User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
COPD: How to manage comorbid depression and anxiety
Mood disorders spell danger for patients with chronic obstructive pulmonary disease (COPD). Comorbid depression and anxiety often complicate or frustrate treatment of this debilitating—and ultimately fatal—respiratory disease (Box 1).
Managing COPD-related psychiatric disorders is crucial to improving patients’ quality of life. This article presents two cases to address:
- common causes of psychiatric symptoms in patients with COPD
- strategies for effectively treating these symptoms while avoiding adverse effects and drug-drug interactions.
CASE REPORT: COPD AND DEPRESSION
Ms. H, age 59, a pack-a-day smoker since age 19, was diagnosed with COPD 3 years ago. Since then, dyspnea has rendered her unable to work, play with her grandchildren, or walk her dog. She has become increasingly apathetic and tired and is not complying with her prescribed pulmonary rehabilitation. Her primary care physician suspects she is depressed and refers her to a psychiatrist.
COPD is the fourth leading cause of death in the United States after heart disease, malignant neoplasms, and cerebrovascular disease. A total of 122,009 COPD-related deaths were reported in 2000.1
Cigarette smoking causes 80 to 90% of COPD cases.2 Occupational exposure to particles of silica, coal dust, and asbestos also can play a significant role. Alpha-1-antitrypsin deficiency—a rare, genetically transmitted enzyme deficiency—accounts for 0.1% of total cases.
Two disease processes are present in most COPD cases:
- emphysema, resulting from destruction of air spaces and their associated pulmonary capillaries (Figure)
- chronic bronchitis, causing airway hyperreactivity and increased mucus production.
The first symptom of COPD may be a chronic, productive cough. As the disease progresses, the patient becomes more prone to pulmonary infections, increasingly dyspneic, and unable to exercise. This results in occupational disability, social withdrawal, decreased mobility, and difficulty performing activities of daily living. Initially, an increased respiratory rate keeps oxygen saturation normal. Over time, however, the disease progresses to chronic hypoxia.
End-stage COPD is characterized by chronic hypoxia and retention of carbon dioxide due to inadequate gas exchange. Death results from respiratory failure or from complications such as infections.
During the psychiatrist’s initial interview, Ms. H exhibits anhedonia, feelings of worthlessness and hopelessness, and low energy. She also reports poor sleep and appetite. Her Beck Depression Inventory score of 30 indicates severe major depression.
She is taking inhaled albuterol and ipratropium, 2 puffs each every 6 hours, and has been taking oral prednisone, 10 mg/d, for 5 years. The psychiatrist adds sertraline, 50 mg/d. Her mood, anhedonia, and subjective energy level improve across 2 months. Her Beck Depression Inventory score improves to 6, but her positive responses indicate continued poor appetite, lack of sex drive, and low energy. She often becomes breathless when she tries to eat. Her body mass index is 18, indicating that she is underweight. Caloric nutritional supplements are initiated tid to increase her weight. Her sertraline dose is continued.
Approximately 1 month later, Ms. H is able to begin a pulmonary rehabilitation program, which includes:
- prescribed exercise to increase her endurance during physical activity
- breathing exercises to decrease her breathlessness.
Ms. H also begins attending a support group for patients with COPD.
After 12 weeks of pulmonary rehabilitation, Ms. H is once again able to walk her dog. The psychiatrist continues sertraline, 50 mg/d, because of her high risk of depression recurrence. She continues to smoke despite repeated counseling.
Discussion. This case illustrates how progressing COPD symptoms can compromise a patient’s ability to work, socialize, and enjoy life. The resulting social isolation and loss of independence and self-esteem can lead to depression.3
Forty to 50% of patients with COPD are believed to have comorbid depression compared with 13% of total patients.4 Small sample sizes have limited many prevalence studies, however.4-6
Long-term corticosteroid therapy may also have fueled Ms. H’s depression. Prednisone is associated with dose-related side effects, including depression, anxiety, mania, irritability, and delirium.7
Ms. H’s case also illustrates how depression can derail COPD treatment and predict poorer outcomes of medical treatment in COPD patients.8 Fatigue, apathy, and hopelessness kept her from following her pulmonary rehabilitation regimen.
Treatment. Selective serotonin reuptake inhibitors (SSRIs) are considered first-line treatment for comorbid depressive or anxiety disorders in patients with COPD. These agents are associated with a relatively low incidence of:
- anticholinergic and other side effects
- interactions with other drugs commonly used by COPD patients.
Sertraline, citalopram, and escitalopram have fewer side effects and affect the cytochrome P (CYP)-450 pathway to a lesser degree than do other SSRIs.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is another first-line option. This agent is associated with dose-dependent increases in blood pressure, so use it with caution in hypertensive patients.
Mirtazapine, which has been shown to stimulate appetite, can be considered for patients with prominent anorexia or if dyspnea frequently interferes with eating.
Tricyclic antidepressants and monoamine oxidase inhibitors are rarely considered first-line for COPD patients but may help in some clinical instances, such as in younger or middle-aged patients with chronic pain. Dosages for chronic pain generally are much lower than therapeutic dosages for depression. For example, amitriptyline is usually given at 25 mg/d for chronic pain and at 50 to 100 mg/dfor depression.
Table 1
Interactions between selected psychotropics and drugs used by COPD patients
| Psychotropic | Potential interactions |
|---|---|
| Alprazolam | Itraconazole, fluconazole, cimetidine increase alprazolam levels |
| Bupropion | Lowers seizure threshold, so use with other drugs with seizure-causing potential (eg, theophylline) requires caution May increase adverse effects of levodopa, amantadine |
| Buspirone | Erythromycin, itraconazole increase buspirone levels |
| Diazepam, lorazepam | Theophylline may decrease serum levels of these drugs |
| Divalproex | May increase prothrombin time and INR* in patients taking warfarin |
| Fluoxetine | May increase prothrombin time and INR in patients taking warfarin |
| Nefazodone | Could increase atorvastatin, simvastatin levels |
| Paroxetine | May interact with warfarin Cimetidine increases paroxetine levels Reports of increased theophylline levels |
| Risperidone | Metabolized by CYP-450 2D6 enzyme; potential exists for interactions, but none reported |
| INR: International normalized ratio, a standardized measurement of warfarin therapy effectiveness. | |
Tricyclics, however, may cause excessive sedation, orthostatic hypotension, confusion, constipation, and urinary retention. These effects can be debilitating in older patients.
Nefazodone is a potent inhibitor of the CYP-450 3A4 isoenzyme and may increase levels of triazolam and alprazolam. Levels of the lipid-lowering agents atorvastatin and simvastatin may increase threefold to fourfold when nefazodone is added. Use nefazodone with caution in patients taking digoxin, because nefazodone is 99% bound to serum proteins and may increase serum digoxin to a dangerous level. Nefazodone also carries a risk of hepatic failure, so hepatic enzyme levels should be monitored.9
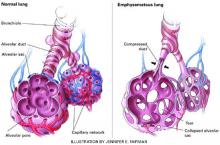
Figure Destruction of air spaces and capillaries in emphysema
Many COPD patients have a mixture of emphysema and chronic bronchitis. Emphysema is characterized by damaged alveoli, loss of elasticity of airways (bronchioles and alveoli), alveoli compression and collapse, tearing of alveoli walls, and bullae formation. In chronic bronchitis, the bronchial walls are inflamed and thickened, with a narrowing and plugging of the bronchial airways.Table 1 lists selected psychotropics and their potential interactions with drugs commonly taken by COPD patients.
CASE REPORT: COPD AND ANXIETY
Ms. P, age 60, is hospitalized for an exacerbation of COPD, which was diagnosed 10 years ago. She is intubated and ventilated after developing pneumonia-related respiratory failure. After a 2-week hospitalization, her pulmonologist tries to wean her off the ventilator, but episodes of panic and dyspnea result in significant oxygen desaturations.
The patient is transferred to a rehabilitation facility. A psychiatrist is consulted and discovers a 10-year history of anxiety that had been managed with lorazepam, 1 mg tid, and sertraline, 50 mg/d.
On evaluation, Ms. P is sweating, tremulous, and hyperventilating. She cannot speak, mouth words, or nod because of her respiratory distress. During her hospitalization she has been receiving albuterol and ipratropium nebulized every 4 hours; intravenous methylprednisolone, weaned from 40 mg to 10 mg every 6 hours; sertraline, 50 mg/d; clonazepam, 1 mg qid; theophylline, 400 mg/d, and several intravenous antibiotics. Ciprofloxacin, 500 mg bid, was recently added for a urinary tract infection.
Table 2
Drugs commonly used to treat COPD and their potential psychiatric side effects
| Drug | Action | Possible psychiatric side effect |
|---|---|---|
| Albuterol | Short-acting bronchodilator | Anxiety |
| Salmeterol | Long-acting bronchodilator | Anxiety, especially if used more than twice daily |
| Ipratropium | Inhaled anticholinergic | None |
| Inhaled corticosteroid (eg, fluticasone, budesonide) | Anti-inflammatory | None |
| Oral corticosteroid (prednisone, methylprednisolone) | Anti-inflammatory | Depression, anxiety, mania, delirium |
| Montelukast tablets or chewable tablets | Possibly both anti-inflammatory and bronchodilator activity | None |
| Theophylline | Anti-inflammatory and respiratory stimulant | Anxiety, especially if blood level is >20 μg/mL |
Ms. P’s mental status alternates between severe anxiety and obtundation. When her anxiety becomes acute, the attending physician prescribes intravenous lorazepam, 1 to 2 mg as needed. Her chart reveals that she has received 4 to 6 mg of lorazepam each day.
A blood test reveals a toxic theophylline level of 20 mg/mL. Acting on the psychiatrist’s suggestion, Ms. P’s physician decreases theophylline to 200 mg/d. Her anxiety improves slightly, but episodes of panic continue to block attempts to wean her from the ventilator. The psychiatrist increases sertraline to 100 mg/d and stops lorazepam. She adds gabapentin, 300 mg every 8 hours.
Within 3 days, Ms. P’s obtundation ceases and she is less tremulous and panicked. She can mouth words and answer questions by nodding. Within 1 week, her anxiety is improved. Five days later, she is weaned from the ventilator. The facility’s psychologist teaches her relaxation, visualization, and breathing exercises to counteract panic and anxiety.
Ms. P is discharged 2 weeks later, after beginning a pulmonary rehabilitation program. Her primary care physician weans her off clonazepam, and her gabapentin and sertraline dosages are continued.
Discussion. Although the estimated prevalence of anxiety among patients with COPD varies widely,10 anxiety is more prevalent in patients with severe lung disease.11
Panic attacks and anxiety in COPD have been linked to hypoxia, hypercapnia, and hypocapnia. Hyperventilation leads to a decrease in pCO2 , causing a respiratory alkalosis that leads to cerebral vasoconstriction. This ultimately results in anxiety symptoms.
Communication with other care team members is crucial to psychiatric treatment of patients with COPD. To ensure proper coordination of care:
- Medication history. Report changes in psychiatric medication to all doctors. Obtain from the primary care physician a complete list of the patient’s medications and medical problems to prevent drug-drug interactions.
- Onset of depression, anxiety. Report warning signs of depression and anxiety to other care team members, and urge doctors to refer patients who exhibit these signs. Primary care physicians often miss these potential warning signs:
- Suicidality. Alert other doctors to the warning signs of suicidality. Patients older than 65 and those with depression or chronic health problems are at increased risk of suicide. Many patients with COPD exhibit the following risk factors:
In patients with severe COPD, chronic hypoventilation increases pCO 2 levels. This has been shown in animals to activate a medullary chemoreceptor, which elicits a panic response by activating neurons in the locus ceruleus.
Lactic acid, formed because of hypoxia, is also linked to panic attacks. Investigators have postulated that persons with both panic disorder and COPD are hypersensitive to lactic acid and hyperventilation.12
In some patients, shortness of breath causes anticipatory anxiety that can further decrease activity and worsen deconditioning.
The crippling fear that comes with an anxiety or panic disorder can also complicate COPD therapy. Panic and anxiety often interfere with weaning from mechanical ventilation, despite treatment with high-dose benzodiazepines in some cases.13 The more frequent or protracted the use of ventilation, the greater the risk of ventilator-associated pneumonia.
COPD drugs that cause anxiety. A comprehensive review of the patient’s medications and lab readings is crucial to planning treatment. Ms. P was concomitantly taking several drugs for COPD that can cause anxiety or panic symptoms (Table 2):
- Bronchodilators such as albuterol are agonists that can increase heart rate and cause anxiety associated with rapid heartbeat.
- Theophylline, which may act as a bronchodilator and respiratory stimulant, can cause anxiety, especially at blood levels >20 mg/mL. In Ms. P’s case, the combination of ciprofloxacin and theophylline caused a CYP-450 interaction that increased her theophylline level. This is because ciprofloxacin and most other quinolone antibiotics are CYP 1A2 inducers, whereas theophylline is a CYP 1A2 substrate.9
- High-dose corticosteroids (eg, methylprednisolone) also may contribute to anxiety.
Treatment. SSRIs are an accepted first-line therapy for COPD-related anxiety. Buspirone may also work in some COPD patients. Anticonvulsants such as gabapentin and divalproex are possible adjuncts to antidepressants.
Routine use of benzodiazepines is not recommended to treat anxiety in COPD for several reasons:
- These agents can cause respiratory depression in higher doses and thus may be dangerous to patients with end-stage COPD. Reports indicate that benzodiazepines may worsen pulmonary status.14
- Rebound anxiety may occur when the drug is cleared from the system. This may accelerate benzodiazepine use, which can lead to excessively high doses and/or addiction.
Antihistamines such as hydroxyzine are a nonaddictive alternative to benzodiazepines for anxiety control. They may be used as an adjunct to antidepressants if alcohol or drug addiction are present. These agents, however, may have sedating and anticholinergic side effects.
Beta blockers, commonly used to treat performance anxiety, may worsen pulmonary status and are contraindicated in COPD patients.
COPD and comorbidities. Many patients with COPD are taking several medications for comorbid hypertension, diabetes, coronary artery disease, or congestive heart failure. These other conditions or medications may contribute to psychiatric symptoms, diminish the effectiveness of psychiatric treatment, or cause an adverse interaction with a psychotropic.
A thorough review of the patient’s medical records is strongly recommended. Communication with other care team members is critical (Box 2).
PSYCHOSOCIAL TREATMENT
Cognitive-behavioral therapy (CBT) may be effective in treating COPD-related anxiety and depression. CBT involves the correction of unrealistic and harmful thought patterns (such as cat-astrophizing shortness of breath) through techniques such as guided imagery and relaxation. Breathing exercises are also used.6
Medically stable patients can be taught “interoceptive exposure” techniques by learning to induce panic symptoms in a controlled setting (such as by hyperventilating in the doctor’s office), then desensitizing themselves to the anxiety. Exposure can also be used in social settings to accustom the patient to feared stimuli.
Support groups can increase social interaction and offer a chance to discuss disease-related medical, psychological, and social issues with other COPD patients.
Pulmonary rehabilitation has been shown to decrease depression and anxiety, increase functioning, and promote independence in patients with COPD.12 Patients are educated about their disease and learn breathing techniques to reduce air hunger and exercises to optimize oxygen use.
Physical exercise figures prominently in pulmonary rehabilitation by improving oxygen consumption efficiency. This in turn improves exercise tolerance.15
COPD AND DELIRIUM
Delirium is common among older patients with COPD. Two or more causes can be at work simultaneously, such as:
- hypoxia and hypercapnia
- reactions to antibiotics, antivirals, and corticosteroids used to treat COPD.
Delirium can simulate depression, anxiety, mania, and psychosis because affective lability, fluctuating levels of consciousness, and impaired reality testing are features of delirium.
A COPD patient’s sudden change in mental status should prompt a careful review of medications and medical conditions and an oxygen saturation measurement. An arterial blood gas reading may also be helpful because hypercapnia can be present without hypoxia. The sudden onset of psychotic symptoms in a patient with COPD should also prompt a thorough search for causes of delirium.16
Related resources
- Braunwald E, Fauci AS, Kasper DL, et al (eds.). Harrison’s principles of internal medicine (15th ed.). New York: McGraw-Hill Medical Publishing, 2001.
- American Lung Association: Around the clock with COPD. http://www.lungusa.org/diseases/copd_clock.html
- National Emphysema Foundation. http://www.emphysemafoundation.org/
Drug brand names
- Albuterol • Proventil, Ventolin
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Atorvastatin • Lipitor
- Budesonide • Pulmicort
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Ciprofloxacin • Ciloxan, Cipro
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Digoxin • Lanoxin
- Divalproex • Depakote
- Erythromycin • Emgel, others
- Escitalopram • Lexapro
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Hydroxyzine • Atarax, Vistaril
- Ipratropium • Atrovent
- Itraconazole • Sporanox
- Levodopa • Sinemet
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Montelukast • Singulair
- Nefazodone • Serzone
- Paroxetine • Paxil
- Propranolol • Inderal
- Risperidone • Risperdal
- Salmeterol • Serevent
- Sertraline • Zoloft
- Simvastatin • Zocor
- Theophylline • Theo-dur, others
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Deaths: Leading causes for 2000. National Vital Statistics Reports. 2002;50(6):8.-Available at: http://www.cdc.gov/nchs. Accessed October 16, 2003.
2. American Lung Association fact sheet: COPD. Available at: http://www.lungusa.org/diseases/copd_factsheet.html. Accessed Sept. 23, 2003.
3. American Lung Association: Breathless in America Available at: http://www.lungusa.org/press/lung_dis/asn_copd21601.html. Accessed Sept. 8, 2003.
4. Gift AG, McCrone SH. Depression in patients with COPD. Heart Lung 1993;22:289-97.
5. Light RW, Merrill EJ, Despars JA, et al. Prevalence of depression and anxiety in patients with COPD. Chest 1985;87:35-8.
6. Dudley DL, Glaser EM, Jorgenson BN, Logan DL. Psychosocial concomitants to rehabilitation in chronic obstructive pulmonary disease. Part 2: psychosocial treatment. Chest 1980;77:544-51.
7. Wise MG, Rundell JR (eds). Textbook of consultation-liaison psychiatry: psychiatry in the medically ill. (2nd ed). Washington, DC: American Psychiatric Press, 2002.
8. Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with chronic obstructive pulmonary disease. 2002;122:1633-7.
9. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
10. Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. Am J Psychiatry 1990;147:200-1.
11. Porzelius J, Vest M, Nochomovitz M. Respiratory function, cognitions, and panic in chronic obstructive pulmonary patients. Behav Res Ther 1992;30:75-7.
12. Smoller JW, Pollack MH. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6-17.
13. Mendel JG, Kahn FA. Psychosocial aspects of weaning from mechanical ventilation. Psychosomatics 1980;21:465-71.
14. Man GCW, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832-6.
15. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823-32.
16. Yudofsky SC, Hales RE (eds). Textbook of neuropsychiatry. (3rd ed). Washington, DC: American Psychiatric Press, 1997;447-70.
Mood disorders spell danger for patients with chronic obstructive pulmonary disease (COPD). Comorbid depression and anxiety often complicate or frustrate treatment of this debilitating—and ultimately fatal—respiratory disease (Box 1).
Managing COPD-related psychiatric disorders is crucial to improving patients’ quality of life. This article presents two cases to address:
- common causes of psychiatric symptoms in patients with COPD
- strategies for effectively treating these symptoms while avoiding adverse effects and drug-drug interactions.
CASE REPORT: COPD AND DEPRESSION
Ms. H, age 59, a pack-a-day smoker since age 19, was diagnosed with COPD 3 years ago. Since then, dyspnea has rendered her unable to work, play with her grandchildren, or walk her dog. She has become increasingly apathetic and tired and is not complying with her prescribed pulmonary rehabilitation. Her primary care physician suspects she is depressed and refers her to a psychiatrist.
COPD is the fourth leading cause of death in the United States after heart disease, malignant neoplasms, and cerebrovascular disease. A total of 122,009 COPD-related deaths were reported in 2000.1
Cigarette smoking causes 80 to 90% of COPD cases.2 Occupational exposure to particles of silica, coal dust, and asbestos also can play a significant role. Alpha-1-antitrypsin deficiency—a rare, genetically transmitted enzyme deficiency—accounts for 0.1% of total cases.
Two disease processes are present in most COPD cases:
- emphysema, resulting from destruction of air spaces and their associated pulmonary capillaries (Figure)
- chronic bronchitis, causing airway hyperreactivity and increased mucus production.
The first symptom of COPD may be a chronic, productive cough. As the disease progresses, the patient becomes more prone to pulmonary infections, increasingly dyspneic, and unable to exercise. This results in occupational disability, social withdrawal, decreased mobility, and difficulty performing activities of daily living. Initially, an increased respiratory rate keeps oxygen saturation normal. Over time, however, the disease progresses to chronic hypoxia.
End-stage COPD is characterized by chronic hypoxia and retention of carbon dioxide due to inadequate gas exchange. Death results from respiratory failure or from complications such as infections.
During the psychiatrist’s initial interview, Ms. H exhibits anhedonia, feelings of worthlessness and hopelessness, and low energy. She also reports poor sleep and appetite. Her Beck Depression Inventory score of 30 indicates severe major depression.
She is taking inhaled albuterol and ipratropium, 2 puffs each every 6 hours, and has been taking oral prednisone, 10 mg/d, for 5 years. The psychiatrist adds sertraline, 50 mg/d. Her mood, anhedonia, and subjective energy level improve across 2 months. Her Beck Depression Inventory score improves to 6, but her positive responses indicate continued poor appetite, lack of sex drive, and low energy. She often becomes breathless when she tries to eat. Her body mass index is 18, indicating that she is underweight. Caloric nutritional supplements are initiated tid to increase her weight. Her sertraline dose is continued.
Approximately 1 month later, Ms. H is able to begin a pulmonary rehabilitation program, which includes:
- prescribed exercise to increase her endurance during physical activity
- breathing exercises to decrease her breathlessness.
Ms. H also begins attending a support group for patients with COPD.
After 12 weeks of pulmonary rehabilitation, Ms. H is once again able to walk her dog. The psychiatrist continues sertraline, 50 mg/d, because of her high risk of depression recurrence. She continues to smoke despite repeated counseling.
Discussion. This case illustrates how progressing COPD symptoms can compromise a patient’s ability to work, socialize, and enjoy life. The resulting social isolation and loss of independence and self-esteem can lead to depression.3
Forty to 50% of patients with COPD are believed to have comorbid depression compared with 13% of total patients.4 Small sample sizes have limited many prevalence studies, however.4-6
Long-term corticosteroid therapy may also have fueled Ms. H’s depression. Prednisone is associated with dose-related side effects, including depression, anxiety, mania, irritability, and delirium.7
Ms. H’s case also illustrates how depression can derail COPD treatment and predict poorer outcomes of medical treatment in COPD patients.8 Fatigue, apathy, and hopelessness kept her from following her pulmonary rehabilitation regimen.
Treatment. Selective serotonin reuptake inhibitors (SSRIs) are considered first-line treatment for comorbid depressive or anxiety disorders in patients with COPD. These agents are associated with a relatively low incidence of:
- anticholinergic and other side effects
- interactions with other drugs commonly used by COPD patients.
Sertraline, citalopram, and escitalopram have fewer side effects and affect the cytochrome P (CYP)-450 pathway to a lesser degree than do other SSRIs.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is another first-line option. This agent is associated with dose-dependent increases in blood pressure, so use it with caution in hypertensive patients.
Mirtazapine, which has been shown to stimulate appetite, can be considered for patients with prominent anorexia or if dyspnea frequently interferes with eating.
Tricyclic antidepressants and monoamine oxidase inhibitors are rarely considered first-line for COPD patients but may help in some clinical instances, such as in younger or middle-aged patients with chronic pain. Dosages for chronic pain generally are much lower than therapeutic dosages for depression. For example, amitriptyline is usually given at 25 mg/d for chronic pain and at 50 to 100 mg/dfor depression.
Table 1
Interactions between selected psychotropics and drugs used by COPD patients
| Psychotropic | Potential interactions |
|---|---|
| Alprazolam | Itraconazole, fluconazole, cimetidine increase alprazolam levels |
| Bupropion | Lowers seizure threshold, so use with other drugs with seizure-causing potential (eg, theophylline) requires caution May increase adverse effects of levodopa, amantadine |
| Buspirone | Erythromycin, itraconazole increase buspirone levels |
| Diazepam, lorazepam | Theophylline may decrease serum levels of these drugs |
| Divalproex | May increase prothrombin time and INR* in patients taking warfarin |
| Fluoxetine | May increase prothrombin time and INR in patients taking warfarin |
| Nefazodone | Could increase atorvastatin, simvastatin levels |
| Paroxetine | May interact with warfarin Cimetidine increases paroxetine levels Reports of increased theophylline levels |
| Risperidone | Metabolized by CYP-450 2D6 enzyme; potential exists for interactions, but none reported |
| INR: International normalized ratio, a standardized measurement of warfarin therapy effectiveness. | |
Tricyclics, however, may cause excessive sedation, orthostatic hypotension, confusion, constipation, and urinary retention. These effects can be debilitating in older patients.
Nefazodone is a potent inhibitor of the CYP-450 3A4 isoenzyme and may increase levels of triazolam and alprazolam. Levels of the lipid-lowering agents atorvastatin and simvastatin may increase threefold to fourfold when nefazodone is added. Use nefazodone with caution in patients taking digoxin, because nefazodone is 99% bound to serum proteins and may increase serum digoxin to a dangerous level. Nefazodone also carries a risk of hepatic failure, so hepatic enzyme levels should be monitored.9
Figure Destruction of air spaces and capillaries in emphysema
Many COPD patients have a mixture of emphysema and chronic bronchitis. Emphysema is characterized by damaged alveoli, loss of elasticity of airways (bronchioles and alveoli), alveoli compression and collapse, tearing of alveoli walls, and bullae formation. In chronic bronchitis, the bronchial walls are inflamed and thickened, with a narrowing and plugging of the bronchial airways.Table 1 lists selected psychotropics and their potential interactions with drugs commonly taken by COPD patients.
CASE REPORT: COPD AND ANXIETY
Ms. P, age 60, is hospitalized for an exacerbation of COPD, which was diagnosed 10 years ago. She is intubated and ventilated after developing pneumonia-related respiratory failure. After a 2-week hospitalization, her pulmonologist tries to wean her off the ventilator, but episodes of panic and dyspnea result in significant oxygen desaturations.
The patient is transferred to a rehabilitation facility. A psychiatrist is consulted and discovers a 10-year history of anxiety that had been managed with lorazepam, 1 mg tid, and sertraline, 50 mg/d.
On evaluation, Ms. P is sweating, tremulous, and hyperventilating. She cannot speak, mouth words, or nod because of her respiratory distress. During her hospitalization she has been receiving albuterol and ipratropium nebulized every 4 hours; intravenous methylprednisolone, weaned from 40 mg to 10 mg every 6 hours; sertraline, 50 mg/d; clonazepam, 1 mg qid; theophylline, 400 mg/d, and several intravenous antibiotics. Ciprofloxacin, 500 mg bid, was recently added for a urinary tract infection.
Table 2
Drugs commonly used to treat COPD and their potential psychiatric side effects
| Drug | Action | Possible psychiatric side effect |
|---|---|---|
| Albuterol | Short-acting bronchodilator | Anxiety |
| Salmeterol | Long-acting bronchodilator | Anxiety, especially if used more than twice daily |
| Ipratropium | Inhaled anticholinergic | None |
| Inhaled corticosteroid (eg, fluticasone, budesonide) | Anti-inflammatory | None |
| Oral corticosteroid (prednisone, methylprednisolone) | Anti-inflammatory | Depression, anxiety, mania, delirium |
| Montelukast tablets or chewable tablets | Possibly both anti-inflammatory and bronchodilator activity | None |
| Theophylline | Anti-inflammatory and respiratory stimulant | Anxiety, especially if blood level is >20 μg/mL |
Ms. P’s mental status alternates between severe anxiety and obtundation. When her anxiety becomes acute, the attending physician prescribes intravenous lorazepam, 1 to 2 mg as needed. Her chart reveals that she has received 4 to 6 mg of lorazepam each day.
A blood test reveals a toxic theophylline level of 20 mg/mL. Acting on the psychiatrist’s suggestion, Ms. P’s physician decreases theophylline to 200 mg/d. Her anxiety improves slightly, but episodes of panic continue to block attempts to wean her from the ventilator. The psychiatrist increases sertraline to 100 mg/d and stops lorazepam. She adds gabapentin, 300 mg every 8 hours.
Within 3 days, Ms. P’s obtundation ceases and she is less tremulous and panicked. She can mouth words and answer questions by nodding. Within 1 week, her anxiety is improved. Five days later, she is weaned from the ventilator. The facility’s psychologist teaches her relaxation, visualization, and breathing exercises to counteract panic and anxiety.
Ms. P is discharged 2 weeks later, after beginning a pulmonary rehabilitation program. Her primary care physician weans her off clonazepam, and her gabapentin and sertraline dosages are continued.
Discussion. Although the estimated prevalence of anxiety among patients with COPD varies widely,10 anxiety is more prevalent in patients with severe lung disease.11
Panic attacks and anxiety in COPD have been linked to hypoxia, hypercapnia, and hypocapnia. Hyperventilation leads to a decrease in pCO2 , causing a respiratory alkalosis that leads to cerebral vasoconstriction. This ultimately results in anxiety symptoms.
Communication with other care team members is crucial to psychiatric treatment of patients with COPD. To ensure proper coordination of care:
- Medication history. Report changes in psychiatric medication to all doctors. Obtain from the primary care physician a complete list of the patient’s medications and medical problems to prevent drug-drug interactions.
- Onset of depression, anxiety. Report warning signs of depression and anxiety to other care team members, and urge doctors to refer patients who exhibit these signs. Primary care physicians often miss these potential warning signs:
- Suicidality. Alert other doctors to the warning signs of suicidality. Patients older than 65 and those with depression or chronic health problems are at increased risk of suicide. Many patients with COPD exhibit the following risk factors:
In patients with severe COPD, chronic hypoventilation increases pCO 2 levels. This has been shown in animals to activate a medullary chemoreceptor, which elicits a panic response by activating neurons in the locus ceruleus.
Lactic acid, formed because of hypoxia, is also linked to panic attacks. Investigators have postulated that persons with both panic disorder and COPD are hypersensitive to lactic acid and hyperventilation.12
In some patients, shortness of breath causes anticipatory anxiety that can further decrease activity and worsen deconditioning.
The crippling fear that comes with an anxiety or panic disorder can also complicate COPD therapy. Panic and anxiety often interfere with weaning from mechanical ventilation, despite treatment with high-dose benzodiazepines in some cases.13 The more frequent or protracted the use of ventilation, the greater the risk of ventilator-associated pneumonia.
COPD drugs that cause anxiety. A comprehensive review of the patient’s medications and lab readings is crucial to planning treatment. Ms. P was concomitantly taking several drugs for COPD that can cause anxiety or panic symptoms (Table 2):
- Bronchodilators such as albuterol are agonists that can increase heart rate and cause anxiety associated with rapid heartbeat.
- Theophylline, which may act as a bronchodilator and respiratory stimulant, can cause anxiety, especially at blood levels >20 mg/mL. In Ms. P’s case, the combination of ciprofloxacin and theophylline caused a CYP-450 interaction that increased her theophylline level. This is because ciprofloxacin and most other quinolone antibiotics are CYP 1A2 inducers, whereas theophylline is a CYP 1A2 substrate.9
- High-dose corticosteroids (eg, methylprednisolone) also may contribute to anxiety.
Treatment. SSRIs are an accepted first-line therapy for COPD-related anxiety. Buspirone may also work in some COPD patients. Anticonvulsants such as gabapentin and divalproex are possible adjuncts to antidepressants.
Routine use of benzodiazepines is not recommended to treat anxiety in COPD for several reasons:
- These agents can cause respiratory depression in higher doses and thus may be dangerous to patients with end-stage COPD. Reports indicate that benzodiazepines may worsen pulmonary status.14
- Rebound anxiety may occur when the drug is cleared from the system. This may accelerate benzodiazepine use, which can lead to excessively high doses and/or addiction.
Antihistamines such as hydroxyzine are a nonaddictive alternative to benzodiazepines for anxiety control. They may be used as an adjunct to antidepressants if alcohol or drug addiction are present. These agents, however, may have sedating and anticholinergic side effects.
Beta blockers, commonly used to treat performance anxiety, may worsen pulmonary status and are contraindicated in COPD patients.
COPD and comorbidities. Many patients with COPD are taking several medications for comorbid hypertension, diabetes, coronary artery disease, or congestive heart failure. These other conditions or medications may contribute to psychiatric symptoms, diminish the effectiveness of psychiatric treatment, or cause an adverse interaction with a psychotropic.
A thorough review of the patient’s medical records is strongly recommended. Communication with other care team members is critical (Box 2).
PSYCHOSOCIAL TREATMENT
Cognitive-behavioral therapy (CBT) may be effective in treating COPD-related anxiety and depression. CBT involves the correction of unrealistic and harmful thought patterns (such as cat-astrophizing shortness of breath) through techniques such as guided imagery and relaxation. Breathing exercises are also used.6
Medically stable patients can be taught “interoceptive exposure” techniques by learning to induce panic symptoms in a controlled setting (such as by hyperventilating in the doctor’s office), then desensitizing themselves to the anxiety. Exposure can also be used in social settings to accustom the patient to feared stimuli.
Support groups can increase social interaction and offer a chance to discuss disease-related medical, psychological, and social issues with other COPD patients.
Pulmonary rehabilitation has been shown to decrease depression and anxiety, increase functioning, and promote independence in patients with COPD.12 Patients are educated about their disease and learn breathing techniques to reduce air hunger and exercises to optimize oxygen use.
Physical exercise figures prominently in pulmonary rehabilitation by improving oxygen consumption efficiency. This in turn improves exercise tolerance.15
COPD AND DELIRIUM
Delirium is common among older patients with COPD. Two or more causes can be at work simultaneously, such as:
- hypoxia and hypercapnia
- reactions to antibiotics, antivirals, and corticosteroids used to treat COPD.
Delirium can simulate depression, anxiety, mania, and psychosis because affective lability, fluctuating levels of consciousness, and impaired reality testing are features of delirium.
A COPD patient’s sudden change in mental status should prompt a careful review of medications and medical conditions and an oxygen saturation measurement. An arterial blood gas reading may also be helpful because hypercapnia can be present without hypoxia. The sudden onset of psychotic symptoms in a patient with COPD should also prompt a thorough search for causes of delirium.16
Related resources
- Braunwald E, Fauci AS, Kasper DL, et al (eds.). Harrison’s principles of internal medicine (15th ed.). New York: McGraw-Hill Medical Publishing, 2001.
- American Lung Association: Around the clock with COPD. http://www.lungusa.org/diseases/copd_clock.html
- National Emphysema Foundation. http://www.emphysemafoundation.org/
Drug brand names
- Albuterol • Proventil, Ventolin
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Atorvastatin • Lipitor
- Budesonide • Pulmicort
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Ciprofloxacin • Ciloxan, Cipro
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Digoxin • Lanoxin
- Divalproex • Depakote
- Erythromycin • Emgel, others
- Escitalopram • Lexapro
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Hydroxyzine • Atarax, Vistaril
- Ipratropium • Atrovent
- Itraconazole • Sporanox
- Levodopa • Sinemet
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Montelukast • Singulair
- Nefazodone • Serzone
- Paroxetine • Paxil
- Propranolol • Inderal
- Risperidone • Risperdal
- Salmeterol • Serevent
- Sertraline • Zoloft
- Simvastatin • Zocor
- Theophylline • Theo-dur, others
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Mood disorders spell danger for patients with chronic obstructive pulmonary disease (COPD). Comorbid depression and anxiety often complicate or frustrate treatment of this debilitating—and ultimately fatal—respiratory disease (Box 1).
Managing COPD-related psychiatric disorders is crucial to improving patients’ quality of life. This article presents two cases to address:
- common causes of psychiatric symptoms in patients with COPD
- strategies for effectively treating these symptoms while avoiding adverse effects and drug-drug interactions.
CASE REPORT: COPD AND DEPRESSION
Ms. H, age 59, a pack-a-day smoker since age 19, was diagnosed with COPD 3 years ago. Since then, dyspnea has rendered her unable to work, play with her grandchildren, or walk her dog. She has become increasingly apathetic and tired and is not complying with her prescribed pulmonary rehabilitation. Her primary care physician suspects she is depressed and refers her to a psychiatrist.
COPD is the fourth leading cause of death in the United States after heart disease, malignant neoplasms, and cerebrovascular disease. A total of 122,009 COPD-related deaths were reported in 2000.1
Cigarette smoking causes 80 to 90% of COPD cases.2 Occupational exposure to particles of silica, coal dust, and asbestos also can play a significant role. Alpha-1-antitrypsin deficiency—a rare, genetically transmitted enzyme deficiency—accounts for 0.1% of total cases.
Two disease processes are present in most COPD cases:
- emphysema, resulting from destruction of air spaces and their associated pulmonary capillaries (Figure)
- chronic bronchitis, causing airway hyperreactivity and increased mucus production.
The first symptom of COPD may be a chronic, productive cough. As the disease progresses, the patient becomes more prone to pulmonary infections, increasingly dyspneic, and unable to exercise. This results in occupational disability, social withdrawal, decreased mobility, and difficulty performing activities of daily living. Initially, an increased respiratory rate keeps oxygen saturation normal. Over time, however, the disease progresses to chronic hypoxia.
End-stage COPD is characterized by chronic hypoxia and retention of carbon dioxide due to inadequate gas exchange. Death results from respiratory failure or from complications such as infections.
During the psychiatrist’s initial interview, Ms. H exhibits anhedonia, feelings of worthlessness and hopelessness, and low energy. She also reports poor sleep and appetite. Her Beck Depression Inventory score of 30 indicates severe major depression.
She is taking inhaled albuterol and ipratropium, 2 puffs each every 6 hours, and has been taking oral prednisone, 10 mg/d, for 5 years. The psychiatrist adds sertraline, 50 mg/d. Her mood, anhedonia, and subjective energy level improve across 2 months. Her Beck Depression Inventory score improves to 6, but her positive responses indicate continued poor appetite, lack of sex drive, and low energy. She often becomes breathless when she tries to eat. Her body mass index is 18, indicating that she is underweight. Caloric nutritional supplements are initiated tid to increase her weight. Her sertraline dose is continued.
Approximately 1 month later, Ms. H is able to begin a pulmonary rehabilitation program, which includes:
- prescribed exercise to increase her endurance during physical activity
- breathing exercises to decrease her breathlessness.
Ms. H also begins attending a support group for patients with COPD.
After 12 weeks of pulmonary rehabilitation, Ms. H is once again able to walk her dog. The psychiatrist continues sertraline, 50 mg/d, because of her high risk of depression recurrence. She continues to smoke despite repeated counseling.
Discussion. This case illustrates how progressing COPD symptoms can compromise a patient’s ability to work, socialize, and enjoy life. The resulting social isolation and loss of independence and self-esteem can lead to depression.3
Forty to 50% of patients with COPD are believed to have comorbid depression compared with 13% of total patients.4 Small sample sizes have limited many prevalence studies, however.4-6
Long-term corticosteroid therapy may also have fueled Ms. H’s depression. Prednisone is associated with dose-related side effects, including depression, anxiety, mania, irritability, and delirium.7
Ms. H’s case also illustrates how depression can derail COPD treatment and predict poorer outcomes of medical treatment in COPD patients.8 Fatigue, apathy, and hopelessness kept her from following her pulmonary rehabilitation regimen.
Treatment. Selective serotonin reuptake inhibitors (SSRIs) are considered first-line treatment for comorbid depressive or anxiety disorders in patients with COPD. These agents are associated with a relatively low incidence of:
- anticholinergic and other side effects
- interactions with other drugs commonly used by COPD patients.
Sertraline, citalopram, and escitalopram have fewer side effects and affect the cytochrome P (CYP)-450 pathway to a lesser degree than do other SSRIs.
Venlafaxine, a serotonin-norepinephrine reuptake inhibitor, is another first-line option. This agent is associated with dose-dependent increases in blood pressure, so use it with caution in hypertensive patients.
Mirtazapine, which has been shown to stimulate appetite, can be considered for patients with prominent anorexia or if dyspnea frequently interferes with eating.
Tricyclic antidepressants and monoamine oxidase inhibitors are rarely considered first-line for COPD patients but may help in some clinical instances, such as in younger or middle-aged patients with chronic pain. Dosages for chronic pain generally are much lower than therapeutic dosages for depression. For example, amitriptyline is usually given at 25 mg/d for chronic pain and at 50 to 100 mg/dfor depression.
Table 1
Interactions between selected psychotropics and drugs used by COPD patients
| Psychotropic | Potential interactions |
|---|---|
| Alprazolam | Itraconazole, fluconazole, cimetidine increase alprazolam levels |
| Bupropion | Lowers seizure threshold, so use with other drugs with seizure-causing potential (eg, theophylline) requires caution May increase adverse effects of levodopa, amantadine |
| Buspirone | Erythromycin, itraconazole increase buspirone levels |
| Diazepam, lorazepam | Theophylline may decrease serum levels of these drugs |
| Divalproex | May increase prothrombin time and INR* in patients taking warfarin |
| Fluoxetine | May increase prothrombin time and INR in patients taking warfarin |
| Nefazodone | Could increase atorvastatin, simvastatin levels |
| Paroxetine | May interact with warfarin Cimetidine increases paroxetine levels Reports of increased theophylline levels |
| Risperidone | Metabolized by CYP-450 2D6 enzyme; potential exists for interactions, but none reported |
| INR: International normalized ratio, a standardized measurement of warfarin therapy effectiveness. | |
Tricyclics, however, may cause excessive sedation, orthostatic hypotension, confusion, constipation, and urinary retention. These effects can be debilitating in older patients.
Nefazodone is a potent inhibitor of the CYP-450 3A4 isoenzyme and may increase levels of triazolam and alprazolam. Levels of the lipid-lowering agents atorvastatin and simvastatin may increase threefold to fourfold when nefazodone is added. Use nefazodone with caution in patients taking digoxin, because nefazodone is 99% bound to serum proteins and may increase serum digoxin to a dangerous level. Nefazodone also carries a risk of hepatic failure, so hepatic enzyme levels should be monitored.9
Figure Destruction of air spaces and capillaries in emphysema
Many COPD patients have a mixture of emphysema and chronic bronchitis. Emphysema is characterized by damaged alveoli, loss of elasticity of airways (bronchioles and alveoli), alveoli compression and collapse, tearing of alveoli walls, and bullae formation. In chronic bronchitis, the bronchial walls are inflamed and thickened, with a narrowing and plugging of the bronchial airways.Table 1 lists selected psychotropics and their potential interactions with drugs commonly taken by COPD patients.
CASE REPORT: COPD AND ANXIETY
Ms. P, age 60, is hospitalized for an exacerbation of COPD, which was diagnosed 10 years ago. She is intubated and ventilated after developing pneumonia-related respiratory failure. After a 2-week hospitalization, her pulmonologist tries to wean her off the ventilator, but episodes of panic and dyspnea result in significant oxygen desaturations.
The patient is transferred to a rehabilitation facility. A psychiatrist is consulted and discovers a 10-year history of anxiety that had been managed with lorazepam, 1 mg tid, and sertraline, 50 mg/d.
On evaluation, Ms. P is sweating, tremulous, and hyperventilating. She cannot speak, mouth words, or nod because of her respiratory distress. During her hospitalization she has been receiving albuterol and ipratropium nebulized every 4 hours; intravenous methylprednisolone, weaned from 40 mg to 10 mg every 6 hours; sertraline, 50 mg/d; clonazepam, 1 mg qid; theophylline, 400 mg/d, and several intravenous antibiotics. Ciprofloxacin, 500 mg bid, was recently added for a urinary tract infection.
Table 2
Drugs commonly used to treat COPD and their potential psychiatric side effects
| Drug | Action | Possible psychiatric side effect |
|---|---|---|
| Albuterol | Short-acting bronchodilator | Anxiety |
| Salmeterol | Long-acting bronchodilator | Anxiety, especially if used more than twice daily |
| Ipratropium | Inhaled anticholinergic | None |
| Inhaled corticosteroid (eg, fluticasone, budesonide) | Anti-inflammatory | None |
| Oral corticosteroid (prednisone, methylprednisolone) | Anti-inflammatory | Depression, anxiety, mania, delirium |
| Montelukast tablets or chewable tablets | Possibly both anti-inflammatory and bronchodilator activity | None |
| Theophylline | Anti-inflammatory and respiratory stimulant | Anxiety, especially if blood level is >20 μg/mL |
Ms. P’s mental status alternates between severe anxiety and obtundation. When her anxiety becomes acute, the attending physician prescribes intravenous lorazepam, 1 to 2 mg as needed. Her chart reveals that she has received 4 to 6 mg of lorazepam each day.
A blood test reveals a toxic theophylline level of 20 mg/mL. Acting on the psychiatrist’s suggestion, Ms. P’s physician decreases theophylline to 200 mg/d. Her anxiety improves slightly, but episodes of panic continue to block attempts to wean her from the ventilator. The psychiatrist increases sertraline to 100 mg/d and stops lorazepam. She adds gabapentin, 300 mg every 8 hours.
Within 3 days, Ms. P’s obtundation ceases and she is less tremulous and panicked. She can mouth words and answer questions by nodding. Within 1 week, her anxiety is improved. Five days later, she is weaned from the ventilator. The facility’s psychologist teaches her relaxation, visualization, and breathing exercises to counteract panic and anxiety.
Ms. P is discharged 2 weeks later, after beginning a pulmonary rehabilitation program. Her primary care physician weans her off clonazepam, and her gabapentin and sertraline dosages are continued.
Discussion. Although the estimated prevalence of anxiety among patients with COPD varies widely,10 anxiety is more prevalent in patients with severe lung disease.11
Panic attacks and anxiety in COPD have been linked to hypoxia, hypercapnia, and hypocapnia. Hyperventilation leads to a decrease in pCO2 , causing a respiratory alkalosis that leads to cerebral vasoconstriction. This ultimately results in anxiety symptoms.
Communication with other care team members is crucial to psychiatric treatment of patients with COPD. To ensure proper coordination of care:
- Medication history. Report changes in psychiatric medication to all doctors. Obtain from the primary care physician a complete list of the patient’s medications and medical problems to prevent drug-drug interactions.
- Onset of depression, anxiety. Report warning signs of depression and anxiety to other care team members, and urge doctors to refer patients who exhibit these signs. Primary care physicians often miss these potential warning signs:
- Suicidality. Alert other doctors to the warning signs of suicidality. Patients older than 65 and those with depression or chronic health problems are at increased risk of suicide. Many patients with COPD exhibit the following risk factors:
In patients with severe COPD, chronic hypoventilation increases pCO 2 levels. This has been shown in animals to activate a medullary chemoreceptor, which elicits a panic response by activating neurons in the locus ceruleus.
Lactic acid, formed because of hypoxia, is also linked to panic attacks. Investigators have postulated that persons with both panic disorder and COPD are hypersensitive to lactic acid and hyperventilation.12
In some patients, shortness of breath causes anticipatory anxiety that can further decrease activity and worsen deconditioning.
The crippling fear that comes with an anxiety or panic disorder can also complicate COPD therapy. Panic and anxiety often interfere with weaning from mechanical ventilation, despite treatment with high-dose benzodiazepines in some cases.13 The more frequent or protracted the use of ventilation, the greater the risk of ventilator-associated pneumonia.
COPD drugs that cause anxiety. A comprehensive review of the patient’s medications and lab readings is crucial to planning treatment. Ms. P was concomitantly taking several drugs for COPD that can cause anxiety or panic symptoms (Table 2):
- Bronchodilators such as albuterol are agonists that can increase heart rate and cause anxiety associated with rapid heartbeat.
- Theophylline, which may act as a bronchodilator and respiratory stimulant, can cause anxiety, especially at blood levels >20 mg/mL. In Ms. P’s case, the combination of ciprofloxacin and theophylline caused a CYP-450 interaction that increased her theophylline level. This is because ciprofloxacin and most other quinolone antibiotics are CYP 1A2 inducers, whereas theophylline is a CYP 1A2 substrate.9
- High-dose corticosteroids (eg, methylprednisolone) also may contribute to anxiety.
Treatment. SSRIs are an accepted first-line therapy for COPD-related anxiety. Buspirone may also work in some COPD patients. Anticonvulsants such as gabapentin and divalproex are possible adjuncts to antidepressants.
Routine use of benzodiazepines is not recommended to treat anxiety in COPD for several reasons:
- These agents can cause respiratory depression in higher doses and thus may be dangerous to patients with end-stage COPD. Reports indicate that benzodiazepines may worsen pulmonary status.14
- Rebound anxiety may occur when the drug is cleared from the system. This may accelerate benzodiazepine use, which can lead to excessively high doses and/or addiction.
Antihistamines such as hydroxyzine are a nonaddictive alternative to benzodiazepines for anxiety control. They may be used as an adjunct to antidepressants if alcohol or drug addiction are present. These agents, however, may have sedating and anticholinergic side effects.
Beta blockers, commonly used to treat performance anxiety, may worsen pulmonary status and are contraindicated in COPD patients.
COPD and comorbidities. Many patients with COPD are taking several medications for comorbid hypertension, diabetes, coronary artery disease, or congestive heart failure. These other conditions or medications may contribute to psychiatric symptoms, diminish the effectiveness of psychiatric treatment, or cause an adverse interaction with a psychotropic.
A thorough review of the patient’s medical records is strongly recommended. Communication with other care team members is critical (Box 2).
PSYCHOSOCIAL TREATMENT
Cognitive-behavioral therapy (CBT) may be effective in treating COPD-related anxiety and depression. CBT involves the correction of unrealistic and harmful thought patterns (such as cat-astrophizing shortness of breath) through techniques such as guided imagery and relaxation. Breathing exercises are also used.6
Medically stable patients can be taught “interoceptive exposure” techniques by learning to induce panic symptoms in a controlled setting (such as by hyperventilating in the doctor’s office), then desensitizing themselves to the anxiety. Exposure can also be used in social settings to accustom the patient to feared stimuli.
Support groups can increase social interaction and offer a chance to discuss disease-related medical, psychological, and social issues with other COPD patients.
Pulmonary rehabilitation has been shown to decrease depression and anxiety, increase functioning, and promote independence in patients with COPD.12 Patients are educated about their disease and learn breathing techniques to reduce air hunger and exercises to optimize oxygen use.
Physical exercise figures prominently in pulmonary rehabilitation by improving oxygen consumption efficiency. This in turn improves exercise tolerance.15
COPD AND DELIRIUM
Delirium is common among older patients with COPD. Two or more causes can be at work simultaneously, such as:
- hypoxia and hypercapnia
- reactions to antibiotics, antivirals, and corticosteroids used to treat COPD.
Delirium can simulate depression, anxiety, mania, and psychosis because affective lability, fluctuating levels of consciousness, and impaired reality testing are features of delirium.
A COPD patient’s sudden change in mental status should prompt a careful review of medications and medical conditions and an oxygen saturation measurement. An arterial blood gas reading may also be helpful because hypercapnia can be present without hypoxia. The sudden onset of psychotic symptoms in a patient with COPD should also prompt a thorough search for causes of delirium.16
Related resources
- Braunwald E, Fauci AS, Kasper DL, et al (eds.). Harrison’s principles of internal medicine (15th ed.). New York: McGraw-Hill Medical Publishing, 2001.
- American Lung Association: Around the clock with COPD. http://www.lungusa.org/diseases/copd_clock.html
- National Emphysema Foundation. http://www.emphysemafoundation.org/
Drug brand names
- Albuterol • Proventil, Ventolin
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Atorvastatin • Lipitor
- Budesonide • Pulmicort
- Bupropion • Wellbutrin
- Buspirone • BuSpar
- Cimetidine • Tagamet
- Ciprofloxacin • Ciloxan, Cipro
- Citalopram • Celexa
- Clonazepam • Klonopin
- Diazepam • Valium
- Digoxin • Lanoxin
- Divalproex • Depakote
- Erythromycin • Emgel, others
- Escitalopram • Lexapro
- Fluconazole • Diflucan
- Fluoxetine • Prozac
- Fluticasone • Flovent
- Gabapentin • Neurontin
- Hydroxyzine • Atarax, Vistaril
- Ipratropium • Atrovent
- Itraconazole • Sporanox
- Levodopa • Sinemet
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Montelukast • Singulair
- Nefazodone • Serzone
- Paroxetine • Paxil
- Propranolol • Inderal
- Risperidone • Risperdal
- Salmeterol • Serevent
- Sertraline • Zoloft
- Simvastatin • Zocor
- Theophylline • Theo-dur, others
- Triazolam • Halcion
- Venlafaxine • Effexor
- Warfarin • Coumadin
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Deaths: Leading causes for 2000. National Vital Statistics Reports. 2002;50(6):8.-Available at: http://www.cdc.gov/nchs. Accessed October 16, 2003.
2. American Lung Association fact sheet: COPD. Available at: http://www.lungusa.org/diseases/copd_factsheet.html. Accessed Sept. 23, 2003.
3. American Lung Association: Breathless in America Available at: http://www.lungusa.org/press/lung_dis/asn_copd21601.html. Accessed Sept. 8, 2003.
4. Gift AG, McCrone SH. Depression in patients with COPD. Heart Lung 1993;22:289-97.
5. Light RW, Merrill EJ, Despars JA, et al. Prevalence of depression and anxiety in patients with COPD. Chest 1985;87:35-8.
6. Dudley DL, Glaser EM, Jorgenson BN, Logan DL. Psychosocial concomitants to rehabilitation in chronic obstructive pulmonary disease. Part 2: psychosocial treatment. Chest 1980;77:544-51.
7. Wise MG, Rundell JR (eds). Textbook of consultation-liaison psychiatry: psychiatry in the medically ill. (2nd ed). Washington, DC: American Psychiatric Press, 2002.
8. Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with chronic obstructive pulmonary disease. 2002;122:1633-7.
9. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
10. Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. Am J Psychiatry 1990;147:200-1.
11. Porzelius J, Vest M, Nochomovitz M. Respiratory function, cognitions, and panic in chronic obstructive pulmonary patients. Behav Res Ther 1992;30:75-7.
12. Smoller JW, Pollack MH. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6-17.
13. Mendel JG, Kahn FA. Psychosocial aspects of weaning from mechanical ventilation. Psychosomatics 1980;21:465-71.
14. Man GCW, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832-6.
15. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823-32.
16. Yudofsky SC, Hales RE (eds). Textbook of neuropsychiatry. (3rd ed). Washington, DC: American Psychiatric Press, 1997;447-70.
1. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. Deaths: Leading causes for 2000. National Vital Statistics Reports. 2002;50(6):8.-Available at: http://www.cdc.gov/nchs. Accessed October 16, 2003.
2. American Lung Association fact sheet: COPD. Available at: http://www.lungusa.org/diseases/copd_factsheet.html. Accessed Sept. 23, 2003.
3. American Lung Association: Breathless in America Available at: http://www.lungusa.org/press/lung_dis/asn_copd21601.html. Accessed Sept. 8, 2003.
4. Gift AG, McCrone SH. Depression in patients with COPD. Heart Lung 1993;22:289-97.
5. Light RW, Merrill EJ, Despars JA, et al. Prevalence of depression and anxiety in patients with COPD. Chest 1985;87:35-8.
6. Dudley DL, Glaser EM, Jorgenson BN, Logan DL. Psychosocial concomitants to rehabilitation in chronic obstructive pulmonary disease. Part 2: psychosocial treatment. Chest 1980;77:544-51.
7. Wise MG, Rundell JR (eds). Textbook of consultation-liaison psychiatry: psychiatry in the medically ill. (2nd ed). Washington, DC: American Psychiatric Press, 2002.
8. Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with chronic obstructive pulmonary disease. 2002;122:1633-7.
9. Physicians’ Desk Reference (57th ed). Montvale, NJ: Thomson Healthcare, 2003.
10. Karajgi B, Rifkin A, Doddi S, Kolli R. The prevalence of anxiety disorders in patients with chronic obstructive pulmonary disease. Am J Psychiatry 1990;147:200-1.
11. Porzelius J, Vest M, Nochomovitz M. Respiratory function, cognitions, and panic in chronic obstructive pulmonary patients. Behav Res Ther 1992;30:75-7.
12. Smoller JW, Pollack MH. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996;154:6-17.
13. Mendel JG, Kahn FA. Psychosocial aspects of weaning from mechanical ventilation. Psychosomatics 1980;21:465-71.
14. Man GCW, Hsu K, Sproule BJ. Effect of alprazolam on exercise and dyspnea in patients with chronic obstructive pulmonary disease. Chest 1986;90:832-6.
15. Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Ann Intern Med 1995;122:823-32.
16. Yudofsky SC, Hales RE (eds). Textbook of neuropsychiatry. (3rd ed). Washington, DC: American Psychiatric Press, 1997;447-70.
Beware the men with toupees
HISTORY: Treatment-refractory depression
Mr. S, age 78, has a history of depression that has not responded to selective serotonin reuptake inhibitors and electroconvulsive therapy (ECT).
According to his niece, Mr. S had become withdrawn, suspicious, and forgetful. Several times over the past year, police found him wandering the streets and brought him to the community hospital’s emergency room.
During one emergency room visit, he complained of decreased appetite, poor sleep, and depressed mood. He was subsequently admitted to the psychiatric unit, where he was treated with ECT and discharged on citalopram, 20 mg/d. His symptoms did not improve and he became ataxic and incontinent of urine.
Mr. S’ family placed him in a nursing home, where he became increasingly paranoid. The attending physician prescribed risperidone, 3 mg/d, with no effect. He was then transferred to our psychiatric facility.
At admission, Mr. S told us that a group of men disguised in toupees and mustaches were out to kill him. He said these men had recently killed his niece—with whom he had just spoken on the phone and had seen at the hospital. He suspected that these men were after his money, hired a woman to impersonate his niece and spy on him, and planned to bury his body and his niece’s in a remote place.
On evaluation, Mr. S was suspicious, guarded, and uncooperative, and often ended conversations abruptly. He denied auditory and visual hallucinations, was not suicidal or homicidal, and denied abusing drugs or alcohol. He said constant fear of his imminent murder left him feeling depressed.
Physical and neurologic exams were unremarkable except for mild ataxia. Mr. S’ Folstein Mini-Mental State Examination score was 19/30, indicating moderate cognitive impairment.
Mr. S’ history and behavior suggest depression with psychotic features. Do we have enough information for a diagnosis?
Dr. Greenberg’s and Tampi’s observations
Mr. S is displaying mood symptoms consistent with his prior diagnosis of depression, but with new-onset psychosis as well.
Because of Mr. S’ neurobiologic symptoms, it is improper to diagnose depression with psychotic features without first performing a full medical and neurologic workup. The differential diagnosis needs to include medical and neurologic diagnoses, including:
- delirium secondary to urinary tract infection
- Alzheimer’s and/or vascular dementia
- normal-pressure hydrocephalus
- substance abuse.
A complete dementia and delirium workup and detailed medical history are imperative.
FURTHER HISTORY: Risky behavior
Further history reveals that Mr. S had been having sexual intercourse with prostitutes since his early teens and that this habit continued into his 70s. He had been diagnosed with syphilis in his teens and again in his 50s. Both times he refused to complete the recommended penicillin regimen because he was embarrassed by the diagnosis and had falsely believed that a single penicillin injection would cure him.
Lab tests showed a white blood cell count of 3.5 and a weakly reactive serum venereal disease research laboratory (VDRL) reading.
Reporting of syphilis cases in the United States began in 1941.1 At about that time, Yale University and the Mayo Clinic began conducting clinical trials of penicillin in syphilis treatment.2
Thanks to the advent of penicillin, syphilis incidence has declined dramatically since 1943, when 575,593 cases were reported.3 Only 5,979 cases were reported to the U.S. Centers for Disease Control and Prevention in 2000.4 A slight increase in cases, mainly among homosexual men, was reported in 2001.1,4
The AIDS epidemic and the emergence of crack/cocaine use5,6 were believed to have triggered a brief increase in cases that peaked in 1990. This was likely caused by the high-risk sexual behavior observed in individuals with sexually transmitted diseases and the practice of exchanging sex for drugs.6
Could Mr. S’ syphilis—inadequately treated in his youth—be causing his depression and paranoia decades later? If so, how would you confirm this finding?
Dr. Greenberg’s and Tampi’s observations
Mr. S has a longstanding history of syphilis secondary to high-risk sexual activity. This, combined with the lab findings and his worsening depression and paranoia, points to possible neurosyphilis.
Syphilis, caused by the spirochete Treponema pallidum., can traverse mucous membranes and abraded skin. Transmission is most common during sexual activity but also occurs through blood transfusions and nonsexual lesion contact and from mother to fetus.
Prevalence
- 6,103 cases reported in 2001
- More prevalent among men than women (2.1:1), probably because of elevated prevalence among homosexual men
- African-Americans accounted for 62% of cases in 2001. Prevalence in African–Americans that year was 16 times greater than in whites
Risk factors
- Presence of HIV infection or other sexually transmitted disease
- Unprotected sex
- Residence in urban areas
- Substance abuse
- Homosexuality
Source: References 5 and 6
Because syphilis and its psychiatric effects are relatively uncommon (Box 1), many psychiatrists do not consider neurosyphilis in high-risk patients who present with depression, dementia, or psychosis (Box 2).
HOW SYPHILIS BECOMES NEUROSYPHILIS
Primary syphilis incubates for 10 to 90 days following infection. After this period, an infectious chancre appears along with regional adenopathy. If untreated, the chancre will disappear but the infection will progress.
Secondary syphilis is characterized by skin manifestations and occasionally affects the joints, eyes, bones, kidneys, liver, and CNS. Common effects include condylomata—highly infectious warty lesions—and a diffuse maculopapular rash on the palms and soles. These lesions disappear if left untreated, but most patients then either enter syphilis’ latent stage or experience a potentially fatal relapse of secondary syphilis.5
Latent syphilis usually remains latent or resolves, but about one-third of patients with latent syphilis slowly progress to tertiary syphilis. Neurosyphilis, one of the main forms of tertiary syphilis, can surface 5 to 35 years after an untreated primary infection.7
There are four categories of neurosyphilis:
- General paresis results in dementia, changes in personality, transient hemiparesis, depression, and psychosis.
- Tabes dorsalis degenerates the posterior columns and dorsal root ganglia of the spinal cord. This results in ataxia, parasthesias, decreased proprioception and vibratory sense, Argyll Robertson pupil (an optical disorder in which the pupil does not react normally to light), neurogenic bladder, and sharp shooting pains throughout the body.
- Meningovascular neurosyphilis can result in cranial nerve abnormalities, symptoms of meningitis, and cerebral infarctions.
- Asymptomatic but with CSF positive for syphilis.
Neurosyphilis is fatal if untreated, and treatment usually does not eliminate symptoms but prevents further progression. Approximately 8% of patients with untreated primary syphilis develop neurosyphilis.5,7
Standard nontreponemal tests, such as the VDRL or rapid plasmin reagin, can be used to screen for syphilis. Because these tests often produce false positives, confirm positive results with a syphilis-specific test, such as the fluorescent treponemal antibody absorption (FTA-ABS) test, microhemagglutination assay for antibodies to T pallidum., and the T pallidum. hemagglutination assay.
If neurosyphilis is suspected, CSF testing is strongly recommended. Diagnostic findings include elevated white blood cell and protein counts and a positive VDRL. If the CSF is negative, refer the patient for treatment anyway because false negatives are common. Patients with consistent neurologic symptoms, positive VDRL and/or FTA-ABS, and negative CSF are diagnosed with neurosyphilis and warrant treatment.7
How would you manage Mr. S’ psychiatric symptoms concomitant with medical treatment of late-stage syphilis?
Dr. Greenberg’s and Tampi’s observations
Although no specific guidelines exist for treating psychosis secondary to neurosyphilis, atypical antipsychotics remain the first-line treatment. Atypicals do not interact significantly with penicillin and can be given safely with syphilis treatment. Atypicals also are better tolerated than typical antipsychotics and produce fewer extrapyramidal symptoms, which are common among older patients and those with neurologic diseases.
Screening for syphilis. Every patient with a history of high-risk sexual behavior who presents with new-onset dementia or psychosis should be screened for syphilis. Sexual history can be difficult to obtain from some patients and family members, so communication between providers becomes crucial. Obtain lab test results from other care team members to monitor compliance, and coordinate patient education with other doctors on safe sexual practices.
TREATMENT: Taking his medicine
Mr. S refused further testing and emergency conservatorship was sought. Citalopram was discontinued and risperidone was gradually increased to 6 mg at bedtime. He remained paranoid and delusional.
A brain MRI showed chronic ischemic small-vessel disease. HIV testing was negative, and serum FTA-ABS was reactive. CSF showed elevated protein and white blood cell count with a nonreactive VDRL and a reactive FTA-ABS. A diagnosis of neurosyphilis was made, and treatment was initiated with aqueous crystalline penicillin G, 4 million units every 4 hours for 2 weeks.
Mr. S was discharged back to the nursing home where his penicillin injections were continued. His paranoia diminished slightly but he remained ataxic, incontinent, and confused. He was discharged from the nursing home but needed confirmative HIV screening and repeated CSF testing to determine if syphilis treatment was effective.
Six months after treatment, Mr. S’ niece reports that his paranoia has decreased. He has not needed additional psychiatric hospitalizations.
Related resources
- Merck Manual. www.merck.com. Search: “syphilis”
- U.S. Centers for Disease Control and Prevention—Syphilis elimination: History in the making. www.cdc.gov. Click on “Health Topics A-Z,” then click on “S” and find “syphilis.”
- National Institute of Allergy and Infectious Disease. www.niaid.nih.gov. Search: “syphilis”
Drug brand names
- Citalopram • Celexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Centers for Disease Control and Prevention. Primary and secondary syphilis—United States, 2000-2001. MMWR Morb Mortal Wkly Rep. 2002;51:971-3.
2. Mandell GL, Petri WA. Antimicrobial agents: penicillins, cephalosporins, and other beta-lactam antibiotics. In:Hardman JG, Limbird LE, Molinoff PB, et al (eds) Goodman and Gilman’s the pharmacological basis of therapeutics. (9th ed). New York: McGraw-Hill, 1996;1073-4.
3. Lukehart SA, Holmes KK. Spirochetal diseases. In: Braunwald E, Fauci AS, Kasper DL, et al (eds). Harrison’s principles of internal medicine. (14th ed). New York: McGraw-Hill, 1998;1023.-
4. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2001 supplement, syphilis surveillance report. Available at: http://www.cdc.gov/std/stats/2001syphilis.htm. Accessed October 10, 2003.
5. Jacobs RA. Infectious diseases: spirochetal. In: Tierney LM, McPhee SJ, Papadakis MA (eds). Current medical diagnosis and treatment. (39th ed). New York: Lange Medical Books/McGraw-Hill, 2000;1376-86.
6. Hutto B. Syphilis in clinical psychiatry: a review. Psychosomatics. 2001;42:453-60.
7. Carpenter CJ, Lederman MM, Salata RA. Sexually transmitted diseases. In: Andreoli TE, Bennett JC, Carpenter CJ, Plum F (eds). Cecil essentials of medicine. (4th ed). Philadelphia: WB Saunders Co, 1997;742-5.
HISTORY: Treatment-refractory depression
Mr. S, age 78, has a history of depression that has not responded to selective serotonin reuptake inhibitors and electroconvulsive therapy (ECT).
According to his niece, Mr. S had become withdrawn, suspicious, and forgetful. Several times over the past year, police found him wandering the streets and brought him to the community hospital’s emergency room.
During one emergency room visit, he complained of decreased appetite, poor sleep, and depressed mood. He was subsequently admitted to the psychiatric unit, where he was treated with ECT and discharged on citalopram, 20 mg/d. His symptoms did not improve and he became ataxic and incontinent of urine.
Mr. S’ family placed him in a nursing home, where he became increasingly paranoid. The attending physician prescribed risperidone, 3 mg/d, with no effect. He was then transferred to our psychiatric facility.
At admission, Mr. S told us that a group of men disguised in toupees and mustaches were out to kill him. He said these men had recently killed his niece—with whom he had just spoken on the phone and had seen at the hospital. He suspected that these men were after his money, hired a woman to impersonate his niece and spy on him, and planned to bury his body and his niece’s in a remote place.
On evaluation, Mr. S was suspicious, guarded, and uncooperative, and often ended conversations abruptly. He denied auditory and visual hallucinations, was not suicidal or homicidal, and denied abusing drugs or alcohol. He said constant fear of his imminent murder left him feeling depressed.
Physical and neurologic exams were unremarkable except for mild ataxia. Mr. S’ Folstein Mini-Mental State Examination score was 19/30, indicating moderate cognitive impairment.
Mr. S’ history and behavior suggest depression with psychotic features. Do we have enough information for a diagnosis?
Dr. Greenberg’s and Tampi’s observations
Mr. S is displaying mood symptoms consistent with his prior diagnosis of depression, but with new-onset psychosis as well.
Because of Mr. S’ neurobiologic symptoms, it is improper to diagnose depression with psychotic features without first performing a full medical and neurologic workup. The differential diagnosis needs to include medical and neurologic diagnoses, including:
- delirium secondary to urinary tract infection
- Alzheimer’s and/or vascular dementia
- normal-pressure hydrocephalus
- substance abuse.
A complete dementia and delirium workup and detailed medical history are imperative.
FURTHER HISTORY: Risky behavior
Further history reveals that Mr. S had been having sexual intercourse with prostitutes since his early teens and that this habit continued into his 70s. He had been diagnosed with syphilis in his teens and again in his 50s. Both times he refused to complete the recommended penicillin regimen because he was embarrassed by the diagnosis and had falsely believed that a single penicillin injection would cure him.
Lab tests showed a white blood cell count of 3.5 and a weakly reactive serum venereal disease research laboratory (VDRL) reading.
Reporting of syphilis cases in the United States began in 1941.1 At about that time, Yale University and the Mayo Clinic began conducting clinical trials of penicillin in syphilis treatment.2
Thanks to the advent of penicillin, syphilis incidence has declined dramatically since 1943, when 575,593 cases were reported.3 Only 5,979 cases were reported to the U.S. Centers for Disease Control and Prevention in 2000.4 A slight increase in cases, mainly among homosexual men, was reported in 2001.1,4
The AIDS epidemic and the emergence of crack/cocaine use5,6 were believed to have triggered a brief increase in cases that peaked in 1990. This was likely caused by the high-risk sexual behavior observed in individuals with sexually transmitted diseases and the practice of exchanging sex for drugs.6
Could Mr. S’ syphilis—inadequately treated in his youth—be causing his depression and paranoia decades later? If so, how would you confirm this finding?
Dr. Greenberg’s and Tampi’s observations
Mr. S has a longstanding history of syphilis secondary to high-risk sexual activity. This, combined with the lab findings and his worsening depression and paranoia, points to possible neurosyphilis.
Syphilis, caused by the spirochete Treponema pallidum., can traverse mucous membranes and abraded skin. Transmission is most common during sexual activity but also occurs through blood transfusions and nonsexual lesion contact and from mother to fetus.
Prevalence
- 6,103 cases reported in 2001
- More prevalent among men than women (2.1:1), probably because of elevated prevalence among homosexual men
- African-Americans accounted for 62% of cases in 2001. Prevalence in African–Americans that year was 16 times greater than in whites
Risk factors
- Presence of HIV infection or other sexually transmitted disease
- Unprotected sex
- Residence in urban areas
- Substance abuse
- Homosexuality
Source: References 5 and 6
Because syphilis and its psychiatric effects are relatively uncommon (Box 1), many psychiatrists do not consider neurosyphilis in high-risk patients who present with depression, dementia, or psychosis (Box 2).
HOW SYPHILIS BECOMES NEUROSYPHILIS
Primary syphilis incubates for 10 to 90 days following infection. After this period, an infectious chancre appears along with regional adenopathy. If untreated, the chancre will disappear but the infection will progress.
Secondary syphilis is characterized by skin manifestations and occasionally affects the joints, eyes, bones, kidneys, liver, and CNS. Common effects include condylomata—highly infectious warty lesions—and a diffuse maculopapular rash on the palms and soles. These lesions disappear if left untreated, but most patients then either enter syphilis’ latent stage or experience a potentially fatal relapse of secondary syphilis.5
Latent syphilis usually remains latent or resolves, but about one-third of patients with latent syphilis slowly progress to tertiary syphilis. Neurosyphilis, one of the main forms of tertiary syphilis, can surface 5 to 35 years after an untreated primary infection.7
There are four categories of neurosyphilis:
- General paresis results in dementia, changes in personality, transient hemiparesis, depression, and psychosis.
- Tabes dorsalis degenerates the posterior columns and dorsal root ganglia of the spinal cord. This results in ataxia, parasthesias, decreased proprioception and vibratory sense, Argyll Robertson pupil (an optical disorder in which the pupil does not react normally to light), neurogenic bladder, and sharp shooting pains throughout the body.
- Meningovascular neurosyphilis can result in cranial nerve abnormalities, symptoms of meningitis, and cerebral infarctions.
- Asymptomatic but with CSF positive for syphilis.
Neurosyphilis is fatal if untreated, and treatment usually does not eliminate symptoms but prevents further progression. Approximately 8% of patients with untreated primary syphilis develop neurosyphilis.5,7
Standard nontreponemal tests, such as the VDRL or rapid plasmin reagin, can be used to screen for syphilis. Because these tests often produce false positives, confirm positive results with a syphilis-specific test, such as the fluorescent treponemal antibody absorption (FTA-ABS) test, microhemagglutination assay for antibodies to T pallidum., and the T pallidum. hemagglutination assay.
If neurosyphilis is suspected, CSF testing is strongly recommended. Diagnostic findings include elevated white blood cell and protein counts and a positive VDRL. If the CSF is negative, refer the patient for treatment anyway because false negatives are common. Patients with consistent neurologic symptoms, positive VDRL and/or FTA-ABS, and negative CSF are diagnosed with neurosyphilis and warrant treatment.7
How would you manage Mr. S’ psychiatric symptoms concomitant with medical treatment of late-stage syphilis?
Dr. Greenberg’s and Tampi’s observations
Although no specific guidelines exist for treating psychosis secondary to neurosyphilis, atypical antipsychotics remain the first-line treatment. Atypicals do not interact significantly with penicillin and can be given safely with syphilis treatment. Atypicals also are better tolerated than typical antipsychotics and produce fewer extrapyramidal symptoms, which are common among older patients and those with neurologic diseases.
Screening for syphilis. Every patient with a history of high-risk sexual behavior who presents with new-onset dementia or psychosis should be screened for syphilis. Sexual history can be difficult to obtain from some patients and family members, so communication between providers becomes crucial. Obtain lab test results from other care team members to monitor compliance, and coordinate patient education with other doctors on safe sexual practices.
TREATMENT: Taking his medicine
Mr. S refused further testing and emergency conservatorship was sought. Citalopram was discontinued and risperidone was gradually increased to 6 mg at bedtime. He remained paranoid and delusional.
A brain MRI showed chronic ischemic small-vessel disease. HIV testing was negative, and serum FTA-ABS was reactive. CSF showed elevated protein and white blood cell count with a nonreactive VDRL and a reactive FTA-ABS. A diagnosis of neurosyphilis was made, and treatment was initiated with aqueous crystalline penicillin G, 4 million units every 4 hours for 2 weeks.
Mr. S was discharged back to the nursing home where his penicillin injections were continued. His paranoia diminished slightly but he remained ataxic, incontinent, and confused. He was discharged from the nursing home but needed confirmative HIV screening and repeated CSF testing to determine if syphilis treatment was effective.
Six months after treatment, Mr. S’ niece reports that his paranoia has decreased. He has not needed additional psychiatric hospitalizations.
Related resources
- Merck Manual. www.merck.com. Search: “syphilis”
- U.S. Centers for Disease Control and Prevention—Syphilis elimination: History in the making. www.cdc.gov. Click on “Health Topics A-Z,” then click on “S” and find “syphilis.”
- National Institute of Allergy and Infectious Disease. www.niaid.nih.gov. Search: “syphilis”
Drug brand names
- Citalopram • Celexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
HISTORY: Treatment-refractory depression
Mr. S, age 78, has a history of depression that has not responded to selective serotonin reuptake inhibitors and electroconvulsive therapy (ECT).
According to his niece, Mr. S had become withdrawn, suspicious, and forgetful. Several times over the past year, police found him wandering the streets and brought him to the community hospital’s emergency room.
During one emergency room visit, he complained of decreased appetite, poor sleep, and depressed mood. He was subsequently admitted to the psychiatric unit, where he was treated with ECT and discharged on citalopram, 20 mg/d. His symptoms did not improve and he became ataxic and incontinent of urine.
Mr. S’ family placed him in a nursing home, where he became increasingly paranoid. The attending physician prescribed risperidone, 3 mg/d, with no effect. He was then transferred to our psychiatric facility.
At admission, Mr. S told us that a group of men disguised in toupees and mustaches were out to kill him. He said these men had recently killed his niece—with whom he had just spoken on the phone and had seen at the hospital. He suspected that these men were after his money, hired a woman to impersonate his niece and spy on him, and planned to bury his body and his niece’s in a remote place.
On evaluation, Mr. S was suspicious, guarded, and uncooperative, and often ended conversations abruptly. He denied auditory and visual hallucinations, was not suicidal or homicidal, and denied abusing drugs or alcohol. He said constant fear of his imminent murder left him feeling depressed.
Physical and neurologic exams were unremarkable except for mild ataxia. Mr. S’ Folstein Mini-Mental State Examination score was 19/30, indicating moderate cognitive impairment.
Mr. S’ history and behavior suggest depression with psychotic features. Do we have enough information for a diagnosis?
Dr. Greenberg’s and Tampi’s observations
Mr. S is displaying mood symptoms consistent with his prior diagnosis of depression, but with new-onset psychosis as well.
Because of Mr. S’ neurobiologic symptoms, it is improper to diagnose depression with psychotic features without first performing a full medical and neurologic workup. The differential diagnosis needs to include medical and neurologic diagnoses, including:
- delirium secondary to urinary tract infection
- Alzheimer’s and/or vascular dementia
- normal-pressure hydrocephalus
- substance abuse.
A complete dementia and delirium workup and detailed medical history are imperative.
FURTHER HISTORY: Risky behavior
Further history reveals that Mr. S had been having sexual intercourse with prostitutes since his early teens and that this habit continued into his 70s. He had been diagnosed with syphilis in his teens and again in his 50s. Both times he refused to complete the recommended penicillin regimen because he was embarrassed by the diagnosis and had falsely believed that a single penicillin injection would cure him.
Lab tests showed a white blood cell count of 3.5 and a weakly reactive serum venereal disease research laboratory (VDRL) reading.
Reporting of syphilis cases in the United States began in 1941.1 At about that time, Yale University and the Mayo Clinic began conducting clinical trials of penicillin in syphilis treatment.2
Thanks to the advent of penicillin, syphilis incidence has declined dramatically since 1943, when 575,593 cases were reported.3 Only 5,979 cases were reported to the U.S. Centers for Disease Control and Prevention in 2000.4 A slight increase in cases, mainly among homosexual men, was reported in 2001.1,4
The AIDS epidemic and the emergence of crack/cocaine use5,6 were believed to have triggered a brief increase in cases that peaked in 1990. This was likely caused by the high-risk sexual behavior observed in individuals with sexually transmitted diseases and the practice of exchanging sex for drugs.6
Could Mr. S’ syphilis—inadequately treated in his youth—be causing his depression and paranoia decades later? If so, how would you confirm this finding?
Dr. Greenberg’s and Tampi’s observations
Mr. S has a longstanding history of syphilis secondary to high-risk sexual activity. This, combined with the lab findings and his worsening depression and paranoia, points to possible neurosyphilis.
Syphilis, caused by the spirochete Treponema pallidum., can traverse mucous membranes and abraded skin. Transmission is most common during sexual activity but also occurs through blood transfusions and nonsexual lesion contact and from mother to fetus.
Prevalence
- 6,103 cases reported in 2001
- More prevalent among men than women (2.1:1), probably because of elevated prevalence among homosexual men
- African-Americans accounted for 62% of cases in 2001. Prevalence in African–Americans that year was 16 times greater than in whites
Risk factors
- Presence of HIV infection or other sexually transmitted disease
- Unprotected sex
- Residence in urban areas
- Substance abuse
- Homosexuality
Source: References 5 and 6
Because syphilis and its psychiatric effects are relatively uncommon (Box 1), many psychiatrists do not consider neurosyphilis in high-risk patients who present with depression, dementia, or psychosis (Box 2).
HOW SYPHILIS BECOMES NEUROSYPHILIS
Primary syphilis incubates for 10 to 90 days following infection. After this period, an infectious chancre appears along with regional adenopathy. If untreated, the chancre will disappear but the infection will progress.
Secondary syphilis is characterized by skin manifestations and occasionally affects the joints, eyes, bones, kidneys, liver, and CNS. Common effects include condylomata—highly infectious warty lesions—and a diffuse maculopapular rash on the palms and soles. These lesions disappear if left untreated, but most patients then either enter syphilis’ latent stage or experience a potentially fatal relapse of secondary syphilis.5
Latent syphilis usually remains latent or resolves, but about one-third of patients with latent syphilis slowly progress to tertiary syphilis. Neurosyphilis, one of the main forms of tertiary syphilis, can surface 5 to 35 years after an untreated primary infection.7
There are four categories of neurosyphilis:
- General paresis results in dementia, changes in personality, transient hemiparesis, depression, and psychosis.
- Tabes dorsalis degenerates the posterior columns and dorsal root ganglia of the spinal cord. This results in ataxia, parasthesias, decreased proprioception and vibratory sense, Argyll Robertson pupil (an optical disorder in which the pupil does not react normally to light), neurogenic bladder, and sharp shooting pains throughout the body.
- Meningovascular neurosyphilis can result in cranial nerve abnormalities, symptoms of meningitis, and cerebral infarctions.
- Asymptomatic but with CSF positive for syphilis.
Neurosyphilis is fatal if untreated, and treatment usually does not eliminate symptoms but prevents further progression. Approximately 8% of patients with untreated primary syphilis develop neurosyphilis.5,7
Standard nontreponemal tests, such as the VDRL or rapid plasmin reagin, can be used to screen for syphilis. Because these tests often produce false positives, confirm positive results with a syphilis-specific test, such as the fluorescent treponemal antibody absorption (FTA-ABS) test, microhemagglutination assay for antibodies to T pallidum., and the T pallidum. hemagglutination assay.
If neurosyphilis is suspected, CSF testing is strongly recommended. Diagnostic findings include elevated white blood cell and protein counts and a positive VDRL. If the CSF is negative, refer the patient for treatment anyway because false negatives are common. Patients with consistent neurologic symptoms, positive VDRL and/or FTA-ABS, and negative CSF are diagnosed with neurosyphilis and warrant treatment.7
How would you manage Mr. S’ psychiatric symptoms concomitant with medical treatment of late-stage syphilis?
Dr. Greenberg’s and Tampi’s observations
Although no specific guidelines exist for treating psychosis secondary to neurosyphilis, atypical antipsychotics remain the first-line treatment. Atypicals do not interact significantly with penicillin and can be given safely with syphilis treatment. Atypicals also are better tolerated than typical antipsychotics and produce fewer extrapyramidal symptoms, which are common among older patients and those with neurologic diseases.
Screening for syphilis. Every patient with a history of high-risk sexual behavior who presents with new-onset dementia or psychosis should be screened for syphilis. Sexual history can be difficult to obtain from some patients and family members, so communication between providers becomes crucial. Obtain lab test results from other care team members to monitor compliance, and coordinate patient education with other doctors on safe sexual practices.
TREATMENT: Taking his medicine
Mr. S refused further testing and emergency conservatorship was sought. Citalopram was discontinued and risperidone was gradually increased to 6 mg at bedtime. He remained paranoid and delusional.
A brain MRI showed chronic ischemic small-vessel disease. HIV testing was negative, and serum FTA-ABS was reactive. CSF showed elevated protein and white blood cell count with a nonreactive VDRL and a reactive FTA-ABS. A diagnosis of neurosyphilis was made, and treatment was initiated with aqueous crystalline penicillin G, 4 million units every 4 hours for 2 weeks.
Mr. S was discharged back to the nursing home where his penicillin injections were continued. His paranoia diminished slightly but he remained ataxic, incontinent, and confused. He was discharged from the nursing home but needed confirmative HIV screening and repeated CSF testing to determine if syphilis treatment was effective.
Six months after treatment, Mr. S’ niece reports that his paranoia has decreased. He has not needed additional psychiatric hospitalizations.
Related resources
- Merck Manual. www.merck.com. Search: “syphilis”
- U.S. Centers for Disease Control and Prevention—Syphilis elimination: History in the making. www.cdc.gov. Click on “Health Topics A-Z,” then click on “S” and find “syphilis.”
- National Institute of Allergy and Infectious Disease. www.niaid.nih.gov. Search: “syphilis”
Drug brand names
- Citalopram • Celexa
- Risperidone • Risperdal
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. U.S. Centers for Disease Control and Prevention. Primary and secondary syphilis—United States, 2000-2001. MMWR Morb Mortal Wkly Rep. 2002;51:971-3.
2. Mandell GL, Petri WA. Antimicrobial agents: penicillins, cephalosporins, and other beta-lactam antibiotics. In:Hardman JG, Limbird LE, Molinoff PB, et al (eds) Goodman and Gilman’s the pharmacological basis of therapeutics. (9th ed). New York: McGraw-Hill, 1996;1073-4.
3. Lukehart SA, Holmes KK. Spirochetal diseases. In: Braunwald E, Fauci AS, Kasper DL, et al (eds). Harrison’s principles of internal medicine. (14th ed). New York: McGraw-Hill, 1998;1023.-
4. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2001 supplement, syphilis surveillance report. Available at: http://www.cdc.gov/std/stats/2001syphilis.htm. Accessed October 10, 2003.
5. Jacobs RA. Infectious diseases: spirochetal. In: Tierney LM, McPhee SJ, Papadakis MA (eds). Current medical diagnosis and treatment. (39th ed). New York: Lange Medical Books/McGraw-Hill, 2000;1376-86.
6. Hutto B. Syphilis in clinical psychiatry: a review. Psychosomatics. 2001;42:453-60.
7. Carpenter CJ, Lederman MM, Salata RA. Sexually transmitted diseases. In: Andreoli TE, Bennett JC, Carpenter CJ, Plum F (eds). Cecil essentials of medicine. (4th ed). Philadelphia: WB Saunders Co, 1997;742-5.
1. U.S. Centers for Disease Control and Prevention. Primary and secondary syphilis—United States, 2000-2001. MMWR Morb Mortal Wkly Rep. 2002;51:971-3.
2. Mandell GL, Petri WA. Antimicrobial agents: penicillins, cephalosporins, and other beta-lactam antibiotics. In:Hardman JG, Limbird LE, Molinoff PB, et al (eds) Goodman and Gilman’s the pharmacological basis of therapeutics. (9th ed). New York: McGraw-Hill, 1996;1073-4.
3. Lukehart SA, Holmes KK. Spirochetal diseases. In: Braunwald E, Fauci AS, Kasper DL, et al (eds). Harrison’s principles of internal medicine. (14th ed). New York: McGraw-Hill, 1998;1023.-
4. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2001 supplement, syphilis surveillance report. Available at: http://www.cdc.gov/std/stats/2001syphilis.htm. Accessed October 10, 2003.
5. Jacobs RA. Infectious diseases: spirochetal. In: Tierney LM, McPhee SJ, Papadakis MA (eds). Current medical diagnosis and treatment. (39th ed). New York: Lange Medical Books/McGraw-Hill, 2000;1376-86.
6. Hutto B. Syphilis in clinical psychiatry: a review. Psychosomatics. 2001;42:453-60.
7. Carpenter CJ, Lederman MM, Salata RA. Sexually transmitted diseases. In: Andreoli TE, Bennett JC, Carpenter CJ, Plum F (eds). Cecil essentials of medicine. (4th ed). Philadelphia: WB Saunders Co, 1997;742-5.
Tics and tourette’s disorder: Which therapies, and when to use them
When managing pediatric tics and Tourette’s disorder, we do not seek to eliminate tic symptoms. Instead—based on evidence and our experience—we use a six-step approach to increase tic control, decrease our patients’ embarrassment and discomfort, and help them function more normally.
Drug therapy is not appropriate for all children and adolescents with tic disorders. Mild transient tics and Tourette’s disorder usually do not require treatment, and medications should not be given to patients whose tics do not impair their quality of life. Treatment is warranted, however, when tics interfere with peer relations, social interactions, academic performance, or activities of daily living.
Standard treatment of pediatric tic disorders is changing. Instead of using typical antipsychotics, many experienced clinicians are using other medications that are safer and more effective, particularly for children and adolescents with psychiatric comorbidities such as attention-deficit/hyperactivity disorder (ADHD). In these patients, it is difficult to avoid drug interactions and exacerbation of non-targeted conditions when you attempt to control the tics.
Table 1
Diagnostic criteria for tic disorders
| Shared characteristics |
|
| Transient tic disorder |
|
| Chronic motor or vocal tic disorder |
|
| Tourette’s disorder |
|
| Source: Adapted from DSM-IV-TR |
TICS’ FLUCTUATING COURSE
Tics and Tourette’s disorder are characterized by a fluctuating course. Tic activity tends to occur in bursts over hours to weeks, followed by relative quiescence—spontaneously varying from one extreme to the other. Tics:
- are often preceded by mounting tension
- occur most frequently without volition, although they can be consciously suppressed
- are influenced by emotional state and tend to worsen during increased stress, excitement, or fatigue.
This variable natural history limits the value of uncontrolled studies, as symptom changes are not necessarily treatment-related.
DSM-IV-TR lists three types of childhood tic disorders (Table 1). Transient tics are seen in up to 10% of children, chronic tics are less common, and Tourette’s disorder has a community prevalence of 0.1 to 0.8%.1 Tic disorders usually present by age 112 and are three times more common in boys than in girls. One-half of cases remit spontaneously by late adolescence or adulthood, with important treatment implications.2
Causes. Neurophysiologic studies suggest disinhibition and dysfunction of dopamine and related serotonergic pathways in the cortico-striatal-thalamic-cortical circuit.3 Corollary neuroimaging studies have found decreased metabolism and blood flow in the basal ganglia—specifically the caudate nucleus, thalamus, globus pallidus, and putamen—and increased activity in the frontotemporal cortex—specifically the prefrontal and supplementary motor areas.4,5
Comorbidities. Tics and Tourette’s disorder rarely occur in isolation. The most common comorbidities and the frequencies with which they occur with tic disorders and Tourette’s disorder are:
- ADHD (50% and 90%)6
- obsessive-compulsive disorder (OCD)(11% and 80%)6
- major depressive disorder (40% and 44%).1,6
Additional comorbid problems include rage attacks, poor impulse control, and learning disorders. Many children with Tourette’s disorder display explosive rage.7
GUIDE TO WORKUP
During initial assessment, clearly delineate the onset, severity, complexity, and course of tics. Use empirically validated instruments—such as the Yale Global Tic Severity Scale8—at baseline and follow-up visits to monitor the natural history and clinical course, including treatment response. Determine predominant sources of distress and domains of impaired function.
Identify comorbid psychiatric illnesses (Box). Often, tics are not impairing9 and take on less clinical importance than the associated disorders. Prioritize target symptoms after considering the youth’s and family’s wishes. Follow a multidisciplinary approach, including behavioral, psychotherapeutic, and drug treatment as needed. Involve patients’ parents, schools, and teachers to help monitor functional impairment and treatment impact.
Use follow-up visits as needed to monitor treatment effectiveness. Follow-up frequency may decrease after tics are controlled to an acceptable level, although comorbid disorders may require continued attention.
PANDAS. Consider a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS) when tics present abruptly with upper respiratory tract illness.10 In this context, throat culture and antibody titers for group A beta hemolytic streptococcal infection may be warranted. Treat aggressively with antibiotics such as penicillin V when tests are positive.
- Assess for comorbid illnesses, then prioritize and treat the most troublesome symptoms
- Aim to decrease rather than eliminate tic-related discomfort
- Medicate only if tics cause distress and dysfunction
- Use one agent when needed at the lowest effective dosage to minimize side effects and drug interactions
- Involve the family and school to monitor progress
- Reassess treatment efficacy often
6-STEP TREATMENT APPROACH
A six-step approach—based on our experience and available evidence—can guide treatment. Tics coexisting with ADHD/disruptive disorders, OCD/anxiety disorders, or major depressive disorder call for specialized strategies (Algorithm).
Step 1: Nondrug therapies. Psychoeducation, supportive therapy, and behavioral therapy are appropriate for all patients with burdensome tics. The unusual behaviors associated with tic disorders may have a far-reaching impact on a child’s functioning, self-esteem, and confidence. These effects can be moderated when children and their families understand tics’ fluctuating nature, including their:
- increase with stress and fatigue
- capacity for brief inhibition
- and high rate of spontaneous remission.
Because of this fluctuating pattern, observe the patient for a few weeks before starting medical treatment, unless dysfunction is severe. Observation is especially useful for initial presentations, in which symptom peaks tend to precede quiescence. Carefully weigh the benefits and potential risks of medical treatment for each patient.
Behavioral options include habit reversal, relaxation training, and self-monitoring. One of the few studies of behavioral therapy found tic symptoms decreased 55% with habit reversal, 44% with self-monitoring, and 32% with relaxation training.11
Step 2: Adrenergic alpha-2 agonists. If medication seems appropriate for moderate to severe tics, we recommend clonidine or guanfacine (Table 2) as first-line therapy. These agents decrease the release of norepinephrine, dopamine, and gluta-mate, and norepinephrine turnover.12 They are commonly used to treat Tourette’s disorder because they are better tolerated than antipsychotics, although controlled studies supporting their use are limited and the FDA has not approved this indication.
Approximately one-fourth of Tourette’s disorder patients respond well to clonidine.6 Pulse and blood pressure need to be monitored when using these medications, which in rare cases cause hypotension, bradycardia, and cardiac conduction delay. Because of its sedating properties, clonidine is frequently given at bedtime to promote sleep. Use caution when giving clonidine with medications that have potential cardiovascular effects—such as propranolol or tricyclic antidepressants.
Guanfacine is similar to clonidine except that it binds alpha-2a receptors more selectively and has a longer half-life. As such, it is associated with lower rates of sedation and hypotension than clonidine.
Step 3: Atypical antipsychotics. If symptoms do not respond adequately to an adrenergic alpha-2 agonist, try an atypical antipsychotic. Atypicals block dopamine (D2) receptors and—as a result of serotonergic-2 blockade—are less likely to cause extrapyramidal symptoms than are older antipsychotics.
Risperidone, the most studied atypical in Tourette’s disorder, has been shown to reduce symptoms by 21 to 61%—an effect significantly greater than placebo13 and similar to that of pimozide14 and clonidine. Because it is also relatively well-tolerated, a risperidone trial is warranted before using typical antipsychotics. Tics may worsen during withdrawal while switching a patient from a typical to an atypical antipsychotic.
In one comparative, crossover study in adults with severe Tourette’s disorder, olanzapine was more effective at 5 and 10 mg/d than pimozide at 2 and 4 mg/d, respectively.15 Weight gain and abnormal glucose tolerance associated with olanzapine may be troublesome side effects. Ziprasidone has demonstrated a 35% decrease in tic symptoms in placebo-controlled studies.16 Its use has been associated with increased risk of QTc interval prolongation, requiring ECG monitoring.
Two case series have reported positive effects when quetiapine was used for tics and Tourette’s disorder.17 Like clozapine (which is ineffective for tics), quetiapine has relatively low D2 antagonist potency, suggesting its efficacy in treating tics may be limited. Unlike clozapine, however, quetiapine has few anticholinergic effects. Aripiprazole’s pharmacodynamic profile suggests similar efficacy, but its use in tic disorders has not been validated.
Algorithm 6-step treatment approach to tics and Tourette’s disorder
Further controlled trials of atypical antipsychotics in children and adolescents with tic disorders are needed. ECGs are recommended to monitor QTc intervals when using these medications.
Step 4: Typical antipsychotics. Haloperidol is the most commonly used medication for treating pediatric Tourette’s disorder13 and one of two drugs (pimozide is the other) approved by the FDA for this indication. These postsynaptic D2 antagonists are the most-studied and most-potent medications for treating tics and Tourette’s disorder. Many other typical antipsychotics such as fluphenazine, thioridazine, trifluoperazine, molindone, and thiothixene also have been used.
In controlled trials, haloperidol improved symptoms by 43 to 66%,18 which was greater than placebo and equal to the effect of fluphenazine and trifluoperazine. Haloperidol, however, demonstrated a higher rate of EPS.
Pimozide’s indication for pediatric Tourette’s disorder applies only to treatmentrefractory cases. In controlled studies, pimozide was at least as effective as haloperidol,18 equal to risperidone14 and less effective than olanzapine.15 Pimozide caused fewer side effects than haloperidol but more than atypical antipsychotics. Pimozide may cause QTc prolongation, and regular ECG monitoring is required.
Despite their efficacy, typical antipsychotics are associated with common and occasionally severe side effects that limit their long-term tolerability.6 Fear of tardive dyskinesia generally limits their use to only severe and treatmentresistant cases.
Step 5: Benzodiazepines. Although controlled trials of tic disorders have not evaluated benzodiazepines, these drugs were effective adjuncts in one case series using haloperidol.6 Anecdotal reports suggest they may reduce tics indirectly by lessening anxiety. Many experienced clinicians use clonazepam, 0.5 to 3 mg/d, or lorazepam, 0.5 to 4 mg/d, to treat Tourette’s disorder. Aside from its anxiolytic effects, clonazepam is also considered a minor mood stabilizer.
Step 6: Other options. Numerous novel medications have been studied in trials of tics and Tourette’s, although most—including the mixed D1/D2 agonist pergolide—have not been proven effective. In an uncontrolled study, the parenteral opioid antagonist naloxone decreased tics at low doses and increased them at higher doses. Botulinum toxin, nicotine, mecamylamine (a nicotine antagonist), baclofen, and flutamide have not proven efficacy in placebo-controlled trials.
Transcranial magnetic stimulation and neurosurgery have been used in patients with severe refractory tics and Tourette’s disorder but are not well-established treatments.
TICS AND COMORBIDITIES
ADHD. When it presents with tics, ADHD is frequently an independent target—or even the main target—of management. Because stimulants may exacerbate tics, nonstimulant medications such as clonidine, guanfacine, or desipramine could be tried first.19 Clonidine has been shown to ameliorate aggression, hyperactivity, and impulsivity but has sedating side effects. Atomoxetine—a nonstimulant ADHD medication—is another option for youth with comorbid tics and ADHD.
In our experience, carefully monitored stimulant trials may also be tried. A recent controlled trial showed that methylphenidate and clonidine, separately and combined, are effective for ADHD and comorbid Tourette’s disorder.20 Stimulants of potential benefit include methylphenidate and amphetamine (not just dextroamphetamine), including their long-acting formulations. Combining stimulants with antipsychotics or clonidine may also be useful.
Table 2
Recommended drugs and dosages for pediatric tics and Tourette’s disorder*
| Class/drug | Starting dosage (mg/d) | Dosage increase interval | Dosage range (mg/d) | Dosing regime | Potency/CYP-450 pathway | Delay to onset | |
|---|---|---|---|---|---|---|---|
| Alpha2 agonists | |||||||
| Clonidine | 0.025-0.05 | 5 to 7 days | 0.1-0.3 | bid or tid (patch every 5 to 7 days) | N/A | 2 to 8 wks | |
| Guanfacine | 0.5 | 5 to 7 days | 0.5-4 | bid to tid | N/A | 2 to 8 wks | |
| Side effects | Common: dry mouth, drowsiness, dizziness, sedation, weakness, skin rashes (patch) Rare: hypotension, bradycardia, conduction delay, rebound symptoms | ||||||
| Atypical antipsychotics | |||||||
| Risperidone | 0.25-1 | 7 to 21 days | 0.5-6 | qd to bid | high/ 2D6, 3A4 | 2 to 4 wks | |
| Olanzapine | 2.5-5 | 7 to 21 days | 2.5-10 | qd to bid | medium/ 1A2, 2D6 | 2 to 4 wks | |
| Ziprasidone | 20 | 7 to 21 days | 40-160 | qd to bid | medium/ 3A4 | 2 to 4 wks | |
| Quetiapine | 12.5-25 | 7 to 21 days | 100-600 | qd to bid | low/ 3A4 | 2 to 4 wks | |
| Side effects | Common: weight gain (especially in youth), sedation Rare: hepatic enzyme elevation, extrapyramidal symptoms (EPS), increased QTc interval (ziprasidone) | ||||||
| Typical antipsychotics | |||||||
| Haloperidol | 0.25-0.5 | 7 to 21 days | 2-10 mg | qd to tid | high/ 2D6, 3A4 | 2 to 4 wks | |
| Pimozide | 0.5 | 7 to 21 days | 1-8 mg | qd to tid | high/ 1A2, 2D6, 3A4 | 2 to 4 wks | |
| Side effects | Common: EPS (acute dystonia, akathisia, parkinsonism), sedation, weight gain, dysphoria, cognitive dulling, increased plasma prolactin Rare: neuroleptic malignant syndrome (potentially fatal: autonomic instability, hyperthermia and muscular rigidity); tardive dyskinesia; increased QTc interval (pimozide) | ||||||
| Tricyclic antidepressants | |||||||
| Desipramine | 25 | 5 to 7 days | 2.5-5 /kg/d | qd to bid | 2D6 | 3 to 6 wks | |
| Nortriptyline | 10 | 5 to 7 days | 0.5-3 /kg/d | qd to bid | 2D6 | 3 to 6 wks | |
| Imipramine | 25 | 5 to 7 days | 2.5-5 /kg/d | qd to bid | 2C19, 2D6, 3A4 | 3 to 6 wks | |
| Clomipramine | 25 | 5 to 7 days | 3 /kg/d | qd to bid | 2C19, 2D6, 3A4 | 3 to 6 wks | |
| Side effects | Common: anticholinergic (dry mouth, blurred vision, constipation); antihistaminic (sedation, weight gain); and antialpha adrenergic (dizziness) Rare: heart palpitations, seizures, hepatic enzyme elevations, increased QTc interval | ||||||
| * Benzodiazepines (clonazepam or lorazepam) may be useful adjuncts; see text for side effects and dosages. Source: Adapted from DSM-IV-TR | |||||||
To control rage attacks, a trial of mood stabilizers or atypical antipsychotics may be combined with standard tic medications.
Tricyclic antidepressants have been used to treat tics—especially in children with comorbid ADHD.21 A case series and controlled study by Singer et al of desipramine and clonidine found no significant impact on tics,22 although this trial was limited by a fixed dose design and few assessment points.
More recently, a double-blind, placebo-controlled trial found a 58% decrease in tic symptoms with desipramine (mean dosage 3.4 mg/kg/d) in patients with tics and ADHD.19 This effect was associated with small increases in heart rate and blood pressure.
Tricyclics’ potential toxicity in overdose and anticholinergic side effects require caution and may limit their use. However, they can be considered as adjuncts in treating chronic tic disorders, especially with comorbid ADHD. Serum levels and ECG monitoring every 3 to 6 months are required to rule out prolonged conduction times and tachycardia. Concurrent methylphenidate use may increase serum desipramine levels, and concurrent pimozide use may increase risk for arrhythmias.
OCD and anxiety disorders. Medically treating anxiety can help indirectly to manage tics, which are sensitive to stress.9 OCD comorbidity is especially common in youth with a family history of Tourette’s disorder.6 Screening for OCD is important, as its secretive symptoms frequently go unnoticed and its prognosis may be poorer with a concurrent tic disorder.
Standard treatment for pediatric OCD is cognitive-behavioral therapy, followed when needed by selective serotonin reuptake inhibitors (SSRIs), then clomipramine. These treatments are added to tic management, with attention to primary and comorbid symptoms. Anecdotal reports suggest that SSRIs occasionally exacerbate tics. Similarly, behavioral side effects are common in younger children treated with SSRIs and may aggravate ADHD symptoms.
Mood disorders. Except for tricyclics, antidepressants have been ineffective at reducing tics/Tourette’s disorder. Tricyclics, however, have not been proven effective in depressed youth, in part because of methodologic limitations in controlled trials. Even so, tricyclics may help some children with tics and major depressive disorder. SSRIs combined with usual tic treatment may also be tried, with monitoring for tic worsening.9 To control rage attacks, a trial of mood stabilizers or atypical antipsychotics may be combined with standard tic medications.
Related resources
- Leckman JF, Cohen DJ (ed). Tourette’s syndrome. Tics, obsessions, compulsions: developmental psychopathology and clinical care. New York: John Wiley & Sons, 1999.
- Jankovic J. Tourette’s syndrome. N Engl J Med 2001;345(16):1184-92.
- Martin A, Scahill L, Charney DS, Leckman JF (ed). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2003.
- Tourette Syndrome Association. www.tsa-usa.org
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Desipramine • Norpramin
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Clonidine • Catapres
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluphenazine • Permitil, Prolixin
- Guanfacine • Tenex
- Haldoperidol • Haldol
- Imipramine • Tofranil
- Lorazepam • Ativan
- Molindone • Moban
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Thiothixine • Navane
- Trifluoperazine • Stelazine
- Ziprasidone • Geodon
Disclosure
Dr. Stewart and Loren Gianini report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Geller receives grant/research support from Eli Lilly and Co. and Forest Laboratories Inc., is a consultant to GlaxoSmithKline, and is a speaker for Eli Lilly and Co., Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Spencer receives research/grant support from and is a speaker or consultant for Abbott Laboratories, Ortho-McNeil Pharmaceutical Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Pfizer Inc., Shire Pharmaceuticals Group, and Wyeth Pharmaceuticals.
1. Sukhodolsky DG, Scahill L, Zhang H, et al. Disruptive behavior in children with Tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry 2003;42(1):98-105.
2. Jankovic J. Tourette’s syndrome. N Engl J Med 2001;345(16):1184-92.
3. Leckman JF, Goodman WK, Anderson GM, et al. CSF biogenic amines in obsessive-compulsive disorder and Tourette’s syndrome. Neuropsychopharmacology 1995;12:73-86.
4. Braun AR, Stoetter B, Randolph C, et al. The functional neuroanatomy of Tourette’s syndrome: An FDG-PET study: I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology 1993;9:277-91.
5. Moriarty J, Campos D, Schmitz B, et al. Brain perfusion abnormalities in Gilles de la Tourette’s syndrome. Br J Psychiatry 1995;167:249-54.
6. Leckman JF. Cohen DJ (eds). Tourette’s syndrome. Tics, obsessions, compulsions: developmental psychopathology and clinical care. New York: John Wiley & Sons, 1999.
7. Budman C, Bruun R, Park K, Olson M. Rage attacks in children and adolescents with Tourette’s disorder: a pilot study. J Clin Psychiatry 1998;59(11):576-80.
8. Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28(4):566-73.
9. Spencer T, Biederman J, Harding M, et al. Disentangling the overlap between Tourette’s disorder and ADHD. J Child Psychol Psychiatry 1999;39:1037-44.
10. Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998;155:264-71.
11. Peterson AL, Azrin NH. An evaluation of behavioral treatments for Tourette syndrome. Behav Res Ther 1992;30(2):167-74.
12. Leckman JF, Hardin MT, Riddle MA, et al. Clonidine treatment of Gilles de la Tourette’s syndrome. Arch Gen Psychiatry 1991;48(4):324-8.
13. Dion Y, Annable L, Sandor P, Chouinard G. Risperidone in the treatment of Tourette syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacology 2002;22(1):31-9.
14. Bruggeman R, van der Linden C, Buitelaar JK, et al. Risperidone versus pimozide in Tourette’s disorder: a comparative double-blind parallel-group study. J Clin Psychiatry 2001;62(1):50-6.
15. Onofrj M, Paci C, D’Andreamatteo G, Toma L. Olanzapine in severe Gilles de la Tourette syndrome: a 52-week double-blind, cross-over study vs. low-dose pimozide. J Neurol 2000;247:443-6.
16. Sallee FR, Kurlan R, Goetz, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry 2000;39(3):292-9.
17. Parraga HC, Parraga MI, Woodward RL, Fenning PA. Quetiapine treatment of children with Tourette’s syndrome: report of two cases. J Child Adolesc Psychopharmacology 2001;11(2):187-91.
18. Sallee FR, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry 1997;154(8):1057-62.
19. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2002;59(7):649-56.
20. Kurlan R. for the Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: A randomized controlled trial. Neurology 2002;58:527-36.
21. Spencer T, Biederman J, Kerman K, et al. Desipramine treatment of children with attention-deficit/hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 1993;32(2):354-60.
22. Singer HS, Brown J, Quaskey S, et al. The treatment of attentiondeficit hyperactivity disorder in Tourette’s syndrome: A double-blind, placebo-controlled study with clonidine and desipramine. Pediatrics 1995;95(1):74-81.
When managing pediatric tics and Tourette’s disorder, we do not seek to eliminate tic symptoms. Instead—based on evidence and our experience—we use a six-step approach to increase tic control, decrease our patients’ embarrassment and discomfort, and help them function more normally.
Drug therapy is not appropriate for all children and adolescents with tic disorders. Mild transient tics and Tourette’s disorder usually do not require treatment, and medications should not be given to patients whose tics do not impair their quality of life. Treatment is warranted, however, when tics interfere with peer relations, social interactions, academic performance, or activities of daily living.
Standard treatment of pediatric tic disorders is changing. Instead of using typical antipsychotics, many experienced clinicians are using other medications that are safer and more effective, particularly for children and adolescents with psychiatric comorbidities such as attention-deficit/hyperactivity disorder (ADHD). In these patients, it is difficult to avoid drug interactions and exacerbation of non-targeted conditions when you attempt to control the tics.
Table 1
Diagnostic criteria for tic disorders
| Shared characteristics |
|
| Transient tic disorder |
|
| Chronic motor or vocal tic disorder |
|
| Tourette’s disorder |
|
| Source: Adapted from DSM-IV-TR |
TICS’ FLUCTUATING COURSE
Tics and Tourette’s disorder are characterized by a fluctuating course. Tic activity tends to occur in bursts over hours to weeks, followed by relative quiescence—spontaneously varying from one extreme to the other. Tics:
- are often preceded by mounting tension
- occur most frequently without volition, although they can be consciously suppressed
- are influenced by emotional state and tend to worsen during increased stress, excitement, or fatigue.
This variable natural history limits the value of uncontrolled studies, as symptom changes are not necessarily treatment-related.
DSM-IV-TR lists three types of childhood tic disorders (Table 1). Transient tics are seen in up to 10% of children, chronic tics are less common, and Tourette’s disorder has a community prevalence of 0.1 to 0.8%.1 Tic disorders usually present by age 112 and are three times more common in boys than in girls. One-half of cases remit spontaneously by late adolescence or adulthood, with important treatment implications.2
Causes. Neurophysiologic studies suggest disinhibition and dysfunction of dopamine and related serotonergic pathways in the cortico-striatal-thalamic-cortical circuit.3 Corollary neuroimaging studies have found decreased metabolism and blood flow in the basal ganglia—specifically the caudate nucleus, thalamus, globus pallidus, and putamen—and increased activity in the frontotemporal cortex—specifically the prefrontal and supplementary motor areas.4,5
Comorbidities. Tics and Tourette’s disorder rarely occur in isolation. The most common comorbidities and the frequencies with which they occur with tic disorders and Tourette’s disorder are:
- ADHD (50% and 90%)6
- obsessive-compulsive disorder (OCD)(11% and 80%)6
- major depressive disorder (40% and 44%).1,6
Additional comorbid problems include rage attacks, poor impulse control, and learning disorders. Many children with Tourette’s disorder display explosive rage.7
GUIDE TO WORKUP
During initial assessment, clearly delineate the onset, severity, complexity, and course of tics. Use empirically validated instruments—such as the Yale Global Tic Severity Scale8—at baseline and follow-up visits to monitor the natural history and clinical course, including treatment response. Determine predominant sources of distress and domains of impaired function.
Identify comorbid psychiatric illnesses (Box). Often, tics are not impairing9 and take on less clinical importance than the associated disorders. Prioritize target symptoms after considering the youth’s and family’s wishes. Follow a multidisciplinary approach, including behavioral, psychotherapeutic, and drug treatment as needed. Involve patients’ parents, schools, and teachers to help monitor functional impairment and treatment impact.
Use follow-up visits as needed to monitor treatment effectiveness. Follow-up frequency may decrease after tics are controlled to an acceptable level, although comorbid disorders may require continued attention.
PANDAS. Consider a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS) when tics present abruptly with upper respiratory tract illness.10 In this context, throat culture and antibody titers for group A beta hemolytic streptococcal infection may be warranted. Treat aggressively with antibiotics such as penicillin V when tests are positive.
- Assess for comorbid illnesses, then prioritize and treat the most troublesome symptoms
- Aim to decrease rather than eliminate tic-related discomfort
- Medicate only if tics cause distress and dysfunction
- Use one agent when needed at the lowest effective dosage to minimize side effects and drug interactions
- Involve the family and school to monitor progress
- Reassess treatment efficacy often
6-STEP TREATMENT APPROACH
A six-step approach—based on our experience and available evidence—can guide treatment. Tics coexisting with ADHD/disruptive disorders, OCD/anxiety disorders, or major depressive disorder call for specialized strategies (Algorithm).
Step 1: Nondrug therapies. Psychoeducation, supportive therapy, and behavioral therapy are appropriate for all patients with burdensome tics. The unusual behaviors associated with tic disorders may have a far-reaching impact on a child’s functioning, self-esteem, and confidence. These effects can be moderated when children and their families understand tics’ fluctuating nature, including their:
- increase with stress and fatigue
- capacity for brief inhibition
- and high rate of spontaneous remission.
Because of this fluctuating pattern, observe the patient for a few weeks before starting medical treatment, unless dysfunction is severe. Observation is especially useful for initial presentations, in which symptom peaks tend to precede quiescence. Carefully weigh the benefits and potential risks of medical treatment for each patient.
Behavioral options include habit reversal, relaxation training, and self-monitoring. One of the few studies of behavioral therapy found tic symptoms decreased 55% with habit reversal, 44% with self-monitoring, and 32% with relaxation training.11
Step 2: Adrenergic alpha-2 agonists. If medication seems appropriate for moderate to severe tics, we recommend clonidine or guanfacine (Table 2) as first-line therapy. These agents decrease the release of norepinephrine, dopamine, and gluta-mate, and norepinephrine turnover.12 They are commonly used to treat Tourette’s disorder because they are better tolerated than antipsychotics, although controlled studies supporting their use are limited and the FDA has not approved this indication.
Approximately one-fourth of Tourette’s disorder patients respond well to clonidine.6 Pulse and blood pressure need to be monitored when using these medications, which in rare cases cause hypotension, bradycardia, and cardiac conduction delay. Because of its sedating properties, clonidine is frequently given at bedtime to promote sleep. Use caution when giving clonidine with medications that have potential cardiovascular effects—such as propranolol or tricyclic antidepressants.
Guanfacine is similar to clonidine except that it binds alpha-2a receptors more selectively and has a longer half-life. As such, it is associated with lower rates of sedation and hypotension than clonidine.
Step 3: Atypical antipsychotics. If symptoms do not respond adequately to an adrenergic alpha-2 agonist, try an atypical antipsychotic. Atypicals block dopamine (D2) receptors and—as a result of serotonergic-2 blockade—are less likely to cause extrapyramidal symptoms than are older antipsychotics.
Risperidone, the most studied atypical in Tourette’s disorder, has been shown to reduce symptoms by 21 to 61%—an effect significantly greater than placebo13 and similar to that of pimozide14 and clonidine. Because it is also relatively well-tolerated, a risperidone trial is warranted before using typical antipsychotics. Tics may worsen during withdrawal while switching a patient from a typical to an atypical antipsychotic.
In one comparative, crossover study in adults with severe Tourette’s disorder, olanzapine was more effective at 5 and 10 mg/d than pimozide at 2 and 4 mg/d, respectively.15 Weight gain and abnormal glucose tolerance associated with olanzapine may be troublesome side effects. Ziprasidone has demonstrated a 35% decrease in tic symptoms in placebo-controlled studies.16 Its use has been associated with increased risk of QTc interval prolongation, requiring ECG monitoring.
Two case series have reported positive effects when quetiapine was used for tics and Tourette’s disorder.17 Like clozapine (which is ineffective for tics), quetiapine has relatively low D2 antagonist potency, suggesting its efficacy in treating tics may be limited. Unlike clozapine, however, quetiapine has few anticholinergic effects. Aripiprazole’s pharmacodynamic profile suggests similar efficacy, but its use in tic disorders has not been validated.
Algorithm 6-step treatment approach to tics and Tourette’s disorder
Further controlled trials of atypical antipsychotics in children and adolescents with tic disorders are needed. ECGs are recommended to monitor QTc intervals when using these medications.
Step 4: Typical antipsychotics. Haloperidol is the most commonly used medication for treating pediatric Tourette’s disorder13 and one of two drugs (pimozide is the other) approved by the FDA for this indication. These postsynaptic D2 antagonists are the most-studied and most-potent medications for treating tics and Tourette’s disorder. Many other typical antipsychotics such as fluphenazine, thioridazine, trifluoperazine, molindone, and thiothixene also have been used.
In controlled trials, haloperidol improved symptoms by 43 to 66%,18 which was greater than placebo and equal to the effect of fluphenazine and trifluoperazine. Haloperidol, however, demonstrated a higher rate of EPS.
Pimozide’s indication for pediatric Tourette’s disorder applies only to treatmentrefractory cases. In controlled studies, pimozide was at least as effective as haloperidol,18 equal to risperidone14 and less effective than olanzapine.15 Pimozide caused fewer side effects than haloperidol but more than atypical antipsychotics. Pimozide may cause QTc prolongation, and regular ECG monitoring is required.
Despite their efficacy, typical antipsychotics are associated with common and occasionally severe side effects that limit their long-term tolerability.6 Fear of tardive dyskinesia generally limits their use to only severe and treatmentresistant cases.
Step 5: Benzodiazepines. Although controlled trials of tic disorders have not evaluated benzodiazepines, these drugs were effective adjuncts in one case series using haloperidol.6 Anecdotal reports suggest they may reduce tics indirectly by lessening anxiety. Many experienced clinicians use clonazepam, 0.5 to 3 mg/d, or lorazepam, 0.5 to 4 mg/d, to treat Tourette’s disorder. Aside from its anxiolytic effects, clonazepam is also considered a minor mood stabilizer.
Step 6: Other options. Numerous novel medications have been studied in trials of tics and Tourette’s, although most—including the mixed D1/D2 agonist pergolide—have not been proven effective. In an uncontrolled study, the parenteral opioid antagonist naloxone decreased tics at low doses and increased them at higher doses. Botulinum toxin, nicotine, mecamylamine (a nicotine antagonist), baclofen, and flutamide have not proven efficacy in placebo-controlled trials.
Transcranial magnetic stimulation and neurosurgery have been used in patients with severe refractory tics and Tourette’s disorder but are not well-established treatments.
TICS AND COMORBIDITIES
ADHD. When it presents with tics, ADHD is frequently an independent target—or even the main target—of management. Because stimulants may exacerbate tics, nonstimulant medications such as clonidine, guanfacine, or desipramine could be tried first.19 Clonidine has been shown to ameliorate aggression, hyperactivity, and impulsivity but has sedating side effects. Atomoxetine—a nonstimulant ADHD medication—is another option for youth with comorbid tics and ADHD.
In our experience, carefully monitored stimulant trials may also be tried. A recent controlled trial showed that methylphenidate and clonidine, separately and combined, are effective for ADHD and comorbid Tourette’s disorder.20 Stimulants of potential benefit include methylphenidate and amphetamine (not just dextroamphetamine), including their long-acting formulations. Combining stimulants with antipsychotics or clonidine may also be useful.
Table 2
Recommended drugs and dosages for pediatric tics and Tourette’s disorder*
| Class/drug | Starting dosage (mg/d) | Dosage increase interval | Dosage range (mg/d) | Dosing regime | Potency/CYP-450 pathway | Delay to onset | |
|---|---|---|---|---|---|---|---|
| Alpha2 agonists | |||||||
| Clonidine | 0.025-0.05 | 5 to 7 days | 0.1-0.3 | bid or tid (patch every 5 to 7 days) | N/A | 2 to 8 wks | |
| Guanfacine | 0.5 | 5 to 7 days | 0.5-4 | bid to tid | N/A | 2 to 8 wks | |
| Side effects | Common: dry mouth, drowsiness, dizziness, sedation, weakness, skin rashes (patch) Rare: hypotension, bradycardia, conduction delay, rebound symptoms | ||||||
| Atypical antipsychotics | |||||||
| Risperidone | 0.25-1 | 7 to 21 days | 0.5-6 | qd to bid | high/ 2D6, 3A4 | 2 to 4 wks | |
| Olanzapine | 2.5-5 | 7 to 21 days | 2.5-10 | qd to bid | medium/ 1A2, 2D6 | 2 to 4 wks | |
| Ziprasidone | 20 | 7 to 21 days | 40-160 | qd to bid | medium/ 3A4 | 2 to 4 wks | |
| Quetiapine | 12.5-25 | 7 to 21 days | 100-600 | qd to bid | low/ 3A4 | 2 to 4 wks | |
| Side effects | Common: weight gain (especially in youth), sedation Rare: hepatic enzyme elevation, extrapyramidal symptoms (EPS), increased QTc interval (ziprasidone) | ||||||
| Typical antipsychotics | |||||||
| Haloperidol | 0.25-0.5 | 7 to 21 days | 2-10 mg | qd to tid | high/ 2D6, 3A4 | 2 to 4 wks | |
| Pimozide | 0.5 | 7 to 21 days | 1-8 mg | qd to tid | high/ 1A2, 2D6, 3A4 | 2 to 4 wks | |
| Side effects | Common: EPS (acute dystonia, akathisia, parkinsonism), sedation, weight gain, dysphoria, cognitive dulling, increased plasma prolactin Rare: neuroleptic malignant syndrome (potentially fatal: autonomic instability, hyperthermia and muscular rigidity); tardive dyskinesia; increased QTc interval (pimozide) | ||||||
| Tricyclic antidepressants | |||||||
| Desipramine | 25 | 5 to 7 days | 2.5-5 /kg/d | qd to bid | 2D6 | 3 to 6 wks | |
| Nortriptyline | 10 | 5 to 7 days | 0.5-3 /kg/d | qd to bid | 2D6 | 3 to 6 wks | |
| Imipramine | 25 | 5 to 7 days | 2.5-5 /kg/d | qd to bid | 2C19, 2D6, 3A4 | 3 to 6 wks | |
| Clomipramine | 25 | 5 to 7 days | 3 /kg/d | qd to bid | 2C19, 2D6, 3A4 | 3 to 6 wks | |
| Side effects | Common: anticholinergic (dry mouth, blurred vision, constipation); antihistaminic (sedation, weight gain); and antialpha adrenergic (dizziness) Rare: heart palpitations, seizures, hepatic enzyme elevations, increased QTc interval | ||||||
| * Benzodiazepines (clonazepam or lorazepam) may be useful adjuncts; see text for side effects and dosages. Source: Adapted from DSM-IV-TR | |||||||
To control rage attacks, a trial of mood stabilizers or atypical antipsychotics may be combined with standard tic medications.
Tricyclic antidepressants have been used to treat tics—especially in children with comorbid ADHD.21 A case series and controlled study by Singer et al of desipramine and clonidine found no significant impact on tics,22 although this trial was limited by a fixed dose design and few assessment points.
More recently, a double-blind, placebo-controlled trial found a 58% decrease in tic symptoms with desipramine (mean dosage 3.4 mg/kg/d) in patients with tics and ADHD.19 This effect was associated with small increases in heart rate and blood pressure.
Tricyclics’ potential toxicity in overdose and anticholinergic side effects require caution and may limit their use. However, they can be considered as adjuncts in treating chronic tic disorders, especially with comorbid ADHD. Serum levels and ECG monitoring every 3 to 6 months are required to rule out prolonged conduction times and tachycardia. Concurrent methylphenidate use may increase serum desipramine levels, and concurrent pimozide use may increase risk for arrhythmias.
OCD and anxiety disorders. Medically treating anxiety can help indirectly to manage tics, which are sensitive to stress.9 OCD comorbidity is especially common in youth with a family history of Tourette’s disorder.6 Screening for OCD is important, as its secretive symptoms frequently go unnoticed and its prognosis may be poorer with a concurrent tic disorder.
Standard treatment for pediatric OCD is cognitive-behavioral therapy, followed when needed by selective serotonin reuptake inhibitors (SSRIs), then clomipramine. These treatments are added to tic management, with attention to primary and comorbid symptoms. Anecdotal reports suggest that SSRIs occasionally exacerbate tics. Similarly, behavioral side effects are common in younger children treated with SSRIs and may aggravate ADHD symptoms.
Mood disorders. Except for tricyclics, antidepressants have been ineffective at reducing tics/Tourette’s disorder. Tricyclics, however, have not been proven effective in depressed youth, in part because of methodologic limitations in controlled trials. Even so, tricyclics may help some children with tics and major depressive disorder. SSRIs combined with usual tic treatment may also be tried, with monitoring for tic worsening.9 To control rage attacks, a trial of mood stabilizers or atypical antipsychotics may be combined with standard tic medications.
Related resources
- Leckman JF, Cohen DJ (ed). Tourette’s syndrome. Tics, obsessions, compulsions: developmental psychopathology and clinical care. New York: John Wiley & Sons, 1999.
- Jankovic J. Tourette’s syndrome. N Engl J Med 2001;345(16):1184-92.
- Martin A, Scahill L, Charney DS, Leckman JF (ed). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2003.
- Tourette Syndrome Association. www.tsa-usa.org
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Desipramine • Norpramin
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Clonidine • Catapres
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluphenazine • Permitil, Prolixin
- Guanfacine • Tenex
- Haldoperidol • Haldol
- Imipramine • Tofranil
- Lorazepam • Ativan
- Molindone • Moban
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Thiothixine • Navane
- Trifluoperazine • Stelazine
- Ziprasidone • Geodon
Disclosure
Dr. Stewart and Loren Gianini report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Geller receives grant/research support from Eli Lilly and Co. and Forest Laboratories Inc., is a consultant to GlaxoSmithKline, and is a speaker for Eli Lilly and Co., Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Spencer receives research/grant support from and is a speaker or consultant for Abbott Laboratories, Ortho-McNeil Pharmaceutical Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Pfizer Inc., Shire Pharmaceuticals Group, and Wyeth Pharmaceuticals.
When managing pediatric tics and Tourette’s disorder, we do not seek to eliminate tic symptoms. Instead—based on evidence and our experience—we use a six-step approach to increase tic control, decrease our patients’ embarrassment and discomfort, and help them function more normally.
Drug therapy is not appropriate for all children and adolescents with tic disorders. Mild transient tics and Tourette’s disorder usually do not require treatment, and medications should not be given to patients whose tics do not impair their quality of life. Treatment is warranted, however, when tics interfere with peer relations, social interactions, academic performance, or activities of daily living.
Standard treatment of pediatric tic disorders is changing. Instead of using typical antipsychotics, many experienced clinicians are using other medications that are safer and more effective, particularly for children and adolescents with psychiatric comorbidities such as attention-deficit/hyperactivity disorder (ADHD). In these patients, it is difficult to avoid drug interactions and exacerbation of non-targeted conditions when you attempt to control the tics.
Table 1
Diagnostic criteria for tic disorders
| Shared characteristics |
|
| Transient tic disorder |
|
| Chronic motor or vocal tic disorder |
|
| Tourette’s disorder |
|
| Source: Adapted from DSM-IV-TR |
TICS’ FLUCTUATING COURSE
Tics and Tourette’s disorder are characterized by a fluctuating course. Tic activity tends to occur in bursts over hours to weeks, followed by relative quiescence—spontaneously varying from one extreme to the other. Tics:
- are often preceded by mounting tension
- occur most frequently without volition, although they can be consciously suppressed
- are influenced by emotional state and tend to worsen during increased stress, excitement, or fatigue.
This variable natural history limits the value of uncontrolled studies, as symptom changes are not necessarily treatment-related.
DSM-IV-TR lists three types of childhood tic disorders (Table 1). Transient tics are seen in up to 10% of children, chronic tics are less common, and Tourette’s disorder has a community prevalence of 0.1 to 0.8%.1 Tic disorders usually present by age 112 and are three times more common in boys than in girls. One-half of cases remit spontaneously by late adolescence or adulthood, with important treatment implications.2
Causes. Neurophysiologic studies suggest disinhibition and dysfunction of dopamine and related serotonergic pathways in the cortico-striatal-thalamic-cortical circuit.3 Corollary neuroimaging studies have found decreased metabolism and blood flow in the basal ganglia—specifically the caudate nucleus, thalamus, globus pallidus, and putamen—and increased activity in the frontotemporal cortex—specifically the prefrontal and supplementary motor areas.4,5
Comorbidities. Tics and Tourette’s disorder rarely occur in isolation. The most common comorbidities and the frequencies with which they occur with tic disorders and Tourette’s disorder are:
- ADHD (50% and 90%)6
- obsessive-compulsive disorder (OCD)(11% and 80%)6
- major depressive disorder (40% and 44%).1,6
Additional comorbid problems include rage attacks, poor impulse control, and learning disorders. Many children with Tourette’s disorder display explosive rage.7
GUIDE TO WORKUP
During initial assessment, clearly delineate the onset, severity, complexity, and course of tics. Use empirically validated instruments—such as the Yale Global Tic Severity Scale8—at baseline and follow-up visits to monitor the natural history and clinical course, including treatment response. Determine predominant sources of distress and domains of impaired function.
Identify comorbid psychiatric illnesses (Box). Often, tics are not impairing9 and take on less clinical importance than the associated disorders. Prioritize target symptoms after considering the youth’s and family’s wishes. Follow a multidisciplinary approach, including behavioral, psychotherapeutic, and drug treatment as needed. Involve patients’ parents, schools, and teachers to help monitor functional impairment and treatment impact.
Use follow-up visits as needed to monitor treatment effectiveness. Follow-up frequency may decrease after tics are controlled to an acceptable level, although comorbid disorders may require continued attention.
PANDAS. Consider a diagnosis of pediatric autoimmune neuropsychiatric disorders associated with Streptococcus (PANDAS) when tics present abruptly with upper respiratory tract illness.10 In this context, throat culture and antibody titers for group A beta hemolytic streptococcal infection may be warranted. Treat aggressively with antibiotics such as penicillin V when tests are positive.
- Assess for comorbid illnesses, then prioritize and treat the most troublesome symptoms
- Aim to decrease rather than eliminate tic-related discomfort
- Medicate only if tics cause distress and dysfunction
- Use one agent when needed at the lowest effective dosage to minimize side effects and drug interactions
- Involve the family and school to monitor progress
- Reassess treatment efficacy often
6-STEP TREATMENT APPROACH
A six-step approach—based on our experience and available evidence—can guide treatment. Tics coexisting with ADHD/disruptive disorders, OCD/anxiety disorders, or major depressive disorder call for specialized strategies (Algorithm).
Step 1: Nondrug therapies. Psychoeducation, supportive therapy, and behavioral therapy are appropriate for all patients with burdensome tics. The unusual behaviors associated with tic disorders may have a far-reaching impact on a child’s functioning, self-esteem, and confidence. These effects can be moderated when children and their families understand tics’ fluctuating nature, including their:
- increase with stress and fatigue
- capacity for brief inhibition
- and high rate of spontaneous remission.
Because of this fluctuating pattern, observe the patient for a few weeks before starting medical treatment, unless dysfunction is severe. Observation is especially useful for initial presentations, in which symptom peaks tend to precede quiescence. Carefully weigh the benefits and potential risks of medical treatment for each patient.
Behavioral options include habit reversal, relaxation training, and self-monitoring. One of the few studies of behavioral therapy found tic symptoms decreased 55% with habit reversal, 44% with self-monitoring, and 32% with relaxation training.11
Step 2: Adrenergic alpha-2 agonists. If medication seems appropriate for moderate to severe tics, we recommend clonidine or guanfacine (Table 2) as first-line therapy. These agents decrease the release of norepinephrine, dopamine, and gluta-mate, and norepinephrine turnover.12 They are commonly used to treat Tourette’s disorder because they are better tolerated than antipsychotics, although controlled studies supporting their use are limited and the FDA has not approved this indication.
Approximately one-fourth of Tourette’s disorder patients respond well to clonidine.6 Pulse and blood pressure need to be monitored when using these medications, which in rare cases cause hypotension, bradycardia, and cardiac conduction delay. Because of its sedating properties, clonidine is frequently given at bedtime to promote sleep. Use caution when giving clonidine with medications that have potential cardiovascular effects—such as propranolol or tricyclic antidepressants.
Guanfacine is similar to clonidine except that it binds alpha-2a receptors more selectively and has a longer half-life. As such, it is associated with lower rates of sedation and hypotension than clonidine.
Step 3: Atypical antipsychotics. If symptoms do not respond adequately to an adrenergic alpha-2 agonist, try an atypical antipsychotic. Atypicals block dopamine (D2) receptors and—as a result of serotonergic-2 blockade—are less likely to cause extrapyramidal symptoms than are older antipsychotics.
Risperidone, the most studied atypical in Tourette’s disorder, has been shown to reduce symptoms by 21 to 61%—an effect significantly greater than placebo13 and similar to that of pimozide14 and clonidine. Because it is also relatively well-tolerated, a risperidone trial is warranted before using typical antipsychotics. Tics may worsen during withdrawal while switching a patient from a typical to an atypical antipsychotic.
In one comparative, crossover study in adults with severe Tourette’s disorder, olanzapine was more effective at 5 and 10 mg/d than pimozide at 2 and 4 mg/d, respectively.15 Weight gain and abnormal glucose tolerance associated with olanzapine may be troublesome side effects. Ziprasidone has demonstrated a 35% decrease in tic symptoms in placebo-controlled studies.16 Its use has been associated with increased risk of QTc interval prolongation, requiring ECG monitoring.
Two case series have reported positive effects when quetiapine was used for tics and Tourette’s disorder.17 Like clozapine (which is ineffective for tics), quetiapine has relatively low D2 antagonist potency, suggesting its efficacy in treating tics may be limited. Unlike clozapine, however, quetiapine has few anticholinergic effects. Aripiprazole’s pharmacodynamic profile suggests similar efficacy, but its use in tic disorders has not been validated.
Algorithm 6-step treatment approach to tics and Tourette’s disorder
Further controlled trials of atypical antipsychotics in children and adolescents with tic disorders are needed. ECGs are recommended to monitor QTc intervals when using these medications.
Step 4: Typical antipsychotics. Haloperidol is the most commonly used medication for treating pediatric Tourette’s disorder13 and one of two drugs (pimozide is the other) approved by the FDA for this indication. These postsynaptic D2 antagonists are the most-studied and most-potent medications for treating tics and Tourette’s disorder. Many other typical antipsychotics such as fluphenazine, thioridazine, trifluoperazine, molindone, and thiothixene also have been used.
In controlled trials, haloperidol improved symptoms by 43 to 66%,18 which was greater than placebo and equal to the effect of fluphenazine and trifluoperazine. Haloperidol, however, demonstrated a higher rate of EPS.
Pimozide’s indication for pediatric Tourette’s disorder applies only to treatmentrefractory cases. In controlled studies, pimozide was at least as effective as haloperidol,18 equal to risperidone14 and less effective than olanzapine.15 Pimozide caused fewer side effects than haloperidol but more than atypical antipsychotics. Pimozide may cause QTc prolongation, and regular ECG monitoring is required.
Despite their efficacy, typical antipsychotics are associated with common and occasionally severe side effects that limit their long-term tolerability.6 Fear of tardive dyskinesia generally limits their use to only severe and treatmentresistant cases.
Step 5: Benzodiazepines. Although controlled trials of tic disorders have not evaluated benzodiazepines, these drugs were effective adjuncts in one case series using haloperidol.6 Anecdotal reports suggest they may reduce tics indirectly by lessening anxiety. Many experienced clinicians use clonazepam, 0.5 to 3 mg/d, or lorazepam, 0.5 to 4 mg/d, to treat Tourette’s disorder. Aside from its anxiolytic effects, clonazepam is also considered a minor mood stabilizer.
Step 6: Other options. Numerous novel medications have been studied in trials of tics and Tourette’s, although most—including the mixed D1/D2 agonist pergolide—have not been proven effective. In an uncontrolled study, the parenteral opioid antagonist naloxone decreased tics at low doses and increased them at higher doses. Botulinum toxin, nicotine, mecamylamine (a nicotine antagonist), baclofen, and flutamide have not proven efficacy in placebo-controlled trials.
Transcranial magnetic stimulation and neurosurgery have been used in patients with severe refractory tics and Tourette’s disorder but are not well-established treatments.
TICS AND COMORBIDITIES
ADHD. When it presents with tics, ADHD is frequently an independent target—or even the main target—of management. Because stimulants may exacerbate tics, nonstimulant medications such as clonidine, guanfacine, or desipramine could be tried first.19 Clonidine has been shown to ameliorate aggression, hyperactivity, and impulsivity but has sedating side effects. Atomoxetine—a nonstimulant ADHD medication—is another option for youth with comorbid tics and ADHD.
In our experience, carefully monitored stimulant trials may also be tried. A recent controlled trial showed that methylphenidate and clonidine, separately and combined, are effective for ADHD and comorbid Tourette’s disorder.20 Stimulants of potential benefit include methylphenidate and amphetamine (not just dextroamphetamine), including their long-acting formulations. Combining stimulants with antipsychotics or clonidine may also be useful.
Table 2
Recommended drugs and dosages for pediatric tics and Tourette’s disorder*
| Class/drug | Starting dosage (mg/d) | Dosage increase interval | Dosage range (mg/d) | Dosing regime | Potency/CYP-450 pathway | Delay to onset | |
|---|---|---|---|---|---|---|---|
| Alpha2 agonists | |||||||
| Clonidine | 0.025-0.05 | 5 to 7 days | 0.1-0.3 | bid or tid (patch every 5 to 7 days) | N/A | 2 to 8 wks | |
| Guanfacine | 0.5 | 5 to 7 days | 0.5-4 | bid to tid | N/A | 2 to 8 wks | |
| Side effects | Common: dry mouth, drowsiness, dizziness, sedation, weakness, skin rashes (patch) Rare: hypotension, bradycardia, conduction delay, rebound symptoms | ||||||
| Atypical antipsychotics | |||||||
| Risperidone | 0.25-1 | 7 to 21 days | 0.5-6 | qd to bid | high/ 2D6, 3A4 | 2 to 4 wks | |
| Olanzapine | 2.5-5 | 7 to 21 days | 2.5-10 | qd to bid | medium/ 1A2, 2D6 | 2 to 4 wks | |
| Ziprasidone | 20 | 7 to 21 days | 40-160 | qd to bid | medium/ 3A4 | 2 to 4 wks | |
| Quetiapine | 12.5-25 | 7 to 21 days | 100-600 | qd to bid | low/ 3A4 | 2 to 4 wks | |
| Side effects | Common: weight gain (especially in youth), sedation Rare: hepatic enzyme elevation, extrapyramidal symptoms (EPS), increased QTc interval (ziprasidone) | ||||||
| Typical antipsychotics | |||||||
| Haloperidol | 0.25-0.5 | 7 to 21 days | 2-10 mg | qd to tid | high/ 2D6, 3A4 | 2 to 4 wks | |
| Pimozide | 0.5 | 7 to 21 days | 1-8 mg | qd to tid | high/ 1A2, 2D6, 3A4 | 2 to 4 wks | |
| Side effects | Common: EPS (acute dystonia, akathisia, parkinsonism), sedation, weight gain, dysphoria, cognitive dulling, increased plasma prolactin Rare: neuroleptic malignant syndrome (potentially fatal: autonomic instability, hyperthermia and muscular rigidity); tardive dyskinesia; increased QTc interval (pimozide) | ||||||
| Tricyclic antidepressants | |||||||
| Desipramine | 25 | 5 to 7 days | 2.5-5 /kg/d | qd to bid | 2D6 | 3 to 6 wks | |
| Nortriptyline | 10 | 5 to 7 days | 0.5-3 /kg/d | qd to bid | 2D6 | 3 to 6 wks | |
| Imipramine | 25 | 5 to 7 days | 2.5-5 /kg/d | qd to bid | 2C19, 2D6, 3A4 | 3 to 6 wks | |
| Clomipramine | 25 | 5 to 7 days | 3 /kg/d | qd to bid | 2C19, 2D6, 3A4 | 3 to 6 wks | |
| Side effects | Common: anticholinergic (dry mouth, blurred vision, constipation); antihistaminic (sedation, weight gain); and antialpha adrenergic (dizziness) Rare: heart palpitations, seizures, hepatic enzyme elevations, increased QTc interval | ||||||
| * Benzodiazepines (clonazepam or lorazepam) may be useful adjuncts; see text for side effects and dosages. Source: Adapted from DSM-IV-TR | |||||||
To control rage attacks, a trial of mood stabilizers or atypical antipsychotics may be combined with standard tic medications.
Tricyclic antidepressants have been used to treat tics—especially in children with comorbid ADHD.21 A case series and controlled study by Singer et al of desipramine and clonidine found no significant impact on tics,22 although this trial was limited by a fixed dose design and few assessment points.
More recently, a double-blind, placebo-controlled trial found a 58% decrease in tic symptoms with desipramine (mean dosage 3.4 mg/kg/d) in patients with tics and ADHD.19 This effect was associated with small increases in heart rate and blood pressure.
Tricyclics’ potential toxicity in overdose and anticholinergic side effects require caution and may limit their use. However, they can be considered as adjuncts in treating chronic tic disorders, especially with comorbid ADHD. Serum levels and ECG monitoring every 3 to 6 months are required to rule out prolonged conduction times and tachycardia. Concurrent methylphenidate use may increase serum desipramine levels, and concurrent pimozide use may increase risk for arrhythmias.
OCD and anxiety disorders. Medically treating anxiety can help indirectly to manage tics, which are sensitive to stress.9 OCD comorbidity is especially common in youth with a family history of Tourette’s disorder.6 Screening for OCD is important, as its secretive symptoms frequently go unnoticed and its prognosis may be poorer with a concurrent tic disorder.
Standard treatment for pediatric OCD is cognitive-behavioral therapy, followed when needed by selective serotonin reuptake inhibitors (SSRIs), then clomipramine. These treatments are added to tic management, with attention to primary and comorbid symptoms. Anecdotal reports suggest that SSRIs occasionally exacerbate tics. Similarly, behavioral side effects are common in younger children treated with SSRIs and may aggravate ADHD symptoms.
Mood disorders. Except for tricyclics, antidepressants have been ineffective at reducing tics/Tourette’s disorder. Tricyclics, however, have not been proven effective in depressed youth, in part because of methodologic limitations in controlled trials. Even so, tricyclics may help some children with tics and major depressive disorder. SSRIs combined with usual tic treatment may also be tried, with monitoring for tic worsening.9 To control rage attacks, a trial of mood stabilizers or atypical antipsychotics may be combined with standard tic medications.
Related resources
- Leckman JF, Cohen DJ (ed). Tourette’s syndrome. Tics, obsessions, compulsions: developmental psychopathology and clinical care. New York: John Wiley & Sons, 1999.
- Jankovic J. Tourette’s syndrome. N Engl J Med 2001;345(16):1184-92.
- Martin A, Scahill L, Charney DS, Leckman JF (ed). Pediatric psychopharmacology: principles and practice. New York: Oxford University Press, 2003.
- Tourette Syndrome Association. www.tsa-usa.org
Drug brand names
- Aripiprazole • Abilify
- Atomoxetine • Strattera
- Desipramine • Norpramin
- Clomipramine • Anafranil
- Clonazepam • Klonopin
- Clonidine • Catapres
- Clozapine • Clozaril
- Desipramine • Norpramin
- Fluphenazine • Permitil, Prolixin
- Guanfacine • Tenex
- Haldoperidol • Haldol
- Imipramine • Tofranil
- Lorazepam • Ativan
- Molindone • Moban
- Nortriptyline • Pamelor
- Olanzapine • Zyprexa
- Pimozide • Orap
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Thioridazine • Mellaril
- Thiothixine • Navane
- Trifluoperazine • Stelazine
- Ziprasidone • Geodon
Disclosure
Dr. Stewart and Loren Gianini report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Geller receives grant/research support from Eli Lilly and Co. and Forest Laboratories Inc., is a consultant to GlaxoSmithKline, and is a speaker for Eli Lilly and Co., Wyeth Pharmaceuticals, Novartis Pharmaceuticals Corp., and Shire Pharmaceuticals Group.
Dr. Spencer receives research/grant support from and is a speaker or consultant for Abbott Laboratories, Ortho-McNeil Pharmaceutical Inc., GlaxoSmithKline, Eli Lilly and Co., Novartis Pharmaceuticals Corp., Pfizer Inc., Shire Pharmaceuticals Group, and Wyeth Pharmaceuticals.
1. Sukhodolsky DG, Scahill L, Zhang H, et al. Disruptive behavior in children with Tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry 2003;42(1):98-105.
2. Jankovic J. Tourette’s syndrome. N Engl J Med 2001;345(16):1184-92.
3. Leckman JF, Goodman WK, Anderson GM, et al. CSF biogenic amines in obsessive-compulsive disorder and Tourette’s syndrome. Neuropsychopharmacology 1995;12:73-86.
4. Braun AR, Stoetter B, Randolph C, et al. The functional neuroanatomy of Tourette’s syndrome: An FDG-PET study: I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology 1993;9:277-91.
5. Moriarty J, Campos D, Schmitz B, et al. Brain perfusion abnormalities in Gilles de la Tourette’s syndrome. Br J Psychiatry 1995;167:249-54.
6. Leckman JF. Cohen DJ (eds). Tourette’s syndrome. Tics, obsessions, compulsions: developmental psychopathology and clinical care. New York: John Wiley & Sons, 1999.
7. Budman C, Bruun R, Park K, Olson M. Rage attacks in children and adolescents with Tourette’s disorder: a pilot study. J Clin Psychiatry 1998;59(11):576-80.
8. Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28(4):566-73.
9. Spencer T, Biederman J, Harding M, et al. Disentangling the overlap between Tourette’s disorder and ADHD. J Child Psychol Psychiatry 1999;39:1037-44.
10. Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998;155:264-71.
11. Peterson AL, Azrin NH. An evaluation of behavioral treatments for Tourette syndrome. Behav Res Ther 1992;30(2):167-74.
12. Leckman JF, Hardin MT, Riddle MA, et al. Clonidine treatment of Gilles de la Tourette’s syndrome. Arch Gen Psychiatry 1991;48(4):324-8.
13. Dion Y, Annable L, Sandor P, Chouinard G. Risperidone in the treatment of Tourette syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacology 2002;22(1):31-9.
14. Bruggeman R, van der Linden C, Buitelaar JK, et al. Risperidone versus pimozide in Tourette’s disorder: a comparative double-blind parallel-group study. J Clin Psychiatry 2001;62(1):50-6.
15. Onofrj M, Paci C, D’Andreamatteo G, Toma L. Olanzapine in severe Gilles de la Tourette syndrome: a 52-week double-blind, cross-over study vs. low-dose pimozide. J Neurol 2000;247:443-6.
16. Sallee FR, Kurlan R, Goetz, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry 2000;39(3):292-9.
17. Parraga HC, Parraga MI, Woodward RL, Fenning PA. Quetiapine treatment of children with Tourette’s syndrome: report of two cases. J Child Adolesc Psychopharmacology 2001;11(2):187-91.
18. Sallee FR, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry 1997;154(8):1057-62.
19. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2002;59(7):649-56.
20. Kurlan R. for the Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: A randomized controlled trial. Neurology 2002;58:527-36.
21. Spencer T, Biederman J, Kerman K, et al. Desipramine treatment of children with attention-deficit/hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 1993;32(2):354-60.
22. Singer HS, Brown J, Quaskey S, et al. The treatment of attentiondeficit hyperactivity disorder in Tourette’s syndrome: A double-blind, placebo-controlled study with clonidine and desipramine. Pediatrics 1995;95(1):74-81.
1. Sukhodolsky DG, Scahill L, Zhang H, et al. Disruptive behavior in children with Tourette’s syndrome: association with ADHD comorbidity, tic severity, and functional impairment. J Am Acad Child Adolesc Psychiatry 2003;42(1):98-105.
2. Jankovic J. Tourette’s syndrome. N Engl J Med 2001;345(16):1184-92.
3. Leckman JF, Goodman WK, Anderson GM, et al. CSF biogenic amines in obsessive-compulsive disorder and Tourette’s syndrome. Neuropsychopharmacology 1995;12:73-86.
4. Braun AR, Stoetter B, Randolph C, et al. The functional neuroanatomy of Tourette’s syndrome: An FDG-PET study: I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology 1993;9:277-91.
5. Moriarty J, Campos D, Schmitz B, et al. Brain perfusion abnormalities in Gilles de la Tourette’s syndrome. Br J Psychiatry 1995;167:249-54.
6. Leckman JF. Cohen DJ (eds). Tourette’s syndrome. Tics, obsessions, compulsions: developmental psychopathology and clinical care. New York: John Wiley & Sons, 1999.
7. Budman C, Bruun R, Park K, Olson M. Rage attacks in children and adolescents with Tourette’s disorder: a pilot study. J Clin Psychiatry 1998;59(11):576-80.
8. Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 1989;28(4):566-73.
9. Spencer T, Biederman J, Harding M, et al. Disentangling the overlap between Tourette’s disorder and ADHD. J Child Psychol Psychiatry 1999;39:1037-44.
10. Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998;155:264-71.
11. Peterson AL, Azrin NH. An evaluation of behavioral treatments for Tourette syndrome. Behav Res Ther 1992;30(2):167-74.
12. Leckman JF, Hardin MT, Riddle MA, et al. Clonidine treatment of Gilles de la Tourette’s syndrome. Arch Gen Psychiatry 1991;48(4):324-8.
13. Dion Y, Annable L, Sandor P, Chouinard G. Risperidone in the treatment of Tourette syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacology 2002;22(1):31-9.
14. Bruggeman R, van der Linden C, Buitelaar JK, et al. Risperidone versus pimozide in Tourette’s disorder: a comparative double-blind parallel-group study. J Clin Psychiatry 2001;62(1):50-6.
15. Onofrj M, Paci C, D’Andreamatteo G, Toma L. Olanzapine in severe Gilles de la Tourette syndrome: a 52-week double-blind, cross-over study vs. low-dose pimozide. J Neurol 2000;247:443-6.
16. Sallee FR, Kurlan R, Goetz, et al. Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry 2000;39(3):292-9.
17. Parraga HC, Parraga MI, Woodward RL, Fenning PA. Quetiapine treatment of children with Tourette’s syndrome: report of two cases. J Child Adolesc Psychopharmacology 2001;11(2):187-91.
18. Sallee FR, Nesbitt L, Jackson C, et al. Relative efficacy of haloperidol and pimozide in children and adolescents with Tourette’s disorder. Am J Psychiatry 1997;154(8):1057-62.
19. Spencer T, Biederman J, Coffey B, et al. A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2002;59(7):649-56.
20. Kurlan R. for the Tourette’s Syndrome Study Group. Treatment of ADHD in children with tics: A randomized controlled trial. Neurology 2002;58:527-36.
21. Spencer T, Biederman J, Kerman K, et al. Desipramine treatment of children with attention-deficit/hyperactivity disorder and tic disorder or Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 1993;32(2):354-60.
22. Singer HS, Brown J, Quaskey S, et al. The treatment of attentiondeficit hyperactivity disorder in Tourette’s syndrome: A double-blind, placebo-controlled study with clonidine and desipramine. Pediatrics 1995;95(1):74-81.
Minding menopause: Psychotropics vs. estrogen? What you need to know now
Psychiatrists are suddenly viewed as experts in treating menopause-related mood problems because of our expertise with using psychotropics. Practically overnight, the Women’s Health Initiative studies1,2 have made women and their doctors think twice about using estrogen. Instead, many are turning to psychiatric medications that have been shown to improve both mood and hot flashes—without estrogen’s potential risks.
Chances are good that after an Ob/Gyn has tried one or two psychotropics without success or with too many side effects, he or she will ask a psychiatrist to consult for certain patients. How well-prepared are you to assume this role?
If your recall of female reproductive physiology from medical school is incomplete, read on about one approach to a perimenopausal patient with depressed mood. This review can help you:
- discuss menopause knowledgeably when other physicians refer their patients to you
- provide effective, up-to-date treatments for menopause-related mood and sexual problems, using psychotropics or hormones, alone or in combination.
Irritable, with no interest in sex
Anne, age 51, has been referred to you for complaints of depressed mood and low libido. She says she has become irritable and snaps easily at her two children and her husband. She has no interest in sex, no urge to masturbate, and has had no sexual intercourse for 6 months.
Table 1
Why mood problems may occur during menopause
| Hypothesis | Explanation |
|---|---|
| Psychodynamic | Onset of menopause is a critical life event and a readjustment of self-concept |
| Sociologic | Mood changes are caused by changing life circumstances at menopause (‘empty nest,’ aging parents, health changes) |
| Domino | Depressed mood is caused by hot flashes due to declining estrogen levels, which cause chronic sleep deprivation with subsequent irritability and memory and mood changes |
| Biochemical | Decreasing estrogen leads to neurochemical changes in the brain (serotonin, dopamine, cholinergic, GABA, norepinephrine) |
Anne also complains of fatigue, dry hair and skin, warm flushes, and painful joints. She has no personal or family history of depression. She is not suicidal but states that she really doesn’t want to live anymore if “this is it.”
HOT FLASHES: A SPARK FOR DEPRESSION
Women who experience their first depression after age 50 do not fit the usual DSM-IV diagnostic criteria for depression. The Massachusetts Women’s Health Study3 found that 52% of women who experience depressed mood in the perimenopause have never had a depression before. This study also found a correlation between a longer perimenopause (>27 months) and increased risk of depressed mood. At the same time, women who have had a prior depression are 4 to 9 times more likely to experience depressive symptoms during perimenopause than those who have never had a depression before.4
The increased mood symptoms may be related to psychodynamic, sociologic, or biochemical factors, or they may result from a domino effect triggered by declining estrogen levels (Table 1). Women who experience vasomotor symptoms such as hot flashes are at 4.6 times greater risk for depression than those who are hot flash-free.5
Hot flashes begin on average at age 51, which is also the average age when natural menopause begins. During menopause, most women (82%) experience hot flashes (suddenly feeling hot and sweating during the day), warm flushes (a sensation of warmth or heat spreading over the skin), and night sweats (Table 2). All women who undergo surgical menopause experience hot flashes.
Hot flashes are moderate to severe for 40% of women who experience them and persist for 5 to 15 years. By definition, moderate to severe hot flashes occur 6 to 10 or more times daily, last 6 to 10 minutes each, and are often preceded by anxiety, palpitations, irritability, nervousness, or panic.
A marriage under stress
Anne says that her husband is angry about the lack of sexual intercourse, and she feels the stress in their marriage. She also is worrying about her children leaving for college and about her mother’s ill health.
She scores 20 on the Beck Depression Inventory, which indicates that she has mild to moderate depression. Her menstrual periods remain regular, but her cycle has shortened from 29 to 24 days. She reports experiencing some hot flashes that wake her at night and says she hasn’t had a good night’s sleep in months.
Laboratory tests show FSH of 25 mIU/mL and inhibin B <45 pg/mL. Her estradiol is 80 pg/mL, which is not yet in the menopausal range of 10 to 20 pg/mL. Her thyroid stimulating hormone (TSH) is normal. Her endocrinologic and reproductive diagnosis is perimenopause.
Table 2
Symptoms of menopause related to decreased estrogen
| Brain | Irritability, mood swings, depressed mood, forgetfulness, low sex interest, sleep problems, decreased well-being |
| Body | Hot flashes, vaginal dryness, painful intercourse, fatigue, joint pain, pain with orgasm, bladder dysfunction |
TREATING HOT FLASHES IMPROVES MOOD
Until July 2002, estrogen was standard treatment for controlling hot flashes in patients such as Anne. Then the Women’s Health Initiative trial reported that estrogen’s health risks—heart attack, stroke, breast cancer, and blood clots—exceeded potential benefits during 5 years of therapy. As a result, fewer women want to take estrogen,6 and many Ob/Gyns are advising patients to get through menopause without hormones if they can.
For mild hot flashes—one to three per day—patients may only need vitamin E, 800 mg/d, and deep relaxation breathing to “rev down” the sympathetic nervous system when a hot flash occurs.
For moderate to severe hot flashes—four to 10 or more per day—estrogen replacement is the most effective therapy. Estradiol, 1 mg/d, reduces hot flashes by approximately 80 to 90%.7 Many small studies have shown that patients’ mood often improves as estrogen reduces their hot flashes.8 The recent Women’s Health Initiative Quality-of-Life study, however, reported that estrogen plus progestin did not improve mood in women ages 50 to 54 with moderate-to-severe vasomotor symptoms, even though hot flashes were reduced and sleep may have improved.9
New drugs of choice. Because of estrogen’s effectiveness in controlling hot flashes, some women and their doctors may choose to use it briefly (18 to 24 months). For others, psychotropics are becoming the drugs of choice for mood disorders with moderate to severe hot flashes.
The serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine, 75 or 150 mg/d, has been shown to reduce hot flashes by 60 to 70%.10 A new trial is investigating whether duloxetine—an SNRI awaiting FDA approval—also reduces hot flashes. Other useful agents that have been shown to reduce hot flashes by 50% or more include:
- selective serotonin reuptake inhibitors (SSRIs) paroxetine CR, 12.5 mg/d to 25 mg/d,11 citalopram, 20 to 60 mg/d,12 and fluoxetine, 20 mg/d13
- gabapentin, 900 mg/d.14
For hot flashes and moderate to major depression, try an SNRI or SSRI first (see Algorithm), but consider the possible effects on sexual function. All SNRIs and SSRIs have sexual side effects, including anorgasmia and loss of libido in women and men. Among the psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
Mood improves, but still no libido
You and Ann decide on a trial of the SNRI venlafaxine, 75 mg/d, to treat her hot flashes and depressed mood. Four weeks later, her hot flashes are reduced by 50% in frequency and her mood has improved (Beck Depression Inventory score is now 10). She is feeling much better and wishes to continue taking the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
TREATING LOW INTEREST IN SEX
Being angry with one’s partner is the number-one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.15 Other agents the FDA is reviewing for erectile dysfunction—such as tadalafil and vardenafil—may also help arousal problems in women.
Understanding how hormones affect female sexual desire also may help you decide what advice to give Anne and how you and her Ob/Gyn coordinate her care. For example, you might treat her sexual complaints and relationship problems while the Ob/Gyn manages symptoms of the vagina, uterus, and breast.
HOW TESTOSTERONE AFFECTS SEXUAL DESIRE
Testosterone is the hormone of sexual desire in men and women. Other female androgens include androstenedione, androstenediol, 5 α-dihydrotestosterone (DHT), dihydroepiandrosterone (DHEA), and its sulfate (DHEA-S). Premenopausal women produce these androgens in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%).
Average daily serum testosterone concentrations decline in women between ages 20 and 50. Lower levels are also seen with estrogen replacement therapy or oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Women who undergo total hysterectomy with bilateral oophorectomy experience a sudden 50% loss of testosterone and an 80% decline in estradiol.16
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—than they had when they were in their 30s.17 The percentage of women reporting low libido increases with age until menopause, from 30% at age 30 to about 50% at age 50. Then the rate declines to 27% in women age 50 to 59.18 After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire.
Oral estrogen replacement therapy reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in a menopausal woman.19 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.20
Female androgen deficiency. A number of papers have been published on female androgen deficiency syndrome (FADS).21 Its diagnosis requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
TREATING TESTOSTERONE DEFICIENCY
Benefits of replacement therapy. Replacing testosterone in women with FADS can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.22 Muscle and bone stimulation and decreased hot flashes are also reported.23 Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Risks of replacement therapy. Androgen replacement therapy does carry some risks, which need to be discussed with the patient. Testosterone may lower levels of beneficial HDL cholesterol, so get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level <45 mg/dL.
Algorithm Managing mood and libido problems during perimenopause
A meta-analysis of eight clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg/d of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone dosages.24
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle one-third of the cycle—on days 10 to 16, counting the first day of menstrual bleeding as day 1.25
- Oral contraceptives also decrease androgen production by the ovary and can result in low libido in some women.26
Tests developed to measure testosterone levels in men are not sensitive enough to accurately measure women’s naturally lower serum concentrations, let alone the even lower levels characteristic of female androgen or testosterone deficiency. New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.27
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEAS. Measuring SHBG will help you determine the free, biologically active testosterone level and calculate the Free Androgen Index (FAI) for women (Table 3).28
Table 3
Free androgen index (FAI) values in women, by age
| Replacing a woman’s bioactive testosterone to the normal free androgen index range for her age may improve low libido. | |
| How to calculate FAI | |
| Total testosterone in nmol/L (total testosterone in ng/ml X 0.0347 X 100), divided by sex hormone-binding globulin (SHBG) in nmol/L. | |
| Age | Normal range |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Source: Guay et al, reference 28. | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 (normal range, 1.78 to 2.86).
In collaboration with her Ob/Gyn, you and Anne decide to start her on testosterone replacement therapy. You prescribe Androgel, starting at 1/7th of a 2.5-mg foil packet (0.35 mg/d of testosterone), and instruct her to rate her sexual energy daily, using a Sexual Energy Scale.
TESTOSTERONE CHOICES FOR WOMEN
Replacing a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options include:
- methyl testosterone sublingual pills, 0.5 mg/d, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels—unless you order the very expensive test that is specific for methyl testosterone.
- 2% vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption
- Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg/d or one-seventh of the 2.5 mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations—including a testosterone patch for women and a gel in female-sized doses.
Using the Sexual Energy Scale. To monitor for a therapeutic response, ask the patient to use the Sexual Energy Scale (Figure 1).29,30 Instruct her to define her “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Her “1” would be when she felt the worst sexually and had the least desire.
Giving supplemental estrogen. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS, which contains 0.625 mg esterified estrogens and 1.25 mg of methyl testosterone. Add a progestin if the patient is postmenopausal and has not had a hysterectomy, to protect the uterus from endometrial hyperplasia.
Women with vaginal dryness also need supplemental estrogen, which can be applied vaginally (such as Premarin cream or Estrace cream). A vaginal lubricant is not sufficient to avoid age-related vaginal atrophy, which may make intercourse difficult or impossible.
Figure 1 How to use the Sexual Energy Scale to monitor response to therapy
Libido improves modestly
Ann returns in 4 weeks with gradually improving sex drive (Sexual Energy Score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but also did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low. You increase the Androgel dosage to 1/5th of a 2.5 mg packet (0.5 mg/day) and continue to monitor Anne’s Sexual Energy Scale ratings at monthly follow-up visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Louann Brizendine, MD
Medicine’s understanding of menopause’s physiologic and psychological consequences is changing, just as the “baby-boom” generation is navigating this passage called the change of life. Many midlife women are unaware that the menopause transition does not begin around age 50 but spans 30 years—from ages 35 to 65. Natural menopause begins 15 years before and ends 15 years after menstruation ceases—as the brain and tissues adjust to first fluctuating then decreased estrogen levels. It occurs in three phases—early menopause, perimenopause, and late menopause—that reflect a progression of hormone changes.
EARLY MENOPAUSE: OVULATION ACCELERATES
At approximately age 35, the ovaries start producing lower levels of inhibin—a glycoprotein that inhibits pituitary production of follicle-stimulating hormone (FSH) (Figure 2). Less inhibin means less negative feedback to the pituitary and an increase in pituitary FSH production. More FSH means more activin—an ovarian glycoprotein that stimulates ripening of eggs—and so ovulation begins to accelerate at approximately age 36. Activin stimulates more and more eggs in the ovary to develop faster and faster.
By age 37, the ovarian egg reserve starts to decline, and—because FSH has increased—the follicle is driven to produce greater amounts of estrogen. Estrogen serum levels in fertile women average 100 pg/mL. During perimenopause, estrogen levels sometimes soar to 300, 400, or even 500 pg/mL, then may crash down to 50 to 80 pg/mL. These wild fluctuations are thought to trigger headaches, sleep disturbance, mood swings, and sexual complaints in some women.
Hysterectomy and mood symptoms. Women in their early 40s are exposed to high levels of estrogen in some menstrual cycles and low levels in others. Excess estrogen thickens the endometrium—causing heavier bleeding—and stimulates fibroid growth, which is the leading reason for hysterectomies.
One in four American women undergoes surgical menopause. The average age of the 700,000 U.S. women who undergo hysterectomy each year is 40 to 44. Women who have had a hysterectomy and have mood symptoms and sexual adjustment problems are likely to see psychiatrists earlier than women who undergo a more gradual natural menopause.
PERIMENOPAUSE: HOT FLASHES, DRY VAGINA
At ages 45 to 55, most women (90%) who have not had a hysterectomy start to cycle irregularly, tending at first toward shorter cycles and then skipping periods. Some periods are heavier and some lighter than usual. The remaining 10% of women continue to cycle regularly until their menstrual periods stop abruptly.
Many women notice temperature dysregulation during perimenopause. When they exercise, their cool-down times may double. Menopausal symptoms such as hot flashes occur when estrogen levels drop below the point that some researchers call a woman’s “estrogen set point.”
Screening for estrogen decline. When you see a patient in your office, you can often determine whether her affective symptoms—irritability, mood swings, depressed mood, and forgetfulness—might be related to estrogen decline by asking two screening questions:
- Are you having any warm flushes or hot flashes?
- Do you have vaginal dryness?
Figure 2 Normal female reproductive cycle: The rhythm of the hypothalamic-pituitary-ovarian axis
LATE MENOPAUSE: ESTROGEN LOW, MOOD UP
During late menopause, approximately age 55 and older, women commonly complain of vaginal dryness, hot flashes, night sweats, sleep problems, and fatigue. Sexual interest may decrease, and a decline in sexual activity can become a problem for some couples. Asking “How is your sex life?” often will open a discussion of the couple’s sexual and emotional relationship.
Other physiologic changes caused by estrogen and androgen deficiency include thinning body hair—including pubic, auxiliary, and leg hair—decreased body odor, thinning skin, wrinkling skin, and decreasing bone density.
Table 4
Three phases of menopause: A 30-year process
| Early Ages 35 to 45 | Middle (perimenopause) Ages 46 to 55 | Late Ages 56 to 65+ |
|---|---|---|
| Physiologic changes | ||
| Ovary starts producing less inhibin 15 years before menses stop | Irregular menstrual cycles, with shorter cycles, skipping periods for 90% of women | Depletion of eggs and follicle |
| Decreased inhibin increases FSH and stimulates follicle to produce more estrogen | Some periods heavier, some lighter than usual | |
| Increased estrogen thickens endometrium and leads to heavier menstrual bleeding and increased risk of fibroids | ||
| Increased FSH produces more activin, which makes eggs develop faster and accelerates egg depletion | ||
| Lab values | ||
| Menses: Normal | Cycle shorter (24-26 days) | None for >12 months |
| FSH day 3: 10-25 mIU/mL | 20-30 mIU/mL | 50-90 mIU/mL |
| Estradiol day 3: 40-200 pg/mL | 40-200 pg/mL | 10-20 pg/mL |
| Inhibin B day 3: Varies | <45 pg/mL | 0 |
| Symptoms | ||
| Headaches, sleep disturbances, mood swings, urinary problems, sexual complaints | Warm flushes, hot flashes, night sweats in 82% of women (moderate to severe in 40%) | Vaginal dryness, hot flashes, night sweats, sleep problems, fatigue, sexual interest changes, thinning body hair (pubic, legs, axillary), decreased body odor, thinning skin, wrinkling skin, decreasing bone density, BUT mood starts to stabilize |
| FSH: follicle-stimulating hormone | ||
Urinary symptoms. Bladder problems and urinary symptoms are persistent symptoms of menopause for 75% of women. Although we psychiatrists don’t review the urinary system, it is important to remember that embarrassment because of urinary incontinence during sex may have a lot to do with a woman’s “loss of interest” in sexual intercourse.
Despite sometimes-difficult physiologic changes, the good news for many women is that mood symptoms start to stabilize after perimenopause (Table 4). Women whose moods are very responsive to hormonal fluctuations—such as those with severe premenstrual syndrome or premenstrual dysphoric disorder—sometimes get much better after menopause.
Related resources
- The Women’s Health Site. Duke Academic Program in Women’s Health. www.thewomenshealthsite.org
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Paroxetine • Paxil
- Sildenafil • Viagra
- Venlafaxine • Effexor
Disclosure
Dr. Brizendine reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric 2003;6(suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med 2003;348:645-50.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002;9(6):392-8.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am 1997;26(2):279-94.
6. Seppa N. Hormone therapy falls out of favor. Science News 2002;162:61.-
7. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am 2003;32(2):325-36.
8. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44(9):798-811.
9. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003;348(19):1839-54.
10. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002;29(1):33-40.
11. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003;289(21):2827-34.
12. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry 2003;64(4):473-9.
13. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20(6):1578-83.
14. Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101(2):337-45.
15. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108(6):623-8.
16. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric 2002;5(4):357-65.
17. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol 2003;85(2-5):363-6.
18. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44.
19. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril 2003;79(6):1341-52.
20. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol 1997;90(6):995-8.
21. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77(4):660-5.
22. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab 2003;17(1):165-75.
23. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol 2002;20(2):106-10.
24. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause 1999;6(3):216-24.
25. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums 2003;8(March):3.-
26. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995;52(6):363-9.
27. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril 2002;77(suppl 4):S83-8.
28. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther 2002;28(suppl 1):129-42.
29. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull 1997;33(4):761-5.
30. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction (poster presentation). Waikoloa, HI: North American Society for Psychosocial Obstetrics and Gynecology, 2001.
Psychiatrists are suddenly viewed as experts in treating menopause-related mood problems because of our expertise with using psychotropics. Practically overnight, the Women’s Health Initiative studies1,2 have made women and their doctors think twice about using estrogen. Instead, many are turning to psychiatric medications that have been shown to improve both mood and hot flashes—without estrogen’s potential risks.
Chances are good that after an Ob/Gyn has tried one or two psychotropics without success or with too many side effects, he or she will ask a psychiatrist to consult for certain patients. How well-prepared are you to assume this role?
If your recall of female reproductive physiology from medical school is incomplete, read on about one approach to a perimenopausal patient with depressed mood. This review can help you:
- discuss menopause knowledgeably when other physicians refer their patients to you
- provide effective, up-to-date treatments for menopause-related mood and sexual problems, using psychotropics or hormones, alone or in combination.
Irritable, with no interest in sex
Anne, age 51, has been referred to you for complaints of depressed mood and low libido. She says she has become irritable and snaps easily at her two children and her husband. She has no interest in sex, no urge to masturbate, and has had no sexual intercourse for 6 months.
Table 1
Why mood problems may occur during menopause
| Hypothesis | Explanation |
|---|---|
| Psychodynamic | Onset of menopause is a critical life event and a readjustment of self-concept |
| Sociologic | Mood changes are caused by changing life circumstances at menopause (‘empty nest,’ aging parents, health changes) |
| Domino | Depressed mood is caused by hot flashes due to declining estrogen levels, which cause chronic sleep deprivation with subsequent irritability and memory and mood changes |
| Biochemical | Decreasing estrogen leads to neurochemical changes in the brain (serotonin, dopamine, cholinergic, GABA, norepinephrine) |
Anne also complains of fatigue, dry hair and skin, warm flushes, and painful joints. She has no personal or family history of depression. She is not suicidal but states that she really doesn’t want to live anymore if “this is it.”
HOT FLASHES: A SPARK FOR DEPRESSION
Women who experience their first depression after age 50 do not fit the usual DSM-IV diagnostic criteria for depression. The Massachusetts Women’s Health Study3 found that 52% of women who experience depressed mood in the perimenopause have never had a depression before. This study also found a correlation between a longer perimenopause (>27 months) and increased risk of depressed mood. At the same time, women who have had a prior depression are 4 to 9 times more likely to experience depressive symptoms during perimenopause than those who have never had a depression before.4
The increased mood symptoms may be related to psychodynamic, sociologic, or biochemical factors, or they may result from a domino effect triggered by declining estrogen levels (Table 1). Women who experience vasomotor symptoms such as hot flashes are at 4.6 times greater risk for depression than those who are hot flash-free.5
Hot flashes begin on average at age 51, which is also the average age when natural menopause begins. During menopause, most women (82%) experience hot flashes (suddenly feeling hot and sweating during the day), warm flushes (a sensation of warmth or heat spreading over the skin), and night sweats (Table 2). All women who undergo surgical menopause experience hot flashes.
Hot flashes are moderate to severe for 40% of women who experience them and persist for 5 to 15 years. By definition, moderate to severe hot flashes occur 6 to 10 or more times daily, last 6 to 10 minutes each, and are often preceded by anxiety, palpitations, irritability, nervousness, or panic.
A marriage under stress
Anne says that her husband is angry about the lack of sexual intercourse, and she feels the stress in their marriage. She also is worrying about her children leaving for college and about her mother’s ill health.
She scores 20 on the Beck Depression Inventory, which indicates that she has mild to moderate depression. Her menstrual periods remain regular, but her cycle has shortened from 29 to 24 days. She reports experiencing some hot flashes that wake her at night and says she hasn’t had a good night’s sleep in months.
Laboratory tests show FSH of 25 mIU/mL and inhibin B <45 pg/mL. Her estradiol is 80 pg/mL, which is not yet in the menopausal range of 10 to 20 pg/mL. Her thyroid stimulating hormone (TSH) is normal. Her endocrinologic and reproductive diagnosis is perimenopause.
Table 2
Symptoms of menopause related to decreased estrogen
| Brain | Irritability, mood swings, depressed mood, forgetfulness, low sex interest, sleep problems, decreased well-being |
| Body | Hot flashes, vaginal dryness, painful intercourse, fatigue, joint pain, pain with orgasm, bladder dysfunction |
TREATING HOT FLASHES IMPROVES MOOD
Until July 2002, estrogen was standard treatment for controlling hot flashes in patients such as Anne. Then the Women’s Health Initiative trial reported that estrogen’s health risks—heart attack, stroke, breast cancer, and blood clots—exceeded potential benefits during 5 years of therapy. As a result, fewer women want to take estrogen,6 and many Ob/Gyns are advising patients to get through menopause without hormones if they can.
For mild hot flashes—one to three per day—patients may only need vitamin E, 800 mg/d, and deep relaxation breathing to “rev down” the sympathetic nervous system when a hot flash occurs.
For moderate to severe hot flashes—four to 10 or more per day—estrogen replacement is the most effective therapy. Estradiol, 1 mg/d, reduces hot flashes by approximately 80 to 90%.7 Many small studies have shown that patients’ mood often improves as estrogen reduces their hot flashes.8 The recent Women’s Health Initiative Quality-of-Life study, however, reported that estrogen plus progestin did not improve mood in women ages 50 to 54 with moderate-to-severe vasomotor symptoms, even though hot flashes were reduced and sleep may have improved.9
New drugs of choice. Because of estrogen’s effectiveness in controlling hot flashes, some women and their doctors may choose to use it briefly (18 to 24 months). For others, psychotropics are becoming the drugs of choice for mood disorders with moderate to severe hot flashes.
The serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine, 75 or 150 mg/d, has been shown to reduce hot flashes by 60 to 70%.10 A new trial is investigating whether duloxetine—an SNRI awaiting FDA approval—also reduces hot flashes. Other useful agents that have been shown to reduce hot flashes by 50% or more include:
- selective serotonin reuptake inhibitors (SSRIs) paroxetine CR, 12.5 mg/d to 25 mg/d,11 citalopram, 20 to 60 mg/d,12 and fluoxetine, 20 mg/d13
- gabapentin, 900 mg/d.14
For hot flashes and moderate to major depression, try an SNRI or SSRI first (see Algorithm), but consider the possible effects on sexual function. All SNRIs and SSRIs have sexual side effects, including anorgasmia and loss of libido in women and men. Among the psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
Mood improves, but still no libido
You and Ann decide on a trial of the SNRI venlafaxine, 75 mg/d, to treat her hot flashes and depressed mood. Four weeks later, her hot flashes are reduced by 50% in frequency and her mood has improved (Beck Depression Inventory score is now 10). She is feeling much better and wishes to continue taking the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
TREATING LOW INTEREST IN SEX
Being angry with one’s partner is the number-one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.15 Other agents the FDA is reviewing for erectile dysfunction—such as tadalafil and vardenafil—may also help arousal problems in women.
Understanding how hormones affect female sexual desire also may help you decide what advice to give Anne and how you and her Ob/Gyn coordinate her care. For example, you might treat her sexual complaints and relationship problems while the Ob/Gyn manages symptoms of the vagina, uterus, and breast.
HOW TESTOSTERONE AFFECTS SEXUAL DESIRE
Testosterone is the hormone of sexual desire in men and women. Other female androgens include androstenedione, androstenediol, 5 α-dihydrotestosterone (DHT), dihydroepiandrosterone (DHEA), and its sulfate (DHEA-S). Premenopausal women produce these androgens in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%).
Average daily serum testosterone concentrations decline in women between ages 20 and 50. Lower levels are also seen with estrogen replacement therapy or oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Women who undergo total hysterectomy with bilateral oophorectomy experience a sudden 50% loss of testosterone and an 80% decline in estradiol.16
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—than they had when they were in their 30s.17 The percentage of women reporting low libido increases with age until menopause, from 30% at age 30 to about 50% at age 50. Then the rate declines to 27% in women age 50 to 59.18 After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire.
Oral estrogen replacement therapy reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in a menopausal woman.19 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.20
Female androgen deficiency. A number of papers have been published on female androgen deficiency syndrome (FADS).21 Its diagnosis requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
TREATING TESTOSTERONE DEFICIENCY
Benefits of replacement therapy. Replacing testosterone in women with FADS can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.22 Muscle and bone stimulation and decreased hot flashes are also reported.23 Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Risks of replacement therapy. Androgen replacement therapy does carry some risks, which need to be discussed with the patient. Testosterone may lower levels of beneficial HDL cholesterol, so get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level <45 mg/dL.
Algorithm Managing mood and libido problems during perimenopause
A meta-analysis of eight clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg/d of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone dosages.24
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle one-third of the cycle—on days 10 to 16, counting the first day of menstrual bleeding as day 1.25
- Oral contraceptives also decrease androgen production by the ovary and can result in low libido in some women.26
Tests developed to measure testosterone levels in men are not sensitive enough to accurately measure women’s naturally lower serum concentrations, let alone the even lower levels characteristic of female androgen or testosterone deficiency. New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.27
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEAS. Measuring SHBG will help you determine the free, biologically active testosterone level and calculate the Free Androgen Index (FAI) for women (Table 3).28
Table 3
Free androgen index (FAI) values in women, by age
| Replacing a woman’s bioactive testosterone to the normal free androgen index range for her age may improve low libido. | |
| How to calculate FAI | |
| Total testosterone in nmol/L (total testosterone in ng/ml X 0.0347 X 100), divided by sex hormone-binding globulin (SHBG) in nmol/L. | |
| Age | Normal range |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Source: Guay et al, reference 28. | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 (normal range, 1.78 to 2.86).
In collaboration with her Ob/Gyn, you and Anne decide to start her on testosterone replacement therapy. You prescribe Androgel, starting at 1/7th of a 2.5-mg foil packet (0.35 mg/d of testosterone), and instruct her to rate her sexual energy daily, using a Sexual Energy Scale.
TESTOSTERONE CHOICES FOR WOMEN
Replacing a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options include:
- methyl testosterone sublingual pills, 0.5 mg/d, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels—unless you order the very expensive test that is specific for methyl testosterone.
- 2% vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption
- Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg/d or one-seventh of the 2.5 mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations—including a testosterone patch for women and a gel in female-sized doses.
Using the Sexual Energy Scale. To monitor for a therapeutic response, ask the patient to use the Sexual Energy Scale (Figure 1).29,30 Instruct her to define her “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Her “1” would be when she felt the worst sexually and had the least desire.
Giving supplemental estrogen. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS, which contains 0.625 mg esterified estrogens and 1.25 mg of methyl testosterone. Add a progestin if the patient is postmenopausal and has not had a hysterectomy, to protect the uterus from endometrial hyperplasia.
Women with vaginal dryness also need supplemental estrogen, which can be applied vaginally (such as Premarin cream or Estrace cream). A vaginal lubricant is not sufficient to avoid age-related vaginal atrophy, which may make intercourse difficult or impossible.
Figure 1 How to use the Sexual Energy Scale to monitor response to therapy
Libido improves modestly
Ann returns in 4 weeks with gradually improving sex drive (Sexual Energy Score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but also did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low. You increase the Androgel dosage to 1/5th of a 2.5 mg packet (0.5 mg/day) and continue to monitor Anne’s Sexual Energy Scale ratings at monthly follow-up visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Louann Brizendine, MD
Medicine’s understanding of menopause’s physiologic and psychological consequences is changing, just as the “baby-boom” generation is navigating this passage called the change of life. Many midlife women are unaware that the menopause transition does not begin around age 50 but spans 30 years—from ages 35 to 65. Natural menopause begins 15 years before and ends 15 years after menstruation ceases—as the brain and tissues adjust to first fluctuating then decreased estrogen levels. It occurs in three phases—early menopause, perimenopause, and late menopause—that reflect a progression of hormone changes.
EARLY MENOPAUSE: OVULATION ACCELERATES
At approximately age 35, the ovaries start producing lower levels of inhibin—a glycoprotein that inhibits pituitary production of follicle-stimulating hormone (FSH) (Figure 2). Less inhibin means less negative feedback to the pituitary and an increase in pituitary FSH production. More FSH means more activin—an ovarian glycoprotein that stimulates ripening of eggs—and so ovulation begins to accelerate at approximately age 36. Activin stimulates more and more eggs in the ovary to develop faster and faster.
By age 37, the ovarian egg reserve starts to decline, and—because FSH has increased—the follicle is driven to produce greater amounts of estrogen. Estrogen serum levels in fertile women average 100 pg/mL. During perimenopause, estrogen levels sometimes soar to 300, 400, or even 500 pg/mL, then may crash down to 50 to 80 pg/mL. These wild fluctuations are thought to trigger headaches, sleep disturbance, mood swings, and sexual complaints in some women.
Hysterectomy and mood symptoms. Women in their early 40s are exposed to high levels of estrogen in some menstrual cycles and low levels in others. Excess estrogen thickens the endometrium—causing heavier bleeding—and stimulates fibroid growth, which is the leading reason for hysterectomies.
One in four American women undergoes surgical menopause. The average age of the 700,000 U.S. women who undergo hysterectomy each year is 40 to 44. Women who have had a hysterectomy and have mood symptoms and sexual adjustment problems are likely to see psychiatrists earlier than women who undergo a more gradual natural menopause.
PERIMENOPAUSE: HOT FLASHES, DRY VAGINA
At ages 45 to 55, most women (90%) who have not had a hysterectomy start to cycle irregularly, tending at first toward shorter cycles and then skipping periods. Some periods are heavier and some lighter than usual. The remaining 10% of women continue to cycle regularly until their menstrual periods stop abruptly.
Many women notice temperature dysregulation during perimenopause. When they exercise, their cool-down times may double. Menopausal symptoms such as hot flashes occur when estrogen levels drop below the point that some researchers call a woman’s “estrogen set point.”
Screening for estrogen decline. When you see a patient in your office, you can often determine whether her affective symptoms—irritability, mood swings, depressed mood, and forgetfulness—might be related to estrogen decline by asking two screening questions:
- Are you having any warm flushes or hot flashes?
- Do you have vaginal dryness?
Figure 2 Normal female reproductive cycle: The rhythm of the hypothalamic-pituitary-ovarian axis
LATE MENOPAUSE: ESTROGEN LOW, MOOD UP
During late menopause, approximately age 55 and older, women commonly complain of vaginal dryness, hot flashes, night sweats, sleep problems, and fatigue. Sexual interest may decrease, and a decline in sexual activity can become a problem for some couples. Asking “How is your sex life?” often will open a discussion of the couple’s sexual and emotional relationship.
Other physiologic changes caused by estrogen and androgen deficiency include thinning body hair—including pubic, auxiliary, and leg hair—decreased body odor, thinning skin, wrinkling skin, and decreasing bone density.
Table 4
Three phases of menopause: A 30-year process
| Early Ages 35 to 45 | Middle (perimenopause) Ages 46 to 55 | Late Ages 56 to 65+ |
|---|---|---|
| Physiologic changes | ||
| Ovary starts producing less inhibin 15 years before menses stop | Irregular menstrual cycles, with shorter cycles, skipping periods for 90% of women | Depletion of eggs and follicle |
| Decreased inhibin increases FSH and stimulates follicle to produce more estrogen | Some periods heavier, some lighter than usual | |
| Increased estrogen thickens endometrium and leads to heavier menstrual bleeding and increased risk of fibroids | ||
| Increased FSH produces more activin, which makes eggs develop faster and accelerates egg depletion | ||
| Lab values | ||
| Menses: Normal | Cycle shorter (24-26 days) | None for >12 months |
| FSH day 3: 10-25 mIU/mL | 20-30 mIU/mL | 50-90 mIU/mL |
| Estradiol day 3: 40-200 pg/mL | 40-200 pg/mL | 10-20 pg/mL |
| Inhibin B day 3: Varies | <45 pg/mL | 0 |
| Symptoms | ||
| Headaches, sleep disturbances, mood swings, urinary problems, sexual complaints | Warm flushes, hot flashes, night sweats in 82% of women (moderate to severe in 40%) | Vaginal dryness, hot flashes, night sweats, sleep problems, fatigue, sexual interest changes, thinning body hair (pubic, legs, axillary), decreased body odor, thinning skin, wrinkling skin, decreasing bone density, BUT mood starts to stabilize |
| FSH: follicle-stimulating hormone | ||
Urinary symptoms. Bladder problems and urinary symptoms are persistent symptoms of menopause for 75% of women. Although we psychiatrists don’t review the urinary system, it is important to remember that embarrassment because of urinary incontinence during sex may have a lot to do with a woman’s “loss of interest” in sexual intercourse.
Despite sometimes-difficult physiologic changes, the good news for many women is that mood symptoms start to stabilize after perimenopause (Table 4). Women whose moods are very responsive to hormonal fluctuations—such as those with severe premenstrual syndrome or premenstrual dysphoric disorder—sometimes get much better after menopause.
Related resources
- The Women’s Health Site. Duke Academic Program in Women’s Health. www.thewomenshealthsite.org
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Paroxetine • Paxil
- Sildenafil • Viagra
- Venlafaxine • Effexor
Disclosure
Dr. Brizendine reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Psychiatrists are suddenly viewed as experts in treating menopause-related mood problems because of our expertise with using psychotropics. Practically overnight, the Women’s Health Initiative studies1,2 have made women and their doctors think twice about using estrogen. Instead, many are turning to psychiatric medications that have been shown to improve both mood and hot flashes—without estrogen’s potential risks.
Chances are good that after an Ob/Gyn has tried one or two psychotropics without success or with too many side effects, he or she will ask a psychiatrist to consult for certain patients. How well-prepared are you to assume this role?
If your recall of female reproductive physiology from medical school is incomplete, read on about one approach to a perimenopausal patient with depressed mood. This review can help you:
- discuss menopause knowledgeably when other physicians refer their patients to you
- provide effective, up-to-date treatments for menopause-related mood and sexual problems, using psychotropics or hormones, alone or in combination.
Irritable, with no interest in sex
Anne, age 51, has been referred to you for complaints of depressed mood and low libido. She says she has become irritable and snaps easily at her two children and her husband. She has no interest in sex, no urge to masturbate, and has had no sexual intercourse for 6 months.
Table 1
Why mood problems may occur during menopause
| Hypothesis | Explanation |
|---|---|
| Psychodynamic | Onset of menopause is a critical life event and a readjustment of self-concept |
| Sociologic | Mood changes are caused by changing life circumstances at menopause (‘empty nest,’ aging parents, health changes) |
| Domino | Depressed mood is caused by hot flashes due to declining estrogen levels, which cause chronic sleep deprivation with subsequent irritability and memory and mood changes |
| Biochemical | Decreasing estrogen leads to neurochemical changes in the brain (serotonin, dopamine, cholinergic, GABA, norepinephrine) |
Anne also complains of fatigue, dry hair and skin, warm flushes, and painful joints. She has no personal or family history of depression. She is not suicidal but states that she really doesn’t want to live anymore if “this is it.”
HOT FLASHES: A SPARK FOR DEPRESSION
Women who experience their first depression after age 50 do not fit the usual DSM-IV diagnostic criteria for depression. The Massachusetts Women’s Health Study3 found that 52% of women who experience depressed mood in the perimenopause have never had a depression before. This study also found a correlation between a longer perimenopause (>27 months) and increased risk of depressed mood. At the same time, women who have had a prior depression are 4 to 9 times more likely to experience depressive symptoms during perimenopause than those who have never had a depression before.4
The increased mood symptoms may be related to psychodynamic, sociologic, or biochemical factors, or they may result from a domino effect triggered by declining estrogen levels (Table 1). Women who experience vasomotor symptoms such as hot flashes are at 4.6 times greater risk for depression than those who are hot flash-free.5
Hot flashes begin on average at age 51, which is also the average age when natural menopause begins. During menopause, most women (82%) experience hot flashes (suddenly feeling hot and sweating during the day), warm flushes (a sensation of warmth or heat spreading over the skin), and night sweats (Table 2). All women who undergo surgical menopause experience hot flashes.
Hot flashes are moderate to severe for 40% of women who experience them and persist for 5 to 15 years. By definition, moderate to severe hot flashes occur 6 to 10 or more times daily, last 6 to 10 minutes each, and are often preceded by anxiety, palpitations, irritability, nervousness, or panic.
A marriage under stress
Anne says that her husband is angry about the lack of sexual intercourse, and she feels the stress in their marriage. She also is worrying about her children leaving for college and about her mother’s ill health.
She scores 20 on the Beck Depression Inventory, which indicates that she has mild to moderate depression. Her menstrual periods remain regular, but her cycle has shortened from 29 to 24 days. She reports experiencing some hot flashes that wake her at night and says she hasn’t had a good night’s sleep in months.
Laboratory tests show FSH of 25 mIU/mL and inhibin B <45 pg/mL. Her estradiol is 80 pg/mL, which is not yet in the menopausal range of 10 to 20 pg/mL. Her thyroid stimulating hormone (TSH) is normal. Her endocrinologic and reproductive diagnosis is perimenopause.
Table 2
Symptoms of menopause related to decreased estrogen
| Brain | Irritability, mood swings, depressed mood, forgetfulness, low sex interest, sleep problems, decreased well-being |
| Body | Hot flashes, vaginal dryness, painful intercourse, fatigue, joint pain, pain with orgasm, bladder dysfunction |
TREATING HOT FLASHES IMPROVES MOOD
Until July 2002, estrogen was standard treatment for controlling hot flashes in patients such as Anne. Then the Women’s Health Initiative trial reported that estrogen’s health risks—heart attack, stroke, breast cancer, and blood clots—exceeded potential benefits during 5 years of therapy. As a result, fewer women want to take estrogen,6 and many Ob/Gyns are advising patients to get through menopause without hormones if they can.
For mild hot flashes—one to three per day—patients may only need vitamin E, 800 mg/d, and deep relaxation breathing to “rev down” the sympathetic nervous system when a hot flash occurs.
For moderate to severe hot flashes—four to 10 or more per day—estrogen replacement is the most effective therapy. Estradiol, 1 mg/d, reduces hot flashes by approximately 80 to 90%.7 Many small studies have shown that patients’ mood often improves as estrogen reduces their hot flashes.8 The recent Women’s Health Initiative Quality-of-Life study, however, reported that estrogen plus progestin did not improve mood in women ages 50 to 54 with moderate-to-severe vasomotor symptoms, even though hot flashes were reduced and sleep may have improved.9
New drugs of choice. Because of estrogen’s effectiveness in controlling hot flashes, some women and their doctors may choose to use it briefly (18 to 24 months). For others, psychotropics are becoming the drugs of choice for mood disorders with moderate to severe hot flashes.
The serotonin and norepinephrine reuptake inhibitor (SNRI) venlafaxine, 75 or 150 mg/d, has been shown to reduce hot flashes by 60 to 70%.10 A new trial is investigating whether duloxetine—an SNRI awaiting FDA approval—also reduces hot flashes. Other useful agents that have been shown to reduce hot flashes by 50% or more include:
- selective serotonin reuptake inhibitors (SSRIs) paroxetine CR, 12.5 mg/d to 25 mg/d,11 citalopram, 20 to 60 mg/d,12 and fluoxetine, 20 mg/d13
- gabapentin, 900 mg/d.14
For hot flashes and moderate to major depression, try an SNRI or SSRI first (see Algorithm), but consider the possible effects on sexual function. All SNRIs and SSRIs have sexual side effects, including anorgasmia and loss of libido in women and men. Among the psychotropics that improve hot flashes and mood, gabapentin is the only one that does not interfere with sexual function.
Mood improves, but still no libido
You and Ann decide on a trial of the SNRI venlafaxine, 75 mg/d, to treat her hot flashes and depressed mood. Four weeks later, her hot flashes are reduced by 50% in frequency and her mood has improved (Beck Depression Inventory score is now 10). She is feeling much better and wishes to continue taking the antidepressant.
She and her husband attempted intercourse once during the past month, although she wasn’t very interested. She did not achieve orgasm, despite adequate vaginal lubrication, and she did not enjoy the experience. “I still have no libido—zero, or even less,” she says.
TREATING LOW INTEREST IN SEX
Being angry with one’s partner is the number-one reason for decreased sexual desire in all studies. Therefore, consider couples therapy for any woman complaining of loss of interest in sex. In addition, eliminate—if possible—any medications she may be taking that have known sexual side effects, such as SSRIs or beta blockers.
If the patient complains of slow or no arousal, vaginal estrogen and/or sildenafil, 25 to 50 mg 1 hour before intercourse, may be beneficial.15 Other agents the FDA is reviewing for erectile dysfunction—such as tadalafil and vardenafil—may also help arousal problems in women.
Understanding how hormones affect female sexual desire also may help you decide what advice to give Anne and how you and her Ob/Gyn coordinate her care. For example, you might treat her sexual complaints and relationship problems while the Ob/Gyn manages symptoms of the vagina, uterus, and breast.
HOW TESTOSTERONE AFFECTS SEXUAL DESIRE
Testosterone is the hormone of sexual desire in men and women. Other female androgens include androstenedione, androstenediol, 5 α-dihydrotestosterone (DHT), dihydroepiandrosterone (DHEA), and its sulfate (DHEA-S). Premenopausal women produce these androgens in the ovaries (25%), adrenal glands (25%), and peripheral tissues (50%).
Average daily serum testosterone concentrations decline in women between ages 20 and 50. Lower levels are also seen with estrogen replacement therapy or oral contraceptives, lactation, anorexia nervosa, and conditions that reduce ovarian function. Women who undergo total hysterectomy with bilateral oophorectomy experience a sudden 50% loss of testosterone and an 80% decline in estradiol.16
Regularly menstruating women in their 40s and early 50s can have very low testosterone levels—at least 50% lower in the first 5 to 7 days of their cycles—than they had when they were in their 30s.17 The percentage of women reporting low libido increases with age until menopause, from 30% at age 30 to about 50% at age 50. Then the rate declines to 27% in women age 50 to 59.18 After natural menopause, luteinizing hormone (LH) continues to stimulate the ovarian hilar cells and interstitial cells to produce androgens, which is why many women at age 50 have adequate testosterone levels to sustain sexual desire.
Oral estrogen replacement therapy reduces bioavailable testosterone by 42% on average, which can induce androgen deficiency in a menopausal woman.19 The increased estrogen inhibits pituitary LH and decreases stimulation of the androgen-producing cells in the ovary.20
Female androgen deficiency. A number of papers have been published on female androgen deficiency syndrome (FADS).21 Its diagnosis requires symptoms of thinning pubic and axillary hair, decreased body odor, lethargy, low mood, diminished well-being, and declining libido and orgasm, despite adequate estrogen but low levels of testosterone and DHEA.
TREATING TESTOSTERONE DEFICIENCY
Benefits of replacement therapy. Replacing testosterone in women with FADS can improve mood, well-being, motivation, cognition, sexual function related to libido, orgasm, sexual fantasies, desire to masturbate, and nipple and clitoral sensitivity.22 Muscle and bone stimulation and decreased hot flashes are also reported.23 Women with androgen deficiency symptoms and low testosterone at menopause should at least be considered for physiologic testosterone replacement.
Risks of replacement therapy. Androgen replacement therapy does carry some risks, which need to be discussed with the patient. Testosterone may lower levels of beneficial HDL cholesterol, so get the cardiologist’s clearance before you give testosterone to a woman with heart disease or an HDL cholesterol level <45 mg/dL.
Algorithm Managing mood and libido problems during perimenopause
A meta-analysis of eight clinical trials found no changes in liver function in menopausal women taking 1.25 to 2.5 mg/d of methyl testosterone. Liver toxicity has been reported in men using 10-fold higher testosterone dosages.24
At the normal level of testosterone, darkening and thickening of facial hair are rare in light-skinned, light-haired women but can occur in dark-skinned, dark-haired women. Increased irritability, excess energy, argumentativeness, and aggressive behavior have been noted if testosterone levels exceed the physiologic range.
Controlled, randomized studies are needed to assess the effects of long-term use (more than 24 months) of testosterone replacement in women.
Challenges in measuring testosterone levels. Serum free testosterone is the most reliable indicator of a woman’s androgen status, but accurately measuring testosterone levels is tricky:
- Only 2% of circulating testosterone is unbound and biologically active; the rest is bound to sex hormone-binding globulin (SHBG) or albumin.
- In ovulating women, serum testosterone levels are higher in the morning than later in the day and vary greatly within the menstrual cycle.
- Levels of androgens and estrogen are highest during the middle one-third of the cycle—on days 10 to 16, counting the first day of menstrual bleeding as day 1.25
- Oral contraceptives also decrease androgen production by the ovary and can result in low libido in some women.26
Tests developed to measure testosterone levels in men are not sensitive enough to accurately measure women’s naturally lower serum concentrations, let alone the even lower levels characteristic of female androgen or testosterone deficiency. New measurements and standardization of normal reference ranges have been developed for women complaining of low libido.27
Tests for androgen deficiency include total testosterone, free testosterone, DHEA, and DHEAS. Measuring SHBG will help you determine the free, biologically active testosterone level and calculate the Free Androgen Index (FAI) for women (Table 3).28
Table 3
Free androgen index (FAI) values in women, by age
| Replacing a woman’s bioactive testosterone to the normal free androgen index range for her age may improve low libido. | |
| How to calculate FAI | |
| Total testosterone in nmol/L (total testosterone in ng/ml X 0.0347 X 100), divided by sex hormone-binding globulin (SHBG) in nmol/L. | |
| Age | Normal range |
| 20 to 29 | 3.72 to 4.96 |
| 30 to 39 | 2.04 to 2.96 |
| 40 to 49 | 1.98 to 2.94 |
| 50 to 59+ | 1.78 to 2.86 |
| Source: Guay et al, reference 28. | |
A candidate for testosterone therapy?
Now that Anne’s mood, sleep, and hot flashes have improved with venlafaxine, she wants help with her lack of sexual interest. You measure her testosterone and SHBG levels and find that her free androgen index is very low at 0.51 (normal range, 1.78 to 2.86).
In collaboration with her Ob/Gyn, you and Anne decide to start her on testosterone replacement therapy. You prescribe Androgel, starting at 1/7th of a 2.5-mg foil packet (0.35 mg/d of testosterone), and instruct her to rate her sexual energy daily, using a Sexual Energy Scale.
TESTOSTERONE CHOICES FOR WOMEN
Replacing a woman’s bioactive testosterone level to the normal free androgen index range for her age group may improve low libido. Some low-dose testosterone replacement options include:
- methyl testosterone sublingual pills, 0.5 mg/d, made by a compounding pharmacy or reduced dosages of oral pills made for men. If you prescribe methyl testosterone, routine lab tests will not accurately measure serum testosterone levels—unless you order the very expensive test that is specific for methyl testosterone.
- 2% vaginal cream, applied topically to increase clitoral and genital sensitivity. It may increase blood levels moderately through absorption
- Androgel, a topical testosterone approved for men. As in Anne’s case, start with 0.35 mg/d or one-seventh of the 2.5 mg packet (ask the pharmacist to place this amount in a syringe). Instruct the patient to apply the gel to hairless skin, such as inside the forearm. Effects last about 24 hours, and you can measure serum levels accurately after 14 days. Vaginal throbbing—a normal response—may occur within 30 minutes of testosterone application.
The FDA is considering other testosterone preparations—including a testosterone patch for women and a gel in female-sized doses.
Using the Sexual Energy Scale. To monitor for a therapeutic response, ask the patient to use the Sexual Energy Scale (Figure 1).29,30 Instruct her to define her “10” as the time in life when she had the most fulfilling sexual life, was the most easily aroused, had the most sexual pleasure, and the best orgasms. Her “1” would be when she felt the worst sexually and had the least desire.
Giving supplemental estrogen. If you prescribe estrogen plus testosterone (Estratest), start with Estratest HS, which contains 0.625 mg esterified estrogens and 1.25 mg of methyl testosterone. Add a progestin if the patient is postmenopausal and has not had a hysterectomy, to protect the uterus from endometrial hyperplasia.
Women with vaginal dryness also need supplemental estrogen, which can be applied vaginally (such as Premarin cream or Estrace cream). A vaginal lubricant is not sufficient to avoid age-related vaginal atrophy, which may make intercourse difficult or impossible.
Figure 1 How to use the Sexual Energy Scale to monitor response to therapy
Libido improves modestly
Ann returns in 4 weeks with gradually improving sex drive (Sexual Energy Score is now 5). She had sexual intercourse twice in the past month and didn’t “dread” it, but also did not enjoy it or reach orgasm. You have told her that venlafaxine may slow or prevent orgasm, but she wants to keep taking it. She reports that her marital relationship is improving.
You order repeat testosterone and SHBG blood levels and find her free androgen index has improved to 1.10, which is still low. You increase the Androgel dosage to 1/5th of a 2.5 mg packet (0.5 mg/day) and continue to monitor Anne’s Sexual Energy Scale ratings at monthly follow-up visits. She has set a Sexual Energy Scale rating of 7 to 8 as her target. Anne says she appreciates your help with—as she puts it—“this embarrassing problem.”
Louann Brizendine, MD
Medicine’s understanding of menopause’s physiologic and psychological consequences is changing, just as the “baby-boom” generation is navigating this passage called the change of life. Many midlife women are unaware that the menopause transition does not begin around age 50 but spans 30 years—from ages 35 to 65. Natural menopause begins 15 years before and ends 15 years after menstruation ceases—as the brain and tissues adjust to first fluctuating then decreased estrogen levels. It occurs in three phases—early menopause, perimenopause, and late menopause—that reflect a progression of hormone changes.
EARLY MENOPAUSE: OVULATION ACCELERATES
At approximately age 35, the ovaries start producing lower levels of inhibin—a glycoprotein that inhibits pituitary production of follicle-stimulating hormone (FSH) (Figure 2). Less inhibin means less negative feedback to the pituitary and an increase in pituitary FSH production. More FSH means more activin—an ovarian glycoprotein that stimulates ripening of eggs—and so ovulation begins to accelerate at approximately age 36. Activin stimulates more and more eggs in the ovary to develop faster and faster.
By age 37, the ovarian egg reserve starts to decline, and—because FSH has increased—the follicle is driven to produce greater amounts of estrogen. Estrogen serum levels in fertile women average 100 pg/mL. During perimenopause, estrogen levels sometimes soar to 300, 400, or even 500 pg/mL, then may crash down to 50 to 80 pg/mL. These wild fluctuations are thought to trigger headaches, sleep disturbance, mood swings, and sexual complaints in some women.
Hysterectomy and mood symptoms. Women in their early 40s are exposed to high levels of estrogen in some menstrual cycles and low levels in others. Excess estrogen thickens the endometrium—causing heavier bleeding—and stimulates fibroid growth, which is the leading reason for hysterectomies.
One in four American women undergoes surgical menopause. The average age of the 700,000 U.S. women who undergo hysterectomy each year is 40 to 44. Women who have had a hysterectomy and have mood symptoms and sexual adjustment problems are likely to see psychiatrists earlier than women who undergo a more gradual natural menopause.
PERIMENOPAUSE: HOT FLASHES, DRY VAGINA
At ages 45 to 55, most women (90%) who have not had a hysterectomy start to cycle irregularly, tending at first toward shorter cycles and then skipping periods. Some periods are heavier and some lighter than usual. The remaining 10% of women continue to cycle regularly until their menstrual periods stop abruptly.
Many women notice temperature dysregulation during perimenopause. When they exercise, their cool-down times may double. Menopausal symptoms such as hot flashes occur when estrogen levels drop below the point that some researchers call a woman’s “estrogen set point.”
Screening for estrogen decline. When you see a patient in your office, you can often determine whether her affective symptoms—irritability, mood swings, depressed mood, and forgetfulness—might be related to estrogen decline by asking two screening questions:
- Are you having any warm flushes or hot flashes?
- Do you have vaginal dryness?
Figure 2 Normal female reproductive cycle: The rhythm of the hypothalamic-pituitary-ovarian axis
LATE MENOPAUSE: ESTROGEN LOW, MOOD UP
During late menopause, approximately age 55 and older, women commonly complain of vaginal dryness, hot flashes, night sweats, sleep problems, and fatigue. Sexual interest may decrease, and a decline in sexual activity can become a problem for some couples. Asking “How is your sex life?” often will open a discussion of the couple’s sexual and emotional relationship.
Other physiologic changes caused by estrogen and androgen deficiency include thinning body hair—including pubic, auxiliary, and leg hair—decreased body odor, thinning skin, wrinkling skin, and decreasing bone density.
Table 4
Three phases of menopause: A 30-year process
| Early Ages 35 to 45 | Middle (perimenopause) Ages 46 to 55 | Late Ages 56 to 65+ |
|---|---|---|
| Physiologic changes | ||
| Ovary starts producing less inhibin 15 years before menses stop | Irregular menstrual cycles, with shorter cycles, skipping periods for 90% of women | Depletion of eggs and follicle |
| Decreased inhibin increases FSH and stimulates follicle to produce more estrogen | Some periods heavier, some lighter than usual | |
| Increased estrogen thickens endometrium and leads to heavier menstrual bleeding and increased risk of fibroids | ||
| Increased FSH produces more activin, which makes eggs develop faster and accelerates egg depletion | ||
| Lab values | ||
| Menses: Normal | Cycle shorter (24-26 days) | None for >12 months |
| FSH day 3: 10-25 mIU/mL | 20-30 mIU/mL | 50-90 mIU/mL |
| Estradiol day 3: 40-200 pg/mL | 40-200 pg/mL | 10-20 pg/mL |
| Inhibin B day 3: Varies | <45 pg/mL | 0 |
| Symptoms | ||
| Headaches, sleep disturbances, mood swings, urinary problems, sexual complaints | Warm flushes, hot flashes, night sweats in 82% of women (moderate to severe in 40%) | Vaginal dryness, hot flashes, night sweats, sleep problems, fatigue, sexual interest changes, thinning body hair (pubic, legs, axillary), decreased body odor, thinning skin, wrinkling skin, decreasing bone density, BUT mood starts to stabilize |
| FSH: follicle-stimulating hormone | ||
Urinary symptoms. Bladder problems and urinary symptoms are persistent symptoms of menopause for 75% of women. Although we psychiatrists don’t review the urinary system, it is important to remember that embarrassment because of urinary incontinence during sex may have a lot to do with a woman’s “loss of interest” in sexual intercourse.
Despite sometimes-difficult physiologic changes, the good news for many women is that mood symptoms start to stabilize after perimenopause (Table 4). Women whose moods are very responsive to hormonal fluctuations—such as those with severe premenstrual syndrome or premenstrual dysphoric disorder—sometimes get much better after menopause.
Related resources
- The Women’s Health Site. Duke Academic Program in Women’s Health. www.thewomenshealthsite.org
- Massachusetts General Hospital Center for Women’s Mental Health. www.womensmentalhealth.org
Drug brand names
- Citalopram • Celexa
- Fluoxetine • Prozac
- Gabapentin • Neurontin
- Paroxetine • Paxil
- Sildenafil • Viagra
- Venlafaxine • Effexor
Disclosure
Dr. Brizendine reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric 2003;6(suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med 2003;348:645-50.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002;9(6):392-8.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am 1997;26(2):279-94.
6. Seppa N. Hormone therapy falls out of favor. Science News 2002;162:61.-
7. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am 2003;32(2):325-36.
8. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44(9):798-811.
9. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003;348(19):1839-54.
10. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002;29(1):33-40.
11. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003;289(21):2827-34.
12. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry 2003;64(4):473-9.
13. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20(6):1578-83.
14. Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101(2):337-45.
15. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108(6):623-8.
16. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric 2002;5(4):357-65.
17. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol 2003;85(2-5):363-6.
18. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44.
19. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril 2003;79(6):1341-52.
20. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol 1997;90(6):995-8.
21. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77(4):660-5.
22. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab 2003;17(1):165-75.
23. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol 2002;20(2):106-10.
24. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause 1999;6(3):216-24.
25. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums 2003;8(March):3.-
26. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995;52(6):363-9.
27. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril 2002;77(suppl 4):S83-8.
28. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther 2002;28(suppl 1):129-42.
29. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull 1997;33(4):761-5.
30. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction (poster presentation). Waikoloa, HI: North American Society for Psychosocial Obstetrics and Gynecology, 2001.
1. Burger H. Hormone replacement therapy in the post-Women’s Health Initiative era. Climacteric 2003;6(suppl 1):11-36.
2. Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone replacement therapy. N Engl J Med 2003;348:645-50.
3. Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause 2002;9(6):392-8.
4. Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001;58(6):529-34.
5. Pearlstein T, Rosen K, Stone AB. Mood disorders and menopause. Endocrinol Metab Clin North Am 1997;26(2):279-94.
6. Seppa N. Hormone therapy falls out of favor. Science News 2002;162:61.-
7. Nieman LK. Management of surgically hypogonadal patients unable to take sex hormone replacement therapy. Endocrinol Metab Clin North Am 2003;32(2):325-36.
8. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry 1998;44(9):798-811.
9. Hays J, Ockene JK, Brunner RL, et al. Women’s Health Initiative Investigators. Effects of estrogen plus progestin on health-related quality of life. N Engl J Med 2003;348(19):1839-54.
10. Barton D, La VB, Loprinzi C, et al. Venlafaxine for the control of hot flashes: results of a longitudinal continuation study. Oncol Nurs Forum 2002;29(1):33-40.
11. Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release in the treatment of menopausal hot flashes: a randomized controlled trial. JAMA 2003;289(21):2827-34.
12. Soares CN, Poitras JR, Prouty J, et al. Efficacy of citalopram as a monotherapy or as an adjunctive treatment to estrogen therapy for perimenopausal and postmenopausal women with depression and vasomotor symptoms. J Clin Psychiatry 2003;64(4):473-9.
13. Loprinzi CL, Sloan JA, Perez EA, et al. Phase III evaluation of fluoxetine for treatment of hot flashes. J Clin Oncol 2002;20(6):1578-83.
14. Guttuso T, Jr, Kurlan R, McDermott MP, Kieburtz K. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2003;101(2):337-45.
15. Caruso S, Intelisano G, Lupo L, Agnello C. Premenopausal women affected by sexual arousal disorder treated with sildenafil: a double-blind, cross-over, placebo-controlled study. BJOG 2001;108(6):623-8.
16. Floter A, Nathorst-Boos J, Carlstrom K, et al. Addition of testosterone to estrogen replacement therapy in oophorectomized women: effects on sexuality and well-being. Climacteric 2002;5(4):357-65.
17. Davison SL, Davis SR. Androgens in women. J Steroid Biochem Mol Biol 2003;85(2-5):363-6.
18. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44.
19. Lobo RA, Rosen RC, Yang HM, et al. Comparative effects of oral esterified estrogens with and without methyltestosterone on endocrine profiles and dimensions of sexual function in postmenopausal women with hypoactive sexual desire. Fertil Steril 2003;79(6):1341-52.
20. Casson PR, Elkind-Hirsch KE, Buster JE, et al. Effect of postmenopausal estrogen replacement on circulating androgens. Obstet Gynecol 1997;90(6):995-8.
21. Bachmann G, Bancroft J, Braunstein G, et al. Female androgen insufficiency: the Princeton consensus statement on definition, classification, and assessment. Fertil Steril 2002;77(4):660-5.
22. Davis SR, Burger HG. The role of androgen therapy. Best Pract Res Clin Endocrinol Metab 2003;17(1):165-75.
23. Guay A, Davis SR. Testosterone insufficiency in women: fact or fiction? World J Urol 2002;20(2):106-10.
24. Gitlin N, Korner P, Yang HM. Liver function in postmenopausal women on estrogen-androgen hormone replacement therapy: a meta-analysis of eight clinical trials. Menopause 1999;6(3):216-24.
25. Warnock JK, Biggs CF. Reproductive life events and sexual functioning in women: case reports. CNS Spectrums 2003;8(March):3.-
26. Graham CA, Ramos R, Bancroft J, et al. The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995;52(6):363-9.
27. Guay AT. Screening for androgen deficiency in women: methodological and interpretive issues. Fertil Steril 2002;77(suppl 4):S83-8.
28. Guay AT, Jacobson J. Decreased free testosterone and dehydroepiandrosterone-sulfate (DHEA-S) levels in women with decreased libido. J Sex Marital Ther 2002;28(suppl 1):129-42.
29. Warnock JK, Bundren JC, Morris DW. Female hypoactive sexual desire disorder due to androgen deficiency: clinical and psychometric issues. Psychopharmacol Bull 1997;33(4):761-5.
30. Warnock JK, Clayton AH, Yates WR, Bundren JC. Sexual Energy Scale (SES): a simple valid screening tool for measuring of sexual dysfunction (poster presentation). Waikoloa, HI: North American Society for Psychosocial Obstetrics and Gynecology, 2001.
Tardive dyskinesia: How to prevent and treat a lingering nemesis
Atypical antipsychotics seldom cause tardive dyskinesia (TD), but we cannot let our guard down when prescribing them. Although they pose a much lower risk of TD than do conventional antipsychotics, atypicals can cause TD in vulnerable patients.
Less worrisome than in the past, TD’s associated problems linger, including insidious onset, tendency for persistence, and lack of reliably effective treatment. It is important, therefore, for psychiatrists to:
- identify patients at risk for developing TD
- recognize extrapyramidal symptoms (EPS) when they occur
- and manage these side effects appropriately.
A CHANGING CLINICAL PICTURE
The term “dyskinesias” covers a variety of abnormal involuntary movements (Box). The incidence and prevalence of TD have dropped markedly in the last 10 years, as:
- more and more older, chronically ill patients are switched from conventional to atypical agents
- younger psychotic patients are usually treated with atypicals as first-line therapy and are never exposed to conventional antipsychotics.
Tardive dyskinesia (TD) tends to develop in patients receiving long-term antipsychotic treatment. Its typical movements are choreiform (jerky) or athetoid (writhing), irregular, and purposeless.
TD onset is usually insidious and may occur during drug therapy or weeks after antipsychotics are discontined. Its signs are usually observed in the face or mouth, and typical orofacial dyskinetic movements are:
Lips: puckering, pouting, smacking
Jaw: chewing, biting, side-to-side movements, jaw openings
Tongue: twisting, rolling, undulations, protrusion, darting (“fly-catching”)
Face: blinking, frowning, grimacing.
The trunk and extremities are involved less often. Choreiform finger and wrist movements, flexion and rotation of the ankle, toe movements, foot tapping, and rocking or twisting of the neck, hip, and trunk may be seen. Patients are often oblivious to these movements, which may be only intermittently present and are absent during sleep. Anxiety and arousal states may aggravate TD.
TD prevalence of about 20%—as shown by earlier studies of long-term conventional agents1—is declining. Newer studies comparing atypicals with conventional antipsychotics demonstrate much lower prevalence rates.2,3
TD incidence—estimated by new cases of TD per year of drug treatment—may have declined 10-fold, from 5% with conventional antipsychotics to 0.5% with atypicals. Likewise, incidence in the elderly may have fallen from 25% to 2.5%.4
Risk factors. Despite these improvements, case reports5-7 demonstrate that TD is possible in patients treated with atypicals, even without previous exposure to a conventional antipsychotic. Besides antipsychotic use, risk factors for developing TD include:
- older age
- negative symptoms of schizophrenia
- affective disorders
- acute EPS
- and diabetes mellitus.8
RECOGNIZING TD SYMPTOMS
Recognizing TD may be complicated by the presence of other EPS, particularly drug-induced parkinsonism (DIP). DIP typically develops early and often when treating patients with conventional antipsychotics (Table 1). TD and DIP may occur simultaneously in the same patient, making accurate diagnosis even more difficult.
Other dyskinesias may complicate the diagnosis. Three common TD variants, which may be acute or tardive (occurring after long-term antipsychotic therapy), are:
- akathisia, a distressing and at times irresistible urge to move the legs or other parts of the body
- dystonia, abnormal muscle tone and posture and muscle spasms
- tics, brief muscle contractions, usually in the face, including vocal tics.
AIMS testing. Defining a “case of TD” by dyskinetic movement severity is somewhat arbitrary. A commonly accepted definition is two area scores of “mild” or one rating of “moderate” using the Abnormal Involuntary Movement Scale (AIMS).9 The AIMS has been widely used in epidemiologic and treatment studies of TD and is easy to administer in a clinical setting (see Related Resources).
A careful drug history is required before TD can be diagnosed definitively. Spontaneous dys kinesias—usually orofacial—are sometimes seen in older patients who are not taking neuroleptics.8 Antidepressants, mood stabilizers, or antihistamines may infrequently trigger neurologic side effects—including dyskinesias, akathisia, and tremor—which are almost invariably reversible after the causative agent is withdrawn.8,10
Table 1
Features that differentiate two common extrapyramidal symptoms
| Tardive dyskinesia (TD) | Drug-induced parkinsonism (DIP) | |
|---|---|---|
| Onset | Late | Early |
| Type of movement | Choreoathetoid | Tremor |
| Amount of movement | Increased | Decreased |
| Muscle tone | Decreased | Increased |
| Most common site | Orofacial | Extremities |
| Response to anticholinergics | Tends to worsen | Tends to improve |
MANAGING MILD TD
Atypical antipsychotics have radically altered the clinical outlook for patients with TD and improved our ability to manage their symptoms. The clinician treating a TD patient today rarely faces the dilemma that exists with conventional antipsychotics: discontinue treatment and risk psychotic relapse, or continue treatment and risk persistent TD.
Using atypicals. Today, patients who need antipsychotic therapy for TD are usually already taking atypicals, which may ameliorate TD and control psychotic symptoms. Case reports and some studies have shown therapeutic effects in patients with TD taking olanzapine,3 risperidone,2 quetiapine,11 ziprasidone,12 aripiprazole,13 or the substituted benzamides (such as sulpiride), which are not marketed in the United States.14
Interestingly, TD triggered by taking one atypical may respond to treatment with another. Suzuki et al15 reported that three patients who had developed early-onset TD while taking risperidone showed TD remission after risperidone was replaced by olanzapine in one patient and by quetiapine in the other two.
The atypicals are well tolerated but not without side effects. Weight gain is the most common problem and one with potentially serious health consequences.16
Using conventional agents. Even though atypicals are available, the clinician may consider continuing therapy with conventional antipsychotics in patients with TD when:
- the patient’s mental status has been satisfactory while taking conventional agents
- TD has been mild and stable over an extended time
- the patient has no side effects other than TD.
The literature supports the clinical experience that mild TD rarely worsens with continued antipsychotic therapy. Studies of 5 years or more tend to show TD stability with continued conventional antipsychotic therapy.17 It is prudent to maintain stable chronic psychotic patients with mild TD on the lowest effective dosages of conventional antipsychotics and to monitor them regularly for changes in dyskinesia and psychiatric status.
MANAGING COMPLICATED TD
Managing severe TD or patients showing dystonia, tics, marked akathisia, or DIP coexisting with TD usually calls for more-aggressive interventions (Algorithm).
Algorithm Clinical management of tardive dyskinesia (TD)
Clozapine remains the first-line treatment for difficult TD; it has a very low propensity for inducing DIP and very rarely causes TD.18 Controlled studies,18,19 case reports, and open trials demonstrate its efficacy for reducing TD of all types and severity at a usual dosage of 300 to 500 mg/d. Clozapine’s antidyskinetic effects may be attributed to the absence of rebound after withdrawal and its greater efficacy in more-severe cases.18
Long-term clozapine therapy is recommended for TD, as symptoms remit slowly. Because weight gain, sedation, and other side effects—as well as mandatory blood monitoring—make clozapine less-than-ideal in clinical practice, researchers are seeking other effective therapies for TD.
Other atypicals. The obvious place to look is the other atypicals, which are simpler than clozapine to administer long-term. To date, however, these drugs have not proven to be as reliably effective as clozapine for TD. A recent review concluded that among the atypicals only clozapine induces less EPS than low-potency conventional antipsychotics.20
Nonantipsychotic agents. Other antidyskinetic drugs have come and gone; none has stood the test of time or proven effective in controlled trials. These agents may benefit some TD patients, but improvement is usually not dramatic.
Vitamin E was found to be effective in some TD treatment studies14 but not more effective than placebo in the largest controlled trial.21 Long-term treatment with dopamine-blocking antipsychotics is thought to cause oxidative stressinduced neurotoxicity in the nigrostriatal system.22 Lipid-soluble antioxidants such as vitamin E decrease free-radical formation, and it is possible that vitamin E may yet emerge as a helpful agent in preventing TD.23
Melatonin, a stronger antioxidant than vitamin E, was found to reduce TD in a 6-week placebocontrolled study,22 but the degree of TD improvement was modest. Melatonin’s value as a therapeutic agent for TD remains dubious.23
Miscellaneous. Case reports and studies with small series of TD patients have advanced numerous compounds as possible therapeutic agents (Table 2). Other drugs that occasionally have shown benefit in TD include buspirone, propranolol, pyridoxine (vitamin B6), ondansetron, clonidine, and the neuropeptide ceruletide.
ECT and diet. Suggested nondrug treatments of TD include electroconvulsive therapy (ECT)14 and a diet of mixed branched-chain amino acids.24
Table 2
Compounds that occasionally show benefit in TD
| Class | Example |
|---|---|
| Cholinergics | Lecithin |
| Catecholamine depletors | Tetrabenazine (investigational orphan drug) |
| Calcium channel blockers | Verapamil |
| Gabaergic compounds | Baclofen |
| Benzodiazepines | Clonazepam |
MANAGING TD VARIANTS
TD variants are notoriously difficult to treat but tend to respond to clozapine.18 In addition:
- Tardive dystonia is often treated with reserpine, tetrabenazine, or high doses of anticholinergic drugs.25 Botulinum toxin A injections into affected muscles may be remarkably effective but must be repeated regularly.25
- Tardive akathisia may improve slowly with clozapine, propranolol, or benzodiazepines.25
Managing severe or atypical TD is usually beyond the expertise of the practicing psychiatrist. Obtaining consultation from a psychopharmacologist or a neurologist experienced in treating movement disorders is highly recommended.
PREVENTING TD
Conventional antipsychotics are still prescribed by psychiatrists, internists, and family physicians and are often given in emergency rooms. Avoiding these drugs whenever possible and using the lowest effective dosages will reduce the risk of TD.26
Patients at relatively high risk for TD—the elderly, those who are very sensitive to acute EPS, and those with affective disorders or diabetes mellitus—are rarely candidates for conventional neuroleptics if a suitable alternative exists. Genetic research may further identify individuals susceptible to TD.27
- Abnormal Involuntary Movement Scale (AIMS). www.dr-bob.org/tips/aims.html
- Bloom FE, Kupfer DJ (eds). Psychopharmacology: The fourth generation of progress. New York: Raven Press, 1995.
- Tandon R, Halbreich U (eds). Atypical antipsychotics: Efficacy and tolerability—achieving the optimal balance. Psychoneuroendocrinology 2003;28(suppl 1).
Drug brand names
- Aripiprazole • Abilify
- Baclofen • Lioresal
- Buspirone • BuSpar
- Clonazepam • Klonopin
- Clonidine • Catapres
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Ondansetron • Zofran
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Verapamil • Calan, others
- Ziprasidone • Geodon
Disclosure
Dr. Gardos receives research grant support from Forest Laboratories.
1. Woerner M, Kane JM, Lieberman JA, et al. The prevalence of tardive dyskinesia, J Clin Psychopharmacology 1991;11:34-42.
2. Caroff SN, Mann SC, Campbell EC, et al. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002;63(suppl 4):12-19.
3. Tollefson GD, Beasley CM, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997;154:457-65.
4. Jeste DV, Lacro JP, Bailey A, et al. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999;47:716-19.
5. Kumet R, Freeman MP. Clozapine and tardive dyskinesia. J Clin Psychiatry 2002;63:167-8.
6. Hong KS, Cheong SS, Woo J-M, Kim E. Risperidone-induced tardive dyskinesia. Am J Psychiatry 1999;156:1290.-
7. Ghaemi SN, Ko JY. Quetiapine-related tardive dyskinesia. Am J Psychiatry 2001;158:1737.-
8. Kane JM. Tardive dyskinesia: epidemiological and clinical presentation. In: Bloom FE, Kupfer DJ (eds.) Psychopharmacology: The fourth generation of progress. New York: Raven Press, Ltd, 1995;1485-95.
9. Guy W. ECDEU assessment manual for psychopharmacology (rev. ed). Washington, DC: Department of Health, Education and Welfare, 1976.
10. Madhusoodanan S, Brenner R. Reversible choreiform dyskinesia and extrapyramidal symptoms associated with sertraline therapy. J Clin Psychopharmacology 1997;17:138-9.
11. Glazer WM, Morgenstern H, Pultz JA, et al. Incidence of tardive dyskinesia is lower with quetiapine treatment than with typical antipsychotics in patients with schizophrenia and schizo-affective disorder. Schizophrenia Res 2000;41:206-7.
12. Hirsch SR, Kissling W, Bauml J, et al. A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophrenia. J Clin Psychiatry 2002;63:516-23.
13. Kujawa M, Sala A, Ingenito GG, et al. Aripiprazole for long-term maintenance treatment of schizophrenia (poster presentation). Montreal, Canada: Collegium Internationale Neuropsychopharmacologicum 23rd congress, June 23-27, 2002.
14. Gupta S, Mosnik D, Black DW, et al. Tardive dyskinesia: review of treatments past, present and future. Ann Clin Psychiatry 1999;11:257-66.
15. Suzuki E, Obata M, Yoshida Y, Miyaoka H. Tardive dyskinesia with risperidone and anticholinergics. Am J Psychiatry 2002;159:1948.-
16. Nasrallah HA. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology 2003;28(suppl 1):83-96.
17. Gardos G, Casey DE, Cole JO, et al. Ten-year outcome of tardive dyskinesia. Am J Psychiatry 1994;151:836-41.
18. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry 1991;158:503-10.
19. Tamminga CA, Thaker GK, Moran M, et al. Clozapine in tardive dyskinesia: observations from human and animal model studies J Clin Psychiatry 1994;55(suppl B):102-6.
20. Leucht S, Wahlbeck C, Hermann J, Kissling W. New-generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet 2003;361:1581-9.
21. Adler LA, Rotrosen J, Edson R, et al. Vitamin E treatment of tardive dyskinesia. Arch Gen Psychiatry 1999;56:836-41.
22. Shamir E, Barak Y, Shalman I, et al. Melatonin treatment for tardive dyskinesia. Arch Gen Psychiatry 2001;58:1046-52.
23. Glazer WM, Woods SW. Should Sisyphus have taken melatonin? Arch Gen Psychiatry 2001;58:1054-5.
24. Richardson MA, Bevans M, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry 2003;160:1117-24.
25. Gardos G, Cole JO. The evaluation and treatment of neurolepticinduced movement disorders. Harvard Rev Psychiatry 1995;3:130-9.
26. Lohr JB, Caligiuri MP, Edson R, et al. Treatment predictors of extrapyramidal side effects in patients with tardive dyskinesia: results from Veterans Affairs Cooperative Study 394. J Clin Psychopharmacol 2002;22:196-200.
27. Casey DE. Effect of clozapine therapy in schizophrenic individuals at risk for tardive dyskinesia. J Clin Psychiatry 1998;59(suppl 3):31-7.
Atypical antipsychotics seldom cause tardive dyskinesia (TD), but we cannot let our guard down when prescribing them. Although they pose a much lower risk of TD than do conventional antipsychotics, atypicals can cause TD in vulnerable patients.
Less worrisome than in the past, TD’s associated problems linger, including insidious onset, tendency for persistence, and lack of reliably effective treatment. It is important, therefore, for psychiatrists to:
- identify patients at risk for developing TD
- recognize extrapyramidal symptoms (EPS) when they occur
- and manage these side effects appropriately.
A CHANGING CLINICAL PICTURE
The term “dyskinesias” covers a variety of abnormal involuntary movements (Box). The incidence and prevalence of TD have dropped markedly in the last 10 years, as:
- more and more older, chronically ill patients are switched from conventional to atypical agents
- younger psychotic patients are usually treated with atypicals as first-line therapy and are never exposed to conventional antipsychotics.
Tardive dyskinesia (TD) tends to develop in patients receiving long-term antipsychotic treatment. Its typical movements are choreiform (jerky) or athetoid (writhing), irregular, and purposeless.
TD onset is usually insidious and may occur during drug therapy or weeks after antipsychotics are discontined. Its signs are usually observed in the face or mouth, and typical orofacial dyskinetic movements are:
Lips: puckering, pouting, smacking
Jaw: chewing, biting, side-to-side movements, jaw openings
Tongue: twisting, rolling, undulations, protrusion, darting (“fly-catching”)
Face: blinking, frowning, grimacing.
The trunk and extremities are involved less often. Choreiform finger and wrist movements, flexion and rotation of the ankle, toe movements, foot tapping, and rocking or twisting of the neck, hip, and trunk may be seen. Patients are often oblivious to these movements, which may be only intermittently present and are absent during sleep. Anxiety and arousal states may aggravate TD.
TD prevalence of about 20%—as shown by earlier studies of long-term conventional agents1—is declining. Newer studies comparing atypicals with conventional antipsychotics demonstrate much lower prevalence rates.2,3
TD incidence—estimated by new cases of TD per year of drug treatment—may have declined 10-fold, from 5% with conventional antipsychotics to 0.5% with atypicals. Likewise, incidence in the elderly may have fallen from 25% to 2.5%.4
Risk factors. Despite these improvements, case reports5-7 demonstrate that TD is possible in patients treated with atypicals, even without previous exposure to a conventional antipsychotic. Besides antipsychotic use, risk factors for developing TD include:
- older age
- negative symptoms of schizophrenia
- affective disorders
- acute EPS
- and diabetes mellitus.8
RECOGNIZING TD SYMPTOMS
Recognizing TD may be complicated by the presence of other EPS, particularly drug-induced parkinsonism (DIP). DIP typically develops early and often when treating patients with conventional antipsychotics (Table 1). TD and DIP may occur simultaneously in the same patient, making accurate diagnosis even more difficult.
Other dyskinesias may complicate the diagnosis. Three common TD variants, which may be acute or tardive (occurring after long-term antipsychotic therapy), are:
- akathisia, a distressing and at times irresistible urge to move the legs or other parts of the body
- dystonia, abnormal muscle tone and posture and muscle spasms
- tics, brief muscle contractions, usually in the face, including vocal tics.
AIMS testing. Defining a “case of TD” by dyskinetic movement severity is somewhat arbitrary. A commonly accepted definition is two area scores of “mild” or one rating of “moderate” using the Abnormal Involuntary Movement Scale (AIMS).9 The AIMS has been widely used in epidemiologic and treatment studies of TD and is easy to administer in a clinical setting (see Related Resources).
A careful drug history is required before TD can be diagnosed definitively. Spontaneous dys kinesias—usually orofacial—are sometimes seen in older patients who are not taking neuroleptics.8 Antidepressants, mood stabilizers, or antihistamines may infrequently trigger neurologic side effects—including dyskinesias, akathisia, and tremor—which are almost invariably reversible after the causative agent is withdrawn.8,10
Table 1
Features that differentiate two common extrapyramidal symptoms
| Tardive dyskinesia (TD) | Drug-induced parkinsonism (DIP) | |
|---|---|---|
| Onset | Late | Early |
| Type of movement | Choreoathetoid | Tremor |
| Amount of movement | Increased | Decreased |
| Muscle tone | Decreased | Increased |
| Most common site | Orofacial | Extremities |
| Response to anticholinergics | Tends to worsen | Tends to improve |
MANAGING MILD TD
Atypical antipsychotics have radically altered the clinical outlook for patients with TD and improved our ability to manage their symptoms. The clinician treating a TD patient today rarely faces the dilemma that exists with conventional antipsychotics: discontinue treatment and risk psychotic relapse, or continue treatment and risk persistent TD.
Using atypicals. Today, patients who need antipsychotic therapy for TD are usually already taking atypicals, which may ameliorate TD and control psychotic symptoms. Case reports and some studies have shown therapeutic effects in patients with TD taking olanzapine,3 risperidone,2 quetiapine,11 ziprasidone,12 aripiprazole,13 or the substituted benzamides (such as sulpiride), which are not marketed in the United States.14
Interestingly, TD triggered by taking one atypical may respond to treatment with another. Suzuki et al15 reported that three patients who had developed early-onset TD while taking risperidone showed TD remission after risperidone was replaced by olanzapine in one patient and by quetiapine in the other two.
The atypicals are well tolerated but not without side effects. Weight gain is the most common problem and one with potentially serious health consequences.16
Using conventional agents. Even though atypicals are available, the clinician may consider continuing therapy with conventional antipsychotics in patients with TD when:
- the patient’s mental status has been satisfactory while taking conventional agents
- TD has been mild and stable over an extended time
- the patient has no side effects other than TD.
The literature supports the clinical experience that mild TD rarely worsens with continued antipsychotic therapy. Studies of 5 years or more tend to show TD stability with continued conventional antipsychotic therapy.17 It is prudent to maintain stable chronic psychotic patients with mild TD on the lowest effective dosages of conventional antipsychotics and to monitor them regularly for changes in dyskinesia and psychiatric status.
MANAGING COMPLICATED TD
Managing severe TD or patients showing dystonia, tics, marked akathisia, or DIP coexisting with TD usually calls for more-aggressive interventions (Algorithm).
Algorithm Clinical management of tardive dyskinesia (TD)
Clozapine remains the first-line treatment for difficult TD; it has a very low propensity for inducing DIP and very rarely causes TD.18 Controlled studies,18,19 case reports, and open trials demonstrate its efficacy for reducing TD of all types and severity at a usual dosage of 300 to 500 mg/d. Clozapine’s antidyskinetic effects may be attributed to the absence of rebound after withdrawal and its greater efficacy in more-severe cases.18
Long-term clozapine therapy is recommended for TD, as symptoms remit slowly. Because weight gain, sedation, and other side effects—as well as mandatory blood monitoring—make clozapine less-than-ideal in clinical practice, researchers are seeking other effective therapies for TD.
Other atypicals. The obvious place to look is the other atypicals, which are simpler than clozapine to administer long-term. To date, however, these drugs have not proven to be as reliably effective as clozapine for TD. A recent review concluded that among the atypicals only clozapine induces less EPS than low-potency conventional antipsychotics.20
Nonantipsychotic agents. Other antidyskinetic drugs have come and gone; none has stood the test of time or proven effective in controlled trials. These agents may benefit some TD patients, but improvement is usually not dramatic.
Vitamin E was found to be effective in some TD treatment studies14 but not more effective than placebo in the largest controlled trial.21 Long-term treatment with dopamine-blocking antipsychotics is thought to cause oxidative stressinduced neurotoxicity in the nigrostriatal system.22 Lipid-soluble antioxidants such as vitamin E decrease free-radical formation, and it is possible that vitamin E may yet emerge as a helpful agent in preventing TD.23
Melatonin, a stronger antioxidant than vitamin E, was found to reduce TD in a 6-week placebocontrolled study,22 but the degree of TD improvement was modest. Melatonin’s value as a therapeutic agent for TD remains dubious.23
Miscellaneous. Case reports and studies with small series of TD patients have advanced numerous compounds as possible therapeutic agents (Table 2). Other drugs that occasionally have shown benefit in TD include buspirone, propranolol, pyridoxine (vitamin B6), ondansetron, clonidine, and the neuropeptide ceruletide.
ECT and diet. Suggested nondrug treatments of TD include electroconvulsive therapy (ECT)14 and a diet of mixed branched-chain amino acids.24
Table 2
Compounds that occasionally show benefit in TD
| Class | Example |
|---|---|
| Cholinergics | Lecithin |
| Catecholamine depletors | Tetrabenazine (investigational orphan drug) |
| Calcium channel blockers | Verapamil |
| Gabaergic compounds | Baclofen |
| Benzodiazepines | Clonazepam |
MANAGING TD VARIANTS
TD variants are notoriously difficult to treat but tend to respond to clozapine.18 In addition:
- Tardive dystonia is often treated with reserpine, tetrabenazine, or high doses of anticholinergic drugs.25 Botulinum toxin A injections into affected muscles may be remarkably effective but must be repeated regularly.25
- Tardive akathisia may improve slowly with clozapine, propranolol, or benzodiazepines.25
Managing severe or atypical TD is usually beyond the expertise of the practicing psychiatrist. Obtaining consultation from a psychopharmacologist or a neurologist experienced in treating movement disorders is highly recommended.
PREVENTING TD
Conventional antipsychotics are still prescribed by psychiatrists, internists, and family physicians and are often given in emergency rooms. Avoiding these drugs whenever possible and using the lowest effective dosages will reduce the risk of TD.26
Patients at relatively high risk for TD—the elderly, those who are very sensitive to acute EPS, and those with affective disorders or diabetes mellitus—are rarely candidates for conventional neuroleptics if a suitable alternative exists. Genetic research may further identify individuals susceptible to TD.27
- Abnormal Involuntary Movement Scale (AIMS). www.dr-bob.org/tips/aims.html
- Bloom FE, Kupfer DJ (eds). Psychopharmacology: The fourth generation of progress. New York: Raven Press, 1995.
- Tandon R, Halbreich U (eds). Atypical antipsychotics: Efficacy and tolerability—achieving the optimal balance. Psychoneuroendocrinology 2003;28(suppl 1).
Drug brand names
- Aripiprazole • Abilify
- Baclofen • Lioresal
- Buspirone • BuSpar
- Clonazepam • Klonopin
- Clonidine • Catapres
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Ondansetron • Zofran
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Verapamil • Calan, others
- Ziprasidone • Geodon
Disclosure
Dr. Gardos receives research grant support from Forest Laboratories.
Atypical antipsychotics seldom cause tardive dyskinesia (TD), but we cannot let our guard down when prescribing them. Although they pose a much lower risk of TD than do conventional antipsychotics, atypicals can cause TD in vulnerable patients.
Less worrisome than in the past, TD’s associated problems linger, including insidious onset, tendency for persistence, and lack of reliably effective treatment. It is important, therefore, for psychiatrists to:
- identify patients at risk for developing TD
- recognize extrapyramidal symptoms (EPS) when they occur
- and manage these side effects appropriately.
A CHANGING CLINICAL PICTURE
The term “dyskinesias” covers a variety of abnormal involuntary movements (Box). The incidence and prevalence of TD have dropped markedly in the last 10 years, as:
- more and more older, chronically ill patients are switched from conventional to atypical agents
- younger psychotic patients are usually treated with atypicals as first-line therapy and are never exposed to conventional antipsychotics.
Tardive dyskinesia (TD) tends to develop in patients receiving long-term antipsychotic treatment. Its typical movements are choreiform (jerky) or athetoid (writhing), irregular, and purposeless.
TD onset is usually insidious and may occur during drug therapy or weeks after antipsychotics are discontined. Its signs are usually observed in the face or mouth, and typical orofacial dyskinetic movements are:
Lips: puckering, pouting, smacking
Jaw: chewing, biting, side-to-side movements, jaw openings
Tongue: twisting, rolling, undulations, protrusion, darting (“fly-catching”)
Face: blinking, frowning, grimacing.
The trunk and extremities are involved less often. Choreiform finger and wrist movements, flexion and rotation of the ankle, toe movements, foot tapping, and rocking or twisting of the neck, hip, and trunk may be seen. Patients are often oblivious to these movements, which may be only intermittently present and are absent during sleep. Anxiety and arousal states may aggravate TD.
TD prevalence of about 20%—as shown by earlier studies of long-term conventional agents1—is declining. Newer studies comparing atypicals with conventional antipsychotics demonstrate much lower prevalence rates.2,3
TD incidence—estimated by new cases of TD per year of drug treatment—may have declined 10-fold, from 5% with conventional antipsychotics to 0.5% with atypicals. Likewise, incidence in the elderly may have fallen from 25% to 2.5%.4
Risk factors. Despite these improvements, case reports5-7 demonstrate that TD is possible in patients treated with atypicals, even without previous exposure to a conventional antipsychotic. Besides antipsychotic use, risk factors for developing TD include:
- older age
- negative symptoms of schizophrenia
- affective disorders
- acute EPS
- and diabetes mellitus.8
RECOGNIZING TD SYMPTOMS
Recognizing TD may be complicated by the presence of other EPS, particularly drug-induced parkinsonism (DIP). DIP typically develops early and often when treating patients with conventional antipsychotics (Table 1). TD and DIP may occur simultaneously in the same patient, making accurate diagnosis even more difficult.
Other dyskinesias may complicate the diagnosis. Three common TD variants, which may be acute or tardive (occurring after long-term antipsychotic therapy), are:
- akathisia, a distressing and at times irresistible urge to move the legs or other parts of the body
- dystonia, abnormal muscle tone and posture and muscle spasms
- tics, brief muscle contractions, usually in the face, including vocal tics.
AIMS testing. Defining a “case of TD” by dyskinetic movement severity is somewhat arbitrary. A commonly accepted definition is two area scores of “mild” or one rating of “moderate” using the Abnormal Involuntary Movement Scale (AIMS).9 The AIMS has been widely used in epidemiologic and treatment studies of TD and is easy to administer in a clinical setting (see Related Resources).
A careful drug history is required before TD can be diagnosed definitively. Spontaneous dys kinesias—usually orofacial—are sometimes seen in older patients who are not taking neuroleptics.8 Antidepressants, mood stabilizers, or antihistamines may infrequently trigger neurologic side effects—including dyskinesias, akathisia, and tremor—which are almost invariably reversible after the causative agent is withdrawn.8,10
Table 1
Features that differentiate two common extrapyramidal symptoms
| Tardive dyskinesia (TD) | Drug-induced parkinsonism (DIP) | |
|---|---|---|
| Onset | Late | Early |
| Type of movement | Choreoathetoid | Tremor |
| Amount of movement | Increased | Decreased |
| Muscle tone | Decreased | Increased |
| Most common site | Orofacial | Extremities |
| Response to anticholinergics | Tends to worsen | Tends to improve |
MANAGING MILD TD
Atypical antipsychotics have radically altered the clinical outlook for patients with TD and improved our ability to manage their symptoms. The clinician treating a TD patient today rarely faces the dilemma that exists with conventional antipsychotics: discontinue treatment and risk psychotic relapse, or continue treatment and risk persistent TD.
Using atypicals. Today, patients who need antipsychotic therapy for TD are usually already taking atypicals, which may ameliorate TD and control psychotic symptoms. Case reports and some studies have shown therapeutic effects in patients with TD taking olanzapine,3 risperidone,2 quetiapine,11 ziprasidone,12 aripiprazole,13 or the substituted benzamides (such as sulpiride), which are not marketed in the United States.14
Interestingly, TD triggered by taking one atypical may respond to treatment with another. Suzuki et al15 reported that three patients who had developed early-onset TD while taking risperidone showed TD remission after risperidone was replaced by olanzapine in one patient and by quetiapine in the other two.
The atypicals are well tolerated but not without side effects. Weight gain is the most common problem and one with potentially serious health consequences.16
Using conventional agents. Even though atypicals are available, the clinician may consider continuing therapy with conventional antipsychotics in patients with TD when:
- the patient’s mental status has been satisfactory while taking conventional agents
- TD has been mild and stable over an extended time
- the patient has no side effects other than TD.
The literature supports the clinical experience that mild TD rarely worsens with continued antipsychotic therapy. Studies of 5 years or more tend to show TD stability with continued conventional antipsychotic therapy.17 It is prudent to maintain stable chronic psychotic patients with mild TD on the lowest effective dosages of conventional antipsychotics and to monitor them regularly for changes in dyskinesia and psychiatric status.
MANAGING COMPLICATED TD
Managing severe TD or patients showing dystonia, tics, marked akathisia, or DIP coexisting with TD usually calls for more-aggressive interventions (Algorithm).
Algorithm Clinical management of tardive dyskinesia (TD)
Clozapine remains the first-line treatment for difficult TD; it has a very low propensity for inducing DIP and very rarely causes TD.18 Controlled studies,18,19 case reports, and open trials demonstrate its efficacy for reducing TD of all types and severity at a usual dosage of 300 to 500 mg/d. Clozapine’s antidyskinetic effects may be attributed to the absence of rebound after withdrawal and its greater efficacy in more-severe cases.18
Long-term clozapine therapy is recommended for TD, as symptoms remit slowly. Because weight gain, sedation, and other side effects—as well as mandatory blood monitoring—make clozapine less-than-ideal in clinical practice, researchers are seeking other effective therapies for TD.
Other atypicals. The obvious place to look is the other atypicals, which are simpler than clozapine to administer long-term. To date, however, these drugs have not proven to be as reliably effective as clozapine for TD. A recent review concluded that among the atypicals only clozapine induces less EPS than low-potency conventional antipsychotics.20
Nonantipsychotic agents. Other antidyskinetic drugs have come and gone; none has stood the test of time or proven effective in controlled trials. These agents may benefit some TD patients, but improvement is usually not dramatic.
Vitamin E was found to be effective in some TD treatment studies14 but not more effective than placebo in the largest controlled trial.21 Long-term treatment with dopamine-blocking antipsychotics is thought to cause oxidative stressinduced neurotoxicity in the nigrostriatal system.22 Lipid-soluble antioxidants such as vitamin E decrease free-radical formation, and it is possible that vitamin E may yet emerge as a helpful agent in preventing TD.23
Melatonin, a stronger antioxidant than vitamin E, was found to reduce TD in a 6-week placebocontrolled study,22 but the degree of TD improvement was modest. Melatonin’s value as a therapeutic agent for TD remains dubious.23
Miscellaneous. Case reports and studies with small series of TD patients have advanced numerous compounds as possible therapeutic agents (Table 2). Other drugs that occasionally have shown benefit in TD include buspirone, propranolol, pyridoxine (vitamin B6), ondansetron, clonidine, and the neuropeptide ceruletide.
ECT and diet. Suggested nondrug treatments of TD include electroconvulsive therapy (ECT)14 and a diet of mixed branched-chain amino acids.24
Table 2
Compounds that occasionally show benefit in TD
| Class | Example |
|---|---|
| Cholinergics | Lecithin |
| Catecholamine depletors | Tetrabenazine (investigational orphan drug) |
| Calcium channel blockers | Verapamil |
| Gabaergic compounds | Baclofen |
| Benzodiazepines | Clonazepam |
MANAGING TD VARIANTS
TD variants are notoriously difficult to treat but tend to respond to clozapine.18 In addition:
- Tardive dystonia is often treated with reserpine, tetrabenazine, or high doses of anticholinergic drugs.25 Botulinum toxin A injections into affected muscles may be remarkably effective but must be repeated regularly.25
- Tardive akathisia may improve slowly with clozapine, propranolol, or benzodiazepines.25
Managing severe or atypical TD is usually beyond the expertise of the practicing psychiatrist. Obtaining consultation from a psychopharmacologist or a neurologist experienced in treating movement disorders is highly recommended.
PREVENTING TD
Conventional antipsychotics are still prescribed by psychiatrists, internists, and family physicians and are often given in emergency rooms. Avoiding these drugs whenever possible and using the lowest effective dosages will reduce the risk of TD.26
Patients at relatively high risk for TD—the elderly, those who are very sensitive to acute EPS, and those with affective disorders or diabetes mellitus—are rarely candidates for conventional neuroleptics if a suitable alternative exists. Genetic research may further identify individuals susceptible to TD.27
- Abnormal Involuntary Movement Scale (AIMS). www.dr-bob.org/tips/aims.html
- Bloom FE, Kupfer DJ (eds). Psychopharmacology: The fourth generation of progress. New York: Raven Press, 1995.
- Tandon R, Halbreich U (eds). Atypical antipsychotics: Efficacy and tolerability—achieving the optimal balance. Psychoneuroendocrinology 2003;28(suppl 1).
Drug brand names
- Aripiprazole • Abilify
- Baclofen • Lioresal
- Buspirone • BuSpar
- Clonazepam • Klonopin
- Clonidine • Catapres
- Clozapine • Clozaril
- Olanzapine • Zyprexa
- Ondansetron • Zofran
- Propranolol • Inderal
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Verapamil • Calan, others
- Ziprasidone • Geodon
Disclosure
Dr. Gardos receives research grant support from Forest Laboratories.
1. Woerner M, Kane JM, Lieberman JA, et al. The prevalence of tardive dyskinesia, J Clin Psychopharmacology 1991;11:34-42.
2. Caroff SN, Mann SC, Campbell EC, et al. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002;63(suppl 4):12-19.
3. Tollefson GD, Beasley CM, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997;154:457-65.
4. Jeste DV, Lacro JP, Bailey A, et al. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999;47:716-19.
5. Kumet R, Freeman MP. Clozapine and tardive dyskinesia. J Clin Psychiatry 2002;63:167-8.
6. Hong KS, Cheong SS, Woo J-M, Kim E. Risperidone-induced tardive dyskinesia. Am J Psychiatry 1999;156:1290.-
7. Ghaemi SN, Ko JY. Quetiapine-related tardive dyskinesia. Am J Psychiatry 2001;158:1737.-
8. Kane JM. Tardive dyskinesia: epidemiological and clinical presentation. In: Bloom FE, Kupfer DJ (eds.) Psychopharmacology: The fourth generation of progress. New York: Raven Press, Ltd, 1995;1485-95.
9. Guy W. ECDEU assessment manual for psychopharmacology (rev. ed). Washington, DC: Department of Health, Education and Welfare, 1976.
10. Madhusoodanan S, Brenner R. Reversible choreiform dyskinesia and extrapyramidal symptoms associated with sertraline therapy. J Clin Psychopharmacology 1997;17:138-9.
11. Glazer WM, Morgenstern H, Pultz JA, et al. Incidence of tardive dyskinesia is lower with quetiapine treatment than with typical antipsychotics in patients with schizophrenia and schizo-affective disorder. Schizophrenia Res 2000;41:206-7.
12. Hirsch SR, Kissling W, Bauml J, et al. A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophrenia. J Clin Psychiatry 2002;63:516-23.
13. Kujawa M, Sala A, Ingenito GG, et al. Aripiprazole for long-term maintenance treatment of schizophrenia (poster presentation). Montreal, Canada: Collegium Internationale Neuropsychopharmacologicum 23rd congress, June 23-27, 2002.
14. Gupta S, Mosnik D, Black DW, et al. Tardive dyskinesia: review of treatments past, present and future. Ann Clin Psychiatry 1999;11:257-66.
15. Suzuki E, Obata M, Yoshida Y, Miyaoka H. Tardive dyskinesia with risperidone and anticholinergics. Am J Psychiatry 2002;159:1948.-
16. Nasrallah HA. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology 2003;28(suppl 1):83-96.
17. Gardos G, Casey DE, Cole JO, et al. Ten-year outcome of tardive dyskinesia. Am J Psychiatry 1994;151:836-41.
18. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry 1991;158:503-10.
19. Tamminga CA, Thaker GK, Moran M, et al. Clozapine in tardive dyskinesia: observations from human and animal model studies J Clin Psychiatry 1994;55(suppl B):102-6.
20. Leucht S, Wahlbeck C, Hermann J, Kissling W. New-generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet 2003;361:1581-9.
21. Adler LA, Rotrosen J, Edson R, et al. Vitamin E treatment of tardive dyskinesia. Arch Gen Psychiatry 1999;56:836-41.
22. Shamir E, Barak Y, Shalman I, et al. Melatonin treatment for tardive dyskinesia. Arch Gen Psychiatry 2001;58:1046-52.
23. Glazer WM, Woods SW. Should Sisyphus have taken melatonin? Arch Gen Psychiatry 2001;58:1054-5.
24. Richardson MA, Bevans M, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry 2003;160:1117-24.
25. Gardos G, Cole JO. The evaluation and treatment of neurolepticinduced movement disorders. Harvard Rev Psychiatry 1995;3:130-9.
26. Lohr JB, Caligiuri MP, Edson R, et al. Treatment predictors of extrapyramidal side effects in patients with tardive dyskinesia: results from Veterans Affairs Cooperative Study 394. J Clin Psychopharmacol 2002;22:196-200.
27. Casey DE. Effect of clozapine therapy in schizophrenic individuals at risk for tardive dyskinesia. J Clin Psychiatry 1998;59(suppl 3):31-7.
1. Woerner M, Kane JM, Lieberman JA, et al. The prevalence of tardive dyskinesia, J Clin Psychopharmacology 1991;11:34-42.
2. Caroff SN, Mann SC, Campbell EC, et al. Movement disorders associated with atypical antipsychotic drugs. J Clin Psychiatry 2002;63(suppl 4):12-19.
3. Tollefson GD, Beasley CM, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997;154:457-65.
4. Jeste DV, Lacro JP, Bailey A, et al. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999;47:716-19.
5. Kumet R, Freeman MP. Clozapine and tardive dyskinesia. J Clin Psychiatry 2002;63:167-8.
6. Hong KS, Cheong SS, Woo J-M, Kim E. Risperidone-induced tardive dyskinesia. Am J Psychiatry 1999;156:1290.-
7. Ghaemi SN, Ko JY. Quetiapine-related tardive dyskinesia. Am J Psychiatry 2001;158:1737.-
8. Kane JM. Tardive dyskinesia: epidemiological and clinical presentation. In: Bloom FE, Kupfer DJ (eds.) Psychopharmacology: The fourth generation of progress. New York: Raven Press, Ltd, 1995;1485-95.
9. Guy W. ECDEU assessment manual for psychopharmacology (rev. ed). Washington, DC: Department of Health, Education and Welfare, 1976.
10. Madhusoodanan S, Brenner R. Reversible choreiform dyskinesia and extrapyramidal symptoms associated with sertraline therapy. J Clin Psychopharmacology 1997;17:138-9.
11. Glazer WM, Morgenstern H, Pultz JA, et al. Incidence of tardive dyskinesia is lower with quetiapine treatment than with typical antipsychotics in patients with schizophrenia and schizo-affective disorder. Schizophrenia Res 2000;41:206-7.
12. Hirsch SR, Kissling W, Bauml J, et al. A 28-week comparison of ziprasidone and haloperidol in outpatients with stable schizophrenia. J Clin Psychiatry 2002;63:516-23.
13. Kujawa M, Sala A, Ingenito GG, et al. Aripiprazole for long-term maintenance treatment of schizophrenia (poster presentation). Montreal, Canada: Collegium Internationale Neuropsychopharmacologicum 23rd congress, June 23-27, 2002.
14. Gupta S, Mosnik D, Black DW, et al. Tardive dyskinesia: review of treatments past, present and future. Ann Clin Psychiatry 1999;11:257-66.
15. Suzuki E, Obata M, Yoshida Y, Miyaoka H. Tardive dyskinesia with risperidone and anticholinergics. Am J Psychiatry 2002;159:1948.-
16. Nasrallah HA. A review of the effect of atypical antipsychotics on weight. Psychoneuroendocrinology 2003;28(suppl 1):83-96.
17. Gardos G, Casey DE, Cole JO, et al. Ten-year outcome of tardive dyskinesia. Am J Psychiatry 1994;151:836-41.
18. Lieberman JA, Saltz BL, Johns CA, et al. The effects of clozapine on tardive dyskinesia. Br J Psychiatry 1991;158:503-10.
19. Tamminga CA, Thaker GK, Moran M, et al. Clozapine in tardive dyskinesia: observations from human and animal model studies J Clin Psychiatry 1994;55(suppl B):102-6.
20. Leucht S, Wahlbeck C, Hermann J, Kissling W. New-generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet 2003;361:1581-9.
21. Adler LA, Rotrosen J, Edson R, et al. Vitamin E treatment of tardive dyskinesia. Arch Gen Psychiatry 1999;56:836-41.
22. Shamir E, Barak Y, Shalman I, et al. Melatonin treatment for tardive dyskinesia. Arch Gen Psychiatry 2001;58:1046-52.
23. Glazer WM, Woods SW. Should Sisyphus have taken melatonin? Arch Gen Psychiatry 2001;58:1054-5.
24. Richardson MA, Bevans M, Read LL, et al. Efficacy of the branched-chain amino acids in the treatment of tardive dyskinesia in men. Am J Psychiatry 2003;160:1117-24.
25. Gardos G, Cole JO. The evaluation and treatment of neurolepticinduced movement disorders. Harvard Rev Psychiatry 1995;3:130-9.
26. Lohr JB, Caligiuri MP, Edson R, et al. Treatment predictors of extrapyramidal side effects in patients with tardive dyskinesia: results from Veterans Affairs Cooperative Study 394. J Clin Psychopharmacol 2002;22:196-200.
27. Casey DE. Effect of clozapine therapy in schizophrenic individuals at risk for tardive dyskinesia. J Clin Psychiatry 1998;59(suppl 3):31-7.
When a mother’s love hurts
INITIAL PRESENTATION: A sickly child
Ms. J, age 35, was referred to a psychiatrist after she was observed endangering her daughter. The child’s pediatrician provided the following history.
The 4-year-old has frequently required medical attention. As a baby, she wore a breathing monitor at home for almost 1 year after her mother expressed fear that she would die of apnea. Throughout her early years, the child was treated for asthma and abdominal pain and for bruises her parents said were caused when she fell out of her crib.
Recently, the child has suffered with recurring skin infections over her arm and shoulders. Her mother treated those with prescribed topical and oral antibiotics and dressings. The wounds would heal briefly, then become inflamed again.
The pediatrician consulted with a child psychiatrist, who suggested that the girl be hospitalized for a day so that doctors could examine the wound. Despite the mother’s protests, her daughter was hospitalized.
That day, Ms. J visited her daughter, unaware that the hospital room was equipped with a concealed video camera. As she was leaving late that evening, she lifted her daughter’s bandages as if to check them, then applied irritants to the wounds.
When the staff confronted her about this destructive behavior toward her daughter, Ms. J denied it had occurred. Upon seeing the videotape, however, she wept profusely, exclaiming, “I didn’t mean any harm.”
Ms. J does not fit the profile of a child abuser. Aside from part-time clerical work at home, she is a full-time mother who is intensely involved in every aspect of her daughter’s life. Before the videotaped incident, her pediatrician’s office staff had described her as caring and loving, and she had brought them thank-you gifts.
On psychiatric evaluation, Ms. J’s speech is coherent and logical, and she has no delusions or hallucinations. In describing her childhood, she recalls her parents arguing constantly. Her father, with whom she had little contact, traveled extensively on business. At home, her mother ruled with an iron fist. Any show of rebellion by Ms. J or her siblings led to a sharp slap on the shoulders.
Ms. J had few friends in grade school. In college, she earned good grades but often visited the infirmary with nonspecific complaints. She was hospitalized twice without a conclusive diagnosis. She twice saw a psychiatrist to address her medical symptoms and feelings of emptiness.
Table
Defining symptoms, features of Munchausen’s syndrome
Major symptoms
|
Secondary features
|
After college, Ms. J worked as a teacher’s aide. At age 30 she married a teacher, and their daughter was born 1 year later. The marriage ended in divorce after 3 years. After divorce the husband tried to visit the daughter but Ms. J interfered, insisting, “I’m enough.”
Ms. J told the psychiatrist that her ex-husband at times accused her of being “too clingy.” If he went to a sporting event, she would repeatedly ask what time he would come home. If she had to go shopping and he was home, she would ask him to drop everything and accompany her.
Does Ms. J’s behavior suggest a straightforward personality disorder, or would you consider a factitious disorder?
Dr. Messer’s observations
Ms. J recalled a childhood fraught with rejection and abuse. This finding, plus the deliberate injury to her daughter, points to a diagnosis of Munchausen’s syndrome by proxy.
Although not listed in DSM-IV-TR, Munchausen’s is the most severe form of factitious disorder (Table). First described in 1951, the disorder is named for Baron Karl von Munchausen, an 18th-century German nobleman known for telling tall tales about his life and exploits.1
In 1977, British pediatrician Roy Meadow described Munchausen’s syndrome by proxy, in which parents induce symptoms in their children—such as by injuring them with drugs or bacterial contaminants—then have doctors treat them.2 Because persons with Munchausen’s tend to frequently change their names and locales, the incidence of Munchausen’s and Munchausen’s by proxy has never been accurately gauged.
Munchausen’s occurs in both sexes, although men tend to exhibit more-dramatic symptoms such as self-induced fevers, bleeding, and seizures. Antisocial behavior is common among men with Munchausen’s, and many are jailed at some point. One man flying from New York to London feigned a heart attack so convincingly that he forced an emergency landing in Halifax, Nova Scotia, where he was hospitalized and released with a referral to his cardiologist.
By contrast, women with Munchausen’s or Munchausen’s by proxy—often nurses or hospital personnel—are more difficult to diagnose because they usually are agreeable and compliant with medical staff. Staff members tend to ask themselves, “Could such a caring person be putting us on?”
Some persons with Munchausen’s visit disease-specific support groups and draw attention or sympathy by faking illness. Having amassed significant medical knowledge through Web searches and frequent doctor visits, they present themselves as both patient and lofty advisor. Their antisocial tendencies can negatively alter group discussions, and the revelation that a fellow group member is an impostor can distress legitimate participants.3
Ms. J exhibited no evidence of a delusional disorder, which occurs rarely among parents with Munchausen’s by proxy. Her allegedly “clingy” (dependent) behavior toward her daughter and ex-husband, however, indicates borderline personality disorder or antisocial personality disorder, which are common in both forms of Munchausen’s. Like Ms. J, these patients
- have strong fears of rejection and abandonment
- are often impulsive
- exhibit damaging behavior to self or others
- have an unstable sense of self
- are unusually willing to comply with clinical tests or procedures
- and describe feelings of emptiness.
How would you confirm a diagnosis of Munchausen’s? What features in Ms J’s case distinguish Munchausen’s from other factitious disorders?Box
- Munchausen’s syndrome by proxy has been reported in women who were sexually abused in their youth. As adults, some of these women become hypervigilant against sexual abuse of their children.
- The disorder has been cited in several legal cases in which a suspect—usually a teacher or religious leader—was falsely accused of sexually molesting a child.4 Psychiatrists involved as expert witnesses or examiners in such cases should consider these allegations as a possible manifestation of Munchausen’s.
- If the accused denies the charges and no evidence is uncovered, the alleged victim’s mother should be interviewed. In some cases the mother was sexually abused and sought help, but the perpetrator was never apprehended. She may harbor resentment toward men and repeatedly ask her child whether he or she has been touched inappropriately. The child—besieged by frequent inquiries or unsure of what constitutes an inappropriate touch—may answer "yes," prompting the mother to press charges against the named individual.
Dr. Messer’s observations
When taking the patient history, look for:
- a pattern of rejection or abuse in youth. In women who have been sexually molested, Munchausen’s by proxy may manifest as allegations that their children have been touched inappropriately (Box).4
- history of multiple surgeries.5 Because Munchausen’s can coexist with genuine physical illness, the psychiatrist and other doctors need to carefully review the findings to determine the existence of medical symptoms or Munchausen’s. Doctors usually choose to operate if there is any suspicion that medical symptoms exist.
- memories of sympathetic and nurturing medical care early in life. A severe emotional crisis can awaken these memories. For example, emotional pain after a divorce or break-up can trigger a Munchausen’s episode in a subconscious attempt to repair this hurt.
Carefully reviewing the history will turn up glaring discrepancies in the patient’s account of his or her illness. Patients with Munchausen’s or Munchausen’s by proxy usually present to numerous clinicians and do not describe their “illness” the same way twice. Sharing information with other members of the care team—many of whom may feel anger after learning that a patient has been feigning illness—is crucial to confirming the diagnosis.
DIFFERENTIAL DIAGNOSIS
Munchausen’s should not be confused with symptoms of other lookalike disorders:
- Hypochondriasis applies to patients who are convinced they have a serious illness despite lack of a medical basis. The person may misinterpret normal bodily functions and suffer severe distress. The symptoms may express inner psychic conflict.
- Somatoform disorder is a physical complaint that cannot be explained as a known medical condition. This may include preoccupation with an imagined defect in appearance or loss of physical capacity. The deficit usually coincides with emotional conflict and—unlike Munchausen’s—there is no fabrication of illness.
- Malingerers purposely fake physical symptoms for specific gain, such as money, hospital care, disability, drugs, or avoiding military duty.
Persons with Munchausen’s have no external incentive for their behavior. Aside from the medical staff’s admiration, Ms. J had nothing to gain from injuring her daughter or portraying her as sickly.
TREATMENT: Coming home
Ms. J’s daughter responded well after being placed in the home of a relative who had a 3-year-old daughter of her own. Ms. J was allowed to see her daughter for 2 hours each day under strict supervision by a retired schoolteacher hired by the family. If Ms. J. violated the agreement, such as by leaving the house with her daughter, she was prohibited from seeing her child the next day.
Ms. J agreed to long-term therapy; in exchange, judicial proceedings were deferred. In most cases, the mother is referred to police and charged with child abuse/endangerment. However, the medical staff in this case felt treatment and supervision would provide ample rehabilitation.
The mother was referred to a psychiatrist trained in psychoanalysis. By making her aware of her unconscious responses, the doctor sought to improve Ms. J’s ability to cope with stress. Patients with a borderline personality disorder seldom respond in psychoanalysis, but Ms. J exhibited significant ego strengths and intelligence—and was motivated to regain custody of her daughter.
Ms. J’s transference response was predictable: She tried to please the doctor in everything she said or did. This reaction was analyzed and the insight helped Ms. J understand why she exaggerated or exacerbated normal childhood illnesses in her daughter. She discovered that she had hungered for approval since childhood and went as far as injuring her daughter to achieve this fulfillment. Early in treatment she developed a mantra: “I have behaved toward my daughter as my mother behaved toward me.”
After 8 months of therapy 3 times a week, Ms. J regained custody. She continued to receive once-weekly psychotherapy for 1 year.
Two years after regaining custody, Ms. J developed a stable, loving relationship with her daughter. She works full-time while her daughter is in high school and participates in many activities. The relative who assumed temporary custody stays in touch, as does her pediatrician.
The girl is seen by her father on alternate weekends. She tells friends she wants to become a nurse.
How can psychiatrists facilitate early diagnosis and treatment of Munchausen’s?
Dr. Messer’s observations
Although primary care physicians or general surgeons treat most patients with Munchausen’s, the disorder is psychiatric in nature.
For that reason, psychiatrists should participate when possible in the early medical workup of a patient who presents with an apparently severe illness. This way, the psychiatrist can:
- help detect Munchausen’s or another factitious disorder sooner
- establish a rapport with the patient early on. If Munchausen’s is subsequently diagnosed, the patient may be less likely to resist treatment or bolt when confronted.
When confronting someone suspected of fabricating or inducing symptoms, tell the patient that he or she might have Munchausen’s syndrome. Describe the disorder and cite cases, then challenge the patient to find a more appropriate way to cope with stress.
Related resources
- Adshead G, Brooke D (eds). Munchausen's syndrome by proxy. Current issues in assessment, treatment and research. Singapore: World Scientific Publishing, 2001.
- Munchausen’s syndrome by proxy. www.gktscientific.com
1. Asher R. Munchausen’s syndrome. Lancet 1951;1:580-5.
2. Meadow R. False allegations of abuse and Munchausen’s syndrome by proxy. Arch Dis Child 1993;68:444-7.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Publishing, 1998.
4. Schreier HA. Repeated false allegations of sexual abuse presenting to sheriffs: when is it Munchausen’s by proxy? Child Abuse Negl 1996;20:985-91.
5. Menninger KA. Polysurgery and polysurgical addiction. Psychoanal Q 1934;3:883-9.
INITIAL PRESENTATION: A sickly child
Ms. J, age 35, was referred to a psychiatrist after she was observed endangering her daughter. The child’s pediatrician provided the following history.
The 4-year-old has frequently required medical attention. As a baby, she wore a breathing monitor at home for almost 1 year after her mother expressed fear that she would die of apnea. Throughout her early years, the child was treated for asthma and abdominal pain and for bruises her parents said were caused when she fell out of her crib.
Recently, the child has suffered with recurring skin infections over her arm and shoulders. Her mother treated those with prescribed topical and oral antibiotics and dressings. The wounds would heal briefly, then become inflamed again.
The pediatrician consulted with a child psychiatrist, who suggested that the girl be hospitalized for a day so that doctors could examine the wound. Despite the mother’s protests, her daughter was hospitalized.
That day, Ms. J visited her daughter, unaware that the hospital room was equipped with a concealed video camera. As she was leaving late that evening, she lifted her daughter’s bandages as if to check them, then applied irritants to the wounds.
When the staff confronted her about this destructive behavior toward her daughter, Ms. J denied it had occurred. Upon seeing the videotape, however, she wept profusely, exclaiming, “I didn’t mean any harm.”
Ms. J does not fit the profile of a child abuser. Aside from part-time clerical work at home, she is a full-time mother who is intensely involved in every aspect of her daughter’s life. Before the videotaped incident, her pediatrician’s office staff had described her as caring and loving, and she had brought them thank-you gifts.
On psychiatric evaluation, Ms. J’s speech is coherent and logical, and she has no delusions or hallucinations. In describing her childhood, she recalls her parents arguing constantly. Her father, with whom she had little contact, traveled extensively on business. At home, her mother ruled with an iron fist. Any show of rebellion by Ms. J or her siblings led to a sharp slap on the shoulders.
Ms. J had few friends in grade school. In college, she earned good grades but often visited the infirmary with nonspecific complaints. She was hospitalized twice without a conclusive diagnosis. She twice saw a psychiatrist to address her medical symptoms and feelings of emptiness.
Table
Defining symptoms, features of Munchausen’s syndrome
Major symptoms
|
Secondary features
|
After college, Ms. J worked as a teacher’s aide. At age 30 she married a teacher, and their daughter was born 1 year later. The marriage ended in divorce after 3 years. After divorce the husband tried to visit the daughter but Ms. J interfered, insisting, “I’m enough.”
Ms. J told the psychiatrist that her ex-husband at times accused her of being “too clingy.” If he went to a sporting event, she would repeatedly ask what time he would come home. If she had to go shopping and he was home, she would ask him to drop everything and accompany her.
Does Ms. J’s behavior suggest a straightforward personality disorder, or would you consider a factitious disorder?
Dr. Messer’s observations
Ms. J recalled a childhood fraught with rejection and abuse. This finding, plus the deliberate injury to her daughter, points to a diagnosis of Munchausen’s syndrome by proxy.
Although not listed in DSM-IV-TR, Munchausen’s is the most severe form of factitious disorder (Table). First described in 1951, the disorder is named for Baron Karl von Munchausen, an 18th-century German nobleman known for telling tall tales about his life and exploits.1
In 1977, British pediatrician Roy Meadow described Munchausen’s syndrome by proxy, in which parents induce symptoms in their children—such as by injuring them with drugs or bacterial contaminants—then have doctors treat them.2 Because persons with Munchausen’s tend to frequently change their names and locales, the incidence of Munchausen’s and Munchausen’s by proxy has never been accurately gauged.
Munchausen’s occurs in both sexes, although men tend to exhibit more-dramatic symptoms such as self-induced fevers, bleeding, and seizures. Antisocial behavior is common among men with Munchausen’s, and many are jailed at some point. One man flying from New York to London feigned a heart attack so convincingly that he forced an emergency landing in Halifax, Nova Scotia, where he was hospitalized and released with a referral to his cardiologist.
By contrast, women with Munchausen’s or Munchausen’s by proxy—often nurses or hospital personnel—are more difficult to diagnose because they usually are agreeable and compliant with medical staff. Staff members tend to ask themselves, “Could such a caring person be putting us on?”
Some persons with Munchausen’s visit disease-specific support groups and draw attention or sympathy by faking illness. Having amassed significant medical knowledge through Web searches and frequent doctor visits, they present themselves as both patient and lofty advisor. Their antisocial tendencies can negatively alter group discussions, and the revelation that a fellow group member is an impostor can distress legitimate participants.3
Ms. J exhibited no evidence of a delusional disorder, which occurs rarely among parents with Munchausen’s by proxy. Her allegedly “clingy” (dependent) behavior toward her daughter and ex-husband, however, indicates borderline personality disorder or antisocial personality disorder, which are common in both forms of Munchausen’s. Like Ms. J, these patients
- have strong fears of rejection and abandonment
- are often impulsive
- exhibit damaging behavior to self or others
- have an unstable sense of self
- are unusually willing to comply with clinical tests or procedures
- and describe feelings of emptiness.
How would you confirm a diagnosis of Munchausen’s? What features in Ms J’s case distinguish Munchausen’s from other factitious disorders?Box
- Munchausen’s syndrome by proxy has been reported in women who were sexually abused in their youth. As adults, some of these women become hypervigilant against sexual abuse of their children.
- The disorder has been cited in several legal cases in which a suspect—usually a teacher or religious leader—was falsely accused of sexually molesting a child.4 Psychiatrists involved as expert witnesses or examiners in such cases should consider these allegations as a possible manifestation of Munchausen’s.
- If the accused denies the charges and no evidence is uncovered, the alleged victim’s mother should be interviewed. In some cases the mother was sexually abused and sought help, but the perpetrator was never apprehended. She may harbor resentment toward men and repeatedly ask her child whether he or she has been touched inappropriately. The child—besieged by frequent inquiries or unsure of what constitutes an inappropriate touch—may answer "yes," prompting the mother to press charges against the named individual.
Dr. Messer’s observations
When taking the patient history, look for:
- a pattern of rejection or abuse in youth. In women who have been sexually molested, Munchausen’s by proxy may manifest as allegations that their children have been touched inappropriately (Box).4
- history of multiple surgeries.5 Because Munchausen’s can coexist with genuine physical illness, the psychiatrist and other doctors need to carefully review the findings to determine the existence of medical symptoms or Munchausen’s. Doctors usually choose to operate if there is any suspicion that medical symptoms exist.
- memories of sympathetic and nurturing medical care early in life. A severe emotional crisis can awaken these memories. For example, emotional pain after a divorce or break-up can trigger a Munchausen’s episode in a subconscious attempt to repair this hurt.
Carefully reviewing the history will turn up glaring discrepancies in the patient’s account of his or her illness. Patients with Munchausen’s or Munchausen’s by proxy usually present to numerous clinicians and do not describe their “illness” the same way twice. Sharing information with other members of the care team—many of whom may feel anger after learning that a patient has been feigning illness—is crucial to confirming the diagnosis.
DIFFERENTIAL DIAGNOSIS
Munchausen’s should not be confused with symptoms of other lookalike disorders:
- Hypochondriasis applies to patients who are convinced they have a serious illness despite lack of a medical basis. The person may misinterpret normal bodily functions and suffer severe distress. The symptoms may express inner psychic conflict.
- Somatoform disorder is a physical complaint that cannot be explained as a known medical condition. This may include preoccupation with an imagined defect in appearance or loss of physical capacity. The deficit usually coincides with emotional conflict and—unlike Munchausen’s—there is no fabrication of illness.
- Malingerers purposely fake physical symptoms for specific gain, such as money, hospital care, disability, drugs, or avoiding military duty.
Persons with Munchausen’s have no external incentive for their behavior. Aside from the medical staff’s admiration, Ms. J had nothing to gain from injuring her daughter or portraying her as sickly.
TREATMENT: Coming home
Ms. J’s daughter responded well after being placed in the home of a relative who had a 3-year-old daughter of her own. Ms. J was allowed to see her daughter for 2 hours each day under strict supervision by a retired schoolteacher hired by the family. If Ms. J. violated the agreement, such as by leaving the house with her daughter, she was prohibited from seeing her child the next day.
Ms. J agreed to long-term therapy; in exchange, judicial proceedings were deferred. In most cases, the mother is referred to police and charged with child abuse/endangerment. However, the medical staff in this case felt treatment and supervision would provide ample rehabilitation.
The mother was referred to a psychiatrist trained in psychoanalysis. By making her aware of her unconscious responses, the doctor sought to improve Ms. J’s ability to cope with stress. Patients with a borderline personality disorder seldom respond in psychoanalysis, but Ms. J exhibited significant ego strengths and intelligence—and was motivated to regain custody of her daughter.
Ms. J’s transference response was predictable: She tried to please the doctor in everything she said or did. This reaction was analyzed and the insight helped Ms. J understand why she exaggerated or exacerbated normal childhood illnesses in her daughter. She discovered that she had hungered for approval since childhood and went as far as injuring her daughter to achieve this fulfillment. Early in treatment she developed a mantra: “I have behaved toward my daughter as my mother behaved toward me.”
After 8 months of therapy 3 times a week, Ms. J regained custody. She continued to receive once-weekly psychotherapy for 1 year.
Two years after regaining custody, Ms. J developed a stable, loving relationship with her daughter. She works full-time while her daughter is in high school and participates in many activities. The relative who assumed temporary custody stays in touch, as does her pediatrician.
The girl is seen by her father on alternate weekends. She tells friends she wants to become a nurse.
How can psychiatrists facilitate early diagnosis and treatment of Munchausen’s?
Dr. Messer’s observations
Although primary care physicians or general surgeons treat most patients with Munchausen’s, the disorder is psychiatric in nature.
For that reason, psychiatrists should participate when possible in the early medical workup of a patient who presents with an apparently severe illness. This way, the psychiatrist can:
- help detect Munchausen’s or another factitious disorder sooner
- establish a rapport with the patient early on. If Munchausen’s is subsequently diagnosed, the patient may be less likely to resist treatment or bolt when confronted.
When confronting someone suspected of fabricating or inducing symptoms, tell the patient that he or she might have Munchausen’s syndrome. Describe the disorder and cite cases, then challenge the patient to find a more appropriate way to cope with stress.
Related resources
- Adshead G, Brooke D (eds). Munchausen's syndrome by proxy. Current issues in assessment, treatment and research. Singapore: World Scientific Publishing, 2001.
- Munchausen’s syndrome by proxy. www.gktscientific.com
INITIAL PRESENTATION: A sickly child
Ms. J, age 35, was referred to a psychiatrist after she was observed endangering her daughter. The child’s pediatrician provided the following history.
The 4-year-old has frequently required medical attention. As a baby, she wore a breathing monitor at home for almost 1 year after her mother expressed fear that she would die of apnea. Throughout her early years, the child was treated for asthma and abdominal pain and for bruises her parents said were caused when she fell out of her crib.
Recently, the child has suffered with recurring skin infections over her arm and shoulders. Her mother treated those with prescribed topical and oral antibiotics and dressings. The wounds would heal briefly, then become inflamed again.
The pediatrician consulted with a child psychiatrist, who suggested that the girl be hospitalized for a day so that doctors could examine the wound. Despite the mother’s protests, her daughter was hospitalized.
That day, Ms. J visited her daughter, unaware that the hospital room was equipped with a concealed video camera. As she was leaving late that evening, she lifted her daughter’s bandages as if to check them, then applied irritants to the wounds.
When the staff confronted her about this destructive behavior toward her daughter, Ms. J denied it had occurred. Upon seeing the videotape, however, she wept profusely, exclaiming, “I didn’t mean any harm.”
Ms. J does not fit the profile of a child abuser. Aside from part-time clerical work at home, she is a full-time mother who is intensely involved in every aspect of her daughter’s life. Before the videotaped incident, her pediatrician’s office staff had described her as caring and loving, and she had brought them thank-you gifts.
On psychiatric evaluation, Ms. J’s speech is coherent and logical, and she has no delusions or hallucinations. In describing her childhood, she recalls her parents arguing constantly. Her father, with whom she had little contact, traveled extensively on business. At home, her mother ruled with an iron fist. Any show of rebellion by Ms. J or her siblings led to a sharp slap on the shoulders.
Ms. J had few friends in grade school. In college, she earned good grades but often visited the infirmary with nonspecific complaints. She was hospitalized twice without a conclusive diagnosis. She twice saw a psychiatrist to address her medical symptoms and feelings of emptiness.
Table
Defining symptoms, features of Munchausen’s syndrome
Major symptoms
|
Secondary features
|
After college, Ms. J worked as a teacher’s aide. At age 30 she married a teacher, and their daughter was born 1 year later. The marriage ended in divorce after 3 years. After divorce the husband tried to visit the daughter but Ms. J interfered, insisting, “I’m enough.”
Ms. J told the psychiatrist that her ex-husband at times accused her of being “too clingy.” If he went to a sporting event, she would repeatedly ask what time he would come home. If she had to go shopping and he was home, she would ask him to drop everything and accompany her.
Does Ms. J’s behavior suggest a straightforward personality disorder, or would you consider a factitious disorder?
Dr. Messer’s observations
Ms. J recalled a childhood fraught with rejection and abuse. This finding, plus the deliberate injury to her daughter, points to a diagnosis of Munchausen’s syndrome by proxy.
Although not listed in DSM-IV-TR, Munchausen’s is the most severe form of factitious disorder (Table). First described in 1951, the disorder is named for Baron Karl von Munchausen, an 18th-century German nobleman known for telling tall tales about his life and exploits.1
In 1977, British pediatrician Roy Meadow described Munchausen’s syndrome by proxy, in which parents induce symptoms in their children—such as by injuring them with drugs or bacterial contaminants—then have doctors treat them.2 Because persons with Munchausen’s tend to frequently change their names and locales, the incidence of Munchausen’s and Munchausen’s by proxy has never been accurately gauged.
Munchausen’s occurs in both sexes, although men tend to exhibit more-dramatic symptoms such as self-induced fevers, bleeding, and seizures. Antisocial behavior is common among men with Munchausen’s, and many are jailed at some point. One man flying from New York to London feigned a heart attack so convincingly that he forced an emergency landing in Halifax, Nova Scotia, where he was hospitalized and released with a referral to his cardiologist.
By contrast, women with Munchausen’s or Munchausen’s by proxy—often nurses or hospital personnel—are more difficult to diagnose because they usually are agreeable and compliant with medical staff. Staff members tend to ask themselves, “Could such a caring person be putting us on?”
Some persons with Munchausen’s visit disease-specific support groups and draw attention or sympathy by faking illness. Having amassed significant medical knowledge through Web searches and frequent doctor visits, they present themselves as both patient and lofty advisor. Their antisocial tendencies can negatively alter group discussions, and the revelation that a fellow group member is an impostor can distress legitimate participants.3
Ms. J exhibited no evidence of a delusional disorder, which occurs rarely among parents with Munchausen’s by proxy. Her allegedly “clingy” (dependent) behavior toward her daughter and ex-husband, however, indicates borderline personality disorder or antisocial personality disorder, which are common in both forms of Munchausen’s. Like Ms. J, these patients
- have strong fears of rejection and abandonment
- are often impulsive
- exhibit damaging behavior to self or others
- have an unstable sense of self
- are unusually willing to comply with clinical tests or procedures
- and describe feelings of emptiness.
How would you confirm a diagnosis of Munchausen’s? What features in Ms J’s case distinguish Munchausen’s from other factitious disorders?Box
- Munchausen’s syndrome by proxy has been reported in women who were sexually abused in their youth. As adults, some of these women become hypervigilant against sexual abuse of their children.
- The disorder has been cited in several legal cases in which a suspect—usually a teacher or religious leader—was falsely accused of sexually molesting a child.4 Psychiatrists involved as expert witnesses or examiners in such cases should consider these allegations as a possible manifestation of Munchausen’s.
- If the accused denies the charges and no evidence is uncovered, the alleged victim’s mother should be interviewed. In some cases the mother was sexually abused and sought help, but the perpetrator was never apprehended. She may harbor resentment toward men and repeatedly ask her child whether he or she has been touched inappropriately. The child—besieged by frequent inquiries or unsure of what constitutes an inappropriate touch—may answer "yes," prompting the mother to press charges against the named individual.
Dr. Messer’s observations
When taking the patient history, look for:
- a pattern of rejection or abuse in youth. In women who have been sexually molested, Munchausen’s by proxy may manifest as allegations that their children have been touched inappropriately (Box).4
- history of multiple surgeries.5 Because Munchausen’s can coexist with genuine physical illness, the psychiatrist and other doctors need to carefully review the findings to determine the existence of medical symptoms or Munchausen’s. Doctors usually choose to operate if there is any suspicion that medical symptoms exist.
- memories of sympathetic and nurturing medical care early in life. A severe emotional crisis can awaken these memories. For example, emotional pain after a divorce or break-up can trigger a Munchausen’s episode in a subconscious attempt to repair this hurt.
Carefully reviewing the history will turn up glaring discrepancies in the patient’s account of his or her illness. Patients with Munchausen’s or Munchausen’s by proxy usually present to numerous clinicians and do not describe their “illness” the same way twice. Sharing information with other members of the care team—many of whom may feel anger after learning that a patient has been feigning illness—is crucial to confirming the diagnosis.
DIFFERENTIAL DIAGNOSIS
Munchausen’s should not be confused with symptoms of other lookalike disorders:
- Hypochondriasis applies to patients who are convinced they have a serious illness despite lack of a medical basis. The person may misinterpret normal bodily functions and suffer severe distress. The symptoms may express inner psychic conflict.
- Somatoform disorder is a physical complaint that cannot be explained as a known medical condition. This may include preoccupation with an imagined defect in appearance or loss of physical capacity. The deficit usually coincides with emotional conflict and—unlike Munchausen’s—there is no fabrication of illness.
- Malingerers purposely fake physical symptoms for specific gain, such as money, hospital care, disability, drugs, or avoiding military duty.
Persons with Munchausen’s have no external incentive for their behavior. Aside from the medical staff’s admiration, Ms. J had nothing to gain from injuring her daughter or portraying her as sickly.
TREATMENT: Coming home
Ms. J’s daughter responded well after being placed in the home of a relative who had a 3-year-old daughter of her own. Ms. J was allowed to see her daughter for 2 hours each day under strict supervision by a retired schoolteacher hired by the family. If Ms. J. violated the agreement, such as by leaving the house with her daughter, she was prohibited from seeing her child the next day.
Ms. J agreed to long-term therapy; in exchange, judicial proceedings were deferred. In most cases, the mother is referred to police and charged with child abuse/endangerment. However, the medical staff in this case felt treatment and supervision would provide ample rehabilitation.
The mother was referred to a psychiatrist trained in psychoanalysis. By making her aware of her unconscious responses, the doctor sought to improve Ms. J’s ability to cope with stress. Patients with a borderline personality disorder seldom respond in psychoanalysis, but Ms. J exhibited significant ego strengths and intelligence—and was motivated to regain custody of her daughter.
Ms. J’s transference response was predictable: She tried to please the doctor in everything she said or did. This reaction was analyzed and the insight helped Ms. J understand why she exaggerated or exacerbated normal childhood illnesses in her daughter. She discovered that she had hungered for approval since childhood and went as far as injuring her daughter to achieve this fulfillment. Early in treatment she developed a mantra: “I have behaved toward my daughter as my mother behaved toward me.”
After 8 months of therapy 3 times a week, Ms. J regained custody. She continued to receive once-weekly psychotherapy for 1 year.
Two years after regaining custody, Ms. J developed a stable, loving relationship with her daughter. She works full-time while her daughter is in high school and participates in many activities. The relative who assumed temporary custody stays in touch, as does her pediatrician.
The girl is seen by her father on alternate weekends. She tells friends she wants to become a nurse.
How can psychiatrists facilitate early diagnosis and treatment of Munchausen’s?
Dr. Messer’s observations
Although primary care physicians or general surgeons treat most patients with Munchausen’s, the disorder is psychiatric in nature.
For that reason, psychiatrists should participate when possible in the early medical workup of a patient who presents with an apparently severe illness. This way, the psychiatrist can:
- help detect Munchausen’s or another factitious disorder sooner
- establish a rapport with the patient early on. If Munchausen’s is subsequently diagnosed, the patient may be less likely to resist treatment or bolt when confronted.
When confronting someone suspected of fabricating or inducing symptoms, tell the patient that he or she might have Munchausen’s syndrome. Describe the disorder and cite cases, then challenge the patient to find a more appropriate way to cope with stress.
Related resources
- Adshead G, Brooke D (eds). Munchausen's syndrome by proxy. Current issues in assessment, treatment and research. Singapore: World Scientific Publishing, 2001.
- Munchausen’s syndrome by proxy. www.gktscientific.com
1. Asher R. Munchausen’s syndrome. Lancet 1951;1:580-5.
2. Meadow R. False allegations of abuse and Munchausen’s syndrome by proxy. Arch Dis Child 1993;68:444-7.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Publishing, 1998.
4. Schreier HA. Repeated false allegations of sexual abuse presenting to sheriffs: when is it Munchausen’s by proxy? Child Abuse Negl 1996;20:985-91.
5. Menninger KA. Polysurgery and polysurgical addiction. Psychoanal Q 1934;3:883-9.
1. Asher R. Munchausen’s syndrome. Lancet 1951;1:580-5.
2. Meadow R. False allegations of abuse and Munchausen’s syndrome by proxy. Arch Dis Child 1993;68:444-7.
3. Feldman MD, Eisendrath SJ. The spectrum of factitious disorders. Washington, DC: American Psychiatric Publishing, 1998.
4. Schreier HA. Repeated false allegations of sexual abuse presenting to sheriffs: when is it Munchausen’s by proxy? Child Abuse Negl 1996;20:985-91.
5. Menninger KA. Polysurgery and polysurgical addiction. Psychoanal Q 1934;3:883-9.
Staying ‘current’
Current Psychiatry is germane and easy to read. The articles offer the insight and depth many professional publications lack.
I was impressed by Dr. Erik Nelson’s and Dr. Susan McElroy’s article on atypical depression (April 2003) and your update on atypical antipsychotics (March 2003).
You and your staff should be proud of the work you do. We in the field need to stay current, and your publication is by far the best way to do it. Keep up the great work.
Ronald O. Asta MD
Third-year psychiatry resident, Meharry Medical College
Elam Mental Health Center, Nashville, TN
Current Psychiatry is germane and easy to read. The articles offer the insight and depth many professional publications lack.
I was impressed by Dr. Erik Nelson’s and Dr. Susan McElroy’s article on atypical depression (April 2003) and your update on atypical antipsychotics (March 2003).
You and your staff should be proud of the work you do. We in the field need to stay current, and your publication is by far the best way to do it. Keep up the great work.
Ronald O. Asta MD
Third-year psychiatry resident, Meharry Medical College
Elam Mental Health Center, Nashville, TN
Current Psychiatry is germane and easy to read. The articles offer the insight and depth many professional publications lack.
I was impressed by Dr. Erik Nelson’s and Dr. Susan McElroy’s article on atypical depression (April 2003) and your update on atypical antipsychotics (March 2003).
You and your staff should be proud of the work you do. We in the field need to stay current, and your publication is by far the best way to do it. Keep up the great work.
Ronald O. Asta MD
Third-year psychiatry resident, Meharry Medical College
Elam Mental Health Center, Nashville, TN
Serotonin toxicity and 5-HT2A receptors
I have some thoughts concerning the article by Harvey Sternbach, MD, on serotonin syndrome (Current Psychiatry, May 2003).
Greater emphasis on the 5-HT2A receptor—both in research and treatment—is justified.1 The most recent evidence, which Dr. Sternbach may not have seen at press time, indicates that severe (life-threatening) cases of serotonin toxicity may benefit from 5-HT2A blockers, such as cyproheptadine or chlorpromazine, rather than propranolol.2,3
Based on recent data, it is difficult to confuse neuroleptic malignant syndrome with serotonin toxicity. Thus, administering chlorpromazine in severe cases is rarely a clinical problem.2,4 In one clinical trial, some 80 patients with moderately severe serotonin toxicity were safely and successfully treated with chlorpromazine or cyproheptadine.2
Recent animal studies have also demonstrated the efficacy of 5-HT2A blockers, but not of propranolol.5,6
Knowledge of serotonin toxicity, or serotonin syndrome, has become increasingly important in psychiatry. Readers wishing to remain current should check out a just-published study4 from the toxicology research group led by Ian Whyte, MBBS. The group’s data, from an analysis of 2,222 cases of serotonergic drug poisoning, increase our understanding of serotonin toxicity. Other data presented by Whyte and colleagues3,4 are summarized on my PsychoTropical Research Web site (www.psychotropical.com/SerotoninToxicity.doc); some are available by searching PubMed.
P. Ken Gillman, MRC Psych
Consultant and director
Department of clinical neuropharmacology
Pioneer Valley Private Hospital
Mackay, Australia
- Gillman PK. Serotonin syndrome: history and risk. Fundam Clin Pharmacol 1998:12:482–91.
- Isbister GK, Dawson AH, et al. Comment: serotonin syndrome and 5-HT2A antagonism. Ann Pharmacother 2001;35:1143–4.
- Isbister GK, Whyte IM. Serotonin toxicity and malignant hyperthermia: role of 5-HT2 receptors. Br J Anaesth 2002;88:603.
- Dunkley E, Isbister G, et al. Hunter serotonin toxicity criteria: a simple and accurate diagnostic decision rule for serotonin toxicity. Q J Med 2003;96:635–42.
- Nisijima K, Shioda K, et al. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003;43:155–64.
- Nisijima K, Yoshino T, et al. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001;890:23–31.
I have some thoughts concerning the article by Harvey Sternbach, MD, on serotonin syndrome (Current Psychiatry, May 2003).
Greater emphasis on the 5-HT2A receptor—both in research and treatment—is justified.1 The most recent evidence, which Dr. Sternbach may not have seen at press time, indicates that severe (life-threatening) cases of serotonin toxicity may benefit from 5-HT2A blockers, such as cyproheptadine or chlorpromazine, rather than propranolol.2,3
Based on recent data, it is difficult to confuse neuroleptic malignant syndrome with serotonin toxicity. Thus, administering chlorpromazine in severe cases is rarely a clinical problem.2,4 In one clinical trial, some 80 patients with moderately severe serotonin toxicity were safely and successfully treated with chlorpromazine or cyproheptadine.2
Recent animal studies have also demonstrated the efficacy of 5-HT2A blockers, but not of propranolol.5,6
Knowledge of serotonin toxicity, or serotonin syndrome, has become increasingly important in psychiatry. Readers wishing to remain current should check out a just-published study4 from the toxicology research group led by Ian Whyte, MBBS. The group’s data, from an analysis of 2,222 cases of serotonergic drug poisoning, increase our understanding of serotonin toxicity. Other data presented by Whyte and colleagues3,4 are summarized on my PsychoTropical Research Web site (www.psychotropical.com/SerotoninToxicity.doc); some are available by searching PubMed.
P. Ken Gillman, MRC Psych
Consultant and director
Department of clinical neuropharmacology
Pioneer Valley Private Hospital
Mackay, Australia
- Gillman PK. Serotonin syndrome: history and risk. Fundam Clin Pharmacol 1998:12:482–91.
- Isbister GK, Dawson AH, et al. Comment: serotonin syndrome and 5-HT2A antagonism. Ann Pharmacother 2001;35:1143–4.
- Isbister GK, Whyte IM. Serotonin toxicity and malignant hyperthermia: role of 5-HT2 receptors. Br J Anaesth 2002;88:603.
- Dunkley E, Isbister G, et al. Hunter serotonin toxicity criteria: a simple and accurate diagnostic decision rule for serotonin toxicity. Q J Med 2003;96:635–42.
- Nisijima K, Shioda K, et al. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003;43:155–64.
- Nisijima K, Yoshino T, et al. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001;890:23–31.
I have some thoughts concerning the article by Harvey Sternbach, MD, on serotonin syndrome (Current Psychiatry, May 2003).
Greater emphasis on the 5-HT2A receptor—both in research and treatment—is justified.1 The most recent evidence, which Dr. Sternbach may not have seen at press time, indicates that severe (life-threatening) cases of serotonin toxicity may benefit from 5-HT2A blockers, such as cyproheptadine or chlorpromazine, rather than propranolol.2,3
Based on recent data, it is difficult to confuse neuroleptic malignant syndrome with serotonin toxicity. Thus, administering chlorpromazine in severe cases is rarely a clinical problem.2,4 In one clinical trial, some 80 patients with moderately severe serotonin toxicity were safely and successfully treated with chlorpromazine or cyproheptadine.2
Recent animal studies have also demonstrated the efficacy of 5-HT2A blockers, but not of propranolol.5,6
Knowledge of serotonin toxicity, or serotonin syndrome, has become increasingly important in psychiatry. Readers wishing to remain current should check out a just-published study4 from the toxicology research group led by Ian Whyte, MBBS. The group’s data, from an analysis of 2,222 cases of serotonergic drug poisoning, increase our understanding of serotonin toxicity. Other data presented by Whyte and colleagues3,4 are summarized on my PsychoTropical Research Web site (www.psychotropical.com/SerotoninToxicity.doc); some are available by searching PubMed.
P. Ken Gillman, MRC Psych
Consultant and director
Department of clinical neuropharmacology
Pioneer Valley Private Hospital
Mackay, Australia
- Gillman PK. Serotonin syndrome: history and risk. Fundam Clin Pharmacol 1998:12:482–91.
- Isbister GK, Dawson AH, et al. Comment: serotonin syndrome and 5-HT2A antagonism. Ann Pharmacother 2001;35:1143–4.
- Isbister GK, Whyte IM. Serotonin toxicity and malignant hyperthermia: role of 5-HT2 receptors. Br J Anaesth 2002;88:603.
- Dunkley E, Isbister G, et al. Hunter serotonin toxicity criteria: a simple and accurate diagnostic decision rule for serotonin toxicity. Q J Med 2003;96:635–42.
- Nisijima K, Shioda K, et al. Diazepam and chlormethiazole attenuate the development of hyperthermia in an animal model of the serotonin syndrome. Neurochem Int 2003;43:155–64.
- Nisijima K, Yoshino T, et al. Potent serotonin (5-HT)(2A) receptor antagonists completely prevent the development of hyperthermia in an animal model of the 5-HT syndrome. Brain Res 2001;890:23–31.
Return phone calls, remain calm, and invest in good billing software
Whether you’ve just completed your residency or have been in practice for years, attention to small details can make or break a practice.
The following five tips can help you start—or improve upon—a successful practice:
- Spell out your billing procedures in writing. List specific fees (eg, consultation fee, 45-minute therapy visit, 15- to 20-minute medical evaluation) and payment schedules.
- Obtain billing software, especially if you employ minimal office help. You’ll need to generate insurance-based invoices on paper and to episodically (weekly to monthly) print out patient bills in batches, depending on your practice’s size and caseload.
- Return phone calls. This may seem basic, but patients expect their doctors to get back to them the same day.
- Be nice to referral sources and to your staff. Niceness is a remarkably powerful tool that can help you forge valuable professional relationships and assist you clinically.
- Provide consultation reports—typewritten and timely—to referral sources. Many doctors keep typed records of all office encounters. Thoroughly documenting the first meeting with a patient is particularly important to establish rapport with referral sources and to refresh your memory of the case when the patient reappears after a hiatus. These reports also help justify consultation fees.
Dr. Vuckovic is assistant clinical professor of psychiatry, Harvard Medical School, Boston, and is medical director of the Pavilion at McLean Hospital, a residential psychiatric evaluation program.
Whether you’ve just completed your residency or have been in practice for years, attention to small details can make or break a practice.
The following five tips can help you start—or improve upon—a successful practice:
- Spell out your billing procedures in writing. List specific fees (eg, consultation fee, 45-minute therapy visit, 15- to 20-minute medical evaluation) and payment schedules.
- Obtain billing software, especially if you employ minimal office help. You’ll need to generate insurance-based invoices on paper and to episodically (weekly to monthly) print out patient bills in batches, depending on your practice’s size and caseload.
- Return phone calls. This may seem basic, but patients expect their doctors to get back to them the same day.
- Be nice to referral sources and to your staff. Niceness is a remarkably powerful tool that can help you forge valuable professional relationships and assist you clinically.
- Provide consultation reports—typewritten and timely—to referral sources. Many doctors keep typed records of all office encounters. Thoroughly documenting the first meeting with a patient is particularly important to establish rapport with referral sources and to refresh your memory of the case when the patient reappears after a hiatus. These reports also help justify consultation fees.
Whether you’ve just completed your residency or have been in practice for years, attention to small details can make or break a practice.
The following five tips can help you start—or improve upon—a successful practice:
- Spell out your billing procedures in writing. List specific fees (eg, consultation fee, 45-minute therapy visit, 15- to 20-minute medical evaluation) and payment schedules.
- Obtain billing software, especially if you employ minimal office help. You’ll need to generate insurance-based invoices on paper and to episodically (weekly to monthly) print out patient bills in batches, depending on your practice’s size and caseload.
- Return phone calls. This may seem basic, but patients expect their doctors to get back to them the same day.
- Be nice to referral sources and to your staff. Niceness is a remarkably powerful tool that can help you forge valuable professional relationships and assist you clinically.
- Provide consultation reports—typewritten and timely—to referral sources. Many doctors keep typed records of all office encounters. Thoroughly documenting the first meeting with a patient is particularly important to establish rapport with referral sources and to refresh your memory of the case when the patient reappears after a hiatus. These reports also help justify consultation fees.
Dr. Vuckovic is assistant clinical professor of psychiatry, Harvard Medical School, Boston, and is medical director of the Pavilion at McLean Hospital, a residential psychiatric evaluation program.
Dr. Vuckovic is assistant clinical professor of psychiatry, Harvard Medical School, Boston, and is medical director of the Pavilion at McLean Hospital, a residential psychiatric evaluation program.
Getting it right about menopause
We in psychiatry have often gotten it wrong about menopause. We have “pathologized” normal perimenopause experience, at times ascribing its mood symptoms to DSM-II’s “involutional melancholia,” or to change of life or empty nest syndrome. At other times we have “normalized” pathological experience, dismissing women’s complaints of depression and anxiety and referring them too quickly to Ob/Gyns.
Sometimes we have relied too heavily on hormonal treatments and at other times have not used them enough. Hormone replacement therapies clearly do benefit many women with psychiatric symptoms ranging from mild to severe. But hormonal treatments do not help all women and they can have harmful side effects, as the Women’s Health Initiative studies of estrogen with progesterone have shown. We clearly need to understand more about hormonal treatments and not just leave their understanding and management to Ob/Gyns.
A step in that direction is “Minding menopause,” by Louann Brizendine, MD. This article presents the current approach to diagnosis and treatment of menopause-related psychiatric symptoms, based on the latest evidence and Dr. Brizendine’s experience as director of the Women’s Mood and Hormone Clinic at Langley Porter Psychiatric Clinic, San Francisco.
Dr. Brizendine’s article portrays psychiatry at its best, changing its approaches to coincide with new data and experience—until we get it right.
We in psychiatry have often gotten it wrong about menopause. We have “pathologized” normal perimenopause experience, at times ascribing its mood symptoms to DSM-II’s “involutional melancholia,” or to change of life or empty nest syndrome. At other times we have “normalized” pathological experience, dismissing women’s complaints of depression and anxiety and referring them too quickly to Ob/Gyns.
Sometimes we have relied too heavily on hormonal treatments and at other times have not used them enough. Hormone replacement therapies clearly do benefit many women with psychiatric symptoms ranging from mild to severe. But hormonal treatments do not help all women and they can have harmful side effects, as the Women’s Health Initiative studies of estrogen with progesterone have shown. We clearly need to understand more about hormonal treatments and not just leave their understanding and management to Ob/Gyns.
A step in that direction is “Minding menopause,” by Louann Brizendine, MD. This article presents the current approach to diagnosis and treatment of menopause-related psychiatric symptoms, based on the latest evidence and Dr. Brizendine’s experience as director of the Women’s Mood and Hormone Clinic at Langley Porter Psychiatric Clinic, San Francisco.
Dr. Brizendine’s article portrays psychiatry at its best, changing its approaches to coincide with new data and experience—until we get it right.
We in psychiatry have often gotten it wrong about menopause. We have “pathologized” normal perimenopause experience, at times ascribing its mood symptoms to DSM-II’s “involutional melancholia,” or to change of life or empty nest syndrome. At other times we have “normalized” pathological experience, dismissing women’s complaints of depression and anxiety and referring them too quickly to Ob/Gyns.
Sometimes we have relied too heavily on hormonal treatments and at other times have not used them enough. Hormone replacement therapies clearly do benefit many women with psychiatric symptoms ranging from mild to severe. But hormonal treatments do not help all women and they can have harmful side effects, as the Women’s Health Initiative studies of estrogen with progesterone have shown. We clearly need to understand more about hormonal treatments and not just leave their understanding and management to Ob/Gyns.
A step in that direction is “Minding menopause,” by Louann Brizendine, MD. This article presents the current approach to diagnosis and treatment of menopause-related psychiatric symptoms, based on the latest evidence and Dr. Brizendine’s experience as director of the Women’s Mood and Hormone Clinic at Langley Porter Psychiatric Clinic, San Francisco.
Dr. Brizendine’s article portrays psychiatry at its best, changing its approaches to coincide with new data and experience—until we get it right.