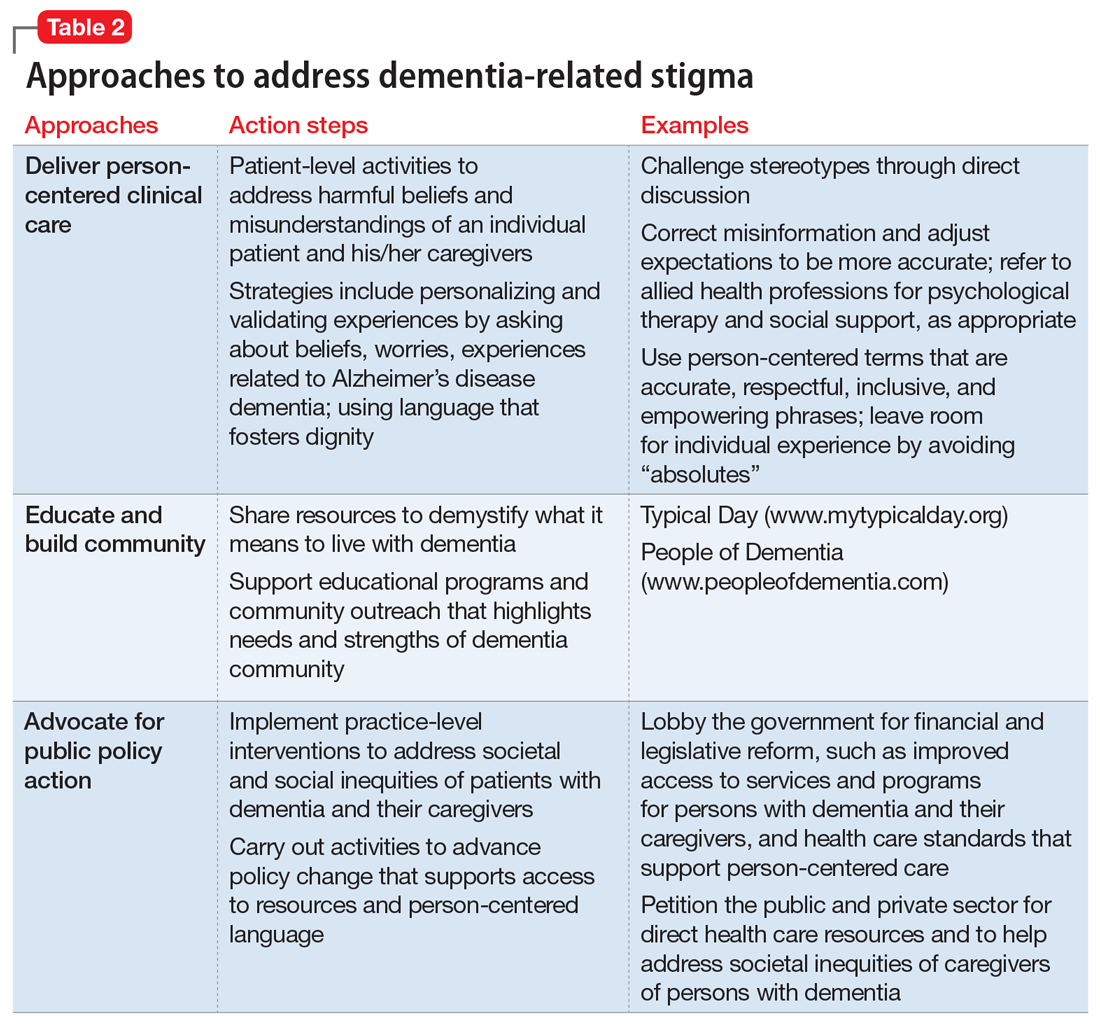
User login
Treatment of delirium: A review of 3 studies
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
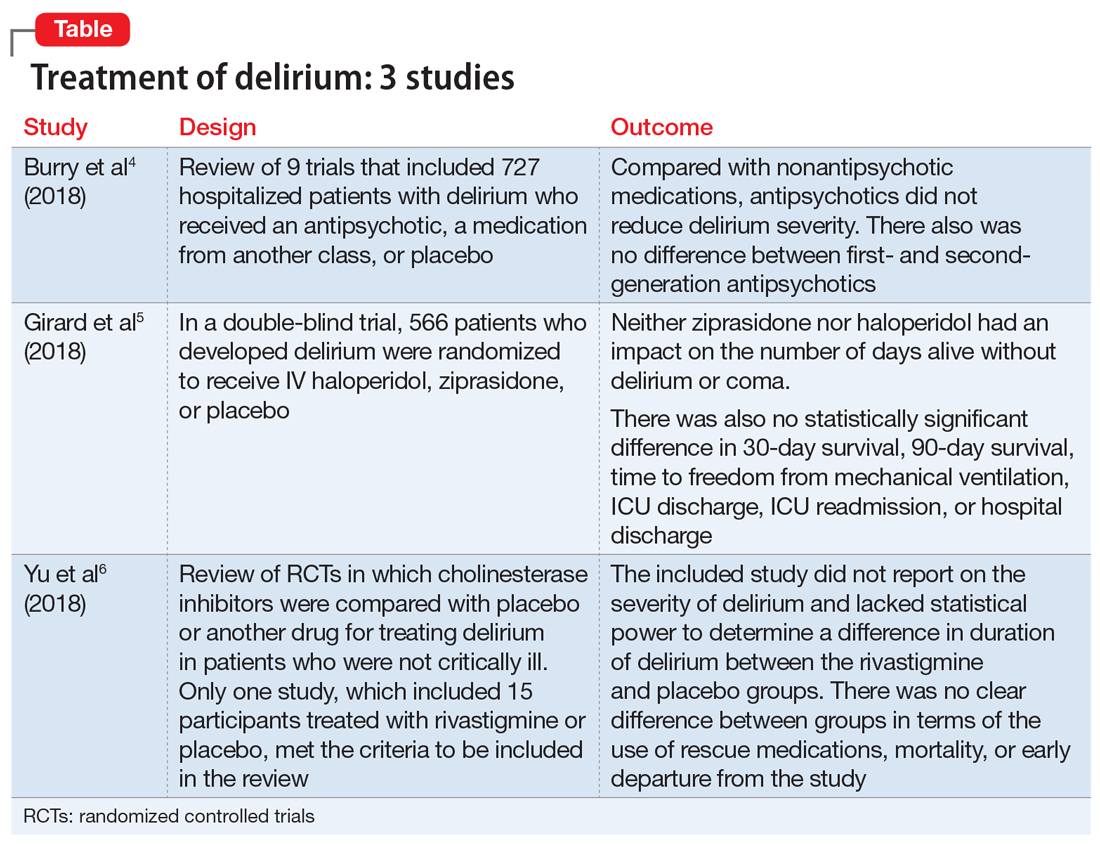
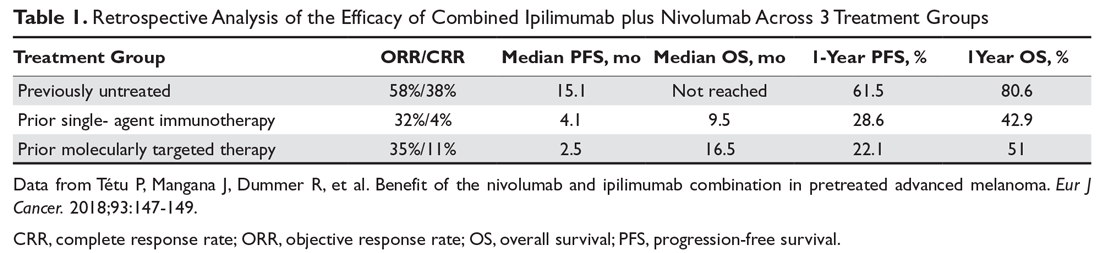
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
Delirium is defined as a disturbance in attention, awareness, and cognition that develops over hours to days as a direct physiological consequence of an underlying medical condition and is not better explained by another neurocognitive disorder.1 This condition is found in up to 31% of general medical patients and up to 87% of critically ill medical patients. Delirium is commonly seen in patients who have undergone surgery, those who are in palliative care, and patients with cancer.2 It is associated with increased morbidity and mortality. Compared with those who do not develop delirium, patients who are hospitalized who develop delirium have a higher risk of longer hospital stays, post-hospitalization nursing facility placement, persistent cognitive dysfunction, and death.3
Thus far, the management and treatment of delirium have been complicated by an incomplete understanding of the pathophysiology of this condition. However, prevailing theories suggest a dysregulation of neurotransmitter synthesis, function, or availability.2 Recent literature reflects this theory; researchers have investigated agents that target dopamine or acetylcholine. Below we review some of this recent literature on treating delirium; these studies are summarized in the Table.4-6
1. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594.
An extensive literature review identified randomized or quasi-randomized trials on the treatment of delirium among non-critically ill hospitalized patients in which antipsychotics were compared with nonantipsychotic medications or placebo, or in which a first-generation antipsychotic (FGA) was compared with a second-generation antipsychotic (SGA).4
Study design
- Researchers conducted a literature review of 9 trials that included 727 hospitalized but not critically ill patients (ie, they were not in an ICU) who developed delirium.
- Four trials compared an antipsychotic with a medication from another drug class or with placebo.
- Seven trials compared a FGA with an SGA.
Outcomes
- Although the intended primary outcome was the duration of delirium, none of the included studies reported on duration of delirium. Secondary outcomes were delirium severity and resolution, mortality, hospital length of stay, discharge disposition, health-related quality of life, and adverse effects.
- Among the secondary outcomes, no statistical difference was observed between delirium severity, delirium resolution, or mortality.
- None of the included studies reported on hospital length of stay, discharge disposition, or health-related quality of life.
- Evidence related to adverse effects was determined to be very low quality due to potential bias, inconsistency, and imprecision.
Conclusion
- A review of 9 randomized trials did not find any evidence supporting the use of antipsychotics for treating delirium. However, most of the studies included were of lower quality because they were single-center trials with insufficient sample sizes, heterogeneous study populations, and risk of bias.
Continue to: 2...
2. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
Study design
- Researchers used the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) to assess 1,183 patients with acute respiratory failure or shock in 16 medical centers in the United States.5
- Overall, 566 patients developed delirium and were randomized in a double-blind fashion to receive IV haloperidol, ziprasidone, or placebo.
- Haloperidol was started at 2.5 mg (age <70) or 1.25 mg (age ≥70) every 12 hours and titrated to a maximum dose of 20 mg/d as tolerated.
- Ziprasidone was started at 5 mg (age <70) or 2.5 mg (age ≥70) every 12 hours and titrated to a maximum dose of 40 mg/d as tolerated.
Outcomes
- The primary endpoint was days alive without delirium or coma. Secondary endpoints included duration of delirium, time to freedom from mechanical ventilation, time to final successful ICU discharge, time to ICU readmission, time to successful hospital discharge, 30-day survival, and 90-day survival.
- Neither ziprasidone nor haloperidol had an impact on number of days alive without delirium or coma.
- There was also no statistically significant difference in 30-day survival, 90-day survival, time to freedom from mechanical ventilation, ICU discharge, ICU readmission, or hospital discharge.
Conclusion
- This study found no evidence supporting haloperidol or ziprasidone for the treatment of delirium. Because all patients in this study were critically ill, it is unclear if these results would be generalizable to other hospitalized patient populations.
3. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Study design
- A literature review identified published and unpublished randomized controlled trials in English and Chinese in which cholinesterase inhibitors were compared with placebo or another drug for treating delirium in non-critically ill patients.6
- Only one study met the criteria to be included in the review. It included 15 participants treated with rivastigmine or placebo.
Outcomes
- The intended primary outcomes were severity of delirium and duration of delirium. However, the included study did not report on the severity of delirium. It also lacked statistical power to determine a difference in duration of delirium between the rivastigmine and placebo groups.
- Secondary outcomes included use of a rescue medication, persistent cognitive impairment, length of hospitalization, institutionalization, mortality, cost of intervention, early departure from the study, and quality of life.
- There was no clear difference between the rivastigmine group and the placebo group in terms of the use of rescue medications, mortality, or early departure from the study. The included study did not report on persistent cognitive impairment, length of hospitalization, institutionalization, cost of intervention, or quality of life.
Conclusion
- This literature review did not find any evidence to support the use of cholinesterase inhibitors for treating delirium. However, because this review included only a single small study, limited conclusions can be drawn from this research.
In summary, delirium is common, especially among patients who are acutely medically ill, and it is associated with poor physical and cognitive clinical outcomes. Because of these poor outcomes, it is important to identify delirium early and intervene aggressively. Clearly, there is a need for further research into short- and long-term treatments for delirium.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
1. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
2. Maldonado JR. Acute brain failure: pathophysiology, diagnosis, management, and sequelae of delirium. Crit Care Clin. 2017;33(3):461-519.
3. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466.
4. Burry L, Mehta S, Perreault MM, et al. Antipsychotics for treatment of delirium in hospitalized non-ICU patients. Cochrane Database Syst Rev. 2018;6:CD005594. doi: 10.1002/14651858.CD005594.pub3.
5. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516.
6. Yu A, Wu S, Zhang Z, et al. Cholinesterase inhibitors for the treatment of delirium in non-ICU settings. Cochrane Database Syst Rev. 2018;6:CD012494.
Nothing to sneeze at: Upper respiratory infections and mood disorders
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
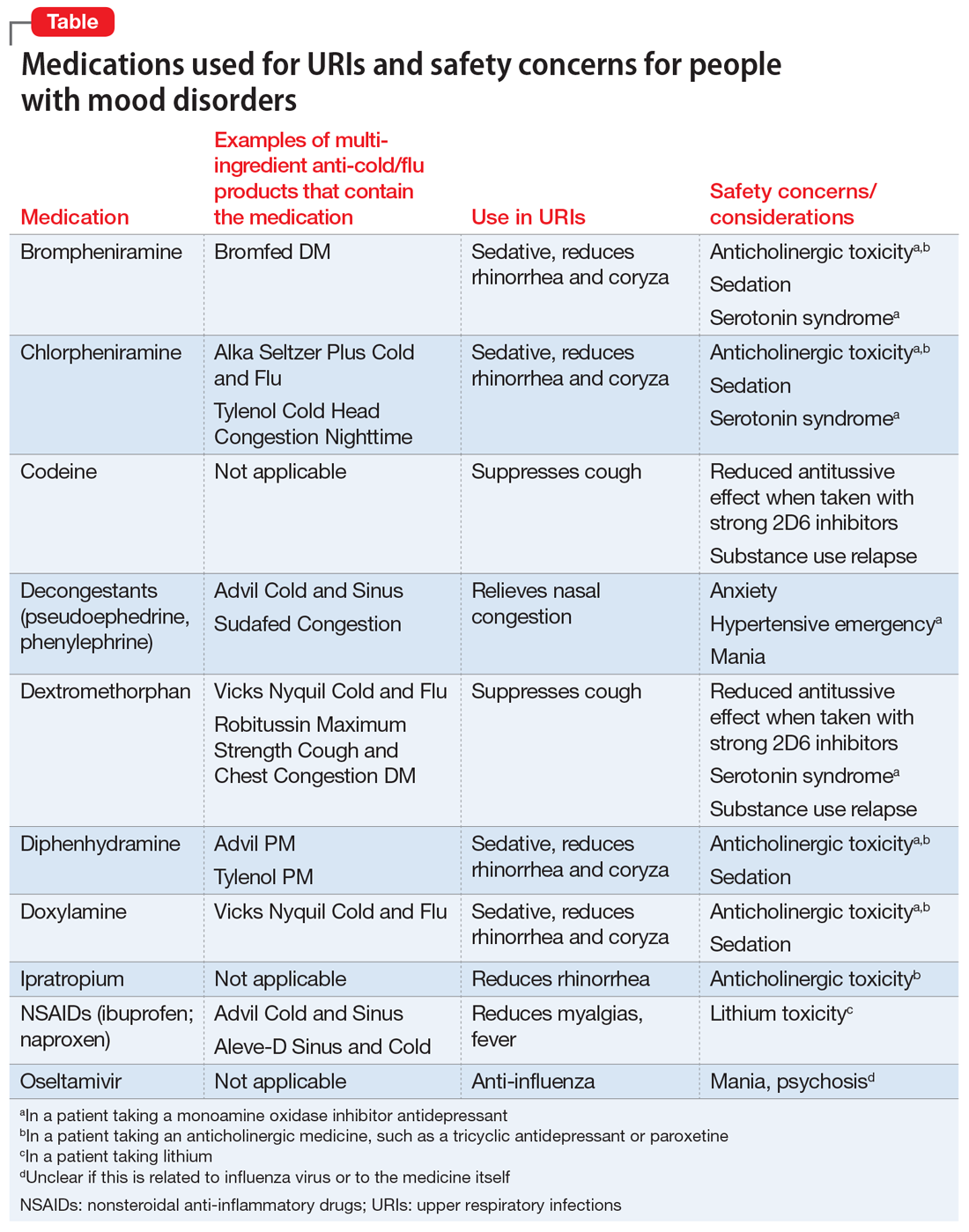
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
Acute upper respiratory infections (URIs) often lead to mild illnesses, but they can be severely destabilizing for individuals with mood disorders. Additionally, the medications patients often take to target symptoms of the common cold or influenza can interact with psychiatric medications to produce dangerous adverse events or induce further mood symptoms. In this article, we describe the relationship between URIs and mood disorders, the psychiatric diagnostic challenges that arise when evaluating a patient with a URI, and treatment approaches that emphasize psychoeducation and watchful waiting, when appropriate.
A bidirectional relationship
Acute upper respiratory infections are the most common human illnesses, affecting almost 25 million people annually in the United States.1 The common cold is caused by >200 different viruses; rhinovirus and coronavirus are the most common. Influenza, which also attacks the upper respiratory tract, is caused by strains of influenza A, B, or C virus.2 The common cold may present initially with mild symptoms of headache, sneezing, chills, and sore throat, and then progress to nasal discharge, congestion, cough, and malaise. When influenza strikes, patients may have a sudden onset of fever, headache, cough, sore throat, myalgia, congestion, weakness, anorexia, and gastrointestinal (GI) symptoms. Production of URI symptoms results from viral cytopathic activity along with immune activation of inflammatory pathways.2,3 The incidence of colds is inversely correlated with age; adults average 2 to 4 colds per year.4,5 Cold symptoms peak at 1 to 3 days and typically last 7 to 10 days, but can persist up to 3 weeks.6 With influenza, fever and other systemic symptoms last for 3 days but can persist up to 8 days, while cough and lethargy can persist for another 2 weeks.7
Upper respiratory infections have the potential to disrupt mood. Large studies of psychiatrically-healthy undergraduate students have found that compared with healthy controls, participants with URIs endorsed a negative affect within the first week of viral illness,8 and that the number and intensity of URI symptoms caused by cold viruses were correlated with the degree of their negative affect.9 A few case reports have documented instances of individuals with no previous personal or family psychiatric history developing full manic episodes in the setting of influenza.10-12 One case report described an influenza-induced manic episode in a patient with pre-existing psychiatric illness.13 There are no published case reports of common cold viruses inducing a full depressive or manic episode. If cold symptom severity correlates with negative affect among individuals with no psychiatric illness, and if influenza can induce manic episodes, then it is reasonable to expect that patients with pre-existing mood disorders could have an elevated risk for mood disturbances when they experience a URI (Box).
Box
Ms. E is a 35-year-old financial analyst with bipolar disorder type I and alcohol use disorder in sustained remission. She had been euthymic for the last 3 years, receiving weekly psychotherapy and taking lamotrigine, 350 mg/d, lithium ER, 900 mg/d (lithium level: 1.0 mmol/L), lurasidone, 60 mg/d, and clonazepam, 1 mg/d. At her most recent quarterly outpatient psychiatrist visit, she says her depression had returned. She reports 1 week of crying spells, initial and middle insomnia, anhedonia, feelings of worthlessness, fatigue, poor concentration, and poor appetite. She denies having suicidal ideation or manic or psychotic symptoms, and she continues to abstain from alcohol, illicit drugs, and tobacco. She has been fully adherent to her medication regimen and has not added any new medications or made any dietary changes since her last visit. She is puzzled as to what brought on this depression recurrence and says she feels defeated by the bipolar illness, a condition she had worked tirelessly to manage. When asked about changes in her health, she reports that about 1.5 weeks ago she developed a cough, nasal congestion, rhinorrhea, and fatigue. Because of her annual goal to run a marathon, she continues to train, albeit at a slower pace, and has not had much time to rest because of her demanding job.
The psychiatrist explains to Ms. E that an upper respiratory infection (URI) can sometimes induce depressive symptoms. Given the patient’s lengthy period of euthymia and the absence of new medicines, dietary changes, or drug/alcohol intake, the psychiatrist suspects that the cause of her mood episode recurrence is related to the URI. Hearing this is a relief for Ms. E. She and the psychiatrist decide to refrain from making any medication changes with the expectation that the URI would soon resolve because it had already persisted for 1.5 weeks. The psychiatrist tells Ms. E that if it does not and her symptoms worsen, she should call him to discuss treatment options. The psychiatrist also encourages Ms. E to take a temporary break from training and allow her body to rest.
Three weeks later, Ms. E returns and reports that both the URI symptoms and the depressive symptoms lifted a few days after her last visit.
Mood disorders may also be a risk factor for contracting URIs. Patients with mood disorders are more likely than healthy controls to be seropositive for markers of influenza A, influenza B, and coronavirus, and those with a history of suicide attempts are more likely to be seropositive for markers of influenza B.14 In a community sample of German adults age 18 to 65, those with mood disorders had a 35% higher likelihood of having had a cold within the last 12 months compared with those without a mood disorder.15 A survey of Korean employees found the odds of having had a cold in the last 4 months were up to 2.5 times greater for individuals with elevated scores on a depression symptom severity scale compared with those with lower scores.16 Because these studies were retrospective, recall bias may have impacted the results, as patients who are depressed are more likely to recall negative recent events.17
Proposed mechanisms
Researchers have proposed several mechanisms to explain the association of URIs with mood episodes. Mood disorders, such as bipolar disorder and major depressive disorder (MDD), are associated with chronic dysregulation of the innate immune system, which leads to elevated levels of cortisol and pro-inflammatory cytokines.18,19 Men with chronic low-grade inflammation are more vulnerable to all types of infection, including those that cause respiratory illnesses.20 High levels of stress,21 a negative affective style,22 and depression23 have all been associated with reduced antibody response and/or cellular-mediated immunity following vaccination, which suggests a possible mechanism for the vulnerability to infection found in individuals with mood disorders. On the other hand, after influenza vaccination, patients with depression produce a greater and more prolonged release of the cytokine interleukin 6, which perpetuates the state of chronic low-grade inflammation.24 Additionally, patients with mood disorders may engage in behaviors that reduce immune functioning, such as using illicit substances, drinking alcohol, smoking cigarettes, consuming an unhealthy diet, or living a sedentary lifestyle.
Conversely, there are several mechanisms by which a URI could induce a mood episode in a patient with a mood disorder. Animal studies have shown that a non-CNS viral infection can lead to depressive behavior by inducing peripheral interferon-beta release. This signaling protein binds to a receptor on the endothelial cells of the blood-brain barrier, inducing the release of additional cytokines that affect neuronal functioning.25 Among patients receiving interferon treatments for hepatitis C, a history of depression increased their likelihood of becoming depressed during their treatment course, which suggests people with mood disorders have a sensitivity to peripheral cytokines.26
Sleep interruptions from nighttime coughing or nasal congestion can increase the risk of a recurrence of hypomania or mania in patients with bipolar disorder,27 or a recurrence of depression in a patient with MDD.28 The stress that comes with missed work days or the inability to take care of other personal responsibilities due to a URI may increase the risk of becoming depressed in a patient with bipolar disorder or MDD. When present, GI symptoms such as vomiting and diarrhea can reduce the absorption of psychotropic medications and increase the risk of a mood recurrence. Finally, the treatments used for URIs may also contribute to mood instability. Case reports have described instances where patients with URIs developed mania or depression when exposed to medications such as intranasal corticosteroids,29 nasal decongestants,30,31 and anti-influenza treatments.32,33
Continue to: A diagnostic challenge
A diagnostic challenge
Making the diagnosis of a major depressive episode can be challenging in patients who present with a URI, particularly in those who are highly vigilant for relapse and seek care soon after mood symptoms emerge. Many symptoms overlap between the conditions, including insomnia, hypersomnia, reduced interest, anhedonia, fatigue, impaired concentration, and anorexia. Symptoms that are more specific for a major depressive episode include depressed mood, pathologic guilt, worthlessness, and suicidal ideation. Of course, a major depressive episode and a URI are not mutually exclusive and can occur simultaneously. However, incorrectly diagnosing recurrence of a major depressive episode in a euthymic patient who has a URI could lead to unnecessary changes to psychiatric treatment.
Psychoeducation is key
Teach patients about the bidirectional relationship between URIs and mood symptoms to reduce anxiety and confusion about the cause of the return of mood symptoms. Telling patients that they can expect their mood symptoms to be of short duration and self-limiting due to the URI can provide helpful reassurance.
Because it is possible that the mood symptoms will be transient, increasing psychotropic doses or adding a new psychotropic medication may not be necessary. The decision to initiate such changes should be made collaboratively with patients and should be based on the severity and duration of the patient’s mood symptoms. Symptoms that may warrant a medication change include psychosis, suicidal ideation, or mania. If a patient taking lithium becomes dehydrated because of excessive vomiting, diarrhea, or anorexia, temporarily reducing the dose or stopping the medication until the patient is hydrated may be appropriate.
When a patient presents with a URI, make basic URI treatment recommendations, including rest, hydration, and the use of over-the-counter (OTC) anti-cold medications and zinc.34 Encourage patients with suspected influenza to visit their primary care physician so that they may receive an anti-influenza medication. However, also remind patients about the psychiatric risks associated with some of these treatments and their potential interactions with psychotropics (Table). For example, many OTC cold formulations contain dextromethorphan or chlorpheniramine, both of which have weak serotonin reuptake properties and should not be combined with a monoamine oxidase inhibitor. Such cold formulations may also contain non-steroidal anti-inflammatory agents, which could elevate lithium levels. Codeine, which is often prescribed to suppress the coughing reflex, can lead a patient with a history of substance use to relapse on their drug of choice.
Also recommend lifestyle modifications to help patients reduce their risk of infection. These includes frequent hand washing, avoiding or limiting alcohol use, avoiding cigarettes, exercising regularly, consuming a Mediterranean diet, and receiving scheduled immunizations. To avoid contracting a URI and infecting patients, wash your hands or use an alcohol-based cleanser after shaking hands with patients. Finally, if a patient does not have a primary care physician, encourage him/her to find one to help manage subsequent infections.
Continue to: Bottom Line
Bottom Line
Patients with mood disorders may have an increased risk of developing an upper respiratory infection (URI), which can worsen their mood. Clinicians must make psychotropic treatment changes cautiously and guide patients to select safe over-the-counter medications for relief of URI symptoms.
Related Resources
- Centers for Disease Control and Prevention. Cold versus flu. www.cdc.gov/flu/about/qa/coldflu.htm.
- Centers for Disease Control and Prevention. Nonspecific upper respiratory tract infection. www.cdc.gov/getsmart/community/materials-references/print-materials/hcp/adult-tract-infection.pdf.
Drug Brand Names
Clonazepam • Klonopin
Ipratropium • Atrovent
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Oseltamivir • Tamiflu
Paroxetine • Paxil
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
1. Gonzales R, Malone DC, Maselli JH, et al. Excessive antibiotic use for acute respiratory infections in the United States. Clin Infect Dis. 2001;33(6):757-762.
2. Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718-725.
3. Passioti M, Maggina P, Megremis S, et al. The common cold: potential for future prevention or cure. Curr Allergy Asthma Rep. 2014;14(2):413.
4. Monto AS, Ullman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA. 1974;227(2):164-169.
5. Monto AS. Studies of the community and family: acute respiratory illness and infection. Epidemiol Rev. 1994;16(2):351-373.
6. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51-59.
7. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697-708.
8. Hall S, Smith A. Investigation of the effects and aftereffects of naturally occurring upper respiratory tract illnesses on mood and performance. Physiol Behav. 1996;59(3):569-577.
9. Smith A, Thomas M, Kent J, et al. Effects of the common cold on mood and performance. Psychoneuroendocrinology. 1998;23(7):733-739.
10. Ayub S, Kanner J, Riddle M, et al. Influenza-induced mania. J Neuropsychiatry Clin Neurosci. 2016;28(1):e17-e18.
11. Maurizi CP. Influenza and mania: a possible connection with the locus ceruleus. South Med J. 1985;78(2):207-209.
12. Steinberg D, Hirsch SR, Marston SD, et al. Influenza infection causing manic psychosis. Br J Psychiatry. 1972;120(558):531-535.
13. Ishitobi M, Shukunami K, Murata T, et al. Hypomanic switching during influenza infection without intracranial infection in an adolescent patient with bipolar disorder. Pediatr Emerg Care. 2011;27(7):652-653.
14. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord. 2011;130(1-2):220-225.
15. Adam Y, Meinlschmidt G, Lieb R. Associations between mental disorders and the common cold in adults: a population-based cross-sectional study. J Psychosom Res. 2013;74(1):69-73.
16. Kim HC, Park SG, Leem JH, et al. Depressive symptoms as a risk factor for the common cold among employees: a 4-month follow-up study. J Psychosom Res. 2011;71(3):194-196.
17. Dalgleish T, Werner-Seidler A. Disruptions in autobiographical memory processing in depression and the emergence of memory therapeutics. Trends Cogn Sci. 2014;18(11):596-604.
18. Rosenblat JD, McIntyre RS. Bipolar disorder and inflammation. Psychiatr Clin North Am. 2016;39(1):125-137.
19. Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075-1091.
20. Kaspersen KA, Dinh KM, Erikstrup LT, et al. Low-grade inflammation is associated with susceptibility to infection in healthy men: results from the Danish Blood Donor Study (DBDS). PLoS One. 2016;11(10):e0164220.
21. Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci U S A. 1996;93(7):3043-3047.
22. Rosenkranz MA, Jackson DC, Dalton KM, et al. Affective style and in vivo immune response: neurobehavioral mechanisms. Proc Natl Acad Sci U S A. 2003;100(19):11148-1152.
23. Irwin MR, Levin MJ, Laudenslager ML, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis. 2013;56(8):1085-1093.
24. Glaser R, Robles TF, Sheridan J, et al. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch Gen Psychiatry. 2003;60(10):1009-1014.
25. Blank T, Detje CN, Spiess A, et al. Brain endothelial- and epithelial-specific interferon receptor chain 1 drives virus-induced sickness behavior and cognitive impairment. Immunity. 2016;44(4):901-912.
26. Smith KJ, Norris S, O’Farrelly C, et al. Risk factors for the development of depression in patients with hepatitis C taking interferon-α. Neuropsychiatr Dis Treat. 2011;7:275-292.
27. Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843.
28. Cho HJ, Lavretsky H, Olmstead R, et al. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. 2008;165(12):1543-1550.
29. Saraga M. A manic episode in a patient with stable bipolar disorder triggered by intranasal mometasone furoate. Ther Adv Psychopharmacol. 2014;4(1):48-49.
30. Kandeger A, Tekdemir R, Sen B, et al. A case report of patient who had two manic episodes with psychotic features induced by nasal decongestant. European Psychiatry. 2017;41(Suppl):S428.
31. Waters BG, Lapierre YD. Secondary mania associated with sympathomimetic drug use. Am J Psychiatry. 1981;138(6):837-838.
32. Ho LN, Chung JP, Choy KL. Oseltamivir-induced mania in a patient with H1N1. Am J Psychiatry. 2010;167(3):350.
33. Jeon SW, Han C. Psychiatric symptoms in a patient with influenza A (H1N1) treated with oseltamivir (Tamiflu): a case report. Clin Psychopharmacol Neurosci. 2015;13(2):209-211.
34. Allan GM, Arroll B. Prevention and treatment of the common cold: making sense of the evidence. CMAJ. 2014;186(3):190-199.
Stigma in dementia: It’s time to talk about it
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
Dementia is a family of disorders characterized by a decline in multiple cognitive abilities that significantly interferes with an individual’s functioning. An estimated 50 million people are living with a dementia worldwide.1 Alzheimer’s disease (AD) is the leading cause of dementia, accounting for approximately two-thirds of dementia cases.1 These numbers are expected to increase dramatically in the upcoming decades.
Sociologist Erving Goffman defined stigma as “an attribute, behaviour, or reputation which is socially discrediting in a particular way: it causes an individual to be mentally classified by others in an undesirable, rejected stereotype rather than in an accepted, normal one.”2 Goffman2 defined 3 broad categories of stigma: public, self, and courtesy (Table 12).
Considerable evidence shows that the combined impact of having dementia and the negative response to the diagnosis significantly undermines an individual’s psychosocial well-being and quality of life.3 Persons with dementia (PwD) commonly report a loss of identity and self-worth, and stigma appears to deepen this distress.3 Stigma also negatively affects individuals associated with PwD, including family members and professionals. In this article, we discuss the impact of dementia-related stigma, and steps you can take to address it, including implementing person-centered clinical practices, promoting anti-stigma messaging campaigns, and advocating for public policy action to improve the lives of PwD and their families.
A pervasive problem
Although the Alzheimer’s Society International and the World Health Organization acknowledge that stigma has a central role in defining the experience of AD, how stigma may present, how clinicians and researchers can recognize and measure stigma, and how to best combat it have been understudied.3-5 A recent systematic literature review examined worldwide evidence on dementia-related stigma over the past decade.6 Hermann et al6 found that health care providers and the general public may hold stigmatizing attitudes toward PwD, and that stigma may be particularly harsh among racial and ethnic minorities, although the literature is scarce in this area. Cultural factors may also worsen stigma, and stigma may be associated with reduced awareness of dementia services and reduced help-seeking among minority groups.7,8 Studies show that stigmatizing attitudes are more pronounced in people with limited knowledge of dementia, in those with little contact with PwD, in men, in younger individuals, and in the context of cultural interpretations of dementia.6 Health care providers can also sometimes contribute to the perpetuation of stigma.6
In terms of standardized scales or instruments for evaluating dementia-related stigma, there is no uniformly accepted “gold standard” measure, which makes it difficult to compare studies.6 In order to effectively study efforts to reduce stigma, researchers need to identify and establish a consensus on rating scales for evaluating stigma among PwD, caregivers, and the general public. Three instruments that may be used for this purpose are the Family Stigma in Alzheimer’s Disease Scale (FS-ADS),9 the Stigma Scale for Chronic Illness (SSCI),10 and the Perceptions Regarding Investigational Screening for Memory in Primary Care (PRISM-PC).11
The detrimental effects of stigma
Burgener et al12 reported that personal stigma impacted functioning and quality of life in PwD. Higher levels of stigma were associated with higher anxiety, depression, and behavioral symptoms and lower self-esteem, social support, participation in activities, personal control, and physical health.12 Personal characteristics that may affect stigma include gender, location (rural vs urban), ethnicity, education level, and living arrangements (alone vs with family).12
In a subset of PwD with early-stage memory loss (n = 22), Burgener and Buckwalter13 found that 42% of participants were reluctant to reveal their diagnosis to others, with some fearing they would no longer be allowed to live alone and would be “sent to a facility.” In addition, 46% indicated they did not want “to be talked about like they were not there.” More than 50% of participants reported changes in their social network after receiving the diagnosis, including reducing activities and limiting types of contacts (ie, telephone only) or interacting only when “people come to me.” Participants were most comfortable with good friends “who understand” and persons within their faith communities. When asked about how they were treated by family members, >50% of participants described being treated differently, including loss of financial independence, more limited contact, and being “treated like a baby” by their children, who in general were uncomfortable talking about the diagnosis.
Continue to: In a recent study...
In a recent study by Harper et al,14 stigma was prevalent in the experience of PwD. One participant disclosed:
“I think there is [are] people I know who don’t ask me to go places or do things ’cause I have a dementia…I think lots of people don’t know what dementia is and I think it scares them ’cause they think of it as crazy. It hurts…”
Another participant said:
“I have had friends for over thirty years. They have turned their backs on me…we used to go for walks and they would phone me and go for coffee. Now I don’t hear from any of them…those aren’t true friends…true friends will stand behind you, not in front of you. That’s why I am not happy.”
Overall, quantitative and qualitative findings indicate multiple, detrimental effects of personal stigma on PwD. These effects fit well with measures of self-stigma, including social rejection (eg, being treated differently, participating in fewer activities, and having fewer friends), internalized shame (eg, being treated like a child, having fewer responsibilities, others acting as if dementia is “contagious”), and social isolation (eg, being less outgoing, feeling more comfortable in small groups, having limited social contacts).15
Continue to: Receiving a diagnosis of dementia...
Receiving a diagnosis of dementia presents patients and their families with psychological and social challenges.16 Many of these challenges are the consequence of stigma. A broad range of efforts are underway worldwide to reduce dementia-related stigma. These efforts include programs to promote public awareness and education, campaigns to develop inclusive social policies, and skills-based training initiatives to promote delivery of patient-centered care by clinicians and educators.3,17,18 Many of these efforts share a common focus on promoting the “dignity” and “personhood” of PwD in order to disrupt stereotypes or fixed, oversimplified beliefs associated with dementia.
Implementing person-centered clinical care
In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Health care communications that call attention to stereotypes may allow PwD to identify stereotypes as well as inaccuracies in those stereotypes. Interventions that validate the value of diversity can help PwD accept the ways in which they may not conform to social norms. This could include language such as “There is no one way to have Alzheimer’s disease. A person’s experience can differ from what others might experience or expect, and that’s okay.” In addition, the use of language that is accurate, respectful, inclusive, and empowering can support PwD and their caregivers.19,20 For example, referring to PwD as “individuals living with dementia” rather than “those who are demented” conveys respect and appreciation for personhood. Other clinicians have provided additional practical suggestions.21
Anti-stigma messaging campaigns
The mass media is a common source of stereotypes about AD and other dementias. They typically present a “worst-case” scenario that promotes ageism, gerontophobia, and negative emotions, which may worsen stigma and discrimination towards PwD and the people who care for them. However, public messaging campaigns are emerging to counter negative messages and stereotypes in the mass media. Projects such as Typical Day, People with Dementia, and other online anti-stigma messaging campaigns allow a broad audience to gain a more nuanced understanding of the lives of PwD and their caregivers. These projects are rich resources that offer education and personal stories that can counter common stereotypes about dementia.
Typical Day is a photography project developed and maintained by clinicians and researchers at the University of Pennsylvania. Since early 2017, the project has provided a forum for individuals with mild cognitive impairment or dementia to document their lives and show what it means to them to live with dementia. Participants in the project photo-document the people, places, and objects that define their daily lives. They review and explain these photos with researchers at Penn Memory Center, who help them tell their stories. The participants’ stories, the photos they capture, and their portraits are available at www.mytypicalday.org.
People of Dementia. Storytelling is a powerful way to raise awareness of and reduce the stigma associated with dementia. For PwD, telling their stories can be an effective and therapeutic way to communicate their emotions and deliver an important message. In the blog People of Dementia (www.peopleofdementia.com),22,23 PwD highlight who they were before the disease and how things have changed, with family members highlighting the challenges of caring for a person with dementia.
Continue to: The common thread is...
The common thread is the enduring “person” behind the exterior that is obscured by dementia. By allowing the audience to form a connection with who the individual was prior to the disease, and understanding the changes that have come as a result of dementia to both PwD and their support network, readers gain a greater appreciation of those affected by dementia. Between May 1, 2017 and May 31, 2019, the blog had more than 3,860 visitors. In an accompanying online survey (N = 57), 79% of respondents agreed/strongly agreed that after visiting the People of Dementia blog, they had a better understanding of the changes that occur as a result of cognitive impairment/dementia (Figure 1). Almost two-thirds of respondents (65%) agreed/strongly agreed that they felt more comfortable interacting with PwD (Figure 2). Additionally, 60% of respondents agreed/strongly agreed that they were more encouraged to work with PwD, and 90% agreed/strongly agreed that they had a greater appreciation of the challenges of being a caregiver for PwD. Overall, these findings suggest that the People of Dementia blog is useful for engaging the public and promoting a better understanding of dementia.
Work for policy changes
Clinicians can support public policy through education and advocacy both in the delivery of care and as spokespersons and stakeholders in their local communities. Public policies are important for providing access to medical and social services to meet the needs of PwD and their caregivers. The absence—real or perceived—of sufficient resources exacerbates dementia-related stigma. In addition to facilitating access to resources, national dementia strategies or legal frameworks, such as the National Alzheimer’s Project Act in the United States, include policy initiatives to identify and promote communication approaches that are effective and sensitive with respect to people living with dementia and their caregivers.
State and local legislators and patient advocates are leading policy efforts to reduce dementia-related stigma. For example, Colorado recently changed statutory references from being specific to diseases that cause dementia to the broader, more inclusive phrase “dementia diseases and related disabilities.”18 In addition to making funds available to support caregiving services for PwD, this legislative change added training for first responders to better meet the needs of missing PwD, and shifted the terminology used to diagnose and communicate about diseases causing dementia. The shift in language added new terminology that was chosen for being more person-centered to replace prior references to “senior senility,” “senility,” and other terms with pejorative meanings.
In Canada, a National Dementia Strategy will commit the Canadian government to action with definitive timelines, targets, reporting structures, and measurable outcomes.24
Table 2 summarizes approaches to a
Continue to: An open discussion
An open discussion
Larger studies and testing of diverse approaches are needed to better understand whether intergenerational initiatives or other approaches can genuinely modify stigmatizing attitudes in various dementia populations, especially considering language, health literacy, cultural preferences, and other needs. The identified effects on physical and mental health, quality of life, self-esteem, and behavioral symptoms further support the extensive, negative effects of self-stigma on PwD, and emphasize the need to develop and test interventions to ameliorate these effects.
We presented at a Stigma Symposium at the 2018 Gerontological Society of America Annual Scientific Meeting in Boston, Massachusetts.25 Attendees of this conference shared our concerns about the detrimental effects of stigma. The main question we were asked was “What can we do to reduce stigma?” Perhaps the most immediate response is that in order to move the stigma dial, clinicians need to recognize that stigma has multiple, broad-reaching, and negative effects on PwD and their families.6 Bringing the discussion into the open and targeting stigma at multiple levels needs to be addressed by clinicians, researchers, administrators, and society at large.
Bottom Line
Stigma has multiple, broad-reaching, and negative effects on persons with dementia and their families. In clinical practice, direct discussion that encourages reflection and the use of effective and sensitive communication can help to limit passing on stigmatizing beliefs and to reduce negative stereotypes associated with the disease. Anti-stigma messaging campaigns and public policy changes also can be used to address societal and social inequities of patients with dementia and their caregivers.
Related Resources
- Khoury R, Shach R, Nair A, et al. Can lifestyle modifications delay or prevent Alzheimer’s disease? Current Psychiatry. 2019;18(1):29-36,38.
- Burke AD, Burke WJ. Antipsychotics for patients with dementia: The road less traveled. Current Psychiatry. 2018;17(10):26-32,35-37.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
1. World Health Organization. Towards a dementia plan: a WHO guide. https://www.who.int/mental_health/neurology/dementia/policy_guidance/en/. Published 2018. Accessed May 28, 2019.
2. Goffman E. Stigma. New York, NY: Prentice-Hall; 1963:1-123.
3. Alzheimer’s Disease International. World Alzheimer Report 2012: overcoming the stigma of dementia. https://www.alz.co.uk/research/WorldAlzheimerReport2012.pdf. Published 2012. Accessed May 28, 2019.
4. Blay SL, Peluso ETP. Public stigma: the community’s tolerance of Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(2):163-171.
5. Piver LC, Nubukpo P, Faure A, et al. Describing perceived stigma against Alzheimer’s disease in a general population in France: the STIG-MA survey. Int J Geriatr Psychiatry. 2013;28(9):933-938.
6. Herrmann LK, Welter E, Leverenz J, et al. A systematic review of dementia-related stigma research: can we move the stigma dial? Am J Geriatr Psychiatry. 2018;26(3):316-331.
7. Eng KJ, Woo BKP. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3-e4. doi:10.1016/j.genhosppsych.2014.11.003.
8. Jang Y, Kim G, Chiriboga D. Knowledge of Alzheimer’s disease, feelings of shame, and awareness of services among Korean American elders. J Aging Health. 2010;22(4):419-433.
9. Werner P, Goldstein D, Heinik J. Development and validity of the Family Stigma in Alzheimer’s disease scale (FS-ADS). Alzheimer Disease & Associated Disorders. 2011;25(1):42-48.
10. Rao D, Choi SW, Victorson D, et al. Measuring stigma across neurological conditions: the development of the stigma scale for chronic illness (SSCI). Qual Life Res. 2009;18(5):585-595.
11. Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23(8):812-820.
12. Burgener SC, Buckwalter K, Perkounkova Y, et al. Perceived stigma in persons with early-stage dementia: longitudinal findings: Part 2. Dementia. 2015;14(5):609-632.
13. Burgener SC, Buckwalter K. The effects of perceived stigma on persons with dementia and their family caregivers. In: Symposium on Stigma: It’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
14. Harper L, Dobbs B, Royan H, et al. The experience of stigma in care partners of people with dementia – results from an exploratory study. In Symposium on stigma: it’s time to talk about it. Boston, MA: Gerontological Society of America 2018 Annual Scientific Meeting; 2018. Session 2805.
15. Burgener S, Berger B. Measuring perceived stigma in persons with progressive neurological disease: Alzheimer’s dementia and Parkinson disease. Dementia. 2008;7(1):31-53.
16. Stites SD, Milne R, Karlawish J. Advances in Alzheimer’s imaging are changing the experience of Alzheimer’s disease. Alzheimer’s & Dementia. 2018;10;285-300.
17. Anderson LA, Egge R. Expanding efforts to address Alzheimer’s disease: the Healthy Brain Initiative. Alzheimer’s Dement. 2014;10(50):S453-S456.
18. Alzheimer’s Association National Plan Milestone Workgroup. Report on the milestones for the US National plan to address Alzheimer’s disease. Alzheimer’s Dementia. 2014;10(Suppl 5);S430-S452. doi:10.1016/j/jalz.2014.08.103.
19. Kirkman AM. Dementia in the news: the media coverage of Alzheimer’s disease. Australasian Journal on Ageing. 2006;25(2):74-79.
20. Swaffer, K. Dementia: stigma, language, and dementia-friendly. Dementia. 2014;13(6):709-716.
21. Stites SD, Karlawish J. Stigma of Alzheimer’s disease dementia: considerations for practice. Practical Neurology. https://practicalneurology.com/articles/2018-june/stigma-of-alzheimers-disease-dementia. Published June 2018. Accessed May 28, 2019.
22. Jamieson J, Dobbs B, Charles L, et al. Forgetful, but not forgotten people of dementia: a novel, technology focused project with a humanistic touch. Geriatric Grand Rounds; October 10, 2017. Edmonton, Alberta, Canada.
23. Dobbs B, Charles L, Chan K, et al. People of Dementia. CGS 37th Annual Scientific Meeting: Integrating Care, Making an Impact. Can Geriatr J. 2017;20(3):220.
24. Government of Canada. Conference report: National Dementia Conference. https://www.canada.ca/en/services/health/publications/diseases-conditions/national-dementia-conference-report.html. Government of Canada. Published August 2018. Accessed May 28, 2019.
25. The Gerontological Society of America. Program Abstracts from the GSA 2018 Annual Scientific Meeting “The Purposes of Longer Lives.” Innovation in Aging. 2018;2(Suppl 1):143.
Modern surgical techniques for gastrointestinal endometriosis
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.

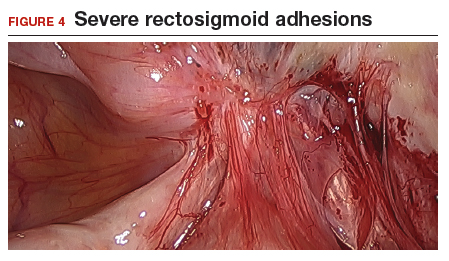

Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.
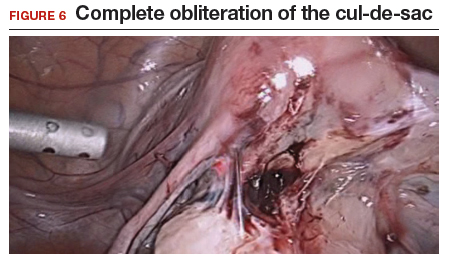
Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2
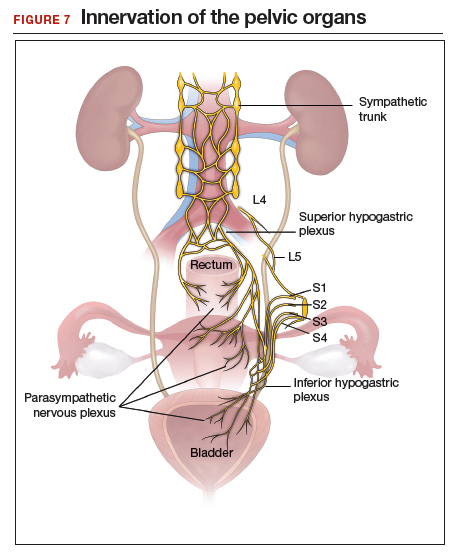
Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
About 10% of all reproductive-aged women and 35% to 50% of women with pelvic pain and infertility are affected by endometriosis.1,2 The disease typically involves the reproductive tract organs, anterior and posterior cul-de-sacs, and uterosacral ligaments. However, disease outside of the reproductive tract occurs frequently and has been found on all organs except the spleen.3
The bowel is the most common site for extragenital endometriosis, affected in an estimated 3.8% to 37% of patients with known endometriosis.4-7 Implants may be superficial, involving the bowel serosa and subserosa (FIGURE 1), or they can manifest as deeply infiltrating lesions involving the muscularis and mucosa (FIGURE 2). The rectosigmoid colon is the most common location for bowel endometriosis, followed by the rectum, ileum, appendix, and cecum4,8 (FIGURES 3, 4, and 5). Case reports also have described endometrial implants on the stomach and transverse colon.9 Although isolated bowel involvement has been recognized, most patients with bowel endometriosis have concurrent disease elsewhere.2,4
Historically, segmental resection was performed regardless of the anatomical location of the lesion.10 Even today, many surgeons continue to routinely perform segmental bowel resection as a first-line surgical approach.11 Unnecessary segmental resection, however, places patients at risk for short- and long-term postoperative morbidity, including the possibility of permanent ostomy. Modern surgical techniques, such as shaving excision and disc resection, have been performed to successfully treat bowel endometriosis with excellent long-term outcomes and fewer complications when compared with traditional segmental resection.2,12-16
In this article, we focus on the clinical indications and surgical techniques for video-laparoscopic management, but first we describe the pathophysiology, clinical presentation, and diagnosis of bowel endometriosis.





Pathophysiology of bowel endometriosis
The pathogenesis of endometriosis remains unknown, as no single mechanism explains all clinical cases of the disease. The most popular proposed theory describes retrograde menstruation through the fallopian tubes.17 Once inside the peritoneal cavity, endometrial cells attach to and invade healthy peritoneum, establishing a blood supply necessary for growth and survival.
In the case of bowel endometriosis, deposition of effluxed endometrial cells may lead to an inflammatory response that increases the risk of adhesion formation, leading to potential cul-de-sac obliteration. Lesions may originate as Allen-Masters peritoneal defects, developing into deeply infiltrative rectovaginal septum lesions. The anatomical shelter theory contributes to lesions within the pelvis, with the rectosigmoid colon blocking the cephalad flow of effluxed menstrual blood from the pelvis, thus leading to a preponderance of lesions in the pelvis and along the rectosigmoid colon.2
Continue to: Clinical presentation and diagnosis...
Clinical presentation and diagnosis
Women presenting with endometriosis of the bowel are typically of reproductive age and commonly report symptoms of dysmenorrhea, chronic pelvic pain, dyspareunia, and dyschezia. Some women also experience catamenial diarrhea, constipation, hematochezia, and bloating.2 The differential diagnosis of these symptoms is broad and includes irritable bowel disease, ischemic colitis, inflammatory bowel disease, diverticulitis, pelvic inflammatory disease, and malignancy.
Because of its nonspecific symptoms, bowel endometriosis is often misdiagnosed and the disease goes untreated for years.18 Therefore, it is imperative that clinicians maintain a high index of suspicion when evaluating reproductive-aged women with gastrointestinal symptoms and pelvic pain.
Physical examination can be helpful in making the diagnosis of endometriosis. During bimanual examination, findings such as a fixed, tender, or retroverted uterus, uterosacral ligament nodularity, or an enlarged adnexal mass representing an ovarian endometrioma may be appreciated. Rectovaginal exam can identify areas of tenderness and nodularity along the rectovaginal septum. Speculum exam may reveal a laterally displaced cervix or blue powder-burn lesions along the cervix or posterior fornix.19 Rarely, endometriosis is found on the perineum within an episiotomy scar.20
Imaging studies can be used in conjunction with physical examination findings to aid in the diagnosis of endometriosis. Images also guide preoperative planning by characterizing lesions based on their size, location, and depth of invasion. Hudelist and colleagues found transvaginal ultrasound (TVUS) to have an overall sensitivity of 71% to 98% and a specificity of 92% to 100%.21 However, it was noted that the accuracy of the diagnosis was directly related to the experience of the sonographer, and lesions above the sigmoid colon were generally unable to be diagnosed. Other imaging modalities that have been reported to have high sensitivity and specificity for diagnosing bowel endometriosis include rectal water contrast TVUS,22,23 rectal endoscopic sonography,22 magnetic resonance imaging,22 and barium enema.24
Medical management
Medical therapy for patients with endometriosis is utilized with the goal of suppressing ovulation, lowering circulating hormone levels, and inducing endometrial atrophy. Medications commonly employed include gonadotropin-releasing hormone agonists and antagonists, anabolic steriods such as danazol, combined oral contraceptive pills, progestins, and aromatase inhibitors.
Continue to: To date, no optimal hormonal regimen...
To date, no optimal hormonal regimen has been established for the treatment of bowel endometriosis. Vercellini and colleagues demonstrated that progestins with and without low-dose estrogen improved symptoms of dysmenorrhea and dyspareunia.25 Ferrero and colleagues reported that 2.5 mg of norethindrone daily resulted in 53% of women with colorectal endometriosis reporting improved gastrointestinal symptoms.26 However, by 12 months of follow-up, 33% of these patients had elected to undergo surgical management.
Gonadotropin-releasing hormone agonists, such as leuprolide acetate, also can be used to mitigate symptoms of bowel endometriosis or to decrease disease burden at the time of surgery, and they can be used with add-back norethindrone acetate. The use of these medications is limited by adverse effects, such as vasomotor symptoms and decreased bone mineral density when used for longer than 6 months.2
Medical therapy is commonly used for patients with mild to moderate symptoms and in those who are poor surgical candidates or decline surgical intervention. Medical therapy is especially useful when employed postoperatively to suppress the regrowth of microscopic ectopic endometrial tissue.
Patients must be counseled, however, that even with medical management, they may still require surgery in the future to control their symptoms and/or to preserve organ function.2
Surgical management
Surgical treatment for bowel endometriosis depends on the disease location, the size and depth of the lesion, the presence or absence of stricture, and the surgeon’s level of expertise.2,12,27-30
In our group, we advocate for video-laparoscopy, with or without robotic as sistance. Minimally invasive surgery offers reduced blood loss, shorter recovery time, and fewer postoperative complications compared with laparotomy.2,16,27,31-33 The conversion rate to laparotomy has been reported to be about 3% when performed by an experienced surgeon.12
Darai and colleagues conducted a randomized trial of 52 patients undergoing surgery for colorectal endometriosis via either laparoscopic or open colon resection.33 Blood loss was significantly lower in the laparoscopy group (1.6 vs 2.7 mg/L, P <.05). No difference was noted in long-term outcomes. In a retrospective study of 436 cases, Ruffo and colleagues showed that those who underwent laparoscopic colorectal resection had higher postoperative pregnancy rates compared with those who had laparotomy (57.6% vs 23.1%, P <.035).32
The goal of surgical management of bowel endometriosis is to remove as many of the endometriotic lesions as possible while minimizing short- and long-term complications. Three surgical approaches have been described: shaving excision, disc resection, and segmental resection.2
Some surgeons prefer traditional segmental resection of the bowel regardless of the anatomical site, citing reduced disease recurrence with this approach; however, traditional segmental resection confers increased risk of complications. Increasingly, in an effort to reduce morbidity, more surgeons are advocating for the less aggressive methods of shaving excision and disc resection.
Aggressive resection at the level of the low rectum requires extensive surgical dissection of the retrorectal space, with the potential for inadvertent injury to surrounding neurovascular structures, such as the pelvic splanchnic nerves and superior and inferior hypogastric plexus.29 Injury to these structures can lead to significant complications, including bowel stenosis, fistula formation, constipation, and urinary retention. Complete resection of other areas, such as the small bowel, do not carry the same risks and may have more significant benefit to the patient than less aggressive techniques.
Our group recommends carefully balancing the risks and benefits of aggressive surgical treatment for each individual and treating the patient with the appropriate technique. Regardless of technique, surgical treatment of bowel endometriosis can lead to long-term improvements in pain and infertility.29,30,34,35
- The clinical presentation of bowel endometriosis is often nonspecific, with a broad differential diagnosis. Maintain a high index of suspicion when reproductive-aged women present for evaluation of dysmenorrhea, chronic pelvic pain, dyspareunia, bloating, dyschezia, or hematochezia.
- Symptomatic patients not desiring fertility, poor surgical candidates, and those declining surgical intervention may benefit from medical management. Patients who fail medical therapy, have severe symptoms, or experience infertility are candidates for surgical intervention.
- Surgical management involves shaving excision, disc resection, and segmental resection. Some surgeons advocate for aggressive segmental resection regardless of the endometriotic lesion's location. Based on our extensive experience, we prefer shaving excision for lesions below the sigmoid to avoid dissection into the retrorectal space and inadvertent injury to nerve tissue controlling bowel and bladder function.
- Following shaving excision, patients experience low complication rates29,39,40 and favorable long-term outcomes.15,40,56 For lesions above the sigmoid colon, including the small bowel, segmental resection or disc resection for smaller lesions are reasonable surgical approaches.
Continue to: Shaving excision...
Shaving excision
The most conservative approach to resection of bowel endometriosis is shaving excision; this involves removing endometriotic tissue layer-by-layer until healthy, underlying tissue is encountered.2 With bowel endometriosis, the goal of shaving excision is to remove as much of the diseased tissue as possible while leaving behind the mucosal layer and a portion of the muscularis.2,15,16,36-38 This is the most conservative of the 3 surgical techniques and is associated with the lowest complication rate.2,14,15,36,37
Our group reported on 185 women who underwent shaving excision for bowel endometriosis. At the time of surgery, 80 women had complete obliteration of the cul-de-sac (FIGURE 6). Of the study patients, 174 patients were available for follow-up, with 93% reporting moderate to complete pain relief.15
In a retrospective analysis of 3,298 surgeries for rectovaginal endometriosis in which shaving excision was used on all but 1% of patients, Donnez and colleagues reported a very low complication rate, with 1 case of rectal perforation, 1 case of fecal peritonitis, and 3 cases of ureteral injury.39
Roman and colleagues described the use of shaving excision for rectal endometriosis using plasma energy (n = 54) and laparoscopic scissors (n = 68).40 Only 4% of patients reported experiencing symptom recurrence, and the pregnancy rate was 65.4%, with 59% of those patients spontaneously conceiving. Two cases of rectal fistula were noted.

Disc resection
Laparoscopic disc excision has been described in the literature since the 1980s, and the technique involves the full-thickness removal of the diseased portion of the bowel, followed by closure of the remaining defect.2,12-14,28,29,31,41-45 To be appropriate for this technique, a lesion should involve only a portion of the bowel wall and, preferably, less than one-half of the bowel circumference.2,42 Disc excision results in excellent outcomes with fewer postoperative complications than segmental resection, but with more complications when compared to shaving excision.2,12,13,29,45,46
We reported on a series of 141 women with bowel endometriosis who underwent disc excision.2 At 1-month follow-up, 87% of patients experienced an improvement in their symptoms. No cases required conversion to laparotomy or were complicated by rectovaginal fistula formation, ureteral injury, bowel perforation, or pelvic abscess.2
Continue to: Segmental resection...
Segmental resection
The most aggressive surgical approach, segmental resection involves complete removal of a diseased portion of bowel, followed by side-to-side or end-to-end reanastomosis of the adjacent segments.2 For this procedure, a multidisciplinary approach is recommended, with involvement of a colorectal surgeon or gynecologic oncologist trained in performing bowel resections. Segmental resection is indicated for lesions that are larger than 3 cm, circumferential, obstructive, or multifocal.
Given the higher complication rate associated with this procedure and the good outcomes associated with less invasive techniques, we avoid segmental resection whenever possible, especially for lesions near the anal verge.2
Complications associated with surgical approach
In 2005, our group reported on a cohort of 178 women who underwent laparoscopic treatment of deeply infiltrative bowel endometriosis with shaving excision (n = 93), disc excision (n = 38), and segmental resection (n = 47).34 The major complication rate was significantly higher for those undergoing segmental resection (12.5%, P <.001); only 7.7% of those who underwent disc resection experienced a major complication; and none were observed in the group treated with shaving excision.
In 2011, De Cicco and colleagues conducted a systematic review of 1,889 patients who underwent segmental bowel resection.35 The major complication rate was 11%, with a leakage rate of 2.7%, fistula rate of 1.8%, major obstruction rate of 2.7%, and hemorrhage rate of 2.5%. Many of these complications, however, occurred in patients who had low rectal resections.
Regardless of surgical approach, the complication rate is related to the surgeon’s ability to preserve the superior and inferior hypogastric plexuses and the sympathetic and parasympathetic nerve bundles (FIGURE 7). Nerve-sparing techniques should be used to decrease the incidence of postoperative bowel, bladder, and sexual function complications.2

Our group’s preferences
In our practice, we emphasize that the choice of surgical technique depends on the location, size, and depth of the lesion, as well as the extent of bowel wall circumferential invasion.2
We categorize lesions by their anatomic location: those above the sigmoid colon, on the sigmoid colon, on the rectosigmoid colon, and on the rectum. For lesions above the sigmoid colon, segmental or disc resection is appropriate.2 We recommend segmental resection for multifocal lesions, lesions larger than 3 cm, or for lesions involving more than one-third of the bowel lumen.37,44,45,47 Disc resection is appropriate for lesions smaller than 3 cm even if the bowel lumen is involved.44,45,48 If endometriosis is encountered in any location along the bowel, appendectomy can be performed even without visible disease, due to a high incidence of occult disease of the appendix.49,50
When lesions involve the sigmoid colon, we prefer utilizing shaving excision when possible to limit dissection of the retrorectal space and pelvic sidewall nerves.2 Segmental resection at or below the sigmoid colon has been associated with postoperative surgical site leakage51 and long-term bowel and bladder dysfunction with risk of permanent colostomy.52,53 For lesions smaller than 3 cm or involving less than one-third of the bowel lumen, disc resection can be performed. Segmental resection is required if multifocal disease or obstruction are present, if lesions are larger than 3 cm, or if more than one-third of the bowel lumen is involved.
For lesions along the rectosigmoid colon, we prefer utilizing shaving excision when possible.2 Disc excision can be performed utilizing a transanal approach, being mindful to minimize dissection of the retroperitoneal space and pelvic sidewall nerves.48 Segmental resection is avoided even with lesions larger than 3 cm, unless prior surgery has failed. Approaches for segmental resection can utilize laparoscopy or the natural orifices of the rectum or vagina.31,51
For lesions on the rectum, we strongly advise shaving excision.2 Evidence fails to show that the benefits of segmental resection outweigh the risks when compared to conservative techniques at the rectum.30,39,54 There is evidence indicating that aggressive surgery 5 to 8 cm from the anal verge is predictive of postoperative complications.55 In our group, we use shaving excision to remove as much disease as possible without compromising the integrity of the bowel wall or surrounding neurovascular structures. We err on the side of caution, leaving some of the disease on the rectum to avoid rectal perforation, and plan for postoperative hormonal suppression in these patients.
For patients desiring fertility, successful pregnancy is often achieved using the shaving technique.41
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
- Giudice LC. Clinical practice. Endometriosis. N Engl J Med. 2010;362:2389-2398.
- Nezhat C, Li A, Falik R, et al. Bowel endometriosis: diagnosis and management. Am J Obstet Gynecol. 2018;218:549-562.
- Markham SM, Carpenter SE, Rock JA. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1989;16:193-219.
- Veeraswamy A, Lewis M, Mann A, et al. Extragenital endometriosis. Clin Obstet Gynecol. 2010;53:449-466.
- Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72:310-315.
- Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69:727-730.
- Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34:41-46.
- Redwine DB. Intestinal endometriosis. In: Redwine DB. Surgical Management of Endometriosis. New York, NY: Martin Dunitz; 2004:196.
- Hartmann D, Schilling D, Roth SU, et al. [Endometriosis of the transverse colon--a rare localization]. Dtsch Med Wochenschr. 2002;127:2317-2320.
- Nezhat C, Nezhat F, Nezhat C. Endometriosis: ancient disease, ancient treatments. Fertil Steril. 2012;98(6 suppl):S1-62.
- Macafee CH, Greer HL. Intestinal endometriosis. A report of 29 cases and a survey of the literature. J Obstet Gynaecol Br Emp. 1960;67:539-555.
- Nezhat C, Nezhat F, Ambroze W, et al. Laparoscopic repair of small bowel and colon. A report of 26 cases. Surg Endosc. 1993;7:88-89.
- Nezhat C, Nezhat F, Pennington E, et al. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994;8:682-685.
- Nezhat C, Nezhat F. Evaluation of safety of videolaseroscopic treatment of bowel endometriosis. Presented at: 44th Annual Meeting of the American Fertility Society; October, 1988; Atlanta, GA.
- Nezhat C, Nezhat F, Pennington E. Laparoscopic treatment of infiltrative rectosigmoid colon and rectovaginal septum endometriosis by the technique of videolaparoscopy and the CO2 laser. Br J Obstet Gynaecol. 1992;99:664-667.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45:778-783.
- Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014;2014:179515.
- Skoog SM, Foxx-Orenstein AE, Levy MJ, et al. Intestinal endometriosis: the great masquerader. Curr Gastroenterol Rep. 2004;6:405-409.
- Alabiso G, Alio L, Arena S, et al. How to manage bowel endometriosis: the ETIC approach. J Minim Invasive Gynecol. 2015;22:517-529.
- Heller DS, Lespinasse P, Mirani N. Endometriosis of the perineum: a rare diagnosis usually associated with episiotomy. J Low Genit Tract Dis. 2016;20:e48-e49.
- Hudelist G, English J, Thomas AE, et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2011;37:257-263.
- Nisenblat V, Bossuyt PM, Farquhar C, et al. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst Rev. 2016;2:CD009591.
- Menada MV, Remorgida V, Abbamonte LH, et al. Transvaginal ultrasonography combined with water-contrast in the rectum in the diagnosis of rectovaginal endometriosis infiltrating the bowel. Fertil Steril. 2008;89:699-700.
- Gordon RL, Evers K, Kressel HY, et al. Double-contrast enema in pelvic endometriosis. AJR Am J Roentgenol. 1982;138:549-552.
- Vercellini P, Pietropaolo G, De Giorgi O, et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil Steril. 2005;84:1375-1387.
- Ferrero S, Camerini G, Ragni N, et al. Norethisterone acetate in the treatment of colorectal endometriosis: a pilot study. Hum Reprod. 2010;25:94-100.
- Nezhat C, Hajhosseini B, King LP. Robotic-assisted laparoscopic treatment of bowel, bladder, and ureteral endometriosis. JSLS. 2011;15:387-392.
- Nezhat C, Hajhosseini B, King LP. Laparoscopic management of bowel endometriosis: predictors of severe disease and recurrence. JSLS. 2011;15:431-438.
- Roman H, Milles M, Vassilieff M, et al. Long-term functional outcomes following colorectal resection versus shaving for rectal endometriosis. Am J Obstet Gynecol. 2016;215:762.e1-762.e9.
- Kent A, Shakir F, Rockall T, et al. Laparoscopic surgery for severe rectovaginal endometriosis compromising the bowel: a prospective cohort study. J Minim Invasive Gynecol. 2016;23:526-534.
- Nezhat F, Nezhat C, Pennington E. Laparoscopic proctectomy for infiltrating endometriosis of the rectum. Fertil Steril. 1992;57:1129-1132.
- Ruffo G, Scopelliti F, Scioscia M, et al. Laparoscopic colorectal resection for deep infiltrating endometriosis: analysis of 436 cases. Surg Endosc. 2010;24:63-67.
- Darai E, Dubernard G, Coutant C, et al. Randomized trial of laparoscopically assisted versus open colorectal resection for endometriosis: morbidity, symptoms, quality of life, and fertility. Ann Surg. 2010;251:1018-1023.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- De Cicco C, Corona R, Schonman R, et al. Bowel resection for deep endometriosis: a systematic review. BJOG. 2011;118:285-291.
- Nezhat C, Nezhat FR. Safe laser endoscopic excision or vaporization of peritoneal endometriosis. Fertil Steril. 1989;52:149-151.
- Donnez J, Squifflet J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum Reprod. 2010;25:1949-1958.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy and videolaseroscopy. Contrib Gynecol Obstet. 1987;16:303-312.
- Donnez J, Jadoul P, Colette S, et al. Deep rectovaginal endometriotic nodules: perioperative complications from a series of 3,298 patients operated on by the shaving technique. Gynecol Surg. 2013;10:31-40.
- Roman H, Moatassim-Drissa S, Marty N, et al. Rectal shaving for deep endometriosis infiltrating the rectum: a 5-year continuous retrospective series. Fertil Steril. 2016;106:1438-1445.e2.
- Mohr C, Nezhat FR, Nezhat CH, et al. Fertility considerations in laparoscopic treatment of infiltrative bowel endometriosis. JSLS. 2005;9:16-24.
- Jerby BL, Kessler H, Falcone T, et al. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999;13:1125-1128.
- Coronado C, Franklin RR, Lotze EC, et al. Surgical treatment of symptomatic colorectal endometriosis. Fertil Steril. 1990;53:411-416.
- Fanfani F, Fagotti A, Gagliardi ML, et al. Discoid or segmental rectosigmoid resection for deep infiltrating endometriosis: a case-control study. Fertil Steril. 2010;94:444-449.
- Landi S, Pontrelli G, Surico D, et al. Laparoscopic disk resection for bowel endometriosis using a circular stapler and a new endoscopic method to control postoperative bleeding from the stapler line. J Am Coll Surg. 2008;207:205-209.
- Slack A, Child T, Lindsey I, et al. Urological and colorectal complications following surgery for rectovaginal endometriosis. BJOG. 2007;114:1278-1282.
- Ceccaroni M, Clarizia R, Bruni F, et al. Nerve-sparing laparoscopic eradication of deep endometriosis with segmental rectal and parametrial resection: the Negrar method. A single-center, prospective, clinical trial. Surg Endosc. 2012;26:2029-2045.
- Roman H, Abo C, Huet E, et al. Deep shaving and transanal disc excision in large endometriosis of mid and lower rectum: the Rouen technique. Surg Endosc. 2016;30:2626-2627.
- Gustofson RL, Kim N, Liu S, et al. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86:298-303.
- Berker B, Lashay N, Davarpanah R, et al. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12:206-209.
- Ret Dávalos ML, De Cicco C, D'Hoore A, et al. Outcome after rectum or sigmoid resection: a review for gynecologists. J Minim Invasive Gynecol. 2007;14:33-38.
- Alves A, Panis Y, Mathieu P, et al; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514.
- Camilleri-Brennan J, Steele RJ. Objective assessment of morbidity and quality of life after surgery for low rectal cancer. Colorectal Dis. 2002;4:61-66.
- Acien P, Núñez C, Quereda F, et al. Is a bowel resection necessary for deep endometriosis with rectovaginal or colorectal involvement? Int J Womens Health. 2013;5:449-455.
- Abrão MS, Petraglia F, Falcone T, et al. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum Reprod Update. 2015;21:329-339.
- Donnez J, Nisolle M, Gillerot S, et al. Rectovaginal septum adenomyotic nodules: a series of 500 cases. Br J Obstet Gynaecol. 1997;104:1014-1018.
2019 Update on menopause
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

Among peri- and postmenopausal women, abnormal bleeding, breast cancer, and mood disorders represent prevalent conditions. In this Update, we discuss data from a review that provides quantitative information on the likelihood of finding endometrial cancer among women with postmenopausal bleeding (PMB). We also summarize 2 recent consensus recommendations: One addresses the clinically important but controversial issue of the treatment of genitourinary syndrome of menopause (GSM) in breast cancer survivors, and the other provides guidance on the management of depression in perimenopausal women.
Endometrial cancer is associated with a high prevalence of PMB
Clarke MA, Long BJ, Del Mar Morillo A, et al. Association of endometrial cancer risk with postmenopausal bleeding in women: a systematic review and meta-analysis. JAMA Intern Med. 2018;178:1210-1222.
Endometrial cancer is the most common gynecologic malignancy and the fourth most common cancer among US women. In recent years, the incidence of and mortality from endometrial cancer have increased.1 Despite the high prevalence of endometrial cancer, population-based screening currently is not recommended.
PMB affects up to 10% of women and can be caused by endometrial atrophy, endometrial polyps, uterine leiomyoma, and malignancy. While it is well known that PMB is a common presenting symptom of endometrial cancer, we do not have good data to guide counseling patients with PMB on the likelihood that endometrial cancer is present. Similarly, estimates are lacking regarding what proportion of women with endometrial cancer will present with PMB.
To address these 2 issues, Clarke and colleagues conducted a comprehensive systematic review and meta-analysis of the prevalence of PMB among women with endometrial cancer (sensitivity) and the risk of endometrial cancer among women with PMB (positive predictive value). The authors included 129 studies--with 34,432 women with PMB and 6,358 with endometrial cancer--in their report.
Cancer prevalence varied with HT use, geographic location
The study findings demonstrated that the prevalence of PMB in women with endometrial cancer was 90% (95% confidence interval [CI], 84%-94%), and there was no significant difference in the occurrence of PMB by cancer stage. The risk of endometrial cancer in women with PMB ranged from 0% to 48%, yielding an overall pooled estimate of 9% (95% CI, 8%-11%). As an editorialist pointed out, the risk of endometrial cancer in women with PMB is similar to that of colorectal cancer in individuals with rectal bleeding (8%) and breast cancer in women with a palpable mass (10%), supporting current guidance that recommends evaluation of women with PMB.2 Evaluating 100 women with PMB to diagnose 9 endometrial cancers does not seem excessive.
Interestingly, among women with PMB, the prevalence of endometrial cancer was significantly higher among women not using hormone therapy (HT) than among users of HT (12% and 7%, respectively). In 7 studies restricted to women with PMB and polyps (n = 2,801), the pooled risk of endometrial cancer was 3% (95% CI, 3%-4%). In an analysis stratified by geographic region, a striking difference was noted in the risk of endometrial cancer among women with PMB in North America (5%), Northern Europe (7%), and in Western Europe (13%). This finding may be explained by regional differences in the approach to evaluating PMB, cultural perceptions of PMB that can affect thresholds to present for care, and differences in risk factors between these populations.
The study had several limitations, including an inability to evaluate the number of years since menopause and the effects of body mass index. Additionally, the study did not address endometrial hyperplasia or endometrial intraepithelial neoplasia.
PMB accounts for two-thirds of all gynecologic visits among perimenopausal and postmenopausal women.3 This study revealed a 9% risk of endometrial cancer in patients experiencing PMB, which supports current practice guidelines to further evaluate and rule out endometrial cancer among all women presenting with PMB4; it also provides reassurance that targeting this high-risk group of women for early detection and prevention strategies will capture most cases of endometrial cancers. However, the relatively low positive predictive value of PMB emphasizes the need for additional triage tests with high specificity to improve management of PMB and minimize unnecessary biopsies in low-risk women.
Treating GSM in breast cancer survivors: New guidance targets QoL and sexuality
Faubion SS, Larkin LC, Stuenkel CA, et al. Management of genitourinary syndrome of menopause in women with or at high risk for breast cancer: consensus recommendations from The North American Menopause Society and The International Society for the Study of Women's Sexual Health. Menopause. 2018;25:596-608.
Given that there is little evidence addressing the safety of vaginal estrogen, other hormonal therapies, and nonprescription treatments for GSM in breast cancer survivors, many survivors with bothersome GSM symptoms are not appropriately treated.
Continue to: Expert panel creates evidence-based guidance...
Expert panel creates evidence-based guidance
Against this backdrop, The North American Menopause Society and the International Society for the Study of Women's Sexual Health convened a group comprised of menopause specialists (ObGyns, internists, and nurse practitioners), specialists in sexuality, medical oncologists specializing in breast cancer, and a psychologist to create evidence-based interdisciplinary consensus guidelines for enhancing quality of life and sexuality for breast cancer survivors with GSM.
Measures to help enhance quality of life and sexuality
The group's key recommendations for clinicians include:
- Sexual function and quality of life (QoL) should be assessed in all women with or at high risk for breast cancer.
- Management of GSM should be individualized based on shared decision-making involving the patient and her oncologist.
- Initial treatment options include:
—over-the-counter vaginal moisturizers used several times weekly on a regular basis
—lubricants used with intercourse
—vaginal dilator therapy
—pelvic floor physical therapy.
- Low-dose vaginal estrogen therapy may be appropriate for select women who have been treated for breast cancer:
—With use of vaginal estrogen, serum estradiol levels remain in the postmenopausal range.
—Based on limited data, use of vaginal estrogen is associated with a minimal risk for recurrence of breast cancer.
—Because their use is associated with the lowest serum estradiol levels, vaginal tablets, rings, or inserts may be preferable to creams.
—Decisions regarding use of vaginal estrogen in breast cancer survivors should involve the woman's oncologist. Appropriate candidates for off-label use of vaginal estrogen may be survivors:
–who are at relatively low risk for recurrence
–with hormone receptor-negative disease
–using tamoxifen rather than an AI
–who are particularly concerned about quality of life.
—Given that AIs prevent recurrence by lowering estrogen levels, oncologists may be reluctant to consider use of vaginal estrogen in survivors using adjuvant agents.
—With respect to use of vaginal estrogen, oncologists may be more comfortable with use in patients taking tamoxifen.
- Neither intravaginal dehydroepiandrosterone (DHEA; prasterone) nor the oral selective estrogen receptor modulator ospemifene has been studied in breast cancer survivors.
In women with metastatic disease, QoL, comfort, and sexual intimacy are key considerations when weighing potential therapies; optimal choices will vary with probability of long-term survival.
Although more data addressing the safety of vaginal estrogen as well as prasterone and ospemifene in breast cancer survivors clearly are needed, these guidelines should help clinicians who care for breast cancer survivors with GSM.
Framework provided for managing depressive disorders in perimenopausal women
Maki PM, Kornstein SG, Joffe H, et al; Board of Trustees for The North American Menopause Society (NAMS) and the Women and Mood Disorders Task Force of the National Network of Depression Centers. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. Menopause. 2018;25:1069-1085.
Although perimenopausal women are more susceptible to the development of depressive symptoms and major depressive episodes (MDE), there is a lack of consensus regarding how to evaluate and treat depression in women during the menopausal transition and postmenopausal period.
Recently, an expert panel comprised of representatives from The North American Menopause Society and the National Network of Depression Centers Women and Mood Disorders Task Group developed clinical guidelines addressing epidemiology, clinical presentation, therapeutic effects of antidepressants, effects of HT, and efficacy of other therapies. Here we provide a summary of the expert panel's findings and guidelines.
Continue to: Certain factors are associated with higher risk for depression...
Certain factors are associated with higher risk for depression
The perimenopause represents a time of increased risk for depressive symptoms and major depressive disorder (MDD), even in women with no prior history of depression. Several characteristics and health factors are associated with the increased risk during the menopause transition. These include a prior history of MDD, current antidepressant use, anxiety, premenstrual depressive symptoms, African American race, high body mass index, younger age, social isolation, upsetting life events, and menopausal sleep disturbances.
Although data are inconclusive on whether surgical menopause increases or decreases the risk for developing depression compared with women who transition through menopause naturally, recent studies show an elevated risk of depression in women following hysterectomy with and without oophorectomy.6,7
Menopausal and depressive symptoms can overlap
Midlife depression presents with classic depressive symptoms that commonly occur in combination with menopausal symptoms, including vasomotor symptoms, sleep and sexual disturbances, and weight and energy changes. These menopausal symptoms can complicate, co-occur, and overlap with the clinical presentation of depression.
Conversely, depression may affect an individual's judgment of the degree of bother from menopausal somatic symptoms, thereby further magnifying the effect of symptoms on quality of life. The interrelationship between depressive symptoms and menopausal symptoms may pose a challenge when attempting to parse out contributing etiologies, relative contributions of each etiology, and the potential additive effects.
Diagnosis and treatment options
Diagnosis involves identifying the menopausal stage, assessing for co-existing psychiatric and menopause symptoms, appreciating the psychosocial factors common in midlife, and considering the differential diagnosis. Validated screening instruments can be helpful. Although a menopause-specific mood disorder scale does not yet exist, several general validated screening measures, such as the Patient Health Questionnaire-9, or PHQ-9, can be used for categorical determination of mood disorder diagnoses during the menopause transition.
Antidepressants, cognitive-behavioral therapy, and other psychotherapies are considered first-line treatments for perimenopausal major depressive episodes. Only desvenlafaxine has been studied in large randomized placebo-controlled trials and has proven efficacious for the treatment of MDD in perimenopausal and postmenopausal women.
A number of small open-label studies of other selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), and mirtazapine to treat MDD in perimenopausal and postmenopausal women have demonstrated a positive effect on mood, and several SSRIs and SNRIs also have the added benefit of improving menopause-related symptoms.
In women with a history of MDD, a prior adequate response to a particular antidepressant should guide treatment selection when MDD recurs during the midlife years.
Although estrogen is not approved by the US Food and Drug Administration specifically for the treatment of mood disturbances, some evidence suggests that unopposed estrogen therapy has efficacy similar to that of antidepressant medications in treating depressive disorders in perimenopausal women,8-11 but it is ineffective in treating depressive disorders in postmenopausal women. Estrogen therapy also may augment the clinical response to antidepressants in midlife and older women.12,13 The data on combined HT (estrogen plus progestogen) or for different progestogens in treating depressive disorders in perimenopausal women are lacking and inconclusive.
The findings from this expert review panel demonstrate that women in the perimenopausal transition are at increased risk for depressive symptoms, major depressive episodes, and major depressive disorder. The interrelationship between symptoms of depression and menopause can complicate, co-occur, overlap, and magnify one another. Clinicians treating perimenopausal women with depression that is unresponsive to conventional antidepressant therapy should consider concurrent use of estrogen-based hormone therapy or referring the patient to a clinician comfortable doing so.

- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Matteson KA, Robison K, Jacoby VL. Opportunities for early detection of endometrial cancer in women with postmenopausal bleeding. JAMA Intern Med. 2018;178:1222-1223.
- van Hanegem N, Breijer MC, Khan KS, et al. Diagnostic evaluation of the endometrium in postmenopausal bleeding: an evidence-based approach. Maturitas. 2011;68:155-164.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion no. 734 summary. The role of transvaginal ultrasonography in evaluating the endometrium of women with postmenopausal bleeding. Obstet Gynecol. 2018; 131:945-946.
- Baumgart J, Nilsson K, Evers AS, et al. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162-168.
- Chou PH, Lin CH, Cheng C, et al. Risk of depressive disorders in women undergoing hysterectomy: a population-based follow-up study. J Psychiatr Res. 2015;68:186-191.
- Wilson L, Pandeya N, Byles J, et al. Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women's Health. Epidemiol Psychiatr Sci. 2018;27:381-392.
- Schmidt PJ, Nieman L, Danaceau MA, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414-420.
- Rasgon NL, Altshuler LL, Fairbanks L. Estrogen-replacement therapy for depression. Am J Psychiatry. 2001;158:1738.
- Soares CN, Almeida OP, Joffe H, et al. Efficacy of estradiol for the treatment of major depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58:529-534.
- Cohen LS, Soares CN, Poitras JR, et al. Short-term use of estradiol for depression in perimenopausal and postmenopausal women: a preliminary report. Am J Psychiatry. 2003;160:1519-1522.
- Schneider LS, Small GW, Hamilton SH, et al. Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Fluoxetine Collaborative Study Group. Am J Geriatr Psychiatry. 1997;5:97-106.
- Schneider LS, Small GW, Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393-399.
What to do when a patient presents with breast pain
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.
Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.
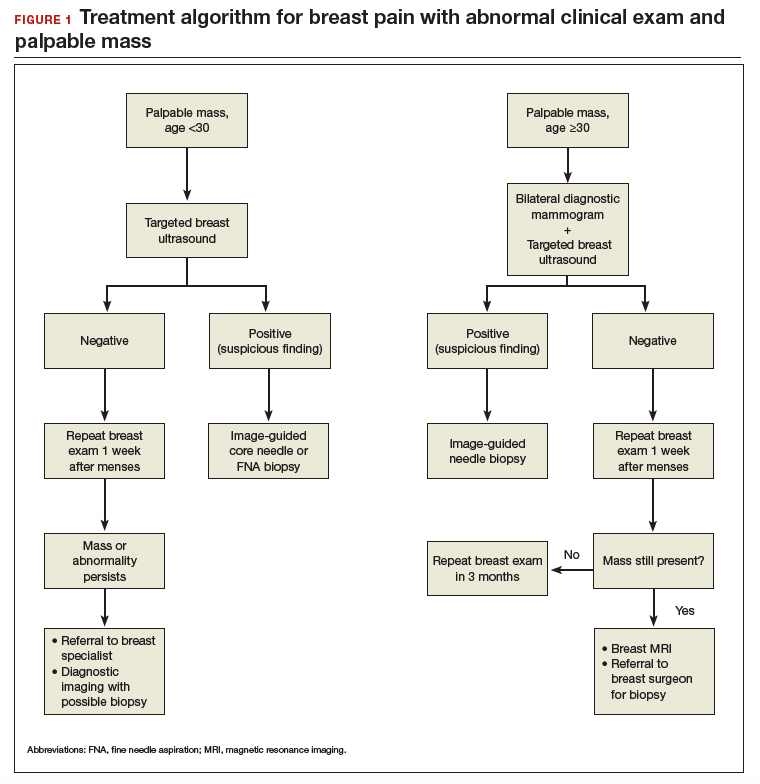
Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...
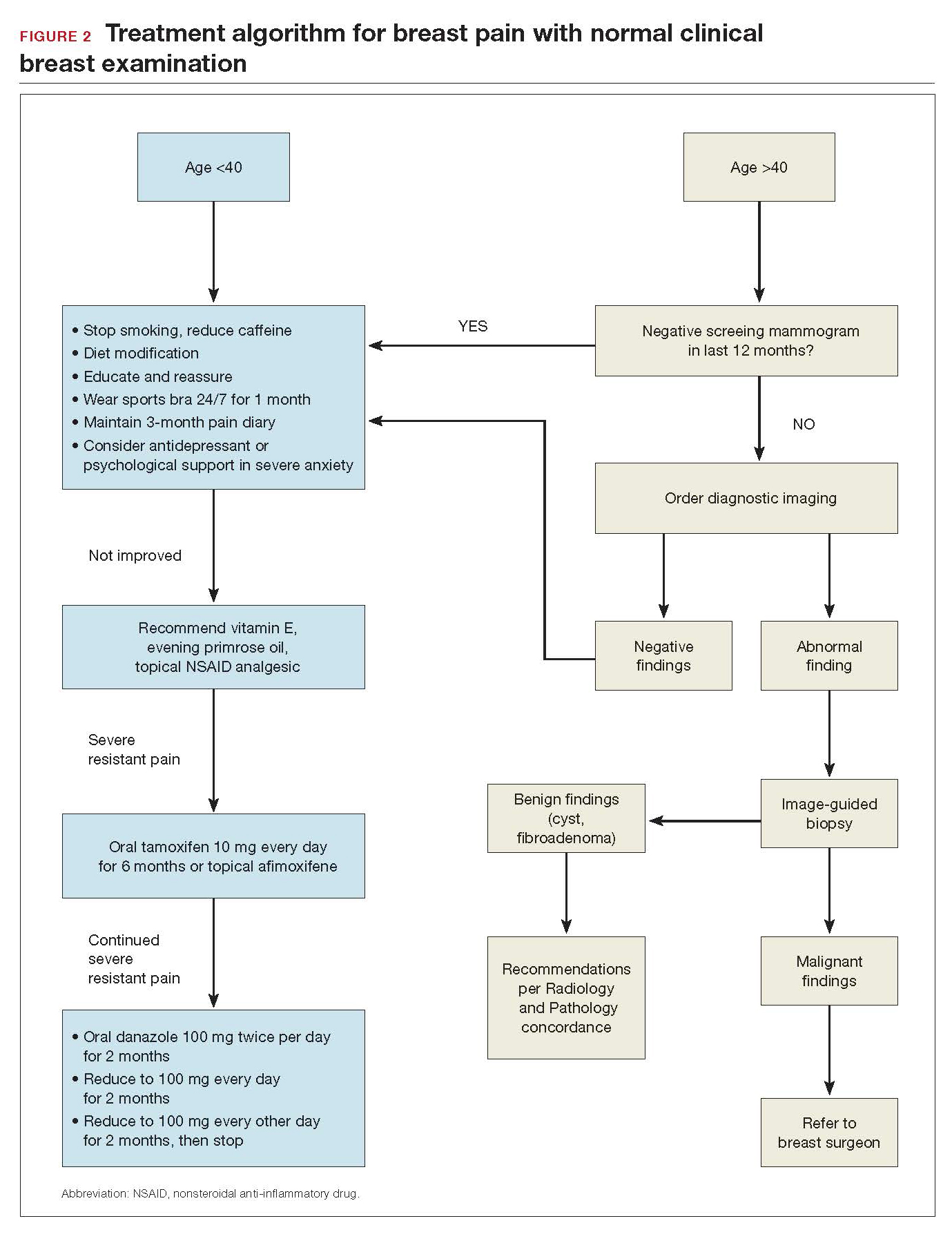
In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.

Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.

Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...

In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
Breast pain is one of the most common breast-related patient complaints and is found to affect at least 50% of the female population.1 Most cases are self-limiting and are related to hormonal and normal fibrocystic changes. The median age of onset of symptoms is 36 years, with most women experiencing pain for 5 to 12 years.2 Because the cause of breast pain is not always clear, its presence can produce anxiety in patients and physicians over the possibility of underlying malignancy. Although breast cancer is not associated with breast pain, many patients presenting with pain are referred for diagnostic imaging (usually with negative results). The majority of women with mastalgia and normal clinical examination findings can be reassured with education about the many benign causes of breast pain.
What are causes of breast pain without an imaging abnormality?
Hormones. Mastalgia can be focal or generalized and is mostly due to hormonal changes. Elevated estrogen can stimulate the growth of breast tissue, which is known as epithelial hyperplasia.3 Fluctuations in hormone levels can occur in perimenopausal women in their forties and can result in new symptoms of breast pain.4 Sometimes starting a new contraceptive medication or hormone replacement therapy can exacerbate the pain. Switching brands or medications may help. Another cause of mastalgia may be elevated prolactin levels, with hypothalamic-pituitary dysfunction.5,6
Diet. There is evidence to link a high-fat diet with breast pain. The pain has been shown to improve when lipid intake is reduced and high- and low-density lipoprotein cholesterol levels are normalized. As estrogen is a steroid hormone that can be synthesized from lipids and fatty acids, elevated lipid metabolism can increase estrogen levels and exacerbate breast pain symptoms.7,8 Essential fatty acids, such as evening primrose oil and vitamin E, have been used to treat mastalgia because they reduce inflammation in fatty breast tissue through the prostaglandin pathway.9,10
Caffeine. Methylxanthines can be found in coffee, tea, and chocolate and can aggravate mastalgia by enhancing the cyclin adenosine monophosphate (cAMP) pathway. This pathway stimulates cellular proliferation and fibrocystic changes which in turn can exacerbate breast pain.11
Smoking. In my clinical practice I have clearly noted a higher incidence of breast pain in patients who smoke. The pain tends to improve significantly when the patient quits or even cuts back on smoking. The exact reasons for smoking’s effects on breast pain are not well known; however, they are thought to be related to acceleration of the cAMP pathway.
Large breast size. Very large breasts will strain and weaken the suspensory ligaments, leading to pain and discomfort. It has been shown that wearing a supportive sports bra during episodes of breast pain is effective.
Types of breast pain
Cyclical
Women with fibrocystic breasts tend to experience more breast pain. Breast sensitivity can be localized to the upper outer quadrants or to the nipple and sub-areolar area. It also can be generalized. The pain tends to peak with ovulation, improve with menses, and to recur every few weeks. Patients who have had partial hysterectomy (with ovaries in situ) or endometrial ablation will be unable to correlate their symptoms to menstruation. Therefore, women are encouraged to keep a diary or calendar of their symptoms to detect any correlation with their ovarian cycle. Such correlation is reassuring.

Continue to: Noncyclical...
Noncyclical
Noncyclical breast pain is not associated with the menstrual cycle and can be unilateral or bilateral. Providers should perform a good history of patients presenting with noncyclicalbreast pain, to include character, onset, duration, location, radiation, alleviating, and aggravating factors. A physical examination may elicit point tenderness at the chest by pushing the breast tissue off of the chest wall while the patient is in supine position and pressing directly over the ribs. Lack of tenderness on palpation of the breast parenchyma, but pain on the chest wall, points to a musculoskeletal etiology. Chest wall pain may be related to muscle spasm or muscle strain, trauma, rib fracture, or costochondritis (Tietze syndrome). Finally, based on history of review of systems and physical examination, referred pain from biliary or cardiac etiology should be considered.
When breast pain occurs with skin changes
Skin changes usually have an underlying pathology. Infectious processes, such as infected epidermal inclusion cyst, hidradenitis of the cleavage and inframammary crease, or breast abscess will present with pain and induration with an acute onset of 5 to 10 days. Large pendulous breasts may develop yeast infection at the inframammary crease. Chronic infectious irritation can lead to hyperpigmentation of that area. Eczema or contact dermatitis frequently can affect the areola and become confused with Paget disease (ductal carcinoma in situ of the nipple). With Paget, the excoriation always starts at the nipple and can then spread to the areola. However, with dermatitis, the rash begins on the peri-areolar skin, without affecting the nipple itself.
When breast pain occurs with nipple discharge
Breast pain with nipple discharge usually is bilateral and more common in patients with significant fibrocystic changes who smoke. If the nipple discharge is bilateral, serous and non-bloody, and multiduct, it is considered benign and physiologic. Physiologic nipple discharge can be multifactorial and hormonal. It may be related to thyroid disorders or medications such as antidepressants, selective serotonin reuptake inhibitors (SSRIs), mood stabilizers, or antipsychotics. The only nipple discharge that is considered pathologic is unilateral spontaneous bloody discharge for which diagnostic imaging and breast surgeon referral is indicated. Women should be discouraged from self-expressing their nipples, as 80% will experience serous nipple discharge upon manual self-expression.
Breast pain is not associated with breast cancer. Most breast cancers do not hurt; they present as firm, painless masses. However, when a woman notices pain in her breast, her first concern is breast cancer. This concern is re-enforced by the medical provider whose first impulse is to order diagnostic imaging. Yet less than 3% of breast cancers are associated with breast pain.
There have been multiple published retrospective and prospective radiologic studies about the utility of breast imaging in women with breast pain without a palpable mass. All of the studies have demonstrated that breast imaging with mammography and ultrasonography in these patients yields mostly negative or benign findings. The incidence of breast cancer during imaging work-up in women with breast pain and no clinical abnormality is only 0.4% to 1.8%.1-3 Some patients may develop future subsequent breast cancer in the symptomatic breast. But this is considered incidental and possibly related to increased cell turnover related to fibrocystic changes. Breast imaging for evaluation of breast pain only provides reassurance to the physician. The patient's reassurance will come from a medical explanation for the symptoms and advice on symptom management from the provider.
Researchers from MD Anderson Cancer Center reported imaging findings and cost analysis for 799 patients presenting with breast pain from 3 large network community-based breast imaging centers in 2014. Breast ultrasound was the initial imaging modality for women younger than age 30. Digital mammography (sometimes with tomosynthesis) was used for those older than age 30 that had not had a mammogram in the last 6 months. Breast magnetic resonance imaging was performed only when ordered by the referring physician. Most of the patients presented for diagnostic imaging, and 95% had negative findings and 5% had a benign finding. Only 1 patient was found to have an incidental cancer in the contralateral breast, which was detected by tomosynthesis. The cost of breast imaging was $87,322 in younger women and $152,732 in women older than age 40, representing overutilization of health care resources and no association between breast pain and breast cancer.4
References
- Chetlan AL, Kapoor MM, Watts MR. Mastalgia: imaging work-up appropriateness. Acad Radiol. 2017;24:345-349.
- Arslan M, Kücükerdem HS, Can H, et al. Retrospective analysis of women with only mastalgia. J Breast Health. 2016;12:151-154.
- Noroozian M, Stein LF, Gaetke-Udager K, et al. Long-term clinical outcomes in women with breast pain in the absence of additional clinical findings: Mammography remains indicated. Breast Cancer Res Treat. 2015;149:417-424.
- Kushwaha AC, Shin K, Kalambo M, et al. Overutilization of health care resources for breast pain. AJR Am J Roentgenol. 2018; 211:217-223.
Management of mastalgia
Appropriate breast pain management begins with a good history and physical examination. The decision to perform imaging should depend on clinical exam findings and not on symptoms of breast pain. If there is a palpable mass, then breast imaging and possible biopsy is appropriate. However, if clinical exam is normal, there is no indication for breast imaging in low-risk women under the age of 40 whose only symptom is breast pain. Women older than age 40 can undergo diagnostic imaging, if they have not had a negative screening mammogram in the past year.
Breast pain with abnormal clinical exam
In the patient who is younger than age 30 with a palpable mass. For this patient order targeted breast ultrasound (US) (FIGURE 1). If results are negative, repeat the clinical examination 1 week after menses. If the mass is persistent, refer the patient to a breast surgeon. If diagnostic imaging results are negative, consider breast MRI, especially if there is a strong family history of breast cancer.
In the patient who is aged 30 and older with a palpable mass. For this patient, bilateral diagnostic mammogram and US are in order. The testing is best performed 1 week after menses to reduce false-positive findings. If imaging is negative and the patient still has a clinically suspicious finding or mass, refer her to a breast surgeon and consider breast MRI. At this point if there is a persistent firm dominant mass, a biopsy is indicated as part of the triple test. If the mass resolves with menses, the patient can be reassured that the cause is most likely benign, with clinical examination repeated in 3 months.

Continue to: Breast pain and normal clinical exam...
Breast pain and normal clinical exam
When women who report breast pain have normal clinical examination findings (and have a negative screening mammogram in the past 12 months if older than age 40), there are several management strategies you can offer (FIGURE 2).
Reassurance and education. The majority of women with breast pain can be managed with reassurance and education, which are often sufficient to reduce their anxieties.
Supportive bra. The most effective intervention is to wear and sleep in a well-fitted supportive sports bra for 4 to 12 weeks. In a nonrandomized single-center trial of danazol versus sports bra, 85% of women reported relief of their breast pain with bra alone (vs 58% with danazol).12 A supportive bra is the first-line management of mastalgia (Level II evidence).
Symptom diary/calendar. Many women do not know whether or not their symptoms correspond to their ovarian cycle or are related to hormonal fluctuations. Therefore, it is reassuring and informative for them to keep a calendar or a diary of their symptoms to determine whether their symptoms occur or are exacerbated in a cyclical pattern.
Diet and lifestyle modification. Women should avoid caffeine (especially when having pain). Studies on methylxanthines have demonstrated some symptom relief with reducing caffeine intake.11,13 One cup of coffee or tea per day most likely will not make a difference. However, if a woman is drinking large quantities of caffeinated beverages throughout the day, it will very likely improve her breast pain if she cuts back. This is especially true during the times of exacerbated pain prior to her menses.
In addition, recommend reduced dietary fat (overall good health). This is good advice for any patient. There were 2 small studies that showed improvement in breast pain with a 15% reduction in dietary fat.7,8
Finally, advise that patients stop smoking. Smoking aggravates and exacerbates fibrocystic changes, and these will lead to more breast pain.
Medical management. Over-the-counter medications that are found in the vitamin section of a local drug store are vitamin E and evening primrose oil. There are no significant adverse effects with these treatments. Their efficacy is theoretical, however; 3 randomized controlled trials demonstrated no significant clinical benefit with evening primrose oil versus placebo for treatment of mastalgia.14
Topical or oral nonsteroidal anti-inflammatory drugs (NSAIDs; Voltaren gel, topical compound pain creams) are useful as second-line management after using a supportive bra. Three randomized controlled trials have demonstrated up to 90% improvement of mastalgia with topical NSAIDs.15-17
Tamoxifen is a selective estrogen-receptor modulator (SERM), which is an antagonist to the estrogen receptor (ER) in the breast and an agonist to the ER in the endometrium. Tamoxifen has been found to reduce symptoms of mastalgia by 70% even at a lower dosage of 10-mg per day (for 6 months), or as a topical gel (afimoxifene). The oral form can have some adverse effects, including hot flashes, and has a low risk for thromboembolic events and endometrial neoplasia.18-20
Danazol is very effective in reducing breast pain symptoms (by 80%), with a higher relapse after stopping the medication. Danazol is less tolerated due to its androgenic effects, such as hirsutism, acne, menorrhagia, and voice changes. Both danazol and tamoxifen can be teratogenic and should be used with caution in women of child-bearing age.21
Finally, bromocriptine inhibits serum prolactin and has been reported to provide 65% improvement in breast pain. Its use for breast pain is not US Food and Drug Administration–approved and adverse effects include nausea, dizziness, and hypotension.22
Tamoxifen, danazol, and bromocriptine can be considered as third-line management options for severe treatment-resistant mastalgia.
Continue to: FIGURE 2 Treatment algorithm for breast pain...

In summary
Evaluation and counseling for breast pain should be managed by women’s health care providers in a primary care setting. Most patients need reassurance and medical explanation of their symptoms. They should be educated that more than 95% of the time breast pain is not caused by an underlying malignancy but rather due to hormonal and fibrocystic changes, which can be managed conservatively. If the clinical breast examination and recent screening mammogram (in women over age 40) results are negative, patients should be educated that their pain is benign and undergo a trial of conservative measures: wear and sleep in a supporting bra; keep a calendar of symptoms to determine any relation to cyclical changes; and avoid nicotine, caffeine, and fatty food. Topical pain creams with diclofenac and evening primrose oil also can be effective in ameliorating the symptoms. Breast pain is not a surgical disease; referral to a surgical specialist and diagnostic imaging can be unnecessary and expensive.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
- Scurr J, Hedger W, Morris P, et al. The prevalence, severity, and impact of breast pain in the general population. Breast J. 2014;20:508-513.
- Davies EL, Gateley CA, Miers M, et al. The long-term course of mastalgia. J R Soc Med. 1998;91:462-464.
- Singletary SE, Robb GL, Hortobagy GN. Advanced Therapy of Breast Disease. 2nd ed. Ontario, Canada: BC Decker Inc.; 2004.
- Gong C, Song E, Jia W, et al. A double-blind randomized controlled trial of toremifen therapy for mastaglia. Arch Surg. 2006;141:43-47.
- Kumar S, Mansel RE, Scanlon MF, et al. Altered responses of prolactin, luteinizing hormone and follicle stimulating hormone secretion to thyrotrophin releasing hormone/gonadotrophin releasing hormone stimulation in cyclical mastalgia. Br J Surg. 1984;71:870-873.
- Mansel RE, Dogliotti L. European multicentre trial of bromocriptine in cyclical mastalgia. Lancet. 1990;335:190-193.
- Rose DP, Boyar AP, Cohen C, et al. Effect of a low-fat diet on hormone levels in women with cystic breast disease. I. Serum steroids and gonadotropins. J Natl Cancer Inst. 1987;78:623-626.
- Goodwin JP, Miller A, Del Giudice ME, et al. Elevated high-density lipoprotein cholesterol and dietary fat intake in women with cyclic mastopathy. Am J Obstet Gynecol. 1998;179:430-437.
- Goyal A, Mansel RE. Efamast Study Group. A randomized multicenter study of gamolenic acid (Efamast) with and without antioxidant vitamins and minerals in the management of mastalgia. Breast J. 2005;11(1):41-47.
- Parsay S, Olfati F, Nahidi S. Therapeutic effects of vitamin E on cyclic mastalgia. Breast J. 2009;15:510-514.
- Allen SS, Froberg DG. The effect of decreased caffeine consumption on benign proliferative breast disease: a randomized clinical trial. Surgery. 1987;101:720-730.
- Hadi MS. Sports brassiere: is it a solution for mastalgia? Breast J. 2000;6:407-409.
- Russell LC. Caffeine restriction as initial treatment for breast pain. Nurse Pract. 1989; 14(2): 36-7.
- Srivastava A, Mansel RE, Arvind N, et al. Evidence-based management of mastalgia: a meta-analysis of randomised trials. Breast. 2007;16:503-512.
- Irving AD, Morrison SL. Effectiveness of topical non-steroidal anti-inflammatory drugs in the management of breast pain. J R Coll Surg Edinb. 1998;43:158-159.
- Colak T, Ipek T, Kanik A, et al. Efficiency of topical nonsteroidal anti-inflammatory drugs in mastalgia treatment. J Am Coll Surg. 2003;196(4):525-530.
- Kaviani A, Mehrdad N, Najafi M, et al. Comparison of naproxen with placebo for the management of noncyclical breast pain: a randomized, double-blind, controlled trial. World J Surg. 2008;32:2464-2470.
- Fentiman IS, Caleffi M, Brame K, et al. Double-blind controlled trial of tamoxifen therapy for mastalgia. Lancet. 1986;1:287-288.
- Jain BK, Bansal A, Choudhary D, et al. Centchroman vs tamoxifen for regression of mastalgia: a randomized controlled trial. Intl J Surg. 2015;15:11-16.
- Mansel R, Goyal A, Le Nestour EL, et al; Afimoxifene (4-OHT) Breast Pain Research group. A phase II trial of Afimoxifene (4-hydroxyamoxifen gel) for cyclical mastalgia in premenopausal women. Breast Cancer Res Treat. 2007;106:389-397.
- O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol. 1999;180:18-23.
- Blichert-Toft M, Andersen AN, Henriksen OB, et al. Treatment of mastalgia with bromocriptine: a double-blind cross-over study. Br Med J. 1979;1:237.
Chronic Myeloid Leukemia: Selecting First-line TKI Therapy
From the Moffitt Cancer Center, Tampa, FL.
Abstract
- Objective: To outline the approach to selecting a tyrosine kinase inhibitor (TKI) for initial treatment of chronic myeloid leukemia (CML) and monitoring patients following initiation of therapy.
- Methods: Review of the literature and evidence-based guidelines.
- Results: The development and availability of TKIs has improved survival for patients diagnosed with CML. The life expectancy of patients diagnosed with chronic-phase CML (CP-CML) is similar to that of the general population, provided they receive appropriate TKI therapy and adhere to treatment. Selection of the most appropriate first-line TKI for newly diagnosed CP-CML requires incorporation of the patient’s baseline karyotype and Sokal or EURO risk score, and a clear understanding of the patient’s comorbidities. The adverse effect profile of all TKIs must be considered in conjunction with the patient’s ongoing medical issues to decrease the likelihood of worsening their current symptoms or causing a severe complication from TKI therapy. After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, close monitoring and follow-up are necessary to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses.
- Conclusion: Given the successful treatments available for patients with CML, it is crucial to identify patients with this diagnosis; ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing; and select the best therapy for each individual patient.
Keywords: chronic myeloid leukemia; CML; tyrosine kinase inhibitor; TKI; cancer; BCR-ABL protein.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2)), resulting in the BCR-ABL1 fusion gene and its protein product, BCR-ABL tyrosine kinase.1 BCR-ABL has constitutive tyrosine kinase activity that promotes growth, replication, and survival of hematopoietic cells through downstream pathways, which is the driving factor in the pathogenesis of CML.1
CML is divided into 3 phases based on the number of myeloblasts observed in the blood or bone marrow: chronic, accelerated, and blast. Most cases of CML are diagnosed in the chronic phase (CP), which is marked by proliferation of primarily the myeloid element.
Typical treatment for CML involves lifelong use of oral BCR-ABL tyrosine kinase inhibitors (TKIs). Currently, 5 TKIs have regulatory approval for treatment of this disease. The advent of TKIs, a class of small molecules targeting the tyrosine kinases, particularly the BCR-ABL tyrosine kinase, led to rapid changes in the management of CML and improved survival for patients. Patients diagnosed with chronic-phase CML (CP-CML) now have a life expectancy that is similar to that of the general population, as long as they receive appropriate TKI therapy and adhere to treatment. As such, it is crucial to identify patients with CML; ensure they receive a complete, appropriate diagnostic workup; and select the best therapy for each patient.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed CP-CML has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the white blood cell differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
Testing
Bone marrow biopsy. There are 3 distinct phases of CML: CP, accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
Diagnostic criteria. The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of 1 of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100,000/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy sample (Table).9
The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosome abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
PCR assay. The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate or high risk scores compared to those with a low risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of CML patients relies on close monitoring and follow-up to ensure they are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.20
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a complete cytogenetic response (CCyR) implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.21
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,22 As noted, the IS has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,23 Molecular responses are based on a log reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that, if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.24 A value of 1% IS is approximately equivalent to a CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival (PFS), and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.24 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,22 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,25
Selecting First-line TKI Therapy
Selection of the most appropriate first-line TKI for newly diagnosed CP-CML patients requires incorporation of many patient-specific factors. These factors include baseline karyotype and confirmation of CP-CML through bone marrow biopsy, Sokal or EURO risk score, and a thorough patient history, including a clear understanding of the patient’s comorbidities. The adverse effect profile of all TKIs must be considered in conjunction with the patient’s ongoing medical issues in order to decrease the likelihood of worsening their current symptoms or causing a severe complication from TKI therapy.
Imatinib
The management of CML was revolutionized by the development and ultimate regulatory approval of imatinib mesylate in 2001. Imatinib was the first small-molecule cancer therapy developed and approved. It acts by binding to the adenosine triphosphate (ATP) binding site in the catalytic domain of BCR-ABL, thus inhibiting the oncoprotein’s tyrosine kinase activity.26
The International Randomized Study of Interferon versus STI571 (IRIS) trial was a randomized phase 3 study that compared imatinib 400 mg daily to interferon alfa (IFNa) plus cytarabine. More than 1000 CP-CML patients were randomly assigned 1:1 to either imatinib or IFNa plus cytarabine and were assessed for event-free survival, hematologic and cytogenetic responses, freedom from progression to AP or BP, and toxicity. Imatinib was superior to the prior standard of care for all these outcomes.21 The long-term follow-up of the IRIS trial reported an 83% estimated 10-year OS and 79% estimated event-free survival for patients on the imatinib arm of this study.15 The cumulative rate of CCyR was 82.8%. Of the 204 imatinib-treated patients who could undergo a molecular response evaluation at 10 years, 93.1% had a MMR and 63.2% had a MR4.5, suggesting durable, deep molecular responses for many patients. The estimated 10-year rate of freedom from progression to AP or BP was 92.1%.
Higher doses of imatinib (600-800 mg daily) have been studied in an attempt to overcome resistance and improve cytogenetic and molecular response rates. The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was a randomized phase 3 study that compared imatinib 800 mg daily to imatinib 400 mg daily. Although the 6-month assessments found increased rates of CCyR and a MMR in the higher-dose imatinib arm, these differences were no longer present at the 12-month assessment. Furthermore, the higher dose of imatinib led to a significantly higher incidence of grade 3/4 hematologic adverse events, and approximately 50% of patients on imatinib 800 mg daily required a dose reduction to less than 600 mg daily because of toxicity.27
The Therapeutic Intensification in De Novo Leukaemia (TIDEL)-II study used plasma trough levels of imatinib on day 22 of treatment with imatinib 600 mg daily to determine if patients should escalate the imatinib dose to 800 mg daily. In patients who did not meet molecular milestones at 3, 6, or 12 months, cohort 1 was dose escalated to imatinib 800 mg daily and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later, and cohort 2 was switched to nilotinib. At 2 years, 73% of patients achieved MMR and 34% achieved MR4.5, suggesting that initial treatment with higher-dose imatinib, followed by a switch to nilotinib in those failing to achieve desired milestones, could be an effective strategy for managing newly diagnosed CP-CML.28
Toxicity. The standard starting dose of imatinib in CP-CML patients is 400 mg. The safety profile of imatinib has been very well established. In the IRIS trial, the most common adverse events (all grades in decreasing order of frequency) were peripheral and periorbital edema (60%), nausea (50%), muscle cramps (49%), musculoskeletal pain (47%), diarrhea (45%), rash (40%), fatigue (39%), abdominal pain (37%), headache (37%), and joint pain (31%). Grade 3/4 liver enzyme elevation can occur in 5% of patients.29 In the event of severe liver toxicity or fluid retention, imatinib should be held until the event resolves. At that time, imatinib can be restarted if deemed appropriate, but this is dependent on the severity of the inciting event. Fluid retention can be managed by the use of supportive care, diuretics, imatinib dose reduction, dose interruption, or imatinib discontinuation if the fluid retention is severe. Muscle cramps can be managed by the use of calcium supplements or tonic water. Management of rash can include topical or systemic steroids, or in some cases imatinib dose reduction, interruption, or discontinuation.19
Grade 3/4 imatinib-induced hematologic toxicity is not uncommon, with 17% of patients experiencing neutropenia, 9% thrombocytopenia, and 4% anemia. These adverse events occurred most commonly during the first year of therapy, and the frequency decreased over time.15,29 Depending on the degree of cytopenias, imatinib dosing should be interrupted until recovery of the absolute neutrophil count or platelet count, and can often be resumed at 400 mg daily. However, if cytopenias recur, imatinib should be held and subsequently restarted at 300 mg daily.19
Dasatinib
Dasatinib is a second-generation TKI that has regulatory approval for treatment of adult patients with newly diagnosed CP-CML or CP-CML in patients with resistance or intolerance to prior TKIs. In addition to dasatinib’s ability to inhibit ABL kinases, it is also known to be a potent inhibitor of Src family kinases. Dasatinib has shown efficacy in patients who have developed imatinib-resistant ABL kinase domain mutations.
Dasatinib was initially approved as second-line therapy in patients with resistance or intolerance to imatinib. This indication was based on the results of the phase 3 CA180-034 trial, which ultimately identified dasatinib 100 mg daily as the optimal dose. In this trial, 74% of patients enrolled had resistance to imatinib and the remainder were intolerant. The 7-year follow-up of patients randomized to dasatinib 100 mg (n = 167) daily indicated that 46% achieved MMR while on study. Of the 124 imatinib-resistant patients on dasatinib 100 mg daily, the 7-year PFS was 39% and OS was 63%. In the 43 imatinib-intolerant patients, the 7-year PFS was 51% and OS was 70%.30
Dasatinib 100 mg daily was compared to imatinib 400 mg daily in newly diagnosed CP-CML patients in the randomized phase 3 DASISION (Dasatinib versus Imatinib Study in Treatment-Naive CML Patients) trial. More patients on the dasatinib arm achieved an EMR of BCR-ABL1 transcripts ≤ 10% IS after 3 months on treatment compared to imatinib (84% versus 64%). Furthermore, the 5-year follow-up reports that the cumulative incidence of MMR and MR4.5 in dasatinib-treated patients was 76% and 42%, and was 64% and 33% with imatinib (P = 0.0022 and P = 0.0251, respectively). Fewer patients treated with dasatinib progressed to AP or BP (4.6%) compared to imatinib (7.3%), but the estimated 5-year OS was similar between the 2 arms (91% for dasatinib versus 90% for imatinib).16 Regulatory approval for dasatinib as first-line therapy in newly diagnosed CML patients was based on results of the DASISION trial.
Toxicity. Most dasatinib-related toxicities are reported as grade 1 or grade 2, but grade 3/4 hematologic adverse events are fairly common. In the DASISION trial, grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 29%, 13%, and 22% of dasatinib-treated patients, respectively. Cytopenias can generally be managed with temporary dose interruptions or dose reductions.
During the 5-year follow-up of the DASISION trial, pleural effusions were reported in 28% of patients, most of which were grade 1/2. This occurred at a rate of approximately ≤ 8% per year, suggesting a stable incidence over time, and the effusions appear to be dose-dependent.16 Depending on the severity, pleural effusion may be treated with diuretics, dose interruption, and, in some instances, steroids or a thoracentesis. Typically, dasatinib can be restarted at 1 dose level lower than the previous dose once the effusion has resolved.19 Other, less common side effects of dasatinib include pulmonary hypertension (5% of patients), as well as abdominal pain, fluid retention, headaches, fatigue, musculoskeletal pain, rash, nausea, and diarrhea. Pulmonary hypertension is typically reversible after cessation of dasatinib, and thus dasatinib should be permanently discontinued once the diagnosis is confirmed. Fluid retention is often treated with diuretics and supportive care. Nausea and diarrhea are generally manageable and occur less frequently when dasatinib is taken with food and a large glass of water. Antiemetics and antidiarrheals can be used as needed. Troublesome rash can be best managed with topical or systemic steroids as well as possible dose reduction or dose interruption.16,19 In the DASISION trial, adverse events led to therapy discontinuation more often in the dasatinib group than in the imatinib group (16% versus 7%).16 Bleeding, particularly in the setting of thrombocytopenia, has been reported in patients being treated with dasatinib as a result of the drug-induced reversible inhibition of platelet aggregation.31
Nilotinib
The structure of nilotinib is similar to that of imatinib; however, it has a markedly increased affinity for the ATP‐binding site on the BCR-ABL1 protein. It was initially given regulatory approval in the setting of imatinib failure. Nilotinib was studied at a dose of 400 mg twice daily in 321 patients who were imatinib-resistant or -intolerant. It proved to be highly effective at inducing cytogenetic remissions in the second-line setting, with 59% of patients achieving a MCyR and 45% achieving a CCyR. With a median follow-up time of 4 years, the OS was 78%.32
Nilotinib gained regulatory approval for use as a first-line TKI after completion of the randomized phase 3 ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients) trial. ENESTnd was a 3-arm study comparing nilotinib 300 mg twice daily versus nilotinib 400 mg twice daily versus imatinib 400 mg daily in newly diagnosed, previously untreated patients diagnosed with CP-CML. The primary endpoint of this clinical trial was rate of MMR at 12 months.33 Nilotinib surpassed imatinib in this regard, with 44% of patients on nilotinib 300 mg twice daily achieving MMR at 12 months versus 43% of nilotinib 400 mg twice daily patients versus 22% of the imatinib-treated patients (P < 0.001 for both comparisons). Furthermore, the rate of CCyR by 12 months was significantly higher for both nilotinib arms compared with imatinib (80% for nilotinib 300 mg, 78% for nilotinib 400 mg, and 65% for imatinib) (P < 0.001).12 Based on this data, nilotinib 300 mg twice daily was chosen as the standard dose of nilotinib in the first-line setting. After 5 years of follow-up on the ENESTnd study, there were fewer progressions to AP/BP CML in nilotinib-treated patients compared with imatinib. MMR was achieved in 77% of nilotinib 300 mg patients compared with 60.4% of patients on the imatinib arm. MR4.5 was also more common in patients treated with nilotinib 300 mg twice daily, with a rate of 53.5% at 5 years versus 31.4% in the imatinib arm.17 In spite of the deeper cytogenetic and molecular responses achieved with nilotinib, this did not translate into a significant improvement in OS. The 5-year OS rate was 93.7% in nilotinib 300 mg patients versus 91.7% in imatinib-treated patients, and this difference lacked statistical significance.17
Toxicity. Although some similarities exist between the toxicity profiles of nilotinib and imatinib, each drug has some distinct adverse events. On the ENESTnd trial, the rate of any grade 3/4 non-hematologic adverse event was fairly low; however, lower-grade toxicities were not uncommon. Patients treated with nilotinib 300 mg twice daily experienced rash (31%), headache (14%), pruritis (15%), and fatigue (11%) most commonly. The most frequently reported laboratory abnormalities included increased total bilirubin (53%), hypophosphatemia (32%), hyperglycemia (36%), elevated lipase (24%), increased alanine aminotransferase (ALT; 66%), and increased aspartate aminotransferase (AST; 40%). Any grade of neutropenia, thrombocytopenia, or anemia occurred at rates of 43%, 48%, and 38%, respectively.33 Although nilotinib has a Black Box Warning from the US Food and Drug Administration for QT interval prolongation, no patients on the ENESTnd trial experienced a QT interval corrected for heart rate greater than 500 msec.12
More recent concerns have emerged regarding the potential for cardiovascular toxicity after long-term use of nilotinib. The 5-year update of ENESTnd reports cardiovascular events, including ischemic heart disease, ischemic cerebrovascular events, or peripheral arterial disease occurring in 7.5% of patients treated with nilotinib 300 mg twice daily, as compared with a rate of 2.1% in imatinib-treated patients. The frequency of these cardiovascular events increased linearly over time in both arms. Elevations in total cholesterol from baseline occurred in 27.6% of nilotinib patients compared with 3.9% of imatinib patients. Furthermore, clinically meaningful increases in low-density lipoprotein cholesterol and glycated hemoglobin occurred more frequently with nilotinib therapy.33
Nilotinib should be taken on an empty stomach; therefore, patients should be made aware of the need to fast for 2 hours prior to each dose and 1 hour after each dose. Given the potential risk of QT interval prolongation, a baseline electrocardiogram (ECG) is recommended prior to initiating treatment to ensure the QT interval is within a normal range. A repeat ECG should be done approximately 7 days after nilotinib initiation to ensure no prolongation of the QT interval after starting. Close monitoring of potassium and magnesium levels is important to decrease the risk of cardiac arrhythmias, and concomitant use of drugs considered strong CYP3A4 inhibitors should be avoided.19
If the patient experiences any grade 3 or higher laboratory abnormalities, nilotinib should be held until resolution of the toxicity, and then restarted at a lower dose. Similarly, if patients develop significant neutropenia or thrombocytopenia, nilotinib doses should be interrupted until resolution of the cytopenias. At that point, nilotinib can be reinitiated at either the same or a lower dose. Rash can be managed by the use of topical or systemic steroids as well as potential dose reduction, interruption, or discontinuation.
Given the concerns for potential cardiovascular events with long-term use of nilotinib, caution is advised when prescribing it to any patient with a history of cardiovascular disease or peripheral arterial occlusive disease. At the first sign of new occlusive disease, nilotinib should be discontinued.19
Bosutinib
Bosutinib is a second-generation BCR-ABL TKI with activity against the Src family of kinases; it was initially approved to treat patients with CP-, AP-, or BP-CML after resistance or intolerance to imatinib. Long-term data has been reported from the phase 1/2 trial of bosutinib therapy in patients with CP-CML who developed resistance or intolerance to imatinib plus dasatinib and/or nilotinib. A total of 119 patients were included in the 4-year follow-up; 38 were resistant/intolerant to imatinib and resistant to dasatinib, 50 were resistant/intolerant to imatinib and intolerant to dasatinib, 26 were resistant/intolerant to imatinib and resistant to nilotinib, and 5 were resistant/intolerant to imatinib and intolerant to nilotinib or resistant/intolerant to dasatinib and nilotinib. Bosutinib 400 mg daily was studied in this setting. Of the 38 patients with imatinib resistance/intolerance and dasatinib resistance, 39% achieved MCyR, 22% achieved CCyR, and the OS was 67%. Of the 50 patients with imatinib resistance/intolerance and dasatinib intolerance, 42% achieved MCyR, 40% achieved CCyR, and the OS was 80%. Finally, in the 26 patients with imatinib resistance/intolerance and nilotinib resistance, 38% achieved MCyR, 31% achieved CCyR, and the OS was 87%.34
Five-year follow-up from the phase 1/2 clinical trial that studied bosutinib 500 mg daily in CP-CML patients after imatinib failure reported data on 284 patients. By 5 years on study, 60% of patients had achieved MCyR and 50% achieved CCyR with a 71% and 69% probability, respectively, of maintaining these responses at 5 years. The 5-year OS was 84%.35 These data led to the regulatory approval of bosutinib 500 mg daily as second-line or later therapy.
Bosutinib was initially studied in the first-line setting in the randomized phase 3 BELA (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia) trial. This trial compared bosutinib 500 mg daily to imatinib 400 mg daily in newly diagnosed, previously untreated CP-CML patients. This trial failed to meet its primary endpoint of increased rate of CCyR at 12 months, with 70% of bosutinib patients achieving this response, compared to 68% of imatinib-treated patients (P = 0.601). In spite of this, the rate of MMR at 12 months was significantly higher in the bosutinib arm (41%) compared to the imatinib arm (27%; P = 0.001).36
A second phase 3 trial (BFORE) was designed to study bosutinib 400 mg daily versus imatinib in newly diagnosed, previously untreated CP-CML patients. This study enrolled 536 patients who were randomly assigned 1:1 to bosutinib versus imatinib. The primary endpoint of this trial was rate of MMR at 12 months. A significantly higher number of bosutinib-treated patients achieved this response (47.2%) compared with imatinib-treated patients (36.9%, P = 0.02). Furthermore, by 12 months 77.2% of patients on the bosutinib arm had achieved CCyR compared with 66.4% on the imatinib arm, and this difference did meet statistical significance (P = 0.0075). A lower rate of progression to AP- or BP-CML was noted in bosutinib-treated patients as well (1.6% versus 2.5%). Based on this data, bosutinib gained regulatory approval for first-line therapy in CP-CML at a dose of 400 mg daily.18
Toxicity. On the BFORE trial, the most common treatment-emergent adverse events of any grade reported in the bosutinib-treated patients were diarrhea (70.1%), nausea (35.1%), increased ALT (30.6%), and increased AST (22.8%). Musculoskeletal pain or spasms occurred in 29.5% of patients, rash in 19.8%, fatigue in 19.4%, and headache in 18.7%. Hematologic toxicity was also reported, but most was grade 1/2. Thrombocytopenia was reported in 35.1%, anemia in 18.7%, and neutropenia in 11.2%.18
Cardiovascular events occurred in 5.2% of patients on the bosutinib arm of the BFORE trial, which was similar to the rate observed in imatinib patients. The most common cardiovascular event was QT interval prolongation, which occurred in 1.5% of patients. Pleural effusions were reported in 1.9% of patients treated with bosutinib, and none were grade 3 or higher.18
If liver enzyme elevation occurs at a value greater than 5 times the institutional upper limit of normal, bosutinib should be held until the level recovers to ≤ 2.5 times the upper limit of normal, at which point bosutinib can be restarted at a lower dose. If recovery takes longer than 4 weeks, bosutinib should be permanently discontinued. Liver enzymes elevated greater than 3 times the institutional upper limit of normal and a concurrent elevation in total bilirubin to 2 times the upper limit of normal are consistent with Hy’s law, and bosutinib should be discontinued. Although diarrhea is the most common toxicity associated with bosutinib, it is commonly low grade and transient. Diarrhea occurs most frequently in the first few days after initiating bosutinib. It can often be managed with over-the-counter antidiarrheal medications, but if the diarrhea is grade 3 or higher, bosutinib should be held until recovery to grade 1 or lower. Gastrointestinal side effects may be improved by taking bosutinib with a meal and a large glass of water. Fluid retention can be managed with diuretics and supportive care. Finally, if rash occurs, this can be addressed with topical or systemic steroids as well as bosutinib dose reduction, interruption, or discontinuation.19
Similar to other TKIs, if bosutinib-induced cytopenias occur, treatment should be held and restarted at the same or a lower dose upon blood count recovery.19
Ponatinib
The most common cause of TKI resistance in CP-CML is the development of ABL kinase domain mutations. The majority of imatinib-resistant mutations can be overcome by the use of second-generation TKIs, including dasatinib, nilotinib, or bosutinib. However, ponatinib is the only BCR-ABL TKI able to overcome a T315I mutation. The phase 2 PACE (Ponatinib Ph-positive ALL and CML Evaluation) trial enrolled patients with CP-, AP-, or BP-CML as well as patients with Ph-positive acute lymphoblastic leukemia who were resistant or intolerant to nilotinib or dasatinib, or who had evidence of a T315I mutation. The starting dose of ponatinib on this trial was 45 mg daily.37 The PACE trial enrolled 267 patients with CP-CML: 203 with resistance or intolerance to nilotinib or dasatinib, and 64 with a T315I mutation. The primary endpoint in the CP cohort was rate of MCyR at any time within 12 months of starting ponatinib. The overall rate of MCyR by 12 months in the CP-CML patients was 56%. In those with a T315I mutation, 70% achieved MCyR, which compared favorably with those with resistance or intolerance to nilotinib or dasatinib, 51% of whom achieved MCyR. CCyR was achieved in 46% of CP-CML patients (40% in the resistant/intolerant cohort and 66% in the T315I cohort). In general, patients with T315I mutations received fewer prior therapies than those in the resistant/intolerant cohort, which likely contributed to the higher response rates in the T315I patients. MR4.5 was achieved in 15% of CP-CML patients by 12 months on the PACE trial.37 The 5-year update to this study reported that 60%, 40%, and 24% of CP-CML patients achieved MCyR, MMR, and MR4.5, respectively. In the patients who achieved MCyR, the probability of maintaining this response for 5 years was 82% and the estimated 5-year OS was 73%.19
Toxicity. In 2013, after the regulatory approval of ponatinib, reports became available that the drug can cause an increase in arterial occlusive events, including fatal myocardial infarctions and cerebrovascular accidents. For this reason, dose reductions were implemented in patients who were deriving clinical benefit from ponatinib. In spite of these dose reductions, ≥ 90% of responders maintained their response for up to 40 months.38 Although the likelihood of developing an arterial occlusive event appears higher in the first year after starting ponatinib than in later years, the cumulative incidence of events continues to increase. The 5-year follow-up to the PACE trial reports 31% of patients experiencing any grade of arterial occlusive event while on ponatinib. Aside from these events, the most common treatment-emergent adverse events in ponatinib-treated patients on the PACE trial included rash (47%), abdominal pain (46%), headache (43%), dry skin (42%), constipation (41%), and hypertension (37%). Hematologic toxicity was also common, with 46% of patients experiencing any grade of thrombocytopenia, 20% experiencing neutropenia, and 20% anemia.38
Patients receiving ponatinib therapy should be monitored closely for any evidence of arterial or venous thrombosis. If an occlusive event occurs, ponatinib should be discontinued. Similarly, in the setting of any new or worsening heart failure symptoms, ponatinib should be promptly discontinued. Management of any underlying cardiovascular risk factors, including hypertension, hyperlipidemia, diabetes, or smoking history, is recommended, and these patients should be referred to a cardiologist for a full evaluation. In the absence of any contraindications to aspirin, low-dose aspirin should be considered as a means of decreasing cardiovascular risks associated with ponatinib. In patients with known risk factors, a ponatinib starting dose of 30 mg daily rather than the standard 45 mg daily may be a safer option, resulting in fewer arterial occlusive events, although the efficacy of this dose is still being studied in comparison to 45 mg daily.19
If ponatinib-induced transaminitis greater than 3 times the upper limit of normal occurs, ponatinib should be held until resolution to less than 3 times the upper limit of normal, at which point it should be resumed at a lower dose. Similarly, in the setting of elevated serum lipase or symptomatic pancreatitis, ponatinib should be held and restarted at a lower dose after resolution of symptoms.19
In the event of neutropenia or thrombocytopenia, ponatinib should be held until blood count recovery and then restarted at the same dose. If cytopenias occur for a second time, the dose of ponatinib should be lowered at the time of treatment reinitiation. If rash occurs, it can be addressed with topical or systemic steroids as well as dose reduction, interruption, or discontinuation.19
Conclusion
With the development of imatinib and the subsequent TKIs, dasatinib, nilotinib, bosutinib, and ponatinib, CP-CML has become a chronic disease with a life expectancy that is similar to that of the general population. Given the successful treatments available for these patients, it is crucial to identify patients with this diagnosis, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated. This is the only way to be certain patients are achieving the desired treatment milestones that correlate with the favorable long-term outcomes that have been observed with TKI-based treatment of CP-CML.
Corresponding author: Kendra Sweet, MD, MS, Department of Malignant Hematology, Moffitt Cancer Center, Tampa, FL.
Financial disclosures: Dr. Sweet has served on the Advisory Board and Speakers Bureau of Novartis, Bristol-Meyers Squibb, Ariad Pharmaceuticals, and Pfizer, and has served as a consultant to Pfizer.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-2340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
21. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
22. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
23. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR]. Medicina (B Aires). 2017;77:61-72.
24. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
25. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
26. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
27. Baccarani M, Druker BJ, Branford S, et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99:616-624.
28. Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125:915-923.
29. Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-2417.
30. Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869-874.
31. Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261-263.
32. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107-112.
33. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-2259.
34. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206-1214.
35. Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298-1307.
36. Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486-3492.
37. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783-1796.
38. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393-404.
From the Moffitt Cancer Center, Tampa, FL.
Abstract
- Objective: To outline the approach to selecting a tyrosine kinase inhibitor (TKI) for initial treatment of chronic myeloid leukemia (CML) and monitoring patients following initiation of therapy.
- Methods: Review of the literature and evidence-based guidelines.
- Results: The development and availability of TKIs has improved survival for patients diagnosed with CML. The life expectancy of patients diagnosed with chronic-phase CML (CP-CML) is similar to that of the general population, provided they receive appropriate TKI therapy and adhere to treatment. Selection of the most appropriate first-line TKI for newly diagnosed CP-CML requires incorporation of the patient’s baseline karyotype and Sokal or EURO risk score, and a clear understanding of the patient’s comorbidities. The adverse effect profile of all TKIs must be considered in conjunction with the patient’s ongoing medical issues to decrease the likelihood of worsening their current symptoms or causing a severe complication from TKI therapy. After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, close monitoring and follow-up are necessary to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses.
- Conclusion: Given the successful treatments available for patients with CML, it is crucial to identify patients with this diagnosis; ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing; and select the best therapy for each individual patient.
Keywords: chronic myeloid leukemia; CML; tyrosine kinase inhibitor; TKI; cancer; BCR-ABL protein.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2)), resulting in the BCR-ABL1 fusion gene and its protein product, BCR-ABL tyrosine kinase.1 BCR-ABL has constitutive tyrosine kinase activity that promotes growth, replication, and survival of hematopoietic cells through downstream pathways, which is the driving factor in the pathogenesis of CML.1
CML is divided into 3 phases based on the number of myeloblasts observed in the blood or bone marrow: chronic, accelerated, and blast. Most cases of CML are diagnosed in the chronic phase (CP), which is marked by proliferation of primarily the myeloid element.
Typical treatment for CML involves lifelong use of oral BCR-ABL tyrosine kinase inhibitors (TKIs). Currently, 5 TKIs have regulatory approval for treatment of this disease. The advent of TKIs, a class of small molecules targeting the tyrosine kinases, particularly the BCR-ABL tyrosine kinase, led to rapid changes in the management of CML and improved survival for patients. Patients diagnosed with chronic-phase CML (CP-CML) now have a life expectancy that is similar to that of the general population, as long as they receive appropriate TKI therapy and adhere to treatment. As such, it is crucial to identify patients with CML; ensure they receive a complete, appropriate diagnostic workup; and select the best therapy for each patient.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed CP-CML has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the white blood cell differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
Testing
Bone marrow biopsy. There are 3 distinct phases of CML: CP, accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
Diagnostic criteria. The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of 1 of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100,000/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy sample (Table).9
The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosome abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
PCR assay. The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate or high risk scores compared to those with a low risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of CML patients relies on close monitoring and follow-up to ensure they are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.20
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a complete cytogenetic response (CCyR) implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.21
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,22 As noted, the IS has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,23 Molecular responses are based on a log reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that, if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.24 A value of 1% IS is approximately equivalent to a CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival (PFS), and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.24 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,22 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,25
Selecting First-line TKI Therapy
Selection of the most appropriate first-line TKI for newly diagnosed CP-CML patients requires incorporation of many patient-specific factors. These factors include baseline karyotype and confirmation of CP-CML through bone marrow biopsy, Sokal or EURO risk score, and a thorough patient history, including a clear understanding of the patient’s comorbidities. The adverse effect profile of all TKIs must be considered in conjunction with the patient’s ongoing medical issues in order to decrease the likelihood of worsening their current symptoms or causing a severe complication from TKI therapy.
Imatinib
The management of CML was revolutionized by the development and ultimate regulatory approval of imatinib mesylate in 2001. Imatinib was the first small-molecule cancer therapy developed and approved. It acts by binding to the adenosine triphosphate (ATP) binding site in the catalytic domain of BCR-ABL, thus inhibiting the oncoprotein’s tyrosine kinase activity.26
The International Randomized Study of Interferon versus STI571 (IRIS) trial was a randomized phase 3 study that compared imatinib 400 mg daily to interferon alfa (IFNa) plus cytarabine. More than 1000 CP-CML patients were randomly assigned 1:1 to either imatinib or IFNa plus cytarabine and were assessed for event-free survival, hematologic and cytogenetic responses, freedom from progression to AP or BP, and toxicity. Imatinib was superior to the prior standard of care for all these outcomes.21 The long-term follow-up of the IRIS trial reported an 83% estimated 10-year OS and 79% estimated event-free survival for patients on the imatinib arm of this study.15 The cumulative rate of CCyR was 82.8%. Of the 204 imatinib-treated patients who could undergo a molecular response evaluation at 10 years, 93.1% had a MMR and 63.2% had a MR4.5, suggesting durable, deep molecular responses for many patients. The estimated 10-year rate of freedom from progression to AP or BP was 92.1%.
Higher doses of imatinib (600-800 mg daily) have been studied in an attempt to overcome resistance and improve cytogenetic and molecular response rates. The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was a randomized phase 3 study that compared imatinib 800 mg daily to imatinib 400 mg daily. Although the 6-month assessments found increased rates of CCyR and a MMR in the higher-dose imatinib arm, these differences were no longer present at the 12-month assessment. Furthermore, the higher dose of imatinib led to a significantly higher incidence of grade 3/4 hematologic adverse events, and approximately 50% of patients on imatinib 800 mg daily required a dose reduction to less than 600 mg daily because of toxicity.27
The Therapeutic Intensification in De Novo Leukaemia (TIDEL)-II study used plasma trough levels of imatinib on day 22 of treatment with imatinib 600 mg daily to determine if patients should escalate the imatinib dose to 800 mg daily. In patients who did not meet molecular milestones at 3, 6, or 12 months, cohort 1 was dose escalated to imatinib 800 mg daily and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later, and cohort 2 was switched to nilotinib. At 2 years, 73% of patients achieved MMR and 34% achieved MR4.5, suggesting that initial treatment with higher-dose imatinib, followed by a switch to nilotinib in those failing to achieve desired milestones, could be an effective strategy for managing newly diagnosed CP-CML.28
Toxicity. The standard starting dose of imatinib in CP-CML patients is 400 mg. The safety profile of imatinib has been very well established. In the IRIS trial, the most common adverse events (all grades in decreasing order of frequency) were peripheral and periorbital edema (60%), nausea (50%), muscle cramps (49%), musculoskeletal pain (47%), diarrhea (45%), rash (40%), fatigue (39%), abdominal pain (37%), headache (37%), and joint pain (31%). Grade 3/4 liver enzyme elevation can occur in 5% of patients.29 In the event of severe liver toxicity or fluid retention, imatinib should be held until the event resolves. At that time, imatinib can be restarted if deemed appropriate, but this is dependent on the severity of the inciting event. Fluid retention can be managed by the use of supportive care, diuretics, imatinib dose reduction, dose interruption, or imatinib discontinuation if the fluid retention is severe. Muscle cramps can be managed by the use of calcium supplements or tonic water. Management of rash can include topical or systemic steroids, or in some cases imatinib dose reduction, interruption, or discontinuation.19
Grade 3/4 imatinib-induced hematologic toxicity is not uncommon, with 17% of patients experiencing neutropenia, 9% thrombocytopenia, and 4% anemia. These adverse events occurred most commonly during the first year of therapy, and the frequency decreased over time.15,29 Depending on the degree of cytopenias, imatinib dosing should be interrupted until recovery of the absolute neutrophil count or platelet count, and can often be resumed at 400 mg daily. However, if cytopenias recur, imatinib should be held and subsequently restarted at 300 mg daily.19
Dasatinib
Dasatinib is a second-generation TKI that has regulatory approval for treatment of adult patients with newly diagnosed CP-CML or CP-CML in patients with resistance or intolerance to prior TKIs. In addition to dasatinib’s ability to inhibit ABL kinases, it is also known to be a potent inhibitor of Src family kinases. Dasatinib has shown efficacy in patients who have developed imatinib-resistant ABL kinase domain mutations.
Dasatinib was initially approved as second-line therapy in patients with resistance or intolerance to imatinib. This indication was based on the results of the phase 3 CA180-034 trial, which ultimately identified dasatinib 100 mg daily as the optimal dose. In this trial, 74% of patients enrolled had resistance to imatinib and the remainder were intolerant. The 7-year follow-up of patients randomized to dasatinib 100 mg (n = 167) daily indicated that 46% achieved MMR while on study. Of the 124 imatinib-resistant patients on dasatinib 100 mg daily, the 7-year PFS was 39% and OS was 63%. In the 43 imatinib-intolerant patients, the 7-year PFS was 51% and OS was 70%.30
Dasatinib 100 mg daily was compared to imatinib 400 mg daily in newly diagnosed CP-CML patients in the randomized phase 3 DASISION (Dasatinib versus Imatinib Study in Treatment-Naive CML Patients) trial. More patients on the dasatinib arm achieved an EMR of BCR-ABL1 transcripts ≤ 10% IS after 3 months on treatment compared to imatinib (84% versus 64%). Furthermore, the 5-year follow-up reports that the cumulative incidence of MMR and MR4.5 in dasatinib-treated patients was 76% and 42%, and was 64% and 33% with imatinib (P = 0.0022 and P = 0.0251, respectively). Fewer patients treated with dasatinib progressed to AP or BP (4.6%) compared to imatinib (7.3%), but the estimated 5-year OS was similar between the 2 arms (91% for dasatinib versus 90% for imatinib).16 Regulatory approval for dasatinib as first-line therapy in newly diagnosed CML patients was based on results of the DASISION trial.
Toxicity. Most dasatinib-related toxicities are reported as grade 1 or grade 2, but grade 3/4 hematologic adverse events are fairly common. In the DASISION trial, grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 29%, 13%, and 22% of dasatinib-treated patients, respectively. Cytopenias can generally be managed with temporary dose interruptions or dose reductions.
During the 5-year follow-up of the DASISION trial, pleural effusions were reported in 28% of patients, most of which were grade 1/2. This occurred at a rate of approximately ≤ 8% per year, suggesting a stable incidence over time, and the effusions appear to be dose-dependent.16 Depending on the severity, pleural effusion may be treated with diuretics, dose interruption, and, in some instances, steroids or a thoracentesis. Typically, dasatinib can be restarted at 1 dose level lower than the previous dose once the effusion has resolved.19 Other, less common side effects of dasatinib include pulmonary hypertension (5% of patients), as well as abdominal pain, fluid retention, headaches, fatigue, musculoskeletal pain, rash, nausea, and diarrhea. Pulmonary hypertension is typically reversible after cessation of dasatinib, and thus dasatinib should be permanently discontinued once the diagnosis is confirmed. Fluid retention is often treated with diuretics and supportive care. Nausea and diarrhea are generally manageable and occur less frequently when dasatinib is taken with food and a large glass of water. Antiemetics and antidiarrheals can be used as needed. Troublesome rash can be best managed with topical or systemic steroids as well as possible dose reduction or dose interruption.16,19 In the DASISION trial, adverse events led to therapy discontinuation more often in the dasatinib group than in the imatinib group (16% versus 7%).16 Bleeding, particularly in the setting of thrombocytopenia, has been reported in patients being treated with dasatinib as a result of the drug-induced reversible inhibition of platelet aggregation.31
Nilotinib
The structure of nilotinib is similar to that of imatinib; however, it has a markedly increased affinity for the ATP‐binding site on the BCR-ABL1 protein. It was initially given regulatory approval in the setting of imatinib failure. Nilotinib was studied at a dose of 400 mg twice daily in 321 patients who were imatinib-resistant or -intolerant. It proved to be highly effective at inducing cytogenetic remissions in the second-line setting, with 59% of patients achieving a MCyR and 45% achieving a CCyR. With a median follow-up time of 4 years, the OS was 78%.32
Nilotinib gained regulatory approval for use as a first-line TKI after completion of the randomized phase 3 ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients) trial. ENESTnd was a 3-arm study comparing nilotinib 300 mg twice daily versus nilotinib 400 mg twice daily versus imatinib 400 mg daily in newly diagnosed, previously untreated patients diagnosed with CP-CML. The primary endpoint of this clinical trial was rate of MMR at 12 months.33 Nilotinib surpassed imatinib in this regard, with 44% of patients on nilotinib 300 mg twice daily achieving MMR at 12 months versus 43% of nilotinib 400 mg twice daily patients versus 22% of the imatinib-treated patients (P < 0.001 for both comparisons). Furthermore, the rate of CCyR by 12 months was significantly higher for both nilotinib arms compared with imatinib (80% for nilotinib 300 mg, 78% for nilotinib 400 mg, and 65% for imatinib) (P < 0.001).12 Based on this data, nilotinib 300 mg twice daily was chosen as the standard dose of nilotinib in the first-line setting. After 5 years of follow-up on the ENESTnd study, there were fewer progressions to AP/BP CML in nilotinib-treated patients compared with imatinib. MMR was achieved in 77% of nilotinib 300 mg patients compared with 60.4% of patients on the imatinib arm. MR4.5 was also more common in patients treated with nilotinib 300 mg twice daily, with a rate of 53.5% at 5 years versus 31.4% in the imatinib arm.17 In spite of the deeper cytogenetic and molecular responses achieved with nilotinib, this did not translate into a significant improvement in OS. The 5-year OS rate was 93.7% in nilotinib 300 mg patients versus 91.7% in imatinib-treated patients, and this difference lacked statistical significance.17
Toxicity. Although some similarities exist between the toxicity profiles of nilotinib and imatinib, each drug has some distinct adverse events. On the ENESTnd trial, the rate of any grade 3/4 non-hematologic adverse event was fairly low; however, lower-grade toxicities were not uncommon. Patients treated with nilotinib 300 mg twice daily experienced rash (31%), headache (14%), pruritis (15%), and fatigue (11%) most commonly. The most frequently reported laboratory abnormalities included increased total bilirubin (53%), hypophosphatemia (32%), hyperglycemia (36%), elevated lipase (24%), increased alanine aminotransferase (ALT; 66%), and increased aspartate aminotransferase (AST; 40%). Any grade of neutropenia, thrombocytopenia, or anemia occurred at rates of 43%, 48%, and 38%, respectively.33 Although nilotinib has a Black Box Warning from the US Food and Drug Administration for QT interval prolongation, no patients on the ENESTnd trial experienced a QT interval corrected for heart rate greater than 500 msec.12
More recent concerns have emerged regarding the potential for cardiovascular toxicity after long-term use of nilotinib. The 5-year update of ENESTnd reports cardiovascular events, including ischemic heart disease, ischemic cerebrovascular events, or peripheral arterial disease occurring in 7.5% of patients treated with nilotinib 300 mg twice daily, as compared with a rate of 2.1% in imatinib-treated patients. The frequency of these cardiovascular events increased linearly over time in both arms. Elevations in total cholesterol from baseline occurred in 27.6% of nilotinib patients compared with 3.9% of imatinib patients. Furthermore, clinically meaningful increases in low-density lipoprotein cholesterol and glycated hemoglobin occurred more frequently with nilotinib therapy.33
Nilotinib should be taken on an empty stomach; therefore, patients should be made aware of the need to fast for 2 hours prior to each dose and 1 hour after each dose. Given the potential risk of QT interval prolongation, a baseline electrocardiogram (ECG) is recommended prior to initiating treatment to ensure the QT interval is within a normal range. A repeat ECG should be done approximately 7 days after nilotinib initiation to ensure no prolongation of the QT interval after starting. Close monitoring of potassium and magnesium levels is important to decrease the risk of cardiac arrhythmias, and concomitant use of drugs considered strong CYP3A4 inhibitors should be avoided.19
If the patient experiences any grade 3 or higher laboratory abnormalities, nilotinib should be held until resolution of the toxicity, and then restarted at a lower dose. Similarly, if patients develop significant neutropenia or thrombocytopenia, nilotinib doses should be interrupted until resolution of the cytopenias. At that point, nilotinib can be reinitiated at either the same or a lower dose. Rash can be managed by the use of topical or systemic steroids as well as potential dose reduction, interruption, or discontinuation.
Given the concerns for potential cardiovascular events with long-term use of nilotinib, caution is advised when prescribing it to any patient with a history of cardiovascular disease or peripheral arterial occlusive disease. At the first sign of new occlusive disease, nilotinib should be discontinued.19
Bosutinib
Bosutinib is a second-generation BCR-ABL TKI with activity against the Src family of kinases; it was initially approved to treat patients with CP-, AP-, or BP-CML after resistance or intolerance to imatinib. Long-term data has been reported from the phase 1/2 trial of bosutinib therapy in patients with CP-CML who developed resistance or intolerance to imatinib plus dasatinib and/or nilotinib. A total of 119 patients were included in the 4-year follow-up; 38 were resistant/intolerant to imatinib and resistant to dasatinib, 50 were resistant/intolerant to imatinib and intolerant to dasatinib, 26 were resistant/intolerant to imatinib and resistant to nilotinib, and 5 were resistant/intolerant to imatinib and intolerant to nilotinib or resistant/intolerant to dasatinib and nilotinib. Bosutinib 400 mg daily was studied in this setting. Of the 38 patients with imatinib resistance/intolerance and dasatinib resistance, 39% achieved MCyR, 22% achieved CCyR, and the OS was 67%. Of the 50 patients with imatinib resistance/intolerance and dasatinib intolerance, 42% achieved MCyR, 40% achieved CCyR, and the OS was 80%. Finally, in the 26 patients with imatinib resistance/intolerance and nilotinib resistance, 38% achieved MCyR, 31% achieved CCyR, and the OS was 87%.34
Five-year follow-up from the phase 1/2 clinical trial that studied bosutinib 500 mg daily in CP-CML patients after imatinib failure reported data on 284 patients. By 5 years on study, 60% of patients had achieved MCyR and 50% achieved CCyR with a 71% and 69% probability, respectively, of maintaining these responses at 5 years. The 5-year OS was 84%.35 These data led to the regulatory approval of bosutinib 500 mg daily as second-line or later therapy.
Bosutinib was initially studied in the first-line setting in the randomized phase 3 BELA (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia) trial. This trial compared bosutinib 500 mg daily to imatinib 400 mg daily in newly diagnosed, previously untreated CP-CML patients. This trial failed to meet its primary endpoint of increased rate of CCyR at 12 months, with 70% of bosutinib patients achieving this response, compared to 68% of imatinib-treated patients (P = 0.601). In spite of this, the rate of MMR at 12 months was significantly higher in the bosutinib arm (41%) compared to the imatinib arm (27%; P = 0.001).36
A second phase 3 trial (BFORE) was designed to study bosutinib 400 mg daily versus imatinib in newly diagnosed, previously untreated CP-CML patients. This study enrolled 536 patients who were randomly assigned 1:1 to bosutinib versus imatinib. The primary endpoint of this trial was rate of MMR at 12 months. A significantly higher number of bosutinib-treated patients achieved this response (47.2%) compared with imatinib-treated patients (36.9%, P = 0.02). Furthermore, by 12 months 77.2% of patients on the bosutinib arm had achieved CCyR compared with 66.4% on the imatinib arm, and this difference did meet statistical significance (P = 0.0075). A lower rate of progression to AP- or BP-CML was noted in bosutinib-treated patients as well (1.6% versus 2.5%). Based on this data, bosutinib gained regulatory approval for first-line therapy in CP-CML at a dose of 400 mg daily.18
Toxicity. On the BFORE trial, the most common treatment-emergent adverse events of any grade reported in the bosutinib-treated patients were diarrhea (70.1%), nausea (35.1%), increased ALT (30.6%), and increased AST (22.8%). Musculoskeletal pain or spasms occurred in 29.5% of patients, rash in 19.8%, fatigue in 19.4%, and headache in 18.7%. Hematologic toxicity was also reported, but most was grade 1/2. Thrombocytopenia was reported in 35.1%, anemia in 18.7%, and neutropenia in 11.2%.18
Cardiovascular events occurred in 5.2% of patients on the bosutinib arm of the BFORE trial, which was similar to the rate observed in imatinib patients. The most common cardiovascular event was QT interval prolongation, which occurred in 1.5% of patients. Pleural effusions were reported in 1.9% of patients treated with bosutinib, and none were grade 3 or higher.18
If liver enzyme elevation occurs at a value greater than 5 times the institutional upper limit of normal, bosutinib should be held until the level recovers to ≤ 2.5 times the upper limit of normal, at which point bosutinib can be restarted at a lower dose. If recovery takes longer than 4 weeks, bosutinib should be permanently discontinued. Liver enzymes elevated greater than 3 times the institutional upper limit of normal and a concurrent elevation in total bilirubin to 2 times the upper limit of normal are consistent with Hy’s law, and bosutinib should be discontinued. Although diarrhea is the most common toxicity associated with bosutinib, it is commonly low grade and transient. Diarrhea occurs most frequently in the first few days after initiating bosutinib. It can often be managed with over-the-counter antidiarrheal medications, but if the diarrhea is grade 3 or higher, bosutinib should be held until recovery to grade 1 or lower. Gastrointestinal side effects may be improved by taking bosutinib with a meal and a large glass of water. Fluid retention can be managed with diuretics and supportive care. Finally, if rash occurs, this can be addressed with topical or systemic steroids as well as bosutinib dose reduction, interruption, or discontinuation.19
Similar to other TKIs, if bosutinib-induced cytopenias occur, treatment should be held and restarted at the same or a lower dose upon blood count recovery.19
Ponatinib
The most common cause of TKI resistance in CP-CML is the development of ABL kinase domain mutations. The majority of imatinib-resistant mutations can be overcome by the use of second-generation TKIs, including dasatinib, nilotinib, or bosutinib. However, ponatinib is the only BCR-ABL TKI able to overcome a T315I mutation. The phase 2 PACE (Ponatinib Ph-positive ALL and CML Evaluation) trial enrolled patients with CP-, AP-, or BP-CML as well as patients with Ph-positive acute lymphoblastic leukemia who were resistant or intolerant to nilotinib or dasatinib, or who had evidence of a T315I mutation. The starting dose of ponatinib on this trial was 45 mg daily.37 The PACE trial enrolled 267 patients with CP-CML: 203 with resistance or intolerance to nilotinib or dasatinib, and 64 with a T315I mutation. The primary endpoint in the CP cohort was rate of MCyR at any time within 12 months of starting ponatinib. The overall rate of MCyR by 12 months in the CP-CML patients was 56%. In those with a T315I mutation, 70% achieved MCyR, which compared favorably with those with resistance or intolerance to nilotinib or dasatinib, 51% of whom achieved MCyR. CCyR was achieved in 46% of CP-CML patients (40% in the resistant/intolerant cohort and 66% in the T315I cohort). In general, patients with T315I mutations received fewer prior therapies than those in the resistant/intolerant cohort, which likely contributed to the higher response rates in the T315I patients. MR4.5 was achieved in 15% of CP-CML patients by 12 months on the PACE trial.37 The 5-year update to this study reported that 60%, 40%, and 24% of CP-CML patients achieved MCyR, MMR, and MR4.5, respectively. In the patients who achieved MCyR, the probability of maintaining this response for 5 years was 82% and the estimated 5-year OS was 73%.19
Toxicity. In 2013, after the regulatory approval of ponatinib, reports became available that the drug can cause an increase in arterial occlusive events, including fatal myocardial infarctions and cerebrovascular accidents. For this reason, dose reductions were implemented in patients who were deriving clinical benefit from ponatinib. In spite of these dose reductions, ≥ 90% of responders maintained their response for up to 40 months.38 Although the likelihood of developing an arterial occlusive event appears higher in the first year after starting ponatinib than in later years, the cumulative incidence of events continues to increase. The 5-year follow-up to the PACE trial reports 31% of patients experiencing any grade of arterial occlusive event while on ponatinib. Aside from these events, the most common treatment-emergent adverse events in ponatinib-treated patients on the PACE trial included rash (47%), abdominal pain (46%), headache (43%), dry skin (42%), constipation (41%), and hypertension (37%). Hematologic toxicity was also common, with 46% of patients experiencing any grade of thrombocytopenia, 20% experiencing neutropenia, and 20% anemia.38
Patients receiving ponatinib therapy should be monitored closely for any evidence of arterial or venous thrombosis. If an occlusive event occurs, ponatinib should be discontinued. Similarly, in the setting of any new or worsening heart failure symptoms, ponatinib should be promptly discontinued. Management of any underlying cardiovascular risk factors, including hypertension, hyperlipidemia, diabetes, or smoking history, is recommended, and these patients should be referred to a cardiologist for a full evaluation. In the absence of any contraindications to aspirin, low-dose aspirin should be considered as a means of decreasing cardiovascular risks associated with ponatinib. In patients with known risk factors, a ponatinib starting dose of 30 mg daily rather than the standard 45 mg daily may be a safer option, resulting in fewer arterial occlusive events, although the efficacy of this dose is still being studied in comparison to 45 mg daily.19
If ponatinib-induced transaminitis greater than 3 times the upper limit of normal occurs, ponatinib should be held until resolution to less than 3 times the upper limit of normal, at which point it should be resumed at a lower dose. Similarly, in the setting of elevated serum lipase or symptomatic pancreatitis, ponatinib should be held and restarted at a lower dose after resolution of symptoms.19
In the event of neutropenia or thrombocytopenia, ponatinib should be held until blood count recovery and then restarted at the same dose. If cytopenias occur for a second time, the dose of ponatinib should be lowered at the time of treatment reinitiation. If rash occurs, it can be addressed with topical or systemic steroids as well as dose reduction, interruption, or discontinuation.19
Conclusion
With the development of imatinib and the subsequent TKIs, dasatinib, nilotinib, bosutinib, and ponatinib, CP-CML has become a chronic disease with a life expectancy that is similar to that of the general population. Given the successful treatments available for these patients, it is crucial to identify patients with this diagnosis, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated. This is the only way to be certain patients are achieving the desired treatment milestones that correlate with the favorable long-term outcomes that have been observed with TKI-based treatment of CP-CML.
Corresponding author: Kendra Sweet, MD, MS, Department of Malignant Hematology, Moffitt Cancer Center, Tampa, FL.
Financial disclosures: Dr. Sweet has served on the Advisory Board and Speakers Bureau of Novartis, Bristol-Meyers Squibb, Ariad Pharmaceuticals, and Pfizer, and has served as a consultant to Pfizer.
From the Moffitt Cancer Center, Tampa, FL.
Abstract
- Objective: To outline the approach to selecting a tyrosine kinase inhibitor (TKI) for initial treatment of chronic myeloid leukemia (CML) and monitoring patients following initiation of therapy.
- Methods: Review of the literature and evidence-based guidelines.
- Results: The development and availability of TKIs has improved survival for patients diagnosed with CML. The life expectancy of patients diagnosed with chronic-phase CML (CP-CML) is similar to that of the general population, provided they receive appropriate TKI therapy and adhere to treatment. Selection of the most appropriate first-line TKI for newly diagnosed CP-CML requires incorporation of the patient’s baseline karyotype and Sokal or EURO risk score, and a clear understanding of the patient’s comorbidities. The adverse effect profile of all TKIs must be considered in conjunction with the patient’s ongoing medical issues to decrease the likelihood of worsening their current symptoms or causing a severe complication from TKI therapy. After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, close monitoring and follow-up are necessary to ensure patients are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses.
- Conclusion: Given the successful treatments available for patients with CML, it is crucial to identify patients with this diagnosis; ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing; and select the best therapy for each individual patient.
Keywords: chronic myeloid leukemia; CML; tyrosine kinase inhibitor; TKI; cancer; BCR-ABL protein.
Chronic myeloid leukemia (CML) is a rare myeloproliferative neoplasm that is characterized by the presence of the Philadelphia (Ph) chromosome and uninhibited expansion of bone marrow stem cells. The Ph chromosome arises from a reciprocal translocation between the Abelson (ABL) region on chromosome 9 and the breakpoint cluster region (BCR) of chromosome 22 (t(9;22)(q34;q11.2)), resulting in the BCR-ABL1 fusion gene and its protein product, BCR-ABL tyrosine kinase.1 BCR-ABL has constitutive tyrosine kinase activity that promotes growth, replication, and survival of hematopoietic cells through downstream pathways, which is the driving factor in the pathogenesis of CML.1
CML is divided into 3 phases based on the number of myeloblasts observed in the blood or bone marrow: chronic, accelerated, and blast. Most cases of CML are diagnosed in the chronic phase (CP), which is marked by proliferation of primarily the myeloid element.
Typical treatment for CML involves lifelong use of oral BCR-ABL tyrosine kinase inhibitors (TKIs). Currently, 5 TKIs have regulatory approval for treatment of this disease. The advent of TKIs, a class of small molecules targeting the tyrosine kinases, particularly the BCR-ABL tyrosine kinase, led to rapid changes in the management of CML and improved survival for patients. Patients diagnosed with chronic-phase CML (CP-CML) now have a life expectancy that is similar to that of the general population, as long as they receive appropriate TKI therapy and adhere to treatment. As such, it is crucial to identify patients with CML; ensure they receive a complete, appropriate diagnostic workup; and select the best therapy for each patient.
Epidemiology
According to SEER data estimates, 8430 new cases of CML were diagnosed in the United States in 2018. CML is a disease of older adults, with a median age of 65 years at diagnosis, and there is a slight male predominance. Between 2011 and 2015, the number of new CML cases was 1.8 per 100,000 persons. The median overall survival (OS) in patients with newly diagnosed CP-CML has not been reached.2 Given the effective treatments available for managing CML, it is estimated that the prevalence of CML in the United States will plateau at 180,000 patients by 2050.3
Diagnosis
Clinical Features
The diagnosis of CML is often suspected based on an incidental finding of leukocytosis and, in some cases, thrombocytosis. In many cases, this is an incidental finding on routine blood work, but approximately 50% of patients will present with constitutional symptoms associated with the disease. Characteristic features of the white blood cell differential include left-shifted maturation with neutrophilia and immature circulating myeloid cells. Basophilia and eosinophilia are often present as well. Splenomegaly is a common sign, present in 50% to 90% of patients at diagnosis. In those patients with symptoms related to CML at diagnosis, the most common presentation includes increasing fatigue, fevers, night sweats, early satiety, and weight loss. The diagnosis is confirmed by cytogenetic studies showing the Ph chromosome abnormality, t(9; 22)(q3.4;q1.1), and/or reverse transcriptase polymerase chain reaction (PCR) showing BCR-ABL1 transcripts.
Testing
Bone marrow biopsy. There are 3 distinct phases of CML: CP, accelerated phase (AP), and blast phase (BP). Bone marrow biopsy and aspiration at diagnosis are mandatory in order to determine the phase of the disease at diagnosis. This distinction is based on the percentage of blasts, promyelocytes, and basophils present as well as the platelet count and presence or absence of extramedullary disease.4 The vast majority of patients at diagnosis have CML that is in the chronic phase. The typical appearance in CP-CML is a hypercellular marrow with granulocytic and occasionally megakaryocytic hyperplasia. In many cases, basophilia and/or eosinophilia are noted as well. Dysplasia is not a typical finding in CML.5 Bone marrow fibrosis can be seen in up to one-third of patients at diagnosis, and may indicate a slightly worse prognosis.6 Although a diagnosis of CML can be made without a bone marrow biopsy, complete staging and prognostication are only possible with information gained from this test, including baseline karyotype and confirmation of CP versus a more advanced phase of CML.
Diagnostic criteria. The criteria for diagnosing AP-CML has not been agreed upon by various groups, but the modified MD Anderson Cancer Center (MDACC) criteria are used in the majority of clinical trials evaluating the efficacy of TKIs in preventing progression to advanced phases of CML. MDACC criteria define AP-CML as the presence of 1 of the following: 15% to 29% blasts in the peripheral blood or bone marrow, ≥ 30% peripheral blasts plus promyelocytes, ≥ 20% basophils in the blood or bone marrow, platelet count ≤ 100,000/μL unrelated to therapy, and clonal cytogenetic evolution in Ph-positive metaphases (Table).7
BP-CML is typically defined using the criteria developed by the International Bone Marrow Transplant Registry (IBMTR): ≥ 30% blasts in the peripheral blood and/or the bone marrow or the presence of extramedullary disease.8 Although not typically used in clinical trials, the revised World Health Organization (WHO) criteria for BP-CML include ≥ 20% blasts in the peripheral blood or bone marrow, extramedullary blast proliferation, and large foci or clusters of blasts in the bone marrow biopsy sample (Table).9
The defining feature of CML is the presence of the Ph chromosome abnormality. In a small subset of patients, additional chromosome abnormalities (ACA) in the Ph-positive cells may be identified at diagnosis. Some reports indicate that the presence of “major route” ACA (trisomy 8, isochromosome 17q, a second Ph chromosome, or trisomy 19) at diagnosis may negatively impact prognosis, but other reports contradict these findings.10,11
PCR assay. The typical BCR breakpoint in CML is the major breakpoint cluster region (M-BCR), which results in a 210-kDa protein (p210). Alternate breakpoints that are less frequently identified are the minor BCR (mBCR or p190), which is more commonly found in Ph-positive acute lymphoblastic leukemia (ALL), and the micro BCR (µBCR or p230), which is much less common and is often characterized by chronic neutrophilia.12 Identifying which BCR-ABL1 transcript is present in each patient using qualitative PCR is crucial in order to ensure proper monitoring during treatment.
The most sensitive method for detecting BCR-ABL1 mRNA transcripts is the quantitative real-time PCR (RQ-PCR) assay, which is typically done on peripheral blood. RQ-PCR is capable of detecting a single CML cell in the presence of ≥ 100,000 normal cells. This test should be done during the initial diagnostic workup in order to confirm the presence of BCR-ABL1 transcripts, and it is used as a standard method for monitoring response to TKI therapy.13 The International Scale (IS) is a standardized approach to reporting RQ-PCR results that was developed to allow comparison of results across various laboratories and has become the gold standard for reporting BCR-ABL1 transcript values.14
Determining Risk Scores
Calculating a patient’s Sokal score or EURO risk score at diagnosis remains an important component of the diagnostic workup in CP-CML, as this information has prognostic and therapeutic implications (an online calculator is available through European LeukemiaNet [ELN]). The risk for disease progression to the accelerated or blast phases is higher in patients with intermediate or high risk scores compared to those with a low risk score at diagnosis. The risk of progression in intermediate- or high-risk patients is lower when a second-generation TKI (dasatinib, nilotinib, or bosutinib) is used as frontline therapy compared to imatinib, and therefore, the National Comprehensive Cancer Network (NCCN) CML Panel recommends starting with a second-generation TKI in these patients.15-19
Monitoring Response to Therapy
After confirming a diagnosis of CML and selecting the most appropriate TKI for first-line therapy, the successful management of CML patients relies on close monitoring and follow-up to ensure they are meeting the desired treatment milestones. Responses in CML can be assessed based on hematologic parameters, cytogenetic results, and molecular responses. A complete hematologic response (CHR) implies complete normalization of peripheral blood counts (with the exception of TKI-induced cytopenias) and resolution of any palpable splenomegaly. The majority of patients will achieve a CHR within 4 to 6 weeks after initiating CML-directed therapy.20
Cytogenetic Response
Cytogenetic responses are defined by the decrease in the number of Ph chromosome–positive metaphases when assessed on bone marrow cytogenetics. A partial cytogenetic response (PCyR) is defined as having 1% to 35% Ph-positive metaphases, a major cytogenetic response (MCyR) as having 0% to 35% Ph-positive metaphases, and a complete cytogenetic response (CCyR) implies that no Ph-positive metaphases are identified on bone marrow cytogenetics. An ideal response is the achievement of PCyR after 3 months on a TKI and a CCyR after 12 months on a TKI.21
Molecular Response
Once a patient has achieved a CCyR, monitoring their response to therapy can only be done using RQ-PCR to measure BCR-ABL1 transcripts in the peripheral blood. The NCCN and the ELN recommend monitoring RQ-PCR from the peripheral blood every 3 months in order to assess response to TKIs.19,22 As noted, the IS has become the gold standard reporting system for all BCR-ABL1 transcript levels in the majority of laboratories worldwide.14,23 Molecular responses are based on a log reduction in BCR-ABL1 transcripts from a standardized baseline. Many molecular responses can be correlated with cytogenetic responses such that, if reliable RQ-PCR testing is available, monitoring can be done using only peripheral blood RQ-PCR rather than repeat bone marrow biopsies. For example, an early molecular response (EMR) is defined as a RQ-PCR value of ≤ 10% IS, which is approximately equivalent to a PCyR.24 A value of 1% IS is approximately equivalent to a CCyR. A major molecular response (MMR) is a ≥ 3-log reduction in BCR-ABL1 transcripts from baseline and is a value of ≤ 0.1% IS. Deeper levels of molecular response are best described by the log reduction in BCR-ABL1 transcripts, with a 4-log reduction denoted as MR4.0, a 4.5-log reduction as MR4.5, and so forth. Complete molecular response (CMR) is defined by the level of sensitivity of the RQ-PCR assay being used.14
The definition of relapsed disease in CML is dependent on the type of response the patient had previously achieved. Relapse could be the loss of a hematologic or cytogenetic response, but fluctuations in BCR-ABL1 transcripts on routine RQ-PCR do not necessarily indicate relapsed CML. A 1-log increase in the level of BCR-ABL1 transcripts with a concurrent loss of MMR should prompt a bone marrow biopsy in order to assess for the loss of CCyR, and thus a cytogenetic relapse; however, this loss of MMR does not define relapse in and of itself. In the setting of relapsed disease, testing should be done to look for possible ABL kinase domain mutations, and alternate therapy should be selected.19
Multiple reports have identified the prognostic relevance of achieving an EMR at 3 and 6 months after starting TKI therapy. Marin and colleagues reported that in 282 imatinib-treated patients, there was a significant improvement in 8-year OS, progression-free survival (PFS), and cumulative incidence of CCyR and CMR in patients who had BCR-ABL1 transcripts < 9.84% IS after 3 months on treatment.24 This data highlights the importance of early molecular monitoring in order to ensure the best outcomes for patients with CP-CML.
The NCCN CML guidelines and ELN recommendations both agree that an ideal response after 3 months on a TKI is BCR-ABL1 transcripts < 10% IS, but treatment is not considered to be failing at this point if the patient marginally misses this milestone. After 6 months on treatment, an ideal response is considered BCR-ABL1 transcripts < 1%–10% IS. Ideally, patients will have BCR-ABL1 transcripts < 0.1%–1% IS by the time they complete 12 months of TKI therapy, suggesting that these patients have at least achieved a CCyR.19,22 Even after patients achieve these early milestones, frequent monitoring by RQ-PCR is required to ensure that they are maintaining their response to treatment. This will help to ensure patient compliance with treatment and will also help to identify a select subset of patients who could potentially be considered for an attempt at TKI cessation (not discussed in detail here) after a minimum of 3 years on therapy.19,25
Selecting First-line TKI Therapy
Selection of the most appropriate first-line TKI for newly diagnosed CP-CML patients requires incorporation of many patient-specific factors. These factors include baseline karyotype and confirmation of CP-CML through bone marrow biopsy, Sokal or EURO risk score, and a thorough patient history, including a clear understanding of the patient’s comorbidities. The adverse effect profile of all TKIs must be considered in conjunction with the patient’s ongoing medical issues in order to decrease the likelihood of worsening their current symptoms or causing a severe complication from TKI therapy.
Imatinib
The management of CML was revolutionized by the development and ultimate regulatory approval of imatinib mesylate in 2001. Imatinib was the first small-molecule cancer therapy developed and approved. It acts by binding to the adenosine triphosphate (ATP) binding site in the catalytic domain of BCR-ABL, thus inhibiting the oncoprotein’s tyrosine kinase activity.26
The International Randomized Study of Interferon versus STI571 (IRIS) trial was a randomized phase 3 study that compared imatinib 400 mg daily to interferon alfa (IFNa) plus cytarabine. More than 1000 CP-CML patients were randomly assigned 1:1 to either imatinib or IFNa plus cytarabine and were assessed for event-free survival, hematologic and cytogenetic responses, freedom from progression to AP or BP, and toxicity. Imatinib was superior to the prior standard of care for all these outcomes.21 The long-term follow-up of the IRIS trial reported an 83% estimated 10-year OS and 79% estimated event-free survival for patients on the imatinib arm of this study.15 The cumulative rate of CCyR was 82.8%. Of the 204 imatinib-treated patients who could undergo a molecular response evaluation at 10 years, 93.1% had a MMR and 63.2% had a MR4.5, suggesting durable, deep molecular responses for many patients. The estimated 10-year rate of freedom from progression to AP or BP was 92.1%.
Higher doses of imatinib (600-800 mg daily) have been studied in an attempt to overcome resistance and improve cytogenetic and molecular response rates. The Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) trial was a randomized phase 3 study that compared imatinib 800 mg daily to imatinib 400 mg daily. Although the 6-month assessments found increased rates of CCyR and a MMR in the higher-dose imatinib arm, these differences were no longer present at the 12-month assessment. Furthermore, the higher dose of imatinib led to a significantly higher incidence of grade 3/4 hematologic adverse events, and approximately 50% of patients on imatinib 800 mg daily required a dose reduction to less than 600 mg daily because of toxicity.27
The Therapeutic Intensification in De Novo Leukaemia (TIDEL)-II study used plasma trough levels of imatinib on day 22 of treatment with imatinib 600 mg daily to determine if patients should escalate the imatinib dose to 800 mg daily. In patients who did not meet molecular milestones at 3, 6, or 12 months, cohort 1 was dose escalated to imatinib 800 mg daily and subsequently switched to nilotinib 400 mg twice daily for failing the same target 3 months later, and cohort 2 was switched to nilotinib. At 2 years, 73% of patients achieved MMR and 34% achieved MR4.5, suggesting that initial treatment with higher-dose imatinib, followed by a switch to nilotinib in those failing to achieve desired milestones, could be an effective strategy for managing newly diagnosed CP-CML.28
Toxicity. The standard starting dose of imatinib in CP-CML patients is 400 mg. The safety profile of imatinib has been very well established. In the IRIS trial, the most common adverse events (all grades in decreasing order of frequency) were peripheral and periorbital edema (60%), nausea (50%), muscle cramps (49%), musculoskeletal pain (47%), diarrhea (45%), rash (40%), fatigue (39%), abdominal pain (37%), headache (37%), and joint pain (31%). Grade 3/4 liver enzyme elevation can occur in 5% of patients.29 In the event of severe liver toxicity or fluid retention, imatinib should be held until the event resolves. At that time, imatinib can be restarted if deemed appropriate, but this is dependent on the severity of the inciting event. Fluid retention can be managed by the use of supportive care, diuretics, imatinib dose reduction, dose interruption, or imatinib discontinuation if the fluid retention is severe. Muscle cramps can be managed by the use of calcium supplements or tonic water. Management of rash can include topical or systemic steroids, or in some cases imatinib dose reduction, interruption, or discontinuation.19
Grade 3/4 imatinib-induced hematologic toxicity is not uncommon, with 17% of patients experiencing neutropenia, 9% thrombocytopenia, and 4% anemia. These adverse events occurred most commonly during the first year of therapy, and the frequency decreased over time.15,29 Depending on the degree of cytopenias, imatinib dosing should be interrupted until recovery of the absolute neutrophil count or platelet count, and can often be resumed at 400 mg daily. However, if cytopenias recur, imatinib should be held and subsequently restarted at 300 mg daily.19
Dasatinib
Dasatinib is a second-generation TKI that has regulatory approval for treatment of adult patients with newly diagnosed CP-CML or CP-CML in patients with resistance or intolerance to prior TKIs. In addition to dasatinib’s ability to inhibit ABL kinases, it is also known to be a potent inhibitor of Src family kinases. Dasatinib has shown efficacy in patients who have developed imatinib-resistant ABL kinase domain mutations.
Dasatinib was initially approved as second-line therapy in patients with resistance or intolerance to imatinib. This indication was based on the results of the phase 3 CA180-034 trial, which ultimately identified dasatinib 100 mg daily as the optimal dose. In this trial, 74% of patients enrolled had resistance to imatinib and the remainder were intolerant. The 7-year follow-up of patients randomized to dasatinib 100 mg (n = 167) daily indicated that 46% achieved MMR while on study. Of the 124 imatinib-resistant patients on dasatinib 100 mg daily, the 7-year PFS was 39% and OS was 63%. In the 43 imatinib-intolerant patients, the 7-year PFS was 51% and OS was 70%.30
Dasatinib 100 mg daily was compared to imatinib 400 mg daily in newly diagnosed CP-CML patients in the randomized phase 3 DASISION (Dasatinib versus Imatinib Study in Treatment-Naive CML Patients) trial. More patients on the dasatinib arm achieved an EMR of BCR-ABL1 transcripts ≤ 10% IS after 3 months on treatment compared to imatinib (84% versus 64%). Furthermore, the 5-year follow-up reports that the cumulative incidence of MMR and MR4.5 in dasatinib-treated patients was 76% and 42%, and was 64% and 33% with imatinib (P = 0.0022 and P = 0.0251, respectively). Fewer patients treated with dasatinib progressed to AP or BP (4.6%) compared to imatinib (7.3%), but the estimated 5-year OS was similar between the 2 arms (91% for dasatinib versus 90% for imatinib).16 Regulatory approval for dasatinib as first-line therapy in newly diagnosed CML patients was based on results of the DASISION trial.
Toxicity. Most dasatinib-related toxicities are reported as grade 1 or grade 2, but grade 3/4 hematologic adverse events are fairly common. In the DASISION trial, grade 3/4 neutropenia, anemia, and thrombocytopenia occurred in 29%, 13%, and 22% of dasatinib-treated patients, respectively. Cytopenias can generally be managed with temporary dose interruptions or dose reductions.
During the 5-year follow-up of the DASISION trial, pleural effusions were reported in 28% of patients, most of which were grade 1/2. This occurred at a rate of approximately ≤ 8% per year, suggesting a stable incidence over time, and the effusions appear to be dose-dependent.16 Depending on the severity, pleural effusion may be treated with diuretics, dose interruption, and, in some instances, steroids or a thoracentesis. Typically, dasatinib can be restarted at 1 dose level lower than the previous dose once the effusion has resolved.19 Other, less common side effects of dasatinib include pulmonary hypertension (5% of patients), as well as abdominal pain, fluid retention, headaches, fatigue, musculoskeletal pain, rash, nausea, and diarrhea. Pulmonary hypertension is typically reversible after cessation of dasatinib, and thus dasatinib should be permanently discontinued once the diagnosis is confirmed. Fluid retention is often treated with diuretics and supportive care. Nausea and diarrhea are generally manageable and occur less frequently when dasatinib is taken with food and a large glass of water. Antiemetics and antidiarrheals can be used as needed. Troublesome rash can be best managed with topical or systemic steroids as well as possible dose reduction or dose interruption.16,19 In the DASISION trial, adverse events led to therapy discontinuation more often in the dasatinib group than in the imatinib group (16% versus 7%).16 Bleeding, particularly in the setting of thrombocytopenia, has been reported in patients being treated with dasatinib as a result of the drug-induced reversible inhibition of platelet aggregation.31
Nilotinib
The structure of nilotinib is similar to that of imatinib; however, it has a markedly increased affinity for the ATP‐binding site on the BCR-ABL1 protein. It was initially given regulatory approval in the setting of imatinib failure. Nilotinib was studied at a dose of 400 mg twice daily in 321 patients who were imatinib-resistant or -intolerant. It proved to be highly effective at inducing cytogenetic remissions in the second-line setting, with 59% of patients achieving a MCyR and 45% achieving a CCyR. With a median follow-up time of 4 years, the OS was 78%.32
Nilotinib gained regulatory approval for use as a first-line TKI after completion of the randomized phase 3 ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients) trial. ENESTnd was a 3-arm study comparing nilotinib 300 mg twice daily versus nilotinib 400 mg twice daily versus imatinib 400 mg daily in newly diagnosed, previously untreated patients diagnosed with CP-CML. The primary endpoint of this clinical trial was rate of MMR at 12 months.33 Nilotinib surpassed imatinib in this regard, with 44% of patients on nilotinib 300 mg twice daily achieving MMR at 12 months versus 43% of nilotinib 400 mg twice daily patients versus 22% of the imatinib-treated patients (P < 0.001 for both comparisons). Furthermore, the rate of CCyR by 12 months was significantly higher for both nilotinib arms compared with imatinib (80% for nilotinib 300 mg, 78% for nilotinib 400 mg, and 65% for imatinib) (P < 0.001).12 Based on this data, nilotinib 300 mg twice daily was chosen as the standard dose of nilotinib in the first-line setting. After 5 years of follow-up on the ENESTnd study, there were fewer progressions to AP/BP CML in nilotinib-treated patients compared with imatinib. MMR was achieved in 77% of nilotinib 300 mg patients compared with 60.4% of patients on the imatinib arm. MR4.5 was also more common in patients treated with nilotinib 300 mg twice daily, with a rate of 53.5% at 5 years versus 31.4% in the imatinib arm.17 In spite of the deeper cytogenetic and molecular responses achieved with nilotinib, this did not translate into a significant improvement in OS. The 5-year OS rate was 93.7% in nilotinib 300 mg patients versus 91.7% in imatinib-treated patients, and this difference lacked statistical significance.17
Toxicity. Although some similarities exist between the toxicity profiles of nilotinib and imatinib, each drug has some distinct adverse events. On the ENESTnd trial, the rate of any grade 3/4 non-hematologic adverse event was fairly low; however, lower-grade toxicities were not uncommon. Patients treated with nilotinib 300 mg twice daily experienced rash (31%), headache (14%), pruritis (15%), and fatigue (11%) most commonly. The most frequently reported laboratory abnormalities included increased total bilirubin (53%), hypophosphatemia (32%), hyperglycemia (36%), elevated lipase (24%), increased alanine aminotransferase (ALT; 66%), and increased aspartate aminotransferase (AST; 40%). Any grade of neutropenia, thrombocytopenia, or anemia occurred at rates of 43%, 48%, and 38%, respectively.33 Although nilotinib has a Black Box Warning from the US Food and Drug Administration for QT interval prolongation, no patients on the ENESTnd trial experienced a QT interval corrected for heart rate greater than 500 msec.12
More recent concerns have emerged regarding the potential for cardiovascular toxicity after long-term use of nilotinib. The 5-year update of ENESTnd reports cardiovascular events, including ischemic heart disease, ischemic cerebrovascular events, or peripheral arterial disease occurring in 7.5% of patients treated with nilotinib 300 mg twice daily, as compared with a rate of 2.1% in imatinib-treated patients. The frequency of these cardiovascular events increased linearly over time in both arms. Elevations in total cholesterol from baseline occurred in 27.6% of nilotinib patients compared with 3.9% of imatinib patients. Furthermore, clinically meaningful increases in low-density lipoprotein cholesterol and glycated hemoglobin occurred more frequently with nilotinib therapy.33
Nilotinib should be taken on an empty stomach; therefore, patients should be made aware of the need to fast for 2 hours prior to each dose and 1 hour after each dose. Given the potential risk of QT interval prolongation, a baseline electrocardiogram (ECG) is recommended prior to initiating treatment to ensure the QT interval is within a normal range. A repeat ECG should be done approximately 7 days after nilotinib initiation to ensure no prolongation of the QT interval after starting. Close monitoring of potassium and magnesium levels is important to decrease the risk of cardiac arrhythmias, and concomitant use of drugs considered strong CYP3A4 inhibitors should be avoided.19
If the patient experiences any grade 3 or higher laboratory abnormalities, nilotinib should be held until resolution of the toxicity, and then restarted at a lower dose. Similarly, if patients develop significant neutropenia or thrombocytopenia, nilotinib doses should be interrupted until resolution of the cytopenias. At that point, nilotinib can be reinitiated at either the same or a lower dose. Rash can be managed by the use of topical or systemic steroids as well as potential dose reduction, interruption, or discontinuation.
Given the concerns for potential cardiovascular events with long-term use of nilotinib, caution is advised when prescribing it to any patient with a history of cardiovascular disease or peripheral arterial occlusive disease. At the first sign of new occlusive disease, nilotinib should be discontinued.19
Bosutinib
Bosutinib is a second-generation BCR-ABL TKI with activity against the Src family of kinases; it was initially approved to treat patients with CP-, AP-, or BP-CML after resistance or intolerance to imatinib. Long-term data has been reported from the phase 1/2 trial of bosutinib therapy in patients with CP-CML who developed resistance or intolerance to imatinib plus dasatinib and/or nilotinib. A total of 119 patients were included in the 4-year follow-up; 38 were resistant/intolerant to imatinib and resistant to dasatinib, 50 were resistant/intolerant to imatinib and intolerant to dasatinib, 26 were resistant/intolerant to imatinib and resistant to nilotinib, and 5 were resistant/intolerant to imatinib and intolerant to nilotinib or resistant/intolerant to dasatinib and nilotinib. Bosutinib 400 mg daily was studied in this setting. Of the 38 patients with imatinib resistance/intolerance and dasatinib resistance, 39% achieved MCyR, 22% achieved CCyR, and the OS was 67%. Of the 50 patients with imatinib resistance/intolerance and dasatinib intolerance, 42% achieved MCyR, 40% achieved CCyR, and the OS was 80%. Finally, in the 26 patients with imatinib resistance/intolerance and nilotinib resistance, 38% achieved MCyR, 31% achieved CCyR, and the OS was 87%.34
Five-year follow-up from the phase 1/2 clinical trial that studied bosutinib 500 mg daily in CP-CML patients after imatinib failure reported data on 284 patients. By 5 years on study, 60% of patients had achieved MCyR and 50% achieved CCyR with a 71% and 69% probability, respectively, of maintaining these responses at 5 years. The 5-year OS was 84%.35 These data led to the regulatory approval of bosutinib 500 mg daily as second-line or later therapy.
Bosutinib was initially studied in the first-line setting in the randomized phase 3 BELA (Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia) trial. This trial compared bosutinib 500 mg daily to imatinib 400 mg daily in newly diagnosed, previously untreated CP-CML patients. This trial failed to meet its primary endpoint of increased rate of CCyR at 12 months, with 70% of bosutinib patients achieving this response, compared to 68% of imatinib-treated patients (P = 0.601). In spite of this, the rate of MMR at 12 months was significantly higher in the bosutinib arm (41%) compared to the imatinib arm (27%; P = 0.001).36
A second phase 3 trial (BFORE) was designed to study bosutinib 400 mg daily versus imatinib in newly diagnosed, previously untreated CP-CML patients. This study enrolled 536 patients who were randomly assigned 1:1 to bosutinib versus imatinib. The primary endpoint of this trial was rate of MMR at 12 months. A significantly higher number of bosutinib-treated patients achieved this response (47.2%) compared with imatinib-treated patients (36.9%, P = 0.02). Furthermore, by 12 months 77.2% of patients on the bosutinib arm had achieved CCyR compared with 66.4% on the imatinib arm, and this difference did meet statistical significance (P = 0.0075). A lower rate of progression to AP- or BP-CML was noted in bosutinib-treated patients as well (1.6% versus 2.5%). Based on this data, bosutinib gained regulatory approval for first-line therapy in CP-CML at a dose of 400 mg daily.18
Toxicity. On the BFORE trial, the most common treatment-emergent adverse events of any grade reported in the bosutinib-treated patients were diarrhea (70.1%), nausea (35.1%), increased ALT (30.6%), and increased AST (22.8%). Musculoskeletal pain or spasms occurred in 29.5% of patients, rash in 19.8%, fatigue in 19.4%, and headache in 18.7%. Hematologic toxicity was also reported, but most was grade 1/2. Thrombocytopenia was reported in 35.1%, anemia in 18.7%, and neutropenia in 11.2%.18
Cardiovascular events occurred in 5.2% of patients on the bosutinib arm of the BFORE trial, which was similar to the rate observed in imatinib patients. The most common cardiovascular event was QT interval prolongation, which occurred in 1.5% of patients. Pleural effusions were reported in 1.9% of patients treated with bosutinib, and none were grade 3 or higher.18
If liver enzyme elevation occurs at a value greater than 5 times the institutional upper limit of normal, bosutinib should be held until the level recovers to ≤ 2.5 times the upper limit of normal, at which point bosutinib can be restarted at a lower dose. If recovery takes longer than 4 weeks, bosutinib should be permanently discontinued. Liver enzymes elevated greater than 3 times the institutional upper limit of normal and a concurrent elevation in total bilirubin to 2 times the upper limit of normal are consistent with Hy’s law, and bosutinib should be discontinued. Although diarrhea is the most common toxicity associated with bosutinib, it is commonly low grade and transient. Diarrhea occurs most frequently in the first few days after initiating bosutinib. It can often be managed with over-the-counter antidiarrheal medications, but if the diarrhea is grade 3 or higher, bosutinib should be held until recovery to grade 1 or lower. Gastrointestinal side effects may be improved by taking bosutinib with a meal and a large glass of water. Fluid retention can be managed with diuretics and supportive care. Finally, if rash occurs, this can be addressed with topical or systemic steroids as well as bosutinib dose reduction, interruption, or discontinuation.19
Similar to other TKIs, if bosutinib-induced cytopenias occur, treatment should be held and restarted at the same or a lower dose upon blood count recovery.19
Ponatinib
The most common cause of TKI resistance in CP-CML is the development of ABL kinase domain mutations. The majority of imatinib-resistant mutations can be overcome by the use of second-generation TKIs, including dasatinib, nilotinib, or bosutinib. However, ponatinib is the only BCR-ABL TKI able to overcome a T315I mutation. The phase 2 PACE (Ponatinib Ph-positive ALL and CML Evaluation) trial enrolled patients with CP-, AP-, or BP-CML as well as patients with Ph-positive acute lymphoblastic leukemia who were resistant or intolerant to nilotinib or dasatinib, or who had evidence of a T315I mutation. The starting dose of ponatinib on this trial was 45 mg daily.37 The PACE trial enrolled 267 patients with CP-CML: 203 with resistance or intolerance to nilotinib or dasatinib, and 64 with a T315I mutation. The primary endpoint in the CP cohort was rate of MCyR at any time within 12 months of starting ponatinib. The overall rate of MCyR by 12 months in the CP-CML patients was 56%. In those with a T315I mutation, 70% achieved MCyR, which compared favorably with those with resistance or intolerance to nilotinib or dasatinib, 51% of whom achieved MCyR. CCyR was achieved in 46% of CP-CML patients (40% in the resistant/intolerant cohort and 66% in the T315I cohort). In general, patients with T315I mutations received fewer prior therapies than those in the resistant/intolerant cohort, which likely contributed to the higher response rates in the T315I patients. MR4.5 was achieved in 15% of CP-CML patients by 12 months on the PACE trial.37 The 5-year update to this study reported that 60%, 40%, and 24% of CP-CML patients achieved MCyR, MMR, and MR4.5, respectively. In the patients who achieved MCyR, the probability of maintaining this response for 5 years was 82% and the estimated 5-year OS was 73%.19
Toxicity. In 2013, after the regulatory approval of ponatinib, reports became available that the drug can cause an increase in arterial occlusive events, including fatal myocardial infarctions and cerebrovascular accidents. For this reason, dose reductions were implemented in patients who were deriving clinical benefit from ponatinib. In spite of these dose reductions, ≥ 90% of responders maintained their response for up to 40 months.38 Although the likelihood of developing an arterial occlusive event appears higher in the first year after starting ponatinib than in later years, the cumulative incidence of events continues to increase. The 5-year follow-up to the PACE trial reports 31% of patients experiencing any grade of arterial occlusive event while on ponatinib. Aside from these events, the most common treatment-emergent adverse events in ponatinib-treated patients on the PACE trial included rash (47%), abdominal pain (46%), headache (43%), dry skin (42%), constipation (41%), and hypertension (37%). Hematologic toxicity was also common, with 46% of patients experiencing any grade of thrombocytopenia, 20% experiencing neutropenia, and 20% anemia.38
Patients receiving ponatinib therapy should be monitored closely for any evidence of arterial or venous thrombosis. If an occlusive event occurs, ponatinib should be discontinued. Similarly, in the setting of any new or worsening heart failure symptoms, ponatinib should be promptly discontinued. Management of any underlying cardiovascular risk factors, including hypertension, hyperlipidemia, diabetes, or smoking history, is recommended, and these patients should be referred to a cardiologist for a full evaluation. In the absence of any contraindications to aspirin, low-dose aspirin should be considered as a means of decreasing cardiovascular risks associated with ponatinib. In patients with known risk factors, a ponatinib starting dose of 30 mg daily rather than the standard 45 mg daily may be a safer option, resulting in fewer arterial occlusive events, although the efficacy of this dose is still being studied in comparison to 45 mg daily.19
If ponatinib-induced transaminitis greater than 3 times the upper limit of normal occurs, ponatinib should be held until resolution to less than 3 times the upper limit of normal, at which point it should be resumed at a lower dose. Similarly, in the setting of elevated serum lipase or symptomatic pancreatitis, ponatinib should be held and restarted at a lower dose after resolution of symptoms.19
In the event of neutropenia or thrombocytopenia, ponatinib should be held until blood count recovery and then restarted at the same dose. If cytopenias occur for a second time, the dose of ponatinib should be lowered at the time of treatment reinitiation. If rash occurs, it can be addressed with topical or systemic steroids as well as dose reduction, interruption, or discontinuation.19
Conclusion
With the development of imatinib and the subsequent TKIs, dasatinib, nilotinib, bosutinib, and ponatinib, CP-CML has become a chronic disease with a life expectancy that is similar to that of the general population. Given the successful treatments available for these patients, it is crucial to identify patients with this diagnosis, ensure they receive a complete, appropriate diagnostic workup including a bone marrow biopsy and aspiration with cytogenetic testing, and select the best therapy for each individual patient. Once on treatment, the importance of frequent monitoring cannot be overstated. This is the only way to be certain patients are achieving the desired treatment milestones that correlate with the favorable long-term outcomes that have been observed with TKI-based treatment of CP-CML.
Corresponding author: Kendra Sweet, MD, MS, Department of Malignant Hematology, Moffitt Cancer Center, Tampa, FL.
Financial disclosures: Dr. Sweet has served on the Advisory Board and Speakers Bureau of Novartis, Bristol-Meyers Squibb, Ariad Pharmaceuticals, and Pfizer, and has served as a consultant to Pfizer.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-2340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
21. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
22. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
23. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR]. Medicina (B Aires). 2017;77:61-72.
24. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
25. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
26. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
27. Baccarani M, Druker BJ, Branford S, et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99:616-624.
28. Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125:915-923.
29. Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-2417.
30. Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869-874.
31. Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261-263.
32. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107-112.
33. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-2259.
34. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206-1214.
35. Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298-1307.
36. Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486-3492.
37. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783-1796.
38. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393-404.
1. Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl J Med. 1999;341:164-172.
2. Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Leukemia - Chronic Myeloid Leukemia (CML). 2018.
3. Huang X, Cortes J, Kantarjian H. Estimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapy. Cancer. 2012;118:3123-3127.
4. Savage DG, Szydlo RM, Chase A, et al. Bone marrow transplantation for chronic myeloid leukaemia: the effects of differing criteria for defining chronic phase on probabilities of survival and relapse. Br J Haematol. 1997;99:30-35.
5. Knox WF, Bhavnani M, Davson J, Geary CG. Histological classification of chronic granulocytic leukaemia. Clin Lab Haematol. 1984;6:171-175.
6. Kvasnicka HM, Thiele J, Schmitt-Graeff A, et al. Impact of bone marrow morphology on multivariate risk classification in chronic myelogenous leukemia. Acta Haematol. 2003;109:53-56.
7. Cortes JE, Talpaz M, O’Brien S, et al. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006;106:1306-1315.
8. Druker BJ. Chronic myeloid leukemia. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. DeVita, Hellman, and Rosenberg’s Cancer Principles & Practice of Oncology. 8th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2007:2267-2304.
9. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391-2405.
10. Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760-6768.
11. Alhuraiji A, Kantarjian H, Boddu P, et al. Prognostic significance of additional chromosomal abnormalities at the time of diagnosis in patients with chronic myeloid leukemia treated with frontline tyrosine kinase inhibitors. Am J Hematol. 2018;93:84-90.
12. Melo JV. BCR-ABL gene variants. Baillieres Clin Haematol. 1997;10:203-222.
13. Kantarjian HM, Talpaz M, Cortes J, et al. Quantitative polymerase chain reaction monitoring of BCR-ABL during therapy with imatinib mesylate (STI571; gleevec) in chronic-phase chronic myelogenous leukemia. Clin Cancer Res. 2003;9:160-166.
14. Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28-37.
15. Hochhaus A, Larson RA, Guilhot F, et al. Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917-927.
16. Cortes JE, Saglio G, Kantarjian HM, et al. Final 5-year study results of DASISION: the Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial. J Clin Oncol. 2016;34:2333-2340.
17. Hochhaus A, Saglio G, Hughes TP, et al. Long-term benefits and risks of frontline nilotinib vs imatinib for chronic myeloid leukemia in chronic phase: 5-year update of the randomized ENESTnd trial. Leukemia. 2016;30:1044-1054.
18. Cortes JE, Gambacorti-Passerini C, Deininger MW, et al. Bosutinib versus imatinib for newly diagnosed chronic myeloid leukemia: results from the randomized BFORE trial. J Clin Oncol. 2018;36:231-237.
19. Radich JP, Deininger M, Abboud CN, et al. Chronic Myeloid Leukemia, Version 1.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:1108-1135.
20. Faderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Ann Intern Med. 1999;131:207-219.
21. O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994-1004.
22. Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122:872-884.
23. Larripa I, Ruiz MS, Gutierrez M, Bianchini M. [Guidelines for molecular monitoring of BCR-ABL1 in chronic myeloid leukemia patients by RT-qPCR]. Medicina (B Aires). 2017;77:61-72.
24. Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232-238.
25. Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23.
26. Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037.
27. Baccarani M, Druker BJ, Branford S, et al. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. Int J Hematol. 2014;99:616-624.
28. Yeung DT, Osborn MP, White DL, et al. TIDEL-II: first-line use of imatinib in CML with early switch to nilotinib for failure to achieve time-dependent molecular targets. Blood. 2015;125:915-923.
29. Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408-2417.
30. Shah NP, Rousselot P, Schiffer C, et al. Dasatinib in imatinib-resistant or -intolerant chronic-phase, chronic myeloid leukemia patients: 7-year follow-up of study CA180-034. Am J Hematol. 2016;91:869-874.
31. Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Tyrosine kinase inhibitor-induced platelet dysfunction in patients with chronic myeloid leukemia. Blood. 2009;114:261-263.
32. Giles FJ, le Coutre PD, Pinilla-Ibarz J, et al. Nilotinib in imatinib-resistant or imatinib-intolerant patients with chronic myeloid leukemia in chronic phase: 48-month follow-up results of a phase II study. Leukemia. 2013;27:107-112.
33. Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-2259.
34. Cortes JE, Khoury HJ, Kantarjian HM, et al. Long-term bosutinib for chronic phase chronic myeloid leukemia after failure of imatinib plus dasatinib and/or nilotinib. Am J Hematol. 2016;91:1206-1214.
35. Gambacorti-Passerini C, Cortes JE, Lipton JH, et al. Safety and efficacy of second-line bosutinib for chronic phase chronic myeloid leukemia over a five-year period: final results of a phase I/II study. Haematologica. 2018;103:1298-1307.
36. Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486-3492.
37. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783-1796.
38. Cortes JE, Kim DW, Pinilla-Ibarz J, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018;132:393-404.
Calculating Risk for Poor Outcomes After Transcatheter Aortic Valve Replacement
From Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City, Kansas City, MO.
Abstract
- Objective: To outline the tools available to help understand the risk of transcatheter aortic valve replacement (TAVR) and the gaps in knowledge regarding TAVR risk estimation.
- Methods: Review of the literature.
- Results: Two models developed and validated by the American College of Cardiology can be used to estimate the risk of short-term mortality, a 6-variable in-hospital model designed for clinical use and a 41-variable 30-day model designed primarily for site comparisons and quality improvement. Importantly, neither model should be used to inform the choice of TAVR versus surgical aortic valve replacement. Regarding long-term outcomes, a risk model to estimate risk of dying or having a persistently poor quality of life at 1 year after TAVR has been developed and validated. Factors that most significantly increase a patient’s risk for poor outcomes are very poor functional status prior to TAVR, requiring home oxygen, chronic renal insufficiency, atrial fibrillation, dependencies in activities of daily living, and dementia. If a patient has ≥ 2 or 3 major risk factors for a poor outcome, this risk and the uncertainty about the degree of recovery expected after TAVR should be discussed with the patient (and family).
- Conclusion: It is important to understand the patient factors that most strongly drive risk of poor outcomes after TAVR and use this information to set appropriate expectations for recovery.
Keywords: aortic valve stenosis; risk factors; postoperative complications; TAVR.
Among patients with severe aortic stenosis,
As with any emerging technology, selecting the appropriate patients for TAVR—a procedure with high initial costs10—has been an area of active investigation. As TAVR was first introduced in patients who were considered inoperable, initial efforts focused on trying to identify the patients who did not improve functionally or live longer following TAVR. Termed Cohort C patients, these patients were thought to have too many comorbidities, be too sick, and have too little reserve to recover from TAVR, and in the early trials, represented a substantial minority of the patients. For example, in pivotal clinical trials of patients at high or extreme surgical risk, approximately 1 in 4 patients who were treated with TAVR were dead at 1 year.1,3,11 Furthermore, a number of patients who received TAVR were alive at 1 year but continued to have significant heart failure symptoms and functional limitations.2,4 Practitioners,12,13 regulators,14 and third-party payers15 have recommended that TAVR should not be offered to patients in whom valve replacement would not be expected to positively impact either their survival or quality of life, but how best to identify these patients has been less clear.
More recently, as the use of TAVR has moved down the risk spectrum, patient selection for TAVR has shifted to understanding which patients should be preferentially treated with TAVR versus SAVR. While patients often prefer a less invasive treatment option with faster recovery—which is what TAVR offers—there are lingering questions about valve longevity, need for a pacemaker (and the associated long-term implications), and the ability to treat other cardiovascular conditions (eg, Maze, mitral valve repair) that potentially make a patient a more appropriate candidate for valve surgery. This review
Short-Term Outcomes
When TAVR was initially introduced, the 30-day mortality rate was 5% to 8%.1,11,16 This high mortality rate was a function of treating very ill patients and more invasive procedures with larger sheath sizes and routine use of general anesthesia, transesophageal echocardiography, pulmonary artery catheterization, and so on. Over time, however, this rate has gone down substantially, with the 30-day mortality rate in intermediate- and low-risk patients now ranging from 0.5% to 1%.8,17-19 Although this low mortality rate indicates that the vast majority of patients will survive to discharge from the hospital,
While 30 days is a better time frame for assessment because outcome is less impacted by differences in local post-acute care facilities, we explicitly did not create a parsimonious 30-day mortality model for clinical use due to concern that having such a model would allow for indirect comparisons with estimated risk of SAVR using the Society of Thoracic Surgeons risk model (http://riskcalc.sts.org/stswebriskcalc). It would be tempting to estimate a patient’s risk of mortality with the TAVR calculator and the SAVR calculator and use those risk estimates to
The decision of selecting SAVR over TAVR is typically driven by factors other than short- or long-term mortality (eg, whether TAVR will be covered by insurance, very young age and concern about durability, need to treat concomitant mitral regurgitation or aortopathy), as clinical trials have shown that survival and quality of life outcomes are at least as good with TAVR compared with SAVR.6,7,9,23 In fact, in an analysis that compared similar patients treated with TAVR versus SAVR and specifically looked for patient factors that might make one treatment preferable to the other, patients who had a prior cardiac operation and those on home oxygen were more likely to do better with TAVR, whereas no patient factors that favored SAVR were found.24 The majority of patients, however, were expected to have similar long-term outcomes regardless of treatment choice, and as such, the benefit of TAVR appears mostly to be an earlier and easier recovery.
Long-Term Outcomes: Estimating the Risk for Failure to Recover
While many patients who undergo TAVR are quite ill prior to the procedure, with substantial limitations due to the fatigue and shortness of breath associated with severe aortic stenosis, most patients recover well after the procedure, with marked improvement in symptoms and functional capacity. Approximately 25% to 35% of patients currently treated with TAVR commercially (ie, intermediate- and high-surgical-risk patients) either die or do not recover a reasonable quality of life after the procedure. Identifying those patients prior to the procedure can be challenging. We have previously developed and externally validated
Beyond clinical factors, frailty negatively impacts both survival and quality of life after TAVR. Frailty is a geriatric syndrome of impaired physiologic reserve and decreased resistance to stressors27 that is characterized by weakness, slowness, exhaustion, wasting, and low activity level. Across a wide variety of clinical situations (eg, pneumonia,28 myocardial infarction,29 general30,31 and cardiac surgery32,33), frailty increases the risk of morbidity and mortality after nearly any intervention34 or clinical insult, independent of traditional demographic and clinical risk factors. Frail patients often do better with less invasive interventions such as TAVR compared with traditional surgery, but nonetheless remain at increased risk for death35-37 or failure to recover quality of life and functional status25,37 after TAVR. However, there are unique challenges in both assessing and managing frailty in patients who are considered potential candidates for TAVR. One challenge is the lack of a laboratory or radiologic test for frailty; instead, the lack of physiologic reserve of frailty is identified through a combination of factors, such as slow gait speed, weak grip strength, and unintentional weight loss. While these factors readily identify frail patients in general elderly populations, in patients with severe symptomatic aortic stenosis, these metrics can be impacted by the disease process itself. This distinction is important as slow gait speed that is due to aortic stenosis will be “fixed” by TAVR, but slow gait speed from frailty would identify a patient who will have a difficult time recovering from the procedure. For example, in the CoreValve High Risk Pivotal Trial, 80% of patients had a slow gait speed and 67% had a weak grip strength,5 and yet 58% of patients in this trial were alive and with a reasonable quality of life 1 year after TAVR.6 A number of studies have attempted to define true frailty within the pre-TAVR population, that which represents decreased physiologic reserve and an impaired ability to recover from an insult, and the factors that appear to be most prognostically important are malnutrition38 or unintentional weight loss25 and the inability to be independent in activities of daily living (eg, dressing, feeding, transferring).25,37
Even with frailty assessments, the ability to predict who is or is not going to have a poor outcome after TAVR (ie, to use pre-procedural factors to identify patients who perhaps should not be offered TAVR because he or she will not recover from the procedure) is exceedingly difficult. The Table shows how to grossly estimate risk using the major factors that impact risk based on the more precise estimates from our models.25,26
The model shown in the Table can be used to estimate a patient’s risk for a poor outcome, but it should be noted that even at the extreme high end of risk, there will be some patients who still do well after TAVR. Furthermore, being high risk for a poor outcome after TAVR does not imply anything about how the patient would do without TAVR, as many of these patients would likely die even sooner or have worse quality of life with medical therapy only. However,
Conclusion
Calculating the risk of TAVR can be complicated. In patients who are electively treated using transfemoral access and a less invasive approach, the short-term risk of mortality is very low. Risk calculators can be used to estimate short-term risk, but the patients who are high risk for in-hospital mortality are often fairly easy to recognize, as the factors that drive that risk are not subtle (eg, the patient is in shock at the time of the procedure). The true risk of TAVR lies in the inability to recover from the procedure—being chronically ill, frail, or debilitated to a degree that the patient either dies or fails to recover a reasonable quality of life. Given
Corresponding author: Suzanne V. Arnold, MD, MHA, 4401 Wornall Rd., Kansas City, MO 64111.
Financial disclosures: This work was funded in part by grant K23HL116799 from the National Institutes of Health.
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(:1964-1972.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol. 2012;60:548-558.
5. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
6. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US Pivotal trial. JACC Cardiovasc Interv. 2015;8:1207-1217.
7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
8. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
9. Baron SJ, Arnold SV, Wang K, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837-845.
10. Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation. 2012;125:1102-1109.
11. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
12. Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2008;29:1463-1470.
13. Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254.
14. US Food and Drug Administration. FDA Executive Summary: Edwards SAPIEN™ Transcatheter Heart Valve. Presented July 20, 2011, Gaithersburg, MD.
15. Centers for Medicare & Medicaid Services. Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N). May 5, 2012.
16. Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069-2077.
17. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218-2225.
18. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
19. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
20. Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885-892.
21. Arnold SV, O’Brien SM, Vemulapalli S, et al. Inclusion of functional status measures in the risk adjustment of 30-day mortality after transcatheter aortic valve replacement: a report from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2018;11:581-589.
22. Brennan JM, Thomas L, Cohen DJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70:439-450.
23. Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113-121.
24. Baron SJ, Cohen DJ, Suchindran S, et al. Development of a risk prediction model for 1-year mortality after surgical vs. transcatheter aortic valve replacement in patients with severe aortic stenosis. Circulation. 2016;134(A20166).
25. Arnold SV, Afilalo J, Spertus JA, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868-1877.
26. Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682-2690.
27. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24.
28. Torres OH, Munoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603-1609.
29. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397-2404.
30. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-908.
31. Hewitt J, Moug SJ, Middleton M, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254-259.
32. Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33-37.
33. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222-228.
34. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157.
35. Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489-496.
36. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684-689.
37. Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269.
38. Goldfarb M, Lauck S, Webb J, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202-2211.
From Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City, Kansas City, MO.
Abstract
- Objective: To outline the tools available to help understand the risk of transcatheter aortic valve replacement (TAVR) and the gaps in knowledge regarding TAVR risk estimation.
- Methods: Review of the literature.
- Results: Two models developed and validated by the American College of Cardiology can be used to estimate the risk of short-term mortality, a 6-variable in-hospital model designed for clinical use and a 41-variable 30-day model designed primarily for site comparisons and quality improvement. Importantly, neither model should be used to inform the choice of TAVR versus surgical aortic valve replacement. Regarding long-term outcomes, a risk model to estimate risk of dying or having a persistently poor quality of life at 1 year after TAVR has been developed and validated. Factors that most significantly increase a patient’s risk for poor outcomes are very poor functional status prior to TAVR, requiring home oxygen, chronic renal insufficiency, atrial fibrillation, dependencies in activities of daily living, and dementia. If a patient has ≥ 2 or 3 major risk factors for a poor outcome, this risk and the uncertainty about the degree of recovery expected after TAVR should be discussed with the patient (and family).
- Conclusion: It is important to understand the patient factors that most strongly drive risk of poor outcomes after TAVR and use this information to set appropriate expectations for recovery.
Keywords: aortic valve stenosis; risk factors; postoperative complications; TAVR.
Among patients with severe aortic stenosis,
As with any emerging technology, selecting the appropriate patients for TAVR—a procedure with high initial costs10—has been an area of active investigation. As TAVR was first introduced in patients who were considered inoperable, initial efforts focused on trying to identify the patients who did not improve functionally or live longer following TAVR. Termed Cohort C patients, these patients were thought to have too many comorbidities, be too sick, and have too little reserve to recover from TAVR, and in the early trials, represented a substantial minority of the patients. For example, in pivotal clinical trials of patients at high or extreme surgical risk, approximately 1 in 4 patients who were treated with TAVR were dead at 1 year.1,3,11 Furthermore, a number of patients who received TAVR were alive at 1 year but continued to have significant heart failure symptoms and functional limitations.2,4 Practitioners,12,13 regulators,14 and third-party payers15 have recommended that TAVR should not be offered to patients in whom valve replacement would not be expected to positively impact either their survival or quality of life, but how best to identify these patients has been less clear.
More recently, as the use of TAVR has moved down the risk spectrum, patient selection for TAVR has shifted to understanding which patients should be preferentially treated with TAVR versus SAVR. While patients often prefer a less invasive treatment option with faster recovery—which is what TAVR offers—there are lingering questions about valve longevity, need for a pacemaker (and the associated long-term implications), and the ability to treat other cardiovascular conditions (eg, Maze, mitral valve repair) that potentially make a patient a more appropriate candidate for valve surgery. This review
Short-Term Outcomes
When TAVR was initially introduced, the 30-day mortality rate was 5% to 8%.1,11,16 This high mortality rate was a function of treating very ill patients and more invasive procedures with larger sheath sizes and routine use of general anesthesia, transesophageal echocardiography, pulmonary artery catheterization, and so on. Over time, however, this rate has gone down substantially, with the 30-day mortality rate in intermediate- and low-risk patients now ranging from 0.5% to 1%.8,17-19 Although this low mortality rate indicates that the vast majority of patients will survive to discharge from the hospital,
While 30 days is a better time frame for assessment because outcome is less impacted by differences in local post-acute care facilities, we explicitly did not create a parsimonious 30-day mortality model for clinical use due to concern that having such a model would allow for indirect comparisons with estimated risk of SAVR using the Society of Thoracic Surgeons risk model (http://riskcalc.sts.org/stswebriskcalc). It would be tempting to estimate a patient’s risk of mortality with the TAVR calculator and the SAVR calculator and use those risk estimates to
The decision of selecting SAVR over TAVR is typically driven by factors other than short- or long-term mortality (eg, whether TAVR will be covered by insurance, very young age and concern about durability, need to treat concomitant mitral regurgitation or aortopathy), as clinical trials have shown that survival and quality of life outcomes are at least as good with TAVR compared with SAVR.6,7,9,23 In fact, in an analysis that compared similar patients treated with TAVR versus SAVR and specifically looked for patient factors that might make one treatment preferable to the other, patients who had a prior cardiac operation and those on home oxygen were more likely to do better with TAVR, whereas no patient factors that favored SAVR were found.24 The majority of patients, however, were expected to have similar long-term outcomes regardless of treatment choice, and as such, the benefit of TAVR appears mostly to be an earlier and easier recovery.
Long-Term Outcomes: Estimating the Risk for Failure to Recover
While many patients who undergo TAVR are quite ill prior to the procedure, with substantial limitations due to the fatigue and shortness of breath associated with severe aortic stenosis, most patients recover well after the procedure, with marked improvement in symptoms and functional capacity. Approximately 25% to 35% of patients currently treated with TAVR commercially (ie, intermediate- and high-surgical-risk patients) either die or do not recover a reasonable quality of life after the procedure. Identifying those patients prior to the procedure can be challenging. We have previously developed and externally validated
Beyond clinical factors, frailty negatively impacts both survival and quality of life after TAVR. Frailty is a geriatric syndrome of impaired physiologic reserve and decreased resistance to stressors27 that is characterized by weakness, slowness, exhaustion, wasting, and low activity level. Across a wide variety of clinical situations (eg, pneumonia,28 myocardial infarction,29 general30,31 and cardiac surgery32,33), frailty increases the risk of morbidity and mortality after nearly any intervention34 or clinical insult, independent of traditional demographic and clinical risk factors. Frail patients often do better with less invasive interventions such as TAVR compared with traditional surgery, but nonetheless remain at increased risk for death35-37 or failure to recover quality of life and functional status25,37 after TAVR. However, there are unique challenges in both assessing and managing frailty in patients who are considered potential candidates for TAVR. One challenge is the lack of a laboratory or radiologic test for frailty; instead, the lack of physiologic reserve of frailty is identified through a combination of factors, such as slow gait speed, weak grip strength, and unintentional weight loss. While these factors readily identify frail patients in general elderly populations, in patients with severe symptomatic aortic stenosis, these metrics can be impacted by the disease process itself. This distinction is important as slow gait speed that is due to aortic stenosis will be “fixed” by TAVR, but slow gait speed from frailty would identify a patient who will have a difficult time recovering from the procedure. For example, in the CoreValve High Risk Pivotal Trial, 80% of patients had a slow gait speed and 67% had a weak grip strength,5 and yet 58% of patients in this trial were alive and with a reasonable quality of life 1 year after TAVR.6 A number of studies have attempted to define true frailty within the pre-TAVR population, that which represents decreased physiologic reserve and an impaired ability to recover from an insult, and the factors that appear to be most prognostically important are malnutrition38 or unintentional weight loss25 and the inability to be independent in activities of daily living (eg, dressing, feeding, transferring).25,37
Even with frailty assessments, the ability to predict who is or is not going to have a poor outcome after TAVR (ie, to use pre-procedural factors to identify patients who perhaps should not be offered TAVR because he or she will not recover from the procedure) is exceedingly difficult. The Table shows how to grossly estimate risk using the major factors that impact risk based on the more precise estimates from our models.25,26
The model shown in the Table can be used to estimate a patient’s risk for a poor outcome, but it should be noted that even at the extreme high end of risk, there will be some patients who still do well after TAVR. Furthermore, being high risk for a poor outcome after TAVR does not imply anything about how the patient would do without TAVR, as many of these patients would likely die even sooner or have worse quality of life with medical therapy only. However,
Conclusion
Calculating the risk of TAVR can be complicated. In patients who are electively treated using transfemoral access and a less invasive approach, the short-term risk of mortality is very low. Risk calculators can be used to estimate short-term risk, but the patients who are high risk for in-hospital mortality are often fairly easy to recognize, as the factors that drive that risk are not subtle (eg, the patient is in shock at the time of the procedure). The true risk of TAVR lies in the inability to recover from the procedure—being chronically ill, frail, or debilitated to a degree that the patient either dies or fails to recover a reasonable quality of life. Given
Corresponding author: Suzanne V. Arnold, MD, MHA, 4401 Wornall Rd., Kansas City, MO 64111.
Financial disclosures: This work was funded in part by grant K23HL116799 from the National Institutes of Health.
From Saint Luke’s Mid America Heart Institute/University of Missouri–Kansas City, Kansas City, MO.
Abstract
- Objective: To outline the tools available to help understand the risk of transcatheter aortic valve replacement (TAVR) and the gaps in knowledge regarding TAVR risk estimation.
- Methods: Review of the literature.
- Results: Two models developed and validated by the American College of Cardiology can be used to estimate the risk of short-term mortality, a 6-variable in-hospital model designed for clinical use and a 41-variable 30-day model designed primarily for site comparisons and quality improvement. Importantly, neither model should be used to inform the choice of TAVR versus surgical aortic valve replacement. Regarding long-term outcomes, a risk model to estimate risk of dying or having a persistently poor quality of life at 1 year after TAVR has been developed and validated. Factors that most significantly increase a patient’s risk for poor outcomes are very poor functional status prior to TAVR, requiring home oxygen, chronic renal insufficiency, atrial fibrillation, dependencies in activities of daily living, and dementia. If a patient has ≥ 2 or 3 major risk factors for a poor outcome, this risk and the uncertainty about the degree of recovery expected after TAVR should be discussed with the patient (and family).
- Conclusion: It is important to understand the patient factors that most strongly drive risk of poor outcomes after TAVR and use this information to set appropriate expectations for recovery.
Keywords: aortic valve stenosis; risk factors; postoperative complications; TAVR.
Among patients with severe aortic stenosis,
As with any emerging technology, selecting the appropriate patients for TAVR—a procedure with high initial costs10—has been an area of active investigation. As TAVR was first introduced in patients who were considered inoperable, initial efforts focused on trying to identify the patients who did not improve functionally or live longer following TAVR. Termed Cohort C patients, these patients were thought to have too many comorbidities, be too sick, and have too little reserve to recover from TAVR, and in the early trials, represented a substantial minority of the patients. For example, in pivotal clinical trials of patients at high or extreme surgical risk, approximately 1 in 4 patients who were treated with TAVR were dead at 1 year.1,3,11 Furthermore, a number of patients who received TAVR were alive at 1 year but continued to have significant heart failure symptoms and functional limitations.2,4 Practitioners,12,13 regulators,14 and third-party payers15 have recommended that TAVR should not be offered to patients in whom valve replacement would not be expected to positively impact either their survival or quality of life, but how best to identify these patients has been less clear.
More recently, as the use of TAVR has moved down the risk spectrum, patient selection for TAVR has shifted to understanding which patients should be preferentially treated with TAVR versus SAVR. While patients often prefer a less invasive treatment option with faster recovery—which is what TAVR offers—there are lingering questions about valve longevity, need for a pacemaker (and the associated long-term implications), and the ability to treat other cardiovascular conditions (eg, Maze, mitral valve repair) that potentially make a patient a more appropriate candidate for valve surgery. This review
Short-Term Outcomes
When TAVR was initially introduced, the 30-day mortality rate was 5% to 8%.1,11,16 This high mortality rate was a function of treating very ill patients and more invasive procedures with larger sheath sizes and routine use of general anesthesia, transesophageal echocardiography, pulmonary artery catheterization, and so on. Over time, however, this rate has gone down substantially, with the 30-day mortality rate in intermediate- and low-risk patients now ranging from 0.5% to 1%.8,17-19 Although this low mortality rate indicates that the vast majority of patients will survive to discharge from the hospital,
While 30 days is a better time frame for assessment because outcome is less impacted by differences in local post-acute care facilities, we explicitly did not create a parsimonious 30-day mortality model for clinical use due to concern that having such a model would allow for indirect comparisons with estimated risk of SAVR using the Society of Thoracic Surgeons risk model (http://riskcalc.sts.org/stswebriskcalc). It would be tempting to estimate a patient’s risk of mortality with the TAVR calculator and the SAVR calculator and use those risk estimates to
The decision of selecting SAVR over TAVR is typically driven by factors other than short- or long-term mortality (eg, whether TAVR will be covered by insurance, very young age and concern about durability, need to treat concomitant mitral regurgitation or aortopathy), as clinical trials have shown that survival and quality of life outcomes are at least as good with TAVR compared with SAVR.6,7,9,23 In fact, in an analysis that compared similar patients treated with TAVR versus SAVR and specifically looked for patient factors that might make one treatment preferable to the other, patients who had a prior cardiac operation and those on home oxygen were more likely to do better with TAVR, whereas no patient factors that favored SAVR were found.24 The majority of patients, however, were expected to have similar long-term outcomes regardless of treatment choice, and as such, the benefit of TAVR appears mostly to be an earlier and easier recovery.
Long-Term Outcomes: Estimating the Risk for Failure to Recover
While many patients who undergo TAVR are quite ill prior to the procedure, with substantial limitations due to the fatigue and shortness of breath associated with severe aortic stenosis, most patients recover well after the procedure, with marked improvement in symptoms and functional capacity. Approximately 25% to 35% of patients currently treated with TAVR commercially (ie, intermediate- and high-surgical-risk patients) either die or do not recover a reasonable quality of life after the procedure. Identifying those patients prior to the procedure can be challenging. We have previously developed and externally validated
Beyond clinical factors, frailty negatively impacts both survival and quality of life after TAVR. Frailty is a geriatric syndrome of impaired physiologic reserve and decreased resistance to stressors27 that is characterized by weakness, slowness, exhaustion, wasting, and low activity level. Across a wide variety of clinical situations (eg, pneumonia,28 myocardial infarction,29 general30,31 and cardiac surgery32,33), frailty increases the risk of morbidity and mortality after nearly any intervention34 or clinical insult, independent of traditional demographic and clinical risk factors. Frail patients often do better with less invasive interventions such as TAVR compared with traditional surgery, but nonetheless remain at increased risk for death35-37 or failure to recover quality of life and functional status25,37 after TAVR. However, there are unique challenges in both assessing and managing frailty in patients who are considered potential candidates for TAVR. One challenge is the lack of a laboratory or radiologic test for frailty; instead, the lack of physiologic reserve of frailty is identified through a combination of factors, such as slow gait speed, weak grip strength, and unintentional weight loss. While these factors readily identify frail patients in general elderly populations, in patients with severe symptomatic aortic stenosis, these metrics can be impacted by the disease process itself. This distinction is important as slow gait speed that is due to aortic stenosis will be “fixed” by TAVR, but slow gait speed from frailty would identify a patient who will have a difficult time recovering from the procedure. For example, in the CoreValve High Risk Pivotal Trial, 80% of patients had a slow gait speed and 67% had a weak grip strength,5 and yet 58% of patients in this trial were alive and with a reasonable quality of life 1 year after TAVR.6 A number of studies have attempted to define true frailty within the pre-TAVR population, that which represents decreased physiologic reserve and an impaired ability to recover from an insult, and the factors that appear to be most prognostically important are malnutrition38 or unintentional weight loss25 and the inability to be independent in activities of daily living (eg, dressing, feeding, transferring).25,37
Even with frailty assessments, the ability to predict who is or is not going to have a poor outcome after TAVR (ie, to use pre-procedural factors to identify patients who perhaps should not be offered TAVR because he or she will not recover from the procedure) is exceedingly difficult. The Table shows how to grossly estimate risk using the major factors that impact risk based on the more precise estimates from our models.25,26
The model shown in the Table can be used to estimate a patient’s risk for a poor outcome, but it should be noted that even at the extreme high end of risk, there will be some patients who still do well after TAVR. Furthermore, being high risk for a poor outcome after TAVR does not imply anything about how the patient would do without TAVR, as many of these patients would likely die even sooner or have worse quality of life with medical therapy only. However,
Conclusion
Calculating the risk of TAVR can be complicated. In patients who are electively treated using transfemoral access and a less invasive approach, the short-term risk of mortality is very low. Risk calculators can be used to estimate short-term risk, but the patients who are high risk for in-hospital mortality are often fairly easy to recognize, as the factors that drive that risk are not subtle (eg, the patient is in shock at the time of the procedure). The true risk of TAVR lies in the inability to recover from the procedure—being chronically ill, frail, or debilitated to a degree that the patient either dies or fails to recover a reasonable quality of life. Given
Corresponding author: Suzanne V. Arnold, MD, MHA, 4401 Wornall Rd., Kansas City, MO 64111.
Financial disclosures: This work was funded in part by grant K23HL116799 from the National Institutes of Health.
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(:1964-1972.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol. 2012;60:548-558.
5. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
6. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US Pivotal trial. JACC Cardiovasc Interv. 2015;8:1207-1217.
7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
8. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
9. Baron SJ, Arnold SV, Wang K, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837-845.
10. Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation. 2012;125:1102-1109.
11. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
12. Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2008;29:1463-1470.
13. Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254.
14. US Food and Drug Administration. FDA Executive Summary: Edwards SAPIEN™ Transcatheter Heart Valve. Presented July 20, 2011, Gaithersburg, MD.
15. Centers for Medicare & Medicaid Services. Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N). May 5, 2012.
16. Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069-2077.
17. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218-2225.
18. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
19. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
20. Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885-892.
21. Arnold SV, O’Brien SM, Vemulapalli S, et al. Inclusion of functional status measures in the risk adjustment of 30-day mortality after transcatheter aortic valve replacement: a report from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2018;11:581-589.
22. Brennan JM, Thomas L, Cohen DJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70:439-450.
23. Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113-121.
24. Baron SJ, Cohen DJ, Suchindran S, et al. Development of a risk prediction model for 1-year mortality after surgical vs. transcatheter aortic valve replacement in patients with severe aortic stenosis. Circulation. 2016;134(A20166).
25. Arnold SV, Afilalo J, Spertus JA, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868-1877.
26. Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682-2690.
27. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24.
28. Torres OH, Munoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603-1609.
29. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397-2404.
30. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-908.
31. Hewitt J, Moug SJ, Middleton M, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254-259.
32. Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33-37.
33. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222-228.
34. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157.
35. Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489-496.
36. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684-689.
37. Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269.
38. Goldfarb M, Lauck S, Webb J, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202-2211.
1. Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597-1607.
2. Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124(:1964-1972.
3. Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187-2198.
4. Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial (Cohort A). J Am Coll Cardiol. 2012;60:548-558.
5. Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790-1798.
6. Arnold SV, Reynolds MR, Wang K, et al. Health status after transcatheter or surgical aortic valve replacement in patients with severe aortic stenosis at increased surgical risk: results from the CoreValve US Pivotal trial. JACC Cardiovasc Interv. 2015;8:1207-1217.
7. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374:1609-1620.
8. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2017;376:1321-1331.
9. Baron SJ, Arnold SV, Wang K, et al. Health status benefits of transcatheter vs surgical aortic valve replacement in patients with severe aortic stenosis at intermediate surgical risk: results from the PARTNER 2 randomized clinical trial. JAMA Cardiol. 2017;2:837-845.
10. Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation. 2012;125:1102-1109.
11. Popma JJ, Adams DH, Reardon MJ, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972-1981.
12. Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2008;29:1463-1470.
13. Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59:1200-1254.
14. US Food and Drug Administration. FDA Executive Summary: Edwards SAPIEN™ Transcatheter Heart Valve. Presented July 20, 2011, Gaithersburg, MD.
15. Centers for Medicare & Medicaid Services. Decision Memo for Transcatheter Aortic Valve Replacement (TAVR) (CAG-00430N). May 5, 2012.
16. Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069-2077.
17. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. Lancet. 2016;387:2218-2225.
18. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380:1695-1705.
19. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380:1706-1715.
20. Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885-892.
21. Arnold SV, O’Brien SM, Vemulapalli S, et al. Inclusion of functional status measures in the risk adjustment of 30-day mortality after transcatheter aortic valve replacement: a report from the Society of Thoracic Surgeons/American College of Cardiology TVT Registry. JACC Cardiovasc Interv. 2018;11:581-589.
22. Brennan JM, Thomas L, Cohen DJ, et al. Transcatheter versus surgical aortic valve replacement: propensity-matched comparison. J Am Coll Cardiol. 2017;70:439-450.
23. Reardon MJ, Adams DH, Kleiman NS, et al. 2-year outcomes in patients undergoing surgical or self-expanding transcatheter aortic valve replacement. J Am Coll Cardiol. 2015;66:113-121.
24. Baron SJ, Cohen DJ, Suchindran S, et al. Development of a risk prediction model for 1-year mortality after surgical vs. transcatheter aortic valve replacement in patients with severe aortic stenosis. Circulation. 2016;134(A20166).
25. Arnold SV, Afilalo J, Spertus JA, et al. Prediction of poor outcome after transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;68:1868-1877.
26. Arnold SV, Reynolds MR, Lei Y, et al. Predictors of poor outcomes after transcatheter aortic valve replacement: results from the PARTNER (Placement of Aortic Transcatheter Valve) trial. Circulation. 2014;129:2682-2690.
27. Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24.
28. Torres OH, Munoz J, Ruiz D, et al. Outcome predictors of pneumonia in elderly patients: importance of functional assessment. J Am Geriatr Soc. 2004;52:1603-1609.
29. Ekerstad N, Swahn E, Janzon M, et al. Frailty is independently associated with short-term outcomes for elderly patients with non-ST-segment elevation myocardial infarction. Circulation. 2011;124:2397-2404.
30. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901-908.
31. Hewitt J, Moug SJ, Middleton M, et al. Prevalence of frailty and its association with mortality in general surgery. Am J Surg. 2015;209:254-259.
32. Sundermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33-37.
33. Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes. 2012;5:222-228.
34. Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr. 2016;16:157.
35. Stortecky S, Schoenenberger AW, Moser A, et al. Evaluation of multidimensional geriatric assessment as a predictor of mortality and cardiovascular events after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2012;5:489-496.
36. Schoenenberger AW, Stortecky S, Neumann S, et al. Predictors of functional decline in elderly patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J. 2013;34:684-689.
37. Green P, Arnold SV, Cohen DJ, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol. 2015;116:264-269.
38. Goldfarb M, Lauck S, Webb J, et al. Malnutrition and mortality in frail and non-frail older adults undergoing aortic valve replacement. Circulation. 2018;138:2202-2211.
Advanced Melanoma: Treatment After Progression on First-line Therapy
The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) for patients with metastatic melanoma. This article reviews current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy. The selection of first-line therapy for metastatic melanoma is reviewed in a separate article.
Case Presentation
A 62-year-old man was diagnosed with stage IIA melanoma after undergoing wide local excision of a right scalp lesion (final staging was consistent with pT3aN0M0). After 3.5 years of follow-up, he developed symptoms of vertigo, diplopia, and recurrent falls prompting medical attention. Magnetic resonance imaging (MRI) brain revealed multiple supratentorial and infratentorial lesions concerning for intracranial metastases and computed tomography (CT) chest/abdomen/pelvis revealed a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He was treated with intravenous dexamethasone and further evaluation with an endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass revealed metastatic melanoma. The patient underwent whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy. Testing showed the melanoma was positive for a BRAF V600K mutation. He was started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially did well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass.
After 3 months of therapy, surveillance PET-CT notes increasing size and FDG avidity of the right lower lobe mass. MRI brain reveals resolution of several previously noted metastases, but with interval development of a new left frontal lobe mass concerning for progressive disease.
What is the general approach to treatment of metastatic melanoma after progression on first-line therapy?
Based on the current evidence, there is no definitive algorithm for the treatment of metastatic melanoma after progression on first-line therapy. Enrollment in clinical trials is encouraged to further elucidate the best sequencing of treatment. The current practice is to typically switch class of agents after progression on front-line therapy to either immunotherapy that has not yet been tried or to molecularly targeted therapy in patients harboring a BRAF V600 mutation. After further progression of disease, retreatment with a previously received agent is possible, and this may be combined with investigational therapies.
Immune Checkpoint Inhibitors in Progressive Disease
The 2 major populations of patients to consider are those with BRAF wild-type melanomas who progress on first-line immunotherapy and those with BRAF V600 mutation–positive melanoma who progress on molecularly targeted therapy with BRAF and MEK inhibitors. There is relatively limited data on the efficacy of immune checkpoint inhibition after progression on anti-programmed cell death 1 (PD-1) monotherapy. A small retrospective study of patients who progressed on anti-PD-1 monotherapy were treated with ipilimumab, with a 10% ORR and another 8% having stable disease for more than 6 months; however, 35% of patients experienced grade 3 to 5 immune-related adverse events.1 The only prospective data supports the efficacy of anti-PD-1 therapy after progression on ipilimumab, as supported by the CheckMate 037 trial (nivolumab versus chemotherapy)2 and KEYNOTE-002 trial (pembrolizumab versus chemotherapy)3,4; however, this is no longer applicable as ipilimumab is no longer given in the first-line setting and has been replaced by anti-PD-1 monotherapy or combination immunotherapy.
Another interesting facet of PD-1 monotherapy is the idea of treatment beyond progression. The concept of pseudoprogression—whereby patients receiving PD-1 inhibitors initially meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria for progression, but then later go on to demonstrate significant decreases in tumor burden on subsequent imaging studies—has been described in melanoma patients receiving such immunotherapies. It is thought that pseudoprogression occurs due to either an initial delay in anti-tumor response to the immunotherapy or from the measured target lesion appearing larger due to surrounding immune/inflammatory infiltrate. In an analysis of individual patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression and had subsequent imaging to evaluate the tumor burden; in these patients, the same target lesion later met RECIST criteria for response, with a greater than 30% reduction in tumor size. Furthermore, of the evaluable cohort, the median OS in patients who did receive treatment beyond progression was 24.4 months compared to 11.2 months in those who did not receive treatment beyond progression.5 While further randomized studies are warranted to characterize the potential benefit, the existing data suggests that selected patients who are doing well clinically despite evidence of radiographic progressive disease may benefit from continued treatment with PD-1 inhibitors.
Combination immunotherapy with both PD-1 and CTLA-4 blockade has been studied retrospectively in the second-line setting. A retrospective analysis of patients who had progressive disease on PD-1 inhibitor monotherapy compared the outcomes of patients who received just ipilimumab to those of patients who received both ipilimumab and nivolumab. The ORR (16% ipilimumab vs 21% combination group) and 1-year OS (54% vs 55%) were similar in both groups,6 and this demonstrated significantly less efficacy with combination therapy when compared to use in the first-line setting, albeit in a separate prospective trial.7 A multicenter, retrospective study by Tétu and colleagues compared outcomes with ipilimumab plus nivolumab across 3 groups that included previously untreated patients, patients who had progressed on single-agent immunotherapy, and patients who had progressed on prior molecularly targeted therapy.8 Despite clearly inferior efficacy in previously treated patients, the results support combination immunotherapy as a viable treatment option in the second-line setting. Outcomes are reported in Table 1 below. Of note, there is an ongoing phase 2 trial to assess the use of combined PD-1 and CTLA-4 inhibitors versus CTLA-4 inhibition alone after progression on first-line PD-1 inhibitor monotherapy (NCT03033576).
For patients with BRAF V600–mutation positive melanoma who progress on front-line molecularly targeted therapy, immune checkpoint inhibitor therapy with either anti-PD-1 monotherapy or combination anti-PD-1 and ipilimumab should be considered. The KEYNOTE-006 trial that demonstrated superiority of pembrolizumab compared to ipilimumab included patients who had received up to 1 prior systemic therapy that was not a PD-1 or CTLA-4 inhibitor, and subgroup analysis demonstrated efficacy with pembrolizumab in patients who had received prior treatment with a BRAF inhibitor.9 The retrospective analysis by Tétu et al (Table 1) noted efficacy of combination nivolumab and ipilimumab in patients treated with prior molecularly targeted therapy, as evidenced by an ORR of 35% and median OS of 16.5 months.8
A retrospective trial by Ackerman et al analyzed ORR, median PFS, and median OS from the time of commencement of BRAF inhibitor therapy (with or without a MEK inhibitor), and the comparison was made between those who received ipilimumab before or after molecularly targeted therapy. While ipilimumab is no longer the first-line immunotherapy agent used in advanced melanoma, the study did highlight some important concepts. First, ORRs to BRAF inhibitors were similar between the 2 treatment groups. The conclusions of the analysis were that there was no significant difference in median PFS or OS in regard to which therapy was given first, but median OS after BRAF inhibitors were discontinued was very short and patients had poor responses to ipilimumab after stopping a BRAF inhibitor. This highlights the concern that patients who have progressive disease on molecularly targeted therapy often have a poor performance status and undergo too rapid of a clinical decline to derive benefit from immunotherapy, which can often take weeks to months to take effect.10
A more recent retrospective study by Johnson et al compared efficacy outcomes in patients who received single-agent anti-PD-1 therapy prior to molecularly targeted therapy (BRAF inhibitor with or without MEK inhibitor) to those who received molecularly targeted therapy prior to anti-PD-1 therapy. The difference in median OS was not statistically significant (27.5 months with PD-1 inhibitor first vs 40.3 months with molecularly targeted therapy first). Both treatments demonstrated second-line efficacy, but outcomes were inferior to those reported when either type of therapy was used in the first-line setting. Interestingly, patients who were maintained on molecularly targeted therapy for more than 6 months prior to progression demonstrated an improved ORR to subsequent anti-PD-1 therapy (34% vs 15%).11
Molecularly Targeted Therapy in Progressive Disease
When melanoma patients with a BRAF V600 mutation are treated initially with immunotherapy and demonstrate progressive disease, molecularly targeted therapy with combined BRAF and MEK inhibition should be considered for second-line therapy. While there are no dedicated prospective trial results with BRAF/MEK inhibitors after progression on immune checkpoint inhibitors, for practical purposes, it may be reasonable to extrapolate outcomes from the currently available first-line studies.12-16 An ongoing study (NCT02224781) in which patients are randomized to receive ipilimumab/nivolumab followed by dabrafenib/trametinib at progression versus the reverse order is designed to help answer the question of optimal sequencing and timing of therapy. Johnson et al’s retrospective analysis of patients receiving single-agent anti-PD-1 therapy prior to molecularly targeted therapy compared to the reverse order concluded that there was no statistically significant difference in median OS.11 Ackerman et al’s retrospective study of patients who had received ipilimumab before or after molecularly targeted therapy noted similar response rates to molecularly targeted therapy in each treatment group.10
The issue of re-treatment with a BRAF/MEK inhibitor in a patient already progressing on targeted therapy is a more challenging situation, and currently available data suggests there is limited benefit. However, select patients may be considered for this approach. The combination of dabrafenib/trametinib demonstrated an ORR of approximately 15% in a cohort of patients who progressed on single-agent BRAF inhibitor therapy, with a suggestion that those patients who had previously derived benefit for more than 6 months may have a more favorable outcome.17
Based on the hypothesis that acquired resistance to BRAF/MEK inhibition may be reversible if the selective pressure of the medication is held for a period of time, a phase 2 trial analyzed outcomes with retreatment. The study included patients with BRAF V600–mutant melanoma who had progressed on prior BRAF inhibition (with or without MEK inhibitor) and required that they had been off of therapy for at least 12 weeks. Of the 25 patients who received dabrafenib plus trametinib as retreatment, 32% demonstrated a partial response and 40% had stable disease.18 While further studies are warranted, retreatment with molecularly targeted therapy may be a viable option, especially in light of the multiple approved BRAF and MEK inhibitor combinations.
Another concept that has been studied is treatment beyond disease progression with molecularly targeted therapy. In a retrospective analysis of patients who had progressed on a single-agent BRAF inhibitor, 39% of those patients were continued on the same BRAF inhibitor and compared to patients who received no subsequent therapy or changed to an alternative systemic therapy. In the multivariable analysis adjusting for other prognostic factors, continued treatment with the BRAF inhibitor was associated with prolonged OS.19
Case Conclusion
The patient is started on second-line therapy with nivolumab and ipilimumab and demonstrates a partial response. One year later he continues to feel well with decreased size of the intracranial and right lower lobe lesions, and without any interval development of new areas of metastatic disease.
Special Considerations
Intralesional Therapies
Talimogene laherparepvec (T-VEC) is a genetically modified herpesvirus-1 oncolytic virus that is injected into melanoma skin lesions and leads to the expression of granulocyte-macrophage colony-stimulating factor. While T-VEC is currently approved for local treatment of unresectable cutaneous, subcutaneous, or nodal recurrences,20 it has also been investigated in combination with other therapies for patients with advanced disease. In patients with previously treated melanoma, T-VEC plus ipilimumab demonstrated superior ORR to ipilimumab alone (39% vs 18%), and the tumor response was not limited to the injected lesions. The observation of systemic response suggests synergy between T-VEC and immune checkpoint blockade in enhancing the anti-tumor immune response.21 The phase 1b MASTERKEY-265 trial combining pembrolizumab and T-VEC led to an ORR of 62% and CR of 33%.22 A phase 3 trial comparing pembrolizumab plus T-VEC to pembrolizumab alone is ongoing (NCT02263508).
Melanoma Brain Metastases
The presence of brain metastases is a common event in patients with metastatic melanoma, and often confers a poor prognosis.23 The approach to the management of brain metastases should be multidisciplinary among medical oncology, neurosurgery, and radiation oncology providers, as treatment algorithms continue to rapidly evolve. Historically, there has been little prospective clinical trial data regarding optimal systemic therapy, and local therapies such as surgery or stereotactic radiation have long been the mainstay of therapy for intracranial disease.24 However, recent data with both immunotherapy and molecularly targeted therapy has demonstrated efficacy with intracranial metastases.
A recent trial of combined nivolumab and ipilimumab as frontline therapy in patients with asymptomatic melanoma brain metastases demonstrated a complete response rate of 26% and partial response rate of 30% in patients with a median follow-up of 14 months.25 In a separate study, ipilimumab plus nivolumab demonstrated better intracranial ORR when compared to nivolumab alone in asymptomatic, previously untreated patients. Outcomes were better in patients presenting with asymptomatic versus symptomatic brain metastases.26 Collectively, these results suggest that systemic immunotherapy alone may be adequate for patients with asymptomatic, previously untreated brain metastases.
For molecularly targeted therapy in patients with BRAF mutations and brain metastases, the BREAK-MB trial demonstrated that an intracranial response was attainable with dabrafenib regardless of whether the patient had previously received local therapy in the form of surgery or radiation.27 The COMBI-MB trial enhanced the preexisting data by testing the intracranial efficacy of dabrafenib plus trametinib in 4 different cohorts of patients, further supporting that systemic molecularly targeted therapy can provide significant intracranial activity in patients with both symptomatic and asymptomatic brain lesions and regardless of prior local therapy (Table 2).28
Conclusion
The treatment of advanced melanoma has been drastically improved over the past decade by the development and study of immune checkpoint inhibitors and molecularly targeted agents. There is still much to learn regarding the optimal combination and sequencing of therapies. Many of these trials are ongoing and will provide additional evidence to guide treatment decisions moving forward.
1. Bowyer S, Prithviraj P, Lorigan P, et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer. 2016;114:1084-1089.
2. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
3. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
4. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
5. Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229-239.
6. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55.
7. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
8. Tétu P, Mangana J, Dummer R, et al. Benefit of the nivolumab and ipilimumab combination in pretreated advanced melanoma. Eur J Cancer. 2018;93:147-149.
9. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
10. Ackerman A, Klein O, McDermott D, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695-1701.
11. Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-pd-1 before and after BRAF inhibition. J Immunother. 2017;40:31-35.
12. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
13. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
14. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-1260.
15. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
16. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
17. Johnson DB, Flaherty KT, Weber, JS et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697-3704.
18. Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017;18:464-472.
19. Chan MM, Haydu LE, Azer MW, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142-3153.
20. Imlygic (talimogene laherparepvec) suspension for intralesional injection [package insert]. Thousand Oaks, CA: BioVex; 2015.
21. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36:1658-1667.
22. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031-1032.
23. Sampson JH, Carter Jr. JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
24. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395.
25. Tawbi HA, Forsyth PA, Hamid O, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722-730.
26. Long GV, Atkinson V, La S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicenter randomised phase 2 study. Lancet Oncol. 2018;19:672-681.
27. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087-1095.
28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicenter, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863-873.
The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) for patients with metastatic melanoma. This article reviews current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy. The selection of first-line therapy for metastatic melanoma is reviewed in a separate article.
Case Presentation
A 62-year-old man was diagnosed with stage IIA melanoma after undergoing wide local excision of a right scalp lesion (final staging was consistent with pT3aN0M0). After 3.5 years of follow-up, he developed symptoms of vertigo, diplopia, and recurrent falls prompting medical attention. Magnetic resonance imaging (MRI) brain revealed multiple supratentorial and infratentorial lesions concerning for intracranial metastases and computed tomography (CT) chest/abdomen/pelvis revealed a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He was treated with intravenous dexamethasone and further evaluation with an endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass revealed metastatic melanoma. The patient underwent whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy. Testing showed the melanoma was positive for a BRAF V600K mutation. He was started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially did well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass.
After 3 months of therapy, surveillance PET-CT notes increasing size and FDG avidity of the right lower lobe mass. MRI brain reveals resolution of several previously noted metastases, but with interval development of a new left frontal lobe mass concerning for progressive disease.
What is the general approach to treatment of metastatic melanoma after progression on first-line therapy?
Based on the current evidence, there is no definitive algorithm for the treatment of metastatic melanoma after progression on first-line therapy. Enrollment in clinical trials is encouraged to further elucidate the best sequencing of treatment. The current practice is to typically switch class of agents after progression on front-line therapy to either immunotherapy that has not yet been tried or to molecularly targeted therapy in patients harboring a BRAF V600 mutation. After further progression of disease, retreatment with a previously received agent is possible, and this may be combined with investigational therapies.
Immune Checkpoint Inhibitors in Progressive Disease
The 2 major populations of patients to consider are those with BRAF wild-type melanomas who progress on first-line immunotherapy and those with BRAF V600 mutation–positive melanoma who progress on molecularly targeted therapy with BRAF and MEK inhibitors. There is relatively limited data on the efficacy of immune checkpoint inhibition after progression on anti-programmed cell death 1 (PD-1) monotherapy. A small retrospective study of patients who progressed on anti-PD-1 monotherapy were treated with ipilimumab, with a 10% ORR and another 8% having stable disease for more than 6 months; however, 35% of patients experienced grade 3 to 5 immune-related adverse events.1 The only prospective data supports the efficacy of anti-PD-1 therapy after progression on ipilimumab, as supported by the CheckMate 037 trial (nivolumab versus chemotherapy)2 and KEYNOTE-002 trial (pembrolizumab versus chemotherapy)3,4; however, this is no longer applicable as ipilimumab is no longer given in the first-line setting and has been replaced by anti-PD-1 monotherapy or combination immunotherapy.
Another interesting facet of PD-1 monotherapy is the idea of treatment beyond progression. The concept of pseudoprogression—whereby patients receiving PD-1 inhibitors initially meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria for progression, but then later go on to demonstrate significant decreases in tumor burden on subsequent imaging studies—has been described in melanoma patients receiving such immunotherapies. It is thought that pseudoprogression occurs due to either an initial delay in anti-tumor response to the immunotherapy or from the measured target lesion appearing larger due to surrounding immune/inflammatory infiltrate. In an analysis of individual patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression and had subsequent imaging to evaluate the tumor burden; in these patients, the same target lesion later met RECIST criteria for response, with a greater than 30% reduction in tumor size. Furthermore, of the evaluable cohort, the median OS in patients who did receive treatment beyond progression was 24.4 months compared to 11.2 months in those who did not receive treatment beyond progression.5 While further randomized studies are warranted to characterize the potential benefit, the existing data suggests that selected patients who are doing well clinically despite evidence of radiographic progressive disease may benefit from continued treatment with PD-1 inhibitors.
Combination immunotherapy with both PD-1 and CTLA-4 blockade has been studied retrospectively in the second-line setting. A retrospective analysis of patients who had progressive disease on PD-1 inhibitor monotherapy compared the outcomes of patients who received just ipilimumab to those of patients who received both ipilimumab and nivolumab. The ORR (16% ipilimumab vs 21% combination group) and 1-year OS (54% vs 55%) were similar in both groups,6 and this demonstrated significantly less efficacy with combination therapy when compared to use in the first-line setting, albeit in a separate prospective trial.7 A multicenter, retrospective study by Tétu and colleagues compared outcomes with ipilimumab plus nivolumab across 3 groups that included previously untreated patients, patients who had progressed on single-agent immunotherapy, and patients who had progressed on prior molecularly targeted therapy.8 Despite clearly inferior efficacy in previously treated patients, the results support combination immunotherapy as a viable treatment option in the second-line setting. Outcomes are reported in Table 1 below. Of note, there is an ongoing phase 2 trial to assess the use of combined PD-1 and CTLA-4 inhibitors versus CTLA-4 inhibition alone after progression on first-line PD-1 inhibitor monotherapy (NCT03033576).
For patients with BRAF V600–mutation positive melanoma who progress on front-line molecularly targeted therapy, immune checkpoint inhibitor therapy with either anti-PD-1 monotherapy or combination anti-PD-1 and ipilimumab should be considered. The KEYNOTE-006 trial that demonstrated superiority of pembrolizumab compared to ipilimumab included patients who had received up to 1 prior systemic therapy that was not a PD-1 or CTLA-4 inhibitor, and subgroup analysis demonstrated efficacy with pembrolizumab in patients who had received prior treatment with a BRAF inhibitor.9 The retrospective analysis by Tétu et al (Table 1) noted efficacy of combination nivolumab and ipilimumab in patients treated with prior molecularly targeted therapy, as evidenced by an ORR of 35% and median OS of 16.5 months.8
A retrospective trial by Ackerman et al analyzed ORR, median PFS, and median OS from the time of commencement of BRAF inhibitor therapy (with or without a MEK inhibitor), and the comparison was made between those who received ipilimumab before or after molecularly targeted therapy. While ipilimumab is no longer the first-line immunotherapy agent used in advanced melanoma, the study did highlight some important concepts. First, ORRs to BRAF inhibitors were similar between the 2 treatment groups. The conclusions of the analysis were that there was no significant difference in median PFS or OS in regard to which therapy was given first, but median OS after BRAF inhibitors were discontinued was very short and patients had poor responses to ipilimumab after stopping a BRAF inhibitor. This highlights the concern that patients who have progressive disease on molecularly targeted therapy often have a poor performance status and undergo too rapid of a clinical decline to derive benefit from immunotherapy, which can often take weeks to months to take effect.10
A more recent retrospective study by Johnson et al compared efficacy outcomes in patients who received single-agent anti-PD-1 therapy prior to molecularly targeted therapy (BRAF inhibitor with or without MEK inhibitor) to those who received molecularly targeted therapy prior to anti-PD-1 therapy. The difference in median OS was not statistically significant (27.5 months with PD-1 inhibitor first vs 40.3 months with molecularly targeted therapy first). Both treatments demonstrated second-line efficacy, but outcomes were inferior to those reported when either type of therapy was used in the first-line setting. Interestingly, patients who were maintained on molecularly targeted therapy for more than 6 months prior to progression demonstrated an improved ORR to subsequent anti-PD-1 therapy (34% vs 15%).11
Molecularly Targeted Therapy in Progressive Disease
When melanoma patients with a BRAF V600 mutation are treated initially with immunotherapy and demonstrate progressive disease, molecularly targeted therapy with combined BRAF and MEK inhibition should be considered for second-line therapy. While there are no dedicated prospective trial results with BRAF/MEK inhibitors after progression on immune checkpoint inhibitors, for practical purposes, it may be reasonable to extrapolate outcomes from the currently available first-line studies.12-16 An ongoing study (NCT02224781) in which patients are randomized to receive ipilimumab/nivolumab followed by dabrafenib/trametinib at progression versus the reverse order is designed to help answer the question of optimal sequencing and timing of therapy. Johnson et al’s retrospective analysis of patients receiving single-agent anti-PD-1 therapy prior to molecularly targeted therapy compared to the reverse order concluded that there was no statistically significant difference in median OS.11 Ackerman et al’s retrospective study of patients who had received ipilimumab before or after molecularly targeted therapy noted similar response rates to molecularly targeted therapy in each treatment group.10
The issue of re-treatment with a BRAF/MEK inhibitor in a patient already progressing on targeted therapy is a more challenging situation, and currently available data suggests there is limited benefit. However, select patients may be considered for this approach. The combination of dabrafenib/trametinib demonstrated an ORR of approximately 15% in a cohort of patients who progressed on single-agent BRAF inhibitor therapy, with a suggestion that those patients who had previously derived benefit for more than 6 months may have a more favorable outcome.17
Based on the hypothesis that acquired resistance to BRAF/MEK inhibition may be reversible if the selective pressure of the medication is held for a period of time, a phase 2 trial analyzed outcomes with retreatment. The study included patients with BRAF V600–mutant melanoma who had progressed on prior BRAF inhibition (with or without MEK inhibitor) and required that they had been off of therapy for at least 12 weeks. Of the 25 patients who received dabrafenib plus trametinib as retreatment, 32% demonstrated a partial response and 40% had stable disease.18 While further studies are warranted, retreatment with molecularly targeted therapy may be a viable option, especially in light of the multiple approved BRAF and MEK inhibitor combinations.
Another concept that has been studied is treatment beyond disease progression with molecularly targeted therapy. In a retrospective analysis of patients who had progressed on a single-agent BRAF inhibitor, 39% of those patients were continued on the same BRAF inhibitor and compared to patients who received no subsequent therapy or changed to an alternative systemic therapy. In the multivariable analysis adjusting for other prognostic factors, continued treatment with the BRAF inhibitor was associated with prolonged OS.19
Case Conclusion
The patient is started on second-line therapy with nivolumab and ipilimumab and demonstrates a partial response. One year later he continues to feel well with decreased size of the intracranial and right lower lobe lesions, and without any interval development of new areas of metastatic disease.
Special Considerations
Intralesional Therapies
Talimogene laherparepvec (T-VEC) is a genetically modified herpesvirus-1 oncolytic virus that is injected into melanoma skin lesions and leads to the expression of granulocyte-macrophage colony-stimulating factor. While T-VEC is currently approved for local treatment of unresectable cutaneous, subcutaneous, or nodal recurrences,20 it has also been investigated in combination with other therapies for patients with advanced disease. In patients with previously treated melanoma, T-VEC plus ipilimumab demonstrated superior ORR to ipilimumab alone (39% vs 18%), and the tumor response was not limited to the injected lesions. The observation of systemic response suggests synergy between T-VEC and immune checkpoint blockade in enhancing the anti-tumor immune response.21 The phase 1b MASTERKEY-265 trial combining pembrolizumab and T-VEC led to an ORR of 62% and CR of 33%.22 A phase 3 trial comparing pembrolizumab plus T-VEC to pembrolizumab alone is ongoing (NCT02263508).
Melanoma Brain Metastases
The presence of brain metastases is a common event in patients with metastatic melanoma, and often confers a poor prognosis.23 The approach to the management of brain metastases should be multidisciplinary among medical oncology, neurosurgery, and radiation oncology providers, as treatment algorithms continue to rapidly evolve. Historically, there has been little prospective clinical trial data regarding optimal systemic therapy, and local therapies such as surgery or stereotactic radiation have long been the mainstay of therapy for intracranial disease.24 However, recent data with both immunotherapy and molecularly targeted therapy has demonstrated efficacy with intracranial metastases.
A recent trial of combined nivolumab and ipilimumab as frontline therapy in patients with asymptomatic melanoma brain metastases demonstrated a complete response rate of 26% and partial response rate of 30% in patients with a median follow-up of 14 months.25 In a separate study, ipilimumab plus nivolumab demonstrated better intracranial ORR when compared to nivolumab alone in asymptomatic, previously untreated patients. Outcomes were better in patients presenting with asymptomatic versus symptomatic brain metastases.26 Collectively, these results suggest that systemic immunotherapy alone may be adequate for patients with asymptomatic, previously untreated brain metastases.
For molecularly targeted therapy in patients with BRAF mutations and brain metastases, the BREAK-MB trial demonstrated that an intracranial response was attainable with dabrafenib regardless of whether the patient had previously received local therapy in the form of surgery or radiation.27 The COMBI-MB trial enhanced the preexisting data by testing the intracranial efficacy of dabrafenib plus trametinib in 4 different cohorts of patients, further supporting that systemic molecularly targeted therapy can provide significant intracranial activity in patients with both symptomatic and asymptomatic brain lesions and regardless of prior local therapy (Table 2).28
Conclusion
The treatment of advanced melanoma has been drastically improved over the past decade by the development and study of immune checkpoint inhibitors and molecularly targeted agents. There is still much to learn regarding the optimal combination and sequencing of therapies. Many of these trials are ongoing and will provide additional evidence to guide treatment decisions moving forward.
The past decade has brought rapid advancements in treatment with immune checkpoint inhibitors and molecularly targeted agents, which have significantly improved objective response rates (ORRs), progression-free survival (PFS), and overall survival (OS) for patients with metastatic melanoma. This article reviews current evidence for immune checkpoint blockade and molecularly targeted agents in the treatment of metastatic melanoma after progression on first-line therapy. The selection of first-line therapy for metastatic melanoma is reviewed in a separate article.
Case Presentation
A 62-year-old man was diagnosed with stage IIA melanoma after undergoing wide local excision of a right scalp lesion (final staging was consistent with pT3aN0M0). After 3.5 years of follow-up, he developed symptoms of vertigo, diplopia, and recurrent falls prompting medical attention. Magnetic resonance imaging (MRI) brain revealed multiple supratentorial and infratentorial lesions concerning for intracranial metastases and computed tomography (CT) chest/abdomen/pelvis revealed a right lower lobe pulmonary mass with right hilar and subcarinal lymphadenopathy. He was treated with intravenous dexamethasone and further evaluation with an endobronchial ultrasound-guided fine-needle aspiration of the right lower lobe mass revealed metastatic melanoma. The patient underwent whole brain radiation therapy for symptomatic relief prior to initiating systemic therapy. Testing showed the melanoma was positive for a BRAF V600K mutation. He was started on combination molecularly targeted therapy with dabrafenib and trametinib. He initially did well, with a partial response noted by resolution of symptoms and decreased size of his intracranial metastases and decreased size of the right lower lobe mass.
After 3 months of therapy, surveillance PET-CT notes increasing size and FDG avidity of the right lower lobe mass. MRI brain reveals resolution of several previously noted metastases, but with interval development of a new left frontal lobe mass concerning for progressive disease.
What is the general approach to treatment of metastatic melanoma after progression on first-line therapy?
Based on the current evidence, there is no definitive algorithm for the treatment of metastatic melanoma after progression on first-line therapy. Enrollment in clinical trials is encouraged to further elucidate the best sequencing of treatment. The current practice is to typically switch class of agents after progression on front-line therapy to either immunotherapy that has not yet been tried or to molecularly targeted therapy in patients harboring a BRAF V600 mutation. After further progression of disease, retreatment with a previously received agent is possible, and this may be combined with investigational therapies.
Immune Checkpoint Inhibitors in Progressive Disease
The 2 major populations of patients to consider are those with BRAF wild-type melanomas who progress on first-line immunotherapy and those with BRAF V600 mutation–positive melanoma who progress on molecularly targeted therapy with BRAF and MEK inhibitors. There is relatively limited data on the efficacy of immune checkpoint inhibition after progression on anti-programmed cell death 1 (PD-1) monotherapy. A small retrospective study of patients who progressed on anti-PD-1 monotherapy were treated with ipilimumab, with a 10% ORR and another 8% having stable disease for more than 6 months; however, 35% of patients experienced grade 3 to 5 immune-related adverse events.1 The only prospective data supports the efficacy of anti-PD-1 therapy after progression on ipilimumab, as supported by the CheckMate 037 trial (nivolumab versus chemotherapy)2 and KEYNOTE-002 trial (pembrolizumab versus chemotherapy)3,4; however, this is no longer applicable as ipilimumab is no longer given in the first-line setting and has been replaced by anti-PD-1 monotherapy or combination immunotherapy.
Another interesting facet of PD-1 monotherapy is the idea of treatment beyond progression. The concept of pseudoprogression—whereby patients receiving PD-1 inhibitors initially meet Response Evaluation Criteria in Solid Tumors (RECIST) criteria for progression, but then later go on to demonstrate significant decreases in tumor burden on subsequent imaging studies—has been described in melanoma patients receiving such immunotherapies. It is thought that pseudoprogression occurs due to either an initial delay in anti-tumor response to the immunotherapy or from the measured target lesion appearing larger due to surrounding immune/inflammatory infiltrate. In an analysis of individual patient data pooled from 8 multicenter clinical trials, 19% of patients were treated beyond initially documented RECIST progression and had subsequent imaging to evaluate the tumor burden; in these patients, the same target lesion later met RECIST criteria for response, with a greater than 30% reduction in tumor size. Furthermore, of the evaluable cohort, the median OS in patients who did receive treatment beyond progression was 24.4 months compared to 11.2 months in those who did not receive treatment beyond progression.5 While further randomized studies are warranted to characterize the potential benefit, the existing data suggests that selected patients who are doing well clinically despite evidence of radiographic progressive disease may benefit from continued treatment with PD-1 inhibitors.
Combination immunotherapy with both PD-1 and CTLA-4 blockade has been studied retrospectively in the second-line setting. A retrospective analysis of patients who had progressive disease on PD-1 inhibitor monotherapy compared the outcomes of patients who received just ipilimumab to those of patients who received both ipilimumab and nivolumab. The ORR (16% ipilimumab vs 21% combination group) and 1-year OS (54% vs 55%) were similar in both groups,6 and this demonstrated significantly less efficacy with combination therapy when compared to use in the first-line setting, albeit in a separate prospective trial.7 A multicenter, retrospective study by Tétu and colleagues compared outcomes with ipilimumab plus nivolumab across 3 groups that included previously untreated patients, patients who had progressed on single-agent immunotherapy, and patients who had progressed on prior molecularly targeted therapy.8 Despite clearly inferior efficacy in previously treated patients, the results support combination immunotherapy as a viable treatment option in the second-line setting. Outcomes are reported in Table 1 below. Of note, there is an ongoing phase 2 trial to assess the use of combined PD-1 and CTLA-4 inhibitors versus CTLA-4 inhibition alone after progression on first-line PD-1 inhibitor monotherapy (NCT03033576).
For patients with BRAF V600–mutation positive melanoma who progress on front-line molecularly targeted therapy, immune checkpoint inhibitor therapy with either anti-PD-1 monotherapy or combination anti-PD-1 and ipilimumab should be considered. The KEYNOTE-006 trial that demonstrated superiority of pembrolizumab compared to ipilimumab included patients who had received up to 1 prior systemic therapy that was not a PD-1 or CTLA-4 inhibitor, and subgroup analysis demonstrated efficacy with pembrolizumab in patients who had received prior treatment with a BRAF inhibitor.9 The retrospective analysis by Tétu et al (Table 1) noted efficacy of combination nivolumab and ipilimumab in patients treated with prior molecularly targeted therapy, as evidenced by an ORR of 35% and median OS of 16.5 months.8
A retrospective trial by Ackerman et al analyzed ORR, median PFS, and median OS from the time of commencement of BRAF inhibitor therapy (with or without a MEK inhibitor), and the comparison was made between those who received ipilimumab before or after molecularly targeted therapy. While ipilimumab is no longer the first-line immunotherapy agent used in advanced melanoma, the study did highlight some important concepts. First, ORRs to BRAF inhibitors were similar between the 2 treatment groups. The conclusions of the analysis were that there was no significant difference in median PFS or OS in regard to which therapy was given first, but median OS after BRAF inhibitors were discontinued was very short and patients had poor responses to ipilimumab after stopping a BRAF inhibitor. This highlights the concern that patients who have progressive disease on molecularly targeted therapy often have a poor performance status and undergo too rapid of a clinical decline to derive benefit from immunotherapy, which can often take weeks to months to take effect.10
A more recent retrospective study by Johnson et al compared efficacy outcomes in patients who received single-agent anti-PD-1 therapy prior to molecularly targeted therapy (BRAF inhibitor with or without MEK inhibitor) to those who received molecularly targeted therapy prior to anti-PD-1 therapy. The difference in median OS was not statistically significant (27.5 months with PD-1 inhibitor first vs 40.3 months with molecularly targeted therapy first). Both treatments demonstrated second-line efficacy, but outcomes were inferior to those reported when either type of therapy was used in the first-line setting. Interestingly, patients who were maintained on molecularly targeted therapy for more than 6 months prior to progression demonstrated an improved ORR to subsequent anti-PD-1 therapy (34% vs 15%).11
Molecularly Targeted Therapy in Progressive Disease
When melanoma patients with a BRAF V600 mutation are treated initially with immunotherapy and demonstrate progressive disease, molecularly targeted therapy with combined BRAF and MEK inhibition should be considered for second-line therapy. While there are no dedicated prospective trial results with BRAF/MEK inhibitors after progression on immune checkpoint inhibitors, for practical purposes, it may be reasonable to extrapolate outcomes from the currently available first-line studies.12-16 An ongoing study (NCT02224781) in which patients are randomized to receive ipilimumab/nivolumab followed by dabrafenib/trametinib at progression versus the reverse order is designed to help answer the question of optimal sequencing and timing of therapy. Johnson et al’s retrospective analysis of patients receiving single-agent anti-PD-1 therapy prior to molecularly targeted therapy compared to the reverse order concluded that there was no statistically significant difference in median OS.11 Ackerman et al’s retrospective study of patients who had received ipilimumab before or after molecularly targeted therapy noted similar response rates to molecularly targeted therapy in each treatment group.10
The issue of re-treatment with a BRAF/MEK inhibitor in a patient already progressing on targeted therapy is a more challenging situation, and currently available data suggests there is limited benefit. However, select patients may be considered for this approach. The combination of dabrafenib/trametinib demonstrated an ORR of approximately 15% in a cohort of patients who progressed on single-agent BRAF inhibitor therapy, with a suggestion that those patients who had previously derived benefit for more than 6 months may have a more favorable outcome.17
Based on the hypothesis that acquired resistance to BRAF/MEK inhibition may be reversible if the selective pressure of the medication is held for a period of time, a phase 2 trial analyzed outcomes with retreatment. The study included patients with BRAF V600–mutant melanoma who had progressed on prior BRAF inhibition (with or without MEK inhibitor) and required that they had been off of therapy for at least 12 weeks. Of the 25 patients who received dabrafenib plus trametinib as retreatment, 32% demonstrated a partial response and 40% had stable disease.18 While further studies are warranted, retreatment with molecularly targeted therapy may be a viable option, especially in light of the multiple approved BRAF and MEK inhibitor combinations.
Another concept that has been studied is treatment beyond disease progression with molecularly targeted therapy. In a retrospective analysis of patients who had progressed on a single-agent BRAF inhibitor, 39% of those patients were continued on the same BRAF inhibitor and compared to patients who received no subsequent therapy or changed to an alternative systemic therapy. In the multivariable analysis adjusting for other prognostic factors, continued treatment with the BRAF inhibitor was associated with prolonged OS.19
Case Conclusion
The patient is started on second-line therapy with nivolumab and ipilimumab and demonstrates a partial response. One year later he continues to feel well with decreased size of the intracranial and right lower lobe lesions, and without any interval development of new areas of metastatic disease.
Special Considerations
Intralesional Therapies
Talimogene laherparepvec (T-VEC) is a genetically modified herpesvirus-1 oncolytic virus that is injected into melanoma skin lesions and leads to the expression of granulocyte-macrophage colony-stimulating factor. While T-VEC is currently approved for local treatment of unresectable cutaneous, subcutaneous, or nodal recurrences,20 it has also been investigated in combination with other therapies for patients with advanced disease. In patients with previously treated melanoma, T-VEC plus ipilimumab demonstrated superior ORR to ipilimumab alone (39% vs 18%), and the tumor response was not limited to the injected lesions. The observation of systemic response suggests synergy between T-VEC and immune checkpoint blockade in enhancing the anti-tumor immune response.21 The phase 1b MASTERKEY-265 trial combining pembrolizumab and T-VEC led to an ORR of 62% and CR of 33%.22 A phase 3 trial comparing pembrolizumab plus T-VEC to pembrolizumab alone is ongoing (NCT02263508).
Melanoma Brain Metastases
The presence of brain metastases is a common event in patients with metastatic melanoma, and often confers a poor prognosis.23 The approach to the management of brain metastases should be multidisciplinary among medical oncology, neurosurgery, and radiation oncology providers, as treatment algorithms continue to rapidly evolve. Historically, there has been little prospective clinical trial data regarding optimal systemic therapy, and local therapies such as surgery or stereotactic radiation have long been the mainstay of therapy for intracranial disease.24 However, recent data with both immunotherapy and molecularly targeted therapy has demonstrated efficacy with intracranial metastases.
A recent trial of combined nivolumab and ipilimumab as frontline therapy in patients with asymptomatic melanoma brain metastases demonstrated a complete response rate of 26% and partial response rate of 30% in patients with a median follow-up of 14 months.25 In a separate study, ipilimumab plus nivolumab demonstrated better intracranial ORR when compared to nivolumab alone in asymptomatic, previously untreated patients. Outcomes were better in patients presenting with asymptomatic versus symptomatic brain metastases.26 Collectively, these results suggest that systemic immunotherapy alone may be adequate for patients with asymptomatic, previously untreated brain metastases.
For molecularly targeted therapy in patients with BRAF mutations and brain metastases, the BREAK-MB trial demonstrated that an intracranial response was attainable with dabrafenib regardless of whether the patient had previously received local therapy in the form of surgery or radiation.27 The COMBI-MB trial enhanced the preexisting data by testing the intracranial efficacy of dabrafenib plus trametinib in 4 different cohorts of patients, further supporting that systemic molecularly targeted therapy can provide significant intracranial activity in patients with both symptomatic and asymptomatic brain lesions and regardless of prior local therapy (Table 2).28
Conclusion
The treatment of advanced melanoma has been drastically improved over the past decade by the development and study of immune checkpoint inhibitors and molecularly targeted agents. There is still much to learn regarding the optimal combination and sequencing of therapies. Many of these trials are ongoing and will provide additional evidence to guide treatment decisions moving forward.
1. Bowyer S, Prithviraj P, Lorigan P, et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer. 2016;114:1084-1089.
2. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
3. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
4. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
5. Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229-239.
6. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55.
7. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
8. Tétu P, Mangana J, Dummer R, et al. Benefit of the nivolumab and ipilimumab combination in pretreated advanced melanoma. Eur J Cancer. 2018;93:147-149.
9. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
10. Ackerman A, Klein O, McDermott D, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695-1701.
11. Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-pd-1 before and after BRAF inhibition. J Immunother. 2017;40:31-35.
12. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
13. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
14. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-1260.
15. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
16. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
17. Johnson DB, Flaherty KT, Weber, JS et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697-3704.
18. Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017;18:464-472.
19. Chan MM, Haydu LE, Azer MW, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142-3153.
20. Imlygic (talimogene laherparepvec) suspension for intralesional injection [package insert]. Thousand Oaks, CA: BioVex; 2015.
21. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36:1658-1667.
22. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031-1032.
23. Sampson JH, Carter Jr. JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
24. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395.
25. Tawbi HA, Forsyth PA, Hamid O, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722-730.
26. Long GV, Atkinson V, La S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicenter randomised phase 2 study. Lancet Oncol. 2018;19:672-681.
27. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087-1095.
28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicenter, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863-873.
1. Bowyer S, Prithviraj P, Lorigan P, et al. Efficacy and toxicity of treatment with the anti-CTLA-4 antibody ipilimumab in patients with metastatic melanoma after prior anti-PD-1 therapy. Br J Cancer. 2016;114:1084-1089.
2. Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384.
3. Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908-918.
4. Hamid O, Puzanov I, Dummer R, et al. Final analysis of a randomised trial comparing pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory advanced melanoma. Eur J Cancer. 2017;86:37-45.
5. Beaver JA, Hazarika M, Mulkey F, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19:229-239.
6. Zimmer L, Apuri S, Eroglu Z, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-1 therapy in advanced melanoma. Eur J Cancer. 2017;75:47-55.
7. Larkin J, Chiarion-Sileni V, Gonazalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23-34.
8. Tétu P, Mangana J, Dummer R, et al. Benefit of the nivolumab and ipilimumab combination in pretreated advanced melanoma. Eur J Cancer. 2018;93:147-149.
9. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2522-2532.
10. Ackerman A, Klein O, McDermott D, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120:1695-1701.
11. Johnson DB, Pectasides E, Feld E, et al. Sequencing treatment in BRAFV600 mutant melanoma: anti-pd-1 before and after BRAF inhibition. J Immunother. 2017;40:31-35.
12. Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicenter, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2015;386:444-451.
13. Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631-1639.
14. Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advanced BRAFV600-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248-1260.
15. Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicenter, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603-615.
16. Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicenter, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315-1327.
17. Johnson DB, Flaherty KT, Weber, JS et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol. 2014;32:3697-3704.
18. Schreuer M, Jansen Y, Planken S, et al. Combination of dabrafenib plus trametinib for BRAF and MEK inhibitor pretreated patients with advanced BRAFV600-mutant melanoma: an open-label, single arm, dual-centre, phase 2 clinical trial. Lancet Oncol. 2017;18:464-472.
19. Chan MM, Haydu LE, Azer MW, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142-3153.
20. Imlygic (talimogene laherparepvec) suspension for intralesional injection [package insert]. Thousand Oaks, CA: BioVex; 2015.
21. Chesney J, Puzanov I, Collichio F, et al. Randomized, open-label phase ii study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced, unresectable melanoma. J Clin Oncol. 2018;36:1658-1667.
22. Ribas A, Dummer R, Puzanov I, et al. Oncolytic virotherapy promotes intratumoral t cell infiltration and improves anti-PD-1 immunotherapy. Cell. 2018;174:1031-1032.
23. Sampson JH, Carter Jr. JH, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11-20.
24. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15:387-395.
25. Tawbi HA, Forsyth PA, Hamid O, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722-730.
26. Long GV, Atkinson V, La S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicenter randomised phase 2 study. Lancet Oncol. 2018;19:672-681.
27. Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicenter, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087-1095.
28. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): a multicenter, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863-873.