User login
Myeloma patients over age 70 can benefit from auto-HC transplant
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
ORLANDO – Age 70 may be the new 60, at least when it comes to outcomes following autologous hematopoietic cell transplantation (auto-HCT) in multiple myeloma.
A large-scale study looking at transplant outcomes across age groups in multiple myeloma patients found similar rates of nonrelapse mortality, relapse/progression, progression-free survival, and overall survival between patients who were aged 70 years and older and those who were aged 60-69 years.
“Age has no implication in terms of the antimyeloma effect of transplant,” Anita D’Souza, MD, of the Medical College of Wisconsin, Milwaukee, said at the annual meeting of the American Society of Hematology.
The study analyzed outcomes from 15,999 multiple myeloma patients aged 20 years or older in the United States who received a single auto-HCT with melphalan conditioning within 12 months of diagnosis between 2013 and 2017. Within that dataset, the researchers compared outcomes from 7,032 patients aged 60-69 years and 2,092 patients aged 70 years and older.
This is the largest study of auto-HCT in older adults with multiple myeloma, the researchers said, and provides important data about the benefit of transplant at any age.
Univariate analysis showed that 100-day nonrelapse mortality was higher in patients aged 70 years and older – at 1% – compared with younger patients (P less than .01). Also, 2-year overall survival was lower in older adults – at 86% – compared with 60- to 69-year-olds (P less than .01).
However, on multivariate analysis with 60- to 69-year-olds as the reference group, patients older than age 70 years had similar nonrelapse mortality (hazard ratio [HR] 1.3, 95% confidence interval [CI] 1, 1.7, P = .06). The same trends were seen for relapse/progression (HR 1.0, 95% CI, 0.9-1, P = .6), progression-free survival (HR 1.1, 95% CI 1-1.2, P = .2), and overall survival (HR 1.2, 95% CI 1-1.4, P = .03). Given the large sample size, a P value of .01 was considered statistically significant.
Over the course of the study period, the percentage of patients aged 70 and older who received a transplant grew each year, rising to 28% by 2017. But Dr. D’Souza said that number is still too low given the safety and efficacy of auto-HCT in these patients.
“Every patient with myeloma should be referred to a transplant center,” she said.
Dr. D’Souza reported financial disclosures related to EDO-Mundapharma, Merck, Prothena, Sanofi, TeneoBio, Prothena, Pfizer, Imbrium, and Akcea. Other study authors reported financial relationships with multiple companies including Celgene, Takeda, BMS, and Janssen.
SOURCE: Munshi PN et al. ASH 2019, Abstract 782.
REPORTING FROM ASH 2019
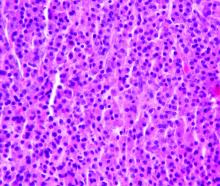
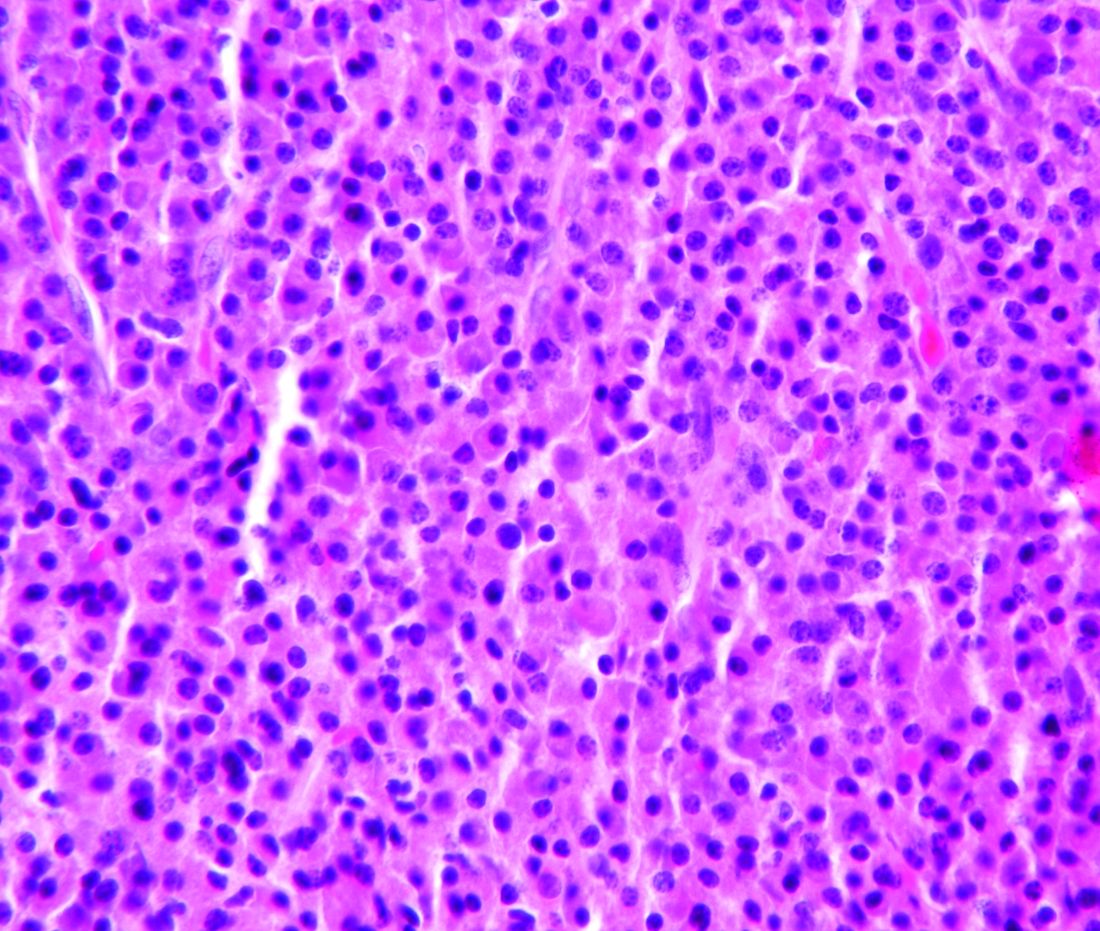
Bispecific CAR T-cells yield high response rate in relapsed/refractory myeloma
ORLANDO – A dual-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated a high overall response rate, a long response duration, and manageable safety in patients with relapsed or refractory multiple myeloma, according to an investigator in a phase 1 study.
The overall response rate exceeded 90%, and about three-quarters of patients remained progression-free at 9 months after treatment with the CAR T-cell therapy, which targets both B-cell maturation antigen (BCMA) and CD38, the study investigator reported.
Grade 3 or greater cytokine release syndrome (CRS) occurred in about one-quarter of the patients, and no neurotoxicity was observed, according to investigator Yu Hu, MD, of Tongji Medical College in Hubei, China.
“,” Dr. Hu said in a press conference.
Short-term relapse has been a “major challenge” with current CAR T-cell therapies currently under investigation for myeloma, most of which target BCMA, according to Dr. Hu.
He said the bispecific CAR T-cell therapy under investigation, known as BM38, was designed to target antigen loss and increase persistence of effector cells. According to the investigator, this was the first study to focus on an anti-BCMA and CD38 dual-targeted CAR T-cell therapy for patients with relapsed or refractory multiple myeloma.
Gary J. Schiller, MD, of UCLA Health, who moderated the press conference, said that while dual-targeting is a potentially “attractive” approach in these hard-to-treat patients, further follow-up is needed to see duration of response and to see if antigen escape re-emerges.
“Cellular therapy is costly, in terms of toxicity as well as financial costs, so you would like to see what the durability of responses is before engaging in that as a late-stage therapy, not to mention moving it up front,” Dr. Schiller said in an interview.
The median progression-free survival (PFS) duration had not been reached at the time of this report, though the 9-month PFS rate was 78.87%, according to the data presented by Dr. Hu.
In the phase 1 study, 22 patients received BM38 CAR T-cell infusions following a fludarabine and cyclophosphamide preconditioning regimen. The median patient age was 59 years, and 50% were male. Nearly three-quarters (72%) had a cytogenetic abnormality, and the median number of prior therapies approached four (range, two to nine prior therapies).
Twenty of the patients (90.9%) had a response: 12 who achieved stringent complete remission, 2 with very good partial response, 5 with partial responses, and 1 with a minimal response.
Of 9 patients with extramedullary disease, 8 achieved partial or complete elimination of tumors, Dr. Hu said in his presentation.
Cytokine release syndrome occurred in 20 patients (90.91%), 5 of whom experienced severe cases (22.73%), according to the reported data. There was no observed neurotoxicity, according to the report, while almost all had hematologic toxicities. Three experienced hepatotoxicity and one had nephrotoxicity, according to Dr. Hu.
The phase 1 study was supported by the National Natural Science Foundation of China, the Major Technological Innovation Special Project Fund of Hubei Province of China, and Cellyan Therapeutics. The senior author of the study was affiliated with Cellyan Therapeutics. Dr. Hu and coauthors reported that they had no relevant conflicts of interest to declare.
SOURCE: Li C et al. ASH 2019. Abstract 930.
ORLANDO – A dual-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated a high overall response rate, a long response duration, and manageable safety in patients with relapsed or refractory multiple myeloma, according to an investigator in a phase 1 study.
The overall response rate exceeded 90%, and about three-quarters of patients remained progression-free at 9 months after treatment with the CAR T-cell therapy, which targets both B-cell maturation antigen (BCMA) and CD38, the study investigator reported.
Grade 3 or greater cytokine release syndrome (CRS) occurred in about one-quarter of the patients, and no neurotoxicity was observed, according to investigator Yu Hu, MD, of Tongji Medical College in Hubei, China.
“,” Dr. Hu said in a press conference.
Short-term relapse has been a “major challenge” with current CAR T-cell therapies currently under investigation for myeloma, most of which target BCMA, according to Dr. Hu.
He said the bispecific CAR T-cell therapy under investigation, known as BM38, was designed to target antigen loss and increase persistence of effector cells. According to the investigator, this was the first study to focus on an anti-BCMA and CD38 dual-targeted CAR T-cell therapy for patients with relapsed or refractory multiple myeloma.
Gary J. Schiller, MD, of UCLA Health, who moderated the press conference, said that while dual-targeting is a potentially “attractive” approach in these hard-to-treat patients, further follow-up is needed to see duration of response and to see if antigen escape re-emerges.
“Cellular therapy is costly, in terms of toxicity as well as financial costs, so you would like to see what the durability of responses is before engaging in that as a late-stage therapy, not to mention moving it up front,” Dr. Schiller said in an interview.
The median progression-free survival (PFS) duration had not been reached at the time of this report, though the 9-month PFS rate was 78.87%, according to the data presented by Dr. Hu.
In the phase 1 study, 22 patients received BM38 CAR T-cell infusions following a fludarabine and cyclophosphamide preconditioning regimen. The median patient age was 59 years, and 50% were male. Nearly three-quarters (72%) had a cytogenetic abnormality, and the median number of prior therapies approached four (range, two to nine prior therapies).
Twenty of the patients (90.9%) had a response: 12 who achieved stringent complete remission, 2 with very good partial response, 5 with partial responses, and 1 with a minimal response.
Of 9 patients with extramedullary disease, 8 achieved partial or complete elimination of tumors, Dr. Hu said in his presentation.
Cytokine release syndrome occurred in 20 patients (90.91%), 5 of whom experienced severe cases (22.73%), according to the reported data. There was no observed neurotoxicity, according to the report, while almost all had hematologic toxicities. Three experienced hepatotoxicity and one had nephrotoxicity, according to Dr. Hu.
The phase 1 study was supported by the National Natural Science Foundation of China, the Major Technological Innovation Special Project Fund of Hubei Province of China, and Cellyan Therapeutics. The senior author of the study was affiliated with Cellyan Therapeutics. Dr. Hu and coauthors reported that they had no relevant conflicts of interest to declare.
SOURCE: Li C et al. ASH 2019. Abstract 930.
ORLANDO – A dual-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated a high overall response rate, a long response duration, and manageable safety in patients with relapsed or refractory multiple myeloma, according to an investigator in a phase 1 study.
The overall response rate exceeded 90%, and about three-quarters of patients remained progression-free at 9 months after treatment with the CAR T-cell therapy, which targets both B-cell maturation antigen (BCMA) and CD38, the study investigator reported.
Grade 3 or greater cytokine release syndrome (CRS) occurred in about one-quarter of the patients, and no neurotoxicity was observed, according to investigator Yu Hu, MD, of Tongji Medical College in Hubei, China.
“,” Dr. Hu said in a press conference.
Short-term relapse has been a “major challenge” with current CAR T-cell therapies currently under investigation for myeloma, most of which target BCMA, according to Dr. Hu.
He said the bispecific CAR T-cell therapy under investigation, known as BM38, was designed to target antigen loss and increase persistence of effector cells. According to the investigator, this was the first study to focus on an anti-BCMA and CD38 dual-targeted CAR T-cell therapy for patients with relapsed or refractory multiple myeloma.
Gary J. Schiller, MD, of UCLA Health, who moderated the press conference, said that while dual-targeting is a potentially “attractive” approach in these hard-to-treat patients, further follow-up is needed to see duration of response and to see if antigen escape re-emerges.
“Cellular therapy is costly, in terms of toxicity as well as financial costs, so you would like to see what the durability of responses is before engaging in that as a late-stage therapy, not to mention moving it up front,” Dr. Schiller said in an interview.
The median progression-free survival (PFS) duration had not been reached at the time of this report, though the 9-month PFS rate was 78.87%, according to the data presented by Dr. Hu.
In the phase 1 study, 22 patients received BM38 CAR T-cell infusions following a fludarabine and cyclophosphamide preconditioning regimen. The median patient age was 59 years, and 50% were male. Nearly three-quarters (72%) had a cytogenetic abnormality, and the median number of prior therapies approached four (range, two to nine prior therapies).
Twenty of the patients (90.9%) had a response: 12 who achieved stringent complete remission, 2 with very good partial response, 5 with partial responses, and 1 with a minimal response.
Of 9 patients with extramedullary disease, 8 achieved partial or complete elimination of tumors, Dr. Hu said in his presentation.
Cytokine release syndrome occurred in 20 patients (90.91%), 5 of whom experienced severe cases (22.73%), according to the reported data. There was no observed neurotoxicity, according to the report, while almost all had hematologic toxicities. Three experienced hepatotoxicity and one had nephrotoxicity, according to Dr. Hu.
The phase 1 study was supported by the National Natural Science Foundation of China, the Major Technological Innovation Special Project Fund of Hubei Province of China, and Cellyan Therapeutics. The senior author of the study was affiliated with Cellyan Therapeutics. Dr. Hu and coauthors reported that they had no relevant conflicts of interest to declare.
SOURCE: Li C et al. ASH 2019. Abstract 930.
REPORTING FROM ASH 2019
High complete response rate seen with novel CAR-T for myeloma
ORLANDO – A novel chimeric antigen receptor T (CAR T) cell construct is associated with deep clinical responses in patients with multiple myeloma for whom prior lines of therapy – some numbering in the double digits – have failed.
Among 29 patients with multiple myeloma enrolled in a phase 1b/2 trial of JNJ-4528, the overall response rate (ORR) at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression at a median of 6 months, reported Deepu Madduri, MD, of Icahn School of Medicine at Mount Sinai, New York.
“These are very heavily pretreated patients, and so getting early and deep responses is quite amazing,” she said at a briefing prior to presentation of the data at the annual meeting of the American Society of Hematology.
JNJ-4528 is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). As previously reported, an identical CAR T cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma. JNJ-4528 was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration on Dec. 6, 2019, and a priority medicines (PRIME) designation by the European Medicines Agency in April 2019.
BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells. Several research groups are currently investigating CAR T cells or monoclonal antibodies targeted to BCMA. The product closest to receiving FDA approval is likely BB2121.
At ASH 2019, Dr. Madduri presented results from the phase 1b portion of the CARTITUDE-1 trial. The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least 3 prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug (IMiD), and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
A total of 29 patients, median age 60, were evaluable for the safety and efficacy endpoints. One-fourth of the patients had a high-risk cytogenetic profile. The patients had received a median of 5 prior lines of therapy, with one patient receiving 18 prior lines. Of the 29 patients, 25 (86%) had previously undergone autologous transplantation.
As noted before, the ORR after a median follow-up of 6 months was 100%, with 69% completer responses, 17% very good partial responses, and 14% partial responses. The median time to complete response was 1 month (range 1 to 9 months). All but two patients remained free of disease progression at the median 6-month follow-up.
Nearly all patients (27) developed cytokine release syndrome (CRS), and one patient with prolonged grade 4 CRS died from related complications 99 days after infusion.
The median time to onset of CRS was 7 days with more than 90% of cases occurring between days 5 and 9.
Neurotoxicities, specifically immune effector cell–associated neurotoxicity syndrome (ICANS), were infrequent in CRS, and when they did occur were generally low grade, with only 1 grade 3 ICANS event.
Asked in an interview whether the impressive response rates seen with JNJ-4528 might persist over time, Dr. Madduri acknowledged that follow-up is still relatively short.
“This product is unique in that has a CD8 central memory phenotype preferentially, and we’re hoping that this would play a central role in the durability of response because they’re memory cells, but I think at this time we don’t know,” she said.
The CARTITUDE-1 trial is funded by Janssen Research & Development. Dr. Madduri disclosed serving as a consultant to Janssen and to Takeda, Foundation Medicine, AbbVie, and Celgene.
SOURCE: Madduri D et al. ASH 2019. Abstract 577.
ORLANDO – A novel chimeric antigen receptor T (CAR T) cell construct is associated with deep clinical responses in patients with multiple myeloma for whom prior lines of therapy – some numbering in the double digits – have failed.
Among 29 patients with multiple myeloma enrolled in a phase 1b/2 trial of JNJ-4528, the overall response rate (ORR) at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression at a median of 6 months, reported Deepu Madduri, MD, of Icahn School of Medicine at Mount Sinai, New York.
“These are very heavily pretreated patients, and so getting early and deep responses is quite amazing,” she said at a briefing prior to presentation of the data at the annual meeting of the American Society of Hematology.
JNJ-4528 is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). As previously reported, an identical CAR T cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma. JNJ-4528 was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration on Dec. 6, 2019, and a priority medicines (PRIME) designation by the European Medicines Agency in April 2019.
BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells. Several research groups are currently investigating CAR T cells or monoclonal antibodies targeted to BCMA. The product closest to receiving FDA approval is likely BB2121.
At ASH 2019, Dr. Madduri presented results from the phase 1b portion of the CARTITUDE-1 trial. The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least 3 prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug (IMiD), and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
A total of 29 patients, median age 60, were evaluable for the safety and efficacy endpoints. One-fourth of the patients had a high-risk cytogenetic profile. The patients had received a median of 5 prior lines of therapy, with one patient receiving 18 prior lines. Of the 29 patients, 25 (86%) had previously undergone autologous transplantation.
As noted before, the ORR after a median follow-up of 6 months was 100%, with 69% completer responses, 17% very good partial responses, and 14% partial responses. The median time to complete response was 1 month (range 1 to 9 months). All but two patients remained free of disease progression at the median 6-month follow-up.
Nearly all patients (27) developed cytokine release syndrome (CRS), and one patient with prolonged grade 4 CRS died from related complications 99 days after infusion.
The median time to onset of CRS was 7 days with more than 90% of cases occurring between days 5 and 9.
Neurotoxicities, specifically immune effector cell–associated neurotoxicity syndrome (ICANS), were infrequent in CRS, and when they did occur were generally low grade, with only 1 grade 3 ICANS event.
Asked in an interview whether the impressive response rates seen with JNJ-4528 might persist over time, Dr. Madduri acknowledged that follow-up is still relatively short.
“This product is unique in that has a CD8 central memory phenotype preferentially, and we’re hoping that this would play a central role in the durability of response because they’re memory cells, but I think at this time we don’t know,” she said.
The CARTITUDE-1 trial is funded by Janssen Research & Development. Dr. Madduri disclosed serving as a consultant to Janssen and to Takeda, Foundation Medicine, AbbVie, and Celgene.
SOURCE: Madduri D et al. ASH 2019. Abstract 577.
ORLANDO – A novel chimeric antigen receptor T (CAR T) cell construct is associated with deep clinical responses in patients with multiple myeloma for whom prior lines of therapy – some numbering in the double digits – have failed.
Among 29 patients with multiple myeloma enrolled in a phase 1b/2 trial of JNJ-4528, the overall response rate (ORR) at 6 months median follow-up was 100%, including 69% complete responses, with 27 patients remaining free of disease progression at a median of 6 months, reported Deepu Madduri, MD, of Icahn School of Medicine at Mount Sinai, New York.
“These are very heavily pretreated patients, and so getting early and deep responses is quite amazing,” she said at a briefing prior to presentation of the data at the annual meeting of the American Society of Hematology.
JNJ-4528 is a second-generation CAR T containing two single-domain antibodies targeted against B-cell maturation protein (BCMA). As previously reported, an identical CAR T cell construct showed a high overall response with manageable toxicities in 74 patients with relapsed/refractory multiple myeloma. JNJ-4528 was granted a breakthrough therapy designation for relapsed/refractory multiple myeloma by the Food and Drug Administration on Dec. 6, 2019, and a priority medicines (PRIME) designation by the European Medicines Agency in April 2019.
BCMA was first described in myeloma in 2004 as a mechanism for the growth and survival of malignant plasma cells. Several research groups are currently investigating CAR T cells or monoclonal antibodies targeted to BCMA. The product closest to receiving FDA approval is likely BB2121.
At ASH 2019, Dr. Madduri presented results from the phase 1b portion of the CARTITUDE-1 trial. The investigators enrolled patients with multiple myeloma with measurable diseases as assessed by M-protein or serum free light chain levels who had experienced disease progression on at least 3 prior lines of therapy, or whose disease was refractory to at least two lines of therapy with a proteasome inhibitor, immunomodulatory drug (IMiD), and an anti-CD38 antibody.
Patients underwent apheresis for T-cell collection, with bridging therapy allowed until the expanded T cells could be delivered.
Following T-cell depletion with cyclophosphamide 300 mg/m2 and fludarabine 30 mg/m2 over 3 days, patients received a single weight-based infusion (compared with fixed-dose infusions used with other CAR T cell constructs).
The dose was targeted at 0.75x106 CAR-positive cells/kg, with a target range of 0.5–1.0x106, administered 5-7 days after the start of the conditioning regimen.
A total of 29 patients, median age 60, were evaluable for the safety and efficacy endpoints. One-fourth of the patients had a high-risk cytogenetic profile. The patients had received a median of 5 prior lines of therapy, with one patient receiving 18 prior lines. Of the 29 patients, 25 (86%) had previously undergone autologous transplantation.
As noted before, the ORR after a median follow-up of 6 months was 100%, with 69% completer responses, 17% very good partial responses, and 14% partial responses. The median time to complete response was 1 month (range 1 to 9 months). All but two patients remained free of disease progression at the median 6-month follow-up.
Nearly all patients (27) developed cytokine release syndrome (CRS), and one patient with prolonged grade 4 CRS died from related complications 99 days after infusion.
The median time to onset of CRS was 7 days with more than 90% of cases occurring between days 5 and 9.
Neurotoxicities, specifically immune effector cell–associated neurotoxicity syndrome (ICANS), were infrequent in CRS, and when they did occur were generally low grade, with only 1 grade 3 ICANS event.
Asked in an interview whether the impressive response rates seen with JNJ-4528 might persist over time, Dr. Madduri acknowledged that follow-up is still relatively short.
“This product is unique in that has a CD8 central memory phenotype preferentially, and we’re hoping that this would play a central role in the durability of response because they’re memory cells, but I think at this time we don’t know,” she said.
The CARTITUDE-1 trial is funded by Janssen Research & Development. Dr. Madduri disclosed serving as a consultant to Janssen and to Takeda, Foundation Medicine, AbbVie, and Celgene.
SOURCE: Madduri D et al. ASH 2019. Abstract 577.
REPORTING FROM ASH 2019
ASH preview: Key themes include tackling CAR T obstacles, sickle cell advances, VTE
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Chimeric antigen receptor (CAR) T-cell therapies have garnered a great deal of attention given their “incredible efficacy” in treating B-cell malignancies, and new findings are taking aim at the drawbacks of therapy, such as the time, expense, and toxicity involved, according to Robert A. Brodsky, MD.
One example, from a study slated for presentation during a plenary session at the upcoming annual meeting of the American Society of Hematology involves the investigational T-cell bispecific antibody mosunetuzumab, which targets both CD20 on the surface of malignant B cells, and CD3 on cytotoxic T cells, engaging the T cells and directing their cytotoxicity against B cells.
In a study (Abstract 6) of 218 non-Hodgkin lymphoma patients, including 23 who had already received CAR T-cell therapy and had relapsed or were refractory to the treatment, 64% responded, 42% had a complete response, and the median duration of response is now out to 9 months, Dr. Brodsky, ASH secretary and director of the division of hematology at Johns Hopkins University, Baltimore, said during a premeeting press conference.
“It’s basically an antibody using the patient’s own T cell to do what a CAR-T cell would do – [a] very exciting study and large study,” he said. “It is an off-the-shelf product, it completely gets around the problem of the time to generate the CAR T-cell product, and because it’s going to be much simpler and faster to produce, it’s likely going to be much cheaper than CAR T cells.”
The preliminary results also suggest it is less toxic than CAR T-cell therapy, he added.
Two other CAR T-cell therapy–related studies highlighted during the press conference address its use for multiple myeloma. One, the phase 1b/2 CARTITUDE study (Abstract 577) uses CAR T cells against the B-cell maturation antigen (BCMA) in the relapsed/refractory setting.
Of 25 patients treated with chemotherapy followed by CAR T-cell infusion and followed for a median of 3 months, 91% responded, two achieved a complete remission, and “many other responses were very deep responses,” Dr. Brodsky said, noting that the second featured multiple myeloma trial (Abstract 930) looked at bispecific CAR T-cell therapy targeting BCMA and CD38 in an effort to reduce resistance to the therapy.
“Again, very interesting preliminary results,” he said, noting that of 16 patients followed for a median of 36 weeks, 87.5% responded, the treatment was well tolerated, and progression-free survival at 9 months was 75%.
In addition to the “key theme” of overcoming CAR T-cell therapy obstacles, three other themes have emerged from among the thousands of abstracts submitted for presentation at ASH. These, as presented during the press conference, include new venous thromboembolism (VTE) therapies and approaches to research; inclusive medicine, with abstracts focused on age- and race-related issues in clinical trials; and new advances in the treatment of sickle cell disease. All of these have potentially practice-changing implications, as do the six late-breaking abstracts selected from 93 abstracts submitted for consideration for oral presentation at ASH, Dr. Brodsky said.
One of the “truly practice-changing” late-breakers is a randomized phase 3 trial (Abstract LBA-1) comparing the bispecific antibody blinatumomab to chemotherapy for post-re-induction therapy in high- and intermediate-risk acute lymphoblastic leukemia (ALL) at first relapse in children, adolescents and young adults.
The study demonstrated the superiority of blinatumomab for efficacy and tolerability, which is particularly encouraging given the challenges in getting relapsed ALL patients back into remission so they can undergo bone marrow transplant, Dr. Brodsky said.
Of 208 patients randomized, 73% vs. 45% in the blinatumomab vs. chemotherapy arms were able to get to transplant – and therefore to potential cure, he said.
“Of note, the blinatumomab arm was less toxic and there was marked improvement in disease-free and overall survival, so this is clearly going to become a new standard of care for relapsed and refractory ALL,” he added.
Early lenalidomide may delay progression of smoldering myeloma
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
Early treatment with lenalidomide may delay disease progression and prevent end-organ damage in patients with high-risk smoldering multiple myeloma (SMM), according to findings from a phase 3 trial.
While observation is the current standard of care in SMM, early therapy may represent a new standard for patients with high-risk disease, explained Sagar Lonial, MD, of Winship Cancer Institute, Emory University, Atlanta, and colleagues. Their findings were published in the Journal of Clinical Oncology.
The randomized, open-label, phase 3 study included 182 patients with intermediate- or high-risk SMM. Study patients were randomly allocated to receive either oral lenalidomide at 25 mg daily on days 1-21 of a 28-day cycle or observation.
Study subjects were stratified based on time since SMM diagnosis – 1 year or less vs. more than 1 year, and all patients in the lenalidomide arm received aspirin at 325 mg on days 1-28. Both interventions were maintained until unacceptable toxicity, disease progression, or withdrawal for other reasons.
The primary outcome was progression-free survival (PFS), measured from baseline to the development of symptomatic multiple myeloma (MM). The criteria for progression included evidence of end-organ damage in relation to MM and biochemical disease progression.
The researchers found that at 1 year PFS was 98% in the lenalidomide group and 89% in the observation group. At 2 years, PFS was 93% in the lenalidomide group and 76% in the observation group. PFS was 91% in the lenalidomide group and 66% in the observation group at 3 years (hazard ratio, 0.28; P = .002).
Among lenalidomide-treated patients, grade 3 or 4 hematologic and nonhematologic adverse events occurred in 36 patients (41%). Nonhematologic adverse events occurred in 25 patients (28%).
Frequent AEs among lenalidomide-treated patients included grade 4 decreased neutrophil count (4.5%), as well as grade 3 infections (20.5%), hypertension (9.1%), fatigue (6.8%), skin problems (5.7%), dyspnea (5.7%), and hypokalemia (3.4%). “In most cases, [adverse events] could be managed with dose modifications,” they wrote.
To reduce long-term toxicity, the researchers recommended a 2-year duration of therapy for patients at highest risk.
“Our results support the use of early intervention in patients with high-risk SMM – as defined by the 20/2/20 criteria where our magnitude of benefit was the greatest – rather than continued observation,” the researchers wrote.
The trial was funded by the National Cancer Institute. The authors reported financial affiliations with AbbVie, Aduro Biotech, Amgen, Bristol-Myers Squibb, Celgene, Juno Therapeutics, Kite Pharma, Sanofi, Takeda, and several other companies.
SOURCE: Lonial S et al. J Clin Oncol. 2019 Oct 25. doi: 10.1200/JCO.19.01740.
FROM JOURNAL OF CLINICAL ONCOLOGY
Foundation launches direct-to-patient registry in multiple myeloma
The Multiple Myeloma Research Foundation (MMRF) recently launched its Direct-to-Patient registry, in what the organization’s leaders are describing as a “disruptive” step toward improving outcomes for patients with multiple myeloma.
The new registry is intended to build upon CoMMpass, a program started 8 years ago that now represents the largest genomic database of any type of cancer. Although CoMMpass includes data from about 1,150 patients with myeloma, it’s not enough information, according to the chief marketing and development officer at the MMRF, Anne Quinn Young.
“For a disease as heterogenous as myeloma is, we need more, particularly because we don’t have all the samples for later-stage disease,” Ms. Quinn Young said in an interview. “And even with the clinical data, given the patient population, both [in terms of] demographics and the nature of the disease, the numbers of patients still living after multiple relapses is rather small.”
In an earlier effort to gather more data, the MMRF first turned to other organizations for help, but this approach fell short because of scarcity of data, and in some cases, unwillingness to share. Steven Labkoff, MD, chief data officer at the MMRF, described this experience in an interview.
“When the MMRF was looking around for different data sources for myeloma data, it was always the claim that, ‘Sure, we have plenty of patients, we have plenty of data, and it’s rich and really complete.’ However, as we approached an array of organizations – big organizations – as we dug into the details and reviewed patient counts or data completeness, they either didn’t have a sufficient number of patients, they didn’t have sufficiently complete data for our needs, and in the case where some did have sufficient numbers and complete data sets, they simply weren’t in a position to share that data outside their institution,” Dr. Labkoff said.
Undeterred, the MMRF switched tactics to the current, patient-centric approach.
“We’re leveraging one aspect of the HIPAA legislation,” Dr. Labkoff said, referring to patients’ rights to request their own medical records and an institution’s legal obligation to provide those records.
In the short-term, the registry will collect three types of data: patient donated data (answers from a patient survey), electronic medical records abstracted from all relevant past providers, and genomic test results. Participating patients will have blood drawn at home by a phlebotomist for the genomic assay. Additional tubes of blood will be concurrently collected and biobanked. This will eventually allow for immune profiling, Dr. Labkoff said.
Future goals include a patient-reported outcomes module and the ability to link data with medical claims.
So far, 79 patients have participated in the pilot program, according to the MMRF. As the database builds, Ms. Quinn Young and Dr. Labkoff anticipate that it will yield answers to a variety of real-world questions.
Dr. Labkoff offered two examples. “Of all the patients who have been exposed to ‘name your drug,’ what were the costs of their therapy, and what were the outcomes?” he said. In addition, researchers will be able to query clinical trial inclusion criteria to search for data on a specific patient profile, such as patients with a 4:14 translocation, who have had a bone marrow transplant in the last 2 years, and have been exposed to a certain drug regimen.
Ms. Quinn Young noted that doctors may be able to use the database to reliably identify high-risk patients and guide agent selection. Common patient questions also will be addressed, she said, including best treatment regimens for certain types of patients.
“For patients who may have run out of all commercially available options, or for patients who are perhaps seen at a community center, where certainly this type of profiling is not standard, it’s opening up a whole new set of options for them,” Ms. Quinn Young said. “And if their physician doesn’t pursue those options, they have the report that they can use to seek a second opinion.”
The Direct-to-Patient registry is unique because it aims to empower patients in a way that hasn’t been done before, Ms. Quinn Young said. “We are committed ... ever since we conceived of this project, to giving results back to patients. That is disruptive because right now that doesn’t exist.”
But the cost of implementing the registry, which has an approximate budget of $20 million, stands in the way of a completely free flow of anonymized data. MMRF leaders are exploring different strategies to sustain funding for the program.
Another MMRF program, CoMMpass, uses a precompetitive consortium model, in which several pharmaceutical companies pay for a preview of the data 6 months in advance of nonprofit researchers. A similar model may be used with the Direct-to-Patient registry, but this has yet to be determined, according to Dr. Labkoff and Ms. Quinn Young.
For now, Ms. Quinn Young said she hopes that physicians will be receptive to the program. “[The short term goal is that] when patients come to their doctors asking about this, that there is support and open-mindedness,” she said.
Looking to the future, Dr. Labkoff described how the registry could accelerate myeloma research, ultimately toward a cure.
“It is generally accepted that it can take 17 years to get something – a therapy, a new drug, or a guideline – from the bench to the bedside,” he said. “It’s my hope that we can take next generation sequencing and the results of this registry and bend that curve, maybe ... to 10 [years], or very aggressively, to 7 or 5 [years], where doctors are able to use the information in these reports for the patients that have literally given themselves, and use this to help guide the choices of their therapy or the trials they apply for, to help them get a better outcome in general.”
The Direct-to-Patient registry is a collaborative effort between the MMRF and multiple organizations, including the health care technology company COTA, the Broad Institute of Harvard and MIT, Prometheus Research, Tempus, and the Dana Farber Cancer Institute.
The Multiple Myeloma Research Foundation (MMRF) recently launched its Direct-to-Patient registry, in what the organization’s leaders are describing as a “disruptive” step toward improving outcomes for patients with multiple myeloma.
The new registry is intended to build upon CoMMpass, a program started 8 years ago that now represents the largest genomic database of any type of cancer. Although CoMMpass includes data from about 1,150 patients with myeloma, it’s not enough information, according to the chief marketing and development officer at the MMRF, Anne Quinn Young.
“For a disease as heterogenous as myeloma is, we need more, particularly because we don’t have all the samples for later-stage disease,” Ms. Quinn Young said in an interview. “And even with the clinical data, given the patient population, both [in terms of] demographics and the nature of the disease, the numbers of patients still living after multiple relapses is rather small.”
In an earlier effort to gather more data, the MMRF first turned to other organizations for help, but this approach fell short because of scarcity of data, and in some cases, unwillingness to share. Steven Labkoff, MD, chief data officer at the MMRF, described this experience in an interview.
“When the MMRF was looking around for different data sources for myeloma data, it was always the claim that, ‘Sure, we have plenty of patients, we have plenty of data, and it’s rich and really complete.’ However, as we approached an array of organizations – big organizations – as we dug into the details and reviewed patient counts or data completeness, they either didn’t have a sufficient number of patients, they didn’t have sufficiently complete data for our needs, and in the case where some did have sufficient numbers and complete data sets, they simply weren’t in a position to share that data outside their institution,” Dr. Labkoff said.
Undeterred, the MMRF switched tactics to the current, patient-centric approach.
“We’re leveraging one aspect of the HIPAA legislation,” Dr. Labkoff said, referring to patients’ rights to request their own medical records and an institution’s legal obligation to provide those records.
In the short-term, the registry will collect three types of data: patient donated data (answers from a patient survey), electronic medical records abstracted from all relevant past providers, and genomic test results. Participating patients will have blood drawn at home by a phlebotomist for the genomic assay. Additional tubes of blood will be concurrently collected and biobanked. This will eventually allow for immune profiling, Dr. Labkoff said.
Future goals include a patient-reported outcomes module and the ability to link data with medical claims.
So far, 79 patients have participated in the pilot program, according to the MMRF. As the database builds, Ms. Quinn Young and Dr. Labkoff anticipate that it will yield answers to a variety of real-world questions.
Dr. Labkoff offered two examples. “Of all the patients who have been exposed to ‘name your drug,’ what were the costs of their therapy, and what were the outcomes?” he said. In addition, researchers will be able to query clinical trial inclusion criteria to search for data on a specific patient profile, such as patients with a 4:14 translocation, who have had a bone marrow transplant in the last 2 years, and have been exposed to a certain drug regimen.
Ms. Quinn Young noted that doctors may be able to use the database to reliably identify high-risk patients and guide agent selection. Common patient questions also will be addressed, she said, including best treatment regimens for certain types of patients.
“For patients who may have run out of all commercially available options, or for patients who are perhaps seen at a community center, where certainly this type of profiling is not standard, it’s opening up a whole new set of options for them,” Ms. Quinn Young said. “And if their physician doesn’t pursue those options, they have the report that they can use to seek a second opinion.”
The Direct-to-Patient registry is unique because it aims to empower patients in a way that hasn’t been done before, Ms. Quinn Young said. “We are committed ... ever since we conceived of this project, to giving results back to patients. That is disruptive because right now that doesn’t exist.”
But the cost of implementing the registry, which has an approximate budget of $20 million, stands in the way of a completely free flow of anonymized data. MMRF leaders are exploring different strategies to sustain funding for the program.
Another MMRF program, CoMMpass, uses a precompetitive consortium model, in which several pharmaceutical companies pay for a preview of the data 6 months in advance of nonprofit researchers. A similar model may be used with the Direct-to-Patient registry, but this has yet to be determined, according to Dr. Labkoff and Ms. Quinn Young.
For now, Ms. Quinn Young said she hopes that physicians will be receptive to the program. “[The short term goal is that] when patients come to their doctors asking about this, that there is support and open-mindedness,” she said.
Looking to the future, Dr. Labkoff described how the registry could accelerate myeloma research, ultimately toward a cure.
“It is generally accepted that it can take 17 years to get something – a therapy, a new drug, or a guideline – from the bench to the bedside,” he said. “It’s my hope that we can take next generation sequencing and the results of this registry and bend that curve, maybe ... to 10 [years], or very aggressively, to 7 or 5 [years], where doctors are able to use the information in these reports for the patients that have literally given themselves, and use this to help guide the choices of their therapy or the trials they apply for, to help them get a better outcome in general.”
The Direct-to-Patient registry is a collaborative effort between the MMRF and multiple organizations, including the health care technology company COTA, the Broad Institute of Harvard and MIT, Prometheus Research, Tempus, and the Dana Farber Cancer Institute.
The Multiple Myeloma Research Foundation (MMRF) recently launched its Direct-to-Patient registry, in what the organization’s leaders are describing as a “disruptive” step toward improving outcomes for patients with multiple myeloma.
The new registry is intended to build upon CoMMpass, a program started 8 years ago that now represents the largest genomic database of any type of cancer. Although CoMMpass includes data from about 1,150 patients with myeloma, it’s not enough information, according to the chief marketing and development officer at the MMRF, Anne Quinn Young.
“For a disease as heterogenous as myeloma is, we need more, particularly because we don’t have all the samples for later-stage disease,” Ms. Quinn Young said in an interview. “And even with the clinical data, given the patient population, both [in terms of] demographics and the nature of the disease, the numbers of patients still living after multiple relapses is rather small.”
In an earlier effort to gather more data, the MMRF first turned to other organizations for help, but this approach fell short because of scarcity of data, and in some cases, unwillingness to share. Steven Labkoff, MD, chief data officer at the MMRF, described this experience in an interview.
“When the MMRF was looking around for different data sources for myeloma data, it was always the claim that, ‘Sure, we have plenty of patients, we have plenty of data, and it’s rich and really complete.’ However, as we approached an array of organizations – big organizations – as we dug into the details and reviewed patient counts or data completeness, they either didn’t have a sufficient number of patients, they didn’t have sufficiently complete data for our needs, and in the case where some did have sufficient numbers and complete data sets, they simply weren’t in a position to share that data outside their institution,” Dr. Labkoff said.
Undeterred, the MMRF switched tactics to the current, patient-centric approach.
“We’re leveraging one aspect of the HIPAA legislation,” Dr. Labkoff said, referring to patients’ rights to request their own medical records and an institution’s legal obligation to provide those records.
In the short-term, the registry will collect three types of data: patient donated data (answers from a patient survey), electronic medical records abstracted from all relevant past providers, and genomic test results. Participating patients will have blood drawn at home by a phlebotomist for the genomic assay. Additional tubes of blood will be concurrently collected and biobanked. This will eventually allow for immune profiling, Dr. Labkoff said.
Future goals include a patient-reported outcomes module and the ability to link data with medical claims.
So far, 79 patients have participated in the pilot program, according to the MMRF. As the database builds, Ms. Quinn Young and Dr. Labkoff anticipate that it will yield answers to a variety of real-world questions.
Dr. Labkoff offered two examples. “Of all the patients who have been exposed to ‘name your drug,’ what were the costs of their therapy, and what were the outcomes?” he said. In addition, researchers will be able to query clinical trial inclusion criteria to search for data on a specific patient profile, such as patients with a 4:14 translocation, who have had a bone marrow transplant in the last 2 years, and have been exposed to a certain drug regimen.
Ms. Quinn Young noted that doctors may be able to use the database to reliably identify high-risk patients and guide agent selection. Common patient questions also will be addressed, she said, including best treatment regimens for certain types of patients.
“For patients who may have run out of all commercially available options, or for patients who are perhaps seen at a community center, where certainly this type of profiling is not standard, it’s opening up a whole new set of options for them,” Ms. Quinn Young said. “And if their physician doesn’t pursue those options, they have the report that they can use to seek a second opinion.”
The Direct-to-Patient registry is unique because it aims to empower patients in a way that hasn’t been done before, Ms. Quinn Young said. “We are committed ... ever since we conceived of this project, to giving results back to patients. That is disruptive because right now that doesn’t exist.”
But the cost of implementing the registry, which has an approximate budget of $20 million, stands in the way of a completely free flow of anonymized data. MMRF leaders are exploring different strategies to sustain funding for the program.
Another MMRF program, CoMMpass, uses a precompetitive consortium model, in which several pharmaceutical companies pay for a preview of the data 6 months in advance of nonprofit researchers. A similar model may be used with the Direct-to-Patient registry, but this has yet to be determined, according to Dr. Labkoff and Ms. Quinn Young.
For now, Ms. Quinn Young said she hopes that physicians will be receptive to the program. “[The short term goal is that] when patients come to their doctors asking about this, that there is support and open-mindedness,” she said.
Looking to the future, Dr. Labkoff described how the registry could accelerate myeloma research, ultimately toward a cure.
“It is generally accepted that it can take 17 years to get something – a therapy, a new drug, or a guideline – from the bench to the bedside,” he said. “It’s my hope that we can take next generation sequencing and the results of this registry and bend that curve, maybe ... to 10 [years], or very aggressively, to 7 or 5 [years], where doctors are able to use the information in these reports for the patients that have literally given themselves, and use this to help guide the choices of their therapy or the trials they apply for, to help them get a better outcome in general.”
The Direct-to-Patient registry is a collaborative effort between the MMRF and multiple organizations, including the health care technology company COTA, the Broad Institute of Harvard and MIT, Prometheus Research, Tempus, and the Dana Farber Cancer Institute.
Multiple Myeloma: A New Treatment Option for Newly Diagnosed, Transplant-Ineligible Patients
Levofloxacin prophylaxis improves survival in newly diagnosed myeloma
Adding levofloxacin to antimyeloma therapy improved survival and reduced infections in patients with newly diagnosed myeloma, findings from a phase 3 trial suggest.
The advantages of levofloxacin prophylaxis appear to offset the potential risks in patients with newly diagnosed disease, explained Mark T. Drayson, MBChB, PhD, of the University of Birmingham (England) and colleagues. The study was published in the Lancet Oncology.
The randomized, placebo-controlled, phase 3 TEAMM study enrolled 977 patients with newly diagnosed myeloma. The effects of antimicrobial prophylaxis on infection risk and infection-related mortality were evaluated across 93 hospitals throughout the United Kingdom.
Study patients were randomly assigned to receive 500 mg of oral levofloxacin once daily or placebo for a total of 12 weeks. If applicable, dose adjustments were made based on estimated glomerular filtration rate.
At baseline, the team collected stool samples and nasal swabs, and follow-up assessment occurred every 4 weeks for up to 1 year. The primary endpoint was time to death (all causes) or first febrile event from the start of prophylactic therapy to 12 weeks.
After a median follow-up of 12 months, first febrile episodes or deaths were significantly lower for patients in the levofloxacin arm (19%), compared with the placebo arm (27%) for a hazard ratio for time to first event of 0.66 (95% confidence interval, 0.51-0.86; P = .0018).
With respect to safety, the rates of serious adverse events were similar between the study arms, with the exception of tendinitis in the levofloxacin group (1%). Among all patients, a total of 597 serious toxicities were observed from baseline to 16 weeks (52% in the levofloxacin arm vs. 48% in the placebo arm).
“To our knowledge, this is the first time that the use of prophylactic antibiotics has shown a survival benefit in patients with newly diagnosed myeloma,” the researchers reported.
One key limitation of the study was the younger patient population relative to the general population. As a result, differences in survival estimates could exist between the trial and real-world populations, they noted.
“Patients with newly diagnosed myeloma could benefit from levofloxacin prophylaxis, although local antibiotic resistance proportions must be considered,” the researchers cautioned.
The study was funded by the National Institute for Health Research in the United Kingdom. The authors reported financial affiliations with Actelion, Astellas, Celgene, Gilead, Janssen, Pfizer, Takeda, and other companies.
SOURCE: Drayson MT et al. Lancet Oncol. 2019 Oct 23. doi: 10.1016/S1470-2045(19)30506-6.
Adding levofloxacin to antimyeloma therapy improved survival and reduced infections in patients with newly diagnosed myeloma, findings from a phase 3 trial suggest.
The advantages of levofloxacin prophylaxis appear to offset the potential risks in patients with newly diagnosed disease, explained Mark T. Drayson, MBChB, PhD, of the University of Birmingham (England) and colleagues. The study was published in the Lancet Oncology.
The randomized, placebo-controlled, phase 3 TEAMM study enrolled 977 patients with newly diagnosed myeloma. The effects of antimicrobial prophylaxis on infection risk and infection-related mortality were evaluated across 93 hospitals throughout the United Kingdom.
Study patients were randomly assigned to receive 500 mg of oral levofloxacin once daily or placebo for a total of 12 weeks. If applicable, dose adjustments were made based on estimated glomerular filtration rate.
At baseline, the team collected stool samples and nasal swabs, and follow-up assessment occurred every 4 weeks for up to 1 year. The primary endpoint was time to death (all causes) or first febrile event from the start of prophylactic therapy to 12 weeks.
After a median follow-up of 12 months, first febrile episodes or deaths were significantly lower for patients in the levofloxacin arm (19%), compared with the placebo arm (27%) for a hazard ratio for time to first event of 0.66 (95% confidence interval, 0.51-0.86; P = .0018).
With respect to safety, the rates of serious adverse events were similar between the study arms, with the exception of tendinitis in the levofloxacin group (1%). Among all patients, a total of 597 serious toxicities were observed from baseline to 16 weeks (52% in the levofloxacin arm vs. 48% in the placebo arm).
“To our knowledge, this is the first time that the use of prophylactic antibiotics has shown a survival benefit in patients with newly diagnosed myeloma,” the researchers reported.
One key limitation of the study was the younger patient population relative to the general population. As a result, differences in survival estimates could exist between the trial and real-world populations, they noted.
“Patients with newly diagnosed myeloma could benefit from levofloxacin prophylaxis, although local antibiotic resistance proportions must be considered,” the researchers cautioned.
The study was funded by the National Institute for Health Research in the United Kingdom. The authors reported financial affiliations with Actelion, Astellas, Celgene, Gilead, Janssen, Pfizer, Takeda, and other companies.
SOURCE: Drayson MT et al. Lancet Oncol. 2019 Oct 23. doi: 10.1016/S1470-2045(19)30506-6.
Adding levofloxacin to antimyeloma therapy improved survival and reduced infections in patients with newly diagnosed myeloma, findings from a phase 3 trial suggest.
The advantages of levofloxacin prophylaxis appear to offset the potential risks in patients with newly diagnosed disease, explained Mark T. Drayson, MBChB, PhD, of the University of Birmingham (England) and colleagues. The study was published in the Lancet Oncology.
The randomized, placebo-controlled, phase 3 TEAMM study enrolled 977 patients with newly diagnosed myeloma. The effects of antimicrobial prophylaxis on infection risk and infection-related mortality were evaluated across 93 hospitals throughout the United Kingdom.
Study patients were randomly assigned to receive 500 mg of oral levofloxacin once daily or placebo for a total of 12 weeks. If applicable, dose adjustments were made based on estimated glomerular filtration rate.
At baseline, the team collected stool samples and nasal swabs, and follow-up assessment occurred every 4 weeks for up to 1 year. The primary endpoint was time to death (all causes) or first febrile event from the start of prophylactic therapy to 12 weeks.
After a median follow-up of 12 months, first febrile episodes or deaths were significantly lower for patients in the levofloxacin arm (19%), compared with the placebo arm (27%) for a hazard ratio for time to first event of 0.66 (95% confidence interval, 0.51-0.86; P = .0018).
With respect to safety, the rates of serious adverse events were similar between the study arms, with the exception of tendinitis in the levofloxacin group (1%). Among all patients, a total of 597 serious toxicities were observed from baseline to 16 weeks (52% in the levofloxacin arm vs. 48% in the placebo arm).
“To our knowledge, this is the first time that the use of prophylactic antibiotics has shown a survival benefit in patients with newly diagnosed myeloma,” the researchers reported.
One key limitation of the study was the younger patient population relative to the general population. As a result, differences in survival estimates could exist between the trial and real-world populations, they noted.
“Patients with newly diagnosed myeloma could benefit from levofloxacin prophylaxis, although local antibiotic resistance proportions must be considered,” the researchers cautioned.
The study was funded by the National Institute for Health Research in the United Kingdom. The authors reported financial affiliations with Actelion, Astellas, Celgene, Gilead, Janssen, Pfizer, Takeda, and other companies.
SOURCE: Drayson MT et al. Lancet Oncol. 2019 Oct 23. doi: 10.1016/S1470-2045(19)30506-6.
FROM LANCET ONCOLOGY
SEER analysis reveals medication adherence factors in newly diagnosed myeloma
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
Black race, polypharmacy, and increasing age were associated with poor adherence to lenalidomide in older patients with newly-diagnosed multiple myeloma, according to an analysis of Surveillance, Epidemiology, and End Results (SEER)–Medicare linked data.
The objective of the study was to examine factors affecting adherence in older adults who received lenalidomide.
Of 793 patients diagnosed and treated between 2007 and 2014, 302 (38%) had poor adherence to lenalidomide, reported Hira Mian, MD, of McMaster University, Hamilton, Ont., and colleagues. The findings were published in Clinical Lymphoma, Myeloma & Leukemia.
The researchers studied patients 65 years and older who had received at least two lenalidomide prescriptions in the first year following diagnosis. Only patients who filled a prescription for lenalidomide within 60 days of a myeloma diagnosis were included.
The median age of the patients was 73 years; 43% were aged 75 years or older. Most of the patients included in the analysis were white.
The medication possession ratio, defined as the “ratio of the number of days the patient had pills in their possession to the number of days in the observation period,” was used to evaluate adherence to therapy. A ratio of less than 90% was deemed poor adherence by the researchers.
After analysis, the researchers found that black race (adjusted odds ratio, 1.72; P = .022), polypharmacy (aOR, 1.04 per drug; P = .008), and increasing age (aOR, 1.03 per year; P = .024) were all significantly associated with poor adherence to lenalidomide.
The mean medication possession ratio among study patients was 89.5%. Overall, 38% of patients in the study had poor adherence to lenalidomide, while just 7% of patients in the study had a medication possession ratio of 100%, indicating “perfect adherence.”
There was a trend toward inferior overall survival among patients with poor adherence to lenalidomide, but it was not statistically significant (hazard ratio 1.10, 95% confidence interval, 0.88-1.38).
“Our study emphasizes the need for both better clinical monitoring of adherence and for future prospective studies in accurately understanding the rates and predictors of adherence while simultaneously developing strategies for improving adherence for patients that are at high risk of nonadherence,” the researchers wrote.
The National Institutes of Health funded the study. No conflicts of interest were reported.
SOURCE: Mian H et al. Clin Lymphoma Myeloma Leuk. 2019 Oct 9. doi: 10.1016/j.clml.2019.09.618.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
What is the optimal duration of maintenance in myeloma?
SAN FRANCISCO – Should patients with multiple myeloma receive maintenance therapy until progression?
Yvonne A. Efebera, MD, of The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital in Columbus, and Nina Shah, MD, of the University of California San Francisco Health, faced off on this question at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Shah said maintenance therapy improves survival in myeloma patients, so it follows that treating them until progression would confer a survival advantage. While Dr. Efebera agreed that maintenance can improve survival, she said the optimal duration of that treatment is unknown.
Treat until progression
Dr. Shah cited studies suggesting that maintenance improves progression-free survival (PFS) and may prolong overall survival (OS) in multiple myeloma.
A meta-analysis of data from the IFM 2005-02, CALGB 100104, and GIMEMA RV-MM-PI-209 trials showed that lenalidomide maintenance prolonged PFS and OS. The median PFS was 52.8 months in patients who received maintenance and 23.5 months in those who received placebo or observation (hazard ratio [HR], 0.48). At a median follow-up of 79.5 months, the median OS was not reached for the maintenance group and was 86.0 months for the no-maintenance group (HR, 0.75; P = .001; J Clin Oncol. 2017 Oct 10;35[29]:3279-89).
In the Myeloma XI trial, maintenance improved PFS, but not OS, in both transplant-eligible and ineligible patients. Overall, the median PFS was 39 months in the lenalidomide maintenance arm and 20 months in the observation arm (P less than .0001). Among transplant-eligible patients, the median PFS was 57 months and 30 months, respectively (P less than .0001). Among transplant-ineligible patients, the median PFS was 26 months and 11 months, respectively (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:57-73).
These data suggest maintenance can improve survival, “but the question is, how long should we have therapy,” Dr. Shah said. “No one has looked at this in a prospective manner, so we really have to look at our retrospective data.”
One study suggested a longer duration of lenalidomide maintenance improves PFS. The HR for progression or death was 0.39 for patients who received maintenance for 12-24 months, compared with those who received maintenance for less than 12 months. The HR was 0.13 for patients who received maintenance for more than 24 months, compared with less than 12 months (Leuk Lymphoma. 2019 Feb;60[2]:511-4).
Dr. Shah also cited a pooled analysis of three phase 3 trials suggesting that continuous therapy is superior to fixed-duration therapy in patients with newly diagnosed myeloma. The median PFS was 32 months with continuous therapy and 16 months with fixed-duration therapy (P less than .001). The 4-year OS was 69% and 60%, respectively (P = .003; J Clin Oncol. 2015 Oct 20;33[30]:3459-66).
These data suggest that “continuous therapy, more therapy, has a survival advantage,” Dr. Shah said.
Don’t treat until progression
Dr. Efebera also discussed data from studies showing that lenalidomide maintenance can prolong survival in multiple myeloma. However, she said, it’s unclear how long maintenance should last.
Different durations of maintenance have proved effective in different trials. In the CALGB 100104 trial, the median duration of maintenance was 31 months (Lancet Haematol. 2017 Sep;4[9]:e431-e442). In the meta-analysis of the CALGB, IFM, and GIMEMA trials, the median duration was 22 months. And in Myeloma XI, the median duration was 18 months.
As there is no randomized trial comparing different durations of maintenance, Dr. Efebera proposed that researchers conduct one. She said this “perfect study” would involve induction with an immunomodulatory agent, a proteasome inhibitor, dexamethasone, and perhaps an anti-CD38 therapy. Transplant-eligible patients would receive four cycles of induction before transplant. Transplant-ineligible patients would receive eight cycles of induction. Then, all patients would be randomized to lenalidomide maintenance for 3 years, 5 years, or 7-10 years.
Until a trial like this reveals the optimal duration of maintenance, we cannot conclude that treating patients until progression is better, Dr. Efebera said.
She added that maintenance has been shown to have detrimental effects, and these should be taken into consideration. For instance, neutropenia, other hematologic adverse events, and second primary malignancies have been shown to be more common among patients who receive lenalidomide maintenance (N Engl J Med. 2012; 366:1782-91).
The cost of maintenance is another factor to consider. Researchers analyzed data from the CALGB 100104 and IFM 2005-02 trials to compare the cost of lenalidomide maintenance with no maintenance. In the CALGB 100104 trial, patients who received lenalidomide maintenance had 5.72 quality-adjusted life years (QALYs), and those who received no maintenance had 4.61 QALYs. The incremental cost-utility ratio (ICUR) was more than 277,000 euros per QALY.
In the IFM2005-02 trial, patients in the lenalidomide group had 5.13 QALYs, and those who didn’t receive maintenance had 4.98 QALYs. The ICUR was more than 1.5 million euros per QALY. The researchers said the high ICURs and budgetary impact add “uncertainty about the maximum prudent duration of the treatment” (Bone Marrow Transplant. 2019 May 31. doi: 10.1038/s41409-019-0574-5).
Dr. Efebera reported relationships with Akcea Therapeutics, Janssen, and Takeda. Dr. Shah reported having no relevant financial relationships.
SAN FRANCISCO – Should patients with multiple myeloma receive maintenance therapy until progression?
Yvonne A. Efebera, MD, of The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital in Columbus, and Nina Shah, MD, of the University of California San Francisco Health, faced off on this question at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Shah said maintenance therapy improves survival in myeloma patients, so it follows that treating them until progression would confer a survival advantage. While Dr. Efebera agreed that maintenance can improve survival, she said the optimal duration of that treatment is unknown.
Treat until progression
Dr. Shah cited studies suggesting that maintenance improves progression-free survival (PFS) and may prolong overall survival (OS) in multiple myeloma.
A meta-analysis of data from the IFM 2005-02, CALGB 100104, and GIMEMA RV-MM-PI-209 trials showed that lenalidomide maintenance prolonged PFS and OS. The median PFS was 52.8 months in patients who received maintenance and 23.5 months in those who received placebo or observation (hazard ratio [HR], 0.48). At a median follow-up of 79.5 months, the median OS was not reached for the maintenance group and was 86.0 months for the no-maintenance group (HR, 0.75; P = .001; J Clin Oncol. 2017 Oct 10;35[29]:3279-89).
In the Myeloma XI trial, maintenance improved PFS, but not OS, in both transplant-eligible and ineligible patients. Overall, the median PFS was 39 months in the lenalidomide maintenance arm and 20 months in the observation arm (P less than .0001). Among transplant-eligible patients, the median PFS was 57 months and 30 months, respectively (P less than .0001). Among transplant-ineligible patients, the median PFS was 26 months and 11 months, respectively (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:57-73).
These data suggest maintenance can improve survival, “but the question is, how long should we have therapy,” Dr. Shah said. “No one has looked at this in a prospective manner, so we really have to look at our retrospective data.”
One study suggested a longer duration of lenalidomide maintenance improves PFS. The HR for progression or death was 0.39 for patients who received maintenance for 12-24 months, compared with those who received maintenance for less than 12 months. The HR was 0.13 for patients who received maintenance for more than 24 months, compared with less than 12 months (Leuk Lymphoma. 2019 Feb;60[2]:511-4).
Dr. Shah also cited a pooled analysis of three phase 3 trials suggesting that continuous therapy is superior to fixed-duration therapy in patients with newly diagnosed myeloma. The median PFS was 32 months with continuous therapy and 16 months with fixed-duration therapy (P less than .001). The 4-year OS was 69% and 60%, respectively (P = .003; J Clin Oncol. 2015 Oct 20;33[30]:3459-66).
These data suggest that “continuous therapy, more therapy, has a survival advantage,” Dr. Shah said.
Don’t treat until progression
Dr. Efebera also discussed data from studies showing that lenalidomide maintenance can prolong survival in multiple myeloma. However, she said, it’s unclear how long maintenance should last.
Different durations of maintenance have proved effective in different trials. In the CALGB 100104 trial, the median duration of maintenance was 31 months (Lancet Haematol. 2017 Sep;4[9]:e431-e442). In the meta-analysis of the CALGB, IFM, and GIMEMA trials, the median duration was 22 months. And in Myeloma XI, the median duration was 18 months.
As there is no randomized trial comparing different durations of maintenance, Dr. Efebera proposed that researchers conduct one. She said this “perfect study” would involve induction with an immunomodulatory agent, a proteasome inhibitor, dexamethasone, and perhaps an anti-CD38 therapy. Transplant-eligible patients would receive four cycles of induction before transplant. Transplant-ineligible patients would receive eight cycles of induction. Then, all patients would be randomized to lenalidomide maintenance for 3 years, 5 years, or 7-10 years.
Until a trial like this reveals the optimal duration of maintenance, we cannot conclude that treating patients until progression is better, Dr. Efebera said.
She added that maintenance has been shown to have detrimental effects, and these should be taken into consideration. For instance, neutropenia, other hematologic adverse events, and second primary malignancies have been shown to be more common among patients who receive lenalidomide maintenance (N Engl J Med. 2012; 366:1782-91).
The cost of maintenance is another factor to consider. Researchers analyzed data from the CALGB 100104 and IFM 2005-02 trials to compare the cost of lenalidomide maintenance with no maintenance. In the CALGB 100104 trial, patients who received lenalidomide maintenance had 5.72 quality-adjusted life years (QALYs), and those who received no maintenance had 4.61 QALYs. The incremental cost-utility ratio (ICUR) was more than 277,000 euros per QALY.
In the IFM2005-02 trial, patients in the lenalidomide group had 5.13 QALYs, and those who didn’t receive maintenance had 4.98 QALYs. The ICUR was more than 1.5 million euros per QALY. The researchers said the high ICURs and budgetary impact add “uncertainty about the maximum prudent duration of the treatment” (Bone Marrow Transplant. 2019 May 31. doi: 10.1038/s41409-019-0574-5).
Dr. Efebera reported relationships with Akcea Therapeutics, Janssen, and Takeda. Dr. Shah reported having no relevant financial relationships.
SAN FRANCISCO – Should patients with multiple myeloma receive maintenance therapy until progression?
Yvonne A. Efebera, MD, of The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital in Columbus, and Nina Shah, MD, of the University of California San Francisco Health, faced off on this question at the National Comprehensive Cancer Network Hematologic Malignancies Annual Congress.
Dr. Shah said maintenance therapy improves survival in myeloma patients, so it follows that treating them until progression would confer a survival advantage. While Dr. Efebera agreed that maintenance can improve survival, she said the optimal duration of that treatment is unknown.
Treat until progression
Dr. Shah cited studies suggesting that maintenance improves progression-free survival (PFS) and may prolong overall survival (OS) in multiple myeloma.
A meta-analysis of data from the IFM 2005-02, CALGB 100104, and GIMEMA RV-MM-PI-209 trials showed that lenalidomide maintenance prolonged PFS and OS. The median PFS was 52.8 months in patients who received maintenance and 23.5 months in those who received placebo or observation (hazard ratio [HR], 0.48). At a median follow-up of 79.5 months, the median OS was not reached for the maintenance group and was 86.0 months for the no-maintenance group (HR, 0.75; P = .001; J Clin Oncol. 2017 Oct 10;35[29]:3279-89).
In the Myeloma XI trial, maintenance improved PFS, but not OS, in both transplant-eligible and ineligible patients. Overall, the median PFS was 39 months in the lenalidomide maintenance arm and 20 months in the observation arm (P less than .0001). Among transplant-eligible patients, the median PFS was 57 months and 30 months, respectively (P less than .0001). Among transplant-ineligible patients, the median PFS was 26 months and 11 months, respectively (P less than .0001; Lancet Oncol. 2019 Jan;20[1]:57-73).
These data suggest maintenance can improve survival, “but the question is, how long should we have therapy,” Dr. Shah said. “No one has looked at this in a prospective manner, so we really have to look at our retrospective data.”
One study suggested a longer duration of lenalidomide maintenance improves PFS. The HR for progression or death was 0.39 for patients who received maintenance for 12-24 months, compared with those who received maintenance for less than 12 months. The HR was 0.13 for patients who received maintenance for more than 24 months, compared with less than 12 months (Leuk Lymphoma. 2019 Feb;60[2]:511-4).
Dr. Shah also cited a pooled analysis of three phase 3 trials suggesting that continuous therapy is superior to fixed-duration therapy in patients with newly diagnosed myeloma. The median PFS was 32 months with continuous therapy and 16 months with fixed-duration therapy (P less than .001). The 4-year OS was 69% and 60%, respectively (P = .003; J Clin Oncol. 2015 Oct 20;33[30]:3459-66).
These data suggest that “continuous therapy, more therapy, has a survival advantage,” Dr. Shah said.
Don’t treat until progression
Dr. Efebera also discussed data from studies showing that lenalidomide maintenance can prolong survival in multiple myeloma. However, she said, it’s unclear how long maintenance should last.
Different durations of maintenance have proved effective in different trials. In the CALGB 100104 trial, the median duration of maintenance was 31 months (Lancet Haematol. 2017 Sep;4[9]:e431-e442). In the meta-analysis of the CALGB, IFM, and GIMEMA trials, the median duration was 22 months. And in Myeloma XI, the median duration was 18 months.
As there is no randomized trial comparing different durations of maintenance, Dr. Efebera proposed that researchers conduct one. She said this “perfect study” would involve induction with an immunomodulatory agent, a proteasome inhibitor, dexamethasone, and perhaps an anti-CD38 therapy. Transplant-eligible patients would receive four cycles of induction before transplant. Transplant-ineligible patients would receive eight cycles of induction. Then, all patients would be randomized to lenalidomide maintenance for 3 years, 5 years, or 7-10 years.
Until a trial like this reveals the optimal duration of maintenance, we cannot conclude that treating patients until progression is better, Dr. Efebera said.
She added that maintenance has been shown to have detrimental effects, and these should be taken into consideration. For instance, neutropenia, other hematologic adverse events, and second primary malignancies have been shown to be more common among patients who receive lenalidomide maintenance (N Engl J Med. 2012; 366:1782-91).
The cost of maintenance is another factor to consider. Researchers analyzed data from the CALGB 100104 and IFM 2005-02 trials to compare the cost of lenalidomide maintenance with no maintenance. In the CALGB 100104 trial, patients who received lenalidomide maintenance had 5.72 quality-adjusted life years (QALYs), and those who received no maintenance had 4.61 QALYs. The incremental cost-utility ratio (ICUR) was more than 277,000 euros per QALY.
In the IFM2005-02 trial, patients in the lenalidomide group had 5.13 QALYs, and those who didn’t receive maintenance had 4.98 QALYs. The ICUR was more than 1.5 million euros per QALY. The researchers said the high ICURs and budgetary impact add “uncertainty about the maximum prudent duration of the treatment” (Bone Marrow Transplant. 2019 May 31. doi: 10.1038/s41409-019-0574-5).
Dr. Efebera reported relationships with Akcea Therapeutics, Janssen, and Takeda. Dr. Shah reported having no relevant financial relationships.
EXPERT ANALYSIS FROM NCCN HEMATOLOGIC MALIGNANCIES