User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Should psychiatry list hubris in DSM-V?
A recent book contends that psychiatry has transformed normal sadness and sorrow into a depressive disorder,1 which would be akin to saying primary care physicians diagnose every mild cough as pneumonia. The book’s premise is not true, of course, but it’s a perfect example of how misunderstood serious mental illness is.
Psychiatric disorders deal with extreme, pathologic changes in behavior, thoughts and emotions, perception, or will. Psychiatrists do not pathologize normal sadness over a missed opportunity nor noticeable grief over the death of a loved one. We do, however, recognize the substantial disruption that clinical depression brings to a person’s life and the potential for suicidal acts during major depressive illness.
Similarly, being happy about a minor achievement does not affect one’s life as much as the giddiness of infatuation and certainly is not as serious as the grandiose euphoria of a manic episode. Psychiatrists would intervene with a diagnosis and treatment plan only if the condition were severe enough to impair social and vocational functioning.
Cataloging human flaws
Let’s assume, just for fun, that psychiatrists did decide to pathologize common human traits. Consider the many categories we would need to add to DSM-V!
Take the worldwide financial meltdown triggered by questionable practices of banking executives who thought they would never fail or be caught on their way to accumulating obscene wealth. They certainly left a lot of wreckage in their wake, so perhaps psychiatry should create new diagnostic entities of “Horrendous Hubris” and “Gargantuan Greed.”
But why stop there? How about “Verbal Diarrhea” for folks who chatter incessantly at a cocktail party or committee meeting, or “Intellectual Constipation” for our friends with exasperating narrow-mindedness. And for the painfully irritating person, “Social Hemorrhoid” might be apropos.
Let’s not forget those who throw temper tantrums when they can’t have their way: they may suffer from “Temperamental Arrhythmia.” And for chronic complainers who never stop whining, let’s go with “Emotive Angina.” I’d better stop here and not go into the garden variety of human flaws: gluttony, sloth, fanaticism, indecisiveness, cowardice, chicanery, snobbishness, rudeness, and plain old stupidity.
Normal vs pathologic
My point is that the social retina of psychiatry does not perceive ordinary human traits and emotions such as normal sadness as pathologic behavior. But psychiatrists certainly are willing to intervene when people seek help on their own for problems such as depressive episodes that are disrupting their lives or are referred by physicians or brought in by family or friends who recognize the potential gravity of their afflictions.
Horwitz AV, Wakefield JC. The loss of sadness: how psychiatry transformed normal sorrow into depressive disorder. New York, NY: Oxford University Press; 2007.
A recent book contends that psychiatry has transformed normal sadness and sorrow into a depressive disorder,1 which would be akin to saying primary care physicians diagnose every mild cough as pneumonia. The book’s premise is not true, of course, but it’s a perfect example of how misunderstood serious mental illness is.
Psychiatric disorders deal with extreme, pathologic changes in behavior, thoughts and emotions, perception, or will. Psychiatrists do not pathologize normal sadness over a missed opportunity nor noticeable grief over the death of a loved one. We do, however, recognize the substantial disruption that clinical depression brings to a person’s life and the potential for suicidal acts during major depressive illness.
Similarly, being happy about a minor achievement does not affect one’s life as much as the giddiness of infatuation and certainly is not as serious as the grandiose euphoria of a manic episode. Psychiatrists would intervene with a diagnosis and treatment plan only if the condition were severe enough to impair social and vocational functioning.
Cataloging human flaws
Let’s assume, just for fun, that psychiatrists did decide to pathologize common human traits. Consider the many categories we would need to add to DSM-V!
Take the worldwide financial meltdown triggered by questionable practices of banking executives who thought they would never fail or be caught on their way to accumulating obscene wealth. They certainly left a lot of wreckage in their wake, so perhaps psychiatry should create new diagnostic entities of “Horrendous Hubris” and “Gargantuan Greed.”
But why stop there? How about “Verbal Diarrhea” for folks who chatter incessantly at a cocktail party or committee meeting, or “Intellectual Constipation” for our friends with exasperating narrow-mindedness. And for the painfully irritating person, “Social Hemorrhoid” might be apropos.
Let’s not forget those who throw temper tantrums when they can’t have their way: they may suffer from “Temperamental Arrhythmia.” And for chronic complainers who never stop whining, let’s go with “Emotive Angina.” I’d better stop here and not go into the garden variety of human flaws: gluttony, sloth, fanaticism, indecisiveness, cowardice, chicanery, snobbishness, rudeness, and plain old stupidity.
Normal vs pathologic
My point is that the social retina of psychiatry does not perceive ordinary human traits and emotions such as normal sadness as pathologic behavior. But psychiatrists certainly are willing to intervene when people seek help on their own for problems such as depressive episodes that are disrupting their lives or are referred by physicians or brought in by family or friends who recognize the potential gravity of their afflictions.
A recent book contends that psychiatry has transformed normal sadness and sorrow into a depressive disorder,1 which would be akin to saying primary care physicians diagnose every mild cough as pneumonia. The book’s premise is not true, of course, but it’s a perfect example of how misunderstood serious mental illness is.
Psychiatric disorders deal with extreme, pathologic changes in behavior, thoughts and emotions, perception, or will. Psychiatrists do not pathologize normal sadness over a missed opportunity nor noticeable grief over the death of a loved one. We do, however, recognize the substantial disruption that clinical depression brings to a person’s life and the potential for suicidal acts during major depressive illness.
Similarly, being happy about a minor achievement does not affect one’s life as much as the giddiness of infatuation and certainly is not as serious as the grandiose euphoria of a manic episode. Psychiatrists would intervene with a diagnosis and treatment plan only if the condition were severe enough to impair social and vocational functioning.
Cataloging human flaws
Let’s assume, just for fun, that psychiatrists did decide to pathologize common human traits. Consider the many categories we would need to add to DSM-V!
Take the worldwide financial meltdown triggered by questionable practices of banking executives who thought they would never fail or be caught on their way to accumulating obscene wealth. They certainly left a lot of wreckage in their wake, so perhaps psychiatry should create new diagnostic entities of “Horrendous Hubris” and “Gargantuan Greed.”
But why stop there? How about “Verbal Diarrhea” for folks who chatter incessantly at a cocktail party or committee meeting, or “Intellectual Constipation” for our friends with exasperating narrow-mindedness. And for the painfully irritating person, “Social Hemorrhoid” might be apropos.
Let’s not forget those who throw temper tantrums when they can’t have their way: they may suffer from “Temperamental Arrhythmia.” And for chronic complainers who never stop whining, let’s go with “Emotive Angina.” I’d better stop here and not go into the garden variety of human flaws: gluttony, sloth, fanaticism, indecisiveness, cowardice, chicanery, snobbishness, rudeness, and plain old stupidity.
Normal vs pathologic
My point is that the social retina of psychiatry does not perceive ordinary human traits and emotions such as normal sadness as pathologic behavior. But psychiatrists certainly are willing to intervene when people seek help on their own for problems such as depressive episodes that are disrupting their lives or are referred by physicians or brought in by family or friends who recognize the potential gravity of their afflictions.
Horwitz AV, Wakefield JC. The loss of sadness: how psychiatry transformed normal sorrow into depressive disorder. New York, NY: Oxford University Press; 2007.
Horwitz AV, Wakefield JC. The loss of sadness: how psychiatry transformed normal sorrow into depressive disorder. New York, NY: Oxford University Press; 2007.
A mysterious physical and mental decline
HISTORY: ‘Not himself’
Mr. C, age 69, presents to the emergency department complaining of intermittent fever of about 100°F, hematuria, headache, weakness, fatigue, and decreased appetite over 2 months. Testing shows acute renal failure, elevated C-reactive protein, and increased sedimentation rate. The attending internist admits Mr. C with a working diagnosis of temporal arteritis and acute renal failure, administers corticosteroids for headache, and orders a right temporal artery biopsy, which shows no signs of vasculitis.
Family members report that Mr. C has not been himself—he has become increasingly withdrawn and “emotionless.” Mr. C’s wife says her husband has needed help with dressing and eating because of short-term memory loss over 9 months. She says he has lost 20 to 30 lb.
The patient’s cognitive function appears to have worsened since he developed these physical symptoms. Mrs. C also reports that he has had weakness and fatigue for 8 months.
One month earlier, the patient was admitted to a different hospital and treated for 2 weeks with IV antibiotics for fever of unknown origin. Results of lumbar puncture and extensive rheumatologic, infectious disease, urologic, and gastroenterologic evaluations were normal.
The internal medicine physician requests a psychiatric consultation. During our interview, Mr. C is cooperative, shows no signs of acute distress, is well groomed and dressed appropriately, and maintains eye contact. Speech rate and volume are low, with normal articulation and coherence, diminished spontaneity, and paucity of language. Mrs. C tells us her husband was lively and talkative before his recent illness. His mood is euthymic, and he is pleasant and cheerful during the evaluation.
The authors’ observations
Initially, we suspect an underlying medical condition is causing Mr. C’s psychiatric symptoms.
Mr. C’s wife reports that her husband stopped drinking 2 years ago after his family expressed concern about his health. Mr. C’s past alcohol use could not be quantified. He has not abused illicit drugs and has no personal or family history of dementia, trauma, or psychiatric or neurologic disorders.
EVALUATION: Impaired memory
Mr. C is afebrile during the initial physical examination, but fever returns within several days. Neurologic examination is normal, and negative rapid plasma reagin rules out syphilis. Vitamin B12 and folate levels are normal, as is thyroid function. Other laboratory findings are outside normal limits (Table).
Urine is cloudy with 2+ protein, 3+ blood, and trace leukocyte esterase. The presence of protein and blood suggests a glomerular disease such as a glomerulonephritis.
A positive leukocyte esterase test results from the presence of white blood cells, either as whole cells or as lysed cells. An abnormal number of leukocytes may appear with upper or lower urinary tract infection or in acute glomerulonephritis.
Chest radiography shows increased bilateral pulmonary vasculature, which can indicate pulmonary hypertension.
Mr. C has fair attention and concentration but impaired recent memory. He cannot recall yesterday’s events without help.
Mr. C’s Mini-Mental State Examination score of 21/30 suggests markedly impaired executive functioning and cognitive deficits. The attending psychiatrist recommends brain MRI.
Table
Mr. C’s laboratory findings
| Value | Normal range | |
|---|---|---|
| WBC | 15.1 | 4.8 to 10.8 cells/μL |
| Hb | 9 | 13.8 to 17.5 g/dL |
| Hct | 25.9% | 40% to 54% |
| MCV | 89.7 | 80 to 94 fL |
| BUN | 119 | 7 to 18 mg/dL |
| Cr | 12.1 | 0.7 to 1.3 mg/dL |
| Na | 125 | 136 to 145 mmol/L |
| K | 6.5 | 3.5 to 5 mEq/dL |
| HCO3 | 13.5 | 22 to 29 mmol/L |
| ECR | 125 | 30 mm/hr |
| WBC: white blood cell; Hb: hemoglobin; Hct: hematocrit; MCV: mean corpuscular volume; BUN: blood urea nitrogen; Cr: creatinine; Na: sodium; K: potassium; HCO3: bicarbonate; ESR: erythrocyte sedimentation rate | ||
The authors’ observations
Mr. C shows markedly impaired cognitive function without significant impairment of attention and concentration despite his progressive deterioration and increasing disability. Urine toxicology shows no illicit substances. Given his lack of a previous mood disorder and his family’s description of him as formerly vibrant and cheerful, he likely does not have a mood disorder.
Based on the history of events, including the symptom pattern, we rule out delirium. We suspect that Mr. C has dementia secondary to a general medical condition. His symptoms seem to be directly related to his medical complaints and do not have a waxing and waning course. The internal medicine physician orders additional laboratory tests.
TESTING: Kidney, lung damage
Over 5 days, Mr. C’s intermittent low-grade fevers continue. Laboratory tests are negative for HIV antibody, hepatitis panel, and antinuclear antibodies (ANA). C-reactive protein is elevated at 27.8 mg/dL (normal range,
Renal ultrasound is normal, but preliminary renal biopsy shows rapidly progressive glomerulonephritis. The internist immediately starts dialysis, cyclophosphamide at 1.5 mg/kg, and prednisone, 1 mg/kg. The pathology report on the renal biopsy describes extensive crescentic glomerular destruction, with inflammatory cells present.
Ten days after admission, Mr. C develops hemoptysis, and chest radiography shows increasing alveolar infiltrates. The attending internist consults pulmonary and critical care services.
The consultant suspects a pulmonary-renal syndrome because of bilateral alveolar infiltrates (diffuse alveolar hemorrhage). The internal medicine team continues high-dose corticosteroids, followed by plasmapheresis.
Brain MRI shows subacute to chronic infarcts involving the right basal ganglia and corona radiate and mild to moderate small vessel ischemic changes. Old areas of hemorrhage are noted within both cerebellar lobes, left temporal lobe, right basal ganglia, right parietal lobe, and right frontal lobe.
During follow-up interviews, Mr. C often cannot recall recent dialysis or plasmapheresis and reports no physical symptoms. His short-term memory continues to deteriorate; he would forget to eat if not cued by family or nursing staff. He shows global cognitive deficits and is increasingly withdrawn and flat.
The authors’ observations
Although few case reports have associated Goodpasture’s syndrome with neurobehavioral changes, the apparent relationship of Mr. C’s medical symptoms with the worsening of his cognitive impairment suggests a link.
Mr. C’s MRI findings also might suggest CNS vasculitis, which affects small arteries of the cerebral and spinal cord leptomeninges and parenchyma, leading to CNS dys-function.6-8 CNS vasculitis can result from primary nervous system involvement or from a secondary systemic process such as Goodpasture’s syndrome.9
We rule out lupus because Mr. C is ANA-negative; this test has 99% sensitivity for lupus.10
Goodpasture’s syndrome, which afflicts 1 Patients typically present with alveolar bleeding, rapidly progressive acute renal failure with proteinuria,1 and pulmonary symptoms such as dyspnea and hemoptysis.2
Possible triggers include:
- viral upper respiratory tract infection (20% to 60% of patients)3
- exposure to hydrocarbon solvents (3,4
Mr. C was exposed to solvents during the 15 years he worked in a factory. Some researchers believe a noxious event among genetically susceptible persons damages basement membrane and exposes an antigen that triggers IgG auto-antibody production.3,4
Malaise, weight loss, and fever are atypical in Goodpasture’s syndrome but could suggest concomitant vasculitis.5
OUTCOME: Ongoing disability
Mr. C is hospitalized for 6 weeks. He receives cyclophosphamide, prednisone, and 10 sessions of plasmapheresis. We prescribe mirtazapine, 15 mg at bedtime, to treat mood symptoms. We chose mirtazapine because of the drug’s sleep-restoring and appetite-stimulating properties.
Mr. C’s fever resolves and pulmonary function soon improves, but his cognitive impairment persists. He has difficulties preparing meals, taking medications, and managing his money.
Mr. C is discharged with a referral to a psychiatrist. He continues taking mirtazapine and a lower dose of prednisone. He requires ongoing hemodialysis and assistance with activities of daily living.
The authors’ observations
Prompt multidisciplinary intervention is critical when patients present with concurrent cognitive and medical symptoms. A thorough psychiatric evaluation can help piece together the illness’ course. The psychiatrist’s role in a multidisciplinary assessment is to:
- document neurocognitive changes
- verify them through collateral information
- correlate these changes with the timing of medical symptoms.
An underlying psychiatric condition can complicate the diagnosis. In these cases, careful interviewing and collateral information can help you discern the chronology of events.
1. Bolton WK. Goodpasture’s syndrome. Kidney Int 1996;50(5):1753-66.
2. Pusey CD. Anti-glomerular basement membrane disease. Kidney Int 2003;64(4):1535-50.
3. Humes HD, DuPont HL. eds. Kelley’s textbook of internal medicine. 4th ed. New York, NY: Lippincott Williams & Wilkins; 2000.
4. Stevenson A, Yaqoob M, Mason H, et al. Biochemical markers of basement membrane disturbances and occupational exposure to hydrocarbons and mixed solvents. QJM 1995;88(1):23-8.
5. Kluth DC, Rees AJ. Anti-glomerular basement membrane disease. J Am Soc Nephrol. 1999;10(11):2446-53.
6. Rydel JJ, Rodby RA. An 18-year-old man with Goodpasture’s syndrome and ANCA-negative central nervous system vasculitis. Am J Kidney Dis 1998;31(2):345-9.
7. Gittins N, Basu A, Eyre J, et al. Cerebral vasculitis in a teenager with Goodpasture’s syndrome. Nephrol Dial Transplant 2004;19(12):3168-71.
8. Garnier P, Deprele C. Cerebral angiitis and Goodpasture’s syndrome. Rev Neurol 2003;159(1):68-70.
9. Calabrese LH, Duna GF, Lie JT. Vasculitis in the central nervous system. Arthritis Rhem 1997;40(7):1189-201.
10. Edworthy SM, Zatarain E, McShane DJ, Bloch DA. Analysis of the 1982 ARA lupus criteria data set by recursive partitioning methodology: new insights into the relative merit of individual criteria. J Rheumatol 1988;15(10):1493-8.
HISTORY: ‘Not himself’
Mr. C, age 69, presents to the emergency department complaining of intermittent fever of about 100°F, hematuria, headache, weakness, fatigue, and decreased appetite over 2 months. Testing shows acute renal failure, elevated C-reactive protein, and increased sedimentation rate. The attending internist admits Mr. C with a working diagnosis of temporal arteritis and acute renal failure, administers corticosteroids for headache, and orders a right temporal artery biopsy, which shows no signs of vasculitis.
Family members report that Mr. C has not been himself—he has become increasingly withdrawn and “emotionless.” Mr. C’s wife says her husband has needed help with dressing and eating because of short-term memory loss over 9 months. She says he has lost 20 to 30 lb.
The patient’s cognitive function appears to have worsened since he developed these physical symptoms. Mrs. C also reports that he has had weakness and fatigue for 8 months.
One month earlier, the patient was admitted to a different hospital and treated for 2 weeks with IV antibiotics for fever of unknown origin. Results of lumbar puncture and extensive rheumatologic, infectious disease, urologic, and gastroenterologic evaluations were normal.
The internal medicine physician requests a psychiatric consultation. During our interview, Mr. C is cooperative, shows no signs of acute distress, is well groomed and dressed appropriately, and maintains eye contact. Speech rate and volume are low, with normal articulation and coherence, diminished spontaneity, and paucity of language. Mrs. C tells us her husband was lively and talkative before his recent illness. His mood is euthymic, and he is pleasant and cheerful during the evaluation.
The authors’ observations
Initially, we suspect an underlying medical condition is causing Mr. C’s psychiatric symptoms.
Mr. C’s wife reports that her husband stopped drinking 2 years ago after his family expressed concern about his health. Mr. C’s past alcohol use could not be quantified. He has not abused illicit drugs and has no personal or family history of dementia, trauma, or psychiatric or neurologic disorders.
EVALUATION: Impaired memory
Mr. C is afebrile during the initial physical examination, but fever returns within several days. Neurologic examination is normal, and negative rapid plasma reagin rules out syphilis. Vitamin B12 and folate levels are normal, as is thyroid function. Other laboratory findings are outside normal limits (Table).
Urine is cloudy with 2+ protein, 3+ blood, and trace leukocyte esterase. The presence of protein and blood suggests a glomerular disease such as a glomerulonephritis.
A positive leukocyte esterase test results from the presence of white blood cells, either as whole cells or as lysed cells. An abnormal number of leukocytes may appear with upper or lower urinary tract infection or in acute glomerulonephritis.
Chest radiography shows increased bilateral pulmonary vasculature, which can indicate pulmonary hypertension.
Mr. C has fair attention and concentration but impaired recent memory. He cannot recall yesterday’s events without help.
Mr. C’s Mini-Mental State Examination score of 21/30 suggests markedly impaired executive functioning and cognitive deficits. The attending psychiatrist recommends brain MRI.
Table
Mr. C’s laboratory findings
| Value | Normal range | |
|---|---|---|
| WBC | 15.1 | 4.8 to 10.8 cells/μL |
| Hb | 9 | 13.8 to 17.5 g/dL |
| Hct | 25.9% | 40% to 54% |
| MCV | 89.7 | 80 to 94 fL |
| BUN | 119 | 7 to 18 mg/dL |
| Cr | 12.1 | 0.7 to 1.3 mg/dL |
| Na | 125 | 136 to 145 mmol/L |
| K | 6.5 | 3.5 to 5 mEq/dL |
| HCO3 | 13.5 | 22 to 29 mmol/L |
| ECR | 125 | 30 mm/hr |
| WBC: white blood cell; Hb: hemoglobin; Hct: hematocrit; MCV: mean corpuscular volume; BUN: blood urea nitrogen; Cr: creatinine; Na: sodium; K: potassium; HCO3: bicarbonate; ESR: erythrocyte sedimentation rate | ||
The authors’ observations
Mr. C shows markedly impaired cognitive function without significant impairment of attention and concentration despite his progressive deterioration and increasing disability. Urine toxicology shows no illicit substances. Given his lack of a previous mood disorder and his family’s description of him as formerly vibrant and cheerful, he likely does not have a mood disorder.
Based on the history of events, including the symptom pattern, we rule out delirium. We suspect that Mr. C has dementia secondary to a general medical condition. His symptoms seem to be directly related to his medical complaints and do not have a waxing and waning course. The internal medicine physician orders additional laboratory tests.
TESTING: Kidney, lung damage
Over 5 days, Mr. C’s intermittent low-grade fevers continue. Laboratory tests are negative for HIV antibody, hepatitis panel, and antinuclear antibodies (ANA). C-reactive protein is elevated at 27.8 mg/dL (normal range,
Renal ultrasound is normal, but preliminary renal biopsy shows rapidly progressive glomerulonephritis. The internist immediately starts dialysis, cyclophosphamide at 1.5 mg/kg, and prednisone, 1 mg/kg. The pathology report on the renal biopsy describes extensive crescentic glomerular destruction, with inflammatory cells present.
Ten days after admission, Mr. C develops hemoptysis, and chest radiography shows increasing alveolar infiltrates. The attending internist consults pulmonary and critical care services.
The consultant suspects a pulmonary-renal syndrome because of bilateral alveolar infiltrates (diffuse alveolar hemorrhage). The internal medicine team continues high-dose corticosteroids, followed by plasmapheresis.
Brain MRI shows subacute to chronic infarcts involving the right basal ganglia and corona radiate and mild to moderate small vessel ischemic changes. Old areas of hemorrhage are noted within both cerebellar lobes, left temporal lobe, right basal ganglia, right parietal lobe, and right frontal lobe.
During follow-up interviews, Mr. C often cannot recall recent dialysis or plasmapheresis and reports no physical symptoms. His short-term memory continues to deteriorate; he would forget to eat if not cued by family or nursing staff. He shows global cognitive deficits and is increasingly withdrawn and flat.
The authors’ observations
Although few case reports have associated Goodpasture’s syndrome with neurobehavioral changes, the apparent relationship of Mr. C’s medical symptoms with the worsening of his cognitive impairment suggests a link.
Mr. C’s MRI findings also might suggest CNS vasculitis, which affects small arteries of the cerebral and spinal cord leptomeninges and parenchyma, leading to CNS dys-function.6-8 CNS vasculitis can result from primary nervous system involvement or from a secondary systemic process such as Goodpasture’s syndrome.9
We rule out lupus because Mr. C is ANA-negative; this test has 99% sensitivity for lupus.10
Goodpasture’s syndrome, which afflicts 1 Patients typically present with alveolar bleeding, rapidly progressive acute renal failure with proteinuria,1 and pulmonary symptoms such as dyspnea and hemoptysis.2
Possible triggers include:
- viral upper respiratory tract infection (20% to 60% of patients)3
- exposure to hydrocarbon solvents (3,4
Mr. C was exposed to solvents during the 15 years he worked in a factory. Some researchers believe a noxious event among genetically susceptible persons damages basement membrane and exposes an antigen that triggers IgG auto-antibody production.3,4
Malaise, weight loss, and fever are atypical in Goodpasture’s syndrome but could suggest concomitant vasculitis.5
OUTCOME: Ongoing disability
Mr. C is hospitalized for 6 weeks. He receives cyclophosphamide, prednisone, and 10 sessions of plasmapheresis. We prescribe mirtazapine, 15 mg at bedtime, to treat mood symptoms. We chose mirtazapine because of the drug’s sleep-restoring and appetite-stimulating properties.
Mr. C’s fever resolves and pulmonary function soon improves, but his cognitive impairment persists. He has difficulties preparing meals, taking medications, and managing his money.
Mr. C is discharged with a referral to a psychiatrist. He continues taking mirtazapine and a lower dose of prednisone. He requires ongoing hemodialysis and assistance with activities of daily living.
The authors’ observations
Prompt multidisciplinary intervention is critical when patients present with concurrent cognitive and medical symptoms. A thorough psychiatric evaluation can help piece together the illness’ course. The psychiatrist’s role in a multidisciplinary assessment is to:
- document neurocognitive changes
- verify them through collateral information
- correlate these changes with the timing of medical symptoms.
An underlying psychiatric condition can complicate the diagnosis. In these cases, careful interviewing and collateral information can help you discern the chronology of events.
HISTORY: ‘Not himself’
Mr. C, age 69, presents to the emergency department complaining of intermittent fever of about 100°F, hematuria, headache, weakness, fatigue, and decreased appetite over 2 months. Testing shows acute renal failure, elevated C-reactive protein, and increased sedimentation rate. The attending internist admits Mr. C with a working diagnosis of temporal arteritis and acute renal failure, administers corticosteroids for headache, and orders a right temporal artery biopsy, which shows no signs of vasculitis.
Family members report that Mr. C has not been himself—he has become increasingly withdrawn and “emotionless.” Mr. C’s wife says her husband has needed help with dressing and eating because of short-term memory loss over 9 months. She says he has lost 20 to 30 lb.
The patient’s cognitive function appears to have worsened since he developed these physical symptoms. Mrs. C also reports that he has had weakness and fatigue for 8 months.
One month earlier, the patient was admitted to a different hospital and treated for 2 weeks with IV antibiotics for fever of unknown origin. Results of lumbar puncture and extensive rheumatologic, infectious disease, urologic, and gastroenterologic evaluations were normal.
The internal medicine physician requests a psychiatric consultation. During our interview, Mr. C is cooperative, shows no signs of acute distress, is well groomed and dressed appropriately, and maintains eye contact. Speech rate and volume are low, with normal articulation and coherence, diminished spontaneity, and paucity of language. Mrs. C tells us her husband was lively and talkative before his recent illness. His mood is euthymic, and he is pleasant and cheerful during the evaluation.
The authors’ observations
Initially, we suspect an underlying medical condition is causing Mr. C’s psychiatric symptoms.
Mr. C’s wife reports that her husband stopped drinking 2 years ago after his family expressed concern about his health. Mr. C’s past alcohol use could not be quantified. He has not abused illicit drugs and has no personal or family history of dementia, trauma, or psychiatric or neurologic disorders.
EVALUATION: Impaired memory
Mr. C is afebrile during the initial physical examination, but fever returns within several days. Neurologic examination is normal, and negative rapid plasma reagin rules out syphilis. Vitamin B12 and folate levels are normal, as is thyroid function. Other laboratory findings are outside normal limits (Table).
Urine is cloudy with 2+ protein, 3+ blood, and trace leukocyte esterase. The presence of protein and blood suggests a glomerular disease such as a glomerulonephritis.
A positive leukocyte esterase test results from the presence of white blood cells, either as whole cells or as lysed cells. An abnormal number of leukocytes may appear with upper or lower urinary tract infection or in acute glomerulonephritis.
Chest radiography shows increased bilateral pulmonary vasculature, which can indicate pulmonary hypertension.
Mr. C has fair attention and concentration but impaired recent memory. He cannot recall yesterday’s events without help.
Mr. C’s Mini-Mental State Examination score of 21/30 suggests markedly impaired executive functioning and cognitive deficits. The attending psychiatrist recommends brain MRI.
Table
Mr. C’s laboratory findings
| Value | Normal range | |
|---|---|---|
| WBC | 15.1 | 4.8 to 10.8 cells/μL |
| Hb | 9 | 13.8 to 17.5 g/dL |
| Hct | 25.9% | 40% to 54% |
| MCV | 89.7 | 80 to 94 fL |
| BUN | 119 | 7 to 18 mg/dL |
| Cr | 12.1 | 0.7 to 1.3 mg/dL |
| Na | 125 | 136 to 145 mmol/L |
| K | 6.5 | 3.5 to 5 mEq/dL |
| HCO3 | 13.5 | 22 to 29 mmol/L |
| ECR | 125 | 30 mm/hr |
| WBC: white blood cell; Hb: hemoglobin; Hct: hematocrit; MCV: mean corpuscular volume; BUN: blood urea nitrogen; Cr: creatinine; Na: sodium; K: potassium; HCO3: bicarbonate; ESR: erythrocyte sedimentation rate | ||
The authors’ observations
Mr. C shows markedly impaired cognitive function without significant impairment of attention and concentration despite his progressive deterioration and increasing disability. Urine toxicology shows no illicit substances. Given his lack of a previous mood disorder and his family’s description of him as formerly vibrant and cheerful, he likely does not have a mood disorder.
Based on the history of events, including the symptom pattern, we rule out delirium. We suspect that Mr. C has dementia secondary to a general medical condition. His symptoms seem to be directly related to his medical complaints and do not have a waxing and waning course. The internal medicine physician orders additional laboratory tests.
TESTING: Kidney, lung damage
Over 5 days, Mr. C’s intermittent low-grade fevers continue. Laboratory tests are negative for HIV antibody, hepatitis panel, and antinuclear antibodies (ANA). C-reactive protein is elevated at 27.8 mg/dL (normal range,
Renal ultrasound is normal, but preliminary renal biopsy shows rapidly progressive glomerulonephritis. The internist immediately starts dialysis, cyclophosphamide at 1.5 mg/kg, and prednisone, 1 mg/kg. The pathology report on the renal biopsy describes extensive crescentic glomerular destruction, with inflammatory cells present.
Ten days after admission, Mr. C develops hemoptysis, and chest radiography shows increasing alveolar infiltrates. The attending internist consults pulmonary and critical care services.
The consultant suspects a pulmonary-renal syndrome because of bilateral alveolar infiltrates (diffuse alveolar hemorrhage). The internal medicine team continues high-dose corticosteroids, followed by plasmapheresis.
Brain MRI shows subacute to chronic infarcts involving the right basal ganglia and corona radiate and mild to moderate small vessel ischemic changes. Old areas of hemorrhage are noted within both cerebellar lobes, left temporal lobe, right basal ganglia, right parietal lobe, and right frontal lobe.
During follow-up interviews, Mr. C often cannot recall recent dialysis or plasmapheresis and reports no physical symptoms. His short-term memory continues to deteriorate; he would forget to eat if not cued by family or nursing staff. He shows global cognitive deficits and is increasingly withdrawn and flat.
The authors’ observations
Although few case reports have associated Goodpasture’s syndrome with neurobehavioral changes, the apparent relationship of Mr. C’s medical symptoms with the worsening of his cognitive impairment suggests a link.
Mr. C’s MRI findings also might suggest CNS vasculitis, which affects small arteries of the cerebral and spinal cord leptomeninges and parenchyma, leading to CNS dys-function.6-8 CNS vasculitis can result from primary nervous system involvement or from a secondary systemic process such as Goodpasture’s syndrome.9
We rule out lupus because Mr. C is ANA-negative; this test has 99% sensitivity for lupus.10
Goodpasture’s syndrome, which afflicts 1 Patients typically present with alveolar bleeding, rapidly progressive acute renal failure with proteinuria,1 and pulmonary symptoms such as dyspnea and hemoptysis.2
Possible triggers include:
- viral upper respiratory tract infection (20% to 60% of patients)3
- exposure to hydrocarbon solvents (3,4
Mr. C was exposed to solvents during the 15 years he worked in a factory. Some researchers believe a noxious event among genetically susceptible persons damages basement membrane and exposes an antigen that triggers IgG auto-antibody production.3,4
Malaise, weight loss, and fever are atypical in Goodpasture’s syndrome but could suggest concomitant vasculitis.5
OUTCOME: Ongoing disability
Mr. C is hospitalized for 6 weeks. He receives cyclophosphamide, prednisone, and 10 sessions of plasmapheresis. We prescribe mirtazapine, 15 mg at bedtime, to treat mood symptoms. We chose mirtazapine because of the drug’s sleep-restoring and appetite-stimulating properties.
Mr. C’s fever resolves and pulmonary function soon improves, but his cognitive impairment persists. He has difficulties preparing meals, taking medications, and managing his money.
Mr. C is discharged with a referral to a psychiatrist. He continues taking mirtazapine and a lower dose of prednisone. He requires ongoing hemodialysis and assistance with activities of daily living.
The authors’ observations
Prompt multidisciplinary intervention is critical when patients present with concurrent cognitive and medical symptoms. A thorough psychiatric evaluation can help piece together the illness’ course. The psychiatrist’s role in a multidisciplinary assessment is to:
- document neurocognitive changes
- verify them through collateral information
- correlate these changes with the timing of medical symptoms.
An underlying psychiatric condition can complicate the diagnosis. In these cases, careful interviewing and collateral information can help you discern the chronology of events.
1. Bolton WK. Goodpasture’s syndrome. Kidney Int 1996;50(5):1753-66.
2. Pusey CD. Anti-glomerular basement membrane disease. Kidney Int 2003;64(4):1535-50.
3. Humes HD, DuPont HL. eds. Kelley’s textbook of internal medicine. 4th ed. New York, NY: Lippincott Williams & Wilkins; 2000.
4. Stevenson A, Yaqoob M, Mason H, et al. Biochemical markers of basement membrane disturbances and occupational exposure to hydrocarbons and mixed solvents. QJM 1995;88(1):23-8.
5. Kluth DC, Rees AJ. Anti-glomerular basement membrane disease. J Am Soc Nephrol. 1999;10(11):2446-53.
6. Rydel JJ, Rodby RA. An 18-year-old man with Goodpasture’s syndrome and ANCA-negative central nervous system vasculitis. Am J Kidney Dis 1998;31(2):345-9.
7. Gittins N, Basu A, Eyre J, et al. Cerebral vasculitis in a teenager with Goodpasture’s syndrome. Nephrol Dial Transplant 2004;19(12):3168-71.
8. Garnier P, Deprele C. Cerebral angiitis and Goodpasture’s syndrome. Rev Neurol 2003;159(1):68-70.
9. Calabrese LH, Duna GF, Lie JT. Vasculitis in the central nervous system. Arthritis Rhem 1997;40(7):1189-201.
10. Edworthy SM, Zatarain E, McShane DJ, Bloch DA. Analysis of the 1982 ARA lupus criteria data set by recursive partitioning methodology: new insights into the relative merit of individual criteria. J Rheumatol 1988;15(10):1493-8.
1. Bolton WK. Goodpasture’s syndrome. Kidney Int 1996;50(5):1753-66.
2. Pusey CD. Anti-glomerular basement membrane disease. Kidney Int 2003;64(4):1535-50.
3. Humes HD, DuPont HL. eds. Kelley’s textbook of internal medicine. 4th ed. New York, NY: Lippincott Williams & Wilkins; 2000.
4. Stevenson A, Yaqoob M, Mason H, et al. Biochemical markers of basement membrane disturbances and occupational exposure to hydrocarbons and mixed solvents. QJM 1995;88(1):23-8.
5. Kluth DC, Rees AJ. Anti-glomerular basement membrane disease. J Am Soc Nephrol. 1999;10(11):2446-53.
6. Rydel JJ, Rodby RA. An 18-year-old man with Goodpasture’s syndrome and ANCA-negative central nervous system vasculitis. Am J Kidney Dis 1998;31(2):345-9.
7. Gittins N, Basu A, Eyre J, et al. Cerebral vasculitis in a teenager with Goodpasture’s syndrome. Nephrol Dial Transplant 2004;19(12):3168-71.
8. Garnier P, Deprele C. Cerebral angiitis and Goodpasture’s syndrome. Rev Neurol 2003;159(1):68-70.
9. Calabrese LH, Duna GF, Lie JT. Vasculitis in the central nervous system. Arthritis Rhem 1997;40(7):1189-201.
10. Edworthy SM, Zatarain E, McShane DJ, Bloch DA. Analysis of the 1982 ARA lupus criteria data set by recursive partitioning methodology: new insights into the relative merit of individual criteria. J Rheumatol 1988;15(10):1493-8.
Is dialectical behavior therapy the right ‘fit’ for your patient?
Strong evidence for the efficacy of dialectical behavior therapy (DBT) for patients with borderline personality disorder (BPD) has brought hope to clinicians and patients alike. By including cognitive therapy, behavioral strategies, skills training, and exposure therapy, DBT addresses more than just self-harm and suicidal behavior (Box 1).1-13 In fact, DBT’s primary interventions—such as skills training in emotion regulation and a straightforward approach to dysfunctional behaviors—could help many people.
Because DBT is so comprehensive and practical, clinicians might be tempted to refer almost anyone who seems even slightly “borderline” for DBT. But some patients—particularly those with mood and anxiety disorders—might benefit more from other treatments. To help you make appropriate evidence-based referrals for DBT and other psychological treatments, this article recommends 4 steps:
- Know what the treatment involves.
- Consider the evidence for the treatment in patients similar to yours.
- Consider why your patient—with unique characteristics and problems—would benefit from these specific interventions.
- Communicate to the patient your reasons for the referral.
Marsha Linehan, PhD, developed dialectical behavior therapy (DBT) in an attempt to devise an effective protocol for highly suicidal women. Over time, she realized that many of these women met criteria for borderline personality disorder (BPD), and DBT evolved to address their emotional, interpersonal, and mental health issues.1
Linehan et al2 published results from the first randomized clinical trial (RCT) of any psychological treatment for BPD. In this study, chronically parasuicidal women who met criteria for BPD received 1 year of DBT or “treatment as usual” in community settings. Those treated with DBT experienced fewer and less severe parasuicidal events, were more likely to remain in treatment, and required fewer days of inpatient care.
Findings from 9 additional RCTs have supported the efficacy of DBT for women with BPD and other populations.3 These RCTs have examined DBT (or adapted versions of DBT) for treating:
- women with BPD and substance dependence4,5
- men and women with BPD in a community setting6
- women veterans with BPD7
- non-BPD women with bulimia8 or binge-eating disorder9
- women with BPD in the Netherlands (53% of study subjects had a substance use disorder)10,11
- depressed older adults12
- suicidal women with BPD.13
Step 1. What does DBT involve?
Difficulty with emotion regulation. DBT is based on a biosocial theory of BPD.1 Within this framework, BPD is caused by the transaction (mutual interplay) of a biologically based vulnerability to emotions with an invalidating rearing environment. The patient with BPD typically experiences strong and long-lasting emotional reactions, often to seemingly small or insignificant events such as a slight look of disappointment on someone’s face or a minor daily hassle. Patients with BPD often are especially attuned to emotional reactions, particularly signs of rejection or disapproval.
Caregivers in an invalidating environment fail to provide the support a highly emotional child needs to learn to manage intense emotions. An invalidating environment:
- indiscriminately rejects the child’s communication of emotions and thoughts as invalid, independent of the validity of the child’s experience
- punishes emotional displays, then intermittently reinforces emotional escalation
- oversimplifies the ease of problem solving or coping.1
As a result, the fledgling BPD individual learns to mistrust and fear emotions and does not learn how to manage them. A patient with BPD is like a car with a powerful “emotional engine” but lacking brakes.
Team treatment. The standard DBT treatment package is an outpatient program run by a team.1 Therapists meet weekly for consultation to help them execute DBT according to the manual, prevent burn-out, and improve skills and motivation to treat patients with multiple, severe problems. The team also maintains the DBT program’s integrity and functioning and ensures that all treatment components—including individual therapy and skills training—are in place.
In individual therapy, the therapist and client collaborate to help the client reduce dysfunctional behaviors, increase motivation, and work toward goals. Because persons with BPD often present with many serious life problems, the therapist organizes session time to address 3 main priorities:
- Life-threatening behavior (intentional self-injury or imminent threat of intentional self-injury, including suicidal crises or threats, severe suicidal ideation or urges, suicide attempts, nonsuicidal self-injury or self-injury urges, or similar behaviors).
- Therapy-interfering behaviors (actions by the therapist or patient that interfere with progress, such as angry outbursts, missed sessions, or tardiness).
- Quality-of-life-interfering behavior (behaviors or problems—such as depression, eating disorders, or substance use disorders; homelessness or financial difficulties; or serious interpersonal discord—that make it hard for the patient to establish a reasonable quality of life).
Additional priorities include skills deficits and secondary targets.1 Each week, the client monitors his or her behaviors, emotions, and actions using a diary card. The therapist uses this information to collaboratively prioritize the focus of each individual therapy session.
Skills training typically occurs weekly in group sessions of 1.5 to 2.5 hours with 1 or 2 therapists. This structured, psycho-educational training focuses on skills that persons with BPD often lack:
- mindfulness (paying attention to the experience of the present moment)
- emotion regulation (regulating or managing distressing emotions)
- distress tolerance (averting crises, tolerating or accepting distressing situations or emotions)
- interpersonal effectiveness (maintaining relationships and asserting needs or wishes).
Therapists often use the first half of group sessions to review each patient’s homework and to provide feedback and coaching on effective skill use. The remaining time is spent teaching new skills. The therapist then assigns homework to practice new skills and closes with a wind-down exercise, often involving relaxation training.
Step 2. Consider the evidence
Before you make a referral for DBT (or any psychological treatment), know what the research says about who is likely to benefit from it. For women with BPD, DBT is the only treatment that can be considered “well-established.”3,14 The literature on DBT includes 10 randomized controlled trials (as well as many uncontrolled trials), and the strongest research supports its use in women with BPD.2,4-13
Based on a detailed review of the literature on DBT, I recommend a basic, evidence-based priority list for referrals (Table 1).3,12,13 Patient groups at the top are most likely to benefit from DBT—according to the most solid, rigorous research—and deserve your strongest consideration for referral. Patient groups further down the list—with fewer rigorous studies of DBT—merit less consideration of DBT as the treatment of choice. Of course to use this list, an accurate diagnosis of your patient’s problems is essential.
DBT’s treatment strategies—exposure therapy, skills training, cognitive therapy, emotion regulation training, and mindfulness—can work for other types of patients. I have noticed, however, that some clinicians refer patients with depression, anxiety disorders, or even bipolar disorder for DBT. Despite DBT’s intuitive appeal, sufficient evidence does not yet support its use in patients with these disorders. Other evidence-based treatments may be more suitable for patients with uncomplicated mood and anxiety disorders (Table 2).3
Table 1
Candidates for DBT: An evidence-based referral priority list*

| Women with BPD who are suicidal or who self-harm (without bipolar disorder, a psychotic disorder, or mental retardation). One randomized clinical trial with suicidal individuals with BPD included men. Two studies excluded participants with substance dependence, but the most recent, largest study13 did not. |
| Women with BPD and substance use problems (without bipolar disorder, a psychotic disorder, or cognitive impairment) | |
| Women with bulimia nervosa or binge-eating disorder (without substance abuse, psychotic disorder, or suicidal ideation). Other empirically supported treatments exist for these patients (Table 2). | |
| Depressed older adults (age ≥60, without bipolar disorder, a psychotic disorder, or cognitive impairment). Investigated treatments included group DBT skills training, telephone consultation, selective serotonin reuptake inhibitor medications, and psychiatric clinical management.12 | |
| Suicidal and nonsuicidal adolescentswith oppositional defiant disorder or bipolar disorder | |
| Incarcerated men and womenwith or without BPD, in high- and low-security forensic settings | |
| Couples and families where 1 member has BPDor where domestic violence occurs in an intimate relationship | |
| * Persons at the top of the list are the ones for whom the most solid, rigorous research has demonstrated the efficacy of DBT. Fewer rigorous studies of DBT have been conducted in persons further down the list. | |
| BPD: borderline personality disorder; DBT: dialectical behavior therapy | |
| Source: References 3,12,13 | |
Table 2
When not to refer a patient for DBT: Evidence is stronger for alternate treatments
| Diagnosis | Treatments with empirical support |
|---|---|
| Major depressive disorder | CBT, behavioral activation, interpersonal therapy, antidepressant medication, mindfulness-based cognitive therapy for depressive relapse |
| Panic disorder/panic disorder with agoraphobia | CBT involving cognitive therapy and exposure-based with agoraphobia interventions |
| Posttraumatic stress disorder | Prolonged exposure therapy, cognitive therapy, EMDR |
| Bulimia nervosa | CBT, interpersonal therapy |
| Primary substance use disorders | CBT, motivational enhancement/motivational interviewing, community reinforcement approach |
| Psychotic disorders | Medication management, social skills training, family-based interventions |
| CBT: cognitive-behavioral therapy; DBT: dialectical behavior therapy; EMDR: eye movement desensitization and reprocessing therapy | |
| Source: Reference 3 | |
Step 3: Would this patient benefit?
Would your patient, with unique struggles and characteristics, benefit from DBT? Consider to what degree DBT’s interventions would solve some of your patient’s problems and whether DBT fits your patient’s needs.
DBT’s target problems. In controlled trials, DBT’s pragmatic approach outperforms comparator treatments in reducing suicidal behaviors and self-injury, and DBT therapists monitor and target these behaviors. Thus, because few treatments reduce self-injury,15,16 you might consider DBT for patients who self-injure even if they do not have BPD.
DBT also includes a strong focus on emotions and emotion regulation. Therefore, if difficulty managing emotions is among your patient’s primary problems, DBT may offer some benefit. DBT also includes structured interpersonal skills training that might be useful for patients who lack assertiveness.
Finally, if you have a patient with multiple diagnoses and severe problems—but not psychosis—the DBT approach to organizing and prioritizing treatment targets may be beneficial. Some multi-diagnosis patients may struggle with aspects of DBT (such as learning new skills), but DBT is set up to incorporate other empirically supported treatment protocols for co-occurring Axis I and II disorders.
Does DBT ‘fit’ your patient? DBT is very structured and involves direct discussions of maladaptive behaviors. If your patient prefers or would benefit from a structured approach, you might consider a referral for DBT.
DBT is an outpatient behavioral treatment that focuses on the here and now. DBT might not be the best fit if your patient:
- views his or her problems as resulting primarily from childhood experiences or relationships with parents
- would prefer insight-oriented therapy.
If, however, your patient would like a practical approach focused on problem-solving, DBT could be an effective choice.
DBT is based in part on a dialectical philosophy, and DBT therapists often seek to bring together or synthesize polarized thinking. If your patient struggles with “black or white” thinking, this dialectical philosophy might be helpful. On the other hand, DBT might not be the best fit if your patient is particularly rigid in thinking or seems to require cognitive therapy to address his or her thought patterns.
DBT is not the treatment of choice for all personality disorders. Most of the evidence examines its use for BPD, and few studies have looked at any other personality disorder. Also, keep in mind that being interpersonally “difficult” does not mean that a patient is “borderline” or needs DBT.
Step 4: Communicate reasons for referral to your patient
Finally, communicate to your patient the reasons you are referring him or her for DBT. Patients often walk into my office for DBT, confused about why they are there. If patients understand why they have been referred for DBT and how it may help them, they may be more likely to follow through and realize its benefits.
A sample explanation of referral that I offer to guide this communication (Box 2) includes 3 main points:
- my diagnosis or conceptualization of the patient’s clinical problems
- a brief description of DBT
- a rationale for why DBT would be a good fit, and what kinds of benefits the patient might receive.
Based on my initial assessment, you seem to meet criteria for a diagnosis of borderline personality disorder, or BPD. A diagnosis is a category for different symptoms or experiences. To receive a BPD diagnosis, a person has to have at least 5 of 9 symptoms, and you seem to have about 6 of them. From what you have said, the main problems you struggle with are roller-coaster emotions and moods, problems with relationships with other people, and self-harm.
A lot of people recover from BPD, and there’s no reason to think you will have these problems for the rest of your life. In fact, there is a very effective treatment for BPD. This treatment is called dialectical behavior therapy, or DBT. I think you’re a great candidate for DBT. Of course, there’s no guarantee that DBT is the ideal treatment for you, but several studies have shown that DBT helps people learn how to manage their emotions, reduce self-harm, and improve their functioning in life.
DBT includes a couple of different things: meeting once a week with a therapist on an individual basis, then meeting once a week with a group. In the group, you will learn how to manage your emotions, pay attention to the present moment, deal with other people, and tolerate being upset without getting into a crisis.
I know some people in town who provide DBT. Is this something you think you might be interested in? If so, what questions do you have?
Related Resources
- Chapman AL, Gratz KL. The borderline personality disorder survival guide: everything you need to know about living with BPD. Oakland, CA: New Harbinger Publications; 2007.
- National Education Alliance for Borderline Personality Disorder. Information for professionals, patients, and families. www.neabpd.org.
- Behavioral Tech, LLC, founded by Marsha Linehan, PhD. DBT training and resources, including a directory of DBT therapists. www.behavioraltech.org.
- Dialectical Behaviour Therapy Centre of Vancouver. www.dbtvancouver.com.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Linehan MM. Cognitive behavior treatment of borderline personality disorder. New York, NY: The Guilford Press; 1993.
2. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991;48:1060-4.
3. Robins CJ, Chapman AL. Dialectical behavior therapy: current status, recent developments, and future directions. J Personal Disord 2004;18:73-9.
4. Linehan MM, Schmidt HI, Dimeff LA, et al. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addictions 1999;8:279-92.
5. Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorder. Cognit Behav Pract 2000;7:413-9.
6. Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend 2002;67:13-26.
7. Koons C, Robins CJ, Tweed JL, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behav Ther 2001;32:371-90.
8. Safer DL, Telch CF, Agras WS. Dialectical behavior therapy for bulimia nervosa. Am J Psychiatry 2001;158:632-4.
9. Telch CF, Agras WS, Linehan MM. Dialectical behavior therapy for binge eating disorder. J Consult Clin Psychol 2001;69:1061-5.
10. van den Bosch LMC, Verheul R, Schippers GM, van den Brink W. Dialectical behavior therapy of borderline patients with and without substance abuse problems: implementation and long-term effects. Addict Behav 2002;27:911-23.
11. Verheul R, van den Bosch LMC, Koeter MWJ, et al. Dialectical behavior therapy for women with borderline personality disorder. Br J Psychiatry 2003;182:135-40.
12. Lynch TR, Morse JQ, Mendelson T, Robins CJ. Dialectical behavior therapy for depressed older adults: a randomized pilot study. Am J Geriatr Psychiatry 2003;11:33-45.
13. Linehan MM, Comtois KA, Murray AM, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs. therapy by experts for suicidal behaviors and borderline personality disorder. Arch Gen Psychiatry 2006;63:757-66.
14. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol 2001;52:685-716.
15. Hawton K, Arensman E, Townsend E, et al. Deliberate self-harm: systematic review of efficacy of psychosocial and pharmacological treatments in preventing repetition. BMJ 1998;317:441-7.
16. Tyrer P, Tom B, Byford S, et al. Differential effects of manual-assisted cognitive behavior therapy in the treatment of recurrent deliberate self-harm and personality disturbance: the POPMACT study. J Personal Disord 2004;18:102-16.
Strong evidence for the efficacy of dialectical behavior therapy (DBT) for patients with borderline personality disorder (BPD) has brought hope to clinicians and patients alike. By including cognitive therapy, behavioral strategies, skills training, and exposure therapy, DBT addresses more than just self-harm and suicidal behavior (Box 1).1-13 In fact, DBT’s primary interventions—such as skills training in emotion regulation and a straightforward approach to dysfunctional behaviors—could help many people.
Because DBT is so comprehensive and practical, clinicians might be tempted to refer almost anyone who seems even slightly “borderline” for DBT. But some patients—particularly those with mood and anxiety disorders—might benefit more from other treatments. To help you make appropriate evidence-based referrals for DBT and other psychological treatments, this article recommends 4 steps:
- Know what the treatment involves.
- Consider the evidence for the treatment in patients similar to yours.
- Consider why your patient—with unique characteristics and problems—would benefit from these specific interventions.
- Communicate to the patient your reasons for the referral.
Marsha Linehan, PhD, developed dialectical behavior therapy (DBT) in an attempt to devise an effective protocol for highly suicidal women. Over time, she realized that many of these women met criteria for borderline personality disorder (BPD), and DBT evolved to address their emotional, interpersonal, and mental health issues.1
Linehan et al2 published results from the first randomized clinical trial (RCT) of any psychological treatment for BPD. In this study, chronically parasuicidal women who met criteria for BPD received 1 year of DBT or “treatment as usual” in community settings. Those treated with DBT experienced fewer and less severe parasuicidal events, were more likely to remain in treatment, and required fewer days of inpatient care.
Findings from 9 additional RCTs have supported the efficacy of DBT for women with BPD and other populations.3 These RCTs have examined DBT (or adapted versions of DBT) for treating:
- women with BPD and substance dependence4,5
- men and women with BPD in a community setting6
- women veterans with BPD7
- non-BPD women with bulimia8 or binge-eating disorder9
- women with BPD in the Netherlands (53% of study subjects had a substance use disorder)10,11
- depressed older adults12
- suicidal women with BPD.13
Step 1. What does DBT involve?
Difficulty with emotion regulation. DBT is based on a biosocial theory of BPD.1 Within this framework, BPD is caused by the transaction (mutual interplay) of a biologically based vulnerability to emotions with an invalidating rearing environment. The patient with BPD typically experiences strong and long-lasting emotional reactions, often to seemingly small or insignificant events such as a slight look of disappointment on someone’s face or a minor daily hassle. Patients with BPD often are especially attuned to emotional reactions, particularly signs of rejection or disapproval.
Caregivers in an invalidating environment fail to provide the support a highly emotional child needs to learn to manage intense emotions. An invalidating environment:
- indiscriminately rejects the child’s communication of emotions and thoughts as invalid, independent of the validity of the child’s experience
- punishes emotional displays, then intermittently reinforces emotional escalation
- oversimplifies the ease of problem solving or coping.1
As a result, the fledgling BPD individual learns to mistrust and fear emotions and does not learn how to manage them. A patient with BPD is like a car with a powerful “emotional engine” but lacking brakes.
Team treatment. The standard DBT treatment package is an outpatient program run by a team.1 Therapists meet weekly for consultation to help them execute DBT according to the manual, prevent burn-out, and improve skills and motivation to treat patients with multiple, severe problems. The team also maintains the DBT program’s integrity and functioning and ensures that all treatment components—including individual therapy and skills training—are in place.
In individual therapy, the therapist and client collaborate to help the client reduce dysfunctional behaviors, increase motivation, and work toward goals. Because persons with BPD often present with many serious life problems, the therapist organizes session time to address 3 main priorities:
- Life-threatening behavior (intentional self-injury or imminent threat of intentional self-injury, including suicidal crises or threats, severe suicidal ideation or urges, suicide attempts, nonsuicidal self-injury or self-injury urges, or similar behaviors).
- Therapy-interfering behaviors (actions by the therapist or patient that interfere with progress, such as angry outbursts, missed sessions, or tardiness).
- Quality-of-life-interfering behavior (behaviors or problems—such as depression, eating disorders, or substance use disorders; homelessness or financial difficulties; or serious interpersonal discord—that make it hard for the patient to establish a reasonable quality of life).
Additional priorities include skills deficits and secondary targets.1 Each week, the client monitors his or her behaviors, emotions, and actions using a diary card. The therapist uses this information to collaboratively prioritize the focus of each individual therapy session.
Skills training typically occurs weekly in group sessions of 1.5 to 2.5 hours with 1 or 2 therapists. This structured, psycho-educational training focuses on skills that persons with BPD often lack:
- mindfulness (paying attention to the experience of the present moment)
- emotion regulation (regulating or managing distressing emotions)
- distress tolerance (averting crises, tolerating or accepting distressing situations or emotions)
- interpersonal effectiveness (maintaining relationships and asserting needs or wishes).
Therapists often use the first half of group sessions to review each patient’s homework and to provide feedback and coaching on effective skill use. The remaining time is spent teaching new skills. The therapist then assigns homework to practice new skills and closes with a wind-down exercise, often involving relaxation training.
Step 2. Consider the evidence
Before you make a referral for DBT (or any psychological treatment), know what the research says about who is likely to benefit from it. For women with BPD, DBT is the only treatment that can be considered “well-established.”3,14 The literature on DBT includes 10 randomized controlled trials (as well as many uncontrolled trials), and the strongest research supports its use in women with BPD.2,4-13
Based on a detailed review of the literature on DBT, I recommend a basic, evidence-based priority list for referrals (Table 1).3,12,13 Patient groups at the top are most likely to benefit from DBT—according to the most solid, rigorous research—and deserve your strongest consideration for referral. Patient groups further down the list—with fewer rigorous studies of DBT—merit less consideration of DBT as the treatment of choice. Of course to use this list, an accurate diagnosis of your patient’s problems is essential.
DBT’s treatment strategies—exposure therapy, skills training, cognitive therapy, emotion regulation training, and mindfulness—can work for other types of patients. I have noticed, however, that some clinicians refer patients with depression, anxiety disorders, or even bipolar disorder for DBT. Despite DBT’s intuitive appeal, sufficient evidence does not yet support its use in patients with these disorders. Other evidence-based treatments may be more suitable for patients with uncomplicated mood and anxiety disorders (Table 2).3
Table 1
Candidates for DBT: An evidence-based referral priority list*

| Women with BPD who are suicidal or who self-harm (without bipolar disorder, a psychotic disorder, or mental retardation). One randomized clinical trial with suicidal individuals with BPD included men. Two studies excluded participants with substance dependence, but the most recent, largest study13 did not. |
| Women with BPD and substance use problems (without bipolar disorder, a psychotic disorder, or cognitive impairment) | |
| Women with bulimia nervosa or binge-eating disorder (without substance abuse, psychotic disorder, or suicidal ideation). Other empirically supported treatments exist for these patients (Table 2). | |
| Depressed older adults (age ≥60, without bipolar disorder, a psychotic disorder, or cognitive impairment). Investigated treatments included group DBT skills training, telephone consultation, selective serotonin reuptake inhibitor medications, and psychiatric clinical management.12 | |
| Suicidal and nonsuicidal adolescentswith oppositional defiant disorder or bipolar disorder | |
| Incarcerated men and womenwith or without BPD, in high- and low-security forensic settings | |
| Couples and families where 1 member has BPDor where domestic violence occurs in an intimate relationship | |
| * Persons at the top of the list are the ones for whom the most solid, rigorous research has demonstrated the efficacy of DBT. Fewer rigorous studies of DBT have been conducted in persons further down the list. | |
| BPD: borderline personality disorder; DBT: dialectical behavior therapy | |
| Source: References 3,12,13 | |
Table 2
When not to refer a patient for DBT: Evidence is stronger for alternate treatments
| Diagnosis | Treatments with empirical support |
|---|---|
| Major depressive disorder | CBT, behavioral activation, interpersonal therapy, antidepressant medication, mindfulness-based cognitive therapy for depressive relapse |
| Panic disorder/panic disorder with agoraphobia | CBT involving cognitive therapy and exposure-based with agoraphobia interventions |
| Posttraumatic stress disorder | Prolonged exposure therapy, cognitive therapy, EMDR |
| Bulimia nervosa | CBT, interpersonal therapy |
| Primary substance use disorders | CBT, motivational enhancement/motivational interviewing, community reinforcement approach |
| Psychotic disorders | Medication management, social skills training, family-based interventions |
| CBT: cognitive-behavioral therapy; DBT: dialectical behavior therapy; EMDR: eye movement desensitization and reprocessing therapy | |
| Source: Reference 3 | |
Step 3: Would this patient benefit?
Would your patient, with unique struggles and characteristics, benefit from DBT? Consider to what degree DBT’s interventions would solve some of your patient’s problems and whether DBT fits your patient’s needs.
DBT’s target problems. In controlled trials, DBT’s pragmatic approach outperforms comparator treatments in reducing suicidal behaviors and self-injury, and DBT therapists monitor and target these behaviors. Thus, because few treatments reduce self-injury,15,16 you might consider DBT for patients who self-injure even if they do not have BPD.
DBT also includes a strong focus on emotions and emotion regulation. Therefore, if difficulty managing emotions is among your patient’s primary problems, DBT may offer some benefit. DBT also includes structured interpersonal skills training that might be useful for patients who lack assertiveness.
Finally, if you have a patient with multiple diagnoses and severe problems—but not psychosis—the DBT approach to organizing and prioritizing treatment targets may be beneficial. Some multi-diagnosis patients may struggle with aspects of DBT (such as learning new skills), but DBT is set up to incorporate other empirically supported treatment protocols for co-occurring Axis I and II disorders.
Does DBT ‘fit’ your patient? DBT is very structured and involves direct discussions of maladaptive behaviors. If your patient prefers or would benefit from a structured approach, you might consider a referral for DBT.
DBT is an outpatient behavioral treatment that focuses on the here and now. DBT might not be the best fit if your patient:
- views his or her problems as resulting primarily from childhood experiences or relationships with parents
- would prefer insight-oriented therapy.
If, however, your patient would like a practical approach focused on problem-solving, DBT could be an effective choice.
DBT is based in part on a dialectical philosophy, and DBT therapists often seek to bring together or synthesize polarized thinking. If your patient struggles with “black or white” thinking, this dialectical philosophy might be helpful. On the other hand, DBT might not be the best fit if your patient is particularly rigid in thinking or seems to require cognitive therapy to address his or her thought patterns.
DBT is not the treatment of choice for all personality disorders. Most of the evidence examines its use for BPD, and few studies have looked at any other personality disorder. Also, keep in mind that being interpersonally “difficult” does not mean that a patient is “borderline” or needs DBT.
Step 4: Communicate reasons for referral to your patient
Finally, communicate to your patient the reasons you are referring him or her for DBT. Patients often walk into my office for DBT, confused about why they are there. If patients understand why they have been referred for DBT and how it may help them, they may be more likely to follow through and realize its benefits.
A sample explanation of referral that I offer to guide this communication (Box 2) includes 3 main points:
- my diagnosis or conceptualization of the patient’s clinical problems
- a brief description of DBT
- a rationale for why DBT would be a good fit, and what kinds of benefits the patient might receive.
Based on my initial assessment, you seem to meet criteria for a diagnosis of borderline personality disorder, or BPD. A diagnosis is a category for different symptoms or experiences. To receive a BPD diagnosis, a person has to have at least 5 of 9 symptoms, and you seem to have about 6 of them. From what you have said, the main problems you struggle with are roller-coaster emotions and moods, problems with relationships with other people, and self-harm.
A lot of people recover from BPD, and there’s no reason to think you will have these problems for the rest of your life. In fact, there is a very effective treatment for BPD. This treatment is called dialectical behavior therapy, or DBT. I think you’re a great candidate for DBT. Of course, there’s no guarantee that DBT is the ideal treatment for you, but several studies have shown that DBT helps people learn how to manage their emotions, reduce self-harm, and improve their functioning in life.
DBT includes a couple of different things: meeting once a week with a therapist on an individual basis, then meeting once a week with a group. In the group, you will learn how to manage your emotions, pay attention to the present moment, deal with other people, and tolerate being upset without getting into a crisis.
I know some people in town who provide DBT. Is this something you think you might be interested in? If so, what questions do you have?
Related Resources
- Chapman AL, Gratz KL. The borderline personality disorder survival guide: everything you need to know about living with BPD. Oakland, CA: New Harbinger Publications; 2007.
- National Education Alliance for Borderline Personality Disorder. Information for professionals, patients, and families. www.neabpd.org.
- Behavioral Tech, LLC, founded by Marsha Linehan, PhD. DBT training and resources, including a directory of DBT therapists. www.behavioraltech.org.
- Dialectical Behaviour Therapy Centre of Vancouver. www.dbtvancouver.com.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Strong evidence for the efficacy of dialectical behavior therapy (DBT) for patients with borderline personality disorder (BPD) has brought hope to clinicians and patients alike. By including cognitive therapy, behavioral strategies, skills training, and exposure therapy, DBT addresses more than just self-harm and suicidal behavior (Box 1).1-13 In fact, DBT’s primary interventions—such as skills training in emotion regulation and a straightforward approach to dysfunctional behaviors—could help many people.
Because DBT is so comprehensive and practical, clinicians might be tempted to refer almost anyone who seems even slightly “borderline” for DBT. But some patients—particularly those with mood and anxiety disorders—might benefit more from other treatments. To help you make appropriate evidence-based referrals for DBT and other psychological treatments, this article recommends 4 steps:
- Know what the treatment involves.
- Consider the evidence for the treatment in patients similar to yours.
- Consider why your patient—with unique characteristics and problems—would benefit from these specific interventions.
- Communicate to the patient your reasons for the referral.
Marsha Linehan, PhD, developed dialectical behavior therapy (DBT) in an attempt to devise an effective protocol for highly suicidal women. Over time, she realized that many of these women met criteria for borderline personality disorder (BPD), and DBT evolved to address their emotional, interpersonal, and mental health issues.1
Linehan et al2 published results from the first randomized clinical trial (RCT) of any psychological treatment for BPD. In this study, chronically parasuicidal women who met criteria for BPD received 1 year of DBT or “treatment as usual” in community settings. Those treated with DBT experienced fewer and less severe parasuicidal events, were more likely to remain in treatment, and required fewer days of inpatient care.
Findings from 9 additional RCTs have supported the efficacy of DBT for women with BPD and other populations.3 These RCTs have examined DBT (or adapted versions of DBT) for treating:
- women with BPD and substance dependence4,5
- men and women with BPD in a community setting6
- women veterans with BPD7
- non-BPD women with bulimia8 or binge-eating disorder9
- women with BPD in the Netherlands (53% of study subjects had a substance use disorder)10,11
- depressed older adults12
- suicidal women with BPD.13
Step 1. What does DBT involve?
Difficulty with emotion regulation. DBT is based on a biosocial theory of BPD.1 Within this framework, BPD is caused by the transaction (mutual interplay) of a biologically based vulnerability to emotions with an invalidating rearing environment. The patient with BPD typically experiences strong and long-lasting emotional reactions, often to seemingly small or insignificant events such as a slight look of disappointment on someone’s face or a minor daily hassle. Patients with BPD often are especially attuned to emotional reactions, particularly signs of rejection or disapproval.
Caregivers in an invalidating environment fail to provide the support a highly emotional child needs to learn to manage intense emotions. An invalidating environment:
- indiscriminately rejects the child’s communication of emotions and thoughts as invalid, independent of the validity of the child’s experience
- punishes emotional displays, then intermittently reinforces emotional escalation
- oversimplifies the ease of problem solving or coping.1
As a result, the fledgling BPD individual learns to mistrust and fear emotions and does not learn how to manage them. A patient with BPD is like a car with a powerful “emotional engine” but lacking brakes.
Team treatment. The standard DBT treatment package is an outpatient program run by a team.1 Therapists meet weekly for consultation to help them execute DBT according to the manual, prevent burn-out, and improve skills and motivation to treat patients with multiple, severe problems. The team also maintains the DBT program’s integrity and functioning and ensures that all treatment components—including individual therapy and skills training—are in place.
In individual therapy, the therapist and client collaborate to help the client reduce dysfunctional behaviors, increase motivation, and work toward goals. Because persons with BPD often present with many serious life problems, the therapist organizes session time to address 3 main priorities:
- Life-threatening behavior (intentional self-injury or imminent threat of intentional self-injury, including suicidal crises or threats, severe suicidal ideation or urges, suicide attempts, nonsuicidal self-injury or self-injury urges, or similar behaviors).
- Therapy-interfering behaviors (actions by the therapist or patient that interfere with progress, such as angry outbursts, missed sessions, or tardiness).
- Quality-of-life-interfering behavior (behaviors or problems—such as depression, eating disorders, or substance use disorders; homelessness or financial difficulties; or serious interpersonal discord—that make it hard for the patient to establish a reasonable quality of life).
Additional priorities include skills deficits and secondary targets.1 Each week, the client monitors his or her behaviors, emotions, and actions using a diary card. The therapist uses this information to collaboratively prioritize the focus of each individual therapy session.
Skills training typically occurs weekly in group sessions of 1.5 to 2.5 hours with 1 or 2 therapists. This structured, psycho-educational training focuses on skills that persons with BPD often lack:
- mindfulness (paying attention to the experience of the present moment)
- emotion regulation (regulating or managing distressing emotions)
- distress tolerance (averting crises, tolerating or accepting distressing situations or emotions)
- interpersonal effectiveness (maintaining relationships and asserting needs or wishes).
Therapists often use the first half of group sessions to review each patient’s homework and to provide feedback and coaching on effective skill use. The remaining time is spent teaching new skills. The therapist then assigns homework to practice new skills and closes with a wind-down exercise, often involving relaxation training.
Step 2. Consider the evidence
Before you make a referral for DBT (or any psychological treatment), know what the research says about who is likely to benefit from it. For women with BPD, DBT is the only treatment that can be considered “well-established.”3,14 The literature on DBT includes 10 randomized controlled trials (as well as many uncontrolled trials), and the strongest research supports its use in women with BPD.2,4-13
Based on a detailed review of the literature on DBT, I recommend a basic, evidence-based priority list for referrals (Table 1).3,12,13 Patient groups at the top are most likely to benefit from DBT—according to the most solid, rigorous research—and deserve your strongest consideration for referral. Patient groups further down the list—with fewer rigorous studies of DBT—merit less consideration of DBT as the treatment of choice. Of course to use this list, an accurate diagnosis of your patient’s problems is essential.
DBT’s treatment strategies—exposure therapy, skills training, cognitive therapy, emotion regulation training, and mindfulness—can work for other types of patients. I have noticed, however, that some clinicians refer patients with depression, anxiety disorders, or even bipolar disorder for DBT. Despite DBT’s intuitive appeal, sufficient evidence does not yet support its use in patients with these disorders. Other evidence-based treatments may be more suitable for patients with uncomplicated mood and anxiety disorders (Table 2).3
Table 1
Candidates for DBT: An evidence-based referral priority list*

| Women with BPD who are suicidal or who self-harm (without bipolar disorder, a psychotic disorder, or mental retardation). One randomized clinical trial with suicidal individuals with BPD included men. Two studies excluded participants with substance dependence, but the most recent, largest study13 did not. |
| Women with BPD and substance use problems (without bipolar disorder, a psychotic disorder, or cognitive impairment) | |
| Women with bulimia nervosa or binge-eating disorder (without substance abuse, psychotic disorder, or suicidal ideation). Other empirically supported treatments exist for these patients (Table 2). | |
| Depressed older adults (age ≥60, without bipolar disorder, a psychotic disorder, or cognitive impairment). Investigated treatments included group DBT skills training, telephone consultation, selective serotonin reuptake inhibitor medications, and psychiatric clinical management.12 | |
| Suicidal and nonsuicidal adolescentswith oppositional defiant disorder or bipolar disorder | |
| Incarcerated men and womenwith or without BPD, in high- and low-security forensic settings | |
| Couples and families where 1 member has BPDor where domestic violence occurs in an intimate relationship | |
| * Persons at the top of the list are the ones for whom the most solid, rigorous research has demonstrated the efficacy of DBT. Fewer rigorous studies of DBT have been conducted in persons further down the list. | |
| BPD: borderline personality disorder; DBT: dialectical behavior therapy | |
| Source: References 3,12,13 | |
Table 2
When not to refer a patient for DBT: Evidence is stronger for alternate treatments
| Diagnosis | Treatments with empirical support |
|---|---|
| Major depressive disorder | CBT, behavioral activation, interpersonal therapy, antidepressant medication, mindfulness-based cognitive therapy for depressive relapse |
| Panic disorder/panic disorder with agoraphobia | CBT involving cognitive therapy and exposure-based with agoraphobia interventions |
| Posttraumatic stress disorder | Prolonged exposure therapy, cognitive therapy, EMDR |
| Bulimia nervosa | CBT, interpersonal therapy |
| Primary substance use disorders | CBT, motivational enhancement/motivational interviewing, community reinforcement approach |
| Psychotic disorders | Medication management, social skills training, family-based interventions |
| CBT: cognitive-behavioral therapy; DBT: dialectical behavior therapy; EMDR: eye movement desensitization and reprocessing therapy | |
| Source: Reference 3 | |
Step 3: Would this patient benefit?
Would your patient, with unique struggles and characteristics, benefit from DBT? Consider to what degree DBT’s interventions would solve some of your patient’s problems and whether DBT fits your patient’s needs.
DBT’s target problems. In controlled trials, DBT’s pragmatic approach outperforms comparator treatments in reducing suicidal behaviors and self-injury, and DBT therapists monitor and target these behaviors. Thus, because few treatments reduce self-injury,15,16 you might consider DBT for patients who self-injure even if they do not have BPD.
DBT also includes a strong focus on emotions and emotion regulation. Therefore, if difficulty managing emotions is among your patient’s primary problems, DBT may offer some benefit. DBT also includes structured interpersonal skills training that might be useful for patients who lack assertiveness.
Finally, if you have a patient with multiple diagnoses and severe problems—but not psychosis—the DBT approach to organizing and prioritizing treatment targets may be beneficial. Some multi-diagnosis patients may struggle with aspects of DBT (such as learning new skills), but DBT is set up to incorporate other empirically supported treatment protocols for co-occurring Axis I and II disorders.
Does DBT ‘fit’ your patient? DBT is very structured and involves direct discussions of maladaptive behaviors. If your patient prefers or would benefit from a structured approach, you might consider a referral for DBT.
DBT is an outpatient behavioral treatment that focuses on the here and now. DBT might not be the best fit if your patient:
- views his or her problems as resulting primarily from childhood experiences or relationships with parents
- would prefer insight-oriented therapy.
If, however, your patient would like a practical approach focused on problem-solving, DBT could be an effective choice.
DBT is based in part on a dialectical philosophy, and DBT therapists often seek to bring together or synthesize polarized thinking. If your patient struggles with “black or white” thinking, this dialectical philosophy might be helpful. On the other hand, DBT might not be the best fit if your patient is particularly rigid in thinking or seems to require cognitive therapy to address his or her thought patterns.
DBT is not the treatment of choice for all personality disorders. Most of the evidence examines its use for BPD, and few studies have looked at any other personality disorder. Also, keep in mind that being interpersonally “difficult” does not mean that a patient is “borderline” or needs DBT.
Step 4: Communicate reasons for referral to your patient
Finally, communicate to your patient the reasons you are referring him or her for DBT. Patients often walk into my office for DBT, confused about why they are there. If patients understand why they have been referred for DBT and how it may help them, they may be more likely to follow through and realize its benefits.
A sample explanation of referral that I offer to guide this communication (Box 2) includes 3 main points:
- my diagnosis or conceptualization of the patient’s clinical problems
- a brief description of DBT
- a rationale for why DBT would be a good fit, and what kinds of benefits the patient might receive.
Based on my initial assessment, you seem to meet criteria for a diagnosis of borderline personality disorder, or BPD. A diagnosis is a category for different symptoms or experiences. To receive a BPD diagnosis, a person has to have at least 5 of 9 symptoms, and you seem to have about 6 of them. From what you have said, the main problems you struggle with are roller-coaster emotions and moods, problems with relationships with other people, and self-harm.
A lot of people recover from BPD, and there’s no reason to think you will have these problems for the rest of your life. In fact, there is a very effective treatment for BPD. This treatment is called dialectical behavior therapy, or DBT. I think you’re a great candidate for DBT. Of course, there’s no guarantee that DBT is the ideal treatment for you, but several studies have shown that DBT helps people learn how to manage their emotions, reduce self-harm, and improve their functioning in life.
DBT includes a couple of different things: meeting once a week with a therapist on an individual basis, then meeting once a week with a group. In the group, you will learn how to manage your emotions, pay attention to the present moment, deal with other people, and tolerate being upset without getting into a crisis.
I know some people in town who provide DBT. Is this something you think you might be interested in? If so, what questions do you have?
Related Resources
- Chapman AL, Gratz KL. The borderline personality disorder survival guide: everything you need to know about living with BPD. Oakland, CA: New Harbinger Publications; 2007.
- National Education Alliance for Borderline Personality Disorder. Information for professionals, patients, and families. www.neabpd.org.
- Behavioral Tech, LLC, founded by Marsha Linehan, PhD. DBT training and resources, including a directory of DBT therapists. www.behavioraltech.org.
- Dialectical Behaviour Therapy Centre of Vancouver. www.dbtvancouver.com.
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Linehan MM. Cognitive behavior treatment of borderline personality disorder. New York, NY: The Guilford Press; 1993.
2. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991;48:1060-4.
3. Robins CJ, Chapman AL. Dialectical behavior therapy: current status, recent developments, and future directions. J Personal Disord 2004;18:73-9.
4. Linehan MM, Schmidt HI, Dimeff LA, et al. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addictions 1999;8:279-92.
5. Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorder. Cognit Behav Pract 2000;7:413-9.
6. Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend 2002;67:13-26.
7. Koons C, Robins CJ, Tweed JL, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behav Ther 2001;32:371-90.
8. Safer DL, Telch CF, Agras WS. Dialectical behavior therapy for bulimia nervosa. Am J Psychiatry 2001;158:632-4.
9. Telch CF, Agras WS, Linehan MM. Dialectical behavior therapy for binge eating disorder. J Consult Clin Psychol 2001;69:1061-5.
10. van den Bosch LMC, Verheul R, Schippers GM, van den Brink W. Dialectical behavior therapy of borderline patients with and without substance abuse problems: implementation and long-term effects. Addict Behav 2002;27:911-23.
11. Verheul R, van den Bosch LMC, Koeter MWJ, et al. Dialectical behavior therapy for women with borderline personality disorder. Br J Psychiatry 2003;182:135-40.
12. Lynch TR, Morse JQ, Mendelson T, Robins CJ. Dialectical behavior therapy for depressed older adults: a randomized pilot study. Am J Geriatr Psychiatry 2003;11:33-45.
13. Linehan MM, Comtois KA, Murray AM, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs. therapy by experts for suicidal behaviors and borderline personality disorder. Arch Gen Psychiatry 2006;63:757-66.
14. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol 2001;52:685-716.
15. Hawton K, Arensman E, Townsend E, et al. Deliberate self-harm: systematic review of efficacy of psychosocial and pharmacological treatments in preventing repetition. BMJ 1998;317:441-7.
16. Tyrer P, Tom B, Byford S, et al. Differential effects of manual-assisted cognitive behavior therapy in the treatment of recurrent deliberate self-harm and personality disturbance: the POPMACT study. J Personal Disord 2004;18:102-16.
1. Linehan MM. Cognitive behavior treatment of borderline personality disorder. New York, NY: The Guilford Press; 1993.
2. Linehan MM, Armstrong HE, Suarez A, et al. Cognitive-behavioral treatment of chronically parasuicidal borderline patients. Arch Gen Psychiatry 1991;48:1060-4.
3. Robins CJ, Chapman AL. Dialectical behavior therapy: current status, recent developments, and future directions. J Personal Disord 2004;18:73-9.
4. Linehan MM, Schmidt HI, Dimeff LA, et al. Dialectical behavior therapy for patients with borderline personality disorder and drug-dependence. Am J Addictions 1999;8:279-92.
5. Turner RM. Naturalistic evaluation of dialectical behavior therapy-oriented treatment for borderline personality disorder. Cognit Behav Pract 2000;7:413-9.
6. Linehan MM, Dimeff LA, Reynolds SK, et al. Dialectical behavior therapy versus comprehensive validation therapy plus 12-step for the treatment of opioid dependent women meeting criteria for borderline personality disorder. Drug Alcohol Depend 2002;67:13-26.
7. Koons C, Robins CJ, Tweed JL, et al. Efficacy of dialectical behavior therapy in women veterans with borderline personality disorder. Behav Ther 2001;32:371-90.
8. Safer DL, Telch CF, Agras WS. Dialectical behavior therapy for bulimia nervosa. Am J Psychiatry 2001;158:632-4.
9. Telch CF, Agras WS, Linehan MM. Dialectical behavior therapy for binge eating disorder. J Consult Clin Psychol 2001;69:1061-5.
10. van den Bosch LMC, Verheul R, Schippers GM, van den Brink W. Dialectical behavior therapy of borderline patients with and without substance abuse problems: implementation and long-term effects. Addict Behav 2002;27:911-23.
11. Verheul R, van den Bosch LMC, Koeter MWJ, et al. Dialectical behavior therapy for women with borderline personality disorder. Br J Psychiatry 2003;182:135-40.
12. Lynch TR, Morse JQ, Mendelson T, Robins CJ. Dialectical behavior therapy for depressed older adults: a randomized pilot study. Am J Geriatr Psychiatry 2003;11:33-45.
13. Linehan MM, Comtois KA, Murray AM, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs. therapy by experts for suicidal behaviors and borderline personality disorder. Arch Gen Psychiatry 2006;63:757-66.
14. Chambless DL, Ollendick TH. Empirically supported psychological interventions: controversies and evidence. Annu Rev Psychol 2001;52:685-716.
15. Hawton K, Arensman E, Townsend E, et al. Deliberate self-harm: systematic review of efficacy of psychosocial and pharmacological treatments in preventing repetition. BMJ 1998;317:441-7.
16. Tyrer P, Tom B, Byford S, et al. Differential effects of manual-assisted cognitive behavior therapy in the treatment of recurrent deliberate self-harm and personality disturbance: the POPMACT study. J Personal Disord 2004;18:102-16.
Driving with dementia: How to assess safety behind the wheel
Mr. D, age 75, presents to your office with a 5-year history of gradually declining memory. His wife reports he is having difficulty with word finding, managing his finances, and shopping, and he needs supervision when using the stove. Nonetheless, he enjoys playing golf and drives himself to the golf course 3 times a week. He is compliant with his chronic medical therapy for hypertension, hypercholesterolemia, and asthma.
Patients with dementia who continue to drive pose a potential danger on the road, worry their families, and present challenges to clinicians. Most people would agree that patients with moderate or severe dementia should not drive, but a careful evaluation is required to assess whether a patient such as Mr. D with mild dementia remains fit to drive.
This article explores how dementia exacerbates age-related changes in driving ability and discusses how to assess driving in patients with dementia. Our goal is to help clinicians sort through data from in-office physical and cognitive assessments, family caregivers/informants’ reports, and (when available) on-road testing. We also discuss:
- guidelines for assessing older drivers that can help balance patients’ need for autonomy with public safety
- strategies for discussing driving cessation with patients and their families.
Driving: A privilege, not a right
Driving is central to older adults’ autonomy, and >75% of persons age ≥75 rely on driving as their primary mode of transportation.1 Driving cessation in this population has been associated with a 3-fold decrease in out-of-home activity2 and a 2.5-fold increase in depressive symptoms.3 Nonetheless, some 4.5 million Americans have Alzheimer’s disease (AD),4 and dementia poses a substantial risk to safe driving.
Although driving must be sacrificed when personal and public safety is at risk, most physicians perceive an uncomfortable conflict of interest between patient confidentiality and public safety.5 Assessing driving safety of patients with dementia can undermine the doctor-patient relationship and pose hardships for patients.
Mr. D has a 5-year history of memory problems that affect his daily functioning, yet he continues to drive. A longitudinal study of persons with dementia found that among the 29% who were driving at baseline, more than one-half were still behind the wheel 2 years later.6
Age and driving safety. Even in the absence of dementia, driving ability declines with aging (Tables 1 and 2).7,8 Older persons may self-regulate and restrict their driving to shorter distances, with fewer trips at night, on high-speed roads, or in unfamiliar situations. Their driving is rarely aggressive and they are unlikely to speed, but they may drive more slowly than other traffic.7,8 Although the overall rate of motor vehicle collisions declines with age:
- the rate of collisions per mile driven increases after age 659
- drivers age >65 have the highest fatality rate per mile driven among adults age ≥25.10
A dementia diagnosis is not sufficient to withdraw driving privileges, according to American Medical Association (AMA)/National Highway Traffic Safety Administration (NHTSA) guidelines. These recommend that you base decisions on the individual’s driving ability, and—when you have concerns—factor in a focused medical assessment and formal assessment of driving skills.10
Table 1
Age-related changes that may affect driving fitness
| Decreased physical capabilities, including declining muscle tone, flexibility, and reaction time |
| Decreased hearing and visual acuity |
| Increased fragility, resulting in longer time to heal should injuries occur |
| Increased medication use with possible side effect of drowsiness |
| Source: References 7,8 |
Table 2
Older drivers’ common traffic violations leading to crashes*
| Failure to obey traffic signals, including stop signs and red lights |
| Unsafe left turns (driver may inaccurately judge speed of oncoming vehicle) |
| Inappropriate turns (such as difficulty judging distance from oncoming cars, wide or narrow turns, or not timing the turn correctly with traffic lights) |
| Unsafe passing |
| Failure to yield |
| * These errors often lead to multivehicle accidents Source: References 7,8 |
CASE CONTINUED: Cognitive deficits quantified
You perform a Mini-Mental State Examination (MMSE). Mr. D scores 24/30, losing 1 point for orientation, 2 points for attention, 2 points for recall, and 1 point for copying. This score, along with his history, indicates mild dementia, although he claims he is a safe driver. On further cognitive testing, Mr. D completes the Trails A test in 90 seconds and Trails B test in 250 seconds (well below 1.5 standard deviations of the norm for his age and education).11 On the clock-drawing task, he drew a poorly organized clock, with unequal spaces between numbers and hands pointing to “10” and “11” instead of properly indicating “10 after 11.”
Mr. D and his wife live in a rural area, 5 miles from the nearest grocery store. His wife never drove, and she relies on him for weekly shopping trips and to drive her to her bridge club. She denies any problems with his driving but states, “Other drivers have become so aggressive; they’re always honking at him.” Their daughter denies that Mr. D has driving problems but admits that for the last 2 years she has refused to allow her child to ride in his car.
Focused in-office assessment
Information to assess driving ability can come from the patient, family caregiver/informant, and clinical judgment. Patients with dementia are notoriously inaccurate in self-reported driving ability, either for lack of insight or as a testament to the importance of driving to their autonomy. Caregivers often are more accurate in describing a patient’s driving, but other agendas may color their responses.
In a study of patients with very mild or mild AD, 94% reported themselves as safe drivers, whereas on-road driving instructors rated <50% of drivers in these groups as safe. Caregivers were better able to classify driving performance, but 36% of their ratings were incorrect.12
Cognitive assessment. To assess older drivers’ cognition, AMA/NHTSA’s Guide to Assessing and Counseling Older Drivers recommends the Trail-Making Test, Part B and the clock-drawing test.10 The Canadian Medical Association suggests the MMSE.13 Both guides say that abnormalities in these tests indicate a need for more detailed testing, including referral to specialized driving assessment and retesting at regular intervals (Algorithm). Retest patients with mild dementia at least every 6 months or sooner when dementia severity increases noticeably14 (Box 1).6,15
The MMSE is widely used to screen for cognitive impairment and identify dementia or delirium, but it is not a diagnostic tool or proxy driving test. A patient with dementia may produce a high MMSE score and yet be an unsafe driver. For example, well-educated patients or those with vascular or frontotemporal dementia may retain cognitive abilities as measured by the MMSE until later in the disease.
Considerable effort has been put into developing tools to help clinicians quickly and accurately differentiate safe from unsafe drivers by assessing cognition. Unfortunately, no consistent link has been found between cognitive test results and driving outcome measures. A systematic review of office-based predictors of fitness to drive in dementia found 5 studies showing an association between MMSE scores and driving and 5 studies showing no such association.16 Thus, although the AMA/NHTSA guide recommends the MMSE, Trails B, and clock-drawing tests, cognitive tests—including these—are not sufficient to assess driving ability.
Severity of dementia. International consensus groups have attempted to create guidelines for patients with dementia who drive. American, Canadian, and Australian groups suggest that a diagnosis of moderate to severe dementia precludes driving, and the driver’s licenses of persons with these conditions should be revoked.17
In general, AD is considered severe when the MMSE score is <10 or the patient becomes dependent on a caregiver for survival.18 AD of moderate severity is more difficult to define, but a Canadian consensus conference suggested a practical approach: Patients with AD would be considered to have moderate to severe dementia and should not drive when they cannot independently perform multiple instrumental activities of daily living or any of the basic activities of daily living.19
Some dementias may impair driving more quickly than AD does. For example, hallucinations may occur early in Lewy body dementia, as may impulsivity in frontotemporal dementia and motor impairment in vascular dementia.
Mrs. Y visits your office for a follow-up regarding mild Alzheimer’s disease (AD), which was diagnosed 2 years ago. She passed an on-road test 3 months ago and has an Mini-Mental State Examination score of 24/30. Over the last month she has become depressed, with insomnia and mild psychomotor retardation. She occasionally has hallucinations.
Behavioral and psychological symptoms such as agitation, aggression, hallucinations, apathy, depression, and anxiety are common neuropsychiatric sequelae of AD. Little is known about the risks these symptoms pose to road safety, but we recommend that clinicians strongly consider the potential for impaired driving.
In a longitudinal study, cognitive impairment and behavioral disturbances—especially agitation, apathy, and hallucinations—were strong predictors of driving cessation among patients with dementia.6 Furthermore, a case crossover study of patients with dementia found a 54% increase in risk of motor vehicle collisions associated with the use of psychotropic medications.15
Consider all aspects of the patient’s clinical status, including neuropsychiatric symptoms, psychotropic medications, comorbid medical conditions (including hearing and vision impairment), and concomitant therapy for medical conditions. Any could change a safe driver with mild dementia into an unsafe driver.
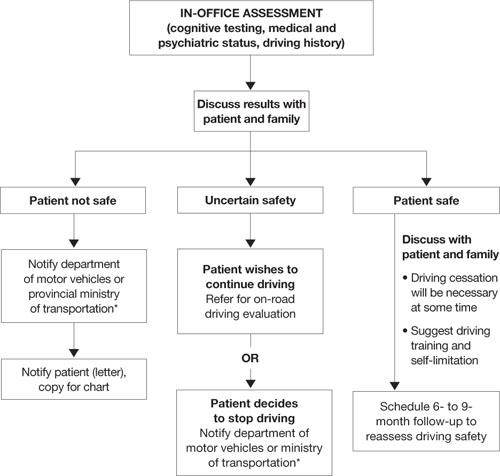
Algorithm: 3 options for drivers with dementia, based on in-office assessment
* Observe legislation or statutes that address reporting unsafe drivers to the department of motor vehicles or ministry of transportation
On-road driving tests
Because some individuals with mild dementia can drive safely for extended periods, international recommendations for assessing the driver with dementia emphasize on-road driving tests.10,13,20–22 American10 and Canadian guidelines13 suggest that a dementia diagnosis is not sufficient to withdraw licensure.
A formal driving assessment is necessary to establish road safety for patients with mild dementia except when the need for license withdrawal is evident, such as when the patient has:
- a history of major driving problems (such as crashes or driving the wrong way on a highway)
- significant contraindications to driving on the history or physical examination (such as severe inattention or psychosis).
Challenges of on-road testing. On-road tests may be the gold standard, but they are not without clinical problems.
Need to retest. Because almost all dementias are progressive and driving skills deteriorate over time, most guidelines recommend periodic retesting. For patients with dementia who pass on-road evaluations, limited evidence supports retesting every 6 months.14 Take an individual approach, however, because of the various rates at which the dementias progress.
Testing vs real world conditions. Structured on-road testing is not equivalent to unstructured real-world driving, in which the patient often must navigate without instruction or assistance.
Rural vs urban driving. Road tests conducted in urban areas assess skills associated with complex conditions and the need to respond quickly to crises. They might not assess as well rural driving, which requires sustained attention on monotonous roads.
Inaccessibility. Cost and lack of availability of on-road tests, particularly in rural areas, limit the number of patients whose performance can be evaluated.
CASE CONTINUED: Distressing results
Mr. D has a history of decline in cognition and function, objective cognitive difficulties, and a subtle history of driving problems. You refer him for a specialized on-road test, and the report indicates that he failed. Errors included wide turns, driving too slowly, getting caught in an intersection twice during red lights while attempting to turn left, driving on the shoulder, and failing to signal lane changes. You review the results with Mr. D and his wife and recommend that he cease driving immediately.
Mr. D is furious, and his wife is dismayed. He demands to know how he can continue to play golf, which is his only form of exercise and recreation. Will she have to give up her bridge club? How will they shop for food? They request permission to at least to drive to the grocery store during the daytime.
You explain that no system allows individuals to drive only at certain times, and for the sake of safety you cannot grant them special permission. You discuss alternatives, such as asking their daughter for assistance with grocery shopping and taking taxis or ride-sharing with friends who play golf and bridge.
Remain firm, but ease the blow
Driving cessation orders distress patients, families, and clinicians. A failed road test clearly indicates unsafe driving, and driving cessation is critical to public safety.
A review by Man-Son-Hing et al23 found that drivers with dementia performed worse than nondemented controls in all studies that examined driving performance (on-road, simulator, or caregiver report). Simulators showed problems such as off-road driving, deviation from posted speed, and more time to negotiate left turns.24
By comparison, only 1 of 3 studies using state crash records showed an increased risk of collisions in persons with dementia compared with controls.23 From a research perspective, however, studies that use state-reported collisions to assess driving risk are confounded by driving restrictions on persons with dementia.
Mr. D wants to continue driving with restrictions. No studies have shown reduced crash rates when drivers with dementia used compensatory strategies such as restrictions, retraining/education, having a passenger “co-pilot,” on-board navigation, or cognitive enhancers.23
If Mr. D had passed the road test, the situation would have been more ambiguous. Two studies have examined on-road driving performance over time in patients with early-stage dementia.25,26 Both studies followed drivers prospectively for 2 years, and those with mild dementia (vs very mild or no dementia) were most likely to show a decline in driving skills:
- All participants with mild dementia were rated as “not safe” by the end of 2 years by Duchek et al.25
- Median time to “failure” (or a rating of unsafe) was 324 days for drivers with mild dementia vs 605 days for those with very mild dementia, as reported by Ott et al.26
Mr. D’s passionate plea for reconsideration highlights the need for communities to develop alternate transportation for seniors whose driving becomes unsafe (Box 2).
Legal liability? Physicians often are concerned about legal responsibilities and risks involved in reporting unsafe drivers. Be aware of local statutes or legislations regarding mandatory reporting of patients you deem unsafe to drive.17 These laws usually protect physicians from lawsuits related to violating patient confidentiality. Civil lawsuits remain possible, however, if clinicians fail to report an unsafe driver who subsequently is involved in a motor vehicle collision.27
1. Meet with family first. Help them assume a positive and supportive role. Explain concretely and empathically your concern for the safety of the patient and others. Clearly outline your findings that the patient is not fit to drive, and explain that the law requires you to report the patient to the authorities.
Remind family members that the goal of driving assessment is to prevent a collision, and they carry some responsibility because they are aware of the potential risk of letting their family member continue to drive. If necessary, have family members witness a repeat performance by the patient on the most revealing test. Discuss the importance of finding alternate transportation to reduce the risk of isolation and depression that can follow driving cessation.
2. Meet with patient. Having the family present can be helpful, but ask them to assume a supportive role. Give the patient a positive role by recognizing that he or she has been a responsible driver, and part of this responsibility is to stop driving before an accident occurs. Acknowledge that it is normal to be unhappy upon learning that one’s driving privileges are being revoked.
Sometimes it helps to give the patient a prescription in their name that says, “Do not drive.” Families who receive a copy may find this very helpful, too, for reminding the patient later about what you said.
If your patient argues with your position, remain firm and do not argue. Indicate that you have made notes on the meeting and are notifying the authorities about the patient’s unsafe driving. You can add that your chart could be subpoenaed and the patient may be legally liable and financially responsible should he or she continue to drive and have a collision.
3. Talk about transportation options. Family members could share driving responsibilities. Taxi rides can cost less than maintaining a car if the patient drives <4,000 km (2,500 miles) per year. Suggest that patients or families find volunteer drivers or contact helpful taxi drivers a day before an outing is planned.
4. If patient refuses to comply, meet with the family again and encourage them to remove the patient’s opportunity to drive (confiscate the keys, disable the car, or remove the car altogether).
Provide a written statement to the patient and family outlining why the patient can no longer drive. Indicate that it is your legal responsibility to report unsafe drivers, and you intend to notify the authorities regarding the patient’s driving status. If the patient remains noncompliant, continue to encourage family to remove the opportunity to drive.
Related resources
- Physician’s guide to assessing and counseling older drivers. American Medical Association and the National Highway Traffic Safety Administration, 2003. www.nhtsa.dot.gov/people/injury/olddrive/OlderDriversBook.
- Determining medical fitness to operate motor vehicles. Canadian Medical Association. 2006. www.cma.ca/index.cfm/ci_id/18223/la_id/1.htm.
- Canadian Driving Research Initiative for Vehicular Safety in the Elderly (CanDRIVE). www.candrive.ca.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rapoport receives grant/research support from the Canadian Institute of Health Research and the Ontario Neurotrauma Foundation.
1. Stowell-Ritter A, Straight A, Evans EL. Understanding senior transportation: report and analysis of a survey of consumers age 50+. Washington, DC: American Association of Retired Persons; 2002.
2. Marottoli RA, de Leon CFM, Glass TA, et al. Consequences of driving cessation: decreased out-of-home activity levels. J Gerontol B Psychol Sci Soc Sci 2000;55(6):S334-40.
3. Marottoli RA, Mendes de Leon CF, Glass TA, et al. Driving cessation and increased depressive symptoms: prospective evidence from the New Haven EPESE. Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc 1997;45(2):202-6.
4. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003;60(8):1119-22.
5. Jang RW, Man-Son-Hing M, Molnar FJ, et al. Family physicians’ attitudes and practices regarding assessments of medical fitness to drive in older persons. J Gen Intern Med 2007;22(4):531-43.
6. Herrmann N, Rapoport MJ, Sambrook R, et al. Predictors of driving cessation in mild-to-moderate dementia. CMAJ 2006;175(6):591-5.
7. Hedlund J. Countermeasures that work: a highway safety countermeasure guide for state highway safety offices. Washington, DC: National Highway Traffic Safety Administration; 2006.
8. Dobbs B. Medical conditions and driving: a review of the literature (1960-2000). Washington, DC: National Highway Traffic Safety Administration; 2005. Available at: http://www.nhtsa.dot.gov/people/injury/research/Medical_Condition_Driving/pages/TRD.html. Accessed September 29, 2008.
9. Li G, Braver ER, Chen LH. Fragility versus excessive crash involvement as determinants of high death rates per vehicle-mile of travel among older drivers. Accid Anal Prev 2003;35(2):227-35.
10. Physician’s guide to assessing and counseling older drivers. Washington, DC: National Highway Traffic Safety Administration; 2003. Available at: http://www.nhtsa.dot.gov/people/injury/olddrive/OlderDriversBook. Accessed September 29, 2008.
11. Yeudall LT, Reddon JR, Gill DM, et al. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. J Clin Psychol 1987;43(3):346-67.
12. Brown LB, Ott BR, Papandonatos GD, et al. Prediction of on-road driving performance in patients with early Alzheimer’s disease. J Am Geriatr Soc 2005;53(1):94-8.
13. Determining medical fitness to operate motor vehicles. CMA driver’s guide. 7th ed. Ottawa, Ontario, Canada: Canadian Medical Association; 2006.
14. Molnar FJ, Patel A, Marshall SC, et al. Systematic review of the optimal frequency of follow-up in persons with mild dementia who continue to drive. Alzheimer Dis Assoc Disord 2006;20(4):295-7.
15. Rapoport MJ, Herrmann N, Molnar FJ, et al. Psychotropic medications and motor vehicle collisions in patients with dementia (letter). J Am Geriatr Soc 2008;56(10):1968-70.
16. Molnar FJ, Patel A, Marshall SC, et al. Clinical utility of office-based cognitive predictors of fitness to drive in persons with dementia: a systematic review. J Am Geriatr Soc 2006;54(12):1809-24.
17. Rapoport MJ, Herrmann N, Molnar FJ, et al. Sharing the responsibility for assessing the risk of the driver with dementia. CMAJ. 2007;177(6):599-601.
18. Herrmann N, Gauthier S, Lysy PG. Clinical practice guidelines for severe Alzheimer’s disease. Alzheimers Dement 2007;3(4):385-97.
19. Hogan DB, Bailey P, Carswell A, et al. Management of mild to moderate Alzheimer’s disease and dementia. Alzheimers Dement 2007;3(4):355-84.
19. Assessing fitness to drive for commercial and private vehicle drivers. Sydney, Australia: National Library of Australia; 2006. Available at: http://www.austroads.com.au/aftd/index.html. Accessed November 4, 2008.
21. Medical aspects of fitness to drive. A guide for medical practitioners. Wellington, New Zealand: Land Transport Safety Authority; 2002. Available at: http://www.transfund.govt.nz/licensing/docs/ltsa-medical-aspects.pdf. Accessed September 29, 2008.
22. At a glance guide to the current medical standards of fitness to drive. Swansea, UK: Drivers Medical Group, Driver and Vehicle Licensing Agency; 2008. Available at: http://www.dvla.gov.uk/medical/ataglance.aspx. Accessed September 29, 2008.
23. Man-Son-Hing M, Marshall SC, Molnar FJ, Wilson KG. Systematic review of driving risk and the efficacy of compensatory strategies in persons with dementia. J Am Geriatr Soc 2007;55(6):878-84.
24. Cox DJ, Quillian WC, Thorndike FP, et al. Evaluating driving performance of outpatients with Alzheimer disease. J Am Board Fam Pract 1998;11(4):264-71.
25. Duchek JM, Carr DB, Hunt L, et al. Longitudinal driving performance in early-stage dementia of the Alzheimer type. J Am Geriatr Soc 2003;51(10):1342-7.
26. Ott BR, Heindel WC, Papandonatos GD, et al. A longitudinal study of drivers with Alzheimer disease. Neurology 2008;70(14):1171-8.
27. Molnar FJ, Byszewski AM, Marshall SC, Man-Son-Hing M. In-office evaluation of medical fitness to drive: practical approaches for assessing older people. Can Fam Physician 2005;51:372-9.
Mr. D, age 75, presents to your office with a 5-year history of gradually declining memory. His wife reports he is having difficulty with word finding, managing his finances, and shopping, and he needs supervision when using the stove. Nonetheless, he enjoys playing golf and drives himself to the golf course 3 times a week. He is compliant with his chronic medical therapy for hypertension, hypercholesterolemia, and asthma.
Patients with dementia who continue to drive pose a potential danger on the road, worry their families, and present challenges to clinicians. Most people would agree that patients with moderate or severe dementia should not drive, but a careful evaluation is required to assess whether a patient such as Mr. D with mild dementia remains fit to drive.
This article explores how dementia exacerbates age-related changes in driving ability and discusses how to assess driving in patients with dementia. Our goal is to help clinicians sort through data from in-office physical and cognitive assessments, family caregivers/informants’ reports, and (when available) on-road testing. We also discuss:
- guidelines for assessing older drivers that can help balance patients’ need for autonomy with public safety
- strategies for discussing driving cessation with patients and their families.
Driving: A privilege, not a right
Driving is central to older adults’ autonomy, and >75% of persons age ≥75 rely on driving as their primary mode of transportation.1 Driving cessation in this population has been associated with a 3-fold decrease in out-of-home activity2 and a 2.5-fold increase in depressive symptoms.3 Nonetheless, some 4.5 million Americans have Alzheimer’s disease (AD),4 and dementia poses a substantial risk to safe driving.
Although driving must be sacrificed when personal and public safety is at risk, most physicians perceive an uncomfortable conflict of interest between patient confidentiality and public safety.5 Assessing driving safety of patients with dementia can undermine the doctor-patient relationship and pose hardships for patients.
Mr. D has a 5-year history of memory problems that affect his daily functioning, yet he continues to drive. A longitudinal study of persons with dementia found that among the 29% who were driving at baseline, more than one-half were still behind the wheel 2 years later.6
Age and driving safety. Even in the absence of dementia, driving ability declines with aging (Tables 1 and 2).7,8 Older persons may self-regulate and restrict their driving to shorter distances, with fewer trips at night, on high-speed roads, or in unfamiliar situations. Their driving is rarely aggressive and they are unlikely to speed, but they may drive more slowly than other traffic.7,8 Although the overall rate of motor vehicle collisions declines with age:
- the rate of collisions per mile driven increases after age 659
- drivers age >65 have the highest fatality rate per mile driven among adults age ≥25.10
A dementia diagnosis is not sufficient to withdraw driving privileges, according to American Medical Association (AMA)/National Highway Traffic Safety Administration (NHTSA) guidelines. These recommend that you base decisions on the individual’s driving ability, and—when you have concerns—factor in a focused medical assessment and formal assessment of driving skills.10
Table 1
Age-related changes that may affect driving fitness
| Decreased physical capabilities, including declining muscle tone, flexibility, and reaction time |
| Decreased hearing and visual acuity |
| Increased fragility, resulting in longer time to heal should injuries occur |
| Increased medication use with possible side effect of drowsiness |
| Source: References 7,8 |
Table 2
Older drivers’ common traffic violations leading to crashes*
| Failure to obey traffic signals, including stop signs and red lights |
| Unsafe left turns (driver may inaccurately judge speed of oncoming vehicle) |
| Inappropriate turns (such as difficulty judging distance from oncoming cars, wide or narrow turns, or not timing the turn correctly with traffic lights) |
| Unsafe passing |
| Failure to yield |
| * These errors often lead to multivehicle accidents Source: References 7,8 |
CASE CONTINUED: Cognitive deficits quantified
You perform a Mini-Mental State Examination (MMSE). Mr. D scores 24/30, losing 1 point for orientation, 2 points for attention, 2 points for recall, and 1 point for copying. This score, along with his history, indicates mild dementia, although he claims he is a safe driver. On further cognitive testing, Mr. D completes the Trails A test in 90 seconds and Trails B test in 250 seconds (well below 1.5 standard deviations of the norm for his age and education).11 On the clock-drawing task, he drew a poorly organized clock, with unequal spaces between numbers and hands pointing to “10” and “11” instead of properly indicating “10 after 11.”
Mr. D and his wife live in a rural area, 5 miles from the nearest grocery store. His wife never drove, and she relies on him for weekly shopping trips and to drive her to her bridge club. She denies any problems with his driving but states, “Other drivers have become so aggressive; they’re always honking at him.” Their daughter denies that Mr. D has driving problems but admits that for the last 2 years she has refused to allow her child to ride in his car.
Focused in-office assessment
Information to assess driving ability can come from the patient, family caregiver/informant, and clinical judgment. Patients with dementia are notoriously inaccurate in self-reported driving ability, either for lack of insight or as a testament to the importance of driving to their autonomy. Caregivers often are more accurate in describing a patient’s driving, but other agendas may color their responses.
In a study of patients with very mild or mild AD, 94% reported themselves as safe drivers, whereas on-road driving instructors rated <50% of drivers in these groups as safe. Caregivers were better able to classify driving performance, but 36% of their ratings were incorrect.12
Cognitive assessment. To assess older drivers’ cognition, AMA/NHTSA’s Guide to Assessing and Counseling Older Drivers recommends the Trail-Making Test, Part B and the clock-drawing test.10 The Canadian Medical Association suggests the MMSE.13 Both guides say that abnormalities in these tests indicate a need for more detailed testing, including referral to specialized driving assessment and retesting at regular intervals (Algorithm). Retest patients with mild dementia at least every 6 months or sooner when dementia severity increases noticeably14 (Box 1).6,15
The MMSE is widely used to screen for cognitive impairment and identify dementia or delirium, but it is not a diagnostic tool or proxy driving test. A patient with dementia may produce a high MMSE score and yet be an unsafe driver. For example, well-educated patients or those with vascular or frontotemporal dementia may retain cognitive abilities as measured by the MMSE until later in the disease.
Considerable effort has been put into developing tools to help clinicians quickly and accurately differentiate safe from unsafe drivers by assessing cognition. Unfortunately, no consistent link has been found between cognitive test results and driving outcome measures. A systematic review of office-based predictors of fitness to drive in dementia found 5 studies showing an association between MMSE scores and driving and 5 studies showing no such association.16 Thus, although the AMA/NHTSA guide recommends the MMSE, Trails B, and clock-drawing tests, cognitive tests—including these—are not sufficient to assess driving ability.
Severity of dementia. International consensus groups have attempted to create guidelines for patients with dementia who drive. American, Canadian, and Australian groups suggest that a diagnosis of moderate to severe dementia precludes driving, and the driver’s licenses of persons with these conditions should be revoked.17
In general, AD is considered severe when the MMSE score is <10 or the patient becomes dependent on a caregiver for survival.18 AD of moderate severity is more difficult to define, but a Canadian consensus conference suggested a practical approach: Patients with AD would be considered to have moderate to severe dementia and should not drive when they cannot independently perform multiple instrumental activities of daily living or any of the basic activities of daily living.19
Some dementias may impair driving more quickly than AD does. For example, hallucinations may occur early in Lewy body dementia, as may impulsivity in frontotemporal dementia and motor impairment in vascular dementia.
Mrs. Y visits your office for a follow-up regarding mild Alzheimer’s disease (AD), which was diagnosed 2 years ago. She passed an on-road test 3 months ago and has an Mini-Mental State Examination score of 24/30. Over the last month she has become depressed, with insomnia and mild psychomotor retardation. She occasionally has hallucinations.
Behavioral and psychological symptoms such as agitation, aggression, hallucinations, apathy, depression, and anxiety are common neuropsychiatric sequelae of AD. Little is known about the risks these symptoms pose to road safety, but we recommend that clinicians strongly consider the potential for impaired driving.
In a longitudinal study, cognitive impairment and behavioral disturbances—especially agitation, apathy, and hallucinations—were strong predictors of driving cessation among patients with dementia.6 Furthermore, a case crossover study of patients with dementia found a 54% increase in risk of motor vehicle collisions associated with the use of psychotropic medications.15
Consider all aspects of the patient’s clinical status, including neuropsychiatric symptoms, psychotropic medications, comorbid medical conditions (including hearing and vision impairment), and concomitant therapy for medical conditions. Any could change a safe driver with mild dementia into an unsafe driver.

Algorithm: 3 options for drivers with dementia, based on in-office assessment
* Observe legislation or statutes that address reporting unsafe drivers to the department of motor vehicles or ministry of transportation
On-road driving tests
Because some individuals with mild dementia can drive safely for extended periods, international recommendations for assessing the driver with dementia emphasize on-road driving tests.10,13,20–22 American10 and Canadian guidelines13 suggest that a dementia diagnosis is not sufficient to withdraw licensure.
A formal driving assessment is necessary to establish road safety for patients with mild dementia except when the need for license withdrawal is evident, such as when the patient has:
- a history of major driving problems (such as crashes or driving the wrong way on a highway)
- significant contraindications to driving on the history or physical examination (such as severe inattention or psychosis).
Challenges of on-road testing. On-road tests may be the gold standard, but they are not without clinical problems.
Need to retest. Because almost all dementias are progressive and driving skills deteriorate over time, most guidelines recommend periodic retesting. For patients with dementia who pass on-road evaluations, limited evidence supports retesting every 6 months.14 Take an individual approach, however, because of the various rates at which the dementias progress.
Testing vs real world conditions. Structured on-road testing is not equivalent to unstructured real-world driving, in which the patient often must navigate without instruction or assistance.
Rural vs urban driving. Road tests conducted in urban areas assess skills associated with complex conditions and the need to respond quickly to crises. They might not assess as well rural driving, which requires sustained attention on monotonous roads.
Inaccessibility. Cost and lack of availability of on-road tests, particularly in rural areas, limit the number of patients whose performance can be evaluated.
CASE CONTINUED: Distressing results
Mr. D has a history of decline in cognition and function, objective cognitive difficulties, and a subtle history of driving problems. You refer him for a specialized on-road test, and the report indicates that he failed. Errors included wide turns, driving too slowly, getting caught in an intersection twice during red lights while attempting to turn left, driving on the shoulder, and failing to signal lane changes. You review the results with Mr. D and his wife and recommend that he cease driving immediately.
Mr. D is furious, and his wife is dismayed. He demands to know how he can continue to play golf, which is his only form of exercise and recreation. Will she have to give up her bridge club? How will they shop for food? They request permission to at least to drive to the grocery store during the daytime.
You explain that no system allows individuals to drive only at certain times, and for the sake of safety you cannot grant them special permission. You discuss alternatives, such as asking their daughter for assistance with grocery shopping and taking taxis or ride-sharing with friends who play golf and bridge.
Remain firm, but ease the blow
Driving cessation orders distress patients, families, and clinicians. A failed road test clearly indicates unsafe driving, and driving cessation is critical to public safety.
A review by Man-Son-Hing et al23 found that drivers with dementia performed worse than nondemented controls in all studies that examined driving performance (on-road, simulator, or caregiver report). Simulators showed problems such as off-road driving, deviation from posted speed, and more time to negotiate left turns.24
By comparison, only 1 of 3 studies using state crash records showed an increased risk of collisions in persons with dementia compared with controls.23 From a research perspective, however, studies that use state-reported collisions to assess driving risk are confounded by driving restrictions on persons with dementia.
Mr. D wants to continue driving with restrictions. No studies have shown reduced crash rates when drivers with dementia used compensatory strategies such as restrictions, retraining/education, having a passenger “co-pilot,” on-board navigation, or cognitive enhancers.23
If Mr. D had passed the road test, the situation would have been more ambiguous. Two studies have examined on-road driving performance over time in patients with early-stage dementia.25,26 Both studies followed drivers prospectively for 2 years, and those with mild dementia (vs very mild or no dementia) were most likely to show a decline in driving skills:
- All participants with mild dementia were rated as “not safe” by the end of 2 years by Duchek et al.25
- Median time to “failure” (or a rating of unsafe) was 324 days for drivers with mild dementia vs 605 days for those with very mild dementia, as reported by Ott et al.26
Mr. D’s passionate plea for reconsideration highlights the need for communities to develop alternate transportation for seniors whose driving becomes unsafe (Box 2).
Legal liability? Physicians often are concerned about legal responsibilities and risks involved in reporting unsafe drivers. Be aware of local statutes or legislations regarding mandatory reporting of patients you deem unsafe to drive.17 These laws usually protect physicians from lawsuits related to violating patient confidentiality. Civil lawsuits remain possible, however, if clinicians fail to report an unsafe driver who subsequently is involved in a motor vehicle collision.27
1. Meet with family first. Help them assume a positive and supportive role. Explain concretely and empathically your concern for the safety of the patient and others. Clearly outline your findings that the patient is not fit to drive, and explain that the law requires you to report the patient to the authorities.
Remind family members that the goal of driving assessment is to prevent a collision, and they carry some responsibility because they are aware of the potential risk of letting their family member continue to drive. If necessary, have family members witness a repeat performance by the patient on the most revealing test. Discuss the importance of finding alternate transportation to reduce the risk of isolation and depression that can follow driving cessation.
2. Meet with patient. Having the family present can be helpful, but ask them to assume a supportive role. Give the patient a positive role by recognizing that he or she has been a responsible driver, and part of this responsibility is to stop driving before an accident occurs. Acknowledge that it is normal to be unhappy upon learning that one’s driving privileges are being revoked.
Sometimes it helps to give the patient a prescription in their name that says, “Do not drive.” Families who receive a copy may find this very helpful, too, for reminding the patient later about what you said.
If your patient argues with your position, remain firm and do not argue. Indicate that you have made notes on the meeting and are notifying the authorities about the patient’s unsafe driving. You can add that your chart could be subpoenaed and the patient may be legally liable and financially responsible should he or she continue to drive and have a collision.
3. Talk about transportation options. Family members could share driving responsibilities. Taxi rides can cost less than maintaining a car if the patient drives <4,000 km (2,500 miles) per year. Suggest that patients or families find volunteer drivers or contact helpful taxi drivers a day before an outing is planned.
4. If patient refuses to comply, meet with the family again and encourage them to remove the patient’s opportunity to drive (confiscate the keys, disable the car, or remove the car altogether).
Provide a written statement to the patient and family outlining why the patient can no longer drive. Indicate that it is your legal responsibility to report unsafe drivers, and you intend to notify the authorities regarding the patient’s driving status. If the patient remains noncompliant, continue to encourage family to remove the opportunity to drive.
Related resources
- Physician’s guide to assessing and counseling older drivers. American Medical Association and the National Highway Traffic Safety Administration, 2003. www.nhtsa.dot.gov/people/injury/olddrive/OlderDriversBook.
- Determining medical fitness to operate motor vehicles. Canadian Medical Association. 2006. www.cma.ca/index.cfm/ci_id/18223/la_id/1.htm.
- Canadian Driving Research Initiative for Vehicular Safety in the Elderly (CanDRIVE). www.candrive.ca.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rapoport receives grant/research support from the Canadian Institute of Health Research and the Ontario Neurotrauma Foundation.
Mr. D, age 75, presents to your office with a 5-year history of gradually declining memory. His wife reports he is having difficulty with word finding, managing his finances, and shopping, and he needs supervision when using the stove. Nonetheless, he enjoys playing golf and drives himself to the golf course 3 times a week. He is compliant with his chronic medical therapy for hypertension, hypercholesterolemia, and asthma.
Patients with dementia who continue to drive pose a potential danger on the road, worry their families, and present challenges to clinicians. Most people would agree that patients with moderate or severe dementia should not drive, but a careful evaluation is required to assess whether a patient such as Mr. D with mild dementia remains fit to drive.
This article explores how dementia exacerbates age-related changes in driving ability and discusses how to assess driving in patients with dementia. Our goal is to help clinicians sort through data from in-office physical and cognitive assessments, family caregivers/informants’ reports, and (when available) on-road testing. We also discuss:
- guidelines for assessing older drivers that can help balance patients’ need for autonomy with public safety
- strategies for discussing driving cessation with patients and their families.
Driving: A privilege, not a right
Driving is central to older adults’ autonomy, and >75% of persons age ≥75 rely on driving as their primary mode of transportation.1 Driving cessation in this population has been associated with a 3-fold decrease in out-of-home activity2 and a 2.5-fold increase in depressive symptoms.3 Nonetheless, some 4.5 million Americans have Alzheimer’s disease (AD),4 and dementia poses a substantial risk to safe driving.
Although driving must be sacrificed when personal and public safety is at risk, most physicians perceive an uncomfortable conflict of interest between patient confidentiality and public safety.5 Assessing driving safety of patients with dementia can undermine the doctor-patient relationship and pose hardships for patients.
Mr. D has a 5-year history of memory problems that affect his daily functioning, yet he continues to drive. A longitudinal study of persons with dementia found that among the 29% who were driving at baseline, more than one-half were still behind the wheel 2 years later.6
Age and driving safety. Even in the absence of dementia, driving ability declines with aging (Tables 1 and 2).7,8 Older persons may self-regulate and restrict their driving to shorter distances, with fewer trips at night, on high-speed roads, or in unfamiliar situations. Their driving is rarely aggressive and they are unlikely to speed, but they may drive more slowly than other traffic.7,8 Although the overall rate of motor vehicle collisions declines with age:
- the rate of collisions per mile driven increases after age 659
- drivers age >65 have the highest fatality rate per mile driven among adults age ≥25.10
A dementia diagnosis is not sufficient to withdraw driving privileges, according to American Medical Association (AMA)/National Highway Traffic Safety Administration (NHTSA) guidelines. These recommend that you base decisions on the individual’s driving ability, and—when you have concerns—factor in a focused medical assessment and formal assessment of driving skills.10
Table 1
Age-related changes that may affect driving fitness
| Decreased physical capabilities, including declining muscle tone, flexibility, and reaction time |
| Decreased hearing and visual acuity |
| Increased fragility, resulting in longer time to heal should injuries occur |
| Increased medication use with possible side effect of drowsiness |
| Source: References 7,8 |
Table 2
Older drivers’ common traffic violations leading to crashes*
| Failure to obey traffic signals, including stop signs and red lights |
| Unsafe left turns (driver may inaccurately judge speed of oncoming vehicle) |
| Inappropriate turns (such as difficulty judging distance from oncoming cars, wide or narrow turns, or not timing the turn correctly with traffic lights) |
| Unsafe passing |
| Failure to yield |
| * These errors often lead to multivehicle accidents Source: References 7,8 |
CASE CONTINUED: Cognitive deficits quantified
You perform a Mini-Mental State Examination (MMSE). Mr. D scores 24/30, losing 1 point for orientation, 2 points for attention, 2 points for recall, and 1 point for copying. This score, along with his history, indicates mild dementia, although he claims he is a safe driver. On further cognitive testing, Mr. D completes the Trails A test in 90 seconds and Trails B test in 250 seconds (well below 1.5 standard deviations of the norm for his age and education).11 On the clock-drawing task, he drew a poorly organized clock, with unequal spaces between numbers and hands pointing to “10” and “11” instead of properly indicating “10 after 11.”
Mr. D and his wife live in a rural area, 5 miles from the nearest grocery store. His wife never drove, and she relies on him for weekly shopping trips and to drive her to her bridge club. She denies any problems with his driving but states, “Other drivers have become so aggressive; they’re always honking at him.” Their daughter denies that Mr. D has driving problems but admits that for the last 2 years she has refused to allow her child to ride in his car.
Focused in-office assessment
Information to assess driving ability can come from the patient, family caregiver/informant, and clinical judgment. Patients with dementia are notoriously inaccurate in self-reported driving ability, either for lack of insight or as a testament to the importance of driving to their autonomy. Caregivers often are more accurate in describing a patient’s driving, but other agendas may color their responses.
In a study of patients with very mild or mild AD, 94% reported themselves as safe drivers, whereas on-road driving instructors rated <50% of drivers in these groups as safe. Caregivers were better able to classify driving performance, but 36% of their ratings were incorrect.12
Cognitive assessment. To assess older drivers’ cognition, AMA/NHTSA’s Guide to Assessing and Counseling Older Drivers recommends the Trail-Making Test, Part B and the clock-drawing test.10 The Canadian Medical Association suggests the MMSE.13 Both guides say that abnormalities in these tests indicate a need for more detailed testing, including referral to specialized driving assessment and retesting at regular intervals (Algorithm). Retest patients with mild dementia at least every 6 months or sooner when dementia severity increases noticeably14 (Box 1).6,15
The MMSE is widely used to screen for cognitive impairment and identify dementia or delirium, but it is not a diagnostic tool or proxy driving test. A patient with dementia may produce a high MMSE score and yet be an unsafe driver. For example, well-educated patients or those with vascular or frontotemporal dementia may retain cognitive abilities as measured by the MMSE until later in the disease.
Considerable effort has been put into developing tools to help clinicians quickly and accurately differentiate safe from unsafe drivers by assessing cognition. Unfortunately, no consistent link has been found between cognitive test results and driving outcome measures. A systematic review of office-based predictors of fitness to drive in dementia found 5 studies showing an association between MMSE scores and driving and 5 studies showing no such association.16 Thus, although the AMA/NHTSA guide recommends the MMSE, Trails B, and clock-drawing tests, cognitive tests—including these—are not sufficient to assess driving ability.
Severity of dementia. International consensus groups have attempted to create guidelines for patients with dementia who drive. American, Canadian, and Australian groups suggest that a diagnosis of moderate to severe dementia precludes driving, and the driver’s licenses of persons with these conditions should be revoked.17
In general, AD is considered severe when the MMSE score is <10 or the patient becomes dependent on a caregiver for survival.18 AD of moderate severity is more difficult to define, but a Canadian consensus conference suggested a practical approach: Patients with AD would be considered to have moderate to severe dementia and should not drive when they cannot independently perform multiple instrumental activities of daily living or any of the basic activities of daily living.19
Some dementias may impair driving more quickly than AD does. For example, hallucinations may occur early in Lewy body dementia, as may impulsivity in frontotemporal dementia and motor impairment in vascular dementia.
Mrs. Y visits your office for a follow-up regarding mild Alzheimer’s disease (AD), which was diagnosed 2 years ago. She passed an on-road test 3 months ago and has an Mini-Mental State Examination score of 24/30. Over the last month she has become depressed, with insomnia and mild psychomotor retardation. She occasionally has hallucinations.
Behavioral and psychological symptoms such as agitation, aggression, hallucinations, apathy, depression, and anxiety are common neuropsychiatric sequelae of AD. Little is known about the risks these symptoms pose to road safety, but we recommend that clinicians strongly consider the potential for impaired driving.
In a longitudinal study, cognitive impairment and behavioral disturbances—especially agitation, apathy, and hallucinations—were strong predictors of driving cessation among patients with dementia.6 Furthermore, a case crossover study of patients with dementia found a 54% increase in risk of motor vehicle collisions associated with the use of psychotropic medications.15
Consider all aspects of the patient’s clinical status, including neuropsychiatric symptoms, psychotropic medications, comorbid medical conditions (including hearing and vision impairment), and concomitant therapy for medical conditions. Any could change a safe driver with mild dementia into an unsafe driver.

Algorithm: 3 options for drivers with dementia, based on in-office assessment
* Observe legislation or statutes that address reporting unsafe drivers to the department of motor vehicles or ministry of transportation
On-road driving tests
Because some individuals with mild dementia can drive safely for extended periods, international recommendations for assessing the driver with dementia emphasize on-road driving tests.10,13,20–22 American10 and Canadian guidelines13 suggest that a dementia diagnosis is not sufficient to withdraw licensure.
A formal driving assessment is necessary to establish road safety for patients with mild dementia except when the need for license withdrawal is evident, such as when the patient has:
- a history of major driving problems (such as crashes or driving the wrong way on a highway)
- significant contraindications to driving on the history or physical examination (such as severe inattention or psychosis).
Challenges of on-road testing. On-road tests may be the gold standard, but they are not without clinical problems.
Need to retest. Because almost all dementias are progressive and driving skills deteriorate over time, most guidelines recommend periodic retesting. For patients with dementia who pass on-road evaluations, limited evidence supports retesting every 6 months.14 Take an individual approach, however, because of the various rates at which the dementias progress.
Testing vs real world conditions. Structured on-road testing is not equivalent to unstructured real-world driving, in which the patient often must navigate without instruction or assistance.
Rural vs urban driving. Road tests conducted in urban areas assess skills associated with complex conditions and the need to respond quickly to crises. They might not assess as well rural driving, which requires sustained attention on monotonous roads.
Inaccessibility. Cost and lack of availability of on-road tests, particularly in rural areas, limit the number of patients whose performance can be evaluated.
CASE CONTINUED: Distressing results
Mr. D has a history of decline in cognition and function, objective cognitive difficulties, and a subtle history of driving problems. You refer him for a specialized on-road test, and the report indicates that he failed. Errors included wide turns, driving too slowly, getting caught in an intersection twice during red lights while attempting to turn left, driving on the shoulder, and failing to signal lane changes. You review the results with Mr. D and his wife and recommend that he cease driving immediately.
Mr. D is furious, and his wife is dismayed. He demands to know how he can continue to play golf, which is his only form of exercise and recreation. Will she have to give up her bridge club? How will they shop for food? They request permission to at least to drive to the grocery store during the daytime.
You explain that no system allows individuals to drive only at certain times, and for the sake of safety you cannot grant them special permission. You discuss alternatives, such as asking their daughter for assistance with grocery shopping and taking taxis or ride-sharing with friends who play golf and bridge.
Remain firm, but ease the blow
Driving cessation orders distress patients, families, and clinicians. A failed road test clearly indicates unsafe driving, and driving cessation is critical to public safety.
A review by Man-Son-Hing et al23 found that drivers with dementia performed worse than nondemented controls in all studies that examined driving performance (on-road, simulator, or caregiver report). Simulators showed problems such as off-road driving, deviation from posted speed, and more time to negotiate left turns.24
By comparison, only 1 of 3 studies using state crash records showed an increased risk of collisions in persons with dementia compared with controls.23 From a research perspective, however, studies that use state-reported collisions to assess driving risk are confounded by driving restrictions on persons with dementia.
Mr. D wants to continue driving with restrictions. No studies have shown reduced crash rates when drivers with dementia used compensatory strategies such as restrictions, retraining/education, having a passenger “co-pilot,” on-board navigation, or cognitive enhancers.23
If Mr. D had passed the road test, the situation would have been more ambiguous. Two studies have examined on-road driving performance over time in patients with early-stage dementia.25,26 Both studies followed drivers prospectively for 2 years, and those with mild dementia (vs very mild or no dementia) were most likely to show a decline in driving skills:
- All participants with mild dementia were rated as “not safe” by the end of 2 years by Duchek et al.25
- Median time to “failure” (or a rating of unsafe) was 324 days for drivers with mild dementia vs 605 days for those with very mild dementia, as reported by Ott et al.26
Mr. D’s passionate plea for reconsideration highlights the need for communities to develop alternate transportation for seniors whose driving becomes unsafe (Box 2).
Legal liability? Physicians often are concerned about legal responsibilities and risks involved in reporting unsafe drivers. Be aware of local statutes or legislations regarding mandatory reporting of patients you deem unsafe to drive.17 These laws usually protect physicians from lawsuits related to violating patient confidentiality. Civil lawsuits remain possible, however, if clinicians fail to report an unsafe driver who subsequently is involved in a motor vehicle collision.27
1. Meet with family first. Help them assume a positive and supportive role. Explain concretely and empathically your concern for the safety of the patient and others. Clearly outline your findings that the patient is not fit to drive, and explain that the law requires you to report the patient to the authorities.
Remind family members that the goal of driving assessment is to prevent a collision, and they carry some responsibility because they are aware of the potential risk of letting their family member continue to drive. If necessary, have family members witness a repeat performance by the patient on the most revealing test. Discuss the importance of finding alternate transportation to reduce the risk of isolation and depression that can follow driving cessation.
2. Meet with patient. Having the family present can be helpful, but ask them to assume a supportive role. Give the patient a positive role by recognizing that he or she has been a responsible driver, and part of this responsibility is to stop driving before an accident occurs. Acknowledge that it is normal to be unhappy upon learning that one’s driving privileges are being revoked.
Sometimes it helps to give the patient a prescription in their name that says, “Do not drive.” Families who receive a copy may find this very helpful, too, for reminding the patient later about what you said.
If your patient argues with your position, remain firm and do not argue. Indicate that you have made notes on the meeting and are notifying the authorities about the patient’s unsafe driving. You can add that your chart could be subpoenaed and the patient may be legally liable and financially responsible should he or she continue to drive and have a collision.
3. Talk about transportation options. Family members could share driving responsibilities. Taxi rides can cost less than maintaining a car if the patient drives <4,000 km (2,500 miles) per year. Suggest that patients or families find volunteer drivers or contact helpful taxi drivers a day before an outing is planned.
4. If patient refuses to comply, meet with the family again and encourage them to remove the patient’s opportunity to drive (confiscate the keys, disable the car, or remove the car altogether).
Provide a written statement to the patient and family outlining why the patient can no longer drive. Indicate that it is your legal responsibility to report unsafe drivers, and you intend to notify the authorities regarding the patient’s driving status. If the patient remains noncompliant, continue to encourage family to remove the opportunity to drive.
Related resources
- Physician’s guide to assessing and counseling older drivers. American Medical Association and the National Highway Traffic Safety Administration, 2003. www.nhtsa.dot.gov/people/injury/olddrive/OlderDriversBook.
- Determining medical fitness to operate motor vehicles. Canadian Medical Association. 2006. www.cma.ca/index.cfm/ci_id/18223/la_id/1.htm.
- Canadian Driving Research Initiative for Vehicular Safety in the Elderly (CanDRIVE). www.candrive.ca.
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Rapoport receives grant/research support from the Canadian Institute of Health Research and the Ontario Neurotrauma Foundation.
1. Stowell-Ritter A, Straight A, Evans EL. Understanding senior transportation: report and analysis of a survey of consumers age 50+. Washington, DC: American Association of Retired Persons; 2002.
2. Marottoli RA, de Leon CFM, Glass TA, et al. Consequences of driving cessation: decreased out-of-home activity levels. J Gerontol B Psychol Sci Soc Sci 2000;55(6):S334-40.
3. Marottoli RA, Mendes de Leon CF, Glass TA, et al. Driving cessation and increased depressive symptoms: prospective evidence from the New Haven EPESE. Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc 1997;45(2):202-6.
4. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003;60(8):1119-22.
5. Jang RW, Man-Son-Hing M, Molnar FJ, et al. Family physicians’ attitudes and practices regarding assessments of medical fitness to drive in older persons. J Gen Intern Med 2007;22(4):531-43.
6. Herrmann N, Rapoport MJ, Sambrook R, et al. Predictors of driving cessation in mild-to-moderate dementia. CMAJ 2006;175(6):591-5.
7. Hedlund J. Countermeasures that work: a highway safety countermeasure guide for state highway safety offices. Washington, DC: National Highway Traffic Safety Administration; 2006.
8. Dobbs B. Medical conditions and driving: a review of the literature (1960-2000). Washington, DC: National Highway Traffic Safety Administration; 2005. Available at: http://www.nhtsa.dot.gov/people/injury/research/Medical_Condition_Driving/pages/TRD.html. Accessed September 29, 2008.
9. Li G, Braver ER, Chen LH. Fragility versus excessive crash involvement as determinants of high death rates per vehicle-mile of travel among older drivers. Accid Anal Prev 2003;35(2):227-35.
10. Physician’s guide to assessing and counseling older drivers. Washington, DC: National Highway Traffic Safety Administration; 2003. Available at: http://www.nhtsa.dot.gov/people/injury/olddrive/OlderDriversBook. Accessed September 29, 2008.
11. Yeudall LT, Reddon JR, Gill DM, et al. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. J Clin Psychol 1987;43(3):346-67.
12. Brown LB, Ott BR, Papandonatos GD, et al. Prediction of on-road driving performance in patients with early Alzheimer’s disease. J Am Geriatr Soc 2005;53(1):94-8.
13. Determining medical fitness to operate motor vehicles. CMA driver’s guide. 7th ed. Ottawa, Ontario, Canada: Canadian Medical Association; 2006.
14. Molnar FJ, Patel A, Marshall SC, et al. Systematic review of the optimal frequency of follow-up in persons with mild dementia who continue to drive. Alzheimer Dis Assoc Disord 2006;20(4):295-7.
15. Rapoport MJ, Herrmann N, Molnar FJ, et al. Psychotropic medications and motor vehicle collisions in patients with dementia (letter). J Am Geriatr Soc 2008;56(10):1968-70.
16. Molnar FJ, Patel A, Marshall SC, et al. Clinical utility of office-based cognitive predictors of fitness to drive in persons with dementia: a systematic review. J Am Geriatr Soc 2006;54(12):1809-24.
17. Rapoport MJ, Herrmann N, Molnar FJ, et al. Sharing the responsibility for assessing the risk of the driver with dementia. CMAJ. 2007;177(6):599-601.
18. Herrmann N, Gauthier S, Lysy PG. Clinical practice guidelines for severe Alzheimer’s disease. Alzheimers Dement 2007;3(4):385-97.
19. Hogan DB, Bailey P, Carswell A, et al. Management of mild to moderate Alzheimer’s disease and dementia. Alzheimers Dement 2007;3(4):355-84.
19. Assessing fitness to drive for commercial and private vehicle drivers. Sydney, Australia: National Library of Australia; 2006. Available at: http://www.austroads.com.au/aftd/index.html. Accessed November 4, 2008.
21. Medical aspects of fitness to drive. A guide for medical practitioners. Wellington, New Zealand: Land Transport Safety Authority; 2002. Available at: http://www.transfund.govt.nz/licensing/docs/ltsa-medical-aspects.pdf. Accessed September 29, 2008.
22. At a glance guide to the current medical standards of fitness to drive. Swansea, UK: Drivers Medical Group, Driver and Vehicle Licensing Agency; 2008. Available at: http://www.dvla.gov.uk/medical/ataglance.aspx. Accessed September 29, 2008.
23. Man-Son-Hing M, Marshall SC, Molnar FJ, Wilson KG. Systematic review of driving risk and the efficacy of compensatory strategies in persons with dementia. J Am Geriatr Soc 2007;55(6):878-84.
24. Cox DJ, Quillian WC, Thorndike FP, et al. Evaluating driving performance of outpatients with Alzheimer disease. J Am Board Fam Pract 1998;11(4):264-71.
25. Duchek JM, Carr DB, Hunt L, et al. Longitudinal driving performance in early-stage dementia of the Alzheimer type. J Am Geriatr Soc 2003;51(10):1342-7.
26. Ott BR, Heindel WC, Papandonatos GD, et al. A longitudinal study of drivers with Alzheimer disease. Neurology 2008;70(14):1171-8.
27. Molnar FJ, Byszewski AM, Marshall SC, Man-Son-Hing M. In-office evaluation of medical fitness to drive: practical approaches for assessing older people. Can Fam Physician 2005;51:372-9.
1. Stowell-Ritter A, Straight A, Evans EL. Understanding senior transportation: report and analysis of a survey of consumers age 50+. Washington, DC: American Association of Retired Persons; 2002.
2. Marottoli RA, de Leon CFM, Glass TA, et al. Consequences of driving cessation: decreased out-of-home activity levels. J Gerontol B Psychol Sci Soc Sci 2000;55(6):S334-40.
3. Marottoli RA, Mendes de Leon CF, Glass TA, et al. Driving cessation and increased depressive symptoms: prospective evidence from the New Haven EPESE. Established Populations for Epidemiologic Studies of the Elderly. J Am Geriatr Soc 1997;45(2):202-6.
4. Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003;60(8):1119-22.
5. Jang RW, Man-Son-Hing M, Molnar FJ, et al. Family physicians’ attitudes and practices regarding assessments of medical fitness to drive in older persons. J Gen Intern Med 2007;22(4):531-43.
6. Herrmann N, Rapoport MJ, Sambrook R, et al. Predictors of driving cessation in mild-to-moderate dementia. CMAJ 2006;175(6):591-5.
7. Hedlund J. Countermeasures that work: a highway safety countermeasure guide for state highway safety offices. Washington, DC: National Highway Traffic Safety Administration; 2006.
8. Dobbs B. Medical conditions and driving: a review of the literature (1960-2000). Washington, DC: National Highway Traffic Safety Administration; 2005. Available at: http://www.nhtsa.dot.gov/people/injury/research/Medical_Condition_Driving/pages/TRD.html. Accessed September 29, 2008.
9. Li G, Braver ER, Chen LH. Fragility versus excessive crash involvement as determinants of high death rates per vehicle-mile of travel among older drivers. Accid Anal Prev 2003;35(2):227-35.
10. Physician’s guide to assessing and counseling older drivers. Washington, DC: National Highway Traffic Safety Administration; 2003. Available at: http://www.nhtsa.dot.gov/people/injury/olddrive/OlderDriversBook. Accessed September 29, 2008.
11. Yeudall LT, Reddon JR, Gill DM, et al. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. J Clin Psychol 1987;43(3):346-67.
12. Brown LB, Ott BR, Papandonatos GD, et al. Prediction of on-road driving performance in patients with early Alzheimer’s disease. J Am Geriatr Soc 2005;53(1):94-8.
13. Determining medical fitness to operate motor vehicles. CMA driver’s guide. 7th ed. Ottawa, Ontario, Canada: Canadian Medical Association; 2006.
14. Molnar FJ, Patel A, Marshall SC, et al. Systematic review of the optimal frequency of follow-up in persons with mild dementia who continue to drive. Alzheimer Dis Assoc Disord 2006;20(4):295-7.
15. Rapoport MJ, Herrmann N, Molnar FJ, et al. Psychotropic medications and motor vehicle collisions in patients with dementia (letter). J Am Geriatr Soc 2008;56(10):1968-70.
16. Molnar FJ, Patel A, Marshall SC, et al. Clinical utility of office-based cognitive predictors of fitness to drive in persons with dementia: a systematic review. J Am Geriatr Soc 2006;54(12):1809-24.
17. Rapoport MJ, Herrmann N, Molnar FJ, et al. Sharing the responsibility for assessing the risk of the driver with dementia. CMAJ. 2007;177(6):599-601.
18. Herrmann N, Gauthier S, Lysy PG. Clinical practice guidelines for severe Alzheimer’s disease. Alzheimers Dement 2007;3(4):385-97.
19. Hogan DB, Bailey P, Carswell A, et al. Management of mild to moderate Alzheimer’s disease and dementia. Alzheimers Dement 2007;3(4):355-84.
19. Assessing fitness to drive for commercial and private vehicle drivers. Sydney, Australia: National Library of Australia; 2006. Available at: http://www.austroads.com.au/aftd/index.html. Accessed November 4, 2008.
21. Medical aspects of fitness to drive. A guide for medical practitioners. Wellington, New Zealand: Land Transport Safety Authority; 2002. Available at: http://www.transfund.govt.nz/licensing/docs/ltsa-medical-aspects.pdf. Accessed September 29, 2008.
22. At a glance guide to the current medical standards of fitness to drive. Swansea, UK: Drivers Medical Group, Driver and Vehicle Licensing Agency; 2008. Available at: http://www.dvla.gov.uk/medical/ataglance.aspx. Accessed September 29, 2008.
23. Man-Son-Hing M, Marshall SC, Molnar FJ, Wilson KG. Systematic review of driving risk and the efficacy of compensatory strategies in persons with dementia. J Am Geriatr Soc 2007;55(6):878-84.
24. Cox DJ, Quillian WC, Thorndike FP, et al. Evaluating driving performance of outpatients with Alzheimer disease. J Am Board Fam Pract 1998;11(4):264-71.
25. Duchek JM, Carr DB, Hunt L, et al. Longitudinal driving performance in early-stage dementia of the Alzheimer type. J Am Geriatr Soc 2003;51(10):1342-7.
26. Ott BR, Heindel WC, Papandonatos GD, et al. A longitudinal study of drivers with Alzheimer disease. Neurology 2008;70(14):1171-8.
27. Molnar FJ, Byszewski AM, Marshall SC, Man-Son-Hing M. In-office evaluation of medical fitness to drive: practical approaches for assessing older people. Can Fam Physician 2005;51:372-9.
Words to the wise: 4 secrets of successful pharmacotherapy
Any medication’s therapeutic success depends on the interaction between its specific biochemical effects and nonspecific factors.1 Thus, clinical trial designers may view the placebo effect as undesirable, but it can be a valuable response that improves treatment outcomes in clinical practice. As Freud stated, “Expectation colored by hope and faith is an effective force with which we have to reckon…in all our attempts at treatment and cure.”2
This article describes how experienced clinicians make use of the placebo effect and 3 other powerful, nonspecific elements of successful pharmacotherapy.
The placebo effect
The placebo effect is any effect attributable to a pill or potion that does not originate from its specific pharmacologic properties.3 Its clinical value has been trivialized, in part because of misconceptions (Table 1). For example, the placebo effect is commonly believed to be short-lived, whereas in fact it can last a long time.4
In clinical practice, our goal is to enhance the placebo effect to maximize a desirable therapeutic outcome (Table 2).5 Therefore, before I prescribe a medication, I tell my patient that I have selected a particular medication because I have had good results with it in many other patients and I believe it will work well for him or her, too.
Too often, doctors feel pessimistic about a medication’s potential therapeutic result and communicate this pessimism. What the patient hears is, “There’s nothing else I can do for you; why not try this medication, even though I don’t believe it’s going to work.” This may create a negative placebo effect6—termed the “nocebo” effect—which gives the patient a negative expectation about the treatment’s outcome. The patient internalizes the doctor’s words and lives out this negative expectation.
Table 1
Correcting misconceptions about the placebo effect
| Misconception | What the evidence shows |
|---|---|
| Placebo effects are short-lived | The placebo effect has been documented to last for a long time |
| Only complaints that are psychologically originated respond to placebo | Changes after placebo have been documented for most symptoms, including those originating from somatic diseases |
| Placebo responders are distinctly different from nonresponders | There is no difference between placebo responders and nonresponders |
| The placebo effect is only about one-third of the total therapeutic effect | The placebo effect can be up to 100% of the total therapeutic effect |
| Only about one-third of the population responds to placebo | The placebo response is context-dependent and may include >90% of the patient population |
| Source: Reference 4 | |
Clinical strategies to enhance the placebo effect
| |
| Source: Reference 5 | |
CASE REPORT: Predicting positive results
Mr. B, age 42, has a history of recurrent depression associated with severe insomnia, poor appetite, significant weight loss, and psychosocial withdrawal with feelings of hopelessness. After I take a detailed history and do a mental status examination, I suggest that he be treated with cognitive-behavioral therapy (CBT) and mirtazapine.
Even though studies of antidepressants rarely show mood improvements within the first 7 days, it is not unusual to hear patients report feeling less depressed within days after they start a new antidepressant. Although the drug’s specific chemical effects on the brain may not be sufficient to explain this phenomenon, the explanation probably lies in nonspecific effects—such as the patient expecting that this medication will make him feel better.
The placebo effect can occur as soon as a patient starts a medication. Experienced clinicians understand the placebo effect’s power and harness it to benefit their patients.
Conditioned responses
Many biological responses can be associated with visual, auditory, tactile, olfactory, or gustatory stimuli. Nonconditioned physiologic responses paired with conditioned stimuli induce the same biological effects of a drug. Evidence supporting this phenomenon includes successful conditioning of the immune system.7-10 Conditioned responses—as demonstrated in glycemia regulation10 and with psychopharmacology11—also can enhance the desirable results of pharmacotherapy.
CASE REPORT: A soothing drink
Ms. L, a 22-year-old college student, suffers from obsessive-compulsive disorder associated with anxiety and depression. She arrives at the appointment hurried and worried that she might be late. She is short of breath and looks stressed. The nurse offers Ms. L a cup of tea or water. She chooses a glass of water and is asked to bring it into her session.
Following a comprehensive interview and mental status examination, I recommend CBT plus medication. Considering Ms. L’s medication history, we agree to start treatment with sertraline. We review its potential benefits and expectations that it will reduce her anxiety, alleviate her ruminating obsessive worries, and improve her mood. I give her a 50-mg sample and inform her that some patients experience positive effects soon after taking the medication. I then ask her to take the first pill, using her glass of water. She does so and thanks me for being attentive to her needs.
I instruct her to call within 1 week and report on her condition, even if she feels better. Seven days later she reports that she is feeling better and is looking forward to her next appointment. She reports no side effects.
Often patients come to my office feeling thirsty. My staff or I offer them a glass of water or a cup of tea. As patients sip from the cup, they swallow and incorporate the liquid into their bodies. At the same time, I use verbal interventions to make them feel listened to and understood. They internalize this emotional experience in connection with swallowing the liquid.
Later, when swallowing the new medication as instructed, the patient re-experiences the positive therapeutic effect that was internalized in the doctor’s office.
The power of suggestion
The power of suggestion has been shown to positively or negatively affect treatment outcomes.12,13 In practice, most clinicians give unintentional suggestions by how and what they communicate to the patient.
CASE REPORT: Predicting improvement
Mrs. J, age 48, has had dysthymic disorder and fibromyalgia for many years. She describes how various specialists have tried to alleviate her depression and chronic pain. Follow-up questions reveal that whenever she received a new prescription the physician would alert her to all the possible side effects and instruct her to call the office if she developed a problem with the new medication.
Invariably, Mrs. J would call as instructed and describe side effects she developed with the new medication. Often the doctor would discontinue the medication, depriving Mrs. J of benefits she might have derived later.
When Mrs. J calls to report on her status, she mentions that she is sleeping better and has begun to feel better during the day. She says that her husband told her she has started to smile again.
This vignette illustrates the importance of suggesting to the patient a positive outcome of pharmacotherapy associated with a particular action (calling the doctor’s office to report results). When the patient promised to call, she internalized the suggestion that calling would be associated with feeling better—and that is what happened. This intervention contrasts with saying to the patient, “Call me if you have a problem with any of these side effects,” which gives the patient a suggestion to call and report a problem.
The suggestion effect also can be used to reframe a predictable side effect as a positive sign that indicates the beginning of change leading to recovery (Box).
Ms. M, age 32 and single, has an anxiety disorder associated with bipolar depression. She has discontinued several psychotropics because of uncomfortable side effects, such as constipation.
After taking a detailed history, I decide to prescribe quetiapine. I tell Ms. M about this medication’s potential benefits and side effects. One common side effect is dry mouth, which often occurs before patients experience therapeutic effects.
I inform Ms. M that a dry mouth will be her sign that the medication has begun to work, and beneficial effects—such as improved sleep, reduced anxiety, and improved mood—will soon follow. I then instruct her to call my office and report when she experiences a dry mouth.
Discussion. In pharmacotherapy, side effects may appear before patients experience a medication’s beneficial/therapeutic effects. Patients’ initial experience often determines whether or not they will continue taking a prescribed medication. I know Ms. M may stop taking quetiapine—as she has done with other medications—if she initially has uncomfortable side effects.
Instructing patients to expect a specific side effect (such as a dry mouth with quetiapine) and associating it with a future therapeutic benefit sets up a road map of expectations. They know their experience is compatible with the doctor’s predictions. For Ms. M, I reframed the side effect as a positive sign that recovery has begun, with more positive changes to come.
Participatory pharmacotherapy
Many patients seek ownership in making decisions about their treatment and medications. In participatory pharmacotherapy, patients provide you with data and valuable information—such as family history, personal medical history, and experience with treatment—and inform you about which medications worked best and which did not work. You invite patients to predict how they see themselves getting better and into recovery.
CASE REPORT: All in the family
Mr. A, age 28 and single, has been diagnosed with a bipolar mood disorder. As part of a detailed family history, he reports that his maternal grandfather, mother, and a maternal uncle were diagnosed with mood swings and were successfully treated with medications, specifically lithium. He states that he believes he has the same condition.
I compliment Mr. A for being so well informed about his grandfather and uncle and educate him about mood stabilizers’ benefits in bipolar disorder. I tell him about the finding that if lithium has helped his relatives, it will probably help him as well.
I also reassure Mr. A that, in deciding what medications to avoid and what medications to use, I will consider his experience with specific antidepressants that did not help him. He thanks me for considering his suggestion about what medication to use for him.
- a successful therapeutic alliance
- adherence with prescribed medications
- the best possible outcome with pharmacotherapy.
In patients with a defiant-oppositional personality, consider framing the treatment decision as a choice between 2 equally efficacious medications. This gives the patient the sense of control in choosing his or her own medication, which is jointly monitored.
Table 3
Choosing patients for participatory pharmacotherapy
| Good candidates | Exclusionary qualities |
|---|---|
| Adults | Children, adolescents, and prison inmates |
| No history of alcoholism or drug addiction | Alcohol dependence or drug addiction |
| Average and above intelligence | Below-average intelligence |
| Intact cognitive function | Cognitive deficits, such as dementia |
| Not psychotic | Actively psychotic |
| Good comprehension of diagnosis and treatment | Poor comprehension of diagnosis and treatment |
| Therapeutic alliance is present | Therapeutic alliance is absent |
| Personality style or disorder with a need to be in control of treatment, such as obsessive-compulsive personality | Passive, dependent personality style or disorder; these patients may view a participatory approach as the doctor’s lack of confidence |
- Brody H. The placebo response: how you can release the body’s inner pharmacy for better health. New York, NY: HarperCollins Publishers; 2000.
- Spiro H. The power of hope: a doctor’s perspective. New Haven, CT: Yale University Press; 1998.
- Ernst E. Placebo: new insights into an old enigma. Drug Discov Today 2007;12:413-8.
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Sertraline • Zoloft
Dr. Torem reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Frank JD, Frank JB. Persuasion and healing. Baltimore, MD: The Johns Hopkins University Press; 1991.
2. Freud S. The complete psychological works of Sigmund Freud. Strachey J, trans-ed. Toronto, Ontario, Canada: Hogarth Press; 1953.
3. Wolf S. The pharmacology of placebos. Pharmacol Rev 1959;11:689-704.
4. Ernst E. Placebo: new insights into an old enigma. Drug Discov Today 2007;12:413-8.
5. Brody H. The placebo response: recent research and implications for family medicine. J Fam Pract 2000;49:649-54.
6. Spiegel H. Nocebo, the power of suggestibility. Prev Med 1997;26:616-21.
7. Ader R, Cohen N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science 1982;215:1534-6.
8. Ader R. The role of conditioning in pharmacotherapy. In: Harrington A, ed. The placebo effect: an interdisciplinary exploration. Cambridge, MA: Harvard University Press; 1997;138-65.
9. Olness K, Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J Dev Behav Pediatr 1992;13:124-5.
10. Stockhorst U, Mahl N, Krueger M, et al. Classical conditioning and conditionability of insulin and glucose effect in healthy humans. Physiol Behav 2004;81:375-88.
11. Wolf S. Effect of suggestion and conditioning on the action of chemical agents in human subjects—the pharmacology of placebos. J Clin Invest 1950;29:100-9.
12. Lown B. The verbal conditioning of angina pectoris during exercise testing. Am J Cardiol 1977;40:630-4.
13. Lown B. Introduction. In: Cousins N. The healing heart. New York, NY: W.W. Norton; 1983:11-28.
14. Watzlawick P. If you desire to see, learn how to act. In: Nardone G, Watzlawick P, eds. The art of change San Francisco, CA: Jossey-Bass; 1993:1-16.
Any medication’s therapeutic success depends on the interaction between its specific biochemical effects and nonspecific factors.1 Thus, clinical trial designers may view the placebo effect as undesirable, but it can be a valuable response that improves treatment outcomes in clinical practice. As Freud stated, “Expectation colored by hope and faith is an effective force with which we have to reckon…in all our attempts at treatment and cure.”2
This article describes how experienced clinicians make use of the placebo effect and 3 other powerful, nonspecific elements of successful pharmacotherapy.
The placebo effect
The placebo effect is any effect attributable to a pill or potion that does not originate from its specific pharmacologic properties.3 Its clinical value has been trivialized, in part because of misconceptions (Table 1). For example, the placebo effect is commonly believed to be short-lived, whereas in fact it can last a long time.4
In clinical practice, our goal is to enhance the placebo effect to maximize a desirable therapeutic outcome (Table 2).5 Therefore, before I prescribe a medication, I tell my patient that I have selected a particular medication because I have had good results with it in many other patients and I believe it will work well for him or her, too.
Too often, doctors feel pessimistic about a medication’s potential therapeutic result and communicate this pessimism. What the patient hears is, “There’s nothing else I can do for you; why not try this medication, even though I don’t believe it’s going to work.” This may create a negative placebo effect6—termed the “nocebo” effect—which gives the patient a negative expectation about the treatment’s outcome. The patient internalizes the doctor’s words and lives out this negative expectation.
Table 1
Correcting misconceptions about the placebo effect
| Misconception | What the evidence shows |
|---|---|
| Placebo effects are short-lived | The placebo effect has been documented to last for a long time |
| Only complaints that are psychologically originated respond to placebo | Changes after placebo have been documented for most symptoms, including those originating from somatic diseases |
| Placebo responders are distinctly different from nonresponders | There is no difference between placebo responders and nonresponders |
| The placebo effect is only about one-third of the total therapeutic effect | The placebo effect can be up to 100% of the total therapeutic effect |
| Only about one-third of the population responds to placebo | The placebo response is context-dependent and may include >90% of the patient population |
| Source: Reference 4 | |
Clinical strategies to enhance the placebo effect
| |
| Source: Reference 5 | |
CASE REPORT: Predicting positive results
Mr. B, age 42, has a history of recurrent depression associated with severe insomnia, poor appetite, significant weight loss, and psychosocial withdrawal with feelings of hopelessness. After I take a detailed history and do a mental status examination, I suggest that he be treated with cognitive-behavioral therapy (CBT) and mirtazapine.
Even though studies of antidepressants rarely show mood improvements within the first 7 days, it is not unusual to hear patients report feeling less depressed within days after they start a new antidepressant. Although the drug’s specific chemical effects on the brain may not be sufficient to explain this phenomenon, the explanation probably lies in nonspecific effects—such as the patient expecting that this medication will make him feel better.
The placebo effect can occur as soon as a patient starts a medication. Experienced clinicians understand the placebo effect’s power and harness it to benefit their patients.
Conditioned responses
Many biological responses can be associated with visual, auditory, tactile, olfactory, or gustatory stimuli. Nonconditioned physiologic responses paired with conditioned stimuli induce the same biological effects of a drug. Evidence supporting this phenomenon includes successful conditioning of the immune system.7-10 Conditioned responses—as demonstrated in glycemia regulation10 and with psychopharmacology11—also can enhance the desirable results of pharmacotherapy.
CASE REPORT: A soothing drink
Ms. L, a 22-year-old college student, suffers from obsessive-compulsive disorder associated with anxiety and depression. She arrives at the appointment hurried and worried that she might be late. She is short of breath and looks stressed. The nurse offers Ms. L a cup of tea or water. She chooses a glass of water and is asked to bring it into her session.
Following a comprehensive interview and mental status examination, I recommend CBT plus medication. Considering Ms. L’s medication history, we agree to start treatment with sertraline. We review its potential benefits and expectations that it will reduce her anxiety, alleviate her ruminating obsessive worries, and improve her mood. I give her a 50-mg sample and inform her that some patients experience positive effects soon after taking the medication. I then ask her to take the first pill, using her glass of water. She does so and thanks me for being attentive to her needs.
I instruct her to call within 1 week and report on her condition, even if she feels better. Seven days later she reports that she is feeling better and is looking forward to her next appointment. She reports no side effects.
Often patients come to my office feeling thirsty. My staff or I offer them a glass of water or a cup of tea. As patients sip from the cup, they swallow and incorporate the liquid into their bodies. At the same time, I use verbal interventions to make them feel listened to and understood. They internalize this emotional experience in connection with swallowing the liquid.
Later, when swallowing the new medication as instructed, the patient re-experiences the positive therapeutic effect that was internalized in the doctor’s office.
The power of suggestion
The power of suggestion has been shown to positively or negatively affect treatment outcomes.12,13 In practice, most clinicians give unintentional suggestions by how and what they communicate to the patient.
CASE REPORT: Predicting improvement
Mrs. J, age 48, has had dysthymic disorder and fibromyalgia for many years. She describes how various specialists have tried to alleviate her depression and chronic pain. Follow-up questions reveal that whenever she received a new prescription the physician would alert her to all the possible side effects and instruct her to call the office if she developed a problem with the new medication.
Invariably, Mrs. J would call as instructed and describe side effects she developed with the new medication. Often the doctor would discontinue the medication, depriving Mrs. J of benefits she might have derived later.
When Mrs. J calls to report on her status, she mentions that she is sleeping better and has begun to feel better during the day. She says that her husband told her she has started to smile again.
This vignette illustrates the importance of suggesting to the patient a positive outcome of pharmacotherapy associated with a particular action (calling the doctor’s office to report results). When the patient promised to call, she internalized the suggestion that calling would be associated with feeling better—and that is what happened. This intervention contrasts with saying to the patient, “Call me if you have a problem with any of these side effects,” which gives the patient a suggestion to call and report a problem.
The suggestion effect also can be used to reframe a predictable side effect as a positive sign that indicates the beginning of change leading to recovery (Box).
Ms. M, age 32 and single, has an anxiety disorder associated with bipolar depression. She has discontinued several psychotropics because of uncomfortable side effects, such as constipation.
After taking a detailed history, I decide to prescribe quetiapine. I tell Ms. M about this medication’s potential benefits and side effects. One common side effect is dry mouth, which often occurs before patients experience therapeutic effects.
I inform Ms. M that a dry mouth will be her sign that the medication has begun to work, and beneficial effects—such as improved sleep, reduced anxiety, and improved mood—will soon follow. I then instruct her to call my office and report when she experiences a dry mouth.
Discussion. In pharmacotherapy, side effects may appear before patients experience a medication’s beneficial/therapeutic effects. Patients’ initial experience often determines whether or not they will continue taking a prescribed medication. I know Ms. M may stop taking quetiapine—as she has done with other medications—if she initially has uncomfortable side effects.
Instructing patients to expect a specific side effect (such as a dry mouth with quetiapine) and associating it with a future therapeutic benefit sets up a road map of expectations. They know their experience is compatible with the doctor’s predictions. For Ms. M, I reframed the side effect as a positive sign that recovery has begun, with more positive changes to come.
Participatory pharmacotherapy
Many patients seek ownership in making decisions about their treatment and medications. In participatory pharmacotherapy, patients provide you with data and valuable information—such as family history, personal medical history, and experience with treatment—and inform you about which medications worked best and which did not work. You invite patients to predict how they see themselves getting better and into recovery.
CASE REPORT: All in the family
Mr. A, age 28 and single, has been diagnosed with a bipolar mood disorder. As part of a detailed family history, he reports that his maternal grandfather, mother, and a maternal uncle were diagnosed with mood swings and were successfully treated with medications, specifically lithium. He states that he believes he has the same condition.
I compliment Mr. A for being so well informed about his grandfather and uncle and educate him about mood stabilizers’ benefits in bipolar disorder. I tell him about the finding that if lithium has helped his relatives, it will probably help him as well.
I also reassure Mr. A that, in deciding what medications to avoid and what medications to use, I will consider his experience with specific antidepressants that did not help him. He thanks me for considering his suggestion about what medication to use for him.
- a successful therapeutic alliance
- adherence with prescribed medications
- the best possible outcome with pharmacotherapy.
In patients with a defiant-oppositional personality, consider framing the treatment decision as a choice between 2 equally efficacious medications. This gives the patient the sense of control in choosing his or her own medication, which is jointly monitored.
Table 3
Choosing patients for participatory pharmacotherapy
| Good candidates | Exclusionary qualities |
|---|---|
| Adults | Children, adolescents, and prison inmates |
| No history of alcoholism or drug addiction | Alcohol dependence or drug addiction |
| Average and above intelligence | Below-average intelligence |
| Intact cognitive function | Cognitive deficits, such as dementia |
| Not psychotic | Actively psychotic |
| Good comprehension of diagnosis and treatment | Poor comprehension of diagnosis and treatment |
| Therapeutic alliance is present | Therapeutic alliance is absent |
| Personality style or disorder with a need to be in control of treatment, such as obsessive-compulsive personality | Passive, dependent personality style or disorder; these patients may view a participatory approach as the doctor’s lack of confidence |
- Brody H. The placebo response: how you can release the body’s inner pharmacy for better health. New York, NY: HarperCollins Publishers; 2000.
- Spiro H. The power of hope: a doctor’s perspective. New Haven, CT: Yale University Press; 1998.
- Ernst E. Placebo: new insights into an old enigma. Drug Discov Today 2007;12:413-8.
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Sertraline • Zoloft
Dr. Torem reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Any medication’s therapeutic success depends on the interaction between its specific biochemical effects and nonspecific factors.1 Thus, clinical trial designers may view the placebo effect as undesirable, but it can be a valuable response that improves treatment outcomes in clinical practice. As Freud stated, “Expectation colored by hope and faith is an effective force with which we have to reckon…in all our attempts at treatment and cure.”2
This article describes how experienced clinicians make use of the placebo effect and 3 other powerful, nonspecific elements of successful pharmacotherapy.
The placebo effect
The placebo effect is any effect attributable to a pill or potion that does not originate from its specific pharmacologic properties.3 Its clinical value has been trivialized, in part because of misconceptions (Table 1). For example, the placebo effect is commonly believed to be short-lived, whereas in fact it can last a long time.4
In clinical practice, our goal is to enhance the placebo effect to maximize a desirable therapeutic outcome (Table 2).5 Therefore, before I prescribe a medication, I tell my patient that I have selected a particular medication because I have had good results with it in many other patients and I believe it will work well for him or her, too.
Too often, doctors feel pessimistic about a medication’s potential therapeutic result and communicate this pessimism. What the patient hears is, “There’s nothing else I can do for you; why not try this medication, even though I don’t believe it’s going to work.” This may create a negative placebo effect6—termed the “nocebo” effect—which gives the patient a negative expectation about the treatment’s outcome. The patient internalizes the doctor’s words and lives out this negative expectation.
Table 1
Correcting misconceptions about the placebo effect
| Misconception | What the evidence shows |
|---|---|
| Placebo effects are short-lived | The placebo effect has been documented to last for a long time |
| Only complaints that are psychologically originated respond to placebo | Changes after placebo have been documented for most symptoms, including those originating from somatic diseases |
| Placebo responders are distinctly different from nonresponders | There is no difference between placebo responders and nonresponders |
| The placebo effect is only about one-third of the total therapeutic effect | The placebo effect can be up to 100% of the total therapeutic effect |
| Only about one-third of the population responds to placebo | The placebo response is context-dependent and may include >90% of the patient population |
| Source: Reference 4 | |
Clinical strategies to enhance the placebo effect
| |
| Source: Reference 5 | |
CASE REPORT: Predicting positive results
Mr. B, age 42, has a history of recurrent depression associated with severe insomnia, poor appetite, significant weight loss, and psychosocial withdrawal with feelings of hopelessness. After I take a detailed history and do a mental status examination, I suggest that he be treated with cognitive-behavioral therapy (CBT) and mirtazapine.
Even though studies of antidepressants rarely show mood improvements within the first 7 days, it is not unusual to hear patients report feeling less depressed within days after they start a new antidepressant. Although the drug’s specific chemical effects on the brain may not be sufficient to explain this phenomenon, the explanation probably lies in nonspecific effects—such as the patient expecting that this medication will make him feel better.
The placebo effect can occur as soon as a patient starts a medication. Experienced clinicians understand the placebo effect’s power and harness it to benefit their patients.
Conditioned responses
Many biological responses can be associated with visual, auditory, tactile, olfactory, or gustatory stimuli. Nonconditioned physiologic responses paired with conditioned stimuli induce the same biological effects of a drug. Evidence supporting this phenomenon includes successful conditioning of the immune system.7-10 Conditioned responses—as demonstrated in glycemia regulation10 and with psychopharmacology11—also can enhance the desirable results of pharmacotherapy.
CASE REPORT: A soothing drink
Ms. L, a 22-year-old college student, suffers from obsessive-compulsive disorder associated with anxiety and depression. She arrives at the appointment hurried and worried that she might be late. She is short of breath and looks stressed. The nurse offers Ms. L a cup of tea or water. She chooses a glass of water and is asked to bring it into her session.
Following a comprehensive interview and mental status examination, I recommend CBT plus medication. Considering Ms. L’s medication history, we agree to start treatment with sertraline. We review its potential benefits and expectations that it will reduce her anxiety, alleviate her ruminating obsessive worries, and improve her mood. I give her a 50-mg sample and inform her that some patients experience positive effects soon after taking the medication. I then ask her to take the first pill, using her glass of water. She does so and thanks me for being attentive to her needs.
I instruct her to call within 1 week and report on her condition, even if she feels better. Seven days later she reports that she is feeling better and is looking forward to her next appointment. She reports no side effects.
Often patients come to my office feeling thirsty. My staff or I offer them a glass of water or a cup of tea. As patients sip from the cup, they swallow and incorporate the liquid into their bodies. At the same time, I use verbal interventions to make them feel listened to and understood. They internalize this emotional experience in connection with swallowing the liquid.
Later, when swallowing the new medication as instructed, the patient re-experiences the positive therapeutic effect that was internalized in the doctor’s office.
The power of suggestion
The power of suggestion has been shown to positively or negatively affect treatment outcomes.12,13 In practice, most clinicians give unintentional suggestions by how and what they communicate to the patient.
CASE REPORT: Predicting improvement
Mrs. J, age 48, has had dysthymic disorder and fibromyalgia for many years. She describes how various specialists have tried to alleviate her depression and chronic pain. Follow-up questions reveal that whenever she received a new prescription the physician would alert her to all the possible side effects and instruct her to call the office if she developed a problem with the new medication.
Invariably, Mrs. J would call as instructed and describe side effects she developed with the new medication. Often the doctor would discontinue the medication, depriving Mrs. J of benefits she might have derived later.
When Mrs. J calls to report on her status, she mentions that she is sleeping better and has begun to feel better during the day. She says that her husband told her she has started to smile again.
This vignette illustrates the importance of suggesting to the patient a positive outcome of pharmacotherapy associated with a particular action (calling the doctor’s office to report results). When the patient promised to call, she internalized the suggestion that calling would be associated with feeling better—and that is what happened. This intervention contrasts with saying to the patient, “Call me if you have a problem with any of these side effects,” which gives the patient a suggestion to call and report a problem.
The suggestion effect also can be used to reframe a predictable side effect as a positive sign that indicates the beginning of change leading to recovery (Box).
Ms. M, age 32 and single, has an anxiety disorder associated with bipolar depression. She has discontinued several psychotropics because of uncomfortable side effects, such as constipation.
After taking a detailed history, I decide to prescribe quetiapine. I tell Ms. M about this medication’s potential benefits and side effects. One common side effect is dry mouth, which often occurs before patients experience therapeutic effects.
I inform Ms. M that a dry mouth will be her sign that the medication has begun to work, and beneficial effects—such as improved sleep, reduced anxiety, and improved mood—will soon follow. I then instruct her to call my office and report when she experiences a dry mouth.
Discussion. In pharmacotherapy, side effects may appear before patients experience a medication’s beneficial/therapeutic effects. Patients’ initial experience often determines whether or not they will continue taking a prescribed medication. I know Ms. M may stop taking quetiapine—as she has done with other medications—if she initially has uncomfortable side effects.
Instructing patients to expect a specific side effect (such as a dry mouth with quetiapine) and associating it with a future therapeutic benefit sets up a road map of expectations. They know their experience is compatible with the doctor’s predictions. For Ms. M, I reframed the side effect as a positive sign that recovery has begun, with more positive changes to come.
Participatory pharmacotherapy
Many patients seek ownership in making decisions about their treatment and medications. In participatory pharmacotherapy, patients provide you with data and valuable information—such as family history, personal medical history, and experience with treatment—and inform you about which medications worked best and which did not work. You invite patients to predict how they see themselves getting better and into recovery.
CASE REPORT: All in the family
Mr. A, age 28 and single, has been diagnosed with a bipolar mood disorder. As part of a detailed family history, he reports that his maternal grandfather, mother, and a maternal uncle were diagnosed with mood swings and were successfully treated with medications, specifically lithium. He states that he believes he has the same condition.
I compliment Mr. A for being so well informed about his grandfather and uncle and educate him about mood stabilizers’ benefits in bipolar disorder. I tell him about the finding that if lithium has helped his relatives, it will probably help him as well.
I also reassure Mr. A that, in deciding what medications to avoid and what medications to use, I will consider his experience with specific antidepressants that did not help him. He thanks me for considering his suggestion about what medication to use for him.
- a successful therapeutic alliance
- adherence with prescribed medications
- the best possible outcome with pharmacotherapy.
In patients with a defiant-oppositional personality, consider framing the treatment decision as a choice between 2 equally efficacious medications. This gives the patient the sense of control in choosing his or her own medication, which is jointly monitored.
Table 3
Choosing patients for participatory pharmacotherapy
| Good candidates | Exclusionary qualities |
|---|---|
| Adults | Children, adolescents, and prison inmates |
| No history of alcoholism or drug addiction | Alcohol dependence or drug addiction |
| Average and above intelligence | Below-average intelligence |
| Intact cognitive function | Cognitive deficits, such as dementia |
| Not psychotic | Actively psychotic |
| Good comprehension of diagnosis and treatment | Poor comprehension of diagnosis and treatment |
| Therapeutic alliance is present | Therapeutic alliance is absent |
| Personality style or disorder with a need to be in control of treatment, such as obsessive-compulsive personality | Passive, dependent personality style or disorder; these patients may view a participatory approach as the doctor’s lack of confidence |
- Brody H. The placebo response: how you can release the body’s inner pharmacy for better health. New York, NY: HarperCollins Publishers; 2000.
- Spiro H. The power of hope: a doctor’s perspective. New Haven, CT: Yale University Press; 1998.
- Ernst E. Placebo: new insights into an old enigma. Drug Discov Today 2007;12:413-8.
- Lithium • Eskalith, Lithobid
- Mirtazapine • Remeron
- Quetiapine • Seroquel
- Sertraline • Zoloft
Dr. Torem reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Frank JD, Frank JB. Persuasion and healing. Baltimore, MD: The Johns Hopkins University Press; 1991.
2. Freud S. The complete psychological works of Sigmund Freud. Strachey J, trans-ed. Toronto, Ontario, Canada: Hogarth Press; 1953.
3. Wolf S. The pharmacology of placebos. Pharmacol Rev 1959;11:689-704.
4. Ernst E. Placebo: new insights into an old enigma. Drug Discov Today 2007;12:413-8.
5. Brody H. The placebo response: recent research and implications for family medicine. J Fam Pract 2000;49:649-54.
6. Spiegel H. Nocebo, the power of suggestibility. Prev Med 1997;26:616-21.
7. Ader R, Cohen N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science 1982;215:1534-6.
8. Ader R. The role of conditioning in pharmacotherapy. In: Harrington A, ed. The placebo effect: an interdisciplinary exploration. Cambridge, MA: Harvard University Press; 1997;138-65.
9. Olness K, Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J Dev Behav Pediatr 1992;13:124-5.
10. Stockhorst U, Mahl N, Krueger M, et al. Classical conditioning and conditionability of insulin and glucose effect in healthy humans. Physiol Behav 2004;81:375-88.
11. Wolf S. Effect of suggestion and conditioning on the action of chemical agents in human subjects—the pharmacology of placebos. J Clin Invest 1950;29:100-9.
12. Lown B. The verbal conditioning of angina pectoris during exercise testing. Am J Cardiol 1977;40:630-4.
13. Lown B. Introduction. In: Cousins N. The healing heart. New York, NY: W.W. Norton; 1983:11-28.
14. Watzlawick P. If you desire to see, learn how to act. In: Nardone G, Watzlawick P, eds. The art of change San Francisco, CA: Jossey-Bass; 1993:1-16.
1. Frank JD, Frank JB. Persuasion and healing. Baltimore, MD: The Johns Hopkins University Press; 1991.
2. Freud S. The complete psychological works of Sigmund Freud. Strachey J, trans-ed. Toronto, Ontario, Canada: Hogarth Press; 1953.
3. Wolf S. The pharmacology of placebos. Pharmacol Rev 1959;11:689-704.
4. Ernst E. Placebo: new insights into an old enigma. Drug Discov Today 2007;12:413-8.
5. Brody H. The placebo response: recent research and implications for family medicine. J Fam Pract 2000;49:649-54.
6. Spiegel H. Nocebo, the power of suggestibility. Prev Med 1997;26:616-21.
7. Ader R, Cohen N. Behaviorally conditioned immunosuppression and murine systemic lupus erythematosus. Science 1982;215:1534-6.
8. Ader R. The role of conditioning in pharmacotherapy. In: Harrington A, ed. The placebo effect: an interdisciplinary exploration. Cambridge, MA: Harvard University Press; 1997;138-65.
9. Olness K, Ader R. Conditioning as an adjunct in the pharmacotherapy of lupus erythematosus. J Dev Behav Pediatr 1992;13:124-5.
10. Stockhorst U, Mahl N, Krueger M, et al. Classical conditioning and conditionability of insulin and glucose effect in healthy humans. Physiol Behav 2004;81:375-88.
11. Wolf S. Effect of suggestion and conditioning on the action of chemical agents in human subjects—the pharmacology of placebos. J Clin Invest 1950;29:100-9.
12. Lown B. The verbal conditioning of angina pectoris during exercise testing. Am J Cardiol 1977;40:630-4.
13. Lown B. Introduction. In: Cousins N. The healing heart. New York, NY: W.W. Norton; 1983:11-28.
14. Watzlawick P. If you desire to see, learn how to act. In: Nardone G, Watzlawick P, eds. The art of change San Francisco, CA: Jossey-Bass; 1993:1-16.
Screening for Chlamydia? New advice for sexually active women
- Ask female psychiatric patients about high-risk sexual behaviors, and recommend Chlamydia screening when appropriate.
- Recommend Chlamydia screening for all sexually active women age <25 years.
- Chlamydia screening decreases incidence of pelvic inflammatory disease, improves pregnancy outcomes, and lowers risk of other sexually transmitted infections.
- Urine nucleic acid amplification tests minimize patient discomfort and remove logistical barriers of speculum or urethra specimens.
- Evidence is insufficient to recommend screening men.
Chlamydia trachomatis is the most common bacterial sexually transmitted infection (STI), with nearly 3 million new cases diagnosed annually in the United States.1 In July 2007 the U.S. Preventive Services Task Force (USPSTF) updated its recommendation on Chlamydia screening of sexually active female adolescents and adults ( Table 1 ).2 Routine screening is not recommended for men because there is not enough data to determine the benefits and risks of screening.
Although the USPSTF recommendation targets the general population, it is important to assess each patient’s sexual behavior. Women exhibiting impulsivity caused by bipolar mania, substance abuse, or personality disorders or who exchange sex for food, shelter, substances, or money are at high risk of infection ( Table 2 ).1,2
The new guideline introduces a screening cutoff at age 25 because women age <25 years are 5 times more likely than women age >30 to have chlamydial infection.2 In women age ≥25, yearly screening is recommended only for those at high risk as indicated by:
- previous chlamydial infection or other STIs
- new or multiple sexual partners
- inconsistent condom use
- being a sex worker ( Table 2 ).2
Chlamydia screening reduces the incidence of pelvic inflammatory disease (PID) in nonpregnant adolescent and adult women, which can cause infertility, ectopic pregnancy, and chronic pelvic pain.2 If untreated, the risk of PID approaches 40%.1 In pregnant women, Chlamydia treatment significantly improves birth outcomes. Pregnant women should be screened during the first prenatal visit and in the third trimester if at continued risk.
Discussant: Glen L. Xiong, MD
Psychological implications of screening. Address possible themes of guilt and shame with patients to clarify and alleviate the burden they may feel about STI screening and treatment. Compared with men, women experience more stigmatization, blame, and denial about the source of infection. Women also report being more concerned about potential threats to their relationships.1
Table 1
U.S. Preventive Services Task Force
screening recommendations for chlamydial infection
| Nonpregnant women* | Pregnant women† | |
|---|---|---|
| Age <25 | Screen | Screen |
| Age ≥25 | Screen those at increased risk | Screen those at increased risk |
| Interval‡ | At least annually | First prenatal visit, third trimester if at continued risk |
| * Grade A recommendation: high quality evidence | ||
| † Grade B recommendation: moderate quality evidence | ||
| ‡ The optimal screening interval remains to be determined | ||
| Source: Reference 2 | ||
Table 2
Risks for chlamydial infection
| • Sexually active women age <25 years |
| • History of chlamydial or other sexually transmitted infection |
| • New or multiple sexual partners |
| • Inconsistent condom use |
| • Exchange of sex for money, drugs, or shelter |
| Other demographic groups at high risk |
| • African-American and Hispanic women |
| • Incarcerated men and women |
| • Military recruits |
| Source: References 1,2 |
Screening test. The Centers for Disease Control and Prevention (CDC) recommends screening with nucleic acid amplification tests (NAATs), which have high specificity (>95%) and sensitivity (80% to 93%) for chlamydial infections. Urine specimens are comparable to cervical and urethral specimens and avert the cost and patient discomfort associated with speculum exams.3 NAATs do not exclude other infections, such as trichomonas, however, and are not sufficient in patients with active urinary or vaginal symptoms.
Clinical presentation and treatment. Most persons with Chlamydia are asymptomatic and may infect new sexual partners. In women, chlamydial infection may cause cervicitis, urethritis, PID, chronic pelvic pain, ectopic pregnancy, miscarriage, preterm labor, and infertility. In men, chlamydial infection may cause urethritis, urethral strictures, and epididymis. In both genders, chlamydial infection increases the risk of acquiring other STIs, such as human immunodeficiency virus.4
The CDC recommends treating chlamydial infection with azithromycin, 1 g/d PO for pregnant and nonpregnant women. Alternatives include amoxicillin, 500 mg tid for 7 days for pregnant women, or doxycycline, 100 mg bid for 7 days for nonpregnant women. Sexual partners of an infected individual should be treated presumptively or tested and then treated. Because Chlamydia NAAT is highly sensitive, patients with a negative test do not need treatment. Patients who test positive for gonorrhea and receive a negative non-NAAT (antigen-based tests that are less sensitive than NAATs) for Chlamydia should be treated for both.5
Related resources
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. www.cdc.gov/std/treatment.
- U.S. Department of Health and Human Services. Agency for Healthcare Research and Quality. Preventive Services. www.preventiveservices.ahrq.gov.
Drug brand names
- Amoxicillin • Amoxil, others
- Azithromycin • Zithromax
- Doxycycline • Vibramycin
Disclosure
Dr. Xiong reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Meyers DS, Halvorson H, Luckhaupt S. Screening for chlamydial infection: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med 2007;147(2):135-42.
2. U.S. Preventive Services Task Force. Screening for chlamydial infection: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2007;147(2):128-34.
3. Cook RI, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoea. Ann Intern Med 2005;142(11):914-25.
4. Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75(1):3-17.
5. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2006: dual therapy for gonococcal and chlamydial infections. Available at: http://www.cdc.gov/std/treatment/2006/urethritis-and-cervicitis.htm#dualtherapy. Accessed June 16, 2008.
Dr. Xiong is assistant clinical professor, departments of internal medicine and psychiatry, University of California, Davis.
- Ask female psychiatric patients about high-risk sexual behaviors, and recommend Chlamydia screening when appropriate.
- Recommend Chlamydia screening for all sexually active women age <25 years.
- Chlamydia screening decreases incidence of pelvic inflammatory disease, improves pregnancy outcomes, and lowers risk of other sexually transmitted infections.
- Urine nucleic acid amplification tests minimize patient discomfort and remove logistical barriers of speculum or urethra specimens.
- Evidence is insufficient to recommend screening men.
Chlamydia trachomatis is the most common bacterial sexually transmitted infection (STI), with nearly 3 million new cases diagnosed annually in the United States.1 In July 2007 the U.S. Preventive Services Task Force (USPSTF) updated its recommendation on Chlamydia screening of sexually active female adolescents and adults ( Table 1 ).2 Routine screening is not recommended for men because there is not enough data to determine the benefits and risks of screening.
Although the USPSTF recommendation targets the general population, it is important to assess each patient’s sexual behavior. Women exhibiting impulsivity caused by bipolar mania, substance abuse, or personality disorders or who exchange sex for food, shelter, substances, or money are at high risk of infection ( Table 2 ).1,2
The new guideline introduces a screening cutoff at age 25 because women age <25 years are 5 times more likely than women age >30 to have chlamydial infection.2 In women age ≥25, yearly screening is recommended only for those at high risk as indicated by:
- previous chlamydial infection or other STIs
- new or multiple sexual partners
- inconsistent condom use
- being a sex worker ( Table 2 ).2
Chlamydia screening reduces the incidence of pelvic inflammatory disease (PID) in nonpregnant adolescent and adult women, which can cause infertility, ectopic pregnancy, and chronic pelvic pain.2 If untreated, the risk of PID approaches 40%.1 In pregnant women, Chlamydia treatment significantly improves birth outcomes. Pregnant women should be screened during the first prenatal visit and in the third trimester if at continued risk.
Discussant: Glen L. Xiong, MD
Psychological implications of screening. Address possible themes of guilt and shame with patients to clarify and alleviate the burden they may feel about STI screening and treatment. Compared with men, women experience more stigmatization, blame, and denial about the source of infection. Women also report being more concerned about potential threats to their relationships.1
Table 1
U.S. Preventive Services Task Force
screening recommendations for chlamydial infection
| Nonpregnant women* | Pregnant women† | |
|---|---|---|
| Age <25 | Screen | Screen |
| Age ≥25 | Screen those at increased risk | Screen those at increased risk |
| Interval‡ | At least annually | First prenatal visit, third trimester if at continued risk |
| * Grade A recommendation: high quality evidence | ||
| † Grade B recommendation: moderate quality evidence | ||
| ‡ The optimal screening interval remains to be determined | ||
| Source: Reference 2 | ||
Table 2
Risks for chlamydial infection
| • Sexually active women age <25 years |
| • History of chlamydial or other sexually transmitted infection |
| • New or multiple sexual partners |
| • Inconsistent condom use |
| • Exchange of sex for money, drugs, or shelter |
| Other demographic groups at high risk |
| • African-American and Hispanic women |
| • Incarcerated men and women |
| • Military recruits |
| Source: References 1,2 |
Screening test. The Centers for Disease Control and Prevention (CDC) recommends screening with nucleic acid amplification tests (NAATs), which have high specificity (>95%) and sensitivity (80% to 93%) for chlamydial infections. Urine specimens are comparable to cervical and urethral specimens and avert the cost and patient discomfort associated with speculum exams.3 NAATs do not exclude other infections, such as trichomonas, however, and are not sufficient in patients with active urinary or vaginal symptoms.
Clinical presentation and treatment. Most persons with Chlamydia are asymptomatic and may infect new sexual partners. In women, chlamydial infection may cause cervicitis, urethritis, PID, chronic pelvic pain, ectopic pregnancy, miscarriage, preterm labor, and infertility. In men, chlamydial infection may cause urethritis, urethral strictures, and epididymis. In both genders, chlamydial infection increases the risk of acquiring other STIs, such as human immunodeficiency virus.4
The CDC recommends treating chlamydial infection with azithromycin, 1 g/d PO for pregnant and nonpregnant women. Alternatives include amoxicillin, 500 mg tid for 7 days for pregnant women, or doxycycline, 100 mg bid for 7 days for nonpregnant women. Sexual partners of an infected individual should be treated presumptively or tested and then treated. Because Chlamydia NAAT is highly sensitive, patients with a negative test do not need treatment. Patients who test positive for gonorrhea and receive a negative non-NAAT (antigen-based tests that are less sensitive than NAATs) for Chlamydia should be treated for both.5
Related resources
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. www.cdc.gov/std/treatment.
- U.S. Department of Health and Human Services. Agency for Healthcare Research and Quality. Preventive Services. www.preventiveservices.ahrq.gov.
Drug brand names
- Amoxicillin • Amoxil, others
- Azithromycin • Zithromax
- Doxycycline • Vibramycin
Disclosure
Dr. Xiong reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
- Ask female psychiatric patients about high-risk sexual behaviors, and recommend Chlamydia screening when appropriate.
- Recommend Chlamydia screening for all sexually active women age <25 years.
- Chlamydia screening decreases incidence of pelvic inflammatory disease, improves pregnancy outcomes, and lowers risk of other sexually transmitted infections.
- Urine nucleic acid amplification tests minimize patient discomfort and remove logistical barriers of speculum or urethra specimens.
- Evidence is insufficient to recommend screening men.
Chlamydia trachomatis is the most common bacterial sexually transmitted infection (STI), with nearly 3 million new cases diagnosed annually in the United States.1 In July 2007 the U.S. Preventive Services Task Force (USPSTF) updated its recommendation on Chlamydia screening of sexually active female adolescents and adults ( Table 1 ).2 Routine screening is not recommended for men because there is not enough data to determine the benefits and risks of screening.
Although the USPSTF recommendation targets the general population, it is important to assess each patient’s sexual behavior. Women exhibiting impulsivity caused by bipolar mania, substance abuse, or personality disorders or who exchange sex for food, shelter, substances, or money are at high risk of infection ( Table 2 ).1,2
The new guideline introduces a screening cutoff at age 25 because women age <25 years are 5 times more likely than women age >30 to have chlamydial infection.2 In women age ≥25, yearly screening is recommended only for those at high risk as indicated by:
- previous chlamydial infection or other STIs
- new or multiple sexual partners
- inconsistent condom use
- being a sex worker ( Table 2 ).2
Chlamydia screening reduces the incidence of pelvic inflammatory disease (PID) in nonpregnant adolescent and adult women, which can cause infertility, ectopic pregnancy, and chronic pelvic pain.2 If untreated, the risk of PID approaches 40%.1 In pregnant women, Chlamydia treatment significantly improves birth outcomes. Pregnant women should be screened during the first prenatal visit and in the third trimester if at continued risk.
Discussant: Glen L. Xiong, MD
Psychological implications of screening. Address possible themes of guilt and shame with patients to clarify and alleviate the burden they may feel about STI screening and treatment. Compared with men, women experience more stigmatization, blame, and denial about the source of infection. Women also report being more concerned about potential threats to their relationships.1
Table 1
U.S. Preventive Services Task Force
screening recommendations for chlamydial infection
| Nonpregnant women* | Pregnant women† | |
|---|---|---|
| Age <25 | Screen | Screen |
| Age ≥25 | Screen those at increased risk | Screen those at increased risk |
| Interval‡ | At least annually | First prenatal visit, third trimester if at continued risk |
| * Grade A recommendation: high quality evidence | ||
| † Grade B recommendation: moderate quality evidence | ||
| ‡ The optimal screening interval remains to be determined | ||
| Source: Reference 2 | ||
Table 2
Risks for chlamydial infection
| • Sexually active women age <25 years |
| • History of chlamydial or other sexually transmitted infection |
| • New or multiple sexual partners |
| • Inconsistent condom use |
| • Exchange of sex for money, drugs, or shelter |
| Other demographic groups at high risk |
| • African-American and Hispanic women |
| • Incarcerated men and women |
| • Military recruits |
| Source: References 1,2 |
Screening test. The Centers for Disease Control and Prevention (CDC) recommends screening with nucleic acid amplification tests (NAATs), which have high specificity (>95%) and sensitivity (80% to 93%) for chlamydial infections. Urine specimens are comparable to cervical and urethral specimens and avert the cost and patient discomfort associated with speculum exams.3 NAATs do not exclude other infections, such as trichomonas, however, and are not sufficient in patients with active urinary or vaginal symptoms.
Clinical presentation and treatment. Most persons with Chlamydia are asymptomatic and may infect new sexual partners. In women, chlamydial infection may cause cervicitis, urethritis, PID, chronic pelvic pain, ectopic pregnancy, miscarriage, preterm labor, and infertility. In men, chlamydial infection may cause urethritis, urethral strictures, and epididymis. In both genders, chlamydial infection increases the risk of acquiring other STIs, such as human immunodeficiency virus.4
The CDC recommends treating chlamydial infection with azithromycin, 1 g/d PO for pregnant and nonpregnant women. Alternatives include amoxicillin, 500 mg tid for 7 days for pregnant women, or doxycycline, 100 mg bid for 7 days for nonpregnant women. Sexual partners of an infected individual should be treated presumptively or tested and then treated. Because Chlamydia NAAT is highly sensitive, patients with a negative test do not need treatment. Patients who test positive for gonorrhea and receive a negative non-NAAT (antigen-based tests that are less sensitive than NAATs) for Chlamydia should be treated for both.5
Related resources
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. www.cdc.gov/std/treatment.
- U.S. Department of Health and Human Services. Agency for Healthcare Research and Quality. Preventive Services. www.preventiveservices.ahrq.gov.
Drug brand names
- Amoxicillin • Amoxil, others
- Azithromycin • Zithromax
- Doxycycline • Vibramycin
Disclosure
Dr. Xiong reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Meyers DS, Halvorson H, Luckhaupt S. Screening for chlamydial infection: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med 2007;147(2):135-42.
2. U.S. Preventive Services Task Force. Screening for chlamydial infection: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2007;147(2):128-34.
3. Cook RI, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoea. Ann Intern Med 2005;142(11):914-25.
4. Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75(1):3-17.
5. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2006: dual therapy for gonococcal and chlamydial infections. Available at: http://www.cdc.gov/std/treatment/2006/urethritis-and-cervicitis.htm#dualtherapy. Accessed June 16, 2008.
Dr. Xiong is assistant clinical professor, departments of internal medicine and psychiatry, University of California, Davis.
1. Meyers DS, Halvorson H, Luckhaupt S. Screening for chlamydial infection: an evidence update for the U.S. Preventive Services Task Force. Ann Intern Med 2007;147(2):135-42.
2. U.S. Preventive Services Task Force. Screening for chlamydial infection: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2007;147(2):128-34.
3. Cook RI, Hutchison SL, Østergaard L, et al. Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoea. Ann Intern Med 2005;142(11):914-25.
4. Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 1999;75(1):3-17.
5. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines 2006: dual therapy for gonococcal and chlamydial infections. Available at: http://www.cdc.gov/std/treatment/2006/urethritis-and-cervicitis.htm#dualtherapy. Accessed June 16, 2008.
Dr. Xiong is assistant clinical professor, departments of internal medicine and psychiatry, University of California, Davis.
Make PROGRESS by supporting adherence
Medication nonadherence challenges psychiatrists in most clinical settings.1 Supportive psychotherapy techniques—outlined in the mnemonic PROGRESS—can improve adherence and strengthen the therapeutic relationship. They also can help restore adaptive living skills, promote patient autonomy, minimize relapse, and improve attitudes toward treatment.
Praise. Reinforce positive behavior with genuine praise. Build an inventory of phrases to congratulate patients when they meet their goals or demonstrate a new effort. Follow praise with a question that elicits feedback from patients about their behavior.
Reassure. Because patients may lose faith in medications’ efficacy, use reassurance to explain the time frame for drugs to reach therapeutic levels. Instill hope and remind patients that psychiatric illnesses can improve. Point out that although medications have limitations they do help reduce symptoms.
Optimize regimens. Find an appropriate dosing and frequency that minimizes side effects and facilitates a daily routine of taking medication.2 This will help alleviate patients’ anxiety and support confidence in managing their medications.
Guide. Provide verbal and written guidance about what patients can expect from their medications. Include information about side effects and explore supplemental treatment options such as healthy eating, psychotherapy, community rehabilitation programs, and refraining from substances. If patients are unsure about why they take medication, help identify their goals and point out how pharmacotherapy might improve their symptoms.
Remind. Brainstorm with patients about how they can set up ambient cues to help them remember to take medications. Environmental associations promote autonomous behavior that can become second nature. For example, patients may learn to associate breakfast with taking medications. If patients place their medications on the table where they eat their meals, this may reinforce breakfast as a cue to take their medications.
Encourage patients to complete tasks that could help them achieve their goals. This includes taking medications as prescribed and communicating with mental healthcare providers to report side effects or during times of crisis.
Solidify strengths. Build on patients’ adaptive skills and strengths. Identifying efforts to improve their illnesses may turn roadblocks into opportunities to build rapport.
Support self-efficacy. Commend patients, for example, when they can explain and break down into steps the complexities of refilling and taking their medications.
1. Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol 2003;23(4):389-99.
2. Heinssen RK. Improving medication compliance of a patient with schizophrenia through collaborative behavioral therapy. Psychiatr Serv 2002;53(3):255-7.
Medication nonadherence challenges psychiatrists in most clinical settings.1 Supportive psychotherapy techniques—outlined in the mnemonic PROGRESS—can improve adherence and strengthen the therapeutic relationship. They also can help restore adaptive living skills, promote patient autonomy, minimize relapse, and improve attitudes toward treatment.
Praise. Reinforce positive behavior with genuine praise. Build an inventory of phrases to congratulate patients when they meet their goals or demonstrate a new effort. Follow praise with a question that elicits feedback from patients about their behavior.
Reassure. Because patients may lose faith in medications’ efficacy, use reassurance to explain the time frame for drugs to reach therapeutic levels. Instill hope and remind patients that psychiatric illnesses can improve. Point out that although medications have limitations they do help reduce symptoms.
Optimize regimens. Find an appropriate dosing and frequency that minimizes side effects and facilitates a daily routine of taking medication.2 This will help alleviate patients’ anxiety and support confidence in managing their medications.
Guide. Provide verbal and written guidance about what patients can expect from their medications. Include information about side effects and explore supplemental treatment options such as healthy eating, psychotherapy, community rehabilitation programs, and refraining from substances. If patients are unsure about why they take medication, help identify their goals and point out how pharmacotherapy might improve their symptoms.
Remind. Brainstorm with patients about how they can set up ambient cues to help them remember to take medications. Environmental associations promote autonomous behavior that can become second nature. For example, patients may learn to associate breakfast with taking medications. If patients place their medications on the table where they eat their meals, this may reinforce breakfast as a cue to take their medications.
Encourage patients to complete tasks that could help them achieve their goals. This includes taking medications as prescribed and communicating with mental healthcare providers to report side effects or during times of crisis.
Solidify strengths. Build on patients’ adaptive skills and strengths. Identifying efforts to improve their illnesses may turn roadblocks into opportunities to build rapport.
Support self-efficacy. Commend patients, for example, when they can explain and break down into steps the complexities of refilling and taking their medications.
Medication nonadherence challenges psychiatrists in most clinical settings.1 Supportive psychotherapy techniques—outlined in the mnemonic PROGRESS—can improve adherence and strengthen the therapeutic relationship. They also can help restore adaptive living skills, promote patient autonomy, minimize relapse, and improve attitudes toward treatment.
Praise. Reinforce positive behavior with genuine praise. Build an inventory of phrases to congratulate patients when they meet their goals or demonstrate a new effort. Follow praise with a question that elicits feedback from patients about their behavior.
Reassure. Because patients may lose faith in medications’ efficacy, use reassurance to explain the time frame for drugs to reach therapeutic levels. Instill hope and remind patients that psychiatric illnesses can improve. Point out that although medications have limitations they do help reduce symptoms.
Optimize regimens. Find an appropriate dosing and frequency that minimizes side effects and facilitates a daily routine of taking medication.2 This will help alleviate patients’ anxiety and support confidence in managing their medications.
Guide. Provide verbal and written guidance about what patients can expect from their medications. Include information about side effects and explore supplemental treatment options such as healthy eating, psychotherapy, community rehabilitation programs, and refraining from substances. If patients are unsure about why they take medication, help identify their goals and point out how pharmacotherapy might improve their symptoms.
Remind. Brainstorm with patients about how they can set up ambient cues to help them remember to take medications. Environmental associations promote autonomous behavior that can become second nature. For example, patients may learn to associate breakfast with taking medications. If patients place their medications on the table where they eat their meals, this may reinforce breakfast as a cue to take their medications.
Encourage patients to complete tasks that could help them achieve their goals. This includes taking medications as prescribed and communicating with mental healthcare providers to report side effects or during times of crisis.
Solidify strengths. Build on patients’ adaptive skills and strengths. Identifying efforts to improve their illnesses may turn roadblocks into opportunities to build rapport.
Support self-efficacy. Commend patients, for example, when they can explain and break down into steps the complexities of refilling and taking their medications.
1. Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol 2003;23(4):389-99.
2. Heinssen RK. Improving medication compliance of a patient with schizophrenia through collaborative behavioral therapy. Psychiatr Serv 2002;53(3):255-7.
1. Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol 2003;23(4):389-99.
2. Heinssen RK. Improving medication compliance of a patient with schizophrenia through collaborative behavioral therapy. Psychiatr Serv 2002;53(3):255-7.
What’s lurking in your waiting room?
When planning your outpatient, office-based practice, you might have overlooked the design of your waiting room while you negotiated a lease, ensured Health Insurance Portability and Accountability Act (HIPAA) compliance, and procured adequate liability insurance. The waiting room is important, however, because it provides a patient’s first impression of you. Aim to make your waiting room as comfortable as possible while avoiding unintended negative meanings.
Privacy
Maintaining privacy and confidentiality is many patients’ primary concern when they see a psychiatrist, given the sensitive nature of their presenting problems and the lingering stigma surrounding mental illness.
Some outpatient psychiatrists provide separate entrances and exits to ensure that successive patients will not see one another. Although this setup depends on the office’s layout, it may be an important factor to consider, especially when choosing new space.
Design
Whether you are a minimalist or favor a more eclectic style, give thought to the waiting room décor. Many physicians decorate their waiting rooms—as they do their offices—with personal works of art. Avoid overly personal pieces, however, such as family pictures, or provocative works of art, such as those with explicit sexual or aggressive content.
Providing magazines on an array of topics is a simple but thoughtful way to make your patient feel at ease. Choose comfortable furniture arranged to ensure adequate personal space and avoid crowding. Some physicians allow pharmaceutical representatives to leave literature in the waiting room, whereas others believe such advertisements are inappropriate.
Etiquette
A busy or shared waiting room can exacerbate the anxiety a patient may feel when meeting you for the first session. One way to maintain confidentiality when you meet a patient is to identify yourself and ask who in the room is waiting to meet with you.
Although some doctors wonder whether to use a patient’s first or last name, we initially refer to patients as Mr., Ms., or Mrs. Inside the office, we ask the patient how he or she would like to be addressed to establish openness and avoid awkwardness.
Body language
Establish eye contact when meeting the patient. A smile and maintaining 1 to 2 arm-lengths of personal space indicates appropriate intimacy without appearing threatening.
Even extending your hand may seem too forward or uncomfortable to some patients. Because some may refuse to shake hands out of religious or cultural observance, wait a moment when in doubt to see if the patient offers his or her hand first.
When planning your outpatient, office-based practice, you might have overlooked the design of your waiting room while you negotiated a lease, ensured Health Insurance Portability and Accountability Act (HIPAA) compliance, and procured adequate liability insurance. The waiting room is important, however, because it provides a patient’s first impression of you. Aim to make your waiting room as comfortable as possible while avoiding unintended negative meanings.
Privacy
Maintaining privacy and confidentiality is many patients’ primary concern when they see a psychiatrist, given the sensitive nature of their presenting problems and the lingering stigma surrounding mental illness.
Some outpatient psychiatrists provide separate entrances and exits to ensure that successive patients will not see one another. Although this setup depends on the office’s layout, it may be an important factor to consider, especially when choosing new space.
Design
Whether you are a minimalist or favor a more eclectic style, give thought to the waiting room décor. Many physicians decorate their waiting rooms—as they do their offices—with personal works of art. Avoid overly personal pieces, however, such as family pictures, or provocative works of art, such as those with explicit sexual or aggressive content.
Providing magazines on an array of topics is a simple but thoughtful way to make your patient feel at ease. Choose comfortable furniture arranged to ensure adequate personal space and avoid crowding. Some physicians allow pharmaceutical representatives to leave literature in the waiting room, whereas others believe such advertisements are inappropriate.
Etiquette
A busy or shared waiting room can exacerbate the anxiety a patient may feel when meeting you for the first session. One way to maintain confidentiality when you meet a patient is to identify yourself and ask who in the room is waiting to meet with you.
Although some doctors wonder whether to use a patient’s first or last name, we initially refer to patients as Mr., Ms., or Mrs. Inside the office, we ask the patient how he or she would like to be addressed to establish openness and avoid awkwardness.
Body language
Establish eye contact when meeting the patient. A smile and maintaining 1 to 2 arm-lengths of personal space indicates appropriate intimacy without appearing threatening.
Even extending your hand may seem too forward or uncomfortable to some patients. Because some may refuse to shake hands out of religious or cultural observance, wait a moment when in doubt to see if the patient offers his or her hand first.
When planning your outpatient, office-based practice, you might have overlooked the design of your waiting room while you negotiated a lease, ensured Health Insurance Portability and Accountability Act (HIPAA) compliance, and procured adequate liability insurance. The waiting room is important, however, because it provides a patient’s first impression of you. Aim to make your waiting room as comfortable as possible while avoiding unintended negative meanings.
Privacy
Maintaining privacy and confidentiality is many patients’ primary concern when they see a psychiatrist, given the sensitive nature of their presenting problems and the lingering stigma surrounding mental illness.
Some outpatient psychiatrists provide separate entrances and exits to ensure that successive patients will not see one another. Although this setup depends on the office’s layout, it may be an important factor to consider, especially when choosing new space.
Design
Whether you are a minimalist or favor a more eclectic style, give thought to the waiting room décor. Many physicians decorate their waiting rooms—as they do their offices—with personal works of art. Avoid overly personal pieces, however, such as family pictures, or provocative works of art, such as those with explicit sexual or aggressive content.
Providing magazines on an array of topics is a simple but thoughtful way to make your patient feel at ease. Choose comfortable furniture arranged to ensure adequate personal space and avoid crowding. Some physicians allow pharmaceutical representatives to leave literature in the waiting room, whereas others believe such advertisements are inappropriate.
Etiquette
A busy or shared waiting room can exacerbate the anxiety a patient may feel when meeting you for the first session. One way to maintain confidentiality when you meet a patient is to identify yourself and ask who in the room is waiting to meet with you.
Although some doctors wonder whether to use a patient’s first or last name, we initially refer to patients as Mr., Ms., or Mrs. Inside the office, we ask the patient how he or she would like to be addressed to establish openness and avoid awkwardness.
Body language
Establish eye contact when meeting the patient. A smile and maintaining 1 to 2 arm-lengths of personal space indicates appropriate intimacy without appearing threatening.
Even extending your hand may seem too forward or uncomfortable to some patients. Because some may refuse to shake hands out of religious or cultural observance, wait a moment when in doubt to see if the patient offers his or her hand first.
12 best Web sites for clinical needs
Nearly 1 in 4 Internet users has searched the Web for mental health information,1 but finding reliable sources is challenging. Wading through poorly organized, variable quality sites to find information you need can be time-consuming and frustrating.2 Also, without your guidance, patients may consult disreputable Web sites and follow advice that is contrary to standard psychiatric care.3
Because less is more when using the Internet, we recommend 1 good Web site for each of the following clinical needs. Each may be useful to you and to recommend to your patients.
Patient education
Medlineplus.gov from the National Library of Medicine and the National Institutes of Health (NIH) is an authoritative source for reliable, unbiased information on medications and illnesses. You will find valuable information on all psychotropic and nonpsychotropic medications and most common psychiatric disorders, including information in Spanish.
You can print out medication information and give it to patients, though we recommend asking patients to visit the Web site to introduce them to this resource. Most important, Medlineplus.gov provides links to other trusted medical Web pages. For consumers, this site provides a variety of information including an illustrated medical encyclopedia and a guide to finding reputable health information on the Web. Medlineplus.gov is an enormous site that alone could satisfy most of your patient education needs.
Formulary information
When prescribing, you often need to know if a patient’s insurance will cover the cost of the drug or if preauthorization is necessary. Fingertipformulary.com, a free and user-friendly site, allows you to select a medication, your patient’s state, and insurance plan to find out if the drug will be covered. This site also tells you authorization requirements, quantity limits, and the medication’s “tier” classification, which specifies the patient’s copayment level.
Patient assistance programs
Needymeds.org is a nonprofit resource center of patient assistance programs (PAP) administered by pharmaceutical companies for individuals who cannot afford their medications. The site links to these programs’ Web sites, application forms, and groups that can help patients fill out necessary paperwork. With this Web site, patients no longer have to request or retain PAP paperwork.
Drug interactions
Enter a drug name into the search box at Epocrates.com to learn about possible drug interactions as well as dosing information, contraindications, black-box warnings, and adverse effects. This free, continually updated Web site is invaluable when treating patients who take a large number of medications.
Clinical trials
When you want to know what clinical trials are being conducted on a particular medication or disorder, visit clinicaltrials.gov. All federally and privately supported clinical trials now must be registered with the NIH and posted at clinicaltrials.gov. The site lists ongoing and completed trials, allows you to search by medication, disorder, and geographic area, and indicates which trials are recruiting volunteers.
Information on drug abuse
The National Institute on Drug Abuse’s Drugabuse.gov provides information about substance abuse for clinicians, patients, parents, and teachers. In addition, the site features new research findings, information in Spanish, and links related to substance abuse. Click on the link for parents and teachers to access a searchable index of substance abuse treatment facilities, with information about insurance plans accepted, available treatments, and contact information.
Medication pricing
When you pull out your prescription pad, patients may ask how much a drug costs or if there is a cheaper way to buy the medication. Visit the pharmacy section of Costco.com to quickly check prices and out-of-pocket expenses, even if the patient does not buy medications from this retailer. Search by drug name to find out how much a formulation of a drug costs and if generic alternatives are available. Often this exercise will help you prescribe tablet strengths or formulations that can save the patient money.
Searching MEDLINE
You can search the National Library of Medicine’s MEDLINE bibliographic database for free by visiting Pubmed.gov. You can read abstracts of all journal articles in the database and full-text of some articles. The number of free full-text articles will increase because all articles based on research funded by the NIH must now be posted on Pubmed.gov.
Support groups
We recommend support groups to most of our psychiatric patients and their families. To find support groups in your area visit:
- Depression and Bipolar Support Alliance (dbsalliance.org) for patients with mood disorders
- Alcoholics Anonymous (aa.org) and Narcotics Anonymous (na.org) for patients with alcohol and other substance abuse problems
- National Alliance on Mental Illness (nami.org) for general support related to severe mental illness.
1. Fox S. Online health search 2006. Washington, DC: Pew Internet & American Life Project. Available at: http://www.pewinternet.org/pdfs/PIP_Online_Health_2006.pdf. Accessed July 29, 2008.
2. Christensen H, Griffiths K. The Internet and mental health practice. Evid Based Ment Health 2003;6(3):66-9.
3. Montgomery R. Are irreputable health sites hurting your patients? Current Psychiatry 2006;5(12):98-100
Nearly 1 in 4 Internet users has searched the Web for mental health information,1 but finding reliable sources is challenging. Wading through poorly organized, variable quality sites to find information you need can be time-consuming and frustrating.2 Also, without your guidance, patients may consult disreputable Web sites and follow advice that is contrary to standard psychiatric care.3
Because less is more when using the Internet, we recommend 1 good Web site for each of the following clinical needs. Each may be useful to you and to recommend to your patients.
Patient education
Medlineplus.gov from the National Library of Medicine and the National Institutes of Health (NIH) is an authoritative source for reliable, unbiased information on medications and illnesses. You will find valuable information on all psychotropic and nonpsychotropic medications and most common psychiatric disorders, including information in Spanish.
You can print out medication information and give it to patients, though we recommend asking patients to visit the Web site to introduce them to this resource. Most important, Medlineplus.gov provides links to other trusted medical Web pages. For consumers, this site provides a variety of information including an illustrated medical encyclopedia and a guide to finding reputable health information on the Web. Medlineplus.gov is an enormous site that alone could satisfy most of your patient education needs.
Formulary information
When prescribing, you often need to know if a patient’s insurance will cover the cost of the drug or if preauthorization is necessary. Fingertipformulary.com, a free and user-friendly site, allows you to select a medication, your patient’s state, and insurance plan to find out if the drug will be covered. This site also tells you authorization requirements, quantity limits, and the medication’s “tier” classification, which specifies the patient’s copayment level.
Patient assistance programs
Needymeds.org is a nonprofit resource center of patient assistance programs (PAP) administered by pharmaceutical companies for individuals who cannot afford their medications. The site links to these programs’ Web sites, application forms, and groups that can help patients fill out necessary paperwork. With this Web site, patients no longer have to request or retain PAP paperwork.
Drug interactions
Enter a drug name into the search box at Epocrates.com to learn about possible drug interactions as well as dosing information, contraindications, black-box warnings, and adverse effects. This free, continually updated Web site is invaluable when treating patients who take a large number of medications.
Clinical trials
When you want to know what clinical trials are being conducted on a particular medication or disorder, visit clinicaltrials.gov. All federally and privately supported clinical trials now must be registered with the NIH and posted at clinicaltrials.gov. The site lists ongoing and completed trials, allows you to search by medication, disorder, and geographic area, and indicates which trials are recruiting volunteers.
Information on drug abuse
The National Institute on Drug Abuse’s Drugabuse.gov provides information about substance abuse for clinicians, patients, parents, and teachers. In addition, the site features new research findings, information in Spanish, and links related to substance abuse. Click on the link for parents and teachers to access a searchable index of substance abuse treatment facilities, with information about insurance plans accepted, available treatments, and contact information.
Medication pricing
When you pull out your prescription pad, patients may ask how much a drug costs or if there is a cheaper way to buy the medication. Visit the pharmacy section of Costco.com to quickly check prices and out-of-pocket expenses, even if the patient does not buy medications from this retailer. Search by drug name to find out how much a formulation of a drug costs and if generic alternatives are available. Often this exercise will help you prescribe tablet strengths or formulations that can save the patient money.
Searching MEDLINE
You can search the National Library of Medicine’s MEDLINE bibliographic database for free by visiting Pubmed.gov. You can read abstracts of all journal articles in the database and full-text of some articles. The number of free full-text articles will increase because all articles based on research funded by the NIH must now be posted on Pubmed.gov.
Support groups
We recommend support groups to most of our psychiatric patients and their families. To find support groups in your area visit:
- Depression and Bipolar Support Alliance (dbsalliance.org) for patients with mood disorders
- Alcoholics Anonymous (aa.org) and Narcotics Anonymous (na.org) for patients with alcohol and other substance abuse problems
- National Alliance on Mental Illness (nami.org) for general support related to severe mental illness.
Nearly 1 in 4 Internet users has searched the Web for mental health information,1 but finding reliable sources is challenging. Wading through poorly organized, variable quality sites to find information you need can be time-consuming and frustrating.2 Also, without your guidance, patients may consult disreputable Web sites and follow advice that is contrary to standard psychiatric care.3
Because less is more when using the Internet, we recommend 1 good Web site for each of the following clinical needs. Each may be useful to you and to recommend to your patients.
Patient education
Medlineplus.gov from the National Library of Medicine and the National Institutes of Health (NIH) is an authoritative source for reliable, unbiased information on medications and illnesses. You will find valuable information on all psychotropic and nonpsychotropic medications and most common psychiatric disorders, including information in Spanish.
You can print out medication information and give it to patients, though we recommend asking patients to visit the Web site to introduce them to this resource. Most important, Medlineplus.gov provides links to other trusted medical Web pages. For consumers, this site provides a variety of information including an illustrated medical encyclopedia and a guide to finding reputable health information on the Web. Medlineplus.gov is an enormous site that alone could satisfy most of your patient education needs.
Formulary information
When prescribing, you often need to know if a patient’s insurance will cover the cost of the drug or if preauthorization is necessary. Fingertipformulary.com, a free and user-friendly site, allows you to select a medication, your patient’s state, and insurance plan to find out if the drug will be covered. This site also tells you authorization requirements, quantity limits, and the medication’s “tier” classification, which specifies the patient’s copayment level.
Patient assistance programs
Needymeds.org is a nonprofit resource center of patient assistance programs (PAP) administered by pharmaceutical companies for individuals who cannot afford their medications. The site links to these programs’ Web sites, application forms, and groups that can help patients fill out necessary paperwork. With this Web site, patients no longer have to request or retain PAP paperwork.
Drug interactions
Enter a drug name into the search box at Epocrates.com to learn about possible drug interactions as well as dosing information, contraindications, black-box warnings, and adverse effects. This free, continually updated Web site is invaluable when treating patients who take a large number of medications.
Clinical trials
When you want to know what clinical trials are being conducted on a particular medication or disorder, visit clinicaltrials.gov. All federally and privately supported clinical trials now must be registered with the NIH and posted at clinicaltrials.gov. The site lists ongoing and completed trials, allows you to search by medication, disorder, and geographic area, and indicates which trials are recruiting volunteers.
Information on drug abuse
The National Institute on Drug Abuse’s Drugabuse.gov provides information about substance abuse for clinicians, patients, parents, and teachers. In addition, the site features new research findings, information in Spanish, and links related to substance abuse. Click on the link for parents and teachers to access a searchable index of substance abuse treatment facilities, with information about insurance plans accepted, available treatments, and contact information.
Medication pricing
When you pull out your prescription pad, patients may ask how much a drug costs or if there is a cheaper way to buy the medication. Visit the pharmacy section of Costco.com to quickly check prices and out-of-pocket expenses, even if the patient does not buy medications from this retailer. Search by drug name to find out how much a formulation of a drug costs and if generic alternatives are available. Often this exercise will help you prescribe tablet strengths or formulations that can save the patient money.
Searching MEDLINE
You can search the National Library of Medicine’s MEDLINE bibliographic database for free by visiting Pubmed.gov. You can read abstracts of all journal articles in the database and full-text of some articles. The number of free full-text articles will increase because all articles based on research funded by the NIH must now be posted on Pubmed.gov.
Support groups
We recommend support groups to most of our psychiatric patients and their families. To find support groups in your area visit:
- Depression and Bipolar Support Alliance (dbsalliance.org) for patients with mood disorders
- Alcoholics Anonymous (aa.org) and Narcotics Anonymous (na.org) for patients with alcohol and other substance abuse problems
- National Alliance on Mental Illness (nami.org) for general support related to severe mental illness.
1. Fox S. Online health search 2006. Washington, DC: Pew Internet & American Life Project. Available at: http://www.pewinternet.org/pdfs/PIP_Online_Health_2006.pdf. Accessed July 29, 2008.
2. Christensen H, Griffiths K. The Internet and mental health practice. Evid Based Ment Health 2003;6(3):66-9.
3. Montgomery R. Are irreputable health sites hurting your patients? Current Psychiatry 2006;5(12):98-100
1. Fox S. Online health search 2006. Washington, DC: Pew Internet & American Life Project. Available at: http://www.pewinternet.org/pdfs/PIP_Online_Health_2006.pdf. Accessed July 29, 2008.
2. Christensen H, Griffiths K. The Internet and mental health practice. Evid Based Ment Health 2003;6(3):66-9.
3. Montgomery R. Are irreputable health sites hurting your patients? Current Psychiatry 2006;5(12):98-100
Examine the evidence on expert witness testimony
The authors of “Psychiatrist/patient boundaries: When it’s OK to stretch the line” (Current Psychiatry, August 2008) cited the American Academy of Psychiatry and the Law’s recommendation that “psychiatrists avoid acting as expert witnesses for their patients or performing patient evaluations for legal purposes… The intrusion of another role into the doctor/patient relationship can alter the treatment process and permanently color future interactions… these situations create the appearance that you have conscripted a vulnerable individual into your practice.”
These statements raise several questions:
- Aren’t most individuals coming into psychiatric practices vulnerable?
- If psychiatrists avoid patient evaluations for legal purposes, who will do them?
- Which opinion carries more weight, that of a psychiatrist who has worked with a patient for years or that of an independent evaluator who has seen the patient for an hour or 2?
- Is it in the best interest of the patient or the therapeutic alliance if the treating psychiatrist declines the patient’s request to testify or write an evaluation because of possible mercenary motivations, particularly when the testimony might make a significant difference?
If the patient and psychiatrist are willing to forego confidentiality, the doctor should have the option to represent the patient’s psychiatric treatment as a witness, not as an independent medical examiner. Salaried psychiatrists testifying for patients treated in public agencies—from city to federal—do not receive fees for testimony. As far as I know, there is not a standard forensic fee for private practice psychiatrists, who can adjust their fees to reflect work done, perhaps applying their hourly office rate for time spent on preparation, travel, and testimony. These psychiatrists also can discuss the fee with patients to prevent undue financial strain.
Hope is a precious commodity that should not be denied to patients because of possible overinterpretation of boundary concepts.
Perhaps I have overinterpreted the authors’ points. Maybe forensic psychiatric testimony should be re-examined in light of its possible harmful effects on psychiatrist/patient relationships. However, I think the material cited by the authors could needlessly deprive patients of assistance. I thought our goal is to use our clinical judgment to serve the best interest of each patient, not merely avoid lawsuits or board complaints as we go.
S. Epstein, MD
Los Angeles, CA
Drs. Marshall, Teston, and Myers respond
Dr. Epstein’s questions imply that in some cases, the legal system might serve as an ally to the therapist. Our assumptions about the legal system are less charitable. Though there may be occasions when treatment goals and a legal ruling may align, risks multiply when therapy moves from the office to the courtroom. Although we agree that our decisions should not be driven by a desire to avoid lawsuits or board complaints, once a case goes to court the therapist surrenders control of the therapeutic process, opening a Pandora’s box of potentially negative consequences.
Although there are unusual circumstances—such as physicians practicing in rural areas—when a physician may assume dual roles, physicians under oath serve the court, not the patient, and litigation is not always driven by the patient’s best interests. Presumably, the treating psychiatrist possesses more information about the patient, but what if the patient has lied? Or what if the psychiatrist reveals sensitive information about the patient, which results in a patient losing a job, losing respect in the community, or getting divorced? In other cases, patients may not be sufficiently competent to “forego confidentiality,” or they might not fully appreciate the risks involved in granting the therapist permission to testify.
As noted, patients are not obliged to maintain boundaries. In fact, they are not even obliged to tell us the truth. Only psychiatrists have the obligation to tell the truth, maintain boundaries, and protect our patients, even—at times—from themselves. Advocate courageously for your patients, but be judicious (no pun intended) where you exercise that courage.
Richard M. Marshall, PhD
Associate professor
University of South Florida Polytechnic
Lakeland, FL
Karen Teston, MD
Staff psychiatrist
Watson Clinic LLP
Lakeland, FL
Wade C. Myers, MD
Professor and director,
forensic psychiatry program
University of South Florida College of Medicine
Tampa, FL
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
The authors of “Psychiatrist/patient boundaries: When it’s OK to stretch the line” (Current Psychiatry, August 2008) cited the American Academy of Psychiatry and the Law’s recommendation that “psychiatrists avoid acting as expert witnesses for their patients or performing patient evaluations for legal purposes… The intrusion of another role into the doctor/patient relationship can alter the treatment process and permanently color future interactions… these situations create the appearance that you have conscripted a vulnerable individual into your practice.”
These statements raise several questions:
- Aren’t most individuals coming into psychiatric practices vulnerable?
- If psychiatrists avoid patient evaluations for legal purposes, who will do them?
- Which opinion carries more weight, that of a psychiatrist who has worked with a patient for years or that of an independent evaluator who has seen the patient for an hour or 2?
- Is it in the best interest of the patient or the therapeutic alliance if the treating psychiatrist declines the patient’s request to testify or write an evaluation because of possible mercenary motivations, particularly when the testimony might make a significant difference?
If the patient and psychiatrist are willing to forego confidentiality, the doctor should have the option to represent the patient’s psychiatric treatment as a witness, not as an independent medical examiner. Salaried psychiatrists testifying for patients treated in public agencies—from city to federal—do not receive fees for testimony. As far as I know, there is not a standard forensic fee for private practice psychiatrists, who can adjust their fees to reflect work done, perhaps applying their hourly office rate for time spent on preparation, travel, and testimony. These psychiatrists also can discuss the fee with patients to prevent undue financial strain.
Hope is a precious commodity that should not be denied to patients because of possible overinterpretation of boundary concepts.
Perhaps I have overinterpreted the authors’ points. Maybe forensic psychiatric testimony should be re-examined in light of its possible harmful effects on psychiatrist/patient relationships. However, I think the material cited by the authors could needlessly deprive patients of assistance. I thought our goal is to use our clinical judgment to serve the best interest of each patient, not merely avoid lawsuits or board complaints as we go.
S. Epstein, MD
Los Angeles, CA
Drs. Marshall, Teston, and Myers respond
Dr. Epstein’s questions imply that in some cases, the legal system might serve as an ally to the therapist. Our assumptions about the legal system are less charitable. Though there may be occasions when treatment goals and a legal ruling may align, risks multiply when therapy moves from the office to the courtroom. Although we agree that our decisions should not be driven by a desire to avoid lawsuits or board complaints, once a case goes to court the therapist surrenders control of the therapeutic process, opening a Pandora’s box of potentially negative consequences.
Although there are unusual circumstances—such as physicians practicing in rural areas—when a physician may assume dual roles, physicians under oath serve the court, not the patient, and litigation is not always driven by the patient’s best interests. Presumably, the treating psychiatrist possesses more information about the patient, but what if the patient has lied? Or what if the psychiatrist reveals sensitive information about the patient, which results in a patient losing a job, losing respect in the community, or getting divorced? In other cases, patients may not be sufficiently competent to “forego confidentiality,” or they might not fully appreciate the risks involved in granting the therapist permission to testify.
As noted, patients are not obliged to maintain boundaries. In fact, they are not even obliged to tell us the truth. Only psychiatrists have the obligation to tell the truth, maintain boundaries, and protect our patients, even—at times—from themselves. Advocate courageously for your patients, but be judicious (no pun intended) where you exercise that courage.
Richard M. Marshall, PhD
Associate professor
University of South Florida Polytechnic
Lakeland, FL
Karen Teston, MD
Staff psychiatrist
Watson Clinic LLP
Lakeland, FL
Wade C. Myers, MD
Professor and director,
forensic psychiatry program
University of South Florida College of Medicine
Tampa, FL
The authors of “Psychiatrist/patient boundaries: When it’s OK to stretch the line” (Current Psychiatry, August 2008) cited the American Academy of Psychiatry and the Law’s recommendation that “psychiatrists avoid acting as expert witnesses for their patients or performing patient evaluations for legal purposes… The intrusion of another role into the doctor/patient relationship can alter the treatment process and permanently color future interactions… these situations create the appearance that you have conscripted a vulnerable individual into your practice.”
These statements raise several questions:
- Aren’t most individuals coming into psychiatric practices vulnerable?
- If psychiatrists avoid patient evaluations for legal purposes, who will do them?
- Which opinion carries more weight, that of a psychiatrist who has worked with a patient for years or that of an independent evaluator who has seen the patient for an hour or 2?
- Is it in the best interest of the patient or the therapeutic alliance if the treating psychiatrist declines the patient’s request to testify or write an evaluation because of possible mercenary motivations, particularly when the testimony might make a significant difference?
If the patient and psychiatrist are willing to forego confidentiality, the doctor should have the option to represent the patient’s psychiatric treatment as a witness, not as an independent medical examiner. Salaried psychiatrists testifying for patients treated in public agencies—from city to federal—do not receive fees for testimony. As far as I know, there is not a standard forensic fee for private practice psychiatrists, who can adjust their fees to reflect work done, perhaps applying their hourly office rate for time spent on preparation, travel, and testimony. These psychiatrists also can discuss the fee with patients to prevent undue financial strain.
Hope is a precious commodity that should not be denied to patients because of possible overinterpretation of boundary concepts.
Perhaps I have overinterpreted the authors’ points. Maybe forensic psychiatric testimony should be re-examined in light of its possible harmful effects on psychiatrist/patient relationships. However, I think the material cited by the authors could needlessly deprive patients of assistance. I thought our goal is to use our clinical judgment to serve the best interest of each patient, not merely avoid lawsuits or board complaints as we go.
S. Epstein, MD
Los Angeles, CA
Drs. Marshall, Teston, and Myers respond
Dr. Epstein’s questions imply that in some cases, the legal system might serve as an ally to the therapist. Our assumptions about the legal system are less charitable. Though there may be occasions when treatment goals and a legal ruling may align, risks multiply when therapy moves from the office to the courtroom. Although we agree that our decisions should not be driven by a desire to avoid lawsuits or board complaints, once a case goes to court the therapist surrenders control of the therapeutic process, opening a Pandora’s box of potentially negative consequences.
Although there are unusual circumstances—such as physicians practicing in rural areas—when a physician may assume dual roles, physicians under oath serve the court, not the patient, and litigation is not always driven by the patient’s best interests. Presumably, the treating psychiatrist possesses more information about the patient, but what if the patient has lied? Or what if the psychiatrist reveals sensitive information about the patient, which results in a patient losing a job, losing respect in the community, or getting divorced? In other cases, patients may not be sufficiently competent to “forego confidentiality,” or they might not fully appreciate the risks involved in granting the therapist permission to testify.
As noted, patients are not obliged to maintain boundaries. In fact, they are not even obliged to tell us the truth. Only psychiatrists have the obligation to tell the truth, maintain boundaries, and protect our patients, even—at times—from themselves. Advocate courageously for your patients, but be judicious (no pun intended) where you exercise that courage.
Richard M. Marshall, PhD
Associate professor
University of South Florida Polytechnic
Lakeland, FL
Karen Teston, MD
Staff psychiatrist
Watson Clinic LLP
Lakeland, FL
Wade C. Myers, MD
Professor and director,
forensic psychiatry program
University of South Florida College of Medicine
Tampa, FL
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.
To comment on articles in this issue or other topics, send letters in care of Erica Vonderheid, Current Psychiatry, 110 Summit Avenue, Montvale, NJ 07645, [email protected] or click here.

