User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
ECT: Effective, but it has an image problem
Yesterday I tried to explain electroconvulsive therapy (ECT) to my 15-year-old son. Of my three children, he comes closest to idealizing me and is most likely to consider medical school. Still, he was a tough sell. His initial reaction to ECT was “that sounds sort of primitive.”
Dr. Max Fink’s article in this issue reviews the overwhelming evidence for ECT’s efficacy in major depressive disorder and the obstacles that prevent ECT from being used as widely as research suggests it should be. Two obstacles are limited availability (few psychiatrists make it part of their practice) and social stigma. The stigma leads to low availability, which makes ECT available only as a last resort, which in turn increases the stigma.
Dr. Fink identifies a third obstacle as “academic low regard,” meaning that academic psychiatrists relegate ECT to a third- or fourth-line therapy and neglect to teach about it. ECT was introduced before psychodynamic therapies and effective medications revolutionized psychiatry. Consequently, psychiatrists trained in psychodynamics and psychopharmacology pay less attention to ECT than the data warrant.
Repetitive transcranial magnetic stimulation (rTMS)—ably reviewed in this issue by Drs. Sheila Dowd and Philip Janicak—has an advantage over ECT in being new and therefore perceived as exciting. It also is less aesthetically problematic because most people have a more positive attitude towards magnets than electric shocks.
Time will tell where rTMS might fit into our treatment algorithms for major depressive disorder. Taken together, however, ECT and rTMS illustrate how psychiatry can advance by keeping established treatments of proven efficacy while embracing new treatments.
Either because I convinced my son of the benefits of ECT—or because he wanted to avoid conflict—he eventually said, “Well, I guess you have to use whatever works, even if you don’t know exactly how it works.” So true.
Yesterday I tried to explain electroconvulsive therapy (ECT) to my 15-year-old son. Of my three children, he comes closest to idealizing me and is most likely to consider medical school. Still, he was a tough sell. His initial reaction to ECT was “that sounds sort of primitive.”
Dr. Max Fink’s article in this issue reviews the overwhelming evidence for ECT’s efficacy in major depressive disorder and the obstacles that prevent ECT from being used as widely as research suggests it should be. Two obstacles are limited availability (few psychiatrists make it part of their practice) and social stigma. The stigma leads to low availability, which makes ECT available only as a last resort, which in turn increases the stigma.
Dr. Fink identifies a third obstacle as “academic low regard,” meaning that academic psychiatrists relegate ECT to a third- or fourth-line therapy and neglect to teach about it. ECT was introduced before psychodynamic therapies and effective medications revolutionized psychiatry. Consequently, psychiatrists trained in psychodynamics and psychopharmacology pay less attention to ECT than the data warrant.
Repetitive transcranial magnetic stimulation (rTMS)—ably reviewed in this issue by Drs. Sheila Dowd and Philip Janicak—has an advantage over ECT in being new and therefore perceived as exciting. It also is less aesthetically problematic because most people have a more positive attitude towards magnets than electric shocks.
Time will tell where rTMS might fit into our treatment algorithms for major depressive disorder. Taken together, however, ECT and rTMS illustrate how psychiatry can advance by keeping established treatments of proven efficacy while embracing new treatments.
Either because I convinced my son of the benefits of ECT—or because he wanted to avoid conflict—he eventually said, “Well, I guess you have to use whatever works, even if you don’t know exactly how it works.” So true.
Yesterday I tried to explain electroconvulsive therapy (ECT) to my 15-year-old son. Of my three children, he comes closest to idealizing me and is most likely to consider medical school. Still, he was a tough sell. His initial reaction to ECT was “that sounds sort of primitive.”
Dr. Max Fink’s article in this issue reviews the overwhelming evidence for ECT’s efficacy in major depressive disorder and the obstacles that prevent ECT from being used as widely as research suggests it should be. Two obstacles are limited availability (few psychiatrists make it part of their practice) and social stigma. The stigma leads to low availability, which makes ECT available only as a last resort, which in turn increases the stigma.
Dr. Fink identifies a third obstacle as “academic low regard,” meaning that academic psychiatrists relegate ECT to a third- or fourth-line therapy and neglect to teach about it. ECT was introduced before psychodynamic therapies and effective medications revolutionized psychiatry. Consequently, psychiatrists trained in psychodynamics and psychopharmacology pay less attention to ECT than the data warrant.
Repetitive transcranial magnetic stimulation (rTMS)—ably reviewed in this issue by Drs. Sheila Dowd and Philip Janicak—has an advantage over ECT in being new and therefore perceived as exciting. It also is less aesthetically problematic because most people have a more positive attitude towards magnets than electric shocks.
Time will tell where rTMS might fit into our treatment algorithms for major depressive disorder. Taken together, however, ECT and rTMS illustrate how psychiatry can advance by keeping established treatments of proven efficacy while embracing new treatments.
Either because I convinced my son of the benefits of ECT—or because he wanted to avoid conflict—he eventually said, “Well, I guess you have to use whatever works, even if you don’t know exactly how it works.” So true.
Therapy-resistant major depression When to consider ECT: Algorithm seeks respect for neglected therapy
Patients with what’s called “therapy-resistant” depression (TRD)—with subtherapeutic response to medications and psychotherapy—are often actually suffering from unrecognized, inadequately treated psychotic depression. Psychiatrists could greatly diminish the clinical challenge of TRD by recognizing psychotic depression and treating it more effectively.1 And the most effective treatment for psychotic depression is neither antidepressants nor antipsychotic drugs but electroconvulsive therapy (ECT).
Despite ECT’s superior efficacy in TRD, however, algorithms for treating major depression relegate ECT to an option of last resort. By not considering ECT sooner, we consign many severely depressed patients to less-effective treatments and the risk of chronic illness.
Table
Diagnostic signs of psychosis in patients with major depression
| Sign | Example |
|---|---|
| Somatic concern | Delusions of fatal illness |
| Grandiosity | Special relation to God or royalty |
| Suspiciousness | Delusions of spousal infidelity |
| Hallucinations | Foul body odor |
| Unusual thought | Bizarre, confused ideation |
| Depressive delusion | Worthlessness, guilt, feelings of deserving death or punishment |
| Source: Based on the Brief Psychiatric Rating Scale.14 | |
It is time for a more realistic algorithm that recommends ECT earlier for major depressive episodes, with or without psychotic features. This article proposes such an algorithm and discusses the supporting evidence.
TREATING PSYCHOTIC DEPRESSION
Patients with delusions or hallucinations were classified as suffering from schizophrenia until the mid-1970s. Researchers then found that depressed patients with psychotic features responded well to ECT but poorly to adequate serum levels of imipramine.2
These observations were confirmed by a large Italian study, in which 437 depressed patients were treated with high-dose imipramine (200 to 350 mg/d). Depression remitted in 244 patients (56%). Those who remained depressed were then treated with ECT, and depression remitted in 136 of 190 (72%). Psychosis was the marker of poor response to imipramine.3 DSM-III codified these findings by separating the syndrome of “major depression with psychosis” (296.34) from “major depression without psychosis” (296.33).
As psychiatry recognized psychotic depression as a distinct form of depression, it became clear that drugs often could not adequately treat it. Less than one-third of patients with psychotic depression respond to tricyclics alone.4-6
Response to antipsychotic monotherapy averaged 50% and increased to 75% with combined antipsychotics and antidepressants. However, high daily dosages —at least 32 mg of perphenazine and 225 mg of amitriptyline—were required for an adequate response,7 and side effects made sustaining such heroic dosing was difficult. The greatest improvement rates were seen with ECT.
Few other drug combinations have been reported to be effective in psychotic depression, but we lack proper studies. Schatzberg8 addressed the use of newer antidepressants and atypical antipsychotics without offering an algorithm based on the data. Evidence on combination therapies consists mainly of case reports, with few designed studies.
EFFICACY OF ECT
ECT is the most effective treatment for psychotic major depression—achieving remission rates >80% within 3 weeks—as demonstrated by the ongoing, four-hospital Consortium for Research in ECT (CORE), supported by the National Institute of Mental Health.
CORE researchers are studying the efficacy of bilateral ECT in treating severe unipolar depression in patients ages 18 to 85 and of continuation treatments with ECT or lithium plus nortriptyline.9 Under the CORE protocol, diagnoses are made by structured clinical interview using DSM-III-R criteria, and remission is defined as >60% reduction in Hamilton Rating Scale for Depression scores, with final scores 10 sustained for 1 week.
In the first 253 CORE patients treated with ECT, depression remitted in 75% and did not remit in 11%; 14% dropped out. Psychotic depression was identified in 30% (77 of 253), and the remission rate among these patients was 83%.
Among patients who completed the full ECT course (at least 12 sessions), remission rates were 96% for psychotic depression and 83% for nonpsychotic depression. The overall remission rate was 87%.
Treatments were given three times per week. Among patients who completed treatment in weeks 1 through 4, remission rates were 5%, 45%, 81%, and 100%, respectively. Psychotic depression remitted more rapidly than nonpsychotic depression.
Suicide risk. CORE findings suggest that ECT also may reduce suicide risk. In item 3 of the Hamilton Rating Scale for Depression, scores of 2 to 4 indicate preoccupation with death or suicide, or a recent suicide attempt. Nearly 60% of 404 patients (237) reported baseline scores of 2 to 4, but their scores dropped rapidly with ECT. Scores of 0 were reported in 68% after 1 week of ECT, in 87% after 2 weeks, and in 93% after 3 weeks.10
Summary. In patients with severe depressive illness, CORE’s remission rates of 95% for psychotic depression and 83% for nonpsychotic depression are remarkable. Another group is independently reporting a 92% remission rate for psychotic depression treated with ECT.11
Psychotic depression is difficult to recognize and treat, even for clinicians with advanced training. For example:
- only 2 of 52 psychotic depressed patients were determined to have been adequately treated before referral to a National Institute of Mental Health-supported study of ECT15
- only 3 of 46 psychotic depressed patients had been adequately treated prior to enrollment in the Consortium for Research in ECT (CORE) study.9
The three most useful diagnostic criteria are spelled out in the Brief Psychiatric Rating Scale:
- any sign of psychosis is sufficient for designating a major depression as “psychotic”
- one well-developed sign is sufficient to prescribe treatment for psychotic depression
- well-developed vegetative signs also indicate the need to treat psychotic depression.14
ROADBLOCKS TO WIDER USE OF ECT
Many eligible patients never receive ECT, despite its track record of producing high remission rates in psychotic depression. Reasons include:
Limited access. Few psychiatrists—less than 8%—offer ECT as a treatment option, and most who do offer it practice in private hospitals.12-14
Academic low regard. Psychiatry’s academic lecturers largely ignore ECT’s efficacy in psychotic depression and therapy-resistant depression. This low regard for ECT is codified in expert algorithms, which cite ECT as an option of last resort.
Social stigma. In a recent essay summarizing medication’s weak effect in treating psychotic depression, Schatzberg states, “While ECT is a remarkably effective treatment for psychotic depression, requirements for its use are stringent, and public perception about the overall appropriateness of shock treatment is negative.”8
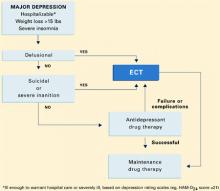
Algorithm Treatment of major depression, with or without psychotic features
ALGORITHM FOR MAJOR DEPRESSION
Because patients with psychotic and nonpsychotic major depression clearly require different treatments, differentiating between these two types is critical. Although psychotic depression can be difficult to diagnose,15,16 commonly recognized criteria are listed in the Table
Assessment. Assess each severely depressed patient for psychotic features, such as delusions and hallucinations. A useful assessment guide is the Brief Psychiatric Rating Scale (Box).17 Also treat those with melancholia, inanition, severe weight loss and insomnia, concentration and memory difficulty, stupor, or suicidal ideation as if they had psychotic depression. These symptoms and signs are evidence that the patient’s neuroendocrine system is disturbed, an indication of severe depression that responds poorly to antidepressant drugs alone.
Treatment. Nonpsychotic depressed patients are best offered antidepressants—tricyclics or selective serotonin reuptake inhibitors (SSRIs)—as recommended by conventional guidelines. Insufficient response to two adequate trials calls for a careful assessment for psychosis and, if found, treatment with effective drug combinations or ECT (Algorithm).18 For patients with psychotic depression—especially those who fail medication trials or whose severe symptoms would likely respond to ECT as a primary treatment—bilateral ECT is the effective standard.19
ECT is the appropriate first option for hospitalized patients with psychotic depression—especially those who are suicidal or require supplementary feeding and sedation. It may also be considered the first option in patients who have:
- attempted suicide
- lost more than 10% of body weight (approximately 15 lbs for adults) in the weeks of their illness
- or show signs of severe melancholia, such as catatonia or pseudodementia.
TREATING NONPSYCHOTIC DEPRESSION
When medications are first-line treatment for nonpsychotic depression, how long should a trial be continued before taking another tack? How many courses should you try before you declare therapy resistance and consider ECT?
Studies of clozapine provide a useful model.20 Because of clozapine’s association with agranulocytosis and induced seizures, patients with schizophrenia usually do not receive this antipsychotic unless two 4- to 6-week trials of other neuroleptics have proven ineffective. Ethicists have deemed two failed medication trials to be sufficient before a more hazardous treatment is offered.
Figure Remission of major depression with ECT
We can apply a similar standard when considering ECT in patients first treated with medication.18 A patient’s depression could be defined as therapy-resistant after an inadequate response to 4-week courses (in either order) of:
- an SSRI at dosages equivalent to fluoxetine, 40 mg/d
- a tricyclic at 200 mg/d.
In depressed patients with bipolar disorder, a trial of lithium or an anticonvulsant may replace an antidepressant.
ECT is appropriate when debilitating depression persists after two adequate medication trials. Only after an adequate ECT trial has failed—and such failure is infrequent—is it reasonable to offer poorly tested augmentation and combination strategies.
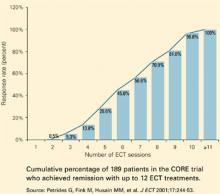
What is ‘adequate’ ECT? For patients with major depression, the definition of an adequate ECT trial is complex. Although many doctors expect six ECT sessions to be sufficient, the CORE studies are finding that only 45% of patients remitted with six ECT, 81% with nine ECT, and almost all with 12 ECT sessions (Figure).9 These patients were treated with bitemporal electrode placement, the more effective form of ECT. When unilateral electrode placements are used, ECT courses may be inadequate, as this form requires special attention to electrical dosing.
Further, the quality of the seizure—like the dosing of medications—directly influences outcome. Seizure monitoring is essential for assessing the adequacy of each treatment and the treatment course.21,22
Related resources
- Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus non-psychotic depressed patients: a report from CORE. J ECT 2001;17:244-53.
Drug brand names
- Amitriptyline • Elavil
- Clozapine • Clozaril
- Imipramine • Tofranil
- Nortriptyline • Pamelor
- Perphenazine • Trilafon
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was aided by grants from the Scion Natural Science Association, Inc., St. James, NY.
1. Thase M. New approaches to managing difficult-to-treat depressions. J Clin Psychiatry 2003;64[suppl1]:3-4.
2. Kantor SJ, Glassman AH. Delusional depressions: natural history and response to treatment. Br J Psychiatry 1977;131:351-6.
3. Avery D, Lubrano A. Depression treated with imipramine and ECT: the deCarolis study reconsidered. Am J Psychiatry 1979;136:559-62.
4. Kroessler D. Relative efficacy rates for therapies of delusional depression. Convuls Ther 1985;1:173-82.
5. Parker G, Roy K, Hadzi-Pavlovic D, Pedic F. Psychotic (delusional) depression: a meta-analysis of physical treatments. J Affect Dis 1992;24:17-24.
6. Wheeler Vega JA, Mortimer AM, Tyson PJ. Somatic treatment of psychotic depression: review and recommendations for practice. J Clin Psychopharmacol 2000;20:504-19.
7. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacologic treatment of delusional depression. Am J Psychiatry 1985;142:430-6.
8. Schatzberg AF. New approaches to managing psychotic depression. J Clin Psychiatry 2003;64[suppl1]:19-23.
9. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus non-psychotic depressed patients: a report from CORE. J ECT 2001;17:244-53.
10. Kellner CH, Fink M, Knapp R, et al. Bilateral ECT rapidly relieves suicidality: findings from phase I of the CORE ECT study. Am J Psychiatry (submitted).
11. Birkenhäger TK, Pluijms EM, Lucius SAP. ECT response in delusional versus non-delusional depressed inpatients. J Affect Dis (in press).
12. Hermann RC, Ettner SL, Dorwart RA, et al. Characteristics of psychiatrists who perform ECT. Am J Psychiatry 1998;155:889-94.
13. Thompson JW, Weiner RD, Myers CP. Use of ECT in the United States in 1975, 1980, and 1986. Am J Psychiatry 1994;151:1657-61.
14. Kramer BA. Use of ECT in California revisited: 1984-1994. J ECT 1999;15:245-51.
15. Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res 1990;31:287-96.
16. Mulsant BH, Haskett RF, Prudic J, et al. Low use of neuroleptic drugs in the treatment of psychotic major depression. Am J Psychiatry 1997;154:559-61.
17. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep 1962;10:799-812.
18. Fink M. Electroconvulsive therapy in medication-resistant depression. In: Amsterdam J, Hornig-Rohan M, Nierenberg A (eds.): Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press, 2001;223-38.
19. Fink M. The efficacy of ECT and “treatment resistance.” J ECT 2002;18:1-2.
20. Lieberman JA, Kane JM, Johns CA. Clozapine: guidelines for clinical management. J Clin Psychiatry 1989;50:329-38.
21. Abrams R. Electroconvulsive therapy. (4th ed). New York: Oxford University Press, 2002.
22. Fink M. Optimizing ECT. Encephale 1994;20:297-302.
Patients with what’s called “therapy-resistant” depression (TRD)—with subtherapeutic response to medications and psychotherapy—are often actually suffering from unrecognized, inadequately treated psychotic depression. Psychiatrists could greatly diminish the clinical challenge of TRD by recognizing psychotic depression and treating it more effectively.1 And the most effective treatment for psychotic depression is neither antidepressants nor antipsychotic drugs but electroconvulsive therapy (ECT).
Despite ECT’s superior efficacy in TRD, however, algorithms for treating major depression relegate ECT to an option of last resort. By not considering ECT sooner, we consign many severely depressed patients to less-effective treatments and the risk of chronic illness.
Table
Diagnostic signs of psychosis in patients with major depression
| Sign | Example |
|---|---|
| Somatic concern | Delusions of fatal illness |
| Grandiosity | Special relation to God or royalty |
| Suspiciousness | Delusions of spousal infidelity |
| Hallucinations | Foul body odor |
| Unusual thought | Bizarre, confused ideation |
| Depressive delusion | Worthlessness, guilt, feelings of deserving death or punishment |
| Source: Based on the Brief Psychiatric Rating Scale.14 | |
It is time for a more realistic algorithm that recommends ECT earlier for major depressive episodes, with or without psychotic features. This article proposes such an algorithm and discusses the supporting evidence.
TREATING PSYCHOTIC DEPRESSION
Patients with delusions or hallucinations were classified as suffering from schizophrenia until the mid-1970s. Researchers then found that depressed patients with psychotic features responded well to ECT but poorly to adequate serum levels of imipramine.2
These observations were confirmed by a large Italian study, in which 437 depressed patients were treated with high-dose imipramine (200 to 350 mg/d). Depression remitted in 244 patients (56%). Those who remained depressed were then treated with ECT, and depression remitted in 136 of 190 (72%). Psychosis was the marker of poor response to imipramine.3 DSM-III codified these findings by separating the syndrome of “major depression with psychosis” (296.34) from “major depression without psychosis” (296.33).
As psychiatry recognized psychotic depression as a distinct form of depression, it became clear that drugs often could not adequately treat it. Less than one-third of patients with psychotic depression respond to tricyclics alone.4-6
Response to antipsychotic monotherapy averaged 50% and increased to 75% with combined antipsychotics and antidepressants. However, high daily dosages —at least 32 mg of perphenazine and 225 mg of amitriptyline—were required for an adequate response,7 and side effects made sustaining such heroic dosing was difficult. The greatest improvement rates were seen with ECT.
Few other drug combinations have been reported to be effective in psychotic depression, but we lack proper studies. Schatzberg8 addressed the use of newer antidepressants and atypical antipsychotics without offering an algorithm based on the data. Evidence on combination therapies consists mainly of case reports, with few designed studies.
EFFICACY OF ECT
ECT is the most effective treatment for psychotic major depression—achieving remission rates >80% within 3 weeks—as demonstrated by the ongoing, four-hospital Consortium for Research in ECT (CORE), supported by the National Institute of Mental Health.
CORE researchers are studying the efficacy of bilateral ECT in treating severe unipolar depression in patients ages 18 to 85 and of continuation treatments with ECT or lithium plus nortriptyline.9 Under the CORE protocol, diagnoses are made by structured clinical interview using DSM-III-R criteria, and remission is defined as >60% reduction in Hamilton Rating Scale for Depression scores, with final scores 10 sustained for 1 week.
In the first 253 CORE patients treated with ECT, depression remitted in 75% and did not remit in 11%; 14% dropped out. Psychotic depression was identified in 30% (77 of 253), and the remission rate among these patients was 83%.
Among patients who completed the full ECT course (at least 12 sessions), remission rates were 96% for psychotic depression and 83% for nonpsychotic depression. The overall remission rate was 87%.
Treatments were given three times per week. Among patients who completed treatment in weeks 1 through 4, remission rates were 5%, 45%, 81%, and 100%, respectively. Psychotic depression remitted more rapidly than nonpsychotic depression.
Suicide risk. CORE findings suggest that ECT also may reduce suicide risk. In item 3 of the Hamilton Rating Scale for Depression, scores of 2 to 4 indicate preoccupation with death or suicide, or a recent suicide attempt. Nearly 60% of 404 patients (237) reported baseline scores of 2 to 4, but their scores dropped rapidly with ECT. Scores of 0 were reported in 68% after 1 week of ECT, in 87% after 2 weeks, and in 93% after 3 weeks.10
Summary. In patients with severe depressive illness, CORE’s remission rates of 95% for psychotic depression and 83% for nonpsychotic depression are remarkable. Another group is independently reporting a 92% remission rate for psychotic depression treated with ECT.11
Psychotic depression is difficult to recognize and treat, even for clinicians with advanced training. For example:
- only 2 of 52 psychotic depressed patients were determined to have been adequately treated before referral to a National Institute of Mental Health-supported study of ECT15
- only 3 of 46 psychotic depressed patients had been adequately treated prior to enrollment in the Consortium for Research in ECT (CORE) study.9
The three most useful diagnostic criteria are spelled out in the Brief Psychiatric Rating Scale:
- any sign of psychosis is sufficient for designating a major depression as “psychotic”
- one well-developed sign is sufficient to prescribe treatment for psychotic depression
- well-developed vegetative signs also indicate the need to treat psychotic depression.14
ROADBLOCKS TO WIDER USE OF ECT
Many eligible patients never receive ECT, despite its track record of producing high remission rates in psychotic depression. Reasons include:
Limited access. Few psychiatrists—less than 8%—offer ECT as a treatment option, and most who do offer it practice in private hospitals.12-14
Academic low regard. Psychiatry’s academic lecturers largely ignore ECT’s efficacy in psychotic depression and therapy-resistant depression. This low regard for ECT is codified in expert algorithms, which cite ECT as an option of last resort.
Social stigma. In a recent essay summarizing medication’s weak effect in treating psychotic depression, Schatzberg states, “While ECT is a remarkably effective treatment for psychotic depression, requirements for its use are stringent, and public perception about the overall appropriateness of shock treatment is negative.”8
Algorithm Treatment of major depression, with or without psychotic features
ALGORITHM FOR MAJOR DEPRESSION
Because patients with psychotic and nonpsychotic major depression clearly require different treatments, differentiating between these two types is critical. Although psychotic depression can be difficult to diagnose,15,16 commonly recognized criteria are listed in the Table
Assessment. Assess each severely depressed patient for psychotic features, such as delusions and hallucinations. A useful assessment guide is the Brief Psychiatric Rating Scale (Box).17 Also treat those with melancholia, inanition, severe weight loss and insomnia, concentration and memory difficulty, stupor, or suicidal ideation as if they had psychotic depression. These symptoms and signs are evidence that the patient’s neuroendocrine system is disturbed, an indication of severe depression that responds poorly to antidepressant drugs alone.
Treatment. Nonpsychotic depressed patients are best offered antidepressants—tricyclics or selective serotonin reuptake inhibitors (SSRIs)—as recommended by conventional guidelines. Insufficient response to two adequate trials calls for a careful assessment for psychosis and, if found, treatment with effective drug combinations or ECT (Algorithm).18 For patients with psychotic depression—especially those who fail medication trials or whose severe symptoms would likely respond to ECT as a primary treatment—bilateral ECT is the effective standard.19
ECT is the appropriate first option for hospitalized patients with psychotic depression—especially those who are suicidal or require supplementary feeding and sedation. It may also be considered the first option in patients who have:
- attempted suicide
- lost more than 10% of body weight (approximately 15 lbs for adults) in the weeks of their illness
- or show signs of severe melancholia, such as catatonia or pseudodementia.
TREATING NONPSYCHOTIC DEPRESSION
When medications are first-line treatment for nonpsychotic depression, how long should a trial be continued before taking another tack? How many courses should you try before you declare therapy resistance and consider ECT?
Studies of clozapine provide a useful model.20 Because of clozapine’s association with agranulocytosis and induced seizures, patients with schizophrenia usually do not receive this antipsychotic unless two 4- to 6-week trials of other neuroleptics have proven ineffective. Ethicists have deemed two failed medication trials to be sufficient before a more hazardous treatment is offered.
Figure Remission of major depression with ECT
We can apply a similar standard when considering ECT in patients first treated with medication.18 A patient’s depression could be defined as therapy-resistant after an inadequate response to 4-week courses (in either order) of:
- an SSRI at dosages equivalent to fluoxetine, 40 mg/d
- a tricyclic at 200 mg/d.
In depressed patients with bipolar disorder, a trial of lithium or an anticonvulsant may replace an antidepressant.
ECT is appropriate when debilitating depression persists after two adequate medication trials. Only after an adequate ECT trial has failed—and such failure is infrequent—is it reasonable to offer poorly tested augmentation and combination strategies.
What is ‘adequate’ ECT? For patients with major depression, the definition of an adequate ECT trial is complex. Although many doctors expect six ECT sessions to be sufficient, the CORE studies are finding that only 45% of patients remitted with six ECT, 81% with nine ECT, and almost all with 12 ECT sessions (Figure).9 These patients were treated with bitemporal electrode placement, the more effective form of ECT. When unilateral electrode placements are used, ECT courses may be inadequate, as this form requires special attention to electrical dosing.
Further, the quality of the seizure—like the dosing of medications—directly influences outcome. Seizure monitoring is essential for assessing the adequacy of each treatment and the treatment course.21,22
Related resources
- Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus non-psychotic depressed patients: a report from CORE. J ECT 2001;17:244-53.
Drug brand names
- Amitriptyline • Elavil
- Clozapine • Clozaril
- Imipramine • Tofranil
- Nortriptyline • Pamelor
- Perphenazine • Trilafon
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was aided by grants from the Scion Natural Science Association, Inc., St. James, NY.
Patients with what’s called “therapy-resistant” depression (TRD)—with subtherapeutic response to medications and psychotherapy—are often actually suffering from unrecognized, inadequately treated psychotic depression. Psychiatrists could greatly diminish the clinical challenge of TRD by recognizing psychotic depression and treating it more effectively.1 And the most effective treatment for psychotic depression is neither antidepressants nor antipsychotic drugs but electroconvulsive therapy (ECT).
Despite ECT’s superior efficacy in TRD, however, algorithms for treating major depression relegate ECT to an option of last resort. By not considering ECT sooner, we consign many severely depressed patients to less-effective treatments and the risk of chronic illness.
Table
Diagnostic signs of psychosis in patients with major depression
| Sign | Example |
|---|---|
| Somatic concern | Delusions of fatal illness |
| Grandiosity | Special relation to God or royalty |
| Suspiciousness | Delusions of spousal infidelity |
| Hallucinations | Foul body odor |
| Unusual thought | Bizarre, confused ideation |
| Depressive delusion | Worthlessness, guilt, feelings of deserving death or punishment |
| Source: Based on the Brief Psychiatric Rating Scale.14 | |
It is time for a more realistic algorithm that recommends ECT earlier for major depressive episodes, with or without psychotic features. This article proposes such an algorithm and discusses the supporting evidence.
TREATING PSYCHOTIC DEPRESSION
Patients with delusions or hallucinations were classified as suffering from schizophrenia until the mid-1970s. Researchers then found that depressed patients with psychotic features responded well to ECT but poorly to adequate serum levels of imipramine.2
These observations were confirmed by a large Italian study, in which 437 depressed patients were treated with high-dose imipramine (200 to 350 mg/d). Depression remitted in 244 patients (56%). Those who remained depressed were then treated with ECT, and depression remitted in 136 of 190 (72%). Psychosis was the marker of poor response to imipramine.3 DSM-III codified these findings by separating the syndrome of “major depression with psychosis” (296.34) from “major depression without psychosis” (296.33).
As psychiatry recognized psychotic depression as a distinct form of depression, it became clear that drugs often could not adequately treat it. Less than one-third of patients with psychotic depression respond to tricyclics alone.4-6
Response to antipsychotic monotherapy averaged 50% and increased to 75% with combined antipsychotics and antidepressants. However, high daily dosages —at least 32 mg of perphenazine and 225 mg of amitriptyline—were required for an adequate response,7 and side effects made sustaining such heroic dosing was difficult. The greatest improvement rates were seen with ECT.
Few other drug combinations have been reported to be effective in psychotic depression, but we lack proper studies. Schatzberg8 addressed the use of newer antidepressants and atypical antipsychotics without offering an algorithm based on the data. Evidence on combination therapies consists mainly of case reports, with few designed studies.
EFFICACY OF ECT
ECT is the most effective treatment for psychotic major depression—achieving remission rates >80% within 3 weeks—as demonstrated by the ongoing, four-hospital Consortium for Research in ECT (CORE), supported by the National Institute of Mental Health.
CORE researchers are studying the efficacy of bilateral ECT in treating severe unipolar depression in patients ages 18 to 85 and of continuation treatments with ECT or lithium plus nortriptyline.9 Under the CORE protocol, diagnoses are made by structured clinical interview using DSM-III-R criteria, and remission is defined as >60% reduction in Hamilton Rating Scale for Depression scores, with final scores 10 sustained for 1 week.
In the first 253 CORE patients treated with ECT, depression remitted in 75% and did not remit in 11%; 14% dropped out. Psychotic depression was identified in 30% (77 of 253), and the remission rate among these patients was 83%.
Among patients who completed the full ECT course (at least 12 sessions), remission rates were 96% for psychotic depression and 83% for nonpsychotic depression. The overall remission rate was 87%.
Treatments were given three times per week. Among patients who completed treatment in weeks 1 through 4, remission rates were 5%, 45%, 81%, and 100%, respectively. Psychotic depression remitted more rapidly than nonpsychotic depression.
Suicide risk. CORE findings suggest that ECT also may reduce suicide risk. In item 3 of the Hamilton Rating Scale for Depression, scores of 2 to 4 indicate preoccupation with death or suicide, or a recent suicide attempt. Nearly 60% of 404 patients (237) reported baseline scores of 2 to 4, but their scores dropped rapidly with ECT. Scores of 0 were reported in 68% after 1 week of ECT, in 87% after 2 weeks, and in 93% after 3 weeks.10
Summary. In patients with severe depressive illness, CORE’s remission rates of 95% for psychotic depression and 83% for nonpsychotic depression are remarkable. Another group is independently reporting a 92% remission rate for psychotic depression treated with ECT.11
Psychotic depression is difficult to recognize and treat, even for clinicians with advanced training. For example:
- only 2 of 52 psychotic depressed patients were determined to have been adequately treated before referral to a National Institute of Mental Health-supported study of ECT15
- only 3 of 46 psychotic depressed patients had been adequately treated prior to enrollment in the Consortium for Research in ECT (CORE) study.9
The three most useful diagnostic criteria are spelled out in the Brief Psychiatric Rating Scale:
- any sign of psychosis is sufficient for designating a major depression as “psychotic”
- one well-developed sign is sufficient to prescribe treatment for psychotic depression
- well-developed vegetative signs also indicate the need to treat psychotic depression.14
ROADBLOCKS TO WIDER USE OF ECT
Many eligible patients never receive ECT, despite its track record of producing high remission rates in psychotic depression. Reasons include:
Limited access. Few psychiatrists—less than 8%—offer ECT as a treatment option, and most who do offer it practice in private hospitals.12-14
Academic low regard. Psychiatry’s academic lecturers largely ignore ECT’s efficacy in psychotic depression and therapy-resistant depression. This low regard for ECT is codified in expert algorithms, which cite ECT as an option of last resort.
Social stigma. In a recent essay summarizing medication’s weak effect in treating psychotic depression, Schatzberg states, “While ECT is a remarkably effective treatment for psychotic depression, requirements for its use are stringent, and public perception about the overall appropriateness of shock treatment is negative.”8
Algorithm Treatment of major depression, with or without psychotic features
ALGORITHM FOR MAJOR DEPRESSION
Because patients with psychotic and nonpsychotic major depression clearly require different treatments, differentiating between these two types is critical. Although psychotic depression can be difficult to diagnose,15,16 commonly recognized criteria are listed in the Table
Assessment. Assess each severely depressed patient for psychotic features, such as delusions and hallucinations. A useful assessment guide is the Brief Psychiatric Rating Scale (Box).17 Also treat those with melancholia, inanition, severe weight loss and insomnia, concentration and memory difficulty, stupor, or suicidal ideation as if they had psychotic depression. These symptoms and signs are evidence that the patient’s neuroendocrine system is disturbed, an indication of severe depression that responds poorly to antidepressant drugs alone.
Treatment. Nonpsychotic depressed patients are best offered antidepressants—tricyclics or selective serotonin reuptake inhibitors (SSRIs)—as recommended by conventional guidelines. Insufficient response to two adequate trials calls for a careful assessment for psychosis and, if found, treatment with effective drug combinations or ECT (Algorithm).18 For patients with psychotic depression—especially those who fail medication trials or whose severe symptoms would likely respond to ECT as a primary treatment—bilateral ECT is the effective standard.19
ECT is the appropriate first option for hospitalized patients with psychotic depression—especially those who are suicidal or require supplementary feeding and sedation. It may also be considered the first option in patients who have:
- attempted suicide
- lost more than 10% of body weight (approximately 15 lbs for adults) in the weeks of their illness
- or show signs of severe melancholia, such as catatonia or pseudodementia.
TREATING NONPSYCHOTIC DEPRESSION
When medications are first-line treatment for nonpsychotic depression, how long should a trial be continued before taking another tack? How many courses should you try before you declare therapy resistance and consider ECT?
Studies of clozapine provide a useful model.20 Because of clozapine’s association with agranulocytosis and induced seizures, patients with schizophrenia usually do not receive this antipsychotic unless two 4- to 6-week trials of other neuroleptics have proven ineffective. Ethicists have deemed two failed medication trials to be sufficient before a more hazardous treatment is offered.
Figure Remission of major depression with ECT
We can apply a similar standard when considering ECT in patients first treated with medication.18 A patient’s depression could be defined as therapy-resistant after an inadequate response to 4-week courses (in either order) of:
- an SSRI at dosages equivalent to fluoxetine, 40 mg/d
- a tricyclic at 200 mg/d.
In depressed patients with bipolar disorder, a trial of lithium or an anticonvulsant may replace an antidepressant.
ECT is appropriate when debilitating depression persists after two adequate medication trials. Only after an adequate ECT trial has failed—and such failure is infrequent—is it reasonable to offer poorly tested augmentation and combination strategies.
What is ‘adequate’ ECT? For patients with major depression, the definition of an adequate ECT trial is complex. Although many doctors expect six ECT sessions to be sufficient, the CORE studies are finding that only 45% of patients remitted with six ECT, 81% with nine ECT, and almost all with 12 ECT sessions (Figure).9 These patients were treated with bitemporal electrode placement, the more effective form of ECT. When unilateral electrode placements are used, ECT courses may be inadequate, as this form requires special attention to electrical dosing.
Further, the quality of the seizure—like the dosing of medications—directly influences outcome. Seizure monitoring is essential for assessing the adequacy of each treatment and the treatment course.21,22
Related resources
- Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus non-psychotic depressed patients: a report from CORE. J ECT 2001;17:244-53.
Drug brand names
- Amitriptyline • Elavil
- Clozapine • Clozaril
- Imipramine • Tofranil
- Nortriptyline • Pamelor
- Perphenazine • Trilafon
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
Preparation of this manuscript was aided by grants from the Scion Natural Science Association, Inc., St. James, NY.
1. Thase M. New approaches to managing difficult-to-treat depressions. J Clin Psychiatry 2003;64[suppl1]:3-4.
2. Kantor SJ, Glassman AH. Delusional depressions: natural history and response to treatment. Br J Psychiatry 1977;131:351-6.
3. Avery D, Lubrano A. Depression treated with imipramine and ECT: the deCarolis study reconsidered. Am J Psychiatry 1979;136:559-62.
4. Kroessler D. Relative efficacy rates for therapies of delusional depression. Convuls Ther 1985;1:173-82.
5. Parker G, Roy K, Hadzi-Pavlovic D, Pedic F. Psychotic (delusional) depression: a meta-analysis of physical treatments. J Affect Dis 1992;24:17-24.
6. Wheeler Vega JA, Mortimer AM, Tyson PJ. Somatic treatment of psychotic depression: review and recommendations for practice. J Clin Psychopharmacol 2000;20:504-19.
7. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacologic treatment of delusional depression. Am J Psychiatry 1985;142:430-6.
8. Schatzberg AF. New approaches to managing psychotic depression. J Clin Psychiatry 2003;64[suppl1]:19-23.
9. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus non-psychotic depressed patients: a report from CORE. J ECT 2001;17:244-53.
10. Kellner CH, Fink M, Knapp R, et al. Bilateral ECT rapidly relieves suicidality: findings from phase I of the CORE ECT study. Am J Psychiatry (submitted).
11. Birkenhäger TK, Pluijms EM, Lucius SAP. ECT response in delusional versus non-delusional depressed inpatients. J Affect Dis (in press).
12. Hermann RC, Ettner SL, Dorwart RA, et al. Characteristics of psychiatrists who perform ECT. Am J Psychiatry 1998;155:889-94.
13. Thompson JW, Weiner RD, Myers CP. Use of ECT in the United States in 1975, 1980, and 1986. Am J Psychiatry 1994;151:1657-61.
14. Kramer BA. Use of ECT in California revisited: 1984-1994. J ECT 1999;15:245-51.
15. Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res 1990;31:287-96.
16. Mulsant BH, Haskett RF, Prudic J, et al. Low use of neuroleptic drugs in the treatment of psychotic major depression. Am J Psychiatry 1997;154:559-61.
17. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep 1962;10:799-812.
18. Fink M. Electroconvulsive therapy in medication-resistant depression. In: Amsterdam J, Hornig-Rohan M, Nierenberg A (eds.): Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press, 2001;223-38.
19. Fink M. The efficacy of ECT and “treatment resistance.” J ECT 2002;18:1-2.
20. Lieberman JA, Kane JM, Johns CA. Clozapine: guidelines for clinical management. J Clin Psychiatry 1989;50:329-38.
21. Abrams R. Electroconvulsive therapy. (4th ed). New York: Oxford University Press, 2002.
22. Fink M. Optimizing ECT. Encephale 1994;20:297-302.
1. Thase M. New approaches to managing difficult-to-treat depressions. J Clin Psychiatry 2003;64[suppl1]:3-4.
2. Kantor SJ, Glassman AH. Delusional depressions: natural history and response to treatment. Br J Psychiatry 1977;131:351-6.
3. Avery D, Lubrano A. Depression treated with imipramine and ECT: the deCarolis study reconsidered. Am J Psychiatry 1979;136:559-62.
4. Kroessler D. Relative efficacy rates for therapies of delusional depression. Convuls Ther 1985;1:173-82.
5. Parker G, Roy K, Hadzi-Pavlovic D, Pedic F. Psychotic (delusional) depression: a meta-analysis of physical treatments. J Affect Dis 1992;24:17-24.
6. Wheeler Vega JA, Mortimer AM, Tyson PJ. Somatic treatment of psychotic depression: review and recommendations for practice. J Clin Psychopharmacol 2000;20:504-19.
7. Spiker DG, Weiss JC, Dealy RS, et al. The pharmacologic treatment of delusional depression. Am J Psychiatry 1985;142:430-6.
8. Schatzberg AF. New approaches to managing psychotic depression. J Clin Psychiatry 2003;64[suppl1]:19-23.
9. Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus non-psychotic depressed patients: a report from CORE. J ECT 2001;17:244-53.
10. Kellner CH, Fink M, Knapp R, et al. Bilateral ECT rapidly relieves suicidality: findings from phase I of the CORE ECT study. Am J Psychiatry (submitted).
11. Birkenhäger TK, Pluijms EM, Lucius SAP. ECT response in delusional versus non-delusional depressed inpatients. J Affect Dis (in press).
12. Hermann RC, Ettner SL, Dorwart RA, et al. Characteristics of psychiatrists who perform ECT. Am J Psychiatry 1998;155:889-94.
13. Thompson JW, Weiner RD, Myers CP. Use of ECT in the United States in 1975, 1980, and 1986. Am J Psychiatry 1994;151:1657-61.
14. Kramer BA. Use of ECT in California revisited: 1984-1994. J ECT 1999;15:245-51.
15. Prudic J, Sackeim HA, Devanand DP. Medication resistance and clinical response to electroconvulsive therapy. Psychiatry Res 1990;31:287-96.
16. Mulsant BH, Haskett RF, Prudic J, et al. Low use of neuroleptic drugs in the treatment of psychotic major depression. Am J Psychiatry 1997;154:559-61.
17. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep 1962;10:799-812.
18. Fink M. Electroconvulsive therapy in medication-resistant depression. In: Amsterdam J, Hornig-Rohan M, Nierenberg A (eds.): Treatment-resistant mood disorders. Cambridge, UK: Cambridge University Press, 2001;223-38.
19. Fink M. The efficacy of ECT and “treatment resistance.” J ECT 2002;18:1-2.
20. Lieberman JA, Kane JM, Johns CA. Clozapine: guidelines for clinical management. J Clin Psychiatry 1989;50:329-38.
21. Abrams R. Electroconvulsive therapy. (4th ed). New York: Oxford University Press, 2002.
22. Fink M. Optimizing ECT. Encephale 1994;20:297-302.
Treating schizophrenia in the ‘real world’
HISTORY: Jesus’ ‘cousin’
Mr. F, age 60, was hospitalized in May 1995 after expressing fear he would hurt—or kill—himself or someone else. He cooperated with admission procedures but refused to participate in ward activities or meetings. His hygiene was poor, he made little eye contact, and reportedly heard voices. Two days after admission, he emphatically denied suicidal or homicidal ideation and was discharged against medical advice.
Two weeks later, Mr. F was readmitted after his symptoms worsened. He said voices told him that he was a cousin to Jesus Christ and that he had telepathic abilities. He also reported visual hallucinations.
Twice divorced, Mr. F has three uncles who have been diagnosed with schizophrenia. His late father had a history of alcohol abuse, and his late mother suffered from Alzheimer’s disease.
Mr. F lived a normal life until 1975, when he began drinking heavily. Three years later, he quit his job of 11 years at the local airport. At that time, he told a psychiatrist that “people are out to get me. I feel nervous a lot.” He was diagnosed as having generalized anxiety disorder and treated with diazepam, 20 mg/d.
Four months later he complained of severe insomnia, was diagnosed with depression, and was prescribed amitriptyline, 100 mg at bedtime. He was hospitalized 1 week later after he complained of chest pain and expressed paranoid thoughts. During the 3-week hospitalization, he experienced persecutory delusions and heard voices telling him he was “damned.” He was diagnosed with paranoid schizophrenia and alcohol dependence. The amitriptyline was stopped, and Mr. F was discharged on chlorpromazine, 300 mg/d.
From 1978 to 1995, Mr. F was hospitalized 35 times, often at his family’s urging after he made threats or became violent at home. He once kicked his elderly father and another time was jailed after a domestic violence incident. Religious delusions characterized his thought content. Thought blocking, flight of ideas, and somatic and sexual delusions were also apparent.
Was Mr. F’s diagnosis accurate, or do his frequent psychotic episodes meet criteria for a type of mania?
Dr. Canive’s observations
Diagnoses of mania or mood disorder with psychotic features were not considered because Mr. F never experienced a distinct period of persistently expansive or depressed mood.
Mr. F’s initial complaints of increased anxiety and depression were considered prodromal symptoms of schizophrenia and may have reflected his inability to discuss or cope with his delusions and hallucinations during the initial evaluation. What’s more, his occupational functioning gradually deteriorated months before his initial mental health assessment.
TREATMENT: Many medications, no progress
At different times from 1978 to 1995, Mr. F had taken chlorpromazine, 100 to 300 mg/d; thioridazine, 50 to 200 mg/d; loxapine, 25 mg/d; fluphenazine, 5 to 10 mg/d; haloperidol, 2 to 4 mg/d, and fluphenazine decanoate, 3.125 to 6.25 mg biweekly, as well as concomitant anticholinergics, benzodiazepines, or other hypnotics.
A closer look at Mr. F’s chart revealed that medication noncompliance often preceded hospitalization. He was extremely prone to antipsychotic-related extrapyramidal symptoms (EPS), even at low dosages. Whenever motor symptoms surfaced, he would stop taking his antipsychotics.
Buccolingual tardive dyskinesia (TD) was first noticed in 1987. Four years later, an Abnormal Involuntary Movement Scale (AIMS) exam revealed mild TD that was managed with vitamin E, 400 IU/d.
While hospitalized, Mr. F many times received injectable antipsychotics and benzodiazepines, mostly to control violence. Depot antipsychotics also were tried in an effort to promote compliance, but recurrent alcohol abuse often triggered a relapse.
How would you confront Mr. F’s history of noncompliance? Can his delusions be controlled without prompting severe motor effects?
Dr. Canive’s observations
For Mr. F, poor tolerability, incomplete efficacy, and variable compliance have repeatedly led to symptom exacerbation and hospitalization. Low dosing because of sensitivity to EPS may partially explain his insufficient response to antipsychotics.
In 1995, after numerous unsuccessful drug treatments, we considered entering Mr. F into a phase II clinical trial of the atypical antipsychotic aripiprazole.
Now FDA-approved for treatment of schizophrenia, aripiprazole decreases dopaminergic transmission in the nigrostriatal and tuberoinfundibular pathways, thus reducing the likelihood of EPS.1,2 Also, aripiprazole’s dopamine-serotonin stabilization effects have been reported in clinical trials to improve tolerability, compliance, and overall effectiveness in patients with schizophrenia.3
Common side effects of aripiprazole are mild nausea, insomnia, and restlessness, although data indicate that these effects have a low prevalence and disappear within 2 weeks. If insomnia and restlessness are prominent, a low-dose, short-acting benzodiazepine may be added, tapered after 1 week, and discontinued at week 2.
Table
Mr. F’s progress while taking aripiprazole, 1995-2003
| Visit | CGI-S | CGI-G | PANSS Positive | PANSS Negative | PANSS total | Clinical correlates |
|---|---|---|---|---|---|---|
| Baseline | 4 | 5 | 24 | 21 | 94 | —- |
| Week 2 | 3 | 2 | N/A | N/A | N/A | Positive, negative symptoms much improved |
| Week 12 | 3 | 2 | 11 | 16 | 56 | Mr. F’s understanding about his illness, life, socioeconomic issues much improved |
| Week 76 | 3 | 2 | 11 | 18 | 56 | Activity level increased; starts doing yard work to supplement disability income |
| Week 88 | 3 | 2 | 12 | 14 | 52 | Volunteers as courier at local hospital; continues to do yard work |
| Week 226 | 2 | 2 | 9 | 12 | 42 | Starts steady work as a janitor and security aid |
| Week 284 | 2 | 2 | 9 | 14 | 45 | Concerned about losing Social Security benefits, since he is working 40 hours a week. |
| Week 328 | 3 | 1 | 11 | 15 | 52 | Discharged from hospital after psychotic relapse. Looking for apartment. |
| Week 384 (Final visit) | 3 | 1 | 9 | 12 | 45 | Father died the previous week. Mr. F accepted his father’s passing well. Open-label study terminated. Patient continued on aripiprazole, 20 mg/d. |
What the scores mean
Clinical Global Impression-Severity of Illness (CGI-S)—Scores range from 1 to 7, with 1 meaning normal (normal, minimal, mild, moderate, moderately severe, severe, among the most extreme).
Clinical Global Impression-Global Improvement (CGI-G)—Scores range from 1 to 7, with 1 meaning very much improved (very much improved, much improved, improved, unchanged, little worse, much worse, very much worse).
Positive and Negative Syndrome Scale (PANSS) Positive—consists of 7 items (delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness/persecution, hostility); scores range from 7 to 49 and decrease as patients improve.
PANSS Negative—consists of 7 items (blunted affect, emotional withdrawal, poor rapport, passive pathetic withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, stereotyped thinking); scores range from 7 to 49 and decrease as patients improve.
PANSS General—consists of 16 items (somatic concern, anxiety, guilt feelings, tension, mannerism and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, active social avoidance). Scores range from 16 to 112 and decrease as patients improve.
CONTINUED TREATMENT: A new trial
Mr. F participated in a 4-week, double-blind, placebo-controlled trial of aripiprazole, 2, 10, or 30 mg/d, versus haloperidol, 10 mg/d.
One month later, he entered a second aripiprazole trial: a 4-day, open-label study starting at 5 mg/d with titration to 20 mg/d. In the interval between the two trials, Mr. F was prescribed thiothixene, 10 mg/d, and benztropine, 2 mg at bedtime.
During the 4-day trial, he complained of insomnia and was given chloral hydrate, 500 to 1,000 mg at bedtime. He also complained of anxiety and was started on lorazepam, 2 mg bid.
After completing the open-label aripiprazole trial, Mr. F exhibited no behavioral problems and complied with ward routine. He was discharged after 17 days, at which time he denied auditory or visual hallucinations. His thinking seemed clear and his insight improved. His Global Assessment of Functioning (GAF) score at discharge was 55, suggesting moderate symptoms and difficulty in social and occupational functioning.
For the next 5 1/2 years, Mr. F was maintained on aripiprazole, 20 mg/d, as part of the same ongoing open-label trial. During that period he also took lorazepam, 1 mg bid prn; oxazepam, 15 mg bid; or clonazepam, 0.5 mg bid, for anxiety.
Mr. F. exhibited significant sustained improvement as measured with the Positive and Negative Symptom Scale (PANSS), Clinical Global Impression scale (CGI), and GAF (Table). His TD remained mild throughout the trial, as determined through AIMS scores. He also reported no EPS, akathisia, or other adverse events.
About 18 months after starting aripiprazole, Mr. F resumed working part time. In September 2001, he stopped receiving disability benefits and started supporting himself again.
FOLLOW-UP: ‘The voices were ugly’
In December 2001, after 6 years without hospitalization, Mr. F was back in the psychiatric ward. One week before admission, he reported that he had been having panic attacks because “the voices were ugly.” He only slept 4 to 5 hours per night.
He then revealed that he had stopped taking aripiprazole for 2 weeks because he had no longer felt ill. He was still taking his lorazepam, however.
Mr. F appeared mildly anxious upon presentation and his affect was blunted. On examination, his thought processes were linear; he was once again hearing voices and experiencing delusions of telepathic control.
The patient was placed back on aripiprazole, 20 mg/d. His behavior on the ward improved dramatically, and the frequency and severity of his delusions and auditory hallucinations decreased gradually.
At discharge, Mr. F’s insight was good, his delusions had disappeared, and auditory hallucinations were rare. He was instructed to continue the aripiprazole and was prescribed clonazepam, 0.5 mg bid, for his anxiety and trazodone, 50 mg at bed-time, to help his sleep.
Since then, Mr. F has lived on his own, is working steadily, and has not required hospitalization. He stopped taking trazodone soon after discharge, but continues taking aripiprazole and clonazepam as prescribed. His hygiene is good, and he is making amends with family members. He attends church every Sunday—free of the messianic delusions that once tormented him. He also stopped abusing alcohol on his own in 1995 and has remained abstinent since.
How can we ensure that patients with schizophrenia keep taking their medications—regardless of whether symptoms are present?
Dr. Canive’s observations
Clinical trials measure a drug’s efficacy under highly controlled circumstances. In the “real world,” however, noncompliance due to intolerability can undermine a medication’s effectiveness.
Too often noncompliance—stemming from abatement of symptoms or the emergence of side effects—derails treatment of schizophrenia. Misdrahi et al found that medication noncompliance accounts for 40% of schizophrenia relapses occurring more than 1 year after patients’ first hospitalization.4
Given aripiprazole’s 75-hour half-life, one might not expect to see symptoms emerge so soon after discontinuation. It is possible that:
- Mr. F. abstained from aripiprazole longer than he realized—or admitted
- Unidentified stressful life events also exacerbated symptoms and precipitated hospitalization.
When Mr. F consistently followed his regimen, his positive symptoms abated and he could attempt to live a normal life.
Our patients must understand that schizophrenia is a lifelong illness and that continued adherence to medication—even when symptoms do not exist—is crucial. A strong therapeutic alliance,5 increased social support, adjunctive cognitive-behavioral therapy, psychosocial interventions,6 and medications with fewer and less-severe side effects may help patients embrace this message.
Related resources
- Tamminga CA. Partial dopamine agonists in the treatment of psychosis. J Neural Transm 2002;109:411-20.
Drug brand names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Chlorpromazine • Thorazine
- Clonazepam • Klonopin
- Diazepam • Valium
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Lorazepam • Ativan
- Loxapine • Loxitane
- Oxazepam • Serax
- Thiothixene • Navane
- Trazodone • Desyrel
Disclosure
The author receives research/grant support from and is a speaker for and consultant to Bristol-Myers Squibb Co. He also receives research/grant support from and/or is a speaker for AstraZeneca Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly and Co.
1. Aripiprazole prescribing information. Bristol-Myers Squibb Co. and Otsuka America Pharmaceutical, 2002.
2. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63:763-71.
3. Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 2002;302:381-9.
4. Misdrahi D, Llorca PM, Lancon C, Bayle FJ. Compliance in schizophrenia: Predictive factors, therapeutical considerations and research implications (French). Encephale 2002;3:266-72.
5. Weiss KA, Smith TE, Hull JW, et al. Predictors of risk of nonadherence in outpatients with schizophrenia and other psychotic disorders. Schizophr Bull 2002;28:341-9.
6. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 2002;63:1121-8.
HISTORY: Jesus’ ‘cousin’
Mr. F, age 60, was hospitalized in May 1995 after expressing fear he would hurt—or kill—himself or someone else. He cooperated with admission procedures but refused to participate in ward activities or meetings. His hygiene was poor, he made little eye contact, and reportedly heard voices. Two days after admission, he emphatically denied suicidal or homicidal ideation and was discharged against medical advice.
Two weeks later, Mr. F was readmitted after his symptoms worsened. He said voices told him that he was a cousin to Jesus Christ and that he had telepathic abilities. He also reported visual hallucinations.
Twice divorced, Mr. F has three uncles who have been diagnosed with schizophrenia. His late father had a history of alcohol abuse, and his late mother suffered from Alzheimer’s disease.
Mr. F lived a normal life until 1975, when he began drinking heavily. Three years later, he quit his job of 11 years at the local airport. At that time, he told a psychiatrist that “people are out to get me. I feel nervous a lot.” He was diagnosed as having generalized anxiety disorder and treated with diazepam, 20 mg/d.
Four months later he complained of severe insomnia, was diagnosed with depression, and was prescribed amitriptyline, 100 mg at bedtime. He was hospitalized 1 week later after he complained of chest pain and expressed paranoid thoughts. During the 3-week hospitalization, he experienced persecutory delusions and heard voices telling him he was “damned.” He was diagnosed with paranoid schizophrenia and alcohol dependence. The amitriptyline was stopped, and Mr. F was discharged on chlorpromazine, 300 mg/d.
From 1978 to 1995, Mr. F was hospitalized 35 times, often at his family’s urging after he made threats or became violent at home. He once kicked his elderly father and another time was jailed after a domestic violence incident. Religious delusions characterized his thought content. Thought blocking, flight of ideas, and somatic and sexual delusions were also apparent.
Was Mr. F’s diagnosis accurate, or do his frequent psychotic episodes meet criteria for a type of mania?
Dr. Canive’s observations
Diagnoses of mania or mood disorder with psychotic features were not considered because Mr. F never experienced a distinct period of persistently expansive or depressed mood.
Mr. F’s initial complaints of increased anxiety and depression were considered prodromal symptoms of schizophrenia and may have reflected his inability to discuss or cope with his delusions and hallucinations during the initial evaluation. What’s more, his occupational functioning gradually deteriorated months before his initial mental health assessment.
TREATMENT: Many medications, no progress
At different times from 1978 to 1995, Mr. F had taken chlorpromazine, 100 to 300 mg/d; thioridazine, 50 to 200 mg/d; loxapine, 25 mg/d; fluphenazine, 5 to 10 mg/d; haloperidol, 2 to 4 mg/d, and fluphenazine decanoate, 3.125 to 6.25 mg biweekly, as well as concomitant anticholinergics, benzodiazepines, or other hypnotics.
A closer look at Mr. F’s chart revealed that medication noncompliance often preceded hospitalization. He was extremely prone to antipsychotic-related extrapyramidal symptoms (EPS), even at low dosages. Whenever motor symptoms surfaced, he would stop taking his antipsychotics.
Buccolingual tardive dyskinesia (TD) was first noticed in 1987. Four years later, an Abnormal Involuntary Movement Scale (AIMS) exam revealed mild TD that was managed with vitamin E, 400 IU/d.
While hospitalized, Mr. F many times received injectable antipsychotics and benzodiazepines, mostly to control violence. Depot antipsychotics also were tried in an effort to promote compliance, but recurrent alcohol abuse often triggered a relapse.
How would you confront Mr. F’s history of noncompliance? Can his delusions be controlled without prompting severe motor effects?
Dr. Canive’s observations
For Mr. F, poor tolerability, incomplete efficacy, and variable compliance have repeatedly led to symptom exacerbation and hospitalization. Low dosing because of sensitivity to EPS may partially explain his insufficient response to antipsychotics.
In 1995, after numerous unsuccessful drug treatments, we considered entering Mr. F into a phase II clinical trial of the atypical antipsychotic aripiprazole.
Now FDA-approved for treatment of schizophrenia, aripiprazole decreases dopaminergic transmission in the nigrostriatal and tuberoinfundibular pathways, thus reducing the likelihood of EPS.1,2 Also, aripiprazole’s dopamine-serotonin stabilization effects have been reported in clinical trials to improve tolerability, compliance, and overall effectiveness in patients with schizophrenia.3
Common side effects of aripiprazole are mild nausea, insomnia, and restlessness, although data indicate that these effects have a low prevalence and disappear within 2 weeks. If insomnia and restlessness are prominent, a low-dose, short-acting benzodiazepine may be added, tapered after 1 week, and discontinued at week 2.
Table
Mr. F’s progress while taking aripiprazole, 1995-2003
| Visit | CGI-S | CGI-G | PANSS Positive | PANSS Negative | PANSS total | Clinical correlates |
|---|---|---|---|---|---|---|
| Baseline | 4 | 5 | 24 | 21 | 94 | —- |
| Week 2 | 3 | 2 | N/A | N/A | N/A | Positive, negative symptoms much improved |
| Week 12 | 3 | 2 | 11 | 16 | 56 | Mr. F’s understanding about his illness, life, socioeconomic issues much improved |
| Week 76 | 3 | 2 | 11 | 18 | 56 | Activity level increased; starts doing yard work to supplement disability income |
| Week 88 | 3 | 2 | 12 | 14 | 52 | Volunteers as courier at local hospital; continues to do yard work |
| Week 226 | 2 | 2 | 9 | 12 | 42 | Starts steady work as a janitor and security aid |
| Week 284 | 2 | 2 | 9 | 14 | 45 | Concerned about losing Social Security benefits, since he is working 40 hours a week. |
| Week 328 | 3 | 1 | 11 | 15 | 52 | Discharged from hospital after psychotic relapse. Looking for apartment. |
| Week 384 (Final visit) | 3 | 1 | 9 | 12 | 45 | Father died the previous week. Mr. F accepted his father’s passing well. Open-label study terminated. Patient continued on aripiprazole, 20 mg/d. |
What the scores mean
Clinical Global Impression-Severity of Illness (CGI-S)—Scores range from 1 to 7, with 1 meaning normal (normal, minimal, mild, moderate, moderately severe, severe, among the most extreme).
Clinical Global Impression-Global Improvement (CGI-G)—Scores range from 1 to 7, with 1 meaning very much improved (very much improved, much improved, improved, unchanged, little worse, much worse, very much worse).
Positive and Negative Syndrome Scale (PANSS) Positive—consists of 7 items (delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness/persecution, hostility); scores range from 7 to 49 and decrease as patients improve.
PANSS Negative—consists of 7 items (blunted affect, emotional withdrawal, poor rapport, passive pathetic withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, stereotyped thinking); scores range from 7 to 49 and decrease as patients improve.
PANSS General—consists of 16 items (somatic concern, anxiety, guilt feelings, tension, mannerism and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, active social avoidance). Scores range from 16 to 112 and decrease as patients improve.
CONTINUED TREATMENT: A new trial
Mr. F participated in a 4-week, double-blind, placebo-controlled trial of aripiprazole, 2, 10, or 30 mg/d, versus haloperidol, 10 mg/d.
One month later, he entered a second aripiprazole trial: a 4-day, open-label study starting at 5 mg/d with titration to 20 mg/d. In the interval between the two trials, Mr. F was prescribed thiothixene, 10 mg/d, and benztropine, 2 mg at bedtime.
During the 4-day trial, he complained of insomnia and was given chloral hydrate, 500 to 1,000 mg at bedtime. He also complained of anxiety and was started on lorazepam, 2 mg bid.
After completing the open-label aripiprazole trial, Mr. F exhibited no behavioral problems and complied with ward routine. He was discharged after 17 days, at which time he denied auditory or visual hallucinations. His thinking seemed clear and his insight improved. His Global Assessment of Functioning (GAF) score at discharge was 55, suggesting moderate symptoms and difficulty in social and occupational functioning.
For the next 5 1/2 years, Mr. F was maintained on aripiprazole, 20 mg/d, as part of the same ongoing open-label trial. During that period he also took lorazepam, 1 mg bid prn; oxazepam, 15 mg bid; or clonazepam, 0.5 mg bid, for anxiety.
Mr. F. exhibited significant sustained improvement as measured with the Positive and Negative Symptom Scale (PANSS), Clinical Global Impression scale (CGI), and GAF (Table). His TD remained mild throughout the trial, as determined through AIMS scores. He also reported no EPS, akathisia, or other adverse events.
About 18 months after starting aripiprazole, Mr. F resumed working part time. In September 2001, he stopped receiving disability benefits and started supporting himself again.
FOLLOW-UP: ‘The voices were ugly’
In December 2001, after 6 years without hospitalization, Mr. F was back in the psychiatric ward. One week before admission, he reported that he had been having panic attacks because “the voices were ugly.” He only slept 4 to 5 hours per night.
He then revealed that he had stopped taking aripiprazole for 2 weeks because he had no longer felt ill. He was still taking his lorazepam, however.
Mr. F appeared mildly anxious upon presentation and his affect was blunted. On examination, his thought processes were linear; he was once again hearing voices and experiencing delusions of telepathic control.
The patient was placed back on aripiprazole, 20 mg/d. His behavior on the ward improved dramatically, and the frequency and severity of his delusions and auditory hallucinations decreased gradually.
At discharge, Mr. F’s insight was good, his delusions had disappeared, and auditory hallucinations were rare. He was instructed to continue the aripiprazole and was prescribed clonazepam, 0.5 mg bid, for his anxiety and trazodone, 50 mg at bed-time, to help his sleep.
Since then, Mr. F has lived on his own, is working steadily, and has not required hospitalization. He stopped taking trazodone soon after discharge, but continues taking aripiprazole and clonazepam as prescribed. His hygiene is good, and he is making amends with family members. He attends church every Sunday—free of the messianic delusions that once tormented him. He also stopped abusing alcohol on his own in 1995 and has remained abstinent since.
How can we ensure that patients with schizophrenia keep taking their medications—regardless of whether symptoms are present?
Dr. Canive’s observations
Clinical trials measure a drug’s efficacy under highly controlled circumstances. In the “real world,” however, noncompliance due to intolerability can undermine a medication’s effectiveness.
Too often noncompliance—stemming from abatement of symptoms or the emergence of side effects—derails treatment of schizophrenia. Misdrahi et al found that medication noncompliance accounts for 40% of schizophrenia relapses occurring more than 1 year after patients’ first hospitalization.4
Given aripiprazole’s 75-hour half-life, one might not expect to see symptoms emerge so soon after discontinuation. It is possible that:
- Mr. F. abstained from aripiprazole longer than he realized—or admitted
- Unidentified stressful life events also exacerbated symptoms and precipitated hospitalization.
When Mr. F consistently followed his regimen, his positive symptoms abated and he could attempt to live a normal life.
Our patients must understand that schizophrenia is a lifelong illness and that continued adherence to medication—even when symptoms do not exist—is crucial. A strong therapeutic alliance,5 increased social support, adjunctive cognitive-behavioral therapy, psychosocial interventions,6 and medications with fewer and less-severe side effects may help patients embrace this message.
Related resources
- Tamminga CA. Partial dopamine agonists in the treatment of psychosis. J Neural Transm 2002;109:411-20.
Drug brand names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Chlorpromazine • Thorazine
- Clonazepam • Klonopin
- Diazepam • Valium
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Lorazepam • Ativan
- Loxapine • Loxitane
- Oxazepam • Serax
- Thiothixene • Navane
- Trazodone • Desyrel
Disclosure
The author receives research/grant support from and is a speaker for and consultant to Bristol-Myers Squibb Co. He also receives research/grant support from and/or is a speaker for AstraZeneca Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly and Co.
HISTORY: Jesus’ ‘cousin’
Mr. F, age 60, was hospitalized in May 1995 after expressing fear he would hurt—or kill—himself or someone else. He cooperated with admission procedures but refused to participate in ward activities or meetings. His hygiene was poor, he made little eye contact, and reportedly heard voices. Two days after admission, he emphatically denied suicidal or homicidal ideation and was discharged against medical advice.
Two weeks later, Mr. F was readmitted after his symptoms worsened. He said voices told him that he was a cousin to Jesus Christ and that he had telepathic abilities. He also reported visual hallucinations.
Twice divorced, Mr. F has three uncles who have been diagnosed with schizophrenia. His late father had a history of alcohol abuse, and his late mother suffered from Alzheimer’s disease.
Mr. F lived a normal life until 1975, when he began drinking heavily. Three years later, he quit his job of 11 years at the local airport. At that time, he told a psychiatrist that “people are out to get me. I feel nervous a lot.” He was diagnosed as having generalized anxiety disorder and treated with diazepam, 20 mg/d.
Four months later he complained of severe insomnia, was diagnosed with depression, and was prescribed amitriptyline, 100 mg at bedtime. He was hospitalized 1 week later after he complained of chest pain and expressed paranoid thoughts. During the 3-week hospitalization, he experienced persecutory delusions and heard voices telling him he was “damned.” He was diagnosed with paranoid schizophrenia and alcohol dependence. The amitriptyline was stopped, and Mr. F was discharged on chlorpromazine, 300 mg/d.
From 1978 to 1995, Mr. F was hospitalized 35 times, often at his family’s urging after he made threats or became violent at home. He once kicked his elderly father and another time was jailed after a domestic violence incident. Religious delusions characterized his thought content. Thought blocking, flight of ideas, and somatic and sexual delusions were also apparent.
Was Mr. F’s diagnosis accurate, or do his frequent psychotic episodes meet criteria for a type of mania?
Dr. Canive’s observations
Diagnoses of mania or mood disorder with psychotic features were not considered because Mr. F never experienced a distinct period of persistently expansive or depressed mood.
Mr. F’s initial complaints of increased anxiety and depression were considered prodromal symptoms of schizophrenia and may have reflected his inability to discuss or cope with his delusions and hallucinations during the initial evaluation. What’s more, his occupational functioning gradually deteriorated months before his initial mental health assessment.
TREATMENT: Many medications, no progress
At different times from 1978 to 1995, Mr. F had taken chlorpromazine, 100 to 300 mg/d; thioridazine, 50 to 200 mg/d; loxapine, 25 mg/d; fluphenazine, 5 to 10 mg/d; haloperidol, 2 to 4 mg/d, and fluphenazine decanoate, 3.125 to 6.25 mg biweekly, as well as concomitant anticholinergics, benzodiazepines, or other hypnotics.
A closer look at Mr. F’s chart revealed that medication noncompliance often preceded hospitalization. He was extremely prone to antipsychotic-related extrapyramidal symptoms (EPS), even at low dosages. Whenever motor symptoms surfaced, he would stop taking his antipsychotics.
Buccolingual tardive dyskinesia (TD) was first noticed in 1987. Four years later, an Abnormal Involuntary Movement Scale (AIMS) exam revealed mild TD that was managed with vitamin E, 400 IU/d.
While hospitalized, Mr. F many times received injectable antipsychotics and benzodiazepines, mostly to control violence. Depot antipsychotics also were tried in an effort to promote compliance, but recurrent alcohol abuse often triggered a relapse.
How would you confront Mr. F’s history of noncompliance? Can his delusions be controlled without prompting severe motor effects?
Dr. Canive’s observations
For Mr. F, poor tolerability, incomplete efficacy, and variable compliance have repeatedly led to symptom exacerbation and hospitalization. Low dosing because of sensitivity to EPS may partially explain his insufficient response to antipsychotics.
In 1995, after numerous unsuccessful drug treatments, we considered entering Mr. F into a phase II clinical trial of the atypical antipsychotic aripiprazole.
Now FDA-approved for treatment of schizophrenia, aripiprazole decreases dopaminergic transmission in the nigrostriatal and tuberoinfundibular pathways, thus reducing the likelihood of EPS.1,2 Also, aripiprazole’s dopamine-serotonin stabilization effects have been reported in clinical trials to improve tolerability, compliance, and overall effectiveness in patients with schizophrenia.3
Common side effects of aripiprazole are mild nausea, insomnia, and restlessness, although data indicate that these effects have a low prevalence and disappear within 2 weeks. If insomnia and restlessness are prominent, a low-dose, short-acting benzodiazepine may be added, tapered after 1 week, and discontinued at week 2.
Table
Mr. F’s progress while taking aripiprazole, 1995-2003
| Visit | CGI-S | CGI-G | PANSS Positive | PANSS Negative | PANSS total | Clinical correlates |
|---|---|---|---|---|---|---|
| Baseline | 4 | 5 | 24 | 21 | 94 | —- |
| Week 2 | 3 | 2 | N/A | N/A | N/A | Positive, negative symptoms much improved |
| Week 12 | 3 | 2 | 11 | 16 | 56 | Mr. F’s understanding about his illness, life, socioeconomic issues much improved |
| Week 76 | 3 | 2 | 11 | 18 | 56 | Activity level increased; starts doing yard work to supplement disability income |
| Week 88 | 3 | 2 | 12 | 14 | 52 | Volunteers as courier at local hospital; continues to do yard work |
| Week 226 | 2 | 2 | 9 | 12 | 42 | Starts steady work as a janitor and security aid |
| Week 284 | 2 | 2 | 9 | 14 | 45 | Concerned about losing Social Security benefits, since he is working 40 hours a week. |
| Week 328 | 3 | 1 | 11 | 15 | 52 | Discharged from hospital after psychotic relapse. Looking for apartment. |
| Week 384 (Final visit) | 3 | 1 | 9 | 12 | 45 | Father died the previous week. Mr. F accepted his father’s passing well. Open-label study terminated. Patient continued on aripiprazole, 20 mg/d. |
What the scores mean
Clinical Global Impression-Severity of Illness (CGI-S)—Scores range from 1 to 7, with 1 meaning normal (normal, minimal, mild, moderate, moderately severe, severe, among the most extreme).
Clinical Global Impression-Global Improvement (CGI-G)—Scores range from 1 to 7, with 1 meaning very much improved (very much improved, much improved, improved, unchanged, little worse, much worse, very much worse).
Positive and Negative Syndrome Scale (PANSS) Positive—consists of 7 items (delusions, conceptual disorganization, hallucinatory behavior, excitement, grandiosity, suspiciousness/persecution, hostility); scores range from 7 to 49 and decrease as patients improve.
PANSS Negative—consists of 7 items (blunted affect, emotional withdrawal, poor rapport, passive pathetic withdrawal, difficulty in abstract thinking, lack of spontaneity and flow of conversation, stereotyped thinking); scores range from 7 to 49 and decrease as patients improve.
PANSS General—consists of 16 items (somatic concern, anxiety, guilt feelings, tension, mannerism and posturing, depression, motor retardation, uncooperativeness, unusual thought content, disorientation, poor attention, lack of judgment and insight, disturbance of volition, poor impulse control, preoccupation, active social avoidance). Scores range from 16 to 112 and decrease as patients improve.
CONTINUED TREATMENT: A new trial
Mr. F participated in a 4-week, double-blind, placebo-controlled trial of aripiprazole, 2, 10, or 30 mg/d, versus haloperidol, 10 mg/d.
One month later, he entered a second aripiprazole trial: a 4-day, open-label study starting at 5 mg/d with titration to 20 mg/d. In the interval between the two trials, Mr. F was prescribed thiothixene, 10 mg/d, and benztropine, 2 mg at bedtime.
During the 4-day trial, he complained of insomnia and was given chloral hydrate, 500 to 1,000 mg at bedtime. He also complained of anxiety and was started on lorazepam, 2 mg bid.
After completing the open-label aripiprazole trial, Mr. F exhibited no behavioral problems and complied with ward routine. He was discharged after 17 days, at which time he denied auditory or visual hallucinations. His thinking seemed clear and his insight improved. His Global Assessment of Functioning (GAF) score at discharge was 55, suggesting moderate symptoms and difficulty in social and occupational functioning.
For the next 5 1/2 years, Mr. F was maintained on aripiprazole, 20 mg/d, as part of the same ongoing open-label trial. During that period he also took lorazepam, 1 mg bid prn; oxazepam, 15 mg bid; or clonazepam, 0.5 mg bid, for anxiety.
Mr. F. exhibited significant sustained improvement as measured with the Positive and Negative Symptom Scale (PANSS), Clinical Global Impression scale (CGI), and GAF (Table). His TD remained mild throughout the trial, as determined through AIMS scores. He also reported no EPS, akathisia, or other adverse events.
About 18 months after starting aripiprazole, Mr. F resumed working part time. In September 2001, he stopped receiving disability benefits and started supporting himself again.
FOLLOW-UP: ‘The voices were ugly’
In December 2001, after 6 years without hospitalization, Mr. F was back in the psychiatric ward. One week before admission, he reported that he had been having panic attacks because “the voices were ugly.” He only slept 4 to 5 hours per night.
He then revealed that he had stopped taking aripiprazole for 2 weeks because he had no longer felt ill. He was still taking his lorazepam, however.
Mr. F appeared mildly anxious upon presentation and his affect was blunted. On examination, his thought processes were linear; he was once again hearing voices and experiencing delusions of telepathic control.
The patient was placed back on aripiprazole, 20 mg/d. His behavior on the ward improved dramatically, and the frequency and severity of his delusions and auditory hallucinations decreased gradually.
At discharge, Mr. F’s insight was good, his delusions had disappeared, and auditory hallucinations were rare. He was instructed to continue the aripiprazole and was prescribed clonazepam, 0.5 mg bid, for his anxiety and trazodone, 50 mg at bed-time, to help his sleep.
Since then, Mr. F has lived on his own, is working steadily, and has not required hospitalization. He stopped taking trazodone soon after discharge, but continues taking aripiprazole and clonazepam as prescribed. His hygiene is good, and he is making amends with family members. He attends church every Sunday—free of the messianic delusions that once tormented him. He also stopped abusing alcohol on his own in 1995 and has remained abstinent since.
How can we ensure that patients with schizophrenia keep taking their medications—regardless of whether symptoms are present?
Dr. Canive’s observations
Clinical trials measure a drug’s efficacy under highly controlled circumstances. In the “real world,” however, noncompliance due to intolerability can undermine a medication’s effectiveness.
Too often noncompliance—stemming from abatement of symptoms or the emergence of side effects—derails treatment of schizophrenia. Misdrahi et al found that medication noncompliance accounts for 40% of schizophrenia relapses occurring more than 1 year after patients’ first hospitalization.4
Given aripiprazole’s 75-hour half-life, one might not expect to see symptoms emerge so soon after discontinuation. It is possible that:
- Mr. F. abstained from aripiprazole longer than he realized—or admitted
- Unidentified stressful life events also exacerbated symptoms and precipitated hospitalization.
When Mr. F consistently followed his regimen, his positive symptoms abated and he could attempt to live a normal life.
Our patients must understand that schizophrenia is a lifelong illness and that continued adherence to medication—even when symptoms do not exist—is crucial. A strong therapeutic alliance,5 increased social support, adjunctive cognitive-behavioral therapy, psychosocial interventions,6 and medications with fewer and less-severe side effects may help patients embrace this message.
Related resources
- Tamminga CA. Partial dopamine agonists in the treatment of psychosis. J Neural Transm 2002;109:411-20.
Drug brand names
- Amitriptyline • Elavil
- Aripiprazole • Abilify
- Benztropine • Cogentin
- Chlorpromazine • Thorazine
- Clonazepam • Klonopin
- Diazepam • Valium
- Fluphenazine • Prolixin
- Haloperidol • Haldol
- Lorazepam • Ativan
- Loxapine • Loxitane
- Oxazepam • Serax
- Thiothixene • Navane
- Trazodone • Desyrel
Disclosure
The author receives research/grant support from and is a speaker for and consultant to Bristol-Myers Squibb Co. He also receives research/grant support from and/or is a speaker for AstraZeneca Pharmaceuticals, Janssen Pharmaceutica, and Eli Lilly and Co.
1. Aripiprazole prescribing information. Bristol-Myers Squibb Co. and Otsuka America Pharmaceutical, 2002.
2. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63:763-71.
3. Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 2002;302:381-9.
4. Misdrahi D, Llorca PM, Lancon C, Bayle FJ. Compliance in schizophrenia: Predictive factors, therapeutical considerations and research implications (French). Encephale 2002;3:266-72.
5. Weiss KA, Smith TE, Hull JW, et al. Predictors of risk of nonadherence in outpatients with schizophrenia and other psychotic disorders. Schizophr Bull 2002;28:341-9.
6. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 2002;63:1121-8.
1. Aripiprazole prescribing information. Bristol-Myers Squibb Co. and Otsuka America Pharmaceutical, 2002.
2. Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2002;63:763-71.
3. Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 2002;302:381-9.
4. Misdrahi D, Llorca PM, Lancon C, Bayle FJ. Compliance in schizophrenia: Predictive factors, therapeutical considerations and research implications (French). Encephale 2002;3:266-72.
5. Weiss KA, Smith TE, Hull JW, et al. Predictors of risk of nonadherence in outpatients with schizophrenia and other psychotic disorders. Schizophr Bull 2002;28:341-9.
6. Perkins DO. Predictors of noncompliance in patients with schizophrenia. J Clin Psychiatry 2002;63:1121-8.
Getting to the bottom of problem drinking: The case for routine screening
Do you know which of your patients have alcohol problems? Though alcohol use disorders may be difficult to detect, self-report and biochemical measures followed by a thorough face-to-face assessment improve diagnostic accuracy. New tools—such as the serum carbohydrate-deficient transferrin (CDT) test—are changing how psychiatrists screen for alcohol problems, provide motivational feedback, and monitor patients for relapse.
FOUR REASONS TO SCREEN
Screening for excessive alcohol consumption is important in psychiatric practice because:
- Alcohol use disorders coexist with many psychiatric problems, most notably affective and anxiety disorders and—not surprisingly—other substance abuse disorders (Table 1).1,2
- Patients with psychiatric comorbidity who abuse alcohol have poorer prognoses, are less adherent to treatment, and are more likely to drop out of treatment than are psychiatric patients who do not have alcohol problems.3
- Alcohol interacts with many psychotropics, and chronic heavy drinking can cause pharmacokinetic changes that affect a patient’s response to medications.
- Alcohol-dependent patients are more likely than nondrinkers to become dependent on anxiolytics and sedative-hypnotics.
Table 1
Overlap of alcohol problems with common psychiatric disorders
| Disorder | Risk of alcohol use disorder (odds ratio) | Source of data (population survey) |
|---|---|---|
| Drug use disorder | 25.1 | NLAES |
| Mania | 5.6 | NCS |
| Major depression | 3.7 | NLAES |
| Obsessive-compulsive disorder | 3.4 | ECA |
| Generalized anxiety disorder | 2.7 | NCS |
| Phobia | 2.3 | NCS |
| Posttraumatic stress disorder | 2.2 | NCS |
| Panic disorder | 1.4 | NCS |
| NLAES: National Longitudinal Alcohol Epidemiological Survey | ||
| NCS: National Comorbidity Survey | ||
| ECA: Epidemiologic Catchment Area | ||
Because alcohol problems are common in psychiatric patients, routine screening for alcohol abuse and dependence at the onset of any treatment can be very useful. Thereafter, screening can be done periodically—perhaps annually or more often if the patient’s functioning declines.
CHOOSING A SELF-REPORT MEASURE
Many self-report alcohol screening scales are available,4 the most popular being the CAGE5 and the Michigan Alcoholism Screening Test (MAST).6 Though both instruments can help identify alcohol problems, each has shortcomings:
- The CAGE performs less reliably in women and adolescents than in men, and its validity depends on the patient’s sensitivity to the emotional impacts of alcohol dependence.
- The MAST is long (25 items), concentrates on late-stage alcoholism symptoms, and uses differential weighting—not validated in subsequent studies—of particular items in deriving the score.
Neither addresses drinking behavior or when symptoms occurred and thus may misclassify recovered alcoholics or former problem drinkers.
AUDIT. A more reliable choice is the Alcohol Use Disorders Identification Test (AUDIT).7 It was designed by the World Health Organization (WHO) to be valid across gender and culture and to identify even early stage problem drinking. The AUDIT’s 10 items deal with drinking behavior, dependence on alcohol, and adverse consequences of drinking during the past year (Box). The survey takes less than 5 minutes; can be administered orally, in writing, or online; and it retains its validity when given as part of a comprehensive health risk appraisal.8
The WHO offers an excellent manual detailing how to administer and interpret the AUDIT (see Related resources). A patient’s score is computed by summing the values associated with his or her responses to each item. A score of 8 or greater indicates excessive alcohol consumption, although some researchers have argued that for women a more accurate threshold might be 6 or 7 points.
Standardized for adults, the AUDIT also appears to accurately gauge drinking behavior in adolescents9 and in psychiatric patients, although only three studies have explored its use in the latter population.10-12 Abbreviated AUDIT versions have been found to be psychometrically sound8 and may be useful in an emergency room or busy primary care clinic. In comparisons with other screening tools, the AUDIT almost always has been found to be more valid.9,13
USING BIOCHEMICAL MEASURES
Self-report screens for alcohol problems, especially the AUDIT, are highly sensitive and specific, though their accuracy depends on the patient's memory, understanding of the questions, and candor. In chronic heavy drinkers, biochemical measures (Table 2) can augment self-reports.14
Self-report and biochemical screens have different strengths and weaknesses (Table 3). It is important to see them as complementary because each contributes to accurate screening.
CDT. Most biomarkers screen indirectly for alcohol problems by measuring damage to an end organ-typically the liver-caused by chronic excessive alcohol consumption. False positive results are common because of nonalcohol-related organ damage, medications, smoking, obesity, and other confounding factors. An exception appears to be the serum test for carbohydrate-deficient transferrin (CDT), a biomarker for heavy drinking approved in kit form 3 years ago by the Food and Drug Administration.
The value of measuring CDT levels is that few conditions other than excessive alcohol consumption elevate them. For unclear reasons,15 average daily consumption of >60 grams of alcohol (about five standard drinks) during the previous 2 weeks causes a higher percent of transferrin—a glycoenzyme that transports iron in the body—to lack its usual carbohydrate content.
Bio-Rad Laboratories (www.bio-rad.com) offers a reagent kit (%CDT Turbidimetric Immunoassay). It quantifies CDT as a percent of total serum transferrin, rather than total CDT, thus correcting for individual variations in transferrin levels. CDT values are obtained from a 100-microliter serum sample. The blood is clotted and the serum separated. The sample may be stored at 2 to 8 °C if the test is to be run within 1 week. Samples must be tested at a reference lab (Bio-Rad offers a list of labs). Results are available in a few days.
Patients who deny problem drinking may need convincing to submit to a blood draw. It may help to explain that alcohol use can exacerbate emotional problems and that the test can provide information on possible risky alcohol use.
GGT. Using a second biochemical marker may improve the sensitivity of CDT to detect heavy drinking.16-18
The most-researched choice for a second marker is gamma glutamyltransferase (GGT). Patients are considered to have tested positive for an alcohol problem if either CDT or GGT levels are elevated. Combining these tests may be especially useful in alcohol-dependent women, in whom the reliability of CDT testing alone has been questioned.
Recommendation. Start with a self-report screening measure. If the patient scores slightly below the threshold for an alcohol problem, follow up with the more costly CDT and GGT tests.
For example, biomarkers might be useful for follow-up in men with AUDIT screening scores of 6 or 7 or women with scores of 5 to 7. Biomarkers also are recommended when you suspect an alcohol problem for another reason or question whether the patient responded accurately to the self-report measure.
- How often do you have a drink containing alcohol?
- How many drinks containing alcohol do you have on a typical day when you are drinking?
- How often do you have 6 or more drinks on one occasion?
- How often during the last year have you found that you were not able to stop drinking once you had started?
- How often during the last year have you failed to do what was normally expected from you because of drinking?
- How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
- How often during the last year have you had a feeling of guilt or remorse after drinking?
- How often during the last year have you been unable to remember what happened the night before because you had been drinking?
- Have you or someone else been injured as a result of your drinking?
- Has a relative, friend, doctor, or other health worker been concerned about your drinking or suggested that you cut down?
The World Health Organization offers a manual on how to administer and interpret AUDIT (http://www.who.int/substance_abuse/pubs_alcohol.htm).
Source: World Health Organization
Table 2
Biochemical markers of heavy drinking
| Marker | Time needed for return to normal limits | Level of drinking characterized | Comments |
|---|---|---|---|
| Gamma glutamyltransferase (GGT) | 2 to 6 weeks of abstinence | ~70 drinks/wk for several weeks | Most common and reliable of the traditional markers of heavy drinking; many sources of false positives |
| Aspartate aminotransferase (AST) (formerly SGOT) | 7 days, but much variability in declines with abstinence | Unknown, but heavy | Present in many organs; many sources of false positives; moderate correlations with GGT |
| Alanine aminotransferase (ALT) (formerly SGPT) | Unknown | Unknown, but heavy | Many sources of false positives and less sensitive than AST; ratio of AST to ALT may be more accurate |
| Macrocytic volume (MCV) | Unknown; half-life ~40 days | Unknown, but regular and heavy | Poor sensitivity and specificity; even with abstinence, very slow return to normal limits and may increase at first; little, if any, gender effect |
| Carbohydrate-deficient transferrin (CDT) | 2 to 4 weeks of abstinence | >60 grams/day for approximately 2 weeks | Few sources of false positives; excellent indicator of relapse |
MONITORING PATIENTS IN TREATMENT
MET. Motivational enhancement therapy (MET) has gained popularity as a means of changing problematic drinking.19 Project MATCH—a multi-site trial on alcohol abuse treatment—studied MET and two other interventions. MET required fewer sessions but equaled the other interventions in reducing drinking days and aver-age amount of alcohol consumed.20
A key component of MET—and other brief interventions—is to provide patients with empathetic, nonjudgmental feedback.19-21 Responses to the first three AUDIT items can provide such feedback to patients with drinking problems. Amazingly, heavy drinkers and alcoholics often do not realize how much more they drink than other people. To help them develop this insight, show them their self-report responses in contrast with national normative data.
Biomarker results can be used similarly, in this case comparing the patient’s score with the test’s reference range values. Kristenson et al22 showed that giving men with elevated scores recurrent biomarker information and advice significantly reduced morbidity and mortality and improved their work performance.
Using visual aids can deepen patients’ under-standing of motivational feedback. For example, displaying sequential test results on a timeline can reinforce motivation by showing how their drinking behavior has improved with continuing treatment and sustained effort.
Table 3
Self-report and biochemical measures of drinking: Pros and cons
| Measure | Strengths | Weaknesses |
|---|---|---|
| Self-report | Noninvasive Inexpensive High validity Flexible window of assessment Immediate results | Easily feigned Accuracy depends on patient’s verbal skills and memory |
| Biochemical | Objective Results may be more compelling to patients than self-reports May reflect organ damage Useful in tracking treatment progress | Window of assessment is limited to recent past Results often not immediately available May be more costly than self-report measures |
DETECTING RELAPSE
Although treatment for alcohol problems is often successful,23 relapse to some level of drinking is not uncommon, especially during the first 3 or 4 months after patients complete treatment.20 Recognizing relapse quickly can help you:
- decrease risk of harm from resumed alcohol use
- reduce the potential for drinking to again become habitual
- identify circumstances and cues that may have triggered the drinking episode, for use in tailoring future interventions.
In clinical practice, relapse is most often revealed via comments from the family, direct observation by the clinician, or voluntary acknowledgment by the patient. Interestingly, CDT has demonstrated a relapse “heralding effect,”24 meaning that it tends to rise well before a patient will admit he or she resumed drinking.24-26 Although other markers may also rise following relapse, their elevation tends to be delayed and less dramatic.
The sensitivity and specificity of CDT alone and with GGT in identifying relapse have been evaluated. Across male-only studies, CDT’s median sensitivity (percent of relapsed patients with elevated scores) was 0.73, with a specificity (percent of those not relapsed who had low scores) of 0.91. In the two female-only studies, median sensitivity and specificity for CDT were 0.32 and 0.86, respectively. For women, using CDT and GGT in combination substantially raised median sensitivity to 0.62, although specificity fell slightly to 0.80.27
Recommendation. When using biomarkers to identify relapse, examine the temporal pattern of test results to date. Assume that an increase of 30% or more above the lowest observed lab value indicates a relapse.28
Frequent testing—probably biweekly—is recommended during the first 3 or 4 months after patients complete treatment. If there is no indication of relapse, testing frequency could be tapered down.
- AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for primary care use. Available at: http://www.who.int/substance_abuse/pubs_alcohol.htm
- Allen JP, Litten RZ. Psychometric and laboratory measures to assist in the treatment of alcoholism. Clin Psychol Rev 1993;13(3):223-39.
- Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999; 19(3):261-71.
Disclosure
Dr. Allen reports that he serves as a consultant to Axis-Shield ASA and Bio-Rad Laboratories, patent holder and U.S. distributor, respectively, of the carbohydrate-deficient transferrin (%CDT) reagent kit.
Dr. Anthenelli reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hall W. What have population surveys revealed about substance use disorders and their co-morbidity with other mental disorders? Drug Alcohol Rev 1996;15(2):157-70.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend 1995;39(3):197-206.
3. Shivani R, Goldsmith RJ, Anthenelli RM. Alcoholism and psychiatric disorders: diagnostic challenges. Alcohol Res Health 2002;26(2):90-8.
4. Allen JP, Columbus M (eds). Assessing alcohol problems: a guide for clinicians and researchers. NIAAA Treatment Handbook, Series 4. Washington, DC: U.S. Department of Health and Human Services, 1995 [NIH publication no. 95-3745].
5. Mayfield D, McLeod G, Hall P. CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131(10):1121-3.
6. Seltzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8.
7. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993;88(6):791-804.
8. Daeppen J, Yersin B, Landry U, et al. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res 2000;24:659-65.
9. Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: a review of recent research. Alcohol Clin Exp Res 2002;26(2):272-9.
10. Maisto SA, Carey MP, Carey KB, et al. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2002;12:186-92.
11. Hulse GK, Saunders JB, Roydhouse RM, et al. Screening for hazardous alcohol use and dependence in psychiatric inpatients using the AUDIT questionnaire. Drug Alcohol Rev 2000;19:291-8.
12. Dawe S, Seinen A, Kavanagh D. An examination of the utility of the AUDIT in people with schizophrenia. J Stud Alcohol 2000;61:744-75.
13. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613-19.
14. Hermansson U, Helander A, Huss A, et al. The Alcohol Use Disorders Identification Test (AUDIT) and carbohydrate-deficient transferrin (CDT) in a routine workplace health examination. Alcohol Clin Exp Res 2000;24:180-7.
15. Sillanaukee P, Strid N, Allen JP, Litten RZ. Possible reasons why heavy drinking increases carbohydrate-deficient transferrin. Alcohol Clin Exp Res 2001;25(1):34-40.
16. Litten RZ, Allen JP, Fertig JB. Gamma-glutamyltranspeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
17. Allen JP, Litten RZ, Fertig JB, Sillanaukee P. Carbohydrate-deficient transferrin, gamma glutamyl transferase and macrocytic volume as biomarkers of alcohol problems in women. Alcohol Clin Exp Res 2000;24(4):492-6.
18. Sillanaukee P, Strid N, Allen JP, Litten RZ. Combining biomarkers to screen for alcohol problems (manuscript submitted for publication).
19. Miller WR, Zweben A, DiClemente CC, et al. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: U.S. Department of Health and Human Services, 1995.
20. Project MATCH research group. Matching alcoholism treatments to client heterogeneity: Project MATCH post-treatment drinking outcomes. J Stud Alcohol 1997;58(1):7-29.
21. Bien TH, Miller WR, Tonigan S. Brief intervention for alcohol problems: a review. Addiction 1993;88:315-36.
22. Kristenson H, Hood B, Peterson B, et al. Prevention of alcohol-related problems in urban middle-aged males. Alcohol 1985;2(3):545-9.
23. Miller WR, Walter ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211-20.
24. Mitchell C, Simpson D, Chick J. Carbohydrate-deficient transferrin in detecting relapse in alcohol dependence. Drug Alcohol Depend 1997;48:97-103.
25. Borg S, Helander A, Voltaire Carlsson A, Hogstrom Brandt AM. Detection of relapses in alcohol-dependent patients using carbohydrate-deficient transferrin: improvement with individualized reference levels during long-term monitoring. Alcohol Clin Exp Res 1995;19(4):961-3.
26. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
27. Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. In: Galanter M (ed). Recent developments in alcoholism: research on alcoholism treatment (vol. XVI). New York: Plenum Press (in press).
28. Anton RF, Lieber C, Tabakoff B, et al. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multi-site study. Alcohol Clin Exp Res 2002;26(8):1215-22.
Do you know which of your patients have alcohol problems? Though alcohol use disorders may be difficult to detect, self-report and biochemical measures followed by a thorough face-to-face assessment improve diagnostic accuracy. New tools—such as the serum carbohydrate-deficient transferrin (CDT) test—are changing how psychiatrists screen for alcohol problems, provide motivational feedback, and monitor patients for relapse.
FOUR REASONS TO SCREEN
Screening for excessive alcohol consumption is important in psychiatric practice because:
- Alcohol use disorders coexist with many psychiatric problems, most notably affective and anxiety disorders and—not surprisingly—other substance abuse disorders (Table 1).1,2
- Patients with psychiatric comorbidity who abuse alcohol have poorer prognoses, are less adherent to treatment, and are more likely to drop out of treatment than are psychiatric patients who do not have alcohol problems.3
- Alcohol interacts with many psychotropics, and chronic heavy drinking can cause pharmacokinetic changes that affect a patient’s response to medications.
- Alcohol-dependent patients are more likely than nondrinkers to become dependent on anxiolytics and sedative-hypnotics.
Table 1
Overlap of alcohol problems with common psychiatric disorders
| Disorder | Risk of alcohol use disorder (odds ratio) | Source of data (population survey) |
|---|---|---|
| Drug use disorder | 25.1 | NLAES |
| Mania | 5.6 | NCS |
| Major depression | 3.7 | NLAES |
| Obsessive-compulsive disorder | 3.4 | ECA |
| Generalized anxiety disorder | 2.7 | NCS |
| Phobia | 2.3 | NCS |
| Posttraumatic stress disorder | 2.2 | NCS |
| Panic disorder | 1.4 | NCS |
| NLAES: National Longitudinal Alcohol Epidemiological Survey | ||
| NCS: National Comorbidity Survey | ||
| ECA: Epidemiologic Catchment Area | ||
Because alcohol problems are common in psychiatric patients, routine screening for alcohol abuse and dependence at the onset of any treatment can be very useful. Thereafter, screening can be done periodically—perhaps annually or more often if the patient’s functioning declines.
CHOOSING A SELF-REPORT MEASURE
Many self-report alcohol screening scales are available,4 the most popular being the CAGE5 and the Michigan Alcoholism Screening Test (MAST).6 Though both instruments can help identify alcohol problems, each has shortcomings:
- The CAGE performs less reliably in women and adolescents than in men, and its validity depends on the patient’s sensitivity to the emotional impacts of alcohol dependence.
- The MAST is long (25 items), concentrates on late-stage alcoholism symptoms, and uses differential weighting—not validated in subsequent studies—of particular items in deriving the score.
Neither addresses drinking behavior or when symptoms occurred and thus may misclassify recovered alcoholics or former problem drinkers.
AUDIT. A more reliable choice is the Alcohol Use Disorders Identification Test (AUDIT).7 It was designed by the World Health Organization (WHO) to be valid across gender and culture and to identify even early stage problem drinking. The AUDIT’s 10 items deal with drinking behavior, dependence on alcohol, and adverse consequences of drinking during the past year (Box). The survey takes less than 5 minutes; can be administered orally, in writing, or online; and it retains its validity when given as part of a comprehensive health risk appraisal.8
The WHO offers an excellent manual detailing how to administer and interpret the AUDIT (see Related resources). A patient’s score is computed by summing the values associated with his or her responses to each item. A score of 8 or greater indicates excessive alcohol consumption, although some researchers have argued that for women a more accurate threshold might be 6 or 7 points.
Standardized for adults, the AUDIT also appears to accurately gauge drinking behavior in adolescents9 and in psychiatric patients, although only three studies have explored its use in the latter population.10-12 Abbreviated AUDIT versions have been found to be psychometrically sound8 and may be useful in an emergency room or busy primary care clinic. In comparisons with other screening tools, the AUDIT almost always has been found to be more valid.9,13
USING BIOCHEMICAL MEASURES
Self-report screens for alcohol problems, especially the AUDIT, are highly sensitive and specific, though their accuracy depends on the patient's memory, understanding of the questions, and candor. In chronic heavy drinkers, biochemical measures (Table 2) can augment self-reports.14
Self-report and biochemical screens have different strengths and weaknesses (Table 3). It is important to see them as complementary because each contributes to accurate screening.
CDT. Most biomarkers screen indirectly for alcohol problems by measuring damage to an end organ-typically the liver-caused by chronic excessive alcohol consumption. False positive results are common because of nonalcohol-related organ damage, medications, smoking, obesity, and other confounding factors. An exception appears to be the serum test for carbohydrate-deficient transferrin (CDT), a biomarker for heavy drinking approved in kit form 3 years ago by the Food and Drug Administration.
The value of measuring CDT levels is that few conditions other than excessive alcohol consumption elevate them. For unclear reasons,15 average daily consumption of >60 grams of alcohol (about five standard drinks) during the previous 2 weeks causes a higher percent of transferrin—a glycoenzyme that transports iron in the body—to lack its usual carbohydrate content.
Bio-Rad Laboratories (www.bio-rad.com) offers a reagent kit (%CDT Turbidimetric Immunoassay). It quantifies CDT as a percent of total serum transferrin, rather than total CDT, thus correcting for individual variations in transferrin levels. CDT values are obtained from a 100-microliter serum sample. The blood is clotted and the serum separated. The sample may be stored at 2 to 8 °C if the test is to be run within 1 week. Samples must be tested at a reference lab (Bio-Rad offers a list of labs). Results are available in a few days.
Patients who deny problem drinking may need convincing to submit to a blood draw. It may help to explain that alcohol use can exacerbate emotional problems and that the test can provide information on possible risky alcohol use.
GGT. Using a second biochemical marker may improve the sensitivity of CDT to detect heavy drinking.16-18
The most-researched choice for a second marker is gamma glutamyltransferase (GGT). Patients are considered to have tested positive for an alcohol problem if either CDT or GGT levels are elevated. Combining these tests may be especially useful in alcohol-dependent women, in whom the reliability of CDT testing alone has been questioned.
Recommendation. Start with a self-report screening measure. If the patient scores slightly below the threshold for an alcohol problem, follow up with the more costly CDT and GGT tests.
For example, biomarkers might be useful for follow-up in men with AUDIT screening scores of 6 or 7 or women with scores of 5 to 7. Biomarkers also are recommended when you suspect an alcohol problem for another reason or question whether the patient responded accurately to the self-report measure.
- How often do you have a drink containing alcohol?
- How many drinks containing alcohol do you have on a typical day when you are drinking?
- How often do you have 6 or more drinks on one occasion?
- How often during the last year have you found that you were not able to stop drinking once you had started?
- How often during the last year have you failed to do what was normally expected from you because of drinking?
- How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
- How often during the last year have you had a feeling of guilt or remorse after drinking?
- How often during the last year have you been unable to remember what happened the night before because you had been drinking?
- Have you or someone else been injured as a result of your drinking?
- Has a relative, friend, doctor, or other health worker been concerned about your drinking or suggested that you cut down?
The World Health Organization offers a manual on how to administer and interpret AUDIT (http://www.who.int/substance_abuse/pubs_alcohol.htm).
Source: World Health Organization
Table 2
Biochemical markers of heavy drinking
| Marker | Time needed for return to normal limits | Level of drinking characterized | Comments |
|---|---|---|---|
| Gamma glutamyltransferase (GGT) | 2 to 6 weeks of abstinence | ~70 drinks/wk for several weeks | Most common and reliable of the traditional markers of heavy drinking; many sources of false positives |
| Aspartate aminotransferase (AST) (formerly SGOT) | 7 days, but much variability in declines with abstinence | Unknown, but heavy | Present in many organs; many sources of false positives; moderate correlations with GGT |
| Alanine aminotransferase (ALT) (formerly SGPT) | Unknown | Unknown, but heavy | Many sources of false positives and less sensitive than AST; ratio of AST to ALT may be more accurate |
| Macrocytic volume (MCV) | Unknown; half-life ~40 days | Unknown, but regular and heavy | Poor sensitivity and specificity; even with abstinence, very slow return to normal limits and may increase at first; little, if any, gender effect |
| Carbohydrate-deficient transferrin (CDT) | 2 to 4 weeks of abstinence | >60 grams/day for approximately 2 weeks | Few sources of false positives; excellent indicator of relapse |
MONITORING PATIENTS IN TREATMENT
MET. Motivational enhancement therapy (MET) has gained popularity as a means of changing problematic drinking.19 Project MATCH—a multi-site trial on alcohol abuse treatment—studied MET and two other interventions. MET required fewer sessions but equaled the other interventions in reducing drinking days and aver-age amount of alcohol consumed.20
A key component of MET—and other brief interventions—is to provide patients with empathetic, nonjudgmental feedback.19-21 Responses to the first three AUDIT items can provide such feedback to patients with drinking problems. Amazingly, heavy drinkers and alcoholics often do not realize how much more they drink than other people. To help them develop this insight, show them their self-report responses in contrast with national normative data.
Biomarker results can be used similarly, in this case comparing the patient’s score with the test’s reference range values. Kristenson et al22 showed that giving men with elevated scores recurrent biomarker information and advice significantly reduced morbidity and mortality and improved their work performance.
Using visual aids can deepen patients’ under-standing of motivational feedback. For example, displaying sequential test results on a timeline can reinforce motivation by showing how their drinking behavior has improved with continuing treatment and sustained effort.
Table 3
Self-report and biochemical measures of drinking: Pros and cons
| Measure | Strengths | Weaknesses |
|---|---|---|
| Self-report | Noninvasive Inexpensive High validity Flexible window of assessment Immediate results | Easily feigned Accuracy depends on patient’s verbal skills and memory |
| Biochemical | Objective Results may be more compelling to patients than self-reports May reflect organ damage Useful in tracking treatment progress | Window of assessment is limited to recent past Results often not immediately available May be more costly than self-report measures |
DETECTING RELAPSE
Although treatment for alcohol problems is often successful,23 relapse to some level of drinking is not uncommon, especially during the first 3 or 4 months after patients complete treatment.20 Recognizing relapse quickly can help you:
- decrease risk of harm from resumed alcohol use
- reduce the potential for drinking to again become habitual
- identify circumstances and cues that may have triggered the drinking episode, for use in tailoring future interventions.
In clinical practice, relapse is most often revealed via comments from the family, direct observation by the clinician, or voluntary acknowledgment by the patient. Interestingly, CDT has demonstrated a relapse “heralding effect,”24 meaning that it tends to rise well before a patient will admit he or she resumed drinking.24-26 Although other markers may also rise following relapse, their elevation tends to be delayed and less dramatic.
The sensitivity and specificity of CDT alone and with GGT in identifying relapse have been evaluated. Across male-only studies, CDT’s median sensitivity (percent of relapsed patients with elevated scores) was 0.73, with a specificity (percent of those not relapsed who had low scores) of 0.91. In the two female-only studies, median sensitivity and specificity for CDT were 0.32 and 0.86, respectively. For women, using CDT and GGT in combination substantially raised median sensitivity to 0.62, although specificity fell slightly to 0.80.27
Recommendation. When using biomarkers to identify relapse, examine the temporal pattern of test results to date. Assume that an increase of 30% or more above the lowest observed lab value indicates a relapse.28
Frequent testing—probably biweekly—is recommended during the first 3 or 4 months after patients complete treatment. If there is no indication of relapse, testing frequency could be tapered down.
- AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for primary care use. Available at: http://www.who.int/substance_abuse/pubs_alcohol.htm
- Allen JP, Litten RZ. Psychometric and laboratory measures to assist in the treatment of alcoholism. Clin Psychol Rev 1993;13(3):223-39.
- Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999; 19(3):261-71.
Disclosure
Dr. Allen reports that he serves as a consultant to Axis-Shield ASA and Bio-Rad Laboratories, patent holder and U.S. distributor, respectively, of the carbohydrate-deficient transferrin (%CDT) reagent kit.
Dr. Anthenelli reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Do you know which of your patients have alcohol problems? Though alcohol use disorders may be difficult to detect, self-report and biochemical measures followed by a thorough face-to-face assessment improve diagnostic accuracy. New tools—such as the serum carbohydrate-deficient transferrin (CDT) test—are changing how psychiatrists screen for alcohol problems, provide motivational feedback, and monitor patients for relapse.
FOUR REASONS TO SCREEN
Screening for excessive alcohol consumption is important in psychiatric practice because:
- Alcohol use disorders coexist with many psychiatric problems, most notably affective and anxiety disorders and—not surprisingly—other substance abuse disorders (Table 1).1,2
- Patients with psychiatric comorbidity who abuse alcohol have poorer prognoses, are less adherent to treatment, and are more likely to drop out of treatment than are psychiatric patients who do not have alcohol problems.3
- Alcohol interacts with many psychotropics, and chronic heavy drinking can cause pharmacokinetic changes that affect a patient’s response to medications.
- Alcohol-dependent patients are more likely than nondrinkers to become dependent on anxiolytics and sedative-hypnotics.
Table 1
Overlap of alcohol problems with common psychiatric disorders
| Disorder | Risk of alcohol use disorder (odds ratio) | Source of data (population survey) |
|---|---|---|
| Drug use disorder | 25.1 | NLAES |
| Mania | 5.6 | NCS |
| Major depression | 3.7 | NLAES |
| Obsessive-compulsive disorder | 3.4 | ECA |
| Generalized anxiety disorder | 2.7 | NCS |
| Phobia | 2.3 | NCS |
| Posttraumatic stress disorder | 2.2 | NCS |
| Panic disorder | 1.4 | NCS |
| NLAES: National Longitudinal Alcohol Epidemiological Survey | ||
| NCS: National Comorbidity Survey | ||
| ECA: Epidemiologic Catchment Area | ||
Because alcohol problems are common in psychiatric patients, routine screening for alcohol abuse and dependence at the onset of any treatment can be very useful. Thereafter, screening can be done periodically—perhaps annually or more often if the patient’s functioning declines.
CHOOSING A SELF-REPORT MEASURE
Many self-report alcohol screening scales are available,4 the most popular being the CAGE5 and the Michigan Alcoholism Screening Test (MAST).6 Though both instruments can help identify alcohol problems, each has shortcomings:
- The CAGE performs less reliably in women and adolescents than in men, and its validity depends on the patient’s sensitivity to the emotional impacts of alcohol dependence.
- The MAST is long (25 items), concentrates on late-stage alcoholism symptoms, and uses differential weighting—not validated in subsequent studies—of particular items in deriving the score.
Neither addresses drinking behavior or when symptoms occurred and thus may misclassify recovered alcoholics or former problem drinkers.
AUDIT. A more reliable choice is the Alcohol Use Disorders Identification Test (AUDIT).7 It was designed by the World Health Organization (WHO) to be valid across gender and culture and to identify even early stage problem drinking. The AUDIT’s 10 items deal with drinking behavior, dependence on alcohol, and adverse consequences of drinking during the past year (Box). The survey takes less than 5 minutes; can be administered orally, in writing, or online; and it retains its validity when given as part of a comprehensive health risk appraisal.8
The WHO offers an excellent manual detailing how to administer and interpret the AUDIT (see Related resources). A patient’s score is computed by summing the values associated with his or her responses to each item. A score of 8 or greater indicates excessive alcohol consumption, although some researchers have argued that for women a more accurate threshold might be 6 or 7 points.
Standardized for adults, the AUDIT also appears to accurately gauge drinking behavior in adolescents9 and in psychiatric patients, although only three studies have explored its use in the latter population.10-12 Abbreviated AUDIT versions have been found to be psychometrically sound8 and may be useful in an emergency room or busy primary care clinic. In comparisons with other screening tools, the AUDIT almost always has been found to be more valid.9,13
USING BIOCHEMICAL MEASURES
Self-report screens for alcohol problems, especially the AUDIT, are highly sensitive and specific, though their accuracy depends on the patient's memory, understanding of the questions, and candor. In chronic heavy drinkers, biochemical measures (Table 2) can augment self-reports.14
Self-report and biochemical screens have different strengths and weaknesses (Table 3). It is important to see them as complementary because each contributes to accurate screening.
CDT. Most biomarkers screen indirectly for alcohol problems by measuring damage to an end organ-typically the liver-caused by chronic excessive alcohol consumption. False positive results are common because of nonalcohol-related organ damage, medications, smoking, obesity, and other confounding factors. An exception appears to be the serum test for carbohydrate-deficient transferrin (CDT), a biomarker for heavy drinking approved in kit form 3 years ago by the Food and Drug Administration.
The value of measuring CDT levels is that few conditions other than excessive alcohol consumption elevate them. For unclear reasons,15 average daily consumption of >60 grams of alcohol (about five standard drinks) during the previous 2 weeks causes a higher percent of transferrin—a glycoenzyme that transports iron in the body—to lack its usual carbohydrate content.
Bio-Rad Laboratories (www.bio-rad.com) offers a reagent kit (%CDT Turbidimetric Immunoassay). It quantifies CDT as a percent of total serum transferrin, rather than total CDT, thus correcting for individual variations in transferrin levels. CDT values are obtained from a 100-microliter serum sample. The blood is clotted and the serum separated. The sample may be stored at 2 to 8 °C if the test is to be run within 1 week. Samples must be tested at a reference lab (Bio-Rad offers a list of labs). Results are available in a few days.
Patients who deny problem drinking may need convincing to submit to a blood draw. It may help to explain that alcohol use can exacerbate emotional problems and that the test can provide information on possible risky alcohol use.
GGT. Using a second biochemical marker may improve the sensitivity of CDT to detect heavy drinking.16-18
The most-researched choice for a second marker is gamma glutamyltransferase (GGT). Patients are considered to have tested positive for an alcohol problem if either CDT or GGT levels are elevated. Combining these tests may be especially useful in alcohol-dependent women, in whom the reliability of CDT testing alone has been questioned.
Recommendation. Start with a self-report screening measure. If the patient scores slightly below the threshold for an alcohol problem, follow up with the more costly CDT and GGT tests.
For example, biomarkers might be useful for follow-up in men with AUDIT screening scores of 6 or 7 or women with scores of 5 to 7. Biomarkers also are recommended when you suspect an alcohol problem for another reason or question whether the patient responded accurately to the self-report measure.
- How often do you have a drink containing alcohol?
- How many drinks containing alcohol do you have on a typical day when you are drinking?
- How often do you have 6 or more drinks on one occasion?
- How often during the last year have you found that you were not able to stop drinking once you had started?
- How often during the last year have you failed to do what was normally expected from you because of drinking?
- How often during the last year have you needed a first drink in the morning to get yourself going after a heavy drinking session?
- How often during the last year have you had a feeling of guilt or remorse after drinking?
- How often during the last year have you been unable to remember what happened the night before because you had been drinking?
- Have you or someone else been injured as a result of your drinking?
- Has a relative, friend, doctor, or other health worker been concerned about your drinking or suggested that you cut down?
The World Health Organization offers a manual on how to administer and interpret AUDIT (http://www.who.int/substance_abuse/pubs_alcohol.htm).
Source: World Health Organization
Table 2
Biochemical markers of heavy drinking
| Marker | Time needed for return to normal limits | Level of drinking characterized | Comments |
|---|---|---|---|
| Gamma glutamyltransferase (GGT) | 2 to 6 weeks of abstinence | ~70 drinks/wk for several weeks | Most common and reliable of the traditional markers of heavy drinking; many sources of false positives |
| Aspartate aminotransferase (AST) (formerly SGOT) | 7 days, but much variability in declines with abstinence | Unknown, but heavy | Present in many organs; many sources of false positives; moderate correlations with GGT |
| Alanine aminotransferase (ALT) (formerly SGPT) | Unknown | Unknown, but heavy | Many sources of false positives and less sensitive than AST; ratio of AST to ALT may be more accurate |
| Macrocytic volume (MCV) | Unknown; half-life ~40 days | Unknown, but regular and heavy | Poor sensitivity and specificity; even with abstinence, very slow return to normal limits and may increase at first; little, if any, gender effect |
| Carbohydrate-deficient transferrin (CDT) | 2 to 4 weeks of abstinence | >60 grams/day for approximately 2 weeks | Few sources of false positives; excellent indicator of relapse |
MONITORING PATIENTS IN TREATMENT
MET. Motivational enhancement therapy (MET) has gained popularity as a means of changing problematic drinking.19 Project MATCH—a multi-site trial on alcohol abuse treatment—studied MET and two other interventions. MET required fewer sessions but equaled the other interventions in reducing drinking days and aver-age amount of alcohol consumed.20
A key component of MET—and other brief interventions—is to provide patients with empathetic, nonjudgmental feedback.19-21 Responses to the first three AUDIT items can provide such feedback to patients with drinking problems. Amazingly, heavy drinkers and alcoholics often do not realize how much more they drink than other people. To help them develop this insight, show them their self-report responses in contrast with national normative data.
Biomarker results can be used similarly, in this case comparing the patient’s score with the test’s reference range values. Kristenson et al22 showed that giving men with elevated scores recurrent biomarker information and advice significantly reduced morbidity and mortality and improved their work performance.
Using visual aids can deepen patients’ under-standing of motivational feedback. For example, displaying sequential test results on a timeline can reinforce motivation by showing how their drinking behavior has improved with continuing treatment and sustained effort.
Table 3
Self-report and biochemical measures of drinking: Pros and cons
| Measure | Strengths | Weaknesses |
|---|---|---|
| Self-report | Noninvasive Inexpensive High validity Flexible window of assessment Immediate results | Easily feigned Accuracy depends on patient’s verbal skills and memory |
| Biochemical | Objective Results may be more compelling to patients than self-reports May reflect organ damage Useful in tracking treatment progress | Window of assessment is limited to recent past Results often not immediately available May be more costly than self-report measures |
DETECTING RELAPSE
Although treatment for alcohol problems is often successful,23 relapse to some level of drinking is not uncommon, especially during the first 3 or 4 months after patients complete treatment.20 Recognizing relapse quickly can help you:
- decrease risk of harm from resumed alcohol use
- reduce the potential for drinking to again become habitual
- identify circumstances and cues that may have triggered the drinking episode, for use in tailoring future interventions.
In clinical practice, relapse is most often revealed via comments from the family, direct observation by the clinician, or voluntary acknowledgment by the patient. Interestingly, CDT has demonstrated a relapse “heralding effect,”24 meaning that it tends to rise well before a patient will admit he or she resumed drinking.24-26 Although other markers may also rise following relapse, their elevation tends to be delayed and less dramatic.
The sensitivity and specificity of CDT alone and with GGT in identifying relapse have been evaluated. Across male-only studies, CDT’s median sensitivity (percent of relapsed patients with elevated scores) was 0.73, with a specificity (percent of those not relapsed who had low scores) of 0.91. In the two female-only studies, median sensitivity and specificity for CDT were 0.32 and 0.86, respectively. For women, using CDT and GGT in combination substantially raised median sensitivity to 0.62, although specificity fell slightly to 0.80.27
Recommendation. When using biomarkers to identify relapse, examine the temporal pattern of test results to date. Assume that an increase of 30% or more above the lowest observed lab value indicates a relapse.28
Frequent testing—probably biweekly—is recommended during the first 3 or 4 months after patients complete treatment. If there is no indication of relapse, testing frequency could be tapered down.
- AUDIT. The Alcohol Use Disorders Identification Test. Guidelines for primary care use. Available at: http://www.who.int/substance_abuse/pubs_alcohol.htm
- Allen JP, Litten RZ. Psychometric and laboratory measures to assist in the treatment of alcoholism. Clin Psychol Rev 1993;13(3):223-39.
- Salaspuro M. Carbohydrate-deficient transferrin as compared to other markers of alcoholism: a systematic review. Alcohol 1999; 19(3):261-71.
Disclosure
Dr. Allen reports that he serves as a consultant to Axis-Shield ASA and Bio-Rad Laboratories, patent holder and U.S. distributor, respectively, of the carbohydrate-deficient transferrin (%CDT) reagent kit.
Dr. Anthenelli reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Hall W. What have population surveys revealed about substance use disorders and their co-morbidity with other mental disorders? Drug Alcohol Rev 1996;15(2):157-70.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend 1995;39(3):197-206.
3. Shivani R, Goldsmith RJ, Anthenelli RM. Alcoholism and psychiatric disorders: diagnostic challenges. Alcohol Res Health 2002;26(2):90-8.
4. Allen JP, Columbus M (eds). Assessing alcohol problems: a guide for clinicians and researchers. NIAAA Treatment Handbook, Series 4. Washington, DC: U.S. Department of Health and Human Services, 1995 [NIH publication no. 95-3745].
5. Mayfield D, McLeod G, Hall P. CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131(10):1121-3.
6. Seltzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8.
7. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993;88(6):791-804.
8. Daeppen J, Yersin B, Landry U, et al. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res 2000;24:659-65.
9. Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: a review of recent research. Alcohol Clin Exp Res 2002;26(2):272-9.
10. Maisto SA, Carey MP, Carey KB, et al. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2002;12:186-92.
11. Hulse GK, Saunders JB, Roydhouse RM, et al. Screening for hazardous alcohol use and dependence in psychiatric inpatients using the AUDIT questionnaire. Drug Alcohol Rev 2000;19:291-8.
12. Dawe S, Seinen A, Kavanagh D. An examination of the utility of the AUDIT in people with schizophrenia. J Stud Alcohol 2000;61:744-75.
13. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613-19.
14. Hermansson U, Helander A, Huss A, et al. The Alcohol Use Disorders Identification Test (AUDIT) and carbohydrate-deficient transferrin (CDT) in a routine workplace health examination. Alcohol Clin Exp Res 2000;24:180-7.
15. Sillanaukee P, Strid N, Allen JP, Litten RZ. Possible reasons why heavy drinking increases carbohydrate-deficient transferrin. Alcohol Clin Exp Res 2001;25(1):34-40.
16. Litten RZ, Allen JP, Fertig JB. Gamma-glutamyltranspeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
17. Allen JP, Litten RZ, Fertig JB, Sillanaukee P. Carbohydrate-deficient transferrin, gamma glutamyl transferase and macrocytic volume as biomarkers of alcohol problems in women. Alcohol Clin Exp Res 2000;24(4):492-6.
18. Sillanaukee P, Strid N, Allen JP, Litten RZ. Combining biomarkers to screen for alcohol problems (manuscript submitted for publication).
19. Miller WR, Zweben A, DiClemente CC, et al. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: U.S. Department of Health and Human Services, 1995.
20. Project MATCH research group. Matching alcoholism treatments to client heterogeneity: Project MATCH post-treatment drinking outcomes. J Stud Alcohol 1997;58(1):7-29.
21. Bien TH, Miller WR, Tonigan S. Brief intervention for alcohol problems: a review. Addiction 1993;88:315-36.
22. Kristenson H, Hood B, Peterson B, et al. Prevention of alcohol-related problems in urban middle-aged males. Alcohol 1985;2(3):545-9.
23. Miller WR, Walter ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211-20.
24. Mitchell C, Simpson D, Chick J. Carbohydrate-deficient transferrin in detecting relapse in alcohol dependence. Drug Alcohol Depend 1997;48:97-103.
25. Borg S, Helander A, Voltaire Carlsson A, Hogstrom Brandt AM. Detection of relapses in alcohol-dependent patients using carbohydrate-deficient transferrin: improvement with individualized reference levels during long-term monitoring. Alcohol Clin Exp Res 1995;19(4):961-3.
26. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
27. Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. In: Galanter M (ed). Recent developments in alcoholism: research on alcoholism treatment (vol. XVI). New York: Plenum Press (in press).
28. Anton RF, Lieber C, Tabakoff B, et al. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multi-site study. Alcohol Clin Exp Res 2002;26(8):1215-22.
1. Hall W. What have population surveys revealed about substance use disorders and their co-morbidity with other mental disorders? Drug Alcohol Rev 1996;15(2):157-70.
2. Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Depend 1995;39(3):197-206.
3. Shivani R, Goldsmith RJ, Anthenelli RM. Alcoholism and psychiatric disorders: diagnostic challenges. Alcohol Res Health 2002;26(2):90-8.
4. Allen JP, Columbus M (eds). Assessing alcohol problems: a guide for clinicians and researchers. NIAAA Treatment Handbook, Series 4. Washington, DC: U.S. Department of Health and Human Services, 1995 [NIH publication no. 95-3745].
5. Mayfield D, McLeod G, Hall P. CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131(10):1121-3.
6. Seltzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971;127:1653-8.
7. Saunders JB, Aasland OG, Babor TF, et al. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction 1993;88(6):791-804.
8. Daeppen J, Yersin B, Landry U, et al. Reliability and validity of the Alcohol Use Disorders Identification Test (AUDIT) imbedded within a general health risk screening questionnaire: results of a survey in 332 primary care patients. Alcohol Clin Exp Res 2000;24:659-65.
9. Reinert DF, Allen JP. The Alcohol Use Disorders Identification Test: a review of recent research. Alcohol Clin Exp Res 2002;26(2):272-9.
10. Maisto SA, Carey MP, Carey KB, et al. Use of the AUDIT and the DAST-10 to identify alcohol and drug use disorders among adults with a severe and persistent mental illness. Psychol Assess 2002;12:186-92.
11. Hulse GK, Saunders JB, Roydhouse RM, et al. Screening for hazardous alcohol use and dependence in psychiatric inpatients using the AUDIT questionnaire. Drug Alcohol Rev 2000;19:291-8.
12. Dawe S, Seinen A, Kavanagh D. An examination of the utility of the AUDIT in people with schizophrenia. J Stud Alcohol 2000;61:744-75.
13. Allen JP, Litten RZ, Fertig JB, Babor T. A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997;21:613-19.
14. Hermansson U, Helander A, Huss A, et al. The Alcohol Use Disorders Identification Test (AUDIT) and carbohydrate-deficient transferrin (CDT) in a routine workplace health examination. Alcohol Clin Exp Res 2000;24:180-7.
15. Sillanaukee P, Strid N, Allen JP, Litten RZ. Possible reasons why heavy drinking increases carbohydrate-deficient transferrin. Alcohol Clin Exp Res 2001;25(1):34-40.
16. Litten RZ, Allen JP, Fertig JB. Gamma-glutamyltranspeptidase and carbohydrate deficient transferrin: alternative measures of excessive alcohol consumption. Alcohol Clin Exp Res 1995;19(6):1541-6.
17. Allen JP, Litten RZ, Fertig JB, Sillanaukee P. Carbohydrate-deficient transferrin, gamma glutamyl transferase and macrocytic volume as biomarkers of alcohol problems in women. Alcohol Clin Exp Res 2000;24(4):492-6.
18. Sillanaukee P, Strid N, Allen JP, Litten RZ. Combining biomarkers to screen for alcohol problems (manuscript submitted for publication).
19. Miller WR, Zweben A, DiClemente CC, et al. Motivational enhancement therapy manual: A clinical research guide for therapists treating individuals with alcohol abuse and dependence. Rockville, MD: U.S. Department of Health and Human Services, 1995.
20. Project MATCH research group. Matching alcoholism treatments to client heterogeneity: Project MATCH post-treatment drinking outcomes. J Stud Alcohol 1997;58(1):7-29.
21. Bien TH, Miller WR, Tonigan S. Brief intervention for alcohol problems: a review. Addiction 1993;88:315-36.
22. Kristenson H, Hood B, Peterson B, et al. Prevention of alcohol-related problems in urban middle-aged males. Alcohol 1985;2(3):545-9.
23. Miller WR, Walter ST, Bennett ME. How effective is alcoholism treatment in the United States? J Stud Alcohol. 2001;62:211-20.
24. Mitchell C, Simpson D, Chick J. Carbohydrate-deficient transferrin in detecting relapse in alcohol dependence. Drug Alcohol Depend 1997;48:97-103.
25. Borg S, Helander A, Voltaire Carlsson A, Hogstrom Brandt AM. Detection of relapses in alcohol-dependent patients using carbohydrate-deficient transferrin: improvement with individualized reference levels during long-term monitoring. Alcohol Clin Exp Res 1995;19(4):961-3.
26. Schmidt LG, Schmidt K, Dufeu P, et al. Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. Am J Psychiatry 1997;154(1):75-80.
27. Allen JP, Anton R. Biomarkers as aids to identification of relapse in alcoholic patients. In: Galanter M (ed). Recent developments in alcoholism: research on alcoholism treatment (vol. XVI). New York: Plenum Press (in press).
28. Anton RF, Lieber C, Tabakoff B, et al. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multi-site study. Alcohol Clin Exp Res 2002;26(8):1215-22.
Serotonin syndrome: How to avoid, identify, & treat dangerous drug interactions
Promptly identifying serotonin syndrome and acting decisively can keep side effects at the mild end of the spectrum. Symptoms of this potentially dangerous syndrome range from minimal in patients starting selective serotonin reuptake inhibitors (SSRIs) to fatal in those combining monoamine oxidase inhibitors (MAOIs) with serotonergic agents.
This article presents the latest evidence on how to:
- reduce the risk of serotonin syndrome
- recognize its symptoms
- and treat patients with mild to life-threatening symptoms.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome is characterized by changes in autonomic, neuromotor, and cognitive-behavioral function (Table 1) triggered by increased serotonergic stimulation. It typically results from pharmacodynamic and/or pharmacokinetic interactions between drugs that increase serotonin activity.1,2
Table 1
How to recognize serotonin syndrome
| System | Clinical signs and symptoms |
|---|---|
| Autonomic | Diaphoresis, hyperthermia, hypertension, tachycardia, pupillary dilatation, nausea, diarrhea, shivering |
| Neuromotor | Hyperreflexia, myoclonus, restlessness, tremor, incoordination, rigidity, clonus, teeth chattering, trismus, seizures |
| Cognitive-behavioral | Confusion, agitation, anxiety, hypomania, insomnia, hallucinations, headache |
The syndrome was first identified in animal studies, followed by case reports in humans. The first review—with suggested diagnostic criteria— was published in 1991.1
Since then, case reports have described serotonin syndrome with many drug combinations, including nonpsychotropics and illicit drugs. Using an irreversible MAOI with a serotonergic agent is the most toxic reported combination, but any drug or combination that increases serotonin can, in theory, cause serotonin syndrome (Table 2). A clinical scale3 is being developed to define and identify this potentially dangerous state, but no consensus has emerged on diagnostic criteria.
Pathophysiology. Serotonin syndrome’s symptoms and signs appear to result from stimulation of specific central and peripheral serotonin receptors, especially 5HT1a and 5HT2. Others—such as 5HT3 and 5HT4—may also be involved in causing GI symptoms and may affect dopaminergic transmission.
Damaged vascular or pulmonary endothelium, atherosclerosis, hypertension, or hypercholesterolemia may increase the risk for serotonin syndrome. In patients with these common medical conditions, reduced endothelial MAO-A activity or reduced ability to secrete endothelium-derived nitric oxide may diminish the ability to metabolize serotonin.2
POTENTIALLY DANGEROUS COMBINATIONS
MAOIs. Serotonin syndrome has been reported as a result of interactions between MAOIs— including selegiline and reversible MAO-A inhibitors (RIMAs)—and various serotonergic compounds. These reports have included fatalities,4 some of which were preceded by severe hyperthermia with complications such as disseminated intravascular coagulation, rhabdomyolysis, and renal failure. Some cases resulted from overdoses, but others did not.
Most disturbingly, some cases occurred after patients had undergone the traditional 2-week washout from the MAOI and then took a serotonergic agent.5-7 In one instance,8 a patient who had discontinued fluoxetine for 6 weeks developed serotonin syndrome after starting tranylcypromine. These cases remind us to be vigilant when switching patients from irreversible MAOIs to serotonergic antidepressants or vice versa—even when recommended wash-out times are observed—and not to combine these agents acutely.
Selegiline is a relatively selective MAO-B inhibitor when used at 5 to 10 mg/d to treat Parkinson’s disease, though it loses MAO-B selectivity when used at higher dosages to treat depression. In a study9 of 4,568 patients with Parkinson’s disease who received selegiline (in dosages selective for MAO-B) plus an antidepressant:
- 11 (0.24%) experienced symptoms “possibly” consistent with serotonin syndrome
- 2 others (0.04%) experienced serious serotonin syndrome symptoms.9
Serotonin syndrome has been reported when MAO-B-selective doses of selegiline were combined with meperidine10 and nortriptyline.11 This underscores the need for caution when combining these agents, especially if transdermal selegiline— which would not be MAO-B-selective—becomes available for treating depression.
Moclobemide is a RIMA used in treating depression and anxiety, with a purported reduced risk of drug and food interactions compared with other MAOIs. Moclobemide is not approved in the United States, but some patients obtain it elsewhere.
Joffe and Bakish reported on safely combining moclobemide with SSRIs,12 and a review of MAOIs—including RIMAs—indicated that moclobemide was involved in only 9 of 226 cases of adverse effects and 3 of 105 cases of defined serotonin syndrome.13 Most moclobemide-SSRI interactions—including fatalities—involved overdoses in suicide attempts, although toxic symptoms have been reported with clomipramine or meperidine taken at normal dosages.14,15
In one study,16 18 healthy controls received fluoxetine, 20 to 40 mg/d, for 23 days, then were given moclobemide, up to 600 mg/d, or placebo and observed for adverse effects. No indication of serotonin syndrome was observed.
Linezolid is an oxazolidinone antibiotic with relatively weak, nonspecific, but reversible MAO inhibition. Cases of potential serotonin syndrome have been reported with linezolid plus paroxetine17 or sertraline.18 Patients in each case were medically ill and taking several other medications, which complicates interpretation of these reports. Nonetheless, physicians should be aware of the potential risk of serotonin syndrome if this antibiotic is combined with serotonergic agents.
Table 2
Serotonergic agents and their actions
| Actions | Agents |
|---|---|
| Inhibit serotonin reuptake | Fluoxetine, sertraline, citalopram, escitalopram, paroxetine, clomipramine, venlafaxine, fluvoxamine, tramadol, trazodone, nefazodone, tricyclic antidepressants, amphetamine, cocaine, dextromethorphan, meperidine, St. John’s wort |
| Increases serotonin synthesis | Tryptophan |
| Inhibit serotonin metabolism | Phenelzine, tranylcypromine, isocarboxazid, selegiline (deprenyl), linezolid, moclobemide |
| Increase serotonin release | MDMA (“Ecstasy”), amphetamine, cocaine, fenfluramine |
| Increase serotonin activity | Lithium, ECT |
| Serotonin receptor agonists | Buspirone, sumatriptan and other “triptans” used for migraine |
Atypical antipsychotics. Original diagnostic criteria for serotonin syndrome excluded the addition of, or increase in, an antipsychotic prior to the syndrome’s onset.1 However, serotonin syndrome has been reported with combinations of risperidone with paroxetine,19 olanzapine with mirtazapine and tramadol,20 and olanzapine with lithium and citalopram.21 The 5HT2 antagonist effect of these atypical antipsychotics may have led indirectly to overactivation of 5HT1a receptors and serotonin syndrome. In each case, neuroleptic malignant syndrome was ruled out.
Table 3
Signs and symptoms that differentiate 5 hyperthermic states
| Hyperthermic state | Symptoms/signs | Lab findings | Cause |
|---|---|---|---|
| Serotonin syndrome | Typically rapid onset with hyperreflexia, tremors, myoclonus, diaphoresis, confusion, agitation, or shivering; muscular rigidity not invariably present | Nonspecific | Increased serotonergic tone |
| Neuroleptic malignant syndrome | Variable rapidity of onset; severe muscular rigidity, diaphoresis, delirium, fluctuating blood pressure, tachycardia, extrapyramidal symptoms | Elevated CPK, leukocytosis | Blockade of dopamine receptors or abrupt withdrawal of a dopamine agonist |
| Lethal catatonia | Muscular rigidity, diaphoresis, delirium, alternating extreme excitement and stupor, tremors, hypertension | Nonspecific | Evidence of pre-existing psychosis (bipolar disorder, schizophrenia) |
| Anticholinergic toxicity | Hot, dry skin, pupillary dilatation, tachycardia, constipation, urinary retention, confusion, hallucinations, muscular relaxation | Nonspecific | Agents that block central and peripheral muscarinic cholinergic receptors |
| Malignant hyperthermia | Rapid onset, severe muscular rigidity, ischemia, hypotension | Elevated CPK, potassium, magnesium; DIC; acidosis; rhabdomyolysis | Inherited disorder with onset after exposure to anesthetic agents that block the neuromuscular junction |
| CPK: creatine phosphokinase | |||
| DIC: disseminated intravascular coagulation | |||
Tramadol is an analgesic with opioid and serotonin-reuptake inhibiting properties that is metabolized by the cytochrome P (CYP)-450 isoenzyme 2D6. Serotonin syndrome has been reported from interactions between tramadol and sertraline22 and fluoxetine.23 Possible causes include SSRI inhibition of CYP 2D6 metabolism of tramadol, tramadol abuse,23 and multiple coadministered medications.22
Sumatriptan is one of the selective 5HT1D agonists used in treating migraine. Gardner and Lynd24 concluded that most patients tolerate sumatriptan with SSRIs or lithium. They felt they could not ensure the safety of sumatriptan with MAOIs, however, because sumatriptan elimination depends on hepatic MAO activity.
Among the 5HT1D agonists, using sumatriptan, zolmitriptan, rizatriptan, or almotriptan with an MAOI or within 2 weeks of discontinuing an MAOI is contraindicated. Naratriptan and frovatriptan appear less likely to interact with MAOIs, based on FDA-approved labeling.
MDMA. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) is widely used as a recreational drug, especially at crowded dances (“raves”) and with other drugs.25 This illicit amphetamine derivative stimulates the release of serotonin and inhibits its reuptake.
Kaskey reported the rapid onset of serotonin syndrome when a patient taking lithium and phenelzine ingested MDMA.26 Signs and symptoms of serotonin syndrome also may develop when MDMA is used alone, facilitated by the high ambient temperatures on crowded dance floors and the dancers’ relative dehydration.
Fatalities have been blamed on complications including disseminated intravascular coagulation, rhabdomyolysis, and acute hepatic, renal, or cardiac failure.25 Cases are difficult to interpret because of uncertainty about whether the victim ingested MDMA or another agent or combination.
St. John’s wort (Hypericum perforatum) contains numerous constituents, including hypericin and hyperforin, which have been found to inhibit the synaptic uptake of monoamines, including serotonin.27 Which constituents are responsible for its clinical effect is unclear. Adverse effects from monotherapy include GI symptoms, confusion, dry mouth, dizziness, headache, fatigue, allergic skin reactions, photosensitivity, and urinary frequency.27
Several cases of purported serotonin syndrome have been associated with St. John’s wort alone28 or in combination with SSRIs, nefazodone, or fenfluramine.29,30 GI symptoms and anxiety were the primary complaints and resolved without complications (adjunctive cyproheptadine was prescribed in two cases, though it is not clear that this agent contributed to resolution).
MISCELLANEOUS COMBINATIONS
Antiretroviral therapy. Five cases of serotonin syndrome were reported in HIV-infected patients taking fluoxetine with antiretroviral therapy.31 In particular, the use or addition of ritonavir—a potent CYP 2D6 inhibitor—was implicated, though saquinavir, efavirenz, or grapefruit juice (all primarily CYP 3A4 inhibitors) were also used, suggesting that pharmacokinetic interactions increased serotonergic stimulation. All five patients were taking multiple additional medications and had complex medical and/or psychiatric histories. Reducing SSRI dosages by one-half when used with ritonavir has been recommended to minimize adverse effects from a pharmacokinetic interaction.
Erythromycin was reported to induce serotonin syndrome in a 12-year-old boy when added to ongoing treatment with sertraline, an effect believed to be secondary to CYP 3A4 inhibition of sertraline metabolism.32
Mirtazapine was reported to induce serotonin syndrome in an elderly man 8 days after it was added to a regimen he had been taking for several years to treat chronic obstructive pulmonary disease.33 Serotonin syndrome also developed in a 12-year-old boy with Ewing’s sarcoma when the 5HT3 antagonist ondansetron was added to mirtazapine and morphine34 and in an 11-year-old girl with acute lymphoblastic leukemia when ondansetron was added to fentanyl. Interestingly, another report35 suggested using mirtazapine to treat serotonin syndrome caused by serotonergic antagonist effects.
Reports have associated the following combinations with serotonin syndrome, perhaps as the result of pharmacodynamic and/or pharmacokinetic interactions:
- paroxetine plus dextromethorphan and pseudoephedrine
- paroxetine plus nefazodone
- fluoxetine plus clomipramine and buspirone
- fluvoxamine plus buspirone
- fluoxetine plus buspirone
- amitriptyline plus meperidine and venlafaxine
- venlafaxine and dextroamphetamine
- fluoxetine plus clomipramine.
Table 4
Clinical signs that distinguish hyperthermic states
| Signs | Possible diagnosis |
|---|---|
| Prominent muscular rigidity | Neuroleptic malignant syndrome, malignant hyperthermia, catatonia |
| Myoclonus/hyperreflexia | Serotonin syndrome |
| Diaphoresis | Serotonin syndrome, neuroleptic malignant syndrome, catatonia |
| Hot dry skin | Anticholinergic toxicity |
| Elevated creatine phosphokinase | Neuroleptic malignant syndrome, malignant hyperthermia |
| Family history of anesthetic-induced hyperthermia | Malignant hyperthermia |
HOW TO RECOGNIZE SEROTONIN SYNDROME
Signs and symptoms of serotonin syndrome can overlap with those seen in neuroleptic malignant syndrome, lethal catatonia, malignant hyperthermia, and anticholinergic toxicity (Table 3),1,36,37 particularly with fever or hyperthermia (>40.5 °C, 105 °F). Fink37 has opined that acute neurotoxic syndromes such as serotonin syndrome and neuroleptic malignant syndrome also meet criteria for catatonia and are therefore subtypes of catatonia. The types of drugs involved and clinical findings can help distinguish the various hyperthermic states (Table 4).
As mentioned above, original diagnostic criteria for serotonin syndrome excluded the addition of, or increase in, an antipsychotic agent. This exclusion was intended to avoid confusion between serotonin syndrome and neuroleptic malignant syndrome. Co-administering antipsychotic and serotonergic agents requires heightened awareness for both neurotoxic syndromes.
TREATING MILD TO SEVERE CASES
If a patient develops serotonin syndrome, immediately discontinue the suspected agent(s) and observe carefully. In most cases, serotonin syndrome will resolve within 24 hours.
In mild cases, lorazepam, 1 to 2 mg slow IV push every 30 minutes until excessive sedation develops, may help. In moderate to severe cases, agents that block serotonin’s action are recommended,2 including:
- cyproheptadine (4 mg po every 4 hours as needed, up to 20 mg in 24 hours)
- propranolol (1 to 3 mg IV every 5 minutes, up to 0.1 mg/kg).
Case reports attest to these agents’ potential benefit. Other clinicians have reported using mirtazapine,35 nitroglycerin,38 and chlorpromazine.1
Serotonin syndrome symptoms resolved within minutes when IV nitroglycerin was used in a patient with serotonin syndrome and cardiac ischemia. The authors hypothesized that nitroglycerin, via nitric acid, provided an “off” signal for serotonin, though they did not advocate this as a routine treatment.38
The rationale for using chlorpromazine is its potential to block serotonin receptors. I would avoid the routine use of any antipsychotic agent in this setting, however, to minimize the risk of neuroleptic malignant syndrome.
Severe cases. Intensive care observation and treatment is required for patients with severe serotonin syndrome, including evidence of hyperthermia, DIC, rhabdomyolysis, renal failure, or aspiration. In cases of hyperthermia, supportive measures and standard treatments include muscle relaxants, cooling, and endotracheal intubation.
Severe complications are most likely with interactions between MAOIs and serotonergic agents, especially in overdose. Therefore, using such combinations requires close observation.
Related resources
- Di Rosa AE, Morgante L, Spina E et al. Epidemiology and pathoetiology of neurological syndromes with hyperthermia. Funct Neurol 1995;10:111-19.
- Radomski, JW, Dursun SM, Reveley MA, et al. An exploratory approach to the serotonin syndrome: an update of clinical phenomenology and revised diagnostic criteria. Med Hypothesis 2000;55: 218-24.
- Lane R, Baldwin D. Selective serotonin reuptake inhibitor-induced serotonin syndrome: review. J Clin Psychopharmacol 1997;17:208-21.
Drug brand names
- Almotriptan • Axert
- Amitriptyline • Elavil
- Buspirone • Buspar
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clomipramine • Anafranil
- Cyproheptadine • Periactin
- Dextroamphetamine • Dexedrine
- Dextromethorphan • Delsym
- Efavirenz • Sustiva
- Escitalopram • Lexapro
- Fenfluramine • Pondimin
- Fentanyl • Sublimaze
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Frovatriptan • Frova
- Isocarboxazid • Marplan
- Linezolid • Zyvox
- Meperidine • Demerol
- Mirtazapine • Remeron
- Moclobemide • Aurorix
- Nortriptyline • Pamelor
- Naratriptan, • Amerge
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Ondansetron • Zofran
- Paroxetine • Paxil
- Phenelzine • Nardil
- Propranolol • Inderal
- Risperidone • Risperdal
- Ritonavir • Norvir
- Rizatriptan • Maxalt
- Saquinavir • Invirase
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Sumatriptan • Imitrex
- Tramadol • Ultram
- Tranylcypromine • Parnate
- Trazodone • Desyrel
- Venlafaxine • Effexor
- Zolmitriptan • Zomig
Disclosure
Dr. Sternbach receives research grants from Otsuka America Pharmaceuticals and Eli Lilly and Co. and owns stock in Merck & Co., Pfizer Inc., and Johnson & Johnson.
1. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148:705-13.
2. Brown TM, Skop BP, Mareth TR. Pathophysiology and management of the serotonin syndrome. Ann Pharmacother 1996;30:527-33.
3. Hegerl U, Bottlender R, Gallinat J, et al. The serotonin syndrome scale: first results on validity. Eur Arch Psychiatry Clin Neurosci 1998;248:96-103.
4. Beasley CM, Jr, Masica DN, Heiligenstein JH, et al. Possible monoamine-oxidase inhibitor-serotonin uptake inhibitor interaction: fluoxetine clinical data and pre-clinical findings. J Clin Psychopharmacol 1993;13:312-20.
5. Ruiz F. Fluoxetine and the serotonin syndrome. Ann Emerg Med 1994;34:983-5.
6. Gitlin MJ. Venlafaxine, monoamine oxidase inhibitors and the serotonin syndrome. J Clin Psychopharmacol 1997;17:66-7.
7. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. Clin Toxicol 1997;35:211-12.
8. Coplan JD, Gorman JM. Detectable levels of fluoxetine metabolites after discontinuation: an unexpected serotonin syndrome. Am J Psychiatry 1993;15:837.-
9. Richard IH, Kurlan R, Tanner C, et al. Serotonin syndrome and the combined use of deprenyl and an antidepressant in Parkinson’s disease. Neurology 1997;48:1070-7.
10. Zornberg GL, Bodkin JA, Cohen BM. Severe adverse interaction between pethidine and selegiline. Lancet 1991;337:246.-
11. Hinds NP, Hillier CE, Wiles CM. Possible serotonin syndrome arising from an interaction between nortriptyline and selegiline in a lady with parkinsonism. J Neurol 2000;247:811.-
12. Joffe RT, Bakish D. Combined SSRI-moclobemide treatment of psychiatric illness. J Clin Psychiatry 1994;55:24-5.
13. Hilton SE, Maradit H, Moller HJ. Serotonin syndrome and drug combinations: focus on MAOI and RIMA. Eur Arch Psychiatry Clin Neurosci 1997;247:113-19.
14. Dardennes RM, Even C, Ballon N, et al. Serotonin syndrome caused by a clomipramine-moclobemide interaction. J Clin Psychiatry 1998;59:382-3.
15. Gillman PK. Possible serotonin syndrome with moclobemide and pethidine. Med J Aust 1995;162:554.-
16. Dingemanse J, Wallnofer A, Gieschke R, et al. Pharmacokinetic and pharmacodynamic interaction between fluoxetine and moclobemide in the investigation of development of the “serotonin syndrome.” Clin Pharmacol Ther 1998;63:403-13.
17. Wigen CL, Goetz MB. Serotonin syndrome and linezolid. Clin Infect Dis 2002;34:1651-2.
18. Lavery S, Ravi H, McDaniel WW, et al. Linezolid and serotonin syndrome. Psychosomatics 2001;42:432-4.
19. Hamilton S, Malone K. Serotonin syndrome during treatment with paroxetine and risperidone. J Clin Psychopharmacol 2000;20:103-5.
20. Duggal HS, Fetchko J. Serotonin syndrome and atypical antipsychotics. Am J Psychiatry 2002;159:672-3.
21. Haslett CD, Kumar S. Can olanzapine be implicated in causing serotonin syndrome? Psychiatry Clin Neurosci 2002;56:533-6.
22. Mason BJ, Blackburn KH. Possible serotonin syndrome associated with tramadol and sertraline coadministration. Ann Pharmacother 1997;31:175-7.
23. Lange-Asschenfeldt C, Weigmann H, Hiemke C, et al. Serotonin syndrome as a result of fluoxetine in a patient with tramadol abuse: plasma level-correlated symptomatology. J Clin Psychopharmacol 2002;22:440-1.
24. Gardner DM, Lynd LD. Sumatriptan contraindications and the serotonin syndrome. Ann Pharmacother 1998;32:33-8.
25. Parrott AC. Recreational Ecstasy/MDMA, the serotonin syndrome and serotonergic neurotoxicity. Pharmacol Biochem Behav 2002;71:837-44.
26. Kaskey GB. Possible interaction between an MAOI and “Ecstasy.” Am J Psychiatry 1992;149:411-12.
27. De Smet PA. Herbal remedies. N Engl J Med 2002;347:2046-56.
28. Parker V, Wong AH, Boon HS, et al. Adverse reactions to St. John’s wort. Can J Psychiatry 2001;46:77-9.
29. Lantz MS, Buchalter E, Giambanco V. St. John’s wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol 1999;12:7-10.
30. Beckman SE, Sommi RW, Switzer J. Consumer use of St. John’s wort: a survey of effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
31. De Silva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS 2001;15:1281-5.
32. Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999;19:894-6.
33. Hernandez JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother 2002;36:641-3.
34. Turkel SB, Nadala JGB, Wincor MZ. Possible serotonin syndrome in association with 5HT3 antagonist agents. Psychosomatics 2001;42:258-60.
35. Hoes MJ, Zeijpveld JH. Mirtazapine as treatment for serotonin syndrome. Pharmacopsychiatry 1996;29:81.-
36. Theoharides TC, Harris RS, Weckstein D. Neuroleptic malignant-like syndrome due to cyclobenzaprine? J Clin Psychopharmacol 1995;15:79-81.
37. Fink M. Toxic serotonin syndrome or neuroleptic malignant syndrome? Pharmacopsychiatry 1996;29:159-61.
38. Brown TM, Skop BP. Nitroglycerin in the treatment of the serotonin syndrome. Ann Pharmacother 1996;30:191-2.
Promptly identifying serotonin syndrome and acting decisively can keep side effects at the mild end of the spectrum. Symptoms of this potentially dangerous syndrome range from minimal in patients starting selective serotonin reuptake inhibitors (SSRIs) to fatal in those combining monoamine oxidase inhibitors (MAOIs) with serotonergic agents.
This article presents the latest evidence on how to:
- reduce the risk of serotonin syndrome
- recognize its symptoms
- and treat patients with mild to life-threatening symptoms.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome is characterized by changes in autonomic, neuromotor, and cognitive-behavioral function (Table 1) triggered by increased serotonergic stimulation. It typically results from pharmacodynamic and/or pharmacokinetic interactions between drugs that increase serotonin activity.1,2
Table 1
How to recognize serotonin syndrome
| System | Clinical signs and symptoms |
|---|---|
| Autonomic | Diaphoresis, hyperthermia, hypertension, tachycardia, pupillary dilatation, nausea, diarrhea, shivering |
| Neuromotor | Hyperreflexia, myoclonus, restlessness, tremor, incoordination, rigidity, clonus, teeth chattering, trismus, seizures |
| Cognitive-behavioral | Confusion, agitation, anxiety, hypomania, insomnia, hallucinations, headache |
The syndrome was first identified in animal studies, followed by case reports in humans. The first review—with suggested diagnostic criteria— was published in 1991.1
Since then, case reports have described serotonin syndrome with many drug combinations, including nonpsychotropics and illicit drugs. Using an irreversible MAOI with a serotonergic agent is the most toxic reported combination, but any drug or combination that increases serotonin can, in theory, cause serotonin syndrome (Table 2). A clinical scale3 is being developed to define and identify this potentially dangerous state, but no consensus has emerged on diagnostic criteria.
Pathophysiology. Serotonin syndrome’s symptoms and signs appear to result from stimulation of specific central and peripheral serotonin receptors, especially 5HT1a and 5HT2. Others—such as 5HT3 and 5HT4—may also be involved in causing GI symptoms and may affect dopaminergic transmission.
Damaged vascular or pulmonary endothelium, atherosclerosis, hypertension, or hypercholesterolemia may increase the risk for serotonin syndrome. In patients with these common medical conditions, reduced endothelial MAO-A activity or reduced ability to secrete endothelium-derived nitric oxide may diminish the ability to metabolize serotonin.2
POTENTIALLY DANGEROUS COMBINATIONS
MAOIs. Serotonin syndrome has been reported as a result of interactions between MAOIs— including selegiline and reversible MAO-A inhibitors (RIMAs)—and various serotonergic compounds. These reports have included fatalities,4 some of which were preceded by severe hyperthermia with complications such as disseminated intravascular coagulation, rhabdomyolysis, and renal failure. Some cases resulted from overdoses, but others did not.
Most disturbingly, some cases occurred after patients had undergone the traditional 2-week washout from the MAOI and then took a serotonergic agent.5-7 In one instance,8 a patient who had discontinued fluoxetine for 6 weeks developed serotonin syndrome after starting tranylcypromine. These cases remind us to be vigilant when switching patients from irreversible MAOIs to serotonergic antidepressants or vice versa—even when recommended wash-out times are observed—and not to combine these agents acutely.
Selegiline is a relatively selective MAO-B inhibitor when used at 5 to 10 mg/d to treat Parkinson’s disease, though it loses MAO-B selectivity when used at higher dosages to treat depression. In a study9 of 4,568 patients with Parkinson’s disease who received selegiline (in dosages selective for MAO-B) plus an antidepressant:
- 11 (0.24%) experienced symptoms “possibly” consistent with serotonin syndrome
- 2 others (0.04%) experienced serious serotonin syndrome symptoms.9
Serotonin syndrome has been reported when MAO-B-selective doses of selegiline were combined with meperidine10 and nortriptyline.11 This underscores the need for caution when combining these agents, especially if transdermal selegiline— which would not be MAO-B-selective—becomes available for treating depression.
Moclobemide is a RIMA used in treating depression and anxiety, with a purported reduced risk of drug and food interactions compared with other MAOIs. Moclobemide is not approved in the United States, but some patients obtain it elsewhere.
Joffe and Bakish reported on safely combining moclobemide with SSRIs,12 and a review of MAOIs—including RIMAs—indicated that moclobemide was involved in only 9 of 226 cases of adverse effects and 3 of 105 cases of defined serotonin syndrome.13 Most moclobemide-SSRI interactions—including fatalities—involved overdoses in suicide attempts, although toxic symptoms have been reported with clomipramine or meperidine taken at normal dosages.14,15
In one study,16 18 healthy controls received fluoxetine, 20 to 40 mg/d, for 23 days, then were given moclobemide, up to 600 mg/d, or placebo and observed for adverse effects. No indication of serotonin syndrome was observed.
Linezolid is an oxazolidinone antibiotic with relatively weak, nonspecific, but reversible MAO inhibition. Cases of potential serotonin syndrome have been reported with linezolid plus paroxetine17 or sertraline.18 Patients in each case were medically ill and taking several other medications, which complicates interpretation of these reports. Nonetheless, physicians should be aware of the potential risk of serotonin syndrome if this antibiotic is combined with serotonergic agents.
Table 2
Serotonergic agents and their actions
| Actions | Agents |
|---|---|
| Inhibit serotonin reuptake | Fluoxetine, sertraline, citalopram, escitalopram, paroxetine, clomipramine, venlafaxine, fluvoxamine, tramadol, trazodone, nefazodone, tricyclic antidepressants, amphetamine, cocaine, dextromethorphan, meperidine, St. John’s wort |
| Increases serotonin synthesis | Tryptophan |
| Inhibit serotonin metabolism | Phenelzine, tranylcypromine, isocarboxazid, selegiline (deprenyl), linezolid, moclobemide |
| Increase serotonin release | MDMA (“Ecstasy”), amphetamine, cocaine, fenfluramine |
| Increase serotonin activity | Lithium, ECT |
| Serotonin receptor agonists | Buspirone, sumatriptan and other “triptans” used for migraine |
Atypical antipsychotics. Original diagnostic criteria for serotonin syndrome excluded the addition of, or increase in, an antipsychotic prior to the syndrome’s onset.1 However, serotonin syndrome has been reported with combinations of risperidone with paroxetine,19 olanzapine with mirtazapine and tramadol,20 and olanzapine with lithium and citalopram.21 The 5HT2 antagonist effect of these atypical antipsychotics may have led indirectly to overactivation of 5HT1a receptors and serotonin syndrome. In each case, neuroleptic malignant syndrome was ruled out.
Table 3
Signs and symptoms that differentiate 5 hyperthermic states
| Hyperthermic state | Symptoms/signs | Lab findings | Cause |
|---|---|---|---|
| Serotonin syndrome | Typically rapid onset with hyperreflexia, tremors, myoclonus, diaphoresis, confusion, agitation, or shivering; muscular rigidity not invariably present | Nonspecific | Increased serotonergic tone |
| Neuroleptic malignant syndrome | Variable rapidity of onset; severe muscular rigidity, diaphoresis, delirium, fluctuating blood pressure, tachycardia, extrapyramidal symptoms | Elevated CPK, leukocytosis | Blockade of dopamine receptors or abrupt withdrawal of a dopamine agonist |
| Lethal catatonia | Muscular rigidity, diaphoresis, delirium, alternating extreme excitement and stupor, tremors, hypertension | Nonspecific | Evidence of pre-existing psychosis (bipolar disorder, schizophrenia) |
| Anticholinergic toxicity | Hot, dry skin, pupillary dilatation, tachycardia, constipation, urinary retention, confusion, hallucinations, muscular relaxation | Nonspecific | Agents that block central and peripheral muscarinic cholinergic receptors |
| Malignant hyperthermia | Rapid onset, severe muscular rigidity, ischemia, hypotension | Elevated CPK, potassium, magnesium; DIC; acidosis; rhabdomyolysis | Inherited disorder with onset after exposure to anesthetic agents that block the neuromuscular junction |
| CPK: creatine phosphokinase | |||
| DIC: disseminated intravascular coagulation | |||
Tramadol is an analgesic with opioid and serotonin-reuptake inhibiting properties that is metabolized by the cytochrome P (CYP)-450 isoenzyme 2D6. Serotonin syndrome has been reported from interactions between tramadol and sertraline22 and fluoxetine.23 Possible causes include SSRI inhibition of CYP 2D6 metabolism of tramadol, tramadol abuse,23 and multiple coadministered medications.22
Sumatriptan is one of the selective 5HT1D agonists used in treating migraine. Gardner and Lynd24 concluded that most patients tolerate sumatriptan with SSRIs or lithium. They felt they could not ensure the safety of sumatriptan with MAOIs, however, because sumatriptan elimination depends on hepatic MAO activity.
Among the 5HT1D agonists, using sumatriptan, zolmitriptan, rizatriptan, or almotriptan with an MAOI or within 2 weeks of discontinuing an MAOI is contraindicated. Naratriptan and frovatriptan appear less likely to interact with MAOIs, based on FDA-approved labeling.
MDMA. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) is widely used as a recreational drug, especially at crowded dances (“raves”) and with other drugs.25 This illicit amphetamine derivative stimulates the release of serotonin and inhibits its reuptake.
Kaskey reported the rapid onset of serotonin syndrome when a patient taking lithium and phenelzine ingested MDMA.26 Signs and symptoms of serotonin syndrome also may develop when MDMA is used alone, facilitated by the high ambient temperatures on crowded dance floors and the dancers’ relative dehydration.
Fatalities have been blamed on complications including disseminated intravascular coagulation, rhabdomyolysis, and acute hepatic, renal, or cardiac failure.25 Cases are difficult to interpret because of uncertainty about whether the victim ingested MDMA or another agent or combination.
St. John’s wort (Hypericum perforatum) contains numerous constituents, including hypericin and hyperforin, which have been found to inhibit the synaptic uptake of monoamines, including serotonin.27 Which constituents are responsible for its clinical effect is unclear. Adverse effects from monotherapy include GI symptoms, confusion, dry mouth, dizziness, headache, fatigue, allergic skin reactions, photosensitivity, and urinary frequency.27
Several cases of purported serotonin syndrome have been associated with St. John’s wort alone28 or in combination with SSRIs, nefazodone, or fenfluramine.29,30 GI symptoms and anxiety were the primary complaints and resolved without complications (adjunctive cyproheptadine was prescribed in two cases, though it is not clear that this agent contributed to resolution).
MISCELLANEOUS COMBINATIONS
Antiretroviral therapy. Five cases of serotonin syndrome were reported in HIV-infected patients taking fluoxetine with antiretroviral therapy.31 In particular, the use or addition of ritonavir—a potent CYP 2D6 inhibitor—was implicated, though saquinavir, efavirenz, or grapefruit juice (all primarily CYP 3A4 inhibitors) were also used, suggesting that pharmacokinetic interactions increased serotonergic stimulation. All five patients were taking multiple additional medications and had complex medical and/or psychiatric histories. Reducing SSRI dosages by one-half when used with ritonavir has been recommended to minimize adverse effects from a pharmacokinetic interaction.
Erythromycin was reported to induce serotonin syndrome in a 12-year-old boy when added to ongoing treatment with sertraline, an effect believed to be secondary to CYP 3A4 inhibition of sertraline metabolism.32
Mirtazapine was reported to induce serotonin syndrome in an elderly man 8 days after it was added to a regimen he had been taking for several years to treat chronic obstructive pulmonary disease.33 Serotonin syndrome also developed in a 12-year-old boy with Ewing’s sarcoma when the 5HT3 antagonist ondansetron was added to mirtazapine and morphine34 and in an 11-year-old girl with acute lymphoblastic leukemia when ondansetron was added to fentanyl. Interestingly, another report35 suggested using mirtazapine to treat serotonin syndrome caused by serotonergic antagonist effects.
Reports have associated the following combinations with serotonin syndrome, perhaps as the result of pharmacodynamic and/or pharmacokinetic interactions:
- paroxetine plus dextromethorphan and pseudoephedrine
- paroxetine plus nefazodone
- fluoxetine plus clomipramine and buspirone
- fluvoxamine plus buspirone
- fluoxetine plus buspirone
- amitriptyline plus meperidine and venlafaxine
- venlafaxine and dextroamphetamine
- fluoxetine plus clomipramine.
Table 4
Clinical signs that distinguish hyperthermic states
| Signs | Possible diagnosis |
|---|---|
| Prominent muscular rigidity | Neuroleptic malignant syndrome, malignant hyperthermia, catatonia |
| Myoclonus/hyperreflexia | Serotonin syndrome |
| Diaphoresis | Serotonin syndrome, neuroleptic malignant syndrome, catatonia |
| Hot dry skin | Anticholinergic toxicity |
| Elevated creatine phosphokinase | Neuroleptic malignant syndrome, malignant hyperthermia |
| Family history of anesthetic-induced hyperthermia | Malignant hyperthermia |
HOW TO RECOGNIZE SEROTONIN SYNDROME
Signs and symptoms of serotonin syndrome can overlap with those seen in neuroleptic malignant syndrome, lethal catatonia, malignant hyperthermia, and anticholinergic toxicity (Table 3),1,36,37 particularly with fever or hyperthermia (>40.5 °C, 105 °F). Fink37 has opined that acute neurotoxic syndromes such as serotonin syndrome and neuroleptic malignant syndrome also meet criteria for catatonia and are therefore subtypes of catatonia. The types of drugs involved and clinical findings can help distinguish the various hyperthermic states (Table 4).
As mentioned above, original diagnostic criteria for serotonin syndrome excluded the addition of, or increase in, an antipsychotic agent. This exclusion was intended to avoid confusion between serotonin syndrome and neuroleptic malignant syndrome. Co-administering antipsychotic and serotonergic agents requires heightened awareness for both neurotoxic syndromes.
TREATING MILD TO SEVERE CASES
If a patient develops serotonin syndrome, immediately discontinue the suspected agent(s) and observe carefully. In most cases, serotonin syndrome will resolve within 24 hours.
In mild cases, lorazepam, 1 to 2 mg slow IV push every 30 minutes until excessive sedation develops, may help. In moderate to severe cases, agents that block serotonin’s action are recommended,2 including:
- cyproheptadine (4 mg po every 4 hours as needed, up to 20 mg in 24 hours)
- propranolol (1 to 3 mg IV every 5 minutes, up to 0.1 mg/kg).
Case reports attest to these agents’ potential benefit. Other clinicians have reported using mirtazapine,35 nitroglycerin,38 and chlorpromazine.1
Serotonin syndrome symptoms resolved within minutes when IV nitroglycerin was used in a patient with serotonin syndrome and cardiac ischemia. The authors hypothesized that nitroglycerin, via nitric acid, provided an “off” signal for serotonin, though they did not advocate this as a routine treatment.38
The rationale for using chlorpromazine is its potential to block serotonin receptors. I would avoid the routine use of any antipsychotic agent in this setting, however, to minimize the risk of neuroleptic malignant syndrome.
Severe cases. Intensive care observation and treatment is required for patients with severe serotonin syndrome, including evidence of hyperthermia, DIC, rhabdomyolysis, renal failure, or aspiration. In cases of hyperthermia, supportive measures and standard treatments include muscle relaxants, cooling, and endotracheal intubation.
Severe complications are most likely with interactions between MAOIs and serotonergic agents, especially in overdose. Therefore, using such combinations requires close observation.
Related resources
- Di Rosa AE, Morgante L, Spina E et al. Epidemiology and pathoetiology of neurological syndromes with hyperthermia. Funct Neurol 1995;10:111-19.
- Radomski, JW, Dursun SM, Reveley MA, et al. An exploratory approach to the serotonin syndrome: an update of clinical phenomenology and revised diagnostic criteria. Med Hypothesis 2000;55: 218-24.
- Lane R, Baldwin D. Selective serotonin reuptake inhibitor-induced serotonin syndrome: review. J Clin Psychopharmacol 1997;17:208-21.
Drug brand names
- Almotriptan • Axert
- Amitriptyline • Elavil
- Buspirone • Buspar
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clomipramine • Anafranil
- Cyproheptadine • Periactin
- Dextroamphetamine • Dexedrine
- Dextromethorphan • Delsym
- Efavirenz • Sustiva
- Escitalopram • Lexapro
- Fenfluramine • Pondimin
- Fentanyl • Sublimaze
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Frovatriptan • Frova
- Isocarboxazid • Marplan
- Linezolid • Zyvox
- Meperidine • Demerol
- Mirtazapine • Remeron
- Moclobemide • Aurorix
- Nortriptyline • Pamelor
- Naratriptan, • Amerge
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Ondansetron • Zofran
- Paroxetine • Paxil
- Phenelzine • Nardil
- Propranolol • Inderal
- Risperidone • Risperdal
- Ritonavir • Norvir
- Rizatriptan • Maxalt
- Saquinavir • Invirase
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Sumatriptan • Imitrex
- Tramadol • Ultram
- Tranylcypromine • Parnate
- Trazodone • Desyrel
- Venlafaxine • Effexor
- Zolmitriptan • Zomig
Disclosure
Dr. Sternbach receives research grants from Otsuka America Pharmaceuticals and Eli Lilly and Co. and owns stock in Merck & Co., Pfizer Inc., and Johnson & Johnson.
Promptly identifying serotonin syndrome and acting decisively can keep side effects at the mild end of the spectrum. Symptoms of this potentially dangerous syndrome range from minimal in patients starting selective serotonin reuptake inhibitors (SSRIs) to fatal in those combining monoamine oxidase inhibitors (MAOIs) with serotonergic agents.
This article presents the latest evidence on how to:
- reduce the risk of serotonin syndrome
- recognize its symptoms
- and treat patients with mild to life-threatening symptoms.
WHAT IS SEROTONIN SYNDROME?
Serotonin syndrome is characterized by changes in autonomic, neuromotor, and cognitive-behavioral function (Table 1) triggered by increased serotonergic stimulation. It typically results from pharmacodynamic and/or pharmacokinetic interactions between drugs that increase serotonin activity.1,2
Table 1
How to recognize serotonin syndrome
| System | Clinical signs and symptoms |
|---|---|
| Autonomic | Diaphoresis, hyperthermia, hypertension, tachycardia, pupillary dilatation, nausea, diarrhea, shivering |
| Neuromotor | Hyperreflexia, myoclonus, restlessness, tremor, incoordination, rigidity, clonus, teeth chattering, trismus, seizures |
| Cognitive-behavioral | Confusion, agitation, anxiety, hypomania, insomnia, hallucinations, headache |
The syndrome was first identified in animal studies, followed by case reports in humans. The first review—with suggested diagnostic criteria— was published in 1991.1
Since then, case reports have described serotonin syndrome with many drug combinations, including nonpsychotropics and illicit drugs. Using an irreversible MAOI with a serotonergic agent is the most toxic reported combination, but any drug or combination that increases serotonin can, in theory, cause serotonin syndrome (Table 2). A clinical scale3 is being developed to define and identify this potentially dangerous state, but no consensus has emerged on diagnostic criteria.
Pathophysiology. Serotonin syndrome’s symptoms and signs appear to result from stimulation of specific central and peripheral serotonin receptors, especially 5HT1a and 5HT2. Others—such as 5HT3 and 5HT4—may also be involved in causing GI symptoms and may affect dopaminergic transmission.
Damaged vascular or pulmonary endothelium, atherosclerosis, hypertension, or hypercholesterolemia may increase the risk for serotonin syndrome. In patients with these common medical conditions, reduced endothelial MAO-A activity or reduced ability to secrete endothelium-derived nitric oxide may diminish the ability to metabolize serotonin.2
POTENTIALLY DANGEROUS COMBINATIONS
MAOIs. Serotonin syndrome has been reported as a result of interactions between MAOIs— including selegiline and reversible MAO-A inhibitors (RIMAs)—and various serotonergic compounds. These reports have included fatalities,4 some of which were preceded by severe hyperthermia with complications such as disseminated intravascular coagulation, rhabdomyolysis, and renal failure. Some cases resulted from overdoses, but others did not.
Most disturbingly, some cases occurred after patients had undergone the traditional 2-week washout from the MAOI and then took a serotonergic agent.5-7 In one instance,8 a patient who had discontinued fluoxetine for 6 weeks developed serotonin syndrome after starting tranylcypromine. These cases remind us to be vigilant when switching patients from irreversible MAOIs to serotonergic antidepressants or vice versa—even when recommended wash-out times are observed—and not to combine these agents acutely.
Selegiline is a relatively selective MAO-B inhibitor when used at 5 to 10 mg/d to treat Parkinson’s disease, though it loses MAO-B selectivity when used at higher dosages to treat depression. In a study9 of 4,568 patients with Parkinson’s disease who received selegiline (in dosages selective for MAO-B) plus an antidepressant:
- 11 (0.24%) experienced symptoms “possibly” consistent with serotonin syndrome
- 2 others (0.04%) experienced serious serotonin syndrome symptoms.9
Serotonin syndrome has been reported when MAO-B-selective doses of selegiline were combined with meperidine10 and nortriptyline.11 This underscores the need for caution when combining these agents, especially if transdermal selegiline— which would not be MAO-B-selective—becomes available for treating depression.
Moclobemide is a RIMA used in treating depression and anxiety, with a purported reduced risk of drug and food interactions compared with other MAOIs. Moclobemide is not approved in the United States, but some patients obtain it elsewhere.
Joffe and Bakish reported on safely combining moclobemide with SSRIs,12 and a review of MAOIs—including RIMAs—indicated that moclobemide was involved in only 9 of 226 cases of adverse effects and 3 of 105 cases of defined serotonin syndrome.13 Most moclobemide-SSRI interactions—including fatalities—involved overdoses in suicide attempts, although toxic symptoms have been reported with clomipramine or meperidine taken at normal dosages.14,15
In one study,16 18 healthy controls received fluoxetine, 20 to 40 mg/d, for 23 days, then were given moclobemide, up to 600 mg/d, or placebo and observed for adverse effects. No indication of serotonin syndrome was observed.
Linezolid is an oxazolidinone antibiotic with relatively weak, nonspecific, but reversible MAO inhibition. Cases of potential serotonin syndrome have been reported with linezolid plus paroxetine17 or sertraline.18 Patients in each case were medically ill and taking several other medications, which complicates interpretation of these reports. Nonetheless, physicians should be aware of the potential risk of serotonin syndrome if this antibiotic is combined with serotonergic agents.
Table 2
Serotonergic agents and their actions
| Actions | Agents |
|---|---|
| Inhibit serotonin reuptake | Fluoxetine, sertraline, citalopram, escitalopram, paroxetine, clomipramine, venlafaxine, fluvoxamine, tramadol, trazodone, nefazodone, tricyclic antidepressants, amphetamine, cocaine, dextromethorphan, meperidine, St. John’s wort |
| Increases serotonin synthesis | Tryptophan |
| Inhibit serotonin metabolism | Phenelzine, tranylcypromine, isocarboxazid, selegiline (deprenyl), linezolid, moclobemide |
| Increase serotonin release | MDMA (“Ecstasy”), amphetamine, cocaine, fenfluramine |
| Increase serotonin activity | Lithium, ECT |
| Serotonin receptor agonists | Buspirone, sumatriptan and other “triptans” used for migraine |
Atypical antipsychotics. Original diagnostic criteria for serotonin syndrome excluded the addition of, or increase in, an antipsychotic prior to the syndrome’s onset.1 However, serotonin syndrome has been reported with combinations of risperidone with paroxetine,19 olanzapine with mirtazapine and tramadol,20 and olanzapine with lithium and citalopram.21 The 5HT2 antagonist effect of these atypical antipsychotics may have led indirectly to overactivation of 5HT1a receptors and serotonin syndrome. In each case, neuroleptic malignant syndrome was ruled out.
Table 3
Signs and symptoms that differentiate 5 hyperthermic states
| Hyperthermic state | Symptoms/signs | Lab findings | Cause |
|---|---|---|---|
| Serotonin syndrome | Typically rapid onset with hyperreflexia, tremors, myoclonus, diaphoresis, confusion, agitation, or shivering; muscular rigidity not invariably present | Nonspecific | Increased serotonergic tone |
| Neuroleptic malignant syndrome | Variable rapidity of onset; severe muscular rigidity, diaphoresis, delirium, fluctuating blood pressure, tachycardia, extrapyramidal symptoms | Elevated CPK, leukocytosis | Blockade of dopamine receptors or abrupt withdrawal of a dopamine agonist |
| Lethal catatonia | Muscular rigidity, diaphoresis, delirium, alternating extreme excitement and stupor, tremors, hypertension | Nonspecific | Evidence of pre-existing psychosis (bipolar disorder, schizophrenia) |
| Anticholinergic toxicity | Hot, dry skin, pupillary dilatation, tachycardia, constipation, urinary retention, confusion, hallucinations, muscular relaxation | Nonspecific | Agents that block central and peripheral muscarinic cholinergic receptors |
| Malignant hyperthermia | Rapid onset, severe muscular rigidity, ischemia, hypotension | Elevated CPK, potassium, magnesium; DIC; acidosis; rhabdomyolysis | Inherited disorder with onset after exposure to anesthetic agents that block the neuromuscular junction |
| CPK: creatine phosphokinase | |||
| DIC: disseminated intravascular coagulation | |||
Tramadol is an analgesic with opioid and serotonin-reuptake inhibiting properties that is metabolized by the cytochrome P (CYP)-450 isoenzyme 2D6. Serotonin syndrome has been reported from interactions between tramadol and sertraline22 and fluoxetine.23 Possible causes include SSRI inhibition of CYP 2D6 metabolism of tramadol, tramadol abuse,23 and multiple coadministered medications.22
Sumatriptan is one of the selective 5HT1D agonists used in treating migraine. Gardner and Lynd24 concluded that most patients tolerate sumatriptan with SSRIs or lithium. They felt they could not ensure the safety of sumatriptan with MAOIs, however, because sumatriptan elimination depends on hepatic MAO activity.
Among the 5HT1D agonists, using sumatriptan, zolmitriptan, rizatriptan, or almotriptan with an MAOI or within 2 weeks of discontinuing an MAOI is contraindicated. Naratriptan and frovatriptan appear less likely to interact with MAOIs, based on FDA-approved labeling.
MDMA. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) is widely used as a recreational drug, especially at crowded dances (“raves”) and with other drugs.25 This illicit amphetamine derivative stimulates the release of serotonin and inhibits its reuptake.
Kaskey reported the rapid onset of serotonin syndrome when a patient taking lithium and phenelzine ingested MDMA.26 Signs and symptoms of serotonin syndrome also may develop when MDMA is used alone, facilitated by the high ambient temperatures on crowded dance floors and the dancers’ relative dehydration.
Fatalities have been blamed on complications including disseminated intravascular coagulation, rhabdomyolysis, and acute hepatic, renal, or cardiac failure.25 Cases are difficult to interpret because of uncertainty about whether the victim ingested MDMA or another agent or combination.
St. John’s wort (Hypericum perforatum) contains numerous constituents, including hypericin and hyperforin, which have been found to inhibit the synaptic uptake of monoamines, including serotonin.27 Which constituents are responsible for its clinical effect is unclear. Adverse effects from monotherapy include GI symptoms, confusion, dry mouth, dizziness, headache, fatigue, allergic skin reactions, photosensitivity, and urinary frequency.27
Several cases of purported serotonin syndrome have been associated with St. John’s wort alone28 or in combination with SSRIs, nefazodone, or fenfluramine.29,30 GI symptoms and anxiety were the primary complaints and resolved without complications (adjunctive cyproheptadine was prescribed in two cases, though it is not clear that this agent contributed to resolution).
MISCELLANEOUS COMBINATIONS
Antiretroviral therapy. Five cases of serotonin syndrome were reported in HIV-infected patients taking fluoxetine with antiretroviral therapy.31 In particular, the use or addition of ritonavir—a potent CYP 2D6 inhibitor—was implicated, though saquinavir, efavirenz, or grapefruit juice (all primarily CYP 3A4 inhibitors) were also used, suggesting that pharmacokinetic interactions increased serotonergic stimulation. All five patients were taking multiple additional medications and had complex medical and/or psychiatric histories. Reducing SSRI dosages by one-half when used with ritonavir has been recommended to minimize adverse effects from a pharmacokinetic interaction.
Erythromycin was reported to induce serotonin syndrome in a 12-year-old boy when added to ongoing treatment with sertraline, an effect believed to be secondary to CYP 3A4 inhibition of sertraline metabolism.32
Mirtazapine was reported to induce serotonin syndrome in an elderly man 8 days after it was added to a regimen he had been taking for several years to treat chronic obstructive pulmonary disease.33 Serotonin syndrome also developed in a 12-year-old boy with Ewing’s sarcoma when the 5HT3 antagonist ondansetron was added to mirtazapine and morphine34 and in an 11-year-old girl with acute lymphoblastic leukemia when ondansetron was added to fentanyl. Interestingly, another report35 suggested using mirtazapine to treat serotonin syndrome caused by serotonergic antagonist effects.
Reports have associated the following combinations with serotonin syndrome, perhaps as the result of pharmacodynamic and/or pharmacokinetic interactions:
- paroxetine plus dextromethorphan and pseudoephedrine
- paroxetine plus nefazodone
- fluoxetine plus clomipramine and buspirone
- fluvoxamine plus buspirone
- fluoxetine plus buspirone
- amitriptyline plus meperidine and venlafaxine
- venlafaxine and dextroamphetamine
- fluoxetine plus clomipramine.
Table 4
Clinical signs that distinguish hyperthermic states
| Signs | Possible diagnosis |
|---|---|
| Prominent muscular rigidity | Neuroleptic malignant syndrome, malignant hyperthermia, catatonia |
| Myoclonus/hyperreflexia | Serotonin syndrome |
| Diaphoresis | Serotonin syndrome, neuroleptic malignant syndrome, catatonia |
| Hot dry skin | Anticholinergic toxicity |
| Elevated creatine phosphokinase | Neuroleptic malignant syndrome, malignant hyperthermia |
| Family history of anesthetic-induced hyperthermia | Malignant hyperthermia |
HOW TO RECOGNIZE SEROTONIN SYNDROME
Signs and symptoms of serotonin syndrome can overlap with those seen in neuroleptic malignant syndrome, lethal catatonia, malignant hyperthermia, and anticholinergic toxicity (Table 3),1,36,37 particularly with fever or hyperthermia (>40.5 °C, 105 °F). Fink37 has opined that acute neurotoxic syndromes such as serotonin syndrome and neuroleptic malignant syndrome also meet criteria for catatonia and are therefore subtypes of catatonia. The types of drugs involved and clinical findings can help distinguish the various hyperthermic states (Table 4).
As mentioned above, original diagnostic criteria for serotonin syndrome excluded the addition of, or increase in, an antipsychotic agent. This exclusion was intended to avoid confusion between serotonin syndrome and neuroleptic malignant syndrome. Co-administering antipsychotic and serotonergic agents requires heightened awareness for both neurotoxic syndromes.
TREATING MILD TO SEVERE CASES
If a patient develops serotonin syndrome, immediately discontinue the suspected agent(s) and observe carefully. In most cases, serotonin syndrome will resolve within 24 hours.
In mild cases, lorazepam, 1 to 2 mg slow IV push every 30 minutes until excessive sedation develops, may help. In moderate to severe cases, agents that block serotonin’s action are recommended,2 including:
- cyproheptadine (4 mg po every 4 hours as needed, up to 20 mg in 24 hours)
- propranolol (1 to 3 mg IV every 5 minutes, up to 0.1 mg/kg).
Case reports attest to these agents’ potential benefit. Other clinicians have reported using mirtazapine,35 nitroglycerin,38 and chlorpromazine.1
Serotonin syndrome symptoms resolved within minutes when IV nitroglycerin was used in a patient with serotonin syndrome and cardiac ischemia. The authors hypothesized that nitroglycerin, via nitric acid, provided an “off” signal for serotonin, though they did not advocate this as a routine treatment.38
The rationale for using chlorpromazine is its potential to block serotonin receptors. I would avoid the routine use of any antipsychotic agent in this setting, however, to minimize the risk of neuroleptic malignant syndrome.
Severe cases. Intensive care observation and treatment is required for patients with severe serotonin syndrome, including evidence of hyperthermia, DIC, rhabdomyolysis, renal failure, or aspiration. In cases of hyperthermia, supportive measures and standard treatments include muscle relaxants, cooling, and endotracheal intubation.
Severe complications are most likely with interactions between MAOIs and serotonergic agents, especially in overdose. Therefore, using such combinations requires close observation.
Related resources
- Di Rosa AE, Morgante L, Spina E et al. Epidemiology and pathoetiology of neurological syndromes with hyperthermia. Funct Neurol 1995;10:111-19.
- Radomski, JW, Dursun SM, Reveley MA, et al. An exploratory approach to the serotonin syndrome: an update of clinical phenomenology and revised diagnostic criteria. Med Hypothesis 2000;55: 218-24.
- Lane R, Baldwin D. Selective serotonin reuptake inhibitor-induced serotonin syndrome: review. J Clin Psychopharmacol 1997;17:208-21.
Drug brand names
- Almotriptan • Axert
- Amitriptyline • Elavil
- Buspirone • Buspar
- Chlorpromazine • Thorazine
- Citalopram • Celexa
- Clomipramine • Anafranil
- Cyproheptadine • Periactin
- Dextroamphetamine • Dexedrine
- Dextromethorphan • Delsym
- Efavirenz • Sustiva
- Escitalopram • Lexapro
- Fenfluramine • Pondimin
- Fentanyl • Sublimaze
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Frovatriptan • Frova
- Isocarboxazid • Marplan
- Linezolid • Zyvox
- Meperidine • Demerol
- Mirtazapine • Remeron
- Moclobemide • Aurorix
- Nortriptyline • Pamelor
- Naratriptan, • Amerge
- Nefazodone • Serzone
- Olanzapine • Zyprexa
- Ondansetron • Zofran
- Paroxetine • Paxil
- Phenelzine • Nardil
- Propranolol • Inderal
- Risperidone • Risperdal
- Ritonavir • Norvir
- Rizatriptan • Maxalt
- Saquinavir • Invirase
- Selegiline • Eldepryl
- Sertraline • Zoloft
- Sumatriptan • Imitrex
- Tramadol • Ultram
- Tranylcypromine • Parnate
- Trazodone • Desyrel
- Venlafaxine • Effexor
- Zolmitriptan • Zomig
Disclosure
Dr. Sternbach receives research grants from Otsuka America Pharmaceuticals and Eli Lilly and Co. and owns stock in Merck & Co., Pfizer Inc., and Johnson & Johnson.
1. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148:705-13.
2. Brown TM, Skop BP, Mareth TR. Pathophysiology and management of the serotonin syndrome. Ann Pharmacother 1996;30:527-33.
3. Hegerl U, Bottlender R, Gallinat J, et al. The serotonin syndrome scale: first results on validity. Eur Arch Psychiatry Clin Neurosci 1998;248:96-103.
4. Beasley CM, Jr, Masica DN, Heiligenstein JH, et al. Possible monoamine-oxidase inhibitor-serotonin uptake inhibitor interaction: fluoxetine clinical data and pre-clinical findings. J Clin Psychopharmacol 1993;13:312-20.
5. Ruiz F. Fluoxetine and the serotonin syndrome. Ann Emerg Med 1994;34:983-5.
6. Gitlin MJ. Venlafaxine, monoamine oxidase inhibitors and the serotonin syndrome. J Clin Psychopharmacol 1997;17:66-7.
7. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. Clin Toxicol 1997;35:211-12.
8. Coplan JD, Gorman JM. Detectable levels of fluoxetine metabolites after discontinuation: an unexpected serotonin syndrome. Am J Psychiatry 1993;15:837.-
9. Richard IH, Kurlan R, Tanner C, et al. Serotonin syndrome and the combined use of deprenyl and an antidepressant in Parkinson’s disease. Neurology 1997;48:1070-7.
10. Zornberg GL, Bodkin JA, Cohen BM. Severe adverse interaction between pethidine and selegiline. Lancet 1991;337:246.-
11. Hinds NP, Hillier CE, Wiles CM. Possible serotonin syndrome arising from an interaction between nortriptyline and selegiline in a lady with parkinsonism. J Neurol 2000;247:811.-
12. Joffe RT, Bakish D. Combined SSRI-moclobemide treatment of psychiatric illness. J Clin Psychiatry 1994;55:24-5.
13. Hilton SE, Maradit H, Moller HJ. Serotonin syndrome and drug combinations: focus on MAOI and RIMA. Eur Arch Psychiatry Clin Neurosci 1997;247:113-19.
14. Dardennes RM, Even C, Ballon N, et al. Serotonin syndrome caused by a clomipramine-moclobemide interaction. J Clin Psychiatry 1998;59:382-3.
15. Gillman PK. Possible serotonin syndrome with moclobemide and pethidine. Med J Aust 1995;162:554.-
16. Dingemanse J, Wallnofer A, Gieschke R, et al. Pharmacokinetic and pharmacodynamic interaction between fluoxetine and moclobemide in the investigation of development of the “serotonin syndrome.” Clin Pharmacol Ther 1998;63:403-13.
17. Wigen CL, Goetz MB. Serotonin syndrome and linezolid. Clin Infect Dis 2002;34:1651-2.
18. Lavery S, Ravi H, McDaniel WW, et al. Linezolid and serotonin syndrome. Psychosomatics 2001;42:432-4.
19. Hamilton S, Malone K. Serotonin syndrome during treatment with paroxetine and risperidone. J Clin Psychopharmacol 2000;20:103-5.
20. Duggal HS, Fetchko J. Serotonin syndrome and atypical antipsychotics. Am J Psychiatry 2002;159:672-3.
21. Haslett CD, Kumar S. Can olanzapine be implicated in causing serotonin syndrome? Psychiatry Clin Neurosci 2002;56:533-6.
22. Mason BJ, Blackburn KH. Possible serotonin syndrome associated with tramadol and sertraline coadministration. Ann Pharmacother 1997;31:175-7.
23. Lange-Asschenfeldt C, Weigmann H, Hiemke C, et al. Serotonin syndrome as a result of fluoxetine in a patient with tramadol abuse: plasma level-correlated symptomatology. J Clin Psychopharmacol 2002;22:440-1.
24. Gardner DM, Lynd LD. Sumatriptan contraindications and the serotonin syndrome. Ann Pharmacother 1998;32:33-8.
25. Parrott AC. Recreational Ecstasy/MDMA, the serotonin syndrome and serotonergic neurotoxicity. Pharmacol Biochem Behav 2002;71:837-44.
26. Kaskey GB. Possible interaction between an MAOI and “Ecstasy.” Am J Psychiatry 1992;149:411-12.
27. De Smet PA. Herbal remedies. N Engl J Med 2002;347:2046-56.
28. Parker V, Wong AH, Boon HS, et al. Adverse reactions to St. John’s wort. Can J Psychiatry 2001;46:77-9.
29. Lantz MS, Buchalter E, Giambanco V. St. John’s wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol 1999;12:7-10.
30. Beckman SE, Sommi RW, Switzer J. Consumer use of St. John’s wort: a survey of effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
31. De Silva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS 2001;15:1281-5.
32. Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999;19:894-6.
33. Hernandez JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother 2002;36:641-3.
34. Turkel SB, Nadala JGB, Wincor MZ. Possible serotonin syndrome in association with 5HT3 antagonist agents. Psychosomatics 2001;42:258-60.
35. Hoes MJ, Zeijpveld JH. Mirtazapine as treatment for serotonin syndrome. Pharmacopsychiatry 1996;29:81.-
36. Theoharides TC, Harris RS, Weckstein D. Neuroleptic malignant-like syndrome due to cyclobenzaprine? J Clin Psychopharmacol 1995;15:79-81.
37. Fink M. Toxic serotonin syndrome or neuroleptic malignant syndrome? Pharmacopsychiatry 1996;29:159-61.
38. Brown TM, Skop BP. Nitroglycerin in the treatment of the serotonin syndrome. Ann Pharmacother 1996;30:191-2.
1. Sternbach H. The serotonin syndrome. Am J Psychiatry 1991;148:705-13.
2. Brown TM, Skop BP, Mareth TR. Pathophysiology and management of the serotonin syndrome. Ann Pharmacother 1996;30:527-33.
3. Hegerl U, Bottlender R, Gallinat J, et al. The serotonin syndrome scale: first results on validity. Eur Arch Psychiatry Clin Neurosci 1998;248:96-103.
4. Beasley CM, Jr, Masica DN, Heiligenstein JH, et al. Possible monoamine-oxidase inhibitor-serotonin uptake inhibitor interaction: fluoxetine clinical data and pre-clinical findings. J Clin Psychopharmacol 1993;13:312-20.
5. Ruiz F. Fluoxetine and the serotonin syndrome. Ann Emerg Med 1994;34:983-5.
6. Gitlin MJ. Venlafaxine, monoamine oxidase inhibitors and the serotonin syndrome. J Clin Psychopharmacol 1997;17:66-7.
7. Kolecki P. Venlafaxine induced serotonin syndrome occurring after abstinence from phenelzine for more than two weeks. Clin Toxicol 1997;35:211-12.
8. Coplan JD, Gorman JM. Detectable levels of fluoxetine metabolites after discontinuation: an unexpected serotonin syndrome. Am J Psychiatry 1993;15:837.-
9. Richard IH, Kurlan R, Tanner C, et al. Serotonin syndrome and the combined use of deprenyl and an antidepressant in Parkinson’s disease. Neurology 1997;48:1070-7.
10. Zornberg GL, Bodkin JA, Cohen BM. Severe adverse interaction between pethidine and selegiline. Lancet 1991;337:246.-
11. Hinds NP, Hillier CE, Wiles CM. Possible serotonin syndrome arising from an interaction between nortriptyline and selegiline in a lady with parkinsonism. J Neurol 2000;247:811.-
12. Joffe RT, Bakish D. Combined SSRI-moclobemide treatment of psychiatric illness. J Clin Psychiatry 1994;55:24-5.
13. Hilton SE, Maradit H, Moller HJ. Serotonin syndrome and drug combinations: focus on MAOI and RIMA. Eur Arch Psychiatry Clin Neurosci 1997;247:113-19.
14. Dardennes RM, Even C, Ballon N, et al. Serotonin syndrome caused by a clomipramine-moclobemide interaction. J Clin Psychiatry 1998;59:382-3.
15. Gillman PK. Possible serotonin syndrome with moclobemide and pethidine. Med J Aust 1995;162:554.-
16. Dingemanse J, Wallnofer A, Gieschke R, et al. Pharmacokinetic and pharmacodynamic interaction between fluoxetine and moclobemide in the investigation of development of the “serotonin syndrome.” Clin Pharmacol Ther 1998;63:403-13.
17. Wigen CL, Goetz MB. Serotonin syndrome and linezolid. Clin Infect Dis 2002;34:1651-2.
18. Lavery S, Ravi H, McDaniel WW, et al. Linezolid and serotonin syndrome. Psychosomatics 2001;42:432-4.
19. Hamilton S, Malone K. Serotonin syndrome during treatment with paroxetine and risperidone. J Clin Psychopharmacol 2000;20:103-5.
20. Duggal HS, Fetchko J. Serotonin syndrome and atypical antipsychotics. Am J Psychiatry 2002;159:672-3.
21. Haslett CD, Kumar S. Can olanzapine be implicated in causing serotonin syndrome? Psychiatry Clin Neurosci 2002;56:533-6.
22. Mason BJ, Blackburn KH. Possible serotonin syndrome associated with tramadol and sertraline coadministration. Ann Pharmacother 1997;31:175-7.
23. Lange-Asschenfeldt C, Weigmann H, Hiemke C, et al. Serotonin syndrome as a result of fluoxetine in a patient with tramadol abuse: plasma level-correlated symptomatology. J Clin Psychopharmacol 2002;22:440-1.
24. Gardner DM, Lynd LD. Sumatriptan contraindications and the serotonin syndrome. Ann Pharmacother 1998;32:33-8.
25. Parrott AC. Recreational Ecstasy/MDMA, the serotonin syndrome and serotonergic neurotoxicity. Pharmacol Biochem Behav 2002;71:837-44.
26. Kaskey GB. Possible interaction between an MAOI and “Ecstasy.” Am J Psychiatry 1992;149:411-12.
27. De Smet PA. Herbal remedies. N Engl J Med 2002;347:2046-56.
28. Parker V, Wong AH, Boon HS, et al. Adverse reactions to St. John’s wort. Can J Psychiatry 2001;46:77-9.
29. Lantz MS, Buchalter E, Giambanco V. St. John’s wort and antidepressant drug interactions in the elderly. J Geriatr Psychiatry Neurol 1999;12:7-10.
30. Beckman SE, Sommi RW, Switzer J. Consumer use of St. John’s wort: a survey of effectiveness, safety, and tolerability. Pharmacotherapy 2000;20:568-74.
31. De Silva KE, Le Flore DB, Marston BJ, et al. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS 2001;15:1281-5.
32. Lee DO, Lee CD. Serotonin syndrome in a child associated with erythromycin and sertraline. Pharmacotherapy 1999;19:894-6.
33. Hernandez JL, Ramos FJ, Infante J, et al. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother 2002;36:641-3.
34. Turkel SB, Nadala JGB, Wincor MZ. Possible serotonin syndrome in association with 5HT3 antagonist agents. Psychosomatics 2001;42:258-60.
35. Hoes MJ, Zeijpveld JH. Mirtazapine as treatment for serotonin syndrome. Pharmacopsychiatry 1996;29:81.-
36. Theoharides TC, Harris RS, Weckstein D. Neuroleptic malignant-like syndrome due to cyclobenzaprine? J Clin Psychopharmacol 1995;15:79-81.
37. Fink M. Toxic serotonin syndrome or neuroleptic malignant syndrome? Pharmacopsychiatry 1996;29:159-61.
38. Brown TM, Skop BP. Nitroglycerin in the treatment of the serotonin syndrome. Ann Pharmacother 1996;30:191-2.
Atomoxetine: A different approach to ADHD
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
Methylphenidate and other amphetamine-based agents are mainstays in treating attention-deficit/hyperactivity disorder (ADHD). Although these stimulants are considered safe, their potentially addictive properties have concerned clinicians, adult patients, and parents of children and adolescents with ADHD.
Table
Atomoxetine: fast facts
| Drug brand name: Strattera |
| Class: Selective norepinephrine reuptake inhibitor |
| FDA-approved indications: Treatment of ADHD in children, adolescents, and adults |
| Manufacturer: Eli Lilly and Co. |
| Dosing forms: 5 mg, 10 mg, 18 mg, 25 mg, 40 mg, and 60 mg capsules |
| Recommended dosage: Determined primarily by body weight; optimal at 1 to 1.2 mg/kg/d |
Atomoxetine—a nonaddictive, nonstimulant medication—has demonstrated efficacy in placebo-controlled trials.
HOW IT WORKS
Atomoxetine enhances synaptic concentrations of norepinephrine via the presynaptic transporter. The agent has a strong affinity with norepinephrine transporters, modest affinity with serotonin transporters, and no affinity with dopamine transporters.1
When applied directly to the prefrontal cortex, however, atomoxetine has been shown to increase both extracellular norepinephrine and dopamine. Sustained levels of norepinephrine and dopamine in the prefrontal cortex may explain why atomoxetine works well beyond its 5.3-hour biologic half-life.1
In contrast, methylphenidate has shown high affinity with dopamine transporters. It produces intense, brief prefrontal increases in norepinephrine and dopamine and sustained dopamine increases in the nucleus accumbens and striatum.2 This might explain methylphenidate’s rewarding properties and its association with stereotypic motor activity and tics. By comparison, atomoxetine has a lower abuse potential and does not affect basal ganglia motor output.3
Atomoxetine’s pharmacokinetics have been evaluated in more than 400 children and adolescents. Its half-life, clearance (0.35 L/hr/kg), and volume of distribution are similar across age groups, and the dose-plasma concentration relationship is linear, suggesting that dosing can be reliably adjusted according to weight. Atomoxetine is rapidly absorbed, food does not appreciably affect absorption, and peak plasma concentrations are achieved within 1 to 2 hours. The drug is distributed mostly in total body water and is highly protein bound.
Atomoxetine is metabolized primarily through the cytochrome P (CYP)-450 2D6 pathway. The major metabolite is 4-hydroxyatomoxetine, which is equipotent to atomoxetine as a norepinephrine transporter inhibitor.
WHAT RESEARCHERS SAY
In an 8-week study, 297 patients ages 8 to 18 received a divided fixed dosage of atomoxetine (0.5, 1.2 or 1.8 mg/kg/d) or placebo. The 1.2 and 1.8 mg/kg/d dosages were more effective than placebo and were equally effective against hyperactivity/impulsivity and inattention symptoms. The 0.5 mg/kg/d dosage was not much more effective than placebo.4
In a 6-week, placebo-controlled study, 85 subjects ages 6 to 16 who received a single dose of atomoxetine each morning (mean dosage 1.3 mg/kg/d) achieved favorable outcomes based on investigator, parent, and teacher ratings and on an ADHD Rating Scale (ADHD-RS) primary outcome measure. The treatment effect size (0.71) was similar to that found in the twice-daily dosing studies, suggesting that single-daily dosing is effective.5
Adults and adolescents >70 kg body weight—Start at 40 mg/d and increase after 3 days to a target dosage of 80 mg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening. If the patient does not respond, wait 2 to 4 more weeks and increase the dosage to 100 mg/d.
Children and adolescents <70 kg body weight—Start at 0.5 mg/kg/d. After 3 days, increase to a target dosage of 1.2 mg/kg/d, either as a single dose in the morning or as evenly divided doses in the morning and late afternoon/early evening.
Caveats—Because atomoxetine is metabolized primarily by CYP 2D6 isoenzymes, patients with hepatic disease, low metabolizers of CYP 2D6, and those taking strong CYP 2D6 inhibitors require lower dosages. Adjust dosages cautiously.
Extensive CYP 2D6 metabolizers may require higher dosages, although atomoxetine has demonstrated no additional benefit at >1.2 mg/kg/d. No systematic safety data exist for single doses >120 mg or total daily doses >150 mg.
Source: Prescribing information, Eli Lilly and Co., 2002.
Two controlled, comparison studies involving 291 subjects ages 7 to 13 with ADHD found that atomoxetine (mean final dosage 1.6 mg/kg/d) compares favorably to methylphenidate with similar effect sizes across ADHD symptom domains (unpublished data). Limited published data indicate that randomized, open-label atomoxetine and methylphenidate are similarly effective across ADHD symptom domains in children.6
Atomoxetine also was shown to improve ADHD symptoms in two placebo-controlled trials involving a total of 536 adults (mean daily divided dose 95 mg).7 Inattention, hyperactivity, and impulsivity—as measured with the Conners Adult ADHD Rating Scale—were reduced among both treatment groups.
DOSING AND ADMINISTRATION
No age- or gender-related differences in response to atomoxetine have been reported, although dosing varies with age and weight (Box).
The agent should be used cautiously in patients with cardiovascular or cerebrovascular disease, as side effects include slight elevation of pulse and blood pressure. Atomoxetine also may exacerbate urinary retention or hesitation in some adults. The drug may impair sexual function; at least 7% of men in placebo-controlled trials experienced erectile disturbance, and 3% experienced impotence.7
In children and adolescents, gastrointestinal discomfort, asthenia, fatigue, mild appetite decreases, and slight weight loss were reported adverse effects.5 Nausea and vomiting were the most troublesome acute side effects in children, with most episodes lasting 1 to 2 days.5
CLINICAL IMPLICATIONS
Atomoxetine may help patients with ADHD who respond inadequately or do not respond to stimulants. Its lack of abuse potential suggests it may be useful in adults with comorbid substance use disorders. Atomoxetine also does not appear to exacerbate insomnia—a potential benefit for ADHD patients with poor sleep quality.
Given its pharmacologic profile, the agent will reduce the impact of comorbidities (such as anxiety and depression) common to adults with ADHD. Research is needed to determine its role in treating more complicated pathologies, such as ADHD with comorbid bipolar disorder.
Whereas some stimulants require multiple daily dosing, atomoxetine is administered once daily. This could save clinicians time by reducing the need for refills, out-of-visit prescribing, and monthly patient visits (our pediatric practice writes 20 to 40 stimulant refills per day)and enhance convenience for patients.
Related resources
- Spencer T, Biederman J, Wilens T, et al. Effectiveness and tolerability of tomoxetine in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1998;155:693-5.
Drug Brand Names
- Methylphenidate • Concerta, Ritalin
Disclosure
The author receives research/grant support from and is a consultant to and speaker for Eli Lilly and Co. He also receives research/grant support from Shire Pharmaceuticals and Johnson & Johnson, and is a consultant to Abbott Laboratories, Merck and Co., Pfizer Inc., and Organon.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
1. Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: A potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27:699-711.
2. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci 2001;21:RC121:1-5.
3. Heil SH, Holmes HW, Bickel WK, et al. Comparison of the subjective, physiological, and psychomotor effects of atomoxetine and methylphenidate in light drug users. Drug Alcohol Depend 2002;67:149-56.
4. Michelson D, Faries D, Wernicke J, et al. and the Atomoxetine ADHD Study Group Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001;108(5):E83.-
5. Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002;159(11):1896-1901.
6. Kratochvil CJ, Heiligenstein JH, Dittmann R, et al. Atomoxetine and methylphenidate treatment in children with ADHD: A prospective, randomized, open-label trial. J Am Acad Child Adolesc Psychiatry 2002;41:776-84.
7. Michelson D, Adler I, Spencer T, et al. Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003;53:112-20.
When patients can’t sleep: Practical guide to using and choosing hypnotic therapy
Careful investigation can often reveal insomnia’s cause1—whether a psychiatric or medical condition or poor sleep habits. Understanding why patients can’t sleep is key to effective therapy.
Acute and chronic sleep deprivation is associated with measurable declines in daytime performance (Box). Some data even suggest that long-term sleeplessness increases the risk of new psychiatric disorders—most notably major depression.3
PSYCHIATRIC DISORDERS AND INSOMNIA
Depression. Many depressed persons—up to 80%—experience insomnia, although no one sleep pattern seems typical.2 Depression may be associated with:
- difficulties in falling asleep
- interrupted nocturnal sleep
- and early morning awakening.
Anxiety disorders. Generalized anxiety disorder (GAD), social phobia, panic attacks, and posttraumatic stress disorder (PTSD) are all associated with disrupted sleep. Patients with GAD experience prolonged sleep latency (time needed to fall asleep after lights out) and fragmented sleep, similar to those with primary insomnia.
One-half of adult Americans experience insomnia during their lives, and 10% report persistent sleep difficulties (longer than 2 weeks). Individuals who complain of insomnia report:
- daytime drowsiness
- diminished memory and concentration
- depression
- strained relationships
- increased risk of accidents
- impaired job performance.
Despite these complaints, a surprising 70% of those with insomnia never seek medical help. Only 6% visit their physicians specifically for insomnia, and 24% address sleep difficulty as a secondary complaint. Many (40%) self-medicate with over-the-counter sleep aids or alcohol.2
Insomnia becomes more frequent with aging, associated with increased rates of medical and psychiatric illness and an age-related deterioration in the brain’s sleep-generating processes.3
Subjective sleep quality may be impaired in patients with social phobia. Some patients experience panic symptoms while sleeping, possibly in association with mild hypercapnia. Patients with sleep panic attacks tend to have earlier onset of panic disorder and a higher likelihood of comorbid mood and other anxiety disorders.4
In patients with PTSD, disturbed sleep continuity and increased REM phasic activity—such as eye movements—are directly correlated with severity of PTSD symptoms. Nightmares and disturbed REM sleep are hypothesized hallmarks of PTSD.5
Schizophrenia. Patients with schizophrenia often have disrupted sleep patterns. These include prolonged sleep latency, fragmented sleep with frequent arousals, decreased slow-wave sleep, variable REM latency, and decreased REM rebound after sleep deprivation. Despite investigations going back to the 1950s, no specific link between REM sleep and psychosis has been found.6 Interestingly, increases in REM sleep time and REM activity have been associated with an increased risk of suicide in patients with schizophrenia.7
Adjustment sleep disorder. Acute emotional stressors—such as bereavement, job loss, or hospitalization—often cause adjustment sleep disorder. Symptoms typically remit soon after the stressors abate, so this transient insomnia usually lasts a few days to a few weeks. Treatment with behavioral therapies and hypnotics8 is warranted if:
- sleepiness and fatigue interfere with daytime functioning
- a pattern of recurring episodes develops.9
Psychophysiologic insomnia. Once initiated—regardless of cause—insomnia may persist well after its precipitating factors resolve. Thus, short-term insomnia may develop into long-term, chronic difficulty with recurring episodes or a constant, daily pattern of insomnia. Sufferers often spend hours in bed awake focused upon—and brooding over—their sleeplessness. which in turn further aggravates their insomnia.
Adjustment sleep disorder and psychophysiologic insomnia are included within DSM-IV’s term “primary insomnia.”
OTHER CAUSES OF INSOMNIA
Medications that may affect sleep quality include antidepressants (Table 1),10,11 antihypertensives, antineoplastic agents, bronchodilators, stimulants, corticosteroids, decongestants, diuretics, histamine-2 receptor blockers, and smoking cessation aids.
Recreational drugs, such as cocaine, often cause insomnia. Hypnotics and anxiolytics can cause insomnia following long-term use and during withdrawal.
Other disorders known to disturb sleep include periodic limb movement disorder (PLMD), restless legs syndrome (RLS), sleep apnea syndrome, disrupted circadian rhythms (as with travel or shift work), cardiopulmonary disorders, chronic pain, diabetes, hyperthyroidism, hot flashes associated with menopause, seizures, dementia, and Parkinson’s disease, to name a few.
WORKUP OF SLEEP COMPLAINTS
Acute. Most short-term insomnias—lasting a few weeks or less—are caused by situational stressors, circadian rhythm alterations, and sleep hygiene violations. A logical initial approach, therefore, is to combine sleep hygiene measures with supportive psychotherapy. Hypnotic agents may be considered for apparent daytime consequences—such as sleepiness and occupational impairment—or if the insomnia seems to be escalating.
Chronic. For longer-term insomnias—lasting more than a few weeks—consider a more thorough evaluation, including medical and psychiatric history, physical examination, and mental status examination. Inquire about cardinal symptoms of disorders associated with insomnia, including:
- snoring or breathing pauses during sleep (sleep apnea syndrome)
- restlessness or twitching in the lower extremities (PLMS/RLS).
Question the bed partner, who may be more aware of such symptoms than the patient. Carefully review sleep patterns on weekdays and weekends, bedtime habits, sleep hygiene habits, and substance and medication use.
Table 1
Antidepressants’ effects on sleep and wakefulness
| Activating agents | Bupropion, protriptyline, most selective serotonin reuptake inhibitors, venlafaxine, monoamine oxidase inhibitors |
| Sedating agents | Amitriptyline, doxepin, trimipramine, nefazodone, trazodone, mirtazapine |
| Neutral agents | Citalopram, escitalopram |
Sleep clinic referrals. Consider an evaluation by a sleep disorders center when:
- the diagnosis remains unclear
- or treatment of the presumed conditions fails after a reasonable time
BEHAVIORAL TREATMENTS
Behavioral treatments—with or without hypnotics—are appropriate for a wide variety of insomnia complaints, including adjustment sleep disorder, psychophysiologic insomnia, and depression. Behavioral measures may take longer to implement than drug therapy, but their effects have been shown to last longer in patients with primary insomnia. In many cases, it may be useful to start with both hypnotic and behavioral treatments and withdraw the hypnotic after behavioral measures take effect.
Sleep hygiene. Many individuals unknowingly engage in habitual behaviors that impair sleep. Those with insomnia, for example, often try to compensate for lost sleep by staying in bed later in the morning or by napping, which further fragment nocturnal sleep. Advise these patients to adhere to a regular awakening time—regardless of how long they slept the night before—and to avoid naps. Other tips for getting a good night’s sleep are outlined in Table 2.12
Caffeine has a plasma half-life of 3 to 7 hours, although individual sensitivity varies widely and caffeine’s erratic absorption can prolong its effects. Advise patients with insomnia to avoid caffeine-containing beverages—including coffee, tea, and soft drinks—after noon.
Relaxation training. Muscle tension can be reduced through electromyography (EMG) biofeedback, abdominal breathing exercises, or progressive muscle relaxation techniques, among others. Relaxation training is usually effective within a few weeks.
Psychotherapy. Cognitive-behavioral therapy can help identify and dispel tension-producing thoughts that are disrupting sleep, such as preoccupation with unpleasant work experiences or school examinations. Reassurance may help patients overcome fears about sleeplessness; suggest that patients deal with anxiety-producing thoughts during therapy sessions and at times other than bedtime.
Insight-oriented psychotherapy may enhance patients’ awareness of psychological conflicts from their past that may be producing anxiety and contributing to sleeplessness.
PRESCRIBING HYPNOTICS
Sedative-hypnotics are indicated primarily for short-term management of insomnia. Most are used prophylactically at bedtime until insomnia dissipates or the physician advises the patient to take a break.
Treatment principles. Because many insomnias are recurrent, prolonged hypnotic treatment given in short bouts is often optimal. Longer treatment—months to years—is not recommended by standard textbooks but is clearly needed by a small number of patients with chronic insomnia. In these cases, carefully monitor for tolerance, as manifested by dosage escalation. Long-term hypnotic treatment is not suitable for patients with drug abuse or dependence histories.
Table 2
How to get a good night’s sleep
|
Although chloral hydrate and barbiturates are effective hypnotics, adverse effects limit their safety and usefulness. Benzodiazepines and more recently introduced agents have milder side effect profiles (Table 3). Choose agents based on the patient’s situation, preferences, and effects of prior trials with similar agents. Guidelines for hypnotics discourage chronic use to minimize abuse, misuse, and habituation (Table 4).
Elimination half-life is the primary pharmacokinetic property that differentiates the hypnotics from each other:13
- longer half-life: flurazepam, quazepam
- intermediate half-life: estazolam, temazepam
- short half-life: triazolam, zolpidem, zaleplon (Table 3).
Table 3
Actions and available doses of common hypnotics
| Class/drug | Onset of action | Half-life (hrs) | Active metabolites | Doses (mg) |
|---|---|---|---|---|
| Benzodiazepines | ||||
| Flurazepam | Rapid | 40 to 250 | Yes | 15, 30 |
| Quazepam | Rapid | 40 to 250 | Yes | 7.5, 15 |
| Estazolam | Rapid | 10 to 24 | Yes | 0.5, 1, 2 |
| Temazepam | Intermediate | 8 to 22 | No | 7.5, 15 |
| Triazolam | Rapid | <6 | No | 0.125, 0.25, 0.5 |
| Imidazopyridine | ||||
| Zolpidem | Rapid | 2.5 | No | 5, 10 |
| Pyrazolopyrimidine | ||||
| Zaleplon | Rapid | 1 | No | 5, 10, 20 |
Whereas benzodiazepines bind to benzodiazepine receptor types 1 and 2, zolpidem and zaleplon (and possibly quazepam) bind selectively to type 1. This selectivity may explain why zolpidem and zaleplon are more easily tolerated.
Hypnotic agents with relatively longer half-lives tend to be associated with greater potential for residual daytime effects such as sedation, motor incoordination, amnesia, and slowed reflexes. These effects may impair performance and increase the risk of auto accidents and injuries, especially hip fractures in the elderly.
Nonbenzodiazepines. Because of its ultra-short half-life, zaleplon is least likely to cause residual daytime effects when administered at bedtime. At 10-mg doses, its side effects seem to last no more than 4 hours following administration. Zaleplon can be safely taken after nocturnal awakenings if the patient remains in bed 4 hours or longer after taking it.14
Some patients feel that taking zaleplon only when needed allows them to use hypnotics more sparingly. On the other hand, zaleplon’s ultrashort half-life makes it less useful for patients who have frequent episodes of sleep-interruption insomnia every night. For them, a longer elimination half-life agent such as zolpidem may be more predictably effective for the entire night.15 Short half-life hypnotics have many advantages, but they do not offer anxiolysis for patients with daytime anxiety, as the longer half-life agents do.
Tolerance and rebound. Tolerance can develop following repeated dosing with benzodiazepines—primarily triazolam—and rebound insomnia can follow abrupt discontinuation. Despite widespread concerns, neither tolerance nor rebound insomnia has been well documented. Nevertheless, both can be minimized by using benzodiazepines at the lowest effective dosages and for brief periods. Gradual tapering when discontinuing the drug can help control rebound.
Tolerance and rebound seem to be less of a concern with the newer hypnotics than with benzodiazepines. In preliminary uncontrolled trials, zolpidem and zaleplon did not show evidence of these problems in 1 year of continued use.
NONHYPNOTIC SLEEP AIDS
Sedating antidepressants. Physicians often prescribe low doses of sedating antidepressants to control insomnia, a practice supported by some controlled clinical trials. For example, polysomnography showed that patients who took doxepin, 25 to 50 mg at bedtime, had enhanced sleep efficiency (ratio of time slept to time spent in bed) yet no change in sleep latency. Liver abnormalities, leukopenia, and thrombopenia developed in a few patients.16 Controlled studies have also shown subjective efficacy of trazodone17 and trimipramine18 in treating insomnia.
Some physicians advocate using the more sedating antidepressants—at dosages needed to treat depression—to control insomnia in depressed patients. Evening dosing can minimize daytime sedation. If you choose an activating antidepressant, the potential side effect of insomnia can be managed by judicious use of hypnotic agents. Little is known about antidepressants’ effects on sleep quality after the first 6 to 8 weeks of treatment.19
Although possibly helpful as sleep aids, antidepressants are also associated with side effects. Trazodone, for example, may cause daytime sedation, orthostatic hypotension, and priapism. As a class, the tricyclics are associated with anticholinergic effects such as dry mouth, urinary flow difficulties, and cardiac dysrhythmias.
Table 4
Guidelines for safe use of hypnotics
|
Alcohol. Patients with insomnia often self-medicate with agents that are not specifically indicated to induce sleep. Alcohol is widely used at bedtime because it enhances sleepiness and induces a more rapid sleep onset.20 Drinking a “nightcap” is a poor choice, however, because alcohol—especially after prolonged use—can impair sleep quality, resulting in daytime somnolence. Alcohol is also associated with rapid development of tolerance.
Patients who use alcohol report unrefreshing and disturbed sleep, with frequent nocturnal awakenings even after prolonged abstinence. Alcohol also can further impair sleep-related respiration in patients with obstructive sleep apnea syndrome.
Antihistamines and over-the-counter products whose main active ingredients are antihistamines—such as doxylamine and diphenhydramine—can cause unpredictable efficacy and side effects such as daytime sedation, confusion, and systemic anticholinergic effects.21
Melatonin is a dietary supplement used in dosages of 0.5 to 3,000 mg. Anecdotal reports indicate it may be efficacious in certain subtypes of insomnia—such as shift work, jetlag, blindness, delayed sleep phase syndrome—and in the elderly. However, melatonin’s efficacy has not been established conclusively and is in doubt. Concerns have been expressed regarding the purity of available preparations and possible coronary artery tissue stimulation, as observed in animal studies of melatonin.
Related resources
- American Academy of Sleep Medicine. Sleep logs, patient education materials. www.aasmnet.org
- American Sleep Apnea Association. www.sleepapnea.org
- National Sleep Foundation. www.sleepfoundation.org
Drug brand names
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Doxepin • Sinequan
- Escitalopram • Lexapro
- Estazolam • Prosom
- Flurazepam • Dalmane
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Protriptyline • Vivactil
- Quazepam • Doral
- Temazepam • Restoril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trimipramine • Surmontil
- Venlafaxine • Effexor
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosure
Dr. Doghramji receives research grant support from Cephalon Inc., GlaxoSmithKline, Merck & Co., and Sanofi-Synthelabo.
1. Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. Sleep 2000;23:243-81.
2. Reynolds CF III, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep 1987;10:199-215.
3. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. JAMA 1989;262:1479-84.
4. Labbate LA, Pollack MH, Otto MW, et al. Sleep panic attacks: an association with childhood anxiety and adult psychopathology. Biol Psychiatry 1994;43:840-2.
5. Ross RJ, Ball WA, Sullivan KA, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989;146:697-707.
6. Neylan TC, Reynolds CF III, Kupfer DJ. Sleep disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry(4th ed). Washington, DC: American Psychiatric Publishing, 2003;978-90.
7. Lewis CF, Tandon R, Shipley JE, et al. Biological predictors of suicidality in schizophrenia. Acta Psychiatr Scand 1996;94:416-20.
8. Spielman AJ, Glovinsky P. The varied nature of insomnia. In: Hauri P (ed). Case studies in insomnia. New York: Plenum Press, 1991;1-15.
9. American Sleep Disorders Association International classification of sleep disorders (rev). Diagnostic and coding manual. Rochester: American Sleep Disorders Association, 1997.
10. Winokur A, Reynolds CF. The effects of antidepressants on sleep physiology. Primary Psychiatry 1994;6:22-7.
11. Gillin JC, Rapaport M, Erman MK, Winokur A, Albala BJ. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. J Clin Psychiatry 1997;58:185-92.
12. Doghramji K. The evaluation and management of sleep disorders. In: Stoudemire A (ed). Clinical psychiatry for medical students (3rd ed). Philadelphia: J.B. Lippincott Co., 1998;783-818.
13. Gillin JC. The long and short of sleeping pills. N Engl J Med 1991;324:1735-7.
14. Corser B, Mayleben D, Doghramji K, et al. No next-day residual sedation four hours after middle-of-the-night treatment with zaleplon. Sleep 2000;23 (S2):A309.-
15. Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865-89.
16. Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psych 2001;62:453-63.
17. Walsh JK, Erman M, Erwin CE, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol 1998;13(3):191-8.
18. Hohagen F, Monero RF, Weiss E, et al. Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci 1994;244(2):65-72.
19. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry 1999;60(suppl 17):28-31.
20. Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep 1998;21:178-86.
21. Gengo F, Gabos C, Miller JK. The pharmacodynamics of diphenhydramine-induced drowsiness and changes in mental performance. Clin Pharmacol Ther 1989;45:15-21.
Careful investigation can often reveal insomnia’s cause1—whether a psychiatric or medical condition or poor sleep habits. Understanding why patients can’t sleep is key to effective therapy.
Acute and chronic sleep deprivation is associated with measurable declines in daytime performance (Box). Some data even suggest that long-term sleeplessness increases the risk of new psychiatric disorders—most notably major depression.3
PSYCHIATRIC DISORDERS AND INSOMNIA
Depression. Many depressed persons—up to 80%—experience insomnia, although no one sleep pattern seems typical.2 Depression may be associated with:
- difficulties in falling asleep
- interrupted nocturnal sleep
- and early morning awakening.
Anxiety disorders. Generalized anxiety disorder (GAD), social phobia, panic attacks, and posttraumatic stress disorder (PTSD) are all associated with disrupted sleep. Patients with GAD experience prolonged sleep latency (time needed to fall asleep after lights out) and fragmented sleep, similar to those with primary insomnia.
One-half of adult Americans experience insomnia during their lives, and 10% report persistent sleep difficulties (longer than 2 weeks). Individuals who complain of insomnia report:
- daytime drowsiness
- diminished memory and concentration
- depression
- strained relationships
- increased risk of accidents
- impaired job performance.
Despite these complaints, a surprising 70% of those with insomnia never seek medical help. Only 6% visit their physicians specifically for insomnia, and 24% address sleep difficulty as a secondary complaint. Many (40%) self-medicate with over-the-counter sleep aids or alcohol.2
Insomnia becomes more frequent with aging, associated with increased rates of medical and psychiatric illness and an age-related deterioration in the brain’s sleep-generating processes.3
Subjective sleep quality may be impaired in patients with social phobia. Some patients experience panic symptoms while sleeping, possibly in association with mild hypercapnia. Patients with sleep panic attacks tend to have earlier onset of panic disorder and a higher likelihood of comorbid mood and other anxiety disorders.4
In patients with PTSD, disturbed sleep continuity and increased REM phasic activity—such as eye movements—are directly correlated with severity of PTSD symptoms. Nightmares and disturbed REM sleep are hypothesized hallmarks of PTSD.5
Schizophrenia. Patients with schizophrenia often have disrupted sleep patterns. These include prolonged sleep latency, fragmented sleep with frequent arousals, decreased slow-wave sleep, variable REM latency, and decreased REM rebound after sleep deprivation. Despite investigations going back to the 1950s, no specific link between REM sleep and psychosis has been found.6 Interestingly, increases in REM sleep time and REM activity have been associated with an increased risk of suicide in patients with schizophrenia.7
Adjustment sleep disorder. Acute emotional stressors—such as bereavement, job loss, or hospitalization—often cause adjustment sleep disorder. Symptoms typically remit soon after the stressors abate, so this transient insomnia usually lasts a few days to a few weeks. Treatment with behavioral therapies and hypnotics8 is warranted if:
- sleepiness and fatigue interfere with daytime functioning
- a pattern of recurring episodes develops.9
Psychophysiologic insomnia. Once initiated—regardless of cause—insomnia may persist well after its precipitating factors resolve. Thus, short-term insomnia may develop into long-term, chronic difficulty with recurring episodes or a constant, daily pattern of insomnia. Sufferers often spend hours in bed awake focused upon—and brooding over—their sleeplessness. which in turn further aggravates their insomnia.
Adjustment sleep disorder and psychophysiologic insomnia are included within DSM-IV’s term “primary insomnia.”
OTHER CAUSES OF INSOMNIA
Medications that may affect sleep quality include antidepressants (Table 1),10,11 antihypertensives, antineoplastic agents, bronchodilators, stimulants, corticosteroids, decongestants, diuretics, histamine-2 receptor blockers, and smoking cessation aids.
Recreational drugs, such as cocaine, often cause insomnia. Hypnotics and anxiolytics can cause insomnia following long-term use and during withdrawal.
Other disorders known to disturb sleep include periodic limb movement disorder (PLMD), restless legs syndrome (RLS), sleep apnea syndrome, disrupted circadian rhythms (as with travel or shift work), cardiopulmonary disorders, chronic pain, diabetes, hyperthyroidism, hot flashes associated with menopause, seizures, dementia, and Parkinson’s disease, to name a few.
WORKUP OF SLEEP COMPLAINTS
Acute. Most short-term insomnias—lasting a few weeks or less—are caused by situational stressors, circadian rhythm alterations, and sleep hygiene violations. A logical initial approach, therefore, is to combine sleep hygiene measures with supportive psychotherapy. Hypnotic agents may be considered for apparent daytime consequences—such as sleepiness and occupational impairment—or if the insomnia seems to be escalating.
Chronic. For longer-term insomnias—lasting more than a few weeks—consider a more thorough evaluation, including medical and psychiatric history, physical examination, and mental status examination. Inquire about cardinal symptoms of disorders associated with insomnia, including:
- snoring or breathing pauses during sleep (sleep apnea syndrome)
- restlessness or twitching in the lower extremities (PLMS/RLS).
Question the bed partner, who may be more aware of such symptoms than the patient. Carefully review sleep patterns on weekdays and weekends, bedtime habits, sleep hygiene habits, and substance and medication use.
Table 1
Antidepressants’ effects on sleep and wakefulness
| Activating agents | Bupropion, protriptyline, most selective serotonin reuptake inhibitors, venlafaxine, monoamine oxidase inhibitors |
| Sedating agents | Amitriptyline, doxepin, trimipramine, nefazodone, trazodone, mirtazapine |
| Neutral agents | Citalopram, escitalopram |
Sleep clinic referrals. Consider an evaluation by a sleep disorders center when:
- the diagnosis remains unclear
- or treatment of the presumed conditions fails after a reasonable time
BEHAVIORAL TREATMENTS
Behavioral treatments—with or without hypnotics—are appropriate for a wide variety of insomnia complaints, including adjustment sleep disorder, psychophysiologic insomnia, and depression. Behavioral measures may take longer to implement than drug therapy, but their effects have been shown to last longer in patients with primary insomnia. In many cases, it may be useful to start with both hypnotic and behavioral treatments and withdraw the hypnotic after behavioral measures take effect.
Sleep hygiene. Many individuals unknowingly engage in habitual behaviors that impair sleep. Those with insomnia, for example, often try to compensate for lost sleep by staying in bed later in the morning or by napping, which further fragment nocturnal sleep. Advise these patients to adhere to a regular awakening time—regardless of how long they slept the night before—and to avoid naps. Other tips for getting a good night’s sleep are outlined in Table 2.12
Caffeine has a plasma half-life of 3 to 7 hours, although individual sensitivity varies widely and caffeine’s erratic absorption can prolong its effects. Advise patients with insomnia to avoid caffeine-containing beverages—including coffee, tea, and soft drinks—after noon.
Relaxation training. Muscle tension can be reduced through electromyography (EMG) biofeedback, abdominal breathing exercises, or progressive muscle relaxation techniques, among others. Relaxation training is usually effective within a few weeks.
Psychotherapy. Cognitive-behavioral therapy can help identify and dispel tension-producing thoughts that are disrupting sleep, such as preoccupation with unpleasant work experiences or school examinations. Reassurance may help patients overcome fears about sleeplessness; suggest that patients deal with anxiety-producing thoughts during therapy sessions and at times other than bedtime.
Insight-oriented psychotherapy may enhance patients’ awareness of psychological conflicts from their past that may be producing anxiety and contributing to sleeplessness.
PRESCRIBING HYPNOTICS
Sedative-hypnotics are indicated primarily for short-term management of insomnia. Most are used prophylactically at bedtime until insomnia dissipates or the physician advises the patient to take a break.
Treatment principles. Because many insomnias are recurrent, prolonged hypnotic treatment given in short bouts is often optimal. Longer treatment—months to years—is not recommended by standard textbooks but is clearly needed by a small number of patients with chronic insomnia. In these cases, carefully monitor for tolerance, as manifested by dosage escalation. Long-term hypnotic treatment is not suitable for patients with drug abuse or dependence histories.
Table 2
How to get a good night’s sleep
|
Although chloral hydrate and barbiturates are effective hypnotics, adverse effects limit their safety and usefulness. Benzodiazepines and more recently introduced agents have milder side effect profiles (Table 3). Choose agents based on the patient’s situation, preferences, and effects of prior trials with similar agents. Guidelines for hypnotics discourage chronic use to minimize abuse, misuse, and habituation (Table 4).
Elimination half-life is the primary pharmacokinetic property that differentiates the hypnotics from each other:13
- longer half-life: flurazepam, quazepam
- intermediate half-life: estazolam, temazepam
- short half-life: triazolam, zolpidem, zaleplon (Table 3).
Table 3
Actions and available doses of common hypnotics
| Class/drug | Onset of action | Half-life (hrs) | Active metabolites | Doses (mg) |
|---|---|---|---|---|
| Benzodiazepines | ||||
| Flurazepam | Rapid | 40 to 250 | Yes | 15, 30 |
| Quazepam | Rapid | 40 to 250 | Yes | 7.5, 15 |
| Estazolam | Rapid | 10 to 24 | Yes | 0.5, 1, 2 |
| Temazepam | Intermediate | 8 to 22 | No | 7.5, 15 |
| Triazolam | Rapid | <6 | No | 0.125, 0.25, 0.5 |
| Imidazopyridine | ||||
| Zolpidem | Rapid | 2.5 | No | 5, 10 |
| Pyrazolopyrimidine | ||||
| Zaleplon | Rapid | 1 | No | 5, 10, 20 |
Whereas benzodiazepines bind to benzodiazepine receptor types 1 and 2, zolpidem and zaleplon (and possibly quazepam) bind selectively to type 1. This selectivity may explain why zolpidem and zaleplon are more easily tolerated.
Hypnotic agents with relatively longer half-lives tend to be associated with greater potential for residual daytime effects such as sedation, motor incoordination, amnesia, and slowed reflexes. These effects may impair performance and increase the risk of auto accidents and injuries, especially hip fractures in the elderly.
Nonbenzodiazepines. Because of its ultra-short half-life, zaleplon is least likely to cause residual daytime effects when administered at bedtime. At 10-mg doses, its side effects seem to last no more than 4 hours following administration. Zaleplon can be safely taken after nocturnal awakenings if the patient remains in bed 4 hours or longer after taking it.14
Some patients feel that taking zaleplon only when needed allows them to use hypnotics more sparingly. On the other hand, zaleplon’s ultrashort half-life makes it less useful for patients who have frequent episodes of sleep-interruption insomnia every night. For them, a longer elimination half-life agent such as zolpidem may be more predictably effective for the entire night.15 Short half-life hypnotics have many advantages, but they do not offer anxiolysis for patients with daytime anxiety, as the longer half-life agents do.
Tolerance and rebound. Tolerance can develop following repeated dosing with benzodiazepines—primarily triazolam—and rebound insomnia can follow abrupt discontinuation. Despite widespread concerns, neither tolerance nor rebound insomnia has been well documented. Nevertheless, both can be minimized by using benzodiazepines at the lowest effective dosages and for brief periods. Gradual tapering when discontinuing the drug can help control rebound.
Tolerance and rebound seem to be less of a concern with the newer hypnotics than with benzodiazepines. In preliminary uncontrolled trials, zolpidem and zaleplon did not show evidence of these problems in 1 year of continued use.
NONHYPNOTIC SLEEP AIDS
Sedating antidepressants. Physicians often prescribe low doses of sedating antidepressants to control insomnia, a practice supported by some controlled clinical trials. For example, polysomnography showed that patients who took doxepin, 25 to 50 mg at bedtime, had enhanced sleep efficiency (ratio of time slept to time spent in bed) yet no change in sleep latency. Liver abnormalities, leukopenia, and thrombopenia developed in a few patients.16 Controlled studies have also shown subjective efficacy of trazodone17 and trimipramine18 in treating insomnia.
Some physicians advocate using the more sedating antidepressants—at dosages needed to treat depression—to control insomnia in depressed patients. Evening dosing can minimize daytime sedation. If you choose an activating antidepressant, the potential side effect of insomnia can be managed by judicious use of hypnotic agents. Little is known about antidepressants’ effects on sleep quality after the first 6 to 8 weeks of treatment.19
Although possibly helpful as sleep aids, antidepressants are also associated with side effects. Trazodone, for example, may cause daytime sedation, orthostatic hypotension, and priapism. As a class, the tricyclics are associated with anticholinergic effects such as dry mouth, urinary flow difficulties, and cardiac dysrhythmias.
Table 4
Guidelines for safe use of hypnotics
|
Alcohol. Patients with insomnia often self-medicate with agents that are not specifically indicated to induce sleep. Alcohol is widely used at bedtime because it enhances sleepiness and induces a more rapid sleep onset.20 Drinking a “nightcap” is a poor choice, however, because alcohol—especially after prolonged use—can impair sleep quality, resulting in daytime somnolence. Alcohol is also associated with rapid development of tolerance.
Patients who use alcohol report unrefreshing and disturbed sleep, with frequent nocturnal awakenings even after prolonged abstinence. Alcohol also can further impair sleep-related respiration in patients with obstructive sleep apnea syndrome.
Antihistamines and over-the-counter products whose main active ingredients are antihistamines—such as doxylamine and diphenhydramine—can cause unpredictable efficacy and side effects such as daytime sedation, confusion, and systemic anticholinergic effects.21
Melatonin is a dietary supplement used in dosages of 0.5 to 3,000 mg. Anecdotal reports indicate it may be efficacious in certain subtypes of insomnia—such as shift work, jetlag, blindness, delayed sleep phase syndrome—and in the elderly. However, melatonin’s efficacy has not been established conclusively and is in doubt. Concerns have been expressed regarding the purity of available preparations and possible coronary artery tissue stimulation, as observed in animal studies of melatonin.
Related resources
- American Academy of Sleep Medicine. Sleep logs, patient education materials. www.aasmnet.org
- American Sleep Apnea Association. www.sleepapnea.org
- National Sleep Foundation. www.sleepfoundation.org
Drug brand names
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Doxepin • Sinequan
- Escitalopram • Lexapro
- Estazolam • Prosom
- Flurazepam • Dalmane
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Protriptyline • Vivactil
- Quazepam • Doral
- Temazepam • Restoril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trimipramine • Surmontil
- Venlafaxine • Effexor
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosure
Dr. Doghramji receives research grant support from Cephalon Inc., GlaxoSmithKline, Merck & Co., and Sanofi-Synthelabo.
Careful investigation can often reveal insomnia’s cause1—whether a psychiatric or medical condition or poor sleep habits. Understanding why patients can’t sleep is key to effective therapy.
Acute and chronic sleep deprivation is associated with measurable declines in daytime performance (Box). Some data even suggest that long-term sleeplessness increases the risk of new psychiatric disorders—most notably major depression.3
PSYCHIATRIC DISORDERS AND INSOMNIA
Depression. Many depressed persons—up to 80%—experience insomnia, although no one sleep pattern seems typical.2 Depression may be associated with:
- difficulties in falling asleep
- interrupted nocturnal sleep
- and early morning awakening.
Anxiety disorders. Generalized anxiety disorder (GAD), social phobia, panic attacks, and posttraumatic stress disorder (PTSD) are all associated with disrupted sleep. Patients with GAD experience prolonged sleep latency (time needed to fall asleep after lights out) and fragmented sleep, similar to those with primary insomnia.
One-half of adult Americans experience insomnia during their lives, and 10% report persistent sleep difficulties (longer than 2 weeks). Individuals who complain of insomnia report:
- daytime drowsiness
- diminished memory and concentration
- depression
- strained relationships
- increased risk of accidents
- impaired job performance.
Despite these complaints, a surprising 70% of those with insomnia never seek medical help. Only 6% visit their physicians specifically for insomnia, and 24% address sleep difficulty as a secondary complaint. Many (40%) self-medicate with over-the-counter sleep aids or alcohol.2
Insomnia becomes more frequent with aging, associated with increased rates of medical and psychiatric illness and an age-related deterioration in the brain’s sleep-generating processes.3
Subjective sleep quality may be impaired in patients with social phobia. Some patients experience panic symptoms while sleeping, possibly in association with mild hypercapnia. Patients with sleep panic attacks tend to have earlier onset of panic disorder and a higher likelihood of comorbid mood and other anxiety disorders.4
In patients with PTSD, disturbed sleep continuity and increased REM phasic activity—such as eye movements—are directly correlated with severity of PTSD symptoms. Nightmares and disturbed REM sleep are hypothesized hallmarks of PTSD.5
Schizophrenia. Patients with schizophrenia often have disrupted sleep patterns. These include prolonged sleep latency, fragmented sleep with frequent arousals, decreased slow-wave sleep, variable REM latency, and decreased REM rebound after sleep deprivation. Despite investigations going back to the 1950s, no specific link between REM sleep and psychosis has been found.6 Interestingly, increases in REM sleep time and REM activity have been associated with an increased risk of suicide in patients with schizophrenia.7
Adjustment sleep disorder. Acute emotional stressors—such as bereavement, job loss, or hospitalization—often cause adjustment sleep disorder. Symptoms typically remit soon after the stressors abate, so this transient insomnia usually lasts a few days to a few weeks. Treatment with behavioral therapies and hypnotics8 is warranted if:
- sleepiness and fatigue interfere with daytime functioning
- a pattern of recurring episodes develops.9
Psychophysiologic insomnia. Once initiated—regardless of cause—insomnia may persist well after its precipitating factors resolve. Thus, short-term insomnia may develop into long-term, chronic difficulty with recurring episodes or a constant, daily pattern of insomnia. Sufferers often spend hours in bed awake focused upon—and brooding over—their sleeplessness. which in turn further aggravates their insomnia.
Adjustment sleep disorder and psychophysiologic insomnia are included within DSM-IV’s term “primary insomnia.”
OTHER CAUSES OF INSOMNIA
Medications that may affect sleep quality include antidepressants (Table 1),10,11 antihypertensives, antineoplastic agents, bronchodilators, stimulants, corticosteroids, decongestants, diuretics, histamine-2 receptor blockers, and smoking cessation aids.
Recreational drugs, such as cocaine, often cause insomnia. Hypnotics and anxiolytics can cause insomnia following long-term use and during withdrawal.
Other disorders known to disturb sleep include periodic limb movement disorder (PLMD), restless legs syndrome (RLS), sleep apnea syndrome, disrupted circadian rhythms (as with travel or shift work), cardiopulmonary disorders, chronic pain, diabetes, hyperthyroidism, hot flashes associated with menopause, seizures, dementia, and Parkinson’s disease, to name a few.
WORKUP OF SLEEP COMPLAINTS
Acute. Most short-term insomnias—lasting a few weeks or less—are caused by situational stressors, circadian rhythm alterations, and sleep hygiene violations. A logical initial approach, therefore, is to combine sleep hygiene measures with supportive psychotherapy. Hypnotic agents may be considered for apparent daytime consequences—such as sleepiness and occupational impairment—or if the insomnia seems to be escalating.
Chronic. For longer-term insomnias—lasting more than a few weeks—consider a more thorough evaluation, including medical and psychiatric history, physical examination, and mental status examination. Inquire about cardinal symptoms of disorders associated with insomnia, including:
- snoring or breathing pauses during sleep (sleep apnea syndrome)
- restlessness or twitching in the lower extremities (PLMS/RLS).
Question the bed partner, who may be more aware of such symptoms than the patient. Carefully review sleep patterns on weekdays and weekends, bedtime habits, sleep hygiene habits, and substance and medication use.
Table 1
Antidepressants’ effects on sleep and wakefulness
| Activating agents | Bupropion, protriptyline, most selective serotonin reuptake inhibitors, venlafaxine, monoamine oxidase inhibitors |
| Sedating agents | Amitriptyline, doxepin, trimipramine, nefazodone, trazodone, mirtazapine |
| Neutral agents | Citalopram, escitalopram |
Sleep clinic referrals. Consider an evaluation by a sleep disorders center when:
- the diagnosis remains unclear
- or treatment of the presumed conditions fails after a reasonable time
BEHAVIORAL TREATMENTS
Behavioral treatments—with or without hypnotics—are appropriate for a wide variety of insomnia complaints, including adjustment sleep disorder, psychophysiologic insomnia, and depression. Behavioral measures may take longer to implement than drug therapy, but their effects have been shown to last longer in patients with primary insomnia. In many cases, it may be useful to start with both hypnotic and behavioral treatments and withdraw the hypnotic after behavioral measures take effect.
Sleep hygiene. Many individuals unknowingly engage in habitual behaviors that impair sleep. Those with insomnia, for example, often try to compensate for lost sleep by staying in bed later in the morning or by napping, which further fragment nocturnal sleep. Advise these patients to adhere to a regular awakening time—regardless of how long they slept the night before—and to avoid naps. Other tips for getting a good night’s sleep are outlined in Table 2.12
Caffeine has a plasma half-life of 3 to 7 hours, although individual sensitivity varies widely and caffeine’s erratic absorption can prolong its effects. Advise patients with insomnia to avoid caffeine-containing beverages—including coffee, tea, and soft drinks—after noon.
Relaxation training. Muscle tension can be reduced through electromyography (EMG) biofeedback, abdominal breathing exercises, or progressive muscle relaxation techniques, among others. Relaxation training is usually effective within a few weeks.
Psychotherapy. Cognitive-behavioral therapy can help identify and dispel tension-producing thoughts that are disrupting sleep, such as preoccupation with unpleasant work experiences or school examinations. Reassurance may help patients overcome fears about sleeplessness; suggest that patients deal with anxiety-producing thoughts during therapy sessions and at times other than bedtime.
Insight-oriented psychotherapy may enhance patients’ awareness of psychological conflicts from their past that may be producing anxiety and contributing to sleeplessness.
PRESCRIBING HYPNOTICS
Sedative-hypnotics are indicated primarily for short-term management of insomnia. Most are used prophylactically at bedtime until insomnia dissipates or the physician advises the patient to take a break.
Treatment principles. Because many insomnias are recurrent, prolonged hypnotic treatment given in short bouts is often optimal. Longer treatment—months to years—is not recommended by standard textbooks but is clearly needed by a small number of patients with chronic insomnia. In these cases, carefully monitor for tolerance, as manifested by dosage escalation. Long-term hypnotic treatment is not suitable for patients with drug abuse or dependence histories.
Table 2
How to get a good night’s sleep
|
Although chloral hydrate and barbiturates are effective hypnotics, adverse effects limit their safety and usefulness. Benzodiazepines and more recently introduced agents have milder side effect profiles (Table 3). Choose agents based on the patient’s situation, preferences, and effects of prior trials with similar agents. Guidelines for hypnotics discourage chronic use to minimize abuse, misuse, and habituation (Table 4).
Elimination half-life is the primary pharmacokinetic property that differentiates the hypnotics from each other:13
- longer half-life: flurazepam, quazepam
- intermediate half-life: estazolam, temazepam
- short half-life: triazolam, zolpidem, zaleplon (Table 3).
Table 3
Actions and available doses of common hypnotics
| Class/drug | Onset of action | Half-life (hrs) | Active metabolites | Doses (mg) |
|---|---|---|---|---|
| Benzodiazepines | ||||
| Flurazepam | Rapid | 40 to 250 | Yes | 15, 30 |
| Quazepam | Rapid | 40 to 250 | Yes | 7.5, 15 |
| Estazolam | Rapid | 10 to 24 | Yes | 0.5, 1, 2 |
| Temazepam | Intermediate | 8 to 22 | No | 7.5, 15 |
| Triazolam | Rapid | <6 | No | 0.125, 0.25, 0.5 |
| Imidazopyridine | ||||
| Zolpidem | Rapid | 2.5 | No | 5, 10 |
| Pyrazolopyrimidine | ||||
| Zaleplon | Rapid | 1 | No | 5, 10, 20 |
Whereas benzodiazepines bind to benzodiazepine receptor types 1 and 2, zolpidem and zaleplon (and possibly quazepam) bind selectively to type 1. This selectivity may explain why zolpidem and zaleplon are more easily tolerated.
Hypnotic agents with relatively longer half-lives tend to be associated with greater potential for residual daytime effects such as sedation, motor incoordination, amnesia, and slowed reflexes. These effects may impair performance and increase the risk of auto accidents and injuries, especially hip fractures in the elderly.
Nonbenzodiazepines. Because of its ultra-short half-life, zaleplon is least likely to cause residual daytime effects when administered at bedtime. At 10-mg doses, its side effects seem to last no more than 4 hours following administration. Zaleplon can be safely taken after nocturnal awakenings if the patient remains in bed 4 hours or longer after taking it.14
Some patients feel that taking zaleplon only when needed allows them to use hypnotics more sparingly. On the other hand, zaleplon’s ultrashort half-life makes it less useful for patients who have frequent episodes of sleep-interruption insomnia every night. For them, a longer elimination half-life agent such as zolpidem may be more predictably effective for the entire night.15 Short half-life hypnotics have many advantages, but they do not offer anxiolysis for patients with daytime anxiety, as the longer half-life agents do.
Tolerance and rebound. Tolerance can develop following repeated dosing with benzodiazepines—primarily triazolam—and rebound insomnia can follow abrupt discontinuation. Despite widespread concerns, neither tolerance nor rebound insomnia has been well documented. Nevertheless, both can be minimized by using benzodiazepines at the lowest effective dosages and for brief periods. Gradual tapering when discontinuing the drug can help control rebound.
Tolerance and rebound seem to be less of a concern with the newer hypnotics than with benzodiazepines. In preliminary uncontrolled trials, zolpidem and zaleplon did not show evidence of these problems in 1 year of continued use.
NONHYPNOTIC SLEEP AIDS
Sedating antidepressants. Physicians often prescribe low doses of sedating antidepressants to control insomnia, a practice supported by some controlled clinical trials. For example, polysomnography showed that patients who took doxepin, 25 to 50 mg at bedtime, had enhanced sleep efficiency (ratio of time slept to time spent in bed) yet no change in sleep latency. Liver abnormalities, leukopenia, and thrombopenia developed in a few patients.16 Controlled studies have also shown subjective efficacy of trazodone17 and trimipramine18 in treating insomnia.
Some physicians advocate using the more sedating antidepressants—at dosages needed to treat depression—to control insomnia in depressed patients. Evening dosing can minimize daytime sedation. If you choose an activating antidepressant, the potential side effect of insomnia can be managed by judicious use of hypnotic agents. Little is known about antidepressants’ effects on sleep quality after the first 6 to 8 weeks of treatment.19
Although possibly helpful as sleep aids, antidepressants are also associated with side effects. Trazodone, for example, may cause daytime sedation, orthostatic hypotension, and priapism. As a class, the tricyclics are associated with anticholinergic effects such as dry mouth, urinary flow difficulties, and cardiac dysrhythmias.
Table 4
Guidelines for safe use of hypnotics
|
Alcohol. Patients with insomnia often self-medicate with agents that are not specifically indicated to induce sleep. Alcohol is widely used at bedtime because it enhances sleepiness and induces a more rapid sleep onset.20 Drinking a “nightcap” is a poor choice, however, because alcohol—especially after prolonged use—can impair sleep quality, resulting in daytime somnolence. Alcohol is also associated with rapid development of tolerance.
Patients who use alcohol report unrefreshing and disturbed sleep, with frequent nocturnal awakenings even after prolonged abstinence. Alcohol also can further impair sleep-related respiration in patients with obstructive sleep apnea syndrome.
Antihistamines and over-the-counter products whose main active ingredients are antihistamines—such as doxylamine and diphenhydramine—can cause unpredictable efficacy and side effects such as daytime sedation, confusion, and systemic anticholinergic effects.21
Melatonin is a dietary supplement used in dosages of 0.5 to 3,000 mg. Anecdotal reports indicate it may be efficacious in certain subtypes of insomnia—such as shift work, jetlag, blindness, delayed sleep phase syndrome—and in the elderly. However, melatonin’s efficacy has not been established conclusively and is in doubt. Concerns have been expressed regarding the purity of available preparations and possible coronary artery tissue stimulation, as observed in animal studies of melatonin.
Related resources
- American Academy of Sleep Medicine. Sleep logs, patient education materials. www.aasmnet.org
- American Sleep Apnea Association. www.sleepapnea.org
- National Sleep Foundation. www.sleepfoundation.org
Drug brand names
- Amitriptyline • Elavil
- Bupropion • Wellbutrin
- Citalopram • Celexa
- Doxepin • Sinequan
- Escitalopram • Lexapro
- Estazolam • Prosom
- Flurazepam • Dalmane
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Protriptyline • Vivactil
- Quazepam • Doral
- Temazepam • Restoril
- Trazodone • Desyrel
- Triazolam • Halcion
- Trimipramine • Surmontil
- Venlafaxine • Effexor
- Zaleplon • Sonata
- Zolpidem • Ambien
Disclosure
Dr. Doghramji receives research grant support from Cephalon Inc., GlaxoSmithKline, Merck & Co., and Sanofi-Synthelabo.
1. Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. Sleep 2000;23:243-81.
2. Reynolds CF III, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep 1987;10:199-215.
3. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. JAMA 1989;262:1479-84.
4. Labbate LA, Pollack MH, Otto MW, et al. Sleep panic attacks: an association with childhood anxiety and adult psychopathology. Biol Psychiatry 1994;43:840-2.
5. Ross RJ, Ball WA, Sullivan KA, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989;146:697-707.
6. Neylan TC, Reynolds CF III, Kupfer DJ. Sleep disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry(4th ed). Washington, DC: American Psychiatric Publishing, 2003;978-90.
7. Lewis CF, Tandon R, Shipley JE, et al. Biological predictors of suicidality in schizophrenia. Acta Psychiatr Scand 1996;94:416-20.
8. Spielman AJ, Glovinsky P. The varied nature of insomnia. In: Hauri P (ed). Case studies in insomnia. New York: Plenum Press, 1991;1-15.
9. American Sleep Disorders Association International classification of sleep disorders (rev). Diagnostic and coding manual. Rochester: American Sleep Disorders Association, 1997.
10. Winokur A, Reynolds CF. The effects of antidepressants on sleep physiology. Primary Psychiatry 1994;6:22-7.
11. Gillin JC, Rapaport M, Erman MK, Winokur A, Albala BJ. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. J Clin Psychiatry 1997;58:185-92.
12. Doghramji K. The evaluation and management of sleep disorders. In: Stoudemire A (ed). Clinical psychiatry for medical students (3rd ed). Philadelphia: J.B. Lippincott Co., 1998;783-818.
13. Gillin JC. The long and short of sleeping pills. N Engl J Med 1991;324:1735-7.
14. Corser B, Mayleben D, Doghramji K, et al. No next-day residual sedation four hours after middle-of-the-night treatment with zaleplon. Sleep 2000;23 (S2):A309.-
15. Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865-89.
16. Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psych 2001;62:453-63.
17. Walsh JK, Erman M, Erwin CE, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol 1998;13(3):191-8.
18. Hohagen F, Monero RF, Weiss E, et al. Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci 1994;244(2):65-72.
19. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry 1999;60(suppl 17):28-31.
20. Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep 1998;21:178-86.
21. Gengo F, Gabos C, Miller JK. The pharmacodynamics of diphenhydramine-induced drowsiness and changes in mental performance. Clin Pharmacol Ther 1989;45:15-21.
1. Sateia MJ, Doghramji K, Hauri PJ, Morin CM. Evaluation of chronic insomnia. Sleep 2000;23:243-81.
2. Reynolds CF III, Kupfer DJ. Sleep research in affective illness: state of the art circa 1987. Sleep 1987;10:199-215.
3. Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. JAMA 1989;262:1479-84.
4. Labbate LA, Pollack MH, Otto MW, et al. Sleep panic attacks: an association with childhood anxiety and adult psychopathology. Biol Psychiatry 1994;43:840-2.
5. Ross RJ, Ball WA, Sullivan KA, et al. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989;146:697-707.
6. Neylan TC, Reynolds CF III, Kupfer DJ. Sleep disorders. In: Hales RE, Yudofsky SC (eds). Textbook of clinical psychiatry(4th ed). Washington, DC: American Psychiatric Publishing, 2003;978-90.
7. Lewis CF, Tandon R, Shipley JE, et al. Biological predictors of suicidality in schizophrenia. Acta Psychiatr Scand 1996;94:416-20.
8. Spielman AJ, Glovinsky P. The varied nature of insomnia. In: Hauri P (ed). Case studies in insomnia. New York: Plenum Press, 1991;1-15.
9. American Sleep Disorders Association International classification of sleep disorders (rev). Diagnostic and coding manual. Rochester: American Sleep Disorders Association, 1997.
10. Winokur A, Reynolds CF. The effects of antidepressants on sleep physiology. Primary Psychiatry 1994;6:22-7.
11. Gillin JC, Rapaport M, Erman MK, Winokur A, Albala BJ. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. J Clin Psychiatry 1997;58:185-92.
12. Doghramji K. The evaluation and management of sleep disorders. In: Stoudemire A (ed). Clinical psychiatry for medical students (3rd ed). Philadelphia: J.B. Lippincott Co., 1998;783-818.
13. Gillin JC. The long and short of sleeping pills. N Engl J Med 1991;324:1735-7.
14. Corser B, Mayleben D, Doghramji K, et al. No next-day residual sedation four hours after middle-of-the-night treatment with zaleplon. Sleep 2000;23 (S2):A309.-
15. Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacy and tolerability in the treatment of insomnia. Drugs 2000;59:865-89.
16. Hajak G, Rodenbeck A, Voderholzer U, et al. Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psych 2001;62:453-63.
17. Walsh JK, Erman M, Erwin CE, et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol 1998;13(3):191-8.
18. Hohagen F, Monero RF, Weiss E, et al. Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci 1994;244(2):65-72.
19. Thase ME. Antidepressant treatment of the depressed patient with insomnia. J Clin Psychiatry 1999;60(suppl 17):28-31.
20. Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep 1998;21:178-86.
21. Gengo F, Gabos C, Miller JK. The pharmacodynamics of diphenhydramine-induced drowsiness and changes in mental performance. Clin Pharmacol Ther 1989;45:15-21.
Birds, butterflies and bullfrogs: When patients ‘see things’
HISTORY: A sudden vision
Ms. K, 73, was in reasonably good health when one day she suddenly noticed red, green, and yellow birds and butterflies covering her wall.
Ms. K, who lives alone, was frightened at first, but she did not immediately alert anyone because she thought she “was just seeing things, and they’ll go away.”
Instead, she saw more visions over the next 3 months. She once “watched” as two doctors and a nun carried a middle-aged burn victim into her apartment. She remembers seeing the doctors put a “patch” over the woman’s body. To Ms. K, this experience seemed so shockingly real that she called 911, reporting, “That woman should have been in the hospital!”
She reports that a pack of butterflies once “followed” her to the market. She vividly recalls how they crawled about her shoes and legs as she entered the store. When asked if anyone noticed her insect-covered extremities, she replied matter-of-factly, “Maybe it’s not for them to see, maybe it’s just for me,” as if her hallucinations were a divine gift.
Ms. K’s hallucinations usually occur at home, where she spends most of her time. She says that the images are fleeting, lasting from a few seconds to several minutes, and that the creatures fly silently around her room.
Ms. K’s daughter grew concerned that the hallucinations were increasingly diminishing her mother’s ability to care for herself. She brought Ms. K into our emergency department, from which the patient was admitted.
On admission, Ms. K said she had lost 20 lbs within 6 months, and that “concentrating on those things in the house” was impairing her sleep. She denied recent illness, trauma, loss of conscious ness, changes in medications, seizures, drug or alcohol use, suicidal or homicidal ideation, or specific stress in her life. She added that she often cooks for herself—only to lose her appetite after seeing bugs and other creatures crawl into her food.
Her medical history includes hypertension, type 2 diabetes mellitus, peripheral vascular disease, urinary incontinence, gastroesophageal reflux, glaucoma in her left eye, and bilateral cataracts. She denies any psychiatric history and adds that she had never experienced hallucinations until about 3 months before hospitalization. She also denies any history of auditory, tactile, or olfactory hallucinations.
Would you suspect a primary psychotic illness? What clinical tests might help us understand Ms. K’s progressively debilitating visual hallucinations?
The authors’ observations
Ms. K’s case places us at the crossroads of psychiatric disturbances and medical conditions that can present as or precipitate apparent psychiatric symptoms. Delirium, dementia, psychosis, endocrinopathies, encephalitis, electrolyte disturbances, drug abuse/withdrawal, and occipital or temporal lobe seizures are all possible differential diagnoses (Table 1).
A cognitive function screening and a battery of laboratory tests, imaging scans, and neurologic and vision exams are needed to uncover the cause of her hallucinations.
EVALUATION: Looking for clues
Ms. K’s left pupil was fixed at 6 mm and did not respond to light, while the right pupil was regular and reactive to light at 3 mm. Using a Snellen eye chart, her visual acuity was poor: 20/100 to 20/200 in her right eye and less than 20/200 in the left eye. She scored a 29 out of 30 on the Folstein Mini-Mental State Examination (MMSE), indicating her cognition was intact. The remainder of the neurologic exam was unremarkable.
At admission, Ms. K’s medications included metoprolol, 100 mg qd, for hypertension; lansoprazole, 30 mg qd, for gastroesophageal reflux; tolterodine, 2 mg bid, and oxybutynin, 10 mg qd, for urinary incontinence; repaglinide, 2 mg bid, for type 2 diabetes; and three ophthalmic agents: brimonidine, prednisolone, and dorzolamide/timolol. The patient had been maintained on these medications for more than 2 years with no recent changes in dosing.
Results of Ms. K’s lab studies were normal, including a basic metabolic panel, CBC, liver function tests, urinalysis, B12, thyroid panel, rapid plasma reagin test, and urine drug screen.
A head CT without contrast revealed chronic small-vessel ischemic white matter disease and a chronic infarct of the left cerebellar hemisphere. No acute intracranial hemorrhages, masses, or other abnormalities were noted. No seizures were seen on EEG.
Table 1
Common causes of visual hallucinations
| Schizophrenia |
| Delirium |
| Dementias |
| Substance-induced psychosis |
| Electrolyte disturbances |
| Occipital and temporal lobe epilepsy |
| Charles Bonnet syndrome |
What do the laboratory and imaging tests reveal about Ms. K’s hallucinations? Is her diagnosis delirium? Alzheimer’s or other type of dementia? Schizophrenia?
The authors’ observations
Visual hallucinations—often of deceased parents or siblings, unknown intruders, and animals—can occur in up to 25% of patients with Alzheimer’s-type dementia.1 Also, patients with Lewy body dementia often present with well-formed visual hallucinations, which are thought to result from temporal lobe involvement by the characteristic Lewy bodies.
To diagnose dementia, DSM-IV requires the presence of multiple cognitive deficits manifested by memory impairment and one or more of the following:
- aphasia
- apraxia
- agnosia
- disturbance of executive functioning.2
Ms. K exhibited none of these characteristics, and she retained full executive function—she could balance her checkbook, buy groceries, and cook for herself. Also, her MMSE score was high.
Ms. K showed no consciousness fluctuations or attention deficits, two features commonly seen in delirium. She was alert and oriented throughout the interview, and her flow of thought, speech, language, and attention were appropriate. Therefore, delirium can be reasonably excluded.
The hallucinations probably do not signal onset of schizophrenia because of Ms. K’s age at presentation, lack of family history of psychotic disorder, and paucity of negative symptoms. Auditory hallucinations are much more common in psychosis, and isolated visual hallucinations rarely occur in schizophrenia.
Finally, Ms. K’s electrophysiologic, laboratory, and imaging studies revealed isolated systolic hypertension, low visual acuity, and a mild gait disturbance. Severe left lens opacification accounted for the patient’s discordant pupillary light reflex. None of these findings explained her visual hallucinations, however.
Is a non-psychiatric disorder causing Ms. K’s hallucinations? What type of medication might alleviate her symptoms?
The authors’ observations
Given Ms. K’s strong cognitive function and poor visual acuity, we concluded that her hallucinations may fit the criteria for Charles Bonnet syndrome (CBS), a poorly understood medical phenomenon.
CBS is characterized by complex visual hallucinations in visually impaired elderly patients without cognitive deficits (Table 2).3,4 Swiss philosopher Charles Bonnet first described the disorder in 1760 to explain the vivid visual hallucinations of his 89-year-old grandfather, who had severe cataracts but no cognitive deficits.3 Bonnet’s grandfather claimed to have visions of men, women, birds, buildings, and tapestries.3
CBS is increasingly recognized and reported, but the medical community has never formed a universally accepted definition for this phenomenon. Persons with CBS react positively or negatively to their hallucinations, and the images may stimulate anxiety, anger, or mild paranoia. Research has focused on prevalence, risk indicators, and treatment.
Table 2
Charles Bonnet syndrome: fast facts
|
Teunisse et al determined that visual hallucinations plague up to 14% of sight-impaired persons.4,5 The hallucinations vary widely: people, animals, flowers, vehicles, buildings, and sometimes complete scenes.4,5 Significant risk factors for CBS include advanced age and low visual acuity.4,5 Loneliness, introversion, and shyness are additional risk indicators in older, visually handicapped persons.6 Therefore, social isolation may be a predisposing factor.
Drug treatment of visual hallucinations in CBS currently includes antipsychotics, such as quetiapine (25 to 100 mg/d) and risperidone (0.25 to 1.0 mg/d).7 However, mixed results have been reported after use of antipsychotics in CBS; one patient’s visual hallucinations were exacerbated after risperidone was initiated.8 Case reports have also described the use of valproate, carbamazepine, and ondansetron in CBS.9-11
Empathy and patient education are the cornerstones of CBS treatment.3 Patients need to be reassured that their visions are benign. For many, simply increasing the amount of ambient light in the home can reduce hallucinations.
TREATMENT A frog in the toilet
Ms. K was started on quetiapine, 25 mg bid, to try to promote restorative sleep and resolve her hallucinations. Up to 18% of persons treated with quetiapine report somnolence as an adverse effect, vs. 3 to 8% of those treated with risperidone.12
During her hospital stay, Ms. K experienced no visual hallucinations during the day but reported seeing a grayish-brown bullfrog in the toilet at night. This hallucination did not frighten her; she would simply close the bathroom door and wait until the bullfrog “disappeared.”
Her sleep improved, as did her appetite. She participated in daily group sessions and socialized with other patients.
After 12 days, Ms. K was discharged. To decrease her social isolation, we encouraged her to participate in a day program for seniors. We also continued her on quetiapine, 25 mg bid.
Five months later, her primary care physician reports that Ms. K remains symptom free while maintaining her quetiapine dosage.
Related resources
- Royal National Institute of the Blind: Fact sheet for Charles Bonnet syndrome. Available at: http://www.rnib.org.uk/info/cbsfin.htm
- Verstraten PFJ. The Charles Bonnet syndrome: Development of a protocol for clinical practice in a multidisciplinary approach from assessment to intervention. Available at: http://www.rehab-syn.enter.iris.se/kc-syn/cb.htm
- Adamczyk DT. Optometric educators. Am I seeing things? Optometry Today June 18, 1999:37-9. Available at: http://www.optometry.co.uk/articles/19990618/Adamczyk.pdf
Drug brand names
- Brimonidine Ophthalmic • Alphagan
- Carbamazepine • Tegretol
- Dorzolamide/Timolol • Cosopt
- Lansoprazole • Prevacid
- Metoprolol • Toprol XL
- Ondansetron • Zofran
- Oxybutynin • Ditropan XL
- Quetiapine • Seroquel
- Repaglinide • Prandin
- Risperidone • Risperdal
- Tolterodine • Detrol
- Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with competing manufacturers.
1. Geldmacher DS, Whitehouse PJ. Current concepts: evaluation of dementia. JAMA 1996;335:330-6.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, rev). Washington, DC: American Psychiatric Press, 2000.
3. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis 1997;185:195-200.
4. Teunisse RJ, Cruysberg , JR, Hoefnagels WH, et al. Risk indicators for the Charles Bonnet syndrome. J Nerv Ment Dis 1998;186:190-2.
5. Teunisse RJ, Cruysberg J, Verbeek A, Zitman FG. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijme. Br J Psychiatry 1995;166(2):254-7.
6. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry 1999;40(4):315-19.
7. Rovner BW. The Charles Bonnet syndrome: Visual hallucinations caused by visual impairment. Geriatrics 2002;57:45-6.
8. Kornreich C, Dan B, Verbanck P, Pelc I. Treating Charles Bonnet syndrome: understanding inconsistency. J Clin Psychopharmacol 2000;20(3):396.-
9. Hori H, Terao T, Shiraishi Y, Nakamura J. Treatment of Charles Bonnet syndrome with valproate. Int Clin Psychopharmacol 2000;15:117-19.
10. Batra A, Bartels M, Wormstall H. Therapeutic options in Charles Bonnet syndrome. Acta Psychiatr Scand 1997;96:129-33.
11. Nevins M. Charles Bonnet syndrome. J Am Geriatr Soc 1997;45:894.-
12. Brown C, Markowitz J, Moore T, Parker N. Atypical antipsychotics, part II: adverse effects, drug interactions, and efficacy. Ann Pharmacother 1999;33:210-17.
HISTORY: A sudden vision
Ms. K, 73, was in reasonably good health when one day she suddenly noticed red, green, and yellow birds and butterflies covering her wall.
Ms. K, who lives alone, was frightened at first, but she did not immediately alert anyone because she thought she “was just seeing things, and they’ll go away.”
Instead, she saw more visions over the next 3 months. She once “watched” as two doctors and a nun carried a middle-aged burn victim into her apartment. She remembers seeing the doctors put a “patch” over the woman’s body. To Ms. K, this experience seemed so shockingly real that she called 911, reporting, “That woman should have been in the hospital!”
She reports that a pack of butterflies once “followed” her to the market. She vividly recalls how they crawled about her shoes and legs as she entered the store. When asked if anyone noticed her insect-covered extremities, she replied matter-of-factly, “Maybe it’s not for them to see, maybe it’s just for me,” as if her hallucinations were a divine gift.
Ms. K’s hallucinations usually occur at home, where she spends most of her time. She says that the images are fleeting, lasting from a few seconds to several minutes, and that the creatures fly silently around her room.
Ms. K’s daughter grew concerned that the hallucinations were increasingly diminishing her mother’s ability to care for herself. She brought Ms. K into our emergency department, from which the patient was admitted.
On admission, Ms. K said she had lost 20 lbs within 6 months, and that “concentrating on those things in the house” was impairing her sleep. She denied recent illness, trauma, loss of conscious ness, changes in medications, seizures, drug or alcohol use, suicidal or homicidal ideation, or specific stress in her life. She added that she often cooks for herself—only to lose her appetite after seeing bugs and other creatures crawl into her food.
Her medical history includes hypertension, type 2 diabetes mellitus, peripheral vascular disease, urinary incontinence, gastroesophageal reflux, glaucoma in her left eye, and bilateral cataracts. She denies any psychiatric history and adds that she had never experienced hallucinations until about 3 months before hospitalization. She also denies any history of auditory, tactile, or olfactory hallucinations.
Would you suspect a primary psychotic illness? What clinical tests might help us understand Ms. K’s progressively debilitating visual hallucinations?
The authors’ observations
Ms. K’s case places us at the crossroads of psychiatric disturbances and medical conditions that can present as or precipitate apparent psychiatric symptoms. Delirium, dementia, psychosis, endocrinopathies, encephalitis, electrolyte disturbances, drug abuse/withdrawal, and occipital or temporal lobe seizures are all possible differential diagnoses (Table 1).
A cognitive function screening and a battery of laboratory tests, imaging scans, and neurologic and vision exams are needed to uncover the cause of her hallucinations.
EVALUATION: Looking for clues
Ms. K’s left pupil was fixed at 6 mm and did not respond to light, while the right pupil was regular and reactive to light at 3 mm. Using a Snellen eye chart, her visual acuity was poor: 20/100 to 20/200 in her right eye and less than 20/200 in the left eye. She scored a 29 out of 30 on the Folstein Mini-Mental State Examination (MMSE), indicating her cognition was intact. The remainder of the neurologic exam was unremarkable.
At admission, Ms. K’s medications included metoprolol, 100 mg qd, for hypertension; lansoprazole, 30 mg qd, for gastroesophageal reflux; tolterodine, 2 mg bid, and oxybutynin, 10 mg qd, for urinary incontinence; repaglinide, 2 mg bid, for type 2 diabetes; and three ophthalmic agents: brimonidine, prednisolone, and dorzolamide/timolol. The patient had been maintained on these medications for more than 2 years with no recent changes in dosing.
Results of Ms. K’s lab studies were normal, including a basic metabolic panel, CBC, liver function tests, urinalysis, B12, thyroid panel, rapid plasma reagin test, and urine drug screen.
A head CT without contrast revealed chronic small-vessel ischemic white matter disease and a chronic infarct of the left cerebellar hemisphere. No acute intracranial hemorrhages, masses, or other abnormalities were noted. No seizures were seen on EEG.
Table 1
Common causes of visual hallucinations
| Schizophrenia |
| Delirium |
| Dementias |
| Substance-induced psychosis |
| Electrolyte disturbances |
| Occipital and temporal lobe epilepsy |
| Charles Bonnet syndrome |
What do the laboratory and imaging tests reveal about Ms. K’s hallucinations? Is her diagnosis delirium? Alzheimer’s or other type of dementia? Schizophrenia?
The authors’ observations
Visual hallucinations—often of deceased parents or siblings, unknown intruders, and animals—can occur in up to 25% of patients with Alzheimer’s-type dementia.1 Also, patients with Lewy body dementia often present with well-formed visual hallucinations, which are thought to result from temporal lobe involvement by the characteristic Lewy bodies.
To diagnose dementia, DSM-IV requires the presence of multiple cognitive deficits manifested by memory impairment and one or more of the following:
- aphasia
- apraxia
- agnosia
- disturbance of executive functioning.2
Ms. K exhibited none of these characteristics, and she retained full executive function—she could balance her checkbook, buy groceries, and cook for herself. Also, her MMSE score was high.
Ms. K showed no consciousness fluctuations or attention deficits, two features commonly seen in delirium. She was alert and oriented throughout the interview, and her flow of thought, speech, language, and attention were appropriate. Therefore, delirium can be reasonably excluded.
The hallucinations probably do not signal onset of schizophrenia because of Ms. K’s age at presentation, lack of family history of psychotic disorder, and paucity of negative symptoms. Auditory hallucinations are much more common in psychosis, and isolated visual hallucinations rarely occur in schizophrenia.
Finally, Ms. K’s electrophysiologic, laboratory, and imaging studies revealed isolated systolic hypertension, low visual acuity, and a mild gait disturbance. Severe left lens opacification accounted for the patient’s discordant pupillary light reflex. None of these findings explained her visual hallucinations, however.
Is a non-psychiatric disorder causing Ms. K’s hallucinations? What type of medication might alleviate her symptoms?
The authors’ observations
Given Ms. K’s strong cognitive function and poor visual acuity, we concluded that her hallucinations may fit the criteria for Charles Bonnet syndrome (CBS), a poorly understood medical phenomenon.
CBS is characterized by complex visual hallucinations in visually impaired elderly patients without cognitive deficits (Table 2).3,4 Swiss philosopher Charles Bonnet first described the disorder in 1760 to explain the vivid visual hallucinations of his 89-year-old grandfather, who had severe cataracts but no cognitive deficits.3 Bonnet’s grandfather claimed to have visions of men, women, birds, buildings, and tapestries.3
CBS is increasingly recognized and reported, but the medical community has never formed a universally accepted definition for this phenomenon. Persons with CBS react positively or negatively to their hallucinations, and the images may stimulate anxiety, anger, or mild paranoia. Research has focused on prevalence, risk indicators, and treatment.
Table 2
Charles Bonnet syndrome: fast facts
|
Teunisse et al determined that visual hallucinations plague up to 14% of sight-impaired persons.4,5 The hallucinations vary widely: people, animals, flowers, vehicles, buildings, and sometimes complete scenes.4,5 Significant risk factors for CBS include advanced age and low visual acuity.4,5 Loneliness, introversion, and shyness are additional risk indicators in older, visually handicapped persons.6 Therefore, social isolation may be a predisposing factor.
Drug treatment of visual hallucinations in CBS currently includes antipsychotics, such as quetiapine (25 to 100 mg/d) and risperidone (0.25 to 1.0 mg/d).7 However, mixed results have been reported after use of antipsychotics in CBS; one patient’s visual hallucinations were exacerbated after risperidone was initiated.8 Case reports have also described the use of valproate, carbamazepine, and ondansetron in CBS.9-11
Empathy and patient education are the cornerstones of CBS treatment.3 Patients need to be reassured that their visions are benign. For many, simply increasing the amount of ambient light in the home can reduce hallucinations.
TREATMENT A frog in the toilet
Ms. K was started on quetiapine, 25 mg bid, to try to promote restorative sleep and resolve her hallucinations. Up to 18% of persons treated with quetiapine report somnolence as an adverse effect, vs. 3 to 8% of those treated with risperidone.12
During her hospital stay, Ms. K experienced no visual hallucinations during the day but reported seeing a grayish-brown bullfrog in the toilet at night. This hallucination did not frighten her; she would simply close the bathroom door and wait until the bullfrog “disappeared.”
Her sleep improved, as did her appetite. She participated in daily group sessions and socialized with other patients.
After 12 days, Ms. K was discharged. To decrease her social isolation, we encouraged her to participate in a day program for seniors. We also continued her on quetiapine, 25 mg bid.
Five months later, her primary care physician reports that Ms. K remains symptom free while maintaining her quetiapine dosage.
Related resources
- Royal National Institute of the Blind: Fact sheet for Charles Bonnet syndrome. Available at: http://www.rnib.org.uk/info/cbsfin.htm
- Verstraten PFJ. The Charles Bonnet syndrome: Development of a protocol for clinical practice in a multidisciplinary approach from assessment to intervention. Available at: http://www.rehab-syn.enter.iris.se/kc-syn/cb.htm
- Adamczyk DT. Optometric educators. Am I seeing things? Optometry Today June 18, 1999:37-9. Available at: http://www.optometry.co.uk/articles/19990618/Adamczyk.pdf
Drug brand names
- Brimonidine Ophthalmic • Alphagan
- Carbamazepine • Tegretol
- Dorzolamide/Timolol • Cosopt
- Lansoprazole • Prevacid
- Metoprolol • Toprol XL
- Ondansetron • Zofran
- Oxybutynin • Ditropan XL
- Quetiapine • Seroquel
- Repaglinide • Prandin
- Risperidone • Risperdal
- Tolterodine • Detrol
- Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with competing manufacturers.
HISTORY: A sudden vision
Ms. K, 73, was in reasonably good health when one day she suddenly noticed red, green, and yellow birds and butterflies covering her wall.
Ms. K, who lives alone, was frightened at first, but she did not immediately alert anyone because she thought she “was just seeing things, and they’ll go away.”
Instead, she saw more visions over the next 3 months. She once “watched” as two doctors and a nun carried a middle-aged burn victim into her apartment. She remembers seeing the doctors put a “patch” over the woman’s body. To Ms. K, this experience seemed so shockingly real that she called 911, reporting, “That woman should have been in the hospital!”
She reports that a pack of butterflies once “followed” her to the market. She vividly recalls how they crawled about her shoes and legs as she entered the store. When asked if anyone noticed her insect-covered extremities, she replied matter-of-factly, “Maybe it’s not for them to see, maybe it’s just for me,” as if her hallucinations were a divine gift.
Ms. K’s hallucinations usually occur at home, where she spends most of her time. She says that the images are fleeting, lasting from a few seconds to several minutes, and that the creatures fly silently around her room.
Ms. K’s daughter grew concerned that the hallucinations were increasingly diminishing her mother’s ability to care for herself. She brought Ms. K into our emergency department, from which the patient was admitted.
On admission, Ms. K said she had lost 20 lbs within 6 months, and that “concentrating on those things in the house” was impairing her sleep. She denied recent illness, trauma, loss of conscious ness, changes in medications, seizures, drug or alcohol use, suicidal or homicidal ideation, or specific stress in her life. She added that she often cooks for herself—only to lose her appetite after seeing bugs and other creatures crawl into her food.
Her medical history includes hypertension, type 2 diabetes mellitus, peripheral vascular disease, urinary incontinence, gastroesophageal reflux, glaucoma in her left eye, and bilateral cataracts. She denies any psychiatric history and adds that she had never experienced hallucinations until about 3 months before hospitalization. She also denies any history of auditory, tactile, or olfactory hallucinations.
Would you suspect a primary psychotic illness? What clinical tests might help us understand Ms. K’s progressively debilitating visual hallucinations?
The authors’ observations
Ms. K’s case places us at the crossroads of psychiatric disturbances and medical conditions that can present as or precipitate apparent psychiatric symptoms. Delirium, dementia, psychosis, endocrinopathies, encephalitis, electrolyte disturbances, drug abuse/withdrawal, and occipital or temporal lobe seizures are all possible differential diagnoses (Table 1).
A cognitive function screening and a battery of laboratory tests, imaging scans, and neurologic and vision exams are needed to uncover the cause of her hallucinations.
EVALUATION: Looking for clues
Ms. K’s left pupil was fixed at 6 mm and did not respond to light, while the right pupil was regular and reactive to light at 3 mm. Using a Snellen eye chart, her visual acuity was poor: 20/100 to 20/200 in her right eye and less than 20/200 in the left eye. She scored a 29 out of 30 on the Folstein Mini-Mental State Examination (MMSE), indicating her cognition was intact. The remainder of the neurologic exam was unremarkable.
At admission, Ms. K’s medications included metoprolol, 100 mg qd, for hypertension; lansoprazole, 30 mg qd, for gastroesophageal reflux; tolterodine, 2 mg bid, and oxybutynin, 10 mg qd, for urinary incontinence; repaglinide, 2 mg bid, for type 2 diabetes; and three ophthalmic agents: brimonidine, prednisolone, and dorzolamide/timolol. The patient had been maintained on these medications for more than 2 years with no recent changes in dosing.
Results of Ms. K’s lab studies were normal, including a basic metabolic panel, CBC, liver function tests, urinalysis, B12, thyroid panel, rapid plasma reagin test, and urine drug screen.
A head CT without contrast revealed chronic small-vessel ischemic white matter disease and a chronic infarct of the left cerebellar hemisphere. No acute intracranial hemorrhages, masses, or other abnormalities were noted. No seizures were seen on EEG.
Table 1
Common causes of visual hallucinations
| Schizophrenia |
| Delirium |
| Dementias |
| Substance-induced psychosis |
| Electrolyte disturbances |
| Occipital and temporal lobe epilepsy |
| Charles Bonnet syndrome |
What do the laboratory and imaging tests reveal about Ms. K’s hallucinations? Is her diagnosis delirium? Alzheimer’s or other type of dementia? Schizophrenia?
The authors’ observations
Visual hallucinations—often of deceased parents or siblings, unknown intruders, and animals—can occur in up to 25% of patients with Alzheimer’s-type dementia.1 Also, patients with Lewy body dementia often present with well-formed visual hallucinations, which are thought to result from temporal lobe involvement by the characteristic Lewy bodies.
To diagnose dementia, DSM-IV requires the presence of multiple cognitive deficits manifested by memory impairment and one or more of the following:
- aphasia
- apraxia
- agnosia
- disturbance of executive functioning.2
Ms. K exhibited none of these characteristics, and she retained full executive function—she could balance her checkbook, buy groceries, and cook for herself. Also, her MMSE score was high.
Ms. K showed no consciousness fluctuations or attention deficits, two features commonly seen in delirium. She was alert and oriented throughout the interview, and her flow of thought, speech, language, and attention were appropriate. Therefore, delirium can be reasonably excluded.
The hallucinations probably do not signal onset of schizophrenia because of Ms. K’s age at presentation, lack of family history of psychotic disorder, and paucity of negative symptoms. Auditory hallucinations are much more common in psychosis, and isolated visual hallucinations rarely occur in schizophrenia.
Finally, Ms. K’s electrophysiologic, laboratory, and imaging studies revealed isolated systolic hypertension, low visual acuity, and a mild gait disturbance. Severe left lens opacification accounted for the patient’s discordant pupillary light reflex. None of these findings explained her visual hallucinations, however.
Is a non-psychiatric disorder causing Ms. K’s hallucinations? What type of medication might alleviate her symptoms?
The authors’ observations
Given Ms. K’s strong cognitive function and poor visual acuity, we concluded that her hallucinations may fit the criteria for Charles Bonnet syndrome (CBS), a poorly understood medical phenomenon.
CBS is characterized by complex visual hallucinations in visually impaired elderly patients without cognitive deficits (Table 2).3,4 Swiss philosopher Charles Bonnet first described the disorder in 1760 to explain the vivid visual hallucinations of his 89-year-old grandfather, who had severe cataracts but no cognitive deficits.3 Bonnet’s grandfather claimed to have visions of men, women, birds, buildings, and tapestries.3
CBS is increasingly recognized and reported, but the medical community has never formed a universally accepted definition for this phenomenon. Persons with CBS react positively or negatively to their hallucinations, and the images may stimulate anxiety, anger, or mild paranoia. Research has focused on prevalence, risk indicators, and treatment.
Table 2
Charles Bonnet syndrome: fast facts
|
Teunisse et al determined that visual hallucinations plague up to 14% of sight-impaired persons.4,5 The hallucinations vary widely: people, animals, flowers, vehicles, buildings, and sometimes complete scenes.4,5 Significant risk factors for CBS include advanced age and low visual acuity.4,5 Loneliness, introversion, and shyness are additional risk indicators in older, visually handicapped persons.6 Therefore, social isolation may be a predisposing factor.
Drug treatment of visual hallucinations in CBS currently includes antipsychotics, such as quetiapine (25 to 100 mg/d) and risperidone (0.25 to 1.0 mg/d).7 However, mixed results have been reported after use of antipsychotics in CBS; one patient’s visual hallucinations were exacerbated after risperidone was initiated.8 Case reports have also described the use of valproate, carbamazepine, and ondansetron in CBS.9-11
Empathy and patient education are the cornerstones of CBS treatment.3 Patients need to be reassured that their visions are benign. For many, simply increasing the amount of ambient light in the home can reduce hallucinations.
TREATMENT A frog in the toilet
Ms. K was started on quetiapine, 25 mg bid, to try to promote restorative sleep and resolve her hallucinations. Up to 18% of persons treated with quetiapine report somnolence as an adverse effect, vs. 3 to 8% of those treated with risperidone.12
During her hospital stay, Ms. K experienced no visual hallucinations during the day but reported seeing a grayish-brown bullfrog in the toilet at night. This hallucination did not frighten her; she would simply close the bathroom door and wait until the bullfrog “disappeared.”
Her sleep improved, as did her appetite. She participated in daily group sessions and socialized with other patients.
After 12 days, Ms. K was discharged. To decrease her social isolation, we encouraged her to participate in a day program for seniors. We also continued her on quetiapine, 25 mg bid.
Five months later, her primary care physician reports that Ms. K remains symptom free while maintaining her quetiapine dosage.
Related resources
- Royal National Institute of the Blind: Fact sheet for Charles Bonnet syndrome. Available at: http://www.rnib.org.uk/info/cbsfin.htm
- Verstraten PFJ. The Charles Bonnet syndrome: Development of a protocol for clinical practice in a multidisciplinary approach from assessment to intervention. Available at: http://www.rehab-syn.enter.iris.se/kc-syn/cb.htm
- Adamczyk DT. Optometric educators. Am I seeing things? Optometry Today June 18, 1999:37-9. Available at: http://www.optometry.co.uk/articles/19990618/Adamczyk.pdf
Drug brand names
- Brimonidine Ophthalmic • Alphagan
- Carbamazepine • Tegretol
- Dorzolamide/Timolol • Cosopt
- Lansoprazole • Prevacid
- Metoprolol • Toprol XL
- Ondansetron • Zofran
- Oxybutynin • Ditropan XL
- Quetiapine • Seroquel
- Repaglinide • Prandin
- Risperidone • Risperdal
- Tolterodine • Detrol
- Valproate • Depakote
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with competing manufacturers.
1. Geldmacher DS, Whitehouse PJ. Current concepts: evaluation of dementia. JAMA 1996;335:330-6.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, rev). Washington, DC: American Psychiatric Press, 2000.
3. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis 1997;185:195-200.
4. Teunisse RJ, Cruysberg , JR, Hoefnagels WH, et al. Risk indicators for the Charles Bonnet syndrome. J Nerv Ment Dis 1998;186:190-2.
5. Teunisse RJ, Cruysberg J, Verbeek A, Zitman FG. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijme. Br J Psychiatry 1995;166(2):254-7.
6. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry 1999;40(4):315-19.
7. Rovner BW. The Charles Bonnet syndrome: Visual hallucinations caused by visual impairment. Geriatrics 2002;57:45-6.
8. Kornreich C, Dan B, Verbanck P, Pelc I. Treating Charles Bonnet syndrome: understanding inconsistency. J Clin Psychopharmacol 2000;20(3):396.-
9. Hori H, Terao T, Shiraishi Y, Nakamura J. Treatment of Charles Bonnet syndrome with valproate. Int Clin Psychopharmacol 2000;15:117-19.
10. Batra A, Bartels M, Wormstall H. Therapeutic options in Charles Bonnet syndrome. Acta Psychiatr Scand 1997;96:129-33.
11. Nevins M. Charles Bonnet syndrome. J Am Geriatr Soc 1997;45:894.-
12. Brown C, Markowitz J, Moore T, Parker N. Atypical antipsychotics, part II: adverse effects, drug interactions, and efficacy. Ann Pharmacother 1999;33:210-17.
1. Geldmacher DS, Whitehouse PJ. Current concepts: evaluation of dementia. JAMA 1996;335:330-6.
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (4th ed, rev). Washington, DC: American Psychiatric Press, 2000.
3. Fernandez A, Lichtshein G, Vieweg WV. The Charles Bonnet syndrome: a review. J Nerv Ment Dis 1997;185:195-200.
4. Teunisse RJ, Cruysberg , JR, Hoefnagels WH, et al. Risk indicators for the Charles Bonnet syndrome. J Nerv Ment Dis 1998;186:190-2.
5. Teunisse RJ, Cruysberg J, Verbeek A, Zitman FG. The Charles Bonnet syndrome: a large prospective study in the Netherlands. A study of the prevalence of the Charles Bonnet syndrome and associated factors in 500 patients attending the University Department of Ophthalmology at Nijme. Br J Psychiatry 1995;166(2):254-7.
6. Teunisse RJ, Cruysberg JR, Hoefnagels WH, et al. Social and psychological characteristics of elderly visually handicapped patients with the Charles Bonnet Syndrome. Compr Psychiatry 1999;40(4):315-19.
7. Rovner BW. The Charles Bonnet syndrome: Visual hallucinations caused by visual impairment. Geriatrics 2002;57:45-6.
8. Kornreich C, Dan B, Verbanck P, Pelc I. Treating Charles Bonnet syndrome: understanding inconsistency. J Clin Psychopharmacol 2000;20(3):396.-
9. Hori H, Terao T, Shiraishi Y, Nakamura J. Treatment of Charles Bonnet syndrome with valproate. Int Clin Psychopharmacol 2000;15:117-19.
10. Batra A, Bartels M, Wormstall H. Therapeutic options in Charles Bonnet syndrome. Acta Psychiatr Scand 1997;96:129-33.
11. Nevins M. Charles Bonnet syndrome. J Am Geriatr Soc 1997;45:894.-
12. Brown C, Markowitz J, Moore T, Parker N. Atypical antipsychotics, part II: adverse effects, drug interactions, and efficacy. Ann Pharmacother 1999;33:210-17.
Benzodiazepines for substance abusers
How should you treat anxiety in substance-abusing patients: deny them benzodiazepines and risk under-treatment, or prescribe benzodiazepines for the anxiolytic effect and risk contributing to addiction?
There is no definitive answer, but one thing is clear: Among psychiatric patients, substance abusers are most likely to abuse benzodiazepines and become addicted to them.
Some argue that the abuse potential is overstated, but only limited data suggest that benzodiazepines can be safely prescribed to patients who are abusing alcohol or drugs. In this article, we discuss benzodiazepine use in these patients and offer a sobriety-based treatment approach.
Table 1
Benzodiazepines’ potency and half-lives, including half-lives of active metabolites
| Potency | Shorter half-life (hr) | Longer half-life (hr) |
|---|---|---|
| High | Alprazolam (6 to 12) Lorazepam (10 to 20) Triazolam (2) | Clonazepam (18 to 50) |
| Low | Oxazepam (4 to 15) Temazepam (8 to 22) | Chlordiazepoxide (5 to 30) [36 to 200]* Clorazepate [36 to 200]* Diazepam (20 to 100) [36 to 200]* Flurazepam [40 to 250]* |
| * [active metabolite] | ||
| Source: Ashton CH. Benzodiazepine equivalence table. Available at www.benzo.org.uk | ||
BENEFITS AND RISKS
Considered a safe substitute for barbiturates, benzodiazepines were heralded as wonder drugs when they were introduced in the 1950s. Reports of their addictive potential surfaced in the 1970s, and since then researchers have disagreed on whether benzodiazepines should be prescribed to substance-abusing or -dependent patients.
Clinical utility. Benzodiazepines are used in many clinical situations because of their:
- anxiolytic, hypnotic, anticonvulsant, antipanic, antidepressant, amnestic, anesthetic, and antispastic effects
- relatively mild side effects, when compared with alternatives such as barbiturates.
In psychiatry, benzodiazepines are used to treat anxiety disorders, agitation, and insomnia. Because of cross-tolerance with alcohol and barbiturates, benzodiazepines also are used to manage alcohol or barbiturate withdrawal.
Interactions. Benzodiazepines can interact with other psychotropics, including lithium, antipsychotics, and selective serotonin reuptake inhibitors (SSRIs). Respiratory arrest has been reported in patients taking both a high-potency benzodiazepine and clozapine.1
Overdose and withdrawal symptoms. Benzodiazepine overdose is characterized by slurred speech, sedation, memory impairment, incoordination, respiratory depression, hypotension, stupor, and coma. Abrupt withdrawal may produce life-threatening delirium, hallucinations, grand mal seizures, and symptoms similar to those of alcohol withdrawal (insomnia, anxiety, tremor, hyperactivity, nausea, vomiting, and psychomotor agitation).1
ABUSE PATTERNS
The few empiric studies examining benzodiazepines’ abuse potential in substance abusers have shown inconsistent results. However, it is generally accepted that:
- long-term benzodiazepine use may lead to tolerance and physiologic dependence
- withdrawal symptoms can occur if benzodiazepines are stopped suddenly, especially after long-term (months to years) use.
Even though most benzodiazepine prescriptions are not abused,2 a history of alcohol and drug abuse suggests high potential for benzodiazepine abuse. Also, long-term users of prescribed benzodiazepines often develop tolerance and may escalate their doses to get the same desired effects. If their supply is threatened, these patients may seek benzodiazepines illicitly.
Benzodiazepines may enhance or prolong the elation (“high”) associated with other drugs or mitigate the depression (“crash”) that follows a stimulant “high.” Sometimes benzodiazepines are the drug of choice, as high doses of potent, short-acting agents may provide a stimulant “high.”
WHO ABUSES BENZODIAZEPINES?
Alcohol and substance abusers tend to ingest benzodiazepines for recreational purposes. Thirty to 50% of alcoholics undergoing detoxification and 44% of IV drug abusers also may be abusing benzodiazepines.3
Benzodiazepines are cross-tolerant with alcohol, and alcoholics may use them with alcohol or as a substitute when alcohol is unavailable. They also may self-medicate with benzodiazepines to ease alcohol’s withdrawal symptoms. Opiate, amphetamine, and cocaine abusers may use benzodiazepines with their drugs of choice, as may younger abusers of MDMA (“Ecstasy”) and LSD.
Even patients who begin taking benzodiazepines for legitimate reasons may end up abusing them. In one study of 2,600 patients prescribed diazepam, up to 60% had abused and/or become dependent on it.4
Benzodiazepine abuse may start with other sedative/hypnotic abuse or as experimentation with drugs or alcohol, typically around age 13 or 14.5 The average benzodiazepine abuser is age 19 to 31, and the male-to-female ratio is about 2:1.6
Multi-drug abuse. Benzodiazepines are usually not the preferred or sole drug of abuse. Roughly 80% of benzodiazepine abuse may be a component of poly-drug abuse, most commonly with opioid addiction.7 A 2-year National Institute on Drug Abuse study of heroin abusers suggested that 15% also abused benzodiazepines daily for more than 1 year, and 73% had abused benzodiazepines several times during the previous week.8 Other studies suggest that up to 90% of methadone users regularly abuse benzodiazepines, often at high doses.9
Table 2
Should benzodiazepines be prescribed to substance abusers?
YES: Arguments for
|
NO: Arguments against
|
ILLICIT USE POTENTIAL
Prescriptions are the primary source of supply for benzodiazepine abusers. These patients are doctor shoppers and often change pharmacies. They visit emergency rooms frequently and may feign symptoms to obtain benzodiazepine prescriptions. They fill prescriptions for personal use or sell the drugs to illicit sources to support their addictions.
On the street, brand-name benzodiazepines are worth much more than generics because they can be identified by photographs of brand-name benzodiazepines on the Internet or in reference books. In many cities, the street value of the Xanax or Klonopin brands may be $5 to $10 per pill. A 5mg tablet of Valium-brand diazepam may sell for $5, and 10-mg tablets are worth up to $10.
Higher abuse potential. All benzodiazepines have abuse potential, and most have been reported in the literature as being abused. Those most likely to be abused have a short half-life10 (Table 1) or rapidly cross the blood brain barrier, such as alprazolam.11
Alprazolam and lorazepam are popular among benzodiazepine abusers. In experienced but nondependent users, 1 mg of alprazolam produces a sense of elation and carries an abuse potential similar to that of 10 mg of dextroamphetamine.12 Lipophilic agents such as diazepam also have a high abuse and addiction potential.
In the United States, diazepam and alprazolam appear to be the most abused benzodi-azepines.13 Flunitrazepam has become popular among high school students and drug addicts, particularly in the south and southwest. This potent benzodiazepine is not approved for use in the United States but is diverted from Latin America or Europe in the illegal drug trade.
Lower abuse potential. Benzodiazepines with longer half-lives generally are less likely to be abused, although diazepam—with a half-life of up to 100 hours—is the exception. Chlordiazepoxide has been reported to produce a lower “high” than other benzodiazepines.14 Among the short half-life benzodiazepines, oxazepam may have a relatively low abuse potential.14 Clonazepam—a high-potency benzodiazepine with a long half-life—is generally safe and may have a lower abuse or addiction potential, although its abuse has been report-ed.15 Similarly, oxazepam, clorazepate, and chlordiazepoxide may be less reinforcing than other benzodiazepines, although reports have linked these agents to abuse as well.16
TO PRESCRIBE OR NOT TO PRESCRIBE
Opponents blame benzodiazepines for promoting the drug culture and argue that prescribing benzodiazepines promotes drug abuse. Advocates of benzodiazepine therapy contend that restricting an effective and safe medication is unethical, even in substance abusers. Arguments from each perspective are summarized in Table 2.17-27
In 1990, an American Psychiatric Association task force concluded that alcohol and substance abusers could be prescribed benzodiazepines with very close monitoring but did not recommend specific standards.28
RECOMMENDATIONS
Prescribing benzodiazepines to substance abusers is not absolutely contraindicated, despite an elevated relative risk of abuse or dependence. In the absence of convincing data, physicians must decide on their own—usually case by case—the merits of using benzodiazepines to treat anxiety in substance abusers.
A sobriety-based approach. Our group at the Substance Abuse Treatment Center, VA Medical Center, Omaha, Nebraska, has developed a treatment algorithm for substance abusers presenting with anxiety (see Algorithm). It is based on clinical experience, more than 200 relevant articles, and the consensus of psychiatrists trained and certified by the American Board of Psychiatry and Neurology and the American Society of Addiction Medicine.
Algorithm Sobriety-based protocol for treating anxiety in substance abusers
Precautions for prescribing benzodiazepines
- Inform patient of planned duration of therapy
- Prescribe for brief periods (weeks to months), with follow-up at least monthly
- No refills without follow-up, and no refills over the phone
- Use random urine toxicology screening every 1 to 3 months to monitor for relapse
- Attempt to taper dosage after 3 to 6 months—even if patient resists—and monitor for objective withdrawal
- If no objective withdrawal, terminate benzodiazepine; continue other medications
- If objective withdrawal, continue benzodiazepine and reattempt taper in 3 to 6 months; continue Alcoholics/Narcotics Anonymous
We suggest that you begin by encouraging sobriety and referring willing patients to detoxification. Because most addicts deny or greatly minimize their substance abuse, investigate all potential drug or alcohol abuse thoroughly and address it appropriately.
If anxiety persists after detoxification, begin drug therapy with nonbenzodiazepines:
- Diphenhydramine, 50 to 100 mg/d, may reduce anxiety and often improves sleep, but consider its anticholinergic side effects before prescribing.
- Some SSRIs and venlafaxine are used to treat anxiety, but they generally take weeks to produce a therapeutic effect and some patients cannot wait that long.
- Mirtazapine, 15 to 30 mg/d, provides relatively rapid sedation and helps with sleep and anxiety.
- Buspirone may reduce anxiety, especially when given at 30 to 60 mg/d.
- Gabapentin, 100 to 300 mg tid or higher, may reduce anxiety and help with sleep.
- Tricyclic antidepressants may be considered, but watch for cardiac and anticholinergic side effects and overdose risks.
If anxiety does not improve with an adequate trial of first-line agents, consider adding long-acting benzodiazepines at sufficient dosages, such as clonazepam, 0.5 to 1 mg bid to tid. Prescribe scheduled doses, rather than “as needed.” Continue the first-line antianxiety agent, and reiterate to the patient that benzodiazepine therapy will be short-term.
Observe prescribing precautions (see Algorithm), and screen patients’ urine randomly every 1 to 3 months to monitor their adherence to substance abuse treatment.
Keep patient records current, with attention to dates of visits and prescriptions and quantity of benzodiazepines prescribed. To ensure proper continued use of benzodiazepines, consider consulting with physicians who have expertise in treating similar patients. Watch for possible signs of benzodiazepine dependence and abuse, such as requests for dose increases or early refills.
NONDRUG TREATMENTS
Nondrug treatments have been shown to reduce substance use and control anxiety in some studies. These include cognitive-behavioral therapy, motivational enhancement therapy, interpersonal therapy, and brief dynamic therapy, among others. Their use requires specific training or referral to more experienced colleagues. For information on these treatments, consult the Web sites of the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism (Related resources).
Group and self-help therapies such as Alcoholics Anonymous or Narcotics Anonymous also have been shown to reduce substance use.
- Parran TV. Prescription drug abuse. A question of balance. Med Clin North Am 1997;81:967-78.
- Lader M, Russell J. Guidelines for the prevention and treatment of benzodiazepine dependence: summary of a report from the Mental Health Foundation. Addiction 1993;88(12):1707-8.
- National Institute on Drug Abuse. www.nida.nih.gov
- National Institute on Alcohol Abuse and Addiction. www.niaaa.nih.gov
Drug brand names
- Alprazolam • Xanax
- Buspirone • BuSpar
- Chlordiazepoxide • Librium
- Clonazepam • Klonopin
- Clorazepate • Tranxene
- Diazepam • Valium
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Oxazepam • Serax
- Temazepam • Restoril
- Triazolam • Halcion
- Venlafaxine • Effexor
Disclosure
This work was supported by the Attorney General’s Office, Commonwealth of Massachusetts and the United States Department of Veterans Affairs.
Dr. Sattar has received grant funding from Abbott Laboratories and is a speaker for AstraZeneca and Eli Lilly and Co.
Dr. Bhatia is a speaker for AstraZeneca, Eli Lilly and Co., Janssen Pharmaceutica, and Bristol-Myers Squibb Co.
Acknowledgment
The authors wish to thank Jennifer Hong, second-year medical student, Creighton University School of Medicine, Omaha, NE, for her assistance in preparing this article for publication.
1. Hales RE, Yudofsky SC. Textbook of clinical psychiatry, 4th ed. Washington, DC: American Psychiatric Publishing, 2003:318-19,493,501,1098.
2. Woods JH, Katz JL, Winger G. Use and abuse of benzodiazepines. Issues relevant to prescribing. JAMA 1988;260(23):3476-80.
3. Shaw M, Brabbins C, Ruben S. Misuse of benzodiazepines. Specify the formulation when prescribing. BMJ 1994;308(6945):1709.-
4. Woody GE, O’Brien CP, Greenstein R. Misuse and abuse of diazepam: an increasingly common medical problem. Int J Addict 1975;10(5):843-8.
5. Pedersen W, Lavik NJ. Adolescents and benzodiazepines: prescribed use, self-medication and intoxication. Acta Psychiatrica Scand 1991;84:94-8.
6. Ruben SM, Morrison CL. Temazepam misuse in a group of injecting drug users. Br J Addict 1992;87:1387-92,
7. Gold MS, Miller NS, Stennie K, Populla-Vardi C. Epidemiology of benzodiazepine use and dependence. Psychiatr Annals 1995;25:146-8.
8. Dumont RL. Abuse of benzodiazepines—the problems and the solutions. A report of a Committee of the Institute for Behavior and Health, Inc. Am J Drug Alcohol Abuse. 1988;14(suppl 1):1-69.
9. Iguchi MY, Griffiths RR, Bickel WK, et al. Relative abuse liability of benzodiazepines in methadone-maintained populations in three cities. In: Harris LS (ed). Problems of drug dependence, 1988. Proceedings of the 50th annual scientific meeting, the Committee on Problems of Drug Dependence, Inc. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute on Drug Abuse, Office of Science, 1989. DHHS publication no. (ADM) 89-1605.
10. Longo LP. Non-benzodiazepine pharmacotherapy of anxiety and panic in substance abusing patients. Psychiatr Annals 1998;28(3):142-53.
11. Roache JD, Meisch RA. Findings from self-administration research on the addiction potential of benzodiazepines. Psychiatr Annals 1995;25(3):153-7.
12. Zawertailo LA, Busto U, Kaplan HL, Sellers EM. Comparative abuse liability of sertraline, alprazolam, and dextroamphetamine in humans. J Clin Psychopharmacol 1995;15(2):117-24.
13. Griffiths RR, McLeod DR, Bigelow GE, et al. Comparison of diazepam and oxazepam: preference, liking and extent of abuse. J Pharmacol Exp Ther 1984;229(2):501-8.
14. Griffiths RR, Wolf B. Relative abuse liability of different benzodiazepines in drug abusers. J Clin Psychopharmacol 1990;10(4):237-43.
15. Albeck JH. Withdrawal and detoxification from benzodiazepine dependence: a potential role for clonazepam. J Clin Psychiatry 1987;48(suppl):43-9.
16. Woods JH, Katz JL, Winger G. Use and abuse of benzodiazepines: issues relevant to prescribing. JAMA 1988;260(23):3476-80.
17. Maletzky BM, Klotter J. Addiction to diazepam. Int J Addict 1976;II(1):95-115.
18. Lader M. Short-term versus long-term benzodiazepine therapy. Curr Med Res Opin 1984(8, suppl 4);120-6.
19. Ciraulo DA, Sands BK, Shader RI. Critical review of liability for benzodiazepine abuse among alcoholics. Am J Psychiatry 1988;145(12):1501-6.
20. Berner R. The patient’s perspective. NYS J Med 1991;91(11, suppl):37S-39S.
21. Schatzberg AF. Benzodiazepines: therapeutic, biological and psychosocial issues. J Psychiatr Res 1990;24(2):1-2.
22. Lader M. Drug development optimization—benzodiazepines. Agents Actions 1990;29:59-69.
23. Sellers EM, Marshman JA, Kaplan Hl, et al. Acute and chronic drug abuse emergencies in metropolitan Toronto. Int J Addict 1981;16(2):283-303.
24. Hamlin M. Guidelines for benzodiazepine prescribing. Br J Hosp Med 1989;42(1):82.-
25. Piesiur Strehlow B, Strehlow U, Poser W. Mortality of patients dependent on benzodiazepines. Acta Psychiatr Scand 1986;73:330-5.
26. Bendtsen P, Hensing G, McKenzie L, Stardsman AK. Prescribing benzodiazepines—a critical incident study of a physician dilemma. Soc Sci Med 1999;49:459-67.
27. Linnoila MI. Benzodiazepines and alcohol. J Psychiatric Res 1990;24(2, suppl):121-7.
28. Benzodiazepine dependence, toxicity and abuse. A task force report. Washington, DC: American Psychiatric Association, 1990.
How should you treat anxiety in substance-abusing patients: deny them benzodiazepines and risk under-treatment, or prescribe benzodiazepines for the anxiolytic effect and risk contributing to addiction?
There is no definitive answer, but one thing is clear: Among psychiatric patients, substance abusers are most likely to abuse benzodiazepines and become addicted to them.
Some argue that the abuse potential is overstated, but only limited data suggest that benzodiazepines can be safely prescribed to patients who are abusing alcohol or drugs. In this article, we discuss benzodiazepine use in these patients and offer a sobriety-based treatment approach.
Table 1
Benzodiazepines’ potency and half-lives, including half-lives of active metabolites
| Potency | Shorter half-life (hr) | Longer half-life (hr) |
|---|---|---|
| High | Alprazolam (6 to 12) Lorazepam (10 to 20) Triazolam (2) | Clonazepam (18 to 50) |
| Low | Oxazepam (4 to 15) Temazepam (8 to 22) | Chlordiazepoxide (5 to 30) [36 to 200]* Clorazepate [36 to 200]* Diazepam (20 to 100) [36 to 200]* Flurazepam [40 to 250]* |
| * [active metabolite] | ||
| Source: Ashton CH. Benzodiazepine equivalence table. Available at www.benzo.org.uk | ||
BENEFITS AND RISKS
Considered a safe substitute for barbiturates, benzodiazepines were heralded as wonder drugs when they were introduced in the 1950s. Reports of their addictive potential surfaced in the 1970s, and since then researchers have disagreed on whether benzodiazepines should be prescribed to substance-abusing or -dependent patients.
Clinical utility. Benzodiazepines are used in many clinical situations because of their:
- anxiolytic, hypnotic, anticonvulsant, antipanic, antidepressant, amnestic, anesthetic, and antispastic effects
- relatively mild side effects, when compared with alternatives such as barbiturates.
In psychiatry, benzodiazepines are used to treat anxiety disorders, agitation, and insomnia. Because of cross-tolerance with alcohol and barbiturates, benzodiazepines also are used to manage alcohol or barbiturate withdrawal.
Interactions. Benzodiazepines can interact with other psychotropics, including lithium, antipsychotics, and selective serotonin reuptake inhibitors (SSRIs). Respiratory arrest has been reported in patients taking both a high-potency benzodiazepine and clozapine.1
Overdose and withdrawal symptoms. Benzodiazepine overdose is characterized by slurred speech, sedation, memory impairment, incoordination, respiratory depression, hypotension, stupor, and coma. Abrupt withdrawal may produce life-threatening delirium, hallucinations, grand mal seizures, and symptoms similar to those of alcohol withdrawal (insomnia, anxiety, tremor, hyperactivity, nausea, vomiting, and psychomotor agitation).1
ABUSE PATTERNS
The few empiric studies examining benzodiazepines’ abuse potential in substance abusers have shown inconsistent results. However, it is generally accepted that:
- long-term benzodiazepine use may lead to tolerance and physiologic dependence
- withdrawal symptoms can occur if benzodiazepines are stopped suddenly, especially after long-term (months to years) use.
Even though most benzodiazepine prescriptions are not abused,2 a history of alcohol and drug abuse suggests high potential for benzodiazepine abuse. Also, long-term users of prescribed benzodiazepines often develop tolerance and may escalate their doses to get the same desired effects. If their supply is threatened, these patients may seek benzodiazepines illicitly.
Benzodiazepines may enhance or prolong the elation (“high”) associated with other drugs or mitigate the depression (“crash”) that follows a stimulant “high.” Sometimes benzodiazepines are the drug of choice, as high doses of potent, short-acting agents may provide a stimulant “high.”
WHO ABUSES BENZODIAZEPINES?
Alcohol and substance abusers tend to ingest benzodiazepines for recreational purposes. Thirty to 50% of alcoholics undergoing detoxification and 44% of IV drug abusers also may be abusing benzodiazepines.3
Benzodiazepines are cross-tolerant with alcohol, and alcoholics may use them with alcohol or as a substitute when alcohol is unavailable. They also may self-medicate with benzodiazepines to ease alcohol’s withdrawal symptoms. Opiate, amphetamine, and cocaine abusers may use benzodiazepines with their drugs of choice, as may younger abusers of MDMA (“Ecstasy”) and LSD.
Even patients who begin taking benzodiazepines for legitimate reasons may end up abusing them. In one study of 2,600 patients prescribed diazepam, up to 60% had abused and/or become dependent on it.4
Benzodiazepine abuse may start with other sedative/hypnotic abuse or as experimentation with drugs or alcohol, typically around age 13 or 14.5 The average benzodiazepine abuser is age 19 to 31, and the male-to-female ratio is about 2:1.6
Multi-drug abuse. Benzodiazepines are usually not the preferred or sole drug of abuse. Roughly 80% of benzodiazepine abuse may be a component of poly-drug abuse, most commonly with opioid addiction.7 A 2-year National Institute on Drug Abuse study of heroin abusers suggested that 15% also abused benzodiazepines daily for more than 1 year, and 73% had abused benzodiazepines several times during the previous week.8 Other studies suggest that up to 90% of methadone users regularly abuse benzodiazepines, often at high doses.9
Table 2
Should benzodiazepines be prescribed to substance abusers?
YES: Arguments for
|
NO: Arguments against
|
ILLICIT USE POTENTIAL
Prescriptions are the primary source of supply for benzodiazepine abusers. These patients are doctor shoppers and often change pharmacies. They visit emergency rooms frequently and may feign symptoms to obtain benzodiazepine prescriptions. They fill prescriptions for personal use or sell the drugs to illicit sources to support their addictions.
On the street, brand-name benzodiazepines are worth much more than generics because they can be identified by photographs of brand-name benzodiazepines on the Internet or in reference books. In many cities, the street value of the Xanax or Klonopin brands may be $5 to $10 per pill. A 5mg tablet of Valium-brand diazepam may sell for $5, and 10-mg tablets are worth up to $10.
Higher abuse potential. All benzodiazepines have abuse potential, and most have been reported in the literature as being abused. Those most likely to be abused have a short half-life10 (Table 1) or rapidly cross the blood brain barrier, such as alprazolam.11
Alprazolam and lorazepam are popular among benzodiazepine abusers. In experienced but nondependent users, 1 mg of alprazolam produces a sense of elation and carries an abuse potential similar to that of 10 mg of dextroamphetamine.12 Lipophilic agents such as diazepam also have a high abuse and addiction potential.
In the United States, diazepam and alprazolam appear to be the most abused benzodi-azepines.13 Flunitrazepam has become popular among high school students and drug addicts, particularly in the south and southwest. This potent benzodiazepine is not approved for use in the United States but is diverted from Latin America or Europe in the illegal drug trade.
Lower abuse potential. Benzodiazepines with longer half-lives generally are less likely to be abused, although diazepam—with a half-life of up to 100 hours—is the exception. Chlordiazepoxide has been reported to produce a lower “high” than other benzodiazepines.14 Among the short half-life benzodiazepines, oxazepam may have a relatively low abuse potential.14 Clonazepam—a high-potency benzodiazepine with a long half-life—is generally safe and may have a lower abuse or addiction potential, although its abuse has been report-ed.15 Similarly, oxazepam, clorazepate, and chlordiazepoxide may be less reinforcing than other benzodiazepines, although reports have linked these agents to abuse as well.16
TO PRESCRIBE OR NOT TO PRESCRIBE
Opponents blame benzodiazepines for promoting the drug culture and argue that prescribing benzodiazepines promotes drug abuse. Advocates of benzodiazepine therapy contend that restricting an effective and safe medication is unethical, even in substance abusers. Arguments from each perspective are summarized in Table 2.17-27
In 1990, an American Psychiatric Association task force concluded that alcohol and substance abusers could be prescribed benzodiazepines with very close monitoring but did not recommend specific standards.28
RECOMMENDATIONS
Prescribing benzodiazepines to substance abusers is not absolutely contraindicated, despite an elevated relative risk of abuse or dependence. In the absence of convincing data, physicians must decide on their own—usually case by case—the merits of using benzodiazepines to treat anxiety in substance abusers.
A sobriety-based approach. Our group at the Substance Abuse Treatment Center, VA Medical Center, Omaha, Nebraska, has developed a treatment algorithm for substance abusers presenting with anxiety (see Algorithm). It is based on clinical experience, more than 200 relevant articles, and the consensus of psychiatrists trained and certified by the American Board of Psychiatry and Neurology and the American Society of Addiction Medicine.
Algorithm Sobriety-based protocol for treating anxiety in substance abusers
Precautions for prescribing benzodiazepines
- Inform patient of planned duration of therapy
- Prescribe for brief periods (weeks to months), with follow-up at least monthly
- No refills without follow-up, and no refills over the phone
- Use random urine toxicology screening every 1 to 3 months to monitor for relapse
- Attempt to taper dosage after 3 to 6 months—even if patient resists—and monitor for objective withdrawal
- If no objective withdrawal, terminate benzodiazepine; continue other medications
- If objective withdrawal, continue benzodiazepine and reattempt taper in 3 to 6 months; continue Alcoholics/Narcotics Anonymous
We suggest that you begin by encouraging sobriety and referring willing patients to detoxification. Because most addicts deny or greatly minimize their substance abuse, investigate all potential drug or alcohol abuse thoroughly and address it appropriately.
If anxiety persists after detoxification, begin drug therapy with nonbenzodiazepines:
- Diphenhydramine, 50 to 100 mg/d, may reduce anxiety and often improves sleep, but consider its anticholinergic side effects before prescribing.
- Some SSRIs and venlafaxine are used to treat anxiety, but they generally take weeks to produce a therapeutic effect and some patients cannot wait that long.
- Mirtazapine, 15 to 30 mg/d, provides relatively rapid sedation and helps with sleep and anxiety.
- Buspirone may reduce anxiety, especially when given at 30 to 60 mg/d.
- Gabapentin, 100 to 300 mg tid or higher, may reduce anxiety and help with sleep.
- Tricyclic antidepressants may be considered, but watch for cardiac and anticholinergic side effects and overdose risks.
If anxiety does not improve with an adequate trial of first-line agents, consider adding long-acting benzodiazepines at sufficient dosages, such as clonazepam, 0.5 to 1 mg bid to tid. Prescribe scheduled doses, rather than “as needed.” Continue the first-line antianxiety agent, and reiterate to the patient that benzodiazepine therapy will be short-term.
Observe prescribing precautions (see Algorithm), and screen patients’ urine randomly every 1 to 3 months to monitor their adherence to substance abuse treatment.
Keep patient records current, with attention to dates of visits and prescriptions and quantity of benzodiazepines prescribed. To ensure proper continued use of benzodiazepines, consider consulting with physicians who have expertise in treating similar patients. Watch for possible signs of benzodiazepine dependence and abuse, such as requests for dose increases or early refills.
NONDRUG TREATMENTS
Nondrug treatments have been shown to reduce substance use and control anxiety in some studies. These include cognitive-behavioral therapy, motivational enhancement therapy, interpersonal therapy, and brief dynamic therapy, among others. Their use requires specific training or referral to more experienced colleagues. For information on these treatments, consult the Web sites of the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism (Related resources).
Group and self-help therapies such as Alcoholics Anonymous or Narcotics Anonymous also have been shown to reduce substance use.
- Parran TV. Prescription drug abuse. A question of balance. Med Clin North Am 1997;81:967-78.
- Lader M, Russell J. Guidelines for the prevention and treatment of benzodiazepine dependence: summary of a report from the Mental Health Foundation. Addiction 1993;88(12):1707-8.
- National Institute on Drug Abuse. www.nida.nih.gov
- National Institute on Alcohol Abuse and Addiction. www.niaaa.nih.gov
Drug brand names
- Alprazolam • Xanax
- Buspirone • BuSpar
- Chlordiazepoxide • Librium
- Clonazepam • Klonopin
- Clorazepate • Tranxene
- Diazepam • Valium
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Oxazepam • Serax
- Temazepam • Restoril
- Triazolam • Halcion
- Venlafaxine • Effexor
Disclosure
This work was supported by the Attorney General’s Office, Commonwealth of Massachusetts and the United States Department of Veterans Affairs.
Dr. Sattar has received grant funding from Abbott Laboratories and is a speaker for AstraZeneca and Eli Lilly and Co.
Dr. Bhatia is a speaker for AstraZeneca, Eli Lilly and Co., Janssen Pharmaceutica, and Bristol-Myers Squibb Co.
Acknowledgment
The authors wish to thank Jennifer Hong, second-year medical student, Creighton University School of Medicine, Omaha, NE, for her assistance in preparing this article for publication.
How should you treat anxiety in substance-abusing patients: deny them benzodiazepines and risk under-treatment, or prescribe benzodiazepines for the anxiolytic effect and risk contributing to addiction?
There is no definitive answer, but one thing is clear: Among psychiatric patients, substance abusers are most likely to abuse benzodiazepines and become addicted to them.
Some argue that the abuse potential is overstated, but only limited data suggest that benzodiazepines can be safely prescribed to patients who are abusing alcohol or drugs. In this article, we discuss benzodiazepine use in these patients and offer a sobriety-based treatment approach.
Table 1
Benzodiazepines’ potency and half-lives, including half-lives of active metabolites
| Potency | Shorter half-life (hr) | Longer half-life (hr) |
|---|---|---|
| High | Alprazolam (6 to 12) Lorazepam (10 to 20) Triazolam (2) | Clonazepam (18 to 50) |
| Low | Oxazepam (4 to 15) Temazepam (8 to 22) | Chlordiazepoxide (5 to 30) [36 to 200]* Clorazepate [36 to 200]* Diazepam (20 to 100) [36 to 200]* Flurazepam [40 to 250]* |
| * [active metabolite] | ||
| Source: Ashton CH. Benzodiazepine equivalence table. Available at www.benzo.org.uk | ||
BENEFITS AND RISKS
Considered a safe substitute for barbiturates, benzodiazepines were heralded as wonder drugs when they were introduced in the 1950s. Reports of their addictive potential surfaced in the 1970s, and since then researchers have disagreed on whether benzodiazepines should be prescribed to substance-abusing or -dependent patients.
Clinical utility. Benzodiazepines are used in many clinical situations because of their:
- anxiolytic, hypnotic, anticonvulsant, antipanic, antidepressant, amnestic, anesthetic, and antispastic effects
- relatively mild side effects, when compared with alternatives such as barbiturates.
In psychiatry, benzodiazepines are used to treat anxiety disorders, agitation, and insomnia. Because of cross-tolerance with alcohol and barbiturates, benzodiazepines also are used to manage alcohol or barbiturate withdrawal.
Interactions. Benzodiazepines can interact with other psychotropics, including lithium, antipsychotics, and selective serotonin reuptake inhibitors (SSRIs). Respiratory arrest has been reported in patients taking both a high-potency benzodiazepine and clozapine.1
Overdose and withdrawal symptoms. Benzodiazepine overdose is characterized by slurred speech, sedation, memory impairment, incoordination, respiratory depression, hypotension, stupor, and coma. Abrupt withdrawal may produce life-threatening delirium, hallucinations, grand mal seizures, and symptoms similar to those of alcohol withdrawal (insomnia, anxiety, tremor, hyperactivity, nausea, vomiting, and psychomotor agitation).1
ABUSE PATTERNS
The few empiric studies examining benzodiazepines’ abuse potential in substance abusers have shown inconsistent results. However, it is generally accepted that:
- long-term benzodiazepine use may lead to tolerance and physiologic dependence
- withdrawal symptoms can occur if benzodiazepines are stopped suddenly, especially after long-term (months to years) use.
Even though most benzodiazepine prescriptions are not abused,2 a history of alcohol and drug abuse suggests high potential for benzodiazepine abuse. Also, long-term users of prescribed benzodiazepines often develop tolerance and may escalate their doses to get the same desired effects. If their supply is threatened, these patients may seek benzodiazepines illicitly.
Benzodiazepines may enhance or prolong the elation (“high”) associated with other drugs or mitigate the depression (“crash”) that follows a stimulant “high.” Sometimes benzodiazepines are the drug of choice, as high doses of potent, short-acting agents may provide a stimulant “high.”
WHO ABUSES BENZODIAZEPINES?
Alcohol and substance abusers tend to ingest benzodiazepines for recreational purposes. Thirty to 50% of alcoholics undergoing detoxification and 44% of IV drug abusers also may be abusing benzodiazepines.3
Benzodiazepines are cross-tolerant with alcohol, and alcoholics may use them with alcohol or as a substitute when alcohol is unavailable. They also may self-medicate with benzodiazepines to ease alcohol’s withdrawal symptoms. Opiate, amphetamine, and cocaine abusers may use benzodiazepines with their drugs of choice, as may younger abusers of MDMA (“Ecstasy”) and LSD.
Even patients who begin taking benzodiazepines for legitimate reasons may end up abusing them. In one study of 2,600 patients prescribed diazepam, up to 60% had abused and/or become dependent on it.4
Benzodiazepine abuse may start with other sedative/hypnotic abuse or as experimentation with drugs or alcohol, typically around age 13 or 14.5 The average benzodiazepine abuser is age 19 to 31, and the male-to-female ratio is about 2:1.6
Multi-drug abuse. Benzodiazepines are usually not the preferred or sole drug of abuse. Roughly 80% of benzodiazepine abuse may be a component of poly-drug abuse, most commonly with opioid addiction.7 A 2-year National Institute on Drug Abuse study of heroin abusers suggested that 15% also abused benzodiazepines daily for more than 1 year, and 73% had abused benzodiazepines several times during the previous week.8 Other studies suggest that up to 90% of methadone users regularly abuse benzodiazepines, often at high doses.9
Table 2
Should benzodiazepines be prescribed to substance abusers?
YES: Arguments for
|
NO: Arguments against
|
ILLICIT USE POTENTIAL
Prescriptions are the primary source of supply for benzodiazepine abusers. These patients are doctor shoppers and often change pharmacies. They visit emergency rooms frequently and may feign symptoms to obtain benzodiazepine prescriptions. They fill prescriptions for personal use or sell the drugs to illicit sources to support their addictions.
On the street, brand-name benzodiazepines are worth much more than generics because they can be identified by photographs of brand-name benzodiazepines on the Internet or in reference books. In many cities, the street value of the Xanax or Klonopin brands may be $5 to $10 per pill. A 5mg tablet of Valium-brand diazepam may sell for $5, and 10-mg tablets are worth up to $10.
Higher abuse potential. All benzodiazepines have abuse potential, and most have been reported in the literature as being abused. Those most likely to be abused have a short half-life10 (Table 1) or rapidly cross the blood brain barrier, such as alprazolam.11
Alprazolam and lorazepam are popular among benzodiazepine abusers. In experienced but nondependent users, 1 mg of alprazolam produces a sense of elation and carries an abuse potential similar to that of 10 mg of dextroamphetamine.12 Lipophilic agents such as diazepam also have a high abuse and addiction potential.
In the United States, diazepam and alprazolam appear to be the most abused benzodi-azepines.13 Flunitrazepam has become popular among high school students and drug addicts, particularly in the south and southwest. This potent benzodiazepine is not approved for use in the United States but is diverted from Latin America or Europe in the illegal drug trade.
Lower abuse potential. Benzodiazepines with longer half-lives generally are less likely to be abused, although diazepam—with a half-life of up to 100 hours—is the exception. Chlordiazepoxide has been reported to produce a lower “high” than other benzodiazepines.14 Among the short half-life benzodiazepines, oxazepam may have a relatively low abuse potential.14 Clonazepam—a high-potency benzodiazepine with a long half-life—is generally safe and may have a lower abuse or addiction potential, although its abuse has been report-ed.15 Similarly, oxazepam, clorazepate, and chlordiazepoxide may be less reinforcing than other benzodiazepines, although reports have linked these agents to abuse as well.16
TO PRESCRIBE OR NOT TO PRESCRIBE
Opponents blame benzodiazepines for promoting the drug culture and argue that prescribing benzodiazepines promotes drug abuse. Advocates of benzodiazepine therapy contend that restricting an effective and safe medication is unethical, even in substance abusers. Arguments from each perspective are summarized in Table 2.17-27
In 1990, an American Psychiatric Association task force concluded that alcohol and substance abusers could be prescribed benzodiazepines with very close monitoring but did not recommend specific standards.28
RECOMMENDATIONS
Prescribing benzodiazepines to substance abusers is not absolutely contraindicated, despite an elevated relative risk of abuse or dependence. In the absence of convincing data, physicians must decide on their own—usually case by case—the merits of using benzodiazepines to treat anxiety in substance abusers.
A sobriety-based approach. Our group at the Substance Abuse Treatment Center, VA Medical Center, Omaha, Nebraska, has developed a treatment algorithm for substance abusers presenting with anxiety (see Algorithm). It is based on clinical experience, more than 200 relevant articles, and the consensus of psychiatrists trained and certified by the American Board of Psychiatry and Neurology and the American Society of Addiction Medicine.
Algorithm Sobriety-based protocol for treating anxiety in substance abusers
Precautions for prescribing benzodiazepines
- Inform patient of planned duration of therapy
- Prescribe for brief periods (weeks to months), with follow-up at least monthly
- No refills without follow-up, and no refills over the phone
- Use random urine toxicology screening every 1 to 3 months to monitor for relapse
- Attempt to taper dosage after 3 to 6 months—even if patient resists—and monitor for objective withdrawal
- If no objective withdrawal, terminate benzodiazepine; continue other medications
- If objective withdrawal, continue benzodiazepine and reattempt taper in 3 to 6 months; continue Alcoholics/Narcotics Anonymous
We suggest that you begin by encouraging sobriety and referring willing patients to detoxification. Because most addicts deny or greatly minimize their substance abuse, investigate all potential drug or alcohol abuse thoroughly and address it appropriately.
If anxiety persists after detoxification, begin drug therapy with nonbenzodiazepines:
- Diphenhydramine, 50 to 100 mg/d, may reduce anxiety and often improves sleep, but consider its anticholinergic side effects before prescribing.
- Some SSRIs and venlafaxine are used to treat anxiety, but they generally take weeks to produce a therapeutic effect and some patients cannot wait that long.
- Mirtazapine, 15 to 30 mg/d, provides relatively rapid sedation and helps with sleep and anxiety.
- Buspirone may reduce anxiety, especially when given at 30 to 60 mg/d.
- Gabapentin, 100 to 300 mg tid or higher, may reduce anxiety and help with sleep.
- Tricyclic antidepressants may be considered, but watch for cardiac and anticholinergic side effects and overdose risks.
If anxiety does not improve with an adequate trial of first-line agents, consider adding long-acting benzodiazepines at sufficient dosages, such as clonazepam, 0.5 to 1 mg bid to tid. Prescribe scheduled doses, rather than “as needed.” Continue the first-line antianxiety agent, and reiterate to the patient that benzodiazepine therapy will be short-term.
Observe prescribing precautions (see Algorithm), and screen patients’ urine randomly every 1 to 3 months to monitor their adherence to substance abuse treatment.
Keep patient records current, with attention to dates of visits and prescriptions and quantity of benzodiazepines prescribed. To ensure proper continued use of benzodiazepines, consider consulting with physicians who have expertise in treating similar patients. Watch for possible signs of benzodiazepine dependence and abuse, such as requests for dose increases or early refills.
NONDRUG TREATMENTS
Nondrug treatments have been shown to reduce substance use and control anxiety in some studies. These include cognitive-behavioral therapy, motivational enhancement therapy, interpersonal therapy, and brief dynamic therapy, among others. Their use requires specific training or referral to more experienced colleagues. For information on these treatments, consult the Web sites of the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism (Related resources).
Group and self-help therapies such as Alcoholics Anonymous or Narcotics Anonymous also have been shown to reduce substance use.
- Parran TV. Prescription drug abuse. A question of balance. Med Clin North Am 1997;81:967-78.
- Lader M, Russell J. Guidelines for the prevention and treatment of benzodiazepine dependence: summary of a report from the Mental Health Foundation. Addiction 1993;88(12):1707-8.
- National Institute on Drug Abuse. www.nida.nih.gov
- National Institute on Alcohol Abuse and Addiction. www.niaaa.nih.gov
Drug brand names
- Alprazolam • Xanax
- Buspirone • BuSpar
- Chlordiazepoxide • Librium
- Clonazepam • Klonopin
- Clorazepate • Tranxene
- Diazepam • Valium
- Flurazepam • Dalmane
- Gabapentin • Neurontin
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Oxazepam • Serax
- Temazepam • Restoril
- Triazolam • Halcion
- Venlafaxine • Effexor
Disclosure
This work was supported by the Attorney General’s Office, Commonwealth of Massachusetts and the United States Department of Veterans Affairs.
Dr. Sattar has received grant funding from Abbott Laboratories and is a speaker for AstraZeneca and Eli Lilly and Co.
Dr. Bhatia is a speaker for AstraZeneca, Eli Lilly and Co., Janssen Pharmaceutica, and Bristol-Myers Squibb Co.
Acknowledgment
The authors wish to thank Jennifer Hong, second-year medical student, Creighton University School of Medicine, Omaha, NE, for her assistance in preparing this article for publication.
1. Hales RE, Yudofsky SC. Textbook of clinical psychiatry, 4th ed. Washington, DC: American Psychiatric Publishing, 2003:318-19,493,501,1098.
2. Woods JH, Katz JL, Winger G. Use and abuse of benzodiazepines. Issues relevant to prescribing. JAMA 1988;260(23):3476-80.
3. Shaw M, Brabbins C, Ruben S. Misuse of benzodiazepines. Specify the formulation when prescribing. BMJ 1994;308(6945):1709.-
4. Woody GE, O’Brien CP, Greenstein R. Misuse and abuse of diazepam: an increasingly common medical problem. Int J Addict 1975;10(5):843-8.
5. Pedersen W, Lavik NJ. Adolescents and benzodiazepines: prescribed use, self-medication and intoxication. Acta Psychiatrica Scand 1991;84:94-8.
6. Ruben SM, Morrison CL. Temazepam misuse in a group of injecting drug users. Br J Addict 1992;87:1387-92,
7. Gold MS, Miller NS, Stennie K, Populla-Vardi C. Epidemiology of benzodiazepine use and dependence. Psychiatr Annals 1995;25:146-8.
8. Dumont RL. Abuse of benzodiazepines—the problems and the solutions. A report of a Committee of the Institute for Behavior and Health, Inc. Am J Drug Alcohol Abuse. 1988;14(suppl 1):1-69.
9. Iguchi MY, Griffiths RR, Bickel WK, et al. Relative abuse liability of benzodiazepines in methadone-maintained populations in three cities. In: Harris LS (ed). Problems of drug dependence, 1988. Proceedings of the 50th annual scientific meeting, the Committee on Problems of Drug Dependence, Inc. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute on Drug Abuse, Office of Science, 1989. DHHS publication no. (ADM) 89-1605.
10. Longo LP. Non-benzodiazepine pharmacotherapy of anxiety and panic in substance abusing patients. Psychiatr Annals 1998;28(3):142-53.
11. Roache JD, Meisch RA. Findings from self-administration research on the addiction potential of benzodiazepines. Psychiatr Annals 1995;25(3):153-7.
12. Zawertailo LA, Busto U, Kaplan HL, Sellers EM. Comparative abuse liability of sertraline, alprazolam, and dextroamphetamine in humans. J Clin Psychopharmacol 1995;15(2):117-24.
13. Griffiths RR, McLeod DR, Bigelow GE, et al. Comparison of diazepam and oxazepam: preference, liking and extent of abuse. J Pharmacol Exp Ther 1984;229(2):501-8.
14. Griffiths RR, Wolf B. Relative abuse liability of different benzodiazepines in drug abusers. J Clin Psychopharmacol 1990;10(4):237-43.
15. Albeck JH. Withdrawal and detoxification from benzodiazepine dependence: a potential role for clonazepam. J Clin Psychiatry 1987;48(suppl):43-9.
16. Woods JH, Katz JL, Winger G. Use and abuse of benzodiazepines: issues relevant to prescribing. JAMA 1988;260(23):3476-80.
17. Maletzky BM, Klotter J. Addiction to diazepam. Int J Addict 1976;II(1):95-115.
18. Lader M. Short-term versus long-term benzodiazepine therapy. Curr Med Res Opin 1984(8, suppl 4);120-6.
19. Ciraulo DA, Sands BK, Shader RI. Critical review of liability for benzodiazepine abuse among alcoholics. Am J Psychiatry 1988;145(12):1501-6.
20. Berner R. The patient’s perspective. NYS J Med 1991;91(11, suppl):37S-39S.
21. Schatzberg AF. Benzodiazepines: therapeutic, biological and psychosocial issues. J Psychiatr Res 1990;24(2):1-2.
22. Lader M. Drug development optimization—benzodiazepines. Agents Actions 1990;29:59-69.
23. Sellers EM, Marshman JA, Kaplan Hl, et al. Acute and chronic drug abuse emergencies in metropolitan Toronto. Int J Addict 1981;16(2):283-303.
24. Hamlin M. Guidelines for benzodiazepine prescribing. Br J Hosp Med 1989;42(1):82.-
25. Piesiur Strehlow B, Strehlow U, Poser W. Mortality of patients dependent on benzodiazepines. Acta Psychiatr Scand 1986;73:330-5.
26. Bendtsen P, Hensing G, McKenzie L, Stardsman AK. Prescribing benzodiazepines—a critical incident study of a physician dilemma. Soc Sci Med 1999;49:459-67.
27. Linnoila MI. Benzodiazepines and alcohol. J Psychiatric Res 1990;24(2, suppl):121-7.
28. Benzodiazepine dependence, toxicity and abuse. A task force report. Washington, DC: American Psychiatric Association, 1990.
1. Hales RE, Yudofsky SC. Textbook of clinical psychiatry, 4th ed. Washington, DC: American Psychiatric Publishing, 2003:318-19,493,501,1098.
2. Woods JH, Katz JL, Winger G. Use and abuse of benzodiazepines. Issues relevant to prescribing. JAMA 1988;260(23):3476-80.
3. Shaw M, Brabbins C, Ruben S. Misuse of benzodiazepines. Specify the formulation when prescribing. BMJ 1994;308(6945):1709.-
4. Woody GE, O’Brien CP, Greenstein R. Misuse and abuse of diazepam: an increasingly common medical problem. Int J Addict 1975;10(5):843-8.
5. Pedersen W, Lavik NJ. Adolescents and benzodiazepines: prescribed use, self-medication and intoxication. Acta Psychiatrica Scand 1991;84:94-8.
6. Ruben SM, Morrison CL. Temazepam misuse in a group of injecting drug users. Br J Addict 1992;87:1387-92,
7. Gold MS, Miller NS, Stennie K, Populla-Vardi C. Epidemiology of benzodiazepine use and dependence. Psychiatr Annals 1995;25:146-8.
8. Dumont RL. Abuse of benzodiazepines—the problems and the solutions. A report of a Committee of the Institute for Behavior and Health, Inc. Am J Drug Alcohol Abuse. 1988;14(suppl 1):1-69.
9. Iguchi MY, Griffiths RR, Bickel WK, et al. Relative abuse liability of benzodiazepines in methadone-maintained populations in three cities. In: Harris LS (ed). Problems of drug dependence, 1988. Proceedings of the 50th annual scientific meeting, the Committee on Problems of Drug Dependence, Inc. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute on Drug Abuse, Office of Science, 1989. DHHS publication no. (ADM) 89-1605.
10. Longo LP. Non-benzodiazepine pharmacotherapy of anxiety and panic in substance abusing patients. Psychiatr Annals 1998;28(3):142-53.
11. Roache JD, Meisch RA. Findings from self-administration research on the addiction potential of benzodiazepines. Psychiatr Annals 1995;25(3):153-7.
12. Zawertailo LA, Busto U, Kaplan HL, Sellers EM. Comparative abuse liability of sertraline, alprazolam, and dextroamphetamine in humans. J Clin Psychopharmacol 1995;15(2):117-24.
13. Griffiths RR, McLeod DR, Bigelow GE, et al. Comparison of diazepam and oxazepam: preference, liking and extent of abuse. J Pharmacol Exp Ther 1984;229(2):501-8.
14. Griffiths RR, Wolf B. Relative abuse liability of different benzodiazepines in drug abusers. J Clin Psychopharmacol 1990;10(4):237-43.
15. Albeck JH. Withdrawal and detoxification from benzodiazepine dependence: a potential role for clonazepam. J Clin Psychiatry 1987;48(suppl):43-9.
16. Woods JH, Katz JL, Winger G. Use and abuse of benzodiazepines: issues relevant to prescribing. JAMA 1988;260(23):3476-80.
17. Maletzky BM, Klotter J. Addiction to diazepam. Int J Addict 1976;II(1):95-115.
18. Lader M. Short-term versus long-term benzodiazepine therapy. Curr Med Res Opin 1984(8, suppl 4);120-6.
19. Ciraulo DA, Sands BK, Shader RI. Critical review of liability for benzodiazepine abuse among alcoholics. Am J Psychiatry 1988;145(12):1501-6.
20. Berner R. The patient’s perspective. NYS J Med 1991;91(11, suppl):37S-39S.
21. Schatzberg AF. Benzodiazepines: therapeutic, biological and psychosocial issues. J Psychiatr Res 1990;24(2):1-2.
22. Lader M. Drug development optimization—benzodiazepines. Agents Actions 1990;29:59-69.
23. Sellers EM, Marshman JA, Kaplan Hl, et al. Acute and chronic drug abuse emergencies in metropolitan Toronto. Int J Addict 1981;16(2):283-303.
24. Hamlin M. Guidelines for benzodiazepine prescribing. Br J Hosp Med 1989;42(1):82.-
25. Piesiur Strehlow B, Strehlow U, Poser W. Mortality of patients dependent on benzodiazepines. Acta Psychiatr Scand 1986;73:330-5.
26. Bendtsen P, Hensing G, McKenzie L, Stardsman AK. Prescribing benzodiazepines—a critical incident study of a physician dilemma. Soc Sci Med 1999;49:459-67.
27. Linnoila MI. Benzodiazepines and alcohol. J Psychiatric Res 1990;24(2, suppl):121-7.
28. Benzodiazepine dependence, toxicity and abuse. A task force report. Washington, DC: American Psychiatric Association, 1990.
Speeding up your Web Search
Search engines are the express lanes of the Internet. Knowing how to navigate them can help you get exactly the information you need in seconds flat.
This article will address the differences between search engines, offer basic and advanced search methods, and discuss Web search technologies and alternatives.
How search engines work
Search engines employ different methods of finding Web pages and ranking them according to relevance of information requested.1
Search engines such as Google and Altavista gather information by “crawling” or “spidering” through the Web. They check all the pages in a site and then proceed through pages linked to that site. As it progresses, it creates an indexed database of the content of each page it finds. Some search engines index more Web pages than others, while others update their indexes more often.
Many search engines pick up on how often and where the key word appears on a Web page.2 Pages on which key words appear more frequently or near the top usually will be deemed more relevant.
Search engines garner additional listings by charging advertisers to list their Web sites (e.g., a pharmaceutical company’s site promoting a particular drug) among the search results. These listings vary from paid placement, where sites are guaranteed a high ranking, to paid inclusion, by which an advertisement might be listed in more search requests.3
Taken together, these methods can generate millions of search results for a user who enters a broad topic. For example, entering “depression” in the Google search field produces 5,420,000 links ranging from product sites, government agencies and academic departments, to advocacy organizations and patients’ blogs. Unless the page relevant to you happens to be among the top five search results, you will need to refine your search. Fortunately, there are many ways to do this.
Refining your search: Basic tips
Say you’re trying to find this article without a link or a URL. Just follow these simple key word tips.4
- First, try the obvious. Enter Psyber Psychiatry.
- Use words likely to appear on a site with the information you want (e.g., psychiatry, John Luo).
- Make key words as specific as possible-for example, Current Psychiatry Psyber vs. Psyber Psychiatry
Google and most search engines assume that you want to find pages with all the words you have entered. Other search engines, such as Teoma, usually generate pages containing all key words preceded by a + (e.g., +Current +Psychiatry will produce a link to this Web site).
If that search produces too many results, you can eliminate sites containing certain key words by inserting a - before the key word (e.g., +current +psychiatry -American -psychology). Your search still may be too broad, however, because the words “current” and “psychiatry” could appear anywhere on the site-including pages you’re trying to rule out. Placing key words within quotation marks (e.g., “Current Psychiatry” “Psyber Psychiatry”) can eliminate still more sites.
Advanced searches
If your search requires even more fine-tuning, consider the following:
Site search. Specifying a site in your query can uncover references to specific topics. For example, enter computers site: www.currentpsychiatry.com in Google to find all pages with computers mentioned on the Current Psychiatry Web site. Other sites such as AltaVista use the term host: instead of site:.
Title search. Every Web page has an HTML title, which is also searched by the spider programs. Because an HTML title usually is indicative of the site’s content, searching through these titles may point to relevant information. Use allintitle: or intitle: to find a site (e.g., intitle:currentpsychiatry).
URL search. Similar to a site and title search, you can also search for words in the URL of a Web page. For example, in Google try entering current inurl:psychiatry or inurl:currentpsychiatry.
Some search engines employ techniques such as “clustering” (prevents a search from finding too many results from the same site), “stemming” (searches for variations of a word), and “find similar” (seeks out other pages with similar information). Some sites offer “related searches” or “search again” options to help users zero in on the desired information.
Confused about which commands and search features work for which search engines? searchenginewatch.com offers a comprehensive list of basic and power search commands, search assistance features, customization and display features, and Boolean commands.
Because Google is the most popular search engine, many search tools have been developed based on the Google engine. Fagan Finder provides a Web-based interface to Google that lets users search by exact phrase, any/all words, subject, author, and many other ways. Visualization technologies such as Touchgraph and Anacubis, both of which are connected to Google, allow searchers to visually explore and navigate relationships between Web sites.
KartOO is a metasearch engine with visual display interfaces. A metasearch engine uses other search engines to find information. After you enter a search term, KartOO launches the query to a set of search engines, compiles the results, and presents them in a series of interactive maps through a proprietary algorithm. Users can narrow the search by clicking on one of the plus/minus icons and categories between the results.
When all else fails …
If you cannot be bothered with search engine tricks and tools, consider Ask Jeeves, a popular site that uses natural language processing to enter a search request. Users simply phrase and enter a query as it would be phrased in conversation-for example, “What are the side effects of lithium?” Using techniques such as tokenization, stemming, parsing, and semantic analysis, Ask Jeeves attempts to determine the information you desire, and then generates results via the Teoma search engine.
If you’re really strapped for time, you could pay someone to do the searching for you.5 For example, Google Answers allows users to submit a question for between $2.50 and $200. Google’s research team will provide an answer, usually within 24 hours.
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Related Resources
Sullivan D. Articles on searchenginewatch.com:
- Search engine math. http://www.searchenginewatch.com/facts/math.html
- Power searching for anyone. http://www.searchenginewatch.com/facts/powersearch.html
- Search assistance features. http://www.searchenginewatch.com/facts/assistance.html
Disclosure:
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
1. Sullivan D. How search engines work. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/work.html. Accessed April 7, 2003.
2. Sullivan D. How search engines rank Web pages. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/rank.html. Accessed April 7, 2003.
3. Sullivan D. Buying your way into search engines. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/paid.html. Accessed April 7, 2003.
4. The basics of Google search. Available at: http://www.google.com/help/basics.html. Accessed April 7, 2003.
5. Arnoldy B. Paying for answers online. Christian Science Monitor July 22, 2002. Available at: http://www.csmonitor.com/2002/0722/p14s01-wmcn.htm. Accessed April 7, 2003.
Search engines are the express lanes of the Internet. Knowing how to navigate them can help you get exactly the information you need in seconds flat.
This article will address the differences between search engines, offer basic and advanced search methods, and discuss Web search technologies and alternatives.
How search engines work
Search engines employ different methods of finding Web pages and ranking them according to relevance of information requested.1
Search engines such as Google and Altavista gather information by “crawling” or “spidering” through the Web. They check all the pages in a site and then proceed through pages linked to that site. As it progresses, it creates an indexed database of the content of each page it finds. Some search engines index more Web pages than others, while others update their indexes more often.
Many search engines pick up on how often and where the key word appears on a Web page.2 Pages on which key words appear more frequently or near the top usually will be deemed more relevant.
Search engines garner additional listings by charging advertisers to list their Web sites (e.g., a pharmaceutical company’s site promoting a particular drug) among the search results. These listings vary from paid placement, where sites are guaranteed a high ranking, to paid inclusion, by which an advertisement might be listed in more search requests.3
Taken together, these methods can generate millions of search results for a user who enters a broad topic. For example, entering “depression” in the Google search field produces 5,420,000 links ranging from product sites, government agencies and academic departments, to advocacy organizations and patients’ blogs. Unless the page relevant to you happens to be among the top five search results, you will need to refine your search. Fortunately, there are many ways to do this.
Refining your search: Basic tips
Say you’re trying to find this article without a link or a URL. Just follow these simple key word tips.4
- First, try the obvious. Enter Psyber Psychiatry.
- Use words likely to appear on a site with the information you want (e.g., psychiatry, John Luo).
- Make key words as specific as possible-for example, Current Psychiatry Psyber vs. Psyber Psychiatry
Google and most search engines assume that you want to find pages with all the words you have entered. Other search engines, such as Teoma, usually generate pages containing all key words preceded by a + (e.g., +Current +Psychiatry will produce a link to this Web site).
If that search produces too many results, you can eliminate sites containing certain key words by inserting a - before the key word (e.g., +current +psychiatry -American -psychology). Your search still may be too broad, however, because the words “current” and “psychiatry” could appear anywhere on the site-including pages you’re trying to rule out. Placing key words within quotation marks (e.g., “Current Psychiatry” “Psyber Psychiatry”) can eliminate still more sites.
Advanced searches
If your search requires even more fine-tuning, consider the following:
Site search. Specifying a site in your query can uncover references to specific topics. For example, enter computers site: www.currentpsychiatry.com in Google to find all pages with computers mentioned on the Current Psychiatry Web site. Other sites such as AltaVista use the term host: instead of site:.
Title search. Every Web page has an HTML title, which is also searched by the spider programs. Because an HTML title usually is indicative of the site’s content, searching through these titles may point to relevant information. Use allintitle: or intitle: to find a site (e.g., intitle:currentpsychiatry).
URL search. Similar to a site and title search, you can also search for words in the URL of a Web page. For example, in Google try entering current inurl:psychiatry or inurl:currentpsychiatry.
Some search engines employ techniques such as “clustering” (prevents a search from finding too many results from the same site), “stemming” (searches for variations of a word), and “find similar” (seeks out other pages with similar information). Some sites offer “related searches” or “search again” options to help users zero in on the desired information.
Confused about which commands and search features work for which search engines? searchenginewatch.com offers a comprehensive list of basic and power search commands, search assistance features, customization and display features, and Boolean commands.
Because Google is the most popular search engine, many search tools have been developed based on the Google engine. Fagan Finder provides a Web-based interface to Google that lets users search by exact phrase, any/all words, subject, author, and many other ways. Visualization technologies such as Touchgraph and Anacubis, both of which are connected to Google, allow searchers to visually explore and navigate relationships between Web sites.
KartOO is a metasearch engine with visual display interfaces. A metasearch engine uses other search engines to find information. After you enter a search term, KartOO launches the query to a set of search engines, compiles the results, and presents them in a series of interactive maps through a proprietary algorithm. Users can narrow the search by clicking on one of the plus/minus icons and categories between the results.
When all else fails …
If you cannot be bothered with search engine tricks and tools, consider Ask Jeeves, a popular site that uses natural language processing to enter a search request. Users simply phrase and enter a query as it would be phrased in conversation-for example, “What are the side effects of lithium?” Using techniques such as tokenization, stemming, parsing, and semantic analysis, Ask Jeeves attempts to determine the information you desire, and then generates results via the Teoma search engine.
If you’re really strapped for time, you could pay someone to do the searching for you.5 For example, Google Answers allows users to submit a question for between $2.50 and $200. Google’s research team will provide an answer, usually within 24 hours.
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Related Resources
Sullivan D. Articles on searchenginewatch.com:
- Search engine math. http://www.searchenginewatch.com/facts/math.html
- Power searching for anyone. http://www.searchenginewatch.com/facts/powersearch.html
- Search assistance features. http://www.searchenginewatch.com/facts/assistance.html
Disclosure:
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
Search engines are the express lanes of the Internet. Knowing how to navigate them can help you get exactly the information you need in seconds flat.
This article will address the differences between search engines, offer basic and advanced search methods, and discuss Web search technologies and alternatives.
How search engines work
Search engines employ different methods of finding Web pages and ranking them according to relevance of information requested.1
Search engines such as Google and Altavista gather information by “crawling” or “spidering” through the Web. They check all the pages in a site and then proceed through pages linked to that site. As it progresses, it creates an indexed database of the content of each page it finds. Some search engines index more Web pages than others, while others update their indexes more often.
Many search engines pick up on how often and where the key word appears on a Web page.2 Pages on which key words appear more frequently or near the top usually will be deemed more relevant.
Search engines garner additional listings by charging advertisers to list their Web sites (e.g., a pharmaceutical company’s site promoting a particular drug) among the search results. These listings vary from paid placement, where sites are guaranteed a high ranking, to paid inclusion, by which an advertisement might be listed in more search requests.3
Taken together, these methods can generate millions of search results for a user who enters a broad topic. For example, entering “depression” in the Google search field produces 5,420,000 links ranging from product sites, government agencies and academic departments, to advocacy organizations and patients’ blogs. Unless the page relevant to you happens to be among the top five search results, you will need to refine your search. Fortunately, there are many ways to do this.
Refining your search: Basic tips
Say you’re trying to find this article without a link or a URL. Just follow these simple key word tips.4
- First, try the obvious. Enter Psyber Psychiatry.
- Use words likely to appear on a site with the information you want (e.g., psychiatry, John Luo).
- Make key words as specific as possible-for example, Current Psychiatry Psyber vs. Psyber Psychiatry
Google and most search engines assume that you want to find pages with all the words you have entered. Other search engines, such as Teoma, usually generate pages containing all key words preceded by a + (e.g., +Current +Psychiatry will produce a link to this Web site).
If that search produces too many results, you can eliminate sites containing certain key words by inserting a - before the key word (e.g., +current +psychiatry -American -psychology). Your search still may be too broad, however, because the words “current” and “psychiatry” could appear anywhere on the site-including pages you’re trying to rule out. Placing key words within quotation marks (e.g., “Current Psychiatry” “Psyber Psychiatry”) can eliminate still more sites.
Advanced searches
If your search requires even more fine-tuning, consider the following:
Site search. Specifying a site in your query can uncover references to specific topics. For example, enter computers site: www.currentpsychiatry.com in Google to find all pages with computers mentioned on the Current Psychiatry Web site. Other sites such as AltaVista use the term host: instead of site:.
Title search. Every Web page has an HTML title, which is also searched by the spider programs. Because an HTML title usually is indicative of the site’s content, searching through these titles may point to relevant information. Use allintitle: or intitle: to find a site (e.g., intitle:currentpsychiatry).
URL search. Similar to a site and title search, you can also search for words in the URL of a Web page. For example, in Google try entering current inurl:psychiatry or inurl:currentpsychiatry.
Some search engines employ techniques such as “clustering” (prevents a search from finding too many results from the same site), “stemming” (searches for variations of a word), and “find similar” (seeks out other pages with similar information). Some sites offer “related searches” or “search again” options to help users zero in on the desired information.
Confused about which commands and search features work for which search engines? searchenginewatch.com offers a comprehensive list of basic and power search commands, search assistance features, customization and display features, and Boolean commands.
Because Google is the most popular search engine, many search tools have been developed based on the Google engine. Fagan Finder provides a Web-based interface to Google that lets users search by exact phrase, any/all words, subject, author, and many other ways. Visualization technologies such as Touchgraph and Anacubis, both of which are connected to Google, allow searchers to visually explore and navigate relationships between Web sites.
KartOO is a metasearch engine with visual display interfaces. A metasearch engine uses other search engines to find information. After you enter a search term, KartOO launches the query to a set of search engines, compiles the results, and presents them in a series of interactive maps through a proprietary algorithm. Users can narrow the search by clicking on one of the plus/minus icons and categories between the results.
When all else fails …
If you cannot be bothered with search engine tricks and tools, consider Ask Jeeves, a popular site that uses natural language processing to enter a search request. Users simply phrase and enter a query as it would be phrased in conversation-for example, “What are the side effects of lithium?” Using techniques such as tokenization, stemming, parsing, and semantic analysis, Ask Jeeves attempts to determine the information you desire, and then generates results via the Teoma search engine.
If you’re really strapped for time, you could pay someone to do the searching for you.5 For example, Google Answers allows users to submit a question for between $2.50 and $200. Google’s research team will provide an answer, usually within 24 hours.
If you have any questions about these products or comments about Psyber Psychiatry, click here to contact Dr. Luo or send an e-mail to [email protected].
Related Resources
Sullivan D. Articles on searchenginewatch.com:
- Search engine math. http://www.searchenginewatch.com/facts/math.html
- Power searching for anyone. http://www.searchenginewatch.com/facts/powersearch.html
- Search assistance features. http://www.searchenginewatch.com/facts/assistance.html
Disclosure:
Dr. Luo reports no financial relationship with any company whose products are mentioned in this article. The opinions expressed by Dr. Luo in this column are his own and do not necessarily reflect those of Current Psychiatry.
1. Sullivan D. How search engines work. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/work.html. Accessed April 7, 2003.
2. Sullivan D. How search engines rank Web pages. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/rank.html. Accessed April 7, 2003.
3. Sullivan D. Buying your way into search engines. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/paid.html. Accessed April 7, 2003.
4. The basics of Google search. Available at: http://www.google.com/help/basics.html. Accessed April 7, 2003.
5. Arnoldy B. Paying for answers online. Christian Science Monitor July 22, 2002. Available at: http://www.csmonitor.com/2002/0722/p14s01-wmcn.htm. Accessed April 7, 2003.
1. Sullivan D. How search engines work. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/work.html. Accessed April 7, 2003.
2. Sullivan D. How search engines rank Web pages. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/rank.html. Accessed April 7, 2003.
3. Sullivan D. Buying your way into search engines. searchenginewatch.com. Available at: http://www.searchenginewatch.com/webmasters/paid.html. Accessed April 7, 2003.
4. The basics of Google search. Available at: http://www.google.com/help/basics.html. Accessed April 7, 2003.
5. Arnoldy B. Paying for answers online. Christian Science Monitor July 22, 2002. Available at: http://www.csmonitor.com/2002/0722/p14s01-wmcn.htm. Accessed April 7, 2003.