User login
Transient Eruption of Verrucous Keratoses During Encorafenib Therapy: Adverse Event or Paraneoplastic Phenomenon?
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
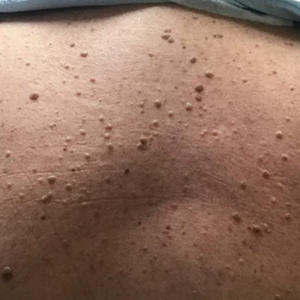
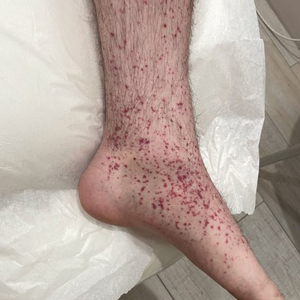
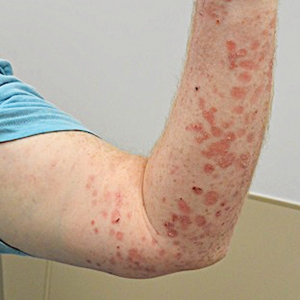
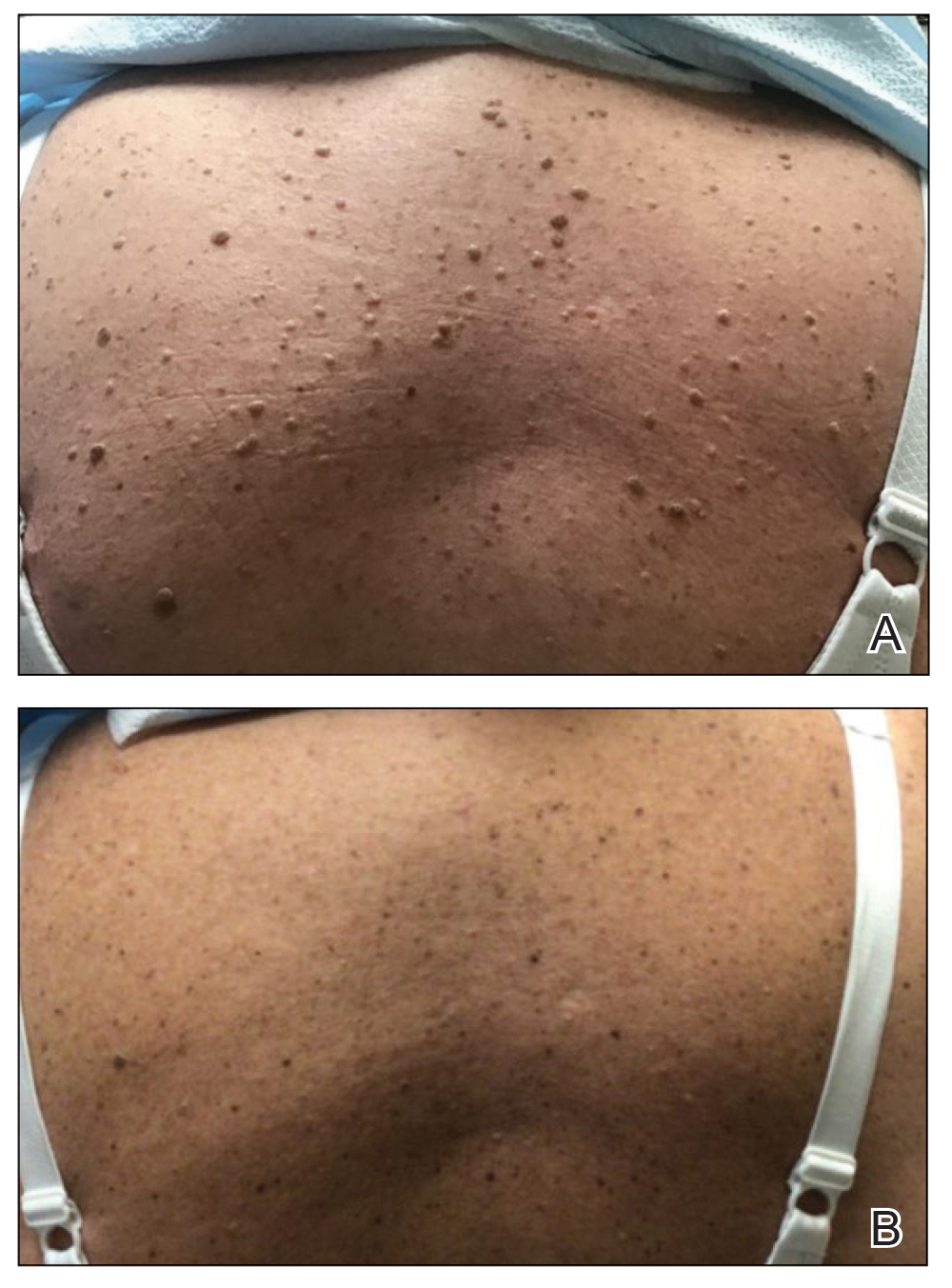
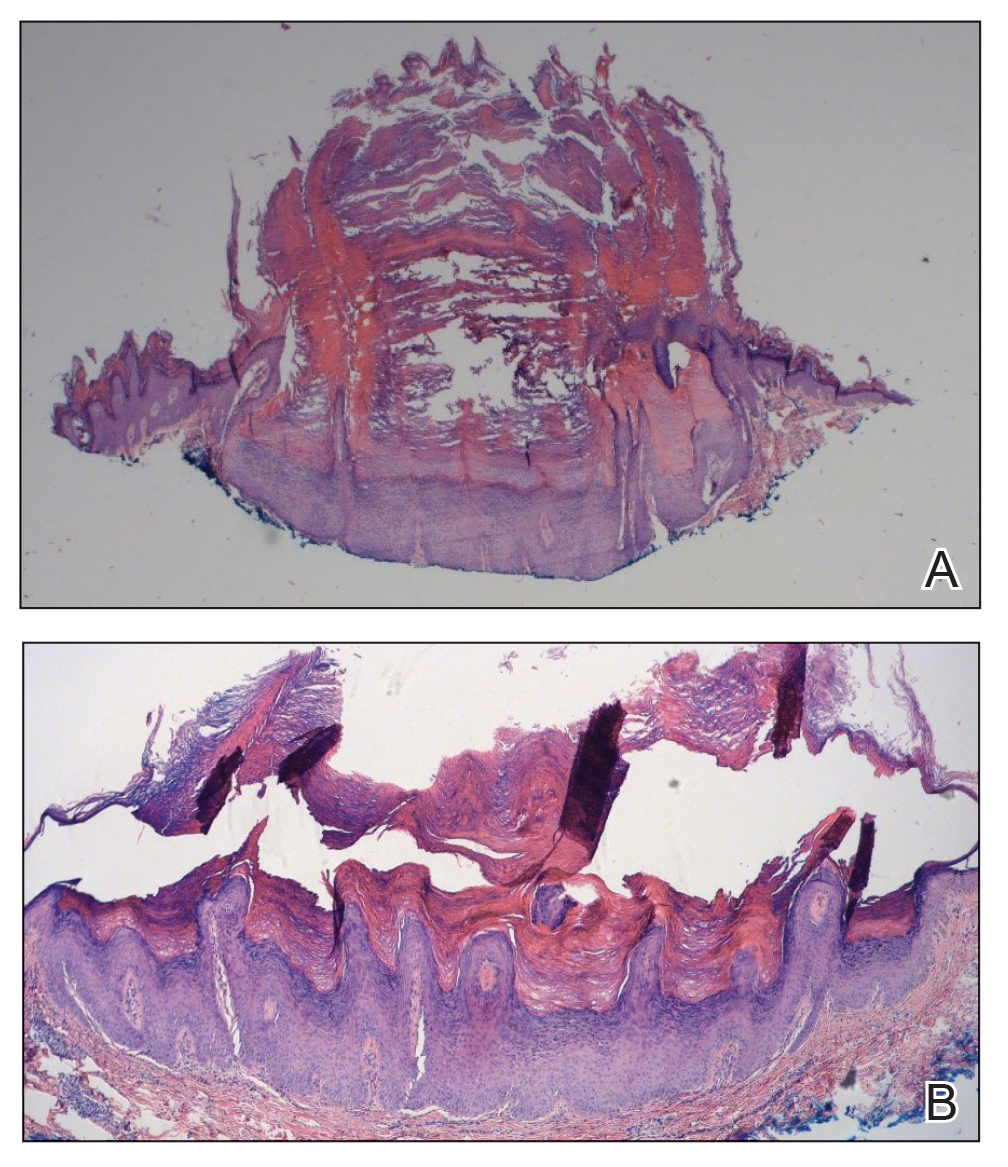
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.
Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1 BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.


Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
Practice Points
- Verrucous keratoses are common cutaneous adverse events (AEs) associated with BRAF inhibitor therapy.
- Verrucous papules may be a paraneoplastic phenomenon and can be differentiated from a treatment-related AE based on the timing and progression in relation to tumor burden.
- Although treatment of particularly bothersome lesions with cryotherapy may be warranted, verrucous papules secondary to BRAF inhibitor therapy may resolve spontaneously.
Rare Case of Photodistributed Hyperpigmentation Linked to Kratom Consumption
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
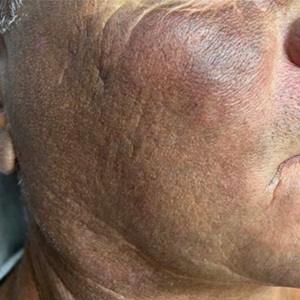
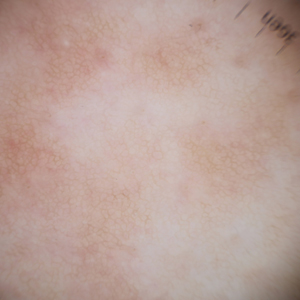
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.
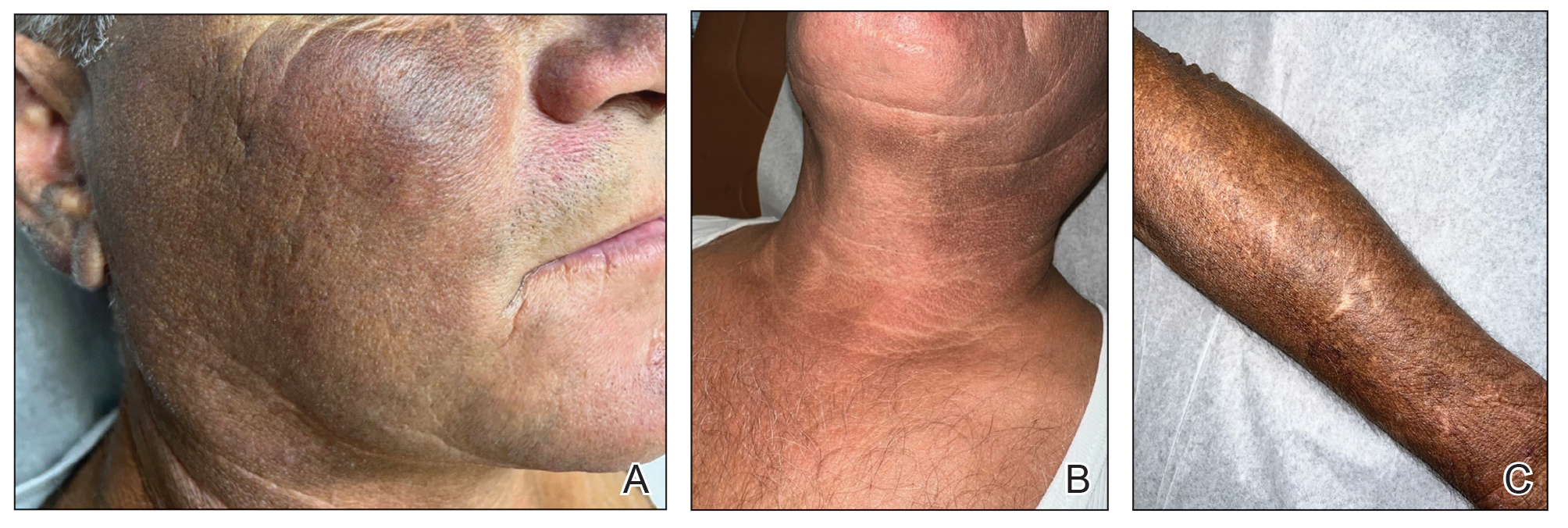
When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
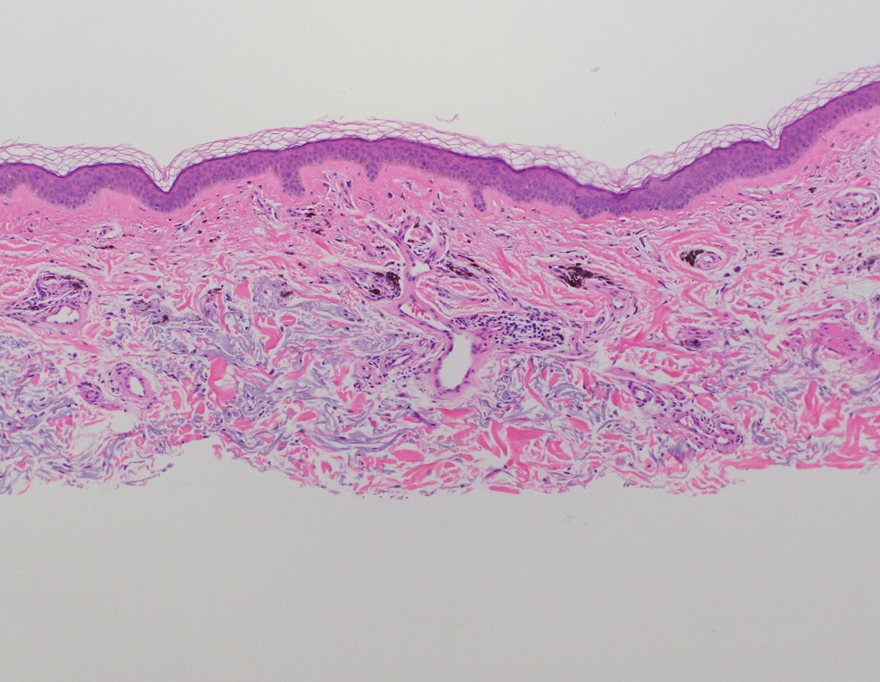
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).
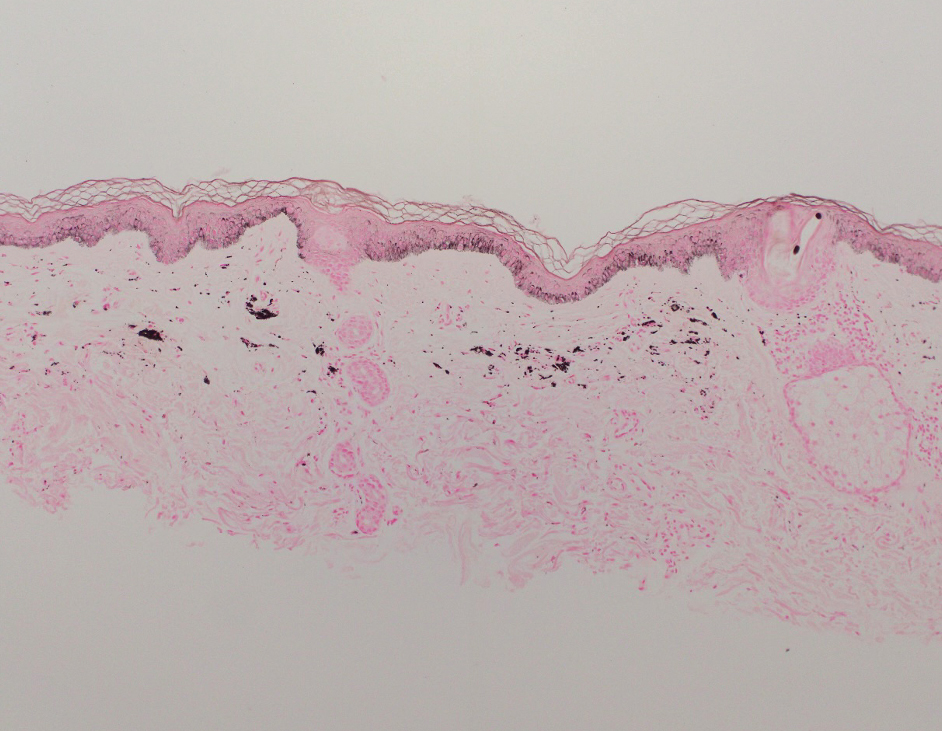
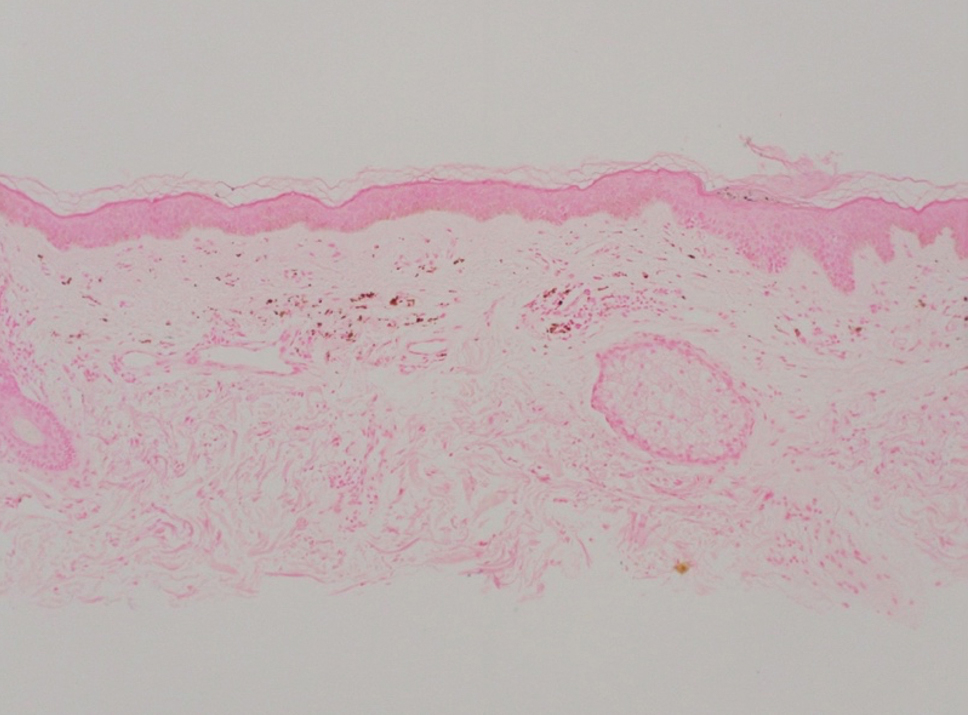
Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
Practice Points
- Clinicians should be aware of photodistributed hyperpigmentation as a potential adverse effect of kratom usage.
- Kratom-induced photodistributed hyperpigmentation should be suspected in patients with hyperpigmented lesions in sun-exposed areas of the skin following kratom use. A biopsy of lesions should be obtained to confirm the diagnosis.
- Cessation of kratom should be recommended.
Successful Treatment of Refractory Extensive Pityriasis Rubra Pilaris With Risankizumab and Acitretin
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare papulosquamous condition with an unknown pathogenesis and limited efficacy data, which can make treatment challenging. Some cases of PRP spontaneously resolve in a few months, which is most common in the pediatric population.1 Pityriasis rubra pilaris in adults is likely to persist for years, and spontaneous resolution is unpredictable. Randomized clinical trials are difficult to perform due to the rarity of PRP.
Although there is no cure and no standard protocol for treating PRP, systemic retinoids historically are considered first-line therapy for moderate to severe cases.2 Additional management approaches include symptomatic control with moisturizers and psychological support. Alternative systemic treatments for moderate to severe cases include methotrexate, phototherapy, and cyclosporine.2
Pityriasis rubra pilaris demonstrates a favorable response to methotrexate treatment, especially in type I cases; however, patients on this alternative therapy should be monitored for severe adverse effects (eg, hepatotoxicity, pancytopenia, pneumonitis).2 Phototherapy should be approached with caution. Narrowband UVB, UVA1, and psoralen plus UVA therapy have successfully treated PRP; however, the response is variable. In some cases, the opposite effect can occur, in which the condition is photoaggravated. Phototherapy is a valid alternative form of treatment when used in combination with acitretin, and a phototest should be performed prior to starting this regimen. Cyclosporine is another immunosuppressant that can be considered for PRP treatment, though there are limited data demonstrating its efficacy.2
The introduction of biologic agents has changed the treatment approach for many dermatologic diseases, including PRP. Given the similar features between psoriasis and PRP, the biologics prescribed for psoriasis therapy also are used for patients with PRP that is challenging to treat, such as anti–tumor necrosis factor α inhibitors and IL inhibitors—specifically IL-17 and IL-23. Remission has been achieved with the use of biologics in combination with retinoid therapy.2
Biologic therapies used for PRP effectively inhibit cytokines and reduce the overall inflammatory processes involved in the development of the scaly patches and plaques seen in this condition. However, most reported clinical experiences are case studies, and more research in the form of randomized clinical trials is needed to understand the efficacy and long-term effects of this form of treatment in PRP. We present a case of a patient with refractory adult subtype I PRP that was successfully treated with the IL-23 inhibitor risankizumab.
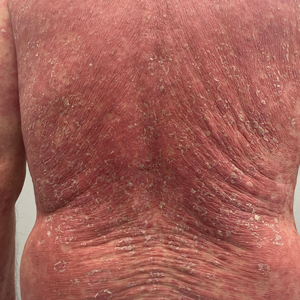
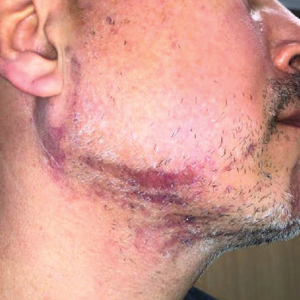
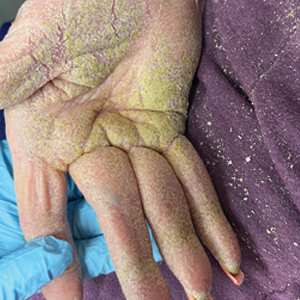
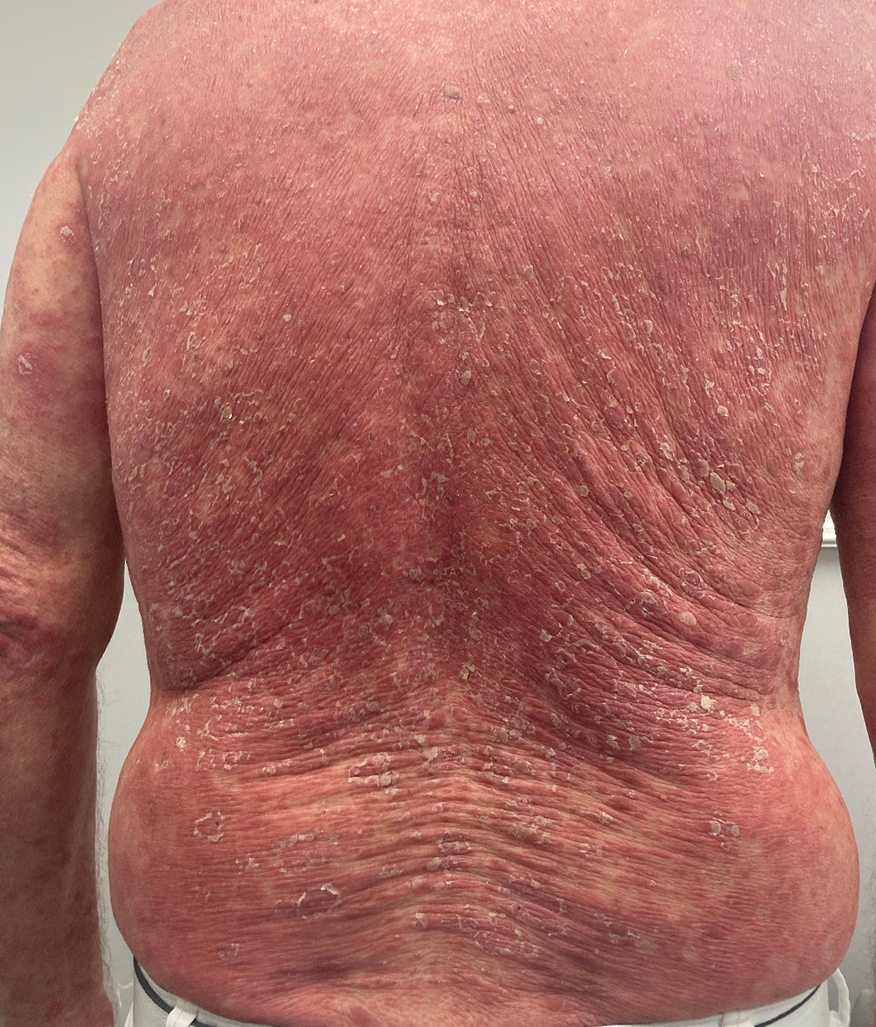
A 65-year-old man was referred to Florida Academic Dermatology Center (Coral Gables, Florida) with biopsy-proven PRP diagnosed 1 year prior. The patient reported experiencing a debilitating quality of life in the year since diagnosis (Figure 1). Treatment attempts with dupilumab, tralokinumab, intramuscular steroid injections, and topical corticosteroids had failed (Figure 2). Following evaluation at Florida Academic Dermatology Center, the patient was started on acitretin 25 mg every other day and received an initial subcutaneous injection of ixekizumab 160 mg (an IL-17 inhibitor) followed 2 weeks later by a second injection of 80 mg. After the 2 doses of ixekizumab, the patient’s condition worsened with the development of pinpoint hemorrhagic lesions. The medication was discontinued, and he was started on risankizumab 150 mg at the approved dosing regimen for plaque psoriasis in combination with the acitretin therapy. Prior to starting risankizumab, the affected body surface area (BSA) was 80%. At 1-month follow-up, he showed improvement with reduction in scaling and erythema and an affected BSA of 30% (Figure 3). At 4-month follow-up, he continued showing improvement with an affected BSA of 10% (Figure 4). Acitretin was discontinued, and the patient has been successfully maintained on risankizumab 150 mg/mL subcutaneous injections every 12 weeks since.
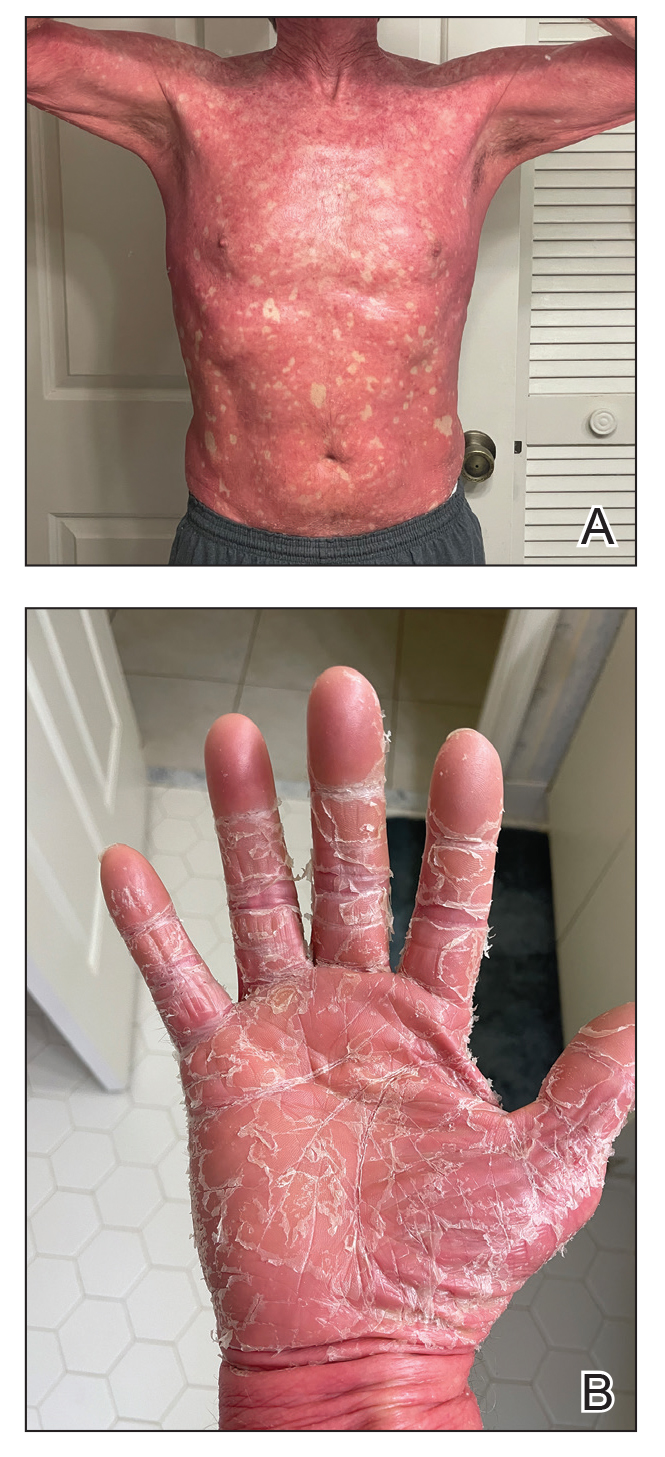
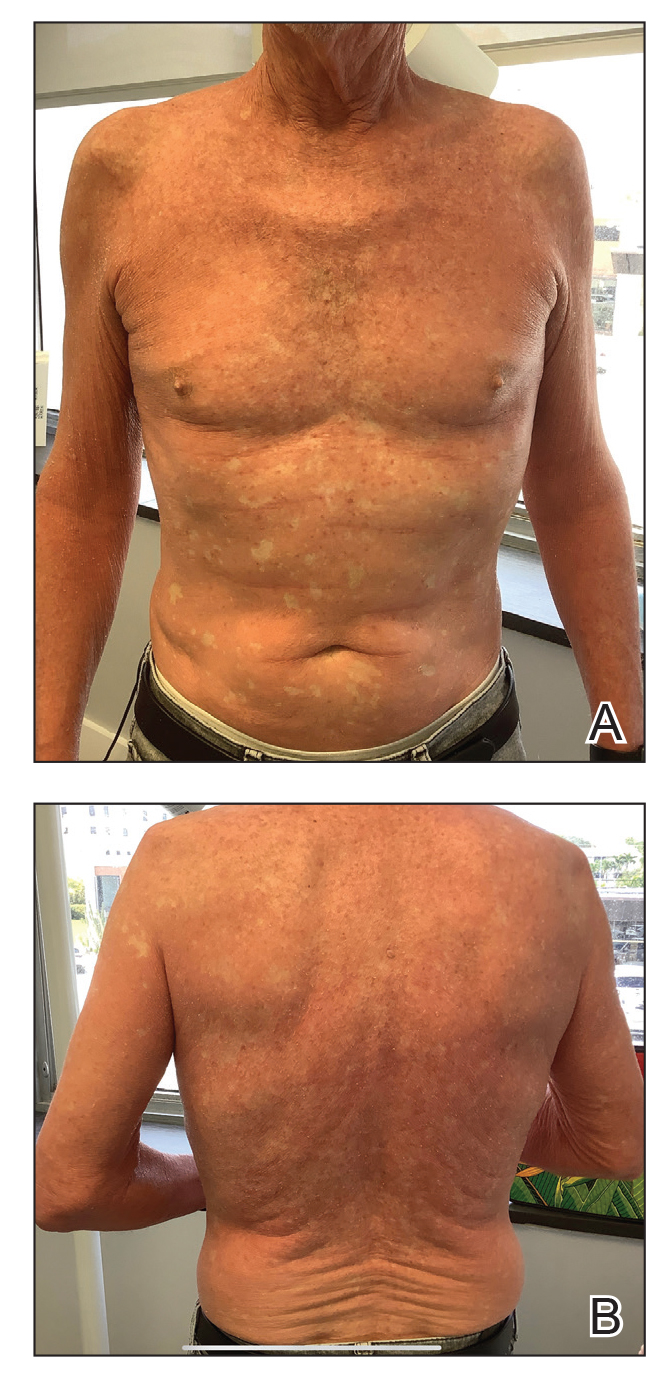
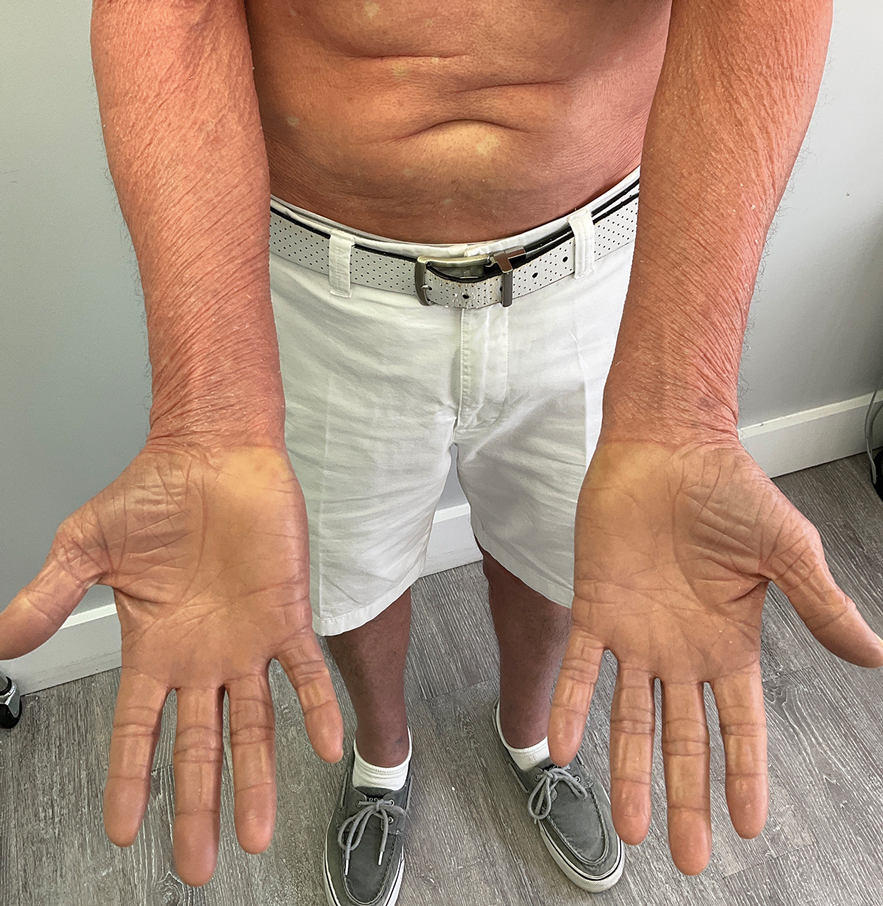
Oral retinoid therapy historically was considered first-line therapy for moderate to severe PRP. A systematic review (N=105) of retinoid therapies showed 83% of patients with PRP who were treated with acitretin plus biologic therapy had a favorable response, whereas only 36% of patients treated with acitretin as monotherapy had the same response, highlighting the importance of dual therapy.3 The use of ustekinumab, ixekizumab, and secukinumab (IL-17 inhibitors) for refractory PRP has been well documented, but a PubMed search of articles indexed for MEDLINE using the search terms risankizumab and pityriasis rubra pilaris yielded only 8 published cases of risankizumab for treatment of PRP.4-8 All patients were diagnosed with refractory PRP, and multiple treatment modalities failed.
Ustekinumab has been shown to create a rapid response and maintain it long term, especially in patients with type 1 PRP who did not respond to systemic therapies or anti–tumor necrosis factor α agents.2 An open-label, single-arm clinical trial found secukinumab was an effective therapy for PRP and demonstrated transcription heterogeneity of this dermatologic condition.9 The researchers proposed that some patients may respond to IL-17 inhibitors but others may not due to the differences in RNA molecules transcribed.9 Our patient demonstrated worsening of his condition with an IL-17 inhibitor but experienced remarkable improvement with risankizumab, an IL-23 inhibitor.
Risankizumab is indicated for the treatment of adults with moderate to severe plaque psoriasis. This humanized IgG1 monoclonal antibody targets the p19 subunit of IL-23, inhibiting its role in the pathogenic helper T cell (TH17) pathway. Research has shown that it is an efficacious and well-tolerated treatment modality for psoriatic conditions.10 It is well known that PRP and psoriasis have similar cytokine activations; therefore, we propose that combination therapy with risankizumab and acitretin may show promise for refractory PRP.
- Gelmetti C, Schiuma AA, Cerri D, et al. Pityriasis rubra pilaris in childhood: a long-term study of 29 cases. Pediatr Dermatol. 1986;3:446-451. doi:10.1111/j.1525-1470.1986.tb00648.x
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351
- Engelmann C, Elsner P, Miguel D. Treatment of pityriasis rubra pilaris type I: a systematic review. Eur J Dermatol. 2019;29:524-537. doi:10.1684/ejd.2019.3641
- Ricar J, Cetkovska P. Successful treatment of refractory extensive pityriasis rubra pilaris with risankizumab. Br J Dermatol. 2021;184:E148. doi:10.1111/bjd.19681
- Brocco E, Laffitte E. Risankizumab for pityriasis rubra pilaris. Clin Exp Dermatol. 2021;46:1322-1324. doi:10.1111/ced.14715
- Duarte B, Paiva Lopes MJ. Response to: ‘Successful treatment of refractory extensive pityriasis rubra pilaris with risankizumab.’ Br J Dermatol. 2021;185:235-236. doi:10.1111/bjd.20061
- Kromer C, Schön MP, Mössner R. Treatment of pityriasis rubra pilaris with risankizumab in two cases. J Dtsch Dermatol Ges. 2021;19:1207-1209. doi:10.1111/ddg.14504
- Kołt-Kamińska M, Osińska A, Kaznowska E, et al. Successful treatment of pityriasis rubra pilaris with risankizumab in children. Dermatol Ther (Heidelb). 2023;13:2431-2441. doi:10.1007/s13555-023-01005-y
- Boudreaux BW, Pincelli TP, Bhullar PK, et al. Secukinumab for the treatment of adult-onset pityriasis rubra pilaris: a single-arm clinical trial with transcriptomic analysis. Br J Dermatol. 2022;187:650-658. doi:10.1111/bjd.21708
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare papulosquamous condition with an unknown pathogenesis and limited efficacy data, which can make treatment challenging. Some cases of PRP spontaneously resolve in a few months, which is most common in the pediatric population.1 Pityriasis rubra pilaris in adults is likely to persist for years, and spontaneous resolution is unpredictable. Randomized clinical trials are difficult to perform due to the rarity of PRP.
Although there is no cure and no standard protocol for treating PRP, systemic retinoids historically are considered first-line therapy for moderate to severe cases.2 Additional management approaches include symptomatic control with moisturizers and psychological support. Alternative systemic treatments for moderate to severe cases include methotrexate, phototherapy, and cyclosporine.2
Pityriasis rubra pilaris demonstrates a favorable response to methotrexate treatment, especially in type I cases; however, patients on this alternative therapy should be monitored for severe adverse effects (eg, hepatotoxicity, pancytopenia, pneumonitis).2 Phototherapy should be approached with caution. Narrowband UVB, UVA1, and psoralen plus UVA therapy have successfully treated PRP; however, the response is variable. In some cases, the opposite effect can occur, in which the condition is photoaggravated. Phototherapy is a valid alternative form of treatment when used in combination with acitretin, and a phototest should be performed prior to starting this regimen. Cyclosporine is another immunosuppressant that can be considered for PRP treatment, though there are limited data demonstrating its efficacy.2
The introduction of biologic agents has changed the treatment approach for many dermatologic diseases, including PRP. Given the similar features between psoriasis and PRP, the biologics prescribed for psoriasis therapy also are used for patients with PRP that is challenging to treat, such as anti–tumor necrosis factor α inhibitors and IL inhibitors—specifically IL-17 and IL-23. Remission has been achieved with the use of biologics in combination with retinoid therapy.2
Biologic therapies used for PRP effectively inhibit cytokines and reduce the overall inflammatory processes involved in the development of the scaly patches and plaques seen in this condition. However, most reported clinical experiences are case studies, and more research in the form of randomized clinical trials is needed to understand the efficacy and long-term effects of this form of treatment in PRP. We present a case of a patient with refractory adult subtype I PRP that was successfully treated with the IL-23 inhibitor risankizumab.
A 65-year-old man was referred to Florida Academic Dermatology Center (Coral Gables, Florida) with biopsy-proven PRP diagnosed 1 year prior. The patient reported experiencing a debilitating quality of life in the year since diagnosis (Figure 1). Treatment attempts with dupilumab, tralokinumab, intramuscular steroid injections, and topical corticosteroids had failed (Figure 2). Following evaluation at Florida Academic Dermatology Center, the patient was started on acitretin 25 mg every other day and received an initial subcutaneous injection of ixekizumab 160 mg (an IL-17 inhibitor) followed 2 weeks later by a second injection of 80 mg. After the 2 doses of ixekizumab, the patient’s condition worsened with the development of pinpoint hemorrhagic lesions. The medication was discontinued, and he was started on risankizumab 150 mg at the approved dosing regimen for plaque psoriasis in combination with the acitretin therapy. Prior to starting risankizumab, the affected body surface area (BSA) was 80%. At 1-month follow-up, he showed improvement with reduction in scaling and erythema and an affected BSA of 30% (Figure 3). At 4-month follow-up, he continued showing improvement with an affected BSA of 10% (Figure 4). Acitretin was discontinued, and the patient has been successfully maintained on risankizumab 150 mg/mL subcutaneous injections every 12 weeks since.




Oral retinoid therapy historically was considered first-line therapy for moderate to severe PRP. A systematic review (N=105) of retinoid therapies showed 83% of patients with PRP who were treated with acitretin plus biologic therapy had a favorable response, whereas only 36% of patients treated with acitretin as monotherapy had the same response, highlighting the importance of dual therapy.3 The use of ustekinumab, ixekizumab, and secukinumab (IL-17 inhibitors) for refractory PRP has been well documented, but a PubMed search of articles indexed for MEDLINE using the search terms risankizumab and pityriasis rubra pilaris yielded only 8 published cases of risankizumab for treatment of PRP.4-8 All patients were diagnosed with refractory PRP, and multiple treatment modalities failed.
Ustekinumab has been shown to create a rapid response and maintain it long term, especially in patients with type 1 PRP who did not respond to systemic therapies or anti–tumor necrosis factor α agents.2 An open-label, single-arm clinical trial found secukinumab was an effective therapy for PRP and demonstrated transcription heterogeneity of this dermatologic condition.9 The researchers proposed that some patients may respond to IL-17 inhibitors but others may not due to the differences in RNA molecules transcribed.9 Our patient demonstrated worsening of his condition with an IL-17 inhibitor but experienced remarkable improvement with risankizumab, an IL-23 inhibitor.
Risankizumab is indicated for the treatment of adults with moderate to severe plaque psoriasis. This humanized IgG1 monoclonal antibody targets the p19 subunit of IL-23, inhibiting its role in the pathogenic helper T cell (TH17) pathway. Research has shown that it is an efficacious and well-tolerated treatment modality for psoriatic conditions.10 It is well known that PRP and psoriasis have similar cytokine activations; therefore, we propose that combination therapy with risankizumab and acitretin may show promise for refractory PRP.
To the Editor:
Pityriasis rubra pilaris (PRP) is a rare papulosquamous condition with an unknown pathogenesis and limited efficacy data, which can make treatment challenging. Some cases of PRP spontaneously resolve in a few months, which is most common in the pediatric population.1 Pityriasis rubra pilaris in adults is likely to persist for years, and spontaneous resolution is unpredictable. Randomized clinical trials are difficult to perform due to the rarity of PRP.
Although there is no cure and no standard protocol for treating PRP, systemic retinoids historically are considered first-line therapy for moderate to severe cases.2 Additional management approaches include symptomatic control with moisturizers and psychological support. Alternative systemic treatments for moderate to severe cases include methotrexate, phototherapy, and cyclosporine.2
Pityriasis rubra pilaris demonstrates a favorable response to methotrexate treatment, especially in type I cases; however, patients on this alternative therapy should be monitored for severe adverse effects (eg, hepatotoxicity, pancytopenia, pneumonitis).2 Phototherapy should be approached with caution. Narrowband UVB, UVA1, and psoralen plus UVA therapy have successfully treated PRP; however, the response is variable. In some cases, the opposite effect can occur, in which the condition is photoaggravated. Phototherapy is a valid alternative form of treatment when used in combination with acitretin, and a phototest should be performed prior to starting this regimen. Cyclosporine is another immunosuppressant that can be considered for PRP treatment, though there are limited data demonstrating its efficacy.2
The introduction of biologic agents has changed the treatment approach for many dermatologic diseases, including PRP. Given the similar features between psoriasis and PRP, the biologics prescribed for psoriasis therapy also are used for patients with PRP that is challenging to treat, such as anti–tumor necrosis factor α inhibitors and IL inhibitors—specifically IL-17 and IL-23. Remission has been achieved with the use of biologics in combination with retinoid therapy.2
Biologic therapies used for PRP effectively inhibit cytokines and reduce the overall inflammatory processes involved in the development of the scaly patches and plaques seen in this condition. However, most reported clinical experiences are case studies, and more research in the form of randomized clinical trials is needed to understand the efficacy and long-term effects of this form of treatment in PRP. We present a case of a patient with refractory adult subtype I PRP that was successfully treated with the IL-23 inhibitor risankizumab.
A 65-year-old man was referred to Florida Academic Dermatology Center (Coral Gables, Florida) with biopsy-proven PRP diagnosed 1 year prior. The patient reported experiencing a debilitating quality of life in the year since diagnosis (Figure 1). Treatment attempts with dupilumab, tralokinumab, intramuscular steroid injections, and topical corticosteroids had failed (Figure 2). Following evaluation at Florida Academic Dermatology Center, the patient was started on acitretin 25 mg every other day and received an initial subcutaneous injection of ixekizumab 160 mg (an IL-17 inhibitor) followed 2 weeks later by a second injection of 80 mg. After the 2 doses of ixekizumab, the patient’s condition worsened with the development of pinpoint hemorrhagic lesions. The medication was discontinued, and he was started on risankizumab 150 mg at the approved dosing regimen for plaque psoriasis in combination with the acitretin therapy. Prior to starting risankizumab, the affected body surface area (BSA) was 80%. At 1-month follow-up, he showed improvement with reduction in scaling and erythema and an affected BSA of 30% (Figure 3). At 4-month follow-up, he continued showing improvement with an affected BSA of 10% (Figure 4). Acitretin was discontinued, and the patient has been successfully maintained on risankizumab 150 mg/mL subcutaneous injections every 12 weeks since.




Oral retinoid therapy historically was considered first-line therapy for moderate to severe PRP. A systematic review (N=105) of retinoid therapies showed 83% of patients with PRP who were treated with acitretin plus biologic therapy had a favorable response, whereas only 36% of patients treated with acitretin as monotherapy had the same response, highlighting the importance of dual therapy.3 The use of ustekinumab, ixekizumab, and secukinumab (IL-17 inhibitors) for refractory PRP has been well documented, but a PubMed search of articles indexed for MEDLINE using the search terms risankizumab and pityriasis rubra pilaris yielded only 8 published cases of risankizumab for treatment of PRP.4-8 All patients were diagnosed with refractory PRP, and multiple treatment modalities failed.
Ustekinumab has been shown to create a rapid response and maintain it long term, especially in patients with type 1 PRP who did not respond to systemic therapies or anti–tumor necrosis factor α agents.2 An open-label, single-arm clinical trial found secukinumab was an effective therapy for PRP and demonstrated transcription heterogeneity of this dermatologic condition.9 The researchers proposed that some patients may respond to IL-17 inhibitors but others may not due to the differences in RNA molecules transcribed.9 Our patient demonstrated worsening of his condition with an IL-17 inhibitor but experienced remarkable improvement with risankizumab, an IL-23 inhibitor.
Risankizumab is indicated for the treatment of adults with moderate to severe plaque psoriasis. This humanized IgG1 monoclonal antibody targets the p19 subunit of IL-23, inhibiting its role in the pathogenic helper T cell (TH17) pathway. Research has shown that it is an efficacious and well-tolerated treatment modality for psoriatic conditions.10 It is well known that PRP and psoriasis have similar cytokine activations; therefore, we propose that combination therapy with risankizumab and acitretin may show promise for refractory PRP.
- Gelmetti C, Schiuma AA, Cerri D, et al. Pityriasis rubra pilaris in childhood: a long-term study of 29 cases. Pediatr Dermatol. 1986;3:446-451. doi:10.1111/j.1525-1470.1986.tb00648.x
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351
- Engelmann C, Elsner P, Miguel D. Treatment of pityriasis rubra pilaris type I: a systematic review. Eur J Dermatol. 2019;29:524-537. doi:10.1684/ejd.2019.3641
- Ricar J, Cetkovska P. Successful treatment of refractory extensive pityriasis rubra pilaris with risankizumab. Br J Dermatol. 2021;184:E148. doi:10.1111/bjd.19681
- Brocco E, Laffitte E. Risankizumab for pityriasis rubra pilaris. Clin Exp Dermatol. 2021;46:1322-1324. doi:10.1111/ced.14715
- Duarte B, Paiva Lopes MJ. Response to: ‘Successful treatment of refractory extensive pityriasis rubra pilaris with risankizumab.’ Br J Dermatol. 2021;185:235-236. doi:10.1111/bjd.20061
- Kromer C, Schön MP, Mössner R. Treatment of pityriasis rubra pilaris with risankizumab in two cases. J Dtsch Dermatol Ges. 2021;19:1207-1209. doi:10.1111/ddg.14504
- Kołt-Kamińska M, Osińska A, Kaznowska E, et al. Successful treatment of pityriasis rubra pilaris with risankizumab in children. Dermatol Ther (Heidelb). 2023;13:2431-2441. doi:10.1007/s13555-023-01005-y
- Boudreaux BW, Pincelli TP, Bhullar PK, et al. Secukinumab for the treatment of adult-onset pityriasis rubra pilaris: a single-arm clinical trial with transcriptomic analysis. Br J Dermatol. 2022;187:650-658. doi:10.1111/bjd.21708
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
- Gelmetti C, Schiuma AA, Cerri D, et al. Pityriasis rubra pilaris in childhood: a long-term study of 29 cases. Pediatr Dermatol. 1986;3:446-451. doi:10.1111/j.1525-1470.1986.tb00648.x
- Moretta G, De Luca EV, Di Stefani A. Management of refractory pityriasis rubra pilaris: challenges and solutions. Clin Cosmet Investig Dermatol. 2017;10:451-457. doi:10.2147/CCID.S124351
- Engelmann C, Elsner P, Miguel D. Treatment of pityriasis rubra pilaris type I: a systematic review. Eur J Dermatol. 2019;29:524-537. doi:10.1684/ejd.2019.3641
- Ricar J, Cetkovska P. Successful treatment of refractory extensive pityriasis rubra pilaris with risankizumab. Br J Dermatol. 2021;184:E148. doi:10.1111/bjd.19681
- Brocco E, Laffitte E. Risankizumab for pityriasis rubra pilaris. Clin Exp Dermatol. 2021;46:1322-1324. doi:10.1111/ced.14715
- Duarte B, Paiva Lopes MJ. Response to: ‘Successful treatment of refractory extensive pityriasis rubra pilaris with risankizumab.’ Br J Dermatol. 2021;185:235-236. doi:10.1111/bjd.20061
- Kromer C, Schön MP, Mössner R. Treatment of pityriasis rubra pilaris with risankizumab in two cases. J Dtsch Dermatol Ges. 2021;19:1207-1209. doi:10.1111/ddg.14504
- Kołt-Kamińska M, Osińska A, Kaznowska E, et al. Successful treatment of pityriasis rubra pilaris with risankizumab in children. Dermatol Ther (Heidelb). 2023;13:2431-2441. doi:10.1007/s13555-023-01005-y
- Boudreaux BW, Pincelli TP, Bhullar PK, et al. Secukinumab for the treatment of adult-onset pityriasis rubra pilaris: a single-arm clinical trial with transcriptomic analysis. Br J Dermatol. 2022;187:650-658. doi:10.1111/bjd.21708
- Blauvelt A, Leonardi CL, Gooderham M, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649-658. doi:10.1001/jamadermatol.2020.0723
Practice Points
- Pityriasis rubra pilaris (PRP) is a rare condition that is challenging to treat due to its unknown pathogenesis and limited efficacy data. Systemic retinoids historically were considered first-line therapy for moderate to severe cases of PRP.
- Biologics may be useful for refractory cases of PRP.
- Risankizumab is approved for moderate to severe plaque psoriasis and can be considered off-label for refractory PRP.
Blaschkolinear Lupus Erythematosus: Strategies for Early Detection and Management
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).
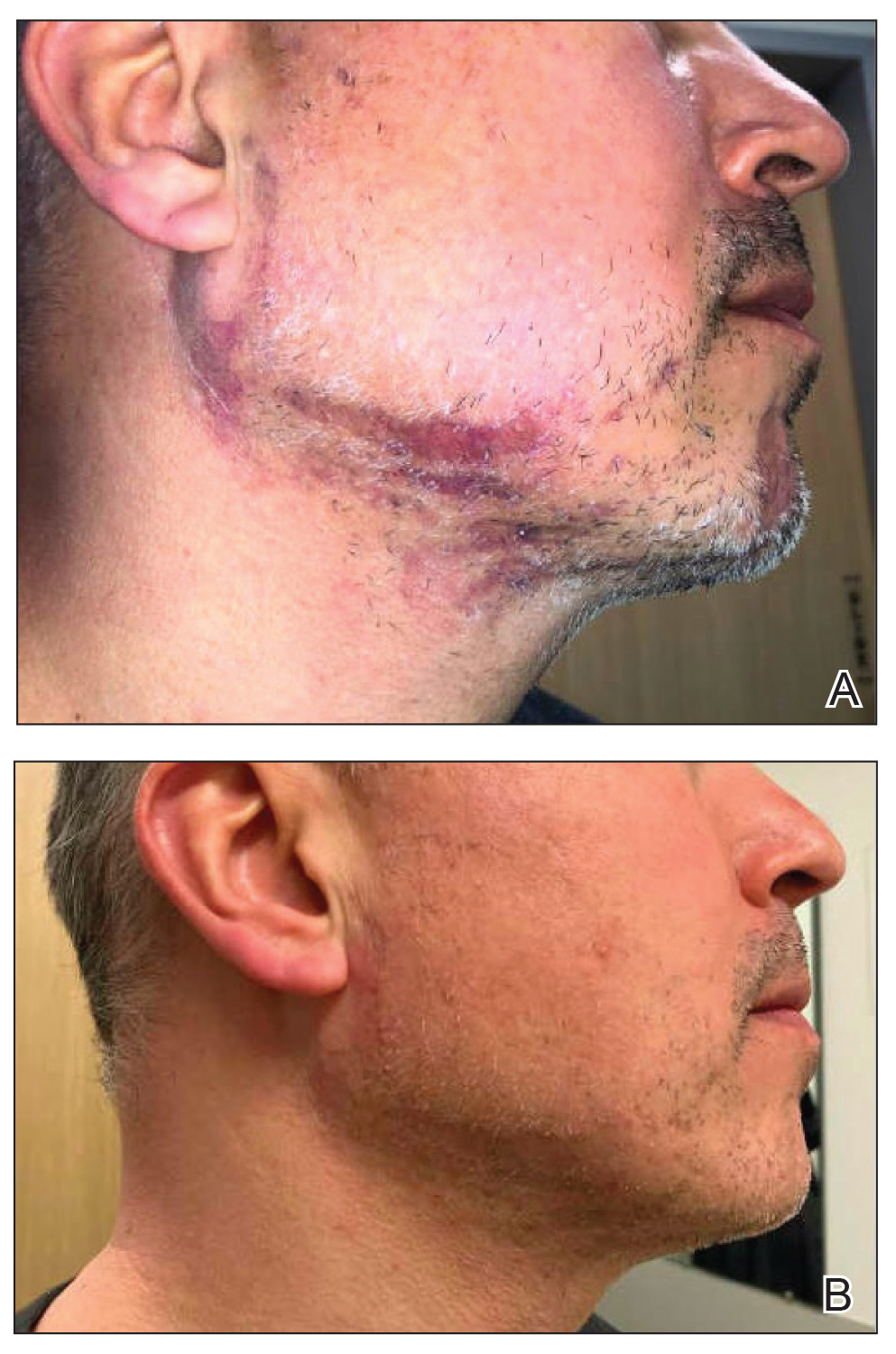
Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).

Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
To the Editor:
Chronic cutaneous lupus erythematosus (CCLE) is an inflammatory condition with myriad cutaneous manifestations. Most forms of CCLE have the potential to progress to systemic lupus erythematosus (SLE).1
Blaschkolinear lupus erythematosus (BLE) is an exceedingly rare subtype of cutaneous lupus erythematosus that usually manifests during childhood as linear plaques along the lines of Blaschko.2,3 Under normal conditions, Blaschko lines are not noticeable; they correspond to the direction of ectodermal cell migration during cutaneous embryogenesis.4,5 The embryonic cells travel ventrolaterally, forming a V-shaped pattern on the back, an S-shaped pattern on the trunk, and an hourglass-shaped pattern on the face with several perpendicular intersections near the mouth and nose.6 During their migration, the cells are susceptible to somatic mutations and clonal expansion, resulting in a monoclonal population of genetically heterogenous cells. This phenomenon is known as somatic mosaicism and may lead to an increased susceptibility to an array of congenital and inflammatory dermatoses, such as cutaneous lupus erythematosus.4 Blaschkolinear entities tend to manifest in a unilateral distribution following exposure to a certain environmental trigger, such as trauma, viral illness, or UV radiation, although a trigger is not always present.7 We report a case of BLE manifesting on the head and neck in an adult patient.
A 46-year-old man presented with a pruritic rash of 3 months’ duration on the right cheek that extended inferiorly to the right upper chest. He had a medical history of well-controlled psoriasis, and he denied any antecedent trauma, fevers, chills, arthralgia, or night sweats. There had been no improvement with mometasone ointment 0.1% applied daily for 2 months as prescribed by his primary care provider. Physical examination revealed indurated, red-brown, atrophic plaques in a blaschkolinear distribution around the nose, right upper jaw, right side of the neck, and right upper chest (Figure, A).

Histopathology of punch biopsies from the right jaw and right upper chest showed an atrophic epidermis with scattered dyskeratotic keratinocytes and vacuolar alteration of the basal cell layer. A superficial and deep perivascular and periadnexal lymphocytic infiltrate was observed in both biopsies. Staining with Verhoeff-van Gieson elastin and periodic acid–Schiff highlighted prominent basement membrane thickening and loss of elastic fibers in the superficial dermis. These findings favored a diagnosis of CCLE, and the clinical blaschkolinear distribution of the rash led to our specific diagnosis of BLE. Laboratory workup for SLE including a complete blood cell count; urine analysis; and testing for liver and kidney function, antinuclearantibodies, complement levels, and erythrocyte sedimentation rate revealed no abnormalities.
The patient started hydroxychloroquine 200 mg twice daily and methotrexate 25 mg weekly along with strict photoprotection measures, including wearing photoprotective clothing and avoiding sunlight during the most intense hours of the day.
Linear lichen planus is an important differential diagnosis to consider in patients with a blaschkolinear eruption.7 Although the clinical manifestations of BLE and linear lichen planus are similar, they differ histopathologically. One study found that only 33.3% of patients (6/18) who clinically presented with blaschkolinear eruptions were correctly diagnosed before histologic examination.7 Visualization of the adnexa as well as the superficial and deep vascular plexuses is paramount in distinguishing between linear lichen planus and BLE; linear lichen planus does not have perivascular and periadnexal infiltration, while BLE does. Thus, in our experience, a punch biopsy—rather than a shave biopsy—should be performed to access the deeper layers of the skin.
Because these 2 entities have noteworthy differences in their management, prognosis, and long-term follow-up, accurate diagnosis is critical. To start, BLE is treated with the use of photoprotection, whereas linear lichen planus is commonly treated with phototherapy. Given the potential for forms of CCLE to progress to SLE, serial monitoring is indicated in patients with BLE. As the risk for progression to SLE is highest in the first 3 years after diagnosis, a review of systems and laboratory testing should occur every 2 to 3 months in the first year after diagnosis (sooner if the disease presentation is more severe).9 Also, treatment with hydroxychloroquine likely delays transformation to SLE and is important in the early management of BLE.10 On the other hand, linear lichen planus tends to self-resolve without progression to systemic involvement, warranting limited follow-up.9
Blaschkolinear lupus erythematosus typically manifests in childhood, but it also can be seen in adults, such as in our patient. Adult-onset BLE is rare but may be underrecognized or underreported in the literature.11 However, dermatologists should consider it in the differential diagnosis for any patient with a blaschkolinear eruption, as establishing the correct diagnosis is key to ensuring prompt and effective treatment for this rare inflammatory condition.
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
- Grönhagen CM, Fored CM, Granath F, et al. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: a population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164:1335-1341. doi:10.1111/j.1365-2133.2011.10272.x
- Requena C, Torrelo A, de Prada I, et al. Linear childhood cutaneous lupus erythematosus following Blaschko lines. J Eur Acad Dermatol Venereol. 2002;16:618-620. doi:10.1046/j.1468-3083.2002.00588.x
- Lim D, Hatami A, Kokta V, et al. Linear cutaneous lupus erythematosus in children-report of two cases and review of the literature: a case report. SAGE Open Med Case Rep. 2020;8:2050313x20979206. doi:10.1177/2050313X20979206
- Jin H, Zhang G, Zhou Y, et al. Old lines tell new tales: Blaschko linear lupus erythematosus. Autoimmun Rev. 2016;15:291-306. doi:10.1016/j.autrev.2015.11.014
- Yu S, Yu H-S. A patient with subacute cutaneous lupus erythematosus along Blaschko lines: implications for the role of keratinocytes in lupus erythematosus. Dermatologica Sinica. 2016;34:144-147. doi:10.1016/j.dsi.2015.12.002
- Kouzak SS, Mendes MST, Costa IMC. Cutaneous mosaicisms: concepts, patterns and classifications. An Bras Dermatol. 2013;88:507-517. doi:10.1590/abd1806-4841.20132015
- Liu W, Vano-Galvan S, Liu J-W, et al. Pigmented linear discoid lupus erythematosus following the lines of Blaschko: a retrospective study of a Chinese series. Indian J Dermatol Venereol Leprol. 2020;86:359-365. doi:10.4103/ijdvl.IJDVL_341_19
- O’Brien JC, Chong BF. Not just skin deep: systemic disease involvement in patients with cutaneous lupus. J Invest Dermatol Symp Proc. 2017;18:S69-S74. doi:10.1016/j.jisp.2016.09.001
- Curtiss P, Walker AM, Chong BF. A systematic review of the progression of cutaneous lupus to systemic lupus erythematosus. Front Immunol. 2022:13:866319. doi:10.3389/fimmu.2022.866319
- Okon LG, Werth VP. Cutaneous lupus erythematosus: diagnosis and treatment. Best Pract Res Clin Rheumatol. 2013;27:391-404. doi:10.1016/j.berh.2013.07.008
- Milosavljevic K, Fibeger E, Virata AR. A case of linear cutaneous lupus erythematosus in a 55-year-old woman. Am J Case Rep. 2020;21:E921495. doi:10.12659/AJCR.921495
Practice Points
- Blaschkolinear lupus erythematosus (BLE), an exceedingly rare subtype of chronic cutaneous lupus erythematosus, usually presents during childhood as linear plaques along the lines of Blaschko.
- It is important to consider linear lichen planus in patients with a blaschkolinear eruption, as the clinical manifestations are similar but there are differences in histopathology, management, prognosis, and long-term follow-up.
- Serial monitoring is indicated in patients with BLE given the potential for progression to systemic lupus erythematosus, which may be delayed with early use of hydroxychloroquine.
Misdiagnosis of Crusted Scabies: Skin Excoriations Resembling Brown Sugar Are Characteristic
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.
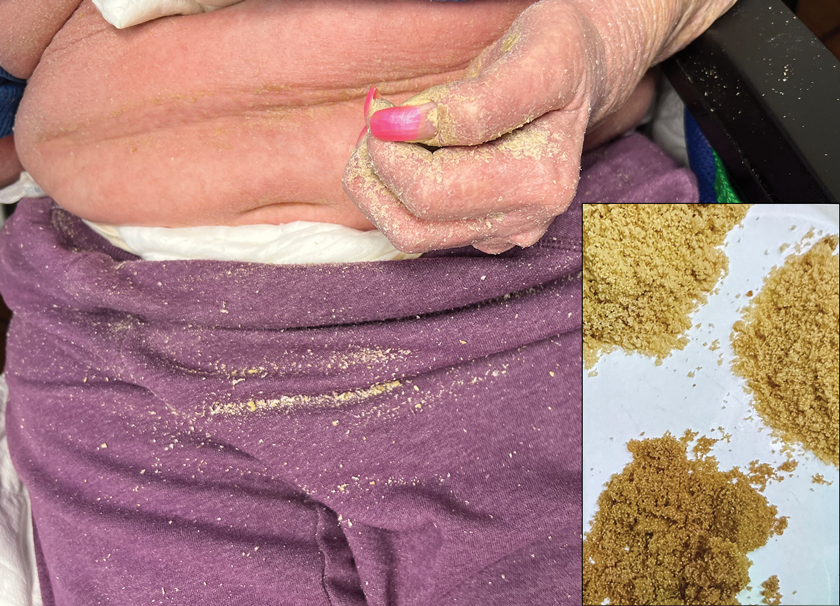
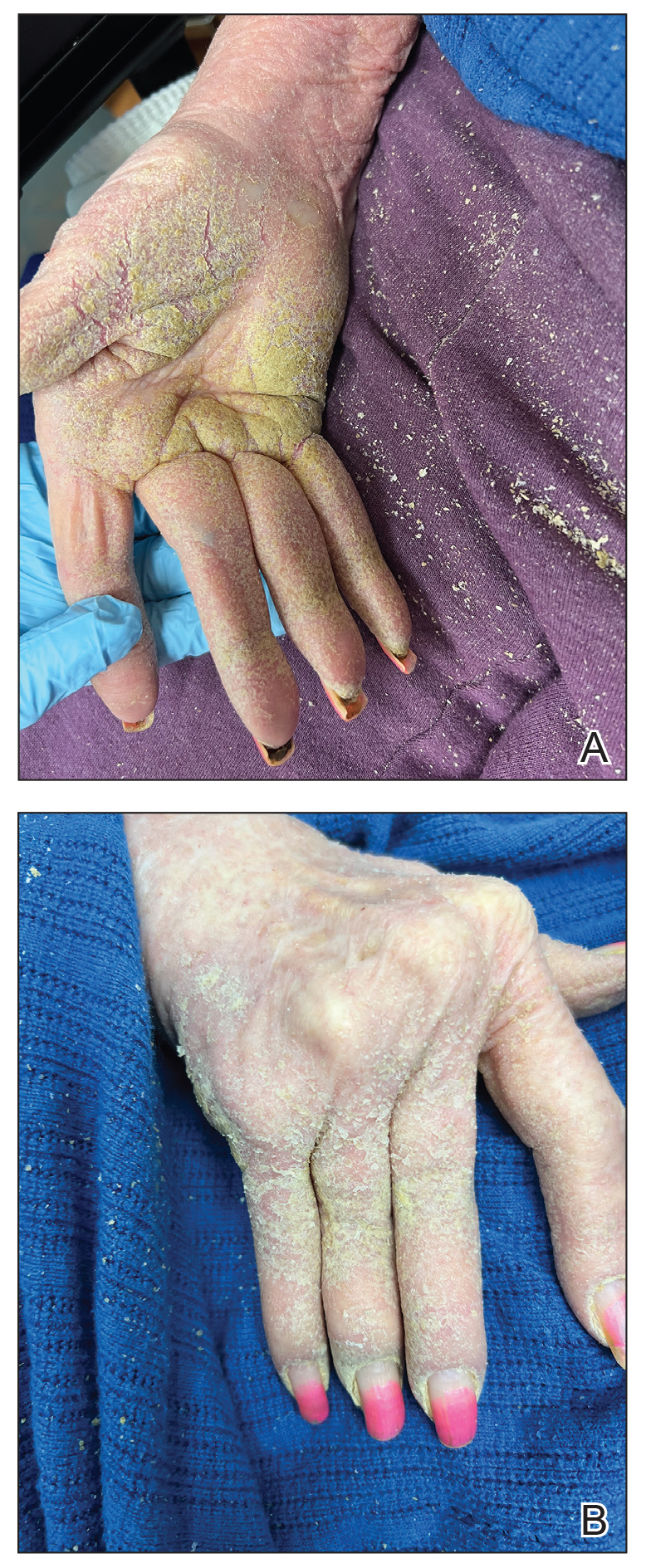
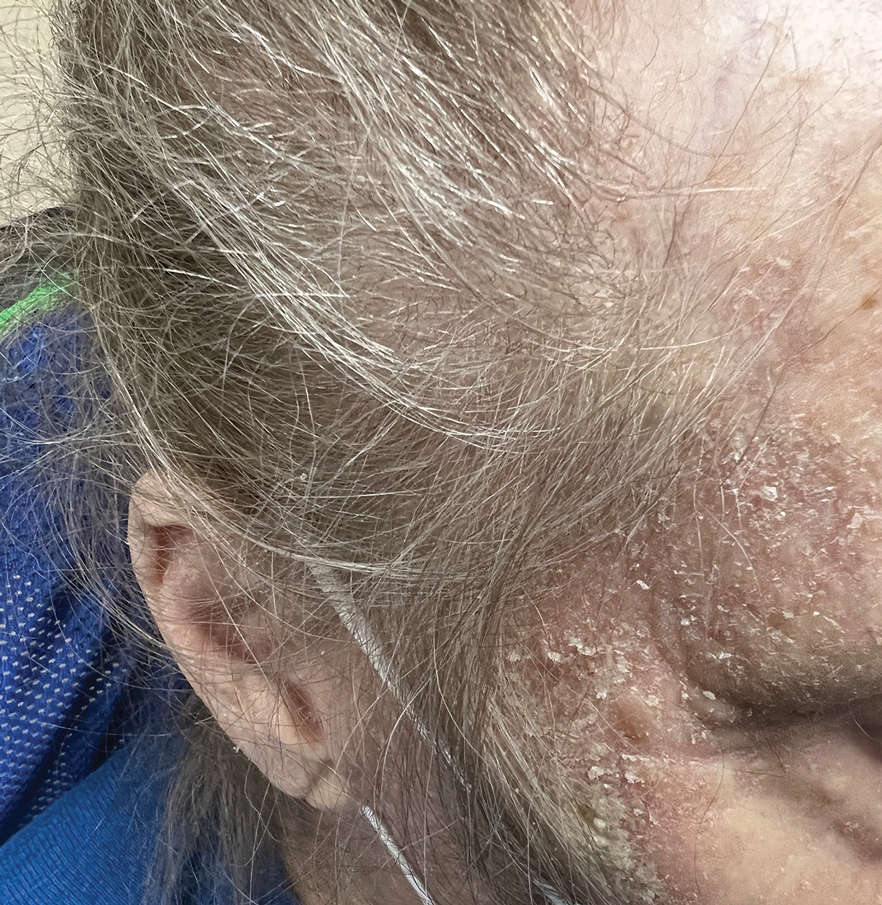
The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.
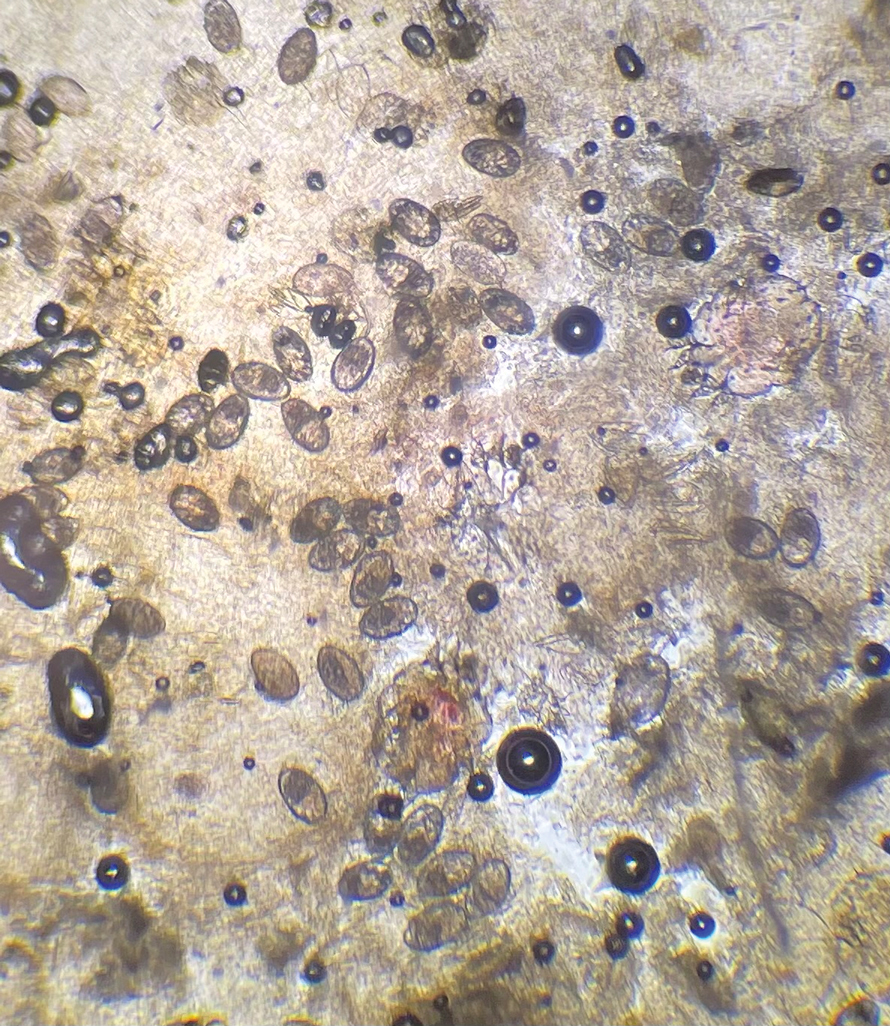
Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.



The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.

Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.



The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.

Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
PRACTICE POINTS
- Crusted scabies often is misdiagnosed because it mimics common dermatologic conditions, such as atopic dermatitis, psoriasis, drug eruption, and seborrhea. A unique feature of crusted scabies is fine or coarse scaling that resembles brown sugar.
- Immunosuppressants, such as topical corticosteroids, worsen the skin’s immune response to classic scabies infestations, which leads to parasitic overgrowth and the development of crusted scabies.
- Treatment of crusted scabies requires topical and oral scabicide; in addition, all clothing, bedding, and towels should be machine-washed and dried with hot water and hot dryer cycles to prevent environmental transmission and reinfection.
Eruptive Syringoma Manifesting as a Widespread Rash in 3 Patients
To the Editor:
Syringoma is a relatively common benign adnexal neoplasm originating in the ducts of eccrine sweat glands. It can be
A 28-year-old man presented with multiple asymptomatic papules on the trunk and upper arms of 20 years’ duration (patient 1). He had been diagnosed with Darier disease 3 years prior to the current presentation and was treated with oral and topical retinoic acid without a response. After 3 months of oral treatment, the retinoic acid was stopped due to elevated liver enzymes. Physical examination at the current presentation revealed multiple smooth, firm, nonfused, 1- to 4-mm
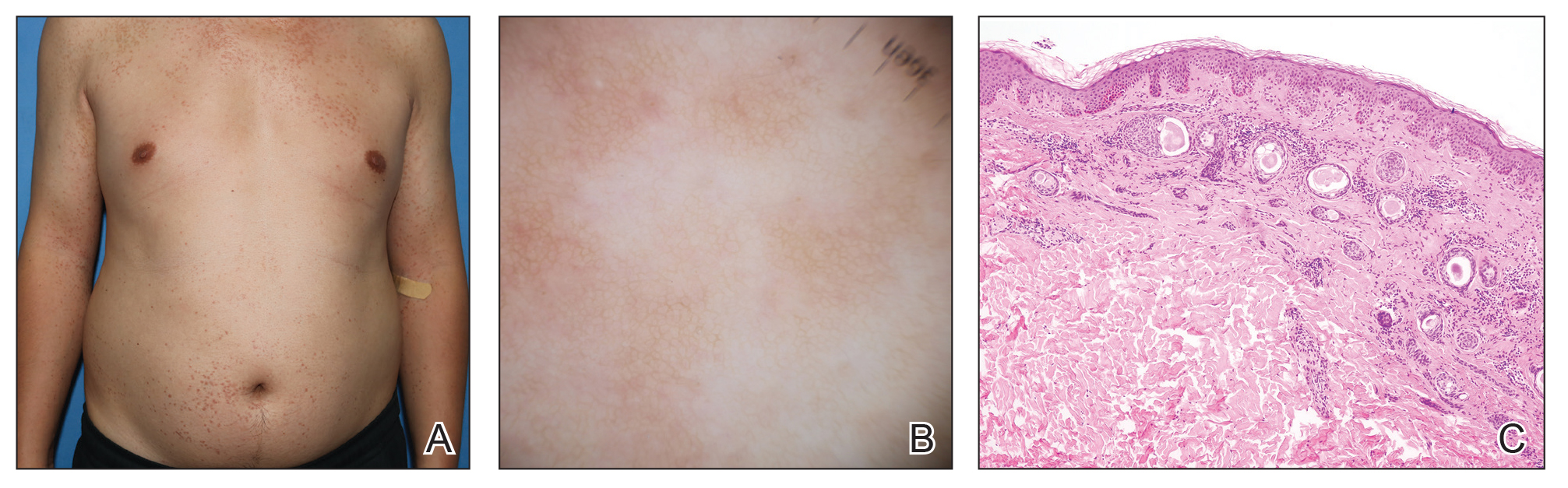
A 27-year-old woman presented with widespread asymptomatic papules

A 43-year-old man who was otherwise healthy presented with brownish flat-topped papules on the chest and abdomen of 19 years’ duration (Figure 3A)(patient 3). The lesions had remained stable and did not progress. He denied any treatment.
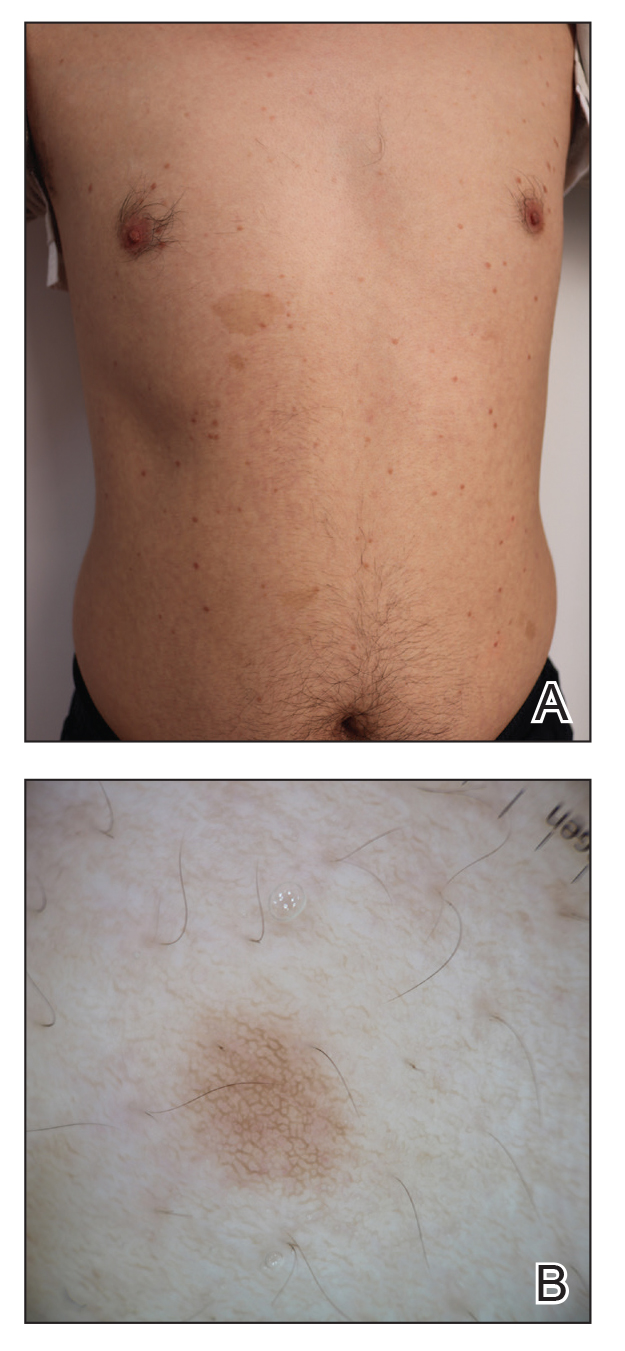
All 3 patients demonstrated classic histopathologic features of syringoma, and none had a family history of similar skin lesions. The clinical and dermoscopic findings along with the histopathology in all 3 patients were consistent with ES.
The pathogenesis of ES is
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.e1239. doi:10.1016/j.jaad.2015.12.006
- Jacquet L, Darier J.
Hidradénomes éruptifs, I.épithéliomes adenoids des glandes sudoripares ou adénomes sudoripares. Ann Dermatol Venerol. 1887;8:317-323. - Huang A, Taylor G, Liebman TN. Generalized eruptive syringomas. Dermatol Online J. 2017;23:13030/qt0hb8q22g..
- Maeda T, Natsuga K, Nishie W, et al. Extensive eruptive syringoma after liver transplantation. Acta Derm Venereol. 2018;98:119-120. doi:10.2340/00015555-2814
- Lerner TH, Barr RJ, Dolezal JF, et al. Syringomatous hyperplasia and eccrine squamous syringometaplasia associated with benoxaprofen therapy. Arch Dermatol. 1987;123:1202-1204. doi:10.1001/archderm.1987.01660330113022
- Ozturk F, Ermertcan AT, Bilac C, et al.
A case report of postpubertal eruptive syringoma triggered with antiepileptic drugs. J Drugs Dermatol. 2010;9:707-710. - Guitart J, Rosenbaum MM, Requena L. ‘Eruptive syringoma’: a misnomer for a reactive eccrine gland ductal proliferation? J Cutan Pathol. 2003;30:202-205. doi:10.1034/j.1600-0560.2003.00023.x
- Dupre A, Carrere S, Bonafe JL, et al. Eruptive generalized syringomas, milium and atrophoderma vermiculata. Nicolau and Balus’ syndrome (author’s transl). Dermatologica. 1981;162:281-286.
- Schepis C, Torre V, Siragusa M, et al. Eruptive syringomas with calcium deposits in a young woman with Down’s syndrome. Dermatology. 2001;203:345-347. doi:10.1159/000051788
- Samia AM, Donthi D, Nenow J, et al. A case study and review of literature of eruptive syringoma in a six-year-old. Cureus. 2021;13:E14634. doi:10.7759/cureus.14634
- Soler-Carrillo J, Estrach T, Mascaró JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246. doi:10.1046/j.1468-3083.2001.00235.x
- Aleissa M, Aljarbou O, AlJasser MI. Dermoscopy of eruptive syringoma. Skin Appendage Disord. 2021;7:401-403. doi:10.1159/000515443
- Botsali A, Caliskan E, Coskun A, et al. Eruptive syringoma: two cases with dermoscopic features. Skin Appendage Disord. 2020;6:319-322. doi:10.1159/000508656
- Dutra Rezende H, Madia ACT, Elias BM, et al. Comment on: eruptive syringoma—two cases with dermoscopic features. Skin Appendage Disord. 2022;8:81-82. doi:10.1159/000518158
- Silva-Hirschberg C, Cabrera R, Rollán MP, et al. Darier disease: the use of dermoscopy in monitoring acitretin treatment. An Bras Dermatol. 2022;97:644-647. doi:10.1016/j.abd.2021.05.021
- Singal A, Kaur I, Jakhar D. Fox-Fordyce disease: dermoscopic perspective. Skin Appendage Disord. 2020;6:247-249. doi:10.1159/000508201
- Brau Javier CN, Morales A, Sanchez JL. Histopathology attributes of Fox-Fordyce disease. Int J Dermatol. 2012;51:1313-1318. doi:10.1159/000508201
- Horie K, Shinkuma S, Fujita Y, et al. Efficacy of N-(3,4-dimethoxycinnamoyl)-anthranilic acid (tranilast) against eruptive syringoma: report of two cases and review of published work. J Dermatol. 2012;39:1044-1046. doi:10.1111/j.1346-8138.2012.01612.x
- Sanchez TS, Dauden E, Casas AP, et al. Eruptive pruritic syringomas: treatment with topical atropine. J Am Acad Dermatol. 2001;44:148-149. doi:10.1067/mjd.2001.109854
To the Editor:
Syringoma is a relatively common benign adnexal neoplasm originating in the ducts of eccrine sweat glands. It can be
A 28-year-old man presented with multiple asymptomatic papules on the trunk and upper arms of 20 years’ duration (patient 1). He had been diagnosed with Darier disease 3 years prior to the current presentation and was treated with oral and topical retinoic acid without a response. After 3 months of oral treatment, the retinoic acid was stopped due to elevated liver enzymes. Physical examination at the current presentation revealed multiple smooth, firm, nonfused, 1- to 4-mm

A 27-year-old woman presented with widespread asymptomatic papules

A 43-year-old man who was otherwise healthy presented with brownish flat-topped papules on the chest and abdomen of 19 years’ duration (Figure 3A)(patient 3). The lesions had remained stable and did not progress. He denied any treatment.

All 3 patients demonstrated classic histopathologic features of syringoma, and none had a family history of similar skin lesions. The clinical and dermoscopic findings along with the histopathology in all 3 patients were consistent with ES.
The pathogenesis of ES is
To the Editor:
Syringoma is a relatively common benign adnexal neoplasm originating in the ducts of eccrine sweat glands. It can be
A 28-year-old man presented with multiple asymptomatic papules on the trunk and upper arms of 20 years’ duration (patient 1). He had been diagnosed with Darier disease 3 years prior to the current presentation and was treated with oral and topical retinoic acid without a response. After 3 months of oral treatment, the retinoic acid was stopped due to elevated liver enzymes. Physical examination at the current presentation revealed multiple smooth, firm, nonfused, 1- to 4-mm

A 27-year-old woman presented with widespread asymptomatic papules

A 43-year-old man who was otherwise healthy presented with brownish flat-topped papules on the chest and abdomen of 19 years’ duration (Figure 3A)(patient 3). The lesions had remained stable and did not progress. He denied any treatment.

All 3 patients demonstrated classic histopathologic features of syringoma, and none had a family history of similar skin lesions. The clinical and dermoscopic findings along with the histopathology in all 3 patients were consistent with ES.
The pathogenesis of ES is
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.e1239. doi:10.1016/j.jaad.2015.12.006
- Jacquet L, Darier J.
Hidradénomes éruptifs, I.épithéliomes adenoids des glandes sudoripares ou adénomes sudoripares. Ann Dermatol Venerol. 1887;8:317-323. - Huang A, Taylor G, Liebman TN. Generalized eruptive syringomas. Dermatol Online J. 2017;23:13030/qt0hb8q22g..
- Maeda T, Natsuga K, Nishie W, et al. Extensive eruptive syringoma after liver transplantation. Acta Derm Venereol. 2018;98:119-120. doi:10.2340/00015555-2814
- Lerner TH, Barr RJ, Dolezal JF, et al. Syringomatous hyperplasia and eccrine squamous syringometaplasia associated with benoxaprofen therapy. Arch Dermatol. 1987;123:1202-1204. doi:10.1001/archderm.1987.01660330113022
- Ozturk F, Ermertcan AT, Bilac C, et al.
A case report of postpubertal eruptive syringoma triggered with antiepileptic drugs. J Drugs Dermatol. 2010;9:707-710. - Guitart J, Rosenbaum MM, Requena L. ‘Eruptive syringoma’: a misnomer for a reactive eccrine gland ductal proliferation? J Cutan Pathol. 2003;30:202-205. doi:10.1034/j.1600-0560.2003.00023.x
- Dupre A, Carrere S, Bonafe JL, et al. Eruptive generalized syringomas, milium and atrophoderma vermiculata. Nicolau and Balus’ syndrome (author’s transl). Dermatologica. 1981;162:281-286.
- Schepis C, Torre V, Siragusa M, et al. Eruptive syringomas with calcium deposits in a young woman with Down’s syndrome. Dermatology. 2001;203:345-347. doi:10.1159/000051788
- Samia AM, Donthi D, Nenow J, et al. A case study and review of literature of eruptive syringoma in a six-year-old. Cureus. 2021;13:E14634. doi:10.7759/cureus.14634
- Soler-Carrillo J, Estrach T, Mascaró JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246. doi:10.1046/j.1468-3083.2001.00235.x
- Aleissa M, Aljarbou O, AlJasser MI. Dermoscopy of eruptive syringoma. Skin Appendage Disord. 2021;7:401-403. doi:10.1159/000515443
- Botsali A, Caliskan E, Coskun A, et al. Eruptive syringoma: two cases with dermoscopic features. Skin Appendage Disord. 2020;6:319-322. doi:10.1159/000508656
- Dutra Rezende H, Madia ACT, Elias BM, et al. Comment on: eruptive syringoma—two cases with dermoscopic features. Skin Appendage Disord. 2022;8:81-82. doi:10.1159/000518158
- Silva-Hirschberg C, Cabrera R, Rollán MP, et al. Darier disease: the use of dermoscopy in monitoring acitretin treatment. An Bras Dermatol. 2022;97:644-647. doi:10.1016/j.abd.2021.05.021
- Singal A, Kaur I, Jakhar D. Fox-Fordyce disease: dermoscopic perspective. Skin Appendage Disord. 2020;6:247-249. doi:10.1159/000508201
- Brau Javier CN, Morales A, Sanchez JL. Histopathology attributes of Fox-Fordyce disease. Int J Dermatol. 2012;51:1313-1318. doi:10.1159/000508201
- Horie K, Shinkuma S, Fujita Y, et al. Efficacy of N-(3,4-dimethoxycinnamoyl)-anthranilic acid (tranilast) against eruptive syringoma: report of two cases and review of published work. J Dermatol. 2012;39:1044-1046. doi:10.1111/j.1346-8138.2012.01612.x
- Sanchez TS, Dauden E, Casas AP, et al. Eruptive pruritic syringomas: treatment with topical atropine. J Am Acad Dermatol. 2001;44:148-149. doi:10.1067/mjd.2001.109854
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234-1240.e1239. doi:10.1016/j.jaad.2015.12.006
- Jacquet L, Darier J.
Hidradénomes éruptifs, I.épithéliomes adenoids des glandes sudoripares ou adénomes sudoripares. Ann Dermatol Venerol. 1887;8:317-323. - Huang A, Taylor G, Liebman TN. Generalized eruptive syringomas. Dermatol Online J. 2017;23:13030/qt0hb8q22g..
- Maeda T, Natsuga K, Nishie W, et al. Extensive eruptive syringoma after liver transplantation. Acta Derm Venereol. 2018;98:119-120. doi:10.2340/00015555-2814
- Lerner TH, Barr RJ, Dolezal JF, et al. Syringomatous hyperplasia and eccrine squamous syringometaplasia associated with benoxaprofen therapy. Arch Dermatol. 1987;123:1202-1204. doi:10.1001/archderm.1987.01660330113022
- Ozturk F, Ermertcan AT, Bilac C, et al.
A case report of postpubertal eruptive syringoma triggered with antiepileptic drugs. J Drugs Dermatol. 2010;9:707-710. - Guitart J, Rosenbaum MM, Requena L. ‘Eruptive syringoma’: a misnomer for a reactive eccrine gland ductal proliferation? J Cutan Pathol. 2003;30:202-205. doi:10.1034/j.1600-0560.2003.00023.x
- Dupre A, Carrere S, Bonafe JL, et al. Eruptive generalized syringomas, milium and atrophoderma vermiculata. Nicolau and Balus’ syndrome (author’s transl). Dermatologica. 1981;162:281-286.
- Schepis C, Torre V, Siragusa M, et al. Eruptive syringomas with calcium deposits in a young woman with Down’s syndrome. Dermatology. 2001;203:345-347. doi:10.1159/000051788
- Samia AM, Donthi D, Nenow J, et al. A case study and review of literature of eruptive syringoma in a six-year-old. Cureus. 2021;13:E14634. doi:10.7759/cureus.14634
- Soler-Carrillo J, Estrach T, Mascaró JM. Eruptive syringoma: 27 new cases and review of the literature. J Eur Acad Dermatol Venereol. 2001;15:242-246. doi:10.1046/j.1468-3083.2001.00235.x
- Aleissa M, Aljarbou O, AlJasser MI. Dermoscopy of eruptive syringoma. Skin Appendage Disord. 2021;7:401-403. doi:10.1159/000515443
- Botsali A, Caliskan E, Coskun A, et al. Eruptive syringoma: two cases with dermoscopic features. Skin Appendage Disord. 2020;6:319-322. doi:10.1159/000508656
- Dutra Rezende H, Madia ACT, Elias BM, et al. Comment on: eruptive syringoma—two cases with dermoscopic features. Skin Appendage Disord. 2022;8:81-82. doi:10.1159/000518158
- Silva-Hirschberg C, Cabrera R, Rollán MP, et al. Darier disease: the use of dermoscopy in monitoring acitretin treatment. An Bras Dermatol. 2022;97:644-647. doi:10.1016/j.abd.2021.05.021
- Singal A, Kaur I, Jakhar D. Fox-Fordyce disease: dermoscopic perspective. Skin Appendage Disord. 2020;6:247-249. doi:10.1159/000508201
- Brau Javier CN, Morales A, Sanchez JL. Histopathology attributes of Fox-Fordyce disease. Int J Dermatol. 2012;51:1313-1318. doi:10.1159/000508201
- Horie K, Shinkuma S, Fujita Y, et al. Efficacy of N-(3,4-dimethoxycinnamoyl)-anthranilic acid (tranilast) against eruptive syringoma: report of two cases and review of published work. J Dermatol. 2012;39:1044-1046. doi:10.1111/j.1346-8138.2012.01612.x
- Sanchez TS, Dauden E, Casas AP, et al. Eruptive pruritic syringomas: treatment with topical atropine. J Am Acad Dermatol. 2001;44:148-149. doi:10.1067/mjd.2001.109854
Practice Points
- Eruptive syringoma (ES) is a benign cutaneous adnexal neoplasm that typically does not require treatment.
- Dermoscopy and biopsy are helpful for the diagnosis of ES, which often is missed or misdiagnosed clinically.
Saxophone Penis: A Forgotten Manifestation of Hidradenitis Suppurativa
To the Editor:
Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease affecting 1% to 4% of Europeans. It is characterized by recurrent inflamed nodules, abscesses, and sinus tracts in intertriginous regions.1 The genital area is affected in 11% of cases2 and usually is connected to severe forms of HS in both men and women.3 The prevalence of HS-associated genital lymphedema remains unknown.
Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation of the major penile lymphatic vessels that cause fibrosis of the surrounding connective tissue. Poor blood flow further causes contracture and distortion of the penile axis.4 Saxophone penis also has been associated with primary lymphedema, lymphogranuloma venereum, filariasis,5 and administration of paraffin injections.6 We describe 3 men with HS who presented with saxophone penis.
A 33-year-old man with Hurley stage III HS presented with a medical history of groin lesions and progressive penoscrotal edema of 13 years’ duration. He had a body mass index (BMI) of 37, no family history of HS or comorbidities, and a 15-year history of smoking 20 cigarettes per day. After repeated surgical drainage of the HS lesions as well as antibiotic treatment with clindamycin 600 mg/d and rifampicin 600 mg/d, the patient was kept on a maintenance therapy with adalimumab 40 mg/wk. Due to lack of response, treatment was discontinued at week 16. Clindamycin and rifampicin 300 mg were immediately reintroduced with no benefit on the genital lesions. The patient underwent genital reconstruction, including penile degloving, scrotoplasty, infrapubic fat pad removal, and perineoplasty (Figure 1). The patient currently is not undergoing any therapies.
A 55-year-old man presented with Hurley stage II HS of 33 years’ duration. He had a BMI of 52; a history of hypertension, hyperuricemia, severe hip and knee osteoarthritis, and orchiopexy in childhood; a smoking history of 40 cigarettes per day; and an alcohol consumption history of 200 mL per day since 18 years of age. He had radical excision of axillary lesions 8 years prior. One year later, he was treated with concomitant clindamycin and rifampicin 300 mg twice daily for 3 months with no desirable effects. Adalimumab 40 mg/wk was initiated. After 12 weeks of treatment, he experienced 80% improvement in all areas except the genital region. He continued adalimumab for 3 years with good clinical response in all HS-affected sites except the genital region.
A 66-year-old man presented with Hurley stage III HS of 37 years’ duration. He had a smoking history of 10 cigarettes per day for 30 years, a BMI of 24.6, and a medical history of long-standing hypertension and hypothyroidism. A 3-month course of clindamycin and rifampicin 600 mg/d was ineffective; adalimumab 40 mg/wk was initiated. All affected areas improved, except for the saxophone penis. He continues his fifth year of therapy with adalimumab (Figure 2).

Hidradenitis suppurativa is associated with chronic pain, purulent malodor, and scarring with structural deformity. Repetitive inflammation causes fibrosis, scar formation, and soft-tissue destruction of lymphatic vessels, leading to lymphedema; primary lymphedema of the genitals in men has been reported to result in a saxophone penis.4
The only approved biologic treatments for moderate to severe HS are the tumor necrosis factor α inhibitor adalimumab and anti-IL-17 secukinumab.1 All 3 of our patients with HS were treated with adalimumab with reasonable success; however, the penile condition remained refractory, which we speculate may be due to adalimumab’s ability to control only active inflammatory lesions but not scars or fibrotic tissue.7 Higher adalimumab dosages were unlikely to be beneficial for their penile condition; some improvements have been reported following fluoroquinolone therapy. To our knowledge, there is no effective medical treatment for saxophone penis. However, surgery showed good results in one of our patients. Among our 3 adalimumab-treated patients, only 1 patient had corrective surgery that resulted in improvement in the penile deformity, further confirming adalimumab’s limited role in genital lymphedema.7 Extensive resection of the lymphedematous tissue, scrotoplasty, and Charles procedure are treatment options.8
Genital lymphedema has been associated with lymphangiectasia, lymphangioma circumscriptum, infections, and neoplasms such as lymphangiosarcoma and squamous cell carcinoma.9 Our patients reported discomfort, hygiene issues, and swelling. One patient reported micturition, and 2 patients reported sexual dysfunction.
Saxophone penis remains a disabling sequela of HS. Early diagnosis and treatment of HS may help prevent development of this condition.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Fertitta L, Hotz C, Wolkenstein P, et al. Efficacy and satisfaction of surgical treatment for hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34:839-845.
- Micieli R, Alavi A. Lymphedema in patients with hidradenitis suppurativa: a systematic review of published literature. Int J Dermatol. 2018;57:1471-1480.
- Maatouk I, Moutran R. Saxophone penis. JAMA Dermatol. 2013;149:802.
- Koley S, Mandal RK. Saxophone penis after unilateral inguinal bubo of lymphogranuloma venereum. Indian J Sex Transm Dis AIDS. 2013;34:149-151.
- D’Antuono A, Lambertini M, Gaspari V, et al. Visual dermatology: self-induced chronic saxophone penis due to paraffin injections. J Cutan Med Surg. 2019;23:330.
- Musumeci ML, Scilletta A, Sorci F, et al. Genital lymphedema associated with hidradenitis suppurativa unresponsive to adalimumab treatment. JAAD Case Rep. 2019;5:326-328.
- Jain V, Singh S, Garge S, et al. Saxophone penis due to primary lymphoedema. J Indian Assoc Pediatr Surg. 2009;14:230-231.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;64:1223-1224.
To the Editor:
Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease affecting 1% to 4% of Europeans. It is characterized by recurrent inflamed nodules, abscesses, and sinus tracts in intertriginous regions.1 The genital area is affected in 11% of cases2 and usually is connected to severe forms of HS in both men and women.3 The prevalence of HS-associated genital lymphedema remains unknown.
Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation of the major penile lymphatic vessels that cause fibrosis of the surrounding connective tissue. Poor blood flow further causes contracture and distortion of the penile axis.4 Saxophone penis also has been associated with primary lymphedema, lymphogranuloma venereum, filariasis,5 and administration of paraffin injections.6 We describe 3 men with HS who presented with saxophone penis.
A 33-year-old man with Hurley stage III HS presented with a medical history of groin lesions and progressive penoscrotal edema of 13 years’ duration. He had a body mass index (BMI) of 37, no family history of HS or comorbidities, and a 15-year history of smoking 20 cigarettes per day. After repeated surgical drainage of the HS lesions as well as antibiotic treatment with clindamycin 600 mg/d and rifampicin 600 mg/d, the patient was kept on a maintenance therapy with adalimumab 40 mg/wk. Due to lack of response, treatment was discontinued at week 16. Clindamycin and rifampicin 300 mg were immediately reintroduced with no benefit on the genital lesions. The patient underwent genital reconstruction, including penile degloving, scrotoplasty, infrapubic fat pad removal, and perineoplasty (Figure 1). The patient currently is not undergoing any therapies.
A 55-year-old man presented with Hurley stage II HS of 33 years’ duration. He had a BMI of 52; a history of hypertension, hyperuricemia, severe hip and knee osteoarthritis, and orchiopexy in childhood; a smoking history of 40 cigarettes per day; and an alcohol consumption history of 200 mL per day since 18 years of age. He had radical excision of axillary lesions 8 years prior. One year later, he was treated with concomitant clindamycin and rifampicin 300 mg twice daily for 3 months with no desirable effects. Adalimumab 40 mg/wk was initiated. After 12 weeks of treatment, he experienced 80% improvement in all areas except the genital region. He continued adalimumab for 3 years with good clinical response in all HS-affected sites except the genital region.
A 66-year-old man presented with Hurley stage III HS of 37 years’ duration. He had a smoking history of 10 cigarettes per day for 30 years, a BMI of 24.6, and a medical history of long-standing hypertension and hypothyroidism. A 3-month course of clindamycin and rifampicin 600 mg/d was ineffective; adalimumab 40 mg/wk was initiated. All affected areas improved, except for the saxophone penis. He continues his fifth year of therapy with adalimumab (Figure 2).

Hidradenitis suppurativa is associated with chronic pain, purulent malodor, and scarring with structural deformity. Repetitive inflammation causes fibrosis, scar formation, and soft-tissue destruction of lymphatic vessels, leading to lymphedema; primary lymphedema of the genitals in men has been reported to result in a saxophone penis.4
The only approved biologic treatments for moderate to severe HS are the tumor necrosis factor α inhibitor adalimumab and anti-IL-17 secukinumab.1 All 3 of our patients with HS were treated with adalimumab with reasonable success; however, the penile condition remained refractory, which we speculate may be due to adalimumab’s ability to control only active inflammatory lesions but not scars or fibrotic tissue.7 Higher adalimumab dosages were unlikely to be beneficial for their penile condition; some improvements have been reported following fluoroquinolone therapy. To our knowledge, there is no effective medical treatment for saxophone penis. However, surgery showed good results in one of our patients. Among our 3 adalimumab-treated patients, only 1 patient had corrective surgery that resulted in improvement in the penile deformity, further confirming adalimumab’s limited role in genital lymphedema.7 Extensive resection of the lymphedematous tissue, scrotoplasty, and Charles procedure are treatment options.8
Genital lymphedema has been associated with lymphangiectasia, lymphangioma circumscriptum, infections, and neoplasms such as lymphangiosarcoma and squamous cell carcinoma.9 Our patients reported discomfort, hygiene issues, and swelling. One patient reported micturition, and 2 patients reported sexual dysfunction.
Saxophone penis remains a disabling sequela of HS. Early diagnosis and treatment of HS may help prevent development of this condition.
To the Editor:
Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease affecting 1% to 4% of Europeans. It is characterized by recurrent inflamed nodules, abscesses, and sinus tracts in intertriginous regions.1 The genital area is affected in 11% of cases2 and usually is connected to severe forms of HS in both men and women.3 The prevalence of HS-associated genital lymphedema remains unknown.
Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation of the major penile lymphatic vessels that cause fibrosis of the surrounding connective tissue. Poor blood flow further causes contracture and distortion of the penile axis.4 Saxophone penis also has been associated with primary lymphedema, lymphogranuloma venereum, filariasis,5 and administration of paraffin injections.6 We describe 3 men with HS who presented with saxophone penis.
A 33-year-old man with Hurley stage III HS presented with a medical history of groin lesions and progressive penoscrotal edema of 13 years’ duration. He had a body mass index (BMI) of 37, no family history of HS or comorbidities, and a 15-year history of smoking 20 cigarettes per day. After repeated surgical drainage of the HS lesions as well as antibiotic treatment with clindamycin 600 mg/d and rifampicin 600 mg/d, the patient was kept on a maintenance therapy with adalimumab 40 mg/wk. Due to lack of response, treatment was discontinued at week 16. Clindamycin and rifampicin 300 mg were immediately reintroduced with no benefit on the genital lesions. The patient underwent genital reconstruction, including penile degloving, scrotoplasty, infrapubic fat pad removal, and perineoplasty (Figure 1). The patient currently is not undergoing any therapies.
A 55-year-old man presented with Hurley stage II HS of 33 years’ duration. He had a BMI of 52; a history of hypertension, hyperuricemia, severe hip and knee osteoarthritis, and orchiopexy in childhood; a smoking history of 40 cigarettes per day; and an alcohol consumption history of 200 mL per day since 18 years of age. He had radical excision of axillary lesions 8 years prior. One year later, he was treated with concomitant clindamycin and rifampicin 300 mg twice daily for 3 months with no desirable effects. Adalimumab 40 mg/wk was initiated. After 12 weeks of treatment, he experienced 80% improvement in all areas except the genital region. He continued adalimumab for 3 years with good clinical response in all HS-affected sites except the genital region.
A 66-year-old man presented with Hurley stage III HS of 37 years’ duration. He had a smoking history of 10 cigarettes per day for 30 years, a BMI of 24.6, and a medical history of long-standing hypertension and hypothyroidism. A 3-month course of clindamycin and rifampicin 600 mg/d was ineffective; adalimumab 40 mg/wk was initiated. All affected areas improved, except for the saxophone penis. He continues his fifth year of therapy with adalimumab (Figure 2).

Hidradenitis suppurativa is associated with chronic pain, purulent malodor, and scarring with structural deformity. Repetitive inflammation causes fibrosis, scar formation, and soft-tissue destruction of lymphatic vessels, leading to lymphedema; primary lymphedema of the genitals in men has been reported to result in a saxophone penis.4
The only approved biologic treatments for moderate to severe HS are the tumor necrosis factor α inhibitor adalimumab and anti-IL-17 secukinumab.1 All 3 of our patients with HS were treated with adalimumab with reasonable success; however, the penile condition remained refractory, which we speculate may be due to adalimumab’s ability to control only active inflammatory lesions but not scars or fibrotic tissue.7 Higher adalimumab dosages were unlikely to be beneficial for their penile condition; some improvements have been reported following fluoroquinolone therapy. To our knowledge, there is no effective medical treatment for saxophone penis. However, surgery showed good results in one of our patients. Among our 3 adalimumab-treated patients, only 1 patient had corrective surgery that resulted in improvement in the penile deformity, further confirming adalimumab’s limited role in genital lymphedema.7 Extensive resection of the lymphedematous tissue, scrotoplasty, and Charles procedure are treatment options.8
Genital lymphedema has been associated with lymphangiectasia, lymphangioma circumscriptum, infections, and neoplasms such as lymphangiosarcoma and squamous cell carcinoma.9 Our patients reported discomfort, hygiene issues, and swelling. One patient reported micturition, and 2 patients reported sexual dysfunction.
Saxophone penis remains a disabling sequela of HS. Early diagnosis and treatment of HS may help prevent development of this condition.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Fertitta L, Hotz C, Wolkenstein P, et al. Efficacy and satisfaction of surgical treatment for hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34:839-845.
- Micieli R, Alavi A. Lymphedema in patients with hidradenitis suppurativa: a systematic review of published literature. Int J Dermatol. 2018;57:1471-1480.
- Maatouk I, Moutran R. Saxophone penis. JAMA Dermatol. 2013;149:802.
- Koley S, Mandal RK. Saxophone penis after unilateral inguinal bubo of lymphogranuloma venereum. Indian J Sex Transm Dis AIDS. 2013;34:149-151.
- D’Antuono A, Lambertini M, Gaspari V, et al. Visual dermatology: self-induced chronic saxophone penis due to paraffin injections. J Cutan Med Surg. 2019;23:330.
- Musumeci ML, Scilletta A, Sorci F, et al. Genital lymphedema associated with hidradenitis suppurativa unresponsive to adalimumab treatment. JAAD Case Rep. 2019;5:326-328.
- Jain V, Singh S, Garge S, et al. Saxophone penis due to primary lymphoedema. J Indian Assoc Pediatr Surg. 2009;14:230-231.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;64:1223-1224.
- Lee EY, Alhusayen R, Lansang P, et al. What is hidradenitis suppurativa? Can Fam Physician. 2017;63:114-120.
- Fertitta L, Hotz C, Wolkenstein P, et al. Efficacy and satisfaction of surgical treatment for hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2020;34:839-845.
- Micieli R, Alavi A. Lymphedema in patients with hidradenitis suppurativa: a systematic review of published literature. Int J Dermatol. 2018;57:1471-1480.
- Maatouk I, Moutran R. Saxophone penis. JAMA Dermatol. 2013;149:802.
- Koley S, Mandal RK. Saxophone penis after unilateral inguinal bubo of lymphogranuloma venereum. Indian J Sex Transm Dis AIDS. 2013;34:149-151.
- D’Antuono A, Lambertini M, Gaspari V, et al. Visual dermatology: self-induced chronic saxophone penis due to paraffin injections. J Cutan Med Surg. 2019;23:330.
- Musumeci ML, Scilletta A, Sorci F, et al. Genital lymphedema associated with hidradenitis suppurativa unresponsive to adalimumab treatment. JAAD Case Rep. 2019;5:326-328.
- Jain V, Singh S, Garge S, et al. Saxophone penis due to primary lymphoedema. J Indian Assoc Pediatr Surg. 2009;14:230-231.
- Moosbrugger EA, Mutasim DF. Hidradenitis suppurativa complicated by severe lymphedema and lymphangiectasias. J Am Acad Dermatol. 2011;64:1223-1224.
Practice Points
- Hidradenitis suppurativa (HS) is a multifactorial chronic inflammatory skin disease.
- Saxophone penis is a specific penile malformation characterized by a saxophone shape due to inflammation.
- Repetitive inflammation within the context of HS may cause structural deformity of the penis, resulting in a saxophone penis.
- Early diagnosis and treatment of HS may help prevent development of this condition.
Anti-Smith and Anti–Double-Stranded DNA Antibodies in a Patient With Henoch-Schönlein Purpura Following COVID-19 Vaccination
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.
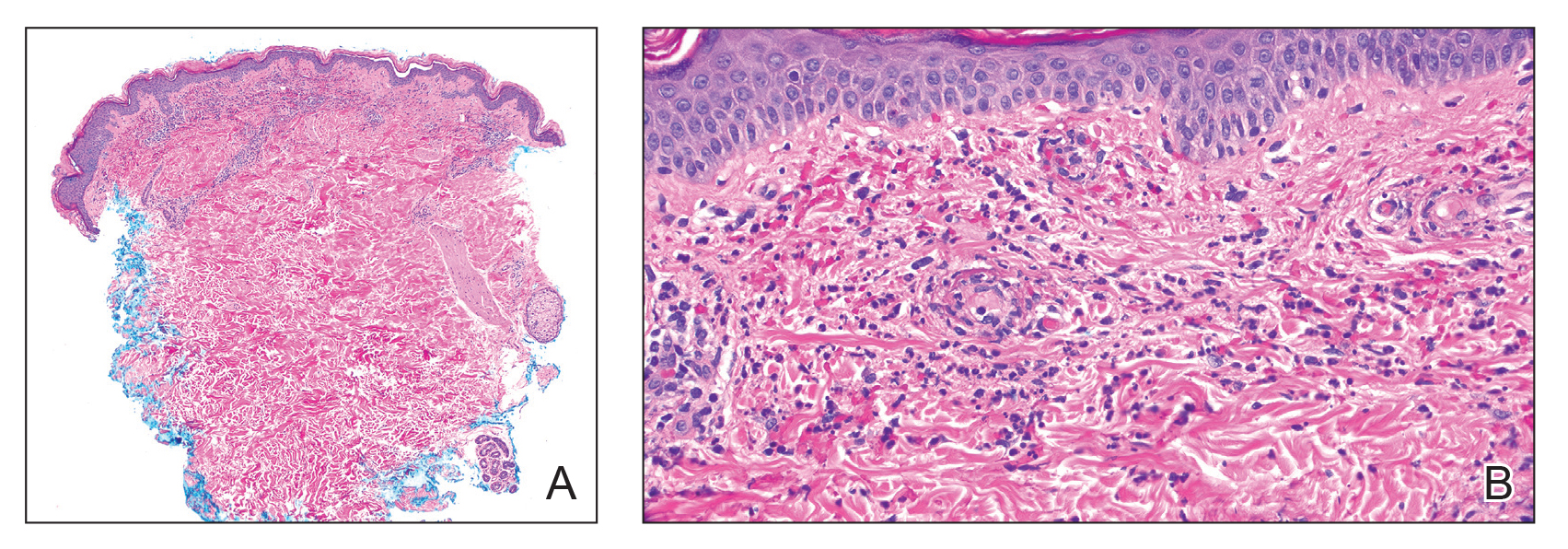
Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
To the Editor:
Henoch-Schönlein purpura (HSP)(also known as IgA vasculitis) is a small vessel vasculitis characterized by deposition of IgA in small vessels, resulting in the development of purpura on the legs. Based on the European Alliance of Associations for Rheumatology criteria,1 the patient also must have at least 1 of the following: arthritis, arthralgia, abdominal pain, leukocytoclastic vasculitis with IgA deposition, or kidney involvement. The disease can be triggered by infection—with more than 75% of patients reporting an antecedent upper respiratory tract infection2—as well as medications, circulating immune complexes, certain foods, vaccines, and rarely cancer.3,4 The disease more commonly occurs in children but also can affect adults.
Several cases of HSP have been reported following COVID-19 vaccination.5 We report a case of HSP developing days after the messenger RNA Pfizer-BioNTech COVID-19 vaccine booster that was associated with anti-Smith and anti–double-stranded DNA (dsDNA) antibodies as well as antineutrophil cytoplasmic antibodies (ANCAs).
A 24-year-old man presented to dermatology with a rash of 3 weeks’ duration that first appeared 1 week after receiving his second booster of the messenger RNA Pfizer-BioNTech COVID-19 vaccine. Physical examination revealed petechiae with nonblanching erythematous macules and papules covering the legs below the knees (Figure 1) as well as the back of the right arm. A few days later, he developed arthralgia in the knees, hands, and feet. The patient denied any recent infections as well as respiratory and urinary tract symptoms. Approximately 10 days after the rash appeared, he developed epigastric abdominal pain that gradually worsened and sought care from his primary care physician, who ordered computed tomography and referred him for endoscopy. Computed tomography with and without contrast was suspicious for colitis. Colonoscopy and endoscopy were unremarkable. Laboratory tests were notable for elevated white blood cell count (17.08×103/µL [reference range, 3.66–10.60×103/µL]), serum IgA (437 mg/dL [reference range, 70–400 mg/dL]), C-reactive protein (1.5 mg/dL [reference range, <0.5 mg/dL]), anti-Smith antibody (28.1 CU [reference range, <20 CU), positive antinuclear antibody with titer (1:160 [reference range, <1:80]), anti-dsDNA (40.4 IU/mL [reference range, <27 IU/mL]), and cytoplasmic ANCA (c-ANCA) titer (1:320 [reference range, <1:20]). Blood urea nitrogen, creatinine, and estimated glomerular filtration rate were all within reference range. Urinalysis with microscopic examination was notable for 2 to 5 red blood cells per high-power field (reference range, 0) and proteinuria of 1+ (reference range, negative for protein).
The patient’s rash progressively worsened over the next few weeks, spreading proximally on the legs to the buttocks and the back of both elbows. A repeat complete blood cell count showed resolution of the leukocytosis. Two biopsies were taken from a lesion on the left proximal thigh: 1 for hematoxylin and eosin stain for histopathologic examination and 1 for direct immunofluorescence examination.
The patient was preliminarily diagnosed with HSP, and dermatology prescribed oral tofacitinib 5 mg twice daily for 5 days, which was supposed to be increased to 10 mg twice daily on the sixth day of treatment; however, the patient discontinued the medication after 4 days based on his primary care physician’s recommendation due to clotting concerns. The rash and arthralgia temporarily improved for 1 week, then relapsed.
Histopathology revealed neutrophils surrounding and infiltrating small dermal blood vessel walls as well as associated neutrophilic debris and erythrocytes, consistent with leukocytoclastic vasculitis (Figure 2). Direct immunofluorescence was negative for IgA antibodies. His primary care physician, in consultation with his dermatologist, then started the patient on oral prednisone 70 mg once daily for 7 days with a plan to taper. Three days after prednisone was started, the arthralgia and abdominal pain resolved, and the rash became lighter in color. After 1 week, the rash resolved completely.
Due to the unusual antibodies, the patient was referred to a rheumatologist, who repeated the blood tests approximately 1 week after the patient started prednisone. The tests were negative for anti-Smith, anti-dsDNA, and c-ANCA but showed an elevated atypical perinuclear ANCA (p-ANCA) titer of 1:80 (reference range [negative], <1:20). A repeat urinalysis was unremarkable. The patient slowly tapered the prednisone over the course of 3 months and was subsequently lost to follow-up. The rash and other symptoms had not recurred as of the patient’s last physician contact. The most recent laboratory results showed a white blood cell count of 14.0×103/µL (reference range, 3.4–10.8×103/µL), likely due to the prednisone; blood urea nitrogen, creatinine, and estimated glomerular filtration rate were within reference range. The urinalysis was notable for occult blood and was negative for protein. C-reactive protein was 1 mg/dL (reference range, 0–10 mg/dL); p-ANCA, c-ANCA, and atypical p-ANCA, as well as antinuclear antibody, were negative. As of his last follow-up, the patient felt well.
The major differential diagnoses for our patient included HSP, ANCA vasculitis, and systemic lupus erythematosus. Although ANCA vasculitis has been reported after SARS-CoV-2 infection,6 the lack of pulmonary symptoms made this diagnosis unlikely.7 Although our patient initially had elevated anti-Smith and anti-dsDNA antibodies as well as mild renal involvement, he fulfilled at most only 2 of the 11 criteria necessary for diagnosing lupus: malar rash, discoid rash (includes alopecia), photosensitivity, ocular ulcers, nonerosive arthritis, serositis, renal disorder (protein >500 mg/24 h, red blood cells, casts), neurologic disorder (seizures, psychosis), hematologic disorders (hemolytic anemia, leukopenia), ANA, and immunologic disorder (anti-Smith). Four of the 11 criteria are necessary for the diagnosis of lupus.8
Torraca et al7 reported a case of HSP with positive c-ANCA (1:640) in a patient lacking pulmonary symptoms who was diagnosed with HSP. Cytoplasmic ANCA is not a typical finding in HSP. However, the additional findings of anti-Smith, anti-dsDNA, and mildly elevated atypical p-ANCA antibodies in our patient were unexpected and could be explained by the proposed pathogenesis of HSP—an overzealous immune response resulting in aberrant antibody complex deposition with ensuing complement activation.5,9 Production of these additional antibodies could be part of the overzealous response to COVID-19 vaccination.


Of all the COVID-19 vaccines, messenger RNA–based vaccines have been associated with the majority of cutaneous reactions, including local injection-site reactions (most common), delayed local reactions, urticaria, angioedema, morbilliform eruption, herpes zoster eruption, bullous eruptions, dermal filler reactions, chilblains, and pityriasis rosea. Less common reactions have included acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, erythema multiforme, Sweet Syndrome, lichen planus, papulovesicular eruptions, pityriasis rosea–like eruptions, generalized annular lesions, facial pustular neutrophilic eruptions, and flares of underlying autoimmune skin conditions.10 Multiple cases of HSP have been reported following COVID-19 vaccination from all the major vaccine companies.5
In our patient, laboratory tests were repeated by a rheumatologist and were negative for anti-Smith and anti-dsDNA antibodies as well as c-ANCA, most likely because he started taking prednisone approximately 1 week prior, which may have resulted in decreased antibodies. Also, the patient’s symptoms resolved after 1 week of steroid therapy. Therefore, the diagnosis is most consistent with HSP associated with COVID-19 vaccination. The clinical presentation, microscopic hematuria and proteinuria, and histopathology were consistent with the European Alliance of Associations for Rheumatology criteria for HSP.1
Although direct immunofluorescence typically is positive for IgA deposition on biopsies, it can be negative for IgA, especially in lesions that are biopsied more than 7 days after their appearance, as shown in our case; a negative IgA on immunofluorescence does not rule out HSP.4 Elevated serum IgA is seen in more than 50% of cases of HSP.11 Although the disease typically is self-limited, glucocorticoids are used if the disease course is prolonged or if there is evidence of kidney involvement.9 The unique combination of anti-Smith and anti-dsDNA antibodies as well as ANCAs associated with HSP with negative IgA on direct immunofluorescence has been reported with lupus.12 Clinicians should be aware of COVID-19 vaccine–associated HSP that is negative for IgA deposition and positive for anti-Smith and anti-dsDNA antibodies as well as ANCAs.
Acknowledgment—We thank our patient for granting permission to publish this information.
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
- Ozen S, Ruperto N, Dillon MJ, et al. EULAR/PReS endorsed consensus criteria for the classification of childhood vasculitides. Ann Rheum Dis. 2006;65:936-941. doi:10.1136/ard.2005.046300
- Rai A, Nast C, Adler S. Henoch–Schönlein purpura nephritis. J Am Soc Nephrol. 1999;10:2637-2644.
- Casini F, Magenes VC, De Sanctis M, et al. Henoch-Schönlein purpura following COVID-19 vaccine in a child: a case report. Ital J Pediatr. 2022;48:158. doi:10.1186/s13052-022-01351-1
- Poudel P, Adams SH, Mirchia K, et al. IgA negative immunofluorescence in diagnoses of adult-onset Henoch-Schönlein purpura. Proc (Bayl Univ Med Cent). 2020;33:436-437. doi:10.1080/08998280.2020.1770526
- Maronese CA, Zelin E, Avallone G, et al. Cutaneous vasculitis and vasculopathy in the era of COVID-19 pandemic. Front Med (Lausanne). 2022;9:996288. doi:10.3389/fmed.2022.996288
- Bryant MC, Spencer LT, Yalcindag A. A case of ANCA-associated vasculitis in a 16-year-old female following SARS-COV-2 infection and a systematic review of the literature. Pediatr Rheumatol Online J. 2022;20:65. doi:10.1186/s12969-022-00727-1
- Torraca PFS, Castro BC, Hans Filho G. Henoch-Schönlein purpura with c-ANCA antibody in adult. An Bras Dermatol. 2016;91:667-669. doi:10.1590/abd1806-4841.20164181
- Agabegi SS, Agabegi ED. Step-Up to Medicine. 4th ed. Wolters Kluwer; 2015.
- Ball-Burack MR, Kosowsky JM. A Case of leukocytoclastic vasculitis following SARS-CoV-2 vaccination. J Emerg Med. 2022;63:E62-E65. doi:10.1016/j.jemermed.2021.10.005
- Tan SW, Tam YC, Pang SM. Cutaneous reactions to COVID-19 vaccines: a review. JAAD Int. 2022;7:178-186. doi:10.1016/j.jdin.2022.01.011
- Calviño MC, Llorca J, García-Porrúa C, et al. Henoch-Schönlein purpura in children from northwestern Spain: a 20-year epidemiologic and clinical study. Medicine (Baltimore). 2001;80:279-290.
- Hu P, Huang BY, Zhang DD, et al. Henoch-Schönlein purpura in a pediatric patient with lupus. Arch Med Sci. 2017;13:689-690. doi:10.5114/aoms.2017.67288
Practice Points
- Dermatologists should be vigilant for Henoch-Schönlein purpura (HSP) despite negative direct immunofluorescence of IgA deposition and unusual antibodies.
- Messenger RNA–based COVID-19 vaccines are associated with various cutaneous reactions, including HSP.
- Anti-Smith and anti–double-stranded DNA antibodies typically are not associated with HSP but may be seen in patients with coexisting systemic lupus erythematosus.
Cyclosporine for Recalcitrant Bullous Pemphigoid Induced by Nivolumab Therapy for Malignant Melanoma
To the Editor:
Immune checkpoint inhibitors have revolutionized the treatment of advanced-stage melanoma, with remarkably improved progression-free survival.1 Anti–programmed death receptor 1 (anti–PD-1) therapies, such as nivolumab and pembrolizumab, are a class of checkpoint inhibitors that have been approved by the US Food and Drug Administration for unresectable metastatic melanoma. Anti–PD-1 agents block the interaction of programmed death-ligand 1 (PD-L1) found on tumor cells with the PD-1 receptor on T cells, facilitating a positive immune response.2
Although these therapies have demonstrated notable antitumor efficacy, they also give rise to numerous immune-related adverse events (irAEs). As many as 70% of patients treated with PD-1/PD-L1 inhibitors experience some type of organ system irAE, of which 30% to 40% are cutaneous.3-6 Dermatologic adverse events are the most common irAEs, specifically spongiotic dermatitis, lichenoid dermatitis, pruritus, and vitiligo.7 Bullous pemphigoid (BP), an autoimmune bullous skin disorder caused by autoantibodies to basement membrane zone antigens, is a rare but potentially serious cutaneous irAE.8 Systemic corticosteroids commonly are used to treat immune checkpoint inhibitor–induced BP; other options include tetracyclines for maintenance therapy and rituximab for corticosteroid-refractory BP associated with anti-PD-1.9 We present a case of recalcitrant BP secondary to nivolumab therapy in a patient with metastatic melanoma who had near-complete resolution of BP following 2 months of cyclosporine.
A 41-year-old man presented with a generalized papular skin eruption of 1 month’s duration. He had a history of stage IIIC malignant melanoma of the lower right leg with positive sentinel lymph node biopsy. The largest lymph node deposit was 0.03 mm without extracapsular extension. Whole-body positron emission tomography–computed tomography showed no evidence of distant disease. The patient was treated with wide local excision with clear surgical margins plus 12 cycles of nivolumab, which was discontinued due to colitis. Four months after the final cycle of nivolumab, the patient developed widespread erythematous papules with hemorrhagic yellow crusting and no mucosal involvement. He was referred to dermatology by his primary oncologist for further evaluation.
A punch biopsy from the abdomen showed parakeratosis with leukocytoclasis and a superficial dermal infiltrate of neutrophils and eosinophils (Figure 1). Direct immunofluorescence revealed linear basement membrane deposits of IgG and C3, consistent with subepidermal blistering disease. Indirect immunofluorescence demonstrated trace IgG and IgG4 antibodies localized to the epidermal roof of salt-split skin and was negative for IgA antibodies. An enzyme-linked immunoassay was positive for BP antigen 2 (BP180) antibodies (98.4 U/mL [positive, ≥9 U/mL]) and negative for BP antigen 1 (BP230) antibodies (4.3 U/mL [positive, ≥9 U/mL]). Overall, these findings were consistent with a diagnosis of BP.
The patient was treated with prednisone 60 mg daily with initial response; however, there was disease recurrence with tapering. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily were added as steroid-sparing agents, as prednisone was discontinued due to mood changes. Three months after the prednisone taper, the patient continued to develop new blisters. He completed treatment with doxycycline and nicotinamide. Rituximab 375 mg weekly was then initiated for 4 weeks.
At 2-week follow-up after completing the rituximab course, the patient reported worsening symptoms and presented with new bullae on the abdomen and upper extremities (Figure 2). Because of the recent history of mood changes while taking prednisone, a trial of cyclosporine 100 mg twice daily (1.37 mg/kg/d) was initiated, with notable improvement within 2 weeks of treatment. After 2 months of cyclosporine, approximately 90% of the rash had resolved with a few tense bullae remaining on the left frontal scalp but no new flares (Figure 3). One month after treatment ended, the patient remained clear of lesions without relapse.
Programmed death receptor 1 inhibitors have shown dramatic efficacy for a growing number of solid and hematologic malignancies, especially malignant melanoma. However, their use is accompanied by nonspecific activation of the immune system, resulting in a variety of adverse events, many manifesting on the skin. Several cases of BP in patients treated with PD-1/PD-L1 inhibitors have been reported.9 Cutaneous irAEs usually manifest within 3 weeks of initiation of PD-1 inhibitor therapy; however, the onset of BP typically occurs later at approximately 21 weeks.4,9 Our patient developed cutaneous manifestations 4 months after cessation of nivolumab.
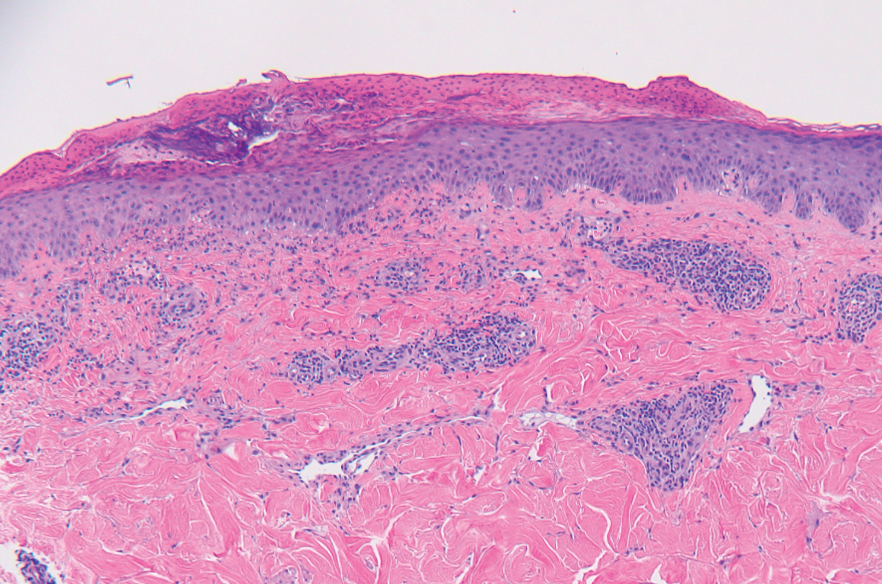
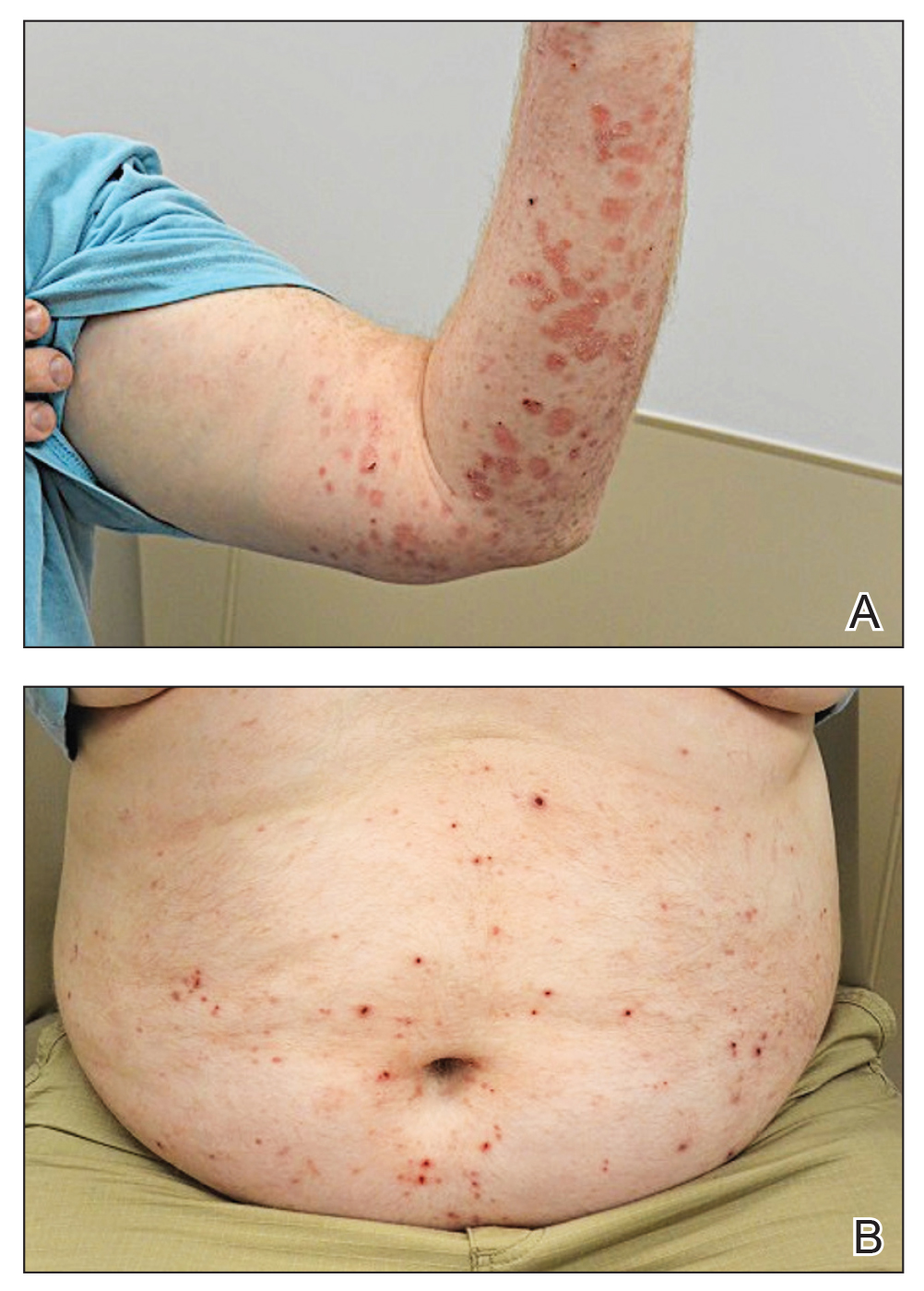
Bullous pemphigoid classically manifests with pruritus and tense bullae. Notably, our patient’s clinical presentation included a widespread eruption of papules without bullae, which was similar to a review by Tsiogka et al,9 which reported that one-third of patients first present with a nonspecific cutaneous eruption. Bullous pemphigoid induced by anti–PD-1 may manifest differently than traditional BP, illuminating the importance of a thorough diagnostic workup.
Although the pathogenesis of immune checkpoint inhibitor–induced BP has not been fully elucidated, it is hypothesized to be caused by increased T cell cytotoxic activity leading to tumor lysis and release of numerous autoantigens. These autoantigens cause priming of abnormal T cells that can lead to further tissue damage in peripheral tissue and to generation of aberrant B cells and subsequent autoantibodies such as BP180 in germinal centers.4,10,11
Cyclosporine is a calcineurin inhibitor that reduces synthesis of IL-2, resulting in reduced cell activation.12 Therefore, cyclosporine may alleviate BP in patients who are being treated, or were previously treated, with an immune checkpoint inhibitor by suppressing T cell–mediated immune reaction and may be a rapid alternative for patients who cannot tolerate systemic steroids.
Treatment options for mild to moderate cases of BP include topical corticosteroids and antihistamines, while severe cases may require high-dose systemic corticosteroids. In recalcitrant cases, rituximab infusion with or without intravenous immunoglobulin often is utilized.8,13 The use of cyclosporine for various bullous disorders, including pemphigus vulgaris and epidermolysis bullosa acquisita, has been described.14 In recent years there has been a shift away from the use of cyclosporine for these conditions following the introduction of rituximab, a monoclonal antibody directed against the CD20 antigen on B lymphocytes. We utilized cyclosporine in our patient after he experienced worsening symptoms 1 month after completing treatment with rituximab.
Improvement from rituximab therapy may be delayed because it can take months to deplete CD20+ B lymphocytes from circulation, which may necessitate additional immunosuppressants or re-treatment with rituximab.15,16 In these instances, cyclosporine may be beneficial as a low-cost alternative in patients who are unable to tolerate systemic steroids, with a relatively good safety profile. The dosage of cyclosporine prescribed to the patient was chosen based on Joint American Academy of Dermatology–National Psoriasis Foundation management guidelines for psoriasis with systemic nonbiologic therapies, which recommends an initial dosage of 1 to 3 mg/kg/d in 2 divided doses.17
As immunotherapy for treating various cancers gains popularity, the frequency of dermatologic irAEs will increase. Therefore, dermatologists must be aware of the array of cutaneous manifestations, such as BP, and potential treatment options. When first-line and second-line therapies are contraindicated or do not provide notable improvement, cyclosporine may be an effective alternative for immune checkpoint inhibitor–induced BP.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi:10.1056/NEJMoa1504030
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
- Puzanov I, Diab A, Abdallah K, et al; . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi:10.1186/s40425-017-0300-z
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575. doi:10.3978/j.issn.2218-6751.2015.06.06
- Kumar V, Chaudhary N, Garg M, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Schauer F, Rafei-Shamsabadi D, Mai S, et al. Hemidesmosomal reactivity and treatment recommendations in immune checkpoint inhibitor-induced bullous pemphigoid—a retrospective, monocentric study. Front Immunol. 2022;13:953546. doi:10.3389/fimmu.2022.953546
- Tsiogka A, Bauer JW, Patsatsi A. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a review of the literature. Acta Derm Venereol. 2021;101:adv00377. doi:10.2340/00015555-3740
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669. doi:10.1111/ijd.13984
- Yang H, Yao Z, Zhou X, et al. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213:108377. doi:10.1016/j.clim.2020.108377
- Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum. 1992;21(6 suppl 3):16-22. doi:10.1016/0049-0172(92)90009-3
- Ahmed AR, Shetty S, Kaveri S, et al. Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: a retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700-708.e3. doi:10.1016/j.jaad.2015.11.030
- Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925-946. doi:10.1016/j.jaad.2010.02.063
- Schmidt E, Hunzelmann N, Zillikens D, et al. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol. 2006;31:503-508. doi:10.1111/j.1365-2230.2006.02151.x
- Kasperkiewicz M, Shimanovich I, Ludwig RJ, et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65:552-558.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
To the Editor:
Immune checkpoint inhibitors have revolutionized the treatment of advanced-stage melanoma, with remarkably improved progression-free survival.1 Anti–programmed death receptor 1 (anti–PD-1) therapies, such as nivolumab and pembrolizumab, are a class of checkpoint inhibitors that have been approved by the US Food and Drug Administration for unresectable metastatic melanoma. Anti–PD-1 agents block the interaction of programmed death-ligand 1 (PD-L1) found on tumor cells with the PD-1 receptor on T cells, facilitating a positive immune response.2
Although these therapies have demonstrated notable antitumor efficacy, they also give rise to numerous immune-related adverse events (irAEs). As many as 70% of patients treated with PD-1/PD-L1 inhibitors experience some type of organ system irAE, of which 30% to 40% are cutaneous.3-6 Dermatologic adverse events are the most common irAEs, specifically spongiotic dermatitis, lichenoid dermatitis, pruritus, and vitiligo.7 Bullous pemphigoid (BP), an autoimmune bullous skin disorder caused by autoantibodies to basement membrane zone antigens, is a rare but potentially serious cutaneous irAE.8 Systemic corticosteroids commonly are used to treat immune checkpoint inhibitor–induced BP; other options include tetracyclines for maintenance therapy and rituximab for corticosteroid-refractory BP associated with anti-PD-1.9 We present a case of recalcitrant BP secondary to nivolumab therapy in a patient with metastatic melanoma who had near-complete resolution of BP following 2 months of cyclosporine.
A 41-year-old man presented with a generalized papular skin eruption of 1 month’s duration. He had a history of stage IIIC malignant melanoma of the lower right leg with positive sentinel lymph node biopsy. The largest lymph node deposit was 0.03 mm without extracapsular extension. Whole-body positron emission tomography–computed tomography showed no evidence of distant disease. The patient was treated with wide local excision with clear surgical margins plus 12 cycles of nivolumab, which was discontinued due to colitis. Four months after the final cycle of nivolumab, the patient developed widespread erythematous papules with hemorrhagic yellow crusting and no mucosal involvement. He was referred to dermatology by his primary oncologist for further evaluation.
A punch biopsy from the abdomen showed parakeratosis with leukocytoclasis and a superficial dermal infiltrate of neutrophils and eosinophils (Figure 1). Direct immunofluorescence revealed linear basement membrane deposits of IgG and C3, consistent with subepidermal blistering disease. Indirect immunofluorescence demonstrated trace IgG and IgG4 antibodies localized to the epidermal roof of salt-split skin and was negative for IgA antibodies. An enzyme-linked immunoassay was positive for BP antigen 2 (BP180) antibodies (98.4 U/mL [positive, ≥9 U/mL]) and negative for BP antigen 1 (BP230) antibodies (4.3 U/mL [positive, ≥9 U/mL]). Overall, these findings were consistent with a diagnosis of BP.
The patient was treated with prednisone 60 mg daily with initial response; however, there was disease recurrence with tapering. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily were added as steroid-sparing agents, as prednisone was discontinued due to mood changes. Three months after the prednisone taper, the patient continued to develop new blisters. He completed treatment with doxycycline and nicotinamide. Rituximab 375 mg weekly was then initiated for 4 weeks.
At 2-week follow-up after completing the rituximab course, the patient reported worsening symptoms and presented with new bullae on the abdomen and upper extremities (Figure 2). Because of the recent history of mood changes while taking prednisone, a trial of cyclosporine 100 mg twice daily (1.37 mg/kg/d) was initiated, with notable improvement within 2 weeks of treatment. After 2 months of cyclosporine, approximately 90% of the rash had resolved with a few tense bullae remaining on the left frontal scalp but no new flares (Figure 3). One month after treatment ended, the patient remained clear of lesions without relapse.
Programmed death receptor 1 inhibitors have shown dramatic efficacy for a growing number of solid and hematologic malignancies, especially malignant melanoma. However, their use is accompanied by nonspecific activation of the immune system, resulting in a variety of adverse events, many manifesting on the skin. Several cases of BP in patients treated with PD-1/PD-L1 inhibitors have been reported.9 Cutaneous irAEs usually manifest within 3 weeks of initiation of PD-1 inhibitor therapy; however, the onset of BP typically occurs later at approximately 21 weeks.4,9 Our patient developed cutaneous manifestations 4 months after cessation of nivolumab.



Bullous pemphigoid classically manifests with pruritus and tense bullae. Notably, our patient’s clinical presentation included a widespread eruption of papules without bullae, which was similar to a review by Tsiogka et al,9 which reported that one-third of patients first present with a nonspecific cutaneous eruption. Bullous pemphigoid induced by anti–PD-1 may manifest differently than traditional BP, illuminating the importance of a thorough diagnostic workup.
Although the pathogenesis of immune checkpoint inhibitor–induced BP has not been fully elucidated, it is hypothesized to be caused by increased T cell cytotoxic activity leading to tumor lysis and release of numerous autoantigens. These autoantigens cause priming of abnormal T cells that can lead to further tissue damage in peripheral tissue and to generation of aberrant B cells and subsequent autoantibodies such as BP180 in germinal centers.4,10,11
Cyclosporine is a calcineurin inhibitor that reduces synthesis of IL-2, resulting in reduced cell activation.12 Therefore, cyclosporine may alleviate BP in patients who are being treated, or were previously treated, with an immune checkpoint inhibitor by suppressing T cell–mediated immune reaction and may be a rapid alternative for patients who cannot tolerate systemic steroids.
Treatment options for mild to moderate cases of BP include topical corticosteroids and antihistamines, while severe cases may require high-dose systemic corticosteroids. In recalcitrant cases, rituximab infusion with or without intravenous immunoglobulin often is utilized.8,13 The use of cyclosporine for various bullous disorders, including pemphigus vulgaris and epidermolysis bullosa acquisita, has been described.14 In recent years there has been a shift away from the use of cyclosporine for these conditions following the introduction of rituximab, a monoclonal antibody directed against the CD20 antigen on B lymphocytes. We utilized cyclosporine in our patient after he experienced worsening symptoms 1 month after completing treatment with rituximab.
Improvement from rituximab therapy may be delayed because it can take months to deplete CD20+ B lymphocytes from circulation, which may necessitate additional immunosuppressants or re-treatment with rituximab.15,16 In these instances, cyclosporine may be beneficial as a low-cost alternative in patients who are unable to tolerate systemic steroids, with a relatively good safety profile. The dosage of cyclosporine prescribed to the patient was chosen based on Joint American Academy of Dermatology–National Psoriasis Foundation management guidelines for psoriasis with systemic nonbiologic therapies, which recommends an initial dosage of 1 to 3 mg/kg/d in 2 divided doses.17
As immunotherapy for treating various cancers gains popularity, the frequency of dermatologic irAEs will increase. Therefore, dermatologists must be aware of the array of cutaneous manifestations, such as BP, and potential treatment options. When first-line and second-line therapies are contraindicated or do not provide notable improvement, cyclosporine may be an effective alternative for immune checkpoint inhibitor–induced BP.
To the Editor:
Immune checkpoint inhibitors have revolutionized the treatment of advanced-stage melanoma, with remarkably improved progression-free survival.1 Anti–programmed death receptor 1 (anti–PD-1) therapies, such as nivolumab and pembrolizumab, are a class of checkpoint inhibitors that have been approved by the US Food and Drug Administration for unresectable metastatic melanoma. Anti–PD-1 agents block the interaction of programmed death-ligand 1 (PD-L1) found on tumor cells with the PD-1 receptor on T cells, facilitating a positive immune response.2
Although these therapies have demonstrated notable antitumor efficacy, they also give rise to numerous immune-related adverse events (irAEs). As many as 70% of patients treated with PD-1/PD-L1 inhibitors experience some type of organ system irAE, of which 30% to 40% are cutaneous.3-6 Dermatologic adverse events are the most common irAEs, specifically spongiotic dermatitis, lichenoid dermatitis, pruritus, and vitiligo.7 Bullous pemphigoid (BP), an autoimmune bullous skin disorder caused by autoantibodies to basement membrane zone antigens, is a rare but potentially serious cutaneous irAE.8 Systemic corticosteroids commonly are used to treat immune checkpoint inhibitor–induced BP; other options include tetracyclines for maintenance therapy and rituximab for corticosteroid-refractory BP associated with anti-PD-1.9 We present a case of recalcitrant BP secondary to nivolumab therapy in a patient with metastatic melanoma who had near-complete resolution of BP following 2 months of cyclosporine.
A 41-year-old man presented with a generalized papular skin eruption of 1 month’s duration. He had a history of stage IIIC malignant melanoma of the lower right leg with positive sentinel lymph node biopsy. The largest lymph node deposit was 0.03 mm without extracapsular extension. Whole-body positron emission tomography–computed tomography showed no evidence of distant disease. The patient was treated with wide local excision with clear surgical margins plus 12 cycles of nivolumab, which was discontinued due to colitis. Four months after the final cycle of nivolumab, the patient developed widespread erythematous papules with hemorrhagic yellow crusting and no mucosal involvement. He was referred to dermatology by his primary oncologist for further evaluation.
A punch biopsy from the abdomen showed parakeratosis with leukocytoclasis and a superficial dermal infiltrate of neutrophils and eosinophils (Figure 1). Direct immunofluorescence revealed linear basement membrane deposits of IgG and C3, consistent with subepidermal blistering disease. Indirect immunofluorescence demonstrated trace IgG and IgG4 antibodies localized to the epidermal roof of salt-split skin and was negative for IgA antibodies. An enzyme-linked immunoassay was positive for BP antigen 2 (BP180) antibodies (98.4 U/mL [positive, ≥9 U/mL]) and negative for BP antigen 1 (BP230) antibodies (4.3 U/mL [positive, ≥9 U/mL]). Overall, these findings were consistent with a diagnosis of BP.
The patient was treated with prednisone 60 mg daily with initial response; however, there was disease recurrence with tapering. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily were added as steroid-sparing agents, as prednisone was discontinued due to mood changes. Three months after the prednisone taper, the patient continued to develop new blisters. He completed treatment with doxycycline and nicotinamide. Rituximab 375 mg weekly was then initiated for 4 weeks.
At 2-week follow-up after completing the rituximab course, the patient reported worsening symptoms and presented with new bullae on the abdomen and upper extremities (Figure 2). Because of the recent history of mood changes while taking prednisone, a trial of cyclosporine 100 mg twice daily (1.37 mg/kg/d) was initiated, with notable improvement within 2 weeks of treatment. After 2 months of cyclosporine, approximately 90% of the rash had resolved with a few tense bullae remaining on the left frontal scalp but no new flares (Figure 3). One month after treatment ended, the patient remained clear of lesions without relapse.
Programmed death receptor 1 inhibitors have shown dramatic efficacy for a growing number of solid and hematologic malignancies, especially malignant melanoma. However, their use is accompanied by nonspecific activation of the immune system, resulting in a variety of adverse events, many manifesting on the skin. Several cases of BP in patients treated with PD-1/PD-L1 inhibitors have been reported.9 Cutaneous irAEs usually manifest within 3 weeks of initiation of PD-1 inhibitor therapy; however, the onset of BP typically occurs later at approximately 21 weeks.4,9 Our patient developed cutaneous manifestations 4 months after cessation of nivolumab.



Bullous pemphigoid classically manifests with pruritus and tense bullae. Notably, our patient’s clinical presentation included a widespread eruption of papules without bullae, which was similar to a review by Tsiogka et al,9 which reported that one-third of patients first present with a nonspecific cutaneous eruption. Bullous pemphigoid induced by anti–PD-1 may manifest differently than traditional BP, illuminating the importance of a thorough diagnostic workup.
Although the pathogenesis of immune checkpoint inhibitor–induced BP has not been fully elucidated, it is hypothesized to be caused by increased T cell cytotoxic activity leading to tumor lysis and release of numerous autoantigens. These autoantigens cause priming of abnormal T cells that can lead to further tissue damage in peripheral tissue and to generation of aberrant B cells and subsequent autoantibodies such as BP180 in germinal centers.4,10,11
Cyclosporine is a calcineurin inhibitor that reduces synthesis of IL-2, resulting in reduced cell activation.12 Therefore, cyclosporine may alleviate BP in patients who are being treated, or were previously treated, with an immune checkpoint inhibitor by suppressing T cell–mediated immune reaction and may be a rapid alternative for patients who cannot tolerate systemic steroids.
Treatment options for mild to moderate cases of BP include topical corticosteroids and antihistamines, while severe cases may require high-dose systemic corticosteroids. In recalcitrant cases, rituximab infusion with or without intravenous immunoglobulin often is utilized.8,13 The use of cyclosporine for various bullous disorders, including pemphigus vulgaris and epidermolysis bullosa acquisita, has been described.14 In recent years there has been a shift away from the use of cyclosporine for these conditions following the introduction of rituximab, a monoclonal antibody directed against the CD20 antigen on B lymphocytes. We utilized cyclosporine in our patient after he experienced worsening symptoms 1 month after completing treatment with rituximab.
Improvement from rituximab therapy may be delayed because it can take months to deplete CD20+ B lymphocytes from circulation, which may necessitate additional immunosuppressants or re-treatment with rituximab.15,16 In these instances, cyclosporine may be beneficial as a low-cost alternative in patients who are unable to tolerate systemic steroids, with a relatively good safety profile. The dosage of cyclosporine prescribed to the patient was chosen based on Joint American Academy of Dermatology–National Psoriasis Foundation management guidelines for psoriasis with systemic nonbiologic therapies, which recommends an initial dosage of 1 to 3 mg/kg/d in 2 divided doses.17
As immunotherapy for treating various cancers gains popularity, the frequency of dermatologic irAEs will increase. Therefore, dermatologists must be aware of the array of cutaneous manifestations, such as BP, and potential treatment options. When first-line and second-line therapies are contraindicated or do not provide notable improvement, cyclosporine may be an effective alternative for immune checkpoint inhibitor–induced BP.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi:10.1056/NEJMoa1504030
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
- Puzanov I, Diab A, Abdallah K, et al; . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi:10.1186/s40425-017-0300-z
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575. doi:10.3978/j.issn.2218-6751.2015.06.06
- Kumar V, Chaudhary N, Garg M, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Schauer F, Rafei-Shamsabadi D, Mai S, et al. Hemidesmosomal reactivity and treatment recommendations in immune checkpoint inhibitor-induced bullous pemphigoid—a retrospective, monocentric study. Front Immunol. 2022;13:953546. doi:10.3389/fimmu.2022.953546
- Tsiogka A, Bauer JW, Patsatsi A. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a review of the literature. Acta Derm Venereol. 2021;101:adv00377. doi:10.2340/00015555-3740
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669. doi:10.1111/ijd.13984
- Yang H, Yao Z, Zhou X, et al. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213:108377. doi:10.1016/j.clim.2020.108377
- Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum. 1992;21(6 suppl 3):16-22. doi:10.1016/0049-0172(92)90009-3
- Ahmed AR, Shetty S, Kaveri S, et al. Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: a retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700-708.e3. doi:10.1016/j.jaad.2015.11.030
- Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925-946. doi:10.1016/j.jaad.2010.02.063
- Schmidt E, Hunzelmann N, Zillikens D, et al. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol. 2006;31:503-508. doi:10.1111/j.1365-2230.2006.02151.x
- Kasperkiewicz M, Shimanovich I, Ludwig RJ, et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65:552-558.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34. doi:10.1056/NEJMoa1504030
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi:10.3389/fphar.2017.00561
- Puzanov I, Diab A, Abdallah K, et al; . Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi:10.1186/s40425-017-0300-z
- Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255-1268. doi:10.1016/j.jaad.2020.03.132
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560-575. doi:10.3978/j.issn.2218-6751.2015.06.06
- Kumar V, Chaudhary N, Garg M, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
- Belum VR, Benhuri B, Postow MA, et al. Characterisation and management of dermatologic adverse events to agents targeting the PD-1 receptor. Eur J Cancer. 2016;60:12-25. doi:10.1016/j.ejca.2016.02.010
- Schauer F, Rafei-Shamsabadi D, Mai S, et al. Hemidesmosomal reactivity and treatment recommendations in immune checkpoint inhibitor-induced bullous pemphigoid—a retrospective, monocentric study. Front Immunol. 2022;13:953546. doi:10.3389/fimmu.2022.953546
- Tsiogka A, Bauer JW, Patsatsi A. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a review of the literature. Acta Derm Venereol. 2021;101:adv00377. doi:10.2340/00015555-3740
- Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664-669. doi:10.1111/ijd.13984
- Yang H, Yao Z, Zhou X, et al. Immune-related adverse events of checkpoint inhibitors: insights into immunological dysregulation. Clin Immunol. 2020;213:108377. doi:10.1016/j.clim.2020.108377
- Russell G, Graveley R, Seid J, et al. Mechanisms of action of cyclosporine and effects on connective tissues. Semin Arthritis Rheum. 1992;21(6 suppl 3):16-22. doi:10.1016/0049-0172(92)90009-3
- Ahmed AR, Shetty S, Kaveri S, et al. Treatment of recalcitrant bullous pemphigoid (BP) with a novel protocol: a retrospective study with a 6-year follow-up. J Am Acad Dermatol. 2016;74:700-708.e3. doi:10.1016/j.jaad.2015.11.030
- Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. 2010;63:925-946. doi:10.1016/j.jaad.2010.02.063
- Schmidt E, Hunzelmann N, Zillikens D, et al. Rituximab in refractory autoimmune bullous diseases. Clin Exp Dermatol. 2006;31:503-508. doi:10.1111/j.1365-2230.2006.02151.x
- Kasperkiewicz M, Shimanovich I, Ludwig RJ, et al. Rituximab for treatment-refractory pemphigus and pemphigoid: a case series of 17 patients. J Am Acad Dermatol. 2011;65:552-558.
- Menter A, Gelfand JM, Connor C, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82:1445-1486. doi:10.1016/j.jaad.2020.02.044
Practice Points
- Bullous pemphigoid is a rare dermatologic immune-related adverse event that can occur secondary to anti–programmed death receptor 1 therapy.
- For cases of immune checkpoint inhibitor–induced bullous pemphigoid that are recalcitrant to corticosteroids and rituximab, cyclosporine might be an effective alternative.
Mycobacterium interjectum Infection in an Immunocompetent Host Following Contact With Aquarium Fish
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.
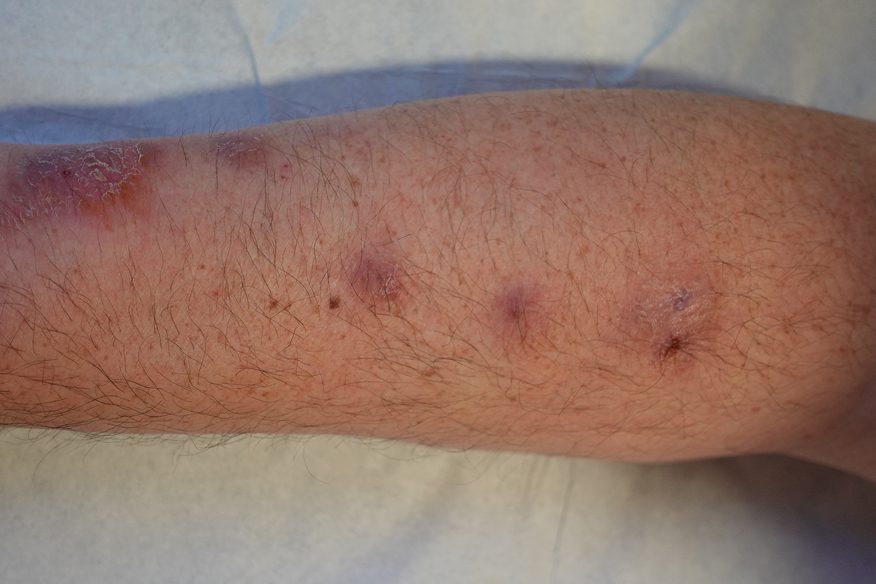
The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.

The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
To the Editor:
A 48-year-old man presented with nodular lesions in a sporotrichoid pattern on the right hand and forearm of 3 months’ duration (Figure). There were no lymphadeno-pathies, and he had no notable medical history. He denied fever and other systemic symptoms. The patient recently had manipulated a warm water fish aquarium. Although he did not recall a clear injury, inadvertent mild trauma was a possibility. He denied other contact or trauma in relation to animals or vegetables.
Histopathology from a punch biopsy of the forearm revealed a granulomatous infiltrate with necrosis at the deep dermis level at the interface with the subcutaneous cellular tissue that was composed of mainly epithelioid cells with a few multinucleated giant cells. No acid-fast bacilli or fungi were observed with special stains.
A polymerase chain reaction assay for atypical mycobacteria was positive for Mycobacterium interjectum. The culture of the skin biopsy was negative for fungi and mycobacteria after long incubation (6 weeks) on 2 occasions, and an antibiogram was not available. Complementary tests including hemogram, HIV serology, and chest and upper extremity radiographs did not reveal any abnormalities.

The patient was treated with rifampicin 600 mg/d, clarithromycin 500 mg every 12 hours, and co-trimoxazole 160/800 mg every 12 hours for 9 months with some resolution but persistence of some residual scarring lesions. There was no recurrence at 6-month follow-up.
Mycobacterium interjectum is a rare, slow-growing, scotochromogenic mycobacteria. Case reports usually refer to lymphadenitis in healthy children and pulmonary infections in immunocompromised or immunocompetent adults.1,2 A case of M interjectum with cutaneous involvement was reported by Fukuoka et al,3 with ulcerated nodules and abscesses on the leg identified in an immunocompromised patient. Our patient did not present with any cause of immunosuppression or clear injury predisposing him to infection. This microorganism has been detected in water, soil,3 and aquarium fish,4 the latter being the most likely source of infection in our patient. Given its slow growth rate and the need for a specific polymerase chain reaction assay, which is not widely available, M interjectum infection may be underdiagnosed.
No standard antibiotic regimen has been established, but M interjectum has proven to be a multidrug-resistant bacterium with frequent therapy failures. Treatment options have ranged from standard tuberculostatic therapy to combination therapy with medications such as amikacin, levofloxacin, rifampicin, and co-trimoxazole.1 Because an antibiogram was not available for our patient, empiric treatment with rifampicin, clarithromycin, and co-trimoxazole was prescribed for 9 months, with satisfactory response and tolerance. These drugs were selected because of their susceptibility profile in the literature.1,5
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
- Sotello D, Hata DJ, Reza M, et al. Disseminated Mycobacterium interjectum infection with bacteremia, hepatic and pulmonary involvement associated with a long-term catheter infection. Case Rep Infect Dis. 2017;2017:1-5.
- Dholakia YN. Mycobacterium interjectum isolated from an immunocompetent host with lung infection. Int J Mycobacteriol. 2017;6:401-403.
- Fukuoka M, Matsumura Y, Kore-eda S, et al. Cutaneous infection due to Mycobacterium interjectum in an immunosuppressed patient with microscopic polyangiitis. Br J Dermatol. 2008;159:1382-1384.
- Zanoni RG, Florio D, Fioravanti ML, et al. Occurrence of Mycobacterium spp. in ornamental fish in Italy. J Fish Dis. 2008;31:433-441.
- Emler S, Rochat T, Rohner P, et al. Chronic destructive lung disease associated with a novel mycobacterium. Am J Respir Crit Care Med. 1994;150:261-265.
Practice Points
- Mycobacterium interjectum can cause cutaneous nodules in a sporotrichoid or lymphocutaneous pattern and may affect immunocompromised and immunocompetent patients.
- This mycobacteria has been detected in water, soil, and aquarium fish. The latter could be a source of infection and should be taken into account in the anamnesis.
- There is no established therapeutic regimen for M interjectum infection. Combination therapy with rifampicin, clarithromycin, and co-trimoxazole could be an option, though it must always be adapted to an antibiogram if results are available.