User login
What to do after basal insulin: 3 Tx strategies for type 2 diabetes
› Intensify diabetes treatment for patients who have a normal fasting glucose, but an HbA1c >7% and daytime hyperglycemia, and for those who are not at goal despite basal insulin doses >0.5 units/kg/d. B
› Consider intensifying diabetes management beyond basal insulin therapy by adding a glucagon-like peptide 1 receptor agonist, insulin prior to one meal each day, or insulin prior to all meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetes mellitus is a complex, progressive disease that affects every family physician’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every 2 to 3 months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many family physicians have become comfortable with using once-daily basal insulin such as glargine or detemir, what to do after basal insulin is much more complex. This review builds upon an earlier article in this journal, “Insulin for type 2 diabetes: How and when to get started,”3 by explaining 3 strategies to consider when basal insulin alone isn't enough.
3 main strategies for intensifying treatment
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated hemoglobin A1c (HbA1c) despite using 2 or more oral agents, or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial HbA1c levels >7% or >8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk of hypoglycemia, effects on cardiovascular risk factors, and other non-glycemic effects should be considered in the shared decision-making process. There are 3 main strategies for intensifying treatment:
1. Basal plus incretin therapy. Add a newer injectable agent such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
2. Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
3. Basal-bolus combination. Add insulin prior to all meals.
TABLE 15-8 provides details of several studies that have documented the efficacy of these 3 strategies.
CLICK IMAGE TO ENLARGE
Monitoring blood glucose to guide the way
Blood glucose monitoring using either a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for 3 meals a day and an additional bedtime reading.9 This is typically performed for 3 to 7 days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to 2 to 3 weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
Basal plus incretin therapy
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
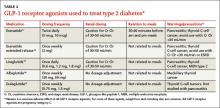
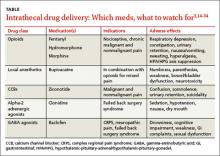
GLP-1RAs can be considered as an add-on therapy for patients whose HbA1c exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL, or for patients with a basal insulin dose >0.5 unit/kg/d. The 5 currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in TABLE 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance <30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Basal plus one strategy
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The “delta” value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤50 mg/dL.17 If the delta for a meal is >75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of <130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the post-meal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 unit per kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one16 (or 10%-20% of TDD per meal for basal-bolus).
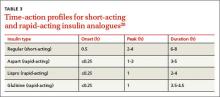
Consider the timing of administration. Rapid-acting insulin analogs exhibit peak pharmacodynamic activity 60 minutes after injection (TABLE 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analog should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 unit/d to a goal of either a 90-minute to 2-hour postprandial glucose of <140 to 180 mg/dL or the next preprandial glucose of <130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of <50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of <50 mg/dL.
Basal-bolus combination
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and physician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen, and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk of hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of <70 mg/dL, and severe hypoglycemia as <50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia, and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for 2 to 3 weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk of hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, hypoglycemia, injection site reactions and, rarely, allergy to insulin or its vehicle.16
CORRESPONDENCE
Jay Shubrook, DO, FAAF P, FACOF P, BC-ADM, Touro University College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected]
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook, J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014; 63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two singledose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management--exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin – Options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. Monthly Prescribing Reference Web site. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Eng J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Family Physician. 2014;1:19-25.
› Intensify diabetes treatment for patients who have a normal fasting glucose, but an HbA1c >7% and daytime hyperglycemia, and for those who are not at goal despite basal insulin doses >0.5 units/kg/d. B
› Consider intensifying diabetes management beyond basal insulin therapy by adding a glucagon-like peptide 1 receptor agonist, insulin prior to one meal each day, or insulin prior to all meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetes mellitus is a complex, progressive disease that affects every family physician’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every 2 to 3 months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many family physicians have become comfortable with using once-daily basal insulin such as glargine or detemir, what to do after basal insulin is much more complex. This review builds upon an earlier article in this journal, “Insulin for type 2 diabetes: How and when to get started,”3 by explaining 3 strategies to consider when basal insulin alone isn't enough.
3 main strategies for intensifying treatment
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated hemoglobin A1c (HbA1c) despite using 2 or more oral agents, or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial HbA1c levels >7% or >8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk of hypoglycemia, effects on cardiovascular risk factors, and other non-glycemic effects should be considered in the shared decision-making process. There are 3 main strategies for intensifying treatment:
1. Basal plus incretin therapy. Add a newer injectable agent such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
2. Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
3. Basal-bolus combination. Add insulin prior to all meals.
TABLE 15-8 provides details of several studies that have documented the efficacy of these 3 strategies.
CLICK IMAGE TO ENLARGE
Monitoring blood glucose to guide the way
Blood glucose monitoring using either a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for 3 meals a day and an additional bedtime reading.9 This is typically performed for 3 to 7 days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to 2 to 3 weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
Basal plus incretin therapy
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
GLP-1RAs can be considered as an add-on therapy for patients whose HbA1c exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL, or for patients with a basal insulin dose >0.5 unit/kg/d. The 5 currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in TABLE 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance <30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Basal plus one strategy
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The “delta” value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤50 mg/dL.17 If the delta for a meal is >75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of <130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the post-meal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 unit per kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one16 (or 10%-20% of TDD per meal for basal-bolus).
Consider the timing of administration. Rapid-acting insulin analogs exhibit peak pharmacodynamic activity 60 minutes after injection (TABLE 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analog should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 unit/d to a goal of either a 90-minute to 2-hour postprandial glucose of <140 to 180 mg/dL or the next preprandial glucose of <130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of <50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of <50 mg/dL.
Basal-bolus combination
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and physician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen, and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk of hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of <70 mg/dL, and severe hypoglycemia as <50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia, and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for 2 to 3 weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk of hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, hypoglycemia, injection site reactions and, rarely, allergy to insulin or its vehicle.16
CORRESPONDENCE
Jay Shubrook, DO, FAAF P, FACOF P, BC-ADM, Touro University College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected]
› Intensify diabetes treatment for patients who have a normal fasting glucose, but an HbA1c >7% and daytime hyperglycemia, and for those who are not at goal despite basal insulin doses >0.5 units/kg/d. B
› Consider intensifying diabetes management beyond basal insulin therapy by adding a glucagon-like peptide 1 receptor agonist, insulin prior to one meal each day, or insulin prior to all meals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Diabetes mellitus is a complex, progressive disease that affects every family physician’s practice. Major diabetes organizations recommend that treatment be ongoing and progressive in order to control the disease. The American Diabetes Association (ADA), the European Association for the Study of Diabetes (EASD), and the American Association of Clinical Endocrinologists recommend that patients be assessed every 2 to 3 months after diagnosis and that treatment should be intensified if the patient is not meeting treatment goals.1,2 Using this approach, all people with type 2 diabetes could be on insulin one year after diagnosis.1,2
While many family physicians have become comfortable with using once-daily basal insulin such as glargine or detemir, what to do after basal insulin is much more complex. This review builds upon an earlier article in this journal, “Insulin for type 2 diabetes: How and when to get started,”3 by explaining 3 strategies to consider when basal insulin alone isn't enough.
3 main strategies for intensifying treatment
Basal insulin is indicated for patients who have glucose toxicity and persistently elevated hemoglobin A1c (HbA1c) despite using 2 or more oral agents, or for those who have not achieved glucose goals one year into treatment.3,4 ADA/EASD recommends initiating a weight-based approach for basal insulin therapy based on initial HbA1c levels >7% or >8%.4 Instructing and encouraging patients to titrate their own insulin dose based on fasting glucose readings provides greater and faster glucose control.1,2
Despite these attempts, some patients will not reach their glucose goals with basal insulin. When intensifying treatment beyond basal insulin therapy, patient preference, cost-effectiveness, safety, tolerability, glycemic efficacy, risk of hypoglycemia, effects on cardiovascular risk factors, and other non-glycemic effects should be considered in the shared decision-making process. There are 3 main strategies for intensifying treatment:
1. Basal plus incretin therapy. Add a newer injectable agent such as a glucagon-like peptide 1 receptor agonist (GLP-1RA).
2. Basal plus one strategy. Add prandial insulin prior to the largest meal of the day.
3. Basal-bolus combination. Add insulin prior to all meals.
TABLE 15-8 provides details of several studies that have documented the efficacy of these 3 strategies.
CLICK IMAGE TO ENLARGE
Monitoring blood glucose to guide the way
Blood glucose monitoring using either a 7-point glucose monitoring technique or staggered glucose checks should guide insulin intensification. A 7-point glucose profile includes pre-meal and post-meal readings for 3 meals a day and an additional bedtime reading.9 This is typically performed for 3 to 7 days prior to an appointment and provides an estimate of a typical full day’s glucose pattern.
Staggered monitoring includes a pair of glucose checks taken immediately before and typically 90 minutes after a meal. This is assigned to a different meal each day in order to obtain the same information as is achieved with 7-point monitoring, but with fewer checks on any given day. It may take up to 2 to 3 weeks to gather the necessary information using the staggered monitoring technique.
In order to optimize insulin strategies for tighter glycemic control, it is important to review blood glucose logs at each office visit with either of the above techniques.
Basal plus incretin therapy
GLP-1RAs are subcutaneously administered injectable incretin agents. They mimic the action of endogenous GLP-1 hormones, which are normally secreted in response to meals by the cells of the small intestine.10 GLP-1 stimulates glucose-dependent insulin secretion, suppresses postprandial glucagon release from pancreatic alpha cells, signals satiety, and slows gastric emptying.10 In other words, GLP-1 appears to be a physiologic regulator of appetite and food intake. GLP-1 is rapidly metabolized and inactivated by dipeptidyl peptidase-4 (DPP-4) enzymes.10 The amplification of insulin secretion elicited by hormones secreted from the gastrointestinal (GI) tract is called the “incretin effect.”10 Obesity, insulin resistance, and type 2 diabetes greatly reduce the incretin effect.10
GLP-1RAs mimic the incretin effect and are not degraded by endogenous DPP-4 enzymes.10 They provide a pharmacologic level of GLP-1 activity, including beneficial glucose effects (via insulin secretion and glucagon suppression), but they also increase GI adverse effects, such as nausea and vomiting.11-15 Further, they can suppress appetite and contribute to weight loss.11-15
GLP-1RAs can be considered as an add-on therapy for patients whose HbA1c exceeds 7% and whose fasting blood glucose ranges from 80 to 130 mg/dL, or for patients with a basal insulin dose >0.5 unit/kg/d. The 5 currently available GLP-1RAs (exenatide, exenatide extended-release, liraglutide, albiglutide, and dulaglutide) are compared in TABLE 2.11-15
Dosing varies with each agent and includes twice daily before meals for exenatide, once daily (independent of meals) for liraglutide, and once weekly for exenatide extended-release, albiglutide, and dulaglutide. These agents should not be used for patients with a history of pancreatitis or a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia type 2. Because exenatide is cleared through the kidneys, its use is contraindicated in patients with a creatinine clearance <30 mL/min or end-stage renal disease. Caution is advised for its use in patients with a creatinine clearance of 30 to 50 mL/min.11
Basal plus one strategy
To best utilize prandial insulin, it is important to know what the patient’s glucose readings are before and after meals as assessed by the 7-point or staggered blood glucose monitoring techniques described earlier. Once you have clarified which meal(s) are raising the patient’s glucose levels, selecting appropriate treatment becomes easier. To reduce the glucose-monitoring burden for the patient, it may be acceptable to allow the patient to omit the fasting glucose measurement (if stable).
The first major decision is whether to treat one meal per day (basal plus one) or all meals (basal-bolus). Adding a rapid-acting insulin prior to one meal a day (usually the largest meal) is a reasonable starting point.16
The meal that produces the highest postprandial glucose readings can be considered the meal of greatest glycemic impact. The “delta” value—the difference between pre-meal glucose and 2-hour postprandial glucose readings—also helps to determine the largest meal of the day.17 The average physiologic delta is ≤50 mg/dL.17 If the delta for a meal is >75 mg/dL, consider initiating prandial insulin prior to that meal and titrating the dose to achieve a target glucose level of <130 mg/dL before the next meal.
Using 4 to 6 units of a rapid-acting insulin per meal is a good initial regimen for a basal plus one (as well as for a basal-bolus) approach.16 If the patient experiences significantly increased insulin demands as indicated by glucose patterns where the post-meal glucose is still consistently above 180 mg/dL, the initial regimen may be modified to 0.1 unit per kg per meal,17-19 and then titrated up to a maximum of 50% of the total daily insulin dose (TDD) for basal plus one16 (or 10%-20% of TDD per meal for basal-bolus).
Consider the timing of administration. Rapid-acting insulin analogs exhibit peak pharmacodynamic activity 60 minutes after injection (TABLE 3).20
Peak carbohydrate absorption following a meal occurs approximately 75 to 90 minutes after eating begins.17,21 Thus, to synchronize the action of insulin with carbohydrate digestion, the analog should be injected 15 minutes before meals. This can be increased by titrating prandial insulin by 1 unit/d to a goal of either a 90-minute to 2-hour postprandial glucose of <140 to 180 mg/dL or the next preprandial glucose of <130 mg/dL.16 The goal is to obtain a near-normal physiologic delta of <50 mg/dL. The drop in delta noted with every unit of insulin added to the current dose can provide a rough approximation of how many additional insulin titrations will be needed to achieve a delta of <50 mg/dL.
Basal-bolus combination
A gradual increase from one injection before a single meal each day to as-needed multiple daily injections (MDIs) is the next step in hyperglycemia management. Starting slow and building up to insulin therapy prior to each meal offers structure, simplicity, and physician-patient confidence in diabetes management. The slow progression from basal plus one to basal-bolus combination allows the patient ease into a complex, labor-intensive regimen of MDIs. Additionally, the stepwise reduction of postprandial hyperglycemia with this slow approach often reduces the incidence of hypoglycemia (more on this in a moment).8
Advanced insulin users can calculate an “insulin-to-carbohydrate ratio” (ICR) to estimate the amount of insulin they need to accommodate the amount of carbohydrates they ingest per meal. An ICR of 1:10 implies that the patient administers 1 unit of insulin for every 10 grams of carbohydrates ingested. For example, if a patient with an ICR of 1:10 concludes that his meal contains a total of 60 grams of carbohydrates, then he would administer 6 units of insulin prior to this meal to address the anticipated post-meal hyperglycemia.
In order to use the ICR regimen, a patient would need to be able to accurately determine the nutritional content of his meals (starch, protein, carbohydrates, and fat) and calculate the appropriate insulin dosage. For successful diabetes management, it is essential to evaluate the patient’s skills in these areas before starting an ICR regimen, and to routinely assess hypoglycemic episodes at follow-up visits.
An ICR approach is usually reserved for patients who require tighter glucose control than that obtained from fixed prandial insulin doses, such as patients with type 1 diabetes, those with variable meal schedules and content, those with a malabsorption syndrome that requires consuming meals with a specific amount of carbohydrates, athletes on a structured diet with specific carbohydrate content, and patients who want flexibility with carbohydrate intake with meals.
The risk of hypoglycemia is a major barrier to initiating basal-bolus insulin therapy. Hypoglycemia is classified as a blood glucose level of <70 mg/dL, and severe hypoglycemia as <50 mg/dL, regardless of whether the patient develops symptoms.22 Symptoms of hypoglycemia include dizziness, difficulty speaking, anxiety, confusion, and lethargy. Hypoglycemia can result in loss of consciousness or even death.22
A patient who has frequent hypoglycemic episodes may lose the protective physiologic response and may not recognize that he is experiencing a hypoglycemic episode (“hypoglycemia unawareness”). This is why it is crucial to ask patients if they have had symptoms of hypoglycemia, and to correlate the timing of these symptoms with blood glucose logs. For example, it is possible for a patient to experience hypoglycemic symptoms for blood glucose readings in the 100 to 200 mg/dL range if his or her average blood glucose has been in the 250 to 300 mg/dL range. Such patient may not realize he is experiencing hypoglycemia until he develops severe symptoms, such as loss of consciousness.
Hypoglycemia unawareness must be addressed immediately by reducing insulin dosing to prevent all hypoglycemic episodes for 2 to 3 weeks. This has been shown to “reset” the normal physiologic response to hypoglycemia, regardless of how long the patient has had diabetes.23,24 Even if your patient is aware of the warning signs of a hypoglycemic episode, it is important to routinely ask about hypoglycemia at all diabetes visits because patients may reduce insulin doses, skip doses, or eat defensively to prevent hypoglycemia.
Other than the risk of hypoglycemia, insulin typically has fewer adverse effects than oral medications used to treat diabetes. Most common concerns include weight gain, hypoglycemia, injection site reactions and, rarely, allergy to insulin or its vehicle.16
CORRESPONDENCE
Jay Shubrook, DO, FAAF P, FACOF P, BC-ADM, Touro University College of Osteopathic Medicine, 1310 Club Drive, Vallejo, CA 94592; [email protected]
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook, J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014; 63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two singledose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management--exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin – Options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. Monthly Prescribing Reference Web site. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Eng J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Family Physician. 2014;1:19-25.
1. Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE Comprehensive Diabetes Management Algorithm 2013. Endocr Pract. 2013;19:327-336.
2. Inzucchi SE, Bergenstal RM, Buse JB, et al; American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient centered approach. A position statement of the ADA and the EASD. Diabetes Care. 2012;35:1364-1379.
3. Shubrook, J. Insulin for type 2 diabetes: How and when to get started. J Fam Pract. 2014; 63:76-81.
4. Nathan D, Buse J, Davidson M, et al; American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Rosenstock J, Fonseca VA, Gross JL, et al. Advancing basal insulin replacement in type 2 diabetes inadequately controlled with insulin glargine plus oral agents: a comparison of adding albiglutide, a weekly GLP-1 receptor agonist, versus thrice daily prandial insulin lispro. Diabetes Care. 2014;37:2317-2325.
6. Owens DR, Luzio SD, Sert-Langeron C, et al. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011;13:1020-1027.
7. Lankisch MR, Ferlinz KC, Leahy JL, et al; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two singledose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008;10:1178-1185.
8. Davidson MB, Raskin P, Tanenberg RJ, et al. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011;17:395-403.
9. Owens DR. Stepwise intensification of insulin therapy in type 2 diabetes management--exploring the concept of basal-plus approach in clinical practice. Diabet Med. 2013;30:276-288.
10. Holst J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409-1439.
11. Byetta [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2015.
12. Bydureon [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2014.
13. Victoza [package insert]. Plainsboro, NJ: Novo Nordisk Inc; 2015.
14. Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline; 2014.
15. Trulicity [package insert]. Indianapolis, IN: Eli Lilly and Company; 2014.
16. Vaidya A, McMahon GT. Initiating insulin for type 2 diabetes: Strategies for success. J Clin Outcomes Manag. 2009;16:127-136.
17. Unger J. Insulin initiation and intensification in patients with T2DM for the primary care physician. Diabetes Metab Syndr Obes. 2011;4:253-261.
18. Sharma MD, Garber AJ. Progression from basal to pre-mixed or rapid-acting insulin – Options for intensification and the use of pumps. US Endocrinology. 2009;5:40-44.
19. Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125-134.
20. Monthly Prescribing Reference (MPR). Insulin. Monthly Prescribing Reference Web site. Available at: http://www.empr.com/insulins/article/123739/. Accessed January 10, 2014.
21. Guyton AC, Hall JE. Insulin, glucagon, and diabetes mellitus. In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology. 11th ed. Philadelphia, PA: Elsevier Saunders; 2006:961-977.
22. Kitabchi AE, Gosmanov AR. Safety of rapid-acting insulin analogs versus regular human insulin. Am J Med Sci. 2012;344:136-141.
23. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Eng J Med. 2004;350:2272-2279.
24. Gehlaut RR, Shubrook JH. Revisiting hypoglycemia in diabetes. Osteopathic Family Physician. 2014;1:19-25.
Speech, language, hearing delays: Time for early intervention?
› Consider using age-specific published milestones, such as those found online at the American Speech-Language-Hearing Association’s site, to evaluate children’s developmental progress. C
› Consult your state’s early intervention agency (cited in this article) for assistance in referring children for further evaluation and possible treatment. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A young mother in your practice arrives with her 2-year-old son for a well-child visit. She remarks that, although her son uses a few single words to indicate hunger and other needs, her sister’s child at the same age had begun using multiple words to ask questions and express her wishes. She’s concerned about whether her son’s behavior is normal. As you start to engage the child, you note that he responds only after you repeat his name a few times. Are these observations indicative of a typical delay in development, or are they clues to a serious medical issue or communication disability? Given the absence of any known medical problem or evident physical or intellectual disability, how would you proceed in this case and in counseling the mother?
Developmental screening minimizes adverse long-term consequences
Speech, language, and hearing delays and disorders in children can lead to learning and socialization problems that may persist into adulthood. Health care providers who monitor speech, language, and hearing development in children can guide parents, as needed, to appropriate services for further assessment or treatment1 and direct them to advocacy programs such as the Center for Parent Information and Resources (formerly the National Dissemination Center for Children with Disabilities).2
A useful tool at well-child visits is the Denver II, a quick developmental screening test to help identify a variety of disorders of intelligence, language, mental health, and motor and self-help skills.3
Suspicion of a developmental delay not likely due to a medical issue or congenital abnormality requiring examination by an otorhinolaryngologist could warrant referral of the child for early intervention (EI).
Communication disorders and their manifestations
Communication—the ability to receive, process, comprehend, and transmit information—is essential for a successful life.4 Speech, language, and hearing impairments affect a child’s ability to send (speak, write, or gesture) and receive (hear, interpret, or decipher) messages.
Speech impairments
Beginning at birth, we systematically develop speech sounds and an ability to use these sounds to convey meaning by forming words and using language.5 Speech and language pathologists make a distinction between speech and language impairments.6
Speech disorders may involve problems of articulation, fluency, voice, or resonance. About 8% to 9% of preschool children have speech disorders, and approximately 5% of school-age children have speech or language impairments.7
Problems of articulation are heard in such instances as substituting a “w” for an “r” (“wabbit” for “rabbit”) or in distorting or omitting sounds or syllables (“tato” for “potato”). Considering that articulation involves the precise coordination of about 70 muscles (tongue, lips, velum, vocal folds, etc), development of this skill normally goes through phases of inaccurate sound productions. Concerns arise when these phases persist or are atypical.
Speech fluency/stuttering is the uncontrollable blocking of speech, sound prolongation (“wwwwater”), or repetition of a sound, syllable, or word during speaking (“pu-pu-pu-puppy”).
Problems of voice include symptoms such as hoarseness, an exceptionally weak voice or one that is too high or too low, or abnormal resonance (hyper- or hyponasality, which gives the impression the child is talking “through the nose” or is constantly congested).
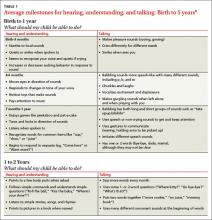
Using common milestones as reference points. The American Speech-Language-Hearing Association (ASHA)8 lists the milestones for speech development (English and Spanish) at http://www.asha.org/public/speech/development/chart.htm. For example, between 12 and 24 months of age, a child should be learning vocabulary (“doggie, nana”), combining 2 words (“mommy car”), asking 2-word questions (“where daddy?”), and producing a variety of speech sounds. These milestones represent an average and some children may not master all the items in a category until they reach the upper limit of the age range (TABLE 1).8 Roth et al9 found that intervention benefited preschoolers with speech and language disabilities when applied earlier than previously recommended. In other words, avoid the “wait-and-see” option. Busari and Weggelaar,10 studying referral recommendations for children who are “slow to speak,” concluded that EI may diminish further consequences later in the child’s life.
ASHA launched a campaign to increase awareness of communication disorders across the lifespan and to encourage early identification (http://identifythesigns.org/).11 The site has basic lists of signs of common speech and language disorders and hearing loss in children from birth to 4 years of age. This period in a child’s life is “an important stage in early detection of communication disorders.”
Language impairments
Language impairments affect 3 domains of language: form (grammar/syntax), content, or use. These domains are governed by rules specific to the language spoken in the home. Language impairment can interfere with comprehension and formulation of messages. About 2% to 3% of preschoolers have language disorders.12
Language impairments may be observed in one or more of the language components, including phonology (rules of the sounds in the language), lexicon (vocabulary), morphology (word markers—eg, final “s” to make the plural of “cat”), syntax (word order) and pragmatics (socially appropriate speech, gesture, eye contact, and language use).
While there is great variability in typical development, atypical language development can be a secondary characteristic of other physical or developmental problems attributed to other conditions such as autism spectrum disorder (ASD), cerebral palsy, childhood apraxia of speech, dysarthria, intellectual disability, or selective mutism.13
Verbal communication difficulties may appear in expressive and receptive language.14Receptive language is the ability to comprehend language communicated by another person.6 Receptive language (processing) skills can be demonstrated as follows. If a child is asked “Do you like cats?” she must first decide to whom the question is directed (to her and not someone else). She must then search her long-term memory for the word/concept “cat” (compared with dog, a similar concept; or with mat, a similar-sounding word), and process the word “like” (compared with “dislike”). Now the child understands the question and can decide on an answer.
Expressive language is a child’s ability to speak; the mental process used to produce speech and communicate a message.6 To answer, “Yes, I like cats,” the child retrieves the concept “cat” from memory (cognition and semantics: the meanings of words, their relationships and usage), finds the right words (vocabulary), puts them in the right order (syntax), uses the right verb tense (grammar/morphology), assembles the right sounds in order, initiates the neuromotor acts (phonology/speech production), and communicates that she understands what cats are and that she likes them (pragmatics; socially/contextually appropriate responses).
Resources on language development. Typical language development, the length of which varies among children, must be practiced in a rich linguistic environment. Some children are adventurous with language. They babble, talk, and communicate in a carefree manner. Others are cautious. They may wait until they are sure of their skills before attempting a new word. Usually, concern about a child’s speech and language development arises if there is no speech, if speech is not clear, or if speech or language is different from that of peers.
The Centers for Disease Control and Prevention, under their “Learn the signs. Act early” campaign (www.cdc.gov/actearly), has published checklists of children’s developmental milestones from 2 months to 5 years of age on social, communication, cognitive, and motor skills. Health care providers helping parents determine if their child’s communication is developing normally can find information and materials at http://www.cdc.gov/ncbddd/actearly/hcp/index.html.15
Hearing impairments
Moeller et al16 surveyed 1968 primary care physicians on their attitude, practices, and knowledge of universal hearing screenings for newborns. They noted limitations in awareness of EI options for infants with hearing loss: proper times and places for referrals, available communication modalities, cochlear implant candidacy, and professionals in their locale with expertise on hearing loss. These knowledge gaps involved some medical issues, such as hearing loss genetics and later-onset hearing loss in infants and children. They also found low confidence in providing information to families about how to proceed with EI and in discussing intervention needs and resources.
Adverse effects of hearing loss. Hearing loss can be unilateral or bilateral, conductive or sensorineural, and can range in severity from mild to profound. According to the National Institute on Deafness and Communication Disorders (NIDCD), one in every 350 infants is born with a significant hearing loss, and others become deaf due to childhood illness or injury.17 According to ASHA,18 hearing loss can affect children in 4 major ways:19 delays in the development of receptive (comprehension) and expressive communication skills; a language deficit that causes learning problems and reduces academic achievement; communication difficulties that often lead to social isolation and poor self-concept; and impact on vocational choices and options.20 NIDCD provides a checklist to determine a child’s hearing status at http://www.nidcd.nih.gov/health/hearing/silence.asp.17
Timing of intervention is significant. EI is critical in minimizing the deleterious effects of hearing loss and in optimizing speech and language development. Severity of hearing loss influences EI outcome, and treatment options depend on the hearing loss having occurred either before language development (prelingually) or after (postlingually). Early management of hearing impairment can improve language, especially for children with a severe or profound hearing loss.21
Devices and methods that promote communication development for students who are deaf or hard of hearing include the use of hearing aids to amplify residual hearing for oral or auditory-oral approaches; the manual approach stressing sign-language (American Sign Language or Signed English); or Total Communication using both the oral and sign-language methods.
With an infant, suspicion of a hearing problem warrants referral to an otorhinolaryngologist or an audiologist for thorough evaluation. In New Jersey, an EI referral typically triggers a referral to one of these specialists. TABLE 2 lists resources for early identification and intervention in communication disorders.
TABLE 2
| Resources for early identification and intervention in communication disorders | |
| Resource | Information |
| American Speech-Language-Hearing Association (ASHA) | Communication skills, milestones, disorders, and treatment resources across the lifespan; for parents and professionals |
| CDC’s “Learn the signs. Act early” campaign
| Children’s developmental milestones from 2 months to 5 years; checklists for parents |
| Children’s developmental milestones and Early Intervention for health care providers | |
| National Institute on Deafness and Other Communication Disorders | Speech and language development checklists |
| National Institute on Deafness and Other Communication Disorders | Hearing loss and its effects on communication; identification and management options |
| Center for Parent Information and Resources | Early Intervention: overview and process |
| National Institute of Mental Health | Autism spectrum disorder; a guide for parents |
| First Signs, Inc | Early warning signs of autism spectrum disorder; for parents and professionals |
| The Autism Screening Test | Autism screening test to identify children 16-30 months who should receive a more thorough assessment for possible early signs of autism spectrum disorder or developmental delay |
Autism spectrum disorder
The fifth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-5)22 has revised its diagnostic codes for ASD. An in-depth analysis of the new diagnosis is beyond the purview of this article, but it deserves a few comments.
The revised diagnosis of ASD consolidates the previously separate diagnoses of autistic disorder, Asperger’s disorder, childhood disintegrative disorder, and pervasive developmental disorder/not otherwise specified.22-24 According to DSM-5, an individual with ASD must have 1) persistent deficits in social communication and social interactions, 2) restricted, repetitive patterns of behavior, interests or activities, 3) symptoms present in early childhood (but may not become fully manifest until social demands exceed limited capacities), and 4) symptoms that limit and impair everyday functioning.22,24
Onset of the disorder must be obvious before age 3 for a child to be eligible for EI. Children who do not qualify for an ASD diagnosis under the new DSM-5 definition may be included in a new category called social communication disorder (SCD) under “Communication Disorders” in DSM-5. SCD is defined as impairment in pragmatics that impacts development of social relationships and comprehension of social conversations. ASD must be ruled out before a diagnosis of SCD can be made.23,24
Early intervention services
Individual states offer EI services to families to facilitate detection of developmental delays in children and to provide a comprehensive system of support designed to reduce the effects of disabilities (or to prevent learning and developmental problems later in life).5 The rationale for EI is that the earlier interventions are started, the less likely later interventions will be needed.25 EI services are provided to children from birth through their third birthday; services are free of charge to eligible families or on a sliding payment scale determined by a family’s income.
Resources for information and referrals. To access EI services on behalf of a family, contact a local hospital or point them to the CPIR (http://www.parentcenterhub.org/repository/disability-landing/).2 For families with disabled children older than 3, consider suggesting that parents contact the local school district (even if the child isn’t enrolled there) to arrange an evaluation under the Individuals with Disabilities Education Act (http://idea.ed.gov/).26 Because individual states’ EI organizations may have slightly different procedures, it is best to consult one’s own state EI site for specific information regarding referrals (TABLE 3).
Efficacy of treatment
The prevalence of specific communication disorders varies widely, as do prognoses, possibly due to the variability of underlying causes (physical/biological/medical or environmental/educational).27 Also, as described earlier, “communication disorder” is an umbrella term inclusive of problems as diverse as resonance (eg, hypernasality due to a submucous palatal cleft), severe language delay (eg, due to Down syndrome), or lisp (misarticulation of the “s” sound).
Is therapy effective? Speech and language pathologists use the National Outcomes Measurement System (NOMS) as an index of the outcomes of treatment on functional communication along 6 scales. The most frequent types of communication problems seen in the prekindergarten children’s NOMS were “articulation” (75% of children), “spoken language production” (61%), and “spoken language comprehension” (42%). Problems in the remaining 3 scales (“pragmatics,” “cognitive orientation,” and “swallowing”) were seen in fewer than 15% of the preschool-age students.28
Articulation therapy yielded improvement in 69.3% of cases, spoken language comprehension therapy in 65.3%, and spoken language production therapy in 65.2%.28 Such outcomes support regular screening of children’s communication development and, as needed, referral for EI.
TABLE 3
CORRESPONDENCE
Christopher Mulrine, EdD; William Paterson University, 1600 Valley Road 3003 Wayne, NJ 07474-0920; [email protected]
1. Wankoff LS. Warning signs in the development of speech, language, and communication: when to refer to a speech-language pathologist. J Child Adolesc Psychiatr Nurs. 2011;24:175-184.
2. Center for Parent Information and Resources. Disabilities. Center for Parent Information and Resources Web site. Available at: http://www.parentcenterhub.org/repository/disability-landing/. Updated June 2014. Accessed January 24, 2015.
3. Denver Developmental Materials, Inc. Denver II Online. Denver Developmental Materials, Inc Web site. Available at: http://denverii.com/denverii/. Accessed January 24, 2015.
4. Guralnick MJ. The Effectiveness of Early Intervention. Baltimore, MD: Brookes Publishing; 2013.
5. Schwartz HD. A Primer on Communication and Communicative Disorders. Boston, MA: Pearson Education; 2012.
6. Heward WL. Exceptional Children: An Introduction to Special Education. 10th ed. Boston, Mass: Pearson Education; 2013.
7. Hallahan DP, Kauffman J M, Pullen PC. Exceptional Learners: An Introduction to Special Education. 13th ed. Upper Saddle River, NJ: Pearson Education; 2015.
8. American Speech-Language-Hearing Association. How does your child hear and talk? American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/speech/development/chart.htm. Accessed January 24, 2015.
9. Roth FP, Troia GA, Worthington CK, et al. Promoting awareness of sounds in speech: An initial report of an early intervention program for children with speech and language impairments. Appl Psycholinguistics. 2002;23:535-565.
10. Busari JO, Weggelaar NM. How to investigate and manage the child who is slow to speak. BMJ. 2004;328:272-276.
11. American Speech-Language-Hearing Association. Identify the Signs of Communication Disorders. Identify the Signs of Communication Disorders Web site. Available at: http://identifythesigns.org/. Accessed January 24, 2015.
12. McLaughlin MR. Speech and language delay in children. Am Fam Physician. 2011;83:1183-1188.
13. American Speech-Language-Hearing Association. Child speech and language. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/speech/disorders/ChildSandL.htm. Accessed January 24, 2015.
14. Bondurant-Utz JA. Practical Guide to Assessing Infants and Preschoolers with Special Needs. Upper Saddle River, NJ: Pearson Education; 2002.
15. Centers for Disease Control and Prevention. Learn the Signs: Act Early. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/actearly/hcp/index.html. Accessed January 24, 2015.
16. Moeller MP, White KR, Shisler L. Primary care physicians’ knowledge, attitudes, and practices related to newborn hearing screening. Pediatrics. 2006;118:1357-1370.
17. National Institute on Deafness and Other Communication Disorders. Your baby’s hearing and communicative checklist. National Institute on Deafness and Other Communication Disorders Web site. Available at: http://www.nidcd.nih.gov/health/hearing/silence.asp. Accessed January 24, 2015.
18. American Speech-Language-Hearing Association. Effects of hearing loss on development. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/hearing/Effects-of-Hearing-Loss-on-Development/. Accessed January 24, 2015.
19. American Speech-Language-Hearing Association. Facts about pediatric hearing loss. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/aud/Facts-about-Pediatric-Hearing-Loss/. Accessed January 24, 2015.
20. Tang BG, Feldman HM, Padden C, et al. Delayed recognition of profound hearing loss in a 7-year-old girl with a neurological condition. J Dev Behav Pediatr. 2010;31(3 suppl):S42-S45.
21. Watkin P, McCann D, Law C, et al. Language ability in children with permanent hearing impairment: the influence of early management and family participation. Pediatrics. 2007;120:e694-e701.
22. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
23. Autism Match. 5 things to know about autism and DSM-5. Austism Match Web site. Available at: https://autismmatch.org/info/news/2012/05/02/5-things-to-know-about-autism-and-dsm-5. Accessed January 24, 2015.
24. American Psychiatric Publishing. Autism Spectrum Disorder. American Psychiatric Association Web site. Available at: http://www.dsm5.org/Documents/Autism%20Spectrum%20Disorder%20Fact%20Sheet.pdf. Accessed January 24, 2015.
25. Law J, Garrett Z, Nye C. Speech and language therapy interventions for children with primary speech and language delay or disorder. Cochrane Database Syst Rev. 2003;(3):CD004110.
26. U.S. Department of Education. Building the legacy: IDEA 2004. Individuals with Disabilities Education Act Web site. Available at: http://idea.ed.gov/. Accessed January 24, 2015.
27. Friend MP. Special Education: Contemporary Perspectives for School Professionals. Boston, MA: Pearson; 2005.
28. Mullen R, Schooling T. The National Outcomes Measurement System for Pediatric Speech-Language Pathology. Lang Speech Hearing Services Schools. 2010;41:44-60.
› Consider using age-specific published milestones, such as those found online at the American Speech-Language-Hearing Association’s site, to evaluate children’s developmental progress. C
› Consult your state’s early intervention agency (cited in this article) for assistance in referring children for further evaluation and possible treatment. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A young mother in your practice arrives with her 2-year-old son for a well-child visit. She remarks that, although her son uses a few single words to indicate hunger and other needs, her sister’s child at the same age had begun using multiple words to ask questions and express her wishes. She’s concerned about whether her son’s behavior is normal. As you start to engage the child, you note that he responds only after you repeat his name a few times. Are these observations indicative of a typical delay in development, or are they clues to a serious medical issue or communication disability? Given the absence of any known medical problem or evident physical or intellectual disability, how would you proceed in this case and in counseling the mother?
Developmental screening minimizes adverse long-term consequences
Speech, language, and hearing delays and disorders in children can lead to learning and socialization problems that may persist into adulthood. Health care providers who monitor speech, language, and hearing development in children can guide parents, as needed, to appropriate services for further assessment or treatment1 and direct them to advocacy programs such as the Center for Parent Information and Resources (formerly the National Dissemination Center for Children with Disabilities).2
A useful tool at well-child visits is the Denver II, a quick developmental screening test to help identify a variety of disorders of intelligence, language, mental health, and motor and self-help skills.3
Suspicion of a developmental delay not likely due to a medical issue or congenital abnormality requiring examination by an otorhinolaryngologist could warrant referral of the child for early intervention (EI).
Communication disorders and their manifestations
Communication—the ability to receive, process, comprehend, and transmit information—is essential for a successful life.4 Speech, language, and hearing impairments affect a child’s ability to send (speak, write, or gesture) and receive (hear, interpret, or decipher) messages.
Speech impairments
Beginning at birth, we systematically develop speech sounds and an ability to use these sounds to convey meaning by forming words and using language.5 Speech and language pathologists make a distinction between speech and language impairments.6
Speech disorders may involve problems of articulation, fluency, voice, or resonance. About 8% to 9% of preschool children have speech disorders, and approximately 5% of school-age children have speech or language impairments.7
Problems of articulation are heard in such instances as substituting a “w” for an “r” (“wabbit” for “rabbit”) or in distorting or omitting sounds or syllables (“tato” for “potato”). Considering that articulation involves the precise coordination of about 70 muscles (tongue, lips, velum, vocal folds, etc), development of this skill normally goes through phases of inaccurate sound productions. Concerns arise when these phases persist or are atypical.
Speech fluency/stuttering is the uncontrollable blocking of speech, sound prolongation (“wwwwater”), or repetition of a sound, syllable, or word during speaking (“pu-pu-pu-puppy”).
Problems of voice include symptoms such as hoarseness, an exceptionally weak voice or one that is too high or too low, or abnormal resonance (hyper- or hyponasality, which gives the impression the child is talking “through the nose” or is constantly congested).
Using common milestones as reference points. The American Speech-Language-Hearing Association (ASHA)8 lists the milestones for speech development (English and Spanish) at http://www.asha.org/public/speech/development/chart.htm. For example, between 12 and 24 months of age, a child should be learning vocabulary (“doggie, nana”), combining 2 words (“mommy car”), asking 2-word questions (“where daddy?”), and producing a variety of speech sounds. These milestones represent an average and some children may not master all the items in a category until they reach the upper limit of the age range (TABLE 1).8 Roth et al9 found that intervention benefited preschoolers with speech and language disabilities when applied earlier than previously recommended. In other words, avoid the “wait-and-see” option. Busari and Weggelaar,10 studying referral recommendations for children who are “slow to speak,” concluded that EI may diminish further consequences later in the child’s life.
ASHA launched a campaign to increase awareness of communication disorders across the lifespan and to encourage early identification (http://identifythesigns.org/).11 The site has basic lists of signs of common speech and language disorders and hearing loss in children from birth to 4 years of age. This period in a child’s life is “an important stage in early detection of communication disorders.”
Language impairments
Language impairments affect 3 domains of language: form (grammar/syntax), content, or use. These domains are governed by rules specific to the language spoken in the home. Language impairment can interfere with comprehension and formulation of messages. About 2% to 3% of preschoolers have language disorders.12
Language impairments may be observed in one or more of the language components, including phonology (rules of the sounds in the language), lexicon (vocabulary), morphology (word markers—eg, final “s” to make the plural of “cat”), syntax (word order) and pragmatics (socially appropriate speech, gesture, eye contact, and language use).
While there is great variability in typical development, atypical language development can be a secondary characteristic of other physical or developmental problems attributed to other conditions such as autism spectrum disorder (ASD), cerebral palsy, childhood apraxia of speech, dysarthria, intellectual disability, or selective mutism.13
Verbal communication difficulties may appear in expressive and receptive language.14Receptive language is the ability to comprehend language communicated by another person.6 Receptive language (processing) skills can be demonstrated as follows. If a child is asked “Do you like cats?” she must first decide to whom the question is directed (to her and not someone else). She must then search her long-term memory for the word/concept “cat” (compared with dog, a similar concept; or with mat, a similar-sounding word), and process the word “like” (compared with “dislike”). Now the child understands the question and can decide on an answer.
Expressive language is a child’s ability to speak; the mental process used to produce speech and communicate a message.6 To answer, “Yes, I like cats,” the child retrieves the concept “cat” from memory (cognition and semantics: the meanings of words, their relationships and usage), finds the right words (vocabulary), puts them in the right order (syntax), uses the right verb tense (grammar/morphology), assembles the right sounds in order, initiates the neuromotor acts (phonology/speech production), and communicates that she understands what cats are and that she likes them (pragmatics; socially/contextually appropriate responses).
Resources on language development. Typical language development, the length of which varies among children, must be practiced in a rich linguistic environment. Some children are adventurous with language. They babble, talk, and communicate in a carefree manner. Others are cautious. They may wait until they are sure of their skills before attempting a new word. Usually, concern about a child’s speech and language development arises if there is no speech, if speech is not clear, or if speech or language is different from that of peers.
The Centers for Disease Control and Prevention, under their “Learn the signs. Act early” campaign (www.cdc.gov/actearly), has published checklists of children’s developmental milestones from 2 months to 5 years of age on social, communication, cognitive, and motor skills. Health care providers helping parents determine if their child’s communication is developing normally can find information and materials at http://www.cdc.gov/ncbddd/actearly/hcp/index.html.15
Hearing impairments
Moeller et al16 surveyed 1968 primary care physicians on their attitude, practices, and knowledge of universal hearing screenings for newborns. They noted limitations in awareness of EI options for infants with hearing loss: proper times and places for referrals, available communication modalities, cochlear implant candidacy, and professionals in their locale with expertise on hearing loss. These knowledge gaps involved some medical issues, such as hearing loss genetics and later-onset hearing loss in infants and children. They also found low confidence in providing information to families about how to proceed with EI and in discussing intervention needs and resources.
Adverse effects of hearing loss. Hearing loss can be unilateral or bilateral, conductive or sensorineural, and can range in severity from mild to profound. According to the National Institute on Deafness and Communication Disorders (NIDCD), one in every 350 infants is born with a significant hearing loss, and others become deaf due to childhood illness or injury.17 According to ASHA,18 hearing loss can affect children in 4 major ways:19 delays in the development of receptive (comprehension) and expressive communication skills; a language deficit that causes learning problems and reduces academic achievement; communication difficulties that often lead to social isolation and poor self-concept; and impact on vocational choices and options.20 NIDCD provides a checklist to determine a child’s hearing status at http://www.nidcd.nih.gov/health/hearing/silence.asp.17
Timing of intervention is significant. EI is critical in minimizing the deleterious effects of hearing loss and in optimizing speech and language development. Severity of hearing loss influences EI outcome, and treatment options depend on the hearing loss having occurred either before language development (prelingually) or after (postlingually). Early management of hearing impairment can improve language, especially for children with a severe or profound hearing loss.21
Devices and methods that promote communication development for students who are deaf or hard of hearing include the use of hearing aids to amplify residual hearing for oral or auditory-oral approaches; the manual approach stressing sign-language (American Sign Language or Signed English); or Total Communication using both the oral and sign-language methods.
With an infant, suspicion of a hearing problem warrants referral to an otorhinolaryngologist or an audiologist for thorough evaluation. In New Jersey, an EI referral typically triggers a referral to one of these specialists. TABLE 2 lists resources for early identification and intervention in communication disorders.
TABLE 2
| Resources for early identification and intervention in communication disorders | |
| Resource | Information |
| American Speech-Language-Hearing Association (ASHA) | Communication skills, milestones, disorders, and treatment resources across the lifespan; for parents and professionals |
| CDC’s “Learn the signs. Act early” campaign
| Children’s developmental milestones from 2 months to 5 years; checklists for parents |
| Children’s developmental milestones and Early Intervention for health care providers | |
| National Institute on Deafness and Other Communication Disorders | Speech and language development checklists |
| National Institute on Deafness and Other Communication Disorders | Hearing loss and its effects on communication; identification and management options |
| Center for Parent Information and Resources | Early Intervention: overview and process |
| National Institute of Mental Health | Autism spectrum disorder; a guide for parents |
| First Signs, Inc | Early warning signs of autism spectrum disorder; for parents and professionals |
| The Autism Screening Test | Autism screening test to identify children 16-30 months who should receive a more thorough assessment for possible early signs of autism spectrum disorder or developmental delay |
Autism spectrum disorder
The fifth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-5)22 has revised its diagnostic codes for ASD. An in-depth analysis of the new diagnosis is beyond the purview of this article, but it deserves a few comments.
The revised diagnosis of ASD consolidates the previously separate diagnoses of autistic disorder, Asperger’s disorder, childhood disintegrative disorder, and pervasive developmental disorder/not otherwise specified.22-24 According to DSM-5, an individual with ASD must have 1) persistent deficits in social communication and social interactions, 2) restricted, repetitive patterns of behavior, interests or activities, 3) symptoms present in early childhood (but may not become fully manifest until social demands exceed limited capacities), and 4) symptoms that limit and impair everyday functioning.22,24
Onset of the disorder must be obvious before age 3 for a child to be eligible for EI. Children who do not qualify for an ASD diagnosis under the new DSM-5 definition may be included in a new category called social communication disorder (SCD) under “Communication Disorders” in DSM-5. SCD is defined as impairment in pragmatics that impacts development of social relationships and comprehension of social conversations. ASD must be ruled out before a diagnosis of SCD can be made.23,24
Early intervention services
Individual states offer EI services to families to facilitate detection of developmental delays in children and to provide a comprehensive system of support designed to reduce the effects of disabilities (or to prevent learning and developmental problems later in life).5 The rationale for EI is that the earlier interventions are started, the less likely later interventions will be needed.25 EI services are provided to children from birth through their third birthday; services are free of charge to eligible families or on a sliding payment scale determined by a family’s income.
Resources for information and referrals. To access EI services on behalf of a family, contact a local hospital or point them to the CPIR (http://www.parentcenterhub.org/repository/disability-landing/).2 For families with disabled children older than 3, consider suggesting that parents contact the local school district (even if the child isn’t enrolled there) to arrange an evaluation under the Individuals with Disabilities Education Act (http://idea.ed.gov/).26 Because individual states’ EI organizations may have slightly different procedures, it is best to consult one’s own state EI site for specific information regarding referrals (TABLE 3).
Efficacy of treatment
The prevalence of specific communication disorders varies widely, as do prognoses, possibly due to the variability of underlying causes (physical/biological/medical or environmental/educational).27 Also, as described earlier, “communication disorder” is an umbrella term inclusive of problems as diverse as resonance (eg, hypernasality due to a submucous palatal cleft), severe language delay (eg, due to Down syndrome), or lisp (misarticulation of the “s” sound).
Is therapy effective? Speech and language pathologists use the National Outcomes Measurement System (NOMS) as an index of the outcomes of treatment on functional communication along 6 scales. The most frequent types of communication problems seen in the prekindergarten children’s NOMS were “articulation” (75% of children), “spoken language production” (61%), and “spoken language comprehension” (42%). Problems in the remaining 3 scales (“pragmatics,” “cognitive orientation,” and “swallowing”) were seen in fewer than 15% of the preschool-age students.28
Articulation therapy yielded improvement in 69.3% of cases, spoken language comprehension therapy in 65.3%, and spoken language production therapy in 65.2%.28 Such outcomes support regular screening of children’s communication development and, as needed, referral for EI.
TABLE 3
CORRESPONDENCE
Christopher Mulrine, EdD; William Paterson University, 1600 Valley Road 3003 Wayne, NJ 07474-0920; [email protected]
› Consider using age-specific published milestones, such as those found online at the American Speech-Language-Hearing Association’s site, to evaluate children’s developmental progress. C
› Consult your state’s early intervention agency (cited in this article) for assistance in referring children for further evaluation and possible treatment. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A young mother in your practice arrives with her 2-year-old son for a well-child visit. She remarks that, although her son uses a few single words to indicate hunger and other needs, her sister’s child at the same age had begun using multiple words to ask questions and express her wishes. She’s concerned about whether her son’s behavior is normal. As you start to engage the child, you note that he responds only after you repeat his name a few times. Are these observations indicative of a typical delay in development, or are they clues to a serious medical issue or communication disability? Given the absence of any known medical problem or evident physical or intellectual disability, how would you proceed in this case and in counseling the mother?
Developmental screening minimizes adverse long-term consequences
Speech, language, and hearing delays and disorders in children can lead to learning and socialization problems that may persist into adulthood. Health care providers who monitor speech, language, and hearing development in children can guide parents, as needed, to appropriate services for further assessment or treatment1 and direct them to advocacy programs such as the Center for Parent Information and Resources (formerly the National Dissemination Center for Children with Disabilities).2
A useful tool at well-child visits is the Denver II, a quick developmental screening test to help identify a variety of disorders of intelligence, language, mental health, and motor and self-help skills.3
Suspicion of a developmental delay not likely due to a medical issue or congenital abnormality requiring examination by an otorhinolaryngologist could warrant referral of the child for early intervention (EI).
Communication disorders and their manifestations
Communication—the ability to receive, process, comprehend, and transmit information—is essential for a successful life.4 Speech, language, and hearing impairments affect a child’s ability to send (speak, write, or gesture) and receive (hear, interpret, or decipher) messages.
Speech impairments
Beginning at birth, we systematically develop speech sounds and an ability to use these sounds to convey meaning by forming words and using language.5 Speech and language pathologists make a distinction between speech and language impairments.6
Speech disorders may involve problems of articulation, fluency, voice, or resonance. About 8% to 9% of preschool children have speech disorders, and approximately 5% of school-age children have speech or language impairments.7
Problems of articulation are heard in such instances as substituting a “w” for an “r” (“wabbit” for “rabbit”) or in distorting or omitting sounds or syllables (“tato” for “potato”). Considering that articulation involves the precise coordination of about 70 muscles (tongue, lips, velum, vocal folds, etc), development of this skill normally goes through phases of inaccurate sound productions. Concerns arise when these phases persist or are atypical.
Speech fluency/stuttering is the uncontrollable blocking of speech, sound prolongation (“wwwwater”), or repetition of a sound, syllable, or word during speaking (“pu-pu-pu-puppy”).
Problems of voice include symptoms such as hoarseness, an exceptionally weak voice or one that is too high or too low, or abnormal resonance (hyper- or hyponasality, which gives the impression the child is talking “through the nose” or is constantly congested).
Using common milestones as reference points. The American Speech-Language-Hearing Association (ASHA)8 lists the milestones for speech development (English and Spanish) at http://www.asha.org/public/speech/development/chart.htm. For example, between 12 and 24 months of age, a child should be learning vocabulary (“doggie, nana”), combining 2 words (“mommy car”), asking 2-word questions (“where daddy?”), and producing a variety of speech sounds. These milestones represent an average and some children may not master all the items in a category until they reach the upper limit of the age range (TABLE 1).8 Roth et al9 found that intervention benefited preschoolers with speech and language disabilities when applied earlier than previously recommended. In other words, avoid the “wait-and-see” option. Busari and Weggelaar,10 studying referral recommendations for children who are “slow to speak,” concluded that EI may diminish further consequences later in the child’s life.
ASHA launched a campaign to increase awareness of communication disorders across the lifespan and to encourage early identification (http://identifythesigns.org/).11 The site has basic lists of signs of common speech and language disorders and hearing loss in children from birth to 4 years of age. This period in a child’s life is “an important stage in early detection of communication disorders.”
Language impairments
Language impairments affect 3 domains of language: form (grammar/syntax), content, or use. These domains are governed by rules specific to the language spoken in the home. Language impairment can interfere with comprehension and formulation of messages. About 2% to 3% of preschoolers have language disorders.12
Language impairments may be observed in one or more of the language components, including phonology (rules of the sounds in the language), lexicon (vocabulary), morphology (word markers—eg, final “s” to make the plural of “cat”), syntax (word order) and pragmatics (socially appropriate speech, gesture, eye contact, and language use).
While there is great variability in typical development, atypical language development can be a secondary characteristic of other physical or developmental problems attributed to other conditions such as autism spectrum disorder (ASD), cerebral palsy, childhood apraxia of speech, dysarthria, intellectual disability, or selective mutism.13
Verbal communication difficulties may appear in expressive and receptive language.14Receptive language is the ability to comprehend language communicated by another person.6 Receptive language (processing) skills can be demonstrated as follows. If a child is asked “Do you like cats?” she must first decide to whom the question is directed (to her and not someone else). She must then search her long-term memory for the word/concept “cat” (compared with dog, a similar concept; or with mat, a similar-sounding word), and process the word “like” (compared with “dislike”). Now the child understands the question and can decide on an answer.
Expressive language is a child’s ability to speak; the mental process used to produce speech and communicate a message.6 To answer, “Yes, I like cats,” the child retrieves the concept “cat” from memory (cognition and semantics: the meanings of words, their relationships and usage), finds the right words (vocabulary), puts them in the right order (syntax), uses the right verb tense (grammar/morphology), assembles the right sounds in order, initiates the neuromotor acts (phonology/speech production), and communicates that she understands what cats are and that she likes them (pragmatics; socially/contextually appropriate responses).
Resources on language development. Typical language development, the length of which varies among children, must be practiced in a rich linguistic environment. Some children are adventurous with language. They babble, talk, and communicate in a carefree manner. Others are cautious. They may wait until they are sure of their skills before attempting a new word. Usually, concern about a child’s speech and language development arises if there is no speech, if speech is not clear, or if speech or language is different from that of peers.
The Centers for Disease Control and Prevention, under their “Learn the signs. Act early” campaign (www.cdc.gov/actearly), has published checklists of children’s developmental milestones from 2 months to 5 years of age on social, communication, cognitive, and motor skills. Health care providers helping parents determine if their child’s communication is developing normally can find information and materials at http://www.cdc.gov/ncbddd/actearly/hcp/index.html.15
Hearing impairments
Moeller et al16 surveyed 1968 primary care physicians on their attitude, practices, and knowledge of universal hearing screenings for newborns. They noted limitations in awareness of EI options for infants with hearing loss: proper times and places for referrals, available communication modalities, cochlear implant candidacy, and professionals in their locale with expertise on hearing loss. These knowledge gaps involved some medical issues, such as hearing loss genetics and later-onset hearing loss in infants and children. They also found low confidence in providing information to families about how to proceed with EI and in discussing intervention needs and resources.
Adverse effects of hearing loss. Hearing loss can be unilateral or bilateral, conductive or sensorineural, and can range in severity from mild to profound. According to the National Institute on Deafness and Communication Disorders (NIDCD), one in every 350 infants is born with a significant hearing loss, and others become deaf due to childhood illness or injury.17 According to ASHA,18 hearing loss can affect children in 4 major ways:19 delays in the development of receptive (comprehension) and expressive communication skills; a language deficit that causes learning problems and reduces academic achievement; communication difficulties that often lead to social isolation and poor self-concept; and impact on vocational choices and options.20 NIDCD provides a checklist to determine a child’s hearing status at http://www.nidcd.nih.gov/health/hearing/silence.asp.17
Timing of intervention is significant. EI is critical in minimizing the deleterious effects of hearing loss and in optimizing speech and language development. Severity of hearing loss influences EI outcome, and treatment options depend on the hearing loss having occurred either before language development (prelingually) or after (postlingually). Early management of hearing impairment can improve language, especially for children with a severe or profound hearing loss.21
Devices and methods that promote communication development for students who are deaf or hard of hearing include the use of hearing aids to amplify residual hearing for oral or auditory-oral approaches; the manual approach stressing sign-language (American Sign Language or Signed English); or Total Communication using both the oral and sign-language methods.
With an infant, suspicion of a hearing problem warrants referral to an otorhinolaryngologist or an audiologist for thorough evaluation. In New Jersey, an EI referral typically triggers a referral to one of these specialists. TABLE 2 lists resources for early identification and intervention in communication disorders.
TABLE 2
| Resources for early identification and intervention in communication disorders | |
| Resource | Information |
| American Speech-Language-Hearing Association (ASHA) | Communication skills, milestones, disorders, and treatment resources across the lifespan; for parents and professionals |
| CDC’s “Learn the signs. Act early” campaign
| Children’s developmental milestones from 2 months to 5 years; checklists for parents |
| Children’s developmental milestones and Early Intervention for health care providers | |
| National Institute on Deafness and Other Communication Disorders | Speech and language development checklists |
| National Institute on Deafness and Other Communication Disorders | Hearing loss and its effects on communication; identification and management options |
| Center for Parent Information and Resources | Early Intervention: overview and process |
| National Institute of Mental Health | Autism spectrum disorder; a guide for parents |
| First Signs, Inc | Early warning signs of autism spectrum disorder; for parents and professionals |
| The Autism Screening Test | Autism screening test to identify children 16-30 months who should receive a more thorough assessment for possible early signs of autism spectrum disorder or developmental delay |
Autism spectrum disorder
The fifth edition of the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-5)22 has revised its diagnostic codes for ASD. An in-depth analysis of the new diagnosis is beyond the purview of this article, but it deserves a few comments.
The revised diagnosis of ASD consolidates the previously separate diagnoses of autistic disorder, Asperger’s disorder, childhood disintegrative disorder, and pervasive developmental disorder/not otherwise specified.22-24 According to DSM-5, an individual with ASD must have 1) persistent deficits in social communication and social interactions, 2) restricted, repetitive patterns of behavior, interests or activities, 3) symptoms present in early childhood (but may not become fully manifest until social demands exceed limited capacities), and 4) symptoms that limit and impair everyday functioning.22,24
Onset of the disorder must be obvious before age 3 for a child to be eligible for EI. Children who do not qualify for an ASD diagnosis under the new DSM-5 definition may be included in a new category called social communication disorder (SCD) under “Communication Disorders” in DSM-5. SCD is defined as impairment in pragmatics that impacts development of social relationships and comprehension of social conversations. ASD must be ruled out before a diagnosis of SCD can be made.23,24
Early intervention services
Individual states offer EI services to families to facilitate detection of developmental delays in children and to provide a comprehensive system of support designed to reduce the effects of disabilities (or to prevent learning and developmental problems later in life).5 The rationale for EI is that the earlier interventions are started, the less likely later interventions will be needed.25 EI services are provided to children from birth through their third birthday; services are free of charge to eligible families or on a sliding payment scale determined by a family’s income.
Resources for information and referrals. To access EI services on behalf of a family, contact a local hospital or point them to the CPIR (http://www.parentcenterhub.org/repository/disability-landing/).2 For families with disabled children older than 3, consider suggesting that parents contact the local school district (even if the child isn’t enrolled there) to arrange an evaluation under the Individuals with Disabilities Education Act (http://idea.ed.gov/).26 Because individual states’ EI organizations may have slightly different procedures, it is best to consult one’s own state EI site for specific information regarding referrals (TABLE 3).
Efficacy of treatment
The prevalence of specific communication disorders varies widely, as do prognoses, possibly due to the variability of underlying causes (physical/biological/medical or environmental/educational).27 Also, as described earlier, “communication disorder” is an umbrella term inclusive of problems as diverse as resonance (eg, hypernasality due to a submucous palatal cleft), severe language delay (eg, due to Down syndrome), or lisp (misarticulation of the “s” sound).
Is therapy effective? Speech and language pathologists use the National Outcomes Measurement System (NOMS) as an index of the outcomes of treatment on functional communication along 6 scales. The most frequent types of communication problems seen in the prekindergarten children’s NOMS were “articulation” (75% of children), “spoken language production” (61%), and “spoken language comprehension” (42%). Problems in the remaining 3 scales (“pragmatics,” “cognitive orientation,” and “swallowing”) were seen in fewer than 15% of the preschool-age students.28
Articulation therapy yielded improvement in 69.3% of cases, spoken language comprehension therapy in 65.3%, and spoken language production therapy in 65.2%.28 Such outcomes support regular screening of children’s communication development and, as needed, referral for EI.
TABLE 3
CORRESPONDENCE
Christopher Mulrine, EdD; William Paterson University, 1600 Valley Road 3003 Wayne, NJ 07474-0920; [email protected]
1. Wankoff LS. Warning signs in the development of speech, language, and communication: when to refer to a speech-language pathologist. J Child Adolesc Psychiatr Nurs. 2011;24:175-184.
2. Center for Parent Information and Resources. Disabilities. Center for Parent Information and Resources Web site. Available at: http://www.parentcenterhub.org/repository/disability-landing/. Updated June 2014. Accessed January 24, 2015.
3. Denver Developmental Materials, Inc. Denver II Online. Denver Developmental Materials, Inc Web site. Available at: http://denverii.com/denverii/. Accessed January 24, 2015.
4. Guralnick MJ. The Effectiveness of Early Intervention. Baltimore, MD: Brookes Publishing; 2013.
5. Schwartz HD. A Primer on Communication and Communicative Disorders. Boston, MA: Pearson Education; 2012.
6. Heward WL. Exceptional Children: An Introduction to Special Education. 10th ed. Boston, Mass: Pearson Education; 2013.
7. Hallahan DP, Kauffman J M, Pullen PC. Exceptional Learners: An Introduction to Special Education. 13th ed. Upper Saddle River, NJ: Pearson Education; 2015.
8. American Speech-Language-Hearing Association. How does your child hear and talk? American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/speech/development/chart.htm. Accessed January 24, 2015.
9. Roth FP, Troia GA, Worthington CK, et al. Promoting awareness of sounds in speech: An initial report of an early intervention program for children with speech and language impairments. Appl Psycholinguistics. 2002;23:535-565.
10. Busari JO, Weggelaar NM. How to investigate and manage the child who is slow to speak. BMJ. 2004;328:272-276.
11. American Speech-Language-Hearing Association. Identify the Signs of Communication Disorders. Identify the Signs of Communication Disorders Web site. Available at: http://identifythesigns.org/. Accessed January 24, 2015.
12. McLaughlin MR. Speech and language delay in children. Am Fam Physician. 2011;83:1183-1188.
13. American Speech-Language-Hearing Association. Child speech and language. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/speech/disorders/ChildSandL.htm. Accessed January 24, 2015.
14. Bondurant-Utz JA. Practical Guide to Assessing Infants and Preschoolers with Special Needs. Upper Saddle River, NJ: Pearson Education; 2002.
15. Centers for Disease Control and Prevention. Learn the Signs: Act Early. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/actearly/hcp/index.html. Accessed January 24, 2015.
16. Moeller MP, White KR, Shisler L. Primary care physicians’ knowledge, attitudes, and practices related to newborn hearing screening. Pediatrics. 2006;118:1357-1370.
17. National Institute on Deafness and Other Communication Disorders. Your baby’s hearing and communicative checklist. National Institute on Deafness and Other Communication Disorders Web site. Available at: http://www.nidcd.nih.gov/health/hearing/silence.asp. Accessed January 24, 2015.
18. American Speech-Language-Hearing Association. Effects of hearing loss on development. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/hearing/Effects-of-Hearing-Loss-on-Development/. Accessed January 24, 2015.
19. American Speech-Language-Hearing Association. Facts about pediatric hearing loss. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/aud/Facts-about-Pediatric-Hearing-Loss/. Accessed January 24, 2015.
20. Tang BG, Feldman HM, Padden C, et al. Delayed recognition of profound hearing loss in a 7-year-old girl with a neurological condition. J Dev Behav Pediatr. 2010;31(3 suppl):S42-S45.
21. Watkin P, McCann D, Law C, et al. Language ability in children with permanent hearing impairment: the influence of early management and family participation. Pediatrics. 2007;120:e694-e701.
22. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
23. Autism Match. 5 things to know about autism and DSM-5. Austism Match Web site. Available at: https://autismmatch.org/info/news/2012/05/02/5-things-to-know-about-autism-and-dsm-5. Accessed January 24, 2015.
24. American Psychiatric Publishing. Autism Spectrum Disorder. American Psychiatric Association Web site. Available at: http://www.dsm5.org/Documents/Autism%20Spectrum%20Disorder%20Fact%20Sheet.pdf. Accessed January 24, 2015.
25. Law J, Garrett Z, Nye C. Speech and language therapy interventions for children with primary speech and language delay or disorder. Cochrane Database Syst Rev. 2003;(3):CD004110.
26. U.S. Department of Education. Building the legacy: IDEA 2004. Individuals with Disabilities Education Act Web site. Available at: http://idea.ed.gov/. Accessed January 24, 2015.
27. Friend MP. Special Education: Contemporary Perspectives for School Professionals. Boston, MA: Pearson; 2005.
28. Mullen R, Schooling T. The National Outcomes Measurement System for Pediatric Speech-Language Pathology. Lang Speech Hearing Services Schools. 2010;41:44-60.
1. Wankoff LS. Warning signs in the development of speech, language, and communication: when to refer to a speech-language pathologist. J Child Adolesc Psychiatr Nurs. 2011;24:175-184.
2. Center for Parent Information and Resources. Disabilities. Center for Parent Information and Resources Web site. Available at: http://www.parentcenterhub.org/repository/disability-landing/. Updated June 2014. Accessed January 24, 2015.
3. Denver Developmental Materials, Inc. Denver II Online. Denver Developmental Materials, Inc Web site. Available at: http://denverii.com/denverii/. Accessed January 24, 2015.
4. Guralnick MJ. The Effectiveness of Early Intervention. Baltimore, MD: Brookes Publishing; 2013.
5. Schwartz HD. A Primer on Communication and Communicative Disorders. Boston, MA: Pearson Education; 2012.
6. Heward WL. Exceptional Children: An Introduction to Special Education. 10th ed. Boston, Mass: Pearson Education; 2013.
7. Hallahan DP, Kauffman J M, Pullen PC. Exceptional Learners: An Introduction to Special Education. 13th ed. Upper Saddle River, NJ: Pearson Education; 2015.
8. American Speech-Language-Hearing Association. How does your child hear and talk? American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/speech/development/chart.htm. Accessed January 24, 2015.
9. Roth FP, Troia GA, Worthington CK, et al. Promoting awareness of sounds in speech: An initial report of an early intervention program for children with speech and language impairments. Appl Psycholinguistics. 2002;23:535-565.
10. Busari JO, Weggelaar NM. How to investigate and manage the child who is slow to speak. BMJ. 2004;328:272-276.
11. American Speech-Language-Hearing Association. Identify the Signs of Communication Disorders. Identify the Signs of Communication Disorders Web site. Available at: http://identifythesigns.org/. Accessed January 24, 2015.
12. McLaughlin MR. Speech and language delay in children. Am Fam Physician. 2011;83:1183-1188.
13. American Speech-Language-Hearing Association. Child speech and language. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/speech/disorders/ChildSandL.htm. Accessed January 24, 2015.
14. Bondurant-Utz JA. Practical Guide to Assessing Infants and Preschoolers with Special Needs. Upper Saddle River, NJ: Pearson Education; 2002.
15. Centers for Disease Control and Prevention. Learn the Signs: Act Early. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/ncbddd/actearly/hcp/index.html. Accessed January 24, 2015.
16. Moeller MP, White KR, Shisler L. Primary care physicians’ knowledge, attitudes, and practices related to newborn hearing screening. Pediatrics. 2006;118:1357-1370.
17. National Institute on Deafness and Other Communication Disorders. Your baby’s hearing and communicative checklist. National Institute on Deafness and Other Communication Disorders Web site. Available at: http://www.nidcd.nih.gov/health/hearing/silence.asp. Accessed January 24, 2015.
18. American Speech-Language-Hearing Association. Effects of hearing loss on development. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/public/hearing/Effects-of-Hearing-Loss-on-Development/. Accessed January 24, 2015.
19. American Speech-Language-Hearing Association. Facts about pediatric hearing loss. American Speech-Language-Hearing Association Web site. Available at: http://www.asha.org/aud/Facts-about-Pediatric-Hearing-Loss/. Accessed January 24, 2015.
20. Tang BG, Feldman HM, Padden C, et al. Delayed recognition of profound hearing loss in a 7-year-old girl with a neurological condition. J Dev Behav Pediatr. 2010;31(3 suppl):S42-S45.
21. Watkin P, McCann D, Law C, et al. Language ability in children with permanent hearing impairment: the influence of early management and family participation. Pediatrics. 2007;120:e694-e701.
22. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013.
23. Autism Match. 5 things to know about autism and DSM-5. Austism Match Web site. Available at: https://autismmatch.org/info/news/2012/05/02/5-things-to-know-about-autism-and-dsm-5. Accessed January 24, 2015.
24. American Psychiatric Publishing. Autism Spectrum Disorder. American Psychiatric Association Web site. Available at: http://www.dsm5.org/Documents/Autism%20Spectrum%20Disorder%20Fact%20Sheet.pdf. Accessed January 24, 2015.
25. Law J, Garrett Z, Nye C. Speech and language therapy interventions for children with primary speech and language delay or disorder. Cochrane Database Syst Rev. 2003;(3):CD004110.
26. U.S. Department of Education. Building the legacy: IDEA 2004. Individuals with Disabilities Education Act Web site. Available at: http://idea.ed.gov/. Accessed January 24, 2015.
27. Friend MP. Special Education: Contemporary Perspectives for School Professionals. Boston, MA: Pearson; 2005.
28. Mullen R, Schooling T. The National Outcomes Measurement System for Pediatric Speech-Language Pathology. Lang Speech Hearing Services Schools. 2010;41:44-60.
Intrathecal analgesia: Time to consider it for your patient?
› Consider continuous intrathecal (IT) analgesia for chronic pain patients with refractory symptoms or intolerance to systemic medication. B
› Explore the possibility of using an IT delivery system
to treat malignant pain syndrome, particularly for patients with a life expectancy of more than 6 months. A
› Do not rule out IT analgesia for patients with refractory nonmalignant pain; while considerations in such cases are more complex, benefits include the efficacy of lower doses and fewer adverse effects. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A switch to hydromorphone 20 mg/d—the physician used the 5:1 morphine-to-hydromorphone conversion ratio, then decreased the dose by 50% to account for incomplete cross-tolerance—left Ms. G lethargic. In addition, her pain score rose to 5, and she began having difficulty swallowing the medication. Prior to the drug rotation, she was able to perform light tasks and was alert enough to interact with her family.
If Ms. G were your patient, what would be your next step?
Continuous intrathecal (IT) drug delivery systems have been in use for more than 30 years.1 And, while IT administration of analgesia has become increasingly useful for patients with refractory chronic pain and spasticity, it remains an underutilized resource.2 Delivered directly into the pre- and post-synaptic opioid receptors in the dorsal horn of the spinal cord, IT analgesia bypasses first-pass metabolism. The result: a higher rate of efficacy, with smaller dosages and fewer adverse effects than systemic delivery.1
The drugs are delivered via a small battery-powered programmable pump that is implanted under the subcutaneous tissue of the abdomen and connected to a catheter tunneled to the site of spinal entry. The device must be refilled periodically—typically every one to 3 months—but this is not a difficult process. It can be done in an office setting or in the patient’s home by a specially trained visiting nurse.3
There is ample reason to consider this approach when systemic analgesics or antispasmodics fail to control pain or cause unacceptable adverse effects. So why isn’t it used more frequently? One factor may be that many primary care physicians—often the first practitioners called upon to manage these complicated cases—know too little about it.
Who is a potential candidate for IT analgesia? What medications can be administered via this route? What is the role of a family physician (FP) in coordinating and overseeing the care of a patient being treated with IT therapy? Our goals in writing this review are to address these questions.
Patient selection: Not just for cancer pain
FPs interested in referring patients for IT therapy have many factors to consider before consulting a pain specialist. Foremost among them are the different criteria for individuals with cancer-related pain and those with chronic nonmalignant pain.
IT analgesia for cancer pain has been shown to improve patients’ quality of life and potentially increase long-term survival due to a decrease in systemic toxicity.4-6 An appropriate candidate is an individual who, like Ms. G, was initially responsive to systemic opioids but later developed refractory symptoms or intolerance.7 Because of the invasive nature and high cost of implantation, subcutaneous IT pumps are typically reserved for patients with a life expectancy of more than 6 months.7 But implantation may be considered for those with a shorter life expectancy if they have severe pain or cannot tolerate the adverse effects of systemic analgesia.
Noncancer pain is more complex
The use of IT analgesia in patients with chronic nonmalignant pain, such as failed back surgery syndrome, spasticity associated with multiple sclerosis, or diabetic neuropathy, is both more controversial and more complex. It is important for FPs to recognize the multidimensional nature of this type of pain, which may be complicated by physical, psychological, and behavioral factors, including the possibility of addiction.8-11
Although IT analgesia is less subject to abuse and diversion than systemic opioids, the dependent relationship associated with a continuous delivery system makes risk stratification a necessity.12 Psychological testing is commonly used to evaluate potential candidates for long-term IT analgesia.
Prior to placement, patients must have had a failed course of conservative pain management and have no surgical options, no medical contraindications (eg, spinal pathology or susceptibility to infection), and no evidence of active addiction.12 A medication history is crucial, too, to identify use of anticoagulation therapy—a relative contraindication—as well as drug allergies and potential drug-drug interactions to guard against.3
An IT trial may be required
It is common practice for patients to undergo an IT analgesia trial prior to implantation of a subcutaneous pump. This involves using an external pump to infuse the selected medication intrathecally and slowly titrating it according to symptoms for 2 to 3 days. During this time frame, the patient records his or her response; a reduction by more than half in VAS pain score is considered a success, indicating that the patient is an appropriate candidate for placement of the device.3,13
Drug choices—a look at the evidence
The US Food and Drug Administration (FDA) has approved 3 medications for continuous IT delivery: morphine, ziconotide, and baclofen. But it is common practice to use alternative agents, such as other opioids, local anesthetics, or alpha 2-adrenergic agonists (TABLE).3,14-34
CASE › Ms. G’s primary care physician referred her to a pain specialist, who thought she would benefit from IT analgesia. After a successful single-shot IT trial with 0.5 mg morphine, the patient underwent implantation. The specialist chose morphine as the IT agent because of Ms. G’s history of successful pain relief with it, and because such a low dose was unlikely to be a problem for a patient with renal failure.
A month later, when she returned to the specialist to have the pump refilled, Ms. G reported a pain score of 3.
Opioids such as morphine exhibit a wider spread of analgesia when administered intrathecally, resulting in fewer adverse effects than systemic opioids.13,35,36 The mu-opioid receptors in the dorsal horn of the spinal cord are the primary target of IT opioids.
In a multicenter randomized trial involving 200 cancer patients on opioids, Smith et al4 compared implantable IT drug delivery systems with comprehensive medical management. The mean VAS pain score in the IT group fell 52% vs a decline of 39% in the medical management group.
The evidence supporting IT opioids for nonmalignant pain is not as strong. This may be due to inherent differences in pain mechanisms. In cancer pain, between 75% and 90% of pain is either nociceptive or mixed nociceptive-neuropathic; the etiology of noncancer pain is more variable.37-39
Although IT opioid therapy is associated with a lower incidence of adverse effects than systemic therapy, this route is not devoid of adverse effects. Opioids delivered intrathecally may still be associated with respiratory depression, constipation, urinary retention, nausea/vomiting, sweating, and hyperalgesia.39 In addition, chronic opioid use suppresses the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis14,40,41—a risk with long-term IT as well as systemic administration.14 Respiratory depression most commonly results from accidental overdosing, and patients must be monitored during initiation and dose escalation of IT opioid therapy.15
Local anesthetics. Numerous studies have documented the favorable outcomes of combining local anesthetics with opioids for patients with cancer16-20 and noncancer pain.21,22 Local anesthetics work via the blockade of voltage-gated sodium channels, interfering with neuron depolarization.17
A retrospective study in which patients with malignant pain and those with failed back surgery syndrome had bupivacaine added to their IT opioid solution found that the combination led to lower pain scores and a 23% reduction in opioid dosage.20 In another retrospective review, researchers demonstrated that the coadministration of IT bupivacaine and an opioid decreased the rate of opioid dose escalation by 65% over the first year in patients with noncancer pain.23
However, a double-blind randomized, crossover multicenter study found that in patients with chronic nonmalignant pain, the addition of bupivacaine to IT opioids failed to produce significant improvement in pain control compared with opioid use alone. Quality of life scores did improve, however, in the group receiving combination therapy.24
Adverse effects of local anesthetics delivered intrathecally include numbness, paresthesias, weakness, bowel/bladder dysfunction, and neurotoxicity.17,19,25
Calcium channel blockers. Found in venom produced by the marine snail Conus magus, ziconotide blocks presynaptic N-type channels. It is the only calcium channel blocker used to manage chronic pain.26 Several trials in patients with malignant and nonmalignant pain have shown a significant decrease in VAS pain scores compared with placebo.25,26 In addition, a multicenter, double-blind placebo-controlled crossover study evaluating IT ziconotide for the treatment of refractory pain in 111 patients with cancer and AIDS found that the treatment group obtained significantly better pain relief than the controls (53% vs 17.5% using a VAS pain intensity score).25 However, 31% of those in the treatment group experienced adverse effects, the most common of which were confusion, somnolence, and urinary retention.
Ziconotide has FDA approval only as monotherapy. But because of its high cost and adverse effect profile, it is mainly used in combination with other IT drugs.27 Ziconotide increases the risk of suicide in patients with a history of depression.28 The prevalence of adverse effects correlates with a higher dose, faster titration rate, and older age.26,28
Alpha-2 adrenergic agonists. Clonidine is the only alpha-2 agonist with FDA approval for epidural use, with several studies supporting its off-label use in combination with IT therapy.22,29 In a prospective open-label study evaluating combination IT therapy in patients with failed back surgery syndrome, 73% reported subjective ratings of good or excellent at 2-year follow-up.22 The most common adverse effects were sedation, hypotension, nausea, and dry mouth.
Gamma-aminobutyric acid (GABA) agonists. Baclofen, a GABA agonist with FDA approval for the treatment of spasticity, has been used intrathecally since the mid-1980s.32 Several studies have supported its effectiveness for this purpose.30,42 Clinical studies have also found IT baclofen to be effective in treating conditions such as complex regional pain syndrome, central pain, and neuropathic pain secondary to failed back surgery syndrome.31,32 In one randomized double-blind crossover trial, 7 women with complex regional pain syndrome were given bolus injections of baclofen or saline. Those treated with baclofen experienced a reduction in pain and regained function.31
In another trial—a double-blind placebo-controlled study of patients with multiple sclerosis and spinal cord injury comparing baclofen with placebo—those treated with baclofen showed significant reductions in dysesthetic and spasm-related pain.32 The most common adverse effects of baclofen are drowsiness, cognitive impairment, weakness, gastrointestinal complaints, and sexual dysfunction.31
Which patients and which drugs? An expert consensus
Due to the potential for inconsistent patient management and the use of therapies with anecdotal evidence, the Polyanalgesic Consensus Conference (PACC)—a panel of experts in IT therapy—convened in 2000, 2003, 2007, and 2011 to develop recommendations for IT therapy and an algorithm for drug selection. PACC’s list of chronic conditions for which IT should be considered includes axial low back pain, postherpetic neuralgia, spinal cord injury, spinal stenosis, pancreatitis, osteoporosis, compression fracture, and phantom limb pain, among others.
The algorithm contains separate arms for neuropathic, nociceptive, and mixed pain states. First-line agents for neuropathic pain include morphine, alone or combined with bupivacaine, and ziconotide. For nociceptive pain, morphine, hydromorphone, fentanyl, and ziconotide are all first-line agents; for mixed pain states, the appropriate choice should be based on the clinical scenario.33
Overseeing IT pain management in primary care
Referring potential candidates for IT therapy to specialists in pain management is just the beginning. While patients typically return to the specialist for pump refills, it is important that they see their primary care physician regularly, as well. Vigilance is required of both the FP and the patient. Any sudden worsening in pain level or acute change in neurologic function must be reported to the pain specialist immediately.
Adverse effects of medications are the most common complications
Kamran and Wright43 performed a retrospective review of their practice’s Intrathecal Drug Delivery Systems database of 122 patients and found that adverse medication effects were most common, accounting for 77% of complications.
Catheter malfunctions were next, at 16%, followed by infections, at 5%.43 In other studies, catheter-related complications were found to have an incidence of 15% to 25%.44,45 Problems include kinking, breaking, leaking, and migration of the catheter. Advise patients to immediately contact their pain specialist for evaluation if they experience a sudden loss of, or change in, pain control.
Infectious complications, which occur infrequently, are usually limited to superficial wounds, although epidural abscesses and meningitis are possible.46 Standard perioperative antibiotic administration helps to minimize the risk of infection. If a patient presents with signs and symptoms of an epidural abscess—back pain, fever, and variable neurologic deficits—emergent initiation of intravenous antibiotics is needed. Magnetic resonance imaging (MRI) with and without gadolinium should be obtained, as well.22
Spinal damage. Although IT catheters are placed under fluoroscopic guidance, there is a risk of direct injury to the spinal cord; this is more common if the catheter is placed above the level of the conus medullaris. Damage to the spinal cord or exiting spinal nerves will manifest as pain, sensory loss, and/or weakness over a dermatomal distribution.43
Neurologic sequelae, ranging from mild symptoms to paraplegia, can result from the formation of a granuloma at the tip of the spinal catheter. A sudden increase in pain usually occurs prior to neurologic deterioration, thereby allowing for early detection and intervention.47 Development of a granuloma appears to be related to the long-term infusion of high-concentration opioids.34 The diagnosis is confirmed by MRI, but physical exam and history are imperative in making the initial diagnosis.
In cases of mild neurologic symptoms, a transition to saline infusion through the pump may allow the granuloma to absorb; more severe cases may require neurosurgical intervention.47
Is your patient scheduled for an IT drug trial?
If a patient of yours is scheduled for an IT drug trial, ideally followed by pump implantation, microdosing—the practice of weaning the individual from oral opioids prior to the procedure so that very low doses of IT opioids will suffice—may play a role.48,49 While this approach appears promising, however, there is little in the way of definitive evidence of efficacy.
CASE › Over time, Ms. G’s maintenance IT dose of morphine had to be slowly increased from 0.5 mg to 1 mg/d. At bimonthly visits with her FP, she consistently reports pain scores of 3 on a scale of 1 to 10. The patient’s function has returned to baseline, and she has minimal adverse effects.
CORRESPONDENCE
Jessica Tsukanov, DO, Montefiore Medical Center, 3347 Steuben Avenue, Bronx, NY 10467; [email protected]
1. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
2. Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18:338.
3. Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
4. Smith TJ, Staats PS, Deer T, et al; Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-4049.
5. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003;4:441-447.
6. Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239-247.
7. Hassenbusch SJ. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology. 1999;13(5 suppl 2):S63-S67.
8. Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain. 2004;109:242-249.
9. Gerber HR. Intrathecal morphine for chronic benign pain. Best Pract Res Clin Anesthesiol. 2003;17:429-442.
10. Tuner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
11. Brown J, Klapow J, Doleys D, et al. Disease-specific and generic health outcomes: a model for the evaluation of long-term intrathecal opioid therapy in noncancer low back pain patients. Clin J Pain. 1999;15:122-131.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2006;6:432-442.
13. Ahmed SU, Martin NM, Chang Y. Patient selection and trial methods for intraspinal drug delivery for chronic pain: a national survey. Neuromodulation. 2005;8:112-120.
14. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
15. Coffey RJ, Owens ML, Broste SK, et al. Mortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer pain. Anesthesiology. 2009;111:881-891.
16. Sjöberg M, Nitescu P, Appelgren L, et al. Long-term intrathecal morphine and bupivacaine in patients with refractory cancer pain. Results from a morphine:bupivacaine dose regimen of 0.5:4.75 mg/ml. Anesthesiology. 1994;80:284-297.
17. Sjöberg M, Appelgen L, Einarsson S, et al. Long-term intrathecal morphine and bupivacaine in “refractory” cancer pain. I. Results from the first series of 52 patients. Acta Anaesthsiol Scand. 1991;35:30-43.
18. Van Dongen RT, Crul BJ, De Bock M. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: a retrospective analysis of 51 cases. Pain. 1993;55:119-123.
19. van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166-172.
20. Deer TR, Caraway DL, Kim CK, et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
21. Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539-548.
22. Rainov NG, Heidecke V, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
23. Veizi IE, Hayek SM, Narouze S, et al. Combination of intrathecal opioids with bupivacaine attenuates opioid dose escalation in chronic noncancer pain patients. Pain Med. 2011;12:1481-1489.
24. Mironer YE, Haasis JC, Chapple I, et al. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: A double blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208-213.
25. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63-70.
26. Rauck RL, Wallace MS, Leong MS, et al; Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393-406.
27. Wallace MS, Rauck R, Fisher R, et al; Ziconotide 98-022 Study Group. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth Analg. 2008;106:628-637.
28. Maier C, Gockel HH, Gruhn K, et al. Increased risk of suicide under intrathecal ziconotide treatment? - a warning. Pain. 2011;152:235-237.
29. Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668-677.
30. Tarrico M, Adone R, Pagliacci C, et al. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000;(2):CD001131.
31. van Hilten BJ, van de Beek WT, Hoff JI, et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343:625-630.
32. Herman RM, D’Luzansky SC, Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions. A pilot study. Clin J Pain. 1992;8:338-345.
33. Deer T, Prager J, Levy R, et al. Polyanalgesic consensus conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436-466.
34. Yaksh TL, Coffey RJ. Spinal opiate toxicity. In: Proceedings of American Society of Regional Anesthesia and Pain Medication Conference; November 18-21, 2004; Phoenix, AZ.
35. Levy MH. Pharmacologic management of cancer pain. Semin Oncol. 1994;21:718-739.
36. Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276-310.
37. Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to a hospice. Palliat Med. 2001;15:243-246.
38. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273-281.
39. Hanks GW, Forbes K. Opioid responsiveness. Acta Anaesthesiol Scan. 1997;41:154-158.
40. Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126-131.
41. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3 suppl 1):S12-S18.
42. Beard S, Hunn A. Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7:iii,ix-x,1-111.
43. Kamran S, Wright BD. Complications of intrathecal drug delivery systems. Neuromodulation. 2001;4:111-115.
44. Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage. 2000;19:209-215.
45. Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related complications. Neuromodulation. 2003;6:32-41.
46. Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
47. Miele VJ, Price KO, Bloomfield S, et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
48. Hayek SM. Intrathecal “microdosing”: reality or artifact? Pain Med. 2012;13:1664-1665.
49. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician. 2011;14:343-351.
› Consider continuous intrathecal (IT) analgesia for chronic pain patients with refractory symptoms or intolerance to systemic medication. B
› Explore the possibility of using an IT delivery system
to treat malignant pain syndrome, particularly for patients with a life expectancy of more than 6 months. A
› Do not rule out IT analgesia for patients with refractory nonmalignant pain; while considerations in such cases are more complex, benefits include the efficacy of lower doses and fewer adverse effects. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A switch to hydromorphone 20 mg/d—the physician used the 5:1 morphine-to-hydromorphone conversion ratio, then decreased the dose by 50% to account for incomplete cross-tolerance—left Ms. G lethargic. In addition, her pain score rose to 5, and she began having difficulty swallowing the medication. Prior to the drug rotation, she was able to perform light tasks and was alert enough to interact with her family.
If Ms. G were your patient, what would be your next step?
Continuous intrathecal (IT) drug delivery systems have been in use for more than 30 years.1 And, while IT administration of analgesia has become increasingly useful for patients with refractory chronic pain and spasticity, it remains an underutilized resource.2 Delivered directly into the pre- and post-synaptic opioid receptors in the dorsal horn of the spinal cord, IT analgesia bypasses first-pass metabolism. The result: a higher rate of efficacy, with smaller dosages and fewer adverse effects than systemic delivery.1
The drugs are delivered via a small battery-powered programmable pump that is implanted under the subcutaneous tissue of the abdomen and connected to a catheter tunneled to the site of spinal entry. The device must be refilled periodically—typically every one to 3 months—but this is not a difficult process. It can be done in an office setting or in the patient’s home by a specially trained visiting nurse.3
There is ample reason to consider this approach when systemic analgesics or antispasmodics fail to control pain or cause unacceptable adverse effects. So why isn’t it used more frequently? One factor may be that many primary care physicians—often the first practitioners called upon to manage these complicated cases—know too little about it.
Who is a potential candidate for IT analgesia? What medications can be administered via this route? What is the role of a family physician (FP) in coordinating and overseeing the care of a patient being treated with IT therapy? Our goals in writing this review are to address these questions.
Patient selection: Not just for cancer pain
FPs interested in referring patients for IT therapy have many factors to consider before consulting a pain specialist. Foremost among them are the different criteria for individuals with cancer-related pain and those with chronic nonmalignant pain.
IT analgesia for cancer pain has been shown to improve patients’ quality of life and potentially increase long-term survival due to a decrease in systemic toxicity.4-6 An appropriate candidate is an individual who, like Ms. G, was initially responsive to systemic opioids but later developed refractory symptoms or intolerance.7 Because of the invasive nature and high cost of implantation, subcutaneous IT pumps are typically reserved for patients with a life expectancy of more than 6 months.7 But implantation may be considered for those with a shorter life expectancy if they have severe pain or cannot tolerate the adverse effects of systemic analgesia.
Noncancer pain is more complex
The use of IT analgesia in patients with chronic nonmalignant pain, such as failed back surgery syndrome, spasticity associated with multiple sclerosis, or diabetic neuropathy, is both more controversial and more complex. It is important for FPs to recognize the multidimensional nature of this type of pain, which may be complicated by physical, psychological, and behavioral factors, including the possibility of addiction.8-11
Although IT analgesia is less subject to abuse and diversion than systemic opioids, the dependent relationship associated with a continuous delivery system makes risk stratification a necessity.12 Psychological testing is commonly used to evaluate potential candidates for long-term IT analgesia.
Prior to placement, patients must have had a failed course of conservative pain management and have no surgical options, no medical contraindications (eg, spinal pathology or susceptibility to infection), and no evidence of active addiction.12 A medication history is crucial, too, to identify use of anticoagulation therapy—a relative contraindication—as well as drug allergies and potential drug-drug interactions to guard against.3
An IT trial may be required
It is common practice for patients to undergo an IT analgesia trial prior to implantation of a subcutaneous pump. This involves using an external pump to infuse the selected medication intrathecally and slowly titrating it according to symptoms for 2 to 3 days. During this time frame, the patient records his or her response; a reduction by more than half in VAS pain score is considered a success, indicating that the patient is an appropriate candidate for placement of the device.3,13
Drug choices—a look at the evidence
The US Food and Drug Administration (FDA) has approved 3 medications for continuous IT delivery: morphine, ziconotide, and baclofen. But it is common practice to use alternative agents, such as other opioids, local anesthetics, or alpha 2-adrenergic agonists (TABLE).3,14-34
CASE › Ms. G’s primary care physician referred her to a pain specialist, who thought she would benefit from IT analgesia. After a successful single-shot IT trial with 0.5 mg morphine, the patient underwent implantation. The specialist chose morphine as the IT agent because of Ms. G’s history of successful pain relief with it, and because such a low dose was unlikely to be a problem for a patient with renal failure.
A month later, when she returned to the specialist to have the pump refilled, Ms. G reported a pain score of 3.
Opioids such as morphine exhibit a wider spread of analgesia when administered intrathecally, resulting in fewer adverse effects than systemic opioids.13,35,36 The mu-opioid receptors in the dorsal horn of the spinal cord are the primary target of IT opioids.
In a multicenter randomized trial involving 200 cancer patients on opioids, Smith et al4 compared implantable IT drug delivery systems with comprehensive medical management. The mean VAS pain score in the IT group fell 52% vs a decline of 39% in the medical management group.
The evidence supporting IT opioids for nonmalignant pain is not as strong. This may be due to inherent differences in pain mechanisms. In cancer pain, between 75% and 90% of pain is either nociceptive or mixed nociceptive-neuropathic; the etiology of noncancer pain is more variable.37-39
Although IT opioid therapy is associated with a lower incidence of adverse effects than systemic therapy, this route is not devoid of adverse effects. Opioids delivered intrathecally may still be associated with respiratory depression, constipation, urinary retention, nausea/vomiting, sweating, and hyperalgesia.39 In addition, chronic opioid use suppresses the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis14,40,41—a risk with long-term IT as well as systemic administration.14 Respiratory depression most commonly results from accidental overdosing, and patients must be monitored during initiation and dose escalation of IT opioid therapy.15
Local anesthetics. Numerous studies have documented the favorable outcomes of combining local anesthetics with opioids for patients with cancer16-20 and noncancer pain.21,22 Local anesthetics work via the blockade of voltage-gated sodium channels, interfering with neuron depolarization.17
A retrospective study in which patients with malignant pain and those with failed back surgery syndrome had bupivacaine added to their IT opioid solution found that the combination led to lower pain scores and a 23% reduction in opioid dosage.20 In another retrospective review, researchers demonstrated that the coadministration of IT bupivacaine and an opioid decreased the rate of opioid dose escalation by 65% over the first year in patients with noncancer pain.23
However, a double-blind randomized, crossover multicenter study found that in patients with chronic nonmalignant pain, the addition of bupivacaine to IT opioids failed to produce significant improvement in pain control compared with opioid use alone. Quality of life scores did improve, however, in the group receiving combination therapy.24
Adverse effects of local anesthetics delivered intrathecally include numbness, paresthesias, weakness, bowel/bladder dysfunction, and neurotoxicity.17,19,25
Calcium channel blockers. Found in venom produced by the marine snail Conus magus, ziconotide blocks presynaptic N-type channels. It is the only calcium channel blocker used to manage chronic pain.26 Several trials in patients with malignant and nonmalignant pain have shown a significant decrease in VAS pain scores compared with placebo.25,26 In addition, a multicenter, double-blind placebo-controlled crossover study evaluating IT ziconotide for the treatment of refractory pain in 111 patients with cancer and AIDS found that the treatment group obtained significantly better pain relief than the controls (53% vs 17.5% using a VAS pain intensity score).25 However, 31% of those in the treatment group experienced adverse effects, the most common of which were confusion, somnolence, and urinary retention.
Ziconotide has FDA approval only as monotherapy. But because of its high cost and adverse effect profile, it is mainly used in combination with other IT drugs.27 Ziconotide increases the risk of suicide in patients with a history of depression.28 The prevalence of adverse effects correlates with a higher dose, faster titration rate, and older age.26,28
Alpha-2 adrenergic agonists. Clonidine is the only alpha-2 agonist with FDA approval for epidural use, with several studies supporting its off-label use in combination with IT therapy.22,29 In a prospective open-label study evaluating combination IT therapy in patients with failed back surgery syndrome, 73% reported subjective ratings of good or excellent at 2-year follow-up.22 The most common adverse effects were sedation, hypotension, nausea, and dry mouth.
Gamma-aminobutyric acid (GABA) agonists. Baclofen, a GABA agonist with FDA approval for the treatment of spasticity, has been used intrathecally since the mid-1980s.32 Several studies have supported its effectiveness for this purpose.30,42 Clinical studies have also found IT baclofen to be effective in treating conditions such as complex regional pain syndrome, central pain, and neuropathic pain secondary to failed back surgery syndrome.31,32 In one randomized double-blind crossover trial, 7 women with complex regional pain syndrome were given bolus injections of baclofen or saline. Those treated with baclofen experienced a reduction in pain and regained function.31
In another trial—a double-blind placebo-controlled study of patients with multiple sclerosis and spinal cord injury comparing baclofen with placebo—those treated with baclofen showed significant reductions in dysesthetic and spasm-related pain.32 The most common adverse effects of baclofen are drowsiness, cognitive impairment, weakness, gastrointestinal complaints, and sexual dysfunction.31
Which patients and which drugs? An expert consensus
Due to the potential for inconsistent patient management and the use of therapies with anecdotal evidence, the Polyanalgesic Consensus Conference (PACC)—a panel of experts in IT therapy—convened in 2000, 2003, 2007, and 2011 to develop recommendations for IT therapy and an algorithm for drug selection. PACC’s list of chronic conditions for which IT should be considered includes axial low back pain, postherpetic neuralgia, spinal cord injury, spinal stenosis, pancreatitis, osteoporosis, compression fracture, and phantom limb pain, among others.
The algorithm contains separate arms for neuropathic, nociceptive, and mixed pain states. First-line agents for neuropathic pain include morphine, alone or combined with bupivacaine, and ziconotide. For nociceptive pain, morphine, hydromorphone, fentanyl, and ziconotide are all first-line agents; for mixed pain states, the appropriate choice should be based on the clinical scenario.33
Overseeing IT pain management in primary care
Referring potential candidates for IT therapy to specialists in pain management is just the beginning. While patients typically return to the specialist for pump refills, it is important that they see their primary care physician regularly, as well. Vigilance is required of both the FP and the patient. Any sudden worsening in pain level or acute change in neurologic function must be reported to the pain specialist immediately.
Adverse effects of medications are the most common complications
Kamran and Wright43 performed a retrospective review of their practice’s Intrathecal Drug Delivery Systems database of 122 patients and found that adverse medication effects were most common, accounting for 77% of complications.
Catheter malfunctions were next, at 16%, followed by infections, at 5%.43 In other studies, catheter-related complications were found to have an incidence of 15% to 25%.44,45 Problems include kinking, breaking, leaking, and migration of the catheter. Advise patients to immediately contact their pain specialist for evaluation if they experience a sudden loss of, or change in, pain control.
Infectious complications, which occur infrequently, are usually limited to superficial wounds, although epidural abscesses and meningitis are possible.46 Standard perioperative antibiotic administration helps to minimize the risk of infection. If a patient presents with signs and symptoms of an epidural abscess—back pain, fever, and variable neurologic deficits—emergent initiation of intravenous antibiotics is needed. Magnetic resonance imaging (MRI) with and without gadolinium should be obtained, as well.22
Spinal damage. Although IT catheters are placed under fluoroscopic guidance, there is a risk of direct injury to the spinal cord; this is more common if the catheter is placed above the level of the conus medullaris. Damage to the spinal cord or exiting spinal nerves will manifest as pain, sensory loss, and/or weakness over a dermatomal distribution.43
Neurologic sequelae, ranging from mild symptoms to paraplegia, can result from the formation of a granuloma at the tip of the spinal catheter. A sudden increase in pain usually occurs prior to neurologic deterioration, thereby allowing for early detection and intervention.47 Development of a granuloma appears to be related to the long-term infusion of high-concentration opioids.34 The diagnosis is confirmed by MRI, but physical exam and history are imperative in making the initial diagnosis.
In cases of mild neurologic symptoms, a transition to saline infusion through the pump may allow the granuloma to absorb; more severe cases may require neurosurgical intervention.47
Is your patient scheduled for an IT drug trial?
If a patient of yours is scheduled for an IT drug trial, ideally followed by pump implantation, microdosing—the practice of weaning the individual from oral opioids prior to the procedure so that very low doses of IT opioids will suffice—may play a role.48,49 While this approach appears promising, however, there is little in the way of definitive evidence of efficacy.
CASE › Over time, Ms. G’s maintenance IT dose of morphine had to be slowly increased from 0.5 mg to 1 mg/d. At bimonthly visits with her FP, she consistently reports pain scores of 3 on a scale of 1 to 10. The patient’s function has returned to baseline, and she has minimal adverse effects.
CORRESPONDENCE
Jessica Tsukanov, DO, Montefiore Medical Center, 3347 Steuben Avenue, Bronx, NY 10467; [email protected]
› Consider continuous intrathecal (IT) analgesia for chronic pain patients with refractory symptoms or intolerance to systemic medication. B
› Explore the possibility of using an IT delivery system
to treat malignant pain syndrome, particularly for patients with a life expectancy of more than 6 months. A
› Do not rule out IT analgesia for patients with refractory nonmalignant pain; while considerations in such cases are more complex, benefits include the efficacy of lower doses and fewer adverse effects. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A switch to hydromorphone 20 mg/d—the physician used the 5:1 morphine-to-hydromorphone conversion ratio, then decreased the dose by 50% to account for incomplete cross-tolerance—left Ms. G lethargic. In addition, her pain score rose to 5, and she began having difficulty swallowing the medication. Prior to the drug rotation, she was able to perform light tasks and was alert enough to interact with her family.
If Ms. G were your patient, what would be your next step?
Continuous intrathecal (IT) drug delivery systems have been in use for more than 30 years.1 And, while IT administration of analgesia has become increasingly useful for patients with refractory chronic pain and spasticity, it remains an underutilized resource.2 Delivered directly into the pre- and post-synaptic opioid receptors in the dorsal horn of the spinal cord, IT analgesia bypasses first-pass metabolism. The result: a higher rate of efficacy, with smaller dosages and fewer adverse effects than systemic delivery.1
The drugs are delivered via a small battery-powered programmable pump that is implanted under the subcutaneous tissue of the abdomen and connected to a catheter tunneled to the site of spinal entry. The device must be refilled periodically—typically every one to 3 months—but this is not a difficult process. It can be done in an office setting or in the patient’s home by a specially trained visiting nurse.3
There is ample reason to consider this approach when systemic analgesics or antispasmodics fail to control pain or cause unacceptable adverse effects. So why isn’t it used more frequently? One factor may be that many primary care physicians—often the first practitioners called upon to manage these complicated cases—know too little about it.
Who is a potential candidate for IT analgesia? What medications can be administered via this route? What is the role of a family physician (FP) in coordinating and overseeing the care of a patient being treated with IT therapy? Our goals in writing this review are to address these questions.
Patient selection: Not just for cancer pain
FPs interested in referring patients for IT therapy have many factors to consider before consulting a pain specialist. Foremost among them are the different criteria for individuals with cancer-related pain and those with chronic nonmalignant pain.
IT analgesia for cancer pain has been shown to improve patients’ quality of life and potentially increase long-term survival due to a decrease in systemic toxicity.4-6 An appropriate candidate is an individual who, like Ms. G, was initially responsive to systemic opioids but later developed refractory symptoms or intolerance.7 Because of the invasive nature and high cost of implantation, subcutaneous IT pumps are typically reserved for patients with a life expectancy of more than 6 months.7 But implantation may be considered for those with a shorter life expectancy if they have severe pain or cannot tolerate the adverse effects of systemic analgesia.
Noncancer pain is more complex
The use of IT analgesia in patients with chronic nonmalignant pain, such as failed back surgery syndrome, spasticity associated with multiple sclerosis, or diabetic neuropathy, is both more controversial and more complex. It is important for FPs to recognize the multidimensional nature of this type of pain, which may be complicated by physical, psychological, and behavioral factors, including the possibility of addiction.8-11
Although IT analgesia is less subject to abuse and diversion than systemic opioids, the dependent relationship associated with a continuous delivery system makes risk stratification a necessity.12 Psychological testing is commonly used to evaluate potential candidates for long-term IT analgesia.
Prior to placement, patients must have had a failed course of conservative pain management and have no surgical options, no medical contraindications (eg, spinal pathology or susceptibility to infection), and no evidence of active addiction.12 A medication history is crucial, too, to identify use of anticoagulation therapy—a relative contraindication—as well as drug allergies and potential drug-drug interactions to guard against.3
An IT trial may be required
It is common practice for patients to undergo an IT analgesia trial prior to implantation of a subcutaneous pump. This involves using an external pump to infuse the selected medication intrathecally and slowly titrating it according to symptoms for 2 to 3 days. During this time frame, the patient records his or her response; a reduction by more than half in VAS pain score is considered a success, indicating that the patient is an appropriate candidate for placement of the device.3,13
Drug choices—a look at the evidence
The US Food and Drug Administration (FDA) has approved 3 medications for continuous IT delivery: morphine, ziconotide, and baclofen. But it is common practice to use alternative agents, such as other opioids, local anesthetics, or alpha 2-adrenergic agonists (TABLE).3,14-34
CASE › Ms. G’s primary care physician referred her to a pain specialist, who thought she would benefit from IT analgesia. After a successful single-shot IT trial with 0.5 mg morphine, the patient underwent implantation. The specialist chose morphine as the IT agent because of Ms. G’s history of successful pain relief with it, and because such a low dose was unlikely to be a problem for a patient with renal failure.
A month later, when she returned to the specialist to have the pump refilled, Ms. G reported a pain score of 3.
Opioids such as morphine exhibit a wider spread of analgesia when administered intrathecally, resulting in fewer adverse effects than systemic opioids.13,35,36 The mu-opioid receptors in the dorsal horn of the spinal cord are the primary target of IT opioids.
In a multicenter randomized trial involving 200 cancer patients on opioids, Smith et al4 compared implantable IT drug delivery systems with comprehensive medical management. The mean VAS pain score in the IT group fell 52% vs a decline of 39% in the medical management group.
The evidence supporting IT opioids for nonmalignant pain is not as strong. This may be due to inherent differences in pain mechanisms. In cancer pain, between 75% and 90% of pain is either nociceptive or mixed nociceptive-neuropathic; the etiology of noncancer pain is more variable.37-39
Although IT opioid therapy is associated with a lower incidence of adverse effects than systemic therapy, this route is not devoid of adverse effects. Opioids delivered intrathecally may still be associated with respiratory depression, constipation, urinary retention, nausea/vomiting, sweating, and hyperalgesia.39 In addition, chronic opioid use suppresses the hypothalamic-pituitary-gonadal axis and the hypothalamic-pituitary-adrenal axis14,40,41—a risk with long-term IT as well as systemic administration.14 Respiratory depression most commonly results from accidental overdosing, and patients must be monitored during initiation and dose escalation of IT opioid therapy.15
Local anesthetics. Numerous studies have documented the favorable outcomes of combining local anesthetics with opioids for patients with cancer16-20 and noncancer pain.21,22 Local anesthetics work via the blockade of voltage-gated sodium channels, interfering with neuron depolarization.17
A retrospective study in which patients with malignant pain and those with failed back surgery syndrome had bupivacaine added to their IT opioid solution found that the combination led to lower pain scores and a 23% reduction in opioid dosage.20 In another retrospective review, researchers demonstrated that the coadministration of IT bupivacaine and an opioid decreased the rate of opioid dose escalation by 65% over the first year in patients with noncancer pain.23
However, a double-blind randomized, crossover multicenter study found that in patients with chronic nonmalignant pain, the addition of bupivacaine to IT opioids failed to produce significant improvement in pain control compared with opioid use alone. Quality of life scores did improve, however, in the group receiving combination therapy.24
Adverse effects of local anesthetics delivered intrathecally include numbness, paresthesias, weakness, bowel/bladder dysfunction, and neurotoxicity.17,19,25
Calcium channel blockers. Found in venom produced by the marine snail Conus magus, ziconotide blocks presynaptic N-type channels. It is the only calcium channel blocker used to manage chronic pain.26 Several trials in patients with malignant and nonmalignant pain have shown a significant decrease in VAS pain scores compared with placebo.25,26 In addition, a multicenter, double-blind placebo-controlled crossover study evaluating IT ziconotide for the treatment of refractory pain in 111 patients with cancer and AIDS found that the treatment group obtained significantly better pain relief than the controls (53% vs 17.5% using a VAS pain intensity score).25 However, 31% of those in the treatment group experienced adverse effects, the most common of which were confusion, somnolence, and urinary retention.
Ziconotide has FDA approval only as monotherapy. But because of its high cost and adverse effect profile, it is mainly used in combination with other IT drugs.27 Ziconotide increases the risk of suicide in patients with a history of depression.28 The prevalence of adverse effects correlates with a higher dose, faster titration rate, and older age.26,28
Alpha-2 adrenergic agonists. Clonidine is the only alpha-2 agonist with FDA approval for epidural use, with several studies supporting its off-label use in combination with IT therapy.22,29 In a prospective open-label study evaluating combination IT therapy in patients with failed back surgery syndrome, 73% reported subjective ratings of good or excellent at 2-year follow-up.22 The most common adverse effects were sedation, hypotension, nausea, and dry mouth.
Gamma-aminobutyric acid (GABA) agonists. Baclofen, a GABA agonist with FDA approval for the treatment of spasticity, has been used intrathecally since the mid-1980s.32 Several studies have supported its effectiveness for this purpose.30,42 Clinical studies have also found IT baclofen to be effective in treating conditions such as complex regional pain syndrome, central pain, and neuropathic pain secondary to failed back surgery syndrome.31,32 In one randomized double-blind crossover trial, 7 women with complex regional pain syndrome were given bolus injections of baclofen or saline. Those treated with baclofen experienced a reduction in pain and regained function.31
In another trial—a double-blind placebo-controlled study of patients with multiple sclerosis and spinal cord injury comparing baclofen with placebo—those treated with baclofen showed significant reductions in dysesthetic and spasm-related pain.32 The most common adverse effects of baclofen are drowsiness, cognitive impairment, weakness, gastrointestinal complaints, and sexual dysfunction.31
Which patients and which drugs? An expert consensus
Due to the potential for inconsistent patient management and the use of therapies with anecdotal evidence, the Polyanalgesic Consensus Conference (PACC)—a panel of experts in IT therapy—convened in 2000, 2003, 2007, and 2011 to develop recommendations for IT therapy and an algorithm for drug selection. PACC’s list of chronic conditions for which IT should be considered includes axial low back pain, postherpetic neuralgia, spinal cord injury, spinal stenosis, pancreatitis, osteoporosis, compression fracture, and phantom limb pain, among others.
The algorithm contains separate arms for neuropathic, nociceptive, and mixed pain states. First-line agents for neuropathic pain include morphine, alone or combined with bupivacaine, and ziconotide. For nociceptive pain, morphine, hydromorphone, fentanyl, and ziconotide are all first-line agents; for mixed pain states, the appropriate choice should be based on the clinical scenario.33
Overseeing IT pain management in primary care
Referring potential candidates for IT therapy to specialists in pain management is just the beginning. While patients typically return to the specialist for pump refills, it is important that they see their primary care physician regularly, as well. Vigilance is required of both the FP and the patient. Any sudden worsening in pain level or acute change in neurologic function must be reported to the pain specialist immediately.
Adverse effects of medications are the most common complications
Kamran and Wright43 performed a retrospective review of their practice’s Intrathecal Drug Delivery Systems database of 122 patients and found that adverse medication effects were most common, accounting for 77% of complications.
Catheter malfunctions were next, at 16%, followed by infections, at 5%.43 In other studies, catheter-related complications were found to have an incidence of 15% to 25%.44,45 Problems include kinking, breaking, leaking, and migration of the catheter. Advise patients to immediately contact their pain specialist for evaluation if they experience a sudden loss of, or change in, pain control.
Infectious complications, which occur infrequently, are usually limited to superficial wounds, although epidural abscesses and meningitis are possible.46 Standard perioperative antibiotic administration helps to minimize the risk of infection. If a patient presents with signs and symptoms of an epidural abscess—back pain, fever, and variable neurologic deficits—emergent initiation of intravenous antibiotics is needed. Magnetic resonance imaging (MRI) with and without gadolinium should be obtained, as well.22
Spinal damage. Although IT catheters are placed under fluoroscopic guidance, there is a risk of direct injury to the spinal cord; this is more common if the catheter is placed above the level of the conus medullaris. Damage to the spinal cord or exiting spinal nerves will manifest as pain, sensory loss, and/or weakness over a dermatomal distribution.43
Neurologic sequelae, ranging from mild symptoms to paraplegia, can result from the formation of a granuloma at the tip of the spinal catheter. A sudden increase in pain usually occurs prior to neurologic deterioration, thereby allowing for early detection and intervention.47 Development of a granuloma appears to be related to the long-term infusion of high-concentration opioids.34 The diagnosis is confirmed by MRI, but physical exam and history are imperative in making the initial diagnosis.
In cases of mild neurologic symptoms, a transition to saline infusion through the pump may allow the granuloma to absorb; more severe cases may require neurosurgical intervention.47
Is your patient scheduled for an IT drug trial?
If a patient of yours is scheduled for an IT drug trial, ideally followed by pump implantation, microdosing—the practice of weaning the individual from oral opioids prior to the procedure so that very low doses of IT opioids will suffice—may play a role.48,49 While this approach appears promising, however, there is little in the way of definitive evidence of efficacy.
CASE › Over time, Ms. G’s maintenance IT dose of morphine had to be slowly increased from 0.5 mg to 1 mg/d. At bimonthly visits with her FP, she consistently reports pain scores of 3 on a scale of 1 to 10. The patient’s function has returned to baseline, and she has minimal adverse effects.
CORRESPONDENCE
Jessica Tsukanov, DO, Montefiore Medical Center, 3347 Steuben Avenue, Bronx, NY 10467; [email protected]
1. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
2. Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18:338.
3. Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
4. Smith TJ, Staats PS, Deer T, et al; Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-4049.
5. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003;4:441-447.
6. Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239-247.
7. Hassenbusch SJ. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology. 1999;13(5 suppl 2):S63-S67.
8. Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain. 2004;109:242-249.
9. Gerber HR. Intrathecal morphine for chronic benign pain. Best Pract Res Clin Anesthesiol. 2003;17:429-442.
10. Tuner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
11. Brown J, Klapow J, Doleys D, et al. Disease-specific and generic health outcomes: a model for the evaluation of long-term intrathecal opioid therapy in noncancer low back pain patients. Clin J Pain. 1999;15:122-131.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2006;6:432-442.
13. Ahmed SU, Martin NM, Chang Y. Patient selection and trial methods for intraspinal drug delivery for chronic pain: a national survey. Neuromodulation. 2005;8:112-120.
14. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
15. Coffey RJ, Owens ML, Broste SK, et al. Mortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer pain. Anesthesiology. 2009;111:881-891.
16. Sjöberg M, Nitescu P, Appelgren L, et al. Long-term intrathecal morphine and bupivacaine in patients with refractory cancer pain. Results from a morphine:bupivacaine dose regimen of 0.5:4.75 mg/ml. Anesthesiology. 1994;80:284-297.
17. Sjöberg M, Appelgen L, Einarsson S, et al. Long-term intrathecal morphine and bupivacaine in “refractory” cancer pain. I. Results from the first series of 52 patients. Acta Anaesthsiol Scand. 1991;35:30-43.
18. Van Dongen RT, Crul BJ, De Bock M. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: a retrospective analysis of 51 cases. Pain. 1993;55:119-123.
19. van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166-172.
20. Deer TR, Caraway DL, Kim CK, et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
21. Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539-548.
22. Rainov NG, Heidecke V, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
23. Veizi IE, Hayek SM, Narouze S, et al. Combination of intrathecal opioids with bupivacaine attenuates opioid dose escalation in chronic noncancer pain patients. Pain Med. 2011;12:1481-1489.
24. Mironer YE, Haasis JC, Chapple I, et al. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: A double blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208-213.
25. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63-70.
26. Rauck RL, Wallace MS, Leong MS, et al; Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393-406.
27. Wallace MS, Rauck R, Fisher R, et al; Ziconotide 98-022 Study Group. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth Analg. 2008;106:628-637.
28. Maier C, Gockel HH, Gruhn K, et al. Increased risk of suicide under intrathecal ziconotide treatment? - a warning. Pain. 2011;152:235-237.
29. Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668-677.
30. Tarrico M, Adone R, Pagliacci C, et al. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000;(2):CD001131.
31. van Hilten BJ, van de Beek WT, Hoff JI, et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343:625-630.
32. Herman RM, D’Luzansky SC, Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions. A pilot study. Clin J Pain. 1992;8:338-345.
33. Deer T, Prager J, Levy R, et al. Polyanalgesic consensus conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436-466.
34. Yaksh TL, Coffey RJ. Spinal opiate toxicity. In: Proceedings of American Society of Regional Anesthesia and Pain Medication Conference; November 18-21, 2004; Phoenix, AZ.
35. Levy MH. Pharmacologic management of cancer pain. Semin Oncol. 1994;21:718-739.
36. Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276-310.
37. Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to a hospice. Palliat Med. 2001;15:243-246.
38. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273-281.
39. Hanks GW, Forbes K. Opioid responsiveness. Acta Anaesthesiol Scan. 1997;41:154-158.
40. Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126-131.
41. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3 suppl 1):S12-S18.
42. Beard S, Hunn A. Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7:iii,ix-x,1-111.
43. Kamran S, Wright BD. Complications of intrathecal drug delivery systems. Neuromodulation. 2001;4:111-115.
44. Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage. 2000;19:209-215.
45. Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related complications. Neuromodulation. 2003;6:32-41.
46. Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
47. Miele VJ, Price KO, Bloomfield S, et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
48. Hayek SM. Intrathecal “microdosing”: reality or artifact? Pain Med. 2012;13:1664-1665.
49. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician. 2011;14:343-351.
1. Wang JK, Nauss LA, Thomas JE. Pain relief by intrathecally applied morphine in man. Anesthesiology. 1979;50:149-151.
2. Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18:338.
3. Krames ES. Intraspinal opioid therapy for chronic nonmalignant pain: current practice and clinical guidelines. J Pain Symptom Manage. 1996;11:333-352.
4. Smith TJ, Staats PS, Deer T, et al; Implantable Drug Delivery Systems Study Group. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. 2002;20:4040-4049.
5. Rauck RL, Cherry D, Boyer MF, et al. Long-term intrathecal opioid therapy with a patient-activated, implanted delivery system for the treatment of refractory cancer pain. J Pain. 2003;4:441-447.
6. Burton AW, Rajagopal A, Shah HN, et al. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239-247.
7. Hassenbusch SJ. Cost modeling for alternate routes of administration of opioids for cancer pain. Oncology. 1999;13(5 suppl 2):S63-S67.
8. Thimineur MA, Kravitz E, Vodapally MS. Intrathecal opioid treatment for chronic non-malignant pain: a 3-year prospective study. Pain. 2004;109:242-249.
9. Gerber HR. Intrathecal morphine for chronic benign pain. Best Pract Res Clin Anesthesiol. 2003;17:429-442.
10. Tuner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180-195.
11. Brown J, Klapow J, Doleys D, et al. Disease-specific and generic health outcomes: a model for the evaluation of long-term intrathecal opioid therapy in noncancer low back pain patients. Clin J Pain. 1999;15:122-131.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2006;6:432-442.
13. Ahmed SU, Martin NM, Chang Y. Patient selection and trial methods for intraspinal drug delivery for chronic pain: a national survey. Neuromodulation. 2005;8:112-120.
14. Abs R, Verhelst J, Maeyaert J, et al. Endocrine consequences of long-term intrathecal administration of opioids. J Clin Endocrinol Metab. 2000;85:2215-2222.
15. Coffey RJ, Owens ML, Broste SK, et al. Mortality associated with implantation and management of intrathecal opioid drug infusion systems to treat noncancer pain. Anesthesiology. 2009;111:881-891.
16. Sjöberg M, Nitescu P, Appelgren L, et al. Long-term intrathecal morphine and bupivacaine in patients with refractory cancer pain. Results from a morphine:bupivacaine dose regimen of 0.5:4.75 mg/ml. Anesthesiology. 1994;80:284-297.
17. Sjöberg M, Appelgen L, Einarsson S, et al. Long-term intrathecal morphine and bupivacaine in “refractory” cancer pain. I. Results from the first series of 52 patients. Acta Anaesthsiol Scand. 1991;35:30-43.
18. Van Dongen RT, Crul BJ, De Bock M. Long-term intrathecal infusion of morphine and morphine/bupivacaine mixtures in the treatment of cancer pain: a retrospective analysis of 51 cases. Pain. 1993;55:119-123.
19. van Dongen RT, Crul BJ, van Egmond J. Intrathecal coadministration of bupivacaine diminishes morphine dose progression during long-term intrathecal infusion in cancer patients. Clin J Pain. 1999;15:166-172.
20. Deer TR, Caraway DL, Kim CK, et al. Clinical experience with intrathecal bupivacaine in combination with opioid for the treatment of chronic pain related to failed back surgery syndrome and metastatic cancer pain of the spine. Spine J. 2002;2:274-278.
21. Krames ES, Lanning RM. Intrathecal infusional analgesia for nonmalignant pain: analgesic efficacy of intrathecal opioid with or without bupivacaine. J Pain Symptom Manage. 1993;8:539-548.
22. Rainov NG, Heidecke V, Burkert W. Long-term intrathecal infusion of drug combinations for chronic back and leg pain. J Pain Symptom Manage. 2001;22:862-871.
23. Veizi IE, Hayek SM, Narouze S, et al. Combination of intrathecal opioids with bupivacaine attenuates opioid dose escalation in chronic noncancer pain patients. Pain Med. 2011;12:1481-1489.
24. Mironer YE, Haasis JC, Chapple I, et al. Efficacy and safety of intrathecal opioid/bupivacaine mixture in chronic nonmalignant pain: A double blind, randomized, crossover, multicenter study by the National Forum of Independent Pain Clinicians (NFIPC). Neuromodulation. 2002;5:208-213.
25. Staats PS, Yearwood T, Charapata SG, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63-70.
26. Rauck RL, Wallace MS, Leong MS, et al; Ziconotide 301 Study Group. A randomized, double-blind, placebo-controlled study of intrathecal ziconotide in adults with severe chronic pain. J Pain Symptom Manage. 2006;31:393-406.
27. Wallace MS, Rauck R, Fisher R, et al; Ziconotide 98-022 Study Group. Intrathecal ziconotide for severe chronic pain: safety and tolerability results of an open-label, long-term trial. Anesth Analg. 2008;106:628-637.
28. Maier C, Gockel HH, Gruhn K, et al. Increased risk of suicide under intrathecal ziconotide treatment? - a warning. Pain. 2011;152:235-237.
29. Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668-677.
30. Tarrico M, Adone R, Pagliacci C, et al. Pharmacological interventions for spasticity following spinal cord injury. Cochrane Database Syst Rev. 2000;(2):CD001131.
31. van Hilten BJ, van de Beek WT, Hoff JI, et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. N Engl J Med. 2000;343:625-630.
32. Herman RM, D’Luzansky SC, Ippolito R. Intrathecal baclofen suppresses central pain in patients with spinal lesions. A pilot study. Clin J Pain. 1992;8:338-345.
33. Deer T, Prager J, Levy R, et al. Polyanalgesic consensus conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436-466.
34. Yaksh TL, Coffey RJ. Spinal opiate toxicity. In: Proceedings of American Society of Regional Anesthesia and Pain Medication Conference; November 18-21, 2004; Phoenix, AZ.
35. Levy MH. Pharmacologic management of cancer pain. Semin Oncol. 1994;21:718-739.
36. Cousins MJ, Mather LE. Intrathecal and epidural administration of opioids. Anesthesiology. 1984;61:276-310.
37. Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in patients with non-malignant terminal disease admitted to a hospice. Palliat Med. 2001;15:243-246.
38. Portenoy RK, Hagen NA. Breakthrough pain: definition, prevalence and characteristics. Pain. 1990;41:273-281.
39. Hanks GW, Forbes K. Opioid responsiveness. Acta Anaesthesiol Scan. 1997;41:154-158.
40. Paice JA, Penn RD, Ryan WG. Altered sexual function and decreased testosterone in patients receiving intraspinal opioids. J Pain Symptom Manage. 1994;9:126-131.
41. Brennan MJ. The effect of opioid therapy on endocrine function. Am J Med. 2013;126(3 suppl 1):S12-S18.
42. Beard S, Hunn A. Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess. 2003;7:iii,ix-x,1-111.
43. Kamran S, Wright BD. Complications of intrathecal drug delivery systems. Neuromodulation. 2001;4:111-115.
44. Follett KA, Naumann CP. A prospective study of catheter-related complications of intrathecal drug delivery systems. J Pain Symptom Manage. 2000;19:209-215.
45. Follett KA, Burchiel K, Deer T, et al. Prevention of intrathecal drug delivery catheter-related complications. Neuromodulation. 2003;6:32-41.
46. Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71-80.
47. Miele VJ, Price KO, Bloomfield S, et al. A review of intrathecal morphine therapy related granulomas. Eur J Pain. 2006;10:251-261.
48. Hayek SM. Intrathecal “microdosing”: reality or artifact? Pain Med. 2012;13:1664-1665.
49. Grider JS, Harned ME, Etscheidt MA. Patient selection and outcomes using a low-dose intrathecal opioid trialing method for chronic nonmalignant pain. Pain Physician. 2011;14:343-351.
What you must know before you recommend a probiotic
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
› Consider probiotics for patients with acute infectious diarrhea, antibiotic-associated diarrhea, or Clostridium difficile-associated diarrhea. A
› Do not recommend probiotics for preventing or treating Crohn’s disease or ulcerative colitis. B
› Consider the probiotic Bifidobacterium bifidum MIMBb75 for patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Probiotics—live micoorganisms that are consumed as supplements or food for purported health benefits—are a popular over-the-counter remedy for various gastrointestinal (GI) ailments and other conditions, but the evidence supporting their use is mixed. Probiotics interact with the normal flora of the human body. They are believed to act by multiple mechanisms to deliver beneficial effects, including providing a protective barrier, altering intestinal pH to favor the growth of nonpathogenic bacteria, enhancing the host’s immunologic response, producing antimicrobial substances, and directly competing with pathogenic bacteria for receptors in the GI tract.1 (See “The normal human intestinal flora.”)
In the United States, Lactobacillus and Bifidobacterium are the probiotic genera that are most commonly used. (For a list of the specific probiotic species found in 5 popular products, see TABLE 1.2-6) The review that follows examines the evidence for using probiotics for select GI ailments, including several types of diarrheal illnesses, inflammatory bowel disease (Crohn’s disease and ulcerative colitis), and irritable bowel syndrome (IBS). These findings are summarized in TABLE 2.1,7-21
The human body contains approximately 1014 prokaryotic organisms, with a biomass of >1 kg. Most of these organisms are indigenous and stable, although transient members such as enteric pathogens can be found.
The gastrointestinal tract is sterile at birth but is colonized immediately, and each individual has marked variations in microbial composition. The complex symbiotic relationship between the normal intestinal flora and the human host is beneficial to both. These microbes utilize complex carbohydrates undigested by the host as energy. Fermentation results in the formation of short-chain fatty acids, which can provide up to 15% of human energy requirements.
In addition to these metabolic benefits, microbial flora dampen the human inflammatory response, induce immunosuppressive T cells (Tregs), and competitively exclude pathogens.
Colonic epithelium is nourished and proliferates in the presence of normal intestinal flora. Disruption of the normal flora can cause disease.
SOURCE: Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65-80.
Probiotics may help with some types of diarrhea
Acute infectious diarrhea. Viruses, bacteria, and parasites cause acute infectious diarrhea, and probiotics are thought to act against these pathogens by competing for available nutrients and pattern recognition receptors in the GI endothelium, acidifying the local environment, and increasing immune responses within the GI tract. In a meta-analysis of 63 studies (N=8014) that used multiple strains and dosages of probiotics, investigators found probiotics shortened the duration of acute infectious diarrhea by approximately 24 hours (95% confidence interval [CI], 15.9-33.6 hours).7 Probiotics also reduced both the risk of diarrhea lasting longer than 4 days (relative risk [RR]=0.41; 95% CI, 0.32-0.53) and stool frequency on Day 2 of illness (mean difference of 0.80 stools; 95% CI, 0.45-1.14).
Traveler’s diarrhea. The incidence of traveler’s diarrhea is >50% when traveling to high-risk areas such as the Middle East, North Africa, Latin America, and Southeast Asia, and 5% to 10% when traveling to areas such as North America, Northern Europe, the United Kingdom, Australia, and New Zealand.8 Traveler’s diarrhea may be caused by ingesting food and liquids contaminated with fecal material. Symptoms include diarrhea, cramps, and nausea that if untreated typically last from 2 to 6 days but can last for as long as a month.8
In a meta-analysis of 12 studies (N=5171) that evaluated various probiotic strains, researchers found probiotics effectively prevented traveler’s diarrhea in US and European travelers who visited a variety of vacation spots (pooled RR=0.85; 95% CI, 0.79-0.91).8 No serious adverse events were reported.
Radiation-induced diarrhea. Radiation treatments to the abdomen and pelvis can damage the lower GI tract and cause diarrhea. The pooled results from a meta-analysis that included 6 studies (N=1449) significantly favored the use of probiotics over placebo for decreasing the incidence of radiation-induced diarrhea (odds ratio [OR]=0.44; 95% CI, 0.21-0.92).9 Probiotics use also was associated with decreased loperamide use (OR=0.29; 95% CI, 0.01-6.80) and decreased incidence of watery stools (OR=0.36; 95% CI, 0.05-2.81), but these outcomes did not reach statistical significance.
Antibiotic-associated diarrhea. Antibiotic use has long been associated with the development of diarrheal illness, sometimes due to the acceleration of GI motility (eg, erythromycin) or by causing osmotic diarrhea by decreasing GI bacteria that assist in carbohydrate breakdown.11 A meta-analysis that evaluated 63 randomized controlled trials (RCTs) (N=11,811) showed that probiotics are effective for treating and preventing antibiotic-associated diarrhea (AAD).1 There was a statistically significant reduction in AAD among patients who received probiotics (RR=0.58; 95% CI, 0.50-0.68; number needed to treat [NNT]=13). Most of the studies in this meta-analysis used a Lactobacillus probiotic alone or in combination with another probiotic. Researchers did not analyze whether the efficacy varied by patient population, probiotic used, causative antibiotic, or duration of treatment.1
Another meta-analysis of 34 studies (N=4138) also found probiotic therapy can prevent AAD.10 The pooled RR for AAD was 0.53 (95% CI, 0.44-0.63) for patients treated with probiotics compared to placebo, with an NNT of 8 (95% CI, 7-11). The effects remained significant when results were grouped by probiotic species, patient age, and duration of antibiotic treatment. Among a subgroup of patients in this meta-analysis who were being treated for Helicobacter pylori, the pooled RR of AAD was 0.37 (95% CI, 0.20-0.69) and the NNT was 5 (95% CI, 4-10).10 However, the 2013 PLACIDE trial (N=17,420) found no significant decrease in AAD rates in hospitalized patients over age 65 years being treated with antibiotics who received probiotics (RR=1.04; 95% CI, 0.84-1.28).22
Clostridium difficile-associated diarrhea. As we know, antibiotics can disrupt the normal GI flora and permit overgrow of Clostridium difficile, which can result in C. difficile-associated diarrhea (CDAD).12 This can occur with oral, parenteral, and even topical antibiotics.11 Researchers have investigated whether probiotics can prevent this opportunistic C. difficile overgrowth.
A 2012 meta-analysis of 20 trials (N=38,180) found probiotic prophylaxis prevented CDAD in both inpatients and outpatients while not increasing the incidence of significant adverse effects.12 Probiotics decreased the incidence of CDAD by 66% (pooled RR=0.34, 95% CI, 0.24-0.49).12 Adverse events occurred in 9.3% of patients taking probiotics, compared with 12.6% of controls (RR=0.82, 95% CI, 0.65-1.05).12
Conversely, a 2008 review of 4 studies (N=336) concluded there is insufficient evidence for using probiotics to treat CDAD, either as monotherapy or adjunct therapy.11 One trial in this meta-analysis (N=124) found patients who received the probiotic Saccharomyces boulardii in addition to antibiotic therapy were significantly less likely to experience CDAD recurrence than those who received placebo (RR=0.59; 95% CI, 0.35-0.98).11 However, this benefit was not found in the other trials in this meta-analysis.11
The PLACIDE trial found probiotics did not prevent CDAD in hospitalized patients over age 65 years; 0.8% of patients who received probiotics developed CDAD, compared to 1.2% in the placebo group (RR=0.71, 95% CI, 0.34-1.47).22
Helicobacter pylori infection. The triple therapy regimen of a proton pump inhibitor plus the antibiotics clarithromycin and amoxicillin is the recommended treatment for H. pylori infection.13 Problems with this treatment include adverse effects such as diarrhea and decreased eradication rates, in part due to antibiotic resistance. Certain Lactobacillus species have been shown to inhibit or kill H. pylori in vitro,13 and evidence from several meta-analyses suggests probiotics should be an adjunct therapy when treating H. pylori.
In a meta-analysis of 10 RCTs (N=963), fermented milk-based probiotics improved H. pylori eradication rates by 5% to 15%.14 In another meta-analysis that evaluated 5 RCTs (N=1307), S. boulardii significantly increased the H. pylori eradication rate when used as an adjunct to triple therapy (RR=1.13; 95% CI, 1.05-1.21) and reduced the rate of treatment-related adverse effects (RR=0.46; 95% CI, 0.3-0.7).13 In a third meta-analysis of 10 trials (N=1469), Lactobacillus supplementation increased H. pylori eradication rates (OR=2.1; 95% CI, 1.4-3.1) while decreasing the overall incidence of adverse effects (OR=0.3; 0.1-0.8).15
For inflammatory bowel disease, probiotics are unlikely to help
Current therapies for Crohn’s disease and ulcerative colitis, such as corticosteroids and other immunosuppressive agents, are effective but have significant adverse events.18 Researchers explored whether probiotics might help treat these diseases by improving immune response, the balance of microbes in the GI tract, and the intestinal barrier.18
Crohn’s disease. In a meta-analysis that was able to identify only one small RCT (N=11), 80% of patients receiving probiotic treatment went into remission, compared to 83% in the placebo group (OR=0.80; 95% CI, 0.04–17.20).16 Researchers concluded there was insufficient evidence for the use of probiotics for inducing remission in Crohn’s disease.
Another meta-analysis of 7 small studies (N=160) found no significant evidence supporting probiotic use for maintaining remission in Crohn’s disease compared with aminosalicylates or azathioprine.17 One small study in this review found there was a benefit to combining S. boulardii with a reduced level of standard maintenance therapy when compared to standard therapy alone, but this difference was not statistically significant.17
Ulcerative colitis. A systematic review of 4 RCTs (N=244) that compared conventional treatment alone to conventional treatment plus probiotics for remission or clinical improvement in patients with active ulcerative colitis found no significant differences between groups.18 Another meta-analysis of 4 studies (N=587) found that compared to placebo or treatment with mesalazine, probiotics had no benefit for maintaining remission in ulcerative colitis.19 The rate of relapse was 40.1% in the probiotics group compared to 34.1% in the mesalazine group. The number of adverse effects was similar in both groups.
Most evidence suggests probiotics are useful for IBS
Research suggests that imbalances in GI flora, along with subsequent dysfunction in intestinal barriers and translocation of intestinal flora, may play a role in symptoms associated with IBS, such as abdominal pain, bloating, and diarrhea/constipation.20 There are few effective therapeutic options for patients suffering with IBS.
In a systematic review of 19 RCTs (N=1650), probiotics were significantly more effective than placebo for patients with IBS, with an NNT of 4 (95% CI, 3-12.5).21 This review did not evaluate the difference between various probiotic species and strains.
In an RCT (N=122), the probiotic strain Bifidobacterium bifidum MIMBb75 was found to be safe and beneficial for treating IBS symptoms and improving patients’ quality of life.20 On a 7-point scale of global assessment of IBS symptoms, the score was reduced by 0.88 points (95% CI, 0.69-1.07) in the group that received B. bifidum MIMBb75 and 0.16 points (95% CI, -0.32-0.00) in the placebo group (P<0.0001). Almost half (47%) of the patients who received B. bifidum MIMBb75 reported adequate relief, compared to 11% in the placebo group (P<.0001).
An RCT (N=179) that compared yogurt that contained probiotics to non-probiotic yogurt found the probiotic yogurt had no benefits for treating IBS symptoms.23 After 4 weeks, 57% of patients who ate the probiotic yogurt reported adequate relief, compared to 53% of those who ate non-probiotic yogurt (P=0.71). After 8 weeks, those numbers were 47% and 68%, respectively.23
CORRESPONDENCE
Erik R. Clauson, DO, Nellis Family Medicine Residency, 99 MDOS/SGOF, 4700 Las Vegas Boulevard North, Nellis Air Force Base, NV 89191; [email protected]
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
1. Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959-1969.
2. Procter & Gamble. Align product information. Procter & Gamble Align Web site. Available at: http://www.aligngi.com/information-on-Align-probiotic-supplement. Accessed February 13, 2015.
3. Bayer HealthCare. Phillip’s Colon Health product information. Bayer HealthCare Phillip’s Colon Health Web site. Available at: http://phillipspro.com/en/home/product-information/index.php. Accessed February 13, 2015.
4. Nature’s Bounty. Nature’s Bounty Acidophilus Probiotic product label. Nature’s Bounty Web site. Available at: http://images.vitaminimages.com/cdn/sd/pdf/L002610-NB.PDF. Accessed February 13, 2015.
5. Dannon. Activia. Dannon Activia Web site. Available at: http://activia.us.com/probiotic-yogurt/activia. Accessed February 13, 2015.
6. Lifeway. Lifeway Kefir frequently asked questions. Lifeway Kefir Web site. Available at: http://lifewaykefir.com/faq/. Accessed February 13, 2015.
7. Allen SJ, Martinez EG, Gregorio GV, et al. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev. 2010;(11):CD003048.
8. McFarland LV. Meta-analysis of probiotics for the prevention of traveler’s diarrhea. Travel Med Infect Dis. 2007;5:97-105.
9. Hamad A, Fragkos KC, Forbes A. A systemic review and meta-analysis of probiotics for the management of radiation induced bowel disease. Clin Nutr. 2013;32:353-360.
10. Videlock EJ, Cremonini F. Meta-analysis: probiotics in antibiotic-associated diarrhoea. Aliment Pharmacol Ther. 2012;35:1355-1369.
11. Pillai A, Nelson RL. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev. 2008;(1):CD004611.
12. Johnston BC, Ma SY, Goldenberg JZ, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea: a systematic review and meta-analysis. Ann Intern Med. 2012;157:878-888.
13. Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther. 2010;32:1069-1079.
14. Sachdeva A, Nagpal J. Effect of fermented milk-based probiotic preparations on Helicobacter pylori eradication: a systematic review and meta-analysis of randomized-controlled trials. Eur J Gastroenterol Hepatol. 2009;21:45-53.
15. Wang ZH, Gao QY, Fang JY. Meta-analysis of the efficacy and safety of Lactobacillus-containing and Bifidobacterium-containing probiotic compound preparation in Helicobacter pylori eradication therapy. J Clin Gastroenterol. 2013;47:25-32.
16. Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2008;16:CD006634.
17. Rolfe VE, Fortun PJ, Hawkey CJ, et al. Probiotics for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2006;(4):CD004826.
18. Mallon P, McKay D, Kirk SJ, et al. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
19. Naidoo K, Gordon M, Fagbemi AO, et al. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2011;(12):CD007443.
20. Guglielmetti S, Mora D, Gschwender M, et al. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–– a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123-1132.
21. Moayyedi P, Ford AC, Talley NJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332.
22. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
23. Roberts LM, McCahon D, Holder R, et al. A randomised controlled trial of a probiotic ‘functional food’ in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45.
Turning team-based care into a winning proposition
› Explore the potential benefits of team-based care by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. A
› Consider partnering with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Institute for Healthcare Improvement’s “Triple Aim” approach to optimizing the delivery of health care in the United States calls for improving the patient’s experience of care, including both quality and satisfaction; improving the health of populations; and reducing the per-capita cost of health care.1 Unfortunately, achieving these goals is being made more challenging by a perfect storm of conditions: The age of the population and the number of people accessing the systems are increasing, while the number of providers available to care for these patients is decreasing. The number of annual office visits to family physicians (FPs) in the United States is projected to increase from 462 million in 2008 to 565 million in 2025, which will require an estimated 51,880 additional FPs.2
One of the health care delivery models that has recently gained traction to help address this is team-based care. By practicing in a team-based care model, physicians and other clinicians can care for more patients, better manage those with high-risk and high-cost needs, and improve overall quality of care and satisfaction for all involved. Here we review the evidence for team-based care and its use for chronic disease management, and offer suggestions for its implementation.
The many providers who comprise the team
There is little consistency in the definition, composition, training, or maintenance of health care teams. Naylor et al3 defined team-based care as “the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”
While the team construct will vary based on the needs of your practice and your patients, developing a high-functioning team is essential to achieving success. Our 12-step checklist for building a successful team is a good starting point (TABLE 1).4-6 Many other resources are available to help with each step of this process (TABLE 2).
Teams should be led by a primary care provider—a physician, nurse practitioner (NP), or physician assistant (PA)—and consist of other members that complement the other’s expertise and roles, such as nurse case managers, clinical pharmacists, social workers, and behavioral health experts. Some practices have large teams with interdisciplinary members, including pharmacists, PAs, and NPs (the “expanded staffing” model), while others form smaller “teamlets” consisting of a physician and a registered nurse (RN) who serves as a health coach.
In the expanded staffing model, RNs and clinical pharmacists assume greater care management, while medical assistants (MAs) and licensed practice nurses (LPNs) are responsible for pre-visit, outreach, and follow-up activities.7 Redefining roles can spread the work among all team members, which allows each member to work to their level of training and licensure and permits the MD/NP/PA to focus on more complex tasks.
The teamlet model has 2 main features: 1) Patient encounters involve a clinician (MD, NP, PA) and a health coach (MA, RN, LPN); and 2) Care is expanded beyond the usual 15-minute visit to include pre-visit, visit, post-visit, and between-visit care.8 Incorporating a health coach puts an increased focus on the patient and self-management support, with the goals of increasing satisfaction for both the patient and the health care team, improving outcomes, and lowering cost due to fewer emergency department (ED) visits and hospital admissions/readmissions.
Smaller teams seem to be more effective and more manageable.8,9 In one example of well-functioning teamlet composed of RNs and MAs, an MA is responsible for patients coming in for timely chronic and preventive care needs, while the RNs focus their efforts on tasks that require their expertise, including health coaching, self-management support, and patient education.9 Although smaller offices may not have the resources of a large academic practice, this model of maximizing the role of the MAs is reasonable and achievable.
Another example of a successful teamlet model is a clinical microsystem, in which a small group of clinicians and support staff work together to provide care to a discrete group of patients.10,11 (For more information on clinical microsystems, go to the Dartmouth Institute Microsystem Academy at https://clinicalmicrosystem.org.)
What are the barriers to creating team-based care?
Many providers and administrators are concerned about the costs of creating a team-based model of care. These include the cost of hiring new staff, retraining current staff, and educating team members and patients, as well as the cost of developing and maintaining the necessary information technology.
There is, of course, always the concern about physicians relinquishing patient care tasks to other team members. The flip side of that is that staff members may not be eager to increase their roles and responsibilities. In addition, developing a high-functioning team requires ongoing efforts to train and retrain, as well as dedicated leadership and an ongoing commitment to team building.12
Team-based care can work well for managing chronic diseases
Despite the challenges of developing and maintaining this approach to care, the evidence suggests that implementing a team-based model can be especially useful for patients with chronic diseases, because it can improve patient outcomes and access to care, decrease costs, and improve clinician satisfaction—as detailed below.
Improved patient outcomes. Initial evidence suggests that implementing a team-based model can improve patients’ health and experience of care.13,14 The most positive findings have been observed for team-based efforts at managing specific diseases, such as diabetes and congestive heart failure (CHF), or specific populations, such as older patients with chronic illness. Studies have shown that using a team approach results in improved metrics, including HbA1c, low-density lipoprotein cholesterol, blood pressure (BP), and body mass index.7,15-20 Team-based models that pair physicians and other primary care providers with a clinical pharmacist have increased patients’ medication adherence and provider adherence to recommended prescribing habits.15,21-23
One small clinical microsystem that focused on self-management support with health coaching increased patients’ ratings of their confidence in self-management from 40% to 60% at baseline to 80% to 90% after one year. This program also increased the proportion of patients in whom BP was controlled by 10% to 15%.10
Despite these successes, some team-based models may not always be “doable” because of the costs of adding an advanced practice clinician to the staff, or the challenges of recruiting the right person for the job. (How to adapt team-based care for smaller practices is discussed below.)
Improved access to care. A preponderance of data shows that team-based care increases the volume of patient visits, thereby improving access to care.7,21,24-28 The critical elements to successfully achieving this are effective training and delegation. In private practice, using well-trained clinical assistants to create a physician-driven team can increase patient visit volume by an estimated 30% (using 1 assistant) to 60% (using 2 assistants).24
Similar increases in visit volume are seen in larger patient-centered medical home (PCMH) models that consist of physicians, PAs or NPs, MAs, LPNs, RNs, and clinical pharmacists.7,25 Teams with defined ratios of assistants to physicians/NPs/PAs see the most patients per day compared to care coordinator models (ie, 1 assistant for multiple physicians) or enhanced traditional models.21 When focusing on disease-specific care, the impact on access can be even greater. A diabetes-specific team-based care program resulted in a >50% increase in daily patient encounters and 4-fold increase in annual office visits.28
In addition to increasing visits, team-based care also increases access to care by decreasing wait times for an appointment and increasing the use of secure messaging and telephone visits.7,25 In a prospective cohort pilot study of more than 2000 patients enrolled in a team-based care model, the average scheduling time for a face-to-face visit for nonurgent care decreased from a mean of 26.5 days to 14 days, compared to a mean of 31.5 days to 17.8 days for controls.25 (The decrease in the control group was likely due to implementation of an electronic medical record in the practice.) Furthermore, a non-controlled evaluation of health plan-based practice groups with very large patient populations (ie, >300,000 patients) reported up to a 3-fold decrease in appointment waiting time when using a team-based model.29
Some studies have found a decrease in office visits after implementing team-based care.7 However, these reports also found a corresponding increase (by as much as 80%) in the use of secure messaging and telephone encounters, which translated to an overall enhanced communication with patients and ultimately increased access to care.7
Decreased costs. Several controlled trials have looked at the financial impact of using team-based care to manage chronic conditions such as asthma, CHF, and diabetes. Rich et al30 found a nurse-directed program of patient self-management support via telephone and home visit follow-up was associated with a 56% reduction in hospital readmissions, which translated to a $460 decrease in cost per patient over a 3-month period compared to a control group. In a study by Domurat,31 hospital stays were 50% shorter for high-risk diabetes patients who were managed by a team that offered planned visits, telephone contact, and group visits; this resulted in a lower cost of care. Katon et al32 found that when a nurse manager was added to a primary care team to enhance self-management support, intensify treatment, and coordinate continuity of care for patients with multiple chronic conditions, outpatient health costs were decreased by $594 per patient over 24 months.
Liu et al33 randomly assigned 354 patients in a VA primary care clinic who met criteria for major depression or dysthymia to usual care or a collaborative care model. The collaborative care model included a mental health care team that provided telephone contact to encourage medication adherence and reviewed and suggested modifications to the treatment plan. After an initial expenditure of $519 per patient, a savings of approximately $33 per patient for total outpatient costs was realized.
A team-based coordinated care program for patients with multiple chronic conditions reduced patient visits to specialists by 24%, ED visits by 13%, and hospitalizations by 39%.34 An internal evaluation found that the program saved money by reducing admissions, including intensive care unit stays and “observational” stays for Medicare fee-for-service patients.35
What about reimbursement? Most studies that have evaluated the financial aspects of implementing team-based care have calculated the cost savings for the health system—rather than for an individual practice—through decreased hospital admissions, readmissions, and ED visits. Efficient, high-quality teams will require a substantial initial investment of time and hiring and training of staff before savings can be realized.
Team-based care may not be financially sustainable unless current reimbursement models are changed. The current US system bases payment on quantity of care instead of quality of care, reimburses only for clinician services, and does not compensate teams.36 The Centers for Medicare and Medicaid Services (CMS) has begun to recognize the need to reimburse for services that are not delivered in face-to-face patient encounters. For example, the agency established a new G-code that can be used for non-face-to-face care management services for Medicare patients with 2 or more significant chronic conditions; this code took effect on January 1, 2015.37
Some insurers are reimbursing practices for obtaining designation as a PCMH. This type of reimbursement could be expanded to include other types of team-based efforts—such as self-management support and health coaching.
Improved team satisfaction. While many primary care providers are experiencing fatigue and burnout,38 support staff in many practices also experience job dissatisfaction, which leads to increased absenteeism and high turnover. Several studies indicate that involving all levels of staff in the improvement process and empowering them to work to their full potential by enhancing their roles and realigning responsibilities can increase satisfaction.7,11,21,38,39 This in turn can lead to increased loyalty, commitment, and productivity, with decreased burnout and turnover.
TABLE 2
| Team-based care: Additional resources | |
| Resource | Comments |
The Dartmouth Institute Microsystem Academy | This site includes assessment tools and strategies for implementing clinical microsystems into practices |
Improving Chronic Illness Care | This site provides information about the chronic care model, care coordination, and patient-centered medical homes |
TeamSTEPPS | TeamSTEPPS is an evidence-based teamwork system to improve communication and teamwork skills among health care professionals. All resources, including training materials, are free and downloadable |
Godfrey MM, Melin CN, Muething SE, et al. Clinical microsystems, Part 3. Transformation of two hospitals using microsystem, mesosystem, and macrosystem strategies. Jt Comm J Qual Patient Saf. 2008;34:591-603. | This article provides resources and strategies to engage all levels of the health system in team-based care |
McKinley KE, Berry SA, Laam LA, et al. Clinical microsystems, Part 4. Building innovative population-specific mesosystems. Jt Comm J Qual Patient Saf. 2008;34:655-663. | This article describes how to engage leadership at the health systems level |
Adapting team-based care for smaller practices
Physicians who practice alone or in small groups may have limited capacity to employ allied health professionals. However, your “team” doesn’t need to be housed only in your office. One innovative approach is the community-based medical home, where physicians with medical homes and/or care teams in their offices refer to, and collaborate with, a network of community-based professionals and agencies for clinical and social service support for their patients.22 Some options are to partner with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools.
While having team-based care strategies is necessary to achieve a PCMH designation, you do not need to seek such designation in order to practice team-based care. Start by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. In addition, determine what you value for your practice and what outcomes you hope for, along with a clear plan of how to measure these outcomes. This will allow you to determine if the estimated cost of the proposed strategy is “worth it” in terms of your individual situation and goals.
CORRESPONDENCE
Michele Q. Zawora, MD, Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Institute of Healthcare Improvement. The IHI Triple Aim. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/offerings/Initiatives/TripleAim/Pages/default.aspx. Accessed February 5, 2015.
2.Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2025. Ann Fam Med. 2012;10:503-509.
3. Naylor MD, Coburn KD, Kurtzman ET, et al. Inter-professional team-based primary care for chronically ill adults: State of the science. White paper presented at: the ABIM Foundation meeting to Advance Team-Based Care for the Chronically Ill in Ambulatory Settings; March 24-25, 2010; Philadelphia, PA.
4.Boult C, Green AF, Boult LB, et al. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the Institute of Medicine’s Retooling for an Aging America report. J Am Geriatr Soc. 2009;57:2328-2337.
5.Kuzel AJ. Keys to high-functioning office teams. Family Practice Management. 2011;18:15-18.
6.Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
7. Reid RJ, Coleman K, Johnson EA, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood). 2010;29:835-843.
8. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
9. Chen EH, Thom DH, Hessler DM, et al. Using the teamlet model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(suppl 4):S610-S614.
10. Wasson JH, Anders SG, Moore LG, et al. Clinical microsystems, part 2. Learning from micro practices about providing patients the care they want and need. Jt Comm J Qual Patient Saf. 2008;34:445-452.
11. Williams I, Dickinson H, Robinson S. Clinical microsystems: An evaluation. Health Services Management Centre, School of Public Policy, University of Birmingham, England; 2007. Available at: http://chain.ulcc.ac.uk/chain/documents/hsmc_evaluation_report_CMS2007final.pdf. Accessed February 20, 2015.
12. Willard R, Bodenheimer T. The building blocks of high-performing primary care: Lessons from the field. April 2012. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/B/PDF%20BuildingBlocksPrimaryCare.pdf. Accessed February 10, 2015.
13. Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569-572.
14. Boult C, Karm L, Groves C. Improving chronic care: the “guided care” model. Perm J. 2008;12:50-54.
15. Smith SM, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560.
16. Bloom FJ, Graf TR, Steele GD. Improved patient outcomes in 3 years with a system of care for diabetes. October 2012. Available at: http://iom.edu/~/media/Files/Perspectives-Files/2012/Commentaries/VSRT-Improved-Patient-Outcomes.pdf. Accessed February 10, 2015.
17. Bloom FJ Jr, Yan X, Stewart WF, et al. Primary care diabetes bundle management: 3-year outcomes for microvascular and macrovascular events. Am J Manag Care. 2014;20:e175-e182.
18. Pape GA, Hunt JS, Butler KL, et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Arch Intern Med. 2011;171:1480-1486.
19. Scanlon DP, Hollenbeak CS, Beich J, et al. Financial and clinical impact of team-based treatment for medicaid enrollees with diabetes in a federally qualified health center. Diabetes Care. 2008;31:2160-2165.
20. Renders CM, Valk GD, Griffin S, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(1):CD001481.
21. Goldberg GD, Beeson T, Kuzel AJ, et al. Team-based care: a critical element of primary care practice transformation. Popul Health Manag. 2013;16:150-156.
22. Lipton HL. Home is where the health is: advancing team-based care in chronic disease management. Arch Intern Med. 2009;169:1945-1948.
23. Farris KB, Côté I, Feeny D, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998-1003.
24. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
25. Grumbach K, Bodenheimer T, Grundy P. The outcomes of implementing patient-centered medical home interventions: A review of the evidence on quality, access and costs from recent prospective evaluation studies, August 2009. Washington, DC; Patient-Centered Primary Care Collaborative; 2009.
26. Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood). 2011;30:646-654.
27. Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;16:955-964.
28. Bray P, Roupe M, Young S, et al. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: the eastern North Carolina experience. Diabetes Educ. 2005;31:712-718.
29. Institute for Healthcare Improvement. Health Partners uses “BestCare” practices to improve care and outcomes, reduce costs. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/engage/initiatives/TripleAim/Documents/IHITripleAimHealthPartnersSummaryofSuccessJul09v2.pdf. Accessed February 5, 2015.
30. Rich MW, Beckman V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190-1195.
31. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
32. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506-514.
33. Liu CF, Hendrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54:698-704.
34. Berenson RA; The Urban Institute. Challenging the status quo in chronic disease care: seven case studies. California Healthcare Foundation: 2006. California HealthCare Foundation Web site. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ChallengingStatusQuoCaseStudies.pdf. Accessed February 5, 2015.
35. Coleman EA, Smith JD, Frank JC, et al. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52:1817-1825.
36. Schneider ME. Medicare finalizes plan for non-face-to-face payments. Family Practice News Web site. Available at: http://www.familypracticenews.com/?id=2633&tx_ttnews%5Btt_news%5D=226457&cHash=2aeafe0585c7156dcf23891d010cd12f. Accessed December 2, 2013.
37. Centers for Medicare & Medicaid Services. Fact sheets: Policy and payment changes to the Medicare Physician Fee Schedule for 2015. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2014-Fact-sheets-items/2014-10-31-7.html. Accessed February 13, 2015.
38. Berry LL, Dunham J. Redefining the patient experience with collaborative care. Harvard Business Review Blog Network. Harvard Business Review Web site. Available at: https://hbr.org/2013/09/redefining-the-patient-experience-with-collaborative-care/. Accessed February 5, 2015.
39. Lyon RK, Slawson J. An organized approach to chronic disease care. Fam Pract Manag. 2011;18:27-31.
› Explore the potential benefits of team-based care by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. A
› Consider partnering with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Institute for Healthcare Improvement’s “Triple Aim” approach to optimizing the delivery of health care in the United States calls for improving the patient’s experience of care, including both quality and satisfaction; improving the health of populations; and reducing the per-capita cost of health care.1 Unfortunately, achieving these goals is being made more challenging by a perfect storm of conditions: The age of the population and the number of people accessing the systems are increasing, while the number of providers available to care for these patients is decreasing. The number of annual office visits to family physicians (FPs) in the United States is projected to increase from 462 million in 2008 to 565 million in 2025, which will require an estimated 51,880 additional FPs.2
One of the health care delivery models that has recently gained traction to help address this is team-based care. By practicing in a team-based care model, physicians and other clinicians can care for more patients, better manage those with high-risk and high-cost needs, and improve overall quality of care and satisfaction for all involved. Here we review the evidence for team-based care and its use for chronic disease management, and offer suggestions for its implementation.
The many providers who comprise the team
There is little consistency in the definition, composition, training, or maintenance of health care teams. Naylor et al3 defined team-based care as “the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”
While the team construct will vary based on the needs of your practice and your patients, developing a high-functioning team is essential to achieving success. Our 12-step checklist for building a successful team is a good starting point (TABLE 1).4-6 Many other resources are available to help with each step of this process (TABLE 2).
Teams should be led by a primary care provider—a physician, nurse practitioner (NP), or physician assistant (PA)—and consist of other members that complement the other’s expertise and roles, such as nurse case managers, clinical pharmacists, social workers, and behavioral health experts. Some practices have large teams with interdisciplinary members, including pharmacists, PAs, and NPs (the “expanded staffing” model), while others form smaller “teamlets” consisting of a physician and a registered nurse (RN) who serves as a health coach.
In the expanded staffing model, RNs and clinical pharmacists assume greater care management, while medical assistants (MAs) and licensed practice nurses (LPNs) are responsible for pre-visit, outreach, and follow-up activities.7 Redefining roles can spread the work among all team members, which allows each member to work to their level of training and licensure and permits the MD/NP/PA to focus on more complex tasks.
The teamlet model has 2 main features: 1) Patient encounters involve a clinician (MD, NP, PA) and a health coach (MA, RN, LPN); and 2) Care is expanded beyond the usual 15-minute visit to include pre-visit, visit, post-visit, and between-visit care.8 Incorporating a health coach puts an increased focus on the patient and self-management support, with the goals of increasing satisfaction for both the patient and the health care team, improving outcomes, and lowering cost due to fewer emergency department (ED) visits and hospital admissions/readmissions.
Smaller teams seem to be more effective and more manageable.8,9 In one example of well-functioning teamlet composed of RNs and MAs, an MA is responsible for patients coming in for timely chronic and preventive care needs, while the RNs focus their efforts on tasks that require their expertise, including health coaching, self-management support, and patient education.9 Although smaller offices may not have the resources of a large academic practice, this model of maximizing the role of the MAs is reasonable and achievable.
Another example of a successful teamlet model is a clinical microsystem, in which a small group of clinicians and support staff work together to provide care to a discrete group of patients.10,11 (For more information on clinical microsystems, go to the Dartmouth Institute Microsystem Academy at https://clinicalmicrosystem.org.)
What are the barriers to creating team-based care?
Many providers and administrators are concerned about the costs of creating a team-based model of care. These include the cost of hiring new staff, retraining current staff, and educating team members and patients, as well as the cost of developing and maintaining the necessary information technology.
There is, of course, always the concern about physicians relinquishing patient care tasks to other team members. The flip side of that is that staff members may not be eager to increase their roles and responsibilities. In addition, developing a high-functioning team requires ongoing efforts to train and retrain, as well as dedicated leadership and an ongoing commitment to team building.12
Team-based care can work well for managing chronic diseases
Despite the challenges of developing and maintaining this approach to care, the evidence suggests that implementing a team-based model can be especially useful for patients with chronic diseases, because it can improve patient outcomes and access to care, decrease costs, and improve clinician satisfaction—as detailed below.
Improved patient outcomes. Initial evidence suggests that implementing a team-based model can improve patients’ health and experience of care.13,14 The most positive findings have been observed for team-based efforts at managing specific diseases, such as diabetes and congestive heart failure (CHF), or specific populations, such as older patients with chronic illness. Studies have shown that using a team approach results in improved metrics, including HbA1c, low-density lipoprotein cholesterol, blood pressure (BP), and body mass index.7,15-20 Team-based models that pair physicians and other primary care providers with a clinical pharmacist have increased patients’ medication adherence and provider adherence to recommended prescribing habits.15,21-23
One small clinical microsystem that focused on self-management support with health coaching increased patients’ ratings of their confidence in self-management from 40% to 60% at baseline to 80% to 90% after one year. This program also increased the proportion of patients in whom BP was controlled by 10% to 15%.10
Despite these successes, some team-based models may not always be “doable” because of the costs of adding an advanced practice clinician to the staff, or the challenges of recruiting the right person for the job. (How to adapt team-based care for smaller practices is discussed below.)
Improved access to care. A preponderance of data shows that team-based care increases the volume of patient visits, thereby improving access to care.7,21,24-28 The critical elements to successfully achieving this are effective training and delegation. In private practice, using well-trained clinical assistants to create a physician-driven team can increase patient visit volume by an estimated 30% (using 1 assistant) to 60% (using 2 assistants).24
Similar increases in visit volume are seen in larger patient-centered medical home (PCMH) models that consist of physicians, PAs or NPs, MAs, LPNs, RNs, and clinical pharmacists.7,25 Teams with defined ratios of assistants to physicians/NPs/PAs see the most patients per day compared to care coordinator models (ie, 1 assistant for multiple physicians) or enhanced traditional models.21 When focusing on disease-specific care, the impact on access can be even greater. A diabetes-specific team-based care program resulted in a >50% increase in daily patient encounters and 4-fold increase in annual office visits.28
In addition to increasing visits, team-based care also increases access to care by decreasing wait times for an appointment and increasing the use of secure messaging and telephone visits.7,25 In a prospective cohort pilot study of more than 2000 patients enrolled in a team-based care model, the average scheduling time for a face-to-face visit for nonurgent care decreased from a mean of 26.5 days to 14 days, compared to a mean of 31.5 days to 17.8 days for controls.25 (The decrease in the control group was likely due to implementation of an electronic medical record in the practice.) Furthermore, a non-controlled evaluation of health plan-based practice groups with very large patient populations (ie, >300,000 patients) reported up to a 3-fold decrease in appointment waiting time when using a team-based model.29
Some studies have found a decrease in office visits after implementing team-based care.7 However, these reports also found a corresponding increase (by as much as 80%) in the use of secure messaging and telephone encounters, which translated to an overall enhanced communication with patients and ultimately increased access to care.7
Decreased costs. Several controlled trials have looked at the financial impact of using team-based care to manage chronic conditions such as asthma, CHF, and diabetes. Rich et al30 found a nurse-directed program of patient self-management support via telephone and home visit follow-up was associated with a 56% reduction in hospital readmissions, which translated to a $460 decrease in cost per patient over a 3-month period compared to a control group. In a study by Domurat,31 hospital stays were 50% shorter for high-risk diabetes patients who were managed by a team that offered planned visits, telephone contact, and group visits; this resulted in a lower cost of care. Katon et al32 found that when a nurse manager was added to a primary care team to enhance self-management support, intensify treatment, and coordinate continuity of care for patients with multiple chronic conditions, outpatient health costs were decreased by $594 per patient over 24 months.
Liu et al33 randomly assigned 354 patients in a VA primary care clinic who met criteria for major depression or dysthymia to usual care or a collaborative care model. The collaborative care model included a mental health care team that provided telephone contact to encourage medication adherence and reviewed and suggested modifications to the treatment plan. After an initial expenditure of $519 per patient, a savings of approximately $33 per patient for total outpatient costs was realized.
A team-based coordinated care program for patients with multiple chronic conditions reduced patient visits to specialists by 24%, ED visits by 13%, and hospitalizations by 39%.34 An internal evaluation found that the program saved money by reducing admissions, including intensive care unit stays and “observational” stays for Medicare fee-for-service patients.35
What about reimbursement? Most studies that have evaluated the financial aspects of implementing team-based care have calculated the cost savings for the health system—rather than for an individual practice—through decreased hospital admissions, readmissions, and ED visits. Efficient, high-quality teams will require a substantial initial investment of time and hiring and training of staff before savings can be realized.
Team-based care may not be financially sustainable unless current reimbursement models are changed. The current US system bases payment on quantity of care instead of quality of care, reimburses only for clinician services, and does not compensate teams.36 The Centers for Medicare and Medicaid Services (CMS) has begun to recognize the need to reimburse for services that are not delivered in face-to-face patient encounters. For example, the agency established a new G-code that can be used for non-face-to-face care management services for Medicare patients with 2 or more significant chronic conditions; this code took effect on January 1, 2015.37
Some insurers are reimbursing practices for obtaining designation as a PCMH. This type of reimbursement could be expanded to include other types of team-based efforts—such as self-management support and health coaching.
Improved team satisfaction. While many primary care providers are experiencing fatigue and burnout,38 support staff in many practices also experience job dissatisfaction, which leads to increased absenteeism and high turnover. Several studies indicate that involving all levels of staff in the improvement process and empowering them to work to their full potential by enhancing their roles and realigning responsibilities can increase satisfaction.7,11,21,38,39 This in turn can lead to increased loyalty, commitment, and productivity, with decreased burnout and turnover.
TABLE 2
| Team-based care: Additional resources | |
| Resource | Comments |
The Dartmouth Institute Microsystem Academy | This site includes assessment tools and strategies for implementing clinical microsystems into practices |
Improving Chronic Illness Care | This site provides information about the chronic care model, care coordination, and patient-centered medical homes |
TeamSTEPPS | TeamSTEPPS is an evidence-based teamwork system to improve communication and teamwork skills among health care professionals. All resources, including training materials, are free and downloadable |
Godfrey MM, Melin CN, Muething SE, et al. Clinical microsystems, Part 3. Transformation of two hospitals using microsystem, mesosystem, and macrosystem strategies. Jt Comm J Qual Patient Saf. 2008;34:591-603. | This article provides resources and strategies to engage all levels of the health system in team-based care |
McKinley KE, Berry SA, Laam LA, et al. Clinical microsystems, Part 4. Building innovative population-specific mesosystems. Jt Comm J Qual Patient Saf. 2008;34:655-663. | This article describes how to engage leadership at the health systems level |
Adapting team-based care for smaller practices
Physicians who practice alone or in small groups may have limited capacity to employ allied health professionals. However, your “team” doesn’t need to be housed only in your office. One innovative approach is the community-based medical home, where physicians with medical homes and/or care teams in their offices refer to, and collaborate with, a network of community-based professionals and agencies for clinical and social service support for their patients.22 Some options are to partner with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools.
While having team-based care strategies is necessary to achieve a PCMH designation, you do not need to seek such designation in order to practice team-based care. Start by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. In addition, determine what you value for your practice and what outcomes you hope for, along with a clear plan of how to measure these outcomes. This will allow you to determine if the estimated cost of the proposed strategy is “worth it” in terms of your individual situation and goals.
CORRESPONDENCE
Michele Q. Zawora, MD, Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
› Explore the potential benefits of team-based care by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. A
› Consider partnering with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Institute for Healthcare Improvement’s “Triple Aim” approach to optimizing the delivery of health care in the United States calls for improving the patient’s experience of care, including both quality and satisfaction; improving the health of populations; and reducing the per-capita cost of health care.1 Unfortunately, achieving these goals is being made more challenging by a perfect storm of conditions: The age of the population and the number of people accessing the systems are increasing, while the number of providers available to care for these patients is decreasing. The number of annual office visits to family physicians (FPs) in the United States is projected to increase from 462 million in 2008 to 565 million in 2025, which will require an estimated 51,880 additional FPs.2
One of the health care delivery models that has recently gained traction to help address this is team-based care. By practicing in a team-based care model, physicians and other clinicians can care for more patients, better manage those with high-risk and high-cost needs, and improve overall quality of care and satisfaction for all involved. Here we review the evidence for team-based care and its use for chronic disease management, and offer suggestions for its implementation.
The many providers who comprise the team
There is little consistency in the definition, composition, training, or maintenance of health care teams. Naylor et al3 defined team-based care as “the provision of health services to individuals, families, and/or their communities by at least two health providers who work collaboratively with patients and their caregivers—to the extent preferred by each patient—to accomplish shared goals within and across settings to achieve coordinated, high-quality care.”
While the team construct will vary based on the needs of your practice and your patients, developing a high-functioning team is essential to achieving success. Our 12-step checklist for building a successful team is a good starting point (TABLE 1).4-6 Many other resources are available to help with each step of this process (TABLE 2).
Teams should be led by a primary care provider—a physician, nurse practitioner (NP), or physician assistant (PA)—and consist of other members that complement the other’s expertise and roles, such as nurse case managers, clinical pharmacists, social workers, and behavioral health experts. Some practices have large teams with interdisciplinary members, including pharmacists, PAs, and NPs (the “expanded staffing” model), while others form smaller “teamlets” consisting of a physician and a registered nurse (RN) who serves as a health coach.
In the expanded staffing model, RNs and clinical pharmacists assume greater care management, while medical assistants (MAs) and licensed practice nurses (LPNs) are responsible for pre-visit, outreach, and follow-up activities.7 Redefining roles can spread the work among all team members, which allows each member to work to their level of training and licensure and permits the MD/NP/PA to focus on more complex tasks.
The teamlet model has 2 main features: 1) Patient encounters involve a clinician (MD, NP, PA) and a health coach (MA, RN, LPN); and 2) Care is expanded beyond the usual 15-minute visit to include pre-visit, visit, post-visit, and between-visit care.8 Incorporating a health coach puts an increased focus on the patient and self-management support, with the goals of increasing satisfaction for both the patient and the health care team, improving outcomes, and lowering cost due to fewer emergency department (ED) visits and hospital admissions/readmissions.
Smaller teams seem to be more effective and more manageable.8,9 In one example of well-functioning teamlet composed of RNs and MAs, an MA is responsible for patients coming in for timely chronic and preventive care needs, while the RNs focus their efforts on tasks that require their expertise, including health coaching, self-management support, and patient education.9 Although smaller offices may not have the resources of a large academic practice, this model of maximizing the role of the MAs is reasonable and achievable.
Another example of a successful teamlet model is a clinical microsystem, in which a small group of clinicians and support staff work together to provide care to a discrete group of patients.10,11 (For more information on clinical microsystems, go to the Dartmouth Institute Microsystem Academy at https://clinicalmicrosystem.org.)
What are the barriers to creating team-based care?
Many providers and administrators are concerned about the costs of creating a team-based model of care. These include the cost of hiring new staff, retraining current staff, and educating team members and patients, as well as the cost of developing and maintaining the necessary information technology.
There is, of course, always the concern about physicians relinquishing patient care tasks to other team members. The flip side of that is that staff members may not be eager to increase their roles and responsibilities. In addition, developing a high-functioning team requires ongoing efforts to train and retrain, as well as dedicated leadership and an ongoing commitment to team building.12
Team-based care can work well for managing chronic diseases
Despite the challenges of developing and maintaining this approach to care, the evidence suggests that implementing a team-based model can be especially useful for patients with chronic diseases, because it can improve patient outcomes and access to care, decrease costs, and improve clinician satisfaction—as detailed below.
Improved patient outcomes. Initial evidence suggests that implementing a team-based model can improve patients’ health and experience of care.13,14 The most positive findings have been observed for team-based efforts at managing specific diseases, such as diabetes and congestive heart failure (CHF), or specific populations, such as older patients with chronic illness. Studies have shown that using a team approach results in improved metrics, including HbA1c, low-density lipoprotein cholesterol, blood pressure (BP), and body mass index.7,15-20 Team-based models that pair physicians and other primary care providers with a clinical pharmacist have increased patients’ medication adherence and provider adherence to recommended prescribing habits.15,21-23
One small clinical microsystem that focused on self-management support with health coaching increased patients’ ratings of their confidence in self-management from 40% to 60% at baseline to 80% to 90% after one year. This program also increased the proportion of patients in whom BP was controlled by 10% to 15%.10
Despite these successes, some team-based models may not always be “doable” because of the costs of adding an advanced practice clinician to the staff, or the challenges of recruiting the right person for the job. (How to adapt team-based care for smaller practices is discussed below.)
Improved access to care. A preponderance of data shows that team-based care increases the volume of patient visits, thereby improving access to care.7,21,24-28 The critical elements to successfully achieving this are effective training and delegation. In private practice, using well-trained clinical assistants to create a physician-driven team can increase patient visit volume by an estimated 30% (using 1 assistant) to 60% (using 2 assistants).24
Similar increases in visit volume are seen in larger patient-centered medical home (PCMH) models that consist of physicians, PAs or NPs, MAs, LPNs, RNs, and clinical pharmacists.7,25 Teams with defined ratios of assistants to physicians/NPs/PAs see the most patients per day compared to care coordinator models (ie, 1 assistant for multiple physicians) or enhanced traditional models.21 When focusing on disease-specific care, the impact on access can be even greater. A diabetes-specific team-based care program resulted in a >50% increase in daily patient encounters and 4-fold increase in annual office visits.28
In addition to increasing visits, team-based care also increases access to care by decreasing wait times for an appointment and increasing the use of secure messaging and telephone visits.7,25 In a prospective cohort pilot study of more than 2000 patients enrolled in a team-based care model, the average scheduling time for a face-to-face visit for nonurgent care decreased from a mean of 26.5 days to 14 days, compared to a mean of 31.5 days to 17.8 days for controls.25 (The decrease in the control group was likely due to implementation of an electronic medical record in the practice.) Furthermore, a non-controlled evaluation of health plan-based practice groups with very large patient populations (ie, >300,000 patients) reported up to a 3-fold decrease in appointment waiting time when using a team-based model.29
Some studies have found a decrease in office visits after implementing team-based care.7 However, these reports also found a corresponding increase (by as much as 80%) in the use of secure messaging and telephone encounters, which translated to an overall enhanced communication with patients and ultimately increased access to care.7
Decreased costs. Several controlled trials have looked at the financial impact of using team-based care to manage chronic conditions such as asthma, CHF, and diabetes. Rich et al30 found a nurse-directed program of patient self-management support via telephone and home visit follow-up was associated with a 56% reduction in hospital readmissions, which translated to a $460 decrease in cost per patient over a 3-month period compared to a control group. In a study by Domurat,31 hospital stays were 50% shorter for high-risk diabetes patients who were managed by a team that offered planned visits, telephone contact, and group visits; this resulted in a lower cost of care. Katon et al32 found that when a nurse manager was added to a primary care team to enhance self-management support, intensify treatment, and coordinate continuity of care for patients with multiple chronic conditions, outpatient health costs were decreased by $594 per patient over 24 months.
Liu et al33 randomly assigned 354 patients in a VA primary care clinic who met criteria for major depression or dysthymia to usual care or a collaborative care model. The collaborative care model included a mental health care team that provided telephone contact to encourage medication adherence and reviewed and suggested modifications to the treatment plan. After an initial expenditure of $519 per patient, a savings of approximately $33 per patient for total outpatient costs was realized.
A team-based coordinated care program for patients with multiple chronic conditions reduced patient visits to specialists by 24%, ED visits by 13%, and hospitalizations by 39%.34 An internal evaluation found that the program saved money by reducing admissions, including intensive care unit stays and “observational” stays for Medicare fee-for-service patients.35
What about reimbursement? Most studies that have evaluated the financial aspects of implementing team-based care have calculated the cost savings for the health system—rather than for an individual practice—through decreased hospital admissions, readmissions, and ED visits. Efficient, high-quality teams will require a substantial initial investment of time and hiring and training of staff before savings can be realized.
Team-based care may not be financially sustainable unless current reimbursement models are changed. The current US system bases payment on quantity of care instead of quality of care, reimburses only for clinician services, and does not compensate teams.36 The Centers for Medicare and Medicaid Services (CMS) has begun to recognize the need to reimburse for services that are not delivered in face-to-face patient encounters. For example, the agency established a new G-code that can be used for non-face-to-face care management services for Medicare patients with 2 or more significant chronic conditions; this code took effect on January 1, 2015.37
Some insurers are reimbursing practices for obtaining designation as a PCMH. This type of reimbursement could be expanded to include other types of team-based efforts—such as self-management support and health coaching.
Improved team satisfaction. While many primary care providers are experiencing fatigue and burnout,38 support staff in many practices also experience job dissatisfaction, which leads to increased absenteeism and high turnover. Several studies indicate that involving all levels of staff in the improvement process and empowering them to work to their full potential by enhancing their roles and realigning responsibilities can increase satisfaction.7,11,21,38,39 This in turn can lead to increased loyalty, commitment, and productivity, with decreased burnout and turnover.
TABLE 2
| Team-based care: Additional resources | |
| Resource | Comments |
The Dartmouth Institute Microsystem Academy | This site includes assessment tools and strategies for implementing clinical microsystems into practices |
Improving Chronic Illness Care | This site provides information about the chronic care model, care coordination, and patient-centered medical homes |
TeamSTEPPS | TeamSTEPPS is an evidence-based teamwork system to improve communication and teamwork skills among health care professionals. All resources, including training materials, are free and downloadable |
Godfrey MM, Melin CN, Muething SE, et al. Clinical microsystems, Part 3. Transformation of two hospitals using microsystem, mesosystem, and macrosystem strategies. Jt Comm J Qual Patient Saf. 2008;34:591-603. | This article provides resources and strategies to engage all levels of the health system in team-based care |
McKinley KE, Berry SA, Laam LA, et al. Clinical microsystems, Part 4. Building innovative population-specific mesosystems. Jt Comm J Qual Patient Saf. 2008;34:655-663. | This article describes how to engage leadership at the health systems level |
Adapting team-based care for smaller practices
Physicians who practice alone or in small groups may have limited capacity to employ allied health professionals. However, your “team” doesn’t need to be housed only in your office. One innovative approach is the community-based medical home, where physicians with medical homes and/or care teams in their offices refer to, and collaborate with, a network of community-based professionals and agencies for clinical and social service support for their patients.22 Some options are to partner with a local pharmacist or with insurers to use their community health workers, nurse case managers, and other self-management support tools.
While having team-based care strategies is necessary to achieve a PCMH designation, you do not need to seek such designation in order to practice team-based care. Start by conducting a full assessment of your practice, including patient panels, payer mix, current finances, regional pay-for-performance programs, leadership support, and your staff’s training and talents. In addition, determine what you value for your practice and what outcomes you hope for, along with a clear plan of how to measure these outcomes. This will allow you to determine if the estimated cost of the proposed strategy is “worth it” in terms of your individual situation and goals.
CORRESPONDENCE
Michele Q. Zawora, MD, Thomas Jefferson University, 1015 Walnut Street, Suite 401, Philadelphia, Pa 19107; [email protected]
1. Institute of Healthcare Improvement. The IHI Triple Aim. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/offerings/Initiatives/TripleAim/Pages/default.aspx. Accessed February 5, 2015.
2.Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2025. Ann Fam Med. 2012;10:503-509.
3. Naylor MD, Coburn KD, Kurtzman ET, et al. Inter-professional team-based primary care for chronically ill adults: State of the science. White paper presented at: the ABIM Foundation meeting to Advance Team-Based Care for the Chronically Ill in Ambulatory Settings; March 24-25, 2010; Philadelphia, PA.
4.Boult C, Green AF, Boult LB, et al. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the Institute of Medicine’s Retooling for an Aging America report. J Am Geriatr Soc. 2009;57:2328-2337.
5.Kuzel AJ. Keys to high-functioning office teams. Family Practice Management. 2011;18:15-18.
6.Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
7. Reid RJ, Coleman K, Johnson EA, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood). 2010;29:835-843.
8. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
9. Chen EH, Thom DH, Hessler DM, et al. Using the teamlet model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(suppl 4):S610-S614.
10. Wasson JH, Anders SG, Moore LG, et al. Clinical microsystems, part 2. Learning from micro practices about providing patients the care they want and need. Jt Comm J Qual Patient Saf. 2008;34:445-452.
11. Williams I, Dickinson H, Robinson S. Clinical microsystems: An evaluation. Health Services Management Centre, School of Public Policy, University of Birmingham, England; 2007. Available at: http://chain.ulcc.ac.uk/chain/documents/hsmc_evaluation_report_CMS2007final.pdf. Accessed February 20, 2015.
12. Willard R, Bodenheimer T. The building blocks of high-performing primary care: Lessons from the field. April 2012. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/B/PDF%20BuildingBlocksPrimaryCare.pdf. Accessed February 10, 2015.
13. Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569-572.
14. Boult C, Karm L, Groves C. Improving chronic care: the “guided care” model. Perm J. 2008;12:50-54.
15. Smith SM, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560.
16. Bloom FJ, Graf TR, Steele GD. Improved patient outcomes in 3 years with a system of care for diabetes. October 2012. Available at: http://iom.edu/~/media/Files/Perspectives-Files/2012/Commentaries/VSRT-Improved-Patient-Outcomes.pdf. Accessed February 10, 2015.
17. Bloom FJ Jr, Yan X, Stewart WF, et al. Primary care diabetes bundle management: 3-year outcomes for microvascular and macrovascular events. Am J Manag Care. 2014;20:e175-e182.
18. Pape GA, Hunt JS, Butler KL, et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Arch Intern Med. 2011;171:1480-1486.
19. Scanlon DP, Hollenbeak CS, Beich J, et al. Financial and clinical impact of team-based treatment for medicaid enrollees with diabetes in a federally qualified health center. Diabetes Care. 2008;31:2160-2165.
20. Renders CM, Valk GD, Griffin S, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(1):CD001481.
21. Goldberg GD, Beeson T, Kuzel AJ, et al. Team-based care: a critical element of primary care practice transformation. Popul Health Manag. 2013;16:150-156.
22. Lipton HL. Home is where the health is: advancing team-based care in chronic disease management. Arch Intern Med. 2009;169:1945-1948.
23. Farris KB, Côté I, Feeny D, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998-1003.
24. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
25. Grumbach K, Bodenheimer T, Grundy P. The outcomes of implementing patient-centered medical home interventions: A review of the evidence on quality, access and costs from recent prospective evaluation studies, August 2009. Washington, DC; Patient-Centered Primary Care Collaborative; 2009.
26. Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood). 2011;30:646-654.
27. Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;16:955-964.
28. Bray P, Roupe M, Young S, et al. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: the eastern North Carolina experience. Diabetes Educ. 2005;31:712-718.
29. Institute for Healthcare Improvement. Health Partners uses “BestCare” practices to improve care and outcomes, reduce costs. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/engage/initiatives/TripleAim/Documents/IHITripleAimHealthPartnersSummaryofSuccessJul09v2.pdf. Accessed February 5, 2015.
30. Rich MW, Beckman V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190-1195.
31. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
32. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506-514.
33. Liu CF, Hendrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54:698-704.
34. Berenson RA; The Urban Institute. Challenging the status quo in chronic disease care: seven case studies. California Healthcare Foundation: 2006. California HealthCare Foundation Web site. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ChallengingStatusQuoCaseStudies.pdf. Accessed February 5, 2015.
35. Coleman EA, Smith JD, Frank JC, et al. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52:1817-1825.
36. Schneider ME. Medicare finalizes plan for non-face-to-face payments. Family Practice News Web site. Available at: http://www.familypracticenews.com/?id=2633&tx_ttnews%5Btt_news%5D=226457&cHash=2aeafe0585c7156dcf23891d010cd12f. Accessed December 2, 2013.
37. Centers for Medicare & Medicaid Services. Fact sheets: Policy and payment changes to the Medicare Physician Fee Schedule for 2015. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2014-Fact-sheets-items/2014-10-31-7.html. Accessed February 13, 2015.
38. Berry LL, Dunham J. Redefining the patient experience with collaborative care. Harvard Business Review Blog Network. Harvard Business Review Web site. Available at: https://hbr.org/2013/09/redefining-the-patient-experience-with-collaborative-care/. Accessed February 5, 2015.
39. Lyon RK, Slawson J. An organized approach to chronic disease care. Fam Pract Manag. 2011;18:27-31.
1. Institute of Healthcare Improvement. The IHI Triple Aim. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/offerings/Initiatives/TripleAim/Pages/default.aspx. Accessed February 5, 2015.
2.Petterson SM, Liaw WR, Phillips RL Jr, et al. Projecting US primary care physician workforce needs: 2010-2025. Ann Fam Med. 2012;10:503-509.
3. Naylor MD, Coburn KD, Kurtzman ET, et al. Inter-professional team-based primary care for chronically ill adults: State of the science. White paper presented at: the ABIM Foundation meeting to Advance Team-Based Care for the Chronically Ill in Ambulatory Settings; March 24-25, 2010; Philadelphia, PA.
4.Boult C, Green AF, Boult LB, et al. Successful models of comprehensive care for older adults with chronic conditions: Evidence for the Institute of Medicine’s Retooling for an Aging America report. J Am Geriatr Soc. 2009;57:2328-2337.
5.Kuzel AJ. Keys to high-functioning office teams. Family Practice Management. 2011;18:15-18.
6.Sinsky CA, Willard-Grace R, Schutzbank AM, et al. In search of joy in practice: a report of 23 high-functioning primary care practices. Ann Fam Med. 2013;11:272-278.
7. Reid RJ, Coleman K, Johnson EA, et al. The Group Health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood). 2010;29:835-843.
8. Bodenheimer T, Laing BY. The teamlet model of primary care. Ann Fam Med. 2007;5:457-461.
9. Chen EH, Thom DH, Hessler DM, et al. Using the teamlet model to improve chronic care in an academic primary care practice. J Gen Intern Med. 2010;25(suppl 4):S610-S614.
10. Wasson JH, Anders SG, Moore LG, et al. Clinical microsystems, part 2. Learning from micro practices about providing patients the care they want and need. Jt Comm J Qual Patient Saf. 2008;34:445-452.
11. Williams I, Dickinson H, Robinson S. Clinical microsystems: An evaluation. Health Services Management Centre, School of Public Policy, University of Birmingham, England; 2007. Available at: http://chain.ulcc.ac.uk/chain/documents/hsmc_evaluation_report_CMS2007final.pdf. Accessed February 20, 2015.
12. Willard R, Bodenheimer T. The building blocks of high-performing primary care: Lessons from the field. April 2012. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/B/PDF%20BuildingBlocksPrimaryCare.pdf. Accessed February 10, 2015.
13. Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320:569-572.
14. Boult C, Karm L, Groves C. Improving chronic care: the “guided care” model. Perm J. 2008;12:50-54.
15. Smith SM, Soubhi H, Fortin M, et al. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012;4:CD006560.
16. Bloom FJ, Graf TR, Steele GD. Improved patient outcomes in 3 years with a system of care for diabetes. October 2012. Available at: http://iom.edu/~/media/Files/Perspectives-Files/2012/Commentaries/VSRT-Improved-Patient-Outcomes.pdf. Accessed February 10, 2015.
17. Bloom FJ Jr, Yan X, Stewart WF, et al. Primary care diabetes bundle management: 3-year outcomes for microvascular and macrovascular events. Am J Manag Care. 2014;20:e175-e182.
18. Pape GA, Hunt JS, Butler KL, et al. Team-based care approach to cholesterol management in diabetes mellitus: two-year cluster randomized controlled trial. Arch Intern Med. 2011;171:1480-1486.
19. Scanlon DP, Hollenbeak CS, Beich J, et al. Financial and clinical impact of team-based treatment for medicaid enrollees with diabetes in a federally qualified health center. Diabetes Care. 2008;31:2160-2165.
20. Renders CM, Valk GD, Griffin S, et al. Interventions to improve the management of diabetes mellitus in primary care, outpatient and community settings. Cochrane Database Syst Rev. 2001;(1):CD001481.
21. Goldberg GD, Beeson T, Kuzel AJ, et al. Team-based care: a critical element of primary care practice transformation. Popul Health Manag. 2013;16:150-156.
22. Lipton HL. Home is where the health is: advancing team-based care in chronic disease management. Arch Intern Med. 2009;169:1945-1948.
23. Farris KB, Côté I, Feeny D, et al. Enhancing primary care for complex patients. Demonstration project using multidisciplinary teams. Can Fam Physician. 2004;50:998-1003.
24. Anderson P, Halley MD. A new approach to making your doctor-nurse team more productive. Fam Pract Manag. 2008;15:35-40.
25. Grumbach K, Bodenheimer T, Grundy P. The outcomes of implementing patient-centered medical home interventions: A review of the evidence on quality, access and costs from recent prospective evaluation studies, August 2009. Washington, DC; Patient-Centered Primary Care Collaborative; 2009.
26. Smith M, Giuliano MR, Starkowski MP. In Connecticut: improving patient medication management in primary care. Health Aff (Millwood). 2011;30:646-654.
27. Kaboli PJ, Hoth AB, McClimon BJ, et al. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;16:955-964.
28. Bray P, Roupe M, Young S, et al. Feasibility and effectiveness of system redesign for diabetes care management in rural areas: the eastern North Carolina experience. Diabetes Educ. 2005;31:712-718.
29. Institute for Healthcare Improvement. Health Partners uses “BestCare” practices to improve care and outcomes, reduce costs. Institute for Healthcare Improvement Web site. Available at http://www.ihi.org/engage/initiatives/TripleAim/Documents/IHITripleAimHealthPartnersSummaryofSuccessJul09v2.pdf. Accessed February 5, 2015.
30. Rich MW, Beckman V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190-1195.
31. Domurat ES. Diabetes managed care and clinical outcomes: the Harbor City, California Kaiser Permanente diabetes care system. Am J Manag Care. 1999;5:1299-1307.
32. Katon W, Russo J, Lin EH, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506-514.
33. Liu CF, Hendrick SC, Chaney EF, et al. Cost-effectiveness of collaborative care for depression in a primary care veteran population. Psychiatr Serv. 2003;54:698-704.
34. Berenson RA; The Urban Institute. Challenging the status quo in chronic disease care: seven case studies. California Healthcare Foundation: 2006. California HealthCare Foundation Web site. Available at: http://www.chcf.org/~/media/MEDIA%20LIBRARY%20Files/PDF/C/PDF%20ChallengingStatusQuoCaseStudies.pdf. Accessed February 5, 2015.
35. Coleman EA, Smith JD, Frank JC, et al. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc. 2004;52:1817-1825.
36. Schneider ME. Medicare finalizes plan for non-face-to-face payments. Family Practice News Web site. Available at: http://www.familypracticenews.com/?id=2633&tx_ttnews%5Btt_news%5D=226457&cHash=2aeafe0585c7156dcf23891d010cd12f. Accessed December 2, 2013.
37. Centers for Medicare & Medicaid Services. Fact sheets: Policy and payment changes to the Medicare Physician Fee Schedule for 2015. Centers for Medicare & Medicaid Services Web site. Available at: http://www.cms.gov/newsroom/mediareleasedatabase/fact-sheets/2014-Fact-sheets-items/2014-10-31-7.html. Accessed February 13, 2015.
38. Berry LL, Dunham J. Redefining the patient experience with collaborative care. Harvard Business Review Blog Network. Harvard Business Review Web site. Available at: https://hbr.org/2013/09/redefining-the-patient-experience-with-collaborative-care/. Accessed February 5, 2015.
39. Lyon RK, Slawson J. An organized approach to chronic disease care. Fam Pract Manag. 2011;18:27-31.
“Doctor, I’m so tired!” Refining your work-up for chronic fatigue
Ms. C says she sleeps well, getting more than 8 hours of sleep per night on weekends but fewer than 7 hours per night during the week. But no matter how long she sleeps, she never awakens feeling refreshed. Ms. C reports that she doesn’t smoke, has no more than 4 alcoholic drinks per month, and adheres to an “average” diet. She is too tired to exercise.
Ms. C is single, with no children. Although she says she has a strong network of family and friends, she increasingly finds she has no energy for socializing. If Ms. C were your patient, what would you do?
Fatigue is a common presenting symptom in primary care, accounting for about 5% of adult visits.1 Defined as a generalized lack of energy, fatigue that persists despite adequate rest or is severe enough to disrupt an individual’s ability to participate in key social and/or occupational activities warrants a thorough investigation.
Because fatigue is a nonspecific symptom that may be linked to a number of medical and psychiatric illnesses or medications used to treat them, determining the cause can be difficult. In about half of all cases, no specific etiology is found.2 This review, which includes the elements of a work-up and management strategies for patients presenting with ongoing fatigue, will help you arrive at the appropriate diagnosis and provide optimal treatment.
Chronic fatigue: Defining the terms
A definition of chronic fatigue syndrome (CFS) was initially published in 1988.3 In subsequent years, the term myalgic encephalomyelitis (ME) became popular. Although the terms are sometimes used interchangeably, ME often refers to patients whose condition is thought to have an infectious cause and for whom postexertional malaise is a hallmark symptom.4
CDC criteria. While several sets of diagnostic criteria for CFS have been developed, the most widely used is that of the Centers for Disease Control and Prevention (CDC), published in 1994 (TABLE 1).5,6 A diagnosis of CFS is made on the basis of exclusion, subjective clinical interpretation, and patient self-report.
When the first 2 criteria—fatigue not due to ongoing exertion or other medical conditions that has lasted ≥6 months and is severe enough to interfere with daily activities—but fewer than 4 of the CDC’s 8 concurrent symptoms (eg, headache, unrefreshing sleep, and postexertion malaise lasting >24 hours) are present, idiopathic fatigue, rather than CFS, is diagnosed.6
International Consensus Criteria (ICC). In 2011, the ICC for ME were proposed in an effort to provide more specific diagnostic criteria (TABLE 2).7 The ICC emphasize fatigability, or what the authors identify as “post-exertional neuroimmune exhaustion.”
The ICC have not yet been broadly researched. But an Australian study of patients with chronic fatigue found that those who met the ICC definition were sicker and more homogeneous, with significantly lower scores for physical and social functioning and bodily pain compared with those who fulfilled the CDC criteria alone.8
Chronic fatigue & neuropsychiatric conditions: Common threads
Recent research has made it clear that depression, somatization, and CFS share some biological underpinnings. These include biomarkers for inflammation, cell-mediated immune activation—which may be related to the symptoms of fatigue—autonomic dysfunction, and hyperalgesia.9 Evidence suggests that up to two-thirds of patients with CFS also meet the criteria for a psychiatric disorder.10 The most common psychiatric conditions are major depressive disorder (MDD), affecting an estimated 22% to 32% of those with CFS; anxiety disorder, affecting about 20%; and somatization disorder, affecting about 10%—at least double the incidence of the general population.10
Others point out, however, that up to half of those with CFS do not have a psychiatric disorder.11 A diagnosis of somatization disorder, in particular, depends largely on a subjective interpretation of whether or not the presenting symptoms have a physical cause.10
CFS and MDD comorbidity. The most widely studied association between CFS and psychiatric disorders involves MDD. Observational studies have found patients with CFS have a lifetime prevalence of MDD of 65%,12,13 which is higher than that of patients with other chronic diseases. Overlapping symptoms include fatigue, sleep disturbance, poor concentration, and memory problems. However, those with CFS have fewer symptoms related to anhedonia, poor self-esteem, guilt, and suicidal ideation compared with individuals with MDD.12,13
There are several possible explanations for CFS and MDD comorbidity, which are not necessarily mutually exclusive.10 One theory is that CFS is an atypical form of depression; another holds that the disability associated with CFS leads to depression, as is the case with many other chronic illnesses; and a third points to overlapping pathophysiology.10
An emerging body of evidence suggests that CFS and MDD have some common oxidative and nitrosative biochemical pathways. Activated by infection, psychological stress, and immune disorders, they are believed to have damaging free radical and nitric oxide effects at the cellular level.14 The cellular effects can result in fatigue, muscle pain, and flu-like malaise.
Cortisol response differs
CFS and MDD might be distinguishable by another pathway—the hypothalamic-pituitary-adrenal (HPA) axis. MDD is classically associated with activation and raised cortisol levels, while CFS is consistently associated with impaired HPA axis functioning and reduced cortisol levels.10 The majority of patients with CFS report symptoms of cognitive decline, with the acquisition of new verbal learning and information-processing speed particularly likely to be impaired.15
A meta-analysis of 50 studies of patients with CFS showed deficits in attention, memory, and reaction time, but not in fine motor speed, vocabulary, or reasoning.16 Autonomic dysfunction has also been observed, including disordered sympathetic activity. The most frequently observed abnormalities on autonomic testing are postural hypotension, tachycardia syndrome, neurally mediated hypotension, and heart rate variability during tilt table testing.16
The link between infection and CFS
Several infectious agents have been associated with ME/CFS, including the Epstein-Barr virus (EBV), herpes simplex virus 6, parvovirus, Q fever, and Lyme disease.8,17,18 Most agents that have been linked to ME/CFS are associated with persistent infection and thus incitement of the immune system.
Numerous observational studies17,18 have documented postinfectious fatigue syndromes after acute viral and bacterial infections and symptoms suggestive of infection, such as fever, myalgias, and respiratory and gastrointestinal distress. In one prospective Australian study,19 investigators identified 253 cases of acute EBV, Ross River virus, and Q fever. Of those 253 patients, 12% went on to develop CFS, with a higher likelihood among those with more severe acute symptoms. No correlation with preexisting psychiatric disorders was found.
Muscle mitochondria studies have demonstrated what appear to be acquired abnormalities in those with CFS.20,21 Signs of increased oxidative stress have been found in both blood and muscle samples from patients with CFS, and longitudinal studies suggest that oxidative stress is greatest during periods of clinical exacerbation.22 Increased lactate levels suggest increased anaerobic metabolism in the central nervous system consistent with mitochondrial dysfunction. Several studies have demonstrated that exercise can precipitate oxidative stress in patients with CFS, in contrast with healthy controls and controls with other chronic illnesses, suggesting a physiologic basis for their postexertional symptoms.17
Autoinflammatory syndrome induced by adjuvants, a rare syndrome associated with vaccine administration, has been linked to postvaccination adverse events, exposure to silicone implants, Gulf War syndrome (related to multiple vaccinations), and macrophagic myofasciitis. All involve exposure to immune adjuvants and have similar clinical manifestations. The corresponding exposures appear to trigger an autoimmune response in susceptible individuals. The hepatitis B vaccine is most often associated with CFS, with symptoms occurring within 90 days of administration.23
The clinical work-up: Putting knowledge into practice
Familiarity with potential causes of and connections with ME/CFS will help you ensure that patients who say they’re always tired receive a thorough work-up. Start with a medical history, inquiring directly about medical and psychiatric disorders that may contribute to fatigue (TABLE 3).5,24,25 Include a medication history, as well, to help determine whether the fatigue is drug-related (TABLE 4).5,25
What to ask. Determine the onset, course, duration, daily pattern, and impact of fatigue on the patient’s daily life. Inquire, too, about related symptoms of daytime sleepiness, dyspnea on exertion, generalized weakness, and depressed mood. The prominence of any of these rather than fatigue, per se, point to a diagnosis of a chronic illness other than ME/CFS.
Keep in mind, too, that patients with an organ-based medical illness tend to associate their fatigue with activities that they are unable to complete, such as shopping or light housework. In contrast, those with fatigue that is not organ-based typically say that they’re tired all the time. Their fatigue is not necessarily related to exertion, nor does it improve with rest.26
To address this distinction, take a sleep history, assessing both the quality and quantity of the patient’s sleep to determine how it affects symptoms.27 Consider using a questionnaire designed to help distinguish between sleepiness—and a primary sleep disorder—and fatigue,28 such as the Fatigue Severity Scale of Sleep Disorders (http://www.healthywomen.org/sites/default/files/FatigueSeverityScale.pdf).
What to rule out. In addition to a medical history, the physical examination should be oriented toward ruling out secondary causes of fatigue. In addition to a system-by-system approach to the differential diagnosis, carefully observe the patient’s general appearance, with attention to his or her level of alertness, grooming, and psychomotor agitation or retardation as possible signs of a psychiatric disorder. To rule out neurologic causes, evaluate muscle bulk, tone, and strength; deep tendon reflexes; and sensory and cranial nerves, as well.29
Lab tests to consider. In most cases of ME/CFS, basic studies—complete blood count with differential, erythrocyte sedimentation rate, blood chemistry, and thyroid-stimulating hormone (TSH) levels—are sufficient. When no medical or psychiatric cause has been found, additional tests may be ordered on a case-by-case basis, although laboratory analysis affects the management of fatigue in less than 5% of such patients.30 Tests to consider include:
- Creatine kinase (for patients who report pain or muscle weakness)
- pregnancy test (for women of childbearing age)
- ferritin testing (for young women who might benefit from iron supplementation for levels <50 ng/mL even if anemia is not present)31
- hepatitis C screening (recommended by the US Preventive Services Task Force for those born between 1945 and 1965)32
- human immunodeficiency virus screening and the purified protein derivative test for tuberculosis (based on patient history).
Forego routine testing for other infections
Routine testing for infectious diseases and conditions associated with fatigue, such as EBV or Lyme disease, immune deficiency (eg, immunoglobulins), inflammatory disease (eg, antinuclear antibodies, rheumatoid factor), celiac disease, vitamin D deficiency, vitamin B12 deficiency, or heavy metal toxicity, is unlikely to be helpful.29 Additional testing simply to reassure a worried patient usually does not accomplish that objective.33
Additional studies, referrals to consider. If you suspect that a patient has a sleep disorder, a referral to a sleep clinic to rule out idiopathic sleep disorders, obstructive sleep apnea, or movement disorders that interfere with sleep may be in order. Spirometry and echocardiography may be helpful for some patients. If you suspect peripheral muscle fatigue, a referral for neuromuscular testing is indicated.
CASE › Ms. C’s medical history reveals that she also suffers from irritable bowel syndrome, which she manages with diet and over-the-counter medication, as needed, for constipation or diarrhea. She denies having any other chronic conditions. Her only other symptoms, she reports, are mild upper back pain after spending long hours at the computer, and arthralgias in her left knee and both hands. She admits to being “somewhat depressed” in the last few months, but denies the presence of anhedonia.
The patient’s physical examination is normal, and her depression screen does not meet the criteria for MDD. Her metabolic chemistry panel, complete blood count, TSH, and sedimentation rate are all normal, as well.
Symptom management and coping strategies
When no specific cause of chronic fatigue is found, the focus shifts from diagnosis to symptom management and coping strategies.
This requires engagement with the patient. It is important to acknowledge the existence of his or her symptoms and to reassure the patient that further investigation may be warranted later, should new symptoms emerge. Advise the patient, too, that periods of remission and relapse are likely.
Strategies designed to motivate patient self-management, as well as the formulation of patient-centered treatment plans, have been shown to reduce symptom scores.34 Participation in a support group, as well as frequent follow-up visits with a primary care physician, a behavioral therapist, or both, may help to provide needed psychological support.
Evidence of the effectiveness of specific therapies for ME/CFS is limited; however, the best-studied approaches are cognitive behavioral therapy (CBT) and graded exercise therapy.35 Exercise should be low intensity, such as walking or cycling for 30 minutes 3 times a week, with a gradual increase in duration and frequency over a period of weeks to months. Patients with cancer-related fatigue may benefit from yoga, group therapy, and stress management.36
Associated mood and pain symptoms should be treated, as well. Bupropion, which is somewhat stimulating, may be considered as an initial treatment for patients with depression and clinically significant fatigue.
Other potentially beneficial approaches include a healthy diet, avoidance of more than nominal amounts of alcohol, relative avoidance of caffeine (no more than one cup of a caffeinated beverage in the morning), and stress reduction techniques.
Attention to good sleep hygiene may be especially beneficial, including a regular bedtime routine and sleep schedule, and elimination of bedroom light and noise. Pharmacologic treatments for insomnia should be used with caution, if at all.
CASE › Ms. C receives a referral for CBT and is scheduled for a return visit in 4 weeks.
At the advice of both her primary care physician and the behavioral therapist, Ms. C gradually makes several lifestyle changes. She begins going to bed earlier on weeknights to ensure that she sleeps for at least 7 hours. She improves her diet, with increasing emphasis on vegetables, fruits, and whole grains. She also starts a walking program, increasing gradually to a total of 3 hours per week. After 4 months she adds a weekly trip to a gym, where she practices resistance training for about 40 minutes.
Ms. C also increases her social activities on weekends, and recently accepted an invitation to join a book club. Six months from her initial visit, Ms. C notes that although she is still more easily fatigued than most people, she has made significant improvement.
CORRESPONDENCE
Linda Speer, MD; University of Toledo, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Nijrolder I, van der Windt DA, van der Horst HE. Prognosis of fatigue and functioning in primary care: a 1-year follow-up study. Ann Fam Med. 2008;6:519-527.
2. Griffith JP, Zarrouf FA. A systematic review of chronic fatigue syndrome: don’t assume it’s depression. Primary Care Companion J Clin Psychiatry. 2008;10:120-128.
3. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387-389.
4. Morris G, Maes M. Case definitions and diagnostic criteria for myalgic encephalomyelitis and chronic fatigue syndrome: from clinical consensus to evidence-based case definitions. Neuro Endocrinol Lett. 2013;34:185-199.
5. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953-959.
6. Centers for Disease Control and Prevention. Chronic fatigue syndrome. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cfs/diagnosis/index.html. Accessed January 6, 2015.
7. Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270:327-338.
8. Johnston SC, Brenu EW, Hardcastle S, et al. A comparison of health status in patients meeting alternative definitions for chronic fatigue syndrome/myalgic encephalomyelitis. Health Qual Life Outcomes. 2014;12:64.
9. Anderson VR, Jason LA, Hlavaty LE, et al. A review and meta-synthesis of qualitative studies on myalgic encephalomyelitis/chronic fatigue syndrome. Patient Educ Couns. 2012;86:147-155.
10. Christley Y, Duffy T, Everall IP, et al. The neuropsychiatric and neuropsychological features of chronic fatigue syndrome: revisiting the enigma. Curr Psychiatry Rep. 2013;15;353.
11. Henningsen P, Zimmermann T, Sattel HH. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528-533.
12. Nater UM, Jones JF, Lin JM, et al. Personality features and personality disorders in chronic fatigue syndrome: a population-based study. Psychother Psychosom. 2010;79:312-318.
13. Taylor RR, Jason LA, Jahn SC. Chronic fatigue and sociodemographic characteristics as predictors of psychiatric disorders in a community-based sample. Psychosom Med. 2003;65:896-901.
14. Leonard B, Maes M. Mechanistic explanations of how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764-785.
15. DeLuca J, Christodoulou C, Diamond BJ, et al. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10:101-109.
16. Cockshell SJ, Mathias JL. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. 2010;40:1253-1267.
17. Komaroff AL, Cho TA. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin Neurol. 2011;31:325-337.
18. Naess H, Sundal E, Myhr KM, et al. Postinfectious and chronic fatigue syndromes: clinical experience from a tertiary-referral centre in Norway. In Vivo. 2010;24:185-188.
19. Hickie I, Davenport T, Wakefield D, et al; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575.
20. Plioplys AV, Plioplys S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology. 1995;32:175-181.
21. Vernon SD, Whistler T, Cameron B, et al. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infect Dis. 2006;6:15.
22. Miwa K, Fujita M. Fluctuation of serum vitamin E (alpha-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels. 2010;25:319-323.
23. Rosenblum H, Shoenfeld Y, Amital H. The common immunogenic etiology of chronic fatigue syndrome: from infections to vaccines via adjuvants to the ASIA syndrome. Infect Dis Clin North Am. 2011;25:851-863.
24. Vincent A, Brimmer DJ, Whipple MO, et al. Prevalence, incidence, and classification of chronic fatigue syndrome in Olmsted County, Minnesota as estimated using the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:1145-1152.
25. Goroll AH, Mulley AG. Primary Care Medicine: Office Evaluation and Management of the Adult Patient. 7th ed. Morrisville, PA: Wolters Kluwer; 2014.
26. Brown RF, Schutte NS. Direct and indirect relationships between emotional intelligence and subjective fatigue in university students. J Psychosom Res. 2006;60:585-593.
27. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54:61-69.
28. Bailes S, Libman E, Baltzan M, et al. Brief and distinct empirical sleepiness and fatigue scales. J Psychosom Res. 2006;60:605-613.
29. National Collaborating Centre for Primary Care (UK). Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (or Encephalopathy): Diagnosis and Management of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (or Encephalopathy) in Adults and Children. London, UK: Royal College of General Practitioners; 2007.
30. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci. 1990;299:313-318.
31. Vaucher P, Druisw PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184:1247-1254.
32. US Preventive Services Task Force. Hepatitis C: Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm. Accessed January 7, 2015.
33. Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: a systematic review and meta-analysis. JAMA Intern Med. 2013;173:407-416.
34. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
35. White PD, Goldsmith KA, Johnson AL, et al; PACE trial management group. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue (PACE): a randomised trial. Lancet. 2011;377:823-836.
36. van Weert E, Hoekstra-Weebers J, Otter R, et al. Cancer-related fatigue: predictors and effects of rehabilitation. Oncologist. 2006;11:184-196.
Ms. C says she sleeps well, getting more than 8 hours of sleep per night on weekends but fewer than 7 hours per night during the week. But no matter how long she sleeps, she never awakens feeling refreshed. Ms. C reports that she doesn’t smoke, has no more than 4 alcoholic drinks per month, and adheres to an “average” diet. She is too tired to exercise.
Ms. C is single, with no children. Although she says she has a strong network of family and friends, she increasingly finds she has no energy for socializing. If Ms. C were your patient, what would you do?
Fatigue is a common presenting symptom in primary care, accounting for about 5% of adult visits.1 Defined as a generalized lack of energy, fatigue that persists despite adequate rest or is severe enough to disrupt an individual’s ability to participate in key social and/or occupational activities warrants a thorough investigation.
Because fatigue is a nonspecific symptom that may be linked to a number of medical and psychiatric illnesses or medications used to treat them, determining the cause can be difficult. In about half of all cases, no specific etiology is found.2 This review, which includes the elements of a work-up and management strategies for patients presenting with ongoing fatigue, will help you arrive at the appropriate diagnosis and provide optimal treatment.
Chronic fatigue: Defining the terms
A definition of chronic fatigue syndrome (CFS) was initially published in 1988.3 In subsequent years, the term myalgic encephalomyelitis (ME) became popular. Although the terms are sometimes used interchangeably, ME often refers to patients whose condition is thought to have an infectious cause and for whom postexertional malaise is a hallmark symptom.4
CDC criteria. While several sets of diagnostic criteria for CFS have been developed, the most widely used is that of the Centers for Disease Control and Prevention (CDC), published in 1994 (TABLE 1).5,6 A diagnosis of CFS is made on the basis of exclusion, subjective clinical interpretation, and patient self-report.
When the first 2 criteria—fatigue not due to ongoing exertion or other medical conditions that has lasted ≥6 months and is severe enough to interfere with daily activities—but fewer than 4 of the CDC’s 8 concurrent symptoms (eg, headache, unrefreshing sleep, and postexertion malaise lasting >24 hours) are present, idiopathic fatigue, rather than CFS, is diagnosed.6
International Consensus Criteria (ICC). In 2011, the ICC for ME were proposed in an effort to provide more specific diagnostic criteria (TABLE 2).7 The ICC emphasize fatigability, or what the authors identify as “post-exertional neuroimmune exhaustion.”
The ICC have not yet been broadly researched. But an Australian study of patients with chronic fatigue found that those who met the ICC definition were sicker and more homogeneous, with significantly lower scores for physical and social functioning and bodily pain compared with those who fulfilled the CDC criteria alone.8
Chronic fatigue & neuropsychiatric conditions: Common threads
Recent research has made it clear that depression, somatization, and CFS share some biological underpinnings. These include biomarkers for inflammation, cell-mediated immune activation—which may be related to the symptoms of fatigue—autonomic dysfunction, and hyperalgesia.9 Evidence suggests that up to two-thirds of patients with CFS also meet the criteria for a psychiatric disorder.10 The most common psychiatric conditions are major depressive disorder (MDD), affecting an estimated 22% to 32% of those with CFS; anxiety disorder, affecting about 20%; and somatization disorder, affecting about 10%—at least double the incidence of the general population.10
Others point out, however, that up to half of those with CFS do not have a psychiatric disorder.11 A diagnosis of somatization disorder, in particular, depends largely on a subjective interpretation of whether or not the presenting symptoms have a physical cause.10
CFS and MDD comorbidity. The most widely studied association between CFS and psychiatric disorders involves MDD. Observational studies have found patients with CFS have a lifetime prevalence of MDD of 65%,12,13 which is higher than that of patients with other chronic diseases. Overlapping symptoms include fatigue, sleep disturbance, poor concentration, and memory problems. However, those with CFS have fewer symptoms related to anhedonia, poor self-esteem, guilt, and suicidal ideation compared with individuals with MDD.12,13
There are several possible explanations for CFS and MDD comorbidity, which are not necessarily mutually exclusive.10 One theory is that CFS is an atypical form of depression; another holds that the disability associated with CFS leads to depression, as is the case with many other chronic illnesses; and a third points to overlapping pathophysiology.10
An emerging body of evidence suggests that CFS and MDD have some common oxidative and nitrosative biochemical pathways. Activated by infection, psychological stress, and immune disorders, they are believed to have damaging free radical and nitric oxide effects at the cellular level.14 The cellular effects can result in fatigue, muscle pain, and flu-like malaise.
Cortisol response differs
CFS and MDD might be distinguishable by another pathway—the hypothalamic-pituitary-adrenal (HPA) axis. MDD is classically associated with activation and raised cortisol levels, while CFS is consistently associated with impaired HPA axis functioning and reduced cortisol levels.10 The majority of patients with CFS report symptoms of cognitive decline, with the acquisition of new verbal learning and information-processing speed particularly likely to be impaired.15
A meta-analysis of 50 studies of patients with CFS showed deficits in attention, memory, and reaction time, but not in fine motor speed, vocabulary, or reasoning.16 Autonomic dysfunction has also been observed, including disordered sympathetic activity. The most frequently observed abnormalities on autonomic testing are postural hypotension, tachycardia syndrome, neurally mediated hypotension, and heart rate variability during tilt table testing.16
The link between infection and CFS
Several infectious agents have been associated with ME/CFS, including the Epstein-Barr virus (EBV), herpes simplex virus 6, parvovirus, Q fever, and Lyme disease.8,17,18 Most agents that have been linked to ME/CFS are associated with persistent infection and thus incitement of the immune system.
Numerous observational studies17,18 have documented postinfectious fatigue syndromes after acute viral and bacterial infections and symptoms suggestive of infection, such as fever, myalgias, and respiratory and gastrointestinal distress. In one prospective Australian study,19 investigators identified 253 cases of acute EBV, Ross River virus, and Q fever. Of those 253 patients, 12% went on to develop CFS, with a higher likelihood among those with more severe acute symptoms. No correlation with preexisting psychiatric disorders was found.
Muscle mitochondria studies have demonstrated what appear to be acquired abnormalities in those with CFS.20,21 Signs of increased oxidative stress have been found in both blood and muscle samples from patients with CFS, and longitudinal studies suggest that oxidative stress is greatest during periods of clinical exacerbation.22 Increased lactate levels suggest increased anaerobic metabolism in the central nervous system consistent with mitochondrial dysfunction. Several studies have demonstrated that exercise can precipitate oxidative stress in patients with CFS, in contrast with healthy controls and controls with other chronic illnesses, suggesting a physiologic basis for their postexertional symptoms.17
Autoinflammatory syndrome induced by adjuvants, a rare syndrome associated with vaccine administration, has been linked to postvaccination adverse events, exposure to silicone implants, Gulf War syndrome (related to multiple vaccinations), and macrophagic myofasciitis. All involve exposure to immune adjuvants and have similar clinical manifestations. The corresponding exposures appear to trigger an autoimmune response in susceptible individuals. The hepatitis B vaccine is most often associated with CFS, with symptoms occurring within 90 days of administration.23
The clinical work-up: Putting knowledge into practice
Familiarity with potential causes of and connections with ME/CFS will help you ensure that patients who say they’re always tired receive a thorough work-up. Start with a medical history, inquiring directly about medical and psychiatric disorders that may contribute to fatigue (TABLE 3).5,24,25 Include a medication history, as well, to help determine whether the fatigue is drug-related (TABLE 4).5,25
What to ask. Determine the onset, course, duration, daily pattern, and impact of fatigue on the patient’s daily life. Inquire, too, about related symptoms of daytime sleepiness, dyspnea on exertion, generalized weakness, and depressed mood. The prominence of any of these rather than fatigue, per se, point to a diagnosis of a chronic illness other than ME/CFS.
Keep in mind, too, that patients with an organ-based medical illness tend to associate their fatigue with activities that they are unable to complete, such as shopping or light housework. In contrast, those with fatigue that is not organ-based typically say that they’re tired all the time. Their fatigue is not necessarily related to exertion, nor does it improve with rest.26
To address this distinction, take a sleep history, assessing both the quality and quantity of the patient’s sleep to determine how it affects symptoms.27 Consider using a questionnaire designed to help distinguish between sleepiness—and a primary sleep disorder—and fatigue,28 such as the Fatigue Severity Scale of Sleep Disorders (http://www.healthywomen.org/sites/default/files/FatigueSeverityScale.pdf).
What to rule out. In addition to a medical history, the physical examination should be oriented toward ruling out secondary causes of fatigue. In addition to a system-by-system approach to the differential diagnosis, carefully observe the patient’s general appearance, with attention to his or her level of alertness, grooming, and psychomotor agitation or retardation as possible signs of a psychiatric disorder. To rule out neurologic causes, evaluate muscle bulk, tone, and strength; deep tendon reflexes; and sensory and cranial nerves, as well.29
Lab tests to consider. In most cases of ME/CFS, basic studies—complete blood count with differential, erythrocyte sedimentation rate, blood chemistry, and thyroid-stimulating hormone (TSH) levels—are sufficient. When no medical or psychiatric cause has been found, additional tests may be ordered on a case-by-case basis, although laboratory analysis affects the management of fatigue in less than 5% of such patients.30 Tests to consider include:
- Creatine kinase (for patients who report pain or muscle weakness)
- pregnancy test (for women of childbearing age)
- ferritin testing (for young women who might benefit from iron supplementation for levels <50 ng/mL even if anemia is not present)31
- hepatitis C screening (recommended by the US Preventive Services Task Force for those born between 1945 and 1965)32
- human immunodeficiency virus screening and the purified protein derivative test for tuberculosis (based on patient history).
Forego routine testing for other infections
Routine testing for infectious diseases and conditions associated with fatigue, such as EBV or Lyme disease, immune deficiency (eg, immunoglobulins), inflammatory disease (eg, antinuclear antibodies, rheumatoid factor), celiac disease, vitamin D deficiency, vitamin B12 deficiency, or heavy metal toxicity, is unlikely to be helpful.29 Additional testing simply to reassure a worried patient usually does not accomplish that objective.33
Additional studies, referrals to consider. If you suspect that a patient has a sleep disorder, a referral to a sleep clinic to rule out idiopathic sleep disorders, obstructive sleep apnea, or movement disorders that interfere with sleep may be in order. Spirometry and echocardiography may be helpful for some patients. If you suspect peripheral muscle fatigue, a referral for neuromuscular testing is indicated.
CASE › Ms. C’s medical history reveals that she also suffers from irritable bowel syndrome, which she manages with diet and over-the-counter medication, as needed, for constipation or diarrhea. She denies having any other chronic conditions. Her only other symptoms, she reports, are mild upper back pain after spending long hours at the computer, and arthralgias in her left knee and both hands. She admits to being “somewhat depressed” in the last few months, but denies the presence of anhedonia.
The patient’s physical examination is normal, and her depression screen does not meet the criteria for MDD. Her metabolic chemistry panel, complete blood count, TSH, and sedimentation rate are all normal, as well.
Symptom management and coping strategies
When no specific cause of chronic fatigue is found, the focus shifts from diagnosis to symptom management and coping strategies.
This requires engagement with the patient. It is important to acknowledge the existence of his or her symptoms and to reassure the patient that further investigation may be warranted later, should new symptoms emerge. Advise the patient, too, that periods of remission and relapse are likely.
Strategies designed to motivate patient self-management, as well as the formulation of patient-centered treatment plans, have been shown to reduce symptom scores.34 Participation in a support group, as well as frequent follow-up visits with a primary care physician, a behavioral therapist, or both, may help to provide needed psychological support.
Evidence of the effectiveness of specific therapies for ME/CFS is limited; however, the best-studied approaches are cognitive behavioral therapy (CBT) and graded exercise therapy.35 Exercise should be low intensity, such as walking or cycling for 30 minutes 3 times a week, with a gradual increase in duration and frequency over a period of weeks to months. Patients with cancer-related fatigue may benefit from yoga, group therapy, and stress management.36
Associated mood and pain symptoms should be treated, as well. Bupropion, which is somewhat stimulating, may be considered as an initial treatment for patients with depression and clinically significant fatigue.
Other potentially beneficial approaches include a healthy diet, avoidance of more than nominal amounts of alcohol, relative avoidance of caffeine (no more than one cup of a caffeinated beverage in the morning), and stress reduction techniques.
Attention to good sleep hygiene may be especially beneficial, including a regular bedtime routine and sleep schedule, and elimination of bedroom light and noise. Pharmacologic treatments for insomnia should be used with caution, if at all.
CASE › Ms. C receives a referral for CBT and is scheduled for a return visit in 4 weeks.
At the advice of both her primary care physician and the behavioral therapist, Ms. C gradually makes several lifestyle changes. She begins going to bed earlier on weeknights to ensure that she sleeps for at least 7 hours. She improves her diet, with increasing emphasis on vegetables, fruits, and whole grains. She also starts a walking program, increasing gradually to a total of 3 hours per week. After 4 months she adds a weekly trip to a gym, where she practices resistance training for about 40 minutes.
Ms. C also increases her social activities on weekends, and recently accepted an invitation to join a book club. Six months from her initial visit, Ms. C notes that although she is still more easily fatigued than most people, she has made significant improvement.
CORRESPONDENCE
Linda Speer, MD; University of Toledo, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
Ms. C says she sleeps well, getting more than 8 hours of sleep per night on weekends but fewer than 7 hours per night during the week. But no matter how long she sleeps, she never awakens feeling refreshed. Ms. C reports that she doesn’t smoke, has no more than 4 alcoholic drinks per month, and adheres to an “average” diet. She is too tired to exercise.
Ms. C is single, with no children. Although she says she has a strong network of family and friends, she increasingly finds she has no energy for socializing. If Ms. C were your patient, what would you do?
Fatigue is a common presenting symptom in primary care, accounting for about 5% of adult visits.1 Defined as a generalized lack of energy, fatigue that persists despite adequate rest or is severe enough to disrupt an individual’s ability to participate in key social and/or occupational activities warrants a thorough investigation.
Because fatigue is a nonspecific symptom that may be linked to a number of medical and psychiatric illnesses or medications used to treat them, determining the cause can be difficult. In about half of all cases, no specific etiology is found.2 This review, which includes the elements of a work-up and management strategies for patients presenting with ongoing fatigue, will help you arrive at the appropriate diagnosis and provide optimal treatment.
Chronic fatigue: Defining the terms
A definition of chronic fatigue syndrome (CFS) was initially published in 1988.3 In subsequent years, the term myalgic encephalomyelitis (ME) became popular. Although the terms are sometimes used interchangeably, ME often refers to patients whose condition is thought to have an infectious cause and for whom postexertional malaise is a hallmark symptom.4
CDC criteria. While several sets of diagnostic criteria for CFS have been developed, the most widely used is that of the Centers for Disease Control and Prevention (CDC), published in 1994 (TABLE 1).5,6 A diagnosis of CFS is made on the basis of exclusion, subjective clinical interpretation, and patient self-report.
When the first 2 criteria—fatigue not due to ongoing exertion or other medical conditions that has lasted ≥6 months and is severe enough to interfere with daily activities—but fewer than 4 of the CDC’s 8 concurrent symptoms (eg, headache, unrefreshing sleep, and postexertion malaise lasting >24 hours) are present, idiopathic fatigue, rather than CFS, is diagnosed.6
International Consensus Criteria (ICC). In 2011, the ICC for ME were proposed in an effort to provide more specific diagnostic criteria (TABLE 2).7 The ICC emphasize fatigability, or what the authors identify as “post-exertional neuroimmune exhaustion.”
The ICC have not yet been broadly researched. But an Australian study of patients with chronic fatigue found that those who met the ICC definition were sicker and more homogeneous, with significantly lower scores for physical and social functioning and bodily pain compared with those who fulfilled the CDC criteria alone.8
Chronic fatigue & neuropsychiatric conditions: Common threads
Recent research has made it clear that depression, somatization, and CFS share some biological underpinnings. These include biomarkers for inflammation, cell-mediated immune activation—which may be related to the symptoms of fatigue—autonomic dysfunction, and hyperalgesia.9 Evidence suggests that up to two-thirds of patients with CFS also meet the criteria for a psychiatric disorder.10 The most common psychiatric conditions are major depressive disorder (MDD), affecting an estimated 22% to 32% of those with CFS; anxiety disorder, affecting about 20%; and somatization disorder, affecting about 10%—at least double the incidence of the general population.10
Others point out, however, that up to half of those with CFS do not have a psychiatric disorder.11 A diagnosis of somatization disorder, in particular, depends largely on a subjective interpretation of whether or not the presenting symptoms have a physical cause.10
CFS and MDD comorbidity. The most widely studied association between CFS and psychiatric disorders involves MDD. Observational studies have found patients with CFS have a lifetime prevalence of MDD of 65%,12,13 which is higher than that of patients with other chronic diseases. Overlapping symptoms include fatigue, sleep disturbance, poor concentration, and memory problems. However, those with CFS have fewer symptoms related to anhedonia, poor self-esteem, guilt, and suicidal ideation compared with individuals with MDD.12,13
There are several possible explanations for CFS and MDD comorbidity, which are not necessarily mutually exclusive.10 One theory is that CFS is an atypical form of depression; another holds that the disability associated with CFS leads to depression, as is the case with many other chronic illnesses; and a third points to overlapping pathophysiology.10
An emerging body of evidence suggests that CFS and MDD have some common oxidative and nitrosative biochemical pathways. Activated by infection, psychological stress, and immune disorders, they are believed to have damaging free radical and nitric oxide effects at the cellular level.14 The cellular effects can result in fatigue, muscle pain, and flu-like malaise.
Cortisol response differs
CFS and MDD might be distinguishable by another pathway—the hypothalamic-pituitary-adrenal (HPA) axis. MDD is classically associated with activation and raised cortisol levels, while CFS is consistently associated with impaired HPA axis functioning and reduced cortisol levels.10 The majority of patients with CFS report symptoms of cognitive decline, with the acquisition of new verbal learning and information-processing speed particularly likely to be impaired.15
A meta-analysis of 50 studies of patients with CFS showed deficits in attention, memory, and reaction time, but not in fine motor speed, vocabulary, or reasoning.16 Autonomic dysfunction has also been observed, including disordered sympathetic activity. The most frequently observed abnormalities on autonomic testing are postural hypotension, tachycardia syndrome, neurally mediated hypotension, and heart rate variability during tilt table testing.16
The link between infection and CFS
Several infectious agents have been associated with ME/CFS, including the Epstein-Barr virus (EBV), herpes simplex virus 6, parvovirus, Q fever, and Lyme disease.8,17,18 Most agents that have been linked to ME/CFS are associated with persistent infection and thus incitement of the immune system.
Numerous observational studies17,18 have documented postinfectious fatigue syndromes after acute viral and bacterial infections and symptoms suggestive of infection, such as fever, myalgias, and respiratory and gastrointestinal distress. In one prospective Australian study,19 investigators identified 253 cases of acute EBV, Ross River virus, and Q fever. Of those 253 patients, 12% went on to develop CFS, with a higher likelihood among those with more severe acute symptoms. No correlation with preexisting psychiatric disorders was found.
Muscle mitochondria studies have demonstrated what appear to be acquired abnormalities in those with CFS.20,21 Signs of increased oxidative stress have been found in both blood and muscle samples from patients with CFS, and longitudinal studies suggest that oxidative stress is greatest during periods of clinical exacerbation.22 Increased lactate levels suggest increased anaerobic metabolism in the central nervous system consistent with mitochondrial dysfunction. Several studies have demonstrated that exercise can precipitate oxidative stress in patients with CFS, in contrast with healthy controls and controls with other chronic illnesses, suggesting a physiologic basis for their postexertional symptoms.17
Autoinflammatory syndrome induced by adjuvants, a rare syndrome associated with vaccine administration, has been linked to postvaccination adverse events, exposure to silicone implants, Gulf War syndrome (related to multiple vaccinations), and macrophagic myofasciitis. All involve exposure to immune adjuvants and have similar clinical manifestations. The corresponding exposures appear to trigger an autoimmune response in susceptible individuals. The hepatitis B vaccine is most often associated with CFS, with symptoms occurring within 90 days of administration.23
The clinical work-up: Putting knowledge into practice
Familiarity with potential causes of and connections with ME/CFS will help you ensure that patients who say they’re always tired receive a thorough work-up. Start with a medical history, inquiring directly about medical and psychiatric disorders that may contribute to fatigue (TABLE 3).5,24,25 Include a medication history, as well, to help determine whether the fatigue is drug-related (TABLE 4).5,25
What to ask. Determine the onset, course, duration, daily pattern, and impact of fatigue on the patient’s daily life. Inquire, too, about related symptoms of daytime sleepiness, dyspnea on exertion, generalized weakness, and depressed mood. The prominence of any of these rather than fatigue, per se, point to a diagnosis of a chronic illness other than ME/CFS.
Keep in mind, too, that patients with an organ-based medical illness tend to associate their fatigue with activities that they are unable to complete, such as shopping or light housework. In contrast, those with fatigue that is not organ-based typically say that they’re tired all the time. Their fatigue is not necessarily related to exertion, nor does it improve with rest.26
To address this distinction, take a sleep history, assessing both the quality and quantity of the patient’s sleep to determine how it affects symptoms.27 Consider using a questionnaire designed to help distinguish between sleepiness—and a primary sleep disorder—and fatigue,28 such as the Fatigue Severity Scale of Sleep Disorders (http://www.healthywomen.org/sites/default/files/FatigueSeverityScale.pdf).
What to rule out. In addition to a medical history, the physical examination should be oriented toward ruling out secondary causes of fatigue. In addition to a system-by-system approach to the differential diagnosis, carefully observe the patient’s general appearance, with attention to his or her level of alertness, grooming, and psychomotor agitation or retardation as possible signs of a psychiatric disorder. To rule out neurologic causes, evaluate muscle bulk, tone, and strength; deep tendon reflexes; and sensory and cranial nerves, as well.29
Lab tests to consider. In most cases of ME/CFS, basic studies—complete blood count with differential, erythrocyte sedimentation rate, blood chemistry, and thyroid-stimulating hormone (TSH) levels—are sufficient. When no medical or psychiatric cause has been found, additional tests may be ordered on a case-by-case basis, although laboratory analysis affects the management of fatigue in less than 5% of such patients.30 Tests to consider include:
- Creatine kinase (for patients who report pain or muscle weakness)
- pregnancy test (for women of childbearing age)
- ferritin testing (for young women who might benefit from iron supplementation for levels <50 ng/mL even if anemia is not present)31
- hepatitis C screening (recommended by the US Preventive Services Task Force for those born between 1945 and 1965)32
- human immunodeficiency virus screening and the purified protein derivative test for tuberculosis (based on patient history).
Forego routine testing for other infections
Routine testing for infectious diseases and conditions associated with fatigue, such as EBV or Lyme disease, immune deficiency (eg, immunoglobulins), inflammatory disease (eg, antinuclear antibodies, rheumatoid factor), celiac disease, vitamin D deficiency, vitamin B12 deficiency, or heavy metal toxicity, is unlikely to be helpful.29 Additional testing simply to reassure a worried patient usually does not accomplish that objective.33
Additional studies, referrals to consider. If you suspect that a patient has a sleep disorder, a referral to a sleep clinic to rule out idiopathic sleep disorders, obstructive sleep apnea, or movement disorders that interfere with sleep may be in order. Spirometry and echocardiography may be helpful for some patients. If you suspect peripheral muscle fatigue, a referral for neuromuscular testing is indicated.
CASE › Ms. C’s medical history reveals that she also suffers from irritable bowel syndrome, which she manages with diet and over-the-counter medication, as needed, for constipation or diarrhea. She denies having any other chronic conditions. Her only other symptoms, she reports, are mild upper back pain after spending long hours at the computer, and arthralgias in her left knee and both hands. She admits to being “somewhat depressed” in the last few months, but denies the presence of anhedonia.
The patient’s physical examination is normal, and her depression screen does not meet the criteria for MDD. Her metabolic chemistry panel, complete blood count, TSH, and sedimentation rate are all normal, as well.
Symptom management and coping strategies
When no specific cause of chronic fatigue is found, the focus shifts from diagnosis to symptom management and coping strategies.
This requires engagement with the patient. It is important to acknowledge the existence of his or her symptoms and to reassure the patient that further investigation may be warranted later, should new symptoms emerge. Advise the patient, too, that periods of remission and relapse are likely.
Strategies designed to motivate patient self-management, as well as the formulation of patient-centered treatment plans, have been shown to reduce symptom scores.34 Participation in a support group, as well as frequent follow-up visits with a primary care physician, a behavioral therapist, or both, may help to provide needed psychological support.
Evidence of the effectiveness of specific therapies for ME/CFS is limited; however, the best-studied approaches are cognitive behavioral therapy (CBT) and graded exercise therapy.35 Exercise should be low intensity, such as walking or cycling for 30 minutes 3 times a week, with a gradual increase in duration and frequency over a period of weeks to months. Patients with cancer-related fatigue may benefit from yoga, group therapy, and stress management.36
Associated mood and pain symptoms should be treated, as well. Bupropion, which is somewhat stimulating, may be considered as an initial treatment for patients with depression and clinically significant fatigue.
Other potentially beneficial approaches include a healthy diet, avoidance of more than nominal amounts of alcohol, relative avoidance of caffeine (no more than one cup of a caffeinated beverage in the morning), and stress reduction techniques.
Attention to good sleep hygiene may be especially beneficial, including a regular bedtime routine and sleep schedule, and elimination of bedroom light and noise. Pharmacologic treatments for insomnia should be used with caution, if at all.
CASE › Ms. C receives a referral for CBT and is scheduled for a return visit in 4 weeks.
At the advice of both her primary care physician and the behavioral therapist, Ms. C gradually makes several lifestyle changes. She begins going to bed earlier on weeknights to ensure that she sleeps for at least 7 hours. She improves her diet, with increasing emphasis on vegetables, fruits, and whole grains. She also starts a walking program, increasing gradually to a total of 3 hours per week. After 4 months she adds a weekly trip to a gym, where she practices resistance training for about 40 minutes.
Ms. C also increases her social activities on weekends, and recently accepted an invitation to join a book club. Six months from her initial visit, Ms. C notes that although she is still more easily fatigued than most people, she has made significant improvement.
CORRESPONDENCE
Linda Speer, MD; University of Toledo, 3000 Arlington Avenue, MS 1179, Toledo, OH 43614; [email protected]
1. Nijrolder I, van der Windt DA, van der Horst HE. Prognosis of fatigue and functioning in primary care: a 1-year follow-up study. Ann Fam Med. 2008;6:519-527.
2. Griffith JP, Zarrouf FA. A systematic review of chronic fatigue syndrome: don’t assume it’s depression. Primary Care Companion J Clin Psychiatry. 2008;10:120-128.
3. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387-389.
4. Morris G, Maes M. Case definitions and diagnostic criteria for myalgic encephalomyelitis and chronic fatigue syndrome: from clinical consensus to evidence-based case definitions. Neuro Endocrinol Lett. 2013;34:185-199.
5. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953-959.
6. Centers for Disease Control and Prevention. Chronic fatigue syndrome. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cfs/diagnosis/index.html. Accessed January 6, 2015.
7. Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270:327-338.
8. Johnston SC, Brenu EW, Hardcastle S, et al. A comparison of health status in patients meeting alternative definitions for chronic fatigue syndrome/myalgic encephalomyelitis. Health Qual Life Outcomes. 2014;12:64.
9. Anderson VR, Jason LA, Hlavaty LE, et al. A review and meta-synthesis of qualitative studies on myalgic encephalomyelitis/chronic fatigue syndrome. Patient Educ Couns. 2012;86:147-155.
10. Christley Y, Duffy T, Everall IP, et al. The neuropsychiatric and neuropsychological features of chronic fatigue syndrome: revisiting the enigma. Curr Psychiatry Rep. 2013;15;353.
11. Henningsen P, Zimmermann T, Sattel HH. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528-533.
12. Nater UM, Jones JF, Lin JM, et al. Personality features and personality disorders in chronic fatigue syndrome: a population-based study. Psychother Psychosom. 2010;79:312-318.
13. Taylor RR, Jason LA, Jahn SC. Chronic fatigue and sociodemographic characteristics as predictors of psychiatric disorders in a community-based sample. Psychosom Med. 2003;65:896-901.
14. Leonard B, Maes M. Mechanistic explanations of how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764-785.
15. DeLuca J, Christodoulou C, Diamond BJ, et al. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10:101-109.
16. Cockshell SJ, Mathias JL. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. 2010;40:1253-1267.
17. Komaroff AL, Cho TA. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin Neurol. 2011;31:325-337.
18. Naess H, Sundal E, Myhr KM, et al. Postinfectious and chronic fatigue syndromes: clinical experience from a tertiary-referral centre in Norway. In Vivo. 2010;24:185-188.
19. Hickie I, Davenport T, Wakefield D, et al; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575.
20. Plioplys AV, Plioplys S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology. 1995;32:175-181.
21. Vernon SD, Whistler T, Cameron B, et al. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infect Dis. 2006;6:15.
22. Miwa K, Fujita M. Fluctuation of serum vitamin E (alpha-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels. 2010;25:319-323.
23. Rosenblum H, Shoenfeld Y, Amital H. The common immunogenic etiology of chronic fatigue syndrome: from infections to vaccines via adjuvants to the ASIA syndrome. Infect Dis Clin North Am. 2011;25:851-863.
24. Vincent A, Brimmer DJ, Whipple MO, et al. Prevalence, incidence, and classification of chronic fatigue syndrome in Olmsted County, Minnesota as estimated using the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:1145-1152.
25. Goroll AH, Mulley AG. Primary Care Medicine: Office Evaluation and Management of the Adult Patient. 7th ed. Morrisville, PA: Wolters Kluwer; 2014.
26. Brown RF, Schutte NS. Direct and indirect relationships between emotional intelligence and subjective fatigue in university students. J Psychosom Res. 2006;60:585-593.
27. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54:61-69.
28. Bailes S, Libman E, Baltzan M, et al. Brief and distinct empirical sleepiness and fatigue scales. J Psychosom Res. 2006;60:605-613.
29. National Collaborating Centre for Primary Care (UK). Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (or Encephalopathy): Diagnosis and Management of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (or Encephalopathy) in Adults and Children. London, UK: Royal College of General Practitioners; 2007.
30. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci. 1990;299:313-318.
31. Vaucher P, Druisw PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184:1247-1254.
32. US Preventive Services Task Force. Hepatitis C: Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm. Accessed January 7, 2015.
33. Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: a systematic review and meta-analysis. JAMA Intern Med. 2013;173:407-416.
34. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
35. White PD, Goldsmith KA, Johnson AL, et al; PACE trial management group. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue (PACE): a randomised trial. Lancet. 2011;377:823-836.
36. van Weert E, Hoekstra-Weebers J, Otter R, et al. Cancer-related fatigue: predictors and effects of rehabilitation. Oncologist. 2006;11:184-196.
1. Nijrolder I, van der Windt DA, van der Horst HE. Prognosis of fatigue and functioning in primary care: a 1-year follow-up study. Ann Fam Med. 2008;6:519-527.
2. Griffith JP, Zarrouf FA. A systematic review of chronic fatigue syndrome: don’t assume it’s depression. Primary Care Companion J Clin Psychiatry. 2008;10:120-128.
3. Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387-389.
4. Morris G, Maes M. Case definitions and diagnostic criteria for myalgic encephalomyelitis and chronic fatigue syndrome: from clinical consensus to evidence-based case definitions. Neuro Endocrinol Lett. 2013;34:185-199.
5. Fukuda K, Straus SE, Hickie I, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953-959.
6. Centers for Disease Control and Prevention. Chronic fatigue syndrome. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cfs/diagnosis/index.html. Accessed January 6, 2015.
7. Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270:327-338.
8. Johnston SC, Brenu EW, Hardcastle S, et al. A comparison of health status in patients meeting alternative definitions for chronic fatigue syndrome/myalgic encephalomyelitis. Health Qual Life Outcomes. 2014;12:64.
9. Anderson VR, Jason LA, Hlavaty LE, et al. A review and meta-synthesis of qualitative studies on myalgic encephalomyelitis/chronic fatigue syndrome. Patient Educ Couns. 2012;86:147-155.
10. Christley Y, Duffy T, Everall IP, et al. The neuropsychiatric and neuropsychological features of chronic fatigue syndrome: revisiting the enigma. Curr Psychiatry Rep. 2013;15;353.
11. Henningsen P, Zimmermann T, Sattel HH. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65:528-533.
12. Nater UM, Jones JF, Lin JM, et al. Personality features and personality disorders in chronic fatigue syndrome: a population-based study. Psychother Psychosom. 2010;79:312-318.
13. Taylor RR, Jason LA, Jahn SC. Chronic fatigue and sociodemographic characteristics as predictors of psychiatric disorders in a community-based sample. Psychosom Med. 2003;65:896-901.
14. Leonard B, Maes M. Mechanistic explanations of how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36:764-785.
15. DeLuca J, Christodoulou C, Diamond BJ, et al. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10:101-109.
16. Cockshell SJ, Mathias JL. Cognitive functioning in chronic fatigue syndrome: a meta-analysis. Psychol Med. 2010;40:1253-1267.
17. Komaroff AL, Cho TA. Role of infection and neurologic dysfunction in chronic fatigue syndrome. Semin Neurol. 2011;31:325-337.
18. Naess H, Sundal E, Myhr KM, et al. Postinfectious and chronic fatigue syndromes: clinical experience from a tertiary-referral centre in Norway. In Vivo. 2010;24:185-188.
19. Hickie I, Davenport T, Wakefield D, et al; Dubbo Infection Outcomes Study Group. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006;333:575.
20. Plioplys AV, Plioplys S. Electron-microscopic investigation of muscle mitochondria in chronic fatigue syndrome. Neuropsychobiology. 1995;32:175-181.
21. Vernon SD, Whistler T, Cameron B, et al. Preliminary evidence of mitochondrial dysfunction associated with post-infective fatigue after acute infection with Epstein Barr virus. BMC Infect Dis. 2006;6:15.
22. Miwa K, Fujita M. Fluctuation of serum vitamin E (alpha-tocopherol) concentrations during exacerbation and remission phases in patients with chronic fatigue syndrome. Heart Vessels. 2010;25:319-323.
23. Rosenblum H, Shoenfeld Y, Amital H. The common immunogenic etiology of chronic fatigue syndrome: from infections to vaccines via adjuvants to the ASIA syndrome. Infect Dis Clin North Am. 2011;25:851-863.
24. Vincent A, Brimmer DJ, Whipple MO, et al. Prevalence, incidence, and classification of chronic fatigue syndrome in Olmsted County, Minnesota as estimated using the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:1145-1152.
25. Goroll AH, Mulley AG. Primary Care Medicine: Office Evaluation and Management of the Adult Patient. 7th ed. Morrisville, PA: Wolters Kluwer; 2014.
26. Brown RF, Schutte NS. Direct and indirect relationships between emotional intelligence and subjective fatigue in university students. J Psychosom Res. 2006;60:585-593.
27. Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: toward improved detection and treatment. J Psychosom Res. 2003;54:61-69.
28. Bailes S, Libman E, Baltzan M, et al. Brief and distinct empirical sleepiness and fatigue scales. J Psychosom Res. 2006;60:605-613.
29. National Collaborating Centre for Primary Care (UK). Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (or Encephalopathy): Diagnosis and Management of Chronic Fatigue Syndrome/Myalgic Encephalomyelitis (or Encephalopathy) in Adults and Children. London, UK: Royal College of General Practitioners; 2007.
30. Lane TJ, Matthews DA, Manu P. The low yield of physical examinations and laboratory investigations of patients with chronic fatigue. Am J Med Sci. 1990;299:313-318.
31. Vaucher P, Druisw PL, Waldvogel S, et al. Effect of iron supplementation on fatigue in nonanemic menstruating women with low ferritin: a randomized controlled trial. CMAJ. 2012;184:1247-1254.
32. US Preventive Services Task Force. Hepatitis C: Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf/uspshepc.htm. Accessed January 7, 2015.
33. Rolfe A, Burton C. Reassurance after diagnostic testing with a low pretest probability of serious disease: a systematic review and meta-analysis. JAMA Intern Med. 2013;173:407-416.
34. Smith RC, Lyles JS, Gardiner JC, et al. Primary care clinicians treat patients with medically unexplained symptoms: a randomized controlled trial. J Gen Intern Med. 2006;21:671-677.
35. White PD, Goldsmith KA, Johnson AL, et al; PACE trial management group. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise therapy, and specialist medical care for chronic fatigue (PACE): a randomised trial. Lancet. 2011;377:823-836.
36. van Weert E, Hoekstra-Weebers J, Otter R, et al. Cancer-related fatigue: predictors and effects of rehabilitation. Oncologist. 2006;11:184-196.
Drowning episodes: Prevention and resuscitation tips
› Recommend swimming lessons for all children ages 4 and older. C
› Consider antibiotics after a drowning event only if the water is known to be contaminated or the victim has aspirated a large volume of water. C
› Monitor asymptomatic patients for at least 4 hours after a drowning event. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A young mother in your practice wants her toddler to begin swimming lessons because her family loves water activities. How would you advise her? In fielding an urgent call about a drowning incident, what priorities would you urge regarding resuscitation at the scene? For a stabilized patient following a drowning episode, when might antibiotics be indicated? This article covers these issues as well as follow-up matters such as assisted ventilation and tiered hypothermia intervention.
Drowning likely occurs more often than is reported
Worldwide, drowning accounts for more than 388,000 deaths annually and is the third leading cause of unintentional injury death. Low- and middle-income countries represent 96% of the yearly total.1 As reported in the United States, nearly 6000 individuals are hospitalized and nearly 4000 die from drowning events annually.2 But these figures likely underestimate the true rate, as many drowning fatalities are officially attributed to floods, boating accidents, or other associated events. Nonfatal drownings often go unreported.
Children under the age of 5 years have the highest drowning mortality worldwide, and drowning is the leading cause of unintentional injury death for this age group in many countries, including the United States.1,2 Men are nearly 4 times as likely to die from drowning than women.2 Predictably, in the United States most drowning happens on the weekend and during summer months. More than half of drownings in children younger than 4 years occur in swimming pools; with increasing age, drowning is more likely to occur in natural bodies of water.2 With adults in higher income countries, alcohol is a significant contributor to drowning events during recreational activities.3-5
Much effort has been made in recent years to standardize the nomenclature and treatment of drowning episodes. The International World Congress on Drowning met in the Netherlands in 2002, and established the definition of drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”6 Submersion refers to the complete submergence of the victim under the water, while immersion implies that the victim’s airway remains above the water.
The Congress recommended that terms such as “wet-drowning,” “dry-drowning,” and “near-drowning” be discontinued in favor of the outcome classifications “death,” “no morbidity,” and “morbidity.” The “morbidity” subgroup was further characterized as “moderately disabled,” “severely disabled,” “vegetative state/coma,” and “brain death.” This meeting established guidelines on the treatment of drowning victims in addition to outlining points for future research.6
Physiologic chain of events in drowning
An unexpected immersion in water, particularly cold water, causes a reflexive inspiratory gasp, and some degree of aspiration occurs in most, if not all, cases of drowning. Aspiration further impairs victims’ ability to hold their breath or breathe normally.5,7,8 It decreases lung compliance due to surfactant washout or intrapulmonary shunting and thereby leads to hypoxia. Aspiration-induced severe laryngospasm can also lead to hypoxia. Pulmonary edema and acute respiratory distress syndrome (ARDS) can follow.
The cardiovascular effects of drowning mirror those seen in hypoxia. Initially, apnea leads to decreased oxygen saturation and precipitates tachycardia and hypertension. Bradycardia and hypotension follow and blood is shunted to vital organs, such as the brain, heart, and lungs.9 This phenomenon is accelerated in cold water and leads to “swimming failure,” the impaired ability of the victim to swim because of decreased perfusion of the extremities.5,7,8,10
“Autonomic conflict” has been proposed as an additional mechanism for morbidity and mortality from drowning episodes. Breath holding and immersion in cold water each can induce cardiac arrhythmias. When combined, these events may increase the risk of an arrhythmogenic state secondary to opposing chronotropic effects: the diving reflex (bradycardia via parasympathetic activation), and the cold shock response (tachycardia via sympathetic activation). This is thought to be an underreported cause of death in drowning, as arrhythmias are undetectable during autopsy.8,11
Drowning prevention
The American Academy of Pediatrics (AAP) recommends swimming lessons for most children ages 4 years and older.12 Previously, swimming lessons were not recommended for children ages 1 to 4 because evidence of benefit was lacking, and there was some concern that it might reduce children’s caution around water and reduce parents’ perceived level of need for supervision. Although data are still conflicting, some reports have since shown benefit in early swimming lessons for
toddlers.13,14
As of 2010, the AAP acknowledges that training may be beneficial for children in this age group, but cautions that not all children will be ready for swimming by this age.12 Infant water safety programs for children under the age of 1 are not recommended because evidence of benefit is lacking.12
Evidence is growing to support teaching basic water survival skills in low- to middle-income countries where water sources are abundant, particularly in Southeast Asia. Specifically, the SwimSafe survival swimming program has yielded impressive results in Bangladesh.15 This program targets children starting at age 5, and involves 20 lessons teaching basic water survival and rescue skills.
Results have shown a 93% reduction in drowning rates for children enrolled in the program, compared with those not enrolled.15 Subsequent analyses have proposed that swimming lessons for children in these parts of the world would be as cost effective as current attempts to prevent diarrheal and respiratory diseases in the same areas.16
Additional preventive measures that are effective in the United States include 4-sided pool fencing, use of personal flotation devices, and bystander cardiopulmonary
resuscitation (CPR).2,5,17
On-scene evaluation and treatment
Drowning victims can appear mottled and have minimal or no peripheral pulses despite a heartbeat. Rescuers may assume the victim is dead when, in fact, there is cardiac function. Because initial assessment in this situation is difficult, CPR should begin, if possible, the moment the victim is out of the water. Successful on-scene resuscitation is the surest predictor of survival.9,18 In fact, delay of CPR until the arrival of emergency personnel lessens the likelihood of survival.19
CPR applied to drowning. For cardiogenic cardiac arrest, chest compressions alone may be better than compressions with rescue breathing. For victims of drowning, though, coordinated compressions and rescue breathing are recommended.20 The
2010 revision of the American Heart Association Guidelines for CPR and Emergency Cardiovascular Care emphasize “compression first” for CPR in cases of cardiogenic cardiac arrest, but continue to support the traditional Airway-Breathing-Chest Compressions sequence for drowning victims in its Special Situations section.21
Ventricular fibrillation (VF) is rare after submersion injury. An external defibrillator should be used when available, but it is unlikely to play a significant role in initial resuscitation.9
Don’t attempt to remove water from the victim’s mouth before resuscitation. The volume of fluid in the oral cavity is usually insignificant, and trying to remove it by abdominal thrusts or Heimlich maneuver will delay CPR and may injure the patient.21
Cervical spine injury is uncommon in drowning episodes, making cervical spine immobilization unnecessary unless the mechanism of injury is known or if there are clinical signs suggesting such injury. Needless cervical spine immobilization can interfere with adequate ventilation.22,23 However, concern for head or cervical spine injury is warranted when recovering an unconscious victim from shallow water, where such injuries are more likely to result from falling or diving into the water.24
Administer oxygen supplementation when available to all spontaneously breathing individuals. Individuals who respond well to initial resuscitation and who don’t require intubation tend to have a very good prognosis overall.25
Total time of submersion and the temperature of the water have bearing on the likelihood of survival. Only in rare cases have victims survived submersion lasting longer than 30 minutes. Ten minutes generally is considered the “point of no return.”9,26 This is consistent with data suggesting 10 minutes of hypoxic insult causes irreversible neurologic damage, with each additional minute rapidly leading to coma.20 However, to complicate matters, unlike cardiac arrest victims, drowning victims can lose cerebral blood flow slowly after respiratory impairment, which makes duration of submersion a potentially unreliable predictor of neurologic outcome.20,26
Does hypothermia have a protective effect? Hypothermia can occur in water 85°F (30°C) or cooler.10 It has been hypothesized that resuscitation can be achieved after longer periods of submersion in cold water. However, the considerable debate on this topic has been based on little more than case reports.
For hypothermia to have a protective effect on neurologic function, cooling must take place rapidly and, ideally, before any hypoxic insult. The water would have to be exceptionally cold, likely less than 50°F (10°C).27 The greater surface-to-volume ratio in children enables more rapid cooling and quicker onset of hypothermia, which may explain why they seem to have better neurologic outcomes than adults after prolonged submersion.28
Hypothermia can also be protective if the victim is breathing when cooling begins, such as while floating or swimming in cold water before drowning.29 This was the likely scenario in a reported case of a Norwegian kayaker who called for help after capsizing in 38°F (3.3°C) seawater. Despite having been in cardiac arrest for over 3 hours, the individual experienced a spontaneous return of circulation and was discharged after 32 days with no neurologic deficits.29
Correcting hypothermia after rescue. Conscious patients with no cardiovascular or respiratory compromise should have wet clothing removed at the scene in exchange for blankets, towels, or warm dry clothing. Advise rescuers to attempt no further rewarming at the scene. With unconscious patients, take only simple measures to prevent further heat loss, and focus on transport and resuscitative efforts.24
Hospital management
Attempts have been made to create a prognostic or predictive scoring system for drowning victims presenting to the emergency department. Factors thought to have bearing on mortality include duration of submersion, victim’s age, Glasgow Coma Scale (GCS) score, pupillary reactivity, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score.23,30,31 Other measurements, such as core temperature, blood pH, and response to painful stimuli, correlate poorly with mortality.31 Hyperkalemia is repeatedly mentioned as a predictor of a poor neurologic outcome, as it is thought to indicate hypoxia before the onset of cooling.8,26,32 The best predictor of a good outcome is consciousness at the time of arrival at the emergency department.4
Continued ventilation assistance is critical. As with prehospital resuscitation efforts, ventilation is critical to in-hospital management. For patients who are breathing spontaneously, continuous positive airway pressure or bi-level positive airway pressure can reduce hypoxia in pulmonary edema. Standard indications of the need for intubation include decreased level of consciousness or concern for ability to protect the airway, hypoxia despite a high fraction of inspired oxygen (FiO2), or persistent hypercapnia even with adequate noninvasive ventilatory support.33
Victims may swallow large amounts of liquid during drowning; for intubated patients, advise orogastric tube placement to prevent aspiration of gastric contents. The reliability of pulse oximetry has been called into question in this setting and may be less accurate for victims who are hypothermic or who have been submerged in cold water.23,34
Tiered intervention for hypothermia. In the hospital setting, passive rewarming is indicated for individuals with a body core temperature of 89.6°F to 95°F (32°C-35°C). Remove wet clothing; cover the victim with warm, dry towels or blankets; and give warm oral fluids and urge movement.
Individuals with core temperatures between 82.4°F to 89.6°F (28°C-32°C) require active external rewarming by applying heat directly to the skin via hot packs, warm blankets, and insulation. These patients should remain in a horizontal position with little movement, if possible, to avoid cold peripheral blood rapidly shifting to the core and precipitating an arrhythmia. Warmed intravenous fluids are appropriate as well.
With a core temperature less than 82.4°F (28°C), aggressive rewarming with extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass (CPB) may be warranted as this individual will likely have unstable or absent vital signs (TABLE).35,36 Some authorities advise that drowning victims with severe hypothermia and cardiac arrest should be resuscitated at facilities with CPB capability.27,28,37
Inducing hypothermia therapeutically is still unproven. Although not tested specifically on drowning victims, therapeutic hypothermia would seem to have theoretical benefit for their resuscitation. In cardiogenic cardiac arrest, good evidence exists for improved neurologic outcomes with therapeutic hypothermia,38 and this benefit might extend to drowning victims given their similar neurologic injury. No specific pharmacologic therapies have shown benefit in preventing loss of cerebral function.9
Monitor for clinical worsening. The concept of “secondary drowning,” a term now abandoned, referred to the phenomenon of clinical worsening hours after the initial drowning episode and resuscitation. This occurrence is now thought to be due to laryngospasm or to the progressive development of pulmonary edema from the aspiration of small amounts of water. Evidence supports monitoring asymptomatic patients; however, the period of suggested monitoring varies between 4 and 24 hours after the incident.8,22,24
Imaging, if delayed, may be useful. Imaging immediately after a drowning episode is an unreliable predictor of outcome and should be sought only if trauma or symptoms dictate. Cranial computed tomography (CT) has yielded normal findings in drowning victims with a GCS score as low as 4.
If CT is performed, any abnormality detected within 2 to 3 days of injury is a strong predictor of a poor neurologic outcome.39 Magnetic resonance imaging can be beneficial when performed more than 24 hours after resuscitation, preferably within a 4- to 7-day window.20 Lung ultrasound has been used as a bedside tool to monitor progression of pulmonary edema, and could serve the same purpose in drowning recovery.40
Anticipate respiratory complications. Since only a small amount of water is usually aspirated during a drowning event, the salinity of the aspirate is unlikely to cause significant disruption in hemodynamic or electrolyte balance.17,20 However, even a small amount of aspirated water, particularly fresh water, can disrupt gas exchange by washing out surfactant. This can rapidly precipitate ARDS. Not surprisingly, the use of exogenous surfactant has been studied in limited case reports and has had positive results.41-43 However, large trials have not yet been conducted, mostly because of the significant cost associated with surfactant therapy.
Antibiotics are rarely indicated prophylactically. Pneumonia after a drowning event is potentially fatal. It is more common in patients who have been intubated, and is therefore thought to be a hospital-acquired infection rather than a direct result of the drowning event.
Frequently, pneumonia after drowning is caused by pathogens native to the upper airway, when a victim is unable to protect his or her upper airway.44 In these cases, start broad spectrum antibiotics, with particular concern for organisms of the upper oropharynx. Also take into consideration species native to the body of water in which the victim was immersed.44
Routine prophylaxis with antibiotics, although common, is not recommended. Exceptions may be victims of drowning in known contaminated water or victims with high volumes of water aspiration.25 Some experts recommend blood cultures for victims who have aspirated, regardless of the presence or absence of infection.24 However, this recommendation seems to be based on opinion.
CORRESPONDENCE
Sean C. Engel, MD, 6600 Excelsior Boulevard Suite 100, St. Louis Park, MN 55426; [email protected]
1. World Health Organization. Drowning. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs347/en/. Accessed December 28, 2014.
2. Centers for Disease Control and Prevention (CDC). Drowning—United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61:344-447.
3. Driscoll TR, Harrison JE, Steenkamp M. Alcohol and drowning in Australia. Inj Control Saf Promot. 2004;11:175-181.
4. Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest. 1997;112:660-665.
5. Hudson D, Ekman R, Svanström L. Survival of immersions during recreational boating events in Alaska, 1999-2004. Accid Anal Prev. 2007;39:437-443.
6. International Life Saving Federation. Drowning Report. International Life Saving Federation Web site. Available at: http://www.ilsf.org/sites/ilsf.org/files/filefield/drowningcongress.doc. Accessed December 28, 2014.
7. Brooks CJ, Howard KA, Neifer SK. How much did cold shock and swimming failure contribute to drowning deaths in the fishing industry in British Columbia 1976-2002? Occup Med (Lond). 2005;55:459-462.
8. Golden FS, Tipton MJ, Scott RC. Immersion, near-drowning and drowning. Br J Anaesth. 1997;79:214-225.
9. Bierens JJ, Knape JT, Gelissen HP. Drowning. Curr Opin Crit Care. 2002;8:578-586.
10. Ducharme MB, Lounsbury DS. Self-rescue swimming in cold water: the latest advice. Appl Physiol Nutr Metab. 2007;32:799-807.
11. Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol. 2012;590(pt 14):3219-3230.
12. Weiss J; American Academy of Pediatrics Committee on Injury, Violence, and Poison Prevention. Prevention of drowning. Pediatrics. 2010;126:e253-e262.
13.Yang L, Nong QQ, Li CL, et al. Risk factors for childhood drowning in rural regions of a developing country: a case-control study. Inj Prev. 2007;13:178-182.
14. Brenner RA, Taneja GS, Haynie DL, et al. The association between swimming lessons and drowning in childhood: a case-control study. Arch Pediatr Adolesc Med. 2009;163:203-210.
15. Rahman A, Rahman F, Hossain J, et al. Survival swimming - effectiveness of SwimSafe in preventing drowning in mid and late childhood. Abstract presented at: World Conference on Drowning Prevention; May 10-13, 2011; Danang, Vietnam.
16. Linnan M, Rahman A, Scarr J, et al. Child drowning: Evidence for a newly recognized cause of child mortality in low and middle income countries in Asia. Florence, Italy: UNICEF Office of Research. UNICEF Web site. Available at: www.unicef-irc.org/publications/pdf/drowning.pdf. Accessed December 28, 2014.
17. Salomez F, Vincent JL. Drowning: a review of epidemiology, pathophysiology, treatment and prevention. Resuscitation. 2004;63:261-268.
18. Venema AM, Groothoff JW, Bierens JJ. The role of bystanders during rescue and resuscitation of drowning victims. Resuscitation. 2010;81:434-439.
19. Pepe PE, Wigginton JG, Mann DM, et al. Prospective, decade-long, population-based study of pediatric drowning related incidents. Acad Emerg Med. 2002;9:516-517.
20. Topjian AA, Berg RA, Bierens JJ, et al. Brain resuscitation in the drowning victim. Neurocrit Care. 2012;17:441-467.
21. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S829-S861.
22. Ibsen LM, Koch T. Submersion and asphyxial injury. Crit Care Med. 2002;30(11 suppl):S402-S408.
23. Schilling UM, Bortolin M. Drowning. Minerva Anethesiol. 2012;78:69-77.
24. Harries M. Near drowning. BMJ. 2003;327:1336-1338.
25. Gregorakos L, Markou N, Psalida V, et al. Near-drowning: clinical course of lung injury in adults. Lung. 2009;187:93-97.
26. Eich C, Bräuer A, Timmermann A, et al. Outcome of 12 drowned children with attempted resuscitation on cardiopulmonary bypass: an analysis of variables based on the “Utstein Style for Drowning”. Resuscitation. 2007;75:42-52.
27. Wollenek G, Honarwar N, Golej J, et al. Cold water submersion and cardiac arrest in treatment of severe hypothermia with cardiopulmonary bypass. Resuscitation. 2002;52:255-263.
28. Letsou GV, Kopf GS, Elefteriades JA, et al. Is cardiopulmonary bypass effective for treatment of hypothermic arrest due to drowning or exposure? Arch Surg. 1992;127:525-528.
29. Lund FK, Torgersen JG, Flaatten HK. Heart rate monitored hypothermia and drowning in a 48-year-old man. survival without sequelae: a case report. Cases J. 2009;2:6204.
30. Ballesteros MA, Gutiérrez-Cuadra M, Muñoz P, et al. Prognostic factors and outcome after drowning in an adult population. Acta Anaesthesiol Scand. 2009;53:935-940.
31. Nichter MA, Everett PB. Childhood near-drowning: is cardiopulmonary resuscitation always indicated? Crit Care Med. 1989;17:993-995.
32. Schaller MD, Fischer AP, Perret CH. Hyperkalemia. A prognostic factor during acute severe hypothermia. JAMA. 1990;264:1842-1845.
33. O’Connor MF, Ovassapian A. Airway management. In: Hall JB, Schmidt GA, Wood LD, eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005.
34. Montenij LJ, de Vries W, Schwarte L, et al. Feasibility of pulse oximetry in the initial prehospital management of victims of drowning: a preliminary study. Resuscitation. 2011;82:1235-1238.
35. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
36. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4:99-103.
37. Coskun KO, Popov AF, Schmitto JD, et al. Extracorporeal circulation for rewarming in drowning and near-drowning pediatric patients. Artif Organs. 2010;34:1026-1030.
38. Arrich J, Holzer M, Havel C, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128.
39. Rafaat KT, Spear RM, Kuelbs C, et al. Cranial computed tomographic findings in a large group of children with drowning: diagnostic, prognostic, and forensic implications. Pediatr Crit Care Med. 2008;9:567-572.
40. Laursen CB, Davidsen JR, Madsen PH. Utility of lung ultrasound in near-drowning victims. BMJ Case Rep. 2012;2012.
41. Ugras M, Guraksin O, Sen TA, et al. Surfactant replacement therapy in a pediatric near-drowning case in manure. Pediatr Emerg Care. 2012;28:913-914.
42. Kapur N, Slater A, McEniery J, et al. Therapeutic bronchoscopy in a child with sand aspiration and respiratory failure from near drowning—case report and literature review. Pediatr Pulmonol. 2009;44:1043-1047.
43. Staudinger T, Bankier A, Strohmaier W, et al. Exogenous surfactant therapy in a patient with adult respiratory distress syndrome after near drowning. Resuscitation. 1997;35:179-182.
44. Tadié JM, Heming N, Serve E, et al. Drowning associated pneumonia: a descriptive cohort. Resuscitation. 2012;83:399-401.
› Recommend swimming lessons for all children ages 4 and older. C
› Consider antibiotics after a drowning event only if the water is known to be contaminated or the victim has aspirated a large volume of water. C
› Monitor asymptomatic patients for at least 4 hours after a drowning event. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A young mother in your practice wants her toddler to begin swimming lessons because her family loves water activities. How would you advise her? In fielding an urgent call about a drowning incident, what priorities would you urge regarding resuscitation at the scene? For a stabilized patient following a drowning episode, when might antibiotics be indicated? This article covers these issues as well as follow-up matters such as assisted ventilation and tiered hypothermia intervention.
Drowning likely occurs more often than is reported
Worldwide, drowning accounts for more than 388,000 deaths annually and is the third leading cause of unintentional injury death. Low- and middle-income countries represent 96% of the yearly total.1 As reported in the United States, nearly 6000 individuals are hospitalized and nearly 4000 die from drowning events annually.2 But these figures likely underestimate the true rate, as many drowning fatalities are officially attributed to floods, boating accidents, or other associated events. Nonfatal drownings often go unreported.
Children under the age of 5 years have the highest drowning mortality worldwide, and drowning is the leading cause of unintentional injury death for this age group in many countries, including the United States.1,2 Men are nearly 4 times as likely to die from drowning than women.2 Predictably, in the United States most drowning happens on the weekend and during summer months. More than half of drownings in children younger than 4 years occur in swimming pools; with increasing age, drowning is more likely to occur in natural bodies of water.2 With adults in higher income countries, alcohol is a significant contributor to drowning events during recreational activities.3-5
Much effort has been made in recent years to standardize the nomenclature and treatment of drowning episodes. The International World Congress on Drowning met in the Netherlands in 2002, and established the definition of drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”6 Submersion refers to the complete submergence of the victim under the water, while immersion implies that the victim’s airway remains above the water.
The Congress recommended that terms such as “wet-drowning,” “dry-drowning,” and “near-drowning” be discontinued in favor of the outcome classifications “death,” “no morbidity,” and “morbidity.” The “morbidity” subgroup was further characterized as “moderately disabled,” “severely disabled,” “vegetative state/coma,” and “brain death.” This meeting established guidelines on the treatment of drowning victims in addition to outlining points for future research.6
Physiologic chain of events in drowning
An unexpected immersion in water, particularly cold water, causes a reflexive inspiratory gasp, and some degree of aspiration occurs in most, if not all, cases of drowning. Aspiration further impairs victims’ ability to hold their breath or breathe normally.5,7,8 It decreases lung compliance due to surfactant washout or intrapulmonary shunting and thereby leads to hypoxia. Aspiration-induced severe laryngospasm can also lead to hypoxia. Pulmonary edema and acute respiratory distress syndrome (ARDS) can follow.
The cardiovascular effects of drowning mirror those seen in hypoxia. Initially, apnea leads to decreased oxygen saturation and precipitates tachycardia and hypertension. Bradycardia and hypotension follow and blood is shunted to vital organs, such as the brain, heart, and lungs.9 This phenomenon is accelerated in cold water and leads to “swimming failure,” the impaired ability of the victim to swim because of decreased perfusion of the extremities.5,7,8,10
“Autonomic conflict” has been proposed as an additional mechanism for morbidity and mortality from drowning episodes. Breath holding and immersion in cold water each can induce cardiac arrhythmias. When combined, these events may increase the risk of an arrhythmogenic state secondary to opposing chronotropic effects: the diving reflex (bradycardia via parasympathetic activation), and the cold shock response (tachycardia via sympathetic activation). This is thought to be an underreported cause of death in drowning, as arrhythmias are undetectable during autopsy.8,11
Drowning prevention
The American Academy of Pediatrics (AAP) recommends swimming lessons for most children ages 4 years and older.12 Previously, swimming lessons were not recommended for children ages 1 to 4 because evidence of benefit was lacking, and there was some concern that it might reduce children’s caution around water and reduce parents’ perceived level of need for supervision. Although data are still conflicting, some reports have since shown benefit in early swimming lessons for
toddlers.13,14
As of 2010, the AAP acknowledges that training may be beneficial for children in this age group, but cautions that not all children will be ready for swimming by this age.12 Infant water safety programs for children under the age of 1 are not recommended because evidence of benefit is lacking.12
Evidence is growing to support teaching basic water survival skills in low- to middle-income countries where water sources are abundant, particularly in Southeast Asia. Specifically, the SwimSafe survival swimming program has yielded impressive results in Bangladesh.15 This program targets children starting at age 5, and involves 20 lessons teaching basic water survival and rescue skills.
Results have shown a 93% reduction in drowning rates for children enrolled in the program, compared with those not enrolled.15 Subsequent analyses have proposed that swimming lessons for children in these parts of the world would be as cost effective as current attempts to prevent diarrheal and respiratory diseases in the same areas.16
Additional preventive measures that are effective in the United States include 4-sided pool fencing, use of personal flotation devices, and bystander cardiopulmonary
resuscitation (CPR).2,5,17
On-scene evaluation and treatment
Drowning victims can appear mottled and have minimal or no peripheral pulses despite a heartbeat. Rescuers may assume the victim is dead when, in fact, there is cardiac function. Because initial assessment in this situation is difficult, CPR should begin, if possible, the moment the victim is out of the water. Successful on-scene resuscitation is the surest predictor of survival.9,18 In fact, delay of CPR until the arrival of emergency personnel lessens the likelihood of survival.19
CPR applied to drowning. For cardiogenic cardiac arrest, chest compressions alone may be better than compressions with rescue breathing. For victims of drowning, though, coordinated compressions and rescue breathing are recommended.20 The
2010 revision of the American Heart Association Guidelines for CPR and Emergency Cardiovascular Care emphasize “compression first” for CPR in cases of cardiogenic cardiac arrest, but continue to support the traditional Airway-Breathing-Chest Compressions sequence for drowning victims in its Special Situations section.21
Ventricular fibrillation (VF) is rare after submersion injury. An external defibrillator should be used when available, but it is unlikely to play a significant role in initial resuscitation.9
Don’t attempt to remove water from the victim’s mouth before resuscitation. The volume of fluid in the oral cavity is usually insignificant, and trying to remove it by abdominal thrusts or Heimlich maneuver will delay CPR and may injure the patient.21
Cervical spine injury is uncommon in drowning episodes, making cervical spine immobilization unnecessary unless the mechanism of injury is known or if there are clinical signs suggesting such injury. Needless cervical spine immobilization can interfere with adequate ventilation.22,23 However, concern for head or cervical spine injury is warranted when recovering an unconscious victim from shallow water, where such injuries are more likely to result from falling or diving into the water.24
Administer oxygen supplementation when available to all spontaneously breathing individuals. Individuals who respond well to initial resuscitation and who don’t require intubation tend to have a very good prognosis overall.25
Total time of submersion and the temperature of the water have bearing on the likelihood of survival. Only in rare cases have victims survived submersion lasting longer than 30 minutes. Ten minutes generally is considered the “point of no return.”9,26 This is consistent with data suggesting 10 minutes of hypoxic insult causes irreversible neurologic damage, with each additional minute rapidly leading to coma.20 However, to complicate matters, unlike cardiac arrest victims, drowning victims can lose cerebral blood flow slowly after respiratory impairment, which makes duration of submersion a potentially unreliable predictor of neurologic outcome.20,26
Does hypothermia have a protective effect? Hypothermia can occur in water 85°F (30°C) or cooler.10 It has been hypothesized that resuscitation can be achieved after longer periods of submersion in cold water. However, the considerable debate on this topic has been based on little more than case reports.
For hypothermia to have a protective effect on neurologic function, cooling must take place rapidly and, ideally, before any hypoxic insult. The water would have to be exceptionally cold, likely less than 50°F (10°C).27 The greater surface-to-volume ratio in children enables more rapid cooling and quicker onset of hypothermia, which may explain why they seem to have better neurologic outcomes than adults after prolonged submersion.28
Hypothermia can also be protective if the victim is breathing when cooling begins, such as while floating or swimming in cold water before drowning.29 This was the likely scenario in a reported case of a Norwegian kayaker who called for help after capsizing in 38°F (3.3°C) seawater. Despite having been in cardiac arrest for over 3 hours, the individual experienced a spontaneous return of circulation and was discharged after 32 days with no neurologic deficits.29
Correcting hypothermia after rescue. Conscious patients with no cardiovascular or respiratory compromise should have wet clothing removed at the scene in exchange for blankets, towels, or warm dry clothing. Advise rescuers to attempt no further rewarming at the scene. With unconscious patients, take only simple measures to prevent further heat loss, and focus on transport and resuscitative efforts.24
Hospital management
Attempts have been made to create a prognostic or predictive scoring system for drowning victims presenting to the emergency department. Factors thought to have bearing on mortality include duration of submersion, victim’s age, Glasgow Coma Scale (GCS) score, pupillary reactivity, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score.23,30,31 Other measurements, such as core temperature, blood pH, and response to painful stimuli, correlate poorly with mortality.31 Hyperkalemia is repeatedly mentioned as a predictor of a poor neurologic outcome, as it is thought to indicate hypoxia before the onset of cooling.8,26,32 The best predictor of a good outcome is consciousness at the time of arrival at the emergency department.4
Continued ventilation assistance is critical. As with prehospital resuscitation efforts, ventilation is critical to in-hospital management. For patients who are breathing spontaneously, continuous positive airway pressure or bi-level positive airway pressure can reduce hypoxia in pulmonary edema. Standard indications of the need for intubation include decreased level of consciousness or concern for ability to protect the airway, hypoxia despite a high fraction of inspired oxygen (FiO2), or persistent hypercapnia even with adequate noninvasive ventilatory support.33
Victims may swallow large amounts of liquid during drowning; for intubated patients, advise orogastric tube placement to prevent aspiration of gastric contents. The reliability of pulse oximetry has been called into question in this setting and may be less accurate for victims who are hypothermic or who have been submerged in cold water.23,34
Tiered intervention for hypothermia. In the hospital setting, passive rewarming is indicated for individuals with a body core temperature of 89.6°F to 95°F (32°C-35°C). Remove wet clothing; cover the victim with warm, dry towels or blankets; and give warm oral fluids and urge movement.
Individuals with core temperatures between 82.4°F to 89.6°F (28°C-32°C) require active external rewarming by applying heat directly to the skin via hot packs, warm blankets, and insulation. These patients should remain in a horizontal position with little movement, if possible, to avoid cold peripheral blood rapidly shifting to the core and precipitating an arrhythmia. Warmed intravenous fluids are appropriate as well.
With a core temperature less than 82.4°F (28°C), aggressive rewarming with extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass (CPB) may be warranted as this individual will likely have unstable or absent vital signs (TABLE).35,36 Some authorities advise that drowning victims with severe hypothermia and cardiac arrest should be resuscitated at facilities with CPB capability.27,28,37
Inducing hypothermia therapeutically is still unproven. Although not tested specifically on drowning victims, therapeutic hypothermia would seem to have theoretical benefit for their resuscitation. In cardiogenic cardiac arrest, good evidence exists for improved neurologic outcomes with therapeutic hypothermia,38 and this benefit might extend to drowning victims given their similar neurologic injury. No specific pharmacologic therapies have shown benefit in preventing loss of cerebral function.9
Monitor for clinical worsening. The concept of “secondary drowning,” a term now abandoned, referred to the phenomenon of clinical worsening hours after the initial drowning episode and resuscitation. This occurrence is now thought to be due to laryngospasm or to the progressive development of pulmonary edema from the aspiration of small amounts of water. Evidence supports monitoring asymptomatic patients; however, the period of suggested monitoring varies between 4 and 24 hours after the incident.8,22,24
Imaging, if delayed, may be useful. Imaging immediately after a drowning episode is an unreliable predictor of outcome and should be sought only if trauma or symptoms dictate. Cranial computed tomography (CT) has yielded normal findings in drowning victims with a GCS score as low as 4.
If CT is performed, any abnormality detected within 2 to 3 days of injury is a strong predictor of a poor neurologic outcome.39 Magnetic resonance imaging can be beneficial when performed more than 24 hours after resuscitation, preferably within a 4- to 7-day window.20 Lung ultrasound has been used as a bedside tool to monitor progression of pulmonary edema, and could serve the same purpose in drowning recovery.40
Anticipate respiratory complications. Since only a small amount of water is usually aspirated during a drowning event, the salinity of the aspirate is unlikely to cause significant disruption in hemodynamic or electrolyte balance.17,20 However, even a small amount of aspirated water, particularly fresh water, can disrupt gas exchange by washing out surfactant. This can rapidly precipitate ARDS. Not surprisingly, the use of exogenous surfactant has been studied in limited case reports and has had positive results.41-43 However, large trials have not yet been conducted, mostly because of the significant cost associated with surfactant therapy.
Antibiotics are rarely indicated prophylactically. Pneumonia after a drowning event is potentially fatal. It is more common in patients who have been intubated, and is therefore thought to be a hospital-acquired infection rather than a direct result of the drowning event.
Frequently, pneumonia after drowning is caused by pathogens native to the upper airway, when a victim is unable to protect his or her upper airway.44 In these cases, start broad spectrum antibiotics, with particular concern for organisms of the upper oropharynx. Also take into consideration species native to the body of water in which the victim was immersed.44
Routine prophylaxis with antibiotics, although common, is not recommended. Exceptions may be victims of drowning in known contaminated water or victims with high volumes of water aspiration.25 Some experts recommend blood cultures for victims who have aspirated, regardless of the presence or absence of infection.24 However, this recommendation seems to be based on opinion.
CORRESPONDENCE
Sean C. Engel, MD, 6600 Excelsior Boulevard Suite 100, St. Louis Park, MN 55426; [email protected]
› Recommend swimming lessons for all children ages 4 and older. C
› Consider antibiotics after a drowning event only if the water is known to be contaminated or the victim has aspirated a large volume of water. C
› Monitor asymptomatic patients for at least 4 hours after a drowning event. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
A young mother in your practice wants her toddler to begin swimming lessons because her family loves water activities. How would you advise her? In fielding an urgent call about a drowning incident, what priorities would you urge regarding resuscitation at the scene? For a stabilized patient following a drowning episode, when might antibiotics be indicated? This article covers these issues as well as follow-up matters such as assisted ventilation and tiered hypothermia intervention.
Drowning likely occurs more often than is reported
Worldwide, drowning accounts for more than 388,000 deaths annually and is the third leading cause of unintentional injury death. Low- and middle-income countries represent 96% of the yearly total.1 As reported in the United States, nearly 6000 individuals are hospitalized and nearly 4000 die from drowning events annually.2 But these figures likely underestimate the true rate, as many drowning fatalities are officially attributed to floods, boating accidents, or other associated events. Nonfatal drownings often go unreported.
Children under the age of 5 years have the highest drowning mortality worldwide, and drowning is the leading cause of unintentional injury death for this age group in many countries, including the United States.1,2 Men are nearly 4 times as likely to die from drowning than women.2 Predictably, in the United States most drowning happens on the weekend and during summer months. More than half of drownings in children younger than 4 years occur in swimming pools; with increasing age, drowning is more likely to occur in natural bodies of water.2 With adults in higher income countries, alcohol is a significant contributor to drowning events during recreational activities.3-5
Much effort has been made in recent years to standardize the nomenclature and treatment of drowning episodes. The International World Congress on Drowning met in the Netherlands in 2002, and established the definition of drowning as “the process of experiencing respiratory impairment from submersion/immersion in liquid.”6 Submersion refers to the complete submergence of the victim under the water, while immersion implies that the victim’s airway remains above the water.
The Congress recommended that terms such as “wet-drowning,” “dry-drowning,” and “near-drowning” be discontinued in favor of the outcome classifications “death,” “no morbidity,” and “morbidity.” The “morbidity” subgroup was further characterized as “moderately disabled,” “severely disabled,” “vegetative state/coma,” and “brain death.” This meeting established guidelines on the treatment of drowning victims in addition to outlining points for future research.6
Physiologic chain of events in drowning
An unexpected immersion in water, particularly cold water, causes a reflexive inspiratory gasp, and some degree of aspiration occurs in most, if not all, cases of drowning. Aspiration further impairs victims’ ability to hold their breath or breathe normally.5,7,8 It decreases lung compliance due to surfactant washout or intrapulmonary shunting and thereby leads to hypoxia. Aspiration-induced severe laryngospasm can also lead to hypoxia. Pulmonary edema and acute respiratory distress syndrome (ARDS) can follow.
The cardiovascular effects of drowning mirror those seen in hypoxia. Initially, apnea leads to decreased oxygen saturation and precipitates tachycardia and hypertension. Bradycardia and hypotension follow and blood is shunted to vital organs, such as the brain, heart, and lungs.9 This phenomenon is accelerated in cold water and leads to “swimming failure,” the impaired ability of the victim to swim because of decreased perfusion of the extremities.5,7,8,10
“Autonomic conflict” has been proposed as an additional mechanism for morbidity and mortality from drowning episodes. Breath holding and immersion in cold water each can induce cardiac arrhythmias. When combined, these events may increase the risk of an arrhythmogenic state secondary to opposing chronotropic effects: the diving reflex (bradycardia via parasympathetic activation), and the cold shock response (tachycardia via sympathetic activation). This is thought to be an underreported cause of death in drowning, as arrhythmias are undetectable during autopsy.8,11
Drowning prevention
The American Academy of Pediatrics (AAP) recommends swimming lessons for most children ages 4 years and older.12 Previously, swimming lessons were not recommended for children ages 1 to 4 because evidence of benefit was lacking, and there was some concern that it might reduce children’s caution around water and reduce parents’ perceived level of need for supervision. Although data are still conflicting, some reports have since shown benefit in early swimming lessons for
toddlers.13,14
As of 2010, the AAP acknowledges that training may be beneficial for children in this age group, but cautions that not all children will be ready for swimming by this age.12 Infant water safety programs for children under the age of 1 are not recommended because evidence of benefit is lacking.12
Evidence is growing to support teaching basic water survival skills in low- to middle-income countries where water sources are abundant, particularly in Southeast Asia. Specifically, the SwimSafe survival swimming program has yielded impressive results in Bangladesh.15 This program targets children starting at age 5, and involves 20 lessons teaching basic water survival and rescue skills.
Results have shown a 93% reduction in drowning rates for children enrolled in the program, compared with those not enrolled.15 Subsequent analyses have proposed that swimming lessons for children in these parts of the world would be as cost effective as current attempts to prevent diarrheal and respiratory diseases in the same areas.16
Additional preventive measures that are effective in the United States include 4-sided pool fencing, use of personal flotation devices, and bystander cardiopulmonary
resuscitation (CPR).2,5,17
On-scene evaluation and treatment
Drowning victims can appear mottled and have minimal or no peripheral pulses despite a heartbeat. Rescuers may assume the victim is dead when, in fact, there is cardiac function. Because initial assessment in this situation is difficult, CPR should begin, if possible, the moment the victim is out of the water. Successful on-scene resuscitation is the surest predictor of survival.9,18 In fact, delay of CPR until the arrival of emergency personnel lessens the likelihood of survival.19
CPR applied to drowning. For cardiogenic cardiac arrest, chest compressions alone may be better than compressions with rescue breathing. For victims of drowning, though, coordinated compressions and rescue breathing are recommended.20 The
2010 revision of the American Heart Association Guidelines for CPR and Emergency Cardiovascular Care emphasize “compression first” for CPR in cases of cardiogenic cardiac arrest, but continue to support the traditional Airway-Breathing-Chest Compressions sequence for drowning victims in its Special Situations section.21
Ventricular fibrillation (VF) is rare after submersion injury. An external defibrillator should be used when available, but it is unlikely to play a significant role in initial resuscitation.9
Don’t attempt to remove water from the victim’s mouth before resuscitation. The volume of fluid in the oral cavity is usually insignificant, and trying to remove it by abdominal thrusts or Heimlich maneuver will delay CPR and may injure the patient.21
Cervical spine injury is uncommon in drowning episodes, making cervical spine immobilization unnecessary unless the mechanism of injury is known or if there are clinical signs suggesting such injury. Needless cervical spine immobilization can interfere with adequate ventilation.22,23 However, concern for head or cervical spine injury is warranted when recovering an unconscious victim from shallow water, where such injuries are more likely to result from falling or diving into the water.24
Administer oxygen supplementation when available to all spontaneously breathing individuals. Individuals who respond well to initial resuscitation and who don’t require intubation tend to have a very good prognosis overall.25
Total time of submersion and the temperature of the water have bearing on the likelihood of survival. Only in rare cases have victims survived submersion lasting longer than 30 minutes. Ten minutes generally is considered the “point of no return.”9,26 This is consistent with data suggesting 10 minutes of hypoxic insult causes irreversible neurologic damage, with each additional minute rapidly leading to coma.20 However, to complicate matters, unlike cardiac arrest victims, drowning victims can lose cerebral blood flow slowly after respiratory impairment, which makes duration of submersion a potentially unreliable predictor of neurologic outcome.20,26
Does hypothermia have a protective effect? Hypothermia can occur in water 85°F (30°C) or cooler.10 It has been hypothesized that resuscitation can be achieved after longer periods of submersion in cold water. However, the considerable debate on this topic has been based on little more than case reports.
For hypothermia to have a protective effect on neurologic function, cooling must take place rapidly and, ideally, before any hypoxic insult. The water would have to be exceptionally cold, likely less than 50°F (10°C).27 The greater surface-to-volume ratio in children enables more rapid cooling and quicker onset of hypothermia, which may explain why they seem to have better neurologic outcomes than adults after prolonged submersion.28
Hypothermia can also be protective if the victim is breathing when cooling begins, such as while floating or swimming in cold water before drowning.29 This was the likely scenario in a reported case of a Norwegian kayaker who called for help after capsizing in 38°F (3.3°C) seawater. Despite having been in cardiac arrest for over 3 hours, the individual experienced a spontaneous return of circulation and was discharged after 32 days with no neurologic deficits.29
Correcting hypothermia after rescue. Conscious patients with no cardiovascular or respiratory compromise should have wet clothing removed at the scene in exchange for blankets, towels, or warm dry clothing. Advise rescuers to attempt no further rewarming at the scene. With unconscious patients, take only simple measures to prevent further heat loss, and focus on transport and resuscitative efforts.24
Hospital management
Attempts have been made to create a prognostic or predictive scoring system for drowning victims presenting to the emergency department. Factors thought to have bearing on mortality include duration of submersion, victim’s age, Glasgow Coma Scale (GCS) score, pupillary reactivity, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score.23,30,31 Other measurements, such as core temperature, blood pH, and response to painful stimuli, correlate poorly with mortality.31 Hyperkalemia is repeatedly mentioned as a predictor of a poor neurologic outcome, as it is thought to indicate hypoxia before the onset of cooling.8,26,32 The best predictor of a good outcome is consciousness at the time of arrival at the emergency department.4
Continued ventilation assistance is critical. As with prehospital resuscitation efforts, ventilation is critical to in-hospital management. For patients who are breathing spontaneously, continuous positive airway pressure or bi-level positive airway pressure can reduce hypoxia in pulmonary edema. Standard indications of the need for intubation include decreased level of consciousness or concern for ability to protect the airway, hypoxia despite a high fraction of inspired oxygen (FiO2), or persistent hypercapnia even with adequate noninvasive ventilatory support.33
Victims may swallow large amounts of liquid during drowning; for intubated patients, advise orogastric tube placement to prevent aspiration of gastric contents. The reliability of pulse oximetry has been called into question in this setting and may be less accurate for victims who are hypothermic or who have been submerged in cold water.23,34
Tiered intervention for hypothermia. In the hospital setting, passive rewarming is indicated for individuals with a body core temperature of 89.6°F to 95°F (32°C-35°C). Remove wet clothing; cover the victim with warm, dry towels or blankets; and give warm oral fluids and urge movement.
Individuals with core temperatures between 82.4°F to 89.6°F (28°C-32°C) require active external rewarming by applying heat directly to the skin via hot packs, warm blankets, and insulation. These patients should remain in a horizontal position with little movement, if possible, to avoid cold peripheral blood rapidly shifting to the core and precipitating an arrhythmia. Warmed intravenous fluids are appropriate as well.
With a core temperature less than 82.4°F (28°C), aggressive rewarming with extracorporeal membrane oxygenation (ECMO) or cardiopulmonary bypass (CPB) may be warranted as this individual will likely have unstable or absent vital signs (TABLE).35,36 Some authorities advise that drowning victims with severe hypothermia and cardiac arrest should be resuscitated at facilities with CPB capability.27,28,37
Inducing hypothermia therapeutically is still unproven. Although not tested specifically on drowning victims, therapeutic hypothermia would seem to have theoretical benefit for their resuscitation. In cardiogenic cardiac arrest, good evidence exists for improved neurologic outcomes with therapeutic hypothermia,38 and this benefit might extend to drowning victims given their similar neurologic injury. No specific pharmacologic therapies have shown benefit in preventing loss of cerebral function.9
Monitor for clinical worsening. The concept of “secondary drowning,” a term now abandoned, referred to the phenomenon of clinical worsening hours after the initial drowning episode and resuscitation. This occurrence is now thought to be due to laryngospasm or to the progressive development of pulmonary edema from the aspiration of small amounts of water. Evidence supports monitoring asymptomatic patients; however, the period of suggested monitoring varies between 4 and 24 hours after the incident.8,22,24
Imaging, if delayed, may be useful. Imaging immediately after a drowning episode is an unreliable predictor of outcome and should be sought only if trauma or symptoms dictate. Cranial computed tomography (CT) has yielded normal findings in drowning victims with a GCS score as low as 4.
If CT is performed, any abnormality detected within 2 to 3 days of injury is a strong predictor of a poor neurologic outcome.39 Magnetic resonance imaging can be beneficial when performed more than 24 hours after resuscitation, preferably within a 4- to 7-day window.20 Lung ultrasound has been used as a bedside tool to monitor progression of pulmonary edema, and could serve the same purpose in drowning recovery.40
Anticipate respiratory complications. Since only a small amount of water is usually aspirated during a drowning event, the salinity of the aspirate is unlikely to cause significant disruption in hemodynamic or electrolyte balance.17,20 However, even a small amount of aspirated water, particularly fresh water, can disrupt gas exchange by washing out surfactant. This can rapidly precipitate ARDS. Not surprisingly, the use of exogenous surfactant has been studied in limited case reports and has had positive results.41-43 However, large trials have not yet been conducted, mostly because of the significant cost associated with surfactant therapy.
Antibiotics are rarely indicated prophylactically. Pneumonia after a drowning event is potentially fatal. It is more common in patients who have been intubated, and is therefore thought to be a hospital-acquired infection rather than a direct result of the drowning event.
Frequently, pneumonia after drowning is caused by pathogens native to the upper airway, when a victim is unable to protect his or her upper airway.44 In these cases, start broad spectrum antibiotics, with particular concern for organisms of the upper oropharynx. Also take into consideration species native to the body of water in which the victim was immersed.44
Routine prophylaxis with antibiotics, although common, is not recommended. Exceptions may be victims of drowning in known contaminated water or victims with high volumes of water aspiration.25 Some experts recommend blood cultures for victims who have aspirated, regardless of the presence or absence of infection.24 However, this recommendation seems to be based on opinion.
CORRESPONDENCE
Sean C. Engel, MD, 6600 Excelsior Boulevard Suite 100, St. Louis Park, MN 55426; [email protected]
1. World Health Organization. Drowning. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs347/en/. Accessed December 28, 2014.
2. Centers for Disease Control and Prevention (CDC). Drowning—United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61:344-447.
3. Driscoll TR, Harrison JE, Steenkamp M. Alcohol and drowning in Australia. Inj Control Saf Promot. 2004;11:175-181.
4. Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest. 1997;112:660-665.
5. Hudson D, Ekman R, Svanström L. Survival of immersions during recreational boating events in Alaska, 1999-2004. Accid Anal Prev. 2007;39:437-443.
6. International Life Saving Federation. Drowning Report. International Life Saving Federation Web site. Available at: http://www.ilsf.org/sites/ilsf.org/files/filefield/drowningcongress.doc. Accessed December 28, 2014.
7. Brooks CJ, Howard KA, Neifer SK. How much did cold shock and swimming failure contribute to drowning deaths in the fishing industry in British Columbia 1976-2002? Occup Med (Lond). 2005;55:459-462.
8. Golden FS, Tipton MJ, Scott RC. Immersion, near-drowning and drowning. Br J Anaesth. 1997;79:214-225.
9. Bierens JJ, Knape JT, Gelissen HP. Drowning. Curr Opin Crit Care. 2002;8:578-586.
10. Ducharme MB, Lounsbury DS. Self-rescue swimming in cold water: the latest advice. Appl Physiol Nutr Metab. 2007;32:799-807.
11. Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol. 2012;590(pt 14):3219-3230.
12. Weiss J; American Academy of Pediatrics Committee on Injury, Violence, and Poison Prevention. Prevention of drowning. Pediatrics. 2010;126:e253-e262.
13.Yang L, Nong QQ, Li CL, et al. Risk factors for childhood drowning in rural regions of a developing country: a case-control study. Inj Prev. 2007;13:178-182.
14. Brenner RA, Taneja GS, Haynie DL, et al. The association between swimming lessons and drowning in childhood: a case-control study. Arch Pediatr Adolesc Med. 2009;163:203-210.
15. Rahman A, Rahman F, Hossain J, et al. Survival swimming - effectiveness of SwimSafe in preventing drowning in mid and late childhood. Abstract presented at: World Conference on Drowning Prevention; May 10-13, 2011; Danang, Vietnam.
16. Linnan M, Rahman A, Scarr J, et al. Child drowning: Evidence for a newly recognized cause of child mortality in low and middle income countries in Asia. Florence, Italy: UNICEF Office of Research. UNICEF Web site. Available at: www.unicef-irc.org/publications/pdf/drowning.pdf. Accessed December 28, 2014.
17. Salomez F, Vincent JL. Drowning: a review of epidemiology, pathophysiology, treatment and prevention. Resuscitation. 2004;63:261-268.
18. Venema AM, Groothoff JW, Bierens JJ. The role of bystanders during rescue and resuscitation of drowning victims. Resuscitation. 2010;81:434-439.
19. Pepe PE, Wigginton JG, Mann DM, et al. Prospective, decade-long, population-based study of pediatric drowning related incidents. Acad Emerg Med. 2002;9:516-517.
20. Topjian AA, Berg RA, Bierens JJ, et al. Brain resuscitation in the drowning victim. Neurocrit Care. 2012;17:441-467.
21. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S829-S861.
22. Ibsen LM, Koch T. Submersion and asphyxial injury. Crit Care Med. 2002;30(11 suppl):S402-S408.
23. Schilling UM, Bortolin M. Drowning. Minerva Anethesiol. 2012;78:69-77.
24. Harries M. Near drowning. BMJ. 2003;327:1336-1338.
25. Gregorakos L, Markou N, Psalida V, et al. Near-drowning: clinical course of lung injury in adults. Lung. 2009;187:93-97.
26. Eich C, Bräuer A, Timmermann A, et al. Outcome of 12 drowned children with attempted resuscitation on cardiopulmonary bypass: an analysis of variables based on the “Utstein Style for Drowning”. Resuscitation. 2007;75:42-52.
27. Wollenek G, Honarwar N, Golej J, et al. Cold water submersion and cardiac arrest in treatment of severe hypothermia with cardiopulmonary bypass. Resuscitation. 2002;52:255-263.
28. Letsou GV, Kopf GS, Elefteriades JA, et al. Is cardiopulmonary bypass effective for treatment of hypothermic arrest due to drowning or exposure? Arch Surg. 1992;127:525-528.
29. Lund FK, Torgersen JG, Flaatten HK. Heart rate monitored hypothermia and drowning in a 48-year-old man. survival without sequelae: a case report. Cases J. 2009;2:6204.
30. Ballesteros MA, Gutiérrez-Cuadra M, Muñoz P, et al. Prognostic factors and outcome after drowning in an adult population. Acta Anaesthesiol Scand. 2009;53:935-940.
31. Nichter MA, Everett PB. Childhood near-drowning: is cardiopulmonary resuscitation always indicated? Crit Care Med. 1989;17:993-995.
32. Schaller MD, Fischer AP, Perret CH. Hyperkalemia. A prognostic factor during acute severe hypothermia. JAMA. 1990;264:1842-1845.
33. O’Connor MF, Ovassapian A. Airway management. In: Hall JB, Schmidt GA, Wood LD, eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005.
34. Montenij LJ, de Vries W, Schwarte L, et al. Feasibility of pulse oximetry in the initial prehospital management of victims of drowning: a preliminary study. Resuscitation. 2011;82:1235-1238.
35. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
36. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4:99-103.
37. Coskun KO, Popov AF, Schmitto JD, et al. Extracorporeal circulation for rewarming in drowning and near-drowning pediatric patients. Artif Organs. 2010;34:1026-1030.
38. Arrich J, Holzer M, Havel C, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128.
39. Rafaat KT, Spear RM, Kuelbs C, et al. Cranial computed tomographic findings in a large group of children with drowning: diagnostic, prognostic, and forensic implications. Pediatr Crit Care Med. 2008;9:567-572.
40. Laursen CB, Davidsen JR, Madsen PH. Utility of lung ultrasound in near-drowning victims. BMJ Case Rep. 2012;2012.
41. Ugras M, Guraksin O, Sen TA, et al. Surfactant replacement therapy in a pediatric near-drowning case in manure. Pediatr Emerg Care. 2012;28:913-914.
42. Kapur N, Slater A, McEniery J, et al. Therapeutic bronchoscopy in a child with sand aspiration and respiratory failure from near drowning—case report and literature review. Pediatr Pulmonol. 2009;44:1043-1047.
43. Staudinger T, Bankier A, Strohmaier W, et al. Exogenous surfactant therapy in a patient with adult respiratory distress syndrome after near drowning. Resuscitation. 1997;35:179-182.
44. Tadié JM, Heming N, Serve E, et al. Drowning associated pneumonia: a descriptive cohort. Resuscitation. 2012;83:399-401.
1. World Health Organization. Drowning. World Health Organization Web site. Available at: http://www.who.int/mediacentre/factsheets/fs347/en/. Accessed December 28, 2014.
2. Centers for Disease Control and Prevention (CDC). Drowning—United States, 2005-2009. MMWR Morb Mortal Wkly Rep. 2012;61:344-447.
3. Driscoll TR, Harrison JE, Steenkamp M. Alcohol and drowning in Australia. Inj Control Saf Promot. 2004;11:175-181.
4. Szpilman D. Near-drowning and drowning classification: a proposal to stratify mortality based on the analysis of 1,831 cases. Chest. 1997;112:660-665.
5. Hudson D, Ekman R, Svanström L. Survival of immersions during recreational boating events in Alaska, 1999-2004. Accid Anal Prev. 2007;39:437-443.
6. International Life Saving Federation. Drowning Report. International Life Saving Federation Web site. Available at: http://www.ilsf.org/sites/ilsf.org/files/filefield/drowningcongress.doc. Accessed December 28, 2014.
7. Brooks CJ, Howard KA, Neifer SK. How much did cold shock and swimming failure contribute to drowning deaths in the fishing industry in British Columbia 1976-2002? Occup Med (Lond). 2005;55:459-462.
8. Golden FS, Tipton MJ, Scott RC. Immersion, near-drowning and drowning. Br J Anaesth. 1997;79:214-225.
9. Bierens JJ, Knape JT, Gelissen HP. Drowning. Curr Opin Crit Care. 2002;8:578-586.
10. Ducharme MB, Lounsbury DS. Self-rescue swimming in cold water: the latest advice. Appl Physiol Nutr Metab. 2007;32:799-807.
11. Shattock MJ, Tipton MJ. ‘Autonomic conflict’: a different way to die during cold water immersion? J Physiol. 2012;590(pt 14):3219-3230.
12. Weiss J; American Academy of Pediatrics Committee on Injury, Violence, and Poison Prevention. Prevention of drowning. Pediatrics. 2010;126:e253-e262.
13.Yang L, Nong QQ, Li CL, et al. Risk factors for childhood drowning in rural regions of a developing country: a case-control study. Inj Prev. 2007;13:178-182.
14. Brenner RA, Taneja GS, Haynie DL, et al. The association between swimming lessons and drowning in childhood: a case-control study. Arch Pediatr Adolesc Med. 2009;163:203-210.
15. Rahman A, Rahman F, Hossain J, et al. Survival swimming - effectiveness of SwimSafe in preventing drowning in mid and late childhood. Abstract presented at: World Conference on Drowning Prevention; May 10-13, 2011; Danang, Vietnam.
16. Linnan M, Rahman A, Scarr J, et al. Child drowning: Evidence for a newly recognized cause of child mortality in low and middle income countries in Asia. Florence, Italy: UNICEF Office of Research. UNICEF Web site. Available at: www.unicef-irc.org/publications/pdf/drowning.pdf. Accessed December 28, 2014.
17. Salomez F, Vincent JL. Drowning: a review of epidemiology, pathophysiology, treatment and prevention. Resuscitation. 2004;63:261-268.
18. Venema AM, Groothoff JW, Bierens JJ. The role of bystanders during rescue and resuscitation of drowning victims. Resuscitation. 2010;81:434-439.
19. Pepe PE, Wigginton JG, Mann DM, et al. Prospective, decade-long, population-based study of pediatric drowning related incidents. Acad Emerg Med. 2002;9:516-517.
20. Topjian AA, Berg RA, Bierens JJ, et al. Brain resuscitation in the drowning victim. Neurocrit Care. 2012;17:441-467.
21. Vanden Hoek TL, Morrison LJ, Shuster M, et al. Part 12: cardiac arrest in special situations: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 suppl 3):S829-S861.
22. Ibsen LM, Koch T. Submersion and asphyxial injury. Crit Care Med. 2002;30(11 suppl):S402-S408.
23. Schilling UM, Bortolin M. Drowning. Minerva Anethesiol. 2012;78:69-77.
24. Harries M. Near drowning. BMJ. 2003;327:1336-1338.
25. Gregorakos L, Markou N, Psalida V, et al. Near-drowning: clinical course of lung injury in adults. Lung. 2009;187:93-97.
26. Eich C, Bräuer A, Timmermann A, et al. Outcome of 12 drowned children with attempted resuscitation on cardiopulmonary bypass: an analysis of variables based on the “Utstein Style for Drowning”. Resuscitation. 2007;75:42-52.
27. Wollenek G, Honarwar N, Golej J, et al. Cold water submersion and cardiac arrest in treatment of severe hypothermia with cardiopulmonary bypass. Resuscitation. 2002;52:255-263.
28. Letsou GV, Kopf GS, Elefteriades JA, et al. Is cardiopulmonary bypass effective for treatment of hypothermic arrest due to drowning or exposure? Arch Surg. 1992;127:525-528.
29. Lund FK, Torgersen JG, Flaatten HK. Heart rate monitored hypothermia and drowning in a 48-year-old man. survival without sequelae: a case report. Cases J. 2009;2:6204.
30. Ballesteros MA, Gutiérrez-Cuadra M, Muñoz P, et al. Prognostic factors and outcome after drowning in an adult population. Acta Anaesthesiol Scand. 2009;53:935-940.
31. Nichter MA, Everett PB. Childhood near-drowning: is cardiopulmonary resuscitation always indicated? Crit Care Med. 1989;17:993-995.
32. Schaller MD, Fischer AP, Perret CH. Hyperkalemia. A prognostic factor during acute severe hypothermia. JAMA. 1990;264:1842-1845.
33. O’Connor MF, Ovassapian A. Airway management. In: Hall JB, Schmidt GA, Wood LD, eds. Principles of Critical Care. 3rd ed. New York, NY: McGraw-Hill; 2005.
34. Montenij LJ, de Vries W, Schwarte L, et al. Feasibility of pulse oximetry in the initial prehospital management of victims of drowning: a preliminary study. Resuscitation. 2011;82:1235-1238.
35. Brown DJ, Brugger H, Boyd J, et al. Accidental hypothermia. N Engl J Med. 2012;367:1930-1938.
36. Durrer B, Brugger H, Syme D; International Commission for Mountain Emergency Medicine. The medical on-site treatment of hypothermia: ICAR-MEDCOM recommendation. High Alt Med Biol. 2003;4:99-103.
37. Coskun KO, Popov AF, Schmitto JD, et al. Extracorporeal circulation for rewarming in drowning and near-drowning pediatric patients. Artif Organs. 2010;34:1026-1030.
38. Arrich J, Holzer M, Havel C, et al. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev. 2012;9:CD004128.
39. Rafaat KT, Spear RM, Kuelbs C, et al. Cranial computed tomographic findings in a large group of children with drowning: diagnostic, prognostic, and forensic implications. Pediatr Crit Care Med. 2008;9:567-572.
40. Laursen CB, Davidsen JR, Madsen PH. Utility of lung ultrasound in near-drowning victims. BMJ Case Rep. 2012;2012.
41. Ugras M, Guraksin O, Sen TA, et al. Surfactant replacement therapy in a pediatric near-drowning case in manure. Pediatr Emerg Care. 2012;28:913-914.
42. Kapur N, Slater A, McEniery J, et al. Therapeutic bronchoscopy in a child with sand aspiration and respiratory failure from near drowning—case report and literature review. Pediatr Pulmonol. 2009;44:1043-1047.
43. Staudinger T, Bankier A, Strohmaier W, et al. Exogenous surfactant therapy in a patient with adult respiratory distress syndrome after near drowning. Resuscitation. 1997;35:179-182.
44. Tadié JM, Heming N, Serve E, et al. Drowning associated pneumonia: a descriptive cohort. Resuscitation. 2012;83:399-401.
7 tools to help patients adopt healthier behaviors
› Determine the patient’s stage of change (Precontemplation, Contemplation, Preparation, Action, Maintenance, or Relapse) before selecting an intervention to help him or her change health-related behaviors. B
› Consider using motivational interviewing or narrative techniques to help patients who aren’t yet ready to change their health-related behaviors or who plan to do so within 6 months. C
› Be aware that patients seldom become motivated to change behaviors by being given information about health risks and benefits; to overcome ambivalence, they need to focus on their core values and goals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Bob G, age 47, has a body mass index of 33, hypertension (blood pressure 150/85 mm Hg), and elevated cholesterol (low-density lipoprotein level, 187 mg/dL) and glucose levels (fasting glucose 122 mg/dL, with an HbA1c of 6.1%). He gets out of breath when he plays with his 2 children. His father has diabetes and had a myocardial infarction (MI) at age 55; Mr. G tells you he is concerned he will develop similar health problems. Mr. G frequents fast food restaurants and eats high-calorie snacks after work, especially when he feels stressed. During a recent office visit, he expresses his desire to “be there” for his children and says he is motivated to lose weight to prevent diabetes and/or an MI.
How would you proceed?
Most health conditions in the United States are directly or indirectly the result of patients’ health-related behaviors.1 Fortunately, family physicians (FPs) and primary care teams are in an excellent position to help their patients make healthy behavior changes by using brief, evidence-based interventions that can be implemented during the typical office visit.
Specifically, the use of the following 7 techniques can build on patients’ own motivations, successes, and life circumstances to improve their satisfaction and self-efficacy:
- the 5 As (Ask, Advise, Assess, Assist, Arrange)
- the FRAMES protocol (Feedback, Responsibility, Advice, Menu, Empathy, and Self-efficacy)
- teachable moments (TM)
- solution-focused brief therapy (SFBT)
- cognitive behavioral therapy (CBT)
- narrative techniques (NT)
- motivational interviewing (MI).
But before we describe the practical application of these 7 techniques, we’ll begin by explaining a few underlying concepts for helping patients change their health-related behaviors.
Understanding what does—and doesn’t—help patients change
Research from the field of psychology and other social sciences has described several important concepts that affect how FPs can best help their patients change to healthier behaviors.2-9 First, several “common factors” have been found to reliably predict behavior change. The likelihood of change is strongly tied to the patient’s strengths, the environment, and the quality of the physician-patient relationship. The patient’s expectations and the techniques a physician uses also predict behavior change, but to a lesser extent.10
Second, patients seldom become motivated to change ingrained behaviors solely by being provided with information about the risks and benefits associated with those behaviors. People overcome ambivalence and develop motivation for change when they align their behaviors with their core values and goals. FPs can help patients link their motivation to change to specific plans and environments. This can then facilitate small changes that can yield large returns by increasing a patient’s self-efficacy and sense of control.2-9
Third, willpower is a finite but renewable resource that increases or decreases based on an individual’s internal and external environments. Reliance on willpower alone to make changes is unlikely to be successful without shaping the environment to support the new behavior.11
A patient’s readiness to change affects choice of technique
Knowing how ready a patient is to change is important for determining which approaches are likely to be effective at a given visit. Prochaska and DiClemente developed a model that defines 6 stages of change: Precontemplation (patient does not intend to change in the next 6 months), Contemplation (patient intends to change within the next 6 months), Preparation (patient intends to change within the next month), Action (patient has made specific changes within the past 6 months), Maintenance (patient works to prevent relapse), and Relapse (patient returns to an earlier stage) (TABLE).12
Patients who identify change as important and are ready to make changes benefit from collaborative work with an FP or other clinicians on the how, when, where, and who (eg, the patient, his or her significant other, family, and friends) of the new behaviors. These individuals are in the Preparation or Action stages of change, which comprise roughly 20% of patients.13 In these circumstances, techniques such as the 5 As, FRAMES, TM, SFBT, and CBT can be effective.
For the estimated 80% of patients who are in the Precontemplation or Contemplation stages and are unsure about the relative importance of changing behaviors and/or lack confidence to make changes, these directive techniques can cause defensiveness, which can make both the patient and the FP uncomfortable. For such patients, approaches that build on the patient’s own motivations and stories, such as MI and NT, may be preferred.
3 techniques that overlap
The FRAMES protocol and TM are based on behavior change theories, and each mixes directive techniques with relationship building to facilitate health behavior change. There is overlap in concepts across the 5 As, FRAMES, and TM, and some evidence suggests these approaches can be adapted for use in primary care settings.14-16
The 5 As is a brief intervention in which the FP sets an agenda and provides advice at the outset. This technique has been shown to improve smoking cessation rates in pregnant women compared with physician recommendations alone.17 It may also help with weight loss for patients who are ready to change and are given support for their
efforts.18
Putting the 5 As into action
CASE › An FP who wants to use the 5 As technique to assist Mr. G might proceed as follows: Ask: “How often do you exercise and follow a diet?” Advise: “I recommend that you start exercising 30 minutes each day and start following a healthier diet. It is one of the most important things you can do for your health.” Assess: “Are you willing to start exercising and trying a diet in the next month?” Assist: “Here is a list of local recreation centers and some information about a healthy diet.” Arrange: “I’d like to have one of the nurses call you in a week to see how things are going and have you return in a month for a follow-up appointment.”
The 6 components of the FRAMES protocol overlap with the 5 As.14 FRAMES utilizes relationship-building by explicitly reinforcing patient autonomy, offering a menu of choices, and acknowledging patient strengths.14
Putting the FRAMES protocol into action
CASE › Using the FRAMES protocol for Mr. G might consist of the following: Feedback: “Your eating habits and lack of exercise have contributed to your weight, high glucose and cholesterol levels, and shortness of breath.” Responsibility: “The decision to lose weight is a choice only you can make.” Advice: “I recommend that you start regularly exercising and eating healthily.” Menu: “Here are some options that many people find helpful when they try to lose weight.” Empathy: “It is challenging to change the way we eat and exercise.” Self-efficacy: “You have been able to overcome a lot of difficult things in your life already and it seems very important to you to make these changes.”
TM begins with the FP linking a patient concern, such as shortness of breath, to a physician concern, such as obesity.15 The FP then provides advice, assesses readiness, and responds based on the patient’s stage of change.16
Putting TM into action
CASE › Using the TM approach to help Mr. G might work as follows: Link a patient concern with specific behavioral change: “I think that your shortness of breath is caused by your weight.” Recommend change, offer support, and ask for commitment: “I recommend that you lose 15 pounds. I’m confident that you can do this, and am here to help you. Are you ready to talk about some specific ways you can do this?” Respond based on the patient’s readiness to change: “All right, let’s talk about healthy food choices and exercise.” (This statement would be appropriate if Mr. G was in the Preparation or Action stage of change.)
Solution-focused brief therapy
SFBT highlights a patient’s previous successes and strengths, as opposed to exploring problems and past failures.19,20 The FP fosters behavior change by using strategic questions to develop an intervention with the patient.21 SFBT involves encouraging patients to find exceptions to current problems and increasing the occurrence of current beneficial behaviors.19 This approach begins with the patient identifying a problem for which he or she would like help. The FP helps the patient explore solutions and/or exceptions to this problem that have worked for the patient previously (or solutions/exceptions that the patient can imagine). The FP does not offer suggestions to solve the patient’s problem. Instead, the patient and FP collaboratively identify and support the patient’s strengths, and they develop a behavioral task to try based on these patient-derived solutions.22
Putting SFBT into action
CASE › A physician who wants to use SFBT to help Mr. G might start by asking an “exception” question (“When have you been able to eat in a more healthy way?”) and following up with a “difference” question (“What was different about those times?”). Perhaps Mr. G remembers that previously he had improved his diet by buying and keeping a bag of apples in the car to snack on. He additionally recalls that he ate less at night if he brushed his teeth right after dinner.
Mr. G decides to revisit the apple and brushing strategies. Mr. G’s physician commends him for wanting to be there for his kids and identifying the apple and brushing strategies. She helps him design a small “experiment” in which he would use these strategies and observe the outcomes. They arrange to speak in one month to discuss how things are going.
Cognitive behavioral therapy
CBT is a practical, goal-directed, action-oriented treatment that focuses on helping patients make changes in their thinking and behavior.23-28 A basic premise of CBT is that emotions are difficult to change directly, so CBT targets distressing emotions by focusing on changing thoughts and/or behaviors that contribute to those emotions. CBT can be useful when the patient and FP can find a link between the patient’s thoughts and a troubling behavior. After thoroughly assessing situations that bother the patient, the FP provides the patient with an empathic summary that captures the essence of the problem. CBT practitioners typically conceptualize problems and plan treatment by working with the patient to gather information on the patient’s thoughts, feelings, and behaviors.
By exploring patterns of thinking that lead to self-destructive behaviors, an FP can help the patient understand and challenge strongly held but often limited patterns of thinking. For example, a patient with depression-related overeating might think, “I am worthless. Nothing ever goes right for me.” A patient with anxiety-related smoking may believe, “I am in danger.” Through a collaborative, respectful relationship, patients learn to test their “hypotheses,” challenge their thoughts, and experiment with alternate ways of thinking and behaving. Patients are given homework assignments, such as tracking their thoughts and behaviors, practicing relaxation techniques, and challenging automatic ways of viewing themselves and the world around them.
Putting CBT into action
CASE › An FP who wants to implement CBT to help Mr. G would begin by trying to understand his patient’s view: “Tell me how your weight is a part of your life.” Next, he would offer Mr. G an empathic summary: “You’re worried that your weight could cause some of the same health problems your dad has and that will prevent you from being the kind of active father you want to be. You sometimes eat when you are feeling stressed and tired, but then you feel worse afterwards.” He would assign Mr. G homework: “Notice and write down your thoughts when you are eating due to stress rather than hunger. Bring this in and we can look at it together.” The FP might also teach Mr. G relaxation breathing, and encourage him to try doing 5 relaxation breaths when he feels stressed and wants to eat.
Narrative techniques
NT can be effective for patients who are in the Precontemplation or Contemplation stages of change.29-33 NT focuses on the patient’s story, context, and language. FPs explore connections, discuss hypotheses, strategize, share power with the patient, and offer reflections in order to understand the patient’s illness experience. This approach fits well with the complex way that many behaviors are woven into the concerns that patients bring to their FPs. As a patient’s story unfolds, the diagnosis and treatment can occur simultaneously. The FP involves the patient in choices about how to proceed and what to focus on together.
NT avoids unsolicited advice and interpretations and rarely imposes the FP’s agenda on the patient. This approach invites the FP and patient to co-create an understanding and narrative of what the symptoms mean, why they are there, and what can be done about them. Ultimately, this approach can result in a new narrative that puts the patient on the path to healing.29-33
Putting NT into action
CASE › A physician might implement an NT approach with Mr. G to co-create a narrative about health and life goals by asking him: “Tell me about how losing weight fits into your goals for being there for your children. Tell me about how you see yourself avoiding some of the health problems your dad has faced.”
Motivational interviewing
For patients who are in the Precontemplation or Contemplation stages of change, MI might be a helpful approach. MI is a person-centered counseling style that addresses ambivalence about change while strengthening internal motivation for, and commitment to, change. It originally was used in addiction treatment, but has since been studied for and applied to a wide variety of medical and psychological conditions.34
MI has an underlying perspective (often called the “spirit” of MI) that includes partnership, acceptance, compassion, and evocation.34 Partnership implies a respectful collaboration between equals—while the FP may be an expert on a particular diagnosis, the patient is the expert on herself. Acceptance is unconditional positive regard and involves a nonjudgmental and person-centered recognition of an individual’s absolute worth and potential that supports autonomy and affirms strengths. Compassion is the sense of actively promoting a patient’s well being and prioritizing his or her needs over your own. Evocation refers to calling forth the patient’s own wisdom based on a realization that the patient has motivation and resources that can be elicited.
In contrast to a deficit model (“You are lacking something; I have it, and I will install it in you”), MI focuses on strengths (“You have what you need, and together we will find it”).
The skills of MI are practiced in a series of 4 sequential and overlapping processes known as Engaging, Focusing, Evoking, and Planning.34 Engaging is establishing a helpful connection and working relationship with a patient. Focusing is developing and maintaining a specific direction toward a goal (or goals). Evoking is eliciting the patient’s own motivations for change. Planning is developing commitment to change and formulating a specific action plan. Five core communication skills are used flexibly and strategically during these 4 processes: asking open questions, affirming, reflective listening, summarizing, and informing and advising with permission.34
Putting MI into action
CASE › An FP who wants to use MI with Mr. G would begin the Engaging and Focusing processes by asking permission: “May I ask you a question about weight loss?” If Mr. G says Yes, the FP would start the process of Evoking using scaling questions, such as: “On a scale of one to 10, where one means it’s not at all important, and 10 means that it’s very important, how important to you is losing weight?" (Mr. G: “I’d say 9, it is very important to me.”) “On a scale of one to 10, where one means that you are not at all confident, and 10 means that you are extremely confident, how confident are you that you can lose weight?” (Mr. G: “I’m a 6.”)
“Why are you at 6 rather than 1?” (Mr. G: “I have lost a few pounds in the past, so I know a little bit about losing weight.”) “What would have to happen for you to get to 7, that is, for you to become just a little bit more confident?” (Mr. G: “I would need to get my family’s support.”)
The FP would implement the Planning process by suggesting that Mr. G talk to his wife about taking a walk with him after dinner and buying skim milk instead of 2%.
CORRESPONDENCE
Michael Raddock, MD, Department of Family Medicine, MetroHealth Medical Center; 2500 MetroHealth Drive, Cleveland, Ohio 44109; [email protected]
1. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
2. Heath C, Heath D. Switch: How to Change Things When Change is Hard. New York, NY: Crown Business; 2010.
3. Achor S. The Happiness Advantage: The Seven Principles of Positive Psychology That Fuel Success and Performance at Work. New York, NY: Crown Business; 2010.
4. Dweck CS. Mindset: The New Psychology of Success. New York, NY: Ballantine Books; 2006.
5. Kotter JP, Cohen DS. The Heart of Change: Real-Life Stories of How People Change Their Organizations. Boston, MA: Harvard Business School Publishing; 2002.
6. Wansink B. Mindless Eating: Why We Eat More Than We Think. New York, NY: Bantam; 2006.
7. Thaler RH, Sunstein CS. Nudge: Improving Decisions About Health, Wealth, and Happiness. New York, NY: Penguin Books; 2009.
8. Maurer R. One Small Step Can Change Your Life: The Kaizen Way. New York, NY: Workman Publishing Company; 2004.
9. Patterson K, Grenny J, Maxfield D, et al. Influencer: The New Science of Leading Change. New York, NY: McGraw-Hill; 2007.
10. Hubble MA, Duncan BL, Miller SD, eds. The Heart and Soul of Change: What Works in Therapy. Washington, DC: American Psychological Association; 1999.
11. Baumeister RF, Tierney J. Willpower: Rediscovering the Greatest Human Strength. New York, NY: Penguin Books; 2011.
12. Prochaska JO, DiClemente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood, IL: Dow Jones/Irwin; 1984.
13. Prochaska JO, Norcross JC. Stages of change. Psychother: Theory, Res, Pract, Training. 2001;38:443-448.
14. Searight HR. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79:277-284.
15. Cohen DJ, Clark EC, Lawson PJ, et al. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85:e8-e15.
16. Flocke SA, Antognoli E, Step MM, et al. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109.
17. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000.
18. Alexander SC, Cox ME, Boling Turer CL, et al. Do the Five A’s work when physicians counsel about weight loss? Fam Med. 2011;43:179-184.
19. Trepper TS, McCollum E, De Jong P, et al. Solution focused therapy treatment manual for working with individuals. Solution Focused Brief Therapy Association Web site. Available at: http://www.sfbta.org/research.pdf. Accessed December 23, 2014.
20. Molnar A, de Shazer S. Solution-focused therapy: Towards the identification of therapeutic tasks. J Marital Fam Ther. 1987;13:349-358.
21. Greenberg G, Ganshorn K, Danilkewich A. Solution-focused therapy. Counseling model for busy family physicians. Can Fam Physician. 2001;47:2289-2295.
22. Giorlando ME, Schilling RJ. On becoming a solution-focused physician: The MED-STAT acronym. Families Syst Health. 1997;15:361-373.
23. Beck AT. Thinking and depression. I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9:324-333.
24. Beck AT. Thinking and depression. II. Theory and therapy. Arch Gen Psychiatry. 1964;10:561-571.
25. Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953-959.
26. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC, Talbott JA, eds. Textbook of Clinical Psychiatry. 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1245-1284.
27. Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York, NY: John Wiley & Sons; 1999.
28. Wright JH, Basco MR, Thase ME. Learning Cognitive-Behavior Therapy: An Illustrated Guide. Arlington, VA: American Psychiatric Publishing; 2006.
29. Launer J. Narrative-based Primary Care: A Practical Guide. Abingdon, United Kingdom: Radcliffe Medical Press; 2002.
30. Engel JD, Zarconi J, Pethtel L, et al. Narrative in Health Care: Healing Patients, Practitioners, Profession, and Community. Abingdon, United Kingdom: Radcliffe Publishing; 2008.
31. Charon R. Narrative Medicine: Honoring the Stories of Illness. New York, NY: Oxford University Press; 2006.
32. Kleinman A. The Illness Narratives: Suffering, Healing & the Human Condition. New York, NY: Basic Books; 1988.
33. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
34. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
› Determine the patient’s stage of change (Precontemplation, Contemplation, Preparation, Action, Maintenance, or Relapse) before selecting an intervention to help him or her change health-related behaviors. B
› Consider using motivational interviewing or narrative techniques to help patients who aren’t yet ready to change their health-related behaviors or who plan to do so within 6 months. C
› Be aware that patients seldom become motivated to change behaviors by being given information about health risks and benefits; to overcome ambivalence, they need to focus on their core values and goals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Bob G, age 47, has a body mass index of 33, hypertension (blood pressure 150/85 mm Hg), and elevated cholesterol (low-density lipoprotein level, 187 mg/dL) and glucose levels (fasting glucose 122 mg/dL, with an HbA1c of 6.1%). He gets out of breath when he plays with his 2 children. His father has diabetes and had a myocardial infarction (MI) at age 55; Mr. G tells you he is concerned he will develop similar health problems. Mr. G frequents fast food restaurants and eats high-calorie snacks after work, especially when he feels stressed. During a recent office visit, he expresses his desire to “be there” for his children and says he is motivated to lose weight to prevent diabetes and/or an MI.
How would you proceed?
Most health conditions in the United States are directly or indirectly the result of patients’ health-related behaviors.1 Fortunately, family physicians (FPs) and primary care teams are in an excellent position to help their patients make healthy behavior changes by using brief, evidence-based interventions that can be implemented during the typical office visit.
Specifically, the use of the following 7 techniques can build on patients’ own motivations, successes, and life circumstances to improve their satisfaction and self-efficacy:
- the 5 As (Ask, Advise, Assess, Assist, Arrange)
- the FRAMES protocol (Feedback, Responsibility, Advice, Menu, Empathy, and Self-efficacy)
- teachable moments (TM)
- solution-focused brief therapy (SFBT)
- cognitive behavioral therapy (CBT)
- narrative techniques (NT)
- motivational interviewing (MI).
But before we describe the practical application of these 7 techniques, we’ll begin by explaining a few underlying concepts for helping patients change their health-related behaviors.
Understanding what does—and doesn’t—help patients change
Research from the field of psychology and other social sciences has described several important concepts that affect how FPs can best help their patients change to healthier behaviors.2-9 First, several “common factors” have been found to reliably predict behavior change. The likelihood of change is strongly tied to the patient’s strengths, the environment, and the quality of the physician-patient relationship. The patient’s expectations and the techniques a physician uses also predict behavior change, but to a lesser extent.10
Second, patients seldom become motivated to change ingrained behaviors solely by being provided with information about the risks and benefits associated with those behaviors. People overcome ambivalence and develop motivation for change when they align their behaviors with their core values and goals. FPs can help patients link their motivation to change to specific plans and environments. This can then facilitate small changes that can yield large returns by increasing a patient’s self-efficacy and sense of control.2-9
Third, willpower is a finite but renewable resource that increases or decreases based on an individual’s internal and external environments. Reliance on willpower alone to make changes is unlikely to be successful without shaping the environment to support the new behavior.11
A patient’s readiness to change affects choice of technique
Knowing how ready a patient is to change is important for determining which approaches are likely to be effective at a given visit. Prochaska and DiClemente developed a model that defines 6 stages of change: Precontemplation (patient does not intend to change in the next 6 months), Contemplation (patient intends to change within the next 6 months), Preparation (patient intends to change within the next month), Action (patient has made specific changes within the past 6 months), Maintenance (patient works to prevent relapse), and Relapse (patient returns to an earlier stage) (TABLE).12
Patients who identify change as important and are ready to make changes benefit from collaborative work with an FP or other clinicians on the how, when, where, and who (eg, the patient, his or her significant other, family, and friends) of the new behaviors. These individuals are in the Preparation or Action stages of change, which comprise roughly 20% of patients.13 In these circumstances, techniques such as the 5 As, FRAMES, TM, SFBT, and CBT can be effective.
For the estimated 80% of patients who are in the Precontemplation or Contemplation stages and are unsure about the relative importance of changing behaviors and/or lack confidence to make changes, these directive techniques can cause defensiveness, which can make both the patient and the FP uncomfortable. For such patients, approaches that build on the patient’s own motivations and stories, such as MI and NT, may be preferred.
3 techniques that overlap
The FRAMES protocol and TM are based on behavior change theories, and each mixes directive techniques with relationship building to facilitate health behavior change. There is overlap in concepts across the 5 As, FRAMES, and TM, and some evidence suggests these approaches can be adapted for use in primary care settings.14-16
The 5 As is a brief intervention in which the FP sets an agenda and provides advice at the outset. This technique has been shown to improve smoking cessation rates in pregnant women compared with physician recommendations alone.17 It may also help with weight loss for patients who are ready to change and are given support for their
efforts.18
Putting the 5 As into action
CASE › An FP who wants to use the 5 As technique to assist Mr. G might proceed as follows: Ask: “How often do you exercise and follow a diet?” Advise: “I recommend that you start exercising 30 minutes each day and start following a healthier diet. It is one of the most important things you can do for your health.” Assess: “Are you willing to start exercising and trying a diet in the next month?” Assist: “Here is a list of local recreation centers and some information about a healthy diet.” Arrange: “I’d like to have one of the nurses call you in a week to see how things are going and have you return in a month for a follow-up appointment.”
The 6 components of the FRAMES protocol overlap with the 5 As.14 FRAMES utilizes relationship-building by explicitly reinforcing patient autonomy, offering a menu of choices, and acknowledging patient strengths.14
Putting the FRAMES protocol into action
CASE › Using the FRAMES protocol for Mr. G might consist of the following: Feedback: “Your eating habits and lack of exercise have contributed to your weight, high glucose and cholesterol levels, and shortness of breath.” Responsibility: “The decision to lose weight is a choice only you can make.” Advice: “I recommend that you start regularly exercising and eating healthily.” Menu: “Here are some options that many people find helpful when they try to lose weight.” Empathy: “It is challenging to change the way we eat and exercise.” Self-efficacy: “You have been able to overcome a lot of difficult things in your life already and it seems very important to you to make these changes.”
TM begins with the FP linking a patient concern, such as shortness of breath, to a physician concern, such as obesity.15 The FP then provides advice, assesses readiness, and responds based on the patient’s stage of change.16
Putting TM into action
CASE › Using the TM approach to help Mr. G might work as follows: Link a patient concern with specific behavioral change: “I think that your shortness of breath is caused by your weight.” Recommend change, offer support, and ask for commitment: “I recommend that you lose 15 pounds. I’m confident that you can do this, and am here to help you. Are you ready to talk about some specific ways you can do this?” Respond based on the patient’s readiness to change: “All right, let’s talk about healthy food choices and exercise.” (This statement would be appropriate if Mr. G was in the Preparation or Action stage of change.)
Solution-focused brief therapy
SFBT highlights a patient’s previous successes and strengths, as opposed to exploring problems and past failures.19,20 The FP fosters behavior change by using strategic questions to develop an intervention with the patient.21 SFBT involves encouraging patients to find exceptions to current problems and increasing the occurrence of current beneficial behaviors.19 This approach begins with the patient identifying a problem for which he or she would like help. The FP helps the patient explore solutions and/or exceptions to this problem that have worked for the patient previously (or solutions/exceptions that the patient can imagine). The FP does not offer suggestions to solve the patient’s problem. Instead, the patient and FP collaboratively identify and support the patient’s strengths, and they develop a behavioral task to try based on these patient-derived solutions.22
Putting SFBT into action
CASE › A physician who wants to use SFBT to help Mr. G might start by asking an “exception” question (“When have you been able to eat in a more healthy way?”) and following up with a “difference” question (“What was different about those times?”). Perhaps Mr. G remembers that previously he had improved his diet by buying and keeping a bag of apples in the car to snack on. He additionally recalls that he ate less at night if he brushed his teeth right after dinner.
Mr. G decides to revisit the apple and brushing strategies. Mr. G’s physician commends him for wanting to be there for his kids and identifying the apple and brushing strategies. She helps him design a small “experiment” in which he would use these strategies and observe the outcomes. They arrange to speak in one month to discuss how things are going.
Cognitive behavioral therapy
CBT is a practical, goal-directed, action-oriented treatment that focuses on helping patients make changes in their thinking and behavior.23-28 A basic premise of CBT is that emotions are difficult to change directly, so CBT targets distressing emotions by focusing on changing thoughts and/or behaviors that contribute to those emotions. CBT can be useful when the patient and FP can find a link between the patient’s thoughts and a troubling behavior. After thoroughly assessing situations that bother the patient, the FP provides the patient with an empathic summary that captures the essence of the problem. CBT practitioners typically conceptualize problems and plan treatment by working with the patient to gather information on the patient’s thoughts, feelings, and behaviors.
By exploring patterns of thinking that lead to self-destructive behaviors, an FP can help the patient understand and challenge strongly held but often limited patterns of thinking. For example, a patient with depression-related overeating might think, “I am worthless. Nothing ever goes right for me.” A patient with anxiety-related smoking may believe, “I am in danger.” Through a collaborative, respectful relationship, patients learn to test their “hypotheses,” challenge their thoughts, and experiment with alternate ways of thinking and behaving. Patients are given homework assignments, such as tracking their thoughts and behaviors, practicing relaxation techniques, and challenging automatic ways of viewing themselves and the world around them.
Putting CBT into action
CASE › An FP who wants to implement CBT to help Mr. G would begin by trying to understand his patient’s view: “Tell me how your weight is a part of your life.” Next, he would offer Mr. G an empathic summary: “You’re worried that your weight could cause some of the same health problems your dad has and that will prevent you from being the kind of active father you want to be. You sometimes eat when you are feeling stressed and tired, but then you feel worse afterwards.” He would assign Mr. G homework: “Notice and write down your thoughts when you are eating due to stress rather than hunger. Bring this in and we can look at it together.” The FP might also teach Mr. G relaxation breathing, and encourage him to try doing 5 relaxation breaths when he feels stressed and wants to eat.
Narrative techniques
NT can be effective for patients who are in the Precontemplation or Contemplation stages of change.29-33 NT focuses on the patient’s story, context, and language. FPs explore connections, discuss hypotheses, strategize, share power with the patient, and offer reflections in order to understand the patient’s illness experience. This approach fits well with the complex way that many behaviors are woven into the concerns that patients bring to their FPs. As a patient’s story unfolds, the diagnosis and treatment can occur simultaneously. The FP involves the patient in choices about how to proceed and what to focus on together.
NT avoids unsolicited advice and interpretations and rarely imposes the FP’s agenda on the patient. This approach invites the FP and patient to co-create an understanding and narrative of what the symptoms mean, why they are there, and what can be done about them. Ultimately, this approach can result in a new narrative that puts the patient on the path to healing.29-33
Putting NT into action
CASE › A physician might implement an NT approach with Mr. G to co-create a narrative about health and life goals by asking him: “Tell me about how losing weight fits into your goals for being there for your children. Tell me about how you see yourself avoiding some of the health problems your dad has faced.”
Motivational interviewing
For patients who are in the Precontemplation or Contemplation stages of change, MI might be a helpful approach. MI is a person-centered counseling style that addresses ambivalence about change while strengthening internal motivation for, and commitment to, change. It originally was used in addiction treatment, but has since been studied for and applied to a wide variety of medical and psychological conditions.34
MI has an underlying perspective (often called the “spirit” of MI) that includes partnership, acceptance, compassion, and evocation.34 Partnership implies a respectful collaboration between equals—while the FP may be an expert on a particular diagnosis, the patient is the expert on herself. Acceptance is unconditional positive regard and involves a nonjudgmental and person-centered recognition of an individual’s absolute worth and potential that supports autonomy and affirms strengths. Compassion is the sense of actively promoting a patient’s well being and prioritizing his or her needs over your own. Evocation refers to calling forth the patient’s own wisdom based on a realization that the patient has motivation and resources that can be elicited.
In contrast to a deficit model (“You are lacking something; I have it, and I will install it in you”), MI focuses on strengths (“You have what you need, and together we will find it”).
The skills of MI are practiced in a series of 4 sequential and overlapping processes known as Engaging, Focusing, Evoking, and Planning.34 Engaging is establishing a helpful connection and working relationship with a patient. Focusing is developing and maintaining a specific direction toward a goal (or goals). Evoking is eliciting the patient’s own motivations for change. Planning is developing commitment to change and formulating a specific action plan. Five core communication skills are used flexibly and strategically during these 4 processes: asking open questions, affirming, reflective listening, summarizing, and informing and advising with permission.34
Putting MI into action
CASE › An FP who wants to use MI with Mr. G would begin the Engaging and Focusing processes by asking permission: “May I ask you a question about weight loss?” If Mr. G says Yes, the FP would start the process of Evoking using scaling questions, such as: “On a scale of one to 10, where one means it’s not at all important, and 10 means that it’s very important, how important to you is losing weight?" (Mr. G: “I’d say 9, it is very important to me.”) “On a scale of one to 10, where one means that you are not at all confident, and 10 means that you are extremely confident, how confident are you that you can lose weight?” (Mr. G: “I’m a 6.”)
“Why are you at 6 rather than 1?” (Mr. G: “I have lost a few pounds in the past, so I know a little bit about losing weight.”) “What would have to happen for you to get to 7, that is, for you to become just a little bit more confident?” (Mr. G: “I would need to get my family’s support.”)
The FP would implement the Planning process by suggesting that Mr. G talk to his wife about taking a walk with him after dinner and buying skim milk instead of 2%.
CORRESPONDENCE
Michael Raddock, MD, Department of Family Medicine, MetroHealth Medical Center; 2500 MetroHealth Drive, Cleveland, Ohio 44109; [email protected]
› Determine the patient’s stage of change (Precontemplation, Contemplation, Preparation, Action, Maintenance, or Relapse) before selecting an intervention to help him or her change health-related behaviors. B
› Consider using motivational interviewing or narrative techniques to help patients who aren’t yet ready to change their health-related behaviors or who plan to do so within 6 months. C
› Be aware that patients seldom become motivated to change behaviors by being given information about health risks and benefits; to overcome ambivalence, they need to focus on their core values and goals. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › Your patient, Bob G, age 47, has a body mass index of 33, hypertension (blood pressure 150/85 mm Hg), and elevated cholesterol (low-density lipoprotein level, 187 mg/dL) and glucose levels (fasting glucose 122 mg/dL, with an HbA1c of 6.1%). He gets out of breath when he plays with his 2 children. His father has diabetes and had a myocardial infarction (MI) at age 55; Mr. G tells you he is concerned he will develop similar health problems. Mr. G frequents fast food restaurants and eats high-calorie snacks after work, especially when he feels stressed. During a recent office visit, he expresses his desire to “be there” for his children and says he is motivated to lose weight to prevent diabetes and/or an MI.
How would you proceed?
Most health conditions in the United States are directly or indirectly the result of patients’ health-related behaviors.1 Fortunately, family physicians (FPs) and primary care teams are in an excellent position to help their patients make healthy behavior changes by using brief, evidence-based interventions that can be implemented during the typical office visit.
Specifically, the use of the following 7 techniques can build on patients’ own motivations, successes, and life circumstances to improve their satisfaction and self-efficacy:
- the 5 As (Ask, Advise, Assess, Assist, Arrange)
- the FRAMES protocol (Feedback, Responsibility, Advice, Menu, Empathy, and Self-efficacy)
- teachable moments (TM)
- solution-focused brief therapy (SFBT)
- cognitive behavioral therapy (CBT)
- narrative techniques (NT)
- motivational interviewing (MI).
But before we describe the practical application of these 7 techniques, we’ll begin by explaining a few underlying concepts for helping patients change their health-related behaviors.
Understanding what does—and doesn’t—help patients change
Research from the field of psychology and other social sciences has described several important concepts that affect how FPs can best help their patients change to healthier behaviors.2-9 First, several “common factors” have been found to reliably predict behavior change. The likelihood of change is strongly tied to the patient’s strengths, the environment, and the quality of the physician-patient relationship. The patient’s expectations and the techniques a physician uses also predict behavior change, but to a lesser extent.10
Second, patients seldom become motivated to change ingrained behaviors solely by being provided with information about the risks and benefits associated with those behaviors. People overcome ambivalence and develop motivation for change when they align their behaviors with their core values and goals. FPs can help patients link their motivation to change to specific plans and environments. This can then facilitate small changes that can yield large returns by increasing a patient’s self-efficacy and sense of control.2-9
Third, willpower is a finite but renewable resource that increases or decreases based on an individual’s internal and external environments. Reliance on willpower alone to make changes is unlikely to be successful without shaping the environment to support the new behavior.11
A patient’s readiness to change affects choice of technique
Knowing how ready a patient is to change is important for determining which approaches are likely to be effective at a given visit. Prochaska and DiClemente developed a model that defines 6 stages of change: Precontemplation (patient does not intend to change in the next 6 months), Contemplation (patient intends to change within the next 6 months), Preparation (patient intends to change within the next month), Action (patient has made specific changes within the past 6 months), Maintenance (patient works to prevent relapse), and Relapse (patient returns to an earlier stage) (TABLE).12
Patients who identify change as important and are ready to make changes benefit from collaborative work with an FP or other clinicians on the how, when, where, and who (eg, the patient, his or her significant other, family, and friends) of the new behaviors. These individuals are in the Preparation or Action stages of change, which comprise roughly 20% of patients.13 In these circumstances, techniques such as the 5 As, FRAMES, TM, SFBT, and CBT can be effective.
For the estimated 80% of patients who are in the Precontemplation or Contemplation stages and are unsure about the relative importance of changing behaviors and/or lack confidence to make changes, these directive techniques can cause defensiveness, which can make both the patient and the FP uncomfortable. For such patients, approaches that build on the patient’s own motivations and stories, such as MI and NT, may be preferred.
3 techniques that overlap
The FRAMES protocol and TM are based on behavior change theories, and each mixes directive techniques with relationship building to facilitate health behavior change. There is overlap in concepts across the 5 As, FRAMES, and TM, and some evidence suggests these approaches can be adapted for use in primary care settings.14-16
The 5 As is a brief intervention in which the FP sets an agenda and provides advice at the outset. This technique has been shown to improve smoking cessation rates in pregnant women compared with physician recommendations alone.17 It may also help with weight loss for patients who are ready to change and are given support for their
efforts.18
Putting the 5 As into action
CASE › An FP who wants to use the 5 As technique to assist Mr. G might proceed as follows: Ask: “How often do you exercise and follow a diet?” Advise: “I recommend that you start exercising 30 minutes each day and start following a healthier diet. It is one of the most important things you can do for your health.” Assess: “Are you willing to start exercising and trying a diet in the next month?” Assist: “Here is a list of local recreation centers and some information about a healthy diet.” Arrange: “I’d like to have one of the nurses call you in a week to see how things are going and have you return in a month for a follow-up appointment.”
The 6 components of the FRAMES protocol overlap with the 5 As.14 FRAMES utilizes relationship-building by explicitly reinforcing patient autonomy, offering a menu of choices, and acknowledging patient strengths.14
Putting the FRAMES protocol into action
CASE › Using the FRAMES protocol for Mr. G might consist of the following: Feedback: “Your eating habits and lack of exercise have contributed to your weight, high glucose and cholesterol levels, and shortness of breath.” Responsibility: “The decision to lose weight is a choice only you can make.” Advice: “I recommend that you start regularly exercising and eating healthily.” Menu: “Here are some options that many people find helpful when they try to lose weight.” Empathy: “It is challenging to change the way we eat and exercise.” Self-efficacy: “You have been able to overcome a lot of difficult things in your life already and it seems very important to you to make these changes.”
TM begins with the FP linking a patient concern, such as shortness of breath, to a physician concern, such as obesity.15 The FP then provides advice, assesses readiness, and responds based on the patient’s stage of change.16
Putting TM into action
CASE › Using the TM approach to help Mr. G might work as follows: Link a patient concern with specific behavioral change: “I think that your shortness of breath is caused by your weight.” Recommend change, offer support, and ask for commitment: “I recommend that you lose 15 pounds. I’m confident that you can do this, and am here to help you. Are you ready to talk about some specific ways you can do this?” Respond based on the patient’s readiness to change: “All right, let’s talk about healthy food choices and exercise.” (This statement would be appropriate if Mr. G was in the Preparation or Action stage of change.)
Solution-focused brief therapy
SFBT highlights a patient’s previous successes and strengths, as opposed to exploring problems and past failures.19,20 The FP fosters behavior change by using strategic questions to develop an intervention with the patient.21 SFBT involves encouraging patients to find exceptions to current problems and increasing the occurrence of current beneficial behaviors.19 This approach begins with the patient identifying a problem for which he or she would like help. The FP helps the patient explore solutions and/or exceptions to this problem that have worked for the patient previously (or solutions/exceptions that the patient can imagine). The FP does not offer suggestions to solve the patient’s problem. Instead, the patient and FP collaboratively identify and support the patient’s strengths, and they develop a behavioral task to try based on these patient-derived solutions.22
Putting SFBT into action
CASE › A physician who wants to use SFBT to help Mr. G might start by asking an “exception” question (“When have you been able to eat in a more healthy way?”) and following up with a “difference” question (“What was different about those times?”). Perhaps Mr. G remembers that previously he had improved his diet by buying and keeping a bag of apples in the car to snack on. He additionally recalls that he ate less at night if he brushed his teeth right after dinner.
Mr. G decides to revisit the apple and brushing strategies. Mr. G’s physician commends him for wanting to be there for his kids and identifying the apple and brushing strategies. She helps him design a small “experiment” in which he would use these strategies and observe the outcomes. They arrange to speak in one month to discuss how things are going.
Cognitive behavioral therapy
CBT is a practical, goal-directed, action-oriented treatment that focuses on helping patients make changes in their thinking and behavior.23-28 A basic premise of CBT is that emotions are difficult to change directly, so CBT targets distressing emotions by focusing on changing thoughts and/or behaviors that contribute to those emotions. CBT can be useful when the patient and FP can find a link between the patient’s thoughts and a troubling behavior. After thoroughly assessing situations that bother the patient, the FP provides the patient with an empathic summary that captures the essence of the problem. CBT practitioners typically conceptualize problems and plan treatment by working with the patient to gather information on the patient’s thoughts, feelings, and behaviors.
By exploring patterns of thinking that lead to self-destructive behaviors, an FP can help the patient understand and challenge strongly held but often limited patterns of thinking. For example, a patient with depression-related overeating might think, “I am worthless. Nothing ever goes right for me.” A patient with anxiety-related smoking may believe, “I am in danger.” Through a collaborative, respectful relationship, patients learn to test their “hypotheses,” challenge their thoughts, and experiment with alternate ways of thinking and behaving. Patients are given homework assignments, such as tracking their thoughts and behaviors, practicing relaxation techniques, and challenging automatic ways of viewing themselves and the world around them.
Putting CBT into action
CASE › An FP who wants to implement CBT to help Mr. G would begin by trying to understand his patient’s view: “Tell me how your weight is a part of your life.” Next, he would offer Mr. G an empathic summary: “You’re worried that your weight could cause some of the same health problems your dad has and that will prevent you from being the kind of active father you want to be. You sometimes eat when you are feeling stressed and tired, but then you feel worse afterwards.” He would assign Mr. G homework: “Notice and write down your thoughts when you are eating due to stress rather than hunger. Bring this in and we can look at it together.” The FP might also teach Mr. G relaxation breathing, and encourage him to try doing 5 relaxation breaths when he feels stressed and wants to eat.
Narrative techniques
NT can be effective for patients who are in the Precontemplation or Contemplation stages of change.29-33 NT focuses on the patient’s story, context, and language. FPs explore connections, discuss hypotheses, strategize, share power with the patient, and offer reflections in order to understand the patient’s illness experience. This approach fits well with the complex way that many behaviors are woven into the concerns that patients bring to their FPs. As a patient’s story unfolds, the diagnosis and treatment can occur simultaneously. The FP involves the patient in choices about how to proceed and what to focus on together.
NT avoids unsolicited advice and interpretations and rarely imposes the FP’s agenda on the patient. This approach invites the FP and patient to co-create an understanding and narrative of what the symptoms mean, why they are there, and what can be done about them. Ultimately, this approach can result in a new narrative that puts the patient on the path to healing.29-33
Putting NT into action
CASE › A physician might implement an NT approach with Mr. G to co-create a narrative about health and life goals by asking him: “Tell me about how losing weight fits into your goals for being there for your children. Tell me about how you see yourself avoiding some of the health problems your dad has faced.”
Motivational interviewing
For patients who are in the Precontemplation or Contemplation stages of change, MI might be a helpful approach. MI is a person-centered counseling style that addresses ambivalence about change while strengthening internal motivation for, and commitment to, change. It originally was used in addiction treatment, but has since been studied for and applied to a wide variety of medical and psychological conditions.34
MI has an underlying perspective (often called the “spirit” of MI) that includes partnership, acceptance, compassion, and evocation.34 Partnership implies a respectful collaboration between equals—while the FP may be an expert on a particular diagnosis, the patient is the expert on herself. Acceptance is unconditional positive regard and involves a nonjudgmental and person-centered recognition of an individual’s absolute worth and potential that supports autonomy and affirms strengths. Compassion is the sense of actively promoting a patient’s well being and prioritizing his or her needs over your own. Evocation refers to calling forth the patient’s own wisdom based on a realization that the patient has motivation and resources that can be elicited.
In contrast to a deficit model (“You are lacking something; I have it, and I will install it in you”), MI focuses on strengths (“You have what you need, and together we will find it”).
The skills of MI are practiced in a series of 4 sequential and overlapping processes known as Engaging, Focusing, Evoking, and Planning.34 Engaging is establishing a helpful connection and working relationship with a patient. Focusing is developing and maintaining a specific direction toward a goal (or goals). Evoking is eliciting the patient’s own motivations for change. Planning is developing commitment to change and formulating a specific action plan. Five core communication skills are used flexibly and strategically during these 4 processes: asking open questions, affirming, reflective listening, summarizing, and informing and advising with permission.34
Putting MI into action
CASE › An FP who wants to use MI with Mr. G would begin the Engaging and Focusing processes by asking permission: “May I ask you a question about weight loss?” If Mr. G says Yes, the FP would start the process of Evoking using scaling questions, such as: “On a scale of one to 10, where one means it’s not at all important, and 10 means that it’s very important, how important to you is losing weight?" (Mr. G: “I’d say 9, it is very important to me.”) “On a scale of one to 10, where one means that you are not at all confident, and 10 means that you are extremely confident, how confident are you that you can lose weight?” (Mr. G: “I’m a 6.”)
“Why are you at 6 rather than 1?” (Mr. G: “I have lost a few pounds in the past, so I know a little bit about losing weight.”) “What would have to happen for you to get to 7, that is, for you to become just a little bit more confident?” (Mr. G: “I would need to get my family’s support.”)
The FP would implement the Planning process by suggesting that Mr. G talk to his wife about taking a walk with him after dinner and buying skim milk instead of 2%.
CORRESPONDENCE
Michael Raddock, MD, Department of Family Medicine, MetroHealth Medical Center; 2500 MetroHealth Drive, Cleveland, Ohio 44109; [email protected]
1. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
2. Heath C, Heath D. Switch: How to Change Things When Change is Hard. New York, NY: Crown Business; 2010.
3. Achor S. The Happiness Advantage: The Seven Principles of Positive Psychology That Fuel Success and Performance at Work. New York, NY: Crown Business; 2010.
4. Dweck CS. Mindset: The New Psychology of Success. New York, NY: Ballantine Books; 2006.
5. Kotter JP, Cohen DS. The Heart of Change: Real-Life Stories of How People Change Their Organizations. Boston, MA: Harvard Business School Publishing; 2002.
6. Wansink B. Mindless Eating: Why We Eat More Than We Think. New York, NY: Bantam; 2006.
7. Thaler RH, Sunstein CS. Nudge: Improving Decisions About Health, Wealth, and Happiness. New York, NY: Penguin Books; 2009.
8. Maurer R. One Small Step Can Change Your Life: The Kaizen Way. New York, NY: Workman Publishing Company; 2004.
9. Patterson K, Grenny J, Maxfield D, et al. Influencer: The New Science of Leading Change. New York, NY: McGraw-Hill; 2007.
10. Hubble MA, Duncan BL, Miller SD, eds. The Heart and Soul of Change: What Works in Therapy. Washington, DC: American Psychological Association; 1999.
11. Baumeister RF, Tierney J. Willpower: Rediscovering the Greatest Human Strength. New York, NY: Penguin Books; 2011.
12. Prochaska JO, DiClemente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood, IL: Dow Jones/Irwin; 1984.
13. Prochaska JO, Norcross JC. Stages of change. Psychother: Theory, Res, Pract, Training. 2001;38:443-448.
14. Searight HR. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79:277-284.
15. Cohen DJ, Clark EC, Lawson PJ, et al. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85:e8-e15.
16. Flocke SA, Antognoli E, Step MM, et al. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109.
17. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000.
18. Alexander SC, Cox ME, Boling Turer CL, et al. Do the Five A’s work when physicians counsel about weight loss? Fam Med. 2011;43:179-184.
19. Trepper TS, McCollum E, De Jong P, et al. Solution focused therapy treatment manual for working with individuals. Solution Focused Brief Therapy Association Web site. Available at: http://www.sfbta.org/research.pdf. Accessed December 23, 2014.
20. Molnar A, de Shazer S. Solution-focused therapy: Towards the identification of therapeutic tasks. J Marital Fam Ther. 1987;13:349-358.
21. Greenberg G, Ganshorn K, Danilkewich A. Solution-focused therapy. Counseling model for busy family physicians. Can Fam Physician. 2001;47:2289-2295.
22. Giorlando ME, Schilling RJ. On becoming a solution-focused physician: The MED-STAT acronym. Families Syst Health. 1997;15:361-373.
23. Beck AT. Thinking and depression. I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9:324-333.
24. Beck AT. Thinking and depression. II. Theory and therapy. Arch Gen Psychiatry. 1964;10:561-571.
25. Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953-959.
26. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC, Talbott JA, eds. Textbook of Clinical Psychiatry. 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1245-1284.
27. Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York, NY: John Wiley & Sons; 1999.
28. Wright JH, Basco MR, Thase ME. Learning Cognitive-Behavior Therapy: An Illustrated Guide. Arlington, VA: American Psychiatric Publishing; 2006.
29. Launer J. Narrative-based Primary Care: A Practical Guide. Abingdon, United Kingdom: Radcliffe Medical Press; 2002.
30. Engel JD, Zarconi J, Pethtel L, et al. Narrative in Health Care: Healing Patients, Practitioners, Profession, and Community. Abingdon, United Kingdom: Radcliffe Publishing; 2008.
31. Charon R. Narrative Medicine: Honoring the Stories of Illness. New York, NY: Oxford University Press; 2006.
32. Kleinman A. The Illness Narratives: Suffering, Healing & the Human Condition. New York, NY: Basic Books; 1988.
33. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
34. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
1. Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245.
2. Heath C, Heath D. Switch: How to Change Things When Change is Hard. New York, NY: Crown Business; 2010.
3. Achor S. The Happiness Advantage: The Seven Principles of Positive Psychology That Fuel Success and Performance at Work. New York, NY: Crown Business; 2010.
4. Dweck CS. Mindset: The New Psychology of Success. New York, NY: Ballantine Books; 2006.
5. Kotter JP, Cohen DS. The Heart of Change: Real-Life Stories of How People Change Their Organizations. Boston, MA: Harvard Business School Publishing; 2002.
6. Wansink B. Mindless Eating: Why We Eat More Than We Think. New York, NY: Bantam; 2006.
7. Thaler RH, Sunstein CS. Nudge: Improving Decisions About Health, Wealth, and Happiness. New York, NY: Penguin Books; 2009.
8. Maurer R. One Small Step Can Change Your Life: The Kaizen Way. New York, NY: Workman Publishing Company; 2004.
9. Patterson K, Grenny J, Maxfield D, et al. Influencer: The New Science of Leading Change. New York, NY: McGraw-Hill; 2007.
10. Hubble MA, Duncan BL, Miller SD, eds. The Heart and Soul of Change: What Works in Therapy. Washington, DC: American Psychological Association; 1999.
11. Baumeister RF, Tierney J. Willpower: Rediscovering the Greatest Human Strength. New York, NY: Penguin Books; 2011.
12. Prochaska JO, DiClemente CC. The Transtheoretical Approach: Crossing Traditional Boundaries of Therapy. Homewood, IL: Dow Jones/Irwin; 1984.
13. Prochaska JO, Norcross JC. Stages of change. Psychother: Theory, Res, Pract, Training. 2001;38:443-448.
14. Searight HR. Realistic approaches to counseling in the office setting. Am Fam Physician. 2009;79:277-284.
15. Cohen DJ, Clark EC, Lawson PJ, et al. Identifying teachable moments for health behavior counseling in primary care. Patient Educ Couns. 2011;85:e8-e15.
16. Flocke SA, Antognoli E, Step MM, et al. A teachable moment communication process for smoking cessation talk: description of a group randomized clinician-focused intervention. BMC Health Serv Res. 2012;12:109.
17. Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000.
18. Alexander SC, Cox ME, Boling Turer CL, et al. Do the Five A’s work when physicians counsel about weight loss? Fam Med. 2011;43:179-184.
19. Trepper TS, McCollum E, De Jong P, et al. Solution focused therapy treatment manual for working with individuals. Solution Focused Brief Therapy Association Web site. Available at: http://www.sfbta.org/research.pdf. Accessed December 23, 2014.
20. Molnar A, de Shazer S. Solution-focused therapy: Towards the identification of therapeutic tasks. J Marital Fam Ther. 1987;13:349-358.
21. Greenberg G, Ganshorn K, Danilkewich A. Solution-focused therapy. Counseling model for busy family physicians. Can Fam Physician. 2001;47:2289-2295.
22. Giorlando ME, Schilling RJ. On becoming a solution-focused physician: The MED-STAT acronym. Families Syst Health. 1997;15:361-373.
23. Beck AT. Thinking and depression. I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry. 1963;9:324-333.
24. Beck AT. Thinking and depression. II. Theory and therapy. Arch Gen Psychiatry. 1964;10:561-571.
25. Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953-959.
26. Wright JH, Beck AT, Thase ME. Cognitive therapy. In: Hales RE, Yudofsky SC, Talbott JA, eds. Textbook of Clinical Psychiatry. 4th ed. Washington, DC: American Psychiatric Publishing; 2003:1245-1284.
27. Clark DA, Beck AT, Alford BA. Scientific Foundations of Cognitive Theory and Therapy of Depression. New York, NY: John Wiley & Sons; 1999.
28. Wright JH, Basco MR, Thase ME. Learning Cognitive-Behavior Therapy: An Illustrated Guide. Arlington, VA: American Psychiatric Publishing; 2006.
29. Launer J. Narrative-based Primary Care: A Practical Guide. Abingdon, United Kingdom: Radcliffe Medical Press; 2002.
30. Engel JD, Zarconi J, Pethtel L, et al. Narrative in Health Care: Healing Patients, Practitioners, Profession, and Community. Abingdon, United Kingdom: Radcliffe Publishing; 2008.
31. Charon R. Narrative Medicine: Honoring the Stories of Illness. New York, NY: Oxford University Press; 2006.
32. Kleinman A. The Illness Narratives: Suffering, Healing & the Human Condition. New York, NY: Basic Books; 1988.
33. Kleinman A, Eisenberg L, Good B. Culture, illness, and care: clinical lessons from anthropologic and cross-cultural research. Ann Intern Med. 1978;88:251-258.
34. Miller WR, Rollnick S. Motivational Interviewing: Helping People Change. 3rd ed. New York, NY: The Guilford Press; 2013.
HIV: 3 cases that hid in plain sight
› Rule out human immunodeficiency virus (HIV) infection when evaluating a patient for thrombocytopenia. A
› Consider HIV testing in patients with herpes zoster, even for those who do not have risk factors for HIV. B
› Recognize that fatigue, weight loss, unexplained rashes, and hematologic disorders are some of ways in which a patient with HIV infection may present. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Roberta K, age 35, was referred by her family physician (FP) to a hematologist in November 2007 after her FP noted a platelet count of 63,000/mcL on a screening complete blood count (CBC; normal, 150,000-400,000/mcL). Ms. K also had asthma, hypothyroidism, depression, and migraine headaches. She was given a diagnosis of idiopathic thrombocytopenic purpura and started on oral prednisone. Her platelet count improved and she was maintained on prednisone 7.5 to 10 mg/d over the next 5 years with periodic dosage increases whenever her platelet count dropped below 50,000/mcL. She saw her FP for regular medical care 3 to 4 times a year and by a hematologist every 6 months.
In April 2012, Ms. K sought treatment from her FP for an acute painful rash consistent with herpes zoster involving the left C5-C6 dermatomes. Due to severe pain and secondary infection, she was admitted to the hospital. During the hospitalization, the inpatient team caring for her obtained a human immunodeficiency virus (HIV) serology, which was positive. Her only HIV risk factor was that she’d had 3 lifetime male sex partners.
Ms. K’s initial CD4+ T-cell count was 224 cells/mm3 (normal, nonimmunocompromised adult, 500–1,2001) and her percentage of CD4+ T-cells was 21% (normal, 30%-60%2). Her HIV RNA level was 71,587 copies/mL; the goal of HIV treatment typically is to get this down to <200 copies/mL. She was started on antiretroviral therapy (ART) consisting of fixed-dose emtricitabine/rilpivirine/
tenofovir (200 mg/25 mg/300 mg) and was weaned off prednisone. Six months after starting ART, her CD4+ T-cell count was 450 cells/mm3 and her HIV RNA level was <20 copies/mL. Her most recent platelet count was 148,000/mcL.
The correct diagnosis: Thrombocytopenia secondary to HIV infection.
CASE 2 › Christian M, age 40, presented to his FP in February 2010 with worsening cough and shortness of breath that he’d had for 4 weeks. He said he had unintentionally lost 20 pounds since the beginning of the year. He had no medical history of note, but had seen his FP on several occasions over the past few years for treatment of acute minor illnesses and an employment physical. He’d had no occupational exposures that might have affected his lungs, and he did not smoke.
He was initially diagnosed with bronchitis and treated with an oral antibiotic. Two weeks later, his symptoms persisted and Mr. M’s FP referred him to a pulmonologist. A chest x-ray showed an “interstitial process possibly consistent with pneumonia” for which the pulmonologist prescribed levofloxacin and oral prednisone for 10 days. At the follow-up visit, Mr. M had clinically improved. The diagnosis noted by the pulmonologist was “probably viral vs atypical pneumonia.”
Approximately 3 weeks later, in April 2010, Mr. M presented to the emergency department (ED) after several days of fever, cough, and worsening shortness of breath. A chest x-ray showed an interstitial pneumonitis that had worsened since the prior radiography. His pulse oximetry was 87% on room air.
A computed tomography (CT) scan of the chest revealed bilateral ground-glass opacities. The patient was admitted to the hospital and the next day underwent bronchoscopy with bronchoalveolar lavage. A Gomori methenamine silver stain for Pneumocystis jirovecii was positive, as was an HIV serology. Mr. M’s only reported risk factor for HIV was heterosexual contact. He had been in a stable relationship for over 14 years.
His baseline CD4+ T-cell count was 5 cells/mm3 (1%) and his HIV RNA level was >500,000 copies/mL. Several weeks later, Mr. M’s spouse tested positive for HIV. Her CD4+ T-cell count was 45 cells/mm3 (10%) and her viral load was 23,258 copies/mL. Although she was asymptomatic at the time of diagnosis, Ms. M was soon started on the same ART regimen as her husband.
The correct diagnosis: Pneumocystis pneumonia with symptoms of acquired immunodeficiency syndrome (AIDS) wasting syndrome.
CASE 3 › Michael L, age 66, was seen by his FP in September 2010 for “preoperative clearance” for elbow surgery. He was in good health but had a platelet count of 67,000/mcL. For unclear reasons, the surgery was cancelled; Mr. L was supposed to be referred to a hematologist for the thrombocytopenia, but this consultation never occurred. The patient did not return to his FP until April 2012, when he complained of feeling “lightheaded and dizzy” for the past few weeks. His examination was remarkable only for mild orthostatic hypotension and he was diagnosed with “dehydration.”
He returned to the office in July 2012 with similar symptoms and a 12-pound weight loss since his last visit. He also complained of short-term memory problems. Lab testing was done and included a chemistry panel, thyroid-stimulating hormone test, and CBC, all of which were normal except for a hemoglobin of 11.1 g/dL, a white blood cell count of 2.4/mcL, and a platelet count of 119,000/mcL. The patient was advised to get a follow-up CBC in one month, but this was not done.
Mr. L returned in November 2012, again complaining of intermittent lightheadedness and fatigue, and said he had been experiencing “mouth sores.” He was given a diagnosis of “probable oral herpes infection” and treated with oral acyclovir. No lab studies were performed.
Mr. L was brought to the ED in February 2013 with fever and mental status changes that had developed over 2 to 3 days. According to a family member, he had also complained of headache for the previous 2 weeks.
A CT scan of his head was normal and he underwent a lumbar puncture. Cerebrospinal fluid revealed a white blood cell count of 270/mcL, glucose of 62 mg/dL, and protein of 15 mg/dL. A gram stain was negative, but an India ink stain was positive for encapsulated yeast forms consistent with Cryptococcus. Mr. L was diagnosed with cryptococcal meningitis and treated with intravenous amphotericin B and oral flucytosine. An HIV serology was positive. His CD4+ T-cell count was 8 cells/mm3 (3%) and his HIV RNA level was >500,000 copies /mL.
He was discharged from the hospital after 2 weeks and transitioned to oral fluconazole 400 mg/d for the meningitis. One week after discharge, he was started on an ART regimen of darunavir 800 mg, ritonavir 100 mg, and fixed-dose tenofovir/emtricitabine (200 mg/300 mg).
After 6 months of ART, he showed significant clinical improvement, his HIV-RNA level was <20 copies/mL and his CD4+ T-cell count was 136 cells/mm3 (12%). His female partner of 11 years tested negative for HIV.
The correct diagnosis: Cryptococcal meningitis; thrombocytopenia secondary to HIV infection.
These 3 cases illustrate what clinicians who treat patients with HIV/AIDS have observed for many years: Physicians often fail to diagnose patients with HIV infection in a timely fashion. HIV can be missed when patients present with clinical signs of immune suppression, such as herpes zoster, as well as when they present with AIDS-defining illnesses such as lymphoma or recurrent pneumonia. Late diagnosis of HIV—typically defined as diagnosis when a patient’s CD4+ T-cell count is <200 cells/mm3—increases morbidity and mortality, as well as health care costs.3
Historically, late HIV testing has been very common in the United States. A Centers for Disease Control and Prevention (CDC) report noted that from 1996 to 2005, 38% of patients diagnosed in 34 states had an AIDS diagnosis within one year of testing positive for HIV.4 Chin et al5 performed a retrospective cohort study of patients seen in an HIV clinic in North Carolina between November 2008 and November 2011. The median CD4+ T-cell count at time of diagnosis was 313 cells/mm3 and one-third of patients had a count of <50 cells/mm3. Current HIV treatment guidelines recommend ART for all patients diagnosed with HIV infection regardless of CD4+ T-cell count.
The mean number of health care visits in the year before diagnosis was 2.75 (range 0-20). These visits occurred in both primary care settings and the ED. Approximately one-third of patients had complained of HIV-associated signs and symptoms, including recurrent respiratory tract infections, unexplained persistent fevers, and generalized lymphadenopathy prior to diagnosis.
FPs must remain cognizant of the many diverse clinical presentations of patients with HIV/AIDS, including fatigue, weight loss, unexplained rashes, and hematologic disorders (TABLE 16 and TABLE 27). In the 3 cases described here, the specific conditions the treatment teams failed to identify as indicators of HIV infection were thrombocytopenia, pneumocystis pneumonia, herpes zoster, and cryptococcal meningitis.
Thrombocytopenia has many causes, including infection, medications, lymphoproliferative disorders, liver disease, and connective tissue diseases. However, low platelet counts are often seen in individuals with HIV infection.
Before the introduction of ART, the incidence of thrombocytopenia in HIV patients was 40%.8 Since then, this condition is less common, but HIV should be ruled out when evaluating a patient for thrombocytopenia or making a diagnosis of “idiopathic thrombocytopenia” (as was Ms. K’s initial diagnosis).
The incidence of cytopenias in general correlates directly with the degree of immunosuppression. However, isolated hematologic abnormalities, including anemia and leukopenia, may be the initial presentation of HIV infection.9 As a result, HIV must be considered in the assessment of all patients who present with any hematologic abnormality.
Pneumocystis pneumonia. Pneumonia caused by the fungus Pneumocystis jirovecii has been a longtime AIDS-defining illness and is the most common opportunistic infection in patients with advanced HIV infection.10 A slow, indolent course is common, with symptoms of cough and dyspnea progressing over weeks to months (as observed in Mr. M). Radiographs will show diffuse or isolated ground-glass opacities. Partial improvement is sometimes seen in patients with unknown HIV infection who are treated with short courses of prednisone and antibiotics.11 Patients with untreated HIV infection and CD4+ T-cell counts <200 cells/mm3 will develop worsening hypoxemia and, in some cases, fulminant respiratory failure.
Herpes zoster is common in older adults and often indicates a weakened immune system. The incidence of zoster among adults with HIV is more than 15-fold higher than it is among age-matched varicella-zoster virus-infected immunocompetent people.12 A study from the early 1990s noted that nearly 30 cases per year were observed for every 1000 HIV-infected adults.12
Zoster tends to occur in patients with CD4+ counts >200 mm3. If HIV is not diagnosed when a patient presents with zoster, it may be several years before the CD4+ T-cell count declines to a level at which the patient will experience an opportunistic infection or malignancy. A diagnosis of herpes zoster should prompt you to consider HIV and test for infection, even in patients who do not have risk factors associated with HIV, as was the case with Ms. K.
Cryptococcal meningitis. Infections caused by Cryptococcus neoformans are now relatively infrequent in the United States but remain a major cause of AIDS-related morbidity and mortality in the developing world.13 Symptoms of cryptococcal meningitis, such as those observed in Mr. L, usually begin in an indolent fashion over one to 2 weeks. The most common presenting symptoms are fever, headache, and malaise. Nuchal rigidity, photophobia, and vomiting occur in only about 25% of patients.13 Mortality remains high for this infection if it is not treated aggressively.
Implement routine HIV screening, avoid “framing bias”
Prompt diagnosis of HIV infection is essential for several reasons. For one, it lowers the risk of life-threatening opportunistic infections and malignancies. For another, it can help to prevent transmission of HIV infection to partners and contacts.
Historically, HIV testing had been considered primarily for individuals with certain high-risk factors that increase their likelihood of infection (TABLE 3). However, in 2006, recognizing that risk-based testing failed to identify a significant number of people with HIV, the CDC began to recommend opt-out routine HIV screening for all adolescents and adults ages 13 to 64 years.14 In November 2012, the US Preventive Services Task Force issued similar recommendations.15
In fact, routine screening would have likely led to earlier identification of HIV in 2 of the 3 patients in the cases described here. However, only 54% of US adults ages 18 to 64 years report ever having been tested for HIV, and among the 1.1 million people living with HIV/AIDS in the United States, approximately 15% do not know they are infected.16
Physicians are frequently subject to “framing bias” in which diagnostic capabilities are limited to how we perceive individual patients. Finn et al11 reported a case of a 65-year-old “grandfather” with COPD who was eventually diagnosed with Pneumocystis jirovecii pneumonia and subsequently found to be HIV-infected, with a CD4+ T-cell count of 5 cells/mm3. A similar case involving an 81-year-old patient was reported in the literature in 2009 and raised the question of whether patients older than the currently recommended age of 64 years should also undergo routine screening for HIV.17
The 3 patients described here illustrate a similar framing bias in that none of the physicians who cared for them in an outpatient setting considered their patient to be at risk for HIV infection.
To avoid this type of bias, we must remain vigilant in assessing risk factors for HIV infection while obtaining a patient’s medical history. However, even under ideal circumstances, our patients may not be forthcoming about their sexual behavior or drug use. Moreover, many others may be unaware that they were exposed to HIV. Consequently, FPs and other primary care providers should continue to incorporate routine HIV screening into their practices but also remember specific HIV risk factors and clinical indicators of disease.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Lancaster General Hospital Comprehensive Care for HIV, 554 North Duke Street, 3rd Floor, Lancaster, PA 17602; [email protected]
1. AIDS.gov. CD4 count. AIDS.gov Web site. Available at: http://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/understand-your-test-results/cd4-count/. Accessed December 9, 2014.
2. International Association of Providers of AIDS Care. CD4 cell tests. AIDS InfoNet Web site. Available at: http://www.aidsinfonet.org/uploaded/factsheets/13_eng_124.pdf. Accessed December 9, 2014.
3. Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013; 64:183-189.
4. Centers for Disease Control and Prevention (CDC). Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009;58:661-665.
5. Chin T, Hicks C, Samsa G, et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS. 2013; 27:392-397.
6. Northfelt DW. Hematologic manifestations of HIV. University of California San Francisco HIV Insite Web site. Available at: http://hivinsite.ucsf.edu/InSite?page=kb-00&doc=kb-04-01-09. Accessed December 10, 2014.
7. Damery S, Nichols L, Holder R, et al. Assessing the predictive value of HIV indicator conditions in general practice: a case-control study using the THIN database. Br J Gen Pract. 2013;63:e370-e377.
8. Morris L, Distenfeld A, Amorosi E, et al. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96(6 pt 1):714-717.
9. Friel TJ, Scadden DT. Hematologic manifestations of HIV infection: Anemia. UpToDate Web site. Available at: http://www.uptodate.com/contents/hematologic-manifestations-of-hiv-infection-anemia. Accessed December 10, 2014.
10. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011; 32:775-782.
11. Finn KM, Ginns LC, Robbins GK, et al. Case records of the Massachusetts General Hospital. Case 20-2014. A 65-year-old man with dyspnea and progressively worsening lung disease. N Engl J Med. 2014; 370:2521-2530.
12. Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992; 166:1153-1156.
13. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. 2014; 6:169-182.
14. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1-17.
15. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
16. The Henry J. Kaiser Family Foundation. HIV testing in the United States. The Henry J. Kaiser Family Foundation Web site. Available at: http://kff.org/hivaids/fact-sheet/hiv-testing-in-the-united-states/. Accessed December 2, 2014.
17. Stone VE, Bounds BC, Muse VV, et al. Case records of the Massachusetts General Hospital. Case 29-2009. An 81-year-old man with weight loss, odynophagia, and failure to thrive. N Engl J Med. 2009; 361:1189-1198.
› Rule out human immunodeficiency virus (HIV) infection when evaluating a patient for thrombocytopenia. A
› Consider HIV testing in patients with herpes zoster, even for those who do not have risk factors for HIV. B
› Recognize that fatigue, weight loss, unexplained rashes, and hematologic disorders are some of ways in which a patient with HIV infection may present. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Roberta K, age 35, was referred by her family physician (FP) to a hematologist in November 2007 after her FP noted a platelet count of 63,000/mcL on a screening complete blood count (CBC; normal, 150,000-400,000/mcL). Ms. K also had asthma, hypothyroidism, depression, and migraine headaches. She was given a diagnosis of idiopathic thrombocytopenic purpura and started on oral prednisone. Her platelet count improved and she was maintained on prednisone 7.5 to 10 mg/d over the next 5 years with periodic dosage increases whenever her platelet count dropped below 50,000/mcL. She saw her FP for regular medical care 3 to 4 times a year and by a hematologist every 6 months.
In April 2012, Ms. K sought treatment from her FP for an acute painful rash consistent with herpes zoster involving the left C5-C6 dermatomes. Due to severe pain and secondary infection, she was admitted to the hospital. During the hospitalization, the inpatient team caring for her obtained a human immunodeficiency virus (HIV) serology, which was positive. Her only HIV risk factor was that she’d had 3 lifetime male sex partners.
Ms. K’s initial CD4+ T-cell count was 224 cells/mm3 (normal, nonimmunocompromised adult, 500–1,2001) and her percentage of CD4+ T-cells was 21% (normal, 30%-60%2). Her HIV RNA level was 71,587 copies/mL; the goal of HIV treatment typically is to get this down to <200 copies/mL. She was started on antiretroviral therapy (ART) consisting of fixed-dose emtricitabine/rilpivirine/
tenofovir (200 mg/25 mg/300 mg) and was weaned off prednisone. Six months after starting ART, her CD4+ T-cell count was 450 cells/mm3 and her HIV RNA level was <20 copies/mL. Her most recent platelet count was 148,000/mcL.
The correct diagnosis: Thrombocytopenia secondary to HIV infection.
CASE 2 › Christian M, age 40, presented to his FP in February 2010 with worsening cough and shortness of breath that he’d had for 4 weeks. He said he had unintentionally lost 20 pounds since the beginning of the year. He had no medical history of note, but had seen his FP on several occasions over the past few years for treatment of acute minor illnesses and an employment physical. He’d had no occupational exposures that might have affected his lungs, and he did not smoke.
He was initially diagnosed with bronchitis and treated with an oral antibiotic. Two weeks later, his symptoms persisted and Mr. M’s FP referred him to a pulmonologist. A chest x-ray showed an “interstitial process possibly consistent with pneumonia” for which the pulmonologist prescribed levofloxacin and oral prednisone for 10 days. At the follow-up visit, Mr. M had clinically improved. The diagnosis noted by the pulmonologist was “probably viral vs atypical pneumonia.”
Approximately 3 weeks later, in April 2010, Mr. M presented to the emergency department (ED) after several days of fever, cough, and worsening shortness of breath. A chest x-ray showed an interstitial pneumonitis that had worsened since the prior radiography. His pulse oximetry was 87% on room air.
A computed tomography (CT) scan of the chest revealed bilateral ground-glass opacities. The patient was admitted to the hospital and the next day underwent bronchoscopy with bronchoalveolar lavage. A Gomori methenamine silver stain for Pneumocystis jirovecii was positive, as was an HIV serology. Mr. M’s only reported risk factor for HIV was heterosexual contact. He had been in a stable relationship for over 14 years.
His baseline CD4+ T-cell count was 5 cells/mm3 (1%) and his HIV RNA level was >500,000 copies/mL. Several weeks later, Mr. M’s spouse tested positive for HIV. Her CD4+ T-cell count was 45 cells/mm3 (10%) and her viral load was 23,258 copies/mL. Although she was asymptomatic at the time of diagnosis, Ms. M was soon started on the same ART regimen as her husband.
The correct diagnosis: Pneumocystis pneumonia with symptoms of acquired immunodeficiency syndrome (AIDS) wasting syndrome.
CASE 3 › Michael L, age 66, was seen by his FP in September 2010 for “preoperative clearance” for elbow surgery. He was in good health but had a platelet count of 67,000/mcL. For unclear reasons, the surgery was cancelled; Mr. L was supposed to be referred to a hematologist for the thrombocytopenia, but this consultation never occurred. The patient did not return to his FP until April 2012, when he complained of feeling “lightheaded and dizzy” for the past few weeks. His examination was remarkable only for mild orthostatic hypotension and he was diagnosed with “dehydration.”
He returned to the office in July 2012 with similar symptoms and a 12-pound weight loss since his last visit. He also complained of short-term memory problems. Lab testing was done and included a chemistry panel, thyroid-stimulating hormone test, and CBC, all of which were normal except for a hemoglobin of 11.1 g/dL, a white blood cell count of 2.4/mcL, and a platelet count of 119,000/mcL. The patient was advised to get a follow-up CBC in one month, but this was not done.
Mr. L returned in November 2012, again complaining of intermittent lightheadedness and fatigue, and said he had been experiencing “mouth sores.” He was given a diagnosis of “probable oral herpes infection” and treated with oral acyclovir. No lab studies were performed.
Mr. L was brought to the ED in February 2013 with fever and mental status changes that had developed over 2 to 3 days. According to a family member, he had also complained of headache for the previous 2 weeks.
A CT scan of his head was normal and he underwent a lumbar puncture. Cerebrospinal fluid revealed a white blood cell count of 270/mcL, glucose of 62 mg/dL, and protein of 15 mg/dL. A gram stain was negative, but an India ink stain was positive for encapsulated yeast forms consistent with Cryptococcus. Mr. L was diagnosed with cryptococcal meningitis and treated with intravenous amphotericin B and oral flucytosine. An HIV serology was positive. His CD4+ T-cell count was 8 cells/mm3 (3%) and his HIV RNA level was >500,000 copies /mL.
He was discharged from the hospital after 2 weeks and transitioned to oral fluconazole 400 mg/d for the meningitis. One week after discharge, he was started on an ART regimen of darunavir 800 mg, ritonavir 100 mg, and fixed-dose tenofovir/emtricitabine (200 mg/300 mg).
After 6 months of ART, he showed significant clinical improvement, his HIV-RNA level was <20 copies/mL and his CD4+ T-cell count was 136 cells/mm3 (12%). His female partner of 11 years tested negative for HIV.
The correct diagnosis: Cryptococcal meningitis; thrombocytopenia secondary to HIV infection.
These 3 cases illustrate what clinicians who treat patients with HIV/AIDS have observed for many years: Physicians often fail to diagnose patients with HIV infection in a timely fashion. HIV can be missed when patients present with clinical signs of immune suppression, such as herpes zoster, as well as when they present with AIDS-defining illnesses such as lymphoma or recurrent pneumonia. Late diagnosis of HIV—typically defined as diagnosis when a patient’s CD4+ T-cell count is <200 cells/mm3—increases morbidity and mortality, as well as health care costs.3
Historically, late HIV testing has been very common in the United States. A Centers for Disease Control and Prevention (CDC) report noted that from 1996 to 2005, 38% of patients diagnosed in 34 states had an AIDS diagnosis within one year of testing positive for HIV.4 Chin et al5 performed a retrospective cohort study of patients seen in an HIV clinic in North Carolina between November 2008 and November 2011. The median CD4+ T-cell count at time of diagnosis was 313 cells/mm3 and one-third of patients had a count of <50 cells/mm3. Current HIV treatment guidelines recommend ART for all patients diagnosed with HIV infection regardless of CD4+ T-cell count.
The mean number of health care visits in the year before diagnosis was 2.75 (range 0-20). These visits occurred in both primary care settings and the ED. Approximately one-third of patients had complained of HIV-associated signs and symptoms, including recurrent respiratory tract infections, unexplained persistent fevers, and generalized lymphadenopathy prior to diagnosis.
FPs must remain cognizant of the many diverse clinical presentations of patients with HIV/AIDS, including fatigue, weight loss, unexplained rashes, and hematologic disorders (TABLE 16 and TABLE 27). In the 3 cases described here, the specific conditions the treatment teams failed to identify as indicators of HIV infection were thrombocytopenia, pneumocystis pneumonia, herpes zoster, and cryptococcal meningitis.
Thrombocytopenia has many causes, including infection, medications, lymphoproliferative disorders, liver disease, and connective tissue diseases. However, low platelet counts are often seen in individuals with HIV infection.
Before the introduction of ART, the incidence of thrombocytopenia in HIV patients was 40%.8 Since then, this condition is less common, but HIV should be ruled out when evaluating a patient for thrombocytopenia or making a diagnosis of “idiopathic thrombocytopenia” (as was Ms. K’s initial diagnosis).
The incidence of cytopenias in general correlates directly with the degree of immunosuppression. However, isolated hematologic abnormalities, including anemia and leukopenia, may be the initial presentation of HIV infection.9 As a result, HIV must be considered in the assessment of all patients who present with any hematologic abnormality.
Pneumocystis pneumonia. Pneumonia caused by the fungus Pneumocystis jirovecii has been a longtime AIDS-defining illness and is the most common opportunistic infection in patients with advanced HIV infection.10 A slow, indolent course is common, with symptoms of cough and dyspnea progressing over weeks to months (as observed in Mr. M). Radiographs will show diffuse or isolated ground-glass opacities. Partial improvement is sometimes seen in patients with unknown HIV infection who are treated with short courses of prednisone and antibiotics.11 Patients with untreated HIV infection and CD4+ T-cell counts <200 cells/mm3 will develop worsening hypoxemia and, in some cases, fulminant respiratory failure.
Herpes zoster is common in older adults and often indicates a weakened immune system. The incidence of zoster among adults with HIV is more than 15-fold higher than it is among age-matched varicella-zoster virus-infected immunocompetent people.12 A study from the early 1990s noted that nearly 30 cases per year were observed for every 1000 HIV-infected adults.12
Zoster tends to occur in patients with CD4+ counts >200 mm3. If HIV is not diagnosed when a patient presents with zoster, it may be several years before the CD4+ T-cell count declines to a level at which the patient will experience an opportunistic infection or malignancy. A diagnosis of herpes zoster should prompt you to consider HIV and test for infection, even in patients who do not have risk factors associated with HIV, as was the case with Ms. K.
Cryptococcal meningitis. Infections caused by Cryptococcus neoformans are now relatively infrequent in the United States but remain a major cause of AIDS-related morbidity and mortality in the developing world.13 Symptoms of cryptococcal meningitis, such as those observed in Mr. L, usually begin in an indolent fashion over one to 2 weeks. The most common presenting symptoms are fever, headache, and malaise. Nuchal rigidity, photophobia, and vomiting occur in only about 25% of patients.13 Mortality remains high for this infection if it is not treated aggressively.
Implement routine HIV screening, avoid “framing bias”
Prompt diagnosis of HIV infection is essential for several reasons. For one, it lowers the risk of life-threatening opportunistic infections and malignancies. For another, it can help to prevent transmission of HIV infection to partners and contacts.
Historically, HIV testing had been considered primarily for individuals with certain high-risk factors that increase their likelihood of infection (TABLE 3). However, in 2006, recognizing that risk-based testing failed to identify a significant number of people with HIV, the CDC began to recommend opt-out routine HIV screening for all adolescents and adults ages 13 to 64 years.14 In November 2012, the US Preventive Services Task Force issued similar recommendations.15
In fact, routine screening would have likely led to earlier identification of HIV in 2 of the 3 patients in the cases described here. However, only 54% of US adults ages 18 to 64 years report ever having been tested for HIV, and among the 1.1 million people living with HIV/AIDS in the United States, approximately 15% do not know they are infected.16
Physicians are frequently subject to “framing bias” in which diagnostic capabilities are limited to how we perceive individual patients. Finn et al11 reported a case of a 65-year-old “grandfather” with COPD who was eventually diagnosed with Pneumocystis jirovecii pneumonia and subsequently found to be HIV-infected, with a CD4+ T-cell count of 5 cells/mm3. A similar case involving an 81-year-old patient was reported in the literature in 2009 and raised the question of whether patients older than the currently recommended age of 64 years should also undergo routine screening for HIV.17
The 3 patients described here illustrate a similar framing bias in that none of the physicians who cared for them in an outpatient setting considered their patient to be at risk for HIV infection.
To avoid this type of bias, we must remain vigilant in assessing risk factors for HIV infection while obtaining a patient’s medical history. However, even under ideal circumstances, our patients may not be forthcoming about their sexual behavior or drug use. Moreover, many others may be unaware that they were exposed to HIV. Consequently, FPs and other primary care providers should continue to incorporate routine HIV screening into their practices but also remember specific HIV risk factors and clinical indicators of disease.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Lancaster General Hospital Comprehensive Care for HIV, 554 North Duke Street, 3rd Floor, Lancaster, PA 17602; [email protected]
› Rule out human immunodeficiency virus (HIV) infection when evaluating a patient for thrombocytopenia. A
› Consider HIV testing in patients with herpes zoster, even for those who do not have risk factors for HIV. B
› Recognize that fatigue, weight loss, unexplained rashes, and hematologic disorders are some of ways in which a patient with HIV infection may present. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE 1 › Roberta K, age 35, was referred by her family physician (FP) to a hematologist in November 2007 after her FP noted a platelet count of 63,000/mcL on a screening complete blood count (CBC; normal, 150,000-400,000/mcL). Ms. K also had asthma, hypothyroidism, depression, and migraine headaches. She was given a diagnosis of idiopathic thrombocytopenic purpura and started on oral prednisone. Her platelet count improved and she was maintained on prednisone 7.5 to 10 mg/d over the next 5 years with periodic dosage increases whenever her platelet count dropped below 50,000/mcL. She saw her FP for regular medical care 3 to 4 times a year and by a hematologist every 6 months.
In April 2012, Ms. K sought treatment from her FP for an acute painful rash consistent with herpes zoster involving the left C5-C6 dermatomes. Due to severe pain and secondary infection, she was admitted to the hospital. During the hospitalization, the inpatient team caring for her obtained a human immunodeficiency virus (HIV) serology, which was positive. Her only HIV risk factor was that she’d had 3 lifetime male sex partners.
Ms. K’s initial CD4+ T-cell count was 224 cells/mm3 (normal, nonimmunocompromised adult, 500–1,2001) and her percentage of CD4+ T-cells was 21% (normal, 30%-60%2). Her HIV RNA level was 71,587 copies/mL; the goal of HIV treatment typically is to get this down to <200 copies/mL. She was started on antiretroviral therapy (ART) consisting of fixed-dose emtricitabine/rilpivirine/
tenofovir (200 mg/25 mg/300 mg) and was weaned off prednisone. Six months after starting ART, her CD4+ T-cell count was 450 cells/mm3 and her HIV RNA level was <20 copies/mL. Her most recent platelet count was 148,000/mcL.
The correct diagnosis: Thrombocytopenia secondary to HIV infection.
CASE 2 › Christian M, age 40, presented to his FP in February 2010 with worsening cough and shortness of breath that he’d had for 4 weeks. He said he had unintentionally lost 20 pounds since the beginning of the year. He had no medical history of note, but had seen his FP on several occasions over the past few years for treatment of acute minor illnesses and an employment physical. He’d had no occupational exposures that might have affected his lungs, and he did not smoke.
He was initially diagnosed with bronchitis and treated with an oral antibiotic. Two weeks later, his symptoms persisted and Mr. M’s FP referred him to a pulmonologist. A chest x-ray showed an “interstitial process possibly consistent with pneumonia” for which the pulmonologist prescribed levofloxacin and oral prednisone for 10 days. At the follow-up visit, Mr. M had clinically improved. The diagnosis noted by the pulmonologist was “probably viral vs atypical pneumonia.”
Approximately 3 weeks later, in April 2010, Mr. M presented to the emergency department (ED) after several days of fever, cough, and worsening shortness of breath. A chest x-ray showed an interstitial pneumonitis that had worsened since the prior radiography. His pulse oximetry was 87% on room air.
A computed tomography (CT) scan of the chest revealed bilateral ground-glass opacities. The patient was admitted to the hospital and the next day underwent bronchoscopy with bronchoalveolar lavage. A Gomori methenamine silver stain for Pneumocystis jirovecii was positive, as was an HIV serology. Mr. M’s only reported risk factor for HIV was heterosexual contact. He had been in a stable relationship for over 14 years.
His baseline CD4+ T-cell count was 5 cells/mm3 (1%) and his HIV RNA level was >500,000 copies/mL. Several weeks later, Mr. M’s spouse tested positive for HIV. Her CD4+ T-cell count was 45 cells/mm3 (10%) and her viral load was 23,258 copies/mL. Although she was asymptomatic at the time of diagnosis, Ms. M was soon started on the same ART regimen as her husband.
The correct diagnosis: Pneumocystis pneumonia with symptoms of acquired immunodeficiency syndrome (AIDS) wasting syndrome.
CASE 3 › Michael L, age 66, was seen by his FP in September 2010 for “preoperative clearance” for elbow surgery. He was in good health but had a platelet count of 67,000/mcL. For unclear reasons, the surgery was cancelled; Mr. L was supposed to be referred to a hematologist for the thrombocytopenia, but this consultation never occurred. The patient did not return to his FP until April 2012, when he complained of feeling “lightheaded and dizzy” for the past few weeks. His examination was remarkable only for mild orthostatic hypotension and he was diagnosed with “dehydration.”
He returned to the office in July 2012 with similar symptoms and a 12-pound weight loss since his last visit. He also complained of short-term memory problems. Lab testing was done and included a chemistry panel, thyroid-stimulating hormone test, and CBC, all of which were normal except for a hemoglobin of 11.1 g/dL, a white blood cell count of 2.4/mcL, and a platelet count of 119,000/mcL. The patient was advised to get a follow-up CBC in one month, but this was not done.
Mr. L returned in November 2012, again complaining of intermittent lightheadedness and fatigue, and said he had been experiencing “mouth sores.” He was given a diagnosis of “probable oral herpes infection” and treated with oral acyclovir. No lab studies were performed.
Mr. L was brought to the ED in February 2013 with fever and mental status changes that had developed over 2 to 3 days. According to a family member, he had also complained of headache for the previous 2 weeks.
A CT scan of his head was normal and he underwent a lumbar puncture. Cerebrospinal fluid revealed a white blood cell count of 270/mcL, glucose of 62 mg/dL, and protein of 15 mg/dL. A gram stain was negative, but an India ink stain was positive for encapsulated yeast forms consistent with Cryptococcus. Mr. L was diagnosed with cryptococcal meningitis and treated with intravenous amphotericin B and oral flucytosine. An HIV serology was positive. His CD4+ T-cell count was 8 cells/mm3 (3%) and his HIV RNA level was >500,000 copies /mL.
He was discharged from the hospital after 2 weeks and transitioned to oral fluconazole 400 mg/d for the meningitis. One week after discharge, he was started on an ART regimen of darunavir 800 mg, ritonavir 100 mg, and fixed-dose tenofovir/emtricitabine (200 mg/300 mg).
After 6 months of ART, he showed significant clinical improvement, his HIV-RNA level was <20 copies/mL and his CD4+ T-cell count was 136 cells/mm3 (12%). His female partner of 11 years tested negative for HIV.
The correct diagnosis: Cryptococcal meningitis; thrombocytopenia secondary to HIV infection.
These 3 cases illustrate what clinicians who treat patients with HIV/AIDS have observed for many years: Physicians often fail to diagnose patients with HIV infection in a timely fashion. HIV can be missed when patients present with clinical signs of immune suppression, such as herpes zoster, as well as when they present with AIDS-defining illnesses such as lymphoma or recurrent pneumonia. Late diagnosis of HIV—typically defined as diagnosis when a patient’s CD4+ T-cell count is <200 cells/mm3—increases morbidity and mortality, as well as health care costs.3
Historically, late HIV testing has been very common in the United States. A Centers for Disease Control and Prevention (CDC) report noted that from 1996 to 2005, 38% of patients diagnosed in 34 states had an AIDS diagnosis within one year of testing positive for HIV.4 Chin et al5 performed a retrospective cohort study of patients seen in an HIV clinic in North Carolina between November 2008 and November 2011. The median CD4+ T-cell count at time of diagnosis was 313 cells/mm3 and one-third of patients had a count of <50 cells/mm3. Current HIV treatment guidelines recommend ART for all patients diagnosed with HIV infection regardless of CD4+ T-cell count.
The mean number of health care visits in the year before diagnosis was 2.75 (range 0-20). These visits occurred in both primary care settings and the ED. Approximately one-third of patients had complained of HIV-associated signs and symptoms, including recurrent respiratory tract infections, unexplained persistent fevers, and generalized lymphadenopathy prior to diagnosis.
FPs must remain cognizant of the many diverse clinical presentations of patients with HIV/AIDS, including fatigue, weight loss, unexplained rashes, and hematologic disorders (TABLE 16 and TABLE 27). In the 3 cases described here, the specific conditions the treatment teams failed to identify as indicators of HIV infection were thrombocytopenia, pneumocystis pneumonia, herpes zoster, and cryptococcal meningitis.
Thrombocytopenia has many causes, including infection, medications, lymphoproliferative disorders, liver disease, and connective tissue diseases. However, low platelet counts are often seen in individuals with HIV infection.
Before the introduction of ART, the incidence of thrombocytopenia in HIV patients was 40%.8 Since then, this condition is less common, but HIV should be ruled out when evaluating a patient for thrombocytopenia or making a diagnosis of “idiopathic thrombocytopenia” (as was Ms. K’s initial diagnosis).
The incidence of cytopenias in general correlates directly with the degree of immunosuppression. However, isolated hematologic abnormalities, including anemia and leukopenia, may be the initial presentation of HIV infection.9 As a result, HIV must be considered in the assessment of all patients who present with any hematologic abnormality.
Pneumocystis pneumonia. Pneumonia caused by the fungus Pneumocystis jirovecii has been a longtime AIDS-defining illness and is the most common opportunistic infection in patients with advanced HIV infection.10 A slow, indolent course is common, with symptoms of cough and dyspnea progressing over weeks to months (as observed in Mr. M). Radiographs will show diffuse or isolated ground-glass opacities. Partial improvement is sometimes seen in patients with unknown HIV infection who are treated with short courses of prednisone and antibiotics.11 Patients with untreated HIV infection and CD4+ T-cell counts <200 cells/mm3 will develop worsening hypoxemia and, in some cases, fulminant respiratory failure.
Herpes zoster is common in older adults and often indicates a weakened immune system. The incidence of zoster among adults with HIV is more than 15-fold higher than it is among age-matched varicella-zoster virus-infected immunocompetent people.12 A study from the early 1990s noted that nearly 30 cases per year were observed for every 1000 HIV-infected adults.12
Zoster tends to occur in patients with CD4+ counts >200 mm3. If HIV is not diagnosed when a patient presents with zoster, it may be several years before the CD4+ T-cell count declines to a level at which the patient will experience an opportunistic infection or malignancy. A diagnosis of herpes zoster should prompt you to consider HIV and test for infection, even in patients who do not have risk factors associated with HIV, as was the case with Ms. K.
Cryptococcal meningitis. Infections caused by Cryptococcus neoformans are now relatively infrequent in the United States but remain a major cause of AIDS-related morbidity and mortality in the developing world.13 Symptoms of cryptococcal meningitis, such as those observed in Mr. L, usually begin in an indolent fashion over one to 2 weeks. The most common presenting symptoms are fever, headache, and malaise. Nuchal rigidity, photophobia, and vomiting occur in only about 25% of patients.13 Mortality remains high for this infection if it is not treated aggressively.
Implement routine HIV screening, avoid “framing bias”
Prompt diagnosis of HIV infection is essential for several reasons. For one, it lowers the risk of life-threatening opportunistic infections and malignancies. For another, it can help to prevent transmission of HIV infection to partners and contacts.
Historically, HIV testing had been considered primarily for individuals with certain high-risk factors that increase their likelihood of infection (TABLE 3). However, in 2006, recognizing that risk-based testing failed to identify a significant number of people with HIV, the CDC began to recommend opt-out routine HIV screening for all adolescents and adults ages 13 to 64 years.14 In November 2012, the US Preventive Services Task Force issued similar recommendations.15
In fact, routine screening would have likely led to earlier identification of HIV in 2 of the 3 patients in the cases described here. However, only 54% of US adults ages 18 to 64 years report ever having been tested for HIV, and among the 1.1 million people living with HIV/AIDS in the United States, approximately 15% do not know they are infected.16
Physicians are frequently subject to “framing bias” in which diagnostic capabilities are limited to how we perceive individual patients. Finn et al11 reported a case of a 65-year-old “grandfather” with COPD who was eventually diagnosed with Pneumocystis jirovecii pneumonia and subsequently found to be HIV-infected, with a CD4+ T-cell count of 5 cells/mm3. A similar case involving an 81-year-old patient was reported in the literature in 2009 and raised the question of whether patients older than the currently recommended age of 64 years should also undergo routine screening for HIV.17
The 3 patients described here illustrate a similar framing bias in that none of the physicians who cared for them in an outpatient setting considered their patient to be at risk for HIV infection.
To avoid this type of bias, we must remain vigilant in assessing risk factors for HIV infection while obtaining a patient’s medical history. However, even under ideal circumstances, our patients may not be forthcoming about their sexual behavior or drug use. Moreover, many others may be unaware that they were exposed to HIV. Consequently, FPs and other primary care providers should continue to incorporate routine HIV screening into their practices but also remember specific HIV risk factors and clinical indicators of disease.
CORRESPONDENCE
Jeffrey T. Kirchner, DO, FAAFP, AAHIVS, Lancaster General Hospital Comprehensive Care for HIV, 554 North Duke Street, 3rd Floor, Lancaster, PA 17602; [email protected]
1. AIDS.gov. CD4 count. AIDS.gov Web site. Available at: http://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/understand-your-test-results/cd4-count/. Accessed December 9, 2014.
2. International Association of Providers of AIDS Care. CD4 cell tests. AIDS InfoNet Web site. Available at: http://www.aidsinfonet.org/uploaded/factsheets/13_eng_124.pdf. Accessed December 9, 2014.
3. Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013; 64:183-189.
4. Centers for Disease Control and Prevention (CDC). Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009;58:661-665.
5. Chin T, Hicks C, Samsa G, et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS. 2013; 27:392-397.
6. Northfelt DW. Hematologic manifestations of HIV. University of California San Francisco HIV Insite Web site. Available at: http://hivinsite.ucsf.edu/InSite?page=kb-00&doc=kb-04-01-09. Accessed December 10, 2014.
7. Damery S, Nichols L, Holder R, et al. Assessing the predictive value of HIV indicator conditions in general practice: a case-control study using the THIN database. Br J Gen Pract. 2013;63:e370-e377.
8. Morris L, Distenfeld A, Amorosi E, et al. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96(6 pt 1):714-717.
9. Friel TJ, Scadden DT. Hematologic manifestations of HIV infection: Anemia. UpToDate Web site. Available at: http://www.uptodate.com/contents/hematologic-manifestations-of-hiv-infection-anemia. Accessed December 10, 2014.
10. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011; 32:775-782.
11. Finn KM, Ginns LC, Robbins GK, et al. Case records of the Massachusetts General Hospital. Case 20-2014. A 65-year-old man with dyspnea and progressively worsening lung disease. N Engl J Med. 2014; 370:2521-2530.
12. Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992; 166:1153-1156.
13. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. 2014; 6:169-182.
14. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1-17.
15. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
16. The Henry J. Kaiser Family Foundation. HIV testing in the United States. The Henry J. Kaiser Family Foundation Web site. Available at: http://kff.org/hivaids/fact-sheet/hiv-testing-in-the-united-states/. Accessed December 2, 2014.
17. Stone VE, Bounds BC, Muse VV, et al. Case records of the Massachusetts General Hospital. Case 29-2009. An 81-year-old man with weight loss, odynophagia, and failure to thrive. N Engl J Med. 2009; 361:1189-1198.
1. AIDS.gov. CD4 count. AIDS.gov Web site. Available at: http://www.aids.gov/hiv-aids-basics/just-diagnosed-with-hiv-aids/understand-your-test-results/cd4-count/. Accessed December 9, 2014.
2. International Association of Providers of AIDS Care. CD4 cell tests. AIDS InfoNet Web site. Available at: http://www.aidsinfonet.org/uploaded/factsheets/13_eng_124.pdf. Accessed December 9, 2014.
3. Farnham PG, Gopalappa C, Sansom SL, et al. Updates of lifetime costs of care and quality-of-life estimates for HIV-infected persons in the United States: late versus early diagnosis and entry into care. J Acquir Immune Defic Syndr. 2013; 64:183-189.
4. Centers for Disease Control and Prevention (CDC). Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009;58:661-665.
5. Chin T, Hicks C, Samsa G, et al. Diagnosing HIV infection in primary care settings: missed opportunities. AIDS Patient Care STDS. 2013; 27:392-397.
6. Northfelt DW. Hematologic manifestations of HIV. University of California San Francisco HIV Insite Web site. Available at: http://hivinsite.ucsf.edu/InSite?page=kb-00&doc=kb-04-01-09. Accessed December 10, 2014.
7. Damery S, Nichols L, Holder R, et al. Assessing the predictive value of HIV indicator conditions in general practice: a case-control study using the THIN database. Br J Gen Pract. 2013;63:e370-e377.
8. Morris L, Distenfeld A, Amorosi E, et al. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96(6 pt 1):714-717.
9. Friel TJ, Scadden DT. Hematologic manifestations of HIV infection: Anemia. UpToDate Web site. Available at: http://www.uptodate.com/contents/hematologic-manifestations-of-hiv-infection-anemia. Accessed December 10, 2014.
10. Gilroy SA, Bennett NJ. Pneumocystis pneumonia. Semin Respir Crit Care Med. 2011; 32:775-782.
11. Finn KM, Ginns LC, Robbins GK, et al. Case records of the Massachusetts General Hospital. Case 20-2014. A 65-year-old man with dyspnea and progressively worsening lung disease. N Engl J Med. 2014; 370:2521-2530.
12. Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992; 166:1153-1156.
13. Sloan DJ, Parris V. Cryptococcal meningitis: epidemiology and therapeutic options. Clin Epidemiol. 2014; 6:169-182.
14. Branson BM, Handsfield HH, Lampe MA, et al; Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1-17.
15. Moyer VA; U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51-60.
16. The Henry J. Kaiser Family Foundation. HIV testing in the United States. The Henry J. Kaiser Family Foundation Web site. Available at: http://kff.org/hivaids/fact-sheet/hiv-testing-in-the-united-states/. Accessed December 2, 2014.
17. Stone VE, Bounds BC, Muse VV, et al. Case records of the Massachusetts General Hospital. Case 29-2009. An 81-year-old man with weight loss, odynophagia, and failure to thrive. N Engl J Med. 2009; 361:1189-1198.
Addressing Alzheimer’s: A pragmatic approach
› Refer patients for formal neuropsychological testing when dementia is suspected but the history, clinical interview, and brief cognitive tests do not result in a definitive diagnosis. C
› Use non-drug therapies as first-line treatment for behavioral symptoms of Alzheimer’s disease (AD), as the adverse effects of drug therapy generally offset any benefit. B
› Recommend against feeding tubes for patients with late-stage AD as they are more apt to cause discomfort than to provide benefit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Alzheimer’s disease (AD), the most common form of dementia, affects more than 5 million Americans.1 Estimates suggest that by 2050, the prevalence could triple, reaching 13 to 16 million.1 To effectively care for patients with AD and their families, family physicians need to be familiar with the latest evidence on all facets of care, from initial detection to patient management and end-of-life care.
This evidence-based review will help you toward that end by answering common questions regarding Alzheimer’s care, including whether routine screening is advisable, what tests should be ordered, which interventions (including nonpharmacologic options) are worth considering, and how best to counsel patients and families about end-of-life care.
Routine screening? Still subject to debate
In considering routine dementia screening in primary care, the key question is whether screening improves outcomes. Advocates note that individuals with dementia may appear unimpaired during office visits and may not report symptoms due to lack of insight; they point out, too, that waiting for an event that makes cognitive impairment obvious, such as a driving mishap, is risky.2 Those who advocate routine screening also note that only about half of those who have dementia are ever diagnosed.3
Others, including the US Preventive Services Task Force (USPSTF), disagree. In its 2014 evidence review, the USPSTF indicated that there is “insufficient evidence to assess the balance of benefits and harms of screening for cognitive impairment in older adults.”4
Mixed messages
The dearth of evidence is also reflected in the conflicting recommendations of the Affordable Care Act (ACA) and the Centers for Medicare and Medicaid Services (CMS). The ACA requires physicians to assess the cognitive function of Medicare patients during their annual wellness visits. CMS, however, instructs providers to screen for dementia only if observation or concerns raised by the patient or family suggest the possibility of impairment, and does not recommend any particular test.5
Cost-effectiveness analyses raise questions about the value of routine screening, as well. Evidence suggests that if a primary care physician screens 300 older patients, 39 will have a positive screen. But only about half of those 39 will agree to a diagnostic evaluation, and no more than 9 will ultimately be diagnosed with dementia. The estimated cost of identifying 9 cases is nearly $40,000—all in the absence of a treatment to cure or stop the progression of the disorder.6
The bottom line: Evidence does not support routine dementia screening of older adults. When cognitive impairment is suspected, however, physicians should conduct a diagnostic evaluation—and consider educating patients and families about the Alzheimer’s Association (AA)’s 10 warning signs of AD.7 (See “Is it Alzheimer’s? 10 warning signs”7 below.) A longer version, available at http://www.alz.org/national/documents/checklist_10signs.pdf, outlines the cognitive changes that are characteristic of healthy aging and compares them to changes suggestive of early dementia.7
Is it Alzheimer’s? 10 warning signs7 1. Memory loss that disrupts daily life |
How to proceed when you suspect AD
Step 1: Screening instrument. The first step in the diagnostic evaluation of a patient with suspected AD is to determine if, in fact, cognitive impairment is present. This can be done by screening with in-office screening instruments, such as the Mini-Cog (available at alz.org/documents_custom/minicog.pdf) or Mini-Mental State Examination (MMSE; health.gov.bc.ca/pharmacare/adti/clinician/pdf/ADTI%20SMMSE-GDS%20Reference%20Card.pdf), among others.8
Step 2: Clinical evaluation. If observation and test results suggest cognitive impairment, the next step is to determine whether clinical findings are consistent with the diagnostic criteria for AD (TABLE 1)9 developed by workgroups from the National Institute on Aging (NIA)/AA in 2011. A work-up is necessary to identify conditions that can mimic dementia (eg, depression) and behaviors that suggest another type of dementia, such as frontotemporal or Lewy body dementia.10 Lab testing should be included to rule out potentially reversible causes of cognitive dysfunction (eg, hypothyroidism, vitamin D deficiency).
Step 3: Neuropsychological evaluation. The NIA/AA recommends neuropsychological testing when the brief cognitive tests, history, and clinical work-up are not sufficient for a definitive diagnosis of dementia.9This generally involves a referral to a neuropsychologist, who conducts a battery of standardized tests to evaluate attention, memory, language, visual-spatial abilities, and executive functions, among others. Neuropsychological testing can confirm the presence of cognitive impairment and aid in the differential diagnosis by comparing the patient’s performance in these domains with characteristic features of different dementia syndromes.
Step 4: Brain imaging with either computed tomography or magnetic resonance imaging can be included in the work-up for patients with suspected AD to rule out abnormalities—eg, metastatic cancer, hydrocephalus, or occult chronic subdural hematoma—that could be causing cognitive impairment.9,10 Clinical features that generally warrant brain imaging include onset of cognitive impairment before age 60; unexplained focal neurologic signs or symptoms; abrupt onset or rapid decline; and/or predisposing conditions, such as cancer or anticoagulant treatment.10
The role of biomarkers and advanced brain imaging
Biomarkers that might provide confirmation of AD in patients who exhibit early symptoms of dementia have been studied extensively.11 The NIA/AA identified 2 categories of AD biomarkers:
- tests for β-amyloid deposition in the brain, including spinal fluid assays for β-amyloid (Aβ42) and positron emission tomography (PET) scans after intravenous injection of florbetapir or flutemetamol, which bind to amyloid in the brain; and
- tests for neuronal degeneration, which would include spinal fluid assays for tau protein and PET scans after injection of fluorodeoxyglucose (FDG), which shows decreased uptake in patients with AD.9
Research reveals the promise of these biomarkers as diagnostic tools, particularly in patients with an atypical presentation of dementia or mild cognitive impairment (MCI) that may be associated with early AD.12 (More on MCI in a moment.) However, the NIA/AA concluded that additional research is needed to validate these tests for routine diagnostic purposes. Medicare covers PET scans with FDG only for the differential diagnosis of AD vs frontotemporal dementia.13
Mild cognitive impairment: How likely that it will progress?
Along with diagnostic criteria for AD, the NIA/AA developed criteria for a symptomatic predementia phase of AD—often referred to as MCI.14 According to the workgroup, MCI is diagnosed when:
2. there is evidence of cognitive impairment, ideally through psychometric testing, revealing performance below expectation based on the patient’s age and education;
3. the patient is able to maintain independent functioning in daily life, despite mild problems or the need for minimal assistance; and
4. there is no significant impairment in social or occupational functioning.14
Progression: Less likely than you might think
Patients with MCI are at risk for progression to overt dementia, with an overall annual conversion rate from MCI to dementia estimated at 10% to 15%.15,16 This estimate must be interpreted with caution, however, because most studies were conducted prior to the 2011 guidelines, when different diagnostic criteria were used. Observers have noted, too, that the numbers largely reflect data collected in specialty clinics and that community-based studies reveal substantially lower conversion rates (3%-6% per year).16 In addition, evidence suggests that many patients with MCI demonstrate long-term stability or even reversal of deficits.17
While there is some consideration of the use of biomarkers and amyloid imaging tests to help determine which patients with MCI will progress to AD, practice guidelines do not currently recommend such testing and it is not covered by Medicare.
When evidence indicates an AD diagnosis
When faced with the need to communicate an AD diagnosis, follow the general recommendations for delivering any bad news or discouraging prognosis:
Prioritize and limit the information you provide, determining not only what the patient and family want to hear, but also how much they are able to comprehend.
Confirm that the patient and family understand the information you’ve provided.
Offer emotional support and recommend additional resources18 (TABLE 2).
Given the progressive cognitive decline that characterizes AD, it is important to address the primary caregiver’s understanding of, and ability to cope with, the disease. It is also important to explore beliefs and attitudes regarding AD. Keep in mind that different cultural groups tend to differ in their beliefs about the nature, cause, and appropriate management of AD, as well as the role of spirituality, help-seeking, and stigma.19,20
The progressive and ultimately fatal nature of AD also makes planning for the future a priority. Ideally, patients should be engaged in discussions regarding end-of-life care as early as possible, while they are still able to make informed decisions and express their preferences. Discussing end-of-life care can be overwhelming for newly diagnosed patients and their families, however, so it is important that you address issues—medical, financial, and legal planning, for example—that families should be considering.
TABLE 2
| Resources for newly diagnosed patients and families | |
| Issue | Resources |
| Education | Alzheimer’s and Dementia Caregiver Center http://www.alz.org/care/overview.asp NIA Alzheimer’s Disease Education and Referral Center http://www.nia.nih.gov/alzheimers/ |
| Planning (medical, financial, legal)/benefits | AARP Caregiving Resource Center http://www.aarp.org/home-family/caregiving Alzheimer’s Association Alzheimer’s Navigator https://www.alzheimersnavigator.org/ National Council on Aging Benefits Checkup https://www.benefitscheckup.org/ |
| Safety | Association for Driver Rehabilitation Specialists: Driving and Alzheimer’s/Dementia https://c.ymcdn.com/sites/www.aded.net/resource/resmgr/fact_Sheets/ADED_alzheimers-Dementia_fac.pdf NIA’s Home Safety for People with Alzheimer’s booklet http://www.nia.nih.gov/alzheimers/publication/home-safety-people-alzheimers-disease |
| Support | Caregiver Action Network http://caregiveraction.org/ |
Drugs address cognitive and behavioral function
No currently available treatments can cure or significantly alter the progression of AD, but 2 classes of medications are used in an attempt to improve cognitive function. One is cholinesterase inhibitors (ChEIs), which potentiate acetylcholine synaptic transmission. The other is N-methyl-D-aspartate (NMDA) glutamate receptor blockers. Other classes of drugs are sometimes used to treat behavioral symptoms of dementia, such as agitation, aggression, mood disorders, and psychosis (eg, delusions and hallucinations).
Cognitive function. Results from studies of pharmacologic management of MCI vary widely, but recent reviews have found no convincing evidence that either ChEIs or NMDA receptor blockers have an effect on progression from MCI to dementia.21,22 Neither class is FDA-approved for treating MCI.
In patients with dementia, the effects of ChEIs and NMDA receptor blockers on cognition are statistically significant but modest, and often of questionable clinical relevance.23 Nonetheless, among ChEIs, donepezil is approved by the US Food and Drug Administration (FDA) for mild, moderate, and severe dementia and galantamine and rivastigmine are approved for mild and moderate dementia. There is no evidence that any one ChEI is more effective than any other,24 and the choice of drugs is often guided by cost, adverse effects, and health plan formularies. Memantine, the only FDA-approved NMDA receptor blocker, is approved for moderate to severe dementia and can be used alone or in combination with a ChEI.
If these drugs are used in an attempt to improve cognition in AD, guidelines recommend the following approach for initial therapy: Prescribe a ChEI for the mild stage, a ChEI plus memantine for the moderate stage, and memantine (with or without a ChEI) for the severe stage.25 The recommendations also include monitoring every 6 months.
There is no consensus about when to discontinue medication. Various published recommendations call for continuing treatment until the patient has “lost all cognitive and functional abilities;”22 until the patient’s MMSE score falls below 10 and there is no indication that the drug is having a “worthwhile effect;”21 or until he or she has reached stage 7 on the Reisberg Functional Assessment Staging scale, indicating nonambulatory status with speech limited to one to 5 words a day.10
Behavioral function. A variety of drugs are used to treat behavioral symptoms in AD. While not FDA-approved for this use, the most widely prescribed agents are second-generation antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone). The main effect of these drugs is often nothing more than sedation, and one large multisite clinical trial concluded that the adverse effects offset the benefits for patients with AD.26 Indeed, the FDA has issued an advisory on the use of second-generation antipsychotics in AD patients, stating that they are associated with an increased risk of death.27 The recently updated Beers Criteria strongly recommend avoiding these drugs for treating behavioral disturbances in AD unless nonpharmacologic options have failed and the patient is a threat to self or others.28
Because of the black-box warning that antipsychotics increase the risk of death, some physicians have advocated obtaining informed consent prior to prescribing such medications.29 At the very least, when family or guardians are involved, a conversation about risks vs benefits should take place and be documented in the medical record.
Other drug classes are also sometimes used in an attempt to improve behavioral function, including anti-seizure medications (valproic acid, carbamazepine), antidepressants (trazodone and selective serotonin reuptake inhibitors), and anxiolytics (benzodiazepines and buspirone). Other than their sedating effects, there is no strong evidence that these drugs are effective for treating dementia-related behavioral disorders. If used, caution is required due to potential adverse effects.
Nonpharmacologic management is “promising”
A recent systematic review of nonpharmacologic interventions for MCI evaluated exercise, training in compensatory strategies, and engagement in cognitively stimulating activities and found “promising but inconclusive” results. The researchers found that studies show mostly positive effects on cognition but have significant methodologic limitations.30 Importantly, there is no evidence of delayed or reduced conversion to dementia.
For patients who already have mild-to-moderate dementia, cognitive stimulation seems to help in the short term.31 There is also some evidence that exercise and occupational therapy may slow functional decline,32 but the effects are small to modest and their actual clinical significance (eg, the ability to delay institutionalization) is unclear. There is promising but preliminary evidence that cognitive rehabilitation (helping patients devise strategies to complete daily activities) may improve functioning in everyday life.33
While behavioral symptoms are often due to the dementia itself, it is important to identify and treat medical and environmental causes that may be contributing, such as infection, pain, and loud or unsafe environments. As noted before, nonpharmacologic treatments are generally preferred for behavioral problems and should be considered prior to drug therapy. Approaches that identify and modify both the antecedents and consequences of problem behaviors and increase pleasant events have empirical support for the management of behavioral symptoms.34 Interventions including massage therapy, aromatherapy, exercise, and music therapy may also be effective in the short term for agitated behavior.35
Caregivers should be encouraged to receive training in these strategies through organizations like AA. Caregiver education and support can reduce caregivers’ distress and increase their self-efficacy and coping skills.36
End-of-life care must be addressed
Perhaps the most important aspect of end-of-life care in AD is assuring that families (or health care proxies) understand that AD is a fatal illness, with most patients dying within 4 to 8 years of diagnosis.1 Evidence indicates that patients whose proxies have a clear recognition of this are less likely to experience “burdensome” interventions such as parenteral therapy, emergency department visits, hospital admissions, and tube feedings in their last 3 months of life.37
Overall, decisions regarding discontinuing medical treatments in advanced AD should be made by balancing the likelihood of benefit with the potential for adverse effects.38 For example, the American Geriatrics Society recently recommended against feeding tubes because they often result in discomfort due to agitation, use of restraints, and worsening pressure ulcers.39
Unfortunately, only a minority of families receives straightforward information on the course and prognosis of AD, including the fact that patients eventually stop eating and that the natural cause of death is often an acute infection. Studies also show that patients with dementia are at risk for inadequate treatment of pain.40 Assuring adequate pain control is an essential component of end-of-life care.
Hospice. End-of-life care can often be improved with hospice care. This service is underused by patients with dementia, even though hospice care is available at no cost through Medicare. Hospice eligibility criteria for patients with AD are shown in
TABLE 3.41,42
Finally, a word about prevention
Numerous risk factors have been associated with an increased risk of AD (TABLE 4)2,3. Some, like age and genetics, are nonmodifiable, while others—particularly cardiovascular risk factors—can be modified.1 There are also factors associated with decreased risk, most notably, physical exercise and participating in cognitively stimulating activities.3 Identification of these factors has led to the hope that addressing them can prevent AD.
But association does not equal causation. In 2010, a report from the National Institutes of Health concluded that, although there are modifiable factors associated with AD, there is insufficient evidence that addressing any of them will actually prevent AD.43 In fact, there is good evidence that some of these factors (eg, statin therapy) are not effective in reducing the incidence of dementia, and that others (eg, vitamin E and estrogen therapy) are potentially harmful.44
The absence of empirically supported preventive interventions does not mean, however, that we should disregard these risks and protective factors. Encouraging social engagement, for example, may improve both emotional health and quality of life. Addressing cardiovascular risk factors can reduce the rate of coronary and cerebrovascular disease, potentially including vascular dementia, even if it does not reduce the rate of AD.
Studies are evaluating the use of monoclonal antibodies with anti-amyloid properties for preventing AD in individuals who have APOE ε4 genotypes or high amyloid loads on neuroimaging.45 It will be several years before results are available, however, and the outcome of these studies is uncertain as the use of anti-amyloid agents for treating established dementia has not been effective.46,47
CORRESPONDENCE
Marisa Menchola, PhD, Department of Psychiatry, University of Arizona College of Medicine, 1501 N. Campbell Ave., 7OPC. Tucson, AZ 85724; [email protected]
1. Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Association Web site. Available at: http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed December 2, 2014.
2. Román GC, Nash DT, Fillit H. Translating current knowledge into dementia prevention. Alzheimer Dis Assoc Disord. 2012;26:295-299.
3. Jak, AJ. The impact of physical and mental activity on cognitive aging. Curr Top Behav Neurosci. 2012;10:273-291.
4. US Preventive Services Task Force. Cognitive impairment in older adults: Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/cognitive-impairment-in-older-adults-screening. Accessed November 28, 2014.
5. Centers for Medicare & Medicaid Services. The guide to Medicare preventive services. 4th ed. 2011. Available at: http://www.curemd.com/fqhc/The%20Guide%20to%20Medicare%20Preventative%20Services%20for%20Physicans,%20Providers%20and%20Suppliers.pdf. Accessed December 2, 2014.
6. Boustani, M. Dementia screening in primary care: not too fast! J Amer Geriatr Soc. 2013;61:1205-1207.
7. Alzheimer’s Association. Know the 10 signs: Early detection matters. Alzheimer’s Association Web site. Available at: http://www.alz.org/national/documents/checklist_10signs.pdf. Accessed December 2, 2014.
8. Cordell CB, Borson S, Boustani M, et al; Medicare Detection of Cognitive Impairment Workgroup. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9:141-150.
9. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
10. The American Geriatrics Society. A guide to dementia diagnosis and treatment. The American Geriatrics Society Web site. Available at: http://dementia.americangeriatrics.org/documents/AGS_PC_Dementia_Sheet_2010v2.pdf. Accessed December
2, 2014.
11. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207-216.
12. Johnson KA, Minoshima S, Bohnen NI, et al; Alzheimer’s Association; Society of Nuclear Medicine and Molecular Imaging; Amyloid Imaging Taskforce. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9:e1-e16.
13. Centers for Medicare and Medicaid Services. National coverage determination (NCD) for FDG PET for dementia and neurodegenerative diseases (220.6.13). Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed December 2, 2014.
14. Albert MS, DeKosky ST, Ruckson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-179.
15. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception studies. Acta Psychiatr Scand. 2009;119:252-265.
16. Farias ST, Mungas D, Reed BR, et al. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151-1157.
17. Bensadon BA, Odenheimer GL. Current management decisions in mild cognitive impairment. Clin Geriatr Med. 2013;29:847-871.
18. Ngo-Metzger Q, August KJ, Srinivasan M, et al. End-of-life care: guidelines for patient-centered communication. Am Fam Physician. 2008;77:167-174.
19. Sayegh P, Knight BG. Cross-cultural differences in dementia: the Sociocultural Health Belief Model. Int Psychogeriatr. 2013;25:517-530.
20. McDaniel SH, Campbell TL, Hepworth J, et al. Family-Oriented Primary Care. 2nd ed. New York, NY: Springer; 2005.
21. Bensadon BA, Odenheimer GL. Current management decisions in mild cognitive impairment. Clin Geriatr Med. 2013:29;847-871.
22. Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9:CD009132.
23. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
24. Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593.
25. Fillit HM, Doody RS, Binaso K, et al. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother. 2006;4(suppl A):S9-S24;quiz S25-S28.
26. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
27. US Food and Drug Administration. Public health advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Published April 11, 2005. Updated August 16, 2013. Accessed December 2, 2014.
28. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
29. Brummel-Smith K. It’s time to require written informed consent when using antipsychotics in dementia. Br J Med Pract. 2008;1:4-6.
30. Huckans M, Hutson L, Twamley E, et al. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychol Rev. 2013;23:63-80.
31. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
32. McLaren AN, Lamantia MA, Callahan CM. Systematic review of non-pharmacologic interventions to delay functional decline in community-dwelling patients with dementia. Aging Ment Health. 2013;17:655-666.
33. Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013;6:CD003260.
34. Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging. 2007;22:28-36.
35. Raetz J. A nondrug approach to dementia. J Fam Pract. 2013;62:548-557.
36. Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychol Aging. 2007;22:37-51.
37. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009:361:1529-1538.
38. Parsons C, Hughes CM, Passmore AP, et al. Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: a neglected problem in the disadvantaged dying? Drugs Aging. 2010;27:435-449.
39. The American Geriatrics Society. Feeding tubes in advanced dementia position statement. The American Geriatrics Society Web site. Available at: http://www.americangeriatrics.org/files/documents/feeding.tubes.advanced.dementia.pdf. Accessed November 19, 2013.
40. Goodman C, Evans C, Wilcock J, et al. End of life care for community dwelling older people with dementia: an integrated review. Int J Geriatr Psychiatry. 2010;25:329-337.
41. Storey CP. A quick-reference guide to the hospice and palliative care training for physicians: UNIPAC self-study program. American Academy of Hospice and Palliative Medicine. Chicago; 2009.
42. Kaszniak AW, Kligman EW. Hospice care for patients with dementia. Elder Care. 2013. Arizona Alzheimer's Consortium Web site. Available at: http://azalz.org/wp-content/uploads/2013/07/Hospice-Care-for-Pts-with-Dementia.pdf. Accessed December 2, 2014.
43. Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: Preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27:1-30.
44. Patterson C, Feightner JW, Garcia A, et al. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ. 2008;178:548-556.
45. Carrillo MC, Brashear HR, Logovinsky V, et al. Can we prevent Alzheimer’s disease? Secondary “prevention” trials in Alzheimer’s disease. Alzheimers Dement. 2013;9:123-131.e1.
46. Salloway S, Sperling R, Fox NC, et al; Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s Disease. N Engl J Med. 2014;370:322-333.
47. Doody RS, Thomas RG, Farlow M, et al; Alheimer’s Disease Cooperative Study Steering Committee; Solanezumab Study Group. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s Disease. N Engl J Med. 2014:370:311-321.
› Refer patients for formal neuropsychological testing when dementia is suspected but the history, clinical interview, and brief cognitive tests do not result in a definitive diagnosis. C
› Use non-drug therapies as first-line treatment for behavioral symptoms of Alzheimer’s disease (AD), as the adverse effects of drug therapy generally offset any benefit. B
› Recommend against feeding tubes for patients with late-stage AD as they are more apt to cause discomfort than to provide benefit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Alzheimer’s disease (AD), the most common form of dementia, affects more than 5 million Americans.1 Estimates suggest that by 2050, the prevalence could triple, reaching 13 to 16 million.1 To effectively care for patients with AD and their families, family physicians need to be familiar with the latest evidence on all facets of care, from initial detection to patient management and end-of-life care.
This evidence-based review will help you toward that end by answering common questions regarding Alzheimer’s care, including whether routine screening is advisable, what tests should be ordered, which interventions (including nonpharmacologic options) are worth considering, and how best to counsel patients and families about end-of-life care.
Routine screening? Still subject to debate
In considering routine dementia screening in primary care, the key question is whether screening improves outcomes. Advocates note that individuals with dementia may appear unimpaired during office visits and may not report symptoms due to lack of insight; they point out, too, that waiting for an event that makes cognitive impairment obvious, such as a driving mishap, is risky.2 Those who advocate routine screening also note that only about half of those who have dementia are ever diagnosed.3
Others, including the US Preventive Services Task Force (USPSTF), disagree. In its 2014 evidence review, the USPSTF indicated that there is “insufficient evidence to assess the balance of benefits and harms of screening for cognitive impairment in older adults.”4
Mixed messages
The dearth of evidence is also reflected in the conflicting recommendations of the Affordable Care Act (ACA) and the Centers for Medicare and Medicaid Services (CMS). The ACA requires physicians to assess the cognitive function of Medicare patients during their annual wellness visits. CMS, however, instructs providers to screen for dementia only if observation or concerns raised by the patient or family suggest the possibility of impairment, and does not recommend any particular test.5
Cost-effectiveness analyses raise questions about the value of routine screening, as well. Evidence suggests that if a primary care physician screens 300 older patients, 39 will have a positive screen. But only about half of those 39 will agree to a diagnostic evaluation, and no more than 9 will ultimately be diagnosed with dementia. The estimated cost of identifying 9 cases is nearly $40,000—all in the absence of a treatment to cure or stop the progression of the disorder.6
The bottom line: Evidence does not support routine dementia screening of older adults. When cognitive impairment is suspected, however, physicians should conduct a diagnostic evaluation—and consider educating patients and families about the Alzheimer’s Association (AA)’s 10 warning signs of AD.7 (See “Is it Alzheimer’s? 10 warning signs”7 below.) A longer version, available at http://www.alz.org/national/documents/checklist_10signs.pdf, outlines the cognitive changes that are characteristic of healthy aging and compares them to changes suggestive of early dementia.7
Is it Alzheimer’s? 10 warning signs7 1. Memory loss that disrupts daily life |
How to proceed when you suspect AD
Step 1: Screening instrument. The first step in the diagnostic evaluation of a patient with suspected AD is to determine if, in fact, cognitive impairment is present. This can be done by screening with in-office screening instruments, such as the Mini-Cog (available at alz.org/documents_custom/minicog.pdf) or Mini-Mental State Examination (MMSE; health.gov.bc.ca/pharmacare/adti/clinician/pdf/ADTI%20SMMSE-GDS%20Reference%20Card.pdf), among others.8
Step 2: Clinical evaluation. If observation and test results suggest cognitive impairment, the next step is to determine whether clinical findings are consistent with the diagnostic criteria for AD (TABLE 1)9 developed by workgroups from the National Institute on Aging (NIA)/AA in 2011. A work-up is necessary to identify conditions that can mimic dementia (eg, depression) and behaviors that suggest another type of dementia, such as frontotemporal or Lewy body dementia.10 Lab testing should be included to rule out potentially reversible causes of cognitive dysfunction (eg, hypothyroidism, vitamin D deficiency).
Step 3: Neuropsychological evaluation. The NIA/AA recommends neuropsychological testing when the brief cognitive tests, history, and clinical work-up are not sufficient for a definitive diagnosis of dementia.9This generally involves a referral to a neuropsychologist, who conducts a battery of standardized tests to evaluate attention, memory, language, visual-spatial abilities, and executive functions, among others. Neuropsychological testing can confirm the presence of cognitive impairment and aid in the differential diagnosis by comparing the patient’s performance in these domains with characteristic features of different dementia syndromes.
Step 4: Brain imaging with either computed tomography or magnetic resonance imaging can be included in the work-up for patients with suspected AD to rule out abnormalities—eg, metastatic cancer, hydrocephalus, or occult chronic subdural hematoma—that could be causing cognitive impairment.9,10 Clinical features that generally warrant brain imaging include onset of cognitive impairment before age 60; unexplained focal neurologic signs or symptoms; abrupt onset or rapid decline; and/or predisposing conditions, such as cancer or anticoagulant treatment.10
The role of biomarkers and advanced brain imaging
Biomarkers that might provide confirmation of AD in patients who exhibit early symptoms of dementia have been studied extensively.11 The NIA/AA identified 2 categories of AD biomarkers:
- tests for β-amyloid deposition in the brain, including spinal fluid assays for β-amyloid (Aβ42) and positron emission tomography (PET) scans after intravenous injection of florbetapir or flutemetamol, which bind to amyloid in the brain; and
- tests for neuronal degeneration, which would include spinal fluid assays for tau protein and PET scans after injection of fluorodeoxyglucose (FDG), which shows decreased uptake in patients with AD.9
Research reveals the promise of these biomarkers as diagnostic tools, particularly in patients with an atypical presentation of dementia or mild cognitive impairment (MCI) that may be associated with early AD.12 (More on MCI in a moment.) However, the NIA/AA concluded that additional research is needed to validate these tests for routine diagnostic purposes. Medicare covers PET scans with FDG only for the differential diagnosis of AD vs frontotemporal dementia.13
Mild cognitive impairment: How likely that it will progress?
Along with diagnostic criteria for AD, the NIA/AA developed criteria for a symptomatic predementia phase of AD—often referred to as MCI.14 According to the workgroup, MCI is diagnosed when:
2. there is evidence of cognitive impairment, ideally through psychometric testing, revealing performance below expectation based on the patient’s age and education;
3. the patient is able to maintain independent functioning in daily life, despite mild problems or the need for minimal assistance; and
4. there is no significant impairment in social or occupational functioning.14
Progression: Less likely than you might think
Patients with MCI are at risk for progression to overt dementia, with an overall annual conversion rate from MCI to dementia estimated at 10% to 15%.15,16 This estimate must be interpreted with caution, however, because most studies were conducted prior to the 2011 guidelines, when different diagnostic criteria were used. Observers have noted, too, that the numbers largely reflect data collected in specialty clinics and that community-based studies reveal substantially lower conversion rates (3%-6% per year).16 In addition, evidence suggests that many patients with MCI demonstrate long-term stability or even reversal of deficits.17
While there is some consideration of the use of biomarkers and amyloid imaging tests to help determine which patients with MCI will progress to AD, practice guidelines do not currently recommend such testing and it is not covered by Medicare.
When evidence indicates an AD diagnosis
When faced with the need to communicate an AD diagnosis, follow the general recommendations for delivering any bad news or discouraging prognosis:
Prioritize and limit the information you provide, determining not only what the patient and family want to hear, but also how much they are able to comprehend.
Confirm that the patient and family understand the information you’ve provided.
Offer emotional support and recommend additional resources18 (TABLE 2).
Given the progressive cognitive decline that characterizes AD, it is important to address the primary caregiver’s understanding of, and ability to cope with, the disease. It is also important to explore beliefs and attitudes regarding AD. Keep in mind that different cultural groups tend to differ in their beliefs about the nature, cause, and appropriate management of AD, as well as the role of spirituality, help-seeking, and stigma.19,20
The progressive and ultimately fatal nature of AD also makes planning for the future a priority. Ideally, patients should be engaged in discussions regarding end-of-life care as early as possible, while they are still able to make informed decisions and express their preferences. Discussing end-of-life care can be overwhelming for newly diagnosed patients and their families, however, so it is important that you address issues—medical, financial, and legal planning, for example—that families should be considering.
TABLE 2
| Resources for newly diagnosed patients and families | |
| Issue | Resources |
| Education | Alzheimer’s and Dementia Caregiver Center http://www.alz.org/care/overview.asp NIA Alzheimer’s Disease Education and Referral Center http://www.nia.nih.gov/alzheimers/ |
| Planning (medical, financial, legal)/benefits | AARP Caregiving Resource Center http://www.aarp.org/home-family/caregiving Alzheimer’s Association Alzheimer’s Navigator https://www.alzheimersnavigator.org/ National Council on Aging Benefits Checkup https://www.benefitscheckup.org/ |
| Safety | Association for Driver Rehabilitation Specialists: Driving and Alzheimer’s/Dementia https://c.ymcdn.com/sites/www.aded.net/resource/resmgr/fact_Sheets/ADED_alzheimers-Dementia_fac.pdf NIA’s Home Safety for People with Alzheimer’s booklet http://www.nia.nih.gov/alzheimers/publication/home-safety-people-alzheimers-disease |
| Support | Caregiver Action Network http://caregiveraction.org/ |
Drugs address cognitive and behavioral function
No currently available treatments can cure or significantly alter the progression of AD, but 2 classes of medications are used in an attempt to improve cognitive function. One is cholinesterase inhibitors (ChEIs), which potentiate acetylcholine synaptic transmission. The other is N-methyl-D-aspartate (NMDA) glutamate receptor blockers. Other classes of drugs are sometimes used to treat behavioral symptoms of dementia, such as agitation, aggression, mood disorders, and psychosis (eg, delusions and hallucinations).
Cognitive function. Results from studies of pharmacologic management of MCI vary widely, but recent reviews have found no convincing evidence that either ChEIs or NMDA receptor blockers have an effect on progression from MCI to dementia.21,22 Neither class is FDA-approved for treating MCI.
In patients with dementia, the effects of ChEIs and NMDA receptor blockers on cognition are statistically significant but modest, and often of questionable clinical relevance.23 Nonetheless, among ChEIs, donepezil is approved by the US Food and Drug Administration (FDA) for mild, moderate, and severe dementia and galantamine and rivastigmine are approved for mild and moderate dementia. There is no evidence that any one ChEI is more effective than any other,24 and the choice of drugs is often guided by cost, adverse effects, and health plan formularies. Memantine, the only FDA-approved NMDA receptor blocker, is approved for moderate to severe dementia and can be used alone or in combination with a ChEI.
If these drugs are used in an attempt to improve cognition in AD, guidelines recommend the following approach for initial therapy: Prescribe a ChEI for the mild stage, a ChEI plus memantine for the moderate stage, and memantine (with or without a ChEI) for the severe stage.25 The recommendations also include monitoring every 6 months.
There is no consensus about when to discontinue medication. Various published recommendations call for continuing treatment until the patient has “lost all cognitive and functional abilities;”22 until the patient’s MMSE score falls below 10 and there is no indication that the drug is having a “worthwhile effect;”21 or until he or she has reached stage 7 on the Reisberg Functional Assessment Staging scale, indicating nonambulatory status with speech limited to one to 5 words a day.10
Behavioral function. A variety of drugs are used to treat behavioral symptoms in AD. While not FDA-approved for this use, the most widely prescribed agents are second-generation antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone). The main effect of these drugs is often nothing more than sedation, and one large multisite clinical trial concluded that the adverse effects offset the benefits for patients with AD.26 Indeed, the FDA has issued an advisory on the use of second-generation antipsychotics in AD patients, stating that they are associated with an increased risk of death.27 The recently updated Beers Criteria strongly recommend avoiding these drugs for treating behavioral disturbances in AD unless nonpharmacologic options have failed and the patient is a threat to self or others.28
Because of the black-box warning that antipsychotics increase the risk of death, some physicians have advocated obtaining informed consent prior to prescribing such medications.29 At the very least, when family or guardians are involved, a conversation about risks vs benefits should take place and be documented in the medical record.
Other drug classes are also sometimes used in an attempt to improve behavioral function, including anti-seizure medications (valproic acid, carbamazepine), antidepressants (trazodone and selective serotonin reuptake inhibitors), and anxiolytics (benzodiazepines and buspirone). Other than their sedating effects, there is no strong evidence that these drugs are effective for treating dementia-related behavioral disorders. If used, caution is required due to potential adverse effects.
Nonpharmacologic management is “promising”
A recent systematic review of nonpharmacologic interventions for MCI evaluated exercise, training in compensatory strategies, and engagement in cognitively stimulating activities and found “promising but inconclusive” results. The researchers found that studies show mostly positive effects on cognition but have significant methodologic limitations.30 Importantly, there is no evidence of delayed or reduced conversion to dementia.
For patients who already have mild-to-moderate dementia, cognitive stimulation seems to help in the short term.31 There is also some evidence that exercise and occupational therapy may slow functional decline,32 but the effects are small to modest and their actual clinical significance (eg, the ability to delay institutionalization) is unclear. There is promising but preliminary evidence that cognitive rehabilitation (helping patients devise strategies to complete daily activities) may improve functioning in everyday life.33
While behavioral symptoms are often due to the dementia itself, it is important to identify and treat medical and environmental causes that may be contributing, such as infection, pain, and loud or unsafe environments. As noted before, nonpharmacologic treatments are generally preferred for behavioral problems and should be considered prior to drug therapy. Approaches that identify and modify both the antecedents and consequences of problem behaviors and increase pleasant events have empirical support for the management of behavioral symptoms.34 Interventions including massage therapy, aromatherapy, exercise, and music therapy may also be effective in the short term for agitated behavior.35
Caregivers should be encouraged to receive training in these strategies through organizations like AA. Caregiver education and support can reduce caregivers’ distress and increase their self-efficacy and coping skills.36
End-of-life care must be addressed
Perhaps the most important aspect of end-of-life care in AD is assuring that families (or health care proxies) understand that AD is a fatal illness, with most patients dying within 4 to 8 years of diagnosis.1 Evidence indicates that patients whose proxies have a clear recognition of this are less likely to experience “burdensome” interventions such as parenteral therapy, emergency department visits, hospital admissions, and tube feedings in their last 3 months of life.37
Overall, decisions regarding discontinuing medical treatments in advanced AD should be made by balancing the likelihood of benefit with the potential for adverse effects.38 For example, the American Geriatrics Society recently recommended against feeding tubes because they often result in discomfort due to agitation, use of restraints, and worsening pressure ulcers.39
Unfortunately, only a minority of families receives straightforward information on the course and prognosis of AD, including the fact that patients eventually stop eating and that the natural cause of death is often an acute infection. Studies also show that patients with dementia are at risk for inadequate treatment of pain.40 Assuring adequate pain control is an essential component of end-of-life care.
Hospice. End-of-life care can often be improved with hospice care. This service is underused by patients with dementia, even though hospice care is available at no cost through Medicare. Hospice eligibility criteria for patients with AD are shown in
TABLE 3.41,42
Finally, a word about prevention
Numerous risk factors have been associated with an increased risk of AD (TABLE 4)2,3. Some, like age and genetics, are nonmodifiable, while others—particularly cardiovascular risk factors—can be modified.1 There are also factors associated with decreased risk, most notably, physical exercise and participating in cognitively stimulating activities.3 Identification of these factors has led to the hope that addressing them can prevent AD.
But association does not equal causation. In 2010, a report from the National Institutes of Health concluded that, although there are modifiable factors associated with AD, there is insufficient evidence that addressing any of them will actually prevent AD.43 In fact, there is good evidence that some of these factors (eg, statin therapy) are not effective in reducing the incidence of dementia, and that others (eg, vitamin E and estrogen therapy) are potentially harmful.44
The absence of empirically supported preventive interventions does not mean, however, that we should disregard these risks and protective factors. Encouraging social engagement, for example, may improve both emotional health and quality of life. Addressing cardiovascular risk factors can reduce the rate of coronary and cerebrovascular disease, potentially including vascular dementia, even if it does not reduce the rate of AD.
Studies are evaluating the use of monoclonal antibodies with anti-amyloid properties for preventing AD in individuals who have APOE ε4 genotypes or high amyloid loads on neuroimaging.45 It will be several years before results are available, however, and the outcome of these studies is uncertain as the use of anti-amyloid agents for treating established dementia has not been effective.46,47
CORRESPONDENCE
Marisa Menchola, PhD, Department of Psychiatry, University of Arizona College of Medicine, 1501 N. Campbell Ave., 7OPC. Tucson, AZ 85724; [email protected]
› Refer patients for formal neuropsychological testing when dementia is suspected but the history, clinical interview, and brief cognitive tests do not result in a definitive diagnosis. C
› Use non-drug therapies as first-line treatment for behavioral symptoms of Alzheimer’s disease (AD), as the adverse effects of drug therapy generally offset any benefit. B
› Recommend against feeding tubes for patients with late-stage AD as they are more apt to cause discomfort than to provide benefit. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Alzheimer’s disease (AD), the most common form of dementia, affects more than 5 million Americans.1 Estimates suggest that by 2050, the prevalence could triple, reaching 13 to 16 million.1 To effectively care for patients with AD and their families, family physicians need to be familiar with the latest evidence on all facets of care, from initial detection to patient management and end-of-life care.
This evidence-based review will help you toward that end by answering common questions regarding Alzheimer’s care, including whether routine screening is advisable, what tests should be ordered, which interventions (including nonpharmacologic options) are worth considering, and how best to counsel patients and families about end-of-life care.
Routine screening? Still subject to debate
In considering routine dementia screening in primary care, the key question is whether screening improves outcomes. Advocates note that individuals with dementia may appear unimpaired during office visits and may not report symptoms due to lack of insight; they point out, too, that waiting for an event that makes cognitive impairment obvious, such as a driving mishap, is risky.2 Those who advocate routine screening also note that only about half of those who have dementia are ever diagnosed.3
Others, including the US Preventive Services Task Force (USPSTF), disagree. In its 2014 evidence review, the USPSTF indicated that there is “insufficient evidence to assess the balance of benefits and harms of screening for cognitive impairment in older adults.”4
Mixed messages
The dearth of evidence is also reflected in the conflicting recommendations of the Affordable Care Act (ACA) and the Centers for Medicare and Medicaid Services (CMS). The ACA requires physicians to assess the cognitive function of Medicare patients during their annual wellness visits. CMS, however, instructs providers to screen for dementia only if observation or concerns raised by the patient or family suggest the possibility of impairment, and does not recommend any particular test.5
Cost-effectiveness analyses raise questions about the value of routine screening, as well. Evidence suggests that if a primary care physician screens 300 older patients, 39 will have a positive screen. But only about half of those 39 will agree to a diagnostic evaluation, and no more than 9 will ultimately be diagnosed with dementia. The estimated cost of identifying 9 cases is nearly $40,000—all in the absence of a treatment to cure or stop the progression of the disorder.6
The bottom line: Evidence does not support routine dementia screening of older adults. When cognitive impairment is suspected, however, physicians should conduct a diagnostic evaluation—and consider educating patients and families about the Alzheimer’s Association (AA)’s 10 warning signs of AD.7 (See “Is it Alzheimer’s? 10 warning signs”7 below.) A longer version, available at http://www.alz.org/national/documents/checklist_10signs.pdf, outlines the cognitive changes that are characteristic of healthy aging and compares them to changes suggestive of early dementia.7
Is it Alzheimer’s? 10 warning signs7 1. Memory loss that disrupts daily life |
How to proceed when you suspect AD
Step 1: Screening instrument. The first step in the diagnostic evaluation of a patient with suspected AD is to determine if, in fact, cognitive impairment is present. This can be done by screening with in-office screening instruments, such as the Mini-Cog (available at alz.org/documents_custom/minicog.pdf) or Mini-Mental State Examination (MMSE; health.gov.bc.ca/pharmacare/adti/clinician/pdf/ADTI%20SMMSE-GDS%20Reference%20Card.pdf), among others.8
Step 2: Clinical evaluation. If observation and test results suggest cognitive impairment, the next step is to determine whether clinical findings are consistent with the diagnostic criteria for AD (TABLE 1)9 developed by workgroups from the National Institute on Aging (NIA)/AA in 2011. A work-up is necessary to identify conditions that can mimic dementia (eg, depression) and behaviors that suggest another type of dementia, such as frontotemporal or Lewy body dementia.10 Lab testing should be included to rule out potentially reversible causes of cognitive dysfunction (eg, hypothyroidism, vitamin D deficiency).
Step 3: Neuropsychological evaluation. The NIA/AA recommends neuropsychological testing when the brief cognitive tests, history, and clinical work-up are not sufficient for a definitive diagnosis of dementia.9This generally involves a referral to a neuropsychologist, who conducts a battery of standardized tests to evaluate attention, memory, language, visual-spatial abilities, and executive functions, among others. Neuropsychological testing can confirm the presence of cognitive impairment and aid in the differential diagnosis by comparing the patient’s performance in these domains with characteristic features of different dementia syndromes.
Step 4: Brain imaging with either computed tomography or magnetic resonance imaging can be included in the work-up for patients with suspected AD to rule out abnormalities—eg, metastatic cancer, hydrocephalus, or occult chronic subdural hematoma—that could be causing cognitive impairment.9,10 Clinical features that generally warrant brain imaging include onset of cognitive impairment before age 60; unexplained focal neurologic signs or symptoms; abrupt onset or rapid decline; and/or predisposing conditions, such as cancer or anticoagulant treatment.10
The role of biomarkers and advanced brain imaging
Biomarkers that might provide confirmation of AD in patients who exhibit early symptoms of dementia have been studied extensively.11 The NIA/AA identified 2 categories of AD biomarkers:
- tests for β-amyloid deposition in the brain, including spinal fluid assays for β-amyloid (Aβ42) and positron emission tomography (PET) scans after intravenous injection of florbetapir or flutemetamol, which bind to amyloid in the brain; and
- tests for neuronal degeneration, which would include spinal fluid assays for tau protein and PET scans after injection of fluorodeoxyglucose (FDG), which shows decreased uptake in patients with AD.9
Research reveals the promise of these biomarkers as diagnostic tools, particularly in patients with an atypical presentation of dementia or mild cognitive impairment (MCI) that may be associated with early AD.12 (More on MCI in a moment.) However, the NIA/AA concluded that additional research is needed to validate these tests for routine diagnostic purposes. Medicare covers PET scans with FDG only for the differential diagnosis of AD vs frontotemporal dementia.13
Mild cognitive impairment: How likely that it will progress?
Along with diagnostic criteria for AD, the NIA/AA developed criteria for a symptomatic predementia phase of AD—often referred to as MCI.14 According to the workgroup, MCI is diagnosed when:
2. there is evidence of cognitive impairment, ideally through psychometric testing, revealing performance below expectation based on the patient’s age and education;
3. the patient is able to maintain independent functioning in daily life, despite mild problems or the need for minimal assistance; and
4. there is no significant impairment in social or occupational functioning.14
Progression: Less likely than you might think
Patients with MCI are at risk for progression to overt dementia, with an overall annual conversion rate from MCI to dementia estimated at 10% to 15%.15,16 This estimate must be interpreted with caution, however, because most studies were conducted prior to the 2011 guidelines, when different diagnostic criteria were used. Observers have noted, too, that the numbers largely reflect data collected in specialty clinics and that community-based studies reveal substantially lower conversion rates (3%-6% per year).16 In addition, evidence suggests that many patients with MCI demonstrate long-term stability or even reversal of deficits.17
While there is some consideration of the use of biomarkers and amyloid imaging tests to help determine which patients with MCI will progress to AD, practice guidelines do not currently recommend such testing and it is not covered by Medicare.
When evidence indicates an AD diagnosis
When faced with the need to communicate an AD diagnosis, follow the general recommendations for delivering any bad news or discouraging prognosis:
Prioritize and limit the information you provide, determining not only what the patient and family want to hear, but also how much they are able to comprehend.
Confirm that the patient and family understand the information you’ve provided.
Offer emotional support and recommend additional resources18 (TABLE 2).
Given the progressive cognitive decline that characterizes AD, it is important to address the primary caregiver’s understanding of, and ability to cope with, the disease. It is also important to explore beliefs and attitudes regarding AD. Keep in mind that different cultural groups tend to differ in their beliefs about the nature, cause, and appropriate management of AD, as well as the role of spirituality, help-seeking, and stigma.19,20
The progressive and ultimately fatal nature of AD also makes planning for the future a priority. Ideally, patients should be engaged in discussions regarding end-of-life care as early as possible, while they are still able to make informed decisions and express their preferences. Discussing end-of-life care can be overwhelming for newly diagnosed patients and their families, however, so it is important that you address issues—medical, financial, and legal planning, for example—that families should be considering.
TABLE 2
| Resources for newly diagnosed patients and families | |
| Issue | Resources |
| Education | Alzheimer’s and Dementia Caregiver Center http://www.alz.org/care/overview.asp NIA Alzheimer’s Disease Education and Referral Center http://www.nia.nih.gov/alzheimers/ |
| Planning (medical, financial, legal)/benefits | AARP Caregiving Resource Center http://www.aarp.org/home-family/caregiving Alzheimer’s Association Alzheimer’s Navigator https://www.alzheimersnavigator.org/ National Council on Aging Benefits Checkup https://www.benefitscheckup.org/ |
| Safety | Association for Driver Rehabilitation Specialists: Driving and Alzheimer’s/Dementia https://c.ymcdn.com/sites/www.aded.net/resource/resmgr/fact_Sheets/ADED_alzheimers-Dementia_fac.pdf NIA’s Home Safety for People with Alzheimer’s booklet http://www.nia.nih.gov/alzheimers/publication/home-safety-people-alzheimers-disease |
| Support | Caregiver Action Network http://caregiveraction.org/ |
Drugs address cognitive and behavioral function
No currently available treatments can cure or significantly alter the progression of AD, but 2 classes of medications are used in an attempt to improve cognitive function. One is cholinesterase inhibitors (ChEIs), which potentiate acetylcholine synaptic transmission. The other is N-methyl-D-aspartate (NMDA) glutamate receptor blockers. Other classes of drugs are sometimes used to treat behavioral symptoms of dementia, such as agitation, aggression, mood disorders, and psychosis (eg, delusions and hallucinations).
Cognitive function. Results from studies of pharmacologic management of MCI vary widely, but recent reviews have found no convincing evidence that either ChEIs or NMDA receptor blockers have an effect on progression from MCI to dementia.21,22 Neither class is FDA-approved for treating MCI.
In patients with dementia, the effects of ChEIs and NMDA receptor blockers on cognition are statistically significant but modest, and often of questionable clinical relevance.23 Nonetheless, among ChEIs, donepezil is approved by the US Food and Drug Administration (FDA) for mild, moderate, and severe dementia and galantamine and rivastigmine are approved for mild and moderate dementia. There is no evidence that any one ChEI is more effective than any other,24 and the choice of drugs is often guided by cost, adverse effects, and health plan formularies. Memantine, the only FDA-approved NMDA receptor blocker, is approved for moderate to severe dementia and can be used alone or in combination with a ChEI.
If these drugs are used in an attempt to improve cognition in AD, guidelines recommend the following approach for initial therapy: Prescribe a ChEI for the mild stage, a ChEI plus memantine for the moderate stage, and memantine (with or without a ChEI) for the severe stage.25 The recommendations also include monitoring every 6 months.
There is no consensus about when to discontinue medication. Various published recommendations call for continuing treatment until the patient has “lost all cognitive and functional abilities;”22 until the patient’s MMSE score falls below 10 and there is no indication that the drug is having a “worthwhile effect;”21 or until he or she has reached stage 7 on the Reisberg Functional Assessment Staging scale, indicating nonambulatory status with speech limited to one to 5 words a day.10
Behavioral function. A variety of drugs are used to treat behavioral symptoms in AD. While not FDA-approved for this use, the most widely prescribed agents are second-generation antipsychotics (aripiprazole, olanzapine, quetiapine, and risperidone). The main effect of these drugs is often nothing more than sedation, and one large multisite clinical trial concluded that the adverse effects offset the benefits for patients with AD.26 Indeed, the FDA has issued an advisory on the use of second-generation antipsychotics in AD patients, stating that they are associated with an increased risk of death.27 The recently updated Beers Criteria strongly recommend avoiding these drugs for treating behavioral disturbances in AD unless nonpharmacologic options have failed and the patient is a threat to self or others.28
Because of the black-box warning that antipsychotics increase the risk of death, some physicians have advocated obtaining informed consent prior to prescribing such medications.29 At the very least, when family or guardians are involved, a conversation about risks vs benefits should take place and be documented in the medical record.
Other drug classes are also sometimes used in an attempt to improve behavioral function, including anti-seizure medications (valproic acid, carbamazepine), antidepressants (trazodone and selective serotonin reuptake inhibitors), and anxiolytics (benzodiazepines and buspirone). Other than their sedating effects, there is no strong evidence that these drugs are effective for treating dementia-related behavioral disorders. If used, caution is required due to potential adverse effects.
Nonpharmacologic management is “promising”
A recent systematic review of nonpharmacologic interventions for MCI evaluated exercise, training in compensatory strategies, and engagement in cognitively stimulating activities and found “promising but inconclusive” results. The researchers found that studies show mostly positive effects on cognition but have significant methodologic limitations.30 Importantly, there is no evidence of delayed or reduced conversion to dementia.
For patients who already have mild-to-moderate dementia, cognitive stimulation seems to help in the short term.31 There is also some evidence that exercise and occupational therapy may slow functional decline,32 but the effects are small to modest and their actual clinical significance (eg, the ability to delay institutionalization) is unclear. There is promising but preliminary evidence that cognitive rehabilitation (helping patients devise strategies to complete daily activities) may improve functioning in everyday life.33
While behavioral symptoms are often due to the dementia itself, it is important to identify and treat medical and environmental causes that may be contributing, such as infection, pain, and loud or unsafe environments. As noted before, nonpharmacologic treatments are generally preferred for behavioral problems and should be considered prior to drug therapy. Approaches that identify and modify both the antecedents and consequences of problem behaviors and increase pleasant events have empirical support for the management of behavioral symptoms.34 Interventions including massage therapy, aromatherapy, exercise, and music therapy may also be effective in the short term for agitated behavior.35
Caregivers should be encouraged to receive training in these strategies through organizations like AA. Caregiver education and support can reduce caregivers’ distress and increase their self-efficacy and coping skills.36
End-of-life care must be addressed
Perhaps the most important aspect of end-of-life care in AD is assuring that families (or health care proxies) understand that AD is a fatal illness, with most patients dying within 4 to 8 years of diagnosis.1 Evidence indicates that patients whose proxies have a clear recognition of this are less likely to experience “burdensome” interventions such as parenteral therapy, emergency department visits, hospital admissions, and tube feedings in their last 3 months of life.37
Overall, decisions regarding discontinuing medical treatments in advanced AD should be made by balancing the likelihood of benefit with the potential for adverse effects.38 For example, the American Geriatrics Society recently recommended against feeding tubes because they often result in discomfort due to agitation, use of restraints, and worsening pressure ulcers.39
Unfortunately, only a minority of families receives straightforward information on the course and prognosis of AD, including the fact that patients eventually stop eating and that the natural cause of death is often an acute infection. Studies also show that patients with dementia are at risk for inadequate treatment of pain.40 Assuring adequate pain control is an essential component of end-of-life care.
Hospice. End-of-life care can often be improved with hospice care. This service is underused by patients with dementia, even though hospice care is available at no cost through Medicare. Hospice eligibility criteria for patients with AD are shown in
TABLE 3.41,42
Finally, a word about prevention
Numerous risk factors have been associated with an increased risk of AD (TABLE 4)2,3. Some, like age and genetics, are nonmodifiable, while others—particularly cardiovascular risk factors—can be modified.1 There are also factors associated with decreased risk, most notably, physical exercise and participating in cognitively stimulating activities.3 Identification of these factors has led to the hope that addressing them can prevent AD.
But association does not equal causation. In 2010, a report from the National Institutes of Health concluded that, although there are modifiable factors associated with AD, there is insufficient evidence that addressing any of them will actually prevent AD.43 In fact, there is good evidence that some of these factors (eg, statin therapy) are not effective in reducing the incidence of dementia, and that others (eg, vitamin E and estrogen therapy) are potentially harmful.44
The absence of empirically supported preventive interventions does not mean, however, that we should disregard these risks and protective factors. Encouraging social engagement, for example, may improve both emotional health and quality of life. Addressing cardiovascular risk factors can reduce the rate of coronary and cerebrovascular disease, potentially including vascular dementia, even if it does not reduce the rate of AD.
Studies are evaluating the use of monoclonal antibodies with anti-amyloid properties for preventing AD in individuals who have APOE ε4 genotypes or high amyloid loads on neuroimaging.45 It will be several years before results are available, however, and the outcome of these studies is uncertain as the use of anti-amyloid agents for treating established dementia has not been effective.46,47
CORRESPONDENCE
Marisa Menchola, PhD, Department of Psychiatry, University of Arizona College of Medicine, 1501 N. Campbell Ave., 7OPC. Tucson, AZ 85724; [email protected]
1. Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Association Web site. Available at: http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed December 2, 2014.
2. Román GC, Nash DT, Fillit H. Translating current knowledge into dementia prevention. Alzheimer Dis Assoc Disord. 2012;26:295-299.
3. Jak, AJ. The impact of physical and mental activity on cognitive aging. Curr Top Behav Neurosci. 2012;10:273-291.
4. US Preventive Services Task Force. Cognitive impairment in older adults: Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/cognitive-impairment-in-older-adults-screening. Accessed November 28, 2014.
5. Centers for Medicare & Medicaid Services. The guide to Medicare preventive services. 4th ed. 2011. Available at: http://www.curemd.com/fqhc/The%20Guide%20to%20Medicare%20Preventative%20Services%20for%20Physicans,%20Providers%20and%20Suppliers.pdf. Accessed December 2, 2014.
6. Boustani, M. Dementia screening in primary care: not too fast! J Amer Geriatr Soc. 2013;61:1205-1207.
7. Alzheimer’s Association. Know the 10 signs: Early detection matters. Alzheimer’s Association Web site. Available at: http://www.alz.org/national/documents/checklist_10signs.pdf. Accessed December 2, 2014.
8. Cordell CB, Borson S, Boustani M, et al; Medicare Detection of Cognitive Impairment Workgroup. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9:141-150.
9. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
10. The American Geriatrics Society. A guide to dementia diagnosis and treatment. The American Geriatrics Society Web site. Available at: http://dementia.americangeriatrics.org/documents/AGS_PC_Dementia_Sheet_2010v2.pdf. Accessed December
2, 2014.
11. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207-216.
12. Johnson KA, Minoshima S, Bohnen NI, et al; Alzheimer’s Association; Society of Nuclear Medicine and Molecular Imaging; Amyloid Imaging Taskforce. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9:e1-e16.
13. Centers for Medicare and Medicaid Services. National coverage determination (NCD) for FDG PET for dementia and neurodegenerative diseases (220.6.13). Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed December 2, 2014.
14. Albert MS, DeKosky ST, Ruckson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-179.
15. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception studies. Acta Psychiatr Scand. 2009;119:252-265.
16. Farias ST, Mungas D, Reed BR, et al. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151-1157.
17. Bensadon BA, Odenheimer GL. Current management decisions in mild cognitive impairment. Clin Geriatr Med. 2013;29:847-871.
18. Ngo-Metzger Q, August KJ, Srinivasan M, et al. End-of-life care: guidelines for patient-centered communication. Am Fam Physician. 2008;77:167-174.
19. Sayegh P, Knight BG. Cross-cultural differences in dementia: the Sociocultural Health Belief Model. Int Psychogeriatr. 2013;25:517-530.
20. McDaniel SH, Campbell TL, Hepworth J, et al. Family-Oriented Primary Care. 2nd ed. New York, NY: Springer; 2005.
21. Bensadon BA, Odenheimer GL. Current management decisions in mild cognitive impairment. Clin Geriatr Med. 2013:29;847-871.
22. Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9:CD009132.
23. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
24. Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593.
25. Fillit HM, Doody RS, Binaso K, et al. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother. 2006;4(suppl A):S9-S24;quiz S25-S28.
26. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
27. US Food and Drug Administration. Public health advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Published April 11, 2005. Updated August 16, 2013. Accessed December 2, 2014.
28. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
29. Brummel-Smith K. It’s time to require written informed consent when using antipsychotics in dementia. Br J Med Pract. 2008;1:4-6.
30. Huckans M, Hutson L, Twamley E, et al. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychol Rev. 2013;23:63-80.
31. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
32. McLaren AN, Lamantia MA, Callahan CM. Systematic review of non-pharmacologic interventions to delay functional decline in community-dwelling patients with dementia. Aging Ment Health. 2013;17:655-666.
33. Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013;6:CD003260.
34. Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging. 2007;22:28-36.
35. Raetz J. A nondrug approach to dementia. J Fam Pract. 2013;62:548-557.
36. Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychol Aging. 2007;22:37-51.
37. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009:361:1529-1538.
38. Parsons C, Hughes CM, Passmore AP, et al. Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: a neglected problem in the disadvantaged dying? Drugs Aging. 2010;27:435-449.
39. The American Geriatrics Society. Feeding tubes in advanced dementia position statement. The American Geriatrics Society Web site. Available at: http://www.americangeriatrics.org/files/documents/feeding.tubes.advanced.dementia.pdf. Accessed November 19, 2013.
40. Goodman C, Evans C, Wilcock J, et al. End of life care for community dwelling older people with dementia: an integrated review. Int J Geriatr Psychiatry. 2010;25:329-337.
41. Storey CP. A quick-reference guide to the hospice and palliative care training for physicians: UNIPAC self-study program. American Academy of Hospice and Palliative Medicine. Chicago; 2009.
42. Kaszniak AW, Kligman EW. Hospice care for patients with dementia. Elder Care. 2013. Arizona Alzheimer's Consortium Web site. Available at: http://azalz.org/wp-content/uploads/2013/07/Hospice-Care-for-Pts-with-Dementia.pdf. Accessed December 2, 2014.
43. Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: Preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27:1-30.
44. Patterson C, Feightner JW, Garcia A, et al. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ. 2008;178:548-556.
45. Carrillo MC, Brashear HR, Logovinsky V, et al. Can we prevent Alzheimer’s disease? Secondary “prevention” trials in Alzheimer’s disease. Alzheimers Dement. 2013;9:123-131.e1.
46. Salloway S, Sperling R, Fox NC, et al; Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s Disease. N Engl J Med. 2014;370:322-333.
47. Doody RS, Thomas RG, Farlow M, et al; Alheimer’s Disease Cooperative Study Steering Committee; Solanezumab Study Group. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s Disease. N Engl J Med. 2014:370:311-321.
1. Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Association Web site. Available at: http://www.alz.org/downloads/facts_figures_2013.pdf. Accessed December 2, 2014.
2. Román GC, Nash DT, Fillit H. Translating current knowledge into dementia prevention. Alzheimer Dis Assoc Disord. 2012;26:295-299.
3. Jak, AJ. The impact of physical and mental activity on cognitive aging. Curr Top Behav Neurosci. 2012;10:273-291.
4. US Preventive Services Task Force. Cognitive impairment in older adults: Screening. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/Page/Topic/recommendation-summary/cognitive-impairment-in-older-adults-screening. Accessed November 28, 2014.
5. Centers for Medicare & Medicaid Services. The guide to Medicare preventive services. 4th ed. 2011. Available at: http://www.curemd.com/fqhc/The%20Guide%20to%20Medicare%20Preventative%20Services%20for%20Physicans,%20Providers%20and%20Suppliers.pdf. Accessed December 2, 2014.
6. Boustani, M. Dementia screening in primary care: not too fast! J Amer Geriatr Soc. 2013;61:1205-1207.
7. Alzheimer’s Association. Know the 10 signs: Early detection matters. Alzheimer’s Association Web site. Available at: http://www.alz.org/national/documents/checklist_10signs.pdf. Accessed December 2, 2014.
8. Cordell CB, Borson S, Boustani M, et al; Medicare Detection of Cognitive Impairment Workgroup. Alzheimer’s Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement. 2013;9:141-150.
9. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
10. The American Geriatrics Society. A guide to dementia diagnosis and treatment. The American Geriatrics Society Web site. Available at: http://dementia.americangeriatrics.org/documents/AGS_PC_Dementia_Sheet_2010v2.pdf. Accessed December
2, 2014.
11. Jack CR, Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207-216.
12. Johnson KA, Minoshima S, Bohnen NI, et al; Alzheimer’s Association; Society of Nuclear Medicine and Molecular Imaging; Amyloid Imaging Taskforce. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement. 2013;9:e1-e16.
13. Centers for Medicare and Medicaid Services. National coverage determination (NCD) for FDG PET for dementia and neurodegenerative diseases (220.6.13). Centers for Medicare and Medicaid Services Web site. Available at: http://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?NCDId=288&ncdver=3&bc=BAABAAAAAAAA&. Accessed December 2, 2014.
14. Albert MS, DeKosky ST, Ruckson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-179.
15. Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia—meta-analysis of 41 robust inception studies. Acta Psychiatr Scand. 2009;119:252-265.
16. Farias ST, Mungas D, Reed BR, et al. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66:1151-1157.
17. Bensadon BA, Odenheimer GL. Current management decisions in mild cognitive impairment. Clin Geriatr Med. 2013;29:847-871.
18. Ngo-Metzger Q, August KJ, Srinivasan M, et al. End-of-life care: guidelines for patient-centered communication. Am Fam Physician. 2008;77:167-174.
19. Sayegh P, Knight BG. Cross-cultural differences in dementia: the Sociocultural Health Belief Model. Int Psychogeriatr. 2013;25:517-530.
20. McDaniel SH, Campbell TL, Hepworth J, et al. Family-Oriented Primary Care. 2nd ed. New York, NY: Springer; 2005.
21. Bensadon BA, Odenheimer GL. Current management decisions in mild cognitive impairment. Clin Geriatr Med. 2013:29;847-871.
22. Russ TC, Morling JR. Cholinesterase inhibitors for mild cognitive impairment. Cochrane Database Syst Rev. 2012;9:CD009132.
23. Sadowsky CH, Galvin JE. Guidelines for the management of cognitive and behavioral problems in dementia. J Am Board Fam Med. 2012;25:350-366.
24. Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593.
25. Fillit HM, Doody RS, Binaso K, et al. Recommendations for best practices in the treatment of Alzheimer’s disease in managed care. Am J Geriatr Pharmacother. 2006;4(suppl A):S9-S24;quiz S25-S28.
26. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525-1538.
27. US Food and Drug Administration. Public health advisory: Deaths with antipsychotics in elderly patients with behavioral disturbances. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm053171.htm. Published April 11, 2005. Updated August 16, 2013. Accessed December 2, 2014.
28. The American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60:616-631.
29. Brummel-Smith K. It’s time to require written informed consent when using antipsychotics in dementia. Br J Med Pract. 2008;1:4-6.
30. Huckans M, Hutson L, Twamley E, et al. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychol Rev. 2013;23:63-80.
31. Woods B, Aguirre E, Spector AE, et al. Cognitive stimulation to improve cognitive functioning in people with dementia. Cochrane Database Syst Rev. 2012;2:CD005562.
32. McLaren AN, Lamantia MA, Callahan CM. Systematic review of non-pharmacologic interventions to delay functional decline in community-dwelling patients with dementia. Aging Ment Health. 2013;17:655-666.
33. Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for mild to moderate Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev. 2013;6:CD003260.
34. Logsdon RG, McCurry SM, Teri L. Evidence-based psychological treatments for disruptive behaviors in individuals with dementia. Psychol Aging. 2007;22:28-36.
35. Raetz J. A nondrug approach to dementia. J Fam Pract. 2013;62:548-557.
36. Gallagher-Thompson D, Coon DW. Evidence-based psychological treatments for distress in family caregivers of older adults. Psychol Aging. 2007;22:37-51.
37. Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009:361:1529-1538.
38. Parsons C, Hughes CM, Passmore AP, et al. Withholding, discontinuing and withdrawing medications in dementia patients at the end of life: a neglected problem in the disadvantaged dying? Drugs Aging. 2010;27:435-449.
39. The American Geriatrics Society. Feeding tubes in advanced dementia position statement. The American Geriatrics Society Web site. Available at: http://www.americangeriatrics.org/files/documents/feeding.tubes.advanced.dementia.pdf. Accessed November 19, 2013.
40. Goodman C, Evans C, Wilcock J, et al. End of life care for community dwelling older people with dementia: an integrated review. Int J Geriatr Psychiatry. 2010;25:329-337.
41. Storey CP. A quick-reference guide to the hospice and palliative care training for physicians: UNIPAC self-study program. American Academy of Hospice and Palliative Medicine. Chicago; 2009.
42. Kaszniak AW, Kligman EW. Hospice care for patients with dementia. Elder Care. 2013. Arizona Alzheimer's Consortium Web site. Available at: http://azalz.org/wp-content/uploads/2013/07/Hospice-Care-for-Pts-with-Dementia.pdf. Accessed December 2, 2014.
43. Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: Preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27:1-30.
44. Patterson C, Feightner JW, Garcia A, et al. Diagnosis and treatment of dementia: 1. Risk assessment and primary prevention of Alzheimer disease. CMAJ. 2008;178:548-556.
45. Carrillo MC, Brashear HR, Logovinsky V, et al. Can we prevent Alzheimer’s disease? Secondary “prevention” trials in Alzheimer’s disease. Alzheimers Dement. 2013;9:123-131.e1.
46. Salloway S, Sperling R, Fox NC, et al; Bapineuzumab 301 and 302 Clinical Trial Investigators. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s Disease. N Engl J Med. 2014;370:322-333.
47. Doody RS, Thomas RG, Farlow M, et al; Alheimer’s Disease Cooperative Study Steering Committee; Solanezumab Study Group. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s Disease. N Engl J Med. 2014:370:311-321.