User login
Child of The New Gastroenterologist
Underserved populations and colorectal cancer screening: Patient perceptions of barriers to care and effective interventions
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6
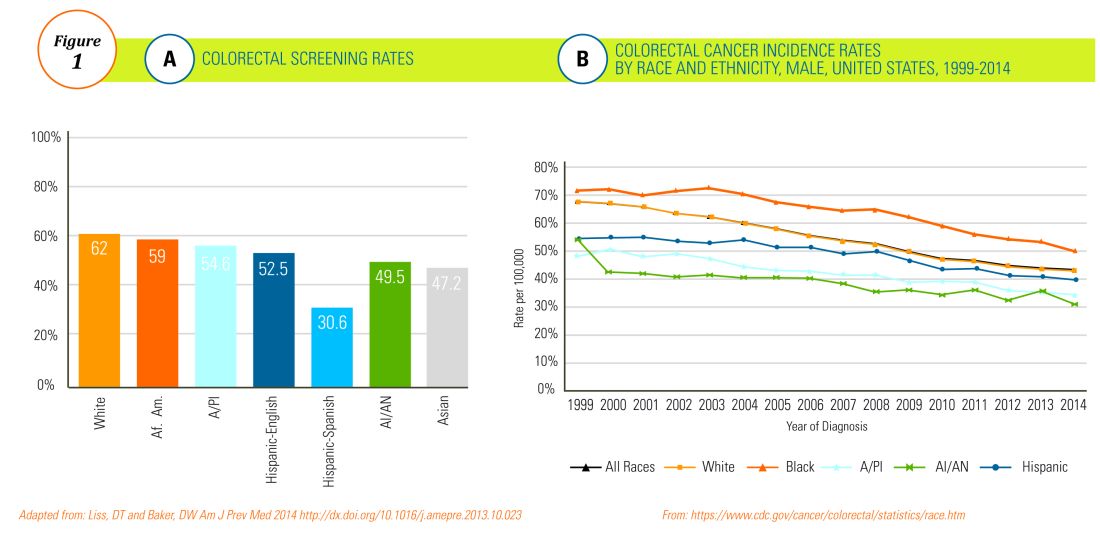
The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.
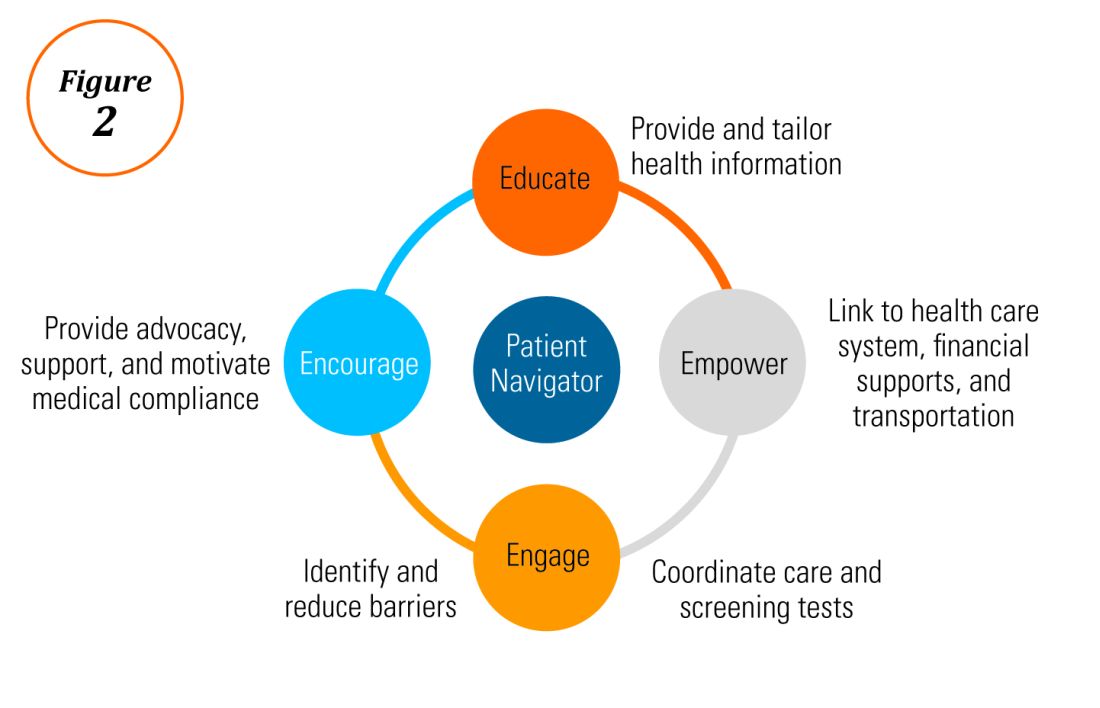
Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6

The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.

Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.
Editor's Note:
Importantly, these barriers often vary between specific population subsets. In this month’s In Focus article, brought to you by The New Gastroenterologist, the members of the AGA Institute Diversity Committee provide an enlightening overview of the barriers affecting underserved populations as well as strategies that can be employed to overcome these impediments. Better understanding of patient-specific barriers will, I hope, allow us to more effectively redress them and ultimately increase colorectal cancer screening rates in all populations.
Bryson W. Katona, MD, PhD
Editor in Chief, The New Gastroenterologist
Despite the positive public health effects of colorectal cancer (CRC) screening, there remains differential uptake of CRC screening in the United States. Minority populations born in the United States and immigrant populations are among those with the lowest rates of CRC screening, and both socioeconomic status and ethnicity are strongly associated with stage of CRC at diagnosis.1,2 Thus, recognizing the economic, social, and cultural factors that result in low rates of CRC screening in underserved populations is important in order to devise targeted interventions to increase CRC uptake and reduce morbidity and mortality in these populations.

What are the facts and figures?
The overall rate of screening colonoscopies has increased in all ethnic groups in the past 10 years but still falls below the goal of 71% established by the Healthy People project (www.healthypeople.gov) for the year 2020.3 According to the Centers for Disease Control and Prevention ethnicity-specific data for U.S.-born populations, 60% of whites, 55% of African Americans (AA), 50% of American Indian/Alaskan natives (AI/AN), 46% of Latino Americans, and 47% of Asians undergo CRC screening (Figure 1A).4 While CRC incidence in non-Hispanic whites age 50 years and older has dropped by 32% since 2000 because of screening, this trend has not been observed in AAs.5,6

The incidence of CRC in AAs is estimated at 49/10,000, one of the highest amongst U.S. populations and is the second and third most common cancer in AA women and men, respectively (Figure 1B).
Similar to AAs, AI/AN patients present with more advanced CRC disease and at younger ages and have lower survival rates, compared with other racial groups, a trend that has not changed in the last decade.7 CRC screening data in this population vary according to sex, geographic location, and health care utilization, with as few as 4.0% of asymptomatic, average-risk AI/ANs who receive medical care in the Indian Health Services being screened for CRC.8
The low rate of CRC screening among Latinos also poses a significant obstacle to the Healthy People project since it is expected that by 2060 Latinos will constitute 30% of the U.S. population. Therefore, strategies to improve CRC screening in this population are needed to continue the gains made in overall CRC mortality rates.
The percentage of immigrants in the U.S. population increased from 4.7% in 1970 to 13.5% in 2015. Immigrants, regardless of their ethnicity, represent a very vulnerable population, and CRC screening data in this population are not as robust as for U.S.-born groups. In general, immigrants have substantially lower CRC screening rates, compared with U.S.-born populations (21% vs. 60%),9 and it is suspected that additional, significant barriers to CRC screening and care exist for undocumented immigrants.
Another often overlooked group, are individuals with physical or cognitive disabilities. In this group, screening rates range from 49% to 65%.10
Finally, while information is available for many health care conditions and disparities faced by various ethnic groups, there are few CRC screening data for the LGBTQ community. Perhaps amplifying this problem is the existence of conflicting data in this population, with some studies suggesting there is no difference in CRC risk across groups in the LGBTQ community and others suggesting an increased risk.11,12 Notably, sexual orientation has been identified as a positive predictor of CRC screening in gay and bisexual men – CRC screening rates are higher in these groups, compared with heterosexual men.13 In contrast, no such difference has been found between homosexual and heterosexual women.14
What are the barriers?
Several common themes contribute to disparities in CRC screening among minority groups, including psychosocial/cultural, socioeconomic, provider-specific, and insurance-related factors. Some patient-related barriers include issues of illiteracy, having poor health literacy or English proficiency, having only grade school education,15,16 cultural misconceptions, transportation issues, difficulties affording copayments or deductibles, and a lack of follow-up for scheduled appointments and exams.17-20 Poor health literacy has a profound effect on exam perceptions, fear of test results, and compliance with scheduling tests and bowel preparation instructions21-25; it also affects one’s understanding of the importance of CRC screening, the recommended screening age, and the available choice of screening tests.
Even when some apparent barriers are mitigated, disparities in CRC screening remain. For example, even among the insured and among Medicare beneficiaries, screening rates and adequate follow-up rates after abnormal findings remain lower among AAs and those of low socioeconomic status than they are among whites.26-28 At least part of this paradox results from the presence of unmeasured cultural/belief systems that affect CRC screening uptake. Some of these factors include fear and/or denial of CRC diagnoses, mistrust of the health care system, and reluctance to undergo medical treatment and surgery.16,29 AAs are also less likely to be aware of a family history of CRC and to discuss personal and/or family history of CRC or polyps, which can thereby hinder the identification of high-risk individuals who would benefit from early screening.15,30
The deeply rooted sense of fatalism also plays a crucial role and has been cited for many minority and immigrant populations. Fatalism leads patients to view a diagnosis of cancer as a matter of “fate” or “God’s will,” and therefore, it is to be endured.23,31 Similarly, in a qualitative study of 44 Somali men living in St. Paul and Minneapolis, believing cancer was more common in whites, believing they were protected from cancer by God, fearing a cancer diagnosis, and fearing ostracism from their community were reported as barriers to cancer screening.32
Perceptions about CRC screening methods in Latino populations also have a tremendous influence and can include fear, stigma of sexual prejudice, embarrassment of being exposed during the exam, worries about humiliation in a male sense of masculinity, a lack of trust in the medical professionals, a sense of being a “guinea pig” for physicians, concerns about health care racism, and expectations of pain.33-37 Studies have reported that immigrants are afraid to seek health care because of the increasingly hostile environment associated with immigration enforcement.38 In addition, the impending dissolution of the Deferred Action for Childhood Arrivals act is likely to augment the barriers to care for Latino groups.39
In addition, provider-specific barriers to care also exist. Racial and ethnic minorities are less likely than whites to receive recommendations for screening by their physician. In fact, this factor alone has been demonstrated to be the main reason for lack of screening among AAs in a Californian cohort.40 In addition, patients from rural areas or those from AI/AN communities are at especially increased risk for lack of access to care because of a scarcity of providers along with patient perceptions regarding their primary care provider’s ability to connect them to subspecialists.41-43 Other cited examples include misconceptions about and poor treatment of the LGBTQ population by health care providers/systems.44
How can we intervene successfully?
Characterization of barriers is important because it promotes the development of targeted interventions. Intervention models include community engagement programs, incorporation of fecal occult testing, and patient navigator programs.45-47 In response to the alarming disparity in CRC screening rates in Latino communities, several interventions have been set in motion in different clinical scenarios, which include patient navigation and a focus on patient education.

Randomized trials have shown that outreach efforts and patient navigation increase CRC screening rates in AAs.48,54,55 Studies evaluating the effects of print-based educational materials on improving screening showed improvement in screening rates, decreases in cancer-related fatalistic attitudes, and patients had a better understanding of the benefits of screening as compared with the cost associated with screening and the cost of advanced disease.56 Similarly, the use of touch-screen computers that tailor informational messages to decisional stage and screening barriers increased participation in CRC screening.57 Including patient navigators along with printed education material was even more effective at increasing the proportion of patients getting colonoscopy screening than providing printed material alone, with more-intensive navigation needed for individuals with low literacy.58 Grubbs et al.reported the success of their patient navigation program, which included wider comprehensive screening and coverage for colonoscopy screening.59 In AAs, they estimated an annual reduction of CRC incidence and mortality of 4,200 and 2,700 patients, respectively.
Among immigrants, there is an increased likelihood of CRC screening in those immigrants with a higher number of primary care visits.60 The intersection of culture, race, socioeconomic status, housing enclaves, limited English proficiency, low health literacy, and immigration policy all play a role in immigrant health and access to health care.61
Therefore, different strategies may be needed for each immigrant group to improve CRC screening. For this group of patients, efforts aimed at mitigating the adverse effects of national immigration policies on immigrant populations may have the additional consequence of improving health care access and CRC screening for these patients.
Data gaps still exist in our understanding of patient perceptions, perspectives, and barriers that present opportunities for further study to develop long-lasting interventions that will improve health care of underserved populations. By raising awareness of the barriers, physicians can enhance their own self-awareness to keenly be attuned to these challenges as patients cross their clinic threshold for medical care.
Additional resources link: www.cdc.gov/cancer/colorectal/
References
1. Klabunde CN et al. Trends in colorectal cancer test use among vulnerable populations in the United States. Cancer Epidemiol Biomarkers Prev. 2011 Aug;20(8):1611-21.
2. Parikh-Patel A et al. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. 2006 Sep;107(5 Suppl):1189-95.
3. Promotion OoDPaH. Healthy People 2020. Cancer. Volume 2017.
4. Centers for Disease Control and Prevention. Cancer screening – United States, 2010. MMWR Morb Mortal Wkly Rep. 2012 Jan 27;61(3):41-5.
5. Doubeni CA et al. Racial and ethnic trends of colorectal cancer screening among Medicare enrollees. Am J Prev Med. 2010 Feb;38(2):184-91.
6. Kupfer SS et al. Reducing colorectal cancer risk among African Americans. Gastroenterology. 2015 Nov;149(6):1302-4.
7. Espey DK et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007 Nov;110(10):2119-52.
8. Day LW et al. Screening prevalence and incidence of colorectal cancer among American Indian/Alaskan natives in the Indian Health Service. Dig Dis Sci. 2011 Jul;56(7):2104-13.
9. Gupta S et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032.
10. Steele CB et al. Colorectal cancer incidence and screening – United States, 2008 and 2010. MMWR Suppl. 2013 Nov 22;62(3):53-60.
11. Boehmer U et al. Cancer survivorship and sexual orientation. Cancer 2011 Aug 15;117(16):3796-804.
12. Austin SB, Pazaris MJ, Wei EK, et al. Application of the Rosner-Wei risk-prediction model to estimate sexual orientation patterns in colon cancer risk in a prospective cohort of US women. Cancer Causes Control. 2014 Aug;25(8):999-1006.
13. Heslin KC et al. Sexual orientation and testing for prostate and colorectal cancers among men in California. Med Care. 2008 Dec;46(12):1240-8.
14. McElroy JA et al. Advancing Health Care for Lesbian, Gay, Bisexual, and Transgender Patients in Missouri. Mo Med. 2015 Jul-Aug;112(4):262-5.
15. Greiner KA et al. Knowledge and perceptions of colorectal cancer screening among urban African Americans. J Gen Intern Med. 2005 Nov;20(11):977-83.
16. Green PM, Kelly BA. Colorectal cancer knowledge, perceptions, and behaviors in African Americans. Cancer Nurs. 2004 May-Jun;27(3):206-15; quiz 216-7.
17. Berkowitz Z et al. Beliefs, risk perceptions, and gaps in knowledge as barriers to colorectal cancer screening in older adults. J Am Geriatr Soc. 2008 Feb;56(2):307-14.
18. Dolan NC et al. Colorectal cancer screening knowledge, attitudes, and beliefs among veterans: Does literacy make a difference? J Clin Oncol. 2004 Jul;22(13):2617-22.
19. Peterson NB et al. The influence of health literacy on colorectal cancer screening knowledge, beliefs and behavior. J Natl Med Assoc. 2007 Oct;99(10):1105-12.
20. Haddock MG et al. Intraoperative irradiation for locally recurrent colorectal cancer in previously irradiated patients. Int J Radiat Oncol Biol Phys. 2001 Apr 1;49(5):1267-74.
21. Jones RM et al. Patient-reported barriers to colorectal cancer screening: a mixed-methods analysis. Am J Prev Med. 2010 May;38(5):508-16.
22. Basch CH et al. Screening colonoscopy bowel preparation: experience in an urban minority population. Therap Adv Gastroenterol. 2013 Nov;6(6):442-6.
23. Davis JL et al. Sociodemographic differences in fears and mistrust contributing to unwillingness to participate in cancer screenings. J Health Care Poor Underserved. 2012 Nov;23(4 Suppl):67-76.
24. Robinson CM et al. Barriers to colorectal cancer screening among publicly insured urban women: no knowledge of tests and no clinician recommendation. J Natl Med Assoc. 2011 Aug;103(8):746-53.
25. Goldman RE et al. Perspectives of colorectal cancer risk and screening among Dominicans and Puerto Ricans: Stigma and misperceptions. Qual Health Res. 2009 Nov;19(11):1559-68.
26. Laiyemo AO et al. Race and colorectal cancer disparities: Health-care utilization vs different cancer susceptibilities. J Natl Cancer Inst. 2010 Apr 21;102(8):538-46.
27. White A et al. Racial disparities and treatment trends in a large cohort of elderly African Americans and Caucasians with colorectal cancer, 1991 to 2002. Cancer. 2008 Dec 15;113(12):3400-9.
28. Doubeni CA et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population – A retrospective cohort study. PLoS One. 2012;7(5):e36392.
29. Tammana VS, Laiyemo AO. Colorectal cancer disparities: Issues, controversies and solutions. World J Gastroenterol. 2014 Jan 28;20(4):869-76.
30. Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci. 2015 Mar;60(3):711-21.
31. Miranda-Diaz C et al. Barriers for Compliance to Breast, Colorectal, and Cervical Screening Cancer Tests among Hispanic Patients. Int J Environ Res Public Health. 2015 Dec 22;13(1):ijerph13010021.
32. Sewali B et al. Understanding cancer screening service utilization by Somali men in Minnesota. J Immigr Minor Health. 2015 Jun;17(3):773-80.
33. Walsh JM et al. Barriers to colorectal cancer screening in Latino and Vietnamese Americans. Compared with non-Latino white Americans. J Gen Intern Med. 2004 Feb;19(2):156-66.
34. Perez-Stable EJ et al. Self-reported use of cancer screening tests among Latinos and Anglos in a prepaid health plan. Arch Intern Med. 1994 May 23;154(10):1073-81.
35. Shariff-Marco S et al. Racial/ethnic differences in self-reported racism and its association with cancer-related health behaviors. Am J Public Health. 2010 Feb;100(2):364-74.
36. Powe BD et al. Comparing knowledge of colorectal and prostate cancer among African American and Hispanic men. Cancer Nurs. 2009 Sep-Oct;32(5):412-7.
37. Jun J, Oh KM. Asian and Hispanic Americans’ cancer fatalism and colon cancer screening. Am J Health Behav. 2013 Mar;37(2):145-54.
38. Hacker K et al. The impact of Immigration and Customs Enforcement on immigrant health: Perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011 Aug;73(4):586-94.
39. Firger J. Rescinding DACA could spur a public health crisis, from lost services to higher rates of depression, substance abuse. Newsweek.
40. May FP et al. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015 Oct;110(10):1388-94.
41. Levy BT et al. Why hasn’t this patient been screened for colon cancer? An Iowa Research Network study. J Am Board Fam Med. 2007 Sep-Oct;20(5):458-68.
42. Rosenblatt RA. A view from the periphery – health care in rural America. N Engl J Med. 2004 Sep 9;351(11):1049-51.
43. Young WF et al. Predictors of colorectal screening in rural Colorado: testing to prevent colon cancer in the high plains research network. J Rural Health. 2007 Summer;23(3):238-45.
44. Kates J et al. Health and Access to Care and Coverage for Lesbian, Gay, Bisexual, and Transgender (LGBT) Individuals in the U.S. In: Foundation KF, ed. Disparities Policy Issue Brief. Volume 2017; Aug 30, 2017.
45. Katz ML et al. Improving colorectal cancer screening by using community volunteers: results of the Carolinas cancer education and screening (CARES) project. Cancer. 2007 Oct 1;110(7):1602-10.
46. Jean-Jacques M et al. Program to improve colorectal cancer screening in a low-income, racially diverse population: A randomized controlled trial. Ann Fam Med. 2012 Sep-Oct;10(5):412-7.
47. Reuland DS et al. Effect of combined patient decision aid and patient navigation vs usual care for colorectal cancer screening in a vulnerable patient population: A randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):967-74.
48. Percac-Lima S et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: a randomized, controlled trial. J Gen Intern Med. 2009 Feb;24(2):211-7.
49. Nash D et al. Evaluation of an intervention to increase screening colonoscopy in an urban public hospital setting. J Urban Health. 2006 Mar;83(2):231-43.
50. Lebwohl B et al. Effect of a patient navigator program on the volume and quality of colonoscopy. J Clin Gastroenterol. 2011 May-Jun;45(5):e47-53.
51. Khankari K et al. Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. J Gen Intern Med. 2007 Oct;22(10):1410-4.
52. Wang JH et al. Recruiting Chinese Americans into cancer screening intervention trials: Strategies and outcomes. Clin Trials. 2014 Apr;11(2):167-77.
53. Katz ML et al. Patient activation increases colorectal cancer screening rates: a randomized trial among low-income minority patients. Cancer Epidemiol Biomarkers Prev. 2012 Jan;21(1):45-52.
54. Ford ME et al. Enhancing adherence among older African American men enrolled in a longitudinal cancer screening trial. Gerontologist. 2006 Aug;46(4):545-50.
55. Christie J et al. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008 Mar;100(3):278-84.
56. Philip EJ et al. Evaluating the impact of an educational intervention to increase CRC screening rates in the African American community: A preliminary study. Cancer Causes Control. 2010 Oct;21(10):1685-91.
57. Greiner KA et al. Implementation intentions and colorectal screening: A randomized trial in safety-net clinics. Am J Prev Med. 2014 Dec;47(6):703-14.
58. Horne HN et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015 Feb;26(2):239-46.
59. Grubbs SS et al. Eliminating racial disparities in colorectal cancer in the real world: It took a village. J Clin Oncol. 2013 Jun 1;31(16):1928-30.
60. Jung MY et al. The Chinese and Korean American immigrant experience: a mixed-methods examination of facilitators and barriers of colorectal cancer screening. Ethn Health. 2017 Feb 25:1-20.
61. Viruell-Fuentes EA et al. More than culture: structural racism, intersectionality theory, and immigrant health. Soc Sci Med. 2012 Dec;75(12):2099-106.
Dr. Oduyebo is a third-year fellow at the Mayo Clinic, Rochester, Minn.; Dr. Malespin is an assistant professor in the department of medicine and the medical director of hepatology at the University of Florida Health, Jacksonville; Dr. Mendoza Ladd is an assistant professor of medicine at Texas Tech University, El Paso; Dr. Day is an associate professor of medicine at the University of California, San Francisco; Dr. Charabaty is an associate professor of medicine and the director of the IBD Center in the division of gastroenterology at Medstar-Georgetown University Center, Washington; Dr. Chen is an associate professor of medicine, the director of patient safety and quality, and the director of the small-bowel endoscopy program in division of gastroenterology at Washington University, St. Louis; Dr. Carr is an assistant professor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia; Dr. Quezada is an assistant dean for admissions, an assistant dean for academic and multicultural affairs, and an assistant professor of medicine in the division of gastroenterology and hepatology at the University of Maryland, Baltimore; and Dr. Lamousé-Smith is a director of translational medicine, immunology, and early development at Janssen Pharmaceuticals Research and Development, Spring House, Penn.
Chronic constipation: Practical approaches and novel therapies
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance
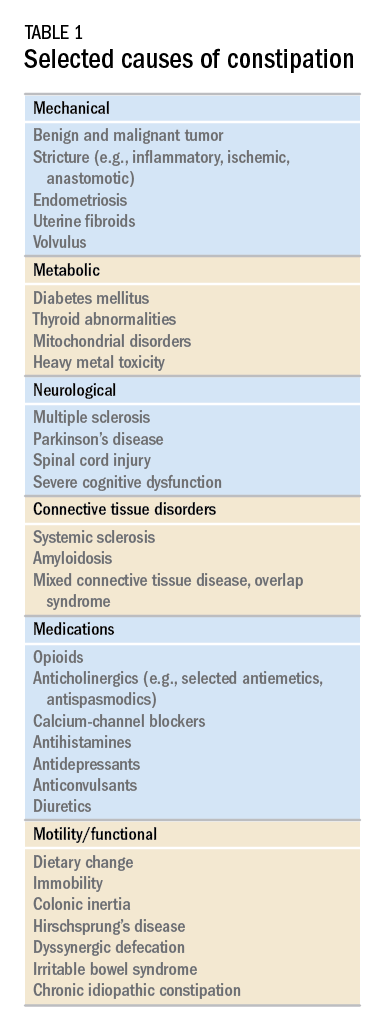
While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
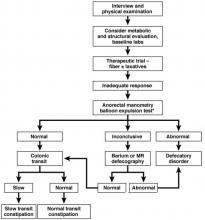
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance

While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.
While constipation is one of the most common symptoms managed by practicing gastroenterologists, it can also be among the most challenging. As a presenting complaint, constipation manifests with widely varying degrees of severity and may be seen in all age groups, ethnicities, and socioeconomic backgrounds. Its implications can include chronic and serious functional impairment as well as protracted and often excessive health care utilization. A growing number of pharmacologic and nonpharmacologic interventions have become available and proven to be effective when appropriately deployed. As such, health care providers and particularly gastroenterologists should strive to develop logical and efficient strategies for addressing this common disorder.
Clinical importance

While there are a variety of etiologies for constipation (Table 1), a large proportion of chronic cases fall within the framework of functional gastrointestinal disorders, a category with a substantial burden of disease across the population. Prevalence estimates vary, but constipation likely affects between 12% and 20% of the North American population.1 Research has demonstrated significant health care expenditures associated with chronic constipation management; U.S. estimates suggest direct costs on the order of hundreds of millions of dollars per year, roughly half of which are attributable to inpatient care.2 The financial burden of constipation also includes indirect costs associated with absenteeism as well as the risks of hospitalization and invasive procedures.3
Physical and emotional complications can be likewise significant and affect all age groups, from newborns to patients in the last days of life. Hirschsprung’s disease, for example, can lead to life-threatening sequelae in infancy, such as spontaneous perforation or enterocolitis, or more prolonged functional impairments when it remains undiagnosed. Severe constipation in childhood can lead to encopresis, translating in turn into ostracism and impaired social functioning. Fecal incontinence associated with overflow diarrhea is common and debilitating, particularly in the elderly population.
The potential mechanical complications of constipation lead to its overlap with a variety of other gastrointestinal complaints. For example, the difficulties of passing inspissated stool can provoke lower gastrointestinal bleeding from irritated hemorrhoids, anal fissures, stercoral ulcers, or prolapsed rectal tissue. Retained stool can also lead to upper gastrointestinal symptoms such as postprandial bloating or early satiety.4 Delayed fecal discharge can promote an increase in fermentative microbiota, associated in turn with the production of short-chain fatty acids, methane, and other gaseous byproducts.
The initial assessment
History
Taking an appropriate history is an essential step toward achieving a successful outcome. Presenting concerns related to constipation can range from hard, infrequent, or small-volume stools; abdominal or rectal pain associated with the process of elimination; and bloating, nausea, or early satiety. A sound diagnosis requires a keen understanding of what patients mean when they indicate that they are constipated, an accurate assessment of its impact on quality of life, and a careful inventory of potentially associated complications.
It is critical to define the duration of the problem. Not infrequently, patients will focus on recent events while failing to reveal that altered bowel habits or other functional symptoms have been problematic for years. Reminding the patients to “begin at the beginning” can aid enormously in contextualizing their complaints. Individuals with longstanding symptoms and previously negative evaluations are much less likely to present with a new organic disease than are those in whom symptoms have truly arisen de novo.
Defining constipation by frequency of bowel eliminations alone has proved inaccurate at predicting actual severity. This is in part because the bowel movement frequency varies widely in healthy individuals (anywhere from thrice daily to once every 3 days) and in part because the primary indicator of effective evacuation is not frequency but volume – a much more difficult quantity for patients to gauge.5 The Bristol Stool Scale is a simple, standardized tool that more accurately evaluates the presence or absence of colonic dysfunction. For example, patients passing Type 1-2 (hard or lumpy) stools often have an element of constipation that needs to be addressed.6 However, the interpretation of stool consistency assessments is still aided by awareness of both frequency and volume. A patient passing multiple small-volume Type 6-7 (loose or watery) stools may be the most constipated, presenting with overflow or paradoxical diarrhea attributable to fecal impaction.
Physical examination
An expert physical exam is another essential aspect of the initial assessment. Alarm features can be elicited in this context as well via signs of pallor, weight loss, blood in the stool, physical abuse, or advanced psychological distress. Attention should also be paid to signs of a systemic disorder that might be associated with gastrointestinal dysmotility including previously unrecognized signs of Raynaud’s syndrome, sclerodactyly, amyloidosis, surgical scars, and joint hypermobility.7,8 Abdominal bloating, a frequently vague symptomatic complaint, can be correlated with the presence or absence of distention as perceived by the patient and/or the examiner.9
Any initial evaluation of constipation should also include a detailed digital rectal exam. A complete examination should include a careful visual assessment of the perianal region for external lesions and of the degree and directional appropriateness of pelvic floor excursion (perineal elevation and descent) during squeeze and simulated defecation maneuvers, respectively. Digital examination should include palpation for the presence or absence of pain as well as stool, blood, or masses in the rectal vault, as well as an assessment of sphincter tone at baseline, with squeeze, and with simulated defecation. Rectal pressure generation with the latter maneuver can also be qualitatively assessed. Research has suggested moderate agreement between the digital rectal examination and formal manometric evaluation in diagnosing dyssynergic defecation, underscoring the former’s utility in guiding initial management decisions.10
Testing
It is reasonable to exclude metabolic, inflammatory, or other secondary etiologies of constipation in patients in whom history or examination raises suspicion. Likewise, colonoscopy should be considered in patients with alarm features or who are due for age-appropriate screening. That said, in the absence of risk factors or ancillary signs and symptoms, a detailed diagnostic work-up is often unnecessary. The AGA’s Medical Position Statement on Constipation recommends a complete blood count as the only test to be ordered on a standard basis in the work-up of constipation.11
In patients new to one’s practice, the diligent retrieval of prior records is one of the most efficient ways to avoid wasting health care resources. Locating an old abdominal radiograph that demonstrates extensive retained stool can not only secure the diagnosis for vague symptomatic complaints but also obviate the need for more extensive testing. One should instead consider how symptom duration and the associated changes in objectives measures such as weight and laboratory parameters can be used to justify or refute the need for repeating costly or invasive studies.
It is important to consider the potential contribution of defecatory dyssynergy to chronic constipation early in a patient’s presentation, and to return to this possibility in the future if initial therapeutic interventions are unsuccessful. An abnormal qualitative assessment on digital rectal examination should trigger a more formal characterization of the patient’s defecatory mechanics via anorectal manometry (ARM) and balloon expulsion testing (BET). Likewise, a lack of response to initial pharmacotherapy should prompt suspicion for outlet dysfunction, which can be queried with functional testing even if a rectal examination is qualitatively unrevealing.
Initial approach to the chronically constipated patient
The aforementioned AGA Medical Position Statement provides a helpful algorithm regarding the diagnostic approach to constipation (Figure 1). In the absence of concern for secondary etiologies of constipation, an initial therapeutic trial of dietary, lifestyle, and medication-based intervention is reasonable for mild symptoms. Patients should be encouraged to strive for 25-30 grams of dietary fiber intake per day. For patients unable to reach this goal via high-fiber foods alone, psyllium husk is a popular supplement, but it should be initiated at modest doses to mitigate the risk of bloating. Fiber may be supplemented with the use of osmotic laxatives (e.g., polyethylene glycol) with instructions that the initial dose may be modified as needed to optimal effectiveness. Selective response to rectal therapies (e.g., bisacodyl or glycerin suppositories) over osmotic laxatives may also suggest utility in early queries of outlet dysfunction.
An abdominal radiograph can be helpful not only to diagnose constipation but also to assess the stool burden present at the time of beginning treatment. For patients presenting with a significant degree of fecal loading, an initial bowel cleanse with four liters of osmotically balanced polyethylene glycol can be a useful means of eliminating background fecal impactions that might have mitigated the effectiveness of initial therapies in the past or that might reduce the effectiveness of daily laxative therapy moving forward.
Patients with a diagnosis of defecatory dyssynergy made via ARM/BET should be referred to pelvic floor physical therapy with biofeedback. Recognizing that courses of therapy are highly individualized in practice, randomized controlled trials suggest symptom improvement in 70%-80% of patients, with the majority also demonstrating maintenance of response.12 Biofeedback appears to be an essential component of this modality based on meta-analysis data and should be requested specifically by the referring provider.13
Pharmacologic agents

For those patients with more severe initial presentations or whose symptoms persist despite initial medical management, there are several pharmacologic agents that may be considered on a prescription basis (Table 2). Linaclotide, a minimally absorbed guanylate cyclase agonist, is approved by the Food and Drug Administration for patients with irritable bowel syndrome with constipation (IBS-C) and chronic idiopathic constipation (CIC). Improvements in constipation tend to occur over a slightly shorter timeline than in abdominal pain, though both have been demonstrated in comparison to placebo.14,15 Plecanatide, a newer agent with a similar mechanism of action, has demonstrated improvements in bowel movement frequency and was recently approved for CIC.16 Lubiprostone, a chloride channel agonist, has demonstrated benefit for IBS-C and CIC as well, though its side effect profile is more varied, including dose-related nausea in up to 30% of patients.17
For patients with opioid-induced constipation who cannot wean from the opioid medications, the peripheral acting mu-opioid receptor antagonists may be quite helpful. These include injectable as well as oral formulations (e.g., methylnaltrexone and naloxegol, respectively) with additional agents under active investigation in particular clinical subsets (e.g., naldemedine for patients with cancer-related pain).18,19 Prucalopride, a selective serotonin receptor agonist, has also demonstrated benefit for constipation; it is available abroad but not yet approved for use in the United States.20 Prucalopride shares its primary mechanism of action (selective agonism of the 5HT4 serotonin receptor) with cisapride, a previously quite popular gastrointestinal motility agent that was subsequently withdrawn from the U.S. market because of arrhythmia risk.21 This risk is likely attributable to cisapride’s dual binding affinity for potassium channels, a feature that prucalopride does not share; as such, cardiotoxicity is not an active concern with the latter agent.22
Still other pharmacologic agents with novel mechanisms of action are currently under investigation. Tenapanor, an inhibitor of a particular sodium/potassium exchanger in the gut lumen, mitigates intestinal sodium absorption, which increases fluid volume and transit. A recent phase 2 study demonstrated significantly increased stool frequency relative to placebo in patients with IBS-C.23 Elobixibat, an ileal bile acid transport inhibitor, promotes colonic retention of bile acids and, in placebo-controlled studies, has led to accelerated colonic transit and an increased number of spontaneous bowel movements in patients with CIC.24
Persistent constipation
In cases of refractory constipation (in practical terms, symptoms that persist despite trials of escalating medical therapy over at least 6 weeks), it is worth revisiting the question of etiology. Querying defecatory dyssynergy via ARM/BET, if not pursued prior to trials of newer pharmacologic agents, should certainly be explored in the event that such trials fail. Inconclusive results of ARM and BET testing, or BET abnormalities that persist despite a course of physical therapy with biofeedback, may raise suspicion for pelvic organ prolapse, which may be formally evaluated with defecography. Additional testing for metabolic or structural predispositions toward constipation may also be reasonable at this juncture.
Formal colonic transit testing via radio-opaque markers, scintigraphy, or the wireless motility capsule is often inaccurate in the setting of dyssynergic defecation and should be pursued only after this entity has been excluded or successfully treated.25 While there are not many practical distinctions at present in the therapeutic management of slow-transit versus normal-transit constipation, the use of novel medications with an explicitly prokinetic mechanism of action may be reasonable to consider in the setting of a document delay in colonic transit. Such delays can also help justify further specialized diagnostic testing (e.g., colonic manometry), and, in rare refractory cases, surgical intervention.
Consideration of colectomy should be reserved for highly selected patients with delayed colonic transit, normal defecatory mechanics, and the absence of potentially explanatory background conditions (e.g., connective tissue disease). Clear evidence of an underlying colonic myopathy or neuropathy may militate in favor of a more targeted surgical intervention (e.g., subtotal colectomy) or guide one’s clinical evaluation toward alternative systemic diagnoses. A diverting loop ileostomy with interval assessment of symptoms may be useful to clarify the potential benefits of colectomy while preserving the option of operative reversal. Proximal transit delays should be definitively excluded before pursuing colonic resections given evidence that multisegment transit delays portend significantly worse postoperative outcomes.26
Conclusion
Constipation is a common, sometimes confusing presenting complaint and the variety of established and emergent options for diagnosis and therapy can lend themselves to haphazard application. Patients and providers both are well served by a clinical approach, rooted in a comprehensive history and examination, that begins to organize these options in thoughtful sequence.
Dr. Ahuja is assistant professor of clinical medicine, division of gastroenterology; Dr. Reynolds is professor of clinical medicine, and director of the program in neurogastroenterology and motility, division of gastroenterology, Perelman School of Medicine, University of Pennsylvania, Philadelphia.
References
1. Higgins P.D., Johanson J.F. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004 Apr;99(4):750-9. PubMed PMID: 15089911.
2. Martin B.C., Barghout V., Cerulli A. Direct medical costs of constipation in the United States. Manage Care Interface. 2006 Dec;19(12):43-9. PubMed PMID: 17274481.
3. Sun S.X., Dibonaventura M., Purayidathil F.W., et al. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig Dis Sc. 2011 Sep;56(9):2688-95. PubMed PMID: 21380761.
4. Heidelbaugh J.J., Stelwagon M., Miller S.A., et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015 Apr;110(4):580-7.
5. Mitsuhashi S., Ballou S., Jiang Z.G., et al. Characterizing normal bowel frequency and consistency in a representative sample of adults in the United States (NHANES). Am J Gastroenterol. 2017 Aug 01. PubMed PMID: 28762379.
6. Saad R.J., Rao S.S., Koch K.L., et al. Do stool form and frequency correlate with whole-gut and colonic transit? Results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010 Feb;105(2):403-11. PubMed PMID: 19888202.
7. Castori M., Morlino S., Pascolini G., et al. Gastrointestinal and nutritional issues in joint hypermobility syndrome/Ehlers-Danlos syndrome, hypermobility type. American Journal of Medical Genetics Part C, Semin Med Genet. 2015 Mar;169C(1):54-75. PubMed PMID: 25821092.
8. Nagaraja V., McMahan Z.H., Getzug T., Khanna D. Management of gastrointestinal involvement in scleroderma. Curr Treatm Opt Rheumatol. 2015 Mar 01;1(1):82-105. PubMed PMID: 26005632. Pubmed Central PMCID: 4437639.
9. Malagelada J.R., Accarino A., Azpiroz F. Bloating and abdominal distension: Old misconceptions and current knowledge. Am J Gastroenterol. 2017 Aug;112(8):1221-31. PubMed PMID: 28508867.
10. Soh J.S., Lee H.J., Jung K.W., et al. The diagnostic value of a digital rectal examination compared with high-resolution anorectal manometry in patients with chronic constipation and fecal incontinence. Am J Gastroenterol. 2015 Aug;110(8):1197-204. PubMed PMID: 26032152.
11. American Gastroenterological Association, Bharucha A.E., Dorn S.D., Lembo A., Pressman A. American Gastroenterological Association medical position statement on constipation. Gastroenterology. 2013 Jan;144(1):211-7. PubMed PMID: 23261064.
12. Skardoon G.R., Khera A.J., Emmanuel A.V., Burgell R.E. Review article: dyssynergic defaecation and biofeedback therapy in the pathophysiology and management of functional constipation. Aliment Pharmacol Therapeut. 2017 Aug;46(4):410-23. PubMed PMID: 28660663.
13. Koh C.E., Young C.J., Young J.M., Solomon M.J. Systematic review of randomized controlled trials of the effectiveness of biofeedback for pelvic floor dysfunction. Br J Surg. 2008 Sep;95(9):1079-87. PubMed PMID: 18655219.
14. Rao S., Lembo A.J., Shiff S.J., et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 Nov;107(11):1714-24; quiz p 25. PubMed PMID: 22986440. Pubmed Central PMCID: 3504311.
15. Lacy B.E., Schey R., Shiff S.J., et al. Linaclotide in chronic idiopathic constipation patients with moderate to severe abdominal bloating: A randomized, controlled trial. PloS One. 2015;10(7):e0134349. PubMed PMID: 26222318. Pubmed Central PMCID: 4519259.
16. Miner P.B., Jr., Koltun W.D., Wiener G.J., et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017 Apr;112(4):613-21. PubMed PMID: 28169285. Pubmed Central PMCID: 5415706.
17. Johanson J.F., Drossman D.A., Panas R., Wahle A., Ueno R. Clinical trial: phase 2 study of lubiprostone for irritable bowel syndrome with constipation. Aliment Pharmacol Therapeut. 2008 Apr;27(8):685-96. PubMed PMID: 18248656.
18. Chey W.D., Webster L., Sostek M., Lappalainen J., Barker P.N., Tack J. Naloxegol for opioid-induced constipation in patients with noncancer pain. N Engl J Med. 2014 Jun 19;370(25):2387-96. PubMed PMID: 24896818.
19. Katakami N., Oda K., Tauchi K., et al. Phase IIb, randomized, double-blind, placebo-controlled study of naldemedine for the treatment of opioid-induced constipation in patients with cancer. J Clin Oncol. 2017 Jun 10;35(17):1921-8. PubMed PMID: 28445097.
20. Sajid M.S., Hebbar M., Baig M.K., Li A., Philipose Z. Use of prucalopride for chronic constipation: A systematic review and meta-analysis of published randomized, controlled trials. J Neurogastroenterol Motil. 2016 Jul 30;22(3):412-22. PubMed PMID: 27127190. Pubmed Central PMCID: 4930296.
21. Quigley E.M. Cisapride: What can we learn from the rise and fall of a prokinetic? J Dig Dis. 2011 Jun;12(3):147-56. PubMed PMID: 21615867.
22. Conlon K., De Maeyer J.H., Bruce C., et al. Nonclinical cardiovascular studies of prucalopride, a highly selective 5-hydroxytryptamine 4 receptor agonist. J Pharmacol Exp Therapeut. 2017 Nov; doi: 10.1124/jpet.117.244079 [epub ahead of print].
23. Chey W.D., Lembo A.J., Rosenbaum D.P. Tenapanor treatment of patients with constipation-predominant irritable bowel syndrome: a phase 2, randomized, placebo-controlled efficacy and safety trial. Am J Gastroenterol. 2017;112:763-74.
24. Simren M., Bajor A., Gillberg P-G, Rudling M., Abrahamsson H. Randomised clinical trial: the ileal bile acid transporter inhibitor A3309 vs. placebo in patients with chronic idiopathic constipation – a double-blind study. Aliment Pharmacol Ther. 2011 Jul;34(1):41-50.
25. Zarate N., Knowles C.H., Newell M., et al. In patients with slow transit constipation, the pattern of colonic transit delay does not differentiate between those with and without impaired rectal evacuation. Am J Gastroenterol. 2008 Feb;103(2):427-34. PubMed PMID: 18070233.
26. Redmond J.M., Smith G.W., Barofsky I., et al. Physiological tests to predict long-term outcome of total abdominal colectomy for intractable constipation. Am J Gastroenterol. 1995 May;90(5):748-53. PubMed PMID: 7733081.
The Light at the End of the Tunnel: Recent Advances in Endoscopic Retrograde Cholangiopancreatograpy
Introduction
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
Introduction
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
Introduction
Direct visualization of the biliary ductal system is quickly gaining importance among gastroenterologists. Since the inception of cholangioscopy in the 1970s, the technology has progressed, allowing for ease of use, better visualization, and a growing number of indications. Conventional endoscopic retrograde cholangiopancreatography (ERCP) is successful for removal of bile duct stones (with success rates over 90%);1 however, its use in the evaluation of potential biliary neoplasia has been somewhat disappointing. The diagnostic yield of ERCP-guided biliary brushings can range from 30% to 40%.2-4 An alternative to ERCP-guided biliary brushings for biliary strictures is endoscopic ultrasound (EUS)-directed fine needle aspiration (FNA), but the reported sensitivity remains poor, ranging from 43% to 77% with negative predictive values of less than 30%.5-7 These results leave much to be desired for diagnostic yield.
Clinical indications
DSOCP appears to have improved accuracy over fiberoptic equipment. In a recent multicenter observational study in patients undergoing digital cholangioscopy, the guided biopsies resulted in adequate tissue for histologic evaluation in 98% of patients. In addition, the sensitivity and specificity of digital cholangioscope-guided biopsies for diagnosis of malignancy was 85% and 100%, respectively.11
Other less common diagnostic indications for DSOCP include evaluation of cystic lesions of the biliary tract, verifying clearance of bile duct stones, bile duct ischemia evaluation after liver transplantation, hemobilia evaluation, removal of a bile duct foreign body, and evaluation of bile duct involvement in the presence of an ampullary adenoma.3,14,15,20,26,27
Risks and complications
Conclusions
Direct visualization of the biliary and pancreatic ductal system with fiber-optic and now digital-based platforms have greatly expanded the diagnostic and therapeutic capabilities available to gastroenterologists in the diagnosis and management of biliary and pancreatic disorders. The digital single-operator cholangiopancreatascope system offers greater diagnostic yield of pancreaticobiliary disorders over conventional diagnostic sampling techniques. In addition, direct visualization has expanded our therapeutic ability in complex stone disease allowing laser-based therapies that are not available with traditional fluoroscopic based techniques. Cholangiopancreatoscopic techniques and indications are rapidly expanding and will continue to expand the diagnostic and therapeutic armamentarium available to gastroenterologists.
Dr. Sonnier is a general gastroenterology fellow, division of gastroenterology, University of South Alabama. Dr. Mizrahi is director of advanced endoscopy, division of gastroenterology, University of South Alabama. Dr. Pleskow, is clinical chief, department of gastroenterology, Beth Israel Deaconess Medical Center, and associate professor of medicine, Harvard Medical School, Boston. Dr. Sonnier and Dr. Mizrahi have no conflicts of interest. Dr. Pleskow serves as a consultant to Boston Scientific.
References
1. Cohen S., et al. Gastrointest Endosc. 2002;56:803–9
2. Lee J.G., et al. Am J Gastroenterol. 1995;90:722-6.
3. De Bellis M., et al. Gastrointest Endosc. 2003;58:176-82
4. Fritcher E.G., et al. Gastroenterology. 2009;136:2180-6.
5. Rosch T., et al. Gastrointest Endosc. 2004;60:390-6.
6. Byrne M.F., et al. Endoscopy. 2004;36:715-9.
7. DeWitt J., et al. Gastrointest Endosc. 2006;64:325-33.
8. Rosch W., Endoscopy. 1976;8:172-5.
9. Takekoshi T., Takagi K. Gastrointest Endosc. 1975;17:678-83.
10. Chen Y.K. Gastrointest Endosc 2007;65:303-11.
11. Navaneethan U., et al. Gastrointest Endosc 2016;84:649-55.
12. Navaneethan U., et al. Gastrointest Endosc 2015;82: 608-14.
13. Chen Y.K., Pleskow DK. Gastrointest Endosc. 2007;65:832-41.
14. Draganov P.V., et al. Gastrointest Endosc. 2011;73:971-9.
15. Ramchandani M., et al. Gastrointest Endosc. 2011;74:511-9.
16. Chen Y.K., et al. Gastrointest Endosc. 2011;74:805-14.
17. Draganov P.V., et al. Gastrointest Endosc. 2012;75:347-53.
18. Classen M., et al. Endoscopy 1988;20:21-6.
19. Parsi M.A., et al. Gastrointest Endosc 2008;67:AB102.
20. Fishman D.S., et al. World J Gastroenterol. 2009;15:1353-8.
21. Maydeo A., et al. Gastrointest Endosc. 2011;74:1308-14.
22. Yamao K., et al. Gastrointest Endosc 2003;57:205-9.
23. Hara T., et al. Gastroenterology 2002;122:34-43.
24. Rösch T., et al. Endoscopy. 2002;34:765–71.
25. Bekkali N.L., et al. Pancreas. 2017;46:528-30.
26. Adwan H., et al. Dig Endosc. 2011;23:199-200.
27. Ransibrahmanakul K., et al. Clin Gastroenterol Hepatol. 2010;8:e9.
28. Pereira P., et al. J Gastrointestin Liver Dis, June 2017;Vol. 26(No 2):165-70.
29. Kawakubo K., et al. Endoscopy 2011;43:E241-2.
Reflux Diagnostics: Modern Techniques and Future Directions
Introduction
Chronic esophageal symptoms attributed to gastroesophageal reflux disease (GERD) are common presenting symptoms in gastroenterology, leading to high healthcare costs and adverse quality of life globally.1,2 The clinical diagnosis of GERD hinges on the presence of “troublesome” compatible typical symptoms (heartburn, acid regurgitation) or evidence of mucosal injury on endoscopy (esophagitis, Barrett’s esophagus, peptic stricture).3 With the growing availability of proton pump inhibitors (PPIs), patients and clinicians often utilize an empiric therapeutic trial of PPI as an initial test, with symptom improvement in the absence of alarm symptoms indicating a high likelihood of GERD.4 A meta-analysis of studies that used objective measures of GERD (in this case, 24-hour pH monitoring) showed that the “PPI test” has a sensitivity of 78%, but a specificity of only 54%, as a diagnostic approach to GERD symptoms.5 Apart from noncardiac chest pain, the diagnostic yield is even lower for atypical and extra-esophageal symptoms such as cough or laryngeal symptoms.6
The “nuts and bolts” of reflux testing
Ambulatory reflux testing assesses esophageal reflux burden and symptom-reflux association (SRA). Individual reflux events are identified as either a drop in esophageal pH to less than 4 (acid reflux events), or a sharp decrease in esophageal impedance measurements in a retrograde fashion (impedance-detected reflux events), with subsequent recovery to the baseline in each instance. Ambulatory reflux testing affords insight into three areas: 1) measurement of esophageal acid exposure time (AET); the cumulative time duration when distal esophageal pH is less than 4 at the recording site, reported as a percentage of the recording period; 2) measurement of the number of reflux events both acidic (from pH monitoring) and weakly acidic/alkaline (from impedance monitoring); and 3) quantitative evaluation of the association between reported symptom episodes and reflux events.
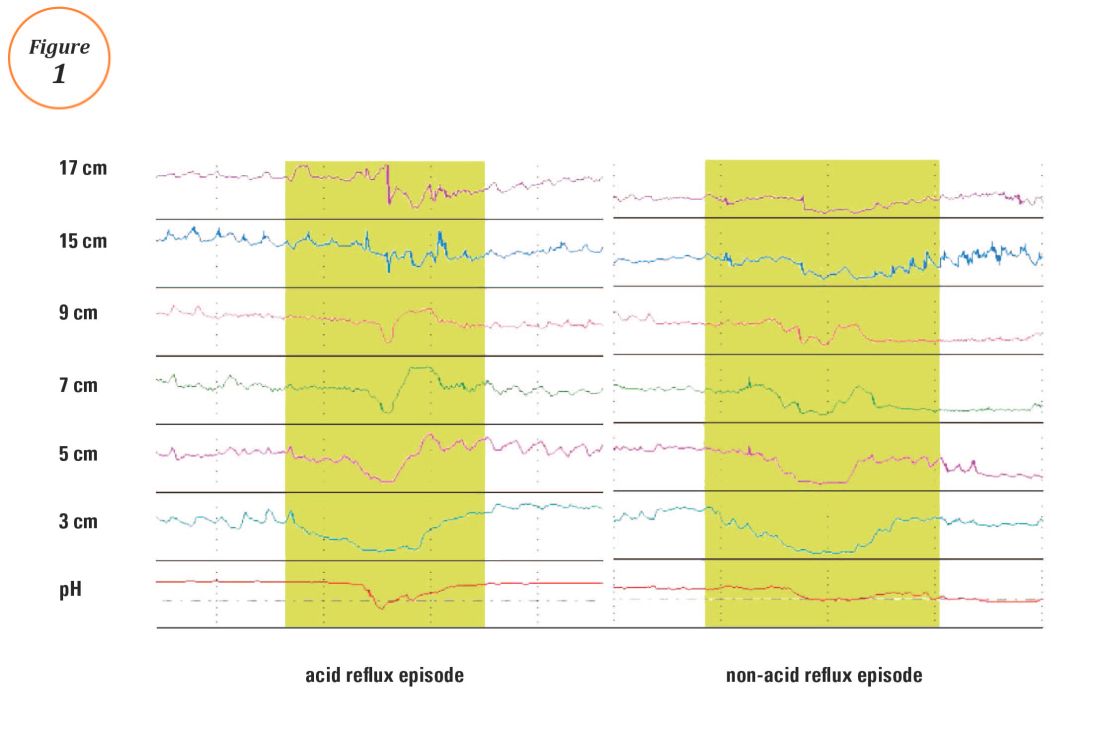
The SI and SAP can be calculated individually for acid-detected reflux events and for impedance-detected reflux events. Since reflux events are better detected with impedance, combined pH-impedance testing increases the yield of detecting positive SRA, especially when performed off PPI therapy.16,17 Because these indices are heavily reliant on patient reporting of symptom episodes, SRA can be overinterpreted;18 positive associations are more clinically useful than negative results in the evaluation of symptoms attributed to GERD.19 Despite these concerns, the two most consistent predictors of symptomatic outcome with antireflux therapy on pH-impedance testing are abnormal AET and positive SAP with impedance-detected reflux events.17
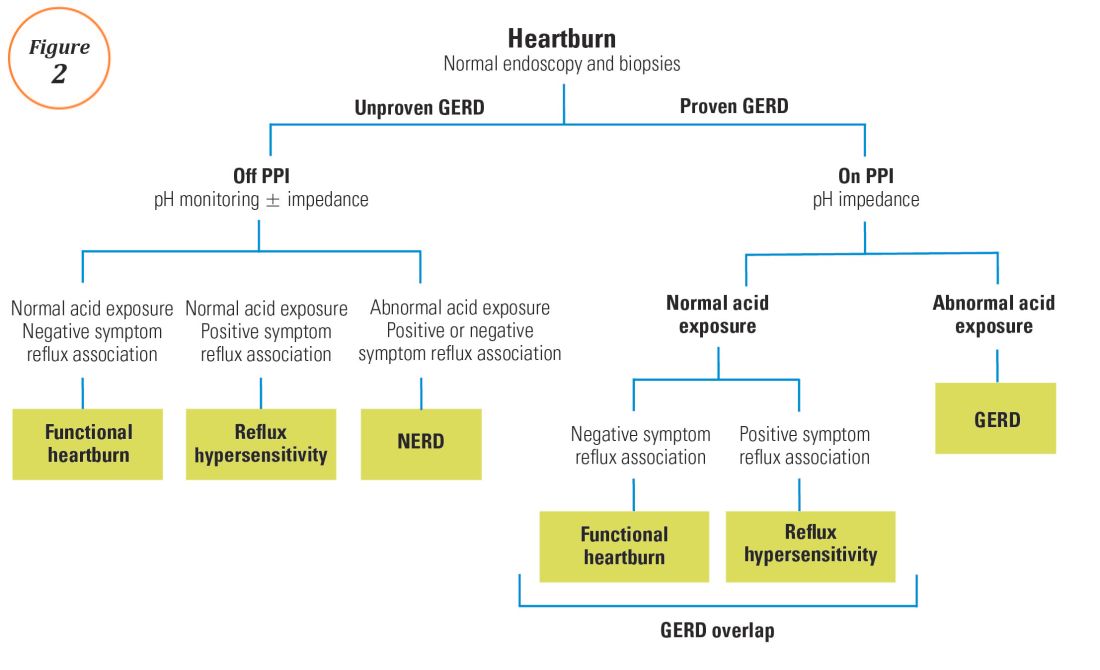
Testing on or off PPI?
For symptoms attributable to GERD that persist despite properly administered PPI therapy, the 2013 American College of Gastroenterology guidelines suggest upper endoscopy with esophageal biopsies for typical symptoms and appropriate referrals for atypical symptoms.24 However, if these evaluations are unremarkable, reflux monitoring is recommended, with PPI status for testing guided by the pre-test probability of GERD: with a low pre-test probability of GERD, reflux testing is best performed off PPI with either pH or combined pH-impedance testing. In contrast, with a high pre-test probability of GERD, testing is best performed on PPI with combined pH-impedance testing. A similar concept is proposed in the Rome IV approach (Figure 2)23 and on GERD consensus guidelines:7 when heartburn or chest pain persists despite PPI therapy and endoscopy and esophageal biopsies are normal, evidence for GERD (past esophagitis, Barrett’s esophagus, peptic stricture, or prior positive reflux testing) prompts pH-impedance monitoring on PPI therapy (i.e., proven GERD). Those without this evidence for proven GERD (i.e., unproven GERD) are best tested off PPI, and the test utilized can be either pH alone or combined pH-impedance.
GERD phenotypes and management
The presence or absence of the two core metrics on ambulatory reflux monitoring – abnormal AET and positive SRA – can stratify symptomatic GERD patients into phenotypes that predict symptomatic improvement with antireflux therapy and guide management of symptoms (Figure 3).25,26 The presence of both abnormal AET and positive SRA suggests “strong” evidence for GERD, for which symptom improvement is likely with maximization of antireflux therapy, which can include BID PPI, baclofen (to decrease transient LES relaxations), alginates (such as Gaviscon), and consideration of endosopic or surgical antireflux procedures such as fundoplication or magnetic sphincter augmentation. Abnormal AET but negative SRA is regarded as “good” evidence for GERD, for which similar antireflux therapies can be advocated. Normal AET but positive SRA is designated as “reflux hypersensitivity,”23 with increasing proportions of patients meeting this phenotype when tested with combined pH-impedance and off PPI therapy.27 Both normal AET and negative SRA suggest equivocal evidence for GERD and the likely presence of a functional esophageal disorder, such as functional heartburn.23 For reflux hypersensitivity and especially functional esophageal disorders, antireflux therapy is unlikely to be as effective and management can include pharmacologic neuromodulation (such as tricyclic antidepressants administered at bedtime) as well as adjunctive nonpharmacologic approaches (such as stress reduction, relaxation, hypnosis, or cognitive-behavioral therapy).
The future of reflux diagnostics

Conclusions
For esophageal symptoms potentially attributable to GERD that persist despite optimized PPI therapy, esophageal testing should be undertaken, starting with endoscopy and biopsies and proceeding to ambulatory reflux monitoring with HRM. The decisions between pH testing alone versus combined pH-impedance monitoring, and between testing on or off PPI therapy, can be guided either by the pre-test probability of GERD or whether GERD has been proven or unproven in prior evaluations (Figure 2). Elevated AET and positive SRA with impedance-detected reflux events can predict the likelihood of successful management outcomes from antireflux therapy. These two core metrics can be utilized to phenotype GERD and guide management approaches for persisting symptoms (Figure 3). Novel impedance metrics (baseline mucosal impedance, postreflux swallow-induced peristaltic wave index) and markers for esophageal mucosal damage continue to be studied as potential markers for evidence of longitudinal reflux exposure.
Dr. Patel is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine and the Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University School of Medicine, St. Louis, Mo.
References
1. Shaheen N.J., et al. Am J Gastroenterol. 2006;101:2128-38.
2. Patel A., Gyawali C.P.. Switzerland: Springer International, 2016.
3. Vakil N., et al. Am J Gastroenterol. 2006;101:1900-20; quiz 1943.
4. Fass R., et al. Arch Intern Med. 1999;159:2161-8.
5. Numans M.E., et al. Ann Intern Med. 2004;140:518-27.
6. Shaheen N.J., et al. Aliment Pharmacol Ther. 2011;33:225-34.
7. Roman S., et al. Neurogastroenterol Motil Mar 31. doi: 10.1111/nmo.13067. [Epub ahead of print] 2017.
8. Dellon E.S., et al. Am J Gastroenterol. 2013;108:679-92; quiz 693.
9. Pandolfino JE, Vela MF. Gastrointest Endosc. 2009;69:917-30, 930 e1.
10. Shay S., et al. Am J Gastroenterol. 2004;99:1037-43.
11. Zerbib F., et al. Clin Gastroenterol Hepatol. 2013;11:366-72.
12. Wiener G.J., et al. Am J Gastroenterol 1988;83:358-61.
13. Weusten B.L., et al. Gastroenterology. 1994;107:1741-5.
14. Ghillebert G., et al. Gut 1990;31:738-44.
15. Kushnir V.M., et al. Aliment Pharmacol Ther. 2012;35(9):1080-7.
16. Bredenoord A.J., et al. Am J Gastroenterol. 2006;101:453-9.
17. Patel A., et al. Clin Gastroenterol Hepatol. 2015;13:884-91.
18. Slaughter J.C., et al. Clin Gastroenterol Hepatol. 2011;9:868-74.
19. Kavitt R.T., et al. Am J Gastroenterol. 2012;107:1826-32.
20. Kahrilas P.J., et al. Gastroenterology 2008;135:1383-91, 1391 e1-5.
21. Kessing B.F., et al. Clin Gastroenterol Hepatol. 2011;9:1020-4.
22. Kahrilas P.J., et al. Neurogastroenterol Motil. 2015;27:160-74.
23. Aziz A, et al. Esophageal disorders. Gastroenterology 2016;150:1368-79.
24. Katz P.O., et al. Am J Gastroenterol. 2013;108:308-28; quiz 329.
25. Boeckxstaens G., et al. Gut 2014;63:1185-93.
26. Patel A., et al. Neurogastroenterol Motil. 2016;28:513-21.
27. Patel A., et al. Neurogastroenterol Motil. 2016;28:1382-90.
28. Martinucci I., et al. Neurogastroenterol Motil. 2014;26:546-55.
29. Ates F., et al. Gastroenterology 2015;148:334-43.
30. Kessing B.F., et al. Am J Gastroenterol. 2011;106:2093-7.
31. Patel A., et al. Aliment Pharmacol Ther. 2016;44:890-8.
32. Frazzoni M., et al. Neurogastroenterol Motil. 2016.
33. Frazzoni M., et al. Neurogastroenterol Motil. 2013;25:399-406, e295.
34. Vela M.F., et al. Am J Gastroenterol. 2011;106:844-50.
Introduction
Chronic esophageal symptoms attributed to gastroesophageal reflux disease (GERD) are common presenting symptoms in gastroenterology, leading to high healthcare costs and adverse quality of life globally.1,2 The clinical diagnosis of GERD hinges on the presence of “troublesome” compatible typical symptoms (heartburn, acid regurgitation) or evidence of mucosal injury on endoscopy (esophagitis, Barrett’s esophagus, peptic stricture).3 With the growing availability of proton pump inhibitors (PPIs), patients and clinicians often utilize an empiric therapeutic trial of PPI as an initial test, with symptom improvement in the absence of alarm symptoms indicating a high likelihood of GERD.4 A meta-analysis of studies that used objective measures of GERD (in this case, 24-hour pH monitoring) showed that the “PPI test” has a sensitivity of 78%, but a specificity of only 54%, as a diagnostic approach to GERD symptoms.5 Apart from noncardiac chest pain, the diagnostic yield is even lower for atypical and extra-esophageal symptoms such as cough or laryngeal symptoms.6
The “nuts and bolts” of reflux testing
Ambulatory reflux testing assesses esophageal reflux burden and symptom-reflux association (SRA). Individual reflux events are identified as either a drop in esophageal pH to less than 4 (acid reflux events), or a sharp decrease in esophageal impedance measurements in a retrograde fashion (impedance-detected reflux events), with subsequent recovery to the baseline in each instance. Ambulatory reflux testing affords insight into three areas: 1) measurement of esophageal acid exposure time (AET); the cumulative time duration when distal esophageal pH is less than 4 at the recording site, reported as a percentage of the recording period; 2) measurement of the number of reflux events both acidic (from pH monitoring) and weakly acidic/alkaline (from impedance monitoring); and 3) quantitative evaluation of the association between reported symptom episodes and reflux events.

The SI and SAP can be calculated individually for acid-detected reflux events and for impedance-detected reflux events. Since reflux events are better detected with impedance, combined pH-impedance testing increases the yield of detecting positive SRA, especially when performed off PPI therapy.16,17 Because these indices are heavily reliant on patient reporting of symptom episodes, SRA can be overinterpreted;18 positive associations are more clinically useful than negative results in the evaluation of symptoms attributed to GERD.19 Despite these concerns, the two most consistent predictors of symptomatic outcome with antireflux therapy on pH-impedance testing are abnormal AET and positive SAP with impedance-detected reflux events.17

Testing on or off PPI?
For symptoms attributable to GERD that persist despite properly administered PPI therapy, the 2013 American College of Gastroenterology guidelines suggest upper endoscopy with esophageal biopsies for typical symptoms and appropriate referrals for atypical symptoms.24 However, if these evaluations are unremarkable, reflux monitoring is recommended, with PPI status for testing guided by the pre-test probability of GERD: with a low pre-test probability of GERD, reflux testing is best performed off PPI with either pH or combined pH-impedance testing. In contrast, with a high pre-test probability of GERD, testing is best performed on PPI with combined pH-impedance testing. A similar concept is proposed in the Rome IV approach (Figure 2)23 and on GERD consensus guidelines:7 when heartburn or chest pain persists despite PPI therapy and endoscopy and esophageal biopsies are normal, evidence for GERD (past esophagitis, Barrett’s esophagus, peptic stricture, or prior positive reflux testing) prompts pH-impedance monitoring on PPI therapy (i.e., proven GERD). Those without this evidence for proven GERD (i.e., unproven GERD) are best tested off PPI, and the test utilized can be either pH alone or combined pH-impedance.
GERD phenotypes and management
The presence or absence of the two core metrics on ambulatory reflux monitoring – abnormal AET and positive SRA – can stratify symptomatic GERD patients into phenotypes that predict symptomatic improvement with antireflux therapy and guide management of symptoms (Figure 3).25,26 The presence of both abnormal AET and positive SRA suggests “strong” evidence for GERD, for which symptom improvement is likely with maximization of antireflux therapy, which can include BID PPI, baclofen (to decrease transient LES relaxations), alginates (such as Gaviscon), and consideration of endosopic or surgical antireflux procedures such as fundoplication or magnetic sphincter augmentation. Abnormal AET but negative SRA is regarded as “good” evidence for GERD, for which similar antireflux therapies can be advocated. Normal AET but positive SRA is designated as “reflux hypersensitivity,”23 with increasing proportions of patients meeting this phenotype when tested with combined pH-impedance and off PPI therapy.27 Both normal AET and negative SRA suggest equivocal evidence for GERD and the likely presence of a functional esophageal disorder, such as functional heartburn.23 For reflux hypersensitivity and especially functional esophageal disorders, antireflux therapy is unlikely to be as effective and management can include pharmacologic neuromodulation (such as tricyclic antidepressants administered at bedtime) as well as adjunctive nonpharmacologic approaches (such as stress reduction, relaxation, hypnosis, or cognitive-behavioral therapy).
The future of reflux diagnostics

Conclusions
For esophageal symptoms potentially attributable to GERD that persist despite optimized PPI therapy, esophageal testing should be undertaken, starting with endoscopy and biopsies and proceeding to ambulatory reflux monitoring with HRM. The decisions between pH testing alone versus combined pH-impedance monitoring, and between testing on or off PPI therapy, can be guided either by the pre-test probability of GERD or whether GERD has been proven or unproven in prior evaluations (Figure 2). Elevated AET and positive SRA with impedance-detected reflux events can predict the likelihood of successful management outcomes from antireflux therapy. These two core metrics can be utilized to phenotype GERD and guide management approaches for persisting symptoms (Figure 3). Novel impedance metrics (baseline mucosal impedance, postreflux swallow-induced peristaltic wave index) and markers for esophageal mucosal damage continue to be studied as potential markers for evidence of longitudinal reflux exposure.
Dr. Patel is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine and the Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University School of Medicine, St. Louis, Mo.
References
1. Shaheen N.J., et al. Am J Gastroenterol. 2006;101:2128-38.
2. Patel A., Gyawali C.P.. Switzerland: Springer International, 2016.
3. Vakil N., et al. Am J Gastroenterol. 2006;101:1900-20; quiz 1943.
4. Fass R., et al. Arch Intern Med. 1999;159:2161-8.
5. Numans M.E., et al. Ann Intern Med. 2004;140:518-27.
6. Shaheen N.J., et al. Aliment Pharmacol Ther. 2011;33:225-34.
7. Roman S., et al. Neurogastroenterol Motil Mar 31. doi: 10.1111/nmo.13067. [Epub ahead of print] 2017.
8. Dellon E.S., et al. Am J Gastroenterol. 2013;108:679-92; quiz 693.
9. Pandolfino JE, Vela MF. Gastrointest Endosc. 2009;69:917-30, 930 e1.
10. Shay S., et al. Am J Gastroenterol. 2004;99:1037-43.
11. Zerbib F., et al. Clin Gastroenterol Hepatol. 2013;11:366-72.
12. Wiener G.J., et al. Am J Gastroenterol 1988;83:358-61.
13. Weusten B.L., et al. Gastroenterology. 1994;107:1741-5.
14. Ghillebert G., et al. Gut 1990;31:738-44.
15. Kushnir V.M., et al. Aliment Pharmacol Ther. 2012;35(9):1080-7.
16. Bredenoord A.J., et al. Am J Gastroenterol. 2006;101:453-9.
17. Patel A., et al. Clin Gastroenterol Hepatol. 2015;13:884-91.
18. Slaughter J.C., et al. Clin Gastroenterol Hepatol. 2011;9:868-74.
19. Kavitt R.T., et al. Am J Gastroenterol. 2012;107:1826-32.
20. Kahrilas P.J., et al. Gastroenterology 2008;135:1383-91, 1391 e1-5.
21. Kessing B.F., et al. Clin Gastroenterol Hepatol. 2011;9:1020-4.
22. Kahrilas P.J., et al. Neurogastroenterol Motil. 2015;27:160-74.
23. Aziz A, et al. Esophageal disorders. Gastroenterology 2016;150:1368-79.
24. Katz P.O., et al. Am J Gastroenterol. 2013;108:308-28; quiz 329.
25. Boeckxstaens G., et al. Gut 2014;63:1185-93.
26. Patel A., et al. Neurogastroenterol Motil. 2016;28:513-21.
27. Patel A., et al. Neurogastroenterol Motil. 2016;28:1382-90.
28. Martinucci I., et al. Neurogastroenterol Motil. 2014;26:546-55.
29. Ates F., et al. Gastroenterology 2015;148:334-43.
30. Kessing B.F., et al. Am J Gastroenterol. 2011;106:2093-7.
31. Patel A., et al. Aliment Pharmacol Ther. 2016;44:890-8.
32. Frazzoni M., et al. Neurogastroenterol Motil. 2016.
33. Frazzoni M., et al. Neurogastroenterol Motil. 2013;25:399-406, e295.
34. Vela M.F., et al. Am J Gastroenterol. 2011;106:844-50.
Introduction
Chronic esophageal symptoms attributed to gastroesophageal reflux disease (GERD) are common presenting symptoms in gastroenterology, leading to high healthcare costs and adverse quality of life globally.1,2 The clinical diagnosis of GERD hinges on the presence of “troublesome” compatible typical symptoms (heartburn, acid regurgitation) or evidence of mucosal injury on endoscopy (esophagitis, Barrett’s esophagus, peptic stricture).3 With the growing availability of proton pump inhibitors (PPIs), patients and clinicians often utilize an empiric therapeutic trial of PPI as an initial test, with symptom improvement in the absence of alarm symptoms indicating a high likelihood of GERD.4 A meta-analysis of studies that used objective measures of GERD (in this case, 24-hour pH monitoring) showed that the “PPI test” has a sensitivity of 78%, but a specificity of only 54%, as a diagnostic approach to GERD symptoms.5 Apart from noncardiac chest pain, the diagnostic yield is even lower for atypical and extra-esophageal symptoms such as cough or laryngeal symptoms.6
The “nuts and bolts” of reflux testing
Ambulatory reflux testing assesses esophageal reflux burden and symptom-reflux association (SRA). Individual reflux events are identified as either a drop in esophageal pH to less than 4 (acid reflux events), or a sharp decrease in esophageal impedance measurements in a retrograde fashion (impedance-detected reflux events), with subsequent recovery to the baseline in each instance. Ambulatory reflux testing affords insight into three areas: 1) measurement of esophageal acid exposure time (AET); the cumulative time duration when distal esophageal pH is less than 4 at the recording site, reported as a percentage of the recording period; 2) measurement of the number of reflux events both acidic (from pH monitoring) and weakly acidic/alkaline (from impedance monitoring); and 3) quantitative evaluation of the association between reported symptom episodes and reflux events.

The SI and SAP can be calculated individually for acid-detected reflux events and for impedance-detected reflux events. Since reflux events are better detected with impedance, combined pH-impedance testing increases the yield of detecting positive SRA, especially when performed off PPI therapy.16,17 Because these indices are heavily reliant on patient reporting of symptom episodes, SRA can be overinterpreted;18 positive associations are more clinically useful than negative results in the evaluation of symptoms attributed to GERD.19 Despite these concerns, the two most consistent predictors of symptomatic outcome with antireflux therapy on pH-impedance testing are abnormal AET and positive SAP with impedance-detected reflux events.17

Testing on or off PPI?
For symptoms attributable to GERD that persist despite properly administered PPI therapy, the 2013 American College of Gastroenterology guidelines suggest upper endoscopy with esophageal biopsies for typical symptoms and appropriate referrals for atypical symptoms.24 However, if these evaluations are unremarkable, reflux monitoring is recommended, with PPI status for testing guided by the pre-test probability of GERD: with a low pre-test probability of GERD, reflux testing is best performed off PPI with either pH or combined pH-impedance testing. In contrast, with a high pre-test probability of GERD, testing is best performed on PPI with combined pH-impedance testing. A similar concept is proposed in the Rome IV approach (Figure 2)23 and on GERD consensus guidelines:7 when heartburn or chest pain persists despite PPI therapy and endoscopy and esophageal biopsies are normal, evidence for GERD (past esophagitis, Barrett’s esophagus, peptic stricture, or prior positive reflux testing) prompts pH-impedance monitoring on PPI therapy (i.e., proven GERD). Those without this evidence for proven GERD (i.e., unproven GERD) are best tested off PPI, and the test utilized can be either pH alone or combined pH-impedance.
GERD phenotypes and management
The presence or absence of the two core metrics on ambulatory reflux monitoring – abnormal AET and positive SRA – can stratify symptomatic GERD patients into phenotypes that predict symptomatic improvement with antireflux therapy and guide management of symptoms (Figure 3).25,26 The presence of both abnormal AET and positive SRA suggests “strong” evidence for GERD, for which symptom improvement is likely with maximization of antireflux therapy, which can include BID PPI, baclofen (to decrease transient LES relaxations), alginates (such as Gaviscon), and consideration of endosopic or surgical antireflux procedures such as fundoplication or magnetic sphincter augmentation. Abnormal AET but negative SRA is regarded as “good” evidence for GERD, for which similar antireflux therapies can be advocated. Normal AET but positive SRA is designated as “reflux hypersensitivity,”23 with increasing proportions of patients meeting this phenotype when tested with combined pH-impedance and off PPI therapy.27 Both normal AET and negative SRA suggest equivocal evidence for GERD and the likely presence of a functional esophageal disorder, such as functional heartburn.23 For reflux hypersensitivity and especially functional esophageal disorders, antireflux therapy is unlikely to be as effective and management can include pharmacologic neuromodulation (such as tricyclic antidepressants administered at bedtime) as well as adjunctive nonpharmacologic approaches (such as stress reduction, relaxation, hypnosis, or cognitive-behavioral therapy).
The future of reflux diagnostics

Conclusions
For esophageal symptoms potentially attributable to GERD that persist despite optimized PPI therapy, esophageal testing should be undertaken, starting with endoscopy and biopsies and proceeding to ambulatory reflux monitoring with HRM. The decisions between pH testing alone versus combined pH-impedance monitoring, and between testing on or off PPI therapy, can be guided either by the pre-test probability of GERD or whether GERD has been proven or unproven in prior evaluations (Figure 2). Elevated AET and positive SRA with impedance-detected reflux events can predict the likelihood of successful management outcomes from antireflux therapy. These two core metrics can be utilized to phenotype GERD and guide management approaches for persisting symptoms (Figure 3). Novel impedance metrics (baseline mucosal impedance, postreflux swallow-induced peristaltic wave index) and markers for esophageal mucosal damage continue to be studied as potential markers for evidence of longitudinal reflux exposure.
Dr. Patel is assistant professor of medicine, division of gastroenterology, Duke University School of Medicine and the Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gyawali is professor of medicine, division of gastroenterology, Washington University School of Medicine, St. Louis, Mo.
References
1. Shaheen N.J., et al. Am J Gastroenterol. 2006;101:2128-38.
2. Patel A., Gyawali C.P.. Switzerland: Springer International, 2016.
3. Vakil N., et al. Am J Gastroenterol. 2006;101:1900-20; quiz 1943.
4. Fass R., et al. Arch Intern Med. 1999;159:2161-8.
5. Numans M.E., et al. Ann Intern Med. 2004;140:518-27.
6. Shaheen N.J., et al. Aliment Pharmacol Ther. 2011;33:225-34.
7. Roman S., et al. Neurogastroenterol Motil Mar 31. doi: 10.1111/nmo.13067. [Epub ahead of print] 2017.
8. Dellon E.S., et al. Am J Gastroenterol. 2013;108:679-92; quiz 693.
9. Pandolfino JE, Vela MF. Gastrointest Endosc. 2009;69:917-30, 930 e1.
10. Shay S., et al. Am J Gastroenterol. 2004;99:1037-43.
11. Zerbib F., et al. Clin Gastroenterol Hepatol. 2013;11:366-72.
12. Wiener G.J., et al. Am J Gastroenterol 1988;83:358-61.
13. Weusten B.L., et al. Gastroenterology. 1994;107:1741-5.
14. Ghillebert G., et al. Gut 1990;31:738-44.
15. Kushnir V.M., et al. Aliment Pharmacol Ther. 2012;35(9):1080-7.
16. Bredenoord A.J., et al. Am J Gastroenterol. 2006;101:453-9.
17. Patel A., et al. Clin Gastroenterol Hepatol. 2015;13:884-91.
18. Slaughter J.C., et al. Clin Gastroenterol Hepatol. 2011;9:868-74.
19. Kavitt R.T., et al. Am J Gastroenterol. 2012;107:1826-32.
20. Kahrilas P.J., et al. Gastroenterology 2008;135:1383-91, 1391 e1-5.
21. Kessing B.F., et al. Clin Gastroenterol Hepatol. 2011;9:1020-4.
22. Kahrilas P.J., et al. Neurogastroenterol Motil. 2015;27:160-74.
23. Aziz A, et al. Esophageal disorders. Gastroenterology 2016;150:1368-79.
24. Katz P.O., et al. Am J Gastroenterol. 2013;108:308-28; quiz 329.
25. Boeckxstaens G., et al. Gut 2014;63:1185-93.
26. Patel A., et al. Neurogastroenterol Motil. 2016;28:513-21.
27. Patel A., et al. Neurogastroenterol Motil. 2016;28:1382-90.
28. Martinucci I., et al. Neurogastroenterol Motil. 2014;26:546-55.
29. Ates F., et al. Gastroenterology 2015;148:334-43.
30. Kessing B.F., et al. Am J Gastroenterol. 2011;106:2093-7.
31. Patel A., et al. Aliment Pharmacol Ther. 2016;44:890-8.
32. Frazzoni M., et al. Neurogastroenterol Motil. 2016.
33. Frazzoni M., et al. Neurogastroenterol Motil. 2013;25:399-406, e295.
34. Vela M.F., et al. Am J Gastroenterol. 2011;106:844-50.
Health Maintenance and Preventive Care in Patients with Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) consists of two chronic inflammatory diseases, Crohn’s disease (CD) and ulcerative colitis (UC), as well as a small category of patients (~10%) who have atypical features called IBD-unclassified (IBD-U) or indeterminate colitis. The prevalence of IBD ranges from 0.3% to 0.5% overall in North America and Europe.1 In North America, the incidences of CD and UC are estimated to be 3.1 to 14.6 per 100,000 person-years and 2.2 to 14.3 cases per 100,000 person-years, respectively; similar rates are seen in Europe.2 However, incidences up to 19.2 and 20.2 per 100,000 for UC and CD, respectively, have been reported in Canada.3,4 The incidences of both UC and CD are increasing over time in Western countries and in rapidly industrializing countries throughout Asia and South America.5-8
Influenza vaccine and pneumococcal vaccine
Influenza A and B outbreaks are commonly seen during the fall and early spring and risk factors for pneumonia and hospitalization include older age, chronic medical conditions, and immunosuppression. The CDC now recommend annual influenza vaccination for all individuals older than six months. For patients on immunosuppression, the vaccine administered should be the inactivated vaccine, as live attenuated vaccines should not be administered to these patients.
In IBD patients, the influenza and pneumococcal vaccines are both well tolerated without an increased rate of adverse effects over the general population and without an increased risk of IBD flares after vaccination.12 A common question for patients on biologic therapy is whether the vaccine should be timed at a specific point in the dose cycle. For infliximab, and likely other biologics, the timing does not change the vaccine immunogenicity and patients should be given these vaccines regardless of where they are in the cycle of administration of their biologic.13 In addition, there is significant response to influenza and pneumococcal vaccines in patients on combination therapy with immunomodulators and anti-TNFs and concerns about a lack of response to vaccines should not discourage vaccination since benefits are still acquired by patients even if immunogenicity is somewhat decreased.14,15
Other vaccinations
In addition to the influenza and pneumococcal vaccines, adult and pediatric patients with IBD should follow the ACIP recommendations for tetanus, diphtheria, pertussis (Tdap), Td boosters, hepatitis A, hepatitis B, human papilloma virus (HPV), and meningococcal vaccinations.16,17
Live vaccines including measles mumps rubella (MMR), varicella, and zoster vaccines are in general contraindicated in immunosuppressed patients on corticosteroids, azathioprine/6-mercaptopurine, methotrexate, anti-TNF, and anti-integrin biologics. An inactive varicella-zoster vaccine will likely be available in the near future and may obviate the need for the live vaccine, which is an important development given the increased risk of zoster in patients with IBD on immunosuppression.18
Osteoporosis screening
Skin cancer screening
Multiple studies have demonstrated that immunosuppression, especially with methotrexate and azathioprine/6-mercaptopurine (6MP) is a risk factor for the development of initial and recurrent non-melanoma skin cancer (NMSC) in IBD patients, the data for biologics are less definitive.23-25 In addition, biologics are associated with increased risk of melanoma in IBD.26 The elevated risk of skin cancer begins in the first year of treatment with thiopurines and may continue after discontinuation. On the basis of this data, screening for melanoma and NMSC is recommended in IBD patients on immunosuppression. Especially for patients on thiopurines it is reasonable for the initial dermatologist visit to occur in the first year of treatment and thereafter with at least annual visits for a full body skin examination. In addition, it is reasonable to recommend regular sunscreen use and protective clothing such as hats.
Cervical cancer screening
A recent meta-analysis shows that women with IBD on immunosuppression have an increased risk of cervical high grade dysplasia and cervical cancer.27 HPV is the major risk factor for cervical cancer and is necessary for its development. The current American College of Gynecology guidelines for women on immunosuppression are to start cervical cancer screening at 21 and annual screening thereafter with Pap and HPV testing.28
Smoking
Smoking has well known associations with poor outcomes in the general population such as increased risk of lung and pancreatic cancers, as well as high risk of cardiovascular disease. In addition, smoking has risks specific to IBD. In CD, smoking is associated with increased disease activity, increased risk of post-operative recurrence, and increased severity of disease.29 Smoking cessation is associated with improved long-term disease outcomes and less risk.30 Making it a point to regularly discuss smoking cessation and partnering with PCPs to offer evidence-based quitting aids may be one of our most significant and beneficial interventions.
Depression and anxiety
Several studies have shown high levels of depression and anxiety in IBD patients and higher levels of depression are associated with increased symptoms, clinical recurrence, poor quality of life and decreased social support.31-33 A recent systematic review of several studies suggested that antidepressants use in IBD patients benefits their mental health and may improve their clinical course as well.34 As such, screening for depression and anxiety regularly and either offering treatment or referral to psychiatrists and psychologists for further management is recommended.10
Conclusion
Patients with IBD frequently develop long-term relationships with their gastroenterologists due to their lifelong chronic disease. It is therefore incumbent on us to be attentive to issues related to IBD patients’ preventive care and collaborate with PCPs to coordinate care for our patients since many of these interventions have both short-term and long-term benefits.
Dr. Chachu is assistant professor and gastroenterologist at Duke University, Durham, N.C.
References
1. Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152(2):313-21.e2.
2. Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17.
3. Bernstein CN, Wajda A, Svenson LW, et al. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. The American journal of gastroenterology. 2006;101(7):1559-68.
4. Lowe AM, Roy PO, M BP, et al. Epidemiology of Crohn’s disease in Quebec, Canada. Inflammatory bowel diseases. 2009;15(3):429-35.
5. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1424-9.
6. Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Digestive diseases and sciences. 2013;58(2):519-25.
7. Ng SC, Kaplan G, Banerjee R, et al. 78 Incidence and Phenotype of Inflammatory Bowel Disease From 13 Countries in Asia-Pacific: Results From the Asia-Pacific Crohn’s and Colitis Epidemiologic Study 2011-2013. Gastroenterology.150(4):S21.
8. Parente JML, Coy CSR, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World Journal of Gastroenterology : WJG. 2015;21(4):1197-206.
9. Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflammatory bowel diseases. 2008;14(2):253-8.
10. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. The American journal of gastroenterology. 2017;112(2):241-58.
11. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. The American journal of gastroenterology. 2013;108(2):240-8.
12. Rahier JF, Papay P, Salleron J, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456-62.
13. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of Influenza Vaccine for Patients with Inflammatory Bowel Disease on Maintenance Infliximab Therapy: A Randomized Trial. Inflammatory bowel diseases. 2016;22(3):638-47.
14. Brezinschek HP, Hofstaetter T, Leeb BF, et al. Immunization of patients with rheumatoid arthritis with antitumor necrosis factor alpha therapy and methotrexate. Current opinion in rheumatology. 2008;20(3):295-9.
15. Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34(2):272-9.
16. Kim DK, Riley LE, Harriman KH, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):136-8.
17. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):134-5.
18. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(12):2392-403.
19. Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut. 2004;53(2):251-5.
20. Casals-Seoane F, Chaparro M, Mate J, et al. Clinical Course of Bone Metabolism Disorders in Patients with Inflammatory Bowel Disease: A 5-Year Prospective Study. Inflammatory bowel diseases. 2016;22(8):1929-36.
21. Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(1):32-44.e5.
22. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International. 2014;25(10):2359-81.
23. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621-28.e1-5.
24. Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(3):268-74.
25. Scott FI, Mamtani R, Brensinger CM, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA dermatology. 2016;152(2):164-72.
26. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390-9.e1.
27. Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflammatory bowel diseases. 2015;21(5):1089-97.
28. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstetrics and gynecology. 2016;128(4):e111-30.
29. Ryan WR, Allan RN, Yamamoto T, et al. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. American journal of surgery. 2004;187(2):219-25.
30. Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120(5):1093-9.
31. Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflammatory bowel diseases. 2006;12(8):697-707.
32. Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. General hospital psychiatry. 1996;18(4):220-9.
33. Mikocka-Walus A, Pittet V, Rossel J-B, et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology.14(6):829-35.e1.
34. Macer BJD, Prady SL, Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflammatory bowel diseases. 2017;23(4):534-50.
Inflammatory bowel disease (IBD) consists of two chronic inflammatory diseases, Crohn’s disease (CD) and ulcerative colitis (UC), as well as a small category of patients (~10%) who have atypical features called IBD-unclassified (IBD-U) or indeterminate colitis. The prevalence of IBD ranges from 0.3% to 0.5% overall in North America and Europe.1 In North America, the incidences of CD and UC are estimated to be 3.1 to 14.6 per 100,000 person-years and 2.2 to 14.3 cases per 100,000 person-years, respectively; similar rates are seen in Europe.2 However, incidences up to 19.2 and 20.2 per 100,000 for UC and CD, respectively, have been reported in Canada.3,4 The incidences of both UC and CD are increasing over time in Western countries and in rapidly industrializing countries throughout Asia and South America.5-8
Influenza vaccine and pneumococcal vaccine
Influenza A and B outbreaks are commonly seen during the fall and early spring and risk factors for pneumonia and hospitalization include older age, chronic medical conditions, and immunosuppression. The CDC now recommend annual influenza vaccination for all individuals older than six months. For patients on immunosuppression, the vaccine administered should be the inactivated vaccine, as live attenuated vaccines should not be administered to these patients.
In IBD patients, the influenza and pneumococcal vaccines are both well tolerated without an increased rate of adverse effects over the general population and without an increased risk of IBD flares after vaccination.12 A common question for patients on biologic therapy is whether the vaccine should be timed at a specific point in the dose cycle. For infliximab, and likely other biologics, the timing does not change the vaccine immunogenicity and patients should be given these vaccines regardless of where they are in the cycle of administration of their biologic.13 In addition, there is significant response to influenza and pneumococcal vaccines in patients on combination therapy with immunomodulators and anti-TNFs and concerns about a lack of response to vaccines should not discourage vaccination since benefits are still acquired by patients even if immunogenicity is somewhat decreased.14,15
Other vaccinations
In addition to the influenza and pneumococcal vaccines, adult and pediatric patients with IBD should follow the ACIP recommendations for tetanus, diphtheria, pertussis (Tdap), Td boosters, hepatitis A, hepatitis B, human papilloma virus (HPV), and meningococcal vaccinations.16,17
Live vaccines including measles mumps rubella (MMR), varicella, and zoster vaccines are in general contraindicated in immunosuppressed patients on corticosteroids, azathioprine/6-mercaptopurine, methotrexate, anti-TNF, and anti-integrin biologics. An inactive varicella-zoster vaccine will likely be available in the near future and may obviate the need for the live vaccine, which is an important development given the increased risk of zoster in patients with IBD on immunosuppression.18
Osteoporosis screening
Skin cancer screening
Multiple studies have demonstrated that immunosuppression, especially with methotrexate and azathioprine/6-mercaptopurine (6MP) is a risk factor for the development of initial and recurrent non-melanoma skin cancer (NMSC) in IBD patients, the data for biologics are less definitive.23-25 In addition, biologics are associated with increased risk of melanoma in IBD.26 The elevated risk of skin cancer begins in the first year of treatment with thiopurines and may continue after discontinuation. On the basis of this data, screening for melanoma and NMSC is recommended in IBD patients on immunosuppression. Especially for patients on thiopurines it is reasonable for the initial dermatologist visit to occur in the first year of treatment and thereafter with at least annual visits for a full body skin examination. In addition, it is reasonable to recommend regular sunscreen use and protective clothing such as hats.
Cervical cancer screening
A recent meta-analysis shows that women with IBD on immunosuppression have an increased risk of cervical high grade dysplasia and cervical cancer.27 HPV is the major risk factor for cervical cancer and is necessary for its development. The current American College of Gynecology guidelines for women on immunosuppression are to start cervical cancer screening at 21 and annual screening thereafter with Pap and HPV testing.28
Smoking
Smoking has well known associations with poor outcomes in the general population such as increased risk of lung and pancreatic cancers, as well as high risk of cardiovascular disease. In addition, smoking has risks specific to IBD. In CD, smoking is associated with increased disease activity, increased risk of post-operative recurrence, and increased severity of disease.29 Smoking cessation is associated with improved long-term disease outcomes and less risk.30 Making it a point to regularly discuss smoking cessation and partnering with PCPs to offer evidence-based quitting aids may be one of our most significant and beneficial interventions.
Depression and anxiety
Several studies have shown high levels of depression and anxiety in IBD patients and higher levels of depression are associated with increased symptoms, clinical recurrence, poor quality of life and decreased social support.31-33 A recent systematic review of several studies suggested that antidepressants use in IBD patients benefits their mental health and may improve their clinical course as well.34 As such, screening for depression and anxiety regularly and either offering treatment or referral to psychiatrists and psychologists for further management is recommended.10
Conclusion
Patients with IBD frequently develop long-term relationships with their gastroenterologists due to their lifelong chronic disease. It is therefore incumbent on us to be attentive to issues related to IBD patients’ preventive care and collaborate with PCPs to coordinate care for our patients since many of these interventions have both short-term and long-term benefits.
Dr. Chachu is assistant professor and gastroenterologist at Duke University, Durham, N.C.
References
1. Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152(2):313-21.e2.
2. Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17.
3. Bernstein CN, Wajda A, Svenson LW, et al. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. The American journal of gastroenterology. 2006;101(7):1559-68.
4. Lowe AM, Roy PO, M BP, et al. Epidemiology of Crohn’s disease in Quebec, Canada. Inflammatory bowel diseases. 2009;15(3):429-35.
5. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1424-9.
6. Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Digestive diseases and sciences. 2013;58(2):519-25.
7. Ng SC, Kaplan G, Banerjee R, et al. 78 Incidence and Phenotype of Inflammatory Bowel Disease From 13 Countries in Asia-Pacific: Results From the Asia-Pacific Crohn’s and Colitis Epidemiologic Study 2011-2013. Gastroenterology.150(4):S21.
8. Parente JML, Coy CSR, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World Journal of Gastroenterology : WJG. 2015;21(4):1197-206.
9. Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflammatory bowel diseases. 2008;14(2):253-8.
10. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. The American journal of gastroenterology. 2017;112(2):241-58.
11. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. The American journal of gastroenterology. 2013;108(2):240-8.
12. Rahier JF, Papay P, Salleron J, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456-62.
13. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of Influenza Vaccine for Patients with Inflammatory Bowel Disease on Maintenance Infliximab Therapy: A Randomized Trial. Inflammatory bowel diseases. 2016;22(3):638-47.
14. Brezinschek HP, Hofstaetter T, Leeb BF, et al. Immunization of patients with rheumatoid arthritis with antitumor necrosis factor alpha therapy and methotrexate. Current opinion in rheumatology. 2008;20(3):295-9.
15. Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34(2):272-9.
16. Kim DK, Riley LE, Harriman KH, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):136-8.
17. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):134-5.
18. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(12):2392-403.
19. Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut. 2004;53(2):251-5.
20. Casals-Seoane F, Chaparro M, Mate J, et al. Clinical Course of Bone Metabolism Disorders in Patients with Inflammatory Bowel Disease: A 5-Year Prospective Study. Inflammatory bowel diseases. 2016;22(8):1929-36.
21. Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(1):32-44.e5.
22. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International. 2014;25(10):2359-81.
23. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621-28.e1-5.
24. Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(3):268-74.
25. Scott FI, Mamtani R, Brensinger CM, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA dermatology. 2016;152(2):164-72.
26. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390-9.e1.
27. Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflammatory bowel diseases. 2015;21(5):1089-97.
28. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstetrics and gynecology. 2016;128(4):e111-30.
29. Ryan WR, Allan RN, Yamamoto T, et al. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. American journal of surgery. 2004;187(2):219-25.
30. Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120(5):1093-9.
31. Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflammatory bowel diseases. 2006;12(8):697-707.
32. Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. General hospital psychiatry. 1996;18(4):220-9.
33. Mikocka-Walus A, Pittet V, Rossel J-B, et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology.14(6):829-35.e1.
34. Macer BJD, Prady SL, Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflammatory bowel diseases. 2017;23(4):534-50.
Inflammatory bowel disease (IBD) consists of two chronic inflammatory diseases, Crohn’s disease (CD) and ulcerative colitis (UC), as well as a small category of patients (~10%) who have atypical features called IBD-unclassified (IBD-U) or indeterminate colitis. The prevalence of IBD ranges from 0.3% to 0.5% overall in North America and Europe.1 In North America, the incidences of CD and UC are estimated to be 3.1 to 14.6 per 100,000 person-years and 2.2 to 14.3 cases per 100,000 person-years, respectively; similar rates are seen in Europe.2 However, incidences up to 19.2 and 20.2 per 100,000 for UC and CD, respectively, have been reported in Canada.3,4 The incidences of both UC and CD are increasing over time in Western countries and in rapidly industrializing countries throughout Asia and South America.5-8
Influenza vaccine and pneumococcal vaccine
Influenza A and B outbreaks are commonly seen during the fall and early spring and risk factors for pneumonia and hospitalization include older age, chronic medical conditions, and immunosuppression. The CDC now recommend annual influenza vaccination for all individuals older than six months. For patients on immunosuppression, the vaccine administered should be the inactivated vaccine, as live attenuated vaccines should not be administered to these patients.
In IBD patients, the influenza and pneumococcal vaccines are both well tolerated without an increased rate of adverse effects over the general population and without an increased risk of IBD flares after vaccination.12 A common question for patients on biologic therapy is whether the vaccine should be timed at a specific point in the dose cycle. For infliximab, and likely other biologics, the timing does not change the vaccine immunogenicity and patients should be given these vaccines regardless of where they are in the cycle of administration of their biologic.13 In addition, there is significant response to influenza and pneumococcal vaccines in patients on combination therapy with immunomodulators and anti-TNFs and concerns about a lack of response to vaccines should not discourage vaccination since benefits are still acquired by patients even if immunogenicity is somewhat decreased.14,15
Other vaccinations
In addition to the influenza and pneumococcal vaccines, adult and pediatric patients with IBD should follow the ACIP recommendations for tetanus, diphtheria, pertussis (Tdap), Td boosters, hepatitis A, hepatitis B, human papilloma virus (HPV), and meningococcal vaccinations.16,17
Live vaccines including measles mumps rubella (MMR), varicella, and zoster vaccines are in general contraindicated in immunosuppressed patients on corticosteroids, azathioprine/6-mercaptopurine, methotrexate, anti-TNF, and anti-integrin biologics. An inactive varicella-zoster vaccine will likely be available in the near future and may obviate the need for the live vaccine, which is an important development given the increased risk of zoster in patients with IBD on immunosuppression.18
Osteoporosis screening
Skin cancer screening
Multiple studies have demonstrated that immunosuppression, especially with methotrexate and azathioprine/6-mercaptopurine (6MP) is a risk factor for the development of initial and recurrent non-melanoma skin cancer (NMSC) in IBD patients, the data for biologics are less definitive.23-25 In addition, biologics are associated with increased risk of melanoma in IBD.26 The elevated risk of skin cancer begins in the first year of treatment with thiopurines and may continue after discontinuation. On the basis of this data, screening for melanoma and NMSC is recommended in IBD patients on immunosuppression. Especially for patients on thiopurines it is reasonable for the initial dermatologist visit to occur in the first year of treatment and thereafter with at least annual visits for a full body skin examination. In addition, it is reasonable to recommend regular sunscreen use and protective clothing such as hats.
Cervical cancer screening
A recent meta-analysis shows that women with IBD on immunosuppression have an increased risk of cervical high grade dysplasia and cervical cancer.27 HPV is the major risk factor for cervical cancer and is necessary for its development. The current American College of Gynecology guidelines for women on immunosuppression are to start cervical cancer screening at 21 and annual screening thereafter with Pap and HPV testing.28
Smoking
Smoking has well known associations with poor outcomes in the general population such as increased risk of lung and pancreatic cancers, as well as high risk of cardiovascular disease. In addition, smoking has risks specific to IBD. In CD, smoking is associated with increased disease activity, increased risk of post-operative recurrence, and increased severity of disease.29 Smoking cessation is associated with improved long-term disease outcomes and less risk.30 Making it a point to regularly discuss smoking cessation and partnering with PCPs to offer evidence-based quitting aids may be one of our most significant and beneficial interventions.
Depression and anxiety
Several studies have shown high levels of depression and anxiety in IBD patients and higher levels of depression are associated with increased symptoms, clinical recurrence, poor quality of life and decreased social support.31-33 A recent systematic review of several studies suggested that antidepressants use in IBD patients benefits their mental health and may improve their clinical course as well.34 As such, screening for depression and anxiety regularly and either offering treatment or referral to psychiatrists and psychologists for further management is recommended.10
Conclusion
Patients with IBD frequently develop long-term relationships with their gastroenterologists due to their lifelong chronic disease. It is therefore incumbent on us to be attentive to issues related to IBD patients’ preventive care and collaborate with PCPs to coordinate care for our patients since many of these interventions have both short-term and long-term benefits.
Dr. Chachu is assistant professor and gastroenterologist at Duke University, Durham, N.C.
References
1. Kaplan GG, Ng SC. Understanding and Preventing the Global Increase of Inflammatory Bowel Disease. Gastroenterology. 2017;152(2):313-21.e2.
2. Loftus EV, Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126(6):1504-17.
3. Bernstein CN, Wajda A, Svenson LW, et al. The Epidemiology of Inflammatory Bowel Disease in Canada: A Population-Based Study. The American journal of gastroenterology. 2006;101(7):1559-68.
4. Lowe AM, Roy PO, M BP, et al. Epidemiology of Crohn’s disease in Quebec, Canada. Inflammatory bowel diseases. 2009;15(3):429-35.
5. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2007;5(12):1424-9.
6. Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Digestive diseases and sciences. 2013;58(2):519-25.
7. Ng SC, Kaplan G, Banerjee R, et al. 78 Incidence and Phenotype of Inflammatory Bowel Disease From 13 Countries in Asia-Pacific: Results From the Asia-Pacific Crohn’s and Colitis Epidemiologic Study 2011-2013. Gastroenterology.150(4):S21.
8. Parente JML, Coy CSR, Campelo V, et al. Inflammatory bowel disease in an underdeveloped region of Northeastern Brazil. World Journal of Gastroenterology : WJG. 2015;21(4):1197-206.
9. Selby L, Kane S, Wilson J, et al. Receipt of preventive health services by IBD patients is significantly lower than by primary care patients. Inflammatory bowel diseases. 2008;14(2):253-8.
10. Farraye FA, Melmed GY, Lichtenstein GR, et al. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. The American journal of gastroenterology. 2017;112(2):241-58.
11. Long MD, Martin C, Sandler RS, et al. Increased risk of pneumonia among patients with inflammatory bowel disease. The American journal of gastroenterology. 2013;108(2):240-8.
12. Rahier JF, Papay P, Salleron J, et al. H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011;60(4):456-62.
13. deBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of Influenza Vaccine for Patients with Inflammatory Bowel Disease on Maintenance Infliximab Therapy: A Randomized Trial. Inflammatory bowel diseases. 2016;22(3):638-47.
14. Brezinschek HP, Hofstaetter T, Leeb BF, et al. Immunization of patients with rheumatoid arthritis with antitumor necrosis factor alpha therapy and methotrexate. Current opinion in rheumatology. 2008;20(3):295-9.
15. Kaine JL, Kivitz AJ, Birbara C, et al. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007;34(2):272-9.
16. Kim DK, Riley LE, Harriman KH, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Adults Aged 19 Years or Older - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):136-8.
17. Robinson CL, Romero JR, Kempe A, et al. Advisory Committee on Immunization Practices Recommended Immunization Schedule for Children and Adolescents Aged 18 Years or Younger - United States, 2017. MMWR Morbidity and mortality weekly report. 2017;66(5):134-5.
18. Cullen G, Baden RP, Cheifetz AS. Varicella zoster virus infection in inflammatory bowel disease. Inflammatory bowel diseases. 2012;18(12):2392-403.
19. Card T, West J, Hubbard R, et al. Hip fractures in patients with inflammatory bowel disease and their relationship to corticosteroid use: a population based cohort study. Gut. 2004;53(2):251-5.
20. Casals-Seoane F, Chaparro M, Mate J, et al. Clinical Course of Bone Metabolism Disorders in Patients with Inflammatory Bowel Disease: A 5-Year Prospective Study. Inflammatory bowel diseases. 2016;22(8):1929-36.
21. Melek J, Sakuraba A. Efficacy and safety of medical therapy for low bone mineral density in patients with inflammatory bowel disease: a meta-analysis and systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(1):32-44.e5.
22. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporosis International. 2014;25(10):2359-81.
23. Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology. 2011;141(5):1621-28.e1-5.
24. Long MD, Herfarth HH, Pipkin CA, et al. Increased risk for non-melanoma skin cancer in patients with inflammatory bowel disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2010;8(3):268-74.
25. Scott FI, Mamtani R, Brensinger CM, et al. Risk of Nonmelanoma Skin Cancer Associated With the Use of Immunosuppressant and Biologic Agents in Patients With a History of Autoimmune Disease and Nonmelanoma Skin Cancer. JAMA dermatology. 2016;152(2):164-72.
26. Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143(2):390-9.e1.
27. Allegretti JR, Barnes EL, Cameron A. Are patients with inflammatory bowel disease on chronic immunosuppressive therapy at increased risk of cervical high-grade dysplasia/cancer? A meta-analysis. Inflammatory bowel diseases. 2015;21(5):1089-97.
28. Practice Bulletin No. 168: Cervical Cancer Screening and Prevention. Obstetrics and gynecology. 2016;128(4):e111-30.
29. Ryan WR, Allan RN, Yamamoto T, et al. Crohn’s disease patients who quit smoking have a reduced risk of reoperation for recurrence. American journal of surgery. 2004;187(2):219-25.
30. Cosnes J, Beaugerie L, Carbonnel F, et al. Smoking cessation and the course of Crohn’s disease: an intervention study. Gastroenterology. 2001;120(5):1093-9.
31. Fuller-Thomson E, Sulman J. Depression and inflammatory bowel disease: findings from two nationally representative Canadian surveys. Inflammatory bowel diseases. 2006;12(8):697-707.
32. Walker EA, Gelfand MD, Gelfand AN, et al. The relationship of current psychiatric disorder to functional disability and distress in patients with inflammatory bowel disease. General hospital psychiatry. 1996;18(4):220-9.
33. Mikocka-Walus A, Pittet V, Rossel J-B, et al. Symptoms of Depression and Anxiety Are Independently Associated With Clinical Recurrence of Inflammatory Bowel Disease. Clinical Gastroenterology and Hepatology.14(6):829-35.e1.
34. Macer BJD, Prady SL, Mikocka-Walus A. Antidepressants in Inflammatory Bowel Disease: A Systematic Review. Inflammatory bowel diseases. 2017;23(4):534-50.
Update on the Management of Acute Pancreatitis and Its Complications
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.
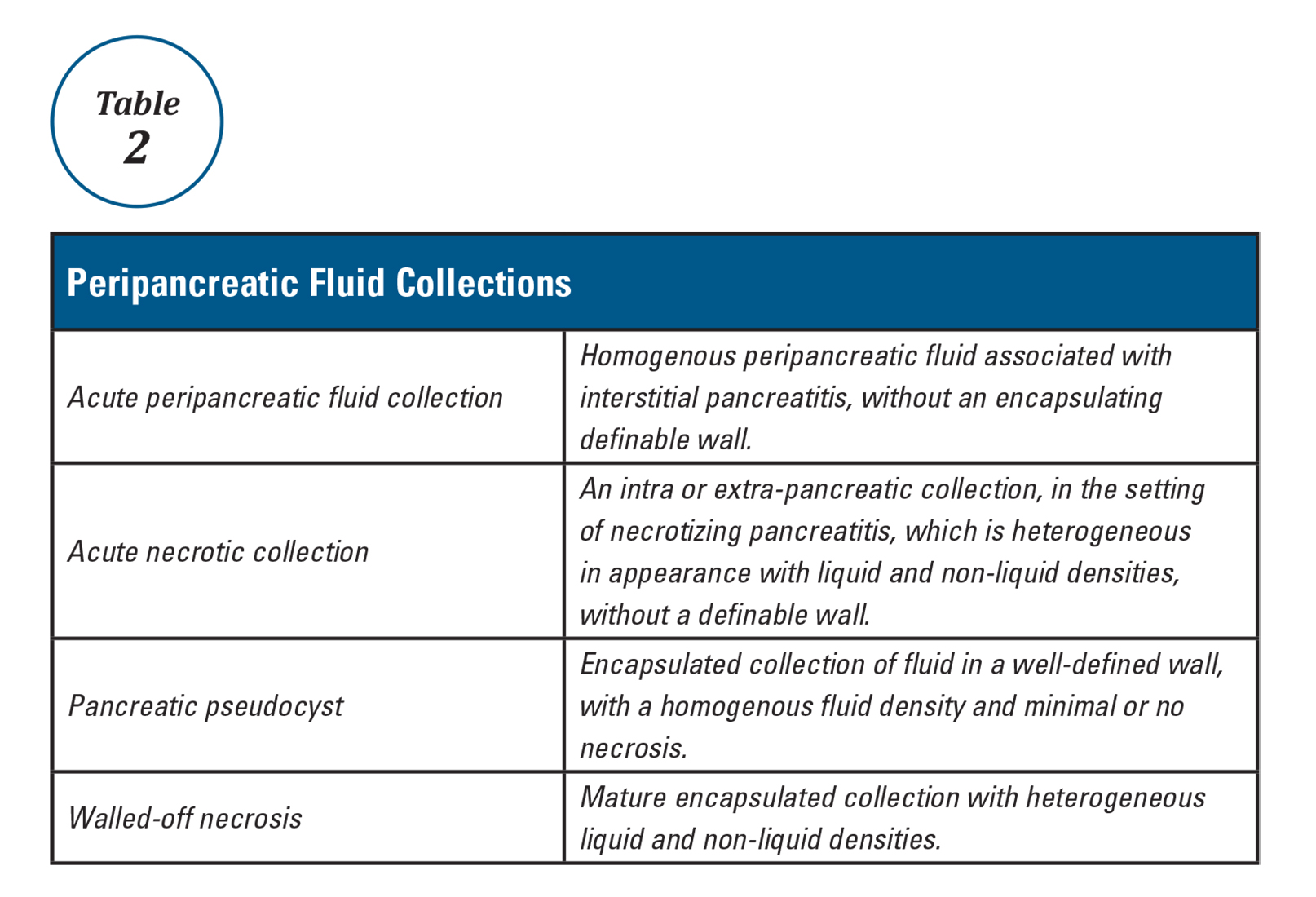
Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.

Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.
Historical perspective
The term “pancreas” derives its name from the Greek words pan (all) and kreas (flesh). Understanding pancreas physiology was first attempted in the 17th century by Regnier de Graaf1. Giovanni Morgagni is credited with the first description of the syndrome of acute pancreatitis (AP) in 17612. Reginald Huber Fitz proposed the first classification of AP into hemorrhagic, gangrenous, and suppurative types in 18893. The distinction of acute from chronic pancreatitis was not well described until the middle of the 20th century when Mandred W. Comfort gave a detailed account of chronic relapsing pancreatitis in 19464.
Diagnosis and classification of severity
The diagnosis of AP is based on the presence of two of the three following criteria: typical abdominal pain (severe, upper abdominal pain frequently radiating to the back), serum amylase and/or lipase levels greater than 3 times the upper limit of normal, and/or characteristic imaging findings.
The original 1992 Atlanta classification provided the first blueprint to standardize how severity of AP was defined5. Over the years, better understanding of AP pathophysiology and its complications led to a greater focus on local and systemic determinants of severity6 and eventually the Revised Atlanta Classification (RAC) in 2013 (Table 1).
Management of acute pancreatitis
Prevention
Determination of etiology
The most common causes of AP are gallstones and alcohol, accounting for more than two-thirds of all cases13. Other etiologies include hypertriglyceridemia, ERCP, drugs induced, familial/hereditary, and post-traumatic. Initial work up includes a thorough history to quantify alcohol consumption and assess for recently started medications, measurement of liver injury tests14 and triglyceride levels, and performance of a transabdominal ultrasound to evaluate for biliary dilation, chole- and choledocholithiasis15.
Assessment of disease severity
Fluid resuscitation
Despite extensive research and trials using medications such as ulinastatin, octreotide, pentoxifylline, gabexate, N-acetyl cysteine, steroids, IL-10, and antibiotics20, no pharmacologic agent has been shown to significantly alter the clinical course/outcomes of AP.
Adequate intravenous hydration remains the cornerstone of early management in AP21. Studies have demonstrated that increased intestinal permeability, secondary to reduced intestinal capillary microcirculation, leads to bacterial translocation and development of SIRS22. Intestinal microcirculation does not become as readily impaired, and there is a certain “latency” to its onset, from the insult that triggers pancreatitis. This gives rise to the concept of a “golden window” of 12-24 hours from the insult to potentially reverse such changes and prevent organ dysfunction. It has been shown that patients who are adequately resuscitated with intravenous fluids have lower risk for local and systemic complications23.
Selecting level of care and ICU management
Patients with predicted severe AP or those with persistent SIRS despite initial fluid resuscitation should be managed in a closely monitored unit, ideally an ICU. Patients with impending respiratory failure require mechanical ventilation, renal failure complicated by metabolic acidosis and/or hyperkalemia requires hemodialysis, and cardiovascular shock requires the initiation of vasopressors and continuous monitoring of blood pressure via an arterial line. A special entity that requires ICU level care is hypertriglyceridemia (HTG)-induced severe AP. HTG should be considered as the etiology of AP in certain clinical scenarios28: previous history of HTG, poorly controlled diabetes mellitus, history of significant alcohol use, third trimester of pregnancy, and use of certain medications associated with HTG such as oral estrogens, tamoxifen, and propofol. Levels of triglyceride greater than 1000 mg/dL strongly point toward HTG being the etiology.
Plasmapheresis, which filters and removes triglycerides from plasma, has been reported as an efficient treatment in such patients based on case series29,30. At this time its use may only be justified in patients with predicted severe AP from HTG, preferably within the first 24 hours of presentation.
Urgent ERCP
Nutrition
Recovery of the gut function is often delayed for several days or weeks in patients with severe AP. Studies have shown that prolonged fasting in such circumstances leads to malnutrition and worse prognosis33,34. Enteral nutrition via a nasogastric (NG) or nasojejunal (NJ) tube is the preferred route of nutritional support, as it is associated with lower risk of infection, multi-organ failure, and mortality when compared to total parenteral nutrition33.
The question of whether NJ feeding offers any additional advantages over NG feeding has not been clearly answered with a recent randomized trial showing NG feeds not to be inferior to NJ feeds35. In regards to the timing of initiation of enteral nutrition, early nasoenteric feeding within 24 hours from presentation was found not to be superior compared to on-demand feeding in patients with predicted severe AP36.
Strategies to decrease risk of recurrent attacks
Management of peripancreatic fluid collections
Patients with AP frequently develop peripancreatic fluid collections (PFCs). Based on the revised Atlanta classification, those are categorized into four types (Table 2, Figures 1-4).
The majority of acute PFCs in patients without evidence of pancreatic necrosis regress within a few weeks and thus intervention is not indicated early in the disease course. Current literature supports delaying the drainage/debridement of such collections for several weeks. The mortality from interventions decreases as the time to intervention from onset of symptoms increases41. Delaying intervention gives more time for recovery from systemic complications and allows the encapsulating wall and contents to organize further.
While surgery is still an option for patients with symptomatic mature PFCs, endoscopic ultrasound-guided drainage in expert hands has been shown to be cost effective, with shorter hospital stay and even decreased risk of cyst recurrence compared with surgical cyst-gastrostomy creation44. Ultrasound or computed tomography-guided drainage of such collections with a percutaneous catheter is an equally efficacious option when compared to the endoscopic approach. However, patients undergoing endotherapy require fewer procedures and imaging studies and shorter length of stay45 when compared with radiological interventions.
Management of pancreatic necrosis
Although this topic has generated much debate, the majority of available evidence shows no clinical benefit from using prophylactic antibiotics to prevent infection in pancreatic necrosis46.

Vascular complications
Vascular complications such as splanchnic vein thrombosis can occur in up to a quarter of AP patients49. Anticoagulation is not usually indicated unless thrombosis is extensive and causes bowel ischemia. Arterial pseudoaneurysms are rare but life threatening complications of AP. They typically require interventional radiology guided coil embolization to prevent massive bleeding50.
Abdominal compartment syndrome
Abdominal compartment syndrome is an end result of third spacing of fluid into the abdominal cavity secondary to inflammation and fluid resuscitation in severe pancreatitis. Abdominal pressure in patients can be monitored by measuring bladder pressures. Intra-abdominal hypertension is defined as a sustained pressure greater than 12 mm Hg, while abdominal compartment syndrome is defined as sustained intra-abdominal pressure greater than 20 mm Hg with new organ failure51. Intra-abdominal hypertension (IAH) is present in up to 75% of patients with severe AP. While all conservative measures to prevent development or worsening of IAH should be implemented (adequate sedation, decompression of bowel in patients with ileus, etc.), current guidelines do not recommend aggressive interventions to treat it. On the other hand, abdominal compartment syndrome is a life-threatening complication that requires urgent intervention to decrease intra-abdominal pressure, such as percutaneous drain placement or surgical fasciotomy52,53.
Conclusion
The key principles in the management of acute pancreatitis are aggressive hydration and preventing development of end organ failure. In the last two decades there has been a paradigm shift in the guidelines for management of peripancreatic fluid collections and pancreatic necrosis. When feasible, drainage of these collections should be delayed and be performed using minimally invasive interventions. There is still an urgent need for developing and testing disease-specific treatments targeting control of the inflammatory response in the early phase of acute pancreatitis and prevention of development of severe disease with end-organ dysfunction.
Dr. Gulati is a gastroenterology and hepatology fellow at Allegheny Health Network, Pittsburgh, and Dr. Papachristou is professor of medicine, University of Pittsburgh School of Medicine, Pittsburgh.
References
1. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, Chapter 55, 923-33.
2. Morgagni G.B. [Fie Books on the Seats and Causes of Diseases as Discovered by the Anatomist]. Venice, Italy: Typographia Remondiniana;1761.
3. Fitz R.H. Boston Med Surg J. 1889;120:181-8.
4. Comfort M., Gambill E., Baggesnstoss A. Gastroenterology. 1946;6:238-76.
5. Bollen T.L., van Santvoort H.C., Besselink M.G., et al. Br J Surg. 2008;95:6–21.
6. Dellinger E.P., Forsmark C.E., Layer P., et al. Ann Surg. 2012 Dec;256[6]:875-80.
7. Kochar B., Akshintala V.S., Afghani E., et al. Gastrointest Endosc. 2015 Jan;81[1]:143-9.
8. Choudhary A., Bechtold M.L., Arif M., et al. Gastrointest Endosc. 2011 Feb;73[2]:275-82.
9. Shi Q.Q., Ning X.Y., Zhan L.L., Tang G.D., Lv X.P. World J Gastroenterol. 2014 Jun 14;20[22]:7040-8.
10. Elmunzer B.J., Waljee A.K., Elta G.H., Taylor J.R., Fehmi S.M., Higgins P.D. Gut. 2008 Sep;57[9]:1262-7.
11. Sethi S., Sethi N., Wadhwa V., Garud S., Brown A. Pancreas. 2014 Mar;43[2]:190-7.
12. Elmunzer B.J., Serrano J., Chak A., et al. Trials. 2016 Mar 3;17[1]:120.
13. Lowenfels A.B., Maisonneuve P., Sullivan T. Curr Gastroenterol Rep. 2009;11:97-103.
14. Agarwal N., Pitchumoni C.S., Sivaprasad A.V. Am J Gastroenterol. 1990;85:356-66.
15. Tenner S., Baillie J., DeWitt J. Vege S.S. Am J Gastroenterol. 2013;108:1400-15.
16. Papachristou G.I., Muddana V., Yadav D., et al. Am J Gastroenterol. 2010;105:435-41.
17. Mounzer R., et al. Gastroenterology 2012;142:1476-82.
18. Working Group IAP/APA Acute Pancreatitis Guidelines. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
19. Koutroumpakis E., Wu B.U., Bakker O.J., et al. Am J Gastroenterol. 2015 Dec;110[12]:1707-16.
20. Bang U.C., Semb S., Nojgaard C., Bendtsen F. World J Gastroenterol. 2008 May 21;14[19]:2968-76.
21. Warndorf M.G., Kurtzman J.T., Bartel M.J., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:705-9.
22. Hotz H.G., Foitzik T., Rohweder J., et al. J Gastrointest Surg. 1998 Nov-Dec;2[6]:518-25.
23. Brown A., Baillargeon J.D., Hughes M.D., et al. Pancreatology 2002;2:104-7.
24. Wu B.U., Hwang J.Q., Gardner T.H., et al. Clin Gastroenterol Hepatol. 2011 Aug;9[8]:710-7.
25. Forsmark C.E., Baillie J., AGA Institute Clinical Practice and Economics Committee, AGA Institute Governing Board. Gastroenterology. 2007 May;132[5]:2022-44.
26. Lankisch P.G., Mahlke R., Blum T., et al. Am J Gastroenterol. 2001;96:2081-5.
27. Wu B.U., Johannes R.S., Sun X., et al. Gastroenterology 2009;137:129-35.
28. Scherer J., Singh V.P., Pitchumoni C.S., Yadav D. J Clin Gastroenterol. 2014 Mar;48[3]:195-203.
29. Gubensek J., Buturovic-Ponikvar J., Romozi K., Ponikvar R. PLoS One. 2014 Jul 21;9[7]:e102748.
30. Chen J.H., Yeh J.H., Lai H.W., Liao C.S. World J Gastroenterol. 2004 Aug 1;10[15]:2272-4.
31. Tse F., Yuan Y. Cochrane Database Syst Rev. 2012 May 16;[5]:CD009779.
32. Folsch U.R., Nitsche R., Ludtke R., et al. N Engl J Med. 1997;336:237-42.
33. Al-Omran M., Albalawi Z.H., Tashkandi M.F., Al-Ansary L.A. Cochrane Database Syst Rev. 2010 Jan 20;[1]:CD002837.
34. Li J.Y., Yu T., Chen G.C., et al. PLoS One. 2013;8[6]:e64926.
35. Singh N., Sharma B., Sharma M., et al. Pancreas. 2012 Jan;41[1]:153-9.
36. Bakker O.J., van Brunschot S., van Santvoort H.C., et al. N Engl J Med. 2014 Nov 20;371[21]:1983-93.
37. Van Baal M.C., Besselink M.G., Bakker O.J., et al. Ann Surg. 2012;255:860–6.
38. Nealon W.H., Bawduniak J., Walser E.M. Ann Surg. 2004 Jun;239[6]:741-9.
39. Sanjay P., Yeeting S., Whigham C., Judson H., Polignano F.M., Tait I.S. Surg Endosc. 2008 Aug;22[8]:1832-7.
40. Nordback I., Pelli H., Lappalainen-Lehto R., Järvinen S., Räty S., Sand J. Gastroenterology. 2009 Mar;136[3]:848-55.
41. Besselink M.G., Verwer T.J., Schoenmaeckers E.J., et al. Arch Surg. 2007;142:1194-201.
42. Besselink M., van Santvoort H., Freeman M. et al. Pancreatology. 2013 Jul-Aug;13(4 Suppl 2):e1-15.
43. Hjalmar C., van Santvoort, H., Besselink M.G., et al. N Engl J Med. 2010;362:1491-502.
44. Varadarajulu S., Bang J.Y., Sutton B.S., et al. Gastroenterology. 2013;145:583-90.e1.
45. Akshintala V.S., Saxena P., Zaheer A., et al. Gastrointest Endosc. 2014 Jun;79[6]:921-8.
46. Jiang K, Huang W, Yang XN., et al. World J Gastroenterol. 2012;18:279–84.
47. Dervenis C., Smailis D., Hatzitheoklitos E. J Hepatobiliary Pancreat Surg. 2003;10[6]:415Y418.
48. Gloor B., Muller C.A., Worni M., et al. Arch Surg. 2001;136[5]:592Y596.
49. Nadkarni N.A., Khanna S., Vege S.S. Pancreas. 2013 Aug;42[6]:924-31.
50. Marshall G.T., Howell D.A., Hansen B.L., Amberson S.M., Abourjaily G.S., Bredenberg C.E. Arch Surg. 1996 Mar;131[3]:278-83.
51. Malbrain M.L., Cheatham M.L., Kirkpatrick A., et al. Intensive Care Med. 2006 Nov;32[11]:1722-32.
52. De Waele J.J. Leppaniemi A.K. World J Surg. 2009;33:1128-33.
53. Kirkpatrick A.W., Roberts D.J., De W.J., et al. Intensive Care Med. 2013 Jul;39[7]1190-206.