trimester was clinically significant for mental health problems in girls, measured at age 4 and 8, when using parent or teacher report.
Fetal alcohol spectrum disorder describes the range of effects that can occur in an individual whose mother drank alcohol during pregnancy. These disorders include fetal alcohol syndrome (FAS), alcohol-related neurodevelopmental disorder (ARND), and alcohol-related birth defects (ARBD).
FAS. Individuals with FAS have a distinct pattern of facial abnormalities, growth deficiency, and evidence of CNS dysfunction. Characteristic facial abnormalities may include a smooth philtrum, thin upper lip, upturned nose, flat nasal bridge and midface, epicanthal folds, small palpebral fissures, and small head circumference. Growth deficiency begins in-utero and continues throughout childhood and into adulthood. CNS abnormalities can include impaired brain growth or abnormal structure, manifested differently depending on age.
ARND. Many individuals affected by alcohol exposure before birth do not have the characteristic facial abnormalities and growth retardation identified with full FAS, yet have significant brain and behavioral impairments. Individuals with ARND have either the facial anomalies, growth retardation, and other physical abnormalities, or a complex pattern of behavioral or cognitive abnormalities inconsistent with developmental level and unexplained by genetic background or environmental conditions (ie, poor impulse control, language deficits, problems with abstraction, mathematical and social perception deficits, learning problems, and impairment in attention, memory, or judgment).b
ARBD. Persons with ARBD have malformations of the skeletal and major organ systems, such as cardiac or renal abnormalities.
Comorbid psychiatric conditions in children with prenatal alcohol exposure are 5 to 16 times more prevalent than in the general population; these children are 38% more likely to have an anger disorder.c O’Connor and Paleyd found that “…mood disorder symptoms were significantly higher for children with parental alcohol exposure compared to children without exposure.” Children with ARND are treated symptomatically depending upon which deficits and behaviors they exhibit.e
References
a. Sayal K, Heron J, Golding J, et al. Binge pattern of alcohol consumption during pregnancy and childhood mental health outcomes: longitudinal population-based study. Pediatrics. 2009;123(2):e289-296.
b. Warren KR, Foudin LL. Alcohol-related birth defects—the past, present, and future. Alcohol Res Health. 2001;25(3):153-158.
c. Burd L, Klug MG, Martsolf JT, et al. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25(6):697-705.
d. O’Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Dev Disabil Res Rev. 2009;15(3):225-234.
e. Paley B, O’Connor MJ. Intervention for individuals with fetal alcohol spectrum disorders: treatment approaches and case management. Dev Disabil Res Rev. 2009;15(3):258-267.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
1. Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470.
2. Youngstrom EA, Birmaher B, Findling RL. Pediatric bipolar disorder: validity, phenomenology, and recommendations for diagnosis. Bipolar Disord. 2008;10(1 Pt 2):194-214.
3. Diagnostic and statistical manual of mental disorders, 4th ed, text rev. Washington, DC: American Psychiatric Association; 2000.
4. Kowatch RA, Fristad MA, Findling RL, et al. Clinical manual for the management of bipolar disorder in children and adolescents. Arlington, VA: American Psychiatric Publishing, Inc.; 2008.
5. Conners CK. Conners’ Parent Rating Scale Long Form (CPRS-R:L) North Tonawanda, NY: Multi-Health Systems, Inc.; 1997.
6. Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50(9):1042-1051.
7. Steiner H, Remsing L. and the Work Group on Quality Issues. Practice parameter for the assessment and treatment of children and adolescents with oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2007;46(1):126-141.
8. Dodge KA, Cole JD. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J Pers Soc Psychol. 1987;53(6):1146-1158.
9. Connor DF, Steingard RJ, Cunningham JA, et al. Proactive and reactive aggression in referred children and adolescents. Am J Orthopsychiatry. 2004;74(2):129-136.
10. Connor DF, Chartier KG, Preen EC, et al. Impulsive aggression in attention-deficit/hyperactivity disorder: symptom severity, co-morbidity, and attention-deficit/hyperactivity disorder subtype. J Child Adolesc Psychopharmacol. 2010;20(2):119-126.
11. Mick E, Spencer T, Wozniak J, et al. Heterogeneity of irritability in attention-deficit/hyperactivity disorder subjects with and without mood disorders. Biol Psychiatry. 2005;58(7):576-582.
12. Orvaschel H. Schizophrenia and Affective Disorders Schedule for children—Epidemiological Version (KSADS-E). Fort Lauderdale, FL: Nova Southeastern University; 1995.
13. Pappadopulos E, Woolston S, Chait A, et al. Pharmacotherapy of aggression in children and adolescents: efficacy and effect size. J Can Acad Child Adolesc Psychiatry. 2006;15(1):27-39.
14. Achenbach TM, Howell CT, McConaughy SH, et al. Six-year predictors of problems in a national sample: IV. Young adult signs of disturbance. J Am Acad Child Adolesc Psychiatry. 1998;37(7):718-727.
15. Birmaher B, Khetarpal S, Brent D, et al. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry. 1997;36:545-553.
16. Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication—Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980-989.
17. Youngstrom EA, Duax J. Evidence-based assessment of pediatric bipolar disorder, part I: base rate and family history. J Am Acad Child Adolesc Psychiatry. 2005;44(7):712-717.
18. Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183.
19. Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988.
20. Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: a review of the existing literature. Dev Psychopathol. 2006;18(4):1023-1035.
21. Birmaher B, Axelson D, Strober M, et al. Comparison of manic and depressive symptoms between children and adolescents with bipolar spectrum disorders. Bipolar Disord. 2009;11(1):52-62.
22. Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804.
Psychiatry behind bars: Practicing in jails and prisons
Discuss this article at http://currentpsychiatry.blogspot.com/2011/02/psychiatry-behind-bars-practicing-in.html#comments
Over the last 2 decades mandatory prison sentences, longer prison terms, and more restrictive release policies have lead to a dramatic increase in the number of persons in jails and prisons. Currently, more than 2 million individuals are incarcerated in the United States.1 Psychiatric illness is over-represented in correctional populations compared with the general population—more than half of all inmates have a mental health diagnosis.2 Correctional facilities are legally obligated to address the medical and mental health needs of the persons committed to them. As a result, more psychiatrists are practicing in jails and prisons.
This article explains correctional facilities’ obligation to provide for inmates’ mental health needs and describes correctional mental health processes and how psychiatrists can play a role in screening, evaluation, and suicide prevention.
Lack of training
Despite the increasing number of psychiatrists working in correctional institutions, most have had little or no training, education, or even orientation to these settings. Forensic psychiatry fellowship requirements include experience in treating acutely and chronically ill patients in correctional systems.3 Although general psychiatric training doesn’t preclude correctional experience, it is not required. The forensic component of general psychiatric residency is limited to evaluation of forensic issues, report writing, and testimony.
Professional organizations—including the American Psychiatric Association,4 the American Public Health Association,5 the National Commission on Correctional Health Care,6 and the American Correctional Health Services Association7—have developed standards and position statements on providing medical and mental health care in correctional facilities. Although psychiatrists’ work in correctional settings generally has been reserved for consultation and medication management, it is important for these clinicians to understand and appreciate the wider landscape and environment in which they practice. Psychiatrists can help develop and implement mental health processes that lead to better services and improved clinical outcomes.
Right to treatment
Convicted persons have a constitutional right to medical and mental health treatment under extension of the Eighth Amendment of the U.S. Constitution, which prohibits cruel and unusual punishment.8 In 1976, the U.S. Supreme Court concluded that “deliberate indifference to serious medical needs of prisoners constitutes the ‘unnecessary and wanton infliction of pain’… proscribed by the Eighth Amendment.”9 This coverage was expanded to mental health needs when the court found “…no underlying distinction between the right to medical care for physical ills and its psychological or psychiatric counterpart.”10 Correctional facilities also are obligated to provide medical and mental health treatment for persons in custody who are not yet convicted of an offense.8 In subsequent litigation, the court formulated 6 components of a minimally adequate correctional mental health treatment program; these are described in Table 1.11
Table 1
Components of minimally adequate mental health system in correctional facilities
| A systematic screening and evaluation program to identify inmates requiring mental health treatment |
| Treatment that encompasses more than simply segregating the mentally ill inmate and increasing correctional supervision |
| Treatment by trained mental health professionals in sufficient numbers to identify and treat inmates suffering from serious mental disorders |
| Maintenance of accurate, complete, and confidential records of the mental health treatment process |
| A suicide prevention program |
| Appropriate use of psychotropic medication (prescription and monitoring by appropriately trained and licensed staff to treat bona fide mental disorders rather than solely as a means of behavioral management) |
| Source: Reference 11 |
Jails vs prisons
The type of psychiatric treatment provided differs based on whether the facility is a jail or a prison, how long inmates are confined, and whether the facility serves a special mission or population, such as serving as a reception center for a prison system or housing only juveniles. Jails generally house inmates for short periods—often <1 year—experience rapid population turnover, and receive admissions day and night. Jails vary in size from a few holding cells to several thousand beds. These factors have implications for screening and evaluation processes, suicide prevention, and coordination of care with community treatment providers. In jails, clinicians’ work focuses on rapid identification of psychiatric illness, assessment, stabilization, and re-linkage to treatment providers in the community. Access to inpatient and ongoing psychiatric care also should be available.
In contrast, prisons house people who have been convicted and sentenced to serve time, generally for >1 year. Turnover is less rapid, admissions and discharges are more predictable, and there is greater opportunity and obligation to develop a continuum of mental health care. Prison systems generally provide or make arrangements for crisis intervention, residential treatment services, and inpatient and outpatient psychiatric care. These services may be provided on the prison grounds, or the inmate may be transferred to another prison within the system that offers specialized treatment or to a community hospital, where the inmate is under the constant supervision of corrections officers. Residential treatment includes intensive, coordinated, and structured mental health services and consists of group and individual therapies, psychoeducation, and therapeutic activities; these services are analogous to intensive day treatment or partial hospitalization programs in the community. In prisons mental health care emphasizes ongoing treatment. As in the community, treatment teams in correctional settings often include mental health professionals such as psychiatric nurses, psychotherapists, and psychology staff in addition to psychiatrists.
Screening and evaluation
Correctional facilities need a systematic screening process that is conducted on all inmates. This preliminary entry or “receiving screening” is intended to identify urgent medical and mental health concerns and persons in need of immediate treatment. A nurse or corrections staff officer who has been trained by medical staff could conduct this screening. Screening consists of observing the inmate’s current condition and conducting a structured inquiry into medical and psychiatric symptoms, psychotropic medications, drug and alcohol use history, and suicide risk. A positive screen leads to immediate action such as instituting drug or alcohol detoxification or initiating suicide precautions or an emergency medical referral and assessment.4,6
Within a few days of an inmate’s arrival, a mental health professional should conduct a more detailed mental health screening to identify non-emergent psychiatric needs. The mental health screening includes:
- a review of accompanying mental health information received from the county jail or arresting/transporting officer
- a self-reported history of psychiatric treatment, such as hospitalization, pharmacotherapy, or outpatient counseling
- current or prior suicidal thoughts or attempts
- intellectual functioning
- history of violence and/or victimization
- a brief mental status examination.4
Records from previous incarcerations should be reviewed. Also, if relevant, obtain the inmate’s consent to collect outside treatment records and/or speak with family or significant others. The results of this brief mental health assessment could prompt a referral for further evaluation and determine the need for psychotropic therapy.
Although usually not directly involved in this systematic screening and evaluation, psychiatrists should be familiar with how and why referrals are made to be sure that they are appropriate and to reduce unnecessary evaluations, leaving more time for medication follow-up, treatment planning, and suicide risk assessment. An efficient and effective mental health screening and assessment process helps ensure that limited psychiatric resources are used to maximal benefit.
Suicide prevention
Suicide prevention programs often include teaching corrections staff to identify suicide risk factors and instructing them to screen at-risk inmates at any time during incarceration. These programs should implement steps to keep inmates safe, such as increasing intensity and frequency of monitoring by corrections staff, removing or limiting access to items that could be used to harm oneself, and moving inmates to a housing area where the means and opportunity for self harm are reduced.4,6 Suicide prevention programs also should include delivery of appropriate mental health interventions to improve the inmate’s clinical condition, resolve the crisis, or otherwise lower suicide risk. These interventions include:
- increased frequency of interaction with mental health staff (more than a brief daily interaction conducted at the cell front)
- treatment of drug and/or alcohol withdrawal
- referral for evaluation and assessment of the need for psychotropic medication or dosage adjustment (Table 2).6
A correctional facility’s policy should allow a low threshold for corrections staff to initiate a suicide prevention watch—it is better to err on the side of caution and institute a watch than to expect non-mental health professionals to conduct clinical risk level assessments. Full assessment of an inmate’s clinical condition and the decision to reduce or discontinue the watch should be left to a trained mental health professional. This function may fall within the psychiatrist’s duties and it is important to be aware of the ramifications of watch discontinuation, such as:
- what type of property is returned
- where the inmate will be housed
- how often the inmate will be monitored by custody staff
- when the next mental health follow-up will occur.
Failure to articulate your expectations to staff members can lead to catastrophic consequences if watches are discontinued without an appropriate plan for monitoring and follow-up.
Psychiatrists can help train corrections staff on signs of suicide risk and also should review suicide attempts and/or completed suicides. This often can be a challenge because a psychiatrist’s time at a facility may be limited, but is an important consideration for quality improvement efforts.
Table 2
Components of a correctional suicide prevention program
| Training for staff on verbal and behavior cues indicating suicide risk and appropriate response |
| Identification of potentially suicidal inmates |
| Referral to mental health providers or facilities |
| Evaluation by qualified mental health professional |
| Housing in safe area of the institution |
| Treatment to address the cause of or reasons for suicidal thoughts |
| Monitoring procedures that permit regular, documented supervision |
| Communication procedures between health care and corrections personnel |
| Intervention procedures addressing how to handle a suicide attempt in progress |
| Notification procedures to ensure appropriate correctional authorities, outside authorities, and family are contacted |
| Reporting procedures for documenting attempted or completed suicides |
| Review of suicides and serious attempts by health care and administrative staff |
| Critical incident debriefing offered to affected personnel and inmates in event of completed suicide |
| Source: Reference 6 |
Pharmacotherapy
Traditionally, the primary role of psychiatrists working in correctional facilities has been psychotropic medication management. Understanding the correctional context and procedures permits more informed prescription choices and recommendations for psychotropics to be included in the formulary.
Antipsychotics, antidepressants, and mood stabilizers should be included in a facility’s formulary. Considerations concerning types of psychotropic medication within a formulary depend on the facility’s size and mission, psychiatric illnesses encountered in the population, and lengths of inmates’ stay.12 A mechanism should be in place to prescribe off-formulary and access other types of psychotropic medication on a case-by-case basis to ensure inmates are not denied appropriate treatment. A psychiatrist may have to advocate strongly for these principles.
Most correctional facilities require that staff administer every dose of psychotropic medication directly to the inmate for whom it is prescribed. In some facilities, only nursing personnel can administer medication, while others use trained corrections staff to deliver medication. Psychiatrists who prescribe psychotropics in correctional institutions must be familiar with the facility’s medication administration procedures, which may impact medication choice and form, dosing frequency, timing of laboratory studies, and inmate medication compliance. Prescribers’ capacity to order emergency or “as needed” medications may be limited or nonexistent if nursing staff is unavailable.
Appropriate use of psychotropic medication for treating psychiatric illness is the standard of care, but is only 1 component of an effective treatment plan for inmates with serious mental illness. Others include group and individual therapy, psychoeducation, and therapeutic activities such as recreational therapy, activity therapy, and opportunities for education and work within the correctional system.
Related Resources
- Scott CL, ed. Handbook of correctional mental health. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Thienhaus OJ, Piasecki M, eds. Correctional psychiatry practice guidelines and strategies. Kingston, NJ: Civic Research Institute, Inc.; 2007.
- National Commission on Correctional Healthcare. www.ncchc.org.
- Society of Correctional Physicians. www.CorrDocs.org.
Disclosure
Dr. Burns reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Glaze LE. Correctional populations in the United States, 2009. Washington, DC: Bureau of Justice Statistics. December 2010. NCJ 231681.
2. James DJ, Glaze LE. Mental health problems of prison and jail inmates. Washington, DC: Bureau of Justice Statistics. September 2006. NCJ 213600.
3. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in forensic psychiatry. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/406pr703_u105.pdf. Accessed December 30, 2010.
4. American Psychiatric Association. Psychiatric services in jails and prisons. 2nd ed. Washington, DC: American Psychiatric Association; 2000.
5. Standards for health services in correctional institutions. 3rd ed. Washington, DC: American Public Health Association; 2003.
6. National Commission on Correctional Health Care. Standards for mental health services in correctional facilities. Chicago, IL: National Commission on Correctional Health Care; 2008.
7. American Correctional Health Services Association. Position statement on forced and involuntary psychotropic medication. Available at: http://www.achsa.org/displaycommon.cfm?an=12. Accessed December 30, 2010.
8. Cohen F. Overview of legal issues. In: Cohen F. The mentally disordered inmate and the law. Kingston, NJ: Civic Research Institute, Inc.; 1998:2-1-2-20.
9. Estelle v Gamble. 429 U.S. 97 (1976).
10. Bowring v Godwin. 551 F2d 44, 47 (4th Cir 1977).
11. Ruiz v Estelle. 503 F Supp 1265 (SD Tex 1980).
12. Burns K. Pharmacotherapy in correctional settings. In: Scott CL, ed. Handbook of correctional mental health. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:321-344.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/02/psychiatry-behind-bars-practicing-in.html#comments
Over the last 2 decades mandatory prison sentences, longer prison terms, and more restrictive release policies have lead to a dramatic increase in the number of persons in jails and prisons. Currently, more than 2 million individuals are incarcerated in the United States.1 Psychiatric illness is over-represented in correctional populations compared with the general population—more than half of all inmates have a mental health diagnosis.2 Correctional facilities are legally obligated to address the medical and mental health needs of the persons committed to them. As a result, more psychiatrists are practicing in jails and prisons.
This article explains correctional facilities’ obligation to provide for inmates’ mental health needs and describes correctional mental health processes and how psychiatrists can play a role in screening, evaluation, and suicide prevention.
Lack of training
Despite the increasing number of psychiatrists working in correctional institutions, most have had little or no training, education, or even orientation to these settings. Forensic psychiatry fellowship requirements include experience in treating acutely and chronically ill patients in correctional systems.3 Although general psychiatric training doesn’t preclude correctional experience, it is not required. The forensic component of general psychiatric residency is limited to evaluation of forensic issues, report writing, and testimony.
Professional organizations—including the American Psychiatric Association,4 the American Public Health Association,5 the National Commission on Correctional Health Care,6 and the American Correctional Health Services Association7—have developed standards and position statements on providing medical and mental health care in correctional facilities. Although psychiatrists’ work in correctional settings generally has been reserved for consultation and medication management, it is important for these clinicians to understand and appreciate the wider landscape and environment in which they practice. Psychiatrists can help develop and implement mental health processes that lead to better services and improved clinical outcomes.
Right to treatment
Convicted persons have a constitutional right to medical and mental health treatment under extension of the Eighth Amendment of the U.S. Constitution, which prohibits cruel and unusual punishment.8 In 1976, the U.S. Supreme Court concluded that “deliberate indifference to serious medical needs of prisoners constitutes the ‘unnecessary and wanton infliction of pain’… proscribed by the Eighth Amendment.”9 This coverage was expanded to mental health needs when the court found “…no underlying distinction between the right to medical care for physical ills and its psychological or psychiatric counterpart.”10 Correctional facilities also are obligated to provide medical and mental health treatment for persons in custody who are not yet convicted of an offense.8 In subsequent litigation, the court formulated 6 components of a minimally adequate correctional mental health treatment program; these are described in Table 1.11
Table 1
Components of minimally adequate mental health system in correctional facilities
| A systematic screening and evaluation program to identify inmates requiring mental health treatment |
| Treatment that encompasses more than simply segregating the mentally ill inmate and increasing correctional supervision |
| Treatment by trained mental health professionals in sufficient numbers to identify and treat inmates suffering from serious mental disorders |
| Maintenance of accurate, complete, and confidential records of the mental health treatment process |
| A suicide prevention program |
| Appropriate use of psychotropic medication (prescription and monitoring by appropriately trained and licensed staff to treat bona fide mental disorders rather than solely as a means of behavioral management) |
| Source: Reference 11 |
Jails vs prisons
The type of psychiatric treatment provided differs based on whether the facility is a jail or a prison, how long inmates are confined, and whether the facility serves a special mission or population, such as serving as a reception center for a prison system or housing only juveniles. Jails generally house inmates for short periods—often <1 year—experience rapid population turnover, and receive admissions day and night. Jails vary in size from a few holding cells to several thousand beds. These factors have implications for screening and evaluation processes, suicide prevention, and coordination of care with community treatment providers. In jails, clinicians’ work focuses on rapid identification of psychiatric illness, assessment, stabilization, and re-linkage to treatment providers in the community. Access to inpatient and ongoing psychiatric care also should be available.
In contrast, prisons house people who have been convicted and sentenced to serve time, generally for >1 year. Turnover is less rapid, admissions and discharges are more predictable, and there is greater opportunity and obligation to develop a continuum of mental health care. Prison systems generally provide or make arrangements for crisis intervention, residential treatment services, and inpatient and outpatient psychiatric care. These services may be provided on the prison grounds, or the inmate may be transferred to another prison within the system that offers specialized treatment or to a community hospital, where the inmate is under the constant supervision of corrections officers. Residential treatment includes intensive, coordinated, and structured mental health services and consists of group and individual therapies, psychoeducation, and therapeutic activities; these services are analogous to intensive day treatment or partial hospitalization programs in the community. In prisons mental health care emphasizes ongoing treatment. As in the community, treatment teams in correctional settings often include mental health professionals such as psychiatric nurses, psychotherapists, and psychology staff in addition to psychiatrists.
Screening and evaluation
Correctional facilities need a systematic screening process that is conducted on all inmates. This preliminary entry or “receiving screening” is intended to identify urgent medical and mental health concerns and persons in need of immediate treatment. A nurse or corrections staff officer who has been trained by medical staff could conduct this screening. Screening consists of observing the inmate’s current condition and conducting a structured inquiry into medical and psychiatric symptoms, psychotropic medications, drug and alcohol use history, and suicide risk. A positive screen leads to immediate action such as instituting drug or alcohol detoxification or initiating suicide precautions or an emergency medical referral and assessment.4,6
Within a few days of an inmate’s arrival, a mental health professional should conduct a more detailed mental health screening to identify non-emergent psychiatric needs. The mental health screening includes:
- a review of accompanying mental health information received from the county jail or arresting/transporting officer
- a self-reported history of psychiatric treatment, such as hospitalization, pharmacotherapy, or outpatient counseling
- current or prior suicidal thoughts or attempts
- intellectual functioning
- history of violence and/or victimization
- a brief mental status examination.4
Records from previous incarcerations should be reviewed. Also, if relevant, obtain the inmate’s consent to collect outside treatment records and/or speak with family or significant others. The results of this brief mental health assessment could prompt a referral for further evaluation and determine the need for psychotropic therapy.
Although usually not directly involved in this systematic screening and evaluation, psychiatrists should be familiar with how and why referrals are made to be sure that they are appropriate and to reduce unnecessary evaluations, leaving more time for medication follow-up, treatment planning, and suicide risk assessment. An efficient and effective mental health screening and assessment process helps ensure that limited psychiatric resources are used to maximal benefit.
Suicide prevention
Suicide prevention programs often include teaching corrections staff to identify suicide risk factors and instructing them to screen at-risk inmates at any time during incarceration. These programs should implement steps to keep inmates safe, such as increasing intensity and frequency of monitoring by corrections staff, removing or limiting access to items that could be used to harm oneself, and moving inmates to a housing area where the means and opportunity for self harm are reduced.4,6 Suicide prevention programs also should include delivery of appropriate mental health interventions to improve the inmate’s clinical condition, resolve the crisis, or otherwise lower suicide risk. These interventions include:
- increased frequency of interaction with mental health staff (more than a brief daily interaction conducted at the cell front)
- treatment of drug and/or alcohol withdrawal
- referral for evaluation and assessment of the need for psychotropic medication or dosage adjustment (Table 2).6
A correctional facility’s policy should allow a low threshold for corrections staff to initiate a suicide prevention watch—it is better to err on the side of caution and institute a watch than to expect non-mental health professionals to conduct clinical risk level assessments. Full assessment of an inmate’s clinical condition and the decision to reduce or discontinue the watch should be left to a trained mental health professional. This function may fall within the psychiatrist’s duties and it is important to be aware of the ramifications of watch discontinuation, such as:
- what type of property is returned
- where the inmate will be housed
- how often the inmate will be monitored by custody staff
- when the next mental health follow-up will occur.
Failure to articulate your expectations to staff members can lead to catastrophic consequences if watches are discontinued without an appropriate plan for monitoring and follow-up.
Psychiatrists can help train corrections staff on signs of suicide risk and also should review suicide attempts and/or completed suicides. This often can be a challenge because a psychiatrist’s time at a facility may be limited, but is an important consideration for quality improvement efforts.
Table 2
Components of a correctional suicide prevention program
| Training for staff on verbal and behavior cues indicating suicide risk and appropriate response |
| Identification of potentially suicidal inmates |
| Referral to mental health providers or facilities |
| Evaluation by qualified mental health professional |
| Housing in safe area of the institution |
| Treatment to address the cause of or reasons for suicidal thoughts |
| Monitoring procedures that permit regular, documented supervision |
| Communication procedures between health care and corrections personnel |
| Intervention procedures addressing how to handle a suicide attempt in progress |
| Notification procedures to ensure appropriate correctional authorities, outside authorities, and family are contacted |
| Reporting procedures for documenting attempted or completed suicides |
| Review of suicides and serious attempts by health care and administrative staff |
| Critical incident debriefing offered to affected personnel and inmates in event of completed suicide |
| Source: Reference 6 |
Pharmacotherapy
Traditionally, the primary role of psychiatrists working in correctional facilities has been psychotropic medication management. Understanding the correctional context and procedures permits more informed prescription choices and recommendations for psychotropics to be included in the formulary.
Antipsychotics, antidepressants, and mood stabilizers should be included in a facility’s formulary. Considerations concerning types of psychotropic medication within a formulary depend on the facility’s size and mission, psychiatric illnesses encountered in the population, and lengths of inmates’ stay.12 A mechanism should be in place to prescribe off-formulary and access other types of psychotropic medication on a case-by-case basis to ensure inmates are not denied appropriate treatment. A psychiatrist may have to advocate strongly for these principles.
Most correctional facilities require that staff administer every dose of psychotropic medication directly to the inmate for whom it is prescribed. In some facilities, only nursing personnel can administer medication, while others use trained corrections staff to deliver medication. Psychiatrists who prescribe psychotropics in correctional institutions must be familiar with the facility’s medication administration procedures, which may impact medication choice and form, dosing frequency, timing of laboratory studies, and inmate medication compliance. Prescribers’ capacity to order emergency or “as needed” medications may be limited or nonexistent if nursing staff is unavailable.
Appropriate use of psychotropic medication for treating psychiatric illness is the standard of care, but is only 1 component of an effective treatment plan for inmates with serious mental illness. Others include group and individual therapy, psychoeducation, and therapeutic activities such as recreational therapy, activity therapy, and opportunities for education and work within the correctional system.
Related Resources
- Scott CL, ed. Handbook of correctional mental health. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Thienhaus OJ, Piasecki M, eds. Correctional psychiatry practice guidelines and strategies. Kingston, NJ: Civic Research Institute, Inc.; 2007.
- National Commission on Correctional Healthcare. www.ncchc.org.
- Society of Correctional Physicians. www.CorrDocs.org.
Disclosure
Dr. Burns reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Discuss this article at http://currentpsychiatry.blogspot.com/2011/02/psychiatry-behind-bars-practicing-in.html#comments
Over the last 2 decades mandatory prison sentences, longer prison terms, and more restrictive release policies have lead to a dramatic increase in the number of persons in jails and prisons. Currently, more than 2 million individuals are incarcerated in the United States.1 Psychiatric illness is over-represented in correctional populations compared with the general population—more than half of all inmates have a mental health diagnosis.2 Correctional facilities are legally obligated to address the medical and mental health needs of the persons committed to them. As a result, more psychiatrists are practicing in jails and prisons.
This article explains correctional facilities’ obligation to provide for inmates’ mental health needs and describes correctional mental health processes and how psychiatrists can play a role in screening, evaluation, and suicide prevention.
Lack of training
Despite the increasing number of psychiatrists working in correctional institutions, most have had little or no training, education, or even orientation to these settings. Forensic psychiatry fellowship requirements include experience in treating acutely and chronically ill patients in correctional systems.3 Although general psychiatric training doesn’t preclude correctional experience, it is not required. The forensic component of general psychiatric residency is limited to evaluation of forensic issues, report writing, and testimony.
Professional organizations—including the American Psychiatric Association,4 the American Public Health Association,5 the National Commission on Correctional Health Care,6 and the American Correctional Health Services Association7—have developed standards and position statements on providing medical and mental health care in correctional facilities. Although psychiatrists’ work in correctional settings generally has been reserved for consultation and medication management, it is important for these clinicians to understand and appreciate the wider landscape and environment in which they practice. Psychiatrists can help develop and implement mental health processes that lead to better services and improved clinical outcomes.
Right to treatment
Convicted persons have a constitutional right to medical and mental health treatment under extension of the Eighth Amendment of the U.S. Constitution, which prohibits cruel and unusual punishment.8 In 1976, the U.S. Supreme Court concluded that “deliberate indifference to serious medical needs of prisoners constitutes the ‘unnecessary and wanton infliction of pain’… proscribed by the Eighth Amendment.”9 This coverage was expanded to mental health needs when the court found “…no underlying distinction between the right to medical care for physical ills and its psychological or psychiatric counterpart.”10 Correctional facilities also are obligated to provide medical and mental health treatment for persons in custody who are not yet convicted of an offense.8 In subsequent litigation, the court formulated 6 components of a minimally adequate correctional mental health treatment program; these are described in Table 1.11
Table 1
Components of minimally adequate mental health system in correctional facilities
| A systematic screening and evaluation program to identify inmates requiring mental health treatment |
| Treatment that encompasses more than simply segregating the mentally ill inmate and increasing correctional supervision |
| Treatment by trained mental health professionals in sufficient numbers to identify and treat inmates suffering from serious mental disorders |
| Maintenance of accurate, complete, and confidential records of the mental health treatment process |
| A suicide prevention program |
| Appropriate use of psychotropic medication (prescription and monitoring by appropriately trained and licensed staff to treat bona fide mental disorders rather than solely as a means of behavioral management) |
| Source: Reference 11 |
Jails vs prisons
The type of psychiatric treatment provided differs based on whether the facility is a jail or a prison, how long inmates are confined, and whether the facility serves a special mission or population, such as serving as a reception center for a prison system or housing only juveniles. Jails generally house inmates for short periods—often <1 year—experience rapid population turnover, and receive admissions day and night. Jails vary in size from a few holding cells to several thousand beds. These factors have implications for screening and evaluation processes, suicide prevention, and coordination of care with community treatment providers. In jails, clinicians’ work focuses on rapid identification of psychiatric illness, assessment, stabilization, and re-linkage to treatment providers in the community. Access to inpatient and ongoing psychiatric care also should be available.
In contrast, prisons house people who have been convicted and sentenced to serve time, generally for >1 year. Turnover is less rapid, admissions and discharges are more predictable, and there is greater opportunity and obligation to develop a continuum of mental health care. Prison systems generally provide or make arrangements for crisis intervention, residential treatment services, and inpatient and outpatient psychiatric care. These services may be provided on the prison grounds, or the inmate may be transferred to another prison within the system that offers specialized treatment or to a community hospital, where the inmate is under the constant supervision of corrections officers. Residential treatment includes intensive, coordinated, and structured mental health services and consists of group and individual therapies, psychoeducation, and therapeutic activities; these services are analogous to intensive day treatment or partial hospitalization programs in the community. In prisons mental health care emphasizes ongoing treatment. As in the community, treatment teams in correctional settings often include mental health professionals such as psychiatric nurses, psychotherapists, and psychology staff in addition to psychiatrists.
Screening and evaluation
Correctional facilities need a systematic screening process that is conducted on all inmates. This preliminary entry or “receiving screening” is intended to identify urgent medical and mental health concerns and persons in need of immediate treatment. A nurse or corrections staff officer who has been trained by medical staff could conduct this screening. Screening consists of observing the inmate’s current condition and conducting a structured inquiry into medical and psychiatric symptoms, psychotropic medications, drug and alcohol use history, and suicide risk. A positive screen leads to immediate action such as instituting drug or alcohol detoxification or initiating suicide precautions or an emergency medical referral and assessment.4,6
Within a few days of an inmate’s arrival, a mental health professional should conduct a more detailed mental health screening to identify non-emergent psychiatric needs. The mental health screening includes:
- a review of accompanying mental health information received from the county jail or arresting/transporting officer
- a self-reported history of psychiatric treatment, such as hospitalization, pharmacotherapy, or outpatient counseling
- current or prior suicidal thoughts or attempts
- intellectual functioning
- history of violence and/or victimization
- a brief mental status examination.4
Records from previous incarcerations should be reviewed. Also, if relevant, obtain the inmate’s consent to collect outside treatment records and/or speak with family or significant others. The results of this brief mental health assessment could prompt a referral for further evaluation and determine the need for psychotropic therapy.
Although usually not directly involved in this systematic screening and evaluation, psychiatrists should be familiar with how and why referrals are made to be sure that they are appropriate and to reduce unnecessary evaluations, leaving more time for medication follow-up, treatment planning, and suicide risk assessment. An efficient and effective mental health screening and assessment process helps ensure that limited psychiatric resources are used to maximal benefit.
Suicide prevention
Suicide prevention programs often include teaching corrections staff to identify suicide risk factors and instructing them to screen at-risk inmates at any time during incarceration. These programs should implement steps to keep inmates safe, such as increasing intensity and frequency of monitoring by corrections staff, removing or limiting access to items that could be used to harm oneself, and moving inmates to a housing area where the means and opportunity for self harm are reduced.4,6 Suicide prevention programs also should include delivery of appropriate mental health interventions to improve the inmate’s clinical condition, resolve the crisis, or otherwise lower suicide risk. These interventions include:
- increased frequency of interaction with mental health staff (more than a brief daily interaction conducted at the cell front)
- treatment of drug and/or alcohol withdrawal
- referral for evaluation and assessment of the need for psychotropic medication or dosage adjustment (Table 2).6
A correctional facility’s policy should allow a low threshold for corrections staff to initiate a suicide prevention watch—it is better to err on the side of caution and institute a watch than to expect non-mental health professionals to conduct clinical risk level assessments. Full assessment of an inmate’s clinical condition and the decision to reduce or discontinue the watch should be left to a trained mental health professional. This function may fall within the psychiatrist’s duties and it is important to be aware of the ramifications of watch discontinuation, such as:
- what type of property is returned
- where the inmate will be housed
- how often the inmate will be monitored by custody staff
- when the next mental health follow-up will occur.
Failure to articulate your expectations to staff members can lead to catastrophic consequences if watches are discontinued without an appropriate plan for monitoring and follow-up.
Psychiatrists can help train corrections staff on signs of suicide risk and also should review suicide attempts and/or completed suicides. This often can be a challenge because a psychiatrist’s time at a facility may be limited, but is an important consideration for quality improvement efforts.
Table 2
Components of a correctional suicide prevention program
| Training for staff on verbal and behavior cues indicating suicide risk and appropriate response |
| Identification of potentially suicidal inmates |
| Referral to mental health providers or facilities |
| Evaluation by qualified mental health professional |
| Housing in safe area of the institution |
| Treatment to address the cause of or reasons for suicidal thoughts |
| Monitoring procedures that permit regular, documented supervision |
| Communication procedures between health care and corrections personnel |
| Intervention procedures addressing how to handle a suicide attempt in progress |
| Notification procedures to ensure appropriate correctional authorities, outside authorities, and family are contacted |
| Reporting procedures for documenting attempted or completed suicides |
| Review of suicides and serious attempts by health care and administrative staff |
| Critical incident debriefing offered to affected personnel and inmates in event of completed suicide |
| Source: Reference 6 |
Pharmacotherapy
Traditionally, the primary role of psychiatrists working in correctional facilities has been psychotropic medication management. Understanding the correctional context and procedures permits more informed prescription choices and recommendations for psychotropics to be included in the formulary.
Antipsychotics, antidepressants, and mood stabilizers should be included in a facility’s formulary. Considerations concerning types of psychotropic medication within a formulary depend on the facility’s size and mission, psychiatric illnesses encountered in the population, and lengths of inmates’ stay.12 A mechanism should be in place to prescribe off-formulary and access other types of psychotropic medication on a case-by-case basis to ensure inmates are not denied appropriate treatment. A psychiatrist may have to advocate strongly for these principles.
Most correctional facilities require that staff administer every dose of psychotropic medication directly to the inmate for whom it is prescribed. In some facilities, only nursing personnel can administer medication, while others use trained corrections staff to deliver medication. Psychiatrists who prescribe psychotropics in correctional institutions must be familiar with the facility’s medication administration procedures, which may impact medication choice and form, dosing frequency, timing of laboratory studies, and inmate medication compliance. Prescribers’ capacity to order emergency or “as needed” medications may be limited or nonexistent if nursing staff is unavailable.
Appropriate use of psychotropic medication for treating psychiatric illness is the standard of care, but is only 1 component of an effective treatment plan for inmates with serious mental illness. Others include group and individual therapy, psychoeducation, and therapeutic activities such as recreational therapy, activity therapy, and opportunities for education and work within the correctional system.
Related Resources
- Scott CL, ed. Handbook of correctional mental health. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2009.
- Thienhaus OJ, Piasecki M, eds. Correctional psychiatry practice guidelines and strategies. Kingston, NJ: Civic Research Institute, Inc.; 2007.
- National Commission on Correctional Healthcare. www.ncchc.org.
- Society of Correctional Physicians. www.CorrDocs.org.
Disclosure
Dr. Burns reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Glaze LE. Correctional populations in the United States, 2009. Washington, DC: Bureau of Justice Statistics. December 2010. NCJ 231681.
2. James DJ, Glaze LE. Mental health problems of prison and jail inmates. Washington, DC: Bureau of Justice Statistics. September 2006. NCJ 213600.
3. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in forensic psychiatry. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/406pr703_u105.pdf. Accessed December 30, 2010.
4. American Psychiatric Association. Psychiatric services in jails and prisons. 2nd ed. Washington, DC: American Psychiatric Association; 2000.
5. Standards for health services in correctional institutions. 3rd ed. Washington, DC: American Public Health Association; 2003.
6. National Commission on Correctional Health Care. Standards for mental health services in correctional facilities. Chicago, IL: National Commission on Correctional Health Care; 2008.
7. American Correctional Health Services Association. Position statement on forced and involuntary psychotropic medication. Available at: http://www.achsa.org/displaycommon.cfm?an=12. Accessed December 30, 2010.
8. Cohen F. Overview of legal issues. In: Cohen F. The mentally disordered inmate and the law. Kingston, NJ: Civic Research Institute, Inc.; 1998:2-1-2-20.
9. Estelle v Gamble. 429 U.S. 97 (1976).
10. Bowring v Godwin. 551 F2d 44, 47 (4th Cir 1977).
11. Ruiz v Estelle. 503 F Supp 1265 (SD Tex 1980).
12. Burns K. Pharmacotherapy in correctional settings. In: Scott CL, ed. Handbook of correctional mental health. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:321-344.
1. Glaze LE. Correctional populations in the United States, 2009. Washington, DC: Bureau of Justice Statistics. December 2010. NCJ 231681.
2. James DJ, Glaze LE. Mental health problems of prison and jail inmates. Washington, DC: Bureau of Justice Statistics. September 2006. NCJ 213600.
3. Accreditation Council for Graduate Medical Education. ACGME program requirements for graduate medical education in forensic psychiatry. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/406pr703_u105.pdf. Accessed December 30, 2010.
4. American Psychiatric Association. Psychiatric services in jails and prisons. 2nd ed. Washington, DC: American Psychiatric Association; 2000.
5. Standards for health services in correctional institutions. 3rd ed. Washington, DC: American Public Health Association; 2003.
6. National Commission on Correctional Health Care. Standards for mental health services in correctional facilities. Chicago, IL: National Commission on Correctional Health Care; 2008.
7. American Correctional Health Services Association. Position statement on forced and involuntary psychotropic medication. Available at: http://www.achsa.org/displaycommon.cfm?an=12. Accessed December 30, 2010.
8. Cohen F. Overview of legal issues. In: Cohen F. The mentally disordered inmate and the law. Kingston, NJ: Civic Research Institute, Inc.; 1998:2-1-2-20.
9. Estelle v Gamble. 429 U.S. 97 (1976).
10. Bowring v Godwin. 551 F2d 44, 47 (4th Cir 1977).
11. Ruiz v Estelle. 503 F Supp 1265 (SD Tex 1980).
12. Burns K. Pharmacotherapy in correctional settings. In: Scott CL, ed. Handbook of correctional mental health. 2nd ed. Arlington, VA: American Psychiatric Publishing, Inc.; 2010:321-344.
Patients' psychosocial concerns after bariatric surgery
A case of returning psychosis
CASE: Agitated and violent
Police bring Ms. Y, age 42, to the emergency room (ER) after her boyfriend calls 911 because she is physically aggressive. The police note that the home is in disarray and several windows are broken. Ms. Y is threatening and violent—she bites and spits at her boyfriend and the police. The ER assessment reports that she is “agitated, confused, and not making sense.” She receives IV haloperidol, 5 mg, for agitation and aggressive behavior, but does not improve and receives a second dose of haloperidol approximately 1 hour later.
On examination she is afebrile. Laboratory results are notable for elevated blood urea nitrogen (27 mg/dL) and creatinine (2.3 mg/dL), suggesting renal failure. Her white blood cell (WBC) count is elevated at 14.7 K/μL with increased neutrophil count. Her creatine phosphokinase (CPK) also is elevated at 2,778 U/L. Other lab results, including liver function tests and a rapid plasma reagin, are within normal limits. Urinalysis reveals WBC >50 and leukocyte esterase 3+ WBC/μL. Urine drug screen is negative for barbiturates, benzodiazepines, opiates, and cocaine and her blood alcohol level is <10 mg/dL. She is overweight, but not obese. Ms. Y is admitted to the medical service for workup of rhabdomyolysis and altered mental status.
When the psychiatric consultation-liaison (CL) service evaluates Ms. Y 12 hours after presentation, she is disheveled, drowsy, and lying in bed, with multiple superficial lacerations on her forearms. She is cooperative but claims to have no recollection of the events leading up to her admission. Her speech is soft with a lack of spontaneity, and she demonstrates substantial psychomotor retardation. Her mood is irritable and affect is restricted. She has a latency of thought and difficulty recalling basic historic information. Ms. Y appears confused and frequently responds to questions with “I don’t remember.” She seems frustrated and distressed by her inability to answer questions. She denies suicidal or homicidal ideation and auditory or visual hallucinations, although she appears to be responding to internal stimuli. We cannot complete a Mini-Mental State Exam because she becomes uncooperative. After 10 minutes, Ms. Y ends the interview, stating that too much is being “demanded” of her.
The authors’ observations
Ms. Y’s acute-onset agitation and confusion could be caused by an infection, such as a urinary tract infection, a frequent culprit in delirium or transient psychosis. Seizure activity with postictal confusion also has to be included in the differential, as well as an endogenous psychotic disorder such as schizophrenia or a manic bipolar episode. Ms. Y’s boyfriend of 16 months indicated that Ms. Y uses alcohol but cannot quantify the amount or frequency. We considered and ruled out other intoxicant use as a potential cause of her transient psychosis. An extended drug screen was negative and her lab values did not suggest heavy alcohol use.
HISTORY: Past psychotic episodes
Ms. Y’s boyfriend reports that she had 2 psychiatric hospitalizations approximately 30 years ago, which were precipitated by psychotic symptoms that she developed while abusing drugs. To the best of his knowledge Ms. Y had not used these agents recently. He stated that Ms. Y did not appear to have ongoing psychotic symptoms and had not received psychiatric treatment since she was a teenager until 6 months ago. He describes the current hospitalization as being “just like 6 months ago.”
Medical records reveal that Ms. Y was admitted to our hospital 6 months ago because she was acting violently and combative. She was “talking out of context,” “stated that she was God,” and had auditory hallucinations. She was admitted to the medical service for rhabdomyolysis, which was thought to be caused by hyperactivity or exertion. Ms. Y indicated that she was taking food supplements, including L-carnitine, to help lose weight. Her psychotic symptoms cleared within 24 hours and she was discharged without any psychiatric medications. Her behavioral disturbance was attributed to ingesting excessive amounts of carnitine supplements, and Ms. Y was counseled to abstain from them.
The authors’ observations
Carnitine is a common dietary supplement that is advertised as being safe and effective.1 It is purported to increase fat oxidation or reduce fat synthesis; however, no trials demonstrate that L-carnitine is effective for weight loss (Box).2-7 Evidence from well-designed randomized, controlled clinical trials indicates that the safe upper limit of long-term intake is 2,000 mg/d of L-carnitine equivalents.8 The data for doses >2,000 mg/d are not sufficient to make a confident conclusion on long-term safety.8
Further evaluation for possible causes of Ms. Y’s symptoms include a chest radiography and blood and urine cultures, which are unremarkable. Results of a lumbar puncture are within normal limits. Computed tomography of the head reveals confluent periventricular hypodensities compatible with moderate to severe non-specific white matter disease.
Carnitine is derived from an amino acid and found in nearly all cells of the body. “Carnitine” is used to refer to several compounds, including L-carnitine, acetyl-L-carnitine, and propionyl-L-carnitine. The natural form and the only one with biologic activity is the geometric isomer L-carnitine. Most endogenous L-carnitine is derived from diet— meat and dairy are the primary sources—and the remainder is synthesized.2-4
Carnitine transports long-chain fatty acids into the mitochondria so they can be oxidized to produce energy and transports toxic compounds out of the mitochondria to prevent them from accumulating. Carnitine is concentrated in tissues that use fatty acids as a dietary fuel, such as skeletal and cardiac muscle.2-4 The body makes enough carnitine to meet most person’s needs and supplementation typically is not required. Some drugs, such as valproic acid and carbamazepine, can reduce carnitine blood concentrations.2
Because of its role in fatty acid oxidation, carnitine often is promoted as a weight loss aid. In addition, it is purported to improve exercise performance and enhance well-being.3,4 However, there is no consistent evidence that carnitine supplements can improve physical performance in healthy individuals.5
At doses of approximately 3 g/d, carnitine supplements can cause nausea, vomiting, abdominal cramps, diarrhea, and a “fishy” body odor. Rare side effects include muscle weakness in uremic patients and seizures in those with a seizure disorder.2-4
In animal studies, carnitine persistently increases dopamine outflow in the nucleus accumbens.6 Dopamine dysregulation in this pathway has been shown to cause psychotic symptoms.7
TREATMENT: Rapid improvement
Ms. Y’s renal dysfunction resolves within 24 hours with aggressive hydration and supportive therapy. Her WBC count normalizes and her CPK decreases.
When Ms. Y becomes more cooperative, the CL team pieces together more of her story with further interviews and collateral information from her cousin. Ms. Y’s family history includes an aunt with schizophrenia. Three years ago Ms. Y moved from the Midwest to a large Southern city with her husband, from whom she is divorced. She has 2 children who were removed from her custody when she was a teenager for unclear reasons. At admission, she lives with her boyfriend, whom she plans on marrying.
Ms. Y says she was taking carnitine to improve her energy and lose weight. She recalls that her physicians advised her to discontinue carnitine supplements, but she continued to take “4 or 5 a day” in an ongoing attempt to lose weight. When asked about other supplements, Ms. Y reports regularly consuming 16-ounce energy drinks, including the day before admission. The label on this drink lists L-carnitine and caffeine as main ingredients. She denies regularly drinking other caffeine-containing beverages, including coffee, tea, or soda.
The authors’ observations
Supplements for weight loss may appeal to people’s desire for a “quick fix” that is less demanding than diets and increased physical activity. Supplements are available without a prescription and despite reports of adverse reactions generally are perceived as being safe and having few side effects. These supplements may be marketed as “natural,” which can be misinterpreted as an assurance of safety and efficacy.
Given the similarities of the current admission to the one 6 months ago, we suspect Ms. Y is experiencing transient psychosis secondary to carnitine intoxication. Based on Ms. Y’s boyfriend’s report and the product labeling, we estimate that Ms. Y took approximately 4,000 mg of carnitine in the 24 hours before admission.
Other causes of transient psychosis, such as infectious, metabolic, and neoplastic processes, were considered and ruled out. Seizures with postictal confusion also was ruled out because Ms. Y does not have a history of seizures and there is no evidence of convulsive activity, incontinence, or buccal lacerations. Given Ms. Y’s family history of schizophrenia and reported history of psychotic symptoms as a teenager we considered that she may have an endogenous psychotic disorder. However, her psychotic symptoms were transient, and Ms. Y returned to her baseline level of functioning between episodes.
OUTCOME: Advice to stop
We start Ms. Y on risperidone, 2 mg/d, at bedtime for her psychotic symptoms. Her psychotic symptoms quickly improve. She seems to return to her baseline state approximately 36 hours after admission and is medically cleared for discharge. Risperidone is discontinued after only 1 dose.
Ms. Y is remorseful over her recent aggressive behavior, and fears that her boyfriend will leave her. She denies suicidal and homicidal ideation and does not require inpatient psychiatric hospitalization. We strongly advise her to discontinue carnitine supplements and energy drinks and to limit her caffeine intake. Because Ms. Y’s had continued to use carnitine supplements despite adverse consequences and against medical advice, we refer her for substance abuse treatment.
The authors’ observations
Although temporal coincidence does not necessarily imply causality, in Ms. Y’s case, the relationship between carnitine ingestion and psychiatric symptoms cannot be ignored. Individuals predisposed to mania or psychosis may be more likely to respond adversely after ingesting nutritional supplements or energy drinks.9 Ms. Y’s past psychotic episodes suggest that she could be vulnerable to future episodes. She also might have a biologic predisposition to psychosis because of her family history of schizophrenia.
The literature contains at least 1 other reported case of carnitine-induced psychosis. A patient with a history of bipolar disorder presented with auditory hallucinations, persecutory delusions, and verbally threatening and physically assaultive behavior 5 days after beginning nutritional supplements containing carnitine.1 There also are reports of patients who experienced acute changes in mental status after consuming other nutritional weight loss supplements (Table).9-17 Chelben et al9 describe 3 patients with known psychiatric illness who showed clinical deterioration leading to psychiatric hospitalization after ingesting nutraceutical preparations. This may be a common but unrecognized cause of decompensation in psychiatric patients who take supplements.
This case highlights the importance of being aware of patients’ use of alternative medications or nutritional supplements. Physicians should routinely inquire about the use of weight loss products, energy drinks, and supplements, and patients should be educated about the risks, including potential to exacerbate pre-existing psychiatric disorders.
Table
Psychiatric effects of common weight loss supplements
| Supplement | Psychiatric effects |
|---|---|
| Caffeine | Depression, anxiety, agitation, aggression, psychosis10-12 |
| Ephedra* | Psychosis, severe depression, mania or agitation, hallucinations, sleep disturbance, suicidal ideation13 |
| Panax (ginseng) | Euphoria, mania14 |
| Amino acid-containing drinks (taurine and inositol) | Euphoria, hypervigilance, insomnia, verbal and physical aggression, impulsive behavior9,15 |
| Hypericum (St. John’s wort) | Mania,16 psychosis17 |
| *FDA removed ephedra from the market in 2003 because of adverse events | |
Related Resources
- National Library of Medicine. Dietary supplements labels database. http://dietarysupplements.nlm.nih.gov/dietary.
- Grossberg G, Fox B. The essential herb-drug-vitamin interaction guide. New York, NY: Broadway Books; 2007.
Drug Brand Names
- Carbamazepine • Tegretol
- Haloperidol • Haldol
- Risperidone • Risperdal
- Valproic acid • Depakene
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Evcimen H, Mania I, Mathews M, et al. Psychosis precipitated by acetyl-L-carnitine in a patient with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2007;9(1):71-72.
2. Office of Dietary Supplements National Institutes of Health. Dietary supplement fact sheet: carnitine. Available at: http://ods.od.nih.gov/factsheets/carnitine.asp. Accessed November 22, 2010.
3. Carnitine: lessons from one hundred years of research Ann NY Acad Sci. 2004;1033:ix-xi.
4. Rebouche CJ, Carnitine. In: Shils ME, Olson JA, Shike M, et al, eds. Modern nutrition in health and disease. 9th ed. Baltimore, MD: Lippincott Williams and Wilkins; 1999: 505-512.
5. Brass EP. Carnitine and sports medicine: use or abuse? Ann NY Acad Sci. 2004;1033:67-78.
6. Scheggi S, Rauggi R, Nanni G, et al. Repeated acetyl-L-carnitine administration increases phosphor-Thr34 DARPP-32 levels and antagonizes cocaine-induced increase in Cdk5 and phosphor-Thr75 DARPP-32 levels in rat striatum. Eur J Neurosci. 2004;19:1609-1620.
7. Howes OD, McDonald C, Cannon M, et al. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(suppl 1):S7-S13.
8. Hathcock JN, Shao A. Risk assessment for carnitine. Regul Toxicol Pharmacol. 2006;46:23-28.
9. Chelben J, Piccone-Sapir A, Ianco I, et al. Effects of amino acid energy drinks leading to hospitalization in individuals with mental illness. Gen Hosp Psychiatry. 2008;30(2):187-189.
10. Hedges DW, Woon FL, Hoopes SP. Caffeine-induced psychosis. CNS Spectr. 2009;14:127-129.
11. Broderick P, Benjamin AB. Caffeine and psychiatric symptoms: a review. J Okla State Med Assoc. 2004;97(12):538-542.
12. Cerimele JM, Stern AP, Jutras-Aswad D. Psychosis following excessive ingestion of energy drinks in a patient with schizophrenia. Am J Psychiatry. 2010;167(3):353.-
13. Maglione M, Miotto K, Iguchi M, et al. Psychiatric effects of ephedra use: an analysis of Food and Drug Administration reports of adverse events. Am J Psychiatry. 2005;162(1):189-191.
14. Engelberg D, McCutcheon A, Wiseman S. A case of ginseng-induced mania. J Clin Psychopharmacol. 2001;21(5):535-537.
15. Machado-Vieira R, Viale CI, Kapczinski F. Mania associated with an energy drink: the possible role of caffeine, taurine and inositol. Can J Psychiatry. 2001;46:454-455.
16. Fahmi M, Huang C, Schweitzer I. A case of mania induced by hypericum. World J Biol Psychiatry. 2002;3(1):58-59.
17. Stevinson C, Ernst E. Can St. John’s wort trigger psychoses? Int J Clin Pharmacol Ther. 2004;42(9):473-480.
CASE: Agitated and violent
Police bring Ms. Y, age 42, to the emergency room (ER) after her boyfriend calls 911 because she is physically aggressive. The police note that the home is in disarray and several windows are broken. Ms. Y is threatening and violent—she bites and spits at her boyfriend and the police. The ER assessment reports that she is “agitated, confused, and not making sense.” She receives IV haloperidol, 5 mg, for agitation and aggressive behavior, but does not improve and receives a second dose of haloperidol approximately 1 hour later.
On examination she is afebrile. Laboratory results are notable for elevated blood urea nitrogen (27 mg/dL) and creatinine (2.3 mg/dL), suggesting renal failure. Her white blood cell (WBC) count is elevated at 14.7 K/μL with increased neutrophil count. Her creatine phosphokinase (CPK) also is elevated at 2,778 U/L. Other lab results, including liver function tests and a rapid plasma reagin, are within normal limits. Urinalysis reveals WBC >50 and leukocyte esterase 3+ WBC/μL. Urine drug screen is negative for barbiturates, benzodiazepines, opiates, and cocaine and her blood alcohol level is <10 mg/dL. She is overweight, but not obese. Ms. Y is admitted to the medical service for workup of rhabdomyolysis and altered mental status.
When the psychiatric consultation-liaison (CL) service evaluates Ms. Y 12 hours after presentation, she is disheveled, drowsy, and lying in bed, with multiple superficial lacerations on her forearms. She is cooperative but claims to have no recollection of the events leading up to her admission. Her speech is soft with a lack of spontaneity, and she demonstrates substantial psychomotor retardation. Her mood is irritable and affect is restricted. She has a latency of thought and difficulty recalling basic historic information. Ms. Y appears confused and frequently responds to questions with “I don’t remember.” She seems frustrated and distressed by her inability to answer questions. She denies suicidal or homicidal ideation and auditory or visual hallucinations, although she appears to be responding to internal stimuli. We cannot complete a Mini-Mental State Exam because she becomes uncooperative. After 10 minutes, Ms. Y ends the interview, stating that too much is being “demanded” of her.
The authors’ observations
Ms. Y’s acute-onset agitation and confusion could be caused by an infection, such as a urinary tract infection, a frequent culprit in delirium or transient psychosis. Seizure activity with postictal confusion also has to be included in the differential, as well as an endogenous psychotic disorder such as schizophrenia or a manic bipolar episode. Ms. Y’s boyfriend of 16 months indicated that Ms. Y uses alcohol but cannot quantify the amount or frequency. We considered and ruled out other intoxicant use as a potential cause of her transient psychosis. An extended drug screen was negative and her lab values did not suggest heavy alcohol use.
HISTORY: Past psychotic episodes
Ms. Y’s boyfriend reports that she had 2 psychiatric hospitalizations approximately 30 years ago, which were precipitated by psychotic symptoms that she developed while abusing drugs. To the best of his knowledge Ms. Y had not used these agents recently. He stated that Ms. Y did not appear to have ongoing psychotic symptoms and had not received psychiatric treatment since she was a teenager until 6 months ago. He describes the current hospitalization as being “just like 6 months ago.”
Medical records reveal that Ms. Y was admitted to our hospital 6 months ago because she was acting violently and combative. She was “talking out of context,” “stated that she was God,” and had auditory hallucinations. She was admitted to the medical service for rhabdomyolysis, which was thought to be caused by hyperactivity or exertion. Ms. Y indicated that she was taking food supplements, including L-carnitine, to help lose weight. Her psychotic symptoms cleared within 24 hours and she was discharged without any psychiatric medications. Her behavioral disturbance was attributed to ingesting excessive amounts of carnitine supplements, and Ms. Y was counseled to abstain from them.
The authors’ observations
Carnitine is a common dietary supplement that is advertised as being safe and effective.1 It is purported to increase fat oxidation or reduce fat synthesis; however, no trials demonstrate that L-carnitine is effective for weight loss (Box).2-7 Evidence from well-designed randomized, controlled clinical trials indicates that the safe upper limit of long-term intake is 2,000 mg/d of L-carnitine equivalents.8 The data for doses >2,000 mg/d are not sufficient to make a confident conclusion on long-term safety.8
Further evaluation for possible causes of Ms. Y’s symptoms include a chest radiography and blood and urine cultures, which are unremarkable. Results of a lumbar puncture are within normal limits. Computed tomography of the head reveals confluent periventricular hypodensities compatible with moderate to severe non-specific white matter disease.
Carnitine is derived from an amino acid and found in nearly all cells of the body. “Carnitine” is used to refer to several compounds, including L-carnitine, acetyl-L-carnitine, and propionyl-L-carnitine. The natural form and the only one with biologic activity is the geometric isomer L-carnitine. Most endogenous L-carnitine is derived from diet— meat and dairy are the primary sources—and the remainder is synthesized.2-4
Carnitine transports long-chain fatty acids into the mitochondria so they can be oxidized to produce energy and transports toxic compounds out of the mitochondria to prevent them from accumulating. Carnitine is concentrated in tissues that use fatty acids as a dietary fuel, such as skeletal and cardiac muscle.2-4 The body makes enough carnitine to meet most person’s needs and supplementation typically is not required. Some drugs, such as valproic acid and carbamazepine, can reduce carnitine blood concentrations.2
Because of its role in fatty acid oxidation, carnitine often is promoted as a weight loss aid. In addition, it is purported to improve exercise performance and enhance well-being.3,4 However, there is no consistent evidence that carnitine supplements can improve physical performance in healthy individuals.5
At doses of approximately 3 g/d, carnitine supplements can cause nausea, vomiting, abdominal cramps, diarrhea, and a “fishy” body odor. Rare side effects include muscle weakness in uremic patients and seizures in those with a seizure disorder.2-4
In animal studies, carnitine persistently increases dopamine outflow in the nucleus accumbens.6 Dopamine dysregulation in this pathway has been shown to cause psychotic symptoms.7
TREATMENT: Rapid improvement
Ms. Y’s renal dysfunction resolves within 24 hours with aggressive hydration and supportive therapy. Her WBC count normalizes and her CPK decreases.
When Ms. Y becomes more cooperative, the CL team pieces together more of her story with further interviews and collateral information from her cousin. Ms. Y’s family history includes an aunt with schizophrenia. Three years ago Ms. Y moved from the Midwest to a large Southern city with her husband, from whom she is divorced. She has 2 children who were removed from her custody when she was a teenager for unclear reasons. At admission, she lives with her boyfriend, whom she plans on marrying.
Ms. Y says she was taking carnitine to improve her energy and lose weight. She recalls that her physicians advised her to discontinue carnitine supplements, but she continued to take “4 or 5 a day” in an ongoing attempt to lose weight. When asked about other supplements, Ms. Y reports regularly consuming 16-ounce energy drinks, including the day before admission. The label on this drink lists L-carnitine and caffeine as main ingredients. She denies regularly drinking other caffeine-containing beverages, including coffee, tea, or soda.
The authors’ observations
Supplements for weight loss may appeal to people’s desire for a “quick fix” that is less demanding than diets and increased physical activity. Supplements are available without a prescription and despite reports of adverse reactions generally are perceived as being safe and having few side effects. These supplements may be marketed as “natural,” which can be misinterpreted as an assurance of safety and efficacy.
Given the similarities of the current admission to the one 6 months ago, we suspect Ms. Y is experiencing transient psychosis secondary to carnitine intoxication. Based on Ms. Y’s boyfriend’s report and the product labeling, we estimate that Ms. Y took approximately 4,000 mg of carnitine in the 24 hours before admission.
Other causes of transient psychosis, such as infectious, metabolic, and neoplastic processes, were considered and ruled out. Seizures with postictal confusion also was ruled out because Ms. Y does not have a history of seizures and there is no evidence of convulsive activity, incontinence, or buccal lacerations. Given Ms. Y’s family history of schizophrenia and reported history of psychotic symptoms as a teenager we considered that she may have an endogenous psychotic disorder. However, her psychotic symptoms were transient, and Ms. Y returned to her baseline level of functioning between episodes.
OUTCOME: Advice to stop
We start Ms. Y on risperidone, 2 mg/d, at bedtime for her psychotic symptoms. Her psychotic symptoms quickly improve. She seems to return to her baseline state approximately 36 hours after admission and is medically cleared for discharge. Risperidone is discontinued after only 1 dose.
Ms. Y is remorseful over her recent aggressive behavior, and fears that her boyfriend will leave her. She denies suicidal and homicidal ideation and does not require inpatient psychiatric hospitalization. We strongly advise her to discontinue carnitine supplements and energy drinks and to limit her caffeine intake. Because Ms. Y’s had continued to use carnitine supplements despite adverse consequences and against medical advice, we refer her for substance abuse treatment.
The authors’ observations
Although temporal coincidence does not necessarily imply causality, in Ms. Y’s case, the relationship between carnitine ingestion and psychiatric symptoms cannot be ignored. Individuals predisposed to mania or psychosis may be more likely to respond adversely after ingesting nutritional supplements or energy drinks.9 Ms. Y’s past psychotic episodes suggest that she could be vulnerable to future episodes. She also might have a biologic predisposition to psychosis because of her family history of schizophrenia.
The literature contains at least 1 other reported case of carnitine-induced psychosis. A patient with a history of bipolar disorder presented with auditory hallucinations, persecutory delusions, and verbally threatening and physically assaultive behavior 5 days after beginning nutritional supplements containing carnitine.1 There also are reports of patients who experienced acute changes in mental status after consuming other nutritional weight loss supplements (Table).9-17 Chelben et al9 describe 3 patients with known psychiatric illness who showed clinical deterioration leading to psychiatric hospitalization after ingesting nutraceutical preparations. This may be a common but unrecognized cause of decompensation in psychiatric patients who take supplements.
This case highlights the importance of being aware of patients’ use of alternative medications or nutritional supplements. Physicians should routinely inquire about the use of weight loss products, energy drinks, and supplements, and patients should be educated about the risks, including potential to exacerbate pre-existing psychiatric disorders.
Table
Psychiatric effects of common weight loss supplements
| Supplement | Psychiatric effects |
|---|---|
| Caffeine | Depression, anxiety, agitation, aggression, psychosis10-12 |
| Ephedra* | Psychosis, severe depression, mania or agitation, hallucinations, sleep disturbance, suicidal ideation13 |
| Panax (ginseng) | Euphoria, mania14 |
| Amino acid-containing drinks (taurine and inositol) | Euphoria, hypervigilance, insomnia, verbal and physical aggression, impulsive behavior9,15 |
| Hypericum (St. John’s wort) | Mania,16 psychosis17 |
| *FDA removed ephedra from the market in 2003 because of adverse events | |
Related Resources
- National Library of Medicine. Dietary supplements labels database. http://dietarysupplements.nlm.nih.gov/dietary.
- Grossberg G, Fox B. The essential herb-drug-vitamin interaction guide. New York, NY: Broadway Books; 2007.
Drug Brand Names
- Carbamazepine • Tegretol
- Haloperidol • Haldol
- Risperidone • Risperdal
- Valproic acid • Depakene
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Agitated and violent
Police bring Ms. Y, age 42, to the emergency room (ER) after her boyfriend calls 911 because she is physically aggressive. The police note that the home is in disarray and several windows are broken. Ms. Y is threatening and violent—she bites and spits at her boyfriend and the police. The ER assessment reports that she is “agitated, confused, and not making sense.” She receives IV haloperidol, 5 mg, for agitation and aggressive behavior, but does not improve and receives a second dose of haloperidol approximately 1 hour later.
On examination she is afebrile. Laboratory results are notable for elevated blood urea nitrogen (27 mg/dL) and creatinine (2.3 mg/dL), suggesting renal failure. Her white blood cell (WBC) count is elevated at 14.7 K/μL with increased neutrophil count. Her creatine phosphokinase (CPK) also is elevated at 2,778 U/L. Other lab results, including liver function tests and a rapid plasma reagin, are within normal limits. Urinalysis reveals WBC >50 and leukocyte esterase 3+ WBC/μL. Urine drug screen is negative for barbiturates, benzodiazepines, opiates, and cocaine and her blood alcohol level is <10 mg/dL. She is overweight, but not obese. Ms. Y is admitted to the medical service for workup of rhabdomyolysis and altered mental status.
When the psychiatric consultation-liaison (CL) service evaluates Ms. Y 12 hours after presentation, she is disheveled, drowsy, and lying in bed, with multiple superficial lacerations on her forearms. She is cooperative but claims to have no recollection of the events leading up to her admission. Her speech is soft with a lack of spontaneity, and she demonstrates substantial psychomotor retardation. Her mood is irritable and affect is restricted. She has a latency of thought and difficulty recalling basic historic information. Ms. Y appears confused and frequently responds to questions with “I don’t remember.” She seems frustrated and distressed by her inability to answer questions. She denies suicidal or homicidal ideation and auditory or visual hallucinations, although she appears to be responding to internal stimuli. We cannot complete a Mini-Mental State Exam because she becomes uncooperative. After 10 minutes, Ms. Y ends the interview, stating that too much is being “demanded” of her.
The authors’ observations
Ms. Y’s acute-onset agitation and confusion could be caused by an infection, such as a urinary tract infection, a frequent culprit in delirium or transient psychosis. Seizure activity with postictal confusion also has to be included in the differential, as well as an endogenous psychotic disorder such as schizophrenia or a manic bipolar episode. Ms. Y’s boyfriend of 16 months indicated that Ms. Y uses alcohol but cannot quantify the amount or frequency. We considered and ruled out other intoxicant use as a potential cause of her transient psychosis. An extended drug screen was negative and her lab values did not suggest heavy alcohol use.
HISTORY: Past psychotic episodes
Ms. Y’s boyfriend reports that she had 2 psychiatric hospitalizations approximately 30 years ago, which were precipitated by psychotic symptoms that she developed while abusing drugs. To the best of his knowledge Ms. Y had not used these agents recently. He stated that Ms. Y did not appear to have ongoing psychotic symptoms and had not received psychiatric treatment since she was a teenager until 6 months ago. He describes the current hospitalization as being “just like 6 months ago.”
Medical records reveal that Ms. Y was admitted to our hospital 6 months ago because she was acting violently and combative. She was “talking out of context,” “stated that she was God,” and had auditory hallucinations. She was admitted to the medical service for rhabdomyolysis, which was thought to be caused by hyperactivity or exertion. Ms. Y indicated that she was taking food supplements, including L-carnitine, to help lose weight. Her psychotic symptoms cleared within 24 hours and she was discharged without any psychiatric medications. Her behavioral disturbance was attributed to ingesting excessive amounts of carnitine supplements, and Ms. Y was counseled to abstain from them.
The authors’ observations
Carnitine is a common dietary supplement that is advertised as being safe and effective.1 It is purported to increase fat oxidation or reduce fat synthesis; however, no trials demonstrate that L-carnitine is effective for weight loss (Box).2-7 Evidence from well-designed randomized, controlled clinical trials indicates that the safe upper limit of long-term intake is 2,000 mg/d of L-carnitine equivalents.8 The data for doses >2,000 mg/d are not sufficient to make a confident conclusion on long-term safety.8
Further evaluation for possible causes of Ms. Y’s symptoms include a chest radiography and blood and urine cultures, which are unremarkable. Results of a lumbar puncture are within normal limits. Computed tomography of the head reveals confluent periventricular hypodensities compatible with moderate to severe non-specific white matter disease.
Carnitine is derived from an amino acid and found in nearly all cells of the body. “Carnitine” is used to refer to several compounds, including L-carnitine, acetyl-L-carnitine, and propionyl-L-carnitine. The natural form and the only one with biologic activity is the geometric isomer L-carnitine. Most endogenous L-carnitine is derived from diet— meat and dairy are the primary sources—and the remainder is synthesized.2-4
Carnitine transports long-chain fatty acids into the mitochondria so they can be oxidized to produce energy and transports toxic compounds out of the mitochondria to prevent them from accumulating. Carnitine is concentrated in tissues that use fatty acids as a dietary fuel, such as skeletal and cardiac muscle.2-4 The body makes enough carnitine to meet most person’s needs and supplementation typically is not required. Some drugs, such as valproic acid and carbamazepine, can reduce carnitine blood concentrations.2
Because of its role in fatty acid oxidation, carnitine often is promoted as a weight loss aid. In addition, it is purported to improve exercise performance and enhance well-being.3,4 However, there is no consistent evidence that carnitine supplements can improve physical performance in healthy individuals.5
At doses of approximately 3 g/d, carnitine supplements can cause nausea, vomiting, abdominal cramps, diarrhea, and a “fishy” body odor. Rare side effects include muscle weakness in uremic patients and seizures in those with a seizure disorder.2-4
In animal studies, carnitine persistently increases dopamine outflow in the nucleus accumbens.6 Dopamine dysregulation in this pathway has been shown to cause psychotic symptoms.7
TREATMENT: Rapid improvement
Ms. Y’s renal dysfunction resolves within 24 hours with aggressive hydration and supportive therapy. Her WBC count normalizes and her CPK decreases.
When Ms. Y becomes more cooperative, the CL team pieces together more of her story with further interviews and collateral information from her cousin. Ms. Y’s family history includes an aunt with schizophrenia. Three years ago Ms. Y moved from the Midwest to a large Southern city with her husband, from whom she is divorced. She has 2 children who were removed from her custody when she was a teenager for unclear reasons. At admission, she lives with her boyfriend, whom she plans on marrying.
Ms. Y says she was taking carnitine to improve her energy and lose weight. She recalls that her physicians advised her to discontinue carnitine supplements, but she continued to take “4 or 5 a day” in an ongoing attempt to lose weight. When asked about other supplements, Ms. Y reports regularly consuming 16-ounce energy drinks, including the day before admission. The label on this drink lists L-carnitine and caffeine as main ingredients. She denies regularly drinking other caffeine-containing beverages, including coffee, tea, or soda.
The authors’ observations
Supplements for weight loss may appeal to people’s desire for a “quick fix” that is less demanding than diets and increased physical activity. Supplements are available without a prescription and despite reports of adverse reactions generally are perceived as being safe and having few side effects. These supplements may be marketed as “natural,” which can be misinterpreted as an assurance of safety and efficacy.
Given the similarities of the current admission to the one 6 months ago, we suspect Ms. Y is experiencing transient psychosis secondary to carnitine intoxication. Based on Ms. Y’s boyfriend’s report and the product labeling, we estimate that Ms. Y took approximately 4,000 mg of carnitine in the 24 hours before admission.
Other causes of transient psychosis, such as infectious, metabolic, and neoplastic processes, were considered and ruled out. Seizures with postictal confusion also was ruled out because Ms. Y does not have a history of seizures and there is no evidence of convulsive activity, incontinence, or buccal lacerations. Given Ms. Y’s family history of schizophrenia and reported history of psychotic symptoms as a teenager we considered that she may have an endogenous psychotic disorder. However, her psychotic symptoms were transient, and Ms. Y returned to her baseline level of functioning between episodes.
OUTCOME: Advice to stop
We start Ms. Y on risperidone, 2 mg/d, at bedtime for her psychotic symptoms. Her psychotic symptoms quickly improve. She seems to return to her baseline state approximately 36 hours after admission and is medically cleared for discharge. Risperidone is discontinued after only 1 dose.
Ms. Y is remorseful over her recent aggressive behavior, and fears that her boyfriend will leave her. She denies suicidal and homicidal ideation and does not require inpatient psychiatric hospitalization. We strongly advise her to discontinue carnitine supplements and energy drinks and to limit her caffeine intake. Because Ms. Y’s had continued to use carnitine supplements despite adverse consequences and against medical advice, we refer her for substance abuse treatment.
The authors’ observations
Although temporal coincidence does not necessarily imply causality, in Ms. Y’s case, the relationship between carnitine ingestion and psychiatric symptoms cannot be ignored. Individuals predisposed to mania or psychosis may be more likely to respond adversely after ingesting nutritional supplements or energy drinks.9 Ms. Y’s past psychotic episodes suggest that she could be vulnerable to future episodes. She also might have a biologic predisposition to psychosis because of her family history of schizophrenia.
The literature contains at least 1 other reported case of carnitine-induced psychosis. A patient with a history of bipolar disorder presented with auditory hallucinations, persecutory delusions, and verbally threatening and physically assaultive behavior 5 days after beginning nutritional supplements containing carnitine.1 There also are reports of patients who experienced acute changes in mental status after consuming other nutritional weight loss supplements (Table).9-17 Chelben et al9 describe 3 patients with known psychiatric illness who showed clinical deterioration leading to psychiatric hospitalization after ingesting nutraceutical preparations. This may be a common but unrecognized cause of decompensation in psychiatric patients who take supplements.
This case highlights the importance of being aware of patients’ use of alternative medications or nutritional supplements. Physicians should routinely inquire about the use of weight loss products, energy drinks, and supplements, and patients should be educated about the risks, including potential to exacerbate pre-existing psychiatric disorders.
Table
Psychiatric effects of common weight loss supplements
| Supplement | Psychiatric effects |
|---|---|
| Caffeine | Depression, anxiety, agitation, aggression, psychosis10-12 |
| Ephedra* | Psychosis, severe depression, mania or agitation, hallucinations, sleep disturbance, suicidal ideation13 |
| Panax (ginseng) | Euphoria, mania14 |
| Amino acid-containing drinks (taurine and inositol) | Euphoria, hypervigilance, insomnia, verbal and physical aggression, impulsive behavior9,15 |
| Hypericum (St. John’s wort) | Mania,16 psychosis17 |
| *FDA removed ephedra from the market in 2003 because of adverse events | |
Related Resources
- National Library of Medicine. Dietary supplements labels database. http://dietarysupplements.nlm.nih.gov/dietary.
- Grossberg G, Fox B. The essential herb-drug-vitamin interaction guide. New York, NY: Broadway Books; 2007.
Drug Brand Names
- Carbamazepine • Tegretol
- Haloperidol • Haldol
- Risperidone • Risperdal
- Valproic acid • Depakene
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Evcimen H, Mania I, Mathews M, et al. Psychosis precipitated by acetyl-L-carnitine in a patient with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2007;9(1):71-72.
2. Office of Dietary Supplements National Institutes of Health. Dietary supplement fact sheet: carnitine. Available at: http://ods.od.nih.gov/factsheets/carnitine.asp. Accessed November 22, 2010.
3. Carnitine: lessons from one hundred years of research Ann NY Acad Sci. 2004;1033:ix-xi.
4. Rebouche CJ, Carnitine. In: Shils ME, Olson JA, Shike M, et al, eds. Modern nutrition in health and disease. 9th ed. Baltimore, MD: Lippincott Williams and Wilkins; 1999: 505-512.
5. Brass EP. Carnitine and sports medicine: use or abuse? Ann NY Acad Sci. 2004;1033:67-78.
6. Scheggi S, Rauggi R, Nanni G, et al. Repeated acetyl-L-carnitine administration increases phosphor-Thr34 DARPP-32 levels and antagonizes cocaine-induced increase in Cdk5 and phosphor-Thr75 DARPP-32 levels in rat striatum. Eur J Neurosci. 2004;19:1609-1620.
7. Howes OD, McDonald C, Cannon M, et al. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(suppl 1):S7-S13.
8. Hathcock JN, Shao A. Risk assessment for carnitine. Regul Toxicol Pharmacol. 2006;46:23-28.
9. Chelben J, Piccone-Sapir A, Ianco I, et al. Effects of amino acid energy drinks leading to hospitalization in individuals with mental illness. Gen Hosp Psychiatry. 2008;30(2):187-189.
10. Hedges DW, Woon FL, Hoopes SP. Caffeine-induced psychosis. CNS Spectr. 2009;14:127-129.
11. Broderick P, Benjamin AB. Caffeine and psychiatric symptoms: a review. J Okla State Med Assoc. 2004;97(12):538-542.
12. Cerimele JM, Stern AP, Jutras-Aswad D. Psychosis following excessive ingestion of energy drinks in a patient with schizophrenia. Am J Psychiatry. 2010;167(3):353.-
13. Maglione M, Miotto K, Iguchi M, et al. Psychiatric effects of ephedra use: an analysis of Food and Drug Administration reports of adverse events. Am J Psychiatry. 2005;162(1):189-191.
14. Engelberg D, McCutcheon A, Wiseman S. A case of ginseng-induced mania. J Clin Psychopharmacol. 2001;21(5):535-537.
15. Machado-Vieira R, Viale CI, Kapczinski F. Mania associated with an energy drink: the possible role of caffeine, taurine and inositol. Can J Psychiatry. 2001;46:454-455.
16. Fahmi M, Huang C, Schweitzer I. A case of mania induced by hypericum. World J Biol Psychiatry. 2002;3(1):58-59.
17. Stevinson C, Ernst E. Can St. John’s wort trigger psychoses? Int J Clin Pharmacol Ther. 2004;42(9):473-480.
1. Evcimen H, Mania I, Mathews M, et al. Psychosis precipitated by acetyl-L-carnitine in a patient with bipolar disorder. Prim Care Companion J Clin Psychiatry. 2007;9(1):71-72.
2. Office of Dietary Supplements National Institutes of Health. Dietary supplement fact sheet: carnitine. Available at: http://ods.od.nih.gov/factsheets/carnitine.asp. Accessed November 22, 2010.
3. Carnitine: lessons from one hundred years of research Ann NY Acad Sci. 2004;1033:ix-xi.
4. Rebouche CJ, Carnitine. In: Shils ME, Olson JA, Shike M, et al, eds. Modern nutrition in health and disease. 9th ed. Baltimore, MD: Lippincott Williams and Wilkins; 1999: 505-512.
5. Brass EP. Carnitine and sports medicine: use or abuse? Ann NY Acad Sci. 2004;1033:67-78.
6. Scheggi S, Rauggi R, Nanni G, et al. Repeated acetyl-L-carnitine administration increases phosphor-Thr34 DARPP-32 levels and antagonizes cocaine-induced increase in Cdk5 and phosphor-Thr75 DARPP-32 levels in rat striatum. Eur J Neurosci. 2004;19:1609-1620.
7. Howes OD, McDonald C, Cannon M, et al. Pathways to schizophrenia: the impact of environmental factors. Int J Neuropsychopharmacol. 2004;7(suppl 1):S7-S13.
8. Hathcock JN, Shao A. Risk assessment for carnitine. Regul Toxicol Pharmacol. 2006;46:23-28.
9. Chelben J, Piccone-Sapir A, Ianco I, et al. Effects of amino acid energy drinks leading to hospitalization in individuals with mental illness. Gen Hosp Psychiatry. 2008;30(2):187-189.
10. Hedges DW, Woon FL, Hoopes SP. Caffeine-induced psychosis. CNS Spectr. 2009;14:127-129.
11. Broderick P, Benjamin AB. Caffeine and psychiatric symptoms: a review. J Okla State Med Assoc. 2004;97(12):538-542.
12. Cerimele JM, Stern AP, Jutras-Aswad D. Psychosis following excessive ingestion of energy drinks in a patient with schizophrenia. Am J Psychiatry. 2010;167(3):353.-
13. Maglione M, Miotto K, Iguchi M, et al. Psychiatric effects of ephedra use: an analysis of Food and Drug Administration reports of adverse events. Am J Psychiatry. 2005;162(1):189-191.
14. Engelberg D, McCutcheon A, Wiseman S. A case of ginseng-induced mania. J Clin Psychopharmacol. 2001;21(5):535-537.
15. Machado-Vieira R, Viale CI, Kapczinski F. Mania associated with an energy drink: the possible role of caffeine, taurine and inositol. Can J Psychiatry. 2001;46:454-455.
16. Fahmi M, Huang C, Schweitzer I. A case of mania induced by hypericum. World J Biol Psychiatry. 2002;3(1):58-59.
17. Stevinson C, Ernst E. Can St. John’s wort trigger psychoses? Int J Clin Pharmacol Ther. 2004;42(9):473-480.
Accepting opposite beliefs
I would like to add to Dr. Henry A. Nasrallah’s editorial (“Are some nonpsychotic psychiatric disorders actually psychotic?” From the Editor, Current Psychiatry, November 2011, p. 16-19) that if we go by his definition of “delusional” then most of us are psychotic, considering that some individuals are atheists, some believe in God or gods, and all of us cannot be right. Politically, some have fixed beliefs and others have exactly the opposite fixed beliefs. Some are right, some are wrong, and some are psychotic. It is true that psychiatric conditions such as depression and anxiety can be considered psychotic and an antipsychotic can help the patient. However, what do we do with those who have fixed beliefs that do not fit into our beliefs? It happened not too long ago in totalitarian regimes where psychiatric treatment was a tool of the oppressors.
Mihai A. Chituc, MD
Psychiatrist
Kaiser Permanente
La Habra, CA
I would like to add to Dr. Henry A. Nasrallah’s editorial (“Are some nonpsychotic psychiatric disorders actually psychotic?” From the Editor, Current Psychiatry, November 2011, p. 16-19) that if we go by his definition of “delusional” then most of us are psychotic, considering that some individuals are atheists, some believe in God or gods, and all of us cannot be right. Politically, some have fixed beliefs and others have exactly the opposite fixed beliefs. Some are right, some are wrong, and some are psychotic. It is true that psychiatric conditions such as depression and anxiety can be considered psychotic and an antipsychotic can help the patient. However, what do we do with those who have fixed beliefs that do not fit into our beliefs? It happened not too long ago in totalitarian regimes where psychiatric treatment was a tool of the oppressors.
Mihai A. Chituc, MD
Psychiatrist
Kaiser Permanente
La Habra, CA
I would like to add to Dr. Henry A. Nasrallah’s editorial (“Are some nonpsychotic psychiatric disorders actually psychotic?” From the Editor, Current Psychiatry, November 2011, p. 16-19) that if we go by his definition of “delusional” then most of us are psychotic, considering that some individuals are atheists, some believe in God or gods, and all of us cannot be right. Politically, some have fixed beliefs and others have exactly the opposite fixed beliefs. Some are right, some are wrong, and some are psychotic. It is true that psychiatric conditions such as depression and anxiety can be considered psychotic and an antipsychotic can help the patient. However, what do we do with those who have fixed beliefs that do not fit into our beliefs? It happened not too long ago in totalitarian regimes where psychiatric treatment was a tool of the oppressors.
Mihai A. Chituc, MD
Psychiatrist
Kaiser Permanente
La Habra, CA
‘Shoebox’ diagnoses
I have always been a strong advocate of antipsychotic treatment, not only for major depressive disorder but also many anxiety disorders, such as posttraumatic stress disorder while in combat and obsessive–compulsive disorder, as well as hypochondriasis and some personality disorders (“Are some nonpsychotic psychiatric disorders actually psychotic?” From the Editor, Current Psychiatry, November 2010, p. 16-19).
Above all, do no harm, but our patients are suffering from severe, disturbing, restrictive, and hurtful illnesses that require any approach we have at hand. I have to admit, it’s not something I do comfortably regardless of the benefit to my patients. There is always a lawyer ready to knock at my door.
When I was practicing as a physician in New Zealand, a patient’s well being was never compromised by fear of being sued, yet I was able to obtain some much-needed relief for many of my patients.
I would like to share with you my thoughts about not forcing patients into a “shoebox” diagnosis that limits a specific treatment, as the DSM tends to do. Keep the government from dictating to doctors and keep doctors from being limited to only FDA-approved indications, when clinically we know which treatment is best, but may be subjected to legal risk. Finally, society as well as lawyers needs to stop expecting perfection, especially with illnesses where remission is not the rule and recurrence is part of the natural history of the disease.
Eduardo Lichi, MD
Major, Army Psychiatrist (Retired)
785th Medical Company, Combat Stress Control
Naples, FL
I have always been a strong advocate of antipsychotic treatment, not only for major depressive disorder but also many anxiety disorders, such as posttraumatic stress disorder while in combat and obsessive–compulsive disorder, as well as hypochondriasis and some personality disorders (“Are some nonpsychotic psychiatric disorders actually psychotic?” From the Editor, Current Psychiatry, November 2010, p. 16-19).
Above all, do no harm, but our patients are suffering from severe, disturbing, restrictive, and hurtful illnesses that require any approach we have at hand. I have to admit, it’s not something I do comfortably regardless of the benefit to my patients. There is always a lawyer ready to knock at my door.
When I was practicing as a physician in New Zealand, a patient’s well being was never compromised by fear of being sued, yet I was able to obtain some much-needed relief for many of my patients.
I would like to share with you my thoughts about not forcing patients into a “shoebox” diagnosis that limits a specific treatment, as the DSM tends to do. Keep the government from dictating to doctors and keep doctors from being limited to only FDA-approved indications, when clinically we know which treatment is best, but may be subjected to legal risk. Finally, society as well as lawyers needs to stop expecting perfection, especially with illnesses where remission is not the rule and recurrence is part of the natural history of the disease.
Eduardo Lichi, MD
Major, Army Psychiatrist (Retired)
785th Medical Company, Combat Stress Control
Naples, FL
I have always been a strong advocate of antipsychotic treatment, not only for major depressive disorder but also many anxiety disorders, such as posttraumatic stress disorder while in combat and obsessive–compulsive disorder, as well as hypochondriasis and some personality disorders (“Are some nonpsychotic psychiatric disorders actually psychotic?” From the Editor, Current Psychiatry, November 2010, p. 16-19).
Above all, do no harm, but our patients are suffering from severe, disturbing, restrictive, and hurtful illnesses that require any approach we have at hand. I have to admit, it’s not something I do comfortably regardless of the benefit to my patients. There is always a lawyer ready to knock at my door.
When I was practicing as a physician in New Zealand, a patient’s well being was never compromised by fear of being sued, yet I was able to obtain some much-needed relief for many of my patients.
I would like to share with you my thoughts about not forcing patients into a “shoebox” diagnosis that limits a specific treatment, as the DSM tends to do. Keep the government from dictating to doctors and keep doctors from being limited to only FDA-approved indications, when clinically we know which treatment is best, but may be subjected to legal risk. Finally, society as well as lawyers needs to stop expecting perfection, especially with illnesses where remission is not the rule and recurrence is part of the natural history of the disease.
Eduardo Lichi, MD
Major, Army Psychiatrist (Retired)
785th Medical Company, Combat Stress Control
Naples, FL
Busting my own myth
I enjoyed the article “From Persephone to psychiatry: Busting psychopharmacology myths” (Pearls, Current Psychiatry, September 2010, p. 40-41) because I think we all have biases, and I wanted to look at my practices. After learning about the use of multiple antipsychotics for schizophrenia despite little evidence it helps, and the fact that most panic disorder patients are not taking a selective serotonin reuptake inhibitor, I began to change what I do.
I recall a recent trial where I tried to follow algorithms until the patient reached remission. In my opinion, these types of algorithms, which are used routinely in the United Kingdom and other government-run health care systems such as the VA, take the guesswork out of our profession and allow us to trust the evidence and give our patients realistic and positive expectations for full remission or recovery regardless of the extent of their disorder. When enacted on a large scale, I also believe that following algorithms and taking out your personal biases leads to improved results by simply making people make almost automated decisions. I also agree that checking evidence and recent articles doesn’t take a lot of time.
Corey Yilmaz, MD
Adult and Child Psychiatrist
Buckeye, AZ
I enjoyed the article “From Persephone to psychiatry: Busting psychopharmacology myths” (Pearls, Current Psychiatry, September 2010, p. 40-41) because I think we all have biases, and I wanted to look at my practices. After learning about the use of multiple antipsychotics for schizophrenia despite little evidence it helps, and the fact that most panic disorder patients are not taking a selective serotonin reuptake inhibitor, I began to change what I do.
I recall a recent trial where I tried to follow algorithms until the patient reached remission. In my opinion, these types of algorithms, which are used routinely in the United Kingdom and other government-run health care systems such as the VA, take the guesswork out of our profession and allow us to trust the evidence and give our patients realistic and positive expectations for full remission or recovery regardless of the extent of their disorder. When enacted on a large scale, I also believe that following algorithms and taking out your personal biases leads to improved results by simply making people make almost automated decisions. I also agree that checking evidence and recent articles doesn’t take a lot of time.
Corey Yilmaz, MD
Adult and Child Psychiatrist
Buckeye, AZ
I enjoyed the article “From Persephone to psychiatry: Busting psychopharmacology myths” (Pearls, Current Psychiatry, September 2010, p. 40-41) because I think we all have biases, and I wanted to look at my practices. After learning about the use of multiple antipsychotics for schizophrenia despite little evidence it helps, and the fact that most panic disorder patients are not taking a selective serotonin reuptake inhibitor, I began to change what I do.
I recall a recent trial where I tried to follow algorithms until the patient reached remission. In my opinion, these types of algorithms, which are used routinely in the United Kingdom and other government-run health care systems such as the VA, take the guesswork out of our profession and allow us to trust the evidence and give our patients realistic and positive expectations for full remission or recovery regardless of the extent of their disorder. When enacted on a large scale, I also believe that following algorithms and taking out your personal biases leads to improved results by simply making people make almost automated decisions. I also agree that checking evidence and recent articles doesn’t take a lot of time.
Corey Yilmaz, MD
Adult and Child Psychiatrist
Buckeye, AZ
The heart of depression: Treating patients who have cardiovascular disease
Mrs. T, age 59, sustained an ST-elevation myocardial infarction (MI) 6 weeks ago. She has a history of hypertension, hyperlipidemia, and major depressive disorder (MDD). Before her MI, Mrs. T’s MDD was well managed with cognitive-behavioral therapy (CBT). She states that her depressive symptoms have worsened since her MI, and clinicians determine that she is experiencing an acute depressive episode severe enough to require pharmacotherapy. Past medication trials for her depression include sertraline, up to 150 mg/d, and duloxetine, 60 mg/d, but her provider determined they were ineffective after an adequate trial duration. Her hypertension is well controlled on her current regimen, which includes lisinopril, 20 mg/d, metoprolol, 50 mg/d, simvastatin, 40 mg/d, and clopidogrel, 75 mg/d. Her father experienced sudden cardiac death and her mother and younger brother have a history of severe MDD.
- Selective serotonin reuptake inhibitors, particularly citalopram and sertraline, are generally well tolerated, effective, and safe to use in patients with cardiovascular disease (CVD), although clinicians must be aware of the risk of drug-drug interactions with these agents.
- Tricyclic antidepressants and monoamine oxidase inhibitors are contraindicated in patients with CVD.
- The FDA warns against using desipramine in patients with cardiovascular disease; fluoxetine and other CYP2C19 inhibitors may reduce the efficacy of clopidogrel.
- Whether pharmacologic or nonpharmacologic treatment of depression improves long-term cardiac outcomes needs to be clarified with sufficiently powered studies.
Depression is more prevalent in patients with cardiovascular disease (CVD) than in the general population, with estimates as high as 23%.1 Possible mechanisms to help explain the relationship between CVD and depression are summarized in Table 1.2 Appropriate antidepressant selection and depression management strategies in patients with CVD, particularly after MI, may reduce the risk for additional cardiac events and reduce mortality.1,2
Table 1
The link between depression and cardiovascular disease
Depressed patients with CVD often exhibit:
|
Other factors that impact cardiovascular risk in patients with depression include:
|
| CRP: C-reactive protein; CVD: cardiovascular disease; TNF: tumor necrosis factor Source: Reference 2 |
Antidepressant choices by class
Many older antidepressants, including tricyclic antidepressants (TCAs), are:
- contraindicated during acute recovery from MI
- cardiotoxic
- lethal in overdose
- not recommended for patients with CVD.1,3
The FDA recently mandated additional labeling for desipramine to alert health care providers to the risk of using this agent in patients with CVD or a family history of sudden death, arrhythmias, or conduction abnormalities.3 Similar to TCAs, monoamine oxidase inhibitors (MAOIs) generally are not recommended for use in this population because of the risk of hypertensive crisis, orthostatic hypotension, tachycardia, and increased QTc interval.2 Trazodone, which might help relieve insomnia, is associated with orthostasis and tachycardia. These effects may occur more frequently in patients with cardiac disease.4
Selective serotonin reuptake inhibitors (SSRIs) are effective antidepressants post-MI, have antiplatelet activity, and may improve surrogate markers of cardiac risk, although further research is needed.5,6 Individual SSRIs vary in their effects on the cytochrome P450 (CYP450) system, and therefore carry different risks of drug-drug interactions (see Related Resources).
The cardiovascular impact of serotonin-norepinephrine reuptake inhibitors is unknown. These agents may be associated with hypertension and tachycardia.7 Additional research on the use of bupropion and mirtazapine in patients with CVD also is warranted. However, bupropion has been used to help patients with CVD stop smoking and likely is safe, although it may be associated with an increase in blood pressure.2,7 Bupropion and mirtazapine also can affect appetite and weight, which require monitoring in CVD patients. The Myocardial Infarction and Depression-Intervention Trial (MIND-IT) reveals that antidepressant treatment with mirtazapine or citalopram does not increase the incidence of cardiac events and does not improve long-term depression status compared with treatment as usual in depressed post-MI patients.8 Orthostatic hypotension is a possible adverse effect of mirtazapine,7 and this antidepressant may reduce the antihypertensive effect of clonidine.
Monitor for interactions
Drug interaction databases—including Micromedex, Lexi-Comp, or Facts and Comparisons—can differ with regard to identifying and classifying drug interactions. Therefore, individual clinicians often carry the burden of recognizing potential drug-drug interactions. Preskorn and Flockhart9 suggest developing a “personal formulary” of the medications clinicians regularly prescribe to minimize drug-drug interactions. This formulary includes knowledge of a drug’s:
- enzymes responsible for elimination
- half-life
- relevant clinical trials
- receptor affinities
- common adverse effects.
Following these recommendations reveals several considerations when selecting an antidepressant for Mrs. T:
- Studies of SSRIs have shown them to be safe and well tolerated in post-MI patients. Because Mrs. T failed only 1 previous SSRI trial (sertraline), it would be reasonable to select an alternate agent within this class.
- An FDA alert highlights the risk of using clopidogrel in combination with drugs such as omeprazole, ketoconazole, fluoxetine, and fluvoxamine.10 These medications are CYP2C19 inhibitors, which can reduce clopidogrel’s effect by inhibiting conversion of the parent drug to its active metabolite.
- Adding a strong CYP2D6 inhibitor, such as fluoxetine or paroxetine, could increase the effects of metoprolol, which is a CYP2D6 substrate.11
Cardiac outcomes
Evidence is insufficient to ascertain whether pharmacologic management of depression can reduce the risk of future cardiac events. Data evaluating SSRIs’ effects on cardiac outcomes are equivocal5,12,13 and limited by inadequate power.14,15 Preliminary evidence suggests patients who respond to antidepressant treatment may have improved cardiovascular outcomes.16,17 Evidence obtained from the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART), the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial, and the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial suggest the SSRIs sertraline and citalopram can be used safely, with minimal bleeding risk, to treat depression in CVD patients (Table 2).14,15,18 When treating depressed patients who have CVD, remember to include nonpharmacologic options, such as psychotherapy, in the treatment plan, although studies have not yet shown improved cardiovascular mortality rates in patients receiving CBT.8,17,18
Although other SSRIs may be helpful for Mrs. T, citalopram is one of the best-studied agents post-MI, with the CREATE study supporting its efficacy and tolerability in this population. Citalopram has negligible drug interactions, although it is a weak inhibitor of CYP2D6 and the possibility of increasing metoprolol’s effects should be monitored. All SSRIs are associated with an increased risk of bleeding in patients receiving antiplatelet therapy; however, in Mrs. T’s case the risks are minimal, which makes citalopram a reasonable option. CBT also could be resumed to optimize Mrs. T’s treatment.
Table 2
SSRIs and cardiovascular disease: Results from RCTs
| Study | Design | Results |
|---|---|---|
| SADHART14 | Randomized, double-blind trial of sertraline vs placebo for 24 weeks for depression following MI or unstable angina (N=369) | Sertraline was more effective than placebo as measured by CGI-I, but not HAM-D in the total sample; both measures demonstrated statistical significance in patients with a history of MDD and those with HAM-D score >18 with 2 past episodes of MDD; incidence of severe cardiovascular events was 14.5% with sertraline and 22.4% with placebo (P=NS) |
| ENRICHD18 | Randomized, double-blind, controlled trial of early CBT supplemented with SSRI (usually sertraline) if necessary vs usual care for depression and low perceived social support after MI (N=2,481) | Intervention had a modest effect on depressive symptoms; antidepressant use reduced the risk of death or nonfatal MI |
| CREATE15 | Randomized, controlled, 12-week, parallel-group trial of 284 depressed patients with coronary artery disease first randomized to weekly interpersonal psychotherapy for 12 weeks plus clinical management or clinical management only, then randomized to citalopram or placebo for 12 weeks | Although the effect size was small, citalopram was more effective for depression than placebo and did not differ in effect on cardiac parameters, such as blood pressure, heart rate, or ECG change (P=.005) |
| CBT: cognitive-behavioral therapy; CGI-I: Clinical Global Impressions-Improvement scale; CREATE: Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy; ENRICHD: Enhancing Recovery in Coronary Heart Disease Patients; HAM-D: Hamilton Depression Rating Scale; MDD: major depressive disorder; MI: myocardial infarction; NS: nonsignificant; RCT: randomized controlled trial; SADHART: Sertraline Antidepressant Heart Attack Randomized Trial; SSRI: selective serotonin reuptake inhibitor | ||
- Summers KM, Martin KE, Watson K. Impact and clinical management of depression in patients with coronary artery disease. Pharmacotherapy. 2010;30:304-322.
- Indiana University School of Medicine. P450 drug interaction table. Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clopidogrel • Plavix
- Desipramine • Norpramin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Lisinopril • Prinivil, Zestril
- Metoprolol • Lopressor, Toprol
- Mirtazapine • Remeron
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Sertraline • Zoloft
- Simvastatin • Zocor
- Trazodone • Desyrel
Disclosure
Dr. Bostwick reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580-592.
2. Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 suppl 2):S20-27.
3. Norpramin [package insert]. Bridgewater, NJ: sanofi-aventis; 2009.
4. American Psychiatric Association. Treatment of patients with major depressive disorder, 3rd ed. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx. Accessed December 2, 2010.
5. Bush DE, Ziegelstein RC, Patel UV, et al. Post-myocardial infarction depression. Summary, evidence report/ technology assessment: number 123. Rockville, MD: Agency for Healthcare Research and Quality; May 2005. AHRQ publication 05-E018-1.
6. Roose SP, Miyazaki M. Pharmacologic treatment of depression in patients with heart disease. Psychosom Med. 2005;(67 suppl 1):S54-57.
7. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23:754-771.
8. van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460-466.
9. Preskorn SH, Flockhart D. 2006 guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
10. U.S. Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/
DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed January 24, 2010.
11. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
12. Von Ruden AE, Adson DE, Kotlyar M. Effect of selective serotonin reuptake inhibitors on cardiovascular morbidity and mortality. J Cardiovasc Pharmacol Ther. 2008;13(1):32-40.
13. Glassman A. Depression and cardiovascular disease. Pharmacopsychiatry. 2008;41(6):221-225.
14. Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
15. Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367-379.
16. de Jonge P, Honig A, van Melle JP, et al. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry. 2007;164:1371-1378.
17. Summers KM, Martin KE, Watson K. Impact and clinical management of depression in patients with coronary artery disease. Pharmacotherapy. 2010;30:304-322.
18. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106-3116.
Mrs. T, age 59, sustained an ST-elevation myocardial infarction (MI) 6 weeks ago. She has a history of hypertension, hyperlipidemia, and major depressive disorder (MDD). Before her MI, Mrs. T’s MDD was well managed with cognitive-behavioral therapy (CBT). She states that her depressive symptoms have worsened since her MI, and clinicians determine that she is experiencing an acute depressive episode severe enough to require pharmacotherapy. Past medication trials for her depression include sertraline, up to 150 mg/d, and duloxetine, 60 mg/d, but her provider determined they were ineffective after an adequate trial duration. Her hypertension is well controlled on her current regimen, which includes lisinopril, 20 mg/d, metoprolol, 50 mg/d, simvastatin, 40 mg/d, and clopidogrel, 75 mg/d. Her father experienced sudden cardiac death and her mother and younger brother have a history of severe MDD.
- Selective serotonin reuptake inhibitors, particularly citalopram and sertraline, are generally well tolerated, effective, and safe to use in patients with cardiovascular disease (CVD), although clinicians must be aware of the risk of drug-drug interactions with these agents.
- Tricyclic antidepressants and monoamine oxidase inhibitors are contraindicated in patients with CVD.
- The FDA warns against using desipramine in patients with cardiovascular disease; fluoxetine and other CYP2C19 inhibitors may reduce the efficacy of clopidogrel.
- Whether pharmacologic or nonpharmacologic treatment of depression improves long-term cardiac outcomes needs to be clarified with sufficiently powered studies.
Depression is more prevalent in patients with cardiovascular disease (CVD) than in the general population, with estimates as high as 23%.1 Possible mechanisms to help explain the relationship between CVD and depression are summarized in Table 1.2 Appropriate antidepressant selection and depression management strategies in patients with CVD, particularly after MI, may reduce the risk for additional cardiac events and reduce mortality.1,2
Table 1
The link between depression and cardiovascular disease
Depressed patients with CVD often exhibit:
|
Other factors that impact cardiovascular risk in patients with depression include:
|
| CRP: C-reactive protein; CVD: cardiovascular disease; TNF: tumor necrosis factor Source: Reference 2 |
Antidepressant choices by class
Many older antidepressants, including tricyclic antidepressants (TCAs), are:
- contraindicated during acute recovery from MI
- cardiotoxic
- lethal in overdose
- not recommended for patients with CVD.1,3
The FDA recently mandated additional labeling for desipramine to alert health care providers to the risk of using this agent in patients with CVD or a family history of sudden death, arrhythmias, or conduction abnormalities.3 Similar to TCAs, monoamine oxidase inhibitors (MAOIs) generally are not recommended for use in this population because of the risk of hypertensive crisis, orthostatic hypotension, tachycardia, and increased QTc interval.2 Trazodone, which might help relieve insomnia, is associated with orthostasis and tachycardia. These effects may occur more frequently in patients with cardiac disease.4
Selective serotonin reuptake inhibitors (SSRIs) are effective antidepressants post-MI, have antiplatelet activity, and may improve surrogate markers of cardiac risk, although further research is needed.5,6 Individual SSRIs vary in their effects on the cytochrome P450 (CYP450) system, and therefore carry different risks of drug-drug interactions (see Related Resources).
The cardiovascular impact of serotonin-norepinephrine reuptake inhibitors is unknown. These agents may be associated with hypertension and tachycardia.7 Additional research on the use of bupropion and mirtazapine in patients with CVD also is warranted. However, bupropion has been used to help patients with CVD stop smoking and likely is safe, although it may be associated with an increase in blood pressure.2,7 Bupropion and mirtazapine also can affect appetite and weight, which require monitoring in CVD patients. The Myocardial Infarction and Depression-Intervention Trial (MIND-IT) reveals that antidepressant treatment with mirtazapine or citalopram does not increase the incidence of cardiac events and does not improve long-term depression status compared with treatment as usual in depressed post-MI patients.8 Orthostatic hypotension is a possible adverse effect of mirtazapine,7 and this antidepressant may reduce the antihypertensive effect of clonidine.
Monitor for interactions
Drug interaction databases—including Micromedex, Lexi-Comp, or Facts and Comparisons—can differ with regard to identifying and classifying drug interactions. Therefore, individual clinicians often carry the burden of recognizing potential drug-drug interactions. Preskorn and Flockhart9 suggest developing a “personal formulary” of the medications clinicians regularly prescribe to minimize drug-drug interactions. This formulary includes knowledge of a drug’s:
- enzymes responsible for elimination
- half-life
- relevant clinical trials
- receptor affinities
- common adverse effects.
Following these recommendations reveals several considerations when selecting an antidepressant for Mrs. T:
- Studies of SSRIs have shown them to be safe and well tolerated in post-MI patients. Because Mrs. T failed only 1 previous SSRI trial (sertraline), it would be reasonable to select an alternate agent within this class.
- An FDA alert highlights the risk of using clopidogrel in combination with drugs such as omeprazole, ketoconazole, fluoxetine, and fluvoxamine.10 These medications are CYP2C19 inhibitors, which can reduce clopidogrel’s effect by inhibiting conversion of the parent drug to its active metabolite.
- Adding a strong CYP2D6 inhibitor, such as fluoxetine or paroxetine, could increase the effects of metoprolol, which is a CYP2D6 substrate.11
Cardiac outcomes
Evidence is insufficient to ascertain whether pharmacologic management of depression can reduce the risk of future cardiac events. Data evaluating SSRIs’ effects on cardiac outcomes are equivocal5,12,13 and limited by inadequate power.14,15 Preliminary evidence suggests patients who respond to antidepressant treatment may have improved cardiovascular outcomes.16,17 Evidence obtained from the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART), the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial, and the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial suggest the SSRIs sertraline and citalopram can be used safely, with minimal bleeding risk, to treat depression in CVD patients (Table 2).14,15,18 When treating depressed patients who have CVD, remember to include nonpharmacologic options, such as psychotherapy, in the treatment plan, although studies have not yet shown improved cardiovascular mortality rates in patients receiving CBT.8,17,18
Although other SSRIs may be helpful for Mrs. T, citalopram is one of the best-studied agents post-MI, with the CREATE study supporting its efficacy and tolerability in this population. Citalopram has negligible drug interactions, although it is a weak inhibitor of CYP2D6 and the possibility of increasing metoprolol’s effects should be monitored. All SSRIs are associated with an increased risk of bleeding in patients receiving antiplatelet therapy; however, in Mrs. T’s case the risks are minimal, which makes citalopram a reasonable option. CBT also could be resumed to optimize Mrs. T’s treatment.
Table 2
SSRIs and cardiovascular disease: Results from RCTs
| Study | Design | Results |
|---|---|---|
| SADHART14 | Randomized, double-blind trial of sertraline vs placebo for 24 weeks for depression following MI or unstable angina (N=369) | Sertraline was more effective than placebo as measured by CGI-I, but not HAM-D in the total sample; both measures demonstrated statistical significance in patients with a history of MDD and those with HAM-D score >18 with 2 past episodes of MDD; incidence of severe cardiovascular events was 14.5% with sertraline and 22.4% with placebo (P=NS) |
| ENRICHD18 | Randomized, double-blind, controlled trial of early CBT supplemented with SSRI (usually sertraline) if necessary vs usual care for depression and low perceived social support after MI (N=2,481) | Intervention had a modest effect on depressive symptoms; antidepressant use reduced the risk of death or nonfatal MI |
| CREATE15 | Randomized, controlled, 12-week, parallel-group trial of 284 depressed patients with coronary artery disease first randomized to weekly interpersonal psychotherapy for 12 weeks plus clinical management or clinical management only, then randomized to citalopram or placebo for 12 weeks | Although the effect size was small, citalopram was more effective for depression than placebo and did not differ in effect on cardiac parameters, such as blood pressure, heart rate, or ECG change (P=.005) |
| CBT: cognitive-behavioral therapy; CGI-I: Clinical Global Impressions-Improvement scale; CREATE: Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy; ENRICHD: Enhancing Recovery in Coronary Heart Disease Patients; HAM-D: Hamilton Depression Rating Scale; MDD: major depressive disorder; MI: myocardial infarction; NS: nonsignificant; RCT: randomized controlled trial; SADHART: Sertraline Antidepressant Heart Attack Randomized Trial; SSRI: selective serotonin reuptake inhibitor | ||
- Summers KM, Martin KE, Watson K. Impact and clinical management of depression in patients with coronary artery disease. Pharmacotherapy. 2010;30:304-322.
- Indiana University School of Medicine. P450 drug interaction table. Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clopidogrel • Plavix
- Desipramine • Norpramin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Lisinopril • Prinivil, Zestril
- Metoprolol • Lopressor, Toprol
- Mirtazapine • Remeron
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Sertraline • Zoloft
- Simvastatin • Zocor
- Trazodone • Desyrel
Disclosure
Dr. Bostwick reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mrs. T, age 59, sustained an ST-elevation myocardial infarction (MI) 6 weeks ago. She has a history of hypertension, hyperlipidemia, and major depressive disorder (MDD). Before her MI, Mrs. T’s MDD was well managed with cognitive-behavioral therapy (CBT). She states that her depressive symptoms have worsened since her MI, and clinicians determine that she is experiencing an acute depressive episode severe enough to require pharmacotherapy. Past medication trials for her depression include sertraline, up to 150 mg/d, and duloxetine, 60 mg/d, but her provider determined they were ineffective after an adequate trial duration. Her hypertension is well controlled on her current regimen, which includes lisinopril, 20 mg/d, metoprolol, 50 mg/d, simvastatin, 40 mg/d, and clopidogrel, 75 mg/d. Her father experienced sudden cardiac death and her mother and younger brother have a history of severe MDD.
- Selective serotonin reuptake inhibitors, particularly citalopram and sertraline, are generally well tolerated, effective, and safe to use in patients with cardiovascular disease (CVD), although clinicians must be aware of the risk of drug-drug interactions with these agents.
- Tricyclic antidepressants and monoamine oxidase inhibitors are contraindicated in patients with CVD.
- The FDA warns against using desipramine in patients with cardiovascular disease; fluoxetine and other CYP2C19 inhibitors may reduce the efficacy of clopidogrel.
- Whether pharmacologic or nonpharmacologic treatment of depression improves long-term cardiac outcomes needs to be clarified with sufficiently powered studies.
Depression is more prevalent in patients with cardiovascular disease (CVD) than in the general population, with estimates as high as 23%.1 Possible mechanisms to help explain the relationship between CVD and depression are summarized in Table 1.2 Appropriate antidepressant selection and depression management strategies in patients with CVD, particularly after MI, may reduce the risk for additional cardiac events and reduce mortality.1,2
Table 1
The link between depression and cardiovascular disease
Depressed patients with CVD often exhibit:
|
Other factors that impact cardiovascular risk in patients with depression include:
|
| CRP: C-reactive protein; CVD: cardiovascular disease; TNF: tumor necrosis factor Source: Reference 2 |
Antidepressant choices by class
Many older antidepressants, including tricyclic antidepressants (TCAs), are:
- contraindicated during acute recovery from MI
- cardiotoxic
- lethal in overdose
- not recommended for patients with CVD.1,3
The FDA recently mandated additional labeling for desipramine to alert health care providers to the risk of using this agent in patients with CVD or a family history of sudden death, arrhythmias, or conduction abnormalities.3 Similar to TCAs, monoamine oxidase inhibitors (MAOIs) generally are not recommended for use in this population because of the risk of hypertensive crisis, orthostatic hypotension, tachycardia, and increased QTc interval.2 Trazodone, which might help relieve insomnia, is associated with orthostasis and tachycardia. These effects may occur more frequently in patients with cardiac disease.4
Selective serotonin reuptake inhibitors (SSRIs) are effective antidepressants post-MI, have antiplatelet activity, and may improve surrogate markers of cardiac risk, although further research is needed.5,6 Individual SSRIs vary in their effects on the cytochrome P450 (CYP450) system, and therefore carry different risks of drug-drug interactions (see Related Resources).
The cardiovascular impact of serotonin-norepinephrine reuptake inhibitors is unknown. These agents may be associated with hypertension and tachycardia.7 Additional research on the use of bupropion and mirtazapine in patients with CVD also is warranted. However, bupropion has been used to help patients with CVD stop smoking and likely is safe, although it may be associated with an increase in blood pressure.2,7 Bupropion and mirtazapine also can affect appetite and weight, which require monitoring in CVD patients. The Myocardial Infarction and Depression-Intervention Trial (MIND-IT) reveals that antidepressant treatment with mirtazapine or citalopram does not increase the incidence of cardiac events and does not improve long-term depression status compared with treatment as usual in depressed post-MI patients.8 Orthostatic hypotension is a possible adverse effect of mirtazapine,7 and this antidepressant may reduce the antihypertensive effect of clonidine.
Monitor for interactions
Drug interaction databases—including Micromedex, Lexi-Comp, or Facts and Comparisons—can differ with regard to identifying and classifying drug interactions. Therefore, individual clinicians often carry the burden of recognizing potential drug-drug interactions. Preskorn and Flockhart9 suggest developing a “personal formulary” of the medications clinicians regularly prescribe to minimize drug-drug interactions. This formulary includes knowledge of a drug’s:
- enzymes responsible for elimination
- half-life
- relevant clinical trials
- receptor affinities
- common adverse effects.
Following these recommendations reveals several considerations when selecting an antidepressant for Mrs. T:
- Studies of SSRIs have shown them to be safe and well tolerated in post-MI patients. Because Mrs. T failed only 1 previous SSRI trial (sertraline), it would be reasonable to select an alternate agent within this class.
- An FDA alert highlights the risk of using clopidogrel in combination with drugs such as omeprazole, ketoconazole, fluoxetine, and fluvoxamine.10 These medications are CYP2C19 inhibitors, which can reduce clopidogrel’s effect by inhibiting conversion of the parent drug to its active metabolite.
- Adding a strong CYP2D6 inhibitor, such as fluoxetine or paroxetine, could increase the effects of metoprolol, which is a CYP2D6 substrate.11
Cardiac outcomes
Evidence is insufficient to ascertain whether pharmacologic management of depression can reduce the risk of future cardiac events. Data evaluating SSRIs’ effects on cardiac outcomes are equivocal5,12,13 and limited by inadequate power.14,15 Preliminary evidence suggests patients who respond to antidepressant treatment may have improved cardiovascular outcomes.16,17 Evidence obtained from the Sertraline Antidepressant Heart Attack Randomized Trial (SADHART), the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial, and the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial suggest the SSRIs sertraline and citalopram can be used safely, with minimal bleeding risk, to treat depression in CVD patients (Table 2).14,15,18 When treating depressed patients who have CVD, remember to include nonpharmacologic options, such as psychotherapy, in the treatment plan, although studies have not yet shown improved cardiovascular mortality rates in patients receiving CBT.8,17,18
Although other SSRIs may be helpful for Mrs. T, citalopram is one of the best-studied agents post-MI, with the CREATE study supporting its efficacy and tolerability in this population. Citalopram has negligible drug interactions, although it is a weak inhibitor of CYP2D6 and the possibility of increasing metoprolol’s effects should be monitored. All SSRIs are associated with an increased risk of bleeding in patients receiving antiplatelet therapy; however, in Mrs. T’s case the risks are minimal, which makes citalopram a reasonable option. CBT also could be resumed to optimize Mrs. T’s treatment.
Table 2
SSRIs and cardiovascular disease: Results from RCTs
| Study | Design | Results |
|---|---|---|
| SADHART14 | Randomized, double-blind trial of sertraline vs placebo for 24 weeks for depression following MI or unstable angina (N=369) | Sertraline was more effective than placebo as measured by CGI-I, but not HAM-D in the total sample; both measures demonstrated statistical significance in patients with a history of MDD and those with HAM-D score >18 with 2 past episodes of MDD; incidence of severe cardiovascular events was 14.5% with sertraline and 22.4% with placebo (P=NS) |
| ENRICHD18 | Randomized, double-blind, controlled trial of early CBT supplemented with SSRI (usually sertraline) if necessary vs usual care for depression and low perceived social support after MI (N=2,481) | Intervention had a modest effect on depressive symptoms; antidepressant use reduced the risk of death or nonfatal MI |
| CREATE15 | Randomized, controlled, 12-week, parallel-group trial of 284 depressed patients with coronary artery disease first randomized to weekly interpersonal psychotherapy for 12 weeks plus clinical management or clinical management only, then randomized to citalopram or placebo for 12 weeks | Although the effect size was small, citalopram was more effective for depression than placebo and did not differ in effect on cardiac parameters, such as blood pressure, heart rate, or ECG change (P=.005) |
| CBT: cognitive-behavioral therapy; CGI-I: Clinical Global Impressions-Improvement scale; CREATE: Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy; ENRICHD: Enhancing Recovery in Coronary Heart Disease Patients; HAM-D: Hamilton Depression Rating Scale; MDD: major depressive disorder; MI: myocardial infarction; NS: nonsignificant; RCT: randomized controlled trial; SADHART: Sertraline Antidepressant Heart Attack Randomized Trial; SSRI: selective serotonin reuptake inhibitor | ||
- Summers KM, Martin KE, Watson K. Impact and clinical management of depression in patients with coronary artery disease. Pharmacotherapy. 2010;30:304-322.
- Indiana University School of Medicine. P450 drug interaction table. Indiana University School of Medicine. http://medicine.iupui.edu/clinpharm/ddis/table.asp.
Drug Brand Names
- Bupropion • Wellbutrin, Zyban
- Citalopram • Celexa
- Clopidogrel • Plavix
- Desipramine • Norpramin
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Ketoconazole • Nizoral
- Lisinopril • Prinivil, Zestril
- Metoprolol • Lopressor, Toprol
- Mirtazapine • Remeron
- Omeprazole • Prilosec
- Paroxetine • Paxil
- Sertraline • Zoloft
- Simvastatin • Zocor
- Trazodone • Desyrel
Disclosure
Dr. Bostwick reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580-592.
2. Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 suppl 2):S20-27.
3. Norpramin [package insert]. Bridgewater, NJ: sanofi-aventis; 2009.
4. American Psychiatric Association. Treatment of patients with major depressive disorder, 3rd ed. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx. Accessed December 2, 2010.
5. Bush DE, Ziegelstein RC, Patel UV, et al. Post-myocardial infarction depression. Summary, evidence report/ technology assessment: number 123. Rockville, MD: Agency for Healthcare Research and Quality; May 2005. AHRQ publication 05-E018-1.
6. Roose SP, Miyazaki M. Pharmacologic treatment of depression in patients with heart disease. Psychosom Med. 2005;(67 suppl 1):S54-57.
7. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23:754-771.
8. van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460-466.
9. Preskorn SH, Flockhart D. 2006 guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
10. U.S. Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/
DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed January 24, 2010.
11. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
12. Von Ruden AE, Adson DE, Kotlyar M. Effect of selective serotonin reuptake inhibitors on cardiovascular morbidity and mortality. J Cardiovasc Pharmacol Ther. 2008;13(1):32-40.
13. Glassman A. Depression and cardiovascular disease. Pharmacopsychiatry. 2008;41(6):221-225.
14. Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
15. Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367-379.
16. de Jonge P, Honig A, van Melle JP, et al. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry. 2007;164:1371-1378.
17. Summers KM, Martin KE, Watson K. Impact and clinical management of depression in patients with coronary artery disease. Pharmacotherapy. 2010;30:304-322.
18. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106-3116.
1. Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55:580-592.
2. Carney RM, Freedland KE. Depression in patients with coronary heart disease. Am J Med. 2008;121(11 suppl 2):S20-27.
3. Norpramin [package insert]. Bridgewater, NJ: sanofi-aventis; 2009.
4. American Psychiatric Association. Treatment of patients with major depressive disorder, 3rd ed. Available at: http://www.psychiatryonline.com/pracGuide/pracGuideChapToc_7.aspx. Accessed December 2, 2010.
5. Bush DE, Ziegelstein RC, Patel UV, et al. Post-myocardial infarction depression. Summary, evidence report/ technology assessment: number 123. Rockville, MD: Agency for Healthcare Research and Quality; May 2005. AHRQ publication 05-E018-1.
6. Roose SP, Miyazaki M. Pharmacologic treatment of depression in patients with heart disease. Psychosom Med. 2005;(67 suppl 1):S54-57.
7. Alvarez W Jr, Pickworth KK. Safety of antidepressant drugs in the patient with cardiac disease: a review of the literature. Pharmacotherapy. 2003;23:754-771.
8. van Melle JP, de Jonge P, Honig A, et al. Effects of antidepressant treatment following myocardial infarction. Br J Psychiatry. 2007;190:460-466.
9. Preskorn SH, Flockhart D. 2006 guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
10. U.S. Food and Drug Administration. Information for healthcare professionals: update to the labeling of clopidogrel bisulfate (marketed as Plavix) to alert healthcare professionals about a drug interaction with omeprazole (marketed as Prilosec and Prilosec OTC). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/
DrugSafetyInformationforHeathcareProfessionals/ucm190787.htm. Accessed January 24, 2010.
11. Spina E, Santoro V, D’Arrigo C. Clinically relevant pharmacokinetic drug interactions with second-generation antidepressants: an update. Clin Ther. 2008;30:1206-1227.
12. Von Ruden AE, Adson DE, Kotlyar M. Effect of selective serotonin reuptake inhibitors on cardiovascular morbidity and mortality. J Cardiovasc Pharmacol Ther. 2008;13(1):32-40.
13. Glassman A. Depression and cardiovascular disease. Pharmacopsychiatry. 2008;41(6):221-225.
14. Glassman AH, O’Connor CM, Califf RM, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701-709.
15. Lespérance F, Frasure-Smith N, Koszycki D, et al. Effects of citalopram and interpersonal psychotherapy on depression in patients with coronary artery disease: the Canadian Cardiac Randomized Evaluation of Antidepressant and Psychotherapy Efficacy (CREATE) trial. JAMA. 2007;297:367-379.
16. de Jonge P, Honig A, van Melle JP, et al. Nonresponse to treatment for depression following myocardial infarction: association with subsequent cardiac events. Am J Psychiatry. 2007;164:1371-1378.
17. Summers KM, Martin KE, Watson K. Impact and clinical management of depression in patients with coronary artery disease. Pharmacotherapy. 2010;30:304-322.
18. Berkman LF, Blumenthal J, Burg M, et al. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) Randomized Trial. JAMA 2003;289:3106-3116.
Lurasidone for schizophrenia
In October 2010, the FDA approved lurasidone for the acute treatment of schizophrenia at a dose of 40 or 80 mg/d administered once daily with food (Table 1).
Table 1
Lurasidone: Fast facts
| Brand name: Latuda |
| Indication: Schizophrenia in adults |
| Approval date: October 28, 2010 |
| Availability date: February 2011 |
| Manufacturer: Sunovion Pharmaceuticals, Inc. |
| Dosing forms: 40 mg and 80 mg tablets |
| Recommended dosage: Starting dose: 40 mg/d. Maximum dose: 80 mg/d |
How it works
Although the drug’s exact mechanism of action is not known, it is thought that lurasidone’s antipsychotic properties are related to its antagonism at serotonin 2A (5-HT2A) and dopamine D2 receptors.1
Similar to most other atypical antipsychotics, lurasidone has high binding affinity for 5-HT2A and D2. Lurasidone has also high binding affinity for 5-HT7, 5-HT1A, and α2C-adrenergic receptors, low affinity for α-1 receptors, and virtually no affinity for H1 and M1 receptors (Table 2). Activity on 5-HT7, 5-HT1A, and α2C-adrenergic receptors is believed to enhance cognition, and 5-HT7 is being studied for a potential role in mood regulation and sensory processing.2,3 Lurasidone’s low activity on α-1, H1, and M1 receptors suggests a low risk of orthostatic hypotension, H1-mediated sedation and weight gain, and H1- and M1-mediated cognitive blunting.
Pharmacokinetics
Lurasidone is absorbed in the gastrointestinal tract. It reaches maximum concentration (Cmax) in 1 to 3 hours. Cmax doubles when lurasidone is administered with food (≥350 calories), but absorption is independent of the meal’s fat content.4 After absorption, the drug is highly bound (99%) to serum proteins (albumin and α-1-glycoprotein). The elimination half-life is 18 hours and steady-state concentration is reached within 7 days.1 Lurasidone is eliminated predominantly through cytochrome P450 (CYP) 3A4 metabolism in the liver.
Efficacy
Lurasidone’s efficacy for treatment of acute schizophrenia was established in four 6-week, randomized placebo-controlled clinical trials.1 The patients were adults (mean age: 38.8; range: 18 to 72) who met DSM-IVTR criteria for schizophrenia, didn’t abuse drugs or alcohol, and had not taken any investigational drug for ≥1 month. Symptoms were measured on the Positive and Negative Syndrome Scale (PANSS); Brief Psychiatric Rating Scale as derived from the PANSS (BPRSd); and the Clinical Global Impressions-Severity scale (CGI-S).
In the first clinical trial, 145 patients were randomized to lurasidone, 40 mg/d or 120 mg/d, or placebo. Treatment with either dose of lurasidone was superior to treatment with placebo on the BPRSd (Least Squares Mean [LSM] difference from placebo in change from baseline: -5.6 on lurasidone 40 mg/d, -6.7 on lurasidone 120 mg/d) and CGI-S.1,5
The second trial randomized 180 patients to lurasidone, 80 mg/d, or placebo. Lurasidone, 80 mg/d, was superior to placebo as measured on the BPRSd (LSM difference from placebo in change from baseline: -4.7 on lurasidone 80 mg/d) and CGI-S.1,6
The third trial randomized 489 patients to lurasidone, 40 mg/d, 80 mg/d, 120 mg/d, or placebo. All lurasidone arms were superior to placebo on PANSS (LSM difference from placebo in change from baseline: -2.1 on 40 mg/d, -6.4 on 80 mg/d, and -3.5 on 120 mg/d) and CGI-S scores. This study also showed that lurasidone appears to have a rapid onset of action (day 3 to 4) and provides sustained improvement of symptoms.1
In the fourth trial, 473 individuals were randomized to lurasidone, 40 mg/d or 120 mg/d, olanzapine, 15 mg/d, or placebo. Olanzapine and both dosages of lurasidone were superior to placebo in improving PANSS scores (LSM difference from placebo in change from baseline: -9.7 on lurasidone 40 mg/d, -7.5 on lurasidone 120 mg/d, and -12.6 on olanzapine 15 mg/d) and CGI-S.1,7 Both doses of lurasidone were not superior to olanzapine but had less negative impact on lipid profile, weight gain, and glycemia.
Tolerability
Tolerability information is extracted from a clinical study database consisting of 2,096 patients with schizophrenia who participated in premarketing clinical trials and were exposed to single or multiple doses of lurasidone, 20 mg, 40 mg, 80 mg, or 120 mg.1 Overall, lurasidone was well tolerated. The rate of discontinuation from clinical trials because of adverse effects was 9.4% for lurasidone vs 5.9% for placebo. Somnolence, akathisia, nausea, parkinsonism, and agitation were the most commonly reported adverse reactions; somnolence and akathisia appear dose-related. Other adverse effects associated with lurasidone were nausea, vomiting, dyspepsia, dystonia, dizziness, insomnia, agitation, and anxiety (Table 2).
Metabolic changes (hyperlipidemia, hyperglycemia, and increased body weight) associated with cardiovascular risk in patients treated with atypical antipsychotics were studied in short-term placebo-controlled trials. Lurasidone is considered to be weight-neutral and does not have significant effects on serum lipids or glucose.2 As is the case with other D2 antagonists, lurasidone is associated with increased prolactin, which appears to be greater in females and is dose-dependent. Lurasidone is not associated with significant QTc prolongation, seizures, transaminases increase, or changes in serum chemistry, hematology, or urinalysis.
Table 2
Lurasidone receptor binding profile and receptor-related effects
| Ki (nM)* | Effects associated with activity on the receptor | |
|---|---|---|
| D2 | 0.994 | Antipsychotic effects. Akathisia (15%), parkinsonism (11%), dystonia (5%), hyperprolactinemia (8.3% for women, 1.9% for men) |
| 5-HT2A | 0.47 | Antipsychotic effects. Improves extrapyramidal symptoms |
| 5-HT7 | 0.495 | Antipsychotic effects. Improves cognition, mood |
| 5-HT1A | 6.38 | Improves cognition, mood. Nausea (12%), vomiting (8%) |
| α-1 | 48 | Orthostatic hypotension (5%), sedation (22%) |
| α-2C | 10.8 | Improves cognition |
| H1 | >1000 | No significant adverse effects mediated through H1 receptor because of low binding affinity |
| M1 | >1000 | No significant adverse effects mediated through M1 receptor because of low binding affinity |
| *Ki dissociation constant; the lower the number, the higher affinity of the compound for the receptor Source: Adapted from reference 1, expert opinion, and lurasidone data on file, 2008 | ||
Contraindications
Lurasidone is contraindicated in patients with known sensitivity to lurasidone hydrochloride. Because of the risk for pharmacokinetic drug-drug interactions, lurasidone is contraindicated for patients who are taking strong CYP3A4 inhibitors (eg, ketoconazole) or inducers (eg, rifampin).
Similar to other medications in its class, lurasidone carries a “black-box” warning of increased mortality in elderly patients with dementia-related psychosis and it is not FDA-approved for treating this condition. Animal teratogenicity studies using lurasidone, 25 mg/kg/d and 50 mg/kg/d, did not show adverse effects during organogenesis, and lurasidone is classified as pregnancy category B (animal studies failed to demonstrate risk to the fetus and there are no adequate and well-controlled studies in pregnant women, or animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester). The use of lurasidone in geriatric and pediatric populations was not studied.1
Dosing
Lurasidone is manufactured as 40 mg and 80 mg tablets. The recommended starting dose is 40 mg/d and the maximum recommended dose is 80 mg/d.1 In clinical trials, lurasidone, 120 mg/d, was associated with increased incidence of adverse effects without added benefit.
Lurasidone doesn’t require initial dose titration and should be given with food that provides ≥350 calories to improve medication absorption. Dose adjustment is recommended for use in patients with moderate or severe renal or hepatic impairment and when coadministered with CYP3A4 moderate inhibitors; the dose in these patients should not exceed 40 mg/d.
Related Resource
- Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2010 Epub ahead of print.
Drug Brand Names
- Ketoconazole • Nizoral
- Lurasidone • Latuda
- Olanzapine • Zyprexa
- Rifampin • Rifadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc.; 2010.
2. Meyer JM, Loebel AD, Schweizer E. Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs. 2009;18(11):1715-1726.
3. Terry AV, Jr, Buccafusco JJ, Wilson C. Cognitive dysfunction in neuropsychiatric, disorders: selected serotonin receptor subtypes as therapeutic targets. Behav Brain Res. 2008;195(1):30-38.
4. Preskorn SH, Yu-Yuan CH, Sarubbi D, et al. Lurasidone pharmacokinetics: Assessment of potential for drug-drug interaction. Abstract presented at: The American College of Neuropsychopharmacology 49th Annual Meeting; December 5-9, 2010; Miami Beach, FL.
5. Loebel A, Cucchiaro J, Ogasa M, et al. Lurasidone for schizophrenia: symptomatic remission during short-term treatment. Abstract presented at: 162nd Annual Meeting of American Psychiatric Association; May 16-21, 2009; San Francisco, CA. Abstract NR1-054.
6. Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829-836.
7. Meltzer H, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of acute schizophrenia: results of the double-blind, placebo-controlled, PEARL 2 trial. Abstract presented at: 48th Annual Meeting of American College of Neuropsychopharmacology; December 6-10, 2009; Hollywood, FL. Abstract 76.
In October 2010, the FDA approved lurasidone for the acute treatment of schizophrenia at a dose of 40 or 80 mg/d administered once daily with food (Table 1).
Table 1
Lurasidone: Fast facts
| Brand name: Latuda |
| Indication: Schizophrenia in adults |
| Approval date: October 28, 2010 |
| Availability date: February 2011 |
| Manufacturer: Sunovion Pharmaceuticals, Inc. |
| Dosing forms: 40 mg and 80 mg tablets |
| Recommended dosage: Starting dose: 40 mg/d. Maximum dose: 80 mg/d |
How it works
Although the drug’s exact mechanism of action is not known, it is thought that lurasidone’s antipsychotic properties are related to its antagonism at serotonin 2A (5-HT2A) and dopamine D2 receptors.1
Similar to most other atypical antipsychotics, lurasidone has high binding affinity for 5-HT2A and D2. Lurasidone has also high binding affinity for 5-HT7, 5-HT1A, and α2C-adrenergic receptors, low affinity for α-1 receptors, and virtually no affinity for H1 and M1 receptors (Table 2). Activity on 5-HT7, 5-HT1A, and α2C-adrenergic receptors is believed to enhance cognition, and 5-HT7 is being studied for a potential role in mood regulation and sensory processing.2,3 Lurasidone’s low activity on α-1, H1, and M1 receptors suggests a low risk of orthostatic hypotension, H1-mediated sedation and weight gain, and H1- and M1-mediated cognitive blunting.
Pharmacokinetics
Lurasidone is absorbed in the gastrointestinal tract. It reaches maximum concentration (Cmax) in 1 to 3 hours. Cmax doubles when lurasidone is administered with food (≥350 calories), but absorption is independent of the meal’s fat content.4 After absorption, the drug is highly bound (99%) to serum proteins (albumin and α-1-glycoprotein). The elimination half-life is 18 hours and steady-state concentration is reached within 7 days.1 Lurasidone is eliminated predominantly through cytochrome P450 (CYP) 3A4 metabolism in the liver.
Efficacy
Lurasidone’s efficacy for treatment of acute schizophrenia was established in four 6-week, randomized placebo-controlled clinical trials.1 The patients were adults (mean age: 38.8; range: 18 to 72) who met DSM-IVTR criteria for schizophrenia, didn’t abuse drugs or alcohol, and had not taken any investigational drug for ≥1 month. Symptoms were measured on the Positive and Negative Syndrome Scale (PANSS); Brief Psychiatric Rating Scale as derived from the PANSS (BPRSd); and the Clinical Global Impressions-Severity scale (CGI-S).
In the first clinical trial, 145 patients were randomized to lurasidone, 40 mg/d or 120 mg/d, or placebo. Treatment with either dose of lurasidone was superior to treatment with placebo on the BPRSd (Least Squares Mean [LSM] difference from placebo in change from baseline: -5.6 on lurasidone 40 mg/d, -6.7 on lurasidone 120 mg/d) and CGI-S.1,5
The second trial randomized 180 patients to lurasidone, 80 mg/d, or placebo. Lurasidone, 80 mg/d, was superior to placebo as measured on the BPRSd (LSM difference from placebo in change from baseline: -4.7 on lurasidone 80 mg/d) and CGI-S.1,6
The third trial randomized 489 patients to lurasidone, 40 mg/d, 80 mg/d, 120 mg/d, or placebo. All lurasidone arms were superior to placebo on PANSS (LSM difference from placebo in change from baseline: -2.1 on 40 mg/d, -6.4 on 80 mg/d, and -3.5 on 120 mg/d) and CGI-S scores. This study also showed that lurasidone appears to have a rapid onset of action (day 3 to 4) and provides sustained improvement of symptoms.1
In the fourth trial, 473 individuals were randomized to lurasidone, 40 mg/d or 120 mg/d, olanzapine, 15 mg/d, or placebo. Olanzapine and both dosages of lurasidone were superior to placebo in improving PANSS scores (LSM difference from placebo in change from baseline: -9.7 on lurasidone 40 mg/d, -7.5 on lurasidone 120 mg/d, and -12.6 on olanzapine 15 mg/d) and CGI-S.1,7 Both doses of lurasidone were not superior to olanzapine but had less negative impact on lipid profile, weight gain, and glycemia.
Tolerability
Tolerability information is extracted from a clinical study database consisting of 2,096 patients with schizophrenia who participated in premarketing clinical trials and were exposed to single or multiple doses of lurasidone, 20 mg, 40 mg, 80 mg, or 120 mg.1 Overall, lurasidone was well tolerated. The rate of discontinuation from clinical trials because of adverse effects was 9.4% for lurasidone vs 5.9% for placebo. Somnolence, akathisia, nausea, parkinsonism, and agitation were the most commonly reported adverse reactions; somnolence and akathisia appear dose-related. Other adverse effects associated with lurasidone were nausea, vomiting, dyspepsia, dystonia, dizziness, insomnia, agitation, and anxiety (Table 2).
Metabolic changes (hyperlipidemia, hyperglycemia, and increased body weight) associated with cardiovascular risk in patients treated with atypical antipsychotics were studied in short-term placebo-controlled trials. Lurasidone is considered to be weight-neutral and does not have significant effects on serum lipids or glucose.2 As is the case with other D2 antagonists, lurasidone is associated with increased prolactin, which appears to be greater in females and is dose-dependent. Lurasidone is not associated with significant QTc prolongation, seizures, transaminases increase, or changes in serum chemistry, hematology, or urinalysis.
Table 2
Lurasidone receptor binding profile and receptor-related effects
| Ki (nM)* | Effects associated with activity on the receptor | |
|---|---|---|
| D2 | 0.994 | Antipsychotic effects. Akathisia (15%), parkinsonism (11%), dystonia (5%), hyperprolactinemia (8.3% for women, 1.9% for men) |
| 5-HT2A | 0.47 | Antipsychotic effects. Improves extrapyramidal symptoms |
| 5-HT7 | 0.495 | Antipsychotic effects. Improves cognition, mood |
| 5-HT1A | 6.38 | Improves cognition, mood. Nausea (12%), vomiting (8%) |
| α-1 | 48 | Orthostatic hypotension (5%), sedation (22%) |
| α-2C | 10.8 | Improves cognition |
| H1 | >1000 | No significant adverse effects mediated through H1 receptor because of low binding affinity |
| M1 | >1000 | No significant adverse effects mediated through M1 receptor because of low binding affinity |
| *Ki dissociation constant; the lower the number, the higher affinity of the compound for the receptor Source: Adapted from reference 1, expert opinion, and lurasidone data on file, 2008 | ||
Contraindications
Lurasidone is contraindicated in patients with known sensitivity to lurasidone hydrochloride. Because of the risk for pharmacokinetic drug-drug interactions, lurasidone is contraindicated for patients who are taking strong CYP3A4 inhibitors (eg, ketoconazole) or inducers (eg, rifampin).
Similar to other medications in its class, lurasidone carries a “black-box” warning of increased mortality in elderly patients with dementia-related psychosis and it is not FDA-approved for treating this condition. Animal teratogenicity studies using lurasidone, 25 mg/kg/d and 50 mg/kg/d, did not show adverse effects during organogenesis, and lurasidone is classified as pregnancy category B (animal studies failed to demonstrate risk to the fetus and there are no adequate and well-controlled studies in pregnant women, or animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester). The use of lurasidone in geriatric and pediatric populations was not studied.1
Dosing
Lurasidone is manufactured as 40 mg and 80 mg tablets. The recommended starting dose is 40 mg/d and the maximum recommended dose is 80 mg/d.1 In clinical trials, lurasidone, 120 mg/d, was associated with increased incidence of adverse effects without added benefit.
Lurasidone doesn’t require initial dose titration and should be given with food that provides ≥350 calories to improve medication absorption. Dose adjustment is recommended for use in patients with moderate or severe renal or hepatic impairment and when coadministered with CYP3A4 moderate inhibitors; the dose in these patients should not exceed 40 mg/d.
Related Resource
- Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2010 Epub ahead of print.
Drug Brand Names
- Ketoconazole • Nizoral
- Lurasidone • Latuda
- Olanzapine • Zyprexa
- Rifampin • Rifadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In October 2010, the FDA approved lurasidone for the acute treatment of schizophrenia at a dose of 40 or 80 mg/d administered once daily with food (Table 1).
Table 1
Lurasidone: Fast facts
| Brand name: Latuda |
| Indication: Schizophrenia in adults |
| Approval date: October 28, 2010 |
| Availability date: February 2011 |
| Manufacturer: Sunovion Pharmaceuticals, Inc. |
| Dosing forms: 40 mg and 80 mg tablets |
| Recommended dosage: Starting dose: 40 mg/d. Maximum dose: 80 mg/d |
How it works
Although the drug’s exact mechanism of action is not known, it is thought that lurasidone’s antipsychotic properties are related to its antagonism at serotonin 2A (5-HT2A) and dopamine D2 receptors.1
Similar to most other atypical antipsychotics, lurasidone has high binding affinity for 5-HT2A and D2. Lurasidone has also high binding affinity for 5-HT7, 5-HT1A, and α2C-adrenergic receptors, low affinity for α-1 receptors, and virtually no affinity for H1 and M1 receptors (Table 2). Activity on 5-HT7, 5-HT1A, and α2C-adrenergic receptors is believed to enhance cognition, and 5-HT7 is being studied for a potential role in mood regulation and sensory processing.2,3 Lurasidone’s low activity on α-1, H1, and M1 receptors suggests a low risk of orthostatic hypotension, H1-mediated sedation and weight gain, and H1- and M1-mediated cognitive blunting.
Pharmacokinetics
Lurasidone is absorbed in the gastrointestinal tract. It reaches maximum concentration (Cmax) in 1 to 3 hours. Cmax doubles when lurasidone is administered with food (≥350 calories), but absorption is independent of the meal’s fat content.4 After absorption, the drug is highly bound (99%) to serum proteins (albumin and α-1-glycoprotein). The elimination half-life is 18 hours and steady-state concentration is reached within 7 days.1 Lurasidone is eliminated predominantly through cytochrome P450 (CYP) 3A4 metabolism in the liver.
Efficacy
Lurasidone’s efficacy for treatment of acute schizophrenia was established in four 6-week, randomized placebo-controlled clinical trials.1 The patients were adults (mean age: 38.8; range: 18 to 72) who met DSM-IVTR criteria for schizophrenia, didn’t abuse drugs or alcohol, and had not taken any investigational drug for ≥1 month. Symptoms were measured on the Positive and Negative Syndrome Scale (PANSS); Brief Psychiatric Rating Scale as derived from the PANSS (BPRSd); and the Clinical Global Impressions-Severity scale (CGI-S).
In the first clinical trial, 145 patients were randomized to lurasidone, 40 mg/d or 120 mg/d, or placebo. Treatment with either dose of lurasidone was superior to treatment with placebo on the BPRSd (Least Squares Mean [LSM] difference from placebo in change from baseline: -5.6 on lurasidone 40 mg/d, -6.7 on lurasidone 120 mg/d) and CGI-S.1,5
The second trial randomized 180 patients to lurasidone, 80 mg/d, or placebo. Lurasidone, 80 mg/d, was superior to placebo as measured on the BPRSd (LSM difference from placebo in change from baseline: -4.7 on lurasidone 80 mg/d) and CGI-S.1,6
The third trial randomized 489 patients to lurasidone, 40 mg/d, 80 mg/d, 120 mg/d, or placebo. All lurasidone arms were superior to placebo on PANSS (LSM difference from placebo in change from baseline: -2.1 on 40 mg/d, -6.4 on 80 mg/d, and -3.5 on 120 mg/d) and CGI-S scores. This study also showed that lurasidone appears to have a rapid onset of action (day 3 to 4) and provides sustained improvement of symptoms.1
In the fourth trial, 473 individuals were randomized to lurasidone, 40 mg/d or 120 mg/d, olanzapine, 15 mg/d, or placebo. Olanzapine and both dosages of lurasidone were superior to placebo in improving PANSS scores (LSM difference from placebo in change from baseline: -9.7 on lurasidone 40 mg/d, -7.5 on lurasidone 120 mg/d, and -12.6 on olanzapine 15 mg/d) and CGI-S.1,7 Both doses of lurasidone were not superior to olanzapine but had less negative impact on lipid profile, weight gain, and glycemia.
Tolerability
Tolerability information is extracted from a clinical study database consisting of 2,096 patients with schizophrenia who participated in premarketing clinical trials and were exposed to single or multiple doses of lurasidone, 20 mg, 40 mg, 80 mg, or 120 mg.1 Overall, lurasidone was well tolerated. The rate of discontinuation from clinical trials because of adverse effects was 9.4% for lurasidone vs 5.9% for placebo. Somnolence, akathisia, nausea, parkinsonism, and agitation were the most commonly reported adverse reactions; somnolence and akathisia appear dose-related. Other adverse effects associated with lurasidone were nausea, vomiting, dyspepsia, dystonia, dizziness, insomnia, agitation, and anxiety (Table 2).
Metabolic changes (hyperlipidemia, hyperglycemia, and increased body weight) associated with cardiovascular risk in patients treated with atypical antipsychotics were studied in short-term placebo-controlled trials. Lurasidone is considered to be weight-neutral and does not have significant effects on serum lipids or glucose.2 As is the case with other D2 antagonists, lurasidone is associated with increased prolactin, which appears to be greater in females and is dose-dependent. Lurasidone is not associated with significant QTc prolongation, seizures, transaminases increase, or changes in serum chemistry, hematology, or urinalysis.
Table 2
Lurasidone receptor binding profile and receptor-related effects
| Ki (nM)* | Effects associated with activity on the receptor | |
|---|---|---|
| D2 | 0.994 | Antipsychotic effects. Akathisia (15%), parkinsonism (11%), dystonia (5%), hyperprolactinemia (8.3% for women, 1.9% for men) |
| 5-HT2A | 0.47 | Antipsychotic effects. Improves extrapyramidal symptoms |
| 5-HT7 | 0.495 | Antipsychotic effects. Improves cognition, mood |
| 5-HT1A | 6.38 | Improves cognition, mood. Nausea (12%), vomiting (8%) |
| α-1 | 48 | Orthostatic hypotension (5%), sedation (22%) |
| α-2C | 10.8 | Improves cognition |
| H1 | >1000 | No significant adverse effects mediated through H1 receptor because of low binding affinity |
| M1 | >1000 | No significant adverse effects mediated through M1 receptor because of low binding affinity |
| *Ki dissociation constant; the lower the number, the higher affinity of the compound for the receptor Source: Adapted from reference 1, expert opinion, and lurasidone data on file, 2008 | ||
Contraindications
Lurasidone is contraindicated in patients with known sensitivity to lurasidone hydrochloride. Because of the risk for pharmacokinetic drug-drug interactions, lurasidone is contraindicated for patients who are taking strong CYP3A4 inhibitors (eg, ketoconazole) or inducers (eg, rifampin).
Similar to other medications in its class, lurasidone carries a “black-box” warning of increased mortality in elderly patients with dementia-related psychosis and it is not FDA-approved for treating this condition. Animal teratogenicity studies using lurasidone, 25 mg/kg/d and 50 mg/kg/d, did not show adverse effects during organogenesis, and lurasidone is classified as pregnancy category B (animal studies failed to demonstrate risk to the fetus and there are no adequate and well-controlled studies in pregnant women, or animal studies have shown an adverse effect, but adequate and well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester). The use of lurasidone in geriatric and pediatric populations was not studied.1
Dosing
Lurasidone is manufactured as 40 mg and 80 mg tablets. The recommended starting dose is 40 mg/d and the maximum recommended dose is 80 mg/d.1 In clinical trials, lurasidone, 120 mg/d, was associated with increased incidence of adverse effects without added benefit.
Lurasidone doesn’t require initial dose titration and should be given with food that provides ≥350 calories to improve medication absorption. Dose adjustment is recommended for use in patients with moderate or severe renal or hepatic impairment and when coadministered with CYP3A4 moderate inhibitors; the dose in these patients should not exceed 40 mg/d.
Related Resource
- Citrome L. Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract. 2010 Epub ahead of print.
Drug Brand Names
- Ketoconazole • Nizoral
- Lurasidone • Latuda
- Olanzapine • Zyprexa
- Rifampin • Rifadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc.; 2010.
2. Meyer JM, Loebel AD, Schweizer E. Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs. 2009;18(11):1715-1726.
3. Terry AV, Jr, Buccafusco JJ, Wilson C. Cognitive dysfunction in neuropsychiatric, disorders: selected serotonin receptor subtypes as therapeutic targets. Behav Brain Res. 2008;195(1):30-38.
4. Preskorn SH, Yu-Yuan CH, Sarubbi D, et al. Lurasidone pharmacokinetics: Assessment of potential for drug-drug interaction. Abstract presented at: The American College of Neuropsychopharmacology 49th Annual Meeting; December 5-9, 2010; Miami Beach, FL.
5. Loebel A, Cucchiaro J, Ogasa M, et al. Lurasidone for schizophrenia: symptomatic remission during short-term treatment. Abstract presented at: 162nd Annual Meeting of American Psychiatric Association; May 16-21, 2009; San Francisco, CA. Abstract NR1-054.
6. Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829-836.
7. Meltzer H, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of acute schizophrenia: results of the double-blind, placebo-controlled, PEARL 2 trial. Abstract presented at: 48th Annual Meeting of American College of Neuropsychopharmacology; December 6-10, 2009; Hollywood, FL. Abstract 76.
1. Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals, Inc.; 2010.
2. Meyer JM, Loebel AD, Schweizer E. Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs. 2009;18(11):1715-1726.
3. Terry AV, Jr, Buccafusco JJ, Wilson C. Cognitive dysfunction in neuropsychiatric, disorders: selected serotonin receptor subtypes as therapeutic targets. Behav Brain Res. 2008;195(1):30-38.
4. Preskorn SH, Yu-Yuan CH, Sarubbi D, et al. Lurasidone pharmacokinetics: Assessment of potential for drug-drug interaction. Abstract presented at: The American College of Neuropsychopharmacology 49th Annual Meeting; December 5-9, 2010; Miami Beach, FL.
5. Loebel A, Cucchiaro J, Ogasa M, et al. Lurasidone for schizophrenia: symptomatic remission during short-term treatment. Abstract presented at: 162nd Annual Meeting of American Psychiatric Association; May 16-21, 2009; San Francisco, CA. Abstract NR1-054.
6. Nakamura M, Ogasa M, Guarino J, et al. Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(6):829-836.
7. Meltzer H, Cucchiaro J, Silva R, et al. Lurasidone in the treatment of acute schizophrenia: results of the double-blind, placebo-controlled, PEARL 2 trial. Abstract presented at: 48th Annual Meeting of American College of Neuropsychopharmacology; December 6-10, 2009; Hollywood, FL. Abstract 76.