User login
A Rare Case of a Splenic Abscess as the Origin of Illness in Exudative Pleural Effusion
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
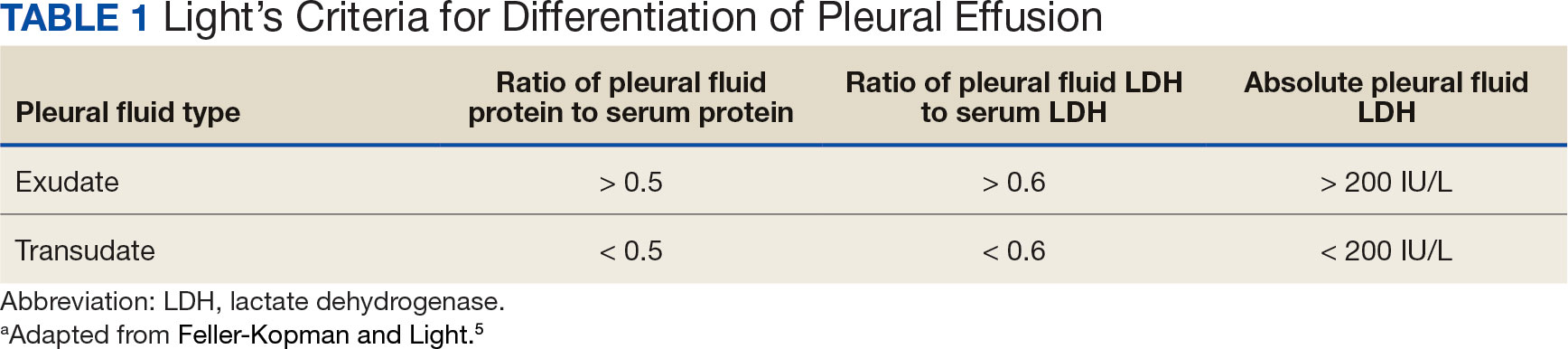
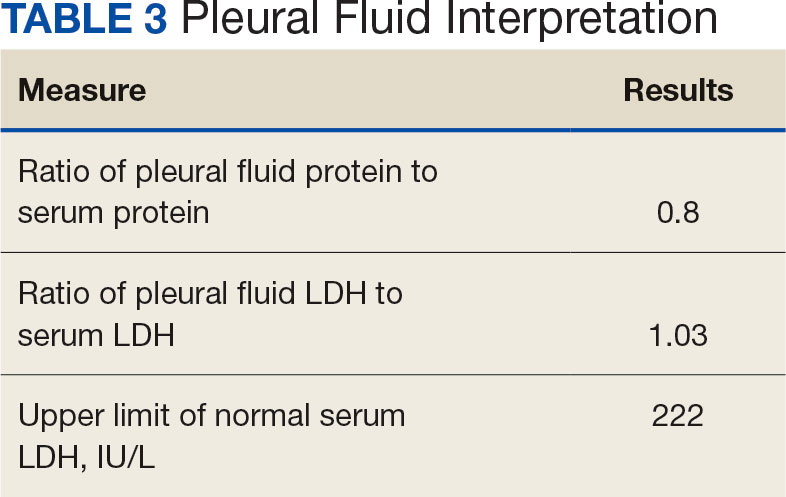
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6
A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
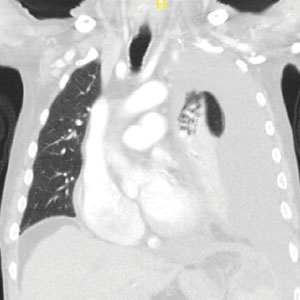
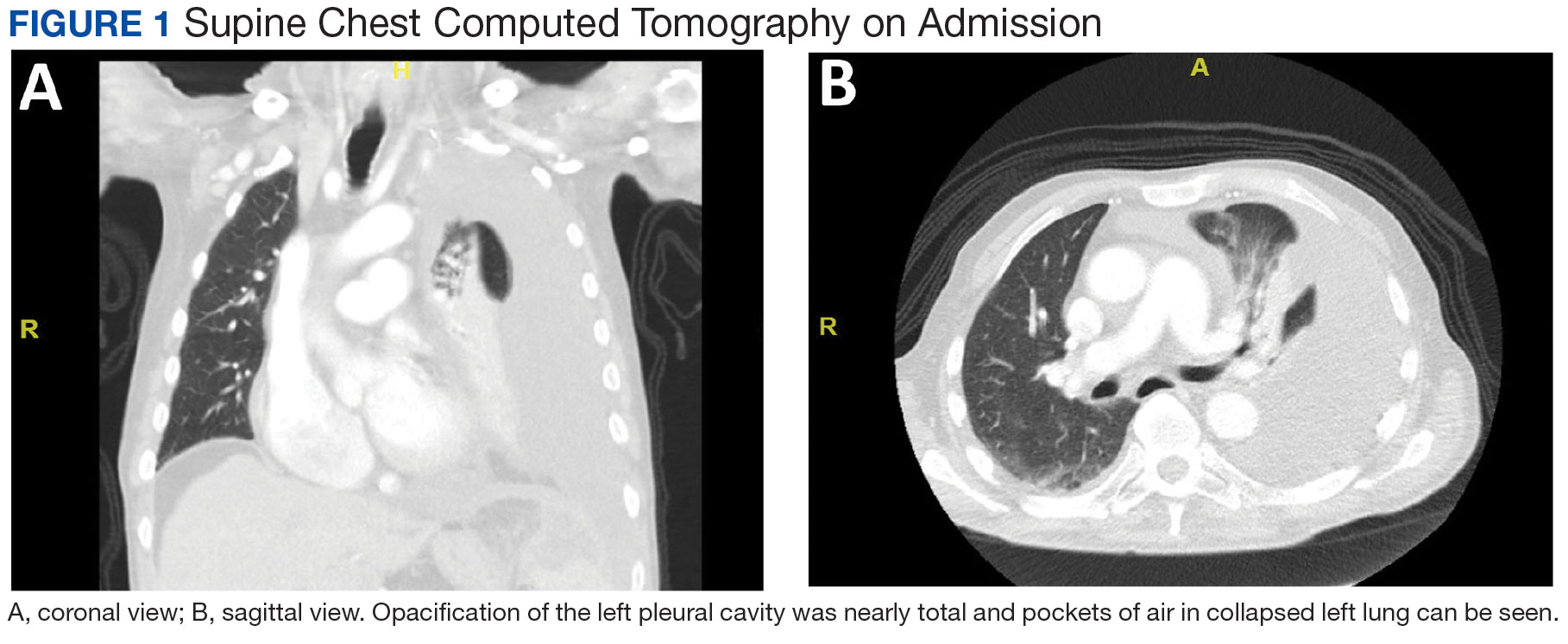
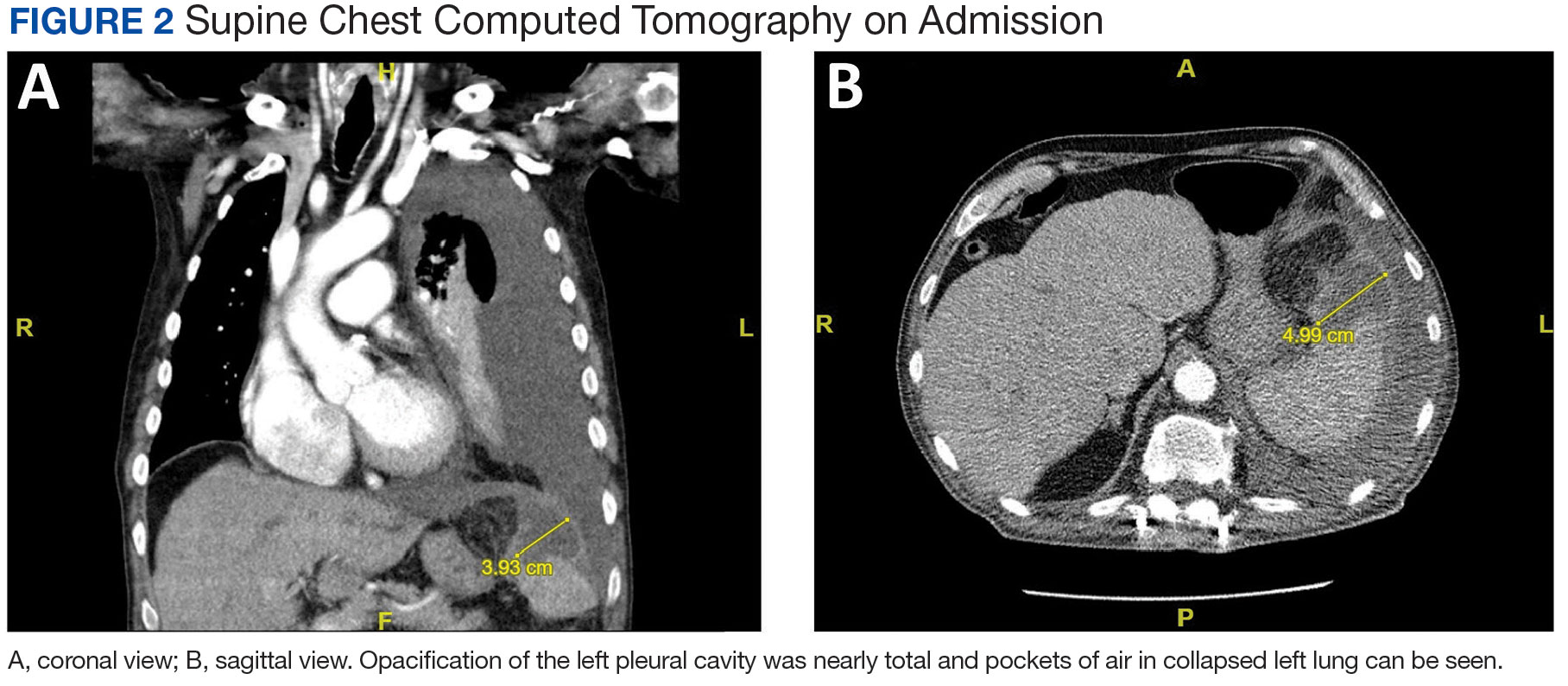
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).
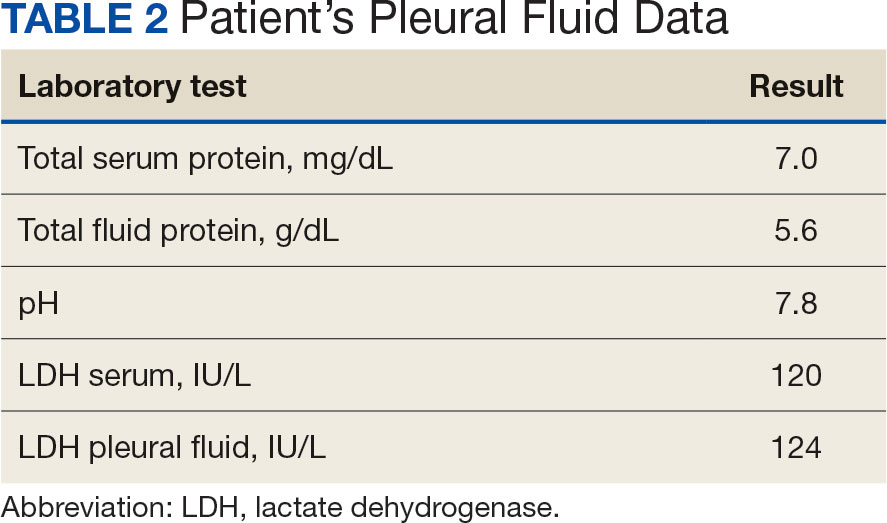
Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.
The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
Splenic abscesses are a rare occurrence that represent a marginal proportion of intra-abdominal infections. One study found splenic abscesses in only 0.14% to 0.70% of autopsies and none of the 540 abdominal abscesses they examined originated in the spleen.1 Patients with splenic abscesses tend to present with nonspecific symptoms such as fevers, chills, and abdominal pain.2 Imaging modalities such as abdominal ultrasound and computed tomography (CT) are vital to the workup and diagnosis identification.2 Splenic abscesses are generally associated with another underlying process, as seen in patients who are affected by endocarditis, trauma, metastatic infection, splenic infarction, or neoplasia.2
Pleural effusions, or the buildup of fluid within the pleural space, is a common condition typically secondary to another disease.3 Clinical identification of the primary condition may be challenging.3 In the absence of a clear etiology, such as obvious signs of congestive heart failure, further differentiation relies upon pleural fluid analysis, beginning with the distinction between exudate (inflammatory) and transudate (noninflammatory). 3,4 This distinction can be made using Light’s criteria, which relies on protein and lactate dehydrogenase (LDH) ratios between the pleural fluid and serum (Table 1).5 Though rare, half of splenic abscesses are associated with pleural effusion.6 As an inflammatory condition, splenic abscesses have been classically described as a cause of exudative pleural effusions.5,6

A myelodysplastic syndrome is a group of diseases that arise from malignant hematopoietic stem cells, leading to the proliferation of the malignant cells and faulty production of other bone marrow products.7 These disorders can range from single to multilineage dysplasia. Cells are often left in an immature blast form, unable to function appropriately, and vulnerable to destruction. Patients with myeloproliferative disorders frequently suffer from leukopenia and infections attributable to known quantitative and qualitative defects of neutrophils.8
CASE PRESENTATION
A male aged 80 years presented to the Central Texas Veterans Affairs Hospital (CTVAH) with shortness of breath, weight loss, and fever. On admission, his medical history was notable for atrial fibrillation, myelodysplastic syndrome, hypertension, hyperlipidemia, stable ascending aortic aneurysm, and Vitamin B12 deficiency. A chest CT showed a large left pleural effusion (Figure 1). Additionally, the radiology report noted a nonspecific 4- to 5-cm lobulated subdiaphragmatic mass within the anterior dome of the spleen with surrounding soft tissue swelling and splenomegaly (Figure 2).


Initial thoracentesis was performed with 1500 mL of straw-colored fluid negative for bacteria, fungi, malignancy, and acid-fast organisms (Tables 2 and 3). The pleural effusion persisted, requiring a second thoracentesis 2 days later that was positive for Escherichia coli (E coli). Given the exudative nature and positive culture, a chest tube was placed, and the pleural effusion was therefore felt to be an empyema, arousing suspicion that the splenic mass seen on CT was an abscess. The site was accessed by interventional radiology, purulent fluid aspirated, and a drain was placed. Cultures grew E coli sensitive to ceftriaxone. Despite receiving intravenous ceftriaxone 2 g daily, the pleural effusion became further complicated due to chest tube obstruction and persistent drainage.


The patient was discharged to Baylor Scott & White Medical Center in Temple, Texas where he underwent decortication with cardiothoracic surgery with several pleural adhesions noted. Following surgery the patient was readmitted to CTVAH and continued ceftriaxone therapy following the infectious disease specialist's recommendation. He was discharged with plans to return to CTVAH for continued care. The patient was readmitted and transitioned to oral levofloxacin 500 mg daily and received physical and occupational therapy. He showed dramatic improvement on this regimen, with a 3-week follow-up CT that indicated only a small left pleural effusion and a 28 mm × 11 mm × 10 mm lesion in the anterior superior spleen. The patient had not returned for further evaluation by thoracic surgery; however, he has continued to see CTVAH primary care without reported recurrence of symptoms.
DISCUSSION
Splenic abscesses are a rare condition typically characterized by hematogenous spread of bacteria from another source, most commonly the endocardium.2 Other differential diagnoses include bacteremia or spread from an intra-abdominal site.2 Staphylococcus aureus and E coli are the most common bacteria seen in splenic abscesses. 2 Treatment includes antibiotics, percutaneous drainage, and, as a last resort, splenectomy.2
Our patient was found to have grown E coli, but no source indicative of spread was identified. He had negative blood cultures, negative findings for intra-abdominal pathologies on CT scans, and a negative echocardiogram for endocarditis. A bronchoscopy showed no evidence of a source from the lungs, and specimens taken from the pleural adhesions were negative for malignancy and bacteria.
This patient had risk factors for the illness, namely his history of being immunocompromised secondary to myelodysplastic syndrome.7 Accordingly, the patient showed persistent leukopenia with neutropenia and lymphocytopenia, which would not be expected for most patients with such an extensive infection. 8 While being immunocompromised undoubtedly contributed to the severity of the patient’s presentation and slow recovery, it does not explain the etiology or origin of his infection. This patient differs from current literature in that his splenic abscess was truly idiopathic rather than resulting from an alternative source.
Complications of splenic abscesses include pleural effusions, as seen with this patient, as well as pneumonia, pneumothorax, hemorrhage, subphrenic abscess, and intraabdominal perforation, among others.2 We determined conclusively that the patient’s pleural effusion was secondary to the splenic abscess, and excluded other bacterial foci strongly suggests that the spleen was the origin of the illness.
CONCLUSIONS
This case suggests splenic abscesses should be considered when evaluating pleural effusion. It further demonstrates that the spleen may be the central source of infection in the absence of iatrogenic inoculation or bacteremia. We hope our findings may lead to earlier identification in similar scenarios and improved patient outcomes in a multidisciplinary approach.
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
- Lee WS, Choi ST, Kim KK. Splenic abscess: a single institution study and review of the literature. Yonsei Med J. 2011;52(2):288-292. doi:10.3349/ymj.2011.52.2.288
- Lotfollahzadeh S, Mathew G, Zemaitis MR. Splenic Abscess. In: StatPearls. StatPearls Publishing; June 3, 2023.
- Jany B, Welte T. Pleural effusion in adults-etiology, diagnosis, and treatment. Dtsch Arztebl Int. 2019;116(21):377- 386. doi:10.3238/arztebl.2019.0377
- Light RW. Pleural effusions. Med Clin North Am. 2011;95(6):1055-1070. doi:10.1016/j.mcna.2011.08.005
- Feller-Kopman D, Light R. Pleural Disease. N Engl J Med. 2018;378(18):1754. doi:10.1056/NEJMc1803858
- Ferreiro L, Casal A, Toubes ME, et al. Pleural effusion due to nonmalignant gastrointestinal disease. ERJ Open Res. 2023;9(3):00290-2022. doi:10.1183/23120541.00290-2022
- Hasserjian RP. Myelodysplastic syndrome updated. Pathobiology. 2019;86(1):7-13. doi:10.1159/000489702
- Toma A, Fenaux P, Dreyfus F, Cordonnier C. Infections in myelodysplastic syndromes. Haematologica. 2012;97(10):1459- 1470. doi:10.3324/haematol2012.063420
Posterior Reversible Encephalopathy Syndrome (PRES) Following Bevacizumab and Atezolizumab Therapy in Hepatocellular Carcinoma (HCC)
Background
Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, is known to inhibit angiogenesis and prevent carcinogenesis. Recent evidence from the IMbrave050 trial indicates that combining bevacizumab with atezolizumab enhances recurrence-free survival (RFS) in high-risk HCC patients undergoing curative treatments. Bevacizumab is notorious for causing endothelial dysfunction that may provoke vasospasm, leading to central hypoperfusion, hypertension, and, albeit rarely, PRES. Similarly, immunotherapy, including atezolizumab, has been implicated in PRES, underscoring a potential risk when these therapies are administered concurrently.
Case Presentation
A 64-year-old woman with a history of hepatitis C and alcoholic cirrhosis was diagnosed with stage II (T2 N0 M0) HCC. Following partial hepatectomy, we proceeded with adjuvant systemic therapy with atezolizumab and bevacizumab (per the IMbrave050 trial). After her 2nd treatment, she developed altered mental status, seizures, and severe hypertension. Labs revealed acute kidney injury and elevated creatinine kinase levels suggesting rhabdomyolysis. Computed tomography head showed no acute findings, but magnetic resonance imaging of the brain identified increased flair attenuated inversion recovery (FLAIR) signal in the brain’s posterior regions, indicating PRES. Symptomatic management with anti-hypertensives and intravenous fluids led to the recovery of mental status to baseline. Further therapy with bevacizumab and atezolizumab was then held off.
Discussion
Therapeutic advances in HCC management through the IMbrave050 trial demonstrate the efficacy of bevacizumab and atezolizumab in reducing RFS, without highlighting the serious side effects like PRES. To our knowledge, this is the first case reported where PRES occurred with the simultaneous use of atezolizumab and bevacizumab. Since both drugs can individually cause PRES, there might be a heightened risk with the co-administration, signaling a critical need for vigilant monitoring and further research into this treatment modality’s long-term safety profile.
Background
Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, is known to inhibit angiogenesis and prevent carcinogenesis. Recent evidence from the IMbrave050 trial indicates that combining bevacizumab with atezolizumab enhances recurrence-free survival (RFS) in high-risk HCC patients undergoing curative treatments. Bevacizumab is notorious for causing endothelial dysfunction that may provoke vasospasm, leading to central hypoperfusion, hypertension, and, albeit rarely, PRES. Similarly, immunotherapy, including atezolizumab, has been implicated in PRES, underscoring a potential risk when these therapies are administered concurrently.
Case Presentation
A 64-year-old woman with a history of hepatitis C and alcoholic cirrhosis was diagnosed with stage II (T2 N0 M0) HCC. Following partial hepatectomy, we proceeded with adjuvant systemic therapy with atezolizumab and bevacizumab (per the IMbrave050 trial). After her 2nd treatment, she developed altered mental status, seizures, and severe hypertension. Labs revealed acute kidney injury and elevated creatinine kinase levels suggesting rhabdomyolysis. Computed tomography head showed no acute findings, but magnetic resonance imaging of the brain identified increased flair attenuated inversion recovery (FLAIR) signal in the brain’s posterior regions, indicating PRES. Symptomatic management with anti-hypertensives and intravenous fluids led to the recovery of mental status to baseline. Further therapy with bevacizumab and atezolizumab was then held off.
Discussion
Therapeutic advances in HCC management through the IMbrave050 trial demonstrate the efficacy of bevacizumab and atezolizumab in reducing RFS, without highlighting the serious side effects like PRES. To our knowledge, this is the first case reported where PRES occurred with the simultaneous use of atezolizumab and bevacizumab. Since both drugs can individually cause PRES, there might be a heightened risk with the co-administration, signaling a critical need for vigilant monitoring and further research into this treatment modality’s long-term safety profile.
Background
Bevacizumab, an anti-vascular endothelial growth factor monoclonal antibody, is known to inhibit angiogenesis and prevent carcinogenesis. Recent evidence from the IMbrave050 trial indicates that combining bevacizumab with atezolizumab enhances recurrence-free survival (RFS) in high-risk HCC patients undergoing curative treatments. Bevacizumab is notorious for causing endothelial dysfunction that may provoke vasospasm, leading to central hypoperfusion, hypertension, and, albeit rarely, PRES. Similarly, immunotherapy, including atezolizumab, has been implicated in PRES, underscoring a potential risk when these therapies are administered concurrently.
Case Presentation
A 64-year-old woman with a history of hepatitis C and alcoholic cirrhosis was diagnosed with stage II (T2 N0 M0) HCC. Following partial hepatectomy, we proceeded with adjuvant systemic therapy with atezolizumab and bevacizumab (per the IMbrave050 trial). After her 2nd treatment, she developed altered mental status, seizures, and severe hypertension. Labs revealed acute kidney injury and elevated creatinine kinase levels suggesting rhabdomyolysis. Computed tomography head showed no acute findings, but magnetic resonance imaging of the brain identified increased flair attenuated inversion recovery (FLAIR) signal in the brain’s posterior regions, indicating PRES. Symptomatic management with anti-hypertensives and intravenous fluids led to the recovery of mental status to baseline. Further therapy with bevacizumab and atezolizumab was then held off.
Discussion
Therapeutic advances in HCC management through the IMbrave050 trial demonstrate the efficacy of bevacizumab and atezolizumab in reducing RFS, without highlighting the serious side effects like PRES. To our knowledge, this is the first case reported where PRES occurred with the simultaneous use of atezolizumab and bevacizumab. Since both drugs can individually cause PRES, there might be a heightened risk with the co-administration, signaling a critical need for vigilant monitoring and further research into this treatment modality’s long-term safety profile.
Rare Gems: Navigating Goblet Cell Appendiceal Cancer
Background
Goblet cell adenocarcinoma (GCA), also known as goblet cell carcinoid, is a rare and distinct type of cancer originating from the appendix. It is characterized by cells that exhibit both mucinous and neuroendocrine differentiation, presenting a more aggressive nature compared to conventional carcinoids and a higher propensity for metastasis.
Case Presentation
A 60-year-old male presented with complaints of abdominal pain, nausea, vomiting, constipation, and weight loss worsening in the last month. He had a history of heavy alcohol intake, smoking, and family history of colon cancer in his grandfather. Initial workup with abdominal CT revealed findings suggestive of early bowel obstruction and possible malignancy. Subsequent EGD showed esophagitis, and colonoscopy identified a cecal mass. Biopsies confirmed malignant cells of enteric type with goblet cell features. Staging CT during hospitalization did not reveal distant metastasis initially. However, diagnostic laparoscopy later identified widespread peritoneal carcinomatosis, precluding surgical intervention. The case was discussed in tumor boards, leading to the initiation of palliative FOLFOX + Bevacizumab chemotherapy. After completing 7 cycles, restaging imaging showed stable disease. Subsequently, the patient experienced worsening obstructive symptoms with CT abdomen and pelvis demonstrating disease progression. Given his condition, decompressive gastrostomy was not feasible. The patient decided to transition to comfort measures only.
Discussion
Goblet cell adenocarcinoma is a rare appendiceal tumor with amphicrine differentiation, occurring at a rate of 0.01–0.05 per 100,000 individuals annually and comprising approximately 15% of all appendiceal neoplasms. These tumors often disseminate within the peritoneum, contributing to their aggressive behavior and challenging management.
Conclusions
Metastatic goblet cell adenocarcinoma presents significant treatment challenges and is associated with a poor prognosis. Tailored treatment strategies, vigilant monitoring, and ongoing research efforts are essential for optimizing patient outcomes and enhancing quality of life in this aggressive cancer
Background
Goblet cell adenocarcinoma (GCA), also known as goblet cell carcinoid, is a rare and distinct type of cancer originating from the appendix. It is characterized by cells that exhibit both mucinous and neuroendocrine differentiation, presenting a more aggressive nature compared to conventional carcinoids and a higher propensity for metastasis.
Case Presentation
A 60-year-old male presented with complaints of abdominal pain, nausea, vomiting, constipation, and weight loss worsening in the last month. He had a history of heavy alcohol intake, smoking, and family history of colon cancer in his grandfather. Initial workup with abdominal CT revealed findings suggestive of early bowel obstruction and possible malignancy. Subsequent EGD showed esophagitis, and colonoscopy identified a cecal mass. Biopsies confirmed malignant cells of enteric type with goblet cell features. Staging CT during hospitalization did not reveal distant metastasis initially. However, diagnostic laparoscopy later identified widespread peritoneal carcinomatosis, precluding surgical intervention. The case was discussed in tumor boards, leading to the initiation of palliative FOLFOX + Bevacizumab chemotherapy. After completing 7 cycles, restaging imaging showed stable disease. Subsequently, the patient experienced worsening obstructive symptoms with CT abdomen and pelvis demonstrating disease progression. Given his condition, decompressive gastrostomy was not feasible. The patient decided to transition to comfort measures only.
Discussion
Goblet cell adenocarcinoma is a rare appendiceal tumor with amphicrine differentiation, occurring at a rate of 0.01–0.05 per 100,000 individuals annually and comprising approximately 15% of all appendiceal neoplasms. These tumors often disseminate within the peritoneum, contributing to their aggressive behavior and challenging management.
Conclusions
Metastatic goblet cell adenocarcinoma presents significant treatment challenges and is associated with a poor prognosis. Tailored treatment strategies, vigilant monitoring, and ongoing research efforts are essential for optimizing patient outcomes and enhancing quality of life in this aggressive cancer
Background
Goblet cell adenocarcinoma (GCA), also known as goblet cell carcinoid, is a rare and distinct type of cancer originating from the appendix. It is characterized by cells that exhibit both mucinous and neuroendocrine differentiation, presenting a more aggressive nature compared to conventional carcinoids and a higher propensity for metastasis.
Case Presentation
A 60-year-old male presented with complaints of abdominal pain, nausea, vomiting, constipation, and weight loss worsening in the last month. He had a history of heavy alcohol intake, smoking, and family history of colon cancer in his grandfather. Initial workup with abdominal CT revealed findings suggestive of early bowel obstruction and possible malignancy. Subsequent EGD showed esophagitis, and colonoscopy identified a cecal mass. Biopsies confirmed malignant cells of enteric type with goblet cell features. Staging CT during hospitalization did not reveal distant metastasis initially. However, diagnostic laparoscopy later identified widespread peritoneal carcinomatosis, precluding surgical intervention. The case was discussed in tumor boards, leading to the initiation of palliative FOLFOX + Bevacizumab chemotherapy. After completing 7 cycles, restaging imaging showed stable disease. Subsequently, the patient experienced worsening obstructive symptoms with CT abdomen and pelvis demonstrating disease progression. Given his condition, decompressive gastrostomy was not feasible. The patient decided to transition to comfort measures only.
Discussion
Goblet cell adenocarcinoma is a rare appendiceal tumor with amphicrine differentiation, occurring at a rate of 0.01–0.05 per 100,000 individuals annually and comprising approximately 15% of all appendiceal neoplasms. These tumors often disseminate within the peritoneum, contributing to their aggressive behavior and challenging management.
Conclusions
Metastatic goblet cell adenocarcinoma presents significant treatment challenges and is associated with a poor prognosis. Tailored treatment strategies, vigilant monitoring, and ongoing research efforts are essential for optimizing patient outcomes and enhancing quality of life in this aggressive cancer
Cholangioblastic Intrahepatic Cholangiocarcinoma: A Rare Case of an Inhibin-Positive Variant Mimicking Neuroendocrine Tumors
Background
Cholangiocarcinoma (CCA) is a rare and aggressive cancer of the biliary system, accounting for 15% of primary liver cancers. Most CCAs arise spontaneously, with risk factors including primary biliary cirrhosis, liver fluke infection, and biliary malformations. A newly described variant, Inhibin-positive Cholangioblastic (solid-tubulocystic) intrahepatic cholangiocarcinoma (iCCA), mimics neuroendocrine tumors (NET). This report presents a case of this new variant.
Case Presentation
A 53-year-old female with a history of alcohol use disorder and no family history of liver cancer presented with watery diarrhea for a month. Blood tests, including tumor markers, were normal. An ultrasound revealed a large mass in the right hepatic lobe. CT and MRI scans suggested a hemangioma. Due to the mass’s size and spontaneous bleeding risk, she underwent surgical resection. The mass was initially thought to be a hemangioma but was later identified as poorly differentiated intrahepatic CCA with a solid and tubulocystic structure. Pathology showed strong staining for Cytokeratin (CK) 7, CK-19, and Inhibin, and weak staining for synaptophysin, confirming a diagnosis of cholangioblastic iCCA. Genetic testing revealed no actionable variations. She was started on capecitabine for 8 cycles. Follow-up imaging showed no disease recurrence or metastasis.
Discussion
CCA often presents at advanced stages with symptoms like weight loss and jaundice. Diagnosis involves clinical assessment, lab work, and imaging, particularly MRI. Cholangioblastic Intrahepatic CCA (iCCA) is a newly described variant of cholangiocarcinoma. There have been 16 reported cases of the disease. Initially, it was thought to be a NET as it expressed Chromogranin, insulinoma-associated protein-1, and Synaptophysin. Almost half of the reported cases were diagnosed as NET initially. One tool clinicians can use to differentiate them is inhibin. Inhibin has been documented in all of the reported cases of Cholangioblastic iCCA. A novel inhibin-positive cholangioblastic iCCA variant with a Nipped-B-like protein and nucleus accumbens associated-1 (NIPBL-NACC1) fusion transcript has been reported recently, further helping differentiate the two. There is no standard of therapy for this variant. It’s managed similarly to CCAs, relying on surgical resection as the primary treatment. Limited data shows varied responses to neoadjuvant and adjuvant therapy.
Background
Cholangiocarcinoma (CCA) is a rare and aggressive cancer of the biliary system, accounting for 15% of primary liver cancers. Most CCAs arise spontaneously, with risk factors including primary biliary cirrhosis, liver fluke infection, and biliary malformations. A newly described variant, Inhibin-positive Cholangioblastic (solid-tubulocystic) intrahepatic cholangiocarcinoma (iCCA), mimics neuroendocrine tumors (NET). This report presents a case of this new variant.
Case Presentation
A 53-year-old female with a history of alcohol use disorder and no family history of liver cancer presented with watery diarrhea for a month. Blood tests, including tumor markers, were normal. An ultrasound revealed a large mass in the right hepatic lobe. CT and MRI scans suggested a hemangioma. Due to the mass’s size and spontaneous bleeding risk, she underwent surgical resection. The mass was initially thought to be a hemangioma but was later identified as poorly differentiated intrahepatic CCA with a solid and tubulocystic structure. Pathology showed strong staining for Cytokeratin (CK) 7, CK-19, and Inhibin, and weak staining for synaptophysin, confirming a diagnosis of cholangioblastic iCCA. Genetic testing revealed no actionable variations. She was started on capecitabine for 8 cycles. Follow-up imaging showed no disease recurrence or metastasis.
Discussion
CCA often presents at advanced stages with symptoms like weight loss and jaundice. Diagnosis involves clinical assessment, lab work, and imaging, particularly MRI. Cholangioblastic Intrahepatic CCA (iCCA) is a newly described variant of cholangiocarcinoma. There have been 16 reported cases of the disease. Initially, it was thought to be a NET as it expressed Chromogranin, insulinoma-associated protein-1, and Synaptophysin. Almost half of the reported cases were diagnosed as NET initially. One tool clinicians can use to differentiate them is inhibin. Inhibin has been documented in all of the reported cases of Cholangioblastic iCCA. A novel inhibin-positive cholangioblastic iCCA variant with a Nipped-B-like protein and nucleus accumbens associated-1 (NIPBL-NACC1) fusion transcript has been reported recently, further helping differentiate the two. There is no standard of therapy for this variant. It’s managed similarly to CCAs, relying on surgical resection as the primary treatment. Limited data shows varied responses to neoadjuvant and adjuvant therapy.
Background
Cholangiocarcinoma (CCA) is a rare and aggressive cancer of the biliary system, accounting for 15% of primary liver cancers. Most CCAs arise spontaneously, with risk factors including primary biliary cirrhosis, liver fluke infection, and biliary malformations. A newly described variant, Inhibin-positive Cholangioblastic (solid-tubulocystic) intrahepatic cholangiocarcinoma (iCCA), mimics neuroendocrine tumors (NET). This report presents a case of this new variant.
Case Presentation
A 53-year-old female with a history of alcohol use disorder and no family history of liver cancer presented with watery diarrhea for a month. Blood tests, including tumor markers, were normal. An ultrasound revealed a large mass in the right hepatic lobe. CT and MRI scans suggested a hemangioma. Due to the mass’s size and spontaneous bleeding risk, she underwent surgical resection. The mass was initially thought to be a hemangioma but was later identified as poorly differentiated intrahepatic CCA with a solid and tubulocystic structure. Pathology showed strong staining for Cytokeratin (CK) 7, CK-19, and Inhibin, and weak staining for synaptophysin, confirming a diagnosis of cholangioblastic iCCA. Genetic testing revealed no actionable variations. She was started on capecitabine for 8 cycles. Follow-up imaging showed no disease recurrence or metastasis.
Discussion
CCA often presents at advanced stages with symptoms like weight loss and jaundice. Diagnosis involves clinical assessment, lab work, and imaging, particularly MRI. Cholangioblastic Intrahepatic CCA (iCCA) is a newly described variant of cholangiocarcinoma. There have been 16 reported cases of the disease. Initially, it was thought to be a NET as it expressed Chromogranin, insulinoma-associated protein-1, and Synaptophysin. Almost half of the reported cases were diagnosed as NET initially. One tool clinicians can use to differentiate them is inhibin. Inhibin has been documented in all of the reported cases of Cholangioblastic iCCA. A novel inhibin-positive cholangioblastic iCCA variant with a Nipped-B-like protein and nucleus accumbens associated-1 (NIPBL-NACC1) fusion transcript has been reported recently, further helping differentiate the two. There is no standard of therapy for this variant. It’s managed similarly to CCAs, relying on surgical resection as the primary treatment. Limited data shows varied responses to neoadjuvant and adjuvant therapy.
Neuroendocrine Tumor of Ampulla of Vater: A Rare Case Report and Review of Literature
Background
Ampulla of Vater is an extremely rare site for neuroendocrine tumors (NET), accounting for less than 0.3% of gastrointestinal (GI) and 2% of ampullary malignancies. This case report highlights the circuitous diagnosis of this rare tumor in a patient with a history of primary biliary cholangitis presenting with epigastric pain and severe pruritis.
Case Presentation
A 58-year-old female with history of sarcoidosis and primary biliary cholangitis status post sphincterotomy eight months prior, presented with worsening epigastric pain, fatigue, and weight loss over 6 months. Physical exam showed right upper quadrant tenderness. Labs revealed elevated alanine and aspartate aminotransferases at 415 and 195 units/L, with bilirubin of 0.3 mg/dl. Computerized tomography (CT) revealed a 2.3x3.2x4.0 cm peripancreatic hypodensity associated with phlegmon, pancreatic ductal dilation and pneumobilia. Magnetic resonance imaging (MRI) demonstrated a pancreatic head mass. Positron emission tomogram (PET) was negative for distant metastases. After discussion of management options, patient opted for Whipple procedure. The surgical pathology was consistent with invasive ampullary ductal carcinoma of the small intestine, pancreaticobiliary type. However, staining for synaptophysin and chromogranin were positive, with Ki-67 < 55%. Tumor board review confirmed neuroendocrine tumor of the ampulla of Vater. NCCN guidelines recommended active surveillance due to locoregional disease without positive margins or lymph nodes, advising routine follow-up and imaging.
Discussion
Neuroendocrine tumors (NET) at the Ampulla of Vater are exceedingly rare. Often manifesting as obstructive jaundice, they pose diagnostic hurdles, especially in patients with anatomical variations like scarring from primary biliary cholangitis. In a case series of 20 ampullary tumors, only one was neuroendocrine, highlighting their rarity. Accurate diagnosis, achieved through surgical biopsy and immunohistochemical testing, is crucial for appropriate management. Following NCCN guidelines for gastrointestinal NETs, our patient avoided unnecessary systemic treatment meant for adenocarcinoma, preserving her quality of life. Reporting such cases is essential for advancing understanding and refining patient care.
Conclusions
This case had evolving diagnoses, altering both the prognosis and treatment standards. Comorbid primary biliary cholangitis and high-grade tumor complexity posed diagnostic challenges, which was finally confirmed by surgical biopsy. Reporting such cases is vital in aiding tumor management and patient outcomes.
Background
Ampulla of Vater is an extremely rare site for neuroendocrine tumors (NET), accounting for less than 0.3% of gastrointestinal (GI) and 2% of ampullary malignancies. This case report highlights the circuitous diagnosis of this rare tumor in a patient with a history of primary biliary cholangitis presenting with epigastric pain and severe pruritis.
Case Presentation
A 58-year-old female with history of sarcoidosis and primary biliary cholangitis status post sphincterotomy eight months prior, presented with worsening epigastric pain, fatigue, and weight loss over 6 months. Physical exam showed right upper quadrant tenderness. Labs revealed elevated alanine and aspartate aminotransferases at 415 and 195 units/L, with bilirubin of 0.3 mg/dl. Computerized tomography (CT) revealed a 2.3x3.2x4.0 cm peripancreatic hypodensity associated with phlegmon, pancreatic ductal dilation and pneumobilia. Magnetic resonance imaging (MRI) demonstrated a pancreatic head mass. Positron emission tomogram (PET) was negative for distant metastases. After discussion of management options, patient opted for Whipple procedure. The surgical pathology was consistent with invasive ampullary ductal carcinoma of the small intestine, pancreaticobiliary type. However, staining for synaptophysin and chromogranin were positive, with Ki-67 < 55%. Tumor board review confirmed neuroendocrine tumor of the ampulla of Vater. NCCN guidelines recommended active surveillance due to locoregional disease without positive margins or lymph nodes, advising routine follow-up and imaging.
Discussion
Neuroendocrine tumors (NET) at the Ampulla of Vater are exceedingly rare. Often manifesting as obstructive jaundice, they pose diagnostic hurdles, especially in patients with anatomical variations like scarring from primary biliary cholangitis. In a case series of 20 ampullary tumors, only one was neuroendocrine, highlighting their rarity. Accurate diagnosis, achieved through surgical biopsy and immunohistochemical testing, is crucial for appropriate management. Following NCCN guidelines for gastrointestinal NETs, our patient avoided unnecessary systemic treatment meant for adenocarcinoma, preserving her quality of life. Reporting such cases is essential for advancing understanding and refining patient care.
Conclusions
This case had evolving diagnoses, altering both the prognosis and treatment standards. Comorbid primary biliary cholangitis and high-grade tumor complexity posed diagnostic challenges, which was finally confirmed by surgical biopsy. Reporting such cases is vital in aiding tumor management and patient outcomes.
Background
Ampulla of Vater is an extremely rare site for neuroendocrine tumors (NET), accounting for less than 0.3% of gastrointestinal (GI) and 2% of ampullary malignancies. This case report highlights the circuitous diagnosis of this rare tumor in a patient with a history of primary biliary cholangitis presenting with epigastric pain and severe pruritis.
Case Presentation
A 58-year-old female with history of sarcoidosis and primary biliary cholangitis status post sphincterotomy eight months prior, presented with worsening epigastric pain, fatigue, and weight loss over 6 months. Physical exam showed right upper quadrant tenderness. Labs revealed elevated alanine and aspartate aminotransferases at 415 and 195 units/L, with bilirubin of 0.3 mg/dl. Computerized tomography (CT) revealed a 2.3x3.2x4.0 cm peripancreatic hypodensity associated with phlegmon, pancreatic ductal dilation and pneumobilia. Magnetic resonance imaging (MRI) demonstrated a pancreatic head mass. Positron emission tomogram (PET) was negative for distant metastases. After discussion of management options, patient opted for Whipple procedure. The surgical pathology was consistent with invasive ampullary ductal carcinoma of the small intestine, pancreaticobiliary type. However, staining for synaptophysin and chromogranin were positive, with Ki-67 < 55%. Tumor board review confirmed neuroendocrine tumor of the ampulla of Vater. NCCN guidelines recommended active surveillance due to locoregional disease without positive margins or lymph nodes, advising routine follow-up and imaging.
Discussion
Neuroendocrine tumors (NET) at the Ampulla of Vater are exceedingly rare. Often manifesting as obstructive jaundice, they pose diagnostic hurdles, especially in patients with anatomical variations like scarring from primary biliary cholangitis. In a case series of 20 ampullary tumors, only one was neuroendocrine, highlighting their rarity. Accurate diagnosis, achieved through surgical biopsy and immunohistochemical testing, is crucial for appropriate management. Following NCCN guidelines for gastrointestinal NETs, our patient avoided unnecessary systemic treatment meant for adenocarcinoma, preserving her quality of life. Reporting such cases is essential for advancing understanding and refining patient care.
Conclusions
This case had evolving diagnoses, altering both the prognosis and treatment standards. Comorbid primary biliary cholangitis and high-grade tumor complexity posed diagnostic challenges, which was finally confirmed by surgical biopsy. Reporting such cases is vital in aiding tumor management and patient outcomes.
Preleukemic Chronic Myeloid Leukemia: A Case Report and Literature Review
Background
CML is usually classified in chronic phase (CP), accelerated phase (AP) and/or Blast phase (BP). Studies have described another provisional entity as Preleukemic phase of CML which precedes CP and is without leukocytosis and blood features of CP phase of CML. Here we will present a case of metastatic prostate cancer, where next-generation sequencing showed BCR-ABL1 but no overt leukocytosis and then later developed clinical CML after 11 months.
Case Presentation
Our patient was 87-yr-old male with history of metastatic prostate cancer, who presented with elevated PSA levels and new bony metastasis. He underwent next-generation-sequencing (foundation one liquid cdx) in April 2022. It did not show reportable alterations related to prostate cancer but BCR-ABL1 (p210) fusion was detected. It also showed ASXL1-S846fs5 and TET2-Q1548 that are also markers of clonal hematopoiesis. In March 2023 (11 months after the finding of BCR-ABL1), he developed asymptomatic leukocytosis, Workup showed BCR-ABL1(210) of 44% in peripheral blood and bone marrow showed 9% blasts. He was started on Imatinib after shared decision-making and considering the toxicity profile and comorbidities. He followed up regularly with improvement in leukocytosis.
Discussion
The diagnosis of CML is first suspected by typical findings in blood and/or bone marrow and then confirmed by presence of Philadelphia chromosome. Along with chronic and accelerated phases, there is another term described in few cases called “preleukemic or smoldering or aleukemic phase.” This is not a wellestablished term but mostly defined as normal leukocyte count with presence of BCR/ABL1 fusion gene/ ph chromosome. Preleukemic phase of CML is mostly underdiagnosed or misdiagnosed. Our case is unique in that BCR/ABL1 fusion was detected incidentally on next-generation sequencing and patient progressed to chronic phase of CML 11 months later. Upon literature review, few case reports and case series documenting aleukemic/preleukemic phase of CML but timing from the appearance of BCR/ABL1 mutation to actual development to leukocytosis is not well documented. Especially in the era of NGS testing, patients with incidental BCR-ABL1 should be evaluated further irrespective of normal WBC. Further studies need to be done to recognize this early and decrease the delay in treatment.
Background
CML is usually classified in chronic phase (CP), accelerated phase (AP) and/or Blast phase (BP). Studies have described another provisional entity as Preleukemic phase of CML which precedes CP and is without leukocytosis and blood features of CP phase of CML. Here we will present a case of metastatic prostate cancer, where next-generation sequencing showed BCR-ABL1 but no overt leukocytosis and then later developed clinical CML after 11 months.
Case Presentation
Our patient was 87-yr-old male with history of metastatic prostate cancer, who presented with elevated PSA levels and new bony metastasis. He underwent next-generation-sequencing (foundation one liquid cdx) in April 2022. It did not show reportable alterations related to prostate cancer but BCR-ABL1 (p210) fusion was detected. It also showed ASXL1-S846fs5 and TET2-Q1548 that are also markers of clonal hematopoiesis. In March 2023 (11 months after the finding of BCR-ABL1), he developed asymptomatic leukocytosis, Workup showed BCR-ABL1(210) of 44% in peripheral blood and bone marrow showed 9% blasts. He was started on Imatinib after shared decision-making and considering the toxicity profile and comorbidities. He followed up regularly with improvement in leukocytosis.
Discussion
The diagnosis of CML is first suspected by typical findings in blood and/or bone marrow and then confirmed by presence of Philadelphia chromosome. Along with chronic and accelerated phases, there is another term described in few cases called “preleukemic or smoldering or aleukemic phase.” This is not a wellestablished term but mostly defined as normal leukocyte count with presence of BCR/ABL1 fusion gene/ ph chromosome. Preleukemic phase of CML is mostly underdiagnosed or misdiagnosed. Our case is unique in that BCR/ABL1 fusion was detected incidentally on next-generation sequencing and patient progressed to chronic phase of CML 11 months later. Upon literature review, few case reports and case series documenting aleukemic/preleukemic phase of CML but timing from the appearance of BCR/ABL1 mutation to actual development to leukocytosis is not well documented. Especially in the era of NGS testing, patients with incidental BCR-ABL1 should be evaluated further irrespective of normal WBC. Further studies need to be done to recognize this early and decrease the delay in treatment.
Background
CML is usually classified in chronic phase (CP), accelerated phase (AP) and/or Blast phase (BP). Studies have described another provisional entity as Preleukemic phase of CML which precedes CP and is without leukocytosis and blood features of CP phase of CML. Here we will present a case of metastatic prostate cancer, where next-generation sequencing showed BCR-ABL1 but no overt leukocytosis and then later developed clinical CML after 11 months.
Case Presentation
Our patient was 87-yr-old male with history of metastatic prostate cancer, who presented with elevated PSA levels and new bony metastasis. He underwent next-generation-sequencing (foundation one liquid cdx) in April 2022. It did not show reportable alterations related to prostate cancer but BCR-ABL1 (p210) fusion was detected. It also showed ASXL1-S846fs5 and TET2-Q1548 that are also markers of clonal hematopoiesis. In March 2023 (11 months after the finding of BCR-ABL1), he developed asymptomatic leukocytosis, Workup showed BCR-ABL1(210) of 44% in peripheral blood and bone marrow showed 9% blasts. He was started on Imatinib after shared decision-making and considering the toxicity profile and comorbidities. He followed up regularly with improvement in leukocytosis.
Discussion
The diagnosis of CML is first suspected by typical findings in blood and/or bone marrow and then confirmed by presence of Philadelphia chromosome. Along with chronic and accelerated phases, there is another term described in few cases called “preleukemic or smoldering or aleukemic phase.” This is not a wellestablished term but mostly defined as normal leukocyte count with presence of BCR/ABL1 fusion gene/ ph chromosome. Preleukemic phase of CML is mostly underdiagnosed or misdiagnosed. Our case is unique in that BCR/ABL1 fusion was detected incidentally on next-generation sequencing and patient progressed to chronic phase of CML 11 months later. Upon literature review, few case reports and case series documenting aleukemic/preleukemic phase of CML but timing from the appearance of BCR/ABL1 mutation to actual development to leukocytosis is not well documented. Especially in the era of NGS testing, patients with incidental BCR-ABL1 should be evaluated further irrespective of normal WBC. Further studies need to be done to recognize this early and decrease the delay in treatment.
Male Patient With a History of Monoclonal B Cell Lymphocytosis Presenting with Breast Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: A Case Report and Literature Review
Background
Monoclonal B cell lymphocytosis (MBL) is defined as presence of clonal b cell population that is fewer than 5 × 10(9)/L B-cells in peripheral blood and no other signs of a lymphoproliferative disorder. Patients with MBL are usually monitored with periodic history, physical exam and blood counts. Here we presented a case of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in breast in a patient with a history of MBL.
Case Presentation
68-year-old male with history of MBL underwent mammogram for breast mass. It showed suspicious 4.4 x 1.6 cm solid and cystic lesion containing a 1.7 x 0.9 x 1.8 cm solid hypervascular mass. Patient underwent left breast mass excision. Histologic sections focus of ADH involving papilloma with uninvolved margins. Lymphoid infiltrates noted had CLL/SLL immunophenotype and that it consists mostly of small B cells positive for CD5, CD20, CD23, CD43, Bcl-2, LEF1. CT CAP and PET/CT were negative for lymphadenopathy. Bone marrow biopsy showed marrow involvement by mature B-cell lymphoproliferative process, immunophenotypically consistent with CLL/SLL. As intra-ductal papilloma completely excised and hemogram was normal tumor board recommended surveillance only for CLL/SLL.
Discussion
MBL can progress to CLL, but it can rarely be presented as an extra-nodal mass in solid organs. We described a case of MBL that progressed to CLL/ SLL in breast mass in a male patient. This is the first reported case in literature where MBL progressed to CLL/ SLL of breast without lymphadenopathy. Upon literature review 8 case reports were found where CLL/SLL were described in breast tissue. 7 of them were in females and 1 one was in male. Two patients had CLL before breast mass but none of them had a history of MBL. 3 described cases in females had CLL/SLL infiltration of breast along with invasive ductal carcinoma. So, a patient with MBL can progress to involve solid organs despite no absolute lymphocytosis and should be considered in differentials of a new mass. Although more common in females, but it can occur in males as well. It’s important to consider the possibility of both CLL/SLL and breast cancer existing simultaneously.
Background
Monoclonal B cell lymphocytosis (MBL) is defined as presence of clonal b cell population that is fewer than 5 × 10(9)/L B-cells in peripheral blood and no other signs of a lymphoproliferative disorder. Patients with MBL are usually monitored with periodic history, physical exam and blood counts. Here we presented a case of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in breast in a patient with a history of MBL.
Case Presentation
68-year-old male with history of MBL underwent mammogram for breast mass. It showed suspicious 4.4 x 1.6 cm solid and cystic lesion containing a 1.7 x 0.9 x 1.8 cm solid hypervascular mass. Patient underwent left breast mass excision. Histologic sections focus of ADH involving papilloma with uninvolved margins. Lymphoid infiltrates noted had CLL/SLL immunophenotype and that it consists mostly of small B cells positive for CD5, CD20, CD23, CD43, Bcl-2, LEF1. CT CAP and PET/CT were negative for lymphadenopathy. Bone marrow biopsy showed marrow involvement by mature B-cell lymphoproliferative process, immunophenotypically consistent with CLL/SLL. As intra-ductal papilloma completely excised and hemogram was normal tumor board recommended surveillance only for CLL/SLL.
Discussion
MBL can progress to CLL, but it can rarely be presented as an extra-nodal mass in solid organs. We described a case of MBL that progressed to CLL/ SLL in breast mass in a male patient. This is the first reported case in literature where MBL progressed to CLL/ SLL of breast without lymphadenopathy. Upon literature review 8 case reports were found where CLL/SLL were described in breast tissue. 7 of them were in females and 1 one was in male. Two patients had CLL before breast mass but none of them had a history of MBL. 3 described cases in females had CLL/SLL infiltration of breast along with invasive ductal carcinoma. So, a patient with MBL can progress to involve solid organs despite no absolute lymphocytosis and should be considered in differentials of a new mass. Although more common in females, but it can occur in males as well. It’s important to consider the possibility of both CLL/SLL and breast cancer existing simultaneously.
Background
Monoclonal B cell lymphocytosis (MBL) is defined as presence of clonal b cell population that is fewer than 5 × 10(9)/L B-cells in peripheral blood and no other signs of a lymphoproliferative disorder. Patients with MBL are usually monitored with periodic history, physical exam and blood counts. Here we presented a case of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) in breast in a patient with a history of MBL.
Case Presentation
68-year-old male with history of MBL underwent mammogram for breast mass. It showed suspicious 4.4 x 1.6 cm solid and cystic lesion containing a 1.7 x 0.9 x 1.8 cm solid hypervascular mass. Patient underwent left breast mass excision. Histologic sections focus of ADH involving papilloma with uninvolved margins. Lymphoid infiltrates noted had CLL/SLL immunophenotype and that it consists mostly of small B cells positive for CD5, CD20, CD23, CD43, Bcl-2, LEF1. CT CAP and PET/CT were negative for lymphadenopathy. Bone marrow biopsy showed marrow involvement by mature B-cell lymphoproliferative process, immunophenotypically consistent with CLL/SLL. As intra-ductal papilloma completely excised and hemogram was normal tumor board recommended surveillance only for CLL/SLL.
Discussion
MBL can progress to CLL, but it can rarely be presented as an extra-nodal mass in solid organs. We described a case of MBL that progressed to CLL/ SLL in breast mass in a male patient. This is the first reported case in literature where MBL progressed to CLL/ SLL of breast without lymphadenopathy. Upon literature review 8 case reports were found where CLL/SLL were described in breast tissue. 7 of them were in females and 1 one was in male. Two patients had CLL before breast mass but none of them had a history of MBL. 3 described cases in females had CLL/SLL infiltration of breast along with invasive ductal carcinoma. So, a patient with MBL can progress to involve solid organs despite no absolute lymphocytosis and should be considered in differentials of a new mass. Although more common in females, but it can occur in males as well. It’s important to consider the possibility of both CLL/SLL and breast cancer existing simultaneously.
Chronic Myeloid Leukemia Presenting as Priapism: A Rare and Acute Initial Presentation in a Young Male
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
Introduction
Priapism, defined as a prolonged and often painful penile erection without sexual arousal, constitutes a urological emergency requiring immediate intervention. While commonly associated with conditions like sickle cell anemia and certain medications, malignancy-related priapism is rare and frequently overlooked. Herein, we present a unique case of a 31-year-old male with no significant medical history, who developed persistent priapism as the initial presentation of chronic myeloid leukemia (CML).
Case Presentation
A 31-year-old male without significant medical history, presented to the emergency department with painless priapism, was evaluated by urology and discharged home with precautions. He returned the following day with persistent, now painful priapism. Upon examination, his vital signs were stable. Urology performed aspiration and injection with Sudafed, resulting in mild symptom improvement. Laboratory findings revealed elevated white blood cell count (563.64 k/mcL), anemia (hemoglobin 8.4 g/dL), and a peripheral blood smear showed immature circulating cells with blast forms. He was transferred to a tertiary care center where conservative management addressed bleeding from the penile injection site, with subsequent treatment including leukapheresis and hydroxyurea for cytoreduction. Imaging revealed severe splenomegaly (36 cm) with abdominal mass effect. Peripheral flow cytometry didn’t show malignancy, but cytogenetic analysis showed a BCR/ABL1 fusion gene, confirming chronic myeloid leukemia (CML). Bone marrow biopsy showed hypercellularity without increased blasts. Treatment with dasatinib reduced the white count to 52,000 k/mcL, and was discharged home.
Discussion
Priapism is a urological emergency necessitating immediate intervention to prevent erectile dysfunction and permanent impotence. Management aims to achieve detumescence and typically involves methods such as irrigation or injection of vasoconstrictors into the penis. Malignancy-associated priapism (MAP) often results from venous obstruction due to hyperviscosity. Studies show that CML accounts for approximately 50% cases presenting with MAP, predominantly affecting younger individuals with a mean onset around 27 years of age. Priapism can occur before, during, or after treatment initiation or splenectomy in these patients. Providers should keep a high threshold of suspicion for MAP in patients with no other risk factors as prompt identification and treatment are needed to avoid permanent injury.
UC as a Culprit for Hemolytic Anemia
Introduction
Autoimmune hemolytic anemia (AIHA) can rarely be seen as an extra-intestinal manifestation (EIM) of inflammatory bowel disease (IBD), mostly ulcerative colitis (UC). This case report describes the clinical significance of recognizing AIHA in the context of UC.
Case Presentation
A 32-year-old male presented with profound fatigue, pallor, and dyspnea on exertion for one month. He also recalled intermittent bloody diarrhea for two years for which he never sought medical attention. Physical examination was unremarkable except for mid-abdominal tenderness. Labs revealed microcytosis, hemoglobin of 3.8 g/dL, total bilirubin 2.9 mg/ dL, indirect bilirubin of 2.0 mg/dL, LDH 132 U/L alk-p 459 U/L AST 98 U/L ALT 22 U/L. Direct Coombs test was positive suggesting warm AIHA with pan-agglutinin positive on the eluate test. Further testing revealed negative hepatitis and HIV panels and positive fecal calprotectin. CT abdomen and pelvis showed ascites, right pleural effusion and hepatosplenomegaly. Colonoscopy confirmed the diagnosis of ulcerative colitis, with extensive involvement of the colon. Mesalamine was initiated. Hematology was consulted for AIHA, who started the patient on methylprednisone leading to resolution of hemolytic anemia and improvement in gastrointestinal symptoms.
Discussion
IBD typically manifests as colitis, and the incidence of EIM as an initial symptom is observed in less than 10% cases. However, over the course of their lifetime, approximately 25% of patients will experience EIM, underscoring their relevance to clinical outcomes. Anemia is very common in IBD patients, mostly iron deficiency anemia (IDA) or anemia of chronic disease (ACD). However, AIHA can represent a rare but significant EIM of ulcerative colitis (UC), often posing diagnostic challenges. The underlying pathophysiological mechanisms linking UC and AIHA remain incompletely understood, necessitating a multidisciplinary approach to management. Treatment strategies focus on controlling both the hemolysis and the underlying IBD, emphasizing the importance of tailored interventions.
Conclusion
This case underscores the clinical significance of AIHA as an EIM of ulcerative colitis (UC), particularly when presenting as the primary symptom. Timely recognition is paramount to optimizing patient outcomes and preventing disease progression. Further research is warranted to elucidate the underlying mechanisms and therapeutic strategies for AIHA in the context of UC.
Introduction
Autoimmune hemolytic anemia (AIHA) can rarely be seen as an extra-intestinal manifestation (EIM) of inflammatory bowel disease (IBD), mostly ulcerative colitis (UC). This case report describes the clinical significance of recognizing AIHA in the context of UC.
Case Presentation
A 32-year-old male presented with profound fatigue, pallor, and dyspnea on exertion for one month. He also recalled intermittent bloody diarrhea for two years for which he never sought medical attention. Physical examination was unremarkable except for mid-abdominal tenderness. Labs revealed microcytosis, hemoglobin of 3.8 g/dL, total bilirubin 2.9 mg/ dL, indirect bilirubin of 2.0 mg/dL, LDH 132 U/L alk-p 459 U/L AST 98 U/L ALT 22 U/L. Direct Coombs test was positive suggesting warm AIHA with pan-agglutinin positive on the eluate test. Further testing revealed negative hepatitis and HIV panels and positive fecal calprotectin. CT abdomen and pelvis showed ascites, right pleural effusion and hepatosplenomegaly. Colonoscopy confirmed the diagnosis of ulcerative colitis, with extensive involvement of the colon. Mesalamine was initiated. Hematology was consulted for AIHA, who started the patient on methylprednisone leading to resolution of hemolytic anemia and improvement in gastrointestinal symptoms.
Discussion
IBD typically manifests as colitis, and the incidence of EIM as an initial symptom is observed in less than 10% cases. However, over the course of their lifetime, approximately 25% of patients will experience EIM, underscoring their relevance to clinical outcomes. Anemia is very common in IBD patients, mostly iron deficiency anemia (IDA) or anemia of chronic disease (ACD). However, AIHA can represent a rare but significant EIM of ulcerative colitis (UC), often posing diagnostic challenges. The underlying pathophysiological mechanisms linking UC and AIHA remain incompletely understood, necessitating a multidisciplinary approach to management. Treatment strategies focus on controlling both the hemolysis and the underlying IBD, emphasizing the importance of tailored interventions.
Conclusion
This case underscores the clinical significance of AIHA as an EIM of ulcerative colitis (UC), particularly when presenting as the primary symptom. Timely recognition is paramount to optimizing patient outcomes and preventing disease progression. Further research is warranted to elucidate the underlying mechanisms and therapeutic strategies for AIHA in the context of UC.
Introduction
Autoimmune hemolytic anemia (AIHA) can rarely be seen as an extra-intestinal manifestation (EIM) of inflammatory bowel disease (IBD), mostly ulcerative colitis (UC). This case report describes the clinical significance of recognizing AIHA in the context of UC.
Case Presentation
A 32-year-old male presented with profound fatigue, pallor, and dyspnea on exertion for one month. He also recalled intermittent bloody diarrhea for two years for which he never sought medical attention. Physical examination was unremarkable except for mid-abdominal tenderness. Labs revealed microcytosis, hemoglobin of 3.8 g/dL, total bilirubin 2.9 mg/ dL, indirect bilirubin of 2.0 mg/dL, LDH 132 U/L alk-p 459 U/L AST 98 U/L ALT 22 U/L. Direct Coombs test was positive suggesting warm AIHA with pan-agglutinin positive on the eluate test. Further testing revealed negative hepatitis and HIV panels and positive fecal calprotectin. CT abdomen and pelvis showed ascites, right pleural effusion and hepatosplenomegaly. Colonoscopy confirmed the diagnosis of ulcerative colitis, with extensive involvement of the colon. Mesalamine was initiated. Hematology was consulted for AIHA, who started the patient on methylprednisone leading to resolution of hemolytic anemia and improvement in gastrointestinal symptoms.
Discussion
IBD typically manifests as colitis, and the incidence of EIM as an initial symptom is observed in less than 10% cases. However, over the course of their lifetime, approximately 25% of patients will experience EIM, underscoring their relevance to clinical outcomes. Anemia is very common in IBD patients, mostly iron deficiency anemia (IDA) or anemia of chronic disease (ACD). However, AIHA can represent a rare but significant EIM of ulcerative colitis (UC), often posing diagnostic challenges. The underlying pathophysiological mechanisms linking UC and AIHA remain incompletely understood, necessitating a multidisciplinary approach to management. Treatment strategies focus on controlling both the hemolysis and the underlying IBD, emphasizing the importance of tailored interventions.
Conclusion
This case underscores the clinical significance of AIHA as an EIM of ulcerative colitis (UC), particularly when presenting as the primary symptom. Timely recognition is paramount to optimizing patient outcomes and preventing disease progression. Further research is warranted to elucidate the underlying mechanisms and therapeutic strategies for AIHA in the context of UC.
The First Patient in the Veteran Affairs System to Receive Chimeric Antigen Receptors T-cell Therapy for Refractory Multiple Myeloma and the Role of Intravenous Immunoglobulin in the Prevention of Therapy-associated Infections
Background
In 3/2021, chimeric antigen receptor (CAR) T-cell therapy was approved for the treatment of multiple myeloma in adult patients with refractory disease. Currently, only the Veterans Affair (VA) center at the Tennessee Valley Healthcare System (TVHS) offers this treatment. Herein, we report a significant healthcare milestone in 2024 when the first patient received CAR T-cell therapy for multiple myeloma in the VA system. Additionally, the rate of hypogammaglobulinemia is the highest for CAR T-cell therapy using idecabtagene vicleucel compared to therapies using other antineoplastic agents (Wat et al, 2021). The complications of hypogammaglobulinemia can be mitigated by intravenous immunoglobulin (IVIG) treatment.
Case Presentation
A 75-year-old male veteran was diagnosed with IgA Kappa multiple myeloma and received induction therapy with bortezomib, lenalidomide, and dexamethasone in 2014. The patient underwent autologous stem cell transplant (SCT) in the same year. His disease recurred in 3/2019, and the patient was started on daratumumab and pomalidomide. He received another autologous SCT in 2/2021, to which he was refractory. The veteran then received treatment with daratumumab and ixazomib, followed by carfilzomib and cyclophosphamide. Starting in 9/2022, the patient also required regular IVIG treatment for hypogammaglobulinemia. He eventually received CAR T-cell therapy with idecabtagene vicleucel at THVS on 4/18/2024. The patient tolerated the treatment well and is undergoing routine disease monitoring. Following CAR T-cell therapy, his hypogammaglobulinemia persists with immunoglobulins level less than 500 mg/dL, and the veteran is still receiving supportive care IVIG.
Discussion
A population estimate of 1.3 million veterans are uninsured and can only access healthcare through the VA (Nelson et al, 2007). This case highlights the first patient to receive CAR T-cell therapy for multiple myeloma in the VA system, indicating that veterans now have access to this life-saving treatment. The rate of hypogammaglobulinemia following CAR T-cell therapy for multiple myeloma is as high as 41%, with an associated infection risk of 70%. Following CAR T-cell therapy with idecabtagene vicleucel, around 61% of patients will require IVIG treatment (Wat el al, 2021). Our case adds to this growing literature on the prevalence of IVIG treatment following CAR T-cell therapy in this patient population.
Background
In 3/2021, chimeric antigen receptor (CAR) T-cell therapy was approved for the treatment of multiple myeloma in adult patients with refractory disease. Currently, only the Veterans Affair (VA) center at the Tennessee Valley Healthcare System (TVHS) offers this treatment. Herein, we report a significant healthcare milestone in 2024 when the first patient received CAR T-cell therapy for multiple myeloma in the VA system. Additionally, the rate of hypogammaglobulinemia is the highest for CAR T-cell therapy using idecabtagene vicleucel compared to therapies using other antineoplastic agents (Wat et al, 2021). The complications of hypogammaglobulinemia can be mitigated by intravenous immunoglobulin (IVIG) treatment.
Case Presentation
A 75-year-old male veteran was diagnosed with IgA Kappa multiple myeloma and received induction therapy with bortezomib, lenalidomide, and dexamethasone in 2014. The patient underwent autologous stem cell transplant (SCT) in the same year. His disease recurred in 3/2019, and the patient was started on daratumumab and pomalidomide. He received another autologous SCT in 2/2021, to which he was refractory. The veteran then received treatment with daratumumab and ixazomib, followed by carfilzomib and cyclophosphamide. Starting in 9/2022, the patient also required regular IVIG treatment for hypogammaglobulinemia. He eventually received CAR T-cell therapy with idecabtagene vicleucel at THVS on 4/18/2024. The patient tolerated the treatment well and is undergoing routine disease monitoring. Following CAR T-cell therapy, his hypogammaglobulinemia persists with immunoglobulins level less than 500 mg/dL, and the veteran is still receiving supportive care IVIG.
Discussion
A population estimate of 1.3 million veterans are uninsured and can only access healthcare through the VA (Nelson et al, 2007). This case highlights the first patient to receive CAR T-cell therapy for multiple myeloma in the VA system, indicating that veterans now have access to this life-saving treatment. The rate of hypogammaglobulinemia following CAR T-cell therapy for multiple myeloma is as high as 41%, with an associated infection risk of 70%. Following CAR T-cell therapy with idecabtagene vicleucel, around 61% of patients will require IVIG treatment (Wat el al, 2021). Our case adds to this growing literature on the prevalence of IVIG treatment following CAR T-cell therapy in this patient population.
Background
In 3/2021, chimeric antigen receptor (CAR) T-cell therapy was approved for the treatment of multiple myeloma in adult patients with refractory disease. Currently, only the Veterans Affair (VA) center at the Tennessee Valley Healthcare System (TVHS) offers this treatment. Herein, we report a significant healthcare milestone in 2024 when the first patient received CAR T-cell therapy for multiple myeloma in the VA system. Additionally, the rate of hypogammaglobulinemia is the highest for CAR T-cell therapy using idecabtagene vicleucel compared to therapies using other antineoplastic agents (Wat et al, 2021). The complications of hypogammaglobulinemia can be mitigated by intravenous immunoglobulin (IVIG) treatment.
Case Presentation
A 75-year-old male veteran was diagnosed with IgA Kappa multiple myeloma and received induction therapy with bortezomib, lenalidomide, and dexamethasone in 2014. The patient underwent autologous stem cell transplant (SCT) in the same year. His disease recurred in 3/2019, and the patient was started on daratumumab and pomalidomide. He received another autologous SCT in 2/2021, to which he was refractory. The veteran then received treatment with daratumumab and ixazomib, followed by carfilzomib and cyclophosphamide. Starting in 9/2022, the patient also required regular IVIG treatment for hypogammaglobulinemia. He eventually received CAR T-cell therapy with idecabtagene vicleucel at THVS on 4/18/2024. The patient tolerated the treatment well and is undergoing routine disease monitoring. Following CAR T-cell therapy, his hypogammaglobulinemia persists with immunoglobulins level less than 500 mg/dL, and the veteran is still receiving supportive care IVIG.
Discussion
A population estimate of 1.3 million veterans are uninsured and can only access healthcare through the VA (Nelson et al, 2007). This case highlights the first patient to receive CAR T-cell therapy for multiple myeloma in the VA system, indicating that veterans now have access to this life-saving treatment. The rate of hypogammaglobulinemia following CAR T-cell therapy for multiple myeloma is as high as 41%, with an associated infection risk of 70%. Following CAR T-cell therapy with idecabtagene vicleucel, around 61% of patients will require IVIG treatment (Wat el al, 2021). Our case adds to this growing literature on the prevalence of IVIG treatment following CAR T-cell therapy in this patient population.