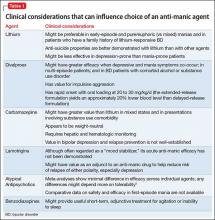
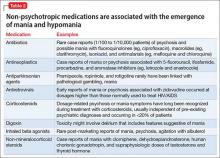
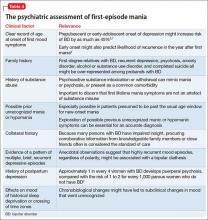
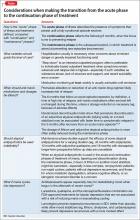
User login
Botulinum toxin for depression? An idea that’s raising some eyebrows
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
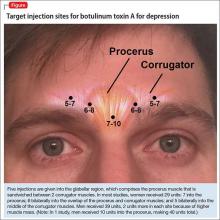
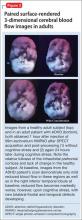
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
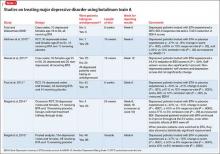
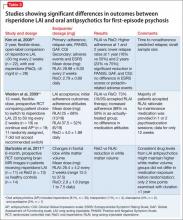
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
Psychiatry is experiencing a major paradigm shift.1 No longer is depression a disease of norepinephrine and serotonin deficiency. Today, we are exploring inflammation, methylation, epigenetics, and neuroplasticity as major players; we are using innovative treatment interventions such as ketamine, magnets, psilocin, anti-inflammatories, and even botulinum toxin.
In 2006, dermatologist Eric Finzi, MD, PhD, reported a case series of 10 depressed patients who were given a single course of botulinum toxin A (BTA, onabotulinum-toxinA) injections in the forehead.2 After 2 months, 9 out of the 10 patients were no longer depressed. The 10th patient, who reported improvement in symptoms but not remission, was the only patient with bipolar depression.
As a psychiatrist (M.M.) and a dermatologist (J.R.), we conducted a randomized controlled trial3 to challenge the difficult-to-swallow notion that a cosmetic intervention could help severely depressed patients. After reporting our positive findings and hearing numerous encouraging patient testimonials, we present a favorable review on the treatment of depression using BTA. We also present the top 10 questions we are asked at lectures about this novel treatment.
A deadly toxin used to treat medical conditions
Botulinum toxin is one of the deadliest substance known to man.4 It was named after the gram-positive bacterium Clostridium botulinum, which causes so-called floppy baby syndrome in infants who eat contaminated honey. Botulinum toxin prevents nerves from releasing acetylcholine, which causes muscle paralysis.
In the wrong hands, botulinum toxin can be exploited for chemical warfare.4 However, doctors are using it to treat >50 medical conditions, including migraine, cervical dystonia, strabismus, overactive bladder, urinary incontinence, excessive sweating, muscle spasm, and now depression.5,6 In 2014, BTA was the top cosmetic treatment in the United States, with >3 million procedures performed, generating more than 1 billion dollars in revenue.7
The most common site injected with BTA for cosmetic treatments is the glabellar region, which is the area directly above and in between the eyebrows (ie, the lower forehead). The glabella comprises 2 main muscles: the central procerus flanked by a pair of corrugators (Figure). When expressing fear, anger, sadness, or anguish, these muscles contract, causing the appearance of 2 vertical wrinkles, referred to as the “11s.” The wrinkles also can form the shape of an upside-down “U,” known as the omega sign.8 BTA prevents contraction of these muscles and therefore prevents the appearance of a furrowed brow. During cosmetic procedures, approximately 20 to 50 units of BTA are spread out over 5 glabellar injection sites.9 A similar technique is being used in studies of BTA for depression2,3,10,11 (Figure).
BTA for depression is new to the mental health world but, before psychiatrists caught on, dermatologists were aware that BTA could improve quality of life,12 reduce negative emotions,13 and increase feelings of well-being.14
The evidence
To date, there have been 2 case series,2,15 3 randomized control trials (RCTs),3,10,11 1 pooled analysis,16,17 and 1 meta-analysis18 looking at botulinum for depression (Table 1).2,10,11,15-17 In each trial, a single treatment of BTA (ie, 1 doctor’s visit; 29 to 40 units of BTA distributed into 5 glabellar injections sites), was the intervention studied.2
The first case series, by Finzi and Wasserman2 is described above. A second case series, published in 2013, describes 50 female patients, one-half depressed and one-half non-depressed, all of whom received 20 units of BTA into the glabella.15 At 12 weeks, depression scores in the depressed group had decreased by 54% (14.9 point drop on Beck Depression Inventory [BDI], P < .001) and self-esteem scores had increased significantly. In non-depressed participants, depression scores and self-esteem scores remained constant throughout the 12 weeks.
A pooled analysis reported results of 3 RCTs16,17 consisting of a total of 134 depressed patients, males and females age 18 to 65 who received BTA (n = 59) or placebo (n = 74) into the glabellar region. At the end of 6 weeks, BDI scores in the depressed group had decreased by 47.4% (14.3 points) compared with a 16.2% decrease (5.1 points) in the placebo group. This corresponds to a 52.5% vs 8.0% response rate and a 42.4% vs 8.0% remission rate, respectively (Table 1,1,2,10,11,15-17). There was no difference between the 2 groups in sex, age, depression severity, and number of antidepressants being taken. Females received 29 units and males received 10 to 11 units more to account for higher muscle mass (Figure).
Depression as measured by the physician-administered Hamilton Depression Rating Scale (HAM-D) and the Montgomery-Åsberg Depression Rating Scale showed similar reduction in overall scores (−45.7% vs −14.6%), response rates (54.2% vs 10.7%) and remission rates (30.5% vs 6.7%) with BTA.
Although these improvements in depression scores do not reach those seen with electroconvulsive therapy,19,20 they are comparable to placebo-controlled studies of antidepressants.21,22
Doesn’t this technique work because people who look better, feel better?
Aesthetic improvement alone is unlikely to explain the entire story. A recent study showed that improvement in wrinkle score did not correlate with improvement in mood.23 Furthermore, some patients in RCTs did not like the cosmetic effects of BTA but still reported feeling less depressed after treatment.10
How might it work?
Several theories about the mechanism of action have been proposed:
• The facial feedback hypothesis dates to Charles Darwin in 1872: Facial movements influence emotional states. Numerous studies have confirmed this. Strack et al24 found that patients asked to smile while reading comics found them to be funnier. Ekman et al25 found that imitating angry facial expressions made body temperature and heart rate rise. Dialectical behavioral therapy expert Marsha Linehan recognized the importance of modifying facial expressions (from grimacing to smiling) and posture (from clenched fists to open hands) when feeling distressed, because it is hard to feel “willful” when your “mind is going one way and your body is going another.”26 Accordingly, for a person who continuously “looks” depressed or distressed, reducing the anguished facial expression using botulinum toxin might diminish the entwined negative emotions.
• A more pleasant facial expression improves social interactions, which leads to improvement in self-esteem and mood. Social biologists argue that (1) we are attracted to those who have more pleasant facial expressions and (2) we steer clear of those who appear angry or depressed (a negative facial expression, such as a growling dog, is perceived as a threat). Therefore, anyone who looks depressed might have less rewarding interpersonal interactions, which can contribute to a poor mood.
On a similar note, mirror neurons are regions in the brain that are activated by witnessing another person’s emotional cues. When our mirror neurons light up, we can feel an observed experience, which is why we often feel nervous around anxious people, or cringe when we see others get hurt, or why we might prefer engaging with people who appear happier. It is possible that, after BTA injection, a person’s social connectivity is improved because of a more positive reciprocal firing of mirror neurons.
• BTA leads to direct and indirect neurochemical changes in the brain that can reduce depression. Functional MRI studies have shown that after glabellar BTA injections, the amygdala was less responsive to negative stimuli.27,28 For example, patients who were treated with BTA and then shown pictures of angry people had an attenuated amygdala response to the photos.
This is an important finding, especially for patients who have been traumatized. After a traumatic event, the amygdala “remembers” what happened, which is good, in some ways (it prevents us from getting into a similar dangerous situation), but bad in others (the traumatized amygdala may falsely perceive a non-threatening stimuli as threatening). A hypervigilant amygdala can lead to an out-of-proportion fear response, depression, and anxiety. Therefore, quelling an overactive amygdala with BTA could improve emotional dysregulation and posttraumatic disorders.
Many of our patients reported that, after BTA injection, “traumatic events didn’t feel as traumatizing,” as one said. The emotional pain and rumination that often follow a life stressor “does not overstay its welcome” and patients are able to “move on” more quickly.
It is unknown why the amygdala is quieted after BTA; researchers hypothesize that BTA suppresses facial feedback signals from the forehead branch of the trigeminal nerve to the brain. Another hypothesis is that BTA is directly transported by the trigeminal nerve into the brain and exerts central pharmacological effects on the amygdala.29 This theory has only been studied in rat models.30
When does it start working? How long does it last?
From what we know, BTA for depression could start working as early as 2 weeks and could last as long as 6 months. In one RCT, the earliest follow-up was 2 weeks,10 at which time the depressed patients had responded to botulinum toxin (P ≤ .05). In the other 2 controlled trials, the earliest follow-up was 3 weeks, at which time a more robust response was seen (P < .001). Aesthetically, BTA usually lasts 3 months. It is unclear how long the antidepressant effects last but, in the longest trial,3 depression symptoms continued to improve at 6 months, after cosmetic effects had worn off.
These findings raise a series of questions:
• Do mood effects outlast cosmetic effects? If so, why?
• Does botulinum toxin start to work sooner than 2 weeks?
• Will adherence improve if a patient has to be treated only every 6 months?
In our clinical experience, depressed patients who responded to BTA injection report a slow resurfacing of depressive symptoms 4 to 6 months after treatment, at which point they usually return for “maintenance treatment” (same dosing, same injection configuration).
Will psychiatrists administer the treatment?
Any physician or physician extender can, when properly trained, inject BTA. The question is: Do psychiatrists want to? Administrating botulinum toxin requires more labor and preparation than prescribing a drug (Table 2,31) and requires placing hands on patients. Depending on the type of psychiatric practice, this may be a “deal-breaker” for some providers, such as those in a psychoanalytic practice who might worry about boundaries.
As a basis for comparison, despite several indications for BTA for headache and neurologic conditions, few neurologists have added botulinum toxin to their practice. Dermatologists who are comfortable seeing psychiatric patients or family practitioners, who are already set up for injection procedures, could become custodians of this intervention.
Which patients are candidates for the treatment?
Patients with anxious or agitated depression might be ideal candidates for BTA injection. A recent study looked at predictors of response: Patients with a high agitation score (as measured on item 9 of the HAM-D) were more likely to respond, with a sensitivity of 100%, a specificity of 56%, and an overall precision of 78%.32 So far, no other predictors of response have been clearly identified. Higher baseline wrinkle scores do not predict better response.23 Sex and age do not have any predictive value. The treatment appears to be equally effective in males and females; because only a handful of males have been treated (n = 14), however, these patients need to be studied further.
Is botulinum toxin better as monotherapy or augmentation strategy?
So far, it appears to be equally effective as monotherapy or augmentation strategy,16 but more studies are needed.
How expensive is it?
Estimates of patient cost include the cost of the product and the professional fee for injection. As a point of reference, for cosmetic purposes, depending on practice location, dermatologists charge $11 to $20 per unit of BTA. Therefore, 1 treatment of BTA for depression (29 to 40 units) can cost a patient $319 to $800.
When treating a patient with BTA for medical indications, such as tension headache, insurance often reimburses the physician for the BTA at cost (paid with a J code: J0585) and pay an injection fee (a procedure code) of $150 to $200. A recent analysis of cost-effectiveness estimated that BTA for depression would cost a patient $1,200 to $1,600 annually.33 Compared with the price of branded medications (eg, $500 to $2,000 annually)33 plus weekly psychotherapy (eg, $2,000 to $5,000 annually), BTA may be a cost-effective option for patients who do not respond to conventional treatments. Of course, for patients who tolerate and respond to generic medications or have a therapist who charges on a sliding scale, BTA is not the most cost-effective option.
What about injecting other areas of the face?
We’ve thought about it but haven’t tried it. There are several muscles around the mouth that allow us to smile and frown. BTA injections in the depressor anguli oris, a muscle around the mouth that is largely responsible for frowning, could treat depression. However, if the mechanism of action is via amygdala desensitization through the trigeminal nerve, treating mouth frown muscles might not work.
Is it safe?
BTA in the glabella has an exceptionally good safety profile.9,31,34 Adverse reactions, which include eyelid droop, pain, bruising, and redness at the injection site, are minor and temporary.9 In addition, BTA has few drug–drug interactions. The biggest complaint for most patients is discomfort upon injection, which often is described as feeling like “an ant bite.”
In the pooled analysis of RCTs, apart from local irritation immediately after injection, temporary headache was the only relevant, and possibly treatment-related, adverse event. Headache occurred in 13.6% (n = 8) of the BTA group and 9.3% (n = 7) of the placebo group (P = .44). Compared with antidepressants such as citalopram, where approximately 38.6% of patients report a moderate or severe side-effect burden,21 BTA is well tolerated.
Are other studies underway?
Larger studies are being conducted,35 mainly to confirm what pilot studies have shown. It would be interesting to discover other predictors of response and if different dosing and injection configurations could strengthen the response rate and extend the duration of effect.
Because of the cosmetic effects of BTA, further studies are needed to address the problem of blinding. In earlier studies, raters were blinded during appointments because patients wore surgical caps that covered their glabellar region.3,10 Patients did not know their treatment intervention, but 52% to 90% of patients guessed correctly.3,10,11 Although unblinding is a common problem in “blinded” trials in which some researchers have reported >75% of participants and raters guessed the intervention correctly,36 it is a particularly sensitive area in studies that involve a change in appearance because it is almost impossible to prevent someone from looking in a mirror.
Summing up
Botulinum toxin for depression is not ready for prime time. The FDA has not approved its use for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure as a treatment for depression. Patients who request BTA for depression must be informed that this use is off-label.
For now, we recommend psychotherapy or medication management, or both, for most patients with major depression. In addition, until larger studies are done, we recommend that patients who are interested in BTA for depression use it as an add-on to conventional treatment. However, if larger studies replicate the findings of the smaller studies we have described, botulinum toxin could become a novel therapeutic agent in the fight against depression.
Bottom Line
In pilot studies, botulinum toxin A (BTA) has shown efficacy in improving symptoms of depression. Although considered safe, BTA is not FDA-approved for psychiatric indications, and Medicare and commercial insurance do not reimburse for this procedure for depression. Larger studies are underway to determine if this novel treatment can be introduced into practice.
Related Resources
• Wollmer MA, Magid M, Kruger THC. Botulinum toxin treatment in depression. In: Bewley A, Taylor RE, Reichenberg JS, et al, eds. Practical psychodermatology. Hoboken, NJ: John Wiley & Sons; 2014:216-219.
• Botox for depression. www.botoxfordepression.com.
• Botox and depression. www.botoxanddepression.com.
Drug Brand Names
Botulinum toxin A • Botox
Citalopram • Celexa
Acknowledgments
We thank the Brain and Behavior Research Foundation for granting Dr. Magid a young investigator award and for continuing to invest in innovative research ideas. We thank Dr. Eric Finzi, MD, PhD, Axel Wollmer, MD, and Tillmann Krüger, MD, for their continued collaboration in this area of research.
Disclosures
In July 2011, Dr. Magid received a young investigator award from the Brain and Behavior Research Foundation for her study on treating depression using botulinum toxin (Grant number 17648). In November 2012, after completion and as a result of the study on treating depression using botulinum toxin, Dr. Magid became a consultant with Allergan to discuss study findings. In September 2015, Dr. Magid became a speaker for IPSEN Innovation. Dr. Reichenberg is married to Dr. Magid. Dr. Reichenberg has no other conflicts of interest to disclose.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
1. Nasrallah HA. 10 Recent paradigm shifts in the neurobiology and treatment of depression. Current Psychiatry. 2015;14(2):10-13.
2. Finzi E, Wasserman E. Treatment of depression with botulinum toxin A: a case series. Dermatol Surg. 2006;32(5):645-649; discussion 649-650.
3. Magid M, Reichenberg JS, Poth PE, et al. Treatment of major depressive disorder using botulinum toxin A: a 24-week randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(8):837-844.
4. Koussoulakos S. Botulinum neurotoxin: the ugly duckling. Eur Neurol. 2008;61(6):331-342.
5. Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4(10):913-939.
6. Bhidayasiri R, Truong DD. Expanding use of botulinum toxin. J Neurol Sci. 2005;235(1-1):1-9.
7. Cosmetic surgery national data bank statistics. American Society for Asethetic Plastic Surgery. http://www.surgery. org/sites/default/files/2014-Stats.pdf. Published 2014. Accessed May 30, 2015.
8. Shorter E. Darwin’s contribution to psychiatry. Br J Psychiatry. 2009;195(6):473-474.
9. Winter L, Spiegel J. Botulinum toxin type-A in the treatment of glabellar lines. Clin Cosmet Investig Dermatol. 2009;3:1-4.
10. Wollmer MA, de Boer C, Kalak N, et al. Facing depression with botulinum toxin: a randomized controlled trial. J Psychiatr Res. 2012;46(5):574-581.
11. Finzi E, Rosenthal NE. Treatment of depression with onabotulinumtoxinA: a randomized, double-blind, placebo controlled trial. J Psychiatr Res. 2014;52:1-6.
12. Hexsel D, Brum C, Porto MD, et al. Quality of life and satisfaction of patients after full-face injections of abobotulinum toxin type A: a randomized, phase IV clinical trial. J Drugs Dermatol. 2013;12(12):1363-1367.
13. Lewis MB, Bowler PJ. Botulinum toxin cosmetic therapy correlates with a more positive mood. J Cosmet Dermatol. 2009;8(1):24-26.
14. Sommer B, Zschocke I, Bergfeld D, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg. 2003;29(5):456-460.
15. Hexsel D, Brum C, Siega C, et al. Evaluation of self‐esteem and depression symptoms in depressed and nondepressed subjects treated with onabotulinumtoxina for glabellar lines. Dermatol Surg. 2013;39(7):1088-1096.
16. Magid M, Reichenberg JS, Finzi E, et al. Treating depression with botulinum toxin: update and meta-analysis from clinic trials. Paper presented at: XVI World Congress of Psychiatry; September 14-18, 2014; Madrid, Spain.
17. Magid M, Finzi E, Kruger TH, et al. Treating depression with botulinum toxin: a pooled analysis of randomized controlled trials. Pharmacopsychiatry. 2015;48(6):205-210.
18. Parsaik A, Mascarenhas S, Hashmi A, et al. Role of botulinum toxin in depression: a systematic review and meta-analysis. J Psychiatr Pract. In press.
19. Scott AI, ed. The ECT handbook, 2nd ed. The third report of the Royal College of Psychiatrists’ Special Committee of ECT. London, United Kingdom: The Royal College of Psychiatrists; 2005.
20. Ren J, Li H, Palaniyappan L, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:181-189.
21. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40.
22. Gibbons RD, Hur K, Brown CH, et al. Benefits from antidepressants: synthesis of 6-week patient-level outcomes from double-blind placebo-controlled randomized trials of fluoxetine and venlafaxine. Arch Gen Psychiatry. 2012;69(6):572-579.
23. Reichenberg JS, Magid M, Keeling B. Botulinum toxin for depression: does the presence of rhytids predict response? Presented at: Texas Dermatology Society; May 2015; Bastrop, Texas.
24. Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: a nonobtrusive test of the facial feedback hypothesis. J Pers Soc Psychol. 1988;54(5):768-777.
25. Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221(4616):1208-1210.
26. Linehan MM. DBT skills training manual, 2nd ed. New York, NY: Guilford Publications; 2014.
27. Hennenlotter A, Dresel C, Castrop F, et al. The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cereb Cortex. 2009;19(3):537-542.
28. Kim MJ, Neta M, Davis FC, et al. Botulinum toxin-induced facial muscle paralysis affects amygdala responses to the perception of emotional expressions: preliminary findings from an A-B-A design. Biol Mood Anxiety Disord. 2014;4:11.
29. Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin A in the central nervous system. Neuroscientist. 2015;21(1):44-61.
30. Antonucci F, Rossi C, Gianfranceschi L, et al. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28(14):3689-3696.
31. U.S. Food and Drug Administration. Medication guide: botox. http://www.fda.gov/downloads/drugs/drugsafety/ucm176360.pdf. Updated September 2013. Accessed June 7, 2015.
32. Wollmer MA, Kalak N, Jung S, et al. Agitation predicts response of depression to botulinum toxin treatment in a randomized controlled trial. Front Psychiatry. 2014;5:36.
33. Beer K. Cost effectiveness of botulinum toxins for the treatment of depression: preliminary observations. J Drugs Dermatol. 2010;9(1):27-30.
34. Brin MF, Boodhoo TI, Pogoda JM, et al. Safety and tolerability of onabotulinumtoxinA in the treatment of facial lines: a meta-analysis of individual patient data from global clinical registration studies in 1678 participants. J Am Acad Dermatol. 2009;61(6):961-970.e1-11.
35. Botulinum toxin and depression. ClinicalTrials.gov. https:// clinicaltrials.gov/ct2/results?term=botulinum+toxin+and+ depression&Search=Search. Accessed June 1, 2015.
36. Rabkin JG, Markowitz JS, Stewart J, et al. How blind is blind? Assessment of patient and doctor medication guesses in a placebo-controlled trial of imipramine and phenelzine. Psychiatry Res. 1986;19(1):75-86.
An under-recognized epidemic of elder abuse needs your awareness and action
In its simplest form, elder abuse refers to the intentional infliction of injury or neglect of an older adult by a caregiver. The 5 primary types of elder abuse include neglect, physical, financial, psychological/emotional, and sexual, with a subtype of social abuse that falls under psychological/emotional abuse.
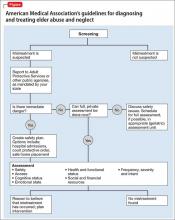
Differentiating abuse from the normal sequelae of physiologic aging can be difficult; therefore, early identification and awareness of risk factors is key, as well as detailed documentation of the patient encounter. As soon as abuse is suspected, clinicians should report it to Adult Protective Services (APS) for further investigation. In terms of prevention, regular screening for elder abuse is still up for debate, but as the incidence of elder abuse continues to rise so should research and preventive efforts to combat this growing public health concern.
What is ‘elder abuse’?According to the Elder Abuse Prevention, Identification, and Treatment Act of 1985, elder abuse is:
…willful infliction of injury, unreasonable confinement, intimidation or cruel punishment with resulting physical harm or pain or mental anguish or the willful deprivation by a caretaker of goods or services which are necessary to avoid physical harm, mental anguish or mental illness.1
There are 2 main components to this definition:
• an older adult has suffered injury or deprivation
• another person is responsible for causing or failing to prevent it.2
Although definitions vary, it generally is accepted that, for elder abuse to occur, it must take place within a relationship of trust.3
An ‘older adult’ is a person age ≥65, representing 14% of the U.S. population.4
According to U.S. Census Bureau data, there were 40 million older adults in 20105;
recent data project that this number will rise to 90 million by 2060 as Baby Boomers age.6 Studies suggest that as many as 10% of older adults in the United States experience elder abuse each year2; one study estimated that 6% of older adults in the community experienced significant abuse in the past month.7
Although elder abuse can occur in any setting, it takes place most often in the community. A survey of state APSs in 2000 showed that 60.7% of abuse was domestic; only 8.3% of incidents occurred in institutional settings.8 The annual direct medical costs associated with elder abuse injury in the United States are estimated at $5.3 billion, which is likely to increase with anticipated growth of the geriatric population.9
Although the number of older adults and the incidence of elder abuse are on the rise, as few as 1 in 14 cases is reported to authorities10; health care providers are some of the least likely of involved parties to report suspected abuse. One study found that 63% of physicians never asked about elder abuse, and only 31% reported encountering it in the previous 12 months.11 A busy clinician—ie, one who sees 20 to 40 geriatric patients a day—has a high likelihood of encountering at least 1 victim of elder abuse,2 yet many cases go unrecognized.
Types of abuseElder abuse comprises 5 categories12:
• neglect (58.5% of cases)
• physical (15.7%)
• financial (12.3%)
• psychological and emotional (7.3%)
• sexual (0.04%).
Social abuse is considered a subtype of psychological and emotional abuse. All “other” types of abuse constitute 5.1% cases; 0.06% are of unknown type.12
Neglect is (1) failure of the caregiver to provide life necessities or (2) the responsible person’s refusal to permit others to provide appropriate care.3 This is one of the most common types of elder abuse in residential facilities. Signs of neglect include dehydration, depression, fecal impaction, and malnutrition (Table).4 The prevalence of dehydration in nursing home patients is reported to be as high as 35%, which may be the result of passive or active withholding of liquids (ie, decreasing hydration to reduce the need to change the resident’s clothing or bedding).4 Other forms of neglect include medication misuse (overdosing or underdosing) and self-neglect, which occurs among people living alone and often is listed as a subtype of neglect, but is controversial because it does not involve another person.
Financial exploitation is the illegal or improper use, or mismanagement, of a person’s money, property, or financial resources3—often, to his (her) detriment. Estimates are that 1 of every 20 older adults has been subject to financial abuse at some point in their life.15 There should be a high index of suspicion for financial exploitation when one notices unexplained changes in power of attorney, wills, or other legal documents; missing checks, money, or belongings.16 In the past, adult children were most likely to be financial abusers; in recent years, however, the abuser is more often a spouse—especially a second spouse.17 Bankers, accountants, and other financial advisors are among those trained to identify risk factors for financial abuse; they are encouraged to caution clients about this possibility.18
Psychological and emotional abuse occurs when a caregiver inflicts mental stress on an older adult by actions and threats that cause fear, violence, isolation, deprivation, or feelings of shame and powerlessness.3 Examples are threatening to put the older adult in a nursing home or verbally abusing him (her). Suspect this type of abuse when a caregiver refuses to leave the older adult or speaks for him, or if the older adult expresses fear in the presence of the caregiver.4 This type of abuse also is prevalent in nursing homes and other long-term care facilities.19
Sexual abuse involves nonconsensual touching or sexual activities (rape, language, exploitive behavior) that are threatened or forced on an older adult.16 Sexual abuse is more common in frail or dependent persons.3 Physical exam findings—particularly dysuria, tender genitalia, and evidence of sexually transmitted infections4—are required to identify sexual abuse, along with signs of depression and display of fear.
Social abuse can be considered a subtype of psychological and emotional abuse, in which a caregiver denies an older adult contact with family and friends or deprives him from access to transportation. Other examples include not allowing the older adult to use the telephone, monitoring phone calls, and claiming that his friends or family are “interfering.”20 Intentionally embarrassing an older adult in front of others also can be considered social abuse.
Technology, particularly smart phones and social media, can complicate and exacerbate elder abuse:
• In July 2013, employees of a Wisconsin nursing home were found with videos and photographs of residents bathing and of a nude resident who had a bowel obstruction being mocked.21
• In May 2014, employees of a nursing home in Massachusetts recorded themselves physically and verbally abusing several older adults with Alzheimer’s disease, including one episode of the employees “hitting the woman on her arms, flicking her ears and then pinching the woman’s nose closed.” The employees also possessed a photograph of her naked.22
• In June 2015, an employee of a nursing home in Indiana was accused of taking
photos of a resident naked and sharing them on the messaging application Snapchat,23 in which images disappear 10 seconds after they are viewed.
As technology evolves, caregivers are finding more cunning ways to abuse older adults. Considering current events and trends in this area, technology as a gateway to elder abuse should be of growing concern.
Risk factorsA 2013 literature review on elder abuse reported that the most important risk factors are related to relationship (family disharmony, poor or conflicting relationships) and environment (a low level of social support),3 although other variables can play a role. Regardless of these findings, it is important to recognize that (1) elder abuse is not a necessary consequence in a family with many risk factors and (2) elder abuse can occur in the absence of any risk factors.
As a whole, women are at a higher risk of abuse, particularly when combined with loneliness, poor social support, cohabitation (especially family members), substance abuse, cognitive impairment, and dementia and other mental health problems.4 Other risk factors include functional deficiency, poor physical health or frailty, low income or wealth, and trauma or past abuse.3
Lower income, poor health, low social support, and belonging to a non-white racial group put an older adult at risk for neglect; female sex is a specific risk factor for sexual assault.15 One study found that, among older adults who suffered physical, mental, or cognitive impairment, 1 of every 4 was at risk of abuse.7
Mental illness. Dementia puts an older adult at higher risk because of increased
caregiver stress resulting from disruptive and aggressive behaviors2; the same is true when the older adult suffers another mental illness, such as anxiety, depression, schizophrenia or bipolar disorder. Presumably, older adults with any of these disorders are at risk of financial and psychological and emotional abuse because of their decreased social support, lack of independence, and inability to hold a job—leaving their caregiver to shoulder more responsibilities and with more opportunities to inflict abuse. In addition, an older adult suffering from depression can feel helpless and unworthy, possibly making him more susceptible to psychological and emotional abuse, and less likely to seek help.
More research is needed to establish racial and ethnic differences in the risk of abuse. Some research states that older adults who are a member of a minority are at greater risk of abuse; however, the difference dissipates after adjusting for variables such as income and social support.24 Cultural confounders, such as varying interpretations of the same set of interactions between older adults, need to be examined further.
Sexual orientation. Identifying one’s self as a lesbian, gay, bisexual, or transgender (LGBT) person is an additional risk factor for elder abuse. In 1997, a report described a nursing home employee who refused to bathe a resident because he didn’t want to “touch the lesbian.”25 Despite evolving attitudes in society toward support and acceptance of sexual orientation, fear of homophobia still prevents some LGBT older adults from seeking help when they have been abused because of their orientation—especially ones who have internalized that
homophobia and feel that they are unworthy of seeking help.25
In addition, health care providers and nursing home staff members might neglect the particular care needs of LGBT older adults, intentionally or unintentionally. APS staff and providers must be cognizant of underlying biases and exhibit respect when assisting LGBT clients.
Approximately 75% of caregivers of older adults are family members; 70% are female26; and most are adult children, spouses, and partners of those receiving care.27 Male caregivers age ≥40 are more likely to be the abuser, however, especially when they possess any of these risk factors: fatigue, burnout, medical illness, mental illness, lack of financial and support services, family history of abusive behavior, and substance abuse.4 People who commit elder abuse also tend to be significantly dependent on the person they are abusing.2 In some cases, and especially when the abuser is financially needy, caregivers turn to elder abuse to obtain resources from the victim.2
From your standpoint as a practitioner, it is important to determine the root cause of elder abuse. According to one review,28 family members with mental illness or a history of substance abuse, or who are stressed by the burden of caregiving, abuse older adults at a higher rate than family members who are not affected in those ways. Depression in particular is a common characteristic of abusers,2 often secondary to the stress of caring for an older adult.
Abuse caused by stress can be addressed by referral to a support group and counseling for the caregiver; psychiatric conditions, such as depression, might be better treated with pharmacotherapy. Evaluate for depression and posttraumatic stress disorder (PTSD) in both the abuser and the abused,29 and for other mental health issues that might compound the situation. It is possible for you to have 2 patients: the older adult and his caregiver. Regardless of the challenge,keep in mind that the older adult’s safety is your priority.
Consequences for the abusedThe abused adult is at risk of a number of serious physical and psychological consequences.30 They tend to have a shorter lifespan, after adjusting for other variables associated with increased mortality.
The reason for shortened lifespan is multifactorial30:
• Bruises, abrasions, and fractures may take longer to heal because of diminished skin and bone regeneration.
• Diseases that affect the heart, lungs, and kidneys might prevent the person from bouncing back from major stressors caused by abuse, such as blood loss, severe injury, and pain.
• Injury from abuse can exacerbate an underlying illness.
• Elder abuse also is associated with increased emergency department use, hospitalization (including readmission within 30 days), and nursing home placement.31
Elder abuse can lead to depression, shame, and guilt; increased isolation; and
increased risk of alcohol abuse and substance use.31 A study found that victims of
elder abuse are significantly more depressed than non-victims.32
In the same study, being a victim of abuse was found to be the second-strongest
predictor of depression, after the state of one’s health.32 Other potential psychiatric
consequences of abuse that need further study include increased risk of developing
fear and anxiety disorders; learned helplessness; and PTSD.33 According to LoFaso,
“depression and anxiety can consume their days and leave them emotionally and
physically frail.”29 Such feelings make these older adults less likely to resolve abuse or break off relations with the abuser.32
Because mental illness can be a risk factor for, and a consequence of, elder abuse,
be aware of such complications and address them appropriately. Keep in mind that older adults are more likely to visit a primary care practitioner than a psychiatrist for a routine health check-up or evaluation of initial cognition-related problems; however, they are more likely to see a psychiatrist for advanced neuropsychiatric problems such as dementia, paranoia, delusions, hallucinations, and insomnia. Adequate education on elder abuse should not be limited to a single medical specialty because it can present in several clinical settings.
Identifying abuseIdentification of elder abuse in the home poses a greater challenge to clinicians than abuse in an institutional setting because it is not directly observable. Compounding this is the lack of unified standards for identifying and dealing with elder abuse. It is first necessary for you to determine the likelihood that abuse or neglect occurred, which can be difficult because the signs of elder abuse and manifestations of normal aging often are similar. You also must establish whether (1) the abused person will accept intervention and (2) the abused person who refuses intervention has the capacity to make that decision. Both of these conditions will guide your approach to management.2
Obtain the history from several sources; review the records; and carefully examine patterns of injury, in particular assessing functional status and level of dependency on the caregiver. Explanations that do not match injuries signal the need for further investigation and examination.
To help differentiate elder abuse from normal physiologic aging, look at the skin for bruises, rashes that do not heal, and ulcers—all of which could be signs of abuse or neglect. Keep in mind that bruising generally is more common in older adults because of the slower turnover rate of epidermal cells; physiologic bruising tends to occur on dorsal aspects of the hands and arms.4 In contrast, bruising secondary to neglect or physical abuse can manifest as a subgaleal hematoma (caused by traumatic hair pulling), tracking in the peritoneum after genital trauma, Battle’s sign, and raccoon eyes, among other findings.4
In addition, larger bruises (>5 cm in diameter) are more likely the result of elder abuse.34 To complicate matters, many older persons are taking anticoagulant therapy, making bruising more likely. In addition, be on the lookout for burns during the physical exam. Evidence suggests that at least 10% of burns caused by battery and assault occur in the context of elder abuse; most burn facilities do not have formal guidelines for screening for abuse and neglect, however.35 According to one retrospective study, the most common causes of burns in older adults are hot water scalds and radiator contact, and the mortality rate of older burn patients in general is higher than among the overall population.36
Falls and fractures are common among older adults, regardless of whether they are
abused, because of polypharmacy, underlying medical conditions, and functional
limitations. Many abusers, however, use these factors to cover up intentional injury
that might have resulted in the older person falling, including overmedication (a form of physical abuse) and withholding a necessary walking aid (a form of neglect). Maintain a high index of suspicion of elder abuse when (1) the caregiver’s and the older adult’s stories of an injury don’t add up and (2) physical findings that might have been caused by abuse are present.
A number of psychiatric and cognitive symptoms suggest other types of elder abuse. Take note of emotional upset, agitation, and unusual behaviors37—especially if you can follow the patient over time to observe marked changes in the presentation. Likewise, be aware of proposed alterations in guardianship, which should be evaluated by a forensic psychiatrist with analysis of medical history, social attachments, home environment, self-care, and finances.38 Such evaluation should provide clues to the motivation behind a change of guardianship and will help to determine if elder abuse should be suspected.
Brandi et al37 provided an informative table that identifies pertinent signs, symptoms, and other findings that clinicians should be aware of to support a suspicion of elder abuse (Table).
Documentation is of utmost importance in evaluating potential elder abuse; keep in mind that the medical record might be used in an investigation of abuse by social workers, law enforcement, and prosecutors. Your records should be legible, clearly indicate who the main caregiver is and what his (her) responsibilities are, and specify who is present at your encounter with the patient.4 Document your observations of patient behavior, reactions to questions, and family dynamics and conflicts16; make note of warning signs such as fear, silence, and inability to interview the patient alone.
In addition to written documentation, take photographs of injuries, with a ruler in the image to record their size. Serial photographs are helpful; so are photographs from a variety of distances (close-up, regional, wholebody) to capture detail and place the wound in the context of a specific area of the body.4
Safety is paramount. Given the findings of the history and physical exam, it is necessary to determine whether it is safe for the patient to return home with the caregiver, or if alternate accommodations or resources, such as a social worker or a support group, are required. Include details of planned follow-up in your evaluation, and offer consideration of possible psychiatric disorders that can develop as a result of such abuse.
ReportingElder abuse is a criminal offense in all states.39 A clinician who has reasonable suspicion that elder abuse occurred must report it, regardless of whether the proof of abuse is concrete.40 At a point of reasonable suspicion, immediately contact APS, law enforcement, and a social worker. Adult Protective Services, modeled after Child Protective Services, is typically administered by local and state health
departments.41
After a report is filed with APS, an assigned social worker makes an in-person home visit to investigate the allegation and determine whether elder abuse is substantiated, partially substantiated, or unsubstantiated.16 In most states, elder abuse reporting is not anonymous because follow-up may be needed to provide additional evidence, especially if the report was made by a health care provider.16
No federal standard exists for states to follow when defining and addressing elder abuse, which can complicate identification and reporting of abuse. Laws governing elder abuse do not allow states to determine the fate of the older adult, who can decide for himself (herself) whether to use or waive protective services.42 Older adults might choose not to report abuse because of shame, intimidation, or fear,43 or to protect a caregiver, who often is a family member.
Elder abuse reports can come from a variety of sources; convincing evidence is, as noted, unnecessary to report it. Health care providers are mandated reporters, but
it is believed that the number of clinicians who report elder abuse based on suspicion is far below what it should be. One study found that 94% of physicians said that they either were unable to prove that the abuse had occurred or decided not to report it.11 Another study found that only 1.4% of elder abuse cases reported to APS come from physicians.44
There are several possible reasons for underreporting elder abuse, including (1) the difficulty of distinguishing elder abuse and neglect from sequelae of normal aging and (2) the fact that cognitive and functional impairment of the abused person makes it difficult, even impossible, to establish the narrative of how the abuse happened. Nursing homes in particular provide a high level of oversight because residents have an average of ≥3 functional deficits.4 Other reasons for underreporting—some of which are difficult to understand, and excuse, in a clinician—are:
• subtlety of signs
• victim denial
• ignorance of reporting procedures
• inadequate training
• lack of information about resources
• concern about losing physician–patient rapport
• concern about involvement in the legal system
• time limitations
• doubt about the effectiveness of APS.16
Assessing capacityThe older adult’s wishes must be respected unless a health care provider or the legal system determines that he lacks functional capacity to make decisions.16
How is capacity evaluated? A capacity evaluation has 3 components:
• Comprehension is a person’s factual understanding of the situation, including
consequences and alternatives
• Free choice is a person’s voluntary decision to accept or reject a proposed treatment, free of coercion (in this setting, free choice is the older adult’s decision whether to report the abuse)
• Reliability is a person’s ability to provide a consistent choice over time.45
Most capacity evaluations are conducted by clinical interview. No single, brief test is used universally, and there is the possibility of inter-rater variability.45 Examples of tests used to assess capacity are the Folstein Mini-Mental Status Examination and the MacArthur Competence Assessment Tool-Treatment45; the latter is a structured interview that incorporates information specific to the individual patient’s decision-making situation.46 Regardless of the approach, the psychiatrist-evaluator ensures that the older adult has been given the appropriate information
to provide informed consent about the situation.47
If the evaluator determines that a person lacks capacity to make decisions, efforts should be made to determine if the cause of that impairment is reversible.47 Older adults who have dementia or other underlying psychiatric condition that impairs cognition might benefit from more education on their situation; ones who appear fearful of consequences should be introduced to a trusted advisor to assist in making competent judgments.47
If the older adult is found to lack capacity, a substitute decision-maker must be sought.47 Many states have statutes specifying the order in which family members are contacted.48 The need to appoint an advisor can become knotty because the suspected abuser often is a family member; clinicians and others involved in identifying a decision-maker to speak on behalf of an older adult should choose carefully.
Prevention and screeningKey to reducing the prevalence of elder abuse in the community is formulating
strategies for prevention and screening. The American Medical Association recommends that clinicians “incorporate routine questions related to elder abuse and neglect into daily practice.”49 Older adults might not admit to abuse or neglect unless they are asked; speak to patients at eye level, keep questions simple, direct, and nonjudgmental, and assure them (1) that discussions are confidential and (2) that their safety is your primary goal.50,51
Comprehensive approaches to questioning patients are available and often recommended for screening for elder abuse.4 However, screening in the office setting often involves short, directly administered questionnaires.49 For example, the Health and Safety Screen developed at the University of Maine comprises 6 questions52:
• Has anyone close to you called you names or insulted you recently?
• Are you afraid of anyone in your life?
• Are you able to use the telephone anytime you want to?
• Has anyone forced you to do things you didn’t want to do?
• Has anyone taken things or money that belong to you without your OK?
• Has anyone close to you tried to hurt you or harm you recently?
Because of time constraints and lack of a universal standard, there is debate whether regular elder abuse screening is time-effective. It often is recommended, therefore, that clinicians in primary care (1) refer older adults with risk factors for abuse to geriatric medical teams trained in these measures and (2) perform periodic follow-up on such patients4 (Figure).
• New York City Elder Abuse Center encourages collaboration among health, mental health, and community justice organizations.28 The program involves a number of resources for addressing elder abuse, such as promoting staff awareness of risk factors for, and signs of, abuse, and screening for mental health problems in the abused.
• The Elder Justice Act, enacted in 2010 to combat elder abuse, provides federal funds and resources to prevent, detect, treat, and intervene to stop abuse and, when appropriate, to prosecute abusers.53
This Web Exclusive Table provides a 7-point summary reference guide for understanding and preventing elder abuse in your practice.
BOTTOM LINEIdentification of elder abuse can be difficult because signs and symptoms of abuse closely resemble physiologic aging. Older adults with identifiable risk factors should be screened for abuse; time constraints make universal screening impossible at this time. In the future, multidisciplinary approaches likely will make elder abuse more easily identifiable through the combined work of health care providers, law enforcement agencies, banks, and other institutions—with the ultimate goal of protecting older adults in the community from abuse.
Related Resources
• Frazão SL, Correia AM, Norton P, et al. Physical abuse against elderly persons in institutional settings. J Forensic Leg Med. 2015;36:54-60.
• Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Disclosures
Ms. Hubert reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Gupta is a member of the speakers’ bureau of Alkermes, Allergan, Avanir Pharmaceuticals, Takeda Pharmaceutical, Lundbeck, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
1. The Elder Abuse Prevention, Identification, and Treatment Act of 1985, HR 1674, 99th Cong (1985).
2. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272.
3. Johannesen M, LoGiudice D. Elder abuse: a systematic review of risk factors in community-dwelling elders. Age Ageing. 2013;42(3):292-298.
4. Gibbs LM, Mosqueda L, eds. Medical implications of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):xv-xvi. doi: 10.1016/j.cger.2014.08.015.
5. Werner CA. The Older Population: 2010. U.S. Census Bureau. http://webcache.googleusercontent.com/search?q=cache:hCCb_pcnO6QJ :ht tps://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf+&cd=1&hl=en&ct=clnk&gl=uss. Issued November 2011. Accessed October 10, 2015.
6. Himes CL. Elderly Americans. Population Bulletin. 2002;56(4):1-41.
7. Cooper C, Selwood A, Livingston G. The prevalence of elder abuse and neglect: a systematic review. Age Ageing. 2008;37(2):151-60.
8. Teaster PB. A response to the abuse of vulnerable adults: the 2000 Survey of State Adult Protective Services. The National Center on Elder Abuse. http://www.ncea.aoa.gov/Resources/Publication/docs/apsreport030703.pdf. 2003.
Accessed October 22, 2015.
9. Mouton CP, Rodabough RJ, Rovi SL, et al. Prevalence and 3-year incidence of abuse among postmenopausal women. Am J Public Health. 2004;94(4):605-612.
10. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. Statistic Brain Research Institute. Elderly abuse statistics. http://www.statisticbrain.com/elderly-abuse-statistics. Accessed June 22, 2015.
13. Lachs MS, Bachman R, Williams CS, et al. Resident-to-resident elder mistreatment and police contact in nursing homes: findings from a population-based cohort. J Am Geriatr Soc. 2007;55(6):840-845.
14. Lachs M, Bachman R, Williams C, et al. Older adults as crime victims, perpetrators, witnesses, and complainants: a population-based study of police interactions. J Elder Abuse Negl. 2005;16(4):25-40.
15. Acierno R, Hernandez-Tejada M, Muzzy W, et al. National Elder Mistreatment Study. Washington, DC: National Institute of Justice; 2009.
16. Dong XQ. Elder abuse: systematic review and implications for practice. J Am Geriatr Soc. 2015;63(6):1214-1238.
17. Freedman M. The growing epidemic of financial elder abuse. The Tax Advisor. http://www.cpa2biz.com/Content/media/PRODUCER_CONTENT/Newsletters/
Articles_2007/Tax/Financial_Elder_Abuse.jsp. Published November 2007. Accessed June 24, 2015.
18. Consumer Financial Protection Bureau. Protection for older Americans. http://www.consumerfinance.gov/olderamericans. Accessed June 22, 2015.
19. Castle NG. Nursing home deficiency citations for abuse. J Appl Gerontol. 2011;30(6):719-743.
20. Elder Abuse Prevention Unit. Social abuse. http://www.eapu.com.au/elder-abuse/social-abuse. Published 2014. Accessed June 24, 2015.
21. Former nursing home employees allegedly photographed naked resident. United Press International. http://www.upi.com/Top_News/US/2013/07/03/Former-nursinghome-employees-allegedly-photographed-nakedresidents/
65801372893020. Published July 3, 2013. Accessed June 24, 2015.
22. Miller N. Two charged with elder assault at an assisted living facility. MetroWest Daily News. http://www.metrowestdailynews.com/article/20140506/
NEWS/140507587. Updated May 7, 2014. Accessed June 24, 2015.
23. Jorgensen J. New charges filed in nursing home case.WHAS11. http://www.whas11.com/story/news/local/2015/06/24/new-charges-filed-in-nursing-homecase/29243183/. Published June 24, 2015. Accessed June 27, 2015.
24. Hermandez-Tejada MA, Amstadter A, Muzzy W, et al. The National Elder Mistreatment Study: race and ethnicity findings. J Elder Abuse Negl. 2013;25(4):281-293.
25. Cooks-Daniels L. Lesbian, gay male, bisexual and transgendered elders: elder abuse and neglect issues. J Elder Abuse Negl. 1998;9(2):35-49.
26. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
27. Tatara T, Blumerman Kuzmeskus L, Duckhorn E, et al. The National Center on Elder Abuse Incidence Study: final report. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed October 19, 2015.
28. Rosen AL. Where mental health and elder abuse intersect. Generations. 2014;38(3):75-79.
29. LoFaso V. The role of the primary physician in assessing and treating the mental health concerns of elder abuse victims. NYC Elder Abuse Center eNewsletter.nyceac.com/wp-content/uploads/2013/03/Exploring-the-IntersectionofElder-Abuse-and-Mental-Health_eNewsletter.pdf. Published March 12, 2013. Accessed August 20, 2015.
30. Lachs MS, Williams CS, O’Brien S, et al. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
31. Dyer CB, Pavlik VN, Murphy KP, et al. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
32. Pillemer K, Prescott D. Psychological effects of elder abuse: a research note. J Elder Abuse Negl. 1988;1(1):65-73.
33. Elder abuse: consequences. Centers for Disease Control and Prevention. http://www.cdc.gov/violenceprevention/elderabuse/consequences.html. Updated June 22, 2015.Accessed August 20, 2015.
34. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196.
35. Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns. 2012;38(5):630-637.
36. 2014 National Burn Repository; report of data between 2004-2013. American Burn Association. http://www.ameriburn.org/2014NBRAnnualReport.pdf. Published 2014. Accessed June 26, 2015.
37. Brandi B, Dyer CB, Heisler CJ, et al. Systemic responses to elder abuse. In: Brandi B, Dyer CB, Heisler CJ, eds. Elder abuse detection and intervention: a collaborative approach. New York, NY: Spring Publishing Company; 2007:79-100.
38. Welner M. Guardianship. The Forensic Panel. http://www.forensicpanel.com/expert_services/psychiatry/civil_law/guardianship.html. Accessed August 20, 2015.
39. Watson E. Elder abuse: definition, types and statistics, and elder abuse (mistreatment and neglect) laws. Journal of Legal Nurse Consulting. 2013;24(2):40-42.
40. National Center on Elder Abuse Administration on Aging. Reporting abuse. http://www.ncea.aoa.gov/Stop_Abuse/Get_Help/Report/index.aspx. Accessed August 18, 2015.
41. Mukherjee D. Organizational structures of elder abuse reporting systems. Administration in Social Work. 2011;35(5):517-531.
42. Costin LB, Karger HJ, Stoesz H. The politics of child abuse in America. New York, NY: Oxford University Press; 1996.
43. Thomson MJ, Lietzau LK, Doty MM, et al. An analysis of elder abuse rates in Milwaukee County. WMJ. 2011;110(6):271-276.
44. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 Survey of State Adult Protective Services: Abuse of Adults 60 Years and Older. http://www.ncea.aoa.gov/Resources/Publication/docs/APS_2004NCEASurvey.pdf. Published March 2007. Accessed October 19, 2015.
45. Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
46. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resources Press; 1998.
47. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;35(18):1834-1840.
48. Wynn S. Decision by surrogates: An overview of surrogate consent laws in the United States. Bifocal: A Journal of the ABA Commission on Bar and Aging. 2014;36(1). http://www.americanbar.org/publications/bifocal/vol_36/
issue_1_october2014/default_surrogate_consent_statutes.html. Accessed October 22, 2015.
49. American Medical Association. Diagnostic and treatment guidelines on elder abuse and neglect. Chicago, IL: American Medical Association; 1992.
50. Harrell R, Toronjo C, McLaughlin J, et al. How geriatricians identify elder abuse and neglect. Am J Med Sci. 2002;323(1):34-38.
51. Ahmad M, Lachs MS. Elder abuse and neglect: what physicians can and should do. Cleve Clin J Med. 2002; 69(10):801-808.
52. Elder abuse screening protocol for physicians: lessons learned from the Maine Partners for Elder Protection Pilot Project. University of Maine Center on Aging. http://umcoa.siteturbine.com/uploaded_files/mainecenteronaging.umaine.edu/files/elderabusescreeningmanual.pdf. Published May 2, 2007. Accessed August 20, 2015.
53. What is the Elder Justice Act? USC Davis School of Gerontology. http://gerontology.usc.edu/resources/articles/what-is-the-elder-justice-act/. Published 2015. Accessed October 20, 2015.
In its simplest form, elder abuse refers to the intentional infliction of injury or neglect of an older adult by a caregiver. The 5 primary types of elder abuse include neglect, physical, financial, psychological/emotional, and sexual, with a subtype of social abuse that falls under psychological/emotional abuse.
Differentiating abuse from the normal sequelae of physiologic aging can be difficult; therefore, early identification and awareness of risk factors is key, as well as detailed documentation of the patient encounter. As soon as abuse is suspected, clinicians should report it to Adult Protective Services (APS) for further investigation. In terms of prevention, regular screening for elder abuse is still up for debate, but as the incidence of elder abuse continues to rise so should research and preventive efforts to combat this growing public health concern.
What is ‘elder abuse’?According to the Elder Abuse Prevention, Identification, and Treatment Act of 1985, elder abuse is:
…willful infliction of injury, unreasonable confinement, intimidation or cruel punishment with resulting physical harm or pain or mental anguish or the willful deprivation by a caretaker of goods or services which are necessary to avoid physical harm, mental anguish or mental illness.1
There are 2 main components to this definition:
• an older adult has suffered injury or deprivation
• another person is responsible for causing or failing to prevent it.2
Although definitions vary, it generally is accepted that, for elder abuse to occur, it must take place within a relationship of trust.3
An ‘older adult’ is a person age ≥65, representing 14% of the U.S. population.4
According to U.S. Census Bureau data, there were 40 million older adults in 20105;
recent data project that this number will rise to 90 million by 2060 as Baby Boomers age.6 Studies suggest that as many as 10% of older adults in the United States experience elder abuse each year2; one study estimated that 6% of older adults in the community experienced significant abuse in the past month.7
Although elder abuse can occur in any setting, it takes place most often in the community. A survey of state APSs in 2000 showed that 60.7% of abuse was domestic; only 8.3% of incidents occurred in institutional settings.8 The annual direct medical costs associated with elder abuse injury in the United States are estimated at $5.3 billion, which is likely to increase with anticipated growth of the geriatric population.9
Although the number of older adults and the incidence of elder abuse are on the rise, as few as 1 in 14 cases is reported to authorities10; health care providers are some of the least likely of involved parties to report suspected abuse. One study found that 63% of physicians never asked about elder abuse, and only 31% reported encountering it in the previous 12 months.11 A busy clinician—ie, one who sees 20 to 40 geriatric patients a day—has a high likelihood of encountering at least 1 victim of elder abuse,2 yet many cases go unrecognized.
Types of abuseElder abuse comprises 5 categories12:
• neglect (58.5% of cases)
• physical (15.7%)
• financial (12.3%)
• psychological and emotional (7.3%)
• sexual (0.04%).
Social abuse is considered a subtype of psychological and emotional abuse. All “other” types of abuse constitute 5.1% cases; 0.06% are of unknown type.12
Neglect is (1) failure of the caregiver to provide life necessities or (2) the responsible person’s refusal to permit others to provide appropriate care.3 This is one of the most common types of elder abuse in residential facilities. Signs of neglect include dehydration, depression, fecal impaction, and malnutrition (Table).4 The prevalence of dehydration in nursing home patients is reported to be as high as 35%, which may be the result of passive or active withholding of liquids (ie, decreasing hydration to reduce the need to change the resident’s clothing or bedding).4 Other forms of neglect include medication misuse (overdosing or underdosing) and self-neglect, which occurs among people living alone and often is listed as a subtype of neglect, but is controversial because it does not involve another person.
Financial exploitation is the illegal or improper use, or mismanagement, of a person’s money, property, or financial resources3—often, to his (her) detriment. Estimates are that 1 of every 20 older adults has been subject to financial abuse at some point in their life.15 There should be a high index of suspicion for financial exploitation when one notices unexplained changes in power of attorney, wills, or other legal documents; missing checks, money, or belongings.16 In the past, adult children were most likely to be financial abusers; in recent years, however, the abuser is more often a spouse—especially a second spouse.17 Bankers, accountants, and other financial advisors are among those trained to identify risk factors for financial abuse; they are encouraged to caution clients about this possibility.18
Psychological and emotional abuse occurs when a caregiver inflicts mental stress on an older adult by actions and threats that cause fear, violence, isolation, deprivation, or feelings of shame and powerlessness.3 Examples are threatening to put the older adult in a nursing home or verbally abusing him (her). Suspect this type of abuse when a caregiver refuses to leave the older adult or speaks for him, or if the older adult expresses fear in the presence of the caregiver.4 This type of abuse also is prevalent in nursing homes and other long-term care facilities.19
Sexual abuse involves nonconsensual touching or sexual activities (rape, language, exploitive behavior) that are threatened or forced on an older adult.16 Sexual abuse is more common in frail or dependent persons.3 Physical exam findings—particularly dysuria, tender genitalia, and evidence of sexually transmitted infections4—are required to identify sexual abuse, along with signs of depression and display of fear.
Social abuse can be considered a subtype of psychological and emotional abuse, in which a caregiver denies an older adult contact with family and friends or deprives him from access to transportation. Other examples include not allowing the older adult to use the telephone, monitoring phone calls, and claiming that his friends or family are “interfering.”20 Intentionally embarrassing an older adult in front of others also can be considered social abuse.
Technology, particularly smart phones and social media, can complicate and exacerbate elder abuse:
• In July 2013, employees of a Wisconsin nursing home were found with videos and photographs of residents bathing and of a nude resident who had a bowel obstruction being mocked.21
• In May 2014, employees of a nursing home in Massachusetts recorded themselves physically and verbally abusing several older adults with Alzheimer’s disease, including one episode of the employees “hitting the woman on her arms, flicking her ears and then pinching the woman’s nose closed.” The employees also possessed a photograph of her naked.22
• In June 2015, an employee of a nursing home in Indiana was accused of taking
photos of a resident naked and sharing them on the messaging application Snapchat,23 in which images disappear 10 seconds after they are viewed.
As technology evolves, caregivers are finding more cunning ways to abuse older adults. Considering current events and trends in this area, technology as a gateway to elder abuse should be of growing concern.
Risk factorsA 2013 literature review on elder abuse reported that the most important risk factors are related to relationship (family disharmony, poor or conflicting relationships) and environment (a low level of social support),3 although other variables can play a role. Regardless of these findings, it is important to recognize that (1) elder abuse is not a necessary consequence in a family with many risk factors and (2) elder abuse can occur in the absence of any risk factors.
As a whole, women are at a higher risk of abuse, particularly when combined with loneliness, poor social support, cohabitation (especially family members), substance abuse, cognitive impairment, and dementia and other mental health problems.4 Other risk factors include functional deficiency, poor physical health or frailty, low income or wealth, and trauma or past abuse.3
Lower income, poor health, low social support, and belonging to a non-white racial group put an older adult at risk for neglect; female sex is a specific risk factor for sexual assault.15 One study found that, among older adults who suffered physical, mental, or cognitive impairment, 1 of every 4 was at risk of abuse.7
Mental illness. Dementia puts an older adult at higher risk because of increased
caregiver stress resulting from disruptive and aggressive behaviors2; the same is true when the older adult suffers another mental illness, such as anxiety, depression, schizophrenia or bipolar disorder. Presumably, older adults with any of these disorders are at risk of financial and psychological and emotional abuse because of their decreased social support, lack of independence, and inability to hold a job—leaving their caregiver to shoulder more responsibilities and with more opportunities to inflict abuse. In addition, an older adult suffering from depression can feel helpless and unworthy, possibly making him more susceptible to psychological and emotional abuse, and less likely to seek help.
More research is needed to establish racial and ethnic differences in the risk of abuse. Some research states that older adults who are a member of a minority are at greater risk of abuse; however, the difference dissipates after adjusting for variables such as income and social support.24 Cultural confounders, such as varying interpretations of the same set of interactions between older adults, need to be examined further.
Sexual orientation. Identifying one’s self as a lesbian, gay, bisexual, or transgender (LGBT) person is an additional risk factor for elder abuse. In 1997, a report described a nursing home employee who refused to bathe a resident because he didn’t want to “touch the lesbian.”25 Despite evolving attitudes in society toward support and acceptance of sexual orientation, fear of homophobia still prevents some LGBT older adults from seeking help when they have been abused because of their orientation—especially ones who have internalized that
homophobia and feel that they are unworthy of seeking help.25
In addition, health care providers and nursing home staff members might neglect the particular care needs of LGBT older adults, intentionally or unintentionally. APS staff and providers must be cognizant of underlying biases and exhibit respect when assisting LGBT clients.
Approximately 75% of caregivers of older adults are family members; 70% are female26; and most are adult children, spouses, and partners of those receiving care.27 Male caregivers age ≥40 are more likely to be the abuser, however, especially when they possess any of these risk factors: fatigue, burnout, medical illness, mental illness, lack of financial and support services, family history of abusive behavior, and substance abuse.4 People who commit elder abuse also tend to be significantly dependent on the person they are abusing.2 In some cases, and especially when the abuser is financially needy, caregivers turn to elder abuse to obtain resources from the victim.2
From your standpoint as a practitioner, it is important to determine the root cause of elder abuse. According to one review,28 family members with mental illness or a history of substance abuse, or who are stressed by the burden of caregiving, abuse older adults at a higher rate than family members who are not affected in those ways. Depression in particular is a common characteristic of abusers,2 often secondary to the stress of caring for an older adult.
Abuse caused by stress can be addressed by referral to a support group and counseling for the caregiver; psychiatric conditions, such as depression, might be better treated with pharmacotherapy. Evaluate for depression and posttraumatic stress disorder (PTSD) in both the abuser and the abused,29 and for other mental health issues that might compound the situation. It is possible for you to have 2 patients: the older adult and his caregiver. Regardless of the challenge,keep in mind that the older adult’s safety is your priority.
Consequences for the abusedThe abused adult is at risk of a number of serious physical and psychological consequences.30 They tend to have a shorter lifespan, after adjusting for other variables associated with increased mortality.
The reason for shortened lifespan is multifactorial30:
• Bruises, abrasions, and fractures may take longer to heal because of diminished skin and bone regeneration.
• Diseases that affect the heart, lungs, and kidneys might prevent the person from bouncing back from major stressors caused by abuse, such as blood loss, severe injury, and pain.
• Injury from abuse can exacerbate an underlying illness.
• Elder abuse also is associated with increased emergency department use, hospitalization (including readmission within 30 days), and nursing home placement.31
Elder abuse can lead to depression, shame, and guilt; increased isolation; and
increased risk of alcohol abuse and substance use.31 A study found that victims of
elder abuse are significantly more depressed than non-victims.32
In the same study, being a victim of abuse was found to be the second-strongest
predictor of depression, after the state of one’s health.32 Other potential psychiatric
consequences of abuse that need further study include increased risk of developing
fear and anxiety disorders; learned helplessness; and PTSD.33 According to LoFaso,
“depression and anxiety can consume their days and leave them emotionally and
physically frail.”29 Such feelings make these older adults less likely to resolve abuse or break off relations with the abuser.32
Because mental illness can be a risk factor for, and a consequence of, elder abuse,
be aware of such complications and address them appropriately. Keep in mind that older adults are more likely to visit a primary care practitioner than a psychiatrist for a routine health check-up or evaluation of initial cognition-related problems; however, they are more likely to see a psychiatrist for advanced neuropsychiatric problems such as dementia, paranoia, delusions, hallucinations, and insomnia. Adequate education on elder abuse should not be limited to a single medical specialty because it can present in several clinical settings.
Identifying abuseIdentification of elder abuse in the home poses a greater challenge to clinicians than abuse in an institutional setting because it is not directly observable. Compounding this is the lack of unified standards for identifying and dealing with elder abuse. It is first necessary for you to determine the likelihood that abuse or neglect occurred, which can be difficult because the signs of elder abuse and manifestations of normal aging often are similar. You also must establish whether (1) the abused person will accept intervention and (2) the abused person who refuses intervention has the capacity to make that decision. Both of these conditions will guide your approach to management.2
Obtain the history from several sources; review the records; and carefully examine patterns of injury, in particular assessing functional status and level of dependency on the caregiver. Explanations that do not match injuries signal the need for further investigation and examination.
To help differentiate elder abuse from normal physiologic aging, look at the skin for bruises, rashes that do not heal, and ulcers—all of which could be signs of abuse or neglect. Keep in mind that bruising generally is more common in older adults because of the slower turnover rate of epidermal cells; physiologic bruising tends to occur on dorsal aspects of the hands and arms.4 In contrast, bruising secondary to neglect or physical abuse can manifest as a subgaleal hematoma (caused by traumatic hair pulling), tracking in the peritoneum after genital trauma, Battle’s sign, and raccoon eyes, among other findings.4
In addition, larger bruises (>5 cm in diameter) are more likely the result of elder abuse.34 To complicate matters, many older persons are taking anticoagulant therapy, making bruising more likely. In addition, be on the lookout for burns during the physical exam. Evidence suggests that at least 10% of burns caused by battery and assault occur in the context of elder abuse; most burn facilities do not have formal guidelines for screening for abuse and neglect, however.35 According to one retrospective study, the most common causes of burns in older adults are hot water scalds and radiator contact, and the mortality rate of older burn patients in general is higher than among the overall population.36
Falls and fractures are common among older adults, regardless of whether they are
abused, because of polypharmacy, underlying medical conditions, and functional
limitations. Many abusers, however, use these factors to cover up intentional injury
that might have resulted in the older person falling, including overmedication (a form of physical abuse) and withholding a necessary walking aid (a form of neglect). Maintain a high index of suspicion of elder abuse when (1) the caregiver’s and the older adult’s stories of an injury don’t add up and (2) physical findings that might have been caused by abuse are present.
A number of psychiatric and cognitive symptoms suggest other types of elder abuse. Take note of emotional upset, agitation, and unusual behaviors37—especially if you can follow the patient over time to observe marked changes in the presentation. Likewise, be aware of proposed alterations in guardianship, which should be evaluated by a forensic psychiatrist with analysis of medical history, social attachments, home environment, self-care, and finances.38 Such evaluation should provide clues to the motivation behind a change of guardianship and will help to determine if elder abuse should be suspected.
Brandi et al37 provided an informative table that identifies pertinent signs, symptoms, and other findings that clinicians should be aware of to support a suspicion of elder abuse (Table).
Documentation is of utmost importance in evaluating potential elder abuse; keep in mind that the medical record might be used in an investigation of abuse by social workers, law enforcement, and prosecutors. Your records should be legible, clearly indicate who the main caregiver is and what his (her) responsibilities are, and specify who is present at your encounter with the patient.4 Document your observations of patient behavior, reactions to questions, and family dynamics and conflicts16; make note of warning signs such as fear, silence, and inability to interview the patient alone.
In addition to written documentation, take photographs of injuries, with a ruler in the image to record their size. Serial photographs are helpful; so are photographs from a variety of distances (close-up, regional, wholebody) to capture detail and place the wound in the context of a specific area of the body.4
Safety is paramount. Given the findings of the history and physical exam, it is necessary to determine whether it is safe for the patient to return home with the caregiver, or if alternate accommodations or resources, such as a social worker or a support group, are required. Include details of planned follow-up in your evaluation, and offer consideration of possible psychiatric disorders that can develop as a result of such abuse.
ReportingElder abuse is a criminal offense in all states.39 A clinician who has reasonable suspicion that elder abuse occurred must report it, regardless of whether the proof of abuse is concrete.40 At a point of reasonable suspicion, immediately contact APS, law enforcement, and a social worker. Adult Protective Services, modeled after Child Protective Services, is typically administered by local and state health
departments.41
After a report is filed with APS, an assigned social worker makes an in-person home visit to investigate the allegation and determine whether elder abuse is substantiated, partially substantiated, or unsubstantiated.16 In most states, elder abuse reporting is not anonymous because follow-up may be needed to provide additional evidence, especially if the report was made by a health care provider.16
No federal standard exists for states to follow when defining and addressing elder abuse, which can complicate identification and reporting of abuse. Laws governing elder abuse do not allow states to determine the fate of the older adult, who can decide for himself (herself) whether to use or waive protective services.42 Older adults might choose not to report abuse because of shame, intimidation, or fear,43 or to protect a caregiver, who often is a family member.
Elder abuse reports can come from a variety of sources; convincing evidence is, as noted, unnecessary to report it. Health care providers are mandated reporters, but
it is believed that the number of clinicians who report elder abuse based on suspicion is far below what it should be. One study found that 94% of physicians said that they either were unable to prove that the abuse had occurred or decided not to report it.11 Another study found that only 1.4% of elder abuse cases reported to APS come from physicians.44
There are several possible reasons for underreporting elder abuse, including (1) the difficulty of distinguishing elder abuse and neglect from sequelae of normal aging and (2) the fact that cognitive and functional impairment of the abused person makes it difficult, even impossible, to establish the narrative of how the abuse happened. Nursing homes in particular provide a high level of oversight because residents have an average of ≥3 functional deficits.4 Other reasons for underreporting—some of which are difficult to understand, and excuse, in a clinician—are:
• subtlety of signs
• victim denial
• ignorance of reporting procedures
• inadequate training
• lack of information about resources
• concern about losing physician–patient rapport
• concern about involvement in the legal system
• time limitations
• doubt about the effectiveness of APS.16
Assessing capacityThe older adult’s wishes must be respected unless a health care provider or the legal system determines that he lacks functional capacity to make decisions.16
How is capacity evaluated? A capacity evaluation has 3 components:
• Comprehension is a person’s factual understanding of the situation, including
consequences and alternatives
• Free choice is a person’s voluntary decision to accept or reject a proposed treatment, free of coercion (in this setting, free choice is the older adult’s decision whether to report the abuse)
• Reliability is a person’s ability to provide a consistent choice over time.45
Most capacity evaluations are conducted by clinical interview. No single, brief test is used universally, and there is the possibility of inter-rater variability.45 Examples of tests used to assess capacity are the Folstein Mini-Mental Status Examination and the MacArthur Competence Assessment Tool-Treatment45; the latter is a structured interview that incorporates information specific to the individual patient’s decision-making situation.46 Regardless of the approach, the psychiatrist-evaluator ensures that the older adult has been given the appropriate information
to provide informed consent about the situation.47
If the evaluator determines that a person lacks capacity to make decisions, efforts should be made to determine if the cause of that impairment is reversible.47 Older adults who have dementia or other underlying psychiatric condition that impairs cognition might benefit from more education on their situation; ones who appear fearful of consequences should be introduced to a trusted advisor to assist in making competent judgments.47
If the older adult is found to lack capacity, a substitute decision-maker must be sought.47 Many states have statutes specifying the order in which family members are contacted.48 The need to appoint an advisor can become knotty because the suspected abuser often is a family member; clinicians and others involved in identifying a decision-maker to speak on behalf of an older adult should choose carefully.
Prevention and screeningKey to reducing the prevalence of elder abuse in the community is formulating
strategies for prevention and screening. The American Medical Association recommends that clinicians “incorporate routine questions related to elder abuse and neglect into daily practice.”49 Older adults might not admit to abuse or neglect unless they are asked; speak to patients at eye level, keep questions simple, direct, and nonjudgmental, and assure them (1) that discussions are confidential and (2) that their safety is your primary goal.50,51
Comprehensive approaches to questioning patients are available and often recommended for screening for elder abuse.4 However, screening in the office setting often involves short, directly administered questionnaires.49 For example, the Health and Safety Screen developed at the University of Maine comprises 6 questions52:
• Has anyone close to you called you names or insulted you recently?
• Are you afraid of anyone in your life?
• Are you able to use the telephone anytime you want to?
• Has anyone forced you to do things you didn’t want to do?
• Has anyone taken things or money that belong to you without your OK?
• Has anyone close to you tried to hurt you or harm you recently?
Because of time constraints and lack of a universal standard, there is debate whether regular elder abuse screening is time-effective. It often is recommended, therefore, that clinicians in primary care (1) refer older adults with risk factors for abuse to geriatric medical teams trained in these measures and (2) perform periodic follow-up on such patients4 (Figure).
• New York City Elder Abuse Center encourages collaboration among health, mental health, and community justice organizations.28 The program involves a number of resources for addressing elder abuse, such as promoting staff awareness of risk factors for, and signs of, abuse, and screening for mental health problems in the abused.
• The Elder Justice Act, enacted in 2010 to combat elder abuse, provides federal funds and resources to prevent, detect, treat, and intervene to stop abuse and, when appropriate, to prosecute abusers.53
This Web Exclusive Table provides a 7-point summary reference guide for understanding and preventing elder abuse in your practice.
BOTTOM LINEIdentification of elder abuse can be difficult because signs and symptoms of abuse closely resemble physiologic aging. Older adults with identifiable risk factors should be screened for abuse; time constraints make universal screening impossible at this time. In the future, multidisciplinary approaches likely will make elder abuse more easily identifiable through the combined work of health care providers, law enforcement agencies, banks, and other institutions—with the ultimate goal of protecting older adults in the community from abuse.
Related Resources
• Frazão SL, Correia AM, Norton P, et al. Physical abuse against elderly persons in institutional settings. J Forensic Leg Med. 2015;36:54-60.
• Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Disclosures
Ms. Hubert reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Gupta is a member of the speakers’ bureau of Alkermes, Allergan, Avanir Pharmaceuticals, Takeda Pharmaceutical, Lundbeck, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
In its simplest form, elder abuse refers to the intentional infliction of injury or neglect of an older adult by a caregiver. The 5 primary types of elder abuse include neglect, physical, financial, psychological/emotional, and sexual, with a subtype of social abuse that falls under psychological/emotional abuse.
Differentiating abuse from the normal sequelae of physiologic aging can be difficult; therefore, early identification and awareness of risk factors is key, as well as detailed documentation of the patient encounter. As soon as abuse is suspected, clinicians should report it to Adult Protective Services (APS) for further investigation. In terms of prevention, regular screening for elder abuse is still up for debate, but as the incidence of elder abuse continues to rise so should research and preventive efforts to combat this growing public health concern.
What is ‘elder abuse’?According to the Elder Abuse Prevention, Identification, and Treatment Act of 1985, elder abuse is:
…willful infliction of injury, unreasonable confinement, intimidation or cruel punishment with resulting physical harm or pain or mental anguish or the willful deprivation by a caretaker of goods or services which are necessary to avoid physical harm, mental anguish or mental illness.1
There are 2 main components to this definition:
• an older adult has suffered injury or deprivation
• another person is responsible for causing or failing to prevent it.2
Although definitions vary, it generally is accepted that, for elder abuse to occur, it must take place within a relationship of trust.3
An ‘older adult’ is a person age ≥65, representing 14% of the U.S. population.4
According to U.S. Census Bureau data, there were 40 million older adults in 20105;
recent data project that this number will rise to 90 million by 2060 as Baby Boomers age.6 Studies suggest that as many as 10% of older adults in the United States experience elder abuse each year2; one study estimated that 6% of older adults in the community experienced significant abuse in the past month.7
Although elder abuse can occur in any setting, it takes place most often in the community. A survey of state APSs in 2000 showed that 60.7% of abuse was domestic; only 8.3% of incidents occurred in institutional settings.8 The annual direct medical costs associated with elder abuse injury in the United States are estimated at $5.3 billion, which is likely to increase with anticipated growth of the geriatric population.9
Although the number of older adults and the incidence of elder abuse are on the rise, as few as 1 in 14 cases is reported to authorities10; health care providers are some of the least likely of involved parties to report suspected abuse. One study found that 63% of physicians never asked about elder abuse, and only 31% reported encountering it in the previous 12 months.11 A busy clinician—ie, one who sees 20 to 40 geriatric patients a day—has a high likelihood of encountering at least 1 victim of elder abuse,2 yet many cases go unrecognized.
Types of abuseElder abuse comprises 5 categories12:
• neglect (58.5% of cases)
• physical (15.7%)
• financial (12.3%)
• psychological and emotional (7.3%)
• sexual (0.04%).
Social abuse is considered a subtype of psychological and emotional abuse. All “other” types of abuse constitute 5.1% cases; 0.06% are of unknown type.12
Neglect is (1) failure of the caregiver to provide life necessities or (2) the responsible person’s refusal to permit others to provide appropriate care.3 This is one of the most common types of elder abuse in residential facilities. Signs of neglect include dehydration, depression, fecal impaction, and malnutrition (Table).4 The prevalence of dehydration in nursing home patients is reported to be as high as 35%, which may be the result of passive or active withholding of liquids (ie, decreasing hydration to reduce the need to change the resident’s clothing or bedding).4 Other forms of neglect include medication misuse (overdosing or underdosing) and self-neglect, which occurs among people living alone and often is listed as a subtype of neglect, but is controversial because it does not involve another person.
Financial exploitation is the illegal or improper use, or mismanagement, of a person’s money, property, or financial resources3—often, to his (her) detriment. Estimates are that 1 of every 20 older adults has been subject to financial abuse at some point in their life.15 There should be a high index of suspicion for financial exploitation when one notices unexplained changes in power of attorney, wills, or other legal documents; missing checks, money, or belongings.16 In the past, adult children were most likely to be financial abusers; in recent years, however, the abuser is more often a spouse—especially a second spouse.17 Bankers, accountants, and other financial advisors are among those trained to identify risk factors for financial abuse; they are encouraged to caution clients about this possibility.18
Psychological and emotional abuse occurs when a caregiver inflicts mental stress on an older adult by actions and threats that cause fear, violence, isolation, deprivation, or feelings of shame and powerlessness.3 Examples are threatening to put the older adult in a nursing home or verbally abusing him (her). Suspect this type of abuse when a caregiver refuses to leave the older adult or speaks for him, or if the older adult expresses fear in the presence of the caregiver.4 This type of abuse also is prevalent in nursing homes and other long-term care facilities.19
Sexual abuse involves nonconsensual touching or sexual activities (rape, language, exploitive behavior) that are threatened or forced on an older adult.16 Sexual abuse is more common in frail or dependent persons.3 Physical exam findings—particularly dysuria, tender genitalia, and evidence of sexually transmitted infections4—are required to identify sexual abuse, along with signs of depression and display of fear.
Social abuse can be considered a subtype of psychological and emotional abuse, in which a caregiver denies an older adult contact with family and friends or deprives him from access to transportation. Other examples include not allowing the older adult to use the telephone, monitoring phone calls, and claiming that his friends or family are “interfering.”20 Intentionally embarrassing an older adult in front of others also can be considered social abuse.
Technology, particularly smart phones and social media, can complicate and exacerbate elder abuse:
• In July 2013, employees of a Wisconsin nursing home were found with videos and photographs of residents bathing and of a nude resident who had a bowel obstruction being mocked.21
• In May 2014, employees of a nursing home in Massachusetts recorded themselves physically and verbally abusing several older adults with Alzheimer’s disease, including one episode of the employees “hitting the woman on her arms, flicking her ears and then pinching the woman’s nose closed.” The employees also possessed a photograph of her naked.22
• In June 2015, an employee of a nursing home in Indiana was accused of taking
photos of a resident naked and sharing them on the messaging application Snapchat,23 in which images disappear 10 seconds after they are viewed.
As technology evolves, caregivers are finding more cunning ways to abuse older adults. Considering current events and trends in this area, technology as a gateway to elder abuse should be of growing concern.
Risk factorsA 2013 literature review on elder abuse reported that the most important risk factors are related to relationship (family disharmony, poor or conflicting relationships) and environment (a low level of social support),3 although other variables can play a role. Regardless of these findings, it is important to recognize that (1) elder abuse is not a necessary consequence in a family with many risk factors and (2) elder abuse can occur in the absence of any risk factors.
As a whole, women are at a higher risk of abuse, particularly when combined with loneliness, poor social support, cohabitation (especially family members), substance abuse, cognitive impairment, and dementia and other mental health problems.4 Other risk factors include functional deficiency, poor physical health or frailty, low income or wealth, and trauma or past abuse.3
Lower income, poor health, low social support, and belonging to a non-white racial group put an older adult at risk for neglect; female sex is a specific risk factor for sexual assault.15 One study found that, among older adults who suffered physical, mental, or cognitive impairment, 1 of every 4 was at risk of abuse.7
Mental illness. Dementia puts an older adult at higher risk because of increased
caregiver stress resulting from disruptive and aggressive behaviors2; the same is true when the older adult suffers another mental illness, such as anxiety, depression, schizophrenia or bipolar disorder. Presumably, older adults with any of these disorders are at risk of financial and psychological and emotional abuse because of their decreased social support, lack of independence, and inability to hold a job—leaving their caregiver to shoulder more responsibilities and with more opportunities to inflict abuse. In addition, an older adult suffering from depression can feel helpless and unworthy, possibly making him more susceptible to psychological and emotional abuse, and less likely to seek help.
More research is needed to establish racial and ethnic differences in the risk of abuse. Some research states that older adults who are a member of a minority are at greater risk of abuse; however, the difference dissipates after adjusting for variables such as income and social support.24 Cultural confounders, such as varying interpretations of the same set of interactions between older adults, need to be examined further.
Sexual orientation. Identifying one’s self as a lesbian, gay, bisexual, or transgender (LGBT) person is an additional risk factor for elder abuse. In 1997, a report described a nursing home employee who refused to bathe a resident because he didn’t want to “touch the lesbian.”25 Despite evolving attitudes in society toward support and acceptance of sexual orientation, fear of homophobia still prevents some LGBT older adults from seeking help when they have been abused because of their orientation—especially ones who have internalized that
homophobia and feel that they are unworthy of seeking help.25
In addition, health care providers and nursing home staff members might neglect the particular care needs of LGBT older adults, intentionally or unintentionally. APS staff and providers must be cognizant of underlying biases and exhibit respect when assisting LGBT clients.
Approximately 75% of caregivers of older adults are family members; 70% are female26; and most are adult children, spouses, and partners of those receiving care.27 Male caregivers age ≥40 are more likely to be the abuser, however, especially when they possess any of these risk factors: fatigue, burnout, medical illness, mental illness, lack of financial and support services, family history of abusive behavior, and substance abuse.4 People who commit elder abuse also tend to be significantly dependent on the person they are abusing.2 In some cases, and especially when the abuser is financially needy, caregivers turn to elder abuse to obtain resources from the victim.2
From your standpoint as a practitioner, it is important to determine the root cause of elder abuse. According to one review,28 family members with mental illness or a history of substance abuse, or who are stressed by the burden of caregiving, abuse older adults at a higher rate than family members who are not affected in those ways. Depression in particular is a common characteristic of abusers,2 often secondary to the stress of caring for an older adult.
Abuse caused by stress can be addressed by referral to a support group and counseling for the caregiver; psychiatric conditions, such as depression, might be better treated with pharmacotherapy. Evaluate for depression and posttraumatic stress disorder (PTSD) in both the abuser and the abused,29 and for other mental health issues that might compound the situation. It is possible for you to have 2 patients: the older adult and his caregiver. Regardless of the challenge,keep in mind that the older adult’s safety is your priority.
Consequences for the abusedThe abused adult is at risk of a number of serious physical and psychological consequences.30 They tend to have a shorter lifespan, after adjusting for other variables associated with increased mortality.
The reason for shortened lifespan is multifactorial30:
• Bruises, abrasions, and fractures may take longer to heal because of diminished skin and bone regeneration.
• Diseases that affect the heart, lungs, and kidneys might prevent the person from bouncing back from major stressors caused by abuse, such as blood loss, severe injury, and pain.
• Injury from abuse can exacerbate an underlying illness.
• Elder abuse also is associated with increased emergency department use, hospitalization (including readmission within 30 days), and nursing home placement.31
Elder abuse can lead to depression, shame, and guilt; increased isolation; and
increased risk of alcohol abuse and substance use.31 A study found that victims of
elder abuse are significantly more depressed than non-victims.32
In the same study, being a victim of abuse was found to be the second-strongest
predictor of depression, after the state of one’s health.32 Other potential psychiatric
consequences of abuse that need further study include increased risk of developing
fear and anxiety disorders; learned helplessness; and PTSD.33 According to LoFaso,
“depression and anxiety can consume their days and leave them emotionally and
physically frail.”29 Such feelings make these older adults less likely to resolve abuse or break off relations with the abuser.32
Because mental illness can be a risk factor for, and a consequence of, elder abuse,
be aware of such complications and address them appropriately. Keep in mind that older adults are more likely to visit a primary care practitioner than a psychiatrist for a routine health check-up or evaluation of initial cognition-related problems; however, they are more likely to see a psychiatrist for advanced neuropsychiatric problems such as dementia, paranoia, delusions, hallucinations, and insomnia. Adequate education on elder abuse should not be limited to a single medical specialty because it can present in several clinical settings.
Identifying abuseIdentification of elder abuse in the home poses a greater challenge to clinicians than abuse in an institutional setting because it is not directly observable. Compounding this is the lack of unified standards for identifying and dealing with elder abuse. It is first necessary for you to determine the likelihood that abuse or neglect occurred, which can be difficult because the signs of elder abuse and manifestations of normal aging often are similar. You also must establish whether (1) the abused person will accept intervention and (2) the abused person who refuses intervention has the capacity to make that decision. Both of these conditions will guide your approach to management.2
Obtain the history from several sources; review the records; and carefully examine patterns of injury, in particular assessing functional status and level of dependency on the caregiver. Explanations that do not match injuries signal the need for further investigation and examination.
To help differentiate elder abuse from normal physiologic aging, look at the skin for bruises, rashes that do not heal, and ulcers—all of which could be signs of abuse or neglect. Keep in mind that bruising generally is more common in older adults because of the slower turnover rate of epidermal cells; physiologic bruising tends to occur on dorsal aspects of the hands and arms.4 In contrast, bruising secondary to neglect or physical abuse can manifest as a subgaleal hematoma (caused by traumatic hair pulling), tracking in the peritoneum after genital trauma, Battle’s sign, and raccoon eyes, among other findings.4
In addition, larger bruises (>5 cm in diameter) are more likely the result of elder abuse.34 To complicate matters, many older persons are taking anticoagulant therapy, making bruising more likely. In addition, be on the lookout for burns during the physical exam. Evidence suggests that at least 10% of burns caused by battery and assault occur in the context of elder abuse; most burn facilities do not have formal guidelines for screening for abuse and neglect, however.35 According to one retrospective study, the most common causes of burns in older adults are hot water scalds and radiator contact, and the mortality rate of older burn patients in general is higher than among the overall population.36
Falls and fractures are common among older adults, regardless of whether they are
abused, because of polypharmacy, underlying medical conditions, and functional
limitations. Many abusers, however, use these factors to cover up intentional injury
that might have resulted in the older person falling, including overmedication (a form of physical abuse) and withholding a necessary walking aid (a form of neglect). Maintain a high index of suspicion of elder abuse when (1) the caregiver’s and the older adult’s stories of an injury don’t add up and (2) physical findings that might have been caused by abuse are present.
A number of psychiatric and cognitive symptoms suggest other types of elder abuse. Take note of emotional upset, agitation, and unusual behaviors37—especially if you can follow the patient over time to observe marked changes in the presentation. Likewise, be aware of proposed alterations in guardianship, which should be evaluated by a forensic psychiatrist with analysis of medical history, social attachments, home environment, self-care, and finances.38 Such evaluation should provide clues to the motivation behind a change of guardianship and will help to determine if elder abuse should be suspected.
Brandi et al37 provided an informative table that identifies pertinent signs, symptoms, and other findings that clinicians should be aware of to support a suspicion of elder abuse (Table).
Documentation is of utmost importance in evaluating potential elder abuse; keep in mind that the medical record might be used in an investigation of abuse by social workers, law enforcement, and prosecutors. Your records should be legible, clearly indicate who the main caregiver is and what his (her) responsibilities are, and specify who is present at your encounter with the patient.4 Document your observations of patient behavior, reactions to questions, and family dynamics and conflicts16; make note of warning signs such as fear, silence, and inability to interview the patient alone.
In addition to written documentation, take photographs of injuries, with a ruler in the image to record their size. Serial photographs are helpful; so are photographs from a variety of distances (close-up, regional, wholebody) to capture detail and place the wound in the context of a specific area of the body.4
Safety is paramount. Given the findings of the history and physical exam, it is necessary to determine whether it is safe for the patient to return home with the caregiver, or if alternate accommodations or resources, such as a social worker or a support group, are required. Include details of planned follow-up in your evaluation, and offer consideration of possible psychiatric disorders that can develop as a result of such abuse.
ReportingElder abuse is a criminal offense in all states.39 A clinician who has reasonable suspicion that elder abuse occurred must report it, regardless of whether the proof of abuse is concrete.40 At a point of reasonable suspicion, immediately contact APS, law enforcement, and a social worker. Adult Protective Services, modeled after Child Protective Services, is typically administered by local and state health
departments.41
After a report is filed with APS, an assigned social worker makes an in-person home visit to investigate the allegation and determine whether elder abuse is substantiated, partially substantiated, or unsubstantiated.16 In most states, elder abuse reporting is not anonymous because follow-up may be needed to provide additional evidence, especially if the report was made by a health care provider.16
No federal standard exists for states to follow when defining and addressing elder abuse, which can complicate identification and reporting of abuse. Laws governing elder abuse do not allow states to determine the fate of the older adult, who can decide for himself (herself) whether to use or waive protective services.42 Older adults might choose not to report abuse because of shame, intimidation, or fear,43 or to protect a caregiver, who often is a family member.
Elder abuse reports can come from a variety of sources; convincing evidence is, as noted, unnecessary to report it. Health care providers are mandated reporters, but
it is believed that the number of clinicians who report elder abuse based on suspicion is far below what it should be. One study found that 94% of physicians said that they either were unable to prove that the abuse had occurred or decided not to report it.11 Another study found that only 1.4% of elder abuse cases reported to APS come from physicians.44
There are several possible reasons for underreporting elder abuse, including (1) the difficulty of distinguishing elder abuse and neglect from sequelae of normal aging and (2) the fact that cognitive and functional impairment of the abused person makes it difficult, even impossible, to establish the narrative of how the abuse happened. Nursing homes in particular provide a high level of oversight because residents have an average of ≥3 functional deficits.4 Other reasons for underreporting—some of which are difficult to understand, and excuse, in a clinician—are:
• subtlety of signs
• victim denial
• ignorance of reporting procedures
• inadequate training
• lack of information about resources
• concern about losing physician–patient rapport
• concern about involvement in the legal system
• time limitations
• doubt about the effectiveness of APS.16
Assessing capacityThe older adult’s wishes must be respected unless a health care provider or the legal system determines that he lacks functional capacity to make decisions.16
How is capacity evaluated? A capacity evaluation has 3 components:
• Comprehension is a person’s factual understanding of the situation, including
consequences and alternatives
• Free choice is a person’s voluntary decision to accept or reject a proposed treatment, free of coercion (in this setting, free choice is the older adult’s decision whether to report the abuse)
• Reliability is a person’s ability to provide a consistent choice over time.45
Most capacity evaluations are conducted by clinical interview. No single, brief test is used universally, and there is the possibility of inter-rater variability.45 Examples of tests used to assess capacity are the Folstein Mini-Mental Status Examination and the MacArthur Competence Assessment Tool-Treatment45; the latter is a structured interview that incorporates information specific to the individual patient’s decision-making situation.46 Regardless of the approach, the psychiatrist-evaluator ensures that the older adult has been given the appropriate information
to provide informed consent about the situation.47
If the evaluator determines that a person lacks capacity to make decisions, efforts should be made to determine if the cause of that impairment is reversible.47 Older adults who have dementia or other underlying psychiatric condition that impairs cognition might benefit from more education on their situation; ones who appear fearful of consequences should be introduced to a trusted advisor to assist in making competent judgments.47
If the older adult is found to lack capacity, a substitute decision-maker must be sought.47 Many states have statutes specifying the order in which family members are contacted.48 The need to appoint an advisor can become knotty because the suspected abuser often is a family member; clinicians and others involved in identifying a decision-maker to speak on behalf of an older adult should choose carefully.
Prevention and screeningKey to reducing the prevalence of elder abuse in the community is formulating
strategies for prevention and screening. The American Medical Association recommends that clinicians “incorporate routine questions related to elder abuse and neglect into daily practice.”49 Older adults might not admit to abuse or neglect unless they are asked; speak to patients at eye level, keep questions simple, direct, and nonjudgmental, and assure them (1) that discussions are confidential and (2) that their safety is your primary goal.50,51
Comprehensive approaches to questioning patients are available and often recommended for screening for elder abuse.4 However, screening in the office setting often involves short, directly administered questionnaires.49 For example, the Health and Safety Screen developed at the University of Maine comprises 6 questions52:
• Has anyone close to you called you names or insulted you recently?
• Are you afraid of anyone in your life?
• Are you able to use the telephone anytime you want to?
• Has anyone forced you to do things you didn’t want to do?
• Has anyone taken things or money that belong to you without your OK?
• Has anyone close to you tried to hurt you or harm you recently?
Because of time constraints and lack of a universal standard, there is debate whether regular elder abuse screening is time-effective. It often is recommended, therefore, that clinicians in primary care (1) refer older adults with risk factors for abuse to geriatric medical teams trained in these measures and (2) perform periodic follow-up on such patients4 (Figure).
• New York City Elder Abuse Center encourages collaboration among health, mental health, and community justice organizations.28 The program involves a number of resources for addressing elder abuse, such as promoting staff awareness of risk factors for, and signs of, abuse, and screening for mental health problems in the abused.
• The Elder Justice Act, enacted in 2010 to combat elder abuse, provides federal funds and resources to prevent, detect, treat, and intervene to stop abuse and, when appropriate, to prosecute abusers.53
This Web Exclusive Table provides a 7-point summary reference guide for understanding and preventing elder abuse in your practice.
BOTTOM LINEIdentification of elder abuse can be difficult because signs and symptoms of abuse closely resemble physiologic aging. Older adults with identifiable risk factors should be screened for abuse; time constraints make universal screening impossible at this time. In the future, multidisciplinary approaches likely will make elder abuse more easily identifiable through the combined work of health care providers, law enforcement agencies, banks, and other institutions—with the ultimate goal of protecting older adults in the community from abuse.
Related Resources
• Frazão SL, Correia AM, Norton P, et al. Physical abuse against elderly persons in institutional settings. J Forensic Leg Med. 2015;36:54-60.
• Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
Disclosures
Ms. Hubert reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products. Dr. Gupta is a member of the speakers’ bureau of Alkermes, Allergan, Avanir Pharmaceuticals, Takeda Pharmaceutical, Lundbeck, Otsuka Pharmaceutical, and Sunovion Pharmaceuticals.
1. The Elder Abuse Prevention, Identification, and Treatment Act of 1985, HR 1674, 99th Cong (1985).
2. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272.
3. Johannesen M, LoGiudice D. Elder abuse: a systematic review of risk factors in community-dwelling elders. Age Ageing. 2013;42(3):292-298.
4. Gibbs LM, Mosqueda L, eds. Medical implications of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):xv-xvi. doi: 10.1016/j.cger.2014.08.015.
5. Werner CA. The Older Population: 2010. U.S. Census Bureau. http://webcache.googleusercontent.com/search?q=cache:hCCb_pcnO6QJ :ht tps://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf+&cd=1&hl=en&ct=clnk&gl=uss. Issued November 2011. Accessed October 10, 2015.
6. Himes CL. Elderly Americans. Population Bulletin. 2002;56(4):1-41.
7. Cooper C, Selwood A, Livingston G. The prevalence of elder abuse and neglect: a systematic review. Age Ageing. 2008;37(2):151-60.
8. Teaster PB. A response to the abuse of vulnerable adults: the 2000 Survey of State Adult Protective Services. The National Center on Elder Abuse. http://www.ncea.aoa.gov/Resources/Publication/docs/apsreport030703.pdf. 2003.
Accessed October 22, 2015.
9. Mouton CP, Rodabough RJ, Rovi SL, et al. Prevalence and 3-year incidence of abuse among postmenopausal women. Am J Public Health. 2004;94(4):605-612.
10. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. Statistic Brain Research Institute. Elderly abuse statistics. http://www.statisticbrain.com/elderly-abuse-statistics. Accessed June 22, 2015.
13. Lachs MS, Bachman R, Williams CS, et al. Resident-to-resident elder mistreatment and police contact in nursing homes: findings from a population-based cohort. J Am Geriatr Soc. 2007;55(6):840-845.
14. Lachs M, Bachman R, Williams C, et al. Older adults as crime victims, perpetrators, witnesses, and complainants: a population-based study of police interactions. J Elder Abuse Negl. 2005;16(4):25-40.
15. Acierno R, Hernandez-Tejada M, Muzzy W, et al. National Elder Mistreatment Study. Washington, DC: National Institute of Justice; 2009.
16. Dong XQ. Elder abuse: systematic review and implications for practice. J Am Geriatr Soc. 2015;63(6):1214-1238.
17. Freedman M. The growing epidemic of financial elder abuse. The Tax Advisor. http://www.cpa2biz.com/Content/media/PRODUCER_CONTENT/Newsletters/
Articles_2007/Tax/Financial_Elder_Abuse.jsp. Published November 2007. Accessed June 24, 2015.
18. Consumer Financial Protection Bureau. Protection for older Americans. http://www.consumerfinance.gov/olderamericans. Accessed June 22, 2015.
19. Castle NG. Nursing home deficiency citations for abuse. J Appl Gerontol. 2011;30(6):719-743.
20. Elder Abuse Prevention Unit. Social abuse. http://www.eapu.com.au/elder-abuse/social-abuse. Published 2014. Accessed June 24, 2015.
21. Former nursing home employees allegedly photographed naked resident. United Press International. http://www.upi.com/Top_News/US/2013/07/03/Former-nursinghome-employees-allegedly-photographed-nakedresidents/
65801372893020. Published July 3, 2013. Accessed June 24, 2015.
22. Miller N. Two charged with elder assault at an assisted living facility. MetroWest Daily News. http://www.metrowestdailynews.com/article/20140506/
NEWS/140507587. Updated May 7, 2014. Accessed June 24, 2015.
23. Jorgensen J. New charges filed in nursing home case.WHAS11. http://www.whas11.com/story/news/local/2015/06/24/new-charges-filed-in-nursing-homecase/29243183/. Published June 24, 2015. Accessed June 27, 2015.
24. Hermandez-Tejada MA, Amstadter A, Muzzy W, et al. The National Elder Mistreatment Study: race and ethnicity findings. J Elder Abuse Negl. 2013;25(4):281-293.
25. Cooks-Daniels L. Lesbian, gay male, bisexual and transgendered elders: elder abuse and neglect issues. J Elder Abuse Negl. 1998;9(2):35-49.
26. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
27. Tatara T, Blumerman Kuzmeskus L, Duckhorn E, et al. The National Center on Elder Abuse Incidence Study: final report. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed October 19, 2015.
28. Rosen AL. Where mental health and elder abuse intersect. Generations. 2014;38(3):75-79.
29. LoFaso V. The role of the primary physician in assessing and treating the mental health concerns of elder abuse victims. NYC Elder Abuse Center eNewsletter.nyceac.com/wp-content/uploads/2013/03/Exploring-the-IntersectionofElder-Abuse-and-Mental-Health_eNewsletter.pdf. Published March 12, 2013. Accessed August 20, 2015.
30. Lachs MS, Williams CS, O’Brien S, et al. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
31. Dyer CB, Pavlik VN, Murphy KP, et al. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
32. Pillemer K, Prescott D. Psychological effects of elder abuse: a research note. J Elder Abuse Negl. 1988;1(1):65-73.
33. Elder abuse: consequences. Centers for Disease Control and Prevention. http://www.cdc.gov/violenceprevention/elderabuse/consequences.html. Updated June 22, 2015.Accessed August 20, 2015.
34. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196.
35. Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns. 2012;38(5):630-637.
36. 2014 National Burn Repository; report of data between 2004-2013. American Burn Association. http://www.ameriburn.org/2014NBRAnnualReport.pdf. Published 2014. Accessed June 26, 2015.
37. Brandi B, Dyer CB, Heisler CJ, et al. Systemic responses to elder abuse. In: Brandi B, Dyer CB, Heisler CJ, eds. Elder abuse detection and intervention: a collaborative approach. New York, NY: Spring Publishing Company; 2007:79-100.
38. Welner M. Guardianship. The Forensic Panel. http://www.forensicpanel.com/expert_services/psychiatry/civil_law/guardianship.html. Accessed August 20, 2015.
39. Watson E. Elder abuse: definition, types and statistics, and elder abuse (mistreatment and neglect) laws. Journal of Legal Nurse Consulting. 2013;24(2):40-42.
40. National Center on Elder Abuse Administration on Aging. Reporting abuse. http://www.ncea.aoa.gov/Stop_Abuse/Get_Help/Report/index.aspx. Accessed August 18, 2015.
41. Mukherjee D. Organizational structures of elder abuse reporting systems. Administration in Social Work. 2011;35(5):517-531.
42. Costin LB, Karger HJ, Stoesz H. The politics of child abuse in America. New York, NY: Oxford University Press; 1996.
43. Thomson MJ, Lietzau LK, Doty MM, et al. An analysis of elder abuse rates in Milwaukee County. WMJ. 2011;110(6):271-276.
44. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 Survey of State Adult Protective Services: Abuse of Adults 60 Years and Older. http://www.ncea.aoa.gov/Resources/Publication/docs/APS_2004NCEASurvey.pdf. Published March 2007. Accessed October 19, 2015.
45. Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
46. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resources Press; 1998.
47. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;35(18):1834-1840.
48. Wynn S. Decision by surrogates: An overview of surrogate consent laws in the United States. Bifocal: A Journal of the ABA Commission on Bar and Aging. 2014;36(1). http://www.americanbar.org/publications/bifocal/vol_36/
issue_1_october2014/default_surrogate_consent_statutes.html. Accessed October 22, 2015.
49. American Medical Association. Diagnostic and treatment guidelines on elder abuse and neglect. Chicago, IL: American Medical Association; 1992.
50. Harrell R, Toronjo C, McLaughlin J, et al. How geriatricians identify elder abuse and neglect. Am J Med Sci. 2002;323(1):34-38.
51. Ahmad M, Lachs MS. Elder abuse and neglect: what physicians can and should do. Cleve Clin J Med. 2002; 69(10):801-808.
52. Elder abuse screening protocol for physicians: lessons learned from the Maine Partners for Elder Protection Pilot Project. University of Maine Center on Aging. http://umcoa.siteturbine.com/uploaded_files/mainecenteronaging.umaine.edu/files/elderabusescreeningmanual.pdf. Published May 2, 2007. Accessed August 20, 2015.
53. What is the Elder Justice Act? USC Davis School of Gerontology. http://gerontology.usc.edu/resources/articles/what-is-the-elder-justice-act/. Published 2015. Accessed October 20, 2015.
1. The Elder Abuse Prevention, Identification, and Treatment Act of 1985, HR 1674, 99th Cong (1985).
2. Lachs MS, Pillemer K. Elder abuse. Lancet. 2004;364(9441):1263-1272.
3. Johannesen M, LoGiudice D. Elder abuse: a systematic review of risk factors in community-dwelling elders. Age Ageing. 2013;42(3):292-298.
4. Gibbs LM, Mosqueda L, eds. Medical implications of elder abuse and neglect. Clin Geriatr Med. 2014;30(4):xv-xvi. doi: 10.1016/j.cger.2014.08.015.
5. Werner CA. The Older Population: 2010. U.S. Census Bureau. http://webcache.googleusercontent.com/search?q=cache:hCCb_pcnO6QJ :ht tps://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf+&cd=1&hl=en&ct=clnk&gl=uss. Issued November 2011. Accessed October 10, 2015.
6. Himes CL. Elderly Americans. Population Bulletin. 2002;56(4):1-41.
7. Cooper C, Selwood A, Livingston G. The prevalence of elder abuse and neglect: a systematic review. Age Ageing. 2008;37(2):151-60.
8. Teaster PB. A response to the abuse of vulnerable adults: the 2000 Survey of State Adult Protective Services. The National Center on Elder Abuse. http://www.ncea.aoa.gov/Resources/Publication/docs/apsreport030703.pdf. 2003.
Accessed October 22, 2015.
9. Mouton CP, Rodabough RJ, Rovi SL, et al. Prevalence and 3-year incidence of abuse among postmenopausal women. Am J Public Health. 2004;94(4):605-612.
10. Acierno R, Hernandez MA, Amstadter AB, et al. Prevalence and correlates of emotional, physical, sexual, and financial abuse and potential neglect in the United States: the National Elder Mistreatment Study. Am J Public Health. 2010;100(2):292-297.
11. Kennedy RD. Elder abuse and neglect: the experience, knowledge, and attitudes of primary care physicians. Fam Med. 2005;37(7):481-485.
12. Statistic Brain Research Institute. Elderly abuse statistics. http://www.statisticbrain.com/elderly-abuse-statistics. Accessed June 22, 2015.
13. Lachs MS, Bachman R, Williams CS, et al. Resident-to-resident elder mistreatment and police contact in nursing homes: findings from a population-based cohort. J Am Geriatr Soc. 2007;55(6):840-845.
14. Lachs M, Bachman R, Williams C, et al. Older adults as crime victims, perpetrators, witnesses, and complainants: a population-based study of police interactions. J Elder Abuse Negl. 2005;16(4):25-40.
15. Acierno R, Hernandez-Tejada M, Muzzy W, et al. National Elder Mistreatment Study. Washington, DC: National Institute of Justice; 2009.
16. Dong XQ. Elder abuse: systematic review and implications for practice. J Am Geriatr Soc. 2015;63(6):1214-1238.
17. Freedman M. The growing epidemic of financial elder abuse. The Tax Advisor. http://www.cpa2biz.com/Content/media/PRODUCER_CONTENT/Newsletters/
Articles_2007/Tax/Financial_Elder_Abuse.jsp. Published November 2007. Accessed June 24, 2015.
18. Consumer Financial Protection Bureau. Protection for older Americans. http://www.consumerfinance.gov/olderamericans. Accessed June 22, 2015.
19. Castle NG. Nursing home deficiency citations for abuse. J Appl Gerontol. 2011;30(6):719-743.
20. Elder Abuse Prevention Unit. Social abuse. http://www.eapu.com.au/elder-abuse/social-abuse. Published 2014. Accessed June 24, 2015.
21. Former nursing home employees allegedly photographed naked resident. United Press International. http://www.upi.com/Top_News/US/2013/07/03/Former-nursinghome-employees-allegedly-photographed-nakedresidents/
65801372893020. Published July 3, 2013. Accessed June 24, 2015.
22. Miller N. Two charged with elder assault at an assisted living facility. MetroWest Daily News. http://www.metrowestdailynews.com/article/20140506/
NEWS/140507587. Updated May 7, 2014. Accessed June 24, 2015.
23. Jorgensen J. New charges filed in nursing home case.WHAS11. http://www.whas11.com/story/news/local/2015/06/24/new-charges-filed-in-nursing-homecase/29243183/. Published June 24, 2015. Accessed June 27, 2015.
24. Hermandez-Tejada MA, Amstadter A, Muzzy W, et al. The National Elder Mistreatment Study: race and ethnicity findings. J Elder Abuse Negl. 2013;25(4):281-293.
25. Cooks-Daniels L. Lesbian, gay male, bisexual and transgendered elders: elder abuse and neglect issues. J Elder Abuse Negl. 1998;9(2):35-49.
26. Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
27. Tatara T, Blumerman Kuzmeskus L, Duckhorn E, et al. The National Center on Elder Abuse Incidence Study: final report. http://aoa.gov/AoA_Programs/Elder_Rights/Elder_Abuse/docs/ABuseReport_Full.pdf. Published September 1998. Accessed October 19, 2015.
28. Rosen AL. Where mental health and elder abuse intersect. Generations. 2014;38(3):75-79.
29. LoFaso V. The role of the primary physician in assessing and treating the mental health concerns of elder abuse victims. NYC Elder Abuse Center eNewsletter.nyceac.com/wp-content/uploads/2013/03/Exploring-the-IntersectionofElder-Abuse-and-Mental-Health_eNewsletter.pdf. Published March 12, 2013. Accessed August 20, 2015.
30. Lachs MS, Williams CS, O’Brien S, et al. The mortality of elder mistreatment. JAMA. 1998;280(5):428-432.
31. Dyer CB, Pavlik VN, Murphy KP, et al. The high prevalence of depression and dementia in elder abuse or neglect. J Am Geriatr Soc. 2000;48(2):205-208.
32. Pillemer K, Prescott D. Psychological effects of elder abuse: a research note. J Elder Abuse Negl. 1988;1(1):65-73.
33. Elder abuse: consequences. Centers for Disease Control and Prevention. http://www.cdc.gov/violenceprevention/elderabuse/consequences.html. Updated June 22, 2015.Accessed August 20, 2015.
34. Wiglesworth A, Austin R, Corona M, et al. Bruising as a marker of physical elder abuse. J Am Geriatr Soc. 2009;57(7):1191-1196.
35. Peck MD. Epidemiology of burns throughout the World. Part II: intentional burns in adults. Burns. 2012;38(5):630-637.
36. 2014 National Burn Repository; report of data between 2004-2013. American Burn Association. http://www.ameriburn.org/2014NBRAnnualReport.pdf. Published 2014. Accessed June 26, 2015.
37. Brandi B, Dyer CB, Heisler CJ, et al. Systemic responses to elder abuse. In: Brandi B, Dyer CB, Heisler CJ, eds. Elder abuse detection and intervention: a collaborative approach. New York, NY: Spring Publishing Company; 2007:79-100.
38. Welner M. Guardianship. The Forensic Panel. http://www.forensicpanel.com/expert_services/psychiatry/civil_law/guardianship.html. Accessed August 20, 2015.
39. Watson E. Elder abuse: definition, types and statistics, and elder abuse (mistreatment and neglect) laws. Journal of Legal Nurse Consulting. 2013;24(2):40-42.
40. National Center on Elder Abuse Administration on Aging. Reporting abuse. http://www.ncea.aoa.gov/Stop_Abuse/Get_Help/Report/index.aspx. Accessed August 18, 2015.
41. Mukherjee D. Organizational structures of elder abuse reporting systems. Administration in Social Work. 2011;35(5):517-531.
42. Costin LB, Karger HJ, Stoesz H. The politics of child abuse in America. New York, NY: Oxford University Press; 1996.
43. Thomson MJ, Lietzau LK, Doty MM, et al. An analysis of elder abuse rates in Milwaukee County. WMJ. 2011;110(6):271-276.
44. Teaster PB, Dugar TA, Mendiondo MS, et al; The National Committee for the Prevention of Elder Abuse; The National Adult Protective Services Association. The 2004 Survey of State Adult Protective Services: Abuse of Adults 60 Years and Older. http://www.ncea.aoa.gov/Resources/Publication/docs/APS_2004NCEASurvey.pdf. Published March 2007. Accessed October 19, 2015.
45. Sorrentino R. Performing capacity evaluations: what’s expected from your consult. Current Psychiatry. 2014;13(1):41-44.
46. Grisso T, Appelbaum PS. MacArthur competence assessment tool for treatment (MacCAT-T). Sarasota, FL: Professional Resources Press; 1998.
47. Appelbaum PS. Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med. 2007;35(18):1834-1840.
48. Wynn S. Decision by surrogates: An overview of surrogate consent laws in the United States. Bifocal: A Journal of the ABA Commission on Bar and Aging. 2014;36(1). http://www.americanbar.org/publications/bifocal/vol_36/
issue_1_october2014/default_surrogate_consent_statutes.html. Accessed October 22, 2015.
49. American Medical Association. Diagnostic and treatment guidelines on elder abuse and neglect. Chicago, IL: American Medical Association; 1992.
50. Harrell R, Toronjo C, McLaughlin J, et al. How geriatricians identify elder abuse and neglect. Am J Med Sci. 2002;323(1):34-38.
51. Ahmad M, Lachs MS. Elder abuse and neglect: what physicians can and should do. Cleve Clin J Med. 2002; 69(10):801-808.
52. Elder abuse screening protocol for physicians: lessons learned from the Maine Partners for Elder Protection Pilot Project. University of Maine Center on Aging. http://umcoa.siteturbine.com/uploaded_files/mainecenteronaging.umaine.edu/files/elderabusescreeningmanual.pdf. Published May 2, 2007. Accessed August 20, 2015.
53. What is the Elder Justice Act? USC Davis School of Gerontology. http://gerontology.usc.edu/resources/articles/what-is-the-elder-justice-act/. Published 2015. Accessed October 20, 2015.
What to do when your depressed patient develops mania
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
When a known depressed patient newly develops signs of mania or hypomania, a cascade of diagnostic and therapeutic questions ensues: Does the event “automatically” signify the presence of bipolar disorder (BD), or could manic symptoms be secondary to another underlying medical problem, a prescribed antidepressant or non-psychotropic medication, or illicit substances?
Even more questions confront the clinician: If mania symptoms are nothing more than an adverse drug reaction, will they go away by stopping the presumed offending agent? Or do symptoms always indicate the unmasking of a bipolar diathesis? Should anti-manic medication be prescribed immediately? If so, which one(s) and for how long? How extensive a medical or neurologic workup is indicated?
And, how do you differentiate ambiguous hypomania symptoms (irritability, insomnia, agitation) from other phenomena, such as akathisia, anxiety, and overstimulation?
In this article, we present an overview of how to approach and answer these key questions, so that you can identify, comprehend, and manage manic symptoms that arise in the course of your patient’s treatment for depression (Box).
Does disease exist on a unipolar−bipolar continuum?
There has been a resurgence of interest in Kraepelin’s original notion of mania and depression as falling along a continuum, rather than being distinct categories of pathology. True bipolar mania has its own identifiable epidemiology, familiality, and treatment, but symptomatic shades of gray often pose a formidable diagnostic and therapeutic challenge.
For example, DSM-5 relaxed its definition of “mixed” episodes of BD to include subsyndromal mania features in unipolar depression. When a patient with unipolar depression develops a full, unequivocal manic episode, there usually isn’t much ambiguity or confusion about initial management: assure a safe environment, stop any antidepressants, rule out drug- or medically induced causes, and begin an acute anti-manic medication.
Next steps can, sometimes, be murkier:
• formulate a definitive, overarching diagnosis
• provide psycho-education
• forecast return to work or school
• discuss prognosis and likelihood of relapse
• address necessary lifestyle modifications (eg, sleep hygiene, elimination of alcohol and illicit drug use)
• determine whether indefinite maintenance pharmacotherapy is indicated— and, if so, with which medication(s).
CASE A diagnostic formulation isn’t always black and white
Ms. J, age 56, a medically healthy woman, has a 10-year history of depression and anxiety that has been treated effectively for most of that time with venlafaxine, 225 mg/d. The mother of 4 grown children, Ms. J has worked steadily for >20 years as a flight attendant for an international airline.
Today, Ms. J is brought by ambulance from work to the emergency department in a paranoid and agitated state. The admission follows her having e-blasted airline corporate executives with a voluminous manifesto that she worked on around the clock the preceding week, in which she explained her bold ideas to revolutionize the airline industry, under her leadership.
Ms. J’s family history is unremarkable for psychiatric illness.
How does one approach a case such as Ms. J’s?
Stark examples of classical mania, as depicted in this case vignette, are easy to recognize but not necessarily straightforward, nosologically. Consider the following not-so-straightforward elements of Ms. J’s case:
• a first-lifetime episode of mania or hypomania is rare after age 50
• Ms. J took a serotonin-norepinephrine reuptake inhibitor (SNRI) for many years without evidence of mood destabilization
• years of repetitive chronobiological stress (including probable frequent time zone changes with likely sleep disruption) apparently did not trigger mood destabilization
• none of Ms. J’s 4 pregnancies led to postpartum mood episodes
• at least on the surface, there are no obvious features that point to likely causes of a secondary mania (eg, drug-induced, toxic, metabolic, or medical)
• Ms. J has no known family history of BD or any other mood disorder.
Approaching a case such as Ms. J’s must involve a systematic strategy that can best be broken into 2 segments: (1) a period of acute initial assessment and treatment and (2) later efforts focused on broader diagnostic evaluation and longer-term relapse prevention.
Initial assessment and treatment
Immediate assessment and management hinges on initial triage and forming a working diagnostic impression. Although full-blown mania usually is obvious (sometimes even without a formal interview), be alert to patients who might minimize or altogether disavow mania symptoms—often because of denial of illness, misidentification of symptoms, or impaired insight about changes in thinking, mood, or behavior.
Because florid mania, by definition, impairs psychosocial functioning, the context of an initial presentation often holds diagnostic relevance. Manic patients who display disruptive behaviors often are brought to treatment by a third party, whereas a less severely ill patient might be more inclined to seek treatment for herself (himself) when psychosis is absent and insight is less compromised or when the patient feels she (he) might be depressed.
It is not uncommon for a manic patient to report “depression” as the chief complaint or to omit elements related to psychomotor acceleration (such as racing thoughts or psychomotor agitation) in the description of symptoms. An accurate diagnosis often requires clinical probing and clarification of symptoms (eg, differentiating simple insomnia with consequent next-day fatigue from loss of the need for sleep with intact or even enhanced next-day energy) or discriminating racing thoughts from anxious ruminations that might be more intrusive than rapid.
Presentations of frank mania also can come to light as a consequence of symptoms, rather than as symptoms per se (eg, conflict in relationships, problems at work, financial reversals).
Particularly in patients who do not have a history of mania, avoid the temptation to begin or modify existing pharmacotherapy until you have performed a basic initial evaluation. Immediate considerations for initial assessment and management include the following:
Provide containment. Ensure a safe setting, level of care, and frequency of monitoring. Evaluate suicide risk (particularly when mixed features are present), and risk of withdrawal from any psychoactive substances.
Engage significant others. Close family members can provide essential history, particularly when a patient’s insight about her illness and need for treatment are impaired. Family members and significant others also often play important roles in helping to restrict access to finances, fostering medication adherence, preventing access to weapons in the home, and sharing information with providers about substance use or high-risk behavior.
Systematically assess for DSM-5 symptoms of mania and depression. DSM-5 modified criteria for mania/hypomania to necessitate increased energy, in addition to change in mood, to make a syndromal diagnosis. Useful during a clinical interview is the popular mnemonic DIGFAST to aid recognition of core mania symptomsa:
• Distractibility
• Indiscretion/impulsivity
• Grandiosity
• Flight of ideas
• Activity increase
• Sleep deficit
• Talkativeness.
aAlso see: “Mnemonics in a mnutshell: 32 aids to psychiatric diagnosis,” in the October 2008 issue Current Psychiatry and in the archive at CurrentPsychiatry.com.
These symptoms should represent a departure from normal baseline characteristics; it often is helpful to ask a significant other or collateral historian how the present symptoms differ from the patient’s usual state.
Assess for unstable medical conditions or toxicity states. When evaluating an acute change in mental status, toxicology screening is relatively standard and the absence of illicit substances should seldom, if ever, be taken for granted—especially because occult substance use can lead to identification of false-positive BD “cases.”1
Stop any antidepressant. During a manic episode, continuing antidepressant medication serves no purpose other than to contribute to or exacerbate mania symptoms. Nonetheless, observational studies demonstrate that approximately 15% of syndromally manic patients continue to receive an antidepressant, often when a clinician perceives more severe depression during mania, multiple prior depressive episodes, current anxiety, or rapid cycling.2
Importantly, antidepressants have been shown to harm, rather than alleviate, presentations that involve a mixed state,3 and have no demonstrated value in preventing post-manic depression. Mere elimination of an antidepressant might ease symptoms during a manic or mixed episode.4
In some cases, it might be advisable to taper, not abruptly stop, a short half-life serotonergic antidepressant, even in the setting of mania, to minimize the potential for aggravating autonomic dysregulation that can result from antidepressant discontinuation effects.
Begin anti-manic pharmacotherapy. Initiation of an anti-manic mood stabilizer, such as lithium and divalproex, has been standard in the treatment of acute mania.
In the 1990s, protocols for oral loading of divalproex (20 to 30 mg/kg/d) gained popularity for achieving more rapid symptom improvement than might occur with lithium. In the current era, atypical antipsychotics have all but replaced mood stabilizers as an initial intervention to contain mania symptoms quickly (and with less risk than first-generation antipsychotics for acute adverse motor effects from so-called rapid neuroleptization).
Because atypical antipsychotics often rapidly subdue mania, psychosis, and agitation, regardless of the underlying process, many practitioners might feel more comfortable initiating them than a mood stabilizer when the diagnosis is ambiguous or provisional, although their longer-term efficacy and safety, relative to traditional mood stabilizers, remains contested. Considerations for choosing from among feasible anti-manic pharmacotherapies are summarized in Table 1.5
Normalize the sleep-wake cycle. Chronobiological and circadian variables, such as irregular sleep patterns, are thought to contribute to the pathophysiology of affective switch in BD. Behavioral and pharmacotherapeutic efforts to impose a normal sleep−wake schedule are considered fundamental to stabilizing acute mania.
Facilitate next steps after acute stabilization. For inpatients, this might involve step-down to a partial hospitalization or intensive outpatient program, alongside taking steps to ensure continued treatment adherence and minimize relapse.
What medical and neurologic workup is appropriate?
Not every first lifetime presentation of mania requires extensive medical and neurologic workup, particularly among patients who have a history of depression and those whose presentation neatly fits the demographic and clinical profile of newly emergent BD. Basic assessment should determine whether any new medication has been started that could plausibly contribute to abnormal mental status (Table 2).
Nevertheless, evaluation of almost all first presentations of mania should include:
• urine toxicology screen
• complete blood count
• comprehensive metabolic panel
• thyroid-stimulating hormone assay
• serum vitamin B12 level assay
• serum folic acid level assay
• rapid plasma reagin test.
Clinical features that usually lead a clinician to pursue a more detailed medical and neurologic evaluation of first-episode mania include:
• onset age >40
• absence of a family history of mood disorder
• symptoms arising during a major medical illness
• multiple medications
• suspicion of a degenerative or hereditary neurologic disorder
• altered state of consciousness
• signs of cortical or diffuse subcortical dysfunction (eg, cognitive deficits, motor deficits, tremor)
• abnormal vital signs.
Depending on the presentation, additional testing might include:
• tests of HIV antibody, immune autoantibodies, and Lyme disease antibody
• heavy metal screening (when suggested by environmental exposure)
• lumbar puncture (eg, in a setting of manic delirium or suspected central nervous system infection or paraneoplastic syndrome)
• neuroimaging (note: MRI provides better visualization than CT of white matter pathology and small vessel cerebrovascular disease) electroencephalography.
Making an overarching diagnosis: Is mania always bipolar disorder?
Mania is considered a manifestation of BD when symptoms cannot be attributed to another psychiatric condition, another underlying medical or neurologic condition, or a toxic-metabolic state (Table 3 and Table 46-9). Classification of mania that occurs soon after antidepressant exposure in patients without a known history of BD continues to be the subject of debate, varying in its conceptualization across editions of DSM.
The National Institute of Mental Health (NIMH) Systematic Treatment Enhancement Program for Bipolar Disorder, or STEP-BD, observed a fairly low (approximately 10%) incidence of switch from depression to mania when an antidepressant is added to a mood stabilizer; the study authors concluded that much of what is presumed to be antidepressant-induced mania might simply be the natural course of illness.10
Notably, several reports suggest that antidepressants might pose a greater risk of mood destabilization in people with BD I than with either BD II or other suspected variants on the bipolar spectrum.
DSM-5 advises that a diagnosis of substance-induced mood disorder appropriately describes symptoms that spontaneously dissipate once an antidepressant has been discontinued, whereas a diagnosis of BD can be made when manic or hypomanic symptoms persist at a syndromal level after an antidepressant has been stopped and its physiological effects are no longer present. With respect to time course, the International Society of Bipolar Disorders proposes that, beyond 12 to 16 weeks after an antidepressant has been started or the dosage has been increased, it is unlikely that new-onset mania/hypomania can reasonably be attributed to “triggering” by an antidepressant11 (although antidepressants should be stopped when symptoms of mania emerge).
Several clinical features have been linked in the literature with an increased susceptibility to BD after an initial depressive episode, including:
• early (pre-adolescent) age at onset of first mood disorder episode6
• family history of BD, highly recurrent depression, or psychosis12,13
• psychosis when depressed.7,14
A number of other characteristics of depressive illness—including seasonal depression, atypical depressive features, suicidality, irritability, anxiety or substance use comorbidity, postpartum mood episodes, and brief recurrent depressive episodes—have been described in the literature as potential correlates of a bipolar diathesis; none have proved to be robust or pathognomonic of a BD diagnosis, as opposed to a unipolar diagnosis.
Data from the NIMH Collaborative Depression Study suggest that recurrent mania/hypomania after an antidepressant-associated polarity switch is greater when a family history of BD is present; other clinical variables might hold less predictive value.15
In addition, although some practitioners consider a history of nonresponse to trials of multiple antidepressants suggestive of an underlying bipolar process, polarity is only one of many variables that must be considered in the differential diagnosis of antidepressant-resistant depression.b Likewise, molecular genetic studies do not support a link between antidepressant nonresponse and the likelihood of a diagnosis of BD.16
bSee “A practical approach to subtyping depression among your patients” in the April 2014 issue of Current Psychiatry or in the archive at CurrentPsychiatry.com.
Indefinite pharmacotherapy for bipolar disorder?
An important but nagging issue when diagnosing BD after a first manic (or hypomanic) episode is the implied need for indefinite pharmacotherapy to sustain remission and prevent relapse and recurrence.
The likelihood of subsequent depression or mania/hypomania remains high after an index manic/hypomanic episode, particularly for 6 to 8 months after recovery.8,17 Natural history data suggest that, during the year that follows a first lifetime mania, approximately 40% of patients experience a second manic episode.8 A second lifetime mania might be especially likely in patients whose index episode involved mood-congruent psychosis, low premorbid work functioning, and an initial manic episode, as opposed to a mixed episode17 or early age at onset.8
In the absence of randomized, placebo-controlled studies of maintenance pharmacotherapy after a first lifetime manic episode, clinical judgment often drives decisions about the duration of continuing pharmacotherapy after initial symptoms resolve. The Texas Medication Algorithm Project for BD advises that:
Similarly, in the most recent (2004) Expert Consensus Guideline Series for the Treatment of Bipolar Disorder,19 84% of practitioner−respondents favored indefinite mood stabilizer therapy after a second lifetime manic episode. No recommendation was made about the duration of maintenance pharmacotherapy after a first lifetime manic/hypomanic episode.
Avoid or reintroduce an antidepressant if depression recurs after a first mania?
Controversies surrounding antidepressant use in BD are extensive; detailed discussion is beyond the scope of this review (Goldberg and Ghaemi provided a broader discussion of risks and benefits of antidepressants in BD20). Although the main clinical concern regarding antidepressant use was, at one time, the potential to induce mania or accelerate the frequency of recurrent episodes, more recent, empirical studies suggest that the greater risk of using antidepressants for BD is lack of efficacy.10,21
If a careful longitudinal history and clinical evaluation reveal that an initial manic episode heralds the onset of BD, decisions about whether to avoid an antidepressant (as opposed to using other, more evidence-based interventions for bipolar depression) depend on a number of variables, including establishing whether the index episode was manic or hypomanic; ruling out current subthreshold mixed features; and clarifying how recently mania developed. Decisions about future antidepressant use (or avoidance) might be less clear if an index manic/hypomanic episode was brief and self-limited once the antidepressant was stopped.
Although some experts eschew antidepressant monotherapy after such occurrences, there is no body of literature to inform decisions about the safety or efficacy of undertaking a future antidepressant trial in such patients. That said, reasonable judgment probably includes several considerations:
• Re-exposure to the same antidepressant that was associated with an induction of mania is likely riskier than choosing a different antidepressant; in general, purely serotonergic antidepressants or bupropion are considered to pose less risk of mood destabilization than is seen with an SNRI or tricyclic antidepressant.
• After a manic episode, a subsequent antidepressant trial generally shouldn’t be attempted without concurrent anti-manic medication.
• Introducing any antidepressant is probably ill-advised in the recent (~2 months) aftermath of acute manic/ hypomanic symptoms.22
• Patients and their significant other should be apprised of the risk of emerging symptoms of mania or hypomania, or mixed features, and should be familiar with key target symptoms to watch for. Prospective mood charting can be helpful.
• Patients should be monitored closely both for an exacerbation of depression and recurrence of mania/hypomania symptoms.
• Any antidepressant should be discontinued promptly at the first sign of psychomotor acceleration or the emergence of mixed features, as defined by DSM-5.
Psychoeducation and forecasting
Functional recovery from a manic episode can lag behind symptomatic recovery. Subsyndromal symptoms often persist after a full episode subsides.
Mania often is followed by a depressive episode, and questions inevitably arise about how to prevent and treat these episodes. Because the median duration of a manic episode is approximately 13 weeks,23 it is crucial for patients and their immediate family to recognize that recovery might be gradual, and that it will likely take time before she (he) can resume full-time responsibilities at work or school or in the home.
Today, a patient who is hospitalized for severe acute mania (as Ms. J was, in the case vignette) seldom remains an inpatient long enough to achieve remission of symptoms; sometimes, she (he) might continue to manifest significant symptoms, even though decisions about the “medical necessity” of ongoing inpatient care tend to be governed mainly by issues of safety and imminent danger. (This web exclusive Table20,24,25 provides considerations when making the transition from the acute phase to the continuation phase of treatment.)
To minimize risk of relapse, psycho-education should include discussion of:
• psychiatrically deleterious effects of alcohol and illicit drug use
• suicide risk, including what to do in an emergency
• protecting a regular sleep schedule and avoiding sleep deprivation
• the potential for poor medication adherence and management of side effects
• the need for periodic laboratory monitoring, as needed
• the role of adjunctive psychotherapy and effective stress management
• familiarity with symptoms that serve as warning signs, and how to monitor their onset.
Bottom Line
When a patient being treated for depression develops signs of mania or hypomania, stop any antidepressant and consider initiating a mood stabilizer, antipsychotic, or both, to contain and stabilize symptoms. Entertain medical and substance-related causes of mania symptoms, and evaluate and treat as suggested by the patient’s presentation. Long-term drug therapy to prevent recurrence of mania/hypomania, as well as risks and benefits of future exposure to antidepressants, should be decided case by case.
Related Resources
• Proudfoot J, Whitton A, Parker G, et al. Triggers of mania and depression in young adults with bipolar disorder. J Affect Disord. 2012;143(1-3):196-202.
• Stange JP, Sylvia LG, Magalhães PV, et al. Extreme attributions predict transition from depression to mania or hypomania in bipolar disorder. J Psychiatr Res. 2013;47(10):1329-1336.
Drug Brand Names
Albuterol • Proventil, Ventolin
Anastrozole • Arimidex
Aripiprazole • Abilify
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Chloroquine • Aralen
Ciprofloxacin • Cipro
Clarithromycin • Biaxin
Clomiphene • Clomid
Digoxin • Digox, Lanoxin
Divalproex • Depakote
5-Fluorouracil • Carac, Efudex
Human chorionic gonadotropin • Novarel, Pregnyl
Ifosfamide • Ifex
Isoniazid • Nydrazid
Lamotrigine • Lamictal
Letrozole • Femara
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Mefloquine • Lariam
Olanzapine • Zyprexa
Olanzapine/fluoxetine combination • Symbyax
ramipexole • Mirapex
Procarbazine • Matulane
Quetiapine • Seroquel
Ropinirole • Requip
Rotigotine • Neupro
Venlafaxine • Effexor
Zidovudine • Retrovir
Disclosures
Dr. Goldberg is a consultant to Merck & Co. and Sunovion. He is a member of the speakers’ bureau of AstraZeneca, Janssen, Merck & Co., Takeda and Lundbeck, and Sunovion.
Dr. Ernst reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
1. Goldberg JF, Garno JL, Callahan AM, et al. Overdiagnosis of bipolar disorder among substance use disorder in patients with mood instability. J Clin Psychiatry. 2008;69(11):1751-1757.
2. Rosa AR, Cruz B, Franco C, et al. Why do clinicians maintain antidepressants in some patients with acute mania? Hints from the European Mania in Bipolar Longitudinal Evaluation of Medication (EMBLEM), a large naturalistic study. J Clin Psychiatry. 2010;71(8):1000-1006.
3. Goldberg JF, Perlis RH, Ghaemi SN, et al. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry. 2007;164(9):1348-1355.
4. Bowers MB Jr, McKay BG, Mazure CM. Discontinuation of antidepressants in newly admitted psychotic patients. J Neuropsychiatr Clin Neurosci. 2003;15(2):227-230.
5. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509-516.
6. Geller B, Zimmerman B, Williams M, et al. Bipolar disorder at prospective follow-up of adults who had prepubertal major depressive disorder. Am J Psychiatry. 2001;158(1):125-127.
7. Goldberg JF, Harrow M, Whiteside JE. Risk for bipolar illness in patients initially hospitalized for unipolar depression. Am J Psychiatry. 2001;158(8):1265-1270.
8. Yatham LN, Kauer-Sant’Anna M, Bond DJ, et al. Course and outcome after the first manic episode in patients with bipolar disorder: prospective 12-month data from the Systematic Treatment Optimization Project for Early Mania project. Can J Psychiatry. 2009;54(2):105-112.
9. Chaudron LH, Pies RW. The relationship between postpartum psychosis and bipolar disorder: a review. J Clin Psychiatry 2003;64(11):1284-1292.
10. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356(17):1711-1722.
11. Tohen M, Frank E, Bowden CL, et al. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord. 2009;11(15):453-473.
12. Schulze TG, Hedeker D, Zandi P, et al. What is familial about familial bipolar disorder? Resemblance among relatives across a broad spectrum of phenotypic characteristics. Arch Gen Psychiatry. 2006;63(12):1368-1376.
13. Song J, Bergen SE, Kuja-Halkola R, et al. Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disord. 2015;7(2):184-193.
14. Goes FS, Sadler B, Toolan J, et al. Psychotic features in bipolar and unipolar depression. Bipolar Disord. 2007;9(8):901-906.
15. Fiedorowicz JG, Endicott J, Solomon DA, et al. Course of illness following prospectively observed mania or hypomania in individuals presenting with unipolar depression. Bipolar Disord. 2007;14(6):664-671.
16. Tansey KE, Guipponi M, Domenici E, et al. Genetic susceptibility for bipolar disorder and response to antidepressants in major depressive disorder. Am J Med Genetics B Neuropsychiatr Genet. 2014;165B(1):77-83.
17. Tohen M, Zarate CA Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160(12):2099-2107.
18. Suppes T, Dennehy EB, Swann AC, et al. Report of the Texas Consensus Conference Panel on medication treatment of bipolar disorder 2000. J Clin Psychiatry. 2002;63(4):288-299.
19. Keck PE Jr, Perlis RH, Otto MW, et al. The Expert Consensus Guideline Series: treatment of bipolar disorder 2004. Postgrad Med Special Report. 2004:1-120.
20. Goldberg JF, Ghaemi SN. Benefits and limitations of antidepressants and traditional mood stabilizers for treatment of bipolar depression. Bipolar Disord. 2005;7(suppl 5):3-12.
21. Sidor MM, MacQueen GM. Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry. 2011;72(2):156-167.
22. MacQueen GM, Trevor Young L, Marriott M, et al. Previous mood state predicts response and switch rates in patients with bipolar depression. Acta Psychiatr Scand. 2002;105(6):414-418.
23. Solomon DA, Leon AC, Coryell WH, et al. Longitudinal course of bipolar I disorder: duration of mood episodes. Arch Gen Psychiatry. 2010;67(4):339-347.
24. Tohen M, Chengappa KN, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br J Psychiatry. 2004;184:337-345.
25. Suppes T, Vieta E, Liu S, et al. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476-488.
What does molecular imaging reveal about the causes of ADHD and the potential for better management?
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common pediatric psychiatric disorders, occurring in approximately 5% of children.1 The disorder persists into adulthood in about one-half of those who are affected in childhood.2 In adults and children, diagnosis continues to be based on the examiner’s subjective assessment. (Box 13-9 describes how ADHD presents a complicated, moving target for the diagnostician.)
Patients who have ADHD are rarely studied with imaging; there are no established imaging findings associated with an ADHD diagnosis. Over the past 20 years, however, significant research has shown that molecular alterations along the dopaminergic−frontostriatal pathways occur in association with the behavioral constellation of ADHD symptoms—suggesting a pathophysiologic mechanism for this disorder.
In this article, we describe molecular findings from nuclear medicine imaging in ADHD. We also summarize imaging evidence for dysfunction of the dopaminergic-frontostriatal neural circuits as central in the pathophysiology of ADHD, with special focus on the dopamine reuptake transporter (DaT). Box 210,11 reviews our key observations and looks at the future of imaging in the management of ADHD.
Dopaminergic theory of ADHD
The executive functions that are disordered in ADHD (impulse control, judgment, maintaining attention) are thought to be centered in the infraorbital, dorsolateral, and medial frontal lobes. Neurotransmitters that have been implicated in the pathophysiology of ADHD include norepinephrine12 and dopamine13; medications that selectively block reuptake of these neurotransmitters are used to treat ADHD.14,15 Only the dopamine system has been extensively evaluated with molecular imaging techniques.
Because methylphenidate, a potent selective dopamine reuptake inhibitor, has been shown to reduce disordered executive functional behaviors in ADHD, considerable imaging research has focused on the dopaminergic neural circuits in the frontostriatal regions of the brain. The dopaminergic theory of ADHD is based on the hypothesis that alterations in the density or function of these circuits are responsible for behaviors that constitute ADHD.
Despite decades of efforts to delineate the underlying pathophysiology and neurochemistry of ADHD, no single unifying theory accounts for all imaging findings in all patients. This might be in part because of imprecision inherent in psychiatric diagnoses that are based on subjective observations. The behavioral criteria for ADHD can manifest in several disorders. For example, anxiety-related symptoms seen in posttraumatic stress disorder, social anxiety disorder, and panic disorder also present as behaviors similar to those in ADHD diagnostic criteria.
Molecular imaging might provide a window into the underlying pathophysiology of ADHD and, by identifying objective findings, (1) allow for patient stratification based on underlying physiologic subtypes, (2) refine diagnostic criteria, and (3) predict treatment response.
Nuclear medicine findings
In general, nuclear medicine investigations of ADHD can be divided into studies of changes in regional cerebral blood flow (rCBF) or glucose metabolism (rCGM) and those that have assessed the concentration of synaptic structures, using highly specific radiolabeled ligands. Both kinds of studies provide limited anatomic resolution, unless co-registered with MRI or CT scans and either single photon emission computed tomography (SPECT) or positron emission tomography (PET).
Synaptic imaging using radiolabeled ligands with high biologic specificity for synaptic structures has high molecular resolution—that is, radiolabeled ligands used for selective imaging of the dopamine transporter or receptor do not identify serotonin transporters or receptors, and vice versa. (Details of SPECT and PET techniques are beyond the scope of this article but can be found in standard nuclear medicine textbooks.)
SPECT and PET of rCBF
Early investigations of rCBF in ADHD were performed using inhaled radioactive xenon-133 gas.16 Later, rCBF was assessed using fat-soluble radiolabeled ligands that rapidly distribute in the brain in proportion to blood flow by crossing the blood−brain barrier. Labeled with radioactive 99m-technetium, these ligands cross rapidly into brain cells after IV injection. Once intracellular, covalent bonds within the ligands cleave into 2 charged particles that do not easily recross the cell membrane. There is little redistribution of tracer after initial uptake.
The imaging data set that results can be reconstructed as (1) surface images, on which defects indicate areas of reduced rCBF, or (2) tomographic slices on which color scales indicate relative rCBF values (Figure 1). Because of the minimal redistribution of the tracer, SPECT images obtained 1 or 2 hours after injection provide a snapshot of rCBF at the time tracer is injected. Patients can be injected under various conditions, such as at rest with eyes and ears open in a dimly lit, quiet room, and then under cognitive stress (Figure 2), such as performing a computer-based attention and impulse control task, or during stimulant treatment.
Numerous investigators have found reduced frontal or striatal rCBF, or both, in patients with ADHD, unilaterally on the right17 or left,18,19 or bilaterally.20 Additionally, with stimulant therapy, normalization of striatal and frontal rCBF has been demonstrated14,19—changes that correlate with resolution of behavioral symptoms of ADHD with stimulant treatment.21
SPECT of 32 boys with previously untreated ADHD. Kim et al21 found that the presence of reduced right or left, or both, frontal rCBF, which normalized with 8 weeks of stimulant therapy, predicted symptom improvement in 85% of patients. Absence of improvement of reduced frontal rCBF had a 75% negative predictive value for treatment response. (Additionally, hyperperfusion of the somatosensory cortex has been demonstrated in children with ADHD,16,22 suggesting increased responsiveness to extraneous environmental input.)
SPECT of 40 untreated pediatric patients compared with 17 age-matched controls. Using SPECT, Lee et al23 reported rCBF reductions in the orbitofrontal cortex and the medial temporal gyrus of participants; reductions corresponded to areas of motor and impulsivity control. The researchers also demonstrated increased rCBF in the somatosensory area.
After methylphenidate treatment, blood flow to these areas normalized, and rCBF to higher visual and superior prefrontal areas decreased. Substantial clinical improvement occurred in 64% of patients—suggesting methylphenidate treatment of ADHD works by (1) increasing function of areas of the brain that control impulses, motor activity, and attention, and (2) reducing function to sensory areas that lead to distraction by extraneous environmental sensory input.
O-15-labeled water PET of 10 adults with ADHD. Schweitzer et al24 found that participants who demonstrated improvement in behavioral symptoms with chronic stimulant therapy had reduced rCBF in the striata at baseline—again, suggesting that baseline hypometabolism in the striata is associated with ADHD.
PET of regional cerebral glucose metabolism
Cerebral metabolism requires a constant supply of glucose; regional differences in cerebral glucose metabolism can be assessed directly with positron-emitting F-18-fluoro-2-deoxyglucose. Although metabolically inert, this agent is transported intracellularly similar to glucose; once phosphorylated within brain cells, however, it can no longer undergo further metabolism or redistribution.
Studies using PET to assess rCGM were some of the earliest molecular imaging applications in ADHD. Zametkin et al25 reported low global cerebral glucose utilization in adults, but not adolescents,26 with ADHD. However, further study, with normalization of the PET data, confirmed reduced rCGM in the left prefrontal cortex in both adolescents26 and adults,27 indicating hypometabolism of cortical areas associated with impulse control and attention in ADHD. In adolescents, symptom severity was inversely related to rCBF in the left anterior frontal cortex.
Synaptic imaging
Nuclear imaging has been used to study several components of the striatal dopaminergic synapse, including:
• dopamine substrates, using fluorine- 18-labeled dopa or carbon-11-labeled dopa
• dopamine receptors, using carbon- 11-labeled raclopride or iodine-123 iodobenzamide
• the tDaT, using iodine-123 ioflupane, 99m-technetium TRODAT, or carbon-11 cocaine (Figure 3).
All of these synaptic imaging agents were used mainly as research tools until 2011, when the FDA approved the SPECT imaging agent iodine-123 ioflupane (DaTscan) for clinical use in assessment of Parkinson’s disease.28 This commercially available agent has high specificity for the DaT, with little background activity noted on SPECT imaging (Figure 4).
Dopamine transporter imaging
Because the site of action of methylphenidate is the DaT, imaging this component of the striatal dopaminergic synapse has been an area of intense investigation in ADHD. Located almost exclusively in the striata, DaT reduces synaptic concentrations of dopamine by means of reuptake channels in the cell membrane.29 By reversibly binding to, and occupying sites on, the DaT, methylphenidate impedes dopamine reuptake, which results in increased availability of dopamine at the synapse.30
By demonstrating an increase in striatal DaT density in patients with ADHD— first reported by Dougherty et al31 using iodine-123 altropane (a dopaminergic uptake inhibitor) in 6 adults with ADHD—investigators have hypothesized that excessive expression of the DaT protein in the striata, which may result from genetic or environmental factors, is a central causative agent of ADHD.32 Subsequent studies, however, have yielded contradictory findings: Hesse et al,33 using SPECT imaging, and Volkow et al,34 using carbon-11 cocaine PET imaging, found reduced DaT density in, respectively, 9 and 26 patients with ADHD.
To clarify the role of DaT levels in the etiology of ADHD and to explain discrepant results, Fusar-Poli et al35 performed a meta-analysis of 9 published papers that reported the results of DaT imaging in a total of 169 ADHD patients and 129 controls. They noted that these studies included 6 different imaging agents and protocols. Patients were stimulant therapy-naïve (n = 137) or drug-free (refrained from stimulant therapy for a time [n = 32]). The team found that the degree of elevation of the striatal DaT concentration correlated with a history of stimulant exposure, and that the drug-naïve group had a reduced DaT level.
Fusar-Poli’s hypothesis? Elevated DaT levels result from up-regulation in the presence of chronic methylphenidate therapy, which accounts for early reports that demonstrated increased striatal DaT density. Clinically, up-regulation might explain the lack of sustained relief of behavioral symptoms with stimulant therapy in 20% of patients with ADHD who showed clinical improvement initially.36
Only limited conclusions can be drawn about the role of DaT levels in ADHD, given the small number of patients studied in published reports. In addition, the Fusar-Poli meta-analysis has come under strong criticism because of methodological errors with improper patient inclusion and characterization of treatment status,37 calling into question the investigators’ conclusions.
Does the DaT level hold promise for practice? Despite a lack of clarity about the significance of DaT level in the etiology of ADHD, knowledge of a patient’s level might prove useful in predicting which patients will respond to methylphenidate. Namely, several researchers have found that:
• an elevated baseline level of DaT (before stimulant therapy) correlates with robust clinical response
• absence of an elevated baseline DaT level suggests that symptomatic improvement with stimulant therapy in unlikely.38-40
Dresel et al38 evaluated 17 drug-naïve adults, newly diagnosed with ADHD, using 99m-technetium TRODAT SPECT before and after methylphenidate therapy. They found a 15% increase in specific DaT binding in patients with ADHD, compared with controls, at baseline. After treatment, the researchers observed a 28% reduction in specific DaT binding—a significant change from baseline that correlated with behavioral response.
Study: SPECT in 18 adults with ADHD given methylphenidate. Krause39 used the same SPECT agent to study 18 adults before they received methylphenidate and 10 weeks after treatment. Participants were categorized as responders or nonresponders based on clinical assessment of ADHD symptoms after those 10 weeks. All 12 responders had an elevated striatal DaT concentration at baseline. Of the 6 nonresponders, 5 had a normal level of striatal DaT compared with age-matched controls.
Study: 22 Adult ADHD patients evaluated with 99m-technetium TRODAT SPECT. The same group of investigators40 presented imaging findings in 22 additional adult patients. Seventeen had an elevated striatal DaT level, 16 of whom responded to stimulant therapy. The remaining 5 patients had reduced striatal DaT at baseline; none had a good clinical response to methylphenidate.
The positive clinical response to methylphenidate in 67%37 and 77%40 of patients is in good agreement with results from larger studies, which reported that approximately 75% of patients with ADHD show prompt clinical improvement with stimulants.41 Improvement might be related to an increase in functioning of the frontostriatal dopaminergic circuit that is seen with stimulant therapy. Increased availability of dopamine at the synapse, resulting from stimulant blockade of the dopamine reuptake transporter, produces increased dopamine neurotransmission and increased activation of frontostriatal circuits.
In another study, rCBF in frontostriatal circuits was determined to be inversely proportional to DaT density; rCBF normalized with stimulant therapy.42
Will imaging pave the way for therapeutic stratification? Baseline determinations of striatal DaT concentration with SPECT imaging might make it possible to stratify patients with ADHD symptoms into those likely to show significant behavioral symptom response to methylphenidate and those who are not likely to respond. There might be an objective imaging finding—striatal DaT density—that allows clinicians to distinguish stimulant-responsive ADHD from stimulant-unresponsive ADHD.
Dopamine substrate imaging
Radiolabeled dopa (carbon-11 or fluorine-18) is transported into presynaptic dopaminergic neurons in the striatum, where it is decarboxylated, converted to radio-dopamine, and stored within vesicles until released in response to neuronal excitation. Semi-quantitative assessment is achieved with calculation of specific (striatal) to nonspecific (background) uptake ratios. Increased values are thought to indicate increased density of dopaminergic neurons.43
Ernst et al44 reported a 50% decrease in specific fluorine-18 dopa uptake in the left prefrontal cortex in 17 drug-naïve adults with ADHD, compared with 23 controls. The same team reported increased midbrain fluorine-18 dopa levels in 10 adolescents with ADHD—48% higher, overall, than what was seen in 10 controls.43 They hypothesized that these opposite results were the results of a reduction in the dopaminergic neuronal density in adults, which might be part of the natural history of ADHD, or a normal age-related reduction in neuronal density, or both. Increased dopa levels in the team’s adolescent group were hypothesized to reflect up-regulation in dopamine synthesis due to low synaptic dopamine concentrations that might result from increased dopamine reuptake.
Dopamine-receptor imaging
The 5 distinct dopamine receptors (D1, D2, D3, D4, and D5) can be grouped into 2 subtypes, based on their coupling with G proteins. D1 and D5 constitute a group; D2, D3, and D4, a second group.
The D1 receptor is the most common dopamine receptor in the brain and is widely distributed in the striatum and prefrontal cerebral cortex. D1 receptor knockout mice demonstrate hyperactivity and poorer performance on learning tasks and are used as an animal model for ADHD.45 D1 has been imaged using C-11 SCH 23390 PET46 in rats, but its role in ADHD has yet to be evaluated. D5 is the most recently cloned and most widely distributed of the known dopamine receptors; however, there are no imaging studies of the D5 receptor.13
D2 receptors are present in presynaptic and postsynaptic neurons47 in the neocortex, substantia nigra, nucleus accumbens, and olfactory tubercle, as well as in other structures.48 Presynaptic D2 receptors act as autoregulators, inhibiting dopaminergic synthesis, firing rate, and release.49
Using C-11 raclopride PET imaging, Lou et al50 reported high D2/3 receptor availability in adolescents who had a history of perinatal cerebral ischemia. They found that this availability is associated with an increase in the severity of ADHD symptoms. They proposed that the increase in “empty” receptor density might have been caused by perinatal ischemia-induced presynaptic dopaminergic neuronal loss or an increase in presynaptic dopamine reuptake (Figure 550). Either mechanism could result in up-regulation in postsynaptic D2/3 receptors.
Volkow et al51 reported that D2 receptor density correlated with methylphenidate-induced changes in rCBF in frontal and temporal lobes in humans. They postulated that the variable therapeutic effects of methylphenidate seen in ADHD patients might be related to variations in baseline D2 receptor availability.
Lou et al50 reported elevated D2 receptor density, demonstrated using carbon-11 raclopride, in children with ADHD, compared with normal adults.
Further support for a relationship between D2-receptor density and symptomatic improvement with methylphenidate in ADHD was presented by Ilgin et al52 using iodine-123 iodobenzamide SPECT. They found elevated D2 receptor levels in 9 drug-naïve children with ADHD, which is 20% to 60% above what is seen in unaffected children. They noted that these patients showed improvement in hyperactivity when treated with methylphenidate.
In a similar study of 20 drug-naïve adults, Volkow et al53 found that durable symptomatic improvement with methylphenidate therapy was associated with increased D2 receptor availability.
Summing up
Striatal DaT is the most likely synaptic target for stratifying patients with ADHD, now that a dopamine transporter imaging agent is available commercially. Stratification might allow for refinement in the diagnostic categorization of ADHD, with introduction of stimulant-responsive and stimulant-unresponsive subtypes that are based on DaT imaging findings.
Bottom Line
Given recent advances showing molecular alterations in the dopaminergic-frontostriatal pathway as central to attention-deficit/hyperactivity disorder, molecular imaging might be useful as an objective study for diagnosis.
Related Resources
• Schweitzer JB, Lee DO, Hanford RB, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28(5):967-973.
• Raz A. Brain imaging data of ADHD. Psychiatric Times. http://www.psychiatrictimes.com/adhd/brain-imaging-data-adhd.
Drug Brand Names
Iodine-123 ioflupane • Methylphenidate • Ritalin DaTscan
Acknowledgment
Kylee M. L. Unsdorfer, a medical student at Northeast Ohio Medical University, helped prepare the manuscript of this article.
Disclosures
Dr. Thacker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Binkovitz received 4 doses of ioflupane I123I (DaTscan) from General Electric for investigator-initiated research, used for animal imaging in 2012.
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Berger I. Diagnosis of attention deficit hyperactivity disorder: much ado about something. Isr Med Assoc J. 2011;13(9):571-574.
5. Schonwald A, Lechner E. Attention deficit/hyperactivity disorder: complexities and controversies. Curr Opin Pediatr. 2006;18(2):189-195.
6. Rousseau C, Measham T, Bathiche-Suidan M. DSM IV, culture and child psychiatry. J Can Acad Child Adolesc Psychiatry. 2008;17(2):69-75.
7. Taylor-Klaus E. Bringing the ADHD debate into sharper focus: part 1. The Huffington Post. http:// www.huffingtonpost.com/elaine-taylorklaus/adhd-debate_b_4571097.html. Updated March 17, 2014. Accessed August 18, 2015.
8. Sweeney CT, Sembower MA, Ertischek MD, et al. Nonmedical use of prescription ADHD stimulants and preexisting patterns of drug abuse. J Addict Dis. 2013;32(1):1-10.
9. Hitt E. Multiple reports of ADHD drug shortages. Medscape. http://www.medscape.com/viewarticle/742686. Published May 13, 2011. Accessed June 4, 2015.
10. Rubia K, Alegria AA, Cubillo AI, et al. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76(8):616-628.
11. Cortese S, Kelly C, Chabernaud C, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169(10):1038-1055.
12. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
13. Wu J, Xiao H, Sun H, et al. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012; 45(3):605-620.
14. Del Campo N, Chamberlain SR, Sahakian BJ, et al. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145-e157.
15. Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e101-e111.
16. Lou HC, Henriksen L, Bruhn P. Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Arch Neurol. 1984;41(8):825-829.
17. Gustafsson P, Thernlund G, Ryding E, et al. Associations between cerebral blood-flow measured by single photon emission computed tomography (SPECT), electro-encephalogram (EEG), behaviour symptoms, cognition and neurological soft signs in children with attention-deficit hyperactivity disorder (ADHD). Acta Paediatr. 2000;89(7):830-835.
18. Sieg KG, Gaffney GR, Preston DF, et al. SPECT brain imaging abnormalities in attention deficit hyperactivity disorder. Clin Nucl Med. 1995;20(1):55-60.
19. Spalletta G, Pasini A, Pau F, et al. Prefrontal blood flow dysregulation in drug naive ADHD children without structural abnormalities. J Neural Transm. 2001;108(10):1203-1216.
20. Amen DG, Carmichael BD. High-resolution brain SPECT imaging in ADHD. Ann Clin Psychiatry. 1997;9(2):81-86.
21. Kim BN, Lee JS, Cho SC, et al. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Med J. 2001;42(1):19-29.
22. Lou HC, Henriksen L, Bruhn P, et al. Striatal dysfunction in attention deficit and hyperkinetic disorder. Arch Neurol. 1989;46(1):48-52.
23. Lee JS, Kim BN, Kang E, et al. Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum Brain Mapp. 2005;24(3):157-164.
24. Schweitzer JB, Lee DO, Hanford RB, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28(5):967-973.
25. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323(20):1361-1366.
26. Zametkin AJ, Liebenauer LL, Fitzgerald GA, et al. Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1993;50(5):333-340.
27. Ernst M, Zametkin AJ, Matochik JA, et al. Effects of intravenous dextroamphetamine on brain metabolism in adults with attention-deficit hyperactivity disorder (ADHD). Preliminary findings. Psychopharmacol Bull. 1994;30(2):219-225.
28. Janssen M. Dopamine transporter (DaT) SPECT imaging. MI Gateway. 2012;6(1):1-3. http://interactive.snm.org/ docs/MI_Gateway_Newsletter_2012-1%20Dopamine%20 Transporter%20SPECT%20Imaging.pdf. Accessed August 18, 2015.
29. Volkow ND, Wang GJ, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325-1331.
30. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121.
31. Dougherty DD, Bonab AA, Spencer TJ, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354(9196):2132-2133.
32. Li JJ, Lee SS. Interaction of dopamine transporter gene and observed parenting behaviors on attention-deficit/ hyperactivity disorder: a structural equation modeling approach. J Clin Child Adolesc Psychol. 2013;42(2):174-186.
33. Hesse S, Ballaschke O, Barthel H, et al. Dopamine transporter imaging in adult patients with attention-deficit/ hyperactivity disorder. Psychiatry Res. 2009;171(2):120-128.
34. Volkow ND, Wang GJ, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084-1091.
35. Fusar-Poli P, Rubia K, Rossi G, et al. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry. 2012;169(3):264-272.
36. Wang GJ, Volkow ND, Wigal T, et al. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One. 2013;8(5):e63023.
37. Spencer TJ, Madras BK, Fischman AJ, et al. Striatal dopamine transporter binding in adults with ADHD. Am J Psychiatry. 2012;169(6):665; author reply 666.
38. Dresel S, Krause J, Krause KH, et al. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27(10):1518-1524.
39. Krause J, la Fougere C, Krause KH, et al. Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):428-431.
40. la Fougère C, Krause J, Krause KH, et al. Value of 99mTc-TRODAT-1 SPECT to predict clinical response to methylphenidate treatment in adults with attention deficit hyperactivity disorder. Nucl Med Commun. 2006;27(9):733-737.
41. MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics. 2004;113(4):754-761.
42. da Silva N Jr, Szobot CM, Anselmi CE, et al. Attention deficit/hyperactivity disorder: is there a correlation between dopamine transporter density and cerebral blood flow? Clin Nucl Med. 2011;36(8):656-660.
43. Ernst M, Zametkin AJ, Matochik JA, et al. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156(8):1209-1215.
44. Ernst M, Zametkin AJ, Matochik JA, et al. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18(15):5901-5907.
45. Xu M, Moratalla R, Gold LH, et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79(4):729-742.
46. Goodwin RJ, Mackay CL, Nilsson A, et al. Qualitative and quantitative MALDI imaging of the positron emission tomography ligands raclopride (a D2 dopamine antagonist) and SCH 23390 (a D1 dopamine antagonist) in rat brain tissue sections using a solvent-free dry matrix application method. Anal Chem. 2011;83(24):9694-9701.
47. Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488(4):464-475.
48. Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6(11):3177-3188.
49. Doi M, Yujnovsky I, Hirayama J, et al. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006;9(6):732-734.
50. Lou HC, Rosa P, Pryds O, et al. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev Med Child Neurol. 2004;46(3):179-183.
51. Volkow ND, Wang GJ, Fowler JS, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154(1):50-55.
52. Ilgin N, Senol S, Gucuyener K, et al. Is increased D2 receptor availability associated with response to stimulant medication in ADHD. Dev Med Child Neurol. 2001;43(11):755-760.
53. Volkow ND, Wang GJ, Tomasi D, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841-849.
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common pediatric psychiatric disorders, occurring in approximately 5% of children.1 The disorder persists into adulthood in about one-half of those who are affected in childhood.2 In adults and children, diagnosis continues to be based on the examiner’s subjective assessment. (Box 13-9 describes how ADHD presents a complicated, moving target for the diagnostician.)
Patients who have ADHD are rarely studied with imaging; there are no established imaging findings associated with an ADHD diagnosis. Over the past 20 years, however, significant research has shown that molecular alterations along the dopaminergic−frontostriatal pathways occur in association with the behavioral constellation of ADHD symptoms—suggesting a pathophysiologic mechanism for this disorder.
In this article, we describe molecular findings from nuclear medicine imaging in ADHD. We also summarize imaging evidence for dysfunction of the dopaminergic-frontostriatal neural circuits as central in the pathophysiology of ADHD, with special focus on the dopamine reuptake transporter (DaT). Box 210,11 reviews our key observations and looks at the future of imaging in the management of ADHD.
Dopaminergic theory of ADHD
The executive functions that are disordered in ADHD (impulse control, judgment, maintaining attention) are thought to be centered in the infraorbital, dorsolateral, and medial frontal lobes. Neurotransmitters that have been implicated in the pathophysiology of ADHD include norepinephrine12 and dopamine13; medications that selectively block reuptake of these neurotransmitters are used to treat ADHD.14,15 Only the dopamine system has been extensively evaluated with molecular imaging techniques.
Because methylphenidate, a potent selective dopamine reuptake inhibitor, has been shown to reduce disordered executive functional behaviors in ADHD, considerable imaging research has focused on the dopaminergic neural circuits in the frontostriatal regions of the brain. The dopaminergic theory of ADHD is based on the hypothesis that alterations in the density or function of these circuits are responsible for behaviors that constitute ADHD.
Despite decades of efforts to delineate the underlying pathophysiology and neurochemistry of ADHD, no single unifying theory accounts for all imaging findings in all patients. This might be in part because of imprecision inherent in psychiatric diagnoses that are based on subjective observations. The behavioral criteria for ADHD can manifest in several disorders. For example, anxiety-related symptoms seen in posttraumatic stress disorder, social anxiety disorder, and panic disorder also present as behaviors similar to those in ADHD diagnostic criteria.
Molecular imaging might provide a window into the underlying pathophysiology of ADHD and, by identifying objective findings, (1) allow for patient stratification based on underlying physiologic subtypes, (2) refine diagnostic criteria, and (3) predict treatment response.
Nuclear medicine findings
In general, nuclear medicine investigations of ADHD can be divided into studies of changes in regional cerebral blood flow (rCBF) or glucose metabolism (rCGM) and those that have assessed the concentration of synaptic structures, using highly specific radiolabeled ligands. Both kinds of studies provide limited anatomic resolution, unless co-registered with MRI or CT scans and either single photon emission computed tomography (SPECT) or positron emission tomography (PET).
Synaptic imaging using radiolabeled ligands with high biologic specificity for synaptic structures has high molecular resolution—that is, radiolabeled ligands used for selective imaging of the dopamine transporter or receptor do not identify serotonin transporters or receptors, and vice versa. (Details of SPECT and PET techniques are beyond the scope of this article but can be found in standard nuclear medicine textbooks.)
SPECT and PET of rCBF
Early investigations of rCBF in ADHD were performed using inhaled radioactive xenon-133 gas.16 Later, rCBF was assessed using fat-soluble radiolabeled ligands that rapidly distribute in the brain in proportion to blood flow by crossing the blood−brain barrier. Labeled with radioactive 99m-technetium, these ligands cross rapidly into brain cells after IV injection. Once intracellular, covalent bonds within the ligands cleave into 2 charged particles that do not easily recross the cell membrane. There is little redistribution of tracer after initial uptake.
The imaging data set that results can be reconstructed as (1) surface images, on which defects indicate areas of reduced rCBF, or (2) tomographic slices on which color scales indicate relative rCBF values (Figure 1). Because of the minimal redistribution of the tracer, SPECT images obtained 1 or 2 hours after injection provide a snapshot of rCBF at the time tracer is injected. Patients can be injected under various conditions, such as at rest with eyes and ears open in a dimly lit, quiet room, and then under cognitive stress (Figure 2), such as performing a computer-based attention and impulse control task, or during stimulant treatment.
Numerous investigators have found reduced frontal or striatal rCBF, or both, in patients with ADHD, unilaterally on the right17 or left,18,19 or bilaterally.20 Additionally, with stimulant therapy, normalization of striatal and frontal rCBF has been demonstrated14,19—changes that correlate with resolution of behavioral symptoms of ADHD with stimulant treatment.21
SPECT of 32 boys with previously untreated ADHD. Kim et al21 found that the presence of reduced right or left, or both, frontal rCBF, which normalized with 8 weeks of stimulant therapy, predicted symptom improvement in 85% of patients. Absence of improvement of reduced frontal rCBF had a 75% negative predictive value for treatment response. (Additionally, hyperperfusion of the somatosensory cortex has been demonstrated in children with ADHD,16,22 suggesting increased responsiveness to extraneous environmental input.)
SPECT of 40 untreated pediatric patients compared with 17 age-matched controls. Using SPECT, Lee et al23 reported rCBF reductions in the orbitofrontal cortex and the medial temporal gyrus of participants; reductions corresponded to areas of motor and impulsivity control. The researchers also demonstrated increased rCBF in the somatosensory area.
After methylphenidate treatment, blood flow to these areas normalized, and rCBF to higher visual and superior prefrontal areas decreased. Substantial clinical improvement occurred in 64% of patients—suggesting methylphenidate treatment of ADHD works by (1) increasing function of areas of the brain that control impulses, motor activity, and attention, and (2) reducing function to sensory areas that lead to distraction by extraneous environmental sensory input.
O-15-labeled water PET of 10 adults with ADHD. Schweitzer et al24 found that participants who demonstrated improvement in behavioral symptoms with chronic stimulant therapy had reduced rCBF in the striata at baseline—again, suggesting that baseline hypometabolism in the striata is associated with ADHD.
PET of regional cerebral glucose metabolism
Cerebral metabolism requires a constant supply of glucose; regional differences in cerebral glucose metabolism can be assessed directly with positron-emitting F-18-fluoro-2-deoxyglucose. Although metabolically inert, this agent is transported intracellularly similar to glucose; once phosphorylated within brain cells, however, it can no longer undergo further metabolism or redistribution.
Studies using PET to assess rCGM were some of the earliest molecular imaging applications in ADHD. Zametkin et al25 reported low global cerebral glucose utilization in adults, but not adolescents,26 with ADHD. However, further study, with normalization of the PET data, confirmed reduced rCGM in the left prefrontal cortex in both adolescents26 and adults,27 indicating hypometabolism of cortical areas associated with impulse control and attention in ADHD. In adolescents, symptom severity was inversely related to rCBF in the left anterior frontal cortex.
Synaptic imaging
Nuclear imaging has been used to study several components of the striatal dopaminergic synapse, including:
• dopamine substrates, using fluorine- 18-labeled dopa or carbon-11-labeled dopa
• dopamine receptors, using carbon- 11-labeled raclopride or iodine-123 iodobenzamide
• the tDaT, using iodine-123 ioflupane, 99m-technetium TRODAT, or carbon-11 cocaine (Figure 3).
All of these synaptic imaging agents were used mainly as research tools until 2011, when the FDA approved the SPECT imaging agent iodine-123 ioflupane (DaTscan) for clinical use in assessment of Parkinson’s disease.28 This commercially available agent has high specificity for the DaT, with little background activity noted on SPECT imaging (Figure 4).
Dopamine transporter imaging
Because the site of action of methylphenidate is the DaT, imaging this component of the striatal dopaminergic synapse has been an area of intense investigation in ADHD. Located almost exclusively in the striata, DaT reduces synaptic concentrations of dopamine by means of reuptake channels in the cell membrane.29 By reversibly binding to, and occupying sites on, the DaT, methylphenidate impedes dopamine reuptake, which results in increased availability of dopamine at the synapse.30
By demonstrating an increase in striatal DaT density in patients with ADHD— first reported by Dougherty et al31 using iodine-123 altropane (a dopaminergic uptake inhibitor) in 6 adults with ADHD—investigators have hypothesized that excessive expression of the DaT protein in the striata, which may result from genetic or environmental factors, is a central causative agent of ADHD.32 Subsequent studies, however, have yielded contradictory findings: Hesse et al,33 using SPECT imaging, and Volkow et al,34 using carbon-11 cocaine PET imaging, found reduced DaT density in, respectively, 9 and 26 patients with ADHD.
To clarify the role of DaT levels in the etiology of ADHD and to explain discrepant results, Fusar-Poli et al35 performed a meta-analysis of 9 published papers that reported the results of DaT imaging in a total of 169 ADHD patients and 129 controls. They noted that these studies included 6 different imaging agents and protocols. Patients were stimulant therapy-naïve (n = 137) or drug-free (refrained from stimulant therapy for a time [n = 32]). The team found that the degree of elevation of the striatal DaT concentration correlated with a history of stimulant exposure, and that the drug-naïve group had a reduced DaT level.
Fusar-Poli’s hypothesis? Elevated DaT levels result from up-regulation in the presence of chronic methylphenidate therapy, which accounts for early reports that demonstrated increased striatal DaT density. Clinically, up-regulation might explain the lack of sustained relief of behavioral symptoms with stimulant therapy in 20% of patients with ADHD who showed clinical improvement initially.36
Only limited conclusions can be drawn about the role of DaT levels in ADHD, given the small number of patients studied in published reports. In addition, the Fusar-Poli meta-analysis has come under strong criticism because of methodological errors with improper patient inclusion and characterization of treatment status,37 calling into question the investigators’ conclusions.
Does the DaT level hold promise for practice? Despite a lack of clarity about the significance of DaT level in the etiology of ADHD, knowledge of a patient’s level might prove useful in predicting which patients will respond to methylphenidate. Namely, several researchers have found that:
• an elevated baseline level of DaT (before stimulant therapy) correlates with robust clinical response
• absence of an elevated baseline DaT level suggests that symptomatic improvement with stimulant therapy in unlikely.38-40
Dresel et al38 evaluated 17 drug-naïve adults, newly diagnosed with ADHD, using 99m-technetium TRODAT SPECT before and after methylphenidate therapy. They found a 15% increase in specific DaT binding in patients with ADHD, compared with controls, at baseline. After treatment, the researchers observed a 28% reduction in specific DaT binding—a significant change from baseline that correlated with behavioral response.
Study: SPECT in 18 adults with ADHD given methylphenidate. Krause39 used the same SPECT agent to study 18 adults before they received methylphenidate and 10 weeks after treatment. Participants were categorized as responders or nonresponders based on clinical assessment of ADHD symptoms after those 10 weeks. All 12 responders had an elevated striatal DaT concentration at baseline. Of the 6 nonresponders, 5 had a normal level of striatal DaT compared with age-matched controls.
Study: 22 Adult ADHD patients evaluated with 99m-technetium TRODAT SPECT. The same group of investigators40 presented imaging findings in 22 additional adult patients. Seventeen had an elevated striatal DaT level, 16 of whom responded to stimulant therapy. The remaining 5 patients had reduced striatal DaT at baseline; none had a good clinical response to methylphenidate.
The positive clinical response to methylphenidate in 67%37 and 77%40 of patients is in good agreement with results from larger studies, which reported that approximately 75% of patients with ADHD show prompt clinical improvement with stimulants.41 Improvement might be related to an increase in functioning of the frontostriatal dopaminergic circuit that is seen with stimulant therapy. Increased availability of dopamine at the synapse, resulting from stimulant blockade of the dopamine reuptake transporter, produces increased dopamine neurotransmission and increased activation of frontostriatal circuits.
In another study, rCBF in frontostriatal circuits was determined to be inversely proportional to DaT density; rCBF normalized with stimulant therapy.42
Will imaging pave the way for therapeutic stratification? Baseline determinations of striatal DaT concentration with SPECT imaging might make it possible to stratify patients with ADHD symptoms into those likely to show significant behavioral symptom response to methylphenidate and those who are not likely to respond. There might be an objective imaging finding—striatal DaT density—that allows clinicians to distinguish stimulant-responsive ADHD from stimulant-unresponsive ADHD.
Dopamine substrate imaging
Radiolabeled dopa (carbon-11 or fluorine-18) is transported into presynaptic dopaminergic neurons in the striatum, where it is decarboxylated, converted to radio-dopamine, and stored within vesicles until released in response to neuronal excitation. Semi-quantitative assessment is achieved with calculation of specific (striatal) to nonspecific (background) uptake ratios. Increased values are thought to indicate increased density of dopaminergic neurons.43
Ernst et al44 reported a 50% decrease in specific fluorine-18 dopa uptake in the left prefrontal cortex in 17 drug-naïve adults with ADHD, compared with 23 controls. The same team reported increased midbrain fluorine-18 dopa levels in 10 adolescents with ADHD—48% higher, overall, than what was seen in 10 controls.43 They hypothesized that these opposite results were the results of a reduction in the dopaminergic neuronal density in adults, which might be part of the natural history of ADHD, or a normal age-related reduction in neuronal density, or both. Increased dopa levels in the team’s adolescent group were hypothesized to reflect up-regulation in dopamine synthesis due to low synaptic dopamine concentrations that might result from increased dopamine reuptake.
Dopamine-receptor imaging
The 5 distinct dopamine receptors (D1, D2, D3, D4, and D5) can be grouped into 2 subtypes, based on their coupling with G proteins. D1 and D5 constitute a group; D2, D3, and D4, a second group.
The D1 receptor is the most common dopamine receptor in the brain and is widely distributed in the striatum and prefrontal cerebral cortex. D1 receptor knockout mice demonstrate hyperactivity and poorer performance on learning tasks and are used as an animal model for ADHD.45 D1 has been imaged using C-11 SCH 23390 PET46 in rats, but its role in ADHD has yet to be evaluated. D5 is the most recently cloned and most widely distributed of the known dopamine receptors; however, there are no imaging studies of the D5 receptor.13
D2 receptors are present in presynaptic and postsynaptic neurons47 in the neocortex, substantia nigra, nucleus accumbens, and olfactory tubercle, as well as in other structures.48 Presynaptic D2 receptors act as autoregulators, inhibiting dopaminergic synthesis, firing rate, and release.49
Using C-11 raclopride PET imaging, Lou et al50 reported high D2/3 receptor availability in adolescents who had a history of perinatal cerebral ischemia. They found that this availability is associated with an increase in the severity of ADHD symptoms. They proposed that the increase in “empty” receptor density might have been caused by perinatal ischemia-induced presynaptic dopaminergic neuronal loss or an increase in presynaptic dopamine reuptake (Figure 550). Either mechanism could result in up-regulation in postsynaptic D2/3 receptors.
Volkow et al51 reported that D2 receptor density correlated with methylphenidate-induced changes in rCBF in frontal and temporal lobes in humans. They postulated that the variable therapeutic effects of methylphenidate seen in ADHD patients might be related to variations in baseline D2 receptor availability.
Lou et al50 reported elevated D2 receptor density, demonstrated using carbon-11 raclopride, in children with ADHD, compared with normal adults.
Further support for a relationship between D2-receptor density and symptomatic improvement with methylphenidate in ADHD was presented by Ilgin et al52 using iodine-123 iodobenzamide SPECT. They found elevated D2 receptor levels in 9 drug-naïve children with ADHD, which is 20% to 60% above what is seen in unaffected children. They noted that these patients showed improvement in hyperactivity when treated with methylphenidate.
In a similar study of 20 drug-naïve adults, Volkow et al53 found that durable symptomatic improvement with methylphenidate therapy was associated with increased D2 receptor availability.
Summing up
Striatal DaT is the most likely synaptic target for stratifying patients with ADHD, now that a dopamine transporter imaging agent is available commercially. Stratification might allow for refinement in the diagnostic categorization of ADHD, with introduction of stimulant-responsive and stimulant-unresponsive subtypes that are based on DaT imaging findings.
Bottom Line
Given recent advances showing molecular alterations in the dopaminergic-frontostriatal pathway as central to attention-deficit/hyperactivity disorder, molecular imaging might be useful as an objective study for diagnosis.
Related Resources
• Schweitzer JB, Lee DO, Hanford RB, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28(5):967-973.
• Raz A. Brain imaging data of ADHD. Psychiatric Times. http://www.psychiatrictimes.com/adhd/brain-imaging-data-adhd.
Drug Brand Names
Iodine-123 ioflupane • Methylphenidate • Ritalin DaTscan
Acknowledgment
Kylee M. L. Unsdorfer, a medical student at Northeast Ohio Medical University, helped prepare the manuscript of this article.
Disclosures
Dr. Thacker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Binkovitz received 4 doses of ioflupane I123I (DaTscan) from General Electric for investigator-initiated research, used for animal imaging in 2012.
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common pediatric psychiatric disorders, occurring in approximately 5% of children.1 The disorder persists into adulthood in about one-half of those who are affected in childhood.2 In adults and children, diagnosis continues to be based on the examiner’s subjective assessment. (Box 13-9 describes how ADHD presents a complicated, moving target for the diagnostician.)
Patients who have ADHD are rarely studied with imaging; there are no established imaging findings associated with an ADHD diagnosis. Over the past 20 years, however, significant research has shown that molecular alterations along the dopaminergic−frontostriatal pathways occur in association with the behavioral constellation of ADHD symptoms—suggesting a pathophysiologic mechanism for this disorder.
In this article, we describe molecular findings from nuclear medicine imaging in ADHD. We also summarize imaging evidence for dysfunction of the dopaminergic-frontostriatal neural circuits as central in the pathophysiology of ADHD, with special focus on the dopamine reuptake transporter (DaT). Box 210,11 reviews our key observations and looks at the future of imaging in the management of ADHD.
Dopaminergic theory of ADHD
The executive functions that are disordered in ADHD (impulse control, judgment, maintaining attention) are thought to be centered in the infraorbital, dorsolateral, and medial frontal lobes. Neurotransmitters that have been implicated in the pathophysiology of ADHD include norepinephrine12 and dopamine13; medications that selectively block reuptake of these neurotransmitters are used to treat ADHD.14,15 Only the dopamine system has been extensively evaluated with molecular imaging techniques.
Because methylphenidate, a potent selective dopamine reuptake inhibitor, has been shown to reduce disordered executive functional behaviors in ADHD, considerable imaging research has focused on the dopaminergic neural circuits in the frontostriatal regions of the brain. The dopaminergic theory of ADHD is based on the hypothesis that alterations in the density or function of these circuits are responsible for behaviors that constitute ADHD.
Despite decades of efforts to delineate the underlying pathophysiology and neurochemistry of ADHD, no single unifying theory accounts for all imaging findings in all patients. This might be in part because of imprecision inherent in psychiatric diagnoses that are based on subjective observations. The behavioral criteria for ADHD can manifest in several disorders. For example, anxiety-related symptoms seen in posttraumatic stress disorder, social anxiety disorder, and panic disorder also present as behaviors similar to those in ADHD diagnostic criteria.
Molecular imaging might provide a window into the underlying pathophysiology of ADHD and, by identifying objective findings, (1) allow for patient stratification based on underlying physiologic subtypes, (2) refine diagnostic criteria, and (3) predict treatment response.
Nuclear medicine findings
In general, nuclear medicine investigations of ADHD can be divided into studies of changes in regional cerebral blood flow (rCBF) or glucose metabolism (rCGM) and those that have assessed the concentration of synaptic structures, using highly specific radiolabeled ligands. Both kinds of studies provide limited anatomic resolution, unless co-registered with MRI or CT scans and either single photon emission computed tomography (SPECT) or positron emission tomography (PET).
Synaptic imaging using radiolabeled ligands with high biologic specificity for synaptic structures has high molecular resolution—that is, radiolabeled ligands used for selective imaging of the dopamine transporter or receptor do not identify serotonin transporters or receptors, and vice versa. (Details of SPECT and PET techniques are beyond the scope of this article but can be found in standard nuclear medicine textbooks.)
SPECT and PET of rCBF
Early investigations of rCBF in ADHD were performed using inhaled radioactive xenon-133 gas.16 Later, rCBF was assessed using fat-soluble radiolabeled ligands that rapidly distribute in the brain in proportion to blood flow by crossing the blood−brain barrier. Labeled with radioactive 99m-technetium, these ligands cross rapidly into brain cells after IV injection. Once intracellular, covalent bonds within the ligands cleave into 2 charged particles that do not easily recross the cell membrane. There is little redistribution of tracer after initial uptake.
The imaging data set that results can be reconstructed as (1) surface images, on which defects indicate areas of reduced rCBF, or (2) tomographic slices on which color scales indicate relative rCBF values (Figure 1). Because of the minimal redistribution of the tracer, SPECT images obtained 1 or 2 hours after injection provide a snapshot of rCBF at the time tracer is injected. Patients can be injected under various conditions, such as at rest with eyes and ears open in a dimly lit, quiet room, and then under cognitive stress (Figure 2), such as performing a computer-based attention and impulse control task, or during stimulant treatment.
Numerous investigators have found reduced frontal or striatal rCBF, or both, in patients with ADHD, unilaterally on the right17 or left,18,19 or bilaterally.20 Additionally, with stimulant therapy, normalization of striatal and frontal rCBF has been demonstrated14,19—changes that correlate with resolution of behavioral symptoms of ADHD with stimulant treatment.21
SPECT of 32 boys with previously untreated ADHD. Kim et al21 found that the presence of reduced right or left, or both, frontal rCBF, which normalized with 8 weeks of stimulant therapy, predicted symptom improvement in 85% of patients. Absence of improvement of reduced frontal rCBF had a 75% negative predictive value for treatment response. (Additionally, hyperperfusion of the somatosensory cortex has been demonstrated in children with ADHD,16,22 suggesting increased responsiveness to extraneous environmental input.)
SPECT of 40 untreated pediatric patients compared with 17 age-matched controls. Using SPECT, Lee et al23 reported rCBF reductions in the orbitofrontal cortex and the medial temporal gyrus of participants; reductions corresponded to areas of motor and impulsivity control. The researchers also demonstrated increased rCBF in the somatosensory area.
After methylphenidate treatment, blood flow to these areas normalized, and rCBF to higher visual and superior prefrontal areas decreased. Substantial clinical improvement occurred in 64% of patients—suggesting methylphenidate treatment of ADHD works by (1) increasing function of areas of the brain that control impulses, motor activity, and attention, and (2) reducing function to sensory areas that lead to distraction by extraneous environmental sensory input.
O-15-labeled water PET of 10 adults with ADHD. Schweitzer et al24 found that participants who demonstrated improvement in behavioral symptoms with chronic stimulant therapy had reduced rCBF in the striata at baseline—again, suggesting that baseline hypometabolism in the striata is associated with ADHD.
PET of regional cerebral glucose metabolism
Cerebral metabolism requires a constant supply of glucose; regional differences in cerebral glucose metabolism can be assessed directly with positron-emitting F-18-fluoro-2-deoxyglucose. Although metabolically inert, this agent is transported intracellularly similar to glucose; once phosphorylated within brain cells, however, it can no longer undergo further metabolism or redistribution.
Studies using PET to assess rCGM were some of the earliest molecular imaging applications in ADHD. Zametkin et al25 reported low global cerebral glucose utilization in adults, but not adolescents,26 with ADHD. However, further study, with normalization of the PET data, confirmed reduced rCGM in the left prefrontal cortex in both adolescents26 and adults,27 indicating hypometabolism of cortical areas associated with impulse control and attention in ADHD. In adolescents, symptom severity was inversely related to rCBF in the left anterior frontal cortex.
Synaptic imaging
Nuclear imaging has been used to study several components of the striatal dopaminergic synapse, including:
• dopamine substrates, using fluorine- 18-labeled dopa or carbon-11-labeled dopa
• dopamine receptors, using carbon- 11-labeled raclopride or iodine-123 iodobenzamide
• the tDaT, using iodine-123 ioflupane, 99m-technetium TRODAT, or carbon-11 cocaine (Figure 3).
All of these synaptic imaging agents were used mainly as research tools until 2011, when the FDA approved the SPECT imaging agent iodine-123 ioflupane (DaTscan) for clinical use in assessment of Parkinson’s disease.28 This commercially available agent has high specificity for the DaT, with little background activity noted on SPECT imaging (Figure 4).
Dopamine transporter imaging
Because the site of action of methylphenidate is the DaT, imaging this component of the striatal dopaminergic synapse has been an area of intense investigation in ADHD. Located almost exclusively in the striata, DaT reduces synaptic concentrations of dopamine by means of reuptake channels in the cell membrane.29 By reversibly binding to, and occupying sites on, the DaT, methylphenidate impedes dopamine reuptake, which results in increased availability of dopamine at the synapse.30
By demonstrating an increase in striatal DaT density in patients with ADHD— first reported by Dougherty et al31 using iodine-123 altropane (a dopaminergic uptake inhibitor) in 6 adults with ADHD—investigators have hypothesized that excessive expression of the DaT protein in the striata, which may result from genetic or environmental factors, is a central causative agent of ADHD.32 Subsequent studies, however, have yielded contradictory findings: Hesse et al,33 using SPECT imaging, and Volkow et al,34 using carbon-11 cocaine PET imaging, found reduced DaT density in, respectively, 9 and 26 patients with ADHD.
To clarify the role of DaT levels in the etiology of ADHD and to explain discrepant results, Fusar-Poli et al35 performed a meta-analysis of 9 published papers that reported the results of DaT imaging in a total of 169 ADHD patients and 129 controls. They noted that these studies included 6 different imaging agents and protocols. Patients were stimulant therapy-naïve (n = 137) or drug-free (refrained from stimulant therapy for a time [n = 32]). The team found that the degree of elevation of the striatal DaT concentration correlated with a history of stimulant exposure, and that the drug-naïve group had a reduced DaT level.
Fusar-Poli’s hypothesis? Elevated DaT levels result from up-regulation in the presence of chronic methylphenidate therapy, which accounts for early reports that demonstrated increased striatal DaT density. Clinically, up-regulation might explain the lack of sustained relief of behavioral symptoms with stimulant therapy in 20% of patients with ADHD who showed clinical improvement initially.36
Only limited conclusions can be drawn about the role of DaT levels in ADHD, given the small number of patients studied in published reports. In addition, the Fusar-Poli meta-analysis has come under strong criticism because of methodological errors with improper patient inclusion and characterization of treatment status,37 calling into question the investigators’ conclusions.
Does the DaT level hold promise for practice? Despite a lack of clarity about the significance of DaT level in the etiology of ADHD, knowledge of a patient’s level might prove useful in predicting which patients will respond to methylphenidate. Namely, several researchers have found that:
• an elevated baseline level of DaT (before stimulant therapy) correlates with robust clinical response
• absence of an elevated baseline DaT level suggests that symptomatic improvement with stimulant therapy in unlikely.38-40
Dresel et al38 evaluated 17 drug-naïve adults, newly diagnosed with ADHD, using 99m-technetium TRODAT SPECT before and after methylphenidate therapy. They found a 15% increase in specific DaT binding in patients with ADHD, compared with controls, at baseline. After treatment, the researchers observed a 28% reduction in specific DaT binding—a significant change from baseline that correlated with behavioral response.
Study: SPECT in 18 adults with ADHD given methylphenidate. Krause39 used the same SPECT agent to study 18 adults before they received methylphenidate and 10 weeks after treatment. Participants were categorized as responders or nonresponders based on clinical assessment of ADHD symptoms after those 10 weeks. All 12 responders had an elevated striatal DaT concentration at baseline. Of the 6 nonresponders, 5 had a normal level of striatal DaT compared with age-matched controls.
Study: 22 Adult ADHD patients evaluated with 99m-technetium TRODAT SPECT. The same group of investigators40 presented imaging findings in 22 additional adult patients. Seventeen had an elevated striatal DaT level, 16 of whom responded to stimulant therapy. The remaining 5 patients had reduced striatal DaT at baseline; none had a good clinical response to methylphenidate.
The positive clinical response to methylphenidate in 67%37 and 77%40 of patients is in good agreement with results from larger studies, which reported that approximately 75% of patients with ADHD show prompt clinical improvement with stimulants.41 Improvement might be related to an increase in functioning of the frontostriatal dopaminergic circuit that is seen with stimulant therapy. Increased availability of dopamine at the synapse, resulting from stimulant blockade of the dopamine reuptake transporter, produces increased dopamine neurotransmission and increased activation of frontostriatal circuits.
In another study, rCBF in frontostriatal circuits was determined to be inversely proportional to DaT density; rCBF normalized with stimulant therapy.42
Will imaging pave the way for therapeutic stratification? Baseline determinations of striatal DaT concentration with SPECT imaging might make it possible to stratify patients with ADHD symptoms into those likely to show significant behavioral symptom response to methylphenidate and those who are not likely to respond. There might be an objective imaging finding—striatal DaT density—that allows clinicians to distinguish stimulant-responsive ADHD from stimulant-unresponsive ADHD.
Dopamine substrate imaging
Radiolabeled dopa (carbon-11 or fluorine-18) is transported into presynaptic dopaminergic neurons in the striatum, where it is decarboxylated, converted to radio-dopamine, and stored within vesicles until released in response to neuronal excitation. Semi-quantitative assessment is achieved with calculation of specific (striatal) to nonspecific (background) uptake ratios. Increased values are thought to indicate increased density of dopaminergic neurons.43
Ernst et al44 reported a 50% decrease in specific fluorine-18 dopa uptake in the left prefrontal cortex in 17 drug-naïve adults with ADHD, compared with 23 controls. The same team reported increased midbrain fluorine-18 dopa levels in 10 adolescents with ADHD—48% higher, overall, than what was seen in 10 controls.43 They hypothesized that these opposite results were the results of a reduction in the dopaminergic neuronal density in adults, which might be part of the natural history of ADHD, or a normal age-related reduction in neuronal density, or both. Increased dopa levels in the team’s adolescent group were hypothesized to reflect up-regulation in dopamine synthesis due to low synaptic dopamine concentrations that might result from increased dopamine reuptake.
Dopamine-receptor imaging
The 5 distinct dopamine receptors (D1, D2, D3, D4, and D5) can be grouped into 2 subtypes, based on their coupling with G proteins. D1 and D5 constitute a group; D2, D3, and D4, a second group.
The D1 receptor is the most common dopamine receptor in the brain and is widely distributed in the striatum and prefrontal cerebral cortex. D1 receptor knockout mice demonstrate hyperactivity and poorer performance on learning tasks and are used as an animal model for ADHD.45 D1 has been imaged using C-11 SCH 23390 PET46 in rats, but its role in ADHD has yet to be evaluated. D5 is the most recently cloned and most widely distributed of the known dopamine receptors; however, there are no imaging studies of the D5 receptor.13
D2 receptors are present in presynaptic and postsynaptic neurons47 in the neocortex, substantia nigra, nucleus accumbens, and olfactory tubercle, as well as in other structures.48 Presynaptic D2 receptors act as autoregulators, inhibiting dopaminergic synthesis, firing rate, and release.49
Using C-11 raclopride PET imaging, Lou et al50 reported high D2/3 receptor availability in adolescents who had a history of perinatal cerebral ischemia. They found that this availability is associated with an increase in the severity of ADHD symptoms. They proposed that the increase in “empty” receptor density might have been caused by perinatal ischemia-induced presynaptic dopaminergic neuronal loss or an increase in presynaptic dopamine reuptake (Figure 550). Either mechanism could result in up-regulation in postsynaptic D2/3 receptors.
Volkow et al51 reported that D2 receptor density correlated with methylphenidate-induced changes in rCBF in frontal and temporal lobes in humans. They postulated that the variable therapeutic effects of methylphenidate seen in ADHD patients might be related to variations in baseline D2 receptor availability.
Lou et al50 reported elevated D2 receptor density, demonstrated using carbon-11 raclopride, in children with ADHD, compared with normal adults.
Further support for a relationship between D2-receptor density and symptomatic improvement with methylphenidate in ADHD was presented by Ilgin et al52 using iodine-123 iodobenzamide SPECT. They found elevated D2 receptor levels in 9 drug-naïve children with ADHD, which is 20% to 60% above what is seen in unaffected children. They noted that these patients showed improvement in hyperactivity when treated with methylphenidate.
In a similar study of 20 drug-naïve adults, Volkow et al53 found that durable symptomatic improvement with methylphenidate therapy was associated with increased D2 receptor availability.
Summing up
Striatal DaT is the most likely synaptic target for stratifying patients with ADHD, now that a dopamine transporter imaging agent is available commercially. Stratification might allow for refinement in the diagnostic categorization of ADHD, with introduction of stimulant-responsive and stimulant-unresponsive subtypes that are based on DaT imaging findings.
Bottom Line
Given recent advances showing molecular alterations in the dopaminergic-frontostriatal pathway as central to attention-deficit/hyperactivity disorder, molecular imaging might be useful as an objective study for diagnosis.
Related Resources
• Schweitzer JB, Lee DO, Hanford RB, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28(5):967-973.
• Raz A. Brain imaging data of ADHD. Psychiatric Times. http://www.psychiatrictimes.com/adhd/brain-imaging-data-adhd.
Drug Brand Names
Iodine-123 ioflupane • Methylphenidate • Ritalin DaTscan
Acknowledgment
Kylee M. L. Unsdorfer, a medical student at Northeast Ohio Medical University, helped prepare the manuscript of this article.
Disclosures
Dr. Thacker reports no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Binkovitz received 4 doses of ioflupane I123I (DaTscan) from General Electric for investigator-initiated research, used for animal imaging in 2012.
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Berger I. Diagnosis of attention deficit hyperactivity disorder: much ado about something. Isr Med Assoc J. 2011;13(9):571-574.
5. Schonwald A, Lechner E. Attention deficit/hyperactivity disorder: complexities and controversies. Curr Opin Pediatr. 2006;18(2):189-195.
6. Rousseau C, Measham T, Bathiche-Suidan M. DSM IV, culture and child psychiatry. J Can Acad Child Adolesc Psychiatry. 2008;17(2):69-75.
7. Taylor-Klaus E. Bringing the ADHD debate into sharper focus: part 1. The Huffington Post. http:// www.huffingtonpost.com/elaine-taylorklaus/adhd-debate_b_4571097.html. Updated March 17, 2014. Accessed August 18, 2015.
8. Sweeney CT, Sembower MA, Ertischek MD, et al. Nonmedical use of prescription ADHD stimulants and preexisting patterns of drug abuse. J Addict Dis. 2013;32(1):1-10.
9. Hitt E. Multiple reports of ADHD drug shortages. Medscape. http://www.medscape.com/viewarticle/742686. Published May 13, 2011. Accessed June 4, 2015.
10. Rubia K, Alegria AA, Cubillo AI, et al. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76(8):616-628.
11. Cortese S, Kelly C, Chabernaud C, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169(10):1038-1055.
12. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
13. Wu J, Xiao H, Sun H, et al. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012; 45(3):605-620.
14. Del Campo N, Chamberlain SR, Sahakian BJ, et al. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145-e157.
15. Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e101-e111.
16. Lou HC, Henriksen L, Bruhn P. Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Arch Neurol. 1984;41(8):825-829.
17. Gustafsson P, Thernlund G, Ryding E, et al. Associations between cerebral blood-flow measured by single photon emission computed tomography (SPECT), electro-encephalogram (EEG), behaviour symptoms, cognition and neurological soft signs in children with attention-deficit hyperactivity disorder (ADHD). Acta Paediatr. 2000;89(7):830-835.
18. Sieg KG, Gaffney GR, Preston DF, et al. SPECT brain imaging abnormalities in attention deficit hyperactivity disorder. Clin Nucl Med. 1995;20(1):55-60.
19. Spalletta G, Pasini A, Pau F, et al. Prefrontal blood flow dysregulation in drug naive ADHD children without structural abnormalities. J Neural Transm. 2001;108(10):1203-1216.
20. Amen DG, Carmichael BD. High-resolution brain SPECT imaging in ADHD. Ann Clin Psychiatry. 1997;9(2):81-86.
21. Kim BN, Lee JS, Cho SC, et al. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Med J. 2001;42(1):19-29.
22. Lou HC, Henriksen L, Bruhn P, et al. Striatal dysfunction in attention deficit and hyperkinetic disorder. Arch Neurol. 1989;46(1):48-52.
23. Lee JS, Kim BN, Kang E, et al. Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum Brain Mapp. 2005;24(3):157-164.
24. Schweitzer JB, Lee DO, Hanford RB, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28(5):967-973.
25. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323(20):1361-1366.
26. Zametkin AJ, Liebenauer LL, Fitzgerald GA, et al. Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1993;50(5):333-340.
27. Ernst M, Zametkin AJ, Matochik JA, et al. Effects of intravenous dextroamphetamine on brain metabolism in adults with attention-deficit hyperactivity disorder (ADHD). Preliminary findings. Psychopharmacol Bull. 1994;30(2):219-225.
28. Janssen M. Dopamine transporter (DaT) SPECT imaging. MI Gateway. 2012;6(1):1-3. http://interactive.snm.org/ docs/MI_Gateway_Newsletter_2012-1%20Dopamine%20 Transporter%20SPECT%20Imaging.pdf. Accessed August 18, 2015.
29. Volkow ND, Wang GJ, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325-1331.
30. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121.
31. Dougherty DD, Bonab AA, Spencer TJ, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354(9196):2132-2133.
32. Li JJ, Lee SS. Interaction of dopamine transporter gene and observed parenting behaviors on attention-deficit/ hyperactivity disorder: a structural equation modeling approach. J Clin Child Adolesc Psychol. 2013;42(2):174-186.
33. Hesse S, Ballaschke O, Barthel H, et al. Dopamine transporter imaging in adult patients with attention-deficit/ hyperactivity disorder. Psychiatry Res. 2009;171(2):120-128.
34. Volkow ND, Wang GJ, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084-1091.
35. Fusar-Poli P, Rubia K, Rossi G, et al. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry. 2012;169(3):264-272.
36. Wang GJ, Volkow ND, Wigal T, et al. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One. 2013;8(5):e63023.
37. Spencer TJ, Madras BK, Fischman AJ, et al. Striatal dopamine transporter binding in adults with ADHD. Am J Psychiatry. 2012;169(6):665; author reply 666.
38. Dresel S, Krause J, Krause KH, et al. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27(10):1518-1524.
39. Krause J, la Fougere C, Krause KH, et al. Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):428-431.
40. la Fougère C, Krause J, Krause KH, et al. Value of 99mTc-TRODAT-1 SPECT to predict clinical response to methylphenidate treatment in adults with attention deficit hyperactivity disorder. Nucl Med Commun. 2006;27(9):733-737.
41. MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics. 2004;113(4):754-761.
42. da Silva N Jr, Szobot CM, Anselmi CE, et al. Attention deficit/hyperactivity disorder: is there a correlation between dopamine transporter density and cerebral blood flow? Clin Nucl Med. 2011;36(8):656-660.
43. Ernst M, Zametkin AJ, Matochik JA, et al. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156(8):1209-1215.
44. Ernst M, Zametkin AJ, Matochik JA, et al. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18(15):5901-5907.
45. Xu M, Moratalla R, Gold LH, et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79(4):729-742.
46. Goodwin RJ, Mackay CL, Nilsson A, et al. Qualitative and quantitative MALDI imaging of the positron emission tomography ligands raclopride (a D2 dopamine antagonist) and SCH 23390 (a D1 dopamine antagonist) in rat brain tissue sections using a solvent-free dry matrix application method. Anal Chem. 2011;83(24):9694-9701.
47. Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488(4):464-475.
48. Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6(11):3177-3188.
49. Doi M, Yujnovsky I, Hirayama J, et al. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006;9(6):732-734.
50. Lou HC, Rosa P, Pryds O, et al. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev Med Child Neurol. 2004;46(3):179-183.
51. Volkow ND, Wang GJ, Fowler JS, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154(1):50-55.
52. Ilgin N, Senol S, Gucuyener K, et al. Is increased D2 receptor availability associated with response to stimulant medication in ADHD. Dev Med Child Neurol. 2001;43(11):755-760.
53. Volkow ND, Wang GJ, Tomasi D, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841-849.
1. Polanczyk G, de Lima MS, Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164(6):942-948.
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211.
3. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
4. Berger I. Diagnosis of attention deficit hyperactivity disorder: much ado about something. Isr Med Assoc J. 2011;13(9):571-574.
5. Schonwald A, Lechner E. Attention deficit/hyperactivity disorder: complexities and controversies. Curr Opin Pediatr. 2006;18(2):189-195.
6. Rousseau C, Measham T, Bathiche-Suidan M. DSM IV, culture and child psychiatry. J Can Acad Child Adolesc Psychiatry. 2008;17(2):69-75.
7. Taylor-Klaus E. Bringing the ADHD debate into sharper focus: part 1. The Huffington Post. http:// www.huffingtonpost.com/elaine-taylorklaus/adhd-debate_b_4571097.html. Updated March 17, 2014. Accessed August 18, 2015.
8. Sweeney CT, Sembower MA, Ertischek MD, et al. Nonmedical use of prescription ADHD stimulants and preexisting patterns of drug abuse. J Addict Dis. 2013;32(1):1-10.
9. Hitt E. Multiple reports of ADHD drug shortages. Medscape. http://www.medscape.com/viewarticle/742686. Published May 13, 2011. Accessed June 4, 2015.
10. Rubia K, Alegria AA, Cubillo AI, et al. Effects of stimulants on brain function in attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Biol Psychiatry. 2014;76(8):616-628.
11. Cortese S, Kelly C, Chabernaud C, et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. Am J Psychiatry. 2012;169(10):1038-1055.
12. Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11(3):203-226.
13. Wu J, Xiao H, Sun H, et al. Role of dopamine receptors in ADHD: a systematic meta-analysis. Mol Neurobiol. 2012; 45(3):605-620.
14. Del Campo N, Chamberlain SR, Sahakian BJ, et al. The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e145-e157.
15. Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69(12):e101-e111.
16. Lou HC, Henriksen L, Bruhn P. Focal cerebral hypoperfusion in children with dysphasia and/or attention deficit disorder. Arch Neurol. 1984;41(8):825-829.
17. Gustafsson P, Thernlund G, Ryding E, et al. Associations between cerebral blood-flow measured by single photon emission computed tomography (SPECT), electro-encephalogram (EEG), behaviour symptoms, cognition and neurological soft signs in children with attention-deficit hyperactivity disorder (ADHD). Acta Paediatr. 2000;89(7):830-835.
18. Sieg KG, Gaffney GR, Preston DF, et al. SPECT brain imaging abnormalities in attention deficit hyperactivity disorder. Clin Nucl Med. 1995;20(1):55-60.
19. Spalletta G, Pasini A, Pau F, et al. Prefrontal blood flow dysregulation in drug naive ADHD children without structural abnormalities. J Neural Transm. 2001;108(10):1203-1216.
20. Amen DG, Carmichael BD. High-resolution brain SPECT imaging in ADHD. Ann Clin Psychiatry. 1997;9(2):81-86.
21. Kim BN, Lee JS, Cho SC, et al. Methylphenidate increased regional cerebral blood flow in subjects with attention deficit/hyperactivity disorder. Yonsei Med J. 2001;42(1):19-29.
22. Lou HC, Henriksen L, Bruhn P, et al. Striatal dysfunction in attention deficit and hyperkinetic disorder. Arch Neurol. 1989;46(1):48-52.
23. Lee JS, Kim BN, Kang E, et al. Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum Brain Mapp. 2005;24(3):157-164.
24. Schweitzer JB, Lee DO, Hanford RB, et al. A positron emission tomography study of methylphenidate in adults with ADHD: alterations in resting blood flow and predicting treatment response. Neuropsychopharmacology. 2003;28(5):967-973.
25. Zametkin AJ, Nordahl TE, Gross M, et al. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323(20):1361-1366.
26. Zametkin AJ, Liebenauer LL, Fitzgerald GA, et al. Brain metabolism in teenagers with attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1993;50(5):333-340.
27. Ernst M, Zametkin AJ, Matochik JA, et al. Effects of intravenous dextroamphetamine on brain metabolism in adults with attention-deficit hyperactivity disorder (ADHD). Preliminary findings. Psychopharmacol Bull. 1994;30(2):219-225.
28. Janssen M. Dopamine transporter (DaT) SPECT imaging. MI Gateway. 2012;6(1):1-3. http://interactive.snm.org/ docs/MI_Gateway_Newsletter_2012-1%20Dopamine%20 Transporter%20SPECT%20Imaging.pdf. Accessed August 18, 2015.
29. Volkow ND, Wang GJ, Fowler JS, et al. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry. 1998;155(10):1325-1331.
30. Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121.
31. Dougherty DD, Bonab AA, Spencer TJ, et al. Dopamine transporter density in patients with attention deficit hyperactivity disorder. Lancet. 1999;354(9196):2132-2133.
32. Li JJ, Lee SS. Interaction of dopamine transporter gene and observed parenting behaviors on attention-deficit/ hyperactivity disorder: a structural equation modeling approach. J Clin Child Adolesc Psychol. 2013;42(2):174-186.
33. Hesse S, Ballaschke O, Barthel H, et al. Dopamine transporter imaging in adult patients with attention-deficit/ hyperactivity disorder. Psychiatry Res. 2009;171(2):120-128.
34. Volkow ND, Wang GJ, Kollins SH, et al. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA. 2009;302(10):1084-1091.
35. Fusar-Poli P, Rubia K, Rossi G, et al. Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry. 2012;169(3):264-272.
36. Wang GJ, Volkow ND, Wigal T, et al. Long-term stimulant treatment affects brain dopamine transporter level in patients with attention deficit hyperactive disorder. PLoS One. 2013;8(5):e63023.
37. Spencer TJ, Madras BK, Fischman AJ, et al. Striatal dopamine transporter binding in adults with ADHD. Am J Psychiatry. 2012;169(6):665; author reply 666.
38. Dresel S, Krause J, Krause KH, et al. Attention deficit hyperactivity disorder: binding of [99mTc]TRODAT-1 to the dopamine transporter before and after methylphenidate treatment. Eur J Nucl Med. 2000;27(10):1518-1524.
39. Krause J, la Fougere C, Krause KH, et al. Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):428-431.
40. la Fougère C, Krause J, Krause KH, et al. Value of 99mTc-TRODAT-1 SPECT to predict clinical response to methylphenidate treatment in adults with attention deficit hyperactivity disorder. Nucl Med Commun. 2006;27(9):733-737.
41. MTA Cooperative Group. National Institute of Mental Health Multimodal Treatment Study of ADHD follow-up: 24-month outcomes of treatment strategies for attention-deficit/hyperactivity disorder. Pediatrics. 2004;113(4):754-761.
42. da Silva N Jr, Szobot CM, Anselmi CE, et al. Attention deficit/hyperactivity disorder: is there a correlation between dopamine transporter density and cerebral blood flow? Clin Nucl Med. 2011;36(8):656-660.
43. Ernst M, Zametkin AJ, Matochik JA, et al. High midbrain [18F]DOPA accumulation in children with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156(8):1209-1215.
44. Ernst M, Zametkin AJ, Matochik JA, et al. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [fluorine-18]fluorodopa positron emission tomographic study. J Neurosci. 1998;18(15):5901-5907.
45. Xu M, Moratalla R, Gold LH, et al. Dopamine D1 receptor mutant mice are deficient in striatal expression of dynorphin and in dopamine-mediated behavioral responses. Cell. 1994;79(4):729-742.
46. Goodwin RJ, Mackay CL, Nilsson A, et al. Qualitative and quantitative MALDI imaging of the positron emission tomography ligands raclopride (a D2 dopamine antagonist) and SCH 23390 (a D1 dopamine antagonist) in rat brain tissue sections using a solvent-free dry matrix application method. Anal Chem. 2011;83(24):9694-9701.
47. Negyessy L, Goldman-Rakic PS. Subcellular localization of the dopamine D2 receptor and coexistence with the calcium-binding protein neuronal calcium sensor-1 in the primate prefrontal cortex. J Comp Neurol. 2005;488(4):464-475.
48. Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6(11):3177-3188.
49. Doi M, Yujnovsky I, Hirayama J, et al. Impaired light masking in dopamine D2 receptor-null mice. Nat Neurosci. 2006;9(6):732-734.
50. Lou HC, Rosa P, Pryds O, et al. ADHD: increased dopamine receptor availability linked to attention deficit and low neonatal cerebral blood flow. Dev Med Child Neurol. 2004;46(3):179-183.
51. Volkow ND, Wang GJ, Fowler JS, et al. Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry. 1997;154(1):50-55.
52. Ilgin N, Senol S, Gucuyener K, et al. Is increased D2 receptor availability associated with response to stimulant medication in ADHD. Dev Med Child Neurol. 2001;43(11):755-760.
53. Volkow ND, Wang GJ, Tomasi D, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. J Neurosci. 2012;32(3):841-849.
How to prevent misuse of psychotropics among college students
Many college students suffer from mental illness (Table 1),1 which can have a negative impact on academic performance. Although psychotropic medications are an important part of treatment for many college students, the potential for misuse always is present. Drug misuse occurs when patients use medications for reasons inconsistent with legal or medical guidelines.2 For example, patients may take a medication that has not been prescribed for them or in a manner that is inconsistent with the prescriber’s instructions, including administration with other substances.3
Misuse of psychotropic drugs is prevalent among college students. A study of 14,175 students from 26 campuses reported that 14.7% of students taking a psychotropic are doing so without a prescription, including stimulants (52.6%), anxiolytics (38.4%), and antidepressants (17.4%).4 Another study states that more than one-third of responders reported misuse of >1 class of medication.5
Psychotropic misuse is concerning because it increases the risk of adverse events. Nearly one-half of medication errors are associated with writing and dispensing the prescription, which means that prescribers can work to reduce these errors.6 However, nonadherence, prescription misuse, and failure to disclose use of over-the-counter drugs, illicit drugs, and herbal products makes preventing most adverse events difficult, if not impossible, for prescribers.7,8
Psychotropic drug misuse among college students is highly variable and unpredictable. Students misuse medications, including stimulants, benzodiazepines, and antidepressants, for a variety of reasons, such as study enhancement, experimentation, intoxication, self-medication, relaxation, and stress management.8 One survey reported that >70% of students taking a psychotropic medication took it with alcohol or another illicit drug.9
However, <20% of those using a psychotropic medication with alcohol or other illicit drugs told their health care provider(s),9 making it impossible for clinicians to predict a patient’s risk of drug− drug interactions and subsequent adverse events. Additionally, additive effects could occur10 and changes in a patient’s presentation could be caused by a reaction to a combination of medications, rather than a new symptom of mental illness.
This article will examine common issues associated with drug misuse among college-age students and review prevention strategies (Table 2).
Stimulants
Stimulants have the highest rate of diversion; 61.7% of college students prescribed stimulants have shared or sold their medication.11 A survey of 115 students from 2 universities reported that the most common reason for stimulant misuse was to enhance academic performance.12 The same survey showed that some students take stimulants with Cannabis (17%) and alcohol (30%).12 As a result, in addition to lowering grade point average (GPA) and other academic difficulties,13 students misusing stimulants are at risk of drug interactions.14
It is critical to ascertain the route of drug administration, because non-oral routes, including crushing then snorting or injecting, are associated with additional health concerns, such as accidental death or blood-borne illnesses.15,16 Cardiac adverse effects of stimulants include hypertension, vasospasm, tachycardia, and dysrhythmia; psychiatric and other effects include serotonin syndrome, hallucinations, anxiety, paranoia, seizures, tics, hyperthermia, and tremor.17 Health care providers prescribing or caring for people taking a stimulant should monitor for these potential effects.
The risk of switch to mania might not be apparent to those who prescribe stimulants or to young people who take non-prescribed stimulants for academic enhancement or to achieve medication-induced euphoria. Adolescent stimulant use is associated with symptoms of early-onset bipolar disorder in patients who have attention-deficit/ hyperactivity disorder (ADHD) and undiagnosed bipolarity.18
The cardiovascular risk associated with stimulant use is debatable. Although several studies have been conducted,19-21 methodological factors limit their applicability. To minimize potential risks, several precautions should be taken before prescribing a stimulant to treat ADHD.
First, obtain a detailed personal and family medical history, asking about possible cardiovascular disease. Second, carefully scrutinize the patient’s cardiovascular system during the physical exam. Third, consider additional testing, such as an electrocardiogram, if the patient’s history or physical exam indicates possible risk.22
As a prescriber, you should be aware of the prevalence of stimulant use among students with and without ADHD, including those who could be feigning ADHD symptoms.15 Diversion could occur through sharing medications or selling them to friends and family.11 It also is possible that these medications may be used with other illicit substances, such as Cannabis, ecstasy, cocaine, and opiates.23 Students also could misuse stimulants by taking more than the prescribed dosage.24
Risk factors for misuse of stimulants include: heavy alcohol use, previous illicit drug use, white race, fraternity or sorority membership, low GPA, increased hyperactivity symptoms, and attendance at a competitive college or university.25-27
Benzodiazepines
Misuse of benzodiazepine is a significant component of prescription drug abuse and often occurs with other medications and alcohol.28 Additional methods of misuse include increased dosage and non-oral routes of administration.29
A 2001 national survey reported that 7.8% of college students have misused benzodiazepines.23 Common characteristics of benzodiazepine abusers include young age, male sex, personality characteristics of impulsivity and hopelessness, and abuse of other drugs, including cocaine and methadone.28,29
Benzodiazepines are prescribed for their anxiolytic and hypnotic properties and students could use these drugs with other agents to augment the euphoric effects or diminish withdrawal symptoms.30 Patients taking benzodiazepines for anxiety might self-medicate with alcohol, which increases sedation and depression, and can contribute to the risk for respiratory depression.10 Misuse of benzodiazepines can result in cognitive and psychomotor impairment and increase the risk of accidents and overdose.29,31
Although overdose with monotherapy is rare, the risk increases when a benzodiazepine is used with alcohol10 or another respiratory depressants, such as opioids, because combination use can produce additive effects.28 You should therefore avoid prescribing benzodiazepines to patients who have a history of significant substance abuse and consider using alternative, non-addictive agents, such as selective serotonin reuptake inhibitors, or non-pharmaceutical treatment when such patients present with an anxiety disorder. The risk of adverse effects of benzodiazepines can be reduced by limiting the dosing and the duration of the treatment, and by using longer-acting rather than the more addictive, shorter-acting, agents.
Antidepressants
Health care providers should be aware that, despite the relative absence of physically addictive properties, antidepressants from most classes are abusable agents sought by young people for non-medical use. In particular, the literature highlights monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and bupropion as the antidepressants most likely to be misused for their amphetamine-like euphoric effects or serotonin-induced dissociative effects.32 However, compared with other drug classes discussed in this article, the rate of antidepressant misuse is relatively low among college students.
Regardless of the antidepressant selected, clinicians should be concerned about alcohol use among college-age patients. Persons with depression are at increased risk of alcoholism compared with the general population.33 This combination can increase depressive symptoms and sedation, and decrease coordination, judgment, and reaction time.33
Excessive alcohol use can increase the risk of seizures in patients taking antidepressants such as buproprion.34 Employ caution when prescribing bupropion to patients who have a predisposing clinical factor that increases seizure risk, such as excessive alcohol use and abrupt cessation, use of other medications that may lower seizure threshold (eg, theophylline, amphetamines, phenothiazines), and a history of head trauma.34
To minimize the risk of seizures with bupropion, titrate up the dosage slowly. Furthermore, using a low dosage during dual therapy for antidepressant augmentation further decreases the risk of seizure.35 For these reasons, we recommend that you avoid bupropion in patients who are at risk of binge drinking, and give careful consideration to providing alternative therapies for them.
Prescribers and patients should also keep in mind that hypertensive crisis could occur if MAOIs are combined with certain types of alcoholic beverages containing tyramine, including some wines and draft beer.33
How you can identify and prevent misuse
Careful communication between health care provider and patient that is necessary to minimize the risk of adverse drug events with psychotropic medications often is lacking. For example, 24% of study college-age participants did not remember if their physician provided a diagnosis and 28.8% could not recall being informed about side effects and, perhaps as a result, many students did not take their medications as prescribed.9
Further, prescribers should ask college-age patients who are undergoing stimulant treatment if they believe that they are being adequately treated. They should inquire about how they are taking their medications.11 These questions can lead to discussion of the need for these medications and reevaluation of their perceived indication.11
Remind patients to take their medication only as directed.36 Highlight the need to:
• store medications in a discreet location
• properly dispose of unused medications
• keep tabs on the quantity of pills
• know how to resist requests for diversion from peers.
The Substance Abuse and Mental Health Services Administration offers additional useful strategies,37 and pharmacists also can be partners in substance use education and prevention.38 These are examples of how health care providers can take an active role in providing patients with a thorough and detailed understanding of (1) their conditions and (2) their prescribed medications to improve efficacy and safety while preventing misuse.8
A study found that the most common method of obtaining these medications without a prescription is acquiring them from peers; 54% of undergraduate patients with stimulant prescriptions have been approached by peers to give, trade, or sell their drugs.25 Other methods include purchasing medications online or faking prescriptions.39 Health care providers should remind patients of the legal ramifications of sharing or selling their prescribed medications. Finally, providers must be vigilant for students who may feign symptoms to obtain a prescription:
• be wary if symptom presentation sounds too “textbook”
• seek collateral history from family. Adults with ADHD should have shown symptoms during childhood
• use external verification such as neuropsychological testing for ADHD. A neuropsychologist can detect deception by analyzing the pattern of responses to questions.
Patient assessment is a key step to in preventing abuse of psychotropic medications. Gentle inquiry about school-related stress and other risk factors for misuse can help practitioners determine if students are at risk of diversion and if additional screening is necessary.
In response to these issues, Stone and Merlo8 have suggested that, in addition to the educational programs held on college campuses on alcohol, illicit drugs, and prescription painkillers, patients should be better informed on the appropriate use of prescription psychiatric medications, instructed to avoid sharing with family and friends, and assessed for abuse risk at regular intervals.
To further protect patients from adverse outcomes during treatment, you can employ conservative and safe prescribing techniques. One strategy might be to keep a personal formulary that lists key medications you use in everyday practice, including knowledge about each drug’s dosage, potential adverse effects, key warnings, and drug−drug interactions.40
Furthermore, maintain healthy caution about newly approved medications and carefully consider how they measure up to existing agents—in other words, practice evidence-based medicine, particularly when students request a particular agent.40,41 Prescribers should evaluate the risk of abuse before prescribing and attempt to prevent misuse by limiting quantities and minimizing polypharmacy.
Last, pharmacists can be key allies for consultation and appropriate medication selection.
Bottom Line
Psychotropic medications are necessary to treat the variety of conditions—anxiety, attention-deficit/hyperactivity disorder, depression, and panic disorder—common among college students. However, students are at risk of combining their prescribed medications with other medications, drugs, and alcohol or could sell or share their medication with peers. Proper counseling and identification of risk factors can be important tools for preventing such events.
Related Resources
• American College Health Association-National College Health Assessment. www.acha-ncha.org.
• Schwartz VI. College mental health: How to provide care for students in need. Current Psychiatry. 2011;10(12):22-29.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Methadone • Methadose, Dolophine
Theophylline • Theo-24, Theolair, Uniphyl
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American College Health Association. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2014. http://www.acha-ncha.org/docs/ACHA-NCHA-II_ReferenceGroup_ExecutiveSummary_ Spring2014.pdf. Published 2014. Accessed January 13, 2015.
2. World Health Organization. Management of substance abuse. http://www.who.int/substance_abuse/terminology/ abuse/en. Accessed June 4, 2015.
3. U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. http://www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm. Published July 28, 2010. Accessed June 18, 2014.
4. Eisenberg D, Hunt J, Speer N, et al. Mental health service utilization among college students in the United States. J Nerv Ment Dis. 2011;199(5):301-308.
5. Peralta RL, Steele JL. Nonmedical prescription drug use among US college students at a Midwest university: a partial test of social learning theory. Subst Use Misuse. 2010;45(6):865-887.
6. Agency for Healthcare Research and Quality. Reducing and preventing adverse drug events to decrease hospital costs: Research in action. http://www.ahrq.gov/research/ findings/factsheets/errors-safety/aderia/index.html. Updated March 2001. Accessed June 21, 2014.
7. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2010;24(7):595-609.
8. Stone AM, Merlo LJ. Attitudes of college students toward mental illness stigma and the misuse of psychiatric medications. J Clin Psychiatry. 2011;72(2):134-139.
9. Oberleitner LM, Tzilos GK, Zumberg KM, et al. Psychotropic drug use among college students: patterns of use, misuse, and medical monitoring. J Am Coll Health. 2011;59(7):658-661.
10. Linnoila MI. Benzodiazepines and alcohol. J Psychiatr Res. 1990;24(suppl 2):121-127.
11. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71(3):262-269.
12. Rabiner DL, Anastopoulos AD, Costello EJ, et al. The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord. 2009;13(2):144-153.
13. Arria AM. Nonmedical use of prescription stimulants and analgesics: associations with social and academic behaviors among college students. J Drug Issues. 2008; 38(4):1045-1060.
14. Arria AM, Caldeira KM, O’Grady KE, et al. Nonmedical use of prescription stimulants among college students: associations with attention-deficit-hyperactivity disorder and polydrug use. Pharmacotherapy. 2008;28(2):156-169.
15. Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep. 2013;15(7):375.
16. Sepúlveda DR, Thomas LM, McCabe SE, et al. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: preliminary analysis among college students. J Pharm Pract. 2011;24(6):551-560.
17. Greydanus DE. Stimulant misuse: strategies to manage a growing problem. http://www.acha.org/Continuing_ Education/docs/ACHA_Use_Misuse_of_Stimulants_ Article2.pdf. Accessed June 29, 2015.
18. Vergne D, Whitham E, Barroilhet S, et al. Adult ADHD and amphetamines: a new paradigm. Neuropsychiatry. 2011;1(6):591-598.
19. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
20. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
21. Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178-185.
22. U.S. Food and Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention-deficit/hyperactivity disorder (ADHD). http://www.fda.gov/Drugs/Drug Safety/PostmarketDrugSafetyInformationforPatients andProviders/DrugSafetyInformationforHeathcare Professionals/ucm165858.htm. Updated August 15, 2013. Accessed June 25, 2014.
23. McCabe SE, Knight JR, Teter CJ, et al. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96-106.
24. McNiel AD, Muzzin KB, DeWald JP, et al. The nonmedical use of prescription stimulants among dental and dental hygiene students. J Dent Educ. 2011;75(3):365-376.
25. McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38(1):43-56.
26. Arria AM, Garnier-Dykstra LM, Caldeira KM, et al. Persistent nonmedical use of prescription stimulants among college students: possible association with ADHD symptoms. J Atten Disord. 2011;15(5):347-356.
27. Teter CJ, McCabe SE, Boyd CJ, et al. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609-617.
28. Hernandez SH, Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther. 2010; 88(3):307-317.
29. McLarnon ME, Monaghan TL, Stewart SH, et al. Drug misuse and diversion in adults prescribed anxiolytics and sedatives. Pharmacotherapy. 2011;31(3):262-272.
30. Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44(2):151-347.
31. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45-58.
32. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
33. Hall-Flavin DK. Why is it bad to mix antidepressants and alcohol? http://www.mayoclinic.com/health/antidepressants-and-alcohol/AN01653. Updated June 12, 2014. Accessed June 20, 2014.
34. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline LLC; 2014.
35. Davidson J. Seizures and bupropion: a review. J Clin Psychiatry. 1989;50(7):256-261.
36. Maddox JC, Levi M, Thompson C. The compliance with antidepressants in general practice. J Psychopharmacol. 1994;8(1):48-52.
37. Substance Abuse and Mental Health Services Administration. You’re in control: using prescription medication responsibly. http://store.samhsa.gov/shin/content/SMA12-4678B3/SMA12-4678B3.pdf. Accessed June 5, 2015.
38. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health Syst Pharm. 2014;71(3):243-246.
39. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription drugs purchased through the internet: who are the end users? Drug Alcohol Depend. 2010;110(1-2):21-29.
40. Preskorn SH, Flockhart D. 2006 Guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
41. Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171(16): 1433-1440.
Many college students suffer from mental illness (Table 1),1 which can have a negative impact on academic performance. Although psychotropic medications are an important part of treatment for many college students, the potential for misuse always is present. Drug misuse occurs when patients use medications for reasons inconsistent with legal or medical guidelines.2 For example, patients may take a medication that has not been prescribed for them or in a manner that is inconsistent with the prescriber’s instructions, including administration with other substances.3
Misuse of psychotropic drugs is prevalent among college students. A study of 14,175 students from 26 campuses reported that 14.7% of students taking a psychotropic are doing so without a prescription, including stimulants (52.6%), anxiolytics (38.4%), and antidepressants (17.4%).4 Another study states that more than one-third of responders reported misuse of >1 class of medication.5
Psychotropic misuse is concerning because it increases the risk of adverse events. Nearly one-half of medication errors are associated with writing and dispensing the prescription, which means that prescribers can work to reduce these errors.6 However, nonadherence, prescription misuse, and failure to disclose use of over-the-counter drugs, illicit drugs, and herbal products makes preventing most adverse events difficult, if not impossible, for prescribers.7,8
Psychotropic drug misuse among college students is highly variable and unpredictable. Students misuse medications, including stimulants, benzodiazepines, and antidepressants, for a variety of reasons, such as study enhancement, experimentation, intoxication, self-medication, relaxation, and stress management.8 One survey reported that >70% of students taking a psychotropic medication took it with alcohol or another illicit drug.9
However, <20% of those using a psychotropic medication with alcohol or other illicit drugs told their health care provider(s),9 making it impossible for clinicians to predict a patient’s risk of drug− drug interactions and subsequent adverse events. Additionally, additive effects could occur10 and changes in a patient’s presentation could be caused by a reaction to a combination of medications, rather than a new symptom of mental illness.
This article will examine common issues associated with drug misuse among college-age students and review prevention strategies (Table 2).
Stimulants
Stimulants have the highest rate of diversion; 61.7% of college students prescribed stimulants have shared or sold their medication.11 A survey of 115 students from 2 universities reported that the most common reason for stimulant misuse was to enhance academic performance.12 The same survey showed that some students take stimulants with Cannabis (17%) and alcohol (30%).12 As a result, in addition to lowering grade point average (GPA) and other academic difficulties,13 students misusing stimulants are at risk of drug interactions.14
It is critical to ascertain the route of drug administration, because non-oral routes, including crushing then snorting or injecting, are associated with additional health concerns, such as accidental death or blood-borne illnesses.15,16 Cardiac adverse effects of stimulants include hypertension, vasospasm, tachycardia, and dysrhythmia; psychiatric and other effects include serotonin syndrome, hallucinations, anxiety, paranoia, seizures, tics, hyperthermia, and tremor.17 Health care providers prescribing or caring for people taking a stimulant should monitor for these potential effects.
The risk of switch to mania might not be apparent to those who prescribe stimulants or to young people who take non-prescribed stimulants for academic enhancement or to achieve medication-induced euphoria. Adolescent stimulant use is associated with symptoms of early-onset bipolar disorder in patients who have attention-deficit/ hyperactivity disorder (ADHD) and undiagnosed bipolarity.18
The cardiovascular risk associated with stimulant use is debatable. Although several studies have been conducted,19-21 methodological factors limit their applicability. To minimize potential risks, several precautions should be taken before prescribing a stimulant to treat ADHD.
First, obtain a detailed personal and family medical history, asking about possible cardiovascular disease. Second, carefully scrutinize the patient’s cardiovascular system during the physical exam. Third, consider additional testing, such as an electrocardiogram, if the patient’s history or physical exam indicates possible risk.22
As a prescriber, you should be aware of the prevalence of stimulant use among students with and without ADHD, including those who could be feigning ADHD symptoms.15 Diversion could occur through sharing medications or selling them to friends and family.11 It also is possible that these medications may be used with other illicit substances, such as Cannabis, ecstasy, cocaine, and opiates.23 Students also could misuse stimulants by taking more than the prescribed dosage.24
Risk factors for misuse of stimulants include: heavy alcohol use, previous illicit drug use, white race, fraternity or sorority membership, low GPA, increased hyperactivity symptoms, and attendance at a competitive college or university.25-27
Benzodiazepines
Misuse of benzodiazepine is a significant component of prescription drug abuse and often occurs with other medications and alcohol.28 Additional methods of misuse include increased dosage and non-oral routes of administration.29
A 2001 national survey reported that 7.8% of college students have misused benzodiazepines.23 Common characteristics of benzodiazepine abusers include young age, male sex, personality characteristics of impulsivity and hopelessness, and abuse of other drugs, including cocaine and methadone.28,29
Benzodiazepines are prescribed for their anxiolytic and hypnotic properties and students could use these drugs with other agents to augment the euphoric effects or diminish withdrawal symptoms.30 Patients taking benzodiazepines for anxiety might self-medicate with alcohol, which increases sedation and depression, and can contribute to the risk for respiratory depression.10 Misuse of benzodiazepines can result in cognitive and psychomotor impairment and increase the risk of accidents and overdose.29,31
Although overdose with monotherapy is rare, the risk increases when a benzodiazepine is used with alcohol10 or another respiratory depressants, such as opioids, because combination use can produce additive effects.28 You should therefore avoid prescribing benzodiazepines to patients who have a history of significant substance abuse and consider using alternative, non-addictive agents, such as selective serotonin reuptake inhibitors, or non-pharmaceutical treatment when such patients present with an anxiety disorder. The risk of adverse effects of benzodiazepines can be reduced by limiting the dosing and the duration of the treatment, and by using longer-acting rather than the more addictive, shorter-acting, agents.
Antidepressants
Health care providers should be aware that, despite the relative absence of physically addictive properties, antidepressants from most classes are abusable agents sought by young people for non-medical use. In particular, the literature highlights monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and bupropion as the antidepressants most likely to be misused for their amphetamine-like euphoric effects or serotonin-induced dissociative effects.32 However, compared with other drug classes discussed in this article, the rate of antidepressant misuse is relatively low among college students.
Regardless of the antidepressant selected, clinicians should be concerned about alcohol use among college-age patients. Persons with depression are at increased risk of alcoholism compared with the general population.33 This combination can increase depressive symptoms and sedation, and decrease coordination, judgment, and reaction time.33
Excessive alcohol use can increase the risk of seizures in patients taking antidepressants such as buproprion.34 Employ caution when prescribing bupropion to patients who have a predisposing clinical factor that increases seizure risk, such as excessive alcohol use and abrupt cessation, use of other medications that may lower seizure threshold (eg, theophylline, amphetamines, phenothiazines), and a history of head trauma.34
To minimize the risk of seizures with bupropion, titrate up the dosage slowly. Furthermore, using a low dosage during dual therapy for antidepressant augmentation further decreases the risk of seizure.35 For these reasons, we recommend that you avoid bupropion in patients who are at risk of binge drinking, and give careful consideration to providing alternative therapies for them.
Prescribers and patients should also keep in mind that hypertensive crisis could occur if MAOIs are combined with certain types of alcoholic beverages containing tyramine, including some wines and draft beer.33
How you can identify and prevent misuse
Careful communication between health care provider and patient that is necessary to minimize the risk of adverse drug events with psychotropic medications often is lacking. For example, 24% of study college-age participants did not remember if their physician provided a diagnosis and 28.8% could not recall being informed about side effects and, perhaps as a result, many students did not take their medications as prescribed.9
Further, prescribers should ask college-age patients who are undergoing stimulant treatment if they believe that they are being adequately treated. They should inquire about how they are taking their medications.11 These questions can lead to discussion of the need for these medications and reevaluation of their perceived indication.11
Remind patients to take their medication only as directed.36 Highlight the need to:
• store medications in a discreet location
• properly dispose of unused medications
• keep tabs on the quantity of pills
• know how to resist requests for diversion from peers.
The Substance Abuse and Mental Health Services Administration offers additional useful strategies,37 and pharmacists also can be partners in substance use education and prevention.38 These are examples of how health care providers can take an active role in providing patients with a thorough and detailed understanding of (1) their conditions and (2) their prescribed medications to improve efficacy and safety while preventing misuse.8
A study found that the most common method of obtaining these medications without a prescription is acquiring them from peers; 54% of undergraduate patients with stimulant prescriptions have been approached by peers to give, trade, or sell their drugs.25 Other methods include purchasing medications online or faking prescriptions.39 Health care providers should remind patients of the legal ramifications of sharing or selling their prescribed medications. Finally, providers must be vigilant for students who may feign symptoms to obtain a prescription:
• be wary if symptom presentation sounds too “textbook”
• seek collateral history from family. Adults with ADHD should have shown symptoms during childhood
• use external verification such as neuropsychological testing for ADHD. A neuropsychologist can detect deception by analyzing the pattern of responses to questions.
Patient assessment is a key step to in preventing abuse of psychotropic medications. Gentle inquiry about school-related stress and other risk factors for misuse can help practitioners determine if students are at risk of diversion and if additional screening is necessary.
In response to these issues, Stone and Merlo8 have suggested that, in addition to the educational programs held on college campuses on alcohol, illicit drugs, and prescription painkillers, patients should be better informed on the appropriate use of prescription psychiatric medications, instructed to avoid sharing with family and friends, and assessed for abuse risk at regular intervals.
To further protect patients from adverse outcomes during treatment, you can employ conservative and safe prescribing techniques. One strategy might be to keep a personal formulary that lists key medications you use in everyday practice, including knowledge about each drug’s dosage, potential adverse effects, key warnings, and drug−drug interactions.40
Furthermore, maintain healthy caution about newly approved medications and carefully consider how they measure up to existing agents—in other words, practice evidence-based medicine, particularly when students request a particular agent.40,41 Prescribers should evaluate the risk of abuse before prescribing and attempt to prevent misuse by limiting quantities and minimizing polypharmacy.
Last, pharmacists can be key allies for consultation and appropriate medication selection.
Bottom Line
Psychotropic medications are necessary to treat the variety of conditions—anxiety, attention-deficit/hyperactivity disorder, depression, and panic disorder—common among college students. However, students are at risk of combining their prescribed medications with other medications, drugs, and alcohol or could sell or share their medication with peers. Proper counseling and identification of risk factors can be important tools for preventing such events.
Related Resources
• American College Health Association-National College Health Assessment. www.acha-ncha.org.
• Schwartz VI. College mental health: How to provide care for students in need. Current Psychiatry. 2011;10(12):22-29.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Methadone • Methadose, Dolophine
Theophylline • Theo-24, Theolair, Uniphyl
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Many college students suffer from mental illness (Table 1),1 which can have a negative impact on academic performance. Although psychotropic medications are an important part of treatment for many college students, the potential for misuse always is present. Drug misuse occurs when patients use medications for reasons inconsistent with legal or medical guidelines.2 For example, patients may take a medication that has not been prescribed for them or in a manner that is inconsistent with the prescriber’s instructions, including administration with other substances.3
Misuse of psychotropic drugs is prevalent among college students. A study of 14,175 students from 26 campuses reported that 14.7% of students taking a psychotropic are doing so without a prescription, including stimulants (52.6%), anxiolytics (38.4%), and antidepressants (17.4%).4 Another study states that more than one-third of responders reported misuse of >1 class of medication.5
Psychotropic misuse is concerning because it increases the risk of adverse events. Nearly one-half of medication errors are associated with writing and dispensing the prescription, which means that prescribers can work to reduce these errors.6 However, nonadherence, prescription misuse, and failure to disclose use of over-the-counter drugs, illicit drugs, and herbal products makes preventing most adverse events difficult, if not impossible, for prescribers.7,8
Psychotropic drug misuse among college students is highly variable and unpredictable. Students misuse medications, including stimulants, benzodiazepines, and antidepressants, for a variety of reasons, such as study enhancement, experimentation, intoxication, self-medication, relaxation, and stress management.8 One survey reported that >70% of students taking a psychotropic medication took it with alcohol or another illicit drug.9
However, <20% of those using a psychotropic medication with alcohol or other illicit drugs told their health care provider(s),9 making it impossible for clinicians to predict a patient’s risk of drug− drug interactions and subsequent adverse events. Additionally, additive effects could occur10 and changes in a patient’s presentation could be caused by a reaction to a combination of medications, rather than a new symptom of mental illness.
This article will examine common issues associated with drug misuse among college-age students and review prevention strategies (Table 2).
Stimulants
Stimulants have the highest rate of diversion; 61.7% of college students prescribed stimulants have shared or sold their medication.11 A survey of 115 students from 2 universities reported that the most common reason for stimulant misuse was to enhance academic performance.12 The same survey showed that some students take stimulants with Cannabis (17%) and alcohol (30%).12 As a result, in addition to lowering grade point average (GPA) and other academic difficulties,13 students misusing stimulants are at risk of drug interactions.14
It is critical to ascertain the route of drug administration, because non-oral routes, including crushing then snorting or injecting, are associated with additional health concerns, such as accidental death or blood-borne illnesses.15,16 Cardiac adverse effects of stimulants include hypertension, vasospasm, tachycardia, and dysrhythmia; psychiatric and other effects include serotonin syndrome, hallucinations, anxiety, paranoia, seizures, tics, hyperthermia, and tremor.17 Health care providers prescribing or caring for people taking a stimulant should monitor for these potential effects.
The risk of switch to mania might not be apparent to those who prescribe stimulants or to young people who take non-prescribed stimulants for academic enhancement or to achieve medication-induced euphoria. Adolescent stimulant use is associated with symptoms of early-onset bipolar disorder in patients who have attention-deficit/ hyperactivity disorder (ADHD) and undiagnosed bipolarity.18
The cardiovascular risk associated with stimulant use is debatable. Although several studies have been conducted,19-21 methodological factors limit their applicability. To minimize potential risks, several precautions should be taken before prescribing a stimulant to treat ADHD.
First, obtain a detailed personal and family medical history, asking about possible cardiovascular disease. Second, carefully scrutinize the patient’s cardiovascular system during the physical exam. Third, consider additional testing, such as an electrocardiogram, if the patient’s history or physical exam indicates possible risk.22
As a prescriber, you should be aware of the prevalence of stimulant use among students with and without ADHD, including those who could be feigning ADHD symptoms.15 Diversion could occur through sharing medications or selling them to friends and family.11 It also is possible that these medications may be used with other illicit substances, such as Cannabis, ecstasy, cocaine, and opiates.23 Students also could misuse stimulants by taking more than the prescribed dosage.24
Risk factors for misuse of stimulants include: heavy alcohol use, previous illicit drug use, white race, fraternity or sorority membership, low GPA, increased hyperactivity symptoms, and attendance at a competitive college or university.25-27
Benzodiazepines
Misuse of benzodiazepine is a significant component of prescription drug abuse and often occurs with other medications and alcohol.28 Additional methods of misuse include increased dosage and non-oral routes of administration.29
A 2001 national survey reported that 7.8% of college students have misused benzodiazepines.23 Common characteristics of benzodiazepine abusers include young age, male sex, personality characteristics of impulsivity and hopelessness, and abuse of other drugs, including cocaine and methadone.28,29
Benzodiazepines are prescribed for their anxiolytic and hypnotic properties and students could use these drugs with other agents to augment the euphoric effects or diminish withdrawal symptoms.30 Patients taking benzodiazepines for anxiety might self-medicate with alcohol, which increases sedation and depression, and can contribute to the risk for respiratory depression.10 Misuse of benzodiazepines can result in cognitive and psychomotor impairment and increase the risk of accidents and overdose.29,31
Although overdose with monotherapy is rare, the risk increases when a benzodiazepine is used with alcohol10 or another respiratory depressants, such as opioids, because combination use can produce additive effects.28 You should therefore avoid prescribing benzodiazepines to patients who have a history of significant substance abuse and consider using alternative, non-addictive agents, such as selective serotonin reuptake inhibitors, or non-pharmaceutical treatment when such patients present with an anxiety disorder. The risk of adverse effects of benzodiazepines can be reduced by limiting the dosing and the duration of the treatment, and by using longer-acting rather than the more addictive, shorter-acting, agents.
Antidepressants
Health care providers should be aware that, despite the relative absence of physically addictive properties, antidepressants from most classes are abusable agents sought by young people for non-medical use. In particular, the literature highlights monoamine oxidase inhibitors (MAOIs), tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and bupropion as the antidepressants most likely to be misused for their amphetamine-like euphoric effects or serotonin-induced dissociative effects.32 However, compared with other drug classes discussed in this article, the rate of antidepressant misuse is relatively low among college students.
Regardless of the antidepressant selected, clinicians should be concerned about alcohol use among college-age patients. Persons with depression are at increased risk of alcoholism compared with the general population.33 This combination can increase depressive symptoms and sedation, and decrease coordination, judgment, and reaction time.33
Excessive alcohol use can increase the risk of seizures in patients taking antidepressants such as buproprion.34 Employ caution when prescribing bupropion to patients who have a predisposing clinical factor that increases seizure risk, such as excessive alcohol use and abrupt cessation, use of other medications that may lower seizure threshold (eg, theophylline, amphetamines, phenothiazines), and a history of head trauma.34
To minimize the risk of seizures with bupropion, titrate up the dosage slowly. Furthermore, using a low dosage during dual therapy for antidepressant augmentation further decreases the risk of seizure.35 For these reasons, we recommend that you avoid bupropion in patients who are at risk of binge drinking, and give careful consideration to providing alternative therapies for them.
Prescribers and patients should also keep in mind that hypertensive crisis could occur if MAOIs are combined with certain types of alcoholic beverages containing tyramine, including some wines and draft beer.33
How you can identify and prevent misuse
Careful communication between health care provider and patient that is necessary to minimize the risk of adverse drug events with psychotropic medications often is lacking. For example, 24% of study college-age participants did not remember if their physician provided a diagnosis and 28.8% could not recall being informed about side effects and, perhaps as a result, many students did not take their medications as prescribed.9
Further, prescribers should ask college-age patients who are undergoing stimulant treatment if they believe that they are being adequately treated. They should inquire about how they are taking their medications.11 These questions can lead to discussion of the need for these medications and reevaluation of their perceived indication.11
Remind patients to take their medication only as directed.36 Highlight the need to:
• store medications in a discreet location
• properly dispose of unused medications
• keep tabs on the quantity of pills
• know how to resist requests for diversion from peers.
The Substance Abuse and Mental Health Services Administration offers additional useful strategies,37 and pharmacists also can be partners in substance use education and prevention.38 These are examples of how health care providers can take an active role in providing patients with a thorough and detailed understanding of (1) their conditions and (2) their prescribed medications to improve efficacy and safety while preventing misuse.8
A study found that the most common method of obtaining these medications without a prescription is acquiring them from peers; 54% of undergraduate patients with stimulant prescriptions have been approached by peers to give, trade, or sell their drugs.25 Other methods include purchasing medications online or faking prescriptions.39 Health care providers should remind patients of the legal ramifications of sharing or selling their prescribed medications. Finally, providers must be vigilant for students who may feign symptoms to obtain a prescription:
• be wary if symptom presentation sounds too “textbook”
• seek collateral history from family. Adults with ADHD should have shown symptoms during childhood
• use external verification such as neuropsychological testing for ADHD. A neuropsychologist can detect deception by analyzing the pattern of responses to questions.
Patient assessment is a key step to in preventing abuse of psychotropic medications. Gentle inquiry about school-related stress and other risk factors for misuse can help practitioners determine if students are at risk of diversion and if additional screening is necessary.
In response to these issues, Stone and Merlo8 have suggested that, in addition to the educational programs held on college campuses on alcohol, illicit drugs, and prescription painkillers, patients should be better informed on the appropriate use of prescription psychiatric medications, instructed to avoid sharing with family and friends, and assessed for abuse risk at regular intervals.
To further protect patients from adverse outcomes during treatment, you can employ conservative and safe prescribing techniques. One strategy might be to keep a personal formulary that lists key medications you use in everyday practice, including knowledge about each drug’s dosage, potential adverse effects, key warnings, and drug−drug interactions.40
Furthermore, maintain healthy caution about newly approved medications and carefully consider how they measure up to existing agents—in other words, practice evidence-based medicine, particularly when students request a particular agent.40,41 Prescribers should evaluate the risk of abuse before prescribing and attempt to prevent misuse by limiting quantities and minimizing polypharmacy.
Last, pharmacists can be key allies for consultation and appropriate medication selection.
Bottom Line
Psychotropic medications are necessary to treat the variety of conditions—anxiety, attention-deficit/hyperactivity disorder, depression, and panic disorder—common among college students. However, students are at risk of combining their prescribed medications with other medications, drugs, and alcohol or could sell or share their medication with peers. Proper counseling and identification of risk factors can be important tools for preventing such events.
Related Resources
• American College Health Association-National College Health Assessment. www.acha-ncha.org.
• Schwartz VI. College mental health: How to provide care for students in need. Current Psychiatry. 2011;10(12):22-29.
Drug Brand Names
Bupropion • Wellbutrin, Zyban
Methadone • Methadose, Dolophine
Theophylline • Theo-24, Theolair, Uniphyl
Disclosures
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American College Health Association. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2014. http://www.acha-ncha.org/docs/ACHA-NCHA-II_ReferenceGroup_ExecutiveSummary_ Spring2014.pdf. Published 2014. Accessed January 13, 2015.
2. World Health Organization. Management of substance abuse. http://www.who.int/substance_abuse/terminology/ abuse/en. Accessed June 4, 2015.
3. U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. http://www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm. Published July 28, 2010. Accessed June 18, 2014.
4. Eisenberg D, Hunt J, Speer N, et al. Mental health service utilization among college students in the United States. J Nerv Ment Dis. 2011;199(5):301-308.
5. Peralta RL, Steele JL. Nonmedical prescription drug use among US college students at a Midwest university: a partial test of social learning theory. Subst Use Misuse. 2010;45(6):865-887.
6. Agency for Healthcare Research and Quality. Reducing and preventing adverse drug events to decrease hospital costs: Research in action. http://www.ahrq.gov/research/ findings/factsheets/errors-safety/aderia/index.html. Updated March 2001. Accessed June 21, 2014.
7. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2010;24(7):595-609.
8. Stone AM, Merlo LJ. Attitudes of college students toward mental illness stigma and the misuse of psychiatric medications. J Clin Psychiatry. 2011;72(2):134-139.
9. Oberleitner LM, Tzilos GK, Zumberg KM, et al. Psychotropic drug use among college students: patterns of use, misuse, and medical monitoring. J Am Coll Health. 2011;59(7):658-661.
10. Linnoila MI. Benzodiazepines and alcohol. J Psychiatr Res. 1990;24(suppl 2):121-127.
11. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71(3):262-269.
12. Rabiner DL, Anastopoulos AD, Costello EJ, et al. The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord. 2009;13(2):144-153.
13. Arria AM. Nonmedical use of prescription stimulants and analgesics: associations with social and academic behaviors among college students. J Drug Issues. 2008; 38(4):1045-1060.
14. Arria AM, Caldeira KM, O’Grady KE, et al. Nonmedical use of prescription stimulants among college students: associations with attention-deficit-hyperactivity disorder and polydrug use. Pharmacotherapy. 2008;28(2):156-169.
15. Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep. 2013;15(7):375.
16. Sepúlveda DR, Thomas LM, McCabe SE, et al. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: preliminary analysis among college students. J Pharm Pract. 2011;24(6):551-560.
17. Greydanus DE. Stimulant misuse: strategies to manage a growing problem. http://www.acha.org/Continuing_ Education/docs/ACHA_Use_Misuse_of_Stimulants_ Article2.pdf. Accessed June 29, 2015.
18. Vergne D, Whitham E, Barroilhet S, et al. Adult ADHD and amphetamines: a new paradigm. Neuropsychiatry. 2011;1(6):591-598.
19. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
20. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
21. Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178-185.
22. U.S. Food and Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention-deficit/hyperactivity disorder (ADHD). http://www.fda.gov/Drugs/Drug Safety/PostmarketDrugSafetyInformationforPatients andProviders/DrugSafetyInformationforHeathcare Professionals/ucm165858.htm. Updated August 15, 2013. Accessed June 25, 2014.
23. McCabe SE, Knight JR, Teter CJ, et al. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96-106.
24. McNiel AD, Muzzin KB, DeWald JP, et al. The nonmedical use of prescription stimulants among dental and dental hygiene students. J Dent Educ. 2011;75(3):365-376.
25. McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38(1):43-56.
26. Arria AM, Garnier-Dykstra LM, Caldeira KM, et al. Persistent nonmedical use of prescription stimulants among college students: possible association with ADHD symptoms. J Atten Disord. 2011;15(5):347-356.
27. Teter CJ, McCabe SE, Boyd CJ, et al. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609-617.
28. Hernandez SH, Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther. 2010; 88(3):307-317.
29. McLarnon ME, Monaghan TL, Stewart SH, et al. Drug misuse and diversion in adults prescribed anxiolytics and sedatives. Pharmacotherapy. 2011;31(3):262-272.
30. Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44(2):151-347.
31. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45-58.
32. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
33. Hall-Flavin DK. Why is it bad to mix antidepressants and alcohol? http://www.mayoclinic.com/health/antidepressants-and-alcohol/AN01653. Updated June 12, 2014. Accessed June 20, 2014.
34. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline LLC; 2014.
35. Davidson J. Seizures and bupropion: a review. J Clin Psychiatry. 1989;50(7):256-261.
36. Maddox JC, Levi M, Thompson C. The compliance with antidepressants in general practice. J Psychopharmacol. 1994;8(1):48-52.
37. Substance Abuse and Mental Health Services Administration. You’re in control: using prescription medication responsibly. http://store.samhsa.gov/shin/content/SMA12-4678B3/SMA12-4678B3.pdf. Accessed June 5, 2015.
38. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health Syst Pharm. 2014;71(3):243-246.
39. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription drugs purchased through the internet: who are the end users? Drug Alcohol Depend. 2010;110(1-2):21-29.
40. Preskorn SH, Flockhart D. 2006 Guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
41. Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171(16): 1433-1440.
1. American College Health Association. American College Health Association-National College Health Assessment II: Reference Group Executive Summary Spring 2014. http://www.acha-ncha.org/docs/ACHA-NCHA-II_ReferenceGroup_ExecutiveSummary_ Spring2014.pdf. Published 2014. Accessed January 13, 2015.
2. World Health Organization. Management of substance abuse. http://www.who.int/substance_abuse/terminology/ abuse/en. Accessed June 4, 2015.
3. U.S. Food and Drug Administration. Combating misuse and abuse of prescription drugs: Q&A with Michael Klein, PhD. http://www.fda.gov/ForConsumers/ConsumerUpdates/ ucm220112.htm. Published July 28, 2010. Accessed June 18, 2014.
4. Eisenberg D, Hunt J, Speer N, et al. Mental health service utilization among college students in the United States. J Nerv Ment Dis. 2011;199(5):301-308.
5. Peralta RL, Steele JL. Nonmedical prescription drug use among US college students at a Midwest university: a partial test of social learning theory. Subst Use Misuse. 2010;45(6):865-887.
6. Agency for Healthcare Research and Quality. Reducing and preventing adverse drug events to decrease hospital costs: Research in action. http://www.ahrq.gov/research/ findings/factsheets/errors-safety/aderia/index.html. Updated March 2001. Accessed June 21, 2014.
7. Procyshyn RM, Barr AM, Brickell T, et al. Medication errors in psychiatry: a comprehensive review. CNS Drugs. 2010;24(7):595-609.
8. Stone AM, Merlo LJ. Attitudes of college students toward mental illness stigma and the misuse of psychiatric medications. J Clin Psychiatry. 2011;72(2):134-139.
9. Oberleitner LM, Tzilos GK, Zumberg KM, et al. Psychotropic drug use among college students: patterns of use, misuse, and medical monitoring. J Am Coll Health. 2011;59(7):658-661.
10. Linnoila MI. Benzodiazepines and alcohol. J Psychiatr Res. 1990;24(suppl 2):121-127.
11. Garnier LM, Arria AM, Caldeira KM, et al. Sharing and selling of prescription medications in a college student sample. J Clin Psychiatry. 2010;71(3):262-269.
12. Rabiner DL, Anastopoulos AD, Costello EJ, et al. The misuse and diversion of prescribed ADHD medications by college students. J Atten Disord. 2009;13(2):144-153.
13. Arria AM. Nonmedical use of prescription stimulants and analgesics: associations with social and academic behaviors among college students. J Drug Issues. 2008; 38(4):1045-1060.
14. Arria AM, Caldeira KM, O’Grady KE, et al. Nonmedical use of prescription stimulants among college students: associations with attention-deficit-hyperactivity disorder and polydrug use. Pharmacotherapy. 2008;28(2):156-169.
15. Rabiner DL. Stimulant prescription cautions: addressing misuse, diversion and malingering. Curr Psychiatry Rep. 2013;15(7):375.
16. Sepúlveda DR, Thomas LM, McCabe SE, et al. Misuse of prescribed stimulant medication for ADHD and associated patterns of substance use: preliminary analysis among college students. J Pharm Pract. 2011;24(6):551-560.
17. Greydanus DE. Stimulant misuse: strategies to manage a growing problem. http://www.acha.org/Continuing_ Education/docs/ACHA_Use_Misuse_of_Stimulants_ Article2.pdf. Accessed June 29, 2015.
18. Vergne D, Whitham E, Barroilhet S, et al. Adult ADHD and amphetamines: a new paradigm. Neuropsychiatry. 2011;1(6):591-598.
19. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683.
20. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904.
21. Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178-185.
22. U.S. Food and Drug Administration. Communication about an ongoing safety review of stimulant medications used in children with attention-deficit/hyperactivity disorder (ADHD). http://www.fda.gov/Drugs/Drug Safety/PostmarketDrugSafetyInformationforPatients andProviders/DrugSafetyInformationforHeathcare Professionals/ucm165858.htm. Updated August 15, 2013. Accessed June 25, 2014.
23. McCabe SE, Knight JR, Teter CJ, et al. Non-medical use of prescription stimulants among US college students: prevalence and correlates from a national survey. Addiction. 2005;100(1):96-106.
24. McNiel AD, Muzzin KB, DeWald JP, et al. The nonmedical use of prescription stimulants among dental and dental hygiene students. J Dent Educ. 2011;75(3):365-376.
25. McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use and diversion of prescription stimulant medication. J Psychoactive Drugs. 2006;38(1):43-56.
26. Arria AM, Garnier-Dykstra LM, Caldeira KM, et al. Persistent nonmedical use of prescription stimulants among college students: possible association with ADHD symptoms. J Atten Disord. 2011;15(5):347-356.
27. Teter CJ, McCabe SE, Boyd CJ, et al. Illicit methylphenidate use in an undergraduate student sample: prevalence and risk factors. Pharmacotherapy. 2003;23(5):609-617.
28. Hernandez SH, Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther. 2010; 88(3):307-317.
29. McLarnon ME, Monaghan TL, Stewart SH, et al. Drug misuse and diversion in adults prescribed anxiolytics and sedatives. Pharmacotherapy. 2011;31(3):262-272.
30. Woods JH, Katz JL, Winger G. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44(2):151-347.
31. Buffett-Jerrott SE, Stewart SH. Cognitive and sedative effects of benzodiazepine use. Curr Pharm Des. 2002;8(1):45-58.
32. Evans EA, Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil. 2014;5:107-120.
33. Hall-Flavin DK. Why is it bad to mix antidepressants and alcohol? http://www.mayoclinic.com/health/antidepressants-and-alcohol/AN01653. Updated June 12, 2014. Accessed June 20, 2014.
34. Wellbutrin [package insert]. Research Triangle Park, NC: GlaxoSmithKline LLC; 2014.
35. Davidson J. Seizures and bupropion: a review. J Clin Psychiatry. 1989;50(7):256-261.
36. Maddox JC, Levi M, Thompson C. The compliance with antidepressants in general practice. J Psychopharmacol. 1994;8(1):48-52.
37. Substance Abuse and Mental Health Services Administration. You’re in control: using prescription medication responsibly. http://store.samhsa.gov/shin/content/SMA12-4678B3/SMA12-4678B3.pdf. Accessed June 5, 2015.
38. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health Syst Pharm. 2014;71(3):243-246.
39. Inciardi JA, Surratt HL, Cicero TJ, et al. Prescription drugs purchased through the internet: who are the end users? Drug Alcohol Depend. 2010;110(1-2):21-29.
40. Preskorn SH, Flockhart D. 2006 Guide to psychiatric drug interactions. Primary Psychiatry. 2006;13(4):35-64.
41. Schiff GD, Galanter WL, Duhig J, et al. Principles of conservative prescribing. Arch Intern Med. 2011;171(16): 1433-1440.
What do >700 letters to a mass murderer tell us about the people who wrote them?
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
Little is known about people who write to criminals incarcerated for a violent crime. However, existence of Web sites such as WriteAPrisoner.com, Meet-An-Inmate.com, and PrisonPenPals.com suggests some appetite among the public for corresponding with the incarcerated. Writers of letters might be drawn to the “bad boy” image of prisoners. Furthermore, much has been written of the willingness of some battered women to remain in an abusive domestic relationship, leading them to correspond with their abusers even after those abusers are incarcerated.1,2
To our knowledge, no examination of letters written to a mass murderer has been published. Therefore, we categorized and analyzed 784 letters sent to a high-profile male mass murderer whose crime was committed during the past decade. Here is a description of the study and what we found, as well as discussion of how our findings might offer utility in a psychiatric practice.
Goals of the study
We hypothesized that a large percentage of those letters could be classified as “Romantic,” given the lay perception that it is women who write to mass murderers. We also sought to evaluate follow-up letters sent by these writers to test the assumption that their individual goals would be constant over time.
We performed this study in the hope that the research could assist psychiatric practitioners in treating patients who seek to associate with a violent person (see “Treatment considerations,”). We thought it might be helpful for practitioners to get a better understanding of the nature of people who write to a violent offender or express a desire to do so.
Methods of study
Two authors (R.S.J. and D.P.G.) evaluated 819 letters that had been written by non-incarcerated, non-family adults to 1 mass murderer. The initial letter and follow-up letters written by each unique writer (n = 333) were categorized as follows:
• state or country from which the letter was sent
• age
• sex
• number of letters sent by each writer
• whether a photograph was enclosed
• whether additional items were enclosed (eg, gifts, drawings)
• whether the letter was rejected by prison authorities
• the writer’s purpose.
The study was approved by the institutional review board of Baylor College of Medicine.
Letters were assigned to 1 of 5 categories:
Acquaintance letters sought ongoing correspondence relationship with the murderer. They focused largely on conveying information about the writer.
Show of support letters also sought an ongoing correspondence relationship with the murderer, but instead focused on him, not the writer.
Romance letters used words that conveyed romantic or non-platonic affection.
Spiritual letters gave advice to the murderer with a religious tone.
Words of wisdom letters offered advice but lacked a religious tone.
Given the nonstandardized nature of categorization and the lack of a formal questionnaire, we were unable to perform an exploratory factor analysis on our categorizations. Inter-rater reliability of letter categorization was 0.79.
Results: Writer profiles, purpose for writing
In all, we reviewed 819 letters:
• Thirty-five letters were excluded because they were written by family members, children, or other prisoners
• Of the remaining 784 letters, there were 333 unique writers
• Two-hundred sixty letters were written by women, 61 by men; 2 were co-written by both sexes; sex could not be determined for 10.
Women were more likely than men to write a letter (P = .014) and to write ≥3 letters (P = .001). The age of the writer was determined for 117 (35.1%) letters; mean age was 27.8 (± 8.9) years (range, 18 to 59 years).
The purpose of the letters differed by sex (P < .001) but not by the writer’s age (P = .058). Women were more likely than men to write letters categorized as “Acquaintance,” “Romance,” and “Show of support”; in contrast, men were more likely than women to write a letter categorized as “Spiritual” (Table 1). Approximately 95% of letters were handwritten. Letters averaged 3 pages (range, 1 to 16 pages).
Two-hundred sixteen writers wrote a single letter; 53 wrote 2 letters; 18 wrote 3 letters; 11 wrote 4 letters; 30 wrote 5 to 10 letters; and 9 wrote 11 to 43 letters. The purpose of follow-up letters was associated with the age of the writer (P < .001) and with the writer’s sex (P < .001). Women were more likely to write “Show of support” and “Romance” follow-up letters; men were more likely to write “Spiritual” follow-up letters (Table 2).
Results suggested that the purpose of the initial letter was a reasonable predictor of the purpose of follow-up letters (P < .001) (Table 3). The murderer never responded to any letters. Letters were most often written from his state of incarceration; next, from contiguous states; then, from non-contiguous states; and, last, from international locations (P < .001).
Of the initial letters from writers who wrote ≥10, 60% were categorized as “Acquaintance” and 20% as “Romance.” The writer who wrote the most letters (43) moved during the course of her letter-writing to live in the same state as the murderer; she stated in her letters that she did so to be closer to him and to be able to attend his court hearings. Four other writers, each of whom wrote >5 letters, stated that they had traveled to the murderer’s state of incarceration to attend some of his hearings in person.
Composite examples of more common categories of letters
Names and other pertinent identifying information have been changed.
Acquaintance. Hi, Steve. I’ve been following your case and just wanted to write you so that maybe we could be friends or keep in touch since you’re probably pretty bored. I’m a 27-year-old college student studying marketing and working at Applebee’s as a waitress (for now) until I can land my dream job. I’ve enclosed a picture of me and my dachshund along with a photo of my favorite beach in the world. Write me back if you want. Jenny.
Show of support. Steve: I’ve been really worried about you since first seeing you on TV. You look different lately and I hope they’re treating you OK and feeding you decent food. In case they’re not, I’ve enclosed a little something to buy yourself a treat. Just know that there are many of us that care about you and are really pulling for you to be strong in this tough situation you’re in. Yours truly, Karen.
Romance. Dearest Steven: My mind has been filled with thoughts of you and of us since I last saw you in my dreams! Be strong, because you are going to beat this once they understand that you are not responsible for what happened! Don’t you see, sweetie, the system failed you, and now you’re caught up in something that you will soon overcome. When I think of the day that you get released, and how we’ll be able to settle down somewhere together, it gets me incredibly excited. You and I are meant to be together, because I understand you and can help you get better. I love you, Steven! Please write me back so that I know we’re on the same page about our plans for the future. Love, ♥ Your sweetie, Rachel.
Spiritual. Dear Child of God: The Lord has a plan for you. I know that things right now might be confusing, and you’re in a black place, but He is there right beside you. If you need some reading materials to give you comfort, just let me know and I can get a Bible to you along with some other books to give you solace and strengthen your walk with Him. God forgives you and he loves you so much! Much love in Christ, Mary.
Discussion
Given that the mass murderer in this study was a young man, it is not surprising that 78% of writers of initial letters were women. However, it is interesting that, among women’s initial letters, 44% were “Acquaintance” letters and only 15% were categorized as “Romance.”
Given the severity of the murderer’s crime, it is remarkable that he received only 1 “Hate mail” letter.
Initial “Spiritual” letters were more likely to be followed by letters of the same category than any other category; “Romance” letters were a close second. This demonstrates the consistent efforts of writers in these 2 categories. Highly persistent writers (≥10 letters) were most likely to fall into “Acquaintance” and “Romance” categories. The persistence of these writers is remarkable, in view of the fact that none of their letters were answered. We hypothesize that the killer did not reply because he had no interest in correspondence.
Similarities to stalking. Given that 9 writers wrote >10 letters each and 2 wrote >20 each, elements of their behavior are not unlike what is seen in stalkers.3 Consistent with the stalking literature and Mullen et al4 stalker typology, many writers in this study appeared to seek intimacy with the perpetrator through “Romance” or “Show of support” letters, and might be akin to Mullen’s so-called intimacy-seeking stalker. Such stalkers’ behavior arises out of loneliness, with a strong desire for a relationship with the target; a significant percentage of such stalkers suffer a delusional disorder.
Mullen’s so-called incompetent suitor stalker is similar to the intimacy-seeking type but, instead, has an interest in a short-term relationship and is far less persistent in his (her) stalking behavior4; this type might apply to the writers in this study who wrote >1 but <10 letters.
Two additional observations also are notable when trying to characterize people who write letters: (1) A high percentage of people who stalk a celebrity suffer a psychotic disorder5,6; (2) 4 letter-writers traveled, and 1 relocated, to the murderer’s state of incarceration to attend his hearings and be closer to him.
This study has limitations:
• categorization of letters is inherently subjective and the categories themselves were created by the researchers
• the nature and categorization of such letters might vary considerably with the age and sex of the violent criminal; our findings in this case are not generalizable.
Last, researchers who plan to study writers of letters to incarcerated criminals should consider sending a personality test and other questionnaires to those writers to understand this population better.
Treatment considerations
Psychiatrists treating patients who seek a romantic attachment with a violent person should consider psychotherapy as a means of treating possible character pathology. The desire for romance with a violent criminal was greater among repeat writers (20%) than in initial letters (15%), suggesting that people who have a strong inclination to associate with a violent person might benefit from exploring romantic feelings in therapy. Specifically, therapists would be wise to explore with such patients the possibility that they experienced violence or verbal abuse in childhood or adulthood.
To the extent that evidence of prior abuse exists, a diagnosis of posttraumatic stress disorder (PTSD) might be appropriate; specialized therapy for men and women with a history of abuse might be indicated. It is important to provide validation for patients who are victims when they describe their abuse, and to stress that they did nothing to provoke the violence. Furthermore, investigation of why the patient feels drawn romantically toward a violent criminal is helpful, as well as an examination of how such behavior is self-defeating.
There might be value in having patients keep a journal in lieu of actually sending letters; there is evidence that “journaling” can reduce substance use recidivism.7 This work can be performed in conjunction with group or individual psychotherapy that addresses any history of abuse and subsequent PTSD.
Many patients are reluctant to discuss their romantic feelings toward a violent criminal until the psychiatrist has established a strong doctor−patient relationship. Last, clinicians should not hesitate to refer these patients to a therapist who specializes in domestic violence.
Related Resource
• Marazziti D, Falaschi V, Lombardi A, et al. Stalking: a neurobiological perspective. Riv Psichiatr. 2015;50(1):12-18.
Disclosures
The authors report no financial relationships with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
1. Mouradian VE. Women’s stay-leave decisions in relationships involving intimate partner violence. Wellesley, MA: Wellesley Centers for Women Publications; 2004:3,4.
2. Bell KM, Naugle AE. Understanding stay/leave decisions in violent relationships: a behavior analytic approach. Behav Soc Issues. 2005;14(1):21-46.
3. Westrup D, Fremouw WJ. Stalking behavior: a literature review and suggested functional analytic assessment technology. Aggression and Violent Behavior. 1998;3: 255-274.
4. Mullen PE, Pathé M, Purcell R, et al. Study of stalkers. Am J Psychiatry. 1999;156(8):1244-1249.
5. West SG, Friedman SH. These boots are made for stalking: characteristics of female stalkers. Psychiatry (Edgmont). 2008;5(8):37-42.
6. Nadkarni R, Grubin D. Stalking: why do people do it? BMJ. 2000;320(7248):1486-1487.
7. Proctor SL, Hoffmann NG, Allison S. The effectiveness of interactive journaling in reducing recidivism among substance-dependent jail inmates. Int J Offender Ther Comp Criminol. 2012;56(2):317-332.
Managing first-episode psychosis: Rationale and evidence for nonstandard first-line treatments for schizophrenia
First-episode psychosis (FEP) in schizophrenia is characterized by high response rates to antipsychotic therapy, followed by frequent antipsychotic discontinuation and elevated relapse rates soon after maintenance treatment begins.1,2 With subsequent episodes, time to response progressively increases and likelihood of response decreases.3,4
To address these issues, this article—the second of 2 parts5—describes the rationale and evidence for using nonstandard first-line antipsychotic therapies to manage FEP. Specifically, we discuss when clinicians might consider monotherapy exceeding FDA-approved maximum dosages, combination therapy, long-acting injectable antipsychotics (LAIA), or clozapine.
Monotherapy beyond FDA-approved dosages
Treatment guidelines for FEP recommend oral antipsychotic dosages in the lower half of the treatment range and lower than those that are required for multi-episode schizophrenia.6-16 Ultimately, clinicians prescribe individualized dosages for their patients based on symptom improvement and tolerability. The optimal dosage at which to achieve a favorable D2 receptor occupancy likely will vary from patient to patient.17
To control symptoms, higher dosages may be needed than those used in FEP clinical trials, recommended by guidelines for FEP or multi-episode patients, or approved by the FDA. Patients seen in everyday practice may be more complicated (eg, have a comorbid condition or history of nonresponse) than study populations. Higher dosages also may be reasonable to overcome drug−drug interactions (eg, cigarette smoking-mediated cytochrome P450 1A2 induction, resulting in increased olanzapine metabolism),18 or to establish antipsychotic failure if adequate trials at lower dosages have resulted in a suboptimal response and the patient is not experiencing tolerability or safety concerns.
In a study of low-, full-, and high-dosage antipsychotic therapy in FEP, an additional 15% of patients responded to higher dosages of olanzapine and risperidone after failing to respond to a standard dosage.19 A study of data from the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program (RAISE-ETP) found that, of participants identified who may benefit from therapy modification, 8.8% were prescribed an antipsychotic (often, olanzapine, risperidone, and haloperidol) at a higher-than-recommended dosage.20 Of note, only olanzapine was prescribed at higher than FDA-approved dosages.
Antipsychotic combination therapy
Prescribing combinations of antipsychotics—antipsychotic polypharmacy (APP)— has a negative connotation because of limited efficacy and safety data,21 and limited endorsement in schizophrenia treatment guidelines.9,13 Caution with APP is warranted; a complex medication regimen may increase the potential for adverse effects, poorer adherence, and adverse drug-drug interactions.9 APP has been shown to independently predict both shorter treatment duration and discontinuation before 1 year.22
Nonetheless, the clinician and patient may share the decision to implement APP and observe whether benefits outweigh risks in situations such as:
• to optimize neuroreceptor occupancy and targets (eg, attempting to achieve adequate D2 receptor blockade while minimizing side effects secondary to binding other receptors)
• to manage co-existing symptom domains (eg, mood changes, aggression, negative symptoms, disorganization, and cognitive deficits)
• to mitigate antipsychotic-induced side effects (eg, initiating aripiprazole to treat hyperprolactinemia induced by another antipsychotic to which the patient has achieved a favorable response).23
Clinicians report using APP to treat as many as 50% of patients with a history of multiple psychotic episodes.23 For FEP patients, 23% of participants in the RAISE-ETP trial who were identified as possibly benefiting from therapy modification were prescribed APP.20 Regrettably, researchers have not found evidence to support a reported rationale for using APP—that lower dosages of individual antipsychotics when used in combination may avoid high-dosage prescriptions.24
Before implementing APP, thoroughly explore and manage reasons for a patient’s suboptimal response to monotherapy.25 An adequate trial with any antipsychotic should be at the highest tolerated dosage for 12 to 16 weeks. Be mindful that response to an APP trial may be the result of additional time on the original antipsychotic.
Long-acting injectable antipsychotics in FEP
Guideline recommendations. Most older guidelines for schizophrenia treatment suggest LAIA after multiple relapses related to medication nonadherence or when a patient prefers injected medication (Table 1).6-13 Expert consensus guidelines also recommend considering LAIA in patients who lack insight into their illness. The Texas Medication Algorithm Project (TMAP) guidelines7 state LAIA can be considered for inadequate adherence at any stage, whereas the 2010 British Association for Psychopharmacology (BAP) guidelines9 express uncertainty about their use in FEP, because of limited evidence. Both the BAP and National Institute for Health and Care Excellence guidelines13 urge clinicians to consider LAIA when avoiding nonadherence is a treatment priority.
Recently, the French Association for Biological Psychiatry and Neuro-psychopharmacology (AFPBN) created expert consensus guidelines12 on using LAIA in practice. They recommend long-acting injectable second-generation antipsychotics (SGAs) as first-line maintenance treatment for schizophrenia and schizoaffective disorder and for individuals experiencing a first recurrent episode. The World Federation of Societies of Biological Psychiatry guidelines contain LAIA dosage recommendations for FEP (Table 2).10
Advances have been made in understanding the serious neurobiological adverse effects of psychotic relapses, including neuroinflammation and oxidative stress, that may explain the atrophic changes observed with psychotic episodes starting with the FEP. Protecting the patient from a second episode has become a vital therapeutic management goal26 (Figure 127).
Concerns. Compared with oral antipsychotics, LAIA offers clinical advantages:
• improved pharmacokinetic profile (lower “peaks” and higher “valleys”)
• more consistent plasma concentrations (no variability related to administration timing or food effects)
• no first-pass metabolism, which can ease the process of finding the lowest effective and safe dosage
• reduced administration burden and objective tracking of adherence with typical dosing every 2 to 4 weeks
• less stigmatizing than oral medication for FEP patients, such as college students living in a dormitory.28,29
Barriers to LAIA use include:
• slow dosage titration and increased time to reach steady state drug level
• oral supplementation for some (eg, risperidone microspheres and aripiprazole long-acting injectable)
• logistical challenges for some (eg, 3-hour post-injection monitoring for delirium sedation syndrome with olanzapine pamoate)
• additional planning to coordinate care for scheduled injections
• higher expenses up front
• local injection site reactions
• dosage adjustment difficulties if adverse effects occur.28,29
Adoption rates of LAIA are low, especially for FEP.30 Most surveys indicate that (1) physicians believe LAIA treatment is ineffective for FEP31 and (2) patients do not prefer injectable to oral antipsychotics,32 despite evidence to the contrary.33,34 A survey of 198 psychiatrists identified 3 factors that influenced their decisions against using LAIA patients with FEP:
• limited availability of SGA depot formulations (4, to date, in the United States)
• frequent rejection by the patient when LAIA is offered without adequate explanation or encouragement
• skepticism of FEP patients (and their family) who lack experience with relapse.35
In reality, when SGA depots were introduced in the United Kingdom, prescribing rates of LAIA did not increase. As for patient rejection being a major reason for not prescribing LAIA, few patients (5% to 36%) are offered depot injections, particularly in FEP.29 Most patients using LAIA are chronic, multi-episode, violent people who are receiving medications involuntarily.29 Interestingly, this survey did not find 2 factors to be influential in psychiatrists’ decision not to use LAIA in FEP:
• guidelines do not explicitly recommend depot treatment in FEP
• treatment in FEP may be limited to 1 year, therefore depot administration is not worthwhile.35
Preliminary evidence. At least a dozen studies have explored LAIA treatment for FEP, with the use of fluphenazine decanoate,36 perphenazine enanthate37 (discontinued), and risperidone microspheres.37-48 The research demonstrates the efficacy and safety of LAIA in FEP as measured by these endpoints:
• improved symptom control38,40-43,46,48
• adherence43,44,48
• reduced relapse rates37,43 and rehospitalizations37,47
• lesser reductions in white matter brain volume45
• no differences in extrapyramidal side effects or prolactin-associated adverse effects.48
A few small studies demonstrate significant differences in outcomes between risperidone LAIA and oral comparator groups (Table 3).43-45 Ongoing studies of LAIA use in FEP are comparing paliperidone palmitate with risperidone microspheres and other oral antipsychotics.49-51 No studies are examining olanzapine pamoate in FEP, likely because several guidelines do not recommended its use. No studies have been published regarding aripiprazole long-acting injectable in FEP. This LAIA formulation was approved in February 2013, and robust studies of the oral formulation in FEP are limited.52
Discussion and recommendations. Psychiatrists relying on subjective measures of antipsychotic adherence may inaccurately assess whether patients meet this criterion for LAIA use.53 LAIA could combat the high relapse rate in FEP, yet depot antipsychotics are prescribed infrequently for FEP patients (eg, for only 9.5% of participants in the RAISE-ETP study).20 Most schizophrenia treatment guidelines do not discuss LAIA use specifically in FEP, although the AFPBN expert consensus guidelines published in 2013 do recommend SGA depot formulations in FEP.12 SGA LAIA may be preferable, given its neuroprotective effects, in contrast to the neurotoxicity concerns of FGA LAIA.54,55
Relapses begin within a few months of illness stabilization after FEP, and >50% of patients relapse within 1 or 2 years2—the recommended minimum treatment duration for FEP.8,9,13 The use of LAIA is advisable in any patient with schizophrenia for whom long-term antipsychotic therapy is indicated.56 LAIA administration requirements objectively track medication adherence, which allows clinicians to be proactive in relapse prevention. Not using an intervention in FEP that improves adherence and decreases relapse rates contradicts our goal of instituting early, effective treatment to improve long-term functional outcomes (Figure 2).29
Considering clozapine in FEP
Guideline recommendations. Schizo-phrenia treatment guidelines and FDA labeling57 reserve clozapine for third-line treatment of refractory schizophrenia after 2 adequate antipsychotic trials have failed despite optimal dosing (Table 1).6-13 Some guidelines specify 1 of the 2 failed antipsychotic trials must include an SGA.6,7,10,11,13-16 Most say clozapine may be considered in patients with chronic aggression or hostility,7-9,14,16 or suicidal thoughts and behaviors.6-8,14,16 TMAP guidelines recommend a clozapine trial with concomitant substance abuse, persistent positive symptoms during 2 years of consistent medication treatment, and after 5 years of inadequate response (“treatment resistance”), regardless of the number of antipsychotic trials.7
Rationale and concerns. Clozapine is a superior choice for treatment-refractory delusions or hallucinations of schizophrenia, because it markedly enhances the response rate to antipsychotic therapy.58 Researchers therefore have investigated whether clozapine, compared with other antipsychotics, would yield more favorable initial and long-term outcomes when used first-line in FEP.
Preliminary evidence. Five studies have explored the use of clozapine as first-line therapy in FEP (Table 4).59-63 Interpreting the results is difficult because clozapine trials may be brief (mostly, 12 to 52 weeks); lack a comparator arm; suffer from a high attrition rate; enroll few patients; and lack potentially important outcome measures such as negative symptoms, suicidality, and functional assessment.
Overall, these studies demonstrate clozapine is as efficacious in this patient population as chlorpromazine (no difference in remission at 1-year, although clozapine-treated patients remitted faster and stayed in remission longer)60,61 or risperidone (no difference in Positive and Negative Syndrome Scale scores).62
At present, clozapine has not been shown superior to other antipsychotics as a first-line treatment for FEP. Research does underscore the importance of a clozapine trial as third-line treatment for FEP patients who have not responded well to 2 SGA trials.63 Many of these nonresponders (77%) have demonstrated a favorable response when promptly switched to clozapine.64
Discussion and recommendations. The limited evidence argues against using clozapine earlier than as third-line treatment in FEP. Perhaps the high treatment response that characterizes FEP creates a ceiling effect that obscures differences in antipsychotic efficacy at this stage.65 Clozapine use as first-line treatment should be re-evaluated with more robust methodology. One approach could be to assess its benefit in FEP by the duration of untreated psychosis.
The odds of achieving remission have been shown to decrease by 15% for each year that psychosis has not been treated.59 Studies exploring the use of clozapine as a second-line agent for FEP also are warranted, as antipsychotic response during subsequent trials is substantially reduced. In fact, the Scottish Intercollegiate Guidelines Network guidelines recommend this as an area for future research.11
For now, clozapine should continue to be reserved as second- or third-line treatment in a patient with FEP. The risks of clozapine’s potentially serious adverse effects (eg, agranulocytosis, seizures, obesity, diabetes, dyslipidemia, myocarditis, pancreatitis, hypotension, sialorrhea, severe sedation, ileus) can be justified only in the treatment of severe and persistent psychotic symptoms.57
Bottom Line
Nonstandard use of antipsychotic monotherapy dosages beyond the approved FDA limit and combination antipsychotic therapy may be reasonable for select first-episode psychosis (FEP) patients. Strongly consider long-acting injectable antipsychotics in FEP to proactively combat the high relapse rate and more easily identify antipsychotic failure. Continue to use clozapine as second- or third-line therapy in FEP: Studies have not found that it is more efficacious than other antipsychotics for first-line use.
Related Resource
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
Drug Brand Names
Aripiprazole • Abilify, Abilify Maintena
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fluphenazine decanoate • Prolixin-D
Haloperidol • Haldol
Haloperidol decanoate • Haldol-D
Olanzapine • Zyprexa
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone microspheres • Risperdal Consta
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products. Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Lieberman JA, Koreen AR, Chakos M, et al. Factors influencing treatment response and outcome of first-episode schizophrenia: implications for understanding the pathophysiology of schizophrenia. J Clin Psychiatry. 1996;57(suppl 9):5-9.
4. Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439-1444.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis. An early stage of schizophrenia with distinct treatment needs. Current Psychiatry. 2015;14(5):32-34,36-40,42.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Texas Department of State Health Services. Texas Medication Algorithm Project (TMAP) Procedural Manual. Schizophrenia Treatment Algorithms. http://www.jpshealthnet.org/sites/default/files/ tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed June 11, 2015.
8. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
9. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
10. Hasan A, Falkai P, Wobrok T, et al; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
11. Scottish Intercollegiate Guidelines Network. SIGN 131: Management of schizophrenia. http://www.sign.ac.uk/ pdf/sign131.pdf. Published March 2013. Accessed June 11, 2015.
12. Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serous mental illness. BMC Psychiatry. 2013;13:340.
13. National Institute for Health and Care Excellence. NICE clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. https://www.nice.org. uk/guidance/cg178/resources/guidance-psychosis-and-schizophrenia-in-adults-treatment-and-management-pdf. Updated March 2014. Accessed June 16, 2015.
14. Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
15. McEvoy JP, Scheifler PL, Frances A. The expert consensus guideline series: treatment of schizophrenia. J Clin Psychiatry. 1999;60(suppl 11):3-80.
16. Marder SR, Essock SM, Miller AL, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
17. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
18. Fankhauser MP. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
19. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
20. Robinson DG, Schooler NR, John M, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172(3):237-248.
21. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
22. Fisher MD, Reilly K, Isenberg K, et al. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC Psychiatry. 2014;14(1):341.
23. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
24. John AP, Dragovic M. Antipsychotic polypharmacy is not associated with reduced dose of individual antipsychotics in schizophrenia. J Clin Psychopharmacol. 2015;35(2):193-195.
25. Nasrallah HA. Treatment-resistant schizophrenia. Current Psychiatry. http://www.currentpsychiatry.com/specialty-focus/schizophrenia-other-psychotic-disorders/article/ treatment-resistant-schizophrenia/9be7bba3713d4a4cd68aa 8c92b79e5b1.html. Accessed June 16, 2015.
26. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
27. Nasrallah HA, Smeltzer DJ. Contemporary diagnosis and management of the patient with schizophrenia. 2nd ed. Newton, PA: Handbooks in Health Care Co; 2011.
28. McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15-18.
29. Agid O, Foussias G, Remington G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin Pharmacother. 2010;11(14):2301-2317.
30. Kirschner M, Theodoridou A, Fusar-Poli P, et al. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode psychosis. Ther Adv Psychophamacol. 2013;3(2):89-99.
31. Heres S, Hamann J, Mendel R, et al. Identifying the profile of optimal candidates for antipsychotic depot therapy: A cluster analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1987-1993.
32. Heres S, Lambert M, Vauth R. Treatment of early episode in patents with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014;29(suppl 2):1409-1413.
33. Heres S, Schmitz FS, Leucht S, et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275-282.
34. Weiden PJ, Schooler NR, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
35. Heres S, Reichhart T, Hamann J, et al. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301.
36. Kane JM, Rifkin A, Quitkin F, et al. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39(1):70-73.
37. Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in a community care after first hospitalization due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224.
38. Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19(suppl 5):5-14.
39. Malla A, Binder C, Chue P. Comparison of long-acting injectable risperidone and oral novel antipsychotic drugs for treatment in early phase of schizophrenia spectrum psychosis. Proceedings of the 61st Annual Convention Society of Biological Psychiatry; Toronto, Canada; 2006.
40. Lasser RA, Bossie CA, Zhu Y, et al. Long-acting risperidone in young adults with early schizophrenia or schizoaffective illness. Ann Clin Psychiatry. 2007;19(2):65-71.
41. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
42. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
43. Kim B, Lee SH, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231-1235.
44. Weiden PJ, Schooler NJ, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
45. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011;132(1):35-41.
46. Napryeyenko O, Burba B, Martinez G, et al. Risperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measures. J Clin Psychopharmacol. 2010;30(2):200-202.
47. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609.
48. Dubois V, Megens J, Mertens C, et al. Long-acting risperidone in early-episode schizophrenia. Acta Psychiatrica Belgica. 2011;111(1):9-21.
49. ClinicalTrials.gov. Oral risperidone versus injectable paliperidone palmitate for treating first-episode schizophrenia. https://clinicaltrials.gov/ct2/show/ NCT01451736. Accessed June 16, 2015.
50. ClinicalTrials.gov. Brain myelination effects of paliperidone palmitate versus oral risperidone in first episode schizophrenia. https://clinicaltrials.gov/ct2/ show/NCT01458379. Accessed June 16, 2015.
51. ClinicalTrials.gov. Effects of paliperidone palmitate versus oral antipsychotics on clinical outcomes and MRI measures. https://clinicaltrials.gov/ct2/show/NCT01359293. Accessed June 16, 2016.
52. U.S. Food and Drug Administration. Drugs@FDA. http:// www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed January 11, 2015.
53. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
54. Nandra KS, Agius M. The difference between typical and atypical antipsychotics: the effects on neurogenesis. Psychiatr Danub. 2012;24(suppl 1):S95-S99.
55. Nasrallah HA. Haloperidol is clearly neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
56. Kane JM, Garcia-Ribora C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;52:S63-S67.
57. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
58. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
59. Woerner MG, Robinson DG, Alvir JMJ, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160(8):1514-1516.
60. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
61. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
62. Sanz-Fuentenebro J, Taboada D, Palomo T, et al. Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149(1-3):156-161.
63. Yang PD, Ji Z. The efficacy and related factors of clozapine on first-episode schizophrenia. Chin J Nerv Ment Dis. 1997;23:155-158.
64. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
65. Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170(2):146-151.
First-episode psychosis (FEP) in schizophrenia is characterized by high response rates to antipsychotic therapy, followed by frequent antipsychotic discontinuation and elevated relapse rates soon after maintenance treatment begins.1,2 With subsequent episodes, time to response progressively increases and likelihood of response decreases.3,4
To address these issues, this article—the second of 2 parts5—describes the rationale and evidence for using nonstandard first-line antipsychotic therapies to manage FEP. Specifically, we discuss when clinicians might consider monotherapy exceeding FDA-approved maximum dosages, combination therapy, long-acting injectable antipsychotics (LAIA), or clozapine.
Monotherapy beyond FDA-approved dosages
Treatment guidelines for FEP recommend oral antipsychotic dosages in the lower half of the treatment range and lower than those that are required for multi-episode schizophrenia.6-16 Ultimately, clinicians prescribe individualized dosages for their patients based on symptom improvement and tolerability. The optimal dosage at which to achieve a favorable D2 receptor occupancy likely will vary from patient to patient.17
To control symptoms, higher dosages may be needed than those used in FEP clinical trials, recommended by guidelines for FEP or multi-episode patients, or approved by the FDA. Patients seen in everyday practice may be more complicated (eg, have a comorbid condition or history of nonresponse) than study populations. Higher dosages also may be reasonable to overcome drug−drug interactions (eg, cigarette smoking-mediated cytochrome P450 1A2 induction, resulting in increased olanzapine metabolism),18 or to establish antipsychotic failure if adequate trials at lower dosages have resulted in a suboptimal response and the patient is not experiencing tolerability or safety concerns.
In a study of low-, full-, and high-dosage antipsychotic therapy in FEP, an additional 15% of patients responded to higher dosages of olanzapine and risperidone after failing to respond to a standard dosage.19 A study of data from the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program (RAISE-ETP) found that, of participants identified who may benefit from therapy modification, 8.8% were prescribed an antipsychotic (often, olanzapine, risperidone, and haloperidol) at a higher-than-recommended dosage.20 Of note, only olanzapine was prescribed at higher than FDA-approved dosages.
Antipsychotic combination therapy
Prescribing combinations of antipsychotics—antipsychotic polypharmacy (APP)— has a negative connotation because of limited efficacy and safety data,21 and limited endorsement in schizophrenia treatment guidelines.9,13 Caution with APP is warranted; a complex medication regimen may increase the potential for adverse effects, poorer adherence, and adverse drug-drug interactions.9 APP has been shown to independently predict both shorter treatment duration and discontinuation before 1 year.22
Nonetheless, the clinician and patient may share the decision to implement APP and observe whether benefits outweigh risks in situations such as:
• to optimize neuroreceptor occupancy and targets (eg, attempting to achieve adequate D2 receptor blockade while minimizing side effects secondary to binding other receptors)
• to manage co-existing symptom domains (eg, mood changes, aggression, negative symptoms, disorganization, and cognitive deficits)
• to mitigate antipsychotic-induced side effects (eg, initiating aripiprazole to treat hyperprolactinemia induced by another antipsychotic to which the patient has achieved a favorable response).23
Clinicians report using APP to treat as many as 50% of patients with a history of multiple psychotic episodes.23 For FEP patients, 23% of participants in the RAISE-ETP trial who were identified as possibly benefiting from therapy modification were prescribed APP.20 Regrettably, researchers have not found evidence to support a reported rationale for using APP—that lower dosages of individual antipsychotics when used in combination may avoid high-dosage prescriptions.24
Before implementing APP, thoroughly explore and manage reasons for a patient’s suboptimal response to monotherapy.25 An adequate trial with any antipsychotic should be at the highest tolerated dosage for 12 to 16 weeks. Be mindful that response to an APP trial may be the result of additional time on the original antipsychotic.
Long-acting injectable antipsychotics in FEP
Guideline recommendations. Most older guidelines for schizophrenia treatment suggest LAIA after multiple relapses related to medication nonadherence or when a patient prefers injected medication (Table 1).6-13 Expert consensus guidelines also recommend considering LAIA in patients who lack insight into their illness. The Texas Medication Algorithm Project (TMAP) guidelines7 state LAIA can be considered for inadequate adherence at any stage, whereas the 2010 British Association for Psychopharmacology (BAP) guidelines9 express uncertainty about their use in FEP, because of limited evidence. Both the BAP and National Institute for Health and Care Excellence guidelines13 urge clinicians to consider LAIA when avoiding nonadherence is a treatment priority.
Recently, the French Association for Biological Psychiatry and Neuro-psychopharmacology (AFPBN) created expert consensus guidelines12 on using LAIA in practice. They recommend long-acting injectable second-generation antipsychotics (SGAs) as first-line maintenance treatment for schizophrenia and schizoaffective disorder and for individuals experiencing a first recurrent episode. The World Federation of Societies of Biological Psychiatry guidelines contain LAIA dosage recommendations for FEP (Table 2).10
Advances have been made in understanding the serious neurobiological adverse effects of psychotic relapses, including neuroinflammation and oxidative stress, that may explain the atrophic changes observed with psychotic episodes starting with the FEP. Protecting the patient from a second episode has become a vital therapeutic management goal26 (Figure 127).
Concerns. Compared with oral antipsychotics, LAIA offers clinical advantages:
• improved pharmacokinetic profile (lower “peaks” and higher “valleys”)
• more consistent plasma concentrations (no variability related to administration timing or food effects)
• no first-pass metabolism, which can ease the process of finding the lowest effective and safe dosage
• reduced administration burden and objective tracking of adherence with typical dosing every 2 to 4 weeks
• less stigmatizing than oral medication for FEP patients, such as college students living in a dormitory.28,29
Barriers to LAIA use include:
• slow dosage titration and increased time to reach steady state drug level
• oral supplementation for some (eg, risperidone microspheres and aripiprazole long-acting injectable)
• logistical challenges for some (eg, 3-hour post-injection monitoring for delirium sedation syndrome with olanzapine pamoate)
• additional planning to coordinate care for scheduled injections
• higher expenses up front
• local injection site reactions
• dosage adjustment difficulties if adverse effects occur.28,29
Adoption rates of LAIA are low, especially for FEP.30 Most surveys indicate that (1) physicians believe LAIA treatment is ineffective for FEP31 and (2) patients do not prefer injectable to oral antipsychotics,32 despite evidence to the contrary.33,34 A survey of 198 psychiatrists identified 3 factors that influenced their decisions against using LAIA patients with FEP:
• limited availability of SGA depot formulations (4, to date, in the United States)
• frequent rejection by the patient when LAIA is offered without adequate explanation or encouragement
• skepticism of FEP patients (and their family) who lack experience with relapse.35
In reality, when SGA depots were introduced in the United Kingdom, prescribing rates of LAIA did not increase. As for patient rejection being a major reason for not prescribing LAIA, few patients (5% to 36%) are offered depot injections, particularly in FEP.29 Most patients using LAIA are chronic, multi-episode, violent people who are receiving medications involuntarily.29 Interestingly, this survey did not find 2 factors to be influential in psychiatrists’ decision not to use LAIA in FEP:
• guidelines do not explicitly recommend depot treatment in FEP
• treatment in FEP may be limited to 1 year, therefore depot administration is not worthwhile.35
Preliminary evidence. At least a dozen studies have explored LAIA treatment for FEP, with the use of fluphenazine decanoate,36 perphenazine enanthate37 (discontinued), and risperidone microspheres.37-48 The research demonstrates the efficacy and safety of LAIA in FEP as measured by these endpoints:
• improved symptom control38,40-43,46,48
• adherence43,44,48
• reduced relapse rates37,43 and rehospitalizations37,47
• lesser reductions in white matter brain volume45
• no differences in extrapyramidal side effects or prolactin-associated adverse effects.48
A few small studies demonstrate significant differences in outcomes between risperidone LAIA and oral comparator groups (Table 3).43-45 Ongoing studies of LAIA use in FEP are comparing paliperidone palmitate with risperidone microspheres and other oral antipsychotics.49-51 No studies are examining olanzapine pamoate in FEP, likely because several guidelines do not recommended its use. No studies have been published regarding aripiprazole long-acting injectable in FEP. This LAIA formulation was approved in February 2013, and robust studies of the oral formulation in FEP are limited.52
Discussion and recommendations. Psychiatrists relying on subjective measures of antipsychotic adherence may inaccurately assess whether patients meet this criterion for LAIA use.53 LAIA could combat the high relapse rate in FEP, yet depot antipsychotics are prescribed infrequently for FEP patients (eg, for only 9.5% of participants in the RAISE-ETP study).20 Most schizophrenia treatment guidelines do not discuss LAIA use specifically in FEP, although the AFPBN expert consensus guidelines published in 2013 do recommend SGA depot formulations in FEP.12 SGA LAIA may be preferable, given its neuroprotective effects, in contrast to the neurotoxicity concerns of FGA LAIA.54,55
Relapses begin within a few months of illness stabilization after FEP, and >50% of patients relapse within 1 or 2 years2—the recommended minimum treatment duration for FEP.8,9,13 The use of LAIA is advisable in any patient with schizophrenia for whom long-term antipsychotic therapy is indicated.56 LAIA administration requirements objectively track medication adherence, which allows clinicians to be proactive in relapse prevention. Not using an intervention in FEP that improves adherence and decreases relapse rates contradicts our goal of instituting early, effective treatment to improve long-term functional outcomes (Figure 2).29
Considering clozapine in FEP
Guideline recommendations. Schizo-phrenia treatment guidelines and FDA labeling57 reserve clozapine for third-line treatment of refractory schizophrenia after 2 adequate antipsychotic trials have failed despite optimal dosing (Table 1).6-13 Some guidelines specify 1 of the 2 failed antipsychotic trials must include an SGA.6,7,10,11,13-16 Most say clozapine may be considered in patients with chronic aggression or hostility,7-9,14,16 or suicidal thoughts and behaviors.6-8,14,16 TMAP guidelines recommend a clozapine trial with concomitant substance abuse, persistent positive symptoms during 2 years of consistent medication treatment, and after 5 years of inadequate response (“treatment resistance”), regardless of the number of antipsychotic trials.7
Rationale and concerns. Clozapine is a superior choice for treatment-refractory delusions or hallucinations of schizophrenia, because it markedly enhances the response rate to antipsychotic therapy.58 Researchers therefore have investigated whether clozapine, compared with other antipsychotics, would yield more favorable initial and long-term outcomes when used first-line in FEP.
Preliminary evidence. Five studies have explored the use of clozapine as first-line therapy in FEP (Table 4).59-63 Interpreting the results is difficult because clozapine trials may be brief (mostly, 12 to 52 weeks); lack a comparator arm; suffer from a high attrition rate; enroll few patients; and lack potentially important outcome measures such as negative symptoms, suicidality, and functional assessment.
Overall, these studies demonstrate clozapine is as efficacious in this patient population as chlorpromazine (no difference in remission at 1-year, although clozapine-treated patients remitted faster and stayed in remission longer)60,61 or risperidone (no difference in Positive and Negative Syndrome Scale scores).62
At present, clozapine has not been shown superior to other antipsychotics as a first-line treatment for FEP. Research does underscore the importance of a clozapine trial as third-line treatment for FEP patients who have not responded well to 2 SGA trials.63 Many of these nonresponders (77%) have demonstrated a favorable response when promptly switched to clozapine.64
Discussion and recommendations. The limited evidence argues against using clozapine earlier than as third-line treatment in FEP. Perhaps the high treatment response that characterizes FEP creates a ceiling effect that obscures differences in antipsychotic efficacy at this stage.65 Clozapine use as first-line treatment should be re-evaluated with more robust methodology. One approach could be to assess its benefit in FEP by the duration of untreated psychosis.
The odds of achieving remission have been shown to decrease by 15% for each year that psychosis has not been treated.59 Studies exploring the use of clozapine as a second-line agent for FEP also are warranted, as antipsychotic response during subsequent trials is substantially reduced. In fact, the Scottish Intercollegiate Guidelines Network guidelines recommend this as an area for future research.11
For now, clozapine should continue to be reserved as second- or third-line treatment in a patient with FEP. The risks of clozapine’s potentially serious adverse effects (eg, agranulocytosis, seizures, obesity, diabetes, dyslipidemia, myocarditis, pancreatitis, hypotension, sialorrhea, severe sedation, ileus) can be justified only in the treatment of severe and persistent psychotic symptoms.57
Bottom Line
Nonstandard use of antipsychotic monotherapy dosages beyond the approved FDA limit and combination antipsychotic therapy may be reasonable for select first-episode psychosis (FEP) patients. Strongly consider long-acting injectable antipsychotics in FEP to proactively combat the high relapse rate and more easily identify antipsychotic failure. Continue to use clozapine as second- or third-line therapy in FEP: Studies have not found that it is more efficacious than other antipsychotics for first-line use.
Related Resource
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
Drug Brand Names
Aripiprazole • Abilify, Abilify Maintena
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fluphenazine decanoate • Prolixin-D
Haloperidol • Haldol
Haloperidol decanoate • Haldol-D
Olanzapine • Zyprexa
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone microspheres • Risperdal Consta
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products. Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
First-episode psychosis (FEP) in schizophrenia is characterized by high response rates to antipsychotic therapy, followed by frequent antipsychotic discontinuation and elevated relapse rates soon after maintenance treatment begins.1,2 With subsequent episodes, time to response progressively increases and likelihood of response decreases.3,4
To address these issues, this article—the second of 2 parts5—describes the rationale and evidence for using nonstandard first-line antipsychotic therapies to manage FEP. Specifically, we discuss when clinicians might consider monotherapy exceeding FDA-approved maximum dosages, combination therapy, long-acting injectable antipsychotics (LAIA), or clozapine.
Monotherapy beyond FDA-approved dosages
Treatment guidelines for FEP recommend oral antipsychotic dosages in the lower half of the treatment range and lower than those that are required for multi-episode schizophrenia.6-16 Ultimately, clinicians prescribe individualized dosages for their patients based on symptom improvement and tolerability. The optimal dosage at which to achieve a favorable D2 receptor occupancy likely will vary from patient to patient.17
To control symptoms, higher dosages may be needed than those used in FEP clinical trials, recommended by guidelines for FEP or multi-episode patients, or approved by the FDA. Patients seen in everyday practice may be more complicated (eg, have a comorbid condition or history of nonresponse) than study populations. Higher dosages also may be reasonable to overcome drug−drug interactions (eg, cigarette smoking-mediated cytochrome P450 1A2 induction, resulting in increased olanzapine metabolism),18 or to establish antipsychotic failure if adequate trials at lower dosages have resulted in a suboptimal response and the patient is not experiencing tolerability or safety concerns.
In a study of low-, full-, and high-dosage antipsychotic therapy in FEP, an additional 15% of patients responded to higher dosages of olanzapine and risperidone after failing to respond to a standard dosage.19 A study of data from the Recovery After an Initial Schizophrenia Episode Project’s Early Treatment Program (RAISE-ETP) found that, of participants identified who may benefit from therapy modification, 8.8% were prescribed an antipsychotic (often, olanzapine, risperidone, and haloperidol) at a higher-than-recommended dosage.20 Of note, only olanzapine was prescribed at higher than FDA-approved dosages.
Antipsychotic combination therapy
Prescribing combinations of antipsychotics—antipsychotic polypharmacy (APP)— has a negative connotation because of limited efficacy and safety data,21 and limited endorsement in schizophrenia treatment guidelines.9,13 Caution with APP is warranted; a complex medication regimen may increase the potential for adverse effects, poorer adherence, and adverse drug-drug interactions.9 APP has been shown to independently predict both shorter treatment duration and discontinuation before 1 year.22
Nonetheless, the clinician and patient may share the decision to implement APP and observe whether benefits outweigh risks in situations such as:
• to optimize neuroreceptor occupancy and targets (eg, attempting to achieve adequate D2 receptor blockade while minimizing side effects secondary to binding other receptors)
• to manage co-existing symptom domains (eg, mood changes, aggression, negative symptoms, disorganization, and cognitive deficits)
• to mitigate antipsychotic-induced side effects (eg, initiating aripiprazole to treat hyperprolactinemia induced by another antipsychotic to which the patient has achieved a favorable response).23
Clinicians report using APP to treat as many as 50% of patients with a history of multiple psychotic episodes.23 For FEP patients, 23% of participants in the RAISE-ETP trial who were identified as possibly benefiting from therapy modification were prescribed APP.20 Regrettably, researchers have not found evidence to support a reported rationale for using APP—that lower dosages of individual antipsychotics when used in combination may avoid high-dosage prescriptions.24
Before implementing APP, thoroughly explore and manage reasons for a patient’s suboptimal response to monotherapy.25 An adequate trial with any antipsychotic should be at the highest tolerated dosage for 12 to 16 weeks. Be mindful that response to an APP trial may be the result of additional time on the original antipsychotic.
Long-acting injectable antipsychotics in FEP
Guideline recommendations. Most older guidelines for schizophrenia treatment suggest LAIA after multiple relapses related to medication nonadherence or when a patient prefers injected medication (Table 1).6-13 Expert consensus guidelines also recommend considering LAIA in patients who lack insight into their illness. The Texas Medication Algorithm Project (TMAP) guidelines7 state LAIA can be considered for inadequate adherence at any stage, whereas the 2010 British Association for Psychopharmacology (BAP) guidelines9 express uncertainty about their use in FEP, because of limited evidence. Both the BAP and National Institute for Health and Care Excellence guidelines13 urge clinicians to consider LAIA when avoiding nonadherence is a treatment priority.
Recently, the French Association for Biological Psychiatry and Neuro-psychopharmacology (AFPBN) created expert consensus guidelines12 on using LAIA in practice. They recommend long-acting injectable second-generation antipsychotics (SGAs) as first-line maintenance treatment for schizophrenia and schizoaffective disorder and for individuals experiencing a first recurrent episode. The World Federation of Societies of Biological Psychiatry guidelines contain LAIA dosage recommendations for FEP (Table 2).10
Advances have been made in understanding the serious neurobiological adverse effects of psychotic relapses, including neuroinflammation and oxidative stress, that may explain the atrophic changes observed with psychotic episodes starting with the FEP. Protecting the patient from a second episode has become a vital therapeutic management goal26 (Figure 127).
Concerns. Compared with oral antipsychotics, LAIA offers clinical advantages:
• improved pharmacokinetic profile (lower “peaks” and higher “valleys”)
• more consistent plasma concentrations (no variability related to administration timing or food effects)
• no first-pass metabolism, which can ease the process of finding the lowest effective and safe dosage
• reduced administration burden and objective tracking of adherence with typical dosing every 2 to 4 weeks
• less stigmatizing than oral medication for FEP patients, such as college students living in a dormitory.28,29
Barriers to LAIA use include:
• slow dosage titration and increased time to reach steady state drug level
• oral supplementation for some (eg, risperidone microspheres and aripiprazole long-acting injectable)
• logistical challenges for some (eg, 3-hour post-injection monitoring for delirium sedation syndrome with olanzapine pamoate)
• additional planning to coordinate care for scheduled injections
• higher expenses up front
• local injection site reactions
• dosage adjustment difficulties if adverse effects occur.28,29
Adoption rates of LAIA are low, especially for FEP.30 Most surveys indicate that (1) physicians believe LAIA treatment is ineffective for FEP31 and (2) patients do not prefer injectable to oral antipsychotics,32 despite evidence to the contrary.33,34 A survey of 198 psychiatrists identified 3 factors that influenced their decisions against using LAIA patients with FEP:
• limited availability of SGA depot formulations (4, to date, in the United States)
• frequent rejection by the patient when LAIA is offered without adequate explanation or encouragement
• skepticism of FEP patients (and their family) who lack experience with relapse.35
In reality, when SGA depots were introduced in the United Kingdom, prescribing rates of LAIA did not increase. As for patient rejection being a major reason for not prescribing LAIA, few patients (5% to 36%) are offered depot injections, particularly in FEP.29 Most patients using LAIA are chronic, multi-episode, violent people who are receiving medications involuntarily.29 Interestingly, this survey did not find 2 factors to be influential in psychiatrists’ decision not to use LAIA in FEP:
• guidelines do not explicitly recommend depot treatment in FEP
• treatment in FEP may be limited to 1 year, therefore depot administration is not worthwhile.35
Preliminary evidence. At least a dozen studies have explored LAIA treatment for FEP, with the use of fluphenazine decanoate,36 perphenazine enanthate37 (discontinued), and risperidone microspheres.37-48 The research demonstrates the efficacy and safety of LAIA in FEP as measured by these endpoints:
• improved symptom control38,40-43,46,48
• adherence43,44,48
• reduced relapse rates37,43 and rehospitalizations37,47
• lesser reductions in white matter brain volume45
• no differences in extrapyramidal side effects or prolactin-associated adverse effects.48
A few small studies demonstrate significant differences in outcomes between risperidone LAIA and oral comparator groups (Table 3).43-45 Ongoing studies of LAIA use in FEP are comparing paliperidone palmitate with risperidone microspheres and other oral antipsychotics.49-51 No studies are examining olanzapine pamoate in FEP, likely because several guidelines do not recommended its use. No studies have been published regarding aripiprazole long-acting injectable in FEP. This LAIA formulation was approved in February 2013, and robust studies of the oral formulation in FEP are limited.52
Discussion and recommendations. Psychiatrists relying on subjective measures of antipsychotic adherence may inaccurately assess whether patients meet this criterion for LAIA use.53 LAIA could combat the high relapse rate in FEP, yet depot antipsychotics are prescribed infrequently for FEP patients (eg, for only 9.5% of participants in the RAISE-ETP study).20 Most schizophrenia treatment guidelines do not discuss LAIA use specifically in FEP, although the AFPBN expert consensus guidelines published in 2013 do recommend SGA depot formulations in FEP.12 SGA LAIA may be preferable, given its neuroprotective effects, in contrast to the neurotoxicity concerns of FGA LAIA.54,55
Relapses begin within a few months of illness stabilization after FEP, and >50% of patients relapse within 1 or 2 years2—the recommended minimum treatment duration for FEP.8,9,13 The use of LAIA is advisable in any patient with schizophrenia for whom long-term antipsychotic therapy is indicated.56 LAIA administration requirements objectively track medication adherence, which allows clinicians to be proactive in relapse prevention. Not using an intervention in FEP that improves adherence and decreases relapse rates contradicts our goal of instituting early, effective treatment to improve long-term functional outcomes (Figure 2).29
Considering clozapine in FEP
Guideline recommendations. Schizo-phrenia treatment guidelines and FDA labeling57 reserve clozapine for third-line treatment of refractory schizophrenia after 2 adequate antipsychotic trials have failed despite optimal dosing (Table 1).6-13 Some guidelines specify 1 of the 2 failed antipsychotic trials must include an SGA.6,7,10,11,13-16 Most say clozapine may be considered in patients with chronic aggression or hostility,7-9,14,16 or suicidal thoughts and behaviors.6-8,14,16 TMAP guidelines recommend a clozapine trial with concomitant substance abuse, persistent positive symptoms during 2 years of consistent medication treatment, and after 5 years of inadequate response (“treatment resistance”), regardless of the number of antipsychotic trials.7
Rationale and concerns. Clozapine is a superior choice for treatment-refractory delusions or hallucinations of schizophrenia, because it markedly enhances the response rate to antipsychotic therapy.58 Researchers therefore have investigated whether clozapine, compared with other antipsychotics, would yield more favorable initial and long-term outcomes when used first-line in FEP.
Preliminary evidence. Five studies have explored the use of clozapine as first-line therapy in FEP (Table 4).59-63 Interpreting the results is difficult because clozapine trials may be brief (mostly, 12 to 52 weeks); lack a comparator arm; suffer from a high attrition rate; enroll few patients; and lack potentially important outcome measures such as negative symptoms, suicidality, and functional assessment.
Overall, these studies demonstrate clozapine is as efficacious in this patient population as chlorpromazine (no difference in remission at 1-year, although clozapine-treated patients remitted faster and stayed in remission longer)60,61 or risperidone (no difference in Positive and Negative Syndrome Scale scores).62
At present, clozapine has not been shown superior to other antipsychotics as a first-line treatment for FEP. Research does underscore the importance of a clozapine trial as third-line treatment for FEP patients who have not responded well to 2 SGA trials.63 Many of these nonresponders (77%) have demonstrated a favorable response when promptly switched to clozapine.64
Discussion and recommendations. The limited evidence argues against using clozapine earlier than as third-line treatment in FEP. Perhaps the high treatment response that characterizes FEP creates a ceiling effect that obscures differences in antipsychotic efficacy at this stage.65 Clozapine use as first-line treatment should be re-evaluated with more robust methodology. One approach could be to assess its benefit in FEP by the duration of untreated psychosis.
The odds of achieving remission have been shown to decrease by 15% for each year that psychosis has not been treated.59 Studies exploring the use of clozapine as a second-line agent for FEP also are warranted, as antipsychotic response during subsequent trials is substantially reduced. In fact, the Scottish Intercollegiate Guidelines Network guidelines recommend this as an area for future research.11
For now, clozapine should continue to be reserved as second- or third-line treatment in a patient with FEP. The risks of clozapine’s potentially serious adverse effects (eg, agranulocytosis, seizures, obesity, diabetes, dyslipidemia, myocarditis, pancreatitis, hypotension, sialorrhea, severe sedation, ileus) can be justified only in the treatment of severe and persistent psychotic symptoms.57
Bottom Line
Nonstandard use of antipsychotic monotherapy dosages beyond the approved FDA limit and combination antipsychotic therapy may be reasonable for select first-episode psychosis (FEP) patients. Strongly consider long-acting injectable antipsychotics in FEP to proactively combat the high relapse rate and more easily identify antipsychotic failure. Continue to use clozapine as second- or third-line therapy in FEP: Studies have not found that it is more efficacious than other antipsychotics for first-line use.
Related Resource
• Recovery After an Initial Schizophrenia Episode (RAISE) Project Early Treatment Program. National Institute of Mental Health. http://raiseetp.org.
Drug Brand Names
Aripiprazole • Abilify, Abilify Maintena
Chlorpromazine • Thorazine
Clozapine • Clozaril
Fluphenazine decanoate • Prolixin-D
Haloperidol • Haldol
Haloperidol decanoate • Haldol-D
Olanzapine • Zyprexa
Olanzapine pamoate • Zyprexa Relprevv
Paliperidone palmitate • Invega Sustenna
Quetiapine • Seroquel
Risperidone • Risperdal
Risperidone microspheres • Risperdal Consta
Disclosures
Dr. Gardner reports no financial relationships with any companies whose products are mentioned in this article or with manufacturers of competing products. Dr. Nasrallah is a consultant to Acadia, Alkermes, Lundbeck, Janssen, Merck, Otsuka, and Sunovion, and is a speaker for Alkermes, Lundbeck, Janssen, Otsuka, and Sunovion.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Lieberman JA, Koreen AR, Chakos M, et al. Factors influencing treatment response and outcome of first-episode schizophrenia: implications for understanding the pathophysiology of schizophrenia. J Clin Psychiatry. 1996;57(suppl 9):5-9.
4. Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439-1444.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis. An early stage of schizophrenia with distinct treatment needs. Current Psychiatry. 2015;14(5):32-34,36-40,42.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Texas Department of State Health Services. Texas Medication Algorithm Project (TMAP) Procedural Manual. Schizophrenia Treatment Algorithms. http://www.jpshealthnet.org/sites/default/files/ tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed June 11, 2015.
8. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
9. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
10. Hasan A, Falkai P, Wobrok T, et al; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
11. Scottish Intercollegiate Guidelines Network. SIGN 131: Management of schizophrenia. http://www.sign.ac.uk/ pdf/sign131.pdf. Published March 2013. Accessed June 11, 2015.
12. Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serous mental illness. BMC Psychiatry. 2013;13:340.
13. National Institute for Health and Care Excellence. NICE clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. https://www.nice.org. uk/guidance/cg178/resources/guidance-psychosis-and-schizophrenia-in-adults-treatment-and-management-pdf. Updated March 2014. Accessed June 16, 2015.
14. Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
15. McEvoy JP, Scheifler PL, Frances A. The expert consensus guideline series: treatment of schizophrenia. J Clin Psychiatry. 1999;60(suppl 11):3-80.
16. Marder SR, Essock SM, Miller AL, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
17. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
18. Fankhauser MP. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
19. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
20. Robinson DG, Schooler NR, John M, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172(3):237-248.
21. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
22. Fisher MD, Reilly K, Isenberg K, et al. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC Psychiatry. 2014;14(1):341.
23. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
24. John AP, Dragovic M. Antipsychotic polypharmacy is not associated with reduced dose of individual antipsychotics in schizophrenia. J Clin Psychopharmacol. 2015;35(2):193-195.
25. Nasrallah HA. Treatment-resistant schizophrenia. Current Psychiatry. http://www.currentpsychiatry.com/specialty-focus/schizophrenia-other-psychotic-disorders/article/ treatment-resistant-schizophrenia/9be7bba3713d4a4cd68aa 8c92b79e5b1.html. Accessed June 16, 2015.
26. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
27. Nasrallah HA, Smeltzer DJ. Contemporary diagnosis and management of the patient with schizophrenia. 2nd ed. Newton, PA: Handbooks in Health Care Co; 2011.
28. McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15-18.
29. Agid O, Foussias G, Remington G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin Pharmacother. 2010;11(14):2301-2317.
30. Kirschner M, Theodoridou A, Fusar-Poli P, et al. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode psychosis. Ther Adv Psychophamacol. 2013;3(2):89-99.
31. Heres S, Hamann J, Mendel R, et al. Identifying the profile of optimal candidates for antipsychotic depot therapy: A cluster analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1987-1993.
32. Heres S, Lambert M, Vauth R. Treatment of early episode in patents with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014;29(suppl 2):1409-1413.
33. Heres S, Schmitz FS, Leucht S, et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275-282.
34. Weiden PJ, Schooler NR, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
35. Heres S, Reichhart T, Hamann J, et al. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301.
36. Kane JM, Rifkin A, Quitkin F, et al. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39(1):70-73.
37. Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in a community care after first hospitalization due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224.
38. Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19(suppl 5):5-14.
39. Malla A, Binder C, Chue P. Comparison of long-acting injectable risperidone and oral novel antipsychotic drugs for treatment in early phase of schizophrenia spectrum psychosis. Proceedings of the 61st Annual Convention Society of Biological Psychiatry; Toronto, Canada; 2006.
40. Lasser RA, Bossie CA, Zhu Y, et al. Long-acting risperidone in young adults with early schizophrenia or schizoaffective illness. Ann Clin Psychiatry. 2007;19(2):65-71.
41. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
42. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
43. Kim B, Lee SH, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231-1235.
44. Weiden PJ, Schooler NJ, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
45. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011;132(1):35-41.
46. Napryeyenko O, Burba B, Martinez G, et al. Risperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measures. J Clin Psychopharmacol. 2010;30(2):200-202.
47. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609.
48. Dubois V, Megens J, Mertens C, et al. Long-acting risperidone in early-episode schizophrenia. Acta Psychiatrica Belgica. 2011;111(1):9-21.
49. ClinicalTrials.gov. Oral risperidone versus injectable paliperidone palmitate for treating first-episode schizophrenia. https://clinicaltrials.gov/ct2/show/ NCT01451736. Accessed June 16, 2015.
50. ClinicalTrials.gov. Brain myelination effects of paliperidone palmitate versus oral risperidone in first episode schizophrenia. https://clinicaltrials.gov/ct2/ show/NCT01458379. Accessed June 16, 2015.
51. ClinicalTrials.gov. Effects of paliperidone palmitate versus oral antipsychotics on clinical outcomes and MRI measures. https://clinicaltrials.gov/ct2/show/NCT01359293. Accessed June 16, 2016.
52. U.S. Food and Drug Administration. Drugs@FDA. http:// www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed January 11, 2015.
53. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
54. Nandra KS, Agius M. The difference between typical and atypical antipsychotics: the effects on neurogenesis. Psychiatr Danub. 2012;24(suppl 1):S95-S99.
55. Nasrallah HA. Haloperidol is clearly neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
56. Kane JM, Garcia-Ribora C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;52:S63-S67.
57. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
58. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
59. Woerner MG, Robinson DG, Alvir JMJ, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160(8):1514-1516.
60. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
61. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
62. Sanz-Fuentenebro J, Taboada D, Palomo T, et al. Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149(1-3):156-161.
63. Yang PD, Ji Z. The efficacy and related factors of clozapine on first-episode schizophrenia. Chin J Nerv Ment Dis. 1997;23:155-158.
64. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
65. Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170(2):146-151.
1. Perkins DO, Gu H, Boteva K, et al. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162(10):1785-1804.
2. Bradford DW, Perkins DO, Lieberman JA. Pharmacological management of first-episode schizophrenia and related nonaffective psychoses. Drugs. 2003;63(21):2265-2283.
3. Lieberman JA, Koreen AR, Chakos M, et al. Factors influencing treatment response and outcome of first-episode schizophrenia: implications for understanding the pathophysiology of schizophrenia. J Clin Psychiatry. 1996;57(suppl 9):5-9.
4. Agid O, Arenovich T, Sajeev G, et al. An algorithm-based approach to first-episode schizophrenia: response rates over 3 prospective antipsychotic trials with a retrospective data analysis. J Clin Psychiatry. 2011;72(11):1439-1444.
5. Gardner KN, Nasrallah HA. Managing first-episode psychosis. An early stage of schizophrenia with distinct treatment needs. Current Psychiatry. 2015;14(5):32-34,36-40,42.
6. Lehman AF, Lieberman JA, Dixon LB, et al; American Psychiatric Association; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(suppl 2):1-56.
7. Texas Department of State Health Services. Texas Medication Algorithm Project (TMAP) Procedural Manual. Schizophrenia Treatment Algorithms. http://www.jpshealthnet.org/sites/default/files/ tmapalgorithmforschizophrenia.pdf. Updated April 2008. Accessed June 11, 2015.
8. Buchanan RW, Kreyenbuhl J, Kelly DL, et al; Schizophrenia Patient Outcomes Research Team (PORT). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71-93.
9. Barnes TR; Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25(5):567-620.
10. Hasan A, Falkai P, Wobrok T, et al; WFSBP Task force on Treatment Guidelines for Schizophrenia. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2-44.
11. Scottish Intercollegiate Guidelines Network. SIGN 131: Management of schizophrenia. http://www.sign.ac.uk/ pdf/sign131.pdf. Published March 2013. Accessed June 11, 2015.
12. Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serous mental illness. BMC Psychiatry. 2013;13:340.
13. National Institute for Health and Care Excellence. NICE clinical guideline 178: Psychosis and schizophrenia in adults: treatment and management. https://www.nice.org. uk/guidance/cg178/resources/guidance-psychosis-and-schizophrenia-in-adults-treatment-and-management-pdf. Updated March 2014. Accessed June 16, 2015.
14. Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry. 2005;50(13 suppl 1):7S-57S.
15. McEvoy JP, Scheifler PL, Frances A. The expert consensus guideline series: treatment of schizophrenia. J Clin Psychiatry. 1999;60(suppl 11):3-80.
16. Marder SR, Essock SM, Miller AL, et al. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schizophr Bull. 2002;28(1):5-16.
17. Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157(4):514-520.
18. Fankhauser MP. Drug interactions with tobacco smoke: implications for patient care. Current Psychiatry. 2013;12(1):12-16.
19. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
20. Robinson DG, Schooler NR, John M, et al. Prescription practices in the treatment of first-episode schizophrenia spectrum disorders: data from the national RAISE-ETP study. Am J Psychiatry. 2015;172(3):237-248.
21. Correll CU, Rummel-Kluge C, Corves C, et al. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35(2):443-457.
22. Fisher MD, Reilly K, Isenberg K, et al. Antipsychotic patterns of use in patients with schizophrenia: polypharmacy versus monotherapy. BMC Psychiatry. 2014;14(1):341.
23. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383-399.
24. John AP, Dragovic M. Antipsychotic polypharmacy is not associated with reduced dose of individual antipsychotics in schizophrenia. J Clin Psychopharmacol. 2015;35(2):193-195.
25. Nasrallah HA. Treatment-resistant schizophrenia. Current Psychiatry. http://www.currentpsychiatry.com/specialty-focus/schizophrenia-other-psychotic-disorders/article/ treatment-resistant-schizophrenia/9be7bba3713d4a4cd68aa 8c92b79e5b1.html. Accessed June 16, 2015.
26. Alvarez-Jiménez M, Parker AG, Hetrick SE, et al. Preventing the second episode: a systematic review and meta-analysis of psychosocial and pharmacological trials in first-episode psychosis. Schizophr Bull. 2011;37(3):619-630.
27. Nasrallah HA, Smeltzer DJ. Contemporary diagnosis and management of the patient with schizophrenia. 2nd ed. Newton, PA: Handbooks in Health Care Co; 2011.
28. McEvoy JP. Risks versus benefits of different types of long-acting injectable antipsychotics. J Clin Psychiatry. 2006;67(suppl 5):15-18.
29. Agid O, Foussias G, Remington G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin Pharmacother. 2010;11(14):2301-2317.
30. Kirschner M, Theodoridou A, Fusar-Poli P, et al. Patients’ and clinicians’ attitude towards long-acting depot antipsychotics in subjects with a first episode psychosis. Ther Adv Psychophamacol. 2013;3(2):89-99.
31. Heres S, Hamann J, Mendel R, et al. Identifying the profile of optimal candidates for antipsychotic depot therapy: A cluster analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(8):1987-1993.
32. Heres S, Lambert M, Vauth R. Treatment of early episode in patents with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. 2014;29(suppl 2):1409-1413.
33. Heres S, Schmitz FS, Leucht S, et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275-282.
34. Weiden PJ, Schooler NR, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
35. Heres S, Reichhart T, Hamann J, et al. Psychiatrists’ attitude to antipsychotic depot treatment in patients with first-episode schizophrenia. Eur Psychiatry. 2011;26(5):297-301.
36. Kane JM, Rifkin A, Quitkin F, et al. Fluphenazine vs placebo in patients with remitted, acute first-episode schizophrenia. Arch Gen Psychiatry. 1982;39(1):70-73.
37. Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in a community care after first hospitalization due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224.
38. Parellada E, Andrezina R, Milanova V, et al. Patients in the early phases of schizophrenia and schizoaffective disorders effectively treated with risperidone long-acting injectable. J Psychopharmacol. 2005;19(suppl 5):5-14.
39. Malla A, Binder C, Chue P. Comparison of long-acting injectable risperidone and oral novel antipsychotic drugs for treatment in early phase of schizophrenia spectrum psychosis. Proceedings of the 61st Annual Convention Society of Biological Psychiatry; Toronto, Canada; 2006.
40. Lasser RA, Bossie CA, Zhu Y, et al. Long-acting risperidone in young adults with early schizophrenia or schizoaffective illness. Ann Clin Psychiatry. 2007;19(2):65-71.
41. Emsley R, Oosthuizen P, Koen L, et al. Remission in patients with first-episode schizophrenia receiving assured antipsychotic medication: a study with risperidone long-acting injection. Int Clin Psychopharmacol. 2008;23(6):325-331.
42. Emsley R, Oosthuizen P, Koen L, et al. Oral versus injectable antipsychotic treatment in early psychosis: post hoc comparison of two studies. Clin Ther. 2008;30(12):2378-2386.
43. Kim B, Lee SH, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231-1235.
44. Weiden PJ, Schooler NJ, Weedon JC, et al. A randomized controlled trial of long-acting injectable risperidone vs continuation on oral atypical antipsychotics for first-episode schizophrenia patients: initial adherence outcome. J Clin Psychiatry. 2009;70(10):1397-1406.
45. Bartzokis G, Lu PH, Amar CP, et al. Long acting injection versus oral risperidone in first-episode schizophrenia: differential impact on white matter myelination trajectory. Schizophr Res. 2011;132(1):35-41.
46. Napryeyenko O, Burba B, Martinez G, et al. Risperidone long-acting injectable in recent-onset schizophrenia examined with clinician and patient self-report measures. J Clin Psychopharmacol. 2010;30(2):200-202.
47. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609.
48. Dubois V, Megens J, Mertens C, et al. Long-acting risperidone in early-episode schizophrenia. Acta Psychiatrica Belgica. 2011;111(1):9-21.
49. ClinicalTrials.gov. Oral risperidone versus injectable paliperidone palmitate for treating first-episode schizophrenia. https://clinicaltrials.gov/ct2/show/ NCT01451736. Accessed June 16, 2015.
50. ClinicalTrials.gov. Brain myelination effects of paliperidone palmitate versus oral risperidone in first episode schizophrenia. https://clinicaltrials.gov/ct2/ show/NCT01458379. Accessed June 16, 2015.
51. ClinicalTrials.gov. Effects of paliperidone palmitate versus oral antipsychotics on clinical outcomes and MRI measures. https://clinicaltrials.gov/ct2/show/NCT01359293. Accessed June 16, 2016.
52. U.S. Food and Drug Administration. Drugs@FDA. http:// www.accessdata.fda.gov/scripts/cder/drugsatfda. Accessed January 11, 2015.
53. Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46; quiz 47-48.
54. Nandra KS, Agius M. The difference between typical and atypical antipsychotics: the effects on neurogenesis. Psychiatr Danub. 2012;24(suppl 1):S95-S99.
55. Nasrallah HA. Haloperidol is clearly neurotoxic. Should it be banned? Current Psychiatry. 2013;12(7):7-8.
56. Kane JM, Garcia-Ribora C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry. 2009;52:S63-S67.
57. Clozaril [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2014.
58. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
59. Woerner MG, Robinson DG, Alvir JMJ, et al. Clozapine as a first treatment for schizophrenia. Am J Psychiatry. 2003;160(8):1514-1516.
60. Lieberman JA, Phillips M, Gu H, et al. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs chlorpromazine. Neuropsychopharmacology. 2003;28(5):995-1003.
61. Girgis RR, Phillips MR, Li X, et al. Clozapine v. chlorpromazine in treatment-naive, first-episode schizophrenia: 9-year outcomes of a randomised clinical trial. Br J Psychiatry. 2011;199(4):281-288.
62. Sanz-Fuentenebro J, Taboada D, Palomo T, et al. Randomized trial of clozapine vs. risperidone in treatment-naïve first-episode schizophrenia: results after one year. Schizophr Res. 2013;149(1-3):156-161.
63. Yang PD, Ji Z. The efficacy and related factors of clozapine on first-episode schizophrenia. Chin J Nerv Ment Dis. 1997;23:155-158.
64. Agid O, Schulze L, Arenovich T, et al. Antipsychotic response in first-episode schizophrenia: efficacy of high doses and switching. Eur Neuropsychopharmacol. 2013;23(9):1017-1022.
65. Remington G, Agid O, Foussias G, et al. Clozapine’s role in the treatment of first-episode schizophrenia. Am J Psychiatry. 2013;170(2):146-151.
Reducing medical comorbidity and mortality in severe mental illness
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
People with serious mental illness (SMI) have a life expectancy that is 25 years less than the general population, according to the Centers for Disease Control and Prevention.1 This disparity is partially a consequence of the lack of primary and preventive medical care for those with psychiatric illness. Decades of research have shown that people with SMI experience higher medical morbidity and mortality in addition to facing the stigma of mental illness.
This article aims to advance the idea that longitudinal “cross education” between primary care providers (PCPs) and behavioral health providers (BHPs) is essential in addressing this problem. BHPs include psychiatry clinics, which often are part of a university or large health systems; county-based community mental health programs; and independent mental health clinics that contract with public and private health plans to provide mental health services.
Although suicide and injury account for 40% of the excess mortality in schizophrenia, 60% can be attributed to cardiovascular disease, diabetes, respiratory diseases, and infection.2 Patients with SMI have 2 to 3 times the risk of diabetes, dyslipidemia, hypertension, and obesity.3,4 Furthermore, those with SMI consume more than one-third of tobacco products,5 and 50% to 80% of people with SMI smoke tobacco, an important reversible risk factor for cardiovascular disease.
Figure 1 shows that people with SMI are at higher risk of dying from a chronic medical condition, such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, and hepatitis C6-8—many of which can be managed by primary and preventive medical interventions. These and other conditions often are not diagnosed or effectively managed in patients with SMI.
The high prevalence of metabolic syndrome and tobacco dependence among people with SMI accelerates development of cardiovascular disease, as shown by several studies. Bobes et al9 found that the prevalence of metabolic syndrome and cardiovascular risk among patients with SMI is similar to what is found in the general population at 10 to 15 years of greater age. Osborn et al10 demonstrated that people with SMI age 18 to 49 had a higher relative risk of death from coronary heart disease, stroke, and lung cancer than age-matched controls (Figure 2).
It can be said, therefore, that patients with SMI seem to “age” and die prematurely. To reduce this disparity, primary and preventive medical care—especially for cardiovascular disease—must be delivered earlier in life for those with SMI.
Iatrogenic causes of morbidity
Many psychiatric medications, especially second-generation antipsychotics (SGAs), could exacerbate cardiovascular and metabolic conditions by increasing the risk of weight gain, insulin resistance, and dyslipidemia. Antipsychotics that generally are considered to be more effective for refractory psychotic illness (eg, clozapine and olanzapine) are associated with the highest risk of metabolic syndrome. Simon et al11 found a dose-response relationship between olanzapine and clozapine serum concentrations and worsening metabolic outcomes. Valproic acid also can cause significant weight gain and could require monitoring similar to what is done with to SGAs, although there has been less clinical and research attention to this mood stabilizer.
The American Diabetes Association et al12 have published guidelines on monitoring antipsychotic-induced obesity and diabetes, but adoption of these guidelines has been slow. Mackin et al13 found that providers are slow to recognize the elevated rate of obesity and dyslipidemia among psychiatric patients, possibly because of “an alarmingly poor rate of monitoring of metabolic parameters.”
Treating adverse metabolic outcomes also seems to lag behind. The same study13 found that physical health parameters among psychiatric patients continue to become worse even when appropriate health care professionals were notified. Rates of nontreatment for diabetes, dyslipidemia, and hypertension were 30%, 60%, and 88% respectively, according to Nasrallah et al.14
Randomized controlled studies have shown that obesity and metabolic syndrome can be effectively managed using lifestyle and pharmacotherapeutic approaches,15,16 but more research is needed to test long-term outcomes and how to best incorporate these interventions. Newcomer et al17 found that gradually switching an antipsychotic with high risk of metabolic adverse effects to one with lower risk could reduce adverse metabolic outcomes; however, some patients returned to their prior antipsychotic because other medications did not effectively treat their schizophrenia symptoms. Therefore, physicians must pay careful attention to the trade-off between benefits and risks of antipsychotics and make treatment decisions on an individual basis.
Barriers to medical care
Research has demonstrated that patients with SMI receive less screening and fewer preventive medical services, especially blood pressure monitoring, vaccinations, mammography, lipid monitoring, and osteoporosis screening, compared with the general population (Table).18 Some barriers to preventive services could exist because of demographic factors and medical insurance coverage19 or medical providers’ discomfort with symptoms of SMI,20 although Mitchell et al21 found that disparities in mammography screening could not be explained by the presence of emotional distress in women with SMI.
DiMatteo et al22 reported that patients with SMI are 3 times more likely to be noncompliant with medical treatment. These patients also are less likely to receive sec ondary preventive medical care and invasive medical procedures. Those with SMI who experience acute myocardial infarction are less likely to receive drug therapy, such as a thrombolytic, aspirin, beta blocker, or angiotensin-converting enzyme inhibitor.23 They also are less likely to receive invasive cardiovascular procedures, including cardiac catheterization, angioplasty, and coronary artery bypass grafting.24
Therefore, not only are patients with SMI less likely to receive preventive care, they are also less likely to receive potentially lifesaving treatments for SMI. Because those with SMI might not be able to advocate for themselves in these matters, psychiatric clinicians can improve their patients’ lives by advocating for appropriate medical care despite multiple barriers.
Bridging the gap: Managing mental health in primary care
Research from the 1970s and 1980s demonstrated that most persons who sought help for depression or anxiety received treatment from their PCP, many of whom felt limited by their lack of behavioral health training. Moreover, many patients failed to receive a psychiatric diagnosis or adequate treatment, despite efforts to educate primary care physicians on appropriate diagnosis and treatment of mental illness.
Katon et al25 at the University of Washington developed the collaborative care model in the early 1990s to help improve treatment of depression in primary care settings. This model involved:
• case load review by psychiatrists
• use of nurses and other support staff to help monitor patients’ adherence and treatment response
• use of standardized tools such as the Patient Health Questionnaire to monitor symptoms
• enhancement of patient education with pamphlets or classes.
Studies evaluating the success of collaborative care models found overall improved outcomes, making it the only evidence-based model for integration of behavioral health and primary care.26 As a result, the collaborative care model has been implemented across the United States in primary care clinics and specialty care settings, such as obstetrics and gynecology.27
Regrettably, access to primary care has been hampered by:
• population growth
• a shortage of PCPs
• enrollment of a flood of new patients into the health care marketplace as a result of mandates of the Affordable Care Act (ACA).
In many settings, a psychiatrist might be the patient’s only consistent care provider, and could be thought of as a “primary care psychiatrist.”
To resolve this predicament, mental health professionals need to recognize the unique medical conditions faced by people with SMI, and also might need to provide treatment of common medical conditions, either directly or through collaborative arrangements. Psychiatrists who are capable of managing core medical issues likely will witness improved psychiatric and overall health outcomes in their patients. Consequently, psychiatrists and mental health professionals are increasingly called on to be advocates to improve access to medical services in patients with SMI and to participate in health systems reform.
Managing medical conditions in mental health settings
Although traditional collaborative care involves mental health providers working at primary care sites, other models have emerged that manage chronic disease in behavioral health settings. Federally funded grants for primary behavioral health care integration have allowed community mental health centers to partner with federally qualified health centers to provide on-site primary care services.28
In these models, care managers in mental health clinics:
• link patients to primary care services
• encourage lifestyle changes to improve their overall health
• identify and overcome barriers to receiving care
• track clinical outcomes in a registry format.
Currently, 126 mental health sites in the United States have received these grants and are working toward greater integration of primary care.
In addition, the ACA provided funding for “health homes” in non-primary care settings, which includes SMI. These health homes cannot provide direct primary care, but can deliver comprehensive care management, care coordination, health promotion, comprehensive transitional care services between facilities, individual and family support, and referral to community social support services. In these health homes, a PCP can act as a consultant to help establish priorities for disease management and improving health status.29 The PCP consultant also can support psychiatric staff and collaborate with providers who want to provide some direct care of medical conditions.30
Last, some behavioral health sites are choosing to apply for Federally Qualified Health Clinic status or add primary care services to their clinics, with the hope that sustainable funding will become available. Without additional funding to cover the limited reimbursement provided by public payers, such as Medicaid and Medicare, these models might be unsustainable. Current innovations in health care funding reform hopefully will offer solutions for sites to provide medical care in the natural “medical home” of the SMI population.
Bottom Line
Psychiatric providers are in a favorable position to develop and oversee a partnership with primary care physicians with the goal of addressing significant and often lethal health disparities among those with mental illness. Psychiatric providers must use evidence-based practices that include assessment and prevention of cardiopulmonary, metabolic, infectious, and oncologic disorders. True primary care–behavioral health integration must include longitudinal “cross education” and changes in health care policy, with an emphasis on decreasing morbidity and mortality in psychiatric patients.
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
1. Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42.
2. Parks J, Svendsen D, Singer P, et al, eds. Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council; 2006.
3. Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA. 2007;298(15):1794-1796.
4. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trails of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19-32.
5. Compton MT, Daumit GL, Druss BG. Cigarette smoking and overweight/obesity among individuals with serious mental illnesses: a preventive perspective. Harv Rev Psychiatry. 2006;14(2):212-222.
6. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64(10):1123-1131.
7. Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156.
8. Carney CP, Jones L, Woolson RF. Medical comorbidity in women and men with schizophrenia: a population-based study. J Gen Intern Med. 2006;21(11):1133-1137.
9. Bobes J, Arango C, Aranda P, et al; CLAMORS Study Collaborative Group. Cardiovascular and metabolic risk in outpatients with schizoaffective disorder treated with antipsychotics; results from the CLAMORS study. Eur Psychiatry. 2012;27(4):267-274.
10. Osborn DP, Levy G, Nazareth I, et al. Relative risk of cardiovascular and cancer mortality in people with severe mental illness from the United Kingdom’s General Practice Research Database [Erratum in: Arch Gen Psychiatry. 2007;64(6):736]. Arch Gen Psychiatry. 2007;64(2):242-249.
11. Simon V, van Winkel R, De Hert M. Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry. 2009;70(7):1041-1050.
12. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
13. Mackin P, Bishop DR, Watkinson HM. A prospective study of monitoring practices for metabolic disease in antipsychotic-treated community psychiatric patients. BMC Psychiatry. 2007;7:28.
14. Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86(1-3):15-22.
15. Alvarez-Jiménez M, Hetrick SE, González-Blanch C, et al. Non-pharmacological management of antipsychotic-induced weight gain: systematic review and meta-analysis of randomized controlled trials. Br J Psychiatry. 2008; 193(2):101-107.
16. Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medication used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35(7):1520-1530.
17. Newcomer JW, Weiden PJ, Buchanan RW. Switching antipsychotic medications to reduce adverse event burden in schizophrenia: establishing evidence-based practice. J Clin Psychiatry. 2013;74(11):1108-1120.
18. Lord O, Malone D, Mitchell AJ. Receipt of preventive medical care and medical screening for patients with mental illness: a comparative analysis. Gen Hosp Psychiatry. 2010;32(5):519-543.
19. Xiong GL, Iosif AM, Bermudes RA, et al. Preventive medical services use among community mental health patients with severe mental illness: the influence of gender and insurance coverage. Prim Care Companion J Clin Psychiatry. 2010;12(5). doi: 10.4088/PCC.09m00927gre.
20. Daub S. Turning toward treating the seriously mentally ill in primary care. Fam Syst Health. 2014;32(1):12-13.
21. Mitchell A, Pereira IE, Yadegarfar M, et al. Breast cancer screening in women with mental illness: comparative meta-analysis of mammography uptake. Br J Psychiatry. 2014;205(6):428-435.
22. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
23. Druss BG, Bradford WD, Rosenheck RA, et al. Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry. 2001;58(6):565-572.
24. Druss BG, Bradford DW, Rosenheck RA, et al. Mental disorders and use of cardiovascular procedures after myocardial infarction. JAMA. 2000;283(4):506-511.
25. Katon W, Unützer J, Wells K, et al. Collaborative depression care: history, evolution and ways to enhance dissemination and sustainability. Gen Hosp Psychiatry. 2010;32(5):456-464.
26. Archer J, Bower P, Gilbody S, et al. Collaborative care for depression and anxiety problems. Cochrane Database Syst Rev. 2012;10:CD006525.
27. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40.
28. Substance Abuse and Mental Health Services Administration. Request for Applications (RFA) No. SM- 09-011. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009.
29. Parks J. Behavioral health homes. In: Integrated care: working at the interface of primary care and behavioral health. Raney LE, ed. Arlington, VA: American Psychiatric Publishing; 2015:195.
30. Raney L. Integrated care: the evolving role of psychiatry in the era of health care reform. Psychiatr Serv. 2013;64(11):1076-1078.
The use of aripiprazole in the management of bipolar disorder during pregnancy
"This patient had presented 2-weeks postpartum in a manic state with psycotic features. She was screened by Ob-Gyn who collaborated with her care while she was admitted to the psychiatric inpatient unit. Patient had been non-compliant with prescribed medications prior to admission and she was started on aripiprazole from day one and the dose was tapered up to 15 mg BID by day 5. Patient's manic symptoms improved slowly as the days progressed by day 8 psychotic symptoms started to subside. As delivery was imminent, patient was transferred to Ob-Gyn service. She delivered a healthy but premature child via csection on day 12. Child did not exhibit any gross or anatomic malformations. She was continued on aripiprazole 15 mg BID after discharge and was seen weeks later in outpatient psychiatry."
Read more from the Poster Abstracts from the 2015 APA Annual Meeting
"This patient had presented 2-weeks postpartum in a manic state with psycotic features. She was screened by Ob-Gyn who collaborated with her care while she was admitted to the psychiatric inpatient unit. Patient had been non-compliant with prescribed medications prior to admission and she was started on aripiprazole from day one and the dose was tapered up to 15 mg BID by day 5. Patient's manic symptoms improved slowly as the days progressed by day 8 psychotic symptoms started to subside. As delivery was imminent, patient was transferred to Ob-Gyn service. She delivered a healthy but premature child via csection on day 12. Child did not exhibit any gross or anatomic malformations. She was continued on aripiprazole 15 mg BID after discharge and was seen weeks later in outpatient psychiatry."
Read more from the Poster Abstracts from the 2015 APA Annual Meeting
"This patient had presented 2-weeks postpartum in a manic state with psycotic features. She was screened by Ob-Gyn who collaborated with her care while she was admitted to the psychiatric inpatient unit. Patient had been non-compliant with prescribed medications prior to admission and she was started on aripiprazole from day one and the dose was tapered up to 15 mg BID by day 5. Patient's manic symptoms improved slowly as the days progressed by day 8 psychotic symptoms started to subside. As delivery was imminent, patient was transferred to Ob-Gyn service. She delivered a healthy but premature child via csection on day 12. Child did not exhibit any gross or anatomic malformations. She was continued on aripiprazole 15 mg BID after discharge and was seen weeks later in outpatient psychiatry."
Read more from the Poster Abstracts from the 2015 APA Annual Meeting
College students with depressive symptoms with and without fatigue: Differences in functioning, suicidality, anxiety, and depressive severity
Nyer et al examined whether fatigue was associated with greater symptomatic burden and functional impairment in 287 college students with depressive symptoms using data from the self-report Beck Depression Inventory (BDI). Students endorsing significant symptoms of depression (BDI score ≥13) were grouped into 3 levels: no fatigue, mild fatigue, or moderate/severe fatigue. Researchers compared the 3 levels of fatigue across a battery of psychiatric and functional outcome measures.
The study found that depressed college students with symptoms of fatigue demonstrated functional impairment and symptomatic burden that worsened with increasing levels of fatigue. The authors call for more attention to assessing and treating symptoms of fatigue within this population.
Nyer et al examined whether fatigue was associated with greater symptomatic burden and functional impairment in 287 college students with depressive symptoms using data from the self-report Beck Depression Inventory (BDI). Students endorsing significant symptoms of depression (BDI score ≥13) were grouped into 3 levels: no fatigue, mild fatigue, or moderate/severe fatigue. Researchers compared the 3 levels of fatigue across a battery of psychiatric and functional outcome measures.
The study found that depressed college students with symptoms of fatigue demonstrated functional impairment and symptomatic burden that worsened with increasing levels of fatigue. The authors call for more attention to assessing and treating symptoms of fatigue within this population.
Nyer et al examined whether fatigue was associated with greater symptomatic burden and functional impairment in 287 college students with depressive symptoms using data from the self-report Beck Depression Inventory (BDI). Students endorsing significant symptoms of depression (BDI score ≥13) were grouped into 3 levels: no fatigue, mild fatigue, or moderate/severe fatigue. Researchers compared the 3 levels of fatigue across a battery of psychiatric and functional outcome measures.
The study found that depressed college students with symptoms of fatigue demonstrated functional impairment and symptomatic burden that worsened with increasing levels of fatigue. The authors call for more attention to assessing and treating symptoms of fatigue within this population.