User login
Can anti-inflammatory medications improve symptoms and reduce mortality in schizophrenia?
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
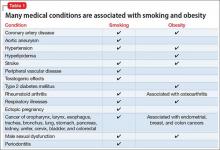
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
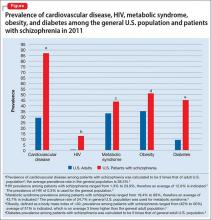
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
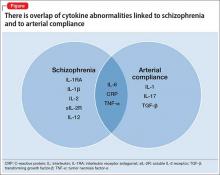
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
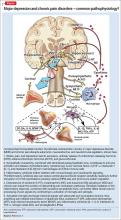
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
Consider 3 observations:
- Evidence is mounting that cytokine abnormalities are present in schizophrenia (Box1-8).
- Reduced arterial compliance (change in volume divided by change in pressure [ΔV/ΔP] in an artery during the cardiac cycle) is an early marker of cardiovascular disease (CVD) and a robust predictor of mortality, and is associated with cytokine abnormalities.
- People with schizophrenia experience increased mortality from CVD.
Taken together, the 3 statements hint at a hypothesis: a common inflammatory process involving cytokine imbalance is associated with symptoms of schizophrenia, reduced arterial compliance, and CVD.
Anti-inflammatory therapeutics that target specific cytokines might both decrease psychiatric symptoms and reduce cardiac mortality in people with schizophrenia. In this article, we (1) highlight the potential role of anti-inflammatory medications in reducing both psychiatric symptoms and cardiac mortality in people with schizophrenia and (2) review the pathophysiological basis of this inflammatory commonality and the evidence for its presence in schizophrenia.
The ‘membrane hypothesis’ of schizophrenia
In this hypothesis, a disturbance in the synthesis and structure of membrane phospholipids results in a subsequent disturbance in the function of neuronal membrane proteins, which might be associated with symptoms and mortality in schizophrenia.9-12 The synaptic vesicle protein synaptophysin, a marker for synaptic density, was found to be decreased in postmortem tissue from the gyrus cinguli in 11 patients with schizophrenia, compared with 13 controls.10 Intracellular phospholipases A2 (inPLA2) act as key enzymes in cell membrane repair and remodeling and in neuroplasticity, neurodevelopment, apoptosis, synaptic pruning, neurodegenerative processes, and neuroinflammation.
In a study, people with first-episode schizophrenia (n = 24) who were drug-naïve or off antipsychotic medication were compared with 25 healthy controls using voxel-based morphometry analysis of T1 high-resolution MRI. inPLA2 activity was increased in the patient group compared with controls; the analysis revealed abnormalities of the frontal and medial temporal cortices, hippocampus, and left-middle and superior temporal gyri in first-episode patients.11 In another study, inPLA2 activity was increased in 35 people with first-episode schizophrenia, compared with 22 controls, and was associated with symptom severity and outcome after 12 weeks of antipsychotic treatment.12
Early CVD mortality in schizophrenia
People with schizophrenia have an elevated rate of CVD compared with the general population; in part, this elevation is linked to magnified risk factors for CVD, including obesity, metabolic syndrome, cigarette smoking, and diabetes13-17; furthermore, most antipsychotics can cause or worsen metabolic syndrome.17
CVD is one of the most common causes of death among people with schizophrenia.17,18 Their life expectancy is reported to be 51 to 61 years—20 to 25 years less than what is seen in the general population.19-21
Arterial compliance in schizophrenia
Reduced arterial compliance has been found to be a robust predictor of atherosclerosis, stroke, and myocardial infarction22-29:
- In 376 subjects who had routine diagnostic coronary angiography associated with coronary stenosis, arterial compliance was reduced significantly—even after controlling for age, sex, smoking, diabetes, hypertension, hyperlipidemia, and obesity.24
In a cross-sectional study, 63 male U.S. veterans age 18 to 70 who had a psychiatric diagnosis (16 taking quetiapine, 19 taking risperidone, and 28 treated in the past but off antipsychotics for 2 months) had significantly reduced compliance in thigh- and calf-level arteries than male controls (n = 111), adjusting for body mass index and Framingham Risk Score (FRS). Of the 63 patients, 23 had a diagnosis of schizophrenia or schizoaffective disorder.30 (The FRS is an estimate of a person’s 10-year cardiovascular risk, calculated using age, sex, total cholesterol, high-density lipoprotein, smoker or not, systolic blood pressure, and whether taking an antihypertensive or not. Compliance was measured using computerized plethysmography). Although not statistically significant, secondary analyses from this data set (n = 77, including men for whom factors for metabolic syndrome were available) showed that calf-level compliance (1.82 vs 2.06 mL) and thigh-level compliance (3.6 vs 4.26 mL; P = .06) were reduced in subjects with schizophrenia, compared with those who had another psychiatric diagnosis.31
- In another study, arterial compliance was significantly reduced in 10 subjects with schizophrenia, compared with 10 healthy controls.32
- Last, reduced total arterial compliance has been shown to be a robust predictor of mortality in older people, compared with reduced local or regional arterial compliance.33
Cytokine abnormalities in arterial compliance
The mechanism by which reduced arterial compliance is associated with cardiovascular pathology is not entirely clear. Arterial compliance is a predictor of cardiovascular disorders independent of hypertension.34 Two studies show that vascular inflammation is associated with reduced arterial compliance.35,36 Reduced arterial compliance is associated with increased angiotensin II activity; increased nicotinamide adenine dinucleotide phosphate oxidase activity; reduced nitric oxide activity; and increased reactive oxygen species.37-39 Angiotensin-II signaling activates transforming growth factor-β, tumor necrosis factor (TNF)-α, interleukin (IL)-1, IL-17, IL-6, and C-reactive protein (CRP)—all of which are associated with reduced arterial compliance.39-46 In addition, high-sensitivity CRP is significantly associated with reduced arterial compliance.47-49
The overlap of cytokine abnormalities linked to schizophrenia and to arterial compliance is depicted in the Figure.
Anti-inflammatory medications and arterial compliance
Evidence suggests that anti-inflammatory medications increase arterial compliance:
- In 10 patients who had coronary artery disease or diabetes, or both, simvastatin (40 mg/d) was administered for 4 months. Arterial compliance improved in all 10 after 2 months of treatment and increased by 34% after 4 months.27
- Evidence also suggests that the use of omega-3 fatty acids was associated with increased arterial compliance in people with dyslipidemia.50
- Last, in people with rheumatoid arthritis, infliximab, a monoclonal antibody against TNF-Symbol Stdα, reduced aortic inflammation; this effect correlated with an increase in aortic compliance.51
Anti-inflammatory medications in schizophrenia
Two studies have yielded notable findings:
- A meta-analysis of 5 randomized controlled trials (RCTs) involving 264 subjects, comprising 4 studies of celecoxib and 1 of acetylsalicylic acid, had an effect size of 0.43 on total symptom severity. Investigators argued that acetylsalicylic acid might have the additional benefit of decreasing the risk of cardiac death in schizophrenia.52
- A review of 26 RCTs examined the efficacy of anti-inflammatory medications on symptom severity in schizophrenia. Acetylsalicylic acid, N-acetylcysteine, and estrogens had an effect size of 0.3, 0.45, and 0.51, respectively.53
Significance of these findings
A revelation that cytokine abnormalities are associated with schizophrenia symptoms and co-occurring somatic illness might offer an important new avenue of therapeutic discovery. On average, people with schizophrenia die 20 to 25 years earlier than the general population; CVD is the major cause of their death. Measuring arterial compliance, a novel noninvasive technology in psychiatry, as well as metabolic parameters, could serve as an early biomarker for assessing risk of CVD.
Implications for psychiatric practice. If inflammation plays a role in CVD in schizophrenia—either independently of factors such as metabolic syndrome, obesity, and smoking, or on the causal pathway linking these factors to reduced arterial compliance and to CVD—treatment with anti-inflammatory medications might reduce the alarming disparity of mortality that accompanies schizophrenia. In short, anti-inflammatory medications may offer a double benefit in this setting. Furthermore, success in this approach could spur clarification of the role of abnormal cytokines in other psychiatric disorders.
At this time, for your patients, consider that anti-inflammatory medications routinely used in medical practice, such as nonsteroidal anti-inflammatory drugs, omega-3 fatty acids, and statins, might alleviate psychiatric symptoms and might reduce cardiovascular mortality in schizophrenia.
Future directions
Perhaps only a limited number of cytokines are common to schizophrenia and reduced arterial compliance. Targeting those specific cytokines might, however, provide the dual benefit in schizophrenia of:
- alleviating symptoms
- reducing the rate of CVD-related mortality.
Studies are warranted to determine the value of (1) anti-inflammatory medications, such as N-acetylcysteine and infliximab and (2) anti-inflammatory combination therapy for this dual purpose. In fact, recruitment of subjects is underway for a study, Anti-Inflammatory Combination Therapy for the Treatment of Schizophrenia, at the University of Maryland (ClinicalTrials.gov Identifier: NCT01514682).
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
1. Potvin S, Stip E, Sepehry AA, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63(8):801-808.
2. Miller BJ, Buckley P, Seabolt W, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663-671.
3. Frydecka D, Misiak B, Pawlak-Adamska E, et al. Interleukin-6: the missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265(6):449-459.
4. Dickerson F, Stallings C, Origoni A, et al. Additive effects of elevated C-reactive protein and exposure to herpes simplex virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134(1):83-88.
5. Dickerson F, Stallings C, Origoni A, et al. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93(1-3):261-265.
6. Asevedo E, Rizzo LB, Gadelha A, et al. Peripheral interleukin-2 level is associated with negative symptoms and cognitive performance in schizophrenia. Physiol Behav. 2014;129:194-198.
7. Miller BJ, Culpepper N, Rapaport MH. C-reactive protein levels in schizophrenia: a review and meta-analysis. Clin Schizophr Relat Psychoses. 2014;7(4):223-230.
8. Micoulaud-Franchi JA, Faugere M, Boyer L, et al. Elevated C-reactive protein is associated with sensory gating deficit in schizophrenia. Schizophr Res. 2015;165(1):94-96.
9. Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophr Res. 1998;30(3):193-208.
10. Landén M, Davidsson P, Gottfries CG, et al. Reduction of the synaptophysin level but normal levels of glycerophospholipids in the gyrus cinguli in schizophrenia. Schizophr Res. 2002;55(1-2):83-98.
11. Smesny S, Milleit B, Nenadic I, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52(4):1314-1327.
12. Smesny S, Kunstmann C, Kunstmann S, et al. Phospholipase A2 activity in first episode schizophrenia: associations with symptom severity and outcome at week 12. World J Biol Psychiatry. 2011;12(8):598-607.
13. Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277-288.
14. Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia, 1987-1996. Schizophr Res. 2002;55(3):277-284.
15. Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291(23):2847-2850.
16. Dickerson FB, Brown CH, Kreyenbuhl JA, et al. Obesity among individuals with serious mental illness. Acta Psychiatr Scand. 2006;113(4):306-313.
17. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(suppl 7):S170-S177.
18. Healy D, Le Noury J, Harris M, et al. Mortality in schizophrenia and related psychoses: data from two cohorts, 1875-1924 and 1994-2010. BMJ Open. 2012;2(5). doi: 10.1136/bmjopen-2012-001810.
19. Newman SC, Bland RC. Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991;36(4):239-245.
20. Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11-53.
21. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
22. Farrar DJ, Bond MG, Riley WA, et al. Anatomic correlates of aortic pulse wave velocity and carotid artery elasticity during atherosclerosis progression and regression in monkeys. Circulation. 1991;83(5):1754-1763.
23. Wada T, Kodaira K, Fujishiro K, et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler Thromb. 1994;14(3):479-482.
24. Herrington DM, Kesler K, Reiber JH, et al. Arterial compliance adds to conventional risk factors for prediction of angiographic coronary artery disease. Am Heart J. 2013;146(4):662-667.
25. Willens HJ, Davis W, Herrington DM, et al. Relationship of peripheral arterial compliance and standard cardiovascular risk factors. Vasc Endovascular Surg. 2003;37(3):197-206.
26. Herrington DM, Brown WV, Mosca L, et al. Relationship between arterial stiffness and subclinical aortic atherosclerosis. Circulation. 2004;110(4):432-437.
27. Saliashvili G, Davis WW, Harris MT, et al. Simvastatin improved arterial compliance in high-risk patients. Vasc Endovascular Surg. 2004;38(6):519-523.
28. Le NA, Brown WV, Davis WW, et al. Comparison of the relation of triglyceride-rich lipoproteins and muscular artery compliance in healthy women versus healthy men. Am J Cardiol. 2005;95(9):1049-1054.
29. Willens HJ, Chirinos JA, Brown WV, et al. Usefulness of arterial compliance in the thigh in predicting exercise capacity in individuals without coronary heart disease. Am J Cardiol. 2005;96(2):306-310.
30. Koola MM, Brown WV, Qualls C, et al. Reduced arterial compliance in patients with psychiatric diagnoses. Schizophr Res. 2012;137(1-3):251-253.
31. Koola MM, Sorkin JD, Fargotstein M, et al. Predictors of calf arterial compliance in male veterans with psychiatric diagnoses. The Primary Care Companion for CNS Disorders. In press.
32. Phillips AA, Warburton DE, Flynn SW, et al. Assessment of arterial stiffness among schizophrenia-spectrum disorders using aortic pulse wave velocity and arterial compliance: a pilot study. Psychiatry Res. 2014;215(1):14-19.
33. Papaioannou TG, Protogerou AD, Stergiopulos N, et al. Total arterial compliance estimated by a novel method and all-cause mortality in the elderly: the PROTEGER study. Age (Dordr). 2014;36(3):9661.
34. Park S, Lakatta EG. Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J. 2012;53(2):258-261.
35. Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107(2):346-354.
36. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932-943.
37. van der Loo B, Labugger R, Skepper JN, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192(12):1731-1744.
38. Csiszar A, Ungvari Z, Edwards JG, et al. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90(11):1159-1166.
39. Wang MC, Tsai WC, Chen JY, et al. Arterial stiffness correlated with cardiac remodelling in patients with chronic kidney disease. Nephrology (Carlton). 2007;12(6):591-597.
40. Belmin J, Bernard C, Corman B, et al. Increased production of tumor necrosis factor and interleukin-6 by arterial wall of aged rats. Am J Physiol. 1995;268(6 pt 2):H2288-2293.
41. Gerli R, Monti D, Bistoni O, et al. Chemokines, sTNF-Rs and sCD30 serum levels in healthy aged people and centenarians. Mech Ageing Dev. 2000;121(1-3):37-46.
42. Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102(18):2165-2168.
43. Torzewski M, Rist C, Mortensen RF, et al. C-reactive protein in the arterial intima: role of C-reactive protein receptor-dependent monocyte recruitment in atherogenesis. Arterioscler Thromb Vasc Biol. 2000;20(9):2094-2099.
44. Venugopal SK, Devaraj S, Yuhanna I, et al. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106(12):1439-1441.
45. Csiszar A, Ungvari Z, Koller A, et al. Aging-induced proinflammatory shift in cytokine expression profile in coronary arteries. FASEB J. 2003;17(9):1183-1185.
46. Spinetti G, Wang M, Monticone R, et al. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24(8):1397-1402.
47. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111-116.
48. Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46(5):1118-1122.
49. Nagano M, Nakamura M, Sato K, et al. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180(1):189-195.
50. Nestel P, Shige H, Pomeroy S, et al. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002;76(2):326-330.
51. Mäki-Petäjä KM, Elkhawad M, Cheriyan J, et al. Anti-tumor necrosis factor-α therapy reduces aortic inflammation and stiffness in patients with rheumatoid arthritis. Circulation. 2012;126(21):2473-2480.
52. Sommer IE, de Witte L, Begemann M, et al. Nonsteroidal anti-inflammatory drugs in schizophrenia: ready for practice or a good start? A meta-analysis. J Clin Psychiatry. 2012;73(4):414-419.
53. Sommer IE, van Westrhenen R, Begemann MJ, et al. Efficacy of anti-inflammatory agents to improve symptoms in patients with schizophrenia: an update. Schizophr Bull. 2014;40(1):181-191.
Is it a 'senior moment' or early dementia? Addressing memory concerns in older patients
Many older patients are concerned about their memory. The “worried well” may come into your office with a list of things they can’t recall, yet they remember each “deficit” quite well. Anticipatory anxiety about one’s own decline is common, and is most often concerned with changes in memory.1,2
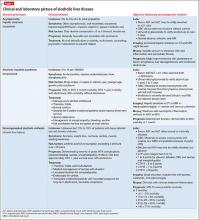
Patients with dementia or early cognitive decline often are oblivious to their cognitive changes, however. Of particular concern is progressive dementia, especially Alzheimer’s disease (AD). Although jokes about “senior moments” are common, concern about AD incurs deep-seated worry. It is essential for clinicians to differentiate normal cognitive changes of aging—particularly those in memory—from early signs of neurodegenerative disease (Table 13).
In this article, we review typical memory changes in persons age >65, and differentiate these from mild cognitive impairment (MCI), an increasingly recognized prodrome of AD. Clinicians armed with knowledge of MCI are able to reassure the worried well, or recommend neuropsychological testing as indicated.
Is memory change inevitable with aging?
Memory loss is a common problem in aging, with variable severity. Research is establishing norms in cognitive functioning through the ninth decade of life.4 Controversy about sampling, measures, and methods abound,5 and drives prolific research on the subject, which is beyond the scope of this article. It has been demonstrated that there are a few “optimally aging” persons who avoid memory decline altogether.5,6 Most researchers and clinicians agree, however, that memory change is pervasive with advancing age.
Memory change follows a gradient with recent memories lost to a greater degree than remote memories (Ribot’s Law).7 Forgetfulness is characteristic of normal aging, and frequently manifests with misplaced objects and short-term lapses. However, this is not pathological—as long as the item or memory is recalled within 24 to 48 hours.
Compared with younger adults, healthy older adults are less efficient at encoding new information. Subsequently, they have more difficulty retrieving data, particularly after a delay. The time needed to learn and use new information increases, which is referred to as processing inefficiency. This influences changes in test performance across all cognitive domains, with decreases in measures of mental processing speed, working memory, and problem-solving.
Many patients who complain about “forgetfulness” are experiencing this normal change. It is not uncommon for a patient to offer a list of things she has forgotten recently, along with the dates and circumstances in which she forgot them. Because she sometimes forgets things, but remembers them later, there likely is nothing to worry about. If reminders—such as her list—help, this too is a good sign, because it shows her resourcefulness in using accommodations. If the patient is managing her normal activities, reassurance is warranted.
Mild cognitive impairment
Since at least 1958,8 clinical observations and research have recognized a prodrome that differentiates cognitive changes predictive of dementia from those that represent typical aging. Several studies and methods have converged toward consensus that MCI is a valid construct for that purpose, with ecological validity and sound predictive value. Clinical value is evident when a patient does not meet criteria for MCI; in this case, the clinician can reassure the worried well with conviction.
Revealing the diagnosis of MCI to patients requires sensitivity and assurance that you will reevaluate the condition annually. Although there is no evidence-based remedy for MCI or means to slow its progression to dementia, data are rapidly accruing regarding the value of lifestyle changes and other nonpharmacologic interventions.9
Recognizing MCI most simply requires 2 criteria:
The patient’s expressed concern about decline in cognitive functioning from a previous level of performance. Alternately, a caretaker’s report is valuable because the patient might lack insight. You are not looking for an inability to perform activities of daily living, which is indicative of frank dementia; rather, you want to determine whether the person’s independence in functional abilities is preserved, although less efficient. Patients might repeatedly report occurrences of new problems, although modest, in some cases. Although problems with memory often are the most frequently reported symptoms, changes can be observed in any cognitive domain. Uncharacteristic inability to understand instructions, frustration with new tasks, and inflexibility are common.
Quantified clinical assessment that the patient’s cognitive decline exceeds norms of his age cohort. Clinicians are already familiar with many of these tests (5-minute recall, clock face drawing, etc.). For MCI, we recommend the Montreal Cognitive Assessment (MoCA), which is specifically designed for MCI.10 It takes only 10 minutes to administer. Multiple versions of the MoCA, and instructions for its administration are available for provider use at www.mocatest.org.
When these criteria are met—a decline in previous functioning and an objective clinical confirmation—referral for neuropsychological testing is recommended. Subtypes of MCI—amnestic and non-amnestic—have been employed to specify the subtype (amnesic) that is most consistent with prodromal AD. However, this dichotomous scheme does not adequately explain or capture the heterogeneity of MCI.11,12
Medical considerations
Just as all domains of cognition are correlated to some degree, the overall health status of a person influences evaluation of memory. Variables, such as fatigue, test anxiety, mood, motivation, visual and auditory acuity, education, language fluency, attention, and pain, affect test performance. In addition, clinician rapport and the manner in which tests are administered must be considered.
Depression can mimic MCI. A depressed patient often has poor expectations of himself and slowed thinking, and might exaggerate symptoms. He might give up on tests or refuse to complete them. His presentation initially could suggest cognitive decline, but depression is revealed when the clinician pays attention to vegetative signs (insomnia, poor appetite) or suicidal ideation. There is growing evidence that subjective complaints of memory loss are more frequently associated with depression than with objective measures of cognitive impairment.13,14
Other treatable conditions can present with cognitive change (the so-called reversible dementias). A deficiency of vitamin B12, thiamine, or folate often is seen because quality of nutrition generally decreases with age. Hyponatremia and dehydration can present with confusion and memory impairment. Other treatable conditions include:
- cerebral vasculitis, which could improve with immune suppressants
- endocrine diseases, which might respond to hormonal or surgical treatment
- normal pressure hydrocephalus, which can be relieved by surgical placement of a shunt.
Take a complete history. What exactly is the nature of the patient or caregiver’s complaint? You need to attempt to engage the patient in conversation, observing his behavior during the evaluation. Is there notable delay in response, difficulty in attention and focus, or in understanding questions?
The content of speech is an indicator of the patient’s information processing. Ask the patient to recite as many animals from the jungle as possible. Most people can come up with at least 15. The person with MCI will likely name fewer animals, but may respond well to cueing, and perform better in recognition (eg, pictures or drawings) vs retrieval. When asked to describe a typical day, the patient may offer a vague, nonchalant response eg, “I keep busy watching the news.” This kind of response may be evidence of confabulation; with further questioning, he is unable to identify current issues of interest.
Substance abuse. It is essential that clinicians recognize that elders are not exempt from alcohol and other drug abuse that affects cognition. Skilled history taking, including attention to non-verbal responses, is indicated. A defensive tone, rolling of eyes, or silent yet affirmative nodding are means by which caregivers offer essential “clues” to the provider.
A quick screening tool for the office is valuable; many clinicians are most familiar with the Mini-Mental State Examination or the Saint Louis University Mental Status Examination, which are known to be sensitive in detecting memory problems and other cognitive defects. As we noted, the MoCA is now recommended for differentiating more subtle changes of MCI.10,15 It is important to remember that common conditions such as an urinary tract infection or trauma after anesthesia for routine procedures such as colonoscopy can cause cognitive impairment. Again, eliciting history from a family member is valuable because the patient may have forgotten vital data.
A good physical exam is important when evaluating for dementia. Look for any neurologic anomaly. Check for disinhibition of primitive reflexes, eg, abnormal grasp or snout response or Babinski sign. Compare the symmetry and strength of deep tendon reflexes. Look for neurologic soft signs. Any pathological reflex response can be an important clue about neurodegeneration or space-occupying lesions. We recall seeing a 62-year-old man whose spouse brought him for evaluation for new-onset reckless driving and marked inattention to personal hygiene that developed over the previous 3 months. On examination, he appeared disheveled and had a dull affect, although disinhibited and careless. His mentation and gait were slowed. He denied distress of any kind. Frontal release signs were noted on exam. An MRI revealed a space-occupying lesion of the frontal lobe measuring 3 cm wide with a thickness of 2 cm, which pathology confirmed as a benign tumor.
Always check for arrhythmia and hypertension. These are significant risk factors for ischemic brain disease, multiple-infarct stroke, or other forms of vascular dementia. A shuffling gait suggests Parkinson’s disease, or even Lewy body dementia, or medication-related conditions, for example, from antipsychotics.
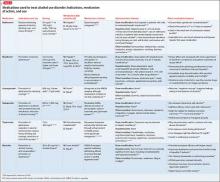
Take a medication history. Many common treatments for anxiety and insomnia can cause symptoms that mimic dementia. Digitalis toxicity results in poor recall and confusion. Combinations of common medicines (antacids, antihistamines, and others) compete for metabolic pathways and lead to altered mental status. Referencing the Beers List16 is valuable; anticholinergics, benzodiazepines, and narcotic analgesics are of special concern. The latter could still be useful for comfort care at the end of life.
It is common for seniors to take a variety of untested and unproven supplements in the hope of preventing or lessening memory problems. In addition to incurring significant costs, the indiscriminate use of supplements poses risks of toxicity, including unintended interactions with prescribed medications. Many older adults do not disclose their use of these supplements to providers because they do not consider them “medicine.”
Labs. The next level of evaluation calls for a basic laboratory workup. Check complete blood count, liver enzymes, thyroid function tests, vitamin D, B12 and folate levels; perform urinalysis and a complete metabolic panel. Look at a general hormone panel; abnormal values could reveal a pituitary adenoma. (In the past 33 years, the first author has found 42 pituitary tumors in the workup of mental status change.)
We use imaging, such as a CT or MRI of the brain, in almost all cases of suspected dementia. Cerebral atrophy, space-occupying lesions, and shifting of the ventricles often correspond with cognitive decline.
Treatment
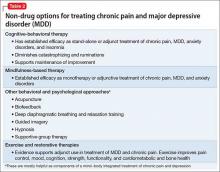
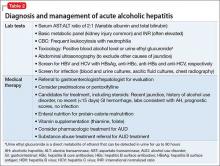
Effective treatment of dementia remains elusive. Other than for the “reversible dementias,” pharmacotherapy has shown less progress than had been expected. Donepezil, galantamine, rivastigmine, and memantine could slow disease progression in some cases. There have been many studies for dementia preventives and treatments. Extensive reviews and meta-analyses, including those of randomized controlled trials17-19 abound for a variety of herbs, supplements, and antioxidants; none have shown compelling results. Table 2 lists Institute of Medicine recommendations supported by evidence that could reduce effects of cognitive aging.20
Recommendations from collaboration between the National Institute on Aging and the Alzheimer’s Association21 state that research should focus on biomarkers, such as neural substrates or genotypes. Indicators of oxidative stress (cytokines) and inflammation (isoprostanes) show promise as measures of brain changes that correspond with increased risk of AD or other dementias.
Summing up
Older adults are a heterogeneous group. Intellectual capacity does not diminish with advancing age. Many elders now exceed expectations for productivity, athletic ability, scientific achievement, and the creative arts. Others live longer with diminished quality of life, their health compromised by progressive neurodegenerative disease.
Age-associated memory change often is exaggerated and feared by older adults and, regrettably, is associated with inevitable functional impairment and is seen as heralding the loss of autonomy. The worried well are anxious, although the stigma associated with cognitive decline may preclude confiding their concerns.
Providers need the tools and acumen to treat patients along an increasingly long continuum of time, including conveyance of evidence-based encouragement toward optimal health and vitality.
1. Serby MJ, Yhap C, Landron EY. A study of herbal remedies for memory complaints. J Neuropsychiatry Clin Neurosci. 2010;22(3):345-347.
2. Jaremka LM, Derry HM, Bornstein R, et al. Omega-3 supplementation and loneliness-related memory problems: secondary analyses of a randomized controlled trial. Psychosom Med. 2014;76(8):650-658.
3. Depp CA, Harmell A, Vania IV. Successful cognitive aging. In: Pardon MC, Bondi MW, eds. Behavioral neurobiology of aging. New York, NY: Springer-Verlag; 2012:35-50.
4. Invik RJ, Malec JF, Smith GE, et al. Mayo’s older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl 1):1-104.
5. Powell DH, Whitla DK. Profiles in cognitive aging. Boston, MA: Harvard University Press; 1994.
6. Negash S, Smith GE, Pankratz SE, et al. Successful aging: definitions and prediction of longevity and conversion to mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(6):581-588.
7. Ribot T. Diseases of memory: an essay in the positive psychology. London, United Kingdom: Kegan Paul Trench; 1882.
8. Kral VA. Neuropsychiatric observations in old peoples home: studies of memory dysfunction in senescence. J Gerontol. 1958;13(2):169-176.
9. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
10. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive assessment. J Am Geriatr Soc. 2005;53(4):695-699.
11. Clark LR, Delano-Wood L, Lisbon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Intl Neuropsychol Soc. 2013;19(6):1-11.
12. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761-767.
13. Bartley M, Bokde AL, Ewers M, et al. Subjective memory complaints in community dwelling older people: the influence of brain and psychopathology. Intl J Geriatr Psychiatry. 2012;27(8):836-843.
14. Chung JC, Man DW. Self-appraised, informant-reported, and objective memory and cognitive function in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(2):187-193.
15. Tsoi KK, Chan JY, Hirai HW, et al. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450-1458.
16. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
17. May BH, Yang AW, Zhang AL, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. 2009;10(2):109-123.
18. Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013;32(1):1-23.
19. Solfrizzi VP, Panza F. Plant-based nutraceutical interventions against cognitive impairment and dementia: meta-analytic evidence of efficacy of a standardized Gingko biloba extract. J Alzheimers Dis. 2015;43(2):605-611.
20. Institute of Medicine. Cognitive aging: progress in understanding and opportunities for action. Washington, DC: National Academies Press; 2015.
21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
Many older patients are concerned about their memory. The “worried well” may come into your office with a list of things they can’t recall, yet they remember each “deficit” quite well. Anticipatory anxiety about one’s own decline is common, and is most often concerned with changes in memory.1,2
Patients with dementia or early cognitive decline often are oblivious to their cognitive changes, however. Of particular concern is progressive dementia, especially Alzheimer’s disease (AD). Although jokes about “senior moments” are common, concern about AD incurs deep-seated worry. It is essential for clinicians to differentiate normal cognitive changes of aging—particularly those in memory—from early signs of neurodegenerative disease (Table 13).
In this article, we review typical memory changes in persons age >65, and differentiate these from mild cognitive impairment (MCI), an increasingly recognized prodrome of AD. Clinicians armed with knowledge of MCI are able to reassure the worried well, or recommend neuropsychological testing as indicated.
Is memory change inevitable with aging?
Memory loss is a common problem in aging, with variable severity. Research is establishing norms in cognitive functioning through the ninth decade of life.4 Controversy about sampling, measures, and methods abound,5 and drives prolific research on the subject, which is beyond the scope of this article. It has been demonstrated that there are a few “optimally aging” persons who avoid memory decline altogether.5,6 Most researchers and clinicians agree, however, that memory change is pervasive with advancing age.
Memory change follows a gradient with recent memories lost to a greater degree than remote memories (Ribot’s Law).7 Forgetfulness is characteristic of normal aging, and frequently manifests with misplaced objects and short-term lapses. However, this is not pathological—as long as the item or memory is recalled within 24 to 48 hours.
Compared with younger adults, healthy older adults are less efficient at encoding new information. Subsequently, they have more difficulty retrieving data, particularly after a delay. The time needed to learn and use new information increases, which is referred to as processing inefficiency. This influences changes in test performance across all cognitive domains, with decreases in measures of mental processing speed, working memory, and problem-solving.
Many patients who complain about “forgetfulness” are experiencing this normal change. It is not uncommon for a patient to offer a list of things she has forgotten recently, along with the dates and circumstances in which she forgot them. Because she sometimes forgets things, but remembers them later, there likely is nothing to worry about. If reminders—such as her list—help, this too is a good sign, because it shows her resourcefulness in using accommodations. If the patient is managing her normal activities, reassurance is warranted.
Mild cognitive impairment
Since at least 1958,8 clinical observations and research have recognized a prodrome that differentiates cognitive changes predictive of dementia from those that represent typical aging. Several studies and methods have converged toward consensus that MCI is a valid construct for that purpose, with ecological validity and sound predictive value. Clinical value is evident when a patient does not meet criteria for MCI; in this case, the clinician can reassure the worried well with conviction.
Revealing the diagnosis of MCI to patients requires sensitivity and assurance that you will reevaluate the condition annually. Although there is no evidence-based remedy for MCI or means to slow its progression to dementia, data are rapidly accruing regarding the value of lifestyle changes and other nonpharmacologic interventions.9
Recognizing MCI most simply requires 2 criteria:
The patient’s expressed concern about decline in cognitive functioning from a previous level of performance. Alternately, a caretaker’s report is valuable because the patient might lack insight. You are not looking for an inability to perform activities of daily living, which is indicative of frank dementia; rather, you want to determine whether the person’s independence in functional abilities is preserved, although less efficient. Patients might repeatedly report occurrences of new problems, although modest, in some cases. Although problems with memory often are the most frequently reported symptoms, changes can be observed in any cognitive domain. Uncharacteristic inability to understand instructions, frustration with new tasks, and inflexibility are common.
Quantified clinical assessment that the patient’s cognitive decline exceeds norms of his age cohort. Clinicians are already familiar with many of these tests (5-minute recall, clock face drawing, etc.). For MCI, we recommend the Montreal Cognitive Assessment (MoCA), which is specifically designed for MCI.10 It takes only 10 minutes to administer. Multiple versions of the MoCA, and instructions for its administration are available for provider use at www.mocatest.org.
When these criteria are met—a decline in previous functioning and an objective clinical confirmation—referral for neuropsychological testing is recommended. Subtypes of MCI—amnestic and non-amnestic—have been employed to specify the subtype (amnesic) that is most consistent with prodromal AD. However, this dichotomous scheme does not adequately explain or capture the heterogeneity of MCI.11,12
Medical considerations
Just as all domains of cognition are correlated to some degree, the overall health status of a person influences evaluation of memory. Variables, such as fatigue, test anxiety, mood, motivation, visual and auditory acuity, education, language fluency, attention, and pain, affect test performance. In addition, clinician rapport and the manner in which tests are administered must be considered.
Depression can mimic MCI. A depressed patient often has poor expectations of himself and slowed thinking, and might exaggerate symptoms. He might give up on tests or refuse to complete them. His presentation initially could suggest cognitive decline, but depression is revealed when the clinician pays attention to vegetative signs (insomnia, poor appetite) or suicidal ideation. There is growing evidence that subjective complaints of memory loss are more frequently associated with depression than with objective measures of cognitive impairment.13,14
Other treatable conditions can present with cognitive change (the so-called reversible dementias). A deficiency of vitamin B12, thiamine, or folate often is seen because quality of nutrition generally decreases with age. Hyponatremia and dehydration can present with confusion and memory impairment. Other treatable conditions include:
- cerebral vasculitis, which could improve with immune suppressants
- endocrine diseases, which might respond to hormonal or surgical treatment
- normal pressure hydrocephalus, which can be relieved by surgical placement of a shunt.
Take a complete history. What exactly is the nature of the patient or caregiver’s complaint? You need to attempt to engage the patient in conversation, observing his behavior during the evaluation. Is there notable delay in response, difficulty in attention and focus, or in understanding questions?
The content of speech is an indicator of the patient’s information processing. Ask the patient to recite as many animals from the jungle as possible. Most people can come up with at least 15. The person with MCI will likely name fewer animals, but may respond well to cueing, and perform better in recognition (eg, pictures or drawings) vs retrieval. When asked to describe a typical day, the patient may offer a vague, nonchalant response eg, “I keep busy watching the news.” This kind of response may be evidence of confabulation; with further questioning, he is unable to identify current issues of interest.
Substance abuse. It is essential that clinicians recognize that elders are not exempt from alcohol and other drug abuse that affects cognition. Skilled history taking, including attention to non-verbal responses, is indicated. A defensive tone, rolling of eyes, or silent yet affirmative nodding are means by which caregivers offer essential “clues” to the provider.
A quick screening tool for the office is valuable; many clinicians are most familiar with the Mini-Mental State Examination or the Saint Louis University Mental Status Examination, which are known to be sensitive in detecting memory problems and other cognitive defects. As we noted, the MoCA is now recommended for differentiating more subtle changes of MCI.10,15 It is important to remember that common conditions such as an urinary tract infection or trauma after anesthesia for routine procedures such as colonoscopy can cause cognitive impairment. Again, eliciting history from a family member is valuable because the patient may have forgotten vital data.
A good physical exam is important when evaluating for dementia. Look for any neurologic anomaly. Check for disinhibition of primitive reflexes, eg, abnormal grasp or snout response or Babinski sign. Compare the symmetry and strength of deep tendon reflexes. Look for neurologic soft signs. Any pathological reflex response can be an important clue about neurodegeneration or space-occupying lesions. We recall seeing a 62-year-old man whose spouse brought him for evaluation for new-onset reckless driving and marked inattention to personal hygiene that developed over the previous 3 months. On examination, he appeared disheveled and had a dull affect, although disinhibited and careless. His mentation and gait were slowed. He denied distress of any kind. Frontal release signs were noted on exam. An MRI revealed a space-occupying lesion of the frontal lobe measuring 3 cm wide with a thickness of 2 cm, which pathology confirmed as a benign tumor.
Always check for arrhythmia and hypertension. These are significant risk factors for ischemic brain disease, multiple-infarct stroke, or other forms of vascular dementia. A shuffling gait suggests Parkinson’s disease, or even Lewy body dementia, or medication-related conditions, for example, from antipsychotics.
Take a medication history. Many common treatments for anxiety and insomnia can cause symptoms that mimic dementia. Digitalis toxicity results in poor recall and confusion. Combinations of common medicines (antacids, antihistamines, and others) compete for metabolic pathways and lead to altered mental status. Referencing the Beers List16 is valuable; anticholinergics, benzodiazepines, and narcotic analgesics are of special concern. The latter could still be useful for comfort care at the end of life.
It is common for seniors to take a variety of untested and unproven supplements in the hope of preventing or lessening memory problems. In addition to incurring significant costs, the indiscriminate use of supplements poses risks of toxicity, including unintended interactions with prescribed medications. Many older adults do not disclose their use of these supplements to providers because they do not consider them “medicine.”
Labs. The next level of evaluation calls for a basic laboratory workup. Check complete blood count, liver enzymes, thyroid function tests, vitamin D, B12 and folate levels; perform urinalysis and a complete metabolic panel. Look at a general hormone panel; abnormal values could reveal a pituitary adenoma. (In the past 33 years, the first author has found 42 pituitary tumors in the workup of mental status change.)
We use imaging, such as a CT or MRI of the brain, in almost all cases of suspected dementia. Cerebral atrophy, space-occupying lesions, and shifting of the ventricles often correspond with cognitive decline.
Treatment
Effective treatment of dementia remains elusive. Other than for the “reversible dementias,” pharmacotherapy has shown less progress than had been expected. Donepezil, galantamine, rivastigmine, and memantine could slow disease progression in some cases. There have been many studies for dementia preventives and treatments. Extensive reviews and meta-analyses, including those of randomized controlled trials17-19 abound for a variety of herbs, supplements, and antioxidants; none have shown compelling results. Table 2 lists Institute of Medicine recommendations supported by evidence that could reduce effects of cognitive aging.20
Recommendations from collaboration between the National Institute on Aging and the Alzheimer’s Association21 state that research should focus on biomarkers, such as neural substrates or genotypes. Indicators of oxidative stress (cytokines) and inflammation (isoprostanes) show promise as measures of brain changes that correspond with increased risk of AD or other dementias.
Summing up
Older adults are a heterogeneous group. Intellectual capacity does not diminish with advancing age. Many elders now exceed expectations for productivity, athletic ability, scientific achievement, and the creative arts. Others live longer with diminished quality of life, their health compromised by progressive neurodegenerative disease.
Age-associated memory change often is exaggerated and feared by older adults and, regrettably, is associated with inevitable functional impairment and is seen as heralding the loss of autonomy. The worried well are anxious, although the stigma associated with cognitive decline may preclude confiding their concerns.
Providers need the tools and acumen to treat patients along an increasingly long continuum of time, including conveyance of evidence-based encouragement toward optimal health and vitality.
Many older patients are concerned about their memory. The “worried well” may come into your office with a list of things they can’t recall, yet they remember each “deficit” quite well. Anticipatory anxiety about one’s own decline is common, and is most often concerned with changes in memory.1,2
Patients with dementia or early cognitive decline often are oblivious to their cognitive changes, however. Of particular concern is progressive dementia, especially Alzheimer’s disease (AD). Although jokes about “senior moments” are common, concern about AD incurs deep-seated worry. It is essential for clinicians to differentiate normal cognitive changes of aging—particularly those in memory—from early signs of neurodegenerative disease (Table 13).
In this article, we review typical memory changes in persons age >65, and differentiate these from mild cognitive impairment (MCI), an increasingly recognized prodrome of AD. Clinicians armed with knowledge of MCI are able to reassure the worried well, or recommend neuropsychological testing as indicated.
Is memory change inevitable with aging?
Memory loss is a common problem in aging, with variable severity. Research is establishing norms in cognitive functioning through the ninth decade of life.4 Controversy about sampling, measures, and methods abound,5 and drives prolific research on the subject, which is beyond the scope of this article. It has been demonstrated that there are a few “optimally aging” persons who avoid memory decline altogether.5,6 Most researchers and clinicians agree, however, that memory change is pervasive with advancing age.
Memory change follows a gradient with recent memories lost to a greater degree than remote memories (Ribot’s Law).7 Forgetfulness is characteristic of normal aging, and frequently manifests with misplaced objects and short-term lapses. However, this is not pathological—as long as the item or memory is recalled within 24 to 48 hours.
Compared with younger adults, healthy older adults are less efficient at encoding new information. Subsequently, they have more difficulty retrieving data, particularly after a delay. The time needed to learn and use new information increases, which is referred to as processing inefficiency. This influences changes in test performance across all cognitive domains, with decreases in measures of mental processing speed, working memory, and problem-solving.
Many patients who complain about “forgetfulness” are experiencing this normal change. It is not uncommon for a patient to offer a list of things she has forgotten recently, along with the dates and circumstances in which she forgot them. Because she sometimes forgets things, but remembers them later, there likely is nothing to worry about. If reminders—such as her list—help, this too is a good sign, because it shows her resourcefulness in using accommodations. If the patient is managing her normal activities, reassurance is warranted.
Mild cognitive impairment
Since at least 1958,8 clinical observations and research have recognized a prodrome that differentiates cognitive changes predictive of dementia from those that represent typical aging. Several studies and methods have converged toward consensus that MCI is a valid construct for that purpose, with ecological validity and sound predictive value. Clinical value is evident when a patient does not meet criteria for MCI; in this case, the clinician can reassure the worried well with conviction.
Revealing the diagnosis of MCI to patients requires sensitivity and assurance that you will reevaluate the condition annually. Although there is no evidence-based remedy for MCI or means to slow its progression to dementia, data are rapidly accruing regarding the value of lifestyle changes and other nonpharmacologic interventions.9
Recognizing MCI most simply requires 2 criteria:
The patient’s expressed concern about decline in cognitive functioning from a previous level of performance. Alternately, a caretaker’s report is valuable because the patient might lack insight. You are not looking for an inability to perform activities of daily living, which is indicative of frank dementia; rather, you want to determine whether the person’s independence in functional abilities is preserved, although less efficient. Patients might repeatedly report occurrences of new problems, although modest, in some cases. Although problems with memory often are the most frequently reported symptoms, changes can be observed in any cognitive domain. Uncharacteristic inability to understand instructions, frustration with new tasks, and inflexibility are common.
Quantified clinical assessment that the patient’s cognitive decline exceeds norms of his age cohort. Clinicians are already familiar with many of these tests (5-minute recall, clock face drawing, etc.). For MCI, we recommend the Montreal Cognitive Assessment (MoCA), which is specifically designed for MCI.10 It takes only 10 minutes to administer. Multiple versions of the MoCA, and instructions for its administration are available for provider use at www.mocatest.org.
When these criteria are met—a decline in previous functioning and an objective clinical confirmation—referral for neuropsychological testing is recommended. Subtypes of MCI—amnestic and non-amnestic—have been employed to specify the subtype (amnesic) that is most consistent with prodromal AD. However, this dichotomous scheme does not adequately explain or capture the heterogeneity of MCI.11,12
Medical considerations
Just as all domains of cognition are correlated to some degree, the overall health status of a person influences evaluation of memory. Variables, such as fatigue, test anxiety, mood, motivation, visual and auditory acuity, education, language fluency, attention, and pain, affect test performance. In addition, clinician rapport and the manner in which tests are administered must be considered.
Depression can mimic MCI. A depressed patient often has poor expectations of himself and slowed thinking, and might exaggerate symptoms. He might give up on tests or refuse to complete them. His presentation initially could suggest cognitive decline, but depression is revealed when the clinician pays attention to vegetative signs (insomnia, poor appetite) or suicidal ideation. There is growing evidence that subjective complaints of memory loss are more frequently associated with depression than with objective measures of cognitive impairment.13,14
Other treatable conditions can present with cognitive change (the so-called reversible dementias). A deficiency of vitamin B12, thiamine, or folate often is seen because quality of nutrition generally decreases with age. Hyponatremia and dehydration can present with confusion and memory impairment. Other treatable conditions include:
- cerebral vasculitis, which could improve with immune suppressants
- endocrine diseases, which might respond to hormonal or surgical treatment
- normal pressure hydrocephalus, which can be relieved by surgical placement of a shunt.
Take a complete history. What exactly is the nature of the patient or caregiver’s complaint? You need to attempt to engage the patient in conversation, observing his behavior during the evaluation. Is there notable delay in response, difficulty in attention and focus, or in understanding questions?
The content of speech is an indicator of the patient’s information processing. Ask the patient to recite as many animals from the jungle as possible. Most people can come up with at least 15. The person with MCI will likely name fewer animals, but may respond well to cueing, and perform better in recognition (eg, pictures or drawings) vs retrieval. When asked to describe a typical day, the patient may offer a vague, nonchalant response eg, “I keep busy watching the news.” This kind of response may be evidence of confabulation; with further questioning, he is unable to identify current issues of interest.
Substance abuse. It is essential that clinicians recognize that elders are not exempt from alcohol and other drug abuse that affects cognition. Skilled history taking, including attention to non-verbal responses, is indicated. A defensive tone, rolling of eyes, or silent yet affirmative nodding are means by which caregivers offer essential “clues” to the provider.
A quick screening tool for the office is valuable; many clinicians are most familiar with the Mini-Mental State Examination or the Saint Louis University Mental Status Examination, which are known to be sensitive in detecting memory problems and other cognitive defects. As we noted, the MoCA is now recommended for differentiating more subtle changes of MCI.10,15 It is important to remember that common conditions such as an urinary tract infection or trauma after anesthesia for routine procedures such as colonoscopy can cause cognitive impairment. Again, eliciting history from a family member is valuable because the patient may have forgotten vital data.
A good physical exam is important when evaluating for dementia. Look for any neurologic anomaly. Check for disinhibition of primitive reflexes, eg, abnormal grasp or snout response or Babinski sign. Compare the symmetry and strength of deep tendon reflexes. Look for neurologic soft signs. Any pathological reflex response can be an important clue about neurodegeneration or space-occupying lesions. We recall seeing a 62-year-old man whose spouse brought him for evaluation for new-onset reckless driving and marked inattention to personal hygiene that developed over the previous 3 months. On examination, he appeared disheveled and had a dull affect, although disinhibited and careless. His mentation and gait were slowed. He denied distress of any kind. Frontal release signs were noted on exam. An MRI revealed a space-occupying lesion of the frontal lobe measuring 3 cm wide with a thickness of 2 cm, which pathology confirmed as a benign tumor.
Always check for arrhythmia and hypertension. These are significant risk factors for ischemic brain disease, multiple-infarct stroke, or other forms of vascular dementia. A shuffling gait suggests Parkinson’s disease, or even Lewy body dementia, or medication-related conditions, for example, from antipsychotics.
Take a medication history. Many common treatments for anxiety and insomnia can cause symptoms that mimic dementia. Digitalis toxicity results in poor recall and confusion. Combinations of common medicines (antacids, antihistamines, and others) compete for metabolic pathways and lead to altered mental status. Referencing the Beers List16 is valuable; anticholinergics, benzodiazepines, and narcotic analgesics are of special concern. The latter could still be useful for comfort care at the end of life.
It is common for seniors to take a variety of untested and unproven supplements in the hope of preventing or lessening memory problems. In addition to incurring significant costs, the indiscriminate use of supplements poses risks of toxicity, including unintended interactions with prescribed medications. Many older adults do not disclose their use of these supplements to providers because they do not consider them “medicine.”
Labs. The next level of evaluation calls for a basic laboratory workup. Check complete blood count, liver enzymes, thyroid function tests, vitamin D, B12 and folate levels; perform urinalysis and a complete metabolic panel. Look at a general hormone panel; abnormal values could reveal a pituitary adenoma. (In the past 33 years, the first author has found 42 pituitary tumors in the workup of mental status change.)
We use imaging, such as a CT or MRI of the brain, in almost all cases of suspected dementia. Cerebral atrophy, space-occupying lesions, and shifting of the ventricles often correspond with cognitive decline.
Treatment
Effective treatment of dementia remains elusive. Other than for the “reversible dementias,” pharmacotherapy has shown less progress than had been expected. Donepezil, galantamine, rivastigmine, and memantine could slow disease progression in some cases. There have been many studies for dementia preventives and treatments. Extensive reviews and meta-analyses, including those of randomized controlled trials17-19 abound for a variety of herbs, supplements, and antioxidants; none have shown compelling results. Table 2 lists Institute of Medicine recommendations supported by evidence that could reduce effects of cognitive aging.20
Recommendations from collaboration between the National Institute on Aging and the Alzheimer’s Association21 state that research should focus on biomarkers, such as neural substrates or genotypes. Indicators of oxidative stress (cytokines) and inflammation (isoprostanes) show promise as measures of brain changes that correspond with increased risk of AD or other dementias.
Summing up
Older adults are a heterogeneous group. Intellectual capacity does not diminish with advancing age. Many elders now exceed expectations for productivity, athletic ability, scientific achievement, and the creative arts. Others live longer with diminished quality of life, their health compromised by progressive neurodegenerative disease.
Age-associated memory change often is exaggerated and feared by older adults and, regrettably, is associated with inevitable functional impairment and is seen as heralding the loss of autonomy. The worried well are anxious, although the stigma associated with cognitive decline may preclude confiding their concerns.
Providers need the tools and acumen to treat patients along an increasingly long continuum of time, including conveyance of evidence-based encouragement toward optimal health and vitality.
1. Serby MJ, Yhap C, Landron EY. A study of herbal remedies for memory complaints. J Neuropsychiatry Clin Neurosci. 2010;22(3):345-347.
2. Jaremka LM, Derry HM, Bornstein R, et al. Omega-3 supplementation and loneliness-related memory problems: secondary analyses of a randomized controlled trial. Psychosom Med. 2014;76(8):650-658.
3. Depp CA, Harmell A, Vania IV. Successful cognitive aging. In: Pardon MC, Bondi MW, eds. Behavioral neurobiology of aging. New York, NY: Springer-Verlag; 2012:35-50.
4. Invik RJ, Malec JF, Smith GE, et al. Mayo’s older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl 1):1-104.
5. Powell DH, Whitla DK. Profiles in cognitive aging. Boston, MA: Harvard University Press; 1994.
6. Negash S, Smith GE, Pankratz SE, et al. Successful aging: definitions and prediction of longevity and conversion to mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(6):581-588.
7. Ribot T. Diseases of memory: an essay in the positive psychology. London, United Kingdom: Kegan Paul Trench; 1882.
8. Kral VA. Neuropsychiatric observations in old peoples home: studies of memory dysfunction in senescence. J Gerontol. 1958;13(2):169-176.
9. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
10. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive assessment. J Am Geriatr Soc. 2005;53(4):695-699.
11. Clark LR, Delano-Wood L, Lisbon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Intl Neuropsychol Soc. 2013;19(6):1-11.
12. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761-767.
13. Bartley M, Bokde AL, Ewers M, et al. Subjective memory complaints in community dwelling older people: the influence of brain and psychopathology. Intl J Geriatr Psychiatry. 2012;27(8):836-843.
14. Chung JC, Man DW. Self-appraised, informant-reported, and objective memory and cognitive function in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(2):187-193.
15. Tsoi KK, Chan JY, Hirai HW, et al. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450-1458.
16. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
17. May BH, Yang AW, Zhang AL, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. 2009;10(2):109-123.
18. Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013;32(1):1-23.
19. Solfrizzi VP, Panza F. Plant-based nutraceutical interventions against cognitive impairment and dementia: meta-analytic evidence of efficacy of a standardized Gingko biloba extract. J Alzheimers Dis. 2015;43(2):605-611.
20. Institute of Medicine. Cognitive aging: progress in understanding and opportunities for action. Washington, DC: National Academies Press; 2015.
21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
1. Serby MJ, Yhap C, Landron EY. A study of herbal remedies for memory complaints. J Neuropsychiatry Clin Neurosci. 2010;22(3):345-347.
2. Jaremka LM, Derry HM, Bornstein R, et al. Omega-3 supplementation and loneliness-related memory problems: secondary analyses of a randomized controlled trial. Psychosom Med. 2014;76(8):650-658.
3. Depp CA, Harmell A, Vania IV. Successful cognitive aging. In: Pardon MC, Bondi MW, eds. Behavioral neurobiology of aging. New York, NY: Springer-Verlag; 2012:35-50.
4. Invik RJ, Malec JF, Smith GE, et al. Mayo’s older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(suppl 1):1-104.
5. Powell DH, Whitla DK. Profiles in cognitive aging. Boston, MA: Harvard University Press; 1994.
6. Negash S, Smith GE, Pankratz SE, et al. Successful aging: definitions and prediction of longevity and conversion to mild cognitive impairment. Am J Geriatr Psychiatry. 2011;19(6):581-588.
7. Ribot T. Diseases of memory: an essay in the positive psychology. London, United Kingdom: Kegan Paul Trench; 1882.
8. Kral VA. Neuropsychiatric observations in old peoples home: studies of memory dysfunction in senescence. J Gerontol. 1958;13(2):169-176.
9. Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308(19):2020-2029.
10. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive assessment. J Am Geriatr Soc. 2005;53(4):695-699.
11. Clark LR, Delano-Wood L, Lisbon DJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Intl Neuropsychol Soc. 2013;19(6):1-11.
12. Ganguli M, Snitz BE, Saxton JA, et al. Outcomes of mild cognitive impairment by definition: a population study. Arch Neurol. 2011;68(6):761-767.
13. Bartley M, Bokde AL, Ewers M, et al. Subjective memory complaints in community dwelling older people: the influence of brain and psychopathology. Intl J Geriatr Psychiatry. 2012;27(8):836-843.
14. Chung JC, Man DW. Self-appraised, informant-reported, and objective memory and cognitive function in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(2):187-193.
15. Tsoi KK, Chan JY, Hirai HW, et al. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med. 2015;175(9):1450-1458.
16. American Geriatrics Society 2012 Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2012;60(4):616-631.
17. May BH, Yang AW, Zhang AL, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. 2009;10(2):109-123.
18. Loef M, Walach H. The omega-6/omega-3 ratio and dementia or cognitive decline: a systematic review on human studies and biological evidence. J Nutr Gerontol Geriatr. 2013;32(1):1-23.
19. Solfrizzi VP, Panza F. Plant-based nutraceutical interventions against cognitive impairment and dementia: meta-analytic evidence of efficacy of a standardized Gingko biloba extract. J Alzheimers Dis. 2015;43(2):605-611.
20. Institute of Medicine. Cognitive aging: progress in understanding and opportunities for action. Washington, DC: National Academies Press; 2015.
21. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270-279.
Technology offers tools for ensuring adherence to medical therapy
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
Nonadherence to medical therapy is a widespread and complex problem that is a significant variable in the treatment of psychiatric illness and in patients’ prognosis. More than 50% of people who have a chronic illness struggle to comply with their medication regimen—for many reasons.1
Many variables predict poor adherence, so it cannot be expected that a single solution will solve the problem entirely.2 Novel adherence technologies are available, as we discuss in this article, and more are in development.
What is nonadherence to medical therapy?
Nonadherence can be defined primarily as not taking prescribed medication in the recommended dosage or frequency, or not taking prescribed medication at all.3 Nonadherence can result in an increased risk of relapse, hospitalization, poor therapeutic response, and delayed remission and recovery.
Secondarily, non-attendance or irregular attendance at appointments with providers is a form of nonadherence that can have a negative impact on treatment outcomes.4
Why is medical adherence important in psychiatry?
Medication nonadherence has major consequences for psychiatric patients5 and for the greater health care system; it is estimated that, in the United States, the cost of nonadherence is as high as $300 billion a year.6 In psychiatry, the rate of nonadherence to medical therapy has been reported to be 11% to 80% of patients with schizophrenia; 12% to 64% with bipolar disorders; and 30% to 60% with depression.7-9 These surprising statistics make it imperative to design treatment strategies that include an effective patient-centric medication adherence plan, based on diagnosis, patient need, education, and support.
Why are patients nonadherent?
Many variables lead to patient nonadherence (Figure 1). The most common reason is that patients simply forget to take their medication.10 Among psychiatric patients, other reasons are:
- lack of insight
- negative emotional reaction to taking medication11
- feeling better and no longer believing that the medication is needed12,13
- distress associated with side effects14,15
- high cost of medication15
- patient’s perception that medication won’t be effective16,17
- concern about substance abuse18
- fear of dependency19
- complicated dosing regimen20
- general lack of motivation.21
Emotional barriers to medication nonadherence are an underestimated area that can benefit greatly from the expertise and understanding of psychiatrists. These barriers include a sense of losing control, self-stigmatization, denial, poor insight, and beliefs about illness and medications.
Additional patient variables that contribute to nonadherence include:
- suboptimal health literacy
- stigma and shame about the need for psychiatric treatment
- lack of patient involvement in treatment decision-making.
Who is responsible for adherence?
Adherence to medical therapy is not the patient’s responsibility, exclusively. Rather, it is a collection of complex components that generally includes physicians and the health care system. Because barriers to medication adherence are complex and varied, solutions to improve adherence must be multifaceted.
Providers. Patients’ care often is managed by multiple physicians, which can lead to communication lapses about complicated drug regimens and potential adverse effects. To assist patients in adhering to their medication regimen, physicians should recognize, and acknowledge to the patient, that many psychiatric patients have difficulty taking their medications and provide advice and information in how to address this problem.
Families. Likewise, it is important to educate patients and their family about the need for medication—helping the patient see that it is his (her) choice and, indeed, his direct responsibility to take his medication and improve his health. The risk–benefit balance of treatment should be explained to the patient and his family, as well as the nature of the psychiatric diagnosis and how effective patient–physician collaboration can help him function and adhere to his medication regimen in a consistent, reliable manner.
The larger system. Health care systems can contribute to medication adherence by reducing time constraints on visits to providers, to allow time to discuss all aspects of medication adherence. Limited visits in the clinic means physicians are not able to (1) spend adequate time discussing the medication regimen to ensure full patient comprehension and (2) conduct an assessment of medication-taking behaviors. Team-based approaches could improve efficiency, patient understanding, adherence, and early detection of adherence issues.22,23
Strategies such as additional clinic visits and reminder calls to discuss adherence carry a cost, but their long-term advantage is that, if patients understand how to better adhere to their medication regimens, their actions will have a positive impact on their health care costs and outcomes and on the wider health economy—as a result of reduced hospital admissions and reduced need to care for patients whose condition deteriorates because of nonadherence. It is imperative that we build strong relationships with other providers to show that we are committed to building supportive, effective adherence support programs that focus on the individual patient’s needs.
What is the available technology?
There is no standard way to measure nonadherence. The most common, and simplest, measure—asking the patient—is unreliable and severely overestimates adherence.
Direct measures of adherence include observing the patient taking his medications and testing for the concentration of those medications in blood or urine. Indirect adherence assessment methods, such as pill counts, a medication diary, self-report, clinician ratings, pharmacy chart review, and electronic devices that monitor the opening of a lid or tablet strip, have all been used; yet reviews of those methods have shown less than favorable results.6
Pre-packaged pill packs have helped some patients with a simple method for medication management.
Electronic monitoring, using a medication vial cap device (Figure 2) that electronically records the date and time of bottle opening, has become common in general medicine and among patients with schizophrenia.6,13,24-26 Diaz et al24 reported that electronic monitoring detected a greater nonadherence rate (57%) than what prescribers reported (7%) or patients self-reported (5%)—demonstrating that prescribers and patients grossly overestimate adherence. In another study that looked at electronic monitoring, researchers reported that adherence was much higher in depressed youth (87%)27 than what had been seen in adults (67%) in a similar study.13
The downside to pill packs and electronic monitoring? There is no guarantee the patient has actually taken the medication despite the data reported by the system.
Event marker-signaling devices. Novel technologies have been developed to measure adherence:
Proteus Digital Health feedback system (www.proteus.com) requires that patients ingest a tablet containing a tiny, dietary mineral-based “ingestible event marker.” Upon contact with gastric fluid electrolytes, the event marker emits a unique signal that is transmitted through bodily tissue to a small receiver in a patch worn on the torso. The receiver then transmits a signal to a cellular phone, indicating the time and date when the medication was ingested (Figure 3).
A 4-week pilot study28 found that the ingestible event marker is feasible and acceptable to patients: 27 of 28 participants (96%) completed the study, with a mean adherence rate of 74%. Although the system identifies ingestible sensors with high accuracy and is easily tolerated by patients, the pilot study was brief; a longer duration of adherence while wearing the patch needs to be studied.
Breath analysis, facial recognition. Even directly observing ingestion of a medication can be problematic: Some patients don’t swallow the medication and spit it out later. One way around that subterfuge is to consider using other advanced medication adherence solutions that are breath-based or use facial recognition technology and confirm ingestion.
Xhale SMART (www.xhale.com/smart) is a handheld device that generates a reminder to the patient to take his medication; afterward, he (she) must blow into the device so that ingestion of the medication is detected (Figure 4). The medication has breath-detectable adherence markers already incorporated. The adherence marker then is released into the stomach and small intestine, where the adherence marker metabolite is transported through the bloodstream into the lungs and exhaled. The patient must breathe into a breath analysis device, which measures medication ingestion compared with a baseline breath print.
Several articles in the literature have reported the accuracy of this device in detecting the ingested metabolite in every participant, without adverse effects.29,30 Clinical data on the use of the breath-based detector is not available to the public at this time.
AiCure (www.aicure.com) is a facial recognition-based technology platform that can work through any smartphone. The device is powered by artificial intelligence software and motion-sensing technology that can detect, in real time, whether the patient is taking the medication as prescribed. Patients who take an incorrect dose, or who do not use the software, are automatically flagged for immediate follow-up. This technology enables real-time intervention by a provider with the nonadherent patient.
An important note: These innovative technological advances are tools that can help clinicians manage an important aspect of treatment, but they do not show the entire picture: The physician−patient relationship and the therapeutic alliance are key to optimal treatment adherence.
Engage and empower the patient
Novel adherence technologies are, as we’ve described, available, and more are being developed. Incorporating these technologies into clinical care requires continued input and support from clinicians and patients. Digital and mobile health applications are multi-beneficial: They can empower patients to self-manage medication regimens and appointments while they also receive social and psychological information and support as needed. Understanding one’s own illness can, ultimately, improve outcomes and significantly reduce health care costs.
Patient empowerment is key. The physician is an important influencer in this regard.
The role of the physician must not be undervalued in maintaining adherence to therapy; she (he) plays a vital role in continued patient engagement and behavioral training. Integrating physician-led oversight, patient education, and commitment, and novel digital mobile adherence technologies will help deliver better outcomes.
The push to engage. A “one size fits all” approach to maintaining adherence won’t be effective. We need to better understand the individual patient’s underlying cause(s) for nonadherence, then to tailor a solution to influence and change that behavior. One way to do this is by interacting and engaging more directly (and in a digital manner) with patients to monitor adherence.
A recent example of the move toward direct patient engagement is the agreement entered by Otsuka Pharmaceuticals and Proteus Digital Health to develop novel digital health products. The FDA has accepted for review the combination product of Otsuka’s brand of aripiprazole and Proteus’s ingestible sensor. If the product is approved by the FDA, physicians will be able to prescribe aripiprazole with the ingestible sensor embedded in the tablet and then measure medication adherence and other patient physiologic metrics (eg, activity, rest) through the wearable sensor patch and medical software application designed specifically for patient and physician use.
This technology could have huge potential in mental health care, where patients struggle with both adhering to their medication regimen and communicating with the health care team. Physicians could measure adherence when treating adults with schizophrenia, bipolar disorders, and major depressive disorder; flag those who are not adhering as having higher risk of disease progression and poorer outcome; and allow decisions to be made more quickly based on treatment need.
Developing and enhancing these collaborative and patient-centric approaches will increase self-monitoring and patient responsibility, and encourage behavior change.
‘All-in’ strategy. By continuing to use the latest technologies and connecting them to the range of stakeholders—physicians, nurses, pharmacists, payers—we will develop an all-inclusive adherence intervention strategy. All patients will be integrated, and all of them, and their family, will be provided with positive psychoeducational care and motivational counseling (Figure 5). In addition, such a support-based patient experience must be aligned with the work of clinical care providers. Compliance therapy and behavioral training, together with active patient engagement, can help improve insight, acceptance of treatment, and, over the long term, adherence.31,32
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
1. World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; 2003.
2. Bosworth HB, Granger BB, Mendys P, et al. Medication adherence: a call for action. Am Heart J. 2011;162(3):412-424.
3. Crowe M, Wilson L, Inder M. Patients’ reports of the factors influencing medication adherence in bipolar disorder – an integrative review of the literature. Int J Nurs Stud. 2011;48(7):894-903.
4. Mert D, Turgut NH, Kelleci M, et al. Perspectives on reasons of medication nonadherence in psychiatric patients. Patient Prefer Adherence. 2015;9:87-93.
5. Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446-452.
6. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487-497.
7. Thompson L, McCabe R. The effect of clinician-patient alliance and communication on treatment adherence in mental health care: a systematic review. BMC Psychiatry. 2012;12:87.
8. Yilmaz S, Buzlu S. Antipsikotik kullanan hastalarda ilaç yan etkileri ve ilaç uyumu. Florence Nightingale Hem˘girelik Dergisi. 2012;20(2):93-103.
9. Kelleci M, Ata EE. Psikiyatri Klini˘ginde yatan hastaların ilaç uyumları ve sosyal destekle iliskisi. [Drug compliance of patients hospitalized in the psychiatry clinic and the relationship with social support]. Psikiyatri Hemsireli˘gi Dergisi. 2011;2(suppl 3):105-110.
10. Bulloch AG, Patten SB. Non-adherence with psychotropic medications in the general population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):47-56.
11. Rosenbaum L. Beyond belief—how people feel about taking medications for heart disease. N Engl J Med. 2015;372(2):183-187.
12. Cramer J, Rosenheck R, Kirk G, et al. Medication compliance feedback and monitoring in a clinical trial: predictions and outcomes. Value Health. 2003;6(5):566-573.
13. Nakonezny PA, Byerly MJ, Rush AJ. Electronic monitoring of antipsychotic medication adherence in outpatients with schizophrenia or schizoaffective disorder: an empirical evaluation of its reliability and predictive validity. Psychiatry Res. 2008;157(1-3):259-263.
14. Fortney JC, Pyne JM, Edlund MJ, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. J Clin Psychiatry. 2011;72(6):827-834.
15. Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553-560.
16. Hoencamp E, Stevens A, Haffmans J. Patients’ attitudes toward antidepressants. Psychiatr Serv. 2002;53(9):1180-1181.
17. Keller MB, Hirschfeld RM, Demyttenaere K, et al. Optimizing outcomes in depression: focus on antidepressant compliance. Int Clin Psychopharmacol. 2002;17(6):265-271.
18. Akerblad AC, Bengtsson F, Holgersson M, et al. Identification of primary care patients at risk of nonadherence to antidepressant treatment. Patient Prefer Adherence. 2008;2:376-386.
19. Brown C, Battista DR, Bruehlman R, et al. Beliefs about antidepressant medications in primary care patients: relationship to self-reported adherence. Med Care. 2005;43(12):1203-1207.
20. Demyttenaere K, Adelin A, Patrick M, et al. Six-month compliance with antidepressant medication in the treatment of major depressive disorder. Int Clin Psychopharmacol. 2008;23(1):36-42.
21. Massand PS. Tolerability and adherence issues in antidepressant therapy. Clin Ther. 2003;25(8):2289-2304.
22. Medicare Prescription Drug, Improvement, and Modernization Act of 2003. Pub L No. 108-173, 117 Stat 2066.
23. Brown MT, Bussell JK. Medication adherence: WHO cares? Mayo Clin Proc. 2011;86(4):304-314.
24. Diaz E, Neuse E, Sullivan MC, et al. Adherence to conventional and atypical antipsychotics after hospital discharge. J Clin Psychiatry. 2004;65(3):354-360.
25. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133.
26. Byerly MJ, Nakonezny PA, Lescouflair E. Antipsychotic medication adherence in schizophrenia. Psychiatr Clin North Am. 2007;30(3):437-452.
27. Nakonezny PA, Hughes CW, Mayes TL, et al. A comparison of various methods of measuring antidepressant medication adherence among children and adolescents with major depressive disorder in a 12-week open trial of fluoxetine. J Child Adolesc Psychopharmacol. 2010;20(5):431-439.
28. Kane JM, Perlis RH, DiCarlo LA, et al. First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J Clin Psychiatry. 2013;74(6):e533-e540. doi: 10.4088/JCP.12m08222.
29. Morey TE, Booth MM, Prather RA, et al. Measurement of ethanol in gaseous breath using a miniature gas chromatograph. J Anal Toxicol. 2011;35(3):134-142.
30. Morey TE, Booth M, Wasdo S, et al. Oral adherence monitoring using a breath test to supplement highly active antiretroviral therapy. AIDS Behav. 2013;17(1):298-306.
31. Torem MS. Participatory pharmacotherapy: 10 strategies for enhancing adherence. Current Psychiatry. 2013;12(7):21-25.
32. Zygmunt A, Olfson M, Boyer CA, et al. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. 2002;159(10):1653-1664.
Patients with severe mental illness can benefit from cognitive remediation training
Cognitive impairment seen in severely mentally ill people is well documented, and has been shown to affect as many as 98% of patients with schizophrenia.1 At this time, there are no FDA-approved medications for treating this cognitive impairment.2
Rusk State Hospital in Rusk, Texas, decided to put greater emphasis on improving cognitive impairment because of an increase in patients with a forensic commitment, either because of (1) not guilty by reason of insanity and (2) restoration of competency to stand trial, which typically require longer lengths of stay. Some of these patients experienced psychotic breaks while earning a college education, and one patient was a member of MENSA (an organization for people with a high IQ) before he became ill. Established programs were not adequate to address cognitive impairment.
How we developed and launched our program
Cognitive remediation is a new focus of psychiatry and is in its infancy; programs include cognitive remediation training (CRT) and cognitive enhancement therapy (CET) (Box3-9). CRT focuses more on practice and rote learning and CET is more inclusive, including aspects such as social skills training. These terms are interchangeable for programs designed to improve cognition. Because there is no standardized model, programs differ in content, length, use of computers vs manuals, social skills training, mentoring, and other modalities.
We could not find a program that could be adapted to our setting because of lack of funding and insufficient patient access to computers. Therefore, we developed our own program to address cognitive impairment in a population of individuals with severe mental illness in a state hospital setting.10 Our CRT program was designed for inpatient psychiatric patients, both on civil and forensic commitments.
The program includes >500 exercises and addresses several cognitive domains. Adding a facilitator or teacher in a group setting introduces an additional dimension to learning. Criteria to participate in the program included:
- behavior stable enough to participate
- ability to read and write English
- no traumatic brain injury that caused cognitive impairment
- the patient had to want to participate in the training program.
We tested each participant at the beginning and end of the 12-week training program, which consisted of 2 one-hour classes a week, with a target group size of 6 to 10 participants. As a rating tool, we used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which has been shown to be an efficient approach to screening for cognitive impairment across several domains.11
We offered 2 levels of training: basic and advanced. Referral was based on the patient’s level of education and current cognitive function. Materials for the advanced group were at a high school or college level; the basic group used materials that were elementary school or mid-high school in scope. Assignment to the basic or advanced training was based on the recovery team’s or psychologist’s recommendation. The training was ongoing, meaning that a participant could begin at any time and continue until he (she) had completed the 12-week training program.
The weekly sessions in the CRT program were based on 12 categories (Table).10
1. Picture Puzzles: Part 1, Odd Man Out. Participants receive a series of 4 pictures and are asked to select the 1 that does not share a common link with the other 3 items. Targeted skills include pattern recognition, visual learning, reasoning, and creativity (looking for non-obvious answers). This plays a role in global cognition and everyday activities that are sight-related.
2. Word Problems. Participants receive math exercises with significant background information presented as text. Targeted skills include calculation, concentration, and reasoning. This helps with making change, figuring out the tip on a bill, balancing a checkbook, and assisting children with homework.
3. Picture Puzzles: Part 2, Matching.Participants view an illustration followed by a series of 4 other pictures, where ≥1 of which will have a close relationship to the example. The participant selects the item with the strongest link. Targeted skills include determining patterns, concentration, visual perception, and reasoning.
4. Verbal Challenge. Participants are provided a variety of word-based problems that involve word usage, definitions, games, and puzzles. Targeted skills include vocabulary, reading comprehension, reasoning, concentration, and global cognition.
5. Picture Puzzles: Part 3, Series Completion. Participants receive a sequence of 3 pictures followed by 4 possible solutions. The participant selects the item that completes the series or shares a common bond. Targeted skills include visual perception, picking up on patterns, creativity, reasoning, and concentration.
6. Mental Arithmetic: Part 1, Coin Counting. Participants are presented math problems related to money that can be solved by simple mental or quick paper calculation. Targeted skills include basic math, speed, concentration, and counting money. This helps with making change and balancing a checkbook.
7. Picture Puzzles: Part 4, Ratio. Participants receive presented analogy questions where the participant has to determine the ratio or proportional relation of the items. Targeted skills include memory, creativity, and decision-making.
8. Mental Arithmetic: Part 2, Potpourri. Participants receive a hodgepodge of math problems, including number sequences and word problems. Targeted skills include reasoning and computation.
9. Visual/spatial. Participants are presented exercises that require them to think in 3 dimensions and see “hidden” areas behind folds or on the other sides of figures. Targeted skills include spatial perception, reasoning, and decision-making.
10. Reasoning. Participants receive problems that involve taking in information, processing the data, analyzing the options based on previous experiences, and coming up with a decision that is factual and rational. Targeted skills include reasoning and decision-making.
11. Memory Exercise, Listening. Participants are provided a reading selection. After the reading, there is 20-minute waiting period during which the participant is engaged in other exercises before returning to answer questions about the reading. Targeted skills include listening, retention, and memory.
12. Speed Training. Participants receive exercises that provide practice in gathering and processing information and making decisions based on the given information. Targeted skills include decision-making, speed, and concentration.
Preliminary results, optimism about good outcomes
In the past 12 months, 28 participants have completed the CRT program: 11 in the basic training class and 17 in the advanced class. Of those, 7 in the basic program and 11 in the advanced program showed significant improvement as measured by the pre- and post-training RBANS; 64% of the participants improved. The average pre-test score in the basic group was 63 and post-test score was 72 (t10 = 3.148, P < .05). The average advanced pre-test score in the advanced class was 75 and post-test score was 80 (t16 = 2.476, P < .05) (Figure 1).
Because this program was developed as a treatment intervention for psychiatric inpatients, not a research study, we did not establish a control group.
In addition to the overall increase in cognitive functioning, individual successes have been noted. One participant who experienced a psychotic break while pursuing a college degree in literature scored 73 on his initial RBANS, indicating moderate impairment. After completing the 12-week program, his RBANS score increased to 95 (Figure 2). One year after completing the CRT program without additional cognitive training, the participant achieved an RBANS score of 104. Since then, the patient has been observed reading the classics in Latin and Greek, as he did before his psychotic break, and has been noted to be making more eye contact and engaging in conversations.
Success also has been noted for participants who did not see an increase in their RBANS scores. One participant historically had shown little interest in any programming or classes, but attended every CRT class, participated, and asked for additional worksheets to take back to the unit. Based on this feedback, each session now includes a worksheet that participants can take back with them.
Further findings of success
Cognitive impairment can be a significant disability in patients with severe mental illness. Longer lengths of stay present an opportunity to provide a CRT program over 12 weeks. However, some increase in cognitive functioning, as measured by the RBANS, was seen with participants who would not or could not complete all 24 classes. In addition to increased cognitive functioning, clinicians have noted improvements in patients’ participation in treatment and self-esteem.
The program engaged patients who previously were uninvolved in activities, and provided a sense of purpose and hope for them. One participant stated that he felt better about himself and had a more optimistic outlook for the future.
This program offers the possibility for participants to clear the mental fog caused by their illness or medication. The exercises stimulate cognitive activity when the goal is not to get the correct answer, but to think about and talk about possible solutions.
CRT, we have found, can greatly increase the quality of life of people with severe mental illness.
1. Keefe R, Easley C, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
2. Nasrallah HA, Keefe RSE, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(suppl 6):S1-S11.
3. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472-485.
4. Baharnoori M, Bartholomeusz C, Boucher A, et al. The 2nd Schizophrenia International Research Society Conference, 10-14 April 2010, Florence, Italy: summaries of oral sessions. Schizophr Res. 2010;124:e1-e62.
5. Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83(1):216-225.
6. Hogarty G, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866-876.
7. Medalia A, Freilich B. The neuropsychological educational approach to cognitive remediation (NEAR) model: practice principles and outcome studies. Am J Psychiatr Rehabil. 2008;11(2):123-143.
8. Hurford IM, Kalkstein S, Hurford MO. Cognitive rehabilitation in schizophrenia. Psychiatric Times. http://www.psychiatrictimes.com/schizophrenia/cognitive-rehabilitation-schizophrenia. Published March 15, 2011. Accessed March 3, 2016.
9. Rogers P, Redoblado-Hodge A. A multi-site trial of cognitive remediation in schizophrenia: an Australian sample. Paper presented at: the 9th annual conference on Cognitive Remediation in Psychiatry; 2004; New York, NY.
10. Bates J. Making your brain hum: 12 weeks to a smarter you. Dallas, TX: Brown Books Publishing Group; 2016.
11. Hobart MP, Goldberg R, Bartko JJ, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia, II: convergent/discriminant validity and diagnostic group comparisons. Am J Psychiatry. 1999;156(12):1951-1957.
Cognitive impairment seen in severely mentally ill people is well documented, and has been shown to affect as many as 98% of patients with schizophrenia.1 At this time, there are no FDA-approved medications for treating this cognitive impairment.2
Rusk State Hospital in Rusk, Texas, decided to put greater emphasis on improving cognitive impairment because of an increase in patients with a forensic commitment, either because of (1) not guilty by reason of insanity and (2) restoration of competency to stand trial, which typically require longer lengths of stay. Some of these patients experienced psychotic breaks while earning a college education, and one patient was a member of MENSA (an organization for people with a high IQ) before he became ill. Established programs were not adequate to address cognitive impairment.
How we developed and launched our program
Cognitive remediation is a new focus of psychiatry and is in its infancy; programs include cognitive remediation training (CRT) and cognitive enhancement therapy (CET) (Box3-9). CRT focuses more on practice and rote learning and CET is more inclusive, including aspects such as social skills training. These terms are interchangeable for programs designed to improve cognition. Because there is no standardized model, programs differ in content, length, use of computers vs manuals, social skills training, mentoring, and other modalities.
We could not find a program that could be adapted to our setting because of lack of funding and insufficient patient access to computers. Therefore, we developed our own program to address cognitive impairment in a population of individuals with severe mental illness in a state hospital setting.10 Our CRT program was designed for inpatient psychiatric patients, both on civil and forensic commitments.
The program includes >500 exercises and addresses several cognitive domains. Adding a facilitator or teacher in a group setting introduces an additional dimension to learning. Criteria to participate in the program included:
- behavior stable enough to participate
- ability to read and write English
- no traumatic brain injury that caused cognitive impairment
- the patient had to want to participate in the training program.
We tested each participant at the beginning and end of the 12-week training program, which consisted of 2 one-hour classes a week, with a target group size of 6 to 10 participants. As a rating tool, we used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which has been shown to be an efficient approach to screening for cognitive impairment across several domains.11
We offered 2 levels of training: basic and advanced. Referral was based on the patient’s level of education and current cognitive function. Materials for the advanced group were at a high school or college level; the basic group used materials that were elementary school or mid-high school in scope. Assignment to the basic or advanced training was based on the recovery team’s or psychologist’s recommendation. The training was ongoing, meaning that a participant could begin at any time and continue until he (she) had completed the 12-week training program.
The weekly sessions in the CRT program were based on 12 categories (Table).10
1. Picture Puzzles: Part 1, Odd Man Out. Participants receive a series of 4 pictures and are asked to select the 1 that does not share a common link with the other 3 items. Targeted skills include pattern recognition, visual learning, reasoning, and creativity (looking for non-obvious answers). This plays a role in global cognition and everyday activities that are sight-related.
2. Word Problems. Participants receive math exercises with significant background information presented as text. Targeted skills include calculation, concentration, and reasoning. This helps with making change, figuring out the tip on a bill, balancing a checkbook, and assisting children with homework.
3. Picture Puzzles: Part 2, Matching.Participants view an illustration followed by a series of 4 other pictures, where ≥1 of which will have a close relationship to the example. The participant selects the item with the strongest link. Targeted skills include determining patterns, concentration, visual perception, and reasoning.
4. Verbal Challenge. Participants are provided a variety of word-based problems that involve word usage, definitions, games, and puzzles. Targeted skills include vocabulary, reading comprehension, reasoning, concentration, and global cognition.
5. Picture Puzzles: Part 3, Series Completion. Participants receive a sequence of 3 pictures followed by 4 possible solutions. The participant selects the item that completes the series or shares a common bond. Targeted skills include visual perception, picking up on patterns, creativity, reasoning, and concentration.
6. Mental Arithmetic: Part 1, Coin Counting. Participants are presented math problems related to money that can be solved by simple mental or quick paper calculation. Targeted skills include basic math, speed, concentration, and counting money. This helps with making change and balancing a checkbook.
7. Picture Puzzles: Part 4, Ratio. Participants receive presented analogy questions where the participant has to determine the ratio or proportional relation of the items. Targeted skills include memory, creativity, and decision-making.
8. Mental Arithmetic: Part 2, Potpourri. Participants receive a hodgepodge of math problems, including number sequences and word problems. Targeted skills include reasoning and computation.
9. Visual/spatial. Participants are presented exercises that require them to think in 3 dimensions and see “hidden” areas behind folds or on the other sides of figures. Targeted skills include spatial perception, reasoning, and decision-making.
10. Reasoning. Participants receive problems that involve taking in information, processing the data, analyzing the options based on previous experiences, and coming up with a decision that is factual and rational. Targeted skills include reasoning and decision-making.
11. Memory Exercise, Listening. Participants are provided a reading selection. After the reading, there is 20-minute waiting period during which the participant is engaged in other exercises before returning to answer questions about the reading. Targeted skills include listening, retention, and memory.
12. Speed Training. Participants receive exercises that provide practice in gathering and processing information and making decisions based on the given information. Targeted skills include decision-making, speed, and concentration.
Preliminary results, optimism about good outcomes
In the past 12 months, 28 participants have completed the CRT program: 11 in the basic training class and 17 in the advanced class. Of those, 7 in the basic program and 11 in the advanced program showed significant improvement as measured by the pre- and post-training RBANS; 64% of the participants improved. The average pre-test score in the basic group was 63 and post-test score was 72 (t10 = 3.148, P < .05). The average advanced pre-test score in the advanced class was 75 and post-test score was 80 (t16 = 2.476, P < .05) (Figure 1).
Because this program was developed as a treatment intervention for psychiatric inpatients, not a research study, we did not establish a control group.
In addition to the overall increase in cognitive functioning, individual successes have been noted. One participant who experienced a psychotic break while pursuing a college degree in literature scored 73 on his initial RBANS, indicating moderate impairment. After completing the 12-week program, his RBANS score increased to 95 (Figure 2). One year after completing the CRT program without additional cognitive training, the participant achieved an RBANS score of 104. Since then, the patient has been observed reading the classics in Latin and Greek, as he did before his psychotic break, and has been noted to be making more eye contact and engaging in conversations.
Success also has been noted for participants who did not see an increase in their RBANS scores. One participant historically had shown little interest in any programming or classes, but attended every CRT class, participated, and asked for additional worksheets to take back to the unit. Based on this feedback, each session now includes a worksheet that participants can take back with them.
Further findings of success
Cognitive impairment can be a significant disability in patients with severe mental illness. Longer lengths of stay present an opportunity to provide a CRT program over 12 weeks. However, some increase in cognitive functioning, as measured by the RBANS, was seen with participants who would not or could not complete all 24 classes. In addition to increased cognitive functioning, clinicians have noted improvements in patients’ participation in treatment and self-esteem.
The program engaged patients who previously were uninvolved in activities, and provided a sense of purpose and hope for them. One participant stated that he felt better about himself and had a more optimistic outlook for the future.
This program offers the possibility for participants to clear the mental fog caused by their illness or medication. The exercises stimulate cognitive activity when the goal is not to get the correct answer, but to think about and talk about possible solutions.
CRT, we have found, can greatly increase the quality of life of people with severe mental illness.
Cognitive impairment seen in severely mentally ill people is well documented, and has been shown to affect as many as 98% of patients with schizophrenia.1 At this time, there are no FDA-approved medications for treating this cognitive impairment.2
Rusk State Hospital in Rusk, Texas, decided to put greater emphasis on improving cognitive impairment because of an increase in patients with a forensic commitment, either because of (1) not guilty by reason of insanity and (2) restoration of competency to stand trial, which typically require longer lengths of stay. Some of these patients experienced psychotic breaks while earning a college education, and one patient was a member of MENSA (an organization for people with a high IQ) before he became ill. Established programs were not adequate to address cognitive impairment.
How we developed and launched our program
Cognitive remediation is a new focus of psychiatry and is in its infancy; programs include cognitive remediation training (CRT) and cognitive enhancement therapy (CET) (Box3-9). CRT focuses more on practice and rote learning and CET is more inclusive, including aspects such as social skills training. These terms are interchangeable for programs designed to improve cognition. Because there is no standardized model, programs differ in content, length, use of computers vs manuals, social skills training, mentoring, and other modalities.
We could not find a program that could be adapted to our setting because of lack of funding and insufficient patient access to computers. Therefore, we developed our own program to address cognitive impairment in a population of individuals with severe mental illness in a state hospital setting.10 Our CRT program was designed for inpatient psychiatric patients, both on civil and forensic commitments.
The program includes >500 exercises and addresses several cognitive domains. Adding a facilitator or teacher in a group setting introduces an additional dimension to learning. Criteria to participate in the program included:
- behavior stable enough to participate
- ability to read and write English
- no traumatic brain injury that caused cognitive impairment
- the patient had to want to participate in the training program.
We tested each participant at the beginning and end of the 12-week training program, which consisted of 2 one-hour classes a week, with a target group size of 6 to 10 participants. As a rating tool, we used the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), which has been shown to be an efficient approach to screening for cognitive impairment across several domains.11
We offered 2 levels of training: basic and advanced. Referral was based on the patient’s level of education and current cognitive function. Materials for the advanced group were at a high school or college level; the basic group used materials that were elementary school or mid-high school in scope. Assignment to the basic or advanced training was based on the recovery team’s or psychologist’s recommendation. The training was ongoing, meaning that a participant could begin at any time and continue until he (she) had completed the 12-week training program.
The weekly sessions in the CRT program were based on 12 categories (Table).10
1. Picture Puzzles: Part 1, Odd Man Out. Participants receive a series of 4 pictures and are asked to select the 1 that does not share a common link with the other 3 items. Targeted skills include pattern recognition, visual learning, reasoning, and creativity (looking for non-obvious answers). This plays a role in global cognition and everyday activities that are sight-related.
2. Word Problems. Participants receive math exercises with significant background information presented as text. Targeted skills include calculation, concentration, and reasoning. This helps with making change, figuring out the tip on a bill, balancing a checkbook, and assisting children with homework.
3. Picture Puzzles: Part 2, Matching.Participants view an illustration followed by a series of 4 other pictures, where ≥1 of which will have a close relationship to the example. The participant selects the item with the strongest link. Targeted skills include determining patterns, concentration, visual perception, and reasoning.
4. Verbal Challenge. Participants are provided a variety of word-based problems that involve word usage, definitions, games, and puzzles. Targeted skills include vocabulary, reading comprehension, reasoning, concentration, and global cognition.
5. Picture Puzzles: Part 3, Series Completion. Participants receive a sequence of 3 pictures followed by 4 possible solutions. The participant selects the item that completes the series or shares a common bond. Targeted skills include visual perception, picking up on patterns, creativity, reasoning, and concentration.
6. Mental Arithmetic: Part 1, Coin Counting. Participants are presented math problems related to money that can be solved by simple mental or quick paper calculation. Targeted skills include basic math, speed, concentration, and counting money. This helps with making change and balancing a checkbook.
7. Picture Puzzles: Part 4, Ratio. Participants receive presented analogy questions where the participant has to determine the ratio or proportional relation of the items. Targeted skills include memory, creativity, and decision-making.
8. Mental Arithmetic: Part 2, Potpourri. Participants receive a hodgepodge of math problems, including number sequences and word problems. Targeted skills include reasoning and computation.
9. Visual/spatial. Participants are presented exercises that require them to think in 3 dimensions and see “hidden” areas behind folds or on the other sides of figures. Targeted skills include spatial perception, reasoning, and decision-making.
10. Reasoning. Participants receive problems that involve taking in information, processing the data, analyzing the options based on previous experiences, and coming up with a decision that is factual and rational. Targeted skills include reasoning and decision-making.
11. Memory Exercise, Listening. Participants are provided a reading selection. After the reading, there is 20-minute waiting period during which the participant is engaged in other exercises before returning to answer questions about the reading. Targeted skills include listening, retention, and memory.
12. Speed Training. Participants receive exercises that provide practice in gathering and processing information and making decisions based on the given information. Targeted skills include decision-making, speed, and concentration.
Preliminary results, optimism about good outcomes
In the past 12 months, 28 participants have completed the CRT program: 11 in the basic training class and 17 in the advanced class. Of those, 7 in the basic program and 11 in the advanced program showed significant improvement as measured by the pre- and post-training RBANS; 64% of the participants improved. The average pre-test score in the basic group was 63 and post-test score was 72 (t10 = 3.148, P < .05). The average advanced pre-test score in the advanced class was 75 and post-test score was 80 (t16 = 2.476, P < .05) (Figure 1).
Because this program was developed as a treatment intervention for psychiatric inpatients, not a research study, we did not establish a control group.
In addition to the overall increase in cognitive functioning, individual successes have been noted. One participant who experienced a psychotic break while pursuing a college degree in literature scored 73 on his initial RBANS, indicating moderate impairment. After completing the 12-week program, his RBANS score increased to 95 (Figure 2). One year after completing the CRT program without additional cognitive training, the participant achieved an RBANS score of 104. Since then, the patient has been observed reading the classics in Latin and Greek, as he did before his psychotic break, and has been noted to be making more eye contact and engaging in conversations.
Success also has been noted for participants who did not see an increase in their RBANS scores. One participant historically had shown little interest in any programming or classes, but attended every CRT class, participated, and asked for additional worksheets to take back to the unit. Based on this feedback, each session now includes a worksheet that participants can take back with them.
Further findings of success
Cognitive impairment can be a significant disability in patients with severe mental illness. Longer lengths of stay present an opportunity to provide a CRT program over 12 weeks. However, some increase in cognitive functioning, as measured by the RBANS, was seen with participants who would not or could not complete all 24 classes. In addition to increased cognitive functioning, clinicians have noted improvements in patients’ participation in treatment and self-esteem.
The program engaged patients who previously were uninvolved in activities, and provided a sense of purpose and hope for them. One participant stated that he felt better about himself and had a more optimistic outlook for the future.
This program offers the possibility for participants to clear the mental fog caused by their illness or medication. The exercises stimulate cognitive activity when the goal is not to get the correct answer, but to think about and talk about possible solutions.
CRT, we have found, can greatly increase the quality of life of people with severe mental illness.
1. Keefe R, Easley C, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
2. Nasrallah HA, Keefe RSE, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(suppl 6):S1-S11.
3. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472-485.
4. Baharnoori M, Bartholomeusz C, Boucher A, et al. The 2nd Schizophrenia International Research Society Conference, 10-14 April 2010, Florence, Italy: summaries of oral sessions. Schizophr Res. 2010;124:e1-e62.
5. Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83(1):216-225.
6. Hogarty G, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866-876.
7. Medalia A, Freilich B. The neuropsychological educational approach to cognitive remediation (NEAR) model: practice principles and outcome studies. Am J Psychiatr Rehabil. 2008;11(2):123-143.
8. Hurford IM, Kalkstein S, Hurford MO. Cognitive rehabilitation in schizophrenia. Psychiatric Times. http://www.psychiatrictimes.com/schizophrenia/cognitive-rehabilitation-schizophrenia. Published March 15, 2011. Accessed March 3, 2016.
9. Rogers P, Redoblado-Hodge A. A multi-site trial of cognitive remediation in schizophrenia: an Australian sample. Paper presented at: the 9th annual conference on Cognitive Remediation in Psychiatry; 2004; New York, NY.
10. Bates J. Making your brain hum: 12 weeks to a smarter you. Dallas, TX: Brown Books Publishing Group; 2016.
11. Hobart MP, Goldberg R, Bartko JJ, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia, II: convergent/discriminant validity and diagnostic group comparisons. Am J Psychiatry. 1999;156(12):1951-1957.
1. Keefe R, Easley C, Poe MP. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57(6):688-691.
2. Nasrallah HA, Keefe RSE, Javitt DC. Cognitive deficits and poor functional outcomes in schizophrenia: clinical and neurobiological progress. Current Psychiatry. 2014;13(suppl 6):S1-S11.
3. Wykes T, Huddy V, Cellard C, et al. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472-485.
4. Baharnoori M, Bartholomeusz C, Boucher A, et al. The 2nd Schizophrenia International Research Society Conference, 10-14 April 2010, Florence, Italy: summaries of oral sessions. Schizophr Res. 2010;124:e1-e62.
5. Antzoulatos EG, Miller EK. Increases in functional connectivity between prefrontal cortex and striatum during category learning. Neuron. 2014;83(1):216-225.
6. Hogarty G, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866-876.
7. Medalia A, Freilich B. The neuropsychological educational approach to cognitive remediation (NEAR) model: practice principles and outcome studies. Am J Psychiatr Rehabil. 2008;11(2):123-143.
8. Hurford IM, Kalkstein S, Hurford MO. Cognitive rehabilitation in schizophrenia. Psychiatric Times. http://www.psychiatrictimes.com/schizophrenia/cognitive-rehabilitation-schizophrenia. Published March 15, 2011. Accessed March 3, 2016.
9. Rogers P, Redoblado-Hodge A. A multi-site trial of cognitive remediation in schizophrenia: an Australian sample. Paper presented at: the 9th annual conference on Cognitive Remediation in Psychiatry; 2004; New York, NY.
10. Bates J. Making your brain hum: 12 weeks to a smarter you. Dallas, TX: Brown Books Publishing Group; 2016.
11. Hobart MP, Goldberg R, Bartko JJ, et al. Repeatable battery for the assessment of neuropsychological status as a screening test in schizophrenia, II: convergent/discriminant validity and diagnostic group comparisons. Am J Psychiatry. 1999;156(12):1951-1957.
Reducing morbidity and mortality from common medical conditions in schizophrenia
Life expectancy for both males and females has been increasing over the past several decades to an average of 76 years. However, the life expectancy among individuals with schizophrenia in the United States is 61 years—a 20% reduction.1 Patients with schizophrenia are known to be at increased risk of several comorbid medical conditions, such as type 2 diabetes mellitus (T2DM), coronary artery disease, and digestive and liver disorders, compared with healthy people (Figure, page 32).2-5 This risk may be heightened by several factors, including sedentary lifestyle, a high rate of cigarette use, poor self-management skills, homelessness, and poor diet.
Although substantial attention is paid to the psychiatric and behavioral management of schizophrenia, many barriers impede the detection and treatment of patients’ medical conditions, which have been implicated in excess unforeseen deaths. Patients with schizophrenia might experience delays in diagnosis, leading to more acute comorbidity at time of diagnosis and premature mortality
Cardiovascular disease is the leading cause of death among psychiatric patients.6 Key risk factors for cardiovascular disease include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more prevalent among patients with schizophrenia.7 In addition, antipsychotics are associated with adverse metabolic effects.8 In general, smoking and obesity are the most modifiable and preventable risk factors for many medical conditions, such as cardiovascular disease, hyperlipidemia, diabetes, and many forms of cancer (Table 1).
In this article, we discuss how to manage common medical comorbidities in patients with schizophrenia. Comprehensive management for all these medical conditions in this population is beyond the scope of this article; we limit ourselves to discussing (1) how common these conditions are in patients with schizophrenia compared with the general population and (2) what can be done in psychiatric practice to manage these medical comorbidities (Box).
Obesity
Obesity—defined as body mass index (BMI) of >30—is common among patients with schizophrenia. The condition leads to poor self-image, decreased treatment adherence, and an increased risk of many chronic medical conditions (Table 1). Being overweight or obese can increase stigma and social discrimination, which will undermine self-esteem and, in turn, affect adherence with medications, leading to relapse.
The prevalence of obesity among patients with schizophrenia is almost double that of the general population9 (Figure2-5). Several factors predispose these patients to overweight or obese, including sedentary lifestyle, lack of exercise, a high-fat diet, medications side effects, and genetic factors. Recent studies report the incidence of weight gain among patients treated with antipsychotics is as high as 80%10 (Table 2).
Mechanisms involved in antipsychotic-induced weight gain are not completely understood, but antagonism of serotonergic (5-HT2C, 5-HT1A), histamine (H1), dopamine (D2), muscarinic, and other receptors are involved in modulation of food intake. Decreased energy expenditure also has been blamed for antipsychotic-induced weight gain.10
Pharmacotherapy and bariatric surgery can be as effective among patients with schizophrenia as they are among the general population. Maintaining a BMI of <25 kg/m2 lowers the risk of cardiovascular disease by 35% to 55%.6 Metformin has modest potential for offsetting weight gain and providing some metabolic control in overweight outpatients with schizophrenia,11 and should be considered early when treating at-risk patients.
Managing obesity. Clinicians can apply several measures to manage obesity in a patient with schizophrenia:
- Educate the patient, and the family, about the risks of being overweight or obese.
- Monitor weight and BMI at each visit.
- Advise smoking cessation.
- When clinically appropriate, switch to an antipsychotic with a lower risk of weight gain—eg, from olanzapine or high-dose quetiapine to a high- or medium-potency typical antipsychotic (eg, haloperidol, perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2, page 36).
- Consider prophylactic use of metformin with an antipsychotic; the drug has modest potential for offsetting weight gain and providing better metabolic control in an overweight patient with schizophrenia.11
- Encourage the patient to engage in modest physical activity; for example, a 20-minute walk, every day, reduces the risk of cardiovascular disease by 35% to 55%.6
- Recommend a formal lifestyle modification program, such as behavioral group-based treatment for weight reduction.12
- Refer the patient and family to a dietitian.
Type 2 diabetes mellitus
There is strong association between T2DM and schizophrenia that is related to abnormal glucose regulation independent of any adverse medication effect.13 Ryan et al14 reported that first-episode, drug-naïve patients with schizophrenia had a higher level of intra-abdominal fat than age- and BMI-matched healthy controls, suggesting that schizophrenia could be associated with changes in adiposity that might increase the risk of insulin resistance, hyperlipidemia, and dyslipidemia. Mechanisms that increase the risk of T2DM in schizophrenia include genetic and environmental factors, such as family history, lack of physical activity, and poor diet.
Diagnosis. All patients with schizophrenia should be evaluated for undiagnosed diabetes. The diagnosis of T2DM is made by documenting:
- a fasting plasma glucose reading of ≥126 mg/dL
- symptoms of T2DM, along with a random plasma glucose reading of ≥200 mg/dL
- 2-hour reading of a plasma glucose level >200 mg/dL on an oral glucose tolerance test.
Recent guidelines also suggest using a hemoglobin A1c value cutoff of ≥6.5% to diagnose T2DM.
In the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, 38% of patients with schizophrenia and diabetes were not receiving any treatment for T2DM.15
Risk factors for T2DM are:
- BMI >25
- a first-degree relative with diabetes
- lack of physical activity
- being a member of a high-risk ethnic group (African American, Hispanic American, Native American, Asian American, or Pacific Islander)
- having delivered a baby >9 lb or having had gestational diabetes
- hypertension
- high-density lipoprotein (HDL) cholesterol level of ≤35 mg/dL
- triglyceride level of ≥250 mg/dL
- history of an abnormal glucose tolerance test
- history of abnormal findings on a fasting plasma glucose test
- history of vascular disease.
Early detection and management.
- Educate the patient and family about signs and symptoms of T2DM, such as polyuria, nocturia, polydipsia, fatigue, visual disturbances, and (in women) vulvitis. Also, psychiatrists should be aware of, and inquire about, symptoms of diabetic ketoacidosis.
- At the start of therapy with any antipsychotic, particularly a second-generation antipsychotic (SGA), ask patients about a family history of diabetes and measure the hemoglobin A1c value.
- Monitor the hemoglobin A1c level 4 months after starting an antipsychotic, then annually, in a patient with significant risk factors for diabetes.
- Monitor blood glucose every 6 months in patients with no change from initial results and more frequently in those with significant risk factors for diabetes and those who gain weight.
- Order a lipid panel and measure the serum glucose level to rule out dyslipidemia and diabetes, because a patient with high lipid levels and diabetes is at higher risk of developing cardiovascular conditions.
- Advocate for smoking cessation.
- Switch to an antipsychotic with a lower risk of diabetes when clinically appropriate, such as switching a patient from olanzapine or high-dose quetiapine to a high- or medium-potency typical antipsychotic (such as haloperidol or perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2).
- Consider prophylactic use of metformin along with antipsychotics. Metformin has been used to improve insulin sensitivity and can lead to weight loss in diabetic and non-diabetic patients. The drug has modest potential for offsetting weight gain and providing better metabolic control in overweight outpatients with schizophrenia.11 Metformin is simple to use, does not lead to hypoglycemia, does not require serum glucose monitoring, and has a favorable safety profile.11
- Educate the patient about modest physical activity. For example, a 20-minute walk every day reduces the risk of cardiovascular disease by 35% to 55%.6
- Refer the patient to a dietitian to develop an appropriate diet plan.
- When diabetes is diagnosed, ensure appropriate follow-up and initiation or continuation of therapy with a general practitioner or an endocrinologist.
- Reinforce the need for ongoing follow-up and compliance with therapy for diabetes.
Hyperlipidemia and dyslipidemia
Elevated cholesterol and triglyceride levels are associated with cardiovascular diseases, such as ischemic heart disease and myocardial infarction. A 10% increase in cholesterol levels is associated with a 20% to 30% increase in the risk of coronary artery disease; lowering cholesterol by 10% decreases the risk by 20% to 30%.16 Triglyceride levels ≥250 mg/dL are associated with 2-fold higher risk of cardiovascular disease.16
The incidence of dyslipidemia is not as well studied as diabetes in patients with schizophrenia. There is increased prevalence of dyslipidemia in patients with schizophrenia compared with the general population because of obesity, lack of physical activity, and poor dietary habits.16
Data regarding the effects of first-generation antipsychotics (FGAs) on lipid levels are limited, but high-potency drugs, such as haloperidol, seem to carry a lower risk of hyperlipidemia than low-potency drugs, such as chlorpromazine and thioridazine.17 A comprehensive review on the effects of SGAs on plasma lipid levels suggested that clozapine, olanzapine, and quetiapine are associated with a higher risk of dyslipidemia17 (Table 2).
In the CATIE study, olanzapine and clozapine were associated with a greater increase in the serum level of cholesterol and triglycerides compared with other antipsychotics, even after adjusting for treatment duration. Furthermore, a retrospective chart review of patients who switched to aripiprazole from other SGAs showed a decrease in levels of total cholesterol and low-density lipoprotein cholesterol15 (Table 2).
Patients with schizophrenia are more likely to have dyslipidemia go undiagnosed, and therefore are less likely to be treated for the disorder. In the CATIE study, 88% of patients with dyslipidemia were not receiving any treatment.15
Management for dyslipidemia.
- Educate the patient and family about risks involved with dyslipidemia.
- Monitor weight and BMI at each visit.
- Monitor lipids to rule out dyslipidemia. Obtain a pretreatment fasting or random lipid profile for any patient receiving an antipsychotic; repeat at least every 6 months after starting the antipsychotic.
- Counsel the patient to quit smoking.
- Switch to an antipsychotic with lower risk of weight gain and dyslipidemia, such as switching from olanzapine or high-dose quetiapine to high- or medium-potency typical antipsychotics (such as, haloperidol or perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2).
- Educate and encourage the patient about modest physical activity. For example, a 20-minute walk everyday will reduce cardiovascular disease risk by 35% to 55%.6
- Refer to a dietitian if indicated.
- Ensure follow-up and initiation of treatment with a general practitioner.
- Educate and encourage the patient about modest physical activity. For example, a 20-minute walk everyday will reduce cardiovascular disease risk by 35% to 55%.
Metabolic syndrome
Metabolic syndrome is cluster of cardiovascular risk factors, including central adiposity, hyperglycemia, dyslipidemia, and hypertension. The National Cholesterol Education Program’s Adult Treatment Panel III report defines metabolic syndrome as the presence of 3 of 5 of the following factors:
- abdominal obesity (waist circumference of >40 inches in men, or >35 inches in women)
- triglyceride level, >150 mg/dL
- HDL cholesterol, <40 mg/dL in men and <50 mg/dL in women
- blood pressure, >130/85 mm Hg
- fasting plasma glucose level, >110 mg/dL.
The presence of metabolic syndrome in the general population is a strong predictor of cardiovascular diseases and diabetes.18 The adverse effects of metabolic syndrome are thought to relate to atherogenic dyslipidemia, higher blood pressure, insulin resistance with or without glucose intolerance, a proinflammatory state, and a prothrombotic state.
The prevalence of metabolic syndrome in patients with schizophrenia is 2- to 3-fold higher than the general population.19 In the CATIE study, approximately one-third of patients met criteria for metabolic syndrome at baseline.15 In a prospective study, De Hert et al20 reported that patients who were started on a SGA had more than twice the rate of developing metabolic syndrome compared with those treated with a FGA (Table 2). Other possible causes of metabolic syndrome are visceral adiposity and insulin resistance.16Management of the metabolic syndrome involves addressing the individual components that have been described in the preceding sections on T2DM and dyslipidemia.
Hepatitis C
Hepatitis C virus (HCV) infection is thought to be the most common blood-borne illness, with an estimated prevalence of 1% of the U.S. population. Some studies suggest that as many as 16% of people with schizophrenia have HCV infection.4 Risk factors for HCV infection include unsafe sexual practices, prostitution, homosexuality, homelessness, and IV drug use.
HCV treatments typically have involved regimens with interferon alfa, which is associated with significant neuropsychiatric side effects, including depression and suicide. There is a dearth of research on treatment of HCV in patients with schizophrenia; however, at least 1 study suggests that there was no increase in psychiatric symptoms in patients treated with interferon-containing regimens.21 There is even less evidence to guide the use of newer, non-interferon–based HCV treatment regimens that are better tolerated and have a higher response rate in the general population; there is reason, however, to be hopeful about their potential in patients with schizophrenia and HCV infection.
Managing HCV infection.
- Educate the patients and family about risk factors associated with contracting HCV.
- Screen for HCV infection in patients with schizophrenia because there is higher prevalence of HCV in these patients compared with the general population.
- When HCV infection is diagnosed, educate the patients and family about available treatments.
- Facilitate referral to an HCV specialist for appropriate treatment.
HIV/AIDS
HIV infection is highly prevalent among people suffering from severe mental illness such as schizophrenia. The incidence of HIV/AIDS in patients with schizophrenia is estimated to be 4% to 23%, compared with 0.6% in the general population.22 Risk factors associated with a higher incidence of HIV/AIDS in patients with schizophrenia are lack of knowledge about contracting HIV, unsafe sexual practices, prostitution, homosexuality, homelessness, and IV drug use.22
Managing HIV/AIDS.
- Educate the patient and family about risk factors associated with contracting HIV/AIDS.
- Educate patients about safe sex practices.
- All patients with schizophrenia should be screened for HIV because there is 10-fold higher HIV prevalence in schizophrenia compared with the general population.
- When HIV infection is diagnosed, facilitate referral to a HIV or infectious disease specialist for treatment.
- Educate the patient in whom HIV/AIDS has been diagnosed about the importance of (1) adherence to his (her) HIV medication regimen and (2) follow-up visits with an infectious disease practitioner and appropriate laboratory tests.
- Educate the patient’s family and significant other about the illness.
- Screen for and treat substance use.
- At each visit, inquire about the patient’s adherence to HIV medical therapy, viral load, and CD4 cell count.
Chronic obstructive pulmonary disease
Patients with schizophrenia are more likely to suffer from respiratory disease, such as chronic obstructive pulmonary disease (COPD) and asthma, compared with the general population.23 Smoking is a major risk factor for COPD. In a study by Dickerson et al,24 64% of people with schizophrenia were current smokers, compared with 19% of those without mental illness.
A high rate of smoking rate among people with schizophrenia suggests a “self-medication” hypothesis: That is, stimulation of CNS nicotinic cholinergic receptors treats the negative symptoms of schizophrenia and overcomes the dopamine blocking effects of antipsychotics.25 Among SGAs, only clozapine has a substantial body of evidence to support its association with decreased smoking behavior.
Managing COPD.
- Educate the patient and family about risk factors associated with COPD and smoking.
- Screen for tobacco use at each visit; try to increase motivation to quit smoking.
- Educate the patients and family about the value and availability of smoking cessation programs.
- Prescribe medication to help with smoking cessation when needed. Bupropion and varenicline have been shown to be effective in patients with schizophrenia; nicotine replacement therapies are safe and can be helpful.
- When treating a patient who is in the process of quitting, encourage and help him to maintain his commitment and enlist support from his family.
- Refer to an appropriate medical provider (primary care provider or pulmonologist) for a patient with an established or suspected diagnosis of COPD.
Cancer
Since 1909, when the Board of Control of the Commissioners in Lunacy for England and Wales noted the possibility of a decreased incidence in cancer among psychiatric patients, this connection has been a matter of controversy.26 Subsequent research has been equivocal; the prevalence of cancer has been reported to be either increased, similar, or decreased compared with the general population.26-28 Risk factors for cancer, including smoking, obesity, poor diet, sedentary lifestyle, and hyperprolactinemia, are more common among patients with schizophrenia.
Genetic factors and a possible protective effect from antipsychotics have been cited as potential causes of decreased prevalence. Clozapine is associated with an increased risk of leukemia. No conclusion can be drawn about the overall prevalence of cancer in schizophrenia.
Managing cancer in a patient with schizophrenia, however, poses a significant challenge29; he might lack capacity to make decisions about cancer treatment. The patient—or his surrogate decision-makers—need to carefully weigh current quality of life against potential benefits of treatment and risks of side effects. Adherence to complex, often toxic, therapies can be challenging for the patient with psychosis. Successful cancer treatment often requires close collaboration between the cancer treatment team and the patient’s support system, including the treating psychiatrist and case management teams.
Bottom Line
Patients with schizophrenia are at higher risk of developing comorbid medical
conditions because of the illness itself, lifestyle behaviors, genetics, and adverse
effects of medications. Because mental health clinicians focus attention on the
psychiatric and behavioral aspect of treatment, often there is delay in screening,
detecting, and treating medical comorbidities. This screening can be done in any
psychiatric practice, which can lead to timely management for those conditions
and preventing premature mortality in patients with schizophrenia.
1. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
2. De Hert M, Correl CU, Bobes J, et al. Physical illness in patients with severe mental disorder. I. Prevalence, impact of medications, and disparities in health care. World Psychiatry. 2011;10(1):52-77.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics update-2011 update. Circulation. 2011;123(4):e18-e209. doi: 10.1161/CIR.0b013e3182009701.
4. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
5. Lovre D, Mauvais-Jarvis F. Trends in prevalence of the metabolic syndrome. JAMA. 2015;314(9):950.
6. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
7. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
8. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
9. Allison DB, Fontaine KR, Heo M et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215-220.
10. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686-1696.
11. Jarskog LF, Hamer RM, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
12. Ganguli R. Behavioral therapy for weight loss in patients with schizophrenia. J Clin Psychiatry. 2007;68(suppl 4):19-25.
13. Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry Suppl. 2004;47:S64-S66.
14. Ryan MC, Flanagan S, Kinsella U, et al. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug naïve patients with schizophrenia. Life Sci. 2004;74(16):1999-2008.
15. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
16. Barnett AH, Mackin P, Chaudhry I, et al. Minimising metabolic and cardiovascular risk in schizophrenia: diabetes, obesity and dyslipidaemia. J Psychopharmacol. 2007;21(4):357-373.
17. Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70(1):1-17.
18. Sacks FM. Metabolic syndrome: epidemiology and consequences. J Clin Psychiatry. 2004;65(suppl 18):3-12.
19. De Hert M, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
20. De Hert M, Hanssens L, Wampers M, et al. Prevalence and incidence rates of metabolic abnormalities and diabetes in a prospective study of patients treated with second-generation antipsychotics. Schizophr Bull. 2007;33:560.
21. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
22. Davidson S, Judd F, Jolley D, et al. Risk factors for HIV/AIDS and hepatitis C among the chronic mentally ill. Aust N Z J Psychiatry. 2001;35(2):203-209.
23. Copeland LA, Mortensen EM, Zeber JE, et al. Pulmonary disease among inpatient decendents: impact of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):720-726.
24. Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64(1):44-50.
25. Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155(11):1490-1501.
26. Hodgson R, Wildgust HJ, Bushe CJ. Cancer and schizophrenia: is there a paradox? J Psychopharmacol. 2010;24(suppl 4):51-60.
27. Hippisley-Cox J, Vinogradova Y, Coupland C, et al. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry. 2007;64(12):1368-1376.
28. Grinshpoon A, Barchana M, Ponizovsky A, et al. Cancer in schizophrenia: is the risk higher or lower? Schizophr Res. 2005;73(2-3):333-341.
29. Hwang M, Farasatpour M, Williams CD, et al. Adjuvant chemotherapy for breast cancer patients with schizophrenia. Oncol Lett. 2012;3(4):845-850.
Life expectancy for both males and females has been increasing over the past several decades to an average of 76 years. However, the life expectancy among individuals with schizophrenia in the United States is 61 years—a 20% reduction.1 Patients with schizophrenia are known to be at increased risk of several comorbid medical conditions, such as type 2 diabetes mellitus (T2DM), coronary artery disease, and digestive and liver disorders, compared with healthy people (Figure, page 32).2-5 This risk may be heightened by several factors, including sedentary lifestyle, a high rate of cigarette use, poor self-management skills, homelessness, and poor diet.
Although substantial attention is paid to the psychiatric and behavioral management of schizophrenia, many barriers impede the detection and treatment of patients’ medical conditions, which have been implicated in excess unforeseen deaths. Patients with schizophrenia might experience delays in diagnosis, leading to more acute comorbidity at time of diagnosis and premature mortality
Cardiovascular disease is the leading cause of death among psychiatric patients.6 Key risk factors for cardiovascular disease include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more prevalent among patients with schizophrenia.7 In addition, antipsychotics are associated with adverse metabolic effects.8 In general, smoking and obesity are the most modifiable and preventable risk factors for many medical conditions, such as cardiovascular disease, hyperlipidemia, diabetes, and many forms of cancer (Table 1).
In this article, we discuss how to manage common medical comorbidities in patients with schizophrenia. Comprehensive management for all these medical conditions in this population is beyond the scope of this article; we limit ourselves to discussing (1) how common these conditions are in patients with schizophrenia compared with the general population and (2) what can be done in psychiatric practice to manage these medical comorbidities (Box).
Obesity
Obesity—defined as body mass index (BMI) of >30—is common among patients with schizophrenia. The condition leads to poor self-image, decreased treatment adherence, and an increased risk of many chronic medical conditions (Table 1). Being overweight or obese can increase stigma and social discrimination, which will undermine self-esteem and, in turn, affect adherence with medications, leading to relapse.
The prevalence of obesity among patients with schizophrenia is almost double that of the general population9 (Figure2-5). Several factors predispose these patients to overweight or obese, including sedentary lifestyle, lack of exercise, a high-fat diet, medications side effects, and genetic factors. Recent studies report the incidence of weight gain among patients treated with antipsychotics is as high as 80%10 (Table 2).
Mechanisms involved in antipsychotic-induced weight gain are not completely understood, but antagonism of serotonergic (5-HT2C, 5-HT1A), histamine (H1), dopamine (D2), muscarinic, and other receptors are involved in modulation of food intake. Decreased energy expenditure also has been blamed for antipsychotic-induced weight gain.10
Pharmacotherapy and bariatric surgery can be as effective among patients with schizophrenia as they are among the general population. Maintaining a BMI of <25 kg/m2 lowers the risk of cardiovascular disease by 35% to 55%.6 Metformin has modest potential for offsetting weight gain and providing some metabolic control in overweight outpatients with schizophrenia,11 and should be considered early when treating at-risk patients.
Managing obesity. Clinicians can apply several measures to manage obesity in a patient with schizophrenia:
- Educate the patient, and the family, about the risks of being overweight or obese.
- Monitor weight and BMI at each visit.
- Advise smoking cessation.
- When clinically appropriate, switch to an antipsychotic with a lower risk of weight gain—eg, from olanzapine or high-dose quetiapine to a high- or medium-potency typical antipsychotic (eg, haloperidol, perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2, page 36).
- Consider prophylactic use of metformin with an antipsychotic; the drug has modest potential for offsetting weight gain and providing better metabolic control in an overweight patient with schizophrenia.11
- Encourage the patient to engage in modest physical activity; for example, a 20-minute walk, every day, reduces the risk of cardiovascular disease by 35% to 55%.6
- Recommend a formal lifestyle modification program, such as behavioral group-based treatment for weight reduction.12
- Refer the patient and family to a dietitian.
Type 2 diabetes mellitus
There is strong association between T2DM and schizophrenia that is related to abnormal glucose regulation independent of any adverse medication effect.13 Ryan et al14 reported that first-episode, drug-naïve patients with schizophrenia had a higher level of intra-abdominal fat than age- and BMI-matched healthy controls, suggesting that schizophrenia could be associated with changes in adiposity that might increase the risk of insulin resistance, hyperlipidemia, and dyslipidemia. Mechanisms that increase the risk of T2DM in schizophrenia include genetic and environmental factors, such as family history, lack of physical activity, and poor diet.
Diagnosis. All patients with schizophrenia should be evaluated for undiagnosed diabetes. The diagnosis of T2DM is made by documenting:
- a fasting plasma glucose reading of ≥126 mg/dL
- symptoms of T2DM, along with a random plasma glucose reading of ≥200 mg/dL
- 2-hour reading of a plasma glucose level >200 mg/dL on an oral glucose tolerance test.
Recent guidelines also suggest using a hemoglobin A1c value cutoff of ≥6.5% to diagnose T2DM.
In the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, 38% of patients with schizophrenia and diabetes were not receiving any treatment for T2DM.15
Risk factors for T2DM are:
- BMI >25
- a first-degree relative with diabetes
- lack of physical activity
- being a member of a high-risk ethnic group (African American, Hispanic American, Native American, Asian American, or Pacific Islander)
- having delivered a baby >9 lb or having had gestational diabetes
- hypertension
- high-density lipoprotein (HDL) cholesterol level of ≤35 mg/dL
- triglyceride level of ≥250 mg/dL
- history of an abnormal glucose tolerance test
- history of abnormal findings on a fasting plasma glucose test
- history of vascular disease.
Early detection and management.
- Educate the patient and family about signs and symptoms of T2DM, such as polyuria, nocturia, polydipsia, fatigue, visual disturbances, and (in women) vulvitis. Also, psychiatrists should be aware of, and inquire about, symptoms of diabetic ketoacidosis.
- At the start of therapy with any antipsychotic, particularly a second-generation antipsychotic (SGA), ask patients about a family history of diabetes and measure the hemoglobin A1c value.
- Monitor the hemoglobin A1c level 4 months after starting an antipsychotic, then annually, in a patient with significant risk factors for diabetes.
- Monitor blood glucose every 6 months in patients with no change from initial results and more frequently in those with significant risk factors for diabetes and those who gain weight.
- Order a lipid panel and measure the serum glucose level to rule out dyslipidemia and diabetes, because a patient with high lipid levels and diabetes is at higher risk of developing cardiovascular conditions.
- Advocate for smoking cessation.
- Switch to an antipsychotic with a lower risk of diabetes when clinically appropriate, such as switching a patient from olanzapine or high-dose quetiapine to a high- or medium-potency typical antipsychotic (such as haloperidol or perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2).
- Consider prophylactic use of metformin along with antipsychotics. Metformin has been used to improve insulin sensitivity and can lead to weight loss in diabetic and non-diabetic patients. The drug has modest potential for offsetting weight gain and providing better metabolic control in overweight outpatients with schizophrenia.11 Metformin is simple to use, does not lead to hypoglycemia, does not require serum glucose monitoring, and has a favorable safety profile.11
- Educate the patient about modest physical activity. For example, a 20-minute walk every day reduces the risk of cardiovascular disease by 35% to 55%.6
- Refer the patient to a dietitian to develop an appropriate diet plan.
- When diabetes is diagnosed, ensure appropriate follow-up and initiation or continuation of therapy with a general practitioner or an endocrinologist.
- Reinforce the need for ongoing follow-up and compliance with therapy for diabetes.
Hyperlipidemia and dyslipidemia
Elevated cholesterol and triglyceride levels are associated with cardiovascular diseases, such as ischemic heart disease and myocardial infarction. A 10% increase in cholesterol levels is associated with a 20% to 30% increase in the risk of coronary artery disease; lowering cholesterol by 10% decreases the risk by 20% to 30%.16 Triglyceride levels ≥250 mg/dL are associated with 2-fold higher risk of cardiovascular disease.16
The incidence of dyslipidemia is not as well studied as diabetes in patients with schizophrenia. There is increased prevalence of dyslipidemia in patients with schizophrenia compared with the general population because of obesity, lack of physical activity, and poor dietary habits.16
Data regarding the effects of first-generation antipsychotics (FGAs) on lipid levels are limited, but high-potency drugs, such as haloperidol, seem to carry a lower risk of hyperlipidemia than low-potency drugs, such as chlorpromazine and thioridazine.17 A comprehensive review on the effects of SGAs on plasma lipid levels suggested that clozapine, olanzapine, and quetiapine are associated with a higher risk of dyslipidemia17 (Table 2).
In the CATIE study, olanzapine and clozapine were associated with a greater increase in the serum level of cholesterol and triglycerides compared with other antipsychotics, even after adjusting for treatment duration. Furthermore, a retrospective chart review of patients who switched to aripiprazole from other SGAs showed a decrease in levels of total cholesterol and low-density lipoprotein cholesterol15 (Table 2).
Patients with schizophrenia are more likely to have dyslipidemia go undiagnosed, and therefore are less likely to be treated for the disorder. In the CATIE study, 88% of patients with dyslipidemia were not receiving any treatment.15
Management for dyslipidemia.
- Educate the patient and family about risks involved with dyslipidemia.
- Monitor weight and BMI at each visit.
- Monitor lipids to rule out dyslipidemia. Obtain a pretreatment fasting or random lipid profile for any patient receiving an antipsychotic; repeat at least every 6 months after starting the antipsychotic.
- Counsel the patient to quit smoking.
- Switch to an antipsychotic with lower risk of weight gain and dyslipidemia, such as switching from olanzapine or high-dose quetiapine to high- or medium-potency typical antipsychotics (such as, haloperidol or perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2).
- Educate and encourage the patient about modest physical activity. For example, a 20-minute walk everyday will reduce cardiovascular disease risk by 35% to 55%.6
- Refer to a dietitian if indicated.
- Ensure follow-up and initiation of treatment with a general practitioner.
- Educate and encourage the patient about modest physical activity. For example, a 20-minute walk everyday will reduce cardiovascular disease risk by 35% to 55%.
Metabolic syndrome
Metabolic syndrome is cluster of cardiovascular risk factors, including central adiposity, hyperglycemia, dyslipidemia, and hypertension. The National Cholesterol Education Program’s Adult Treatment Panel III report defines metabolic syndrome as the presence of 3 of 5 of the following factors:
- abdominal obesity (waist circumference of >40 inches in men, or >35 inches in women)
- triglyceride level, >150 mg/dL
- HDL cholesterol, <40 mg/dL in men and <50 mg/dL in women
- blood pressure, >130/85 mm Hg
- fasting plasma glucose level, >110 mg/dL.
The presence of metabolic syndrome in the general population is a strong predictor of cardiovascular diseases and diabetes.18 The adverse effects of metabolic syndrome are thought to relate to atherogenic dyslipidemia, higher blood pressure, insulin resistance with or without glucose intolerance, a proinflammatory state, and a prothrombotic state.
The prevalence of metabolic syndrome in patients with schizophrenia is 2- to 3-fold higher than the general population.19 In the CATIE study, approximately one-third of patients met criteria for metabolic syndrome at baseline.15 In a prospective study, De Hert et al20 reported that patients who were started on a SGA had more than twice the rate of developing metabolic syndrome compared with those treated with a FGA (Table 2). Other possible causes of metabolic syndrome are visceral adiposity and insulin resistance.16Management of the metabolic syndrome involves addressing the individual components that have been described in the preceding sections on T2DM and dyslipidemia.
Hepatitis C
Hepatitis C virus (HCV) infection is thought to be the most common blood-borne illness, with an estimated prevalence of 1% of the U.S. population. Some studies suggest that as many as 16% of people with schizophrenia have HCV infection.4 Risk factors for HCV infection include unsafe sexual practices, prostitution, homosexuality, homelessness, and IV drug use.
HCV treatments typically have involved regimens with interferon alfa, which is associated with significant neuropsychiatric side effects, including depression and suicide. There is a dearth of research on treatment of HCV in patients with schizophrenia; however, at least 1 study suggests that there was no increase in psychiatric symptoms in patients treated with interferon-containing regimens.21 There is even less evidence to guide the use of newer, non-interferon–based HCV treatment regimens that are better tolerated and have a higher response rate in the general population; there is reason, however, to be hopeful about their potential in patients with schizophrenia and HCV infection.
Managing HCV infection.
- Educate the patients and family about risk factors associated with contracting HCV.
- Screen for HCV infection in patients with schizophrenia because there is higher prevalence of HCV in these patients compared with the general population.
- When HCV infection is diagnosed, educate the patients and family about available treatments.
- Facilitate referral to an HCV specialist for appropriate treatment.
HIV/AIDS
HIV infection is highly prevalent among people suffering from severe mental illness such as schizophrenia. The incidence of HIV/AIDS in patients with schizophrenia is estimated to be 4% to 23%, compared with 0.6% in the general population.22 Risk factors associated with a higher incidence of HIV/AIDS in patients with schizophrenia are lack of knowledge about contracting HIV, unsafe sexual practices, prostitution, homosexuality, homelessness, and IV drug use.22
Managing HIV/AIDS.
- Educate the patient and family about risk factors associated with contracting HIV/AIDS.
- Educate patients about safe sex practices.
- All patients with schizophrenia should be screened for HIV because there is 10-fold higher HIV prevalence in schizophrenia compared with the general population.
- When HIV infection is diagnosed, facilitate referral to a HIV or infectious disease specialist for treatment.
- Educate the patient in whom HIV/AIDS has been diagnosed about the importance of (1) adherence to his (her) HIV medication regimen and (2) follow-up visits with an infectious disease practitioner and appropriate laboratory tests.
- Educate the patient’s family and significant other about the illness.
- Screen for and treat substance use.
- At each visit, inquire about the patient’s adherence to HIV medical therapy, viral load, and CD4 cell count.
Chronic obstructive pulmonary disease
Patients with schizophrenia are more likely to suffer from respiratory disease, such as chronic obstructive pulmonary disease (COPD) and asthma, compared with the general population.23 Smoking is a major risk factor for COPD. In a study by Dickerson et al,24 64% of people with schizophrenia were current smokers, compared with 19% of those without mental illness.
A high rate of smoking rate among people with schizophrenia suggests a “self-medication” hypothesis: That is, stimulation of CNS nicotinic cholinergic receptors treats the negative symptoms of schizophrenia and overcomes the dopamine blocking effects of antipsychotics.25 Among SGAs, only clozapine has a substantial body of evidence to support its association with decreased smoking behavior.
Managing COPD.
- Educate the patient and family about risk factors associated with COPD and smoking.
- Screen for tobacco use at each visit; try to increase motivation to quit smoking.
- Educate the patients and family about the value and availability of smoking cessation programs.
- Prescribe medication to help with smoking cessation when needed. Bupropion and varenicline have been shown to be effective in patients with schizophrenia; nicotine replacement therapies are safe and can be helpful.
- When treating a patient who is in the process of quitting, encourage and help him to maintain his commitment and enlist support from his family.
- Refer to an appropriate medical provider (primary care provider or pulmonologist) for a patient with an established or suspected diagnosis of COPD.
Cancer
Since 1909, when the Board of Control of the Commissioners in Lunacy for England and Wales noted the possibility of a decreased incidence in cancer among psychiatric patients, this connection has been a matter of controversy.26 Subsequent research has been equivocal; the prevalence of cancer has been reported to be either increased, similar, or decreased compared with the general population.26-28 Risk factors for cancer, including smoking, obesity, poor diet, sedentary lifestyle, and hyperprolactinemia, are more common among patients with schizophrenia.
Genetic factors and a possible protective effect from antipsychotics have been cited as potential causes of decreased prevalence. Clozapine is associated with an increased risk of leukemia. No conclusion can be drawn about the overall prevalence of cancer in schizophrenia.
Managing cancer in a patient with schizophrenia, however, poses a significant challenge29; he might lack capacity to make decisions about cancer treatment. The patient—or his surrogate decision-makers—need to carefully weigh current quality of life against potential benefits of treatment and risks of side effects. Adherence to complex, often toxic, therapies can be challenging for the patient with psychosis. Successful cancer treatment often requires close collaboration between the cancer treatment team and the patient’s support system, including the treating psychiatrist and case management teams.
Bottom Line
Patients with schizophrenia are at higher risk of developing comorbid medical
conditions because of the illness itself, lifestyle behaviors, genetics, and adverse
effects of medications. Because mental health clinicians focus attention on the
psychiatric and behavioral aspect of treatment, often there is delay in screening,
detecting, and treating medical comorbidities. This screening can be done in any
psychiatric practice, which can lead to timely management for those conditions
and preventing premature mortality in patients with schizophrenia.
Life expectancy for both males and females has been increasing over the past several decades to an average of 76 years. However, the life expectancy among individuals with schizophrenia in the United States is 61 years—a 20% reduction.1 Patients with schizophrenia are known to be at increased risk of several comorbid medical conditions, such as type 2 diabetes mellitus (T2DM), coronary artery disease, and digestive and liver disorders, compared with healthy people (Figure, page 32).2-5 This risk may be heightened by several factors, including sedentary lifestyle, a high rate of cigarette use, poor self-management skills, homelessness, and poor diet.
Although substantial attention is paid to the psychiatric and behavioral management of schizophrenia, many barriers impede the detection and treatment of patients’ medical conditions, which have been implicated in excess unforeseen deaths. Patients with schizophrenia might experience delays in diagnosis, leading to more acute comorbidity at time of diagnosis and premature mortality
Cardiovascular disease is the leading cause of death among psychiatric patients.6 Key risk factors for cardiovascular disease include smoking, obesity, hypertension, dyslipidemia, diabetes, and lack of physical activity, all of which are more prevalent among patients with schizophrenia.7 In addition, antipsychotics are associated with adverse metabolic effects.8 In general, smoking and obesity are the most modifiable and preventable risk factors for many medical conditions, such as cardiovascular disease, hyperlipidemia, diabetes, and many forms of cancer (Table 1).
In this article, we discuss how to manage common medical comorbidities in patients with schizophrenia. Comprehensive management for all these medical conditions in this population is beyond the scope of this article; we limit ourselves to discussing (1) how common these conditions are in patients with schizophrenia compared with the general population and (2) what can be done in psychiatric practice to manage these medical comorbidities (Box).
Obesity
Obesity—defined as body mass index (BMI) of >30—is common among patients with schizophrenia. The condition leads to poor self-image, decreased treatment adherence, and an increased risk of many chronic medical conditions (Table 1). Being overweight or obese can increase stigma and social discrimination, which will undermine self-esteem and, in turn, affect adherence with medications, leading to relapse.
The prevalence of obesity among patients with schizophrenia is almost double that of the general population9 (Figure2-5). Several factors predispose these patients to overweight or obese, including sedentary lifestyle, lack of exercise, a high-fat diet, medications side effects, and genetic factors. Recent studies report the incidence of weight gain among patients treated with antipsychotics is as high as 80%10 (Table 2).
Mechanisms involved in antipsychotic-induced weight gain are not completely understood, but antagonism of serotonergic (5-HT2C, 5-HT1A), histamine (H1), dopamine (D2), muscarinic, and other receptors are involved in modulation of food intake. Decreased energy expenditure also has been blamed for antipsychotic-induced weight gain.10
Pharmacotherapy and bariatric surgery can be as effective among patients with schizophrenia as they are among the general population. Maintaining a BMI of <25 kg/m2 lowers the risk of cardiovascular disease by 35% to 55%.6 Metformin has modest potential for offsetting weight gain and providing some metabolic control in overweight outpatients with schizophrenia,11 and should be considered early when treating at-risk patients.
Managing obesity. Clinicians can apply several measures to manage obesity in a patient with schizophrenia:
- Educate the patient, and the family, about the risks of being overweight or obese.
- Monitor weight and BMI at each visit.
- Advise smoking cessation.
- When clinically appropriate, switch to an antipsychotic with a lower risk of weight gain—eg, from olanzapine or high-dose quetiapine to a high- or medium-potency typical antipsychotic (eg, haloperidol, perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2, page 36).
- Consider prophylactic use of metformin with an antipsychotic; the drug has modest potential for offsetting weight gain and providing better metabolic control in an overweight patient with schizophrenia.11
- Encourage the patient to engage in modest physical activity; for example, a 20-minute walk, every day, reduces the risk of cardiovascular disease by 35% to 55%.6
- Recommend a formal lifestyle modification program, such as behavioral group-based treatment for weight reduction.12
- Refer the patient and family to a dietitian.
Type 2 diabetes mellitus
There is strong association between T2DM and schizophrenia that is related to abnormal glucose regulation independent of any adverse medication effect.13 Ryan et al14 reported that first-episode, drug-naïve patients with schizophrenia had a higher level of intra-abdominal fat than age- and BMI-matched healthy controls, suggesting that schizophrenia could be associated with changes in adiposity that might increase the risk of insulin resistance, hyperlipidemia, and dyslipidemia. Mechanisms that increase the risk of T2DM in schizophrenia include genetic and environmental factors, such as family history, lack of physical activity, and poor diet.
Diagnosis. All patients with schizophrenia should be evaluated for undiagnosed diabetes. The diagnosis of T2DM is made by documenting:
- a fasting plasma glucose reading of ≥126 mg/dL
- symptoms of T2DM, along with a random plasma glucose reading of ≥200 mg/dL
- 2-hour reading of a plasma glucose level >200 mg/dL on an oral glucose tolerance test.
Recent guidelines also suggest using a hemoglobin A1c value cutoff of ≥6.5% to diagnose T2DM.
In the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, 38% of patients with schizophrenia and diabetes were not receiving any treatment for T2DM.15
Risk factors for T2DM are:
- BMI >25
- a first-degree relative with diabetes
- lack of physical activity
- being a member of a high-risk ethnic group (African American, Hispanic American, Native American, Asian American, or Pacific Islander)
- having delivered a baby >9 lb or having had gestational diabetes
- hypertension
- high-density lipoprotein (HDL) cholesterol level of ≤35 mg/dL
- triglyceride level of ≥250 mg/dL
- history of an abnormal glucose tolerance test
- history of abnormal findings on a fasting plasma glucose test
- history of vascular disease.
Early detection and management.
- Educate the patient and family about signs and symptoms of T2DM, such as polyuria, nocturia, polydipsia, fatigue, visual disturbances, and (in women) vulvitis. Also, psychiatrists should be aware of, and inquire about, symptoms of diabetic ketoacidosis.
- At the start of therapy with any antipsychotic, particularly a second-generation antipsychotic (SGA), ask patients about a family history of diabetes and measure the hemoglobin A1c value.
- Monitor the hemoglobin A1c level 4 months after starting an antipsychotic, then annually, in a patient with significant risk factors for diabetes.
- Monitor blood glucose every 6 months in patients with no change from initial results and more frequently in those with significant risk factors for diabetes and those who gain weight.
- Order a lipid panel and measure the serum glucose level to rule out dyslipidemia and diabetes, because a patient with high lipid levels and diabetes is at higher risk of developing cardiovascular conditions.
- Advocate for smoking cessation.
- Switch to an antipsychotic with a lower risk of diabetes when clinically appropriate, such as switching a patient from olanzapine or high-dose quetiapine to a high- or medium-potency typical antipsychotic (such as haloperidol or perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2).
- Consider prophylactic use of metformin along with antipsychotics. Metformin has been used to improve insulin sensitivity and can lead to weight loss in diabetic and non-diabetic patients. The drug has modest potential for offsetting weight gain and providing better metabolic control in overweight outpatients with schizophrenia.11 Metformin is simple to use, does not lead to hypoglycemia, does not require serum glucose monitoring, and has a favorable safety profile.11
- Educate the patient about modest physical activity. For example, a 20-minute walk every day reduces the risk of cardiovascular disease by 35% to 55%.6
- Refer the patient to a dietitian to develop an appropriate diet plan.
- When diabetes is diagnosed, ensure appropriate follow-up and initiation or continuation of therapy with a general practitioner or an endocrinologist.
- Reinforce the need for ongoing follow-up and compliance with therapy for diabetes.
Hyperlipidemia and dyslipidemia
Elevated cholesterol and triglyceride levels are associated with cardiovascular diseases, such as ischemic heart disease and myocardial infarction. A 10% increase in cholesterol levels is associated with a 20% to 30% increase in the risk of coronary artery disease; lowering cholesterol by 10% decreases the risk by 20% to 30%.16 Triglyceride levels ≥250 mg/dL are associated with 2-fold higher risk of cardiovascular disease.16
The incidence of dyslipidemia is not as well studied as diabetes in patients with schizophrenia. There is increased prevalence of dyslipidemia in patients with schizophrenia compared with the general population because of obesity, lack of physical activity, and poor dietary habits.16
Data regarding the effects of first-generation antipsychotics (FGAs) on lipid levels are limited, but high-potency drugs, such as haloperidol, seem to carry a lower risk of hyperlipidemia than low-potency drugs, such as chlorpromazine and thioridazine.17 A comprehensive review on the effects of SGAs on plasma lipid levels suggested that clozapine, olanzapine, and quetiapine are associated with a higher risk of dyslipidemia17 (Table 2).
In the CATIE study, olanzapine and clozapine were associated with a greater increase in the serum level of cholesterol and triglycerides compared with other antipsychotics, even after adjusting for treatment duration. Furthermore, a retrospective chart review of patients who switched to aripiprazole from other SGAs showed a decrease in levels of total cholesterol and low-density lipoprotein cholesterol15 (Table 2).
Patients with schizophrenia are more likely to have dyslipidemia go undiagnosed, and therefore are less likely to be treated for the disorder. In the CATIE study, 88% of patients with dyslipidemia were not receiving any treatment.15
Management for dyslipidemia.
- Educate the patient and family about risks involved with dyslipidemia.
- Monitor weight and BMI at each visit.
- Monitor lipids to rule out dyslipidemia. Obtain a pretreatment fasting or random lipid profile for any patient receiving an antipsychotic; repeat at least every 6 months after starting the antipsychotic.
- Counsel the patient to quit smoking.
- Switch to an antipsychotic with lower risk of weight gain and dyslipidemia, such as switching from olanzapine or high-dose quetiapine to high- or medium-potency typical antipsychotics (such as, haloperidol or perphenazine), ziprasidone, aripiprazole, iloperidone, and lurasidone (Table 2).
- Educate and encourage the patient about modest physical activity. For example, a 20-minute walk everyday will reduce cardiovascular disease risk by 35% to 55%.6
- Refer to a dietitian if indicated.
- Ensure follow-up and initiation of treatment with a general practitioner.
- Educate and encourage the patient about modest physical activity. For example, a 20-minute walk everyday will reduce cardiovascular disease risk by 35% to 55%.
Metabolic syndrome
Metabolic syndrome is cluster of cardiovascular risk factors, including central adiposity, hyperglycemia, dyslipidemia, and hypertension. The National Cholesterol Education Program’s Adult Treatment Panel III report defines metabolic syndrome as the presence of 3 of 5 of the following factors:
- abdominal obesity (waist circumference of >40 inches in men, or >35 inches in women)
- triglyceride level, >150 mg/dL
- HDL cholesterol, <40 mg/dL in men and <50 mg/dL in women
- blood pressure, >130/85 mm Hg
- fasting plasma glucose level, >110 mg/dL.
The presence of metabolic syndrome in the general population is a strong predictor of cardiovascular diseases and diabetes.18 The adverse effects of metabolic syndrome are thought to relate to atherogenic dyslipidemia, higher blood pressure, insulin resistance with or without glucose intolerance, a proinflammatory state, and a prothrombotic state.
The prevalence of metabolic syndrome in patients with schizophrenia is 2- to 3-fold higher than the general population.19 In the CATIE study, approximately one-third of patients met criteria for metabolic syndrome at baseline.15 In a prospective study, De Hert et al20 reported that patients who were started on a SGA had more than twice the rate of developing metabolic syndrome compared with those treated with a FGA (Table 2). Other possible causes of metabolic syndrome are visceral adiposity and insulin resistance.16Management of the metabolic syndrome involves addressing the individual components that have been described in the preceding sections on T2DM and dyslipidemia.
Hepatitis C
Hepatitis C virus (HCV) infection is thought to be the most common blood-borne illness, with an estimated prevalence of 1% of the U.S. population. Some studies suggest that as many as 16% of people with schizophrenia have HCV infection.4 Risk factors for HCV infection include unsafe sexual practices, prostitution, homosexuality, homelessness, and IV drug use.
HCV treatments typically have involved regimens with interferon alfa, which is associated with significant neuropsychiatric side effects, including depression and suicide. There is a dearth of research on treatment of HCV in patients with schizophrenia; however, at least 1 study suggests that there was no increase in psychiatric symptoms in patients treated with interferon-containing regimens.21 There is even less evidence to guide the use of newer, non-interferon–based HCV treatment regimens that are better tolerated and have a higher response rate in the general population; there is reason, however, to be hopeful about their potential in patients with schizophrenia and HCV infection.
Managing HCV infection.
- Educate the patients and family about risk factors associated with contracting HCV.
- Screen for HCV infection in patients with schizophrenia because there is higher prevalence of HCV in these patients compared with the general population.
- When HCV infection is diagnosed, educate the patients and family about available treatments.
- Facilitate referral to an HCV specialist for appropriate treatment.
HIV/AIDS
HIV infection is highly prevalent among people suffering from severe mental illness such as schizophrenia. The incidence of HIV/AIDS in patients with schizophrenia is estimated to be 4% to 23%, compared with 0.6% in the general population.22 Risk factors associated with a higher incidence of HIV/AIDS in patients with schizophrenia are lack of knowledge about contracting HIV, unsafe sexual practices, prostitution, homosexuality, homelessness, and IV drug use.22
Managing HIV/AIDS.
- Educate the patient and family about risk factors associated with contracting HIV/AIDS.
- Educate patients about safe sex practices.
- All patients with schizophrenia should be screened for HIV because there is 10-fold higher HIV prevalence in schizophrenia compared with the general population.
- When HIV infection is diagnosed, facilitate referral to a HIV or infectious disease specialist for treatment.
- Educate the patient in whom HIV/AIDS has been diagnosed about the importance of (1) adherence to his (her) HIV medication regimen and (2) follow-up visits with an infectious disease practitioner and appropriate laboratory tests.
- Educate the patient’s family and significant other about the illness.
- Screen for and treat substance use.
- At each visit, inquire about the patient’s adherence to HIV medical therapy, viral load, and CD4 cell count.
Chronic obstructive pulmonary disease
Patients with schizophrenia are more likely to suffer from respiratory disease, such as chronic obstructive pulmonary disease (COPD) and asthma, compared with the general population.23 Smoking is a major risk factor for COPD. In a study by Dickerson et al,24 64% of people with schizophrenia were current smokers, compared with 19% of those without mental illness.
A high rate of smoking rate among people with schizophrenia suggests a “self-medication” hypothesis: That is, stimulation of CNS nicotinic cholinergic receptors treats the negative symptoms of schizophrenia and overcomes the dopamine blocking effects of antipsychotics.25 Among SGAs, only clozapine has a substantial body of evidence to support its association with decreased smoking behavior.
Managing COPD.
- Educate the patient and family about risk factors associated with COPD and smoking.
- Screen for tobacco use at each visit; try to increase motivation to quit smoking.
- Educate the patients and family about the value and availability of smoking cessation programs.
- Prescribe medication to help with smoking cessation when needed. Bupropion and varenicline have been shown to be effective in patients with schizophrenia; nicotine replacement therapies are safe and can be helpful.
- When treating a patient who is in the process of quitting, encourage and help him to maintain his commitment and enlist support from his family.
- Refer to an appropriate medical provider (primary care provider or pulmonologist) for a patient with an established or suspected diagnosis of COPD.
Cancer
Since 1909, when the Board of Control of the Commissioners in Lunacy for England and Wales noted the possibility of a decreased incidence in cancer among psychiatric patients, this connection has been a matter of controversy.26 Subsequent research has been equivocal; the prevalence of cancer has been reported to be either increased, similar, or decreased compared with the general population.26-28 Risk factors for cancer, including smoking, obesity, poor diet, sedentary lifestyle, and hyperprolactinemia, are more common among patients with schizophrenia.
Genetic factors and a possible protective effect from antipsychotics have been cited as potential causes of decreased prevalence. Clozapine is associated with an increased risk of leukemia. No conclusion can be drawn about the overall prevalence of cancer in schizophrenia.
Managing cancer in a patient with schizophrenia, however, poses a significant challenge29; he might lack capacity to make decisions about cancer treatment. The patient—or his surrogate decision-makers—need to carefully weigh current quality of life against potential benefits of treatment and risks of side effects. Adherence to complex, often toxic, therapies can be challenging for the patient with psychosis. Successful cancer treatment often requires close collaboration between the cancer treatment team and the patient’s support system, including the treating psychiatrist and case management teams.
Bottom Line
Patients with schizophrenia are at higher risk of developing comorbid medical
conditions because of the illness itself, lifestyle behaviors, genetics, and adverse
effects of medications. Because mental health clinicians focus attention on the
psychiatric and behavioral aspect of treatment, often there is delay in screening,
detecting, and treating medical comorbidities. This screening can be done in any
psychiatric practice, which can lead to timely management for those conditions
and preventing premature mortality in patients with schizophrenia.
1. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
2. De Hert M, Correl CU, Bobes J, et al. Physical illness in patients with severe mental disorder. I. Prevalence, impact of medications, and disparities in health care. World Psychiatry. 2011;10(1):52-77.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics update-2011 update. Circulation. 2011;123(4):e18-e209. doi: 10.1161/CIR.0b013e3182009701.
4. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
5. Lovre D, Mauvais-Jarvis F. Trends in prevalence of the metabolic syndrome. JAMA. 2015;314(9):950.
6. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
7. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
8. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
9. Allison DB, Fontaine KR, Heo M et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215-220.
10. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686-1696.
11. Jarskog LF, Hamer RM, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
12. Ganguli R. Behavioral therapy for weight loss in patients with schizophrenia. J Clin Psychiatry. 2007;68(suppl 4):19-25.
13. Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry Suppl. 2004;47:S64-S66.
14. Ryan MC, Flanagan S, Kinsella U, et al. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug naïve patients with schizophrenia. Life Sci. 2004;74(16):1999-2008.
15. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
16. Barnett AH, Mackin P, Chaudhry I, et al. Minimising metabolic and cardiovascular risk in schizophrenia: diabetes, obesity and dyslipidaemia. J Psychopharmacol. 2007;21(4):357-373.
17. Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70(1):1-17.
18. Sacks FM. Metabolic syndrome: epidemiology and consequences. J Clin Psychiatry. 2004;65(suppl 18):3-12.
19. De Hert M, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
20. De Hert M, Hanssens L, Wampers M, et al. Prevalence and incidence rates of metabolic abnormalities and diabetes in a prospective study of patients treated with second-generation antipsychotics. Schizophr Bull. 2007;33:560.
21. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
22. Davidson S, Judd F, Jolley D, et al. Risk factors for HIV/AIDS and hepatitis C among the chronic mentally ill. Aust N Z J Psychiatry. 2001;35(2):203-209.
23. Copeland LA, Mortensen EM, Zeber JE, et al. Pulmonary disease among inpatient decendents: impact of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):720-726.
24. Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64(1):44-50.
25. Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155(11):1490-1501.
26. Hodgson R, Wildgust HJ, Bushe CJ. Cancer and schizophrenia: is there a paradox? J Psychopharmacol. 2010;24(suppl 4):51-60.
27. Hippisley-Cox J, Vinogradova Y, Coupland C, et al. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry. 2007;64(12):1368-1376.
28. Grinshpoon A, Barchana M, Ponizovsky A, et al. Cancer in schizophrenia: is the risk higher or lower? Schizophr Res. 2005;73(2-3):333-341.
29. Hwang M, Farasatpour M, Williams CD, et al. Adjuvant chemotherapy for breast cancer patients with schizophrenia. Oncol Lett. 2012;3(4):845-850.
1. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
2. De Hert M, Correl CU, Bobes J, et al. Physical illness in patients with severe mental disorder. I. Prevalence, impact of medications, and disparities in health care. World Psychiatry. 2011;10(1):52-77.
3. Roger VL, Go AS, Lloyd-Jones DM, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics update-2011 update. Circulation. 2011;123(4):e18-e209. doi: 10.1161/CIR.0b013e3182009701.
4. Rosenberg SD, Goodman LA, Osher FC, et al. Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness. Am J Public Health. 2001;91(1):31-37.
5. Lovre D, Mauvais-Jarvis F. Trends in prevalence of the metabolic syndrome. JAMA. 2015;314(9):950.
6. Hennekens CH, Hennekens AR, Hollar D, et al. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. 2005;150(6):1115-1121.
7. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical point. J Psychopharmacol. 2010;24(suppl 4):17-25.
8. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
9. Allison DB, Fontaine KR, Heo M et al. The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry. 1999;60(4):215-220.
10. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686-1696.
11. Jarskog LF, Hamer RM, Catellier DJ, et al; METS Investigators. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. Am J Psychiatry. 2013;170(9):1032-1040.
12. Ganguli R. Behavioral therapy for weight loss in patients with schizophrenia. J Clin Psychiatry. 2007;68(suppl 4):19-25.
13. Kohen D. Diabetes mellitus and schizophrenia: historical perspective. Br J Psychiatry Suppl. 2004;47:S64-S66.
14. Ryan MC, Flanagan S, Kinsella U, et al. The effects of atypical antipsychotics on visceral fat distribution in first episode, drug naïve patients with schizophrenia. Life Sci. 2004;74(16):1999-2008.
15. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
16. Barnett AH, Mackin P, Chaudhry I, et al. Minimising metabolic and cardiovascular risk in schizophrenia: diabetes, obesity and dyslipidaemia. J Psychopharmacol. 2007;21(4):357-373.
17. Meyer JM, Koro CE. The effects of antipsychotic therapy on serum lipids: a comprehensive review. Schizophr Res. 2004;70(1):1-17.
18. Sacks FM. Metabolic syndrome: epidemiology and consequences. J Clin Psychiatry. 2004;65(suppl 18):3-12.
19. De Hert M, Schreurs V, Vancampfort D, et al. Metabolic syndrome in people with schizophrenia: a review. World Psychiatry. 2009;8(1):15-22.
20. De Hert M, Hanssens L, Wampers M, et al. Prevalence and incidence rates of metabolic abnormalities and diabetes in a prospective study of patients treated with second-generation antipsychotics. Schizophr Bull. 2007;33:560.
21. Huckans M, Mitchell A, Pavawalla S, et al. The influence of antiviral therapy on psychiatric symptoms among patients with hepatitis C and schizophrenia. Antivir Ther. 2010;15(1):111-119.
22. Davidson S, Judd F, Jolley D, et al. Risk factors for HIV/AIDS and hepatitis C among the chronic mentally ill. Aust N Z J Psychiatry. 2001;35(2):203-209.
23. Copeland LA, Mortensen EM, Zeber JE, et al. Pulmonary disease among inpatient decendents: impact of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):720-726.
24. Dickerson F, Stallings CR, Origoni AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999-2011. Psychiatr Serv. 2013;64(1):44-50.
25. Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155(11):1490-1501.
26. Hodgson R, Wildgust HJ, Bushe CJ. Cancer and schizophrenia: is there a paradox? J Psychopharmacol. 2010;24(suppl 4):51-60.
27. Hippisley-Cox J, Vinogradova Y, Coupland C, et al. Risk of malignancy in patients with schizophrenia or bipolar disorder: nested case-control study. Arch Gen Psychiatry. 2007;64(12):1368-1376.
28. Grinshpoon A, Barchana M, Ponizovsky A, et al. Cancer in schizophrenia: is the risk higher or lower? Schizophr Res. 2005;73(2-3):333-341.
29. Hwang M, Farasatpour M, Williams CD, et al. Adjuvant chemotherapy for breast cancer patients with schizophrenia. Oncol Lett. 2012;3(4):845-850.
Chronic pain and depression: Treatment of 2 culprits in common
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
Patients who have chronic pain and those with a major depressive disorder (MDD) share clinical features, including fatigue, cognitive complaints, and functional limitation. Sleep disturbance and anxiety are common with both disorders. Because pain and depression share common neurobiological pathways (see Part 1 of this article in the February 2016 issue and at CurrentPsychiatry.com) and clinical manifestations, you can use similar strategies and, often, the same agents to treat both conditions when they occur together (Table 1).
What are the medical options?
Antidepressants. Using an antidepressant to treat chronic pain is a common practice in primary care and specialty practice. Antidepressants that modulate multiple neurotransmitters appear to be more efficacious than those with a single mechanism of action.1 Convergent evidence from preclinical and clinical studies supports the use of serotonin-norepinephrine reuptake inhibitors (SNRIs) as more effective analgesic agents, compared with the mostly noradrenergic antidepressants, which, in turn, are more effective than selective serotonin reuptake inhibitors (SSRIs).2 The mechanism of the analgesic action of antidepressants appears to rely on their inhibitory effects of norepinephrine and serotonin reuptake, thereby elevating the performance of endogenous descending pain regulatory pathways.3
Tricyclic antidepressants (TCAs), primarily amitriptyline, nortriptyline, and desipramine, have the advantage of years of clinical experience and low cost. Their side effect burden, however, is higher, especially in geriatric patients. Dose-dependent side effects include sedation, constipation, dry mouth, urinary retention, and orthostatic hypotension.
TCAs must be used with caution in patients with suicidal ideation because of the risk of a potentially lethal intentional overdose.
The key to using a TCA is to start with a low dosage, followed by slow titration. Typically, the dosages of TCAs used in clinical trials that focused on pain have been lower (25 to 100 mg/d of amitriptyline or equivalent) than the dosage typically necessary for treating depression; however, some experts have found that titrating TCAs to higher dosages with an option of monitoring serum levels may benefit some patients.4
SNRIs are considered first-line agents for both neuropathic pain and fibromyalgia. Duloxetine has been shown to be effective in both conditions5; venlafaxine also has shown efficacy in neuropathic pain.6 Milnacipran, another SNRI that blocks 5-HT, and norepinephrine equally and exerts a mild N-methyl-D-aspartate inhibition, has proven efficacy in fibromyalgia.7,8
SSRIs for alleviating central pain or neuropathic pain are supported by minimal evidence only.9 A review of the effectiveness of various antidepressants on pain in diabetic neuropathy concluded that fluoxetine was no more effective than placebo.10,11 Schreiber and Pick11 evaluated the antinociceptive properties of several SSRIs and offered the opinion that fluoxetine, fluvoxamine, and citalopram were, at best, weak antinociceptors.
Opioids. Data on the long-term benefits of opioids are limited, except for use in carefully selected patients; in any case, risk of abuse, diversion, and even death with these agents is quite high.12 Also, there is evidence that opioid-induced hyperalgesia might limit the usefulness of opioids for controlling chronic pain.13
Gabapentin and pregabalin, both anticonvulsants, act by binding to the α-2-σ subunit of voltage-gated calcium channels within the CNS.14 By reducing calcium influx at nerve terminals, the drugs diminish the release of several neurotransmitters, including glutamate, noradrenaline, and substance P. This mechanism is thought to be the basis for the analgesic, anticonvulsant, and anxiolytic effects of these drugs.15
Gabapentin and pregabalin have been shown to decrease pain intensity and improve quality of life and function in patients with neuropathic pain conditions. Pregabalin also has shown efficacy in treating central neuropathic pain and fibromyalgia.16
Added benefits of these drugs is that they have (1) a better side effect profile than TCAs and (2) fewer drug interactions when they are used as a component of combination therapy. Pregabalin has the additional advantage of less-frequent dosing, linear pharmacokinetics, and a predictable dose-response relationship.17
Addressing other comorbid psychiatric conditions
Sleep disturbance is common among patients with chronic pain. Sleep deprivation causes a hyperexcitable state that amplifies the pain response.18
When a patient presents with chronic pain, depression, and disturbed sleep, consider using a sedating antidepressant, such as a TCA. Alternatively, gabapentin or pregabalin can be added to an SNRI; anticonvulsants have been shown to improve quality of sleep.19 Cognitive-behavioral interventions targeting sleep disturbance may be a helpful adjunct in these patients.20
When anxiety is comorbid with chronic pain, antidepressants with proven efficacy in treating anxiety disorders, such as duloxetine or venlafaxine, can be used. When chronic pain coexists with a specific anxiety disorder (social anxiety disorder, obsessive-compulsive disorder, panic disorder), an SSRI might be more advantageous than an SNRI,21 especially if it is combined with a more efficacious analgesic.
Benzodiazepines should be avoided as a routine treatment for comorbid anxiety and pain, because these agents can produce sedation and cognitive interference, and carry the potential for dependence.
Fatigue. In patients who, in addition to pain and depression, complain of fatigue, an activating agent such as milnacipran or adjunct bupropion might be preferable to other agents. Modafinil has been shown to be a well-tolerated and potentially effective augmenting agent for antidepressants when fatigue and sleepiness are present as residual symptoms22; consider them as adjuncts when managing patients who have chronic pain and depression that manifests as excessive sleepiness and/or fatigue.
Cognitive complaints. We have noted that disturbances of cognition are common in patients with depression and chronic pain, and that cognitive dysfunction might improve after antidepressant treatment.
Studies suggest that SSRIs, duloxetine, and other antidepressants, such as bupropion, might exert a positive effect on learning, memory, and executive function in depressed patients.23 Beneficial effects of antidepressants may be “pseudo-specific,” however—that is, predominantly a reflection of overall improvement in mood, not on specific amelioration of the cognitive disturbance.
Vortioxetine has shown promise in improving cognitive function in adults with MDD; its cognitive benefits are largely independent of its antidepressant effect.24 The utility of vortioxetine in chronic pain patients has not been studied, but its positive impact on mood, anxiety, sleep, and cognition might make it a consideration for patients with comorbid depression—although it is uncertain at this time whether putative noradrenergic activity makes it suitable for use in chronic pain disorders.
Last, avoid TCAs in patients who have cognitive complaints. These agents have anticholinergic effects that can have an adverse impact on cognitive function.
Cautions: Drug−drug interactions, suicide risk, disrupted sleep
Avoiding drug−drug interactions is an important consideration when treating comorbid disorders. Many chronic pain patients take over-the-counter or prescribed nonsteroidal anti-inflammatory drugs for analgesia; these agents can increase the risk of gastrointestinal bleeding when they are combined with an SSRI or an SNRI.
The use of the opioid tramadol with an SNRI or a TCA is discouraged because of the risk of serotonin syndrome.
Combining a sedating antidepressant, such as a TCA, with gabapentin or pregabalin can increase the risk of CNS depression and psychomotor impairment, especially in geriatric patients. An opioid analgesic is likely to amplify these effects.
Suicidal ideation is not uncommon in patients with chronic pain and depression. To minimize the risk of suicide in patients with a chronic pain disorder, you should ensure optimal pain control by combining the most efficacious analgesic agent with psychotherapeutic interventions and optimal antidepressant treatment. Furthermore, cognitive-behavioral therapy (CBT) (see the discussion below) might not only improve pain coping skills, but also ameliorate catastrophizing, anxiety, and concomitant sleep disturbance.
Complaints of sleep disturbance and anxiety can compound the risk of suicide in a chronic pain patient. When possible, these complex patients should be treated by a multidisciplinary team that includes a pain management specialist, psychotherapist, and primary care clinician. It is important to strengthen the clinician−patient relationship to facilitate close monitoring of symptoms and to provide a trusting environment in which patients feel free to discuss thoughts of suicide or self-harm. For such patients, prescribing opiates and TCAs in small quantities is a prudent action.
When a patient struggles with suicidal thoughts, his (her) family might need to dispense these medications. Most important, if a patient is actively suicidal, consider referral to an inpatient facility or intensive outpatient program, where aggressive treatment of depressive symptoms and intensive monitoring and support can be provided.25
Usefulness of non-drug interventions
There is, of course, a diversity of non-drug treatments for MDD and for chronic pain; discussion here focuses primarily on modalities with established efficacy in both disease states (Table 2). On rare occasions, non-drug treatments can constitute a stand-alone approach; most often, they are incorporated into a multimodal treatment plan or applied as an adjunct intervention.26
Psychotherapy. The most robust evidence supports the use of CBT in addressing MDD and chronic pain—occurring individually and comorbidly.26-28 Efficacy is well established in MDD, as monotherapy and adjunct treatment, spanning acute and maintenance phases.
Furthermore, CBT also has support from randomized trials, meta-analyses, and treatment guidelines, either as monotherapy or co-therapy for both short-term relief and long-term pain reduction. Additionally, CBT has demonstrated value for relieving pain-related disability.26,28
Combination of a special form of CBT, rumination-focused CBT with ongoing pharmacological therapy over a 26-week period in a group of medication-refractory MDD patients produced a remission rate of 62%, compared with 21% in a treatment-as-usual group.29 This is of particular interest in chronic pain patients, because rumination-related phenomena of pain catastrophizing and avoidance facilitate a transition from acute to chronic pain, while augmenting pain severity and associated disability.30
Catastrophizing also has been implicated in mediating the relationship between pain and sleep disturbance. Not surprisingly, a randomized controlled study demonstrated the benefit of 8-week, Internet-delivered CBT in patients suffering from comorbid chronic pain, depression, and anxiety. Treatment significantly diminished pain catastrophizing, depression, and anxiety; maintenance of improvement was demonstrated after 1 year of follow-up.31
Other behavioral and psychological approaches. Biofeedback, mindfulness-based stress reduction, relaxation training and diaphragmatic breathing, guided imagery, hypnosis, and supportive groups might play an important role as components of an integrated mind−body approach to chronic pain,28,32,33 while also providing mood benefits.
Exercise. The role of exercise as a primary treatment of MDD continues to be controversial, but its benefits as an add-on intervention are indisputable. Exercise not only complements pharmacotherapy to produce greater reduction in depressive scores and improvement in quality of life, it might aid in reestablishing social contacts when conducted in a group setting—an effect that can be of great value in both MDD and chronic pain.34
Exercise and restorative therapies provide several benefits for chronic pain patients, including:
- improved pain control, cognition, and mood
- greater strength and endurance
- cardiovascular and metabolic benefits
- improved bone health and functionality.26,28,32,33,35
To achieve optimal benefit, an exercise program must be customized to fit the patient’s physical condition, level of fitness, and specific type of pain.35 Preliminary evidence suggests that, beyond improvement in pain and functionality, exercise might reduce depressive symptoms in chronic pain patients.36
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
1. Sharp J, Keefe B. Psychiatry in chronic pain: a review and update. Curr Psychiatry Rep. 2005;7(3):213-219.
2. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
3. Schug SA, Goddard C. Recent advances in the pharmacological management of acute and chronic pain. Ann Palliat Med. 2014;3(4):263-275.
4. Kroenke K, Krebs EE, Bair MJ. Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry. 2009;31(3):206-219.
5. Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. doi: 10.1002/14651858.CD007115.pub3.
6. Rowbotham MC, Goli V, Kunz NR, et al. Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697-706.
7. Kranzler JD, Gendreau JF, Rao SG. The psychopharmacology of fibromyalgia: a drug development perspective. Psychopharmacol Bull. 2002;36(1):165-213.
8. Pae CU, Marks DM, Shah M, et al. Milnacipran: beyond a role of antidepressant. Clin Neuropharmacol. 2009;32(6):355-363.
9. Depression and pain. J Clin Psychiatry. 2008;69(12):1970-1978.
10. Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326(19):1250-1256.
11. Schreiber S, Pick CG. From selective to highly selective SSRIs: a comparison of the antinociceptive properties of fluoxetine, fluvoxamine, citalopram and escitalopram. Eur Neuropsychopharmacol. 2006;16(6):464-468.
12. Freynhagen R, Geisslinger G, Schug SA. Opioids for chronic non-cancer pain. BMJ. 2013;346:f2937. doi: 10.1136/bmj.f2937.
13. Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679-684.
14. Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit α2σ-1 to presynaptic terminals in neuropathic pain is inhibited by the α2σ ligand pregabalin. J Neurosci. 2009;29(13):4076-4088.
15. Dooley DJ, Taylor CP, Donevan S, et al. Ca2+ channel α2σ ligands: novel modulators of neurotransmission [Erratum in: Trends Pharmacol Sci. 2007;28(4):151]. Trends Pharmacol Sci. 2007;28(2):75-82.
16. Wiffen PJ, Derry S, Moore RA, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia - an overview of Cochrane reviews. Cochrane Database Syst Rev. 2013;11:CD010567. doi: 10.1002/14651858.CD010567.pub2.
17. Finnerup NB, Otto M, Jensen TS, et al. An evidence-based algorithm for the treatment of neuropathic pain. MedGenMed. 2007;9(2):36.
18. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. 2004;5(suppl 1):S9-S27.
19. Sammaritano M, Sherwin A. Effect of anticonvulsants on sleep. Neurology. 2000;54(5 suppl 1):S16-S24.
20. Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005-2015.
21. Fishbain DA. Polypharmacy treatment approaches to the psychiatric and somatic comorbidities found in patients with chronic pain. Am J Phys Med Rehabil. 2005;84(suppl 3):S56-S63.
22. Fava M, Thase ME, DeBattista C. A multicenter, placebo-controlled study of modafinil augmentation in partial responders to selective serotonin reuptake inhibitors with persistent fatigue and sleepiness. J Clin Psychiatry. 2005;66(1):85-93.
23. Baune BT, Renger L. Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res. 2014;219(1):25-50.
24. McIntyre RS, Lophaven S, Olsen CK. A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol. 2014;17(10):1557-1567.
25. Cheatle MD. Depression, chronic pain, and suicide by overdose: on the edge. Pain Med. 2011;12(suppl 2):S43-S48.
26. Chang KL, Fillingim R, Hurley RW, et al. Chronic pain management: nonpharmacological therapies for chronic pain. FP Essent. 2015;432:21-26.
27. Cuijpers P, Smit F, Bohlmeijer E, et al. Efficacy of cognitive-behavioural therapy and other psychological treatments for adult depression: meta-analytic study of publication bias. Br J Psychiatry. 2010;196(3):173-178.
28. Lambert M. ICSI releases guideline on chronic pain assessment and management. Am Fam Physician. 2010;82(4):434-439.
29. Watkins ER, Mullan E, Wingrove J, et al. Rumination-focused cognitive-behavioural therapy for residual depression: phase II randomised controlled trial. Br J Psychiatry. 2011;199(4):317-322.
30. Turk DC, Wilson HD. Fear of pain as a prognostic factor in chronic pain: conceptual models, assessment, and treatment implications. Curr Pain Headache Rep. 2010;14(2):88-95.
31. Buhrman M, Syk M, Burvall O, et al. Individualized guided Internet-delivered cognitive-behavior therapy for chronic pain patients with comorbid depression and anxiety: a randomized controlled trial. Clin J Pain. 2015;31(6):504-516.
32. American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112(4):810-833.
33. Theadom A, Cropley M, Smith HE, et al. Mind and body therapy for fibromyalgia. Cochrane Database Syst Rev. 2015;4:CD001980. doi: 10.1002/14651858.CD001980.pub3.
34. Mura G, Moro MF, Patten SB, et al. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496-508.
35. Kroll HR. Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):263-281.
36. Liang H, Zhang H, Ji H, et al. Effects of home-based exercise intervention on health-related quality of life for patients with ankylosing spondylitis: a meta-analysis. Clin Rheumatol. 2015;34(10):1737-1744.
Chronic pain and depression: Understanding 2 culprits in common
Any discussion of the relationship between major depressive disorder (MDD) and chronic pain encounters an obstacle immediately: Neither has a singular pathophysiology. Furthermore, MDD and, to a significant extent, chronic pain are defined more by their symptoms than by a presumed etiology and pathogenesis.
Why does this matter to a busy clinician?
Explicitly or implicitly, we often align our treatment approaches with what we assume is the underlying pathophysiology of the conditions we are addressing. An overview of shared pathophysiology of chronic pain conditions and MDD therefore can be useful in practice.
What is chronic pain? Defined as “pain that persists past the healing phase following an injury,”1 chronic pain often is subdivided into 4 types2,3:
- nociceptive (caused by a lesion or potential tissue damage)
- inflammatory
- neuropathic (spontaneous pain or hypersensitivity to pain related to neurologic illness or injury)
- functional (hypersensitivity to pain due to abnormal central processing of a normal input).
Although fibromyalgia often is categorized as a dysfunctional pain syndrome, persons who suffer from it, much like those who suffer neuropathic pain, commonly report hyperalgesia (augmented sensitivity to painful stimuli), allodynia (abnormal pain response to non-noxious stimuli), and paresthesias. These shared clinical features of fibromyalgia and neuropathic pain are consistent with central sensitization, which suggests overlapping pathophysiology.4
Comorbidity between depression and pain is common. A 30% to 60% co-occurrence rate of MDD and chronic pain has been reported.5 Some subtypes of chronic pain, such as fibromyalgia, are so commonly comorbid with psychiatric conditions that they have spawned a scientific debate as to whether the conditions are most parsimoniously considered (1) separate illnesses with high comorbidity or (2) different symptomatic manifestations of a single underlying condition.6 Moreover, cumulative evidence suggests that chronic pain and depression do not just co-occur; each one facilitates development of the other, such that chronic pain is a strong predictor of subsequent onset of MDD, and vice versa.
When pain and depression are comorbid, they also tend to make treatment of each condition more difficult. For example, pain presents (1) a major obstacle to achieving remission when treating depression7,8 and (2) significant risk of relapse.9 A 3-year longitudinal study showed that painful symptoms substantially reduced the chance of recovery in a group of older depressed patients (n = 327). A substantially greater percentage of patients with MDD alone attained recovery (47%), compared with only 9% in whom MDD and painful symptoms were comorbid.10 Furthermore, a higher level of pain can delay remission when treating MDD,11 thus reducing the likelihood of an optimal outcome.12
Understanding shared processes. Recent developments in neuroscience and psycho-immunology point to the fact that comorbid pain and depression might be driven by overlapping pathophysiological processes in the brain and body. In the 2 parts of this article, we (1) review scientific understanding of these shared processes and (2) demonstrate how recent advances in the epidemiology, phenomenology, and etiology of chronic pain and MDD provide important clues for more effective diagnosis (Part 1) and treatment (Part 2, March 2016)—and, therefore, better outcomes. Our focus is primarily on the relationship between MDD and the best-studied comorbid chronic pain conditions: fibromyalgia, neuropathic pain, chronic back pain, and rheumatoid arthritis.
The societal burden of chronic pain conditions is enormous
A recent epidemiological study13 projected that as many as 100 million people in the United States—30.7% of the population—suffer some form of chronic pain, including arthritis and joint pain. A World Health Organization survey yielded a similar (and staggering) 37% prevalence of chronic pain in the population of 10 developed countries.14
Estimates are that various forms of neuropathic pain, including diabetic neuropathy, postherpetic neuralgia, trigeminal neuralgia, spinal cord injury, and radiculopathy, alone afflict as many as 26 million people worldwide, including approximately 1.5% of the U.S. population.15,16
Chronic low back pain is epidemic. With a projected point prevalence of 30%, the condition is the most common cause of activity limitation among people age <45, and the most frequent reason in the United States for visiting a physician.1
Functional somatic syndromes, including fibromyalgia and irritable bowel syndrome, impose an astounding strain on health care: These syndromes account for 25% to 50% of all outpatient visits, or approximately 400 million clinic visits annually in the United States.17
Why should you care about these numbers? The answer is that comorbidity among chronic pain, mood disorders, anxiety disorders, sleep disorders, cognitive impairment, fatigue, and chronic stress presents an enormous clinical challenge because it not only complicates the diagnosis of these conditions but also compromises treatment outcomes and imposes severe limitations on daily functioning and quality of life of those afflicted.5,17-24
A complex relationship and a daunting clinical challenge
Chronic pain enhances the risk of MDD by 2-fold to 5-fold. The risk appears to be mediated by the number of pain conditions rather than by the severity of pain.23 Some authors have noted a kind of dose-response relationship among pain, depression, and anxiety. Among patients who experienced chronic pain that affected 1 body region, the prevalence of generalized anxiety disorder (GAD) and MDD was 30% and 20%, respectively; in patients who experienced pain in ≥2 regions, the prevalence of GAD and MDD was elevated to 54% and 32%.25 Moreover, patients with fibromyalgia were 4.3 times more likely than healthy controls to develop MDD at some point in their lives and 4.7 times more likely to develop an anxiety disorder.26
Although women are more likely to suffer from fibromyalgia, the risk for people of either sex of developing subsequent MDD is comparable once the condition has developed.27 Overall, depression and anxiety are among the most common comorbidities of fibromyalgia, with prevalence ranging from 20% to 80% and 13% to 63.8%, respectively.28
High comorbidity between depression and pain also is relevant for patients with neuropathic pain. A survey from Australia reported depression in 34% and anxiety in 25% of patients with neuropathic pain.29 Pain severity tended to be enduring and associated with significantly impaired functioning. A significant percentage of patients suffering from rheumatoid arthritis and systemic lupus erythematosus tend to manifest anxiety and depression (93% to 94%), cognitive impairment (66%), fatigue (40%), and sleep disorders (72%).22
The relationship between depression and pain appears to be bidirectional. For example, recent studies demonstrate that 30% to 60% of depressed patients also suffer from a painful condition.5
The complex history of patients presenting with concomitant complaints of depression, anxiety, chronic pain, sleep disturbance, cognitive impairment, and fatigue present a daunting diagnostic task. Pain tends to be associated with greater fatigue and sleep disturbance, which in turn depletes a patient’s ability to enjoy life and enhances negative affect.19,20,30 The take-home message might be to screen all chronic pain patients for MDD, anxiety, and sleep disorders, and vice versa.
Furthermore, comorbidity among chronic pain, MDD, anxiety, and sleep disorders can introduce specific intricacies into our treatment approach. Although, in general, comorbidities tend to have a negative impact on treatment outcomes, many pharmacotherapeutic and non-drug interventions targeting chronic pain might ameliorate sleep problems, low energy, anxiety, depression, and anhedonia.18,20,30-32 On the other hand, we should consider that opioid treatment for chronic pain might represent a risk factor for subsequent depression. It is conceivable that chronic opioid treatment and associated sedation can erode self-efficacy and social relationships, thereby compromising sources of support.33,34 It is equally important to keep in mind that, even if we are successful in attaining remission when treating depression and pain, residual pain symptoms might persist, requiring more specific interventions.24
MDD and chronic pain each have, on their own, a well-established association with suicide attempts and completion. Researchers are investigating whether a pathophysiologic suicide-promoting synergy between the 2 disorders exists when they are comorbid (Box35-37).
Shared genetics and pathophysiology
Several candidate genes have been identified as risk genes for chronic pain, depression, and anxiety. One of those studied the most is 5-HTTLPR, involved in regulating synthesis of serotonin transporter. The short form of this gene has been implicated in a diverse set of conditions, including MDD, anxiety disorders, and substance abuse—and fibromyalgia. Other genes associated with the risk of MDD and pain disorders are ones that code for:
- serotonin 5-HT2A and 5-HT1A receptors
- catechol-O-methyltransferase, an enzyme involved in catecholamine metabolism
- dopamine D4 receptor
- proinflammatory cytokines interleukin-1 and interleukin-6.4
Both monoamines and inflammatory cytokines play a role in modulating γ-aminobutyric acid (GABA) and glutamate neurons, as well as glia cells constituting peripheral pain pathways and central circuits that participate in the pain response and regulation of mood.4,17,38
The ‘pain matrix’
Brain circuitry that is involved in processing pain stimuli—often referred to as the pain matrix—shares many structural components with circuitry involved in the stress response and emotional modulation.4 Emerging evidence indicates that the pain matrix might not be pain-specific but, instead, a complex aggregate of interconnected brain structures involved in evoking defensive responses to a number of offending stimuli, including pain, threat, danger, loss, and social rejection or isolation.
It is remarkable, in this regard, that imaging studies show that the dorsal anterior cingulate, central to experiencing negative affect in response to physical pain, also mediates distress in response to the “pain” of social exclusion.39 Emerging functional and structural imaging provides evidence of continuous reorganization of prefrontal cortices as a consequence of enduring chronic pain.1 Of particular interest are findings of (1) a reduction of gray matter in the dorsolateral prefrontal cortex (DLPFC) and (2) functional activation of the medial prefrontal cortex (mPFC), both of which correlate with the duration and experience of chronic back pain.1 It is tempting to speculate that structural decline of the DLPFC, observed in MDD and chronic pain, is linked to cognitive and executive function deficits, which are readily observed in patients with either disorder—given that DLPFC is a “hub” of the so-called “cognitive-executive functional network.”1,4
Likewise, the mPFC is a key component of the default mode network (DMN), a functional network also comprising the posterior cingulate cortex and hippocampus. DMN performs a diverse set of activities, including self-reflection, daydreaming, reminiscing, planning, processing of social information, and creative thinking. Negative neuroplastic changes in the DMN are a common finding in MDD and chronic pain, and might be associated with a tendency toward rumination and catastrophizing—key clinical manifestations of MDD and chronic pain—and linked with pervasive negative affect and sleep disturbance.4,32
Furthermore, functional and structural changes in the amygdala and hippocampus have been described in MDD, fibromyalgia, and neuropathic pain.4 Dysfunction of these limbic formations may be a contributing factor in the disruption of neuroendocrine, autonomic, and immune function, which could further contribute to aggravated mood and pain symptoms.4,17,40
Consequently, excessive hypothalamic-pituitary-adrenal axis and sympathetic activation, combined with elevation of proinflammatory cytokine production and release, likely plays a role in the pathophysiology of MDD and chronic pain disorders.4,17,40 Moreover, at cellular, subcellular, and molecular levels, chronic pain and MDD are associated with:
- perturbed neuron-glia relationships
- altered glutamatergic, GABA, glycine, substance-P, opioid, 5-HT, norepinephrine, and dopamine signaling
- dysfunction of intracellular signaling cascades and neurotrophic signaling.4,20,30,31,38
The Figure that describes how homeostatic function of prefrontal cortical-limbic circuitry is compromised in MDD and chronic pain—thus disrupting autonomic, neuroendocrine, and neuroimmune regulation.
Disturbance in monoamine signaling in chronic pain and MDD might give rise to profound anhedonia, cognitive impairment, anxiety, insomnia, sensitivity to stress, and inadequate functioning of descending pain-regulatory pathways, which primarily use norepinephrine and 5-HT.4,9,20,30,31,38 Using pharmacotherapeutic agents that successfully modulate monoamines, therefore, might ameliorate the function of brain networks innervated by neurotransmitter systems involved in the regulation of pain, mood, cognition, stress response, and sleep. Notably, the same monoamines serve as transmitters in descending pain pathways.
In summary, convergent evidence indicates that MDD and chronic pain states amplify each other, thus contributing to treatment resistance in both disorders.
On the bright side, timely and effective treatment of MDD might optimize the chance of remission and minimize the risk of enduring structural brain changes in MDD and chronic pain.1,4,31,32 The obverse is also true: Emphasizing the importance of the resolution of painful symptoms in the context of MDD, a study reported a significantly greater remission rate of 36.2% in those who had >50% reduction of pain on a visual analogue scale following treatment with a serotonin-norepinephrine reuptake inhibitor, compared with a 17.8% remission rate in persons who experienced <50% pain reduction on the scale.3
Editors’ note: In Part 2 of this article (March 2016), the authors review pharmacotherapeutic and non-drug strategies for managing comorbid chronic pain conditions and MDD.
1. Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87(2):81-97.
2. Verdu B, Decosterd I, Buclin T, et al. Antidepressants for the treatment of chronic pain. Drugs. 2008;68(18):2611-2632.
3. Woolf CJ; American College of Physicians, American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
4. Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci (Landmark Ed). 2009;14:5291-5338.
5. Bair MJ, Wu J, Damush TM, et al. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
6. Cho HJ, Skowera A, Cleare A, et al. Chronic fatigue syndrome: an update focusing on phenomenology and pathophysiology. Curr Opin Psychiatry. 2006;19(1):67-73.
7. Fava M. Depression with physical symptoms: treating to remission. J Clin Psychiatry. 2003;64(suppl 7):24-28.
8. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
9. Ohayon MM. Specific characteristics of the pain/depression association in the general population. J Clin Psychiatry. 2004;65(suppl 12):5-9.
10. Geerlings SW, Twisk JW, Beekman AT, et al. Longitudinal relationship between pain and depression in older adults: sex, age and physical disability. Soc Psychiatry Psychiatr Epidemiol. 2002;37(1):23-30.
11. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
12. Spijker J, de Graaf R, Bijl RV, et al. Determinants of persistence of major depressive episodes in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). J Affect Disord. 2004;81(3):231-240.
13. Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230-1239.
14. Dzau VJ, Pizzo PA. Relieving pain in America: insights from an Institute of Medicine committee. JAMA. 2014;312(15):1507-1508.
15. Butera JA. Current and emerging targets to treat neuropathic pain. J Med Chem. 2007;50(11):2543-2546.
16. Offenbaecher M, Ackenheil M. Current trends in neuropathic pain treatments with special reference to fibromyalgia. CNS Spectr. 2005;10(4):285-297.
17. Goldenberg DL. Pain/depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med. 2010;123(8):675-682.
18. Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007;23(1):15-22.
19. Zautra AJ, Fasman R, Parish BP, et al. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128(1-2):128-135.
20. Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17(3):173-183.
21. Senba E. A key to dissect the triad of insomnia, chronic pain, and depression. Neurosci Lett. 2015;589:197-199.
22. Torta R, Pennazio F, Ieraci V. Anxiety and depression in rheumatologic diseases: the relevance of diagnosis and management. Reumatismo. 2014;66(1):92-97.
23. Howe CQ, Robinson JP, Sullivan MD. Psychiatric and psychological perspectives on chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):283-300.
24. Gerrits MM, van Marwijk HW, van Oppen P, et al. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
25. Manchikanti L, Pampati V, Beyer C, et al. Do number of pain conditions influence emotional status? Pain Physician. 2002;5(2):200-205.
26. Arnold LM. Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis Res Ther. 2006;8(4):212.
27. Weir PT, Harlan GA, Nkoy FL, et al. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J Clin Rheumatol. 2006;12(3):124-128.
28. Fietta P, Fietta P, Manganelli P. Fibromyalgia and psychiatric disorders. Acta Biomed. 2007;78(2):88-95.
29. Gustorff B, Dorner T, Likar R, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008;52(1):132-136.
30. Boakye PA, Olechowski C, Rashiq S, et al. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption [published online May 28, 2015]. Clin J Pain. doi: 10.1097/ AJP.0000000000000260.
31. Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007;27(11):1571-1587.
32. Nekovarova T, Yamamotova A, Vales K, et al. Common mechanisms of pain and depression: are antidepressants also analgesics? Front Behav Neurosci. 2014;8:99.
33. Smith K, Mattick RP, Bruno R, et al. Factors associated with the development of depression in chronic non-cancer pain patients following the onset of opioid treatment for pain. J Affect Disord. 2015;184:72-80.
34. Scherrer JF, Svrakic DM, Freedland KE, et al. Prescription opioid analgesics increase the risk of depression. J Gen Intern Med. 2014;29(3):491-499.
35. Fishbain DA, Lewis JE, Gao J. The pain suicidality association: a narrative review. Pain Med. 2014;15(11):1835-1849.
36. Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1-27.
37. Olié E, Guillaume S, Jaussent I, et al. Higher psychological pain during a major depressive episode may be a factor of vulnerability to suicidal ideation and act. J Affect Disord. 2010;120(1-3):226-230.
38. Han C, Pae CU. Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 2015;12(1):1-8.
39. Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290-292.
40. Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression [published online November 19, 2011]. Pain Res Treat. 2012;2012:486590. doi: 10.1155/2012/486590.
Any discussion of the relationship between major depressive disorder (MDD) and chronic pain encounters an obstacle immediately: Neither has a singular pathophysiology. Furthermore, MDD and, to a significant extent, chronic pain are defined more by their symptoms than by a presumed etiology and pathogenesis.
Why does this matter to a busy clinician?
Explicitly or implicitly, we often align our treatment approaches with what we assume is the underlying pathophysiology of the conditions we are addressing. An overview of shared pathophysiology of chronic pain conditions and MDD therefore can be useful in practice.
What is chronic pain? Defined as “pain that persists past the healing phase following an injury,”1 chronic pain often is subdivided into 4 types2,3:
- nociceptive (caused by a lesion or potential tissue damage)
- inflammatory
- neuropathic (spontaneous pain or hypersensitivity to pain related to neurologic illness or injury)
- functional (hypersensitivity to pain due to abnormal central processing of a normal input).
Although fibromyalgia often is categorized as a dysfunctional pain syndrome, persons who suffer from it, much like those who suffer neuropathic pain, commonly report hyperalgesia (augmented sensitivity to painful stimuli), allodynia (abnormal pain response to non-noxious stimuli), and paresthesias. These shared clinical features of fibromyalgia and neuropathic pain are consistent with central sensitization, which suggests overlapping pathophysiology.4
Comorbidity between depression and pain is common. A 30% to 60% co-occurrence rate of MDD and chronic pain has been reported.5 Some subtypes of chronic pain, such as fibromyalgia, are so commonly comorbid with psychiatric conditions that they have spawned a scientific debate as to whether the conditions are most parsimoniously considered (1) separate illnesses with high comorbidity or (2) different symptomatic manifestations of a single underlying condition.6 Moreover, cumulative evidence suggests that chronic pain and depression do not just co-occur; each one facilitates development of the other, such that chronic pain is a strong predictor of subsequent onset of MDD, and vice versa.
When pain and depression are comorbid, they also tend to make treatment of each condition more difficult. For example, pain presents (1) a major obstacle to achieving remission when treating depression7,8 and (2) significant risk of relapse.9 A 3-year longitudinal study showed that painful symptoms substantially reduced the chance of recovery in a group of older depressed patients (n = 327). A substantially greater percentage of patients with MDD alone attained recovery (47%), compared with only 9% in whom MDD and painful symptoms were comorbid.10 Furthermore, a higher level of pain can delay remission when treating MDD,11 thus reducing the likelihood of an optimal outcome.12
Understanding shared processes. Recent developments in neuroscience and psycho-immunology point to the fact that comorbid pain and depression might be driven by overlapping pathophysiological processes in the brain and body. In the 2 parts of this article, we (1) review scientific understanding of these shared processes and (2) demonstrate how recent advances in the epidemiology, phenomenology, and etiology of chronic pain and MDD provide important clues for more effective diagnosis (Part 1) and treatment (Part 2, March 2016)—and, therefore, better outcomes. Our focus is primarily on the relationship between MDD and the best-studied comorbid chronic pain conditions: fibromyalgia, neuropathic pain, chronic back pain, and rheumatoid arthritis.
The societal burden of chronic pain conditions is enormous
A recent epidemiological study13 projected that as many as 100 million people in the United States—30.7% of the population—suffer some form of chronic pain, including arthritis and joint pain. A World Health Organization survey yielded a similar (and staggering) 37% prevalence of chronic pain in the population of 10 developed countries.14
Estimates are that various forms of neuropathic pain, including diabetic neuropathy, postherpetic neuralgia, trigeminal neuralgia, spinal cord injury, and radiculopathy, alone afflict as many as 26 million people worldwide, including approximately 1.5% of the U.S. population.15,16
Chronic low back pain is epidemic. With a projected point prevalence of 30%, the condition is the most common cause of activity limitation among people age <45, and the most frequent reason in the United States for visiting a physician.1
Functional somatic syndromes, including fibromyalgia and irritable bowel syndrome, impose an astounding strain on health care: These syndromes account for 25% to 50% of all outpatient visits, or approximately 400 million clinic visits annually in the United States.17
Why should you care about these numbers? The answer is that comorbidity among chronic pain, mood disorders, anxiety disorders, sleep disorders, cognitive impairment, fatigue, and chronic stress presents an enormous clinical challenge because it not only complicates the diagnosis of these conditions but also compromises treatment outcomes and imposes severe limitations on daily functioning and quality of life of those afflicted.5,17-24
A complex relationship and a daunting clinical challenge
Chronic pain enhances the risk of MDD by 2-fold to 5-fold. The risk appears to be mediated by the number of pain conditions rather than by the severity of pain.23 Some authors have noted a kind of dose-response relationship among pain, depression, and anxiety. Among patients who experienced chronic pain that affected 1 body region, the prevalence of generalized anxiety disorder (GAD) and MDD was 30% and 20%, respectively; in patients who experienced pain in ≥2 regions, the prevalence of GAD and MDD was elevated to 54% and 32%.25 Moreover, patients with fibromyalgia were 4.3 times more likely than healthy controls to develop MDD at some point in their lives and 4.7 times more likely to develop an anxiety disorder.26
Although women are more likely to suffer from fibromyalgia, the risk for people of either sex of developing subsequent MDD is comparable once the condition has developed.27 Overall, depression and anxiety are among the most common comorbidities of fibromyalgia, with prevalence ranging from 20% to 80% and 13% to 63.8%, respectively.28
High comorbidity between depression and pain also is relevant for patients with neuropathic pain. A survey from Australia reported depression in 34% and anxiety in 25% of patients with neuropathic pain.29 Pain severity tended to be enduring and associated with significantly impaired functioning. A significant percentage of patients suffering from rheumatoid arthritis and systemic lupus erythematosus tend to manifest anxiety and depression (93% to 94%), cognitive impairment (66%), fatigue (40%), and sleep disorders (72%).22
The relationship between depression and pain appears to be bidirectional. For example, recent studies demonstrate that 30% to 60% of depressed patients also suffer from a painful condition.5
The complex history of patients presenting with concomitant complaints of depression, anxiety, chronic pain, sleep disturbance, cognitive impairment, and fatigue present a daunting diagnostic task. Pain tends to be associated with greater fatigue and sleep disturbance, which in turn depletes a patient’s ability to enjoy life and enhances negative affect.19,20,30 The take-home message might be to screen all chronic pain patients for MDD, anxiety, and sleep disorders, and vice versa.
Furthermore, comorbidity among chronic pain, MDD, anxiety, and sleep disorders can introduce specific intricacies into our treatment approach. Although, in general, comorbidities tend to have a negative impact on treatment outcomes, many pharmacotherapeutic and non-drug interventions targeting chronic pain might ameliorate sleep problems, low energy, anxiety, depression, and anhedonia.18,20,30-32 On the other hand, we should consider that opioid treatment for chronic pain might represent a risk factor for subsequent depression. It is conceivable that chronic opioid treatment and associated sedation can erode self-efficacy and social relationships, thereby compromising sources of support.33,34 It is equally important to keep in mind that, even if we are successful in attaining remission when treating depression and pain, residual pain symptoms might persist, requiring more specific interventions.24
MDD and chronic pain each have, on their own, a well-established association with suicide attempts and completion. Researchers are investigating whether a pathophysiologic suicide-promoting synergy between the 2 disorders exists when they are comorbid (Box35-37).
Shared genetics and pathophysiology
Several candidate genes have been identified as risk genes for chronic pain, depression, and anxiety. One of those studied the most is 5-HTTLPR, involved in regulating synthesis of serotonin transporter. The short form of this gene has been implicated in a diverse set of conditions, including MDD, anxiety disorders, and substance abuse—and fibromyalgia. Other genes associated with the risk of MDD and pain disorders are ones that code for:
- serotonin 5-HT2A and 5-HT1A receptors
- catechol-O-methyltransferase, an enzyme involved in catecholamine metabolism
- dopamine D4 receptor
- proinflammatory cytokines interleukin-1 and interleukin-6.4
Both monoamines and inflammatory cytokines play a role in modulating γ-aminobutyric acid (GABA) and glutamate neurons, as well as glia cells constituting peripheral pain pathways and central circuits that participate in the pain response and regulation of mood.4,17,38
The ‘pain matrix’
Brain circuitry that is involved in processing pain stimuli—often referred to as the pain matrix—shares many structural components with circuitry involved in the stress response and emotional modulation.4 Emerging evidence indicates that the pain matrix might not be pain-specific but, instead, a complex aggregate of interconnected brain structures involved in evoking defensive responses to a number of offending stimuli, including pain, threat, danger, loss, and social rejection or isolation.
It is remarkable, in this regard, that imaging studies show that the dorsal anterior cingulate, central to experiencing negative affect in response to physical pain, also mediates distress in response to the “pain” of social exclusion.39 Emerging functional and structural imaging provides evidence of continuous reorganization of prefrontal cortices as a consequence of enduring chronic pain.1 Of particular interest are findings of (1) a reduction of gray matter in the dorsolateral prefrontal cortex (DLPFC) and (2) functional activation of the medial prefrontal cortex (mPFC), both of which correlate with the duration and experience of chronic back pain.1 It is tempting to speculate that structural decline of the DLPFC, observed in MDD and chronic pain, is linked to cognitive and executive function deficits, which are readily observed in patients with either disorder—given that DLPFC is a “hub” of the so-called “cognitive-executive functional network.”1,4
Likewise, the mPFC is a key component of the default mode network (DMN), a functional network also comprising the posterior cingulate cortex and hippocampus. DMN performs a diverse set of activities, including self-reflection, daydreaming, reminiscing, planning, processing of social information, and creative thinking. Negative neuroplastic changes in the DMN are a common finding in MDD and chronic pain, and might be associated with a tendency toward rumination and catastrophizing—key clinical manifestations of MDD and chronic pain—and linked with pervasive negative affect and sleep disturbance.4,32
Furthermore, functional and structural changes in the amygdala and hippocampus have been described in MDD, fibromyalgia, and neuropathic pain.4 Dysfunction of these limbic formations may be a contributing factor in the disruption of neuroendocrine, autonomic, and immune function, which could further contribute to aggravated mood and pain symptoms.4,17,40
Consequently, excessive hypothalamic-pituitary-adrenal axis and sympathetic activation, combined with elevation of proinflammatory cytokine production and release, likely plays a role in the pathophysiology of MDD and chronic pain disorders.4,17,40 Moreover, at cellular, subcellular, and molecular levels, chronic pain and MDD are associated with:
- perturbed neuron-glia relationships
- altered glutamatergic, GABA, glycine, substance-P, opioid, 5-HT, norepinephrine, and dopamine signaling
- dysfunction of intracellular signaling cascades and neurotrophic signaling.4,20,30,31,38
The Figure that describes how homeostatic function of prefrontal cortical-limbic circuitry is compromised in MDD and chronic pain—thus disrupting autonomic, neuroendocrine, and neuroimmune regulation.
Disturbance in monoamine signaling in chronic pain and MDD might give rise to profound anhedonia, cognitive impairment, anxiety, insomnia, sensitivity to stress, and inadequate functioning of descending pain-regulatory pathways, which primarily use norepinephrine and 5-HT.4,9,20,30,31,38 Using pharmacotherapeutic agents that successfully modulate monoamines, therefore, might ameliorate the function of brain networks innervated by neurotransmitter systems involved in the regulation of pain, mood, cognition, stress response, and sleep. Notably, the same monoamines serve as transmitters in descending pain pathways.
In summary, convergent evidence indicates that MDD and chronic pain states amplify each other, thus contributing to treatment resistance in both disorders.
On the bright side, timely and effective treatment of MDD might optimize the chance of remission and minimize the risk of enduring structural brain changes in MDD and chronic pain.1,4,31,32 The obverse is also true: Emphasizing the importance of the resolution of painful symptoms in the context of MDD, a study reported a significantly greater remission rate of 36.2% in those who had >50% reduction of pain on a visual analogue scale following treatment with a serotonin-norepinephrine reuptake inhibitor, compared with a 17.8% remission rate in persons who experienced <50% pain reduction on the scale.3
Editors’ note: In Part 2 of this article (March 2016), the authors review pharmacotherapeutic and non-drug strategies for managing comorbid chronic pain conditions and MDD.
Any discussion of the relationship between major depressive disorder (MDD) and chronic pain encounters an obstacle immediately: Neither has a singular pathophysiology. Furthermore, MDD and, to a significant extent, chronic pain are defined more by their symptoms than by a presumed etiology and pathogenesis.
Why does this matter to a busy clinician?
Explicitly or implicitly, we often align our treatment approaches with what we assume is the underlying pathophysiology of the conditions we are addressing. An overview of shared pathophysiology of chronic pain conditions and MDD therefore can be useful in practice.
What is chronic pain? Defined as “pain that persists past the healing phase following an injury,”1 chronic pain often is subdivided into 4 types2,3:
- nociceptive (caused by a lesion or potential tissue damage)
- inflammatory
- neuropathic (spontaneous pain or hypersensitivity to pain related to neurologic illness or injury)
- functional (hypersensitivity to pain due to abnormal central processing of a normal input).
Although fibromyalgia often is categorized as a dysfunctional pain syndrome, persons who suffer from it, much like those who suffer neuropathic pain, commonly report hyperalgesia (augmented sensitivity to painful stimuli), allodynia (abnormal pain response to non-noxious stimuli), and paresthesias. These shared clinical features of fibromyalgia and neuropathic pain are consistent with central sensitization, which suggests overlapping pathophysiology.4
Comorbidity between depression and pain is common. A 30% to 60% co-occurrence rate of MDD and chronic pain has been reported.5 Some subtypes of chronic pain, such as fibromyalgia, are so commonly comorbid with psychiatric conditions that they have spawned a scientific debate as to whether the conditions are most parsimoniously considered (1) separate illnesses with high comorbidity or (2) different symptomatic manifestations of a single underlying condition.6 Moreover, cumulative evidence suggests that chronic pain and depression do not just co-occur; each one facilitates development of the other, such that chronic pain is a strong predictor of subsequent onset of MDD, and vice versa.
When pain and depression are comorbid, they also tend to make treatment of each condition more difficult. For example, pain presents (1) a major obstacle to achieving remission when treating depression7,8 and (2) significant risk of relapse.9 A 3-year longitudinal study showed that painful symptoms substantially reduced the chance of recovery in a group of older depressed patients (n = 327). A substantially greater percentage of patients with MDD alone attained recovery (47%), compared with only 9% in whom MDD and painful symptoms were comorbid.10 Furthermore, a higher level of pain can delay remission when treating MDD,11 thus reducing the likelihood of an optimal outcome.12
Understanding shared processes. Recent developments in neuroscience and psycho-immunology point to the fact that comorbid pain and depression might be driven by overlapping pathophysiological processes in the brain and body. In the 2 parts of this article, we (1) review scientific understanding of these shared processes and (2) demonstrate how recent advances in the epidemiology, phenomenology, and etiology of chronic pain and MDD provide important clues for more effective diagnosis (Part 1) and treatment (Part 2, March 2016)—and, therefore, better outcomes. Our focus is primarily on the relationship between MDD and the best-studied comorbid chronic pain conditions: fibromyalgia, neuropathic pain, chronic back pain, and rheumatoid arthritis.
The societal burden of chronic pain conditions is enormous
A recent epidemiological study13 projected that as many as 100 million people in the United States—30.7% of the population—suffer some form of chronic pain, including arthritis and joint pain. A World Health Organization survey yielded a similar (and staggering) 37% prevalence of chronic pain in the population of 10 developed countries.14
Estimates are that various forms of neuropathic pain, including diabetic neuropathy, postherpetic neuralgia, trigeminal neuralgia, spinal cord injury, and radiculopathy, alone afflict as many as 26 million people worldwide, including approximately 1.5% of the U.S. population.15,16
Chronic low back pain is epidemic. With a projected point prevalence of 30%, the condition is the most common cause of activity limitation among people age <45, and the most frequent reason in the United States for visiting a physician.1
Functional somatic syndromes, including fibromyalgia and irritable bowel syndrome, impose an astounding strain on health care: These syndromes account for 25% to 50% of all outpatient visits, or approximately 400 million clinic visits annually in the United States.17
Why should you care about these numbers? The answer is that comorbidity among chronic pain, mood disorders, anxiety disorders, sleep disorders, cognitive impairment, fatigue, and chronic stress presents an enormous clinical challenge because it not only complicates the diagnosis of these conditions but also compromises treatment outcomes and imposes severe limitations on daily functioning and quality of life of those afflicted.5,17-24
A complex relationship and a daunting clinical challenge
Chronic pain enhances the risk of MDD by 2-fold to 5-fold. The risk appears to be mediated by the number of pain conditions rather than by the severity of pain.23 Some authors have noted a kind of dose-response relationship among pain, depression, and anxiety. Among patients who experienced chronic pain that affected 1 body region, the prevalence of generalized anxiety disorder (GAD) and MDD was 30% and 20%, respectively; in patients who experienced pain in ≥2 regions, the prevalence of GAD and MDD was elevated to 54% and 32%.25 Moreover, patients with fibromyalgia were 4.3 times more likely than healthy controls to develop MDD at some point in their lives and 4.7 times more likely to develop an anxiety disorder.26
Although women are more likely to suffer from fibromyalgia, the risk for people of either sex of developing subsequent MDD is comparable once the condition has developed.27 Overall, depression and anxiety are among the most common comorbidities of fibromyalgia, with prevalence ranging from 20% to 80% and 13% to 63.8%, respectively.28
High comorbidity between depression and pain also is relevant for patients with neuropathic pain. A survey from Australia reported depression in 34% and anxiety in 25% of patients with neuropathic pain.29 Pain severity tended to be enduring and associated with significantly impaired functioning. A significant percentage of patients suffering from rheumatoid arthritis and systemic lupus erythematosus tend to manifest anxiety and depression (93% to 94%), cognitive impairment (66%), fatigue (40%), and sleep disorders (72%).22
The relationship between depression and pain appears to be bidirectional. For example, recent studies demonstrate that 30% to 60% of depressed patients also suffer from a painful condition.5
The complex history of patients presenting with concomitant complaints of depression, anxiety, chronic pain, sleep disturbance, cognitive impairment, and fatigue present a daunting diagnostic task. Pain tends to be associated with greater fatigue and sleep disturbance, which in turn depletes a patient’s ability to enjoy life and enhances negative affect.19,20,30 The take-home message might be to screen all chronic pain patients for MDD, anxiety, and sleep disorders, and vice versa.
Furthermore, comorbidity among chronic pain, MDD, anxiety, and sleep disorders can introduce specific intricacies into our treatment approach. Although, in general, comorbidities tend to have a negative impact on treatment outcomes, many pharmacotherapeutic and non-drug interventions targeting chronic pain might ameliorate sleep problems, low energy, anxiety, depression, and anhedonia.18,20,30-32 On the other hand, we should consider that opioid treatment for chronic pain might represent a risk factor for subsequent depression. It is conceivable that chronic opioid treatment and associated sedation can erode self-efficacy and social relationships, thereby compromising sources of support.33,34 It is equally important to keep in mind that, even if we are successful in attaining remission when treating depression and pain, residual pain symptoms might persist, requiring more specific interventions.24
MDD and chronic pain each have, on their own, a well-established association with suicide attempts and completion. Researchers are investigating whether a pathophysiologic suicide-promoting synergy between the 2 disorders exists when they are comorbid (Box35-37).
Shared genetics and pathophysiology
Several candidate genes have been identified as risk genes for chronic pain, depression, and anxiety. One of those studied the most is 5-HTTLPR, involved in regulating synthesis of serotonin transporter. The short form of this gene has been implicated in a diverse set of conditions, including MDD, anxiety disorders, and substance abuse—and fibromyalgia. Other genes associated with the risk of MDD and pain disorders are ones that code for:
- serotonin 5-HT2A and 5-HT1A receptors
- catechol-O-methyltransferase, an enzyme involved in catecholamine metabolism
- dopamine D4 receptor
- proinflammatory cytokines interleukin-1 and interleukin-6.4
Both monoamines and inflammatory cytokines play a role in modulating γ-aminobutyric acid (GABA) and glutamate neurons, as well as glia cells constituting peripheral pain pathways and central circuits that participate in the pain response and regulation of mood.4,17,38
The ‘pain matrix’
Brain circuitry that is involved in processing pain stimuli—often referred to as the pain matrix—shares many structural components with circuitry involved in the stress response and emotional modulation.4 Emerging evidence indicates that the pain matrix might not be pain-specific but, instead, a complex aggregate of interconnected brain structures involved in evoking defensive responses to a number of offending stimuli, including pain, threat, danger, loss, and social rejection or isolation.
It is remarkable, in this regard, that imaging studies show that the dorsal anterior cingulate, central to experiencing negative affect in response to physical pain, also mediates distress in response to the “pain” of social exclusion.39 Emerging functional and structural imaging provides evidence of continuous reorganization of prefrontal cortices as a consequence of enduring chronic pain.1 Of particular interest are findings of (1) a reduction of gray matter in the dorsolateral prefrontal cortex (DLPFC) and (2) functional activation of the medial prefrontal cortex (mPFC), both of which correlate with the duration and experience of chronic back pain.1 It is tempting to speculate that structural decline of the DLPFC, observed in MDD and chronic pain, is linked to cognitive and executive function deficits, which are readily observed in patients with either disorder—given that DLPFC is a “hub” of the so-called “cognitive-executive functional network.”1,4
Likewise, the mPFC is a key component of the default mode network (DMN), a functional network also comprising the posterior cingulate cortex and hippocampus. DMN performs a diverse set of activities, including self-reflection, daydreaming, reminiscing, planning, processing of social information, and creative thinking. Negative neuroplastic changes in the DMN are a common finding in MDD and chronic pain, and might be associated with a tendency toward rumination and catastrophizing—key clinical manifestations of MDD and chronic pain—and linked with pervasive negative affect and sleep disturbance.4,32
Furthermore, functional and structural changes in the amygdala and hippocampus have been described in MDD, fibromyalgia, and neuropathic pain.4 Dysfunction of these limbic formations may be a contributing factor in the disruption of neuroendocrine, autonomic, and immune function, which could further contribute to aggravated mood and pain symptoms.4,17,40
Consequently, excessive hypothalamic-pituitary-adrenal axis and sympathetic activation, combined with elevation of proinflammatory cytokine production and release, likely plays a role in the pathophysiology of MDD and chronic pain disorders.4,17,40 Moreover, at cellular, subcellular, and molecular levels, chronic pain and MDD are associated with:
- perturbed neuron-glia relationships
- altered glutamatergic, GABA, glycine, substance-P, opioid, 5-HT, norepinephrine, and dopamine signaling
- dysfunction of intracellular signaling cascades and neurotrophic signaling.4,20,30,31,38
The Figure that describes how homeostatic function of prefrontal cortical-limbic circuitry is compromised in MDD and chronic pain—thus disrupting autonomic, neuroendocrine, and neuroimmune regulation.
Disturbance in monoamine signaling in chronic pain and MDD might give rise to profound anhedonia, cognitive impairment, anxiety, insomnia, sensitivity to stress, and inadequate functioning of descending pain-regulatory pathways, which primarily use norepinephrine and 5-HT.4,9,20,30,31,38 Using pharmacotherapeutic agents that successfully modulate monoamines, therefore, might ameliorate the function of brain networks innervated by neurotransmitter systems involved in the regulation of pain, mood, cognition, stress response, and sleep. Notably, the same monoamines serve as transmitters in descending pain pathways.
In summary, convergent evidence indicates that MDD and chronic pain states amplify each other, thus contributing to treatment resistance in both disorders.
On the bright side, timely and effective treatment of MDD might optimize the chance of remission and minimize the risk of enduring structural brain changes in MDD and chronic pain.1,4,31,32 The obverse is also true: Emphasizing the importance of the resolution of painful symptoms in the context of MDD, a study reported a significantly greater remission rate of 36.2% in those who had >50% reduction of pain on a visual analogue scale following treatment with a serotonin-norepinephrine reuptake inhibitor, compared with a 17.8% remission rate in persons who experienced <50% pain reduction on the scale.3
Editors’ note: In Part 2 of this article (March 2016), the authors review pharmacotherapeutic and non-drug strategies for managing comorbid chronic pain conditions and MDD.
1. Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87(2):81-97.
2. Verdu B, Decosterd I, Buclin T, et al. Antidepressants for the treatment of chronic pain. Drugs. 2008;68(18):2611-2632.
3. Woolf CJ; American College of Physicians, American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
4. Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci (Landmark Ed). 2009;14:5291-5338.
5. Bair MJ, Wu J, Damush TM, et al. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
6. Cho HJ, Skowera A, Cleare A, et al. Chronic fatigue syndrome: an update focusing on phenomenology and pathophysiology. Curr Opin Psychiatry. 2006;19(1):67-73.
7. Fava M. Depression with physical symptoms: treating to remission. J Clin Psychiatry. 2003;64(suppl 7):24-28.
8. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
9. Ohayon MM. Specific characteristics of the pain/depression association in the general population. J Clin Psychiatry. 2004;65(suppl 12):5-9.
10. Geerlings SW, Twisk JW, Beekman AT, et al. Longitudinal relationship between pain and depression in older adults: sex, age and physical disability. Soc Psychiatry Psychiatr Epidemiol. 2002;37(1):23-30.
11. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
12. Spijker J, de Graaf R, Bijl RV, et al. Determinants of persistence of major depressive episodes in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). J Affect Disord. 2004;81(3):231-240.
13. Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230-1239.
14. Dzau VJ, Pizzo PA. Relieving pain in America: insights from an Institute of Medicine committee. JAMA. 2014;312(15):1507-1508.
15. Butera JA. Current and emerging targets to treat neuropathic pain. J Med Chem. 2007;50(11):2543-2546.
16. Offenbaecher M, Ackenheil M. Current trends in neuropathic pain treatments with special reference to fibromyalgia. CNS Spectr. 2005;10(4):285-297.
17. Goldenberg DL. Pain/depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med. 2010;123(8):675-682.
18. Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007;23(1):15-22.
19. Zautra AJ, Fasman R, Parish BP, et al. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128(1-2):128-135.
20. Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17(3):173-183.
21. Senba E. A key to dissect the triad of insomnia, chronic pain, and depression. Neurosci Lett. 2015;589:197-199.
22. Torta R, Pennazio F, Ieraci V. Anxiety and depression in rheumatologic diseases: the relevance of diagnosis and management. Reumatismo. 2014;66(1):92-97.
23. Howe CQ, Robinson JP, Sullivan MD. Psychiatric and psychological perspectives on chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):283-300.
24. Gerrits MM, van Marwijk HW, van Oppen P, et al. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
25. Manchikanti L, Pampati V, Beyer C, et al. Do number of pain conditions influence emotional status? Pain Physician. 2002;5(2):200-205.
26. Arnold LM. Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis Res Ther. 2006;8(4):212.
27. Weir PT, Harlan GA, Nkoy FL, et al. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J Clin Rheumatol. 2006;12(3):124-128.
28. Fietta P, Fietta P, Manganelli P. Fibromyalgia and psychiatric disorders. Acta Biomed. 2007;78(2):88-95.
29. Gustorff B, Dorner T, Likar R, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008;52(1):132-136.
30. Boakye PA, Olechowski C, Rashiq S, et al. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption [published online May 28, 2015]. Clin J Pain. doi: 10.1097/ AJP.0000000000000260.
31. Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007;27(11):1571-1587.
32. Nekovarova T, Yamamotova A, Vales K, et al. Common mechanisms of pain and depression: are antidepressants also analgesics? Front Behav Neurosci. 2014;8:99.
33. Smith K, Mattick RP, Bruno R, et al. Factors associated with the development of depression in chronic non-cancer pain patients following the onset of opioid treatment for pain. J Affect Disord. 2015;184:72-80.
34. Scherrer JF, Svrakic DM, Freedland KE, et al. Prescription opioid analgesics increase the risk of depression. J Gen Intern Med. 2014;29(3):491-499.
35. Fishbain DA, Lewis JE, Gao J. The pain suicidality association: a narrative review. Pain Med. 2014;15(11):1835-1849.
36. Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1-27.
37. Olié E, Guillaume S, Jaussent I, et al. Higher psychological pain during a major depressive episode may be a factor of vulnerability to suicidal ideation and act. J Affect Disord. 2010;120(1-3):226-230.
38. Han C, Pae CU. Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 2015;12(1):1-8.
39. Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290-292.
40. Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression [published online November 19, 2011]. Pain Res Treat. 2012;2012:486590. doi: 10.1155/2012/486590.
1. Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87(2):81-97.
2. Verdu B, Decosterd I, Buclin T, et al. Antidepressants for the treatment of chronic pain. Drugs. 2008;68(18):2611-2632.
3. Woolf CJ; American College of Physicians, American Physiological Society. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441-451.
4. Maletic V, Raison CL. Neurobiology of depression, fibromyalgia and neuropathic pain. Front Biosci (Landmark Ed). 2009;14:5291-5338.
5. Bair MJ, Wu J, Damush TM, et al. Association of depression and anxiety alone and in combination with chronic musculoskeletal pain in primary care patients. Psychosom Med. 2008;70(8):890-897.
6. Cho HJ, Skowera A, Cleare A, et al. Chronic fatigue syndrome: an update focusing on phenomenology and pathophysiology. Curr Opin Psychiatry. 2006;19(1):67-73.
7. Fava M. Depression with physical symptoms: treating to remission. J Clin Psychiatry. 2003;64(suppl 7):24-28.
8. Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17-22.
9. Ohayon MM. Specific characteristics of the pain/depression association in the general population. J Clin Psychiatry. 2004;65(suppl 12):5-9.
10. Geerlings SW, Twisk JW, Beekman AT, et al. Longitudinal relationship between pain and depression in older adults: sex, age and physical disability. Soc Psychiatry Psychiatr Epidemiol. 2002;37(1):23-30.
11. Karp JF, Scott J, Houck P, et al. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591-597.
12. Spijker J, de Graaf R, Bijl RV, et al. Determinants of persistence of major depressive episodes in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS). J Affect Disord. 2004;81(3):231-240.
13. Johannes CB, Le TK, Zhou X, et al. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230-1239.
14. Dzau VJ, Pizzo PA. Relieving pain in America: insights from an Institute of Medicine committee. JAMA. 2014;312(15):1507-1508.
15. Butera JA. Current and emerging targets to treat neuropathic pain. J Med Chem. 2007;50(11):2543-2546.
16. Offenbaecher M, Ackenheil M. Current trends in neuropathic pain treatments with special reference to fibromyalgia. CNS Spectr. 2005;10(4):285-297.
17. Goldenberg DL. Pain/depression dyad: a key to a better understanding and treatment of functional somatic syndromes. Am J Med. 2010;123(8):675-682.
18. Argoff CE. The coexistence of neuropathic pain, sleep, and psychiatric disorders: a novel treatment approach. Clin J Pain. 2007;23(1):15-22.
19. Zautra AJ, Fasman R, Parish BP, et al. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2007;128(1-2):128-135.
20. Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17(3):173-183.
21. Senba E. A key to dissect the triad of insomnia, chronic pain, and depression. Neurosci Lett. 2015;589:197-199.
22. Torta R, Pennazio F, Ieraci V. Anxiety and depression in rheumatologic diseases: the relevance of diagnosis and management. Reumatismo. 2014;66(1):92-97.
23. Howe CQ, Robinson JP, Sullivan MD. Psychiatric and psychological perspectives on chronic pain. Phys Med Rehabil Clin N Am. 2015;26(2):283-300.
24. Gerrits MM, van Marwijk HW, van Oppen P, et al. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
25. Manchikanti L, Pampati V, Beyer C, et al. Do number of pain conditions influence emotional status? Pain Physician. 2002;5(2):200-205.
26. Arnold LM. Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis Res Ther. 2006;8(4):212.
27. Weir PT, Harlan GA, Nkoy FL, et al. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J Clin Rheumatol. 2006;12(3):124-128.
28. Fietta P, Fietta P, Manganelli P. Fibromyalgia and psychiatric disorders. Acta Biomed. 2007;78(2):88-95.
29. Gustorff B, Dorner T, Likar R, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiol Scand. 2008;52(1):132-136.
30. Boakye PA, Olechowski C, Rashiq S, et al. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption [published online May 28, 2015]. Clin J Pain. doi: 10.1097/ AJP.0000000000000260.
31. Jann MW, Slade JH. Antidepressant agents for the treatment of chronic pain and depression. Pharmacotherapy. 2007;27(11):1571-1587.
32. Nekovarova T, Yamamotova A, Vales K, et al. Common mechanisms of pain and depression: are antidepressants also analgesics? Front Behav Neurosci. 2014;8:99.
33. Smith K, Mattick RP, Bruno R, et al. Factors associated with the development of depression in chronic non-cancer pain patients following the onset of opioid treatment for pain. J Affect Disord. 2015;184:72-80.
34. Scherrer JF, Svrakic DM, Freedland KE, et al. Prescription opioid analgesics increase the risk of depression. J Gen Intern Med. 2014;29(3):491-499.
35. Fishbain DA, Lewis JE, Gao J. The pain suicidality association: a narrative review. Pain Med. 2014;15(11):1835-1849.
36. Elman I, Borsook D, Volkow ND. Pain and suicidality: insights from reward and addiction neuroscience. Prog Neurobiol. 2013;109:1-27.
37. Olié E, Guillaume S, Jaussent I, et al. Higher psychological pain during a major depressive episode may be a factor of vulnerability to suicidal ideation and act. J Affect Disord. 2010;120(1-3):226-230.
38. Han C, Pae CU. Pain and depression: a neurobiological perspective of their relationship. Psychiatry Investig. 2015;12(1):1-8.
39. Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302(5643):290-292.
40. Gracely RH, Ceko M, Bushnell MC. Fibromyalgia and depression [published online November 19, 2011]. Pain Res Treat. 2012;2012:486590. doi: 10.1155/2012/486590.
What psychiatrists must know to make the mandated transition to ICD-10
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Just as psychiatrists are adapting to DSM-5, they have to cope with implementation of the 10th edition of the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This challenge raises questions: What is the importance of understanding ICD-10? How will it affect the practice of psychiatry?
Furthermore, how does ICD-10 relate to DSM-5 and Current Procedural Terminology (CPT)? How does it differ from ICD-9? What are the ICD-10-Clinical Modification (CM) and ICD-10-Procedures (PCS)?Learning the essence of the changes, and understanding what impact they have on your clinical work, are necessary to ensure that your practice keeps pace with professional and legal standards of care. The effort involved is not onerous, however, and can improve the quality and efficiency of your care and how you document it.
In this article, we provide you with an overview of ICD-10; highlight major changes of the new classification; explain its relevance to clinical practice; and offer guidelines for implementing it effectively. We also emphasize that a good understanding of DSM-5 facilitates appreciation of ICD-10 and makes its implementation fairly easy and straightforward.
To begin, we provide a glossary of ICD-related terms and a review of additional definitions, distinctions, and dates (Box).1-6
Major changes from ICD-9
No question: ICD-10 is going to significantly influence your practice and your reimbursement. Furthermore, a number of revisions in ICD-10 have the potential to meaningfully improve clinical documentation and communication and to enhance your ability to precisely describe the complexity of your patients—with implications for billing.
ICD-10 differs from ICD-9 in organization, structure, code composition, and level of detail. In addition, ICD-10 makes some changes in terminology and definitions, with the goal of improving precision.
ICD-10 also is much larger than ICD-9.The total number of medical diagnostic codes has increased more than 5-fold—from approximately 13,000 to 69,000. This expansion allows for greater specificity in diagnosis and enables differentiation of an initial clinical encounter from a subsequent encounter.
To accommodate the expansion in the number of codes, the 5-digit numeric codes used in ICD-9 have been replaced in ICD-10 by 7-digit alphanumeric codes:
- the first digit always is a letter
- the second and third digits are numbers followed by a decimal point
- the fourth though seventh digits can be letters or numbers
- the first 3 digits denote the diagnostic category
- the fourth through sixth digits provide diagnostic detail
- the seventh digit provides information about the nature of the encounter (eg, initial, subsequent, or sequel, denoted respectively by “A,” “D,” and “S” in the seventh digit).
The number of 3-digit categories for psychiatric disorders has increased from 30 in ICD-9 (290-319) to 100 in ICD-10 (F00-F99). Only the first 5 digits are used for the section on mental disorders in ICD-10, with the first digit always “F” and the second digit a number denoting the broad type of disorders. The second and third digits in conjunction define the major category of the disorder; the fourth and fifth digits provide additional descriptive detail about the disorder (Table).
ICD-9 ‘V’ codes are out
What were called “V” codes in ICD-9—factors that influence health status and contact with health services—have been replaced by “Z” codes in ICD-10. These “Z” codes provide greater detail and precision than “V” codes provided.
Examples of “Z” codes relevant to psychiatry are:
Z00 General psychiatric examination (eg, of a person who does not have a complaint or diagnosis)
Z03 Examination for suspected mental and behavioral disorder
Z04 Examination for medicolegal or other purposes; Z04.8 is relevant laboratory testing, such as drug testing of urine or blood
Z50 Care involving rehabilitation (substance use disorder, etc.)
Z60 Problem related to social environment
Z61 Problem related to negative life events in childhood
Z63 Problem related to primary support group, including family circumstances
Z64-Z65 Problem related to other psychosocial circumstances
Z70-Z71 Condition requiring counseling, not elsewhere classified
Z73 Problem related to difficulty with life management (burnout, stress, role conflict, etc.)
Z75 Problem related to medical facilities and other aspects of health care (eg, awaiting admission)
Z81 Family history of mental or behavioral disorders
Z85-Z91 Personal history of various disorders (must be absent or in full remission at the moment); Z86.51, for example, refers to a history of combat and operational stress reaction.
Greater precision is now possible when coding for treatment-related adverse effects. A particular adverse effect now is coded under the relevant system, along with its attribution to the specific substance. Obesity attributable to antipsychotic treatment,7,8 for example, is coded as E66.1.
Integrating DSM-5 and ICD-10
Because DSM-5 lists corresponding ICD-10-CM codes for all disorders, you will find it much easier than other physicians to implement ICD-10. DSM-5 includes ICD-9-CM and ICD-10-CM codes for each DSM-5 disorder (for example, the ICD-9-CM code for schizophrenia is 295.x; the ICD-10-CM code is F20.9).9
Furthermore, a number of changes from ICD-9-CM to ICD-10-CM enable documentation of greater diagnostic specificity; for example, DSM-5 schizoaffective disorder, bipolar type, and schizoaffective disorder, depressive type, are distinctly coded as F25.0 and F25.1, respectively, in ICD-10-CM, whereas both were coded as 295.7 in ICD-9-CM.10
You will continue to use DSM-5 criteria to guide your diagnostic process, translating the DSM-5 diagnosis (diagnoses) into corresponding ICD-10-CM codes. Experience with DSM-5 substantially simplifies the transition to ICD-10.
Key differences between DSM-5 and ICD-10
There are notable differences in organization and content between DSM-5 and ICD-10.
The 20 chapters in DSM-5 begin with neurodevelopmental disorders; neurocognitive disorders are toward the end (ie, childhood to late life). In contrast, neurocognitive disorders (ie, “dementia”) appear at the beginning of ICD-10; neurodevelopmental disorders are at the end.
Elimination of schizophrenia subtypes in DSM-5 necessitates coding of all schizophrenia as F20.9 in ICD-10-CM because F20.0-F20.8 are specific subtypes. DSM-5 schizophreniform disorder is coded F20.81.
Substance abuse and substance dependence continue to be separate in ICD-10-CM, but they are combined in a single category of substance use disorders in DSM-5. The correct ICD-10-CM code (ie, abuse vs dependence) is determined by the severity of the substance use disorder: “Mild” coding as abuse (F1x.1) and “moderate” and “severe” coding as dependence (F2x.2), with x denoting the substance abused.
There can be multiple applicable diagnoses associated with a clinical encounter, as there was with ICD-9-CM. Give precedence to the diagnosis that best represents the nature of the presenting problem; list other diagnoses in the order of their relevance. DSM-5 and ICD-10-CM are similar in this regard.
ICD-10-CM uses only subtypes, in contrast to the use of subtypes and specifiers in DSM-5 to describe variability in disorders across patients. It is possible, however, to code certain DSM-5 specifiers in ICD-10-CM. (This is discussed in the “Recording Procedures” section of the DSM-5 text and summarized at the beginning of the manual, and appears in the “Appendix.”) To code the catatonia specifier in the context of schizoaffective disorder, depressive type, for example, use ICD-10-CM code F25.1 for the disorder and add code F06.1 for the catatonia specifier.11
How will ICD-10 affect your practice?
As of October 1, 2015, all health care facilities were to have become ICD-10 compliant. Furthermore, any Health Insurance Portability and Accountability Act-covered entity must use ICD-10-CM codes if it expects to be reimbursed for health care services.
Mental health practitioners might think that the transition from ICD-9-CM to ICD-10-CM involves only billers and coders, not them. They are wrong. All clinicians are responsible for documenting their diagnostic and treatment services properly. Medical records must contain adequate information to support any diagnostic (ICD-10-CM) and treatment (CPT) codes that are applied to a given clinical encounter.
The greater detail and specificity that are provided by ICD-10-CM allow more accurate recording of clinical complexity, which, in turn, influences reimbursement. However, good documentation is necessary for proper coding. Because clinicians are ultimately responsible for proper diagnostic coding, good understanding of ICD-10-CM is essential to be able to code properly.
Similar to the expansion of ICD-10-CM (from volumes 1 and 2 of ICD-9-CM), ICD-10-PCS has undergone similar expansion (from volume 3 of ICD-9-CM), with a corresponding increase in specificity. For example, there are now 5 distinct codes for electroconvulsive therapy (GZB0ZZZ-GZB4ZZZ) that distinguish unilateral from bilateral electrode placement and single from multiple stimulations.
DSM-5 will continue to be the frameworkfor psychiatric assessment and diagnosis. ICD-10-CM will be the coding system to accurately denote DSM-5 diagnoses. The Centers for Medicare and Medicaid Services (CMS) and the National Center for Health Statistics recognize DSM-5 as the means to identify proper ICD-10-CM codes for mental disorders. CMS also has announced that, although ICD-10-CM codes are necessary for reimbursement, use of an incorrect code will not be the basis for denying a Medicare claim for 1 year.
Making ICD-10 part of practice
Here are several keys to implementing ICD-10 with minimum pain and maximum benefit.
Multiple diagnosis codes should be listed in the order of their relevance to the clinical encounter.
Visit type. The seventh character of the ICD-10-CM code denotes the type of visit (initial, subsequent, or sequela) and must be provided:
- An initial encounter is one in which the patient first receives active treatment.
- A subsequent encounter refers to a follow-up visit in which the patient receives routine care during the healing or recovery phase.
- A sequel encounter is one in which a patient receives treatment for complications or conditions that arise as a direct result of the initial condition.
The transition to ICD-10 should be facilitated by adoption of DSM-5. Continue using DSM-5 to determine the correct diagnosis or diagnoses of the mental disorder, then apply the corresponding ICD-10-CM code(s). The better you understand and apply DSM-5, the more precise you can be in utilizing the greater specificity and accuracy afforded by ICD-10-CM coding.
Document well. Good understanding of the structure and organization of ICD-10-CM facilitates efficient, comprehensive documentation. This, in turn, will foster better clinical communication and appropriate reimbursement.
Know your payers—in particular, their policies regarding differential reimbursement for clinical complexity (based on ICD-10-CM/PCS). Medical practices that are part of an accountable care organization, and those that have risk-adjusted contracts must pay special attention to documenting clinical complexity when coding.
Know your electronic health care record, understand what tools it offers to efficiently translate DSM-5 diagnoses into appropriate ICD-10-CM codes, and use those tools efficiently.
Review your medical record documentation for the top 20 conditions in your practice, in the context of their definition in ICD-10-CM.
If you have coders who do ICD-10-CM coding for you, review a few patient charts with them to compare your sense of the patient’s clinical complexity and their coding based on your documentation.
Changes in DSM-5 have encouraged clinicians to improve their assessment of patients and provide measurement-based care. The significant changes in ICD-10-CM should provide the impetus for you to hone your ability to provide documentation. Sufficient flexibility exists within guidelines to permit individualization of the style of documentation.
Because all DSM-5 diagnoses map to appropriate ICD-10-CM codes, effective use of DSM-5 should make the transition to ICD-10 easy.
Bottom Line
Compared with ICD-9, definitions of mental health diagnoses have been improved in ICD-10, and more elaborate code descriptions in ICD-10-CM provide for greater precision when you report a diagnosis. The result? More accurate and efficient documentation of the care you provide and better reimbursement. Understanding what impact the changes in ICD-10 will have on your clinical work will ensure that your practice keeps pace with professional and legal standards of care.
Related Resources
• Blue Cross Blue Shield of Michigan ICD-10 update: mental and behavioral health ICD-10-CM codes. http://www.bcbsm.com/content/dam/public/Providers/Documents/help/faqs/icd10-update-mentalhealth.pdf.
• American Psychiatric Association ICD-10 tutorial. http://www.psychiatry.org/psychiatrists/practice/dsm/icd-10.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
1. Diagnostic and statistical manual of mental disorders, 5th edition. Washington DC: American Psychiatric Association; 2013.
2. World Health Organization. The ICD-10 classification of mental and behavioral disorders: clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization; 1992.
3. American Medical Association. ICD-10-CM 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
4. American Medical Association. CPT-2016, professional edition. Chicago, IL: American Medical Association; 2015.
5. American Medical Association. ICD-10-CM expert for physicians 2016: the complete official code set. Chicago, IL: American Medical Association; 2015.
6. American Medical Association. ICD-10-PCS mapping to ICD-9-CM volume 3. Chicago, IL: American Medical Association; 2015.
7. Tandon R, Halbreich U. The second-generation ‘atypical’ antipsychotics: similar efficacy but different neuroendocrine side-effects. Psychoneuroendocrinology. 2003;28(suppl 1):1-7.
8. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(suppl 1):4-8.
9. Tandon R, Gaebel W, Barch DM, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. 2013;150(1):3-10.
10. Malaspina D, Owens MJ, Heckers S, et al. Schizoaffective disorder in the DSM-5. Schizophr Res. 2013;150(1):21-25.
11. Tandon R, Heckers S, Bustillo J, et al. Catatonia in DSM-5. Schizophr Res. 2013;150(1):26-30.
Preventing drinking relapse in patients with alcoholic liver disease
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
Alcohol use disorder (AUD) is a mosaic of psychiatric and medical symptoms. Alcoholic liver disease (ALD) in its acute and chronic forms is a common clinical consequence of long-standing AUD. Patients with ALD require specialized care from professionals in addiction, gastroenterology, and psychiatry. However, medical specialists treating ALD might not regularly consider medications to treat AUD because of their limited experience with the drugs or the lack of studies in patients with significant liver disease.1 Similarly, psychiatrists might be reticent to prescribe medications for AUD, fearing that liver disease will be made worse or that they will cause other medical complications. As a result, patients with ALD might not receive care that could help treat their AUD (Box).
Given the high worldwide prevalence and morbidity of ALD,2 general and subspecialized psychiatrists routinely evaluate patients with AUD in and out of the hospital. This article aims to equip a psychiatrist with:
• a practical understanding of the natural history and categorization of ALD
• basic skills to detect symptoms of ALD
• preparation to collaborate with medical colleagues in multidisciplinary management of co-occurring AUD and ALD
• a summary of the pharmacotherapeutics of AUD, with emphasis on patients with clinically apparent ALD.
Categorization and clinical features
Alcoholic liver damage encompasses a spectrum of disorders, including alcoholic fatty liver, acute alcohol hepatitis (AH), and cirrhosis following varying durations and patterns of alcohol use. Manifestations of ALD vary from asymptomatic fatty liver with minimal liver enzyme elevation to severe acute AH with jaundice, coagulopathy, and high short-term mortality (Table 1). Symptoms seen in patients with AH include fever, abdominal pain, anorexia, jaundice, leukocytosis, and coagulopathy.3
Patients with chronic ALD often develop cirrhosis, persistent elevation of the serum aminotransferase level (even after prolonged alcohol abstinence), signs of portal hypertension (ascites, encephalopathy, variceal bleeding), and profound malnutrition. The survival of ALD patients with chronic liver failure is predicted in part by a Model for End-Stage Liver Disease (MELD) score that incorporates their serum total bilirubin level, creatinine level, and international normalized ratio. The MELD score, which ranges from 6 to 40, also is used to gauge the need for liver transplantation; most patients who have a MELD score >15 benefit from transplant. To definitively determine the severity of ALD, a liver biopsy is required but usually is not performed in clinical practice.
All patients who drink heavily or suffer with AUD are at risk of developing AH; women and binge drinkers are particularly vulnerable.4 Liver dysfunction and malnutrition in ALD patients compromise the immune system, increasing the risk of infection. Patients hospitalized with AH have a 10% to 30% risk of inpatient mortality; their 1- and 2-month post-discharge survival is 50% to 65%, largely determined by whether the patient can maintain sobriety.5 Psychiatrists’ contribution to ALD treatment therefore has the potential to save lives.
Screening and detection of ALD
Because of the high mortality associated with AH and cirrhosis, symptom recognition and collaborative medical and psychiatric management are critical (Table 2). A psychiatrist evaluating a jaundiced patient who continues to drink should arrange urgent medical evaluation. While gathering a history, mental health providers might hear a patient refer to symptoms of gastrointestinal bleeding (vomiting blood, bloody or dark stool), painful abdominal distension, fevers, or confusion that should prompt a referral to a gastroenterologist or the emergency department. Testing for urinary ethyl glucuronide—a direct metabolite of ethanol that can be detected for as long as 90 hours after ethanol ingestion—is useful in detecting alcohol use in the past 4 or 5 days.
Medical management of ALD
Corticosteroids are a mainstay in pharmacotherapy for severe AH. There is evidence for improved outcomes in patients with severe AH treated with prednisolone for 4 to 6 weeks.5 Prognostic models such as the Maddrey’s Discriminant Function, Lille Model, and the MELD score help determine the need for steroid use and identify high-risk patients. Patients with active infection or bleeding are not a candidate for steroid treatment. An experienced gastroenterologist or hepatologist should initiate medical intervention after thorough evaluation.
Liver transplantation. A select group of patients with refractory liver failure are considered for liver transplantation. Although transplant programs differ in their criteria for organ listing, many require patients to demonstrate at least 6 months of verified abstinence from alcohol and illicit drugs as well as adherence to a formal AUD treatment and rehabilitation plan. The patient’s psychological health and prognosis for sustained sobriety are central to candidacy for organ listing, which highlights the key role of psychiatrists.
Further considerations. Thiamine and folate often are given to patients with ALD. Abdominal imaging and screening for HIV and viral hepatitis—identified in 10% to 20% of ALD patients—is routine. Alcohol abstinence remains central to survival because relapse increases the risk of recurrent, severe liver disease. Regrettably, many physical symptoms of liver disease, such as portal hypertension, ascites, and jaundice, can take months to improve with abstinence.
Treating AUD in patients with ALD
Successful treatment is multifaceted and includes more than just medications. Initial management often includes addressing alcohol withdrawal in dependent patients.6
Behavioral interventions are effective and indispensable components in preventing relapse,7 including a written relapse prevention plan that formally outlines the patient’s commitment to change, identifies triggers, and outlines a discrete plan of action. Primary psychiatric pathology, including depression and anxiety, often are comorbid with AUD; concurrent treatment of these disorders could improve patient outcomes.8
Benzodiazepines often are used during acute alcohol withdrawal. They should not be used for relapse prevention in ALD because of their additive interactions with alcohol, cognitive and psychomotor side effects, and abuse potential.9,10 Many of these drugs are cleared by the liver and generally are not recommended for use in patients with ALD.
Other agents, further considerations. Drug trials in AUD largely have been conducted in small, heterogeneous populations and revealed modest and, at times, conflicting drug effect sizes.6,11,12 The placebo effect among the AUD population is pronounced.6,7,13 Despite these caveats, several agents have been studied and validated by the FDA to treat AUD. Additional agents with promising pilot data are being investigated. Table 31,7,10,11,13-43 summarizes drugs used to treat AUD—those with and without FDA approval—with a focus on how they might be used in patients with ALD. Of note, several of these agents do not rely on the liver for metabolism or excretion.
There is no agreed-upon algorithm or safety profile to guide a prescriber’s decision making about drug or dosage choices when treating AUD in patients with ALD. Because liver function can vary among patients as well as during an individual patient’s disease course, treatment decisions should be made on a clinical, collaborative, and case-by-case basis.
That being said, the AUD treatment literature suggests that specific drugs might be more useful in patients with varying severity of disease and during different phases of recovery:
• Acamprosate has been found to be effective in supporting abstinence in sober patients.14,44
• Naltrexone has been shown to be useful in patients with severe alcohol cravings. By modulating alcohol’s rewarding effects, naltrexone also reduces heavy alcohol consumption in patients who are drinking.14,15,44
• Disulfiram generally is not recommended for use in patients with clinically apparent hepatic insufficiency, such as decompensated cirrhosis or preexisting jaundice.
Although alcohol abstinence remains the treatment goal and a requirement for liver transplant, providers must recognize that some patients might not be able to maintain long-term sobriety. Therefore, harm reduction models are important companions to abstinence-only models of AUD treatment.45 The array of behavioral, pharmacological, and philosophical approaches to AUD treatment underlines the need for an individualized approach to relapse prevention.
Collaboration between medicine and psychiatry
When AUD and ALD are comorbid, psychiatrists might worry about making the patient’s medical condition worse by prescribing additional psychoactive medications—particularly ones that are cleared by the liver. Remember that AUD confers a substantial mortality rate that is more than 3 times that of the general population, along with severe medical46 and psychosocial31 effects. Although prescribers must remain vigilant for adverse drug effects, medications easily can be blamed for what might be the natural progression and symptoms of AUD in patients with ALD.26 This erroneous conclusion can lead to premature medication discontinuation and under-treatment of AUD.
In the end, keeping the patient sober and mentally well might be more beneficial than eliminating the burden of any medication side effects. Collaborative medical and psychiatric management of ALD patients can ensure that clinicians properly weigh the risks, benefits, and duration of treatment unique to each patient.
Starting AUD treatment promptly after alcohol relapse is essential and entails a multidisciplinary effort between medicine and psychiatry, both in and out of the hospital. Because the relapsing, ill ALD patient most often will be admitted to a medical specialist, AUD might not receive enough attention during the medical admission. Psychiatrists can help in initiating AUD treatment in the acute medical setting, which has been shown to improve the outpatient course.6 For medically stable ALD patients admitted for inpatient psychiatric care or presenting a clinic, the mental health clinician should be aware of key laboratory and physical exam findings.
Bottom Line
Patients with alcoholic liver disease (ALD) require collaborative care from specialists in addiction, gastroenterology, and psychiatry. Psychiatrists have a role in identifying signs of ALD, prescribing medication to treat alcohol use disorder, and encouraging abstinence. There is some evidence supporting specific medications for varying severity of disease and different phases of recovery. Pharmacotherapy decisions should be made case by case.
Related Resources
• Khan A, Tansel A, White DL, et al. Efficacy of psychosocial interventions in inducing and maintaining alcohol abstinence in patients with chronic liver disease: a systematic review [published online August 6, 2015]. Clin Gastroenterol Hepatol. doi: 10.1016/j.cgh.2015.07.047.
• Vuittonet CL, Halse M, Leggio L, et al. Pharmacotherapy for alcoholic patients with alcoholic liver disease. Am J Health Syst Pharm. 2014;71(15):1265-1276.
Drug Brand Names
Acamprosate • Campral
Baclofen • Lioresal
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone • ReVia, Vivitrol
Pentoxifylline • Trental
Prednisolone • Prelone
Rifaximin • Xifaxan
Topiramate • Topamax
Disclosures
Dr. Winder and Dr. Mellinger report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Fontana receives research funding from Bristol Myers Squibb, Gilead, and Janssen and consults for the Chronic Liver Disease Foundation.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
1. Gache P, Hadengue A. Baclofen improves abstinence in alcoholic cirrhosis: still better to come? J Hepatol. 2008;49(6):1083-1085.
2. Rehm J, Mathers C, Popova S, et al. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223-2233.
3. Singal AK, Kamath PS, Gores GJ, et al. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12(4):555-564; quiz e31-32.
4. Becker U, Deis A, Sørensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. 1996;23(5):1025-1029.
5. Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(suppl 1):S39-S45.
6. Mann K, Lemenager T, Hoffmann S, et al; PREDICT Study Team. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18(6):937-946.
7. Anton RF, O’Malley SS, Ciraulo DA, et al; COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003-2017.
8. Kranzler HR, Rosenthal RN. Dual diagnosis: alcoholism and co-morbid psychiatric disorders. Am J Addict. 2003;12(suppl 1):S26-S40.
9. Book SW, Myrick H. Novel anticonvulsants in the treatment of alcoholism. Expert Opin Investig Drugs. 2005;14(4):371-376.
10. Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700.
11. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488.
12. Krystal JH, Cramer JA, Krol WF, et al; Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734-1739.
13. Petrakis IL, Poling J, Levinson C, et al; VA New England VISN I MIRECC Study Group. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128-1137.
14. Maisel NC, Blodgett JC, Wilbourne PL, et al. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108(2):275-293.
15. Pettinati HM, O’Brien CP, Rabinowitz AR, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26(6):610-625.
16. Anton RF, Myrick H, Wright TM, et al. Gabapentin combined with naltrexone for the treatment of alcohol dependence. Am J Psychiatry. 2011;168(7):709-717.
17. Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2005(1):CD001867.
18. Naltrexone. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
19. Soyka M, Chick J. Use of acamprosate and opioid antagonists in the treatment of alcohol dependence: a European perspective. Am J Addict. 2003;12(suppl 1):S69-S80.
20. Turncliff RZ, Dunbar JL, Dong Q, et al. Pharmacokinetics of long-acting naltrexone in subjects with mild to moderate hepatic impairment. J Clin Pharmacol. 2005;45(11):1259-1267.
21. United States National Library of Medicine. Naltrexone. http://livertox.nlm.nih.gov/Naltrexone.htm. Updated September 30, 2015. Accessed November 10, 2015.
22. Terg R, Coronel E, Sordá J, et al. Efficacy and safety of oral naltrexone treatment for pruritus of cholestasis, a crossover, double blind, placebo-controlled study. J Hepatol. 2002;37(6):717-722.
23. Skinner MD, Lahmek P, Pham H, et al. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis [published online February 10, 2014]. PLoS One. 2014;9(2):e87366. doi: 10.1371/journal.pone.0087366.
24. Disulfiram. 2014. http://www.micromedexsolutions.com. Accessed January 31, 2015.
25. Björnsson E, Nordlinder H, Olsson R. Clinical characteristics and prognostic markers in disulfiram-induced liver injury. J Hepatol. 2006;44(4):791-797.
26. Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug Saf. 1999;20(5):427-435.
27. Campral [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc.; 2012.
28. Brower KJ, Myra Kim H, Strobbe S, et al. A randomized double-blind pilot trial of gabapentin versus placebo to treat alcohol dependence and comorbid insomnia. Alcohol Clin Exp Res. 2008;32(8):1429-1438.
29. Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77.
30. Neurontin [package insert]. New York, NY: Pfizer; 2015.
31. Johnson BA, Ait-Daoud N, Akhtar FZ, et al. Oral topiramate reduces the consequences of drinking and improves the quality of life of alcohol-dependent individuals: a randomized controlled trial. Arch Gen Psychiatry. 2004;61(9):905-912.
32. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41.
33. Rubio G, Ponce G, Jiménez-Arriero MA, et al. Effects of topiramate in the treatment of alcohol dependence. Pharmacopsychiatry. 2004;37(1):37-40.
34. Topamax [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2009.
35. De Sousa AA, De Sousa J, Kapoor H. An open randomized trial comparing disulfiram and topiramate in the treatment of alcohol dependence. J Subst Abuse Treat. 2008;34(4):460-463.
36. Kampman KM, Pettinati HM, Lynch KG, et al. A double-blind, placebo-controlled trial of topiramate for the treatment of comorbid cocaine and alcohol dependence. Drug Alcohol Depend. 2013;133(1):94-99.
37. Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46(3):312-317.
38. Balcofen [package insert]. Concord, NC: McKesson Packing Services; 2013.
39. United States National Library of Medicine. Baclofen. 2015. http://livertox.nlm.nih.gov/Baclofen.htm. Accessed November 7, 2015.
40. Addolorato G, Leggio L, Ferrulli A, et al. Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: randomised, double-blind controlled study. Lancet. 2007;370(9603):1915-1922.
41. Leggio L, Ferrulli A, Zambon A, et al. Baclofen promotes alcohol abstinence in alcohol dependent cirrhotic patients with hepatitis C virus (HCV) infection. Addict Behav. 2012;37(4):561-564.
42. Franchitto N, Pelissier F, Lauque D, et al. Self-intoxication with baclofen in alcohol-dependent patients with co-existing psychiatric illness: an emergency department case series. Alcohol Alcohol. 2014;49(1):79-83.
43. Brennan JL, Leung JG, Gagliardi JP, et al. Clinical effectiveness of baclofen for the treatment of alcohol dependence: a review. Clin Pharmacol. 2013;5:99-107.
44. Rösner S, Leucht S, Lehert P, et al. Acamprosate supports abstinence, naltrexone prevents excessive drinking: evidence from a meta-analysis with unreported outcomes. J Psychopharmacol. 2008;22(1):11-23.
45. Marlatt GA, Witkiewitz K. Harm reduction approaches to alcohol use: health promotion, prevention, and treatment. Addict Behav. 2002;27(6):867-886.
46. O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33.
Awareness and management of obstetrical complications of depression
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
When a patient who has a preexisting medical illness seeks prenatal care, the obstetrician asks herself (himself) 2 questions:
• What impact will the illness have on the pregnancy?
• What impact will the pregnancy have on the illness?
Depression is both a pregnancy-associated and pregnancy-independent illness, which, in the setting of a pregnant woman who has a depressive disorder, makes these questions particularly difficult to answer. In such a case, coordination of care with a mental health provider is essential.
Awareness of the obstetrical complications associated with depression during pregnancy, as well as their implications for the future health of the mother–infant dyad, is important for the entire care team. This article reviews the associations and interconnectedness of depression with complications of pregnancy, childbirth, and the neonatal period.
Diagnosis of depression during prenatal care
The American College of Obstetricians and Gynecologists (ACOG) states that evidence is insufficient to support a recommendation for universal screening for depression among prenatal patients, although such screening should be considered.1 There is considerable variability among obstetrical providers regarding the practice of depression screening; tools to be used if such screening is done; and screening frequency through the pregnancy.
Discernment of depression is difficult. Many somatic symptoms of depression overlap with common prenatal complaints and, consequentially, can be overlooked. Among a sample of 700 pregnant women, for example, 56% complained of lack of energy; 19%, of insomnia; and 19%, of appetite changes.2 Weight change, of course, is universal.
The 10-question self-rating Edinburgh Postnatal Depression Scale has been validated for use during pregnancy and postnatally. This screening instrument can be helpful for differentiating purely physical complaints from mental distress due to depressive symptoms.2,3
When an obstetrical provider suspects a depressive disorder, or one has been diagnosed, she (he) faces the problem of what to do with that information. Women of low socioeconomic status and victims of domestic violence are at increased risk of depression during pregnancy, but barriers to appropriate referral can seem nearly insurmountable because they lack insurance and social support.4-9
In addition, within the setting of numerous tasks that need attending during the relatively short prenatal period, it is common for women newly given a diagnosis of depression to fail to follow up on a referral to a mental health provider.
Although most providers will “check in” with a depressed or at-risk patient at each prenatal visit about her mood, any effort at follow-up can be overshadowed by tangible physical concerns, such as preterm contractions, fetal growth restriction, and coordination of routine testing that has been delayed because of scant prenatal care. All these physical concerns and circumstances of care are associated with maternal depression, as we will discuss.
Preterm labor and birth
Preterm labor is defined as uterine contractions that lead to cervical change before 37 weeks gestational age. Preterm labor increases the risk of preterm birth; preterm labor precedes 50% of preterm births. Preterm birth is the leading cause of neonatal mortality in the United States, and rates of morbidity and mortality increase as gestational age decreases.10 Common neonatal complications related to prematurity are shown in the Figure.11
Women who suffer from depression have an increased risk of preterm labor and preterm birth, as many studies of treated and untreated depressed pregnant women have shown.12-20 The causative mechanism is unknown; it has been proposed that the increase in maternal cortisol production associated with depression and distress triggers overproduction of placental cortisol releasing hormone, which is thought to be involved in initiation of parturition.21,22 Depression also is associated with other risk factors for preterm birth, such as low socioeconomic status, substance use, and smoking.
Intrauterine growth restriction
Women who have depression during pregnancy have an increased risk of intrauterine growth restriction (IUGR), which leads to delivery of an infant who is small for gestational age (SGA) or of low birth weight (LBW) (weighing <2,500 g at birth), or both.23 Again, the basis of the association between depression and IUGR and SGA is unknown; it is theorized that increased levels of cortisol and catecholamines associated with maternal distress might, by increasing blood pressure and inducing vasoconstriction, cause placental hypoperfusion.24,25
It also is possible that the association of depression with other risk factors for IUGR, such as smoking, substance use, obesity, and poor prenatal care, puts the infants of depressed women at risk of growth restriction.26 Several large-scale studies showed that the association between LBW and depression is lost when smoking and substance use are accounted for; other studies, however, found a persistent association in untreated depressed women when smokers, substance users, and drinkers were excluded.17,26,27
IUGR infants are at increased risk of iatrogenic prematurity and stillbirth. Fetuses that weigh <10th percentile for their gestational age are delivered no later than 40 weeks; delivery can be indicated as early as 32 weeks, depending on the results of other antenatal tests. Women who have a growth-restricted infant have a higher risk of cesarean delivery because growth-restricted infants often have less reserve and poorer tolerance of labor.
Preeclampsia and eclampsia
Preeclampsia is defined as blood pressure >140/90 mm HG on at least 2 occasions, with proteinuria, that occurs later than the twentieth week of pregnancy in women who did not have hypertension or renal dysfunction at baseline. Preeclampsia is a progressive disease that can cause severe maternal morbidity, including renal failure, stroke, hepatic rupture, pulmonary edema, and heart failure.
Eclampsia refers to onset of seizures in the setting of preeclampsia. These 2 hypertensive disorders are the third leading world wide cause of maternal mortality.28
Depressed women have an elevated risk of preeclampsia. The association between preeclampsia and depression might be caused by the presence of increased levels of inflammatory mediators29,30; other comorbidities, such as increased body mass index, also might be involved, but the risk for preeclampsia in depressed women still is increased after controlling for obesity.31
The presence of preeclampsia is responsible for a high percentage of iatrogenic preterm births, because the cure for the disorder is delivery—even at early or previable gestational age. Complication rates for mother and infant are high.
The presence of preeclampsia is a significant risk factor for intrauterine fetal demise. Treating the mother after delivery involves administration of IV magnesium for 24 hours; often, the mother is separated from her infant for a day after birth.
Impact on prenatal care
Depression increases odds that women will have fewer prenatal visits.32 During pregnancy, women typically initiate prenatal care during the first trimester, when pregnancy-dating ultrasonography and early screening tests for chromosomal abnormalities are performed. Prenatal visits occur monthly until the third trimester, then every 2 weeks between 32 and 36 weeks’ gestation, increasing to weekly after 36 weeks’ gestation.
The increased number of visits in late pregnancy allows for early detection and treatment of hypertensive disorders; assesses fetal well-being; and decreases the risks of morbidity and mortality for mother and fetus.33 Because women who suffer from depression are at increased risk of an array of adverse pregnancy outcomes, the importance of regular and timely prenatal care cannot be understated.
In addition, the prenatal visit gives the obstetrician the opportunity to connect women with other specialists for management of any unmet medical needs. One study showed that, when women have adequate prenatal care (measured by the number of visits), the association between preterm birth and self-reported maternal depression was eliminated.34
Substance use
Substance use and depression often co-exist.35,36 Unlike screening for depression, screening for substance use is universal during prenatal care. Studies have shown that women who screen positive for depression are at higher risk of a number of comorbidities, including substance use.37,38 Conversely, women who use substances are more likely to screen positive for depression.
Evidence suggests that best practice might be to screen for depression in any woman who has a positive drug screen, if a provider is not routinely screening their general patient population.39 Substance use in pregnancy is associated with a number of poor outcomes, including placental abruption (cocaine use); dysmorphic facies and congenital anomalies (alcohol); and neonatal abstinence syndrome (heroin).
Antidepressants in pregnancy
A full discussion of the risks and benefits associated with pharmacotherapy for depression in pregnancy is beyond the scope of this article. Generally, antidepressant use is fraught with concerns over teratogenicity and adverse fetal outcomes. Although ACOG states that (1) pharmacotherapy for depression should be individualized and (2) most selective serotonin reuptake inhibitors (SSRIs) are not considered major teratogenic agents, many obstetricians and patients feel uncomfortable using these medications in pregnancy.40 Often, pre-pregnancy antidepressants are discontinued in the first trimester; one large population-based study found that only 0.9% of women who had depression filled their antidepressant prescription consistently throughout their pregnancy.41
It is unclear whether antidepressant use in pregnancy contributes to the risk of preterm birth seen in women who have depression. In a large population-based study, use of antidepressants in the second trimester was associated with preterm delivery but severe depression was not.18 A recent meta-analysis revealed an increased risk of preterm birth in women who used an antidepressant, compared with healthy women and untreated depressed women.42
Research limits, unanswered questions. Regrettably, it is difficult to untangle risk factors for preterm birth among depressed women without randomized controlled studies that are not ethically feasible. It cannot be said with certainty whether antidepressant pharmacotherapy is associated with a higher risk of preterm birth than depression alone.
Likewise, it is difficult to clarify the extent to which antidepressants contribute to infant growth restriction, if at all. Two recent meta-analyses concluded that exposure to antidepressants is associated with a statistically significant risk of LBW.42,43 However, increased severity of depressive symptoms generally is associated with exposure to antidepressants during pregnancy, and a randomized controlled trial is, again, impossible to conduct for ethical reasons.
Whereas a plausible biological mechanism associating IUGR, SGA, and LBW with depression exists, the same cannot be said for antidepressants. In one study, exposure to maternal depression altered the expression of certain placental genes but exposure to SSRIs did not cause further changes. This suggests that, on a cellular level, placental function might differ in depressed women.44 Although antidepressants do cross the placenta, it remains to be seen whether fetal growth is impacted as a result. One study found decreased fetal head circumference in infants who had been exposed to antidepressants during pregnancy, but no increased risk for having a SGA or LWB infant.45
Obstetrical management and mental health implications
Treated or not, women who suffer depression are a high-risk group when it comes to preterm birth and a host of other pregnancy comorbidities. Women with serious complications of pregnancy often are hospitalized for observation, and can undergo a prolonged stay when close proximity to medical services or a surgical suite is required.
For example, hospitalization until delivery is the standard of care for women who have preterm premature rupture of membranes or preeclampsia before 34 weeks’ gestation. Prolonged inpatient admissions and associated restriction of activity is profoundly deleterious on mood, with depression and anxiety significantly correlated with length of stay.46,47 Given the associations between depression and preterm birth, it might be reasonable to consider screening antenatal inpatients at risk of preterm birth for depression on a regular basis, so that treatment can be initiated if needed.
Depression during pregnancy is relatively common; an estimated 12.7% of pregnant women are affected at some time between conception and birth.48 Not only does depression appear to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother.
Bottom Line
Awareness of obstetrical complications associated with depression in pregnancy is important for the entire care team, including the psychiatrist and obstetrician. Depression not only appears to have deleterious effects on pregnancy outcomes, it also plays a pivotal role in the qualitative experience of pregnancy for the mother. Antidepressant use generally is fraught with concerns over teratogenicity and adverse fetal outcomes.
Related Resources
• Freeman MP. Some SSRIs are better than others for pregnant women (audio interview). Current Psychiatry. 2014;13(7). http://www.currentpsychiatry.com/specialty-focus/practice-trends/article/some-ssris-are-better-thanothers-for-pregnant-women/e3adb4704e25492f3e15331fc1cc058d.html.
• Freeman MP, Joffe H, Cohen LS. Postpartum depression: Help patients find the right treatment. Current Psychiatry. 2012;11(11):14-16,19-21.
Disclosures
Dr. Habecker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Freeman is a member of the advisory board of JDS Therapeutics, Sunovion Pharmaceuticals, Inc., and Takeda Pharmaceutical Co. She receives research grant support from Takeda Pharmaceutical Co.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.
1. American College of Obstetricians and Gynecologists. Committee on Obstetric Practice. Committee opinion no. 630. 2015;125:1268-1271.
2. Apter G, Devouche E, Garez V, et al. Pregnancy, somatic complaints and depression: a French population-based study. Eur J Obstet Gynecol Reprod Biol. 2013;171(1):35-39.
3. Murray D, Cox JL. Screening for depression during pregnancy with the Edinburgh Depression Scale (EDDS). J Reprod Infant Psychol. 1990;8(2):99-107.
4. Gotlib IH, Whiffen VE, Mount JH, et al. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. J Consult Clin Psychol. 1989;57(2):269-274.
5. Melville JL, Gavin A, Guo Y, et al. Depressive disorders during pregnancy: prevalence and risk factors in a large urban sample. Obstet Gynecol. 2010;116(5):1064-1070.
6. Leddy M, Haaga D, Gray J, et al. Postpartum mental health screening and diagnosis by obstetrician-gynecologists. J Psychosom Obstet Gynaecol. 2011;32(1):27-34.
7. McFarlane J, Maddoux J, Cesario S, et al. Effect of abuse during pregnancy on maternal and child safety and functioning for 24 months after delivery. Obstet Gynecol. 2014;123(4):839-847.
8. Vesga-López O, Bianco C, Keyes K, et al. Psychiatric disorders in pregnant and postpartum women in the United States. Arch Gen Psychiatry. 2008;65(7):805-815.
9. Farr SL, Bitsko RH, Hayes DK, et al. Mental health and access to services among US women of reproductive age. Am J Obstet Gynecol. 2010;203(6):542.e1-e542.e9. doi: 10.1016/j.ajog.2010.07.007.
10. Committee on Practice Bulletins—Obstetrics; The American College of Obstetricians and Gynecologists. Practice bulletin no. 130: prediction and prevention of preterm birth. Obstet Gynecol. 2012;120(4):964-973.
11. Stoll BJ, Hansen NI, Bell EF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443-456.
12. Steer RA, Scholl TO, Hediger ML, et al. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45(10):1093-1099.
13. Goldenberg RL, Cliver SP, Mulvihill FX, et al. Medical, psychosocial, and behavioral risk factors do not explain the increased risk for low birth weight among black women. Am J Obstet Gynecol. 1996;175(5):1317-1324.
14. Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156(9):797-802.
15. Dayan J, Creveuil C, Marks MN, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med. 2006;68(6):938-946.
16. Goedhart G, Snijders AC, Hesselink AE, et al. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med. 2010;72(8):769-776.
17. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024.
18. Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies [published online April 30, 2012]. Am J Obstet Gynecol. 2012;207(1):49.e1-49.e9. doi: 10.1016/j. ajog.2012.04.028.
19. McDonagh MS, Matthews A, Phillipi C, et al. Depression drug treatment outcomes in pregnancy and the postpartum period: a systematic review and meta-analysis. Obstet Gynecol. 2014;124(3):526-534.
20. Sahingöz M, Yuksel G, Karsidag C, et al. Birth weight and preterm birth in babies of pregnant women with major depression in relation to treatment with antidepressants. J Clin Psychopharmacol. 2014;34(2):226-229.